Abstract
The adherence of Staphylococcus aureus to human endothelial cells (EC) is probably an important step in the pathogenesis of systemic staphylococcal infections. We examined the influence of type 5 capsular polysaccharide (CP5) production, the global regulator agr, and the bacterial growth phase on S. aureus adherence to EC. Whereas S. aureus Newman showed maximal adherence to EC in the logarithmic phase of growth, an isogenic agr mutant showed maximal adherence in the stationary growth phase. S. aureus adherence to EC and CP5 expression were negatively correlated: a mutation in the agr locus diminished CP5 production and led to increased adherence. Likewise, induction of CP5 expression by addition of NaCl to the growth medium resulted in reduced staphylococcal adherence to EC. S. aureus Newman cells that adhered to EC did not express CP5. A Newman cap5O mutant was acapsular and showed significantly greater adherence to EC than the parental strain did (P < 0.005). Complementation of the cap5O mutation in trans restored CP5 expression and reduced EC adherence to a level similar to that of the parental strain. The enhanced adherence shown by the cap5O mutant was similar in magnitude to that of the agr mutant or the cap5O agr double mutant. Cells of the cap5O mutant and cap5O agr double mutant harvested from stationary-phase cultures adhered significantly better than did cells harvested in the exponential growth phase. These data are consistent with the postexponential and agr-independent expression by S. aureus of at least one putative EC adhesin, whose binding domain may be masked by CP5.
Staphylococcus aureus is responsible for a broad range of human diseases, including foreign body infections, bacteremia, abscesses, and wound infections (41). The ability of S. aureus to adhere to extracellular matrix proteins is thought to be essential for colonization and the establishment of infections (17, 41, 52). The interaction of S. aureus with endothelial cells (EC) may play a major role in the pathogenesis of endovascular infections, including endocarditis and metastatic infections (26, 41).
S. aureus can express several cell wall-associated adhesins specific for recognizing matrix proteins; these adhesins are referred to as MSCRAMMs (microbial surface components recognizing adhesive matrix molecules) (52). MSCRAMMs with specific binding activity toward fibronectin (31, 62), fibrinogen (45, 47), and collagen (53) have been cloned and biochemically characterized. Whether MSCRAMMs are also responsible for the adherence of S. aureus to host cells is still unclear. A role for fibronectin-binding proteins in staphylococcal adherence to bovine epithelial cells (13) and mammary gland cells (36) was recently described; however, other factors were also postulated to mediate epithelial cell adherence (13).
The expression of most cell wall adhesins and extracellular virulence determinants in S. aureus is coordinately regulated during the growth cycle (57). Some of the growth cycle-dependent expression patterns can be attributed to the global regulator agr, which acts as a quorum-sensing system (4, 28, 29). The capsular polysaccharide (CP) is an important surface component that is positively regulated by agr (10). More than 90% of S. aureus strains express 1 of 11 CP types (33, 65), and most strains colonizing and infecting humans produce either CP type 5 (CP5) or CP8 (2, 24). Specific environmental and/or host signals result in variation of CP expression both in vitro (9, 40, 55, 68, 70) and in vivo (23, 35, 40).
It is likely that specific adhesins mediating adherence to EC are differentially expressed and/or masked by differentially expressed extracellular surface structures such as CP. In this study, we report the influence of agr, CP production, and bacterial growth conditions on the adherence of S. aureus to EC. Our findings indicate that mutations and growth conditions that reduce or eliminate CP expression enhance the adherence of S. aureus to EC. In the absence of CP expression, S. aureus adherence to EC is enhanced in the postexponential growth phase but does not appear to be influenced by the agr regulatory locus. The importance of differential expression and exposure of an EC adhesin(s) to the pathogenesis of staphylococcal infections is discussed.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The strains and plasmids used in this study are listed in Table 1. S. aureus type 5 strain Newman was grown in medium composed of 1% Casamino Acids, 1% yeast extract, 0.5% NaCl, 0.5% glucose, and 60 mM glycerophosphate (CYGP) at 37°C with shaking. Chloramphenicol (10 μg/ml) or tetracycline (5 μg/ml) was added to the culture medium when indicated. Bacteria were inoculated to an initial optical density at 600 nm of 0.05 in CYGP and grown to the exponential phase (optical density at 600 nm = 0.6; 2.5 h) or the stationary phase (14 h) of the growth cycle. Before each experiment, the bacteria were washed twice and resuspended in sterile phosphate-buffered saline (PBS).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or phenotype | Source or reference |
|---|---|---|
| S. aureus strains | ||
| Newman | Wild type, CP5 positive | NCTC 8178 (12) |
| ALC355 | Newman Δagr::tetM, reduced CP5 expression | 76 |
| KKO22 | Newman Δcap5O, CP5 negative | This study |
| CW22 | Newman Δagr::tetM, Δcap5O, CP5 negative | This study |
| Plasmids | ||
| pLI50 | Shuttle vector (Apr, Cmr) | 37 |
| pKBK22 | 3.4-kb BamHI-EcoRI fragment (cap5MNP Δcap5O) in pTS1 | Portoles et al., submitted |
| pKBK24 | pLI50 containing a 2.4-kb PCR amplicon carrying cap5O and flanking DNA | Portoles et al., submitted |
Construction of the S. aureus cap5O mutant.
pKBK22 was constructed as described previously (M. Portoles, K. B. Kisier, N. Bhasin, K. H. N. Chan, and J. C. Lee, submitted for publication). In brief, a 4.1-kb DNA fragment comprising cap5MNOP was subcloned from the cap5 gene region of S. aureus Reynolds. A 727-bp HpaI deletion, encompassing nucleotides 9 to 734 of the 1,260-bp cap5O gene (61), resulted in a +1 frameshift. Thus, pKBK22 contains a 3.4-kb insert comprising the cap5O deletion and flanking cap5 sequences (cap5MN and cap5P) in the temperature-sensitive shuttle vector pTS1.
pKBK22 was electrotransformed into S. aureus RN4220 and then transduced with phage 80 alpha into S. aureus Newman, in both instances selecting for Cmr colonies at 30°C. The mutation was introduced into the chromosome by allelic exchange. In brief, plasmid integrants in the chromosome were selected by plating of cells on tryptic soy agar plus chloramphenicol (5 μg/ml) at 42°C. Single colonies were passaged at 30°C (no antibiotic selection) and then grown at 42°C to eliminate the excised plasmids. Cms colonies were screened for CP5 production by colony immunoblotting with CP5-specific polyclonal rabbit antiserum. CP5-negative colonies were examined by PCR and Southern blotting to confirm the presence of the deletion in cap5O. The CP5-negative cap5O mutant of strain Newman was designated KKO22. Lack of CP5 production by KKO22 was confirmed by immunodiffusion of capsular extracts with CP5-specific rabbit antiserum. The mutant strain did not differ in other phenotypic markers, e.g., hemolytic activity, from the parental strain.
