Abstract
STUDY QUESTION
Can mice serve as a translational model to examine the reproductive consequences of pubertal suppression with GnRH agonist (GnRHa) followed by testosterone (T) administration, a typical therapy in peripubertal transmasculine youth?
SUMMARY ANSWER
An implanted depot with 3.6 mg of GnRHa followed by T enanthate at 0.45 mg weekly can be used in peripubertal female mice for investigating the impact of gender-affirming hormone therapy in transmasculine youth.
WHAT IS KNOWN ALREADY
There is limited knowledge available in transgender medicine to provide evidence-based fertility care, with the current guidelines being based on the assumption of fertility loss. We recently successfully developed a mouse model to investigate the reproductive consequences of T therapy given to transgender men. On the other hand, to our knowledge, there is no mouse model to assess the reproductive outcomes in peripubertal transmasculine youth.
STUDY DESIGN, SIZE, DURATION
A total of 80 C57BL/6N female mice were used in this study, with n = 7 mice in each experimental group.
PARTICIPANTS/MATERIALS, SETTING, METHODS
We first assessed the effectiveness of GnRHa in arresting pubertal development in the female mice. In this experiment, 26-day-old female mice were subcutaneously implanted with a GnRHa (3.6 mg) depot. Controls underwent a sham surgery. Animals were euthanized at 3, 9, 21 and 28 days after the day of surgery. In the second experiment, we induced a transmasculine youth mouse model. C57BL/6N female mice were subcutaneously implanted with a 3.6 mg GnRHa depot on postnatal day 26 for 21 days and this was followed by weekly injections of 0.45 mg T enanthate for 6 weeks. The control for the GnRH treatment was sham surgery and the control for T treatment was sesame oil vehicle injections. Animals were sacrificed 0.5 weeks after the last injection. The data collected included the day of the vaginal opening and first estrus, daily vaginal cytology, weekly and terminal reproductive hormones levels, body/organ weights, ovarian follicular distribution and corpora lutea (CL) counts.
MAIN RESULTS AND THE ROLE OF CHANCE
GnRHa implanted animals remained in persistent diestrus and had reduced levels of FSH (P = 0.0013), LH (P = 0.0082) and estradiol (P = 0.0155), decreased uterine (P < 0.0001) and ovarian weights (P = 0.0002), and a lack of CL at 21 days after GnRHa implantation. T-only and GnRHa+T-treated animals were acyclic throughout the treatment period, had sustained elevated levels of T, suppressed LH levels (P < 0.0001), and an absence of CL compared to controls (P < 0.0001). Paired ovarian weights were reduced in the T-only and GnRHa+T groups compared with the control and GnRHa-only groups.
LARGE SCALE DATA
N/A.
LIMITATIONS, REASONS FOR CAUTION
Although it is an appropriate tool to provide relevant findings, precaution is needed to extrapolate mouse model results to mirror human reproductive physiology.
WIDER IMPLICATIONS OF THE FINDINGS
To our knowledge, this study describes the first mouse model mimicking gender-affirming hormone therapy in peripubertal transmasculine youth. This model provides a tool for researchers studying the effects of GnRHa-T therapy on other aspects of reproduction, other organ systems and transgenerational effects. The model is supported by GnRHa suppressing puberty and maintaining acyclicity during T treatment, lower LH levels and absence of CL. The results also suggest GnRHa+T therapy in peripubertal female mice does not affect ovarian reserve, since the number of primordial follicles was not affected by treatment.
STUDY FUNDING/COMPETING INTEREST(S)
This work was supported by the Michigan Institute for Clinical and Health Research grants KL2 TR 002241 and UL1 TR 002240 (C.D.C.); National Institutes of Health grants F30-HD100163 and T32-HD079342 (H.M.K.); University of Michigan Office of Research funding U058227 (A.S.); American Society for Reproductive Medicine/Society for Reproductive Endocrinology and Infertility grant (M.B.M.); and National Institutes of Health R01-HD098233 (M.B.M.). The University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core Facility was supported by the Eunice Kennedy Shriver NICHD/NIH grants P50-HD028934 and R24-HD102061. The authors declare that they have no competing interests.
Keywords: mouse model, GnRH agonist, testosterone, transmasculine, ovaries
Introduction
Survey studies indicate there are at least 1.4 million transgender adults and 150 000 transgender youth (age 13–17 years) living in the USA (Crissman et al., 2017; Herman et al., 2017). Many transgender and non-binary people seek medical therapy as part of their transition to their affirmed gender, which may include hormonal treatment and/or surgery (De Sutter, 2002; Wierckx et al., 2012). Transmasculine youth presenting for gender-affirming treatment at Tanner stage 2–3 may receive GnRH agonist (GnRHa) to suppress further pubertal progression incongruent with their gender identity. At age 16, or earlier in some cases, gender-affirming T is then started to induce changes congruent with their gender identity (Coleman et al., 2012; Hembree et al., 2017; Ethics Committee of the American Society for Reproductive Medicine, 2021). This strategy may also allow an extended diagnostic period to explore the adolescent’s gender identity prior to starting testosterone therapy.
Puberty is a critical transitional developmental period characterized by sexual maturation, development of secondary sexual characteristics, and achievement of reproductive capacity. This process is driven by GnRH, a pulsatile hormone secreted from the hypothalamus to stimulate pituitary production and secretion of LH and FSH, which stimulates the production of gonadal steroids such as estrogen, progesterone and testosterone (Herbison, 2016; Lopez-Rodriguez et al., 2021). Puberty is marked by breast development in girls, followed by pubic hair development and menarche (Lopez-Rodriguez et al., 2021). In female mice and rats, puberty onset is characterized by vaginal opening, first estrus and ovulation (Cheung et al., 1997, 2001; Simavli et al., 2015; Witchel and Plant, 2021). The suppression of GnRH pulsatility (e.g. with GnRHa) suppresses the hypothalamic–pituitary–gonadal (HPG) axis and results in delayed pubertal progression in humans, mice and rats (Dipalma, 1990; Gill et al., 2010).
