Abstract
Hutchinson–Gilford progeria syndrome (HGPS) is a rare, invariably fatal childhood premature aging disorder caused by a pre-messenger RNA (mRNA) splicing defect in the LMNA gene. We used combined in vitro screening and in vivo validation to systematically explore the effects of target sequence, backbone chemistry and mechanism of action to identify optimized antisense oligonucleotides (ASOs) for therapeutic use in HGPS. In a library of 198 ASOs, the most potent ASOs targeted the LMNA exon 12 junction and acted via non-RNase H-mediated mechanisms. Treatment with an optimized lead candidate resulted in extension of lifespan in a mouse model of HGPS. Progerin mRNA levels were robustly reduced in vivo, but the extent of progerin protein reduction differed between tissues, suggesting a long half-life and tissue-specific turnover of progerin in vivo. These results identify a novel therapeutic agent for HGPS and provide insight into the HGPS disease mechanism.
HGPS is a pediatric premature aging disorder1,2 characterized by failure to thrive and multi-organ symptoms, including loss of subcutaneous fat, alopecia, joint contractures and cardiac disease2. HGPS is caused by a point mutation in the LMNA gene that encodes for the intermediate filament proteins lamin A and lamin C by alternative pre-mRNA splicing3,4. The most common disease-causing mutation C1824T is located in LMNA exon 11 where it activates an exonic pre-mRNA splice site resulting in the elimination of 150 nucleotides (nts) of exon 11 (Fig. 1a)3,4. The mutation gives rise to the progerin protein, which contains an internal 50-amino acid deletion toward its C terminus1. The deleted region also encompasses an endoproteolytic cleavage site that removes the farnesylated C terminus of the pre-lamin A protein. It is thought that the incorporation of the permanently farnesylated progerin protein into the nuclear lamina contributes to the cellular defects observed in HGPS cells, including altered nuclear morphology, increased DNA damage and abnormal patterns of epigenetic modifications1.
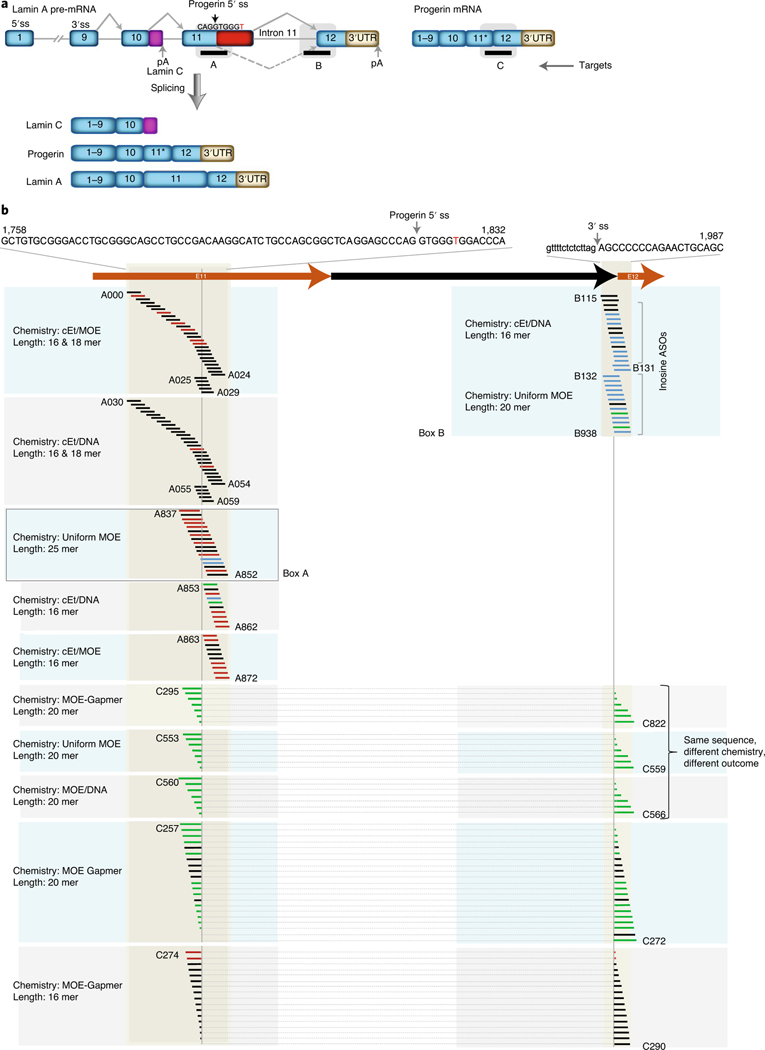
Fig. 1 |. ASOs screen for human lamin A RNA target.
a, Schematic drawing of lamin A, lamin C and progerin mRNA produced through alternative splicing of LMNA. Target A, ASOs targeted to progerin alternative 5′ splice site; Target B, LMNA intron 11–exon 12 splice junction; Target C, progerin-specific E11–E12 splice junction; E11*, exon 11 lacking the terminal 150 nts. Dashed arrow indicates alternative splicing leading to progerin mRNA. Nucleotide in red indicates the HGPS disease-causing point mutation; pA, polyadenylation signal. b, Schematic diagram of ASO libraries. ASOs with different chemistries and various lengths targeted to different targeting regions are indicated. Color code for progerin mRNA reduction: black 0–50%, blue 50–75%, green 75–95% green and red >50% increase. ASOs connected with dotted lines are targeted to progerin mRNA and E11*E12. cEt, 2′, 4′-constrained ethyl; MOE, 2′-O-methoxyethyl. For sequence information of all ASOs, see Supplementary Table 2. ss, splice site; UTR, untranslated region.
Therapeutic approaches to HGPS have primarily targeted the progerin protein1. Most prominently, the farnesyltransferase inhibitor (FTI) lonafarnib prevents the addition of the farnesyl group to the C terminus of progerin and has some benefit in clinical trials5,6. Similarly, the mTOR inhibitor everolimus, which promotes progerin turnover7, is currently under clinical investigation. Many other small molecules have been suggested as potential anti-HGPS agents via reduction of progerin protein levels8–18.
Because HGPS is caused by a pre-mRNA splicing mutation, ASOs are of interest as therapeutic agents in HGPS. ASO-mediated strategies have been used in a variety of diseases, including muscular dystrophy and spinal muscular atrophy19–21. Therapeutically active ASOs function either via RNase H-mediated degradation of the target RNA by attacking DNA–RNA hybrids formed between the target RNA and the ASO or via non-RNase H-mediated mechanisms typically through sterically blocking access of the RNA processing machinery to its target sequences21.
Proof-of-principle studies for antisense-mediated reversal of cellular defects in HGPS have been reported using a morpholino ASO targeting the mutated splice site in LMNA exon 11 in vitro22 and in vivo where the ASO reduced physiological defects and extended lifespan in a mouse model of HGPS23,24. Although previous efforts using ASOs for therapeutic approaches in HGPS used rationally designed ASOs complementary to the mutated exonic splice site in exon 11, it is not evident that directly targeting the progerin splice site presents the most effective therapeutic approach22,23,25.
To identify optimized ASOs for potential use as therapeutic agents for HGPS in a largely unbiased fashion, we combined in vitro and in vivo screens of a diverse library of ASOs. We found non-obvious effects of target sequence, backbone chemistry, ASO length and mechanisms of action on their potency. In an HGPS mouse model, long-term treatment with a candidate ASO resulted in reduction of mRNA, tissue-specific effects on progerin protein levels and extension of lifespan.
Results
A comprehensive ASO screen targeting aberrant LMNA splicing in HGPS.
To identify ASOs that are effective in reducing progerin mRNA and protein, we performed a systematic screen of a library of 198 ASOs with distinct target sequences, lengths, chemistries and mechanisms of action (Fig. 1 and Supplementary Tables 1 and 2). Each ASO at 50–100 nM was delivered by lipid-mediated transfection into immortalized HGPS skin fibroblasts as previously described22 (Methods), and normalized progerin, lamin A and lamin C mRNA levels were measured 24–48 h after transfection by probe-based droplet digital polymerase chain reaction (ddPCR) (Methods). An effective ASO was defined as eliciting a reduction of progerin mRNA by at least 50% compared to untreated control and no concomitant decrease in lamin C mRNA.
We first tested 97 non-RNase H ASOs targeting ~100 nts around the progerin alternative 5′ splice site (Fig. 1a, Target A: nts 1,743–1,841 of LMNA mRNA; Supplementary Table 2). Remarkably, only five of 97 ASOs were effective in reducing progerin mRNA by >50%, whereas 27 ASOs resulted in a ≥50% increase in progerin mRNA, similarly to previous observations26 (Fig. 1b). These effects were not due to interference of the residual ASOs in the ddPCR assay as confirmed by semiquantitative RT–PCR assay using distal upstream and downstream primers (Supplementary Fig. 1).
