Abstract
Pre-implantation embryo movement is crucial to pregnancy success, but the role of ovarian hormones in modulating embryo movement is not understood. We ascertain the effects of altered hormonal environment on embryo location using two delayed implantation mouse models: natural lactational diapause (ND); and artificially induced diapause (AD), a laboratory version of ND generated by ovary removal and provision of supplemental progesterone (P4). Previously, we showed that embryos in a natural pregnancy (NP) first display unidirectional clustered movement, followed by bidirectional scattering and spacing movement. In the ND model, we discovered that embryos are present as clusters near the oviductal–uterine junction for ∼24 h longer than NP, followed by locations consistent with a unidirectional scattering and spacing movement. Intriguingly, the AD model resembles embryo location in NP and not ND. When measuring serum hormone levels, unlike the popular paradigm of reduced estrogen (E2) levels in diapause, we observed that E2 levels are comparable across NP, ND and AD. P4 levels are reduced in ND and highly increased in AD when compared to NP. Further, exogenous administration of E2 or P4 modifies embryo location during the unidirectional phase, while E2 treatment also affects embryo location in the bidirectional phase. Taken together, our data suggest that embryo movement can be modulated by both P4 and E2. Understanding natural hormonal adaptation in diapause provides an opportunity to determine key players that regulate embryo location, thus impacting implantation success. This knowledge can be leveraged to understand pregnancy survival and implantation success in hormonally altered conditions in the clinic.
Keywords: embryo movement, ovarian hormones, estrogen, progesterone, diapause, implantation, lactational delay of implantation, induced delay of implantation
Introduction
Ovarian hormones, estrogen (E2) and progesterone (P4), play essential roles in modulating the uterus for embryo attachment during early pregnancy (Psychoyos, 1973; McCormack and Greenwald, 1974; Wang and Dey, 2006; Cha and Dey, 2014). While hormonal control of receptivity has been studied in depth, details on hormonal regulation of uterine embryo transport are sparse. This embryo movement through the uterus is essential for pregnancy success in mammals such as mice, rats, rabbits, cats, dogs, pigs and horses (Sittmann, 1973; Dziuk, 1985; Leith and Ginther, 1985; McDowell et al., 1988; Tsutsui et al., 1989, 2002). In small multiparous mammals (mice, rats and rabbits), embryo movement is important for even embryo distribution and is critical to avoid competition for maternal resources. Furthermore, in larger animals, such as pigs (Dziuk, 1985) and uniparous horses (Leith and Ginther, 1985), restricting embryo mobility by ligating the uterus results in pregnancy loss, suggesting that embryo movement along the uterine lining is essential for pregnancy success regardless of multiple embryo competition.
Hormonal regulation of egg transport suggests an influence of both E2 and P4 in regulating contractions and tubal (oviductal) transport (Bylander et al., 2015). In the mouse oviduct, treatment with a P4 antagonist halts tubal egg movement, although it seems the importance lies in the presence of P4 rather than the absolute levels of P4 (Kendle and Lee, 1980). In the mare, prostaglandin (PG) F2α treatment disrupts the corpus luteum and thus the luteal P4, which then abrogates embryo mobility (Kastelic et al., 1987). In turn, embryo migration appears to prevent luteolysis (Evans and Ganjam, 2011) thus maintaining corpus luteum P4 production and this is necessary for continuation of pregnancy in equids (Aurich and Budik, 2015). In the rabbit, P4 plays a role in embryo movement by toning the muscle contractions (Boving, 1956). Uterine embryo movement in response to E2 has not been investigated extensively, likely because levels of E2 are high during ovulation but basal or low during uterine embryo movement (Ma et al., 2003). An assessment of hormonal regulation of embryo movement is necessary to provide insights into how altered hormonal profiles in clinical scenarios such as hyperstimulation in ART (Gidley-Baird et al., 1986) or increased E2 in women with polycystic ovary syndrome (van Houten and Visser, 2014) result in less optimal implantation with an unsuccessful pregnancy.
Naturally existing alterations in hormonal conditions represent evolutionarily selected regimens that support pregnancy success despite variation in hormone levels. One such example in mammals is diapause. Diapause is a delay in implantation, induced in response to stress that leads to modification of embryo growth and the uterine environment to pause pregnancy and prevent implantation until conditions are more optimal for survival of the young at birth (Mead, 1993; Cha and Dey, 2014; Fenelon et al., 2014). While there has been extensive research on embryo attachment and uterine receptivity, and some evaluation of hormonal changes in response to diapause, if and how diapause conditions affect embryo location in the uterus remains understudied. Two types of diapause occur naturally: obligate and facultative diapause. In obligate diapause, which occurs in minks, skunks, bears and some wallabies, every pregnancy is paused to align the birth of the offspring with a favorable season for survival (Lopes et al., 2004). In both minks and skunks, compared to post-implantation gestation, P4 levels are reduced during diapause (Mead, 1981; Douglas et al., 1998). Facultative diapause, on the other hand, is induced by external factors, such as metabolic stress, access to resources or lactation and occurs in rodents (rats and mice) and marsupials (wallabies and kangaroos) (Lopes et al., 2004). In lactational diapause, referred to here as natural diapause (ND), lactation stimulated by the first litter regulates ovarian hormone levels to delay embryo attachment. Mechanistically, in the Tammar wallaby, suckling young cause prolactin secretion, which suppresses the activity of the corpus luteum, causing low levels of P4. When the suckling pouch with the pups is removed, prolactin levels decrease, so corpus luteum and blastocyst activity resume (Renfree and Shaw, 2014). Female mice enter post-partum estrus upon delivering the first litter, and this rise in E2 leads to a mating event causing lactational diapause. Unlike the mink, skunk and wallaby, lactating mice are thought to have an active corpus luteum (Whitten, 1958), and it is proposed that continued P4 production from the corpus luteum (in the absence of E2) maintains the pregnancy in a paused state while preventing embryo implantation (Mead, 1993). Further, there have been studies where a single injection of E2 induces implantation in lactating mice, and this has led to a supposition that P4 levels are normal and E2 levels are lower in the ND model of pregnancy (McLaren, 1968). However, ovarian weights of lactating mice are lower than nonlactating mice (Whitten, 1955), indicating that although the corpora lutea are active, P4 production could be affected in mice undergoing lactational diapause. In support of this idea, exogenous P4 injections in lactating mice or rats, similar to E2, can induce implantation (Yoshinaga, 1961; McLaren, 1971). However, the levels of E2 and P4, and their effects (if any) on the trajectory of embryo movement, have not been systematically assessed in the ND mouse model of pregnancy.
Diapause is an important model for understanding how the embryo and the uterine environment are modulated to naturally delay pregnancy in the mouse but generating this model in the laboratory is time consuming and costly. Thus, an induced delay model is more commonly used to study diapause in the laboratory setting. In this model, referred to here as artificial diapause (AD), the ovaries are surgically removed during the pre-implantation period to avoid the E2 surge, followed by P4 injections to keep the uterus in the pre-receptive state and to allow embryo survival. It is postulated that the lack of ovaries, which is the source of nidatory E2, prevents embryo attachment (Yoshinaga and Adams, 1966; McLaren, 1971). The embryos survive in the uterus for several days, and potentially weeks, as long as P4 is present, and implantation can be induced by a single dose of E2 (Cha and Dey, 2014). Although E2 is known to induce attachment, there is additional evidence that if P4 is injected at the same time as removal of the ovary in the AD model, implantation can occur in the absence of E2 (Yoshinaga and Adams, 1966; McLaren, 1971).
Although the AD model is presumed to match the ND model, the two diapause models have not been compared for serum levels of E2 and P4 or for embryo location during the pause period. We recently demonstrated that in a natural virgin mouse pregnancy (NP), the embryos display three phases of movement: embryo entry, unidirectional clustered movement and bidirectional scattering and spacing movement (Flores et al., 2020). Using the AD model, it has been suggested that during the pause, embryos space out evenly but attachment does not occur until after E2 is administered (Nilsson, 1974). However, embryo location has not been assessed in lactating ND mice and little is known about the location of the embryo during the pause period prior to attachment. Additionally, a comparison of hormone levels between the two diapause models, ND and AD, is missing. In this study, we evaluate embryo location in both ND and AD as well as the respective E2 and P4 levels at different time points and compare them to NP. We find that serum P4 levels in each model are differ greatly, whereas serum E2 levels are relatively similar. In addition, we discover that the ND and AD models of diapause differ in embryo location patterns. Further, unlike the NP model, the ND model displays embryo entry followed by a single unidirectional scattering and spacing movement. Finally, with the ND and AD models of pregnancy and exogenous hormone administration in the NP model of pregnancy (Fig. 1), we show that both P4 and E2 can modulate different phases of uterine embryo movement.
Figure 1.
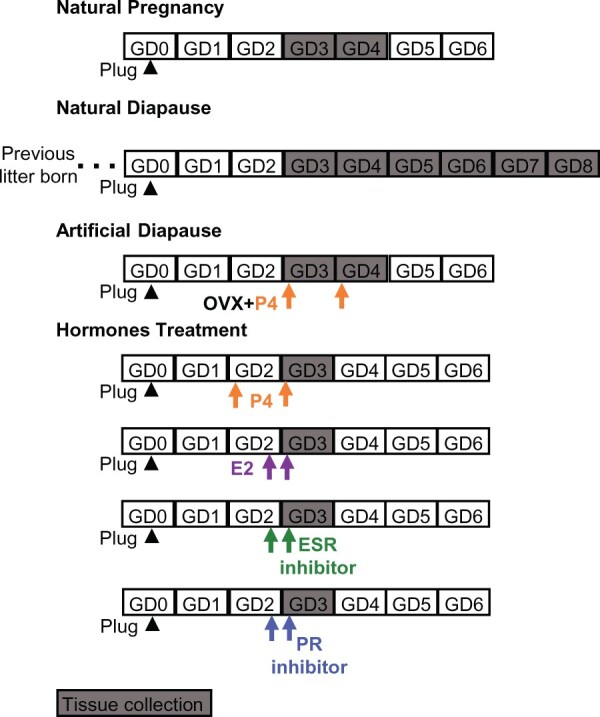
Schematic of mouse models used to study the effects of ovarian hormone modulation on embryo location. Natural pregnancy analysis is performed in the first pregnancy of a virgin post-pubertal mouse. For natural (lactational) diapause, the female mouse gives birth to its first litter and mates with a male within 48 h owing to post-partum estrous, and the resulting pregnancy is analyzed. Artificial diapause is a delay in implantation induced under laboratory conditions by removing the ovaries during the first pregnancy of a virgin mouse, and only progesterone (P4) is injected to keep the pregnancy active. To corroborate results obtained from these models, we also use exogenous hormone (estrogen (E2) or P4) administration or estrogen receptor (ESR) and P4 receptor (PR) inhibitor on gestational day (GD) 2 or/and GD3 to understand their effect on embryo movement. Arrowhead is GD0 1200 h when plug is identified. Arrow indicates the injection time. OVX: ovariectomy.
