Abstract
Studies tracing parental origins of human mutations by means of cytogenetic polymorphisms and RFLPs show that most trisomics arise out of maternal errors of segregation at the first meiotic division in oocytes. Temporal disturbance of meiotic progression seems likely to underly aneuploidy production in the female mouse, and this could equally be true in women, most especially as they approach the menopause when irregular cyclicity sets in. For human monosomy X, a high proportion of cases show loss of the paternal sex chromosome, and from experimental data giving similar findings in the mouse, it seems likely that the error could arise at the pronuclear stage after sperm entry into the egg, rather than at meiosis in the male. For human point mutations and structural rearrangements, a bias exists towards paternal origins. Errors arising during spermatogonial proliferation in men could contribute point mutations, these accumulating over a lifetime to give paternal age effects. For structural rearrangements, the hypersensitive stage is likely to be the post-meiotic differentiating spermatid, a stage not subject to germinal selection, and one which in Drosophila has been shown to combine high breakability with enhanced repair. Lack of a comparable cell type to the condensing spermatid of the male might be a reason why balanced structural rearrangements are produced rather rarely in females, at least in the mouse.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander M L. Radiosensitivity at Specific Autosomal Loci in Mature Sperm and Spermatogonial Cells of Drosophila Melanogaster. Genetics. 1960 Aug;45(8):1019–1022. doi: 10.1093/genetics/45.8.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonarakis S. E., Chakravarti A., Warren A. C., Slaugenhaupt S. A., Wong C., Halloran S. L., Metaxotou C. Reduced recombination rate on chromosomes 21 that have undergone nondisjunction. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):185–190. doi: 10.1101/sqb.1986.051.01.022. [DOI] [PubMed] [Google Scholar]
- Butler M. G., Meaney F. J., Palmer C. G. Clinical and cytogenetic survey of 39 individuals with Prader-Labhart-Willi syndrome. Am J Med Genet. 1986 Mar;23(3):793–809. doi: 10.1002/ajmg.1320230307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspersson T., Lomakka G., Zech L. The 24 fluorescence patterns of the human metaphase chromosomes - distinguishing characters and variability. Hereditas. 1972;67(1):89–102. doi: 10.1111/j.1601-5223.1971.tb02363.x. [DOI] [PubMed] [Google Scholar]
- Chamberlin J., Magenis R. E. Parental origin of de novo chromosome rearrangements. Hum Genet. 1980;53(3):343–347. doi: 10.1007/BF00287054. [DOI] [PubMed] [Google Scholar]
