Abstract
Toxoplasma gondii is an obligate intracellular parasite that actively invades a wide variety of vertebrate cells, although the basis of this pervasive cell recognition is not understood. We demonstrate here that binding to the substratum and to host cells is partially mediated by interaction with sulfated glycosaminoglycans (GAGs). Addition of excess soluble GAGs blocked parasite attachment to serum-coated glass, thereby preventing gliding motility of extracellular parasites. Similarly, excess soluble GAGs decreased the attachment of parasites to human host cells from a variety of lineages, including monocytic, fibroblast, endothelial, epithelial, and macrophage cells. The inhibition of parasite attachment by GAGs was observed with heparin and heparan sulfate and also with chondroitin sulfates, indicating that the ligands for attachment are capable of recognizing a broad range of GAGs. The importance of sulfated proteoglycan recognition was further supported by the demonstration that GAG-deficient mutant host cells, and wild-type cells treated enzymatically to remove GAGs, were partially resistant to parasite invasion. Collectively, these studies reveal that sulfated proteoglycans are one determinant used for substrate and cell recognition by Toxoplasma. The widespread distribution of these receptors may contribute to the broad host and tissue ranges of this highly successful intracellular parasite.
Toxoplasma gondii is one of the most abundant protozoan parasites, chronically infecting approximately 25% of the global human population. This parasite exhibits an extremely broad host range and commonly infects a variety of wild and domestic animals (7). Within its many hosts, the parasite must gain entry into nucleated host cells for survival and replication. Toxoplasma cells are polarized and initially attach by their apical ends prior to actively penetrating the host cell. Invasion is a rapid process (21) that culminates in the formation of a fusion-resistant vacuole (19, 20), which is derived from the host cell plasma membrane (28). This unique mode of entry is driven by an actinomyosin motor operating beneath the parasite plasma membrane (5, 6). The motility of the parasite occurs by a novel process of gliding along the substrate, which also relies on an actinomyosin motor (17).
Despite its impressive ability to invade nearly all types of mammalian cells, little is known about the parasite proteins or host cell receptors that mediate Toxoplasma attachment and invasion. Its wide host range suggests that Toxoplasma may recognize abundant components of the extracellular matrix or widely distributed surface molecules, such as proteoglycans. Several recent reports indicate that recognition of cell surface carbohydrates may contribute to Toxoplasma invasion. For example, removal of surface sialic acid residues from host cells, either enzymatically or by using mutant cell lines, was shown to significantly reduce invasion by Toxoplasma (18). Carbohydrate recognition evidently requires multivalent interactions, as addition of soluble monosaccharides, including sialic acid, had no effect on parasite invasion (3). Several lectin-like activities have been described in Toxoplasma, including binding of whole cells to bovine serum albumin (BSA)-glucosamide (25) and identification of several parasite proteins that bind to red blood cells in a heparin-sensitive manner (22).
Glycosaminoglycan (GAG) moieties on heparan sulfate proteoglycans (HSPGs) decorate the surfaces of members of nearly all vertebrate cell lineages (11, 16). A variety of pathogens have evolved strategies for their recognition, including Chlamydia (30), Trypanosoma cruzi (23), and Plasmodium (the etiologic agent of malaria) (12, 24, 27). A recent study suggested that GAG recognition is also involved in cell attachment by Toxoplasma based on the observations that low concentrations of GAGs increased invasion of human fibroblasts and mutant CHO cells lacking in cell surface sulfated proteoglycans were less susceptible to invasion (22). While these studies implicated GAG recognition in parasite attachment to fibroblastic cells, they did not examine the potential roles of the variety of different sulfated glycans that occur on other cell lineages.
Recognizing that the broad host range of Toxoplasma implies the recognition of a common host cell determinant, we have investigated the interaction of the parasite with GAGs on a wide range of human cell types. Furthermore, because gliding motility is also contact dependent, we have examined whether the interaction with the substratum is influenced by GAGs. Our studies demonstrate that a variety of GAGs may function as receptors for Toxoplasma adhesion to the substratum during gliding and for attachment to host cells during invasion.
MATERIALS AND METHODS
Strain, cells, and culture conditions.
Toxoplasma strain RH and the lacZ-expressing clone 2F were cultured in human foreskin fibroblast (HFF) monolayers as previously described (21). Chinese hamster ovary cells (CHO-K1) and a GAG-deficient mutant called pgsA-745 (8) were obtained from the American Type Culture Collection (ATCC) and maintained by serial passage in Ham's F12K medium with 2 mM l-glutamine, 1.5 g of sodium bicarbonate/liter, and 10% fetal bovine serum (FBS). Additional cell lines, including HEp-2, HUV-EC-C, U373, G361, and U937 cells, were obtained from the ATCC and maintained as recommended. GAGs were obtained from Sigma (St. Louis, Mo.) and dissolved in phosphate-buffered saline (PBS) or complete medium. Heparinase I and heparinase III enzymes (Sigma) and chondroitinase ABC (Seikagaku, Associates of Cape Cod, Falmouth, Mass.) were resuspended in Dulbecco's modified Eagle medium (DMEM), aliquoted, and frozen at −70°C until use.
Gliding assays.
