Abstract
In 2022, over 3 million people died of chronic obstructive pulmonary disease (COPD) and the global burden of the disease is expected to increase over the coming decades. Recommendations for the treatment and management of patients with COPD are published by the Global Initiative for Chronic Obstructive Lung Disease, and updated annually with scientific evidence-based recommendations. The 2023 updates, published in November 2022, contain key changes to recommendations for diagnosis and treatment of COPD that are anticipated to have a significant impact on clinical practice for patients with COPD. Updates to how COPD is defined and diagnosed, including the expansion of contributing factors beyond tobacco use, have the potential to lead to the diagnosis of more patients and to allow for the implementation of early interventions for patients during early stages of the disease. Simplification of the treatment algorithms, and placement of triple therapy within these algorithms, will support clinicians in providing appropriate, timely treatment for patients with COPD with a focus on reducing the risk of future exacerbations. Finally, recognition of mortality reduction as a treatment goal in COPD supports an increase in the use of triple therapy, the only pharmacological intervention that has been demonstrated to improve survival for patients with COPD. Although further guidance and clarification are needed in some areas, such as use of blood eosinophil counts in guiding treatment decisions and implementation of treatment protocols following hospitalizations, recent updates to the GOLD recommendations will support clinicians in addressing current gaps in patient care. Clinicians should utilize these recommendations to drive the early diagnosis of patients with COPD, the identification of exacerbations, and the selection of appropriate, timely treatments for patients.
Keywords: chronic obstructive pulmonary disease, COPD, respiratory, implications, clinical practice, primary care, guidelines
Plain Language Summary
Chronic obstructive pulmonary disease, or COPD, is a long-term lung disease caused by blockage of the airways. It is one of the main causes of death worldwide and, therefore, early diagnosis and personalized treatments are important in reducing the risk of death.
Every year, a group of experts from across the world, known as the Global Initiative for Chronic Obstructive Lung Disease, or GOLD, provide an update of their practical, science-based advice on how to treat people with COPD. The latest recommendations were released in November 2022. The updated recommendations guide healthcare providers to diagnose patients with COPD at an earlier stage of disease than is currently occurring in many cases. Moreover, the updated guidelines encourage treatments to be started before patients become seriously ill. Treatment pathways have also been updated and simplified. Some treatments are now recommended to be used at an earlier stage of disease to prevent COPD flare ups, known as exacerbations. The recommendations also now recognize evidence that some treatment options, such as triple therapy (a combination of three COPD medicines delivered together in a single inhaler), may reduce risk of death in patients with COPD. As such, more emphasis is now placed on the importance of patient survival as a treatment goal and earlier delivery of treatments that may reduce risk of death earlier in the treatment pathway. This article discusses these important changes and how they might affect the care of people living with COPD.
Introduction
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death worldwide, accounting for approximately 3 million deaths globally in 2019.1 The global burden of COPD is expected to increase over the coming decades, owing to the aging population and continued exposure to COPD risk factors.2 Despite this, COPD continues to be inadequately managed, with underdiagnosis, misdiagnosis, and undertreatment occurring in some areas, and overtreatment, typically with systemic corticosteroids, in other areas.3,4
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) was established in 1998 to increase awareness of the burden of COPD and support the prevention and management of COPD worldwide. The GOLD report, first published in 2001, provides scientific evidence-based recommendations on the diagnosis of COPD, the management of stable disease and exacerbations, and the role of comorbidities.5 Adherence to the GOLD recommendations by healthcare professionals may improve outcomes for patients with COPD and reduce COPD-related healthcare-resource utilization (HCRU).6,7
The GOLD recommendations are reviewed and updated annually to reflect the most recent advances in different areas of the disease, particularly treatment and management. The 2023 update, published in November 2022, is the fifth major revision of the report.5 Below, we discuss the key changes in the GOLD 2023 report and the impact that these updates may have on clinical practice, particularly within the primary care setting.
Key Changes in the GOLD 2023 Report
The full updates to the GOLD 2023 report are summarized in Table 1; key changes that are likely to impact clinical practice are discussed in further detail below.
Table 1.
