Abstract
Purpose:
We sought to evaluate whether bilateral prostate cancer detected at active surveillance (AS) enrollment is associated with progression to Grade Group (GG) ≥2 and to compare the efficacy of combined targeted biopsy plus systematic biopsy (Cbx) vs systematic biopsy (Sbx) or targeted biopsy alone to detect bilateral disease.
Materials and Methods:
A prospectively maintained database of patients referred to our institution from 2007–2020 was queried. The study cohort included all AS patients with GG1 on confirmatory Cbx and followup of at least 1 year. Cox proportional hazard analysis identified baseline characteristics associated with progression to ≥GG2 at any point throughout followup.
Results:
Of 579 patients referred, 103 patients had GG1 on Cbx and were included in the study; 49/103 (47.6%) patients progressed to ≥GG2, with 30/72 (41.7%) patients with unilateral disease progressing and 19/31 (61.3%) patients with bilateral disease progressing. Median time to progression was 68 months vs 52 months for unilateral and bilateral disease, respectively (p=0.006). Both prostate specific antigen density (HR 1.72, p=0.005) and presence of bilateral disease (HR 2.21, p=0.012) on confirmatory biopsy were associated with AS progression. At time of progression, GG and risk group were significantly higher in patients with bilateral versus unilateral disease. Cbx detected 16% more patients with bilateral disease than Sbx alone.
Conclusions:
Bilateral disease and prostate specific antigen density at confirmatory Cbx conferred greater risk of earlier AS progression. Cbx was superior to Sbx for identifying bilateral disease. AS risk-stratification protocols may benefit from including presence of bilateral disease and should use Cbx to detect bilateral disease.
Keywords: multiparametric magnetic resonance imaging, prostatic neoplasms, watchful waiting
Active surveillance (AS) is increasingly utilized to manage low-risk prostate cancer because it reduces treatment-related morbidity while minimizing risk of mortality.1,2 Longitudinal outcomes of patients on AS for Grade Group (GG) 1 disease show a prostate cancer specific mortality of less than 1%.3 The traditional diagnostic methods for detecting candidates for AS (elevated prostate specific antigen [PSA] (>4 ng/ml) followed by 12-core systematic biopsies) are currently limited by the risk of over-diagnosis of clinically insignificant prostate cancers and underdiagnosis of higher-grade cancers.4 Overall, moderate rates of AS progression within 1–2 years of AS initiation indicate that current methods of initial screening may be missing more aggressive cancers.5
Currently, multiparametric magnetic resonance imaging (mpMRI)-guided targeted biopsy (Tbx) with combined extended sextant 12-core systematic biopsy (Sbx) has been defined as combined biopsy (Cbx) and has been documented as the most accurate method to detect clinically significant prostate cancers when compared to either method alone.4,6 Accordingly, Cbx has been shown to improve the selection of AS patients to minimize risks of AS failure at followup.7 As imaging and diagnostic methods improve and more patients with low-risk prostate cancers opt for AS, it is imperative to utilize additional patient variables to risk-stratify patients at diagnosis and to personalize a monitoring strategy. Currently, there is no universal consensus on biopsy criteria for AS enrollment. Studies utilizing Sbx for risk stratification suggest grade progression on AS is associated with baseline characteristics of the biopsy including number of positive cores, presence of bilateral disease and tumor volume.3,8 However, there is a paucity of evidence regarding the utility of Cbx for detecting bilateral disease and its possible association with subsequent AS failure. In this study we aimed to determine the risk of AS failure associated with the presence of bilateral prostate cancer at AS enrollment and further describe the diagnostic value of Cbx vs Sbx only and vs Tbx only in detection of bilateral disease.
METHODS
Patient Selection
The cohort was identified from a prospectively maintained database of patients referred to a tertiary referral center for consideration for AS between July 2007 and January 2020 who were enrolled in trial NCT00102544 (IRB No. 05-CC-0091). Eligible patients had a diagnosis of prostate cancer on Sbx prior to referral, underwent prostate mpMRI at our institution and received confirmatory Cbx at our institution at time of enrollment. For this study, patients were excluded if their disease was upgraded to ≥GG2 on our confirmatory Cbx or if they did not have any followup biopsy at our institution. Patients with GG2 on outside biopsy and GG1 on confirmatory biopsy were included as GG1 at AS enrollment. Following confirmatory Cbx, patients received yearly followup, including PSA and physical examination. Surveillance mpMRI and Cbx were offered every 1–2 years. The current study’s cohort is a subgroup of patients identified from our AS cohort, which has been previously reported on.7
Imaging and Biopsy Protocol
mpMRI images were obtained using a 3T MRI with a 16-channel surface coil and, in some cases, endorectal coil. MRI pulse sequences obtained included T2-weighted, dynamic contrast enhanced, and diffusion-weighted imaging including a high b-value image and apparent diffusion coefficient maps. Images were prospectively read by 2 expert genitourinary radiologists on consensus (PC and BT). Patient mpMRI studies were also assigned a 1–5 Likert suspicion score (National Institutes of Health [NIH] Suspicion Score), which has been previously described, and MRI scans after 2015 were assigned Prostate Imaging–Reporting and Data System™ (PI-RADS™) v2 scores.9,10 Based on mpMRI images, suspicious lesions (defined as PI-RADS >2/NIH Suspicion>Low) underwent targeted MRI-transrectal ultrasound image fused guided transrectal biopsies taking cores in both the axial and sagittal planes with the aid of electromagnetic tracking using software guidance with the UroNav™ MR/Ultrasound fusion biopsy device (Philips Healthcare).9 Patients subsequently underwent systematic extended-sextant biopsy collecting 12-cores from both medial and lateral aspects of the apex, mid and base of each lobe of the gland.
