Abstract
Elementary bodies (EBs) of the obligate intracellular bacterium Chlamydia trachomatis are responsible for the first step of attachment to host cells. We have studied the effects of EBs on human sperm protein tyrosine phosphorylation, which is important to sperm function. Indirect immunofluorescence using antiphosphotyrosine antibodies showed that serovar E, but not LGV, caused increased tyrosine phosphorylation which was localized to the sperm tail region. Immunoblotting revealed that serovar E caused a marked increase in tyrosine phosphorylation of 80- and 95-kDa sperm proteins, whereas serovar LGV caused increased phosphorylation of only the 80-kDa moiety. Considering the importance of tyrosine phosphorylation for sperm capacitation and other aspects of sperm function, we conclude that EBs may affect these events.
Chlamydia trachomatis is an obligate intracellular bacterium which is responsible for sexually transmitted disease worldwide (3, 28). Two distinct morphological forms of the microorganism can be seen during its unique life cycle: the intracellular reticulate bodies and the extracellular (infective) form called elementary bodies (EBs). One of the earliest and perhaps most important events in chlamydial pathogenesis is the attachment of EBs to host cells, for which a number of mechanisms have been proposed (19, 28, 32, 34). The specificity of the attachment may determine the next step, which is entry of chlamydiae into the target cells and formation of inclusions or vacuoles (12). The mechanism for this is unknown, but this unique process protects EBs from host cellular defence mechanisms (13). This is typical of intracellular pathogens, which have evolved diverse strategies for evasion of host cellular defence mechanisms associated with adaptations for survival in distinct intracellular compartments (18).
Attachment of C. trachomatis to human spermatozoa in vitro was first reported by Wolner-Hanssen and Mardh (31), who used fluorescence and transmission electron microscopy. They reported that the number of EBs attached to spermatozoa was dependent on the concentration of EBs with which the spermatozoa were incubated. Another in vitro study using transmission electron microscopy has suggested the adherence of C. trachomatis to different regions of spermatozoa (23), and there is also one report analyzing patient spermatozoa following chlamydial infection in vivo (17). However, none of these results have conclusively demonstrated the attachment of C. trachomatis to spermatozoa.
Following ejaculation and deposition in the female reproductive tract, human spermatozoa are incapable of fertilization and need to undergo a series of events before fertilization is possible (see reference 33 for a review). Collectively known as capacitation, these events are poorly understood, but it is now well established that tyrosine phosphorylation of sperm proteins is closely associated with capacitation in vitro (reviewed in reference 30). There is some evidence that attachment of C. trachomatis to target cells may also involve interactions with host signal transduction pathways (2, 19). In one study, changes in tyrosine phosphorylation of host-cell proteins could be detected as early as 10 min postinfection (19).
Therefore, to shed further light on possible C. trachomatis-spermatozoon interactions we have investigated whether coincubation results in altered sperm signal transduction. Specifically, we have investigated the effects on human sperm protein tyrosine phosphorylation by immunoblotting and indirect immunofluorescence.
MATERIALS AND METHODS
Semen samples were obtained from the University Research Laboratory (Jessop Hospital, Sheffield, United Kingdom) from proven fertile donors by masturbation. All of the samples were screened and shown to be free from sexually transmitted diseases, including human immunodeficiency virus, in accordance with British Andrology Society guidelines (1). The ejaculates of these donors had a high sperm concentration (>60 × 106 sperm/ml), with >30% ideal morphological forms (by the World Health Organization 1992 criteria) and no evidence of antisperm antibodies. They were also of proven fertility, either with their own partner or through the use of their cryopreserved semen in donor insemination treatment cycles. The samples were also screened for the current presence of C. trachomatis infection by a plasmid PCR (11) and shown to be free from chlamydial infection. Fast, progressively motile spermatozoa were purified and washed by a Percoll gradient technique (24), and the samples which yielded more than 90% motile spermatozoa (swimming at >10 μm s−1) were used and adjusted to 5 × 106 ml−1 in Earle's balanced salt solution (Sigma Chemical Co., Poole, United Kingdom) containing 0.3% (wt/vol) bovine serum albumin.