Summary of Changes in GOLD 2023 Report
| Summary of Changes (Including Location in GOLD 2023 Report) |
| Definition and taxonomy |
|
|
|
|
| Diagnosis |
|
|
|
Exacerbation management
|
| Disease management: pharmacological therapies |
|
|
|
|
|
| Disease management: non-pharmacological therapies |
|
|
|
| Comorbidities |
|
Notes: Adapted from the GOLD 2023 Report Highlights, Global Initiative for Chronic Obstructive Lung Disease (GOLD): Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease report 2023.5 Chapters and page numbers from the GOLD 2023 Report are included in the table for ease of reference.
Abbreviations: COPD, chronic obstructive pulmonary disease; CT, computed tomography; ICS, inhaled corticosteroid(s); LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist.
Definition of COPD
The definition of COPD has been amended to emphasize the persistent and progressive airflow obstruction and heterogeneity of manifestations, etiopathology, and structural abnormalities associated with the disease (Table 1). However, a post-bronchodilator forced expiratory volume in 1 second / forced vital capacity (FEV1/FVC) of ≤0.7 as measured by spirometry remains the key diagnostic criterion for COPD when used within the appropriate clinical context. This context includes the presence of chronic respiratory symptoms, structural abnormalities of the airways, and/or COPD risk factors.
Additional criteria for the identification and diagnosis of patients at increased risk of developing COPD have been introduced. These criteria capture patients who do not present with airflow obstruction but have structural lung lesions (eg emphysema), respiratory symptoms, and/or physiological abnormalities (eg low-normal FEV1, gas trapping, hyperinflation, reduced lung diffusing capacity, or rapid FEV1 decline). Such patients will be labeled as pre-COPD or Preserved Ratio Impaired Spirometry (PRISm), dependent on spirometry findings.
In addition, the taxonomy of COPD has been expanded to include non-smoking-related etiotypes (Table 2), reflecting a move away from the traditional idea that COPD is a single disease caused by tobacco smoking. Non-smoking etiotypes include COPD caused by genetic factors, abnormal lung development, environmental factors (such as exposure to biomass), infection, and asthma, including the impact of asthma on lung function and the potential mislabeling of poor childhood lung development as asthma. The recognition of other causes and the updated taxonomy highlight the need to explore the effectiveness of therapies in different etiotypes of COPD.8
Table 2.
Summary of New Classification of COPD Etiotypes
| Classification | Description |
|---|---|
| Genetically determined COPD (COPD-G) | Alpha-1 antitrypsin deficiency Other genetic variations with smaller effects acting in combination |
| COPD due to abnormal lung development (COPD-D) | Early life events, including premature birth and low birth weight, among others |
| Environmental COPD | |
| Cigarette smoking COPD (COPD-C) |
|
| Biomass and pollution exposure COPD (COPD-P) | Exposure to household pollution, ambient air pollution, wildfire smoke, occupational hazards |
| COPD due to infections (COPD-I) | Childhood infections, tuberculosis-associated COPD, WHIV-associated COPD |
| COPD and asthma (COPD-A) | Particularly childhood asthma |
| COPD of unknown cause (COPD-U) | - |
Notes: Adapted from the Global Initiative for Chronic Obstructive Lung Disease (GOLD): Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease report 2023, Celli et al 2022, and Stolz et al 2022.5,8,9 ©2022 Global Strategy for Diagnosis, Management and Prevention of COPD all rights reserved. Use is by express license from the owner.
Abbreviations: COPD, chronic obstructive pulmonary disease; WHIV, women with human immunodeficiency virus.
