Abstract
The stx-flanking regions of 49 Shiga toxin-producing Escherichia coli strains and nine Shigella dysenteriae serotype 1 strains containing either stx, stx1, stx2, or stx2 variant genes, were examined. We analyzed these regions by PCR using a set of primers with one primer specific for the respective stx gene and a second primer complementary to sequences of Stx phages H-19B and 933W. We further characterized the amplification products by restriction endonuclease digestion and nucleotide sequencing. PCR products of stx1-containing E. coli strains of serogroups O157, O26, and 0103 showed the same lengths and similar restriction patterns. However, we failed to amplify the 3′ stx-flanking region in stx1-harboring E. coli O111:H− strains. Stx2-producing E. coli strains revealed amplification products of different lengths and restriction patterns, suggesting greater heterogeneity than in stx1-positive strains. We also obtained specific PCR products for two Stx2c-producing and seven Stx2f-producing E. coli strains when they were subjected to PCR analysis. In nine S. dysenteriae type 1 strains, H-19B- and 933W-specific primers amplified only the 3′ stx-flanking region. The results of our study demonstrate that the stx genes of all strains investigated are continuous with phage sequences. Whereas almost all strains except E. coli O111:H− strains were associated with a S-like gene, association with Q could not be demonstrated in nine S. dysenteriae type 1 strains and three E. coli strains. Furthermore, we showed that the organization of the stx-flanking regions is similar in all strains investigated, whereas fine-structure analysis showed subtle differences among the sequences examined. Our results support the hypothesis that stx genes in E. coli and S. dysenteriae are generally phage-borne.
Shiga toxin (Stx)-producing Escherichia coli (STEC) can cause a broad spectrum of human diseases ranging from watery diarrhea to hemorrhagic colitis and the hemolytic-uremic syndrome (5). Shiga toxins have been generally accepted to be the main agent in the development of these diseases (5, 16).
Two Stx subgroups have been described in E. coli: Stx1 and Stx2. The structural genes for Stx1 and Stx2 are encoded in the genome of temperate, lambdoid bacteriophages in E. coli O157 and O26 strains (9, 28–30). Such Stx-encoding phages of E. coli O157 and O26 strains were reported to have similar morphologies with regular hexagonal heads and short tails (20). E. coli O157 phages are similar in genome size and have highly related restriction fragment length polymorphism (RFLP) patterns (17). stx RFLP pattern analysis of O157 and non-O157 STEC strains suggested a more heterogeneous structure of phage DNA flanking the stx genes in E. coli O157:H7 than in non-O157 STEC strains (23). Recently, the whole genome of the Stx2-encoding phage 933W and a 17.3-kb fragment of the Stx1-encoding phage H-19B were determined by nucleotide sequencing (15, 19).
Sequence analysis of a part of the Stx1-encoding phage H-19B has shown that the stxA1 and stxB1 genes are located in the late-phase region, downstream of and in the same transcriptional orientation as a λ Q homologue and upstream of the λ homologue genes S, R, and Rz, which are necessary for the release of phage particles. A similar structure was found in the Stx2-encoding phage 933W (19).
In bacteriophage λ, the Q protein functions as a transcription antiterminator by modifying transcription complexes initiating at the late promoter PR′. Neely and Friedman could show a direct effect of the phage H-19B Q protein on the levels of Stx expression, as well as expression of the downstream lysis genes, and concluded that the H-19B antiterminator Q acts as a transcriptional activator by supporting the readthrough of the transcription terminator (14, 15).
The stx1 genes are separated from the lysis genes by a 3-kb region (15), whereas the distance of the stx2 genes to the lysis genes is 3.5 kb (9, 19). The region between stx genes and lysis genes contains some open reading frames, the sequence of which show only marginal homology with other genes in searches using sequence database libraries. Only one open reading frame, L0105 (19), has been described for the Stx2-encoding phage 933W in this region, which encodes a protein with 50.8% identity to YjhS of E. coli K-12. The function of this gene is yet unknown, but it is described as a part of a fimbrial synthesis and iron transport gene cluster. A similar 849-bp open reading frame whose putative gene product shows 20.5% identity to YjhS is found in the stx-flanking region of the Stx1-encoding phage H-19B.
In this study, we investigated the stx-flanking regions of a large set of distinct Stx-producing E. coli and S. dysenteriae strains to study the heterogeneity in this region and to look for stx-continuous phage sequences.
MATERIALS AND METHODS
Bacterial strains and plasmids.
E. coli strains 6061/96, 2969/99, 2791/97, 6366/97, 4865/96, 893/98, 5951/97, and 6480/96 were isolated at the Institute of Hygiene and Microbiology, University of Würzburg, by routine methods. The characteristics of these strains and of all other E. coli isolates used in this study are presented in Table 1. E. coli C600(H19J) and C600(933W) are laboratory strains lysogenized with phage H19J (29) and phage 933W (17). S. dysenteriae serovar 1 isolates RIMD3101010, H2540-34/82, H1250-36/74, H2765-34/81, H3104-34/89, H4435-34/89, 1971, SZ 340/95, and W206 were kindly provided by Jochen Bockemühl, Nationales Referenzzentrum für Enteritiserreger, Hygiene Institut, Hamburg, Germany.
TABLE 1.
Characteristics of E. coli strains used in this study
| Strain | Serotype | stx Genotype | Disease association or sourcea | Source or reference |
|---|---|---|---|---|
| EDL933 | O157:H7 | stx1stx2 | BD | 18 |
| A8993 CS2 | O157:H7 | stx1stx2 | BD | 8 |
| HUSCL8 | O157:H7 | stx1stx2 | HUS | 10 |
| H19 | O26:H11 | stx1 | 28 | |
| C600(933W) | stx2 | Laboratory strain | ||
| C600(H19J) | stx1 | Laboratory strain | ||
| 1858/96 | O103:H2 | stx1 | D | 23 |
| 2905/96 | O103:H2 | stx1 | D | 23 |
| 3942/96 | O103:H2 | stx1 | HUS | 23 |
| PMK3 | O103:H2 | stx1 | HUS | 12 |
| UTI | O103:H2 | stx1 | HUS | 31 |
| TB-325A | O26:H− | stx1 | D | 2 |
| ED-17 | O26:H− | stx1 | HUS | 21 |
| 5080/97 | O26:H− | stx1stx2 | HUS | 23 |
| TB334 | O85:H− | stx1 | D | 2 |
| SK004 | O26:H− | stx1 | BD | 23 |
| 5236/96 | O26:H11 | stx1 | D | 23 |
| 1639/77 | O111:H− | stx1 | BD | 23 |
| ED-31 | O111:H− | stx1 | HUS | 21 |
| 78/92 | O111:H− | stx1 | HUS | 21 |
| SK003 | O111:H− | stx1stx2 | D | 23 |
| 3574/92 | O157:H7 | stx2 | HUS | 3 |
| 702/88 | O157:H− | stx2 | HUS | 1 |
| 1448/97 | O26:H11 | stx2 | D | This study |
| ED-147 | O26:H11 | stx2 | HUS | 21 |
| 4993/96 | O26:H11 | stx2 | D | 21 |
| 6061/96 | O26:H11 | stx2 | D | This study |
| 3639/96 | O26:H11 | stx2 | D | 21 |
| 7828/95 | O103:H2 | stx1stx2 | HUS | 23 |
| 2969/99 | O103:H2 | stx2 | HUS | This study |
| 2791/97 | O103:H− | stx2 | HUS | This study |
| 6366/97 | O111:H− | stx1stx2 | D | This study |
| 6833/96 | O11:H2 | stx2 | D | 23 |
| 893/98 | O145:H− | stx2 | HUS | This study |
| 5951/97 | O145:H− | stx2 | HUS | This study |
| 6480/96 | O8:H− | stx2 | D | This study |
| E32511 | O157:H− | stx2c | HUS | 27 |
| 4865/96 | O145:H− | stx2c | HUS | This study |
| H.I.8 | O128:B12 | stx2f | D | 4 |
| T4/97 | O128:H2 | stx2f | Pigeon | 26 |
| E-D 365 | O18a,b:HND | stx2f | Pigeon | 26 |
| E-D 371 | O45:HND | stx2f | Pigeon | 26 |
| E-D 373 | O45:HND | stx2f | Pigeon | 26 |
| T4/97 | O128:H2 | stx2f | Pigeon | 26 |
| 5598/97 | O128:H2 | stx2f | Pigeon | 26 |
D, diarrhea; BD, bloody diarrhea; HUS, hemolytic-uremic syndrome; HND, H type not determined.