Coverslips were coated by incubation in 50% FBS diluted in PBS or with 100 μg of BSA for 1 h at 37°C followed by rinsing in PBS. Freshly harvested tachyzoites were resuspended at approximately 107/ml in Hank's balanced salt solution containing 10 mM HEPES and 0.1 mM EGTA, added to precoated Lab-Tek four-chamber glass slides or glass coverslips, and incubated at 37°C for 15 min. The slides were briefly rinsed and fixed in 2.5% formalin–PBS for 10 min. Trails were visualized by indirect immunofluorescence (IF) using the monoclonal antibody (MAb) DG52 diluted 1:500 to detect the surface protein SAG1 (1) (kindly provided by John Boothroyd, Stanford University). Following incubation in primary antibodies, the slides were rinsed and stained with goat-anti mouse immunoglobulin G (IgG) conjugated to fluorescein isothiocyanate at 1:500 (Jackson ImmunoResearch Laboratories, West Grove, Pa.). The slides were rinsed and mounted in 20% glycerol–PBS and examined with a Zeiss Axioscope equipped for phase-contrast and epifluorescence microscopy. Ten representative fields were scored (at ×400) to evaluate the presence of parasites attached to the substrate and to monitor the absolute number and length of trails as estimated in parasite body lengths (approximately 5 to 7 μm). Experiments were repeated three times independently, and the results are tabulated as the percentage of control plus or minus standard error (SE).
Host cell attachment assays.
Toxoplasma attachment to and invasion of HFF, HEp-2, HUV-EC-C, U373, G361, and U937 monolayers were quantified by colorimetric detection of β-galactosidase (β-Gal) activity expressed by the parasite strain 2F as previously described (6). Briefly, purified parasites were incubated with HFF monolayers in invasion medium (DMEM plus 3% FBS, 20 mM HEPES, and 0.2% NaH2CO3) for 10 or 20 min at 37°C. Following extensive washing in PBS, the monolayers were lysed, and β-Gal activity was determined using the substrate chlorophenol red β-d-galactopyranoside (9). Parasite numbers were determined by comparison of enzyme levels to dilution standards that were produced by lysis of parasites that had been counted with a hemocytometer.
IF detection of cell attachment versus invasion.
Differential IF staining was used to examine parasite invasion of HFFs or CHO-K1 or pgsA-745 cells. To discriminate between extracellular and intracellular parasites, differentially stained (red versus green) antibodies to the surface protein SAG1 were added before and after detergent permeabilization, respectively. Briefly, host cells were grown on Permanox chamber slides (Nalge Nunc International, Milwaukee, Wis.) and infected with parasites as described above. The infected monolayers were washed with PBS and then fixed with 2.5% formaldehyde–0.02% gluteraldehyde in PBS. After being blocked for 30 min with 10% FBS, the slides were stained with rabbit anti-SAG1 antibody at 1:2,000 diluted in 1% FBS–1% normal goat serum. The monolayers were then permeabilized by incubation for 10 min in 0.01% saponin and then incubated with MAb DG52 to SAG1 diluted 1:1,000 in 1% FBS–1% normal goat serum. After being rinsed, the slides were incubated with a mixture of Texas red-conjugated goat anti-rabbit IgG (Jackson Laboratories) (1:500) and fluorescein isothiocyanate-conjugated goat anti-mouse IgG (Jackson Laboratories) (1:500). The slides were examined by epifluorescence microscopy, and the total number of cell-associated parasites was determined and scored as being outside (red or orange staining) versus inside (green staining) the cells. Experiments were repeated three times independently, and the results were tabulated to obtain a mean plus or minus standard deviation.
Enzymatic removal of cell surface proteoglycans.
HFFs were grown to confluency in 96-well plates, and separate groups of five wells were treated for 3 h at 37°C with each of the following: heparinase I, heparinase III (at 1.7 × 10−3 or 1.7 × 10−4 IU/ml [corresponding to 1.0 and 0.1 Sigma units, respectively]) and chondroitinase ABC (at 0.1 and 1.0 IU) in DMEM. The cells were then rinsed with warm invasion medium and challenged with 5 × 106 parasites of the 2F strain of Toxoplasma/ml. The parasites were allowed to invade for 30 min and then washed extensively with warm PBS containing 1 mM CaCl2 and 1 mM MgCl2. Wells that did not contain HFFs were also inoculated with 2F and treated in the same manner and were used as controls for washing. The cells were then lysed and developed for β-Gal activity as described above. The data were calculated as the number of parasite cells bound per treatment (minus the washing control) and are reported as the mean of two experiments ±SE.
To evaluate the efficiency of enzymatic treatments, a comparative enzyme-linked immunosorbent assay was used to monitor the expression of heparan sulfate epitopes (recognized by the MAb 10E4) versus the core syndecan protein (recognized by the MAb 3G10) (4). HFF monolayers in 96-well plates were treated with 200 μl of 1.7 × 10−3-IU/ml heparatinase (heparinase III)/well in DMEM lacking serum for 3 h at 37°C in a CO2 incubator. The enzyme suspension was removed, and the cells were fixed for 15 min at 4°C in 10% neutral buffered formalin solution (Sigma). The cells were blocked for 30 min at room temperature in 10% normal goat serum in PBS and incubated for 1 h at room temperature in primary antibodies diluted 1:1,000. After the cells were washed five times with PBS–0.05% Tween, horseradish peroxidase-conjugated goat anti-mouse IgG plus IgM was added at 1:10,000 dilution and the plate was incubated for 1 h at room temperature. After the plate was washed five times with PBS–0.05% Tween, 200 μl of o-phenylenediamine substrate was added per well, and the reaction product was read at 490 nm using an enzyme-linked immunosorbent assay plate reader.
RESULTS
Soluble GAGs disrupt substrate attachment and prevent gliding motility.
