Abstract
Context
5-Fluorouracil (5-FU)-injured stromal cells may cause chronic bone marrow suppression; however, the underlying mechanism remains unclear. Angelica sinensis polysaccharide (ASP), the main biologically active ingredient of the Chinese herb, Angelica sinensis (Oliv.) Diels (Apiaceae), may enrich the blood and promote antioxidation.
Objective
This study investigated the protective antioxidative effects of ASP on perivascular mesenchymal progenitors (PMPs) and their interactions with hematopoietic cells.
Materials and methods
PMPs were dissociated from C57BL/6 mouse femur and tibia and were subsequently divided into the control, ASP (0.1 g/L), 5-FU (0.025 g/L), and 5-FU + ASP (pre-treatment with 0.1 g/L ASP for 6 h, together with 0.025 g/L 5-FU) then cultured for 48 h. Hematopoietic cells were co-cultured on these feeder layers for 24 h. Cell proliferation, senescence, apoptosis, and oxidative indices were detected, along with stromal osteogenic and adipogenic differentiation potentials. Intercellular and intracellular signaling was analyzed by real-time quantitative reverse transcription polymerase chain reaction and Western blotting.
Results
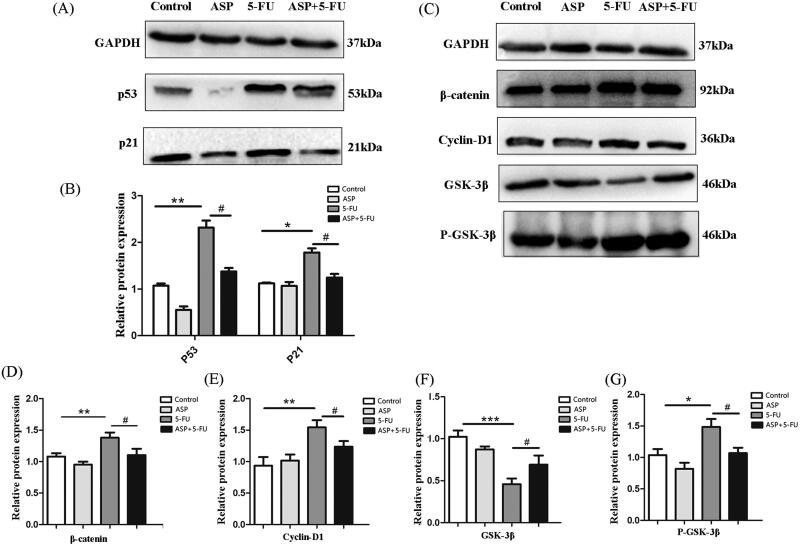
ASP ameliorated the reactive oxygen species production/scavenge balance in PMPs; improved osteogenic differentiation; increased SCF, CXCL12, VLA-4/VCAM-1, ICAM-1/LFA1, and TPO/MPL, Ang-1/Tie-2 gene expression. Further, the ASP-treated feeder layer alleviated hematopoietic cells senescence (from 21.9 ± 1.47 to 12.1 ± 1.13); decreased P53, P21, p-GSK-3β, β-catenin and cyclin-D1 protein expression, and increased glycogen synthase kinase (GSK)-3β protein expression in co-cultured hematopoietic cells.
Discussion and conclusions
ASP delayed oxidative stress-induced premature senescence of 5-FU-treated feeder co-cultured hematopoietic cells via down-regulation of overactivated Wnt/β-catenin signaling. These findings provide a new strategy for alleviating myelosuppressive stress.
Keywords: Hematopoietic niche, oxidative stress, premature senescence of hematopoietic cells
Introduction
5-Fluorouracil (5-FU) is widely used in the treatment of solid cancers, particularly colorectal and breast cancers (Pittman et al. 1993; Vodenkova et al. 2020). Large doses or long-term use of 5-FU may lead to acute bone marrow suppression owing to hematopoietic progenitor cell injury. Notably, if the injury interferes with bone marrow stromal cells and hematopoietic stem cells (HSCs), potentially incurable bone marrow suppression may occur, critically affecting the bone marrow’s hematopoietic function (Parchment et al. 1998). It has been proposed that HSCs localize mainly in the endosteal niche, which is close to the bone and is composed of osteoblasts (Van Laere et al. 2003; Zhang et al. 2003), and in the vascular niche, which includes sinusoids and their endothelial cells (Kiel et al. 2005). However, these two niches may not be distinct from each other because large amounts of vasculature, including an endowment of arterioles and sinusoids, are very close to the endosteum. Recently, it has been demonstrated that a population of mesenchymal progenitors with osteogenic and adipogenic potential plays a vital role in HSC maintenance (Ding et al. 2012; Sugimura et al. 2017; Pinho and Frenette 2019). These mesenchymal progenitors reside perivascularly and travel to the endosteal surface where they differentiate into osteoblasts. Therefore, the osteoblastic niche may be closely related to the function of perivascular mesenchymal progenitors (Zhang et al. 2003; Méndez-Ferrer et al. 2010; Ding et al. 2012). Our previous findings confirmed that 5-FU causes oxidative damage to bone marrow stromal cells, indirectly leading to the inhibition of hematopoietic progenitor growth (Xiao et al. 2017). However, whether 5-FU targets the perivascular hematopoietic niche to exert its toxic effects on hematopoiesis remains unclear. In the current study, we isolated mesenchymal progenitors with a population of perivascular niche genes, including Lepr, Cxcl12 and Nestin, from the endosteum of mouse femur and tibia, and explored the toxicity of 5-FU on hematopoietic supporting function.
The maintenance of hematopoietic stem cells and the differentiation of committed progenitors occur in highly specialized niches. These distinct niches produce a broad range of soluble factors and adhesion molecules that modulate the fate of hematopoietic stem/progenitor cells (HSPCs) fate during normal hematopoiesis and bone marrow regeneration (Méndez-Ferrer et al. 2010; Ding and Morrison 2013; Richter et al. 2017). C-X-C motif chemokine 12 (CXCL12) and stem cell factor (SCF) are two key chemokines secreted by hematopoietic niche cells that play important roles in maintaining hematopoietic stem cell quiescence and self-renewal (Ponomaryov et al. 2000; Tzeng et al. 2011). In addition, osteopontin is involved in the maintenance of the HSPCs compartment and the protection of HSPCs from myelosuppressive stress (Zhang et al. 2003; Zhou et al. 2014). Ang-1/Tie2, thrombopoietin/myeloproliferative leukemia protein (TPO/MPL), and Jagged-1/Notch signaling molecules, vascular cell adhesion molecule-1/very late antigen-4 (VCAM-1/VLA-4) and lymphocyte function-associated antigen-1/intercellular adhesion molecule-1 (LFA-1/ICAM-1) adhesion molecule pairs mediating hematopoietic niche stroma and HSPC interactions are also involved in hematopoietic regulation (Arai et al. 2004; Bigas and Espinosa 2012). Angiopoietin (Ang) and its receptor, tyrosine kinase (Tie2), are required for the maintenance of quiescent HSCs while the TPO/MPL interaction upregulates β-integrin and cyclin-dependent kinase inhibitors in HSPCs and induces quiescence of long-term HSPCs. In contrast, the inhibition of the TPO/MPL pathway with an anti-MPL-neutralizing antibody reduces the number of quiescent HSPCs. The inhibition of Notch signaling also compromises reconstitution and increases HSC differentiation in vivo (Sahin and Buitenhuis 2012). The adhesion molecule receptor on the hematopoietic cell surface and the adhesion molecule ligand on the stromal cell surface mediate adhesion between hematopoietic and stromal cells as well as signal transduction between cells. VCAM-1 and ICAM-1 play important roles in hematopoietic cell mobilization by assisting hematopoietic cells in adhering to and locating the hematopoietic niche and participating in the formation of aggregates between hematopoietic and stromal cells (Chen et al. 2011). Hence, in this study, we focused on the effects of chemotherapeutic drugs on the regulatory function of the perivascular hematopoietic niche and preventative drug treatment.