Unfortunately, little is known about the reproductive effects of blockade of pubertal progression followed directly by gender-affirming T (GnRHa+T), despite research showing that many transmasculine individuals desire children (Wierckx et al., 2012; American Psychiatric Association, 2013; De Roo et al., 2017; Hembree et al., 2017). Accordingly, the World Professional Association for Transgender Health (Coleman et al., 2012), the American Society for Reproductive Medicine (Ethics Committee of the American Society for Reproductive Medicine, 2015, 2021) and the Endocrine Society (Hembree et al., 2017) all recommend counseling of transgender and non-binary individuals about fertility preservation prior to initiating hormone therapy. Currently, fertility preservation options for prepubertal transgender youth are limited to ovarian tissue cryopreservation (Cheng et al., 2019); however, the cost, surgical risk and potential loss of oocytes during the cryopreservation process may be unacceptable to families. As such, information is needed for clinical counseling as to whether fertility preservation is necessary for producing genetically related offspring in the future.
We recently developed a mouse model to mimic gender-affirming T therapy in transmasculine adults. These mice showed defects in ovarian architecture and alterations in folliculogenesis (Kinnear et al., 2019), similar to that in humans. The purpose of the present study was to develop a translational mouse model to examine the consequences of T administration after pretreatment with peripubertal GnRHa on the reproductive phenotype, mimicking gender-affirming hormone therapy in peripubertal transmasculine youth.
Materials and methods
Mice
Prepubertal 26-day-old C57BL/6N female mice (n = 80) (Envigo, Indianapolis, IN, USA) were maintained in ventilated cages under standard housing conditions (ad libitum access to food and water, photoperiod 12 h light and 12 h dark) at the University of Michigan, Ann Arbor. All animal management procedures were carried out in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the University of Michigan Institutional Animal Care and Use Committee (PRO00009635).
Experiment 1: Validating pubertal suppression using a GnRHa
GnRHa is clinically used to suppress puberty in peripubertal transmasculine youth, generally initiated in Tanner Stage 2–3 (Hembree et al., 2017). To validate that our choice of GnRHa was appropriate for arresting pubertal development in mice, prepubertal C57BL/6N females 26 days old (n = 7 mice/group) were subcutaneously implanted with GnRHa (Goserelin acetate implant 3.6 mg (Zoladex®), AstraZeneca, UK Limited). Controls had a sham implant placed. The age chosen for the GnRHa implant was selected to be before vaginal opening in this strain of mice, which occurs around 33 days of age (Hoyer et al., 2019).
For this validation study, to determine the duration of pubertal suppression with GnRHa, the timing of the vaginal opening and first estrus were recorded. Following vaginal opening, daily vaginal cytology was performed to assess estrous cyclicity, and weekly blood was collected for FSH assessment to evaluate HPG axis suppression. Animals were sacrificed at four different time points: 3, 9, 21 and 28 days after the day of implantation.
On the day of sacrifice, body, uterine and paired ovarian weights were recorded, and the left ovary was harvested for histology. The right ovary was harvested and frozen for later molecular analyses. Terminal blood was collected to assess LH, FSH and estradiol levels.
Experiment 2: Establishing a transmasculine adolescent mouse model: pausing pubertal progression by treating with GnRHa, followed by T therapy
To mimic gender-affirming hormone therapy in peripubertal transmasculine youth, C57BL/6N female mice (n = 7 mice/group) received GnRHa or a sham implant on postnatal day (PND) 26. At 21 days after the implantation (time chosen on the basis of efficacy of GnRHa to suppress the HPG axis from Experiment 1), mice received weekly midback 100 µl subcutaneous injections of 0.45 mg Testosterone Enanthate in sesame oil (USP/NF grade, Spectrum Chemical MFG Corp, Gardena, CA, USA) (Kinnear et al., 2019). Control mice received 100 µl of sesame oil only. Sesame oil was sterile filtered prior to T preparation for injections. Animals were assigned to four different groups: Control (sham surgery + sesame oil), GnRHa-only (GnRHa implant + sesame oil), T-only (sham + T enanthate) and GnRHa+T (GnRHa implant + T enanthate), n = 7 mice/group. Daily vaginal cytology was assessed throughout the study. Weekly blood was collected for FSH and testosterone (T) levels.
Animals were sacrificed after 6 weeks of T or oil injections. Anogenital distance was measured using a caliper as an indicator of masculinization (Dela Cruz and Pereira, 2012). The preputial gland was identified, dissected and weighed. Paired ovaries were collected and weighed. The left ovary was used for histology while the right ovary was frozen for future analysis. Terminal blood was collected to assess T, LH, FSH and estradiol levels.
Vaginal opening, first estrous and assessment of estrous cycle
Mice were monitored daily to assess vaginal opening. One day after the vaginal opening, vaginal cytology was performed daily throughout the study. The estrous cycle stage was determined by light microscopy analysis of the vaginal epithelial cellular distribution and was characterized based on the presence of cornified cells, nucleated epithelial cells and leukocytes (Cora et al., 2015).
Weekly blood collection and serum hormone analysis
Weekly blood was collected from the lateral tail vein at the midpoint between injections, up to 0.5% of body weight. At the time of sacrifice (3 days after the last T or oil injection), terminal blood was collected via decapitation. Samples were kept at 4°C overnight, centrifuged for 10 min (9200×g), and stored at −20°C until analysis. Peptide hormone measurements of LH and FSH and steroid hormone measures of testosterone and estradiol were performed at the Ligand Assay and Analysis Core Facility, University of Virginia Center for Research in Reproduction. The reportable ranges were established with a coefficient of variation of 0.2–14.8% for estradiol and 1.0–5.5% for testosterone. The reportable dose range for LH Mouse and Rat in-house protocol radioimmunoassay was 0.04–75.0 ng/ml for both experiments. The reportable dose range for FSH Mouse and Rat in-house protocol radioimmunoassay was 2.1–45 ng/ml for both experiments. In the Testosterone Mouse and Rat enzyme-linked immunosorbent assay (Immuno-Biological Laboratories, Inc., Minneapolis, MN, USA), the reportable dose range was 0.10–16 ng/ml (or 0.20–32 ng/ml, with a 2× dilution). The Mouse/Rat Estradiol ELISA (Calbiotech) detection limit was 3–300 pg/ml for Experiment 1, and the Mouse/Rat Estradiol ELISA (ALPCO) detection limit was 5.00–3200.00 pg/ml for Experiment 2. All immunoassays were performed in singlets.
Ovarian histology
Left ovaries were fixed in Bouin’s fixative at 4°C overnight, transferred and stored in 70% ethanol at 4°C. All samples were sent to the Histology Core in the School of Dentistry at the University of Michigan for processing. Samples were embedded in paraffin and serially sectioned at 5 µm thickness with five sections per slide, and every other slide was stained with hematoxylin and eosin.
