Abstract
BACKGROUND:
Swedish National Quality Registers (NQRs) play an important role in collecting large amounts of diagnosis-specific data, symptoms, and treatments. The subset of data, Parkinson's Registry, has been in use for more than 20 years and represents all counties and hospitals in Sweden where neurological care is provided.
OBJECTIVE:
To study the differences between genders regarding diagnostic tools, pharmacological interventions, and self-reported symptoms in patients with symptoms originating from basal ganglia disease, either idiopathic or secondary Parkinsonism (PD).
METHODS:
PD-diagnosed patients from a mix of urban and rural locations were chosen from the NQR and sorted by gender. Self-reported, first-experienced PD-related symptoms defined the debut point of PD.
RESULTS:
In all, data from 1,217 patients were analyzed: 502 (41%) females/715 (59%) males. A total of 493 imaging investigations were performed, where of 239 (48% females/52% males) had a CT scan performed, 120 (24% females/29% males) had a dopamine transporter scans, and 134 (23% females/26% males) had a magnetic resonance imaging performed (Fisher's exact test, P = 0.19). The average time in years from symptom onset to start of first treatment, and from first to second added treatment was 2;7/2;9 (females) and 5;1/5;2 (males). Nonmotor symptoms were more prominent among males, especially in memory and gastrointestinal domains, including drooling and obstipation. Significantly more sexual problems were reported from males; 26% versus 7% (Fisher's exact test, P < 0.0001).
CONCLUSIONS:
Differences between genders were identified in this study. Sexual problems and cognitive decline were more frequent among males. More advanced diagnostic imaging techniques were performed among males. The time point for a second added medication was earlier for males than females.
Keywords: Disease progression, drug therapy, gender, Parkinson disease, quality registers
Introduction
Over the past decades, knowledge of the natural history and progression of Parkinson's disease (PD) and different forms of Parkinsonism has increased. Huge efforts have been made to recognize the earliest, premotor, and phases of the disease. Incidence and prevalence rates differ somewhat between studies,[1] to some degree dependent on insufficient systematic registration. In the era of digitization, it has been possible to expand the self-reported experiences of disease expressions.
The incidence of PD rapidly increases with age, with only about 4% of cases being below 50 years of age. Many studies indicate a lower prevalence and incidence rate in women than in men, with age-standardized male: female incidence ratios ranging from 1.3 to 2.0, and in 2016 systematic worldwide, epidemiological studies reported in an M: F age-standardized prevalence of 1.5.[2,3]
Males seem to be diagnosed with PD 2–3 years earlier than females.[4] Differences in disability and quality-of-life (QoL) reporting have been noted, with women showing greater disability and reduced QoL. Gender differences have also been shown in the response to treatment of PD, for example, women have greater levodopa bioavailability and a lower mean body mass index.[5]
In earlier stage of the disease it has been shown a 90% prevalence of hypokinetic dysarthria.[6] During the progression of the disease, due to less control of articulatory organs, such as the tongue, jaw, and lips, the quality of speech signals deteriorates. Periodic medical evaluations are very important for PD patients. However, mapping information of the dysarthria level into the neurological state of patients and vice versa would be of great value to register and take into consideration during the course of the disease. Nothing seems to be known of gender differences in this aspect but could easily be added to larger nation-based registries.
Survival in PD is reduced by approximately 5%.[7] In this systematic review, 17/21 studies reported male gender as an independent risk factor. However, no differences in mortality ratios across gender or age group were seen in a long-term follow-up of the DATA-TOP study.[8] Changes of mortality rates between genders over time were presented in a recent study from Finland. A decrease in mortality rate among males but not females was shown over the last decade. Pneumonia was the most common immediate cause of death in both genders.[9]
General aspects of differences between genders
It is well known that in the general population in industrialized societies, males have a shorter average length of life than females. However, over their lifespan, women report poorer health than men. Differences in biological and acquired risks have been discussed as causes for, as well as variations of, symptoms or conditions in different phases of the lifecycle.[10]
In an early paper by Macintyre from 1996,[11] two large British population surveys were examined. These revealed a larger complexity than earlier studies had shown in the description of population health and differences between genders. The studies reflected a consistency of reporting more illness, poorer self-evaluation of health, and higher rates of psychosocial malaise among females than males.