Statistical Analysis
AS failure was defined as grade progression to GG ≥2 on any repeat biopsy after initial confirmatory biopsy at time of enrollment. Baseline patient characteristics were compared using Mann-Whitney U or chi-square tests for continuous and categorical variables, respectively. Fisher’s exact tests were used where chi-square approximation was not accurate. Multivariate analysis with a Cox proportional hazard analysis was conducted to identify clinical variables at the time of AS enrollment that significantly associated with AS progression. Clinical variables used in the multivariate model were selected using a lasso regression analysis and included: Age, PSA, PSA density (PSAD; the number of positive cores on systematic biopsy, and the presence of unilateral or bilateral disease on confirmatory biopsy. The proportional hazards assumption was verified for all variables included in the model using scaled Schoenfeld residuals testing. PSAD was calculated from the PSA immediately prior to confirmatory biopsy divided by the volume of the prostate on mpMRI. Each patient’s risk group at the time of progression was calculated based on the National Comprehensive Cancer Network© (NCCN©) prostate cancer risk stratification. A Kaplan-Meier estimator was used to create time to AS progression curves for patients with unilateral and bilateral disease, and curves were compared using a log-rank test. Chi-squared test was used to compare the distribution of GG and NCCN risk categories at time of progression between patients with unilateral and bilateral disease. All tests were 2-sided, and results with p <0.05 were considered statistically significant. Statistical analyses were performed using R version 4.0.2 (R Project for Statistical Computing, Vienna, Austria).
RESULTS
Patient Characteristics
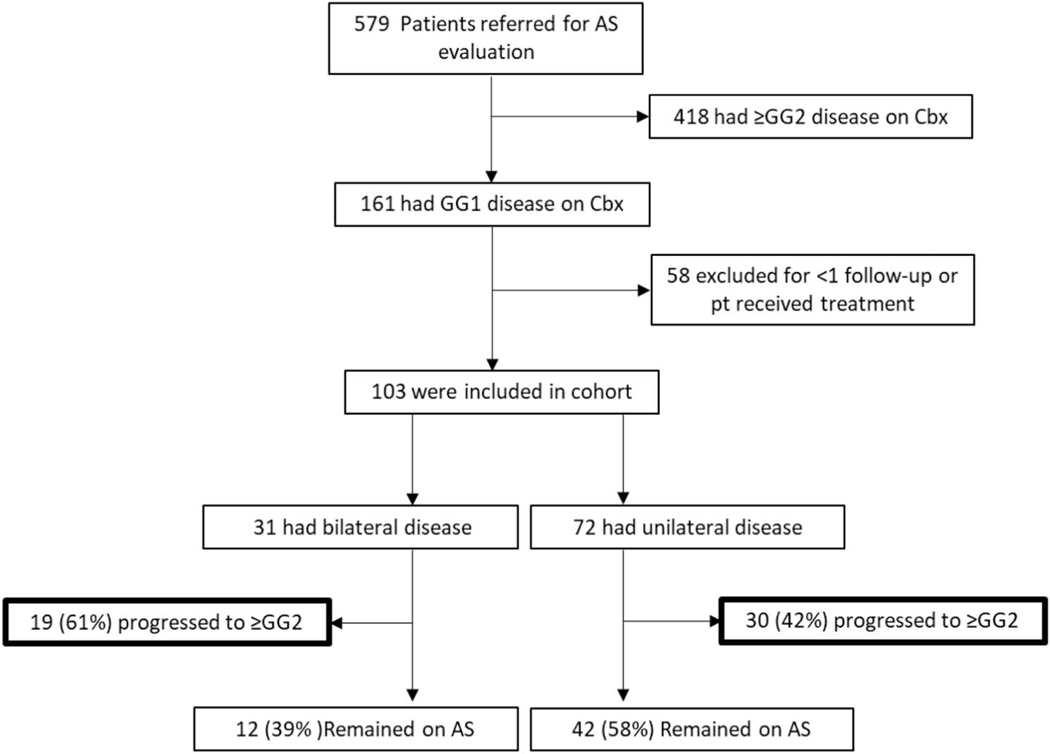
A total of 579 patients with an outside diagnosis of GG1 or GG2 prostate cancer were seen in the NIH Clinical Center between July 2007 and January 2020. On confirmatory Cbx, 27.8% (161/579) of patients had a diagnosis of GG1 and 33.0% (191/579) of patients had a diagnosis of GG2. A total of 58 patients did not enroll in our AS clinical trial. As a result, 103 patients with GG1 disease on AS had at least 1 followup biopsy at the NIH and were included in the final analysis (fig. 1). Median followup time was 38 months in these 103 patients with GG1 disease. Characteristics of these patients and their AS followup are detailed in tables 1 and 2. Median age was 62 years (quartiles: 58–67). The median PSA and PSAD were 4.81 ng/ml (quartiles: 3.7–7.1) and 0.096 ng/ml2 (quartiles: 0.068–0.142), respectively. Median prostate volume was 48.25 ml (quartiles: 38.75–66.25). The number of MRI-visible lesions ranged from 1 to 6, with a median of 1.5 lesions, and there were no demographic differences among patients with unilateral and bilateral disease (table 1). Most patients (96.12%) had 4 or fewer lesions visible on MRI, and there was no difference in the number of lesions between patients with bilateral vs unilateral disease (supplementary table 1, https://www.jurology.com). One patient with bilateral disease had no visible lesion on mpMRI and cancer was detected on Sbx alone.