Follicle counting
Follicle counts were performed for every 10th section throughout the left ovary from each mouse using a light microscope (DM1000, Leica, Germany). Primordial follicles were counted by examining the slides at 20× and 40× magnification. Primary and secondary follicles were counted using a 10× and 20× magnification. Antral follicles (AFs) and corpora lutea (CL) were counted by examining 5× images alongside each other to avoid repeat counting of same follicle.
Total numbers of primordial follicles, primary follicles, secondary follicles, AFs, atretic follicles and CL were recorded. A primordial follicle was defined as an oocyte surrounded by one layer of granulosa cells with no visible space between granulosa cells and the oocyte. An oocyte surrounded by a single layer of cuboidal granulosa cells was identified as a primary follicle, while secondary follicles had multiple layers (two or more) of cuboidal granulosa cells. Primary and secondary follicles were counted when a nucleus was present. An AF was recognized by the presence of a fragmented or continuous antral cavity within the granulosa cell layers. AFs were identified as not having an oocyte connected to granulosa cells and had an attenuated granulosa cell layer (Kinnear et al., 2019). CL were identified as discrete round structures with increased pink cytoplasmic staining with hematoxylin and eosin.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 9. The results were analyzed by descriptive statistics for determination of normal data distributions and Student’s t-test was performed for two-group comparisons. No transformation was required. All remaining analyses utilized two-way ANOVA followed by Tukey’s post hoc test. The level of significance was defined as P < 0.05. For analysis purposes, hormone levels below the detection level were treated as the value set for the lower limit of quantification. All data were presented as mean ± SD.
Results
GnRHa caused a flare effect followed by suppression of the HPG axis
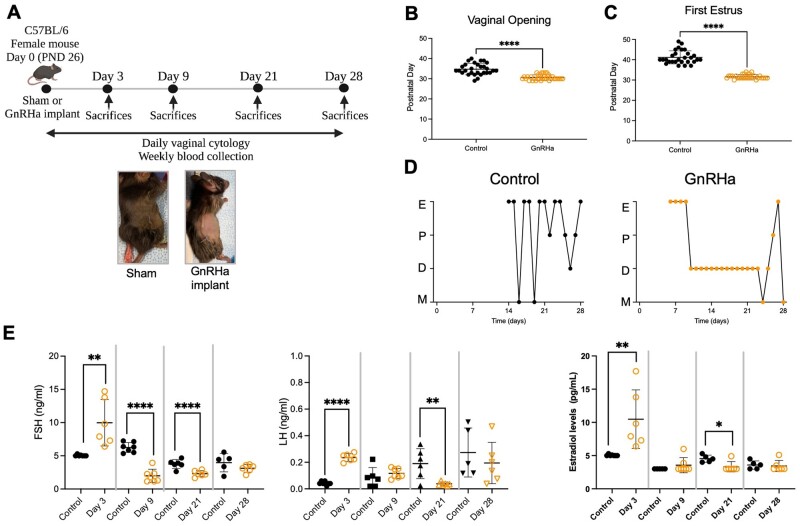
GnRHa implantation on PND 26 (Fig. 1A) advanced the timing of the vaginal opening (P < 0.0001) and first estrus (P < 0.0001) compared to controls (Fig. 1B and C). The initial GnRHa-induced estrous lasted ∼4 days, then all GnRHa-treated animals remained acyclic in diestrus until Day 21 post-implantation. Some of the GnRHa-treated mice resumed cycling between Days 21 and 28, likely corresponding to metabolization of the GnRHa implant. In contrast, control animals cycled continually after their first estrus (Fig. 1D). On Day 3 post-GnRHa implantation, GnRHa-treated animals showed increased FSH (P = 0.0032), LH (P < 0.0001) and estradiol (P = 0.0073) levels compared to controls. Subsequently, FSH levels were suppressed by Day 9 post-GnRHa implantation relative to the control group (P < 0.0001). The HPG axis remained suppressed by Day 21 post-GnRHa implantation with suppression of FSH (P = 0.0013), LH (P = 0.0082) and estradiol levels (P = 0.0155). However, by Day 28 post-GnRHa implantation, FSH (P = 0.2058), LH (P = 0.4599) and estradiol (P = 0.7550) levels returned to levels comparable to that of the controls (Fig. 1E).
Figure 1.
Experimental design and characterization of GnRHa-treated animals. (A) Experimental design showing the implantation day followed by the time points at which animals were evaluated. (B) Day of vaginal opening (C) and first estrus in the two groups. (D) Vaginal cytology from control mice and GnRHa-treated mice until Day 28. Control mice progressed through the estrous cycle, while GnRHa-treated animals showed a persistent diestrus until Day 21, which did not reliably last until Day 28. E, estrus; P, proestrus; D, diestrus; M, metestrus. (E) Levels of FSH, LH and estradiol on Days 3, 9, 21 and 28 after GnRHa or sham implantation. Black circles represent data from control and orange circles represent data from GnRHa-treated animals. Data are expressed as mean + SD, Student’s t-test. *P < 0.0155, **P < 0.0032, ****P < 0.0001.
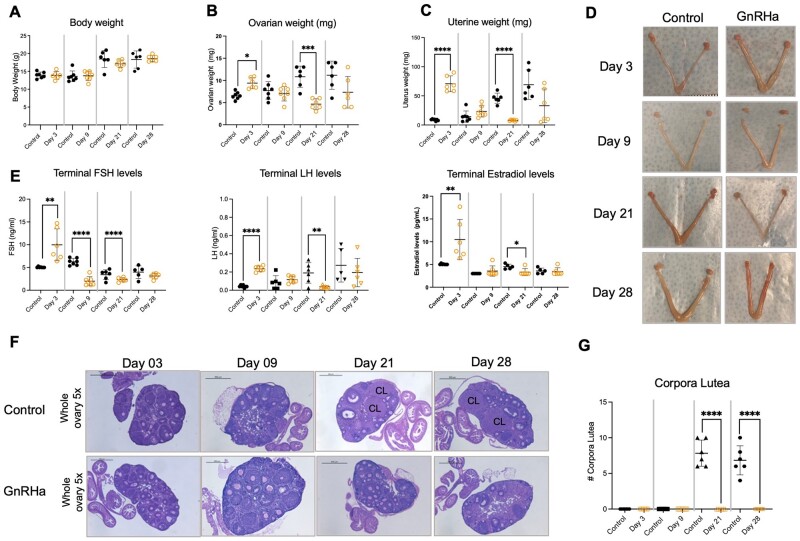
Changes in ovarian and uterine weights occurred in parallel with hormonal changes. During the initial flare, an increase in paired ovarian (P = 0.0005) and uterine (P < 0.0001) weights were observed at Day 3 after GnRHa implantation compared to controls (Fig. 2B–D), when increased in LH, FSH and estradiol levels were evident (Fig. 2E). During the subsequent suppression, decreases in paired ovarian (P = 0.0002) and uterine (P < 0.0001) weights were observed by Day 21 in GnRHa-treated animals, in parallel with the observed decrease in hormone levels. This decrease in uterine and ovarian weight was not consistently sustained on Day 28 (Fig. 2B–D), at which point the hormonal levels were comparable to that of controls (Fig. 2E). Analyses of histological sections of ovaries collected from GnRHa-treated mice demonstrated an absence of CL in all animals, GnRHa treated and control, on Days 3 and 9. On Days 21 and 28, CL suggestive of ovulation were observed in ovaries from control animals, while GnRHa-treated animals did not have CL on either day (Fig. 2F and G), reflecting the lower ovarian weight.
