Abstract
The domestication of plants has commonly resulted in the loss of plant defense metabolites, with important consequences for the plants' interactions with herbivores and their natural enemies. Squash domestication started 10′000 years ago and has led to the loss of cucurbitacins, which are highly toxic triterpenes. The banded cucumber beetle (Diabrotica balteata), a generalist herbivore, is adapted to feed on plants from the Cucurbitaceae and is known to sequester cucurbitacins, supposedly for its own defense. However, the evidence for this is inconclusive. In this study we tested the impact of squash domestication on the chemical protection of D. balteata larvae against a predatory rove beetle (Dalotia coriaria). We found that cucurbitacins do not defend the larvae against this common soil dwelling predator. In fact, D. balteata larvae were less attacked when they fed on cucurbitacin‐free roots of domesticated varieties compared to high‐cucurbitacin roots of wild plants. This study appears to be the first to look at the consequences of plant domestication on belowground tritrophic interactions. Our results challenge the generalized assumption that sequestered cucurbitacins protect this herbivore against natural enemies, and instead reveals an opposite effect that may be due to a tradeoff between coping with cucurbitacins and avoiding predation.
Keywords: cucurbitacins, Diabrotica, domestication, predator, sequestration, squash
1. INTRODUCTION
For thousands of years, humans have imposed strong selection on domesticated crops. This has drastically altered crop phenotypes as compared to their wild relatives (Evans, 1993, Gepts, 2004). Domesticated plants have larger plant structures, are more nutritious and have greater yields under cultivation conditions. In addition, crop selection has reduced plant architectural complexity (Chen and Welter, 2005, Andow and Prokrym, 1990) and plant specialized metabolite diversity (Gols et al., 2008, Milla et al., 2015). Empirical evidence shows that domestication has decreased plant defenses against herbivores (Turcotte et al., 2014, Chen et al., 2015, Whitehead et al., 2017, Fernandez et al., 2021). Indeed, one of the most important consequences of plant domestication is the loss or reduction of plant specialized compounds that are toxic for herbivores (Meyer et al., 2012, Chen et al., 2015, Chacón‐Fuentes et al., 2015, Rodriguez‐Saona et al., 2011), therefore making plants more vulnerable to herbivory. For different crops, domestication has a direct impact on herbivore oviposition preferences (Idris and Grafius, 1996, Cardona et al., 1990, Bellota et al., 2013, Dávila‐Flores et al., 2013), growth (Cardona et al., 1990, Szczepaniec et al., 2013, Benrey et al., 1998), and survival (Cardona et al., 1990, Gols et al., 2008, Chen and Welter, 2005). Variation in host plant quality as a result of plant domestication will indirectly affect the third trophic level, such as predators and parasitoids that rely on the nutrients ingested by their victims (Chen et al., 2015, Chen et al., 2015). Effects on these natural enemies are expected to be particularly relevant for specialized insects that sequester defense compounds from their host plants, as these are often the types of compounds that are reduced in domesticated plants.
Studies that have investigated the effect of plant domestication on natural enemies show that, overall, they perform better on herbivores that feed on domesticated crops than on their wild counterparts (Benrey et al., 1998, Gols et al., 2008, Campan and Benrey, 2004, Harvey et al., 2011, Li et al., 2018). However, most of these studies have focused on aboveground herbivores and their associated natural enemies (βet al., 2015). Little is known about the impact of plant domestication on belowground plant‐insect interactions, particularly on how altered chemical traits in domesticated plants have influenced the susceptibility of belowground herbivores to soil predators.
One classical example of reduction in chemical defense as a result of crop domestication involves species of squash, Cucurbita spp.. There is ample knowledge about the history of domestication of Cucurbita (Smith, 1997, Nee, 1990, Barrera‐Redondo et al., 2021, Kates et al., 2021, Castellanos‐Morales et al., 2018), but the consequences of squash domestication for the interaction between herbivores and their natural enemies have hardly been explored. Cucurbita plants were domesticated during different independent events in the American continent (Sanjur et al., 2002, Kates et al., 2017, Chomicki et al., 2019). The oldest evidence for squash domestication dates to about 10,000 years ago in Mesoamerica along with maize and beans (Lira et al., 2016). One of the main traits altered during the domestication of squash is the loss of cucurbitacins in the different plant organs (Nee, 1990). These defense metabolites are oxygenated tetracyclic triterpenes that are extremely bitter and render plants toxic or unpalatable to many invertebrates and vertebrate herbivores, including humans (Da Costa and Jones, 1971, Ferguson and Metcalf, 1985). However, cucurbitacins can also serve as kairomones (semiochemicals that benefit certain receivers) (Metcalf, 1986) for a number of specialized phytophagous chrysomelid beetles of the Old World tribe Luperini, in the genus Aulacophora (Jaccard et al., 2021, Chambliss and Jones, 1966, Castellanos‐Morales et al., 2018, Lewis and Metcalf, 1996). Both squash and Diabroticine beetles share origins in Mesoamerica and have coevolved over an extended period of time (Metcalf, 1979, Metcalf, 1986, Metcalf and Lampman, 1989). Earlier studies have shown that those beetles have overcome the chemical defense of Cucurbita via physiological adaptations to bitter and toxic cucurbitacins and use them as attractants and possibly as feeding stimulants (Metcalf, 1979). Moreover, it has been hypothesized that species of Diabrotica actively seek bitter squash plants to accumulate cucurbitacins in their body and use them as a defense against natural enemies (Howe et al., 1976, Ferguson and Metcalf, 1985). This hypothesis was first supported by Ferguson and Metcalf (1985), who showed that a significant proportion of laboratory‐reared adult beetles of Diabrotica species (D. balteata, D. undecimpunctata howardi, and D. virgifera virgifera) and of Acalymma vittatum fed on varieties of bitter squash, were rejected by Chinese praying mantis. Cucurbitacins were also shown to be efficient against pathogenic fungi that infect eggs and larvae of Diabrotica undecimpunctata howardi (Tallamy et al., 1998, Brusti & Barbercheck, 1992). However, the evidence that cucurbitacins effectively protect herbivores against natural enemies is scarce and has been challenged (Gould and Massey, 1984, Brusti and Barbercheck, 1992, Barbercheck, 1993, Barbercheck et al., 1995). Indeed, while several studies showed that herbivores can sequester cucurbitacins from their host plants, they did not find evidence for their protective role against a diverse array of natural enemies (arthropods, birds, mice, and toads) (Brusti & Barbercheck, 1992, Gould and Massey, 1984). For example, Barbercheck et al. (1995) found that infection by entomopathogenic nematodes was not different between rootworm larvae (D. undecimpunctata howardi) fed on bitter or non‐bitter squash, although the nematodes' fecundity was lower when their hosts fed on bitter squash compared to corn, peanuts, or non‐bitter squash. Other studies have mostly examined the role of herbivore‐sequestered cucurbitacins against natural enemies in adult beetles that feed on aboveground tissues, and/or their eggs. However, for most squash species, the content of cucurbitacins is higher in the roots than in aboveground tissues (Jaccard et al., 2021). Moreover, the earlier studies exclusively used domesticated varieties of cucurbits (either cucumber or squash) with varying levels of cucurbitacin but in general with very low levels of these compounds (Nee, 1990, Chomicki et al., 2019) as compared to their wild counterparts (Jaccard et al., unpublished data).
In this study, we first confirmed the hypothesis that cucurbitacins levels are reduced in the roots of domesticated Cucurbita argyrosperma, and next tested if this affects the susceptibility of a root larval‐herbivore to a generalist predator. To do this, we compared the preference and performance of a generalist soil predator (Dalotia coriaria, formerly known as Atheta coriaria) when offered larvae of Diabrotica balteata fed on roots of wild C. argyrosperma with expected high cucurbitacin content or on roots of related domesticated varieties where the cucurbitacins ought to be nearly absent.
2. MATERIAL AND METHODS
2.1. Plants
Cucurbita argyrosperma, known as “calabaza pipiana” is an important crop in local agricultural systems in Mexico (Sánchez‐de la Vega et al., 2018). The oldest evidence of its domestication is ~8600 years old from the Xihuatoxtla shelter, in the state of Guerrero (Ranere et al., 2009). Plants can be found in tropical and semi desertic regions from the Southeastern United States through Mexico and Northern Central America (Lira et al., 2016). This is a diverse species in form, color and size of its seeds and fruits (Lira‐Saade, 1995).
