Abstract
Background:
The purpose of this review article is to understand tooth root development and its regulation through evolution and epigenetics as well as future implications involving root regeneration and tissue engineering.
Types of Studies Reviewed:
we performed a comprehensive PubMed search to review all published studies related to the molecular regulation of tooth root development and regeneration until August 2022. Articles selected include original research studies and reviews.
Results:
Epigenetic regulation strongly influences dental tooth root patterning and development. One study highlights how genes such as Ezh2 and Arid1a are crucial components in the development of tooth root furcation patterning. Another study shows that loss of Arid1a ultimately leads to shortened root morphology. Furthermore, researchers are utilizing information about root development and stem cells to find alternative treatments in replacing missing teeth through a stem cell-mediated bioengineered tooth root (bio-root).
Practical Implications:
Dentistry values preserving natural tooth morphology. Presently, implants are the best treatment for replacing missing teeth, but alternative future treatments might include tissue engineering/bio-root regeneration to restore our dentition.
Keywords: Molecular Regulation of Root Development, Regenerative Medicine, Genes, Tooth Root
Introduction
The dentition within the human mouth is important for everyday physiological functions, playing roles in speech, expression, and mastication. Each tooth is comprised of two major anatomical components, the crown and the root. Though each have a critical purpose, the root is essential for the proper function of the dentition because it acts as an anchor for the tooth within the maxilla or mandible. Consequently, the loss of a tooth root leads to lessened bone support and, therefore, disruption of the tooth’s function.1 Maintaining the natural dentition and preventing tooth loss is the primary responsibility of dentists.2 Furthermore, though a natural crown may be replaced by an artificial one, the root of a tooth is the most important part functionally.3 This is because during mastication and resting states, the root transmits occlusal forces through the periodontal ligament to the jaw bone. Additionally, the root provides a passageway for the neurovascular bundle that supplies nutrition, sensation, and blood flow to the tooth in order to maintain dental tissue homeostasis.1
The loss of natural teeth can have physical and mental consequences with adverse effects on the quality of life.4 Recently, researchers have sought to understand the molecular regulatory mechanisms that underlie tooth root development, as well as the epigenetic mechanisms that have led to changes in root morphology during the course of human evolution. Although the molecular regulation of early tooth development, which leads to crown formation, has been the subject of numerous studies, researchers are still seeking to understand how root development is regulated.5 Understanding the epigenetic regulation of root development can provide clinicians with valuable insights, in addition to laying the foundation for future treatment strategies to replace missing teeth through strategies such as stem cell-based dental regeneration. As it stands, implant dentistry is currently the most popular method for replacing missing teeth. Single-tooth dental implants have proven to be very successful long-term, but their success depends upon the presence of high-quality bony architecture.4 This is because implants are anchored to the surrounding bone through osseointegration. This also means that implants lack a periodontal ligament interface with the bone and cannot provide biofeedback through periodontal mechanoreceptors or nerve innervation. This lack of sensorimotor regulation can lead to implant fractures, especially for patients with multiple-unit implant restorations.6 Though the complexities of the dentition and current lack of strategies to successfully induce eruption of engineered dental tissues have thus far precluded the regeneration of an entire tooth, the regeneration of a living tooth root (bio-root) is a promising alternative to dental implants that can anchor either a natural or post-supported crown.4
This review article discusses root development, morphology, and function and how the tooth root has evolved over time through epigenetic regulation. We will then discuss how researchers are using this information on root development and epigenetics to work towards a future where clinicians might be able to restore teeth through tissue engineering and bio-root regeneration.
Root Development, Morphology, Morphogenesis and Function
In order to understand the evolution and epigenetic regulation of tooth roots, we must first take a look at the primary functional purpose of tooth roots, after which we will discuss how tooth roots develop morphologically.
