Abstract
The US-affiliated Pacific Island countries (USAPI) is an endemic region for hepatitis B virus (HBV) infection. Universal infant hepatitis B vaccination was introduced in the USAPI in the mid-1980s to mitigate the HBV burden. We assessed the impact of universal infant vaccination on the HBV infection prevalence over time among children born in the 1980s, 1990s, and 2000s in the USAPI. Demographic and serologic data from serial sero-surveys conducted between 1985 and 2015 were obtained. Descriptive statistics and analysis of variance were performed. From data obtained from 4827 children (2–11 years), HBV prevalence decreased markedly: 8.4% in the 1980s; 2.5% in the 1990s; and 0.2% in the 2000s (P < 0.0001) as vaccination coverage increased: 76.4% in the 1980s; 87.3% in the 1990s; and 97.5% in the 2000s (P < 0.0001). These findings underscore the protective effect of universal infant hepatitis B vaccination over time on the HBV burden in an HBV endemic region.
Keywords: Hepatitis B virus infection, HBV, HBsAg, Hepatitis B vaccination, Children, Pacific Islands
1. Introduction
Hepatitis B virus (HBV) infection is a vaccine-preventable disease and a global health problem, especially in the World Health Organization Western Pacific Region (WPRO) [1,2]. WPRO accounts for more than half of HBV-infected persons worldwide yet makes up 28% of the world population [2,3]. The United States-affiliated Pacific Island countries (USAPI) are part of WPRO and include American Samoa, the Commonwealth of the Northern Mariana Islands, Guam, the Federated States of Micronesia (FSM), the Republic of the Marshall Islands, and the Republic of Palau. The USAPI are disproportionately burdened by HBV infection [4,5]. All the islands and territories that make up the USAPI are considered intermediate (2–7% hepatitis B surface antigen [HBsAg] prevalence) or highly (>7% HBsAg prevalence) endemic regions for HBV infection [4]. Chronic HBV infection leads to liver complications (liver cirrhosis and hepatocellular cancer [HCC]) and up to 25% of chronically-infected people will die prematurely from complications of chronic HBV infection [6]. Chronic HBV infection is an important risk factor for HCC in Guam [7].
Perinatal and early horizontal transmission of HBV infections are central to the high prevalence of HBV in the USAPI [8]. The risk of chronic infection is 90% among those acquiring HBV infection during infancy and 25–50% among those acquiring infection during childhood [9]. Because HBV infection during childhood is less likely to be symptomatic and the consequences of chronic HBV infection such as cirrhosis and HCC are not usually evident until adulthood, HBV infection among children usually goes unrecognized. Infection with HBV and the attendant complications can be prevented with a hepatitis B vaccination series commencing at birth. Hepatitis B vaccination is the most effective means of preventing HBV infection [10]. The hepatitis B vaccine confers a protective antibody response that prevents HBV infection and its complications in greater than 90% of healthy persons who receive the complete series [11]. Immunity from the hepatitis B vaccine can last three decades or longer [12]. In response to the burden of HBV infection, hepatitis B vaccination was introduced into the routine infant vaccination schedule in the USAPI beginning in the mid-to-late 1980s. In this study, we evaluate the impact of universal infant vaccination on the prevalence of HBV infection over time among children born in the 1980, 1990s, and 2000s in the USAPI from data obtained between 1985 and 2015.
2. Methods
2.1. Data collection and measures
Demographic and serological data were obtained from published and unpublished serial cross-sectional sero-prevalence surveys conducted in the six USAPI countries. Data were obtained from children aged 2–11 years in 1985, 1986, 1991, 1995, 2000, 2007, 2010, 2013, and 2015. School-aged (5–11 years) participants were recruited from all (private and public) schools on the participating islands while data from children less than five years old that were included in this study were obtained from sero-surveys of households in the USAPI [8,13]. Some data from FSM were obtained by population proportionate sampling of households [8] while some data from American Samoa from the 1980s were obtained from randomly selected households [13]. Demographic data that were collected included year of birth, age, and sex of each participating child. Based on year of birth, we categorized all participating children into three birth cohorts. Children born between 1980 and 1989 were in the 1980 birth cohort; children born between 1990 and 1999 were in the 1990 birth cohort; and children born between 2000 and 2010 were in the 2000 birth cohort. Vaccination status for each child was obtained from vaccination registry records. Participants who received at least the birth dose of the hepatitis B vaccine were considered vaccinated. Blood samples were obtained from all participants and tested for HBsAg by rapid test or standard ELISA test [8,13]. A positive HBsAg test was classified as a current HBV infection. Informed consent for data and blood sample collection was obtained from parents or guardians of the participating children. All studies were reviewed and approved by the Ethics Review Board of the World Health Organization’s Western Pacific Regional Office or the Institutional Review Board of the Centers for Disease Control and Prevention.
