Abstract
BACKGROUND
Coffee is one of the most commonly consumed beverages in the world, but the acute health effects of coffee consumption remain uncertain.
METHODS
We conducted a prospective, randomized, case-crossover trial to examine the effects of caffeinated coffee on cardiac ectopy and arrhythmias, daily step counts, sleep minutes, and serum glucose levels. A total of 100 adults were fitted with a continuously recording electrocardiogram device, a wrist-worn accelerometer, and a continuous glucose monitor. Participants downloaded a smartphone application to collect geolocation data. We used daily text messages, sent over a period of 14 days, to randomly instruct participants to consume caffeinated coffee or avoid caffeine. The primary outcome was the mean number of daily premature atrial contractions. Adherence to the randomization assignment was assessed with the use of real-time indicators recorded by the participants, daily surveys, reimbursements for date-stamped receipts for coffee purchases, and virtual monitoring (geofencing) of coffee-shop visits.
RESULTS
The mean (±SD) age of the participants was 39±13 years; 51% were women, and 51% were non-Hispanic White. Adherence to the random assignments was assessed to be high. The consumption of caffeinated coffee was associated with 58 daily premature atrial contractions as compared with 53 daily events on days when caffeine was avoided (rate ratio, 1.09; 95% confidence interval [CI], 0.98 to 1.20; P = 0.10). The consumption of caffeinated coffee as compared with no caffeine consumption was associated with 154 and 102 daily premature ventricular contractions, respectively (rate ratio, 1.51; 95% CI, 1.18 to 1.94); 10,646 and 9665 daily steps (mean difference, 1058; 95% CI, 441 to 1675); 397 and 432 minutes of nightly sleep (mean difference, 36; 95% CI, 25 to 47); and serum glucose levels of 95 mg per deciliter and 96 mg per deciliter (mean difference, −0.41; 95% CI, −5.42 to 4.60).
CONCLUSIONS
In this randomized trial, the consumption of caffeinated coffee did not result in significantly more daily premature atrial contractions than the avoidance of caffeine. (Funded by the University of California, San Francisco, and the National Institutes of Health; CRAVE ClinicalTrials.gov number, NCT03671759.)
Coffee is one of the most commonly consumed beverages in the world, yet its acute health effects remain largely unknown.1 Despite the common admonition that coffee should be avoided owing to associated proarrhythmic effects,2,3 evidence in support of this warning is conflicting.4–8 The relevance of this frequently consumed substance to cardiac ectopy has become more salient in light of observations that, in a population-based cohort of older adults, more premature atrial contractions predicted new-onset atrial fibrillation9 and more premature ventricular contractions were associated with a heightened risk of heart failure.10,11 In observational studies, coffee consumption has been associated with lower rates of diabetes and death, a finding that is often attributed to the possibility that caffeine stimulates increased physical activity.12–15 Coffee may suppress effective sleep, but objective measurements of this relationship in natural environments are scarce.16
We conducted the Coffee and Real-time Atrial and Ventricular Ectopy (CRAVE) trial to assess the acute effects of coffee consumption on cardiac ectopy, physical activity, sleep, and glucose levels by using continuously recording, wearable sensors to assess healthy participants who were randomly assigned to alternate between consuming and avoiding caffeinated coffee.
METHODS
TRIAL DESIGN AND PARTICIPANTS
In this prospective, randomized, single-center, 14-day trial, we used a case-crossover design to evaluate the effect of caffeinated coffee on health defined as cardiac ectopy, activity levels, sleep minutes, and glucose levels. The trial was approved by the institutional review board at the University of California, San Francisco, and all the participants provided written informed consent. The contributions of the authors are listed in Section S2 in the Supplementary Appendix, available with the full text of this article at NEJM.org. The authors vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol (available at NEJM.org).
Participants were recruited through the use of flyers, word of mouth, and social media. Eligible participants were at least 18 years of age, consumed caffeinated coffee at least once a year, owned a smartphone with either an iOS or Android operating system, were able to use the Eureka smartphone application (app), and were willing to abstain from coffee, caffeinated products, or minimally caffeinated products (decaffeinated coffee) for at least 2 consecutive days as instructed over the 14-day trial period. We excluded persons who had a history of atrial fibrillation or heart failure; had an implanted pacemaker or implantable cardioverter–defibrillator; had been prescribed beta-blockers, nondihydropyridine calcium-channel blockers or Vaughn–Williams class 1 or 3 antiarrhythmic medications; or had a medical reason to avoid coffee.
TRIAL PROCEDURES
Participants were randomly assigned to consume caffeinated coffee or to avoid caffeine during 2-day periods. The random assignments were made by means of daily text messages sent over a 14-day period. In order to avoid cumulative effects and enhance enrollment and retention, we constructed the randomization in pairs of off–on or on–off days to ensure that a participant had no more than two consecutive days of coffee consumption or abstinence. The participants received their assignments at 8 p.m. for the next day and received a follow-up reminder on the assignment day at 8 a.m. (The assignment instructions and a description of the daily survey regarding coffee consumption are provided in Section S3.)