Figure 2.
Body measurements, hormone levels and ovarian histology analysis of GnRHa-treated animals. (A) Body weight, (B) paired ovarian weight, (C) uterine weight of control and GnRH-treated mice at different time points. (D) Representative ovarian and uterine pictures from Control and GnRHa-treated mice at different time points. (E) FSH, LH and estradiol levels of control and GnRH-treated mice at different time points. (F) Representative comparison of hematoxylin and eosin-stained ovaries from controls and GnRHa-treated animals at different time points. CL, corpora lutea. (G) Number of corpora lutea. Black circles represent data from control and orange circles represent data from GnRHa-treated animals. Data are expressed as mean + SD, Student’s t-test. *P < 0.05, **P < 0.03, ***P < 0.01, ****P < 0.001.
GnRHa+T treatment established and maintained suppression of the HPG axis
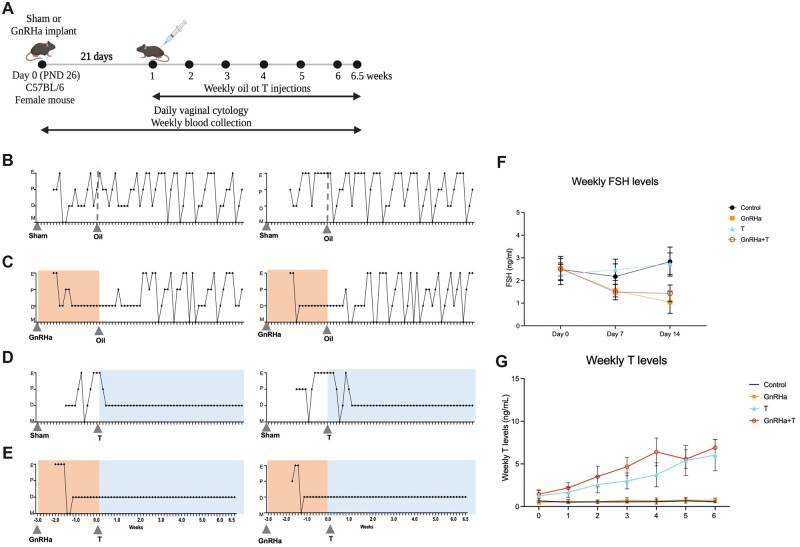
To create a model mimicking the gender-affirming hormone paradigm for peripubertal transmasculine youth, a GnRHa or sham implant was inserted on PND 26, and weekly T or oil injections started 21 days later (PND 46; Experimental design, Fig. 3A). Control animals (sham implant + oil injections) had consistent estrous cycles throughout the entire study (Fig. 3B). After the initial flare response to GnRHa treatment, GnRHa-only (GnRHa implant + oil injections) treated animals remained acyclic for 21 ± 5 days and resumed cycling thereafter, after the oil injections were initiated (Fig. 3C). T-only (sham implant + T injections) treated animals were cyclic throughout treatment with the sham implant, then became acyclic 7 ± 3 days after the start of T treatment (Fig. 3D). In contrast, after the initial flare response to GnRHa implantation, GnRHa+T treatment was effective in suppressing estrus cyclicity, with the animals remaining in constant diestrus throughout the study (Fig. 3E). Weekly FSH levels during the GnRHa treatment in groups GnRHa+T (P = 0.0025) and GnRHa only (P = 0.0040) were lower than that of the T-only and control animals. This suppression was sustained until the end of GnRHa treatment in both the GnRHa-only and GnRHa+T groups (P < 0.0001) in comparison to sham implant groups (T-only and control) (Fig. 3F). Weekly T levels (ng/ml) were elevated throughout T treatment in GnRHa+T (range 1.4–6.9) and T-only (range 1.2–6.0) groups, in comparison to the GnRHa-only (range 0.4–0.7) and control groups (range 0.5–0.6) groups (Fig. 3D). The levels of T achieved with T treatment were similar to the levels seen in age-matched C57BL/6 male mice (Supplementary Fig. S1, range 1.0–4.9 ng/ml).
Figure 3.
Experimental design and phenotype of GnRHa+T-treated animals. (A) Experimental design for the transmasculine adolescent mouse model: GnRH analog treatment followed by T therapy, showing time of treatment and samples collection time points. (B–E) Estrus Cyclicity. (B) Control animals went through all estrous cycle phases. E, estrus; P, Proestrus; D, diestrus; M, Metestrus. (C) GnRHa-treated mice showed an expected flare and subsequent persistent diestrus, and resumed cycling after the GnRHa implant likely finished. T animals presented persistent diestrus after initiating T treatment. GnRHa+T animals showed persistent diestrus during the entire experimental period after the initial flare. (F) Weekly FSH levels were suppressed in GnRHa-treated animals. (G) Weekly testosterone levels for mice over 6 weeks of T treatment. Data are expressed as mean + SD, ANOVA followed by Tukey test.