The senescence of HSCs is the main cause of chronic myelosuppression following chemotherapy. Studies have shown distinct biological effects of Wnt/β-catenin on hematopoietic activity. Self-renewal of HSCs is predominantly stroma-mediated activation. In contrast, overactivation of Wnt/β-catenin in HSCs may compromise HSC maintenance, and lead to hematopoietic failure due to rapid loss of repopulating HSCs and multilineage hematopoietic differentiation block (Staal et al. 2016). In this study, we found that 5-FU induces apoptosis and senescence in perivascular mesenchymal progenitor cells due to oxidative stress. Importantly, 5-FU drives the adipogenic differentiation of mesenchymal progenitor cells at the cost of osteogenic differentiation, thus impairing the perivascular hematopoietic niche function to facilitate proliferation, adhering to HSPCs, and delivering signals to HSPCs. Subsequently, the damaged niche leads to hematopoietic cell oxidative stress-induced premature senescence, and the underlying mechanism may be associated with the overactivation of the Wnt/β-catenin pathway.
Angelica sinensis (Oliv.) Diels (Apiaceae) is a traditional Chinese herb that enrich blood and regulate blood circulation. Angelica sinensis polysaccharides (ASP), the main bioactive ingredients, have the effects of antioxidation, anti-aging, and antitumor (Liu et al. 2010; Ai et al. 2013; Wei et al. 2016). Our previous studies have demonstrated that ASP plays a significant role in antagonizing 5-FU to preserve the reactive oxygen species (ROS) production/scavenge balance, thus maintaining the physiological function of bone marrow stromal cells, HSCs, and hepatocytes (Mu et al. 2017; Zeng et al. 2021). Herein, we further suggest that the protective antioxidant effects of ASP on perivascular mesenchymal progenitors, the pivotal components of the hematopoietic niche, may play a role in delaying hematopoietic cell oxidative stress-induced premature senescence.
Materials and methods
Reagents
ASP was purchased from Ci Yuan Biotechnology Co., Ltd. (China), dissolved in saline to a concentration of 20 g/L and sterilized by ultrafiltration. According to Ci Yuan Biotechnology Co., Ltd., ASP was extracted from Angelica sinensis roots through pressurized hot water extraction, and proteins were removed from the crude polysaccharides using the repeated freeze-thaw method to obtain a mixture of polysaccharides. The purity of derived ASP was approximately 98%. Its monosaccharides include glucose, galactose, arabinose, rhamnose, mannose, and xylose (Mu et al. 2017; Ren et al. 2018; Cheng et al. 2019; Chen et al. 2020; Zeng et al. 2021). 5-FU was obtained from Sigma-Aldrich Co., Ltd. (USA) and dissolved in dimethyl sulfoxide (DMSO) to prepare a storage solution at a concentration of 0.025 g/L. Dulbecco’s Modified Eagle Medium (DMEM) medium/nutrient mixture F-12 was purchased from Gibco (USA). Fetal bovine serum (FBS) was purchased from MRC (Australia). Both the superoxide dismutase (SOD) assay kit and malondialdehyde (MDA) assay kit were obtained from Jiancheng Bioengineering Institute (China). RNAiso reagent, SYBR Green I, and the reverse transcription kit were purchased from TAKARA Biotechnology (Japan). β-catenin, glycogen synthase kinase (GSK)-3β, p-GSK-3β and cyclin D1 antibodies were purchased from Cell Signaling Technology (USA). The antibodies against P53, P21, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were purchased from Boster Biological Technology (Beijing, China).
Cell culture and treatment
C57BL/6 mice (6–8 weeks old) were purchased from the Medical and Laboratory Animal Center of Chongqing Medical University. All animal experiments were approved by the Chongqing Medical University Animal Care and Use Committee and performed in accordance with institutional and national guidelines. Bone marrow cells were aseptically removed by flushing the femur and tibia of C57BL/6 mice with DMEM (Sigma-Aldrich) using a 5 mL syringe needle. The bone obtained was cut into approximately 1 mm fragments. The perivascular mesenchymal progenitor cells were isolated from mouse femurs and tibial fragments using type II collagenase twice for 20 min each time at 37 °C. Cells were then cultured in DMEM F/12 medium with 10% FBS and 100 U/mL penicillin and 100 μg/mL streptomycin in a humidified atmosphere in a 35 mm2 dish with 5% CO2 at 37 °C. Cells were passaged to the third generation for subsequent experiments. The cells were subsequently divided into four groups: (1) the control group, routinely cultured; (2) the 5-FU group, 0.025 g/L 5-FU administered for 48 h; (3) the ASP group, 0.1 g/L ASP treated for 48 h; (4) the 5-FU + ASP group, pre-treatment with a single dose of 0.1 g/L ASP for 6 h combined with 0.025 g/L 5-FU for 48 h.
In the co-culture system, bone marrow cells were flushed from mouse femurs and tibias, and mononuclear cells were isolated with 1.077 g/mL Ficoll and cultured in Iscove’s Modified Dulbecco’s Medium (IMDM) supplemented with 10% FBS. After 6 h, the non-adherent hematopoietic cells (2.5 × 109/L) were collected and plated on four groups of perivascular mesenchymal progenitor cell (2.5 × 108/L) feeder layers as described above, using IMDM medium with 10% FBS. After 24 h of co-culturing, the suspended hematopoietic cells were isolated by centrifugation and adherent perivascular mesenchymal progenitor cells were collected by 0.25% trypsin digestion. The two groups of collected cells were subjected to the following tests.
Agarose gel electrophoresis
Total RNA was extracted from the cells using the TRIzol reagent, and cDNA was obtained by reverse transcription. Taq was added to the PCR amplification apparatus (primer sequences are listed in Table 1). The amplification procedure was as follows: 94 °C pre-denaturation for 30 s, 98 °C denaturation for 10 s, 55 °C annealing for 30 s, 72 °C extension for 1 min, 72 °C final extension for 2 min, and denaturation to extension cycle 35 times. DNA was separated on a 2% agarose gel. The gel bands were analyzed using Quantity-One 1D gel imaging software (Bio-Rad, Hercules, CA, USA).
Table 1.
Primer sequences of DNA.
| Gene | Direction | Primer sequence (5′→3′) | product length (bp) |
|---|---|---|---|
| Nestin | Forward | TAAGCAGTGGGGGTCGGATA | 485 |
| Reverse | AGGTGCTGGTCCTCTGGTAT | ||
| LepR | Forward | CTGCCAGTTAGGCTAGGCAT | 201 |
| Reverse | AACTGGTCGACGTTCTTCCC | ||
| CXCL12 | Forward | GCACTTTCACTCTCGGTCCA | 366 |
| Reverse | GCACTTCGCAGAGCACAAAA | ||
| OPN | Forward | TAACCGGATTCAACATGGGCA | 298 |
| Reverse | GAGCGTTGGTGTTGTACTGC | ||
| Runx2 | Forward | CCTAAATAGCCCCCACCGTC | 410 |
| Reverse | CCACGAGCTGACAAGCTGTA | ||
| PPAR-γ | Forward | TCTCTTACCGCCTCCGAGAT | 220 |
| Reverse | CGCCCTGGAAACTCAGTACA |
Trypan blue detects cell survival
In the co-culture system, suspended hematopoietic cells and adherent perivascular mesenchymal progenitor cells were collected, and the number of viable cells was counted using Trypan blue staining. A 0.4% Trypan blue solution was added into the cell suspension (1:10 dilution) for about 2 min. Unstained cells were counted as viable under a light microscope.
Cell counting kit (CCK)-8 cell proliferation assay
Perivascular mesenchymal progenitor cells were plated in 96-well plates at 3 × 103 cells per well and cultured for 48 h. Subsequently, 20 μL CCK-8 reagents were added to the plates per well. After incubation for 2 h, the optical density (OD) was measured at 450 nm using a microplate reader. Cell viability was calculated as follows:
Senescence-Associated β-Galactosidase (SA-β-gal) staining
Aging perivascular mesenchymal progenitor cells and senescent co-cultured hematopoietic cells were detected respectively by the SA-β-gal staining kit according to the manufacturer’s instructions. Quantifying the percentage of SA-β-gal positive cells and at least 1000 cells in 10 random fields were scored.
Flow cytometry
Apoptosis was analyzed using flow cytometry. Suspended hematopoietic cells were isolated by centrifugation, and adherent perivascular mesenchymal progenitor cells were collected by trypsin digestion. The cell concentration was adjusted to 3 × 106/mL. The cells were resuspended in a binding buffer and incubated with annexin V and propidium iodide (PI) at room temperature in the dark. Cells were detected using a flow cytometer.
Detection of oxidative indicators
To detect SOD activity and MDA content, the suspended hematopoietic cells and adherent perivascular mesenchymal progenitor cells were harvested, lysed and centrifuged to collect the supernatant. SOD activity and MDA content were detected by chemical colorimetric analysis, according to the manufacturer’s instructions.