Standard DNA techniques.
Plasmid DNA was prepared with Qiagen plasmid mini and midi kits (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Genomic DNA was prepared as described previously (7). DNA was digested with restriction endonucleases (New England Biolabs, Schwalbach, Germany; Gibco-BRL, Karlsruhe, Germany), and restriction fragments were analyzed by separation on 0.6 to 0.9% agarose gels in 0.5-fold-concentrated Tris-borate-EDTA buffer and stained with ethidium bromide. Purification of DNA fragments from agarose gels was performed using a Prep-a-Gene kit (Bio-Rad, Munich, Germany) or a QIAex kit (Qiagen). If not indicated otherwise, transformation of plasmid DNA was performed according to standard protocols (22). For Southern blot hybridization, DNA was prepared and separated electrophoretically and subsequently transferred from agarose gels to Zeta-Probe GT blotting membranes (Bio-Rad). Hybridization assays were performed with a digoxigenin-labeled PCR generated stx1B-specific DNA probe (24) under stringent conditions in accordance with the manufacturer's instructions (Boehringer GmbH, Mannheim, Germany). Specific washing steps were performed twice, each for 5 min at 60°C in 1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) and 0.1% (wt/vol) sodium dodecyl sulfate.
PCR.
Amplification was carried out in a total volume of 100 μl containing each deoxynucleoside triphosphate at 200 μM, 30 pmol of each primer, 10 μl of 10-fold-concentrated Expand High Fidelity buffer (with 10-fold-concentrated MgCl2), and 2.6 U of Expand High Fidelity Polymerase (Boehringer GmbH) according to the manufacturer's instructions.
Template DNA (500 to 1,000 ng) was denatured at 94°C for 30 s, annealed at the appropriate temperature for 60 s, and then extended for 3 min at 68°C. This amplification step was carried out for 10 cycles. In the following 20 cycles the elongation time was 3 min plus a 20-s elongation step for each cycle. A final extension step of 7 min at 72°C was also conducted. The PCR products were separated on a 0.8% agarose gel, and gel slices with the appropriate DNA fragment were excised. DNA was eluted and purified with the QIAquick PCR purification kit (Qiagen). The purified DNA amplificates were digested either with PvuII or with EcoRI and separated again on a 0.8% agarose gel.
Oligonucleotides were purchased from ARK Scientific GmbH (Darmstadt, Germany) and were designed with the Oligo 4.06 program (National Biosciences, Inc.). All primers used in this study are listed in Table 2.
TABLE 2.
Nucleotide sequences of primers used for PCR analysis of stx-flanking regions
| Primer | Nucleotide sequence | Target sequence | Source or reference |
|---|---|---|---|
| stxB1-f | 5′-ACGCCTGATTGTGTAACTGGA-3′ | stxB1 | This study |
| 85a-stx1-b | 5′-GACCACCACATCACCTTCTGC-3′ | orf85a | This study |
| 282-stx1-b | 5′-TACCACTGATGAAGGCTGAGG-3′ | orf282 | This study |
| s-stx-b | 5′-ACCTGTTGTGATTTTTTCCAT-3′ | Holin S gene | This study |
| s-stx-b1 | 5′-CCAAACAGCAGACTCCCCAG-3′ | Holin S gene | This study |
| Q-stx-f | 5′-CGGAGGGGATTGTTGAAGGC-3′ | Antiterminator Q | This study |
| 21 | 5′-CCCGGATCCATGAAAAAAACATTATTAATAGC-3′ | stxA1 subunit | This study |
| 285 | 5′-CCTTCGCCACCACATTAACTG-3′ | stxA1 subunit | 25 |
| 595 | 5′-CCGAAGAAAAACCCAGTAACAG-3′ | stxA2 subunit | 25 |
| 545 | 5′-CATGAAGAAGATGTTTATGGCG-3′ | stxB2 subunit | This study |
| 457 | 5′-CACCCCGTTCTCATCCGTCATG-3′ | stx-flanking region | This study |
| 422 | 5′-GGTCTTTTGCATCTGTATGG-3′ | stx-flanking region | This study |
| NinG/526 | 5′-CACAAGCAATGCGTGGTGTGC-3′ | NinG region | This study |
| roi-f1 | 5′-CATTGAAATCGCTGAGTTGGT-3′ | Roi region | This study |
| roi-f2 | 5′-TCGCAGCACCTAAAGTTGAGT-3′ | Roi region | This study |
| 128-1 | 5′-AGAATTGGGCGTCATTCACTGGTTG-3′ | stxA2f subunit | 26 |
| stx2f-s-b | 5′-GAAGCCCAGAACCAGACTCC-3′ | Holin S (T4/97) | This study |
| O111fl-1 | 5′-GGGCAATGATGACCACAC-3′ | stx-flanking region | This study |
| O111fl-2 | 5′-CAGTCGGTACAGTTTTGAGATT-3′ | stx-flanking region | This study |
| O111fl-3 | 5′-CAATAAAACGCACACCATCC-3′ | stx-flanking region | This study |
| IS600-f | 5′-ATGAGCAGAAAAACCCAACG-3′ | IS600 element | This study |
Cloning of PCR products.
PCR products were ligated into pCR 2.1 vector (Original TA Cloning Kit, pCR II, pCR 2.1; Invitrogen) and transformed into E. coli InvVαF′ competent cells according to the manufacturer's instructions.
Nucleotide sequencing.