Tooth roots serve multiple functions. Attachment of the tooth to the surrounding jawbone is one of the most important roles and is achieved by a specialized support apparatus consisting of periodontal ligament (PDL), cementum and alveolar bone. The PDL is a highly specialized connective tissue that communicates between the tooth and the alveolar bone. The main purpose of the PDL is to affix the tooth to the jaw while also allowing the tooth to withstand significant masticatory forces. The PDL-cementum-bone framework additionally provides important sensory function, allowing us to feel when something contacts our teeth.7 This sensorimotor regulation provides the brain with feedback that ultimately controls masticatory movements and forces. The receptors located within the PDL that transmit mechanical stimuli are known as mechanoreceptors. During mastication, the motor output is adjusted based on these somatosensory signals from the PDL to the brain. When chewing hard substances, the body overcomes the resistance of the food by increasing jaw muscle activity and optimizing the bite forces to the location of the food relative to the teeth.6 In addition to the peripheral sensory nerves that allow for sensorimotor regulation, the tooth root serves to provide the tooth with blood vessels, oxygen supply, nutrients, and the ability to dispose of waste products.8 This neurovascular bundle located within the PDL is surrounded by stem cells that act in response to mechanical forces, providing the tooth with the ability to undergo tissue healing and repair.9
Tooth development begins during the sixth week of gestation and involves a cascade of epithelial–mesenchymal interactions and a series of morphological stages.10 Following crown formation, the development of the root begins in the advanced bell stage once the enamel and dentin form to the point of the future cementoenamel junction. Here, the enamel organ forms Hertwig’s epithelial root sheath (HERS), which determines the shape of the roots while initiating dentinal formation. HERS is comprised of inner and outer enamel epithelia and contains cells that are responsible for inducing connective tissue cells to differentiate into odontoblasts. After the odontoblasts deposit the first layer of dentin, the epithelial root sheath incompletely disintegrates. The remnants of HERS persist as the epithelial rests of Malassez in the periodontal space.11
The development of HERS within single-rooted teeth such as incisors is remarkably dissimilar from its development within multi-rooted teeth such as molars or premolars. Prior to root formation, HERS forms an epithelial diaphragm. Here, the bilayered enamel epithelia turn inwards towards the eventual cementoenamel junction. This movement narrows the cervical opening of the tooth germ and lengthens the root sheath, which then signals the differentiation of odontoblasts and formation of dentin. Simultaneously, the connective tissue of the dental sac enveloping the root sheath proliferates and proceeds to break down the epithelial bilayer into a meshwork of epithelial strands. Next, the epithelium moves away from the surface of the dentin such that connective tissue cells come into contact with the outer surface of the dentin, where they differentiate into cementoblasts and deposit a layer of cementum. Histologically, HERS does not appear as a continuous layer on the developing surface of the root due to this rapid proliferation and destruction. Late in root development, the proliferation of the epithelial diaphragm is less rapid than that of the pulp connective tissue. The apical foramen narrows, remaining only as wide as the diaphragmatic opening, and is later reduced further by the apposition of cementum and dentin at the root’s apex.11
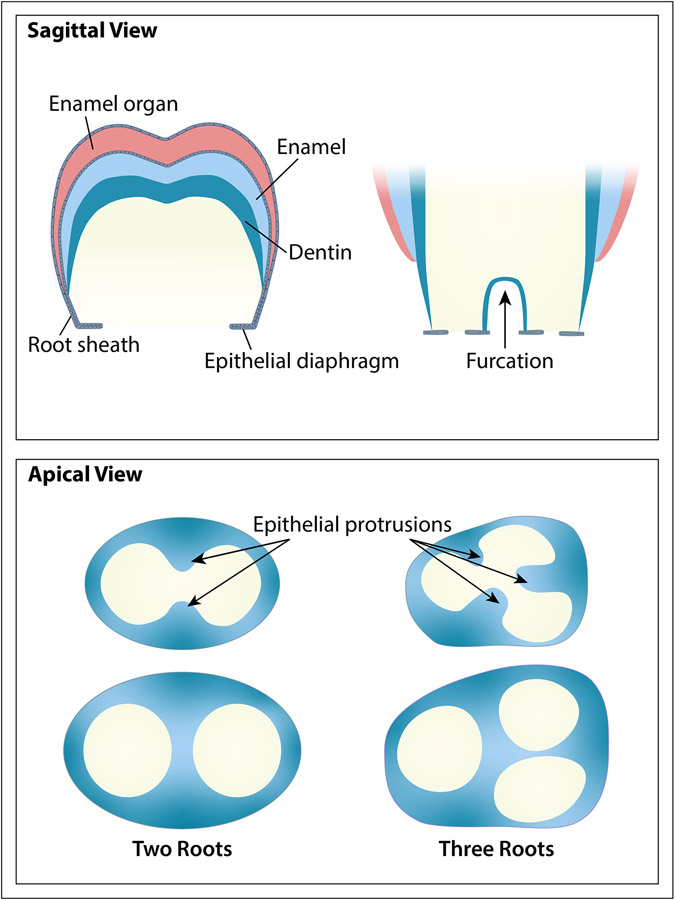
In multi-rooted teeth, HERS plays a significant role in determining the number of roots that form through differential growth of the epithelial diaphragm, which results in the root trunk dividing into two or three branches (See figure 1). As the enamel develops, the cervical opening expands and the diaphragm forms long, horizontal epithelial flaps: two in the mandibular and three in the maxillary molars. Prior to the division of the root trunk, the ends of these flaps grow towards one another and ultimately fuse, such that there are now two or three cervical openings. Dentin starts to form on the pulpal surface of each of the dividing epithelial bridges, and root formation proceeds from each of the openings in the same way as described above for single-rooted teeth.11
Figure 1: Tooth Root Furcation Development.