Figure 1.
Flowchart of patient enrollment on active surveillance with bilateral and unilateral disease.
Table 1.
Clinical features of patients with GG1 disease at time of enrollment into National Cancer Institute AS program
| Total (103 pts) | Unilat (72 pts) | Bilat (31 pts) | p Value | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Median yrs age (IQR) | 62 | (58–67) | 61.5 | (57–67) | 63 | (58–66) | 0.75 |
| No. yrs age (%): | |||||||
| <50 | 4 | (3.88) | 3 | (4.17) | 1 | (3.23) | * |
| 50–60 | 37 | (35.92) | 27 | (37.50) | 10 | (32.26) | 0.78 |
| >60 | 62 | (60.19) | 42 | (58.33) | 20 | (64.52) | 0.71 |
| No. race (%): | |||||||
| White | 88 | (85.43) | 61 | (84.72) | 27 | (87.10) | 0.99 |
| Black | 9 | (8.74) | 7 | (9.72) | 2 | (6.45) | † |
| Asian | 4 | (3.88) | 3 | (4.17) | 1 | (3.23) | * |
| Hispanic | 0 | 0 | 0 | ||||
| Other | 2 | (1.94) | 1 | (1.39) | 1 | (3.23) | * |
| Median ng/ml PSA (IQR) | 4.81 (3.695–7.115) | 4.82 (3.70–7.12) | 4.81 (4.02–7.54) | 0.60 | |||
| No. ng/ml PSA (%): | |||||||
| <4 | 32 | (31.07) | 24 | (33.33) | 8 | (25.81) | 0.60 |
| 4–10 | 65 | (63.11) | 43 | (59.72) | 22 | (70.97) | 0.39 |
| >10 | 6 | (5.83) | 5 | (6.94) | 1 | (3.23) | * |
| Median ng/ml PSAD (IQR) | 0.096 (0.068–0.142) | 0.088 (0.062–0.134) | 0.114 (0.085–0.151) | 0.026† | |||
| Median ml total prostate vol (IQR) | 48.25 (38.75–66.25) | 50 (40–68) | 45.4 (33.85–55) | 0.105 | |||
| No. total prostate vol (%): | |||||||
| <30 ml | 11 | (11.00) | 6 | (8.70) | 5 | (16.13) | * |
| 30–60 ml | 63 | (60.00) | 41 | (55.07) | 22 | (70.97) | 0.26 |
| >60 ml | 29 | (29.00) | 25 | (36.23) | 4 | (12.90) | 0.03‡ |
| Median mm lesion size on MRI (IQR) | 10.0 (8.0–13.0) | 10.0 (8.5–14) | 11.00 (7.0–13.0) | 0.90 | |||
| No. NIH Suspicion Score on MRI (%):§ | |||||||
| Very low | 7 | (6.80) | 4 | (5.56) | 3 | (9.68) | * |
| Low | 16 | (15.53) | 11 | (15.28) | 5 | (16.13) | * |
| Low-moderate | 5 | (4.85) | 5 | (6.94) | 0 | * | |
| Moderate | 65 | (63.11) | 43 | (59.72) | 22 | (70.97) | 0.39 |
| Moderate-high | 4 | (3.88) | 4 | (5.56) | 0 | * | |
| High | 5 | (4.85) | 4 | (5.56) | 1 | (3.23) | * |
Not significant using Fisher’s exact test.
Chi-square test statistic, p <0.05.
Fisher’s exact test, p <0.05.
NIH Suspicion Score Scale has been shown to correlate with PI-RADS.
Table 2.