T and GnRHa+T-treatment reduced ovarian weight and LH levels
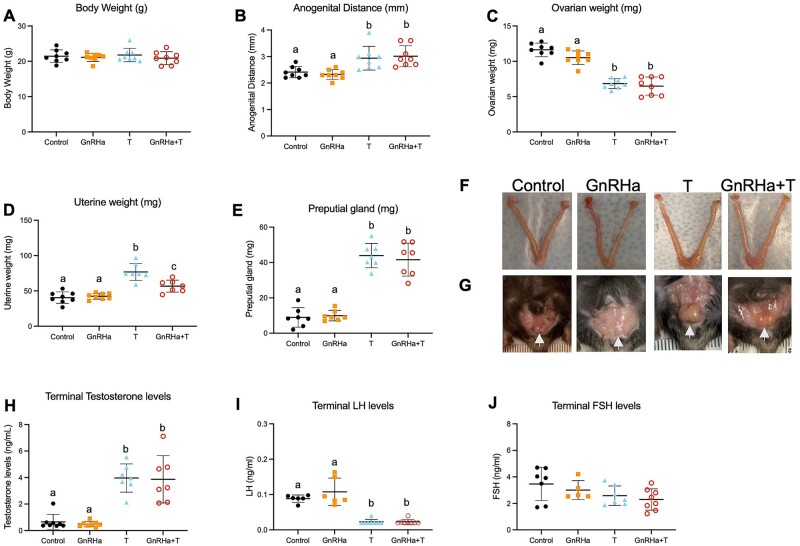
GnRHa+T-treated animals showed an increase in anogenital distance (P = 0.0041), a decrease in paired ovarian weight (P < 0.0001) and increases in uterine (P < 0.0001) and preputial gland (female mouse clitoris) (P < 0.0001) weights compared to GnRHa-only and control groups. T-only animals also presented an increase in anogenital distance (P = 0.0146), a decrease in paired ovarian weight (P < 0.0001) and increases in uterine (P < 0.0001) and preputial gland (P < 0.0001) weights compared to GnRHa-only and control groups (Fig. 4B–E). As expected, terminal T levels were elevated in GnRHa+T (P < 0.0001) and T-only (P < 0.0001) treated animals compared to GnRHa only and controls groups, presenting similar levels to that of aged-matched male mice (ranged levels: 2.0–11.7 ng/ml, Fig. 4F, Supplementary Fig. S1). Terminal LH levels were suppressed in the GnRHa+T (P < 0.0001) and T-only (P < 0.0001) groups compared to GnRHa-only and control groups (Fig. 4G).
Figure 4.
Body measurement and hormones levels of GnRHa+T-treated animals. (A) Body weight, (B) anogenital distance, (C) paired ovarian weight, (D) uterine weight, (E) preputial gland weight and (F) Terminal T, (G) LH and (H) FSH levels in control, GnRHa-only, T-only and GnRHa+T mice. (I) Representative ovarian, uterine and (J) preputial gland images from control, GnRHa-only, T-only and GnRHa+T animals. Data are expressed as mean + SD, ANOVA followed by Tukey test; letters (a, b, c) denote significance.
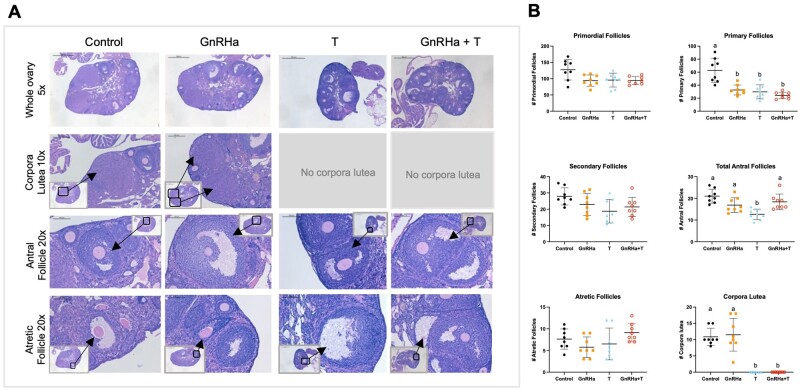
T-only and GnRHa+T treatments altered ovarian follicular distribution and blocked corpora lutea formation
The number of primary follicles in the GnRHa+T (P < 0.0001), GnRHa-only (P = 0.0008) and T-only (P = 0.0003) groups were significantly lower than in the control group. The T-only group showed a decrease in total AFs (P = 0.0004) in comparison to the GnRHa+T group and controls. The GnRHa+T and T-only animals showed an absence of CL compared to the GnRHa-only and control groups. There were no differences in number of primordial follicles between groups (Fig. 5A and B).
Figure 5.
Ovarian histology and follicle counts of GnRHa+T-treated animals. (A) Representative comparison of hematoxylin and eosin-stained ovaries from controls, GnRHa-only, T-only and GnRHA+T-treated mice at different time points. (B) Numbers of primordial, primary, secondary, total antral and atretic follicles, and corpora lutea counts in controls, GnRHa-only, T-only and GnRHA+T-treated mice at different time points. Data expressed as mean + SD, ANOVA followed by Tukey test; letters (a and b) denote significance, P < 0.05.
Discussion
This study establishes a translational model that mimics pubertal suppression followed by gender-affirming testosterone therapy in transmasculine youth. Animals treated with GnRHa in early puberty showed the expected signs of a flare effect at 3 days, characterized by increased FSH, LH and estradiol levels, and increased ovarian and uterine weight. After this initial period, GnRHa treatment caused suppression of the HPG axis, and animals showed decreased levels of FSH, LH and estradiol and lower ovarian and uterine weight as compared to age-matched controls. The absence of CL during the initial flare-up and later suppression supports continued anovulation in GnRHa-treated animals. GnRHa+T-treated female mice remained acyclic throughout GnRHa and T treatment period, with increased testosterone levels during the T-treatment period (with levels similar to age-matched male mice), lower ovarian weight and suppressed LH levels.
In humans, GnRHa interrupts endogenous GnRH input to the pituitary by binding GnRH receptors and effectively blocking the HPG axis in both physiological and pathological conditions (Maggi et al., 2016). Upon initial binding, a flare effect is observed, characterized by hyperstimulation of the GnRH receptors in the pituitary, resulting in an increase in the production and secretion of LH and FSH from the pituitary, and downstream estradiol, progesterone and testosterone release from the gonads. This effect is followed by a downregulation of the GnRH receptors and suppression of the HPG axis (Akaza, 2011). A similar flare-suppression progression was seen in our model.
