Abstract
Background
Jaundice is a very common condition in newborns, affecting up to 60% of term newborns and 80% of preterm newborns in the first week of life. Jaundice is caused by increased bilirubin in the blood from the breakdown of red blood cells. The gold standard for measuring bilirubin levels is obtaining a blood sample and processing it in a laboratory. However, noninvasive transcutaneous bilirubin (TcB) measurement devices are widely available and used in many settings to estimate total serum bilirubin (TSB) levels.
Objectives
To determine the diagnostic accuracy of transcutaneous bilirubin measurement for detecting hyperbilirubinaemia in newborns.
Search methods
We searched CENTRAL, MEDLINE, Embase, CINAHL and trial registries up to 18 August 2022. We also checked the reference lists of all included studies and relevant systematic reviews for other potentially eligible studies.
Selection criteria
We included cross‐sectional and prospective cohort studies that evaluated the accuracy of any TcB device compared to TSB measurement in term or preterm newborn infants (0 to 28 days postnatal age). All included studies provided sufficient data and information to create a 2 × 2 table for the calculation of measures of diagnostic accuracy, including sensitivities and specificities. We excluded studies that only reported correlation coefficients.
Data collection and analysis
Two review authors independently applied the eligibility criteria to all citations from the search and extracted data from the included studies using a standard data extraction form. We summarised the available results narratively and, where possible, we combined study data in a meta‐analysis.
Main results
We included 23 studies, involving 5058 participants. All studies had low risk of bias as measured by the QUADAS 2 tool. The studies were conducted in different countries and settings, included newborns of different gestational and postnatal ages, compared various TcB devices (including the JM 101, JM 102, JM 103, BiliChek, Bilitest and JH20‐1C) and used different cutoff values for a positive result. In most studies, the TcB measurement was taken from the forehead, sternum, or both. The sensitivity of various TcB cutoff values to detect significant hyperbilirubinaemia ranged from 74% to 100%, and specificity ranged from 18% to 89%.
Authors' conclusions
The high sensitivity of TcB to detect hyperbilirubinaemia suggests that TcB devices are reliable screening tests for ruling out hyperbilirubinaemia in newborn infants. Positive test results would require confirmation through serum bilirubin measurement.
Keywords: Humans; Infant; Infant, Newborn; Bilirubin; Cross-Sectional Studies; Hyperbilirubinemia; Hyperbilirubinemia/diagnosis; Jaundice, Neonatal; Jaundice, Neonatal/diagnosis; Neonatal Screening; Neonatal Screening/methods; Prospective Studies
Plain language summary
Is measuring bilirubin levels through the skin a reliable alternative to measuring bilirubin levels in the blood of newborns?
Key messages
The studies included in this review suggest that measuring bilirubin levels through the skin without a needle can identify high bilirubin levels in newborns.
Why is it important to diagnose high bilirubin levels in newborns?
Bilirubin is a substance produced through the breakdown of red blood cells. Jaundice is a very common problem in the newborn period and results from high levels of bilirubin in the blood (hyperbilirubinaemia). It is important to detect hyperbilirubinaemia early to prevent unwanted consequences such as brain damage.
What is transcutaneous bilirubin measurement?
The usual procedure for measuring bilirubin in newborns is to collect a sample of blood (by making a small cut in the heel or inserting a needle into a vein, which can be painful for the infant) and test it in the laboratory (total serum bilirubin measurement). However, there are devices that measure bilirubin by sending a flash of light through the skin (transcutaneous bilirubin measurement). This method is painless and gives an almost immediate result.
What did we want to find out?
We wanted to find out whether transcutaneous bilirubin measurement devices could accurately diagnose hyperbilirubinaemia.
What did we do?
We searched for studies that had investigated the accuracy of transcutaneous bilirubin measurement compared with total serum bilirubin measurement. We had intended to combine the results across studies using statistical methods but were unable to; instead, we presented the results narratively.
What did we find?
We found 23 studies (5058 participants) that were conducted in different countries and settings, used different transcutaneous bilirubin measuring devices, and defined hyperbilirubinaemia with different bilirubin values. Some of the infants were premature and others were born at term (from 37 weeks' pregnancy); their ages ranged from birth to one month of life. Overall, the findings of the studies suggest that transcutaneous bilirubin measurement is a good screening tool for detecting hyperbilirubinaemia in newborns. The included studies found different degrees or levels of accuracy for the use of transcutaneous bilirubin measurement. However, due to the differences between studies, we could not provide an overall combined summary of the accuracy of the different tests. The differences in these studies included factors like the threshold values for hyperbilirubinaemia, the types of transcutaneous bilirubin measuring devices, and age and ethnicity/skin colour of the included infants.
What are the limitations of the evidence?
The included studies were of high methodological quality. However, we reported the results narratively and did not formally evaluate the quality of evidence using GRADE.
How up to date is this evidence?
The evidence is up to date to August 2022.
Summary of findings
Summary of findings 1. Summary of included studies.
| Study ID | Ethnicity or race | GA and PNA | Sample size and number of paired results | TcB device and site of measurement | TSB measurement and time interval | Indication for bilirubin | Phototherapy use | Cutoff values | Accuracy outcomes |
| Bhutta 1991 | Pakistani newborns, race not specified | GA: >34 weeks (term and late preterm) PNA: not reported |
63 newborns; 100 paired comparisons | JM 101: forehead | Photometry; ≤ 2 hours | Clinical jaundice | No prior phototherapy | TcB index of 17 to detect TSB > 12.5 mg/dL | Sensitivity 87%, specificity 53%, NPV 86.5%, PPV 55.6% |
| Bilgen 1998 | Turkish newborns, race not specified | GA: > 37 weeks (term) PNA: 1–5 days |
96 newborns; 96 paired comparisons | JM 101; forehead | Direct spectrophotometry; ≤ 30 minutes | Clinical jaundice | No prior phototherapy | TcB 13 mg/dL to detect TSB 12.9 mg/dL | Sensitivity 100%, specificity 55%, NPV 100%, PPV 32% |
| Christo 1988 | South Indian | GA: >37 weeks (term) and 32–37 weeks (preterm) PNA: not reported |
138 newborns; 138 paired comparisons | JM 101; forehead and sternum (average of the 2 readings) | Spectrophotometry; 30 minutes | Infants from whom blood specimen was drawn for bilirubin or other reasons | No prior phototherapy | TcB 18 mg/dL to detect TSB > 13 mg/dL | Sensitivity 95%, specificity 83%, NPV 98%, PPV 69% |
| Karrar 1989 | Saudi newborns (Riyadh, Saudi Arabia) | GA: > 37 weeks (term) PNA: 4–10 days |
155 newborns; 155 paired comparisons | JM 101; forehead | American optical bilirubinometer; time interval not reported (probably 30 minutes) | Clinical jaundice | No prior phototherapy | TcB index of 21 to detect TSB ≥ 12.5 mg/dL | Sensitivity 74.0%, specificity 90%, NPV 88.0%, PPV 78.0% |
| Maisels 1982 | White newborns | GA: > 37 weeks (term) PNA: not reported |
157 newborns; 292 paired comparisons | JM 101; forehead and sternum | Diazo method; ≤ 30 minutes | Routine bilirubin measurement except in 11 infants (measurement obtained on clinical grounds) | No prior phototherapy | Forehead TcB index of 24 to detect TSB > 12.9 mg/dL. Sternum TcB index of 23 to detect TSB > 12.9 mg/dL |
Forehead TcB: sensitivity 100%, specificity 97%, NPV 100%, PPV 58%. Sternum TcB: sensitivity 100%, specificity 96%, NPV 100%, PPV 44% |
| Schumacher 1985 | "Caucasian" (presumed white) | GA: > 36 weeks; PNA: 1–5 days |
106 newborns; 106 paired comparisons | JM 101; sternum | Direct spectrophotometry; ≤ 30 minutes | Clinical jaundice | No prior phototherapy | TcB 20 mg/dL to detect TSB > 12.9 mg/dL | Sensitivity 94%, specificity 77.5%, NPV 99%, PPV 44% |
| Taha 1984 | Saudi newborns | GA: > 37 weeks (term) PNA: 3–12 days |
68 newborns; 120 paired comparisons | JM 101; forehead | Direct spectrophotometry; near simultaneous measurements | Clinical jaundice | No prior phototherapy | TcB index of 22.5 to detect TSB > 12.9 mg/dL | Sensitivity 69%, specificity 92%, NPV 95%, PPV 58% |
| Wong 2002 | Mostly white, 6 non‐white | GA > 31 weeks (term and preterm) PNA: not reported |
64 newborns; 64 paired comparisons | JM 102 and BiliChek; forehead | Photometry; ≤ 30 minutes | Clinical jaundice | No prior phototherapy | JM 102: TcB > 170 μmol/L (10 mg/dL) to detect TSB > 250 μmol/L (14.6 mg/dL) Bilichek: TcB > 150 μmol/L (8.8 mg/dL) to detect TSB > 250 μmol/L (14.6 mg/dL) |
JM 102: sensitivity 100%, specificity 32%, NPV 100%, PPV 35% Bilichek: sensitivity 100%, specificity 21.3%, NPV 100%, PPV 31.5% |
| Bental 2009 | Mixed (Ashkenazi and Sephardic Ethiopian) at Natanya Hospital, Israel | GA: ≥ 35 weeks (late preterm) and > 37 weeks (term) PNA: ≤ 7 days |
628 newborns; 1091 paired comparisons | JM 103; forehead and sternum (mean from 2 sites was calculated) | Colorimetric method; ≤ 90 minutes | Clinical jaundice | No prior phototherapy | TcB > P75 to predict TSB > P95 on the nomogram | At 24–48 hours: sensitivity 83.3%, specificity 77.5%, NPV 99.2%, PPV 12.2% At 48–72 hours: sensitivity 100%, specificity 75.9%, NPV 100%, PPV 4.5% |
| Kitsommat 2013 | Asian newborns (not specified) | GA term and late preterm PNA: 5–14 days |
405 newborns; 455 paired comparisons | JM‐103 forehead | Diazo method; ≤ 30 minutes | Clinical jaundice | No prior phototherapy, or at least 24 hours since phototherapy | TcB 204 μmol/L (11.9 mg/dL) to detect | Sensitivity 96.0%, specificity 58.0%, NPV 97.4%, PPV 45.3% |
| Lam 2008 | Chinese newborns (Hong Kong) | GA: > 35 weeks (term and late preterm) PNA: 3–7 days |
113 newborns; 113 paired comparisons | JM 103; forehead and sternum | Spectrophotometry; ≤ 5 minutes | Clinical jaundice | No prior phototherapy | TcB 230 μmol/L (13.5 mg/dL) and 298 μmol/L (17.7mg/dL) to predict TSB > 250 μmol/L (14.6 mg/dL) | Sensitivity 100%, specificity 100%, NPV 100%, PPV 100% (for both cutoff values) |
| Mendoza‐Chuctaya 2021 | Cusco, Peru | GA: ≥ 37 weeks (term) PNA: not reported |
123 newborns; 123 paired comparisons | JM 103; forehead and sternum | TSB measurement method not reported; samples obtained 30 minutes | Clinical jaundice | No prior phototherapy | TcB > P95 and > P75 to predict TSB > P95 on the nomogram | Sternum TcB ≥ P95: sensitivity 100%, specificity 80%, NPV 100%, PPV 81% Sternum TcB ≥ P75: sensitivity 100%, specificity 26%, NPV 100%, PPV 54% Forehead TcB ≥ P95: sensitivity 93%, specificity 89%, NPV 94%, PPV 88% Forehead TcB ≥ P75: sensitivity 100%, specificity 33%, NPV 100%, PPV 56% |
| Yaser 2014 | South African newborns | GA: 24–34 weeks (preterm) PNA: < 8 days |
122 newborns; 122 paired comparisons | JM 103; forehead, sternum, interscapular area | TSB measurement method not reported; time interval not reported | Clinical jaundice | No prior phototherapy | Single cutoff values not reported as phototherapy initiation was based on unit phototherapy guidelines ("photo line") | Forehead: sensitivity 80.6%, specificity 87.3%, NPV 78.7%, PPV 88.5% Chest: sensitivity 70.2%, specificity 96.4%, NPV 72.6%, PPV 95.9% Interscapular region: sensitivity 94.0%, specificity 72.7%, NPV 90.9%, PPV 80.8% |
| Boo 2007 | Malay, Chinese, and Indian newborns | GA: 37 weeks (term) PNA: not reported |
345 newborns; 345 paired comparisons | BiliChek; forehead and sternum | Direct spectrophotometry; ≤ 30 minutes | Clinical jaundice | No prior phototherapy | Forehead: TcB 250 μmol/L (14.9 mg/dL) to detect TSB of 300 μmol/L (17.5 mg/dL). Sternum: TcB 200 μmol/L (11.7 mg/dL) to detect TSB 300 μmol/L (17.5 mg/dL) |
Forehead: sensitivity 100%, specificity 39.2%, NPV 100%, PPV 38.5%. Sternum: sensitivity 100%, specificity 33.6%, NPV 100%, PPV 36.4% |
| Campbell 2011 | White, Black Asian, Indian, Latino (Canada) | GA: ≥ 35 weeks (late preterm and term) PNA: not reported |
430 newborns; 430 paired comparisons | BiliChek; forehead | Direct spectrophotometry; ≤ 30 minutes | Clinical jaundice | No prior phototherapy | TcB 180 μmol/L (10.5 mg/dL) to detect TSB > 200 μmol/L (11.7 mg/dL) TcB 200 μmol/L to detect TSB 250 μmol/L (14.6 mg/dL) |
TcB 180 μmol/L to detect TSB 250 μmol/L: sensitivity 96%, specificity 55%, NPV 96%, PPV 64% TcB 200 μmol/L to detect TSB 250 μmol/L: sensitivity 96%, specificity 57%, NPV 97%, PPV 34% |
| Hemmati 2013 | Iranian newborns | GA: ≥ 35 weeks (term and late preterm, most infants) and ≤ 35 weeks (preterm, 5 infants) PNA: 1–15 days |
560 newborns; paired comparisons not reported | BiliChek; forehead | Spectrophotometry and Diazo method; time interval reported | Clinical jaundice | No prior phototherapy | TcB 15 mg/dL 256 μmol/L) to detect TSB > 15mg/dL (256 μmol/L) | Sensitivity 96.6%, specificity 99.0% NPV 99.8% PPV 95.7% |
| Karon 2008 | 146 "Caucasian" (presumed white) 19 Asian, 9 Hispanic, 3 African American | GA: > 32 weeks (term and late preterm) PNA: 1–4 days |
177 newborns; 177 paired comparisons | BiliChek; forehead | Diazo and vitros method; 30 minutes | Clinical jaundice | No prior phototherapy | High or high‐intermediate TcB to predict high or high‐intermediate TSB | Diazo: sensitivity 98%, specificity 40%, NPV 98%, PPV 44% Vitros: sensitivity 94%, specificity 55%, NPV 90%, PPV 68% |
| Knupfer 2001 | 128 "Caucasian" (presumed white), 7 Asian | GA: 23‐36 weeks (preterm) PNA: 2–6 days |
135 newborns; 400 paired comparisons | BiliChek; forehead | Diazo method; time interval not reported | Indication for bilirubin measurement not reported | No prior phototherapy | TcB to correctly identify various threshold for phototherapy; specific cutoff values not reported | Sensitivity 88%, specificity 89.1%, NPV 97.4%, PPV 62.2% |
| Kolman 2007 | Hispanic newborns (Phoenix, AZ) | GA: > 35 weeks (term and late preterm) PNA: 0–144 hours |
192 newborns; 192 paired comparisons | BiliChek; forehead | Diazo method; ≤ 30 minutes | Universal bilirubin screening | No prior phototherapy | TcB > P75 to predict TSB > P95 on the nomogram | Sensitivity 100%, specificity 66%, NPV 100%, PPV 16% |
| Neocleous 2014 | White newborns | GA: ≥ 37 weeks (term) PNA: 24– 96 hours |
222 newborns; 368 paired comparisons | BiliChek; forehead | Direct spectrophotometry; ≤ 20 minutes | Clinical jaundice | Prior use of phototherapy not reported | TcB 207 μmol/L (12 mg/dL) to detect TSB 205 μmol/L (12 mg/dL) TcB 265 μmol/L (15 mg/dL) to detect TSB 240 μmol/L (14 mg/dL) |
TcB 207 μmol/L to detect TSB 205 μmol/L: sensitivity 95.4%, specificity 18.6%, NPV 55.2%, PPV 79.4% (at 24 hours) TcB 265 μmol/L to detect TSB 240 μmol/L: sensitivity 94.2%, specificity 22.5%. NPV 73.8%, PPV 62.5% (at 48 hours) |
| Samanta 2004 | Race/population not reported | GA: ≥ 33 weeks (preterm) and ≥ 37 weeks (term) PNA: 1–11 days |
300 newborns; 300 paired comparisons | BiliChek; site of measurement not reported | Diazo method; near simultaneous measurements with TcB | Clinical jaundice | No prior phototherapy | TcB 195 μmol/L (11.4 mg/dL) to detect TSB 250 μmol/L (14.6 mg/dL) | Sensitivity 91% specificity 66%, NPV 96% PPV 42% |
| Bertini 2008 | Italian/white (Florence, Italy) | GA: > 32 weeks (preterm and term) PNA: not reported |
241 newborns; 241 paired comparisons | Bilitest BB 77; forehead | Spectrophotometry; 10 minutes | Clinical jaundice | No prior phototherapy | TcB 13 mg/dL to detect TSB 15 mg/dL | Sensitivity 85.7%, specificity 97.8%, NPV 99.1%, PPV 70.6% |
| Ercan 2018 | Turkish newborns, race not specified | GA: ≥ 35 weeks (term and late preterm) PNA: 2–30 days |
218 newborns; 271 paired comparisons | JH20‐1C; forehead | Spectrophotometry ≤ 10 minutes | Clinical jaundice | No prior phototherapy | TcB 205 μmol/L (12 mg/dL) to detect TSB ≥ 222 μmol/L (13 mg/dL) | Sensitivity 95.9%, specificity 59.4%, NPV 90%, PPV 77.7%; |
GA: gestational age; NPV: negative predictive value; PNA: postnatal age; PPV: positive predictive value; Px: xth percentile TcB: transcutaneous bilirubin; TSB: total serum bilirubin.
Background
Target condition being diagnosed
Hyperbilirubinaemia is a term used to describe excess of bilirubin in the blood. In newborns, hyperbilirubinaemia becomes clinically apparent as jaundice, a yellow coloration of the skin and sclera (Woodgate 2015). This condition is very common in both term and preterm newborns (affecting around 60% of newborns in the first week of life) and is caused by a predisposition to the production of bilirubin and limited ability to excrete it (Lauer 2011). Most cases of newborn jaundice are mild and resolve spontaneously (Srgo 2006). However, in rare cases, babies have very high levels of bilirubin that can lead to bilirubin encephalopathy and kernicterus (Ebbesen 2005; Srgo 2006). The acute‐phase signs of kernicterus are poor feeding, lethargy, high‐pitched cry, hypertonia or hypotonia, opisthotonos and seizures (Johnson 2002). Chronic manifestations include athetoid cerebral palsy, motor delay, gaze palsy, dental dysplasia, mental retardation and sensorineural hearing loss (AAP 2004). Studies from high‐income countries have reported incidence rates of kernicterus ranging from 0.4 to 2 per 100,000 live births (Burke 2009; Mannig 2007; Srgo 2006). However, studies from low‐income countries suggest that the incidence may be much higher (Nair 2003; Owa 2009). Following guidelines issued by the American Academy of Pediatrics for the management of jaundice in neonates, clinicians are moving away from the long‐established critical cutoff value of 20 mg/dL (342 µmol/L) total serum bilirubin (TSB) in favour of a plot of TSB against time (hours) when deciding which infants require therapy (Kemper 2022). The newer method involves comparing each infant's plot to the age‐specific nomogram to determine the line of management (Higgins 2012). Current treatments for hyperbilirubinaemia include phototherapy and, in severe cases, exchange transfusion (Woodgate 2015).
Index test(s)
Transcutaneous bilirubin (TcB) measurement is a non‐invasive method for measuring serum bilirubin level (Dai 1997). Transcutaneous bilirubinometry works by directing light into the skin and measuring the intensity of the wavelength of light that is returned (Boo 2007). This method is based on optical spectroscopy, which relates the amount of light absorption by bilirubin to the concentration of bilirubin in the skin. The technology was first introduced in 1980 (Yamanouchi 1980). In most cases, the healthcare provider takes the measurement by gently pressing the TcB meter against the sternum or forehead. The result appears in under one minute (Dai 1997). Using this point‐of‐care device saves time and may reduce costs compared to TSB measurement (Maisels 1997). However, gestational age, bodyweight and skin colour may affect the accuracy of TcB results (Knüpfer 2001). For example, TcB tends to underestimate TSB in light and medium skin colours and overestimate TSB in dark skin colours (Samiee‐Zafarghandy 2014). Many TcB devices are available, including the BiliChek device, JM 103 and JM 105 (Grohmann 2006).
Clinical pathway
Nursing staff and physicians routinely monitor newborns for the development of jaundice in the first few hours of life and before discharge from the newborn nursery. This usually involves visual inspection and skin blanching to assess for yellowish discolouration. Visual estimation of bilirubin level is unreliable (Barrington 2007). Therefore, it is necessary to obtain an objective TcB or TSB measurement. Some centres measure TcB or TSB in all infants before hospital discharge, or in all infants with risk factors for severe hyperbilirubinaemia, which include breastfeeding, ABO/Rhesus incompatibility, glucose‐6‐phosphate dehydrogenase deficiency, use of oxytocin during delivery, vacuum‐assisted delivery, prematurity and history of jaundice in a sibling (AAP 2004; Bhutani 2010; Keren 2005). Other centres only perform TcB or TSB measurement in newborns that are visibly jaundiced; the value is plotted on a nomogram to assess the need for treatment (AAP 2004). Healthcare providers can also take measurements in newborns undergoing phototherapy to decide when to stop treatment. The bilirubin levels are interpreted based on the infant's gestational age and postnatal age (Kemper 2022).
Role of index test(s)
TcB assay is a non‐invasive method for measuring bilirubin levels that may help to reduce the risk of anaemia and trauma associated with blood sampling for TSB measurement (Dai 1997). Studies have shown that TcB measurement works well in both hospital and outpatient settings, and is better than visual inspection for estimation of hyperbilirubinaemia (De Luca 2008a; Wainer 2012). Additionally, TcB measurement ensures an immediate result for rapid clinical decision‐making while reducing the risk of infection associated with all invasive procedures (Jangaard 2006). Healthcare professionals can use the TcB meter as a screening tool to estimate the serum bilirubin level in newborns who are not clinically jaundiced, or as a diagnostic tool in jaundiced newborns to assess the need for treatment (Kemper 2022).
Alternative test(s)
There are various methods for determining bilirubin levels in newborns. These include visual assessment, direct spectrophotometric methods (requiring capillary blood) and use of an icterometer (Higgins 2012). Visual assessment for jaundice is common in newborn nurseries and outpatient settings, such as physicians' offices (Harrison 1989). However, studies have shown that this method is ineffective for assessing the severity of jaundice. The icterometer is a specialised ruler marked with different shades of yellow; the operator presses it against the newborn's skin to estimate their bilirubin level (Akman 2000).
Rationale
Bilirubin measurement is one of the most frequently performed tests in newborn infants (Donzelli 2000; Madsen 2000). The current reference standard involves chemical processing. This requires repeated blood sampling, which can be painful for the newborn, costly and time consuming. TcB measurement may be a more cost‐effective and less traumatic method of measuring bilirubin levels in newborns (Dai 1996). However, to justify routine use of TcB devices, we need to systematically review all available evidence from well‐designed studies on the accuracy of TcB measurements in newborn infants. A clear understanding of the diagnostic test accuracy (DTA) of transcutaneous bilirubinometry using a variety of instruments in a variety of populations (including preterm and term infants as well as infants with various racial backgrounds) would be invaluable for understanding the usefulness of TcB measurement in newborns.
Objectives
To determine the diagnostic accuracy of transcutaneous bilirubin measurement for detecting hyperbilirubinaemia in newborns.
Methods
Criteria for considering studies for this review
Types of studies
We included DTA studies with cross‐sectional or prospective cohort design comparing TcB and TSB measurement for hyperbilirubinaemia in newborns.
We excluded randomised controlled trials, retrospective studies, case‐control studies, case reports and any studies that did not provide data for true positives, true negatives, false positives and false negatives, or studies that only reported correlation coefficients between TcB and TSB.
Participants
We included studies that evaluated TcB measurement, performed as part of universal screening or due to visible jaundice, in term or preterm infants aged 0 to 28 days with or without prior phototherapy. We included studies conducted in different clinical settings, such as neonatal intensive care units, paediatric emergency units and paediatric wards; and studies that recruited infants from home or in the community.
Index tests
The index test was TcB measurement in newborns with the use of any TcB device. Normal and abnormal TcB results were as defined by the study authors.
Target conditions
The target condition was hyperbilirubinaemia requiring phototherapy or exchange transfusion.
Reference standards
The reference standard was TSB measured in the laboratory, which requires blood sampling from the newborn. Various methods are available for measuring TSB, including high‐performance liquid chromatography (HPLC), diazo‐based methods, direct spectrophotometry and capillary electrophoresis (Higgins 2012; Kazmierczak 2002). TSB measurement by HPLC is not subject to interference from haemoglobin or lipaemia. However, this method is costly, labour‐intensive and not practical for routine use (Kazmierczak 2004). The diazo‐based methods are the most frequently used laboratory assays, but they may be affected by haemolysis (el‐Beshbishi 2009). The blood sampling required for TSB measurement causes pain and trauma to the neonate, and repeated blood sampling can cause anaemia, especially in preterm infants. There is evidence showing inter‐ and intralaboratory variability with this technique (Lo 2011). For this review, we used the threshold values for hyperbilirubinaemia as defined by the study authors.
Search methods for identification of studies
Electronic searches
The Neonatal Information Specialist (MF) designed the search strategies, which we ran without date, language, or publication type restrictions in August 2022. We searched the following databases.
Cochrane Central Register of Controlled Trials (CENTRAL) via CRS Web (2022, Issue 8)
Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process, In‐Data‐Review & Other Non‐Indexed Citations, Daily and Versions (1946 to 18 August 2022)
Embase (1974 to 18 August 2022)
CINAHL Complete via EBSCOhost (18 August 2022)
We searched the following clinical trial registries for ongoing or recently completed trials.
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP; who.int/ictrp/search/en)
U.S. National Library of Medicine (NIH) ongoing trials register ClinicalTrials.gov (clinicaltrials.gov)
ISRCTN Registry (isrctn.com)
Search strategies are available in Appendix 1, Appendix 2, Appendix 3, Appendix 4 and Appendix 5. We managed search results using Endnote. We identified and removed duplicates using Endnote and Covidence.
Searching other resources
We checked the reference lists of all included studies and relevant systematic reviews for other potentially eligible studies.
Data collection and analysis
Selection of studies
Two review authors (CO and AO) independently screened the titles and abstracts of the records recovered by the searches, excluding those they considered clearly ineligible. We retrieved the full‐text reports of all remaining records, and the same two review authors assessed them against our eligibility criteria. We resolved any disagreement through discussion or by involving a third review author, if necessary. We used Covidence for the screening process.
Data extraction and management
Two review authors (CO and AO) independently extracted data on study characteristics using a standard piloted data extraction form. Where possible, we computed 2 × 2 tables of true positives, false positives, true negatives, and false negatives for the index tests at the thresholds reported in the primary study. For each included study, we extracted the following information.
First author's last name and year of publication
Country of study and race/ethnicity of participants
Gestational age of included newborns
Number of enrolled infants and number of paired TcB and TSB results
Type of TcB device and site of measurement
Time interval between TcB and TSB measurement
Prior use of phototherapy
Accuracy outcomes (sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV)).
Table 2 presents the information we extracted from each study.
1. Data extraction form.
| [Study ID] | First author, year of publication |
| Type of study | Journal article, unpublished data |
| Participants | Sample size Country of study Baseline characteristics (gestational age, postnatal age, race/ethnicity, bodyweight) |
| Study design | Retrospective/prospective design Sample (consecutive, random, or unclear) |
| Study criteria | Inclusion criteria Exclusion criteria |
| Reference standard | Name of assay/manufacturer Name of instrument Cutoff values Time between reference standard performance and TcB measurement Blinding of operator to TcB result |
| Index test | Name of device Cutoff values Operator training Site of measurement (forehead, sternum) |
| Target condition | Universal screening of newborns for hyperbilirubinaemia Diagnostic determination of hyperbilirubinaemia in visibly jaundiced newborns |
| Data | Number of true positives and false positives Number of true negatives and false negatives Number of undetermined/uninterpretable results Sensitivity and specificity of index test Number of missing results for index test Number of missing results for reference standard |
| Notes | Source of funding (whether any author is affiliated with the manufacturer of the index test; the study was directly funded by the manufacturer; whether authors reported conflicts of interests related to the manufacturer or other funding sources) |
TcB: transcutaneous bilirubin.
Assessment of methodological quality
Two review authors (CO and AO) independently assessed the methodological quality of each study using the Quality Assessment of Diagnostic Accuracy Studies‐2 (QUADAS‐2) tool, which consists of four domains: patient selection, index tests, reference standard and flow and timing (Whiting 2011). We developed a rating guideline to ensure consistency when assessing each domain. We assessed risk of bias in all four domains, and we assessed applicability in the first three domains. We piloted our review‐specific QUADAS‐2 tool on five studies to identify possible areas of discrepancy between review authors. We resolved any discrepancies by consensus. Appendix 6 presents the items of the QUADAS‐2 tool and our scoring interpretations for each item.
Statistical analysis and data synthesis
We performed statistical analysis according to Cochrane guidelines for DTA reviews (Macaskill 2010). We included studies that reported sufficient data to construct a 2 × 2 table. For all included studies, we used the data in the 2 x 2 tables to calculate the sensitivity of TcB measurement for detecting hyperbilirubinaemia, need for phototherapy or categorization into various hyperbilirubinaemia risk categories on the TSB nomograms. To calculate specificity, we divided the number of true negatives by the number of participants without the target condition (true negatives plus false positives). Higher specificity at a particular cutoff value represents greater accuracy of the test to correctly identify individuals who do not have the target condition. We had planned to use the 2 × 2 tables to calculate sensitivity and specificity for each study and to meta‐analyse sensitivities and specificities, where appropriate, using the bivariate model (if studies used same threshold for positivity). However, because the studies used different index tests and cutoff values, we were unable to perform a meta‐analysis and have reported the results narratively.
Investigations of heterogeneity
We had planned to investigate heterogeneity by visual examination of both the ROC plot of raw data and the forest plots of sensitivities and specificities. However, we were unable to do this because meta‐analysis was not appropriate.
Sensitivity analyses
We did not perform any sensitivity analyses because we did not combine data from different studies in a meta‐analysis.
Assessment of reporting bias
We had not planned to use funnel plots to evaluate the impact of publication bias or other biases associated with small studies.
Results
Results of the search
Database searches identified 2056 references. After removing 876 duplicates, we screened the titles/abstract of 1180 records, of which we excluded 960. We retrieved and evaluated 220 full‐text articles and excluded 197 (Characteristics of excluded studies). We included 23 studies (Characteristics of included studies). Figure 1 presents the study selection process in a flow diagram.
1.
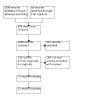
Flow diagram
Methodological quality of included studies
We assessed the risk of bias of each included study using the QUADAS‐2 tool. We judged most studies at low risk of bias across the four domains (Figure 2; Figure 3). The Characteristics of included studies table provides a detailed description of the risk of bias judgements. Applicability concerns were low in all studies across the domains of patient selection, index test and reference standard.
2.

Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies.
3.

Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study.
Findings
The 23 included studies have a total sample size of 5058 newborns. We were unable to ascertain the total number of paired comparisons of TcB and TSB because one study did not provide this information (Hemmati 2013). In the remaining 22 studies, there were 5750 paired comparisons of TcB and TSB. The number of comparisons is higher than the number of newborns because some studies obtained more than one paired TcB and TSB result (Bental 2009; Bhutta 1991; Ercan 2018; Kitsommart 2013; Knupfer 2001; Maisels 1982; Neocleous 2014). The studies were published between 1982 and 2021 and varied in terms of population characteristics/ethnicity, gestational age and postnatal age of the included infants, sample size and number of paired measurements of TcB and TSB, the TcB device used, site of TcB measurement, type of method used for serum bilirubin assay in the laboratory, time interval between TcB and TSB measurement, indication for measurement of bilirubin, and whether the participants received phototherapy or not prior to measurement of TcB and TSB. Most studies took place in well‐baby nurseries; a few were conducted in outpatient settings or the emergency department (Kitsommart 2013; Lam 2008; Maisels 1982). Different race groups were represented across the studies. Some studies included a mixture of infants from different ethnic backgrounds, while others focused on newborns from a specific ethic group. The different ethnic groups/races included, but were not limited to, white infants (Bertini 2008: Maisels 1982; Schumacher 1985), Chinese infants (Lam 2008), African infants (Yaser 2014), and Hispanic infants (Kolman 2007). The gestational age of the newborns also differed across the included studies. Most studies included late preterm newborns (from 35 weeks' gestational age) and term newborns (Bental 2009; Bhutta 1991; Campbell 2011; Ercan 2018; Hemmati 2013; Kitsommart 2013; Kolman 2007; Lam 2008; Schumacher 1985), while other studies included preterm infants (under 35 weeks' gestation; Knupfer 2001; Yaser 2014), or only term newborns (Bilgen 1998; Boo 2007; Karrar 1989; Maisels 1982; Mendoza‐Chuctaya 2021; Neocleous 2014; Taha 1984).
Postnatal age varied significantly across the studies and ranged from birth to 28 days of life. Some studies did not report postnatal age of the infants. Sample sizes ranged from 63 infants (Bhutta 1991) to 628 infants (Bental 2009), and the number of paired measurements of TcB and TSB ranged from 63 (Bhutta 1991) to 1091 (Bental 2009). Some studies included multiple paired measurements of TcB and TSB from the same participants. The number of paired TcB and TSB measurements corresponded to the number of newborns in eight studies (Bertini 2008; Bilgen 1998; Boo 2007; Campbell 2011; Christo 1988; Kolman 2007; Lam 2008; Schumacher 1985).
The studies assessed TcB measurement using various devices (JM 101, JM 102, JM 103, BiliChek, Bilitest and JH20‐1C) compared with TSB measurement in the laboratory by different methods (including HPLC, direct spectrophotometry and diazo method). All studies reported measures of accuracy such as sensitivity or specificity using a specific TSB threshold as diagnostic for hyperbilirubinaemia. The Pearson correlation coefficient is a measure of association and not a valid measure for diagnostic accuracy. Therefore, we did not report the findings of the Pearson correlation coefficient from the included studies. We focused on studies that provided estimates of sensitivity and specificity at specified thresholds, even if the studies did not report association between TcB and TSB using the Pearson correlation coefficient. The remainder of this section provides a narrative summary of the study findings, including details of the TcB devices used, study populations and the DTA results for various reported threshold values. The studies reported the TcB or TSB result either in mg/dL or in μmol/L.
JM 101 transcutaneous device
Seven studies measured TcB with the JM 101 jaundice meter (Bhutta 1991; Bilgen 1998; Christo 1988; Karrar 1989; Maisels 1982; Schumacher 1985; Taha 1984). See Figure 4 and Figure 5.
4.

Forest plot of Analysis 1 (JM 101).
5.

Summary ROC Plot of Analysis 1 (JM 101).
Bhutta 1991 included 63 late preterm and term Pakistani newborns infants with no prior phototherapy, and assessed the accuracy of the JM 101 applied to the forehead to predict a serum bilirubin of 12.5 mg/dL or greater. TSB measurement was by spectrophotometry using an autoanalyser, and the blood sample was obtained at the time of TcB measurement. There were 100 paired comparisons of TcB and TSB from the 63 newborns. At TSB 12.5 mg/dL, the TcB index was 17, with the following accuracy outcomes.
Sensitivity 88%
Specificity 53%
NPV 85.6%
PPV 55.6%
The study authors did not undertake ROC curve analysis to generate a cutoff with maximal sensitivity and specificity.
Bilgen 1998 included 96 term Turkish newborns between one and five days of life with no prior phototherapy, and assessed the accuracy of the JM 101 applied to the forehead to detect significant hyperbilirubinaemia (TSB above 12.9 mg/dL). TSB measurement was by spectrophotometry, and the blood sample was obtained within 30 minutes of TcB measurement. There were 96 paired comparisons of TcB and TSB. A TcB cutoff of 13 mg/dL could detect TSB levels greater than 12.9 mg/dL with the following accuracy outcomes.
Sensitivity 100%
Specificity 56%
NPV 100%
PPV 32%
The study authors did not undertake ROC curve analysis to evaluate different cutoff values.
Christo 1988 included 138 preterm (32 to 37 weeks' gestation) and term South Indian newborns with no prior phototherapy, and assessed the accuracy of the JM 101 applied to the forehead and sternum. The investigators took the average of two readings, one each from the sternum and forehead. TSB measurement was by spectrophotometry using the Technicon RA1000 Autoanalyser, and the blood sample was obtained within 30 minutes of TcB measurement. There were 138 paired comparisons of TcB and TSB. A TcB cutoff of 18 mg/dL to detect a TSB level above 13 mg/dL had the following accuracy outcomes.
Sensitivity 95%
Specificity 83%
NPV 98%
PPV 69%
Karrar 1989 included 155 Saudi term newborns between four and 10 days of life with no prior phototherapy, and assessed the accuracy of the JM 101 applied to the forehead. TSB measurement was by optical bilirubinometry, and the blood sample was obtained within 30 minutes of TcB measurement. There were 155 paired comparisons of TcB and TSB. A TcB index of 21 to detect a TSB level of 12.5 mg/dL or greater had the following accuracy outcomes.
Sensitivity 74%
Specificity 90%
NPV 78%
PPV 88%
Maisels 1982 included 157 white term newborns with no prior phototherapy, and assessed the accuracy of the JM 101 applied to the forehead and sternum. TSB measurement was by the diazo method, and the blood sample was obtained within 30 minutes of TcB measurement. There were 292 paired comparisons of TcB and TSB. There were different reported sensitivities and specificities for different TcB cutoffs. For TcB measured at the forehead, a TcB index of 24 to detect a TSB level above 12.9 mg/dL had the following accuracy outcomes.
Sensitivity 100%
Specificity 97%
NPV 100%
PPV 58%
For TcB measured at the sternum, a TcB index of 23 to detect a TSB level above 12.9 mg/dL had the following accuracy outcomes.
Sensitivity 100%
Specificity 96%
NPV 100%
PPV 44%
Schumacher 1985 included 106 white late preterm and term newborns (more than 36 weeks' gestation) between one and five days of life with no prior phototherapy, and assessed the accuracy of the JM 101 applied to the sternum. TSB measurement was by direct spectrophotometry, and the blood sample was obtained within 30 minutes of TcB measurement. There were 106 paired comparisons of TcB and TSB. A TcB cutoff of 20 mg/dL to detect a TSB level above 12.9 mg/dL had the following accuracy outcomes.
Sensitivity 94%
Specificity 77.5%
NPV 99%
PPV 44%
Taha 1984 included 68 Saudi term newborns between three and 12 days of life with no prior phototherapy, and assessed the accuracy of the JM 101 applied to the forehead. TSB measurement was by spectrophotometry using the AO bilirubinometer, and the blood sample was obtained at the time of TcB measurement. There were 120 paired comparisons of TcB and TSB. A TcB index of 22.5 mg/dL to detect a TSB level above 12.9 mg/dL had the following accuracy outcomes.
Sensitivity 69%
Specificity 92%
NPV 95%
PPV 58%
JM 102 transcutaneous device
Only Wong 2002 evaluated the JM 102 transcutaneous device. Wong 2002 included 64 mostly white preterm (less than 31 weeks' gestation) and term newborns between one and 17 days old with no prior phototherapy, and assessed the accuracy of the JM 102 applied to the forehead. TSB measurement was by spectrophotometry, and the blood sample was obtained within 30 minutes of TcB measurement. There were 64 paired comparisons of TcB and TSB. A TcB cutoff of 170 μmol/L to detect a TSB level above 250 μmol/L (14.6 mg/dL) had the following accuracy outcomes.
Sensitivity 100%
Specificity 32%
NPV 100%
PPV 35%
JM 103 transcutaneous device
Five studies evaluated the JM 103 TcB measurement device (Bental 2009; Kitsommart 2013; Lam 2008; Mendoza‐Chuctaya 2021; Yaser 2014).
Bental 2009 included 628 late preterm (more than 35 weeks' gestation) and term newborns in the first week of life with no prior phototherapy, and assessed the accuracy of the JM 103 applied to the forehead and sternum. TSB measurement was by colorimetric assay, and the blood sample was obtained within 90 minutes of TcB measurement. There were 1091 paired comparisons of TcB and TSB. The study authors assessed the ability of TcB above the 75th percentile for age to predict significant hyperbilirubinaemia (TSB level above the 95th percentile for age) between 24 and 48 hours of life and between 48 and 72 hours of life. A TcB level above the 75th percentile between 24 and 48 hours of life had the following accuracy outcomes.
Sensitivity 83.3%
Specificity 77.5%
NPV 99.2%
PPV 12.2%
Between 48 and 72 hours of life, the accuracy outcomes of a TcB level above the 75th percentile were as follows.
Sensitivity 100%
Specificity 75.9%
NPV 100%
PPV 4.5%
Kitsommart 2013 included 405 Asian late preterm (from 35 weeks' gestation) and term newborns between five and 14 days of life with no prior phototherapy, and assessed the accuracy of the JM 103 applied to the forehead. TSB measurement was by the diazo method, and the blood sample was obtained within 30 minutes of TcB measurement. There were 455 paired comparisons of TcB and TSB. The study authors undertook a ROC curve analysis, finding an area under the curve for TcB (average of three measurements) of 0.86, and an area under the curve for a single TcB measurement of 0.85. They did not assess the optimal TcB cutoff with the greatest sensitivity and specificity to detect TSB levels of 255 μmol/L (14.9 mg/dL) or greater. The JM 103 device was found to have the following accuracy outcomes.
Sensitivity 96%
Specificity 58%
NPV 97.4%
PPV 45.3%
Lam 2008 included 133 Chinese late preterm (from 35 weeks' gestation) and term newborns between three and seven days of life with no prior phototherapy, and assessed the accuracy of the JM 103 applied to the forehead and sternum. TSB measurement was by spectrophotometry, and the blood sample was obtained within five minutes of TcB measurement. There were 113 paired comparisons of TcB and TSB. TcB cutoff values of 230 μmol/L (13.5 mg/dL) and 298 μmol/L (17.7mg/dL) to detect a TSB level of 250 μmol/L (14.6 mg/dL) or greater had the following accuracy outcomes.
Sensitivity 100%
Specificity 100%
NPV 100%
PPV 100%
Mendoza‐Chuctaya 2021 included 123 term newborns without prior phototherapy; it did not report the postnatal age or ethnicity of the infants. The study assessed the accuracy of JM 103 applied to the forehead and sternum. The TSB measurement method was not specified. There were 123 paired comparisons of TcB and TSB. A sternum TcB cutoff value of 95th percentile to predict a TSB level of the 95th percentile or higher had the following accuracy outcomes.
Sensitivity 100%
Specificity 80%
NPV 100%
PPV 81%
A sternum TcB cutoff value of 75th percentile to predict a TSB level of the 95th percentile or higher had the following accuracy outcomes:
Sensitivity 100%
Specificity 26%
NPV 100
PPV 54%
A forehead TcB cutoff value of 95th percentile to predict a TSB level of the 95th percentile or higher had the following accuracy outcomes
Sensitivity 93%
Specificity 89%
NPV 94%
PPV 88%
A forehead TcB cutoff value of 75th percentile to predict a TSB level of the 95th percentile or higher had the following accuracy outcomes.
Sensitivity 100%
Specificity 33%
NPV 100%
PPV 56%
Yaser 2014 included 122 preterm neonates (less than 35 weeks' gestation) aged under 8 days in whom serum bilirubin had been requested and phototherapy had not been initiated. The study assessed the accuracy of JM 103 applied to different body sites of TcB to detect the need for phototherapy. The study did not report single cutoff values, as phototherapy initiation was based on unit phototherapy guidelines ("photo line"). The forehead TcB had the following accuracy outcomes.
Sensitivity 80.6 %
Specificity 87.3%
NPV 78.7%
PPV 88.5%
TcB measured at the chest had the following accuracy outcomes.
Sensitivity 70.2%
Specificity 96.4%
NPV 72.6%
PPV 95.9%
TcB measured at the interscapular region had the following accuracy outcomes.
Sensitivity 94.0%
Specificity 72.7%
NPV 90.9%
PPV 80.8%
BiliChek transcutaneous device
Nine studies evaluated BiliChek (Boo 2007; Campbell 2011; Hemmati 2013; Karon 2008;og Knupfer 2001; Kolman 2007; Neocleous 2014; Samanta 2004; Wong 2002).
Boo 2007 included 345 term neonates (from 37 weeks' gestation) and preterm neonates (33 to 37 weeks' gestation) with no prior use of phototherapy. The study assessed the accuracy of the Bilichek applied to the sternum and the forehead. There were 345 paired comparisons of TcB and TSB. TcB measured at the forehead with a cutoff value of 250 μmol/L to detect TSB of 300 μmol/L or greater had the following accuracy outcomes.
Sensitivity 100%
Specificity 39.2%
NPV 100%
PPV 38.5%
TcB measured at the sternum with cutoff value of 200 μmol/L to detect TSB of 300 μmol/L or more had the following accuracy outcomes.
Sensitivity 100%
Specificity 33.6%
NPV 100%
PPV 36.4%
Campbell 2011 included 430 late preterm infants (up to 35 weeks' gestation) and term neonates (from 37 weeks' gestation) with no prior use of phototherapy. This study did not report the postnatal age of the infants. It assessed the accuracy of BiliChek applied to the forehead. A TcB cutoff value of 180 μmol/L had the following accuracy outcomes to detect a TSB value of 200 μmol/L or greater.
Sensitivity 96%
Specificity 55%
NPV 96%
PPV 64%
A TcB cutoff value of 200 μmol/L had the following accuracy outcomes to detect a TSB value of 250 μmol/L or greater.
Sensitivity 96%
Specificity 57%
NPV 97%
PPV 34%
Hemmati 2013 included 560 preterm neonates (up to 35 weeks' gestation), late preterm and term newborns (from 35 weeks' gestation) aged between 1 and 15 days with no prior use of phototherapy. The study assessed the accuracy of Bilichek applied to the forehead. The number of paired comparisons between TcB and TSB was not reported. A TcB cutoff of 256 μmol/L to detect a TSB value of 256 μmol/L had the following accuracy outcomes.
Sensitivity 96.6%
Specificity 99.0%
NPV 99.8%
PPV 95.7%
Karon 2008 included 177 preterm and term newborns (from 32 weeks' gestation) between one and four days of life with no prior use of phototherapy. The study assessed the accuracy of TcB measured at the forehead. TcB measured from the forehead in the high or high‐intermediate range to predict TSB in the high or high‐intermediate range according to the diazo method had the following accuracy outcomes.
Sensitivity 98%
Specificity 40%
NPV 98%
PPV 44%
TcB measured from the forehead in the high or high‐intermediate range to predict TSB in the high or high‐intermediate range according to the vitros method had the following accuracy outcomes.
Sensitivity 94%
Specificity 55%
NPV 90%
PPV 68%
Knupfer 2001 included 135 preterm newborns (23 to 36 weeks' gestation) between two and six days of life with no prior use of phototherapy. The study assessed the accuracy of Bilichek applied to the forehead, and did not report any specific cutoff values. There were 400 paired comparisons of TcB and TSB. TcB measured from the forehead had the following accuracy measures to detect TSB level at or above the threshold for phototherapy.
Sensitivity 88%
Specificity 89.1%
NPV 97.4%
PPV 62.2%
Kolman 2007 included 192 late preterm and term newborns (from 35 weeks' gestation) between birth and six days of life with no prior use of phototherapy. The study assessed the accuracy of Bilichek applied to the forehead. There were 192 paired comparisons of TcB and TSB. A TcB cutoff of 75th percentile on the nomogram had the following accuracy measures to predict a TSB above the 95th percentile.
Sensitivity 100%
Specificity 66%
NPV 100%
PPV 16%
Neocleous 2014 included 222 term newborns (from 37 weeks' gestation) between one and four days of life with no prior use of phototherapy. The study assessed the accuracy of Bilichek applied to the forehead. There were 368 paired comparisons of TcB and TSB. A TcB cutoff of 207 μmol/L had the following accuracy outcomes to detect a TSB level of 205 μmol/L or greater.
Sensitivity 95.4%
Specificity 18.6%
NPV 55.2%
PPV 79.4%
A TcB cutoff of 265 μmol/L had the following accuracy outcomes to detect a TSB level of 240 μmol/L or greater.
Sensitivity 94.2%
Specificity 22.5%
NPV 73.8%
PPV 62.5%
Samanta 2004 included 300 preterm newborns (from 33 weeks' gestation) and term newborns (from 37 weeks' gestation) aged between one and 11 days with no prior use of phototherapy. The study assessed the accuracy of Bilichek (measurement site not specified). There were 300 paired comparisons between TcB and TSB. A TcB cutoff of 195 μmol/L to detect a TSB level of 250 μmol/L or greater had the following accuracy outcomes.
Sensitivity 91%
Specificity 66%
NPV 96%
PPV 42%
Wong 2002 included 64 preterm and term newborns (from 31 weeks' gestation) with no prior use of phototherapy. The study did not report the postnatal age of the infants. The study assessed the accuracy of Bilichek applied to the forehead. There were 64 paired comparisons between TcB and TSB. A TcB cutoff of 150 μmol/L to detect a TSB value of 250 μmol/L or greater had the following accuracy outcomes.
Sensitivity 100%
Specificity 21.3%
NPV 100%
PPV 31.5%
6.