The complexity of recent population studies in the field of gender differences seems to make them more difficult to understand than older studies. Over time, life expectancy, as well as differences in health, has changed.
If we are to make progress toward a better understanding of whether social, psychological, or biological features dominate experienced differences between genders in health aspects, it is important to pay attention to the social and historical context of the observations. Moreover, it is also important to take a more differentiated age-specific and condition-specific view of health' when examining differences between genders.[12]
The national quality registers in Sweden
The National Quality Registers[13] have been used progressively in Sweden for several years and contain individualized data concerning self-reported problems, medical interventions, outcomes, and experiences during treatment (Patient Reported Outcomes/Experiences Measures referred to as “PROM” and “PREM”). The registers are monitored annually and approved for financial support by an executive committee.
The individually based data in the registers also provide the opportunity to evaluate compliance to the “Swedish National Treatment Guidelines.”[14]
Swedish Neuro Registries are represented in all counties and all hospitals where neurological care is provided and have subgroups for eight different neurological diagnoses, of which Parkinsonian disorders is one.
Aims of the study
This study was performed with the aim to identify any differences between genders in the time point of start of pharmacological treatments, type of medication, utilization and choice of diagnostic tools, and differences in self-reported symptoms in Parkinsonian disorders.
Methods
The registrations used in the study were all performed by professional staff working with neurological conditions where movement disorders are at least a part of the daily work. All registrars have a personal log in/identification code and are offered education annually.
Inclusion criteria
All participants in the Swedish Neuro Registries whohave given their written consent to participate. The permission is a written part of each patient's protocol in the register. In addition, the heads of each site have given written informed consent to the study group for data extraction.
Exclusion criteria
Patients diagnosed with atypical Parkinsonism and also data from those who only have given permission for data collection but not extraction from the Parkinson's registry.
Data were extracted using the built-in report generator within the platform. Each patient was assigned a unique computer-generated anonymous identification number to facilitate merging the data from different sections/tables of the registries. To maximize the most representative samples, urban and rural living individuals were collected, with a mix from the University Hospitals of Örebro and Malmö. Jonkoping, Eksjö and Kristianstad represented county hospitals.
The following variables were extracted: age, gender, diagnosis, time point for first Parkinsonian symptom, chosen imaging technique (computed tomography, magnetic resonance imaging (MRI), and/or dopamine transporter scan), the questionnaires EQ5D, Clinical Impression of Severity Index for Parkinson's Disease (CISI-PD), pharmacological treatments, and nonmotor symptoms (NMS).
Questionnaires
NMS Questionnaires (NMSQ) were registered using the NMSQ established in 2007. The questions were answered by yes or no (exist/not exist).[15,16] NMS data were extracted at two time points: NMS reported within 5 years from the appearance of first reported symptom, in this study reported as early symptoms (n = 84; 40/44 [f/m]); and NMS reported later than 5 years from first appearance, here called late symptoms (n = 287; 127/160 [f/m]). The partition is purely semantic and due to the sample sizes. It is not to be confused with early and late stages of PD, which most often refers to the appearance of motor complications.
Clinical impression of severity index for Parkinson's disease
Motor signs, disability, motor complications, and cognitive impairment summarized using clinical assessment are all combined in the CISI-PD.[17]
EQ5D-3L and EQ5D-5L
EQ5D, one of the generic instruments for QoL, was developed for the estimation of the state of health by the EuroQol Group in 1996. The items included are usual activities, self-care, anxiety-depression, mobility, and pain/discomfort. Furthermore, a visual analog scale ranging from 0 (worst) to 100 (best imaginable health) is a part of the instrument.[18] In the evolution of the registries, two different versions of EQ5D were used: EQ5D-3 L, with three response categories, and EQ5D-5 L, with five response categories on each item.
In the study, EQ5D-5 L was condensed to EQ5D-3 L for the actual extraction of reports. Hence, “no symptoms” and “slight symptoms” (one or two), “severe symptoms,” and “unable to perform” (three or four) were merged to one or three, respectively, in this study.
