Abstract
Gastric cancer (GC) is defined as the primary epithelial malignancy derived from the stomach, and it is a complicated and heterogeneous disease with multiple risk factors. Despite its overall declining trend of incidence and mortality in various countries over the past few decades, GC remains the fifth most common malignancy and the fourth leading cause of cancer-related death globally. Although the global burden of GC has shown a significant downward trend, it remains severe in certain areas, such as Asia. GC ranks third in incidence and mortality among all cancer types in China, and it accounts for nearly 44.0% and 48.6% of new GC cases and GC-related deaths in the world, respectively. The regional differences in GC incidence and mortality are obvious, and annual new cases and deaths are increasing rapidly in some developing regions. Therefore, early preventive and screening strategies for GC are urgently needed. The clinical efficacies of conventional treatments for GC are limited, and the developing understanding of GC pathogenesis has increased the demand for new therapeutic regimens, including immune checkpoint inhibitors, cell immunotherapy and cancer vaccines. The present review describes the epidemiology of GC worldwide, especially in China, summarizes its risk and prognostic factors, and focuses on novel immunotherapies to develop therapeutic strategies for the management of GC patients.
Keywords: Gastric cancer, Epidemiology, Risk factors, Prognosis, Treatment, Immunotherapy
Core Tip: As a malignant disease with decreasing trends in incidence and mortality, gastric cancer (GC) remains a public health issue worldwide. Various risk factors have been suggested, and the prognosis of GC is related to various factors, such as tumor location, lymph node metastasis, gene polymorphisms and therapeutic strategies. Therefore, novel treatments have been proposed, and immunotherapy has attracted more attention. The present review discusses the epidemiology, risk and prognostic factors of GC with a focus on immunotherapy to better inform the management of GC patients.
INTRODUCTION
Despite its global declines in incidence and mortality over the past several decades, gastric cancer (GC) remains responsible for 1.089 million new cancer cases and 0.769 million deaths in 2020 worldwide, which makes it the fifth-most common malignancy and the fourth leading cause of cancer-related deaths, according to Global Cancer Statistics (GLOBOCAN) 2020[1]. The global age-standardized incidence and mortality rates for GC were 11.1/100000 and 7.7/100000 in 2020, with wide geographical variations[2]. These data highlight that GC remains a major global health challenge. As a primary epithelial malignancy derived from the stomach, the initiation of GC is a multistage process and is generally associated with various risk factors[3], and some elements are related to its prognosis and survival[4,5]. Thanks to advances in preventative, screening and therapeutic strategies, the incidence and mortality of GC has been decreasing gradually worldwide. However, certain challenges still exist in the management of GC, such as the clinical applications of surgical treatment and chemotherapy. Recent immunotherapy for GC has drawn much attention, and it improved the current therapeutic situation. The present review describes the epidemiology of GC in different regions in the world, especially in China, summarizes its risk and prognostic factors, and focuses on new immunotherapies to develop therapeutic strategies for the management of GC patients.
GLOBAL EPIDEMIOLOGY OF GC
Incidence and mortality rates of GC around the world
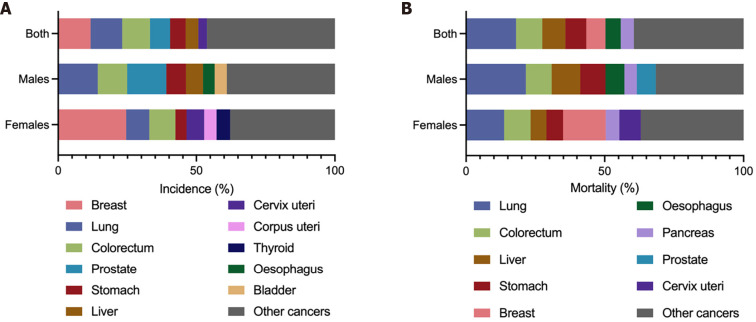
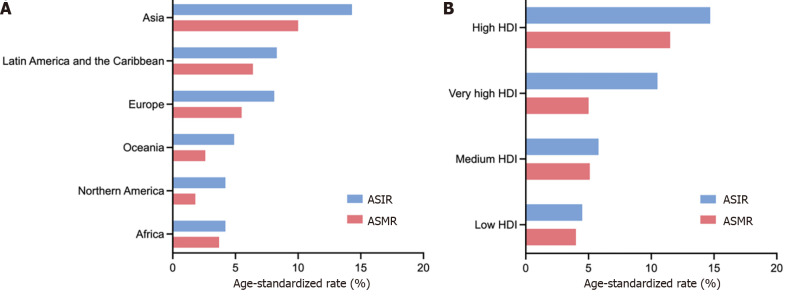
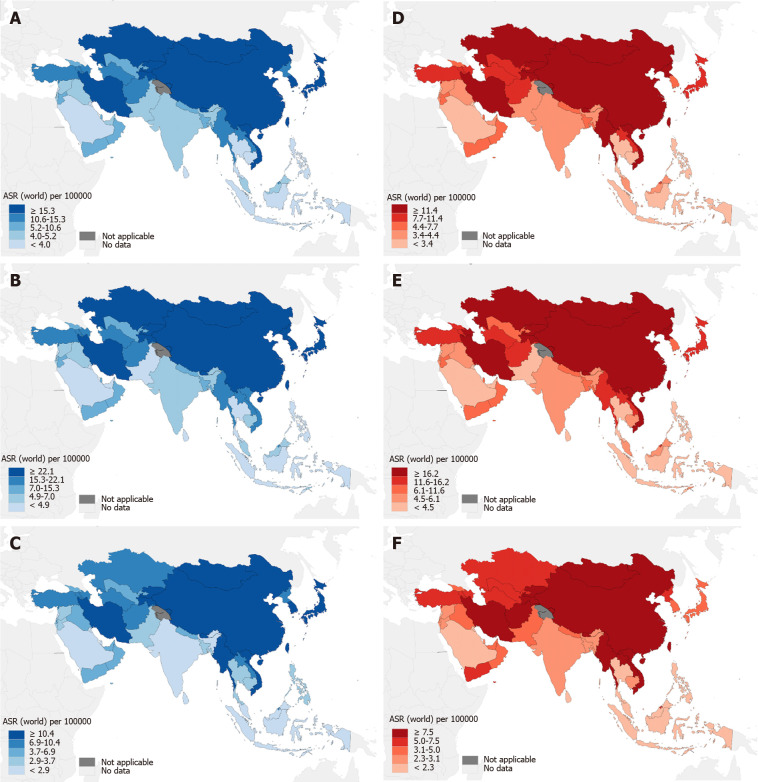
The GLOBOCAN 2020 database (https://gco.iarc.fr/)[1] estimated that there were 1089103 newly diagnosed GC cases in 185 countries, with GC ranking fifth in incidence among all cancer types globally (Figure 1A). According to the anatomical locations, GC is classified as cardia GC and noncardia GC with different epidemiological profiles[1], and noncardia GC is the most common subtype[6]. There is a significant difference in GC incidence in sex distribution, and the age-standardized incidence rate (ASIR) is 15.8/100000 in males and 7.0/100000 in females, which indicates that GC incidence is approximately 2-fold higher in males than females[1]. GC incidence ranked fourth in males and seventh in females among all cancer types[1] (Figure 1A). Geographic variations in the ASIR in GC are up to 1- to 4-fold worldwide[7]. The ASIR is highest in Asia (14.3/100000), followed by Latin America and the Caribbean, Europe and Oceania, and it is lowest in Africa and North America[7] (Figure 2A). Most GC cases are diagnosed in countries with a high and very high human development index (HDI), such as eastern and southeastern Asian countries, central and eastern European and South American countries, and the ASIRs in these countries were higher than countries with a medium and low HDI[2] (Figure 2B). The five countries with the highest ASIRs in Asia were Mongolia (32.5/100000), Japan (31.6/100000), Republic of Korea (27.9/100000), Tajikistan (23.4/100000) and China (20.6/100000) (Figure 3A), which indicated that greater than 69% of the total GC cases in 2020 occurred in eastern and south-central Asia. In addition, Figure 3B and C show the detailed information about the male and female ASIRs in Asia.
Figure 1.
The composition of incidences and mortalities of all cancer types in 2020 globally. A: The composition of incidences of all cancer types in 2020 globally; B: The composition of mortalities of all cancer types in 2020 globally. Bar plots show the composition of incidences or mortalities of all cancer types in both sexes, males and females, respectively. Citation: Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021; 71: 209-249. Copyright ©International Agency for Research on Cancer 2020. Published by International Agency for Research on Cancer[1].
Figure 2.
Age-standardized incidence and mortality rates of gastric cancer in 2020 worldwide. A: Age-standardized incidence and mortality rates of gastric cancer (GC) in 2020 in the five continents; B: Age-standardized incidence and mortality rates of GC in countries classified by human development index in 2020 worldwide. ASIR: Age-standardized incidence rate (1/100000); ASMR: Age-standardized mortality rate (1/100000); HDI: Human development index. Citation: Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021; 71: 209-249. Copyright ©International Agency for Research on Cancer 2020. Published by International Agency for Research on Cancer[1].
Figure 3.
Estimated age-standardized incidence and mortality rates of gastric cancer in 2020 in Asian countries. A: Estimated age-standardized incidence rates of gastric cancer (GC) in 2020 in Asian countries; B: Estimated age-standardized incidence rates of GC in males in 2020 in Asian countries; C: Estimated age-standardized incidence rates of GC in females in 2020 in Asian countries; D: Estimated age-standardized mortality rates of GC in 2020 in Asian countries; E: Estimated age-standardized mortality rates of GC in males in 2020 in Asian countries; F: Estimated age-standardized mortality rates of GC in females in 2020 in Asian countries. ASIR: Age-standardized incidence rate (1/100000); ASMR: Age-standardized mortality rate (1/100000). Citation: Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021; 71: 209-249. Copyright ©International Agency for Research on Cancer 2020. Published by International Agency for Research on Cancer[1].
GC is the fourth most common cause of mortality among all cancer types, followed by lung, colorectal and liver cancers[1] (Figure 1B). A total of 768793 deaths were estimated to be related to GC, with an overall mortality of 7.7/100000 globally, and sex differences exist with males being twice as likely as females to exhibit the disease (Figure 1B)[1]. The age-standardized mortality rate (ASMR) of GC was highest in Asia (10.0/100000) (Figure 2A). Countries with a very high HDI have higher mortality rates, and countries with a medium and low HDI have lower mortality rates, which is consistent with GC incidence (Figure 2B). The five Asian countries with the highest ASMRs were Mongolia (24.6/100000), Tajikistan (19.7/100000), China (15.9/100000), Bhutan (15.9/100000) and Kyrgyzstan (15.7/100000) (Figure 3D), and the male and female ASMRs in Asia are shown in Figure 3E and F, respectively. Mongolia has the highest incidence and mortality rates, primarily due to the lack of endoscopy and professional endoscopists[8].
