Abstract
An increasing concentration of lead (Pb) in urban contaminated soil due to anthropogenic activities has been a global issue threatening human health. The use of urban ornamental plants as phytoremediation of Pb‐contaminated soil is a new choice. In the present experiment, the physiological and biochemical response of five ornamental plants to increase in concentrations of C4H6O4Pb·H2O in the soil were measured to investigate these plans’ Pb tolerance strategies and abilities. Our results showed that Pb stress significantly inhibited the growth and the biomass of all the plants. The root activity (RA), net photosynthetic rate (P n), and chlorophyll (Chl) content in Pb‐stressed leaves were significantly decreased, whereas the leaf proline (Pro), soluble sugar (SS), and membrane stability index (MSI) were remarkable increased compared with those in the control group. By application of all‐subsets regression and linear regression, the reduction in photosynthetic capacity in the five plants is mainly due to the decrease in the leaf Chl content caused by Pb stress. The bioconcentration factor (BCF) in Canna generalis was greater than 1, while in the other plants were lower than 1, suggesting that Canna generalis had the highest Pb accumulation ability. The translocation factor (TF) in all the plants were lower than 1, suggesting that Pb preferentially accumulated in the external part of roots. By calculating the comprehensive evaluation value (CEV), Iris germanica L. was found to be the most sensitive species, and Canna generalis was the most tolerant species, to Pb stress among the five ornamental plants.
Keywords: comprehensive evaluation value, ornamental plants, phytoremediation, soil lead, stress tolerance
1. INTRODUCTION
Heavy metal pollutants released by series of anthropogenic activities including metalliferous mining, use of chemical fertilizers with heavy metal impurities, sewage disposal, municipal landfills, road traffic, and agricultural activities, have become a global environmental problem (Gichner, Žnidar, & Száková, 2008; Kang et al., 2015; Khuzestani & Souri, 2013; Laidlaw, Zahran, Mielke, Taylor, & Filippelli, 2012; Ray & George, 2010; Sarma, Islam, & Prasad, 2017; Verma, Das, & Kumar, 2017). Unlike other pollutants, the heavy metals are not biodegradable and can remain in soil and water at high concentrations for hundreds of years (Verma et al., 2017), ultimately resulting in serious damage to the biotic communities (Auger et al., 2013; Sarma et al., 2017).
Lead (Pb), which is highly phytotoxic, has been recognized as one of the most abundant heavy metals worldwide, and its accumulation is regarded as irreversible and highly toxic to plants (Qin et al., 2018; Shahid, Pinelli, Pourrut, Silvestre, & Dumat, 2011). Although Pb is not an essential element for plants, it is found to have strong affinity to organic and/or inorganic ligands in the soil (Cunningham, Berti, & Huang, 1995; Rodríguez‐Seijo, Andrade, & Vega, 2017), and it can be easily absorbed by plant roots and accumulated in plant tissues even at a low level (Chandrasekhar & Ray, 2019; Johnson, 1998). The hazardous effects of Pb on plant morphological, metabolic, physiological, and biochemical processes have been widely studied (Rucinska, Sobkowiak, & Gwozdz, 2004; Strubińska & Hanaka, 2011; Verma & Dubey, 2003; Yongsheng, Qihui, & Qian, 2011). Studies have shown that excessive amounts of Pb in soil produce excessive reactive oxygen species (ROS; Reddy, Kumar, Jyothsnakumari, Thimmanaik, & Sudhakar, 2005), increase in catalytic enzyme activities (such as polyphenol oxidase, shikimate dehydrogenase and phenylalanine ammonialyase; Wang et al., 2011) and membrane permeability (Sharma & Dubey, 2005), induce leaf chlorosis (Chettri, Cook, Vardaka, Sawidis, & Lanaras, 1998) and cell organelle degeneration (Gratão, Polle, Lea, & Azevedo, 2005), block root elongation (Kopittke, Asher, Blamey, & Menzies, 2007; Liu, Reid, & Smith, 2000), delay seed germination (Lamhamdi, Bakrim, Aarab, Lafont, & Sayah, 2011), disturb the uptake and translocation of nutrient elements (Gopal & Rizvi, 2008), and inhibit plant photosynthesis and respiration (Islam et al., 2008; Rucińska, Waplak, & Gwóźdź, 1999), resulting in a considerable reduction of both vegetative and reproductive plant growth (Hadi, Bano, & Fuller, 2010; Johnson & Eaton, 1980; Sharma & Dubey, 2005; Yongsheng et al., 2011).
The non‐biodegradable and immobile features of Pb due to its strong bond formation with soil organic and/or inorganic ligands makes its removal from contaminated soils very difficult (Cunningham et al., 1995; Dávila et al., 2011a,2011b). Until now, many physical, chemical, and biological technologies have been developed to remediate metalliferous sites. However, only a few technologies are both economical and cost effective. Among those remediation methods, phytoremediation is an eco‐friendly and successful approach for the remediation of toxic metals in polluted soils (Chandrasekhar & Ray, 2019; Cunningham & Berti, 1993; Noufal, Maalla, Noufal, & Hossean, 2017; Salazar & Pignata, 2014). It is a plant‐based technology that utilizes specific hyper‐accumulator plants to retain, remove or reduce toxic metals and metalloids in soil. However, this technology will fail if the concentration of available metal in the soil exceeds its permissible limits (Muszynska, Hanus‐Fajerska, & Ciarkowska, 2018). Hence, the identification of native plant species with metal tolerance, high metal exaction capacity and high biomass yield is essential for the phytoremediation of heavy metal‐contaminated soils (Asgari, Ghorbanpour, & Nikabadi, 2017; Chandrasekhar & Ray, 2017; Gerhardt, Gerwing, & Greenberg, 2017; Macek, Macková, & Káš, 2000).
Urban soil differs from natural soil, because anthropogenic activities can intensely affect its characteristics, and human activities account for 90% of environmental pollution (Gąsiorek, Kowalska, Mazurek, & Pająk, 2017; Puskás & Farsang, 2009). In China, for example, it has been reported that the average concentrations of As, Cd, Cr, Cu, Hg, Ni, Pb, and Zn in the 31 Chinese provincial capital cities reached 11.03, 0.25, 67.92, 32.80, 0.21, 27.23, 36.28, and 99.21 mg/kg, respectively (Zhang, Zha, Guo, Meng, & Zhou, 2018), posing a serious threat to public health. Ornamental plants planted on urban roadsides have great potential for phytoremediation because the absorbed Pb by plant roots cannot enter the food chain to cause Pb toxicity to humans. Thus, identifying native ornamental plants that are able to survive in severe Pb contaminated soil and that show robust Pb accumulation is a new approach for the effective remediation of urban Pb‐contaminated soils. In this study, physiological and biochemical parameters of five Pb‐stressed ornamental plants (Hemerocallis fulva, Iris germanica L., Canna generalis, Pennisetum clandestinum, and Miscanthus sinensis), which have been widely used for urban greening in China, were measured and analyzed to investigate and compare their Pb tolerance strategies and abilities. Overall, the major objective of the present experimental study was (a) to delineate the growth responses and physiological and biochemical changes in five ornamental plants in reponse to increasing levels of Pb in the soil within a six‐week period; (b) to assess the Pb accumulation potential of the five different plants, and (c) to explore the physicochemical mechanisms of plants with tolerance to extensive Pb stress.
2. MATERIALS AND METHODS
2.1. Plant material and growth conditions
The experiment was conducted at Shandong Agriculture University (36°09′N, 117°09′E, 128 m above sea level) in Taian, Shandong Province, China. Healthy and uniformity seeds of Hemerocallis fulva, Iris germanica L., Canna generalis, Pennisetum clandestinum, and Miscanthus sinensis were purchased from a local market. All seeds were soaked in distilled water to improve the germination percentage. After soaking in distilled water for at least 24 hr, the seeds were placed in germinating beds consisting of 5 cm diameter Petri dishes, filled with disinfected peat. Each Petri dish contained one seed. The dishes were incubated at 25°C and 75% relative humidity in an artificial climate chamber (RXZ‐380, made by Ningbo Southeast Instrument Company), with light period and intensity of 14 hr/day and 1,200 mol m−2 day−1, respectively. After three weeks of germination, each individual seedling with 4–5 leaves was transplanted into a plastic plot (18 cm in diameter and 22 cm in depth) which was filled with 5.5 kg Pb‐treated soils. The soil in each plot was watered with deionized water to 60% soil moisture.
The soil samples were collected from the local surface soil (0–20 cm). After air drying inside the laboratory for two weeks, the soil samples were sieved through a 2‐mm mesh. The soil filled pots were kept for 5 days for maturation followed by planting. The soil was classified as Alfisols under an Ultisol in USDA taxonomy and the soil pH was 7.5, the organic matter content 10.5 g/kg, the total nitrogen 0.65 g/kg, available phosphorus content 0.032 g/kg, available potassium content 0.032 g/kg and available Pb concentration was 9.85 mg/kg.
2.2. Experimental design
Six treatments (0, 50, 100, 200, 500, and 1,000 Pb mg/kg) with three replicates were set up and designated as Pb0, Pb50, Pb100, Pb200, Pb500, and Pb1000 in the experiment, respectively. Altogether 90 individual pots were maintained in the study. Each of the treatment levels was prepared by dissolving the respective concentrations of Pb equivalent to C4H6O4Pb·H2O in 500 ml of distilled water. The soil in each plot was spiked with the required levels of Pb solutions. Before transferring the plant seedling to the plot, the Pb‐spiked soil was left for two weeks to reach equilibration.
2.3. Determination of chlorophyll (Chl) content
Chl a, b and total Chl contents in fresh leaves were extracted by acetone and measured according to the method described by Hiscox and Israelstam (1979).
2.4. Determination of proline (Pro)
The extraction procedure and colorimetric determination of Pro in plant cells was carried out according to Bates, Waldren, and Teare (1973). Briefly, 1.0 g leaf sample was homogenized in 3% (w/v) aqueous sulfosalicylic acid. The homogenate was then filtered through two layers of filter paper to obtain a clear filtrate. After addition of the glacial acetic acid and acid ninhydrin mixture to 1 ml of the filtrate, the reaction mixture was heated in a in 100°C water bath for 1.0 hr. The reaction was terminated in an ice bath. The absorbance was read at a wavelength of 546 nm. The Pro concentration was calculated using a standard curve.
2.5. Determination of soluble sugars (SS)
The SS in leaves were extracted and identified following the method of Nelson (1944). Briefly, 0.5 g of fresh leaves were ground in 80% neutral aqueous ethanol and heated in a 100°C water bath for 10 min. The extract was centrifuged at 5,000 rpm for 10 min, and 1.0 ml supernatant was added to 4 ml anthrone reagent followed by heating in a 100°C water bath for 10 min. The reaction was stopped by incubation in a water bath at room temperature (20°C) for 5 min. The absorbance was measured at 630 nm.
2.6. Measurements of the leaf net photosynthetic rate (P n)
Healthy and fully expanded leaves in three plants from each Pb treatment from different pots were chosen to measure the leaf P n using a portable photosynthesis system (LI‐6400, Li‐COR Inc.) with a red–blue LED light source as the illumination. The measurements were conducted from 9:00 to 11:00 a.m. on a sunny day.
2.7. Membrane thermostability index (MTI)
The MTI was determined in 10 leaf discs from fully expanded young leaves by measuring electrolyte leakage according to the method of Sullivan and Ross (1979). After washing in deionized water, 20 ml deionized water was added to the leaf discs in a capped tube and incubated at 25°C for 24 hr. The values of electrical conductivity (EC1) were measured in the samples. The samples were then heated in a boiling water bath for 20 min. After the temperature of the samples reached 25°C, the electrical conductivity (EC2) was again measured. The MTI is expressed as follows: MTI = (1 − (EC1/EC2)) × 100.
2.8. Determination of plant Pb content
The Pb content in plant shoots and roots was determined according to the procedure described by Wang et al. (2009). After washing with deionized water, the shoot and root samples were dried at 75°C for 24 hr. Then, 0.1 g of ground shoots and roots of dried samples were digested in 10 ml HNO3:HClO4 solution and heated in an oven at 100–200°C until near dryness. Subsequently, 5 ml of 5% HNO3 was added to dissolve the cooled residue in addition to ddH2O to a volume of 20 ml. ICP‐MS (Agilent ICP‐MS 7700ce, Agilent Technologies) was used to determine the plant Pb content.
2.9. Determination of root metabolic activity (RA)
The RA was measured according to the method of Liu, Wei, and Li (2014). Briefly, 0.5 g fresh root sample was immersed in 10 ml of a mixed liquid of 0.4% TTC (2,3,5‐triphenyitetrazolium chloride) and 66 mmol/L phosphate buffer solution. The reaction solution was maintained at 37°C for 3 hr, followed by the addition of 2 ml sulfuric acid (1 mol/L) to terminate the reaction. The root was removed and ground in 2 ml ethyl acetate to extract TTF (1,3,5‐triphenylformazan). The absorbance of the extract was measured at a wavelength of 485 nm.
2.10. Plant growth parameters
At the end of the experiment, the plant height (PH), leaf area (LA), leaf numbers per plant (LN), and tiller numbers per plant (TN) of each species subjected to each Pb treatment were measured. The plant height, LN, and TN of each plant were determined prior to harvest. The LA was determined based on the leaf area meter (CI 202, rea meter, CID Incorporated).
2.11. Plant harvest
After 6 weeks of cultivation, all plants were harvested. The plant biomass was separated into two parts: the root biomass and aboveground biomass. The plant material (leaves, stems and roots) was heated in an oven at 105°C for 30 min and dried at 75°C for 48 hr. After the weight of the samples reached a constant value, their dry weight was recorded.
2.12. Bioconcentration factor (BCF) and translocation factor (TF)
The BCF and TF were calculated to evaluate the accumulation of Pb in plants and the translocation from roots to aboveground tissue, respectively. BCF was calculated by dividing the Pb concentration of the root biomass by the Pb concentration of the soil. TF was calculated by dividing the Pb concentration of the plant shoot by the Pb concentration of plant root.
2.13. Calculation of comprehensive evaluation value (CEV)
The CEV was used to figure out the most sensitive/tolerant ornamental plant species under different soil Pb treatments. The development of CEV was based on the combination of the cluster analysis and standard deviation coefficient allocation weighted method described by Zhong et al. (2019) with modifications. Four steps were followed to calculate the CEV.
Principal component analysis
Principal component analysis (PCA) was applied to extract a common factor with a cumulative variance contribution rate ≥ 85%. The factor scores (FCs) of standardized parameters were calculated. The variance contribution rate of the principal component was set as the index weight (W) for each common factor. The principal component score (PCS) could then be calculated by FC multiplied by W.