This figure shows schematics of tooth root furcation development in two rooted molars and three rooted molars from a sagittal and apical view. From the sagittal view, the outer two layers represent the epithelium-derived tissues including enamel and HERS. The teal blue inner layer represents dentin. The apical view reveals how the epithelial protrusions eventually develop into the bifurcation or trifurcation.
Defects in tooth root formation can occur due to misplacement of HERS or disruptions to its development. For instance, enamel pearls might form on the root surface when HERS cells remain adherent to the dentinal surface, causing differentiation into enamel-producing ameloblasts. These enamel pearls are often found in the root furcation area of permanent molars. Additionally, if the continuity of HERS is not well established, or if it is broken prior to dentin formation, a defect can form in the dentinal wall. These defects can be found anywhere on the root. Practitioners performing endodontic therapy might recognize these defects clinically as accessory canals that open to the periodontal surface of the root.11
Molecular Regulation of Tooth Root Development
Though we have an understanding of how tooth roots develop, it was not until recently that researchers began investigating exactly why tooth roots develop the way that they do. For instance, how does the body know to signal the development of a three-rooted molar vs. a one-rooted incisor?
There has been considerable study of the molecular regulation of early tooth morphogenesis, which leads to crown formation. A large body of work has revealed that crown development is regulated by several major signaling pathways, including the transforming growth factor beta (TGF-β), bone morphogenic protein (Bmp), fibroblast growth factor (Fgf), wingless/integrated (Wnt) and sonic hedgehog (Shh) pathways, which act at different stages of early tooth morphogenesis.1 Additionally, it is known that epithelial-mesenchymal interactions are essential for root development and for the integration of the root with the jawbone. As HERS guides root development, the cranial neural crest-derived mesenchyme forms the dental follicle and dental papilla. The inner layer of HERS then interacts with the mesenchyme of the apical papilla to differentiate into dentin-forming odontoblasts.5 Additionally, HERS interacts with the dental follicle which produces the PDL, cementum, and surrounding alveolar bone. Root development defects can occur due to disruptions in the interactions between HERS and the dental follicle or dental papilla. However, the exact network that controls the genetic and epigenetic regulation of root formation and later stages of tooth development more generally is still a subject of investigation.1
Recent studies have revealed important epigenetic mechanisms that regulate tooth root development. Specifically, one study examining root patterning and development in mice found that antagonistic interaction between the epigenetic regulators Ezh2 and Arid1a is required for the patterning of the root furcation. Ezh2 is a gene that is known to be involved in facial bone formation, but it was not previously known that this gene specifically regulates tooth root development. This study found that loss of Ezh2 causes mouse molars to form an improper number of roots while also causing the furcation area to develop incorrectly. It was also found that another gene, Arid1a, which regulates the cell fate and differentiation of different populations including hematopoietic stem cells, embryonic stem cells, intestinal stem cells and cardiac progenitor cells, opposes the function of Ezh2. Based on this finding, the researchers concluded that epigenetic regulation needs to be delicately balanced in order for tooth root patterning and development to occur properly.5 Another study exemplified the importance of epigenetic regulation by demonstrating that loss of Arid1a can lead to shortened tooth roots by regulating Hh signaling in a way that affects the differentiation-associated cell cycle arrest of tooth root progenitors.12