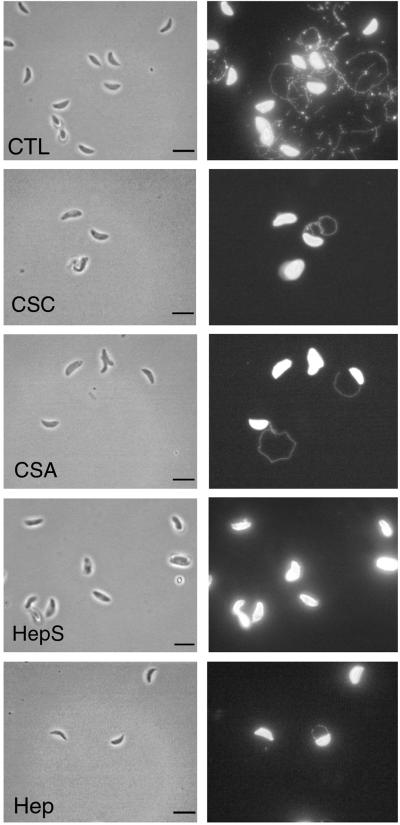
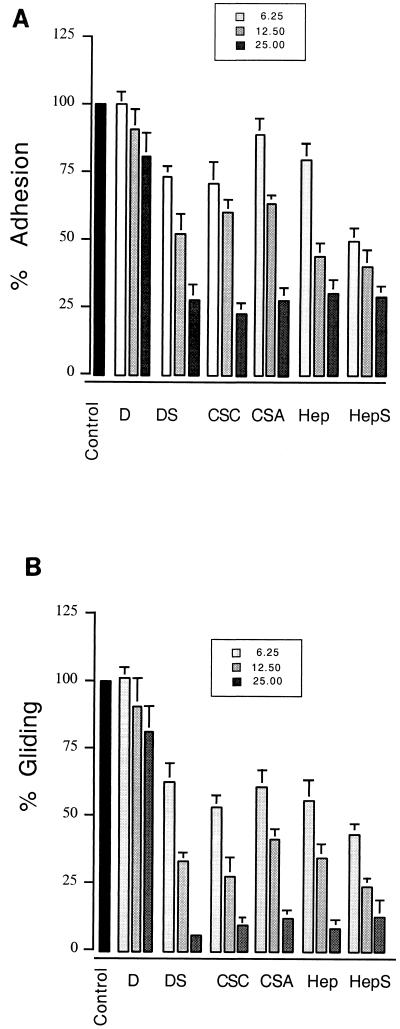
Gliding motility in Toxoplasma is easily visualized by staining for the trails consisting of membrane proteins that are left on the substrate (17). We examined the effect of increasing concentrations of soluble GAGs on Toxoplasma gliding on serum- or BSA-coated slides by staining for the presence of trails using an antibody to the major surface protein SAG1. Heparin, heparan sulfate, and chondroitin sulfate A (CSA) and CSC significantly decreased the attachment of the parasite to protein-coated slides (Fig. 1). Concomitantly, the frequency of gliding as evidenced by the density of trails left on the substrate decreased in a dose-dependent fashion (Fig. 1). When these effects were examined quantitatively, heparin, heparan sulfate, CSA, and CSC showed a dose-dependent inhibition in the average number of parasites attached to the substrate (Fig. 2A) and the average number of trails produced (Fig. 2B). Consistent with these results being mediated by the negatively charged sulfate groups on the molecules, the synthetic GAG substitute dextran sulfate also inhibited attachment and gliding while no difference was observed with dextran alone. In the presence of high levels of heparin, heparan sulfate, and CSA and CSC, those parasites that were able to successfully attach produced very short trails (Fig. 1 and Table 1). The decreased number and length of trails observed with inhibitors is likely a result of competitive displacement from the substrate rather than a direct effect on motility, inasmuch as substrate binding is a prerequisite for gliding (17).
FIG. 1.
IF detection of trails produced by gliding Toxoplasma on protein-coated glass. Addition of heparin (Hep), heparan sulfate (HepS), CSA, and CSC at 25 mg/ml significantly inhibited both substrate attachment and the formation of trails. The trails were detected by staining for the surface antigen SAG1 as described in Materials and Methods. CTL, gliding in medium in the absence of additions. Bars, 5 μm.
FIG. 2.
GAGs inhibit gliding motility. Incubation of parasites with increasing concentrations (6.2, 12.5, and 25 mg/ml) of heparin (Hep), heparan sulfate (HepS), CSA, CSC, and dextran sulfate (DS), but not dextran (D), resulted in a significant inhibition of attachment to serum-coated glass (A) and relative gliding as monitored by the formation of trails (B). Control represents the extent of attachment or gliding in medium lacking additives. The data are reported as average percentages of control plus SE; n = 3.
TABLE 1.
Effects of soluble GAGs on trail lengths produced by gliding Toxoplasma
| Compound | Trail lengtha at GAG concnb of:
|
|||
|---|---|---|---|---|
| 0 | 6.25 | 12.5 | 25.0 | |
| Dextran | 9.7 | 9.1 | 9.2 | 8.8 |
| Dextran sulfate | 9.6 | 8.5 | 8.3 | 4.2c |
| Heparin | 10.2 | 9.3 | 7.9 | 4.7c |
| Heparan sulfate | 9.2 | 8.3 | 7.8 | 4.4c |
| CSA | 9.4 | 8.8 | 8.2 | 4.6c |
| CSC | 9.5 | 8.8 | 8.4 | 4.6c |
Mean trail length in parasite body lengths (approximately 5 to 7 μm).
In milligrams per milliliter.
Significantly different from control (P ≤ 0.05; Student's t test).
Soluble GAGs disrupt parasite binding to human fibroblasts.