Definition of an Exacerbation
The definition of a COPD exacerbation has been updated to include specific symptoms and their duration, as well as introducing more objective clinical variables to define the severity of the event (Table 1). Local and systemic inflammation have also been emphasized as important aspects of a COPD exacerbation. This latest definition is aligned with the Rome proposal published in 2021 and addresses the lack of objective supportive elements in the previous definition.9,10 The GOLD report continues to recognize the critical importance of timely identification of exacerbations, owing to their association with loss of lung function and an increased risk of future exacerbations and mortality.11–13
ABE Initial Assessment Tool and Revised Follow-Up Treatment Algorithms
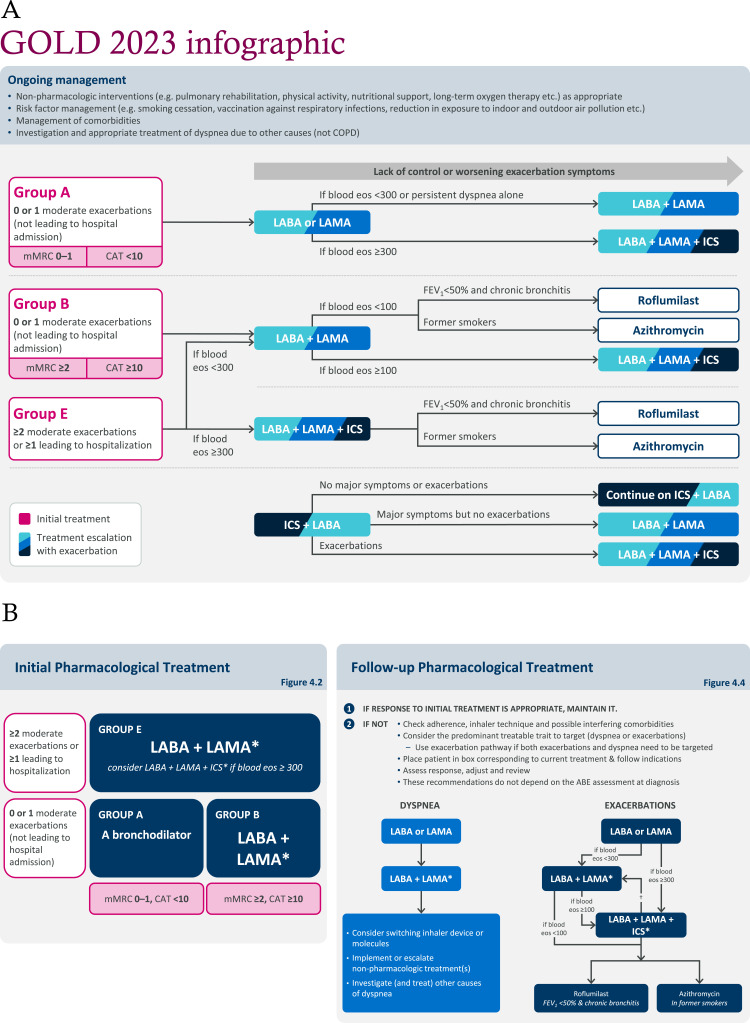
Treatment algorithms have been updated and simplified for patients who are starting pharmacological treatment and for those who experience continued symptoms or exacerbations despite appropriate initial therapy (Figure 1). A key update includes the replacement of the ABCD initial assessment tool, which has remained unchanged since its introduction in 2011, with a new ABE model. Group E, which merges the previous Group C and Group D, comprises patients with a history of either two or more moderate exacerbations or any severe exacerbation (defined as one requiring hospitalization) in the previous year, irrespective of symptom burden.
Figure 1.
(A) Consolidated representation of the updated GOLD treatment algorithms, as interpreted by the authors. (B) Initial Pharmacological Treatment and Follow-up Pharmacological Treatment algorithms as presented in the Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease report 2023.5
Notes: Clinicians should classify treatment-naïve patients as either Groups A, B, or E and prescribe the appropriate treatment as indicated by the algorithm. Patients already receiving treatment should begin at the most appropriate step in the algorithm. (A) adapted from Pharmacological treatment of stable COPD, Figure 4.2, and Figure 4.4, in Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease report 2023.5 (B) as presented in Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease report 20235 (Figures 4.2 and 4.4). ©2022 Global Strategy for Diagnosis, Management and Prevention of COPD all rights reserved. Use is by express license from the owner. GOLD states that the eosinophil levels in its recommendations are estimates and not precise cutoffs. A holistic evaluation of exacerbation risk should be used to decide when initiating or escalating to ICS-containing therapy. *Single inhaler therapy may be more convenient and effective than multiple inhalers; †consider de-escalation of ICS if pneumonia or other considerable side-effects. In case of blood eosinophils ≥300 cells/μL de-escalation is more likely to be associated with the development of exacerbations.
Abbreviations: CAT, COPD Assessment Test; COPD, chronic obstructive pulmonary disease; eos, eosinophils; ICS, inhaled corticosteroid(s); FEV1, forced expiratory volume in 1 second; GOLD, Global Initiative for Chronic Obstructive Lung Disease; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; mMRC, modified Medical Research Council.