Calculation of the subordinate function value
The PCS was normalized according to a subordinator function (see Equations 1 and 2) to obtain the subordinate function value (SFV).
| (1) |
| (2) |
where SFV(PCS ij ) is the subordinate function value among the PCS ij values for the jth variable, PCS ij is the value of the principal component score among the PCS ij values for the jth variable, i represents different plant genotypes, j represents different Pb treatments, PCSmax denotes the maximal value among the PCS ij values for the jth variable, and PCSmin denotes the minimal value among the PCS ij values for the jth variable. If the measured parameter had a positive relationship with plant Pb tolerance, the subordinator function should be expressed by Equation (1). In contrast, if there is a negative relationship between the measured parameter and plant Pb tolerance, Equation (2) should be used.
Calculation of the comprehensive evaluation value
The comprehensive evaluation value was calculated using Equation (3):
| (3) |
where Wi is the factor weight, which is the ratio of the index weight of each extracted score to the weighted summations of all extracted scores in the ith plant.
Reorder of the comprehensive evaluation value
With the calculated comprehensive evaluation value, the responses of different ornamental genotypes to Pb stress could be reflected by a single value. The values of five plant species were arranged in descending order and labelled a, b, c, d, and e, respectively, where a denotes the most Pb‐tolerant and e denotes the most Pb‐sensitive plant species.
2.14. Statistical analysis
The experiment was conducted using a completely randomized block design with three replicates. All the parameters described above were measured at six weeks after the plants were subjected to soil Pb treatments. The data for Miscanthus sinensis exposed to Pb1000 treatment are not shown in the study because this plant could not survive under these severe Pb stress conditions. The SPSS 18.0 statistical software package (SPSS) was used to perform the statistical analyses. The mean with standard deviation (±SD) is shown for each treatment in the tables and figures. The parameters were analyzed by one‐/two‐way analysis of variance (ANOVA) followed by Duncan's multiple range test at p < .05. Figures 1, 2 and 3 were performed with the help of Origin 9.0 software (Origin Lab). The heatmap in Figure 4 was constructed by R 3.6.1 (Bell Laboratories).
FIGURE 1.
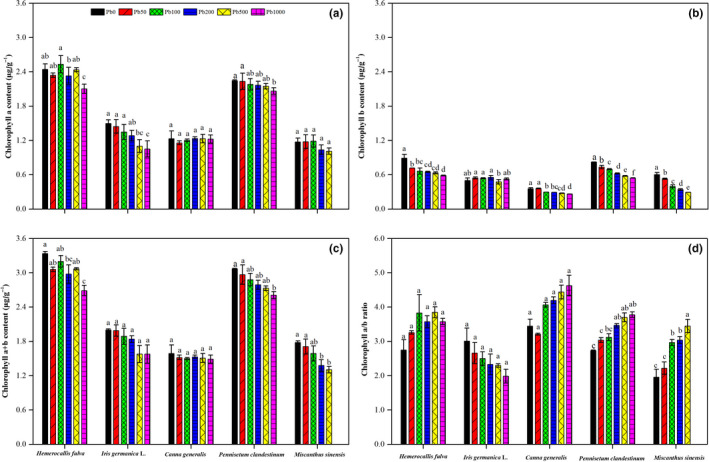
Effects of Pb treatment on chlorophyll a (a), chlorophyll b (b), chlorophyll a + b (c), and chlorophyll a/b (d) in leaves of Hemerocallis fulva, Iris germanica L., Canna generalis, Pennisetum clandestinum, and Miscanthus sinensis. Vertical bars represent ± SD of the mean (n = 3); different letters on the SD bars indicate significant differences among the Pd treatments (p < .05). Pb, Pb treatment; PS, plant species. **Significant differences at p < .01
FIGURE 2.
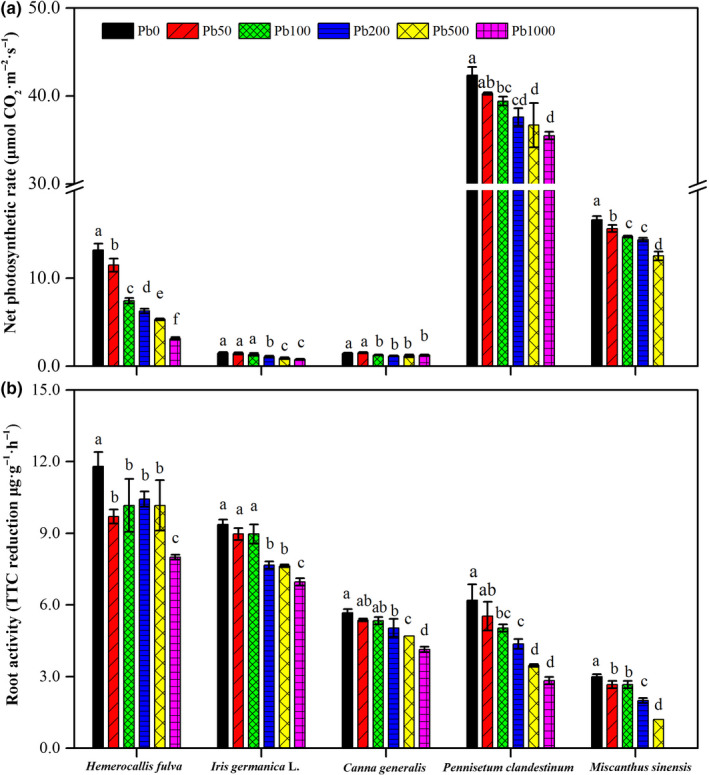
Effects of Pb treatment on leaf net photosynthetic rate (a) and root activity (b) of Hemerocallis fulva, Iris germanica L., Canna generalis, Pennisetum clandestinum, and Miscanthus sinensis. Vertical bars represent ± SD of the mean (n = 3); different letters on the SD bars indicate significant differences among the Pd treatments (p < .05). Pb, Pb treatment; PS, plant species. **Significant differences at p < .01
FIGURE 3.
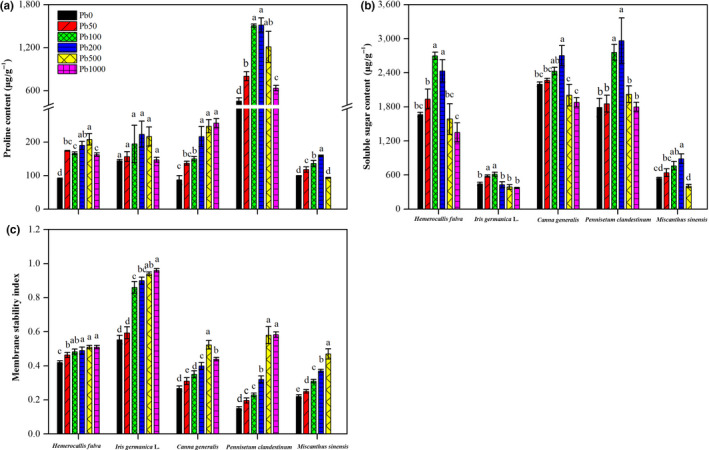
Effects of Pb treatment on proline (a), soluble sugar (b) and membrane stability index (c) in leaves of Hemerocallis fulva, Iris germanica L., Canna generalis, Pennisetum clandestinum, and Miscanthus sinensis. Vertical bars represent ± SD of the mean (n = 3); different letters on the SD bars indicate significant differences among the Pd treatments (p < .05). Pb, Pb treatment; PS, plant species. **Significant differences at p < .01
FIGURE 4.
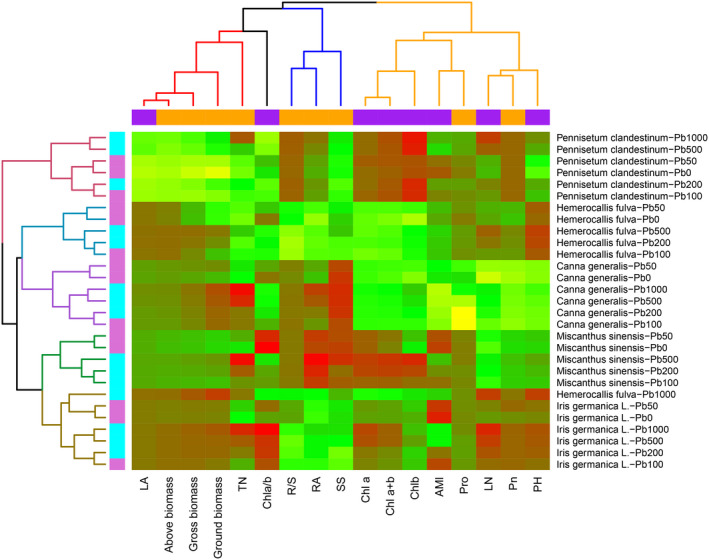
Heatmap based on the Spearman correlation matrix of the 17 variables (plant physiological and biochemical parameters) in different Pb‐treated ornamental plants. AB, above biomass of plant; Chl a + b, chlorophyll a + chlorophyll b; Chl a/b, chlorophyll a/chlorophyll b; Chla, chlorophyll a; Chlb, chlorophyll b; GB, gross biomass of plant; LA, leaf area; LN, leaf number per plant; MTI, membrane thermostability index; PH, plant height; P n, net photosynthetic rate; Pro, proline; R/S, root biomass/aboveground biomass; RA, root activity; RB, root biomass of plant; SS, soluble sugar; TN, tiller number per plant
3. RESULTS
3.1. Plant growth and biomass
The changes in plant growth parameters (c.v. PH, LN, LA, and TN) and biomass (c.v. AB, RB, GB, and R/S) in different ornamental plants under six soil Pb treatments are shown in Table 1. For Hemerocallis fulva, Pb stress significantly decreased PH, LN, LA, TN, AB, RB, and GB. As for the R/S ratio of Hemerocallis fulva, the Pb50 and Pb1000 treatment had no significant difference with the Pb0 treatment, but the other Pb treatments (Pb100, Pb200 and Pb500) significantly increased the R/S ratio by 74.6%–84.6%. For Iris germanica L., different Pb treatments significantly decreased PH by 2.2%–38.1%, LN by 3.7%–20.2%, TN by 11.3%–49.1%, AB by 3.5%–20.1%, RB by 4.7%–28.8%, and GB by 3.9%–24.5. However, Pb stress showed no significant effect on the LA and the R/S ratio in Iris germanica L. All the plant growth parameters (except for LA) and biomass of Canna generalis were significantly affected by Pb stress and decreased with increasing Pb in the soil. Excluding the Pb treatments that had no significant effect on LN in Pennisetum clandestinum and PH and LN in Miscanthus sinensis, the changes in other parameters in Pennisetum clandestinum and Miscanthus sinensis under different Pb treatments exhibited similar trends to Canna generalis.
TABLE 1.
