Abstract
An infectious Shiga toxin (Stx) 2e-converting bacteriophage (φP27) was isolated from Stx2e-producing Escherichia coli ONT:H− isolate 2771/97 originating from a patient with diarrhea. The phage could be transduced to E. coli laboratory strain DH5α, and we could show that lysogens were able to produce biologically active toxin in a recA-dependent manner. By DNA sequence analysis of a 6,388-bp HindIII restriction fragment of φP27, we demonstrated that the stx2e gene was located directly downstream of ileZ and argO tRNA genes. Although no analogue of an antiterminator Q encoding gene was present on this fragment, a lysis cassette comprising two holin genes which are related to the holin genes of Pseudomonas aeruginosa phage φCTX and a gene homologous to the endolysin gene gp19 of phage PS3 were detected. The results of our study demonstrated for the first time that Stx2e can be encoded in the genome of an infectious bacteriophage.
Shiga toxin (Stx)-producing Escherichia coli (STEC) strains are a worldwide cause of diarrhea, hemorrhagic colitis, and the hemolytic-uremic syndrome (HUS) (8). Stx identified in human STEC isolates comprise Stx1, Stx2 and variants of Stx2, including Stx2c, Stx2d, and Stx2e (21, 31, 35). STEC strains associated with diarrhea and HUS usually produce Stx1, Stx2, and Stx2c, either alone or in various combinations (6, 24). In contrast, Stx2d was frequently identified in STEC isolates from asymptomatic carriers (21). Stx2e is typically produced by STEC strains that cause pig edema disease and belong to serogroups O138, O139, and O141 (15). However, Stx2e-producing STEC strains have also been isolated, albeit rarely, from patients with diarrhea (20) and HUS (35). These human isolates belonged to serogroups O101 and O9 that have not been reported in STEC strains associated with pig edema disease. Interestingly, Stx2e-producing STEC belonging to serogroup O101 have been isolated from slaughtered healthy pigs (2), suggesting this animal species as a potential reservoir of human infections. Several stx2e genes have been cloned and sequenced from STEC O101 isolates originating from a patient with diarrhea and from healthy pigs, respectively (4), and were demonstrated to be identical or almost identical to stx2e present in an STEC O139 isolate from a pig with edema disease (36).
Stx1 and Stx2 are encoded in the genome of temperate bacteriophages (11, 32, 33). Phages that contain the structural genes for Stx1 and Stx2 have been isolated from STEC O157 and O26 strains, and their morphology, genome sizes, and restriction fragment length polymorphism patterns have been characterized (23, 32, 38). Moreover, the Stx1-converting phage H-19B isolated from STEC O26:H11 strain H19 and the Stx2-converting phage 933W originating from STEC O157:H7 strain EDL 933 (19) have been characterized by nucleotide sequencing and shown to have a genetic structure related to that of bacteriophage λ (18, 22). Analysis of a 17-kb region of the genome of phage H-19B demonstrated that the stx1 gene was located downstream of a gene encoding an analogue of the transcription activator Q of lambdoid phages and upstream of the analogues of λ genes encoding lysis functions (18). Phage 933W and phage VT2-Sakai had a related structure in this region (14, 22). Functional studies in phage H-19B suggested a role for the Q protein and the lysis genes in the release of toxin from the bacterial cell (18).
In contrast to stx1 and stx2, the stx2e genes in STEC strains associated with pig edema disease have been reported to be located in the chromosome because no Stx-converting phages could be isolated from such strains (15, 37). No data have been provided in the literature on the location of stx2e genes in sporadic Stx2e-producing STEC isolates of human origin described until now (20, 35). The lack of information on the localization of the stx2e gene prompted us to investigate the presence of Stx2e-converting bacteriophages in stx2e-harboring E. coli strains isolated from patients in our laboratory. We were able to isolate and characterize such a phage from the human STEC isolate 2771/97.
MATERIALS AND METHODS
Bacterial strains, phages, plasmids, and growth media.
Eleven Stx2e-producing E. coli strains were included in this study. Eight of them were isolated in 1997 and 1998 from patients with diarrhea in Würzburg, Germany. These strains were found to be positive by PCR with primers LP43 and LP44 (stxA2) and were then determined to contain stx2e by PCR with primers FK9 and FK10 (4). Strain VUB-EH60 was isolated from a patient with diarrhea in Belgium (20), strain E-D53 was isolated from a healthy pig (2), and strain E57 was isolated from a pig with diarrhea (12). E. coli laboratory strain DH5α (Gibco-BRL) was used as a host for bacteriophage φP27 and recombinant plasmids. Phage 933W isolated from E. coli O157:H7 strain EDL 933 (19) and bacteriophage λ (ATCC 97537) were used as controls in transduction experiments and plaque assays. Recombinant plasmid pIM10 containing the recA gene of E. coli (5) was a kind gift of G. Blum-Oehler and J. Hacker, Würzburg, Germany. Bacteria were routinely grown in Luria-Bertani (LB) broth or on LB agar plates. When required, media were supplemented with 100 μg of ampicillin (Sigma-Aldrich, Deisenhofen, Germany) per ml.
PCR.
Primer pairs FK9-FK10 and FK1-FK2 were used to amplify the stxA2e and stxB2e subunit genes, respectively, as described by Franke et al. (3, 4). A 5-μl volume of each PCR product was separated on 1% agarose gels, and the bands were visualized by staining with ethidium bromide. The presence of stx1, stx2, stx2c, stx2d, eae, and enterohemorrhagic E. coli hly genes was investigated using PCR primers and the conditions described previously (21, 28, 30).
