Abstract
Scanning electrochemical probe microscopy (SEPM) techniques can disclose the local electrochemical reactivity of interfaces in single-entity and sub-entity studies. Operando SEPM measurements consist of using a SEPM tip to investigate the performance of electrocatalysts, while the reactivity of the interface is simultaneously modulated. This powerful combination can correlate electrochemical activity with changes in surface properties, e.g., topography and structure, as well as provide insight into reaction mechanisms. The focus of this review is to reveal the recent progress in local SEPM measurements of the catalytic activity of a surface toward the reduction and evolution of O2 and H2 and electrochemical conversion of CO2. The capabilities of SEPMs are showcased, and the possibility of coupling other techniques to SEPMs is presented. Emphasis is given to scanning electrochemical microscopy (SECM), scanning ion conductance microscopy (SICM), electrochemical scanning tunneling microscopy (EC-STM), and scanning electrochemical cell microscopy (SECCM).
1. Introduction
Today, electrocatalysis plays a key role in achieving defossilization in minimizing carbon emission and hence in supporting the ambitious goals necessary to fight climate change. The electrochemical conversion of abundant small molecules like H2O, N2, O2, and CO2 into chemical feedstocks or energy carriers using renewable electricity is considered a promising approach to the global supply of sustainable energy.1−6 Consequently, the development of (electro)catalysts that can maximize the rates of reaction and minimize the overpotentials of these conversions is crucial for the large-scale industrialization of this green technology.7,8 To enable a rational design of electrocatalysts, an in-depth understanding of the complex chemical occurrences at the electrochemical interface during a reaction (e.g., adsorption and desorption, charge and electron transfer, solvation and desolvation, and electrostatic interactions) is of high importance and the basis for engineering and optimizing electrocatalytic systems.2,7,9−11
A holistic study of the interfacial processes demands measuring kinetic and thermodynamic parameters.12 One strategy to enhance the selectivity and to investigate the interfacial electrode–electrolyte composition is to couple methods for real-time analysis, enabling so-called operando measurements. The goal of most operando measurements is to gain in-depth insight into the mechanism of a reaction. To this end, working under operando conditions has been proposed as a method that bridges experimental gaps in measurement conditions between instrumental requirements and realistic electrocatalytic reactions.13 This approach combines techniques for simultaneously recording independent signals, where one technique is employed as an actuator for altering the interface properties (e.g., structure, morphology, activity, mechanism), while a second technique acts as a spectator to monitor the resulting changes.14 General aspects of operando measurements are illustrated in Scheme 1. As such, changes in chemical or structural compositions are typically monitored using spectroscopic methods while controlling the reactivity of the surface with e.g., electrochemical methods. Most applied operando characterization methods including transmission electron microscopy (TEM), X-ray absorption spectroscopy (XAS), X-ray photoelectron spectroscopy (XPS), X-ray diffraction (XRD), Raman spectroscopy, infrared (IR) spectroscopy, mass spectroscopy, online inductively coupled plasma mass spectrometry (ICP-MS), among others, have been developed to study electrocatalysts under realistic reaction conditions.15−17 However, each approach shows its advantages and limitations. For instance, operando XAS is sensitive to the local coordination structure and oxidation state of elements in the catalysts, but it is a bulk technique failing to reflect detailed information on reactions occurring at the catalyst surface.18 While operando XRD has been well-developed, it is often challenging to detect amorphous structures at the surface of a reconstructed OER electrocatalyst.19 Considering the advantages and disadvantages, local investigation of exclusively the catalytically active interface is one important key to obtain a complete picture of the reaction. In this regard, scanning electrochemical probe microscopy (SEPM) has been largely explored in electrocatalysis to shed light on the mechanism of a catalytic reaction and on the activity of the catalyst.13,18,20 Besides the advantage of its inherent high lateral resolution, the use of micro-nanoelectrochemistry in SEPM studies of electrocatalytic activity circumvents the limiting mass transport condition of conventional/traditional techniques due to the hemispherical diffusion conditions.10,21−23
Scheme 1. Diagram Exemplifying a General Operando Measurement Which Involves a Spectator and an Actuator Method Performed Simultaneously, with the Spectator Response Depending on the Actuator Modulation in Real-Time.
Although “operando” is not a typical wording used in the majority of SEPM investigations, the combination of measurements to invoke kinetics changes on a surface while monitoring the subsequent effect is a common aspect in many in situ SEPM analyses. Hence, to clarify this, we here refer to operando SEPM for SEPM experiments when simultaneously: (i) an actuator measurement is applied to tailor changes at the investigated surface, and (ii) the SEPM tip is employed as a spectator to monitor local changes at the investigated surface by measuring processes directly or indirectly related to the surface reaction. In the general case of operando SEPM, two electrochemical reactions are controlled independently and simultaneously, where the reaction of interest is invoked at the sample surface and the SEPM response depends in real-time on the modulation of the response at the sample surface. In contrast with macroscopic techniques, the use of operando SEPM can help to disclose the interfacial dynamics that influence electrode kinetics and reaction mechanisms. Moreover, the correlation of intrinsic electrochemical reactivity with the physical and chemical properties of an electrocatalyst is possible by SEPM mapping or coupling with other techniques.
The literature is replete with review papers on applications of SEPM for imaging electrochemical processes at interfaces locally.24−26 Hence, the focus of this review is on the advances made thus far using SEPM techniques in the field of electrocatalysis however exclusively concerning operando measurements, i.e., the simultaneous readout of an actuator process and a spectator process usually at the tip of the SEPM method. The discussed SEPM methods comprise scanning electrochemical microscopy (SECM), scanning ion conductance microscopy (SICM), scanning electrochemical cell microscopy (SECCM), and electrochemical scanning tunneling microscopy (EC-STM).
The review is organized in two sections considering the preknowledge of potentially interested readers. In the first section, each SEPM method is presented with respect to principles and modes of operation as well as potential applicability. This is complemented by a description of the prerequisites for operando SEPM measurement conditions with examples to enable the reader to appreciate the potential and current limitations of each technique. Moreover, the diversity of the applicability of the different SEPM techniques is covered to provide an overview of the limitations of each technique for the evaluation of interfacial processes. The second section focuses on the progress in operando SEPM for electrocatalysis. Measurements as applied to monitor electrocatalytic processes such as the oxygen reduction reaction (ORR), the hydrogen evolution reaction (HER), the oxygen evolution reaction (OER), and the CO2 reduction reaction (CO2RR) are comprehensively discussed.
2. Backstage: Principles and Applicability of Operando SEPM
SEPM represents a family of techniques that use an electrochemical tip or probe to investigate the local properties of an interface.27 The tip interacts with the surface under investigation and physical quantities such as current or potential are determined. SEPM has been employed for electrochemical studies in corrosion,28−31 single-cells,32−41 batteries,42−48 bioelectrocatalysis,49−51 and electrocatalysis.52,53 Generally, the working principle of all SEPM techniques hinges on the precise positioning of a localized probe (the SEPM tip) to interact with a surface under interrogation, allowing one to image and manipulate the surface with submicron, nanometer and/or atomic scale precision.54−56 To this end, the SEPM tip plays a central role in SEPM measurements because its properties (size and shape) define the resolution and applicability of the particular SEPM technique.54 Note, millimeter resolution and nonscanning techniques such as the scanning vibrating electrode technique (SVET)57−59 and single nanoparticle electrochemical impact (SNEI) measurements,60−63 which, although powerful tools used to investigate corrosion and electron-transfer processes, are not included in this review.
SEPM tips can be divided into solid and pipette-based probes. For instance, tips used for EC-STM measurements consist of a very-sharp metal or an alloy that allows resolutions down to the atomic level.64−67 Likewise, SECM tips are solid-based and predominantly made of platinum, gold, and carbon-based disk-shaped electrodes allowing resolutions in the micrometer to nanometer range. Surface-modified micro/nanoelectrodes are also employed in SECM to increase selectivity during amperometric or potentiometric measurements.26,54,68−72 Electrolyte-filled pipettes are employed in SICM and SECCM in which case the resolution (which reaches the nanometer range) depends on the aperture of the pipette.73,74 In coupled SEPM techniques (e.g., SECM-SICM), solid- and pipette-based probes are merged into a single tip.75 Besides the SEPM tip, the positioning unit which enables precise control of tip movement in the x-, y-, and z-directions relative to the investigated surface is of high importance.54,68,76 The positioned tip monitors signals whose magnitude corresponds to the tip-to-surface distance, which need to be compared with a reference value, known as the set point or feedback signal. For image generation the set point needs to be established, and as the tip scans the surface of interest, a feedback loop compares the measured signals (i.e., faradaic or capacitive signals for SECM and SECCM, ion current for SICM, and tunneling current for EC-STM) with the set point.54,68,73−77
The main goal is to demonstrate the general principles and the limitations associated with the use of operando SEPM. Table 1 and Scheme 2 resume the general aspects and cell configurations of each SEPM technique discussed in this review. The intention is to present a snapshot of the general principles and to give the reader an overview of the capability of each SEPM technique for performing in situ measurements as well as the possibility of simultaneously coupling independent techniques to achieve operando conditions. The applicability of operando SEPM is exemplified also with studies not addressing specifically electrocatalysis to give the reader a general overview of principle operando capabilities, while examples of operando SEPM electrocatalysis are covered in the next section.
Table 1. General Aspects of Scanning Electrochemical Probe Microscopy (More Details Are Discussed in Each SEPM Technique Section).
| SEPM (resolution) | set point for the feedback position | main application | main limitation | operation modes and hybrid techniques |
|---|---|---|---|---|
| SECM54,78 (sub-μm) | The set point is typically achieved at the distance range where the diffusion layer (faradaic process) or electrical double layer of the tip is disturbed physically or chemically by the investigated surface. The range of the set point distance is wide, and the tip current depends on electrochemical processes and the difference in surface topography. | SECM is applied for the investigation of local electron-transfer processes at interfaces (liquid/solid, liquid/gas, liquid/liquid). It is the most adaptable SEPM technique covered in this review. Because the sample can be independently polarized, investigation with good chemical specificity is achievable. | Imaging is typically done at a constant distance. However, the distance between the tip and the sample can vary with changes in the sample topography. The user should use a hybrid SECM technique to decouple the topographic effect from electroactivity. The complexity associated with the fabrication of smaller tips limits the resolution achievable in SECM measurements. Besides that, SECM tips of few nm was already reported (>3 nm).79,80 Hybrid techniques should be coupled to deconvolute the topographic contribution on the tip response. | The option to use a large variability of different SECM tips such as modified electrodes surfaces, potentiometric tips, or ion-selective electrodes makes SECM a versatile tool. |
| Modes: feedback (FB), tip collector/substrate generator (TC-SG) and substrate collector/tip generator (SC-TG), redox competition (RC), AC-SECM (or local EIS), direct mode, and surface interrogation (SI). | ||||
| Hybrid techniques: SECM/SICM, SECM/Raman, SECM/SPR, SECM/FTIR, SECM/AFM, SECM/EQCM, SECM/SVET, SPECM. | ||||
| SICM73 (few nm) | The set point is defined by a distance at which the ion current is disturbed by the underlying surface. The ion current is caused by the movement of ions through the pipette aperture. | SICM is used to acquire topographical images of soft surfaces such as biological samples. In contrast to STM, the tip never touches the sample surface. | SICM has low chemical specificity, which can be rectified by coupling the SICM to other SEPM techniques like SECM (SECM-SICM) or by using ion current rectification (ICR). | Modes: DC and AC (oscillating) modes, and ICR. |
| Hybrid techniques: SICM/SECM, SICM/AFM, SICM/potentiometric sensor. | ||||
| EC-STM76,81 (sub-nm, atomic resolution) | The tunneling current is used as set point, and the feedback signal is reached when the sharp tip is positioned at some atomic distances to the sample surface. A potential is applied between the tip and sample surface to generate the tunneling current. | EC-STM is the technique with the highest resolution (atomic resolution) and is used to simultaneously probe the electrochemical activity and topography of surfaces. | The main limitations are the inability to follow rough surfaces, prolonged scanning times, and the restriction to cover large areas. | Modes: constant current and constant height. |
| Hybrid techniques: EC-STM/SECM, EC-STM/DEMS | ||||
| SECCM74,82 (few nm) | SECCM pipette confines the electrochemical cell size to the contact of the hanging droplet with the surface. The set point is reached when the meniscus of the droplet touches the surface. Typically, a potential is applied between the surface and the QRCE inserted inside the pipette. | SECCM is used to record both topographical and electrochemical activity, at the same time. It exhibits high lateral resolution and yields high-throughput information. | Its is hard to control the integrity of the droplet at the SECCM tip. Additionally, because the set point is defined when the droplet touches the surface, and experimentation begins when the surface is polarized, coupling with other techniques is limited. | Modes: SECCM is a direct method to probe the surface because the desired electrochemical reaction occurs at the sample surface. Single- or double-barrel pipettes are used to interrogate the surface. |
| Hybrid techniques: SECCM/optical methods. |
Scheme 2. Typical Electrochemical Cell Configuration for the Operation of SEPM Techniques.
