Abstract
Leaf level gas‐exchange measurements can be made on detached foliage to address the challenge of access to the crown of tall trees. However, detachment may impact leaf gas exchange. This necessitates the study of gas‐exchange characteristics of foliage on detached branches to assess the feasibility of using detached branches for gas‐exchange analysis. We compared photosynthetic parameters and stomatal conductance in foliage of attached and detached branches of balsam fir [Abies balsamea (L.) Mill.] during the growing season. Data were analyzed using a linear mixed‐effect model, with fixed and random effects (branch status and measurement month, and tree number, respectively). Branch detachment had no significant effects on: (i) photosynthesis at the current ambient CO2 concentration (400 µmol mol−1, A 400); (ii) maximum rates of Ribulose‐1,5‐bisphosphate (RuBP) carboxylation (V cmax) and regeneration (J max); (iii) the ratio of J max to V cmax (i.e., J max:V cmax), and (iv) stomatal conductance (g s) during the study period (p = 0.120–0.335). There was a strong seasonal effect on all gas‐exchange variables (p ≤ 0.001–0.015). Gas‐exchange measurements made on detached foliage during the warm summer months should be performed with care. Reliable gas‐exchange measurements can be obtained using balsam fir foliage on detached branches 50–80 cm in length, in cooler growing‐season months, up to 30 min after detachment.
Keywords: balsam fir physiology, carbon dioxide, detached foliage, photosynthetic response, stomatal conductance
1. INTRODUCTION
Gas‐exchange measurements benefit studies in tree physiology, facilitating the understanding of tree response to changes in environmental conditions. In situ gas‐exchange measurements, with the foliage attached to the branch are readily done in seedlings or small saplings because of ease‐of‐access. However, access to foliage becomes difficult when trees grow past the sapling stage (Gauthier & Jacobs, 2018). Experiments could be done with ladders, scaffolding, towers, etc. to reach foliage in the crown, several tens of meters above the ground. Choice of means of access to the tree crown is informed by the safety of researchers, effort and associated logistics required, flexibility of the system, and cost, etc. (McCarthy, 1988). Difficulties associated with canopy access would limit the number of measurements, many of which are required, in view of within‐tree crown and between‐tree variation. A trade‐off option in conducting gas‐exchange experiments could be accomplished by detaching foliage from tall trees and conveniently taking measurements on the ground (Meng & Arp, 1993; Richardson & Berlyn, 2002).
The process of detaching foliage from trees, that is, branch cutting, the interval between branch cutting and gas‐exchange measurements, or both, can affect the variables being assessed (Richardson & Berlyn, 2002). The photosynthetic capacity of cut foliage may be affected by resultant changes in cell turgor or stomatal aperture (Clark, 1954; Lundegardh, 1931), and drawdown of moisture which may occur because of continued transpiration (Meng & Arp, 1993). However, detachment is still a viable method for measuring gas exchange (see researchers listed in Table 1), but few studies have reported on its effects on gas‐exchange measurements (Clark, 1954; Gauthier & Jacobs, 2018; Koike & Sakagami, 1984; Meng & Arp, 1993).
TABLE 1.
Studies reporting V cmax and J max from detached foliage
| Source | Species studied |
|---|---|
| Niinemets et al. (1998) | Populus tremula L., Fraxinus excelsior L., Tilia cordata Mill., Corylus avellana L. |
| Medlyn et al. (2002) | Pinus pinstar Aiton. |
| Warren et al. (2003) | Pinus sylvestris L. |
| Ethier et al. (2006) | Pseudotsuga menziesii (Mirb.) Franco |
| Goodine et al. (2008) | Abies balsamea (L.) Mill. |
| Wang et al. (2008) a | Fagus crenata Blume |
| Merilo et al. (2009) | Picea abies L. |
| Woodruff et al. (2009) | Pseudotsuga menziesii |
| Drake et al. (2010) | Pinus taeda L. |
| Whitehead et al. (2011) a | Nothofagus solandrii (Hook.f.) Oerst. |
| Raim et al. (2012) | Picea abies |
| Katahata et al. (2014) | Daphniphyllum humile Maxim |
Studies that only report V cmax.
Clark (1954), Koike and Sakagami (1984), Meng and Arp (1993), and Gauthier and Jacobs (2018) studied the effect of detachment on gas exchange in Norway and red spruce [Picea abies (L.) Karst., P. rubens Sarg.], species of birch (i.e., Betula ermanii Cham., B. platyphylla Sukatchev. and B. maximowicziana Sukatchev), black walnut (Juglans nigra L.), red and white oak (Quercus rubra L. and Q. alba L.) and found that the period within which gas‐exchange rates remained unchanged ranged from 3 to 20 min in foliage of differing sizes.
Gas‐exchange studies available in the literature have generally assessed the impacts of detachment on the rate of photosynthesis (A) in tree foliage, and even fewer, on stomatal conductance (g s). This study reports on the impacts of detachment on photosynthetic parameters, including maximum rate of Ribulose‐1,5‐bisphosphate (RuBP) carboxylation (V cmax; μmol m−2 s−1) and the maximum rate of RuBP regeneration (J max; μmol m−2 s−1), derived from measurements of A (μmol m−2 s−1) relative to intercellular carbon dioxide (C i; μmol mol−1), in response to changing levels of carbon dioxide (alias CO2 response or A‐C i curves). This is important because the Farquhar et al. (1980) model is the most widely used in the analysis of CO2‐photosynthesis response in trees. Two key parameters in the model, that is, V cmax and J max, have been reported on, in several in situ gas‐exchange studies (e.gDiaz‐Espejo et al., 2006; Fujita et al., 2012; Wilson et al., 2000; Xu & Baldocchi, 2003). Several researchers have reported on these parameters derived from measurements directly obtained from detached foliage (Table 1). Studies by Wang et al. (2008) and Whitehead et al. (2011) only reported V cmax.
Warren et al. (2003), Ethier et al. (2006), Goodine et al. (2008), Merilo et al. (2009), Woodruff et al. (2009), Drake et al. (2010), and Katahata et al. (2014) referred to preliminary measurements conducted in attached and detached foliage to compare gas‐exchange rates prior to conducting measurements on which their studies were based. The study by Drake et al. (2010) is the only one we are aware of that has reported on the effects of foliage detachment on V cmax and J max. To our knowledge, this is the first study that assesses V cmax and J max of attached and detached foliage in balsam fir, and the response of these parameters in such foliage to seasonal variation. The objective of this study was to report field observations of the parameters of photosynthesis and stomatal conductance before and after branch detachment during early summer‐to‐fall conditions.