In addition to relatively modest effects on progerin mRNA production, ASOs targeting the progerin alternative 5′ splice site showed poor specificity for reduction of progerin mRNA, and most of them also affected wild-type lamin A and lamin C production (Fig. 2a). This effect is likely due to high sequence homology (6 of 7 bases) with the normal 5′ splice donor site in exon 11 located 150 nts downstream of the progerin 5′ splice site27 (Fig. 1a). In support, in an analysis of a set of 17 non-RNase H, uniform 2′-O-methoxyethyl (MOE) chemistry ASOs targeting the progerin 5′ splice site, none resulted in an exclusive effect on progerin mRNA without affecting lamin A mRNA (Fig. 2a). Nine ASOs reduced only lamin A mRNA, as previously observed12,22,23, and two ASOs (A848 and A849) decreased both progerin and lamin A mRNA (Fig. 2a). The effects of individual ASOs were also difficult to predict based on their target location. For example, for two ASOs (A848 and A849) that reduced both progerin and lamin A mRNA, shifting the target sequence by as few as 2 nts upstream or downstream (ASOs A847 and A850) resulted in a reduction of lamin A mRNA but only a minimal effect (<10% reduction) or no effect on progerin mRNA (Fig. 2a).
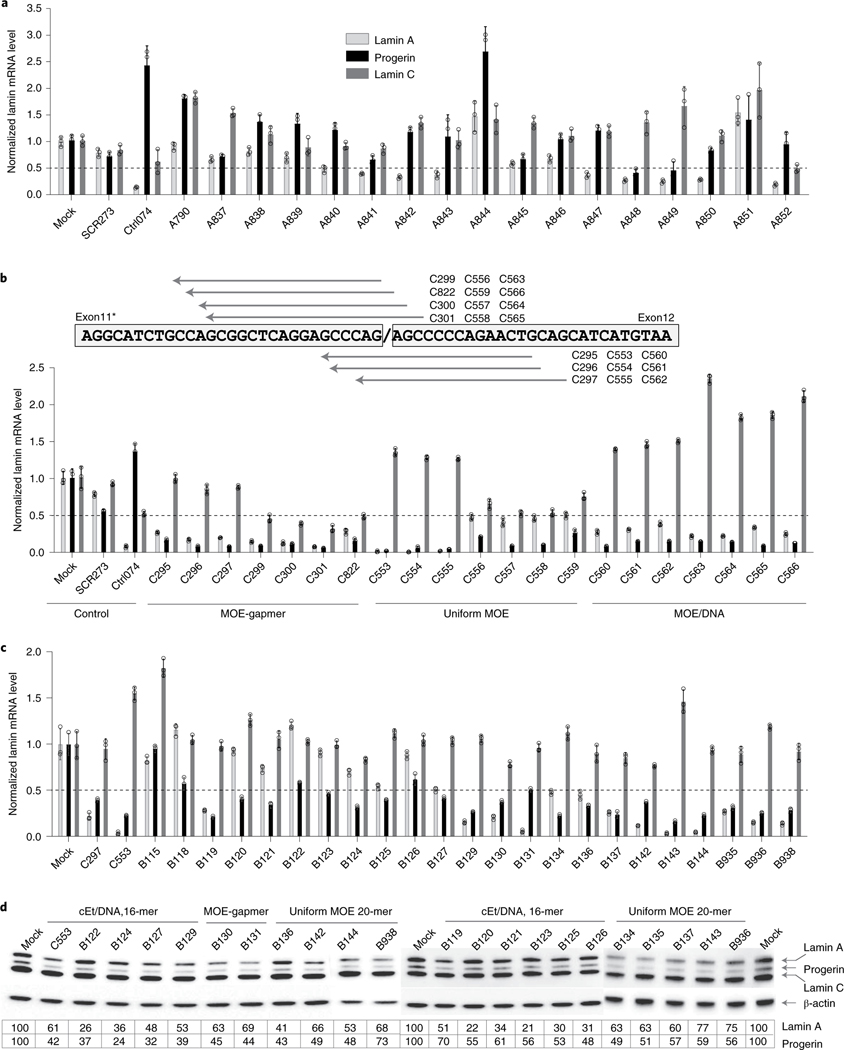
Fig. 2 |. in vitro ASO screen.
a, Assessment of the specificity of ASOs and their effects on progerin, lamin A and lamin C mRNA by ddPCR. ASOs were transfected into immortalized HGPS cells and analyzed 24–48 h after transfection (Methods). The data were normalized to housekeeping gene TBP and expressed as relative to control. Data are mean ± s.d. from three experiments. Data for representative ASOs indicated in Fig. 1., Box A, are shown. b, ASO chemistry significantly affects ASO potency. ASOs with the same sequence were synthesized in three different chemistries (top) and tested in HGPS cells (Methods). Levels of lamin A, progerin and lamin C mRNA were quantified by ddPCR, and the data were normalized to housekeeping gene TBP and expressed as relative to control (bottom). Data are mean ± s.d. from three experiments. c, Elimination of ‘G-quartets’ results in highly effective oligos. Inosine-substituted ASOs were tested in HGPS cells, and levels of human lamin A, progerin and lamin C mRNA levels were quantified by ddPCR (Methods). The data were normalized to housekeeping gene TBP and expressed as relative to control. Data are mean ± s.d. from three experiments. Data for representative ASOs indicated in Fig. 1., Box B, are shown. d, Western blot analysis. Total protein extracts were prepared from HGPS cells transfected with inosine ASOs, and the lamin A isoforms (lamin A, progerin and lamin C) were detected using mouse anti-lamin A/C antibody (Methods). The data are representative of two experiments, are normalized to housekeeping β-actin and are expressed as relative to control.
Next, we screened a set of 71 ASOs against the progerin-specific exon 11–exon 12 mRNA (Fig. 1a, Target C, and Supplementary Table 2), including 57 designed to mediate their effect via RNase H and 14 via non-RNase H (Fig. 1b). Of these, 35 were effective in reducing progerin mRNA by >50%, with 21 acting via RNase H, whereas all 14 non-RNase H ASOs were effective (Fig. 1b). Of the non-RNase H ASOs, most also reduced lamin A mRNA to a considerable extent (>60%) but showed variable effects toward lamin C. RNase H ASOs were generally effective in reducing progerin mRNA, and most of them also reduced lamin A and lamin C mRNA, as expected because these RNAs are generated from the same transcript (Fig. 1a).
We used the above set of ASOs to explore the effects of backbone chemistry on the activity of ASOs. To this end, we tested corresponding sets of ASO containing the same sequences but with a gapmer-2′-O-methoxyethyl (MOE-gapmer) backbone, a uniform MOE or an MOE interrupted with DNA base (MOE/DNA) (Fig. 2b and Supplementary Table 2). Although the latter two sets act via non-RNase H chemistry through steric hinderance, MOE-gapmers act via RNase H-mediated degradation. All three chemistries showed robust effects, leading to 75–90% progerin mRNA reduction (Fig. 2b). However, we found distinct effects of the backbone chemistry on lamin A and lamin C production (Fig. 2b). Uniform MOE ASOs had a tendency to increase lamin A, particularly when targeting the downstream regions (Fig. 2b). In contrast, all ASOs containing MOE chemistry interrupted with DNA base (MOE/DNA) showed a significant increase in lamin C mRNA. These results demonstrate that the effect of ASOs is determined not only by the target sequence but also by the ASO chemistry28.
Finally, we generated a set of 30 ASOs targeting the LMNA intron 11–exon 12 splice junction (Fig. 1, Target B, and Supplementary Table 2). Owing to the presence of G-quartet sequences in exon 12, known to elicit cellular toxicity29, we replaced guanosines with inosines, which serve as universal wobble bases to reduce cytotoxicity30. Inosine ASOs, regardless of the backbone chemistry or ASO length, were well tolerated and highly effective in reducing progerin mRNA. Of the 30 ASOs targeting the LMNA intron 11–exon 12 junction, 22 ASOs were effective in reducing progerin mRNA, five of which showed some reduction (<25%) of lamin A, whereas most ASOs either maintained or elicited an increase in lamin C mRNA (Fig. 2c). Similarly to the sets of ASOs directed toward the other target regions, the effect of the intron 11–exon 12 junction ASOs on progerin mRNA was mirrored at the protein level as determined by western blotting (Fig. 2d).
Based on these observations, we conclude that phosphorothioate-modified ASOs targeting the progerin alternative splice site directly were generally not very effective in reducing progerin levels, consistent with previous studies26, and that RNase H-mediated ASOs were not desirable for therapeutic applications in HGPS because they also reduced levels of the essential lamin C mRNA. The limited effectiveness of ASOs that directly target the progerin splice site in exon 11 might be due, in part, to reduced accessibility of this target site via formation of an RNA hairpin, which includes the progerin splice site and a complementary downstream RNA region (Extended Data Fig. 1)27. In support, mutation of the downstream sequence to open the hairpin enhanced the effectiveness of ASOs targeted to the exon 11 progerin splice site by several fold (Extended Data Fig. 1).
