Abstract
Background
Early coronavirus disease 2019 (COVID-19) vaccine trials excluded pregnant women, resulting in limited data about immunogenicity and maternal–fetal antibody transfer, particularly by gestational timing of vaccination.
Methods
In this multicenter observational immunogenicity study, pregnant and nonpregnant women receiving COVID-19 vaccines were prospectively enrolled. Participants had sera collected before vaccination, at 14–28 days after each vaccine dose, at delivery (umbilical cord and peripheral), and from their infants at 3 and 6 months. Geometric mean titers (GMTs) of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) ID50 neutralizing antibody (nAb) against D614G-like viruses were compared by participant characteristics.
Results
Overall, 23 nonpregnant and 85 pregnant participants (trimester of first vaccine dose: 10 first, 47 second, 28 third) were enrolled. Ninety-three percent (76/82 with blood samples) of pregnant participants had detectable SARS-CoV-2 nAb after 2 vaccine doses, but GMTs (95% confidence intervals) were lower in pregnant participants than nonpregnant participants (1722 [1136–2612] vs 4419 [2012–9703]; P = .04). By 3 and 6 months, 28% and 74% of infants, respectively, of vaccinated participants had no detectable nAb to D614G-like viruses. Among the 71 pregnant participants without detectable nAb before vaccination, cord blood GMTs at delivery were 5-fold higher among participants vaccinated during the third versus first trimester, and cord blood nAb titers appeared inversely correlated with weeks since first vaccine dose (R2 = 0.06, P = .06).
Conclusions
Though most pregnant women develop nAb after 2 doses of mRNA COVID-19 vaccines, this analysis suggests that infant protection from maternal vaccination varies by gestational timing of vaccination and wanes. Additional prevention strategies such as caregiver vaccination may warrant consideration to optimize infant protection.
Keywords: COVID-19 vaccine, immunogenicity, infant, pregnancy
Although pregnant individuals are no more or less likely to contract a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection than are other working-age adults [1], pregnancy increases the risk of severe coronavirus disease 2019 (COVID-19) requiring hospitalization, intensive care, and mechanical ventilation [2–4] and may be associated with increased risks of some adverse pregnancy outcomes [2, 4, 5]. In addition, infants aged <6 months are the only age group for whom COVID-19 vaccines are not authorized, leaving them vulnerable to SARS-CoV-2 infection. Although young infants appeared to be at relatively low risk for severe COVID-19 during the early pandemic [6], more recent data suggest that the burden of SARS-CoV-2 infections has shifted, with an increasing impact on younger age groups during recent SARS-CoV-2 waves [7].
Prenatal vaccination has been used as a strategy to protect both pregnant women and their infants from infection and associated complications from other pathogens such as influenza and pertussis [8, 9]. COVID-19 vaccines have been available and recommended in the United States (US) for pregnant women in recommended target groups, such as essential workers, since December 2020 [10]. In August 2021, the Centers for Disease Control and Prevention (CDC) recommended COVID-19 vaccines specifically for pregnant women [11]. However, early trials of COVID-19 vaccines excluded pregnant women. Thus, data remain limited about the immunogenicity and efficacy of COVID-19 vaccines during pregnancy and about transfer and durability of maternal vaccine-induced antibodies in infants of vaccinated women [12]. In particular, there are few data about whether the amount and durability of maternal antibody transferred to infants varies by gestational timing of vaccination during pregnancy [13], because available studies are small and have focused on COVID-19 vaccination during later pregnancy [14–16].
We conducted a multicenter observational immunogenicity study of pregnant and nonpregnant women who received messenger RNA (mRNA) COVID-19 vaccines as well as the infants of women who received these vaccines during pregnancy. We used study data to compare SARS-CoV-2 neutralizing antibody (nAb) titers after vaccination by pregnancy status and to compare nAb over time among pregnant women and their infants up through 6 months of age by maternal prevaccination nAb status and gestational timing of vaccination (stratified by trimester). We also used data from a concurrent SARS-CoV-2 surveillance study among pregnant women from the same source populations to compare SARS-CoV-2 nAb titers at delivery after prenatal COVID-19 vaccination versus natural infection. For all analyses, we assessed nAb responses against the D614G strain representative of the original SARS-CoV-2 strain that circulated before the emergence of variants. We also assessed nAb against 5 variants that have circulated regionally or nationally in the US (Alpha B.1.1.7, Delta B.1.617.2, Iota B.1.526, Omicron B.1.1.529, and Omicron B.1.1.529.2).
METHODS
Participants and Study Setting
This analysis included participants who were enrolled in 2 concurrent studies conducted at 3 US medical centers located in Salt Lake City, Utah; New York City, New York; and Birmingham, Alabama. During August 2020–February 2021, sites enrolled a cohort of pregnant women into a prospective SARS-CoV-2 surveillance study called the Epidemiology of SARS-CoV-2 in Pregnancy and Infancy (ESPI) study. As previously described, women in the ESPI cohort participated in systematic surveillance for asymptomatic and symptomatic SARS-CoV-2 infection confirmed by reverse-transcription polymerase chain reaction (RT-PCR) testing and provided information about receipt of COVID-19 vaccine, which was verified with electronic medical record documentation. All women in the ESPI cohort reached end of pregnancy by October 2021, before the Omicron variants emerged and began circulating in the US.
During March–July 2021, sites enrolled pregnant women from the ESPI cohort and a convenience sample of pregnant and nonpregnant women from the broader community into an observational COVID-19 immunogenicity study. To be eligible for the immunogenicity study, women had to be aged 18–50 years and able to speak and read in English or Spanish; additional eligibility criteria were specific as to whether women were pregnant or not pregnant and, if pregnant, whether they were already enrolled in the ESPI cohort (Supplementary Methods). Women were excluded if they were previously diagnosed with COVID-19, had been told they had antibodies that indicated a previous infection with SARS-CoV-2, or were already enrolled in a COVID-19 vaccine trial. Pregnant women in the immunogenicity substudy could choose whether to consent to infant blood collection.