2. MATERIALS AND METHODS
2.1. Study site
The study was conducted at the University of New Brunswick Woodlot (45° 56ʹ N, 66° 40ʹ W), New Brunswick, Canada, which lies within the Maritime Lowlands Ecoregion of the Atlantic Maritime Ecozone (Ecological Stratification Working Group, 1996). Humo‐ferric podzols and gray luvisols are the dominant soil types in the Ecoregion, with significant areas of gleysols, fibrisols, and mesisols (Ecological Stratification Working Group, 1996). The soils are well‐drained, sandy‐clay loams, from the soil surface to a depth of 30 cm with <20% coarse fragments. The depth to the compacted layer ranges from <30 to 30–65 cm (Ecological Stratification Working Group, 1996; Canadian Soil Information Service, 2019).
2.2. Gas‐exchange measurements
Nine balsam fir trees, ranging in age from 15 to 20 years, with diameter at breast heights (DBH) between 9 and 19 cm, were selected. Photosynthesis measurements were made in July, August, October, and November of 2019 with respective mean monthly temperature and total precipitation as follows: 20℃ and 70.7 mm; 18.2℃ and 79.9 mm; 8.2℃ and 105.1 mm; −0.6℃ and 118.1 mm (after Environment & Climate Change Canada, 2019).
The development of CO2 response curves and measurement of stomatal conductance commenced each day at 09:00 h (Local Atlantic Daylight Time) using a CIRAS‐2 Photosynthesis System (PP Systems). Measurements were made, first on 1‐year‐old foliage which were totally enclosed in the conifer chamber (cuvette), while branches were attached to each tree, then again on the same foliage, following detachment, each detached branch was around 50‐ to 80‐cm long. Measurements were made under saturating light, with photosynthetically active radiation (PAR) ranging from 1190 to 1200 μmol m−2 s−1 supplied by a tungsten halogen light unit (PP Systems). Reference CO2 concentration (C ref) was changed in descending steps of 400, 200, 100, and 50, and ascending steps of 400, 800, 1,000, 1,400, and 1,800 μmol mol−1. Flow rate was set at 400 ml min−1, chamber temperature at 18℃, and relative humidity at 70%. Chamber temperature was set at 18℃ because conifers are known to undergo optimal photosynthetic activity at temperatures lower than 25℃ (Lin et al., 2012; Wieser et al., 2010), the reference temperature on which the Farquhar et al., (1980) model is based. Before starting each response curve, the foliage was acclimated to cuvette conditions for 3 min. Two minutes were allowed for stabilization of A after each change in CO2 concentration before the variable was measured. Stomatal conductance (mmol m−2 s−1) was measured simultaneously. Each CO2 response curve was completed within 30 min. Fifty‐six response curves were developed over the study period.
On completion of the response curves for both attached and detached foliage, all needles on each foliage (n = 30–50) were removed, and projected leaf area (½ of total leaf area) was determined by scanning the needles with a CanoScanLiDE 110 Flatbed Scanner (Canon Canada Inc). The image was then analyzed with Image J software (National Institutes of Health). Projected leaf area was subsequently used to calculate A on a leaf‐area basis.
2.3. Data analysis
The Farquhar et al., (1980) photosynthesis model is comprised of two parts. The first part is
| (1) |
where A c is the Ribulose‐1,5‐bisphosphatecarboxylase/oxygenase (Rubisco)‐limited net photosynthesis (μmol m−2 s−1), K c and K o the Michaelis–Menten constants for carboxylation and oxygenation (404 μmol mol−1 and 248 mmol mol−1, respectively; von Caemmerer et al., 1994), O i the oxygen concentration at the site of carboxylation (210 mmol mol−1), and R d dark respiration (μmol m−2 s−1). The second part is as follows:
| (2) |
where A r is the Ribulose‐1,5‐bisphosphate (RuBP)‐limited net photosynthesis (μmol m−2 s−1), C i the intercellular CO2 concentration at the current ambient CO2 concentration, ~400 μmol mol−1, and Г* the CO2 compensation point in the absence of dark respiration (μmol mol−1), at which point there is no net assimilation. The CO2 compensation point in the absence of dark respiration is determined by the following equation:
| (3) |
where k c and k o are the turnover rates for RuBP carboxylase and RuBP oxygenase (2.5s−1 and 0.55 s−1 or 0.22k c; after Farquhar et al., 1980).
The key parameters V cmax and J max in eqn.’s 1 and 2 were estimated from regression analysis of A‐C i curves (Wullschleger, 1993), which necessitates the designation a priori of a C i‐threshold, at which there is a switch between RuBP‐saturated and limited portions of the curve (Manter & Kerrigan, 2004). Generally, it is assumed that at low C i, A is solely limited by V cmax, whereas at high C i, A is limited by J max. The parameters V cmax and J max were estimated using the lower section of the response curve, when C i was approximately ≤300 µmol mol−1, and the entire curve, respectively (Farquhar et al., 1980; Wullschleger, 1993; Xu & Baldocchi, 2003), that is,
| (4) |
| (5) |
| (6) |
after Farquhar et al., 1980 and von Caemmerer, 2000), where CE and V c in eqn.’s 4 and 5 are the carboxylation efficiency, representing the initial slope of the CO2 response curve and the rate of carboxylation, respectively. The equation constants, that is, K c, K o, and Г*, having been derived at an ambient temperature of 25℃ were converted to values at the reference temperature (i.e., 18℃) with the following equations:
| (7) |
| (8) |
| (9) |
Lambers et al., (1998), where T is the reference temperature at which the CO2 response curve was developed.
The linear mixed‐effects model option in SPSS Statistical software (ver. 24.0, IBM Corp.) was used to analyze the maximum rate of photosynthesis at the current ambient CO2 concentration (i.e., photosynthesis at 400 µmol mol−1, A 400), V cmax, J max, ratio of J max to V cmax (i.e., J max:V cmax), and mean g s. Branch status (attached or detached foliage) and measurement month (July, August, October, and November) were the fixed factors, and tree number was the random factor. This analysis was done as a result of the non‐independence of measurements made on both foliage types from each tree, and repeated sampling during the study period.