2.2. Analysis
We generated descriptive statistics to characterize the demographic characteristics of the sample and estimated the prevalence of current HBV infection and hepatitis B vaccination coverage for each birth cohort. A one-way analysis of variance was done to determine whether there were significant differences in the prevalence estimates of current HBV infection and hepatitis B vaccination coverage across the three birth cohorts. A two-tailed level of statistical significance was set at P < 0.05 and all analyses were conducted using Statistical Analysis System (SAS) version 9.3 (SAS Institute, Cary, NC).
3. Results
Table 1 shows the demographic characteristics, prevalence of HBV infection and hepatitis B vaccination coverage by birth cohort. A total of 4827 children took part in the surveys: 1323 (27.4%) were in the 1980 birth cohort; 362 (7.5%) were in the 1990 birth cohort; and 3142 (65.1%) were in the 2000 birth cohort (Table 1). Mean age by birth cohort was 7.8 years (1980 birth cohort), 4.8 years (1990 birth cohort) and 6.7 years (2000 birth cohort). Females made up 58.3% of the 1980 birth cohort, 50% of the 1990 birth cohort, and 53.6% of the 2000 birth cohort. The prevalence of HBV infection progressively decreased over time by birth cohort starting at 8.4% (111/1323) in the 1980 birth cohort, then 2.5% (9/362) in the 1990 birth cohort, and down to 0.2% (6/3142) in the 2000 birth cohort (Fig. 1). Correspondingly, the prevalence of hepatitis B vaccination progressively increased from 76.4% (1011/1323) in the 1980 birth cohort, to 87.3% (316/362) in the 1990 birth cohort, and to 97.5% (3063/3142) in the 2000 birth cohort (Fig. 1). There were significant differences in the prevalence of HBV infection and hepatitis B vaccination across all three birth cohorts (p < 0.001).
Table 1.
Demographic characteristics, prevalence of HBV infection and birth dose of hepatitis B vaccination by birth cohort, N = 4827.
| Variable | 1980 birth cohort | 1990 birth cohort | 2000 birth cohort |
|---|---|---|---|
| Number of participants | n = 1323 (27.4%) | n = 362 (7.5%) | n = 3142 (65.1%) |
| Sex | |||
| Male | 267 (41.7%) | 181 (50%) | 1457 (46.4%) |
| Female | 373 (58.3%) | 181 (50%) | 1685 (53.6%) |
| Mean age (range) | 7.8 (5–11) | 4.8 (2–6) | 6.7 (5–10) |
| Prevalence of HBV infection | 8.4% (111/1323) | 2.5% (9/362) | 0.2% (6/3142)a |
| Prevalence of hepatitis B vaccination | 76.4% (1011/1323) | 87.3% (316/362) | 97.5% (3063/3142)a |
| USAPI | American Samoa, Federated States of Micronesia, Republic of the Marshall Islands, Guam, Palau | Federated States of Micronesia (Chuuk and Pohnpei) | American Samoa, Republic of the Marshall Islands, Commonwealth of the Northern Mariana Islands, Guam, Palau |
| Data collection years | 1985, 1986, 1991 | 2000 | 2007, 2010, 2013, 2015 |
| Birth cohort | 1980–1989 | 1990–1999 | 2000–2010 |
Difference significant at p < 0.0001.
Fig. 1.