AS followup characteristics of patients with GG1 disease at time of enrollment into National Cancer Institute AS program
| Total (103 pts) | Unilat (72 pts) | Bilat (31 pts) | p Value | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Median mos on AS (IQR) | 34 (23.5–52.5) | 36.5 (27.75–57.25) | 25 (14–42) | 0.0046* | |||
| Followup biopsies: | |||||||
| Total No. | 203 | 145 | 56 | ||||
| Median (IQR) | 2 | (1–3) | 2 | (1–3) | 2 | (1–2) | 0.35 |
| 1 (%) | 48 | (46.6) | 34 | (23.45) | 15 | (26.79) | 0.99 |
| 2 (%) | 25 | (24.27) | 14 | (9.66) | 11 | (19.64) | 0.14 |
| 3 (%) | 20 | (19.42) | 16 | (11.03) | 3 | (5.36) | 0.22 |
| >4 (%) | 10 | (9.71) | 8 | (5.52) | 2 | (3.57) | 0.71 |
| Median mos time between each biopsy (IQR) | 16 | (12–27) | 16 | (12–29) | 15 | (12–25) | 0.44 |
| Median mos time between each MRI (IQR) | 13 | (12–16) | 13 | (12–16) | 13 | (12–15) | 0.85 |
| No. pts with AS grade progression on followup biopsy (%): | |||||||
| Total | 49 | (47.5) | 30 | (41.67) | 19 | (61.29) | 0.11† |
| Upgrade on Sbx only | 9 | (18.4) | 4 | (13.3) | 5 | (26.3) | 0.17 |
| Upgrade on Tbx only | 14 | (28.6) | 12 | (40.0) | 2 | (10.5) | 0.29 |
| Upgrade on both | 26 | (53.1) | 14 | (46.7) | 12 | (63.2) | 0.07 |
Mann-Whitney U test, p <0.05.
Chi-squared statistic.
Time to Progression to GG2 or Greater
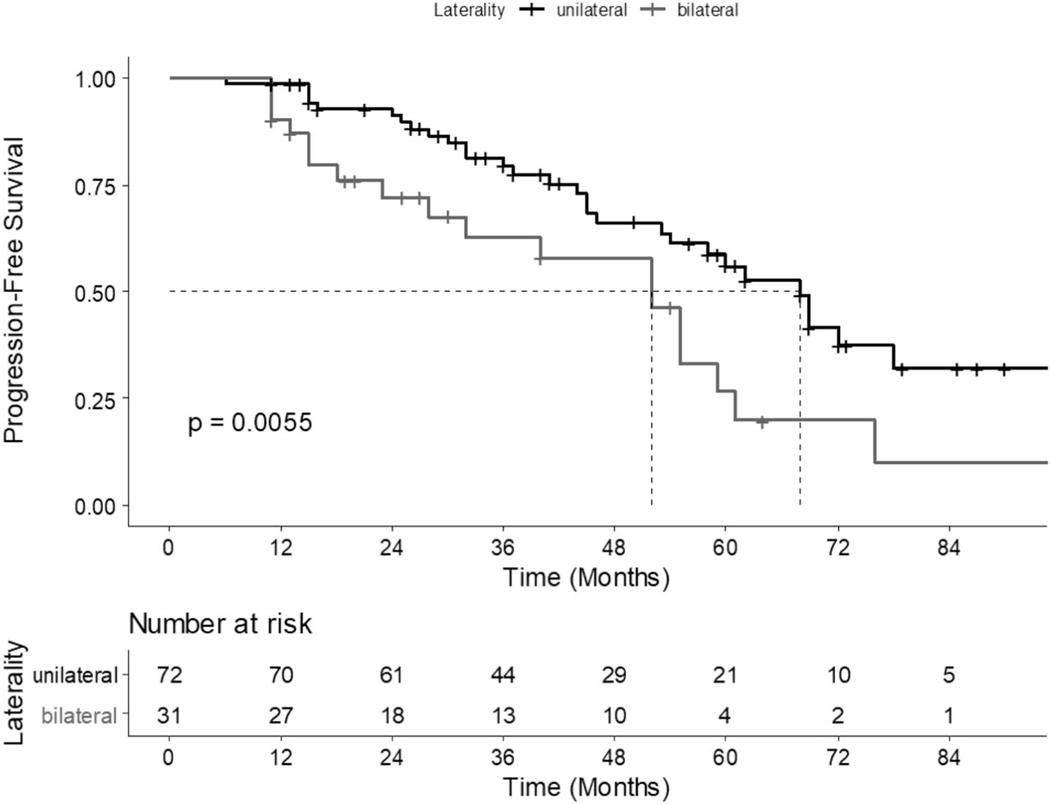
Out of 103 patients, 30% (31/103) had bilateral disease identified on combined biopsy and 70% (72/103) had unilateral disease. Overall, 47% (49/103) of patients progressed to ≥GG2 disease on followup biopsy; 42% (30/72) of patients with unilateral disease progressed, and 61% (19/31) of patients with bilateral disease progressed (table 2). On multivariate cox proportional hazard analysis, both PSAD (HR=2.21; 95% CI 1.41–3.48) and presence of bilateral disease (HR=3.06; 95% CI 1.31–7.13) at time of confirmatory biopsy were significantly associated with later progression to ≥GG2 disease (table 3). On Kaplan-Meier survival estimation, median time from Cbx to progression ≥GG2 for the whole cohort was 52 months (95% CI 44–68). The median time to progression for patients with unilateral and bilateral disease was 68 months and 52 months, respectively (log-rank test, p=0.006; fig. 2).
Table 3.