WE, working electrode; RE, reference electrode; CE, counter electrode; QRCE, quasi reference-counter electrode; colored curved arrows indicate an electron-transfer reaction recorded at the WE 1 (orange arrows) and WE 2 (red arrows). (a) Four-electrode SECM cell, where a disk-shaped micro/nanoelectrode is the SECM tip (WE 2). An electron-transfer reaction at the tip generates the SECM tip current, which is modulated by the underlying surface (WE 1). (b) SICM two-electrode configuration, in which one QRCE is inserted inside the filled nanopipette (SICM tip), while the other QRCE is immersed in the sample solution. A potential is applied between the QRCEs, and the SICM tip current is related to the ion flux through the aperture of the capillary. (c) A sharp EC-STM tip is employed as working electrode (WE 2) in a four-electrode cell configuration. The tunneling current is the set point for the EC-STM, due to the electron-transfer tunneling between the surface (WE 1) and the tip. (d) In the case of SECCM, the electron-transfer reaction occurs directly on the investigated surface (WE 1). A single-barrel or double-barrel pipette is filled with the electrolyte, and a QRCE is inserted in each channel. The SECCM tip droplet confines the electrochemical cell. In the double-barrel configuration, a potential is applied between the two QRCEs to achieve the set point, while the sample potential is applied between the sample and the QRCEs.
2.1. Scanning Electrochemical Microscopy
SECM was first reported in 198954,78 and has since become a robust and largely explored SEPM technique for electrochemical investigations. The SECM technique uses a micro/nanoelectrode as a probe, typically a metallic disk-shaped electrode encased in an insulating body. Scheme 2a shows a SECM electrochemical cell in which WE1 and WE2 are controlled independently. The principle of SECM is to register the electrochemical conversion of free-diffusing species under diffusion-limited conditions, such that the current recorded at the tip during the lateral and vertical interrogation of the interfacial region is invariable with time. The SECM tip current results either from a faradaic process or a non-faradaic process that occurs at the tip surface and is dependent on the distance between the tip and the sample.54 Thus, one of the limitations of SECM is that when the tip scans laterally (x- and y-directions) over the surface at a constant height without variations in the z-position of the tip, the recorded current contains contributions from the sample topography. Deconvolution of these two contributions is the primary motivation for coupling SECM with other techniques to independently adjust the working distance and obtain a constant distance mapping condition. The tip–sample interaction condition occurs when the polarized microelectrode is positioned very close to the sample surface such that the diffusion layer of the tip is modulated by the presence of the sample. The sample, on the one hand, acts as a physical barrier to the diffusion of species to the tip. Furthermore, the sample can locally perturb the composition/flux of species reaching the tip by consuming or releasing material. Under this condition, the sample surface can be unbiased or polarized (WE2) simultaneously with the tip, ensuring that the operando condition is intrinsically achieved as the tip and surface are modulated independently. The analytical aspects of SECM,83 its versatility for nanoscale studies,84 applications in heterogeneous electron transfer,23,26 and biological processes have been reviewed.85,86 The operation of SECM modes depends on the electrochemical processes occurring at the tip and the sample surface. In the ensuing sections, the main SECM modes and hybrid techniques are described. We also highlight the conditions that qualify a particular mode as an operando measurement, where the SECM tip acts as a spectator while the WE1 is employed as an actuator (Scheme 1).
2.1.1. Feedback SECM Mode
The feedback mode (FB) is the most commonly used operational mode of SECM, where due to the feedback effect, the sample does not necessarily require polarization to enable the local biased tip-to-sample electron-transfer kinetics.68,87 The tip current depends on faradaic processes involving electroactive species like freely diffusing reversible redox couples (redox mediators) in the electrolyte. In the bulk solution, when a disk-shaped microelectrode is polarized at a potential, at which the mass transport is limited by diffusion of the species, then the current follows the equation for hemispherical diffusion:54
| 1 |
where ibulk is the measured current at the tip at positions far from the sample surface, n is the number of transferred electrons involved in the electrochemical reaction, F is the Faraday constant (s·A·mol–1), D is the diffusion coefficient of the electroactive species (m2·s–1), C is the concentration of the electroactive species in the bulk (mol·m–3), and r is the radius of the electroactive surface of probe/tip/microelectrode (m).
When the SECM tip is moved toward the sample surface, the species generated at the tip during the electrochemical process diffuse to the surface of the sample. Once the species of the reversible redox couple are confined within a thin layer, a small overpotential is generated, sufficiently high to facilitate electrochemical reactions at the surface of a conductive sample. This phenomenon is called positive feedback, as countless cycles occur between the tip and the conductive surface, leading to a higher SECM tip current in comparison to the value measured at far working distance.54,78 The extension of the feedback effect depends on the kinetics of the electrochemical conversion on the conductive surface and can be calculated.68,88,89 When the substrate is an insulator or the tip reaction yields a non-electroactive species, the SECM tip current decreases as it approaches the substrate, because the diffusion of the electroactive species is hindered by the insulating walls of the SECM tip and the sample surface. This effect is known as hindered diffusion or negative feedback and is entirely dependent on the size of the microelectrode.54,90 The negative feedback is used to map topographical features, while the positive feedback mode is used to assess the electron-transfer ability of the sample surface.54
As the FB mode does not necessarily require that the sample is polarized, an external or additional method/technique must be incorporated simultaneously to conduct operando measurements. As an example, operando FB mode was used to follow the growth of the insulating character of the solid-electrolyte interphase on battery materials.91 The SECM tip was positioned at a working distance using the feedback effect on an anatase TiO2 paste, and the oxidation of ferrocene was performed at the tip while the potential at the battery surface was scanned to invoke the formation of the solid-electrolyte interphase. The potential at which the electrochemical processes on the TiO2 paste occurred to generate the insulating film, were monitored by the drastic changes of the feedback current at the tip.91
2.1.2. Generation–Collection SECM Modes: Substrate Generation–Tip Collection and Tip Generation/Substrate Collection Modes
The generation–collection (G-C)87,92,93 mode of SECM is carried out in a four-electrode electrochemical cell or with two separate electrochemical cells. The principle of the G-C mode relies on the generation of an electroactive species at a biased substrate (or tip), which diffuses and gets detected or collected electrochemically by a simultaneously polarized tip (or substrate). Thus, the G-C mode is, in principle, considered operando, even though the SECM community rarely used this term. During the substrate generation–tip collection (SG-TC) mode experiments, the SECM tip is polarized at a defined potential to collect substrate-generated species while the potential at the substrate is scanned.93
The SG-TC mode was explored extensively to map the local electron transfer at electrode surfaces.94,95 Interfacial processes during the OER on irradiated semiconductor materials for photoelectrochemical cell applications have been studied via the SG-TC mode to screen n-type W-doped BiVO4, and to determine the effective heterogeneous electron-transfer constant of semiconductor materials.96,97 Moreover, the SECM tip can also be employed as a local generator in the TG-SC mode, and most of the species generated at the small tip are collected by the polarized sample surface. Under this condition, the collection efficiency is about 1, which is a difficult value to be reached with conventional rotating ring-disk electrodes.98−102 The SG-TC-SECM mode has also been used to monitor the activity of biological samples like cells and enzymes,103,104 although the increased complexity associated with modulating the activity of the biological samples makes it difficult to meet the operando condition.
2.1.3. Redox Competition SECM Mode
Although previously reported in the context of numerical simulation at a heptode SECM tip,105 the feasibility of the RC-SECM mode was first employed experimentally to locally visualize the catalytic activity of surface-confined noble metal catalysts toward the ORR.106 In the RC mode both the SECM tip and the substrate compete for the same electroactive species present in the electrolyte.106−109 The RC mode, like the two G-C modes, is an “intrinsic” operando measurement because two independent signals are employed to modulate the reaction on the substrate and the SECM tip simultaneously. The RC mode was used to study corrosion processes,110,111 enzyme activity,112−114 ORR activity of catalysts,115−117 and to evaluate the rates of respiration in cells and biological entities.36−38,118−120 In a particular study, local oxygen consumption rates calculated by SECM measurements via the RC mode showed that the reproductive organ of C. elegans was responsible for the observed oxygen consumption, indicating the high energy demand of reproduction for adult worms.37 A similar approach was used to follow oxygen consumption due to mitochondrial activity.38 Specific mitochondria inhibitors were added to the electrolyte containing the cells and the measured SECM tip current showed a variation in the oxygen consumption rate by the cells.
2.1.4. AC-SECM or Local Electrochemical Impedance Spectroscopy (LEIS)
In the AC-SECM mode, a sine potential wave (AC) is applied to the SECM tip enabling the acquisition of localized electrochemical impedance spectra as a function of spatial position.121−125 The tip response in the AC-SECM mode also depends on the working distance between the electrode and the sample surface. During AC-SECM, when the tip is brought closer to an insulating surface, the electric field lines are blocked by the tip leading to a significant increase in the measured impedance. Conversely, when the tip is moved toward a conducting surface, the field lines can pass through the conducting sample to the counter electrode thereby reducing the measured impedance. The observed effect depends on the properties of the conducting surface and the resistance of the solution. In the case of low salt concentration, the pathway of lowest resistance for charge transfer is through the conducting surface, especially at high frequencies. Consequently, the measured impedance at the tip decreases as a function of the working distance. In any case, adequate fitting to equivalent circuits is necessary to extract quantitative and physically meaningful information. An advantage of the AC-SECM is that, unlike the FB mode, no redox mediator is required to map the topography of the sample.123,126 Hence, AC-SECM measurements can be used to decouple topographical effects during kinetic evaluations of surfaces.