Runt-related transcription factor 2 (RUNX2) is an additional subject of research. This gene encodes a transcription factor primarily known for its role in tooth development and osteogenesis. In humans, mutations of RUNX2 can lead to cleidocranial dysplasia (CCD), an autosomal dominant syndrome that affects the bones and teeth. Affected individuals may have short stature, atypical clavicle formation, delayed cranial suture closure, and dental anomalies such as supernumerary teeth and delayed tooth eruption. A recent study discovered that RUNX2 is expressed within tooth root progenitor cells, and loss of this expression adversely affects root development.13
Potential Evolutionary Impact
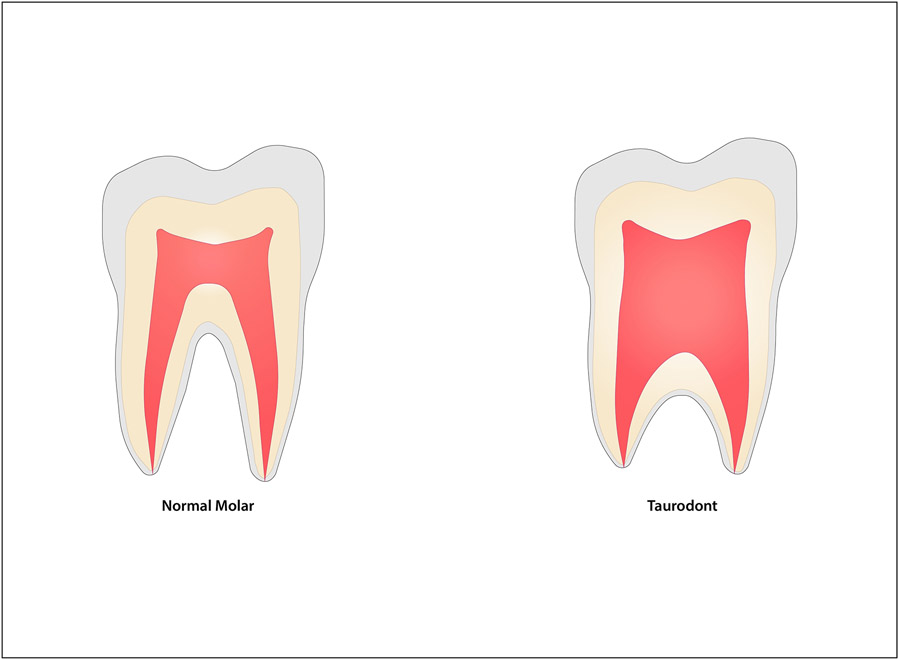
It is clear, not only from a developmental perspective but also from an evolutionary one, that epigenetic regulation is crucial for root patterning and development.5 Neanderthals, the closest extinct relatives to anatomically modern humans, had distinct dental features.14 The tooth roots of Neanderthals exhibit a taurodont phenotype (See figure 2) with an apically displaced root trunk and later binary or ternary branching of the roots. Jing and colleagues saw the emergence of this taurodont phenotype in mice lacking expression of Ezh2 during dental development, indicating the potential role of the human homolog of this gene in the evolution of human teeth.5
Figure 2: Normal Tooth Root vs. Taurodont.
On the left, this schematic represents the normal tooth root morphology for a mandibular molar. On the right, the taurodont tooth displays an apically displaced furcation with an elongated root trunk and pulp chamber.
The mandibular premolars offer an additional perspective on how tooth roots have evolved over time. One study reported that mandibular first premolars in Kenyan specimens of Paranthropus boisei and Australopithecus afarensis, both extinct members of the Hominidae family, show a strong, more circularly shaped mesial/buccal root alongside a large, “plate-like” distal root. In these same hominins, the mandibular second premolars appear more reminiscent of a mandibular molar, with strong mesial and distal roots. More generally in the Pan-Homo clade, which includes chimpanzees and hominins, multi-rooted mandibular premolars are considered the normal root phenotype.15 We speculate that the root attachment area has adapted over time due to the differing occlusal loads and changes in diet during human evolution. For example, primates that exhibit larger root surfaces are known to feed on more mechanically resistant foods. Similarly, Neanderthals are considered to have been primarily carnivores based on studies of the microwear signatures of their molar occlusal surfaces.14 There is also an evolutionary trend in primates to shorten the face and jaws, likely due to an adaptation to arboreal life and the ability of the prehensile hand to grasp food to transport to the oral cavity.16,17 It’s also hypothesized that the well-separated dental roots in modern humans exist to stabilize the dentition while accommodating for the shorter jaw.5
Though more research about the genetic and epigenetic regulation of tooth roots is needed, understanding of how tooth root patterning and development are regulated can provide us with intriguing clues about human evolution in the past as well as possible therapeutic strategies for tooth regeneration in the future.
