Abstract
Most acute phase antipsychotic drug trials in schizophrenia last only a few weeks, but patients must usually take these drugs much longer. We examined the long‐term efficacy of antipsychotic drugs in acutely ill patients using network meta‐analysis. We searched the Cochrane Schizophrenia Group register up to March 6, 2022 for randomized, blinded trials of at least 6‐month duration on all second‐generation and 18 first‐generation antipsychotics. The primary outcome was change in overall symptoms of schizophrenia; secondary outcomes were all‐cause discontinuation; change in positive, negative and depressive symptoms; quality of life, social functioning, weight gain, antiparkinson medication use, akathisia, serum prolactin level, QTc prolongation, and sedation. Confidence in the results was assessed by the CINeMA (Confidence in Network Meta‐Analysis) framework. We included 45 studies with 11,238 participants. In terms of overall symptoms, olanzapine was on average more efficacious than ziprasidone (standardized mean difference, SMD=0.37, 95% CI: 0.26‐0.49), asenapine (SMD=0.33, 95% CI: 0.21‐0.45), iloperidone (SMD=0.32, 95% CI: 0.15‐0.49), paliperidone (SMD=0.28, 95% CI: 0.11‐0.44), haloperidol (SMD=0.27, 95% CI: 0.14‐0.39), quetiapine (SMD=0.25, 95% CI: 0.12‐0.38), aripiprazole (SMD=0.16, 95% CI: 0.04‐0.28) and risperidone (SMD=0.12, 95% CI: 0.03‐0.21). The 95% CIs for olanzapine versus aripiprazole and risperidone included the possibility of trivial effects. The differences between olanzapine and lurasidone, amisulpride, perphenazine, clozapine and zotepine were either small or uncertain. These results were robust in sensitivity analyses and in line with other efficacy outcomes and all‐cause discontinuation. Concerning weight gain, the impact of olanzapine was higher than all other antipsychotics, with a mean difference ranging from –4.58 kg (95% CI: –5.33 to –3.83) compared to ziprasidone to –2.30 kg (95% CI: –3.35 to –1.25) compared to amisulpride. Our data suggest that olanzapine is more efficacious than a number of other antipsychotic drugs in the longer term, but its efficacy must be weighed against its side effect profile.
Keywords: Antipsychotics, schizophrenia, long‐term efficacy, olanzapine, positive symptoms, negative symptoms, all‐cause discontinuation, weight gain
Schizophrenia is a mental disorder which ranks among the 20 top causes of disability according to the World Health Organization 1 , and affects about 1% of the population. Antipsychotic drugs are the mainstay of its treatment. Although acute episodes must often be treated with antipsychotics for several months (and these drugs are frequently continued as maintenance treatment thereafter), most antipsychotic drug trials are short‐term. Indeed, the median study duration in a recent network meta‐analysis on acute schizophrenia was only 6 weeks, and the maximum duration was restricted to 13 weeks 2 .
This discrepancy between the usual course of the disorder and the duration of trials of its main treatment has rightly been criticized 3 . Some side effects of antipsychotics, such as weight gain, may accumulate over time and can therefore be adequately assessed only in longer‐term studies. The efficacy findings of meta‐analyses based on short‐term trials can be biased as well. Some drugs – e.g., olanzapine, quetiapine and clozapine – have a strong affinity for histamine receptors, whose blockage leads to sedation 4 . This may confound assessment of actual antipsychotic efficacy. As initial sedation often subsides when patients get used to their medication, longer‐term studies in initially acutely ill patients are likely to better reflect the true efficacy of antipsychotics.
Long‐term relapse prevention studies which have been summarized in other network meta‐analyses 5 , 6 cannot really fill the above gap, because they include patients stabilized on antipsychotics for several months before randomization. Side effects may have already reached a plateau at study start, and the outcome assessed in such studies is re‐exacerbation of symptoms (relapse) rather than reduction of symptoms.
Thus, the missing link is to examine improvement of symptoms and side effects in longer‐term randomized controlled trials (RCTs) conducted in acutely ill patients with schizophrenia. In the current report, we filled this gap by a network meta‐analysis of the efficacy and tolerability of antipsychotics including only RCTs of at least 6‐month duration in patients with an acute episode of the illness.
METHODS
We report our results following the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines. The protocol was registered at PROSPERO (CRD42014014919).
Participants
We included studies on adults with initially acute symptoms of schizophrenia or related disorders (i.e., schizophreniform or schizoaffective disorder). To comply with the transitivity requirement of network meta‐analysis, we excluded studies which by their inclusion criteria were restricted to the following patient subgroups: initially stable patients (relapse prevention studies), children and adolescents, elderly, first‐episode patients, treatment‐resistant patients, and patients with predominant negative or depressive symptoms, or concomitant substance abuse, or concomitant medical illness.
Interventions
We included all second‐generation antipsychotics (SGAs) available in Europe or the US, and a selection of first‐generation antipsychotics (FGAs) guided by a survey among international schizophrenia experts 7 , 8 (i.e., benperidol, chlorpromazine, clopenthixol, flupenthixol, fluphenazine, haloperidol, levomepromazine, loxapine, molindone, penfluridol, perazine, perphenazine, pimozide, sulpiride, thioridazine, thiothixene, trifluoperazine and zuclopenthixol). We considered all formulations (including long‐acting injectables, LAIs), except short‐acting intramuscular ones (because they are primarily used in emergency situations).
We included all flexible‐dose studies, because the investigators can titrate to the optimum dose for the individual patient. In fixed‐dose studies, we included target‐to‐maximum doses according to the International Consensus Study of Antipsychotic Dosing 9 . If studies used several doses, we averaged the results of the individual arms with appropriate methods 10 .
