Abstract
Background:
Irrigation is commonly used as an adjuvant treatment during the intralesional curettage of bone tumors. The goal of the present study was to analyze the in vitro cytotoxicity of commonly used irrigation solutions on chondrosarcoma and giant cell tumor (GCT) cells as there is no consensus on which solution leads to the greatest amount of cell death.
Methods:
An in vitro evaluation was performed by exposing human GCT and human chondrosarcoma cell lines to 0.9% saline solution, sterile water, 70% ethanol, 3% hydrogen peroxide, 0.05% chlorhexidine gluconate (CHG), and 0.3% povidone iodine solutions independently for 2 and 5 minutes. A low-cytotoxicity control (LCC) and a high-cytotoxicity control (HCC) were established to determine the mean cytotoxicity of each solution and each solution’s superiority to LCC and non-inferiority to HCC.
Results:
The present study demonstrated that 0.05% CHG was non-inferior to the HCC when chondrosarcoma was exposed for 5 minutes and when GCT was exposed for 2 and 5 minutes (mean cytotoxicity, 99% to 102%) (p < 0.003 for all). Sterile water was superior to the LCC when chondrosarcoma was exposed for 5 minutes and when GCT was exposed for 2 minutes (mean, 28% to 37%) (p < 0.05). Sterile water (mean, 18% to 38%) (p < 0.012) and 3% hydrogen peroxide (mean, 7% to 16%) (p < 0.001) were both inferior to the HCC. The 3 other solutions were non-superior to the LCC (mean, −24% to −5%) (p < 0.023).
Conclusions:
In vitro irrigation in 0.05% CHG provided high cytotoxicity, comparable with the HCC. Therefore, the use of a 0.05% CHG solution clinically could serve as a potential chemical adjuvant during intralesional curettage of chondrosarcoma and GCT.
Clinical Relevance:
In an effort to reduce the burden of residual tumor cells, irrigation solutions are often utilized as adjuvant local therapy. Use of a 0.05% CHG solution clinically could serve as a potential chemical adjuvant to intralesional curettage of chondrosarcoma and GCT. Further in vivo studies may be indicated to assess clinical outcomes and safety associated with the use of 0.05% CHG in the treatment of chondrosarcoma and GCT.
Giant cell tumor (GCT) of bone and chondrosarcoma account for approximately 17% to 24% and 8% of bone tumors, respectively1,2. The most commonly utilized technique for the surgical treatment of GCT of bone and low-grade chondrosarcoma is intralesional curettage, which is known to generate cell shedding3,4. Release of these cells can lead to seeding and subsequent recurrence of the tumor within the surgical bed3. In an effort to reduce the burden of residual tumor cells, irrigation solutions are often utilized as adjuvant local therapy to kill residual cells that are shed or remain in the intertrabecular space5. Common irrigation solutions that have been used during the surgical treatment of multiple neoplasms include 0.9% saline solution, water (H2O), 70% ethanol (EtOH), 3% hydrogen peroxide (H2O2), 0.05% chlorhexidine gluconate (CHG), and 0.3% povidone iodine5,6.
In addition to physically separating residual cells from the tumor bed, solutions that are more cytotoxic to neoplastic cells theoretically may prevent local recurrence due to the presence of residual viable cells in the wound bed, although the clear clinical superiority of any single agent has not been demonstrated7. Although prior investigations have explored the in vitro cytotoxic effects of various irrigation solutions on primary bone tumors8,9, we are not aware of any studies that have explored the cytotoxic effect of 0.05% CHG. We do not routinely use phenol because of its potential toxicity to adjacent tissues. On the basis of the previously demonstrated clinical safety of 0.05% CHG when used intraoperatively as an anti-microbial10,11 and its theoretical efficacy in this application, we elected to compare the efficacy of 0.05% CHG with that of other solutions that are readily available for this application at our center.
To that end, the purpose of the present study was to determine the cytotoxicity of commonly used irrigation solutions against chondrosarcoma and GCT cells in an in vitro environment.
Materials and Methods
The flowchart of the study procedures is shown in Figure 1. Human GCT cells (TIB-223; ATCC) were cultured in T75 cell culture flasks and were maintained in a DMEM (Dulbecco’s Modified Eagle Medium), high glucose (with glutamine) (Gibco) and supplemented with 10% fetal bovine serum (FBS), heat inactivated (Gibco), and 1% penicillin-streptomycin. The JJ human chondrosarcoma cell lines12,13 were cultured in T75 cell culture flasks and were maintained in RPMI-1640 medium (Gibco) supplemented with 10% FBS (Gibco) and 1% penicillin-streptomycin (Gibco). Both cell lines were incubated in 5% CO2 at 37°C. Cells were grown to 80% confluency and were passaged following trypsinization. For experiments, cells were counted with use of a hemocytometer and the GCT and chondrosarcoma cells were plated in 96-well plates at a concentration of 10,000 cells per well. After plating, cells were given 24 to 36 hours to adhere to the plates. After adherence to the wells, the medium was changed and cells were incubated for an additional 24 to 36 hours to recover.
Fig. 1.