Summary ROC plot of Analysis 4: BiliChek (cutoff > 250 μmol/L).
7.

Summary ROC plot of Analysis 5: BiliChek (cutoff > 200 μmol/L)
Bilitest transcutaneous device
Bertini 2008 included 241 preterm and term newborns (from 32 weeks' gestation) with no prior phototherapy, and assessed the accuracy of Bilitest BB 77 applied to the forehead. TSB measurement was by spectrophotometry, and the blood sample was obtained within ten minutes of TcB measurement. There were different reported sensitivities and specificities for different TcB cutoffs. A TcB cutoff of 222 μmol/L (13 mg/dL) to detect a TSB level of 256 μmol/L (15 mg/dL) or greater had the following accuracy outcomes.
Sensitivity 85.7%
Specificity 97.8%
NPV 99.1%
PPV 70.6%
JH20‐1C transcutaneous device
Ercan 2018 included 218 late preterm and term newborns between two and 30 days of life. The study authors reported multiple cutoffs in their paper. The main result of interest is that a TcB cutoff of 205 μmol/L (12 mg/dL) determined a TSB level of at least 222 μmol/L (13 mg/dL) with the following accuracy outcomes.
Sensitivity 95.9%
Specificity 59.4%
NPV 90%
PPV 77.7%
Discussion
The American Academy of Pediatrics and the Canadian Paediatric Society recommend screening newborns for hyperbilirubinaemia with either TcB or TSB before discharge from hospital. Findings from our review support the AAP guideline that recommends TcB as a suitable alternative measure in newborns (AAP 2004). Unlike authors of previous reviews, we did not restrict our search to newborns of a particular gestational age or from a specific ethnic background or race. Another difference between out review and previous reviews is that we reported valid DTA measures (sensitivity, specificity, NPV and PPV) and not correlation between TcB and TSB (Moey 2016; Nagar 2013; Nagar 2016).
Summary of main results
We tabulated and summarised the main results from this review of studies on the accuracy of various TcB devices in different populations of newborns (Table 1). The studies compared TcB with TSB for measuring bilirubin in both term and preterm newborns. The studies evaluated the accuracy of TcB measurement performed for different reasons, including to predict significant hyperbilirubinaemia (TSB above 95th percentile on the Bhutani nomogram) or to predict TSB levels above specific thresholds for hyperbilirubinaemia that require phototherapy. Findings from this review demonstrate that there is extensive evidence on the use of various TcB devices in newborns from different ethnic groups and in different settings. The JM‐101, JM‐102, JM 103 and BiliChek all showed high sensitivity and specificity compared with the different methods for TSB measurement in the laboratory. We thus conclude that TcB measurement is an accurate method for detecting clinically significant hyperbilirubinaemia in newborns.
Strengths and weaknesses of the review
To the best of our knowledge, this is the first systematic review to evaluate the accuracy of TcB measurement in newborn infants. We conducted a comprehensive systematic review to evaluate the use of TcB measurement in newborns of varying gestational and postnatal age and from different ethnic backgrounds and races. Our search strategies identified over 200 studies evaluating the accuracy of TcB devices. However, most of these studies did not meet the inclusion criteria and were excluded. Unlike many previous systematic reviews, we summarised results from studies that reported measures of accuracy such as sensitivity and specificity and excluded studies that only reported the correlation between TcB and TSB, which is not a clinically meaningful measure to recommend the use of TcB for estimating serum bilirubin levels (Hynes 2020; Nagar 2013).
Applicability of findings to the review question
Diagnosis of hyperbilirubinaemia in newborns is dependent on gestational and chronological age. There is no set bilirubin cutoff level for diagnosis of hyperbilirubinaemia in all newborns. The threshold serum bilirubin for phototherapy or exchange blood transfusion also varies with the gestational and chronological age of the newborn. Various nomograms have been developed to assess risk classification for subsequent severe hyperbilirubinaemia in newborns. Given the clinical heterogeneity of the populations of the included studies, it is not surprising that they evaluated different thresholds for hyperbilirubinaemia. We presented the results narratively because this clinical heterogeneity precluded meta‐analysis.
Authors' conclusions
Implications for practice.
An increasing number of newborn nurseries are using transcutaneous bilirubin (TcB) devices. It is important to determine the accuracy of TcB devices in relation to total serum bilirubin (TSB) measurement. There is substantial published evidence on the accuracy of TcB devices in different settings and in different populations of newborns. Guidelines published by the American Academy of Pediatrics and the Canadian Paediatric Society suggest that TcB measurement is a suitable substitute for TSB for evaluating risk of significant neonatal hyperbilirubinaemia in healthy newborns (AAP 2004; CPS 2007). A variety of TcB devices are being used in many countries and settings. TcB measurement is a non‐invasive, painless method of measuring bilirubin, whereas TSB requires blood sampling through heel stick or venipuncture. Furthermore, TcB results are available almost instantaneously. Previous guidelines suggested that there was insufficient evidence to recommend one device over another (Kazmierczak 2006).
Implications for research.
We screened over 1100 studies and included 23 studies with 4836 participants. Detailed assessment of the included studies enabled us to identify specific implications for future studies or research on TcB accuracy. Future studies should assess the impact of various factors, including gestational age, chronological age, and race or skin tone, on the accuracy of TcB measurement. It is important to evaluate true measures of accuracy, such as sensitivity and specificity, using prespecified threshold values for hyperbilirubinaemia in specific population of newborns. Several studies of the JM devices suggest that sternum measurements correlate better with TSB compared to forehead measurements (Yamauchi 1991). However, the device manufacturers suggests that either location is acceptable. Evidence from future studies could help to clarify the best site for TcB measurement and the reasons for any differences between forehead and sternum measurements. In addition, there is a need to ascertain the impact of phototherapy on TcB measurement accuracy, and to evaluate the optimal timing after phototherapy when TcB readings are comparable to those obtained in newborns without prior phototherapy exposure.
History
Protocol first published: Issue 5, 2017
Acknowledgements
We would like to acknowledge the South African Medical Research Council for providing funding to conduct this review. We would also like to acknowledge the Cochrane Incentive fund.
We would like to thank the following members of Cochrane Neonatal for providing editorial and administrative support: Michelle Fiander, Managing Editor; Colleen Ovelman, former Managing Editor; Roger Soll and Bill McGuire, Co‐ordinating Editors. Michelle Fiander, Information Specialist, designed and ran the literature searches.
We thank the Cochrane DTA Editorial Team for reviewing this manuscript.
We thank Julia Turner for copy editing the manuscript.
Appendices
Appendix 1. Cochrane CRS strategy
| Cochrane CRS 19 August 2022 | ||
| # | Searches | Results |
| 1 | ((cutaneous* or dermal* or epicutaneous* or percutaneous* or skin or transcutaneous* or trans‐cutaneous or subcutaneous* or sub‐cutaneous*) adj4 bilirubin*) AND CENTRAL:TARGET | 131 |
| 2 | MESH DESCRIPTOR Bilirubin EXPLODE ALL AND CENTRAL:TARGET | 924 |
| 3 | (bilirubin* or bliverdin*) AND CENTRAL:TARGET | 10431 |
| 4 | MESH DESCRIPTOR Administration, Cutaneous EXPLODE ALL AND CENTRAL:TARGET | 3992 |
| 5 | ((cutaneous* or dermal* or epicutaneous* or percutaneous* or skin or transcutaneous* or trans‐cutaneous or subcutaneous* or sub‐cutaneous*) adj3 (administ* or device or devices or measur* or meter* or metre* or monitor? or test*)) AND CENTRAL:TARGET | 31790 |
| 6 | (#2 OR #3) AND (#4 OR #5) | 292 |
| 7 | ((cutaneous* or dermal* or epicutaneous* or percutaneous* or skin or transcutaneous* or trans‐cutaneous or subcutaneous* or sub‐cutaneous*) adj3 bilirubin* adj3 (device or devices or measur* or meter* or metre* or monitor? or test*)) AND CENTRAL:TARGET | 43 |
| 8 | (TcB or bilicare? or bilicheck* or bilichek or Bilicheck* or BiliMed or bilmeter* or JM‐103* or JM103* or JM‐105 or bilirubinometre* or bilirubinometer* or Bilitest or jaundice meter* or jaundicemeter* or icterometer*) AND CENTRAL:TARGET | 113 |
| 9 | #8 OR #7 | 138 |
| 10 | MESH DESCRIPTOR Infant, Newborn EXPLODE ALL AND CENTRAL:TARGET | 17947 |
| 11 | MESH DESCRIPTOR Intensive Care, Neonatal EXPLODE ALL AND CENTRAL:TARGET | 361 |
| 12 | MESH DESCRIPTOR Intensive Care units, Neonatal EXPLODE ALL AND CENTRAL:TARGET | 905 |
| 13 | MESH DESCRIPTOR Gestational Age EXPLODE ALL AND CENTRAL:TARGET | 2938 |
| 14 | MESH DESCRIPTOR Infant, Premature EXPLODE ALL AND CENTRAL:TARGET | 4263 |
| 15 | MESH DESCRIPTOR Hyperbilirubinemia, Neonatal EXPLODE ALL AND CENTRAL:TARGET | 377 |
| 16 | (babe or babes or baby* or babies or gestational age? or infant? or infantile or infancy or low birth weight or low birthweight or neonat* or neo‐nat* or newborn* or new born? or newly born or premature or pre‐mature or pre‐matures or prematures or prematurity or pre‐maturity or preterm or preterms or pre term? or preemie or preemies or premies or premie or VLBW or VLBWI or VLBW‐I or VLBWs or LBW or LBWI or LBWs or ELBW or ELBWI or ELBWs or NICU or NICUs) AND CENTRAL:TARGET | 102544 |
| 17 | #15 OR #14 OR #13 OR #12 OR #11 OR #10 OR #16 | 102544 |
| 18 | #1 AND #17 | 111 |
| 19 | #6 AND #17 | 64 |
| 20 | #9 AND #17 | 85 |
| 21 | #18 OR #19 OR #20 | 144 |
Appendix 2. MEDLINE strategy
| Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process, In‐Data‐Review & Other Non‐Indexed Citations, Daily and Versions 1946 to 18 August 2022 | ||
| # | Searches | Results |
| 1 | ((cutaneous* or dermal* or epicutaneous* or percutaneous* or skin or transcutaneous* or trans‐cutaneous or subcutaneous* or sub‐cutaneous*) adj4 bilirubin*).ti,ab,kw,kf. [Keyword Transcutaneous Bilirubinometry and Synonyms] | 610 |
| 2 | exp bilirubin/ [no Mesh for bilirubin blood level] | 25906 |
| 3 | (bilirubin* or bliverdin?).ti,ab,kw,kf. | 43249 |
| 4 | Administration, Cutaneous/ or ((cutaneous* or dermal* or epicutaneous* or percutaneous* or skin or transcutaneous* or trans‐cutaneous or subcutaneous* or sub‐cutaneous*) adj3 (administ* or device or devices or measur* or meter* or metre* or monitor? or test*)).ti,ab,kw,kf. | 101466 |
| 5 | (or/2‐3) and 4 [Bilirubin and Cutaneous Administration] | 369 |
| 6 | ((cutaneous* or dermal* or epicutaneous* or percutaneous* or skin or transcutaneous* or trans‐cutaneous or subcutaneous* or sub‐cutaneous*) adj3 bilirubin* adj3 (device or devices or measur* or meter* or metre* or monitor? or test*)).ti,ab,kw,kf. | 243 |
| 7 | (TcB or bilicare? or bilicheck* or bilichek or Bilicheck* or BiliMed or bilmeter* or JM‐103* or JM103* or JM‐105 or bilirubinometre* or bilirubinometer* or Bilitest or jaundice meter* or jaundicemeter* or icterometer*).ti,ab,kw,kf. | 1463 |
| 8 | or/6‐7 [Cutaneous Bilirubin OR devices] | 1524 |
| 9 | exp Infant, Newborn/ or Intensive Care, Neonatal/ or Intensive Care Units, Neonatal/ or Gestational Age/ or exp Infant, Premature, Diseases/ or exp Hyperbilirubinemia, Neonatal/ | 710340 |
| 10 | (babe or babes or baby* or babies or gestational age? or infant? or infantile or infancy or low birth weight or low birthweight or neonat* or neo‐nat* or newborn* or new born? or newly born or premature or pre‐mature or pre‐matures or prematures or prematurity or pre‐maturity or preterm or preterms or pre term? or preemie or preemies or premies or premie or VLBW or VLBWI or VLBW‐I or VLBWs or LBW or LBWI or LBWs or ELBW or ELBWI or ELBWs or NICU or NICUs).ti,ab,kw,kf. | 1006385 |
| 11 | or/9‐10 [Filter: Neonatal Population 04‐2022‐MEDLINE] | 1317137 |
| 12 | exp animals/ not humans/ | 5038073 |
| 13 | (1 and 11) not 12 [Cutaneous Bilirubinometry Keyword] | 543 |
| 14 | (5 and 11) not 12 [Bilirubin and Cutaneous Administration and Neonate] | 273 |
| 15 | (8 and 11) not 12 [Cutaneous Bilirubin OR devices AND Neonate] | 479 |
| 16 | or/13‐15 | 635 |
Appendix 3. Embase strategy
| Embase 1974 to 2022 August 18 | ||
| # | Searches | Results |
| 1 | ((cutaneous* or dermal* or epicutaneous* or percutaneous* or skin or transcutaneous* or trans‐cutaneous or subcutaneous* or sub‐cutaneous*) adj4 bilirubin*).ti,ab,kw,kf. [Keyword Transcutaneous Bilirubinometry and Synonyms] | 865 |
| 2 | exp bilirubin/ or exp bilirubin blood level/ | 106013 |
| 3 | cutaneous drug administration/ or ((cutaneous* or dermal* or epicutaneous* or percutaneous* or skin or transcutaneous* or trans‐cutaneous or subcutaneous* or sub‐cutaneous*) adj3 (administ* or device or devices or measur* or meter* or metre* or monitor? or test*)).ti,ab,kw,kf. | 113275 |
| 4 | 2 and 3 [Bilirubin and Cutaneous Administration] | 600 |
| 5 | ((cutaneous* or dermal* or epicutaneous* or percutaneous* or skin or transcutaneous* or trans‐cutaneous or subcutaneous* or sub‐cutaneous*) adj3 bilirubin* adj3 (device or devices or measur* or meter* or metre* or monitor? or test*)).ti,ab,kw,kf. | 342 |
| 6 | (TcB or bilicheck* or bilichek or Bilicheck* or BiliMed or bilmeter* or JM‐103* or JM103* or JM‐105 or bilirubinometre* or bilirubinometer* or Bilitest or jaundice meter* or jaundicemeter* or icterometer*).ti,ab,kw,kf. | 1929 |
| 7 | or/4‐6 [Biirubin, cutaneous OR devices] | 2286 |
| 8 | newborn/ or prematurity/ or newborn intensive care/ or newborn care/ or gestational age/ | 753504 |
| 9 | (babe or babes or baby* or babies or gestational age? or infant? or infantile or infancy or low birth weight or low birthweight or neonat* or neo‐nat* or newborn* or new born? or newly born or premature or pre‐mature or pre‐matures or prematures or prematurity or pre‐maturity or preterm or preterms or pre term? or preemie or preemies or premies or premie or VLBW or VLBWI or VLBW‐I or VLBWs or LBW or LBWI or LBWs or ELBW or ELBWI or ELBWs or NICU or NICUs).ti,ab,kw,kf. | 1187262 |
| 10 | neonatal hyperbilirubinemia/ or newborn jaundice/ | 7762 |
| 11 | or/8‐10 [Filter: Neonatal Population 03‐2022‐OVID EMBASE] | 1446934 |
| 12 | (exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/) and (human/ or normal human/ or human cell/) | 23985429 |
| 13 | (exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/) not 12 | 6960458 |
| 14 | (1 and 11) not 13 [ Keyword Transcutaneous Bilirubinometry AND Neonate] | 739 |
| 15 | (4 and 11) not 13 [Bilirubin and Cutaneous Administration AND Neonate] | 353 |
| 16 | (7 and 11) not 13 [Biirubin, cutaneous OR devices AND Neonate] | 680 |
| 17 | or/14‐16 [Results‐Embase] | 849 |
Appendix 4. CINAHL strategy
| CINAHL Complete EBSCOhost 18 August 2022 | ||
| # | Searches | Results |
| 1 | TI ( ((cutaneous* or dermal* or epicutaneous* or percutaneous* or skin or transcutaneous* or trans‐cutaneous or subcutaneous* or sub‐cutaneous*) N4 bilirubin*) ) OR AB ( ((cutaneous* or dermal* or epicutaneous* or percutaneous* or skin or transcutaneous* or trans‐cutaneous or subcutaneous* or sub‐cutaneous*) N4 bilirubin*) ) | 257 |
| 2 | (MH "Bilirubin") | 3263 |
| 3 | TI ( (bilirubin* or bliverdin*) ) OR AB ( (bilirubin* or bliverdin*) ) | 5841 |
| 4 | TI ( ((cutaneous* or dermal* or epicutaneous* or percutaneous* or skin or transcutaneous* or trans‐cutaneous or subcutaneous* or sub‐cutaneous*) N3 (administ* or device or devices or measur* or meter* or metre* or monitor? or test*)) ) OR AB ( ((cutaneous* or dermal* or epicutaneous* or percutaneous* or skin or transcutaneous* or trans‐cutaneous or subcutaneous* or sub‐cutaneous*) N3 (administ* or device or devices or measur* or meter* or metre* or monitor? or test*)) ) | 12693 |
| 5 | (S2 OR S3) AND S4 | 142 |
| 6 | TI ( ((cutaneous* or dermal* or epicutaneous* or percutaneous* or skin or transcutaneous* or trans‐cutaneous or subcutaneous* or sub‐cutaneous*) N4 bilirubin* N3 (device or devices or measur* or meter* or metre* or monitor? or test*)) ) OR AB ( ((cutaneous* or dermal* or epicutaneous* or percutaneous* or skin or transcutaneous* or trans‐cutaneous or subcutaneous* or sub‐cutaneous*) N4 bilirubin* N3 (device or devices or measur* or meter* or metre* or monitor? or test*)) ) | 118 |
| 7 | TI ( (TcB or bilicare? or bilicheck* or bilichek or Bilicheck* or BiliMed or bilmeter* or JM‐103* or JM103* or JM‐105 or bilirubinometre* or bilirubinometer* or Bilitest or jaundice meter* or jaundicemeter* or icterometer*) ) OR AB ( (TcB or bilicare? or bilicheck* or bilichek or Bilicheck* or BiliMed or bilmeter* or JM‐103* or JM103* or JM‐105 or bilirubinometre* or bilirubinometer* or Bilitest or jaundice meter* or jaundicemeter* or icterometer*) ) | 235 |
| 8 | S6 OR S7 | 277 |
| 9 | S1 OR S5 OR S8 | 362 |
Appendix 5. Trial registry strategies
| Date | Site | Terms | Results |
| August‐18‐2022 | clinicaltrials.gov | transcutaneous bilirubin* [Other terms] | 34 |
| August‐18‐2022 | clinicaltrials.gov | cutaneous bilirubin* [Other terms] AND Child brith‐17 | 11 |
| August‐18‐2022 | clinicaltrials.gov | transcutaneous bilirubinometry [Other terms] | 11 |
| August‐18‐2022 | clinicaltrials.gov | bilicheck [Intervention] | 0 |
| August‐18‐2022 | clinicaltrials.gov | bilicare [Intervention] | 0 |
| August‐18‐2022 | clinicaltrials.gov | bilicheck [other terms] | 2 |
| August‐18‐2022 | clinicaltrials.gov | BiliMed [Other terms] | 0 |
| August‐18‐2022 | ICTRP | transcutaneous bilirubin [Intervention] | 6 |
| August‐18‐2022 | ICTRP | transcutaneous bilirubinometry [Intervention] | 1 |
| August‐18‐2022 | ICTRP | bilichek [Intervention] | 0 |
| August‐18‐2022 | ICTRP | jaundice meter [Intervention] | 0 |
| August‐18‐2022 | ICTRP | bilicare [Intervention] | 1 |
| August‐18‐2022 | ICTRP | bilicheck [Intervention] | 0 |
| August‐18‐2022 | ISRCTN | transcutaneous bilirubinometry [Text search] | 0 |
| August‐18‐2022 | ISRCTN | transcutaneous bilirubinometry [Intervention | 0 |
| August‐18‐2022 | ISRCTN | bilicheck [Text] | 0 |
| August‐18‐2022 | ISRCTN | bilichek [Text] | 0 |
| August‐18‐2022 | ISRCTN | bilicare [Text or Intervention] | 0 |
| Total | 66 | ||
Appendix 6. QUADAS‐2 tool
QUADAS‐2 tool: risk of bias and applicability judgements
| Domain 1: Patient selection | |
| A. Risk of bias | |
| Describe methods of patient selection: | |
| 1. Was a consecutive or random sample of patients enrolled? | Yes/No/Unclear "Yes" if it is clearly stated in the paper that a consecutive or a random sample of patients was enrolled. "No" if the patients were not recruited consecutively or the sample was not random. "Unclear" if there is insufficient information to answer "yes" or "no". |
| 2. Was a case‐control design avoided? | Yes/No/Unclear The answer will always be "yes", as this review will exclude case‐control studies. |
| 3. Did the study avoid inappropriate exclusions? | Yes/No/Unclear "Yes" if the stated inclusion and exclusion criteria are clear and appropriate. "No" if the stated inclusion and exclusion criteria include inappropriate subjects. "Unclear" if insufficient information is available to answer "yes" or "no". |
| Could the selection of patients have introduced bias? | RISK: Yes/No/Unclear "No" if questions 1 and 3 are answered "yes" (Low risk) "Yes" if questions 1 or 3 is answered "no" (High risk) "Unclear" if insufficient information is available to answer questions 1 or 3. |
| B. Concerns regarding applicability | |
| Describe included patients (prior testing, presentation, intended use of index test and setting): | |
| Is there concern that the included patients do not match the review question? | CONCERN: Yes/No/Unclear "No" when the study population represents an unselected sample of newborns expected to receive TcB assessment for hyperbilirubinaemia (Low). "Yes" if included patients are inherently different from those expected to receive TcB assessment for hyperbilirubinaemia (High). "Unclear" if there is insufficient information to make a judgement on the patient inclusion (Unclear). |
| Domain 2: Index test(s) (if more than 1 index test was used, please complete for each test) | |
| A. Risk of bias | |
| Describe the index test and how it was conducted and interpreted: | |
| 1. Were the index test results interpreted without knowledge of the results of the reference standard? | Yes/No/Unclear "Yes" if the paper states that the index test is interpreted by individual(s) who were unaware of the results of the reference test(s). "No" if the results of the index test were known by the individuals performing the reference test, or if the same individual performed both tests. "Unclear" if there is insufficient information to answer "yes" or "no". |
| 2. If a threshold was used, was it pre‐specified? | Yes/No/Unclear "Yes" if a prespecified positivity threshold was stated. "No" if a threshold was not prespecified. "Unclear" if there is insufficient information to answer "yes" or "no". |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: Low/High/Unclear "No" if questions 1 and 2 are answered "yes" (Low risk). "Yes" if questions 1 or 2 is answered "no" (High risk). "Unclear" if there is insufficient information available to answer "yes" or "no" (Unclear risk). |
| B. Concerns regarding applicability | |
| Is there concern that the index test, its conduct or interpretation differ from the review question? | CONCERN: Low/High/Unclear "No" if there are no concerns based on the information available (Low). "Yes" if the index test is not TcB measurement for hyperbilirubinaemia in newborns or if the conduct of the test or its interpretation is not applicable to the review question (High). "Unclear" if there is insufficient information to answer "yes" or "no". |
| Domain 3: Reference standard | |
| A. Risk of bias | |
| Describe the reference standard and how it was conducted and interpreted: | |
| 1. Is the reference standard likely to correctly classify the target condition? | Yes/No/Unclear "Yes" if the reference standard is TSB measured by one of the laboratory methods mentioned in this protocol. "No" if the above condition is not met. "Unclear" if there is insufficient information to answer "yes" or "no". |
| 2. Were the reference standard results interpreted without knowledge of the results of the index test? | Yes/No/Unclear "Yes" if it is stated that the individual performing/interpreting the results of the reference standard was kept unaware of the results of the index test. "No" if the results of the TcB measurement were known to the individual performing/interpreting the reference standard. "Unclear" if there is insufficient information to answer "yes" or "no". |
| Could the reference standard, its conduct or its interpretation have introduced bias? | RISK: Low/High/Unclear "No" if questions 1 and 2 are answered "yes" (Low risk). "Yes" if question 1 or 2 is answered "no" (High risk). "Unclear" if there is insufficient information to answer "yes" or "no". |
| B. Concerns regarding applicability | |
| Is there concern that the target condition as defined by the reference standard does not match the review question? | CONCERN: Low/High/Unclear "No" if the target condition is hyperbilirubinaemia in newborns (Low). "Yes" if the target condition is not hyperbilirubinaemia in newborns, or it is not clearly stated (High). "Unclear" if there is insufficient information available to answer "yes" or "no" (Unclear). |
| Domain 4: Flow and timing | |
| A. Risk of bias | |
| Describe any patients who did not receive the index test(s) and/or reference standard or who were excluded from the 2 × 2 table (refer to flow diagram): Describe the time interval and any interventions between index test(s) and reference standard: | |
| 1. Was there an appropriate interval between index test(s) and reference standard? | Yes/No/Unclear "Yes" if the time between the index test and the reference standard is less than 30 minutes. "No" if the time between the index test and the reference standard is longer than 30 minutes for a significant proportion of the patients. "Unclear" if insufficient information is available to answer "yes" or "no". |
| 2. Did all patients receive a reference standard? | Yes/No/Unclear "Yes" if all the patients who received the index test received a reference standard. "No" if not all the patients who received the index test received a reference standard. "Unclear" if there is insufficient information to answer "yes" or "no". |
| 3. Did patients receive the same reference standard? | Yes/No/Unclear "Yes" if the same reference standard was used for all patients. "No" if different reference standards were used. "Unclear" if there is insufficient information to answer "yes" or "no". |
| 4. Were all patients included in the analysis? | Yes/No/Unclear "Yes" if all patients were included in the analysis, or if any withdrawals or exclusions are adequately explained with a flow chart. "No" if withdrawals or exclusions are not explained or accounted for. "Unclear" if there is insufficient information to answer "yes" or "no". |
| Could the patient flow have introduced bias? | RISK: Low/High/Unclear "No" if questions 1, 2, 3 and 4 are answered "yes" (Low risk). "Yes" if any of the four questions is answered "no" (High risk). "Unclear" if there is insufficient information to answer "yes" or "no" (Unclear risk). |
TcB: transcutaneous bilirubin; TSB: total serum bilirubin.
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
| Test | No. of studies | No. of participants |
|---|---|---|
| 1 JM 101 | 7 | 859 |
| 2 JM 102 | 1 | 64 |
| 3 BiliChek (cutoff > 250 μmol/L) | 4 | 1263 |
| 4 BiliChek (cutoff > 200 μmol/L) | 3 | 1143 |
| 5 JH20‐1C | 1 | 217 |
| 6 BiliChek HR; diazo | 2 | 369 |
| 7 BiliChek HR; vitros | 1 | 131 |
| 8 JM 103 (TcB > 204 μmol/L) | 1 | 455 |
| 9 JM 103 (TcB > 230 μmol/L) | 2 | 568 |
| 10 JM 103 (sternum TcB P95) | 1 | 123 |
| 11 JM 103 (sternum TcB P75) | 2 | 455 |
| 12 JM 103 (forehead TcB P95) | 1 | 123 |
| 13 JM 103 (forehead TcB P75) | 1 | 123 |
| 14 BiliChek (need for phototherapy) | 1 | 400 |
| 15 Bilitest (TSB > 15 mg/dL) | 1 | 241 |
1. Test.