The model equations used are as follows,
| (10) |
| (11) |
In eqn.’s 10 and 11, Y is the gas‐exchange parameter of interest, β0 , β1 , β2 , β 3, and b 1 are regression coefficients to be estimated, and ε is the regression error term.
3. RESULTS
As shown in Figure 1a–e, A increased with varying C ref (from 50 to 1800 µmol mol−1) and ranged from 0.00 to 30.79 µmol m−2 s−1 and from 0.00 to 30.50 µmol m−2 s−1 in attached and detached foliage, respectively. The parameter A 400 ranged from 5.30 to 14.70 µmol m−2 s−1 and from 4.30 to 14.60 µmol m−2 s−1 in attached and detached foliage, respectively. The highest mean A 400 values for attached and detached foliage were 10.80 ± 1.56 and 10.66 ± 1.61 µmol m−2 s−1, respectively, and were found to occur in October. The range for V cmax in attached and detached foliage, was 14.58–53.36 and 11.62–52.78 µmol m−2 s−1, respectively. The highest mean V cmax in attached and detached foliage was 35.79 ± 9.44 and 33.59 ± 9.29 µmol m−2 s−1, respectively, also occurred in October. The range for J max in attached and detached foliage, was 16.28–81.33 and 12.44–72.71 µmol m−2 s−1. The mean J max value in November of 48.20 ± 14.37 µmol m−2 s−1 was the highest in attached foliage. In detached foliage, this occurred in October, with a value of 44.49 ± 13.98 µmol m−2 s−1. The values of J max:V cmax in attached and detached foliage ranged from 1.06 to 1.90 (non‐dimensional). The highest mean J max:V cmax were 1.51 ± 0.33 and 1.35 ± 0.12 for attached and detached foliage, and occurred in July. Stomatal conductance (g s) in both attached and detached foliage ranged from 20 to 150 mmol m−2 s−1. Some exceptions between 150 and 230 mmol m−2 s−1 coincided with periods of active rainfall that increased foliage surface wetness and cuvette relative humidity, which led to higher‐than‐normal readings of g s. In attached foliage, mean g s at the lowest C ref, 50 µmol mol−1 ranged from 74.49 to 117.24 mmol m−2 s−1, and at the highest C ref, 1800 µmol mol−1 it ranged from 67.71 to 106.5 mmol m−2 s−1. In detached foliage, mean g s at 50 mol mol−1 ranged from 67.57 to 119.01 mmol m−2 s−1, and at 1800 µmol mol−1 it ranged from 60.20 to 117.13 mmol m−2 s−1. Across C ref levels, mean g s in attached and detached foliage samples ranged from 36.80 to 139.27 and from 35.74 to 145.79 mmol m−2 s−1, respectively. The highest mean g s in attached and detached foliage samples, 106.42 ± 22.45 and 110.96 ± 24.09 mmol m−2 s−1, respectively, also occurred in October (Figures 2, 3, 4). The branch status (fixed effect) and tree number (random effect) did not have significant effects on A 400, V cmax, J max, J max:V cmax, and mean g s (p = 0.217, 0.181, 0.120, 0.164, 0.335, respectively). The month of measurement did have a statistically significant effect on A 400, V cmax, and J max (p < 0.001, respectively), but branch status ×month of measurement had no significant effect on the parameters (p = 0.916, 0.984, 0.980, respectively). Month of measurement only had a significant effect on J max:V cmax and mean g s when the main effects of the fixed factors were assessed (p = 0.013, 0.009, respectively), as the introduction of the interaction term, which was not significant (p = 0.688, 0.793, respectively) rendered the effect of month on J max:V cmax and mean g s statistically non‐significant. With the interaction between the fixed factors having no significant effect on all gas‐exchange parameters, the interaction term was ignored (Tables 2 and 3).
FIGURE 1.
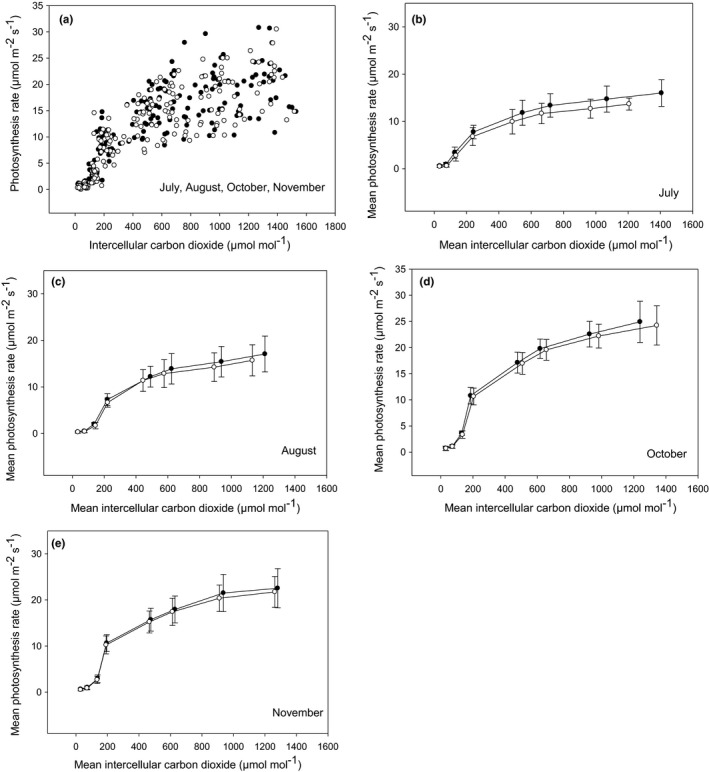
The response of maximum photosynthesis (A; µmol m−2 s−1) to intercellular CO2 (C i; µmol mol−1) of attached (closed circles) and detached balsam fir foliage (open circles) assessed in July through to November 2019 (a). The mean monthly response ±the standard deviation (error bars; panels b–e
FIGURE 2.