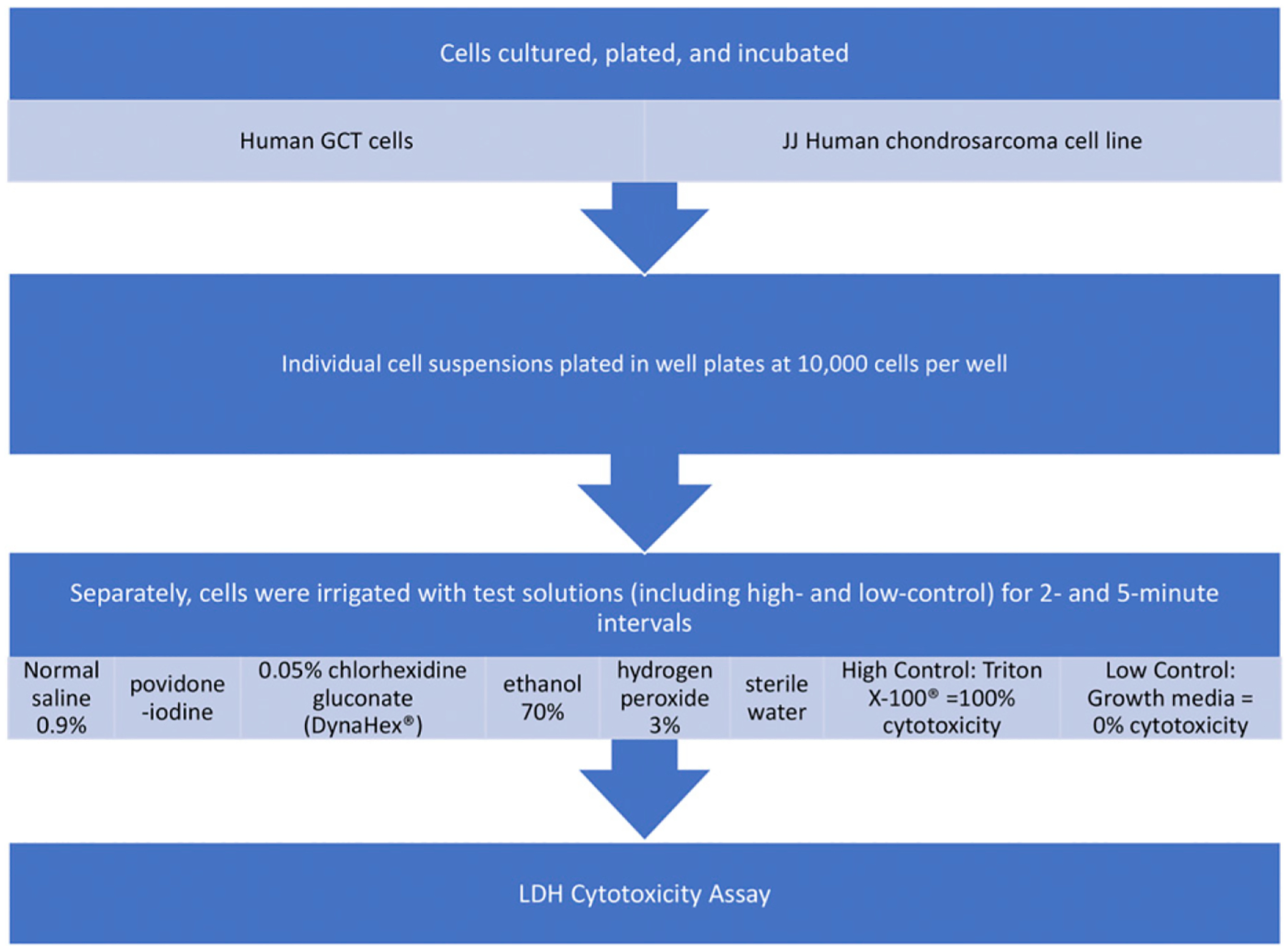
Flowchart of study procedures.
Cytotoxicity was then assessed by comparing each solution (0.9% normal saline solution, 0.3% povidone-iodine, 0.05% CHG [Dyna-Hex], 70% ethanol, 3% hydrogen peroxide, and sterile water) with the low-cytotoxicity control (LCC) of standard growth medium (expected to cause essentially no cell lysis) and the high-cytotoxicity control (HCC), Triton X-100 (a surfactant that lyses cell membranes and is expected to cause near-complete cell death). The growth medium in each well was aspirated carefully prior to exposure to these solutions so as not to dislodge the cell layer. Six wells of the GCT cells were then exposed to 100 μL of 0.9% normal saline solution for 2 minutes. This process was repeated with 0.3% povidone-iodine, 0.05% CHG, 70% ethanol, 3% hydrogen peroxide, and sterile water; 6 separate wells were exposed to each for 2 minutes, simultaneously. An HCC and LCC were established with use of Triton X-100 and the GCT growth medium, respectively, following the same protocol. After 2 minutes of exposure to the solutions, 100 μL of GCT growth medium was added to effectively reduce exposure concentrations on the cells. Next, 100 μL of this solution was transferred from each well to a fresh 96-well plate. The lactate dehydrogenase (LDH) cytotoxicity assay was then performed with use of the Sigma-Aldrich cytotoxicity assay following the established protocol from the kit. A 0.05% CHG background effect was also established as a control following these guidelines. Absorbance was measured at 490 nm. The cytotoxicity percentage was calculated following the kit protocol: Cytotoxicity (%) = 100% × (mean experimental value − mean LCC value)/(mean HCC value − mean LCC value). For this calculation, the mean of the 6 LCC absorbances was used in the equation as well as the mean of the 6 HCC absorbances. This experiment was then repeated with GCT cells at a 5-minute exposure interval. This assay was repeated for the JJ chondrosarcoma cells at 2 and 5-minute exposure intervals, with chondrosarcoma growth medium used in place of the GCT growth medium.