The initial treatment recommended for patients in Group E is dual bronchodilation with long-acting muscarinic antagonists (LAMAs) and long-acting β2-agonists (LABAs), regardless of the intensity of dyspnea or quality of life scores. For patients in Group E who are at high risk of exacerbations based on blood eosinophils of ≥300 cells/μL, initial treatment with triple therapy (inhaled corticosteroids [ICS] + LABA + LAMA) is recommended. Clinicians should also remain aware that patients with COPD who also have a diagnosis of asthma should be treated according to guidelines for asthma, with the mandatory use of ICS.
Triple therapy is also recommended as a follow-up treatment in patients with blood eosinophils of ≥100 cells/μL who continue to exacerbate while receiving LABA + LAMA and in patients with blood eosinophils ≥300 cells/μL who continue to exacerbate while receiving monotherapy. Patients receiving ICS + LABA who continue to experience exacerbations are also recommended to be escalated to triple therapy.
Finally, ICS + LABA has been removed from the initial treatment algorithm and the follow-up treatment algorithms for both dyspnea and exacerbations. However, it is recommended that ICS + LABA is an option for those patients with stable disease who are currently receiving this therapy combination. Escalation to other therapies should be considered for patients who have further exacerbations or major symptoms. ICS + LABA may also be appropriate for those patients in areas with limited access to triple therapy. Other recommendations within the initial and follow-up treatment algorithms remain unchanged.
Mortality Reduction as a Treatment Goal
A final significant change is an emphasis on mortality reduction as a treatment goal, with the addition of a new section, “Therapeutic interventions to reduce COPD mortality”. The GOLD 2023 report recognizes that triple therapy reduces mortality and exacerbations in patients with moderate-to-very-severe COPD and a prior history of exacerbations.14–17 The impact of non-pharmacological interventions on mortality, including pulmonary rehabilitation and long-term oxygen therapy, is also highlighted.
Implications for Clinical Practice
Definition of COPD
The latest updates to the definition of COPD may improve the management of patients with pre-COPD and PRISm. Smoking cessation and avoidance of risk factors are important in this group; dual bronchodilator therapy has been demonstrated to have little impact on respiratory symptoms in patients with a smoking history.18 Additional research and novel clinical trials are needed to identify interventions that will benefit this patient population.
Spirometry is essential for the diagnosis of COPD but remains underused and/or inaccessible in some settings, such as primary care.19–21 GOLD does not recommend the use of spirometry for the routine screening of patients with COPD. Instead, GOLD advocates the use of case-finding tools, such as PUMA, CAPTURE, COLA-6, and LFQ, in primary care to identify patients suitable for spirometric assessment.22,23 In contrast with screening, case finding is conducted among targeted groups (with symptoms and/or risk factors) instead of large populations, which is expected to increase the yield of COPD diagnoses and the cost-effectiveness of spirometry. Healthcare system approaches that may improve patient outcomes, particularly in primary care, include the timely identification of patients needing spirometry (based on their risk profile) and early control of risk factors (both smoking and non-smoking related), combined with appropriate treatment. Considering the use of accessible digital solutions and other technology to deploy case-finding tools would be helpful for clinicians.
The GOLD report highlights that, in addition to tobacco smoking, environmental pollutants and health inequality are significant contributing factors to COPD. The prevalence of these factors varies by region and/or setting, but in some regions, non-smoking related factors contribute approximately 50% of the attributable risk for COPD.5,24 The management of non-smoking related risk factors, including reduction of biomass exposure and air pollution, may support the reduction of COPD development and progression. The medical community should strongly consider engaging policymakers to create system-level policies to address these risk factors, with the goal of reducing the burden of COPD on health systems.
The GOLD 2023 report emphasizes that patients with COPD are at increased risk of other medical conditions including cardiovascular conditions such as heart failure, ischemic heart disease, and myocardial infarction, as well as pneumonia and gastroesophageal reflux disease. These can mimic the non-specific symptoms of COPD and exacerbations and are associated with poorer outcomes for patients. All clinicians involved in COPD care should remain vigilant of these increased risks and appropriately address cardiovascular conditions and other ailments with similar symptoms to maximize patients’ overall health.
Definition of an Exacerbation
Timely identification of an exacerbation and defining the severity of the event remains an important task for clinicians, as GOLD recommendations base treatment choices on exacerbation history and severity. Exacerbations are associated with an increased risk of future exacerbations and mortality; therefore, appropriate treatment has the potential to improve outcomes for patients.12,13 Clearly defining a COPD exacerbation using the new parameters outlined will support clinicians to make timely decisions on treatment escalation and potentially reduce overall mortality.