- Chandley A. C. Asymmetry in chromosome pairing: a major factor in de novo mutation and the production of genetic disease in man. J Med Genet. 1989 Sep;26(9):546–552. doi: 10.1136/jmg.26.9.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryja T. P., Mukai S., Petersen R., Rapaport J. M., Walton D., Yandell D. W. Parental origin of mutations of the retinoblastoma gene. Nature. 1989 Jun 15;339(6225):556–558. doi: 10.1038/339556a0. [DOI] [PubMed] [Google Scholar]
- Griffin C. S., Tease C. Gamma-ray-induced numerical and structural chromosome anomalies in mouse immature oocytes. Mutat Res. 1988 Nov;202(1):209–213. doi: 10.1016/0027-5107(88)90184-4. [DOI] [PubMed] [Google Scholar]
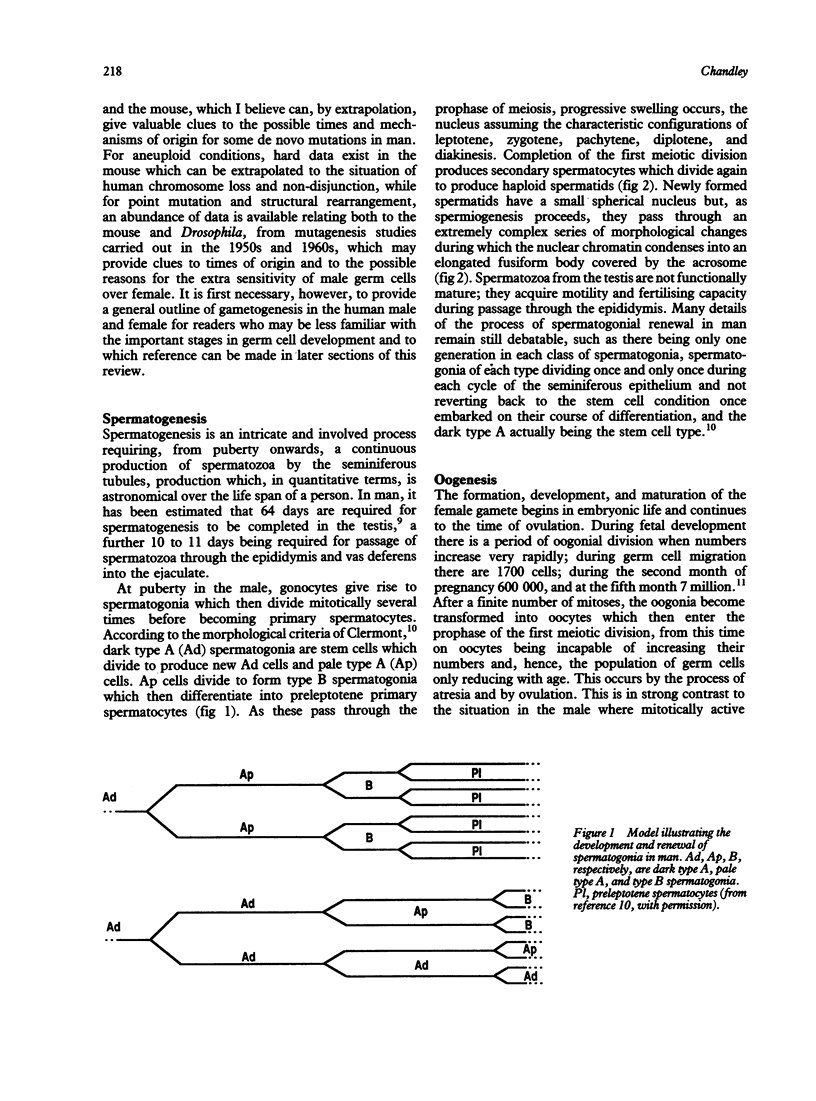
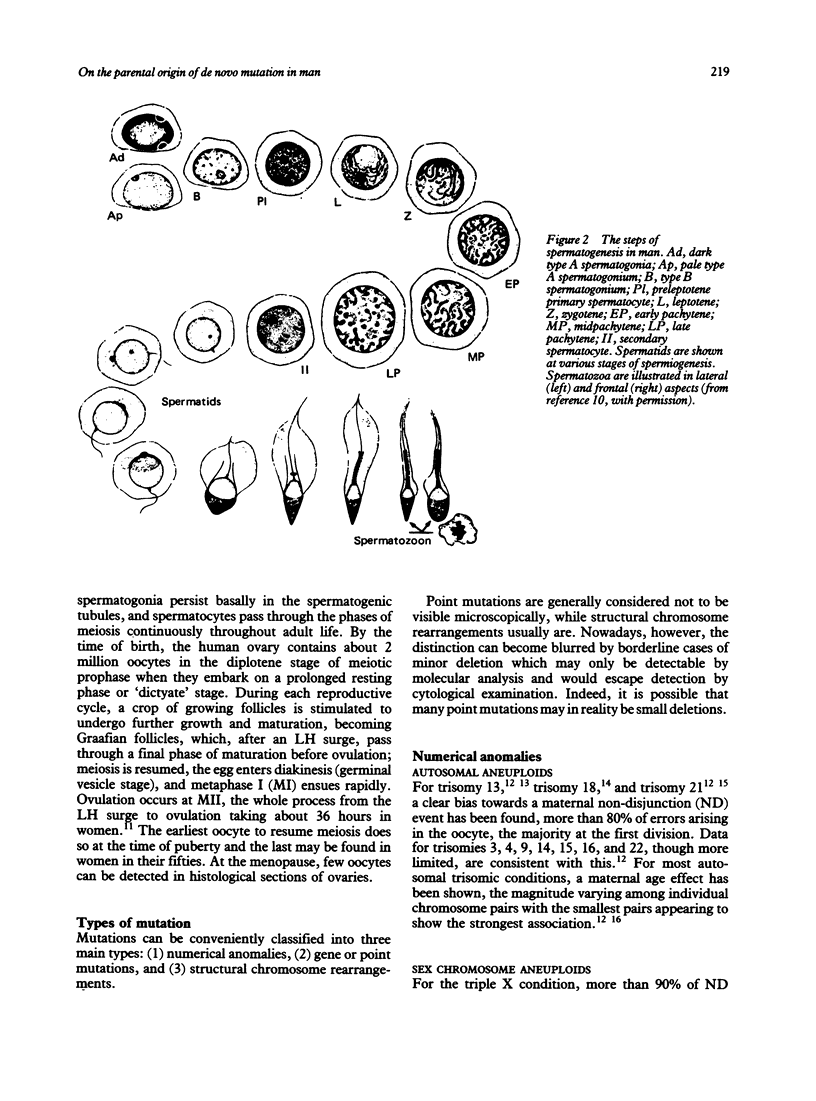
- HELLER C. G., CLERMONT Y. Spermatogenesis in man: an estimate of its duration. Science. 1963 Apr 12;140(3563):184–186. doi: 10.1126/science.140.3563.184. [DOI] [PubMed] [Google Scholar]
- Hall J. G. Genomic imprinting: review and relevance to human diseases. Am J Hum Genet. 1990 May;46(5):857–873. [PMC free article] [PubMed] [Google Scholar]
- Hassold T., Benham F., Leppert M. Cytogenetic and molecular analysis of sex-chromosome monosomy. Am J Hum Genet. 1988 Apr;42(4):534–541. [PMC free article] [PubMed] [Google Scholar]
- Hassold T., Chiu D., Yamane J. A. Parental origin of autosomal trisomies. Ann Hum Genet. 1984 May;48(Pt 2):129–144. doi: 10.1111/j.1469-1809.1984.tb01008.x. [DOI] [PubMed] [Google Scholar]
- Hassold T., Jacobs P. A., Leppert M., Sheldon M. Cytogenetic and molecular studies of trisomy 13. J Med Genet. 1987 Dec;24(12):725–732. doi: 10.1136/jmg.24.12.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassold T., Jacobs P., Kline J., Stein Z., Warburton D. Effect of maternal age on autosomal trisomies. Ann Hum Genet. 1980 Jul;44(Pt 1):29–36. doi: 10.1111/j.1469-1809.1980.tb00943.x. [DOI] [PubMed] [Google Scholar]
- Heslop-Harrison J. S., Leitch A. R., Schwarzacher T., Smith J. B., Atkinson M. D., Bennett M. D. The volumes and morphology of human chromosomes in mitotic reconstructions. Hum Genet. 1989 Dec;84(1):27–34. doi: 10.1007/BF00210666. [DOI] [PubMed] [Google Scholar]
- Huff V., Meadows A., Riccardi V. M., Strong L. C., Saunders G. F. Parental origin of de novo constitutional deletions of chromosomal band 11p13. Am J Hum Genet. 1990 Jul;47(1):155–160. [PMC free article] [PubMed] [Google Scholar]
- Jacobs P. A., Hassold T. J., Whittington E., Butler G., Collyer S., Keston M., Lee M. Klinefelter's syndrome: an analysis of the origin of the additional sex chromosome using molecular probes. Ann Hum Genet. 1988 May;52(Pt 2):93–109. doi: 10.1111/j.1469-1809.1988.tb01084.x. [DOI] [PubMed] [Google Scholar]
- Jadayel D., Fain P., Upadhyaya M., Ponder M. A., Huson S. M., Carey J., Fryer A., Mathew C. G., Barker D. F., Ponder B. A. Paternal origin of new mutations in von Recklinghausen neurofibromatosis. Nature. 1990 Feb 8;343(6258):558–559. doi: 10.1038/343558a0. [DOI] [PubMed] [Google Scholar]