Nucleotide sequencing was carried out with universal and reverse primers for pUC/M13 vectors and customized primers. Separation of sequencing products was performed on 7% denaturing polyacrylamide gels in a 377 automatic sequencer (Perkin-Elmer/Applied Biosystems GmbH, Weiterstadt, Germany). Nucleotide sequence analysis of PCR products was performed with two clones obtained independently. DNA sequences were analyzed with the DNASIS program, version 2.0, from Hitachi Software (San Bruno, Calif.). Searches for homologies of DNA sequences were performed using the EMBL-GenBank database library.
Nucleotide sequence accession numbers.
The nucleotide sequences determined and analyzed during this study were submitted to the EMBL data library. Characteristics and accession numbers of these sequences are presented in Table 3.
TABLE 3.
Characteristics and accession numbers of sequences determined in this study
| Strain | Sequence length (bp) | Description | Accession no. |
|---|---|---|---|
| 1858/96 | 3,199 | stxB1 gene, orf75, orf616, partial S gene | AJ251754 |
| 1639/77 | 5,467 | Partial Q gene, stxA1, stxB1, orf101, orf155, orf120, orf68, orf61 | AJ251325 |
| 1448/97 | 3,919 | stxB2 gene, orf107, orf645, orf59, orf84, orf49, partial S gene | AJ250954 |
| ED-147 | 3,896 | stxB2 gene, orf107, orf645, orf81 | AJ251483 |
| 3985/96 | 3,920 | stxB2 gene, orf107, orf645, orf59, orf90, partial S gene | AJ251520 |
| E32511 | 3,920 | stxB2c gene, orf107, orf645, orf59, partial S gene | AJ251452 |
| 4865/96 | 3,922 | stxB2c gene, orf645, orf59, partial S gene | AJ271063 |
| H.I.8 | 1,892 | stxA2f, stxB2f, orf60, partial S gene | AJ271139 |
| T4/97 | 2,411 | stxA2f, stxB2f, orf67, S gene | AJ270998 |
| H2765-34/81 | 6,014 | IS600, stxA, stxB, orf109, orf536, orf60, orf87, S gene | AJ271153 |
RESULTS
Analysis of the stx-flanking region of Stx1-positive STEC isolates.
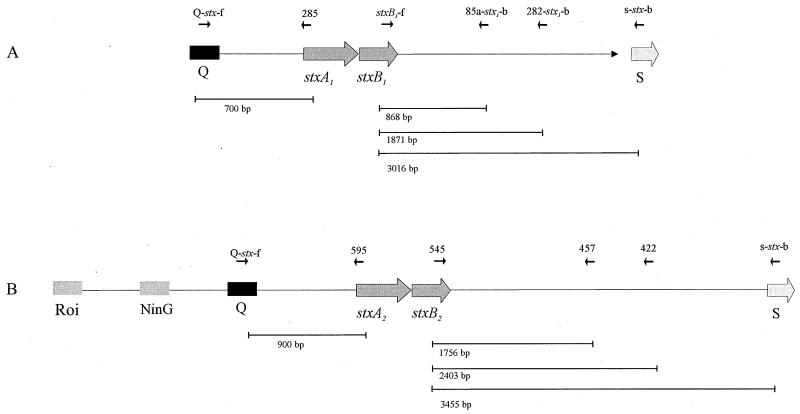
Twenty Stx1-producing E. coli (STEC) isolates representing the major non-O157 STEC serotypes were selected to analyze their stx1-flanking sequences (Tables 1 and 4). We designed a set of primer pairs, with one primer specific for stx1 and the second primer specific for the stx1-flanking sequences (Fig. 1A). These primers were derived from the sequence of the Stx1-encoding phage H-19B (15). We analyzed the 3′-stx-flanking region of the published sequence of phage H-19B for open reading frames and found four open reading frames, designated as orf85a, orf85b, orf282, and orf102. Primers specific for the 3′-stx-flanking regions were chosen from the open reading frames orf85a and orf282 (Table 2, Fig. 2A). To verify the linkage between stx1 and a Q-like gene, PCR amplification was performed using primer pair Q-stx-f and 285 (see Fig. 1). We further characterized the amplification products by subsequent restriction endonuclease digestion with PvuII.
TABLE 4.
Results of PCR and restriction analysis of Stx1-positive E. coli strains used in this study with specific primersa
| Strain | Serotype | stx genotypec | PCR resultb with primer:
|
Sizes (bp) of PvuII fragments of stxB1-f/s-stx-b | |||
|---|---|---|---|---|---|---|---|
| Q-stx-f/285 (700 bp) | stxB1-f/85a-stx1-b (868 bp) | stxB1-f/282-stx1-b (1,871 bp) | stxB1-f/s-stx-b (3,016 bp) | ||||
| EDL933 | O157:H7 | 1, 2 | + | + | + | + | 560, 1,200, 1,300 |
| A8993CS2 | O157:H7 | 1, 2 | + | + | + | + | 560, 1,200, 1,300 |
| HUSCL8 | O157:H7 | 1, 2 | + | + | + | + | 560, 1,200, 1,300 |
| RO10 | O26:H11 | 1 | + | + | + | + | 560, 1,200, 1,300 |
| 1858/96 | O103:H2 | 1 | + | + | + | + | 1,000, 2,000 |
| 2905/96 | O103:H2 | 1 | + | + | + | + | 1,000, 2,000 |
| 3942/96 | O103:H2 | 1 | + | + | + | + | 1,000, 2,000 |
| PMK3 | O103:H2 | 1 | + | + | + | + | 1,000, 2,000 |
| UTI | O103:H2 | 1 | + | + | + | + | 1,000, 2,000 |
| TB-325A | O26:H− | 1 | + | + | + | + | 1,000, 2,000 |
| ED-17 | O26:H− | 1 | + | + | + | + | 1,000, 2,000 |
| 5080/97 | O26:H− | 1, 2 | + | + | + | + | 1,000, 2,000 |
| TB334 | O26:H− | 1 | + | + | + | + | 1,000, 2,000 |
| SK004 | O26:H− | 1 | + | + | + | + | 1,000, 2,000 |
| 5236/96 | O26:H11 | 1 | + | + | + | + | 1,000, 2,000 |
| 1639/97 | O111:H− | 1 | + | − | − | − | |
| ED-31 | O111:H− | 1 | + | − | − | − | |
| 78/92 | O111:H− | 1 | + | − | − | − | |
| SK003 | O111:H− | 1, 2 | + | − | − | − | |
| C600(933W) | 2 | − | − | − | − | ||
For primer description see Table 2.
PCR results: +, product obtained; −, no product obtained.
1, stx1; 2, stx2.
FIG. 1.
Structure of the stx-flanking regions of Stx phages H-19B (A) and 933W (B) analyzed in this study. The arrows indicate the positions and directions of PCR and sequencing primers. The phage genes Roi, NinG, Q, stxA1, stxB1, stxA2, stxB2, and S are depicted by boxes. Lines indicate the expected PCR product length.
