Abstract
Introduction: Many people infected with coronavirus disease 2019 (COVID-19) have developed post-COVID-19 symptoms, which are defined as symptoms and signs (e.g., anosmia and ageusia) that persist for more than 12 weeks after getting infected with COVID-19. These symptoms may appear after or during the infection and cannot be explained by any alternative disease. In this study, we aim to investigate the factors that affect the duration of anosmia and ageusia in Saudi Arabia.
Methods: We conducted a nationwide, cross-sectional study using an online survey in Saudi Arabia from 14 February 2022 to 23 July 2022. The electronic survey was distributed using social media platforms, such as Twitter, WhatsApp, and Telegram.
Result: The study enrolled 2497 individuals who were infected with COVID-19. A total of 60.1% of the participants showed symptoms of anosmia, ageusia, or both after getting infected with COVID-19. According to our data, we found that being a female and not having a repeated COVID-19 infection were risk factors (independent predictors) of the long duration of anosmia after COVID-19 recovery (p = <0.05). While being a male patient, a smoker, and being admitted to the ICU were risk factors (independent predictors) of long duration of ageusia after COVID-19 recovery (p = <0.05).
Conclusion: In conclusion, the prevalence of chemosensory dysfunction symptoms, both olfactory and gustatory, after COVID-19 infection among the Saudi population was high. However, several factors can influence their duration, including gender, smoking, and severity of the infection.
Keywords: anosmia, covid-19, factors, duration, post-covid-19, chemosensory dysfunction, loss of taste, ageusia, loss of smell
Introduction
The coronavirus disease 2019 (COVID-19) outbreak is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which resulted in a worldwide crisis of a highly infectious disease [1].
The clinical picture of COVID-19 symptoms is a dynamic process in which healthcare workers get a clearer image by observing numerous manifestations affecting different organs. Anosmia (loss of smell) and ageusia (loss of taste) are predictive symptoms and the most common chemosensory dysfunction complaints in post-COVID-19 syndrome. This syndrome is defined by persistent clinical signs and symptoms that appear while or after suffering from COVID-19 infection for more than 12 weeks and cannot be explained by an alternative diagnosis [2,3].
As there are many viruses, such as Epstein-Barr virus, rhinovirus, and para influenza virus, which can lead to smell and taste disorders, different studies have reported that SARS-CoV-2 can also result in chemosensory dysfunctions [4,5]. Hence, several pieces of evidence revealed that olfactory disorder seems to be more prevalent than gustatory dysfunctions [4,6].
The exact pathogenesis behind the mechanism of olfactory and gustatory dysfunctions following the infection remains unclear. However, few studies have reported different hypotheses, in which the virus affects mainly the neurons present in the cerebral cortex and hypothalamus [2,5].
Evidence shows that both anosmia and ageusia are highly prevalent in Saudi Arabia during and after the COVID-19 infection [7,8]. Additionally, patients with anosmia and ageusia may experience a marked decline in quality of life [9]. Therefore, we aimed in this study to investigate the factors affecting the duration of these olfactory and gustatory dysfunctions following the infection.
Materials and methods
Study design and participants
This descriptive cross-sectional study was conducted among patients with a confirmed diagnosis of COVID-19 by rhino‐pharyngeal swab and patients who presented with symptoms of anosmia and ageusia in the northern, southern, eastern, western, and central regions of Saudi Arabia in 2022. We excluded patients who did not confirm the diagnosis by rhino‐pharyngeal swab.
Ethical consideration and sampling strategy
After structuring the questionnaire using Google Forms (Google, Mountain View, CA), it was publicized to the Saudi community on social media platforms such as WhatsApp, Telegram, and Twitter, and data were collected from 14 February 2022 to 23 July 2022. Ethical approval was obtained from the Biomedical Ethics Committee at Umm Al-Qura University (UQU), College of Medicine, Makkah, KSA, on 14 February 2022 (IRB: HAPO-02-K-012-2022-02-955).
Using Raosoft Sample Size Calculator software (Raosoft, Inc., Seattle, WA) based on the Saudi Arabia population, the minimum sample size to accomplish a precision of 5% with a 95% confidence interval (CI) was obtained as 385 patients [10]. However, our final sample size during data collection included 2497 participants who were infected by COVID-19.
Study tool
The study included 2497 participants. A web-based questionnaire was used. The survey was adapted based on a previously published study [2]. It was formulated in Arabic and English. Furthermore, to ensure clarity and simplicity, a pilot study was conducted. The results of the pilot study were excluded from the final analysis.
The questionnaire was composed of four parts, including consent for participation in the study, demographics data, data about COVID-19 infection, such as COVID-19 severity, vaccination status, and recurrent COVID-19 infection, and the last part collected data on risk factors of the long duration of chemosensory dysfunction.
Statistical analysis
The obtained data were initially gathered in an Excel sheet (Microsoft Corporation, Redmond, WA). Afterward, we used SPSS software version 26 (IBM Corp., Armonk, NY) for data analysis. The chi-squared test (χ2) was applied to qualitative data that were expressed as numbers and percentages to examine the relationship between the variables. The Mann-Whitney and Kruskal-Wallis tests were used to analyze the relationship between the quantitative non-parametric variables that were expressed as mean and standard deviation (mean ± SD). Spearman's test was used for correlation analysis. Multivariate logistic regression analysis was done to determine risk factors of long-duration of anosmia and ageusia after COVID-19 recovery. The odds ratio was calculated at a CI of 95%. A p-value of less than 0.05 was regarded as statistically significant.