Clinical variables at time of confirmatory biopsy associated with subsequent AS progression to GG ≥2
| Multivariate | ||
|---|---|---|
|
|
||
| Variables | HR | p Value |
|
| ||
| Age | 1.03 (0.99–1.08) | 0.15 |
| PSA | 0.96 (0.86–1.08) | 0.49 |
| PSAD | 2.21 (1.41–3.48) | <0.001 * |
| Pos cores on systematic biopsy | 0.94 (0.75–1.18) | 0.61 |
| Bilat disease | 3.06 (1.31–7.13) | 0.009 * |
Values in bold indicate statistical significance (p <0.05).
Figure 2.
Kaplan-Meier curve demonstrating time to AS progression for patients found to have unilateral and bilateral disease on confirmatory biopsy (log-rank test: p=0.0055).
Combined Biopsy vs Systematic and MRI-Targeted Biopsy for Detecting Bilateral Disease
Of the 31 patients who had bilateral disease on Cbx, Sbx identified bilateral disease in 83.9% (26/31) of patients, resulting in an added value of 16.1% for Cbx in detecting bilateral disease; 65% (20/31) of patients with bilateral cancer were detected by Sbx alone, 9.7% (3/31) of patients with bilateral cancer were detected by mpMRI-targeted biopsy alone, 6.5% (2/31) of patients with bilateral cancer were detected by Cbx alone (eg a right-sided lesion was only detected by targeted biopsy and a left-sided lesion was only detected by systematic biopsy, or vice versa), and 19% (6/31) of patients with bilateral cancer were detected by both MRI and Sbx. Grade progression was detected by SBx only in 9/49 (18.4%) patients, detected by Tbx only in 14/49 (28.6%) patients, and detected by both methods in 26/49 (53.1%) patients (table 2).
NCCN Risk and Grade Group at Time of Progression
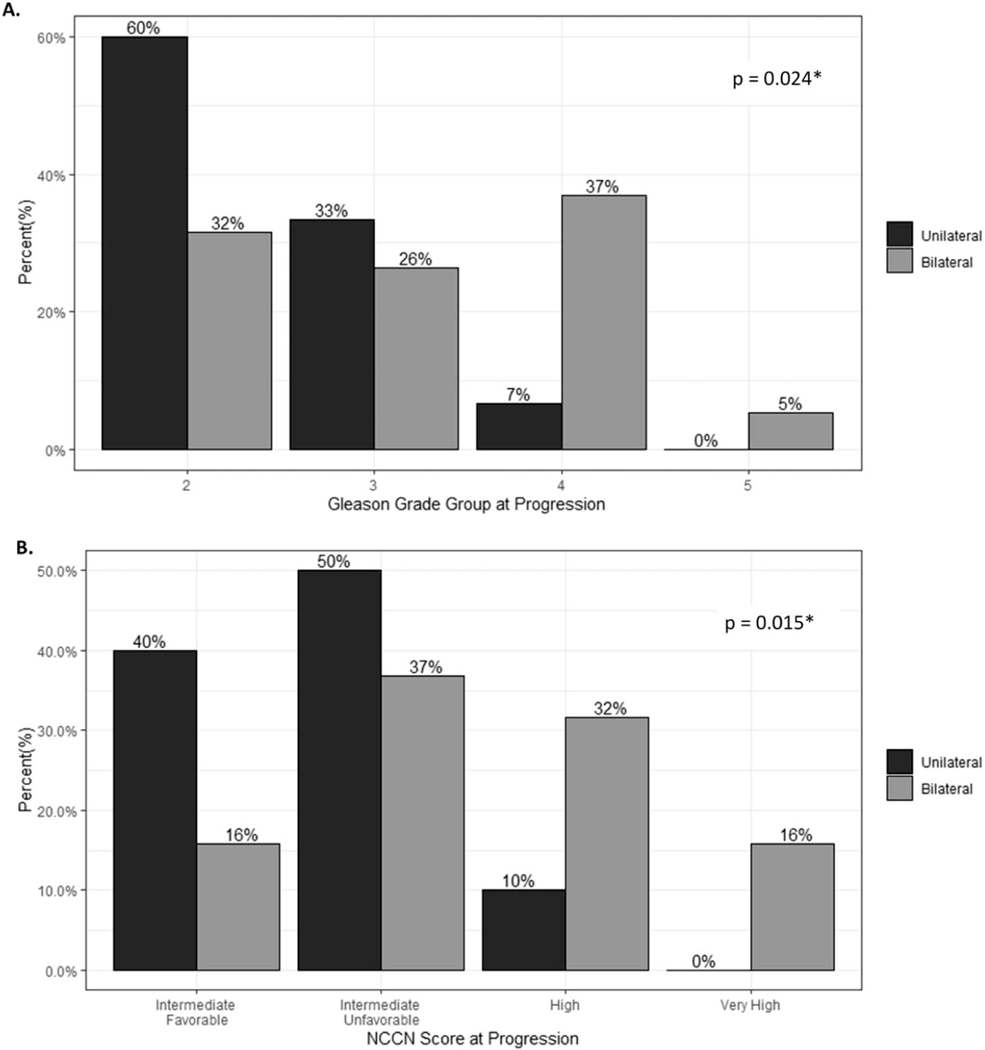
We further assessed grade group and NCCN risk group of cancer after progression. Among the 103 patients diagnosed with GG1 on confirmatory biopsy, the baseline NCCN risk groups on confirmatory biopsy ranged from very low risk to favorable intermediate risk. There were no significant differences in baseline risk groups between patients with unilateral and bilateral disease. For patients who progressed on AS, the risk groups at the time of progression varied between favorable intermediate to very high risk with most of the unilateral and bilateral cohorts progressing to unfavorable intermediate risk disease (50% and 37%, respectively). The proportion of patients with bilateral disease and high-risk or very high-risk disease was greater than that of patients with unilateral disease (48% vs 10% for bilateral and unilateral, respectively). Bilateral disease conferred greater risk of being upgraded to an NCCN risk category of high or very high on upgraded biopsy (relative risk: 3.16, 95% CI 1.004–9.932). NCCN risk category (p=0.015) and GG (p=0.024) at time of progression were significantly higher among patients with bilateral disease compared with unilateral disease on confirmatory biopsy (fig. 3). We additionally repeated cox proportional hazard analysis for the risk of upgrading to GG ≥3 which demonstrated that bilateral disease (HR: 4.89; CI 1.60–15.02), PSAD (HR: 2.86; CI 1.65–4.97), and patient age (HR=1.1; CI 1.02–1.19) predicted risk of upgrading (supplementary table 2, https://www.jurology.com).