2.1.5. Surface Interrogation SECM Mode and Direct Mode of SECM
The surface interrogation (SI) mode was first described in 2008 to quantify surface-adsorbed species at the substrate electrode.127 The technique involves first the generation of a surface intermediate on the substrate by biasing the substrate, followed by a switch of the substrate to open circuit potential and finally the polarization of the tip to generate a redox pair which then reacts with the surface-adsorbed species.128−130 The SECM tip follows a positive feedback effect due to the diverse redox cycles occurring between the tip-generated species and the adsorbed species. The SI-SECM mode can quantify an interfacial modification when the redox cycle is complete or when the depletion of adsorbed species on the sample surface occurs.131 The SI-SECM mode was applied to quantify adsorbed CO,132,133 hydroxyl radicals during water oxidation,134,135 and other molecules.136−138
The direct mode of SECM is used to pattern surfaces and also acquire topographic images.139−142 In this mode, the SECM tip works as a counter electrode while the substrate is employed as the working electrode. The reaction taking place at the tip locally modifies the sample surface, making the direct mode an ideal strategy for constructing microstructures on films or surfaces.143−145 To the best of our knowledge, no operando studies with SI and direct SECM modes have been reported.
2.1.6. SECM Hybrid Techniques
SECM is a dynamic technique and coupling it to other techniques allows the acquisition of a plethora of information in a single experiment. Complementary SECM techniques can be applied to correlate an additional property (structural or chemical information) to the electrochemical property. Contrary to the use of several techniques in sequence to analyze the interface, we focused here on the coupling of a technique with SECM to perform operando measurements. As explained before, SECM is normally coupled with other independent methods to deconvolute the topographic contribution of the SECM tip response during mapping. AFM, SICM, and shear force positioning have been coupled with SECM to overcome this bottleneck.75,146−166 Such approaches have been essential in the study of biological materials.167−169 Strategies to deconvolute SECM tip topographic contribution from the electrochemical activity are still hot topics under discussion.170,171 Although the clear advantage of coupling methods to deconvolute the topographic contribution, the independent additional method is mostly performed in a sequence of measurements with the SECM and not simultaneously, therefore the measurement is not intrinsically an operando method. The coupling of SECM with potentiometric sensors and double-barrel electrodes, SECM tips have been employed to control the tip positioning while mapping local information with the tip. Local information such as pH, magnesium ions, potassium ions or metal ions concentrations has been mapped using this approach.172−174 Recently, a multibarrel SECM tip containing a pH sensor and a Mg2+ ion-selective electrode was employed to map the spontaneous corrosion process at the interface of a magnesium alloy.175
A combination of SECM and quartz crystal microbalance (EQCM) was proposed as an operando approach to correlate the changes in mass with the local electrochemical activity.176−179 Copper ions were monitored using the SECM tip positioned in the vicinity of an aluminum alloy interface, while a cyclic voltammogram was performed at the substrate and the EQCM results were correlated to the corrosion rate.180 A similar strategy was adopted to monitor the evolution of iron pitting corrosion.181,182
SECM was also coupled to Raman spectroscopy to analyze the changes in chemical properties while modulating electrochemical processes on the surface.183−186 The operando SECM-Raman analysis was recently applied to interrogate a self-assembled monolayer with high structural-pH sensitivity.185 The local pH was modulated by the HER on the SECM tip and the Raman analysis registered changes of the protonated state of a modified self-assembled monolayer on the surface. Additionally, SECM was combined with optical techniques, such as surface plasmon resonance (SPR)187−189 and infrared,190,191 fluorescence, and chemiluminescence spectroscopies192−195 to study molecular changes on surfaces, electrodeposition processes, or film thickness changes.
Strategies to modify the tip functionality were also used to derive information with an enhancement in selectivity. In scanning photoelectrochemical microscopy (SPECM) a focalized light source is coupled to locally illuminate the SECM analysis.196−201 For instance, photocurrents of an irradiated BiVO4/FTO semiconductor surface were monitored while the SECM tip measured the local photoelectrocatalytic activity through the oxygen evolved from the semiconductor surface.196
The above discussions show how SECM/hybrid techniques can be employed to correlate interfacial processes to local electroactivity.
2.2. Scanning Ion Conductance Microscopy (SICM)
SICM was introduced by Hansma and colleagues in 1989, as a high-resolution and noncontact imaging SEPM technique for the investigation of samples.73 The technique uses an electrolyte-filled nanopipette as a scanning probe and relies on the flow of ionic current between an electrode inside the nanopipette and a second electrode immersed in the bulk solution or electrochemical cell to create a surface-sensitive feedback signal. Topographical information on the sample is obtained by scanning the nanopipette or probe over the interface in the x- and y-directions. Fluctuations in the flow of ionic current are used to monitor the position of the tip relative to the substrate surface.73
During operation, a DC or an AC potential is applied between two wires: one wire, typically Ag/AgCl, is inserted into the SICM pipette and the other wire is immersed into the electrolyte of the electrochemical cell outside of the nanopipette channel. Scheme 2b illustrates the SICM tip and the two wires as QRCE, with the blue arrow representing the ions movement and no electron-transfer reaction occurring at the pipette aperture. The resulting current is caused by the movement of ions through the SICM tip aperture, which serves as a resistor to the ionic flux.202 Once the pipette is moved toward a surface, the ion flux is physically blocked when the aperture is some nanometers close to the substrate. At this point, the monitored ion current decreases due to the increased resistance and this variation defines the set point for controlling the SICM tip position.203,204 SICM topographic maps are obtained by correcting the tip height (z-direction) to reach the set point. In contrast to SECM microelectrodes, the simple SICM pipette fabrication allows the preparation of nanometer-sized tips. For this reason, SICM is a high-resolution technique, capable of measuring the topography of nonconductive surfaces,205 and mapping activities of soft samples such as cells,205−210 in contrast to AFM.211
2.2.1. SICM Modes of Operation
In addition to the DC or AC modulation of the ion flux into the SICM tip strategies are employed to allow imaging beyond highly resolved topographic maps. The capability to map the topography of highly ordered porous silicon functionalized with lipid membranes was shown using noncontact DC-SICM including the manipulation of the soft membranes.212 Due to drifts encountered using the DC-SICM current response, the positioning stability can be increased by employing AC-SICM. During AC modulation, the set point is reached when a small variation of the tip response is detected. Typically the AC modulation is imposed using an AC potential signal or a physical tip vibration in the vertical direction.203,213,214 The variation in AC is coupled to topographic acquisition while the variation of the DC ion current is related to the charge transfer property of the surface. As an example, vibrations in the vertical direction were used to record topographic images of pores on polymeric membranes while the DC ion current was correlated to the transport of KCl through the pores.215 Instrumental advances have made it possible to carry out electrochemical impedance experiments to image the local capacitance and topography of a gold nanoparticle.216 Advances in DC-SICM and pulse-mode SICM have been proposed as routes to improve the SICM mapping and applied to monitor volume changes in cells.217,218 In parallel, modified nanopipettes were developed to record the ion current rectification (ICR).219−223 With this approach, the SICM sensitivity is increased due to specific ion interaction with the modified aperture.219−223 The correlation of changes in the ion current of functionalized nanopipette pores to the presence of ions was demonstrated by measuring variations in ICR due to a KCl concentration gradient224 and at different pH conditions.225 Moreover, a modified lipid bilayer SICM pore was reported and used to map the diffusion of β-cyclodextrin through a glass micropore.226 Furthermore, the possibility to set up an SICM experiment in a configuration similar to the patch-clamp technique paves the way for SICM to be largely explored in the biophysical analysis of cells.227−231 Recently, SICM tips were inserted into a macrophage cell, and intracellular events such as nanoparticles and vesicle collisions were monitored.232
Although the high capability of SICM to record topography with high resolution is widely explored, the correlation of electrochemical activity to surface properties is complex using SICM alone. A strategy adopted to overcome this is to conduct the measurement in a low ionic strength electrolyte, allowing the double layer of the SICM tip to interact with the charged substrate.220,233 This way, the SICM tip response can then be correlated to surface reactivity.
The use of surface charge mapping was demonstrated on living cells,234−238 films,239,240 and electrode surfaces.241−243 Recently, operando SICM was employed to simultaneously map the surface charge, reactivity and topography of a carbon fiber microelectrode surface.242 In this operando SICM case, the SICM tip is employed as the spectator while the surface activity is modulated by an electrochemical method (Scheme 1). Moreover, Unwin’s group showed that electroosmotic flow controls the flux of uncharged molecules within the SICM tip, in contrast to the flux of charged molecules, which is easily controlled through the migration effect. The role of a biased underlying surface on the ion current was also interrogated and the mass transport and flow rate of species were quantified using simulation tools. This work showed that besides voltage, pipette wall charge, and working distance, the ion flux through the SICM tip also depends on the substrate surface charge.242 Besides the richness on the information on charge mapping, the exploration of using operando SICM during modulation of the surface was not extended to any electrocatalysis study.
2.2.2. SICM Hybrid Techniques
As pointed out before, SICM is a great tool to provide stable and great control of the tip positioning. Due to this unique functionality, SICM was coupled with other techniques like SECM, as this compensates for the lack of selectivity of the SICM measurements, and also serves as a strategy to deconvolute the topographic contributions from the SECM tip response.75,157,244 As discussed in the SECM section 2.1, to adjust the tip height by means of the SICM response and acquisition of the electrochemical information by SECM response is commonly performed sequentially (not operando).
The same hybrid strategy was used by coupling a potentiometric sensor to the SICM tip,245,246 and in a typical example, a dual function tip was used to record the local pH value and the topography: one channel of the double-barrel electrode was modified with a potentiometric iridium oxide sensor to record the pH map, while the SICM tip channel corrected the working distance over a calcite microcrystal.247
SICM hybrid techniques were also employed to image living cells by coupling SICM to near-field optical microscopy248 and confocal microscopy.249−254 The confocal microscopy SICM approach was used to target fluorescing molecules and to correlate changes in the topography of cells. A hybrid confocal microscopy SICM was applied to monitor the topographical changes due to a virus-like particle assembly on a cellular membrane.255 A protein that induces the formation of a virus-like particle was labeled with fluorescent molecules. The topographic changes were correlated to the virus-like particle growth mechanism, as well as the release event. The obtained results were an important step in the elucidation of the replication mechanism of the virus.