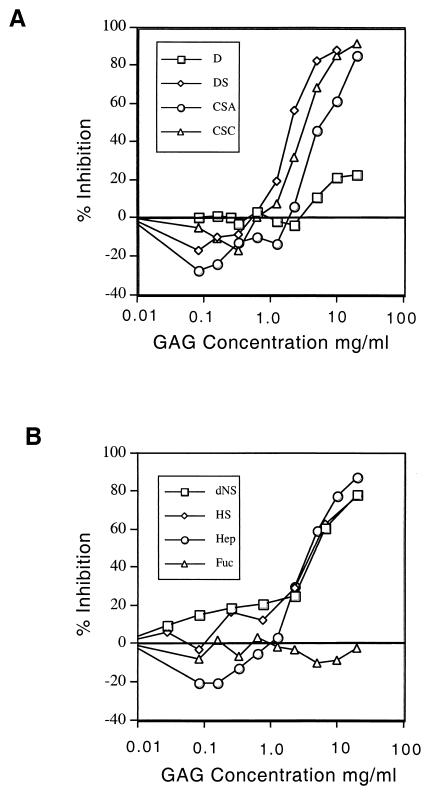
We investigated whether Toxoplasma recognizes ubiquitous receptors such as GAGs, which are expressed on cell surface HSPGs. To disrupt potential interactions between the parasite and cell surface HSPGs, we incubated HFFs with increasing concentrations of soluble GAGs before challenging them with parasites. At low concentrations (<1 mg/ml), GAGs enhanced parasite binding to host cells up to 30% (Fig. 3), an effect that was seen with heparin, dextran sulfate, and CSA and CSC but not with dextran or fucoidin. In contrast, at higher concentrations (1 to 20 mg/ml), heparin, heparan sulfate, de-N-sulfated heparin, CSC, CSA, and dextran sulfate each dramatically inhibited parasite attachment in a dose-dependent manner. Despite the high levels needed to observe this inhibition, the effects of sulfated glycans were not due to toxicity, as incubation of extracellular parasites with high levels of GAGs followed by washing to remove the soluble glycan had no residual effect on invasion (data not shown). Unsulfated dextran and fucoidin, a sulfated l-fucose oligosaccharide, were significantly less potent in blocking parasite binding to HFFs.
FIG. 3.
Soluble GAGs disrupt Toxoplasma attachment to human fibroblasts in a biphasic, dose-dependent manner. (A) CSC, CSA, or the synthetic polyanion dextran sulfate (DS) slightly enhanced attachment at concentrations of <1 mg/ml but then markedly inhibited attachment at higher concentrations, whereas nonsulfated dextran (D) had little effect. (B) Heparin (Hep) also showed a biphasic response, enhancing binding at low doses and inhibiting it at higher concentrations. Heparan sulfate (HS) as well as de-N-sulfated heparin (dNS) competed for binding of parasites to host cell monolayers only at higher concentrations, while fucoidin (Fuc) (a polymer of sulfated l-fucose) had little affect. HFF monolayers in 96-well plates were preincubated with 0 to 20 mg of GAGs/ml, and the attachment of parasites was quantified using a colorimetric assay for β-Gal activity. The data are reported as mean percent inhibition, where untreated controls represent 100%; n = 3.
Host cell GAG-deficient mutants are less susceptible to parasite invasion.
To further explore the role of HSPGs as receptors for Toxoplasma attachment, we investigated the ability of Toxoplasma to bind to pgsA-745 cells, which do not express GAGs due to a mutation in xylose transferase (8). Attachment to wild-type CHO-K1 cells was decreased by excess soluble heparin and dextran sulfate, similar to the effects observed for HFFs described above. Despite the overall reduction in cell-associated parasites, no difference was observed in the proportion of parasites that were outside versus inside the cells (data not shown). Adhesion of Toxoplasma to pgsA-745 cells was reduced by approximately 40% compared to adhesion to wild-type CHO-K1 cells (Fig. 4). Although similar results were reported previously (22), an important additional control included here was to determine whether invasion of pgsA-745 cells was sensitive to GAG inhibition. We observed that residual attachment to pgsA-745 cells was unaffected by heparin or dextran sulfate at a concentration (5 mg/ml) that inhibits attachment to CHO-K1 cells by ∼70% (Fig. 4). These results confirm that inhibition of attachment by soluble GAGs is specific and that alternative receptors used by Toxoplasma are likely structurally unrelated to GAGs.
FIG. 4.
GAG-deficient host cells (pgsA-745) were ∼40% less susceptible to infection by parasites than wild-type CHO-K1 host cells (Ctl), and their residual capacity for attachment was unaffected by soluble GAGs. Wild-type and mutant host cells were pretreated with medium alone or 5 mg of heparin (H), dextran sulfate (DS), or dextran (D) per ml, and host-cell-associated parasites were quantified by IF microscopy. The data are expressed as the mean number of parasites per host cell plus SE; n = 3. ∗∗, statistically significant at a P value of ≤0.01 (Student's t test).
Enzymatic treatments.