The effectiveness of GnRHa in pausing puberty in transgender youth was demonstrated by a retrospective study that showed the LH, FSH and estradiol levels in this population were similar to patients on GnRHa therapy for precocious puberty (Mejia-Otero et al., 2021). Similarly, cohort studies have shown that girls on GnRHa treatment for precocious puberty have decreased ovarian and uterine size during treatment (Heger et al., 2006; Pasquino et al., 2008; Carswell and Roberts, 2017). In line with this, our results demonstrate that GnRHa treatment has similar effects on the reproductive organs of female mice. Other studies on the effect of GnRHa treatment in transgender youth have evaluated metabolic and cardiovascular systems but not reproductive outcomes (Jarin et al., 2017; Perl et al., 2020; Grimstad et al., 2021; Mullins et al., 2021).
The animal model described here is necessary because existing transmasculine mouse models were developed in the context of gender-affirming T in adults, which does not include the pubertal suppression prescribed in peripubertal adolescents. Our group has previously described an adult model where female mice were treated with T for 6 weeks and showed suppression of LH levels, persistent diestrus and absence of CL. Ovaries from T-treated animals showed an increased number of atretic cyst-like late AFs, but similar numbers of early-stage follicles (Kinnear et al., 2019). In the present study, females treated with GnRHa+T and T-only also showed lower levels of LH and demonstrated acyclicity. The histological analysis that we performed showed no differences in follicular distribution barring a decrease in number of primary follicles and complete absence of CL in the GnRHa+T animals compared to controls. The reduced ovarian weight is likely related to the absence of CL and is consistent with these animals not reaching puberty or ovulating. The primordial follicle pool is assembled early in life in humans and rodents. These non-responsive gonadotropin follicles serve as the source of developing follicles and oocytes for the entire reproductive lifespan, and hence serves as an ovarian reserve marker (Ford et al., 2020; Wang et al., 2020). At puberty, the increase in gonadotrophin production enables follicles to progress to preovulatory state and ovulation (Kerr et al., 2013; Monniaux et al., 2014; Findlay et al., 2015). In the present study, GnRHa+T treatment did not alter the number of primordial follicles, indicating that ovarian reserve was not compromised.
The ovarian follicular growth and development can be classified as non-gonadotropin and gonadotropin dependent. During this development, FSH is responsible for driving follicular recruitment and growth to preovulaory stages, and LH is required for inducing ovulation of mature oocytes (Webb et al., 1999; Findlay et al., 2015). The decrease in the number of AFs with T treatment in our study may be a function of timing of start of T treatment. During puberty, there is an initial surge in testosterone in controls, which would have led to enhancement of follicular recruitment, and a relative decrease in AFs seen with continuous T treatment. Interestingly, the number of AFs in the GnRHa+T treatment group did not decrease compared to the controls. As anticipated, prepubertal GnRHa treatment suppressed follicle activation and growth, arresting the follicular pool at the same stage (Hsueh et al., 1996; Yuan and Giudice, 1997). However, the subsequent T treatment prevented ovulation, demonstrated by the acyclicity and absence of CL. As the GnRHa inhibition wore off in the GnRH+T treatment group, a large number of previously suppressed gonadotropin-dependent follicles would have entered the growing pool and resumed growth, in addition to the follicles that had been in the gonadotropin-independent stage of folliculogenesis progressing to gonadotropin-dependent folliculogenesis. The circulating FSH levels were similar across all the groups, suggesting that the growing follicles in the GnRHa+T group had a sufficient gonadotropic drive. T treatment in this group likely arrested follicular development, preventing follicles from proceeding to pre-ovulatory follicles, leading to a large number of AFs. Thus the greater number of AFs present in the GnRHa+T group compared to T-only group may be explained by the sheer number of follicles starting to grow at the same time and persisting without ovulation. Additional experiments are needed to address whether T and/or GnRHa+T treatment impacts oocyte quality and IVF outcomes. Importantly, from a fertility preservation perspective, GnRHa+T treatment did not have detrimental effects on the number of primordial follicles or ovarian reserve.
The increase in uterine weight in both T-only and GnRHa+T groups was unexpected. Our understanding of the role of androgens in uterine function is still limited (Gibson et al., 2020). Recent studies have demonstrated a local regulation of steroids in the endometrial layer suggesting that aromatase (Cyp19A1) may contribute to local conversion of testosterone to estrogen (Das et al., 2009; Gibson et al., 2013, 2018), which may explain the higher uterine weight in T and GnRHa+T groups. However, additional studies are needed to address the functional consequences of increase in uterine weight.
Conclusion
In conclusion, we have described the first mouse model mimicking gender-affirming hormone therapy in peripubertal transmasculine youth. We demonstrate that this model can be used to study the reproductive consequences of GnRHa+T, of which very little is currently known. Additional studies are necessary to address the possible reversibility of effects from GnRHa+T treatment in reproductive organs and consequences for IVF outcomes. We recognize that a mouse model may not always translate directly to human outcomes, so precaution is needed when interpreting results using the model. Nonetheless, this mouse model has the advantage of being easy to reproduce, and will hopefully provide a mechanism for future research on other aspects of reproduction, as well as metabolic consequences of GnRHa+T therapy.
Supplementary Material
Contributor Information
Cynthia Dela Cruz, Department of Obstetrics and Gynecology, University of Michigan, Ann Arbor, MI, USA; Postdoctoral Translational Scholar Program, Michigan Institute for Clinical & Health Research, University of Michigan, Ann Arbor, MI, USA.
Hadrian M Kinnear, Program in Cellular and Molecular Biology, University of Michigan, Ann Arbor, MI, USA; Medical Scientist Training Program, University of Michigan, Ann Arbor, MI, USA.
Prianka H Hashim, Department of Obstetrics and Gynecology, University of Michigan, Ann Arbor, MI, USA.
Abigail Wandoff, Department of Biomedical Engineering, University of Michigan, Ann Arbor, MI, USA.
Likitha Nimmagadda, Department of Biomedical Engineering, University of Michigan, Ann Arbor, MI, USA.
Alexis L Chang, Department of Biomedical Engineering, University of Michigan, Ann Arbor, MI, USA.
Vasantha Padmanabhan, Department of Obstetrics and Gynecology, University of Michigan, Ann Arbor, MI, USA; Department of Pediatrics and Communicable Diseases, University of Michigan, Ann Arbor, MI, USA.
Ariella Shikanov, Department of Obstetrics and Gynecology, University of Michigan, Ann Arbor, MI, USA; Program in Cellular and Molecular Biology, University of Michigan, Ann Arbor, MI, USA; Department of Biomedical Engineering, University of Michigan, Ann Arbor, MI, USA.