2.3. Electrochemical Scanning Tunneling Microscopy (EC-STM)
Scanning tunneling microscopy (STM) was proposed by Binnig and Rohrer at the beginning of 1980 as a technique for imaging the topography of samples with atomic resolution.76 For that, a sharp metallic tip is slightly polarized against the underlying conductive sample, and when the distance between the very end of the STM tip and the surface reaches a distance in the atomic range, electrons can flow due to the tunneling effect.256,257 The electron flow initiates a tunneling current which is adopted as the set point to control the working distance. The STM technique can image surfaces with atomic resolution because of the very sensitive interaction between atoms of the STM tip and the sample surface.258 Advances in STM have enabled measurements in aqueous solution, making it possible to monitor structural changes due to surface polarization.81,259 This condition is called electrochemical STM (EC-STM or ESTM) and it was proposed to monitor redox processes on surfaces by adding a high-impedance reference electrode in a four-electrode cell configuration (2 WE, 1 CE, 1 RE) and illustrated in Scheme 2c.260−263 In this configuration, the potential can be applied independently at the conductive surface and the STM tip, in a similar manner as in the G-C mode of the SECM. One limitation of the EC-STM is the difficulty to deconvolute the tunneling current from the faradaic process at the tip. A strategy frequently used to offset this challenge is to partially coat the body of the EC-STM probe (but not the tip) with an insulating layer, such as nail varnish or Apiezon wax,154,264 to reduce the area exposed to the electrolyte. The insulating layer is illustrated as a black cover on the EC-STM tip in the Scheme 2c). As faradaic and double-layer charge–discharge currents depend on the surface area, this approach is adopted to minimize such currents with respect to the tunneling current.265,266 Another strategy is to keep the tip at the same position while the potential on the surface is scanned. The scanning tunneling spectroscopy (STS) is a deviation of EC-STM, where the applied potentials on the sample are interpreted as energy levels and scanned to obtain an electronic spectrogram. This strategy was widely utilized to study electron-transfer reactions on Au substrates, where the enhancement of the tunneling current was correlated to the reactivity of the surface.267−271
2.3.1. EC-STM Modes of Operation
The typical operation of EC-STM is based on approaching the STM tip toward the surface until a tunneling current is detected and used as the set point. The main feature of STM is to acquire the morphology of the investigated surface with atomic resolution.272 The STM map is built by adjusting the STM position in the vertical direction (z-axis) to reach the same set point value. In contrast, the STM image can also be recorded by keeping the vertical distance (z-axis) constant and recording the tunneling current at each x–y-position. This strategy reduces the image acquisition time, however, the surface morphology should not be rough. Most of the EC-STM measurements are done by using the constant tunneling current mode, which is a powerful tool for investigating electrochemical processes like adsorption,273−275 passivation,276−278 and corrosion279 of surfaces. One of the limitations of EC-STM is the slow scanning process, which makes it difficult to couple measurements simultaneously for operando studies. For this reason, in situ analysis is often used to evaluate morphological changes on surfaces, such as to investigate protein/enzyme covered surfaces,280−284 metallic crystalline interfaces,272,285 electrocatalysts,286−288 and battery materials.289,290 EC-STM was employed to study the topographical changes on highly oriented pyrolytic graphite (HOPG), that was polarized to invoke intercalation of Li ions.289,290 The results indicated that the exfoliation process took place at the HOPG edges instead of at the basal planes. Recently, Wan and Wang used the approach of sequentially polarizing the surface and intermittently acquiring EC-STM images to study the mechanisms of CO2RR and ORR on an adlayer of a cobalt-phthalocyanine catalyst on a gold surface.291,292 In parallel, Itaya’s group used EC-STM to investigate the correlation between the morphology and applied potentials in the formation of regularly patterned adlayers of Zn(II)phthalocyanine and a zinc metalloporphyrin on the crystalline surfaces of Au(111) or Au(100).293 It was shown that the assembly of fullerene molecules was dependent on the packing arrangement of the adlayer, which is also influenced by the crystallographic orientation of Au. Another interesting study used in situ EC-STM to follow the changes in the HOPG morphology during the solid-electrolyte interphase (SEI) formation.294 The topographical changes during electrolyte reduction and film deposition were monitored, as well as the intercalation processes of Li ions and solvent molecules into the resulting surface. Moreover, the operando conditions employed allowed for the concurrent acquisition of STM images and potential programs on the HOPG surface. The overlay of the STM image with the potential scans showed a clear potential dependence of the surface processes, such as material deposition and surface morphological changes. After sequentially scanning the potential of the surface, a potential limit was found where the changes in surface processes became irreversible, which was attributed to the reduction process of the electrolyte anion.
These studies demonstrated the capability of the EC-STM to correlate morphological changes to the electrochemical activity of interrogated surfaces.
2.3.2. EC-STM Hybrid Techniques
One of the limitations of EC-STM is the convolution of the tunneling current with the faradaic processes occurring at the EC-STM tip. Consequently, EC-STM has been coupled with SECM to overcome this bottleneck.295 In this case, a single tip is employed with a dual function of acting as an EC-STM tip to image the surface morphology and as an SECM tip to register the local electroactivity of the surface. After the positioning step, the tip is retracted to a working distance where there is no tunneling effect but is still in the range of the SECM set point. The applicability of SECM/EC-STM to image the topography and reactivity was demonstrated on a self-assembled monolayer (SAM) on gold by the feedback effect.295 Soriaga’s group employed a hybrid EC-STM/DEMS technique to follow changes on a polycrystalline Cu surface in basic medium and under an applied potential to promote CO reduction.296 The products of the reduction reaction were detected by differential electrochemical mass spectrometry (DEMS). The hybrid EC-STM/DEMS analysis was used to correlate the observed low activity of ethanol generation to the ordering process of the Cu(100) lattice over Cu(110), in comparison to the Cu(110) on the polycrystalline surface.296
2.4. Scanning Electrochemical Cell Microscopy
Scanning electrochemical cell microscopy (SECCM), sometimes also called scanning micropipette contact method (SMCM), is a pipette-based tip technique capable of imaging surfaces with a lateral resolution in the nanometer ranges. As a result, SECCM has become a powerful tool for investigating electrochemical properties in single entity/sub-entity studies.297−299 The principle of the technique is to confine the electrochemical cell in a droplet (protruding from the end of the nanopipette aperture), which is brought in contact with the interrogated surface. In contrast to SECM, SICM, and EC-STM, SECCM operates in air or an immiscible solution. Hence, the sample is not entirely immersed in the electrolyte before the local measurement is made. The colored background in Scheme 2a–c represents the electrolyte, indicating the absence of an electrolyte covering the sample in the case of SECCM (Scheme 2d). The electrochemical processes in SECCM take place exclusively on the probed surface providing direct electrochemical activity imaging. The SECCM tip is typically a single-barrel micro- or nanopipette containing the electrolyte and a quasi-reference counter electrode (QRCE). During measurements, a potential is applied between the QRCE and the conductor/semiconductor surface under interrogation. The set point is reached with the flow of a non-faradaic current caused by the contact of the hanging droplet to the surface (note: the pipette tip never touches the surface). Like in the SICM, an AC-modulated current caused by tip vibration is implemented in SECCM measurement for tip positioning. In this configuration, a double-barrel pipette (theta capillary) is used as SECCM tip with QRCEs inserted in each channel (see Scheme 2d). A potential is applied between the QRCEs, and the modulated ion current is then used as a set point. Before the contact between the hanging droplet and the surface is achieved, the modulated ion current is constant, and once there is contact, the ion current changes in its magnitude and oscillates with the same frequency as the tip vibration due to the droplet deformation. The magnitude of the resulting AC current is used to control the tip-to-surface distance. In the case that the interrogated surface is a semiconductor/conductor, an additional (floating) potential can be applied between the QRCEs and the sample. By adjusting this potential, the electrochemical processes occurring at the sample surface, which is wetted by the droplet, can be measured. Details of SECCM instrumentation have been reviewed.74,299,300 Scanning is performed either in a constant distance mode by adjusting the magnitude of the ion current signal with the SECCM tip position, or in a hopping scanning mode where the tip is withdrawn far from the surface and reapproached at each x–y-position. The latter is essential for single-barrel SECCM configurations because it prevents tip crash on rough surfaces.301
The general limitations of all SPEMs such as long scanning time and noise level in single-entity studies are also present in SECCM. The use of smaller SECCM tips leads to a reduction in the noise level because such tips wet smaller electrochemical areas, which increase the signal-to-noise ratio. This approach was explored to record the electron-transfer reaction on nanoparticles82,302,303 and events of single nanoparticle electrochemical impacts (SNEI).304,305 A strategy of coupling optical microscopy to SECCM to follow events during electrochemical measurements was proposed by Hill et al.306,307 for reducing the typical extended scanning time. The optical image was employed to visualize Au nanorods on a transparent electrode surface, i.e., indium tin oxide (ITO). By using a LABVIEW program, the electrochemical measurements were performed just where a particle was visualized. The strategy reduced the scanning time and improved the sample throughput compared to the typical scanning-tip approach.306
Developments in SECCM are still in progress, and the power of the technique is enhanced when local electrochemical data in tandem with data treatment and simulations are used to calculate intrinsic properties. In addition, some technical and instrumental improvements are still under discussion. For example, recently strategies were shown to measure electrochemical impedance spectra,308 acquire high-speed images,309 and use amplified signals to achieve high temporal resolution.310 Moreover, effects of experimental parameters such as the impact of the ohmic drop on the applied potential311 or the possibility of contamination from the Ag-QRCE312 were described.
2.4.1. SECCM Operation Modes and Applicability
During SECCM measurement, the tip performs a direct electrochemical interrogation of electron or charge transfer reactions on the sample surface in a single electrochemical cell configuration (see Scheme 2d). This is in contrast to SECM, SICM, and EC-STM, where the SEPM tip works generally as a spectator to probe the surface properties. The intrinsic operation of SECCM complicates the possibility of combining it with other techniques to perform operando measurements (Scheme 1). Although SECCM, hitherto, has not been coupled with other electrochemical method for operando measurements, the use of optical probes to modulate surface activity was suggested as actuator method. In this approach, the sample surface is irradiated to induce changes in surface activity while the SECCM tip acts as a spectator. The operando optical/SECCM approach was explored for studying the photoelectrochemical activity of semiconductors. Differences in the photoelectrochemical activity of TiO2 and transition metal dichalcogenides such as MoS2 were evaluated by performing measurements in dark and light conditions in the presence of a redox mediator.313−315 The lack of operando SECCM is most likely due to the high complexity of the experimental design of coupling techniques.
It is worth noting that its applicability has been extensively demonstrated to directly probe electrochemical processes on surfaces at high resolution.316 The electrochemical activity was probed using reversible redox species (e.g., hexaammineruthenium(III), ferrocenium, ferrocene trimethylammonium) to establish the structure–activity relationship in single entity studies.317 SECCM was extensively used to acquire high spatial resolution maps to elucidate differences in electrochemical activity between phases and grain boundaries of many polycrystalline materials such as platinum,318,319 palladium,320 gold,321−323 copper,28 or boron-doped diamond324−327 surfaces. SECCM in tandem with simulations was used to spatially map electron-transfer kinetics parameters, like the Tafel slope and the heterogeneous electron-transfer rate constant.328−330 The sensitivity of the SECCM for local activity measurement was demonstrated and used to differentiate the surface activities of glassy carbon331 and graphite.332−335 Two-dimensional materials, such as graphene,336−339 MoS2,340,341 and other transition metal dichalcogenides341,342 were also investigated by SECCM. The studies demonstrated the presence of heterogeneous activity at different sites such as defects, edges, and basal planes. The results further showed that the local activity depends on the number of layers of the 2D material. These findings reveal the capability of SECCM to disclose the role of surface conditions on the activity of materials.