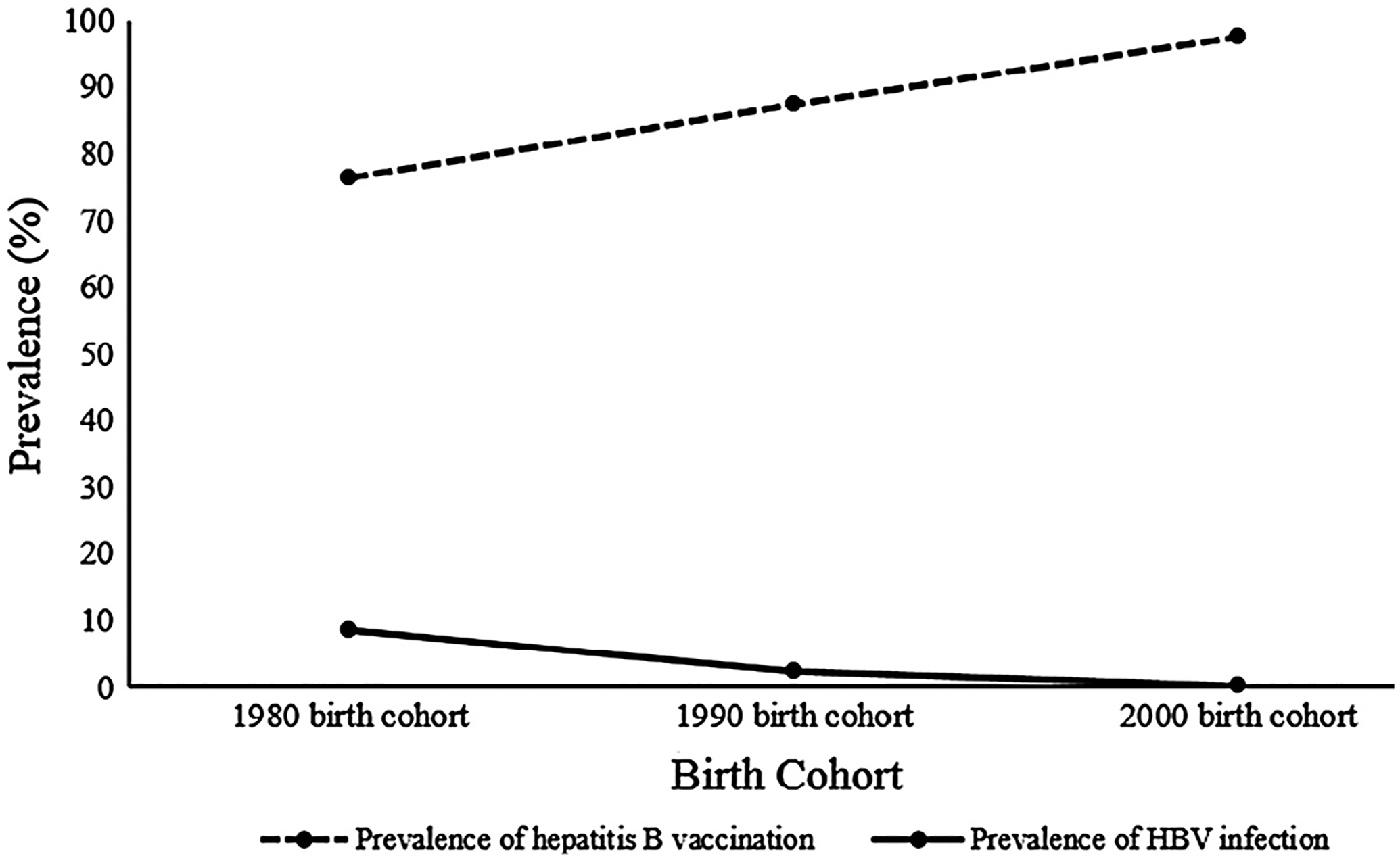
Trends in the prevalence estimates of HBV infection and hepatitis B vaccination by birth cohort.