Preparation of phage particles.
Fresh-grown colonies of stx2e-harboring E. coli strains were suspended in LB broth and incubated with vigorous shaking until they reached an optical density at 600 nm (OD600) of 0.1 to 0.3. After adjusting the cultures to a final mitomycin C (Sigma-Aldrich) concentration of 0.5 μg/ml, the bacterial suspensions were incubated overnight. The cultures were then centrifuged at 16,000 × g for 10 min, and the supernatants were filtered through membrane filters with a pore size of 0.22 μm (Schleicher & Schuell, Dassel, Germany). The filtrates were diluted 10-fold from 1:102 to 1:106. Portions (100 μl) of each dilution were mixed with 100 μl of 0.1 M CaCl2 and 300 μl of a log-phase culture of E. coli DH5α and then investigated by a plaque assay using a double-layer agar method (30). Plaques were counted after overnight incubation at 37°C and subsequent plaque hybridization with a stxA2e probe.
Plaque hybridization.
Plaques were transferred to a nylon membrane (Zeta-Probe GT; Bio-Rad, Munich, Germany) according to a standard procedure (26) and hybridized with a digoxigenin-labeled stxA2e probe as described below.
Southern blot hybridization.
Genomic DNA was isolated from E. coli 2771/97 as described by Heuvelink et al. (9) and digested with EcoRI and ClaI (Gibco BRL, Eggenstein, Germany). Restriction fragments were separated on 0.6% agarose gels. Digested DNA was then transferred to a nylon membrane by capillary blotting (26) and fixed with a UV cross-linker (Stratalinker; Stratagene, Heidelberg, Germany) using the autocross-link mode (120 mJ/cm2). A 1,004-bp DNA fragment of the stxA2e gene resulting from amplification with primers FK9 and FK10 (3, 4) was labeled with digoxigenin and used as a probe. The probe labeling was performed by incorporating digoxigenin-11-deoxyuridine-triphosphate (Boehringer GmbH, Mannheim, Germany) during PCR as described previously (29). Stringent hybridization was achieved with the DIG DNA Labeling and Detection Kit (Boehringer GmbH) according to the manufacturer's instructions.
Enzyme immunoassay.
Stx was detected with an enzyme immunoassay (Premier EHEC; Meridian Diagnostics, Inc., Cincinnati, Ohio, for the detection of Stx1 and Stx2). Briefly, fresh-grown colonies of Stx2e-producing E. coli strains were suspended in LB broth, supplemented with 5 mM CaCl2, and incubated with vigorous shaking until they reached an OD600 of 0.1 to 0.3. The number of CFU was determined by plating 10-fold dilutions on LB agar. The cultures were divided in two samples. One sample was treated with mitomycin C as described above; the other was processed without an inducing agent. After overnight incubation, the cultures were centrifuged in a microcentrifuge for 10 min with 13,000 × g. Portions (100 μl) of the supernatants, either undiluted or diluted 1:10, 1:50, 1:100, 1:500, 1:1,000, or 1:2,000 in LB medium, were used in the enzyme immunoassay according to the manufacturer's instructions. Duplicate samples were run in parallel, and the whole experiment was performed twice. Absorbance measurements were performed bichromatically at 450 and 620 nm with a BioFlow Multiskan MCC/340 ELISA Reader. Calculations were performed with OD values of between 0.1 and 1.0. Specific Stx2e concentrations were expressed as absorbance values divided by the number of bacteria (in CFU/milliliters) present in the suspensions at the time of induction.
Electron microscopy.
A φP27-containing suspension was isolated from a stxA2e probe-positive plaque and purified by cesium chloride centrifugation (26). A drop of the phage suspension was deposited on copper grids with carbon-coated Formvar films and stained with 2% KOH phosphotungstic acid (pH 7.2) for 2.5 min. Samples were examined in a Phillips E.M. 301 electron microscope operating at 80 kV.
Transduction of the stx2e gene.
To determine the ability of phage φP27 to transduce the stx2e gene into E. coli DH5α, a plaque assay was performed. A soft agar layer displaying confluent lysis was harvested into 1 ml of SM buffer (26). Tenfold dilutions of such suspensions containing DH5α were plated onto LB agar and incubated overnight at 37°C. Plates containing 100 to 150 colonies were analyzed by colony hybridization (25) using the stxA2e probe prepared as described above. E. coli DH5α colonies which hybridized with the stx2e probe were subcultured three times on LB agar plates by single colony streaking and tested for the presence of stx2e after each subculture using PCR with primer pairs FK9-FK10 and FK1-FK2.
To confirm lysogenic conversion of DH5α recipients that remained positive for the stx2e gene after the last subculture, putative transductants were tested for immunity to superinfection with phage φP27 using a plaque assay. For this purpose, plates with soft agar layers containing each of the suspected lysogens as a host strain were prepared. A plate containing E. coli DH5α as a host strain was used as a control. Portions (15 μl) of suspensions of φP27, phage 933W, and bacteriophage λ containing approximately 106 PFU were dropped onto each plate. The presence of plaques was determined after incubation for 18 h at 37°C.
Vero cell assay.