Statistics
Data were presented as numbers, percentages, means, standard errors (for continuous variables), and median with percentiles (for ordered categorical variables) where suitable.
Chi-Square and Fisher's exact test were used to analyze comparisons between categorical variables. A significance level ≤5% was considered statistically significant. However, the statistical significance must always be valued in the light of clinical impact. The statistical analyses utilized SAS® ver. 9.41, Copyright ©2002–2012 by SAS Institute Inc., Cary, NC, USA.
Ethics
The Swedish Neuro Registries are approved by the Swedish Ethical Committee.
This study was approved by the Ethics Committees at the University of Stockholm Department 3 medicine 2019-02-0620 19-00577, Protocol: 2019/3:2. The study followed the Declaration of Helsinki of ethical principles for medical research of humans. Clinical Trials.gov identifier is NCT04713306.
Results
Only statistically significant differences were printed out in the text.
Data extraction
In May 2020, about 6,500 patients (39% female, 61% male) of the estimated 22,000 Swedish PD patients were registered in the PD registry in the form of “Active patients with given consent to participate”. The total number of analyzed data was 1,217 individuals, 502 females/716 males. These included 697 (57%) from the University Hospitals of Malmö and Örebro and 520 (43%) from the General Hospitals of Kristianstad, Jönköping, and Eksjö.
Basic characteristics are illustrated in Table 1. A significant number of dropouts were registered. Very few individuals had completed data input. To understand this, it is important to recognize the circumstances for registration. In parallel to input of data, there is a mandatory journal entry. So far, there is no digital transmission of data and only chosen selections of data were registered for each patient.
Table 1.
Basic characteristics of the study population, ages at first experienced symptom of parkinsonism, time from onset of first parkinsonism-symptom to start of first treatment, and imaging techniques recorded
| Age at inclusion to registry (years) | 25th percentile | Median | 75th percentile | Mean (95% CI) |
|---|---|---|---|---|
| All patients (n=1,215) | 69 | 74 | 80 | 74 (73.3-74.3) |
| Females (n=502) | 70 | 75 | 80 | 74 (73.7-75.3) |
| Males (n=715) | 68 | 74 | 79 | 73 (72.7-74.0) |
| Age at data outtake from registry | ||||
| All patients (n=1,217) | 72 | 78 | 83 | 77 (76.4-77.3) |
| Females | 73 | 79 | 83 | 78 (76.9-78.4) |
| Males | 71 | 77 | 82 | 76 (75.6-76.9) |
| Age at onset of first symptom | 61 | 67 | 73 | 66 (65.8-66.9) |
| Females | 62 | 68 | 74 | 67 (66.2-67.9) |
| Males | 60 | 67 | 73 | 66 (65.1-66.6) |
| Time from onset to start of 1st pharmacological treatment (years) | 1.0 | 1.9 | 3.5 | 2.8 (2.6-3.1) |
| Females | 1.0 | 1.8 | 3.4 | 2.8 |
| Males | 0.9 | 2.0 | 3.6 | 2.9 |
|
| ||||
| Imaging (no information for 729 patients) | Total registered (n) | Females (n) (row %/column %) | Males (n) (row %/column %) | |
|
| ||||
| CT scans | 239 | 115 (48/53) | 124 (52/45) | |
| MRIs | 120 | 50 (42/23) | 70 (58/25) | |
| DaT scans | 134 | 52 (39/24) | 82 (61/30) | |
| Total | 483 | 217 | 276 | |
CI: Confidence interval, CT: Computerized tomography, MRI: Magnetic resonance imaging, DaT: Dopamine transporter scans, (No significant differences were identified between groups)
Within 5 years from first reported Parkinsonian symptom, 77/91 (f/m) EQ5D-3 L measures were performed among the studied PD patients.
Of 168 patients, 70 females and 92 males experienced some problems with their daily activities at first measure (43%/56%, respectively), and out of those, 28 patients (12/16, 43%/57%, f/m) at the second measure reported that they could not, or only with great difficulties, perform their daily activities.
Pharmaceutical treatments
Five hundred and fourteen (222/292; f/m) patients were included.