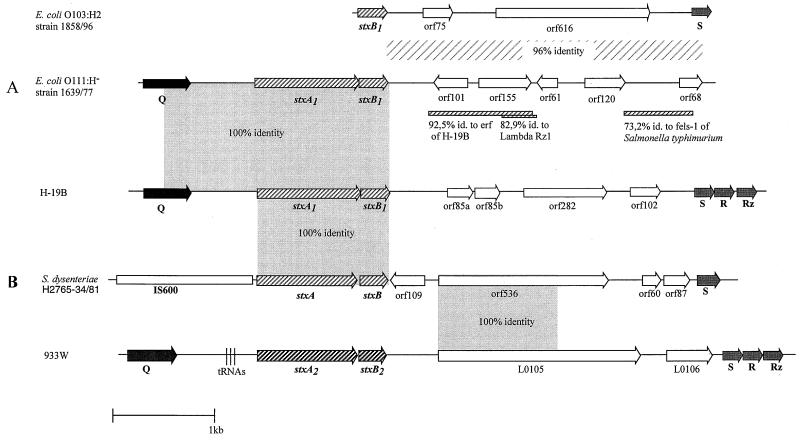
FIG. 2.
Structure of stx genes and stx-flanking sequences present in selected E. coli and S. dysenteriae strains. (A) Comparison of phage H-19B, E. coli O103:H2 strain 1858/96 and E. coli O111:H− strain 1639/77. (B) Comparison of phage H-19B, S. dysenteriae type 1 strain H2765-34/81, and phage 933W. Arrows indicate the lengths and directions of open reading frames. The percentage of sequence identity of strain 1858/96 to phage H-19B is depicted as a lightly shaded box. The bar represents a length of 1 kb. Shaded bars below E. coli O111:H− strain 1639/77 represent regions with sequence identities to phage H-19B, lambda, and S. enterica serovar Typhimurium. Gene designations are described in the text.
The PCR and restriction analysis results for the 20 selected Stx1-producing strains are shown in Table 4. We were able to detect Stx-phage-specific sequences in each of the strains tested. However, with E. coli O111:H− strains, we revealed only PCR products with primers Q-stx-f and 285 in the 5′ region and no products from the 3′-stx region (Table 4).
Nucleotide sequence analysis was performed from the stx-flanking region spanning the stx1 gene and the S gene using E. coli O103:H2 1858/96. Two PCR products of this strain revealed with primer pair stxB1-f/s–stx-b1 were independently cloned and sequenced. The fragments obtained were 3,199 bp in length. A scheme of this sequence is shown in Fig. 2A. The sequence starts with the stxB1 subunit gene. Downstream of stxB1, two open reading frames of 228 bp (orf75) and 1,851 bp (orf616) could be identified, and the sequence ended with the S gene. The overall identity of this sequence to H-19B phage is 96%.
Since no PCR products were revealed from the investigated E. coli O111 strains, we determined the nucleotide sequence of the 3′-stx-flanking region. We prepared genomic DNA of E. coli O111:H− strain 1639/77, digested it with restriction endonuclease SphI, and separated the fragments on an agarose gel. Hybridization with a stxB1-specific probe revealed a signal band of ca. 5 kb. DNA bands of this size were then excised from an agarose gel, ligated in a SphI-digested pBR322 vector, and transformed into E. coli DH5α. Up to 600 transformants were spotted onto a nylon membrane and were hybridized with an stxB1-specific probe. Two of the transformants reacted with this probe, and one of these was investigated further. Using universal and customized primers we could determine the whole sequence of the fragment of 5,467 bp. The 5′ end of 1,849 bp was identical to the respective sequence of Stx1 phage H-19B. This sequence comprises a part of the Q gene and the whole stx1 gene (Fig. 2A).
The sequence downstream of stx1 of 3,618 bp shows only 45% identity to the corresponding sequences of H-19B and 933W. Located 400 bp downstream of the stxB1 subunit was a region with 92% identity to the essential recombination function (erf) gene of phage H-19B; in that phage the erf gene is located upstream of the kil and cIII genes in the early regulatory phase region. A short stretch of 200 bp was 82.9% identical to the lambda Rz1 gene; in the lambda genome this stretch is part of the lysis cassette. We also detected a sequence of 1,042 bp with 73.2% identity to the sequence of the Salmonella enterica serovar Typhimurium LT2 (strain 1196-T3) phage fels-1 (Fig. 2A).
Primers O111fl-1, O111fl-2, and O111fl-3 (Table 2) were designed from the 3′ stx1 region of strain 1639/77 and used for PCR with strains ED-31, 78/92, and SK003. PCR products of approximately 1,000, 2,000, and 3,600 bp were revealed for all strains tested. This finding demonstrates that E. coli O111:H− strains carry a similar stx1-flanking region which is distinct from that of phages H-19B and 933W.
Analysis of the stx-flanking region of Stx2-positive STEC isolates.
In order to perform similar investigations on stx2 genes, we selected 20 Stx2-positive STEC strains (Tables 1 and 5). The primers used were derived from the Stx2-encoding phage 933W (9) and are depicted in Table 2 and Fig. 1B.
TABLE 5.
Results of PCR and restriction analysis of Stx2-positive E. coli strains used in this study with specific primersa
| Strain | Serotype | stx genotype | PCR resultb with primer:
|
Sizes (bp) of EcoRI fragments of 545/s-stx-b | |||
|---|---|---|---|---|---|---|---|
| Q-stx-f/595 (900 bp) | 545/457 (1,756 bp)c | 545/422 (2,403 bp)c | 545/s-stx-b (3,455 bp)c | ||||
| EDL933 | O157:H7 | 1, 2 | + | + | + | + | 1,400, 2,100 |
| C600(933W) | O157:H7 | 2 | + | + | + | + | 1,400, 2,100 |
| A8993 | O157:H7 | 1, 2 | + | + | + | + | 1,400, 2,100 |
| HUSCL8 | O157:H7 | 1, 2 | + | + | + | + | 1,400, 2,100 |
| 3574/92 | O157:H7 | 2 | + | + | + | + | 1,400, 2,100 |
| 702/88 | O157:H− | 2 | + | + | + | + | 1,400, 2,100 |
| 1448/97 | O26:H11 | 2 | + | + | + | + | 1,800, 2,100 |
| ED-147 | O26:H11 | 2 | − | + | + | + | 1,800, 2,100 |
| 4993/96 | O26:H11 | 2 | + | + | + | + | 1,800, 2,100 |
| 6061/96 | O26:H11 | 2 | + | + | + | + | 1,400, 2,100 |
| 3639/96 | O26:H11 | 2 | + | + | + | + | 1,400, 2,100 |
| 7828/95 | O103:H2 | 1, 2 | + | + | + | + | 1,800, 2,100 |
| 2969/99 | O103:H2 | 2 | + | + | + | + | 1,400, 2,100 |
| 2791/97 | O103:H− | 2 | + | + | + | + | 1,400, 2,100 |
| 6366/97 | O111:H− | 1, 2 | + | + | + | + | 1,400, 2,100 |
| 6833/96 | O11:H2 | 2 | −** | + | + | + | 1,400, 2,100 |
| 3985/96 | O145:H− | 2 | −* | + | + | + | 1,800, 2,100 |
| 893/98 | O145:H− | 2 | −* | + | + | + | 1,800, 2,100 |
| 5951/97 | O145:H− | 2 | + | + | + | + | 1,400, 2,100 |
| 6480/96 | O8:H− | 2 | + | + | + | + | 1,800, 2,100 |
| RO10 | O26:H11 | 1 | − | − | − | − | |
For primer description see Table 2.