Effects of different Pb treatments on the plant growth and biomass of Hemerocallis fulva, Iris germanica L., Canna generalis, Pennisetum clandestinum, and Miscanthus sinensis
| Plant species | Treatment | PH (cm) | LN | LA (cm−2· plant) | TN | AB (g) | RB (g) | GB (g) | R/S |
|---|---|---|---|---|---|---|---|---|---|
| Hemerocallis fulva | P0 | 16.95 ± 1.24 a | 16.7 ± 0.33 a | 39.5 ± 1.83 a | 6.7 ± 0.21 a | 6.7 ± 0.5 a | 41.2 ± 2.0 a | 47.9 ± 2.7 a | 6.15 ± 0.46 b |
| P50 | 14.78 ± 2.25 a | 17.0 ± 0.58 a | 38.5 ± 4.44 ab | 6.3 ± 0.88 a | 6.1 ± 0.4 a | 40.7 ± 0.1 a | 46.8 ± 2.2 a | 6.63 ± 0.17 b | |
| P100 | 13.76 ± 1.34 a | 16.3 ± 0.33 ab | 34.8 ± 3.64 ab | 5.7 ± 0.33 ab | 2.4 ± 0.3 b | 26.6 ± 2.3 b | 29.0 ± 3.2 b | 11.07 ± 0.33 a | |
| P200 | 9.91 ± 1.26 b | 15.3 ± 0.33 bc | 29.1 ± 1.38 ab | 6.3 ± 0.3 a | 1.8 ± 0.5 b | 20.3 ± 2.3 c | 22.1 ± 2.3 c | 11.54 ± 0.57 a | |
| P500 | 8.28 ± 2.28 b | 14.7 ± 0.33 cd | 29.6 ± 2.7 ab | 4.7 ± 0.88 bc | 1.6 ± 0.2 b | 17.3 ± 2.1 c | 18.9 ± 2.4 c | 10.74 ± 1.06 a | |
| P1000 | 7.97 ± 2.21 b | 13.7 ± 0.67 d | 28.6 ± 1.88 b | 3.7 ± 0.33 c | 1.8 ± 0.4 b | 10.1 ± 0.9 d | 11.9 ± 2.2 d | 5.53 ± 0.56 b | |
| Iris germanica L. | P0 | 24.08 ± 1.24 a | 16.3 ± 0.89 a | 53.3 ± 0.65 a | 5.3 ± 0.33 a | 16.9 ± 0.7 a | 19.1 ± 1.2 a | 35.9 ± 1.6 a | 1.12 ± 0.07 a |
| P50 | 20.02 ± 2.18 b | 15.7 ± 0.67 ab | 51.4 ± 1.72 a | 4.7 ± 0.33 ab | 16.3 ± 0.5 ab | 18.2 ± 0.6 ab | 34.5 ± 1.4 ab | 1.12 ± 0.06 a | |
| P100 | 19.44 ± 3.22 b | 15.0 ± 0.58 abc | 45.5 ± 3.44 a | 4.3 ± 0.88 abc | 15.8 ± 0.2 ab | 17.6 ± 0.5 b | 33.4 ± 1.1 b | 1.11 ± 0.05 a | |
| P200 | 15.44 ± 2.75 c | 14.3 ± 0.3 bcd | 44.9 ± 4.06 a | 3.7 ± 0.33 abc | 15.1 ± 0.6 abc | 15.7 ± 0.4 c | 30.8 ± 0.8 c | 1.04 ± 0.07 a | |
| P500 | 14.10 ± 1.10 c | 13.7 ± 0.33 cd | 44.2 ± 2.83 a | 3.3 ± 0.33 bc | 14.5 ± 0.48 bc | 15.0 ± 0.4 c | 29.5 ± 1.7 c | 1.04 ± 0.08 a | |
| P1000 | 12.67 ± 1.37 c | 13.0 ± 0.58 d | 43.6 ± 5.08 a | 2.7 ± 0.67 c | 13.5 ± 0.5 c | 13.6 ± 0.3 d | 27.1 ± 0.4 d | 1.01 ± 0.07 a | |
| Canna generalis | P0 | 83.52 ± 3.54 a | 17.3 ± 0.67 a | 683.3 ± 20.77 a | 6.7 ± 0.67 a | 80.2 ± 2.9 a | 63.7 ± 1.4 a | 143.9 ± 2.1 a | 0.79 ± 0.02 a |
| P50 | 82.17 ± 2.80 ab | 17.0 ± 0.58 a | 657.3 ± 46.32 a | 6.3 ± 0.33 ab | 76.3 ± 0.5 ab | 55.4 ± 1.2 b | 131.7 ± 3.7 b | 0.73 ± 0.00 b | |
| P100 | 79.14 ± 6.11 ab | 16.3 ± 0.33 ab | 631.3 ± 27.69 a | 6.0 ± 0.58 ab | 75.3 ± 0.2 ab | 51.4 ± 2.2 b | 126.7 ± 0.7 c | 0.68 ± 0.00 c | |
| P200 | 77.66 ± 7.53 ab | 15.7 ± 0.33 bc | 640.6 ± 38.65 a | 5.3 ± 0.67 ab | 74.9 ± 0.5 ab | 43.3 ± 2.8 c | 118.2 ± 3.4 d | 0.58 ± 0.04 d | |
| P500 | 73.83 ± 3.80 bc | 14.7 ± 0.33 cd | 508.6 ± 38.97 b | 4.7 ± 0.33 bc | 70.3 ± 2.8 bc | 38.4 ± 0.9 cd | 108.7 ± 1.5 e | 0.55 ± 0.00 d | |
| P1000 | 67.75 ± 4.40 c | 13.7 ± 0.33 d | 531.4 ± 6.33 b | 3.3 ± 0.33 c | 65.7 ± 3.4 c | 32.8 ± 4.8 d | 98.5 ± 3.3 f | 0.50 ± 0.02 e | |
| Pennisetum clandestinum | P0 | 72.222.22 a | 25.3 ± 0.88 a | 134.78 ± 12.66 a | 5.3 ± 0.88 a | 13.3 ± 1.3 a | 25.7 ± 2.2 a | 39.0 ± 3.3 a | 1.93 ± 0.18 a |
| P50 | 56.86 ± 3.63 b | 23.7 ± 1.20 a | 123.4 ± 5.06 ab | 5.0 ± 1.53 ab | 12.3 ± 0.3 ab | 20.0 ± 1.2 b | 32.3 ± 1.6 b | 1.62 ± 0.05 b | |
| P100 | 32.42 ± 2.42 d | 22.3 ± 0.67 a | 114.1 ± 6.09 ab | 3.7 ± 0.67 abc | 12.7 ± 0.9 ab | 15.5 ± 1.3 c | 28.2 ± 2.3 c | 1.22 ± 0.05 c | |
| P200 | 37.83 ± 2.84 c | 20.3 ± 2.73 a | 111.6 ± 6.09 ab | 3.7 ± 0.88 abc | 11.5 ± 0.54 abc | 15.2 ± 0.8 c | 26.7 ± 1.7 cd | 1.32 ± 0.05 c | |
| P500 | 32.20 ± 2.20 d | 19.3 ± 2.67 a | 94.8 ± 7.02 bc | 2.7 ± 0.67 bc | 10.4 ± 0.5 bc | 13.6 ± 0.8 c | 24.0 ± 1.8 de | 1.31 ± 0.09 c | |
| P1000 | 25.93 ± 1.95 e | 19.0 ± 3.00 a | 87.2 ± 1.29 c | 2.3 ± 0.33 c | 9.3 ± 0.3 c | 12.4 ± 0.6 c | 21.7 ± 1.3 e | 1.34 ± 0.04 c | |
| Miscanthus sinensis | P0 | 35.59 ± 15.45 a | 21.7 ± 2.85 a | 152.9 ± 6.48 a | 5.0 ± 0.58 a | 21.2 ± 0.9 a | 29.3 ± 2.0 a | 50.4 ± 5.1 a | 1.40 ± 0.11 a |
| P50 | 42.67 ± 3.23 a | 21.0 ± 2.52 a | 152.1 ± 3.21 ab | 4.3 ± 0.88 ab | 20.0 ± 0.5 a | 28.6 ± 0.7 a | 48.6 ± 1.8 a | 1.43 ± 0.03 a | |
| P100 | 39.68 ± 3.21 a | 20.7 ± 2.85 a | 146.6 ± 6.09 ab | 4.0 ± 0.58 ab | 19.1 ± 0.3 ab | 26.6 ± 1.2 ab | 45.7 ± 1.3 a | 1.39 ± 0.01 ab | |
| P200 | 36.16 ± 4.16 a | 19.3 ± 3.18 a | 143.2 ± 7.00 b | 3.3 ± 0.33 ab | 17.9 ± 0.93 b | 23.0 ± 0.7 bc | 40.9 ± 0.9 b | 1.28 ± 0.03 b | |
| P500 | 31.41 ± 3.22 a | 19.0 ± 2.52 a | 140.3 ± 10.53 b | 2.3 ± 0.88 b | 16.0 ± 0.8 c | 21.2 ± 0.4 c | 37.2 ± 1.1 b | 1.32 ± 0.03 ab |
Values are the mean of three replicates. Different letters indicate that values are significantly different from each other at p ≤ .05.
3.2. Chlorophyll content
The chlorophyll contents of Hemerocallis fulva, Iris germanica L., Canna generalis, Pennisetum clandestinum, and Miscanthus sinensis are shown in Figure 1. Different Pb treatments had no noticeable effect on Chl a of Canna generalis and Miscanthus sinensis (Figure 1a). For Hemerocallis fulva and Pennisetum clandestinum, compared with the control, Chl a was not significantly impacted by the Pb50, Pb100, Pb200, and P500 treatments, but it was decreased by 13.9% and 8.1% in response to Pb1000, respectively. For Iris germanica L., Chl a was not significantly affected by the Pb50, Pb100 and Pb200 treatments, but it decreased significantly by 26.4% upon Pb500 treatment and by 29.8% upon Pb1000 treatment. The content of Chl b was much lower than Chl a in all the plants (Figure 1b). Excluding Chl b in leaves of Iris germanica L., soil Pb treatments significantly reduced Chl b in the leaves of the other four plants. Compared with Pb0, Pb stress decreased the levels of Chl b by 19.6%–34.2% in Hemerocallis fulva, 17.1%–25.8% in Canna generalis, 10.7%–33.7% in Pennisetum clandestinum and 12.0%–51.3% in Miscanthus sinensis, respectively. Although Chl a + b decreased in all plants with the increase in Pb content in soil, no significant differences were observed in Pb‐treated Iris germanica L. and Canna generalis (Figure 1c). For Hemerocallis fulva, Pennisetum clandestinum, and Miscanthus sinensis, compared to untreated plants, Pb50 through Pb1000 treatments decreased Chl a + b by 4.1%–19.4%, 3.3%–15.0%, and 3.9%–26.6%, respectively. Regarding Chl a/b, compared with Pb0, increasing Pb stress enhanced the values in Hemerocallis fulva, Canna generalis, Pennisetum clandestinum, and Miscanthus sinensis but decreased the values in Iris germanica L. (Figure 1d).
3.3. Leaf net photosynthetic rate and root activity
The leaf net photosynthetic rate and root activity of Hemerocallis fulva, Iris germanica L., Canna generalis, Pennisetum clandestinum, and Miscanthus sinensis in response to various soil Pb treatments are illustrated in Figure 2. The values obtained for the net photosynthetic rate in leaves of different plants revealed noticeable differences (Figure 2a). Under the Pb0 condition, the highest P n value of 13.2 μmol CO2 m−2 s−1 was found in leaves of Pennisetum clandestinum and the lowest value of 1.5 μmol CO2 m−2 s−1 in leaves of Iris germanica L. and Canna generalis, respectively. Under Pb stress conditions, the P n values in the leaves of five plants decreased with increasing levels of Pb in the soil. Compared with Pb0, the Pb treatments decreased P n in the leaves of Hemerocallis fulva, Iris germanica L., Canna generalis, Pennisetum clandestinum, and Miscanthus sinensis by 13.1%–76.2%, 3.4%–78.2%, 13.4%–20.0%, 4.9%–16.2%, and 6.0%–24.6%, respectively.
The root activities in Hemerocallis fulva, Iris germanica L., Canna generalis, Pennisetum clandestinum, and Miscanthus sinensis were significant effected by Pb stress and decreased with the increasing of Pb contents in the soil (Figure 2b). The lowest RA values of Hemerocallis fulva, Iris germanica L. Canna generalis, and Pennisetum clandestinum in the Pb1000 treatment were 8.0 μg g−1 hr−1, 7.0 μg g−1 hr−1, 4.1 μg g−1 hr−1, and 2.8 in μg g−1 hr−1. The lowest RA values of Miscanthus sinensis in the Pb500 treatment was 1.2 μg g−1 hr−1.
3.4. Proline, soluble sugar and membrane stability index
Changes in Pro, SS and MTI in leaves of Hemerocallis fulva, Iris germanica L., Canna generalis, Pennisetum clandestinum, and Miscanthus sinensis are shown in Figure 3. For Iris germanica L., although Pb stress increased Pro by 1.9%–50.4%, the difference in Pro following the different Pb treatments was not significant. For the other plants, compared with Pb0, Pb stress significantly increased Pro by 80.5%–130.8 in Hemerocallis fulva, 56.8%–193.9% in Canna generalis, and 3.9%–231.6% in Pennisetum clandestinum. The change in Pro in Miscanthus sinensis was small. Pb50, Pb100, and Pb200 treatment significantly increased Pro in Miscanthus sinensis, where Pb500 treatment decreased Pro by 4.9% compared with Pb0 treatment.
The content of SS in all plants showed similar changes (Figure 3b). SS significantly increased in the presence of a low soil Pb content (50–200 mg/kg) but not a high soil Pb content (500–1,000 mg/kg). Compared with the Pb0 treatment, the Pb50, Pb100, and Pb200 treatments increased SS by 16.4%, 62.1% and 46.1% in Hemerocallis fulva, 3.1%, 37.2%, and 22.9% in Canna generalis and 18.1%, 40.4, and 63.6% in Miscanthus sinensis. On the other hand, compared with the Pb0 treatment, the Pb500 and Pb1000 treatments decreased SS by 4.7% and 18.8% in Hemerocallis fulva, and by 8.8% and 14.4% in Canna generalis. As for Miscanthus sinensis, SS decreased by 25.6% in the Pb500 treatment. For Pennisetum clandestinum, all soil Pb treatments enhanced SS by 0.6%–65.9%. For Iris germanica L., SS increased to 134.0 μg/g following Pb50 treatment and 166.7 μg/g following Pb100 treatment, but it decreased to 16.6 μg/g in response to Pb200 treatment, 57.5 μg/g in response to Pb500 treatment and 76.1 μg/g in response to Pb1000 treatment, respectively.
MTI was significantly influenced in all plants by soil Pb stress and increased with increasing levels of Pb in the soil (Figure 3c). The highest values of MTI were 0.51 in Hemerocallis fulva, 0.96 in Iris germanica L., and 0.58 in Pennisetum clandestinum in response to Pb1000 treatment and 0.52 in Canna generalis and 0.47 in Miscanthus sinensis in response to Pb500 treatment, respectively.
3.5. Concentration of Pb in plant tissues
Soil Pb stress notably enhanced the Pb concentration either in shoots or roots of Hemerocallis fulva, Iris germanica L., Canna generalis, Pennisetum clandestinum, and Miscanthus sinensis (Table 2). In non‐Pb‐contaminated soils, the lowest Pb concentrations were detected in shoots (9.64 μg/g DW) and roots (13.9 μg/g DW) of Miscanthus sinensis. In contrast, Canna generalis had the highest shoot and root Pb concentrations, which were four‐fold those of Miscanthus sinensis. Under Pb stress conditions, the Pb contents in plant shoots and roots increased with Pb stress, and the highest values were detected in response to Pb1000 treatment in Hemerocallis fulva, Iris germanica L., Canna generalis, and Pennisetum clandestinum, and in response to Pb500 treatment in Miscanthus sinensis. Moreover in response to the same Pb treatment, the concentrations of Pb in shoots (110.74 μg/g DW ~ 1,032.76μg/g) and roots (151.34–1,904.33 μg/g) were much higher in Canna generalis than other plants.
TABLE 2.
Concentrations of Pb in shoots and roots of Hemerocallis fulva, Iris germanica L., Canna generalis, Pennisetum clandestinum and Miscanthus sinensis
| Plant species | Pb treatments | Pb concentrations in shoot (μg/g DW) | Pb concentrations in root (μg/g DW) | BCF | TF |
|---|---|---|---|---|---|
| Hemerocallis fulva | Pb0 | 12.01 ± 0.12 f | 20.13 ± 0.43 f | 0.60 ± 0.01 a | |
| Pb50 | 35.24 ± 1.77 e | 66.49 ± 0.44 e | 0.70 ± 0.0a a | 0.53 ± 0.03 b | |
| Pb100 | 65.65 ± 0.76 d | 129.10 ± 2.26 d | 0.66 ± 0.01 a | 0.51 ± 0.01 b | |
| Pb200 | 102.61 ± 2.17 c | 229.75 ± 3.00 c | 0.51 ± 0.01 b | 0.45 ± 0.01 c | |
| Pb500 | 203.37 ± 2.02 b | 507.07 ± 13.29 b | 0.41 ± 0.01 c | 0.40±0.01 d | |
| Pb1000 | 301.42 ± 6.37 a | 788.32 ± 7.84 a | 0.30 ± 0.02 d | 0.38 ± 0.01 d | |
| Iris germanica L. | Pb0 | 17.13 ± 0.62 f | 22.47 ± 0.24 f | 0.76 ± 0.03 a | |
| Pb50 | 25.17 ± 0.89 e | 42.81 ± 0.40 e | 0.50 ± 0.02 a | 0.59 ± 0.02 b | |
| Pb100 | 42.11 ± 0.68 d | 75.31 ± 0.96 d | 0.42 ± 0.01 b | 0.56 ± 0.01 b | |
| Pb200 | 72.78 ± 0.60 c | 142.05 ± 2.05 c | 0.36 ± 0.01 bc | 0.51 ± 0.02 c | |
| Pb500 | 162.85 ± 1.97 b | 336.29 ± 5.23 b | 0.33 ± 0.01 cd | 0.48 ± 0.01 cd | |
| Pb1000 | 251.67 ± 3.43 a | 561.28 ± 2.24 a | 0.25 ± 0.01 d | 0.45 ± 0.01 d | |
| Canna generalis | Pb0 | 40.66 ± 1.18 f | 51.15 ± 1.52 f | 0.80 ± 0.01 a | |
| Pb50 | 110.74 ± 2.24 e | 151.34 ± 3.09 e | 2.21 ± 0.04 a | 0.73 ± 0.02 b | |
| Pb100 | 199.83 ± 1.54 d | 287.34 ± 3.00 d | 2.00 ± 0.02 b | 0.69 ± 0.01 c | |
| Pb200 | 312.93 ± 6.98 c | 485.18 ± 8.17 c | 1.56 ± 0.04 c | 0.64 ± 0.01 d | |
| Pb500 | 694.79 ± 10.67 b | 1,215.25 ± 27.86 b | 1.39 ± 0.02 c | 0.57 ± 0.01 e | |
| Pb1000 | 1,032.76 ± 15.98 a | 1,904.33 ± 25.66 a | 1.03 ± 0.02 d | 0.54 ± 0.01 f | |
| Pennisetum clandestinum | Pb0 | 13.41 ± 0.03 f | 22.27 ± 0.28 f | 0.60 ± 0.01 a | |
| Pb50 | 20.63 ± 0.33 e | 40.24 ± 0.49 e | 0.41 ± 0.01 a | 0.51 ± 0.01 b | |
| Pb100 | 28.83 ± 1.06 d | 60.55 ± 2.42 d | 0.29 ± 0.01 b | 0.48 ± 0.01 c | |
| Pb200 | 39.72 ± 1.45 c | 96.49 ± 1.47 c | 0.20 ± 0.01 c | 0.41 ± 0.02 d | |
| Pb500 | 76.72 ± 1.44 b | 199.62 ± 3.86 b | 0.15 ± 0.01 d | 0.39 ± 0.01 d | |
| Pb1000 | 85.15 ± 0.73 a | 249.45 ± 7.75 a | 0.09 ± 0.01 e | 0.34 ± 0.01 e | |
| Miscanthus sinensis | Pb0 | 9.64 ± 0.27 d | 13.90 ± 0.33 d | 0.69 ± 0.01 a | |
| Pb50 | 11.23 ± 0.19 d | 17.27 ± 0.31 d | 0.55 ± 0.01 a | 0.65 ± 0.02 a | |
| Pb100 | 14.64 ± 0.83 c | 30.44 ± 1.22 c | 0.42 ± 0.01 b | 0.48 ± 0.01 b | |
| Pb200 | 21.97 ± 0.30 b | 50.72 ± 0.66 b | 0.23 ± 0.01 c | 0.43 ± 0.01 b | |
| Pb500 | 49.49 ± 0.87 a | 131.13 ± 2.99 a | 0.12 ± 0.01 d | 0.38 ± 0.01 c |
Values are the mean of three replicates. Different letters indicate that values are significantly different from each other at p ≤ .05.