Spermatozoa were either used immediately (noncapacitated), capacitated on a shaker for 3 h at 37°C in 5% CO2 in air, or capacitated in the presence of C. trachomatis serovar E or LGV. Serovar E was isolated from a clinical source (cervical swab from the Department of Genitourinary Medicine, Royal Hallamshire Hospital, Sheffield, United Kingdom), and strain LGV1 was kindly provided by M. Ward (The University of Southampton). Cultures of these chlamydiae were prepared by growing them in McCoy cells, and chlamydial EBs were washed and purified from infected cells by density gradient centrifugation as previously described (8). EBs were titrated, and 105 infective EBs were incubated with 106 spermatozoa in the experiments. These amounts correspond to <30 ng and approximately 15 μg of protein, respectively, as detected by the Bradford assay (data not shown).
Different patterns of tyrosine phosphorylation of proteins in human spermatozoa were assessed by an indirect immunofluorescence technique. Aliquots of spermatozoa (105) were smeared on precleaned microscope slides, allowed to air dry overnight, fixed in methanol for 45 min, and left to air dry before being incubated at 37°C for 1 h in a wet chamber with antiphosphotyrosine monoclonal antibody clone 4G10 (TCS Biologicals Ltd., Botolph Claydon, United Kingdom; 1:500 dilution in phosphate-buffered saline (PBS, 137 mmol of NaCl liter−1, 2.7 mmol of KCl liter−1, 10 mmol of Na2HPO4 liter−1, 1.76 mmol of KH2PO4 liter−1; pH 7.4)). After being washed twice in PBS (fresh PBS was used each time) the slides were further incubated at 37°C for 30 min with anti-mouse immunoglobulin G fluorescein isothiocyanate conjugate (whole antibody raised in sheep). After further washes the slides were mounted using MOWIOL (Calbiochem)/DABCO [1,4-diazobicyclo-(2,2,2)-octane] (15), and fluorescence patterns on the spermatozoa were assessed by epifluorescence microscopy (Olympus, Tokyo, Japan; BH-2 with UV filter [492 nm], ×100 objective magnification). The results were analyzed by one-way analysis of variance (ANOVA) on the transformed data.
To identify the molecular weight of the tyrosine-phosphorylated proteins, prepared samples were subjected to sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and immunoblotting as previously described (5) but with the following changes. Immediately following incubation (see above), the sperm samples were centrifuged at 8,000 × g for 5 min and the pellet was washed with Tris-buffered saline (20 mM Tris HCl 500 mM NaCl; pH 7.4), resuspended in 40 μl of reducing dissociation buffer, and heated at 100°C for 5 min. Samples were prepared and run in duplicate to confirm that loading was consistent. SDS-polyacrylamide gel electrophoresis was performed using 7.5% (vol/vol) acrylamide resolving gels and prestained SDS-molecular weight marker proteins (10 μl; Sigma Chemical Co.), and a positive control of epidermal growth factor (EGF)-stimulated A431 cell lysate (5 μg; Upstate Biotechnology) was also included. Control samples of EBs only were also prepared (1 × 106 to 1.5 × 106). Immunoblotting onto Immobilon-P polyvinylidene difluoride transfer membrane (Millipore Ltd., Watford, United Kingdom) was performed using a wet blotter run at 400 mA for 90 min.
Following transfer, the blotted membranes were blocked overnight and washed before being incubated overnight with antiphosphotyrosine monoclonal antibody (1:4,000 dilution). After the washing, the membranes were incubated for 90min with anti-mouse immunoglobulin G horseradish peroxidase-linked whole sheep antibody (Amersham Pharmacia Biotech, St. Albans, United Kingdom; 1:1,000 dilution) before being subjected to another series of washes. Antibody localization was visualized using the enhanced chemiluminescence technique (Amersham Pharmacia Biotech), and the blots were exposed to photographic film for 10 s to provide optimal detection of bands (all bands visible but not overexposed) (5). Analysis of the resulting bands on the developed photographic film scanned in transmission mode was achieved using 1D Prime Image Master gel analysis software (Amersham Pharmacia Biotech). The results were analyzed by one-way ANOVA on the transformed data.
RESULTS
Indirect immunofluorescence.
Indirect immunofluorescence assessment revealed that 12.5% of noncapacitated spermatozoa displayed fluorescence (indicating the presence of tyrosine-phosphorylated proteins) and that this increased to 26.8% following in vitro capacitation (P < 0.001) (Table 1). When spermatozoa were capacitated in the presence of serovar E, a significantly greater number of spermatozoa displayed fluorescence than those capacitated without EBs (P < 0.01). However, there was no significant increase for spermatozoa capacitated with serovar LGV (P > 0.05).