To complement the mutation in KKO22, the cap5O gene and flanking cap5 sequences were amplified by PCR with primers KK14 (5′-ACAAGGATCCAAAAAGTTCGCTGAACAAGCATTACAAG-3′) and KK15 (5′-ACAAGGATCCAGTAATAAAGATACGCTCTTTGTCTTTG-3′) and the ELONGASE enzyme mix (Life Technologies, Inc., Gaithersburg, Md.) for 25 cycles of 94°C for 30 s, 60°C for 1 min, and 68°C for 3 min. (The BamHI sites in primers KK14 and KK15 are underlined.) The 2.4-kb amplicon was digested with BamHI and ligated into pLI50 to create plasmid pKBK24. pLI50 and pKBK24 were each transformed into RN4220 and then transduced into Newman or KKO22, as described above.
Construction of the S. aureus cap5O agr double mutant.
The agr mutation was transduced from agr mutant ALC355 into strain Newman(pLI50) and cap5O mutant KKO22(pLI50) with φ11. Transductants were selected on medium containing tetracycline and chloramphenicol. Successful construction of the mutants was confirmed by Southern blotting with agr-specific probes. The agr deletion resulted in decreased hemolytic activity compared to those of the parental strains.
EC culture.
EC were obtained from human umbilical cord veins by the method of Jaffe et al. (27) with modified protease digestion. In brief, cells were isolated with 0.025% trypsin–0.01% EDTA (Cell Systems, St. Katharinen, Germany), washed, and grown in endothelial growth medium (Cell Systems) in 0.2% gelatin-coated tissue culture flasks (Becton Dickinson, Heidelberg, Germany). The cells were maintained at 37°C in an atmosphere containing 5% CO2. They were seeded in 24-well plates (Becton Dickinson) or culture slides (Becton Dickinson), and only confluent monolayers of the third to sixth passages were used for adherence assays.
Adherence assays.
Confluent monolayers of EC (∼105 EC per well) were washed three times with M199 (Sigma Chemical Co., Deisenhofen, Germany) to remove antibiotics and incubated for 20 min at 37°C with 3% bovine serum albumin (Sigma) in M199 to minimize background adherence. The EC were then incubated with bacterial inocula ranging from ∼106 to ∼109 CFU of S. aureus at 37°C in an atmosphere containing 5% CO2. To exclude contamination, negative controls with uninfected EC were included in each experiment. The supernatants were removed after 2 h, and nonadherent bacteria were analyzed for CP production by immunofluorescence. EC were subsequently washed three times to remove remaining nonadherent bacteria. The plates were treated with trypsin-EDTA and 0.25% Triton X-100 (Sigma) to lyse EC and release adherent bacteria. Adherent bacteria released by EC lysis were evaluated for CP5 production by immunofluorescence, and serial dilutions were plated onto sheep blood agar for enumeration of CFU. The results of representative experiments are shown. Each assay was repeated at least once, and within one experiment all samples were processed at least in quadruplicate.
For microscopic evaluation, adherence assays were performed on culture slides containing confluent monolayers. Washed monolayers with adherent bacteria were fixed with 50% methanol and stained with crystal violet. Bacterium-to-EC ratios were determined by counting the number of adherent bacteria in three microscopic fields with 50 EC per field.
Evaluation of CP5 production.
CP5 production was detected by a modified indirect immunofluorescence technique (23). In brief, bacteria were fixed by heat, incubated for 30 min with human immunoglobulin G (IgG) (Sigma) (0.2 mg/ml in PBS–0.05% Tween 20), and washed. The bacteria were incubated for 1 h at ambient temperature with mouse IgM monoclonal antibodies to CP5 (25) diluted 1:50 in PBS–0.05% Tween 20. Bacteria were washed three times before being incubated with fluorescein isothiocyanate-conjugated rabbit F(ab)2 fragments to mouse IgM (Dako, Hamburg, Germany). In a subsequent step, the bacteria were stained with 4′,6-diamidino-2-phenylindole (DAPI; 2 μg/ml) (Sigma) for 5 min at room temperature. Three microscopic fields with 50 bacteria per field were evaluated, and the percentage of CP5-positive bacteria was determined by comparing the number of fluorescent bacteria with the total number of DAPI-stained bacteria. To control for nonspecific reactions of antibodies with staphylococcal protein A, immunofluorescence studies were also performed with the cap5O mutant and with the parental strain and the second antibody only.
Statistical analysis.
The data were analyzed by Student's t test for unpaired data to determine statistically significant differences.
RESULTS
Effect of agr and growth phase on adherence of S. aureus to EC.
In preliminary studies of staphylococcal adherence to EC, we observed that S. aureus Newman adhered to EC but that in contrast to the invasive strain 8325-4, <1% of the inoculum invaded the EC. Similarly, the agr mutant ALC355 was noninvasive. After incubation for 2 h with the staphylococci, no obvious changes in EC morphology were evident microscopically.
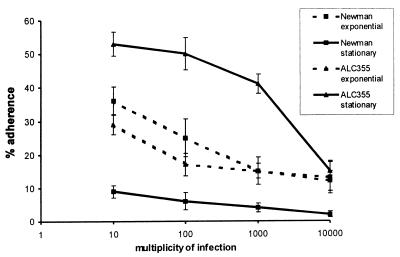
We compared the adherence of S. aureus Newman cells and isogenic agr mutant ALC355 cells harvested from different phases of the growth cycle. As shown in Fig. 1, strain Newman cells harvested during the exponential growth phase showed significantly (P < 0.05) greater adherence than those harvested in the stationary growth phase. This effect was apparent at multiplicities of infection ranging from 10:1 to 10,000:1 (ratio of CFU to EC). In contrast, the agr mutant grown to stationary phase showed greater dose-dependent adherence than did the same strain grown to exponential phase. The agr mutant ALC355 was significantly (P < 0.001) more adherent than the parental strain Newman in the stationary growth phase, but the two strains showed similar levels of adherence in the exponential growth phase (Fig. 1).
FIG. 1.
Effect of S. aureus growth phase and agr on adherence. Approximately 105 EC were incubated for 2 h with S. aureus cells at multiplicities of infection ranging from 10 to 10,000 CFU per cell. Strain Newman and the agr mutant ALC355 were grown to the exponential or stationary growth phase. The percent adherence was expressed as 100 × (CFU of adherent bacteria/CFU of inoculated bacteria). Each point represents the mean of four determinations, with the error bars representing the standard deviations.
