ABSTRACT
Introduction: Cold Weather Injury (CWI) represents a spectrum of pathology, the two main divisions being Freezing Cold Injury (FCI) and Non-Freezing Cold Injury (NFCI). Both are disabling conditions associated with microvascular and nerve injury often treated hours after initial insult when presenting to a healthcarestablishment. Given that iloprost is used for the treatment of FCI, could it be used in a forward operating environment to mitigate treatment delay? Is there a role for its use in the forward treatment of NFCI? This review sought to evaluate the strength of evidence for the potential use of iloprost in a forward operating environment.
Methods: Literature searches were undertaken using the following question for both FCI and NFCI: in [patients with FCI/NFCI] does [the use of iloprost] compared to [standard care] reduce the incidence of [long-term complications]. Medline, CINAHL and EMBASE databases were searched using the above question and relevant alternative terminology. Abstracts were reviewed before full articles were requested.
Results: The FCI search yielded 17 articles that were found to refer to the use of iloprost and FCI. Of the 17, one referred to pre-hospital treatment of frostbite at K2 base camp; however, this was utilising tPA. No articles referred to pre-hospital use in either FCI or NFCI.
Discussion: Although evidence exists to support the use of iloprost in the treatment of FCI, its use to date has been in hospital. A common theme is delayed treatment due to the challenges of evacuating casualties from a remote location. There may be a role for iloprost in the treatment of FCI; however, further study is required to better understand the risk of its use.
KEYWORDS: Frostbite, iloprost, NFCI, military, cold injury
Cold weather injury
Frostbite or freezing cold injury (FCI) has impacted those operating at reach since the heroic age of Antarctic exploration and reference to it can be found in some of the earliest military medical journals [1]. It has also been described in several campaign histories through modern case series relating to those who work or operate in sub-zero temperatures [2–4].
Non-freezing cold injury (NFCI) is similarly not a new condition, having been documented during the Napoleonic Wars, throughout the First World War and Falklands Conflict [5]. “Trench foot”, as it was known, was debilitating for those deployed to cold, damp conditions where prolonged exposure was unavoidable. It has also been described as “immersion foot” for those rescued from shipwrecks having spent protracted periods in cold water [6].
Freezing cold injury
FCI is a freezing cold thermal injury that occurs when tissues are exposed to a temperature of less than −0.55°C. At this point, intracellular fluid freezes beginning the cascade of what can be an extensive injury [7]. FCI that fully resolves within 30 min of re-warming is classified as “frostnip” and regarded as a superficial FCI. Injuries that do not recover within this time are regarding as a deeper injury or “frostbite”. For the purposes of this paper, FCI will refer to “frostbite” rather than the more superficial “frostnip”.
The pathophysiology behind FCI is often regarded as two different injury patterns or phases of injury [4,7]. The first phase describes the formation of ice crystals leading to cellular death. The second phase results in reperfusion injury and progressive dermal ischaemia. If tissues are exposed to repeated cycles of these processes, regarded as freeze-thaw cycles, the injury can be far greater and level of destruction extensive [6,7]. The more extensive the injury, the greater the risk of amputation as outlined in Table 1.
Table 1.
Classification of Frostbite (Cauchy et al.) [8].
| Grade of Frostbite | Appearance after Rewarming | Amputation Risk |
|---|---|---|
| Grade 1 | Absence of cyanosis | No amputation of bone |
| Grade 2 | Cyanosis on distal phalanx | Moderate risk of amputation |
| Grade 3 | Cyanosis up to middle phalanx joint | High risk of amputation |
| Grade 4 | Cyanosis proximal to middle phalanx joint | Risk of amputation 100% |
Basic field care
The basics of field care are to ensure adequate hydration and nutrition, protection from the elements and, if appropriate, re-warming [4,7]. The decision to re-warm will be guided by the level of exposure and proximity to a treatment facility. If there is any risk of re-freezing, re-warming should not be undertaken due to compounding effect of recurrent ice crystal formation and further tissue destruction [4]. Any potential treatments should be given as part of a re-warming phase [4]. If possible, the affected limb should be immobilised but if there is no choice to mobilise the casualty, this may be preferable to the limb freezing further during a period of immobility [7,9].
Freezing cold injury-specific treatment
Once the FCI casualty is transferred to a treatment facility, further assessment and management of the injury can be undertaken. Present FCI treatment requires significant infrastructure which cannot be delivered in austere conditions.
Re-warming should occur for an initial period of 30–60 min at a temperature of 37–39°C and patients offered ibuprofen 12 mg/kg/day up to a maximum dose of 2.4 g [6,7,9–11]. Analgesia should be offered in addition to this, and choice of agent may depend on local protocol or treatment directive.
Tetanus prophylaxis should be administered and depending on the extent of the injury and antibiotics considered [9,10], there remains significant discussion regarding the role of amputation, local nerve blockade and sympathectomy for affected limbs or digits [9,10].
Non-freezing cold injury
NFCI forms part of the spectrum of cold weather injury (CWI) that includes FCI and frostnip [10,12]. Deployed populations are at risk when operating in areas below 15°C or with periods of immersion in wet conditions where prolonged exposure can trigger maximal vasoconstriction and limb ischaemia [10,13].