The participants were fitted with a continuously recording electrocardiogram (ECG) patch (Zio XT Patch, iRhythm), a clinical-grade monitor that used an algorithm that had been validated against overreads performed by cardiologists17; arrhythmias were confirmed by manual review by a board-certified cardiac electrophysiologist who was unaware of the consumption assignments. The participants were encouraged to keep the patch in place for the duration of the trial and were provided with a transparent adhesive dressing to place over the device in case the device adhesive failed. The participants downloaded the Eureka app (developed and maintained by investigators at the University of California, San Francisco) to their smartphones to continuously monitor their geolocation in order to track visits made to coffee shops. They were also fitted with a Bluetooth-enabled, wrist-worn accelerometer to quantify step counts18,19 and duration of sleep20,21 (inferred from actigraphy measured with the use of Fitbit INSPIRE devices), with data collected by means of an automated programming interface that was integrated with the Eureka app, and a Bluetooth-enabled, continuously recording glucose monitoring device routinely used in clinical practice by patients with diabetes to calculate insulin doses22,23 (Dexcom G6, Dexcom), with data collected by means of the Dexcom mobile app.
Participant adherence to the randomization assignment was assessed in four ways. Participants were instructed to press an activator button on the ECG patch when they had consumed a standard 8-ounce cup of coffee or two standard 1-ounce shots of espresso. Pressing the button assigned a time stamp to every drink consumed; this process had been previously validated for real-time assessment of consumption of alcoholic drinks.24 Participants were queried each morning about their coffee consumption on the previous day and were instructed that the time stamps and daily survey were to include every coffee drink they consumed, whether at home or elsewhere. Participants were offered reimbursement for coffee drinks they purchased for immediate consumption if they provided date-stamped receipts, regardless of how those dates corresponded to their assigned consumption. For participants who reported at baseline that they visited particular coffee shops for the sole purpose of coffee consumption, the Eureka app was used to track visits to those locations with the use of smartphone-based geofencing.25
DNA SAMPLE COLLECTION AND ANALYSIS
DNA was collected and purified from saliva and genotyped for seven caffeine-related, single-nucleotide polymorphisms with the use of a real-time polymerase-chain-reaction assay (TaqMan Master Mix and Applied Biosystems 7300).26–28
TRIAL OUTCOMES
The primary outcome was the number of premature atrial contractions per 24-hour period. Secondary outcomes were the daily number of premature ventricular contractions, daily number of episodes of supraventricular tachycardia or ventricular tachycardia, daily step counts, daily minutes of sleep, mean daily serum glucose levels, and interactions between each outcome and caffeine-metabolism–related genetic variants.26,27
STATISTICAL ANALYSIS
Normally distributed continuous variables are reported as means ±SD. Analyses were conducted according to the intention-to-treat principle. As-treated effects were also assessed according to the number of coffee drinks consumed as measured by the activation of the ECG monitor button by the participant.
We used negative binomial models with robust standard errors to account for clustering among participants and with adjustment for day of the week as a fixed effect to assess outcomes that involved counts (to calculate rate ratios of mean daily counts of premature atrial or ventricular contractions on days participants were assigned to coffee versus no caffeine). Logistic models with robust standard errors were used to assess the presence or absence of outcome measures. We assessed normally distributed continuous outcomes (step counts, minutes asleep, and glucose levels) with the use of generalized linear mixed models with random effects to account for clustering within the participant population and adjusted for day of the week. We examined heterogeneity by tertiles of the caffeine-metabolism–related polygenic score (classified as slow, intermediate, and fast caffeine metabolizers) between randomization assignment and outcomes. Treatment of missing data is described in Section S4 and Tables S1 through S6 in the Supplementary Appendix. To acknowledge the possibility that data were not missing completely at random and to address incomplete ECG monitoring data in some participants, the primary outcome analysis was conducted with the use of inverse probability weighting.
Assuming seven cycles each of coffee exposure and caffeine avoidance and using a two-tailed alpha of 0.05, we calculated that 80 participants would provide the trial with 80% power to detect a clinically relevant increase of 30% in premature atrial contractions9 (Table S7). A two-tailed P value of less than 0.05 was considered to indicate statistical significance. The widths of the confidence intervals have not been adjusted for multiplicity and thus should not be used to reject or not reject the effects of coffee consumption. Statistical analyses were performed with the use of Stata software, version 16 (StataCorp).
RESULTS
PARTICIPANTS
Of 113 potential participants screened for the trial, 13 did not meet the entry criteria and were excluded (Table S8). The first participant underwent randomization on May 23, 2019, and the final date of observation for the last participant was March 25, 2020. The characteristics of the 100 participants at baseline are shown in Table 1. The ages of the participants included each decade of life from 20 years of age to more than 70 years (Table S9). Demographic characteristics of the participants were representative of the population of the city of San Francisco (Table S10). The amount of coffee that the participants reported consuming at baseline varied, with the largest proportion of participants consuming one coffee drink a day. However, coffee consumption ranged from less than one coffee drink per month to four or five coffee drinks per day.
Table 1.