JM 101
2. Test.

JM 102
3. Test.

BiliChek (cutoff > 250 μmol/L)
4. Test.

BiliChek (cutoff > 200 μmol/L)
5. Test.

JH20‐1C
6. Test.

BiliChek HR; diazo
7. Test.

BiliChek HR; vitros
8. Test.

JM 103 (TcB > 204 μmol/L)
9. Test.

JM 103 (TcB > 230 μmol/L)
10. Test.

JM 103 (sternum TcB P95)
11. Test.

JM 103 (sternum TcB P75)
12. Test.

JM 103 (forehead TcB P95)
13. Test.

JM 103 (forehead TcB P75)
14. Test.

BiliChek (need for phototherapy)
15. Test.

Bilitest (TSB > 15 mg/dL)
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bental 2009.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional study, consecutive sampling | ||
| Patient characteristics and setting |
Sample size: 628 Gestational age: term (> 37 weeks) and late preterm (> 35 weeks) Race/ethnicity: mixed Prior phototherapy: no Setting: Laniado Hospital, Natanya, Israel |
||
| Index tests |
TcB device: JM 103 Site of TcB measurement: forehead and sternum |
||
| Target condition and reference standard(s) |
Target condition: neonatal jaundice TSB measurement method: colorimetric assay |
||
| Flow and timing |
Time interval between tests: ≤ 90 minutes Number of paired results: 1091 |
||
| Comparative | |||
| Notes | Participants were healthy newborns. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Bertini 2008.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional study, consecutive sampling | ||
| Patient characteristics and setting |
Sample size: 241 Gestational age: > 32 weeks Race/ethnicity: "Caucasian" (presumed white; 100%) Prior phototherapy: no Setting: neonatal nursery of Careggi University Hospital, Florence, Italy |
||
| Index tests |
TcB device: Bilitest BB 77 Site of TcB measurement: forehead |
||
| Target condition and reference standard(s) |
Target condition: neonatal jaundice TSB measurement method: spectrophotometry |
||
| Flow and timing |
Time interval between tests: ≤ 10 minutes Number of paired results: not reported |
||
| Comparative | |||
| Notes | Participants were healthy newborns. TSB was determined according to the judgement of the physician in charge. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Bhutta 1991.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional study, consecutive sampling | ||
| Patient characteristics and setting |
Sample size: 63 Gestational age: term and preterm (> 34 weeks) Race/ethnicity: Pakistani Prior phototherapy: not reported Setting: newborn nursery of Aga Khan University Hospital, Karachi, Pakistan |
||
| Index tests |
TcB device: JM 101 Site of TcB measurement: forehead |
||
| Target condition and reference standard(s) |
Target condition: neonatal jaundice TSB measurement method: photometry |
||
| Flow and timing |
Time interval between tests: ≤ 2 hours Number of paired results: 100 |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Bilgen 1998.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional study, consecutive sampling | ||
| Patient characteristics and setting |
Sample size: 96 Gestational age: term Race/ethnicity: not specified Prior phototherapy: no Setting: Bakirköy Maternity and Children's Hospital, Marmara, Türkiye |
||
| Index tests |
TcB device: not specified, probably JM 101 Site of TcB measurement: forehead |
||
| Target condition and reference standard(s) |
Target condition: neonatal hyperbilirubinaemia TSB measurement method: spectrophotometry |
||
| Flow and timing |
Time interval between tests: ≤ 30 minutes Number of paired results: 96 |
||
| Comparative | |||
| Notes | Participants included term infants from 1 to 5 days of life who were clinically jaundiced and required blood draw for serum bilirubin measurement. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Boo 2007.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional study, consecutive sampling | ||
| Patient characteristics and setting |
Sample size: 345 Gestational age: > 37 weeks Race/ethnicity: Malay, Chinese, Indian Prior phototherapy: no Setting: postnatal ward and NICU at the Hospital Universitii Kebangsaan, Malaysia |
||
| Index tests |
TcB device: BiliChek Site of TcB measurement: sternum and forehead |
||
| Target condition and reference standard(s) |
Target condition: neonatal jaundice TSB measurement method: diazo method |
||
| Flow and timing |
Time interval between tests: ≤ 30 minutes Number of paired results: 345 |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Campbell 2011.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional study, consecutive sampling | ||
| Patient characteristics and setting |
Sample size: 430 Gestational age: term and preterm (35–37 weeks) Race/ethnicity: diverse population (white, Black, Asian, Indian, Latino) Prior phototherapy: no Setting: Academic Hospital in Toronto, Ontario, Canada |
||
| Index tests |
TcB device: BiliChek Site of TcB measurement: forehead |
||
| Target condition and reference standard(s) |
Target condition: neonatal hyperbilirubinaemia TSB measurement method: spectrophotometry |
||
| Flow and timing |
Time interval between tests: ≤ 30 minutes Number of paired results: 430 |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Christo 1988.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional study, consecutive sampling | ||
| Patient characteristics and setting |
Sample size: 138 Gestational age: term and preterm Race/ethnicity: South Indian Prior phototherapy: in some infants Setting: Kasturba Hospital, India |
||
| Index tests |
TcB device: JM101 Site of TcB measurement: forehead and sternum |
||
| Target condition and reference standard(s) |
Target condition: neonatal jaundice TSB measurement method: spectrophotometry |
||
| Flow and timing |
Time interval between tests: ≤ 30 minutes Number of paired results: 138 |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Ercan 2018.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional study, consecutive sampling | ||
| Patient characteristics and setting |
Sample size: 218 newborns Gestational age: ≥ 35 weeks Race/ethnicity: not reported Prior phototherapy: no Setting: not reported |
||
| Index tests |
TcB device: JH20‐1C Site of TcB measurement: forehead |
||
| Target condition and reference standard(s) |
Target condition: neonatal jaundice TSB measurement method: spectrophotometry |
||
| Flow and timing |
Time interval between tests: ≤ 10 minutes Number of paired results: 271 |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Hemmati 2013.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional study, consecutive sampling | ||
| Patient characteristics and setting |
Sample size: 560 Gestational age: mostly term and late preterm (≥ 35 weeks), only 5 infants ≤ 35 weeks Race/ethnicity: Iranian Prior phototherapy: no Setting: neonatal ward of Nemazee Hospital, Shiraz, Iran |
||
| Index tests |
TcB device: BiliChek Site of TcB measurement: forehead |
||
| Target condition and reference standard(s) |
Target condition: neonatal hyperbilirubinaemia TSB measurement method: spectrophotometry and diazo method |
||
| Flow and timing |
Time interval between tests: ≤ 30 minutes Number of paired results: not reported |
||
| Comparative | |||
| Notes | Participants were healthy newborns. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Karon 2008.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional study, consecutive sampling | ||
| Patient characteristics and setting |
Sample size: 177 Gestational age: preterm (> 32 weeks) and term (> 37 weeks) Race/ethnicity: "Caucasian" (presumed white), Asian, Hispanic, African American Prior phototherapy use: no Setting: well‐infant nursery at the Mayo Clinic (Rochester, MN) |
||
| Index tests |
TcB device: BiliChek Site of TcB measurement: forehead |
||
| Target condition and reference standard(s) |
Target condition: neonatal hyperbilirubinaemia TSB measurement method: diazo and vitro method |
||
| Flow and timing |
Time intervalbetween tests: ≤ 30 minutes Number of paired results: 177 |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Karrar 1989.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional study, consecutive sampling | ||
| Patient characteristics and setting |
Sample size: 155 Gestational age: term (> 37 weeks) Race/ethnicity: Saudi newborns Prior phototherapy: no Setting: King Abdulaziz and King Khaled teaching hospitals, Riyadh, Saudi Arabia |
||
| Index tests |
TcB device: JM 101 Site of TcB measurement: forehead |
||
| Target condition and reference standard(s) |
Target condition: neonatal jaundice TSB measurement method: American optical bilirubinometer |
||
| Flow and timing |
Time interval between tests: not reported (probably ≤ 30 minutes) Number of paired results: 155 |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Kitsommart 2013.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional study, consecutive sampling | ||
| Patient characteristics and setting |
Sample size: 405 Gestational age: preterm (≥ 34 weeks) and term (≥ 37 weeks) Race/ethnicity: Asian newborns Prior phototherapy: in some infants Setting: outpatient paediatric department at Siriraj Hospital, Bangkok, Thailand |
||
| Index tests |
TcB Device: JM 103 Site of TcB measurement: forehead |
||
| Target condition and reference standard(s) |
Target condition: neonatal hyperbilirubinaemia TSB measurement method: diazo method |
||
| Flow and timing |
Time interval between tests: ≤ 30 minutes Number of paired results: 455 |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Knupfer 2001.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional study, consecutive sampling | ||
| Patient characteristics and setting |
Sample size: 135 Gestational age: preterm (23–36 weeks) Race/ethnicity: "Caucasians" (presumed white) and Asians Prior phototherapy: in some infants Setting: obstetrics department and NICU at the University of Leipzig, Leipzig, Germany |
||
| Index tests |
TcB Device: BiliChek Site of TcB measurement: forehead |
||
| Target condition and reference standard(s) |
Target condition: neonatal hyperbilirubinaemia TSB measurement method: diazo method (unclear if reference standard interpreted without knowledge of results of index test) |
||
| Flow and timing |
Time interval between tests: not reported Number of paired results: 400 |
||
| Comparative | |||
| Notes | . | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Kolman 2007.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional study, consecutive sampling | ||
| Patient characteristics and setting |
Sample size: 192 Gestational age: preterm (≥ 35 weeks) and term Race/ethnicity: Hispanic newborns Prior phototherapy: no Setting: newborn nursery at Maricopa Medical Center in Phoenix, AZ |
||
| Index tests |
TcB device: BiliChek Site of TcB measurement: forehead |
||
| Target condition and reference standard(s) |
Target condition: neonatal hyperbilirubinaemia TSB measurement method: diazo method |
||
| Flow and timing |
Time interval between tests: ≤ 30 minutes Number of paired results: 192 |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Lam 2008.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional study, consecutive sampling | ||
| Patient characteristics and setting |
Sample size: 113 Gestational age: term and late preterm (> 35 weeks) Race/ethnicity: Asian (Chinese) Prior phototherapy: no Setting: accident and emergency department of a regional hospital in Hong Kong |
||
| Index tests |
TcB device: JM 103 Site of TcB measurement: mid‐sternum and forehead |
||
| Target condition and reference standard(s) |
Target condition: neonatal hyperbilirubinaemia TSB measurement method: spectrophotometry |
||
| Flow and timing |
Time interval between tests: ≤ 5 minutes Number of paired results: 113 |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Maisels 1982.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional study, consecutive sampling | ||
| Patient characteristics and setting |
Sample size: 157 Gestational age: term (≥ 37 weeks) Race/ethnicity: white Prior phototherapy: no Setting: well‐baby nursery of Milton S. Hershey Medical Center, Hershey, PA |
||
| Index tests |
TcB device: JM 101 Site of TcB measurement: forehead and sternum |
||
| Target condition and reference standard(s) |
Target condition: neonatal hyperbilirubinaemia TSB measurement method: diazo method |
||
| Flow and timing |
Time interval between tests: not reported (probably near simultaneous measurement) Number of paired results: 292 |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Low risk | ||
Mendoza‐Chuctaya 2021.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional study, consecutive sampling | ||
| Patient characteristics and setting |
Sample size: 123 Gestational age: term Race/ethnicity: not reported Prior phototherapy: no Setting: Hospital Regional in Cusco‐Peru |
||
| Index tests |
TcB Device: JM 103 Site of TcB measurement: sternum and forehead |
||
| Target condition and reference standard(s) |
Target condition: neonatal hyperbilirubinaemia TSB measurement method: not specified |
||
| Flow and timing |
Time interval between tests: ≤ 30 minutes Number of paired results: 123 |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Neocleous 2014.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional study, consecutive sampling | ||
| Patient characteristics and setting |
Sample size: 222 Gestational age: term (> 37 weeks) Race/ethnicity: Greek; "Caucasian" (presumed white) Prior phototherapy use: not reported Setting: department of medical paediatrics, General Hospital, Drama, Greece |
||
| Index tests |
TcB device: BiliChek Site of TcB measurement: forehead |
||
| Target condition and reference standard(s) |
Target condition: neonatal hyperbilirubinaemia TSB measurement method: spectrophotometry |
||
| Flow and timing |
Time interval between tests: ≤ 20 minutes Number of paired results: 368 |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Samanta 2004.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional study, consecutive sampling | ||
| Patient characteristics and setting |
Sample size: 300 Gestational age: preterm (≥ 33 weeks) and term (≥ 37 weeks) Race/ethnicity: not reported Prior phototherapy: no Setting: postnatal wards at Liverpool Women’s Hospital, Liverpool, UK |
||
| Index tests |
TcB device: BiliChek Site of TcB measurement: not reported |
||
| Target condition and reference standard(s) |
Target condition: neonatal hyperbilirubinaemia TSB measurement method: diazo method (unclear if reference standard interpreted without knowledge of results of index test) |
||
| Flow and timing |
Time interval between tests: near simultaneous measurements Number of paired results: 300 |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Schumacher 1985.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional study, consecutive sampling | ||
| Patient characteristics and setting |
Sample size: 106 Gestational age: > 36 weeks Race/ethnicity: white Prior phototherapy: no Setting: not stated |
||
| Index tests |
TcB device: JM 101 Site of TcB measurement: sternum |
||
| Target condition and reference standard(s) |
Target condition: neonatal hyperbilirubinaemia TSB measurement method: direct spectrophotometry |
||
| Flow and timing |
Time interval between tests: ≤ 30 minutes Number of paired results: 106 |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Taha 1984.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional study, consecutive sampling | ||
| Patient characteristics and setting |
Sample size: 68 Gestational age: term (> 37 weeks) Race/ethnicity: Saudi newborns Prior phototherapy: no Setting: newborn nursery at King Abdul Aziz University Hospital Riyadh, Saudi Arabia |
||
| Index tests |
TcB device: JM 101 Site of TcB measurement: forehead |
||
| Target condition and reference standard(s) |
Target condition: neonatal hyperbilirubinaemia TSB measurement method: direct spectrophotometry |
||
| Flow and timing |
Time interval between tests: near simultaneous measurements Number of paired results: 120 |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Wong 2002.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional study, consecutive sampling | ||
| Patient characteristics and setting |
Sample size: 64 Gestational age: term and preterm (31–42 weeks) Race/ethnicity: mostly white, 6 non‐white Prior phototherapy: no Setting: tertiary neonatal service in South‐East Scotland |
||
| Index tests |
TcB device: JM 102 and BiliChek Site of TcB measurement: forehead |
||
| Target condition and reference standard(s) |
Target condition: neonatal hyperbilirubinaemia TSB measurement method: spectrophotometry |
||
| Flow and timing |
Time interval between tests: ≤ 30 minutes Number of paired results: 64 |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Yaser 2014.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional study, consecutive sampling | ||
| Patient characteristics and setting |
Sample size: 122 Gestational age: preterm (< 35 weeks) Race/ethnicity: not reported Phototherapy use: no prior phototherapy use Setting: level III neonatal care unit (Groote Schuur Hospital) in Cape Town, South Africa |
||
| Index tests |
TcB device: JM 103 Site of TcB measurement: forehead, sternum, interscapular area |
||
| Target condition and reference standard(s) |
Target condition: neonatal hyperbilirubinaemia TSB measurement method: not reported |
||
| Flow and timing |
Time interval between tests: ≤ 30 minutes Number of paired results: 122 for each TcB site |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
HPLC: high‐performance liquid chromatography; NICU: neonatal intensive care unit; TcB: transcutaneous bilirubin; TSB: total serum bilirubin.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adriaansen 2020 | Insufficient information to generate 2 × 2 table; correlation coefficients reported. |
| Afanetti 2014 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Agrawal 2019 | Insufficient information to generate 2 × 2 table. |
| Ahmed 2010 | Study used a series of cut‐offs points to generate ROC curve; but there was insufficient information for any specific cut‐off point to generate a 2 × 2 table |
| Ahn 2003 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Akahira‐Azuma 2013 | Insufficient information to generate 2 × 2 table. |
| Alsaedi 2016 | Insufficient information to generate 2 × 2 table. |
| Alsaedi 2018 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Amato 1985 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Amato 1990 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Arman 2020 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Babaei 2012 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Badiee 2012 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Beck 2003 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Berget 1984 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Bhat 1987 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Bhutani 2000 | Study evaluated BiliChek for predicting risk of subsequent excessive hyperbilirubinaemia (not a focus of this review). |
| Boo 1984 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Bosschaart 2012 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Bourchier 1987 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Briscoe 2002 | TcB device and unit of measurement for TcB not reported. |
| Carbonell 1999 | Duplicate (See Carbonell 2001). |
| Carbonell 2001 | Study evaluated predictive ability of TcB measurement for subsequent hyperbilirubinaemia (not a focus of this review). |
| Carceller 2006 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Casnocha 2021 | Insufficient information to generate 2 × 2 table. |
| Castro 2019 | Wrong study design. |
| Cat 2021 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Chang 2006 | We were unable to find the full text article. |
| Chawla 2014 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Chimhini 2018 | Insufficient information to generate 2 × 2 table. |
| Chokemungmeepisarn 2020 | Insufficient information to generate 2 × 2 table. |
| Conceicao 2014 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Dagli 2018 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Dai 1996 | Retrospective study. |
| De Luca 2007 | Insufficient information to generate 2 × 2 table. |
| De Luca 2008b | Insufficient information to generate 2 × 2 table. |
| Dianova 2021 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Dominguez 1993 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Donzelli 2000 | Insufficient information to generate 2 × 2 table. |
| Ebbesen 2002 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Ebbesen 2012 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| El‐Kabbany 2017 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Engle 2002 | Multiple cut‐off values for TSB; insufficient information to generate 2 × 2 table. |