Fruits of two wild C. argyrosperma populations (Wild Umar [WU] and Wild Bacocho [WB]) were collected in the region of Puerto Escondido along the Pacific coast in the state of Oaxaca, Mexico in January 2018, and their seeds were harvested (WU: 15°92′49.2” N, 97°15′09.77”W and WB: 15°86′44.6”N, −97°08′11.4″). The weather conditions in this region are hot and humid with an average temperature of 27°C and 84% relative humidity. Seeds of domesticated varieties were purchased from the KCB‐Samen GmbH Bottmingen, Schweiz. We used four varieties that have been selected for two different purposes, two varieties selected for fruit consumption: Vera Cruz Pepita (FVP) and Silver Edge (FSE) and two varieties selected as ornamentals: Cushaw Tricolor (OCT) and Navajo calabacita (ONC). These varieties had been previously used in a related study in which we found differences in their cucurbitacin content (Jaccard et al. unpublished results).
Depending on the experiment, either one individual seed or seven seeds of domesticated varieties were germinated in one individual plastic pot filled with a mixture of soil: sand 70:30 (Einheitserde, Sinntal‐Altengronau, Germany). As wild seeds take longer to germinate, they were planted 10 days before the domesticated seeds. Also, wild seeds were subjected to a specific germination procedure to enhance their germination rate: seed coats were pierced, scratched on both sides and placed in groups of 10 in a square Petri dish with wet cotton for 1 week in an incubator at 28 °C degrees in the dark. Germinated wild seeds were transferred following the same procedure as seeds from domesticated varieties. Plants with two cotyledons and two fully developed leaves (15 day‐old) were used for all the experiments. All plants were grown under controlled conditions in a greenhouse (24 ± 5°C, L, D 16:8 h) and watered every other day.
2.2. Insects
The banded cucumber beetle (Diabrotica balteata (LeConte, 1865, Coleoptera: Chrysomelidae) originates from the tropical Americas (Teng et al., 1984, Moreira et al., 2015, Pitre & Kantack, 1962) and is considered a pest of agricultural crops including beans, sweet potatoes, and cucurbits. Larvae feed belowground on roots, while adults eat leaves and flowers (Pitre & Kantack, 1962, Teng et al., 1984). Eggs used to establish our in‐house colony were supplied by Syngenta (Stein, Switzerland). Emerging larvae were maintained in soil (Sinntal‐Altengronau, Deutschland), fed on roots of 4‐day‐old maize seedlings (Hybrid DFI 45321, DEFI genetics AG, Switzerland), and kept at 25 ± 2°C, 60% RH, and 16:8 h L/D cycles. First and second instar larvae were used in all experiments.
The rove beetle Dalotia coriaria Kraatz, Coleoptera: Staphylinidae (formerly Atheta coriaria, [Gusarov, 2003]), is a soil‐dwelling predator used as a biological control agent for certain greenhouse pests (Birken and Cloyd, 2007). Both larvae and adults feed on various arthropod pests (Helyer et al., 2003). It is a generalist predator that did not coevolve with chrysomelids and is not expected to be adapted to the potentially deterrent and toxic effects of cucurbitacins (Miller and Williams, 1983). Eggs were purchased from Andermatt Biocontrol (AG, Switzerland) and a laboratory rearing was established. Predators were kept in controlled conditions (24°C, 60% R.H. and L:D 16:8 h) with coconut fiber and vermiculite as a substrate and fed on oat and dog pellets. Adults of D. coriaria were used in all experiments.
2.3. Cucurbitacin content in roots of wild and domesticated plants and in larvae of D. balteata
To verify the cucurbitacin content in the squash roots of wild accessions and domesticated varieties and in the larvae that fed on these roots, we quantified the cucurbitacin content in the roots of the different plants (N = 4 plants/host plant treatment) and in the larvae of D. balteata (N = 3 per time point) fed on roots of either wild or domesticated plants. The cucurbitacin content in the larvae was measured with dead larvae, because the feeding preference bioassays were performed with frozen larvae.
Roots of 2‐week‐old plants of C. argyrosperma were harvested, immediately flash frozen and ground to a fine powder in liquid nitrogen. Then 100 mg of sample powder was weighed with a microbalance (Mettler Toledo XP&, Columbus, Ohio, USA) and put in a 1.5‐ml Eppendorf tube. We added 1 ml of MeOH 100% and five glass beads per tube.
Larvae of D. balteata fed on roots for 7 days were collected and immediately frozen at −80°C. Larvae were taken out of the freezer and we quantified the content of cucurbitacins in the larval body 2 h and 24 h later. Twenty mg (12 larvae in average) per sample was weighed with a microbalance (Mettler Toledo XP&) and put in a 1.5‐ml Eppendorf tube to which 250 μL of MeOH 100% was added. The samples were ground with a pellet pestle, then another 250 μL of MeOH 100% were added (total of 500 μL in the tube per sample).
All samples (roots and larvae) were vortexed and centrifuged at 4°C (10 min, 9000 rpm). The supernatants from the larvae samples were filtered (13 mm Syringe filter, PTFE hydrophilic, 0.22 μm, BGB, CHE) before the analysis. Supernatants were used for analysis by liquid chromatography‐mass spectrometry as described by Jaccard et al. (2021).
Briefly, the analysis of cucurbitacins was performed by UHPLC‐QTOFMS using an Acquity UPLC™ coupled to a Synapt G2 high‐resolution mass spectrometer (Waters). The column used for chromatography was an Acquity UPLC BEH C18 1.7 μm, 2.1 × 50 mmm (Waters). Data acquisition was performed with the software Masslynx™ v.4.1 (Waters). Cucurbitacins were identified based on their molecular formula and fragmentation patterns provided by accurate mass measurements, and using available databases such as the Dictionary of Natural Product (CRC Press). In some cases, the presence of several possible cucurbitacin isomers prevented full identification. Peaks corresponding to known cucurbitacins were automatically integrated using TargetLynx XS™ with a 0.1‐min chromatographic window centered on the retention time of each component and a 0.02‐Da mass window centered on the (M + HCOO) ion. Quantification of all cucurbitacins was done by external calibration using cucurbitacin B solutions at 0.02, 0.08, 0.4, 2, 5, and 10 μg/ml The cucurbitacin concentration was expressed in μg per g of fresh plant or larva material.
2.4. Preferences of D. coriaria for D. balteata larvae fed on different wild populations and cultivated varieties of squash
With this experiment, we tested the hypothesis that predators will preferentially select D. balteata larvae that were fed on the low‐cucurbitacin plants. For this, we evaluated the preference of D. coriaria adults for D. balteata larvae fed on roots either of wild populations or domesticated varieties in a choice test. Second instar larvae were fed on high‐cucurbitacin roots of two wild populations of C. argyrosperma (WB and WU) and no‐cucurbitacin roots of four domesticated varieties (FVP, FSE, OCT, and ONC) for 7 days. Then, larvae were frozen at −80°C. The predation assay was performed with dead‐frozen larvae to allow us to standardize the size of the larvae and distinguish the high‐cucurbitacin larvae versus no‐cucurbitacin larvae in their marked spot in the Petri dish. The experiment was performed in a red Petri dish to simulate darkness (as in the soil) (Sarstedt, Ø 8 cm). To maintain humidity, 850 μL of water was added on the filter paper placed in the Petri dish. Adults were starved for 12 h prior to use. One D. balteata larva fed on roots of wild squash (either WB or WU) with cucurbitacins was placed on one side on the Petri dish, and on the opposite side (approximatively 7 cm distant from each other), one larva fed with no‐cucurbitacin roots (either FSE, FVP, OCT, or ONC). One adult rove beetle was released in the center of the arena. Petri dishes were sealed with parafilm to prevent the predator from escaping. A total of eight combinations were performed, always one high‐cucurbitacin larva against one no‐cucurbitacin larva: WB × ONC, WB × OCT, WB × FSE, WB × FVP, WU × ONC, WU × OCT, WU × FSE, WU × FVP with 20 replicates per combination and the experiment was performed twice. The predation rate was recorded after 24 h. A predation event was recorded when the hemolymph of the larva was found leaking from the body or when body parts were removed (Figure S1). Larvae without any signs of damage/attack were considered not predated. As comparison (control) for the predation damage/attack, to account for natural deterioration of the dead larvae, we also had five Petri dishes without a predator.