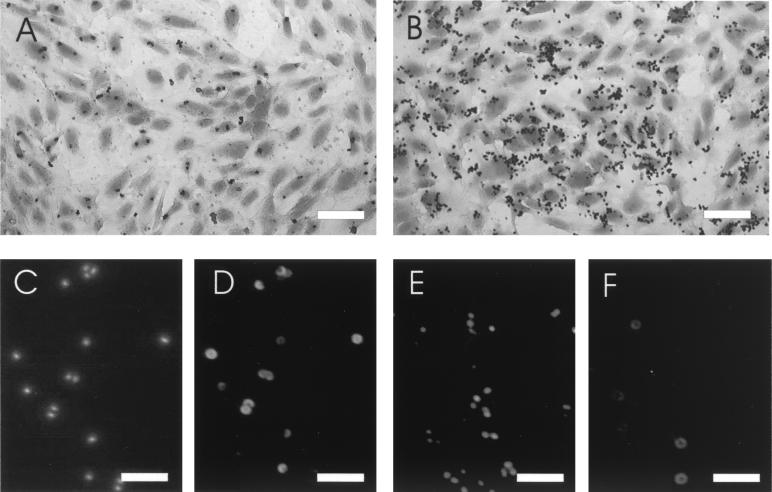
For microscopic evaluation, adherence assays were performed on culture slides with S. aureus strains harvested in the stationary phase (Fig. 2A and B). Quantification of adherent bacteria per EC yielded results that correlated with those obtained by the plating technique (Fig. 1); e.g., at a multiplicity of infection of 100, 11 ± 2.5 strain Newman bacteria per cell and 49 ± 3.2 agr mutant bacteria per cell were counted. For both S. aureus strains, some EC had many adherent bacteria whereas other cells were totally free of bacteria.
FIG. 2.
Microscopic evaluation of staphylococcal adherence and capsule expression. Culture slides were stained with crystal violet to show the distribution of bacteria adherent to EC (A and B). Strain Newman (A, C, and D) and the agr mutant ALC355 (B, E, and F) were grown to stationary phase. DNA from S. aureus cells of the inoculum were stained with DAPI (C and E), and CP was detected by indirect immunofluorescence (D and F). Magnifications, ×200 (bar, 50 μm) in panels A and B and ×1,000 (bar, 10 μm) in panels C to F.
Inverse correlation between CP expression and adherence.
The decreased adherence of stationary-phase bacteria might be explained by an inhibitory factor produced late in the growth cycle. S. aureus CP, which is expressed postexponentially (11), is a candidate for such an inhibitory factor; moreover, CP5 expression is positively regulated by agr (10). We confirmed the agr-mediated activation of CP5 expression by indirect immunofluorescence experiments with monoclonal antibodies to CP5. If the bacterial cells from the stationary growth phase were examined, CP5 was detected on 39% of strain Newman cells but on only 6% of agr mutant cells (Fig. 3). In contrast to the heterogeneous distribution of CP5 on S. aureus Newman cells, expression of protein A (evaluated without blocking with human serum and with the use of fluorescein isothiocyanate-labeled rabbit IgG) was uniform within the bacterial population (data not shown).
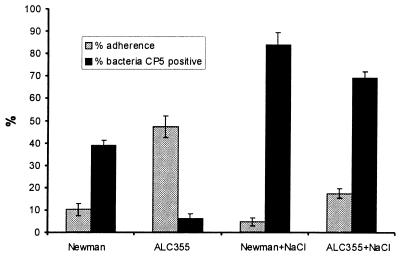
FIG. 3.
Inverse correlation between CP expression and adherence. Strain Newman and the agr mutant ALC355 were grown to stationary phase in culture medium containing 0.5 or 5% NaCl. Adherence assays were performed by incubation of ∼105 EC with 107 CFU of S. aureus for 2 h. The percent adherence was expressed as 100 × (CFU of adherent bacteria/CFU of inoculated bacteria). CP5 expression was determined by indirect immunofluorescence and given as a percentage of all bacteria being DAPI positive. The error bars represent the standard deviations of the means of four (adherence) or three (CP5 expression) determinations.
As shown in Fig. 3, we could modulate CP5 expression by varying the salt concentration in the medium. The addition of 5% NaCl to CYGP culture medium resulted in a significant increase in the numbers of CP5-positive Newman cells (P < 0.005) and agr mutant ALC355 cells (P < 0.0001) over the numbers when the same strains were grown in CYGP containing 0.5% NaCl. Most strain Newman cells cultivated in the presence of added NaCl were encapsulated but poorly adherent (Fig. 3). In contrast, the agr mutant cultivated under low-salt conditions showed minimal CP5 expression and maximal adherence. These results suggest that CP5 inhibits the adherence of S. aureus Newman to EC.
Detection of CP5 on adherent and nonadherent S. aureus.
To determine whether there was a difference in CP5 expression by adherent and nonadherent S. aureus cells, nonadherent bacteria were separated from cell-associated bacteria after the adherence assay. As shown in Table 2, all adherent bacteria were negative for CP5 by indirect immunofluorescence whereas nonadherent bacteria showed higher CP5 expression than that of the input inoculum; this result reflects a high affinity of the unencapsulated bacteria for EC. The inhibitory effect of CP5 on adherence may have led to the separation of a heterogeneous bacterial population such that encapsulated bacteria were nonadherent and unencapsulated cells were adherent.
TABLE 2.
CP5 expression by S. aureus strain Newman and agr mutant ALC355 before (inoculum) and after the adherence assay
| Strain | % of bacteria staining positive for CP5a
|
||
|---|---|---|---|
| Inoculum | Adherent | Nonadherent | |
| Newman | 39 (1.4) | 0 (0.0) | 70 (1.7) |
| ALC355 | 6 (2.1) | 0 (0.0) | 41 (1.9) |
| Newman (NaCl)b | 84 (1.6) | 0 (0.0) | 95 (2.4) |
| ALC355 (NaCl)b | 69 (1.2) | 0 (0.0) | 88 (1.6) |
Means of three determinations given as the percentage of bacteria found to be DAPI positive. Standard deviations are given in parentheses.
Growth medium supplemented with 5% NaCl.
Adherence of the cap5O mutant to EC.