Results
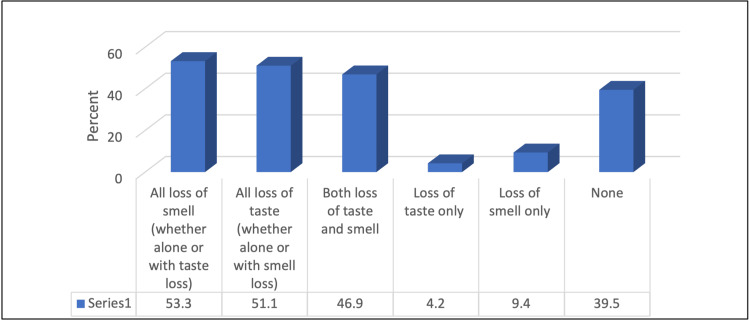
A total of 4158 individuals participated in the study. We included 2497 participants who confirmed the diagnosis of COVID-19 by rhino‐pharyngeal swab. Among those patients, 53.3% had anosmia and 51.1% had ageusia, as shown in Figure 1. Around 38.4% of the participants were aged ≤ 24 years. In addition, most of the participants were females (67.7%). Their mean BMI was 26.19 ± 6.46 kg/m2. The most common blood group was O+ (43.6%). Only 11.5% of participants were smokers, with a mean smoking duration of 10.39 ± 9.79 years and a mean number of daily smoked cigarettes of 11.24 ± 9.36. Only 1% were drinking alcohol. In addition, 34.6% had chronic diseases, and the most common diseases were diabetes mellitus (34.3%) and hypertension (30.9%). Only 18.5% were receiving medications. Furthermore, 15.9% of the study participants were infected with COVID-19 repeatedly, with a mean of 2.09 ± 0.29 times. A total of 76.5% of them were immunized against COVID-19. The majority (29.5%) had two doses with a mean number of doses of 1.62 ± 0.108 doses. A total of 4.6% of patients had chronic rhinosinusitis in the past. Study characteristics are provided in detail in Tables 1, 2.
Table 1. Distribution of studied participants according to their age, gender, blood group, smoking, alcohol consumption, chronic diseases, and medication.
| Variable | No. (%) |
| Age (years) | |
| ≤24 | 960 (38.4) |
| 25-40 | 800 (32) |
| 41-60 | 691 (27.7) |
| >60 | 46 (1.8) |
| Gender | |
| Female | 1691 (67.7) |
| Male | 806 (32.3) |
| BMI (kg/m2) | 26.19 ± 6.46 |
| Blood group | |
| A+ | 765 (30.6) |
| A- | 63 (2.5) |
| B+ | 328 (13.1) |
| B- | 35 (1.4) |
| O+ | 1088 (43.6) |
| O- | 112 (4.5) |
| AB+ | 97 (3.9) |
| AB- | 9 (0.4) |
| Smoker | |
| Yes | 287 (11.5) |
| No | 2210 (88.5) |
| If a smoker: smoking duration (years) | 10.39 ± 9.79 |
| Number of smoked cigarettes (daily) | 11.24 ± 9.36 |
| Do you drink alcohol? | |
| Yes | 24 (1) |
| No | 2473 (99) |
| Chronic diseases | |
| Yes | 864 (34.6) |
| No | 1633 (65.4) |
| If yes, what disease? (No.: 864) | |
| Chronic allergy | 182 (21) |
| Psychological disease | 51 (5.9) |
| Obesity | 166 (19.2) |
| Diabetes mellitus | 297 (34.3) |
| Thyroid disorder | 117 (13.5) |
| Hypertension | 267 (30.9) |
| Respiratory disorder | 183 (21.1) |
| Psoriasis | 17 (1.9) |
| Immunological disorder | 16 (1.8) |
| Cardiovascular diseases | 66 (7.6) |
| Are you receiving any chronic medications? | |
| Yes | 463 (18.5) |
| No | 2034 (81.5) |
Table 2. Distribution of studied participants according to circumstances related to COVID-19 infection.