The calculated BCF and TF values in Hemerocallis fulva, Iris germanica L., Canna generalis, Pennisetum clandestinum, and Miscanthus sinensis decreased with the increasing Pb contents in the soil (Table 3). Excluding the BCF in Canna generalis, which ranged from 2.21 to 1.03, all the BCFs in the other four plants were <1.0. The TFs in all five plants were <1.0, and the TF values decreased from 0.60 to 0.38 in Hemerocallis fulva, 0.76 to 0.45 in Iris germanica L., 0.80 to 0.54 in Canna generalis, 0.60 to 0.34 in Pennisetum clandestinum, and 0.69 to 0.38 in Miscanthus sinensis, respectively.
TABLE 3.
Multiple range test of the effects of soil Pb stress and plant species on plant physiological and biochemical parameters based on one‐/two‐way ANOVA
| Plant parameters | Pb stress | Plant species | Pb stress × Plant species | ||||||
|---|---|---|---|---|---|---|---|---|---|
| df | F | p | df | F | p | df | F | p | |
| PH | 4 | 664.9 | .00 | 5 | 23.5 | .00 | 19 | 11.3 | 0 |
| LN | 4 | 64.1 | .00 | 5 | 13.0 | .00 | 19 | 0.6 | .924 |
| LA | 4 | 4,215.6 | .00 | 5 | 26.0 | .00 | 19 | 10.9 | .00 |
| TN | 4 | 103.7 | .00 | 5 | 114.2 | .00 | 19 | 2.0 | .021 |
| Above biomass | 4 | 15,695.4 | .00 | 5 | 87.0 | .00 | 19 | 10.3 | .000 |
| Root biomass | 4 | 1,117.0 | .00 | 5 | 278.0 | .00 | 19 | 29.2 | .000 |
| Gross biomass | 4 | 5,582.3 | .00 | 5 | 246.2 | .00 | 19 | 22.4 | .000 |
| R/S | 4 | 1,790.4 | .00 | 5 | 27.3 | .00 | 19 | 19.8 | .001 |
| Chl a | 4 | 699.2 | .00 | 5 | 11.6 | .00 | 19 | 2.9 | .000 |
| Chl b | 4 | 834.7 | .00 | 5 | 133.9 | .00 | 19 | 18.7 | .000 |
| Chl a + b | 4 | 1,045.9 | .00 | 5 | 34.8 | .00 | 19 | 3.5 | .000 |
| Chl a/b | 4 | 152.1 | .00 | 5 | 32.4 | .00 | 19 | 11.8 | .000 |
| RA | 4 | 1,102.1 | .00 | 5 | 77.7 | .00 | 19 | 4.4 | .000 |
| P n | 4 | 11,607.2 | .00 | 5 | 104.4 | .00 | 19 | 21.1 | .000 |
| Pro | 4 | 921,766.0 | .00 | 5 | 66,364.8 | .00 | 19 | 41,186.1 | .000 |
| SS | 4 | 1,454.4 | .00 | 5 | 153.8 | .00 | 19 | 18.4 | .000 |
| MTI | 4 | 1,597.2 | .00 | 5 | 471.2 | .00 | 19 | 44.7 | .000 |
3.6. Analysis of variance on plant parameters
The effects of different Pb treatments, plant species and their interactions on PH, LN, LA, TN, AB, RB, TB, R/S, Chl a, Chl b, Chl a + b, Chl a/b, RA, Pn, Pro, SS, and MSI are shown in Table 3. All the measured parameters were significantly affected (p < .05) by the Pb treatments or the plant species. The interactive effect of the Pb treatments and plants species on LN was not significant (p = .924), but it had a notable effect on the other parameters (p < .01).
3.7. Identification of Pb tolerant plant species
The CEV of the different plants in response to the six Pb treatments are listed in Table 4. Under non‐Pb‐stressed conditions, Hemerocallis fulva had the highest value (0.64) and Iris germanica L. had the lowest value (0.07). Under Pb50 conditions, the highest value (0.66) and lowest value (0.10) of CEV was detected in Pennisetum clandestinum and Iris germanica L., respectively. Following treatment with Pb100, Pb200, Pb 500, and Pb1000, Iris germanica L. retained the lowest values (0.05, 0.07, 0.08, and 0.12, respectively), but the highest values (0.71, 0.67, 0.72, and 0.67, respectively) were detected in Canna generalis.
TABLE 4.
Comprehensive evaluation of Pb tolerance for five ornamental plant species by principal component analysis (PCA)
| Pb treatments | Plant species | FS1 | FS2 | FS3 | W1 | W2 | W3 | PCS1 | PCS2 | PCS3 | SFV1 | SFV2 | SFV3 | CEV | Order |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pb0 | Hemerocallis fulva | 1.52 | 0.24 | −0.89 | 2.785 | 2.182 | 1.844 | 4.24 | 0.51 | −1.65 | 1.00 | 0.53 | 0.00 | 0.64 | a |
| Iris germanica L. | −0.57 | −1.20 | −0.58 | 2.785 | 2.182 | 1.844 | −1.58 | −2.62 | −1.08 | 0.09 | 0.00 | 0.12 | 0.07 | e | |
| Canna generalis | 0.52 | −0.31 | 1.68 | 2.785 | 2.182 | 1.844 | 1.45 | −0.69 | 3.10 | 0.56 | 0.32 | 1.00 | 0.56 | b | |
| Pennisetum clandestinum | −0.70 | 1.53 | −0.01 | 2.785 | 2.182 | 1.844 | −1.95 | 3.34 | −0.01 | 0.03 | 1.00 | 0.34 | 0.39 | c | |
| Miscanthus sinensis | −0.78 | −0.25 | −0.20 | 2.785 | 2.182 | 1.844 | −2.16 | −0.55 | −0.36 | 0.00 | 0.35 | 0.27 | 0.16 | d | |
| Pb50 | Hemerocallis fulva | −0.40 | −0.50 | 1.41 | 2.746 | 2.212 | 1.977 | −1.11 | −1.11 | 2.79 | 0.11 | 0.17 | 1.00 | 0.35 | c |
| Iris germanica L. | −0.68 | −0.96 | −0.26 | 2.746 | 2.212 | 1.977 | −1.87 | −2.12 | −0.51 | 0.00 | 0.00 | 0.40 | 0.10 | e | |
| Canna generalis | −0.29 | 1.67 | 0.27 | 2.746 | 2.212 | 1.977 | −0.79 | 3.69 | 0.53 | 0.16 | 1.00 | 0.59 | 0.52 | b | |
| Pennisetum clandestinum | 1.77 | −0.21 | −0.04 | 2.746 | 2.212 | 1.977 | 4.86 | −0.47 | −0.09 | 1.00 | 0.28 | 0.48 | 0.66 | a | |
| Miscanthus sinensis | −0.40 | 0.00 | −1.37 | 2.746 | 2.212 | 1.977 | −1.09 | 0.00 | −2.73 | 0.12 | 0.36 | 0.00 | 0.16 | d | |
| Pb100 | Hemerocallis fulva | −0.89 | 0.14 | 1.33 | 7.755 | 2.379 | 1.773 | −6.90 | 0.34 | 2.36 | 0.00 | 0.45 | 1.00 | 0.35 | c |
| Iris germanica L. | −0.88 | −1.04 | −0.46 | 7.755 | 2.379 | 1.773 | −6.85 | −2.48 | −0.81 | 0.00 | 0.00 | 0.25 | 0.05 | e | |
| Canna generalis | 1.49 | −0.24 | 0.76 | 7.755 | 2.379 | 1.773 | 11.57 | −0.58 | 1.35 | 1.00 | 0.30 | 0.76 | 0.71 | a | |
| Pennisetum clandestinum | −0.14 | 1.62 | −0.60 | 7.755 | 2.379 | 1.773 | −1.10 | 3.84 | −1.06 | 0.31 | 1.00 | 0.18 | 0.53 | b | |
| Miscanthus sinensis | 0.42 | −0.47 | −1.04 | 7.755 | 2.379 | 1.773 | 3.29 | −1.12 | −1.84 | 0.55 | 0.21 | 0.00 | 0.33 | d | |
| Pb200 | Hemerocallis fulva | −0.69 | −0.29 | 1.31 | 2.656 | 2.478 | 1.818 | −1.84 | −0.71 | 2.38 | 0.07 | 0.24 | 1.00 | 0.32 | c |
| Iris germanica L. | −0.87 | −0.93 | −0.40 | 2.656 | 2.478 | 1.818 | −2.32 | −2.29 | −0.73 | 0.00 | 0.00 | 0.35 | 0.07 | e | |
| Canna generalis | 1.59 | −0.21 | 0.58 | 2.656 | 2.478 | 1.818 | 4.23 | −0.52 | 1.05 | 1.00 | 0.27 | 0.72 | 0.67 | a | |
| Pennisetum clandestinum | −0.34 | 1.71 | −0.16 | 2.656 | 2.478 | 1.818 | −0.90 | 4.24 | −0.28 | 0.22 | 1.00 | 0.45 | 0.55 | b | |
| Miscanthus sinensis | 0.31 | −0.29 | −1.33 | 2.656 | 2.478 | 1.818 | 0.84 | −0.73 | −2.42 | 0.48 | 0.24 | 0.00 | 0.30 | d | |
| Pb500 | Hemerocallis fulva | −0.84 | −0.31 | 1.19 | 2.632 | 2.393 | 1.833 | −2.20 | −0.73 | 2.19 | 0.00 | 0.24 | 1.00 | 0.30 | d |
| Iris germanica L. | −0.78 | −0.95 | −0.45 | 2.632 | 2.393 | 1.833 | −2.07 | −2.27 | −0.83 | 0.02 | 0.00 | 0.34 | 0.08 | e | |
| Canna generalis | 1.52 | −0.14 | 0.78 | 2.632 | 2.393 | 1.833 | 4.00 | −0.32 | 1.43 | 1.00 | 0.31 | 0.84 | 0.72 | a | |
| Pennisetum clandestinum | −0.38 | 1.70 | −0.22 | 2.632 | 2.393 | 1.833 | −0.99 | 4.07 | −0.39 | 0.19 | 1.00 | 0.44 | 0.53 | b | |
| Miscanthus sinensis | 0.48 | −0.31 | −1.31 | 2.632 | 2.393 | 1.833 | 1.26 | −0.75 | −2.40 | 0.56 | 0.24 | 0.00 | 0.33 | c | |
| Pb1000 | Hemerocallis fulva | −0.89 | −0.42 | 1.13 | 2.883 | 2.630 | 1.331 | −2.57 | −1.10 | 1.50 | 0.00 | 0.18 | 1.00 | 0.18 | c |
| Iris germanica L. | −0.30 | −0.83 | −1.21 | 2.883 | 2.630 | 1.331 | −0.87 | −2.19 | −1.61 | 0.25 | 0.00 | 0.00 | 0.12 | d | |
| Canna generalis | 1.43 | −0.20 | 0.40 | 2.883 | 2.630 | 1.331 | 4.13 | −0.52 | 0.53 | 1.00 | 0.28 | 0.69 | 0.67 | a | |
| Pennisetum clandestinum | −0.24 | 1.45 | −0.31 | 2.883 | 2.630 | 1.331 | −0.69 | 3.81 | −0.42 | 0.28 | 1.00 | 0.38 | 0.58 | b |
Abbreviations: CEV, Comprehensive evaluation value; FS, Factor score obtained by PCA method; PCS, Principal component score; SFV, Subordinate function value; W, Index weight.
4. DISCUSSION
Pb toxicity has been studied widely in many higher plants for evaluations of Pb tolerance or a high Pb uptake potential of plants (Andra et al., 2009), aiming to select candidate plants for the phytoremediation of Pb‐contaminated regions (Gupta & Chandra, 1994). Although the negative effect of Pb on plant physiological and biochemical activities has been widely accepted (Jayasri & Suthindhiran, 2017; Sharma & Dubey, 2005; Verma & Dubey, 2003), some other studies have provided opposite conclusions (Chandrasekhar & Ray, 2019). For example, Sidhu, Singh, Batish, and Kohli (2017) reported that soil Pb stress enhanced the growth, protein, and carbohydrate levels of Coronopus didymus L. Hou et al. (2018) also found that the maximum photochemical efficiency of photosystem II in Pogonatherum crinitum was enhanced by Pb2+. The different results indicate that different plant species exhibit a range of responses to the Pb concentration, which strongly depends on the soil characteristics, Pb concentration, stress exposure time, and plant genotype (Gao et al., 2010; Pourrut, Shahid, Dumat, Winterton, & Pinelli, 2011; Shu, Yin, Zhang, & Wang, 2012). Furthermore, the types of Pb compound (e.g., Pb(NO3)2, PbCl2, and (CH3COO)2Pb·3H2O) used in the soil Pb treatments may also lead to a different response to Pb stress (Kurtyka, Burdach, Siemieniuk, & Karcz, 2018; Qin et al., 2018; Verma & Dubey, 2003). According to the the results of One‐way ANOVA (Table 3), our present results revealed that ornamental plants subjected to soil Pb stress showed a pronounced decrease in the growth traits (plant height, leaf area, leaf number and tiller number) and in plant biomass compared with non‐Pb‐treated plants, and reduction in growth, physiology and yield with increasing Pb content in the soil (Table 1) indicated that Pb stress posed a serious threat to the normal growth and metabolic activities of the plants. Similar findings have been reported by Hussain et al. (2013).