The diagnosis of NFCI in the field can be challenging due to a lack of external evidence plus a protracted timeline for symptom development [6,10]. The disease process is thought to have three phases – pre-hyperaemic, hyperaemic and post-hyperaemic. These phases start as cold, numb and swollen digits developing over hours with progressive vascular, sensory and motor disturbances lasting several weeks. The post-hyperaemic phase appears as cold-sensitive digits with signs of muscle atrophy after a period of months [14]. Signs and symptoms can overlap between phases but are underpinned by having been exposed to sub 15ºC temperatures without history or evidence of tissues freezing [6,14].
Treating non-freezing cold injury
Treatment for NFCI is somewhat more challenging compared to that of FCI given the difficulties in its diagnosis [10,11]. Prevention of NFCI remains paramount as is the case with FCI, ensuring adequate hydration, nutrition and mitigation of risk for exposure to temperatures below 15ºC [11]. Where this is unavoidable, measures should be taken to ensure that activity undertaken is appropriate to continue in the conditions [11].
Initial field care for NFCI is like that of FCI but re-warming should be slow through exposure to warm, dry air alone and not through warm water immersion [10]. Initial care should also ensure adequate hydration and nutrition as well as preventing further exposure to the affected limb [6,10,11].
Rather than aspirin or anti-inflammatory agents, initial analgesic treatment of NFCI should be amitriptyline 10 mg at night, increasing to a maximum of 75 mg [11,14]. Escalation beyond these doses should be with specialist input as part of CWI services [14].
Care should also be provided to protect the affected limb, preventing progression to tissue freezing or further exacerbation of the NFCI as this can precipitate digital ischaemia and conversion to tissue death [11,14].
Iloprost
Iloprost is a prostacyclin analogue with vasodilatory properties that are similar to the effects of sympathectomy. It also reduces platelet aggregation and capillary permeability whilst activating fibrinolysis, all of which can reduce the incidence of microvascular occlusion [4,9,10].
The role of iloprost in FCI
Given its properties, iloprost has been utilised for the treatment of FCI with early case series suggesting a promising effect with none of the casualties studied requiring amputation [15]. This low amputation rate has been replicated in a smaller trial, further demonstrating the potential role of iloprost in the treatment of FCI [16].
Iloprost is administered as an intravenous (IV) infusion at an initial rate of 0.5 ng/kg/min increasing by 0.5 ng/kg/min every 30 min to a maximum dose of 2 ng/kg/min or until the patient develops side effects such as headache or flushing [4,9,10]. The infusion is then continued for 6 h per day for five to eight days at the previously determined maximal dose [9]. The infusion can be commenced up to 24 h after the injury and may also be utilised in the context of trauma [4,8–10]. As outlined in Table 2, “major trauma” is listed as a contra-indication for iloprost use. To determine the extent of concurrent traumatic injury in which iloprost may be suitable for use requires further investigation [17]. For the context of this paper, traumatic injury has been considered as minor trauma without significant injury causing haemodynamic instability or catastrophic haemorrhage.
Table 2.
Treatment protocol for hospital use of iloprost (Cauchy et al.) [8].
| Administration and Monitoring | Dilute 1 vial 0.5 mL iloprost in 24.5 mL NaCl 9% Syringe pump: 25 mL – speed: 1 mL/h for 30 min, then 2 mL/h for 30 min, then 3 mL/h for 30 min, then 4 mL/h for weight<75 kg or 5 mL/h for weight>75 kg Continue until 25 mL is delivered; all patients receive 1 vial Monitor HR and BP every 30 min |
| Complications and their Management | In case of side effects decrease to previous step If systolic BP <90 mmHg, decrease to lower step |
| Contraindications | Hypotension, hypersensitivity, pulmonary oedema, cardiac arrhythmia, active ulcer disease, major trauma; unknown effects on pregnancy |
| Precautions | Anticipate nausea and vomiting, pain and hypotension, keep patient supine |
Given its vasodilatory properties, a recognised side effect of its use is hypotension. Whilst working in remote conditions or indeed, needing to provide treatment in the field, countering these effects could be challenging. Although the administration of the drug is straightforward when using a syringe driver, countering drug-induced hypotension may be less so [4]. Other side effects can include cardiac dysrhythmia and pulmonary oedema which could be an introduction of unnecessary risk in the management of the FCI casualty [8].
If, however, a deployed clinician or clinical team had the training and capability to manage potential adverse side effects of the drug, then theoretically it could be pushed further forward.
Iloprost is not the only treatment considered a novel pharmacological treatment for FCI. Other treatments have been suggested in the literature as possible therapies for FCI patients attending larger installations for treatment immediately after rescue.
Alternative pharmacological treatments for freezing cold injury
When considering treatments for the FCI patient, the benefit of treatment in a hospital setting is the expanse of resources that are available when compared to the remote or forward operating setting. It is in these settings that several case series have suggested the use of therapies other than iloprost have offered improvement in the outcomes for FCI patients.
Hyperbaric oxygen
The use of hyperbaric oxygen (HBO) has been considered as a possible treatment for FCI [8–10]. Given its association with increased capillary formation and white cell function, the saturation of tissues with HBO may yield benefits in the treatment of FCI. However, the evidence base is at present very limited and although hyperbaric therapy can be provided in portable chambers, evacuating a casualty to a centre for sustained therapy would likely prove challenging [10].