Outcomes
The primary outcome was change in overall symptoms of schizophrenia, as measured by rating scales such as the Positive and Negative Syndrome Scale (PANSS) 11 , the Brief Psychiatric Rating Scale (BPRS) 12 , or any other published scale.
Secondary outcomes were all‐cause discontinuation; change in positive, negative and depressive symptoms; quality of life, social functioning, weight gain, antiparkinson medication use, akathisia, serum prolactin level, QTc prolongation, and sedation.
Study design
We included published and unpublished RCTs reported to be single‐blind or double‐blind. The minimum study duration was 6 months (following the criteria of the Cochrane Schizophrenia Group 10 to define long‐term studies). Studies with a high risk of bias in sequence generation, according to the Cochrane Collaboration risk of bias tool Version 1 13 , were excluded.
We excluded studies from mainland China due to serious quality concerns 8 ; trials in which antipsychotics were used in combination; those in which patients could change the antipsychotic during the triale.g., 14 , or long‐term extensions in which only acute‐phase responders were followed up (since this design corrupts randomization). We included RCT extensions in which all patients could be followed up.
Search strategy
We started from the searches of previous meta‐analyses by our group 15 , 16 , and made update searches of the Cochrane Schizophrenia Group specialized register on June 14, 2021, on September 21, 2021, and until March 6, 2022 (see supplementary information).
Study selection and data extraction
At least two reviewers screened the update search results independently, retrieved full text articles, and checked inclusion criteria. In case of doubt, a third reviewer was involved. Two reviewers independently extracted data and entered them in electronic forms in Microsoft Access 2010. An algorithm checked for conflicting data entries. Differences were discussed and, if consensus was not reached, a third reviewer was involved. Study authors were contacted in case of important missing or unclear information.
For extracting data on the outcomes, we preferred results based on mixed models of repeated measurements or multiple imputation rather than last‐observation‐carried‐forward or completer‐only analyses. Missing standard deviations were estimated from test statistics or imputed as the mean standard deviation of the included studies. We also extracted data on age, sex, baseline severity (PANSS total score), publication year, study duration, pharmaceutical sponsor, and whether only a completer analysis was conducted. Risk of bias was independently assessed using the Cochrane Collaboration's risk of bias tool Version 1 13 .
Data analysis
We conducted a network meta‐analysis in a frequentist framework with the netmeta R package 17 . The effect size for continuous, scale‐derived outcomes was the standardized mean difference (SMD). Mean differences (MDs) were used for weight gain, serum prolactin level and QTc prolongation. Odds ratios (ORs) were used for dichotomous outcomes. All values are presented with 95% confidence intervals (CIs).
All relative treatment effects were estimated against the drug with most trials (olanzapine). We present and interpret treatment effects considering the mean estimate and the width of the 95% CIs, avoiding terms such as “statistically significant” and other ways of dichotomizing results based on p values. To enhance interpretability, the final estimated ORs have been transformed to relative risks (RRs) using the event rate of the outcome in the olanzapine arms (see also supplementary information).
The plausibility of the transitivity assumption was evaluated by comparing the distribution of potential effect modifiers of the primary outcome across studies grouped by comparison. We assumed a common heterogeneity parameter across all treatment comparisons, and presented the between‐study variance (τ 2 ) for each outcome. We characterized the amount of heterogeneity as low, moderate or high using the first and third quantiles of their empirical distributions 18 , 19 . To check the network for inconsistency, we performed the SIDE‐test 20 for each comparison (more than 10% of tests with p<0.1 considered important) and the design‐by‐treatment interaction test for the overall network (p<0.1 considered important) 21 .
We undertook sensitivity analyses by excluding studies with high risk of bias 2 , studies with completer‐only analyses, placebo‐controlled trials, studies with unfair dose comparisons, sponsored trials, studies with duration shorter than one year, and studies with imputed standard deviations. In post‐hoc sensitivity analyses, we excluded single‐blind studies, long‐term extensions of RCTs, and the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study 22 ; and we analyzed LAIs and oral drugs separately. To investigate the presence of small‐study effects (potentially associated with publication bias), we examined the comparison‐adjusted funnel plot for the primary outcome, ordering the treatments from the newest to the oldest.
The certainty of evidence for the primary outcome was evaluated using the CINeMA (Confidence in Network Meta‐Analysis) framework, which allows to grade the confidence in the results into high, moderate, low and very low 23 . We set the minimum relevant SMD to ±0.1 for this purpose.
RESULTS
We screened 2,432 records and included 45 studies with 11,238 participants (see supplementary information). Forty‐one studies were double‐blind, and four rater‐blind. The mean study duration was 42 weeks (interquartile range, IQR: 26 to 52). The participants’ mean age was 37.2 years (IQR: 35.2 to 39.1); 40% were women.
The RCTs examined amisulpride, aripiprazole, asenapine, chlorpromazine, clozapine, fluphenazine, fluspirilene, haloperidol, iloperidone, loxapine, lurasidone, olanzapine, paliperidone, penfluridol, perphenazine, pimozide, quetiapine, risperidone, thioridazine, tiotixene, trifluoperazine, ziprasidone, zotepine, and placebo. Very few participants were available for FGAs, except haloperidol and perphenazine (all others had fewer than 100 participants, except tiotixene which contributed 105 participants to all‐cause discontinuation). The characteristics of individual studies and the risk of bias assessment are presented in the supplementary information.