The non‐uniform distribution of Pb in different parts of the plants and relatively higher levels of heavy metals in edible tissues have become a great challenge (Han, Gao, Geng, Li, & Wang, 2018; Natasha et al., 2018). In the present study, Pb treatment enhanced the Pb concentration in both plant roots and shoots in all the plants, and the content of Pb increased with increasing levels of Pb in the soil (Table 2). However, although Pb bioaccumulated in all Pb‐stressed plants, the concentration of Pb ion in various plant species and the distribution of Pb in different tissues in the same plant were different. As shown in Table 2, the Pb concentration was much higher in Canna generalis than in the other four plant species, indicating that Canna generalis might have potential for phytoremediation of metal‐contaminated soils. To further understand the exact degree of Pb accumulated in the plants, BCF and TF were used as an indication of phytoremediation in the study. According to the study of Yoon, Cao, Zhou, and Ma (2006), the BCF value was reported under the category of hyperaccumulators, with a value < 1 denoting metal excluders. In our research, BCF was greater than 1 in Canna generalis, while the values of BCF were less than 1 in the other plants (Table 2), indicating that Canna generalis is the most suitable plant for use in phytoremediation. TFs were less than 1 in all plants (Table 2), indicating that Pb preferentially accumulated in the external part of the roots and did not translocate to aerial parts, which may be useful information with regard to phyto‐stabilizing Pb traits together with ornamental plants. Similar results have also been also reported by Pal, Banerjee, and Kundu (2013) and Zhao, Xiong, Li, and Zhu (2009), who showed that Pb accumulated mostly in roots, while a small quantity was translocated to shoots. In addition, the lower R/S, which was expressed as the root biomass/aboveground biomass for most plants in Pb‐contaminated soil (Table 1), confirmed the sequestration of Pb in roots and consequent severe inhibition of plant root activities (Figure 2b).
To identify the degree of injury of plant exposed to Pb stress, MTI (expressed by the electrolyte leakage) was determined. MTI, as an indirect measure of plant cell membrane damage, has been widely used to quantify plant cell membrane permeability under environment stress conditions, with a higher membrane thermostability index indicating a loss of membrane permeability (Habiba et al., 2015; Yan et al., 2010). In the present work, the higher Pb levels in soil significantly escalated the leaf MTI (Figure 3c) and enhanced the permeability of membranes, resulting in a loss of membrane integrity in the leaves of all ornamental plants. Similar results have been reported demonstrating that soil Pb increases the conductivity of electrolyte leakage in wheat (Chen, Chen, & Liu, 2017; Kaur, Singh, Batish, & Kohli, 2012), sesame (Mehmood et al., 2018), rice (Ashraf & Tang, 2017), pepper (Kaya, Akram, Sürücü, & Ashraf, 2019), and Coronopus didymus (Sidhu, Singh, Batish, & Kohli, 2016). There are two possible explanations for the increase in MTI. One explanation is the generation of ROS, such as hydrogen peroxide, in Pb‐stressed plant leaves caused lipid and protein degradation/oxidation (Dias, Mariz Ponte, & Santos c, 2019). The other one explanation is the binding of the metal to the sulfhydryl group and the destabilization of the membrane by forming disulfide bonds, causing an alteration in the organization and function of membrane ion channels (Aravind & Prasad, 2005). Furthermore, the highest MTI values in response to the same soil Pb treatment were found in the leaves of Iris germanica L., indicating that Pb stress posed a higher threat to Iris germanica L. than the other ornamental plants.
When suffered environment stress, plants had a self‐protection and stress tolerant ability in avoiding damage caused by ROS. In our study, two parameters, Pro and SS, were determined to identify the physicochemical mechanisms of plant resistance and tolerance to soil Pb stress. The accumulation of Pro and SS stimulated by various metal ions such as Cd2+, Pb2+, Zn2+, Cu2+, and Al3+ has been widely reported in several studies as one of the most commonly induced adaptive responses of plants (Jia, Zhang, Zhao, Liu, & He, 2018; Li, Yang, Jia, Chen, & Wei, 2013; Nedjimi & Daoud, 2009; Sandra, Marija, Dragan, Vibor, & Branka, 2010; Shevyakova, Netronina, Aronova, & Kuznetsov, 2003), and this effect was greater in shoots than in roots (Tian, Guo, & Yan, 2007; Verma & Dubey, 2001). The accumulation of Pro in the cytosol under heavy metal exposure is not only regarded as an indicator of environmental stress but also to play an important protective role against heavy metal stress (Alia & Saradhi, 1991; Sun, Zhou, Sun, & Jin, 2007) by maintaining the osmotic equilibrium within plant cells (Szabados & Savourcb, 2010), scavenging hydroxyl radicals and singlet oxygen (Kavi & Sreenivasulu, 2014; Matysik, Bhalu, Mohanty, & Bohrweg, 2002; Pal et al., 2013), chelating metal ions in plants and forming a nontoxic metal‐proline complex (Sharma, Schat, & Vooijs, 1998). In our study, the Pro contents in the leaves of Hemerocallis fulva, Iris germanica L., Canna generalis, and Pennisetum clandestinum were enhanced consistently with the application of 50, 100, and 200 and 500 mg/kg Pb (Figure 3a). However, at a high concentration of Pb (1,000 mg/kg), excluding Canna generalis, the contents of Pro in the leaves of the other three ornamental plants (Hemerocallis fulva, Iris germanica L., and Pennisetum clandestinum) showed a remarkable decline. For Miscanthus sinensis, the leaf proline contents increased in response to the 50, 100, and 200 mg/kg Pb treatments but significantly decreased following 500 mg/kg Pb treatment. These results indicated that the application of 500 mg/kg Pb to Miscanthus sinensis and 1,000 mg/kg Pb to Hemerocallis fulva, Iris germanica L., Pennisetum clandestinum, and Miscanthus sinensis exceeded their abilities to protect themselves against ROS‐induced cell damage (Matysik et al., 2002). The accumulation of SS, which acts as an osmotic agent in stressed plants, has also been considered to be a resistance mechanism to the stress condition (Roitsch, 1999; Wu & Xia, 2006) and to play a pivotal role in the osmotic adjustment in plants (Sánchez, Manzanares, Andres, Tenorio, & Ayerbe, 1998; Zhou & Yu, 2009). Lehner (2008) found that SS could detoxify ROS and was related to ROS‐producing metabolic pathways in wheat. In this study, we found that the contents of SS in leaves of ornamental plants subjected to low levels of Pb (50–200 mg/kg) were significantly higher than in the control group, suggesting that mild Pb stress could enhance the synthesis of carbohydrates to eliminate ROS by reinforcing the antioxidant system and protecting the cells from damage (Nguyen, Hailstones, Wilkes, & Sutton, 2010). Our results are in line with the findings of John, Ahmad, Gadgil, and Sharma (2008), who reported a significant increase in SS contents in Lemna polyrrhiza following exposure to low Pb concentration stress. Conversely, high levels (500–1,000 mg/kg) of Pb toxicity reduced soluble sugar concentrations in the leaves of all ornamental plants. The decrease of soluble sugar contents at the high levels of lead in soil might be attributed to the lead‐induced detrimental effect on the structure and function of the photosynthetic apparatus, inhibited the plant carbon assimilation ability, and reduced the synthesis of leaf carbohydrate in the end (Jiang, Wang, Dong, & Yan, 2019). Similar results have also been reported by Bhardwaj, Chaturvedi, and Pratti (2009), Sinha (2004), and Ali et al. (2014), demonstrating the dose dependence of the soluble sugar concentration in ornamental plants exposed to Pb treatment.
Photosynthetic abilities of plants have been shown to be vulnerable to heavy metals (Boucher & Carpentier, 1999; Tanyolaç, Ekmekçi, & Ünalan, 2007). Several authors have reported negative effects on photosynthetic capacity in different species grown in media with high concentrations of Pb (Briat & Lebrun, 1999; Ferreyroa, Lagorio, Trinelli, Lavado, & Molina, 2017; Legocka, Sobieszczuk‐Nowicka, Wojtyla, & Samardakiewicz, 2015; Yang et al., 2015; Zhou, Jiang, Ma, Yang, & Wei, 2017). Our study provided similar results showing that soil Pb even at low doses, and despite the small amount of translocation from root s to shoots (validated by TF < 1, Table 2), inhibited the photosynthetic rate in the leaves of all ornamental plants (Figure 2a). The inhibition mechanisms of Pb stress on plant carbon assimilation ability are associated with the destruction of the chloroplast ultrastructure, alteration of the photosynthetic apparatus functionality, and reduction of antioxidant catalase activity, among others (Arena et al., 2017; Islam et al., 2008; Tian et al., 2014). However, the primary restriction factor for plant photosynthetic capacity under Pb stress conditions was species‐specific (Bouchereau, Aziz, Larher, & Martin‐Tanguy, 1999; Sánchez et al., 2001; Wang & Rainbow, 2006; Yang et al., 2011). In the present study, methods of all‐subsets regression and linear regression were used to elucidate the relationship of P n with the Pro, SS, MSI, and Chl contents in the leaves of different ornamental plants. According to the linear relationship between P n and other parameters, there was remarkable evidence that plant photosynthesis had a significant positive relationship with the leaf Chl content, especially Chl b, for all ornamental plants (Table 5). These results indicated that chloroplasts might be the primary target for heavy metal toxicity in terms of the plant photosynthetic capacity of ornamental plants, which is consistent with the findings of a study on Chlorella kessleri (Sabatini et al., 2009). In fact, Chl content has been widely demonstrated to play a crucial role and act as an important indicator of plant photosynthetic potential under heavy metal stress conditions (Piotrowska‐Niczyporuk, Bajguz, Zambrzycka, & Godlewska‐Żyłkiewicz, 2012). Many mechanisms have been developed to explore the decrease in Chl content caused by Pb stress. According to the study of Mishra et al. (2006), the inhibition of photosynthetic pigment synthesis, disruption of the uptake of essential ions such as Mg2+, Fe2+, and Mn2+, and increase in chlorophyllase activity catalyzing chlorophyll degradation resulted in the reduction of Chl. The other explanation for the decrease in Chl content was that the heavy metal ion substitutes for Mg2+ in the tetrapyrrole ring of the chlorophyll molecule, destroying the chloroplast apparatus (Çelekli, Kapı, & Bozkurt, 2013). Although the reduction of chlorophyll a + b content had been widely detected in leaves of plants, such as Vallisneria natans (Wang, Zhang, Wang, & Lu, 2012), Ceratophyllum demersum L. (Mishra et al., 2006), and Elodea canadensis (Dogan, Saygideger, & Colak, 2009), the susceptibilities of chloroplast a and b were different in different plant species. Gajewska, Skłodowska, Słaba, and Mazur (2006) and Xiong, Zhao, and Li (2010) revealed a more significant decline in Chl b than Chl a under heavy metal stress, while Hu et al. (2012) reports a contradictory result. Our results are consistent with former studies showing a more sensitive response of Chl b than Chl a in the leaves of ornamental plants exposed to Pb stress. The results showed that most soil Pb treatments had no significant effect on the Chl a concentration (Figure 1a) in leaves of Hemerocallis fulva, Iris germanica L., Pennisetum clandestinum, and Miscanthus sinensis, but the Chl b concentration was markedly reduced in most ornamental plants (excluding Iris germanica L.), even at a low Pb concentration (50–100 Pb mg/kg; Figure 1b), causing a significantly lower total pigment concentration in Pb‐treated plants than the control (Figure 1c).
TABLE 5.
Linear relationship of the net photosynthetic rate with chlorophyll and soluble sugar contents in leaves of Hemerocallis fulva, Iris germanica L., Canna generalis, Pennisetum clandestinum, and Miscanthus sinensis
| Plant species | Linear equation | R 2 | p |
|---|---|---|---|
| Hemerocallis fulva | P n = −21.7 – 4.1 × Chl b | .82 | <.01 |
| Iris germanica L. | P n = −1.1 + 1.2 × Chl a + 1.2 × Chl b | .75 | <.01 |
| Canna generalis | P n = 0.3 + 3.4 × Chl b | .67 | <.01 |
| Pennisetum clandestinum | P n = 22.7 + 23.8 × Chl b | .83 | <.01 |
| Miscanthus sinensis | P n = 8.3 + 1.1 × Chl b + 0.0025 × SS | .91 | <.01 |
The identification of tolerant native plant species is essential for the phytoremediation of heavy metal‐contaminated soils (Asgari Lajayer et al., 2017). The evaluation of Pb tolerance was complicated and revealed species specificity in different plants. Although numerous parameters, such as the catalytic activities of antioxidant enzymes (superoxide dismutase, ascorbate peroxidase, and guaiacol peroxidase) in Vallisneria natans (Wang et al., 2011), K+/Na+ ratio in Helianthus annuus L. (Hao, Zhou, Li, & Jiang, 2012), growth tolerance index in Dianthus carthusianorum L. (Muszynska et al., 2018), leaf malondialdehyde level in Pogonatherum crinitum seedlings (Hou et al., 2018), plant growth rates in Nerium oleander L. (Trigueros, Mingorance, & Rossini Oliva, 2012), organic acid in shoots of Echium vulgare L. (Dresler et al., 2017), were used to distinguish plant Pb tolerance, the use of a single or a few evaluation indicators may not comprehensively reflect the abilities of plants to adapt to Pb stress conditions. To solve this limitation, in our research, CEV, which is based on the combination of cluster analysis and the standard deviation coefficient allocation weighted method, was used to provide a comprehensive evaluation of the Pb tolerance of five ornamental plants. Based on the comprehensive evaluation value for each ornamental plant species shown in Table 4, Iris germanica L. had the lowest value among all Pb treatments, suggesting that Iris germanica L. had the weakest ability to tolerate Pb stress. In contrast, Canna generalis and Pennisetum clandestinum had a higher value in response to all Pb treatments compared with the other ornamental plants, indicating that they were the two most tolerant species to Pb stress.