| Variable | No. (%) |
| Have you been infected with COVID-19 repeatedly? | |
| Yes | 398 (15.9) |
| No | 2099 (84.1) |
| If yes, how many times have you contracted COVID-19? | 2.09 ± 0.29 |
| Status of immunization against COVID-19 at the time of COVID-19 infection? | |
| Not vaccinated | 586 (23.5) |
| Vaccinated | 1911 (76.5) |
| If vaccinated, how many doses? (No.: 1911) | |
| 2 doses | 980 (39.5) |
| 3 doses | 575 (23) |
| 1 dose | 356 (14.3) |
| Number of doses (mean ± SD) | 1.62 ± 0.108 |
| What are the symptoms of COVID-19 that you have? | |
| Headache | 1974 (79) |
| Fever | 1683 (67.4) |
| Cough | 1344 (53.8) |
| Nausea vomiting | 501 (20) |
| Nose congestion | 1484 (59.4) |
| Sore throat | 1558 (62.3) |
| Runny nose | 1466 (58.7) |
| Dyspnea | 686 (27.4) |
| Body ache | 1083 (43.3) |
| Fatigue | 660 (26.4) |
| Diarrhea | 383 (15.3) |
| Loss of taste | 106 (4.2) |
| Loss of smell | 234 (9.4) |
| What was the first symptom to appear? | |
| Sore throat | 525 (21) |
| Dyspnea | 60 (2.4) |
| Cough | 199 (7.9) |
| Body aches | 24 (0.9) |
| Fatigue | 108 (4.3) |
| Nose congestion | 46 (1.8) |
| Fever | 507 (20.3) |
| Diarrhea | 15 (0.6) |
| Runny nose | 108 (4.3) |
| Headache | 381 (15.2) |
| Loss of taste | 56 (2.2) |
| Loss of smell | 77 (3.1) |
| How long do COVID-19 symptoms last? | |
| 1-3 days | 846 (33.9) |
| 4-6 days | 1031 (41.3) |
| ≥7 days | 620 (24.8) |
| Past history | |
| Nasal fracture | 12 (0.5) |
| Chronic rhinosinusitis | 116 (4.6) |
| Smell alteration before COVID | 55 (2.2) |
| Taste alteration before COVID | 34 (1.4) |
| Malnutrition | 53 (2.1) |
| Surgery or radiotherapy in oral or nasal cavities | 16 (0.6) |
| Antibiotics use | 14 (0.6) |
| Allergic rhinitis | 43 (1.7) |
| Stroke | 2 (0.1) |
| Nasal polyps | 10 (0.4) |
| Multiple sclerosis | 1 (0.01) |
| Head injury | 2 (0.1) |
| Epilepsy | 2 (0.1) |
| Did you need to be hospitalized due to COVID-19? | |
| Yes | 65 (2.6) |
| No | 2432 (97.4) |
| If the answer is yes, please mention the number of days spent in the hospital | 7.22 ± 11.57 |
| Did you need ICU admission? | |
| Yes | 13 (0.5) |
| No | 2484 (99.5) |
| If yes, for what duration (days) | 13.57 ± 2.81 |
| Did you need therapeutic oxygen? | |
| Yes | 245 (9.8) |
| No | 2252 (90.2) |
| Did the sense of taste change in certain foods only after confirming the infection with COVID-19? (Who lost taste only or both = 1282) | |
| Yes | 413 (32.2) |
| No | 869 (67.8) |
| If the answer is yes, please mention the foods: (No.: 413) | |
| All food | 330 (79.9) |
| Fruits | 4 (0.9) |
| Poultry and protein food | 19 (4.8) |
| Salty and spicy | 41 (9.9) |
| Sweets | 13 (3.1) |
| Vegetables | 5 (1.2) |
| Vegetables and fruits | 1 (0.2) |
| How long did the change in the sense of taste toward this type of food last? | 34.93 ± 79.49 |
| How long did the complete loss of sense of smell last after recovering from COVID-19? | 3.48 ± 1.54 |
| How long did the complete loss of taste last after recovering from COVID-19? | 3.17 ± 0.1.38 |
| Have you received any treatment for loss of sense of smell? (1282) | |
| No treatment | 1311 (83.8) |
| Cortisone | 164 (12.7) |
| Zink | 14 (1) |
| Vitamin C | 4 (0.3) |
| Herbal treatment | 28 (2.2) |
Figure 1. Percentage distribution of the participants according to anosmia and ageusia associated with COVID-19 infection.
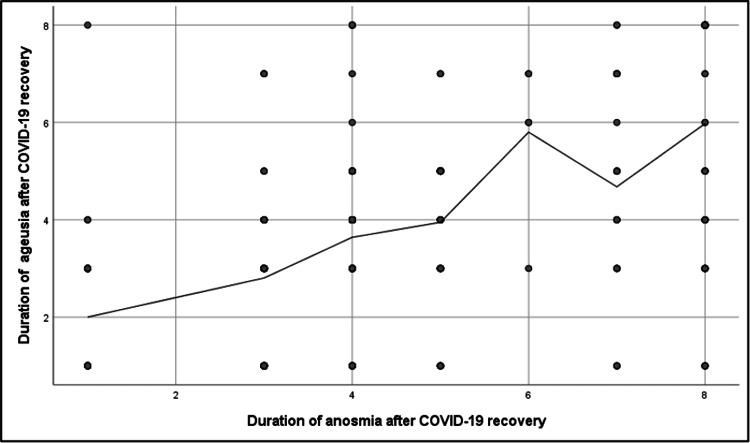
A highly significant positive correlation was found between anosmia and ageusia durations after COVID-19 recovery (r = 0.6, p = <0.001) (Figure 2). Regarding the duration of anosmia after COVID-19 recovery, patients aged 41-60 years, females, those without repeated COVID-19 infection, and patients who were not vaccinated against COVID-19 or took only one dose had a significantly higher mean duration of anosmia (p = <0.05). Additionally, patients who had COVID-19 symptoms for ≥ seven days and patients who needed hospitalization or therapeutic oxygen also had a significantly higher mean duration of anosmia (p = <0.05). Considering the duration of ageusia after COVID-19 recovery, male patients, patients who were not vaccinated against COVID-19, and patients who had COVID-19 symptoms for ≥ seven days had a significantly longer mean duration of ageusia (p = <0.05). Furthermore, patients who needed hospitalization or therapeutic oxygen and patients who had head injuries had a significantly longer mean duration of ageusia (p = <0.05) (Tables 3, 4).
Table 3. Relationship between duration of anosmia and ageusia and participants' age, gender, blood group, smoking, alcohol consumption, chronic diseases, medication, and past history.