| Engle 2005 | Multiple cut‐off values for TSB; insufficient information to generate 2 × 2 table. |
| Fabris 1984 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Faridi 1998 | No full‐text article available. |
| Felc 2005 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Fisher 2015 | No full‐text article available at time of writing of this review. |
| Foged 1983 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Fok 1986 | Insufficient information to generate 2 × 2 table. |
| Fonseca 2012 | Wrong study design. |
| Goldman 1982 | TcB used to identify which infants required TSB determinations rather than to predict actual bilirubin values. |
| Gondale 2013 | TcB device not reported. |
| Gothwal 2021 | No accuracy outcomes such as sensitivity and specificity. |
| Grabenhenrich 2014 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Greco 2017 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Gunaseelan 2017 | Insufficient information to generate 2 × 2 table. |
| Harish 1998 | Multiple cutoff points; insufficient information to generate 2 × 2 table. |
| Hasani 2019 | No full‐text article available. |
| Hegyi 1981 | Sensitivity and specificity not reported. |
| Heick 1982 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Hemmati 2021 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Hill 2016 | We were unable to find the full‐text article. |
| Ho 2006 | Insufficient information to generate 2 × 2 table. |
| Ho 2006a | Retrospective study. |
| Holland 2009 | Study used multiple cutoff points and 3 different gold standards, making it difficult to choose a specific cutoff for comparison of TcB for any of the gold standards. |
| Hoppenot 2012 | Retrospective study. |
| Hulzebos 2019 | Multiple threshold values for TSB for multiple different categories of participants based on the birth weight: difficult to choose a specific threshold for comparison. |
| Itoh 2001 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Jahadi 2013 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Jalalodini 2019 | Insufficient information to generate 2 × 2 table; participants were already receiving phototherapy. |
| Jangaard 2006 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Janjindamai 2005 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Jegathesan 2017 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Jegathesan 2021 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Jeon 2020 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Jnah 2018 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Johnson 2020 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Jones 2017 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Joseph 2014 | No full‐text article available. |
| Juster‐Reicher 2014 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Karen 2009 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Karolyi 2004 | Insufficient information to generate 2 × 2 table. |
| Kaynak‐Turkmen 2011 | Multiple cutoff values; insufficient information to generate 2 × 2 table. |
| Kazmierczak 2004 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Keshini 2018 | No full‐text article available |
| Keshishian 1994 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Khajehei 2021 | Insufficient information to generate 2 × 2 table. |
| Knudsen 1996 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Konana 2021 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Kosarat 2013 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Kumar 1994 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Kumar 2017 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Kumar 2020 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Kumra 2017 | Insufficient information to generate 2 × 2 table. |
| Laeeq 1993 | Insufficient information to generate 2 × 2 table. |
| Lebrun 1982 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Leite 2007 | Insufficient information to generate 2 × 2 table. |
| Lin 1993 | Insufficient information to generate 2 × 2 table. |
| López‐Garrido 2015 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Mahajan 2005 | Wide range of participants (28–42 weeks' gestational age); number of paired comparisons for TcB and TSB not reported; method of TSB measurement not reported. |
| Mahram 2015 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Maisels 2004 | Multiple TcB threshold values; insufficient information to generate 2 × 2 table. |
| Maisels 2006 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Maisels 2011 | Multiple target points and cutoff values for TSB and TcB. |
| Marco 2009 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Maya‐Enero 2021 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Mercanti 2007 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Mohamed 2022 | Insufficient information to generate 2 × 2 table. |
| Moscicka 1994 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Murli 2017 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Nahar 2017 | Multiple cutoff values; insufficient information to generate 2 × 2 table. |
| Namba 2007 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Nanjundaswamy 2004 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Nanjundaswamy 2005 | Wrong study design; infants were already receiving phototherapy. |
| Narang 1983 | No full‐text article available. |
| Ochoa 2000 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Oyapero 2017 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Pallas 1993 | We were unable to find the full‐text article. |
| Palmer 1982 | We were unable to find the full‐text article. |
| Panburana 2010 | Multiple cutoff values; insufficient information to generate 2 × 2 table. |
| Pendse 2017 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Poland 2004 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Povaluk 2011 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Pratesi 2016 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Qandalji 2012 | Poster presentation, no full‐text article available. |
| Qualter 2011 | Insufficient information to generate 2 × 2 table. |
| Quist 2016 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Raba 2020 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Radfar 2016 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Raimondi 2012 | Insufficient information to generate 2 × 2 table. |
| Reyes 2008 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Robertson 2002 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Rodriguez‐Capote 2009 | Insufficient information to generate 2 × 2 table. |
| Rohsiswatmo 2018 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Romagnoli 2012 | Study evaluated the use of TcB measurements plotted on bilirubin nomogram to predict subsequent significant hyperbilirubinaemia or need for phototherapy. |
| Romagnoli 2013 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Romo‐Pinales 1984 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Roy 2010 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Rubaltelli 2001 | Insufficient information to generate 2 × 2 table. |
| Rubio 2017 | Insufficient information to generate 2 × 2 table. |
| Rush 2011 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Rylance 2014 | Insufficient information to generate 2 × 2 table. |
| Sajjadian 2012 | Multiple cutoffs precluded extraction of data for 2 × 2 table. |
| Samiee‐Zafarghandy 2014 | Insufficient information to generate 2 × 2 table. |
| Sanpavat 2004 | Multiple cutoffs precluded extraction of data for 2 × 2 table. |
| Sanpavat 2005 | Insufficient information to generate 2 × 2 table. |
| Sanpavat 2007 | Insufficient information to generate 2 × 2 table. |
| Sardar 2021 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Sarici 2014 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Schmidt 2009 | Insufficient information to generate 2 × 2 table. |
| Sellitto 2010 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Sharma 1988 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Sheridan‐Pereira 1982 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Shihadeh 2016 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Siu 2010 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Slusher 2004 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Soffer 2018 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Spence 2016 | No accuracy outcomes like sensitivity and specificity. |
| Srinivas 2016 | Insufficient information to generate 2 × 2 table. |
| Stillova 2007 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Stillova 2009 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Suckling 1995 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Szabo 2004a | Inappropriate exclusions (newborns with visible jaundice in first 36 hours of life). |
| Szabo 2004b | Insufficient information to generate 2 × 2 table. |
| Tan 1982 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Tan 1985 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Tan 1988 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Tan 1996 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Tan 2003 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Tayaba 1998 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Taylor 2015 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Teran 2011 | We were unable to find a full‐text article. |
| Timalsina 2017 | We were unable to find a full‐text article. |
| Vocel 1985 | Article published in Czech and not in English. |
| Vocel 1987 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Vocel 1988 | Article published in Czech and not in English. |
| Wainer 2009 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Weber 2021 | Multiple cutoff value; insufficient information to generate 2 × 2 table. |
| Wickremasinghe 2011 | Study assessed the use of inpatient TcB measurement to predict subsequent bilirubin levels of high and high‐intermediate risk on Bhutani nomogram. |
| Willems 2004 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Willemsen 2007 | Study not published in English. |
| Williams 2011 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Yamanouchi 1980 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Yamauchi 1988 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Yamauchi 1989a | Study only reported correlation coefficient, not sensitivity and specificity. |
| Yamauchi 1989b | Study only reported correlation coefficient, not sensitivity and specificity. |
| Yamauchi 1989c | Study only reported correlation coefficient, not sensitivity and specificity. |
| Yamauchi 1990 | This study evaluated use of TcB measurement for prediction of subsequent hyperbilirubinaemia > 15 mg/dL. |
| Yamauchi 1991 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Yang 2019 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Yap 2002 | Study only reported correlation coefficient, not sensitivity and specificity. |
| Yasuda 2003 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Ying 2020 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Zecca 2009 | Study reported correlation coefficients without reporting sensitivity and specificity of TcB measurement. |
| Zhan 2016 | Study only reported correlation coefficient, not sensitivity and specificity. |
TcB: transcutaneous bilirubin; TSB: total serum bilirubin.
Differences between protocol and review
In the protocol, we stated we would search DARE and MEDION databases; however, we were unable to access these databases, and we omitted them from the Search methods for identification of studies section of the review (Okwundu 2017).
Contributions of authors
Conception of review: CO Screening and data extraction: AO, CO, and OU Statistical analysis: CO Drafting of protocol and review: CO Writing and approval of review: AO, CO, OU, KG, JS, CW, VB Search strategies; results of search; PRISMA: MF
Sources of support
Internal sources
No sources of support provided
External sources
-
Vermont Oxford Network, USA
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
-
The South African Medical Research Council, South Africa
The South African Medical Research Council provided funding to conduct this review.
-
The Cochrane Incentive Fund, UK
An annual scheme whereby small incentive payments are offered to Cochrane Review Groups (CRGs) for preparing key new or updated Cochrane reviews.
Declarations of interest
AO: none known CO: none known OU: none known KG has declared he has no conflict of interest; but he is an Editor with Cochrane Neonatal Group. He was not be involved in the editorial process of this manuscript. JS: none known CW: none know VB: none known MF is an Information Specialist and Managing Editor with the Cochrane Neonatal Group. She was not involved in the editorial process of this manuscript.
New
References
References to studies included in this review
Bental 2009 {published data only}
- Bental YA, Shiff Y, Dorsht N, Litig E, Tuval L, Mimouni FB. Bhutani-based nomograms for the prediction of significant hyperbilirubinaemia using transcutaneous measurements of bilirubin. Acta Paediatrica 2009;98(12):1902-8. [DOI: 10.1111/j.1651-2227.2009.01385.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bertini 2008 {published data only}
- Bertini G, Pratesi S, Cosenza E, Dani C. Transcutaneous bilirubin measurement: evaluation of Bilitest. Neonatology 2008;93(2):101-5. [DOI: 10.1159/000107351] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bhutta 1991 {published data only}
- Bhutta ZA, Yusuf K. Transcutaneous bilirubinometry in Pakistani newborns: a preliminary report. Journal of the Pakistan Medical Association 1991;41(7):155-6. [PMID: ] [PubMed] [Google Scholar]
Bilgen 1998 {published data only}
- Bilgen H, Ince Z, Ozek E, Bekiroglu N, Ors R. Transcutaneous measurement of hyperbilirubinaemia: comparison of the Minolta jaundice meter and the Ingram icterometer. Annals of Tropical Paediatrics 1998;18(4):325-8. [DOI: 10.1080/02724936.1998.11747968] [PMID: ] [DOI] [PubMed] [Google Scholar]
Boo 2007 {published data only}
- Boo NY, Ishak S. Prediction of severe hyperbilirubinaemia using the Bilicheck transcutaneous bilirubinometer. Journal of Paediatrics and Child Health 2007;43(4):297-302. [DOI: 10.1111/j.1440-1754.2007.01062.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Campbell 2011 {published data only}
- Campbell DM, Danayan KC, McGovern V, Cheema S, Stade B, Sgro M. Transcutaneous bilirubin measurement at the time of hospital discharge in a multiethnic newborn population. Paediatrics & Child Health 2011;16(3):141-5. [DOI: 10.1093/pch/16.3.141] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Christo 1988 {published data only}
- Christo GG, Kamath S, Aroor AR, Venkatesh A. Transcutaneous bilirubinometry in newborns. Indian Pediatrics 1988;25(11):1073-7. [PMID: ] [PubMed] [Google Scholar]
Ercan 2018 {published data only}
- Ercan Ş, Özgün G. The accuracy of transcutaneous bilirubinometer measurements to identify the hyperbilirubinemia in outpatient newborn population. Clinical biochemistry 2018;55:69-74. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hemmati 2013 {published data only}
- Hemmati F, Kiyani Rad NA. The value of bilicheck(R) as a screening tool for neonatal jaundice in the South of Iran. Iranian Journal of Medical Sciences 2013;38(2):122-8. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Karon 2008 {published data only}
- Karon BS, Teske A, Santrach PJ, Cook WJ. Evaluation of the BiliChek noninvasive bilirubin analyzer for prediction of serum bilirubin and risk of hyperbilirubinemia. American Journal of Clinical Pathology 2008;130(6):976-82. [DOI: 10.1309/AJCPRX1E3NWCXHMZ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Karrar 1989 {published data only}
- Karrar Z, al Habib S, al Basit OB, Ashong F, Osundwa V. Transcutaneous bilirubin measurements in Saudi infants: the use of the jaundice meter to identify significant jaundice. Annals of Tropical Paediatrics 1989;9(1):59-61. [DOI: 10.1080/02724936.1989.11748598] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kitsommart 2013 {published data only}
- Kitsommart R, Pornladnun P, Chomchai C, Urujchutchairut P, Paes B. Accuracy and precision of transcutaneous bilirubinometry in postdischarge Asian neonates. European Journal of Pediatrics 2013;172(6):781-6. [DOI: 10.1007/s00431-013-1960-4] [PMID: ] [DOI] [PubMed] [Google Scholar]
Knupfer 2001 {published data only}
- Knupfer M, Pulzer F, Braun L, Heilmann A, Robel-Tillig E, Vogtmann C. Transcutaneous bilirubinometry in preterm infants. Acta Paediatrica 2001;90(8):899-903. [PMID: ] [PubMed] [Google Scholar]
Kolman 2007 {published data only}
- Kolman KB, Mathieson KM, Frias C. A comparison of transcutaneous and total serum bilirubin in newborn Hispanic infants at 35 or more weeks of gestation. Journal of the American Board of Family Medicine 2007;20(3):266-71. [DOI: 10.3122/jabfm.2007.03.060172] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lam 2008 {published data only}
- Lam TS, Tsui KL, Kam CW. Evaluation of a point-of-care transcutaneous bilirubinometer in Chinese neonates at an accident and emergency department. Hong Kong Medical Journal 2008;14(5):356-60. [PMID: ] [PubMed] [Google Scholar]
Maisels 1982 {published data only}
- Maisels MJ, Conrad S. Transcutaneous bilirubin measurements in full-term infants. Pediatrics 1982;70(3):464-7. [PMID: ] [PubMed] [Google Scholar]
Mendoza‐Chuctaya 2021 {published data only}
- Mendoza-Chuctaya G, Ramos-Chuctaya KR, Maraza-Aquino EJ, Ruiz-Esquivel JE, Velazquez-Cordova LAS. Accuracy of transcutaneous bilirubin measurement in full-term newborns at 3400 meters above sea level. Boletin medico del Hospital Infantil de Mexico 2021;78(2):116-22. [PMID: ] [DOI] [PubMed] [Google Scholar]
Neocleous 2014 {published data only}
- Neocleous C, Adramerina A, Limnaios S, Symeonidis S, Spanou C, Malakozi M, et al. A comparison between transcutaneous and total serum bilirubin in healthy-term Greek neonates with clinical jaundice. Prague Medical Report 2014;115(1-2):33-42. [DOI: 10.14712/23362936.2014.4] [PMID: ] [DOI] [PubMed] [Google Scholar]
Samanta 2004 {published data only}
- Samanta S, Tan M, Kissack C, Nayak S, Chittick R, Yoxall CW. The value of Bilicheck as a screening tool for neonatal jaundice in term and near-term babies. Acta Paediatrica 2004;93(11):1486-90. [DOI: 10.1080/08035250410033042] [PMID: ] [DOI] [PubMed] [Google Scholar]
Schumacher 1985 {published data only}
- Schumacher RE, Thornbery JM, Gutcher GR. Transcutaneous bilirubinometry: a comparison of old and new methods. Pediatrics 1985;76(1):10-4. [PMID: ] [PubMed] [Google Scholar]
Taha 1984 {published data only}
- Taha SA, Karrar ZA, Dost SM. Transcutaneous bilirubin measurement in evaluating neonatal jaundice among Saudi newborns. Annals of Tropical Paediatrics 1984;4(4):229-31. [DOI: 10.1080/02724936.1984.11748341] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wong 2002 {published data only}
- Wong CM, Dijk PJ, Laing IA. A comparison of transcutaneous bilirubinometers: SpectRx BiliCheck versus Minolta AirShields. Archives of Disease in Childhood. Fetal and Neonatal Edition 2002;87(2):F137-40. [DOI: 10.1136/fn.87.2.f137] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yaser 2014 {published data only}
- Yaser A, Tooke L, Rhoda N. Interscapular site for transcutaneous bilirubin measurement in preterm infants: a better and safer screening site. Journal of Perinatology 2014;34(3):209-12. [DOI: 10.1038/jp.2013.167] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adriaansen 2020 {published data only}
- Adriaansen M, Williams S, den Boom J. Evaluation of two transcutaneous bilirubin devices for the assessment of neonatal jaundice in a diverse New Zealand population. New Zealand Journal of Medical Laboratory Science 2020;74(1):11-16. [Google Scholar]
Afanetti 2014 {published data only}
- Afanetti M, Eleni Dit Trolli S, Yousef N Jrad I, Mokhtari M. Transcutaneous bilirubinometry is not influenced by term or skin color in neonates. Early Human Development 2014;90(8):417-20. [DOI: 10.1016/j.earlhumdev.2014.05.009] [PMID: ] [DOI] [PubMed] [Google Scholar]
Agrawal 2019 {published data only}
- Agrawal G, Garg K, Sitaraman S, Sarna A. Comparison of diagnostic accuracy of different sites for transcutaneous bilirubin measurement in early preterm infants. Indian Journal of Pediatrics 2019;86(1):32-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ahmed 2010 {published data only}
- Ahmed M, Mostafa S, Fisher G, Reynolds TM. Comparison between transcutaneous bilirubinometry and total serum bilirubin measurements in preterm infants <35 weeks gestation. Annals of Clinical Biochemistry 2010;47 Pt 1:72-7. [DOI: 10.1258/acb.2009.009072] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ahn 2003 {published data only}
- Ahn YM, Kim MR, Lee SM, Jun YH. Assessment of neonatal hyperbilirubinemia using a transcutaneous bilirubinometry. Taehan Kanho Hakhoe Chi 2003;33(1):51-9. [DOI: 10.4040/jkan.2003.33.1.51] [PMID: ] [DOI] [PubMed] [Google Scholar]