Moreover, SECCM is a powerful technique for monitoring the activity of electrocatalysts, allowing the correlation of topography/structure with electrocatalytic activity. The high-throughput characterization potential of SECCM helps to study the intrinsic activity and the kinetic of electron-transfer processes. Catalyst materials for the HER that have been interrogated include transition metal dichalcogenides,323,331,341−345 bimetallic materials such as FeNi,346 Au,323,347,348 Pt,303,349−354 Zn,355 ITO, doped graphene,339,356 boron-doped diamond,326 and low-carbon steel.357 SECCM has also been used to study the ORR,28,82,323,326,358−361 the OER,309,362−364 and the CO2RR.321,322,365,366
In addition to decreasing the probed area, SECCM tip droplets can also be employed as a tool to modify surfaces with high spatial precision. Such strategies have been used for local deposition,367,368 etching,369,370 as well as to fabricate thin film metal oxides371,372 and polymers.373−375 The small area confined by the SECCM tip droplet is an important feature that allows high mass transport rates of materials coming from the pipette aperture to the interrogated surface. For this reason, SECCM is used to study slow kinetic processes, such as charge transfer on lithium-ion battery materials.302,376−382 Adaptations of the experimental conditions of SECCM are in progress to enable further studies for lithium-ion batteries materials. Recently, SECCM studies were carried out in an argon-filled glovebox,383 and using aprotic electrolytes.384 Moreover, SECCM was also employed to monitor corrosion processes by recording polarization curves and revealing the susceptibility of crystallographic phases to the corrosion of polycrystalline low-carbon steel.355,385−389
3. Operando SEPM Applications in Electrocatalysis
In this section, we discuss the application of operando SEPMs during electrocatalysis (ORR, OER, HER, CO2RR, and other important reactions). In selecting the papers that have been reviewed, particular attention was given to those in which operando conditions, as delineated in the introductory and earlier sections of this paper, are met. These studies comprise an actuator measurement or potential, which is applied to stimulate changes at the investigated surface or substrate, while a simultaneously biased SEPM tip is employed as a spectator to detect or monitor local changes on the investigated surface.
Overall, SECM is the most explored SEPM technique for operando investigation of local electrocatalytic activity and interfacial electron-transfer processes during the ORR, OER, HER, and CO2RR. This extended utility stems from the versatility of SECM due to its unique ability to characterize a wide variety of samples for various applications, adapt with other SEPMs (to improve and augment the information available for SECM only) and employ amperometric, potentiometric, as well as multifunctional probes during investigations. Furthermore, compared to the other SEPMs, which require coupling to other techniques to perform operando studies, the intrinsic operational modes of the SECM (SG-TC, TG-SC, and RC) are by default operando measurements.
3.1. Investigation of ORR Activity at the Sample Surface
The electrochemical reduction of molecular oxygen (ORR) has been extensively investigated because of its central role in fuel cells, metal-air batteries, sealed storage batteries, corrosion, and industrial electrocatalytic processes.390,391 Generally, depending on the cathode material, electrode potential, and electrolyte composition,392 it is believed (though debatable) that ORR proceeds in either of two pathways:391,392 a highly efficient four-electron pathway in one step to generate H2O or OH– or a sluggish peroxide-intermediate-based two-electron pathway in two steps to form H2O2. ORR reaction pathways have been extensively reviewed.393−398 The ORR follows either a direct four-electron pathway, or a two-electron-transfer pathway with hydrogen peroxide as intermediate.391,392
ORR investigations are typically performed using Pt-based electrocatalysts because Pt can reduce both O2 and H2O2 to H2O, and more importantly, it avoids the formation of H2O2 and/or other aggressive oxygenated species.399 The high cost, scarcity, and easy poisoning associated with Pt-based materials discourage their use as ORR electrocatalysts on a large scale.399 Addressing these challenges has triggered an intensive search for active electrocatalysts that are less expensive, earth-abundant, poisoning-tolerant, and can achieve ORR overpotentials in the range of that of Pt-based materials.400 Some advances made in this direction include the alloying of Pt with less expensive metals, use of non-platinum metal combinations, transition metal oxides, chalcogenides, inorganic and organometallic complexes, and enzyme electrodes for ORR.400 Despite these research leaps, the detailed mechanism of the ORR process, even on Pt, remains elusive.400 Besides the difficulty encountered in O2 activation, and O–O bond cleavage, which results in the observed sluggish kinetics,401 the pronounced irreversibility of the cathodic reaction, the different possible reaction pathways and the possible generation of a wide spectrum of oxygenated intermediates, are considered as factors that make the detailed study of ORR mechanism an arduous task. Furthermore, the dependence of the reaction rate on the state of the electrode surface, coupled with the lack of a rational approach to the design of new electrocatalysts that can strictly follow the direct four-electron reduction pathway makes studying ORR even more difficult.99,400 As an alternative to traditional macroscopic RDE and RRDE measurements, operando SEPMs, particularly SECM, have been employed to study the ORR (Scheme 3). Maps of chemical reactivity and topographic images were obtained.99 Furthermore, the coupling of SECM with other analytical methods was proposed already by Hillier and Bard in 2003 as an effective approach for studying the ORR and screening of electrocatalysts.99 The general principle for probing the ORR with operando SEPM involves biasing the substrate of interest to invoke the ORR while a simultaneously polarized SEPM tip is used to locally monitor the substrate-generated species, such as H2O2, HO2–, or OH– (SG-TC, TG-SC, and EC-STM in Scheme 3) or compete (RC-SECM on the Scheme 3) with the substrate for O2 in the gap between them. Kinetic parameters, such as the heterogeneous electron-transfer rate, can then be extracted by fitting the experimental tip approach curves to developed quantitative theories for the different operational modes in the case of SECM techniques.23,68,93 In the ensuing paragraphs, the advances made thus far in ORR investigations with SEPMs are recounted, highlighting the operando conditions and the uniqueness of SEPMs as qualitative and/or quantitative electroanalytical characterization tools during ORR electrocatalysis.
Scheme 3. Schematic Drawing Representing the Main Operando SEPM Configurations for ORR Interrogation.

The SEPM tip acts as the spectator while a modulation occurs on the electrocatalyst’s activity by an actuator method. The SEPM tip can be polarized to (i) reduce oxygen (orange arrows) in RC-SECM; (ii) reduce (green arrow) H2O2, an ORR product when n = 2, in SG-TC-SECM; (iii) oxidize H2O2 in TG-SC-SECM; (iv) map with high-resolution SEPM, EC-STM.
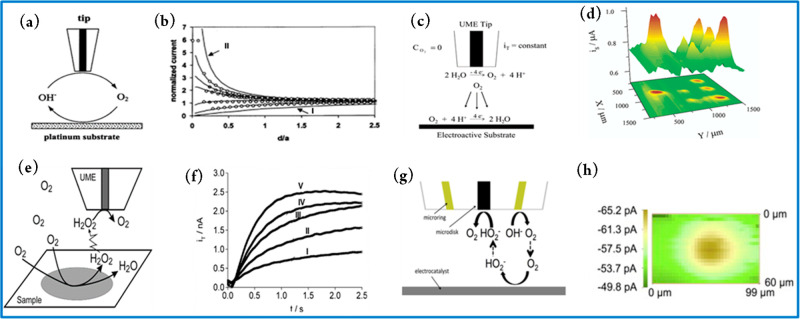
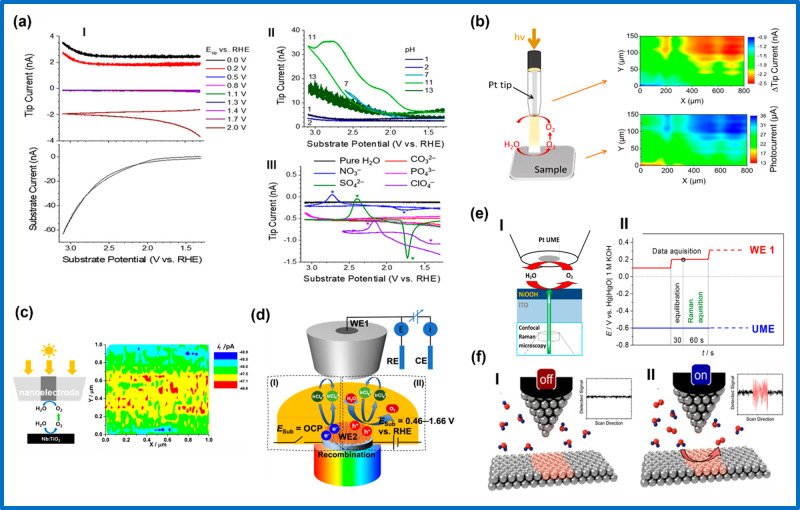
One of the pioneering studies of ORR with the SECM was performed by Liu and Bard402 under operando conditions using the FB operational mode (Figure 1a) to estimate the rate constant of the ORR on a Pt electrode in alkaline solution. A Au UME was employed as SECM tip which was polarized to 1.2 V vs Hg/HgSO4 (i.e., at the diffusion limiting potential for OH– oxidation). The polarized tip was then moved toward the surface of the Pt substrate which was simultaneously held at different potentials to obtain the SECM approach curves (Figure 1b). The Tafel slope and rate constant agreed with previously reported values derived from RDE and other electrochemical techniques. However, the operation of this technique was limited to a short pH range (9–12), where OH– oxidation at the tip could be used. As such, the study of ORR in acid or neutral media is impossible with the FB mode because the feedback diffusion of the tip reactant (H2O) does not induce modulations in the tip current.93,396 Perhaps this observation explains why the FB mode was less explored in operando ORR SECM studies. The Bard group in 2003 proposed the TG-SC mode (Figure 1c) as an alternative to the FB mode to analyze and compare the electrocatalytic activity of an array of finely dispersed catalyst spots of Pt, Ru, and Au for the ORR in acidic medium (0.5 M H2SO4).99 In this mode, the substrate (Pt, Ru, and Au catalyst spots) was polarized to a potential (<1.23 V vs Hg/HgSO4) to facilitate the reduction of O2 to H2O while an oxidation current (between 10 and 220 nA) was applied to the Pt SECM tip to oxidize H2O to O2 and ensure a constant flow and diffusion of tip-generated O2 to the substrate. Figure 1d represents an ORR image obtained in the TG-SC mode of an array of Pt and Ru spots on glassy carbon (GC). The main advantage of the TG-SC mode is that it assumes no feedback contribution and hence allows for the study of reactions inaccessible to the FB mode. Additionally, working in the TG-SC mode allows for studies in solutions with varying concentrations as opposed to the stringent conditions needed in the FB mode to reach diffusion control at the UME tip. As an added advantage, TG-SC is ideal for the rapid screening of large arrays of multicomponent electrocatalysts.99,400 The TG-SC mode was later used to study and screen the electrocatalytic activity of binary and ternary combinations of Pd, Au, Ag, and Co (or Cu) deposited on GC as substrates for the ORR in acidic medium using Pt as SECM tip.400 A drawback of the TG-SC mode lies in the inability to precisely quantify the products/intermediates formed during the ORR.100,399 The detection and quantification of H2O2, for instance, is very important because its formation has been associated with undesirable processes like membrane degradations, corrosion of metals, polymer fittings, and carbon materials, as well as a reduction in the efficiencies of fuel cells.399 Hence there is a need for methods that can directly investigate the formation of H2O2. In 2008, Shen et al.399 proposed and used a transient SG-TC mode to detect H2O2 produced during the ORR at Au, Pt, and PdCo alloy-modified GC electrodes in acidic medium using a Pt-UME as amperometric H2O2 sensor. Figure 1e shows the principle of the transient SG-TC mode: the substrate is first stepped from a potential where no faradaic processes happen, to a value within the limiting-current region of the ORR, while the tip is kept at a fixed potential of 1.1 V vs Ag/AgCl (Figure 1f) to detect and oxidize H2O2.399 Sánchez-Sánchez and co-workers demonstrated a new approach to quantify reaction intermediates based on the SG-TC mode but using smaller substrates (≤200 μm diameter) to induce stationary-state reaction conditions.403 As a demonstration, they employed the technique to quantify H2O2 during ORR at a Hg on Au substrate (100 μm) electrode in acidic media using Pt or Au as SECM tips. The use of smaller substrate electrodes (100 μm diameter) allowed a relatively high collection efficiency at the SECM tip, making it possible to detect and quantify the substrate generated H2O2 as well as to estimate the number of transferred electrons (n) during the ORR. The value of n was in the range of 2.12 to 2.19, and thus the two-electron pathway was clearly revealed as the predominant reaction pathway.404 Bard and co-workers employed SG-TC-SECM in a fundamental study of the ORR mechanism at a Pt SECM tip in alkaline media. Operando SG-TC-SECM was capable to estimate n = 2 when the ORR occurred at NaOH concentration <2 M, and n = 1 in the case of concentrations >6 M with formation of O2•–.405 Related ORR studies with the SG-TC mode were reported for different electrocatalysts including Hg, Au, Ag, Cu, Pt, Pd, Pd80Co20, Au60Cu40,403 Pt and Pd nano/microstructures embedded in multilayer polyelectrolyte films,406 different Fe porphyrins on GC,407 nanoporous Au and flat Au substrates,408 and cobalt metalloids (CoxB and CoxP) in a nitrogen-doped carbon matrix.409 Johnson and Walsh suggested a novel “tip generation–substrate collection–tip collection” (TG-SC-TC) sequence for screening the activity of ORR catalysts while detecting H2O2 simultaneously with the aid of a microring-disk SECM tip (Figure 1g).100 As a proof of concept, the technique was employed to measure the activity of a Au electrocatalyst toward the ORR in alkaline media, while simultaneously monitoring the formation of H2O2. Oxygen is generated at the microring of the SECM tip at a constant current, the O2 generated at the microring diffuses to the substrate (which is polarized at −0.5 V vs Ag/AgCl) to reduce oxygen to HO2–. The substrate-generated HO2– then diffuses to the microdisk (which is biased at a sufficiently anodic potential of 0.2 V vs Ag/AgCl) and HO2– gets oxidized to O2. Figure 1h is an SECM image recorded when the substrate was polarized at −0.50 V vs Ag/AgCl. The advantage of this method lies in the fact that no potential programming of tip or substrate is needed. Furthermore, taking continuous SECM tip scans of the substrate generates maps that correlate to activity and reaction mechanism.