Tooth Root Regeneration
Knowing the importance of the tooth root and the role of epigenetic regulation, we will now discuss how researchers are utilizing stem cell-based regenerative strategies and tissue engineering with dental follicle progenitor/stem cells to regenerate orofacial tissues such as bio-roots. Though we know epigenetic regulation plays a role in these regenerative strategies, more research is needed to understand the exact mechanisms.
Current research seeks to understand how different types of stem cells may aid in the advancement of regenerative dental medicine. The dental follicle is one subject of interest. The dental follicle is a connective tissue that surrounds the enamel organ and dental papilla throughout the beginning stages of tooth development. The dental follicle has its origins in the cranial neural crest and is capable of giving rise to periodontal tissues including alveolar bone, cementum and PDL. As research has advanced, growing attention has been drawn to a population of mesenchymal stem cells that resides within the dental follicle. These cells are known as dental follicle progenitor/stem cells, and they are a type of dental mesenchymal stem cells capable of multipotent differentiation.18,19 Dental follicle progenitor/stem cells are promising candidates for regenerative medicine and tissue engineering due to their many advantages over mesenchymal stem cells from other origins. For starters, dental follicle progenitor/stem cells can be easily accessed and detached from unimpacted third molars. Though the third molars need to be extracted, this is a routine, minimally invasive procedure that is typically harmless to normal dentition. Second, dental follicle progenitor/stem cells have strong differentiation potential, as they are adult stem cells that can be acquired from developing tissue. Finally, studies have shown that dental follicle progenitor/stem cells have a higher proliferation capability than dental pulp stem cells. Due to their high pluripotency, dental follicle progenitor/stem cells can differentiate into cementoblasts, adipocytes, PDL cells, osteocytes, adipocytes and neuronal cells, suggesting they may find multiple important tissue engineering applications in the future.18,19
Stem cell–based strategies for tissue engineering offer the promise of alternative treatment strategies for oral health care clinicians to replace tissues lost to trauma, congenital defects, or disease. Recent headway in this field of research has facilitated the regeneration of living teeth. Due to the complex structure of teeth and the current lack of successful strategies for inducing tooth eruption, it has been very challenging to regenerate an entire tooth including both crown and root. However, regeneration of the tooth root alone might be a feasible strategy in the future. As previously mentioned, the root serves the important function of anchoring the tooth to the jawbone, and a regenerated root could be used to fulfil this role in support of an artificial crown. Researchers have been able to utilize allogeneic dental stem cells to form a fully functional bioengineered tooth root (bio-root) in a miniature pig without eliciting immune rejection.4
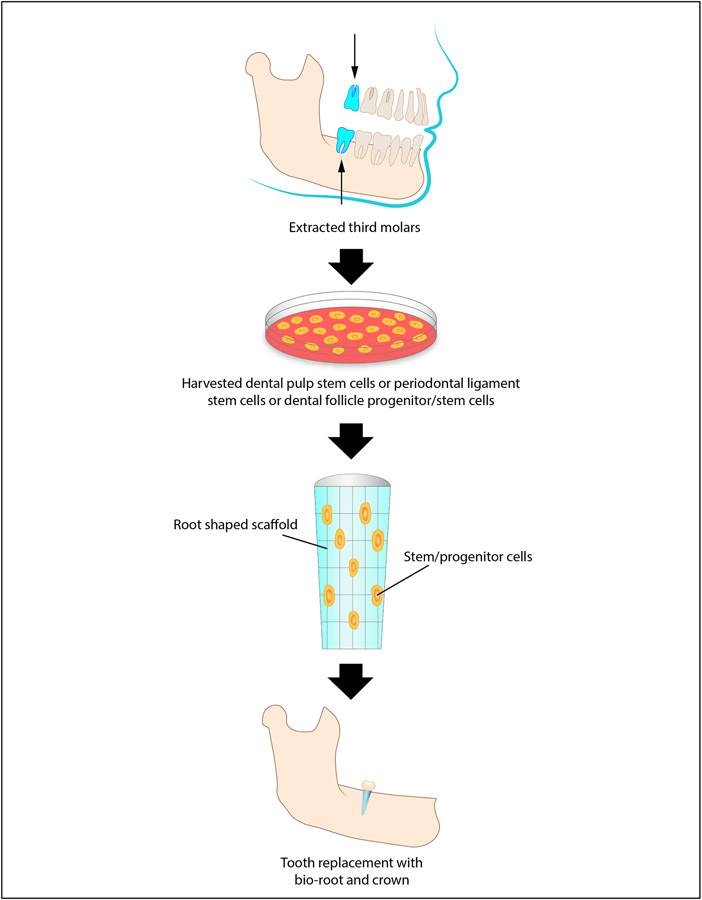
In one study, researchers utilized a miniature pig model to demonstrate the clinical potential of bio-roots as compared to implants for restoring missing teeth. Utilizing a scaffold of periodontal ligament stem cells (PDLSCs) and dental pulp stem cells (DPSCs), a tissue engineering approach (See figure 3) was used to methodically construct the bio-root. Six months after implantation of the bio-root scaffold, the regenerated tissue demonstrated a dentin-like, hard matrix structure as well as a PDL-like structure, confirming that these tissue engineering protocols could indeed regenerate a functional bio-root.4