Cluster and heatmap analyses as useful tools were used in the present study for measurement data reduction (Gu et al., 2012). Agglomerative hierarchical cluster analysis was applied, in which 29 variables (Pb‐treated plant species) were selected and a two‐dimensional visualization technique (heatmap) based on the Spearman correlation matrix of the 17 variables (plant physiological and biochemical parameters) was generated, as shown in Figure 4, to select key variables for different plant species. In this figure, weak correlations between variables are displayed in green and yellow, while stronger correlations are shown in red. The cluster analysis classified different Pb‐treated plant species into five subtypes, which were believed to best represent each cluster. Interestingly, the five classified subtypes presented a very close homologies with plant genotypes under different soil Pb conditions, indicating that different ornamental plant species presented unique physiological and biochemical responses to soil Pb stress. Based on the heatmap, a key variable was selected for each plant. According to the red colour in the heatmap, the key parameter in the responses to different soil Pb treatments was plant height for Hemerocallis fulva, Chl a/b for Iris germanica L., soluble sugar for Canna generalis, Chl b for Pennisetum clandestinum, and root activity for Miscanthus sinensis.
5. CONCLUSIONS
In this study, several physiological and biochemical parameters were determined and analyzed along with the Pb uptake and tolerance abilities in Hemerocallis fulva, Iris germanica L., Canna generalis, Pennisetum clandestinum, and Miscanthus sinensis, with the purpose of testing the negative effect of Pb stress on ornamental plants, exploring plant tolerance mechanisms in Pb toxicity, and elucidating the most suitable plant for use in phytoremediation. Figure 5 shows the Pb toxicity towards the shoots and roots of ornamental plants and their tolerance mechanisms under conditions of Pb stress. Although Pb ion mainly accumulated in plant roots and the aerial parts of plants had a relatively low Pb content, six weeks of soil Pb treatments caused acute Pb toxicity in both shoots and roots. Pb stress decreased all plant growth traits (e.g., plant height, leaf area, leaf number, and tiller number), reduced root activity, enhanced leaf membrane permeability, and inhibited plant photosynthetic capacity by reducing the leaf Chl content, resulting in a significant reduction of plant growth and biomass in all the ornamental plants. Proline and soluble sugars were also accumulated in the Pb‐stressed leaves of ornamental plants in all Pb treatments, but their self‐protective abilities against Pb stress were downregulated at high Pb levels (500–1,000 mg/kg). By calculating the CEV, Iris germanica L. was found to be the most sensitive plant to Pb stress, and Canna generalis was the most suitable plant for use in phytoremediation.
FIGURE 5.
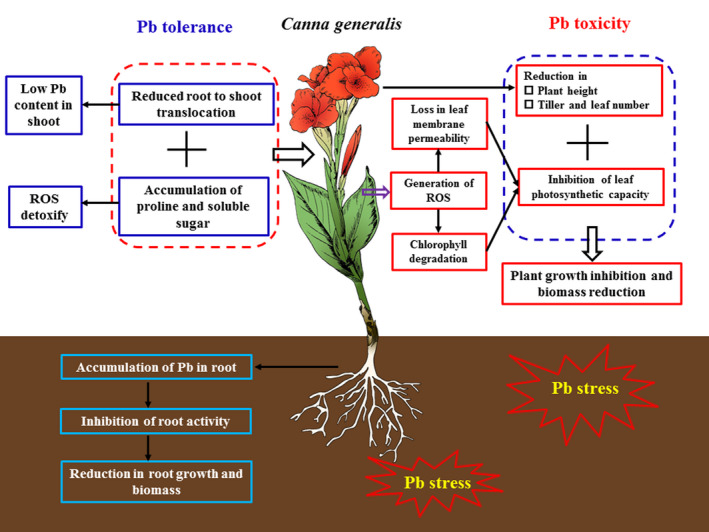
Schematic representation of the Pb toxicity on the shoot and root of ornamental plants and the plant tolerance mechanisms under Pb stress conditions
CONFLICT OF INTEREST
The authors declare no conflict of interest.
[Correction added on 24 May 2021, after first online publication: Conflict of Interest statement added to provide full transparency.]
AUTHOR CONTRIBUTIONS
WF Chen and CX Zhang conceived and conducted the experiment. XL Song, YH Zhu, and YY Wang analyzed the results. and XL Song wrote the article. All authors reviewed the manuscript.
ACKNOWLEDGEMENTS
This work was supported by Shandong Provincial Key Research and Development Program [grant number 2017CXGC0308]. We thank Haibin Li for his help during the experiment.
Song X, Zhang C, Chen W, Zhu Y, Wang Y. Growth responses and physiological and biochemical changes in five ornamental plants grown in urban lead‐contaminated soils. Plant‐Environment Interactions. 2020;1:29–47. 10.1002/pei3.10013
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Ali, B. , Xi, X. , Gill, R. A. , Su, Y. , Ali, S. , Tahir, M. , & Zhou, W. (2014). Promotive role of 5‐aminolevulinic acid on mineral nutrients and antioxidative defense system under lead toxicity in Brassica napus. Industrial Crops & Products, 52(1), 617–626. 10.1016/j.indcrop.2013.11.033 [DOI] [Google Scholar]
- Alia, & Saradhi, P. P. (1991). Proline accumulation under heavy metal stress. Journal of Plant Physiology, 138(5), 554–558. 10.1016/S0176-1617(11)80240-3 [DOI] [Google Scholar]
- Andra, S. S. , Datta, R. , Sarkar, D. , Makris, K. C. , Mullens, C. P. , Sahi, S. V. , & Bach, S. B. (2009). Induction of lead‐binding phytochelatins in vetiver grass [Vetiveria zizanioides (L.)]. Journal of Environmental Quality, 38(3), 868–877. [DOI] [PubMed] [Google Scholar]
- Aravind, P. , & Prasad, M. N. V. (2005). Cadmium‐Zinc interactions in a hydroponic system using Ceratophyllum demersum L.: Adaptive ecophysiology, biochemistry and molecular toxicology. Brazilian Journal of Plant Physiology, 17, 3–20. 10.1590/S1677-04202005000100002 [DOI] [Google Scholar]
- Arena, C. , Figlioli, F. , Sorrentino, M. C. , Izzo, L. G. , Capozzi, F. , Giordano, S. , & Spagnuolo, V. (2017). Ultrastructural, protein and photosynthetic alterations induced by Pb and Cd in Cynara cardunculus L., and its potential for phytoremediation. Ecotoxicology and Environmental Safety, 145, 83–89. 10.1016/j.ecoenv.2017.07.015 [DOI] [PubMed] [Google Scholar]
- Asgari Lajayer, B. , Ghorbanpour, M. , & Nikabadi, S. (2017). Heavy metals in contaminated environment: Destiny of secondary metabolite biosynthesis, oxidative status and phytoextraction in medicinal plants. Ecotoxicology and Environmental Safety, 145, 377–390. 10.1016/j.ecoenv.2017.07.035 [DOI] [PubMed] [Google Scholar]
- Ashraf, U. , & Tang, X. (2017). Yield and quality responses, plant metabolism and metal distribution pattern in aromatic rice under lead (Pb) toxicity. Chemosphere, 176, 141–155. 10.1016/j.chemosphere.2017.02.103 [DOI] [PubMed] [Google Scholar]
- Auger, C. , Han, S. , Appanna, V. P. , Thomas, S. C. , Ulibarri, G. , & Appanna, V. D. (2013). Metabolic reengineering invoked by microbial systems to decontaminate aluminum: Implications for bioremediation technologies. Biotechnology Advances, 31(2), 266–273. 10.1016/j.biotechadv.2012.11.008 [DOI] [PubMed] [Google Scholar]
- Bates, L. S. , Waldren, R. P. , & Teare, I. D. (1973). Rapid determination of free proline for water‐stress studies. Plant & Soil, 39(1), 205–207. 10.1007/BF00018060 [DOI] [Google Scholar]
- Bhardwaj, P. , Chaturvedi, A. , & Pratti, P. (2009). Effect of enhanced lead and cadmium in soil on physiological and biochemical attributes of Phaseolus vulgaris L. National Ascientific, 7, 63–75. [Google Scholar]
- Boucher, N. , & Carpentier, R. (1999). Hg2+, Cu2+, and Pb2+ ‐induced changes in photosystem II photochemical yield and energy storage in isolated thylakoid membranes: A study using simultaneous fluorescence and photoacoustic measurements. Photosynthesis Research, 59(2), 167–174. [Google Scholar]
- Bouchereau, A. , Aziz, A. , Larher, F. , & Martin‐Tanguy, J. (1999). Polyamines and environmental challenges: Recent development. Plant Science, 140(2), 103–125. 10.1016/S0168-9452(98)00218-0 [DOI] [Google Scholar]
- Briat, J. F. , & Lebrun, M. (1999). Plant responses to metal toxicity. Comptes Rendus De L'académie Des Sciences ‐ Series III ‐ Sciences De La Vie, 322(1), 43–54. 10.1016/S0764-4469(99)80016-X [DOI] [PubMed] [Google Scholar]
- Çelekli, A. , Kapı, M. , & Bozkurt, H. (2013). Effect of cadmium on biomass, pigmentation, malondialdehyde, and proline of scenedesmus quadricauda var. longispina. Bulletin of Environmental Contamination and Toxicology, 91(5), 571–576. 10.1007/s00128-013-1100-x [DOI] [PubMed] [Google Scholar]
- Chandrasekhar, C. , & Ray, J. G. (2017). Copper accumulation, localization and antioxidant response in Eclipta alba L. in relation to quantitative variation of the metal in soil. Acta Physiologiae Plantarum, 39(9), 205. [Google Scholar]
- Chandrasekhar, C. , & Ray, J. G. (2019). Lead accumulation, growth responses and biochemical changes of three plant species exposed to soil amended with different concentrations of lead nitrate. Ecotoxicology and Environmental Safety, 171, 26–36. 10.1016/j.ecoenv.2018.12.058 [DOI] [PubMed] [Google Scholar]
- Chen, Y. P. , Chen, D. , & Liu, Q. (2017). Exposure to a magnetic field or laser radiation ameliorates effects of Pb and Cd on physiology and growth of young wheat seedlings. Journal of Photochemistry & Photobiology B Biology, 169, 171–177. 10.1016/j.jphotobiol.2017.03.012 [DOI] [PubMed] [Google Scholar]
- Chettri, M. K. , Cook, C. M. , Vardaka, E. , Sawidis, T. , & Lanaras, T. (1998). The effect of Cu, Zn and Pb on the chlorophyll content of the lichens Cladonia convoluta and Cladonia rangiformis . Environmental and Experimental Botany, 39(1), 1–10. 10.1016/S0098-8472(97)00024-5 [DOI] [Google Scholar]
- Cunningham, S. D. , & Berti, W. R. (1993). Remediation of contaminated soils with green plants: An overview. Vitro Cellular & Developmental Biology Plant, 29(4), 207–212. [Google Scholar]
- Cunningham, S. D. , Berti, W. R. , & Huang, J. W. (1995). Phytoremediation of contaminated soils. Trends in Biotechnology, 13(9), 393–397. 10.1016/S0167-7799(00)88987-8 [DOI] [Google Scholar]
- Dávila Costa, J. S. , Albarracín, V. H. , & Abate, C. M. (2011a). Cupric reductase activity in copper‐resistant amycolatopsis tucumanensis. Water, Air, & Soil Pollution, 216(1), 527–535. 10.1007/s11270-010-0550-6 [DOI] [Google Scholar]
- Dávila Costa, J. S. , Albarracín, V. H. , & Abate, C. M. (2011b). Responses of environmental Amycolatopsis strains to copper stress. Ecotoxicology and Environmental Safety, 74(7), 2020–2028. 10.1016/j.ecoenv.2011.06.017 [DOI] [PubMed] [Google Scholar]
- Dias, M. , Mariz Ponte, N. , & Santos, C. (2019). Lead induces oxidative stress in Pisum sativum plants and changes the levels of phytohormones with antioxidant role. Plant Physiology and Biochemistry, 137, 121–129. 10.1016/j.plaphy.2019.02.005 [DOI] [PubMed] [Google Scholar]
- Dogan, M. , Saygideger, S. D. , & Colak, U. (2009). Effect of lead toxicity on aquatic macrophyte elodea canadensis michx. Bulletin of Environmental Contamination and Toxicology, 83(2), 249–254. 10.1007/s00128-009-9733-5 [DOI] [PubMed] [Google Scholar]
- Dresler, S. , Wójciak‐Kosior, M. , Sowa, I. , Stanisławski, G. , Bany, I. , & Wójcik, M. (2017). Effect of short‐term Zn/Pb or long‐term multi‐metal stress on physiological and morphological parameters of metallicolous and nonmetallicolous Echium vulgare L. populations. Plant Physiology and Biochemistry, 115, 380–389. 10.1016/j.plaphy.2017.04.016 [DOI] [PubMed] [Google Scholar]
- Ferreyroa, G. V. , Lagorio, M. G. , Trinelli, M. A. , Lavado, R. S. , & Molina, F. V. (2017). Lead effects on Brassica napus photosynthetic organs. Ecotoxicology and Environmental Safety, 140, 123–130. 10.1016/j.ecoenv.2017.02.031 [DOI] [PubMed] [Google Scholar]
- Gajewska, E. , Skłodowska, M. , Słaba, M. , & Mazur, J. (2006). Effect of nickel on antioxidative enzyme activities, proline and chlorophyll contents in wheat shoots. Biologia Plantarum, 50(4), 653–659. 10.1007/s10535-006-0102-5 [DOI] [Google Scholar]
- Gao, Y. , Miao, C. , Mao, L. , Zhou, P. , Jin, Z. , & Shi W. (2010) Improvement of phytoextraction and antioxidative defense in Solanum nigrum L. under cadmium stress by application of cadmium‐resistant strain and citric acid. Journal of Hazardous Materials, 181(1), 771–777. [DOI] [PubMed] [Google Scholar]
- Gąsiorek, M. , Kowalska, J. , Mazurek, R. , & Pająk, M. (2017). Comprehensive assessment of heavy metal pollution in topsoil of historical urban park on an example of the Planty Park in Krakow (Poland). Chemosphere, 179, 148–158. 10.1016/j.chemosphere.2017.03.106 [DOI] [PubMed] [Google Scholar]
- Gerhardt, K. E. , Gerwing, P. D. , & Greenberg, B. M. (2017). Opinion: Taking phytoremediation from proven technology to accepted practice. Plant Science, 256, 170–185. 10.1016/j.plantsci.2016.11.016 [DOI] [PubMed] [Google Scholar]
- Gichner, T. , Žnidar, I. , & Száková, J. (2008). Evaluation of DNA damage and mutagenicity induced by lead in tobacco plants. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 652(2), 186–190. 10.1016/j.mrgentox.2008.02.009 [DOI] [PubMed] [Google Scholar]