Data and Sample Collection Procedures
Women provided written informed consent to study participation. At enrollment, all participants completed web-based or telephone surveys about their sociodemographic characteristics, past medical and obstetrical histories, and prenatal care. During the study periods, participants were asked about receipt of COVID-19 vaccine. Vaccination information was verified with electronic medical records and/or local vaccine registries.
Participants in the ESPI cohort self-collected swab specimens every week during pregnancy [1]. They used study-provided kits and materials to ship specimens on ice packs by overnight courier to a central laboratory for testing for SARS-CoV-2 by RT-PCR. Participants also collected additional swab specimens if they experienced onset of COVID-19–like illness symptoms, defined as 1 or more of measured or subjective fever, cough, shortness of breath, sore throat, diarrhea, muscle aches, chills, or change in taste or smell.
Participants in the ESPI cohort had blood collected at enrollment, at approximately 26–30 weeks’ gestation (if enrolled at <24 0/7 weeks’ gestation), and at end of pregnancy (delivery of a live infant, pregnancy termination, stillbirth, or miscarriage); blood was not collected from infants under the ESPI cohort protocol. Participants in the COVID-19 vaccine immunogenicity substudy had baseline blood samples collected within 7 days before (or within 28 days before, if in the ESPI cohort) to 1 day after the first dose of COVID-19 vaccine, postvaccination at 14–28 days after each dose, and within 7 days before or after end of pregnancy (if pregnant). Pregnant participants in the immunogenicity substudy also had umbilical cord blood collected at the end of pregnancy and their infants had blood collected at 3 and 6 months of age if they consented to infant blood collection.
Laboratory Methods
Blood samples were processed for serum collection. Sera were stored at −80°C, thawed, and heat-inactivated for 60 minutes at 56°C. Sera were tested at Labcorp–Monogram Biosciences for SARS-CoV-2 nAb against the following strains: D614G (original Wuhan strain), B.1.1.7 (Alpha variant), B.1.617.2 (Delta variant), B.1.526 (Iota variant), B.1.1.529 (Omicron variant), and B.1.1.529.2 (Omicron variant). The B.1.526 variant was included because it was the predominant strain that circulated in parts of New York City during the ESPI cohort study period.
Testing was performed using the PhenoSense SARS-CoV-2 nAb assay. The assay uses lentivirus particles pseudotyped with full-length SARS-CoV-2 spike protein and a firefly luciferase reporter gene for quantitative measurements of infection by relative luminescence units, as previously described [17]. Neutralization was performed in 96-well plates by incubating pseudovirus with 10 serial 3-fold dilutions of serum samples for 1 hour at 37°C. The dilution series was based on a 1:20 starting dilution, which was reported as 1:40 after addition of virus. Neutralization titers represent the inhibitory dilution of serum samples at which relative luminescence units were reduced by 50% (ID50) compared to virus alone (no serum samples) and are presented in international units per milliliter.
Analytic Populations and Statistical Analysis
Two participant populations were included in this analysis: (1) pregnant and nonpregnant participants in the COVID-19 vaccine immunogenicity substudy who had blood collected after each vaccine dose and (2) pregnant participants in the ESPI cohort who had RT-PCR–confirmed SARS-CoV-2 infection identified by study surveillance, did not receive prenatal COVID-19 vaccine, and had blood collected at the end of pregnancy. Participants’ baseline characteristics, self-reported breastfeeding practices at 4–6 weeks postpartum, and blood collection time intervals relative to vaccination were summarized using descriptive statistics.
SARS-CoV-2 nAb titers in international units per milliliter were log10 transformed for analyses. A titer of 1:5 was assigned to participants with undetectable (<1:20) nAb responses to vaccine. Geometric mean titers (GMTs) at each timepoint were first examined by selected participant characteristics (age group, presence of underlying medical conditions, and prepregnancy body mass index) to confirm that nAb responses were similar across groups (data not shown). GMTs were then compared using Student t test for the following comparisons: (1) nAb responses after COVID-19 vaccination among pregnant versus nonpregnant participants; (2) nAb titers at delivery among pregnant women who received a 2-dose series of vaccine after excluding those with prevaccination nAb (ie, prior infection) versus those with prenatal RT-PCR–confirmed SARS-CoV-2 infection; and (3) nAb titers after COVID-19 vaccination among pregnant women and their infants at 3 and 6 months of age by maternal prevaccination nAb status (positive vs negative), trimester of first maternal COVID-19 vaccine dose, and variant target. For infants with a reported diagnosis of COVID-19 during the infant's first 6 months of life, blood samples collected after the diagnosis date were excluded (n = 4 samples collected at 6 months of age). Participants with detectable prevaccination nAb titers were excluded from the analyses stratified by trimester of vaccination because the proportion of participants with prevaccination nAb titers varied by trimester of vaccination. SARS-CoV-2–positive respiratory specimens from participants with infections were not sequenced, precluding analysis of nAb responses by homologous versus heterologous virus in the group with natural infection.
The antibody transfer ratio after prenatal COVID-19 vaccination was defined as the GMT ratio of nAb in umbilical cord blood divided by maternal peripheral blood at delivery. Antibody transfer ratios were stratified by trimester of vaccination among women who had both types of blood samples collected.
All tests were 2-tailed with a level of significance of .05. Analyses were conducted in SAS version 9.4 software (SAS Institute, Cary, North Carolina).
Patient Consent Statement
The study protocol was reviewed and approved by the Columbia University Irving Medical Center Institutional Review Board (IRB), which served as the central IRB for all study sites. The CDC IRB relied on the review of the Columbia University Irving Medical Center IRB (see 45 Code of Federal Regulations [C.F.R.] part 46; 21 C.F.R. part 56). Informed consent was obtained from all study participants.