Time and resource constraints in primary care should be considered. Of the six variables that can be used to determine exacerbation severity, four are readily available (intensity of dyspnea, respiratory rate, heart rate, and oxygen saturation) and are sufficient from a clinical judgment perspective. The use of the remaining two variables (measurement of C-reactive protein and arterial blood gases) is also suggested in appropriate patients and settings; however, the use of these assessments should not replace the clinical assessment of an exacerbation.10
ABE Initial Assessment Tool and Revised Follow-Up Treatment Algorithms
The simplification of treatment algorithms in the GOLD 2023 report (Figure 1) should facilitate implementation of the recommendations in the clinical setting. Careful follow up is needed after the initiation of any therapy to monitor response to treatment and make adjustments as required.
The current recommendations continue to suggest that blood eosinophils are used to guide treatment decisions on ICS use. From a clinical perspective, although white blood cell counts are obtained in most patients, there is variability worldwide in the proportion of patients with COPD who have their blood eosinophils reviewed.25 Further education for clinicians may be required on the use of blood eosinophils for the management of patients with COPD.
For patients receiving corticosteroids, it will be critical for GOLD to define the point at which blood eosinophils should be measured, as ICS have been associated with lowering blood eosinophils.26 The GOLD 2023 report recognizes that blood eosinophil thresholds of ≥100 cells/µL and ≥300 cells/µL are not definitive cutoffs but reflect the continuum of response to ICS-containing therapies. This is compatible with GOLD favoring the addition of ICS to long-acting bronchodilators if patients have blood eosinophils of 100–300 cells/μL and strongly favoring the use of ICS in patients with blood eosinophils of ≥300 cells/μL. Future studies may clarify the relationship between blood eosinophils and the response to ICS treatment.
GOLD recommends consideration of triple therapy as an initial treatment in patients with recent exacerbations and blood eosinophils ≥300 cells/μL. In addition, escalation to triple therapy is recommended if exacerbations occur in patients with blood eosinophils ≥300 cells/µL receiving monotherapy or patients with blood eosinophils ≥100 cells/μL receiving LABA + LAMA. This is important, as escalation to triple therapy has been shown to reduce future exacerbations, HCRU, and mortality.27
The decision to use an ICS is a careful balance of risk and benefit, as the chronic use of ICS in patients with COPD is associated with an increased risk of pneumonia, influenced by both the dose and duration of ICS use.28,29 A holistic evaluation of exacerbation risk alongside assessment of blood eosinophils is recommended when making treatment decisions.
Although ICS + LABA therapy is no longer included in the treatment algorithms in the GOLD 2023 report, it is recommended that clinicians maintain ICS + LABA if a patient’s response on this therapy is adequate. Patients receiving ICS + LABA who experience exacerbations, with or without additional symptoms, are recommended to be escalated to triple therapy where this is available, either as fixed-dose or open therapy. Patients receiving ICS + LABA who experience major symptoms in the absence of exacerbations are recommended to be switched to LABA + LAMA. However, clarification is needed from GOLD on the definition of major symptoms. With up to 37% of patients with COPD receiving ICS + LABA globally, it is important for clinicians to understand this update on the placement, or lack thereof, of ICS + LABA in the treatment algorithms (Figure 1).25,30–32
Recognition of Mortality Reduction as a Treatment Goal
GOLD has increased the emphasis on the importance of mortality reduction as a treatment goal for patients with COPD. As such, clinicians should consider triple therapy for high-risk patients, as supported by two large studies demonstrating the mortality benefits of triple therapy compared with LABA + LAMA in patients with severe airflow obstruction and a history of exacerbations.14,15 Non-pharmacological interventions, such as smoking cessation and pulmonary rehabilitation, have also been demonstrated to reduce mortality in patients with COPD.33,34
Areas of Controversy and Potential Guideline Evolution
Although the updated GOLD report introduces several welcome changes, questions remain in some areas, and further guidance is required.
The GOLD 2023 report recommends that triple therapy be considered as an initial treatment only for patients with blood eosinophils ≥300 cells/μL and who have had ≥2 moderate or ≥1 severe exacerbation. Recent evidence indicates that patients already receiving therapy and with blood eosinophils of between 100 and 300 cells/μL, particularly those who have experienced a hospitalization, may also benefit from the addition of an ICS to their existing treatment regimens.35 Further research is needed to explore the use of lower thresholds for triple therapy initiation, thus extending the benefits of this treatment to a broader population.