Materials and methods
Animals
All animal research was carried out under the Michigan State University Institutional Animal Care and Use Committee guidelines. CD1 (ICR) mice aged 6–12 weeks were maintained on a 12 h light/dark cycle. For NP, adult females were mated with fertile wild-type males to induce pregnancy. For ND, the female would carry pups to term and deliver. After delivery, female and male mice were in the same cages as the suckling pups. We identify the start of a pregnancy as gestational day (GD) 0 0000 h. The appearance of a vaginal plug, which is identified about 12 h from when mating occurs, is termed GD0 1200 h in all pregnancy models. Uterine dissections were performed at various time points from GD3 to GD8. A total of 4–6 ND mice were evaluated at each time point. For detecting implantation sites in ND on GD5 to GD8, 200 µl of 0.5% Evans blue dye (ICN15110805, MP Biomedicals, Irvine, CA, USA) in PBS was injected into the lateral tail vein of the pregnant mouse 15 min prior to sacrificing the mouse. Uteri were then photographed in white light to observe implantation sites (Psychoyos, 1965).
AD procedure
For inducing AD, different methods are proposed in the literature that differ in timing of P4 treatment once the ovaries are removed (Yoshinaga and Adams, 1966; McLaren, 1971; Paria et al., 1993; Cha and Dey, 2014). To minimize the variables in our study, we chose the method that allows implantation to occur despite the removal of ovaries (Yoshinaga and Adams, 1966; McLaren, 1971). Thus, AD was induced by removing both ovaries on the morning of GD3 (0600 h), leaving the oviduct intact. An injection of 2 mg of P4 s.c. (P0130, Sigma-Aldrich, St. Louis, MO, USA) in 100 µl of sesame oil (AC241002500, Acros Organics, Thermo Fisher Scientific, NJ, USA) was given at the time of surgery. Two groups of mice were dissected either on GD3 at 1200 or 2200 h. The third group of mice was injected with a second dose of 2 mg P4 the following morning on GD4 (0600–0800 h) and was dissected in the afternoon on GD4 (1500–1700 h). We evaluated 3–4 AD mice on GD3 and 7 AD mice on GD4.
Hormone and inhibitor treatments
The hormones 17β-estradiol (E8875, Sigma-Aldrich, St. Louis, MO, USA) and progesterone (P0130, Sigma-Aldrich, St. Louis, MO, USA) were dissolved in sesame oil and injected at 25 ng s.c. (Ma et al., 2003) and 4 mg s.c. (Liang et al., 2018), respectively. Mice in the vehicle groups received injections of sesame oil alone. E2 was administered either on GD2 2200 h and GD3 0800h and uteri from these mice were analyzed at GD3 1200 h, or E2 was administered on GD3 0800 h and uteri were analyzed at GD3 1500 h and GD3 2200 h. P4 was administered on GD2 at 1000 h and uteri from these mice were analyzed at GD3 0300 h or P4 was administered on GD2 at 1000 h and GD3 at 1000 h and uteri from these mice were analyzed at GD3 1500 h and GD3 2200 h. The E2 inhibitor ICI 182,780 (104710, Tocris Bioscience, Minneapolis, MN, USA) was dissolved in dimethylsulphoxide (DMSO); this stock solution was diluted in sesame oil and then 50 µg was injected s.c. (Cha et al., 2013). Mice in the vehicle group received s.c. injections of 0.1 ml sesame oil. Either E2 inhibitor or vehicle was administered on GD2 2200 h, and mice were sacrificed and dissected at GD3 1500 h. An additional group of mice was injected at GD2 2200 h and again at GD3 1000 h, then dissected at GD3 2200 h. Mifepristone (M8046, Sigma-Aldrich, St. Louis, MO, USA) was dissolved in 5% DMSO in sesame oil. Mice were injected with Mifepristone at a dose of 1.2 mg/kg s.c. either at GD2 2100 h and dissected at GD3 0900 h, or injected at GD3 0900 h and dissected at GD3 2100 h. Vehicle-treated mice received 5% DMSO in sesame oil. Three to eight mice were used for each treatment.
Blood collection and serum preparation
At the time of dissection, mouse blood was collected by cardiac puncture. The serum isolation protocol was modified from the University of Virginia Ligand Assay and Analysis Core. Briefly, blood was allowed to clot for ∼30 min. Then a plastic pipette tip was used to disrupt the clot before centrifuging at 2000×g for 15 min at room temperature. Serum was separated from the cells and stored in an Eppendorf tube at −20°C. E2 and P4 levels were assayed by the University of Virginia Center for Research in Reproduction Ligand Core. E2 was determined by commercial ELISA (ES180S-100, Calbiotech, El Cajon, CA, USA). E2 assay characteristics were as follows: sensitivity = 3 pg/ml; intra-assay coefficient of variation (CV) = 7.5%; inter-assay CV = 10.1%. P4 was determined by commercial ELISA (IB79105, Immuno-biological laboratories, Minneapolis, MN, USA). P4 assay characteristics were as follows: sensitivity = 6 ng/ml; intra-assay CV = 7.0%; inter-assay CV = 11.2%. More information on the steroid assay validation can be found at https://med.virginia.edu/research-in-reproduction/wp-content/uploads/sites/311/2017/09/Steroid-Method-Valid-ProcR.pdf. Serum hormone levels were analyzed in 3–7 mice at different time points.
Whole-mount immunofluorescence
Whole-mount immunofluorescence staining was performed as described previously (Arora et al., 2016). Uteri were fixed in DMSO: methanol (1:4) after dissection. For staining, the uteri were rehydrated for 15 min in 1:1, Methanol: PBST (PBS, 1% Triton X-100) solution, followed by a 15 min wash in 100% PBST solution. Samples were then incubated with Hoechst (B2261, Sigma-Aldrich, St. Louis, MO, USA) diluted in PBST (1:500) for two nights at 4°C. The uteri were then washed once for 15 min and three times for 45 min each using PBST. The uteri were then stretched in 100% methanol, followed by 30 min dehydration in 100% methanol, an overnight incubation in 3% H2O2 solution diluted in methanol and a final dehydration step for 60 min in 100% methanol. Finally, samples were cleared using a 1:2 mixture of benzyl alcohol: benzyl benzoate (108006, B6630, Sigma-Aldrich, St. Louis, MO, USA).
Confocal microscopy
Confocal imaging procedures were carried out as previously described (Flores et al., 2020). Stained uteri were imaged using a Leica TCS SP8 X Confocal Laser Scanning Microscope System (Leica Microsystems) with a white-light laser, using a 10× air objective. For each uterine horn, z-stacks were generated with a 7.0 µm increment, and tiled scans were set up to image the entire length and depth of the uterine horn (Arora et al., 2016). Images were merged using Leica software LASX version 3.5.5 (Leica Microsystems).
Image analysis for embryo location
Image analysis was carried out using commercial software Imaris v9.2.1 (Bitplane, Zurich, Switzerland). Embryo location was assessed as described previously (Flores et al., 2020). Briefly, confocal LIF files were imported into the Surpass mode of Imaris, and Surface module 3D renderings were used to create structures for the oviductal–uterine junctions, embryos and horns. The 3D Cartesian co-ordinates of the center of each surface were identified and stored using the measurement module. The distance between the oviductal–uterine junction and an embryo (OE), the distance between adjacent embryos (EE), and the horn length was calculated using the orthogonal projection onto the XY plane. All distances were normalized to the length of their respective uterine horn. Horns with less than three embryos were excluded from the analysis. These distances were used to map the location of the embryos relative to the length of the uterine horn. The uterine horn was divided into three equally spaced segments—closest to the oviduct, middle and closest to the cervix. These segments were quantified for the percentage of embryos present in each section. Embryos in the oviductal region close to the oviductal–uterine junction were accounted for in the first oviductal segment.
Statistical analysis
For serum hormone levels, the unpaired two-tailed Student’s t-test was performed with Welch’s correction, while for ND and AD models, ANOVA was used to analyze OE and EE distances amongst uterine horns and different time points, as described previously (Flores et al., 2020). Statistical analyses were performed with Graph Pad Prism (Dotmatics). To compare the vehicle and the hormone or inhibitor treatments, linear mixed-effects models (Laird and Ware, 1982) were conducted to study the effect of treatment, time and their interaction on OE and EE distances, while controlling for repeated measures within a horn using a random intercept term. Multiple comparisons of means were conducted using Tukey contrasts with the Holm method adjustment for P-values. Analysis was conducted using Graph Pad Prism, with advanced statistical analysis conducted using R Statistical Software (R Development Core Team, 2014) and the nlme package (Pinheiro et al., 2022). P-value <0.05 was considered significant indicating differences between comparisons.