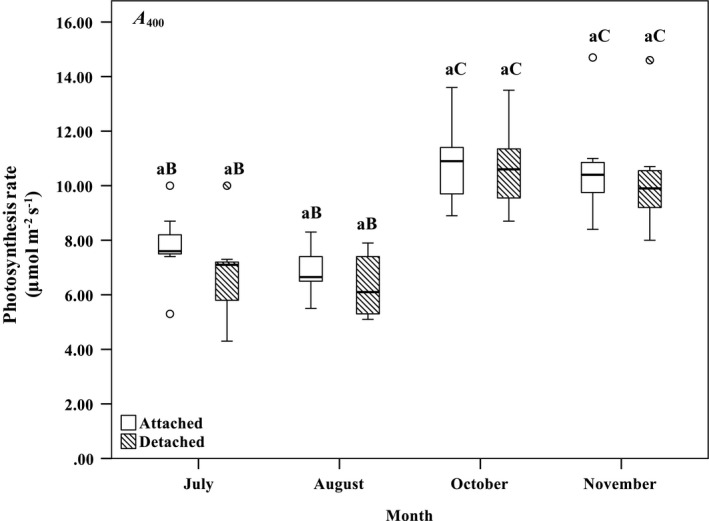
Box plots of rate of photosynthesis at 400 µmol mol−1 (A 400; µmol m−2 s−1) of attached and detached balsam fir foliage assessed in July through to November 2019. The thick horizontal lines in the boxes represent the median of plotted values. The bottom and top edges of the boxes denote the 25th and 75th percentiles, whereas the ends of the whiskers correspond to the 10th and 90th percentiles. Similar lowercase letters indicate no significant difference regarding branch status (p > 0.05). Differences in uppercase letters indicate significant differences regarding measurement month (p < 0.05). Comparisons were made in relation to reference values for branch status and measurement month
FIGURE 3.
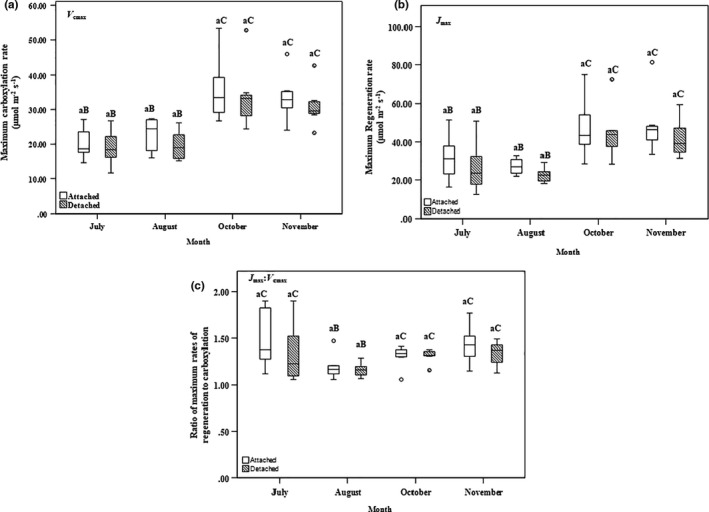
Box plots of (a) the maximum rate of ribulose‐1,5‐bisphosphate carboxylation (V cmax; µmol m−2 s−1), (b) the maximum rate of ribulose‐1,5‐bisphosphate regeneration (J max; µmol m−2 s−1), and (c) the ratio of the maximum rates of ribulose‐1,5‐bisphosphate regeneration (J max;µmol m−2 s−1) to carboxylation (V cmax;µmol m−2 s−1) of attached and detached balsam fir foliage assessed in July through to November 2019. See Figure 2 for box‐plot descriptions. Similar lowercase letters above box plots indicate no significant difference regarding branch status (p > 0.05). Differences in uppercase letters above box plots indicate significant differences regarding measurement month (p < 0.05). Comparisons were made in relation to reference values for branch status and measurement month
FIGURE 4.
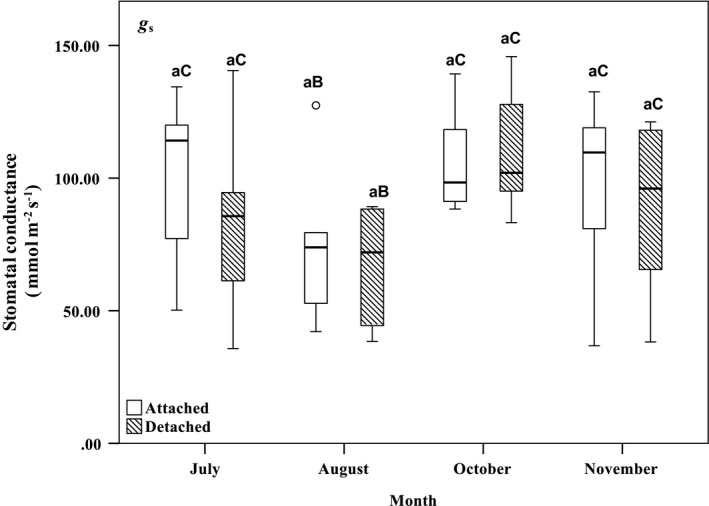
Box plots of mean stomatal conductance (g s; mmol m−2 s−1) of attached and detached balsam fir foliage assessed in July through to November 2019, varying C ref from 50 to 1800 µmol mol−1. The thick horizontal lines in the boxes represent the median of plotted values. See Figure 2 for box‐plot descriptions. Similar lowercase letters indicate no significant difference regarding branch status (p > 0.05). Differences in uppercase letters indicate significant differences regarding measurement month (p < 0.05). Comparisons were made in relation to reference values for branch status and measurement month
TABLE 2.