The overall GC incidence and mortality rates have steadily declined in most countries during the past several decades, with evident decreases in males and females[1,9-13], as preventative, screening and therapeutic programs have been implemented worldwide[14-16]. For example, the ASIR of GC in Korea decreased significantly from 2011 (ASIR 43.0) to 2019 (ASIR 29.6)[17]. Similar to most other cancers, GC is generally rare in adults aged < 50 years, and its incidence increases with aging[9,10]. However, GC incidence has presented an increasing trend in younger generations (below age 50 years) in high- and low-incidence areas, such as the United States and the United Kingdom, compared to older individuals who exhibited a decreasing trend in GC incidence[9,10,18]. One United States study reported a more pronounced increase in incidence in younger females than males and predicted that the overall incidence may no longer be decreasing, and the GC incidence in females may exceed males if this pattern continues[18]. The 5-year overall survival (OS) rate shows that GC survival has improved due to advances in diagnostic and therapeutic strategies, especially with early detection from national screening programs using endoscopic and/or radiographic methods[9,19]. For example, one study found that the 5-year survival rate of GC in Korea increased from 55.7% in 1999-2005 to 77% in 2013-2019[17], which is consistent with the previous cancer statistics in Korea in 2015[20]. However, GC maintains a high case fatality rate, and it is a main contributor to the global burden[21].
Epidemiology of GC in China
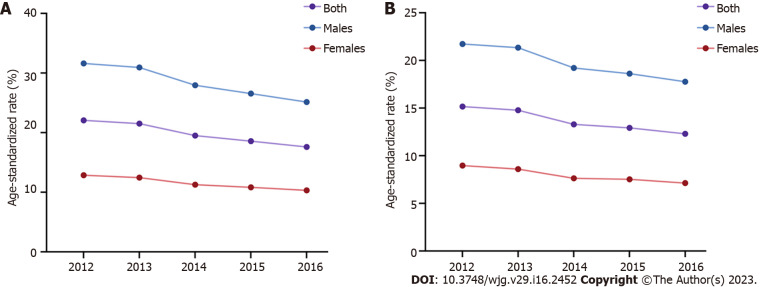
GC is also one of the major malignances in China. The ASIR and ASMR of GC were higher in 2012 (ASIR 22.06/100000, ASMR 15.16/100000) than 2016 (ASIR 17.59/100000, ASMR 12.30/100000), with a decreasing trend from 2012 to 2016 and decreasing trends in males and females[22-26] (Figure 4). The National Cancer Center of China reported 396500 new GC cases and 288500 GC-leading deaths in China in 2016[26], and the ASIR and ASMR of GC ranked fifth (17.59/100000) and third (12.30/100000) among all cancer types, respectively[26,27]. The number of new GC cases in males was nearly 276300 (ASIR 25.14/100000), and GC-related deaths (200200, ASMR 17.77/100000) accounted for approximately 13% of all male cancer-related deaths. The number of new GC cases in females was approximately 120200 (ASIR 10.31/100000), and GC-related deaths (88400, ASMR 7.13/100000) accounted for approximately 10% of all female cancer-related deaths[26]. GLOBOCAN 2020 indicated 479000 new GC cases and 374000 GC-related deaths in China in 2020, which ranked third in incidence and mortality among all cancer types[28] and accounted for 44.0% and 48.6% of new GC cases and GC-related deaths worldwide, respectively[1]. Current data in China (https://gco.iarc.fr/) showed that the ASIR and ASMR of GC were greater than 2-fold in males (ASIR 29.5/100000, ASMR 22.8/100000) than females (ASIR 12.3/100000, ASMR 9.5/100000) in 2022, and more GC cases and deaths occurred in patients over 60 years. Notably, geographic variations also exist in different areas of China. Specifically, the ASIRs and ASMRs of GC in urban areas were higher than rural areas[26]. The ASIR and ASMR were highest in northwestern China (ASIR 25.8/100000, ASMR 18.1/100000), followed by eastern (ASIR 21.9/100000, ASMR 14.8/100000) and central (ASIR 18.7/100000, ASMR 13.8/100000) areas of China, and southern China had the lowest ASIR of 9.2/100000 and ASMR of 6.4/100000[26].
Figure 4.
Trends in age-standardized incidence and mortality rates of gastric cancer according to the National Central Cancer Registry of China in 2012-2016[22-26]. A: Trends in age-standardized incidence rates of gastric cancer (GC) according to the National Central Cancer Registry (NCCR) of China in 2012-2016; B: Trends in age-standardized mortality rates of GC according to the NCCR of China in 2012-2016.
Because the early symptoms of GC are insidious, most GC patients are metastatic in the advanced stage at the time of diagnosis[6]. The proportion of GC patients diagnosed in the early stage was lower than advanced GC patients in China in the past[28]. However, the survival time is closely related to the stage at diagnosis for GC patients[19]. Thereafter, China proposed a series of guidelines aimed at improving GC screening, early detection and therapeutic strategies based on the popularity of gastrointestinal endoscopy, and the proportion of early GCs increased in recent years[28,29]. The 5-year OS rate of GC increased from 27.4% in 2003-2005 to 35.1% in 2012-2015 with an ascending trend in China[29], but it is significantly lower than Japan (81.0%) in 2004-2007[19] and South Korea (75.4%) in 2011-2015[20], which may be related to the different preventive, early screening, diagnostic and therapeutic strategies in individual countries[1].
RISK FACTORS
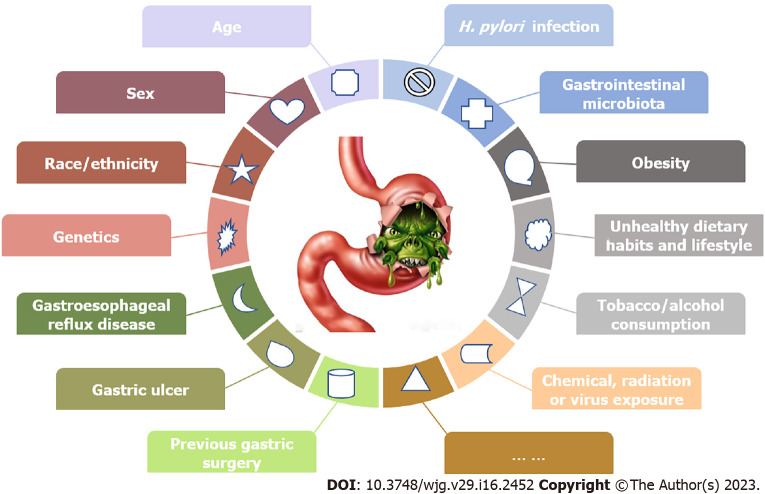
The pathogenesis of GC is complicated, and more attention should be given to individuals with higher GC risks for surveillance. Various factors may synthetically affect GC occurrence and development (Figure 5). Some risk factors are nonmodifiable, such as age, sex, race/ethnicity and genetics[6,28,30,31], and other controllable risk factors may include Helicobacter pylori (H. pylori) infection, gastrointestinal microbiota, obesity, unhealthy dietary habits and lifestyle, tobacco and alcohol consumption, and chemical, radiation or virus exposure[6,16,28,32-34]. Several relatively rare risk factors may also participate in GC pathogenesis, such as gastroesophageal reflux disease, gastric ulcer or previous gastric surgery[30].
Figure 5.
Summarization of risk factors of gastric cancer. H. pylori: Helicobacter pylori.
One study reported that 1.8% of GC cases occurred in individuals younger than 34 years, 38.6% occurred in adults between 35 and 64 years old, and 59.6% occurred in elderly individuals over 65 years from 2015 to 2019[35], with a median age at diagnosis of 68 years, which indicate that GC risk increases with aging. Sex differences exist in GC incidence, which is almost twofold higher in males than females[1,35]. These data suggest the protective effect of sex steroid hormones in GC pathogenesis[36]. Males tend to have higher risks of H. pylori infection than females, which may also lead to sex differences[37,38]. Approximately 10% of GC cases exhibited familial aggregation, which indicates that a family history of GC may be an independent risk factor[39,40]. A total of 1%-3% of GC patients may have germline mutations, and the underlying molecular mechanisms have not been fully clarified[41].
Chronic H. pylori infection is the major confirmed cause of GC, and it may be related to approximately 90% of noncardia GC cases[10,42-44]. H. pylori is a Gram-negative pathogenic bacterium and an indigenous member of the gastric microbiota[18,45]. The prevalence of H. pylori infection in adults exceeds 50% of the human population with regional variations globally[1,45,46]. H. pylori infection is easily acquired during childhood[47], and it is generally carried asymptomatically for a lifetime. Since 1994, H. pylori has been classified as a class I carcinogen by the World Health Organization. The long-term colonization of H. pylori in the gastric mucosa contributes to the development of various gastric diseases, such as persistent inflammation, chronic gastritis, gastric mucosal atrophy and intestinal metaplasia, with its different genes encoding virulence factors[45,48]. Chronic H. pylori infection also induces epigenetic and genetic changes in gastric epithelial cells, which suggests the genetic instability of these cells[48]. Therefore, H. pylori infection is etiologically related to GC, and the duration also predisposes individuals toward GC later in life[48]. Because H. pylori infection is closely related to noncardia GC, its eradication significantly decreased the incidence of noncardia GC[48]. However, H. pylori infection is only necessary, but not sufficient, in the pathogenesis of GC[48]. Although controversial[18], H. pylori screening and eradication has been proposed as a preventive strategy for GC[48]. Data from healthy asymptomatic infected Asians showed that eradicating H. pylori reduced GC incidence[49], and population-based screening and eradication of H. pylori infection was cost-effective[50]. The efficacy of eradicating H. pylori to prevent GC also depends on other risk factors, such as the time of H. pylori eradication, intragastric acidity, resistance to antimicrobial agents and the compliance of infected patients[48,51,52]. In contrast, a similar screening strategy may not be economical and is generally unwarranted in some countries with low GC incidences[2]. Anderson et al[18] once indicated that population-based H. pylori eradication in the United States may raise certain unanswered questions about safety, efficacy, unanticipated consequences and failure to reduce the GC burden. Less than 5% of H. pylori-infected individuals may develop GC due to the genetics of H. pylori and the host, duration of H. pylori infection and certain environmental factors[1,53]. The efficacy of H. pylori eradication and its cost-effectiveness should be further investigated in different geographic regions[48].
With the progression of molecular biological technologies, such as next-generation sequencing, a series of studies examined the correlations between GC and gastric microbiota other than H. pylori[54,55]. Ferreira et al[56] performed 16S rRNA gene sequencing analysis of gastric microbiota for 54 GC patients and 81 gastritis controls, and found that the gastric microbiota of GC had reduced microbial diversities, reduced abundance of H. pylori, and the increment of other microbial genera that were similar to intestinal microbiota; besides, the nitrosating functions and genotoxic potential were also increased. He et al[57] investigated the characteristics of the intestinal microbiota in fecal samples from GC patients and healthy individuals using 16S rRNA gene sequencing technology, and showed that the relative abundances of Faecalibacterium, Bifidobacterium, Subdoligranulum, Enterococcus, Streptococcus and Bacteroides were closely associated with GC risk and occurrence. Aziz et al[58] found four types of microbial proteins in serum and tissue biopsy specimens of GC patients, including Acinetobacter baumannii, Escherichia coli, Fusobacterium nucleatum and Bacteroides fragilis, as well as H. pylori. Overall, microbiota-related GC studies indicated an alteration of the gastric microbiota during gastric carcinogenesis, which was distinct from patients with chronic gastritis and healthy individuals[59]. Therefore, it is necessary to further analyze the gastric microorganisms and explore the possible underlying pathogenesis of microbial dysbiosis in the progression to carcinoma to provide guidance for preventative, screening and therapeutic strategies for GC.