Akahira‐Azuma 2013 {published data only}
- Akahira-Azuma M, Yonemoto N, Ganzorig B, Mori R, Hosokawa S, Matsushita T, et al. Validation of a transcutaneous bilirubin meter in Mongolian neonates: comparison with total serum bilirubin. BMC Pediatrics 2013;13:151. [DOI: 10.1186/1471-2431-13-151] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Alsaedi 2016 {published data only}
- Alsaedi Saad A. Transcutaneous bilirubin measurement in healthy Saudi term newborns. Saudi medical journal 2016;37(2):142-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alsaedi 2018 {published data only}
- Alsaedi SA. Transcutaneous bilirubin measurements can be used to measure bilirubin levels during phototherapy. International journal of pediatrics 2018;2018:4856390. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Amato 1985 {published data only}
- Amato M, Pasquier S, Muralt G. Transcutaneous bilirubin determination: correlation in white premature infants weighing less than 1500 gm. Schweizerische Medizinische Wochenschrift 1985;115(27-28):937-8. [PMID: ] [PubMed] [Google Scholar]
Amato 1990 {published data only}
- Amato M, Huppi P, Markus D. Assessment of neonatal jaundice in low birth weight infants comparing transcutaneous, capillary and arterial bilirubin levels. European Journal of Pediatrics 1990;150(1):59-61. [DOI: 10.1007/bf01959483] [PMID: ] [DOI] [PubMed] [Google Scholar]
Arman 2020 {published data only}
- Arman D, Topcuoglu S, Gursoy T, Ovali F, Karatekin G. The accuracy of transcutaneous bilirubinometry in preterm infants. Journal of Perinatology 2020;40(2):212-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Babaei 2012 {published data only}
- Babaei H, Alipour A, Sheikhi S. Comparison of transcutaneous and serum bilirubin level in term neonates with jaundice. Scientific Journal of Kurdistan University of Medical 2012;17(4):10-16. [Google Scholar]
Badiee 2012 {published data only}
- Badiee Z, Mohammadizadeh M, Shamee M. Diagnostic usefulness of transcutaneous bilirubinometry in very preterm newborns. International Journal of Preventive Medicine 2012;3(4):262-5. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Beck 2003 {published data only}
- Beck M, Kau N, Schlebusch H. Transcutaneous bilirubin measurement in newborn infants: evaluation of a new spectrophotometric method. Archives of Disease in Childhood. Fetal and neonatal edition 2003;88(4):F350-1. [DOI: 10.1136/fn.88.4.f350-b] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Berget 1984 {published data only}
- Berget M, Finne PH. Transcutaneous bilirubinometry in newborn infants. Tidsskrift for den Norske Laegeforening 1984;104(14):984-6. [PMID: ] [PubMed] [Google Scholar]
Bhat 1987 {published data only}
- Bhat V, Srinivasan S, Usha TS, Puri RK. Correlation of transcutaneous bilirubinometry with serum bilirubin in south Indian neonates. Indian Journal of Medical Research 1987;86:49-52. [PMID: ] [PubMed] [Google Scholar]
Bhutani 2000 {published data only}
- Bhutani VK, Gourley GR, Adler S, Kreamer B, Dalin C, Johnson LH. Noninvasive measurement of total serum bilirubin in a multiracial predischarge newborn population to assess the risk of severe hyperbilirubinemia. Pediatrics 2000;106(2):E17. [DOI: 10.1542/peds.106.2.e17] [PMID: ] [DOI] [PubMed] [Google Scholar]
Boo 1984 {published data only}
- Boo NY, Bakar AA. Transcutaneous bilirubinometry in Malay, Chinese and Indian term neonates. Medical Journal of Malaysia 1984;39(1):35-7. [PMID: ] [PubMed] [Google Scholar]
Bosschaart 2012 {published data only}
- Bosschaart N, Kok JH, Newsum AM, Ouweneel DM, Mentink R, Leeuwen TG, et al. Limitations and opportunities of transcutaneous bilirubin measurements. Pediatrics 2012;129(4):689-94. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bourchier 1987 {published data only}
- Bourchier D, Cull AB, Oettli PE. Transcutaneous bilirubinometry: 22 months experience at Waikato Women's Hospital. New Zealand Medical Journal 1987;100(832):599-600. [PMID: ] [PubMed] [Google Scholar]
Briscoe 2002 {published data only}
- Briscoe L, Clark S, Yoxall CW. Can transcutaneous bilirubinometry reduce the need for blood tests in jaundiced full term babies? Archives of Disease in Childhood. Fetal and Neonatal Edition 2002;86(3):F190-2. [DOI: 10.1136/fn.86.3.f190] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carbonell 1999 {published data only}
- Carbonell Estrany X, Botet Mussons F, Figueras Aloy J, Riu Godo A. Hyperbilirubinemia in full-term newborns. Predictive factors. Anales Espanoles de Pediatria 1999;50(4):389-92. [PMID: ] [PubMed] [Google Scholar]
Carbonell 2001 {published data only}
- Carbonell X, Botet F, Figueras J, Riu-Godo A. Prediction of hyperbilirubinaemia in the healthy term newborn. Acta Paediatrica 2001;90(2):166-70. [DOI: 10.1080/080352501300049343] [PMID: ] [DOI] [PubMed] [Google Scholar]
Carceller 2006 {published data only}
- Carceller AM, Delvin E, Gonthier M, Gregoire MC, Cousineau J, Alexandrov L. Evaluation of transcutaneous bilirubin measurement and agreement with bilirubinemia. Annales de Biologie Clinique 2006;64(6):575-9. [PMID: ] [PubMed] [Google Scholar]
Casnocha 2021 {published data only}
- Casnocha LL, Zibolenova J, Matasova K, Docekalova L, Zibolen M. Accuracy of enhanced transcutaneous bilirubinometry according to various measurement sites. Turkish Archives of Pediatrics 2021;56(1):15-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Castro 2019 {published data only}
- Castro A, Zozaya C, Cuesta MT, Gonzalez M, Villar G, Alcaraz A. Usefulness of transcutaneous bilirubin assessment measured in non-photo-exposed skin to guide the length of phototherapy: an observational study. Journal of Perinatal Medicine 2019;47(5):568-73. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cat 2021 {published data only}
- Cat FC, Cat A, Cicek T, Gulec SG. Evaluation of the relationship between transcutaneous bilirubin measurement and total serum bilirubin in neonatal patients followed for jaundice. Sisli Etfal Hastanesi tip bulteni 2021;55(2):262-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chang 2006 {published data only}
- Chang YH, Hsieh WS, Chou HC, Chen CY, Wu JY, Tsao PN. The effectiveness of a noninvasive transcutaneous bilirubin meter in reducing the need for blood sampling in Taiwanese neonates. Clinical Neonatology 2006;13(2):60-3. [DOI: 10.7098/CN.200612.0060] [DOI] [Google Scholar]
Chawla 2014 {published data only}
- Chawla D, Jain S, Kaur G, Sinhmar V, Guglani V. Accuracy of transcutaneous bilirubin measurement in preterm low-birth-weight neonates. European Journal of Pediatrics 2014;173(2):173-9. [DOI: 10.1007/s00431-013-2142-0] [PMID: ] [DOI] [PubMed] [Google Scholar]
Chimhini 2018 {published data only}
- Chimhini GLT, Chimhuya S, Chikwasha V. Evaluation of transcutaneous bilirubinometer (DRAEGER JM 103) use in Zimbabwean newborn babies. Maternal Health, Neonatology and Perinatology 2018;4:1. [DOI: 10.1186/s40748-017-0070-0] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chokemungmeepisarn 2020 {published data only}
- Chokemungmeepisarn P, Tantiprabha W, Kosarat S, Manopunya S. Accuracy of the Bilicare transcutaneous bilirubinometer as the predischarge screening tool for significant hyperbilirubinemia in healthy term and late preterm neonates. Journal of Maternal-fetal & Neonatal Medicine 2020;33(1):57-61. [PMID: ] [DOI] [PubMed] [Google Scholar]
Conceicao 2014 {published data only}
- Conceicao CM, Dornaus MF, Portella MA, Deutsch AD, Rebello CM. Influence of assessment site in measuring transcutaneous bilirubin. Einstein 2014;12(1):11-5. [DOI: 10.1590/s1679-45082014ao2711] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dagli 2018 {published data only}
- Dagli P, Vasava S. Accuracy of transcutaneous bilirubinometry in neonates receiving phototherapy. Journal of Nepal Paediatric Society 2018;38(1):1-7. [Google Scholar]
Dai 1996 {published data only}
- Dai J, Krahn J, Parry DM. Clinical impact of transcutaneous bilirubinometry as an adjunctive screen for hyperbilirubinemia. Clinical Biochemistry 1996;29(6):581-6. [DOI: 10.1016/s0009-9120(96)00104-x] [PMID: ] [DOI] [PubMed] [Google Scholar]
De Luca 2007 {published data only}
- De Luca D, Zecca E, Turris P, Barbato G, Marras M, Romagnoli C. Using BiliCheck for preterm neonates in a sub-intensive unit: diagnostic usefulness and suitability. Early Human Development 2007;83(5):313-7. [DOI: 10.1016/j.earlhumdev.2006.06.006] [PMID: ] [DOI] [PubMed] [Google Scholar]
De Luca 2008b {published data only}
- De Luca D, Zecca E, Corsello M, Tiberi E, Semeraro C, Romagnoli C. Attempt to improve transcutaneous bilirubinometry: a double-blind study of Medick BiliMed versus Respironics BiliCheck. Archives of Disease in Childhood. Fetal and Neonatal Edition 2008;93(2):F135-9. [DOI: 10.1136/adc.2007.121053] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dianova 2021 {published data only}
- Dianova E, Fogel J, Verma R. Predictability of transcutaneous bilirubinometry in late preterm and term infants at risk for pathological hyperbilirubinemia. Journal of Neonatal Perinatal Medicine 2021;14(2):261-7. [DOI] [PubMed] [Google Scholar]
Dominguez 1993 {published data only}
- Dominguez Ortega F, Ormazabal Ramos JC, Martin Zarza M, Domenech Martinez E. Transcutaneous bilirubinometry: correlation of the reading site obtained with spectrophotometry and diazo reaction technique. Anales Espanoles de Pediatria 1993;39(5):438-40. [PMID: ] [PubMed] [Google Scholar]
Donzelli 2000 {published data only}
- Donzelli G, Pratesi S. Transcutaneous bilirubinometry in healthy preterm newborns. Clinical Biochemistry 2000;33(6):505-8. [DOI: 10.1016/s0009-9120(00)00140-5] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ebbesen 2002 {published data only}
- Ebbesen F, Rasmussen LM, Wimberley PD. A new transcutaneous bilirubinometer, BiliCheck, used in the neonatal intensive care unit and the maternity ward. Acta Paediatrica 2002;91(2):203-11. [DOI: 10.1080/080352502317285225] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ebbesen 2012 {published data only}
- Ebbesen F, Vandborg P K, Trydal T. Comparison of the transcutaneous bilirubinometers BiliCheck and Minolta JM-103 in preterm neonates. Acta Paediatrica 2012;101(11):1128-33. [DOI: 10.1111/j.1651-2227.2012.02797.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
El‐Kabbany 2017 {published data only}
- El-Kabbany ZA, Toaima NN, Shedid AM. Implementation and validating transcutaneous bilirubinometry for neonates. Egyptian Pediatric Association Gazette 2017;65(2):38-42. [Google Scholar]
Engle 2002 {published data only}
- Engle WD, Jackson GL, Sendelbach D, Manning D, Frawley WH. Assessment of a transcutaneous device in the evaluation of neonatal hyperbilirubinemia in a primarily Hispanic population. Pediatrics 2002;110(1 Pt 1):61-7. [DOI: 10.1542/peds.110.1.61] [PMID: ] [DOI] [PubMed] [Google Scholar]
Engle 2005 {published data only}
- Engle WD, Jackson GL, Stehel EK, Sendelbach DM, Manning MD. Evaluation of a transcutaneous jaundice meter following hospital discharge in term and near-term neonates. Journal of Perinatology 2005;25(7):486-90. [DOI: 10.1038/sj.jp.7211333] [PMID: ] [DOI] [PubMed] [Google Scholar]
Fabris 1984 {published data only}
- Fabris C, Licata D, Roberi P, Miglio C, Garzena E, Cavo L, et al. Evaluation of transcutaneous bilirubinometry in newborn infants. Minerva Pediatrica 1984;36(11):565-70. [PMID: ] [PubMed] [Google Scholar]
Faridi 1998 {published data only}
- Faridi MM. Transcutaneous bilirubinometry in neonates. Indian Pediatrics 1998;35(6):568-9. [PMID: ] [PubMed] [Google Scholar]
Felc 2005 {published data only}
- Felc Z. Improvement of conventional transcutaneous bilirubinometry results in term newborn infants. American journal of perinatology 2005;22(4):173-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fisher 2015 {published data only}
- Fisher BG, Lakshman R. Transcutaneous bilirubin measurement in neonates with jaundice requiring phototherapy. Archives of Disease in Childhood 2015;100(Suppl 3):A139. [Google Scholar]
Foged 1983 {published data only}
- Foged N, Kristensen GB, Kamper J. Transcutaneous bilirubinometry. A non-invasive method of measuring physiological jaundice. Ugeskrift for Laeger 1983;145(38):2935-7. [PMID: ] [PubMed] [Google Scholar]
Fok 1986 {published data only}
- Fok TF, Lau SP, Hui CW, Fung KP, Wan CW. Transcutaneous bilirubinometer: its use in Chinese term infants and the effect of haematocrit and phototherapy on the TcB index. Australian Paediatric Journal 1986;22(2):107-9. [DOI: 10.1111/j.1440-1754.1986.tb00199.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Fonseca 2012 {published data only}
- Fonseca R, Kyralessa R, Malloy M, Richardson J, Jain SK. Covered skin transcutaneous bilirubin estimation is comparable with serum bilirubin during and after phototherapy. Journal of Perinatology 2012;32(2):129-31. [DOI: 10.1038/jp.2011.66] [PMID: ] [DOI] [PubMed] [Google Scholar]
Goldman 1982 {published data only}
- Goldman SL, Penalver A, Penaranda R. 21818063. Journal of Pediatrics 1982;101(2):253-6. [DOI: 10.1038/jp.2011.66] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gondale 2013 {published data only}
- Gondale G, Taksande A, Vilhekar K. Assessment of a transcutaneous bilirubinometer in the evaluation of neonatal Hyperbilirubinemia in hospitalized neonates. American Journal of Advances in Medical Science 2013;1(2):7-12. [Google Scholar]
Gothwal 2021 {published data only}
- Gothwal S, Singh N, Sitaraman S, Choudhary R, Meena KK, Bairwa GS, et al. Efficacy of transcutaneous bilirubinometry as compared to serum bilirubin in preterm newborn during phototherapy. European Journal of Pediatrics 2021;180(8):2629-36. [PMID: ] [DOI] [PubMed] [Google Scholar]
Grabenhenrich 2014 {published data only}
- Grabenhenrich J, Grabenhenrich L, Buhrer C, Berns M. Transcutaneous bilirubin after phototherapy in term and preterm infants. Pediatrics 2014;134(5):e1324-9. [DOI: 10.1542/peds.2014-1677] [PMID: ] [DOI] [PubMed] [Google Scholar]
Greco 2017 {published data only}
- Greco C, Iskander IF, Akmal DM, El Houchi SZ, Khairy DA, Bedogni G, et al. Comparison between Bilistick System and transcutaneous bilirubin in assessing total bilirubin serum concentration in jaundiced newborns. Journal of Perinatology 2017;37(9):1028-31. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gunaseelan 2017 {published data only}
- Gunaseelan Sushil, Devadas Sahana, Pai Nandita. Correlation of transcutaneous bilirubin and serum bilirubin concentration in term and late preterm newborns. Journal of Clinical Neonatology 2017;6(3):154-8. [Google Scholar]
Harish 1998 {published data only}
- Harish R, Sharma DB. Transcutaneous bilirubinometry in neonates: evaluation of Minolta Air shields jaundicemeter. Indian Pediatrics 1998;35(3):264-7. [PMID: ] [PubMed] [Google Scholar]
Hasani 2019 {published data only}
- Hasani M, Ghiasi A, Kheirkhah M, Sabuteh M, Karimian Z, Zaheri H, et al. Predictive value of transcutaneous bilirubinometry on third day of birth in diagnosis of hyperbilirubinemia in term and near-term neonates. Koomesh 2019;21(1):19-24. [Google Scholar]
Hegyi 1981 {published data only}
- Hegyi T, Hiatt IM, Indyk L. Transcutaneous bilirubinometry. I. Correlations in term infants. Journal of Pediatrics 1981;98(3):454-7. [DOI: 10.1016/s0022-3476(81)80721-4] [PMID: ] [DOI] [PubMed] [Google Scholar]
Heick 1982 {published data only}
- Heick C, Mieth D, Fallenstein F, Schubiger G, Nars PW, Amato M. Transcutaneous bilirubin determination in the newborn infant. Recommendations of the Swiss Neonatology Group. Helvetica Paediatrica Acta 1982;37(6):589-97. [PMID: ] [PubMed] [Google Scholar]
Hemmati 2021 {published data only}
- Hemmati F, Hashemi Z, Yazdani N. Comparing transcutaneous bilirubin versus serum bilirubin measurement using spectrophotometry for jaundice screening of full-term neonates. Tehran University Medical Journal 2021;78(12):835-842. [Google Scholar]
Hill 2016 {published data only}
- Hill S, Yamamoto L, Simons J, Jameson M, Field J, Antwi-Boasiako E. Can transcutaneous bilirubin measurement safely replace plasma bilirubin in well newborns greater than 35 weeks gestation? Clinical Biochemistry 2016;49(18):1434-5. [Google Scholar]
Ho 2006 {published data only}
- Ho EY, Lee SY, Chow CB, Chung JW. BiliCheck transcutaneous bilirubinometer: a screening tool for neonatal jaundice in the Chinese population [Xianggang yi xue za zhi]. Hong Kong Medical Journal 2006;12(2):99-102. [PMID: ] [PubMed] [Google Scholar]
Ho 2006a {published data only}
- Ho HT, Ng TK, Tsui KC, Lo YC. Evaluation of a new transcutaneous bilirubinometer in Chinese newborns. Archives of Disease in Childhood. Fetal and Neonatal Edition 2006;91(6):F434-8. [DOI: 10.1136/adc.2005.090217] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Holland 2009 {published data only}
- Holland L, Blick K. Implementing and validating transcutaneous bilirubinometry for neonates. American Journal of Clinical Pathology 2009;132(4):555-61. [DOI: 10.1309/AJCPN9BMFW8COTWP] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hoppenot 2012 {published data only}
- Hoppenot C, Emmett GA. Neonatal bilirubin triage with transcutaneous meters: when is a blood draw necessary? Hospital Pediatrics 2012;2(4):215-20. [DOI: 10.1542/hpeds.2012-0012] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hulzebos 2019 {published data only}
- Hulzebos CV, Vader-van ID, Bos AF, Dijk PH. Should transcutaneous bilirubin be measured in preterm infants receiving phototherapy? The relationship between transcutaneous and total serum bilirubin in preterm infants with and without phototherapy. PLOS One 2019;14(6):e0218131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Itoh 2001 {published data only}
- Itoh S, Kondo M, Kusaka T, Isobe K, Onishi S. Differences in transcutaneous bilirubin readings in Japanese term infants according to feeding method. Pediatrics International 2001;43(1):12-5. [DOI: 10.1046/j.1442-200x.2001.01339.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Jahadi 2013 {published data only}
- Jahadi R, Sajjadian N. Trans cutaneous bilirubinometry for preterm. Intensive Care Medicine 2013;39:S144-S145. [Google Scholar]
Jalalodini 2019 {published data only}
- Jalalodini A, Ghaljaei F. Comparison of transcutaneous bilirubin measurement with total serum bilirubin levels in term neonates with hyperbilirubinemia: a descriptive-analytical study. Journal of Comprehensive Pediatrics 2019;10(4):10(4):e84720. [Google Scholar]
Jangaard 2006 {published data only}
- Jangaard K, Curtis H, Goldbloom R. Estimation of bilirubin using BiliChektrade mark, a transcutaneous bilirubin measurement device: effects of gestational age and use of phototherapy. Paediatrics & Child Health 2006;11(2):79-83. [DOI: 10.1093/pch/11.2.79] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Janjindamai 2005 {published data only}
- Janjindamai W, Tansantiwong T. Accuracy of transcutaneous bilirubinometer estimates using BiliCheck in Thai neonates [Chotmaihet Thangphaet]. Journal of the Medical Association of Thailand 2005;88(2):187-90. [PMID: ] [PubMed] [Google Scholar]
Jegathesan 2017 {published data only}
- Jegathesan T, Campbell D, Debono M, Shah V, Twiss J, Sgro M. Investigating the accuracy of an upgraded transcutaneous bilirubin screening tool by site of testing. Paediatrics & Child Health 2017;22(Suppl 1):e41. [Google Scholar]
Jegathesan 2021 {published data only}
- Jegathesan T, Campbell DM, Ray JG, Shah V, Berger H, Hayeems RZ, et al. Transcutaneous versus total serum bilirubin measurements in preterm Infants. Neonatology 2021;118(4):443-53. [DOI] [PubMed] [Google Scholar]
Jeon 2020 {published data only}
- Jeon J, Lim G, Oh KW, Lee NM, Park HW, Chung ML. The forehead is a better site than the sternum to check transcutaneous bilirubin during phototherapy in sick infants. BMC Pediatrics 2020;20:1-5. [DOI] [PMC free article] [PubMed]
Jnah 2018 {published data only}
- Jnah A, Newberry DM, Eisenbeisz E. Comparison of transcutaneous and serum bilirubin measurements in neonates 30 to 34 weeks' gestation before, during, and after phototherapy. Advances in Neonatal Care 2018;18(2):144-53. [PMID: ] [DOI] [PubMed] [Google Scholar]
Johnson 2020 {published data only}
- Johnson SM, Vasu V, Marseille C, Hill C, Janvier L, Toussaint P, et al. Validation of transcutaneous bilirubinometry during phototherapy for detection and monitoring of neonatal jaundice in a low-income setting. Paediatrics and International Child Health 2020;40(1):25-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jones 2017 {published data only}
- Jones DF, McRea AR, Knowles JD, Lin FC, Burnette E, Reller LA, et al. A prospective comparison of transcutaneous and serum bilirubin within brief time intervals. Clinical Pediatrics 2017;56(11):1013-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Joseph 2014 {published data only}