For each irrigation solution, time interval, and cell line, a formal statistical approach was used to determine whether the associated cytotoxicity could be considered superior, non-superior, or indeterminate relative to the LCC (nominally 0% cytotoxicity) and non-inferior, inferior, or indeterminate relative to the HCC (nominally 100% cytotoxicity)14,15. Results similar to the LCC values are considered ineffective for killing tumor cells in vitro, and results similar to the HCC are considered highly effective for killing tumor cells in vitro.
A margin of 10% relative to the nominal values of 0% (LCC) and 100% (HCC) was used to define superiority to LCC (i.e., mean cytotoxicity >10%) and non-inferiority to HCC (i.e., mean cytotoxicity >90%). A 2-sided 95% confidence interval (CI) was calculated for the mean cytotoxicity of each treatment. A treatment was designated as superior or not to the LCC and non-inferior or not to the HCC by comparing the lower and upper bounds of the 95% CI to 10% cytotoxicity (comparison to the LCC) and to 90% cytotoxicity (comparison to the HCC). The specific classification rules are listed in Table I.
TABLE I.
Rules for Using the 95% CI of the Mean Cytotoxicity to Designate a Substance as Superior or Not to LCC and as Non-Inferior or Not to HCC*
| Interpretation | |
|---|---|
| Investigation of superiority to LCC | |
| 95% CI lower bound >10% cytotoxicity | Superior to LCC |
| 95% CI upper bound <10% cytotoxicity | Non-superior to LCC |
| 95% CI includes 10% | Indeterminate (may or may not be superior to LCC) |
| Investigation of non-inferiority to HCC | |
| 95% CI lower bound >90% cytotoxicity | Non-inferior to HCC |
| 95% CI upper bound <90% cytotoxicity | Inferior to HCC |
| 95% CI includes 90% | Indeterminate (may or may not be non-inferior to HCC) |
LCC = low-cytotoxicity control, HCC = high-cytotoxicity control.
These comparisons are equivalent to 2-sided hypothesis tests using a 5% significance level. Specifically, the equivalent test for superiority to the LCC is a 1-sample t test of the mean cytotoxicity with a null hypothesis of 10% (the superiority margin over the nominal LCC value of 0%). The equivalent test for non-inferiority to the HCC is a 1-sample t test of the mean cytotoxicity with a null hypothesis of 90% (the non-inferiority margin below the nominal HCC value of 100%). In both cases, results were considered indeterminate if the p value was >0.05, equivalent to the 95% CI containing 10% or 90%.
For each solution and duration, the standard error (SE) of the mean percentage cytotoxicity needed to calculate the 95% CI and t test results was estimated with use of the delta method16. The delta method is a general approach to calculating the SE of a statistic that is a function of 1 or more other statistics. In particular, this method properly combines the variance of each of the constituent statistics into a total variance for the combined statistic. As shown in the formula above, cytotoxicity is a function of 3 statistics: the mean absorbances of the solution of interest, the mean LCC, and the mean HCC, each of which exhibits experimental variation that needs to be accounted for. Last, the corresponding number of degrees of freedom for each t-based CI and test of cytotoxicity was calculated with use of the Welch-Satterthwaite formula17.
Theoretically, the true mean percentage cytotoxicity for any substance must lie between 0% and 100%, inclusive. Values outside the range from 0% to 100% are physically impossible but can be observed in experimental data because of the measurement error that can occur in any physical quantity.
Source of Funding
The authors received grants from the Department of Orthopaedics and Sports Medicine, University of Washington, Seattle (M.J.T.) and grant AR057025 from the NIH/NIAMS (National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases) (R.J.F.).