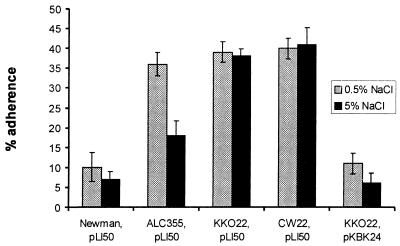
We hypothesized that CP production was responsible for the reduced adherence of the agr-positive strain compared with that of the agr mutant (Fig. 1). Therefore, we investigated the influence of a cap5O mutation on adherence in both agr-positive (KKO22) and agr-negative (CW22) genetic backgrounds. The cap5O gene encodes a UDP-N-acetylmannosamine dehydrogenase, an essential enzyme in the CP5 biosynthetic pathway (Portoles et al., submitted). A cap5O mutation was created by deletion of 727 bp in the 5′ end of the cap5O gene and was introduced by allelic exchange into the chromosome of S. aureus Newman. The mutant KKO22 was determined to be CP5 negative by immunodiffusion of capsular extracts, immunofluorescence, and colony immunoblotting. Adherence assays were performed with staphylococci harvested from the stationary growth phase in CYGP medium supplemented with either 0.5 or 5% NaCl (Fig. 4). Like the agr mutant ALC355, the cap5O mutant KKO22 showed significantly (P < 0.005) greater adherence than the parental strain Newman did. Complementation of the cap5O mutation with pKBK24 (carrying an intact cap5O gene) restored CP5 expression to strain KKO22 and resulted in the reduction of EC adherence to a level similar to that of the wild-type strain (Fig. 4). The cap5O agr double mutant showed no further increase in adherence. In contrast to that of CP-positive strains, the adherence of the CP-negative strains KKO22 and CW22 was unaffected by the addition of NaCl to the growth medium.
FIG. 4.
Influence of CP5 expression on S. aureus adherence to EC. Strains were grown to stationary phase in culture medium containing 0.5 or 5% NaCl to the culture medium. The adherence assay was performed by incubation of ∼105 EC with 107 CFU of S. aureus for 2 h. Percent adherence was expressed as 100 × (CFU of adherent bacteria/CFU of inoculated bacteria). The error bars represent the standard deviations of the means of four determinations.
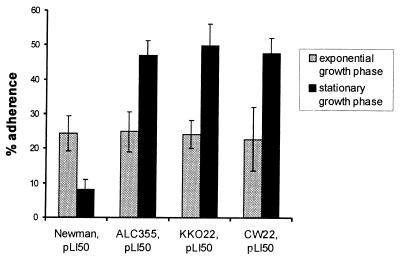
Adherence of S. aureus from exponential and stationary growth phases in a CP-negative background.
To reinvestigate the impact of bacterial growth phase on the adherence of S. aureus to EC, we compared the adherence of strain Newman with that of isogenic strains with mutations in agr and/or cap5O. As shown in Fig. 5, all bacterial strains harvested from the exponential growth phase showed similar levels of adherence. This finding is consistent with the observation that little CP5 is expressed by cells in the exponential phase. However, mutants defective in agr and/or cap5O adhered significantly (P < 0.05) better to EC if they were derived from stationary-phase rather than exponential-phase cultures. This observation suggests postexponential activation of at least one adhesin that is masked by CP in the parental strain.
FIG. 5.
Effect of staphylococcal growth phase on adherence to EC. S. aureus strains were grown to exponential or stationary growth phase. The adherence assay was performed by incubation of ∼105 EC with 107 CFU of S. aureus for 2 h. The percent adherence was expressed as 100 × (CFU of adherent bacteria/CFU of inoculated bacteria). The error bars represent the standard deviations of the means of four determinations.
DISCUSSION
In this study we analyzed the role of CP, growth phase, and agr on the adherence of S. aureus Newman to human EC. The evidence suggests that CP5 inhibits the adherence of bacteria to EC by masking the major cell wall adhesin(s). This possibility is supported by our findings that (i) adherence was negatively correlated with CP expression, (ii) only unencapsulated bacterial cells were adherent, and (iii) the CP5-negative isogenic cap5O mutant showed significantly greater adherence than the parental strain did. Our results are in direct contrast to those of Soell et al. (64), who reported that S. aureus CP5 and CP8 can bind to EC. We believe that the putative adhesin may have been a contaminant of their CP preparation.
Previous studies have shown that the expression of S. aureus extracellular polysaccharide(s) interferes with the adherence process by masking adhesins (8, 18, 20, 42–44, 50). However, masking was seen only for heavily encapsulated strains belonging to capsular type 1 or 2 or was due to an uncharacterized capsule-like material detected after growth of strains in milk whey (43). This periodate-sensitive material might be the recently described poly-N-succinyl-β1→6-glucosamine of S. aureus, which was preferentially expressed during infection (46); alternatively, it might be CP5 or CP8, each of which is released from whole bacterial cells by periodate treatment (J. Lee, unpublished observations). Evidence to support the masking of MSCRAMMs by microcapsules such as CP5 is scant (18, 48, 63). Whereas CP did not influence staphylococcal binding to collagen (18, 63), capsular mutants derived from strain Reynolds showed a tendency to bind more fibronectin and bone sialoprotein than did the parental strain (48). Since the major adhesin responsible for adherence to EC is not known, we can only speculate that the respective binding domain of this putative adhesin is covered by the capsular material.
Masking effects of extracellular polysaccharides leading to decreased adherence to host cells have been described for other pathogenic bacteria, such as Streptococcus pneumoniae (69), group A streptococci (21), Klebsiella pneumoniae (16), Neisseria meningitidis (66), Escherichia coli (59), and Haemophilus influenzae type b (67). Because bacterial adherence is thought to be a prerequisite for infection, the CP-mediated inhibition of adherence suggests that encapsulated strains are less virulent. However, CP exhibits antiphagocytic activity (32, 48, 70) that protects the bacterium during infection. The relative virulence of the clinically prominent CP types 5 and 8 and their acapsular mutants depends on the animal model of infection chosen for study. Early studies indicated that microencapsulated S. aureus strains were no more virulent for rodents than were unencapsulated mutants (1, 3, 38). More recently, a protective effect of specific antibodies to CP5 has been documented in different animal models of staphylococcal infection (15, 39). In addition, mortality was higher (48, 70) and bacteremia (70) and arthritis (48) were more frequent among mice inoculated with serotype 5 strain Reynolds than among mice inoculated with unencapsulated mutants. The results of virulence studies were influenced by the method used for preparation of the challenge inoculum, i.e., growth on solid medium or in liquid growth medium that affects CP expression (70). CP expression in vitro is highly sensitive to various environmental signals (9, 23, 40, 55, 56, 68) and is probably influenced by the environment in vivo as well. Recently, we showed that CP expression is down-regulated during chronic lung infections in patients with cystic fibrosis (23) but is up-regulated in a rat model of endocarditis (40) and in a mouse model of nasal colonization (35). Furthermore, we and others (23, 56) found S. aureus cultured in broth to be heterogeneous for CP expression. Although the molecular basis for the heterogeneity of CP expression by S. aureus is not known, phase variation in the expression of extracellular polysaccharides has been described for other bacteria (22, 34, 58, 77) and is thought to be important in bacterial immune evasion by antigenic variation. Encapsulated and unencapsulated S. aureus cells may occupy different niches in the host by virtue of their different adherence capacities. Furthermore, the ability to adhere may be more important for the onset of infections than later, when the bacterial population is organized in a biofilm community (57). Whether this correlates with the exponential and stationary growth phases in vitro remains to be proven. Therefore, studies evaluating the conditions of cell adherence as well as CP expression during an actual staphylococcal infection are needed. Kiser et al. demonstrated that S. aureus bacteria colonizing the nares of mice were CP5 positive (35). However, whether the CP5-positive bacteria in the nose were cell associated was not addressed.