Output of mixed‐effect model analysis (fixed effects; branch status and month) of A 400, V cmax, J max, J max:V cmax, and g s in attached and detached balsam fir foliage assessed in July through to November 2019
| Parameter a | Estimate | Std. Error | df | t‐value | p‐value |
|---|---|---|---|---|---|
| A 400 | |||||
| Branch status | |||||
| Attached | 0.525 | 0.418 | 42.571 | 1.254 | 0.217 |
| Detached b | 0 | 0 | . | . | . |
| Month | |||||
| July | −3.222 | 0.577 | 47.417 | −5.585 | 0.000 |
| August | −3.447 | 0.604 | 48.842 | −5.707 | 0.000 |
| October | 0.310 | 0.574 | 44.376 | .539 | 0.593 |
| November b | 0 | 0 | . | . | . |
| V cmax | |||||
| Branch status | |||||
| Attached | 2.285 | 1.684 | 46.837 | 1.357 | 0.181 |
| Detached b | 0 | 0 | . | . | . |
| Month | |||||
| July | −12.439 | 2.309 | 48.971 | −5.388 | 0.000 |
| August | −10.885 | 2.409 | 49.028 | −4.518 | 0.000 |
| October | 2.547 | 2.307 | 47.840 | 1.104 | 0.275 |
| November b | 0 | 0 | . | . | . |
| J max | |||||
| Branch status | |||||
| Attached | 4.811 | 3.038 | 47.968 | 1.584 | 0.120 |
| Detached b | 0 | 0 | . | . | . |
| Month | |||||
| July | −15.849 | 4.170 | 49.280 | −3.800 | <0.001 |
| August | −19.942 | 4.352 | 49.322 | −4.582 | <0.001 |
| October | 1.286 | 4.165 | 48.604 | 0.309 | 0.759 |
| November b | 0 | 0 | . | . | . |
| J max:V cmax | |||||
| Branch status | |||||
| Attached | 0.074 | 0.052 | 45.300 | 1.415 | 0.164 |
| Detached b | 0 | 0 | . | . | . |
| Month | |||||
| July | 0.466 | 0.072 | 48.500 | 0.647 | 0.520 |
| August | −0.205 | 0.075 | 48.930 | −2.729 | 0.009 |
| October | −0.071 | 0.072 | 46.580 | −0.994 | 0.325 |
| November b | 0 | 0 | . | . | . |
| g s | |||||
| Branch status | |||||
| Attached | 7.240 | 7.435949 | 47.159 | .974 | 0.335 |
| Detached b | 0 | 0 | . | . | . |
| Month | |||||
| July | −4.216 | 10.274 | 49.189 | −0.410 | 0.683 |
| August | −25.650 | 10.897 | 50.262 | −2.354 | 0.023 |
| October | 14.272 | 10.206 | 47.800 | 1.398 | 0.168 |
| November b | 0 | 0 | . | . | . |
A 400 = rate of photosynthesis at 400 µmol mol−1; V cmax = maximum rate of ribulose‐1,5‐bisphosphate carboxylation; J max = maximum rate of ribulose‐1,5‐bisphosphate regeneration; J max:V cmax = ratio of the maximum rates of ribulose‐1,5‐bisphosphate regeneration to carboxylation; g s = stomatal conductance; df = degrees of freedom.
Reference set to zero.
TABLE 3.
Output of mixed‐effect model analysis (random effect; tree number) of A 400, V cmax, J max, J max:V cmax, and g s in attached and detached balsam fir foliage samples assessed in July through to November 2019
| Parameter | Estimate | Std. Error | Wald Z | p‐value |
|---|---|---|---|---|
| A 400 | 0.090 | 0.283 | 0.319 | 0.749 |
| V cmax | 0.244 | 2.414 | 0.101 | 0.919 |
| J max | 2.933 | 7.884 | 0.372 | 0.710 |
| J max:V cmax | 0.002 | 0.003 | 0.449 | 0.654 |
| g s | 108.231 | 325.566 | 0.332 | 0.740 |
See Table 2 for parameter definitions.
4. DISCUSSION
The results of this study showed that gas exchange in detached foliage did not differ significantly from those in attached foliage 30 min after detachment (p = 0.120–0.335), the period required for completion of a single CO2 response curve. This may be because the water status of the detached foliage was not affected by detachment during the development of individual response curves. Hydraulically efficient stems ensure adequate water supply to the leaves such that the water loss through transpiration can be replaced readily, ensuring that deficits in leaf water supply are minimized, and stomata remain open for CO2 uptake (Bucci et al., 2019; Xiong et al., 2018). Photosynthesis has been documented among the primary physiological processes affected by water stress (Galmes et al., 2011). Detachment does not initially affect the rate of net photosynthesis and stomatal conductance of detached foliage, but over a prolonged period, cumulative moisture losses from leaves following detachment cause reductions. Nevertheless, a small and immediate increase in measurement variations occurs following twig detachment (Meng & Arp, 1993).
The opening and closing of stomata embedded in the epidermis simultaneously control plants’ water loss during transpiration and their uptake of CO2. Water loss through plant leaves during transpiration, which results in stomatal closure, occurs from an imbalance between water effluxes and influxes. During transpiration, water‐conducting elements contain water columns, which are under tension. Consequently, a water‐potential gradient between mesophyll tissue and xylem elements occurs, ensuring the movement of water against flow resistances (Buckley, 2005; Heber et al., 1986). Stomatal response to foliage detachment is a consequence of the positive relationship between stomatal aperture and turgor pressure of the guard cells, which form the pore, but it has a negative relationship with turgor pressure of adjacent epidermal cells, the more effective, of these two opposing pressures, in regulating aperture (Buckley, 2005).
The rate at which CO2 enters the leaf is regulated by resistances caused by the opening and closing of the stomata, and the internal pathways within the leaf mesophyll. The reduction in plant photosynthesis arising from moisture stress is generally explained by an increase in the resistance to the movement of CO2 through the stomata and mesophyll pathways to the site of fixation in the chloroplast. These resistances are substantially increased under such conditions because of low leaf water potentials (Beadle et al., 1973; Brix, 1962; Crafts, 1968; Puritch, 1973; Slatyer, 1967).
Generally, an increase in water stress results in a two‐phase photosynthetic response, with a xylem water potential threshold, above which little or no change in photosynthesis occurs, and below which it rapidly decreases. This two‐phase response varies among species (Melzack et al., 1985) and has been observed in conifers in studies of the effects of water stress in Pinus taeda L., (Brix, 1962) four species of the genus Abies [i.e., A. balsamea, A. amabilis (Doug) ex. Loud., A. Lasiocarpa (Hook.) Nutt., and A. Grandis (Doug) Lindl.; Puritch, 1973], and Picea sitchensis (Bong.) Carr. (Watts & Neilson, 1978). Brix (1962) found that photosynthesis in P. taeda, under water stress was stable until it increased to 405 kPa, following which there was a decline. Puritch (1973) reported similar stability of photosynthesis under water stress in the four species of the genus Abies studied, up until stresses between 900 and 1,100 kPa followed by a decline. Watts and Neilson (1978) observed stable photosynthesis in P. sitchensis under water stress up until 1500 kPa, after which there was a decline. The results of this study may, therefore, be an indication that the period during which a CO2 response curve was assessed for detached foliage, occurred at a xylem water potential that allowed photosynthesis to remain at rates that were largely like those in attached foliage samples.