Tissue plasminogen activator
Similar to iloprost, tissue plasminogen activator (tPA) has often been regarding as a novel therapy for the treatment of FCI [16]. tPA is a thrombolytic agent utilised in the breakdown of clots within the microvascular circulation. It is occasionally utilised in conjunction with intravenous vasodilators such as nitroglycerine or papaverine to counter any vasospasm that occurs due to the injury process [9].
tPA is administered within 24 h of injury, after rapid re-warming and used in conjunction with heparin to reduce the recurrence of microvascular thrombosis [6,8]. There are also recommendations for angiography prior to administration (particularly prior to intra-arterial administration) as well as repeated staging angiography at 12 to 24-h intervals to monitor effect [8–10]. Additionally, its use is contra-indicated in trauma which may restrict its use in those casualties who sustain FCI as part of remote operations [9].
When considering tPA and iloprost, both have the potential to support the treatment of FCI casualties when received in the hospital setting. The use of both agents forms part of the Helsinki Protocol, utilised in the treatment of a number of FCI patients [18]. When considering this treatment protocol, both therapies are administered in a hospital setting and iloprost is used in cases with contraindications to tPA. In the use of iloprost, angiography is not required, and it can be utilised in the context of minor trauma. Considering the population at risk as part of military deployments to remote, austere, cold locations, could iloprost be utilised at the point of wounding? It should also be considered how long it takes for FCI patients to arrive at a centre that can provide treatment and therefore could the use of pre-hospital iloprost reduce delay to treatment?
The use of iloprost in the remote setting
Given the role of iloprost in FCI, and potential overlap in the pathophysiology of NFCI, literatures search was performed to review if there was evidence to support the forward use of iloprost [6,10,14].
Search methods
Two literature searches were undertaken on 22 January 2022 using the same question for each of the specific pathologies (FCI or NFCI): in [patients with FCI] or [in patients with NFCI] does [the use of iloprost] compared to [standard care] reduce the incidence of [long-term complications]. Medline, CINAHL and EMBASE databases were searched using the above question and relevant alternative terminology (NFCI, FCI, CWI, Trench Foot, Frostnip, Frostbite, immersion injury and cold injury). There was no restriction of search for language or date. Abstracts were then reviewed for relevance to the use of iloprost in the management of FCI before full articles were requested for further analysis.
Search results
The original search yielded 17 articles that referred to the use of iloprost and FCI, and 22 articles that referred to the use of iloprost and NFCI. Review of articles references, BestBETs and Cochrane database did not yield any further relevant articles. Following review of the abstracts, full-text articles were subsequently reviewed. Paper review methodology is contained in Figures 1 and 2. The articles were then categorised into three broader categories, those being: technical papers discussing the utilisation of therapies and historic treatments; case series and case reports of FCI and NFCI casualties treated with iloprost; and systematic reviews.
Figure 1.
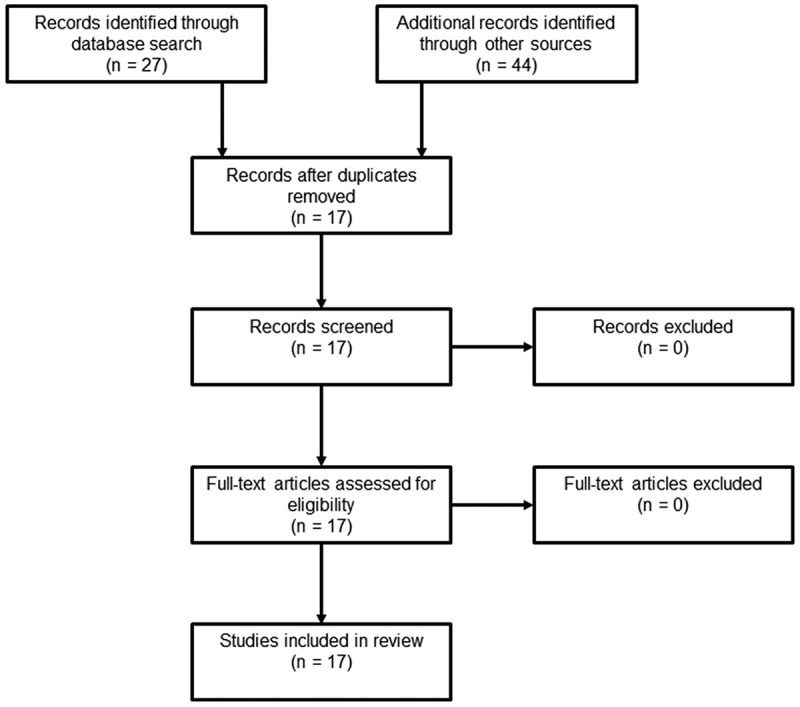
Methodology Flow Diagram (Fci).
Figure 2.