Osteogenic potential detection
The osteogenic differentiation potential of perivascular mesenchymal progenitor cells was detected by Alizarin Red S (ARS) staining. Cells were plated in 6-well plates at a density of 2 × 105 cells/well and cultured in an osteogenic differentiation medium. The culture medium was replaced every two days. After 14 days, the cells were fixed with 4% paraformaldehyde, stained with Alizarin Red S solution for 5 min, and observed under a microscope. For quantitative analysis, 100 μL isopropyl alcohol was added to each well to dissolve the Alizarin Red dye, then the optical density (OD) at 490 nm was analyzed.
Adipogenic potential detection
To test the adipogenic potential of the perivascular mesenchymal progenitor cells, Oil Red O staining was performed. Cells were plated in 6-well plates at a density of 2 × 105 cells/well and cultured in an adipogenic differentiation medium (Cyagen Biotechnology, China). The culture medium was replaced every two days. After 22 d, the cells were fixed with 4% paraformaldehyde and stained with Oil Red O solution for 30 min. For quantitative analysis, isopropyl alcohol was used to dissolve the Oil Red O dye, and the OD value at 490 nm was analyzed.
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
The adherent perivascular mesenchymal progenitor cells and co-cultured suspended hematopoietic cells were collected. Total RNA was extracted using the TRIzol reagent (TAKARA, Japan) according to the manufacturer’s protocol. cDNA was synthesized using a reverse transcription kit (TAKARA, Shiga, Japan). The qRT-PCR was performed using a TaqMan assay. GAPDH was used as an internal control, and the relative quantification values for each gene were calculated by the 2−ΔΔCT method. Primers used are listed in Table 2.
Table 2.
A detailed list of primer sequences, species, gene bank numbers and polymerase chain reaction (PCR) product lengths used in real-time reverse transcription polymerase chain reaction (RT-PCR).
| Gene | Direction | Primer sequence (5′→3′) | GeneBank accession number |
PCR product size (bp) |
|---|---|---|---|---|
| Runx2 | Forward | AACAGCAGCAGCAGCAGCAG | NM_001271631.1 | 192 |
| Reverse | GCACCGAGCACAGGAAGTTGG | |||
| Osterix | Forward | GCGGCAAGGTGTATGGCAAGG | XM_006520519.5 | 176 |
| Reverse | GCAGAGCAGGCAGGTGAACTTC | |||
| OPN | Forward | AAGAGCGGTGAGTCTAAGGAGTCC | NM_001204203.1 | 91 |
| Reverse | TGCCCTTTCCGTTGTTGTCCTG | |||
| Ang-1 | Forward | TAACCGGATTCAACATGGGCA | NM_001286062.1 | 101 |
| Reverse | GAGCGTTGGTGTTGTACTGC | |||
| CXCL12 | Forward | TGACGGTAAACCAGTCAGCC | NM_001012477.2 | 129 |
| Reverse | CGTGCAACAATCTGAAGGGC | |||
| ICAM1 | Forward | CGGAGGATCACAAACGAAGC | NM_010493.3 | 149 |
| Reverse | CTCTTGCCAGGTCCAGTTCC | |||
| VCAM1 | Forward | GTTGTTCTGACGTGTGCTGC | NM_011693.3 | 129 |
| Reverse | CACAGAGCTCAACACAAGCG | |||
| VLA4 | Forward | TGTCCTACTGGTCCCGACAT | NM_010578.2 | 87 |
| Reverse | CCAAATCAGCAGCAAGGCAA | |||
| LFA1 | Forward | GAAGCTGAGCAGCCTTGTCT | NM_001253873.1 | 102 |
| Reverse | CTTGGAGAGTTCCACGGTCC | |||
| SCF | Forward | CGGGATGGATGTTTTGCCTA | XM_006513315.4 | 188 |
| Reverse | TCTTCGGTGCGTTTTCTTCC | |||
| MPL | Forward | GCCCAGATGGACTACCGAAG | NM_005373.3 | 110 |
| Reverse | TTGGCAATGTGGGTGGTACA | |||
| TPO | Forward | CTGGTTATGGCCTGCACAGA | XM_024453093.2 | 110 |
| Reverse | TTGCTTTCCTCCAAGACGCT | |||
| Tie-2 | Forward | CGACTGTAGCCGTCCTTGTA | XM_006502930.4 | 118 |
| Reverse | CGGCTCACAAGCTTTCTCAC | |||
| Notch | Forward | GCTCCGAGGAGATCAACGAG | NM_008714.3 | 268 |
| Reverse | TTGACATCACCCTCACACCG | |||
| Jagged-1 | Forward | CCATGCAGAACGTGAATGGAG | NM_013822.5 | 139 |
| Reverse | GTGACGCGGGACTGATACTC |
Western blotting analysis
The co-cultured hematopoietic cells were then collected. The samples were subjected to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride (PVDF) membranes. PVDF membranes were blocked with 5% skimmed milk powder at room temperature for 1 h and incubated overnight at 4 °C with β-catenin, GSK-3β, p-GSK-3β, Cyclin D1, P53, P21 and GAPDH primary antibodies. Membranes were then incubated with secondary antibodies at room temperature for 1 h. Protein bands were visualized using an electrochemiluminescence (ECL) kit (Biosharp, Anhui Province, China). Relative expression levels of the target proteins were determined using the ratio of the target protein grey value to the GAPDH protein grey value.
Statistical analysis
All experiments were independently repeated at least three-times. Data are expressed as mean ± standard deviation (SD). Results were analyzed by one-way analysis of variance (ANOVA) using SPSS 20.0 (IBM Corp., Armonk, NY, USA). p < 0.05 was considered statistically significant.
Results
Perivascular mesenchymal progenitor identification
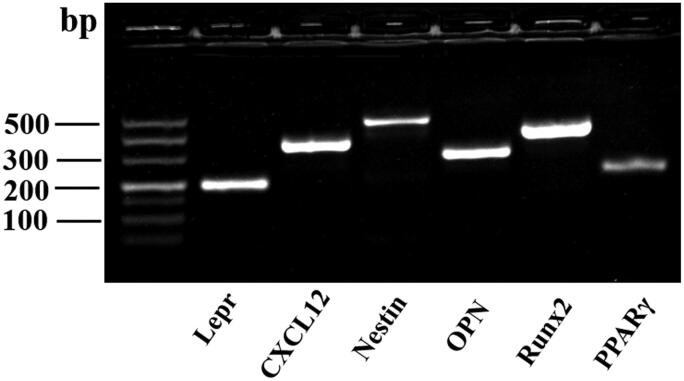
As shown in Figure 1, mouse femur and tibial bone-disassociated cells were identified with an abundance of perivascular hematopoietic niche-related genes by agarose gel electrophoresis, and the results showed a high positive expression of Lepr, Cxcl12, and Nestin. Meanwhile, a high expression of osteogenic differentiation-related genes, Runx2 and OPN, and a low expression of the adipogenic differentiation-related gene, PPARγ, indicated the strong osteogenic potential of bone-disassociated cells. These indicate that the bone-disassociated cells contain a large population of perivascular mesenchymal progenitors.
Figure 1.
The bone-disassociated cells were isolated and cultured to the third generation in vitro, and agarose gel electrophoresis was used to identify perivascular hematopoietic niche-related gene expression.