Figure 3: Bio-Root Tissue Engineering.
This schematic represents bio-root tissue engineering. First, third molars are extracted. Next, stem cells are harvested from the extracted tooth including dental pulp stem cells, periodontal ligament stem cells or dental follicle progenitor/stem cells. Afterwards, a tooth root shaped scaffold is formed with stem cells positioned throughout. Finally, the scaffold can be used to engineer a biocompatible bio-root within the mandible or maxilla. Once the bio-root has developed, tooth replacement can be achieved with a crown.
Future Perspectives in Dental Tissue Engineering
Dental implants are commonly used for replacing missing teeth in today’s clinical dentistry. Single-tooth dental implants have proven to be very successful long-term, however, high-quality bone is a prerequisite for implant placement. Many patients with missing teeth have bony defects and/or poor bone quality, and therefore necessitate bone reconstruction through oral surgery prior to implantation. In addition, implant failures are known to occur due to concentrated stress on the implant. This stress can be attributed to a mismatch between the mechanical properties of the metal implant and those of the natural bone, which can cause bone resorption and ultimately implant failure. Stem cell-mediated root regeneration instead offers clinicians the ability to maintain a physiologically more naturally functioning tooth by regenerating the bio-root and its associated periodontal tissues, providing a biological alternative to dental implants. 4
Following the discovery of the bio-root, researchers continued to compare the relevant mechanical properties of bio-roots with those of conventional dental implants, including compressive strength, modulus of elasticity, and torsional force. Compressive strength is relevant to the ability to bear occlusal force. The modulus of elasticity measures the ability of a structure to resist deformation elastically upon the application of force. Torsional force is typically defined in evaluating the osseointegration of implants as the greatest rotational force that an implant can endure before it is extracted from bone. These biomechanical properties are all critical for implant restorations because uncoupled mechanics between the implant and bone can lead to implant failure or alveolar ridge resorption.4
Bio-roots appear superior to dental implants insofar as their elemental composition and biomechanical properties are more similar to natural tooth roots in terms of compressive strength, modulus of elasticity and torsional force. Dental implants have properties relatively unlike natural tooth roots, with a much higher compressive strength, a higher modulus of elasticity, and a higher torsional force. From a histological standpoint, the periodontal tissues and dentin of the regenerated bio-root also appeared very similar to their natural counterparts.4 Although these results demonstrate that regeneration of a tissue-engineered bio-root is possible using this strategy, the success rate remains far lower than what is typically achieved for dental implants. Reasons for the failure of bio-root regeneration are likely multifaceted. A more optimal scaffold may be needed for this specific dental application. There is also some difficulty in maintaining dental stem cell activity after implantation, and other factors (both known and yet to be discovered) may hinder the regenerative process. With improvements in the scaffold, stem cell quality control, surgical techniques, and overall tissue engineering strategy, it is possible that bio-roots will be used as a competitive alternative treatment strategy for missing teeth in the future.4 It certainly offers a more biological solution for replacing missing teeth.