After incubation of LB broth cultures for 18 to 24 h at 37°C with agitation, bacteria were sedimented by centrifugation for 10 min at 4°C at 16,000 × g, and the supernatant was filter sterilized. After diluting the supernatants 1:25 with minimal essential medium (MEM) cell culture medium (Earle's MEM containing 1% of a solution containing 10,000 U of penicillin-10,000 U of streptomycin, plus 5% fetal calf serum, 1% nonessential amino acids, 1% MEM vitamins, and 1 mM Na-pyruvate; Biochrom, Berlin, Germany), 100-μl aliquots were transferred into the wells of a microtiter plate containing Vero cell monolayers (104 cells/well). Cytotoxic effects were determined after 24 to 48 h of incubation at 37°C in 5% CO2 by microscopic examination of the Vero cells and confirmed macroscopically by staining residual Vero cells with crystal violet (7).
Cloning and sequencing of the stx2e-flanking regions.
Phage φP27 DNA was purified according to a standard procedure (26) and digested by using different restriction enzymes. The digested DNA was transferred to a nylon membrane and hybridized with an stxA2e probe as described above. A 6.4-kb HindIII fragment which hybridized with the probe was chosen for cloning. It was excised from the gel, purified with a QIAquick gel extraction kit (Qiagen GmbH), ligated into a HindIII-digested pBluescript II KS(+) (Stratagene), and transformed into E. coli DH5α according to standard methods (26).
Nucleotide sequencing was performed on both strands with an automated DNA sequencer (model 377; Perkin-Elmer/Applied Biosystems, Weiterstadt, Germany) using universal and reverse primers for pUC/M13 vectors and customized primers. Each nucleotide was determined an average of three times. Contig alignments were made with PREGAP 4 and GAP 4 (34). Searches for open reading frames (ORFs) and prediction of translation start positions were performed with the “GeneMark.hmm” software (1). Searches for homologous DNA sequences in the EMBL-GenBank database libraries were performed with the BLAST software (National Center for Biotechnology Information, Bethesda, Md.). tRNA genes and tRNA structure were determined with tRNAscan-SE (version 1.11) (13). Transmembrane regions of putative holins were predicted with TMpred (10).
Nucleotide sequence accession number.
The nucleotide sequence a 6,388-bp fragment of φP27 DNA containing stx2e and stx2e-flanking regions was submitted to the EMBL database library and assigned accession no. AJ249351.
RESULTS
Eight E. coli isolates from patients with diarrhea, which were originally detected as stx2e-harboring STEC by PCR with primers FK9 and FK10 during routine diagnostic work in Würzburg, a patient isolate from Belgium, and two Stx2e-producing E. coli strains isolated from pigs were investigated for the presence of Stx-encoding phages. Liquid cultures of all strains were induced with mitomycin C, and culture supernatants were tested for the presence of infectious phage particles by a plaque assay using E. coli DH5α as a host. Of 11 supernatants, 8 caused plaque formation on DH5α. Plaques were transferred to a nylon membrane and hybridized with a stxA2e probe. Only the culture supernatant of 1 of the 11 stx2e-containing isolates, E. coli ONT:H− strain 2771/97, caused formation of plaques on DH5α that hybridized with the stxA2e probe. These plaques show a uniform and turbid morphology and were substantially smaller than the plaques formed by Stx2-converting phage 933W. Plaques were difficult to discern by visual inspection, and only the plaque hybridization approach allowed us to evaluate precisely the number of plaques formed by this phage on a lawn of E. coli DH5α.
We decided to further investigate this STEC strain and determined at first its virulence spectrum. Beside the stxA2e and stxB2e genes, which could be detected by PCR with primer pairs FK9-FK10 and FK1-FK2, other stx types, eae, or e-hly were not present.
Without inducing agent, we could prepare a phage lysate containing ca. 102 PFU/ml, indicating a low-level release of Stx2e phages. After this, we prepared a high-titer phage lysate (4.7 × 108 PFU/ml) of φP27 using mitomycin C induction. After performing a plaque assay with the high-titer lysate and subsequent plaque hybridization, we could show that all plaques hybridized with the stxA2e probe. This suggested the presence of only one inducible phage in STEC strain 2771/97, which was designated φP27. Electron microscopic investigation revealed that the phage particles were approximately 140 nm long and had regular hexagonal heads ca. 45 to 50 nm in diameter and long, wide tails (Fig. 1).
FIG. 1.
Transmission electron micrograph of bacteriophage φP27. Bar, 100 nm.
Lysogenization of E. coli DH5α with φP27.
We wanted to know whether it is possible to lysogenize E. coli DH5α with φP27 and thereby to convert it to the production of Stx2e. Thus, 100-μl aliquots of a 1:10 dilution of the φP27 phage lysate were mixed with 300-μl aliquots of a E. coli DH5α log-phase culture and plated by the double-layer method. On such plates, confluent lysis was observed. The soft agar layers from two of these plates were harvested and suspended in 1 ml of SM buffer each (26). Tenfold dilutions of these suspensions were plated on LB agar and incubated overnight at 37°C. We selected plates resulting from a 10−6 dilution which contained 121 and 135 colonies, respectively. These were in turn subjected to colony blot hybridization (25) with an stxA2e probe. Of 121 colonies, 24 (19.8%) from the first plate and of 135 colonies, 13 (9.6%) from the second plate hybridized with the probe.
The 37 stx2e-positive isolates were subcultured three times and each time were analyzed by PCR for the presence of the stxA2e and stxB2e genes. After three subcultures, 35 of the 37 isolates were found to be negative by stxA2e PCR, and only 2 isolates, T9 and T21, retained stx2e, suggesting their lysogenization with bacteriophage φP27.