Results
To better understand the interplay between serum hormones and their regulation of embryo location, we used three different models of mouse pregnancy (Fig. 1): NP—generated by natural mating of virgin female mice; ND—generated by mating female mice that had just given birth to a litter and display diapause due to lactation; and AD—a laboratory version of ND generated by ovariectomy and P4 treatment. We determined embryo location and serum hormone levels in each of these models. We also treated NP mice with exogenous ovarian hormones E2 and P4 (Fig. 1) to better understand the role of each hormone in the regulation of pre-implantation embryo movement.
Ovarian hormones in NP
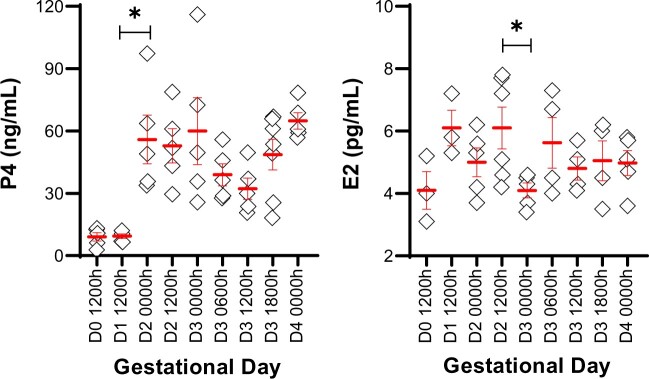
In the NP model, we found that P4 levels are low on GD0 and GD1, begin to rise on GD2 and remain high until implantation (GD4 0000 h) (Fig. 2). On the other hand, serum E2 levels stayed constant throughout the pre-implantation phase of pregnancy, and we did not observe a nidatory rise in the serum levels of E2 (Fig. 2).
Figure 2.
Ovarian hormones in a natural pregnancy in mice. P4 and E2 were measured in mouse serum from gestational day (D) 0 to D4. P4 levels begin to rise on D2 (0000 h), whereas serum E2 levels remain similar throughout early pregnancy. Each diamond represents data from one mouse. Mean ± SEM displayed in red. *P < 0.05. For serum hormone levels, the unpaired two-tailed Student’s t-test was performed with Welch’s correction. P4: Progesterone; E2: Estrogen. Three to seven mice were evaluated for serum hormones at each time point.
The ND model displays two pauses separated by embryo location, congruent with a unidirectional movement pattern
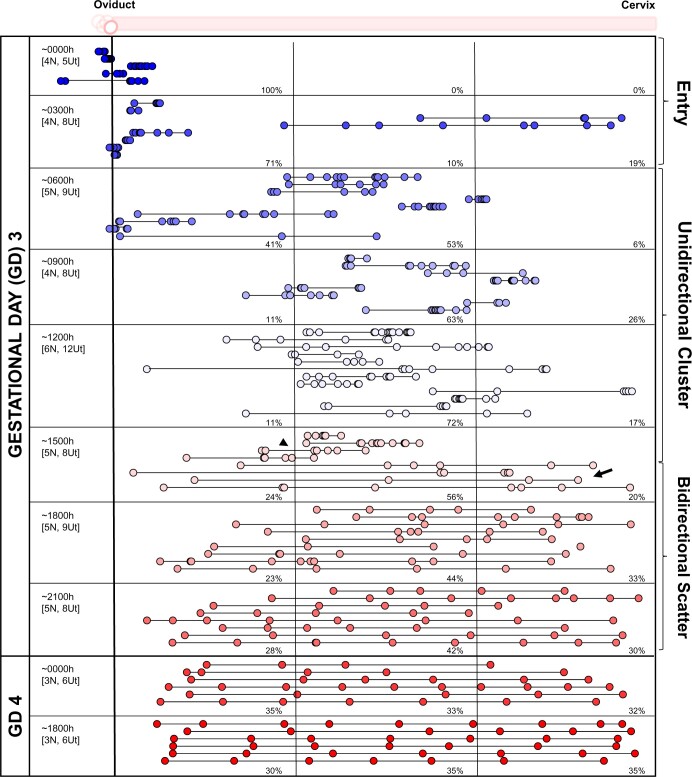
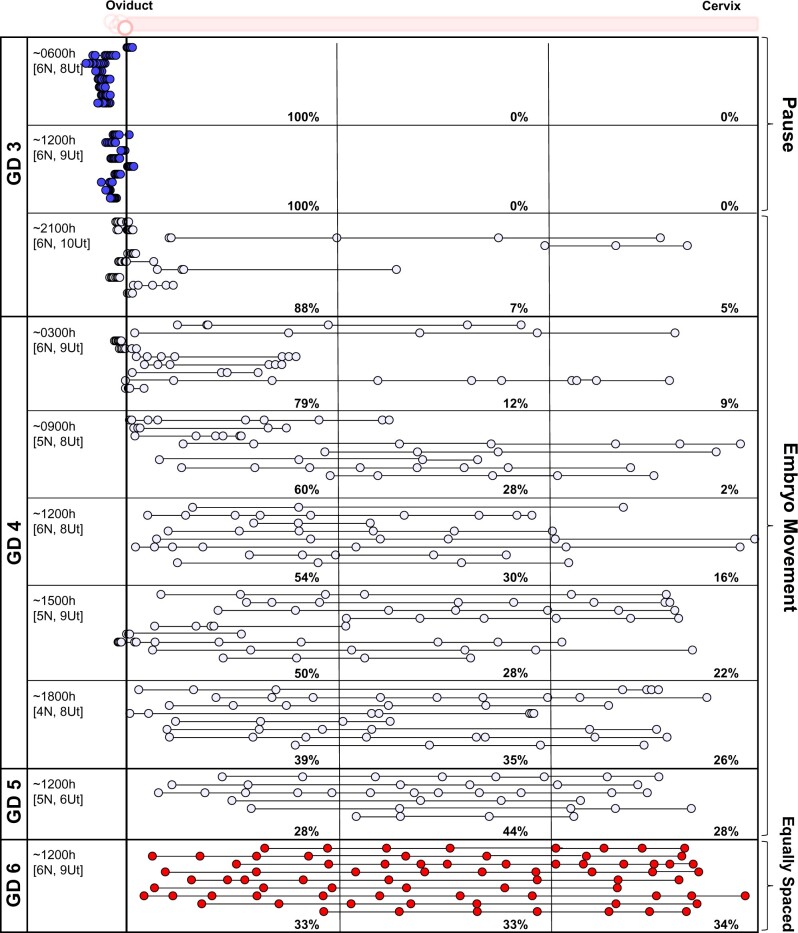
Using a recently established methodology employing confocal imaging and 3D location analysis (Flores et al., 2020), we determined the location of embryos at different time points in the ND pregnancy. The data from an NP pregnancy are reproduced here for side-by-side comparison (Fig. 3). In the ND model, on GD3 between 0600 and 1200 h, 100% of the embryos remain clustered in the oviduct (Fig. 4). At GD3 2100 h, ∼88% of the embryos are still near the oviductal–uterine junction, with only 12% of the embryos displaying a displacement away from the oviduct. Thus, for most of GD3, embryo location is closer to the oviduct in the ND model. We compared the distribution of the embryos in the three segments of the uterus (near the oviduct—oviductal, middle and near the cervix—cervical) and observed that on GD4, embryos appear to scatter and space unidirectionally throughout the length of the horn. At GD4 0900 h, 60% of embryos are present in the oviductal segment of the horn, 28% in the middle and 2% in the cervical segment, while by the evening of GD4 (1800 h), 39% of embryos are in the oviductal segment, 35% in the middle and 26% in the cervical segment (Fig. 4). The percentage of embryos steadily increases in the middle and cervical segments until an equal distribution is achieved on GD6 (Fig. 4). These data suggest that embryo clusters in all mice undergoing ND are paused near the oviduct-uterine junction on GD3, followed by robust embryo movement on GD4 and fine embryo spacing by GD6 (Fig. 4).
Figure 3.
Embryo movement analysis in a natural pregnancy in mice. Location of embryos in uterine horns at fixed time intervals on GD3 and GD4 of a natural pregnancy. Each circle represents an embryo, and circles connected with a line are embryos from the same uterine horn. Blue-white-red colors signify time scale, where blue is the earliest time point on GD3, white is mid-day of GD3, and red is the latest time point on GD4. The left-hand column indicates the time of dissection in hours (h). ‘N’ represents the number of mice, and ‘Ut’ represents the number of uterine horns analyzed for each time point. Dotted lines divide the uterine horns into three equal segments, and percentages for each time point signify the percentage of embryos in each segment. On GD3 at 1500 h, embryos clusters (arrowhead) and embryo scattering (arrow) can be observed. GD: gestational day. Reprinted with permissions from Flores et al. (2020).
Figure 4.
Embryo movement analysis in a lactational natural diapause pregnancy in mice. Location of embryos in uterine horns at fixed time intervals on GD3–GD6 in ND. Each circle represents an embryo, and circles connected with a line are embryos from the same uterine horn. Blue-white-red colors signify time scale where blue represents time points on GD3 when embryos are at the oviductal–uterine junction, white represents embryo movement on GD3, GD4, and GD5, and red represents time points when embryos are equally spaced out and attachment is first observed in some ND pregnancies. The left-hand column indicates the time of dissection in hours (h). ‘N’ represents the number of mice, and ‘Ut’ represents the number of uterine horns analyzed for each time point. Dotted lines divide the uterine horns into three equal segments, and percentages for each time point signify the percentage of embryos in each segment. ND: natural diapause; GD: gestational day.