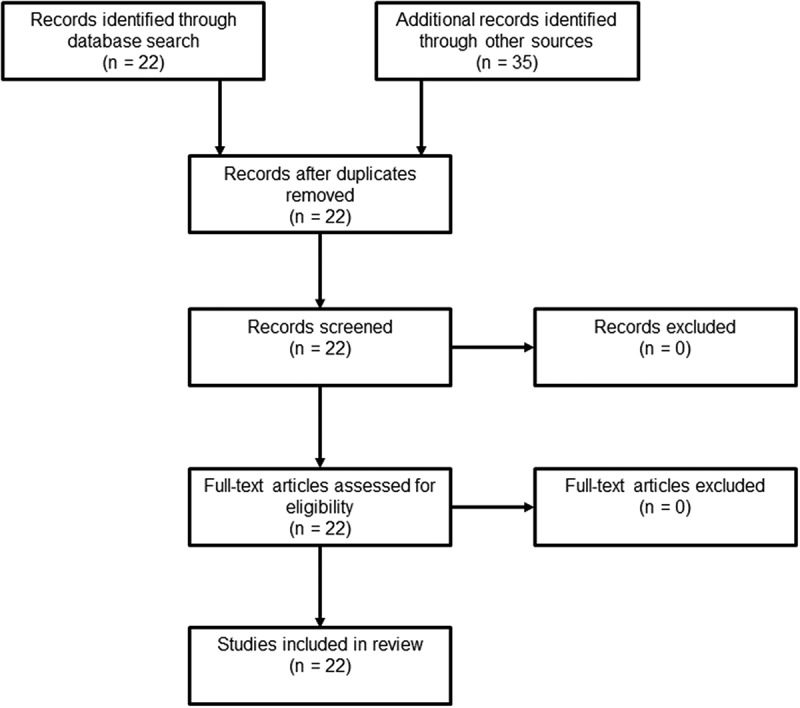
Methodology flow diagram (Nfci).
The remote use of Iloprost in Freezing Cold Injury
The assessment and management of FCI
Kuht et al. discuss the use of iloprost in the management of FCI but share the concerns raised in previous papers about the potential side effects and risk to the patient outside of a hospital setting [6,8]. Its use is again compared to tPA accepting that both have a risk profile that may exceed the capabilities of a deployed healthcare setting if utilised outside of a hospital [6]. A similar reference is made by Imray and Oakley in their summary paper on the treatment of military patients with FCI but its use remains as a potential topic for research in further animal studies [19].
Handford et al. also discuss the use of iloprost as novel therapy for the treatment of FCI [4]. It is described as an agent for utilisation during the hospital phase of treatment of FCI due to the risk profile and potential adverse effect if administered in the pre-hospital environment. Handford recognises the administration of iloprost in the remote or pre-hospital setting as step forward in the management of an FCI patient, but the risks of its use must be acknowledged. The additional benefits of the ability to potentially use iloprost in those causalities with traumatic injuries and without angiography is regarded as an advantage when compared with tPA [4].
Case reports and case series
Looking first at the UK Armed Forces, Heil et al. present a 13-year review of FCI cases from multiple theatres [20]. Over the 13-year period, 149 cases have a diagnosis of FCI. However, of those patients, none received iloprost and the immediate care given in the field is not discussed. Of the 149 cases, 10 had surgical intervention ranging from blister aspiration to amputation. There is no discussion of the time between suspected onset of injury and reception at a facility able to provide care for the patients. The paper acknowledges a role for iloprost; however, at the time of publication, it was not available as a treatment within the context of military medical doctrine.
The SOS-frostbite study was a cross-border collaboration between several hyperbaric chambers in Geneva, Lyon and Mont Blanc comparing outcomes between a retrospective control group treated with iloprost versus a prospective intervention cohort receiving both iloprost and HBO [21]. Due to the nature of the study, all administration of iloprost was in the hospital or hyperbaric centre setting, with a statistically significant reduction in the rate of amputation in those in the intervention group. Of note, Magnan et al. captured the data on delays to treatment from point of injury. 10% of the 100 patients (control and intervention group) received treatment within 6 h of injury but the remaining 90% had delays of 6–72 h before receiving iloprost [21].
The Kathmandu Iloprost experienced by Pandey et al. offers valuable insight into the use of iloprost with protracted evacuation timelines and in an area with limited capabilities [22]. Although not utilised in the pre-hospital environment, Pandey describes the use of iloprost in five patients with different severity of injuries and times to presentation, which ranged from an estimated 32 h to 72 h. The evacuation from Mount Everest Camp 2 can be undertaken by helicopter but due to weather and distance from the camp, evacuation times can vary. Pandey describes few adverse events besides a single episode of a drop in systolic blood pressure of 20 mm Hg. Pandey’s case series demonstrates that iloprost can be utilised more than 24 h after injury, with few side effects and minimal need for monitoring. It is also acknowledged that there may be a role for iloprost further forward towards the point of injury [22].
Poole, Gautier and MacLennan have produced a number of case series based on the experiences of managing FCI in northern Canada [23–25]. The initial case series in 2016 described two patients given iloprost by the same protocol as described by Cauchy with complete healing but with persisting hypersensitivity [8,23]. Both patients were seen more than 24 h after cold exposure with the second patient attending more than 48 h after initially noting symptoms with no adverse outcomes described following iloprost administration. The Yukon case series describes a retrospective analysis of 22 patients treated for grade 2–4 FCI at the Whitehorse General Hospital in the Yukon Territory [24]. Of those patients with grade 2–3 FCI, all digits were salvaged as were 50% of those affected with grade 4 FCI. Poole outlines a cold exposure time of 20 ± 18 h and two patients experiencing a freeze-thaw-refreeze cycle. Although the treatment outcomes were positive, adverse events such as headache and flushing were described in 73% of patients. Two patients were described as having significant adverse events. One event was facial bleeding. However, this was felt to be related to recent physical altercation. Another was of a perforated duodenal ulcer in a patient who was also taking ibuprofen without gastric protection. A further case report describes the use of fluorescence to visualise response to iloprost treatment [25]. The patient presented 8 h after initial injury with grade 2 frostbite. There were no adverse outcomes noted and the patient made a full recovery.