The diverse risk and protective factors of GC are dietary or lifestyle-related factors[60], and unhealthy dietary habits and lifestyle factors may account for 33%-50% of all GC cases[10,61]. Lower intake of fruit and vegetables, higher intake of salt or salted/processed food, and tobacco and alcohol consumption are GC risk factors[60,62,63]. For example, a 5 g/day increase in salt intake increased the risk of GC by 12%[53]. Excessive salt intake may destroy the gastric mucosa and increase DNA synthesis and cell proliferation to promote the development of GC[64], and excessive salt intake also acts synergistically with H. pylori to increase GC incidence[60,65,66]. However, no scientific evidence has confirmed a definite causal association between excessive salt intake and GC risk[60]. Tobacco consumption is also an important behavioral risk factor for GC and may increase GC risk by approximately 50% in males and 20% in females according to relevant data[32,67,68]. Tobacco consumption may induced chronic inflammation in the gastrointestinal tract, alter mucosal cell proliferation, promote immune dysfunction, and increase the risks of bacterial or viral infections, which leads to the carcinogenesis of GC[69]. Alcohol consumption also positively correlates with GC risk[69-71]. These two factors, tobacco and alcohol consumption, affect GC development independently in high-risk populations, and modification of these unhealthy choices may significantly reduce the incidence and mortality of GC[72]. In contrast, increased fresh fruit and vegetable consumption may inhibit GC development and reduce its risk[53]. Specifically, a systematic review and dose-response meta-analysis found that a 100 g/day increase in fruit consumption inversely associated with a 5% reduction in GC risk[53]. Another meta-analysis also indicated that the relationship between intake of citrus fruit and risk of cardia GC was statistically significant, and daily intake of 100 g citrus fruit reduced GC risk by 40%[73], which suggests that phytochemicals in fruit may have antioxidant effects, prevent or reduce DNA oxidation, and regulate cell proliferation and apoptosis[74]. The history of medication may affect GC pathogenesis, and the use of aspirin and other nonsteroidal anti-inflammatory drugs may lower GC risk[75].
In summary, various known and unknown factors may be related to GC risk, and understanding the underlying mechanisms will facilitate appropriate preventative and screening strategies to reduce GC incidence.
PROGNOSTIC FACTORS
Patient- and tumor-related prognostic factors
Although the incidence of GC in males is significantly higher than females[1], few studies have focused on sex differences in GC prognosis. The relationships between race, gene polymorphism and GC prognosis have been explored in many studies[76,77]. Current data show high GC incidences in Asian populations and better GC prognosis in Asian GC patients than Caucasian populations, even after controlling for other well-known prognostic factors[78-80]. However, whether differences exist due to different management strategies or distinct races and tumor biology is not clear. Increasing evidence indicates that genetic polymorphisms are associated with GC survival, and single nucleotide polymorphisms (SNPs) are novel biomarkers of cancer susceptibility, progression and prognosis[81,82]. For example, Gonzalez-Hormazabal et al[83] found that allele A carriers of IL-8 rs4073 were associated with lower OS in GC. The role of ERCC1 SNPs was also extensively studied in patients with gastrointestinal tumors receiving oxaliplatin-based chemotherapy, and ERCC1 rs11615 polymorphisms were closely associated with the clinical outcomes of GC[84]. Wang et al[85] showed that lncRNA H19 rs2839698 was also related to the OS of GC patients. However, more prospective studies with larger sample sizes are needed to verify the above conclusions and elucidate the underlying mechanisms of SNPs in GC prognosis.
The Lauren classification[86] is globally recognized as the classification system for GC. According to the Lauren classification[86], GC is classified into intestinal and diffuse types, and diffuse GC generally has a poor prognosis compared to intestinal GC[87-89]. The survival of GC is strongly related to the stage at diagnosis. For patients with early GC, the cancerous area is localized with no local or distant metastasis, and these patients generally have a better prognosis than patients with advanced GC[4]. The cancer site in advanced GC patients is not localized and generally accompanied by metastasis[90]. GC easily recurs even after surgical resection, and it generally has a poor prognosis[91]. Tumor size, depth of invasion, lymph node metastasis (LNM), and tumor-node-metastasis stage are important prognostic factors[5]. For example, the 5-year survival rate of patients with proximal GC is lower than patients with distal GC, which indicated the correlation of tumor location and GC prognosis[5]. Notably, a Chinese study[92] recruited 611 patients with early GC and showed no significant difference in 5-year survival between mucosal and submucosal cancers. Notably, LNM may be a significant prognostic factor for early GC[92,93], and the 5-year survival rate may be twice as high in patients without LNM than patients with LNM[94].
Treatment-related prognostic factors
A variety of factors affect GC prognosis, including patient-related factors (gender, age, race), tumor-related factors (tumor location, histological type, depth of invasion, and metastasis) and treatment-related factors. We primarily focused on the prognostic factors related to GC treatment.
Surgery is the basis of GC treatment, and it is the only procedure that completely eradicates GC lesions[3,91,95]. Surgical options primarily include endoscopic mucosal resection, distal esophagectomy, subtotal gastrectomy or total gastrectomy[96]. The surgical strategies depend on the tumor location and invasion depth, and may vary in different institutions. For early GC with a low LNM risk, endoscopic treatment or surgery alone may be effective, and patients with advanced GC may benefit from broad lymph node dissection and multimodal therapies[97]. In terms of the extent of lymph node dissection (D), current data indicate that D2 lymph node dissection is the most recommended surgical procedure for advanced GC, and GC patients may have a higher 5-year survival rate after D2 lymph node dissection than after D1 dissection[98]. Accurate preoperative staging and resection evaluation are key factors in successful surgery for GC[95]. The precision medicine of surgical strategies for GC, by the concept of accelerated rehabilitation, includes minimally invasive surgery, precise operation of intraoperative fluorescence navigation and precise perioperative management[95]. Compared to traditional surgery, minimally invasive surgery, including laparoscopy and da Vinci robot-assisted surgery, may reduce surgical trauma and improve recovery after surgery[99]. Although no significant differences in postoperative outcomes were found between laparoscopic and robotic gastrectomies, robotic gastrectomy may require a longer surgical duration and greater financial cost, and is not superior to laparoscopic procedures in perioperative surgical outcomes[99]. Patients with advanced GC need lymph node dissection, which is difficult and complicated. Therefore, whether laparoscopy is suitable for these patients needs further evaluation[95].
Because most GC patients are in advanced stages at initial diagnosis, surgery alone may not be sufficient to treat this malignancy, and therapeutic methods other than surgery should also be performed to improve the survival of operable GC patients[95]. As another important conventional treatment, chemotherapy is classified as perioperative, neoadjuvant or adjuvant, and palliative chemotherapy[28]. The OS of GC patients was significantly improved with perioperative chemotherapy compared to GC patients after surgical resection alone, and perioperative chemotherapy also increased the OS compared to postoperative chemotherapy[100,101]. Specifically, there are several combined options for chemotherapy strategies[28,102,103], including neoadjuvant triple chemotherapy, such as docetaxel, oxaliplatin and S-1 (DOS) or Epirubicin, cisplatin and 5-fluorouracil (ECF), and double chemotherapy, such as capecitabine and oxaliplatin (XELOX), tegafur and cisplatin (SP) or 5-fluorouracil and oxaliplatin (FOLFOX). The ToGA trial[104] showed that trastuzumab in combination with chemotherapy improved survival for HER2-positive GC patients, and an anti-HER2 targeting strategy was proposed as a standard option for HER2-positive GC patients. For example, trastuzumab plus XELOX, trastuzumab in combination with capecitabine, or bevacizumab and trastuzumab combined with docetaxel, oxaliplatin and capecitabine achieved encouraging efficacy and safety for patients with HER2-positive advanced GC, and may be considered first-line treatments for these patients, but further evaluation is warranted[105-107]. However, trastuzumab combined with DOS showed less effectiveness than trastuzumab in combination with XELOX, and further investigation is ongoing[108]. The use of radiotherapy in GC treatment has become more common with the advancement of radiotherapy-related technology[109]. Radiotherapy is generally combined with surgery, chemotherapy, molecular targeted therapy or other treatments, and these combinations eventually benefit GC patients[28]. Radiotherapy may be used as an important adjuvant therapy in the perioperative period for patients with advanced operable GC, especially for some patients after D2 Lymphadenectomy, and it effectively improved progression-free survival time and reduced the local recurrence rate[109]. Notably, perioperative chemotherapy or postoperative chemotherapy plus radiotherapy are listed as preferred strategies in certain guidelines[30]. For GC patients in the early-stage with LNM, OS may be improved after the adoption of adjuvant chemotherapy and radiotherapy, but the benefit was less certain for adequately staged GC patients without LNM[110].
Immune-based therapy for GC
Although surgery, chemotherapy, molecular targeted therapy, radiotherapy or combined modality treatment improved the survival of GC patients[30,91], these treatments have limited efficacies in treating patients with advanced GC, and potential therapeutic strategies are urgently needed for these advanced GC patients. A number of recent studies[111,112] found that immune-based therapy for solid malignancies produced good results and significantly prolonged survival, and immunotherapy showed certain positive efficacies for GC patients compared to other traditional therapies[113-115], which may bring new hope to GC patients[116]. There are three main immunotherapeutic options for GC, including immune checkpoint inhibitors (ICIs), cellular immunotherapy and cancer vaccines.
As a co-suppressor molecule, the immune checkpoint regulates the survival, proliferation, differentiation or response to homologous antigens of T cells via the major histocompatibility complex-T-cell receptor, prevent excessive immune responses, and maintain the immune homeostasis in the human body[117,118]. Tumor cells in patients with malignant diseases could inhibit T cells then escape immune responses via the mechanisms described above[119]. Immunosuppressants are primarily used to reactivate the immune responses of T cells to tumor cells by blocking immune checkpoints or corresponding ligands/receptors with antibodies[120]. ICIs have been widely investigated[121], and these monoclonal antibodies[30] primarily target programmed cell death protein-1 (PD-1), programmed death ligand-1 (PD-L1) or cytotoxic T-lymphocyte antigen 4 (CTLA-4). PD-1 is a co-inhibitory receptor that is primarily expressed on the surface of activated T cells, Treg cells and monocytes. As the ligand of PD-1, PD-L1 binds to the PD-1 receptor and induces the inhibition or apoptosis of related immune cells, which helps tumor cells escape immune responses[122]. Moreover, PD-L1 is overexpressed in advanced GC, and its expression may relate to the tumor size, depth of invasion and LNM in 25%-65% of GCs[122-124]. Furthermore, clinical trials indicated that anti-PD-1 therapy for GC patients may have certain effectiveness. The first randomized phase III study demonstrated that the anti-PD-1 monoclonal antibody, nivolumab, effectively improved survival of patients with advanced gastric or gastric-oesophageal junction cancer[125]. Another famous phase II clinical KEYNOTE-059 trial recruited 259 patients from 16 countries and showed that the PD-1/PD-L1 inhibitor pembrolizumab also had similar efficacy and safety for advanced GC patients[126]. Researchers in the phase III CheckMate-649 trial found that nivolumab combined with fluorouracil and platinum as first-line medications improved the OS of patients with advanced HER2-negative GC, gastroesophageal junction cancer or esophageal adenocarcinoma[127]. Mid-term analysis of the KEYNOTE-811 test showed that the combination of pembrolizumab and trastuzumab plus chemotherapy improved the overall response rate (ORR) of patients with advanced HER2-positive GC[128]. CTLA-4 is also found on the surface of activated T cells and may interact with B7-1/B7-2 on the surface of antigen-presenting cells, which results in inhibition of the CD28 signaling pathway, and plays a key role in T-cell activation[119,129]. A monoclonal anti-CTLA-4 antibody targets T-cell co-inhibitory receptor and reactivates T-cell anti-tumor immune activity[130]. However, preliminary studies indicated that some GC patients showed ineffectiveness or even remission after treatment with monoclonal anti-CTLA-4 antibodies, and the objective response rate with antibodies alone was not satisfactory because only one of 18 patients (5.5%) reached the primary endpoint[131]. Besides, the monoclonal anti-CTLA-4 antibody ipilimumab alone as the maintenance therapy did not show any improvements in FPS for patients with unresectable locally advanced or metastatic GC compared with the best supportive care in a phase II clinical trial[132]. One study, focused on the therapeutic efficacies of PD-1 and CTLA4 inhibitors, found that the ORR of patients with metastatic GC who received nivolumab plus ipilimumab was higher than patients who received only nivolumab[133]. However, Shitara et al[134] recently found that nivolumab plus ipilimumab did not improve the OS of HER2-negative patients compared to the chemotherapy regimen in advanced GC patients.