P-values in bold indicate statistical significance.
| Variable | Duration of anosmia after COVID-19 recovery (weeks) | Test (p-value) | Duration of ageusia after COVID-19 recovery (weeks) | Test (p-value) |
| Age (years) | ||||
| ≤24 | 3.36 ± 1.37 | 3 (0.025) | 3.13 ± 1.29 | 3 (0.668) |
| 25-40 | 3.54 ± 1.56 | 3.21 ± 1.47 | ||
| 41-60 | 3.58 ± 1.42 | 3.18 ± 1.4 | ||
| >60 | 3.08 ± 1.38 | 3.08 ± 1.14 | ||
| Gender | ||||
| Female | 3.61 ± 1.55 | 5.67 (<0.001) | 3.29 ± 1.49 | 5.01 (<0.001) |
| Male | 3.2 ± 1.17 | 2.9 ± 1.07 | ||
| Blood group | ||||
| A+ | 3.43 ± 1.45 | 7 (0.368) | 3.15 ± 1.35 | 7 (0.41) |
| A- | 3.13 ± 1.07 | 2.93 ± 1.21 | ||
| B+ | 3.45 1.35 | 3.09 ± 1.23 | ||
| B- | 3.32 ± 1.35 | 2.91 ± 1.54 | ||
| O+ | 3.52 ± 1.45 | 3.21 ± 1.49 | ||
| O- | 3.52 ± 1.35 | 3.23 ± 1.28 | ||
| AB+ | 3.54 ± 1.12 | 3.26 ± 1.06 | ||
| AB- | 3.8 ± 0.38 | 6.6 ± 0.89 | ||
| Smoker | ||||
| Yes | 3.54 ± 1.51 | 0.49 (0.618) | 3.29 ± 1.44 | 1.16 (0.245) |
| No | 3.47 ± 1.45 | 3.15 ± 1.37 | ||
| Do you drink alcohol? | ||||
| Yes | 3.89 ± 2.02 | 0.75 (0.451) | 3.67 ± 1.6 | 1.31 (0.188) |
| No | 3.47 ± 1.45 | 3.16 ± 1.8 | ||
| Chronic diseases | ||||
| Yes | 3.47 ± 1.49 | 0.31 (0.757) | 3.12 ± 1.4 | 1.73 (0.082) |
| No | 3.48 ± 1.44 | 3.19 ± 1.37 | ||
| Do you receive treatment for any of the following chronic diseases? | ||||
| Yes | 3.37 ± 1.34 | 1.46 (0.143) | 3.13 ± 1.41 | 0.78 (0.436) |
| No | 3.5 ± 1.48 | 3.17 ± 1.38 | ||
| Past history | ||||
| Nasal fracture | ||||
| Yes | 3.5 ± 0.75 | 0.08 (0.1934) | 1.13 ± 0.25 | 0.44 (0.659) |
| No | 3.48 ± 1.46 | 1.03 ± 0.1 | ||
| Chronic rhinosinusitis | ||||
| Yes | 3.35 ± 1.27 | 1.03 (0.3) | 1.05 ± 0.21 | 1.11 (0.267) |
| No | 3.42 ± 1.3 | 1.11 ± 0.3 | ||
| Smell alteration before COVID-19 | ||||
| Yes | 3.53 ± 1.6 | 0.57 (0.563) | 1.12 ± 0.32 | 0.76 (0.447) |
| No | 4.38 ± 1.54 | 1.08 ± 0.21 | ||
| Taste alteration before COVID-19 | ||||
| Yes | 3.76 ± 1.7 | 1.07 (0.281) | 1.1 ± 0.3 | 0.21 (0.826) |
| No | 4.47 ± 1.45 | 1.03 ± 0.21 | ||
| Malnutrition | ||||
| Yes | 3.64 ± 2.76 | 0.65 (0.516) | 1.06 ± 0.24 | 0.45 (0.648) |
| No | 3.47 ± 1.44 | 1.08 ± 0.42 | ||
| Surgery or radiotherapy in oral or nasal cavities | ||||
| Yes | 4.08 ± 1.29 | 1.46 (0.144) | 1.17 ± 0.38 | 1.06 (0.286) |
| No | 4.37 ± 1.53 | 1.07 ± 0.23 | ||
| Antibiotics use | ||||
| Yes | 3.25 ± 2.18 | 0.08 (0.934) | 1.02 ± 0.03 | 0.84 (0.396) |
| No | 3.48 ± 1.45 | 1.07 ± 0.25 | ||
| Allergic rhinitis | ||||
| Yes | 3.71 ± 1.32 | 0.14 (0.886) | 1.11 ± 0.31 | 0.48 (0.628) |
| No | 3.47 ± 1.46 | 1.08 ± 0.27 | ||
| Stroke | ||||
| Yes | 3.14 ± 1.76 | 0.01 (0.998) | 1 ± 0.3 | 0.01 (0.976) |
| No | 3.38 ± 1.52 | 1.01 ± 0.4 | ||
| Nasal polyps | ||||
| Yes | 3.5 ± 2.34 | 0.39 (0.696) | 1.17 ± 0.4 | 0.75 (0.45) |
| No | 3.48 ± 1.6 | 1 ± 0.03 | ||
| Multiple sclerosis | ||||
| Yes | 3.16 ± 1.2 | 0.03 (0.869) | 1.6 ± 0.76 | 0.04 (0.667) |
| No | 3.22 ± 1.7 | 1.01 ± 0.2 | ||
| Head injury | ||||
| Yes | 6 ± 2.82 | 1.7 (0.089) | 1.5 ± 0.7 | 2.15 (0.03) |
| No | 3.47 ± 1.45 | 1.03 ± 0.6 | ||
| Epilepsy | ||||
| Yes | 3 ± 1.01 | 0.51 (0.609) | 1 ± 0.01 | 0.42 (0.672) |
| No | 3.4 ± 1.3 | 1.03 ± 0.3 | ||
Table 4. Distribution of studied participants according to circumstances related to COVID-19 infection.