Results
Figures 2 and 3 show the mean cytotoxicity and corresponding 95% CI for each solution under each condition and how the 95% CIs compare with the superiority and non-inferiority margins. Among the different solutions tested, only 0.05% CHG was superior to the LCC under both exposure intervals (2 minutes and 5 minutes) and for both cell lines (chondrosarcoma and GCT) (p < 0.003 for all) (Tables II and III). The 0.05% CHG solution was also non-inferior to the Triton X-100 HCC for chondrosarcoma when the cells were exposed for 5 minutes (mean, 99%; 95% CI, 95% to 104%; p = 0.003), GCT when the cells were exposed for 2 minutes (mean, 102%; 95% CI, 99% to 106%; p < 0.001), and GCT when the cells were exposed for 5 minutes (mean, 102%; 95% CI, 98% to 105%; p < 0.001). The cytotoxicity of 0.05% CHG for chondrosarcoma was high when the cells were exposed for 2 minutes, but non-inferiority to the HCC was indeterminate because the 95% CI included the non-inferiority threshold of 90% cytotoxicity (mean, 129%; 95% CI, 77% to 180%; p = 0.11). Sterile water was superior to the LCC for chondrosarcoma when the cells were exposed for 5 minutes (mean, 37%; 95% CI, 23% to 51%; p = 0.004) and GCT when the cells were exposed for 2 minutes (mean, 28%; 95% CI, 11% to 45%; p = 0.039), whereas its superiority to the LCC was indeterminate for chondrosarcoma when the cells were exposed for 2 minutes (mean, 18%; 95% CI, 9% to 28%; p = 0.087) and for GCTwhen the cells were exposed for 5 minutes (mean, 38%; 95% CI, 3% to 73%; p = 0.092). However, under both exposure intervals and for both cell lines, the cytotoxicity of sterile water was inferior to the HCC (p < 0.001 for all) (Tables II and III). The 3% hydrogen peroxide solution had a mean cytotoxicity close to the 10% cytotoxicity threshold and thus had indeterminate cytotoxic superiority relative to the LCC under all 4 conditions (mean, 7% to 16%; p > 0.28 for all), although this solution was also inferior to the HCC (p < 0.001 for all). All other solutions (normal saline solution, 70% ethanol, and iodine) were non-superior to the LCC (<10% cytotoxicity) under both time intervals and for both cell lines (mean, − 24% to 5%; p < 0.023 for all) (Tables II and III). Because 0.05% CHG was found to have strong cytotoxicity, a separate experiment was carried out to determine if the 0.05% CHG solution itself (in the absence of cancer cells) had an impact on absorbance. The experiment showed a very small and statistically nonsignificant difference in the mean absorbance values between the background solution alone and 0.05% CHG with the background solution (mean, 0.1285 and 0.1301, respectively; difference, −0.0016; p = 0.42 [t test]; 95% CI for the difference, −0.0047 to 0.0016).
Fig. 2.
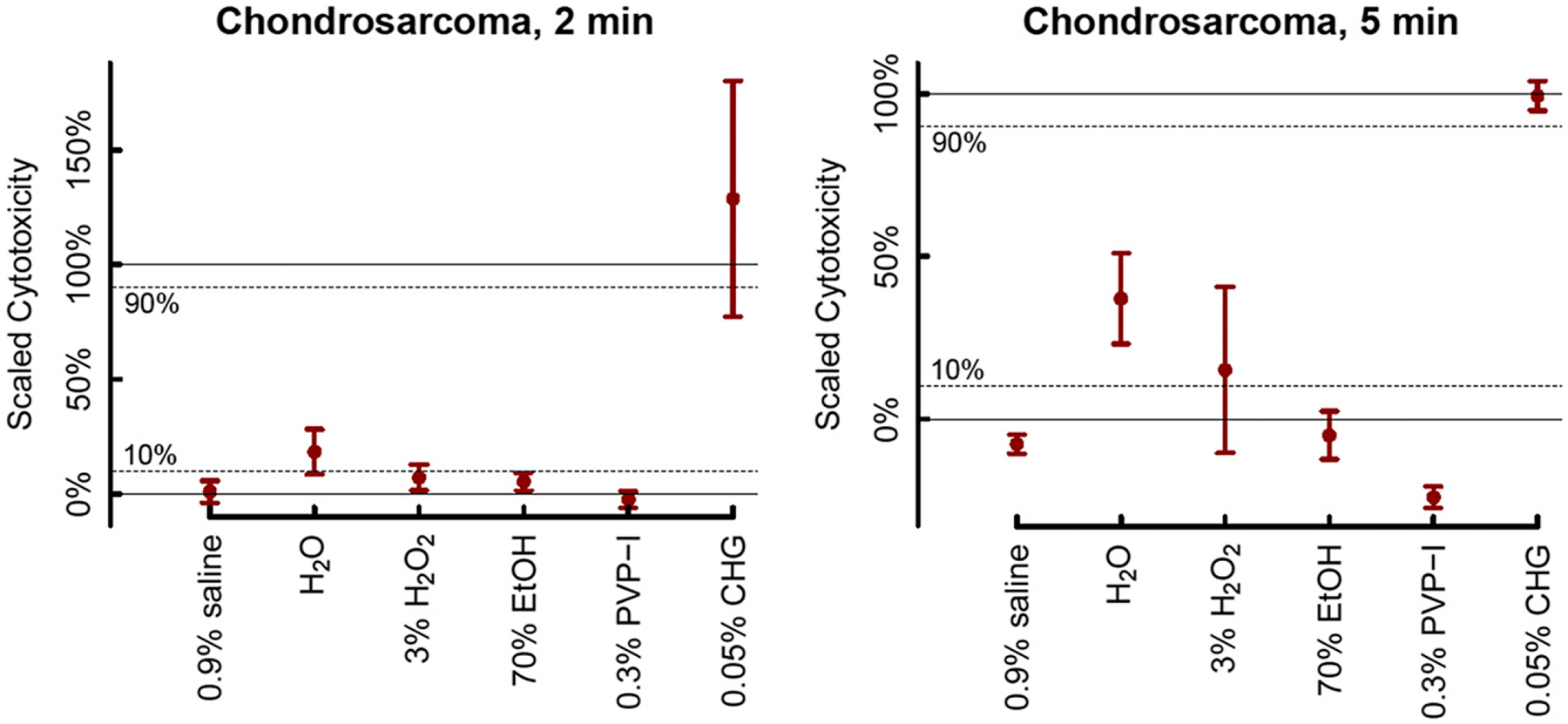
Estimated mean cytotoxicity percentage for 6 commonly used irrigation solutions tested on chondrosarcoma cells at 2 durations (2 minutes and 5 minutes). The 95% CIs for the means are shown. Dashed lines at 10% and 90% represent the 10% margin permitted for superiority to the LCC and non-inferiority to the HCC, respectively. Table I shows how solutions were classified as superior or non-superior to the LCC and non-inferior or inferior to the HCC using the 95% CIs. EtOH = ethanol, PVP-I = povidone-iodine, CHG = chlorohexidine gluconate.