The formulations of levodopa are traditionally divided into fast acting or immediate release (Madopar Quick®) and medium fast acting Levodopa (Madopar®). This latter was by far the most used anti-Parkinson's drug treatment among both genders (62%/62%), as well as dopamine agonists (18, 7%/18, 3%) out of 393/300 (n = f/m).
Out of the 514 included patients, a lead time of 2 years and 8 months versus 2 years and 11 months from first reported Parkinsonian symptom to onset of the first pharmacological treatment was reported [Figure 1]. The difference is not statistically significant. The mean time from the first prescribed drug to the second prescribed drug differed between genders 29, 4/24, 7 months (mean; f/m). The median time was 17, 1/13, 1 (f/m); 25%/75% percentiles: 4, 1/41, 4 months (f) and 4, 2/33, 4 months (m). This was not statistically significant (P = 0, 16; t-test).
Figure 1.
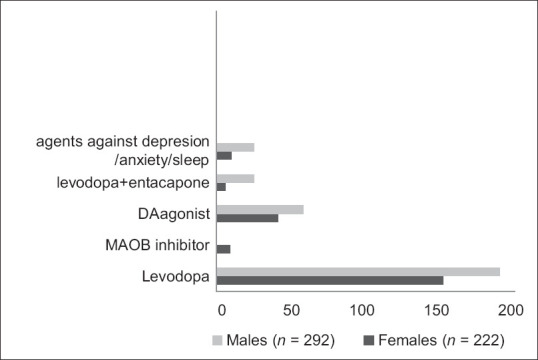
First reported prescribed anti-Parkinson's medication. (Explanations: levodopa + entacapone = levodopa + the entacapone component added for a longer duration of each tablet. Levodopa = levodopa medium fast release, DAagonist = dopaminergic drugs, MAO-B inhibitor = monoaminoxidase inhibitors)
A second treatment was added later for females than for males: Five years and 1 month versus 5 years and 3 months from time point of first reported symptom.
Entacapone was more often prescribed to males than females; 9%/5%, respectively. More differentiated prescriptions in terms of fast acting/slow acting formulas of levodopa were also seen in males.
Advanced treatments
Thirty-one (19/12; f/m) patients were included.
Differences were seen between genders in the use of subcutaneous, intrajejunal, and intracerebral therapy forms; prescriptions are illustrated in Table 2.
Table 2.
Prescriptions of apomorphine pump, levodopa/carbidopa intestinal gel infusion, and deep brain stimulation among 12 males and 19 females
| Apomorphine pump | LCI | DBS | |
|---|---|---|---|
| Males | 1 | 7 | 4 |
| Females | 10 | 6 | 3 |
| Total | 11 | 13 | 7 |
Statistically significant difference (Fischer's exact test, P=0.04) was seen in the distribution between genders of prescribed apomorphine pump therapy. LCI: Levodopa/carbidopa intestinal, DBS: Deep brain stimulation
Nonmotor symptoms
Anxiety, depression, and psychosis
The reported frequency of these neuropsychiatric symptoms are illustrated in [Table 3].
Table 3.
Neuropsychiatric symptoms reported in the register
| sleep | anxiety | depression | dementia | Pseudohallucinations* | |
|---|---|---|---|---|---|
| Males | 19 | 8 | 39 | 18 | 12 |
| Females | 12 | 6 | 32 | 7 | 6 |
| Total | 31 | 14 | 71 | 25 | 18 |
*pseudohallucinations=Mostly visual experiences with retained insight of reality
EQ5D-3L
Seven hundred and forty two (381/361; f/m) patients were included.
EQ5D-3 L was measured at different time points after the first experienced PD symptom. The first measure was performed 5 years after the first reported symptom of PD. The median in months: 64/65 (females/males) 16 with (25th/75th) percentiles: (38/38; 110/109)
The second measure was performed almost 1 year after the first one etc.
The ability for self-care was worse in both genders at the second measure; no female reported severe problems at the first measure. Severe mobility problems were registered more often in males than in females between the first and the second measure. Severe anxiety/depression and severe pain/discomfort was more frequently reported in the second measure in females.
Males reported more severe problems concerning ordinary activities at both measures and mobility problems at the second measure in comparison to females. Severe problems with pain were more frequently reported in females at the second measure. Moderate problems with self-care were more common in males than females at both measures.