P-values in bold indicate statistical significance.
| Variable | Duration of anosmia after COVID-19 recovery (weeks) | Test (p-value) | Duration of ageusia after COVID-19 recovery (weeks) | Test (p-value) |
| Have you been infected with COVID-19 repeatedly? | ||||
| No | 3.69 ± 1.49 | 3.17 (0.001) | 3.3 ± 1.45 | 1.61 (0.106) |
| Yes | 3.42 ± 1.44 | 3.13 ± 1.36 | ||
| Status of immunization against COVID-19 at the time of COVID-19 infection? | ||||
| Not vaccinated | 3.75 ± 1.69 | 3.57 (<0.001) | 3.36 ± 1.52 | 3.57 (<0.001) |
| Vaccinated | 3.36 ± 1.32 | 3.08 ± 1.31 | ||
| If vaccinated, how many doses? (No.: 1911) | ||||
| 1 dose | 3.75 ± 1.69 | 2 (0.003) | 3.63 ± 1.52 | 2 (0.556) |
| 2 doses | 3.38 ± 1.32 | 3.18 ± 1.29 | ||
| 3 doses | 3.32 ± 1.22 | 3.04 ± 1.22 | ||
| How long do COVID-19 symptoms last? | ||||
| 1-3 days | 3.3 ± 1.51 | 2 (<0.001) | 3 ± 1.38 | 1 (0.001) |
| 4-6 days | 3.44 ± 1.39 | 3.14 ± 1.31 | ||
| ≥7 days | 3.69 ± 1 47 | 3.36 ± 1.46 | ||
| Did you need to be hospitalized due to COVID-19? | ||||
| Yes | 3.78 ± 1.58 | 2.13 (0.032) | 3.5 ± 1.66 | 1.51 (0.131) |
| No | 3.47 ± 1.45 | 3.15 ± 1.37 | ||
| Did you need ICU admission? | ||||
| Yes | 3.92 ± 1.78 | 0.93 (0.35) | 3.15 ± 1.37 | 2.22 (0.026) |
| No | 3.47 ± 1.45 | 4.42 ± 2.15 | ||
| Did you need therapeutic oxygen? | ||||
| Yes | 3.79 ± 1.56 | 3.51 (<0.001) | 3.51 ± 1.54 | 3.36 (0.001) |
| No | 3.43 ± 1.43 | 3.12 ± 1.35 | ||
Figure 2. Spearman's test correlation analysis between anosmia and ageusia duration after COVID-19 recovery.
Table 5 illustrates a significant positive correlation between the duration of anosmia after COVID-19 recovery and patients' age and duration of ageusia after COVID-19 recovery (p = <0.05). At the same time, a significant negative correlation was found between the duration of ageusia after COVID-19 recovery and the number of vaccination doses (p = <0.05) (Table 6).
Table 5. Distribution of studied participants according to the severity of COVID-19 infection and associated duration of anosmia.
P-values in bold indicate statistical significance.
| Variable | Duration of anosmia after COVID-19 recovery | |
| r | p-value | |
| Age | 0.06 | 0.015 |
| BMI | -0.004 | 0.881 |
| Smoking duration | -0.04 | 0.555 |
| Number of smoked cigarettes | 0.02 | 0.882 |
| How many times have you contracted COVID-19? | -0.1 | 0.065 |
| Number of vaccination doses | -0.07 | 0.002 |
| Hospital stay (days) | 0.1 | 0.572 |
| ICU duration (days) | 0.4 | 0.428 |
| Duration of ageusia after COVID-19 recovery | 0.2 | <0.001 |
Table 6. Distribution of studied participants according to the severity of COVID-19 infection and associated duration of ageusia.
The p-value in bold indicates statistical significance.
| Variable | Duration of ageusia after COVID-19 recovery | |
| r | p-value | |
| Age | 0.02 | 0.252 |
| BMI | -0.1 | 0.672 |
| Smoking duration | -0.002 | 0.975 |
| Number of smoked cigarettes | 0.01 | 0.814 |
| How many times have you contracted COVID-19? | -0.08 | 0.139 |
| Number of vaccination doses | -0.07 | 0.002 |
| Hospital stay (days) | 0.17 | 0.342 |
| ICU duration (days) | 0.49 | 0.321 |
Multivariate logistic regression analysis assessed the risk factors (independent predictors) of the long duration of anosmia and ageusia after COVID-19 recovery among studied patients (Table 7). It was found that being a female and not having a repeated COVID-19 infection were risk factors (independent predictors) of the long duration of anosmia after COVID-19 recovery (p = <0.05). While being a male patient, a smoker, and being admitted to the ICU were risk factors (independent predictors) of long duration of ageusia after COVID-19 recovery (p = <0.05).
Table 7. Multivariate logistic regression analysis of risk factors of long duration of anosmia and ageusia after COVID-19 recovery.