RESULTS
Participant Characteristics
Overall, 186 participants were included in the analysis: 85 pregnant participants who received prenatal COVID-19 vaccine (including 44 with infant blood collection from 47 infants), 23 nonpregnant participants who received vaccine, and 78 pregnant participants who had prenatal RT-PCR–confirmed SARS-CoV-2 infection (Supplementary Figure 1). Baseline demographic and health characteristics of participants are summarized in Table 1. Among the 108 vaccinated participants, all received mRNA COVID-19 vaccines (104 BNT162b2 [Pfizer] and 4 mRNA-1273 [Moderna]), and 105 (97%) received a complete 2-dose series.
Table 1.
Baseline Characteristics of Participants by Pregnancy and Coronavirus Disease 2019 Vaccination or Severe Acute Respiratory Syndrome Coronavirus 2 Infection Status
| Characteristic | Pregnant and Vaccinated | Not Pregnant and Vaccinated | Pregnant and Infected | |||
|---|---|---|---|---|---|---|
| (n = 85) | (n = 23) | (n = 78) | ||||
| Baseline characteristics | ||||||
| Age, y, median (IQR) | 32 | (28–34) | 27 | (24–31) | 30 | (26–32) |
| Race/ethnicity | ||||||
| Hispanic/Latina | 26 | (31) | 6 | (26) | 32 | (41) |
| White, non-Hispanic | 37 | (43) | 7 | (30) | 35 | (45) |
| Black, non-Hispanic | 10 | (12) | 8 | (35) | 6 | (8) |
| Other, non-Hispanic | 9 | (11) | 2 | (9) | 1 | (1) |
| Missing | 3 | (3) | 0 | (0) | 4 | (5) |
| Underlying medical conditionsa | ||||||
| None | 68 | (80) | 15 | (65) | 68 | (87) |
| At least 1 | 17 | (20) | 7 | (30) | 9 | (12) |
| Missing | 0 | (0) | 1 | (4) | 1 | (1) |
| Pregnancy characteristics | ||||||
| Trimester at vaccine dose 1 or infection | ||||||
| First (0 to 13 6/7 wk) | 10 | (12) | … | … | 4 | (5) |
| Second (14 to 27 6/7 wk) | 47 | (55) | … | … | 44 | (56) |
| Early second (14 to 19 6/7 wk) | 20 | (24) | … | … | 13 | (17) |
| Late second (21 to 27 6/7 wk) | 27 | (32) | … | … | 31 | (40) |
| Third (≥28 wk) | 28 | (33) | … | … | 30 | (39) |
| Early third (28 to 31 6/7 wk) | 26 | (31) | … | … | 14 | (18) |
| Late third (≥32 wk) | 2 | (2) | … | … | 16 | (21) |
| Trimester when fully vaccinated | ||||||
| First (0 to 13 6/7 wk gestation) | 1 | (1) | … | … | … | … |
| Second (13 6/7 to 27 6/7 wk gestation) | 45 | (53) | … | … | … | … |
| Third (≥28 wk gestation) | 36 | (42) | … | … | … | … |
| Not fully vaccinated | 3 | (4) | … | … | … | … |
| Self-reported breastmilk and formula feeding practices at 4–6 wk postpartum | ||||||
| Only breastmilk | 35 | (41) | … | … | 29 | (37) |
| Mostly breastmilk or equivalent amounts of breastmilk and formula | 20 | (24) | … | … | 20 | (26) |
| Mostly formula but some breastmilk | 3 | (4) | … | … | 11 | (14) |
| Only formula | 9 | (11) | … | … | 6 | (8) |
| Missing | 18 | (21) | … | … | 12 | (15) |
| Multiple gestation pregnancy | ||||||
| Yes | 4 | (5) | … | … | 1 | (1) |
| No | 81 | (95) | … | … | 77 | (99) |
| Gestational age at delivery, wk, mean (range) | 38 | (32–41) | … | … | 38 | (28–41) |
| Vaccination characteristics | ||||||
| Vaccine type (dose 1) | ||||||
| BNT162b2 (Pfizer) | 81 | (95) | 23 | (100) | … | … |
| mRNA-1273 (Moderna) | 4 | (5) | 0 | (0) | … | … |
| No. of doses | ||||||
| 1 | 3 | (4) | 0 | (0) | … | … |
| 2 | 82 | (96) | 23 | (100) | … | … |
| Blood collection intervals, mean (range) | ||||||
| Dose 1 to postvaccination blood draw | 18 | (10–28) | 18 | (14–27) | … | … |
| Dose 2 to postvaccination blood draw | 20 | (13–77) | 18 | (9–28) | … | … |
| Last vaccine dose or infection to delivery blood collection | 89 | (19–203) | … | … | 94 | (7–208) |
Data are presented as No. (column %) unless otherwise indicated.
Abbreviation: IQR, interquartile range.
Underlying medical conditions classified by the Centers for Disease Control and Prevention as conferring an increased risk for severe COVID-19, including cancer, chronic kidney disease, chronic lung disease, dementia or neurological conditions, diabetes (types 1 or 2), Down syndrome, heart conditions including hypertension, human immunodeficiency virus (HIV) infection, immunocompromised state, liver disease, sickle cell disease or thalassemia, solid-organ or blood stem cell transplant, and stroke or cerebrovascular disease. Centers for Disease Control and Prevention list available at: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html.