Primary outcome: change in overall symptoms
A total of 23 studies with 9,814 participants on 14 antipsychotics were available for the network meta‐analysis of the primary outcome: change in overall symptoms. The comparisons were reasonably transitive (see supplementary information).
Figure 1 presents the network plot, and Figure 2 the results of the network meta‐analysis. Olanzapine was on average more efficacious than ziprasidone (SMD=0.37, 95% CI: 0.26‐0.49), asenapine (SMD=0.33, 95% CI: 0.21‐0.45), iloperidone (SMD=0.32, 95% CI: 0.15‐0.49), paliperidone (SMD=0.28, 95% CI: 0.11‐0.44), haloperidol (SMD=0.27, 95% CI: 0.14‐0.39), quetiapine (SMD=0.25, 95% CI: 0.12‐0.38), aripiprazole (SMD=0.16, 95% CI: 0.04‐0.28), and risperidone (SMD=0.12, 95% CI: 0.03‐0.21). The 95% CIs for olanzapine versus aripiprazole and risperidone included the possibility of trivial effects. The differences between olanzapine and lurasidone, amisulpride, perphenazine, clozapine and zotepine were either small or uncertain.
Figure 1.
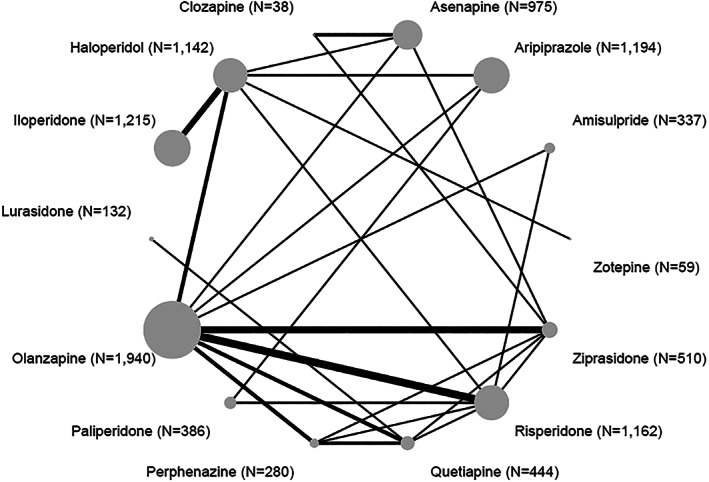
Network plot for change in overall symptoms (primary outcome). The numbers in parentheses are those of participants in the trials.
Figure 2.
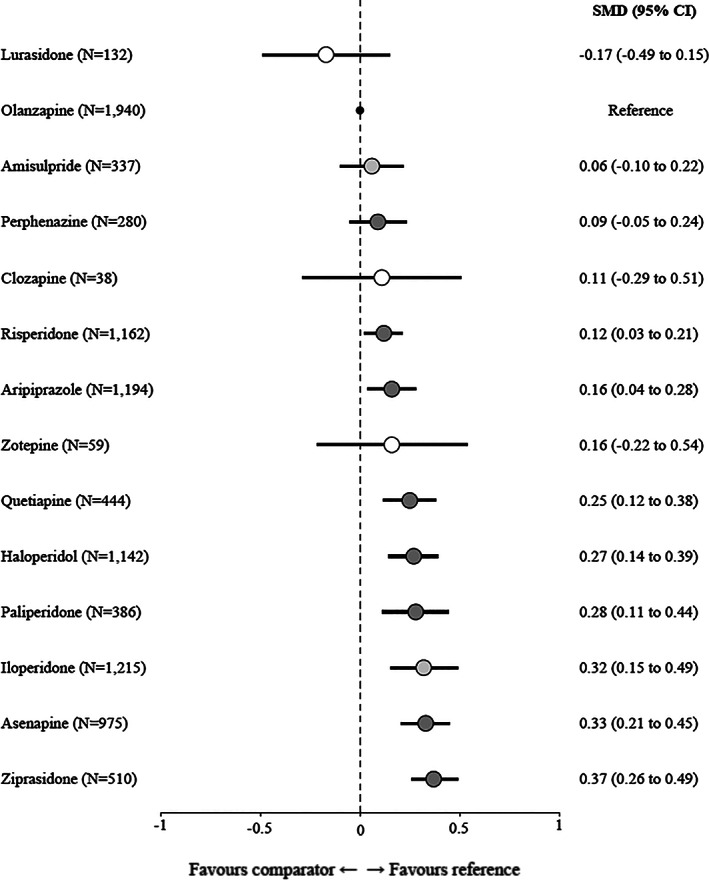
Forest plot for change in overall symptoms (primary outcome). Olanzapine was used as a reference. The numbers in parentheses are those of participants in the trials. The colors represent the confidence in the evidence according to CINeMA (dark grey – moderate confidence, light grey – low confidence, white – very low confidence). SMD – standardized mean difference, CI – confidence interval.
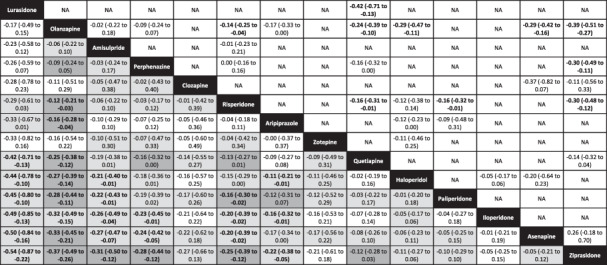
Table 1 shows further results of the network meta‐analysis (left lower part) as well as the results of pairwise meta‐analyses (right upper part). Lurasidone, amisulpride, perphenazine, risperidone and aripiprazole were on average more efficacious than several other drugs, with 95% CIs making opposite effects unlikely. Confidence in the evidence of these comparisons was between moderate and very low (see Table 1 and supplementary information).