PCR result: +, product obtained; ∗, NinG positive; ∗∗, NinG-PCR and Roi positive; −, no PCR product obtained.
The PCR product sizes in parentheses were obtained only with E. coli O157 strains. The PCR products of non-O157 strains differed slightly in size.
The results of these experiments are shown in Table 5. For E. coli O157 strains amplification products of 1,756, 2,403, and 3,455 bp were obtained with these primers. Restriction of the latter PCR product with EcoRI revealed two fragments of 1,400 and 2,100 bp in all six E. coli O157 strains. On closer inspection of the 3′-stx-flanking region of non-O157 STEC, we found two PCR product sizes of ca. 3,455 and 3,800 bp and then found two different restriction patterns for the 545/s-stx-b PCR product, which could not be clearly linked to particular serotypes.
An association with a Q-like gene was revealed in 16/20 strains using primers Q-stx-f and 595 (Table 5). In four cases, this PCR failed to generate a PCR product. Since these results gave no evidence for the presence of phage DNA in the 5′ direction of stx2, we looked for sequences which in phage 933W are located upstream of the Q-like gene. We designed PCR primers complementary to NinG and Roi and used these primers in combination with primer 595 (Fig. 1). A NinG-specific PCR product of 1,600 bp was revealed with strains 6833/96, 3985/96, and 893/98; an Roi-specific PCR product was revealed only with strain 6833/96. The other strain remained negative with both primer pairs.
Nucleotide sequence analysis was performed for the stx2-flanking regions of E. coli O26:H11 strains 1448/97 and ED-147 and of E. coli O145:H− strain 3985/96.
PCR products spanning the stx2 gene and the S gene were cloned and sequenced from each strain. The PCR product of E. coli strain O26:H11 strain 1448/97 was 3,919 bp in length. The sequence starts with the stxB2 subunit gene (Fig. 3A). Downstream of the stxB2 gene, we could identify five open reading frames of 325 bp (orf107), 1,938 bp (orf645), 180 bp (orf59), 255 bp (orf84), and 150 bp (orf49) (Fig. 3A). Searches for homologous sequences revealed that orf645 had 98% nucleotide sequence identity to L0105 of the Stx2-encoding phage 933. L0105 may encode a protein with 50.8% identity to E. coli K-12 YjhS (19). All other open reading frames failed to match a sequence of the database library.
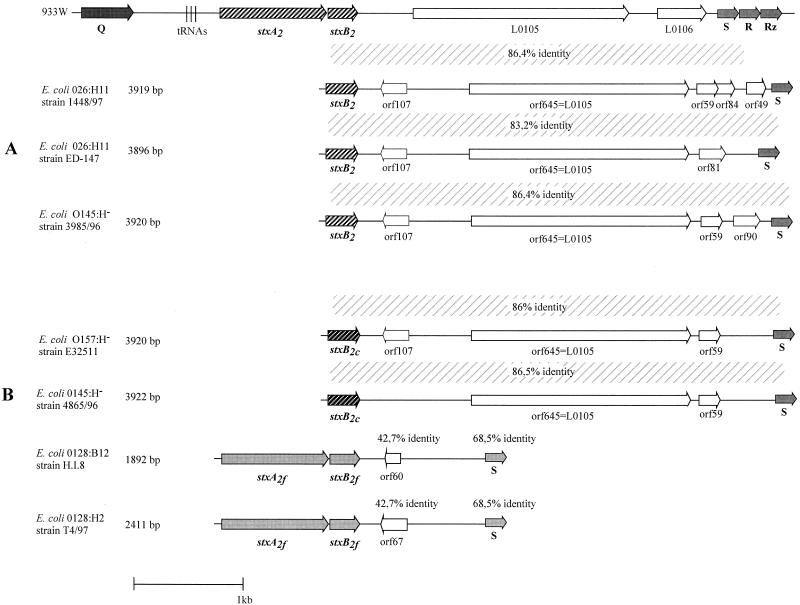
FIG. 3.
Structure of stx2 and stx2 variant genes and stx-flanking sequences present in selected E. coli strains. (A) Comparison of phage 933W with E. coli O26:H11 strain 1448/97, E. coli O26:H11 strain ED-147, and E. coli O145:H− strain 3985/96. (B) Comparison of phage 933W with E. coli O157:H− strain E32511, E. coli O145:H− strain 4865/96, E. coli O128:B12 strain H.I.8, and E. coli O128:H2 T4/97. Symbols are the same as in Fig. 2.
The fragment of E. coli strain O145:H− strain 3985/96 was 3,920 bp. The sequence also starts with the stxB2 subunit gene (Fig. 3A). Downstream of the stxB2 gene, we identified four open reading frames of 325 bp (orf107), 1,938 bp (orf645), 180 bp (orf59), and 273 bp (orf90). orf645 showed 99.3% identity to orf645 of E. coli O26:H11 strain 1448/97 and 92.1% identity to L0105 of phage 933W (19). The other three open reading frames did not show significant identities to published sequences.
The PCR product obtained with E. coli O26:H11 strain ED-147 was 3,896 bp long. The sequence also starts with the stxB2 subunit gene (Fig. 3A). Between the stxB2 gene and the S gene we found three open reading frames of 325 bp (orf107), 1,938 bp (orf645), and 246 bp (orf81). orf645 also showed 92.5% identity to L0105 of phage 933W. All other open reading frames failed to match any database library sequences.
The sequence identity between the 3.9-kb fragments obtained with both E. coli O26:H11 strains was 94.8%. Whereas the identity between E. coli O26:H11 strain 1448/97 and E. coli O145:H− strain 3985/96 was 99.6%, the similarity between E. coli O111:H− ED-147 and E. coli O145:H− strain 3985/96 was only 94.7%.
PCR and nucleotide sequence analysis of the stx-flanking region of stx2 variants.
We extended our studies on some variants of stx2 and carried out PCR and nucleotide sequence analysis on stx2c and its flanking regions harbored by E. coli O157:H− strain E32511 and E. coli O145:H− strain 4865/96 and on six stx2f variant genes from E. coli pigeon isolates (Table 1). PCR of the stx2c variants was performed using oligonucleotide 545 (Table 2), which is specific for the stxB2 subunit gene, and oligonucleotide s-stx-b, which is specific for the S gene. Amplification products were cloned and sequenced.