4. Conclusion
This analysis demonstrated a progressive decline in the prevalence of HBV infection among a sample of children born after the implementation of universal infant vaccination in the USAPI that corresponded with a progressive increase in hepatitis B vaccination coverage. These findings point to the success of the introduction of universal infant hepatitis B vaccination in the USAPI. The high prevalence of HBV infection and low prevalence of hepatitis B vaccine among older children in this study (children born in the 1980s) just as universal infant hepatitis B vaccination was started and the progressive decline in the prevalence of HBV infection and concurrent increase in vaccination coverage among younger children (born in the 1990s and 2000s) as universal infant hepatitis B vaccination became established in the USAPI support this. The efficacy of the hepatitis B vaccine in preventing mother-to-child transmission ranges from 75% to 95% [14–16] and the vaccine offers long-term protection from HBV infection [12], making vaccination at birth essential for prevention [1]. Hepatitis B vaccination reduces the risk of HBV infection by almost fourfold in infants born to infected mothers [16]; therefore, maintaining a high level of hepatitis B vaccination coverage can result in a significant reduction in the burden of HBV infection. Given that the endemicity of HBV infection in the USAPI was driven by new HBV infections among infants and children [8], it is likely that with continued vaccination of infants and children, the USAPI can attain and maintain the WPRO country goal of hepatitis B surface antigen prevalence of less than 1%. High infant vaccination coverage can confer long-term immunity from HBV infection in the population [17,18]. As these infants become adults, the risk of horizontal transmission in the population is significantly reduced, women of reproductive age are at decreased risk of infection, and this prevents vertical transmission of HBV to the next generation of children.
Maximal hepatitis B vaccination coverage is essential to sustaining the decline in HBV infection in the USAPI. Institutionalizing policies and standing orders in all hospitals and healthcare centers to ensure that all newborn infants receive the hepatitis B vaccination is critical to increasing and maintaining high levels of hepatitis B vaccination coverage. Improving access to skilled delivery care at the time of childbirth is another effective strategy to increase hepatitis B vaccine birth dose coverage [15,16]. Although most infants are born in hospitals in the USAPI [19,20], infants that are not born in hospitals are unlikely to be vaccinated against HBV or to receive the birth dose [21]. Home visits to provide timely vaccination and integration of vaccination at the first postnatal visit of these infants would be beneficial to improving hepatitis B vaccination coverage [2,22].
There are limitations to this study. Data were collected using different methodologies and we did not sample participants from the same islands across all three birth cohorts. This may have affected the estimates of HBV prevalence or hepatitis B vaccination coverage, thereby limiting the generalizability of the study findings. The estimates of hepatitis B vaccination and HBV infection in this study could also be limited because not all children in the participating schools took part in the sero-prevalence survey. We also used the birth dose of hepatitis B vaccine as a proxy for hepatitis B vaccination coverage; however, the complete vaccine series confers optimal protection from HBV infection [11,12].
In conclusion, the study findings show that in an HBV endemic region, high hepatitis B vaccine coverage corresponded with a significant decrease in HBV infection over time. The progressive decline in the prevalence of HBV infection points to the protective effect of the hepatitis B vaccine on disease burden and transmission risk when hepatitis B vaccination coverage is high. With continued universal hepatitis B vaccination, HBV elimination in the USAPI is an achievable goal.
Footnotes
Conflict of interest statement
No financial disclosures were reported by the authors of this paper.
Disclaimer
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- [1].Centers for Disease Control and Prevention. Vaccine preventable deaths and the Global Immunization Vision and Strategy, 2006–2015. MMWR Morb Mort Wkly Rep.; 2006. 55: 511. [PubMed] [Google Scholar]
- [2].Hennessey K, Mendoza-Aldana J, Bayutas B, Lorenzo-Mariano KM, Diorditsa S. Hepatitis B control in the World Health Organization’s Western Pacific Region: targets, strategies, status. Vaccine 2013;31:J85–92. [DOI] [PubMed] [Google Scholar]