Another stem cell population of interest for dental clinicians is that of the deciduous dental pulp. These stem cells offer high pluripotency paired with a remarkable capability for cell proliferation. One study demonstrated how these stem cells can salvage immature permanent teeth after injury. Generally, when an immature developing tooth becomes necrotic due to trauma or carious exposure, apexification is indicated. In this study, researchers compared treatment methods for patients with pulpal necrosis following traumatic dental injury. One group received traditional apexification treatment while the other group received treatment with human deciduous pulp stem cells. The researchers demonstrated that implantation with the human deciduous pulp stem cells, but not apexification therapy, resulted in regeneration of pulpal tissue complete with sensory nerves and blood vessels at 12 months post-treatment. Additionally, the roots of the teeth treated with stem cells increased in length and the apical foramen was reduced in size when compared to the group that received apexification. The study concluded that human deciduous pulp stem cells are capable of regenerating dental pulp and may be a promising therapy in the future for treating traumatic tooth injuries.20
In conclusion, tooth root development and morphology are orchestrated by an intricate network of genetic and epigenetic regulators. Epigenetic mechanisms play a key role in stem cell-based regeneration. Dentistry places a strong value on preserving natural tooth root morphology to protect physiological functions of our dentition. Presently, implants are the treatment of choice for replacing missing teeth, but implants require pre-existing, high-quality bony architecture in which to anchor. Additionally, metal implants are a mechanical solution, not a biological one, and do not function similarly to a normal tooth with the associated periodontal apparatus that is crucial for sensation and mechanotransduction. Though more research is needed, the standard of care in the future might involve biological solutions made possible by tissue engineering and dental stem cell-mediated bio-root regeneration.
Acknowledgements
We would like to express our gratitude to Dr. Bridget Samuels for thoughtfully reading and critiquing this manuscript; and Kimi Nakaki for her contributions in developing the artwork.
Funding
This work was supported by the National Institute of Dental and Craniofacial Research, National Institutes of Health (R01 DE012711, R01 DE022503).
Biography
Natalie Black
Natalie is a fourth-year dental student and member of the DDS Class of 2023 at the Herman Ostrow School of Dentistry at the University of Southern California. She partakes in research at the USC Center for Craniofacial Molecular Biology within Dr. Yang Chai’s laboratory. She is passionate about endodontics, sustainability within dentistry, and dental education.
Contributor Information
Natalie Black, University of Southern California Herman Ostrow School of Dentistry, Los Angeles, California UNITED STATES.
Yang Chai, Center for Craniofacial Molecular Biology at the University of Southern California Herman Ostrow School of Dentistry, Los Angeles, California UNITED STATES.
References
- 1.Li J, Parada C, & Chai Y (2017). Cellular and molecular mechanisms of tooth root development. Development (Cambridge, England), 144(3), 374–384. 10.1242/dev.137216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torabinejad M, Fouad AF, Shabahang S, & Walton RE (2021). Preface. In Endodontics: Principles and practice (6th ed., pp. vi–vii). Elsevier. 18. [Google Scholar]
- 3.Wei F, Song T, Ding G, Xu J, Liu Y, Liu D, Fan Z, Zhang C, Shi S, & Wang S (2013). Functional tooth restoration by allogeneic mesenchymal stem cell-based bio-root regeneration in swine. Stem cells and development, 22(12), 1752–1762. 10.1089/scd.2012.0688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao ZH, Hu L, Liu GL, Wei FL, Liu Y, Liu ZH, Fan ZP, Zhang CM, Wang JS, & Wang SL (2016). Bio-Root and Implant-Based Restoration as a Tooth Replacement Alternative. Journal of dental research, 95(6), 642–649. 10.1177/0022034516639260 [DOI] [PubMed] [Google Scholar]