Fig. 3.
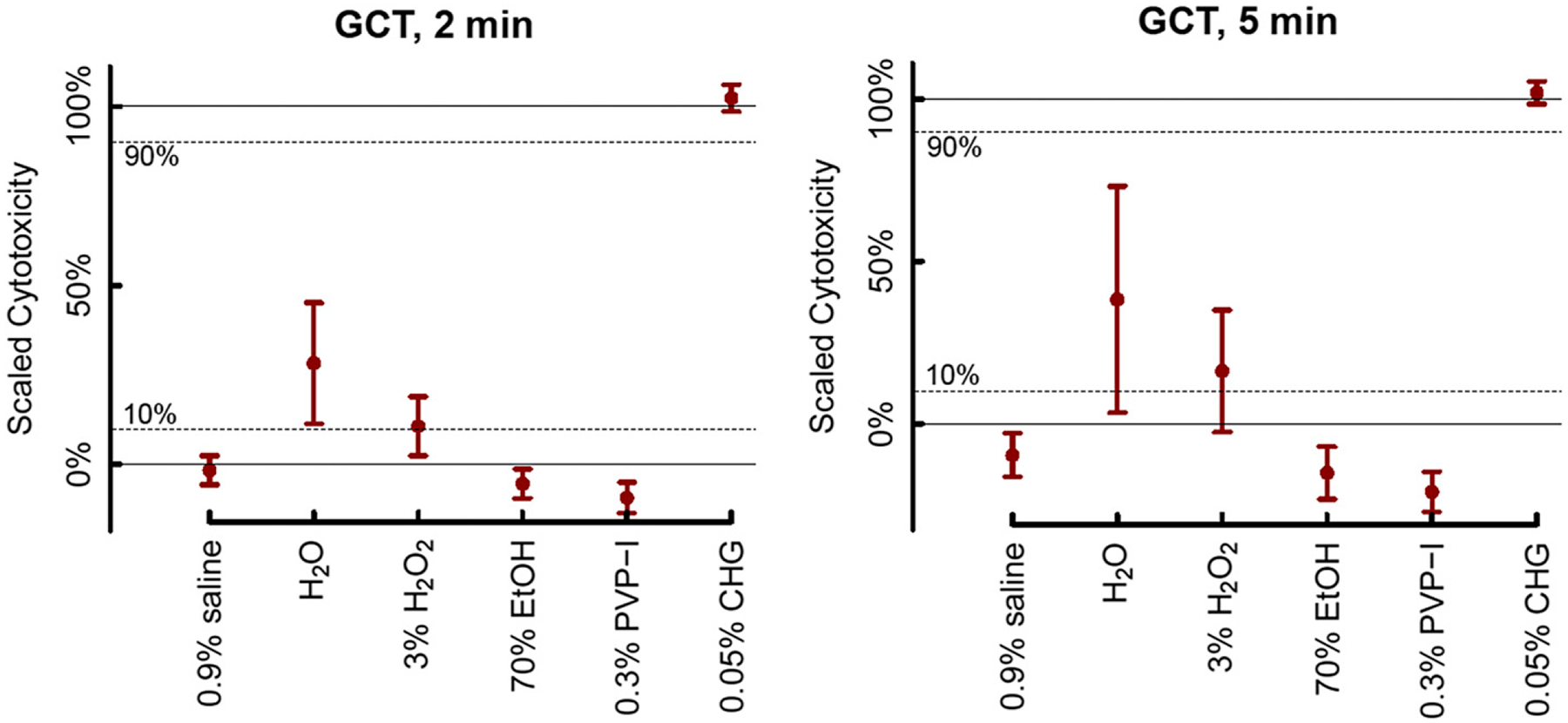
Estimated mean cytotoxicity percentage for 6 commonly used irrigation solutions tested on GCT cells at 2 durations (2 minutes and 5 minutes). The 95% CIs for the means are displayed. Dashed lines at 10% and 90% represent the 10% margin permitted for superiority and non-inferiority, respectively. Table I shows how solutions were classified as superior or non-superior to the LCC and non-inferior or inferior to the HCC using the 95% CIs. EtOH = ethanol, PVP-I = povidone-iodine, CHG = chlorohexidine gluconate.
TABLE II.