The values of A 400, V cmax, J max, and J max:V cmax for attached and detached foliage all showed strong seasonal variation (p ≤ 0.001–0.013). The studies by Xu and Baldocchi (2003) and Wilson et al., (2000) showed seasonal variation in the gas‐exchange parameters measured, with peak values recorded for the gas‐exchange parameters measured in spring and summer, respectively, with declines in subsequent seasons. In this study, however, A 400, V cmax, and J max increased from summer to fall. This is attributable to the fact that Xu and Baldocchi (2003) worked on Q. douglasii Hook & Arn, and Wilson et al., (2000) worked on Q. prinus L., Q. alba, Acer rubrum L., A. saccharum Marsh, and Nyssa sylvatica Marsh, all deciduous species. Conifers and deciduous species have different photosynthetic responses to changing atmospheric temperature, with conifers (including balsam fir) attaining higher photosynthetic rates at lower temperatures compared to deciduous species (Lin et al., 2012), such as obtained during fall. The pattern of seasonal variation in J max:V cmax, in attached and detached samples showed that the highest value in both foliage types occurred in July. Though the pattern of seasonal variation in J max:V cmax is a departure from those seen in A 400, V cmax, and J max, the values ranged between 1 and 3, the range within which J max:V cmax is reported to fluctuate, and reflects the balance between RuBP carboxylation and regeneration (Kattge & Knorr, 2007; Onoda et al., 2005; Robakowski et al., 2002; Walcroft et al., 1997; Wullschleger, 1993). Mean g s across C ref‐levels showed a seasonal trend and peaked in October. A similar trend was reported by Beadle et al., (1985) in their study of Pinus sylvestris L. (another conifer). The seasonal effect on detached foliage also varied in relation to prevailing atmospheric conditions with a general trend of significantly lower gas‐exchange parameters in August compared to those in October and November. This is because of higher temperatures and accompanying greater vapor pressure deficit between foliage and the air in the summer, than in the fall (Berry & Bjorkman, 1980).
5. CONCLUSIONS
A comparison was made of photosynthetic parameters and stomatal conductance in foliage of attached and detached branches of balsam fir at different times during the growing season, using a linear mixed‐effect model. Branch detachment did not have a significant effect on gas‐exchange parameters studied, in particular A 400, V cmax, J max, J max:V cmax, and g s. There was, however, a strong seasonal effect on the parameters. The trend of the data from this study indicates that the photosynthetic parameters and rate of photosynthesis were observed to be highest in October and November, an indication that the optimum temperature of balsam fir is low. The impact of detachment on g s during July and August indicated that caution is required when gas‐exchange measurements are made over long durations, during warm summer months. We can conclude that detachment had a negligible impact on gas‐exchange measurements in balsam fir foliage when carried out on branches 50‐ to 80‐cm long, for up to 30 min. The results from balsam fir are more reliable when gas‐exchange measurements are done during cooler months.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTION
MEA, FRM, and CPAB conceived the ideas; MEA collected the data, designed the methods used, did the data analysis, and led the writing of the manuscript; FRM and CPAB reviewed the drafts, made amendments to the manuscript, and gave final approval for its publication.
ACKNOWLEDGMENT
This study was supported with funding from the Natural Sciences and Engineering Research Council of Canada (NSERC) in the form of a Discovery Grant to CPAB.
Akalusi, M. E. , Meng, F.‐R. , & P.‐A. Bourque, C. (2021). Photosynthetic parameters and stomatal conductance in attached and detached balsam fir foliage. Plant‐Environment Interactions, 2, 206–215. 10.1002/pei3.10059
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in figshare at https://doi.org/10.6084/m9.figshare.14791716.
REFERENCES
- Beadle, C. L. , Neilson, R. E. , Talbot, H. , & Jarvis, P. G. (1985). Stomatal conductance and photosynthesis in a mature Scots pine forest. I. Diurnal, seasonal and spatial variation in shoots. Journal of Applied Ecology, 22(2), 557–571. [Google Scholar]
- Beadle, C. L. , Stevenson, K. R. , Neumann, H. H. , Thurtell, G. W. , & King, K. M. (1973). Diffusive resistance, transpiration, and photosynthesis in single leaves of corn and sorghum in relation to leaf water potential. Canadian Journal of Plant Science, 53(3), 537–544. 10.4141/cjps73-103. [DOI] [Google Scholar]
- Berry, J. , & Bjorkman, O. (1980). Photosynthetic response and adaptation to temperature in higher plants. Annual Review of Plant Physiology, 31(1), 491–543. 10.1146/annurev.pp.31.060180.002423. [DOI] [Google Scholar]
- Brix, H. (1962). The effect of water stress on the rates of photosynthesis and respiration in tomato plants and loblolly pine seedlings. Physiologia Plantarum, 15(1), 10–20. 10.1111/j.1399-3054.1962.tb07982.x. [DOI] [Google Scholar]
- Bucci, S. J. , CarbonellSilletta, L. M. , Garre, A. , Cavallaro, A. , Efron, S. T. , Arias, N. S. , Goldstein, G. , & Scholz, F. G. (2019). Functional relationships between hydraulic traits and the timing of diurnal depression of photosynthesis. Plant, Cell and Environment, 42(5), 1603–1614. 10.1111/pce.13512. [DOI] [PubMed] [Google Scholar]
- Buckley, T. N. (2005). The control of stomata by water balance. New Phytologist, 168(2), 275–292. 10.1111/j.1469-8137.2005.01543.x. [DOI] [PubMed] [Google Scholar]
- Canadian Soil Information Service . (2019). Forest soils of New Brunswick. http://sis.agr.gc.ca/cansis/publications/index.html
- Clark, J. (1954). The immediate effect of severing on the photosynthetic rate of Norway spruce branches. Plant Physiology, 29(5), 489–490. 10.1104/pp.29.5.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crafts, A. S. (1968). Water deficits and physiological processes. In Kozlowski T. T. (Ed.), Water deficits and plant growth (pp. 85–133). Academic Press. [Google Scholar]
- Diaz‐Espejo, A. , Walcroft, A. S. , Fernandez, J. E. , Hafidi, B. , Palomo, M. J. , & Giron, I. F. (2006). Modeling photosynthesis in olive leaves under drought conditions. Tree Physiology, 26(11), 1445–1456. 10.1093/treephys/26.11.1445. [DOI] [PubMed] [Google Scholar]
- Drake, J. E. , Raetz, L. M. , Davis, S. C. , & Delucia, E. H. (2010). Hydraulic limitation not declining nitrogen availability causes the age‐related photosynthetic decline in loblolly pine (Pinus taeda L.). Plant, Cell and Environment, 33(10), 1756–1766. 10.1111/j.1365-3040.2010.02180.x. [DOI] [PubMed] [Google Scholar]
- Ecological Stratification Working Group . (1996). A national ecological framework for Canada. Environment Canada. 76 p.