P-values in bold indicate statistical significance.
| Variable | Duration of anosmia after COVID-19 recovery | |||
| B | Wald | p-value | Odds ratio (CI: 95%) | |
| Age (years) | 0.03 | 1.81 | 0.37 | 1.13 (0.13-1.3) |
| Gender | 0.09 | 12.42 | <0.001 | 1.31 (2.31-4.09) |
| BMI | 0.15 | 1.26 | 0.334 | 1.06 (0.94-1.22) |
| Blood group | 0.22 | 0.56 | 0.78 | 1.43 (0.98-1.89) |
| Smoker | 0.36 | 3.06 | 0.087 | 0.66 (0.31-1.98) |
| Do you drink alcohol? | 1.4 | 5.19 | 0.189 | 0.23 (0.17-0.28) |
| Chronic diseases | 0.14 | 0.78 | 0.211 | 0.9 (0.1-1.09) |
| Are you receiving any chronic medications? | 0.1 | 0.3 | 0.443 | 1 (0.12-1.12) |
| Have you been infected with COVID-19 repeatedly? | 0.45 | 9.7 | <0.001 | 1.9 (2.4-5.09) |
| Status of immunization against COVID-19 at the time of COVID-19 infection? | 0.14 | 0.11 | 0.05 | 0.9 (0.06-1.44) |
| If vaccinated, how many doses? | 0.5 | 1.22 | 0.181 | 0.7 (0.7-1.09) |
| How long do COVID-19 symptoms last? | 0.12 | 0.23 | 0.546 | 1.03 (0.09-1.3) |
| Did you need to be hospitalized due to COVID-19? | 0.23 | 0.45 | 0.051 | 0.9 (0.11-1.34) |
| Did you need ICU admission? | 0.4 | 0.34 | 0.675 | 0.32 (0.13-2.09) |
| Did you need therapeutic oxygen? | 0.08 | 1.67 | 0.126 | 0.61 (0.22-1.08) |
| Nasal fracture | 0.02 | 0.002 | 0.968 | 0.98 (0.38-2.53) |
| Chronic rhinosinusitis | 0.49 | 1.58 | 0.209 | 0.61 (0.28-1.31) |
| Smell alteration before COVID | 0.2 | 0.12 | 0.728 | 0.81 (0.25-2.62) |
| Taste alteration before COVID | 0.6 | 0.48 | 0.485 | 0.54 (1-2.99) |
| Malnutrition | 1 | 0.04 | 0.834 | 1.1 (0.42-2.89) |
| Surgery or radiotherapy in oral or nasal cavities | 0.3 | 0.14 | 0.706 | 1.35 (0.28-6.53) |
| Antibiotics use | 1.11 | 2.59 | 0.107 | 3.06 (0.87-1.93) |
| Allergic rhinitis | 0.54 | 1.32 | 0.249 | 1.71 (0.68-4.31) |
| Stroke | 1.85 | 0.92 | 0.571 | 0.2 (0.31-1.06) |
| Nasal polyps | 0.12 | 0.95 | 0.813 | 0.03 (0.14-10.86) |
| Multiple sclerosis | 0.3 | 0.15 | 0.913 | 0.91 (0.9-0.12) |
| Head injury | 0.65 | 0.16 | 0.501 | 0.14 (0.64-1.053) |
| Epilepsy | 1.13 | 1.56 | 0.601 | 1.09 (0.05-1.13) |
| Duration of ageusia after COVID-19 recovery | ||||
| Age (years) | 0.08 | 0.13 | 0.561 | 1.02 (0.76-1.06) |
| Gender | 0.9 | 12.06 | <0.001 | 1.9 (2.09-4.03) |
| BMI | 0.12 | 2.98 | 0.0.91 | 0.04 (0.33-1.09) |
| Blood group | 0.14 | 0.49 | 0.39 | 1.22 (0.09-1.9) |
| Smoker | 0.81 | 3.05 | 0.001 | 1.3 (1.6-3.67) |
| Do you drink alcohol? | 0.31 | 0.04 | 0.301 | 0.8 (0.1-1.08) |
| Chronic diseases | 0.15 | 0.25 | 0.225 | 0.13 (0.01-2.09) |
| Are you receiving any chronic medications? | 0.20 | 0.13 | 0.513 | 0.93 (0.13-1.09) |
| Have you been infected with COVID-19 repeatedly? | 0.12 | 1.9 | 0.098 | 0.14 (0.13-0.98) |
| Status of immunization against COVID-19 at the time of COVID-19 infection? | 0.09 | 0.15 | 0.112 | 1.09 (0.49-2.39) |
| If vaccinated, how many doses? | 0.11 | 1.23 | 0.77 | 0.01 (0.94-1.89) |
| How long do COVID-19 symptoms last? | 0.12 | 0.211 | 0.708 | 1.03 (0.89-1.49) |
| Did you need to be hospitalized due to COVID-19? | 0.18 | 0.34 | 0.115 | 0.05 (0.09-1) |
| Did you need ICU admission? | 0.98 | 2.32 | 0.016 | 1.08 (1.13-3.29) |
| Did you need therapeutic oxygen? | 0.12 | 1.98 | 0.221 | 0.17 (0.09-1.1) |
| Nasal fracture | 0.52 | 0.69 | 0.405 | 1.68 (0.49.107) |
| Chronic rhinosinusitis | 1.71 | 0.32 | 0.116 | 0.19 (0.09-1.12) |
| Smell alteration before COVID | 0.01 | 0.31 | 0.115 | 0.01 (0.39-1) |
| Taste alteration before COVID | 1.09 | 0.1 | 0.41 | 0.11 (0.31.11) |
| Malnutrition | 0.45 | 0.93 | 0.331 | 0.15 (0.3-1.31) |
| Surgery or radiotherapy in oral or nasal cavities | 0.5 | 0.61 | 0.912 | 0.30 (0.4-1.05) |
| Antibiotics use | 0.91 | 0.87 | 0.304 | 0.02 (0.11-2.01) |
| Allergic rhinitis | 0.4 | 0.51 | 0.201 | 0.19 (0.13-1.2) |
| Stroke | 0.9 | 1.03 | 0.701 | 1.09 (0.9-2.07) |
| Nasal polyps | 0.41 | 0.93 | 0.132 | 0.5 (0.19-0.9) |
| Multiple sclerosis | 0.51 | 1.08 | 0.414 | 0.9 (0.39-1.07) |
| Head injury | 1.2 | 0.31 | 0.611 | 0.8 (0.38-1.33) |
| Epilepsy | 1.35 | 0.91 | 0.667 | 0.3 (0.11-0.91) |
Discussion
Loss of smell and taste are the most common chemosensory complaints in patients who were infected with COVID-19, and they may present without any nasal symptoms [4]. Our survey-based study investigated 2497 individuals infected with COVID-19 and showed that more than half of them (60.5%) had presented with chemosensory complaints. As there is an increasing number of studies indicating that the most common otolaryngologic symptoms in post-COVID-19 patients are smell and taste dysfunctions [4], therefore, we aimed in this study to investigate the factors that affected these symptoms' duration.