The 2022 National Comprehensive Cancer Network Guidelines proposed PD-1/PD-L1 inhibitors as first-line/second-line medications for GC treatment, but anti-CTLA4 immunotherapy was not suggested in GC treatment. However, clinical trials on anti-CTLA4 antibodies (ipilimumab and tremelimumab) are being performed[135]. Clinical trials on many other immunosuppressants, such as LAG3, Tim3, TIGIT and OX40, are also being performed. LAG3 and Tim3 are in phase I and II clinical trials, and TIGIT and OX40 are in the early research stage[136]. Although the toxicity of ICIs may limit their efficacy and clinical application[137], current evidence indicates that the combined modality of ICIs with other treatments may be more effective and applicable in GC, especially when combined with chemotherapy for advanced GC patients with drug resistance[116,138].
Cellular immunotherapy uses immune cytotoxic cells to recognize and attack tumor cells, and induce an effective antitumor response[138]. These immune cells may be expanded T cells and nature killer cells in vitro or gene-engineered T-cell receptor (TCR) T cells (TCR-Ts) and chimeric antigen receptor (CAR) T cells (CAR-Ts)[138,139]. TCR-T/CAR-T immunotherapies, as modified T-cell-based immunotherapeutic approaches, are targeted cellular therapies that take advantage of the cytotoxic potential of T cells to attack tumor cells in an antigen-specific manner[140]. For TCR-T immunotherapy, the target TCR genes that recognize specific tumor-associated antigens (TAAs) are transduced into peripheral blood T cells collected from patients, and these modified T cells are reinjected into the patients’ circulation[138]. TAAs are presented by major histocompatibility complex-I (MHC-I) to TCR-Ts within the patient, and the combination of TAAs with TCR could activate these T cells to release cytokines and attack tumor cells[141]. TCR-Ts expressing KK-LC-1 (encoded by CT83) TCR recognized CT83+ tumor cells in vitro, and KK-LC-1 is frequently expressed in human epithelial tumors, including GC with the highest expression[142]. NY-ESO-1 antibody positivity was also found in GC, which indicated that NY-ESO-1 may be another target for TCR-T immunotherapy of GC[143]. Although TCR-T immunotherapy is being applied in clinical treatment, there are still some challenges. For example, it is difficult to generate a universal TCR for immunotherapy because of the extreme polymorphism of the MHC locus[141]. To resolve these issues, CAR was developed based on antibody recognition specificities[141], and CAR-Ts are considered a promising class of antitumor treatment[144]. T cells are collected from autologous peripheral blood, genetically modified to produce specific CARs, namely CAR-Ts, then reinjected into the patient’s circulation[145-147]. CAR-Ts recognize and combine the specific antigen on the surface of tumor cells by the extracellular single-chain fragment variable domain, which results in the immobilization and clustering of CARs, the formation of nonclassical immune synapses and activation of CAR-Ts[141]. In contrast to MHC-restricted TCR-Ts, CAR-Ts are typically designed and engineered to recognize non-MHC cell surface proteins[141]. The density of the target antigen is particularly important in the modulation of CAR-T-cell signaling compared to TCR-Ts[141]. Only one kind of targeted tumor antigen may not be sufficient to obtain satisfying antitumor responses, and the expression of targeted antigen in other body cells inevitably results in transient and reversible harmful effects, such as cellular toxicity[144]. Several potential targets, such as NKG2D, FOLR1, HER2, MSLN and CLDN18.2, were found, and the real therapeutic efficacies need further evaluation[148-152]. Therefore, targeting GC-specific antigens remains a challenge, and the lack of truly GC-specific antigens limits the clinical application of CAR-T immunotherapy[144].
As an active antitumor immunotherapy, cancer vaccines are designed to enhance body immune function by inducing humoral and/or cellular immune responses[139,153]. Cancer vaccines primarily include autologous tumor cell vaccines, dendritic cell vaccines, peptide vaccines and genetically engineered vaccines[138]. Patients with gastroesophageal adenocarcinoma or untreated metastatic GC may have higher median OS rates after receiving the G17DT (Aphton) vaccine[154], and vaccination improved the OS of GC patients with good safety and tolerance, especially when combined with chemotherapy[116]. The immune responses (e.g., changes in immune milieu and tumor immune escape mechanisms) to cancer vaccines may be rapidly and accurately monitored using molecular sequencing, artificial intelligence or cellular engineering, which could optimize the design of cancer vaccines and facilitate their clinical application[155].
CONCLUSION
The incidence and mortality of GC have shown a downward trend worldwide, which suggests that GC may become a rare disease in the future[16]. However, GC incidence and mortality rank fifth and fourth, respectively, among all cancer types worldwide, and it remains a major health challenge[16]. With regard to the identified GC risk factors, such as H. pylori infection and unhealthy dietary habits and lifestyle, preventive strategies could effectively reduce GC incidence. Therefore, more attention should be given to individuals at higher risk, and unified guidelines for GC surveillance should be established. Due to the different staging and therapeutic strategies used in different regions, the prognosis of GC patients varies greatly. Most GC cases are found in advanced stages at diagnosis, which limits the clinical application and efficacy of surgery[156]. Although chemotherapy has significantly improved the prognosis of advanced GC patients, enormous challenges remain, such as drug resistance and toxicity[107]. With the emergence and promising development of immunotherapy, its clinical application and efficacy have been evaluated in GC patients, especially in advanced GC patients. However, due to the complicated tumor microenvironment and the complex interactions between the immune system and tumor cells, more clinical trials on immunotherapy are needed to verify their efficacy and safety in GC patients.
ACKNOWLEDGEMENTS
We thank the International Agency for Research on Cancer/World Health Organization (IARC/WHO) for the copyright permission to re-use related data and reprint related illustrations.
Footnotes
Conflict-of-interest statement: Authors declare no conflict of interests for this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: December 26, 2022
First decision: March 8, 2023
Article in press: April 7, 2023
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Endo S, Japan; Lieto E, Italy S-Editor: Zhang H L-Editor: A P-Editor: Yu HG
Contributor Information
Wen-Juan Yang, Department of Clinical Laboratory, Honghui Hospital, Xi'an Jiaotong University, Xi'an 710054, Shaanxi Province, China.
He-Ping Zhao, Department of Clinical Laboratory, Honghui Hospital, Xi'an Jiaotong University, Xi'an 710054, Shaanxi Province, China.
Yan Yu, Department of Clinical Laboratory, Honghui Hospital, Xi'an Jiaotong University, Xi'an 710054, Shaanxi Province, China.
Ji-Han Wang, Institute of Medical Research, Northwestern Polytechnical University, Xi'an 710072, Shaanxi Province, China.
Lei Guo, Department of Spinal Surgery, Honghui Hospital, Xi'an Jiaotong University, Xi'an 710054, Shaanxi Province, China.
Jun-Ye Liu, Department of Clinical Laboratory, Honghui Hospital, Xi'an Jiaotong University, Xi'an 710054, Shaanxi Province, China.
Jie Pu, Department of Cardiology, Shaanxi Provincial People’s Hospital, Xi'an 710068, Shaanxi Province, China.