- Kligerman A. D., Campbell J. A., Erexson G. L., Allen J. W., Shelby M. D. Sister chromatid exchange analysis in lung and peripheral blood lymphocytes of mice exposed to methyl isocyanate by inhalation. Environ Mutagen. 1987;9(1):29–36. doi: 10.1002/em.2860090105. [DOI] [PubMed] [Google Scholar]
- Kupke K. G., Müller U. Parental origin of the extra chromosome in trisomy 18. Am J Hum Genet. 1989 Oct;45(4):599–605. [PMC free article] [PubMed] [Google Scholar]
- Lyon M. F., Meredith R. Autosomal translocations causing male sterility and viable aneuploidy in the mouse. Cytogenetics. 1966;5(5):335–354. doi: 10.1159/000129909. [DOI] [PubMed] [Google Scholar]
- Matsunaga E., Minoda K., Sasaki M. S. Parental age and seasonal variation in the births of children with sporadic retinoblastoma: a mutation-epidemiologic study. Hum Genet. 1990 Jan;84(2):155–158. doi: 10.1007/BF00208931. [DOI] [PubMed] [Google Scholar]
- Mattei M. G., Souiah N., Mattei J. F. Chromosome 15 anomalies and the Prader-Willi syndrome: cytogenetic analysis. Hum Genet. 1984;66(4):313–334. doi: 10.1007/BF00287636. [DOI] [PubMed] [Google Scholar]
- May K. M., Jacobs P. A., Lee M., Ratcliffe S., Robinson A., Nielsen J., Hassold T. J. The parental origin of the extra X chromosome in 47,XXX females. Am J Hum Genet. 1990 Apr;46(4):754–761. [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen M., Poulsen H., Grinsted J., Lange A. Non-disjunction in trisomy 21: study of chromosomal heteromorphisms in 110 families. Ann Hum Genet. 1980 Jul;44(Pt 1):17–28. doi: 10.1111/j.1469-1809.1980.tb00942.x. [DOI] [PubMed] [Google Scholar]
- Mitelman F., Heim S. Consistent involvement of only 71 of the 329 chromosomal bands of the human genome in primary neoplasia-associated rearrangements. Cancer Res. 1988 Dec 15;48(24 Pt 1):7115–7119. [PubMed] [Google Scholar]
- Nicholls R. D., Knoll J. H., Glatt K., Hersh J. H., Brewster T. D., Graham J. M., Jr, Wurster-Hill D., Wharton R., Latt S. A. Restriction fragment length polymorphisms within proximal 15q and their use in molecular cytogenetics and the Prader-Willi syndrome. Am J Med Genet. 1989 May;33(1):66–77. doi: 10.1002/ajmg.1320330109. [DOI] [PubMed] [Google Scholar]
- Oster I I. Modification of X-Ray Mutagenesis in Drosophila. I. Reunion of Chromosomes Irradiated during Spermiogenesis. Genetics. 1955 Sep;40(5):692–696. doi: 10.1093/genetics/40.5.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overhauser J., McMahon J., Oberlender S., Carlin M. E., Niebuhr E., Wasmuth J. J., Lee-Chen J. Parental origin of chromosome 5 deletions in the cri-du-chat syndrome. Am J Med Genet. 1990 Sep;37(1):83–86. doi: 10.1002/ajmg.1320370119. [DOI] [PubMed] [Google Scholar]
- PENROSE L. S. Parental age and mutation. Lancet. 1955 Aug 13;269(6885):312–313. doi: 10.1016/s0140-6736(55)92305-9. [DOI] [PubMed] [Google Scholar]