SARS-CoV-2 nAb Responses After Vaccination by Pregnancy Status
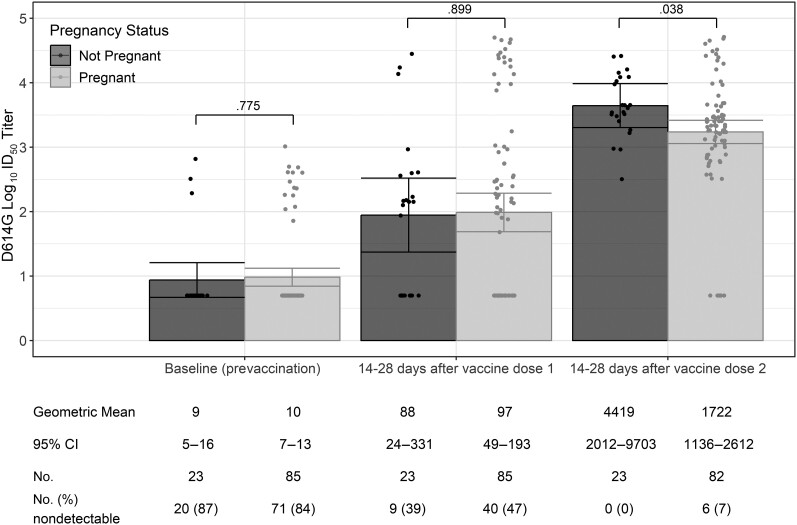
Among the 108 participants who received COVID-19 vaccine, the proportions of participants with detectable nAb to D614G-like viruses at baseline were similar among pregnant and nonpregnant participants (16% [14/85] vs 13% [3/23], P = .78; Figure 1). The mean days from vaccination to postvaccination blood collection for each dose was also similar among pregnant and nonpregnant participants (after vaccine dose 1: 18 vs 18 days, P = .77; after vaccine dose 2: 20 vs 18 days, P = .40; Table 1). Compared to nonpregnant participants, pregnant participants had similar nAb GMTs against D614G-like viruses after dose 1 (88 [95% confidence interval {CI}, 24–331] vs 97 [95% CI, 49–193]; P = .90) but lower GMTs after dose 2 (4419 [95% CI, 2012–9703] vs 1722 [95% CI, 1136–2612]; P = .04) (Figure 1). After vaccine dose 2, 6 of 82 (7%) pregnant participants had nondetectable nAb titers, whereas none of the nonpregnant participants had nondetectable titers.
Figure 1.
Severe acute respiratory syndrome coronavirus 2 neutralizing antibody titers to D614G-like viruses at baseline (prevaccination) and 14–28 d after each coronavirus disease 2019 vaccine dose among pregnant and nonpregnant participants (n = 108). A titer of 1:5 was assigned to participants with undetectable (<1:20) neutralizing antibody responses to vaccine. Error bars indicate the 95% confidence intervals of the log10 titers. P values compare the log10 titer between pregnant and nonpregnant participants at each timepoint. Abbreviations: CI, confidence interval; ID50, inhibitory dilution of serum samples at which relative luminescence units were reduced by 50%.
SARS-CoV-2 nAb Responses at Delivery After Prenatal Vaccination Versus Infection
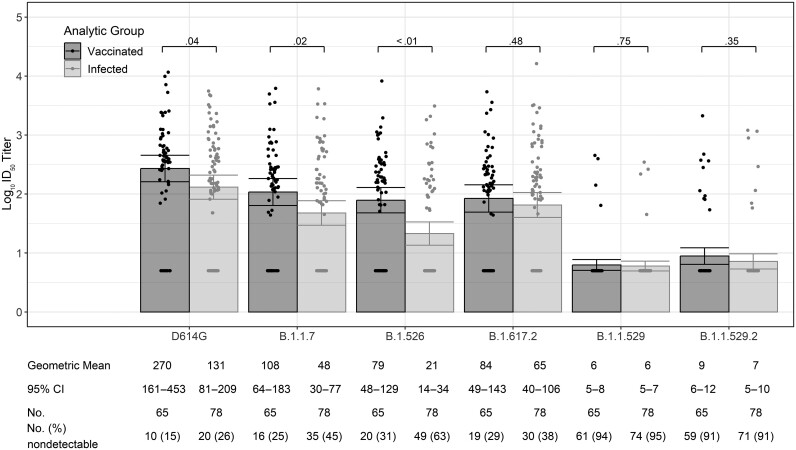
Of the 85 participants who received prenatal COVID-19 vaccine, 20 were excluded from analyses comparing nAb at delivery after prenatal vaccination versus after SARS-CoV-2 infection (16 with detectable prevaccination SARS-CoV-2 nAb to any analysis lineage type [14 to D614G and 2 to other lineages], 3 who received only 1 dose of COVID-19 vaccine, and 1 without a delivery blood sample). Among the remaining 65 COVID-19–vaccinated pregnant participants and the 78 participants with RT-PCR–confirmed SARS-CoV-2 infection (60 symptomatic and 18 asymptomatic), the mean number of days between last vaccine dose or SARS-CoV-2 infection to end of pregnancy blood collection was similar (92 vs 94 days, P = .80; data not shown). Compared to participants with prenatal SARS-CoV-2 infection, those who received prenatal COVID-19 vaccine had higher nAb GMTs at the end of pregnancy against D614G-like viruses (131 [95% CI, 81–209] vs 270 [95% CI, 161–453]; P = .04) and against the Alpha B.1.1.7 and Iota B.1.526 strains; titers among the 2 groups were similar against Delta B.1.617.2 and were similar and low against Omicron B.1.1.529 and Omicron B.1.1.529.2 (Figure 2).
Figure 2.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) neutralizing antibody titers to D614G-like and variant viruses at delivery among pregnant participants who received coronavirus disease 2019 vaccine or had prenatal SARS-CoV-2 infection confirmed by reverse-transcription polymerase chain reaction testing (n = 143). Variant names: B.1.1.7 (Alpha), B.1.617.2 (Delta), B.1.526 (Iota), B.1.1.529 (Omicron BA.1), B.1.1.529.2 (Omicron BA.2). A titer of 1:5 was assigned to participants with undetectable (<1:20) neutralizing antibody responses to vaccine. Error bars indicate the 95% confidence intervals of the log10 titers. P values compare the log10 titer between pregnant and nonpregnant participants at each timepoint. Abbreviations: CI, confidence interval; ID50, inhibitory dilution of serum samples at which relative luminescence units were reduced by 50%.