Figure 1.
(a) Feedback mode SECM used in the study of O2 reduction in alkaline solution. (b) SECM approach curves: solid lines “a” and “b” are theoretical approach curves at conducting and insulating substrates, respectively. Circular symbols are experimental approach curves, and solid lines are theoretical curves at a Pt substrate with its potential held at, from top to bottom, −0.9, −0.8, −0.7, −0.6, and −0.5 V vs Hg/HgSO4. Reproduced from ref (402). Copyright 2002 American Chemical Society. (c) Scheme of the modified TG-SC mode for the study of the ORR in acidic medium. (d) ORR images obtained by the TG-SC mode of an array of Pt (left spot and right row) and Ru (middle row) spots supported on glassy carbon. Reproduced from ref (99). Copyright 2003 American Chemical Society. (e) Schematic illustration of the SECM operation in the pulsed SG-TC mode. (f) UME transient currents at ET = 1.1 V for the collection of H2O2 produced during ORR at a Au electrode. The potential of the Au substrate was stepped from +0.4 V to (I) +0.1, (II) +0.05, (III) 0, (IV) −0.05, and (V) −0.1 V. Reproduced from ref (399). Copyright 2008 American Chemical Society. (g) ‘‘Tip generation–substrate collection–tip collection’’ mode SECM using a microring-disk SECM tip for simultaneous ORR electrocatalyst screening and HO2– detection. (h) SECM image recorded at the microring-disk SECM tip (held at 0.2 V to oxidize HO2–) while scanned over the Au substrate polarized at −0.5 V vs Ag/AgCl. Reproduced with permission from ref (100). Copyright 2012 Elsevier Ltd.
In a quest to further improve the lateral resolution of the SECM the feasibility of a transient RC mode, which was mentioned in an earlier heptode SECM tip simulation study,410 was introduced. In the RC mode, local catalytic information at the substrate is transduced through a current measured at the tip while simultaneously avoiding the need to apply potential pulses to the substrate. The technique was applied to qualitatively monitor the lateral activity of Pt-spots on a GC support during the ORR using a Pt-UME as SECM tip. During the study, both tip and substrate were biased to a reductive potential (e.g., −0.60 V vs Ag/AgCl/3 M KCl) to compete for O2 in the gap between them. Since the diffusion of bulk O2 to the gap between the tip and substrate is hindered during measurement, a predefined potential pulse (1.4 V vs Ag/AgCl/3 M KCl) is applied to the tip to avoid complete depletion of O2. The activity of catalyst spots with different Pt loadings within a single spot was successfully visualized. The inability to extract quantitative kinetic conclusions was reported as an inherent limitation of the RC mode that needed to be addressed to enable the detection of H2O2. Furthermore, it was suggested that by combining the RC-mode with the shearforce-based constant distance mode of SECM, the chronoamperometric features of the RC mode can provide a detailed insight into the catalytic activity at tip-to-substrate distances below 100 nm.106 Regardless of the noted drawbacks, the transient RC mode was successfully used to visualize the local ORR catalytic activity of Pt–Ag nanoparticles with different Ag content,411 patterned carbon nanotubes decorated with Pt nanoparticles,412 undoped and nitrogen-doped CNTs,413 metal nanoclusters (Pt, Au, Ru, and Rh and their co-deposits), CoS2,414 and lanthanide-based oxides (La0.6M0.4Ni0.6Cu0.4O3, M = Ag, Ba, Ce).415
In 2007, Eckhard and Schuhmann suggested a sequential dual imaging mode of SECM making it possible not only to monitor local activity but also to determine the selectivity of electrocatalysts concurrently. The principle as depicted in Figure 2a involves combining three different SECM modes (TG-SC, SG-TC, and RC) and applying a sequential pulse program. Thus, two images are obtained in parallel; one gives information about the activity of the catalyst while the other monitors H2O2 (if any) produced during the ORR (see Figure 2b,c). The local activity and selectivity of Pt and Au spots for ORR were visualized using a Pt-UME as SECM tip. The RC mode enabled effective visualization of the local activity of substrates and the SG-TC mode allowed H2O2 detection and a better understanding of the branching between the two-electron and four-electron pathway of ORR at a given potential.115 The same technique was employed to visualize the catalytic activity and selectivity of different electropolymerized metalloporphyrins,416 tetratolyl porphyrins (with Mn, Fe, and Co as central metal ions),108 to screen Pd–Pt and Pd–Au co-deposits for H2O2 reduction,417 bifunctional catalysts based on cobalt and nickel oxides embedded in nitrogen-doped carbon (CoxOy/NC, NixOy/NC), bifunctional catalyst materials (mixed Ni0.9Co0.1Fe2O4 oxide) in alkaline media,418 multiwalled carbon nanotubes (MWCNTs), cobalt protoporphyrin (CoP) and their composite (MWCNTs/CoP) in neutral solution419 during ORR.
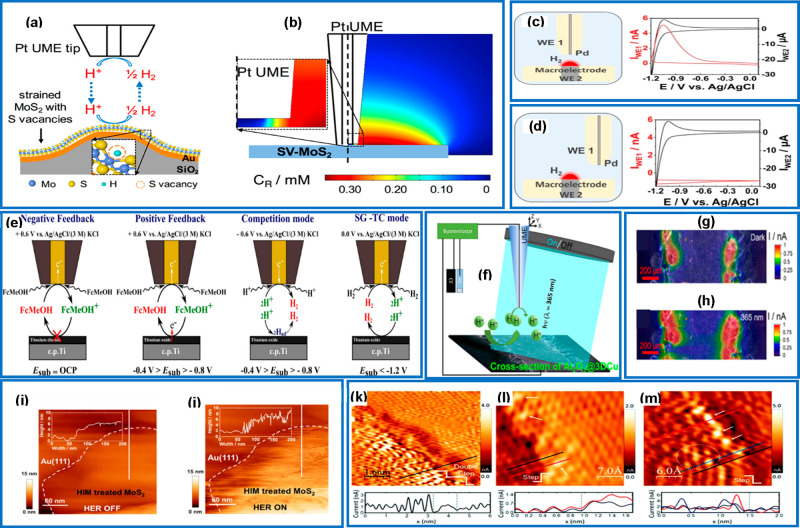
Figure 2.
(a) Schematic representations of different detection modes in SECM. (I) Tip generation–sample collection (TG-SC) mode. The scanning microtip oxidizes water to produce O2. The O2 reduction current at the sample is the analytical signal. (II) Sample generation–tip collection (SG-TC) mode. The sample is polarized to reduce O2. The tip oxidizes H2O2 which may be formed as a byproduct at the sample. The analytical signal is the peroxide oxidation current detected at the tip. (III) Redox competition mode (RC-SECM). The O2 reduction current is detected at the tip. Depletion of this current is correlated to a locally diminished oxygen concentration caused by a competing O2-reducing catalyst site of the sample, 3D false color representations of the obtained SECM images when the substrate was polarized at −400 V. (b) Reduction current at the tip due to competition for oxygen. Darker shaded blue areas correspond to a diminished current flow due to competition for the available O2 with active sites of the substrate. (c) Oxidation current of H2O2 at the tip concurrently registered. Reproduced with permission from ref (115). Copyright 2007 Elsevier Ltd. (d) Tip generation–redox competition–SECM images for O2 reduction on FeN4/rGO modified microelectrode. Color contrasts correspond to the measured cathodic tip current. Reproduced with permission from ref (420). Copyright 2016 Springer Nature. (e) Monitoring local ion activity changes in the water and hydroxide diffusion layer of an operating GDE and in dependence of its ORR rate is realized by voltammetric detection of the PtO reduction peak at a positioned Pt microelectrode. Reproduced with permission from ref (422). Copyright 2018 John Wiley & Sons, Ltd. (f) AFM/SECM mapping on Pt NPs performed using a Au-c-Pt tip: (I) topography, (II) oxygen reduction currents, and (III) peroxide oxidation currents recorded in N2-saturated on HOPG. Reproduced with permission from ref (423). Copyright 2019 John Wiley & Sons, Ltd.