S. aureus adherence properties are influenced by additional factors other than capsule production. The global regulator agr is thought to be an inhibitor of adhesin expression (57), given the up-regulation of cell wall-associated protein A (51) and fibronectin-binding proteins in agr mutants (60). However, we found no evidence that agr regulates the adhesin(s) responsible for the adherence of strain Newman to EC, since no difference in adherence was apparent between the cap5O mutant and the cap5O agr double mutant. The increased adherence capacity of the agr mutant can be fully explained by decreased CP5 expression, which would serve to unmask an adhesin that might be buried beneath the capsular layer. Other investigators have characterized the adherence of S. aureus 8325-4 and its agr mutant to mesothelial cells (54) and to mammary gland epithelial cells (36) and have described the internalization of strain 8325-4 by EC (75). However, strains Newman and 8325-4 differ in numerous properties. Although S. aureus 8325-4 is negative for CP expression (74), it shows greater adherence to EC than does the capsule-negative mutant of strain Newman (unpublished observations). Whether different adhesins or different quantities of the same adhesin account for these strain-specific adherence properties is under investigation.
The agr operon acts as a quorum-sensing system that regulates growth phase-dependent expression of virulence factors and adhesins. However, the growth phase of the bacterial inoculum is not described in most reports of S. aureus adherence to EC (5, 30, 49, 72), while in other studies the assays were performed only with bacteria harvested in the stationary growth phase (6, 7, 14). Tompkins et al. (71) reported that S. aureus cells from the exponential growth phase were more adherent to human EC than were bacteria harvested in the stationary growth phase. Similarly, we found that a greater percentage of strain Newman cells from the exponential growth phase than from a later point in the growth cycle (when capsule production was maximal) adhered to EC. In contrast, the cap5O mutant and the CP5-deficient agr mutant were more adherent to EC when harvested in the stationary growth phase. Thus, the major EC adhesin(s) in strain Newman appears to be expressed postexponentially. Accordingly, other investigators have shown that some S. aureus strains exhibit an increased adherence to cultured cells when derived from the stationary growth phase (73). Whether the different, strain-dependent patterns of adhesion to cell monolayers are due to differential expression of various adhesins or CP remains to be determined.
We conclude that strain Newman produces at least one adhesin that is masked by CP, is expressed postexponentially, and is independent of agr. Although we believe that this adhesin is probably none of the known MSCRAMMs, our observations do not exclude the possibility that other adhesins may mediate EC adherence early in the growth cycle. In addition to the exponential growth phase-dependent expression of fibronectin-binding proteins (60), collagen-binding protein (19), and clumping factor B (47), other adhesins—such as clumping factor A (a fibrinogen-binding protein)—are preferentially expressed in the stationary phase (76). The relevance of these in vitro findings to the pathogenesis of staphylococcal infections is uncertain and requires further investigation.
ACKNOWLEDGMENTS
The work was supported by the Deutsche Forschungsgemeinschaft (Wo573/2-1) and U.S. Public Health Service (grant AI 29040).
We thank S. Heller for supplying us with umbilical cords and Silvia Herbert for providing us with the information about induction of CP5 by NaCl. We acknowledge C. Dehio and Marlene Röttgen for technical assistance in establishing the endothelial cell cultures.
REFERENCES
- 1.Albus A, Arbeit R D, Lee J C. Virulence of Staphylococcus aureus mutants altered in type 5 capsule production. Infect Immun. 1991;59:1008–1014. doi: 10.1128/iai.59.3.1008-1014.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albus A, Fournier J M, Wolz C, Boutonnier A, Ranke M, Hoiby N, Hochkeppel H, Döring G. Staphylococcus aureus capsular types and antibody response to lung infection in patients with cystic fibrosis. J Clin Microbiol. 1988;26:2505–2509. doi: 10.1128/jcm.26.12.2505-2509.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baddour L M, Lowrance C, Albus A, Lowrance J H, Anderson S K, Lee J C. Staphylococcus aureus microcapsule expression attenuates bacterial virulence in a rat model of experimental endocarditis. J Infect Dis. 1992;165:749–753. doi: 10.1093/infdis/165.4.749. [DOI] [PubMed] [Google Scholar]
- 4.Balaban N, Novick R P. Autocrine regulation of toxin synthesis by Staphylococcus aureus. Proc Natl Acad Sci USA. 1995;92:1619–1623. doi: 10.1073/pnas.92.5.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bengualid V, Hatcher V B, Diamond B, Blumberg E A, Lowy F D. Staphylococcus aureus infection of human endothelial cells potentiates Fc receptor expression. J Immunol. 1990;145:4279–4283. [PubMed] [Google Scholar]
- 6.Blumberg E A, Hatcher V B, Lowy F D. Acidic fibroblast growth factor modulates Staphylococcus aureus adherence to human endothelial cells. Infect Immun. 1988;56:1470–1474. doi: 10.1128/iai.56.6.1470-1474.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung A L, Krishnan M, Jaffe E A, Fischetti V A. Fibrinogen acts as a bridging molecule in the adherence of Staphylococcus aureus to cultured human endothelial cells. J Clin Investig. 1991;87:2236–2245. doi: 10.1172/JCI115259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cifrian E, Guidry A J, O'Brien C N, Marquardt W W. Effect of alpha-toxin and capsular exopolysaccharide on the adherence of Staphylococcus aureus to cultured teat, ductal and secretory mammary epithelial cells. Res Vet Sci. 1995;58:20–25. doi: 10.1016/0034-5288(95)90083-7. [DOI] [PubMed] [Google Scholar]