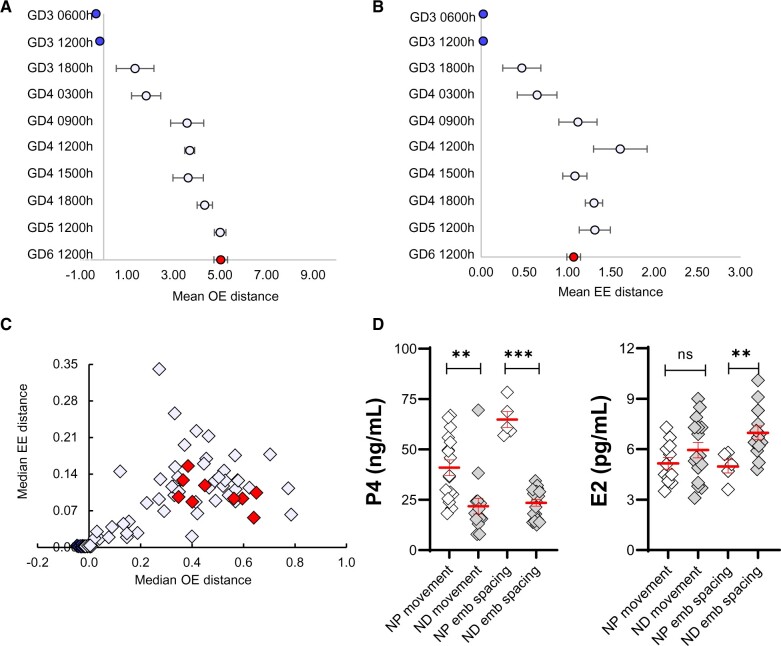
We examined the median OE distance and median EE distance per horn (Flores et al., 2020) and compared the two to interpret embryo movement in ND. In the initial stages, the embryos display smaller OE and EE distances (Fig. 5A–C), supporting the presence of embryo clusters near the oviduct. At later time points on GD4, both OE (Fig. 5A) and EE (Fig. 5B) distances increase simultaneously until equal spacing is achieved (Fig. 5C). These data further suggest a unidirectional scattering and spacing pattern of embryo movement towards the cervix (Boving, 1956) in ND.
Figure 5.
Embryo movement pattern and serum hormone levels in a lactational natural diapause pregnancy differ from a natural pregnancy in mice. (A) Mean OE distance and (B) Mean EE distance, with whiskers representing standard error for embryo location (from Fig. 4) and is color-matched for GD. (C) OE versus EE analysis of embryo location (data from Fig. 4). Each diamond represents the median value from individual uterine horns (from Fig. 4) and is color-matched for GD. (D) Comparison of serum P4 and E2 in NP and ND. Time points are combined, and hormone levels are compared during the movement phase or after embryo spacing. P4 levels are lower both during embryo movement and spacing. E2 levels in ND pregnancy are similar to NP at the start of embryo movement but are significantly higher than NP during embryo spacing. Mean ± SEM displayed in red. ns: not significant, **P < 0.01, ***P < 0.001. For serum hormone levels, the unpaired two-tailed Student’s t-test was performed with Welch’s correction. OE: oviduct-embryo distance; EE: embryo-embryo distance; GD: gestational day; ND: natural diapause; P4: progesterone; E2: estrogen.
The uteri of NP mice display a blue dye reaction, indicating vascular permeability, on the evening of GD4, confirming implantation (Restall and Bindon, 1971). However, as expected, when the blue dye was administered to ND mice, we did not see a blue dye reaction on GD4 (data not shown) and GD5 (Supplementary Fig. S1), suggesting that in ND, implantation initiates after these time points. The first signs of a blue dye reaction were observed on GD6, where 40% of the mice displayed a positive blue dye reaction (Supplementary Fig. S1). This suggests that there is a minimum 2-day delay in attachment initiation, which is in line with the minimum observed delay in delivery (Mantalenakis and Ketchel, 1966; Norris and Adams, 1981). At GD7, 43%, and on GD8, 89% of the ND mice displayed a positive blue dye reaction (Supplementary Fig. S1). These data also suggest that 40% of the animals in ND will display embryos paused once at the oviductal–uterine junction, followed by slow movement, embryo spacing and attachment at GD6. The remaining 60% of animals in ND display a second pause after the embryos are spaced out and until they implant (beyond GD6).
Serum P4 levels are lower while E2 levels are higher in ND compared to NP
To compare ovarian hormone levels in NP and ND models, we evaluated serum P4 and E2 levels during embryo movement and after embryos achieved equidistant spacing. For NP, values of time points for embryo movement ranging from GD3 0600 h to GD3 1800 h (Flores et al., 2020) were pooled, and for post-embryo spacing, GD4 0000 h was used. For ND, values of time points for embryo movement (based on Fig. 2) ranging from GD3 2200 h to GD5 1200 h were pooled, and for post-embryo spacing time points from GD6 1200 h to GD8 1200 h were pooled. Surprisingly, we found that the levels of serum P4 in ND were lower than NP both during embryo movement (mean 21.85 ng/ml versus 40.95 ng/ml, respectively, P < 0.01), and after embryo spacing (mean P4 25.05 ng/ml versus 64.82 ng/ml, respectively, P < 0.0001) (Fig. 5D, Supplementary Fig. S2). Between NP and ND, E2 levels were comparable during embryo movement but were found to be different post-embryo spacing. Further, the trend was toward higher E2 levels in ND (mean: 6.97 pg/ml) compared to NP (mean: 4.98 pg/ml, P < 0.01) (Fig. 5D, Supplementary Fig. S2).
Exogenous E2 affects embryo location in both the unidirectional and bidirectional phases of embryo movement
In addition to differences in ovarian hormones, the uterine milieu in the ND model is recovering from the prior pregnancy and is likely affected by wound repair factors and the presence of immune cells (Mackler et al., 2000). To distinguish between the effects of ovarian hormones and changes in the uterine milieu related to wound healing, we tested the specific effects of E2 and P4 on embryo location by exogenous hormone treatment in NP. E2, P4, E2 inhibitor and P4 inhibitor exogenous treatments were performed at times when the endogenous hormones or their receptors are normally present (Diao et al., 2015). This was to ensure that exogenous treatment would merely amplify or inhibit the hormone receptor signaling.
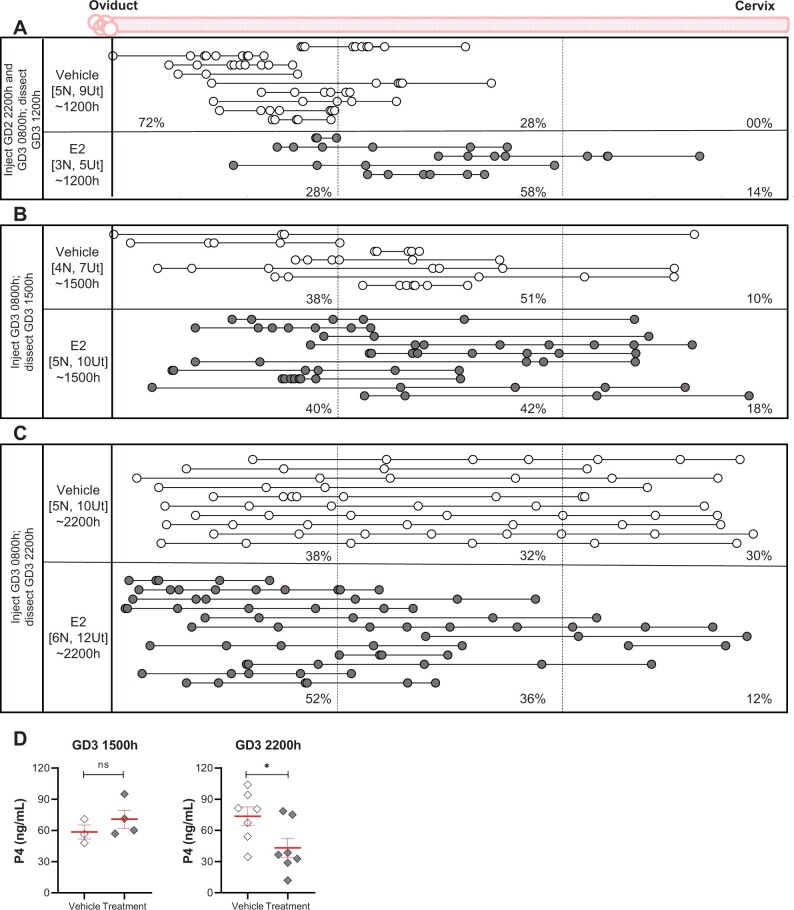
We treated NP mice with 25 ng E2 s.c. This dose of E2 is shown to be disruptive of uterine receptivity in an ovariectomized mouse model and thus we reasoned that it was more than the natural amount of E2 present during pre-implantation embryo movement in a natural pregnancy (Ma et al., 2003). One set of mice was treated with E2 on GD2 (2200 h) prior to embryo entry, and GD3 (0800h) to assess the effect on unidirectional clustered movement at GD3 1200 h. At GD3 1200 h, in both vehicle and E2 treatment, the embryos were observed in clusters. However, embryos accumulated primarily in the middle segment upon E2 treatment compared to the first segment for vehicle treatment (Fig. 6A). Both the mean OE and EE distances for E2 treatment were larger than vehicle treatment (P < 0.005). This suggests that E2 may increase the velocity of embryo movement in the unidirectional phase. Interestingly, when compared with the NP model at GD3 1200 h (Fig. 3), the vehicle-treated mice displayed a slower movement for the embryo clusters (Fig. 6A). We predict that this may be an effect of the vehicle, sesame oil, on muscle contractions and thus embryo movement.
Figure 6.