Angelica sinensis polysaccharides alleviated the inhibitory effect of 5-FU on perivascular mesenchymal progenitor growth
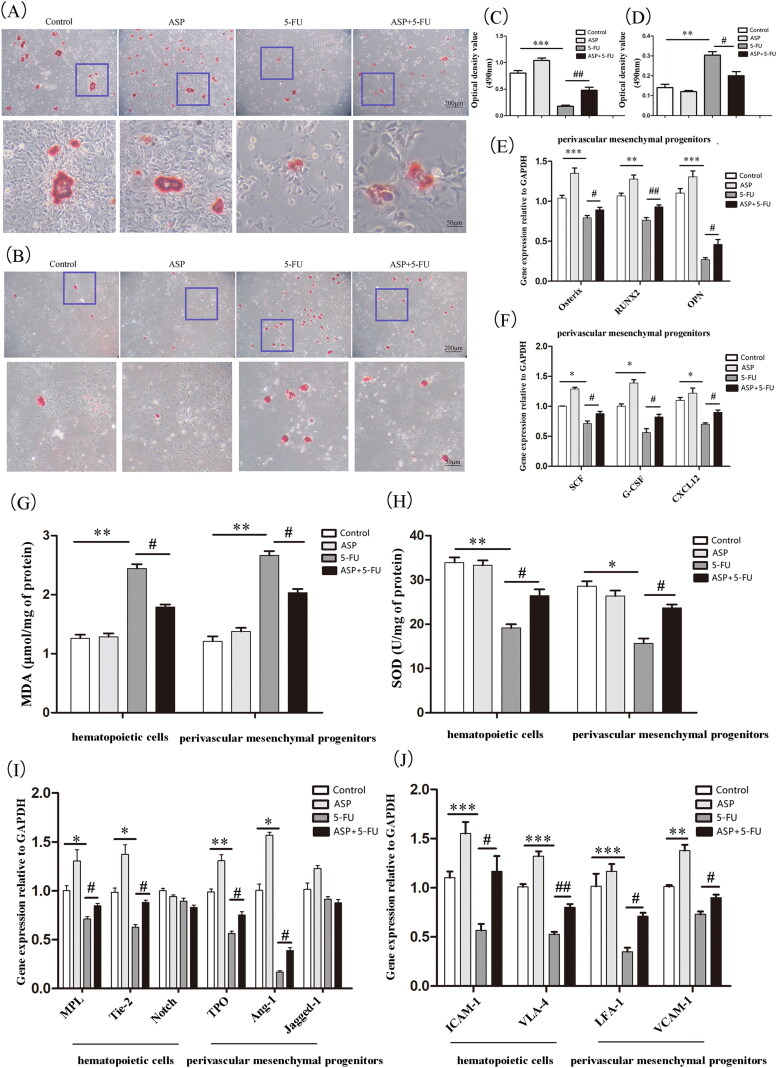
Femur and tibial bone-disassociated cells were cultured until the third generation in vitro. As demonstrated by Trypan blue staining, the number of viable cells in the 5-FU group was significantly lower than that in the control group, whereas the cellularity increased in the 5-FU + ASP group compared to that in the 5-FU group (Figure 2(A)). Cell growth was assessed using the CCK-8 assay. As shown in Figure 2(B), 25 μg/mL of 5-FU treatment at different intervals could inhibit cell growth. However, 100 μg/mL of ASP pre-treatment before 5-FU administration significantly decreased the inhibition rate compared to that of the 5-FU group. These results suggest that ASP alleviates the inhibitory effects of 5-FU on perivascular mesenchymal progenitor growth. To elucidate the underlying mechanism, senescence-related SA-β-gal staining and flow cytometric apoptosis assay were performed. The results showed that the 5-FU group demonstrated a widespread SA-β-gal positive staining, whereas the 5-FU + ASP group revealed a sporadic distribution of positive cells (Figure 2(C,D)). The apoptosis rate in the 5-FU group was significantly higher than that in the control group. However, ASP treatment rescued apoptosis (Figure 2(E,F)). These results suggest that 5-FU inhibits perivascular mesenchymal progenitor growth via both senescent and apoptotic mechanisms. However, ASP pre-treatment may protect perivascular mesenchymal progenitors from senescence and apoptosis.
Figure 2.
Angelica sinensis polysaccharides rescued perivascular mesenchymal progenitor growth inhibition via anti-senescence and anti-apoptosis effects. (A) Perivascular mesenchymal progenitors were cultured in 35 mm2 dishes in different groups. The viable cells were counted by Trypan blue staining (***p < 0.001 vs. control group; #p < 0.05 vs. 5-fluorouracil (5-FU) group, n = 3). (B) A cell counting kit (CCK)-8 assay was performed to detect perivascular mesenchymal progenitor growth (***p < 0.001 **p < 0.01 vs. control group; ##p < 0.01 #P < 0.05 vs. 5-FU group, n = 3). (C) The representative images of senescent perivascular mesenchymal progenitors are presented by senescence-associated β-galactosidase (SA-β-gal) staining. Senescent cells are blue-green stained (scale bar = 50 μm, n = 3). (D) The histogram of SA-β-gal staining positive rate of perivascular mesenchymal progenitors (***p < 0.001 vs. control group; #p < 0.05 vs. 5-FU group). (E) Annexin V-FITC/PI double staining was employed to detect cell apoptosis by flow cytometry. LL represented normal living cells, LR and UR represented early and late apoptotic cells, respectively. (F) The histogram of apoptosis rate of perivascular mesenchymal (***p < 0.001 vs. control group; ##p < 0.01 vs. 5-FU group).
Angelica sinensis polysaccharides restored 5-FU damaged perivascular mesenchymal progenitor niche function
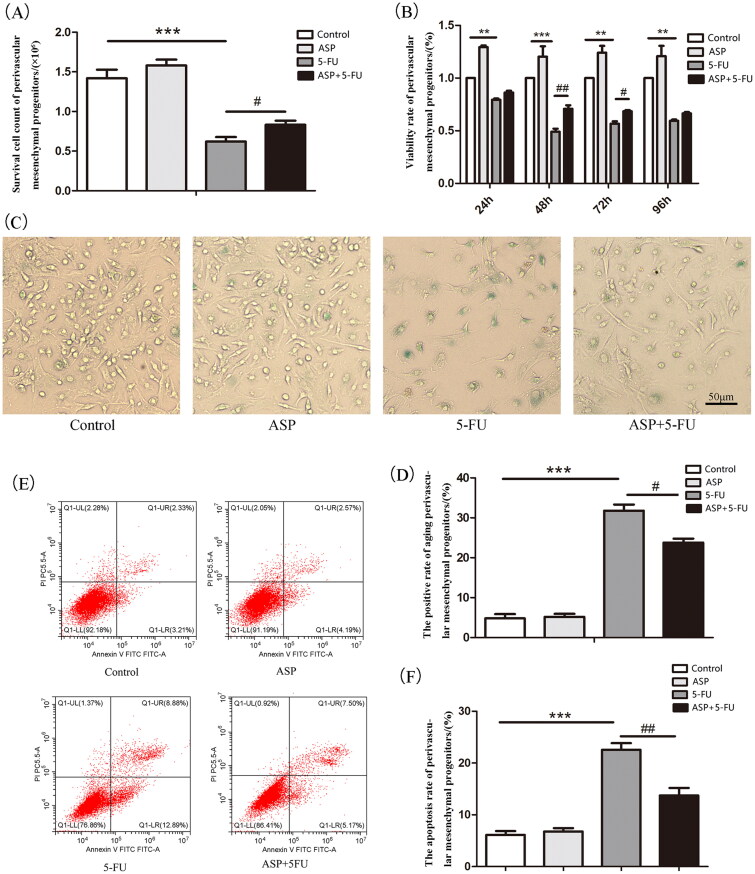
Perivascular mesenchymal progenitors have both osteogenic and adipogenic differentiation potentials, whereas the osteolineage can promote the formation or maintenance of HSC niches. In our study, although there was no significant difference in osteogenic and adipogenic differentiation between the control group and the ASP single-treated group, ASP treatment reversed a decrease in bone nodules caused by 5-FU toxicity (Figure 3(A,C)). In contrast, ASP reduced 5-FU-induced lipid droplet formation and volume (Figure 3(B,D)). In addition, the RT-PCR results revealed that ASP treatment antagonized the 5-FU-induced down-regulation of the mRNA expression of the osteogenesis-related factors Osterix, Runx2, and OPN (Figure 3(E)). These results imply that the toxicity of 5-FU drives perivascular mesenchymal progenitors at the cost of osteoblastic differentiation and that ASP can adjust the imbalance of osteogenic/adipogenic differentiation.
Figure 3.