- 9.Dassy B, Fournier J M. Respiratory activity is essential for post-exponential-phase production of type 5 capsular polysaccharide by Staphylococcus aureus. Infect Immun. 1996;64:2408–2414. doi: 10.1128/iai.64.7.2408-2414.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dassy B, Hogan T, Foster T J, Fournier J M. Involvement of the accessory gene regulator (agr) in expression of type 5 capsular polysaccharide. J Gen Microbiol. 1993;139:1301–1306. doi: 10.1099/00221287-139-6-1301. [DOI] [PubMed] [Google Scholar]
- 11.Dassy B, Stringfellow W T, Lieb M, Fournier J M. Production of type 5 capsular polysaccharide by Staphylococcus aureus grown in a semi-synthetic medium. J Gen Microbiol. 1991;137:1155–1162. doi: 10.1099/00221287-137-5-1155. [DOI] [PubMed] [Google Scholar]
- 12.Duthie E S, Lorenz L L. Staphylococcal coagulase: mode of action and antigenicity. J Gen Microbiol. 1952;6:95–107. doi: 10.1099/00221287-6-1-2-95. [DOI] [PubMed] [Google Scholar]
- 13.Dziewanowska K, Patti J M, Deobald C F, Bayles K W, Trumble W R, Bohach G A. Fibronectin binding protein and host cell tyrosine kinase are required for internalization of Staphylococcus aureus by epithelial cells. Infect Immun. 1999;67:4673–4678. doi: 10.1128/iai.67.9.4673-4678.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elliott D A, Hatcher V B, Lowy F D. A 220-kilodalton glycoprotein in yeast extract inhibits Staphylococcus aureus adherence to human endothelial cells. Infect Immun. 1991;59:2222–2223. doi: 10.1128/iai.59.6.2222-2223.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fattom A I, Sarwar J, Ortiz A, Naso R. A Staphylococcus aureus capsular polysaccharide (CP) vaccine and CP-specific antibodies protect mice against bacterial challenge. Infect Immun. 1996;64:1659–1665. doi: 10.1128/iai.64.5.1659-1665.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Favre-Bonte S, Joly B, Forestier C. Consequences of reduction of Klebsiella pneumoniae capsule expression on interactions of this bacterium with epithelial cells. Infect Immun. 1999;67:554–561. doi: 10.1128/iai.67.2.554-561.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foster T J, McDevitt D. Surface-associated proteins of Staphylococcus aureus: their possible roles in virulence. FEMS Microbiol Lett. 1994;118:199–205. doi: 10.1111/j.1574-6968.1994.tb06828.x. [DOI] [PubMed] [Google Scholar]
- 18.Gillaspy A F, Lee C Y, Sau S, Cheung A L, Smeltzer M S. Factors affecting the collagen binding capacity of Staphylococcus aureus. Infect Immun. 1998;66:3170–3178. doi: 10.1128/iai.66.7.3170-3178.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gillaspy A F, Patti J M, Smeltzer M S. Transcriptional regulation of the Staphylococcus aureus collagen adhesion gene, cna. Infect Immun. 1997;65:1536–1540. doi: 10.1128/iai.65.4.1536-1540.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haagen I A, Heezius H C, Verkooyen R P, Verhoef J, Verbrugh H A. Adherence of peritonitis-causing staphylococci to human peritoneal mesothelial cell monolayers. J Infect Dis. 1990;161:266–273. doi: 10.1093/infdis/161.2.266. [DOI] [PubMed] [Google Scholar]
- 21.Hagman M M, Dale J B, Stevens D L. Comparison of adherence to and penetration of a human laryngeal epithelial cell line by group A streptococci of various M protein types. FEMS Immunol Med Microbiol. 1999;23:195–204. doi: 10.1111/j.1574-695X.1999.tb01239.x. [DOI] [PubMed] [Google Scholar]
- 22.Hammerschmidt S, Muller A, Sillmann H, Muhlenhoff M, Borrow R, Fox A, Zollinger W D, Gerardy S R, Frosch M. Capsule phase variation in Neisseria meningitidis serogroup B by slipped-strand mispairing in the polysialytransferase gene (siaD): correlation with bacterial invasion and the outbreak of meningococcal disease. Mol Microbiol. 1996;20:1211–1220. doi: 10.1111/j.1365-2958.1996.tb02641.x. [DOI] [PubMed] [Google Scholar]
- 23.Herbert S, Worlitzsch D, Dassy B, Boutonnier A, Fournier J M, Bellon G, Dalhoff A, Döring G. Regulation of Staphylococcus aureus capsular polysaccharide type 5: CO2 inhibition in vitro and in vivo. J Infect Dis. 1997;176:431–438. doi: 10.1086/514061. [DOI] [PubMed] [Google Scholar]
- 24.Hochkeppel H K, Braun D G, Vischer W, Imm A, Sutter S, Staeubli U, Guggenheim R, Kaplan E L, Boutonnier A, Fournier J M. Serotyping and electron microscopy studies of Staphylococcus aureus clinical isolates with monoclonal antibodies to capsular polysaccharide types 5 and 8. J Clin Microbiol. 1987;25:526–530. doi: 10.1128/jcm.25.3.526-530.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoeger P H, Lenz W, Boutonnier A, Fournier J M. Staphylococcal skin colonization in children with atopic dermatitis: prevalence, persistence, and transmission of toxigenic and nontoxigenic strains. J Infect Dis. 1992;165:1064–1068. doi: 10.1093/infdis/165.6.1064. [DOI] [PubMed] [Google Scholar]
- 26.Ing M B, Baddour L M, Bayer A S. Bacteremia and infective endocarditis: pathogenesis, diagnosis and complications. In: Crossley K B, Archer G L, editors. The staphylococci in human disease. New York, N.Y: Churchill Livingstone; 1997. pp. 331–354. [Google Scholar]
- 27.Jaffe E A, Nachman R L, Becker C G, Minick C R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Investig. 1973;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ji G, Beavis R, Novick R P. Bacterial interference caused by autoinducing peptide variants. Science. 1997;276:2027–2030. doi: 10.1126/science.276.5321.2027. [DOI] [PubMed] [Google Scholar]
- 29.Ji G, Beavis R C, Novick R P. Cell density control of staphylococcal virulence mediated by an octapeptide pheromone. Proc Natl Acad Sci USA. 1995;92:12055–12059. doi: 10.1073/pnas.92.26.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson C M. Staphylococcus aureus binding to cardiac endothelial cells is partly mediated by a 130 kilodalton glycoprotein. J Lab Clin Med. 1993;121:675–682. [PubMed] [Google Scholar]
- 31.Jönsson K, Signäs C, Müller H-P, Lindberg M. Two different genes encode fibronectin binding proteins in Staphylococcus aureus. The complete nucleotide sequence and characterization of the second gene. Eur J Biochem. 1991;202:1041–1048. doi: 10.1111/j.1432-1033.1991.tb16468.x. [DOI] [PubMed] [Google Scholar]