The current follow-up treatment algorithm for patients experiencing exacerbations does not specify the severity or frequency of their exacerbations. Given the association between exacerbation severity and the risk of future exacerbations and/or mortality, clarity is needed on how exacerbation severity should impact treatment decisions. Considering severity and not just blood eosinophils may present an opportunity to alleviate the pathobiological and financial complications of further exacerbations.
Recommendations on treatment strategies following hospitalization would also be welcomed. There is strong evidence to suggest that hospitalizations are associated with an increased risk of poor outcomes, including future hospitalizations and mortality.12,13 Triple therapy has been found to reduce future hospitalizations following both moderate and severe exacerbations.15,16,36 We would support the inclusion of recommendations standardizing the use of triple therapy for patients who have been hospitalized for an exacerbation. The timely use of pulmonary rehabilitation in patients who are admitted to hospital also remains an area that needs improvement and further clarification in guidelines.37
Conclusions
The updates to the GOLD 2023 report provide a simplified approach to the management of COPD, which will support clinicians in addressing current gaps in patient care.
Further research and clarification are needed in several areas, including the identification of optimal treatments for patients with different etiotypes, the cutoffs of blood eosinophils to guide treatment decisions, and the use of triple therapy after COPD hospitalizations.
It will remain critical for clinicians to interpret how best to implement the latest GOLD recommendations in clinical practice, particularly within the primary care setting, to ensure patients receive optimal, personalized care. We call on clinicians to utilize these recommendations to assist in diagnosing patients with COPD earlier, identifying more exacerbations and stratifying their severity, and reducing mortality by selecting the appropriate life-saving interventions.
Acknowledgments
Medical writing support was provided by Clare Stretton, PhD, and Leanne Miller, PhD, of Helios Medical Communications, Macclesfield, UK, and funded by AstraZeneca.
Funding Statement
This article was funded by AstraZeneca. AstraZeneca provided all necessary scientific bibliography and funded medical writing support and publication charges. The authors did not receive direct funding for the writing of this article.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Diana R Tamondong-Lachica has received consultancy fees from AstraZeneca. Neil Skolnik has received speaker/consultancy fees from Abbott, AstraZeneca, Bayer, Boehringer Ingelheim, Lilly, Genentech, GlaxoSmithKline, Idorsia, Merck, Novartis, Sanofi, Sanofi Pasteur, and Teva; and research funding from AstraZeneca, Bayer, GlaxoSmithKline, Novo Nordisk, and Sanofi. John R Hurst has received speaker/consultancy fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, and Takeda. Nathaniel Marchetti has received speaker/consultancy fees from AstraZeneca; grants from CSL Behring and NIH; and research funding from AstraZeneca, Chiesi, GlaxoSmithKline, and Sanofi. Adrian Paul J Rabe is an employee of AstraZeneca. Maria Montes de Oca has received speaker fees from AstraZeneca and GlaxoSmithKline. Bartolome R Celli has received speaker/consultancy fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Gala Therapeutics, GlaxoSmithKline, Menarini, Novartis, Pulmonx, and Sanofi-Aventis; neither he, nor any member of his family, has shares or interest in any company; and he has not received or had any relationship with money from the tobacco industry. The authors report no other conflicts of interest in this work.
References
- 1.World Health Organization. The top 10 causes of death; 2020.Available from: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death. Accessed April 27, 2021.
- 2.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. doi: 10.1371/journal.pmed.0030442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casas A, Montes de Oca M, Menezes AM, et al. Respiratory medication used in COPD patients from seven Latin American countries: the LASSYC study. Int J Chron Obstruct Pulmon Dis. 2018;13:1545–1556. doi: 10.2147/COPD.S154097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lamprecht B, Soriano JB, Studnicka M, et al. Determinants of underdiagnosis of COPD in national and international surveys. Chest. 2015;148(4):971–985. doi: 10.1378/chest.14-2535 [DOI] [PubMed] [Google Scholar]
- 5.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease report; 2023. Availble from: https://goldcopd.org/2023-gold-report-2/. Accessed April 12, 2023.