Estrogen treatment has an immediate effect on unidirectional and a delayed effect on bidirectional embryo location in mice. Treating the NP with E2 prior to embryo entry alters the embryo movement pattern in the unidirectional phase (A) (P < 0.005 for OE and EE distances between vehicle and E2 treatment). Treatment with E2 after embryo entry does not alter embryo location at the beginning of the bidirectional phase (B), but there is a delayed impact on embryo location in the latter half of the bidirectional phase (C) (P < 0.05 for OE distances between vehicle and E2 treatment). For OE and EE comparison of vehicle and treatment multiple comparisons of means were conducted using Tukey contrasts with the Holm method adjustment for P-values. Each circle represents an embryo, and circles connected with a line are embryos from the same uterine horn. White circles represent mice treated with vehicle and grey circles represent mice treated with E2. The left-hand column indicates the time of dissection in hours (h). ‘N’ represents the number of mice, and ‘Ut’ represents the number of uterine horns analyzed for each time point. Dotted lines divide the uterine horns into three equal segments, and percentages for each time point signify the percentage of embryos in each segment. (D) P4 levels were measured after E2 treatment and were similar in vehicle and treatment groups in the first half of bidirectional movement (GD3 1500h) but were reduced at 2200 h. Mean ± SEM displayed in red. ns: not significant, *P < 0.05. For serum hormone levels, the unpaired two-tailed Student’s t-test was performed with Welch’s correction. NP: natural pregnancy; E2: estrogen; P4: progesterone; GD: gestational day.
Another set of mice was treated with E2 on the morning of GD3 (0800 h) to assess effects on bidirectional movement (GD3 1500 and 2200 h). At GD3 1500 h, the majority of the embryos were present as clusters in the middle segment of the uterine horn in the vehicle and treatment groups (51% and 42%, respectively, Fig. 6B). On the other hand, when evaluated later in the day (GD3 2200 h), more embryos were present in the oviductal segment (52%) and fewer embryos were found in the cervical segment (12%) as compared to a nearly equal distribution of embryos in all three segments in the vehicle-treated animals (Fig. 6C). Further, while the mean EE distance was similar in vehicle and E2 treatment at GD3 2200 h, the mean OE distance was significantly different (vehicle mean OE = 4.736 ± 0.34, treatment mean OE = 3.372 ± 0.28 normalized units, P < 0.05), supporting altered embryo distribution along the uterine horn. To address if the localization of embryos near the oviduct is observed at implantation, we treated mice with 25 ng E2 at GD3 0800 h and assessed embryo implantation using the blue dye procedure at GD4 1200 h. We noted that the embryos had spaced out, away from the oviduct and throughout the uterine horn (Supplementary Fig. S3). Thus, although there is a delayed effect on embryo movement with exogenous E2 treatment during the bidirectional phase of movement, eventually the embryos scatter throughout the horn by the time of embryo implantation.
To address the altered embryo location with E2 treatment at GD3 2200 h, we evaluated P4 levels in vehicle and E2-treated mice. While mean P4 levels were comparable between vehicle and treatment at GD3 1500 h (58.52 and 70.86 ng/ml, respectively), P4 levels at GD3 2200 h were higher in vehicle (73.7 ng/ml) than after E2 treatment (43.19 ng/ml, P < 0.05) (Fig. 6D). These data suggest a delayed impact of E2 treatment on the bidirectional phase of embryo movement, possibly through a reduction in P4 signaling.
Inhibiting E2 activity does not affect unidirectional or bidirectional embryo movement
Next, we tested the effect of inhibiting E2 activity on embryo location patterns. We treated NP mice with a commonly used estrogen receptor (ESR) inhibitor, ICI 182,780, either before embryo entry at GD2 2200 h followed by evaluation of embryo location at GD3 1500 h or treating twice with the inhibitor at GD2 2200 h and GD3 1000 h and evaluation of embryo location at GD3 (2200 h). Inhibiting E2 activity by blocking ESR did not impact embryo location in the NP model of pregnancy (Supplementary Fig. S4).
Modulating P4 affects only the unidirectional phase of embryo movement
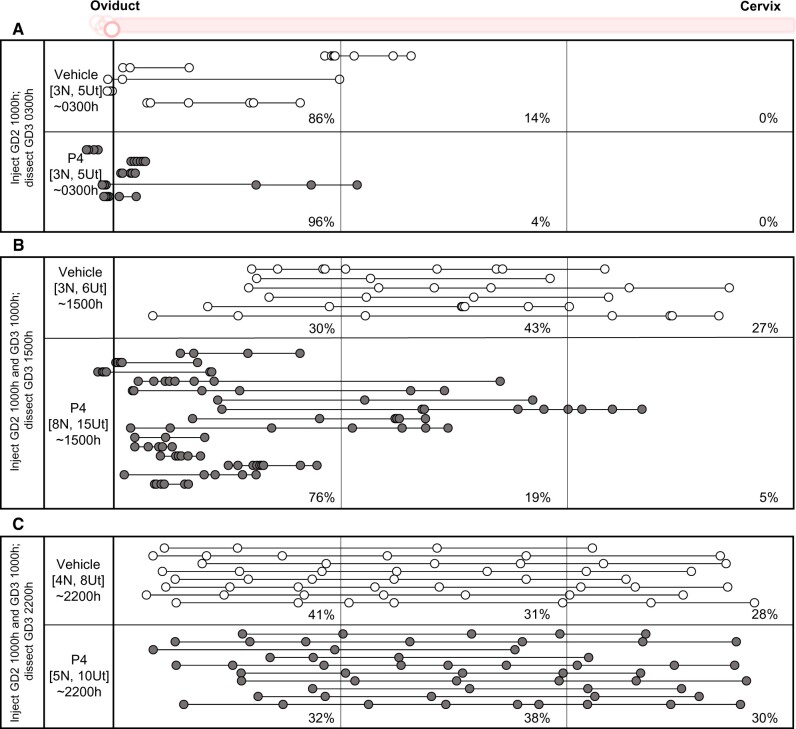
To assess the effects of excess P4, we treated females mated for the NP with 4 mg P4 s.c. (a higher dose of P4 that is known to impact uterine receptivity (Liang et al., 2018)) (Fig. 7). First, to address the effect of P4 treatment on embryo entry, we treated mice with P4 on GD2 1000 h and assessed embryo location on GD3 0300 h. Embryo location was equivalent in both vehicle and P4 treatment, suggesting an identical pattern for embryo entry (Fig. 7A). Next, we treated mice with P4 on GD2 1000 h and GD3 1000 h and evaluated them at GD3 1500 h or 2200 h. On GD3 1500 h, most of the embryos (72%) in the P4 treatment group were present near the oviductal–uterine junction and in the oviductal segment of the uterus, as compared to vehicle-treated controls. Both mean OE and EE distances were significantly different between vehicle and P4-treated mice (P < 0.0001 and P < 0.005, respectively). This embryo distribution was reminiscent of a unidirectional scattering and spacing embryo movement (Fig. 7B). When uteri were evaluated later in the day at GD3 2200 h, the embryos appeared to be spaced out evenly, similar to the vehicle-treated controls (Fig. 7C), suggesting that P4 affects the earlier unidirectional clustered movement, but the embryos space out eventually.
Figure 7.
Progesterone treatment has an immediate effect on mouse embryo location that resolves over time. Treating the NP with P4 does not affect embryo entry (A). P4 treatment for NP induces an embryo location concordant with a slower unidirectional embryo movement pattern on GD3 1500 h (B) (P < 0.0001 and P < 0.005 for OE and EE distances between vehicle and P4 treatment, respectively). (C) Although the embryos initially display an altered movement pattern, they eventually space out at the end of bidirectional movement on GD3 2200 h. For OE and EE comparison of vehicle and treatment multiple comparisons of means were conducted using Tukey contrasts with the Holm method adjustment for P-values. Each circle represents an embryo, and circles connected with a line are embryos from the same uterine horn. White circles represent mice treated with vehicle and grey circles represent mice treated with P4. The left-hand column indicates the time of dissection in hours (h). ‘N’ represents the number of mice, and ‘Ut’ represents the number of uterine horns analyzed for each time point. Dotted lines divide the uterine horns into three equal segments, and percentages for each time point signify the percentage of embryos in each segment. NP: natural pregnancy; E2: estrogen; P4: progesterone; GD: gestational day.
To evaluate the effect of reduced P4 signaling on embryo location, we used a well-known inhibitor of P4 signaling, mifepristone. As mifepristone treatment can affect embryo viability (Roblero et al., 1987), we titrated the dosage to minimize the effects on embryo survival. We administered low-dose mifepristone (1.2 mg/kg) prior to the unidirectional phase of movement at GD2 2100 h and evaluated embryo location prior to the bidirectional scattering and spacing phase (GD3 0900 h) or administered low-dose mifepristone prior to the bidirectional phase of movement at GD3 0900 h and evaluated embryo location closer to the end of scattering phase (GD3 2100 h). Comparing vehicles and treatment, we observed no effect on embryo location in either case (Supplementary Fig. S5). Our data suggest that reducing P4 signaling with low-dose mifepristone does not impact embryo movement. Intriguingly, similar to the results shown in Fig. 6A, vehicle treatment with oil alone slowed down the clustered embryo movement in the first phase of embryo movement (Supplementary Fig. S5A).
AD mimics NP in embryo location patterns
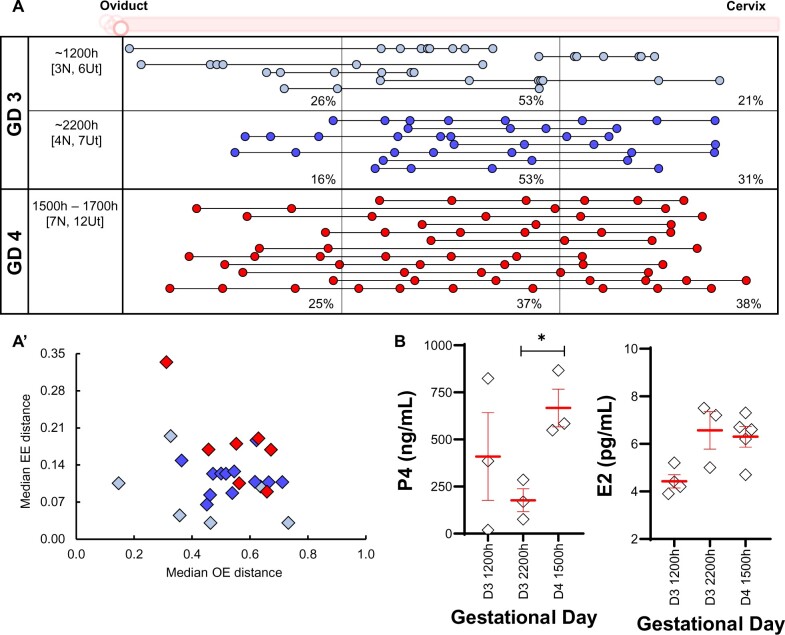
We evaluated embryo location in the AD model of pregnancy that represents a laboratory-induced delay in embryo attachment. AD was induced on the morning of GD3, and embryo location was evaluated in these mice at different time points. On GD3 1200 h, we observed that embryos are primarily found in the middle segment of the uterine horn, with 26% of embryos in the oviductal segment, 53% in the middle segment and 21% in the cervical segment. Further, when evaluated at GD3 2200 h, the embryos largely remained in the middle segment (Fig. 8A). The embryos had achieved even spacing by GD4 mid-day (1500–1700 h) (Fig. 8A and A’), suggesting that embryo spacing occurs normally in the AD model.