Table 1.
League table for the change in overall symptoms (primary outcome)
|
|
The left lower field presents the results of the network meta‐analysis; the right upper field presents the results of pairwise meta‐analyses. Treatments are in order of their point estimate compared to olanzapine. Each cell provides the standardized mean difference and the corresponding 95% confidence interval (CI) of a comparison (treatment in column vs. treatment in row for the network meta‐analysis; treatment in row vs. treatment in column for the pairwise meta‐analysis). Bold prints indicate 95% CIs excluding opposite effects. Please note that in Figure 2 olanzapine was always the reference, which explains why in that figure and in the text the sign of all comparisons with olanzapine was always + except for lurasidone. For the results of the network meta‐analysis, the background colors of the cells reflect confidence in the estimates, with dark grey representing moderate confidence, light grey low confidence, and white very low confidence. NA – not available.
Fluphenazine, fluspirilene, pimozide, loxapine and chlorpromazine were disconnected from the network (see supplementary information for pairwise meta‐analyses involving these drugs).
In the sensitivity analyses, the results did not materially change. When studies conducted by the manufacturer of olanzapine were excluded, the differences of olanzapine compared to risperidone, aripiprazole, haloperidol and iloperidone were no longer clear, in that the 95% CIs included a possibility of opposite effects, but the direction of the differences remained the same as in the main analysis. In the analysis of oral versus LAI formulations, the few RCTs on LAI formulations were disconnected from the network. Comparison‐adjusted funnel plots did not suggest small‐trial effects (see supplementary information).
All‐cause discontinuation
Concerning all‐cause discontinuation, based on 26 RCTs and 8,882 participants, olanzapine was superior to fluphenazine (RR=2.00, 95% CI: 1.44‐2.28), pimozide (RR=1.93, 95% CI: 1.04‐2.32), quetiapine (RR=1.55, 95% CI: 1.35‐1.72), fluspirilene (RR=1.53, 95% CI: 0.36‐2.30), tiotixene (RR=1.51, 95% CI: 1.07‐1.88), paliperidone (RR=1.47, 95% CI: 1.29‐1.65), asenapine (RR=1.46, 95% CI: 1.31‐1.60), ziprasidone (RR=1.44, 95% CI: 1.29‐1.58), haloperidol (RR=1.44, 95% CI: 1.29‐1.58), zotepine (RR=1.41, 95% CI: 0.95‐1.82), and perphenazine (RR=1.27, 95% CI: 1.08‐1.46). Amisulpride, aripiprazole and risperidone were also superior to several other antipsychotics, with 95% CIs making opposite effects unlikely (see Figure 3 and supplementary information).
Figure 3.
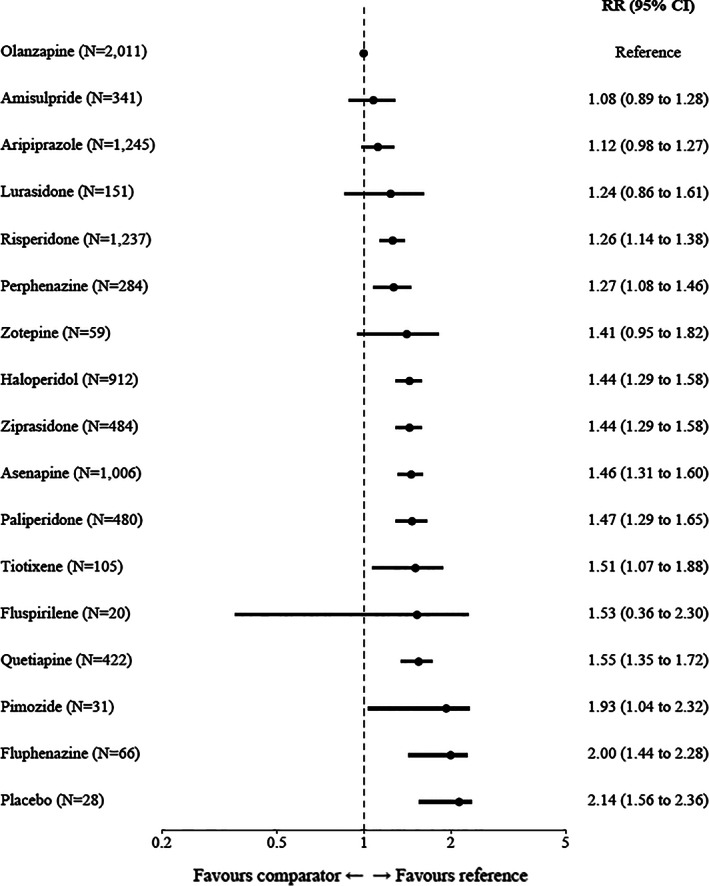
Forest plot for all‐cause study discontinuation. Olanzapine was used as a reference. The numbers in parentheses are those of participants in the trials. RR – relative risk, CI – confidence interval.
Positive and negative symptoms
The results concerning positive and negative symptoms, based on 14 RCTs with 6,155 participants, were similar to those for overall symptoms.