A global systematic review pooled the prevalence of olfactory and gustatory dysfunction among patients who were infected with COVID-19, with percentages of 52.73% and 43.93%, respectively [11]. In comparison to our study, among 2497 patients who recovered from the infection, 53.3% had olfactory dysfunction and 51.1% had gustatory dysfunctions, respectively (Figure 1). These findings are important as they support the association between chemosensory dysfunctions and COVID-19 infection.
The sociodemographic data of a nationwide study showed a significant association between the female sex and the incidence and persistence of anosmia. This aligns with our female participants, who have a significantly higher mean duration of anosmia (p = <0.05). Furthermore, in the mentioned study, females were found to be more susceptible to experiencing ageusia, while this study's data showed that male participants are more prone to have a higher mean duration of ageusia (p = <0.05) [6,12].
Anosmia and ageusia affected the majority of the population in our study aged 24 years and younger (Table 3). The association between age and duration of anosmia after COVID-19 was significantly high (p = 0.025) (Table 3). This was also reported in a previous study, which indicates that younger patients were more susceptible to the infection [6]. On the other hand, the relation between age and duration of ageusia after infection recovery was not as significant (p = 0.668).
Smoking adversely affects the health state of the lungs and the human immune system. Therefore, smokers are more vulnerable to acquiring infectious conditions. Previously published studies revealed that smoking has a highly statistically significant association with anosmia and ageusia [13,14]. Our study reported a low prevalence of smokers in the present study, with a percentage of 11.5%. Despite this small percentage, smokers showed to have a significantly higher mean only in ageusia duration (p = 0.001).
Our results disagree with a recent nationwide cross-sectional study that involves 7520 patients, which shows an association between chronic illnesses and post-COVID-19 symptoms, including taste and smell dysfunctions [5]. In addition, several studies have reported different comorbidities, including diabetes and hypertension, that matter in post-COVID-19 symptoms [4]. Yet, our study revealed that chronic diseases do not have a strong relationship with the duration of anosmia or ageusia after infection recovery. This can be explained by the range difference in age groups participating in the mentioned studies and ours. The majority of the population in Saudi Arabia ranges between 20 and 39 years of age with a percentage of 37.77% [15]. Based on different national studies, the prevalence of diabetes mellitus, hypertension, and respiratory conditions in Saudi Arabia is higher in individuals aged 46 years and older [16-18]. Most of our participants were aged 24 years and younger, which explains the disagreement with previous studies on the relationship between chemosensory dysfunction and their duration after COVID-19 recovery.
Interestingly, patients with mild COVID-19 symptoms and those who were hospitalized were more prone to COVID-19 symptoms [19], and the length of hospital stay and ICU admission can play a significant role in developing these symptoms [2]. Therefore, our data revealed a strong relationship between the duration of ageusia after recovery and ICU admission (p = 0.047). Meanwhile, ICU admission and hospitalization did not have a big impact on the duration of anosmia after COVID-19 infection (p = 0.175 and p = 0.493, respectively).
Limitations
The questionnaire was self-administered, which might lead to self-reporting bias and recall bias. Moreover, due to the nature of this study being cross-sectional and insufficient data collection, more in-depth clinical information from the participants is needed. Therefore, these survey-based findings should be taken with caution.
Conclusions
In conclusion, chemosensory dysfunction symptoms are important manifestations of COVID-19 diagnosis, especially in the early stages of the infection.
Our study has reported the high prevalence of chemosensory dysfunctions after COVID-19 infection among the Saudi population. Moreover, our study shows that gender, smoking, ICU admission, and first-time infection with COVID-19 do have a significant impact on the duration of these symptoms after COVID-19 infection. Therefore, these findings may help in future research on COVID-19 infection consequences and treatment. Moreover, healthcare professionals should take these data under consideration in suspected or confirmed COVID-19 cases.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study. Biomedical Ethics Committee at Umm Al-Qura University (UQU), College of Medicine, Makkah, KSA issued approval HAPO-02-K-012-2022-02-955. The committee has accordingly granted the Principal Investigator final approval concerning the ethics of scientific research.