To confirm the importance of host cell surface GAGs in parasite attachment, we also treated wild-type HFFs with enzymes that recognize and cleave specific classes of sulfated glycans. Treatment with heparinase I, which preferentially cleaves heparin versus heparan sulfate, resulted in a 35% reduction in parasite binding when heparinase I was used at 1.7 × 10−3 IU/ml and 29% reduction at 1.7 × 10−4 IU/ml (Fig. 5). This decrease in invasion is similar in magnitude to that observed for GAG-deficient cells, suggesting that heparin is the major constituent of HSPGs that is recognized by Toxoplasma. Treatment with heparinase III, which preferentially cleaves heparan sulfate versus heparins, reduced parasite binding by 21% and 15% for treatment with 1.7 × 10−3 IU/ml and 1.7 × 10−4 IU/ml, respectively (Fig. 5). Treatment with chondroitinase at 1 IU/ml resulted in only a 10% reduction in parasite binding, although this effect was highly reproducible (Fig. 5). To verify that the enzymatic treatments were effective, we monitored the loss of an epitope that is specific to heparan sulfate (detected with MAb 10E4) and the increase in exposure of an epitope that is exposed only upon enzyme digestion (detected with MAb 3G10 [4]). Treatment with 1 U of heparinase III, which preferentially cleaves heparan sulfate, per ml resulted in a 75% reduction in the signal detected with MAb 10E4 with a corresponding 200-fold increase in the signal detected with MAb 3G10. While a similar test is not feasible for heparinase I or chondroitinases due to the lack of antibodies that specifically recognize these moities, these results indicate that the enzyme treatments were largely effective in removing surface GAGs from HFFs.
FIG. 5.
Inhibition of parasite attachment to HFFs following enzymatic treatments to remove cell surface GAGs. Treatment with heparinase I (HepI) at 1.7 × 10−3 IU/ml or 1.7 × 10−4 IU/ml significantly reduced parasite attachment (∗∗, P ≤ 0.01). Treatment with heparinase III (HepIII) reduced parasite attachment at 1.7 × 10−3 IU/ml and to a lesser extent at 1.7 × 10−4 IU/ml (∗, P ≤ 0.05). Treatment with chondroitinase ABC (Case-ABC) at 1 IU/ml reduced parasite attachment only slightly. The data are expressed as the mean plus SE; n = 2.
Soluble GAGs disrupt parasite binding to a variety of human cell types.
Because Toxoplasma infects a wide range of cell types in vivo, we also tested the effects of soluble GAGs on cell attachment to different human cell types. The same panel of GAGs, and again dextran sulfate but not dextran, blocked parasite attachment to human cells derived from epithelial, endothelial, monocytic, neural, and melanocytic human cell lines (Table 2). Interestingly, fucoidin produced highly varied inhibitory effects: it was a potent inhibitor of invasion of G361 melanocytes (50% inhibitory concentration [IC50], 0.3 mg/ml) yet was relatively ineffective on HEp-2 epithelial cells (IC50, 19.5 mg/ml). This may indicate that fucoidin, while not normally produced by vertebrates, is structurally similar to a receptor that is preferentially used by Toxoplasma for invasion of melanocytes.
TABLE 2.
Inhibition by soluble GAGs of T. gondii attachment to mammalian cellsa
| Cell line | Cell type | IC50 (mg/ml)a
|
|||||
|---|---|---|---|---|---|---|---|
| Heparin | CSC | CSA | Dextran sulfate | Dextran | Fucoidin | ||
| HFF | Fibroblast | 4.8 ± 2.0 | 4.1 ± 1.4 | 7.3 ± 1.7 | 2.2 ± 0.4 | >20 | >20 |
| HEp-2b | Epithelial | 5.5 ± 1.6 | 4.5 ± 0.4 | 6.3 ± 2.3 | 5.7 ± 2.3 | 14.1 ± 2.1 | 19.5 ± 0.2 |
| HUV-EC-Cb | Endothelial | 1.8 ± 0.2 | 1.6 ± 0.4 | 2.9 ± 1.5 | 1.0 ± 0.4 | 9.7 ± 3.4 | 4.1 ± 1.8 |
| U373b | Glial or astrocyte | 3.0 ± 1.9 | 3.0 ± 0.8 | 1.1 ± 0.4 | 2.1 ± 0.4 | 7.4 ± 3.8 | 16.0 ± 3.7 |
| G361b | Melanocyte | 4.0 ± 0.3 | 2.0 ± 0.1 | 3.0 ± 1.9 | 4.6 ± 0.4 | 8.8 ± 4.7 | 0.3 ± 0.1 |
| U937b | Macrophage | 2.0 ± 0.3 | 1.8 ± 0.8 | 2.6 ± 1.5 | 6.7 ± 2.6 | 7.0 ± 2.7 | 13.2 ± 4.9 |
| Averagec | 3.5 ± 1.4 | 2.8 ± 1.1 | 3.9 ± 2.2 | 3.7 ± 2.1 | 11.2 ± 4.6 | 11.7 ± 6.2 | |
Mean ±SE; n = 2 or 3.
Obtained from ATCC.
Average values (±SE) for all cell lines tested.
DISCUSSION
Toxoplasma is able to efficiently attach to and invade a wide range of vertebrate cells, suggesting that it recognizes ubiquitous receptors. Here we provide the following evidence that sulfated proteoglycans are used by Toxoplasma for substrate and cell attachment: (i) soluble GAGs inhibit parasite attachment to the substrate, thereby decreasing motility; (ii) soluble GAGs inhibit parasite attachment to a variety of cell types which express HSPGs; (iii) host cell mutants that lack GAG chains are less susceptible to parasite invasion; and (iv) enzyme treatments that removed GAGs from the surfaces of wild-type HFFs reduced parasite attachment. The recognition of GAGs by Toxoplasma involves both glucosaminoglycans (GAGs) (heparin and heparan sulfates) and galactosaminoglycans (CSA and CSC), and the relative importance of these interactions varies with cell type. Thus, sulfated protoglycans are one class of receptors used for host cell attachment by Toxoplasma, and the widespread distribution of these molecules on the surfaces of mammalian cells may contribute to the broad specificity of host cells susceptible to invasion by this parasite.