TABLE 1.
Effect of C. trachomatis serovars E and LGV on the extent of tyrosine phosphorylation in human spermatozoa assessed by indirect immunofluorescence
| Treatmenta | Proportion of spermatozoa displaying fluorescence (%)b |
|---|---|
| NCap | 12.5 ± 2.0 |
| Cap | 26.8 ± 2.1c |
| Cap+E | 45.7 ± 2.1cd |
| Cap+LGV | 30.8 ± 2.5ce |
NCap, noncapacitated spermatozoa; Cap, capacitated spermatozoa; Cap+E, spermatozoa capacitated with serovar E; Cap+LGV, spermatozoa capacitated with serovar LGV.
The results are presented as means ± standard errors of the means (n = 12, with 100 spermatozoa scored for each treatment). These values were analyzed by one-way ANOVA.
Significantly different from noncapacitated spermatozoa (P < 0.001).
Significantly different from capacitated spermatozoa (P < 0.01).
Not significantly different from capacitated spermatozoa (P > 0.05).
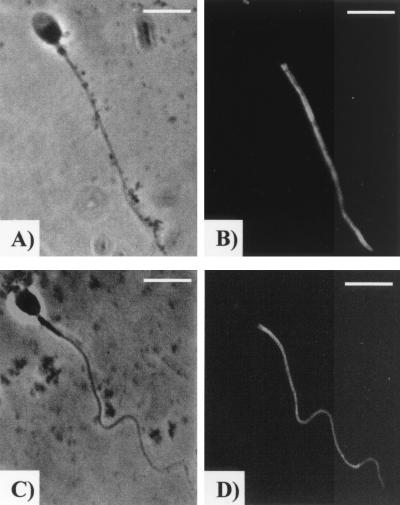
The major immunofluorescence pattern in all of the samples was localized to the sperm tail region (both the principal and end pieces), and this is illustrated in Fig. 1B and D. This represented >90% of patterns observed in all of the samples, and there were no significant differences between the samples. In the minority of spermatozoa that displayed other patterns, tail fluorescence was still observed, but in these instances there was also fluorescence in the midpiece and/or the anterior acrosomal region. Additionally it was observed that fixed spermatozoa incubated with EBs often displayed a bend about halfway down the flagellum (Fig. 1C and D), whereas control sperm were always straight (Fig. 1A and B).
FIG. 1.
Immunolocalization of tyrosine-phosphorylated proteins on human spermatozoa. Displayed are representative examples of the major fluorescence pattern typically observed in capacitated spermatozoa (B) and spermatozoa capacitated in the presence of serovar E (D). Panels A and C are the phase-contrast images for panels B and D, respectively. Bar = 5 μm.
Immunoblotting.
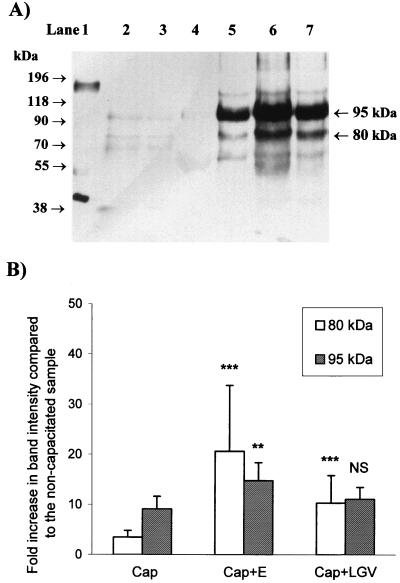
Typical results for the immunoblotting experiments are shown in Fig. 2A, and analysis of this experiment and four others is presented in Fig. 2B. A marked increase in the tyrosine phosphorylation of 80- ± 5- and 95- ± 5-kDa sperm proteins was seen following capacitation. When spermatozoa were capacitated in the presence of serovar E there was a significantly greater increase in the tyrosine phosphorylation of both the 80- and 95-kDa proteins than in that of capacitated spermatozoa (P < 0.01 and P < 0.001, respectively), but for serovar LGV the increase was significant for the 80-kDa protein (P < 0.001) but not for the 95-kDa protein. Results similar to these were obtained when capacitated spermatozoa were incubated for a short (10-min) period with either serovar E or LGV (data not shown). Negative controls containing only serovars E and LGV (10 to 15 times as much as was incubated with spermatozoa) were also included and showed no bands that reacted strongly with the antibody.
FIG. 2.