Jing Lv, Department of Clinical Laboratory, Honghui Hospital, Xi'an Jiaotong University, Xi'an 710054, Shaanxi Province, China. lvjing-1219@163.com.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Thrift AP, El-Serag HB. Burden of Gastric Cancer. Clin Gastroenterol Hepatol. 2020;18:534–542. doi: 10.1016/j.cgh.2019.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635–648. doi: 10.1016/S0140-6736(20)31288-5. [DOI] [PubMed] [Google Scholar]
- 4.Ohman U, Emås S, Rubio C. Relation between early and advanced gastric cancer. Am J Surg. 1980;140:351–355. doi: 10.1016/0002-9610(80)90166-x. [DOI] [PubMed] [Google Scholar]
- 5.Yu X, Hu F, Li C, Yao Q, Zhang H, Xue Y. Clinicopathologic characteristics and prognosis of proximal and distal gastric cancer. Onco Targets Ther. 2018;11:1037–1044. doi: 10.2147/OTT.S157378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol. 2019;14:26–38. doi: 10.5114/pg.2018.80001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sitarz R, Skierucha M, Mielko J, Offerhaus GJA, Maciejewski R, Polkowski WP. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res. 2018;10:239–248. doi: 10.2147/CMAR.S149619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dondov G, Amarbayasgalan D, Batsaikhan B, Badamjav T, Batbaatar B, Tuvdenjamts B, Tumurbat N, Davaa B, Purevdorj E, Nyamaa B, Lonjid T. Diagnostic performances of pepsinogens and gastrin-17 for atrophic gastritis and gastric cancer in Mongolian subjects. PLoS One. 2022;17:e0274938. doi: 10.1371/journal.pone.0274938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnold M, Park JY, Camargo MC, Lunet N, Forman D, Soerjomataram I. Is gastric cancer becoming a rare disease? Gut. 2020;69:823–829. doi: 10.1136/gutjnl-2019-320234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong MCS, Huang J, Chan PSF, Choi P, Lao XQ, Chan SM, Teoh A, Liang P. Global Incidence and Mortality of Gastric Cancer, 1980-2018. JAMA Netw Open. 2021;4:e2118457. doi: 10.1001/jamanetworkopen.2021.18457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo G, Zhang Y, Guo P, Wang L, Huang Y, Li K. Global patterns and trends in stomach cancer incidence: Age, period and birth cohort analysis. Int J Cancer. 2017;141:1333–1344. doi: 10.1002/ijc.30835. [DOI] [PubMed] [Google Scholar]
- 12.Arnold M, Karim-Kos HE, Coebergh JW, Byrnes G, Antilla A, Ferlay J, Renehan AG, Forman D, Soerjomataram I. Recent trends in incidence of five common cancers in 26 European countries since 1988: Analysis of the European Cancer Observatory. Eur J Cancer. 2015;51:1164–1187. doi: 10.1016/j.ejca.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Ferro A, Peleteiro B, Malvezzi M, Bosetti C, Bertuccio P, Levi F, Negri E, La Vecchia C, Lunet N. Worldwide trends in gastric cancer mortality (1980-2011), with predictions to 2015, and incidence by subtype. Eur J Cancer. 2014;50:1330–1344. doi: 10.1016/j.ejca.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 14.Rahman R, Asombang AW, Ibdah JA. Characteristics of gastric cancer in Asia. World J Gastroenterol. 2014;20:4483–4490. doi: 10.3748/wjg.v20.i16.4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan X, Qin X, Zhang Y, Li Z, Zhou T, Zhang J, You W, Li W, Pan K. Screening for gastric cancer in China: Advances, challenges and visions. Chin J Cancer Res. 2021;33:168–180. doi: 10.21147/j.issn.1000-9604.2021.02.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun DQ, Yang F, Li H, Cao MM, Yan XX, He SY, Zhang SL, Xia CF, Chen WQ. [Regional disparities in trends of global gastric cancer incidence and mortality from 1990 to 2019] Zhonghua Zhong Liu Za Zhi. 2022;44:950–954. doi: 10.3760/cma.j.cn112152-20220120-00049. [DOI] [PubMed] [Google Scholar]
- 17.Park SH, Kang MJ, Yun EH, Jung KW. Epidemiology of Gastric Cancer in Korea: Trends in Incidence and Survival Based on Korea Central Cancer Registry Data (1999-2019) J Gastric Cancer. 2022;22:160–168. doi: 10.5230/jgc.2022.22.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson WF, Rabkin CS, Turner N, Fraumeni JF Jr, Rosenberg PS, Camargo MC. The Changing Face of Noncardia Gastric Cancer Incidence Among US Non-Hispanic Whites. J Natl Cancer Inst. 2018;110:608–615. doi: 10.1093/jnci/djx262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ito Y, Miyashiro I, Ishikawa T, Akazawa K, Fukui K, Katai H, Nunobe S, Oda I, Isobe Y, Tsujitani S, Ono H, Tanabe S, Fukagawa T, Suzuki S, Kakeji Y, Sasako M, Bilchik A, Fujita M. Determinant Factors on Differences in Survival for Gastric Cancer Between the United States and Japan Using Nationwide Databases. J Epidemiol. 2021;31:241–248. doi: 10.2188/jea.JE20190351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung KW, Won YJ, Kong HJ, Lee ES Community of Population-Based Regional Cancer Registries. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2015. Cancer Res Treat. 2018;50:303–316. doi: 10.4143/crt.2018.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soerjomataram I, Lortet-Tieulent J, Parkin DM, Ferlay J, Mathers C, Forman D, Bray F. Global burden of cancer in 2008: a systematic analysis of disability-adjusted life-years in 12 world regions. Lancet. 2012;380:1840–1850. doi: 10.1016/S0140-6736(12)60919-2. [DOI] [PubMed] [Google Scholar]
- 22.Chen W, Zheng R, Zuo T, Zeng H, Zhang S, He J. National cancer incidence and mortality in China, 2012. Chin J Cancer Res. 2016;28:1–11. doi: 10.3978/j.issn.1000-9604.2016.02.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen W, Zheng R, Zhang S, Zeng H, Zuo T, Xia C, Yang Z, He J. Cancer incidence and mortality in China in 2013: an analysis based on urbanization level. Chin J Cancer Res. 2017;29:1–10. doi: 10.21147/j.issn.1000-9604.2017.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen WQ, Li H, Sun KX, Zheng RS, Zhang SW, Zeng HM, Zou XN, Gu XY, He J. [Report of Cancer Incidence and Mortality in China, 2014] Zhonghua Zhong Liu Za Zhi. 2018;40:5–13. doi: 10.3760/cma.j.issn.0253-3766.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Zhang S, Sun K, Zheng R, Zeng H, Wang S, Chen R, Wei W, He J. Cancer incidence and mortality in China, 2015. J Natl Cancer Cent . 2021;1:2–11. doi: 10.1016/j.jncc.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng RS, Zhang SW, Zeng HM, Wang SM, Sun KX, Chen R, Li L, Wei WQ, He J. Cancer incidence and mortality in China, 2016. J Natl Cancer Cent. 2022;2:1–9. doi: 10.1016/j.jncc.2022.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maomao C, He L, Dianqin S, Siyi H, Xinxin Y, Fan Y, Shaoli Z, Changfa X, Lin L, Ji P, Wanqing C. Current cancer burden in China: epidemiology, etiology, and prevention. Cancer Biol Med. 2022;19:1121–1138. doi: 10.20892/j.issn.2095-3941.2022.0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Health Commission of the People's Republic of China. National guidelines for diagnosis and treatment of gastric cancer 2022 in China. Chin J Cancer Res . 2022;34:207–237. doi: 10.21147/j.issn.1000-9604.2022.03.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeng H, Chen W, Zheng R, Zhang S, Ji JS, Zou X, Xia C, Sun K, Yang Z, Li H, Wang N, Han R, Liu S, Mu H, He Y, Xu Y, Fu Z, Zhou Y, Jiang J, Yang Y, Chen J, Wei K, Fan D, Wang J, Fu F, Zhao D, Song G, Jiang C, Zhou X, Gu X, Jin F, Li Q, Li Y, Wu T, Yan C, Dong J, Hua Z, Baade P, Bray F, Jemal A, Yu XQ, He J. Changing cancer survival in China during 2003-15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Health. 2018;6:e555–e567. doi: 10.1016/S2214-109X(18)30127-X. [DOI] [PubMed] [Google Scholar]
- 30.Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA Cancer J Clin. 2021;71:264–279. doi: 10.3322/caac.21657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clinton SK, Giovannucci EL, Hursting SD. The World Cancer Research Fund/American Institute for Cancer Research Third Expert Report on Diet, Nutrition, Physical Activity, and Cancer: Impact and Future Directions. J Nutr . 2020;150:663–671. doi: 10.1093/jn/nxz268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ladeiras-Lopes R, Pereira AK, Nogueira A, Pinheiro-Torres T, Pinto I, Santos-Pereira R, Lunet N. Smoking and gastric cancer: systematic review and meta-analysis of cohort studies. Cancer Causes Control. 2008;19:689–701. doi: 10.1007/s10552-008-9132-y. [DOI] [PubMed] [Google Scholar]
- 33.Fortunato L, Rushton L. Stomach cancer and occupational exposure to asbestos: a meta-analysis of occupational cohort studies. Br J Cancer. 2015;112:1805–1815. doi: 10.1038/bjc.2014.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh S, Jha HC. Status of Epstein-Barr Virus Coinfection with Helicobacter pylori in Gastric Cancer. J Oncol. 2017;2017:3456264. doi: 10.1155/2017/3456264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Surveillance E, and End Results Program. Cancer Stat Facts: Stomach Cancer. [cited 26 December 2022]. In: National Cancer Institute. Available from: https://seer.cancer.gov/statfacts/html/stomach.html .
- 36.Wesołowska M, Pawlik P, Jagodziński PP. The clinicopathologic significance of estrogen receptors in human gastric carcinoma. Biomed Pharmacother. 2016;83:314–322. doi: 10.1016/j.biopha.2016.06.048. [DOI] [PubMed] [Google Scholar]
- 37.Replogle ML, Glaser SL, Hiatt RA, Parsonnet J. Biologic sex as a risk factor for Helicobacter pylori infection in healthy young adults. Am J Epidemiol. 1995;142:856–863. doi: 10.1093/oxfordjournals.aje.a117725. [DOI] [PubMed] [Google Scholar]
- 38.Ibrahim A, Morais S, Ferro A, Lunet N, Peleteiro B. Sex-differences in the prevalence of Helicobacter pylori infection in pediatric and adult populations: Systematic review and meta-analysis of 244 studies. Dig Liver Dis. 2017;49:742–749. doi: 10.1016/j.dld.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 39.Zanghieri G, Di Gregorio C, Sacchetti C, Fante R, Sassatelli R, Cannizzo G, Carriero A, Ponz de Leon M. Familial occurrence of gastric cancer in the 2-year experience of a population-based registry. Cancer. 1990;66:2047–2051. doi: 10.1002/1097-0142(19901101)66:9<2047::aid-cncr2820660934>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 40.Eto K, Ohyama S, Yamaguchi T, Wada T, Suzuki Y, Mitsumori N, Kashiwagi H, Anazawa S, Yanaga K, Urashima M. Familial clustering in subgroups of gastric cancer stratified by histology, age group and location. Eur J Surg Oncol. 2006;32:743–748. doi: 10.1016/j.ejso.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 41.Oliveira C, Pinheiro H, Figueiredo J, Seruca R, Carneiro F. Familial gastric cancer: genetic susceptibility, pathology, and implications for management. Lancet Oncol. 2015;16:e60–e70. doi: 10.1016/S1470-2045(14)71016-2. [DOI] [PubMed] [Google Scholar]