- Joseph R, Chinnadurai A, Yeo W, Nkouibert P, George M, Chan, E. Reducing serum bilirubin determinations in outpatient newborns by screening with transcutaneous bilirubinometry. Archives of Disease in Childhood 2014;99:A459-A460.
Juster‐Reicher 2014 {published data only}
- Juster-Reicher A, Flidel-Rimon O, Rozin I, Shinwell ES. Correlation of transcutaneous bilirubinometry (TcB) and total serum bilirubin (TsB) levels after phototherapy. Journal of Maternal-Fetal & Neonatal Medicine 2014;88(2):1-3. [PMID: ] [DOI] [PubMed] [Google Scholar]
Karen 2009 {published data only}
- Karen T, Bucher HU, Fauchere JC. Comparison of a new transcutaneous bilirubinometer (Bilimed) with serum bilirubin measurements in preterm and full-term infants. BMC Pediatrics 2009;9:70. [DOI: 10.1186/1471-2431-9-70] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Karolyi 2004 {published data only}
- Karolyi L, Pohlandt F, Muche R, Franz AR, Mihatsch WA. Transcutaneous bilirubinometry in very low birthweight infants. Acta Paediatrica 2004;93(7):941-4. [DOI: 10.1111/j.1651-2227.2004.tb02693.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kaynak‐Turkmen 2011 {published data only}
- Kaynak-Turkmen M, Aydogdu SA, Gokbulut C, Yenisey C, Soz O, Cetinkaya-Cakmak B. Transcutaneous measurement of bilirubin in Turkish newborns: comparison with total serum bilirubin. Turkish Journal of Pediatrics 2011;53(1):67-74. [PMID: ] [PubMed] [Google Scholar]
Kazmierczak 2004 {published data only}
- Kazmierczak SC, Robertson AF, Briley KP, Kreamer B, Gourley GR. Transcutaneous measurement of bilirubin in newborns: comparison with an automated Jendrassik-Grof procedure and HPLC. Clinical Chemistry 2004;50(2):433-5. [DOI: 10.1373/clinchem.2003.027326] [PMID: ] [DOI] [PubMed] [Google Scholar]
Keshini 2018 {published data only}
- Keshini N, John S, Srinivas B. The accuracy of transcutaneous bilirubin measurement in preterm neonates. Journal of Paediatrics and Child Health 2018;54(S1):84. [Google Scholar]
Keshishian 1994 {published data only}
- Keshishian ES, Ovanesov EN, Prishchepa MI. Use of the method of transcutaneous bilirubinometry in hyperbilirubinemia in the newborn. Klinicheskaia Laboratornaia Diagnostika 1994;5(5):17-20. [PMID: ] [PubMed] [Google Scholar]
Khajehei 2021 {published data only}
- Khajehei M, Chua SC, Gidaszewski B, Swain J. Comparing JM-105 and MBJ-20 transcutaneous bilirubinometers according to the area tested in ethnically diverse late-preterm and term neonates. Journal of Perinatal & Neonatal Nursing 2021;35(3):E30-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Knudsen 1996 {published data only}
- Knudsen A, Ebbesen F. Transcutaneous bilirubinometry in neonatal intensive care units. Archives of Disease in Childhood. Fetal and Neonatal Edition 1996;75(1):F53-6. [DOI: 10.1136/fn.75.1.f53] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Konana 2021 {published data only}
- Konana OS, Bahr TM, Strike HR, Coleman J, Snow GL, Christensen RD. Decision accuracy and safety of transcutaneous bilirubin screening at Intermountain Healthcare. Journal of Pediatrics 2021;228:53-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kosarat 2013 {published data only}
- Kosarat S, Khuwuthyakorn V. Accuracy of transcutaneous bilirubin measurement in term newborns [Chotmaihet Thangphaet]. Journal of the Medical Association of Thailand 2013;96(2):172-7. [PMID: ] [PubMed] [Google Scholar]
Kumar 1994 {published data only}
- Kumar A, Faridi MM, Singh N, Ahmad SH. Transcutaneous bilirubinometry in the management of bilirubinemia in term neonates. Indian Journal of Medical Research 1994;99:227-30. [PMID: ] [PubMed] [Google Scholar]
Kumar 2017 {published data only}
- Kumar V, Kahlon P, Singh P, Singh K, Sharma A. Utility of transcutaneous bilirubinometer in tertiary care hospital. Journal of Nepal Paediatric Society 2017;37(1):72-78. [Google Scholar]
Kumar 2020 {published data only}
- Kumar D, Kumar D. A prospective comparison of serum and transcutaneous bilirubin in Indian neonates (in press). Journal of Pediatric Intensive Care 2020;11(02):100-104. [DOI: 10.1055/s-0040-1721067] [EMBASE: 633608577] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kumra 2017 {published data only}
- Kumra T, Weaver SSJ, Prather K, Garnepudi L Bartlett EL, Crocetti M. Correlation of transcutaneous and serum bilirubin measurements in the outpatient setting. Clinical Pediatrics 2017;57(2):231-4. [DOI] [PubMed] [Google Scholar]
Laeeq 1993 {published data only}
- Laeeq A, Yasin M, Chaudhry AR. Transcutaneous bilirubinometry: clinical application. Journal of the Pakistan Medical Association 1993;43(2):28-30. [PMID: ] [PubMed] [Google Scholar]
Lebrun 1982 {published data only}
- Lebrun F, Sender A, Goujard J, Francoual C, Amiel-Tison C. Clinical evaluation of transcutaneous bilirubinometry. Annales de Pediatrie 1982;29(7):494-8. [PMID: ] [PubMed] [Google Scholar]
Leite 2007 {published data only}
- Leite M, Granato Vde A, Facchini FP, Marba ST. Comparison of transcutaneous and plasma bilirubin measurement. Jornal de Pediatria 2007;83(3):283-6. [DOI: 10.2223/JPED.1619] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lin 1993 {published data only}
- Lin YJ, Ju SH, Lin CH. The clinical application of transcutaneous bilirubinometry in full-term Chinese infants. Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi 1993;34(2):69-76. [PMID: ] [PubMed] [Google Scholar]
López‐Garrido 2015 {published data only}
- López-Garrido E, Chávez-Gutiérrez CA, Rivera-Vázquez P. Transcutaneous and serum bilirubin metering correlation in newborns at term and late preterm. Revista Mexicana de Pediatria 2015;82(5):159-64. [Google Scholar]
Mahajan 2005 {published data only}
- Mahajan G, Kaushal RK, Sankhyan N, Sharma RL, Nakra M. Transcutaneous bilirubinometer in assessment of neonatal jaundice in northern India. Indian Pediatrics 2005;42(1):41-5. [PMID: ] [PubMed] [Google Scholar]
Mahram 2015 {published data only}
- Mahram M, Oveisi S, Jaberi N. Trans-cutaneous bilirubinometery versus serum bilirubin in neonatal jaundice. Acta Medica Iranica 2015;53(12):764-9. [PMID: ] [PubMed] [Google Scholar]
Maisels 2004 {published data only}
- Maisels MJ, Ostrea EMJ, Touch S, Clune SE, Cepeda E, Kring E, et al. Evaluation of a new transcutaneous bilirubinometer. Pediatrics 2004;113(6):1628-35. [DOI: 10.1542/peds.113.6.1628] [PMID: ] [DOI] [PubMed] [Google Scholar]
Maisels 2006 {published data only}
- Maisels MJ, Kring E. Transcutaneous bilirubin levels in the first 96 hours in a normal newborn population of > or = 35 weeks' gestation. Pediatrics 2006;117(4):1169-73. [DOI: 10.1542/peds.2005-0744] [PMID: ] [DOI] [PubMed] [Google Scholar]
Maisels 2011 {published data only}
- Maisels MJ, Engle WD, Wainer S, Jackson GL, McManus S, Artinian F. Transcutaneous bilirubin levels in an outpatient and office population. Journal of Perinatology 2011;31(9):621-4. [DOI: 10.1038/jp.2011.5] [PMID: ] [DOI] [PubMed] [Google Scholar]
Marco 2009 {published data only}
- Marco Lozano N, Vizcaino Diaz C, Quiles Dura JL, Alos Munoz A, Vargas Torcal F. Neonatal jaundice: clinical evaluation of a transcutaneous bilirubinometer. Anales de Pediatria 2009;71(2):157-60. [DOI: 10.1016/j.anpedi.2009.02.006] [PMID: ] [DOI] [PubMed] [Google Scholar]
Maya‐Enero 2021 {published data only}
- Maya-Enero S, Candel-Pau J, Garcia-Garcia J, Duran-Jorda X. Reliability of transcutaneous bilirubin determination based on skin color determined by a neonatal skin color scale of our own. European Journal of Pediatrics 2021;180(2):607-16. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mercanti 2007 {published data only}
- Mercanti I, Michel F, Thomachot L, Loundou DA, Nicaise C, Vialet R, et al. Transcutaneous bilirubin measurement in preterm infants. Archives de Pediatrie 2007;14(7):875-80. [DOI: 10.1016/j.arcped.2007.02.091] [PMID: ] [DOI] [PubMed] [Google Scholar]
Mohamed 2022 {published data only}
- Mohamed M, Ibrahim NR, Ramli N, Abdul Majid N, Yacob NM, Nasir A. Comparison between the Transcutaneous and Total Serum Bilirubin Measurement in Malay Neonates with Neonatal Jaundice. Malaysian Journal of Medical Sciences 2022;29(1):43-54. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moscicka 1994 {published data only}
- Moscicka A, Gadzinowski J, Moscicki A, Breborowicz GH, Opala T. Usefulness of transcutaneous measurements of bilirubin in infants with jaundice. Ginekologia Polska 1994;65(6):271-5. [PMID: ] [PubMed] [Google Scholar]
Murli 2017 {published data only}
- Murli L, Thukral A, Sankar MJ, Vishnubhatla S, Deorari AK, Paul VK, et al. Reliability of transcutaneous bilirubinometry from shielded skin in neonates receiving phototherapy: a prospective cohort study. Journal of Perinatology 2017;37(2):182-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Nahar 2017 {published data only}
- Nahar N, Mannan MA, Dey AC, Ahmed F, Khan KA, Jahan I, et al. Comparison of serum bilirubin with transcutaneous bilirubinometry in late preterm and term newborn. Mymensingh Medical Journal 2017;26(3):621-7. [PMID: ] [PubMed] [Google Scholar]
Namba 2007 {published data only}
- Namba F, Kitajima H. Utility of a new transcutaneous jaundice device with two optical paths in premature infants. Pediatrics International 2007;49(4):497-501. [DOI: 10.1111/j.1442-200X.2007.02386.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Nanjundaswamy 2004 {published data only}
- Nanjundaswamy S, Petrova A, Mehta R, Bernstein W, Hegyi T. The accuracy of transcutaneous bilirubin measurements in neonates: a correlation study. Biology of the Neonate 2004;85(1):21-5. [DOI: 10.1159/000074953] [PMID: ] [DOI] [PubMed] [Google Scholar]
Nanjundaswamy 2005 {published data only}
- Nanjundaswamy S, Petrova A, Mehta R, Hegyi T. Transcutaneous bilirubinometry in preterm infants receiving phototherapy. American Journal of Perinatology 2005;22(3):127-31. [DOI: 10.1055/s-2005-863785] [PMID: ] [DOI] [PubMed] [Google Scholar]
Narang 1983 {published data only}
- Narang A, Buche VB. Evaluation of the Minolta Jaundice Meter as a screening device in Indian babies: a preliminary communication. Indian Pediatrics 1983;20(8):583-5. [PMID: ] [PubMed] [Google Scholar]
Ochoa 2000 {published data only}
- Ochoa Sangrador C, Marugan Isabel VM, Tesoro Gonzalez R, Garcia Rivera MT, Hernandez Calvo MT. Evaluation of a transcutaneous bilirubinometer [Evaluacion de un instrumento de medicion de la bilirrubina transcutanea]. Anales Espanoles de Pediatria 2000;52(6):561-8. [PMID: ] [PubMed] [Google Scholar]
Oyapero 2017 {published data only}
- Oyapero Oyejoke, Njokanma O, Disu E. Evaluation of transcutaneous bilirubinometry in term neonates at Lagos State University Teaching Hospital, Ikeja, Lagos. Journal of Clinical Neonatology 2017;6(4):213-9. [Google Scholar]
Pallas 1993 {published data only}
- Pallas Alonso C, Martin Puerto MJ, Mendoza Soto A, Bustos Lozano G, Flores Anton B, Orbea Gallardo C. Transcutaneous bilirubin measurement in neonates. Anales Espanoles de Pediatria 1993;38(1):33-7. [PMID: ] [PubMed] [Google Scholar]
Palmer 1982 {published data only}
- Palmer DC, Zenner EM, Drew JH. Transcutaneous bilirubinometry: use in Australia. Australian Paediatric Journal 1982;18(4):273-6. [PMID: ] [PubMed] [Google Scholar]
Panburana 2010 {published data only}
- Panburana J, Boonkasidach S, Rearkyai S. Accuracy of transcutaneous bilirubinometry compare to total serum bilirubin measurement [Chotmaihet Thangphaet]. Journal of the Medical Association of Thailand 2010;93 Suppl 2:S81-6. [PMID: ] [PubMed] [Google Scholar]
Pendse 2017 {published data only}
- Pendse A, Jasani B, Nanavati R, Kabra N. Comparison of transcutaneous bilirubin measurement with total serum bilirubin levels in preterm neonates receiving phototherapy. Indian Pediatrics 2017;54(8):641-3. [PMID: ] [DOI] [PubMed] [Google Scholar]
Poland 2004 {published data only}
- Poland RL, Hartenberger C, McHenry H, Hsi A. Comparison of skin sites for estimating serum total bilirubin in in-patients and out-patients: chest is superior to brow. Journal of Perinatology 2004;24(9):541-3. [DOI: 10.1038/sj.jp.7211141] [PMID: ] [DOI] [PubMed] [Google Scholar]
Povaluk 2011 {published data only}
- Povaluk P, Shwetz E, Kliemann R. Comparative study between plasma and transcutaneous bilirubin measurements in newborns. Revista Paulista de Pediatria 2011;29(1):1-12. [Google Scholar]
Pratesi 2016 {published data only}
- Pratesi S, Boni L, Tofani L, Berti E, Sollai S, Dani C. Comparison of the transcutaneous bilirubinometers BiliCare and Minolta JM-103 in late preterm and term neonates. Journal of Maternal-fetal & Neonatal Medicine 2016;29(18):3014-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Qandalji 2012 {published data only}
- Qandalji BR. 1329 Comparison of transcutaneous bilirubinometer with serum bilirubin in neonates. Archives of Disease in Childhood 2012;97(Suppl 2):A378. [Google Scholar]
Qualter 2011 {published data only}
- Qualter YM, Allen NM, Corcoran JD, O'Donovan DJ. Transcutaneous bilirubin – comparing the accuracy of BiliChek(R) and JM 103(R) in a regional postnatal unit. Journal of Maternal-Fetal & Neonatal Medicine 2011;24(2):267-70. [DOI: 10.3109/14767058.2010.484471] [PMID: ] [DOI] [PubMed] [Google Scholar]
Quist 2016 {published data only}
- Quist FK, Bapat R, Kuch-Kunich HK, Ezeanolue K, Keeni S, Thomas R, et al. Clinical utility of transcutaneous bilirubinometer (TcB) in very low birth weight (VLBW) infants. Journal of Perinatal Medicine 2016;44(8):933-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Raba 2020 {published data only}
- Raba AA, O'Sullivan A, Miletin J. Transcutaneous bilirubinometry during and after phototherapy in preterm infants: a prospective observational study. BMJ Paediatrics Open 2020;4(1):e000681. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Radfar 2016 {published data only}
- Radfar M, Hashemieh M, Shirvani F, Madani R. Transcutaneous bilirubinometry in preterm and term newborn infants before and during phototherapy. Archives of Iranian Medicine 2016;19(5):323-8. [PMID: ] [PubMed] [Google Scholar]
Raimondi 2012 {published data only}
- Raimondi F, Lama S, Landolfo F, Sellitto M, Borrelli AC, Maffucci R, et al. Measuring transcutaneous bilirubin: a comparative analysis of three devices on a multiracial population. BMC Pediatrics 2012;12:70. [DOI: 10.1186/1471-2431-12-70] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reyes 2008 {published data only}
- Reyes CA, Stednitz DR, Hahn C, Mutchie KD, McCullough SR, Kronberg K. Evaluation of the BiliChek being used on hyperbilirubinemic newborns undergoing home phototherapy. Archives of Pathology & Laboratory Medicine 2008;132(4):684-9. [DOI: 10.1043/1543-2165(2008)132[684:EOTBBU]2.0.CO;2] [PMID: ] [DOI] [PubMed] [Google Scholar]
Robertson 2002 {published data only}
- Robertson A, Kazmierczak S, Vos P. Improved transcutaneous bilirubinometry: comparison of SpectR(X) BiliCheck and Minolta Jaundice Meter JM-102 for estimating total serum bilirubin in a normal newborn population. Journal of Perinatology 2002;22(1):12-4. [DOI: 10.1038/sj.jp.7210592] [PMID: ] [DOI] [PubMed] [Google Scholar]
Rodriguez‐Capote 2009 {published data only}
- Rodriguez-Capote K, Kim K, Paes B, Turner D, Grey V. Clinical implication of the difference between transcutaneous bilirubinometry and total serum bilirubin for the classification of newborns at risk of hyperbilirubinemia. Clinical Biochemistry 2009;42(3):176-9. [DOI: 10.1016/j.clinbiochem.2008.09.108] [PMID: ] [DOI] [PubMed] [Google Scholar]
Rohsiswatmo 2018 {published data only}
- Rohsiswatmo R, Oswari H, Amandito R, Sjakti HA, Windiastuti E, Roeslani R, et al. Agreement test of transcutaneous bilirubin and bilistick with serum bilirubin in preterm infants receiving phototherapy. BMC Pediatrics 2018;18(1):315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Romagnoli 2012 {published data only}
- Romagnoli C, Zecca E, Catenazzi P, Barone G, Zuppa AA. Transcutaneous bilirubin measurement: comparison of Respironics BiliCheck and JM-103 in a normal newborn population. Clinical Biochemistry 2012;45(9):659-62. [DOI: 10.1016/j.clinbiochem.2012.03.014] [PMID: ] [DOI] [PubMed] [Google Scholar]
Romagnoli 2013 {published data only}
- Romagnoli C, Catenazzi P, Barone G, Giordano L, Riccardi R, Zuppa AA, et al. BiliCheck vs JM-103 in identifying neonates not at risk of hyperbilirubinaemia. Italian Journal of Pediatrics 2013;39:46. [DOI: 10.1186/1824-7288-39-46] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Romo‐Pinales 1984 {published data only}
- Romo-Pinales J, Orozco-Gutiérrez A, Udaeta-Mora E, Graham-Pontones S. Preliminary study in Mexico on the use of the transcutaneous bilirubinometer [Estudio preliminar en Mexico sobre la utilizacion del bilirrubinometro transcutaneo]. Boletin Medico del Hospital Infantil de Mexico 1984;41(10):536-8. [PMID: ] [PubMed] [Google Scholar]
Roy 2010 {published data only}
- Roy B, Coakley J, Badawi N. Is there a place for transcutaneous bilirubinometery in the NICU? Journal of Paediatrics and Child Health 2010;46:36. [Google Scholar]
Rubaltelli 2001 {published data only}
- Rubaltelli FF, Gourley GR, Loskamp N, Modi N, Roth-Kleiner M, Sender A, et al. Transcutaneous bilirubin measurement: a multicenter evaluation of a new device. Pediatrics 2001;107(6):1264-71. [DOI: 10.1542/peds.107.6.1264] [PMID: ] [DOI] [PubMed] [Google Scholar]
Rubio 2017 {published data only}
- Rubio A, Epiard C, Gebus M, Deiber M, Samperiz S, Genty C, et al. Diagnosis accuracy of transcutaneous bilirubinometry in very preterm newborns. Neonatology 2017;111(1):1-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rush 2011 {published data only}
- Rush M, Wiley C. Performance evaluation of the Minolta/Drager JM-103 jaundice meter. Clinical Chemistry 2011;57(10):A52. [Google Scholar]
Rylance 2014 {published data only}
- Rylance S, Yan J, Molyneux E. Can transcutaneous bilirubinometry safely guide phototherapy treatment of neonatal jaundice in Malawi? Paediatrics and International Child Health 2014;34(2):101-7. [DOI: 10.1179/2046905513Y.0000000050] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sajjadian 2012 {published data only}
- Sajjadian N, Shajari H, Saalehi Z, Esphahani F, Alizadeh Taheri P. Transcutaneous bilirubin measurement in preterm neonates. Acta Medica Iranica 2012;50(11):765-70. [PMID: ] [PubMed] [Google Scholar]
Samiee‐Zafarghandy 2014 {published data only}
- Samiee-Zafarghandy S, Feberova J, Williams K, Yasseen AS, Perkins SL, Lemyre B. Influence of skin colour on diagnostic accuracy of the jaundice meter JM 103 in newborns. Archives of Disease in Childhood. Fetal and Neonatal Edition 2014;99(6):F480-4. [DOI: 10.1136/archdischild-2013-305699] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sanpavat 2004 {published data only}
- Sanpavat S, Nuchprayoon I. Noninvasive transcutaneous bilirubin as a screening test to identify the need for serum bilirubin assessment. Journal of the Medical Association of Thailand 2004;87(10):1193-8. [PMID: ] [PubMed] [Google Scholar]
Sanpavat 2005 {published data only}
- Sanpavat S, Nuchprayoon I. Comparison of two transcutaneous bilirubinometers – Minolta AirShields Jaundice Meter JM103 and Spectrx Bilicheck--in Thai neonates. Southeast Asian Journal of Tropical Medicine and Public Health 2005;36(6):1533-7. [PMID: ] [PubMed] [Google Scholar]
Sanpavat 2007 {published data only}
- Sanpavat S, Nuchprayoon I. Transcutaneous bilirubin in the pre-term infants. Journal of the Medical Association of Thailand [Chotmaihet Thangphaet] 2007;90(9):1803-8. [PMID: ] [PubMed] [Google Scholar]
Sardar 2021 {published data only}
- Sardar S, Sarkar N, Ghosh M, Pal S. An observational prospective study to compare transcutaneous bilirubin with serum bilirubin in preterm newborn requiring phototherapy. Journal of Clinical Neonatology 2021;10(2):59-67. [Google Scholar]
Sarici 2014 {published data only}
- Sarici SU, Koklu E, Babacan O. Comparison of two transcutaneous bilirubinometers in term and near-term neonates. Neonatal Network 2014;33(3):138-42. [DOI: 10.1891/0730-0832.33.3.138] [PMID: ] [DOI] [PubMed] [Google Scholar]
Schmidt 2009 {published data only}
- Schmidt ET, Wheeler CA, Jackson GL, Engle WD. Evaluation of transcutaneous bilirubinometry in preterm neonates. Journal of Perinatology 2009;29(8):564-9. [DOI: 10.1038/jp.2009.38] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sellitto 2010 {published data only}
- Sellitto M, De Rosa M, Internicola M, Pisanti R, Del Buono D, Iovino A et al. Measuring transcutaneous bilirubin: a comparative analysis of three devices. Journal of Maternal-Fetal and Neonatal Medicine 2010;23:560-1. [Google Scholar]
Sharma 1988 {published data only}
- Sharma JN, Singh RN, Lodha A, Singh J. Transcutaneous bilirubinometry in newborns. Indian Pediatrics 1988;25(8):757-60. [PMID: ] [PubMed] [Google Scholar]