2.5. Predator survival on D. balteata larvae fed on different wild populations and cultivated varieties of squash
We tested the hypothesis that cucurbitacins sequestered by D. balteata larvae can be deleterious for the predators. Thus, we evaluated D. coriaria survival after they were fed on dead D. balteata larvae that had been reared on wild populations (high‐cucurbitacin roots) or on domesticated varieties (no‐cucurbitacin roots) of C. argyrosperma for 7 days. The predators were individually placed in cells of a tray (each with 32 cells) and fed with one of seven diet treatments (n = 32; see Supplementary Figure 2): D. balteata larvae that fed on either roots of two wild populations (WB and WU) or roots of four domesticated varieties (FVP, FSE, OCT, and ONC) and one control diet consisting of frozen eggs of Ephestia sp. (Pyralidae) (Andermatt Biocontrol, Switzerland). Transparent stickers were used to cover the top of each cell to prevent predator escape. We added a moist filter paper on the bottom of each cell as a source of water and to maintain humidity. For 7 days, every 24 h, predator survival was evaluated and the diet was replaced. The trays were kept in the dark to simulate soil darkness. If a predator escaped the cell, the replicate was removed from the analysis (between one to five replicates were lost per treatment).
2.6. Performance and survival of D. balteata larvae on roots of wild populations and cultivated varieties in the presence of a predator
In the previous experiments the predators were exposed to D. balteata larvae after they fed on the different plant treatments. In this experiment, we wanted to examine the effect of the plant on the predator’s feeding behavior and survival under more realistic conditions. Thus, larvae were exposed to the predator while still feeding on the plant. We tested two different hypotheses. The first hypothesis was that larvae reared on roots of wild populations will suffer lower predation than larvae fed on domesticated varieties, and the second hypothesis was that predator survival will be higher when offered larvae fed with roots of domesticated varieties with lower cucurbitacin content. In contrast, to the previous experiments, here we used living larvae. We carefully placed the root system of 2‐week‐old squash plants (n = 20, Figure S3) from all plants (two wild populations and four domesticated varieties) into a plastic bag. The plants were left for 3 days under laboratory conditions (L: D 16:8 h) to acclimate to the new environment. Then, we added 10, second instar larvae of D. balteata in each plastic bag and allowed them to move and feed freely on the roots. Bags were closed under the cotyledons with elastic film (Parafilm; Pechiney Plastic Packaging; Menasha) to prevent larvae from escaping (Figure S2). Larvae were left in the bags for 5 days, then on day 6, we added 20 predators (D. coriaria) in half of the bags of each plant treatment. The bags without predators were used as a control for survival and growth of D. balteata in the absence of predators. Bags were maintained in the lab at room temperature (22°C ± 2°C) and natural light conditions (L: D 16:8 h) for 4 days. At the end of this period, the bags were opened inside an insect rearing cage to prevent the predators from escaping. The presence and number of both D. balteata larvae and predators were recorded. The 4‐day period was chosen to allow enough time for predation and before the pupation of D. balteata larvae.
To calculate D. balteata larval relative growth rate (RGR), the total weight of the 10 larvae was recorded at the start of the experiment and then 9 days later. For both time points, the individual mean larval weight was calculated by dividing the sum of the weight of the initial (start of the experiment) or recovered larvae (end of the experiment) by the total number of larvae present.
2.7. Statistical analyses
Statistical tests were carried out with Sigma Plot software (v. 11; Systat Software Inc.) and R statistical software (v. 4.0.0; R Development Core Team, 2020) and its complementary console R‐studi, using Analysis of Deviance (ANODEV; a maximum likelihood equivalent of ANOVA), followed by residual analysis to verify suitability of distributions of the tested models (Shapiro–Wilk test). We installed the extra R packages “lme4”, “lmerTest”, and “emmeans” to analyze our data.
Krustal–Wallis test followed by a pairwise comparison using Wilcoxon rank sum test with continuity correction were used to analyze the total amount of cucurbitacin content in the plant’s roots. The cucurbitacin concentration in larval bodies was first log transformed before the analysis and then analyzed with a Linear Model (lm) followed by least squares means (LSMeans) to compare among host plant food source (wild populations vs. domesticated varieties) for the two different time points. A partial least squares discriminant analyses (PLS‐DA) and hierarchical clustering heatmap were carried using MetaboAnalyst 5.0 to check for differences in cucurbitacin profile among wild population of C. argyrosperma and D. balteata larvae.
Predator feeding preference (choice‐test) was analyzed for each treatment combination with a generalized linear model (glm) with binomial distribution. Least Squares Means (LSMeans) were used to compare differences among treatments.
The survival of the predator on D. balteata larvae previously fed on different squash roots and Ephestia eggs (control) was analyzed using Kaplan–Meier estimator by log‐rank method with the Sigma Plot software.
Relative growth rate (RGR) and survival rate of D. balteata larvae in the presence or absence of the predator was first log‐transformed, then analyzed with a linear model (LM) with variety/population as explanatory variables followed by least squares means (LSMeans) to compare among host plant treatments. The RGR and survival rate differences among larvae fed on wild squash plants and domesticated varieties selected for different purposes (consumption or ornamental) was analyzed with a generalized linear mixed model (lmer) with purpose of domestication as the explanatory variable and wild populations and domesticated varieties as random factors.
3. RESULTS
3.1. Cucurbitacin content in roots of wild and domesticated plants and in larvae of D. balteata
The total amount of cucurbitacins in the roots were significantly different between wild populations and domesticated varieties (χ 2 [5] = 26.805, p = 0.0001, Figure 1a). Roots of all four domesticated varieties, did not contain any cucurbitacins, whereas the roots of the two wild populations contained high concentrations of cucurbitacins (more than 10 μg/g). No significant differences in cucurbitacin content were found between the two wild populations (WB‐WU: p = 0.264, Figure 1a).
FIGURE 1.
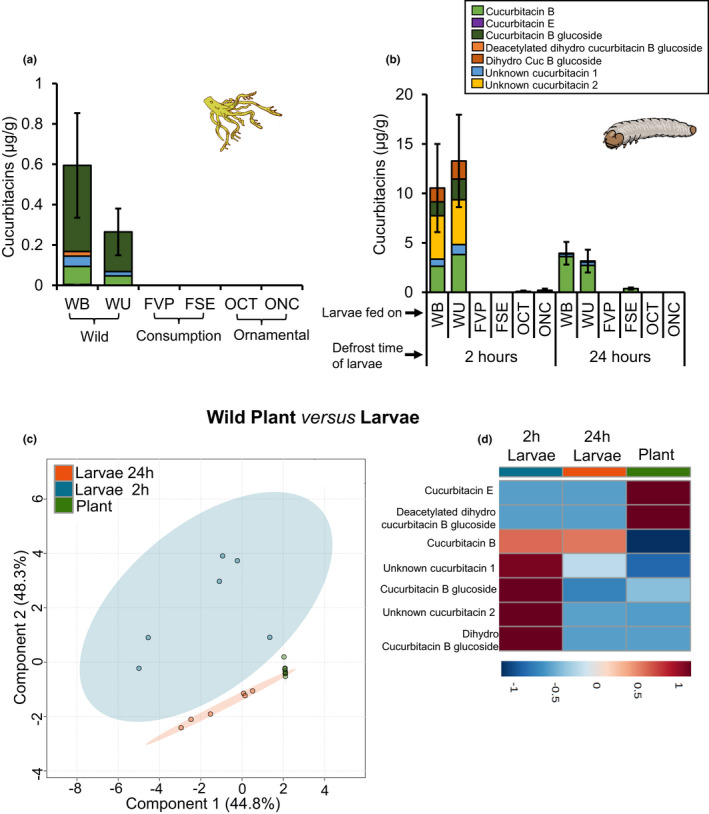
Differences in cucurbitacins profiled in C. argyrosperma roots and D. balteata larvae. (a) Cucurbitacin levels in squash roots (n = 4) of 2‐week‐old plants of wild populations (WU and WB), consumption (FVP and FSE) and ornamental varieties (OCT and ONC). (b) Cucurbitacin content in 2 and 24 h defrosted larvae (n = 3) that fed on roots of wild plants, consumption and ornamental varieties for 7 days. Bars represent the mean (±SE). (c) Results of a discriminant analysis (PLS‐DA) and (d) hierarchical clustering heatmaps of the cucurbitacins present in roots of squash and larvae that fed on these roots. Different letters indicate significant differences between treatments within each time point (p values are given for treatments [generalized linear model (family, Gaussian)] followed by pairwise comparisons of least squares means (LSMeans). **p < 0.01, ***p < 0.001
In accordance with the cucurbitacin content found in the plants, we found that 2 h after defrosting, larvae that had fed on roots of wild plants contained cucurbitacins in their body, while larvae that had fed on the domesticated varieties did not (F 3,12 = 10.61, p = 0.001; Figure 1b). No significant difference was found in cucurbitacin content between larvae that had fed on roots of the two wild populations (t = 0.615, p = 0.925, Figure 1b). After 24 h outside the freezer, the cucurbitacin content in the larvae drastically decreased and the difference between larvae fed on wild plants and domesticated varieties was less evident (F 3,12 = 3.481, p = 0.038). Results clearly show that the larvae actively sequester and accumulate the cucurbitacins, since the levels in their body were much higher compared to the amount found in the roots (Figure 1a,b). Interestingly, the cucurbitacin profiles differed between the roots and the larvae that fed on these roots (Figure 1c,d). Two cucurbitacins (Cucurbitacin E and deacetylated dihydro cucurbitacin B glucoside (or isomer) were exclusively found in the roots (Table S1).