Figure 3.
Distribution of GG (A) and NCCN risk groups (B) at progression among patients who progressed.
DISCUSSION
In this study we report on a cohort of patients with biopsy-proven prostate cancer for enrollment in an AS prostate cancer clinical protocol. We found that the presence of bilateral prostate cancer at time of enrollment in AS was associated with higher risk for disease progression. In addition, we found significant added value in Cbx for detecting bilateral disease at time of confirmatory biopsy. The current literature on risks associated with the presence of bilateral disease in patients on AS is sparse.11 In a retrospective study published in 2019, Wang et al observed that bilateral disease detected on Sbx was the strongest risk factor for AS failure.12 On AS reclassification-free survival analysis, they found that bilateral disease was associated with earlier reclassification on followup biopsy (32 months for bilateral disease vs 119 months for unilateral disease). We expand on this work by adding that detection of bilateral disease using mpMRI of the prostate and Cbx, rather than Sbx alone, was significantly associated with disease progression in patients on AS and finding that PSAD at the time of AS enrollment additionally predicted future progression. Despite this difference in biopsy technique, we similarly found that both the presence of bilateral disease at the time of confirmatory biopsy and PSAD were significant predictors of progression on AS. Currently, the number of positive cores on systematic biopsy is used as proxy for tumor volume or tumor multifocality.13 Bilaterality similarly estimates gland involvement, and in the current study, bilaterality had stronger predictive value for progression than positive systematic biopsy cores.
Our analysis did not identify any significant baseline differences in age, PSA, prostate volume, or number of follow up biopsies between the patient populations. In this cohort, the median time to progression was 52 months and 68 months for bilateral and unilateral disease, respectively. Studies with longer followup may find an even greater difference in the time to progression.14–16
Few studies have examined the risk of bilateral disease for adverse outcomes. One retrospective cohort study of 63 patients found that bilateral disease was significantly associated with positive surgical margins on local treatment.17 A later study by Sfoungaristos et al reported that patients with bilateral multifocal disease at biopsy are more likely to have adverse pathological features such as extraprostatic extension at prostatectomy.18 Similarly, in our cohort, GG and NCCN risk groups among upgraded patients at time of progression were significantly higher in patients with bilateral disease despite being the same at baseline. Taken together, bilateral prostate cancer, even of low grade, may be an indicator of poorer prognosis, and patients with bilateral disease detected at enrollment may harbor more aggressive cancer.
Despite these increased risks among patients with bilateral disease, the implications still remain unclear. Our results confirm previous work that Cbx is the preferred method for AS risk stratification at diagnosis as this led to a 16% increase in cancer detection rate of bilateral disease compared with systematic alone SBx alone underestimates bilateral disease.19 Multiple studies have reported that systematic detection of unilateral cancer among low-risk patients is largely unreliable, with ranges of 48% to 80% of patients classified as possessing unilateral disease ultimately having bilateral disease.19–21 Use of mpMRI guidance in combination with Sbx improves the sensitivity and specificity of bilateral disease.6 Our study indicates that the use of Cbx confers additional benefits for risk stratification beyond cancer detection and could further direct treatment such as electing AS in a specific cohort of patients.
It is worth noting that 1 patient in our cohort who was upgraded to ≥GG2 on followup biopsy had no MRI-visible lesion. The significance of such cases is unclear, as previous studies have shown, having no MRI-visible lesions is not predictive of prostate cancer upstaging nor is it associated with AS failure.22 Thus, MRI-visible bilateral lesions may be more important than simply detecting bilateral disease at biopsy.