Interaction with GAGs disrupts substrate attachment and hence parasite motility.
Gliding is a novel form of motility that, like cell invasion, relies on an actinomyosin motor in the parasite (17). Gliding is substrate dependent and results in the deposit of membrane trails on the substrate, often in spiral or circular patterns. Gliding motility likely underlies cell entry and egress and tissue migrations that occur during dissemination in vivo. During gliding in vitro, the recognition of the surface is highly permissive, and gliding occurs on glass coated with serum, BSA, and a variety of extracellular matrix proteins (17; S. Håkansson and L. D. Sibley, unpublished data). We show here that excess soluble GAGs disrupt parasite binding to both serum- and BSA-coated glass, thus reducing the formation of trails. It is likely that the effect of GAGs in reducing gliding is mainly due to their disruption of parasite attachment rather than to an effect on motility directly. Because GAGs are common constituents of both serum and the extracellular matrix, parasite recognition of these compounds may contribute to substrate attachment that occurs during tissue migration and/or travel between cells in vivo.
Toxoplasma adhesion is partially mediated by binding to host cell HSPGs.
We also explored the influence of soluble GAGs on parasite attachment to HFFs, a cell line commonly used for the propagation of Toxoplasma. We directly monitored parasites that were cell associated after a short infection pulse, using the expression of an exogenous reporter, β-Gal, to enumerate the parasites. At doses from 0.01 to 1.0 mg of soluble GAGs/ml we observed a modest enhancement of attachment, while at higher doses (>1 mg/ml) a pronounced inhibition was observed. The biphasic effect of soluble GAGs on parasite attachment to host cells suggests that parasite binding can occur through a soluble GAG molecule acting as a bridge to receptors on the cell surface or by direct binding of a parasite lectin with cell surface proteoglycans. The enhancement of parasite binding to HFFs that occurs at lower concentrations of GAGs was also observed previously, although a notable difference between our findings and those of the earlier authors was the observation that fucoidin, a branched, sulfated fucose polymer, enhanced attachment to a greater extent than GAGs (22). The reason for this discrepancy is unknown but may be related to differences in the expression of HSPGs by different populations of HFFs which are not clonal and are derived independently from different individual donors. The attachment to host cells by Toxoplasma was disrupted by high doses of a variety of sulfated polysaccharides, including heparin, de-N-sulfated heparin, and heparan sulfate. The inhibition by de-N-sulfated heparin suggests that the N sulfation is not a requirement for recognition but rather that O sulfation may play an important role in binding. This conclusion is also supported by the finding that CSA and CSC, which lack N sulfation but have various degrees of 4-O sulfation and 6-O sulfation, also blocked parasite binding. High doses of fucoidin markedly inhibited parasite attachment only to G361 melanocytes, suggesting that it mimics the structure of a receptor on G361 cells utilized by Toxoplasma.
We have extended the finding that Toxoplasma recognizes host cell sulfated proteoglycans by examining a wide range of human cell types. Excess soluble GAGs were capable of inhibiting parasite invasion of a variety of different lineages, including fibroblastic, epithelial, endothelial, monocytic, melanocytic, and macrophage cell types. Moreover, this inhibition was apparent when using heparin, dextran sulfate, and CSA and CSC. This finding suggests that ligands on the parasite are capable of recognizing both highly sulfated glycans like heparin, consisting of glucuronic or iduronic acid and sulfated glucosamine, and the relatively less sulfated chondroitins, consisting of glucuronic acid and variably sulfated N-acetylgalactosamine. The relative effectiveness of inhibition with these GAG compounds varies with cell type, suggesting that a range of HSPGs on different cell lines are used for attachment by Toxoplasma. For example, CSA was significantly less potent at inhibiting Toxoplasma invasion of the fibroblastic HFF cell line (I.C.50, 7.3 ± 1.7 mg/ml) than the monocytic U373 cell line (I.C.50, 1.1 ± 0.4 mg/ml). Consistent with this, treatment with chondroitinase had little effect on invasion of HFFs. Notably, CSA and CSC were more potent inhibitors of invasion of HUV-EC-C cells, an endothelial cell derived from the umbilical cord. Such differences may influence tissue migrations that occur in vivo and may underlie the specific pathologies of toxoplasmosis in congenital infections.
Further evidence for the role of sulfated proteoglycans in cell recognition was provided by GAG-deficient host cells, which were less susceptible to invasion by Toxoplasma, a result that is similar to that of a recent study (22). To determine if this effect was due to decreased binding or decreased invasion, we employed a two-color fluorescence assay which directly distinguishes parasites that are attached but extracellular from those which have entered the host cell following a short infectious pulse. We observed that the major effect of soluble GAGs is to decrease parasite attachment to the host cells, while parasites that were still able to attach under these conditions displayed a similar rate of internalization. In addition to demonstrating a decrease in the susceptibility of these mutants, we observed that the residual invasion of these cells is no longer affected by soluble GAGs. This result further establishes the specificity of blocking experiments, in which relatively high levels of GAGs are necessary to decrease parasite attachment to wild-type host cells. Finally, enzymatic treatment of wild-type HFFs revealed that removal of either heparin or heparan sulfate significantly reduced the binding of parasites. Despite their important role in cell attachment, HSPGs are not the only class of receptors used for adhesion, as evidenced by the ability of Toxoplasma to invade GAG-deficient host cells and the relatively modest decrease in infectivity following enzymatic treatments. Host cell mutants that are deficient in sulfated proteoglycan synthesis may be useful for identifying alternative receptors for parasite invasion.