On positive symptoms, olanzapine was more efficacious than chlorpromazine (SMD=0.51, 95% CI: 0.09‐0.93), ziprasidone (SMD=0.37, 95% CI: 0.21‐0.54), paliperidone (SMD=0.32, 95% CI: 0.12‐0.52), asenapine (SMD=0.27, 95% CI: 0.14‐0.41), zotepine (SMD=0.19, 95% CI: –0.19 to 0.56) and aripiprazole (SMD=0.18, 95% CI: 0.05‐0.31). No data on perphenazine, clozapine and iloperidone were available. Based on a single study 24 , disconnected from the network, lurasidone improved positive symptoms more than quetiapine (see supplementary information).
On negative symptoms, olanzapine was more efficacious than chlorpromazine (SMD=2.35, 95% CI: 1.84‐2.87), ziprasidone (SMD=0.33, 95% CI: 0.17‐0.50), haloperidol (SMD=0.27, 95% CI: 0.14‐0.40), asenapine (SMD=0.22, 95% CI: 0.08‐0.35), and risperidone (SMD=0.21, 95% CI: 0.07‐0.34). Again, no data on perphenazine, clozapine and iloperidone were available (see supplementary information).
Chlorpromazine had the lowest symptom reduction, but the results were based on a single small study 25 with only 50 participants.
Depressive symptoms
Concerning depressive symptoms, 11 RCTs and 6,686 participants were available for network meta‐analysis. Most results were uncertain according to 95% CIs. Lurasidone appeared to be more efficacious than a number of other drugs, but these findings stemmed from the above‐mentioned single RCT comparing it with quetiapine 24 , with the remaining evidence being indirect (see supplementary information).
Quality of life and social functioning
Eight RCTs with 2,949 participants yielded no clear differences in quality of life (see supplementary information). There were no inconsistent comparisons according to the SIDE‐test 20 , but the design‐by‐treatment interaction test suggested some inconsistency of the overall network (p=0.092) 26 . Similarly, in five RCTs with 1,390 participants, there were no clear differences in social functioning (see supplementary information).
Weight gain
Concerning weight gain, there was low‐to‐moderate heterogeneity (common tau = 1.05), and the network based on 16 RCTs with 7,542 participants was inconsistent (12.5% inconsistent comparisons, design‐by‐treatment interaction test: p=0.0002). We, therefore, present only the results of the pairwise meta‐analyses comparing olanzapine with the other antipsychotics.
Olanzapine produced more weight gain than all other antipsychotics. MDs ranged from –4.58 kg (95% CI: –5.33 to –3.83) versus ziprasidone, to –3.90 kg (95% CI: –6.73 to –1.08) versus perphenazine, –3.76 (95% CI: –4.89 to –2.63) versus quetiapine, –3.37 (95% CI: –7.21 to 0.47) versus haloperidol, –3.30 (95% CI: –4.20 to –2.40) versus asenapine, –3.16 (95% CI: –4.06 to –2.26) versus aripiprazole, –2.37 (95% CI: –3.70 to –1.03) versus risperidone, and –2.30 (95% CI: –3.35 to –1.25) versus amisulpride (see Figure 4 and supplementary information).
Figure 4.
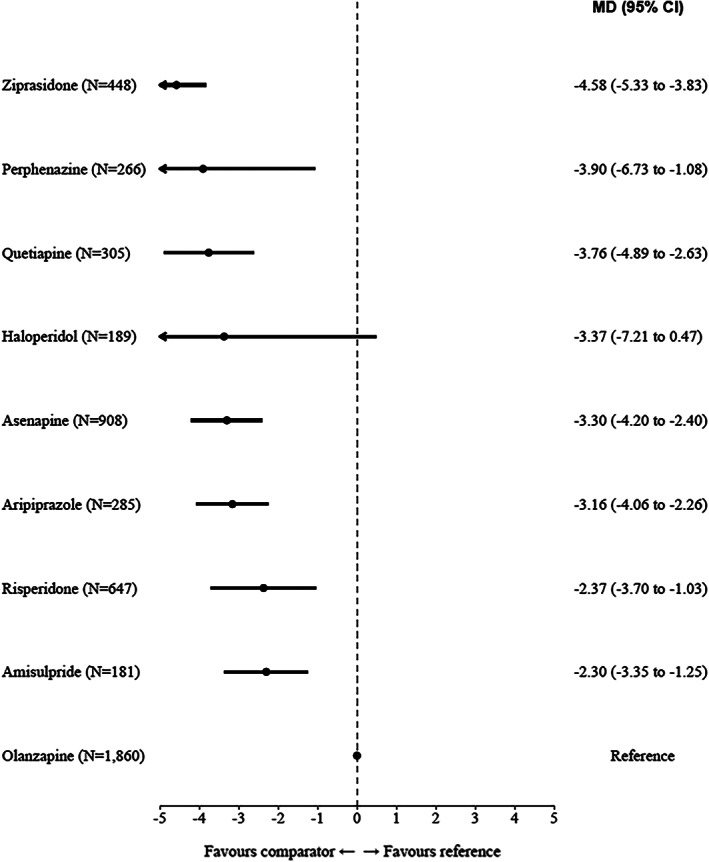
Forest plot for weight gain (pairwise meta‐analyses). The numbers in parentheses are those of participants in the trials. MD – mean difference, CI – confidence interval.
Antiparkinson medication
Concerning use of antiparkinson medication, 14 RCTs with 7,794 participants provided data. Aripiprazole (RR=0.71, 95% CI: 0.54‐0.96) and quetiapine (RR=0.56, 95% CI: 0.29‐1.04) outperformed olanzapine. Zotepine (RR=0.92, 95% CI: 0.43‐1.85, N=59) was about as prone as olanzapine to be associated with the use of that medication, while amisulpride (RR=1.32, 95% CI: 0.90‐1.89), risperidone (RR=1.57, 95% CI: 1.27‐1.94), paliperidone (RR=1.59, 95% CI: 1.13‐2.18), ziprasidone (RR=1.59, 95% CI: 1.11‐2.23), perphenazine (RR=1.63, 95% CI: 1.07‐2.40), haloperidol (RR=2.35, 95% CI: 1.87‐2.92), and asenapine (RR=3.05, 95% CI: 1.51‐5.10) were associated with a greater use (see also supplementary information).