The PCR product of E. coli E32511 was 3,920 bp and started with the stx2cB subunit gene (see Fig. 3B). Between the stxB2c subunit and the S gene, we found three open reading frames of 325 bp (orf107), 1,938 bp (orf645), and 180 bp (orf59) (Fig. 3B). Searches for homologous sequences revealed a 92.2% identity of orf645 to L0105 of the Stx2-encoding phage 933W. We found an overall identity to the 3′-flanking region of phage 933W of 86%.
The 545/s-stx-b1 PCR product of E. coli O145:H− strain 4865/96 was 3,922 bp. Downstream of the stxB2c subunit gene we could identify two open reading frames of 1,938 bp (orf645) and 180 bp (orf59). orf645 of E. coli O145:H− strain 4865/96 showed 92.3% identity to L0105 of the Stx2-encoding phage 933W. We found an overall identity to the 3′-flanking region to E. coli O157:H− strain E32511 of 86% and an 86.5% overall identity to the 3′-flanking region of phage 933W.
Furthermore, we investigated the stx-flanking regions of six E. coli strains containing the stx2f variant by PCR. The primers used were derived from E. coli T4/97 (26). Analyses were performed using oligonucleotide 128-1 (Table 2), which is specific for the stxA2f subunit gene in combination with oligonucleotide stx2f-s-b, which is complementary to the S-like gene of E. coli strain O128:H2 strain T4/97. Using Stx2f-positive E. coli strains T4/97, E-D 365, E-D 371, E-D 373, 5598/97, and H.I.8 as templates, we could amplify PCR products of 2 kb from each of the strains (Fig. 3B).
Nucleotide sequence analysis was performed from the stx2f-flanking regions of E. coli O128:H2 isolate T4/97 and O128:B12 isolate H.I.8. PCR products from both strains, spanning from stx2 to the S gene, were cloned and completely sequenced. The sequence achieved from E. coli O128:H2 isolate T4/97 was 2,411 bp in length. This sequence starts directly adjacent to the stxA2f subunit gene (Fig. 3B). Downstream of the stxB2f subunit we could identify one open reading frame, orf67, of 204 bp with unknown function. We also found an S-like gene with 68.5% identity to the S gene of phage 933W and 71% identity to the S gene of phage H-19B. The overall identities of the stx-flanking region to the corresponding regions of phage 933W and phage H-19B were 42 and 43%, respectively.
The PCR product of E. coli O128:B12 strain H.I.8 was 1,892 bp (Fig. 3B). Downstream of stxB2f we could detect one open reading frame, designated orf60, with unknown function and an S-like gene. The sequence showed an overall identity of 99% to the corresponding sequence of the E. coli O128:H2 isolate T4/97, but a single base-pair exchange resulting in a stop codon in orf60 revealed a putative truncated protein (Fig. 3B).
Analysis of the stx-flanking region of S. dysenteriae serovar 1 isolates.
We have sequenced a 6,014-bp stretch of stx-flanking DNA of S. dysenteriae H2765-34/81. A map of this fragment and the comparison to the corresponding sequence of H-19B and 933W are shown in Fig. 2B. We did not find a Q-like gene close to the stxA subunit in the 5′ direction. Instead of this there is an insertion element with high sequence homology to IS600 of S. sonnei. In the 3′ direction of stx we found five open reading frames. Three of them, orf109, orf60, and orf87, did not show significant sequence identities to known genes. The first 1,140 bp of orf 536 were identical to those of L0105, as described by Plunkett et al. (19). However, homology ceased in the last third of the open reading frame, and it was shorter than that of L0105.
When we looked for overall homology of the stx-S region, we found 68.2% sequence identity with 933W and only 54% with H-19B. The S-like gene demonstrated 95.4% sequence identity with the S-gene of phage 933W and 91.8% with that of H-19B.
We selected S. dysenteriae type 1 isolates RMD3101010, H2540-34/82, H1250-36/74, H3104, 34/89, H4435-34/89, B1971, HSZ340/95, and W206 and subjected them together with H2765-34/81 to PCR analyses.
Amplification products of approximately 2,400 bp were obtained with primer pair stxB1-f/422 for each S. dysenteriae type 1 isolate, and amplification products of approximately 3000 bp length were obtained with primer pair stxB1-f/s-stx-b1. Restriction of the latter PCR product with restriction endonuclease SphI revealed two fragments of 1,000 and 2,000 bp for eight of the nine strains. The S. dysenteriae type 1 H2765-34/81 isolate showed a different restriction pattern, with two products of 1,300 and 2,000 bp. No amplification products were obtained with primer pair stxB1-f/457. Moreover, for all investigated S. dysenteriae type 1 isolates no amplification products were revealed with primer pairs stxB1-f/85a-stx1-b, stxB1-f/282-stx1-b, 545/457, 545/422, and 545/s-stx-b. To confirm a linkage between stx genes and an IS600 element, PCR amplification was performed using the primer pair of IS600-f and 285. An association with an IS600 element was revealed in seven of nine strains. In the cases of H2540-34/82 and B1971, we failed to generate a PCR product using the latter primer pair.
DISCUSSION
Although it was demonstrated earlier that particular stx genes are phage encoded or at least are adjacent to phage sequences (3, 9, 15, 19), we could show with our present experiments that in addition to stx1 and stx2, the stx2 variants stx2c, stx2f, and stx of S. dysenteriae are also flanked by phage sequences. Moreover, fine structure analysis of the stx-flanking regions yielded similarities as well as crucial differences in different Stx-converting bacteriophages.
The results of our study indicate that the physical linkage of stx and phage sequences may be a general feature of Stx-producing bacteria, regardless of the serotype or the species. In most strains, stx is located between a Q-like antiterminator gene and a holin S gene. These genes represent the central region of the late-phase region of phage lambda or other lambdoid bacteriophages. Restriction analysis of PCR products suggested that this regulatory region contained a similar structure in most of the Stx1-positive E. coli strains except E. coli O111.
Different amplification product lengths and different restriction patterns in Stx2-positive E. coli strains indicate a greater sequence divergence than was observed in Stx1-positive strains. However, these different patterns could not be correlated to a specific STEC serotype. Divergence in Stx2 phages isolated from different STEC serotypes was also observed in a study performed by Wagner et al. (32). They demonstrated that Stx phages isolated from seven STEC strains could be placed into five distinct groups by phage genomic RFLP. In addition, when E. coli K-12 was lysogenized with these phages, toxin and phage production differed markedly compared to the RFLP groups.
Comparison of the nucleotide sequence of the 3′-stx-flanking region of three Stx2-positive strains with the corresponding region of phage 933W showed an overall identity of 86% (Fig. 3A). For each of the E. coli strains we could identify the same open reading frame, orf645 (Fig. 3A), with high homology to L0105, which is described as being in the region between the stx genes and the lysis genes for phage 933W (19). This open reading frame may encode a protein with 50.8% identity to E. coli K-12 YjhS (19).