Taken together, screening of a large ASO library indicates that the most effective ASOs to eliminate progerin RNA target the exon 12 splice junction of LMNA, have a phosphorothioate backbone with uniform MOE modification with inosine substitution for guanosine and are of non-RNase H chemistry.
In vivo screening of ASOs.
Although in vitro screening can rapidly assess the effect of a relatively large number of ASOs, cell-based assays are limited in that they do not assess features that are relevant for in vivo application, such as ASO stability, delivery efficiency and tissue targeting. To compare in vitro and in vivo behaviors of ASOs, we tested the ten most effective ASOs from the in vitro screen based on their ability to reduce progerin RNA without reducing lamin C levels (Fig. 3a and Supplementary Table 2) in vivo in an LMNAG/+ HGPS transgenic mouse model, which expresses a human HGPS LMNA transgene and displays several clinical features of human disease31. ASOs were efficiently delivered to all tissues analyzed, and total blood chemistry results indicated that ASO treatment was well tolerated (Supplementary Fig. 2) and did not affect body weight gain compared to mice treated with PBS or ASO targeted to the Malat1 long non-coding RNA as controls (Supplementary Figs. 2 and 3).
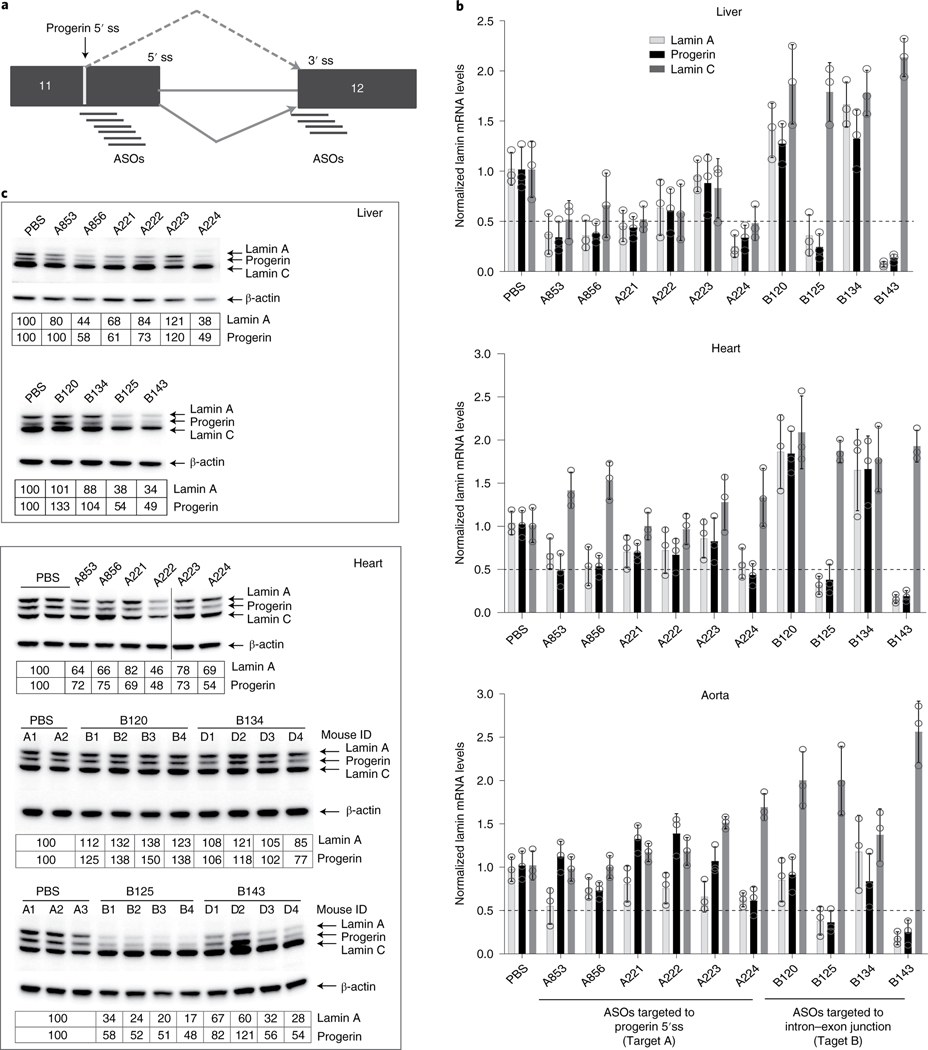
Fig. 3 |. in vivo screen to identify efficient LMNA ASO.
The top ten ASOs from in vitro screen were re-tested in G608G transgenic mice. a, Schematic diagram of alternative progerin 5′ splice site and intron 11–exon 12 splice junction target sites. b, Quantification of lamin A, progerin and lamin C mRNA by ddPCR. LMNAG/+ mice (n = 4 per group) were treated with PBS or LMNA ASOs (50 mg kg−1) as described for 4 weeks (Methods). Total RNA from liver, heart and aorta was isolated, and lamin A, progerin and lamin C mRNAs were measured by ddPCR using human-specific primers and probe (Methods). Data are from at least three mice per group averaged from two separate assays, are normalized to housekeeping gene mTfrc and are presented as mean ± s.d. c, Western blot analysis. Total protein extract from heart and liver was prepared, and the expression of lamin A, progerin and lamin C was measured using anti-lamin A/C antibody (sc376248) (Methods). The data are representative of two experiments, are normalized to housekeeping β-actin and are expressed relative to control (PBS) treatment. ss, splice site.
After 4 weeks of treatment, three ASOs (A224, B125 and B143) showed a robust effect on progerin mRNA in all tissues relative to control mice, leading to 50–80% reduction of both lamin A and progerin mRNA, demonstrating efficient delivery and effectiveness of ASOs in vivo (Fig. 3b). Of the remaining seven ASOs, six reduced progerin mRNA by >50% in liver, four in heart and three in aorta. In addition, consistent with in vitro observations, loss of lamin A mRNA was detected for all ASOs, and every ASO targeting the LMNA intron 11–exon 12 splice junction induced lamin C mRNA in all tissues (Fig. 3b). On the other hand, ASOs targeting the progerin alternative 5′ splice site reduced lamin C mRNA in liver, with no change or a slight increase in lamin C in heart and aorta (Fig. 3b). ddPCR results were validated by semiquantitative RT–PCR using primers located outside of the ASO binding region (Supplementary Fig. 4). ASOs induced >50% reduction of progerin protein after 4 weeks of treatment in liver and up to 40% reduction in heart (Fig. 3c). The effect of ASOs on lamin C protein levels was more variable, with ASOs targeting the progerin alternative 5′ splice site, resulting in no change or a slight reduction in lamin C protein, whereas ASOs targeting the LMNA intron 11–exon 12 splice junction caused an increase in lamin C protein with (Fig. 3c; aorta was not analyzed owing to scarcity of tissue). These results demonstrate that the in vitro behavior of ASOs is generally a good predictor of the in vivo effect on progerin mRNA and protein.
ASO-mediated reduction of progerin mRNA and protein in an HGPS mouse model.
Based on in vivo screening results, ASO B143 targeting LMNA intron 11–exon 12 (Ensembl transcript ID: LMNA-206 ENST00000368300.9, nts 24386–24367) proved the most effective ASO, resulting in progerin mRNA reduction of 83%, 74% and 78% in liver, heart and aorta, respectively (Fig. 3). We, thus, assessed its long-term effect in vivo in a homozygous LMNAG/G transgenic model of HGPS32 (Fig. 4). To enhance cellular uptake and stability, the ASO was conjugated with C16-lipid33,34 to generate L-B143, which was delivered subcutaneously into LMNAG/G transgenic mice at either 17 mg kg−1 or 50 mg kg−1 twice in the first week, followed by weekly dosing. A scrambled control ASO SCR760 (50 mg kg−1) and PBS were used as controls. L-B143 dosage was based on pilot dosing experiments (Supplementary Fig. 5).