In the 2 h‐defrosted larvae we found in total five compounds, but after 24 h only three. The main differences observed between roots and larvae were after 2 h, the larvae had high amounts of an isomer of a dihydro form of Cuc B glucoside and an unknown Cuc (Figure 1a–d). However, these compounds disappeared after 24 h. This difference could be due to either degradation or transformation during the defrosting process.
3.2. Cucurbitacin content in D. balteata larvae does not affect the feeding preference of D. coriaria
The predator D. coriaria did not distinguish between D. balteata larvae that had fed on high‐cucurbitacin roots (WB and WU) or on no‐cucurbitacin roots (FSE, FVP, OCT, and ONC) (Figure 2). The predators were apparently not deterred by the cucurbitacins present in the larvae. Predator preference was recorded as significant for two of the combinations, both include an ornamental variety and the wild population WU. Nevertheless, the preference appears not to be linked to cucurbitacin‐content, as in one case predators significantly chose larvae fed with domesticated roots (OCT, no‐cucurbitacin roots) over the larvae that ate wild squash roots (WU) (p = 0.04) and in the other case they showed the opposite pattern, larvae that fed on wild (WU, high‐cucurbitacin roots) over larvae that fed on domesticated roots (ONC) (p = 0.03).
FIGURE 2.
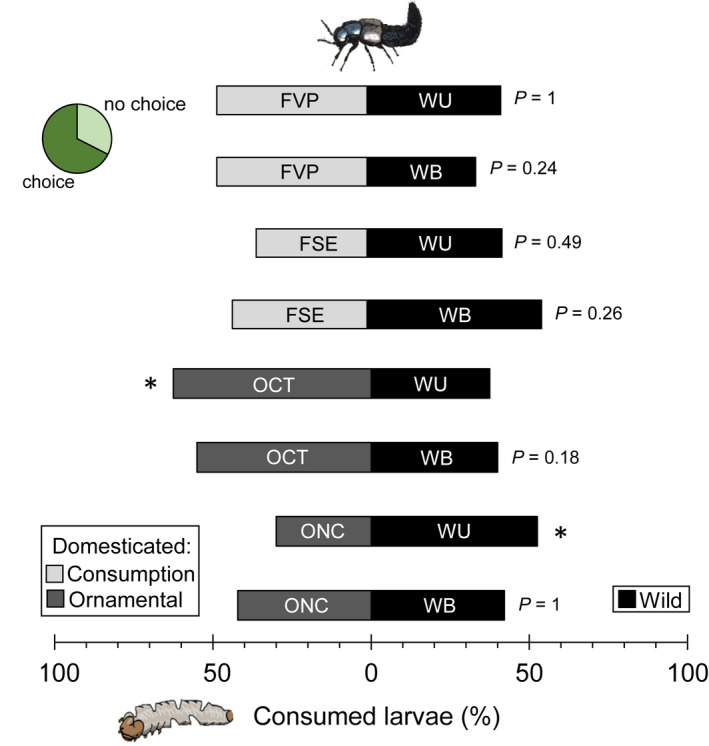
Predation preference by D. coriaria for D. balteata larvae that fed on roots of wild populations (n = 40, WU and WB = black) with cucurbitacins and larvae that fed on domesticated varieties (fruit consumption: FVP and FSE and ornamental: OCT and ONC, shades of gray, n = 40) without cucurbitacins. Feeding preference was evaluated after 24 h. Bars are the percentages of beaten larvae. Pie chart indicate the overall proportion of predators that ate or not during the assay for all treatment combine. Bonferroni corrected p values are given for treatment comparisons [generalized linear model (family, Binomial)], followed by pairwise comparisons of least squares means (LSM). *p < 0.05
3.3. Predator survival is not affected by the content of cucurbitacins in D. balteata larvae
The survival of the predator (D. coriaria) was not affected by the content of cucurbitacins in D. balteata larvae fed with roots of wild or domesticated plants (p = 0.214; Figure 3). After 7 days, predator survival was between 76% and 93% and no significant differences were found among plant treatments as compared to the control.
FIGURE 3.
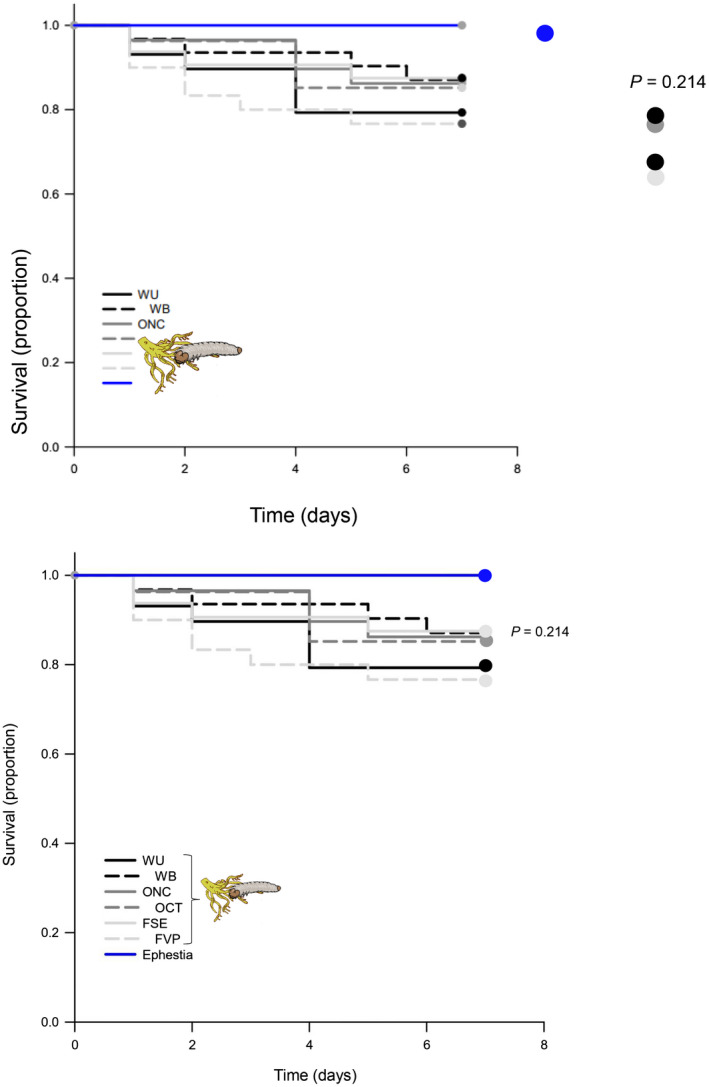
Survival of D. coriaria exposed to D. balteata larvae fed with roots of wild populations (WU: Black (n = 29), WB: Black (n = 31)) and domesticated varieties (fruit consumption: FVP (n = 30) and FSE (n = 32) and ornamental: OCT (n = 27) and ONC (n = 30), shades of gray) and Ephestia eggs used as control (n = 30, blue). The y axis indicates the proportion of survived predators. The x axis represents the time in days. Larvae were frozen 2 h after collection from the squash roots and defrosted 30 min before the start of the experiment. The effect of treatment on predator survival was analyzed using Kaplan–Meier estimator by log‐rank method
3.4. Predation of D. balteata by D. coriaria is affected by larval plant‐diet
The survival of D. coriaria was high when placed in the bags with the D. balteata larvae feeding on squash roots. Between 18 and 20 predators were consistently recovered from the bags at the end of each experiment. The fact that the predators did eat the larvae of D. balteata (Figure S4), indicates that the cucurbitacins present in the roots of wild plants did not affect their survival.