- 6.Gayle A, Dickinson S, Morris K, Poole C, Mathioudakis AG, Vestbo J. What is the impact of GOLD 2017 recommendations in primary care? – a descriptive study of patient classifications, treatment burden and costs. Int J Chron Obstruct Pulmon Dis. 2018;13:3485–3492. doi: 10.2147/COPD.S173664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palli SR, Zhou S, Shaikh A, Willey VJ. Effect of compliance with GOLD treatment recommendations on COPD health care resource utilization, cost, and exacerbations among patients with COPD on maintenance therapy. J Manag Care Spec Pharm. 2021;27(5):625–637. doi: 10.18553/jmcp.2021.20390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Celli B, Fabbri L, Criner G, et al. Definition and nomenclature of chronic obstructive pulmonary disease: time for its revision. Am J Respir Crit Care Med. 2022;206(11):1317–1325. doi: 10.1164/rccm.202204-0671PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stolz D, Mkorombindo T, Schumann DM, et al. Towards the elimination of chronic obstructive pulmonary disease: a Lancet Commission. Lancet. 2022;400(10356):921–972. doi: 10.1016/S0140-6736(22)01273-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Celli B, Fabbri L, Aaron S, et al. An updated definition and severity classification of chronic obstructive pulmonary disease exacerbations: the Rome proposal. Am J Respir Crit Care Med. 2021;204(11):1251–1258. doi: 10.1164/rccm.202108-1819PP [DOI] [PubMed] [Google Scholar]
- 11.Dransfield MT, Kunisaki KM, Strand MJ, et al. Acute exacerbations and lung function loss in smokers with and without chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;195(3):324–330. doi: 10.1164/rccm.201605-1014OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whittaker H, Rubino A, Müllerová H, et al. Frequency and severity of exacerbations of COPD associated with future risk of exacerbations and mortality: a UK routine health care data study. Int J Chron Obstruct Pulmon Dis. 2022;17:427–437. doi: 10.2147/COPD.S346591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haughney J, Lee AJ, Nath M, et al. The long-term clinical impact of COPD exacerbations: a 3-year observational study (SHERLOCK). Ther Adv Respir Dis. 2022;16:17534666211070140. doi: 10.1177/17534666211070139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lipson DA, Crim C, Criner GJ, et al. Reduction in all-cause mortality with fluticasone furoate/umeclidinium/vilanterol in COPD patients. Am J Respir Crit Care Med. 2020;201(12):1508–1516. doi: 10.1164/rccm.201911-2207OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rabe KF, Martinez FJ, Ferguson GT, et al. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020;383(1):35–48. doi: 10.1056/NEJMoa1916046 [DOI] [PubMed] [Google Scholar]
- 16.Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671–1680. doi: 10.1056/NEJMoa1713901 [DOI] [PubMed] [Google Scholar]
- 17.Papi A, Vestbo J, Fabbri L, et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. Lancet. 2018;391(10125):1076–1084. doi: 10.1016/S0140-6736(18)30206-X [DOI] [PubMed] [Google Scholar]
- 18.Han MK, Ye W, Wang D, et al. Bronchodilators in tobacco-exposed persons with symptoms and preserved lung function. N Engl J Med. 2022;387(13):1173–1184. doi: 10.1056/NEJMoa2204752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diab N, Gershon AS, Sin DD, et al. Underdiagnosis and overdiagnosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;198(9):1130–1139. doi: 10.1164/rccm.201804-0621CI [DOI] [PubMed] [Google Scholar]
- 20.Rossaki FM, Hurst JR, van Gemert F, et al. Strategies for the prevention, diagnosis and treatment of COPD in low- and middle- income countries: the importance of primary care. Expert Rev Respir Med. 2021;15(12):1563–1577. doi: 10.1080/17476348.2021.1985762 [DOI] [PubMed] [Google Scholar]