Characteristics of 100 Participants at Baseline.*
| Characteristic | Value |
|---|---|
| Mean (±SD) age — yr | 39±13 |
| Female sex — % | 51 |
| Race or ethnic group — %† | |
| Non-Hispanic White | 51 |
| Black | 8 |
| Asian | 34 |
| Pacific Islander | 1 |
| Hispanic | 8 |
| Other | 6 |
| Median BMI (interquartile range)‡ | 24 (22–26) |
| Hypertension — % | 3 |
| Diabetes mellitus — % | 1 |
| Coronary artery disease | 0 |
| Usual coffee consumption — % | |
| <1 cup per mo | 5 |
| 1–3 cups per mo | 6 |
| 2–5 cups per wk | 14 |
| 6–7 cups per wk | 21 |
| 1 cup per day | 29 |
| 2–3 cups per day | 22 |
| 4–5 cups per day | 3 |
Because 100 participants were enrolled, numbers and percentages are the same.
Race or ethnic group was reported by the participants, who could choose more than one group.
Body-mass index (BMI) is the weight in kilograms divided by the square of the height in meters.
Assessments of adherence to the randomization assignments revealed imperfect adherence, but the majority of participants followed the instructions to consume caffeinated coffee or avoid caffeine on the majority of days (Tables S11 through S16 and Fig. S1). Participants who were adherent to the assignments as ascertained by real-time activation of the ECG time-stamp button were more likely to be White and to consume coffee at a lower usual frequency than participants who did not adhere to the assignments (Table S4). When adherence was assessed with the use of daily surveys, the participants who were more likely to adhere to the random assignments were those who were observed to usually consume coffee less frequently than those who were not adherent to the assignments. On the basis of the responses to the daily surveys, we observed no between-group differences according to race in the adherence to assignments (Table S5). The participants often drank more than their baseline usual amounts of coffee on days when they were assigned to consume coffee (Table S17).
OUTCOMES
The participants wore the ECG monitor for a median of 13.3 days (interquartile range, 12.2 to 13.8). In the intention-to-treat analyses, days on which participants were assigned to coffee consumption were associated with a mean of 58 premature atrial contractions per day, as compared with a mean of 53 premature atrial contractions per day on caffeine-avoidance days (rate ratio, 1.09; 95% confidence interval [CI], 0.98 to 1.20; P=0.10) (Table 2). On days on which participants were assigned to coffee consumption, the mean number of premature ventricular contractions per day was 154, as compared with 102 on days they were instructed to consume no caffeine (rate ratio, 1.51; 95% CI, 1.18 to 1.94). The number of episodes of nonsustained supraventricular tachycardia was similar on coffee-consumption days and caffeine-avoidance days (mean, 0.17 episodes and 0.20 episodes, respectively; rate ratio, 0.83; 95% CI, 0.68 to 1.02), as was the number of episodes of nonsustained ventricular tachycardia (mean, 0.01 episodes and 0.01 episodes, respectively; rate ratio, 1.14; 95% CI, 0.43 to 2.99). The frequencies of observed arrhythmias are shown in Table S18.
Table 2.
Daily Frequency of Arrhythmia.
| Outcome | Coffee Consumption | Caffeine Avoidance | Treatment Effect* |
|---|---|---|---|
| daily mean no. | |||
| Premature atrial contractions | 58 | 53 | 1.09 (0.98–1.20)† |
| Premature ventricular contractions | 154 | 102 | 1.51 (1.18–1.94) |
| Episodes of nonsustained supraventricular tachycardia | 0.17 | 0.20 | 0.83 (0.68–1.02) |
| Episodes of nonsustained ventricular tachycardia | 0.01 | 0.01 | 1.14 (0.43–2.99)‡ |
The estimates are adjusted for the day of the week. The widths of the confidence intervals have not been adjusted for multiplicity and cannot be used to reject or not reject effects of coffee consumption. The treatment effect for episodes of nonsustained ventricular tachycardia is shown as an odds ratio; the other treatment effects are rate ratios.
P=0.10.
An insufficient number of episodes of nonsustained ventricular tachycardia precluded an accurate negative binomial model, so a logistic model with robust standard errors for the presence or absence of an episode was used.
The as-treated analyses suggested similar relationships between the number of coffee drinks consumed and episodes of premature atrial contractions and arrhythmias. Among participants who drank more than one coffee drink per day, the as-treated analysis suggested that the relationship between the number of coffee drinks consumed per day and premature ventricular contractions appeared similar to that shown in the intention-to-treat analysis; however, among participants who consumed only one coffee drink per day, the as-treated analysis suggested a lack of relationship between the number of coffee drinks per day and episodes of premature ventricular contractions (Table S19).
Step-count data were available from 86 participants, sleep data from 81, and glucose data from 87; missing data were the result of technical problems in every case. In the intention-to-treat analysis, assignment to consume coffee was associated with a daily mean of 10,646 steps (recorded by a Fitbit) as compared with 9665 steps recorded on days on which participants were assigned to avoid caffeine (mean difference, 1058; 95% CI, 441 to 1675) (Fig. 1). The as-treated analyses also suggested an association between more caffeinated coffee consumed and more steps. (Details of the as-treated analyses are provided in Section S5.)