- Environment and Climate Change Canada . (2019). Monthly Climate Summaries. http://climate.weather.gc.ca/
- Ethier, G. J. , Livingston, N. J. , Harrison, D. L. , Black, T. A. , & Moran, J. A. (2006). Low stomatal and internal conductance to CO2 versus Rubisco deactivation as determinants of the photosynthetic decline of ageing evergreen leaves. Plant, Cell and Environment, 29(12), 2168–2184. [DOI] [PubMed] [Google Scholar]
- Farquhar, G. D. , von Caemmerer, S. , & Berry, J. A. (1980). A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta, 149(1), 78–90. 10.1007/BF00386231. [DOI] [PubMed] [Google Scholar]
- Fujita, N. , Noma, N. , Shirakawa, H. , & Kikuzawa, K. (2012). Annual photosynthetic activities of temperate evergreen and deciduous broadleaf tree species with simultaneous and successive leaf emergence in response to altitudinal air temperature. Ecological Research, 27(6), 1027–1039. 10.1007/s11284-012-0983-z. [DOI] [Google Scholar]
- Galmes, J. , Ribas‐Carbo, M. , Medrano, H. , & Flexas, J. (2011). Rubisco activity in Mediterranean species is regulated by the chloroplastic CO2 concentration under water stress. Journal of Experimental Botany, 62(2), 653–665. 10.1093/jxb/erq303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier, M. M. , & Jacobs, D. F. (2018). Reductions in net photosynthesis and stomatal conductance vary with time since leaf detachment in three deciduous angiosperms. Trees, 32(5), 1247–1252. 10.1007/s00468-018-1706-z. [DOI] [Google Scholar]
- Goodine, G. K. , Lavigne, M. B. , & Krasowski, M. J. (2008). Springtime resumption of photosynthesis in balsam fir (Abies balsamea). Tree Physiology, 28(7), 1069–1076. 10.1093/treephys/28.7.1069. [DOI] [PubMed] [Google Scholar]
- Heber, U. , Neimanis, S. , & Lange, O. L. (1986). Stomatal aperture, photosythesis and water fluxes in mesophyll cells as affected by the abscission of leaves. Simultaneous measurements of gas exchange, light scattering and chlorophyll fluorescence. Planta, 167(4), 554–562. [DOI] [PubMed] [Google Scholar]
- Katahata, S. I. , Han, Q. , Naramoto, M. , Kakubari, Y. , & Mukai, Y. (2014). Seasonal changes in temperature response of photosynthesis and its contribution to annual carbon gain in Daphniphyllum humile, an evergreen understorey shrub. Plant Biology, 16(2), 345–353. [DOI] [PubMed] [Google Scholar]
- Kattge, J. , & Knorr, W. (2007). Temperature acclimation in a biochemical model of photosynthesis: a reanalysis of data from 36 species. Plant, Cell and Environment, 30(9), 1176–1190. 10.1111/j.1365-3040.2007.01690.x. [DOI] [PubMed] [Google Scholar]
- Koike, T. , & Sakagami, Y. (1984). Examination of methods of measuring photosynthesis with detached parts of three species of birch in Hokkaido. Journal of the Japanese Forestry Society, 66(8), 337–340. [Google Scholar]
- Lambers, H. , Chapin, F. S. III , & Pons, T. L. (1998). Plant physiological. Ecology. Springer‐Verlag. [Google Scholar]
- Lin, Y. S. , Medlyn, B. E. , & Ellsworth, D. S. (2012). Temperature responses of leaf net photosynthesis: the role of component processes. Tree Physiology, 32(2), 219–231. 10.1093/treephys/tpr141. [DOI] [PubMed] [Google Scholar]
- Lundegardh, H. (1931). Environment and plant development (p. 35). Translated and edited by Ashby E.. Edward Arnold and Co. [Google Scholar]
- Manter, D. K. , & Kerrigan, J. (2004). A/C i‐curve analysis across a range of woody plant species: influence of regression analysis parameters and mesophyll conductance. Journal of Experimental Botany, 55(408), 2581–2588. 10.1093/jxb/erh260. [DOI] [PubMed] [Google Scholar]
- McCarthy, B. C. (1988). A method of access into the crowns of subcanopy and canopy trees. Canadian Journal of Forest Research, 18(5), 646–649. 10.1139/x88-096. [DOI] [Google Scholar]
- Medlyn, B. E. , Loustau, D. , & Delzon, S. (2002). Temperature response of parameters of a biochemically based model of photosynthesis. I. Seasonal changes in mature maritime pine (Pinus pinaster Ait.). Plant, Cell and Environment, 25(9), 1155–1165. [Google Scholar]
- Melzack, R. N. , Bravdo, B. , & Riov, J. (1985). The effect of water stress on photosynthesis and related parameters in Pinus halepensis . Physiologia Plantarum, 64(3), 295–300. 10.1111/j.1399-3054.1985.tb03343.x. [DOI] [Google Scholar]
- Meng, F. R. , & Arp, P. A. (1993). Net photosynthesis and stomatal conductance of red spruce twigs before and after twig detachment. Canadian Journal of Forest Research, 23(4), 16–721. 10.1139/x93-093. [DOI] [Google Scholar]
- Merilo, E. , Tulva, I. , Raim, O. , Kukit, A. , Sellin, A. , & Kull, O. (2009). Changes in needle nitrogen partitioning and photosynthesis during 80 years of tree ontogeny in Picea abies . Trees, 23(5), 951–958. [Google Scholar]
- Niinemets, U. , Kull, O. , & Tenhunen, J. D. (1998). An analysis of light effects on foliar morphology, physiology, and light interception in temperate deciduous woody species of contrasting shade tolerance. Tree Physiology, 18(10), 681–696. 10.1093/treephys/18.10.681. [DOI] [PubMed] [Google Scholar]