Descriptive Statistics, 95% CIs, and Superiority and Non-Inferiority Designations for 6 Commonly Used Irrigation Solutions Tested on Chondrosarcoma Cells in Vitro at 2 and 5-Minute Durations*
| Cytotoxicity (%) | Test of Superiority to LCC | Test of Non-Inferiority to HCC | ||||||
|---|---|---|---|---|---|---|---|---|
| Solution and Duration | Mean | SE | 95% CI | P Value | Conclusion† | P Value | Conclusion† | |
| 2 minutes | ||||||||
| 0.9% saline solution | 0.93 | 2.10 | −3.84 | 5.70 | 0.002 | Non-superior | <0.001 | Inferior |
| H2O | 18.37 | 4.45 | 8.56 | 28.19 | 0.087 | Indeterminate | <0.001 | Inferior |
| 3% H2O2 | 7.15 | 2.58 | 1.45 | 12.84 | 0.29 | Indeterminate | <0.001 | Inferior |
| 70% EtOH | 5.33 | 1.82 | 1.42 | 9.23 | 0.022 | Non-superior | <0.001 | Inferior |
| 0.3% PVP-I | −2.45 | 1.65 | −6.08 | 1.18 | <0.001 | Non-superior | <0.001 | Inferior |
| 0.05% CHG | 128.73 | 20.21 | 77.22 | 180.24 | 0.002 | Superior | 0.11 | Indeterminate |
| 5 minutes | ||||||||
| 0.9% saline solution | −7.72 | 1.23 | −10.61 | −4.84 | <0.001 | Non-superior | <0.001 | Inferior |
| H2O | 37.04 | 5.47 | 23.11 | 50.98 | 0.004 | Superior | <0.001 | Inferior |
| 3% H2O2 | 15.11 | 9.97 | −10.41 | 40.64 | 0.63 | Indeterminate | <0.001 | Inferior |
| 70% EtOH | −5.00 | 3.06 | −12.37 | 2.38 | 0.002 | Non-superior | <0.001 | Inferior |
| 0.3% PVP-I | −24.06 | 1.32 | −27.33 | −20.79 | <0.001 | Non-superior | <0.001 | Inferior |
| 0.05% CHG | 99.35 | 1.84 | 94.79 | 103.92 | <0.001 | Superior | 0.003 | Non-inferior |
Classification rules are shown in Table I. The mean and CI of the cytotoxicity percentage for each irrigation solution are plotted in Figure 2. SE = standard error, CI = confidence interval, LCC = low-cytotoxicity control, HCC = high-cytotoxicity control, EtOH = ethanol, PVP-I = povidone-iodine, CHG = chlorohexidine gluconate.
See Table I for rules for determining conclusions based on the 95% CI.
TABLE III.
Descriptive Statistics, 95% CIs, and Superiority and Non-Inferiority Designations for 6 Commonly Used Irrigation Solutions Tested on GCT Cells in Vitro at 2 and 5-minute Durations*
| Cytotoxicity (%) | Test of Superiority to LCC | Test of Non-Inferiority to HCC | ||||||
|---|---|---|---|---|---|---|---|---|
| Solution and Duration | Mean | SE | 95% CI | P Value | Conclusion† | P Value | Conclusion† | |
| 2 minutes | ||||||||
| 0.9% saline solution | −1.61 | 1.73 | −5.66 | 2.44 | <0.001 | Non-superior | <0.001 | Inferior |
| H2O | 28.26 | 6.70 | 11.34 | 45.18 | 0.039 | Superior | <0.001 | Inferior |
| 3% H2O2 | 10.68 | 3.48 | 2.39 | 18.97 | 0.85 | Indeterminate | <0.001 | Inferior |
| 70% EtOH | −5.38 | 1.66 | −9.49 | −1.26 | <0.001 | Non-superior | <0.001 | Inferior |
| 0.3% PVP-I | −9.30 | 1.77 | −13.58 | −5.01 | <0.001 | Non-superior | <0.001 | Inferior |
| 0.05% CHG | 102.35 | 1.67 | 98.55 | 106.15 | <0.001 | Superior | <0.001 | Non-inferior |
| 5 minutes | ||||||||
| 0.9% saline solution | −9.73 | 3.02 | −16.46 | −2.99 | <0.001 | Non-superior | <0.001 | Inferior |
| H2O | 38.22 | 13.64 | 3.34 | 73.10 | 0.092 | Indeterminate | 0.012 | Inferior |
| 3% H2O2 | 16.14 | 7.50 | −2.61 | 34.90 | 0.45 | Indeterminate | <0.001 | Inferior |
| 70% EtOH | −15.18 | 3.60 | −23.23 | −7.13 | <0.001 | Non-superior | <0.001 | Inferior |
| 0.3% PVP-I | −21.01 | 2.43 | −27.20 | −14.82 | <0.001 | Non-superior | <0.001 | Inferior |
| 0.05% CHG | 101.94 | 1.47 | 98.44 | 105.44 | <0.001 | Superior | <0.001 | Non-inferior |
Classification rules are shown in Table I. The mean and confidence bounds of the cytotoxicity percentage for each irrigation solution are plotted in Figure 3. SE = standard error, CI = confidence interval, LCC = low-cytotoxicity control, HCC = high-cytotoxicity control, EtOH = ethanol, PVP-I = povidone-iodine, CHG = chlorohexidine gluconate.
See Table I for rules for determining conclusions based on the 95% CI.