Angelica sinensis polysaccharides protected the hematopoietic niche function of perivascular mesenchymal progenitors. (A) The representative image of induced osteogenic differentiation of perivascular mesenchymal progenitors in vitro is visualized by alizarin red staining. (B) The representative image of induced adipogenic differentiation in vitro of perivascular mesenchymal progenitors is stained by oil red O. (C) Alizarin red dye was dissolved in isopropyl alcohol, and the absorbance was measured at 490 nm, and quantitative analysis was performed by Image J software (n = 3). (D) The absorbance of dissolved oil red O dye at 490 nm was analyzed by Image J software. (n = 3) (E) Quantitative analysis of osteogenic-related mRNA expression (n = 3). (F) Quantitative analysis of hematopoiesis growth factor mRNA expression (n = 3). (G) Analysis of malondialdehyde (MDA) levels in perivascular mesenchymal progenitors and co-cultured hematopoietic cells. (H) Analysis of superoxide dismutase (SOD) levels in perivascular mesenchymal progenitors and co-cultured hematopoietic cells. (I) Quantitative analysis of mRNA relative expression of hematopoietic cell and perivascular mesenchymal progenitors interaction signaling molecules (n = 3). (J) Analysis of mRNA expression of adhesion molecules between hematopoietic cells and perivascular mesenchymal progenitors. (***p < 0.001 **p < 0.01 *p < 0.05 vs. control group; ##p < 0.01 #p < 0.05 vs. 5-fluorouracil (5-FU) group, n = 3).
The balance of stromal osteogenic/adipogenic differentiation is strictly regulated by ROS. Hence, we were able to detect these classic oxidative indicators. The present study showed that 5-FU caused perivascular mesenchymal progenitors to be in an oxidative stress state as lipid oxidative production of malondialdehyde (MDA) increased, whereas the antioxidant enzyme SOD level declined. As expected, ASP treatment alleviated the oxidative burden on perivascular mesenchymal progenitors by significantly increasing SOD and decreasing MDA levels (Figure 3(G,H)).
Evidence has demonstrated that hematopoietic growth factors, including CXCL12 and SCF, as well as the interaction of Ang-1/tie 2, TPO/MPL, Jagged-1/Notch signaling molecules, VCAM-1/VLA-4, and LFA-1/ICAM-1 adhesion molecule pairs between hematopoietic niche stroma and HSPCs are closely related to osteoblasts in the endosteal niche and play a vital role in hematopoietic regulation. Therefore, we analyzed the toxic effects of 5-FU on the expression of hematopoietic factors. In the current study, we demonstrated that except for Notch and Jagged-1, 5-FU significantly reduced most hematopoiesis-related factors, including Ang-1, Tie2, TPO, MPL, CXCL12, and SCF. However, ASP treatment restored the expression of hematopoietic factors (Figure 3(F,I)). It has also been found that 5-FU destroys cell adhesion molecules between hematopoietic cells and perivascular mesenchymal progenitors. Notably, ASP significantly restored the number of the corresponding pairs of adhesion molecules (Figure 3(J)). These results indicate that ASP treatment enhances the antioxidant capacity of perivascular mesenchymal progenitors, thus protecting the niche function of hematopoietic activity from 5-FU-induced oxidative damage.
ASP-treated perivascular mesenchymal progenitor feeder layer protected co-cultured hematopoietic cells from oxidative stress-induced premature senescence
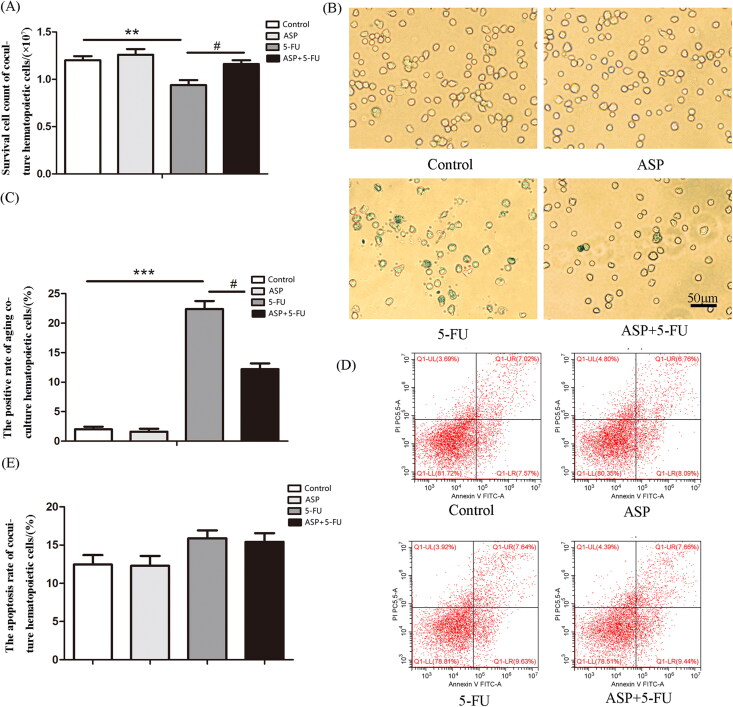
To elucidate whether injured perivascular mesenchymal progenitors negatively affect hematopoietic cells, a hematopoietic cell and conditional perivascular mesenchymal progenitor feeder layer co-culture system was established. The results demonstrated that the number of hematopoietic cells co-cultured with a 5-FU administrated feeder layer was significantly reduced than that in the control group due to senescence rather than apoptosis. Notably, the ASP-treated perivascular mesenchymal progenitor feeder layer rescued senescence, thus restoring the growth of co-cultured hematopoietic cells compared with the 5-FU group (Figure 4(A–E)). Further analysis of underlying mechanisms indicated that the ASP-treated perivascular mesenchymal progenitor feeder layer alleviated the 5-FU administrated layer-caused oxidative burden on hematopoietic cells (Figure 3(G,H)), suggesting that ASP may indirectly alleviate 5-FU-caused oxidative injury in hematopoietic cells via the protective mechanism of hematopoietic niche crosstalk.
Figure 4.
The Angelica sinensis polysaccharide (ASP)-treated perivascular mesenchymal progenitor feeder layer reversed 5-fluorouracil (5-FU)-induced cell growth inhibition and senescence of co-cultured hematopoietic cells. (A) Survival cell count of co-cultured hematopoietic cells is demonstrated by Trypan blue staining (**p < 0.001 vs. control group; #p < 0.05 vs. 5-FU group, n = 5). (B) The representative image of senescence-associated β-galactosidase (SA-β-gal) staining of co-cultured hematopoietic cells. The senescent hematopoietic cells were positively stained to be blue-green (scale bar = 50 μm). (C) The percentage of SA-β-gal positive co-cultured hematopoietic cells is presented as mean ± standard deviation (SD) (***p < 0.001 vs. control group; #p < 0.05 vs. 5-FU group, n = 3). (D) The representative image of Annexin V-FITC/PI double staining by flow cytometry. (E) Results of co-cultured hematopoietic cell apoptosis are presented as mean ± SD (n = 5).
Angelica sinensis polysaccharides antagonized oxidative stress-induced premature senescence of hematopoietic cells via Wnt/β-catenin signaling
Consequently, we found that the senescence of co-cultured hematopoietic cells was related to Wnt/β-catenin signaling. The results showed that the 5-FU-administrated feeder layer up-regulated P53 and P21 protein expression in co-cultured hematopoietic cells; however, ASP pre-treatment down-regulated the pivotal signal for early senescence (Figure 5(A,B)). Furthermore, the expression of β-catenin, phospho-GSK-3β, and cyclin-D1 in co-cultured hematopoietic cells in the 5-FU group was up-regulated, while the expression of GSK-3β protein was down-regulated. In contrast, compared with the 5-FU group, the expression of β-catenin, phospho-GSK-3β and cyclin-D1 of the co-cultured hematopoietic cells in the ASP + 5-FU group was down-regulated, whereas the expression of GSK-3β protein was up-regulated (Figure 5(C–G)). These data implied that ASP-treated perivascular mesenchymal progenitors may protect surrounding hematopoietic cells from oxidative stress-induced premature senescence by inhibiting overactivation of the Wnt/β-catenin signaling pathway.
Figure 5.
Angelica sinensis polysaccharides antagonized oxidative stress-induced premature senescence of hematopoietic cells via Wnt/β-catenin signaling. (A) The representative image of P53 and P21 protein expression in hematopoietic cells as detected by Western blotting. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was probed as loading control. (B) The histograms of P53 and P21 protein expression are presented. Data are presented as mean ± standard deviation (SD) (**p < 0.01 *p < 0.05 vs. control group; #p < 0.05 vs. 5-fluorouracil (5-FU) group, n = 3). (C) The Wnt signaling-related protein expression in hematopoietic cells was detected by Western blot. GAPDH was probed as a loading control. (D–G) The histograms of Wnt signaling-related protein expression are presented. Data are presented as mean ± SD (***p < 0.001 **p < 0.01 *p < 0.05 vs. control group; #p < 0.05 vs. 5-FU group, n = 3).