Lysogenization of T9 and T21 isolates with phage φP27 was confirmed by demonstrating their immunity to superinfection with this phage. None of the lysogens supported plaque formation with phage φP27 when used as a host strain in the plaque assay. In contrast, both lysogens remained sensitive to bacteriophage λ and to phage 933W.
Surprisingly, no phage particles were detected by the plaque assay, and no phage DNA could be isolated from culture supernatants of the lysogens after mitomycin C induction. Transformation with pIM10 restored the ability of the lysogens to release free phage particles (see also below).
Production of Stx2e by E. coli 2771/97 and the lysogens T9 and T21.
We have determined the ability to induce Stx2e production by mitomycin C in wild-type strain 2771/97 and the lysogens T9 and T21. Stx-enzyme immunoassay was performed with supernatants of induced and noninduced cultures. The results of this experiments are shown in Fig. 2.
FIG. 2.
Production of Stx2e before and after mitomycin C induction in E. coli 2771/97; the lysogens T9, T9(pIM10), T21, and T21(pIM10); and E. coli strain DH5α(pIM10) as a negative control. Plasmid pIM10 contains the functional recA gene of E. coli. − Mitomycin C, not induced with mitomycin; + mitomycin C, induced with 0.5 μg of mitomycin C per ml. y axis, logarithmic scale of specific Stx2e concentration expressed as absorbance values divided by the number of bacteria (in CFU/milliliters) present in the suspensions at the time of induction.
E. coli wild-type strain 2771/97 produced low-level amounts of Stx2e which increased 50-fold after induction with mitomycin C. We could not detect Stx2e production in the lysogens T9 and T21, either when induced or when noninduced. Since we suspected that the failure to produce Stx2e depended on the recA-negative genotype of host strain DH5α, we transformed the lysogens with the recA-positive plasmid pIM10. As can be seen in Fig. 2, the ability to produce Stx2e was restored in the lysogens containing a functional recA gene. In lysogens T9 and T21, Stx2e production increased 50- to 100-fold after induction by mitomycin C.
To investigate whether the lysogens T9 and T21 produce biologically active toxin, we performed a Vero cell assay. All recA-positive strains which were positive in the Stx-enzyme immunoassay [2771/97, T9(pIM10), T21(pIM10)] were also cytotoxic in the Vero cell assay, whereas control strains DH5α(pIM10) and the transductants without pIM10 did not show cytotoxicity to Vero cells under the conditions described.
Analysis of stx2e-flanking regions of φP27.
Genomic DNA of φP27 was digested with HindIII, separated by agarose gel electrophoresis, and hybridized with an stxA2e probe. A 6.4-kb HindIII restriction fragment hybridized with the probe. This fragment was excised from the gel, purified, and cloned as described above. The cloned fragment was labeled with digoxigenin and rehybridized with HindIII-restricted chromosomal DNA from 2771/97. Only one 6.4-kb fragment hybridized with the probe, suggesting that the cloned fragment is not a recombinant. Nucleotide sequencing of the fragment revealed a length of 6,388 bp. We used the GeneMark.hmm software for the search for ORFs and prediction of appropriate translation start positions. ORFs proposed by GeneMark.hmm were translated and compared with database libraries. For the final definition of ORFs, we used translation start codons in our sequence that were predicted by GeneMark.hmm with a high probability and which fit best with the start positions of genes encoding homologues proteins found in the database libraries. The results of these searches are shown in Table 1, and a scheme of the sequence features present on the fragment is shown in Fig. 3.
TABLE 1.
Characteristics of the 6,388-bp HindIII fragment of bacteriophage φP27 DNA containing stx2e
| Sequence feature | Position (bp) | Size (bp) of ORF | Direction of ORFa | Size of product (aa)b | Homology to the gene product (% identity; % similarity)c | Comments |
|---|---|---|---|---|---|---|
| orf1 | 128–1357 | 1,230 | D | 409 | NSH | |
| orf2 | 1573–1770 | 198 | D | 65 | NSH | |
| orf3 | 2041–2979 | 939 | D | 312 | gp52 (48; 61)* | Adenine-specific modification methylase of phage N15 |
| tRNA | 3020–3095 | 76 | D | ileZ (98.7)† | Proposed tRNAIle | |
| tRNA | 3197–3273 | 77 | D | argO (97,4)† | Proposed tRNAArg | |
| orf4 | 3364–4323 | 960 | D | 319 | stxA2e (99.3; 99.3)* | Shiga toxin 2e (A subunit) |
| orf5 | 4336–4599 | 264 | D | 87 | stxB2e (100; 100)* | Shiga toxin 2e (B subunit) |
| orf6 | 4650–4847 | 198 | C | 65 | NSH | |
| orf7 | 5018–5410 | 393 | D | 130 | orf9 (27; 48)* | Putative holin of φCTX |
| orf8 | 5400–5675 | 276 | D | 91 | orf10 (30; 49)* | Putative holin of φCTX |
| orf9 | 5678–6055 | 378 | D | 125 | gp19 (46; 64)* | Phage PS3 endolysin |
| orf10 | 5998–6213 | 216 | D | 71 | NSH |
D, direct strand; C, complementary strand.
aa, amino acids.
†, Identity was calculated on nucleotide level; ∗, identity and similarity were calculated at the amino acid level. NSH, no significant homology detected.