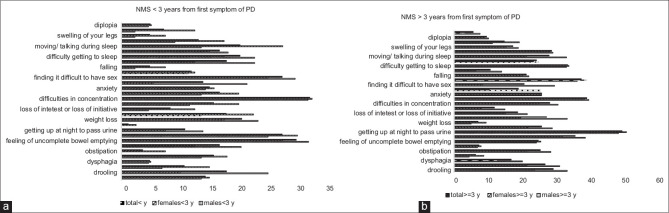
The items in Figure 2a and b follow the NMS questionnaire, a validated scale that identifies the most common NMS in Parkinsonian disorders. As these symptoms alter over time, the results are divided at 3 years after the first reported PD symptom.
Figure 2.
NMS experienced <5 years (a) and >5 years (b) after first PD reported symptom, respectively. Above horizontal bars in black represents reported severe problems. NMS: Nonmotor symptoms
In total, 285 patients (126 females and 159 males) have at least one completed NMS questionnaire equal to or more than 3 years from indicated time point for first experienced symptom.
NMS was more prominent reported as problems among males, especially in the memory and gastrointestinal domains, including drooling and obstipation. Also sexual problems were much more prominent, 26% versus 7% among females (Fishers Exact test, P < 0.0001). These symptoms were increasingly reported after 3 years. On the other hand, excessive sweating, weight loss, and nausea were more commonly reported among females, even more so at later stages of disease. The progression of NMS is illustrated in Figure 2a and b.
Cognition
In total, 225 females and 275 males were recorded. Normal cognition (0) over all measure points was identified among 31 (14%) females and 22 (8%) males. Severe inactivation, helplessness, and need of complete assistance after 10 years were identified among 0.9% of females and 0.7% of males [Figure 3].
Figure 3.
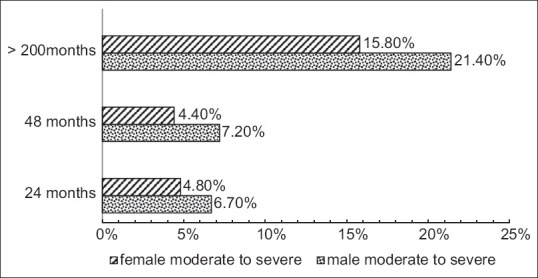
Time from onset of PD symptoms and reporting of moderate to severe cognitive problems, split by gender (%) (Cisi-PD). Cisi-PD: Clinical impression of severity index for Parkinson's disease
Limitations of the study
Dropouts of individual data were considerable in numbers. This is probably due to the lack of time for completing the registration or choosing just to register specified items in the registry. As permission was needed from each regional member of staff, a more limited number of regional data were analyzed. This study was in fact the first attempt to perform comparisons of a large amount of material. The intention was to describe differences seen in a sample of data, extracted directly from a registry with active data.
Discussion
General aspects
Gender-related differences are apparent in brain anatomy and function. From other studies, we know that estrogen influences NMS.[19] It is also well-known that females more often report tremor and worse instability scores as initial symptoms of PD.[20]
Studies have shown that men with LRRK2 mutations express PD symptomatology with milder symptoms, fewer motor complications, and fewer cognitive deficits than women with the same mutation.[21]
There is some evidence for gender differences in PD patients with GBA1 mutations concerning neuropsychiatric symptoms.[22]
An interesting difference concerning biomarkers for PD is the fact that higher uric acid concentrations in blood indicates a lower risk for PD in men only, perhaps due to differences in the purine pathways.[23]
The idea that development of Parkinson's disease may involve different pathological mechanisms and yield distinct prognosis in males and females was supported in a study[24] using diffusion-weighted MRI in de novo Parkinson's disease patients (149/83; f/m) with comparable clinical severity. Atrophy of different cortical areas was seen in males compared to females. Local efficiency of white matter connectivity also showed greater disruption in males than females. These findings may have implications for research into neuroprotection and stratification for clinical trials.
Nonmotor symptoms
Vivid dreams, hyposmia, depression, or obstipation are seldom or never recognized as early symptoms of PD. These symptoms are vague and often unknown to the patient as possible early prediagnostic symptoms.