Silva and colleagues, employed a TG-RC mode to map the activity of iron(III) tetra(N-methyl-4-pyridyl)porphyrin/reduced graphene oxide composite (FeN4/rGO) toward the ORR using a Pt-UME as SECM tip.420 The tip was first polarized at 1.25 V vs Ag/AgCl to generate O2 in the substrate–tip gap followed by an immediate polarization of both tip and substrate at sufficiently cathodic potentials to consume the O2 generated. Figure 2d shows the SECM image obtained for the ORR when the substrate was polarized at −0.50 V vs Ag/AgCl.420 Schulte and co-workers used the RC mode combined with shearforce-based constant distance positioning (4D-SF/SECM) to examine the topography and activity of gas diffusion electrodes toward the ORR.421 Botz and colleagues developed a method to monitor the activity of other redox species in parallel with the ORR (Figure 2(e)) which they implemented to visualize the changes in the local activities of OH– ions and H2O on the surface of an oxygen-depolarized cathode during the ORR in high alkaline medium.422 The substrate was polarized to induce the ORR consuming O2 and H2O and producing OH– while recording CV scans at a Pt-UME SECM tip for the detection of the OH– and H2O activities. The reduction of PtO on the surface of the tip was used to quantify local OH– and H2O activities which is a relevant information for the properties of high-current-density GDEs.422
Besides the use of shear force-based systems,421,424 SECM was hyphenated with other probe techniques to minimize the effect of sample topography on the measured tip current during ORR measurements. O’Connell et al. coupled SECM and SICM (SECM-SICM) to study localized H2O2 generation at individual Au nanoparticles (AuNPs) within ensemble electrodes during the ORR.425 They fabricated a theta (double-barrel) pipette which they used as the imaging probe and employed the SG-TC-SECM mode to detect H2O2 during the ORR at individual AuNPs. The potential of the substrate was varied to induce ORR while the SECM tip was biased at +1 V vs RHE to oxidize H2O2. The SICM hoping mode was employed to yield topographical information about the substrate.425 AFM-SECM operating in the noncontact mode was used to simultaneously map topography, oxygen reduction and detect peroxide intermediates on a bare highly oriented pyrolytic graphite (HOPG) surface and isolated Pt particles during ORR, using a Au-coated SiO2/Pt tip.423 During AFM-SECM image acquisition (Figure 2(f)) the substrate was polarized at 0.7 V vs SHE (for the ORR) and tip at 0.98 V vs SHE (for H2O2 oxidation), and it was concluded that the ORR on isolated Pt particles proceeds selectively via the two-electron pathway.423
Following the first successful application of the STM in the study of an electrode/electrolyte interface,81 EC-STM has found a wide range of applications in electrode processes during ORR electrocatalysis. In situ EC-STM (operated in constant-current mode with tungsten STM tips) was used to successfully investigate the effect on ORR of adlayer structures of metal porphyrin and phthalocyanine substrates including cobalt(II) and copper(II) phthalocyanines,429 iron octaethylporphyrin,430 iron phthalocyanine,287 and 5,10,15,20-tetraphenyl-21H,23H-porphine cobalt(II).426 Cai and colleagues used EC-STM operated under in situ conditions enabling them to monitor the interfacial reactions on the adlayer of metal porphyrins (CoTPP) on a Au(111) single-crystal electrode in gas-saturated (O2, N2 and air) 0.1 M HClO4 solution.426 During the ORR, the substrate was kept at 0.326 V vs SCE (to hinder the ORR) resulting in the formation of high-contrast adsorbed species in oxygen saturated electrolyte (suspected to be CoTPP-O2 complexes) before invoking ORR (by switching the potential to −0.074 V vs SCE). They observed that the final shift to the cathodic potential (invoking ORR) resulted in a shift from CoTPP adsorbates the initial high-contrast CoTPP adsorbates to low contrast, as shown in Figure 3a. This particular study demonstrates the ability of EC-STM to provide direct evidence of the catalytic role of metal porphyrins toward the ORR at the molecular level, information essential for designing such catalysts.426 It is known that when EC-STM is carried out under reaction conditions, the recorded analytical signal shows higher fluctuations (noise) at active sites compared to nonactive sites, which has been suggested as a valid tool to identify the location of active sites.81
Figure 3.
(a) Sequential STM images of a CoTPP monolayer on Au(111) in 0.1 M HClO4 saturated with oxygen at different potentials. Reproduced with permission from ref (426). Copyright 2016 John Wiley & Sons, Ltd. (b) Scheme explaining the concept. When the local environment between the STM tip and the sample changes (in this case, when the tip is over a terrace (I) versus a step (II)), the tunneling barrier also changes in a way that is driven by the changes in approaching and departing reactants and products. In this scenario, increased tunneling-current noise is likely to appear when the tip is over a step edge, which is more active than the terrace sites. If the STM is operated in constant-current mode, then the noise is revealed in the measured z-position (the height of the STM tip over the sample). (III) STM line scans (constant-current mode) obtained over a Pt(111) surface in 0.1 M HClO4 when the potential of the sample is either sufficiently negative or too positive to initiate the HER (which is therefore either “ON” or “OFF”). (IV) Typical STM line scan over the Pt(111) surface in 0.1 M HClO4 under HER conditions. (c) Histograms of the tunneling-current noise over a polycrystalline Au surface (I) and corresponding histograms obtained over a polycrystalline Pt surface (II), results of repetitive ORR “ON” and “OFF” STM noise experiments at the same terrace of a Pt(111) sample (III), STM line scans at the surface of a Pt(111) electrode under ORR conditions, where both terraces and steps can be visualized (IV). Reproduced with permission from ref (427). Copyright 2017 Springer Nature. (d) n-EC-STM measurement of a Pt(111) step and adjacent terraces. With decreasing potential, the overall noise level increases. For data evaluation, the terrace data (on the left) are used for calibration for the relation between noise level and activity on this sample (I), histograms after separating the data for terrace (black) and step (red). At every applied sample potential, the step shows a higher noise level relative to the terrace, as evident from the broader and less intense distribution. All potentials given are referred to the Pt quasi-reference electrode (II). Reproduced with permission from ref (428). Copyright 2021 John Wiley & Sons, Ltd.
In 2017, Pfisterer proposed and demonstrated a method to readily map and quantitatively distinguish the catalytic activity of active sites on electrocatalysts with high spatial resolution by monitoring relative changes in the tunneling current noise.427 The monitoring of noise in EC-STM (n-EC-STM) allowed them to directly evaluate the significance and relative contribution of different defects and sites to the overall catalyst activity of materials toward ORR and HER. Figure 3b is a sketch of the concept. To probe the performance of the STM noise measurement approach in mapping local catalytic activity during ORR, the authors deposited catalytically active platinum nanoparticles on less-active polycrystalline gold which they used as the substrate in 0.1 M HClO4. In the noise measurement, tunneling currents were recorded during the ORR on the pure Pt part as well as on the pure Au part by adjusting potentials to either induce ORR (ON) or hinder ORR (OFF), as shown in Figure 3c. This work portrays the superb ability of STM noise analysis in locating active sites even under the demanding conditions associated with electrochemical interfaces, although the results obtained were only qualitative.427 n-EC-STM has recently been applied to monitor and distinguish the activity levels to link structure to the ORR activity of Pt5Gd and Pt5Pr in acidic medium,52 and Pt(111)-based surfaces in different electrolytes.431 Haid and colleagues showed in 2020 that the hitherto qualitative n-EC-STM method could be extended to obtain quantitative information on the local activity. They demonstrated this by using the well-studied Pt(111) as an ORR catalyst in acidic media as model system. During the n-EC-STM measurements, the potential of the sample was set such that ORR occurred at the Pt(111) surface and the tip potential was gradually increased, from −0.05 V to −0.20 V vs Pt causing faradaic reactions at the tip. The authors were able to link the recorded noise, from ORR at a well-defined active site on the Pt(111) surface to the corresponding TOF estimated from the current recorded at the substrate, thus establishing a direct link between the variations in the STM signal or noise over an active site and the activity levels or rate of the reaction (Figure 3d).428
Table 2. Summary of Experimental Conditions for ORR Studies with SEPMs.
| SEPM | mode | substrate for ORR | tip | tip reaction | ref |
|---|---|---|---|---|---|
| SECM | FB | Pt | Au disk UME (Ø, 25 μm) | OER | (402) |
| SECM | TG-SC | Pt, Ru, and Au | Pt disk UME (Ø, 25 μm) | H2O oxidation | (99) |
| SECM | TG-SC | binary and tertiary combinations of Pd, Au, Ag, and Co (or Cu) on GC | W or Au disk UME (Ø, 25 μm) | H2O oxidation | (400) |
| SECM | SG-TC | Au, Pt, and PdCo alloy-modified GC | Pt disk UME (Ø, 25 μm) | H2O oxidation | (399) |
| SECM | SG-TC | Hg on Au | Pt or Au disk UME (Ø, 25 or 50 μm) | H2O2 oxidation | (404) |
| SECM | SG-TC | Hg, Au, Ag, Cu, Pt, Pd, Pd80Co20, and Au60Cu40 | Pt disk UME (Ø, 25 μm) | H2O2 oxidation | (403) |
| SECM | SG-TC | Pt and Pd nano/microstructure | Pt disk UME (Ø, 25 μm) | H2O2 oxidation | (406) |
| SECM | SG-TC | Fe porphyrins on GC | Au disk UME (Ø, 25 μm) | FeIII(OO2–) oxidation | (407)a |
| SECM | SG-TC | nanoporous Au and flat Au substrates | Pt disk UME (Ø, 25 μm) | H2O2 oxidation | (408) |
| SECM | TG-SC-TC | Au microdisk | microring-disk (ring Au thickness 750 nm, disk Pt Ø, 1 μm) | HO2– oxidation | (100) |
| SECM | RC | Pt spots on GC | Pt disk UME (Ø, 10 μm) | ORR | (106) |
| SECM | RC | Pt–Ag NPs (varying Ag content) | Pt disk UME (Ø, 25 μm) | ORR | (411) |
| SECM | RC | patterned CNTs decorated with Pt NPs | Pt disk UME (Ø, 25 μm) | ORR, H2O oxidation | (412) |
| SECM | RC | undoped and N-doped CNTs | Pt disk UME (Ø, 25 μm) | ORR | (413) |
| SECM | RC | Pt, Au, Ru, and Rh and their co-deposits | Pt disk UME (Ø, 25 or 50 μm) | ORR, H2O oxidation | (432) |
| SECM | RC | CoS2 | Pt disk UME (Ø, 10 μm) | ORR | (414)a |
| SECM | RC | La0.6M0.4Ni0.6Cu0.4O3 (M = Ag, Ba, Ce) | Pt disk UME (Ø, 10 μm) | ORR | (415)a |
| SECM | RC | tetratolyl porphyrins (containing Mn, Fe, and Co as central metals) | Pt disk UME (Ø, 25 μm) | ORR | (108) |
| SECM | RC, SG-TC | Pd–Pt and Pd–Au electrodeposits on graphite | Pt disk UME (Ø, 25 μm) | H2O2 oxidation | (417) |
| SECM | TG-SC, SG-TC, RC | Pt and Au on GC | Pt disk UME | ORR | (115) |
| SECM | RC, SG-TC | CoxOy/NC and NixOy/NC | Pt disk UME (Ø, 25 μm) | H2O2 oxidation | (433) |
| SECM | RC | Ni0.9Co0.1Fe2O4 mixed oxide | Pt disk UME (Ø, 25 μm) | ORR | (418) |
| SECM | RC | MWCNTs, CoP, and MWCNTs/CoP | Pt disk UME (Ø, 25 μm) | ORR | (419) |
| SECM | TG-RC | FeN4/rGO | Pt disk UME (Ø, 10 μm) | ORR | (420) |
| SECM | RC (4D-SF/SECM) | GDEs | Pt disk UME (Ø, ∼1.8 μm) | ORR | (421) |
| SECM | 4D-SF/SECM | GDEs | Pt disk UME (Ø, ∼1 μm) | PtO reduction | (422)a |
| SECM/SICM | SG-TC, hopping | Au NPs | Theta pipette filled with (aperture Ø, ∼200 nm) | H2O2 oxidation | (425) |
| AFM/SECM | noncontact | HOPG and isolated Pt particles | Au-coated SiO2 Pt (edge size 100 nm) | H2O2 oxidation | (423)a |
| EC-STM | noncontact | cobalt(II) and copper(II) phthalocyanines | W coated with nail polish | ORR tunneling current | (429) |
| EC-STM | constant current | iron octaethyiporphyrin, | W coated with nail polish | ORR tunneling current | (430) |
| EC-STM | noncontact | iron phthalocyanine | W coated with nail polish | ORR tunneling current | (287) |
| EC-STM | CoTPP on Au | W coated with nail polish | ORR tunneling current | (426) | |
| n-EC-STM | constant current | Pt on Au | W coated with nail polish | ORR noise | (427) |
| n-EC-STM | noncontact | Pt5Gd and Pt5Pr | ripped Pt/Ir alloy wire insulated with Apiezon | ORR noise | (52)a |
| n-EC-STM | noncontact | Pt(111)-based surfaces | ripped Pt/Ir alloy wire insulated with Apiezon | ORR noise | (431)a |
| n-EC-STM | noncontact | Pt(111) | Pt/Ir alloy wire insulated with Apiezon | ORR noise | (428)a |
Works published in the last 5 years.