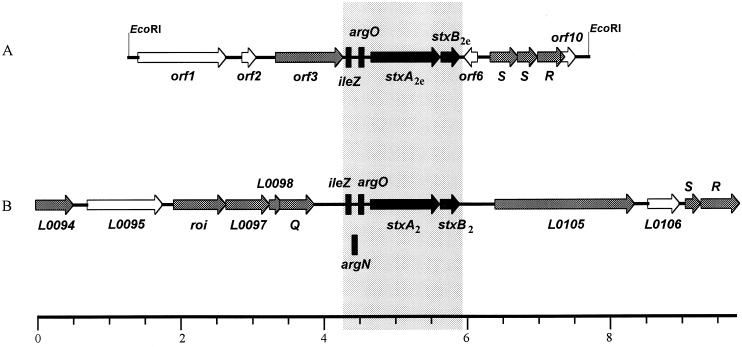
FIG. 3.
Genetic organization of the 6,388-bp region of φP27 DNA analyzed in this study (A) and comparison with a corresponding region of 9,760 bp of phage 933W (B). The arrows indicate the length and direction of transcription of ORFs. Black arrows indicate homologues DNA sequences of φP27 and 933W. Gray-shaded arrows represent ORFs with homologies to EMBL-GenBank sequences. ORFs labeled with white arrows had no significant homologies to sequences present in database libraries. The ORF labels of the 933W sequence were the same as those used by Plunkett et al. (22). tRNAs are depicted as black rectangles. The DNA regions marked with a lightly shaded rectangle represent 93% sequence identity. The scale is in kilobases.
The putative gene products of orf1, orf2, orf6, and orf10 did not show significant homologies with proteins present in the EMBL and GenBank database libraries. However, the nucleotide sequence of orf6 demonstrated significant homology to a sequence downstream of stx2e in STEC isolate S1191 (37). orf3 may encode a protein with a sequence identity of 48% and similarity of 61% to the product of gp52 of bacteriophage N15 (Table 1), which is a putative adenine-specific methylase. Whereas the predicted protein of φP27 is 312 amino acids in length, the N15 methylase comprises only 284 amino acids. We detected downstream of the gene 52 homologue two tRNA genes with high sequence identity to ileZ and argO of E. coli (see Table 1). The next two ORFs in 3′ direction of argO showed a high degree of homology with stxA2e and stxB2e sequences.
The nucleotide sequence of stxA2e of φP27 showed 99.8% identity to the stxA2e subunit gene of Stx2e-producing strains ED-53 (accession no. X81416); 99.7% to that of ED-68 (accession no. X81415), 412 (accession no. M36727), and S1191 (accession no. M21534); 99.4% to that of ED-43 (accession no. X81417); 99.3% to that of ED-42 (accession no. X81418); and 99.1% to stxA2e of R107 (accession no. U72191). The nucleotide sequences of stxB2e of φP27 were identical to the corresponding sequences of S1191, 412, ED-43, ED-53, and ED-68 and 99.2% identical to those of ED-42 and R107.
Moreover, the stxA2e and stxB2e subunit genes carried by φP27 shared 94.0 and 87.5% nucleotide sequence identity, respectively, with the A and B subunits of the stx2 gene of phage 933W (22).
Downstream of the stx2e genes and orf6 we found two tandemly arrayed ORFs which could encode two holins (Fig. 3). The amino acid sequences of both of the putative proteins encoded by orf7 and orf8 demonstrated 27 and 30% amino acid identities, respectively, to the putative holins (encoded by orf9 and orf10) of the Pseudomonas aeruginosa phage φCTX (17). Since these low homologies did not give strong evidence for the presence of holin genes, we performed a search for transmembrane domains according to the suggestions proposed by Young et al. (39).
In each of the putative φP27 holins we found three transmembrane helices which spanned positions 28 to 51, 53 to 76, and 84 to 105 in the first one (orf7) and positions 7 to 24, 33 to 55, and 60 to 77 in the second one (orf8). The structure predicted by TMpred suggested a typical N-out–C-in structure which is characteristic for class I holins of the lambda group (39).
The predicted protein of orf9 is 46% identical to Gp19 encoded by phage PS3 (accession no. AJ011579) and may constitute, together with the putative holin genes, a lysis cassette necessary for the release of infectious φP27 phage particles.
Comparison of the structure of the stx2e-flanking region of φP27 with the corresponding region of phage 933W and the stx2e-flanking region of STEC strain S1191.
Comparison of homologues stx2e-flanking sequences of φP27 and phage 933W demonstrated similarities as well as crucial differences. In Fig. 3, a scheme of the 6,388-bp fragment of φP27 (A) and a corresponding segment of 9,760 bp of the stx2-flanking region 933W (B) is shown.
Similar to the organization in stx2 phage 933W, stx2e of φP27 is also preceded by tRNA genes. We found two putative tRNA genes which are highly homologues to ileZ and argO (Table 1). Moreover, we found sequences related to a third one, argN, which is present in phage 933W. Since a middle portion (ca. 30 bp) of the latter sequence is substituted with heterologous nucleotides in φP27, no tRNA structure could be observed.
We found downstream of the stx2e operon a lysis cassette which was different from the lysis cassettes of phage 933W or phage H-19B. We could identify the holin genes by computer analysis of the tertiary structure of their products but not by homology searches. In the 5′ direction of the tRNA genes, we found a methylase gene which is not present in this area in 933W. Interestingly, a Q-like gene was not present on our fragment.