Discussion
The use of alkylating agents, pyrimidine analogs, anthracyclines, anthraquinones, nitrosoureas, methotrexate, hydroxyurea, and mitomycin C chemotherapeutic drugs causing cytotoxicity, bone marrow stromal rejection of stem cell transplants, and cell-adhesion-mediated drug resistance, is a frequent source of hematopoietic dysfunction after chemotherapy (Schepers et al. 2015; Yamashita et al. 2020). Myelosuppression is the most common complication of chemotherapy (Wang et al. 2015; Atkins and He 2019). Acute myelosuppression often occurs because of the death of hematopoietic progenitors and hematopoietic cells, whereas long-term bone marrow suppression is much more serious because of a decrease in HSC reserves and a defect in HSC self-renewal, which are usually closely related to injury of the hematopoietic niche (Yamashita et al. 2020). In a previous study (Xiao et al. 2017), we demonstrated that 5-FU causes oxidative damage to bone marrow stromal cells. However, it is still not elucidated whether 5-FU injures the hematopoietic niche.
Although the concepts of hematopoietic niche composition and niche function are controversial, recent evidence has demonstrated that distinct hematopoietic progenitors have distinct niches (Richter et al. 2017; Tikhonova et al. 2019). As previously suggested, osteoblasts do not directly regulate HSC maintenance. However, they also regulate B-lineage progenitors to support B lymphopoiesis. In addition to endothelial cells, perivascular mesenchymal cells around the bone marrow sinusoids and arterioles promote HSC maintenance and produce pivotal hematopoietic cytokines, such as SCF and CXCL12, to support primitive hematopoietic cells (Pinho and Frenette 2019). These perivascular mesenchymal cells are adjacent to bone surfaces in trabecular-rich areas and are also termed perivascular mesenchymal progenitors as they have both osteogenic and adipogenic differentiation potentials, of which the osteogenic potential may be sufficient to create bony ossicles that are invested in by the host vasculature and HSCs. Thus, perivascular mesenchymal progenitors from the perivascular niche promote HSC formation and maintenance (Pinho et al. 2013; Zhou et al. 2014; Rodeheffer and Horowitz 2016). In the present study, we treated femoral and tibial fragments with type II collagenase and successfully isolated perivascular mesenchymal progenitors, which highly expressed Nestin, Lepr, Cxcl12 niche genes, OPN, and the osteogenesis-related gene Runt-related transcription factor 2 (Runx2). Therefore, we focused on whether 5-FU negatively affects the perivascular niche in the bone marrow.
Previous studies have shown that under physiological circumstances, the accumulation of adipocytes accompanied by ROS in the bone marrow progresses with age, particularly in humans, and causes dysfunction in bone marrow microenvironments, thereby compromising hematopoiesis (Ambrosi et al. 2017). Additionally, long-term chemotherapy may impair the hematopoietic microenvironment, resulting in bone marrow adiposity, which may be related to oxidative stress (Naveiras et al. 2009). In this study, we first demonstrated that 5-FU causes oxidative stress in perivascular mesenchymal progenitors, leading to apoptosis or senescence, thus retarding growth. Moreover, oxidative stress impairs diverse facets of hematopoietic niche function. First, it has been suggested that oxidative stress drives the adipogenic differentiation of perivascular mesenchymal progenitors. Previous studies suggested that the immature state of perivascular mesenchymal progenitors is important for HSC maintenance. Osteolineage cells promote hematopoiesis. In contrast, adipocytes inhibit hematopoiesis. Adipogenic differentiation relies on the expression of peroxisome proliferator-activated receptor γ (PPARγ), and agonists of the PPARγ pathway promote the formation of adipocytes at the expense of osteoblasts (Rosen et al. 1999). However, osteogenic differentiation cannot proceed without the expression of RUNX2 and its downstream effects on osterix expression (Komori 2019). In a study combining OPN-deficient mice with exogenous OPN, Stier et al. (2005) demonstrated that OPN modifies primitive hematopoietic cell numbers and functions in a stem cell-non-autonomous manner. In the present study, after 5-FU treatment, the number of bone nodules originating from perivascular mesenchymal progenitors decreased, whereas the number of lipid droplets increased. In addition, the gene expression of Runx2, osterix, and OPN dramatically decreased, suggesting that the intoxicated perivascular mesenchymal progenitors are driven towards adipogenic differentiation at the cost of osteogenic differentiation.
It is commonly known that SCF is required for the maintenance of HSCs and c-kit(+)-restricted progenitors as well as erythropoiesis, mast cell development, and lymphopoiesis (Ponomaryov et al. 2000; Waskow et al. 2002). CXCL12 is another chemokine required for the maintenance and retention of HSCs in the bone marrow. Global deletion of CXCL12 or the gene encoding its receptor, Cxcr4, depletes HSCs from the bone marrow (Tzeng et al. 2011). Importantly, evidence has shown that CXCL12 and SCF are primarily expressed by perivascular mesenchymal progenitors, that is, CAR cells (CXCL12 abundant reticular cells) and Nestin+, Lepr+, or Prx-1+ cells, at 100-fold higher levels than endothelial cells and 1000-fold higher levels than osteoblasts (Zhou et al. 2014). Here, we show that 5-FU blunts CXCL12 and SCF secretion in perivascular mesenchymal progenitors.
Arai et al. (2004) demonstrated that HSCs expressing the receptor Tie2 are quiescent. Ang-1, the ligand for Tie2, enhances the ability of HSCs to become quiescent and induces their adhesion to bone, protecting them from stresses that suppress hematopoiesis. TPO and its receptor MPL are the master regulators of megakaryopoiesis and HSCs. TPO has been shown to increase HSC interactions with the osteoblastic niche and support HSC quiescence and expansion post-transplantation (Fox et al. 2002; Qian et al. 2007; Bigas and Espinosa 2012). Mice deficient in TPO or MPL display increased HSC cycling and progressive age-related loss (Qian et al. 2007; Yoshihara et al. 2007). In addition, it has been reported that the co-culture of murine or human HSCs with immobilized Notch ligands or feeder cells expressing such ligands can maintain or even enhance HSC self-renewal (Stier et al. 2002). Hence, in this study, we focused on three pairs of ligand receptors. It was found that 5-FU decreased the expression of Ang-1 and TPO in perivascular mesenchymal progenitors and decreased Tie2 and MPL expression in hematopoietic cells, thus abrogating these hematopoietic signaling pathways and interfering with the cross-talk between the stroma and hematopoietic cells.
Cell-cell interactions between stromal cells and HSCs and cell-matrix interactions in the bone marrow are additional important factors in HSC maintenance. Integrins and selectins are essential mediators (Pillozzi and Becchetti 2012; Ding and Morrison 2013). Membrane-bound receptors of the immunoglobulin superfamily, including intercellular adhesion molecule-1 (ICAM-1; CD54), vascular cell adhesion molecule-1 (VCAM-1; CD105), and CD166, also constitute the bone marrow niche. ICAM-1/LFA-1 and VCAM-1/VLA-4 adhesion pairs are important for hematopoietic stem cell proliferation in mice (Chen et al. 2011). Overexpression or downregulation of these adhesion molecules affects HSC function. In the current study, we analyzed stromal and hematopoietic cell adhesion molecules. It was further demonstrated that 5-FU toxicity dramatically impaired adhesion between perivascular mesenchymal progenitors and hematopoietic cells. In summary, 5-FU caused oxidative damage to perivascular mesenchymal progenitors and destroyed diverse facets of perivascular hematopoietic niche function. We hypothesized that this might result in an ensuing stress response in the surrounding hematopoietic cells.