- [3].Goldstein ST, Zhou F, Hadler SC, Bell BP, Mast EE, Margolis HS. A mathematical model to estimate global hepatitis B disease burden and vaccination impact. Int J Epidemiol 2005;34:1329–39. [DOI] [PubMed] [Google Scholar]
- [4].Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet 2015;386:1546–55. [DOI] [PubMed] [Google Scholar]
- [5].Vogt TM, Goldstein ST, Kuartei S. Endemic hepatitis B virus infection and chronic liver disease mortality in the Republic of Palau, 1990–2002. Trans R Soc Trop Med Hyg 2006;100:1130–4. [DOI] [PubMed] [Google Scholar]
- [6].Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatol 2009;50:661–2. [DOI] [PubMed] [Google Scholar]
- [7].Haddock RL, Paulino YC, Bordallo R. Viral hepatitis and liver cancer on the Island of Guam. Asian Pac J Cancer Prev 2013;14(5):3175–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bialek SR, Helgenberger L, Fischer GE, Bower W, Konelios M, Chaine J, et al. Impact of routine hepatitis B immunization on the prevalence of chronic hepatitis B virus infection in the Marshall Islands and the Federated States of Micronesia. Ped Infect Dis J 2010;29:18–22. [DOI] [PubMed] [Google Scholar]
- [9].Shepard CW, Simard EP, Finelli L, Fiore AE, Bell BP. Hepatitis B virus infection: epidemiology and vaccination. Epidemiol Rev 2006;28:112–25. [DOI] [PubMed] [Google Scholar]
- [10].Lok AS. Prevention of hepatitis B virus–related hepatocellular carcinoma. Gastroenterology 2004;127:S303–9. [DOI] [PubMed] [Google Scholar]
- [11].Venters C, Graham W, Cassidy W. Recombivax-HB: perspectives past, present and future. Exp Rev Vaccines 2004;3:119–29. [DOI] [PubMed] [Google Scholar]
- [12].Bruce MG, Bruden D, Hurlburt D, Zanis C, Thonpson G, Rea L, et al. Antibody levels and protection after hepatitis B vaccine: Results of a 30-year follow-up study and response to a booster dose (epub ahead of print). J Infect Dis 2016. 10.1093/infdis/jiv748. [DOI] [PubMed] [Google Scholar]
- [13].Mahoney FJ, Woodruff BA, Erben JJ, Coleman PJ, Reid EC, Schatz GC, et al. Effect of a hepatitis B vaccination program on the prevalence of hepatitis B virus infection. J Infect Dis 1993;167:203–7. [DOI] [PubMed] [Google Scholar]
- [14].Beasley RP, George CYL, Roan CH, Hwang LY, Lan CC, Huang FY, et al. Prevention of perinatally transmitted hepatitis B virus infections with hepatitis B immune globulin and hepatitis B vaccine. Lancet 1983;322 (8359):1099–102. [DOI] [PubMed] [Google Scholar]
- [15].World Health Organization. Preventing mother-to-child transmission of hepatitis B: operational field guidelines for delivery of the birth dose of hepatitis B vaccine. 2006. [cited 2016 Sep 25]. <http://www.wpro.who.int/hepatitis/resource/hepb_operationalfieldguidelines.pdf?ua=1 >.
- [16].Lee C, Gong Y, Brok J, Boxall EH, Gluud C. Hepatitis B immunisation for newborn infants of hepatitis B surface antigen-positive mothers. Cochrane Syst Rev 2006;2. 10.1002/14651858.CD004790.pub2. [DOI] [PubMed] [Google Scholar]
- [17].Simons BC, Spradling PR, Bruden DJ, Zanis C, Case S, Choromanski TL, et al. A longitudinal hepatitis B vaccine cohort demonstrates long-lasting hepatitis B cellular immunity despite loss of antibody against hepatitis B surface antigen (epub ahead of print). J Infect Dis 2016. 10.1093/infdis/jiw142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Spradling PR, Xing J, Williams R, Masunu-Faleafaga Y, Dulski T, Mahamud A, et al. Immunity to hepatitis B virus (HBV) infection two decades after implementation of universal infant HBV vaccination: association of detectable residual antibodies and response to a single HBV challenge dose. Clin Vaccine Immunol 2013;20:559–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hawley NL, Johnson W, Hart CN, Triche EW, Ching J, Muasau-Howard B, et al. Gestational weight gain among American Samoan women and its impact on delivery and infant outcomes. BMC Preg Childbirth 2015;15:10–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].National Vital Statistics Report. Births: Final data for 2014. 2015. [cited 2016 Sep 25]. <http://www.cdc.gov/nchs/data/nvsr/nvsr64/nvsr64_12.pdf>. [PubMed]
- [21].Hawley NL, Brown C, Nu’usolia O, Ah-Ching J, Muasau-Howard B, McGarvey ST. Barriers to adequate prenatal care utilization in American Samoa. Mat Child Health J 2014;18:2284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].World Health Organization. Practices to improve coverage of the hepatitis B birth dose vaccine; 2013. <http://apps.who.int/iris/bitstream/10665/78616/1/WHO_IVB_12.11_eng.pdf> [cited 2016 Sep 25].