Our study confirms more reported problems with memory, pseudo-hallucinations, sexuality, and urgency among male gender patients and these increased after 3 years. Excessive sweating, weight loss, and nausea were more common among females, and more symptoms were reported at later stages of disease.
Other studies also have described sadness, anxiety, lack of motivation, and obstipation more frequently reported among females, whereas drooling, urinary symptoms, and excessive daytime sleepiness seem to be more common among males.[25]
More prominent dysautonomic symptoms and greater decrease in olfactory function are reported among males, but rem sleep behavior disorder seems to be more frequent in females.[26]
Neuroimaging
The data extraction from the Parkinson Register in this study suggests that there seem to be more DaTscans performed among males than females. DaTscan is an example of an advanced supplementary technique to confirm the diagnosis of dopaminergic deficiency. However, the small numbers of registrations due to the significant number of dropouts makes it difficult to prove significant differences.
Imaging studies have pointed out better preserved presynaptic pathways in women throughout the course of the disease and are possible partial explanations for the differences in clinical symptoms, complications, and course of disease between genders.
Concerning imaging biomarkers, a better cortical and subcortical connectivity of sensorimotor networks is shown in drug naïve PD females compared to males.[27] Higher levels of nigrostriatal dopamine pathways have been shown in women than in men at symptom onset and during the course of PD.
Quality of life
In later stages of the disease males reported significantly more prominent decline in cognitive functions and sexual problems. Trends toward more weight loss and excessive sweating were reported among females, suggesting that there can be more prominent differences in the pathophysiology of the disease.
Males do not seem to experience negative impacts of HRQoL in terms of NMS, but fatigue and depression are described to have negative impacts in females. However, Lubomski et al. showed a greater disease burden in males when comparing UPDRS scores.[28]
Medication
Small, but still important, differences in time from first symptom to second added pharmacological treatment were also recognized, with males receiving their first add-on therapy earlier than females. By far the most commonly used pharmaceutical drug was levodopa (Madopar). This is a formulation with medium controlled pharmacokinetic release. The formulations are differentiated into fast-acting (Madopar Quick mite@) and slow-acting levodopa (Madopar Depot@). This was utilized more in males than in females.
Overall, the use of different dopaminergic drugs was similar in terms of gender. In Sweden, levodopa is the dominating anti-Parkinson's drug used. The data did not include experienced side effects, a limitation of the registries themselves. However, in another recently published longitudinal study, females were shown to develop dyskinesias earlier in the course of the disease than males.[29]
Advanced therapies
In the actual data extraction, only 19 females and 12 males were registered with advanced therapy. There was, however, a considerable difference in the use of apomorphine: 10 females and only one male. The difference is highly significant. Is this difference due to different expectations of effects? Or is it due to experienced reversibility of the regimens? This difference should be further studied.
Limitations of the study
The choice of starting point of a slowly progressive disease, that for several years presents itself with no or weak symptoms for the patient, is precarious. Time point for diagnosis is also a weak indicator and tied to huge variations in time points for the first meeting with the neurologist. The challenges when extracting substantial amounts of data from National Registers are well known. The explanation for the missing data of various magnitude in the extractions from the register is, of course, that filling in complete data for each patient is never mandatory, which is problematic when attempting to process the data in a statistically accurate and safe way. This is why we had to filter the longitudinal data and thus create quite enormous amounts of missing data, which is not common in other scientific work.
Conclusions
Overall, there are great advantages to collecting data in a National Registry. National and international comparisons are possible. Prerequisites for subgrouping of different phenotypes and regional comparisons for “best in practice” can be reached.
This study confirms trends toward differences between genders in chosen aspects of mixed urban and rural living Parkinsonian patients in Sweden.
Some of the results visualize obvious differences: the use of advanced neuroimaging techniques and time point to first add-on therapy favors males. The self-reported symptoms differ over time, and the decline of cognition is more attenuated in males than in females.
To identify subgroups of Parkinsonian patients who are suitable for specific new treatments, extraction of nationwide data can be of great value.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
Leszek Stawiarz, Project manager, Clinical Neuroscience, Karolinska Institute, Campus North, Stockholm, Sweden.