Discussion
The surgical treatment of GCT of bone commonly consists of extended intralesional curettage. The treatment of low-grade chondrosarcoma is more controversial. Chen et al., in a recent analysis in which intralesional curettage was compared with wide excision for the treatment of both appendicular and axial grade-I chondrosarcoma, observed that both methods were associated with equivalent rates of local control but that curettage was associated with a decreased risk of complications18. Nonetheless, it is our impression that most orthopaedic oncologists maintain that intralesional treatment of grade-I chondrosarcoma is appropriate only for the management of appendicular (and not axial) tumors. We chose to evaluate a higher-grade cartilaginous cell line for because accurate histologic classification of cartilaginous tumors is challenging19, and, in light of this, we aimed to test the efficacy of local adjuvants against a scenario of highest risk for local recurrence (i.e., the clinical and/or histologic classification is not reflective of the potential biologic behavior).
Intralesional curettage can result in residual tumor cells within the surgical bed, leading to subsequent recurrence of disease. In an effort to reduce the burden of residual disease, multiple adjuvants are utilized during intralesional curettage, including irrigation with chemical solutions. The purpose of the present study was to determine which irrigation solutions would lead to the greatest degree of cytotoxicity against GCT and chondrosarcoma cells when exposed for common intraoperative surgical irrigation times in an in vitro environment. We found that, of the irrigation solutions tested, 0.05% CHG was the most cytotoxic solution against both GCT and chondrosarcoma cells.
The present study had several limitations. The results of several analyses (specifically, sterile water compared with the LCC during the 2-minute chondrosarcoma and 5-minute GCT experiments, 3% hydrogen peroxide compared with the LCC during the chondrosarcoma and GCT 2-minute and 5-minute experiments, and 0.05% CHG compared with the HCC during the 2-minute chondrosarcoma experiment) were statistically indeterminate. In those analyses, the associated 95% CIs for mean cytotoxicity included values above and below the superiority or non-inferiority margin (10% in the comparisons with the LCC and 90% in the comparisons with the HCC). Additional experiments could be conducted with more wells and fewer irrigation solutions limited to the comparison of 0.05% CHG, sterile water, and 3% hydrogen peroxide. This increase in sample size would increase the power of the study and would allow for more precise estimates of mean cytotoxicity (i.e., narrower 95% CIs) to determine on which side of the superiority and non-inferiority margins the mean cytotoxicity lies. Furthermore, this study did not take into account the mechanical debridement resulting from the intraoperative use of irrigation solutions, whether cytotoxic or not, and we cannot comment on the extent to which these solutions enter into the intertrabecular space. Other limitations of this study include inherent human errors in timing (e.g., seconds of difference in the application of solutions) as well as slight variety in the cell population or concentration per well, causing some wells to be overrepresented or underrepresented in terms of cell death. Every attempt was made to account for this potential limitation by dividing cells equally into the wells in the 96-well plates, based on the cell density in solution, as well as by growing cells for the same duration after plating in the wells.
The present study showed that, of the irrigation solutions tested, 0.05% CHG was the most cytotoxic against both GCT and chondrosarcoma cells. The results of this study are consistent with those of prior in vitro studies that have shown 0.05% CHG to be an effective cytotoxic agent against various humanoid cells, including chondrocytes, fibroblasts, myoblasts, and osteoblasts11,20. One possible concern when using 0.05% CHG as an irrigation solution is the potential damage to surrounding healthy tissue as CHG has been found to inhibit fibroblast cell division and protein synthesis, which could adversely impact wound-healing. However, these effects are seen at greater concentrations and during prolonged exposure in vitro21. Dilute (0.05%) CHG has been shown to be safe and effective as an anti-microbial irrigation solution in the settings of both primary total joint replacement and the treatment of periprosthetic joint infections10,20. Whether in vitro toxicity to non-neoplastic cells results in clinically meaningful outcomes in the treatment of musculoskeletal tumors is not known and will require additional study.
Overall, these findings indicate that 0.05% CHG solution is a potential chemical adjuvant for use during intralesional curettage of chondrosarcoma and GCT. This solution is commonly used and readily available, with demonstrated in vivo safety in other surgical applications and a lower predicted toxicity compared with some currently used agents. Irrigation times as short as 2 and 5 minutes may result in death of residual neoplastic cells in contact with the solution under the conditions of this experiment. Future studies may assess the uniformity of cytotoxicity in additional cell lines and/or clinical outcomes and safety of these irrigation solutions when used in vivo for chondrosarcoma and GCT.
Acknowledgments
The authors would like to acknowledge Seth Pollack at Fred Hutchinson Cancer Research Institute and Dr. Joel Block at Rush University for providing the chondrosarcoma cell line used in this work.
Footnotes
Disclosure: The Disclosure of Potential Conflicts of Interest forms are provided with the online version of the article (http://links.lww.com/JBJS/H220).