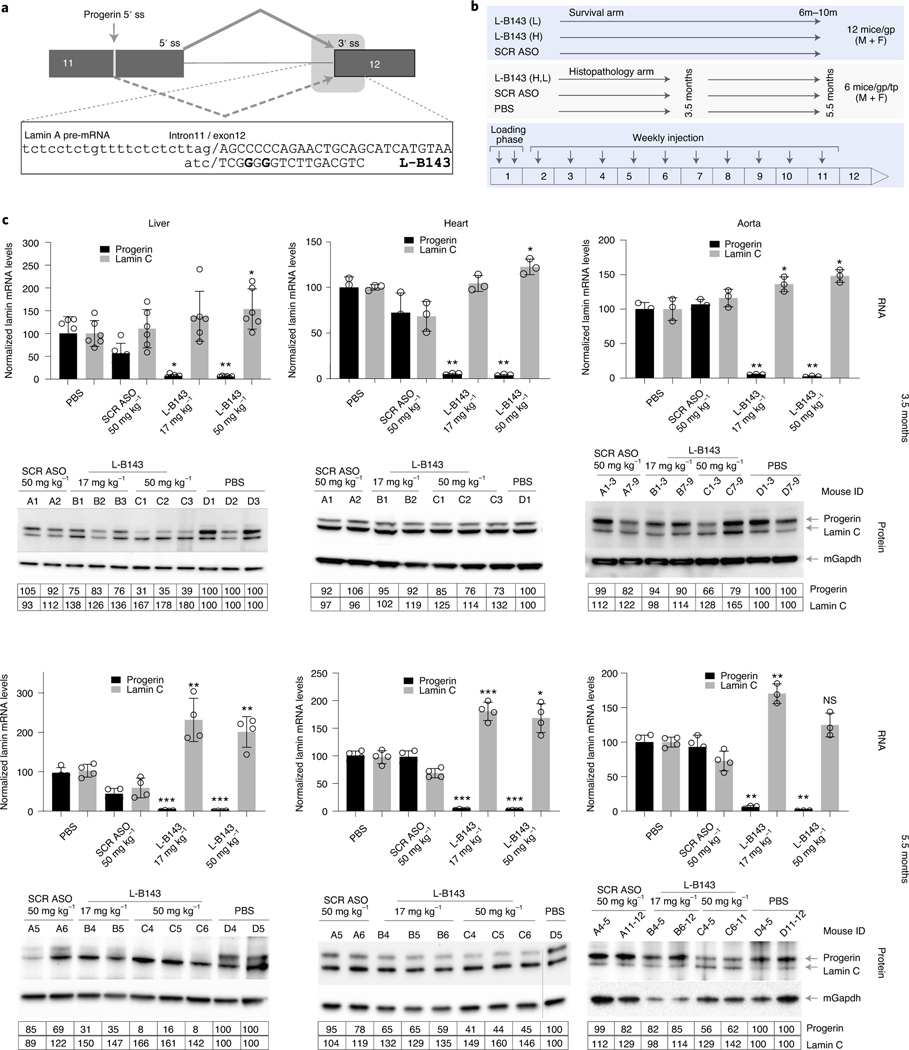
Fig. 4 |. Efficient reduction of progerin mRNA and protein in LMNAG/G mice treated with LMNA ASO.
a, Schematic drawing showing the binding site of L-B143 targeting the intron 11–exon 12 splice junction. Nucleotides in bold indicate that the nucleotides substituted with inosine to minimize ‘G-quartet’-related cellular toxicity. b, Schematic illustration of the animal study design. L, low dose, 17 mg kg−1; H, high dose, 50 mg kg−1; SCR, scrambled ASO760; mice/gp/tp, mice per group per time point; M, male; F, female. c, Efficient reduction of progerin mRNA and protein. LMNAG/G mice (n = 6 animals per group) were treated with PBS, control scrambled ASO SCR760 (50 mg kg−1) or LMNA ASO L-B143 (17 mg kg−1 and 50 mg kg−1) for 3.5 and 5.5 months, respectively (Methods). Top, ddPCR analysis of total RNA from liver, heart and aorta. The data are from 3–6 mice per group averaged from two separate assays, normalized to housekeeping gene mTfrc and presented as mean ± s.d. *P ≤ 0.05, ** P ≤ 0.005 and ***P ≤ 0.0005 indicate P values derived using paired t-test and are indicated above each bar in graph. Bottom, western blot analysis of total protein extract from liver, heart and aorta using human-specific anti-lamin A/C antibody (JOL2, ab40567) to detect progerin and lamin C. The data are normalized to housekeeping mouse GapDH gene (ab8245) and expressed relative to control (PBS) treatment. NS, not significant; ss, splice site.
L-B143 showed a robust effect at the RNA level in all tissues analyzed (liver, heart and aorta), leading to significant reduction of progerin mRNA compared to mice treated with scrambled ASO SCR760 (Fig. 4c). In all tissues, progerin mRNA was reduced by >90% at both doses at 3.5 months of treatment (Fig. 4c), and reduction was maintained until the study endpoint at 5.5 months (Fig. 4c), demonstrating efficient delivery of the ASO. Consistent with in vitro observations, an equal reduction of mouse lamin A mRNA was detected (Supplementary Fig. 6). In addition, as observed in vitro, L-B143 treatment of LMNAG/G mice resulted in a 50%–300% increase of lamin C mRNA compared to scrambled ASO SCR760 or the PBS control (Fig. 4c).
Although progerin mRNA reduction was extensive at all time points and both doses in all tissues analyzed, the extent of progerin protein reduction differed between tissues (Fig. 4c). In liver at 3.5 months of treatment, <25% progerin protein reduction was observed at the 17 mg kg−1 dose compared to 65% reduction at the 50 mg kg−1 dose (Fig. 4c). At 5.5 months, 67% and 91% progerin protein reduction was achieved in liver with the low and high dose of L-B143, respectively. Similarly, in heart, <12% and 37% progerin protein reduction was detected at the lower dose at 3.5 months and 5.5 months, respectively, compared to 25% and 56% reductions with the higher dose at the same time points (Fig. 4c). Similar dose responses occurred in the aorta, and the highest reduction of 41% was detected with the 50 mg kg−1 dose at 5.5 months (Fig. 4c).
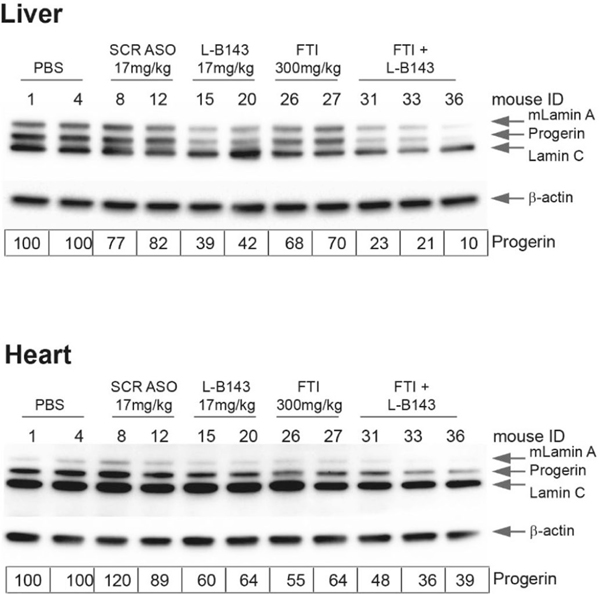
These results demonstrate a discrepancy between the levels of reduction of progerin mRNA and protein. Although part of this effect might be due to higher basal levels of progerin RNA and protein in heart and aorta (Supplementary Fig. 7 and Supplementary Table 3), the persistence of progerin after long-term reduction of progerin mRNA points to a very long half-life of progerin in vivo. To determine whether slow turnover of progerin in vivo contributes to its persistence, LMNAG/G mice were treated for 6.5 months with a combination of L-B143 and the FTI lonafarnib, which prevents the C-terminal farnesylation of progerin and promotes its turnover35. The combination of FTI and L-B143 resulted in more extensive reduction of progerin levels in heart and liver compared to single treatment with either compound (Extended Data Fig. 2). Collectively, these observations strongly indicate that progerin has a long half-life in vivo. These results confirm that ASO treatment is well tolerated and that L-B143 is effective in LMNAG/G mice in reducing progerin mRNA and protein in vivo.
Lifespan extension upon treatment with LMNA ASO L-B143.