- 21.Johns DP, Walters JAE, Walters EH. Diagnosis and early detection of COPD using spirometry. J Thorac Dis. 2014;6(11):1557–1569. doi: 10.3978/j.issn.2072-1439.2014.08.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siddharthan T, Pollard SL, Quaderi SA, et al. Discriminative accuracy of chronic obstructive pulmonary disease screening instruments in 3 low- and middle-income country settings. JAMA. 2022;327(2):151–160. doi: 10.1001/jama.2021.23065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopez Varela MV, Montes de Oca M, Wehrmeister FC, Rodriguez C, Ramirez L, Menezes A. External validation of the PUMA COPD diagnostic questionnaire in a general practice sample and the PLATINO study population. Int J Chron Obstruct Pulmon Dis. 2019;14:1901–1911. doi: 10.2147/COPD.S206250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang IA, Jenkins CR, Salvi SS. Chronic obstructive pulmonary disease in never-smokers: risk factors, pathogenesis, and implications for prevention and treatment. Lancet Respir Med. 2022;10(5):497–511. doi: 10.1016/S2213-2600(21)00506-3 [DOI] [PubMed] [Google Scholar]
- 25.Vogelmeier CF, Kostikas K, Fang J, et al. Evaluation of exacerbations and blood eosinophils in UK and US COPD populations. Respir Res. 2019;20(1):178. doi: 10.1186/s12931-019-1130-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kreindler JL, Watkins ML, Lettis S, Tal-Singer R, Locantore N. Effect of inhaled corticosteroids on blood eosinophil count in steroid-naïve patients with COPD. BMJ Open Respir Res. 2016;3(1):e000151. doi: 10.1136/bmjresp-2016-000151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tkacz J, Evans KA, Touchette DR, et al. PRIMUS - Prompt initiation of maintenance therapy in the US: a real-world analysis of clinical and economic outcomes among patients initiating triple therapy following a COPD exacerbation. Int J Chron Obstruct Pulmon Dis. 2022;17:329–342. doi: 10.2147/COPD.S347735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh S, Amin AV, Loke YK. Long-term use of inhaled corticosteroids and the risk of pneumonia in chronic obstructive pulmonary disease: a meta-analysis. Arch Intern Med. 2009;169(3):219–229. doi: 10.1001/archinternmed.2008.550 [DOI] [PubMed] [Google Scholar]
- 29.Janson C, Johansson G, Ställberg B, et al. Identifying the associated risks of pneumonia in COPD patients: ARCTIC an observational study. Respir Res. 2018;19(1):172. doi: 10.1186/s12931-018-0868-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bloom CI, Douglas I, Usmani OS, Quint JK. Inhaled corticosteroid treatment regimens and health outcomes in a UK COPD population study. Int J Chron Obstruct Pulmon Dis. 2020;15:701–710. doi: 10.2147/COPD.S241568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng W, Duan J, Zhou A, et al. Real-world effectiveness of inhalation therapy among patients with symptomatic COPD in China: a multicenter prospective study. Front Pharmacol. 2021;12:753653. doi: 10.3389/fphar.2021.753653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bloom CI, Montonen J, Jöns O, Garry EM, Bhatt SP. Treatment transitions in chronic obstructive pulmonary disease: retrospective analyses of US and UK healthcare databases. Pulm Ther. 2022;8(1):75–93. doi: 10.1007/s41030-021-00180-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anthonisen NR, Skeans MA, Wise RA, et al. The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med. 2005;142(4):233–239. doi: 10.7326/0003-4819-142-4-200502150-00005 [DOI] [PubMed] [Google Scholar]
- 34.Ryrsø CK, Godtfredsen NS, Kofod LM, et al. Lower mortality after early supervised pulmonary rehabilitation following COPD-exacerbations: a systematic review and meta-analysis. BMC Pulm Med. 2018;18(1):154. doi: 10.1186/s12890-018-0718-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bafadhel M, Peterson S, De Blas MA, et al. Predictors of exacerbation risk and response to budesonide in patients with chronic obstructive pulmonary disease: a post-hoc analysis of three randomised trials. Lancet Respir Med. 2018;6(2):117–126. doi: 10.1016/S2213-2600(18)30006-7 [DOI] [PubMed] [Google Scholar]
- 36.Evans KA, Pollack M, Portillo E, et al. Prompt initiation of triple therapy following hospitalization for a chronic obstructive pulmonary disease exacerbation in the United States: an analysis of the PRIMUS study. J Manag Care Spec Pharm. 2022;28(12):1366–1377. doi: 10.18553/jmcp.2022.28.12.1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lindenauer PK, Stefan MS, Pekow PS, et al. Association between initiation of pulmonary rehabilitation after hospitalization for COPD and 1-year survival among Medicare beneficiaries. JAMA. 2020;323(18):1813–1823. doi: 10.1001/jama.2020.4437 [DOI] [PMC free article] [PubMed] [Google Scholar]