Figure 1. Daily Step Count for Each Participant on Days of Coffee Consumption and Days of Caffeine Avoidance.
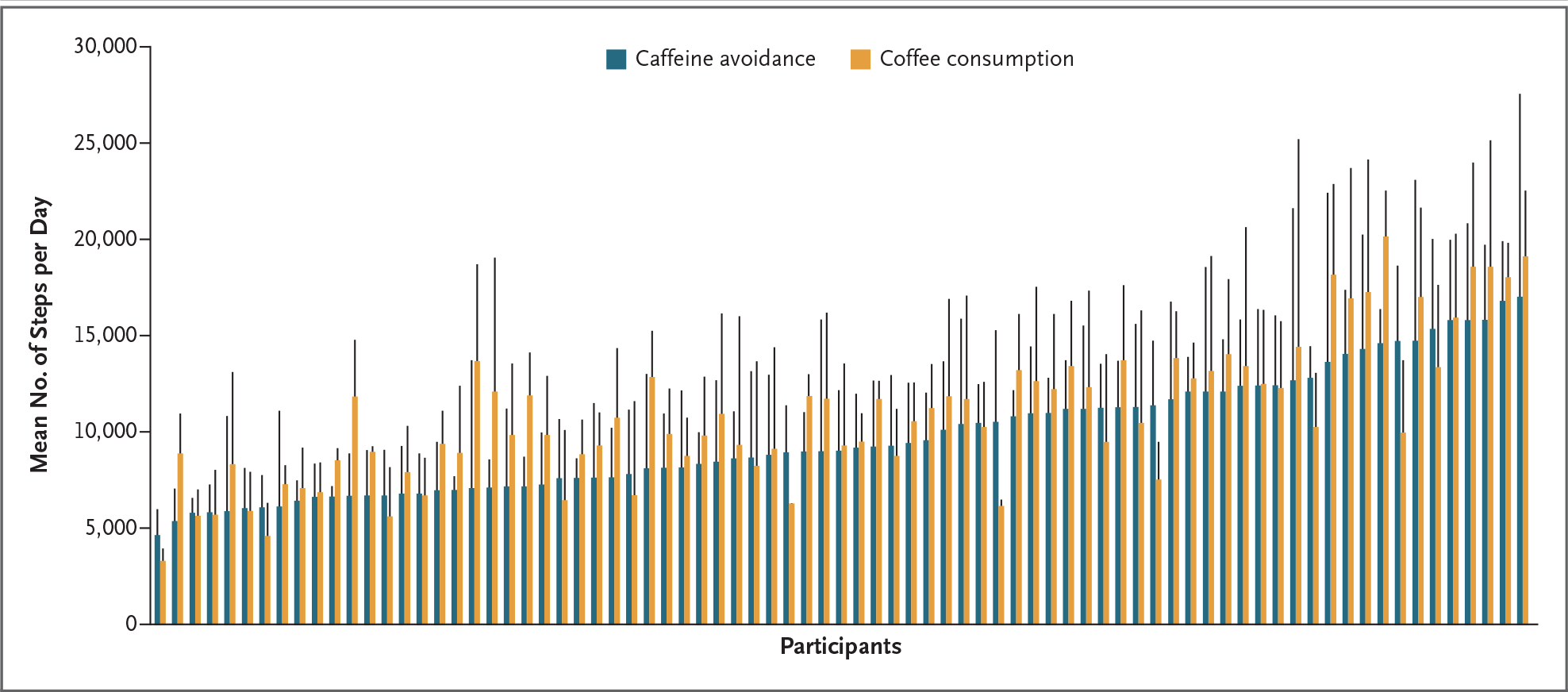
The x axis shows the mean daily step count for each participant for all days on which the participant was randomly assigned to avoid caffeine (blue) or to consume coffee (orange). Data for each participant are grouped in pairs and ordered according to the magnitude of the participants’ step counts on days they were randomly assigned to avoid caffeine. The vertical black bars indicate standard deviations. Step-count data were available for 86 of the 100 participants.
In the intention-to-treat analysis, assignment to coffee consumption was associated with a mean of 397 minutes of sleep per night, as compared with 432 minutes on caffeine-avoidance days (mean difference, 36 fewer minutes of sleep per night; 95% CI, 25 to 47) (Fig. 2). In the as-treated analyses, consumption of more coffee was associated with less sleep.
Figure 2. Minutes Asleep for Each Participant after Coffee Consumption and Caffeine Avoidance.