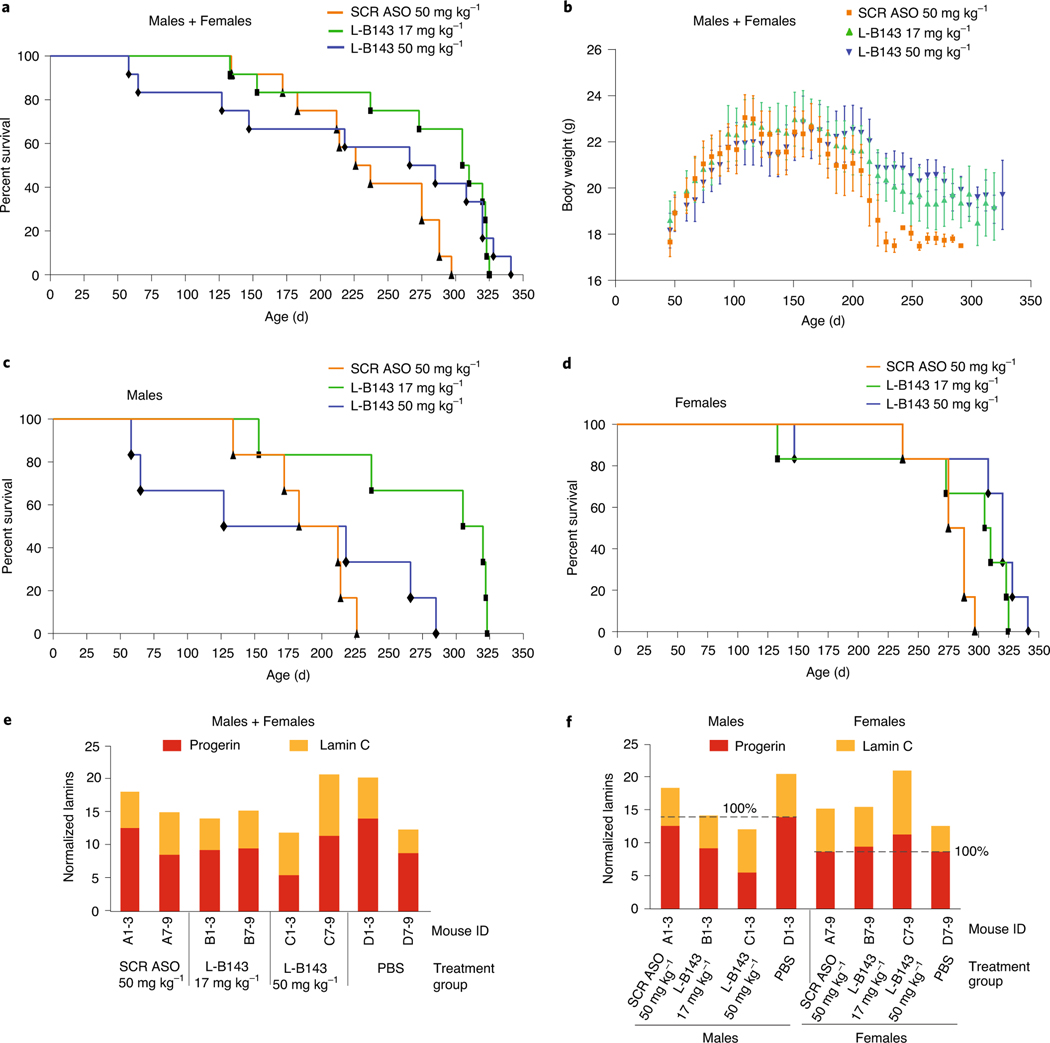
To assess the effect of L-B143 on lifespan, LMNAG/G transgenic mice were treated with L-B143 at 17 mg kg−1 or 50 mg kg−1 or with scrambled ASO SCR760 at 50 mg kg−1 for up to 10 months. L-B143 treatment of LMNAG/G mice produced an improvement in median survival from 232 d for control animals treated with scrambled ASO SCR760 compared to 308 d (33%) and 275 d (19%) at 17 mg kg−1 (P = 0.003) or 50 mg kg−1 (P = 0.13), respectively (Fig. 5a). The increased survival at lower dose implies some toxicity of the L-B143 at higher dose. Concomitant with improvement in lifespan, LMNAG/G mice treated with L-B143 showed an increase in overall body weight as compared to the control group treated with scrambled ASO SCR760 (Fig. 5b; P = 0.0065).
Fig. 5 |. Sex-specific LMNA ASO effect in LMNAG/G mice.
One-month-old LMNAG/G mice (n = 12 per group) were treated with control scrambled ASO SCR760 (50 mg kg−1) or LMNA ASO L-B143 (17 mg kg−1 and 50 mg kg−1) for up to 10 months (Methods). a, Kaplan–Meier survival plots of the entire treatment group (male+female). b, Total body weights of LMNAG/G mice treated with control scrambled ASO SCR760 (50 mg kg−1) or LMNA ASO L-B143 (17 mg kg−1 and 50 mg kg−1) for up to 10 months. Mean values are represented, and error bars indicate s.d. c, d, Kaplan–Meier survival plots based on the sex showing a significant improvement in male lifespan (n = 12) of LMNA ASO L-B143-treated mice compared to females (n = 12). e, f, Protein detection. Total protein extract from pooled aortas treated with PBS, control scrambled ASO SCR760 or LMNA ASO L-B143 (3.5-month time point) and probed using human-specific anti-lamin A/C antibody (JOL2, ab40567). The data were normalized to housekeeping mouse GapDH gene (ab8245). e, Normalized aorta protein data treatment group (males+females) and (f) sex-specific analysis. SCR ASO, scrambled ASO760.
The effect of L-B143 on lifespan was remarkably sex specific, with a substantial enhancement in lifespan of males at the lower dose (increase in mean survival, 26–44%; P = 0.009) compared to a limited lifespan extension in females (increase in mean survival, 10–15%; P = 0.08) (Fig. 5c,d). This difference was not due to sex-specific differences in the basal levels of progerin RNA or protein in untreated mice (Supplementary Table 4) but might be due to the significantly longer lifespan of female control animals compared to males (285 d versus 221 d, 50% survival; Fig. 5c,d). However, the sex-specific effects on survival correlated with the larger extent of reduction of progerin protein level in the aortas of males compared to females, suggesting that sex-specific effects were mediated by progerin (Fig. 5e,f). LMNAG/G males that showed longer survival after treatment with L-B143 produced 34% and 61% less progerin protein at 17 mg kg−1 and 50 mg kg−1, respectively, whereas no or very little progerin protein reduction occurred in females that had shorter survival times. Histopathology analysis demonstrated no adverse effects of ASO treatment on any of the analyzed tissues (Supplementary Fig. 8). The improvement in survival and overall increase in body weights in L-B143-treated LMNAG/G mice were accompanied by a reduction in incidence and severity of progeria-induced hypertrophy of the media in interstitial arteries of the heart after 5.5 months of treatment (Supplementary Fig. 8), but there was no evidence of improvement in HGPS-associated morphological defects in the histology of ascending and descending aortas with Movat and hematoxylin & eosin (H&E) stains (Supplementary Fig. 9), suggesting that the survival effects might be due to non-vascular mechanisms. Taken together, these results identify L-B143 as a promising new candidate agent in HGPS.
Discussion
We used a combined in vitro and in vivo screening approach to identify optimized, therapeutically useful ASOs targeting LMNA pre-mRNA in the premature aging disorder HGPS. The advantage of using combined screening is the ability to evaluate relatively large numbers of ASOs in vitro in cell culture and then narrow down the candidate list for in vivo screening in an HGPS animal model. Most ASOs identified in vitro were active in vivo, suggesting that cell-based screening assays are an effective means to identify candidates for further validation. On the basis of our screening results, we identified several features that are common to effective ASOs directed toward LMNA pre-mRNA. First, the most effective target region in the LMNA pre-mRNA is the exon 12 splice junction rather than the alternative splice site in exon 11 itself, which contains the mutation. One possible explanation might be the nature of the three-dimensional conformation of the progerin pre-mRNA, which shows that the progerin alternative splice site resides in a stem loop formed by extensive pairing with a complementary downstream region, which might limit the accessibility of ASOs to the target site27.
Targeting of progerin RNA by ASOs with high specificity is complicated by two circumstances. First, the LMNA pre-mRNA also gives rise to lamin C mRNA via use of an alternative polyadenylation site in exon 10 upstream of the progerin mutation, and, consequently, RNase H-mediated ASOs reduce all LMNA isoforms, including lamin C, as observed in our experiments. Although lamin A is dispensable in a mouse model, loss of lamin C has negative effects in vivo24, making ASOs that reduce lamin C levels non-ideal for therapeutic pursuit. Second, the wild-type exon 11 splice donor site located 150 nts downstream of the disease-causing mutation is highly homologous (>80%) to the alternative exon 11 splice site created by the progerin mutation. In line with significant homology between these donor sites, we found that most ASOs targeting the alternative 5′ splice site also reduced wild-type lamin A RNA and protein, as reported previously22,23.
Although we were able to identify some features of ASOs that make them more effective in reducing progerin mRNA, precisely predicting the effect of ASOs remains challenging. For example, we found that two ASOs of identical length and backbone chemistry, but shifted in their targeting sequence by as few as 2 nts, showed considerably distinct effects, consistent with other published examples of ASO screens36,37. We also found that the chemistry of ASOs alters their behavior, as demonstrated by our observation that ASOs with distinct chemistries targeting the same sequence showed different effects. The influence of ASO chemistry might also explain our finding that the most effective ASOs in our libraries targeted the intron 11–exon 12 splice site rather than the mutated progerin splice site in exon 11. This is in contrast to earlier studies22,23, including from us, and the accompanying study38, which shows progerin suppression in vivo by targeting the mutated progerin splice site. These studies used morpholino-based chemistry, whereas our set of ASOs is based on phosphorothioate or phosphodiester linkages in an attempt to circumvent the generally poor delivery of morpholinos in patients. The effect of ASO chemistry is likely due to differential recruitment of RNA-binding proteins to the targeted RNA or the displacement of RNA-binding proteins from the target by the bound ASO28. These findings further highlight that unbiased screening approaches have value to identify optimized, therapeutically active ASOs.