SARS-CoV-2 nAb Responses After Prenatal COVID-19 Vaccine
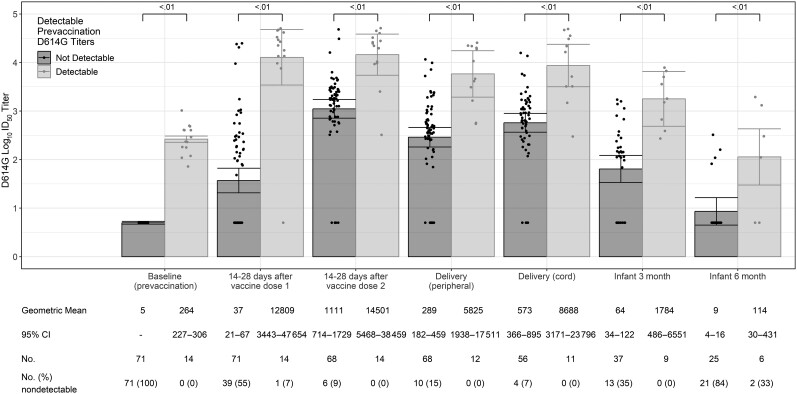
Among the 85 pregnant participants who received prenatal COVID-19 vaccine, 14 had detectable prevaccination nAb to D614G-like viruses despite no history of known prior infection. Compared to participants without prevaccination nAb, those with prevaccination nAb had higher GMTs against D614G-like viruses after each COVID-19 vaccine dose and at delivery (Figure 3). Similarly, infants of participants with detectable prevaccination nAb had higher GMTs at 3 and 6 months of age compared to infants of participants without detectable prevaccination nAb. Overall, SARS-CoV-2 nAb GMTs declined over time after COVID-19 vaccine dose 2 among both participants and their infants, and maternal postvaccination titers were higher than infant titers (Figure 4). Among infants of all 85 vaccinated pregnant women (including those with detectable prevaccination nAb), by 3 and 6 months of age, 28% (13/46) and 74% (23/31), respectively, had no detectable nAb to D614G-like viruses (Figure 3).
Figure 3.
Severe acute respiratory syndrome coronavirus 2 neutralizing antibody titers to D614G-like viruses among coronavirus disease 2019–vaccinated pregnant participants and their infants by maternal baseline (prevaccination) neutralizing antibody status (n = 85). A titer of 1:5 was assigned to participants with undetectable (<1:20) neutralizing antibody responses to vaccine. Error bars indicate the 95% confidence intervals of the log10 titers. P values compare the log10 titer between pregnant and nonpregnant participants at each timepoint. Abbreviations: CI, confidence interval; ID50, inhibitory dilution of serum samples at which relative luminescence units were reduced by 50%.
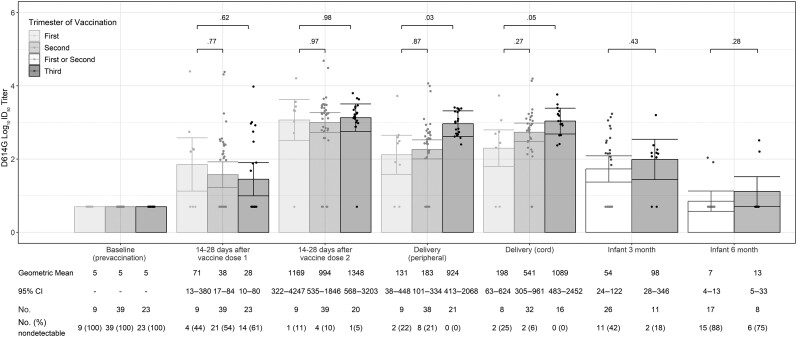
Figure 4.
Severe acute respiratory syndrome coronavirus 2 neutralizing antibody titers to D614G-like viruses among coronavirus disease 2019 (COVID-19)–vaccinated pregnant participants and their infants by trimester of receipt of first COVID-19 vaccine dose (n = 71). Fourteen COVID-19–vaccinated participants with detectable nAb to D614G-like viruses at baseline (prevaccination) were excluded from this analysis. A titer of 1:5 was assigned to participants with undetectable (<1:20) neutralizing antibody responses to vaccine. Error bars indicate the 95% confidence intervals of the log10 titers. P values compare the log10 titer between pregnant and nonpregnant participants at each timepoint. Infants of participants who received their first doses of COVID-19 vaccine during the first and second trimesters were combined because of small sample sizes (at 3 mo: 1 infant with maternal first-trimester vaccination and 25 with second-trimester vaccination; at 6 mo: 3 infants with maternal first-trimester vaccination and 15 with second-trimester vaccination). Abbreviations: CI, confidence interval; ID50, inhibitory dilution of serum samples at which relative luminescence units were reduced by 50%.
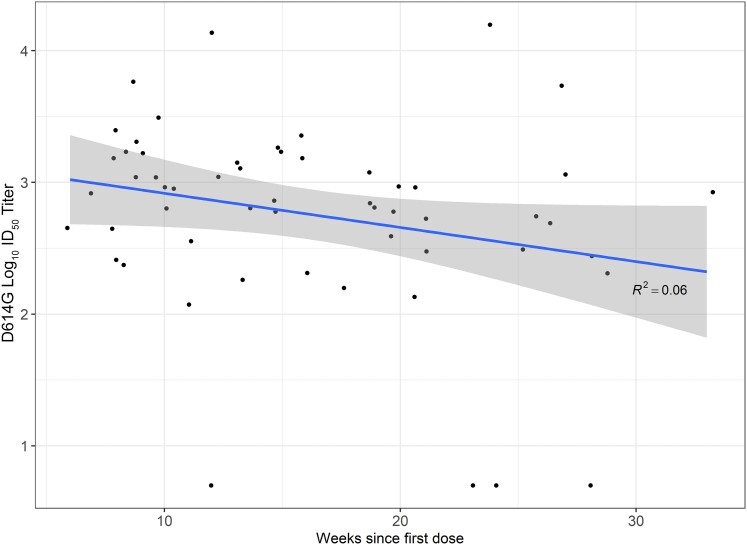
Among the 85 pregnant participants who received COVID-19 vaccine, 10 (12%) received their first dose during the first trimester, 47 (55%) during the second trimester, and 28 (33%) during the third trimester (Table 1). The proportion of COVID-19–vaccinated pregnant participants with detectable prevaccination SARS-CoV-2 nAb to D614G-like viruses varied by trimester of vaccination (1/10 [10%] among those who received their first doses in the first trimester, 8/47 [17%] second trimester, and 5/28 [25%] third trimester; data not shown). After excluding the 14 participants with prevaccination nAb to D614G-like viruses, 71 participants were included in the analysis of nAb responses by trimester of vaccination. Neutralizing antibody GMTs were similar by trimester of vaccination at 14–28 days after each vaccine dose, but umbilical cord blood GMTs at delivery were 5-fold higher among participants vaccinated during the third versus first trimester (1089 [95% CI, 483–2452] vs 198 [95% CI, 63–624]; P = .048) (Figure 4). End of pregnancy umbilical cord blood log10 titers were inversely correlated with weeks since first COVID-19 vaccination dose (R2 = 0.06, P = .06), although the correlation did not reach statistical significance (Figure 5). However, there was no significant difference in umbilical cord/parenteral antibody transfer ratios by trimester of maternal vaccination (first trimester: 1.25 [95% CI, .8–1.75] vs second trimester: 1.45 [95% CI, 1.2–1.7], P = .75; or vs third trimester: 1.05 [95% CI .7–1.4], P = .80; data not shown).