The RGR of D. balteata larvae was not significantly different among wild populations and domesticated varieties with or without predators (with predator: F 5,46 = 0.75, p = 0.59, Figure 4a, without predator: (F 5,49 = 0.82, p = 0.53, Figure 4b). Similarly, when pooled by domestication status or purpose of domestication (wild, fruit consumption, and ornamental use), we did not find significant differences in larval RGR (F 2,49 = 1.664, p = 0.199).
FIGURE 4.
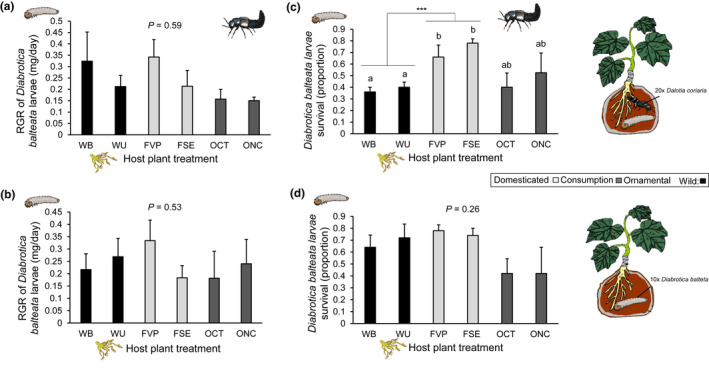
Relative growth rate of D. balteata larvae feeding on roots of wild populations of C. argyrosperma (WB and WU = black, n = 10) or domesticated varieties (FVP and FSE selected for fruit consumption, OCT and ONC selected for an ornamental use, shades of gray, n = 10) with (a) and without (b) predators after 9 days. Survival of D. balteata larvae with (c) and without (d) predators. Larvae fed on roots of wild populations of C. argyrosperma (WB and WU = black, n = 10) or with domesticated varieties (FVP and FSE selected for fruit consumption, OCT and ONC selected for an ornamental use, shades of gray, n = 10) after 9 days. Bars are RGR (A and B) or the proportion of survived larvae (C and D) (+SE). Letters indicate the difference on herbivore survival among host plant treatments with predator presence. p‐values are given for differences among host plant treatments (wild populations and domesticated varieties) [generalized linear model]. *** indicates significant differences between wild populations and domesticated varieties selected for fruit consumption
In contrast, predator presence had a significant effect on larval survival across plant treatments (F 5,48 = 4.23, p = 0.002, Figure 4c). When pooled by domestication status and purpose, survival of D. balteata larvae was lower when feeding on roots of wild populations than on roots of domesticated varieties selected for consumption (F 2,51 = 10.506, p = 0.0001, Figure 4c), but not different from larvae feeding on ornamental plants. However, in the absence of predators in the bag, no significant differences in larval survival were found among host plant treatments (F 5,50 = 1.059, p = 0.394, Figure 4d) nor among purpose of domestication (F 2,53 = 2.428, p = 0.09).
4. DISCUSSION
The most important trait selected during the domestication of the genus Cucurbita (squash) is the absence of cucurbitacins in the fruits (Navot et al., 1990, Gry, 2006). We confirm here for C. argyrosperma that cucurbitacins were also selected out from the roots of domesticated varieties (Figure 1a).
Diabrotica balteata, as many other species of Chrysomelidae in the subfamily Galerucinae is capable of sequestering cucurbitacins through the ingestion of plant tissue (Metcalf, 1979, Metcalf, 1986, Metcalf and Lampman, 1989). The assumed reason for this is that sequestered cucurbitacins provide protection against natural enemies (Nishida and Fukami, 1990, Nishida et al., 1992). Because of the lower levels of cucurbitacins in domesticated squash, we hypothesized that D. balteata larvae feeding on roots of wild plants would be more protected from predators than larvae feeding on no‐cucurbitacin roots of domesticated varieties. We tested this for the predatory rove beetle D. coriaria expecting lower preference for, and survival on, beetle larvae fed with roots of wild plants than when offered larvae that had fed on roots of domesticated varieties.
Our analyses of cucurbitacin content in the larvae showed that they sequester cucurbitacins when feeding on these roots. Yet, contrary to our prediction, larvae of D. balteata fed on roots of wild squash were not protected from predation by D. coriaria. D. coriaria survival was not different when offered beetle larvae fed with high‐cucurbitacin roots (wild populations) or no‐cucurbitacins roots (domesticated varieties). Moreover, in the choice‐test the larvae from the different plant treatments were equally attacked by the predator. The sequestration ability of D. balteata larvae was further confirmed in a recent study with bitter and non‐bitter cucumber plants (Bruno et al. 2021, unpublished data) in which it was also found that this does not provide any protection against this and other natural enemies, as detailed below.
Of further interest is the fact that cucurbitacin profiles were different between plants and sequestering larvae (Table S1). Diabrotica balteata larvae contained high amounts of an isomer of a dihydro form of Cucurbitacin B glucoside and an unknown cucurbitacin, but these two compounds were not found in the plants. This implies that the cucurbitacins ingested by D. balteata larvae are converted into different compounds and then stored in their tissues. These results are in line with those of numerous studies that found that herbivores that sequester plant compounds transform them into different compounds (Trigo et al., 1994, Hartmann et al., 2005). We also found that the concentrations of cucurbitacins in the larvae were much higher than in the roots, indicating true sequestration. At the beginning of the predator survival and choice bioassays, the larvae from wild squash contained more than 10 μg/g cucurbitacins, whereas larvae that had fed on domesticated squash contained barely detectable amounts (Figure 1b). After being defrosted for 24 h, the difference was still evident, but cucurbitacin content had diminished to less than 5 μg/g, most likely due to larval decomposition.
Another unexpected result of this study was that the RGR of D. balteata larvae was similar when feeding on roots of wild plants and of domesticated varieties. In a different study using these same and other wild populations and domesticated varieties of C. argyrosperma, we found very large differences in RGR rate; D. balteata larvae grew slower on wild roots than on roots of domesticated varieties (Jaccard et al., unpublished results). In the current study, treatments consisted of feeding the larvae on plants with high (wild) or no‐cucurbitacin (domesticated) content. It is possible that not only presence or absence, but also small variations in cucurbitacins between cultivars and wild plants, as well as within plant treatments may account for differences between experiments. Recent studies have documented the importance of chemical variation in plant defenses for herbivore and natural enemy performance in unpredictable and nonlinear ways (Pearse et al., 2018, Paul et al., 2021, Hauri et al., 2021). For instance, Hauri et al. (2021) found that the performance of generalist caterpillars of the cabbage looper Trichoplusia ni and their interaction with the generalist predator Podisus maculiventris, depended not so much on the cultivar’s chemotype but rather on the chemotype mixture. They also found differences between the performance of males and females of T. ni when predators were present, suggesting that differences between the sexes in their needs for growth and development could force larger male caterpillars to move more and feed on more chemotypes than smaller females. Future studies on the effects of cucurbitacins on D. balteata and its interactions with natural enemies should consider possible differences in the effects on male and females throughout the insects’ development.