Effect of C. trachomatis serovars E and LGV on protein tyrosine phosphorylation in human spermatozoa. (A) Typical Western blotting result. Human spermatozoa (106 per lane) were incubated under noncapacitating (lane 4) or capacitating (lanes 5 through 7) conditions in the absence of C. trachomatis (lane 5) or with serovar E (lane 6) or with serovar LGV (lane 7) C. trachomatis. Samples containing only serovar E or LGV (lanes 2 and 3, respectively; 10 to 15 times more than incubated with the spermatozoa) were run as negative controls. A positive control (EGF-stimulated A231 cell lysate containing EGF receptor, 5 μg) was also included (lane 1). The apparent molecular masses of prestained markers are shown to the left of the blot, and the major 95- and 80-kDa epitopes are labeled on the right of the blot. (B) Histogram of the relative intensities of the bands identified in Fig. 1A and four similar experiments. The increase in the relative intensity (background-subtracted integrated optical density, in pixels) of each band compared to the noncapacitated sample is presented (mean ± standard error of the mean, n = 5). The results from the samples incubated in the presence of C. trachomatis were compared with those from the samples incubated under capacitating conditions in the absence of C. trachomatis by one-way ANOVA. ∗∗∗, significantly different from capacitated spermatozoa (P < 0.001); ∗∗, significantly different from capacitated spermatozoa (P < 0.01); NS, not significantly different from capacitated spermatozoa (P > 0.05).
DISCUSSION
There is evidence in the literature to show that chlamydial infection causes increased tyrosine phosphorylation of host cell proteins in cultured cell lines (2, 19). Therefore, the rationale behind our investigations was to establish whether incubation of human spermatozoa with C. trachomatis caused alterations in the normal tyrosine phosphorylation of sperm proteins.
Immunofluorescence studies on aliquots of spermatozoa from the immunoblotting experiments showed tyrosine phosphorylation localized to the tail region in capacitated spermatozoa incubated without EBs. This immunolocalization was the same as that previously reported, and the proportion of spermatozoa displaying fluorescence was also broadly similar (9). Importantly, these studies showed that only serovar E, and not LGV, caused a significant increase in the number of spermatozoa displaying fluorescence.
When tyrosine phosphorylation was assessed by immunoblotting, a significant increase in phosphorylation of the 80-kDa protein was observed with both serovars, but an increase of the 95-kDa protein occurred only in response to serovar E. The fact that spermatozoa incubated with C. trachomatis serovar E in these experiments showed increased protein tyrosine phosphorylation (determined by immunofluorescence and immunoblotting) suggests that this serovar is able to attach to spermatozoa. Interestingly, although both serovars are a cause of genital chlamydial infection initially, serovar E is considered the most prevalent cause of localized genital chlamydial infection (14), whereas LGV is primarily associated with infections of the lymphatic system and is rarely seen in the developed world (25). Furthermore, differences between serovars have also been reported by other workers (19, 34). These findings may therefore reflect differences in the mechanisms of attachment of serovars E and LGV, although further studies are required as these investigations were carried out on only one strain of each serovar.
There is good evidence from previous studies employing immunoblotting that spermatozoa exhibit little or no protein tyrosine phosphorylation in the noncapacitated state and display major 95-kDa and minor 80-kDa phosphotyrosine proteins following capacitation in vitro (5, 9, 16). We also routinely observed similar results in these experiments for the noncapacitated and capacitated sperm samples. This was used as an indication that the spermatozoa were functioning normally, and all of the experiments analyzed displayed this typical pattern. Negative controls containing only serovars E and LGV (10 to 15 times as much as was incubated with spermatozoa) were also included to confirm that the patterns of tyrosine phosphorylation following incubation were the result of C. trachomatis-spermatozoon interactions rather than from EBs alone.