- Gopal, R. , & Rizvi, A. H. (2008). Excess lead alters growth, metabolism and translocation of certain nutrients in radish. Chemosphere, 70(9), 1539–1544. 10.1016/j.chemosphere.2007.08.043 [DOI] [PubMed] [Google Scholar]
- Gratão, P. L. , Polle, A. , Lea, P. J. , & Azevedo, R. A. (2005). Making the life of heavy metal‐stressed plants a little easier. Functional Plant Biology, 32(6), 481–494. 10.1071/FP05016 [DOI] [PubMed] [Google Scholar]
- Gu, J. , Pitz, M. , Breitner, S. , Birmili, W. , von Klot, S. , Schneider, A. , … Cyrys, J. (2012). Selection of key ambient particulate variables for epidemiological studies – Applying cluster and heatmap analyses as tools for data reduction. Science of the Total Environment, 435–436, 541–550. 10.1016/j.scitotenv.2012.07.040 [DOI] [PubMed] [Google Scholar]
- Gupta, M. , & Chandra, P. (1994). Lead accumulation and toxicity in Vallisneria spiralis (L.) and Hvdrilla vertieillata (l.f.) Royle. Journal of Environmental Science and Health. Part A: Environmental Science and Engineering and Toxicology, 29, 503–516. [Google Scholar]
- Habiba, U. , Ali, S. , Farid, M. , Shakoor, M. B. , Rizwan, M. , Ibrahim, M. , … Ali, B. (2015). EDTA enhanced plant growth, antioxidant defense system, and phytoextraction of copper by Brassica napus L. Environmental Science & Pollution Research, 22(2), 1534–1544. 10.1007/s11356-014-3431-5 [DOI] [PubMed] [Google Scholar]
- Hadi, F. , Bano, A. , & Fuller, M. P. (2010). The improved phytoextraction of lead (Pb) and the growth of maize (Zea mays L.): The role of plant growth regulators (GA3 and IAA) and EDTA alone and in combinations. Chemosphere, 80(4), 457–462. [DOI] [PubMed] [Google Scholar]
- Han, W. , Gao, G. , Geng, J. , Li, Y. , & Wang, Y. (2018). Ecological and health risks assessment and spatial distribution of residual heavy metals in the soil of an e‐waste circular economy park in Tianjin, China. Chemosphere, 197, 325–335. 10.1016/j.chemosphere.2018.01.043 [DOI] [PubMed] [Google Scholar]
- Hao, X.‐Z. , Zhou, D.‐M. , Li, D.‐D. , & Jiang, P. (2012). Growth, cadmium and zinc accumulation of ornamental sunflower (Helianthus annuus L.) in contaminated soil with different amendments. Pedosphere, 22(5), 631–639. [Google Scholar]
- Hiscox, J. D. , & Israelstam, G. F. (1979). A method for the extraction of chlorophyll from leaf tissue without maceration. Canadian Journal of Botany, 57(12), 1332–1334. [Google Scholar]
- Hou, X. , Han, H. , Cai, L. , Liu, A. , Ma, X. , Zhou, C. , … Meng, F. (2018). Pb stress effects on leaf chlorophyll fluorescence, antioxidative enzyme activities, and organic acid contents of Pogonatherum crinitum seedlings. Flora, 240, 82–88. 10.1016/j.flora.2018.01.006 [DOI] [Google Scholar]
- Hu, R. , Sun, K. , Su, X. , Pan, Y. X. , Zhang, Y. F. , & Wang, X. P. (2012). Physiological responses and tolerance mechanisms to Pb in two xerophils: Salsola passerina Bunge and Chenopodium album L. Journal of Hazardous Materials, 205–206, 131–138. 10.1016/j.jhazmat.2011.12.051 [DOI] [PubMed] [Google Scholar]
- Hussain, A. , Abbas, N. , Arshad, F. , Akram, M. W. , Iqbal Khan, Z. , Ahmad, K. , … Mirzaei, F. (2013). Effects of diverse doses of lead (Pb) on different growth attributes of Zea‐mays L. Scientific Research, 4, 262–265. 10.4236/as.2013.45037 [DOI] [Google Scholar]
- Islam, E. , Liu, D. , Li, T. , Yang, X. , Jin, X. , Mahmood, Q. , … Li, J. (2008). Effect of Pb toxicity on leaf growth, physiology and ultrastructure in the two ecotypes of Elsholtzia argyi. Journal of Hazardous Materials, 154(1), 914–926. 10.1016/j.jhazmat.2007.10.121 [DOI] [PubMed] [Google Scholar]
- Jayasri, M. A. , & Suthindhiran, K. (2017). Effect of zinc and lead on the physiological and biochemical properties of aquatic plant Lemna minor: Its potential role in phytoremediation. Applied Water Science, 7(3), 1247–1253. 10.1007/s13201-015-0376-x [DOI] [Google Scholar]
- Jia, X. , Zhang, C. , Zhao, Y. , Liu, T. , & He, Y. (2018). Three years of exposure to lead and elevated CO2 affects lead accumulation and leaf defenses in Robinia pseudoacacia L. seedlings. Journal of Hazardous Materials, 349(2018), 215–223. 10.1016/j.jhazmat.2018.02.002 [DOI] [PubMed] [Google Scholar]
- Jiang, D. , Wang, Y. Y. , Dong, X. W. , & Yan, S. C. (2019). Inducible defense responses in Populus alba berolinensis to Pb stress. South African Journal of Botany, 119, 295–300. 10.1016/j.sajb.2018.09.030 [DOI] [Google Scholar]
- John, R. , Ahmad, P. , Gadgil, K. , & Sharma, S. (2008). Effect of cadmium and lead on growth, biochemical parameters and uptake in Lemna polyrrhiza L. Plant Soil and Environment, 54, 262–270. 10.17221/2787-PSE [DOI] [Google Scholar]
- Johnson, F. M. (1998). The genetic effects of environmental lead. Mutation Research/Reviews in Mutation Research, 410(2), 123–140. 10.1016/S1383-5742(97)00032-X [DOI] [PubMed] [Google Scholar]
- Johnson, M. S. , & Eaton, J. W. (1980). Environmental contamination through residual trace metal dispersal from a derelict lead‐zinc Mine1. Journal of Environmental Quality, 9, 175–179. 10.2134/jeq1980.00472425000900020001x [DOI] [Google Scholar]
- Kang, C.‐H. , Oh, S. J. , Shin, Y. , Han, S.‐H. , Nam, I.‐H. , & So, J.‐S. (2015). Bioremediation of lead by ureolytic bacteria isolated from soil at abandoned metal mines in South Korea. Ecological Engineering, 74, 402–407. 10.1016/j.ecoleng.2014.10.009 [DOI] [Google Scholar]
- Kaur, G. , Singh, H. P. , Batish, D. R. , & Kohli, R. K. (2012). A time course assessment of changes in reactive oxygen species generation and antioxidant defense in hydroponically grown wheat in response to lead ions (Pb2+). Protoplasma, 249(4), 1091–1100. 10.1007/s00709-011-0353-7 [DOI] [PubMed] [Google Scholar]
- Kavi Kishor, P. B. , & Sreenivasulu, N. (2014). Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant Cell & Environment, 37(2), 300–311. [DOI] [PubMed] [Google Scholar]
- Kaya, C. , Akram, N. A. , Sürücü, A. , & Ashraf, M. (2019). Alleviating effect of nitric oxide on oxidative stress and antioxidant defence system in pepper (Capsicum annuum L.) plants exposed to cadmium and lead toxicity applied separately or in combination. Scientia Horticulturae, 255, 52–60. 10.1016/j.scienta.2019.05.029 [DOI] [Google Scholar]
- Khuzestani, R. B. , & Souri, B. (2013). Evaluation of heavy metal contamination hazards in nuisance dust particles, in Kurdistan Province, western Iran. Journal of Environmental Sciences, 25(7), 1346–1354. [DOI] [PubMed] [Google Scholar]
- Kopittke, P. M. , Asher, C. J. , Blamey, F. P. C. , & Menzies, N. W. (2007). Toxic effects of Pb2+ on the growth and mineral nutrition of signal grass (Brachiaria decumbens) and Rhodes grass (Chloris gayana). Plant and Soil, 300(1), 127–136. 10.1007/s11104-007-9395-1 [DOI] [Google Scholar]
- Kurtyka, R. , Burdach, Z. , Siemieniuk, A. , & Karcz, W. (2018). Single and combined effects of Cd and Pb on the growth, medium pH, membrane potential and metal contents in maize (Zea mays L.) coleoptile segments. Ecotoxicology and Environmental Safety, 161, 8–16. 10.1016/j.ecoenv.2018.05.046 [DOI] [PubMed] [Google Scholar]
- Laidlaw, M. A. S. , Zahran, S. , Mielke, H. W. , Taylor, M. P. , & Filippelli, G. M. (2012). Re‐suspension of lead contaminated urban soil as a dominant source of atmospheric lead in Birmingham, Chicago, Detroit and Pittsburgh, USA. Atmospheric Environment, 49, 302–310. 10.1016/j.atmosenv.2011.11.030 [DOI] [Google Scholar]
- Lamhamdi, M. , Bakrim, A. , Aarab, A. , Lafont, R. , & Sayah, F. (2011). Lead phytotoxicity on wheat (Triticum aestivum L.) seed germination and seedlings growth. Comptes Rendus Biologies, 334(2), 118–126. 10.1016/j.crvi.2010.12.006 [DOI] [PubMed] [Google Scholar]
- Legocka, J. , Sobieszczuk‐Nowicka, E. , Wojtyla, Ł. , & Samardakiewicz, S. (2015). Lead‐stress induced changes in the content of free, thylakoid‐ and chromatin‐bound polyamines, photosynthetic parameters and ultrastructure in greening barley leaves. Journal of Plant Physiology, 186–187, 15–24. 10.1016/j.jplph.2015.07.010 [DOI] [PubMed] [Google Scholar]
- Lehner, B. (2008). Selection to minimise noise in living systems and its implications for the evolution of gene expression. Molecular Systems Biology, 4(1), 170. 10.1038/msb.2008.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. , Yang, Y. , Jia, L. , Chen, H. , & Wei, X. (2013). Zinc‐induced oxidative damage, antioxidant enzyme response and proline metabolism in roots and leaves of wheat plants. Ecotoxicology & Environmental Safety, 89(11), 150–157. 10.1016/j.ecoenv.2012.11.025 [DOI] [PubMed] [Google Scholar]
- Liu, J. , Reid, R. J. , & Smith, F. A. (2000). The mechanism of cobalt toxicity in mung beans. Physiologia Plantarum, 110(1), 104–110. 10.1034/j.1399-3054.2000.110114.x [DOI] [Google Scholar]
- Liu, J. J. , Wei, Z. , & Li, J. H. (2014). Effects of copper on leaf membrane structure and root activity of maize seedling. Botanical Studies, 55(1), 47. 10.1186/s40529-014-0047-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macek, T. , Macková, M. , & Káš, J. (2000). Exploitation of plants for the removal of organics in environmental remediation. Biotechnology Advances, 18(1), 23–34. 10.1016/S0734-9750(99)00034-8 [DOI] [PubMed] [Google Scholar]
- Matysik, J. , Bhalu, B. A. , Mohanty, P. , & Bohrweg, N. (2002). Molecular mechanism of quenching of reactive oxygen species by proline under stress in plants. Current Science, 82(5), 525–532. [Google Scholar]
- Mehmood, S. , Saeed, D. A. , Rizwan, M. , Khan, M. N. , Aziz, O. , Bashir, S. , … Shaheen, A. (2018). Impact of different amendments on biochemical responses of sesame (Sesamum indicum L.) plants grown in lead‐cadmium contaminated soil. Plant Physiology and Biochemistry, 132, 345–355. 10.1016/j.plaphy.2018.09.019 [DOI] [PubMed] [Google Scholar]
- Mishra, S. , Srivastava, S. , Tripathi, R. D. , Kumar, R. , Seth, C. S. , & Gupta, D. K. (2006). Lead detoxification by coontail (Ceratophyllum demersum L.) involves induction of phytochelatins and antioxidant system in response to its accumulation. Chemosphere, 65(6), 1027–1039. [DOI] [PubMed] [Google Scholar]
- Muszynska, E. , Hanus‐Fajerska, E. , & Ciarkowska, K. (2018). Studies on lead and cadmium toxicity in Dianthus carthusianorum calamine ecotype cultivated in vitro. Plant Biology (Stuttgart, Germany), 20(3), 474–482. [DOI] [PubMed] [Google Scholar]
- Natasha, Shahid, M. , Niazi, N. K. , Khalid, S. , Murtaza, B. , Bibi, I. , & Rashid, M. I. (2018). A critical review of selenium biogeochemical behavior in soil‐plant system with an inference to human health. Environmental Pollution, 234, 915–934. 10.1016/j.envpol.2017.12.019 [DOI] [PubMed] [Google Scholar]
- Nedjimi, B. , & Daoud, Y. (2009). Cadmium accumulation in Atriplex halimus subsp. schweinfurthii and its influence on growth, proline, root hydraulic conductivity and nutrient uptake. Flora, 204(4), 316–324. [Google Scholar]
- Nelson, N. (1944). A photometric adaptation of the Somogyi method to determination of glucose. Journal of Biological Chemistry, 153(2), 375–380. [Google Scholar]
- Nguyen, G. N. , Hailstones, D. L. , Wilkes, M. , & Sutton, B. G. (2010). Role of carbohydrate metabolism in drought‐induced male sterility in rice anthers. Journal of Agronomy & Crop Science, 196(5), 346–357. [Google Scholar]
- Noufal, M. J. , Maalla, Z. A. , Noufal, D. J. , & Hossean, A. A. (2017). Removal of lead from the polluted water by phytoremediation technique (Eclipta prostrata plant). International Journal of Energy and Environment, 8(1), 97–104. [Google Scholar]
- Pal, R. , Banerjee, A. , & Kundu, R. (2013). Responses of castor bean (Ricinus communis L.) to lead Stress. Proceedings of the National Academy of Sciences, India Section B: Biological Sciences, 83(4), 643–650. 10.1007/s40011-013-0180-z [DOI] [Google Scholar]
- Piotrowska‐Niczyporuk, A. , Bajguz, A. , Zambrzycka, E. , & Godlewska‐Żyłkiewicz, B. (2012). Phytohormones as regulators of heavy metal biosorption and toxicity in green alga Chlorella vulgaris (Chlorophyceae). Plant Physiology and Biochemistry, 52, 52–65. 10.1016/j.plaphy.2011.11.009 [DOI] [PubMed] [Google Scholar]
- Pourrut, B. , Shahid, M. , Dumat, C. , Winterton, P. , & Pinelli, E. (2011). Lead uptake, toxicity, and detoxification in plants. In Whitacre D. M. (Ed.), Reviews of environmental contamination and toxicology (Vol. 213, pp. 113–136). New York, NY: Springer. [DOI] [PubMed] [Google Scholar]