Akathisia
In 16 RCTs with 7,916 participants, paliperidone (RR=0.82, 95% CI: 0.50‐1.48), amisulpride (RR=0.95, 95% CI: 0.54‐1.69), quetiapine (RR=1.03, 95% CI: 0.58‐1.79) and aripiprazole (RR=1.09, 95% CI: 0.78‐1.52) were associated with approximately the same risk of akathisia as olanzapine. Risperidone (RR=1.32, 95% CI: 0.96‐1.81), perphenazine (RR=1.34, 95% CI: 0.76‐2.30), ziprasidone (RR=1.43, 95% CI: 0.97‐2.06), haloperidol (RR=2.39, 95% CI: 1.72‐3.27), asenapine (RR=2.57, 95% CI: 1.54‐4.12) and lurasidone (RR=4.69, 95% CI: 1.21‐11.01) were associated with higher risk. The results of risperidone, perphenazine and ziprasidone were uncertain, because 95% CIs left some possibility of opposite effect. The 95% CI for lurasidone versus olanzapine was very wide. Results on fluphenazine, trifluoperazine, tiotixene and thioridazine were disconnected from the network (see also supplementary information).
Serum prolactin level
The network of 10 RCTs and 5,152 participants was inconsistent (20% inconsistent loops, common tau = 6.15, design‐by‐treatment interaction test: p=0.001). Based on pairwise meta‐analyses, several drugs were associated with lower average prolactin levels than olanzapine: aripiprazole (MD=–8.89 ng/ml, 95% CI: –14.87 to –2.91), asenapine (MD=–4.00 ng/ml, 95% CI: –7.68 to –0.32) and quetiapine (MD=–3.20, 95% CI: –6.81 to 0.41). Ziprasidone (MD=2.36, 95% CI: –0.75 to 5.48), perphenazine (MD=6.50, 95% CI: 2.42‐10.58), haloperidol (MD=7.36, 95% CI: 0.52‐14.20) and risperidone (MD=30.50, 95% CI: 19.36‐41.65) were associated with higher average prolactin levels than olanzapine (see also supplementary information).
QTc prolongation
In the network meta‐analysis of 7 RCTs with 4,060 participants, paliperidone (MD=–2.22 msec, 95% CI: –7.13 to 2.68), risperidone (MD=–0.12 msec, 95% CI: –3.94 to 3.69), asenapine (MD=0.40 msec, 95% CI: –1.83 to 2.63), perphenazine (MD=0.68 msec, 95% CI: –4.10 to 5.46) and ziprasidone (MD=0.71 msec, 95% CI: –1.98 to 3.39) were associated with a similar average QTc prolongation as olanzapine. The values for amisulpride (MD=5.00 msec, 95% CI: –1.81 to 11.81), quetiapine (MD=5.18 msec, 95% CI: 0.55‐9.81) and lurasidone (MD=8.38 msec, 95% CI: –0.03 to 16.79) were a bit larger, but the 95% CIs were wide and included opposite effects for lurasidone and amisulpride. The data on aripiprazole and haloperidol were disconnected from the network (see also supplementary information).
Sedation
The network meta‐analysis of 16 RCTs with 8,096 participants did not indicate clear differences between antipsychotics, because almost all results had wide 95% CIs. The only exception was aripiprazole, which had less risk of sedation than olanzapine (RR=0.58, 95% CI: 0.38‐0.86) and several other drugs. Data on fluphenazine, fluspirilene, chlorpromazine, thioridazine and tiotixene were disconnected from the network (see also supplementary information).
DISCUSSION
It is an important criticism that most antipsychotic drug trials in acutely ill patients with schizophrenia last only six weeks, although these drugs usually need to be taken much longer. Relapse prevention studies in remitted or stable patients cannot fill this gap, because they are conducted in a different phase of the illness, have different outcomes and usually follow drug‐withdrawal designs 5 , 6 . In this network meta‐analysis, we examined studies in initially symptomatic patients with schizophrenia who were subsequently followed up for at least six months.
The main result was that olanzapine is more efficacious than several other FGAs and SGAs, with SMD point estimates between very small (0.12 vs. risperidone) and small to medium (0.37 vs. ziprasidone), and is associated with the lowest all‐cause discontinuation rate. The results were robust to sensitivity analyses (in the analysis excluding studies from the manufacturer of olanzapine, some differences were no longer clear, but their direction remained the same as in the main analysis). On the other hand, on pairwise meta‐analyses, the impact of olanzapine in terms of weight gain was higher than all other antipsychotics, with an MD ranging from –4.58 kg compared to ziprasidone to –2.30 kg compared to amisulpride.
Olanzapine was among the most efficacious drugs in recent network meta‐analyses in short‐term acute phase studies, and long‐term relapse prevention studies 2 , 6 . It was also superior to other antipsychotics in several trials which lasted between 14 and 22 weeks 27 , 28 and, therefore, were not included either in the current network meta‐analysis of long acute‐phase trials or in the previous analysis of short acute‐phase RCTs 2 . The superiority of this drug to other antipsychotics in three large trials of 6‐month duration, which were excluded because conducted in patients with predominant depressive 29 or negative symptoms 30 , 31 , should also be mentioned. Olanzapine, therefore, appears to be a particularly efficacious antipsychotic drug across the different phases of treatment of schizophrenia.