Lindford et al. described a retrospective case series of 20 patients seen between 2013 and 2016 presenting to the Helsinki Burns Centre for treatment according to the Helsinki Frostbite Management Protocol [18]. The protocol utilises iloprost for those patients who present after more than 48 h since injury or if there are contraindications to fibrinolysis with tPA. Those treated with iloprost had an average longer times from point of injury (greater than 48 h) and lower salvage rate compared to the tPA group (81.1% vs 78.0%). It should be noted that of those patients who received iloprost, no complications were noted compared to those who received tPA, with the most significant complications being catheter site pseudoaneurysm and soft tissue haematoma.
Previously discussed for its reference of the use of iloprost and widely adopted treatment protocol, Cauchy et al. described a case series of two patients who received tPA at base camp of K24. The tPA treatment protocol utilised differed from the more commonly utilised doses but no adverse effects of the administration were described. Although not describing the use of iloprost in the pre-hospital environment, Cauchy does outline the role for these agents at the point of injury to potentially increase salvage rates for patients with FCI, particular in those patients with trauma and protracted times from point of injury [8].
Perhaps, the most significant case report yielded as part of the search is that of a single case described by Irarrazaval et al [26]. After a challenging evacuation, a patient was treated with iloprost 75 h after initial symptoms. The patient had multiple contacts with medical professionals prior to evacuation to Kathmandu; however, no additional treatment could be administered until arrival at hospital due to a lack of iloprost in the pre-hospital environment. It was felt by the authors that administration if iloprost prior to evacuation may have reduced the extent of the patient’s injury.
Systematic Reviews
A short cut review undertaken by Kaller describes four studies utilising iloprost for the treatment of FCI [27]. It is acknowledged that population sizes of any study or case series are low, but the number of amputations were low in those presenting early for treatment. No adverse outcomes were described or time from point of injury to treatment initiation recorded. All four studies occurred in the hospital setting but again support the use of iloprost in the management of FCI [27].
Handford et al. reviewed literature on the treatment of frostbite from January 1969 to July 2013 [9]. Two studies were reviewed with respect to the in-hospital use of iloprost describing good outcomes with low amputation rates for those receiving treatment. Handford also acknowledges the benefits of iloprost when compared to tPA due to its ability to be used in the context of trauma and in those patients with exposure times greater than 24 h.
Heil et al. undertook a comprehensive literature search to review all articles published in English in peer-reviewed journals reporting information on FCI [10]. A single study described a reduction in amputation rates from 40% to 3% but there was no discussion on the pre-hospital utilisation of iloprost. Heil also outlines the benefit of iloprost when compared to tPA given its use in trauma and administration beyond 24 h from initial injury.
The literature search also yielded a single systematic review from the Cochrane Library by Lorentzen et al [28]. The review describes the 2011 randomised control trial (RCT) by Cauchy et al. involving 47 people who were randomised to one of three treatment arms after the confirmation of FCI and initial management with aspirin and buflomedil [16]. Of the three treatment arms, group 1 received further buflomedil, group 2 iloprost, and group 3 iloprost and tPA. Those in the iloprost and iloprost-tPA groups reported fewer amputations but no difference between the two groups. Adverse effects were recorded but they were not felt to be caused by the drug administration. These groups were again treated in hospital establishments with no pre-hospital administration of iloprost.
Evidence of field use
The initial literature search did not yield any recorded instances of the use of iloprost in the pre-hospital environment or at significant reach aligned to the point of injury. Given the potential risk of adverse effects from the use of iloprost outside a hospital environment, the risks in providing treatment in the remote, austere environment are significant, and further research is required to confirm the safety of its use in the first instance. Some agents, such as tPA, have been used infrequently and at reduced doses to mitigate for side effects.
If, however, we consider a team deployed at significant reach; the risk for both FCI and traumatic injury is high. In this instance, iloprost may be both safe to use and may offer significant benefit in the reduction of requirement for amputation in those with grades 2 to 4 FCI. Its use would require minimal monitoring and equipment when compared to tPA and, if within the skill set of a deployed physician, any adverse effects could be managed effectively even if operating at significant reach. A deployed Emergency Physician in support of a Role 1 or Role 2 would be expected to have both the skillset and resources to counter the adverse effects of iloprost thus minimising the risks of its use [29]. Iloprost, having fewer contraindications and a broader therapeutic window, could be considered safe in the forward setting so long as its use was supported by the appropriately resourced and trained clinical team.