Interestingly, when larvae were exposed to the predators under more natural conditions in the soil (bag experiment), predation was higher on larvae feeding on the wild plants. This further supports the findings that the predator was neither deterred, nor harmed by the cucurbitacins present in the herbivore’s body. The higher predation rates on wild‐fed larvae may have something to do with these larvae developing slower and/or being weaker. However, it would be surprising if early development of D. balteata larvae on cucurbitacins containing plants is slower, because it is known that cucurbitacins have a phagostimulant effect on D. balteata (Eichenseer and Mullin, 1996, Halaweish et al., 1999, Howe et al., 1976, Tallamy and Halaweish, 1993). For example, when pure cucurbitacin B was added to soybean leaves, Diabrotica beetles exhibited enhanced feeding on this plant, which it normally rarely attacks (Metcalf et al., 1980). Similarly, Kim and Mullin (2003) found that adding cucurbitacin B (0.2 nmol/disk) on a cellulose disk stimulated feeding by the western corn rootworm (D. virgifera virgifera). Lang et al. (2013) found that for another cucurbit specialist, the coccinellid Epilachna paenulata, the phagostimulation properties of cucurbitacins were due to only one type of cucurbitacin among 28 tested. Diabrotica beetles share similar stimulatory thresholds to various cucurbitacins irrespective of their degree of Cucurbitaceae specialization, with cucurbitacin B believed to be the most phagostimulatory compound (Metcalf et al., 1982, Tallamy et al., 1997). Given that there appear to be no advantages for D. balteata larvae to feed on cucurbitacin‐containing roots, it remains unclear why their feeding is stimulated by these compounds.
Based on the inconsistent preference of the predator for larvae from the high or free‐cucurbitacin treatment, their similar survival on the two types of larvae and the higher predation on D. balteata larvae from high‐cucurbitacin roots, we can conclusively reject the idea that cucurbitacins provide protection against this predator. Evidence from earlier studies corroborates this conclusion (Ferguson and Metcalf, 1985, Tallamy et al., 1998, Metcalf, 1986). Moreover, it has never been shown that cucurbitacins have a lethal effect on any invertebrate and the only indication of their toxicity is the increased mortality in some adult chrysomelids when consuming these compounds at high doses and in vertebrates such as, cows and sheep (Metcalf, 1979, Metcalf, 1986, Metcalf and Lampman, 1989, Breyer‐Brandwijk, 1962). Thus, to date the earlier study by Ferguson and Metcalf (1985) is the only one showing a negative effect of herbivore‐sequestered cucurbitacins on a predator. In a recent study we tested a wide range of natural enemies including entomopathogenic nematodes, insect predators (also D. coriaria), and pathogens (fungi and bacteria) and found that sequestered‐cucurbitacins by D. balteata larvae fed on bitter cucumber varieties did not provide any protection against any of these natural enemies (Bruno et al. 2021, unpublished data). It is therefore increasingly evident, that this commonly assumed protection is not the reason for Diabrotica larvae to preferentially feed on Cucurbitaceae and sequester cucurbitacins.
In conclusion, our results show that for C. argyrosperma, the dramatic reduction of cucurbitacins as a result of domestication, does not increase the susceptibility of root‐feeding larvae to predatory rove beetles. Instead, larvae feeding on high‐cucurbitacin roots suffered higher predation. Thus, our results do not support the common assumption that sequestered cucurbitacins confer protection against natural enemies. This logically leads to the rejection of our hypothesis that squash domestication has had a positive effect on the third trophic level. The effects of domestication on the third trophic level, particularly pertaining belowground organisms, have been largely neglected. These types of studies can help to identify specific plant traits that could enhance direct and indirect crop resistance to insect pests. Moreover, the fact that D. coriaria is not negatively affected by cucurbitacins, could make it a suitable natural enemy in biological control efforts against D. balteata and other squash pests.
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AUTHOR CONTRIBUTIONS
C.J, C.C.M.A, and B.B. originally formulated the idea. C.J., B.B. C.C.M.A, and N.M. designed the experiments. C.J. and N.M performed all the experiments and wrote the first version of the manuscript. G.C and P.B developed the cucurbitacin extraction and analysis method and identified the cucurbitacins. N.M, C.C.M.A, and C.J analyzed the data. N.M did the drawings. All co‐authors contributed to the writing of the last version of the manuscript.
Supporting information
Supplementary Material S1
ACKNOWLEDGMENTS
The authors thank Olivier Kindler and his team at Syngenta Crop protection for providing eggs of Diabrotica balteata. The authors also thank Anthony Pignal and Audrey Duhin for their technical help during the experiments. This research was financed by a grant from the Swiss National Science Foundation (Project No: 310030_197463) awarded to Betty Benrey.
Jaccard, C. , Marguier, N. T. , Arce, C. C. , Bruno, P. , Glauser, G. , Turlings, T. C. & Benrey, B. (2022). The effect of squash domestication on a belowground tritrophic interaction. Plant‐Environment Interactions, 3, 28–39. 10.1002/pei3.10071
DATA AVAILABILITY STATEMENT
All the data are presented in figures, tables, and Supporting Information.
REFERENCES
- Andow, D. , & Prokrym, D. (1990). Plant structural complexity and host‐finding by a parasitoid. Oecologia, 82, 162–165. [DOI] [PubMed] [Google Scholar]
- Barbercheck, M. E. (1993). Tritrophic level effects on entomopathogenic nematodes. Environmental Entomology, 22, 1166–1171. [Google Scholar]
- Barbercheck, M. E. , Wang, J. , & Hirsh, I. (1995). Host plant effects on entomopathogenic nematodes. Journal of Invertebrate Pathology, 66, 169–177. [Google Scholar]
- Barrera‐Redondo, J. , Sanchez‐De La Vega, G. , Aguirre‐Liguori, J. A. , Castellanos‐Morales, G. , Gutiérrez‐Guerrero, Y. T. , Aguirre‐Dugua, X. , Aguirre‐Planter, E. , Tenaillon, M. I. , Lira‐Saade, R. , & Eguiarte, L. E. (2021). The domestication of Cucurbita argyrosperma as revealed by the genome of its wild relative. Horticulture Research, 8, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellota, E. , Medina, R. F. , & Bernal, J. S. (2013). Physical leaf defenses–altered by Zea life‐history evolution, domestication, and breeding–mediate oviposition preference of a specialist leafhopper. Entomologia Experimentalis et Applicata, 149, 185–195. [Google Scholar]
- Benrey, B. , Callejas, A. , Rios, L. , Oyama, K. , & Denno, R. F. (1998). The effects of domestication of brassica and Phaseolus on the interaction between phytophagous insects and parasitoids. Biological Control, 11, 130–140. [Google Scholar]
- Birken, E. M. , & Cloyd, R. A. (2007). Food preference of the rove beetle, Atheta coriaria Kraatz (coleoptera: Staphylinidae) under laboratory conditions. Insect Sci., 14, 53–56. [Google Scholar]
- Breyer‐Brandwijk, M. G . 1962. The Medicinal and Poisonous Plants of Southern and Eastern Africa being an Account of their Medicinal and other Uses, Chemical Composition, Pharmacological Effects and Toxicology in Man and Animal. The Medicinal and Poisonous Plants of Southern and Eastern Africa being an Account of their Medicinal and other Uses, Chemical Composition, Pharmacological Effects and Toxicology in Man and Animal.