References
- 1.Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA, et al. Incidence of Parkinson's disease: Variation by age, gender, and race/ethnicity. Am J Epidemiol. 2003;157:1015–22. doi: 10.1093/aje/kwg068. [DOI] [PubMed] [Google Scholar]
- 2.Wooten GF, Currie LJ, Bovbjerg VE, Lee JK, Patrie J. Are men at greater risk for Parkinson's disease than women? J Neurol Neurosurg Psychiatry. 2004;75:637–9. doi: 10.1136/jnnp.2003.020982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meoni S, Macerollo A, Moro E. Sex differences in movement disorders. Nat Rev Neurol. 2020;16:84–96. doi: 10.1038/s41582-019-0294-x. [DOI] [PubMed] [Google Scholar]
- 4.Picillo M, Nicoletti A, Fetoni V, Garavaglia B, Barone P, Pellecchia MT. The relevance of gender in Parkinson's disease: A review. J Neurol. 2017;264:1583–607. doi: 10.1007/s00415-016-8384-9. [DOI] [PubMed] [Google Scholar]
- 5.Shulman LM. Gender differences in Parkinson's disease. Gend Med. 2007;4:8–18. doi: 10.1016/s1550-8579(07)80003-9. [DOI] [PubMed] [Google Scholar]
- 6.Biswajit K, Sahu SS, Orozco-Arroyave JR. An investigation about the relationship between dysarthria level of speech and the neurological state of Parkinson's patients. Biocybern Biomed Eng. 2022 [Google Scholar]
- 7.Macleod AD, Taylor KS, Counsell CE. Mortality in Parkinson's disease: A systematic review and meta-analysis. Mov Disord. 2014;29:1615–22. doi: 10.1002/mds.25898. [DOI] [PubMed] [Google Scholar]
- 8.Marras C, McDermott MP, Rochon PA, Tanner CM, Naglie G, Rudolph A, et al. Survival in Parkinson disease: Thirteen-year follow-up of the DATATOP cohort. Neurology. 2005;64:87–93. doi: 10.1212/01.WNL.0000148603.44618.19. [DOI] [PubMed] [Google Scholar]
- 9.Kuusimäki T, Kurki S, Sipilä JO, Salminen-Mankonen H, Carpén O, Kaasinen V. Sex-dependent improvement in survival of Parkinson's disease patients. Mov Disord Clin Pract. 2020;7:516–20. doi: 10.1002/mdc3.12954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weber AM, Cislaghi B, Meausoone V, Abdalla S, Mejía-Guevara I, Loftus P, et al. Gender norms and health: Insights from global survey data. Lancet. 2019;393:2455–68. doi: 10.1016/S0140-6736(19)30765-2. [DOI] [PubMed] [Google Scholar]
- 11.Macintyre S. Gender differences in longevity and health in Eastern and Western Europe. In: Platt S, Thomas H, Scott S, Williams G, editors. Locating Health: Sociological and Historical Explanations. Avebury, Aldershot: Routledge; 2018. pp. 57–74. [Google Scholar]
- 12.Wingard DL, Cohn BA, Kaplan GA, Cirillo PM, Cohen RD. Sex differentials in morbidity and mortality risks examined by age and cause in the same cohort. Am J Epidemiol. 1989;130:601–10. doi: 10.1093/oxfordjournals.aje.a115374. [DOI] [PubMed] [Google Scholar]
- 13. [Last accessed on 2020 Nov 01]. Available from: http://kvalitetsregister.se/englishpages/findaregistry/registerarkivenglish/nationalqualityregistryforneurologicalcareneuroregpreviouslyswedishmsregistry.2283html .