Figure 8.
Embryo movement analysis in artificially induced diapause pregnancy in mice. (A) Ovariectomy and treatment with P4 on the morning of GD3 to generate an AD model results in embryo location consistent with unidirectional clustered movement (A). On GD4, embryos eventually space out. Each circle represents an embryo, and circles connected with a line are embryos from the same uterine horn. Blue color signifies early (GD3) dissection time points and red color signifies later (GD4) dissection time points. The left-hand column indicates the time of dissection in hours (h). ‘N’ represents the number of mice, and ‘Ut’ represents the number of uterine horns analyzed for each time point. Dotted lines divide the uterine horns into three equal segments, and percentages for each time point signify the percentage of embryos in each segment. (A’) OE and EE distribution of uterine horns from (A). (B) Serum P4 and E2 levels in the AD model. Mean ± SEM displayed in red. *P < 0.05. For serum hormone levels, the unpaired two-tailed Student’s t-test was performed with Welch’s correction. P4: progesterone; GD: gestational day; AD: artificial diapause; OE: oviduct-embryo distance; EE: embryo-embryo distance; NP: natural pregnancy.
Serum P4, but not E2, levels differ between NP and AD
We further evaluated the serum P4 and E2 levels in the AD model during embryo movement and compared them with NP and ND models of pregnancy. In the AD model, we found that a 2 mg dose of P4 induced ∼10-fold higher P4 concentration (∼385.40 ng/ml) (Fig. 8B) compared to the NP model during embryo movement (mean 40.95 ng/ml) and ∼20-fold higher than the ND model during embryo movement (mean 21.85 ng/ml). In contrast, serum E2 levels appeared to be similar across NP, ND and AD in the embryo movement period, showing mean E2 values of 5.16, 5.95 and 5.34 pg/ml, respectively. Thus, E2 levels in the AD model point to an alternate source of E2 in the ovariectomized mice and, contrary to the prevalent notion, P4 and not E2 differs between all three models of pregnancy.
Discussion
Hormonal regulation of the uterine milieu is known to impact multiple aspects of pregnancy including oviductal transport, implantation and decidualization. However, ovarian hormone regulation of uterine embryo transport is poorly understood. Alternate models of pregnancy where differential levels of ovarian hormones have been evolutionarily selected to help induce a pause and to regulate pregnancy progression are excellent model systems to better understand the effects of ovarian hormones on early stages of embryo–uterine interactions, including uterine transport. Here, using different mouse models of pregnancy and exogenous hormone and inhibitor treatments, we show that both the ovarian hormones E2 and P4 are capable of modulating uterine embryo movement patterns during the pre-implantation stages of the pregnancy.
Differences between embryo movement in ND and NP models
We observed that embryo location at different time points (used here as a proxy for embryo movement patterns) differed between the first virgin pregnancy (NP) and the lactational diapause pregnancy (ND). Embryos in NP are found near the oviductal–uterine junction on the morning of GD3 and undergo clustered, scattering and spacing movements on Day 3 of pregnancy before attaching at GD4 (Flores et al., 2020). Similar to NP, embryos in the ND model of pregnancy arrive at the oviductal–uterine junction on GD3 but, unlike NP, they stay near the junction for an entire day. This suggests that in the ND model, there is a 24 h initial pause around the time of embryo entry into the uterus, prior to embryo movement. This first pause in the ND mouse model resembles the diapausing mink, where blastocysts are clustered near the anterior portion of the uterus (Fenelon et al., 2014) and the diapausing armadillo, where blastocysts are in the portion of the uterus closer to the oviductal opening (Enders and Buchanan, 1959). In the mink, embryo spacing occurs once the pregnancy is reactivated. However, we noted that in the ND mouse model, the embryos enter the uterus and begin to scatter after the first 24 h pause. Unlike the unidirectional clustered movement followed by the bidirectional scattering embryo movement in the NP model (Flores et al., 2020), embryo movement in the ND model is consistent with a unidirectional scattering and spacing pattern. Embryo scattering begins on GD4, but even spacing is slowly acquired by GD6, followed by a second pause in more than half of the ND mice, until embryo attachment occurs. This pattern of movement and spacing is reminiscent of embryo movement in a rabbit natural pregnancy (Boving, 1956).
While the earliest signs of attachment in the NP model are observed at GD3 2100 h (Restall and Bindon, 1971), the earliest attachment in ND was observed at GD6, indicating a minimum 2-day delay. This is consistent with the 24 h pause followed by slow embryo movement in ND. These data are consistent with mouse breeding observations where first litters are observed at ∼19–20 days, but second litters are observed ∼21–22 days after the first litter of pups is born (Mantalenakis and Ketchel, 1966; Norris and Adams, 1981). The number of suckling pups affects the time delay in attachment in the ND model (McLaren, 1968; Norris and Adams, 1981). We did not normalize the number of pups for our ND pregnancies, thus a variable number of pups could explain differing timing of implantation in our ND model (Mantalenakis and Ketchel, 1966).
Similarities between embryo movement patterns in AD and NP
The AD model is derived from the NP model and although it is assumed to mimic the ND model, we found that the AD model displays embryo location parameters similar to NP. In both the NP and AD models, embryos move as clusters to accumulate in the center of the horn before bidirectionally scattering and spacing out (Flores et al., 2020). AD mice are generated by surgically removing the ovaries on the morning of GD3, when embryos have already entered the uterine horn as clusters (Flores et al., 2020). The removal of the ovaries and exogenous supplementation of P4 in AD permits embryo spacing, consistent with previous data (Nilsson, 1974). Thus, the AD pregnancy displays only a single pause after embryo spacing but prior to embryo attachment.
Serum P4 levels differ across NP, ND and AD
Implantation in NP is thought to result from the nidatory peak of E2 (Ma et al., 2003). Our analysis of the different models of pregnancy suggests that serum E2 levels tend to stay basal across all models during embryo entry, embryo movement and the paused states before embryo attachment. The lack of observed E2 variation in our study could be due to limited sensitivity of the assay to detect differences in serum E2 levels. Also, our data were collected at 6 h intervals. Thus, it could be that the peak of E2 in NP is transient and short lived such that E2 peaks and drops in <6 h. Alternatively, E2 levels in the serum may stay basal, and there may be a direct route of E2 delivery through the ovarian circulation into the uterus (Tourgeman et al., 2001). Further, in women, endometrial ovarian hormones have also been implicated in uterine receptivity as opposed to serum hormone levels (Labarta et al., 2021). However, to date, there are no such reports in mice.
In contrast to E2, P4 levels in ND are roughly half that of NP, and in AD, they are ∼10 times that of NP. Thus, in the ND model lower P4, but not E2, may be the reason for altered unidirectional movement of embryos and the delay in implantation; the latter observation is also supported by studies where, similar to exogenous E2, exogenous P4 injections can also support implantation both in the ND rat model (Yoshinaga, 1961) and in the ND and AD mouse model (McLaren, 1971). As part of post-partum endometrial regeneration (Yoshii et al., 2014), the ND model must be characterized by wound healing responses, including secretion of PGs and cytokines in the uterus, to repair placental scars (Mackler et al., 2000). These factors along with the scars may impact embryo movement in the ND model and are not accounted for in our study. Nevertheless, similar levels of serum E2 across the different pregnancy models with varying embryo movement patterns suggest that P4 may be the primary regulator of embryo movement in the uterus.
AD and ND models: using caution while comparing results
AD has been used as a model to study delayed implantation for decades (McLaren, 1971; Paria et al., 1993; Cha and Dey, 2014). Owing to the ease and cost-effectiveness of generating AD animals, it has been used as a proxy to understand the transcriptional, proteomic and structural changes in the embryo or the uterus that support implantation (McLaren, 1971; Nilsson, 1974; Hamatani et al., 2004; Fu et al., 2014; He et al., 2019). However, when comparing embryo location and hormone levels between ND and AD, we noted stark differences. The ND model displays two pauses whereas the AD model only has a single pause. Thus, the AD model cannot be used to study the state of the embryo or the uterus in the first pause in the ND model. Even after embryos are spaced out, serum P4 levels in the ND model are much lower than the aberrantly high P4 levels in the AD model. Thus, the AD model can still be used to understand aspects of delayed implantation (ND); however, it is important to recognize the differences between the two models and exercise caution when interpreting the results.
Excess E2 and P4 influence embryo movement patterns
Ovarian hormone levels are crucial for a receptive uterus and for uterine muscle (myometrial) contractions. Our data support the idea that excess levels of E2 and P4 during pre-implantation embryo movement can alter embryo movement, however inhibiting ESR and partially inhibiting PR function does not affect the movement. This suggests that basal levels of E2 present during peri-implantation likely do not play a role in embryo movement. It is impossible to completely abrogate P4 function using the PR inhibitor because this can cause embryo demise. However, in future studies, tissue-specific deletion of the conditional allele of PR using uterine cell type-specific Cre transgenes can help address the compartment-specific roles of PR during pre-implantation embryo movement.