- Brusti, G. E. , & Barbercheck, M. E. (1992). Effect of dietary cucurbitacin C on southern corn rootworm (coleoptera: Chrysomelidae) egg survival. Environmental Entomology, 21, 1466–1471. [Google Scholar]
- Campan, E. , & Benrey, B. (2004). Behavior and performance of a specialist and a generalist parasitoid of bruchids on wild and cultivated beans. Biological Control, 30, 220–228. [Google Scholar]
- Cardona, C. , Kornegay, J. , Posso, C. E. , Morales, F. , & Ramirez, H. (1990). Comparative value of four arcelin variants in the development of dry bean lines resistant to the Mexican bean weevil. Entomologia Experimentalis et Applicata, 56, 197–206. [Google Scholar]
- Castellanos‐Morales, G. , Paredes‐TORRES, L. M. , Gámez, N. , Hernández‐Rosales, H. S. , Sánchez‐de la Vega, G. , Barrera‐Redondo, J. , Aguirre‐Planter, E. , Vázquez‐Lobo, A. , Montes‐Hernández, S. , & Lira‐Saade, R. (2018). Historical biogeography and phylogeny of Cucurbita: Insights from ancestral area reconstruction and niche evolution. Molecular Phylogenetics and Evolution, 128, 38–54. [DOI] [PubMed] [Google Scholar]
- Chacón‐Fuentes, M. , Parra, L. , Rodriguez‐Saona, C. , Seguel, I. , Ceballos, R. , & Quiroz, A. (2015). Domestication in murtilla (Ugni molinae) reduced defensive flavonol levels but increased resistance against a native herbivorous insect. Environmental Entomology, 44, 627–637. [DOI] [PubMed] [Google Scholar]
- Chambliss, O. L. , & Jones, C. M. (1966). Cucurbitacins: Specific insect attractants in Cucurbitaceae. Science, 153, 1392–1393. [DOI] [PubMed] [Google Scholar]
- Chen, Y. H. , Gols, R. , & Benrey, B. (2015). Crop domestication and its impact on naturally selected trophic interactions. Annual Review of Entomology, 60, 35–58. [DOI] [PubMed] [Google Scholar]
- Chen, Y. H. , Gols, R. , Stratton, C. A. , Brevik, K. A. , & Benrey, B. (2015). Complex tritrophic interactions in response to crop domestication: Predictions from the wild. Entomologia Experimentalis et Applicata, 157, 40–59. [Google Scholar]
- Chen, Y. H. , & Welter, S. C. (2005). Crop domestication disrupts a native tritrophic interaction associated with the sunflower, Helianthus annuus (Asterales: Asteraceae). Ecological Entomology, 30, 673–683. [Google Scholar]
- Chomicki, G. , Schaefer, H. , & Renner, S. S. (2019). Origin and domestication of Cucurbitaceae crops: Insights from phylogenies, genomics and archaeology. New Phytologist., 226(1240), 1255. [DOI] [PubMed] [Google Scholar]
- Da Costa, C. P. , & Jones, C. (1971). Cucumber beetle resistance and mite susceptibility controlled by the bitter gene in Cucumis sativus L. Science, 172, 1145–1146. [DOI] [PubMed] [Google Scholar]
- Dávila‐Flores, A. M. , Dewitt, T. J. , & Bernal, J. S. (2013). Facilitated by nature and agriculture: Performance of a specialist herbivore improves with host‐plant life history evolution, domestication, and breeding. Oecologia, 173, 1425–1437. [DOI] [PubMed] [Google Scholar]
- Eichenseer, H. , & Mullin, C. A. (1996). Maxillary appendages used by western corn rootworms, Diabrotica virgifera virgifera, to discriminate between a phagostimulant and‐deterrent. Entomologia Experimentalis et Applicata, 78, 237–242. [Google Scholar]
- Evans, L. (1993). Crop evolution, adaptation and yield (p. 24). Cambridge University Press. [Google Scholar]
- Ferguson, J. , & Metcalf, R. (1985). Cucurbitacins. Journal of chemical. Ecology, 11, 311–318. [DOI] [PubMed] [Google Scholar]
- Fernandez, A. R. , Sáez, A. , Quintero, C. , Gleiser, G. , & Aizen, M. A. (2021). Intentional and unintentional selection during plant domestication: Herbivore damage, plant defensive traits and nutritional quality of fruit and seed crops. New Phytologist., 231(1586), 1598. [DOI] [PubMed] [Google Scholar]
- Gepts, P. (2004). Crop domestication as a long‐term selection experiment. Plant breeding reviews, 24, 1–44. [Google Scholar]
- Gols, R. , Bukovinszky, T. , Van Dam, N. M. , Dicke, M. , Bullock, J. M. , & Harvey, J. A. (2008). Performance of generalist and specialist herbivores and their endoparasitoids differs on cultivated and wild brassica populations. Journal of Chemical Ecology, 34, 132–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould, F. , & Massey, A. (1984). Cucurbitacins and predation of the spotted cucumber beetle, Diabrotica undecimpunctata howardi. Entomologia Experimentalis et Applicata, 36, 273–278. [Google Scholar]
- Gry, J. . 2006. Cucurbitacins in plant food, Nordic Council of Ministers.
- Gusarov, V. I. (2003). Revision of some types of north American aleocharines (coleoptera: Staphylinidae: Aleocharinae), with synonymic notes. Zootaxa, 353, 1–134‐1–134. [Google Scholar]
- Halaweish, F. T. , Tallamy, D. W. , & Santana, E. (1999). Cucurbitacins: A role in cucumber beetle steroid nutrition? Journal of Chemical Ecology, 25, 2373–2383. [Google Scholar]
- Hartmann, T. , Theuring, C. , Beuerle, T. , Bernays, E. , & Singer, M. (2005). Acquisition, transformation and maintenance of plant pyrrolizidine alkaloids by the polyphagous arctiid Grammia geneura. Insect Biochemistry and Molecular Biology, 35, 1083–1099. [DOI] [PubMed] [Google Scholar]
- Harvey, J. A. , Van Dam, N. M. , Raaijmakers, C. E. , Bullock, J. M. , & Gols, R. (2011). Tri‐trophic effects of inter‐and intra‐population variation in defence chemistry of wild cabbage (Brassica oleracea). Oecologia, 166, 421–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauri, K. C. , Glassmire, A. E. , & Wetzel, W. C. (2021). Chemical diversity rather than cultivar diversity predicts natural enemy control of herbivore pests. Ecological Applications, 31, e02289. [DOI] [PubMed] [Google Scholar]
- Helyer, N. , Brown, K. & Cattlin, N. D. 2003. A color handbook of biological control in plant protection.
- Howe, W. , Sanborn, J. , & Rhodes, A. (1976). Western corn rootworm adult and spotted cucumber beetle associations with Cucurbita and cucurbitacins. Environmental Entomology, 5, 1043–1048. [Google Scholar]
- Idris, A. , & Grafius, E. (1996). Effects of wild and cultivated host plants on oviposition, survival, and development of diamondback moth (lepidoptera: Plutellidae) and its parasitoid Diadegma insulare (Hymenoptera: Ichneumonidae). Environmental Entomology, 25, 825–833. [Google Scholar]
- Jaccard, C. , Cuny, M. A. , Bustos‐Segura, C. , Arce, C. C. M. , Giollo, L. , Glauser, G. & Benrey, B . 2021. Squash varieties domesticated for different purposes differ in chemical and physical defense against leaf and root herbivores. Frontiers in Agronomy, 3.