- 5.Jing J, Feng J, Li J, Han X, He J, Ho TV, Du J, Zhou X, Urata M, & Chai Y (2019). Antagonistic interaction between Ezh2 and Arid1a coordinates root patterning and development via Cdkn2a in mouse molars. eLife, 8, e46426. 10.7554/eLife.46426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trulsson M, van der Bilt A, Carlsson GE, Gotfredsen K, Larsson P, Müller F, Sessle BJ, & Svensson P (2012). From brain to bridge: masticatory function and dental implants. Journal of oral rehabilitation, 39(11), 858–877. 10.1111/j.1365-2842.2012.02340.x [DOI] [PubMed] [Google Scholar]
- 7.Nanci A Development of the Tooth and Its Supporting Tissues. In Ten Cate's oral histology: Development, structure, and function (pp. 70–94). Elsevier. 10.1016/B978-0-323-07846-7.00005-7. [DOI] [Google Scholar]
- 8.Shadad O, Chaulagain R, Luukko K, & Kettunen P (2019). Establishment of tooth blood supply and innervation is developmentally regulated and takes place through differential patterning processes. Journal of anatomy, 234(4), 465–479. 10.1111/joa.12950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Men Y, Wang Y, Yi Y, Jing D, Luo W, Shen B, Stenberg W, Chai Y, Ge WP, Feng JQ, & Zhao H (2020). Gli1+ Periodontium Stem Cells Are Regulated by Osteocytes and Occlusal Force. Developmental Cell, 54(5), 639–654.e6. 10.1016/j.devcel.2020.06.006 [DOI] [PubMed] [Google Scholar]
- 10.Paulsen DF (2010). The Digestive Tract. In Histology & Cell Biology Examination & Board Review (5th ed.). McGraw-Hill Medical. https://accessmedicine-mhmedical-com.libproxy1.usc.edu/content.aspx?bookid=563§ionid=42045310 [Google Scholar]
- 11.Orban BJ, & Bhaskar SN (1990). Development and Growth of Teeth. In Orban's Oral Histology and Embryology (11th ed., pp. 34–45). Mosby Year Book. [Google Scholar]
- 12.Du J, Jing J, Yuan Y, Feng J, Han X, Chen S, Li X, Peng W, Xu J, Ho TV, Jiang X, & Chai Y (2021). Arid1a-Plagl1-Hh signaling is indispensable for differentiation-associated cell cycle arrest of tooth root progenitors. Cell reports, 35(1), 108964. 10.1016/j.celrep.2021.108964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wen Q, Jing J, Han X, Feng J, Yuan Y, Ma Y, Chen S, Ho TV, & Chai Y (2020). Runx2 Regulates Mouse Tooth Root Development Via Activation of WNT Inhibitor NOTUM. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research, 35(11), 2252–2264. 10.1002/jbmr.4120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kupczik K, & Hublin JJ (2010). Mandibular molar root morphology in Neanderthals and Late Pleistocene and recent Homo sapiens. Journal of human evolution, 59(5), 525–541. 10.1016/j.jhevol.2010.05.009 [DOI] [PubMed] [Google Scholar]
- 15.Shields ED (2005). Mandibular premolar and second molar root morphological variation in modern humans: What root number can tell us about tooth morphogenesis. American journal of physical anthropology, 128(2), 299–311. 10.1002/ajpa.20110 [DOI] [PubMed] [Google Scholar]
- 16.Butler P (2000). The evolution of tooth shape and tooth function in primates. In Teaford M, Meredith Smith M, & Ferguson M (Eds.), Development, Function and Evolution of Teeth (pp. 201–211). Cambridge: Cambridge University Press. doi: 10.1017/CBO9780511542626.014 [DOI] [Google Scholar]
- 17.Teaford M, Meredith Smith M, & Ferguson M (Eds.). (2000). Development, Function and Evolution of Teeth. Cambridge: Cambridge University Press. https://doi-org.libproxy2.usc.edu/10.1017/CBO9780511542626 [Google Scholar]
- 18.Bi R, Lyu P, Song Y, Li P, Song D, Cui C, & Fan Y (2021). Function of Dental Follicle Progenitor/Stem Cells and Their Potential in Regenerative Medicine: From Mechanisms to Applications. Biomolecules, 11(7), 997. 10.3390/biom11070997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jing J, Feng J, Yuan Y, Guo T, Lei J, Pei F, Ho TV, & Chai Y (2022). Spatiotemporal single-cell regulatory atlas reveals neural crest lineage diversification and cellular function during tooth morphogenesis. Nature communications, 13(1), 4803. 10.1038/s41467-022-32490-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xuan K, Li B, Guo H, Sun W, Kou X, He X, Zhang Y, Sun J, Liu A, Liao L, Liu S, Liu W, Hu C, Shi S, & Jin Y (2018). Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Science translational medicine, 10(455), eaaf3227. 10.1126/scitranslmed.aaf3227 [DOI] [PubMed] [Google Scholar]