Figure 5.
Umbilical cord blood log10 titers against D614G-like virus at delivery by weeks since first dose of prenatal coronavirus disease 2019 vaccine. Abbreviations: CI, confidence interval; ID50, inhibitory dilution of serum samples at which relative luminescence units were reduced by 50%.
Supplementary Figure 2 summarizes nAb GMTs by variant type among COVID-19–vaccinated pregnant participants and their infants. In general, nAb GMTs against all variant types trended lower than GMTs against D614G-like viruses after 2 doses of vaccine, at delivery, and among infants at 3 months of age with the lowest nAb GMTs against the Omicron B.1.1.529 and B.1.1.529.2 strains.
DISCUSSION
In this multicenter observational immunogenicity study, we examined the relationship between prenatal COVID-19 vaccination timing and delivery and infant nAb titers at 3 and 6 months of age. There was a trend toward an inverse correlation between time since prenatal COVID-19 vaccination and delivery umbilical cord blood nAb titers. In addition, by 3 months of age, 1 in 3 infants of vaccinated pregnant women had no detectable SARS-CoV-2 nAb to D614G-like viruses represented in the vaccine, and by 6 months of age, most infants had no detectable nAb. These findings add to the growing body of evidence that although maternal COVID-19 vaccination provides protection to young infants, protection wanes and infants should receive recommended COVID-19 vaccines when they become eligible for vaccination at 6 months of age. In addition, other prevention strategies such as vaccination of caregivers and household contacts may warrant consideration as adjuncts to maternal COVID-19 vaccination to optimize protection of infants during their first 6 months of life. Further exploration of novel maternal COVID-19 vaccination approaches to protect young infants, such as COVID-19 booster vaccination during the third trimester, might also be warranted [18].
We also examined SARS-CoV-2 nAb titers after vaccination with monovalent mRNA COVID-19 vaccines among pregnant women and their infants and nonpregnant women of reproductive age. After a 2-dose series of COVID-19 vaccine, postvaccination nAb titers to D614G-like viruses were lower among pregnant persons than nonpregnant persons for unclear reasons. Two previous small observational studies also found lower postvaccination antibody levels among pregnant versus nonpregnant persons [19, 20]. One of these studies also reported differences in antibody kinetics and profiles between pregnant and nonpregnant persons, suggesting that pregnancy may influence the type and magnitude of immune response to vaccination [19]. In contrast, previous studies comparing antibody responses to seasonal influenza vaccination in pregnant versus nonpregnant persons of childbearing age have generally reported similar responses in the 2 groups [21]. Despite these findings, our study and others show that pregnant persons still mount relatively robust postvaccination nAb responses to the vaccine strain (D614G) even if titers are lower than in nonpregnant persons. Additionally, multiple studies have shown that prenatal COVID-19 vaccination is associated with a subsequent lower risk of severe illness from SARS-CoV-2 during pregnancy [22–25]. One of these studies also found that COVID-19 vaccination later in pregnancy was associated with more protection against SARS-CoV-2 hospitalizations in infants <6 months of age [26], which is consistent with the trend that we observed toward an inverse correlation between umbilical cord blood SARS-CoV-2 nAb titers and weeks since vaccination.
In this study, compared to prenatal natural infection, prenatal COVID-19 vaccination elicited higher nAb titers to D614G-like viruses and the Alpha and Iota variants at delivery. This is consistent with findings from other studies in both pregnant [27] and nonpregnant populations [28] documenting higher nAb responses to COVID-19 vaccination compared to infection. However, pregnant women with detectable prevaccination SARS-CoV-2 nAb suggestive of previous infection had higher nAb titers after 2 doses of vaccine than those without prevaccination nAb, which is also consistent with data from other studies in nonpregnant persons [29–32]. This suggests that natural infection primes the immune response, and importantly, indicates that those with a history of natural infection still benefit from COVID-19 vaccination through a boost in nAb titers. Delivery nAb titers after prenatal COVID-19 vaccination or SARS-CoV-2 infection were low against the more recently circulating Omicron variants (B.1.1.529 and B.1.1.529.2), underscoring the importance of booster vaccination with more recently available bivalent COVID-19 vaccines that contain components from the original D614G-like strain and Omicron sublineages.