- 42.Plummer M, Franceschi S, Vignat J, Forman D, de Martel C. Global burden of gastric cancer attributable to Helicobacter pylori. Int J Cancer. 2015;136:487–490. doi: 10.1002/ijc.28999. [DOI] [PubMed] [Google Scholar]
- 43.González CA, Megraud F, Buissonniere A, Lujan Barroso L, Agudo A, Duell EJ, Boutron-Ruault MC, Clavel-Chapelon F, Palli D, Krogh V, Mattiello A, Tumino R, Sacerdote C, Quirós JR, Sanchez-Cantalejo E, Navarro C, Barricarte A, Dorronsoro M, Khaw KT, Wareham N, Allen NE, Tsilidis KK, Bas Bueno-de-Mesquita H, Jeurnink SM, Numans ME, Peeters PHM, Lagiou P, Valanou E, Trichopoulou A, Kaaks R, Lukanova-McGregor A, Bergman MM, Boeing H, Manjer J, Lindkvist B, Stenling R, Hallmans G, Mortensen LM, Overvad K, Olsen A, Tjonneland A, Bakken K, Dumeaux V, Lund E, Jenab M, Romieu I, Michaud D, Mouw T, Carneiro F, Fenge C, Riboli E. Helicobacter pylori infection assessed by ELISA and by immunoblot and noncardia gastric cancer risk in a prospective study: the Eurgast-EPIC project. Ann Oncol. 2012;23:1320–1324. doi: 10.1093/annonc/mdr384. [DOI] [PubMed] [Google Scholar]
- 44.Lochhead P, El-Omar EM. Helicobacter pylori infection and gastric cancer. Best Pract Res Clin Gastroenterol. 2007;21:281–297. doi: 10.1016/j.bpg.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 45.Chmiela M, Gonciarz W. Molecular mimicry in Helicobacter pylori infections. World J Gastroenterol. 2017;23:3964–3977. doi: 10.3748/wjg.v23.i22.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peleteiro B, Bastos A, Ferro A, Lunet N. Prevalence of Helicobacter pylori infection worldwide: a systematic review of studies with national coverage. Dig Dis Sci. 2014;59:1698–1709. doi: 10.1007/s10620-014-3063-0. [DOI] [PubMed] [Google Scholar]
- 47.Jafar S, Jalil A, Soheila N, Sirous S. Prevalence of helicobacter pylori infection in children, a population-based cross-sectional study in west iran. Iran J Pediatr. 2013;23:13–18. [PMC free article] [PubMed] [Google Scholar]
- 48.Graham DY. Helicobacter pylori update: gastric cancer, reliable therapy, and possible benefits. Gastroenterology. 2015;148:719–31.e3. doi: 10.1053/j.gastro.2015.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ford AC, Forman D, Hunt RH, Yuan Y, Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: systematic review and meta-analysis of randomised controlled trials. BMJ. 2014;348:g3174. doi: 10.1136/bmj.g3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Areia M, Carvalho R, Cadime AT, Rocha Gonçalves F, Dinis-Ribeiro M. Screening for gastric cancer and surveillance of premalignant lesions: a systematic review of cost-effectiveness studies. Helicobacter. 2013;18:325–337. doi: 10.1111/hel.12050. [DOI] [PubMed] [Google Scholar]
- 51.Yang JC, Lu CW, Lin CJ. Treatment of Helicobacter pylori infection: current status and future concepts. World J Gastroenterol. 2014;20:5283–5293. doi: 10.3748/wjg.v20.i18.5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu Y, Zhu Y, Lu NH. Recent progress in Helicobacter pylori treatment. Chin Med J (Engl) 2020;133:335–343. doi: 10.1097/CM9.0000000000000618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fang X, Wei J, He X, An P, Wang H, Jiang L, Shao D, Liang H, Li Y, Wang F, Min J. Landscape of dietary factors associated with risk of gastric cancer: A systematic review and dose-response meta-analysis of prospective cohort studies. Eur J Cancer. 2015;51:2820–2832. doi: 10.1016/j.ejca.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 54.Wu J, Zhang C, Xu S, Xiang C, Wang R, Yang D, Lu B, Shi L, Tong R, Teng Y, Dong W, Zhang J. Fecal Microbiome Alteration May Be a Potential Marker for Gastric Cancer. Dis Markers. 2020;2020:3461315. doi: 10.1155/2020/3461315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Erawijantari PP, Mizutani S, Shiroma H, Shiba S, Nakajima T, Sakamoto T, Saito Y, Fukuda S, Yachida S, Yamada T. Influence of gastrectomy for gastric cancer treatment on faecal microbiome and metabolome profiles. Gut. 2020;69:1404–1415. doi: 10.1136/gutjnl-2019-319188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ferreira RM, Pereira-Marques J, Pinto-Ribeiro I, Costa JL, Carneiro F, Machado JC, Figueiredo C. Gastric microbial community profiling reveals a dysbiotic cancer-associated microbiota. Gut. 2018;67:226–236. doi: 10.1136/gutjnl-2017-314205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.He F, Wang F, Yang J, Yang S. Explore and Analyze the Composition and Characteristics of Intestinal Microbiota between Gastric Cancer Patients and Healthy People. Evid Based Complement Alternat Med. 2022;2022:5834293. doi: 10.1155/2022/5834293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aziz S, Rasheed F, Akhter TS, Zahra R, König S. Microbial Proteins in Stomach Biopsies Associated with Gastritis, Ulcer, and Gastric Cancer. Molecules. 2022;27 doi: 10.3390/molecules27175410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bessède E, Mégraud F. Microbiota and gastric cancer. Semin Cancer Biol. 2022;86:11–17. doi: 10.1016/j.semcancer.2022.05.001. [DOI] [PubMed] [Google Scholar]
- 60.D'Elia L, Rossi G, Ippolito R, Cappuccio FP, Strazzullo P. Habitual salt intake and risk of gastric cancer: a meta-analysis of prospective studies. Clin Nutr. 2012;31:489–498. doi: 10.1016/j.clnu.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 61.Chan AOO, Wong B. Risk factors for gastric cancer. [cited 26 December 2022]. In: UpToDate. Available from: https://www.uptodate.com/contents/risk-factors-for-gastric-cancer .
- 62.Tsugane S, Sasazuki S, Kobayashi M, Sasaki S. Salt and salted food intake and subsequent risk of gastric cancer among middle-aged Japanese men and women. Br J Cancer. 2004;90:128–134. doi: 10.1038/sj.bjc.6601511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kurosawa M, Kikuchi S, Xu J, Inaba Y. Highly salted food and mountain herbs elevate the risk for stomach cancer death in a rural area of Japan. J Gastroenterol Hepatol. 2006;21:1681–1686. doi: 10.1111/j.1440-1746.2006.04290.x. [DOI] [PubMed] [Google Scholar]
- 64.Bouras E, Tsilidis KK, Triggi M, Siargkas A, Chourdakis M, Haidich AB. Diet and Risk of Gastric Cancer: An Umbrella Review. Nutrients. 2022;14 doi: 10.3390/nu14091764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Toyoda T, Tsukamoto T, Hirano N, Mizoshita T, Kato S, Takasu S, Ban H, Tatematsu M. Synergistic upregulation of inducible nitric oxide synthase and cyclooxygenase-2 in gastric mucosa of Mongolian gerbils by a high-salt diet and Helicobacter pylori infection. Histol Histopathol. 2008;23:593–599. doi: 10.14670/HH-23.593. [DOI] [PubMed] [Google Scholar]
- 66.Loh JT, Torres VJ, Cover TL. Regulation of Helicobacter pylori cagA expression in response to salt. Cancer Res. 2007;67:4709–4715. doi: 10.1158/0008-5472.CAN-06-4746. [DOI] [PubMed] [Google Scholar]
- 67.Sjödahl K, Lu Y, Nilsen TI, Ye W, Hveem K, Vatten L, Lagergren J. Smoking and alcohol drinking in relation to risk of gastric cancer: a population-based, prospective cohort study. Int J Cancer. 2007;120:128–132. doi: 10.1002/ijc.22157. [DOI] [PubMed] [Google Scholar]
- 68.Nomura AM, Wilkens LR, Henderson BE, Epplein M, Kolonel LN. The association of cigarette smoking with gastric cancer: the multiethnic cohort study. Cancer Causes Control. 2012;23:51–58. doi: 10.1007/s10552-011-9854-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li LF, Chan RL, Lu L, Shen J, Zhang L, Wu WK, Wang L, Hu T, Li MX, Cho CH. Cigarette smoking and gastrointestinal diseases: the causal relationship and underlying molecular mechanisms (review) Int J Mol Med. 2014;34:372–380. doi: 10.3892/ijmm.2014.1786. [DOI] [PubMed] [Google Scholar]
- 70.Deng W, Jin L, Zhuo H, Vasiliou V, Zhang Y. Alcohol consumption and risk of stomach cancer: A meta-analysis. Chem Biol Interact. 2021;336:109365. doi: 10.1016/j.cbi.2021.109365. [DOI] [PubMed] [Google Scholar]
- 71.Rota M, Pelucchi C, Bertuccio P, Matsuo K, Zhang ZF, Ito H, Hu J, Johnson KC, Palli D, Ferraroni M, Yu GP, Muscat J, Lunet N, Peleteiro B, Ye W, Song H, Zaridze D, Maximovitch D, Guevara M, Fernández-Villa T, Vioque J, Navarrete-Muñoz EM, Wolk A, Orsini N, Bellavia A, Håkansson N, Mu L, Persiani R, Kurtz RC, Lagiou A, Lagiou P, Galeone C, Bonzi R, Boffetta P, Boccia S, Negri E, La Vecchia C. Alcohol consumption and gastric cancer risk-A pooled analysis within the StoP project consortium. Int J Cancer. 2017;141:1950–1962. doi: 10.1002/ijc.30891. [DOI] [PubMed] [Google Scholar]
- 72.Moy KA, Fan Y, Wang R, Gao YT, Yu MC, Yuan JM. Alcohol and tobacco use in relation to gastric cancer: a prospective study of men in Shanghai, China. Cancer Epidemiol Biomarkers Prev. 2010;19:2287–2297. doi: 10.1158/1055-9965.EPI-10-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bae JM, Kim EH. Dietary intakes of citrus fruit and risk of gastric cancer incidence: an adaptive meta-analysis of cohort studies. Epidemiol Health. 2016;38:e2016034. doi: 10.4178/epih.e2016034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu RH. Health-promoting components of fruits and vegetables in the diet. Adv Nutr. 2013;4:384S–392S. doi: 10.3945/an.112.003517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23:700–713. doi: 10.1158/1055-9965.EPI-13-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao L, Niu P, Zhao D, Chen Y. Regional and racial disparity in proximal gastric cancer survival outcomes 1996-2016: Results from SEER and China National Cancer Center database. Cancer Med. 2021;10:4923–4938. doi: 10.1002/cam4.4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Toledo-Stuardo K, Ribeiro CH, Canals A, Morales M, Gárate V, Rodríguez-Siza J, Tello S, Bustamante M, Armisen R, Matthies DJ, Zapata-Torres G, González-Hormazabal P, Molina MC. Major Histocompatibility Complex Class I-Related Chain A (MICA) Allelic Variants Associate With Susceptibility and Prognosis of Gastric Cancer. Front Immunol. 2021;12:645528. doi: 10.3389/fimmu.2021.645528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang J, Sun Y, Bertagnolli MM. Comparison of gastric cancer survival between Caucasian and Asian patients treated in the United States: results from the Surveillance Epidemiology and End Results (SEER) database. Ann Surg Oncol. 2015;22:2965–2971. doi: 10.1245/s10434-015-4388-4. [DOI] [PubMed] [Google Scholar]