However, the difference between olanzapine and risperidone concerning change in overall symptoms was statistically significant but very small (SMD=0.12), and the differences of olanzapine vs. amisulpride and perphenazine were not significant (SMDs of 0.06 and 0.09, respectively). Perphenazine is an important FGA, because it induces fewer extrapyramidal symptoms than haloperidol and little weight gain, but the data concerning this drug stem almost entirely from the CATIE study 22 . This was a very large, industry‐independent trial, but, if only one study is available, a replication is necessary. The results on clozapine (38 participants), zotepine (59 participants) and all FGAs except haloperidol and perphenazine are uninterpretable, because too few data were available.
Lurasidone ranked (non‐significantly) higher than olanzapine in overall efficacy (Figure 2). However, it was only examined in a single RCT in which it was superior to quetiapine 24 . Thus, its difference compared to drugs other than quetiapine was entirely derived from indirect evidence, and the confidence in these results was often very low.
Taking together the current and previous evidence, risperidone and amisulpride can be currently considered the best alternatives to olanzapine with respect to efficacy in patients with schizophrenia.
The results from the side effect analysis matched with previous findings 2 , 5 , 6 . Risperidone and paliperidone produce most prolactin increase, and partial dopamine agonists are most benign in this regard 2 , 5 , 6 . High‐potency FGAs such as haloperidol cause most extrapyramidal side effects. The main problem with olanzapine is weight gain, which it produces more than all antipsychotics it has been compared to. This side effect is particularly relevant because it is associated with cardiovascular events and may increase mortality in the long term 32 . Therefore, olanzapine is not a drug that can be recommended without reservations for all patients. If more benign antipsychotics are an option, they should be preferred and, in case olanzapine is used, monitoring of cardiovascular risk factors as well as countermeasures to weight gain should be considered. Adjunctive metformin had the best evidence in a Cochrane review 33 , and lifestyle interventions such as diet and physical activity were found effective as well 34 .
Our analysis has limitations. First, compared to our recent meta‐analysis of short‐term trials 2 , the current database is smaller. However, the number of participants was substantial. For several drugs more than 1,000 participants were available for the primary outcome, a threshold which makes results robust 35 . In contrast, clozapine, zotepine and lurasidone had approximately 100 participants or less, and FGAs other than haloperidol and perphenazine were not connected to the network or had no data at all.
Second, quality of life and social functioning are particularly important long‐term outcomes, but the evidence is too scarce to allow firm recommendations. Third, there were several comparisons which lay outside the general networks. Finally, confidence in the evidence was generally moderate to low according to our evaluation with CINeMA 23 .
We conclude that olanzapine is more efficacious than a number of other antipsychotics in the longer‐term treatment of acutely ill patients with schizophrenia. Its superior efficacy must be balanced with its risk for weight gain and, when it is used, monitoring of cardiovascular risk factors as well as initiation of relevant preventive measures appear advisable.
ACKNOWLEDGEMENTS
S. Leucht and J. Schneider‐Thoma are joint first authors, and G. Salanti and J.M. Davis are joint last authors of this paper. The authors are grateful to F. Shokraneh for the literature search, and to S. Siafis, J. Tiang and M. Qin for their help in study selection. The project was funded by the German Ministry of Education and Research (grant no. FKZ01KG1406). Supplementary information on the study is available at https://ebmpp.org/fileadmin/resources/files/00eAppendixAll.pdf.
REFERENCES
- 1. Vos T, Flaxman AD, Naghavi M et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990‐2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2163‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huhn M, Nikolakopoulou A, Schneider‐Thoma J et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi‐episode schizophrenia: a systematic review and network meta‐analysis. Lancet 2019;394:939‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thornley B, Adams C. Content and quality of 2000 controlled trials in schizophrenia over 50 years. BMJ 1998;317:1181‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Correll CU. From receptor pharmacology to improved outcomes: individualising the selection, dosing, and switching of antipsychotics. Eur Psychiatry 2010;25:S12‐21. [DOI] [PubMed] [Google Scholar]
- 5. Ostuzzi G, Bertolini F, Tedeschi F et al. Oral and long‐acting antipsychotics for relapse prevention in schizophrenia‐spectrum disorders: a network meta‐analysis of 92 randomized trials including 22,645 participants. World Psychiatry 2022;21:295‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schneider‐Thoma J, Chalkou K, Dorries C et al. Comparative efficacy and tolerability of 32 oral and long‐acting injectable antipsychotics for the maintenance treatment of adults with schizophrenia: a systematic review and network meta‐analysis. Lancet 2022;399:824‐36. [DOI] [PubMed] [Google Scholar]
- 7. Leucht S, Huhn M, Rothe P et al. Which are the most important first‐generation antipsychotic drugs? Survey of international schizophrenia experts. NPJ Schizophr 2016;2:25. [Google Scholar]
- 8. Leucht S, Davis JM. Which first‐generation antipsychotics should be “repurposed” for the treatment of schizophrenia. Eur Arch Psychiatry Clin Neurosci 2022;272:1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gardner DM, Murphy AL, O'Donnell H et al. International consensus study of antipsychotic dosing. Am J Psychiatry 2010;167:686‐93. [DOI] [PubMed] [Google Scholar]
- 10. Higgins JPT, Thomas J, Chandler J et al. Cochrane handbook for systematic reviews of interventions Version 6.1. www.training.cochrane.org/handbook .