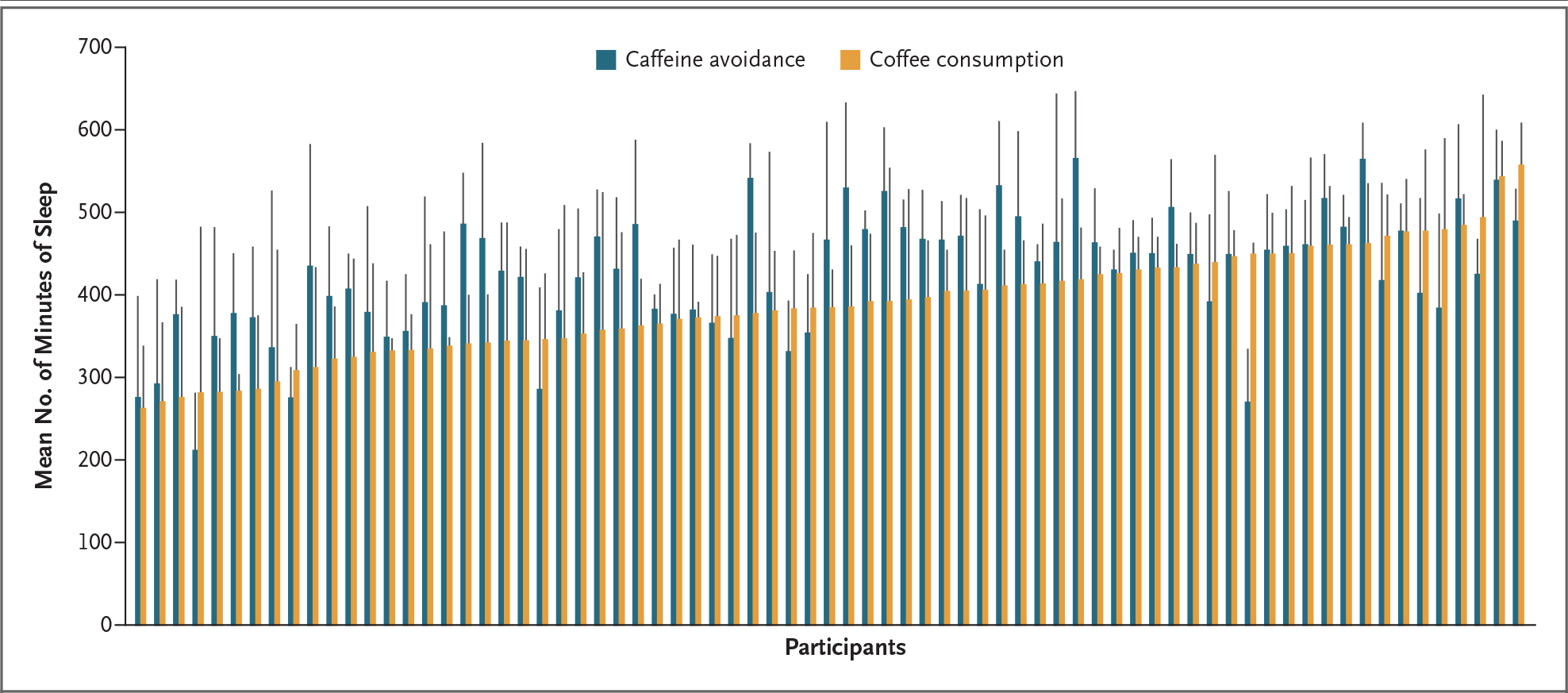
The x axis shows the mean number of minutes of sleep for each participant after all days on which the participant was randomly assigned to avoid caffeine (blue) and to consume coffee (orange). Data for each participant are grouped in pairs and ordered according to the magnitude of the time participants were asleep on days on which they were randomly assigned to consume coffee. The vertical black bars indicate standard deviations. Sleep data were available for 81 of the 100 participants.
On days when participants consumed coffee, the daily average glucose levels were 95 mg per deciliter, as compared with 96 mg per deciliter on caffeine-avoidance days (mean difference, −0.41; 95% CI, −5.42 to 4.60). No relationships between assigned consumption or as-treated consumption and daily average glucose levels were observed (Table S20). Results remained consistent among participants who routinely consumed at least one coffee drink per day at baseline and participants who did not (Table S21).
No substantial differences according to caffeine-related genotype were observed with regard to the amount of coffee consumed (Table S22). The associations between the variants and premature atrial contractions, premature ventricular contractions, daily steps, and daily sleep minutes are shown in Table S23. There was no consistent pattern observed in the associations between the variants and the health measures. Participants with genetic variants that are associated with faster caffeine metabolism appeared to have more premature ventricular contractions when they consumed coffee, and participants with genetic variants that are associated with slower caffeine metabolism appeared to have greater reductions in sleep minutes when they consumed coffee.
DISCUSSION
Among 100 healthy adults, the consumption of caffeinated coffee did not result in more daily premature atrial contractions than the avoidance of caffeine. Our results further suggest that both assignment to daily coffee consumption and greater-than-normal coffee consumption were associated with more recorded daily steps taken, fewer minutes of sleep per night, and potentially more daily premature ventricular contractions among persons who consumed more than one coffee drink per day.