- 79.Strong VE, Song KY, Park CH, Jacks LM, Gonen M, Shah M, Coit DG, Brennan MF. Comparison of gastric cancer survival following R0 resection in the United States and Korea using an internationally validated nomogram. Ann Surg. 2010;251:640–646. doi: 10.1097/SLA.0b013e3181d3d29b. [DOI] [PubMed] [Google Scholar]
- 80.Nelson R, Ko EB, Arrington A, Lee W, Kim J, Garcia-Aguilar J. Race and correlations between lymph node number and survival for patients with gastric cancer. J Gastrointest Surg. 2013;17:471–481. doi: 10.1007/s11605-012-2125-x. [DOI] [PubMed] [Google Scholar]
- 81.Zhou Y, Li N, Zhuang W, Liu GJ, Wu TX, Yao X, Du L, Wei ML, Wu XT. P53 codon 72 polymorphism and gastric cancer: a meta-analysis of the literature. Int J Cancer. 2007;121:1481–1486. doi: 10.1002/ijc.22833. [DOI] [PubMed] [Google Scholar]
- 82.Katoh M. Dysregulation of stem cell signaling network due to germline mutation, SNP, Helicobacter pylori infection, epigenetic change and genetic alteration in gastric cancer. Cancer Biol Ther. 2007;6:832–839. doi: 10.4161/cbt.6.6.4196. [DOI] [PubMed] [Google Scholar]
- 83.Gonzalez-Hormazabal P, Romero S, Musleh M, Bustamante M, Stambuk J, Pisano R, Lanzarini E, Chiong H, Rojas J, Castro VG, Jara L, Berger Z. IL-8-251T>A (rs4073) Polymorphism Is Associated with Prognosis in Gastric Cancer Patients. Anticancer Res. 2018;38:5703–5708. doi: 10.21873/anticanres.12907. [DOI] [PubMed] [Google Scholar]
- 84.Ma SC, Zhao Y, Zhang T, Ling XL, Zhao D. Association between the ERCC1 rs11615 polymorphism and clinical outcomes of oxaliplatin-based chemotherapies in gastrointestinal cancer: a meta-analysis. Onco Targets Ther. 2015;8:641–648. doi: 10.2147/OTT.S80913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang W, Yang Q, Huang Q, Zhang H, Zhang Z, Gao J, Ren W, Hu Y, Lin Y, Dang Y, Zhang F, Wang W, Wang L. The rs2839698 Single Nucleotide Polymorphism of lncRNA H19 is Associated with Post-Operative Prognosis in T3 Gastric Adenocarcinoma. Clin Lab. 2018;64:105–112. doi: 10.7754/Clin.Lab.2017.170706. [DOI] [PubMed] [Google Scholar]
- 86.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 87.Qu JL, Qu XJ, Li Z, Zhang JD, Liu J, Teng YE, Jin B, Zhao MF, Yu P, Shi J, Fu LY, Wang ZN, Liu YP. Prognostic Model Based on Systemic Inflammatory Response and Clinicopathological Factors to Predict Outcome of Patients with Node-Negative Gastric Cancer. PLoS One. 2015;10:e0128540. doi: 10.1371/journal.pone.0128540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu L, Wang ZW, Ji J, Zhang JN, Yan M, Zhang J, Liu BY, Zhu ZG, Yu YY. A cohort study and meta-analysis between histopathological classification and prognosis of gastric carcinoma. Anticancer Agents Med Chem. 2013;13:227–234. doi: 10.2174/1871520611313020007. [DOI] [PubMed] [Google Scholar]
- 89.Chen YC, Fang WL, Wang RF, Liu CA, Yang MH, Lo SS, Wu CW, Li AF, Shyr YM, Huang KH. Clinicopathological Variation of Lauren Classification in Gastric Cancer. Pathol Oncol Res. 2016;22:197–202. doi: 10.1007/s12253-015-9996-6. [DOI] [PubMed] [Google Scholar]
- 90.Meyerhardt JA, Fuchs CS. Adjuvant therapy in gastric cancer: can we prevent recurrences? Oncology (Williston Park) 2003;17:714–721, 728; discussion 728. [PubMed] [Google Scholar]
- 91.Johnston FM, Beckman M. Updates on Management of Gastric Cancer. Curr Oncol Rep. 2019;21:67. doi: 10.1007/s11912-019-0820-4. [DOI] [PubMed] [Google Scholar]
- 92.Wang J, Wang L, Li S, Bai F, Xie H, Shan H, Liu Z, Ma T, Tang X, Tang H, Qin A, Lei S, Zuo C. Risk Factors of Lymph Node Metastasis and Its Prognostic Significance in Early Gastric Cancer: A Multicenter Study. Front Oncol. 2021;11:649035. doi: 10.3389/fonc.2021.649035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kinami S, Nakamura N, Tomita Y, Miyata T, Fujita H, Ueda N, Kosaka T. Precision surgical approach with lymph-node dissection in early gastric cancer. World J Gastroenterol. 2019;25:1640–1652. doi: 10.3748/wjg.v25.i14.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Haist T, Pritzer H, Pauthner M, Fisseler-Eckhoff A, Lorenz D. Prognostic risk factors of early gastric cancer-a western experience. Langenbecks Arch Surg. 2016;401:667–676. doi: 10.1007/s00423-016-1395-2. [DOI] [PubMed] [Google Scholar]
- 95.Wang Y, Zhang L, Yang Y, Lu S, Chen H. Progress of Gastric Cancer Surgery in the era of Precision Medicine. Int J Biol Sci. 2021;17:1041–1049. doi: 10.7150/ijbs.56735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sexton RE, Al Hallak MN, Diab M, Azmi AS. Gastric cancer: a comprehensive review of current and future treatment strategies. Cancer Metastasis Rev. 2020;39:1179–1203. doi: 10.1007/s10555-020-09925-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li GZ, Doherty GM, Wang J. Surgical Management of Gastric Cancer: A Review. JAMA Surg. 2022;157:446–454. doi: 10.1001/jamasurg.2022.0182. [DOI] [PubMed] [Google Scholar]
- 98.Tan Z. Recent Advances in the Surgical Treatment of Advanced Gastric Cancer: A Review. Med Sci Monit. 2019;25:3537–3541. doi: 10.12659/MSM.916475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim HI, Han SU, Yang HK, Kim YW, Lee HJ, Ryu KW, Park JM, An JY, Kim MC, Park S, Song KY, Oh SJ, Kong SH, Suh BJ, Yang DH, Ha TK, Kim YN, Hyung WJ. Multicenter Prospective Comparative Study of Robotic Versus Laparoscopic Gastrectomy for Gastric Adenocarcinoma. Ann Surg. 2016;263:103–109. doi: 10.1097/SLA.0000000000001249. [DOI] [PubMed] [Google Scholar]
- 100.Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, Smith DB, Langley RE, Verma M, Weeden S, Chua YJ, MAGIC Trial Participants. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 101.Saunders JH, Bowman CR, Reece-Smith AM, Pang V, Dorrington MS, Mumtaz E, Soomro I, Kaye P, Madhusudan S, Parsons SL. The role of adjuvant platinum-based chemotherapy in esophagogastric cancer patients who received neoadjuvant chemotherapy prior to definitive surgery. J Surg Oncol. 2017;115:821–829. doi: 10.1002/jso.24601. [DOI] [PubMed] [Google Scholar]
- 102.Park I, Ryu MH, Choi YH, Kang HJ, Yook JH, Park YS, Kim HJ, Jung HY, Lee GH, Kim KC, Kim BS, Kang YK. A phase II study of neoadjuvant docetaxel, oxaliplatin, and S-1 (DOS) chemotherapy followed by surgery and adjuvant S-1 chemotherapy in potentially resectable gastric or gastroesophageal junction adenocarcinoma. Cancer Chemother Pharmacol. 2013;72:815–823. doi: 10.1007/s00280-013-2257-z. [DOI] [PubMed] [Google Scholar]
- 103.Satake H, Kondo M, Mizumoto M, Kotake T, Okita Y, Ogata T, Hatachi Y, Yasui H, Miki A, Imai Y, Ichikawa C, Murotani K, Kotaka M, Kato T, Kaihara S, Tsuji A. Phase I Study of Neoadjuvant Chemotherapy with Capecitabine and Oxaliplatin for Locally Advanced Gastric Cancer. Anticancer Res. 2017;37:3703–3710. doi: 10.21873/anticanres.11742. [DOI] [PubMed] [Google Scholar]
- 104.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 105.Gong J, Liu T, Fan Q, Bai L, Bi F, Qin S, Wang J, Xu N, Cheng Y, Bai Y, Liu W, Wang L, Shen L. Optimal regimen of trastuzumab in combination with oxaliplatin/ capecitabine in first-line treatment of HER2-positive advanced gastric cancer (CGOG1001): a multicenter, phase II trial. BMC Cancer. 2016;16:68. doi: 10.1186/s12885-016-2092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Meulendijks D, Beerepoot LV, Boot H, de Groot JW, Los M, Boers JE, Vanhoutvin SA, Polee MB, Beeker A, Portielje JE, de Jong RS, Goey SH, Kuiper M, Sikorska K, Beijnen JH, Tesselaar ME, Schellens JH, Cats A. Trastuzumab and bevacizumab combined with docetaxel, oxaliplatin and capecitabine as first-line treatment of advanced HER2-positive gastric cancer: a multicenter phase II study. Invest New Drugs. 2016;34:119–128. doi: 10.1007/s10637-015-0309-4. [DOI] [PubMed] [Google Scholar]
- 107.Wagner AD, Syn NL, Moehler M, Grothe W, Yong WP, Tai BC, Ho J, Unverzagt S. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2017;8:CD004064. doi: 10.1002/14651858.CD004064.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Qin S, Ji J, Xu RH, Wang W, Tang Y, Bi F, Li J, Wang K, Xu JM, Fan Q, Su W, Shen L. Treatment Patterns and Outcomes in Chinese Patients with Gastric Cancer by HER2 Status: A Noninterventional Registry Study (EVIDENCE) Oncologist. 2021;26:e1567–e1580. doi: 10.1002/onco.13826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang N, Fei Q, Gu J, Yin L, He X. Progress of preoperative and postoperative radiotherapy in gastric cancer. World J Surg Oncol. 2018;16:187. doi: 10.1186/s12957-018-1490-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Datta J, McMillan MT, Ruffolo L, Lowenfeld L, Mamtani R, Plastaras JP, Dempsey DT, Karakousis GC, Drebin JA, Fraker DL, Roses RE. Multimodality Therapy Improves Survival in Resected Early Stage Gastric Cancer in the United States. Ann Surg Oncol. 2016;23:2936–2945. doi: 10.1245/s10434-016-5224-1. [DOI] [PubMed] [Google Scholar]
- 111.Jung KW, Won YJ, Kong HJ, Oh CM, Lee DH, Lee JS. Prediction of cancer incidence and mortality in Korea, 2014. Cancer Res Treat. 2014;46:124–130. doi: 10.4143/crt.2014.46.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shi L, Chen S, Yang L, Li Y. The role of PD-1 and PD-L1 in T-cell immune suppression in patients with hematological malignancies. J Hematol Oncol. 2013;6:74. doi: 10.1186/1756-8722-6-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jin X, Liu Z, Yang D, Yin K, Chang X. Recent Progress and Future Perspectives of Immunotherapy in Advanced Gastric Cancer. Front Immunol. 2022;13:948647. doi: 10.3389/fimmu.2022.948647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rosenberg SA. Cancer immunotherapy comes of age. Nat Clin Pract Oncol. 2005;2:115. doi: 10.1038/ncponc0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kono K. Advances in cancer immunotherapy for gastroenterological malignancy. Ann Gastroenterol Surg. 2018;2:244–245. doi: 10.1002/ags3.12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Abozeid M, Rosato A, Sommaggio R. Immunotherapeutic Strategies for Gastric Carcinoma: A Review of Preclinical and Clinical Recent Development. Biomed Res Int. 2017;2017:5791262. doi: 10.1155/2017/5791262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ito A, Kondo S, Tada K, Kitano S. Clinical Development of Immune Checkpoint Inhibitors. Biomed Res Int. 2015;2015:605478. doi: 10.1155/2015/605478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang DH, Guo L, Wu XH. Checkpoint inhibitors in immunotherapy of ovarian cancer. Tumour Biol. 2015;36:33–39. doi: 10.1007/s13277-014-2848-2. [DOI] [PubMed] [Google Scholar]