PCR analysis of Stx2c-positive and Stx2f-positive E. coli strains demonstrated for the first time a linkage of stx2 variants to the holin S gene. Sequence comparisons of two Stx2c-positive E. coli strains revealed a high overall homology in the 3′-flanking region and in the presence of orf645. A similar result was obtained with two Stx2f-positive strains. The stx-flanking region of a human and a pigeon isolate differed in only one nucleotide. Although both PCR products were nearly identical, exchange of one nucleotide caused a truncated open reading frame in E. coli O128:B12 strain H.I.8. The distance between the A-subunit gene and the S gene is shorter in stx2 variants than in classical stx2-positive strains.
E. coli H.I.8 was isolated from a patient with infant diarrhea in the United States and was described in 1977 (4, 11), whereas E. coli T4/97 was isolated from a feral pigeon in Würzburg, Germany, in 1999 (26). Our analysis of the stx-flanking regions demonstrated high similarity in that region, indicating close relationship. Since the Stx2f variant was found as an exclusive toxin type in pigeons (26), one could conclude that either horizontal gene transfer may have occurred or that STEC isolates have been transferred between pigeons and humans. One should also keep in mind that pigeons may serve as a reservoir to enlarge the gene pool which may be used by pathogenic enterobacteria.
Analysis of the similar region of S. dysenteriae type 1 which was performed by our group and also independently published by McDonough and Butterton (13) also revealed a linkage of stx with phage sequences. However, Q-like genes were not obtained. Instead of this, an IS600 element was located close to the stxA subunit gene.
With the investigations described here, we could clearly show that stx of S. dysenteriae is linked with phage sequences. However, phages could not be induced in such strains. McDonough and Butterton (13) have analyzed the location of the stx gene in S. dysenteriae type 1 3818T and found genes with high sequence identity to genes of lambdoid phages that were close in proximity to stx. Compared with the gene order found in phage 933W, attL, int, and xis genes seem to be present in functional form, but the right side containing the head and tail genes is not present in this strain. Those authors suggested that recombination events between insertion sequences led to deletions and gene rearrangements. This investigation is in agreement with our study. However, in some isolates we did not find an IS600 element upstream of stx.
From our results, we conclude that a specific genetic order in the vicinity of stx genes is generally present in Stx phages. This could be necessary for controlled expression and release of the toxins together with infectious phage particles. This assumption is in agreement with results published by Neely and Friedman, who have shown the direct interaction between the antiterminator Q and the transcription of the Shiga toxins for phage H-19B (14, 15).
Regarding the region between the stx genes and the S gene, we found a typical mosaic structure, which is common for lambdoid, double-stranded DNA phages (6). Although phages share a similar morphology, a similar mode of replication, and a similar genomic structure they can be unrelated at the nucleotide level. It has been proposed that double-stranded tailed phages share common ancestries and that they undergo a profuse exchange of their functional genetic elements drawn from a large common genetic pool. This is also supported by our finding that the E. coli O111 strains analyzed contained sequences which originally stem from S. enterica serovar Typhimurium phage fels-1, thus providing evidence for exchange of genetic material between S. enterica serovar Typhimurium and E. coli.
The results of our study clearly show that in all analyzed Stx-producing bacteria the stx genes are linked with phage sequences, regardless of the serotype, geographical distribution, animal or human origin, stx genotype, or the species. We suggest that stx genes can be generally considered to be phage-borne. However, location of stx adjacent to phage genes does not necessarily mean that Stx is encoded by intact, functional phages, or that it can be transduced via release of maturated phage particles.
The analysis of the stx-flanking regions is a convenient method that gives us important information on the genetic background in which stx genes are located. However, to more precisely learn about the infection characteristics such as receptor proteins and integration sites, complete phage sequences need to be determined.
ACKNOWLEDGMENTS
We thank Beatrix Henkel for excellent technical assistance. We further thank Michael McClelland, San Diego, Calif., for his interest and helpful discussions. We also thank Lisa Durso, Lincoln, Nebr., for critical reading of the manuscript.
This work was supported by grants from the Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Aleksic S, Karch H, Bockemühl J. A biotyping scheme for Shiga-like (Vero) toxin-producing Escherichia coli O157 and a list of serological cross-reactions between O157 and other gram-negative bacteria. Int J Med Microbiol Virol Parasitol Infect Dis. 1992;276:221–230. doi: 10.1016/s0934-8840(11)80009-5. [DOI] [PubMed] [Google Scholar]
- 2.Bokete T N, Whittam T S, Wilson R A, Clausen C R, O'Callahan C M, Moseley S L, Fritsche T R, Tarr P I. Genetic and phenotypic analysis of Escherichia coli with enteropathogenic characteristics isolated from Seattle children. J Infect Dis. 1997;175:1382–1389. doi: 10.1086/516470. [DOI] [PubMed] [Google Scholar]
- 3.Datz M, Janetzki M C, Franke S, Gunzer F, Schmidt H, Karch H. Analysis of the enterohemorrhagic Escherichia coli O157 DNA region containing lambdoid phage gene p and Shiga-like toxin structural genes. Appl Environ Microbiol. 1996;62:791–797. doi: 10.1128/aem.62.3.791-797.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gannon V P, Teerling C, Masri S A, Gyles C L. Molecular cloning and nucleotide sequence of another variant of the Escherichia coli Shiga-like toxin II family. J Gen Microbiol. 1990;136:1125–1135. doi: 10.1099/00221287-136-6-1125. [DOI] [PubMed] [Google Scholar]
- 5.Griffin P M, Tauxe R V. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol Rev. 1991;13:60–98. doi: 10.1093/oxfordjournals.epirev.a036079. [DOI] [PubMed] [Google Scholar]