There is a slight possibility that the phosphorylated proteins identified following coincubation are derived from chlamydiae and not spermatozoa. This suggestion could be made given that it was shown in a previous study that a 90-kDa protein that was tyrosine phosphorylated upon incubation with enteropathogenic Escherichia coli was bacterial in origin rather than derived from the host cells (22). However, the following is convincing evidence that the proteins identified in the blotting experiments were derived from spermatozoa rather than chlamydiae. The molecular weight of the proteins with increased tyrosine phosphorylation is the same as those in capacitated spermatozoa. It is very unlikely, but not impossible, that there would be two chlamydial proteins that would coincidentally have the same apparent molecular masses (80 and 95 kDa) as the two sperm proteins which are phosphorylated during capacitation. In the coincubation samples 105 EBs (less than 30 ng of protein) were incubated with 106 spermatozoa (approximately 15 μg of protein). Therefore, in terms of protein present there was at least 500 times more sperm protein than EB protein. In our hands the amount of EB protein present in these samples, even if it was all tyrosine phosphorylated, is beyond the detection limit of this assay under the conditions used and could not account for the marked changes observed in the tyrosine phosphorylation of the 80- and 95-kDa bands. It should be stressed that the lanes loaded only with EBs contained 10 to 15 times as many EBs as the coincubation samples and that the faint bands observed were possible as this would be close to the limit of detection. Finally, the fact that increased tyrosine phosphorylation was observed on spermatozoa localized to the tails as shown by immunofluorescence detection is also good evidence that the increased tyrosine phosphorylation is caused by sperm proteins rather than chlamydia.
Since successful conception in vivo relies upon an appropriately capacitated population of spermatozoa being present at the site of fertilization once ovulation has occurred (21), any factor which may prematurely capacitate spermatozoa may lead to a failure of conception. As tyrosine phosphorylation is closely associated with capacitation, chlamydial infection in either partner could therefore lead to a sperm function-mediated cause of infertility by the premature tyrosine phosphorylation of spermatozoa.
Fully capacitated human spermatozoa display a distinctive vigorous hyperactivated motility which increases their thrust (6). This is thought to be important for releasing spermatozoa from contact with the endosalpinx of the Fallopian tube (26) and is probably necessary for penetration of the zona pellucida (33). The increased tyrosine phosphorylation observed following incubation with serovar E is interesting and suggests that chlamydial infection might also affect sperm motility due to the localization to the tail. In addition, the observation that those spermatozoa incubated with serovar E displayed structurally altered (twisted) tails also suggests that serovar E might affect sperm motility. It is technically difficult to analyze sperm motility in detail, but we are addressing this aspect in further investigations. However, previous studies have suggested that chlamydial infection in men may reduce the motility of human spermatozoa as observed in semen (10). These results also suggest that chlamydial infection in the female partner could interfere with subtle changes in motility, such as the onset of hyperactivation within the Fallopian tubes.
Finally, the identity of the 80- and 95-kDa sperm proteins which are tyrosine phosphorylated in response to chlamydiae is currently unknown. Interestingly, proteins of similar molecular weight were also phosphorylated following chlamydial infection of cultured cell lines (2, 19). Whether these represent proteins common to both spermatozoa and the cultured cell lines will require further investigation. It is also possible that the bands identified represent several different proteins of similar molecular weight (5). As well as being tyrosine phosphorylated following in vitro capacitation, there is also controversial evidence showing that a 95-kDa sperm protein is tyrosine phosphorylated in the presence of egg extracellular matrix (zona pellucida) proteins at fertilization (5, 7, 27). As such, this 95-kDa tyrosine kinase receptor is one of the best candidates for the key sperm protein involved in gamete recognition and sperm activation at fertilization (4). It is therefore possible to speculate that chlamydiae might attach these essential moieties and activate signal transduction pathways normally involved in triggering the sperm acrosome reaction during sperm activation. Such premature sperm activation would prevent normal sperm-egg interactions at fertilization and might therefore also be a significant factor in infertility caused by chlamydiae.
In conclusion, these experiments have provided indirect evidence to suggest that C. trachomatis serovar E may attach to human spermatozoa and also may influence the function of human spermatozoa within the female reproductive tract. As such it could be speculated that this represents an additional method by which chlamydial infection might lead to infertility and clearly warrants further investigation.
ACKNOWLEDGMENT
We thank the staff of the University Research Laboratories at the Jessop Hospital for Women for their assistance in obtaining donor semen samples for these experiments.