To validate our screening approach, we tested the lead candidate ASO L-B143 targeting LMNA intron 11–exon 12 in a homozygous HGPS animal model31. We found efficient reduction of progerin mRNA in several tissues, resulting in extension of lifespan. Despite the beneficial effects on lifespan, we did not see any overt correction of vascular morphology, which is a hallmark of HGPS. It is possible that beneficial survival effects can be generated without full morphological recovery of the vascular defects or that the observed prolongation of lifespan occurred via non-vascular effects.
Our experiments also provided insight into the in vivo behavior of the progerin protein. Remarkably, despite robust loss of progerin mRNA in all tissues analyzed upon ASO treatment, we found only partial reduction and considerable variability in the extent of progerin protein removal among different tissues. For example, although the mRNA levels were reduced by more than 90% in all tissues, we found only a 20–30% reduction of progerin protein in heart and aorta after 3.5 months of treatment at low dose of ASO but more than a 60% reduction of progerin protein in liver. These effects are not due to limited delivery of the ASO, because progerin mRNA was effectively reduced in all tissues. The persistence of progerin protein despite robust reduction of its mRNA might suggest that the progerin protein has a long half-life in vivo. In support, we demonstrate that combination treatment of ASO and the FTI lonafarnib, which promotes progerin turnover, results in significant reduction of progerin protein in vivo. Our observation of a prolonged half-life of progerin in vivo is in line with the existence of very long-lived proteins in the nucleus, including proteins of the nuclear pore complex39,40. Previous measurements of the half-life of lamin A wild type in cultured cells have suggested half-lives on the order of ~7 d41, although it is not clear how well cultured cells reflect the behavior of progerin in vivo, and our findings suggest that the half-lives of lamin A and progerin are considerably longer in vivo. An alternative, and not mutually exclusive, possibility is that the persistent presence of progerin in tissues is due to elevated protein translation in HGPS cells, as previously reported41. It will be imperative to measure progerin turnover in vivo to assess the potential of therapeutic strategies for HGPS that target progerin mRNA or protein.
In this study, we used a systematic approach to discover therapeutically relevant ASOs for HGPS. We describe several key features of effective ASOs in HGPS, and our efforts led to the identification of a novel, high-priority candidate ASO for therapeutic development for HGPS. Our observation of additive effects of ASOs in combination with an FTI points to considerable therapeutic potential of ASO combination therapy with compounds, such as FTIs or everolimus, that are currently used for patients with HGPS5,42. Finally, our results provide novel insights into the biology of HGPS and of lamin proteins. In particular, our finding of an apparently long half-life of progerin will be important for the understanding of its in vivo behavior and the development of effective therapeutic agents against HGPS.
Online content
Any methods, additional references, Nature Research reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at https://doi.org/10.1038/s41591–021-01262–4.
Methods
Cell culture and transfection.
Human immortalized HGPS skin fibroblasts (Coriell Cell Repository, AG06297-hTert) were cultured in minimum essential medium (Life Technologies) supplemented with 15% heat-inactivated FBS (Hyclone), 2 mM L-glutamine and 50 U ml−1 of penicillin–streptomycin (Invitrogen) at 37 °C in 5% CO2. Medium was changed every other day, and the cells were passaged every 3–4 d.
ASOs were designed and synthesized by Ionis Pharmaceuticals. For details of ASO backbone chemistries and lengths evaluated, see Supplementary Tables 1 and 2. Transfections were performed using Lipofectamine 2000 transfection reagent (Thermo Fisher Scientific) per manufacturer instructions. Briefly, ~1.0 × 105 cells were plated per well in six-well plates 24 h before transfection. ASOs (50–100 nM) were mixed with Lipofectamine 2000 (10 μl/ml) in Opti-MEM medium and incubated per manufacturer recommendations, and cells were transfected and, in some experiments, again at 48 h. After 5 h, the transfection medium was replaced with fresh complete medium, and cells were harvested between 24 and 48 h for RNA and protein analysis.
RNA isolation, complementary DNA synthesis and ddPCR.
Total RNA from fibroblasts and/or from flash-frozen tissues was isolated using RNeasy Plus Mini Kit (Qiagen), and the integrity of the RNA was assessed by Bioanalyzer using RNA 6000 Nano Chip (Agilent). RNA was reverse transcribed using iScript cDNA Synthesis Kit containing a blend of oligo(dT) and random hexamer primers and iScript Reverse Transcriptase according to manufacturer recommendation (Bio-Rad).
Master mix (22 μl) containing the primers (900 nM) and probe (250 nM) for the gene of interest (labeled with FAM) and housekeeping gene (labeled with HEX), template (variable concentration), deoxyuridine triphosphates and 2× ddPCR Supermix were prepared according to manufacturer guidelines (Bio-Rad). Droplets were generated from the reaction mixture using a QX200 Auto Droplet Generator, and the droplet mixture was then amplified using the following PCR cycling protocol: 95 °C enzyme activation step for 5 min followed by 40 cycles of a two-step cycling protocol (95 °C for 30 s and 59 °C for 1 min), and the final enzyme deactivation step and signal stabilization steps were performed at 98 °C for 10 min and 4 °C, respectively, in accordance with manufacturer instructions (Bio-Rad). The amplification signal was measured using a QX200 Droplet Reader, and data analysis was performed using QuantaSoft software version 1.7.4 (Bio-Rad). After user determination of negative signal baseline, the relative quantity (raw transcript count per microliter) was calculated, and the data were normalized to the TFRC or TBP housekeeping gene using the PrimePCR ddPCR Expression Probe Assay (Bio-Rad). The data are reported as relative to the control.
ddPCR primer–probe pairs (F-forward, P-probe and R-reverse).
Human progerin. hLMNA-RTspecM3F: 5′-GCGTCAGGAGCCCTGAGC-3′hLMNA-RTspec12R: 5′-GACGCAGGAAGCCTCCAC-3′ hLMNA-Probe: 5′-AGCATCATGTAATCTGGGACC-3′
Human lamin A. hLMNAwt_F: 5′-CAGCTTCGGGGACAATCTG-3′ hLMNAwt_R: 5′-GGCATGAGGTGAGGAGGAC-3′ hLMNAwt_P: 5′-GTCACCCGCTCCTACCTCCT-3′
Human lamin C. hlamin9F1: 5′-ACGGCTCTCATCAACTCCAC-3′ hlamin10R: 5′-GCGGCGGCTACCACTCAC-3′ hlaminC-P: 5′-GGTTGAGGACGACGAGGATG-3′
Human semiquantitative RT–PCR primers. hLMNAwt 103_F: 5′-GTGGTTGAGGACGACGAGGAT-3′ hLMNAwt 101_R: 5′-GAGGAGGACGCAGGAAGCCTCCA-3′
Mouse lamin A. mlamin A wt_F1: 5′-ATCCATCTCCTCTGGCTCTT-3′ mlamin A wt_R1: 5′-ACATGATGCTGCAGTTCTGG-3′ mlamin A wt_P1: 5′-TCCAGTCCCCGGAGCCAGAGCT-3′
Mouse lamin C. mlamin C_F1: 5′-ACCGCTCTCATCAACTCCAC-3′ mlamin C_R1: 5′-GCGGCGGCTGCCACTCAC-3′ mlamin C_P1: 5′-GGTTGAGGACAATGAGGATG-3′
Western blotting.
Whole-cell extracts from HGPS fibroblasts were prepared by solubilizing cells in lysis buffer. Briefly, cells were rinsed 1× with ice-cold PBS, and ~100,000 cells were lysed in 100 μl of 2× Laemmli Sample Buffer containing 5% β-mercaptoethanol (Bio-Rad). Total protein extracts from liver, heart and aorta were prepared using either urea buffer (4 M urea buffer containing 10 mM Tris-HCl, pH 7.4; 1% Triton X-100, 300 mM KCl and Protease Inhibitor Cocktail Set III; Roche) or SDS buffer (100 mM Tris-HCl, pH 7.4; 50 mM EDTA, 2% SDS and Protease Inhibitor Cocktail Set III; Roche). Approximately 25–50 mg of ground tissue in 300 μl of buffer was homogenized using Bullet Blender, 0.9–2.0-mm stainless steel beads for 3 min, setting 12, at 4 °C. The homogenate was incubated on ice for 5 min, followed by centrifugation at 3,000 r.p.m. for 5 min. The supernatant was transferred into a clean 1.5-ml tube and treated with Benzonase at 37 °C for 15 min. The protein extract was heated at 95 °C for 5 min and centrifuged at 12,000 r.p.m. for 10 min at 4 °C, and the supernatant was transferred to a fresh 1.5-ml tube. Protein concentration was determined using Pierce BCA Protein Assay Kit (Thermo Fisher Scientific), mixed with 4× Laemmli sample buffer for gel analysis. Equal amounts of protein extract from cells and/or tissue extracts were separated on 4%–12% gradient SDS-PAGE gels (Invitrogen) and transferred onto Immobilon-P 0.45-μm membranes (Millipore). The membrane was blocked with 5% BSA or 5% milk, incubated overnight at 4 °C with mouse monoclonal anti-lamin A/C (sc-376248, Santa Cruz Biotechnology, or ab40567, Abcam) or mouse-anti-β-actin (A2228, Sigma-Aldrich) or mouse-anti-GapDH (ab8245) primary antibody, washed 3× with TBST (Tris-buffer saline, 0.25% Tween-20), incubated for 1 h with horseradish peroxidase-conjugated secondary antibody (rabbit-anti-mouse, ab97046) and again washed 3× with TBST. Proteins were detected using the ECL system (GE Healthcare) and imaged using a Bio-Rad ChemiDoc imaging system and ImageLab 6.0.1 software. The data were normalized to β-actin or GapDH and expressed as relative to control.