On closer inspection of a 1,890-bp stx2e-flanking sequence of STEC strain S1191, which was available in the database (accession no. M21534), we found a structure similar to that described for the φP27. The fragment started with an argO tRNA gene, followed by stx2e. Downstream of stxB2e, we found an ORF with high sequence identity to orf6 of φP27. However, this ORF was shorter than orf6 due to an internal 39 bp in-frame deletion. The published sequence of STEC strain S1191 ended with 30 nucleotides which are identical to the 5′ end of the putative holin gene orf7. All in all, the nucleotide sequence of this region is almost identical to that of φP27.
DISCUSSION
In this study, we demonstrated that Stx2e of STEC patient isolate 2771/97 is encoded in the genome of an infectious bacteriophage. The morphology of this phage is distinct from that of phages 933W and H-19B. The latter phages displayed either elongated hexagonal heads and long, thin, flexible tails as phage H-19B (38) or regular hexagonal heads and short, thin tails as phage 933W (22). In contrast, phage φP27 consists of a regular hexagonal head in combination with a long and wide tail (Fig. 1).
The presence of an infectious Stx2e-converting phage and the ability to convert a new host strain to the production of Stx supports the hypothesis that Stx phages play a role in horizontal gene transfer and the emergence of new STEC pathotypes. We could show in an earlier study that a derivative of a Stx2-converting phage from E. coli O157:H7 was able to lysogenize different enteropathogenic E. coli strains (27). Therefore, phages may be considered as highly mobile genetic elements with the capacity to spread stx genes among E. coli strains.
However, we were not able to detect infectious phage particles from the other 10 stx2e-harboring strains. The reasons for that phenomenon are not known. Either the stx2e genes of these strains could be encoded in the chromosome (37) or important sequences for maturation of the phage particles could be deleted, as is the case in Shigella dysenteriae type 1 (16), or else inactivated by insertion of insertion elements, as is speculated for the VT2-Sakai phage (14).
Interestingly, we could show that the published stx2e-flanking sequence of STEC strain S1191 contains the same structural elements as φP27. A tRNA gene as well as a part of a putative holin gene could be detected on this fragment. This finding raises the question of whether stx2e of S1191 is located in the chromosome as described previously (37) or in the genome of a φP27-related prophage.
The genetic organization of phage DNA flanking the stx genes has been demonstrated to be conserved in Stx1- and Stx2-converting bacteriophages H-19B and 933W (18, 22). The structural genes for Stx1 and Stx2 are integrated in the late-phase regions of the genomes of bacteriophages H-19B and 933W, respectively, both in identical positions between functional analogues of the λ Q transcription activator gene and the holin S gene of the lysis cassette (18, 22). Functional studies of bacteriophage H-19B demonstrated that the expression of stx1 and the release of the toxin from the bacterial cell are dependent on this late-phase region (18). Similarly, the expression of the stx2 gene in phage 933W has been explained as a part of the Q-dependent late transcript of the lysis genes (22).
The sequence analysis of stx2e-flanking regions of φP27 DNA shows an organization that differs from that described for phages H-19B and 933W. Although important structural elements of the late regulatory region of lambdoid phages (i.e., lysis cassette) were detected on the sequenced fragment of φP27, others, such as the Q antiterminator gene, could not be found. A possible explanation for the latter finding could be that the Q analogue is located in the φP27 genome more upstream of the stx2e gene, in a region which was not analyzed in our study.
A gene whose product is related to an adenine-specific modification methylase encoded by gp52 of bacteriophage N15 was identified 385 bp upstream of the stx2e gene. Interestingly, a methylase gene (L0094) was also found 4,100 bp upstream of the stx2 gene in phage 933W (22).
The results presented here allow us to suggest that φP27, although belonging to the lambdoid group of phages, is not closely related to H-19B or 933W. Phages may be considered important vehicles for the spread of stx genes among E. coli strains living in the same or different ecological niches. Since 10 Stx2e-producing strains included in our study could not be demonstrated to release infectious Stx-converting phage particles after induction, further studies are needed to clarify whether such stx2e genes are located in a defective phage genome or whether they are not associated with phage sequences.
ACKNOWLEDGMENTS
This work was supported by grants from the Deutsche Forschungsgemeinschaft and, partially, by a grant from the Fundació Comptes de Barcelona, Barcelona, Spain. Maite Muniesa is a recipient of a scholarship from the Alexander von Humboldt Foundation (no. 15851).
We thank Barbara Plaschke, Olga Böhler, and Beatrix Henkel for excellent technical assistance.