In women undergoing IVF, uterine contraction frequency is reduced when higher P4 levels are present on the day of embryo transfer (ET). Furthermore, ovulatory E2 promotes uterine contractions but E2 treatment in the presence of P4 may not affect uterine contractility (de Ziegler et al., 2001; Bulletti and de Ziegler, 2006; Sebag-Peyrelevade and Fanchin, 2015). Hormonal regulation of uterine contractility could directly impact embryo movement. In our study, blocking E2 signaling using an ESR inhibitor did not affect embryo movement in the unidirectional or bidirectional phase of the NP model. For exogenous hormone treatment, E2 accelerated while P4 slowed the clustered embryo movement after entry into the uterus. These effects could be attributed to ovarian hormone modulation of uterine contractility. The acceleration observed with high E2 is in line with pig studies where E2-coated beads allowed the beads to travel farther in the uterus (Pope et al., 1982). Similar to contractions in women undergoing IVF and ET, higher P4 levels following exogenous P4 treatment in our study correlate with the slow initial embryo movement, although embryos do space out evenly eventually. These data are reminiscent of our recently published work, where modulating muscle contractions in the unidirectional clustered phase prevents embryo movement (Flores et al., 2020). On the other hand, modulating P4 (this study) and uterine contractions (Flores et al., 2020) in the bidirectional phase does not affect embryo movement. These observations lead us to hypothesize that P4 and E2 may regulate embryo movement by modulating uterine contractions during early pregnancy.
The bidirectional phase of movement appears to be dependent on embryo–uterine interactions (Flores et al., 2020). Our study showed a delayed effect of E2 treatment on the bidirectional phase of embryo movement. Since relaxing the muscle does not affect the bidirectional phase of embryo movement, the delayed effect of E2 could be due to embryo–uterine signaling triggered by the transcriptional activity of E2. Alternatively, P4 levels were reduced in E2-treated mice and could signal either a loss of pregnancy or mimic a state of diapause (McLaren, 1971) and account for altered embryo movement patterns. In order to further establish whether E2 and P4 regulate embryo movement by modulating muscle contractions in the first phase of movement and alternate mechanisms are involved in the second phase of movement, hormone receptors should be depleted in the uterine smooth muscle compartment using appropriate Cre transgenic lines (Ghosh et al., 2020). This will be the subject of future studies.
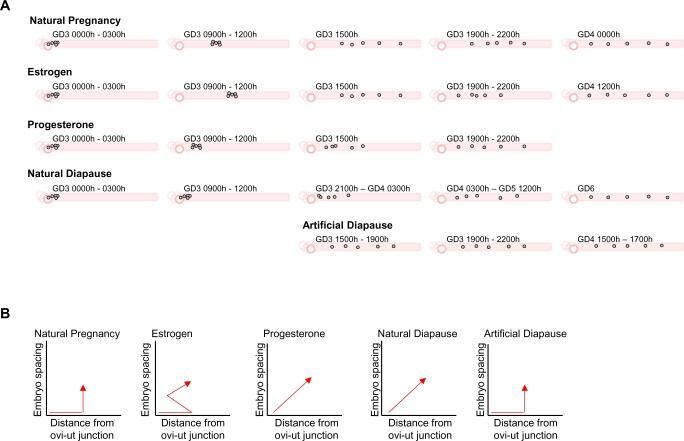
In conclusion, we show that ND, AD and NP are distinct models of pregnancy with variable serum P4 but similar E2 levels. Embryo movement patterns are distinct between the first virgin pregnancy (NP) and lactational diapause (ND), and also when exogenous hormones are administered, implicating at least partial hormonal regulation of embryo movement in the uterus (Fig. 9). These results have clinical significance in ovarian hyperstimulation and ART, where higher P4 levels during blastocyst transfer could impact embryo movement, thereby affecting embryo location during implantation and eventually pregnancy outcomes.
Figure 9.
Schematic of mouse embryo movement trajectories under hormonally altered conditions. (A) In all models of pregnancy, the embryos enter as a cluster through the oviductal–uterine junction. In natural pregnancy, embryos enter as a cluster, undergo unidirectional clustered movement and then bidirectionally scatter and space out. Estrogen treatment speeds up the unidirectional clustered movement and biases the scattering movement toward the oviduct. However, by the time of implantation, the embryos are spread throughout the horn. Progesterone slows down the clustered embryo movement during the unidirectional phase but does not affect the movement in the scattering phase. Natural diapause, characterized by reduced levels of progesterone, causes the embryos to unidirectionally scatter after embryo entry into the uterus. Artificial diapause is characterized by patterns similar to natural pregnancy. (B) Discerning the patterns of embryo movement under different hormonal conditions based on embryo movement along the oviduct and embryo spacing away from each other.
Supplementary Material
Acknowledgments
This research is dedicated to the memory of our dear student Zach Raider who was instrumental in getting this project started.
We thank Prof. Asgerally Fazleabas and Dr. Gregory Burns for scientific discussions for the manuscript.
Authors’ roles
H.L., D.F. and R.A. designed the experiments; H.L., D.F., M.M., M.D. and Z.R. performed experiments; H.L., D.F., Z.R., A.C., M.D., D.S. and R.A. analyzed the data. H.L., D.F. and R.A. interpreted the results; H.L., D.F. and R.A. wrote the article.
Funding
This research was supported in part by the March of Dimes grant #5-FY20-209 to R.A., the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under award# R01HD109152 to R.A. and award# R24 HD102061 to the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core.
Conflict of interest
The authors declare no conflicts of interest.
Contributor Information
Hannah Lufkin, Department of Obstetrics, Gynecology and Reproductive Biology, Michigan State University, East Lansing, MI, USA; Institute for Quantitative Health Science and Engineering, Michigan State University, East Lansing, MI, USA.
Diana Flores, Department of Obstetrics, Gynecology and Reproductive Biology, Michigan State University, East Lansing, MI, USA; Institute for Quantitative Health Science and Engineering, Michigan State University, East Lansing, MI, USA.
Zachary Raider, Department of Obstetrics, Gynecology and Reproductive Biology, Michigan State University, East Lansing, MI, USA; Institute for Quantitative Health Science and Engineering, Michigan State University, East Lansing, MI, USA.
Manoj Madhavan, Department of Obstetrics, Gynecology and Reproductive Biology, Michigan State University, East Lansing, MI, USA; Institute for Quantitative Health Science and Engineering, Michigan State University, East Lansing, MI, USA.
Madeline Dawson, Department of Obstetrics, Gynecology and Reproductive Biology, Michigan State University, East Lansing, MI, USA; Institute for Quantitative Health Science and Engineering, Michigan State University, East Lansing, MI, USA.
Anna Coronel, Department of Obstetrics, Gynecology and Reproductive Biology, Michigan State University, East Lansing, MI, USA; Institute for Quantitative Health Science and Engineering, Michigan State University, East Lansing, MI, USA.
Dhruv Sharma, Center for Statistical Training & Consulting, Michigan State University, East Lansing, MI, USA.
Ripla Arora, Department of Obstetrics, Gynecology and Reproductive Biology, Michigan State University, East Lansing, MI, USA; Institute for Quantitative Health Science and Engineering, Michigan State University, East Lansing, MI, USA.
Supplementary data
Supplementary data are available at Molecular Human Reproduction online.
Data availability
Data generated in the article is available upon reasonable request to the corresponding author.