Molly B Moravek, Department of Obstetrics and Gynecology, University of Michigan, Ann Arbor, MI, USA; Division of Reproductive Endocrinology and Infertility, University of Michigan, Ann Arbor, MI, USA; Department of Urology, University of Michigan, Ann Arbor, MI, USA.
Data Availability
The data underlying this article are available in the article and in its Supplementary Material.
Authors’ roles
Study design: C.D.C., H.M.K., A.S., V.P. and M.B.M. Data acquisition: C.D.C., H.M.K., P.H.H., A.W., L.N. and F.L.C. Data analysis: C.D.C., H.M.K., V.P., A.S. and M.B.M. Funding acquisition: C.D.C., A.S. and M.B.M. Supervision: V.P., A.S. and M.B.M. Writing of original draft: C.D.C., V.P., A.S. and M.B.M. Review and editing: C.D.C., H.M.K., P.H.H., A.W., L.N., F.L.C., V.P., A.S. and M.B.M.
Funding
This work was supported by the Michigan Institute for Clinical and Health Research grants KL2 TR 002241 and UL1 TR 002240 (C.D.C.); National Institutes of Health grants F30-HD100163 and T32-HD079342 (H.M.K.); University of Michigan Office of Research funding U058227 (A.S.); American Society for Reproductive Medicine/Society for Reproductive Endocrinology and Infertility Grant (M.B.M.); and National Institutes of Health R01-HD098233 (M.B.M.). The University of Virginia Center for Research in Reproduction, Ligand Assay and Analysis Core Facility was supported by the Eunice Kennedy Shriver NICHD/NIH grants P50-HD028934 and R24-HD102061.
Conflict of interest
The authors declare that they have no competing interests.
References
- Akaza H. Combined androgen blockade for prostate cancer: review of efficacy, safety and cost-effectiveness. Cancer Sci 2011;102:51–56. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th edn. Arlington: World Professional Association for Transgender Health, 2013. [Google Scholar]
- Carswell JM, Roberts SA.. Induction and maintenance of amenorrhea in transmasculine and nonbinary adolescents. Transgend Health 2017;2:195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng PJ, Pastuszak AW, Myers JB, Goodwin IA, Hotaling JM.. Fertility concerns of the transgender patient. Transl Androl Urol 2019;8:209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung CC, Thornton JE, Kuijper JL, Weigle DS, Clifton DK, Steiner RA.. Leptin is a metabolic gate for the onset of puberty in the female rat. Endocrinology 1997;138:855–858. [DOI] [PubMed] [Google Scholar]
- Cheung CC, Thornton JE, Nurani SD, Clifton DK, Steiner RA.. A reassessment of leptin's role in triggering the onset of puberty in the rat and mouse. Neuroendocrinology 2001;74:12–21. [DOI] [PubMed] [Google Scholar]
- Coleman EB, Bockting W, Botzer M, Cohen-Kettenis P, DeCuypere G, Feldman J, Fraser L, Green J, Knudson G, Meyer WJ. et al. Standards of care for the health of transsexual,transgender, and gender-nonconforming people. Int J Transgend 2012;13:67. [Google Scholar]
- Cora MC, Kooistra L, Travlos G.. Vaginal cytology of the laboratory rat and mouse: review and criteria for the staging of the estrous cycle using stained vaginal smears. Toxicol Pathol 2015;43:776–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crissman HP, Berger MB, Graham LF, Dalton VK.. Transgender demographics: a household probability sample of US adults, 2014. Am J Public Health 2017;107:213–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Mantena SR, Kannan A, Evans DB, Bagchi MK, Bagchi IC.. De novo synthesis of estrogen in pregnant uterus is critical for stromal decidualization and angiogenesis. Proc Natl Acad Sci USA 2009;106:12542–12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Roo C, Lierman S, Tilleman K, Peynshaert K, Braeckmans K, Caanen M, Lambalk CB, Weyers S, T'Sjoen G, Cornelissen R. et al. Ovarian tissue cryopreservation in female-to-male transgender people: insights into ovarian histology and physiology after prolonged androgen treatment. Reprod Biomed Online 2017;34:557–566. [DOI] [PubMed] [Google Scholar]
- De Sutter P, Verschoor A, Hotimsky A, Kira K.. The desire to have children and the preservation of fertility in transsexual women: a survey. Int J Transgend 2002;6:3. [Google Scholar]
- Dela Cruz C, Pereira OC.. Prenatal testosterone supplementation alters puberty onset, aggressive behavior, and partner preference in adult male rats. J Physiol Sci 2012;62:123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipalma JR. Tartrazine sensitivity. Am Fam Physician 1990;42:1347–1350. [PubMed] [Google Scholar]
- Ethics Committee of the American Society for Reproductive Medicine. Access to fertility services by transgender persons: an Ethics Committee opinion. Fertil Steril 2015;104:1111–1115. [DOI] [PubMed] [Google Scholar]
- Ethics Committee of the American Society for Reproductive Medicine. Access to fertility services by transgender and nonbinary persons: an Ethics Committee opinion. Fertil Steril 2021;115:874–878. [DOI] [PubMed] [Google Scholar]
- Findlay JK, Hutt KJ, Hickey M, Anderson RA.. How is the number of primordial follicles in the ovarian reserve established? Biol Reprod 2015;93:111. [DOI] [PubMed] [Google Scholar]
- Ford EA, Beckett EL, Roman SD, McLaughlin EA, Sutherland JM.. Advances in human primordial follicle activation and premature ovarian insufficiency. Reproduction 2020;159:R15–R29. [DOI] [PubMed] [Google Scholar]
- Gibson DA, McInnes KJ, Critchley HO, Saunders PT.. Endometrial intracrinology-generation of an estrogen-dominated microenvironment in the secretory phase of women. J Clin Endocrinol Metab 2013;98:E1802–E1806. [DOI] [PubMed] [Google Scholar]
- Gibson DA, Simitsidellis I, Collins F, Saunders PTK.. Androgens, oestrogens and endometrium: a fine balance between perfection and pathology. J Endocrinol 2020;246:R75–R93. [DOI] [PubMed] [Google Scholar]