References
- 1.Bovée J. Bone: Chondrosarcoma. Atlas Genet Cytogenet Oncol Haematol. 2002; 6(3):235–8. [Google Scholar]
- 2.Cleven A,Bovée J. Bone: Giant cell tumour of bone. Atlas Genet Cytogenet Oncol Haematol. 2018;22(10):459–62. [Google Scholar]
- 3.Abuhejleh H, Wunder JS, Ferguson PC, Isler MH, Mottard S, Werier JA, Griffin AM, Turcotte RE. Extended intralesional curettage preferred over resection-arthrodesis for giant cell tumour of the distal radius. Eur J Orthop Surg Traumatol. 2020. Jan; 30(1):11–7. [DOI] [PubMed] [Google Scholar]
- 4.Zoccali C, Baldi J, Attala D, Rossi B, Anelli V, Annovazzi A, Ferraresi V. Intralesional vs. extralesional procedures for low-grade central chondrosarcoma: a systematic review of the literature. Arch Orthop Trauma Surg. 2018. Jul; 138(7):929–37. [DOI] [PubMed] [Google Scholar]
- 5.Lodhia KA, Dale OT, Winter SC. Irrigation solutions in head and neck cancer surgery: a preclinical efficacy study. Ann Otol Rhinol Laryngol. 2015. Jan;124(1): 68–71. [DOI] [PubMed] [Google Scholar]
- 6.Manzano BR, Santaella NG, Oliveira MA, Rubira CMF, Santos P. Retrospective study of osteoradionecrosis in the jaws of patients with head and neck cancer. J Korean Assoc Oral Maxillofac Surg. 2019;45(1):21–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bickels J, Campanacci DA. Local Adjuvant Substances Following Curettage of Bone Tumors. J Bone Joint Surg Am. 2020. Jan 15;102(2):164–74. [DOI] [PubMed] [Google Scholar]
- 8.Gortzak Y, Kandel R, Deheshi B, Werier J, Turcotte RE, Ferguson PC, Wunder JS. The efficacy of chemical adjuvants on giant-cell tumour of bone. An in vitro study. J Bone Joint Surg Br. 2010. Oct;92(10):1475–9. [DOI] [PubMed] [Google Scholar]
- 9.Nicholson NC, Ramp WK, Kneisl JS, Kaysinger KK. Hydrogen peroxide inhibits giant cell tumor and osteoblast metabolism in vitro. Clin Orthop Relat Res. 1998. Feb;(347):250–60. [PubMed] [Google Scholar]
- 10.Frisch NB, Kadri OM, Tenbrunsel T, Abdul-Hak A, Qatu M, Davis JJ. Intraoperative chlorhexidine irrigation to prevent infection in total hip and knee arthroplasty. Arthroplast Today. 2017;3(4):294–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu JX, Werner J, Kirsch T, Zuckerman JD, Virk MS. Cytotoxicity evaluation of chlorhexidine gluconate on human fibroblasts, myoblasts, and osteoblasts. J Bone Jt Infect. 2018;3(4):165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Block JA, Inerot SE, Gitelis S, Kimura JH. The effects of long term monolayer culture on the proteoglycan phenotype of a clonal population of mature human malignant chondrocytes. Connect Tissue Res. 1991;26(4):295–313. [DOI] [PubMed] [Google Scholar]
- 13.Block JA, Inerot SE, Gitelis S, Kimura JH. Synthesis of chondrocytic keratan sulphate-containing proteoglycans by human chondrosarcoma cells in long-term cell culture. J Bone Joint Surg Am. 1991. Jun;73(5):647–58. [PubMed] [Google Scholar]
- 14.Christensen E Methodology of superiority vs. equivalence trials and non-inferiority trials. J Hepatol. 2007. May;46(5):947–54. [DOI] [PubMed] [Google Scholar]
- 15.Xu W, Huang SH, Su J, Gudi S, O’Sullivan B. Statistical fundamentals on cancer research for clinicians: Working with your statisticians. Clin Transl Radiat Oncol. 2021;27:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ver Hoef J Who Invented the Delta Method? Am Stat. 2012;66(2):124–7. [Google Scholar]
- 17.Welch BL. The generalisation of Student’s problems when several different population variances are involved. Biometrika. 1947;34(1–2):28–35. [DOI] [PubMed] [Google Scholar]
- 18.Chen YC, Wu PK, Chen CF, Chen WM. Intralesional curettage of central low-grade chondrosarcoma: A midterm follow-up study. J Chin Med Assoc. 2017. Mar; 80(3):178–182. [DOI] [PubMed] [Google Scholar]
- 19.Eefting D, Schrage YM, Geirnaerdt MJ, Le Cessie S, Taminiau AH, Bovée JV, Hogendoorn PC; EuroBoNeT consortium. Assessment of interobserver variability and histologic parameters to improve reliability in classification and grading of central cartilaginous tumors. Am J Surg Pathol. 2009. Jan;33(1):50–7. [DOI] [PubMed] [Google Scholar]
- 20.Goswami K, Cho J, Foltz C, Manrique J, Tan TL, Fillingham Y, Higuera C, Della Valle C, Parvizi J. Polymyxin and Bacitracin in the Irrigation Solution Provide No Benefit for Bacterial Killing in Vitro. J Bone Joint Surg Am. 2019. Sep 18;101(18):1689–97. [DOI] [PubMed] [Google Scholar]
- 21.Pucher JJ, Daniel JC. The effects of chlorhexidine digluconate on human fibroblasts in vitro. J Periodontol. 1992. Jun;63(6):526–32. [DOI] [PubMed] [Google Scholar]