References
- Arora R, Fries A, Oelerich K, Marchuk K, Sabeur K, Giudice LC, Laird DJ.. Insights from imaging the implanting embryo and the uterine environment in three dimensions. Development 2016;143:4749–4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurich C, Budik S.. Early pregnancy in the horse revisited—does exception prove the rule? J Anim Sci Biotechnol 2015;6:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boving BG. Rabbit blastocyst distribution. Am J Anat 1956;98:403–434. [DOI] [PubMed] [Google Scholar]
- Bulletti C, de Ziegler D.. Uterine contractility and embryo implantation. Curr Opin Obstet Gynecol 2006;18:473–484. [DOI] [PubMed] [Google Scholar]
- Bylander A, Gunnarsson L, Shao R, Billig H, Larsson DG.. Progesterone-mediated effects on gene expression and oocyte-cumulus complex transport in the mouse fallopian tube. Reprod Biol Endocrinol 2015;13:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha J, Dey SK.. Cadence of procreation: orchestrating embryo-uterine interactions. Semin Cell Dev Biol 2014;34:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha J, Sun X, Bartos A, Fenelon J, Lefèvre P, Daikoku T, Shaw G, Maxson R, Murphy BD, Renfree MB. et al. A new role for muscle segment homeobox genes in mammalian embryonic diapause. Open Biol 2013;3:130035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ziegler D, Bulletti C, Fanchin R, Epiney M, Brioschi PA.. Contractility of the nonpregnant uterus: the follicular phase. Ann N Y Acad Sci 2001;943:172–184. [DOI] [PubMed] [Google Scholar]
- Diao H, Li R, El Zowalaty AE, Xiao S, Zhao F, Dudley EA, Ye X.. Deletion of lysophosphatidic acid receptor 3 (Lpar3) disrupts fine local balance of progesterone and estrogen signaling in mouse uterus during implantation. Biol Reprod 2015;93:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas DA, Houde A, Song JH, Farookhi R, Concannon PW, Murphy BD.. Luteotropic hormone receptors in the ovary of the mink (Mustela vison) during delayed implantation and early-postimplantation gestation. Biol Reprod 1998;59:571–578. [DOI] [PubMed] [Google Scholar]
- Dziuk P. Effect of migration, distribution and spacing of pig embryos on pregnancy and fetal survival. J Reprod Fertil Suppl 1985;33:57–63. [PubMed] [Google Scholar]
- Enders AC, Buchanan GD.. The reproductive tract of the female nine-banded armadillo. Tex Rep Biol Med 1959;17:323–340. [PubMed] [Google Scholar]
- Evans TJ, Ganjam VK.. Reproductive anatomy and physiology. Reprod Dev Toxicol 2011;7–32. [Google Scholar]
- Fenelon JC, Banerjee A, Murphy BD.. Embryonic diapause: development on hold. Int J Dev Biol 2014;58:163–174. [DOI] [PubMed] [Google Scholar]
- Flores D, Madhavan M, Wright S, Arora R.. Mechanical and signaling mechanisms that guide pre-implantation embryo movement. Development 2020;147: [DOI] [PubMed] [Google Scholar]
- Fu Z, Wang B, Wang S, Wu W, Wang Q, Chen Y, Kong S, Lu J, Tang Z, Ran H. et al. Integral proteomic analysis of blastocysts reveals key molecular machinery governing embryonic diapause and reactivation for implantation in mice. Biol Reprod 2014;90:52. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Syed SM, Kumar M, Carpenter TJ, Teixeira JM, Houairia N, Negi S, Tanwar PS.. In vivo cell fate tracing provides no evidence for mesenchymal to epithelial transition in adult fallopian tube and uterus. Cell Rep 2020;31:107631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidley-Baird AA, O’Neill C, Sinosich MJ, Porter RN, Pike IL, Saunders DM.. Failure of implantation in human in vitro fertilization and embryo transfer patients: the effects of altered progesterone/estrogen ratios in humans and mice. Fertil Steril 1986;45:69–74. [DOI] [PubMed] [Google Scholar]
- Hamatani T, Daikoku T, Wang H, Matsumoto H, Carter MG, Ko MS, Dey SK.. Global gene expression analysis identifies molecular pathways distinguishing blastocyst dormancy and activation. Proc Natl Acad Sci USA 2004;101:10326–10331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Zhang H, Wang J, Liu M, Sun Y, Guo C, Lu J, Wang H, Kong S.. Blastocyst activation engenders transcriptome reprogram affecting X-chromosome reactivation and inflammatory trigger of implantation. Proc Natl Acad Sci USA 2019;116:16621–16630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastelic JP, Adams GP, Ginther OJ.. Role of progesterone in mobility, fixation, orientation, and survival of the equine embryonic vesicle. Theriogenology 1987;27:655–663. [DOI] [PubMed] [Google Scholar]
- Kendle KE, Lee B.. Investigation of the influence of progesterone on mouse embryo transport by using antiprogestational steroids. J Reprod Fertil 1980;58:253–258. [DOI] [PubMed] [Google Scholar]
- Labarta E, Sebastian-Leon P, Devesa-Peiro A, Celada P, Vidal C, Giles J, Rodriguez-Varela C, Bosch E, Diaz-Gimeno P.. Analysis of serum and endometrial progesterone in determining endometrial receptivity. Hum Reprod 2021;36:2861–2870. [DOI] [PubMed] [Google Scholar]
- Laird NM, Ware JH.. Random-effects models for longitudinal data. Biometrics 1982;38:963–974. [PubMed] [Google Scholar]
- Leith GS, Ginther OJ.. Mobility of the conceptus and uterine contractions in the mare. Theriogenology 1985;24:701–711. [Google Scholar]
- Liang YX, Liu L, Jin ZY, Liang XH, Fu YS, Gu XW, Yang ZM.. The high concentration of progesterone is harmful for endometrial receptivity and decidualization. Sci Rep 2018;8:712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes FL, Desmarais JA, Murphy BD.. Embryonic diapause and its regulation. Reproduction 2004;128:669–678. [DOI] [PubMed] [Google Scholar]
- Ma WG, Song H, Das SK, Paria BC, Dey SK.. Estrogen is a critical determinant that specifies the duration of the window of uterine receptivity for implantation. Proc Natl Acad Sci USA 2003;100:2963–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackler AM, Green LM, McMillan PJ, Yellon SM.. Distribution and activation of uterine mononuclear phagocytes in peripartum endometrium and myometrium of the mouse. Biol Reprod 2000;62:1193–1200. [DOI] [PubMed] [Google Scholar]
- Mantalenakis SJ, Ketchel MM.. Frequency and extent of delayed implantation in lactating rats and mice. J Reprod Fertil 1966;12:391–394. [DOI] [PubMed] [Google Scholar]
- McCormack JT, Greenwald GS.. Evidence for a preimplantation rise in oestradiol-17beta levels on day 4 of pregnancy in the mouse. J Reprod Fertil 1974;41:297–301. [DOI] [PubMed] [Google Scholar]
- McDowell KJ, Sharp DC, Grubaugh W, Thatcher WW, Wilcox CJ.. Restricted conceptus mobility results in failure of pregnancy maintenance in mares. Biol Reprod 1988;39:340–348. [DOI] [PubMed] [Google Scholar]
- McLaren A. A study of balstocysts during delay and subsequent implantation in lactating mice. J Endocrinol 1968;42:453–463. [DOI] [PubMed] [Google Scholar]
- McLaren A. Blastocysts in the mouse uterus: the effect of ovariectomy, progesterone and oestrogen. J Endocrinol 1971;50:515–526. [DOI] [PubMed] [Google Scholar]
- Mead RA. Delayed implantation in mustelids, with special emphasis on the spotted skunk. J Reprod Fertil Suppl 1981;29:11–24. [PubMed] [Google Scholar]
- Mead RA. Embryonic diapause in vertebrates. J Exp Zool 1993;266:629–641. [DOI] [PubMed] [Google Scholar]
- Nilsson O. The morphology of blastocyst implantation. J Reprod Fertil 1974;39:187–194. [DOI] [PubMed] [Google Scholar]
- Norris ML, Adams CE.. Concurrent lactation and reproductive performance in CFLP mice mated post partum. Lab Anim 1981;15:273–275. [DOI] [PubMed] [Google Scholar]
- Paria BC, Huet-Hudson YM, Dey SK.. Blastocyst's state of activity determines the "window" of implantation in the receptive mouse uterus. Proc Natl Acad Sci USA 1993;90:10159–10162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D; Team RC. nlme: Linear and Nonlinear Mixed Effects Models. 2022. https://cran.r-project.org/web/packages/nlme/nlme.pdf (November 2021, date last accessed).
- Pope WF, Maurer RR, Stormshak F.. Intrauterine migration of the porcine embryo: influence of estradiol-17 beta and histamine. Biol Reprod 1982;27:575–579. [DOI] [PubMed] [Google Scholar]
- Psychoyos A. [CONTROL OF NIDATION IN MAMMALS]. Arch Anat Microsc Morphol Exp 1965;54:85–104. [PubMed] [Google Scholar]
- Psychoyos A. Hormonal control of ovoimplantation. Vitam Horm 1973;31:201–256. [DOI] [PubMed] [Google Scholar]
- Renfree MB, Shaw G.. Embryo-endometrial interactions during early development after embryonic diapause in the marsupial tammar wallaby. Int J Dev Biol 2014;58:175–181. [DOI] [PubMed] [Google Scholar]
- Restall BJ, Bindon BM.. The timing and variation of pre-implantation events in the mouse. J Reprod Fertil 1971;24:423–426. [DOI] [PubMed] [Google Scholar]
- Roblero LS, Fernández O, Croxatto HB.. The effect of RU486 on transport, development and implantation of mouse embryos. Contraception 1987;36:549–555. [DOI] [PubMed] [Google Scholar]
- Sebag-Peyrelevade S, Fanchin R.. Uterine contractility and embryo transfer. In: Allahbadia GN, Chillik CF (eds). Human Embryo Transfer. New Delhi, India: Springer Nature, 2015, 61–68. [Google Scholar]
- Sittmann K. INTRAUTERINE MIGRATION OF PIG EMBRYOS IN LITTERS WITHOUT LOSSES. Can J Anim Sci 1973;53:71–74. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2014.
- Tourgeman DE, Boostanfar R, Chang L, Lu J, Stanczyk FZ, Paulson RJ.. Is there evidence for preferential delivery of ovarian estradiol to the endometrium? Fertil Steril 2001;75:1156–1158. [DOI] [PubMed] [Google Scholar]
- Tsutsui T, Amano T, Shimizu T, Murao I, Stabenfeldt GH.. Evidence for transuterine migration of embryos in the domestic cat. Nihon Juigaku Zasshi 1989;51:613–617. [DOI] [PubMed] [Google Scholar]
- Tsutsui T, Shimizu T, Hori T, Kawakami E.. Factors affecting transuterine migration of canine embryos. J Vet Med Sci 2002;64:1117–1121. [DOI] [PubMed] [Google Scholar]
- van Houten EL, Visser JA.. Mouse models to study polycystic ovary syndrome: a possible link between metabolism and ovarian function? Reprod Biol 2014;14:32–43. [DOI] [PubMed] [Google Scholar]
- Wang H, Dey SK.. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet 2006;7:185–199. [DOI] [PubMed] [Google Scholar]
- Whitten WK. Endocrine studies on delayed implantation in lactating mice. J Endocrinol 1955;13:1–6. [DOI] [PubMed] [Google Scholar]
- Whitten WK. Endocrine studies on delayed implantation in lactating mice; role of the pituitary in implantation. J Endocrinol 1958;16:435–440. [DOI] [PubMed] [Google Scholar]
- Yoshii A, Kitahara S, Ueta H, Matsuno K, Ezaki T.. Role of uterine contraction in regeneration of the murine postpartum endometrium. Biol Reprod 2014;91:32. [DOI] [PubMed] [Google Scholar]
- Yoshinaga K. Effect of local application of ovarian hormones on the delay in implantation in lactating rats. J Reprod Fertil 1961;2:35–41. [DOI] [PubMed] [Google Scholar]
- Yoshinaga K, Adams CE.. Delayed implantation in the spayed, progesterone treated adult mouse. J Reprod Fertil 1966;12:593–595. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data generated in the article is available upon reasonable request to the corresponding author.