REFERENCES
- 1.Besemer J, Borodovsky M. Heuristic approach to deriving models for gene finding. Nucleic Acids Res. 1999;27:3911–3920. doi: 10.1093/nar/27.19.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caprioli A, Nigrelli A, Gatti R, Zavanella M, Blando A M, Minelli F, Donelli G. Characterisation of verocytotoxin-producing Escherichia coli isolated from pigs and cattle in northern Italy. Vet Rec. 1993;133:323–324. doi: 10.1136/vr.133.13.323. [DOI] [PubMed] [Google Scholar]
- 3.Franke S, Gunzer F, Wieler L H, Baljer G, Karch H. Construction of recombinant Shiga-like toxin-IIv (SLT-IIv) and its use in monitoring the SLT-IIv antibody status of pigs. Vet Microbiol. 1995;43:41–52. doi: 10.1016/0378-1135(94)00071-4. [DOI] [PubMed] [Google Scholar]
- 4.Franke S, Harmsen D, Caprioli A, Pierard D, Wieler L H, Karch H. Clonal relatedness of Shiga-like toxin-producing Escherichia coli O101 strains of human and porcine origin. J Clin Microbiol. 1995;33:3174–3178. doi: 10.1128/jcm.33.12.3174-3178.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuchs S, Mühldorfer I, Donohue-Rolfe A, Kerenyi M, Emody L, Alexiev R, Nenkov P, Hacker J. Influence of RecA on in vivo virulence and Shiga toxin 2 production in Escherichia coli pathogens. Microb Pathog. 1999;27:13–23. doi: 10.1006/mpat.1999.0279. [DOI] [PubMed] [Google Scholar]
- 6.Fürst S, Scheef J, Bielaszewska M, Russmann H, Schmidt H, Karch H. Identification and characterisation of Escherichia coli strains of O157 and non-O157 serogroups containing three distinct Shiga toxin genes. J Med Microbiol. 2000;49:383–386. doi: 10.1099/0022-1317-49-4-383. [DOI] [PubMed] [Google Scholar]
- 7.Gentry M K, Dalrymple J M. Quantitative microtiter cytotoxicity assay for Shigella toxin. J Clin Microbiol. 1980;12:361–366. doi: 10.1128/jcm.12.3.361-366.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griffin P M, Tauxe R V. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol Rev. 1991;13:60–98. doi: 10.1093/oxfordjournals.epirev.a036079. [DOI] [PubMed] [Google Scholar]
- 9.Heuvelink A E, van de Kar N C, Meis J F, Monnens L A, Melchers W J. Characterization of verocytotoxin-producing Escherichia coli O157 isolates from patients with haemolytic uraemic syndrome in Western Europe. Epidemiol Infect. 1995;115:1–14. doi: 10.1017/s0950268800058064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hofmann K, Stoffel W. TMbase—a database of membrane-spanning protein segments. Biol Chem Hoppe-Seyler. 1993;347:166. [Google Scholar]
- 11.Karch H, Schmidt H, Janetzki-Mittmann C, Scheef J, Kröger M. Shiga toxins even when different are encoded at identical positions in the genomes of related temperate bacteriophages. Mol Gen Genet. 1999;262:600–607. doi: 10.1007/s004380051122. [DOI] [PubMed] [Google Scholar]
- 12.Konowalchuk J, Speirs J I, Stavric S. Vero response to a cytotoxin of Escherichia coli. Infect Immun. 1977;18:775–779. doi: 10.1128/iai.18.3.775-779.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lowe T M, Eddy S R. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997;25:955–964. doi: 10.1093/nar/25.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Makino K, Yokoyama K, Kubota Y, Yutsudo C H, Kimura S, Kurokawa K, Ishii K, Hattori M, Tatsuno I, Abe H, Iida T, Yamamoto K, Onishi M, Hayashi T, Yasunaga T, Honda T, Sasakawa C, Shinagawa H. Complete nucleotide sequence of the prophage VT2-Sakai carrying the verotoxin 2 genes of the enterohemorrhagic Escherichia coli O157:H7 derived from the Sakai outbreak. Genes Genet Syst. 1999;74:227–239. doi: 10.1266/ggs.74.227. [DOI] [PubMed] [Google Scholar]
- 15.Marques L R, Pieris J S M, Cryz S J, O'Brien A D. Escherichia coli strains isolated from pigs with edema disease produce a variant of Shiga-like toxin II. FEMS Microbiol Lett. 1987;44:33–38. [Google Scholar]
- 16.McDonough M A, Butterton J R. Spontaneous tandem amplification and deletion of the Shiga toxin operon in Shigella dysenteriae 1. Mol Microbiol. 2000;34:1058–1069. doi: 10.1046/j.1365-2958.1999.01669.x. [DOI] [PubMed] [Google Scholar]
- 17.Nakayama K, Kanaya S, Ohnishi M, Terawaki Y, Hayashi T. The complete nucleotide sequence of phi CTX, a cytotoxin-converting phage of Pseudomonas aeruginosa: implications for phage evolution and horizontal gene transfer via bacteriophages. Mol Microbiol. 1999;31:399–419. doi: 10.1046/j.1365-2958.1999.01158.x. [DOI] [PubMed] [Google Scholar]
- 18.Neely M N, Friedman D I. Functional and genetic analysis of regulatory regions of coliphage H-19B: location of Shiga-like toxin and lysis genes suggest a role for phage functions in toxin release. Mol Microbiol. 1998;28:1255–1267. doi: 10.1046/j.1365-2958.1998.00890.x. [DOI] [PubMed] [Google Scholar]
- 19.O'Brien A D, Newland J W, Miller S F, Holmes R K, Smith H W, Formal S B. Shiga-like toxin-converting phages from Escherichia coli strains that cause hemorrhagic colitis or infantile diarrhea. Science. 1984;226:694–696. doi: 10.1126/science.6387911. [DOI] [PubMed] [Google Scholar]
- 20.Pierard D, Huyghens L, Lauwers S, Lior H. Diarrhoea associated with Escherichia coli producing porcine oedema disease verotoxin. Lancet. 1991;338:762. doi: 10.1016/0140-6736(91)91487-f. [DOI] [PubMed] [Google Scholar]