- RUSSELL L. B., RUSSELL W. L. Genetic analysis of induced deletions and of spontaneous nondisjunction involving chromosome 2 of the mouse. J Cell Comp Physiol. 1960 Nov;56(Suppl 1):169–188. doi: 10.1002/jcp.1030560415. [DOI] [PubMed] [Google Scholar]
- Race R. R., Sanger R. Xg and sex-chromosome abnormalities. Br Med Bull. 1969 Jan;25(1):99–103. doi: 10.1093/oxfordjournals.bmb.a070677. [DOI] [PubMed] [Google Scholar]
- Rinchik E. M., Bangham J. W., Hunsicker P. R., Cacheiro N. L., Kwon B. S., Jackson I. J., Russell L. B. Genetic and molecular analysis of chlorambucil-induced germ-line mutations in the mouse. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1416–1420. doi: 10.1073/pnas.87.4.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell L. B. Definition of functional units in a small chromosomal segment of the mouse and its use in interpreting the nature of radiation-induced mutations. Mutat Res. 1971 Jan;11(1):107–123. doi: 10.1016/0027-5107(71)90036-4. [DOI] [PubMed] [Google Scholar]
- Russell L. B., Hunsicker P. R., Cacheiro N. L., Bangham J. W., Russell W. L., Shelby M. D. Chlorambucil effectively induces deletion mutations in mouse germ cells. Proc Natl Acad Sci U S A. 1989 May;86(10):3704–3708. doi: 10.1073/pnas.86.10.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell W. L. Mutation frequencies in female mice and the estimation of genetic hazards of radiation in women. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3523–3527. doi: 10.1073/pnas.74.8.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STERN C. Colour-blindness in Klinefelter's syndrome. Nature. 1959 May 23;183(4673):1452–1453. doi: 10.1038/1831452a0. [DOI] [PubMed] [Google Scholar]
- Sanger R., Tippett P., Gavin J., Teesdale P., Daniels G. L. Xg groups and sex chromosome abnormalities in people of northern European ancestry: an addendum. J Med Genet. 1977 Jun;14(3):210–211. doi: 10.1136/jmg.14.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage J. R. Classification and relationships of induced chromosomal structual changes. J Med Genet. 1976 Apr;13(2):103–122. doi: 10.1136/jmg.13.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle A. G., Beechey C. V. Cytogenetic effects of X-rays and fission neutrons in female mice. Mutat Res. 1974 Aug;24(2):171–186. doi: 10.1016/0027-5107(74)90130-4. [DOI] [PubMed] [Google Scholar]
- Stewart G. D., Hassold T. J., Berg A., Watkins P., Tanzi R., Kurnit D. M. Trisomy 21 (Down syndrome): studying nondisjunction and meiotic recombination by using cytogenetic and molecular polymorphisms that span chromosome 21. Am J Hum Genet. 1988 Feb;42(2):227–236. [PMC free article] [PubMed] [Google Scholar]
- Zhu X. P., Dunn J. M., Phillips R. A., Goddard A. D., Paton K. E., Becker A., Gallie B. L. Preferential germline mutation of the paternal allele in retinoblastoma. Nature. 1989 Jul 27;340(6231):312–313. doi: 10.1038/340312a0. [DOI] [PubMed] [Google Scholar]