- 6.Hendrix R W, Smith M C, Burns R N, Ford M E, Hatfull G F. Evolutionary relationships among diverse bacteriophages and prophages: all the world's a phage. Proc Natl Acad Sci USA. 1999;96:2192–2197. doi: 10.1073/pnas.96.5.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heuvelink A E, N. van-de K, Meis J F, Monnens L A, Melchers W J. Characterization of verocytotoxin-producing Escherichia coli O157 isolates from patients with haemolytic uraemic syndrome in Western Europe. Epidemiol Infect. 1995;115:1–14. doi: 10.1017/s0950268800058064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karch H, Böhm H, Schmidt H, Gunzer F, Aleksic S, Heesemann J. Clonal structure and pathogenicity of Shiga-like toxin-producing, sorbitol-fermenting Escherichia coli O157:H−. J Clin Microbiol. 1993;31:1200–1205. doi: 10.1128/jcm.31.5.1200-1205.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karch H, Schmidt H, Janetzki-Mittmann C, Scheef J, Kröger M. Shiga toxins even when different are encoded at identical positions in the genomes of related temperate bacteriophages. Mol Gen Genet. 1999;262:600–607. doi: 10.1007/s004380051122. [DOI] [PubMed] [Google Scholar]
- 10.Karmali M A, Petric M, Louie S, Cheung R. Antigenic heterogeneity of Escherichia coli verotoxins. Lancet. 1986;i:164–165. doi: 10.1016/s0140-6736(86)92307-x. [DOI] [PubMed] [Google Scholar]
- 11.Konowalchuk J, Speirs J I, Stavric S. Vero response to a cytotoxin of Escherichia coli. Infect Immun. 1977;18:775–779. doi: 10.1128/iai.18.3.775-779.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mariani K P, Denamur E, Milon A, Picard B, Cave H, Lambert Z N, Loirat C, Goullet P, Sansonetti P J, Elion J. Identification of a clone of Escherichia coli O103:H2 as potential agent of hemolytic-uremic syndrome in France. J Clin Microbiol. 1994;32:860. doi: 10.1128/jcm.32.3.860-.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDonough M A, Butterton J R. Spontaneous tandem amplification and deletion of the Shiga toxin operon in Shigella dysenteriae 1. Mol Microbiol. 2000;34:1058–1069. doi: 10.1046/j.1365-2958.1999.01669.x. [DOI] [PubMed] [Google Scholar]
- 14.Neely M N, Friedman D I. Arrangement and functional identification of genes in the regulatory region of lambdoid phage H-19B, a carrier of a Shiga-like toxin. Gene. 1998;223:105–113. doi: 10.1016/s0378-1119(98)00236-4. [DOI] [PubMed] [Google Scholar]
- 15.Neely M N, Friedman D I. Functional and genetic analysis of regulatory regions of coliphage H-19B: location of Shiga-like toxin and lysis genes suggest a role for phage functions in toxin release. Mol Microbiol. 1998;28:1255–1267. doi: 10.1046/j.1365-2958.1998.00890.x. [DOI] [PubMed] [Google Scholar]
- 16.O'Brien A D, Holmes R K. Shiga and Shiga-like toxins. Microbiol Rev. 1987;51:206–220. doi: 10.1128/mr.51.2.206-220.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Brien A D, Newland J W, Miller S F, Holmes R K, Smith H W, Formal S B. Shiga-like toxin-converting phages from Escherichia coli strains that cause hemorrhagic colitis or infantile diarrhea. Science. 1984;226:694–696. doi: 10.1126/science.6387911. [DOI] [PubMed] [Google Scholar]
- 18.O'Brien A O, Lively T A, Chen M E, Rothman S W, Formal S B. Escherichia coli O157:H7 strains associated with haemorrhagic colitis in the United States produce a Shigella dysenteriae 1 (SHIGA)-like cytotoxin. Lancet. 1983;i:702. doi: 10.1016/s0140-6736(83)91987-6. [DOI] [PubMed] [Google Scholar]
- 19.Plunkett G, Rose D J, Durfee T J, Blattner F R. Sequence of Shiga toxin 2 phage 933W from Escherichia coli O157:H7: Shiga toxin as a phage late-gene product. J Bacteriol. 1999;181:1767–1778. doi: 10.1128/jb.181.6.1767-1778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rietra P J, Willshaw G A, Smith H R, Field A M, Scotland S M, Rowe B. Comparison of Vero-cytotoxin-encoding phages from Escherichia coli of human and bovine origin. J Gen Microbiol. 1989;135:2307–2318. doi: 10.1099/00221287-135-8-2307. [DOI] [PubMed] [Google Scholar]
- 21.Rüssmann H, Kothe E, Schmidt H, Franke S, Harmsen D, Caprioli A, Karch H. Genotyping of Shiga-like toxin genes in non-O157 Escherichia coli strains associated with haemolytic uraemic syndrome. J Med Microbiol. 1995;42:404–410. doi: 10.1099/00222615-42-6-404. [DOI] [PubMed] [Google Scholar]
- 22.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 23.Schmidt H, Geitz C, Tarr P I, Frosch M, Karch H. Non-O157:H7 pathogenic Shiga toxin-producing Escherichia coli: phenotypic and genetic profiling of virulence traits and evidence for clonality. J Infect Dis. 1999;179:115–123. doi: 10.1086/314537. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt H, Scheef J, Huppertz H I, Frosch M, Karch H. Escherichia coli O157:H7 and O157:H− strains that do not produce Shiga toxin: phenotypic and genotypic characterization of isolates associated with diarrhea and hemolytic-uremic syndrome. J Clin Microbiol. 1999;37:3491–3496. doi: 10.1128/jcm.37.11.3491-3496.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidt H, Scheef J, Janetzki M C, Datz M, Karch H. An ileX tRNA gene is located close to the Shiga toxin II operon in enterohemorrhagic Escherichia coli O157 and non-O157 strains. FEMS Microbiol Lett. 1997;149:39–44. doi: 10.1111/j.1574-6968.1997.tb10305.x. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt H, Scheef J, Morabito S, Caprioli A, Wieler L, Karch H. A new Shiga toxin 2 variant (Stx2f) from Escherichia coli isolated from pigeons. Appl Environ Microbiol. 2000;66:1205–1208. doi: 10.1128/aem.66.3.1205-1208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmitt C K, McKee M L, O'Brien A D. Two copies of Shiga-like toxin II-related genes common in enterohemorrhagic Escherichia coli strains are responsible for the antigenic heterogeneity of the O157:H− strain E32511. Infect Immun. 1991;59:1065–1073. doi: 10.1128/iai.59.3.1065-1073.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scotland S M, Smith H R, Willshaw G A, Rowe B. Vero cytotoxin production in strain of Escherichia coli is determined by genes carried on bacteriophage. Lancet. 1983;i:216. doi: 10.1016/s0140-6736(83)90192-7. [DOI] [PubMed] [Google Scholar]
- 29.Smith H W, Green P, Parsell Z. Vero cell toxins in Escherichia coli and related bacteria: transfer by phage and conjugation and toxic action in laboratory animals, chickens and pigs. J Gen Microbiol. 1983;129:3121–3137. doi: 10.1099/00221287-129-10-3121. [DOI] [PubMed] [Google Scholar]
- 30.Strockbine N A, Marques L R, Newland J W, Smith H W, Holmes R K, O'Brien A D. Two toxin-converting phages from Escherichia coli O157:H7 strain 933 encode antigenically distinct toxins with similar biologic activities. Infect Immun. 1986;53:135–140. doi: 10.1128/iai.53.1.135-140.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tarr P I, Fouser L S, Stapleton A E, Wilson R A, Kim H H, Vary J C, Clausen C R. Hemolytic-uremic syndrome in a six-year-old girl after a urinary tract infection with Shiga-toxin-producing Escherichia coli O103:H2. N Engl J Med. 1996;335:635–638. doi: 10.1056/NEJM199608293350905. [DOI] [PubMed] [Google Scholar]
- 32.Wagner P L, Acheson D W, Waldor M K. Isogenic lysogens of diverse Shiga toxin 2-encoding bacteriophages produce markedly different amounts of Shiga toxin. Infect Immun. 1999;67:6710–6714. doi: 10.1128/iai.67.12.6710-6714.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]