Cardiac ectopy is a nearly universal human phenomenon, but recent evidence from a population-based cohort of older adults showed that a greater frequency of premature atrial contractions predicts new-onset atrial fibrillation9,29 and that persons who have more premature ventricular contractions are at an increased risk for heart failure.10,29 Thus, interest has focused on identifying common exposures that might influence the frequency of common cardiac ectopy. Despite admonitions that the avoidance of caffeinated beverages minimizes arrhythmias,3,30 randomized trials of the acute effects of coffee on cardiac ectopy were lacking. Our trial produced no evidence of statistically significant relationships between coffee consumption and the frequency of premature atrial contractions, findings that are consistent with those of previous observational studies that used evidence derived from patient-reported general coffee consumption and 24-hour Holter monitoring.5 Evidence from recent observational studies suggests that coffee drinkers have a lower risk of atrial fibrillation than nondrinkers,6,8 a finding that has been attributed to the antiinflammatory or antivagal effects of coffee, given evidence that supports the roles of inflammation31 and vagal tone32,33 as harbingers of atrial arrhythmias.
Caffeine is a nonselective adenosine receptor antagonist, an inhibitor of phosphodiesterases, and a direct agonist of the ryanodine receptor, which enhances calcium release from the sarcoplasmic reticulum. Such calcium release is known to result in afterdepolarizations, which, if large enough, can result in a premature ventricular contraction.11,34 One of the few reports to describe a positive relationship between coffee and arrhythmias showed that the larger the daily consumption of coffee, the greater the likelihood of premature ventricular contractions as shown on a 2-minute ECG,4 a finding that is consistent with our current findings. Our analyses of as-treated coffee consumption indicated that there was a lack of association between a single cup of coffee and premature ventricular contractions but suggested that there might be a possible association between consumption of more than one coffee drink per day and premature ventricular contractions in our trial participants.
Coffee drinkers have consistently had reduced mortality in prospective cohort studies, but the mechanism for this result remains unknown.12,13,35 Because caffeine may enhance physical performance,14,36 some investigators have speculated that coffee consumption may lead to more physical activity, one of the most important determinants of overall health and longevity.37 Random assignment to coffee consumption resulted in a median of 1000 more steps per participant per day in our trial. An additional 1000 steps per day has been associated with a 6% to 15% reduction in mortality,38 effect sizes that are remarkably similar to the magnitude of mortality benefit observed among coffee drinkers.12,13,35 However, the current trial was not equipped to determine precisely why participants took more steps when they consumed more coffee.
Sleep has emerged as an important factor in multiple health outcomes, including the risk of cardiovascular disease, cancer, and cognition issues, as well as in overall mortality.39,40 Coffee notoriously disrupts sleep; however, observational studies are not well-suited to quantify this relationship owing to confounding by indication (persons with the worst caffeine-induced insomnia are more likely to minimize consumption). Although an hour less sleep per night correlates with substantially worse outcomes, the effects of losing 30 minutes of sleep, as observed in our trial, are less certain.41 However, for persons with difficulty sleeping, these data provide evidence from among participants studied in their natural environments that coffee consumption can indeed influence this crucial activity. The results raise the possibility that coffee consumption had little effect in participants who were fast caffeine metabolizers, whereas those who were the slowest caffeine metabolizers had the most exaggerated effects. These findings suggest that an individualized approach to coffee consumption may be the most appropriate method for determining the effects on health.
Despite epidemiologic evidence that coffee drinkers have a lower risk of diabetes than persons who avoid coffee,13 we found no evidence that coffee consumption was associated with mean daily glucose levels. This finding may have occurred because coffee consumption was accompanied by other intake rich in simple carbohydrates. It may also suggest that the consequences of coffee consumption on diabetes risk are long term and may reflect such factors as antiinflammatory effects or ramifications of more regular physical activity.
It is important to acknowledge several limitations of our trial. First, the trial was relatively small; however, the case-crossover design allowed for generally more than 10 different assessments per participant. The trial was not blinded, so we cannot exclude the possibility that other influences concomitant with coffee exposure were the actual causal factors. Regarding the relationship between coffee exposure and the outcome measures, the effects of caffeine withdrawal may have been operative; therefore, although withdrawal probably increased the magnitude of the measurements observed, changes attributed to such phenomena would further support the role of coffee (or caffeine) itself as the culprit source. The trial population was relatively young and healthy, so extrapolation to other populations, including those with frequent premature atrial or ventricular contractions, may not be appropriate.
Although the continuously recording devices facilitate complete data capture for outcome assessments, those outcomes were included only while (or if) those devices were worn. However, given the case-crossover nature of the analyses (for which each participant served as the control), these missing outcome data would primarily limit generalizability and should pose less of a threat to internal validity. Adherence assessments were also not complete for all participants, but the four approaches that were used relied on different methods of ascertainment and consistently favored general compliance with the randomization assignment. The Fitbit is not a clinical-grade device, so the estimates regarding sleep durations that were obtained from the device may not fully quantify the exact amount of time asleep,21 and markers of sleep efficiency other than sleep duration were not captured. Finally, although participants were instructed specifically to consume caffeinated coffee, we cannot disentangle the effects specific to caffeine as compared with other ingredients in the various types of coffee drinks that were allowed.