This study has some limitations. As our institution is a referral center, our cohort was established based on outside referrals from a variety of centers, thereby introducing a potential selection bias. Additionally, it was previously reported that the overall agreement between our center’s confirmatory biopsy and referral center biopsy was as low as 30%, suggesting that access to mpMRI or provider experience may additionally affect detection of bilateral disease.7 PI-RADS v2 (introduced in 2015) and its updated version V2.1 introduced in 2019 may improve the accuracy and diagnostic value of mpMRI among radiologists. Although our institution strictly applies PI-RADS routinely, robust analysis of PI-RADS within this cohort was not possible because many of the patients were scanned before PI-RADS was operational. Finally, with 49 events in the study, the sample size is relatively small which limited the number of factors screened for the multivariate analysis.
CONCLUSIONS
The presence of bilateral disease and PSAD at time of enrollment on AS are associated with prostate cancer progression, and combined MRI-targeted plus systematic biopsy improves the detection rate of bilateral disease. Incorporation of the presence of bilateral disease on combined prostate biopsy could contribute to risk stratification and help guide management from the time of entry on AS.
Supplementary Material
Study Need and Importance:
The traditional diagnostic methods for detecting candidates for active surveillance (AS) are elevated prostate specific antigen (>4 ng/ml) followed by 12-core systematic biopsies. This approach is currently limited by the risk of over diagnosis of clinically insignificant prostate cancers and under diagnosis of higher-grade cancers. Moderate rates of AS progression within 1–2 years of AS initiation indicate that current methods of initial screening may be missing more aggressive cancers. Use of combined targeted and systematic biopsy has improved selection of patients for AS, but additional patient variables like prostate specific antigen density (PSAD) and lesion volume may improve identification for patients most at risk for AS failure. This retrospective study was designed to identify patient variables at the time of enrollment that conferred risk of grade progression on AS.
What We Found:
The presence of bilateral vs unilateral prostate cancer detected by combined biopsy at AS initiation significantly increased the risk of progressing from grade group (GG) 1 to ≥GG2 during active surveillance. Of the patients with unilateral disease 41.7% progressed and 61.3% with bilateral disease progressed. Higher PSAD was also associated with AS progression. Patients with bilateral disease also progressed to higher GG and risk groups at the time of progression. Combined biopsy detected 16% more patients with bilateral disease than systematic biopsy alone.
Limitations:
This study may be limited by selection bias. Our institution is a referral center, and our cohort was established based on outside referrals from a variety of centers. Additionally, although our institution strictly applies Prostate Imaging–Reporting and Data System™ (PI-RADS™) routinely, robust analysis of PI-RADS within this cohort was not possible because many of the patients were scanned before PI-RADS was operational. PI-RADS version 2 (v2) was introduced in 2015 and its updated version (v2.1) was introduced in 2019. Finally, with 49 events in the study the sample size is relatively small which limited the number of factors screened for the multivariate analysis.
Interpretation for Patient Care:
Patients with GG1 cancer who are eligible for AS may be at increased risk of progression if they harbor disease in both prostatic lobes or have high PSAD. Combined biopsy is a superior method for identifying bilateral prostate cancer. Incorporation of these patient variables could be considered in evaluation of patients eligible for AS.
Funding:
This research was supported in part by the Intramural Research Program of the National Cancer Institute, NIH and the National Institutes of Health (NIH) Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the National Institutes of Health from the Doris Duke Charitable Foundation, the American Association for Dental Research, the Colgate-Palmolive Company, Genentech, alumni of student research programs and other individual supporters via contributions to the Foundation for the National Institutes of Health.
Abbreviations and Acronyms
- AS
active surveillance
- Cbx
mpMRI-guided targeted biopsy (Tbx) with combined extended sextant 12-core systematic biopsy (Sbx)
- GG
Grade Group
- NCCN©
National Comprehensive Cancer Network©
- mpMRI
multiparametric MRI
- MRI
magnetic resonance imaging
- NIH
National Institutes of Health
- PI-RADS™
Prostate Imaging–Reporting and Data System™
- PSA
prostate specific antigen
- PSAD
prostate specific antigen density
- Sbx
extended sextant 12-core systematic biopsy
- Tbx
MRI-targeted prostate biopsy
Footnotes
Ethics approval: This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Institutional Review Board of the National Cancer Institute (IRB No. 05-CC-0091).
Conflicts of interest/Competing interests: NIH and Philips have a Cooperative Research and Development Agreement. NIH has intellectual property in the field, including among other patents and patent applications, Patent: “System, methods, and instrumentation for image guided prostate treatment” US Patent number: 8948845, with inventors/authors including PLC, BW and PP. NIH and Philips (InVivo Inc) have a licensing agreement. NIH and authors PLC, BW and PP receive royalties from the U.S. government for a licensing agreement with Philips/InVivo Inc. NIH does not endorse or recommend any commercial products, processes or services. The views and personal opinions of authors expressed herein do not necessarily reflect those of the U.S. Government, nor reflect any official recommendation nor opinion of the NIH nor NCI.
Availability of data and material:
All data will be made available upon request to the corresponding author.