- 11. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 1987;13:261‐76. [DOI] [PubMed] [Google Scholar]
- 12. Overall JE, Gorham DR. The brief psychiatric rating scale. Psychol Rep 1962;10:799‐812. [Google Scholar]
- 13. Higgins JP, Altman DG, Gøtzsche PC et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rosenheck R, Perlick D, Bingham S et al. Effectiveness and cost of olanzapine and haloperidol in the treatment of schizophrenia: a randomized controlled trial. JAMA 2003;290:2693‐702. [DOI] [PubMed] [Google Scholar]
- 15. Leucht S, Chaimani A, Krause M et al. The response of schizophrenia subgroups to different antipsychotic drugs: systematic review and meta‐analysis. Lancet Psychiatry 2022;9:884‐93. [DOI] [PubMed] [Google Scholar]
- 16. Burschinski A, Schneider‐Thoma J, Chiocchia V et al. Metabolic side effects in persons with schizophrenia during mid‐ to long‐term treatment with antipsychotics: a network meta‐analysis of randomized controlled trials. World Psychiatry 2023;22:116‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rücker G, Krahn U, König J et al. Package ‘netmeta’: network meta‐analysis using frequentist methods. mran.revolutionanalytics.com.
- 18. Rhodes KM, Turner RM, Higgins JP. Empirical evidence about inconsistency among studies in a pair‐wise meta‐analysis. Res Synth Methods 2016;7:346‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Turner RM, Davey J, Clarke MJ et al. Predicting the extent of heterogeneity in meta‐analysis, using empirical data from the Cochrane Database of Systematic Reviews. Int J Epidemiol 2012;41:818‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dias S, Welton NJ, Caldwell DM et al. Checking consistency in mixed treatment comparison meta‐analysis. Stat Med 2010;29:932‐44. [DOI] [PubMed] [Google Scholar]
- 21. Higgins JP, Jackson D, Barrett JK et al. Consistency and inconsistency in network meta‐analysis: concepts and models for multi‐arm studies. Res Synth Methods 2012;3:98‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lieberman JA, Stroup TS, McEvoy JP et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;353:1209‐23. [DOI] [PubMed] [Google Scholar]
- 23. Nikolakopoulou A, Higgins JPT, Papakonstantinou T et al. CINeMA: an approach for assessing confidence in the results of a network meta‐analysis. PLoS Med 2020;17:e1003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Loebel A, Cucchiaro J, Xu J et al. Effectiveness of lurasidone vs. quetiapine XR for relapse prevention in schizophrenia: a 12‐month, double‐blind, noninferiority study. Schizophr Res 2013;147:95‐102. [DOI] [PubMed] [Google Scholar]
- 25. Singam AP, Mamarde A, Behere PB. A single blind comparative clinical study of the effects of chlorpromazine and risperidone on positive and negative symptoms in patients of schizophrenia. Indian J Psychol Med 2011;33:134‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cipriani A, Higgins JP, Geddes JR et al. Conceptual and technical challenges in network meta‐analysis. Ann Intern Med 2013;159:130‐7. [DOI] [PubMed] [Google Scholar]
- 27. Dossenbach MRK, Folnegovic‐Smalc V, Hotujac L et al. Double‐blind, randomized comparison of olanzapine versus fluphenazine in the long‐term treatment of schizophrenia. Prog Neuro‐Psychopharmacol Biol Psychiatry 2004;28:311‐8. [DOI] [PubMed] [Google Scholar]
- 28. Tran PV, Tollefson GD, Sanger TM et al. Olanzapine versus haloperidol in the treatment of schizoaffective disorder. Acute and long‐term therapy. Br J Psychiatry 1999;174:15‐22. [DOI] [PubMed] [Google Scholar]
- 29. Kinon BJ, Lipkovich I, Edwards SB et al. A 24‐week randomized study of olanzapine versus ziprasidone in the treatment of schizophrenia or schizoaffective disorder in patients with prominent depressive symptoms. J Clin Psychopharmacol 2006;26:157‐62. [DOI] [PubMed] [Google Scholar]
- 30. Buchanan RW, Panagides J, Zhao J et al. Asenapine versus olanzapine in people with persistent negative symptoms of schizophrenia. J Clin Psychopharmacol 2012;32:36‐45. [DOI] [PubMed] [Google Scholar]
- 31. Kinon BJ, Noordsy DL, Liu‐Seifert H et al. Randomized, double‐blind 6‐month comparison of olanzapine and quetiapine in patients with schizophrenia or schizoaffective disorder with prominent negative symptoms and poor functioning. J Clin Psychopharmacol 2006;26:453‐61. [DOI] [PubMed] [Google Scholar]
- 32. Fleischhacker WW, Cetkovich‐Bakmas M, De Hert M et al. Comorbid somatic illnesses in patients with severe mental disorders: clinical, policy, and research challenges. J Clin Psychiatry 2008;69:514‐9. [DOI] [PubMed] [Google Scholar]
- 33. Agarwal SM, Stogios N, Ahsan ZA et al. Pharmacological interventions for prevention of weight gain in people with schizophrenia. Cochrane Database Syst Rev 2022;10:CD013337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bradley T, Campbell E, Dray J et al. Systematic review of lifestyle interventions to improve weight, physical activity and diet among people with a mental health condition. Syst Rev 2022;11:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Trikalinos TA, Churchill R, Ferri M et al. Effect sizes in cumulative meta‐analyses of mental health randomized trials evolved over time. J Clin Epidemiol 2004;57:1124‐30. [DOI] [PubMed] [Google Scholar]