- Puskás, I. , & Farsang, A. (2009). Diagnostic indicators for characterizing urban soils of Szeged, Hungary. Geoderma, 148(3), 267–281. 10.1016/j.geoderma.2008.10.014 [DOI] [Google Scholar]
- Qin, F. , Liu, G. , Huang, G. , Dong, T. , Liao, Y. , & Xu, X. (2018). Zinc application alleviates the adverse effects of lead stress more in female Morus alba than in males. Environmental and Experimental Botany, 146, 68–76. 10.1016/j.envexpbot.2017.10.003 [DOI] [Google Scholar]
- Ray, J. G. , & George, J. (2010). Lead in soils and tolerant herbs on roadsides in search of the metal accumulation patterns in diverse species. International Journal of Applied Environmental Sciences, 5(2), 227–244. [Google Scholar]
- Radić, S. , Babić, M. , Škobić, D. , Roje, V. , & Pevalek‐Kozlina, B. (2010). Ecotoxicological effects of aluminum and zinc on growth and antioxidants in Lemna minor L. Ecotoxicology & Environmental Safety, 73(3), 336–342. [DOI] [PubMed] [Google Scholar]
- Reddy, A. M. , Kumar, S. G. , Jyothsnakumari, G. , Thimmanaik, S. , & Sudhakar, C. (2005). Lead induced changes in antioxidant metabolism of horsegram (Macrotyloma uniflorum (Lam.) Verdc.) and bengalgram (Cicer arietinum L.). Chemosphere, 60(1), 97–104. [DOI] [PubMed] [Google Scholar]
- Rodríguez‐Seijo, A. , Andrade, M. L. , & Vega, F. A. (2017). Origin and spatial distribution of metals in urban soils. Journal of Soils and Sediments, 17(5), 1514–1526. 10.1007/s11368-015-1304-2 [DOI] [Google Scholar]
- Roitsch, T. (1999). Source‐sink regulation by sugar and stress. Current Opinion in Plant Biology, 2(3), 198–206. 10.1016/S1369-5266(99)80036-3 [DOI] [PubMed] [Google Scholar]
- Rucinska, R. , Sobkowiak, R. , & Gwozdz, E. A. (2004). Genotoxicity of lead in lupin root cells as evaluated by the comet assay. Cellular & Molecular Biology Letters, 9(3), 519–528. [PubMed] [Google Scholar]
- Rucińska, R. , Waplak, S. , & Gwóźdź, E. A. (1999). Free radical formation and activity of antioxidant enzymes in lupin roots exposed to lead. Plant Physiology and Biochemistry, 37(3), 187–194. 10.1016/S0981-9428(99)80033-3 [DOI] [Google Scholar]
- Sabatini, S. E. , Juárez, Á. B. , Eppis, M. R. , Bianchi, L. , Luquet, C. M. , de Molina, R. , & Ríos de Molina, M. D. C. (2009). Oxidative stress and antioxidant defenses in two green microalgae exposed to copper. Ecotoxicology and Environmental Safety, 72(4), 1200–1206. 10.1016/j.ecoenv.2009.01.003 [DOI] [PubMed] [Google Scholar]
- Salazar, M. J. , & Pignata, M. L. (2014). Lead accumulation in plants grown in polluted soils. Screening of native species for phytoremediation. Journal of Geochemical Exploration, 137, 29–36. 10.1016/j.gexplo.2013.11.003 [DOI] [Google Scholar]
- Sánchez, E. , López‐Lefebre, L. R. , García, P. C. , Rivero, R. M. , Ruiz, J. M. , & Romero, L. (2001). Proline metabolism in response to highest nitrogen dosages in green bean plants (Phaseolus vulgaris L. cv. Strike). Journal of Plant Physiology, 158(5), 593–598. 10.1078/0176-1617-00268 [DOI] [Google Scholar]
- Sánchez, F. J. , Manzanares, M. A. , Andres, E. F. D. , Tenorio, J. L. , & Ayerbe, L. (1998). Turgor maintenance, osmotic adjustment and soluble sugar and proline accumulation in 49 pea cultivars in response to water stress. Field Crops Research, 59(3), 225–235. 10.1016/S0378-4290(98)00125-7 [DOI] [Google Scholar]
- Sarma, H. , Islam, N. F. , & Prasad, M. N. V. (2017). Plant‐microbial association in petroleum and gas exploration sites in the state of Assam, north‐east India‐significance for bioremediation. Environmental Science and Pollution Research, 24(9), 8744–8758. 10.1007/s11356-017-8485-8 [DOI] [PubMed] [Google Scholar]
- Shahid, M. , Pinelli, E. , Pourrut, B. , Silvestre, J. , & Dumat, C. (2011). Lead‐induced genotoxicity to Vicia faba L. roots in relation with metal cell uptake and initial speciation. Ecotoxicology and Environmental Safety, 74(1), 78–84. [DOI] [PubMed] [Google Scholar]
- Sharma, P. , & Dubey, R. S. (2005). Lead toxicity in plants. Brazilian Journal of Plant Physiology, 17, 35–52. 10.1590/S1677-04202005000100004 [DOI] [Google Scholar]
- Sharma, S. S. , Schat, H. , & Vooijs, R. (1998). In vitro alleviation of heavy metal‐induced enzyme inhibition by proline. Phytochemistry, 49(6), 1531–1535. 10.1016/S0031-9422(98)00282-9 [DOI] [PubMed] [Google Scholar]
- Shevyakova, N. I. , Netronina, I. A. , Aronova, E. E. , & Kuznetsov, V. V. (2003). Compartmentation of cadmium and iron in mesembryanthemum crystallinum plants during the adaptation to cadmium stress. Russian Journal of Plant Physiology, 50(5), 678–685. [Google Scholar]
- Shu, X. , Yin, L. Y. , Zhang, Q. F. , & Wang, W. B. (2012). Effect of Pb toxicity on leaf growth, antioxidant enzyme activities, and photosynthesis in cuttings and seedlings of. Environmental Science & Pollution Research, 19(3), 893–902. [DOI] [PubMed] [Google Scholar]
- Sidhu, G. P. S. , Singh, H. P. , Batish, D. R. , & Kohli, R. K. (2016). Effect of lead on oxidative status, antioxidative response and metal accumulation in Coronopus didymus . Plant Physiology & Biochemistry, 105, 290–296. 10.1016/j.plaphy.2016.05.019 [DOI] [PubMed] [Google Scholar]
- Sidhu, G. P. S. , Singh, H. P. , Batish, D. R. , & Kohli, R. K. (2017). Alterations in photosynthetic pigments, protein, and carbohydrate metabolism in a wild plant Coronopus didymus L. (Brassicaceae) under lead stress. Acta Physiologiae Plantarum, 39(8), 176. [Google Scholar]
- Sinha, P. (2004). Detrimental effects of lead phytotoxicity on growth, yield, and metabolism of rice. Communications in Soil Science & Plant Analysis, 35(1–2), 255–265. [Google Scholar]
- Strubińska, J. , & Hanaka, A. (2011). Adventitious root system reduces lead uptake and oxidative stress in sunflower seedlings. Biologia Plantarum, 55, 771–774. 10.1007/s10535-011-0185-5 [DOI] [Google Scholar]
- Sullivan, C. Y. , & Ross, W. M. (1979). Selecting for drought and heat resistance in grain sorghum. Field Crops Research, 198, 263–281. 10.1016/j.fcr.2016.03.015 [DOI] [Google Scholar]
- Sun, R. L. , Zhou, Q. X. , Sun, F. H. , & Jin, C. X. (2007). Antioxidative defense and proline/phytochelatin accumulation in a newly discovered Cd‐hyperaccumulator, Solanum nigrum L. Environmental & Experimental Botany, 60(3), 468–476. 10.1016/j.envexpbot.2007.01.004 [DOI] [Google Scholar]
- Szabados, L. L. , & Savourcb, A. (2010). Proline: A multifunctional amino acid. Trends in Plant Science, 15(2), 89–97. [DOI] [PubMed] [Google Scholar]
- Tanyolaç, D. , Ekmekçi, Y. , & Ünalan, Ş. (2007). Changes in photochemical and antioxidant enzyme activities in maize (Zea mays L.) leaves exposed to excess copper. Chemosphere, 67(1), 89–98. [DOI] [PubMed] [Google Scholar]
- Tian, R. G. , Guo, P. Z. , & Yan, H. Z. (2007). Physiological changes in barley plants under combined toxicity of aluminum, copper and cadmium. Colloids & Surfaces B Biointerfaces, 57(2), 182–188. [DOI] [PubMed] [Google Scholar]
- Tian, T. , Ali, B. , Qin, Y. , Malik, Z. , Gill, R. A. , Ali, S. , & Zhou, W. (2014). Alleviation of lead toxicity by 5‐aminolevulinic acid is related to elevated growth, photosynthesis, and suppressed ultrastructural damages in oilseed rape. BioMed Research International, 2014, 530642. 10.1155/2014/530642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trigueros, D. , Mingorance, M. D. , & Rossini Oliva, S. (2012). Evaluation of the ability of Nerium oleander L. to remediate Pb‐contaminated soils. Journal of Geochemical Exploration, 114, 126–133. 10.1016/j.gexplo.2012.01.005 [DOI] [Google Scholar]
- Verma, C. , Das, A. , & Kumar, R. (2017). PGPR‐assisted phytoremediation of cadmium: An advancement towards clean environment. Current Science, 113, 715–724. 10.18520/cs/v113/i04/715-724 [DOI] [Google Scholar]
- Verma, S. , & Dubey, R. S. (2001). Effect of cadmium on soluble sugars and enzymes of their metabolism in rice. Biologia Plantarum, 44(1), 117–123. [Google Scholar]
- Verma, S. , & Dubey, R. S. (2003). Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Science, 164(4), 645–655. 10.1016/S0168-9452(03)00022-0 [DOI] [Google Scholar]
- Wang, C. , Lu, J. , Zhang, S. , Wang, P. , Hou, J. , & Qian, J. (2011). Effects of Pb stress on nutrient uptake and secondary metabolism in submerged macrophyte Vallisneria natans . Ecotoxicology and Environmental Safety, 74(5), 1297–1303. 10.1016/j.ecoenv.2011.03.005 [DOI] [PubMed] [Google Scholar]
- Wang, C. , Zhang, S. H. , Wang, P. F. , Hou, J. , Zhang, W. J. , Li, W. , & Lin, Z. P. (2009). The effect of excess Zn on mineral nutrition and antioxidative response in rapeseed seedlings. Chemosphere, 75(11), 1468–1476. 10.1016/j.chemosphere.2009.02.033 [DOI] [PubMed] [Google Scholar]
- Wang, P. F. , Zhang, S. H. , Wang, C. , & Lu., J. (2012). Effects of Pb on the oxidative stress and antioxidant response in a Pb bioaccumulator plant Vallisneria natans. Ecotoxicology and Environmental Safety, 78, 8–34. [DOI] [PubMed] [Google Scholar]
- Wang, W. X. , & Rainbow, P. S. (2006). Subcellular partitioning and the prediction of cadmium toxicity to aquatic organisms. Environmental Chemistry, 3(6), 395–399. [Google Scholar]
- Wu, Q. S. , & Xia, R. X. (2006). Arbuscular mycorrhizal fungi influence growth, osmotic adjustment and photosynthesis of citrus under well‐watered and water stress conditions. Journal of Plant Physiology, 163(4), 417–425. [DOI] [PubMed] [Google Scholar]
- Xiong, Z. T. , Zhao, F. , & Li, M. J. (2010). Lead toxicity in Brassica pekinensis Rupr.: Effect on nitrate assimilation and growth. Environmental Toxicology, 21, 147–153. [DOI] [PubMed] [Google Scholar]
- Yan, K. , Wei, C. , He, X. , Zhang, G. , Sheng, X. , & Wang, L. (2010). Responses of photosynthesis, lipid peroxidation and antioxidant system in leaves of Quercus mongolica to elevated O3 . Environmental & Experimental Botany, 69(2), 198–204. 10.1016/j.envexpbot.2010.03.008 [DOI] [Google Scholar]
- Yang, Y. , Han, X. , Liang, Y. , Ghosh, A. , Chen, J. , & Tang, M. (2015). The combined effects of arbuscular mycorrhizal fungi (AMF) and lead (Pb) stress on Pb accumulation, plant growth parameters, photosynthesis, and antioxidant enzymes in Robinia pseudoacacia L. PLoS ONE, 10(12), e0145726. 10.1371/journal.pone.0145726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y. , Zhang, Y. , Wei, X. , You, J. , Wang, W. , Lu, J. , & Shi, R. (2011). Comparative antioxidative responses and proline metabolism in two wheat cultivars under short term lead stress. Ecotoxicology and Environmental Safety, 74(4), 733–740. 10.1016/j.ecoenv.2010.10.035 [DOI] [PubMed] [Google Scholar]
- Yongsheng, W. , Qihui, L. , & Qian, T. (2011). Effect of Pb on growth, accumulation and quality component of tea plant. Procedia Engineering, 18, 214–219. 10.1016/j.proeng.2011.11.034 [DOI] [Google Scholar]
- Yoon, J. , Cao, X. , Zhou, Q. , & Ma, L. Q. (2006). Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Science of the Total Environment, 368(2), 456–464. 10.1016/j.scitotenv.2006.01.016 [DOI] [PubMed] [Google Scholar]
- Zhang, X. , Zha, T. , Guo, X. , Meng, G. , & Zhou, J. (2018). Spatial distribution of metal pollution of soils of Chinese provincial capital cities. Science of the Total Environment, 643, 1502–1513. 10.1016/j.scitotenv.2018.06.177 [DOI] [PubMed] [Google Scholar]
- Zhao, L. , Xiong, J. , Li, L. P. , & Zhu, C. (2009). Low concentration of exogenous abscisic acid increases lead tolerance in rice seedlings. Biologia Plantarum, 53(4), 728. 10.1007/s10535-009-0132-x [DOI] [Google Scholar]
- Zhong, Y. P. , Qi, X. J. , Chen, J. Y. , Li, Z. , Bai, D. F. , Wei, C. G. , & Fanf, J. B. (2019). Growth and physiological responses of four kiwifruit genotypes to salt stress and resistance evaluation. Journal of Integrative Agriculture, 18(1), 87–99. 10.1016/S2095-3119(18)62011-8 [DOI] [Google Scholar]
- Zhou, J. , Jiang, Z. , Ma, J. , Yang, L. , & Wei, Y. (2017). The effects of lead stress on photosynthetic function and chloroplast ultrastructure of Robinia pseudoacacia seedlings. Environmental Science & Pollution Research, 24(11), 10718–10726. 10.1007/s11356-017-8713-2 [DOI] [PubMed] [Google Scholar]
- Zhou, Q. , & Yu, B. J. (2009). Accumulation of inorganic and organic osmolytes and their role in osmotic adjustment in NaCl‐stressed vetiver grass seedlings. Russian Journal of Plant Physiology, 56(5), 678–685. 10.1134/S1021443709050148 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