REFERENCES
- 1.Mohler JL and Antonarakis ES: NCCN guidelinesupdates: management of prostate cancer. J Natl Compr Canc Netw 2019; 17: 583. [DOI] [PubMed] [Google Scholar]
- 2.Hamdy FC, Donovan JL, Lane JA et al. : 10-Yearoutcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. New Engl J Med 2016; 375: 1415. [DOI] [PubMed] [Google Scholar]
- 3.Tosoian JJ, Mamawala M, Epstein JI et al. : Active surveillance of grade group 1 prostate cancer: long-term outcomes from a large prospective cohort. Eur Urol 2020; 77: 675. [DOI] [PubMed] [Google Scholar]
- 4.Ahdoot M, Wilbur AR, Reese SE et al. : MRI-targeted, systematic, and combined biopsy for prostate cancer diagnosis. New Engl J Med 2020; 382: 917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sierra PS, Damodaran S and Jarrard D: Clinicaland pathologic factors predicting reclassification in active surveillance cohorts. Int Braz J Urol 2018; 44: 440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rouvière O, Puech P, Renard-Penna R et al. : Use of prostate systematic and targeted biopsy on the basis of multiparametric MRI in biopsy-naive patients (MRI-FIRST): a prospective, multicentre, paired diagnostic study. Lancet Oncol 2019; 20: 100. [DOI] [PubMed] [Google Scholar]
- 7.O’Connor LP, Wang AZ, Yerram NK, et al. : Combined MRI-targeted plus systematic confirmatory biopsy improves risk stratification for patients enrolling on active surveillance for prostate cancer. Urology 2020; 144: 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klotz L, Vesprini D, Sethukavalan P et al. : Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol 2015; 33: 272. [DOI] [PubMed] [Google Scholar]
- 9.Turkbey B, Xu S, Kruecker J et al. : Documentingthe location of prostate biopsies with image fusion. BJU Int 2011; 107: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turkbey B, Rosenkrantz AB, Haider MA et al. : Prostate Imaging Reporting and Data System version 2.1: 2019 update of Prostate Imaging Reporting and Data System version 2. Eur Urol 2019; 76: 340. [DOI] [PubMed] [Google Scholar]
- 11.Tareen B, Godoy G, Sankin A et al. : Lateralityalone should not drive selection of candidates for hemi-ablative focal therapy. J Urol 2009; 181: 1082. [DOI] [PubMed] [Google Scholar]
- 12.Wang JH, Sierra P, Richards KA et al. : Impact ofbilateral biopsy-detected prostate cancer on an active surveillance population. BMC Urol 2019; 19: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schaeffer E, Srinivas S, Antonarakis ES et al. : NCCN guidelines insights: prostate cancer, version 1.2021. J Natl Compr Canc Netw 2021; 19: 134. [DOI] [PubMed] [Google Scholar]
- 14.San Francisco IF, Werner L, Regan MM et al. : Risk stratification and validation of prostate specific antigen density as independent predictor of progression in men with low risk prostate cancer during active surveillance. J Urol 2011; 185: 471. [DOI] [PubMed] [Google Scholar]
- 15.Cary KC, Cowan JE, Sanford M et al. : Predictorsof pathologic progression on biopsy among men on active surveillance for localized prostate cancer: the value of the pattern of surveillance biopsies. Eur Urol 2014; 66: 337. [DOI] [PubMed] [Google Scholar]
- 16.Bul M, Zhu X, Valdagni R et al. : Active surveillance for low-risk prostate cancer worldwide: the PRIAS study. Eur Urol 2013; 63: 597. [DOI] [PubMed] [Google Scholar]
- 17.Gao X, Mohideen N, Flanigan RC et al. : Theextent of biopsy involvement as an independent predictor of extraprostatic extension and surgical margin status in low risk prostate cancer: implications for treatment selection. J Urol 2000; 164: 1982. [PubMed] [Google Scholar]
- 18.Sfoungaristos S and Perimenis P: Bilateral cancer in prostate biopsy associates with the presence of extracapsular disease and positive surgical margins in low risk patients: a consideration for bilateral nerve sparing radical prostatectomy decision. Urol J 2013; 10: 966. [PubMed] [Google Scholar]
- 19.Gallina A, Maccagnano C, Suardi N et al. : Unilateral positive biopsies in low risk prostate cancer patients diagnosed with extended transrectal ultrasound-guided biopsy schemes do not predict unilateral prostate cancer at radical prostatectomy. BJU Int 2012; 110: E64. [DOI] [PubMed] [Google Scholar]
- 20.Mouraviev V, Mayes JM, Sun L et al. : Prostatecancer laterality as a rationale of focal ablative therapy for the treatment of clinically localized prostate cancer. Cancer 2007; 110: 906. [DOI] [PubMed] [Google Scholar]
- 21.Johnson DC, Yang JJ, Kwan L et al. : Do contemporary imaging and biopsy techniques reliably identify unilateral prostate cancer? Implications for hemiablation patient selection. Cancer 2019; 125: 2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gallagher KM, Christopher E, Cameron AJ et al. : Four-year outcomes from a multiparametric magnetic resonance imaging (MRI)-based active surveillance programme: PSA dynamics and serial MRI scans allow omission of protocol biopsies. BJU Int 2019; 123: 429. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data will be made available upon request to the corresponding author.