Consumption of caffeinated coffee was not associated with a change in the number of daily premature atrial contractions among participants. Findings from secondary analyses that should be interpreted as hypothesis-generating suggest that coffee consumption may be associated with more premature ventricular contractions and more physical activity but less sleep. No apparent relationship between coffee consumption and serum glucose levels was observed. These findings suggest protean health-related consequences of consuming this common beverage and provide both clinicians and patients with information that may assist in customizing consumption of coffee to appropriately fit with individual health goals.
Supplementary Material
Acknowledgments
Supported by a Cardiology Innovation Award from the University of California, San Francisco, and by the National Institutes of Health; Dexcom donated continuous glucose monitors for the trial.
Footnotes
Contributor Information
Gregory M. Marcus, Division of Cardiology, University of California, San Francisco, San Francisco.
Gregory Nah, Division of Cardiology, University of California, San Francisco, San Francisco.
Eric Vittinghoff, Department of Epidemiology and Biostatistics, University of California, San Francisco, San Francisco.
Christina Fang, University of California, Irvine, School of Medicine, Irvine.
Kelsey Ogomori, School of Medicine, University of California, San Francisco, San Francisco.
Sean Joyce, School of Medicine, University of California, San Francisco, San Francisco.
Defne Yilmaz, University of California, Berkeley, Berkeley.
Vivian Yang, School of Medicine, University of California, San Francisco, San Francisco.
Tara Kessedjian, University of California, Berkeley, Berkeley.
Emily Wilson, Division of Cardiology, University of California, San Francisco, San Francisco.
Michelle Yang, Division of Cardiology, University of California, San Francisco, San Francisco.
Kathleen Chang, Division of Cardiology, University of California, San Francisco, San Francisco.
Grace Wall, Division of Cardiology, University of California, San Francisco, San Francisco.
Jeffrey E. Olgin, Division of Cardiology, University of California, San Francisco, San Francisco
References
- 1.van Dam RM, Hu FB, Willett WC. Coffee, caffeine, and health. N Engl J Med 2020;383:3 69–78. [DOI] [PubMed] [Google Scholar]
- 2.Blomström-Lundqvist C, Scheinman MM, Aliot EM, et al. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias — executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients with Supraventricular Arrhythmias) developed in collaboration with NASPE-Heart Rhythm Society. J Am Coll Cardiol 2003;42:1493–531. [DOI] [PubMed] [Google Scholar]
- 3.Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2018;138(13):e210–e271. [DOI] [PubMed] [Google Scholar]
- 4.Prineas RJ, Jacobs DR Jr, Crow RS, Blackburn H. Coffee, tea and VPB. J Chronic Dis 1980;33:67–72. [DOI] [PubMed] [Google Scholar]
- 5.Dixit S, Stein PK, Dewland TA, et al. Consumption of caffeinated products and cardiac ectopy. J Am Heart Assoc 2016;5(1):e002503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim E-J, Hoffmann TJ, Nah G, Vittinghoff E, Delling F, Marcus GM. Coffee consumption and incident tachyarrhythmias: reported behavior, mendelian randomization, and their interactions. JAMA Intern Med 2021;181:1185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bodar V, Chen J, Gaziano JM, Albert C, Djoussé L. Coffee consumption and risk of atrial fibrillation in the Physicians’ Health Study. J Am Heart Assoc 2019;8(15):e011346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caldeira D, Martins C, Alves LB, Pereira H, Ferreira JJ, Costa J. Caffeine does not increase the risk of atrial fibrillation: a systematic review and meta-analysis of observational studies. Heart 2013;99:1383–9. [DOI] [PubMed] [Google Scholar]
- 9.Dewland TA, Vittinghoff E, Mandyam MC, et al. Atrial ectopy as a predictor of incident atrial fibrillation: a cohort study. Ann Intern Med 2013;159:721–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dukes JW, Dewland TA, Vittinghoff E, et al. Ventricular ectopy as a predictor of heart failure and death. J Am Coll Cardiol 2015;6 6:101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marcus GM. Evaluation and management of premature ventricular complexes. Circulation 2020;41:1404–18. [DOI] [PubMed] [Google Scholar]
- 12.Ding M, Satija A, Bhupathiraju SN, et al. Association of coffee consumption with total and cause-specific mortality in 3 large prospective cohorts. Circulation 2015;132:2305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poole R, Kennedy OJ, Roderick P, Fallowfield JA, Hayes PC, Parkes J. Coffee consumption and health: umbrella review of meta-analyses of multiple health outcomes. BMJ 2017;359:j5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clarke ND, Kirwan NA, Richardson DL. Coffee ingestion improves 5 km cycling performance in men and women by a similar magnitude. Nutrients 2019;11:2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tabrizi R, Saneei P, Lankarani KB, et al. The effects of caffeine intake on weight loss: a systematic review and dose-response meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr 2019;59:2688–96. [DOI] [PubMed] [Google Scholar]