Strengths of this study include enrollment of a diverse multicenter population of pregnant women and high study completion rates including infant blood collection from >50% of pregnant vaccinated persons. This study also included pregnant women who received COVID-19 vaccine during the first and early second trimesters, a population that has been underrepresented in previous immunogenicity studies of prenatal COVID-19 vaccination. However, several limitations should be considered. First, the overall sample size in this analysis was relatively small as study sites reported that many pregnant persons at their centers declined COVID-19 vaccination during the study period, particularly during early pregnancy. Nonetheless, findings from our analysis are largely consistent with and add to the growing body of evidence related to humoral immune responses to COVID-19 vaccination during pregnancy. Second, this analysis examined responses to monovalent mRNA COVID-19 vaccines relatively early in the course of vaccine availability to the general population in the US. Thus, results may not be directly generalizable to the bivalent COVID-19 vaccine or other COVID-19 vaccine types and to persons who are pregnant now, who are more likely to have had previous natural infection. Third, infant COVID-19 infection status may have been misclassified in instances of undiagnosed infection because infection status was identified based on parental report of COVID-19 diagnosis.
In this multicenter observational immunogenicity study, we found that prenatal COVID-19 vaccination elicited detectable umbilical cord blood antibody titers in most vaccinated women, but a third of infants of women who received COVID-19 vaccine during pregnancy had no detectable nAb by 3 months of age, and infant nAb titers were highest in those whose mothers received their first doses of vaccine later in pregnancy in the third trimester. These findings suggest that timing of maternal vaccination during pregnancy is an important predictor of protective nAb titers in infants of vaccinated women and infant protection from maternal COVID-19 vaccination wanes during the early months of life. Thus, other prevention strategies such as vaccination of caregivers and household contacts of young infants may warrant consideration as adjuncts to maternal COVID-19 vaccination to optimize protection of infants <6 months of age. We also found that a 2-dose series of monovalent mRNA COVID-19 vaccine elicited higher nAb responses than natural infection against multiple strains of SARS-CoV-2 that circulated during the study period, but nAb responses to both COVID-19 vaccine and natural infection elicited similar and low nAb responses to more recently circulating Omicron variants by the time of delivery. These findings highlight the importance of booster vaccination for pregnant persons to optimize their protection against severe illness from more recently circulating SARS-CoV-2 viruses.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Fatimah S Dawood, Influenza Division, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Alan Tita, Center for Women's Reproductive Health and Division of Maternal-Fetal Medicine, Department of Obstetrics and Gynecology, University of Alabama at Birmingham, Birmingham, Alabama, USA.
Melissa S Stockwell, New York–Presbyterian Hospital, New York, New York, USA; Division of Child and Adolescent Health, Department of Pediatrics, Columbia University Irving Medical Center, New York, New York, USA; Department of Population and Family Health, Mailman School of Public Health, Columbia University Irving Medical Center, New York, New York, USA.
Gabriella Newes-Adeyi, Abt Associates, Rockville, Maryland, USA.
Kristina Wielgosz, Influenza Division, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Cynthia Gyamfi-Bannerman, New York–Presbyterian Hospital, New York, New York, USA; Division of Maternal-Fetal Medicine, Department of Obstetrics and Gynecology, Columbia University Irving Medical Center, New York, New York, USA; Department of Obstetrics, Gynecology, and Reproductive Sciences, University of California, San Diego, California, USA.
Ashley Battarbee, Center for Women's Reproductive Health and Division of Maternal-Fetal Medicine, Department of Obstetrics and Gynecology, University of Alabama at Birmingham, Birmingham, Alabama, USA.
Lawrence Reichle, Abt Associates, Rockville, Maryland, USA.
Natalie Thornburg, Influenza Division, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Sascha Ellington, Influenza Division, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Romeo R Galang, Influenza Division, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Kelly Vorwaller, Department of Obstetrics and Gynecology, University of Utah Health Sciences Center, Salt Lake City, Utah, USA.
Celibell Y Vargas, New York–Presbyterian Hospital, New York, New York, USA.
Tyler Morrill, Abt Associates, Rockville, Maryland, USA.
Mickey Parks, Center for Women's Reproductive Health and Division of Maternal-Fetal Medicine, Department of Obstetrics and Gynecology, University of Alabama at Birmingham, Birmingham, Alabama, USA.
Emily Powers, Division of Maternal-Fetal Medicine, Department of Obstetrics and Gynecology, Columbia University Irving Medical Center, New York, New York, USA.
Marie Gibson, Department of Obstetrics and Gynecology, University of Utah Health Sciences Center, Salt Lake City, Utah, USA.
Michael Varner, Department of Obstetrics and Gynecology, University of Utah Health Sciences Center, Salt Lake City, Utah, USA.
Notes
Acknowledgments. The authors acknowledge the participants in the Epidemiology of SARS-CoV-2 in Pregnancy and Infancy (ESPI) cohort and vaccine immunogenicity studies. The authors also acknowledge and thank Raúl A. Silverio and Casandra Almonte from Columbia University Irving Medical Center, Kathy Harvey and Melanie Fiuza from the University of Utah Health Sciences Center, and collaborators at Labcorp–Monogram Biosciences, South San Francisco, California. The article focuses on pregnancy-related or associated events. It makes use of concepts or descriptions that align with traditional gender definitions by using concepts such as “maternal,” “pregnant women,” or “women.” However, the concepts described are translatable to all persons that experience a pregnancy, regardless of their gender identity.
Disclaimer. Centers for Disease Control and Prevention (CDC)–affiliated authors were involved in study design; data collection, analysis, and interpretation; report writing; and the decision to submit the manuscript for publication. The corresponding author had full access to all data used in the analysis and has final responsibility for the decision to submit for publication. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC. Any references to specific commercial products are for identification purposes only and do not constitute an endorsement by the CDC.
Financial support. This study was funded by the Centers for Disease Control and Prevention (contract number 75D30120C08150 with Abt Associates). Study data were collected and managed using Research Electronic Data Capture (REDCap) electronic data capture tools hosted at Vanderbilt University Medical Center with grant support from the National Center for Advancing Translational Sciences of the National Institutes of Health (UL1 TR000445).