The remote iloprost use in non-freezing cold injury
The assessment and management of NFCI
A significant challenge in the assessment of NFCI is associated with the time to presentation [6]. Multiple NFCI patients present late with established disease which can impact the assessment undertaken and options for immediate treatment [6]. Kuht and Imray both note the same challenges in the diagnosis of NFCI; however, neither suggest the use of iloprost nor field treatments besides that of re-warming and analgesia [6,19,30].
Glennie and Whitaker have reviewed the significance of NFCI within military populations and the issues associated with its long-term impact [14,31]. From a historical perspective, iloprost had not been considered as a potential treatment. Mistry et al. refer the potential role of iloprost as a treatment for NFCI but this is not considered for administration in the pre-hospital environment [32]. Ingram et al. suggest a similarity between NFCI and other vascular phenomena of the peripheral limb such as Raynaud’s Disease, but they do not discuss the potential overlap between the role of iloprost in the two disease processes [33]. Zafren also discusses the previous role of sympathectomy in the treatment of NFCI but does not make any reference to iloprost [34].
Case series and case reports
Irwin presents a single case of a patient presenting after three weeks’ duration of sleeping on the streets of an urban centre [35]. This presentation has a duration of injury phase beyond any of the FCI cases previously reviewed and no reference is made to iloprost in the treatment of this patient. In a case series described by Longman et al. in long-distance polar rowers, they discuss patients who presented several days after injury but again with no reference to iloprost [36]. These series highlight the fact that NFCI cases with irreversible damage and lasting disability have presented considerably later after point of injury when compared to FCI cases.
Jorum et al. and Tek et al. present similar case series in military populations with NFCI [37,38]. Neither population received treatment with iloprost, but these series highlight the significant incidence of NFCI within military populations. Similarly, Williamson and Kuht et al. present case series from the UK Armed Forces [39–41]. Although there is no reference to iloprost, the papers do highlight the lasting chronic effects of NFCI including significant pain which can be challenging to manage. To that end, Anand et al. have considered the use of novel topical therapies such as capsaicin in the NFCI population to good effect [42]. These patients showed good response to single treatment topical application but were all known to have established disease and had not received treatment at the point of wounding.
Arguably of most significance to this review is the case report by Ionescu et al. discussing the use of iloprost in the treatment of an established NFCI patient for the management of chronic pain [13]. The patient received iloprost treatment for chronic pain secondary to NFCI following initial injury 20 years prior to presentation. Initial response to therapy was good, reporting reduced pain. However, subsequent treatment exacerbated symptoms requiring additional analgesia to mitigate the increased pain until baseline pain levels were reached.
Systematic reviews
Systematic reviews published by Heil, Imray and Melamed did not discuss the use of iloprost for the treatment of NFCI or for utilisation for treatment of the disease in the pre-hospital environment [10,43,44].
Considering iloprost at the point of wounding
To first consider the use of iloprost in NFCI, current literature regarding its use is limited and available case reports are in established cases rather than at the point of injury or immediately following diagnosis [13].
To then consider FCI, iloprost has an established history of use with success in the reduction of need for amputation and increase in limb salvage [9]. When considering the future treatment of FCI and the projection of operational capability further forward, early diagnosis and treatment administration can play an important role in ensuring good outcomes for patients and minimal disability.
Suitability for the deployed setting
Iloprost requires the least healthcare infrastructure given no requirement for angiography, technetium scanning or concurrent administration of intravenous heparin. As a stand-alone agent, besides basic physiological monitoring, it can be administered at greater intervals from the point of wounding compared to other novel agents such as tPA and may be suitable for use in patients with concurrent traumatic injury [4,6,10,29].
Considering the infrastructure of a deployed Role 1 or Role 2 setting, these requirements are easily met, and its administration facilitated by those operational staff supporting these establishments [29]. All case reports suggest treatment was administered at windows from the point of wounding between 6 and 72 h timelines that could be greatly reduced if treatment was available further forward.
Management of side effects in the deployed environment
The greatest concern when discussing the administration of intravenous iloprost in the pre-hospital environment or at reach is that of managing adverse events or drug side effects [8,16,19]. From the case series reviewed (accepting that all patients received treatment within a hospital setting), those adverse events that were recorded were headache, flushing and a single recorded drop in systolic blood pressure [22].
A number of these side effects were mitigated by reducing the rate of the iloprost infusion however, the concern for significant adverse events persisted throughout all case series. It was also accepted that the administration of iloprost may have reduced the extent of tissue loss had it been given closer to the point of wounding [26].
Knowing this, the skills required to manage such adverse outcomes or indeed, to support patients with more adverse effects from treatment, should sit well within the skill set of the deployed emergency physician and that of the Role 1e or Role 2 establishment [29]. If appropriate medical assets are deployed further forward with those operating at reach, there should be capability to mitigate those risks outlined in previous papers if iloprost was carried for use at the point of wounding.
Further research
Although there are multiple cases of CWI and treatment described, all the reviewed literature acknowledges that fact that there are limited case series for review, without any prolonged observational study or pre-hospital randomised control trial. The single randomised control trial by Cauchy et al. is based on hospital treatment. It is a small open-label study in a single centre over a prolonged period [16]. Although the outcomes in this trial are positive, it stands in isolation- suggesting once again that further research is required to truly understand the effect of iloprost and if there is a greater role for it as part of field care, closer to the point of wounding.