- 120.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yoneda A, Kuroki T, Eguchi S. Immunotherapeutic advances in gastric cancer. Surg Today. 2021;51:1727–1735. doi: 10.1007/s00595-021-02236-2. [DOI] [PubMed] [Google Scholar]
- 122.Jacob JA. Cancer Immunotherapy Researchers Focus on Refining Checkpoint Blockade Therapies. JAMA. 2015;314:2117–2119. doi: 10.1001/jama.2015.10795. [DOI] [PubMed] [Google Scholar]
- 123.Zhang M, Dong Y, Liu H, Wang Y, Zhao S, Xuan Q, Zhang Q. The clinicopathological and prognostic significance of PD-L1 expression in gastric cancer: a meta-analysis of 10 studies with 1,901 patients. Sci Rep. 2016;6:37933. doi: 10.1038/srep37933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kawazoe A, Kuwata T, Kuboki Y, Shitara K, Nagatsuma AK, Aizawa M, Yoshino T, Doi T, Ohtsu A, Ochiai A. Clinicopathological features of programmed death ligand 1 expression with tumor-infiltrating lymphocyte, mismatch repair, and Epstein-Barr virus status in a large cohort of gastric cancer patients. Gastric Cancer. 2017;20:407–415. doi: 10.1007/s10120-016-0631-3. [DOI] [PubMed] [Google Scholar]
- 125.Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK, Yeh KH, Yoshikawa T, Oh SC, Bai LY, Tamura T, Lee KW, Hamamoto Y, Kim JG, Chin K, Oh DY, Minashi K, Cho JY, Tsuda M, Chen LT. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461–2471. doi: 10.1016/S0140-6736(17)31827-5. [DOI] [PubMed] [Google Scholar]
- 126.Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, Sun W, Jalal SI, Shah MA, Metges JP, Garrido M, Golan T, Mandala M, Wainberg ZA, Catenacci DV, Ohtsu A, Shitara K, Geva R, Bleeker J, Ko AH, Ku G, Philip P, Enzinger PC, Bang YJ, Levitan D, Wang J, Rosales M, Dalal RP, Yoon HH. Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol. 2018;4:e180013. doi: 10.1001/jamaoncol.2018.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T, Campos Bragagnoli A, Liu T, Schenker M, Yanez P, Tehfe M, Kowalyszyn R, Karamouzis MV, Bruges R, Zander T, Pazo-Cid R, Hitre E, Feeney K, Cleary JM, Poulart V, Cullen D, Lei M, Xiao H, Kondo K, Li M, Ajani JA. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398:27–40. doi: 10.1016/S0140-6736(21)00797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Janjigian YY, Kawazoe A, Yañez P, Li N, Lonardi S, Kolesnik O, Barajas O, Bai Y, Shen L, Tang Y, Wyrwicz LS, Xu J, Shitara K, Qin S, Van Cutsem E, Tabernero J, Li L, Shah S, Bhagia P, Chung HC. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature. 2021;600:727–730. doi: 10.1038/s41586-021-04161-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 130.Ma ES, Wang ZX, Zhu MQ, Zhao J. Immune evasion mechanisms and therapeutic strategies in gastric cancer. World J Gastrointest Oncol. 2022;14:216–229. doi: 10.4251/wjgo.v14.i1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ralph C, Elkord E, Burt DJ, O'Dwyer JF, Austin EB, Stern PL, Hawkins RE, Thistlethwaite FC. Modulation of lymphocyte regulation for cancer therapy: a phase II trial of tremelimumab in advanced gastric and esophageal adenocarcinoma. Clin Cancer Res. 2010;16:1662–1672. doi: 10.1158/1078-0432.CCR-09-2870. [DOI] [PubMed] [Google Scholar]
- 132.Bang YJ, Cho JY, Kim YH, Kim JW, Di Bartolomeo M, Ajani JA, Yamaguchi K, Balogh A, Sanchez T, Moehler M. Efficacy of Sequential Ipilimumab Monotherapy versus Best Supportive Care for Unresectable Locally Advanced/Metastatic Gastric or Gastroesophageal Junction Cancer. Clin Cancer Res. 2017;23:5671–5678. doi: 10.1158/1078-0432.CCR-17-0025. [DOI] [PubMed] [Google Scholar]
- 133.Janjigian YY, Bendell J, Calvo E, Kim JW, Ascierto PA, Sharma P, Ott PA, Peltola K, Jaeger D, Evans J, de Braud F, Chau I, Harbison CT, Dorange C, Tschaika M, Le DT. CheckMate-032 Study: Efficacy and Safety of Nivolumab and Nivolumab Plus Ipilimumab in Patients With Metastatic Esophagogastric Cancer. J Clin Oncol. 2018;36:2836–2844. doi: 10.1200/JCO.2017.76.6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shitara K, Ajani JA, Moehler M, Garrido M, Gallardo C, Shen L, Yamaguchi K, Wyrwicz L, Skoczylas T, Bragagnoli AC, Liu T, Tehfe M, Elimova E, Bruges R, Zander T, de Azevedo S, Kowalyszyn R, Pazo-Cid R, Schenker M, Cleary JM, Yanez P, Feeney K, Karamouzis MV, Poulart V, Lei M, Xiao H, Kondo K, Li M, Janjigian YY. Nivolumab plus chemotherapy or ipilimumab in gastro-oesophageal cancer. Nature. 2022;603:942–948. doi: 10.1038/s41586-022-04508-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Fanta P, Farjah F, Gerdes H, Gibson MK, Hochwald S, Hofstetter WL, Ilson DH, Keswani RN, Kim S, Kleinberg LR, Klempner SJ, Lacy J, Ly QP, Matkowskyj KA, McNamara M, Mulcahy MF, Outlaw D, Park H, Perry KA, Pimiento J, Poultsides GA, Reznik S, Roses RE, Strong VE, Su S, Wang HL, Wiesner G, Willett CG, Yakoub D, Yoon H, McMillian N, Pluchino LA. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:167–192. doi: 10.6004/jnccn.2022.0008. [DOI] [PubMed] [Google Scholar]
- 136.Cai H, Li M, Deng R, Wang M, Shi Y. Advances in molecular biomarkers research and clinical application progress for gastric cancer immunotherapy. Biomark Res. 2022;10:67. doi: 10.1186/s40364-022-00413-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ramos-Casals M, Brahmer JR, Callahan MK, Flores-Chávez A, Keegan N, Khamashta MA, Lambotte O, Mariette X, Prat A, Suárez-Almazor ME. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. 2020;6:38. doi: 10.1038/s41572-020-0160-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li K, Zhang A, Li X, Zhang H, Zhao L. Advances in clinical immunotherapy for gastric cancer. Biochim Biophys Acta Rev Cancer. 2021;1876:188615. doi: 10.1016/j.bbcan.2021.188615. [DOI] [PubMed] [Google Scholar]
- 139.Subhash VV, Yeo MS, Tan WL, Yong WP. Strategies and Advancements in Harnessing the Immune System for Gastric Cancer Immunotherapy. J Immunol Res. 2015;2015:308574. doi: 10.1155/2015/308574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lim WA, June CH. The Principles of Engineering Immune Cells to Treat Cancer. Cell. 2017;168:724–740. doi: 10.1016/j.cell.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wu L, Wei Q, Brzostek J, Gascoigne NRJ. Signaling from T cell receptors (TCRs) and chimeric antigen receptors (CARs) on T cells. Cell Mol Immunol. 2020;17:600–612. doi: 10.1038/s41423-020-0470-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Marcinkowski B, Stevanović S, Helman SR, Norberg SM, Serna C, Jin B, Gkitsas N, Kadakia T, Warner A, Davis JL, Rooper L, Hinrichs CS. Cancer targeting by TCR gene-engineered T cells directed against Kita-Kyushu Lung Cancer Antigen-1. J Immunother Cancer. 2019;7:229. doi: 10.1186/s40425-019-0678-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fujiwara S, Wada H, Kawada J, Kawabata R, Takahashi T, Fujita J, Hirao T, Shibata K, Makari Y, Iijima S, Nishikawa H, Jungbluth AA, Nakamura Y, Kurokawa Y, Yamasaki M, Miyata H, Nakajima K, Takiguchi S, Nakayama E, Mori M, Doki Y. NY-ESO-1 antibody as a novel tumour marker of gastric cancer. Br J Cancer. 2013;108:1119–1125. doi: 10.1038/bjc.2013.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Guedan S, Calderon H, Posey AD Jr, Maus MV. Engineering and Design of Chimeric Antigen Receptors. Mol Ther Methods Clin Dev. 2019;12:145–156. doi: 10.1016/j.omtm.2018.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Singh H, Moyes JS, Huls MH, Cooper LJ. Manufacture of T cells using the Sleeping Beauty system to enforce expression of a CD19-specific chimeric antigen receptor. Cancer Gene Ther. 2015;22:95–100. doi: 10.1038/cgt.2014.69. [DOI] [PubMed] [Google Scholar]
- 146.Hartmann J, Schüßler-Lenz M, Bondanza A, Buchholz CJ. Clinical development of CAR T cells-challenges and opportunities in translating innovative treatment concepts. EMBO Mol Med. 2017;9:1183–1197. doi: 10.15252/emmm.201607485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Newick K, O'Brien S, Moon E, Albelda SM. CAR T Cell Therapy for Solid Tumors. Annu Rev Med. 2017;68:139–152. doi: 10.1146/annurev-med-062315-120245. [DOI] [PubMed] [Google Scholar]
- 148.Tao K, He M, Tao F, Xu G, Ye M, Zheng Y, Li Y. Development of NKG2D-based chimeric antigen receptor-T cells for gastric cancer treatment. Cancer Chemother Pharmacol. 2018;82:815–827. doi: 10.1007/s00280-018-3670-0. [DOI] [PubMed] [Google Scholar]
- 149.Kim M, Pyo S, Kang CH, Lee CO, Lee HK, Choi SU, Park CH. Folate receptor 1 (FOLR1) targeted chimeric antigen receptor (CAR) T cells for the treatment of gastric cancer. PLoS One. 2018;13:e0198347. doi: 10.1371/journal.pone.0198347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Song Y, Tong C, Wang Y, Gao Y, Dai H, Guo Y, Zhao X, Wang Z, Han W, Chen L. Effective and persistent antitumor activity of HER2-directed CAR-T cells against gastric cancer cells in vitro and xenotransplanted tumors in vivo. Protein Cell. 2018;9:867–878. doi: 10.1007/s13238-017-0384-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jiang H, Shi Z, Wang P, Wang C, Yang L, Du G, Zhang H, Shi B, Jia J, Li Q, Wang H, Li Z. Claudin18.2-Specific Chimeric Antigen Receptor Engineered T Cells for the Treatment of Gastric Cancer. J Natl Cancer Inst. 2019;111:409–418. doi: 10.1093/jnci/djy134. [DOI] [PubMed] [Google Scholar]
- 152.Lv J, Zhao R, Wu D, Zheng D, Wu Z, Shi J, Wei X, Wu Q, Long Y, Lin S, Wang S, Wang Z, Li Y, Chen Y, He Q, Chen S, Yao H, Liu Z, Tang Z, Yao Y, Pei D, Liu P, Zhang X, Zhang Z, Cui S, Chen R, Li P. Mesothelin is a target of chimeric antigen receptor T cells for treating gastric cancer. J Hematol Oncol. 2019;12:18. doi: 10.1186/s13045-019-0704-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Xie J, Fu L, Jin L. Immunotherapy of gastric cancer: Past, future perspective and challenges. Pathol Res Pract. 2021;218:153322. doi: 10.1016/j.prp.2020.153322. [DOI] [PubMed] [Google Scholar]
- 154.Rahma OE, Khleif SN. Therapeutic vaccines for gastrointestinal cancers. Gastroenterol Hepatol (N Y) 2011;7:517–564. [PMC free article] [PubMed] [Google Scholar]
- 155.Miho E, Yermanos A, Weber CR, Berger CT, Reddy ST, Greiff V. Computational Strategies for Dissecting the High-Dimensional Complexity of Adaptive Immune Repertoires. Front Immunol. 2018;9:224. doi: 10.3389/fimmu.2018.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Durães C, Almeida GM, Seruca R, Oliveira C, Carneiro F. Biomarkers for gastric cancer: prognostic, predictive or targets of therapy? Virchows Arch. 2014;464:367–378. doi: 10.1007/s00428-013-1533-y. [DOI] [PubMed] [Google Scholar]