- Kates, H. R. , Anido, F. L. , Sánchez‐de la Vega, G. , Eguiarte, L. E. , Soltis, P. S. , & Soltis, D. E. (2021). Targeted sequencing suggests wild‐crop gene flow is central to different genetic consequences of two independent pumpkin domestications. Frontiers in Ecology and Evolution, 9, 618380. [Google Scholar]
- Kates, H. R. , Soltis, P. S. , & Soltis, D. E. J. M. P. (2017). Evolutionary and domestication history of Cucurbita (pumpkin and squash) species inferred from 44 nuclear loci. Molecular Phylogenetics Evolution, 111, 98–109. [DOI] [PubMed] [Google Scholar]
- Kim, J. H. , & Mullin, C. A. (2003). Antifeedant effects of proteinase inhibitors on feeding behaviors of adult western corn rootworm (Diabrotica virgifera virgifera). Journal of Chemical Ecology, 29, 795–810. [DOI] [PubMed] [Google Scholar]
- Lang, K. L. , Deagosto, E. , Zimmermann, L. A. , Machado, V. R. , Campos Bernardes, L. S. , Schenkel, E. P. , Duran, F. J. , Palermo, J. , & Rossini, C. (2013). Chemical modification produces species‐specific changes in cucurbitacin antifeedant effect. Journal of Agricultural and Food Chemistry, 61, 5534–5539. [DOI] [PubMed] [Google Scholar]
- Lewis, P. A. , & Metcalf, R. L. (1996). Behavior and ecology of Old World Luperini beetles of the genus Aulacophora (coleoptera: Chrysomelidae). Chemoecology, 7, 150–155. [Google Scholar]
- Li, X. , Garvey, M. , Kaplan, I. , Li, B. , & Carrillo, J. (2018). Domestication of tomato has reduced the attraction of herbivore natural enemies to pest‐damaged plants. Agricultural and Forest Entomology, 20, 390–401. [Google Scholar]
- Lira‐Saade, R. (1995). Estudios taxonomicos ecogeograficos de las Cucurbitaceae Latinoamericanas de importancia economica. International Plant Genetic Resources Institute Roma. [Google Scholar]
- Lira, R. , Eguiarte, L. , Montes, S. , Zizumbo‐Villarreal, D. , Marín, P. C.‐G. , & Quesada, M. (2016). Homo sapiens–Cucurbita interaction in mesoamerica: Domestication, dissemination, and diversification. Ethnobotany of Mexico, 389–401. 10.1007/978-1-4614-6669-7_15 [DOI] [Google Scholar]
- Metcalf, R. , & Lampman, R. (1989). The chemical ecology of Diabroticites and Cucurbitaceae. Experientia, 45, 240–247. [Google Scholar]
- Metcalf, R. L. (1979). Plants, chemicals, and insects: Some aspects of coevolution. Bulletin of the ESA, 25, 30–35. [Google Scholar]
- Metcalf, R. L. (1986). Coevolutionary adaptations of rootworm beetles (coleoptera: Chrysomelidae) to cucurbitacins. Journal of Chemical Ecology, 12, 1109–1124. [DOI] [PubMed] [Google Scholar]
- Metcalf, R. L. , Metcalf, R. A. , & Rhodes, A. (1980). Cucurbitacins as kairomones for diabroticite beetles. Proceedings of the National Academy of Sciences, 77, 3769–3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf, R. L. , Rhodes, A. , Metcalf, R. A. , Ferguson, J. , Metcalf, E. R. , & Lu, P.‐Y. (1982). Cucurbitacin contents and diabroticite (coleoptera: Chrysomelidae) feeding upon Cucurbita spp. Environmental Entomology, 11, 931–937. [Google Scholar]
- Meyer, R. S. , Duval, A. E. , & Jensen, H. R. (2012). Patterns and processes in crop domestication: An historical review and quantitative analysis of 203 global food crops. New Phytologist, 196, 29–48. [DOI] [PubMed] [Google Scholar]
- Milla, R. , Osborne, C. P. , Turcotte, M. M. , & Violle, C. (2015). Plant domestication through an ecological lens. Trends in Ecology & Evolution, 30, 463–469. [DOI] [PubMed] [Google Scholar]
- Miller, K. V. , & Williams, R. N. (1983). Biology and host preference of Atheta coriaria (coleoptera: Staphylinidae), an egg predator of Nitidulidae and Muscidae. Annals of the Entomological Society of America, 76, 158–161. [Google Scholar]
- Moreira, X. , Abdala‐Roberts, L. , Hernández‐Cumplido, J. , Cuny, M. A. , Glauser, G. , & Benrey, B. (2015). Specificity of induced defenses, growth, and reproduction in lima bean (Phaseolus lunatus) in response to multispecies herbivory. American Journal of Botany, 102, 1300–1308. [DOI] [PubMed] [Google Scholar]
- Navot, N. , Sarfatti, M. , & Zamir, D. (1990). Linkage relationships of genes affecting bitterness and flesh color in watermelon. Journal of Heredity, 81, 162–165. [Google Scholar]
- Nee, M. (1990). The domestication of Cucurbita (Cucurbitaceae). Economic Botany, 44, 56. [Google Scholar]
- Nishida, R. , & Fukami, H. (1990). Sequestration of distasteful compounds by some pharmacophagous insects. Journal of Chemical Ecology, 16, 151–164. [DOI] [PubMed] [Google Scholar]
- Nishida, R. , Yokoyama, M. , & Fukami, H. (1992). Sequestration of cucurbitacin analogs by New and Old World chrysomelid leaf beetles in the tribe Luperini. Chemoecology, 3, 19–24. [Google Scholar]
- Paul, R. L. , Pearse, I. S. , & Ode, P. J. (2021). Fine‐scale plant defence variability increases top‐down control of an herbivore. Functional Ecology, 35, 1437–1447. [Google Scholar]
- Pearse, I. S. , Paul, R. & Ode, P. J. 2018. Variation in plant defense suppresses herbivore performance. Current Biology, 28, 1981‐1986. e2. [DOI] [PubMed]
- Pitre, H. N., Jr. , & Kantack, E. J. (1962). Biology of the banded cucumber beetle, Diabrotica balteata, in Louisiana. Journal of Economic Entomology, 55, 904–906. [Google Scholar]
- Ranere, A. J. , Piperno, D. R. , Holst, I. , Dickau, R. , & Iriarte, J. (2009). The cultural and chronological context of early Holocene maize and squash domestication in the Central Balsas River Valley, Mexico. Proceedings of the National Academy of Sciences, 106, 5014–5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez‐Saona, C. , Vorsa, N. , Singh, A. P. , Johnson‐Cicalese, J. , Szendrei, Z. , Mescher, M. C. , & Frost, C. J. (2011). Tracing the history of plant traits under domestication in cranberries: potential consequences on anti‐herbivore defences. Journal of Experimental Botany, 62, 2633–2644. [DOI] [PubMed] [Google Scholar]
- Sánchez‐de la Vega, G. , Castellanos‐Morales, G. , Gámez, N. , Hernández‐Rosales, H. S. , Vázquez‐Lobo, A. , Aguirre‐Planter, E. , Jaramillo‐Correa, J. P. , Montes‐Hernández, S. , Lira‐Saade, R. , & Eguiarte, L. E. (2018). Genetic resources in the “Calabaza Pipiana” squash (Cucurbita argyrosperma) in Mexico: genetic diversity, genetic differentiation and distribution models. Frontiers in plant science, 9, 400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjur, O. I. , Piperno, D. R. , Andres, T. C. , & Wessel‐Beaver, L. (2002). Phylogenetic relationships among domesticated and wild species of Cucurbita (Cucurbitaceae) inferred from a mitochondrial gene: Implications for crop plant evolution and areas of origin. Proceedings of the National Academy of Sciences, 99, 535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, B. D. (1997). The initial domestication of Cucurbita pepo in the Americas 10,000 years ago. Science, 276, 932–934. [Google Scholar]
- Szczepaniec, A. , Widney, S. E. , Bernal, J. S. , & Eubanks, M. D. (2013). Higher expression of induced defenses in teosintes (Zea spp.) is correlated with greater resistance to fall armyworm, S podoptera frugiperda . Entomologia Experimentalis et Applicata, 146, 242–251. [Google Scholar]
- Tallamy, D. W. , & Halaweish, F. T. (1993). Effects of age, reproductive activity, sex, and prior exposure on sensitivity to cucurbitacins in southern corn rootworm (Coleoptera: Chrysomelidae). Environmental entomology, 22, 925–932. [Google Scholar]
- Tallamy, D. W. , Stull, J. , Ehresman, N. P. , Gorski, P. M. , & Mason, C. E. (1997). Cucurbitacins as feeding and oviposition deterrents to insects. Environmental Entomology, 26, 678–683. [Google Scholar]
- Tallamy, D. W. , Whittington, D. P. , Defurio, F. , Fontaine, D. A. , Gorski, P. M. , & Gothro, P. W. (1998). Sequestered cucurbitacins and pathogenicity of Metarhizium anisopliae (Moniliales: Moniliaceae) on spotted cucumber beetle eggs and larvae (Coleoptera: Chrysomelidae). Environmental Entomology, 27, 366–372. [Google Scholar]
- Teng, H. , Waddill, V. , Slansky, F. , & Strayer, J. (1984). Performance and host preference of adult banded cucumber beetles, Diabrotica balteata, when offered several crops. Journal of Agricultural Entomology, 1, 330–338. [Google Scholar]
- Trigo, J. R. , Barata, L. E. S. , & Brown, K. S. (1994). Stereochemical inversion of pyrrolizidine alkaloids by Mechanitis polymnia (Lepidoptera: Nymphalidae: Ithomiinae): Specificity and evolutionary significance. Journal of chemical ecology, 20, 2883–2899. [DOI] [PubMed] [Google Scholar]
- Turcotte, M. M. , Turley, N. E. , & Johnson, M. T. (2014). The impact of domestication on resistance to two generalist herbivores across 29 independent domestication events. New Phytologist, 204, 671–681. [DOI] [PubMed] [Google Scholar]
- Whitehead, S. R. , Turcotte, M. M. , & Poveda, K. (2017). Domestication impacts on plant–herbivore interactions: a meta‐analysis. Philosophical Transactions of the Royal Society B: Biological Sciences, 372, 20160034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material S1
Data Availability Statement
All the data are presented in figures, tables, and Supporting Information.