Although it is beyond the scope of this paper, there needs to be consideration for the incidence of adverse events in the use of iloprost for other conditions. This would be to assist with modelling and prediction of the extent and impact of any potential side effects that may occur in the treatment of CWI. This would assist in the planning and preparation of mitigations to support CWI casualties who may have adverse events following its administration.
The focus of this paper was not to demonstrate or determine the role of iloprost in the management of FCI or NFCI – the focus was to explore if there were a role to push iloprost further forward to enhance the care of patients operating at the greatest reach. The concerns of all authors are valid, that the use of vaso-active agents or drugs with the potential to cause changes in patient physiology are of greatest risk when operating in a remote environment. As we continue to enhance forward capabilities and increase the skillset of those deploying at reach, the ability to counter physiological side effects increases and the risk of adverse event decreases. This is in keeping with the modern medical concept of forward deployment of critical care capability, out of hospital settings, with the ability to deliver advanced interventions at the point and time of patient need.
Although more research is required to define any adverse effect and indeed, if earlier administration of iloprost improves patient outcomes, it may have a role as a future forward capability.
Acknowledgments
The authors wish to express their thanks for the support of the Burnett Library, Defence Medical Services, Whittington Barracks for sourcing all papers required for this literature review.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Author contributions
All authors developed the scope and search criteria for this review as well as critically reviewing all drafts of the manuscript, edited and approved the final draft for submission.
References
- [1].Atkinson E. The British antarctic expedition, 1910-1913. J R Nav Med Serv. 1915;1:1–11. [Google Scholar]
- [2].Mills WJ. A study of frostbite treatment. J Royal Nav Med Serv. 1963;49:237–243. [PubMed] [Google Scholar]
- [3].Thomas RJ, Heil K, Johnson K, et al. Frostbite: an ancient affliction – have we made progress? A case series and review of best practice. J Royal Nav Med Serv. 2017;103:114–119. [Google Scholar]
- [4].Handford C, Thomas O, Imray C. Frostbite. Emerg Med Clin N Am. 2017;35:281–299. [DOI] [PubMed] [Google Scholar]
- [5].Francis TJ Non freezing cold injury: a historical review. J R Nav Med Serv. Winter 1984;70(3):134–139. [PubMed] [Google Scholar]
- [6].Kuht J, Smith B, Brown A. Field recognition and management of freezing and non-freezing cold injuries. J R Nav Med Serv. Spring 2018;104(1):41–46. [Google Scholar]
- [7].Ministry of Defence . Joint Service Publication (JSP) 375 Chapter 42 Cold Injury Prevention. 2022. [Cited on 2022 Jul 30] Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/930667/JSP_375_Chapter_42_V1.0_Oct_2020.pdf
- [8].Cauchy E, Davis C, Paquier M, et al. A New Proposal for Management of Severe Frostbite in the Austere Environment. Wildern Environ Med. 2016. 03;27(1):92–99. [DOI] [PubMed] [Google Scholar]
- [9].Handford C, Buxton P, Russell K, et al. Frostbite: a practical approach to hospital management. Extrem Physiol Med. 2014;3:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Heil K, Thomas R, Robertson G, et al. Freezing and non-freezing cold weather injuries: a systematic review. Br Med Bull. 2016. 03;117(1):79–93. [DOI] [PubMed] [Google Scholar]
- [11].Ministry of Defence . Joint Service Publication (JSP) 539 Part 2. 2022. Available at: https://www.gov.uk/government/publications/prevention-of-climatic-injuries-in-the-armed-forces-medical-policy/jsp-539-heat-illness-and-cold-injury-medical-management-part-2-guidance-accessible-version-february-2021 [Accessed on 30 Jul 2022]
- [12].Francis T, Golden F. Non-freezing cold injury. J Roy Nay Med Serv. 1985;71:3–8. [PubMed] [Google Scholar]
- [13].Ionescu A, Hutchinson S, Ahmad M, et al. Potential new treatment for non-freezing cold injury: is Iloprost the way forward?. J R Army Med Corps. 2017. 10;163(5):361. [DOI] [PubMed] [Google Scholar]
- [14].Glennie JS, Milner R. Non-freezing cold injury. J R Nav Med Serv. 2014;100(3):268–271. [PubMed] [Google Scholar]
- [15].Groechenig E. Treatment of frostbite with iloprost. Lancet. 1994;344(8930):1152–1153. [DOI] [PubMed] [Google Scholar]
- [16].Cauchy E, Cheguillaume B, Chetaille E. A controlled trial of a prostacyclin and rt-PA in the treatment of severe frostbite. N Engl J Med. 2011;362(2):189–190. [DOI] [PubMed] [Google Scholar]