- 32.Karakawa W W, Sutton A, Schneerson R, Karpas A, Vann W F. Capsular antibodies induce type-specific phagocytosis of capsulated Staphylococcus aureus by human polymorphonuclear leukocytes. Infect Immun. 1988;56:1090–1095. doi: 10.1128/iai.56.5.1090-1095.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karakawa W W, Vann W. Capsular polysaccharides of Staphylococcus aureus. Semin Infect Dis. 1982;4:285–293. [Google Scholar]
- 34.Kim J O, Weiser J N. Association of intrastrain phase variation in quantity of capsular polysaccharide and teichoic acid with the virulence of Streptococcus pneumoniae. J Infect Dis. 1998;177:368–377. doi: 10.1086/514205. [DOI] [PubMed] [Google Scholar]
- 35.Kiser K B, Cantey-Kiser J M, Lee J C. Development and characterization of a Staphylococcus aureus nasal colonization model in mice. Infect Immun. 1999;67:5001–5006. doi: 10.1128/iai.67.10.5001-5006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lammers A, Nuijten P J M, Smith H E. The fibronectin binding proteins of Staphylococcus aureus are required for adhesion to and invasion of bovine mammary gland cells. FEMS Microbiol Lett. 1999;180:103–109. doi: 10.1111/j.1574-6968.1999.tb08783.x. [DOI] [PubMed] [Google Scholar]
- 37.Lee C Y, Buranen S L, Ye Z H. Construction of single-copy integration vectors for Staphylococcus aureus. Gene. 1991;103:101–105. doi: 10.1016/0378-1119(91)90399-v. [DOI] [PubMed] [Google Scholar]
- 38.Lee J C, Betley M J, Hopkins C A, Perez N E, Pier G B. Virulence studies, in mice, of transposon-induced mutants of Staphylococcus aureus differing in capsule size. J Infect Dis. 1987;156:741–750. doi: 10.1093/infdis/156.5.741. [DOI] [PubMed] [Google Scholar]
- 39.Lee J C, Park J S, Shepherd S E, Carey V, Fattom A. Protective efficacy of antibodies to the Staphylococcus aureus type 5 capsular polysaccharide in a modified model of endocarditis in rats. Infect Immun. 1997;65:4146–4151. doi: 10.1128/iai.65.10.4146-4151.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee J C, Takeda S, Livolsi P J, Paoletti L C. Effects of in vitro and in vivo growth conditions on expression of type 8 capsular polysaccharide by Staphylococcus aureus. Infect Immun. 1993;61:1853–1858. doi: 10.1128/iai.61.5.1853-1858.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lowy F D. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 42.Mamo W, Froman G, Wadstrom T. Interaction of sub-epithelial connective tissue components with Staphylococcus aureus and coagulase-negative staphylococci from bovine mastitis. Vet Microbiol. 1988;18:163–176. doi: 10.1016/0378-1135(88)90062-4. [DOI] [PubMed] [Google Scholar]
- 43.Mamo W, Lindahl M, Jonsson P. Binding of fibronectin and type II collagen to Staphylococcus aureus from bovine mastitis: reduction of binding after growth in milk whey. Microb Pathog. 1992;12:443–449. doi: 10.1016/0882-4010(92)90007-b. [DOI] [PubMed] [Google Scholar]
- 44.Mamo W, Rozgonyi F, Brown A, Hjerten S, Wadstrom T. Cell surface hydrophobicity and charge of Staphylococcus aureus and coagulase-negative staphylococci from bovine mastitis. J Appl Bacteriol. 1987;62:241–249. doi: 10.1111/j.1365-2672.1987.tb02405.x. [DOI] [PubMed] [Google Scholar]
- 45.McDevitt D, Francois P, Vaudaux P, Foster T J. Cloning and sequencing of the clumping factor of Staphylococcus aureus. Mol Microbiol. 1994;11:237–248. doi: 10.1111/j.1365-2958.1994.tb00304.x. [DOI] [PubMed] [Google Scholar]
- 46.McKenney D, Pouliot K L, Wang Y, Murthy V, Ulrich M, Doring G, Lee J C, Goldmann D A, Pier G B. Broadly protective vaccine for Staphylococcus aureus based on an in vivo-expressed antigen. Science. 1999;284:1523–1527. doi: 10.1126/science.284.5419.1523. [DOI] [PubMed] [Google Scholar]
- 47.Ni Eidhin D, Perkins S, Francois P, Vaudaux P, Hook M, Foster T J. Clumping factor B (ClfB), a new surface-located fibrinogen-binding adhesin of Staphylococcus aureus. Mol Microbiol. 1998;30:245–257. doi: 10.1046/j.1365-2958.1998.01050.x. [DOI] [PubMed] [Google Scholar]
- 48.Nilsson I M, Lee J C, Bremell T, Ryden C, Tarkowski A. The role of staphylococcal polysaccharide microcapsule expression in septicemia and septic arthritis. Infect Immun. 1997;65:4216–4221. doi: 10.1128/iai.65.10.4216-4221.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ogawa S K, Yurberg E R, Hatcher V B, Levitt M A, Lowy F D. Bacterial adherence to human endothelial cells in vitro. Infect Immun. 1985;50:218–224. doi: 10.1128/iai.50.1.218-224.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ohtomo T, Yoshida K. Adhesion of Staphylococcus aureus to fibrinogen, collagen and lectin in relation to cell surface structure. Zentbl Bakteriol Mikrobiol Hyg Ser A. 1988;268:325–340. doi: 10.1016/s0176-6724(88)80017-8. [DOI] [PubMed] [Google Scholar]
- 51.Patel A H, Kornblum J, Kreiswirth B, Novick R, Foster T J. Regulation of the protein A-encoding gene in Staphylococcus aureus. Gene. 1992;114:25–34. doi: 10.1016/0378-1119(92)90703-r. [DOI] [PubMed] [Google Scholar]
- 52.Patti J M, Allen B L, McGavin M J, Hook M. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu Rev Microbiol. 1994;48:585–617. doi: 10.1146/annurev.mi.48.100194.003101. [DOI] [PubMed] [Google Scholar]
- 53.Patti J M, Jönsson K, Guss B, Switalski L M, Wiberg K, Lindberg M, Hook M. Molecular characterization and expression of a gene encoding a Staphylococcus aureus collagen adhesin. J Biol Chem. 1992;267:4766–4772. [PubMed] [Google Scholar]
- 54.Poston S M, Glancey G R, Wyatt J E, Hogan T, Foster T J. Co-elimination of mec and spa genes in Staphylococcus aureus and the effect of agr and protein A production on bacterial adherence to cell monolayers. J Med Microbiol. 1993;39:422–428. doi: 10.1099/00222615-39-6-422. [DOI] [PubMed] [Google Scholar]