- 14.Nationella Riktlinjer för Vård Vid Multipel Skleros Och Parkinsons Sjukdom. Sweden: The National Board of Health and Welfare; 2016. [Google Scholar]
- 15.Martinez-Martin P, Schapira AH, Stocchi F, Sethi K, Odin P, MacPhee G, et al. Prevalence of nonmotor symptoms in Parkinson's disease in an international setting; Study using nonmotor symptoms questionnaire in 545 patients. Mov Disord. 2007;22:1623–9. doi: 10.1002/mds.21586. [DOI] [PubMed] [Google Scholar]
- 16.Chaudhuri KR, Martinez-Martin P, Brown RG, Sethi K, Stocchi F, Odin P, et al. The metric properties of a novel non-motor symptoms scale for Parkinson's disease: Results from an international pilot study. Mov Disord. 2007;22:1901–11. doi: 10.1002/mds.21596. [DOI] [PubMed] [Google Scholar]
- 17.Martínez-Martín P, Forjaz MJ, Cubo E, Frades B, de Pedro Cuesta J, ELEP Project Members Global versus factor-related impression of severity in Parkinson's disease: A new clinimetric index (CISI-PD) Mov Disord. 2006;21:208–14. doi: 10.1002/mds.20697. [DOI] [PubMed] [Google Scholar]
- 18.Brooks R. EuroQol: The current state of play. Health Policy. 1996;37:53–72. doi: 10.1016/0168-8510(96)00822-6. [DOI] [PubMed] [Google Scholar]
- 19.Georgiev D, Hamberg K, Hariz M, Forsgren L, Hariz GM. Gender differences in Parkinson's disease: A clinical perspective. Acta Neurol Scand. 2017;136:570–84. doi: 10.1111/ane.12796. [DOI] [PubMed] [Google Scholar]
- 20.Haaxma CA, Bloem BR, Borm GF, Oyen WJ, Leenders KL, Eshuis S, et al. Gender differences in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2007;78:819–24. doi: 10.1136/jnnp.2006.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen W, Yan X, Lv H, Liu Y, He Z, Luo X. Gender differences in prevalence of LRRK2-associated Parkinson disease: A meta-analysis of observational studies. Neurosci Lett. 2020;715:134609. doi: 10.1016/j.neulet.2019.134609. [DOI] [PubMed] [Google Scholar]
- 22.Riboldi GM, Di Fonzo AB. GBA, Gaucher disease, and Parkinson's disease: From genetic to clinic to new therapeutic approaches. Cells. 2019;8:364. doi: 10.3390/cells8040364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oh YS, Kim JS, Yoo SW, Hwang EJ, Lyoo CH, Lee KS. Gender difference in the effect of uric acid on striatal dopamine in early Parkinson's disease. Eur J Neurol. 2020;27:258–64. doi: 10.1111/ene.14070. [DOI] [PubMed] [Google Scholar]
- 24.Tremblay C, Abbasi N, Zeighami Y, Yau Y, Dadar M, Rahayel S, et al. Sex effects on brain structure in de novo Parkinson's disease: A multimodal neuroimaging study. Brain. 2020;143:3052–66. doi: 10.1093/brain/awaa234. [DOI] [PubMed] [Google Scholar]
- 25.Yoon JE, Kim JS, Jang W, Park J, Oh E, Youn J, et al. Gender Differences of nonmotor symptoms affecting quality of life in Parkinson disease. Neurodegener Dis. 2017;17:276–80. doi: 10.1159/000479111. [DOI] [PubMed] [Google Scholar]
- 26.Nalls MA, McLean CY, Rick J, Eberly S, Hutten SJ, Gwinn K, et al. Diagnosis of Parkinson's disease on the basis of clinical and genetic classification: A population-based modelling study. Lancet Neurol. 2015;14:1002–9. doi: 10.1016/S1474-4422(15)00178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yadav SK, Kathiresan N, Mohan S, Vasileiou G, Singh A, Kaura D, et al. Gender-based analysis of cortical thickness and structural connectivity in Parkinson's disease. J Neurol. 2016;263:2308–18. doi: 10.1007/s00415-016-8265-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lubomski M, Louise Rushworth R, Lee W, Bertram KL, Williams DR. Sex differences in Parkinson's disease. J Clin Neurosci. 2014;21:1503–6. doi: 10.1016/j.jocn.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 29.Iwaki H, Blauwendraat C, Leonard HL, Makarious MB, Kim JJ, Liu G, et al. Differences in the presentation and progression of Parkinson's disease by sex. Mov Disord. 2021;36:106–17. doi: 10.1002/mds.28312. [DOI] [PMC free article] [PubMed] [Google Scholar]