- [17].Johansson PI, Eriksen CF, Schmal H, et al. Efficacy and safety of iloprost in trauma patients with haemorrhagic shock-induced endotheliopathy-Protocol for the multicentre randomized, placebo-controlled, blinded, investigator-initiated shine-trauma trial. Acta Anaesthesiol Scand. 2021;65(4):551–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lindford A, Valtonen J, Hult M, et al. The evolution of the Helsinki frostbite management protocol. Burns. 2017. Nov;43(7):1455–1463. [DOI] [PubMed] [Google Scholar]
- [19].Imray CHE, Oakley EHN. Cold still kills: cold-related illnesses in military practice freezing and non-freezing cold injury. J R Army Med Corps. 2005. 12;151(4):218–222. [DOI] [PubMed] [Google Scholar]
- [20].Heil KM, Oakley EHN, Wood AM. British Military freezing cold injuries: a 13-year review. J R Army Med Corps. 2016. 12;162(6):413–418. [DOI] [PubMed] [Google Scholar]
- [21].Magnan M, Gayet-Ageron A, Louge P, et al. Hyperbaric Oxygen Therapy with Iloprost Improves Digit Salvage in Severe Frostbite Compared to Iloprost Alone. Medicina. 2021;57(11):1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pandey P, Vadlamudi R, Pradhan R, et al. Case Report: Severe Frostbite in Extreme Altitude Climbers-The Kathmandu Iloprost Experience. Wildern Environ Med. 2018. 09;29(3):366–374. [DOI] [PubMed] [Google Scholar]
- [23].Poole A, Gauthier J. Treatment of severe frostbite with iloprost in northern Canada. CMAJ. 2016;188(17–18):1255–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Poole A, Gauthier J, MacLennan M. Management of severe frostbite with iloprost, alteplase and heparin: a Yukon case series. CMAJ Open. 2021;9(2):E585–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].MacLennan M, Poole A, Gauthier J. Use of fluorescence to visualize response to iloprost treatment for frostbite. CMAJ. 2021;193(31):E1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Irarrázaval S, Besa P, Cauchy E, et al. Case Report of Frostbite with Delay in Evacuation: Field Use of Iloprost Might Have Improved the Outcome. High Alt Med Biol. 2018. 12;19(4):382–387. [DOI] [PubMed] [Google Scholar]
- [27].Kaller M. BET 2: Treatment of frostbite with iloprost. Emerg Med J. 2017. 10;34(10):689–690. [DOI] [PubMed] [Google Scholar]
- [28].Lorentzen AK, Davis C, Penninga L. Interventions for frostbite injuries. Cochrane Database Syst Rev. 2020;12:CD012980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].NATO STANDARD AJP-4.10 . Allied Joint Doctrine for Medical Support Edition C Version 1. North Atlantic Treaty Organization Allied Joint Publication. NATO Standardization Office (NSO). September 2019.
- [30].Imray CH. Non-freezing cold injury. J R Army Med Corps. 2019. 12;165(6):388–389. [DOI] [PubMed] [Google Scholar]
- [31].Whitaker J. Non-freezing cold injury, lessons from history for future prevention. TRAUMA. 2016. 07;18(3):178–185. [Google Scholar]
- [32].Mistry K, Ondhia C, Levell NJ. A review of trench foot: a disease of the past in the present. Clin Exp Dermatol. 2020. 01;45(1):10–14. [DOI] [PubMed] [Google Scholar]
- [33].Ingram BJ, Raymond TJ. Recognition and treatment of freezing and non-freezing cold injuries. Curr Sports Med Rep. 2013;12(2):125–130. [DOI] [PubMed] [Google Scholar]
- [34].Zafren K. Non-freezing Cold Injury (Trench Foot). Int j environ res public health. 2021;18(19):10482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Irwin MS, Sanders R, Green CJ, et al. Neuropathy in non-freezing cold injury (trench foot). J R Soc Med. 1997;90(8):433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].. Longman DP, Brown EL, Imray CHE. Non-freezing Cold Injuries Among Long-Distance Polar Rowers. Wildern Environ Med. 2020. 06;31(2):209–214. [DOI] [PubMed] [Google Scholar]
- [37].Jørum E, Opstad P. A 4-year follow-up of non-freezing cold injury with cold allodynia and neuropathy in 26 naval soldiers. Scand J Pain. 2019;19(3):441–451. [DOI] [PubMed] [Google Scholar]
- [38].Tek D, Mackey S. Non-freezing cold injury in a Marine infantry battalion. J Wildern Med. 1993;4(4):353–357. [Google Scholar]
- [39].Williamson K, Izard R. Epidemic of non-freezing cold injury in the British Army. J R Army Med Corps. 2007. 06;153(2):143. [PubMed] [Google Scholar]
- [40].Kuht JA, Woods D, Hollis S. Case series of non-freezing cold injury: epidemiology and risk factors. J R Army Med Corps. 2019. 12;165(6):400–404. [DOI] [PubMed] [Google Scholar]
- [41].Kuht JA, Woods D, Hollis S. Case series of non-freezing cold injury: the modern clinical syndrome. BMJ Mil Health. 2020. 10;166(5):324–329. [DOI] [PubMed] [Google Scholar]
- [42].Anand P, Privitera R, Donatien P, et al. Capsaicin 8% Patch Treatment in Non-Freezing Cold Injury: Evidence for Pain Relief and Nerve Regeneration. Front Neurol. 2021;12:722875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Imray C, Grieve A, Dhillon S. Cold damage to the extremities: frostbite and non-freezing cold injuries. Postgrad Med J. 2009. 09;85(1007):481–488. [DOI] [PubMed] [Google Scholar]
- [44].Melamed E, Glassberg E. Non-freezing cold injury in soldiers. Harefuah. 2002;141(12):1050–1054, 1090. [PubMed] [Google Scholar]