- Gibson DA, Simitsidellis I, Collins F, Saunders PTK.. Endometrial intracrinology: oestrogens, androgens and endometrial disorders. Int J Mol Sci 2018;19:3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill JC, Wang O, Kakar S, Martinelli E, Carroll RS, Kaiser UB.. Reproductive hormone-dependent and -independent contributions to developmental changes in kisspeptin in GnRH-deficient hypogonadal mice. PLoS One 2010;5:e11911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimstad F, Kremen J, Shim J, Charlton BM, Boskey ER.. Breakthrough bleeding in transgender and gender diverse adolescents and young adults on long-term testosterone. J Pediatr Adolesc Gynecol 2021;34:706–716. [DOI] [PubMed] [Google Scholar]
- Heger S, Muller M, Ranke M, Schwarz HP, Waldhauser F, Partsch CJ, Sippell WG.. Long-term GnRH agonist treatment for female central precocious puberty does not impair reproductive function. Mol Cell Endocrinol 2006;254–255:217–220. [DOI] [PubMed] [Google Scholar]
- Hembree WC, Cohen-Kettenis PT, Gooren L, Hannema SE, Meyer WJ, Murad MH, Rosenthal SM, Safer JD, Tangpricha V, T'Sjoen GG.. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an Endocrine Society Clinical Practice Guideline. Endocr Pract 2017;23:1437. [DOI] [PubMed] [Google Scholar]
- Herbison AE. Control of puberty onset and fertility by gonadotropin-releasing hormone neurons. Nat Rev Endocrinol 2016;12:452–466. [DOI] [PubMed] [Google Scholar]
- Herman JF, Brown TNT, Wilson BDM, Conron KJ.. Age of Individuals Who Identify as Transgender in the United States. Los Angeles, CA: The Williams Institute UCLA School of Law, 2017. [Google Scholar]
- Hoyer PB, Rice PF, Howard CC, Koevary JW, Dominguez Cooks JP, Hutchens GV, Chambers SK, Craig ZR, Connolly DC, Barton JK.. Comparison of reproductive function in female TgMISIIR-TAg transgenic and wildtype C57BL/6 mice. Comp Med 2019;69:16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsueh AJ, Eisenhauer K, Chun SY, Hsu SY, Billig H.. Gonadal cell apoptosis. Recent Prog Horm Res 1996;51:433–455; discussion 455–436. [PubMed] [Google Scholar]
- Jarin J, Pine-Twaddell E, Trotman G, Stevens J, Conard LA, Tefera E, Gomez-Lobo V.. Cross-sex hormones and metabolic parameters in adolescents with gender dysphoria. Pediatrics 2017;139:e20163173. [DOI] [PubMed] [Google Scholar]
- Kerr JB, Myers M, Anderson RA.. The dynamics of the primordial follicle reserve. Reproduction 2013;146:R205–R215. [DOI] [PubMed] [Google Scholar]
- Kinnear HM, Constance ES, David A, Marsh EE, Padmanabhan V, Shikanov A, Moravek MB.. A mouse model to investigate the impact of testosterone therapy on reproduction in transgender men. Hum Reprod 2019;34:2009–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Rodriguez D, Franssen D, Heger S, Parent AS.. Endocrine-disrupting chemicals and their effects on puberty. Best Pract Res Clin Endocrinol Metab 2021;35:101579. [DOI] [PubMed] [Google Scholar]
- Maggi R, Cariboni AM, Marelli MM, Moretti RM, Andre V, Marzagalli M, Limonta P.. GnRH and GnRH receptors in the pathophysiology of the human female reproductive system. Hum Reprod Update 2016;22:358–381. [DOI] [PubMed] [Google Scholar]
- Mejia-Otero JD, White P, Lopez X.. Effectiveness of puberty suppression with gonadotropin-releasing hormone agonists in transgender youth. Transgend Health 2021;6:31–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monniaux D, Clément F, Dalbiès-Tran R, Estienne A, Fabre S, Mansanet C, Monget P.. The ovarian reserve of primordial follicles and the dynamic reserve of antral growing follicles: what is the link? Biol Reprod 2014;90:85. [DOI] [PubMed] [Google Scholar]
- Mullins ES, Geer R, Metcalf M, Piccola J, Lane A, Conard LAE, Mullins TLK.. Thrombosis risk in transgender adolescents receiving gender-affirming hormone therapy. Pediatrics 2021;147:e2020023549. [DOI] [PubMed] [Google Scholar]
- Pasquino AM, Pucarelli I, Accardo F, Demiraj V, Segni M, Di Nardo R.. Long-term observation of 87 girls with idiopathic central precocious puberty treated with gonadotropin-releasing hormone analogs: impact on adult height, body mass index, bone mineral content, and reproductive function. J Clin Endocrinol Metab 2008;93:190–195. [DOI] [PubMed] [Google Scholar]
- Perl L, Segev-Becker A, Israeli G, Elkon-Tamir E, Oren A.. Blood pressure dynamics after pubertal suppression with gonadotropin-releasing hormone analogs followed by testosterone treatment in transgender male adolescents: a pilot study. LGBT Health 2020;7:340–344. [DOI] [PubMed] [Google Scholar]
- Simavli S, Thompson IR, Maguire CA, Gill JC, Carroll RS, Wolfe A, Kaiser UB, Navarro VM.. Substance p regulates puberty onset and fertility in the female mouse. Endocrinology 2015;156:2313–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JJ, Ge W, Zhai QY, Liu JC, Sun XW, Liu WX, Li L, Lei CZ, Dyce PW, De Felici M. et al. Single-cell transcriptome landscape of ovarian cells during primordial follicle assembly in mice. PLoS Biol 2020;18:e3001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb R, Campbell BK, Garverick HA, Gong JG, Gutierrez CG, Armstrong DG.. Molecular mechanisms regulating follicular recruitment and selection. J Reprod Fertil Suppl 1999;54:33–48. [PubMed] [Google Scholar]
- Wierckx K, Mueller S, Weyers S, Van Caenegem E, Roef G, Heylens G, T'Sjoen G.. Long-term evaluation of cross-sex hormone treatment in transsexual persons. J Sex Med 2012;9:2641–2651. [DOI] [PubMed] [Google Scholar]
- Witchel SF, Plant TM.. Neurobiology of puberty and its disorders. Handb Clin Neurol 2021;181:463–496. [DOI] [PubMed] [Google Scholar]
- Yuan W, Giudice LC.. Programmed cell death in human ovary is a function of follicle and corpus luteum status. J Clin Endocrinol Metab 1997;82:3148–3155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its Supplementary Material.