To elucidate the direct regulatory role of dysfunctional perivascular mesenchymal progenitors in hematopoietic cells, perivascular mesenchymal progenitors and hematopoietic cell co-culture systems were established. In accordance with our expectations, the 5-FU treated feeder layer caused an oxidative burden on hematopoietic cells, and ROS scavenging was impaired, which is in line with our previous study that showed that the 5-FU-treated HS-5 feeder layer decreased the expression level of Cx43 gap junction channels, thus reducing ROS transfer scavenging from HSCs to stromal cells. Interestingly, in the current study, it was found that the direct effect of the 5-FU treated feeder layer on hematopoietic cells is senescence rather than apoptosis, and the underlying mechanism involves the overactivated Wnt/β-catenin signaling pathway in hematopoietic cells. The Wnt/β-catenin signaling pathway is one of the crucial pathways in hematopoietic regulation, and many signaling molecules are involved (Nemeth and Bodine 2007; Ahmadzadeh et al. 2016). Moreover, Wnt/β-catenin signaling pathway is closely related to cellular senescence (Brack et al. 2007; Zhang et al. 2013). β-catenin is a representative protein of Wnt/β-catenin signaling pathway, and its expression level is a critical indicator of the activated Wnt/β-catenin signaling pathway. Inversely, glycogen synthase kinase-3β (GSK-3β) is a pivotal negative regulator, which is involved in the formation of a complex that phosphorylates and degrades β-catenin. Gsk-3β activity is regulated by phospho-GSK-3β, which activates the Wnt/β-catenin signaling pathway (Kim et al. 2013; Ahmadzadeh et al. 2016). Cyclin-d1 is a key downstream target of β-catenin, which regulates the cell cycle. Based on our studies, 5-FU damaged perivascular mesenchymal progenitors led to oxidative stress-induced premature senescence of co-cultured hematopoietic cells via the Wnt/β-catenin signaling pathway overactivation.
Angelica sinensis polysaccharides (ASP) are the main biologically active ingredients of the traditional Chinese medicine Angelica sinensis and are commonly used to enrich blood and antioxidant bioactivity (Zhao et al. 2012). In the present study, it was further suggested that the direct antioxidative protective role of ASP might be to maintain perivascular niche function via the regulation of hematopoietic cytokines, cell adhesion molecules, and hematopoietic cell-stromal cell interaction signaling molecules, thus providing a homeostatic microenvironment for HSPCs regeneration. Also, ASP may indirectly protect hematopoietic cells via inhibition of the overactivation of oxidative stress-induced Wnt/β-catenin signaling pathway after chemotherapy. The underlying mechanism may be that ASP antagonizes chemotherapeutic drugs and enhances oxidative stress transfer via perivascular mesenchymal progenitors. Thus, ASP may be a promising ingredient for the therapeutic prevention of chemoradiation.
Conclusions
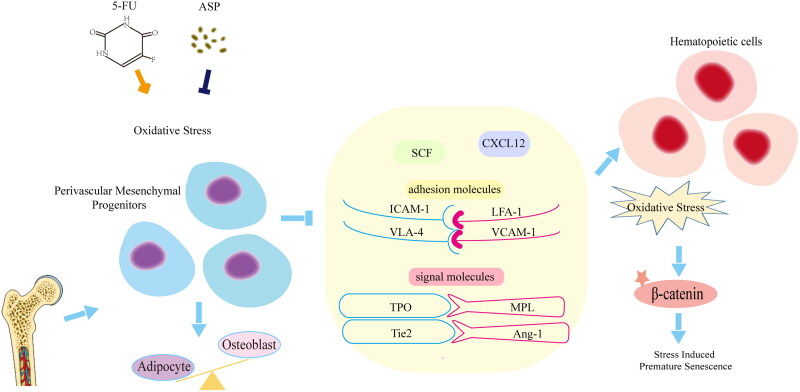
5-FU exerts toxic effects on the perivascular niche. 5-FU induces oxidative damage in perivascular mesenchymal progenitors, thus altering the osteogenic/adipogenic balance and downregulating the expression of hematopoietic cytokines, cell adhesion molecules, and cellular interaction signaling, resulting in stress-induced premature senescence of co-cultured hematopoietic cells. ASP may play a direct protective role by improving oxidative damage, and regulating perivascular niche function, thus indirectly inhibited the Wnt/β-catenin signaling pathway-mediated premature senescence of hematopoietic cells (Figure 6).
Figure 6.
Mechanism of Angelica sinensis polysaccharides (ASP) retarding the premature senescence of hematopoietic cells caused by 5-fluorouracil (5-FU) via maintaining niche function of perivascular mesenchymal progenitor cells.
Funding Statement
This study was supported by the General Program of the Natural Science Foundation of Chongqing, China (grant number: cstc2021jcyj-msxmX0669).
Authors’ contributions
Yilin Niu, Hanxianzhi Xiao and Biyao Wang performed the experiments. Yilin Niu analyzed the data and wrote the manuscript. Wang and Du contributed to the preparation of the analysis tools and reagents. Yaping Wang provided technical support. Lu Wang conceived the experiments and revised the manuscript. All the study authors have read and approved the final version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Ahmadzadeh A, Norozi F, Shahrabi S, Shahjahani M, Saki N.. 2016. Wnt/β-catenin signaling in bone marrow niche. Cell Tissue Res. 363(2):321–335. [DOI] [PubMed] [Google Scholar]
- Ai S, Fan X, Fan L, Sun Q, Liu Y, Tao X, Dai K.. 2013. Extraction and chemical characterization of Angelica sinensis polysaccharides and its antioxidant activity. Carbohydr Polym. 94(2):731–736. [DOI] [PubMed] [Google Scholar]
- Ambrosi TH, Scialdone A, Graja A, Gohlke S, Jank AM, Bocian C, Woelk L, Fan H, Logan DW, Schürmann A, et al. 2017. Adipocyte accumulation in the bone marrow during obesity and aging impairs stem cell-based hematopoietic and bone regeneration. Cell Stem Cell. 20(6):771–784.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T.. 2004. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 118(2):149–161. [DOI] [PubMed] [Google Scholar]
- Atkins S, He F.. 2019. Chemotherapy and beyond: infections in the era of old and new treatments for hematologic malignancies. Infect Dis Clin North Am. 33(2):289–309. [DOI] [PubMed] [Google Scholar]
- Bigas A, Espinosa L.. 2012. Hematopoietic stem cells: to be or notch to be. Blood. The J Am Soc Hematol. 119(14):3226–3235. [DOI] [PubMed] [Google Scholar]
- Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA.. 2007. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 317(5839):807–810. [DOI] [PubMed] [Google Scholar]
- Chen L, Zhao Q, Wang XL, You R, Zhang YH, Ji H, Lai YS.. 2011. ZLJ-6, a novel COX/5-LOX inhibitor, attenuates TNF-α-induced endothelial E-selectin, ICAM-1 and VCAM-1 expression and monocyte-endothelial interactions via a COX/5-LOX-independent mechanism. Vascul Pharmacol. 55(5–6):135–142. [DOI] [PubMed] [Google Scholar]
- Chen Z, Cheng L, Zhang J, Cui X.. 2020. Angelica sinensis polysaccharide prevents mitochondrial apoptosis by regulating the Treg/Th17 ratio in aplastic anemia. BMC Complement Med Ther. 20(1):192. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Cheng X, Yao H, Xiang Y, Chen L, Xiao M, Wang Z, Xiao H, Wang L, Wang S, Wang Y.. 2019. Effect of Angelica polysaccharide on brain senescence of Nestin-GFP mice induced by d-galactose. Neurochem Int. 122:149–156. [DOI] [PubMed] [Google Scholar]
- Ding L, Morrison SJ.. 2013. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 495(7440):231–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Saunders TL, Enikolopov G, Morrison SJ.. 2012. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 481(7382):457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox N, Priestley G, Papayannopoulou T, Kaushansky K.. 2002. Thrombopoietin expands hematopoietic stem cells after transplantation. J Clin Invest. 110(3):389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ.. 2005. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 121(7):1109–1121. [DOI] [PubMed] [Google Scholar]
- Kim W, Kim M, Jho EH.. 2013. Wnt/β-catenin signalling: from plasma membrane to nucleus. Biochem J. 450(1):9–21. [DOI] [PubMed] [Google Scholar]
- Komori T. 2019. Regulation of proliferation, differentiation and functions of osteoblasts by Runx2. IJMS. 20(7):1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu PJ, Hsieh WT, Huang SH, Liao HF, Chiang BH.. 2010. Hematopoietic effect of water-soluble polysaccharides from Angelica sinensis on mice with acute blood loss. Exp Hematol. 38(6):437–445. [DOI] [PubMed] [Google Scholar]
- Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS.. 2010. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 466(7308):829–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu X, Zhang Y, Li J, Xia J, Chen X, Jing P, Song X, Wang L, Wang Y.. 2017. 2017: 1687-966X. Angelica sinensis polysaccharide prevents hematopoietic stem cells senescence in d-galactose-induced aging mouse model. Stem Cells Int. 2017:3508907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ.. 2009. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 460(7252):259–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth MJ, Bodine DM.. 2007. Regulation of hematopoiesis and the hematopoietic stem cell niche by Wnt signaling pathways. Cell Res. 17(9):746–758. [DOI] [PubMed] [Google Scholar]
- Parchment RE, Gordon M, Grieshaber CK, Sessa C, Volpe D, Ghielmini M.. 1998. Predicting hematological toxicity (myelosuppression) of cytotoxic drug therapy from in vitro tests. Ann Oncol. 9(4):357–364. [DOI] [PubMed] [Google Scholar]
- Pillozzi S, Becchetti A.. 2012. Ion channels in hematopoietic and mesenchymal stem cells. Stem Cells Int. 2012:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinho S, Frenette PS.. 2019. Haematopoietic stem cell activity and interactions with the niche. Nat Rev Mol Cell Biol. 20(5):303–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinho S, Lacombe J, Hanoun M, Mizoguchi T, Bruns I, Kunisaki Y, Frenette PS.. 2013. PDGFRα and CD51 mark human nestin + sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. J Exp Med. 210(7):1351–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman K, Perren T, Ward U, Primrose J, Slevin M, Patel N, Selby P.. 1993. Pharmacokinetics of 5-fluorouracil in colorectal cancer patients receiving interferon. Ann Oncol. 4(6):515–516. [DOI] [PubMed] [Google Scholar]
- Ponomaryov T, Peled A, Petit I, Taichman RS, Habler L, Sandbank J, Arenzana-Seisdedos F, Magerus A, Caruz A, Fujii N, et al. 2000. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J Clin Invest. 106(11):1331–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian H, Buza-Vidas N, Hyland CD, Jensen CT, Antonchuk J, Månsson R, Thoren LA, Ekblom M, Alexander WS, Jacobsen SEW.. 2007. Critical role of thrombopoietin in maintaining adult quiescent hematopoietic stem cells. Cell Stem Cell. 1(6):671–684. [DOI] [PubMed] [Google Scholar]
- Ren F, Li J, Wang Y, Wang Y, Feng S, Yuan Z, Qian X.. 2018. The effects of Angelica sinensis polysaccharide on tumor growth and iron metabolism by regulating hepcidin in tumor-bearing mice. Cell Physiol Biochem. 47(3):1084–1094. [DOI] [PubMed] [Google Scholar]
- Richter R, Forssmann W, Henschler R.. 2017. Current developments in mobilization of hematopoietic stem and progenitor cells and their interaction with niches in bone marrow. Transfus Med Hemother. 44(3):151–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodeheffer MS, Horowitz MC.. 2016. Fat decisions: leptin regulates bone versus fat in the marrow. Cell Stem Cell. 18(6):684–686. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM.. 1999. PPARγ is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 4(4):611–617. [DOI] [PubMed] [Google Scholar]
- Sahin AO, Buitenhuis M.. 2012. Molecular mechanisms underlying adhesion and migration of hematopoietic stem cells. Cell Adh Migr. 6(1):39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepers K, Campbell TB, Passegué E.. 2015. Normal and leukemic stem cell niches: insights and therapeutic opportunities. Cell Stem Cell. 16(3):254–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal FJT, Chhatta A, Mikkers H.. 2016. Caught in a Wnt storm: complexities of Wnt signaling in hematopoiesis. Exp Hematol. 44(6):451–457. [DOI] [PubMed] [Google Scholar]
- Stier S, Cheng T, Dombkowski D, Carlesso N, Scadden DT.. 2002. Notch1 activation increases hematopoietic stem cell self-renewal in vivo and favors lymphoid over myeloid lineage outcome. Blood. 99(7):2369–2378. [DOI] [PubMed] [Google Scholar]
- Stier S, Ko Y, Forkert R, Lutz C, Neuhaus T, Grünewald E, Cheng T, Dombkowski D, Calvi LM, Rittling SR, et al. 2005. Osteopontin is a hematopoietic stem cell niche component that negatively regulates stem cell pool size. J Exp Med. 201(11):1781–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimura R, Jha DK, Han A, Soria-Valles C, Da Rocha EL, Lu YF, Goettel JA, Serrao E, Rowe RG, Malleshaiah M, et al. 2017. Haematopoietic stem and progenitor cells from human pluripotent stem cells. Nature. 545(7655):432–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikhonova AN, Dolgalev I, Hu H, Sivaraj KK, Hoxha E, Cuesta-Domínguez Á, Pinho S, Akhmetzyanova I, Gao J, Witkowski M, et al. 2019. The bone marrow microenvironment at single-cell resolution. Nature. 569(7755):222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng YS, Li H, Kang YL, Chen WC, Cheng WC, Lai DM.. 2011. Loss of CXCL12/Sdf-1 in adult mice decreases the quiescent state of hematopoietic stem/progenitor cells and alters the pattern of hematopoietic regeneration after myelosuppression. Blood. 117(2):429–439. [DOI] [PubMed] [Google Scholar]
- Van Laere AS, Nguyen M, Braunschweig M, Nezer C, Collette C, Moreau L, Archibald AL, Haley CS, Buys N, Tally M, et al. 2003. A regulatory mutation in IGF2 causes a major QTL effect on muscle growth in the pig. Nature. 425(6960):832–836. [DOI] [PubMed] [Google Scholar]
- Vodenkova S, Buchler T, Cervena K, Veskrnova V, Vodicka P, Vymetalkova V.. 2020. 5-Fluorouracil and other fluoropyrimidines in colorectal cancer: past, present and future. Pharmacol Ther. 206:107447. [DOI] [PubMed] [Google Scholar]
- Wang S, Zheng G, Tian S, Zhang Y, Shen L, Pak Y, Shen Y, Qian J.. 2015. Echinacoside improves hematopoietic function in 5-FU-induced myelosuppression mice. Life Sci. 123:86–92. [DOI] [PubMed] [Google Scholar]
- Waskow C, Paul S, Haller C, Gassmann M, Rodewald HR.. 2002. Viable c-Kit(W/W) mutants reveal pivotal role for c-kit in the maintenance of lymphopoiesis. Immunity. 17(3):277–288. [DOI] [PubMed] [Google Scholar]
- Wei WL, Zeng R, Gu CM, Qu Y, Huang LF.. 2016. Angelica sinensis in China-A review of botanical profile, ethnopharmacology, phytochemistry and chemical analysis. J Ethnopharmacol. 190:116–141. [DOI] [PubMed] [Google Scholar]
- Xiao H, Xiong L, Song X, Jin P, Chen L, Chen X, Yao H, Wang Y, Wang L.. 2017. Angelica sinensis polysaccharides ameliorate stress-induced premature senescence of hematopoietic cell via protecting bone marrow stromal cells from oxidative injuries caused by 5-fluorouracil. IJMS. 18(11):2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M, Dellorusso PV, Olson OC, Passegué E.. 2020. Dysregulated haematopoietic stem cell behaviour in myeloid leukaemogenesis. Nat Rev Cancer. 20(7):365–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara H, Arai F, Hosokawa K, Hagiwara T, Takubo K, Nakamura Y, Gomei Y, Iwasaki H, Matsuoka S, Miyamoto K, et al. 2007. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell. 1(6):685–697. [DOI] [PubMed] [Google Scholar]
- Zeng D, Wang Y, Chen Y, Li D, Li G, Xiao H, Hou J, Wang Z, Hu L, Wang L, et al. 2021. Angelica polysaccharide antagonizes 5-FU-induced oxidative stress injury to reduce apoptosis in the liver through Nrf2 pathway. Front Oncol. 11:720620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DY, Pan Y, Zhang C, Yan BX, Yu SS, Wu DL, Shi MM, Shi K, Cai XX, Zhou SS, et al. 2013. Wnt/β-catenin signaling induces the aging of mesenchymal stem cells through promoting the ROS production. Mol Cell Biochem. 374(1-2):13–20. [DOI] [PubMed] [Google Scholar]
- Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, et al. 2003. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 425(6960):836–841. [DOI] [PubMed] [Google Scholar]
- Zhao L, Wang Y, Shen HL, Shen XD, Nie Y, Wang Y, Han T, Yin M, Zhang QY.. 2012. Structural characterization and radioprotection of bone marrow hematopoiesis of two novel polysaccharides from the root of Angelica sinensis (Oliv.) Diels. Fitoterapia. 83(8):1712–1720. [DOI] [PubMed] [Google Scholar]
- Zhou BO, Yue R, Murphy MM, Peyer JG, Morrison SJ.. 2014. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell. 15(2):154–168. [DOI] [PMC free article] [PubMed] [Google Scholar]