References
- 1. Dawood FS, Varner M, Tita A, et al. Incidence and clinical characteristics of and risk factors for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection among pregnant individuals in the United States. Clin Infect Dis 2022; 74:2218–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ 2020; 370:m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ellington S, Strid P, Tong VT, et al. Characteristics of women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22–June 7, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:769–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McClymont E, Albert AY, Alton GD, et al. Association of SARS-CoV-2 infection during pregnancy with maternal and perinatal outcomes. JAMA 2022; 327:1983–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Karasek D, Baer RJ, McLemore MR, et al. The association of COVID-19 infection in pregnancy with preterm birth: a retrospective cohort study in California. Lancet Reg Health Am 2021; 2:100027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Griffin I, Irving S, Arriola C, et al. Incidence rates of medically attended COVID-19 in infants less than 6 months of age. Pediatr Infect Dis J 2023; 42:315–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hamid S, Woodworth K, Pham H, et al. COVID-19–associated hospitalizations among U.S. infants aged <6 months—COVID-NET, 13 states, June 2021–August 2022. MMWR Morb Mortal Wkly Rep 2022; 71:1442–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nunes MC, Madhi SA. Influenza vaccination during pregnancy for prevention of influenza confirmed illness in the infants: a systematic review and meta-analysis. Hum Vaccin Immunother 2018; 14:758–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vilajeliu A, Garcia-Basteiro AL, Bayas JM. Protecting newborns against pertussis: the value of vaccinating during pregnancy. Expert Rev Vaccines 2015; 14:1051–3. [DOI] [PubMed] [Google Scholar]
- 10. Dooling K, Marin M, Wallace M, et al. The Advisory Committee on Immunization Practices’ updated interim recommendation for allocation of COVID-19 vaccine—United States, December 2020. MMWR Morb Mortal Wkly Rep 2021; 69:1657–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Centers for Disease Control and Prevention . New CDC data: COVID-19 vaccination safe for pregnant people. 2021. Available at:https://www.cdc.gov/media/releases/2021/s0811-vaccine-safe-pregnant.html#:∼:text=A%20new%20CDC%20analysis%20of,before%2020%20weeks%20of%20pregnancy. Accessed 19 April 2023.
- 12. Beigi RH, Krubiner C, Jamieson DJ, et al. The need for inclusion of pregnant women in COVID-19 vaccine trials. Vaccine 2021; 39:868–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Badell ML, Dude CM, Rasmussen SA, Jamieson DJ. Covid-19 vaccination in pregnancy. BMJ 2022; 378:e069741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Collier AY, McMahan K, Yu J, et al. Immunogenicity of COVID-19 mRNA vaccines in pregnant and lactating women. JAMA 2021; 325:2370–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kugelman N, Nahshon C, Shaked-Mishan P, et al. Third trimester messenger RNA COVID-19 booster vaccination upsurge maternal and neonatal SARS-CoV-2 immunoglobulin G antibody levels at birth. Eur J Obstet Gynecol Reprod Biol 2022; 274:148–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Prahl M, Golan Y, Cassidy AG, et al. Evaluation of transplacental transfer of mRNA vaccine products and functional antibodies during pregnancy and infancy. Nat Commun 2022; 13:4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang Y, Borisov O, Kee JJ, et al. Calibration of two validated SARS-CoV-2 pseudovirus neutralization assays for COVID-19 vaccine evaluation. Sci Rep 2021; 11:23921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang YJ, Murphy EA, Singh S, et al. Association of gestational age at coronavirus disease 2019 (COVID-19) vaccination, history of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, and a vaccine booster dose with maternal and umbilical cord antibody levels at delivery. Obstet Gynecol 2022; 139:373–80. [DOI] [PubMed] [Google Scholar]
- 19. Atyeo C, DeRiso EA, Davis C, et al. COVID-19 mRNA vaccines drive differential antibody Fc-functional profiles in pregnant, lactating, and nonpregnant women. Sci Transl Med 2021; 13:eabi8631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Peretz S B, Regev N, Novick L, et al. Short-term outcome of pregnant women vaccinated with BNT162b2 mRNA COVID-19 vaccine. Ultrasound Obstet Gynecol 2021; 58:450–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kay AW, Blish CA. Immunogenicity and clinical efficacy of influenza vaccination in pregnancy. Front Immunol 2015; 6:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stock SJ, Carruthers J, Calvert C, et al. SARS-CoV-2 infection and COVID-19 vaccination rates in pregnant women in Scotland. Nat Med 2022; 28:504–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dagan N, Barda N, Biron-Shental T, et al. Effectiveness of the BNT162b2 mRNA COVID-19 vaccine in pregnancy. Nat Med 2021; 27:1693–5. [DOI] [PubMed] [Google Scholar]
- 24. Schrag SJ, Verani JR, Dixon BE, et al. Estimation of COVID-19 mRNA vaccine effectiveness against medically attended COVID-19 in pregnancy during periods of Delta and Omicron variant predominance in the United States. JAMA Netw Open 2022; 5:e2233273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Prasad S, Kalafat E, Blakeway H, et al. Systematic review and meta-analysis of the effectiveness and perinatal outcomes of COVID-19 vaccination in pregnancy. Nat Commun 2022; 13:2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Halasa NB, Olson SM, Staat MA, et al. Maternal vaccination and risk of hospitalization for Covid-19 among infants. N Engl J Med 2022; 387:109–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Beharier O, Plitman Mayo R, Raz T, et al. Efficient maternal to neonatal transfer of antibodies against SARS-CoV-2 and BNT162b2 mRNA COVID-19 vaccine. J Clin Invest 2021; 131:e154834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Karachaliou M, Moncunill G, Espinosa A, et al. SARS-CoV-2 infection, vaccination, and antibody response trajectories in adults: a cohort study in Catalonia. BMC Med 2022; 20:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Anichini G, Terrosi C, Gandolfo C, et al. SARS-CoV-2 antibody response in persons with past natural infection. N Engl J Med 2021; 385:90–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wise J. Covid-19: people who have had infection might only need one dose of mRNA vaccine. BMJ 2021; 372:n308. [DOI] [PubMed] [Google Scholar]
- 31. Manisty C, Otter AD, Treibel TA, et al. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet 2021; 397:1057–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Krammer F, Srivastava K, Alshammary H, et al. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med 2021; 384:1372–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.