Animal experiments.
This study was carried out according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The mice were fed a chow diet and housed in a virus-free barrier facility with a 12-h light/dark cycle. Body weights of LMNAG/G transgenic mice were measured weekly. Complete pathological examination included liver, heart and aorta. In addition, the tolerability of the ASOs was assessed by running a full plasma chemistry panel, including bilirubin, total cholesterol, triglycerides, blood urea nitrogen, alanine aminotransferase and aspartate aminotransferase.
HGPS LMNAG/G or LMNAG/+ transgenic mice expressing human progerin31,32 were injected subcutaneously with L-B143 (17 mg kg−1 or 50 mg kg−1), scrambled control ASO SCR760 (50 mg kg−1) or PBS beginning at 5–6 weeks of age. ASOs were conjugated to C16-lipid to enhance uptake, as previously described33,34. ASOs were dissolved in PBS and injected with a loading dose twice during the first week and then continued with a weekly dosing in alternated flanks. The mice were sacrificed at the indicated time points, and tissues were collected for analysis. The effects of ASOs at the RNA level were quantified by ddPCR, and the effects on progerin protein were determined by western blotting, as described above. For histopathology, the aortas were dissected, and the aortic arch was processed for western blotting, and a segment of the ascending aorta was collected for paraffin embedding and H&E staining31.
Histochemistry and vessel scoring analysis.
Tissues were fixed in 2% paraformaldehyde and embedded in paraffin. Cross-sections were stained with H&E and Movat pentachrome using standard procedure. Images were captured using a Hamamatsu NanoZoomer S60. Analysis of the ascending and descending aorta sections were combined, and measurements were separated by sex. The inner and outer adventitia thickness was measured, and the number of elastic layers was counted. Movat images were scored using semiquantitative assessment (score 1–5) measuring the proteoglycan, collagen and smooth muscle content, with 1 indicating no pathology and 5 indicating severe pathology.
Reporting Summary.
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Extended Data
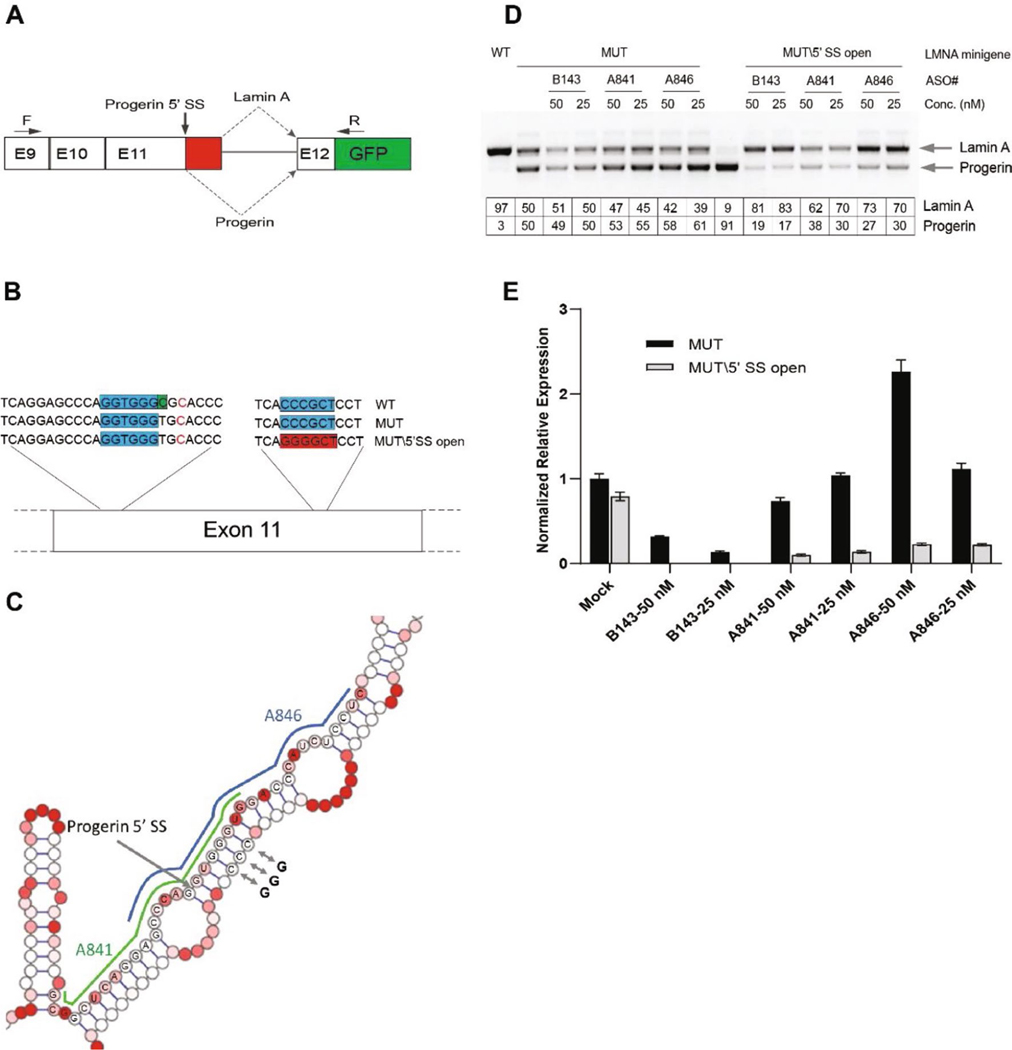
Extended Data Fig. 1 |. Enhanced effect of progerin-targeted ASOs upon relaxation of inhibitory downstream RNA structure.
A, Schematic representation of the LMNA-GFP minigene reporter used to study the effect of RNA structure on alternative splicing as previously described27. The minigene consist of LMNA exons 9–12 and intron 11/exon12. Splicing events are represented by dotted lines. Forward primer F and reverse primer R are, indicated by arrows, allow for amplification of only the minigene RNA, not endogenous LMNA RNA. B, Exon 11 contains a potential dimerization region between the 5’ SS region and downstream sequence. Blue boxes indicate double strand complex region. Green Box indicates the progerin point mutation. Red box indicates tri-nucleotide C-G mutations which disrupt dimerization region and opens up the region in the MUT\5’SS-Open minigene. C, SHAPE-MaP diagram of LMNA (HGPS mutation) indicating the target location of ASOs A841 and A846 (green, blue lines, respectively). The progerin 5’ splice site (SS) is indicated by an arrow. The position of the C>G mutations in the MUT\5’ SS-Open minigene are indicated by double arrows. D, RT-PCR analysis for LMNA and progerin expression. 293T cells were transfected with 500 ng of the indicated mini-gene plasmids and 24 h later with the indicated ASOs. RNA was isolated at 48 h post the later transfection. Table show quantification of mRNA isoforms expressed as relative usage of the normal vs. alternative 5′ SS. E, Quantitative PCR results for LMNA and progerin expression levels for the same samples tested in (D). Expression levels are normalized to expression of TBP. Data are expressed as means ± SEM. For details of constructs and methods see ref. 27.
Extended Data Fig. 2 |. Combination treatment of LMNA-specific ASO and lonafarnib (FTi).
LMNAG/G mice (n = 6–8 per group) were treated with PBS, control scrambled ASO SCR760 (50 mg/kg) or LMNA specific ASO L-B143 (17 mg/kg) for 6.5 months. ASOs were administered as described in Methods. Mice treated with lonafarnib alone or combination treatment were singly housed to accurately measure food consumption. Lonafarnib (300 mg/kg) was mixed with transgenic dough (BioServ, Inc.,) and was orally administered. 5 g (once a day) or 10 g (once in 2 days) of transgenic dough was fed to each mouse and the leftover dough was recorded to asses food consumption. Animals were started on the diet and drugs at approximately 1 month of age. Western blot analysis of total protein extract from liver (top panel), and heart (bottom panel) using anti-Lamin A/C monoclonal antibody (sc376248) to detect lamin A and progerin. The data was normalized to housekeeping mouse β-actin and expressed relative to control (PBS) treatment. Values represent individual animals.