- 55.Poutrel B, Gilbert F B, Lebrun M. Effects of culture conditions on production of type 5 capsular polysaccharide by human and bovine Staphylococcus aureus strains. Clin Diagn Lab Immunol. 1995;2:166–171. doi: 10.1128/cdli.2.2.166-171.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Poutrel B, Rainard P, Sarradin P. Heterogeneity of cell-associated CP5 expression on Staphylococcus aureus strains demonstrated by flow cytometry. Clin Diagn Lab Immunol. 1997;4:275–278. doi: 10.1128/cdli.4.3.275-278.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Projan S J, Novick R P. The molecular basis of pathogenicity. In: Crossley K B, Archer G L, editors. The staphylococci in human disease. New York, N.Y: Churchill Livingstone; 1997. pp. 55–82. [Google Scholar]
- 58.Roche R J, Moxon E R. Phenotypic variation of carbohydrate surface antigens and the pathogenesis of Haemophilus influenzae infections. Trends Microbiol. 1995;3:304–309. doi: 10.1016/s0966-842x(00)88959-3. [DOI] [PubMed] [Google Scholar]
- 59.Runnels P L, Moon H W. Capsule reduces adherence of enterotoxigenic Escherichia coli to isolated intestinal epithelial cells of pigs. Infect Immun. 1984;45:737–740. doi: 10.1128/iai.45.3.737-740.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saravia-Otten P, Müller H-P, Arvidson S. Transcription of Staphylococcus aureus fibronectin binding protein genes is negatively regulated by agr and an agr-independent mechanism. J Bacteriol. 1997;179:5259–5263. doi: 10.1128/jb.179.17.5259-5263.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sau S, Bhasin N, Wann E R, Lee J C, Foster T J, Lee C Y. The Staphylococcus aureus allelic genetic loci for serotype 5 and 8 capsule expression contain type-specific genes flanked by common genes. Microbiology. 1997;143:2395–2405. doi: 10.1099/00221287-143-7-2395. [DOI] [PubMed] [Google Scholar]
- 62.Signäs C, Raucci G, Jönsson K, Lindgren P E, Anantharamaiah G M, Höök M, Lindberg M. Nucleotide sequence of the gene for a fibronectin-binding protein from Staphylococcus aureus: use of this peptide sequence in the synthesis of biologically active peptides. Proc Natl Acad Sci USA. 1989;86:699–703. doi: 10.1073/pnas.86.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Snodgrass J L, Mohamed N, Ross J M, Sau S, Lee C Y, Smeltzer M S. Functional analysis of the Staphylococcus aureus collagen adhesin B domain. Infect Immun. 1999;67:3952–3959. doi: 10.1128/iai.67.8.3952-3959.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soell M, Diab M, Haan A G, Beretz A, Herbelin C, Poutrel B, Klein J P. Capsular polysaccharide types 5 and 8 of Staphylococcus aureus bind specifically to human epithelial (KB) cells, endothelial cells, and monocytes and induce release of cytokines. Infect Immun. 1995;63:1380–1386. doi: 10.1128/iai.63.4.1380-1386.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sompolinsky D, Samra Z, Karakawa W W, Vann W F, Schneerson R, Malik Z. Encapsulation and capsular types in isolates of Staphylococcus aureus from different sources and relationship to phage types. J Clin Microbiol. 1985;22:828–834. doi: 10.1128/jcm.22.5.828-834.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stephens D S, Spellman P A, Swartley J S. Effect of the (alpha 2→8)-linked polysialic acid capsule on adherence of Neisseria meningitidis to human mucosal cells. J Infect Dis. 1993;167:475–479. doi: 10.1093/infdis/167.2.475. [DOI] [PubMed] [Google Scholar]
- 67.St. Geme J W, III, Falkow S. Loss of capsule expression by Haemophilus influenzae type b results in enhanced adherence to and invasion of human cells. Infect Immun. 1991;59:1325–1333. doi: 10.1128/iai.59.4.1325-1333.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stringfellow W T, Dassy B, Lieb M, Fournier J M. Staphylococcus aureus growth and type 5 capsular polysaccharide production in synthetic media. Appl Environ Microbiol. 1991;57:618–621. doi: 10.1128/aem.57.2.618-621.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Talbot U M, Paton A W, Paton J C. Uptake of Streptococcus pneumoniae by respiratory epithelial cells. Infect Immun. 1996;64:3772–3777. doi: 10.1128/iai.64.9.3772-3777.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thakker M, Park J S, Carey V, Lee J C. Staphylococcus aureus serotype 5 capsular polysaccharide is antiphagocytic and enhances bacterial virulence in a murine bacteremia model. Infect Immun. 1998;66:5183–5189. doi: 10.1128/iai.66.11.5183-5189.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tompkins D C, Blackwell L J, Hatcher V B, Elliott D A, O'Hagan Sotsky C, Lowy F D. Staphylococcus aureus proteins that bind to human endothelial cells. Infect Immun. 1992;60:965–969. doi: 10.1128/iai.60.3.965-969.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tompkins D C, Hatcher V B, Patel D, Orr G A, Higgins L L, Lowy F D. A human endothelial cell membrane protein that binds Staphylococcus aureus in vitro. J Clin Investig. 1990;85:1248–1254. doi: 10.1172/JCI114560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van Wamel W J, Vandenbroucke G C, Verhoef J, Fluit A C. The effect of culture conditions on the in vitro adherence of methicillin-resistant Staphylococcus aureus. J Med Microbiol. 1998;47:705–709. doi: 10.1099/00222615-47-8-705. [DOI] [PubMed] [Google Scholar]
- 74.Wann E R, Dassy B, Fournier J M, Foster T J. Genetic analysis of the cap5 locus of Staphylococcus aureus. FEMS Microbiol Lett. 1999;170:97–103. doi: 10.1111/j.1574-6968.1999.tb13360.x. [DOI] [PubMed] [Google Scholar]
- 75.Wesson C A, Liou L E, Todd K M, Bohach G A, Trumble W R, Bayles K W. Staphylococcus aureus Agr and Sar global regulators influence internalization and induction of apoptosis. Infect Immun. 1998;66:5238–5243. doi: 10.1128/iai.66.11.5238-5243.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wolz C, McDevitt D, Foster T J, Cheung A L. Influence of agr on fibrinogen binding in Staphylococcus aureus Newman. Infect Immun. 1996;64:3142–3147. doi: 10.1128/iai.64.8.3142-3147.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ziebuhr W, Krimmer V, Rachid S, Lößner I, Götz F, Hacker J. A novel mechanism of phase variation of virulence in Staphylococcus epidermidis: evidence for control of the polysaccharide intercellular adhesin synthesis by alternating insertion and excision of the insertion sequence element IS256. Mol Microbiol. 1999;32:345–356. doi: 10.1046/j.1365-2958.1999.01353.x. [DOI] [PubMed] [Google Scholar]