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): a global pandemic and treatment strategies. Sharma A, Tiwari S, Deb MK, Marty JL. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7286265/ Int J Antimicrob Agents. 2020;56:106054. doi: 10.1016/j.ijantimicag.2020.106054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Determinants of post-COVID-19 conditions among SARS-CoV-2-infected patients in Saudi Arabia: a web-based cross-sectional study. Samannodi M, Alwafi H, Naser AY, et al. Diseases. 2022;10:55. doi: 10.3390/diseases10030055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Post-COVID-19 syndrome: epidemiology, diagnostic criteria and pathogenic mechanisms involved. Carod-Artal FJ. Rev Neurol. 2021;72:384–396. doi: 10.33588/rn.7211.2021230. [DOI] [PubMed] [Google Scholar]
- 4.Prevalence of anosmia and ageusia symptoms among long-term effects of COVID-19. Moraschini V, Reis D, Sacco R, Calasans-Maia MD. Oral Dis. 2022;28:2533–2537. doi: 10.1111/odi.13919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olfactory and gustatory dysfunctions due to the coronavirus disease (COVID-19): a review of current evidence. Mehraeen E, Behnezhad F, Salehi MA, Noori T, Harandi H, Seyed Alinaghi S. Eur Arch Otorhinolaryngol. 2021;278:307–312. doi: 10.1007/s00405-020-06120-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Investigation on the factors associated with the persistence of anosmia and ageusia in Saudi COVID-19 patients. Algahtani SN, Alzarroug AF, Alghamdi HK, Algahtani HK, Alsywina NB, Bin Abdulrahman KA. Int J Environ Res Public Health. 2022;19:1047. doi: 10.3390/ijerph19031047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Persistent symptoms post-COVID-19: an observational study at King Abdulaziz Medical City, Jeddah, Saudi Arabia. Jabali MA, Alsabban AS, Bahakeem LM, Zwawy MA, Bagasi AT, Bagasi HT, Aldosary TA. Cureus. 2022;14:0. doi: 10.7759/cureus.24343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prevalence of anosmia among COVID-19 patients in Taif City, Kingdom of Saudi Arabia. Mubaraki AA, Alrbaiai GT, Sibyani AK, Alhulayfi RM, Alzaidi RS, Almalki HS. Saudi Med J. 2021;42:38–43. doi: 10.15537/smj.2021.1.25588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Impact of the smell loss on the quality of life and adopted coping strategies in COVID-19 patients. Elkholi SM, Abdelwahab MK, Abdelhafeez M. https://doi.org/10.1007/s00405-020-06575-7. Eur Arch Otorhinolaryngol. 2021;278:3307–3314. doi: 10.1007/s00405-020-06575-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raosoft sample size calculator. 2004. http://www.raosoft.com/samplesize.html http://www.raosoft.com/samplesize.html
- 11.The prevalence of olfactory and gustatory dysfunction in COVID-19 patients: a systematic review and meta-analysis. Tong JY, Wong A, Zhu D, Fastenberg JH, Tham T. Otolaryngol Head Neck Surg. 2020;163:3–11. doi: 10.1177/0194599820926473. [DOI] [PubMed] [Google Scholar]
- 12.Anosmia and olfactory tract neuropathy in a case of COVID-19. Li CW, Syue LS, Tsai YS, et al. J Microbiol Immunol Infect. 2021;54:93–96. doi: 10.1016/j.jmii.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prevalence of anosmia and ageusia in patients with COVID-19 at a primary health center, Doha, Qatar. Al-Ani RM, Acharya D. Indian J Otolaryngol Head Neck Surg. 2022;74:2703–2709. doi: 10.1007/s12070-020-02064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prevalence of anosmia or ageusia in patients with COVID-19 among United Arab Emirates population. Al-Rawi NH, Sammouda AR, AlRahin EA, et al. https://doi.org/10.1016/j.identj.2021.05.006. Int Dent J. 2022;72:249–256. doi: 10.1016/j.identj.2021.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.General Authority for Statistics. Population and housing census. 2020. https://www.stats.gov.sa/en/13 https://www.stats.gov.sa/en/13
- 16.Diabetes mellitus in Saudi Arabia. Al-Nozha MM, Al-Maatouq MA, Al-Mazrou YY, et al. https://www.researchgate.net/profile/Nazeer-Khan/publication/305398808_Diabetes_mellitus_in_Saudi_Arabia/links/09e41509131170d25b000000/Diabetes-mellitus-in-Saudi-Arabia.pdf. Saudi Med J. 2004;25:1603–1610. [PubMed] [Google Scholar]
- 17.Hypertension in Saudi Arabia. Al-Nozha MM, Abdullah M, Arafah MR, et al. https://pubmed.ncbi.nlm.nih.gov/17206295/#:~:text=The%20prevalence%20of%20hypertension%20was,%25%20(p%3C0.001) Saudi Med J. 2007;28:77–84. [PubMed] [Google Scholar]
- 18.Prevalence of respiratory diseases in hospitalized patients in Saudi Arabia: a 5 years study 1996-2000. Alamoudi OS. Ann Thorac Med. 2006;1:76–80. [Google Scholar]
- 19.Post-COVID syndrome in non-hospitalised patients with COVID-19: a longitudinal prospective cohort study. Augustin M, Schommers P, Stecher M, et al. Lancet Reg Health Eur. 2021;6:100122. doi: 10.1016/j.lanepe.2021.100122. [DOI] [PMC free article] [PubMed] [Google Scholar]