Supplementary Material
Acknowledgements
We thank M. Erdos and F. Collins for providing HGPS mice and U. Tavarez for technical help. Lonafarnib was provided by Merck through The Progeria Research Foundation’s Pre-Clinical Drug Supply Program. Research in the Misteli lab was supported by funding from the Intramural Research Program of the National Institutes of Health, National Cancer Institute and Center for Cancer Research (1-ZIA-BC010309) and the Progeria Research Foundation (grants PRF 2012–40, 2012–45 and 2016–66). L.G. was funded by the Progeria Research Foundation.
Footnotes
Competing interests
M.P., A.S., T.M. and L.G. declare no competing interests. C.F.B., F.R., K.S. and M.J. are employees of Ionis Pharmaceuticals and hold stock in the company.
Additional information
Extended data is available for this paper at https://doi.org/10.1038/s41591-021-01262-4.
Supplementary information The online version contains supplementary material available at https://doi.org/10.1038/s41591-021-01262-4.
Peer review information Nature Medicine thanks Thomas Glover and the other, anonymous, reviewer for their contribution to the peer review of this work. Joao Monteiro was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Reprints and permissions information is available at www.nature.com/reprints.
Data availability
No large-scale, minable datasets were generated in this study. Accession codes for genes are indicated. Primary data are available from the authors upon reasonable request. Source data are provided with this paper.
References
- 1.Gordon LB, Rothman FG, Lopez-Otin C. & Misteli T. Progeria: a paradigm for translational medicine. Cell 156, 400–407 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ullrich NJ & Gordon LB Hutchinson–Gilford progeria syndrome. Handb. Clin. Neurol 132, 249–264 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Eriksson M. et al. Recurrent de novo point mutations in lamin A cause Hutchinson–Gilford progeria syndrome. Nature 423, 293–298 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Sandre-Giovannoli A. et al. Lamin a truncation in Hutchinson–Gilford progeria. Science 300, 2055 (2003). [DOI] [PubMed] [Google Scholar]
- 5.Gordon LB et al. Clinical trial of a farnesyltransferase inhibitor in children with Hutchinson–Gilford progeria syndrome. Proc. Natl Acad. Sci. USA 109, 16666–16671 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gordon LB et al. Association of lonafarnib treatment vs no treatment with mortality rate in patients with Hutchinson–Gilford progeria syndrome. JAMA 319, 1687–1695 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DuBose AJ et al. Everolimus rescues multiple cellular defects in laminopathy-patient fibroblasts. Proc. Natl Acad. Sci. USA 115, 4206–4211 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pellegrini C. et al. All-trans retinoic acid and rapamycin normalize Hutchinson–Gilford progeria fibroblast phenotype. Oncotarget 6, 29914–29928 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lo Cicero A. et al. A high throughput phenotypic screening reveals compounds that counteract premature osteogenic differentiation of HGPS iPS-derived mesenchymal stem cells. Sci. Rep 6, 34798 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kubben N, Brimacombe KR, Donegan M, Li Z. & Misteli T. A high-content imaging-based screening pipeline for the systematic identification of anti-progeroid compounds. Methods 96, 46–58 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kreienkamp R. et al. Vitamin D receptor signaling improves Hutchinson–Gilford progeria syndrome cellular phenotypes. Oncotarget 7, 30018–30031 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee SJ et al. Interruption of progerin–lamin A/C binding ameliorates Hutchinson–Gilford progeria syndrome phenotype. J. Clin. Invest 126, 3879–3893 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiong ZM et al. Anti-aging potentials of methylene blue for human skin longevity. Sci. Rep 7, 2475 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guilbert SM, Cardoso D, Levy N, Muchir A. & Nissan X. Hutchinson–Gilford progeria syndrome: rejuvenating old drugs to fight accelerated ageing. Methods 10.1016/j.ymeth.2020.04.005 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Gabriel D, Roedl D, Gordon LB & Djabali K. Sulforaphane enhances progerin clearance in Hutchinson–Gilford progeria fibroblasts. Aging Cell 14, 78–91 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blondel S. et al. Drug screening on Hutchinson–Gilford progeria pluripotent stem cells reveals aminopyrimidines as new modulators of farnesylation. Cell Death Dis. 7, e2105 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larrieu D, Britton S, Demir M, Rodriguez R. & Jackson SP Chemical inhibition of NAT10 corrects defects of laminopathic cells. Science 344, 527–532 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clements CS et al. Presence and distribution of progerin in HGPS cells is ameliorated by drugs that impact on the mevalonate and mTOR pathways. Biogerontology 20, 337–358 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mann CJ et al. Antisense-induced exon skipping and synthesis of dystrophin in the mdx mouse. Proc. Natl Acad. Sci. USA 98, 42–47 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Passini MA et al. Antisense oligonucleotides delivered to the mouse CNS ameliorate symptoms of severe spinal muscular atrophy. Sci. Transl. Med 3, 72ra18 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bennett CF Therapeutic antisense oligonucleotides are coming of age. Annu. Rev. Med 70, 307–321 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Scaffidi P. & Misteli T. Reversal of the cellular phenotype in the premature aging disease Hutchinson–Gilford progeria syndrome. Nat. Med 11, 440–445 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osorio FG et al. Splicing-directed therapy in a new mouse model of human accelerated aging. Sci. Transl. Med 3, 106ra107 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Fong LG et al. Prelamin A and lamin A appear to be dispensable in the nuclear lamina. J. Clin. Invest 116, 743–752 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JM et al. Modulation of LMNA splicing as a strategy to treat prelamin A diseases. J. Clin. Invest 126, 1592–1602 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fong LG et al. Activating the synthesis of progerin, the mutant prelamin A in Hutchinson–Gilford progeria syndrome, with antisense oligonucleotides. Hum. Mol. Genet 18, 2462–2471 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shilo A, Tosto FA, Rausch JW, Le Grice SFJ & Misteli T. Interplay of primary sequence, position and secondary RNA structure determines alternative splicing of LMNA in a pre-mature aging syndrome. Nucleic Acids Res. 47, 5922–5935 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rigo F. et al. Synthetic oligonucleotides recruit ILF2/3 to RNA transcripts to modulate splicing. Nat. Chem. Biol 8, 555–561 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goodchild A. et al. Cytotoxic G-rich oligodeoxynucleotides: putative protein targets and required sequence motif. Nucleic Acids Res. 35, 4562–4572 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin FH, Castro MM, Aboul-ela F. & Tinoco I Jr. Base pairing involving deoxyinosine: implications for probe design. Nucleic Acids Res. 13, 8927–8938 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varga R. et al. Progressive vascular smooth muscle cell defects in a mouse model of Hutchinson–Gilford progeria syndrome. Proc. Natl Acad. Sci. USA 103, 3250–3255 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cubria MB et al. Evaluation of musculoskeletal phenotype of the G608G progeria mouse model with lonafarnib, pravastatin, and zoledronic acid as treatment groups. Proc. Natl Acad. Sci. USA 117, 12029–12040 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chappell AE et al. Mechanisms of palmitic acid-conjugated antisense oligonucleotide distribution in mice. Nucleic Acids Res. 48, 4382–4395 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ostergaard ME et al. Conjugation of hydrophobic moieties enhances potency of antisense oligonucleotides in the muscle of rodents and non-human primates. Nucleic Acids Res. 47, 6045–6058 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gabriel D, Shafry DD, Gordon LB & Djabali K. Intermittent treatment with farnesyltransferase inhibitor and sulforaphane improves cellular homeostasis in Hutchinson–Gilford progeria fibroblasts. Oncotarget 8, 64809–64826 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hua Y, Vickers TA, Baker BF, Bennett CF & Krainer AR Enhancement of SMN2 exon 7 inclusion by antisense oligonucleotides targeting the exon. PLoS Biol. 5, e73 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skotte NH et al. Allele-specific suppression of mutant huntingtin using antisense oligonucleotides: providing a therapeutic option for all Huntington disease patients. PLoS ONE 9, e107434 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Erdos MR et al. A targeted antisense therapeutic approach for Hutchinson-Gilford progeria syndrome. Nat. Med 10.1038/s41591-021-01274-0 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toyama BH & Hetzer MW Protein homeostasis: live long, won’t prosper. Nat. Rev. Mol. Cell Biol 14, 55–61 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toyama BH et al. Identification of long-lived proteins reveals exceptional stability of essential cellular structures. Cell 154, 971–982 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buchwalter A. & Hetzer MW Nucleolar expansion and elevated protein translation in premature aging. Nat. Commun 8, 328 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gordon LB, Kieran MW, Kleinman ME & Misteli T. The decision-making process and criteria in selecting candidate drugs for progeria clinical trials. EMBO Mol. Med 8, 685–687 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No large-scale, minable datasets were generated in this study. Accession codes for genes are indicated. Primary data are available from the authors upon reasonable request. Source data are provided with this paper.