REFERENCES
- 1.Barratt C L, Matson D L, Holt W. British Andrology Society guidelines for the screening of semen donors for donor insemination. Hum Reprod. 1993;8:1521–1523. doi: 10.1093/oxfordjournals.humrep.a138291. [DOI] [PubMed] [Google Scholar]
- 2.Birkelund S, Johnsen H, Christiansen G. Chlamydia trachomatis serovar L2 induces protein tyrosine phosphorylation during uptake by HeLa cells. Infect Immun. 1994;62:4900–4908. doi: 10.1128/iai.62.11.4900-4908.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black C M. Current methods of laboratory diagnosis of Chlamydia trachomatis infections. Clin Microbiol Rev. 1997;10:160–184. doi: 10.1128/cmr.10.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brewis I A, Moore H D M. Molecular mechanisms of gamete recognition and fusion at fertilization. Hum Reprod. 1997;12(Natl. Suppl. J. Br. Fertil. Soc. 2):156–165. [PubMed] [Google Scholar]
- 5.Brewis I A, Clayton R, Browes C E, Martin M, Barratt C L R, Hornby D P, Moore H D M. Tyrosine phosphorylation of a 95 kDa protein and induction of the acrosome reaction in human spermatozoa by recombinant human zona pellucida glycoprotein 3. Mol Hum Reprod. 1998;4:101–109. doi: 10.1093/molehr/4.12.1136. [DOI] [PubMed] [Google Scholar]
- 6.Burkman L J. The motility of human spermatozoa before and after capacitation. In: Grudzinskas J G, Yovich J L, editors. Gametes—the spermatozoon. Cambridge, United Kingdom: Cambridge University Press; 1995. pp. 122–139. [Google Scholar]
- 7.Burks D J, Carballada R, Moore H D M, Saling P M. Interaction of a tyrosine kinase from human sperm with the zona pellucida at fertilisation. Science. 1995;269:83–86. doi: 10.1126/science.7541556. [DOI] [PubMed] [Google Scholar]
- 8.Caldwell H D, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981;31:1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carrera A, Moos J, Ning X P, Gerton G L, Tesarik J, Kopf G S, Moss S B. Regulation of protein tyrosine phosphorylation in human sperm by a calcium/calmodulin-dependent mechanism: identification of a kinase anchor proteins as major substrates for tyrosine phosphorylation. Dev Biol. 1996;180:284–296. doi: 10.1006/dbio.1996.0301. [DOI] [PubMed] [Google Scholar]
- 10.Cengiz T, Aydoganli L, Baykam M, Mungan N A, Tuncbilek E, Dincer M, Yakupoglu K, Akalin Z. Chlamydial infection and male infertility. Int Urol Nephrol. 1997;29:687–693. doi: 10.1007/BF02552187. [DOI] [PubMed] [Google Scholar]
- 11.Claas H C, Melchers W J, de Bruijn I H, de Graaf M, van Dijk W C, Lindeman T. Detection of Chlamydia trachomatis in clinical specimens by the polymerase chain reaction. Eur J Clin Microbiol Infect Dis. 1990;9:864–868. doi: 10.1007/BF01967500. [DOI] [PubMed] [Google Scholar]
- 12.Eissenberg L G, Wyrick P B. Inhibition of phagolysosome fusion is localized to Chlamydia psittaci-laden vacuoles. Infect Immun. 1981;32:889–896. doi: 10.1128/iai.32.2.889-896.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eissenberg L G, Wyrick P B, Davis C H, Rumpp J W. Chlamydia psittaci elementary body envelopes: ingestion and inhibition of phagolysosome fusion. Infect Immun. 1983;40:741–751. doi: 10.1128/iai.40.2.741-751.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eley A, Khalili M, Abbott M. Epidemiology of Chlamydia trachomatis using nested PCR. Genitourin Med. 1993;69:239–243. doi: 10.1136/sti.69.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellis D H, Hartman T D, Moore H D M. Maturation and function of the hamster spermatozoon probed with monoclonal antibodies. J Reprod Immunol. 1985;7:695–711. doi: 10.1016/0165-0378(85)90025-7. [DOI] [PubMed] [Google Scholar]
- 16.Emiliozzi C, Fenichel P. Protein tyrosine phosphorylation is associated with capacitation of human sperm in vitro but is not sufficient for its completion. Biol Reprod. 1997;56:674–679. doi: 10.1095/biolreprod56.3.674. [DOI] [PubMed] [Google Scholar]