Sheridan‐Pereira 1982 {published data only}
- Sheridan-Pereira M, Gorman W. Transcutaneous bilirubinometry: an evaluation. Archives of Disease in Childhood 1982;57(9):708-10. [DOI: 10.1136/adc.57.9.708] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shihadeh 2016 {published data only}
- Shihadeh M, Kheyami D, Al Jufairi M, Jaradat A, Ansari E. Transcutaneous bilirubin measurement correlation with total serum bilirubin in healthy newborns. Bahrain Medical Bulletin 2016;38(4):208-210. [Google Scholar]
Siu 2010 {published data only}
- Siu LY, Siu LW, Au SK, LI KW, Tsui TK, Chang YY, et al. Evaluation of a transcutaneous bilirubinometer with two optical paths in Chinese preterm infants. Hong Kong Journal of Paediatrics 2010;15(2):132-40. [Google Scholar]
Slusher 2004 {published data only}
- Slusher TM, Angyo IA, Bode-Thomas F, Akor F, Pam SD, Adetunji AA, et al. Transcutaneous bilirubin measurements and serum total bilirubin levels in indigenous African infants. Pediatrics 2004;113(6):1636-41. [DOI: 10.1542/peds.113.6.1636] [PMID: ] [DOI] [PubMed] [Google Scholar]
Soffer 2018 {published data only}
- Soffer OD, Barran D, Roth P. Transcutaneous bilirubin on premature neonates, can we rely on it? Pediatrics 2018;141:567. [Google Scholar]
Spence 2016 {published data only}
- Spence J, Bevan C, Walton E. G236(P) Introduction of a transcutaneous bilirubinometer into children's ED – a good initiative? Archives of Disease in Childhood 2016;101(Suppl 1):A129. [Google Scholar]
Srinivas 2016 {published data only}
- Srinivas GL, Cuff CD, Ebeling MD, Mcelligott JT. Transcutaneous bilirubinometry is a reliably conservative method of assessing neonatal jaundice. Journal of Maternal-Fetal & Neonatal Medicine 2016;29(16):2635-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Stillova 2007 {published data only}
- Stillova L, Matasova K, Mikitova T, Stilla J, Kolarovszka H, Zibolen M. Evaluation of transcutaneous bilirubinometry in preterm infants of gestational age 32-34 weeks. Biomedical Papers of the Medical Faculty of the University Palacky, Olomouc, Czechoslovakia 2007;151(2):267-71. [DOI: 10.5507/bp.2007.045] [PMID: ] [DOI] [PubMed] [Google Scholar]
Stillova 2009 {published data only}
- Stillova L, Matasova K, Zibolen M, Stilla J, Kolarovszka H. Transcutaneous bilirubinometry in preterm neonates. Indian Pediatrics 2009;46(5):405-8. [DOI: 10.5507/bp.2007.045] [PMID: ] [DOI] [PubMed] [Google Scholar]
Suckling 1995 {published data only}
- Suckling RJ, Laing IA, Kirk JM. Transcutaneous bilirubinometry as a screening tool for neonatal jaundice. Scottish Medical Journal 1995;40(1):14-5. [DOI: 10.1177/003693309504000106] [PMID: ] [DOI] [PubMed] [Google Scholar]
Szabo 2004a {published data only}
- Szabo P, Wolf M, Bucher HU, Fauchere JC, Haensse D, Arlettaz R. Detection of hyperbilirubinaemia in jaundiced full-term neonates by eye or by bilirubinometer? European Journal of Pediatrics 2004;163(12):722-7. [DOI: 10.1007/s00431-004-1533-7] [PMID: ] [DOI] [PubMed] [Google Scholar]
Szabo 2004b {published data only}
- Szabo P, Wolf M, Bucher HU, Haensse D, Fauchere JC, Arlettaz R. Assessment of jaundice in preterm neonates: comparison between clinical assessment, two transcutaneous bilirubinometers and serum bilirubin values. Acta Paediatrica 2004;93(11):1491-5. [DOI: 10.1080/08035250410018328] [PMID: ] [DOI] [PubMed] [Google Scholar]
Tan 1982 {published data only}
- Tan KL. Transcutaneous bilirubinometry in fullterm Chinese and Malay infants. Acta Paediatrica Scandinavica 1982;71(4):593-6. [DOI: 10.1111/j.1651-2227.1982.tb09480.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Tan 1985 {published data only}
- Tan KL. Transcutaneous bilirubinometry in Chinese and Malay neonates. Annals of the Academy of Medicine, Singapore 1985;14(4):591-4. [PMID: ] [PubMed] [Google Scholar]
Tan 1988 {published data only}
- Tan KL, Mylvaganam A. Transcutaneous bilirubinometry in preterm very low birthweight infants. Acta paediatrica Scandinavica 1988;77(6):796-801. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tan 1996 {published data only}
- Tan KL, Chia HP, Koh BC. Transcutaneous bilirubinometry in Chinese, Malay and Indian infants. Acta Paediatrica 1996;85(8):986-90. [DOI: 10.1111/j.1651-2227.1996.tb14199.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Tan 2003 {published data only}
- Tan KL, Dong F. Transcutaneous bilirubinometry during and after phototherapy. Acta Paediatrica 2003;92(3):327-31. [PMID: ] [PubMed] [Google Scholar]
Tayaba 1998 {published data only}
- Tayaba R, Gribetz D, Gribetz I, Holzman IR. Noninvasive estimation of serum bilirubin. Pediatrics 1998;102(3):E28. [DOI: 10.1542/peds.102.3.e28] [PMID: ] [DOI] [PubMed] [Google Scholar]
Taylor 2015 {published data only}
- Taylor JA, Burgos AE, Flaherman V, Chung EK, Simpson EA, Goyal NK, et al. Discrepancies between transcutaneous and serum bilirubin measurements. Pediatrics 2015;135(2):224-31. [DOI: 10.1542/peds.2014-1919] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Teran 2011 {published data only}
- Teran CG, Mohamed T, Casey J. Transcutaneous bilirubinometry: comparison of two multiwavelength devices in healthy term newborns. European Journal of Pediatrics 2011;170(11):1485. [DOI: 10.1007/s00431-011-1510-x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Timalsina 2017 {published data only}
- Timalsina S, Shrestha S. Utility and accuracy of transcutaneous bilirubin as a screening test to identify the need for serum bilirubin assessment in neonates in Nepal. Clinical Chemistry 2017;63 2017;63:(Supplement 1):S259-60. [Google Scholar]
Vocel 1985 {published data only}
- Vocel J, Mrastikova H, Reitschlagerova V. Transcutaneous bilirubinometry using the Minolta/Air-schield in neonates. Ceskoslovenska Pediatrie 1985;40(11):649-51. [PMID: ] [PubMed] [Google Scholar]
Vocel 1987 {published data only}
- Vocel J, Jezkova Z, Reitschlagerova V. Transcutaneous bilirubinometry using the Minolta/Air-Shields apparatus in neonates with a low birthweight. Ceskoslovenska pediatrie 1987;42(1):20-2. [PubMed] [Google Scholar]
Vocel 1988 {published data only}
- Vocel J, Petrova L, Concha Gonzales J, Paulova M, Reitschlagerova V. Transcutaneous bilirubinometry in neonates with hyperbilirubinemia treated with phototherapy. Ceskoslovenska Pediatrie 1988;43(12):742-3. [PMID: ] [PubMed] [Google Scholar]
Wainer 2009 {published data only}
- Wainer S, Rabi Y, Parmar SM, Allegro D, Lyon M. Impact of skin tone on the performance of a transcutaneous jaundice meter. Acta Paediatrica 2009;98(12):1909-15. [DOI: 10.1111/j.1651-2227.2009.01497.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Weber 2021 {published data only}
- Weber J, Vadasz-Chates N, Wade C, Micetic B, Gerkin R, Rao S. Transcutaneous bilirubin monitoring in preterm infants of 23 to 34 weeks' gestation. American Journal of Perinatology 2021;Epub:ahead of print. [DOI: 10.1055/s-0041-1731277] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wickremasinghe 2011 {published data only}
- Wickremasinghe AC, Karon BS, Cook WJ. Accuracy of neonatal transcutaneous bilirubin measurement in the outpatient setting. Clinical Pediatrics 2011;50(12):1144-9. [DOI: 10.1177/0009922811417292] [PMID: ] [DOI] [PubMed] [Google Scholar]
Willems 2004 {published data only}
- Willems WA, den Berg LM, Wit H, Molendijk A. Transcutaneous bilirubinometry with the Bilicheck in very premature newborns. Journal of Maternal-Fetal & Neonatal Medicine 2004;16(4):209-14. [PMID: ] [DOI] [PubMed] [Google Scholar]
Willemsen 2007 {published data only}
- Willemsen MJ, Korver CR. Transcutaneous bilirubinometry useful in the determination of hyperbilirubinaemia in icteric neonates. Nederlands Tijdschrift voor Geneeskunde 2007;151(6):359-63. [PMID: ] [PubMed] [Google Scholar]
Williams 2011 {published data only}
- Williams C, Holland L, Blick K. Transcutaneous bilirubin comparison studies: BiliChek versus drager methods. Clinical Chemistry 2011;57(10):A137. [Google Scholar]
Yamanouchi 1980 {published data only}
- Yamanouchi I, Yamauchi Y, Igarashi I. Transcutaneous bilirubinometry: preliminary studies of noninvasive transcutaneous bilirubin meter in the Okayama National Hospital. Pediatrics 1980;65(2):195-202. [PMID: ] [PubMed] [Google Scholar]
Yamauchi 1988 {published data only}
- Yamauchi Y, Yamanouchi I. Transcutaneous bilirubinometry. Evaluation of accuracy and reliability in a large population. Acta Paediatrica Scandinavica 1988;77(6):791-5. [DOI: 10.1111/j.1651-2227.1988.tb10757.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Yamauchi 1989a {published data only}
- Yamauchi Y, Yamanouchi I. Transcutaneous bilirubinometry. Interinstrumental variability of TcB instruments. Acta Paediatrica Scandinavica 1989;78(6):844-7. [DOI: 10.1111/j.1651-2227.1989.tb11161.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Yamauchi 1989b {published data only}
- Yamauchi Y, Yamanouchi I. Transcutaneous bilirubinometry in normal Japanese infants. Acta Paediatrica Japonica 1989;31(1):65-72. [DOI: 10.1111/j.1442-200x.1989.tb01271.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Yamauchi 1989c {published data only}
- Yamauchi Y, Yamanouchi I. Transcutaneous bilirubinometry: serum bilirubin measurement using transcutaneous bilirubinometer (TcB). A preliminary study. Biology of the Neonate 1989;56(5):257-62. [DOI: 10.1159/000243132] [PMID: ] [DOI] [PubMed] [Google Scholar]
Yamauchi 1990 {published data only}
- Yamauchi Y, Yamanouchi I. Clinical application of transcutaneous bilirubin measurement. Early prediction of hyperbilirubinemia. Acta Paediatrica Scandinavica 1990;79(4):385-90. [DOI: 10.1111/j.1651-2227.1990.tb11481.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Yamauchi 1991 {published data only}
- Yamauchi Y, Yamanouchi I. Transcutaneous bilirubinometry: effect of postnatal age. Acta Paediatrica Japonica 1991;33(5):663-7. [DOI: 10.1111/j.1442-200x.1991.tb01883.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Yang 2019 {published data only}
- Yang ST, Liu FC, Chen HL. Comparison of transcutaneous and serum bilirubin before, under, and after phototherapy in term and late-preterm infants. The Kaohsiung Journal of Medical Sciences 2019;35(11):715-24. [PMID: ] [DOI] [PubMed] [Google Scholar]
Yap 2002 {published data only}
- Yap SH, Mohammad I, Ryan CA. Avoiding painful blood sampling in neonates by transcutaneous bilirubinometry. Irish Journal of Medical Science 2002;171(4):188-90. [DOI: 10.1007/bf03170276] [PMID: ] [DOI] [PubMed] [Google Scholar]
Yasuda 2003 {published data only}
- Yasuda S, Itoh S, Isobe K, Yonetani M, Nakamura H, Nakamura M, et al. New transcutaneous jaundice device with two optical paths. Journal of Perinatal Medicine 2003;31(1):81-8. [DOI: 10.1515/JPM.2003.012] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ying 2020 {published data only}
- Ying Q, You X, You J, Wang J. The accuracy of transcutaneous bilirubin to identify hyperbilirubinemia in jaundiced neonates. The Journal of Maternal-fetal & Neonatal Medicine 2020;35(22):1-8. [DOI: 10.1080/14767058.2020.1849112] [PMID: ] [DOI] [PubMed] [Google Scholar]
Zecca 2009 {published data only}
- Zecca E, Barone G, De Luca D, Marra R, Tiberi E, Romagnoli C. Skin bilirubin measurement during phototherapy in preterm and term newborn infants. Early Human Development 2009;85(8):537-40. [DOI: 10.1016/j.earlhumdev.2009.05.010] [PMID: ] [DOI] [PubMed] [Google Scholar]
Zhan 2016 {published data only}
- Zhan C, Pan J, Du J, Yu Y, Zheng J, Chen L. Evaluation of the Bilichek transcutaneous bilirubinometer in the Chinese new-borns. Biomedical Research 2016;27(4):1390-1394. [Google Scholar]
Additional references
AAP 2004
- American Academy of Pediatrics, Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 2004;114(1):297-316. [DOI: 10.1542/peds.114.1.297] [PMID: ] [DOI] [PubMed] [Google Scholar]
Akman 2000
- Akman I, Arika Ç, Bilgen H, Kalaça S, Özek E. Transcutaneous measurement of bilirubin by icterometer during phototherapy on a bilibed. Turkish Journal of Medical Sciences 2000;32(2):165-8. [Google Scholar]
Barrington 2007
- Barrington KJ, Sankaran K, Canadian Paediatric Society, Fetus and Newborn Committee. Guidelines for detection, management and prevention of hyperbilirubinemia in term and late preterm newborn infants. Paediatric Child Health 2007;12(Suppl B):1B-12B. [Google Scholar]
Bhutani 2010
- Bhutani VK, Vilms RJ, Hamerman-Johnson L. Universal bilirubin screening for severe neonatal hyperbilirubinemia. Journal of Perinatology 2010;30 Suppl:S6-15. [DOI: 10.1038/jp.2010.98] [PMID: ] [DOI] [PubMed] [Google Scholar]
Burke 2009
- Burke BL, Robbins JM, Bird TM, Hobbs CA, Nesmith C, Tilford JM. Trends in hospitalizations for neonatal jaundice and kernicterus in the United States, 1988–2005. Pediatrics 2009;123(2):524-32. [DOI: 10.1542/peds.2007-2915] [PMID: ] [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Melbourne, Australia: Veritas Health Innovation, Accessed August 2022. Available at covidence.org.
CPS 2007
- Barrington KJ, Sankaran K. Canadian Paediatric Society, Fetus and Newborn Committee Guidelines for detection, management and prevention of hyperbilirubinemia in term and late preterm newborn infants (35 or more weeks’ gestation). www.cps.ca/en/documents/position/hyperbilirubinemia-newborn (accessed October 3, 2018).
Dai 1997
- Dai J, Parry DM, Krahn J. Transcutaneous bilirubinometry: its role in the assessment of neonatal jaundice. Clinical Biochemistry 1997;30(1):1-9. [DOI: 10.1016/s0009-9120(96)00131-2] [PMID: ] [DOI] [PubMed] [Google Scholar]
De Luca 2008a
- De Luca D, Zecca E, Zuppa AA, Romagnoli C. The joint use of human and electronic eye: visual assessment of jaundice and transcutaneous bilirubinometry. Turkish Journal of Pediatrics 2008;50(5):456-61. [PMID: ] [PubMed] [Google Scholar]
Ebbesen 2005
- Ebbesen F, Andersson C, Verder H, Grytter C, Pedersen-Bjergaard L, Petersen JR, et al. Extreme hyperbilirubinaemia in term and near-term infants in Denmark. Acta Paediatrica 2005;94(1):59-64. [DOI: 10.1111/j.1651-2227.2005.tb01789.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
el‐Beshbishi 2009
- el-Beshbishi SN, Shattuck KE, Mohammad AA, Petersen JR. Hyperbilirubinemia and transcutaneous bilirubinometry. Clinical Chemistry 2009;55(7):1280-7. [DOI: 10.1373/clinchem.2008.121889] [PMID: ] [DOI] [PubMed] [Google Scholar]
Grohmann 2006
- Grohmann K, Roser M, Rolinski B, Kadow I, Müller C, Goerlach-Graw A, et al. Bilirubin measurement for neonates: comparison of 9 frequently used methods. Pediatrics 2006;117(4):1174-83. [DOI: 10.1542/peds.2005-0590] [PMID: ] [DOI] [PubMed] [Google Scholar]
Harrison 1989
- Harrison SP, Barlow IM. Three direct spectrophotometric methods for determination of total bilirubin in neonatal and adult serum, adapted to the Technicon RA-1000 analyzer. Clinical Chemistry 1989;35(9):1980-6. [PMID: ] [PubMed] [Google Scholar]
Higgins 2012
- Higgins T, Eckfeldt JH, Barton JC, Doumas BT. Hemoglobin, iron and bilirubin. In: Burtis CA, Ashwood ER, Bruns DE, editors(s). Tietz Textbook of Clinical Chemistry and Molecular Diagnostics. 4th edition. Missouri: Elsevier Saunders, 2012:985-1030. [Google Scholar]
Hynes 2020
- Hynes S, Moore Z, Patton D, O'Connor T, Nugent L. Accuracy of transcutaneous bilirubin versus serum bilirubin measurement in preterm infants receiving phototherapy: a systematic review. Advances in Neonatal Care 2020;20(6):E118-26. [PMID: ] [DOI] [PubMed] [Google Scholar]
Johnson 2002
- Johnson LH, Bhutani VK, Brown AK. System-based approach to management of neonatal jaundice and prevention of kernicterus. Journal of Pediatrics 2002;140(4):396-403. [DOI: 10.1067/mpd.2002.123098] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kazmierczak 2002
- Kazmierczak SC, Robertson AF, Catrou PG, Briley KP, Kreamer BL, Gourley GR. Direct spectrophotometric method for measurement of bilirubin in newborns: comparison with HPLC and an automated diazo method. Clinical Chemistry 2002;48(7):1096-7. [PMID: ] [PubMed] [Google Scholar]
Kazmierczak 2006
- Kazmierczak S, Bhutani VK, Gourley G, Kerr S, Lo S, Roberston A, et al. Transcutaneous Bilirubin Testing. Laboratory Medicine Practice Guidelines: Evidence-based Practice for Point-of-care Testing. Washington (DC): National Academy of Clinical Biochemistry (NACB), 2006. [Google Scholar]
Kemper 2022
Keren 2005
- Keren R, Bhutani VK, Luan X, Nihtianova S, Cnaan A, Schwartz JS. Identifying newborns at risk of significant hyperbilirubinaemia: a comparison of two recommended approaches. Archives of Disease in Childhood 2005;90(4):415-21. [DOI: 10.1136/adc.2004.060079] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Knüpfer 2001
- Knüpfer M, Pulzer F, Braun L, Heilmann A, Robel-Tillig E, Vogtmann C. Transcutaneous bilirubinometry in preterm infants. Acta Paediatrica 2001;90(8):899-903. [PMID: ] [PubMed] [Google Scholar]
Lauer 2011
- Lauer BJ, Spector ND. Hyperbilirubinemia in the newborn. Pediatrics in Review 2011;32(8):341-9. [DOI: 10.1542/pir.32-8-341] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lo 2011
- Lo SF, Doumas BT. The status of bilirubin measurements in U.S. laboratories: why is accuracy elusive? Seminars in Perinatology 2011;35(3):141-7. [DOI: 10.1053/j.semperi.2011.02.008] [PMID: ] [DOI] [PubMed] [Google Scholar]
Macaskill 2010
- Macaskill P, Gatsonis C, Deeks JJ, Harbord RM, Takwoingi Y. Chapter 10: Analysing and presenting results. In: Deeks JJ, Bossuyt PM, Gatsonis C (editors), Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0. The Cochrane Collaboration, 2010. Available from: methods.cochrane.org/sdt/handbook-dta-reviews.
Madsen 2000
- Madsen LP, Rasmussen MK, Bjerregaard LL, Nohr SB, Ebbesen F. Impact of blood sampling in very preterm infants. Scandinavian Journal of Clinical and Laboratory Investigation 2000;60(2):125-32. [DOI: 10.1080/00365510050184949] [PMID: ] [DOI] [PubMed] [Google Scholar]
Maisels 1997
- Maisels MJ, Kring E. Transcutaneous bilirubinometry decreases the need for serum bilirubin measurements and saves money. Pediatrics 1997;99(4):599-601. [DOI: 10.1542/peds.99.4.599] [PMID: ] [DOI] [PubMed] [Google Scholar]
Mannig 2007
- Manning D, Todd P, Maxwell M, Platt M. Prospective surveillance study of severe hyperbilirubinaemia in the newborn in the UK and Ireland. Archives of Disease in Childhood. Fetal and Neonatal Edition 2007;92(5):342-6. [DOI: 10.1136/adc.2006.105361] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moey 2016
- Moey PK. Transcutaneous bilirubin measurement to estimate serum bilirubin in neonates in a multi-ethnic cohort: a literature review. Proceedings of Singapore Healthcare 2016;26(1):42-57. [DOI: 10.1177/2010105816665854] [DOI] [Google Scholar]
Nagar 2013
- Nagar G, Vandermeer B, Campbell S, Kumar M. Reliability of transcutaneous bilirubin devices in preterm infants: a systematic review. Pediatrics 2013;132(5):871-81. [DOI: 10.1542/peds.2013-1713] [PMID: ] [DOI] [PubMed] [Google Scholar]
Nagar 2016
- Nagar G, Vandermeer B, Campbell S, Kumar M. Effect of phototherapy on the reliability of transcutaneous bilirubin devices in term and near-term infants: a systematic review and meta-analysis. Neonatology 2016;109(3):203-12. [DOI: 10.1159/000442195] [PMID: ] [DOI] [PubMed] [Google Scholar]
Nair 2003
- Nair PA, Al Khusaiby SM. Kernicterus and G6PD deficiency – a case series from Oman. Journal of Tropical Pediatrics 2003;49(2):74-7. [DOI: 10.1093/tropej/49.2.74] [PMID: ] [DOI] [PubMed] [Google Scholar]
Owa 2009
- Owa JA, Ogunlesi TA. Why we are still doing so many exchange blood transfusion for neonatal jaundice in Nigeria. World Journal of Pediatrics 2009;5(1):51-5. [DOI: 10.1007/s12519-009-0009-2] [PMID: ] [DOI] [PubMed] [Google Scholar]
Srgo 2006
- Sgro M, Campbell D, Shah V. Incidence and causes of severe neonatal hyperbilirubinemia in Canada. Canadian Medical Association Journal 2006;175(6):587-90. [DOI: 10.1503/cmaj.060328] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wainer 2012
- Wainer S, Parmar SM, Allegro D, Rabi Y, Lyon ME. Impact of a transcutaneous bilirubinometry program on resource utilization and severe hyperbilirubinemia. Pediatrics 2012;129(1):77-86. [DOI: 10.1542/peds.2011-0599] [PMID: ] [DOI] [PubMed] [Google Scholar]
Whiting 2011
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine 2011;155(8):529-36. [DOI: 10.7326/0003-4819-155-8-201110180-00009] [PMID: ] [DOI] [PubMed] [Google Scholar]
Woodgate 2015
- Woodgate P, Jardine LA. Neonatal jaundice: phototherapy. BMJ Clinical Evidence 2015;22:0319. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Okwundu 2017
- Okwundu CI, Uthman OA, Suresh G, Smith J, Wiysonge CS, Bhutani VK. Transcutaneous bilirubinometry versus total serum bilirubin measurement for newborns. Cochrane Database of Systematic Reviews 2017, Issue 5. Art. No: CD012660. [DOI: 10.1002/14651858.CD012660] [DOI] [PMC free article] [PubMed] [Google Scholar]