Potential parasite ligands that recognize host cell surface proteoglycans.
We have recently provided evidence that microneme proteins, which are discharged during the earliest steps of host cell binding, are involved in cell attachment (2). Micronemes contain several proteins implicated in binding to host cell surface HSPGs. For example, MIC2 contains a single integrin-like I domain (also known as A domain) and six tandemly arranged thrombospondin type 1-like (TSP) repeats (29). Independently, we have demonstrated that MIC2 binds tightly to host cells (2) and that purified MIC2 recognizes both GAGs and a subset of extracellular matrix proteins (V. B. Carruthers, unpublished data). The Toxoplasma MIC1 protein also contains two degenerate TSP repeats, and although this protein binds to host cells in vitro, the receptor(s) that it recognizes has not been characterized (10). Recently, several heparin binding proteins were identified in lysates of Toxoplasma based on their ability to agglutinate red blood cells in a heparin-sensitive manner (22). These heparin binding activities were localized to an intracellular, apical compartment in the parasite, although the identities of the molecules remain to be established.
Similarities to cell adhesion by Plasmodium.
Recognition of sulfated glycans on cell surface HSPGs is a common theme among intracellular parasites, and there are a number of parallels between our findings for Toxoplasma and those previously established for the related parasite Plasmodium. For example, similar inhibition of sporozoite binding to hepatocytes has been reported using excess soluble GAGs (24). Similar to our observations with Toxoplasma, the interactions with GAGs account for only part of cell binding by Plasmodium sporozoites, and while reduced binding is observed in GAG-deficient or enzymatically treated cells, invasion still occurs (13). In malaria, the residual binding activities have been attributed to low-density lipoprotein receptor-related protein (LRP), which plays a scavenging role on hepatocytes (26). LRP is also abundant on CHO cells, although it is not known if it contributes to the binding of Toxoplasma. It is equally plausible that the non-GAG binding of Toxoplasma occurs through the previously described interactions with extracellular matrix proteins, such as laminin (14, 15), or with host cell glycoproteins bearing sialic acid residues (18).
The ability of Toxoplasma to infect a variety of tissues and cells within its many vertebrate hosts appears to result in part from the ubiquitous expression of HSPG receptors. Identification of the parasite molecules that mediate this interaction will provide molecular confirmation of the importance of this pathway in parasite attachment to and invasion of host cells.
ACKNOWLEDGMENTS
We thank Amy Crawford for expert assistance in cell culture and Antonio Barragan for helpful comments in the revision of the manuscript.
This work was supported by the National Institutes of Health (AI 36034).
REFERENCES
- 1.Burg J L, Perlman D, Kasper L H, Ware P L, Boothroyd J C. Molecular analysis of the gene encoding the major surface antigen of Toxoplasma gondii. J Immunol. 1988;141:3584–3591. [PubMed] [Google Scholar]
- 2.Carruthers V B, Giddings O K, Sibley L D. Secretion of micronemal proteins is associated with Toxoplasma invasion of host cells. Cell Microbiol. 1999;1:225–236. doi: 10.1046/j.1462-5822.1999.00023.x. [DOI] [PubMed] [Google Scholar]
- 3.Crane M J, Dvorak J A. Influence of monosaccharides on the infection of vertebrate cells by Trypanosoma cruzi and Toxoplasma gondii. Mol Biochem Parasitol. 1982;3:333–341. doi: 10.1016/0166-6851(82)90040-8. [DOI] [PubMed] [Google Scholar]
- 4.David G, Bai X M, Van der Schueren B, Cassiman J J, Van den Berghe H. Developmental changes in heparan sulfate expression: in situ detection with mAbs. J Cell Biol. 1992;119:961–975. doi: 10.1083/jcb.119.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dobrowolski J M, Carruthers V B, Sibley L D. Participation of myosin in gliding motility and host cell invasion by Toxoplasma gondii. Mol Microbiol. 1997;26:163–173. doi: 10.1046/j.1365-2958.1997.5671913.x. [DOI] [PubMed] [Google Scholar]
- 6.Dobrowolski J M, Sibley L D. Toxoplasma invasion of mammalian cells is powered by the actin cytoskeleton of the parasite. Cell. 1996;84:933–939. doi: 10.1016/s0092-8674(00)81071-5. [DOI] [PubMed] [Google Scholar]
- 7.Dubey J P. Toxoplasma, Hammondia, Besniotia, Sarcocystis, and other tissue cyst-forming coccidia of man and animals. In: Kreier J P, editor. Parasitic protozoa. New York, N.Y: Academic Press; 1977. pp. 101–237. [Google Scholar]
- 8.Esko J D, Rostand K S, Weinke J L. Tumor formation dependent on proteoglycan biosynthesis. Science. 1988;241:1092–1096. doi: 10.1126/science.3137658. [DOI] [PubMed] [Google Scholar]
- 9.Eustice D C, Feldman P A, Colberg-Poley A M, Buckery R M, Neubauer R H. A sensitive method for the detection of β-galactosidase in transfected mammalian cells. Biotechnology. 1991;11:739–742. [PubMed] [Google Scholar]