- Onoda, Y. , Hikosaka, K. , & Hirose, T. (2005). Seasonal change in the balance between capacities of RuBP carboxylation and RuBP regeneration affects CO2 response of photosynthesis in Polygonum cuspidatum . Journal of Experimental Botany, 56(412), 755–763. 10.1093/jxb/eri052. [DOI] [PubMed] [Google Scholar]
- Puritch, G. S. (1973). Effect of water stress on photosynthesis, respiration, and transpiration of four Abies species. Canadian Journal of Forest Research, 3(2), 293–298. [Google Scholar]
- Raim, O. , Kaurilind, E. , Hallik, L. , & Merilo, E. (2012). Why does needle photosynthesis decline with tree height in Norway spruce? Plant Biology, 14(2), 306–314. 10.1111/j.1438-8677.2011.00503.x. [DOI] [PubMed] [Google Scholar]
- Richardson, A. D. , & Berlyn, G. P. (2002). Changes in foliar spectral reflectance and chlorophyll fluorescence of four temperate species following branch cutting. Tree Physiology, 22(7), 499–506. 10.1093/treephys/22.7.499. [DOI] [PubMed] [Google Scholar]
- Robakowski, P. , Montpied, P. , & Dreyer, E. (2002). Temperature response of photosynthesis of silver fir (Abies alba Mill.) seedlings. Annals of Forest Science, 59(2), 163–170. [Google Scholar]
- Slatyer, R. O. (1967). Plant‐water relationships. Academic Press. [Google Scholar]
- von Caemmerer, S. (2000). Biochemical models of leaf photosynthesis. CSIRO Publishing. [Google Scholar]
- von Caemmerer, S. , Evans, J. R. , Hudson, G. S. , & Andrews, T. J. (1994). The kinetics of ribulose‐l,5 bisphosphate carboxylase/oxygenase in vivo inferred from measurements of photosynthesis in leaves of transgenic tobacco. Planta, 195(1), 88–97. [Google Scholar]
- Walcroft, A. S. , Whitehead, D. , Silvester, W. B. , & Kelliher, F. M. (1997). The response of photosynthetic model parameters to temperature and nitrogen concentration in Pinus radiata D. Don. Plant, Cell and Environment, 20(11), 1338–1348. 10.1046/j.1365-3040.1997.d01-31.x. [DOI] [Google Scholar]
- Wang, Q. , Iio, A. , Tenhunen, J. , & Kakubari, Y. (2008). Annual and seasonal variations in photosynthetic capacity of Fagus crenata along an elevation gradient in the Naeba Mountains, Japan. Tree Physiology, 28(2), 277–285. 10.1093/treephys/28.2.277. [DOI] [PubMed] [Google Scholar]
- Warren, C. R. , Dreyer, E. , & Adams, M. A. (2003). Photosynthesis‐Rubisco relationships in foliage of Pinus sylvestris in response to nitrogen supply and the proposed role of Rubisco and amino acids as nitrogen stores. Trees, 17(4), 359–366. 10.1007/s00468-003-0246-2. [DOI] [Google Scholar]
- Watts, W. R. , & Neilson, R. E. (1978). Photosynthesis in Sitka Spruce (Picea sitchensis (Bong.) Carr.). VIII. Measurements of stomatal conductance and 14CO2 uptake in controlled environments. Journal of Applied Ecology, 15(1), 245–255. [Google Scholar]
- Whitehead, D. , Barbour, M. M. , Griffin, K. L. , Turnbull, M. H. , & Tissue, D. T. (2011). Effects of leaf age and tree size on stomatal and mesophyll limitations to photosynthesis in mountain beech (Nothofagus solandrii var. cliffortiodes). Tree Physiology, 31(9), 985–996. 10.1093/treephys/tpr021. [DOI] [PubMed] [Google Scholar]
- Wieser, G. , Oberhuber, W. , Walder, L. , Spieler, D. , & Gruber, A. (2010). Photosynthetic temperature adaptation of Pinus cembra within the timberline ecotone of the Central Austrian Alps. Annals of Forest Science, 67(2), 201. 10.1051/forest/2009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, K. B. , Baldocchi, D. D. , & Hanson, P. J. (2000). Spatial and seasonal variability of photosynthetic parameters and their relationship to leaf nitrogen in a deciduous forest. Tree Physiology, 20(9), 565–578. 10.1093/treephys/20.9.565. [DOI] [PubMed] [Google Scholar]
- Woodruff, D. R. , Meinzer, F. C. , Lachenbruch, B. , & Johnson, D. M. (2009). Coordination of leaf structure and gas exchange along height gradient in a tall conifer. Tree Physiology, 29(2), 261–272. [DOI] [PubMed] [Google Scholar]
- Wullschleger, S. D. (1993). Biochemical Limitations to Carbon Assimilation in C3 plants‐a retrospective analysis of the A/Ci curves from 109 Species. Journal of Experimental Botany, 44(5), 907–920. [Google Scholar]
- Xiong, D. , Douthe, C. , & Flexas, J. (2018). Differential coordination of stomatal conductance, mesophyll conductance, and leaf hydraulic conductance in response to changing light across species. Plant, Cell and Environment, 41(2), 436–450. 10.1111/pce.13111. [DOI] [PubMed] [Google Scholar]
- Xu, L. , & Baldocchi, D. D. (2003). Seasonal trends in photosynthetic parameters and stomatal conductance of blue oak (Quercus douglasii) under prolonged summer drought and high temperature. Tree Physiology, 23(13), 865–877. 10.1093/treephys/23.13.865. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available in figshare at https://doi.org/10.6084/m9.figshare.14791716.