- 17.Erbengi T. Ultrastructural observation on the entry of C. trachomatis into human spermatozoa. Hum Reprod. 1993;3:416–421. doi: 10.1093/oxfordjournals.humrep.a138063. [DOI] [PubMed] [Google Scholar]
- 18.Falkow S, Isberg R R, Portnoy D A. The interaction of bacteria with mammalian cells. Annu Rev Cell Biol. 1992;8:333–363. doi: 10.1146/annurev.cb.08.110192.002001. [DOI] [PubMed] [Google Scholar]
- 19.Fawaz F S, van Ooij C, Homola E, Mutka S C, Engel J N. Infection with Chlamydia trachomatis alters the tyrosine phosphorylation and/or localization of several host cell proteins including cortactin. Infect Immun. 1997;65:5301–5308. doi: 10.1128/iai.65.12.5301-5308.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gutierrez-Martin C B, Ojcius D M, Hsia R C, Hellio R, Bavoil P M, Dautry-varsat A. Heparin-mediated inhibition of Chlamydia psittaci adherence to HeLa cells. Microb Pathog. 1997;22:47–57. doi: 10.1006/mpat.1996.0090. [DOI] [PubMed] [Google Scholar]
- 21.Hunter R H F. Ovarian regulation of sperm progression in the Fallopian tubes. Zygote. 1994;2:363–366. doi: 10.1017/s0967199400002227. [DOI] [PubMed] [Google Scholar]
- 22.Kenny B, De Vinney R, Stein M, Reinscheid D J, Frey E A, Finlay B B. Enteropathogenic E.coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell. 1997;91:511–520. doi: 10.1016/s0092-8674(00)80437-7. [DOI] [PubMed] [Google Scholar]
- 23.Mavrov G I. An electron microscopic investigation of interaction between Chlamydia trachomatis and human spermatozoa. Mikrobiol Zlt. 1995;57:74–79. [PubMed] [Google Scholar]
- 24.Moohan J M, Lindsay K S. Spermatozoa selected by a discontinuous percoll density gradient exhibit better motion characteristics, more hyperactivation, and longer survival than direct swim-up. Fertil Steril. 1995;64:160–165. [PubMed] [Google Scholar]
- 25.Moulder J W. Interaction of chlamydiae and host cells in vitro. Microbiol Rev. 1991;55:143–190. doi: 10.1128/mr.55.1.143-190.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pacey A A, Davies N, Warren M A, Barratt C L R, Cooke I D. Hyperactivation may assist human spermatozoa to detach from intimate association with the endosalpinx. Hum Reprod. 1995;10:2603–2609. doi: 10.1093/oxfordjournals.humrep.a135754. [DOI] [PubMed] [Google Scholar]
- 27.Saling P, Burks D J, Tomes C N. Sperm-zona pellucida interaction: a model for zona receptor kinase-mediated signaling. In: Hansson V, Levy F D, Tasken K, editors. Signal transduction in testicular cells. Berlin, Germany: Springer-Verlag KG; 1996. pp. 247–270. [Google Scholar]
- 28.Stamm W E. Chlamydia trachomatis infections: progress and problems. J Infect Dis. 1999;179:S380–S383. doi: 10.1086/513844. [DOI] [PubMed] [Google Scholar]
- 29.Stephens R S. Molecular mimicry and Chlamydia trachomatis infection of eukaryotic cells. Trends Microbiol. 1994;2:99–101. doi: 10.1016/0966-842x(94)90543-6. [DOI] [PubMed] [Google Scholar]
- 30.Visconti P E, Kopf G S. Regulation of protein phosphorylation during sperm capacitation. Biol Reprod. 1998;59:1–6. doi: 10.1095/biolreprod59.1.1. [DOI] [PubMed] [Google Scholar]
- 31.Wolner-Hanssen W H, Mardh P-A. In vitro tests of the adherence of Chlamydia trachomatis to human spermatozoa. Fertil Steril. 1984;42:102–107. doi: 10.1016/s0015-0282(16)47966-5. [DOI] [PubMed] [Google Scholar]
- 32.Wyrick P B. Cell biology of chlamydial infection: a journey in the host epithelial cell by the ultimate cellular microbiologist. In: Stephens R S, et al., editors. Chlamydial infections. Proceedings of the Ninth International Symposium on Human Chlamydial Infection. San Francisco, Calif: International Chlamydia Symposium; 1998. pp. 69–78. [Google Scholar]
- 33.Yanagimachi R. Mammalian fertilisation. In: Knobil E, Neill J, editors. The physiology of reproduction. 2nd ed. New York, N.Y: Raven Press Ltd.; 1994. pp. 189–317. [Google Scholar]
- 34.Zhang J P, Stephens R S. Mechanism of C. trachomatis attachment to eukaryotic cells. Cell. 1992;69:861–869. doi: 10.1016/0092-8674(92)90296-o. [DOI] [PubMed] [Google Scholar]