- 21.Pierard D, Muyldermans G, Moriau L, Stevens D, Lauwers S. Identification of new verocytotoxin type 2 variant B-subunit genes in human and animal Escherichia coli isolates. J Clin Microbiol. 1998;36:3317–3322. doi: 10.1128/jcm.36.11.3317-3322.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plunkett G, Rose D J, Durfee T J, Blattner F R. Sequence of Shiga toxin 2 phage 933W from Escherichia coli O157:H7: Shiga toxin as a phage late-gene product. J Bacteriol. 1999;181:1767–1778. doi: 10.1128/jb.181.6.1767-1778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rietra P J, Willshaw G A, Smith H R, Field A M, Scotland S M, Rowe B. Comparison of Vero-cytotoxin-encoding phages from Escherichia coli of human and bovine origin. J Gen Microbiol. 1989;135:2307–2318. doi: 10.1099/00221287-135-8-2307. [DOI] [PubMed] [Google Scholar]
- 24.Rüssmann H, Kothe E, Schmidt H, Franke S, Harmsen D, Caprioli A, Karch H. Genotyping of Shiga-like toxin genes in non-O157 Escherichia coli strains associated with haemolytic uraemic syndrome. J Med Microbiol. 1995;42:404–410. doi: 10.1099/00222615-42-6-404. [DOI] [PubMed] [Google Scholar]
- 25.Rüssmann H, Schmidt H, Heesemann J, Caprioli A, Karch H. Variants of Shiga-like toxin II constitute a major toxin component in Escherichia coli O157 strains from patients with haemolytic uraemic syndrome. J Med Microbiol. 1994;40:338–343. doi: 10.1099/00222615-40-5-338. [DOI] [PubMed] [Google Scholar]
- 26.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 27.Schmidt H, Bielaszewska M, Karch H. Transduction of enteric Escherichia coli isolates with a derivative of Shiga toxin 2-encoding bacteriophage φ3538 isolated from Escherichia coli O157:H7. Appl Environ Microbiol. 1999;65:3855–3861. doi: 10.1128/aem.65.9.3855-3861.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt H, Geitz C, Tarr P I, Frosch M, Karch H. Non-O157:H7 pathogenic Shiga toxin-producing Escherichia coli: phenotypic and genetic profiling of virulence traits and evidence for clonality. J Infect Dis. 1999;179:115–123. doi: 10.1086/314537. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt H, Plaschke B, Franke S, Russmann H, Schwarzkopf A, Heesemann J, Karch H. Differentiation in virulence patterns of Escherichia coli possessing eae genes. Med Microbiol Immunol Berlin. 1994;183:23–31. doi: 10.1007/BF00193628. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt H, Scheef J, Huppertz H I, Frosch M, Karch H. Escherichia coli O157:H7 and O157:H− strains that do not produce Shiga toxin: phenotypic and genotypic characterization of isolates associated with diarrhea and hemolytic-uremic syndrome. J Clin Microbiol. 1999;37:3491–3496. doi: 10.1128/jcm.37.11.3491-3496.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmitt C K, McKee M L, O'Brien A D. Two copies of Shiga-like toxin II-related genes common in enterohemorrhagic Escherichia coli strains are responsible for the antigenic heterogeneity of the O157:H− strain E32511. Infect Immun. 1991;59:1065–1073. doi: 10.1128/iai.59.3.1065-1073.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scotland S M, Smith H R, Willshaw G A, Rowe B. Vero cytotoxin production in strain of Escherichia coli is determined by genes carried on bacteriophage. Lancet. 1983;i:216. doi: 10.1016/s0140-6736(83)90192-7. [DOI] [PubMed] [Google Scholar]
- 33.Smith H W, Green P, Parsell Z. Vero cell toxins in Escherichia coli and related bacteria: transfer by phage and conjugation and toxic action in laboratory animals, chickens and pigs. J Gen Microbiol. 1983;129:3121–3137. doi: 10.1099/00221287-129-10-3121. [DOI] [PubMed] [Google Scholar]
- 34.Staden R, Beal K F, Bonfield J K. The Staden package, 1998. Methods Mol Biol. 2000;132:115–130. doi: 10.1385/1-59259-192-2:115. [DOI] [PubMed] [Google Scholar]
- 35.Thomas A, Cheasty T, Chart H, Rowe B. Isolation of Vero cytotoxin-producing Escherichia coli serotypes O9ab:H- and O101:H-carrying VT2 variant gene sequences from a patient with haemolytic uraemic syndrome. Eur J Clin Microbiol Infect Dis. 1994;13:1074–1076. doi: 10.1007/BF02111832. [DOI] [PubMed] [Google Scholar]
- 36.Weinstein D L, Holmes R K, O'Brien A D. Effects of iron and temperature on Shiga-like toxin I production by Escherichia coli. Infect Immun. 1988;56:106–111. doi: 10.1128/iai.56.1.106-111.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weinstein D L, Jackson M P, Samuel J E, Holmes R K, O'Brien A D. Cloning and sequencing of a Shiga-like toxin type II variant from Escherichia coli strain responsible for edema disease of swine. J Bacteriol. 1988;170:4223–4230. doi: 10.1128/jb.170.9.4223-4230.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Willshaw G A, Smith H R, Scotland S M, Field A M, Rowe B. Heterogeneity of Escherichia coli phages encoding Vero cytotoxins: comparison of cloned sequences determining VT1 and VT2 and development of specific gene probes. J Gen Microbiol. 1987;133:1309–1317. doi: 10.1099/00221287-133-5-1309. [DOI] [PubMed] [Google Scholar]
- 39.Young R, Wang I N, Roof W D. Phages will out: strategies of host cell lysis. Trends Microbiol. 2000;8:120–127. doi: 10.1016/s0966-842x(00)01705-4. [DOI] [PubMed] [Google Scholar]