- 10.Fourmaux M N, Achbarou A, Mercereau-Puijalon O, Bderre C, Brache I, Loyens A, Odberg-Ferragut C, Camus D, Dubremetz J F. The MIC1 microneme protein of Toxoplasma gondii contains a duplicated receptor-like domain and binds to host cell surface. Mol Biochem Parasitol. 1996;83:201–210. doi: 10.1016/s0166-6851(96)02773-9. [DOI] [PubMed] [Google Scholar]
- 11.Frazier W A. Thrombospondin: a modular adhesive glycoprotein of platelets and nucleated cells. J Cell Biol. 1987;105:625–632. doi: 10.1083/jcb.105.2.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frevert U. Malaria sporozoite-hepatocyte interactions. Exp Parasitol. 1994;79:206–210. doi: 10.1006/expr.1994.1082. [DOI] [PubMed] [Google Scholar]
- 13.Frevert U, Sinnis P, Esko J D, Nussenzweig V. Cell surface glycosaminoglycans are not obligatory for Plasmodium berghei sporozoite invasion in vitro. Mol Biochem Parasitol. 1996;76:257–266. doi: 10.1016/0166-6851(95)02563-4. [DOI] [PubMed] [Google Scholar]
- 14.Furtado G C, Cao Y, Joiner K A. Laminin on Toxoplasma gondii mediates parasite binding to the β1 integrin receptor α6/β1 on human foreskin fibroblasts and Chinese hamster ovary cells. Infect Immun. 1992;60:4925–4931. doi: 10.1128/iai.60.11.4925-4931.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furtado G C, Slowik M, Kleinman H K, Joiner K A. Laminin enhances binding of Toxoplasma gondii tachyzoites to J774 murine macrophage cells. Infect Immun. 1992;60:2337–2342. doi: 10.1128/iai.60.6.2337-2342.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo N-H, Krutzsch H C, Negre E, Zabrenetzky V S, Roberts D D. Heparin-binding peptides from the type I repeats of thrombospondin. J Biol Chem. 1992;267:19349–19355. [PubMed] [Google Scholar]
- 17.Håkansson S, Morisaki H, Heuser J E, Sibley L D. Time-lapse video microscopy of gliding motility in Toxoplasma gondii reveals a novel, biphasic mechanism of cell locomotion. Mol Biol Cell. 1999;10:225–235. doi: 10.1091/mbc.10.11.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monteiro V G, Soares C P, de Souza W. Host cell surface sialic acid residues are involved in the process of penetration of Toxoplasma gondii into mammalian cells. FEMS Microbiol Lett. 1998;164:323–327. doi: 10.1111/j.1574-6968.1998.tb13105.x. [DOI] [PubMed] [Google Scholar]
- 19.Mordue D, Håkansson S, Niesman I, Sibley L D. Toxoplasma gondii resides in a vacuole that avoids fusion with host cell endocytic and exocytic vesicular trafficking pathways. Exp Parasitol. 1999;92:87–99. doi: 10.1006/expr.1999.4412. [DOI] [PubMed] [Google Scholar]
- 20.Mordue D G, Sibley L D. Intracellular fate of vacuoles containing Toxoplasma gondii is determined at the time of formation and depends on the mechanism of entry. J Immunol. 1997;159:4452–4459. [PubMed] [Google Scholar]
- 21.Morisaki J H, Heuser J E, Sibley L D. Invasion of Toxoplasma gondii occurs by active penetration of the host cell. J Cell Sci. 1995;108:2457–2464. doi: 10.1242/jcs.108.6.2457. [DOI] [PubMed] [Google Scholar]
- 22.Ortega-Barria E, Boothroyd J C. A Toxoplasma lectin-like activity specific for sulfated polysaccharides is involved in host cell infection. J Biol Chem. 1999;274:1267–1276. doi: 10.1074/jbc.274.3.1267. [DOI] [PubMed] [Google Scholar]
- 23.Ortega-Barria E, Pereira M E A. A novel T. cruzi heparin-binding protein promotes fibroblast adhesion and penetration of engineered bacteria and trypanosomes into mammalian cells. Cell. 1991;67:411–421. doi: 10.1016/0092-8674(91)90192-2. [DOI] [PubMed] [Google Scholar]
- 24.Pancake S J, Holt G D, Mellouk S, Hoffman S L. Malaria sporozoites and circumsporozoite proteins bind specifically to sulfated glycoconjugates. J Cell Biol. 1992;117:1351–1357. doi: 10.1083/jcb.117.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robert R, Jarrige P L, Mahaza C, Cottin J, Marot-Leblond A, Senet J M. Specific binding of neoglycoproteins to Toxoplasma gondii tachyzoites. Infect Immun. 1991;59:4670–4673. doi: 10.1128/iai.59.12.4670-4673.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shakibaei M, Frevert U. Dual interaction of the malaria circumsporozoite protein with the low density lipoprotein receptor-related protein (LRP) and heparin sulfate proteoglycans. J Exp Med. 1996;184:1699–1711. doi: 10.1084/jem.184.5.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sinnis P, Clavijo P, Fenyo D, Chait B T, Cerami C, Nussenzweig V. Structural and functional properties of region II-plus of the malaria circumsporozoite protein. J Exp Med. 1994;180:297–306. doi: 10.1084/jem.180.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suss-Toby E, Zimmerberg J, Ward G E. Toxoplasma invasion: the parasitophorous vacuole is formed from host cell plasma membrane and pinches off via a fusion pore. Proc Natl Acad Sci USA. 1996;93:8413–8418. doi: 10.1073/pnas.93.16.8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wan K L, Carruthers V B, Sibley L D, Ajioka J W. Molecular characterisation of an expressed sequence tag locus of Toxoplasma gondii encoding the micronemal protein MIC2. Mol Biochem Parasitol. 1996;84:203–214. doi: 10.1016/s0166-6851(96)02796-x. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J P, Stephens R S. Mechanism of C. trachomatis attachment to eukaryotic cells. Cell. 1992;69:861–869. doi: 10.1016/0092-8674(92)90296-o. [DOI] [PubMed] [Google Scholar]