- 16.Grandner MA. Sleep, health, and society. Sleep Med Clin 2017;12:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hannun AY, Rajpurkar P, Haghpanahi M, et al. Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network. Nat Med 2019;25:65–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maganja SA, Clarke DC, Lear SA, Mackey DC. Formative evaluation of consumer-grade activity monitors worn by older adults: test-retest reliability and criterion validity of step counts. JMIR Form Res 2020;4(8):e16537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tedesco S, Sica M, Ancillao A, Tim-mons S, Barton J, O’Flynn B. Validity evaluation of the Fitbit Charge2 and the Garmin vivosmart HR+ in free-living environments in an older adult cohort. JMIR Mhealth Uhealth 2019;7(6):e13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beattie Z, Oyang Y, Statan A, et al. Estimation of sleep stages in a healthy adult population from optical plethysmography and accelerometer signals. Physiol Meas 2017;38:1968–79. [DOI] [PubMed] [Google Scholar]
- 21.Stone JD, Rentz LE, Forsey J, et al. Evaluations of commercial sleep technologies for objective monitoring during routine sleeping conditions. Nat Sci Sleep 2020;12:821–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah VN, Laffel LM, Wadwa RP, Garg SK. Performance of a factory-calibrated real-time continuous glucose monitoring system utilizing an automated sensor applicator. Diabetes Technol Ther 2018;20:428–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah VN, DuBose SN, Li Z, et al. Continuous glucose monitoring profiles in healthy nondiabetic participants: a multicenter prospective study. J Clin Endocrinol Metab 2019;104:4356–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marcus GM, Vittinghoff E, Whitman IR, et al. Acute consumption of alcohol and discrete atrial fibrillation events. Ann Intern Med 2021;174:1503–9. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen KT, Olgin JE, Pletcher MJ, et al. Smartphone-based geofencing to ascertain hospitalizations. Circ Cardiovasc Qual Outcomes 2017;10(3):e003326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cornelis MC, Monda KL, Yu K, et al. Genome-wide meta-analysis identifies regions on 7p21 (AHR) and 15q24 (CYP1A2) as determinants of habitual caffeine consumption. PLoS Genet 2011;7(4):e1002033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coffee and Caffeine Genetics Consortium, Cornelis MC, Byrne EM, et al. Genome-wide meta-analysis identifies six novel loci associated with habitual coffee consumption. Mol Psychiatry 2015;20:647–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou A, Hyppönen E. Long-term coffee consumption, caffeine metabolism genetics, and risk of cardiovascular disease: a prospective analysis of up to 347,077 individuals and 8368 cases. Am J Clin Nutr 2019;109:509–16. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen KT, Vittinghoff E, Dewland TA, et al. Ectopy on a single 12-lead ECG, incident cardiac myopathy, and death in the community. J Am Heart Assoc 2017;6(8):e006028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blomström-Lundqvist C, Scheinman MM, Aliot EM, et al. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias — executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Supraventricular Arrhythmias). Circulation 2003;108:1871–909. [DOI] [PubMed] [Google Scholar]
- 31.Aviles RJ, Martin DO, Apperson-Hansen C, et al. Inflammation as a risk factor for atrial fibrillation. Circulation 2003;108:3006–10. [DOI] [PubMed] [Google Scholar]
- 32.Liu L, Nattel S. Differing sympathetic and vagal effects on atrial fibrillation in dogs: role of refractoriness heterogeneity. Am J Physiol 1997;273:H805–H816. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi Y, Jaïs P, Hocini M, et al. Shortening of fibrillatory cycle length in the pulmonary vein during vagal excitation. J Am Coll Cardiol 2006;47:774–80. [DOI] [PubMed] [Google Scholar]
- 34.Schlotthauer K, Bers DM. Sarcoplasmic reticulum Ca(2+) release causes myocyte depolarization: underlying mechanism and threshold for triggered action potentials. Circ Res 2000;87:774–80. [DOI] [PubMed] [Google Scholar]
- 35.Kim Y, Je Y, Giovannucci E. Coffee consumption and all-cause and cause-specific mortality: a meta-analysis by potential modifiers. Eur J Epidemiol 2019;34:731–52. [DOI] [PubMed] [Google Scholar]
- 36.Karayigit R, Naderi A, Akca F, et al. Effects of different doses of caffeinated coffee on muscular endurance, cognitive performance, and cardiac autonomic modulation in caffeine naive female athletes. Nutrients 2020;13:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reiner M, Niermann C, Jekauc D, Woll A. Long-term health benefits of physical activity — a systematic review of longitudinal studies. BMC Public Health 2013;13:813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee I-M, Shiroma EJ, Kamada M, Bassett DR, Matthews CE, Buring JE. Association of step volume and intensity with all-cause mortality in older women. JAMA Intern Med 2019;179:1105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li J, Cao D, Huang Y, et al. Sleep duration and health outcomes: an umbrella review. Sleep Breath 2022;26:1479–501. [DOI] [PubMed] [Google Scholar]
- 40.Itani O, Jike M, Watanabe N, Kaneita Y. Short sleep duration and health outcomes: a systematic review, meta-analysis, and meta-regression. Sleep Med 2017;32:246–56. [DOI] [PubMed] [Google Scholar]
- 41.Gallicchio L, Kalesan B. Sleep duration and mortality: a systematic review and meta-analysis. J Sleep Res 2009;18:148–58. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