3.2. Investigation of OER Activity at the Substrate Surface
The oxygen evolution reaction is another of the most studied reactions in electrochemistry. OER is involved in the generation process of many energy vectors which are considered promising for sustainable energy supply as well as in other industrial electrochemical processes such as cathodic protection, and metal recovery.434 For example, the OER represents a bottleneck in the production of hydrogen gas through electrochemical water splitting as it limits the overall efficiency of the process.435 The reason lies in the fact that this reaction has a complex mechanism based on four coupled electron–proton transfer steps, which results in sluggish kinetics and high overpotentials. The general mechanism pathways as proposed in the literature for the OER in different electrolyte media evidence the complexity of the process with multiple chemical and electrochemical steps and intermediates.436,437
The development of OER catalysts is of great importance for the future of eco-friendly power generation technologies. The state-of-the-art electrocatalysts for OER are noble metal-based materials like RuO2 and IrO2, but their scarcity and cost definitively hinder their large-scale application.438 Transition-metal oxide-based electrocatalysts (TMOs) are promising alternatives with comparable OER activity and robustness while being inexpensive and earth-abundant.439,440
Considerable progress has been attained in preparing OER catalysts, as materials science has been able to prepare an extensive variety of electrocatalysts in terms of compositions, structures, and morphologies.441 Nevertheless, the rational design of OER active materials is still challenging owing to the lack of knowledge about the mechanisms and the correlation between structure and catalytic performance.442 Electrocatalytic surfaces often present nonuniform properties which lead to high complexity for characterization and benchmarking as their activity depends on many parameters such as the preparative conditions, the aging, the used precursors, and their processing.443 Despite exhaustive work has been done on this matter, one can find a lot of contradictions in the published data; probably due to the variability in experimental conditions.15 Moreover, the structures and surface chemical properties of TMO-based electrocatalysts inherently change during the OER, whose intermediate states play an important role in the process.443 This highlights the need for studies in operando conditions which can provide information about the structure–activity relationship during the OER.442 As already discussed, information about the intrinsic activity of the active centers and their distribution across the surface is vital for catalyst optimization. SEPM techniques are powerful tools to shed light on the characterization of heterogeneous surfaces and the analysis of local electrochemical activity. In this section, we will in-depth gather and review the works that report operando mode SEPM techniques for the study of the OER along with the strategies to achieve an ever more accurate data acquisition. Among the SEPM techniques, SECM was definitively the most employed for the study of OER, possibly due to the same reasons stated in the ORR section (Scheme 4). Even though several modes are available, the SG-TC is by far the most employed in OER studies, which is by default an operando method. Scheme 4i–iv summarizes the operando SEPM configuration for OER evaluation, where the reaction products (O2 and H2O2) are collected at the SEPM tip. Since the reactant in OER is water (or OH– in alkaline conditions), which is already present in the solution, other SECM modes such as FB, TG-SC, and RC were not largely explored for studying the OER. A typical experiment in the SG-TC mode is based on the polarization of the catalyst-modified substrate to drive the OER reaction while the tip scans the catalyst surface. The tip is simultaneously polarized to continuously detect the formed products, generally O2. Figure 4a displays a scheme representing the working principle of an SECM experiment in operando SG-TC mode for the OER.444
Scheme 4. Schematic Drawing Representing the Main Operando SEPM Configurations for OER Interrogation.
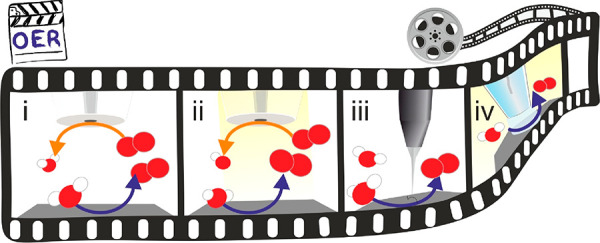
The SEPM tip acts as the spectator and the electrocatalyst’s activity is tailored by an actuator method. The SEPM tip can employed for (i) collecting the OER products formed at the sample in SG-TC-SECM (ORR at the tip, orange arrows); (ii) selectively collecting the OER product when the sample surface is locally irradiated in SPECM; (iii) atomic-resolution mapping of crystalline surfaces; (iv) evaluating the electroactivity of semiconductors with SECCM coupled with light source.
Figure 4.
(a) Schematic representation of the SECM setup to study OER in operando conditions in the SG-TC mode. Reproduced with permission from ref (444). Copyright 2014 Elsevier. (b) Current–potential curves for microcavity electrodes filled with RuO2. The sample current (black) and SECM tip current (gray) are plotted versus the applied potential (SECM tip: Pt disk electrode with 50 μm diameter, 0.1 M NaOH; v = 1 mV/s). Reproduced with permission from ref (448). Copyright 2015 Elsevier. (c) Pictorial representation of the operando SG-TC-SECM methodology used (from black to gray, indicating different chemical compositions), based on a double pulse potential profile applied to the substrate. Reproduced from ref (95). Copyright 2015 American Chemical Society. (d) High-throughput investigation of an array of MnCoFeNi oxide samples with different compositions using SG-TC-SECM. Reproduced with permission from ref (449). Copyright 2022 Elsevier. (e) Characterization of an industrial DSA anode under OER conditions (Esub = 0.700 V, Etip = 0.400 V) with the noise mode of SG-TC-SECM: SG-TC mode image (I) and corresponding amplitude-by-frequency parameter image of the same electrode area (II). Reproduced with permission from ref (450). Copyright 2012 Royal Society of Chemistry. (f) Schematic representation of the experiments: (i) positive feedback produced by oxidation/reduction of ferrocenemethanol and (ii) SG-TC for oxygen detection at a NiO nanosheet. SECM imaging of NiO nanosheets with defect holes: (iii) feedback mode (III) and SG-TC mode SECM images of the same area of the NiO nanosheet (IV). The substrate was either at (III) open-circuit potential or (IV) biased at ESub = 0.9 V. Reproduced with permission from ref (451). Copyright 2019 PNAS. (g) Scheme of the SG-TC-SECM study of a nickel foam toward both HER and OER. Reproduced with permission from ref (452). Copyright 2020 Royal Society of Chemistry.
One of the first works in which OER is studied by SECM in the SG-TC mode was published in the early 2000s. Fushimi et al.445 studied the anodic oxidation of a titanium electrode in acidic media and measured the simultaneously produced O2 by conducting ORR at the SECM tip, a 10 μm diameter Pt UME. They found that OER preferentially takes place on Ti grains covered by a thin oxide film. Also, they could observe that the oxide film of twin grains is easily broken, becoming an active site. Some years later, in 2007, Kang and co-workers studied the oxidation process on a Ni electrode surface in alkaline electrolyte using SECM in the SG-TC mode, gaining insight into Ni surface transformations prior to the OER.446 The tip was not polarized to directly collect the O2 and the oxidation/reduction voltammetric profile of a Pt UME was used to determine the local pH changes near the Ni surface as a result of the electron-transfer processes occurring in the solid phase. The potential of the Ni substrate was swept to promote the formation of surface oxides and OER while the tip monitored the consequent transient pH variation near the substrate’s surface.
Using this nonconventional SG-TC mode, the researchers could study the electrochemical oxidation of the Ni surface and determine the overpotential of the OER at the formed Ni oxidehydroxide electrocatalyst surface. The OER overpotential is used as a benchmarking parameter to compare the performance of different electrocatalysts and, therefore, its accurate determination is of paramount importance. Snook et al.447 reported the use of the SG-TC mode to accurately indicate the OER overpotential for different Ni/Ni(OH)2-based electrode materials. The capability of the SECM was demonstrated compared to macroscale cyclic voltammetry, which failed to discriminate between the Ni oxidation potential and the OER overpotential. SECM in the SG-TC mode was also employed to measure the overpotential of perovskites as OER catalysts such as La0.6Sr0.4Fe0.6Co0.4O3, La0.6Sr0.4FeO3, La0.74Sr0.2Fe0.8Co0.2O3, and La0.6Sr0.4FeO3.448 A Pt UME was employed as SECM tip to monitor the evolved O2 at the electrocatalysts surface and the high sensitivity and accuracy of the SG-TC-SECM mode was demonstrated by comparison with RuO2 as internal standard. Figure 4a summarizes the principles of this strategy.444 The accuracy in the determination of the OER overpotential was further improved.448 On the one hand, microcavity electrodes (MCE) filled with the interrogated catalyst (La0.6Sr0.4FeO3, La0.74Sr0.2Fe0.8Co0.2O3, or La0.6Sr0.4FeO) to avoid the use of binders or other additives that may influence the overpotential. Also, a Pt nanotip was accurately and reproducibly positioned close to the catalyst surface using the shearforce-based constant distance mode of SECM (SF/CD-SECM). Double-barrel MCEs were developed, which allowed for the simultaneous study of the OER with the material of interest and a benchmark material like RuO2, used as an internal standard. This approach ensures a fair comparison between the materials as the measurements are conducted under the same experimental conditions. Figure 4b shows the overlapping of the substrate and the tip signals versus the potential applied to the substrate, which accurately reveals the OER overpotential.
The investigation of different OER catalysts in the same experiment is of great interest to achieve a valid comparison and consistent benchmarking. SECM enables high-throughput investigations by rapidly screening electrocatalysts libraries with varying compositions or structures, all prepared on the same support. Nevertheless, some precautions need to be considered when using the SG-TC mode in high-throughput experiments with an array of samples since the overlap of the diffusion profiles between neighboring spots have to be avoided. In the specific case, the tip current would be a consequence of the O2 produced by surrounding spots under investigation, thus misrepresenting the local activity and compromising the resolution. Bard and colleagues reported some strategies to mitigate this issue.453 In 2008, Minguzzi et al. introduced a tip shield consisting of a gold layer deposited on the external wall of the SECM tip.453 The tip Au shield was polarized along with the tip Au-UME to consume the oxygen coming from the neighboring spots and guaranteed the correct measurement of the OER activity of the underlying sample area. Experimental and simulation studies allowed for a successful screening of the OER activity of different combinations of mixed Sn–Ir oxides. Nevertheless, the fabrication of the shielded tip was tedious and time-consuming. For this reason, the same research group reported in 2015 a simpler strategy for the rapid screening of an array with similar mixed Sn–Ir oxides.95 The new method was based on the application of a series of double-potential steps (up to 10) to the substrate, switching between potentials of OER activity and inactivity. The dual potential step is applied shortly to minimize the O2 diffusion layer and thus the influence of the response from surrounding spots. A regular disk-shaped Au UME was employed as SECM tip, and the response is coaligned to the underlying electrocatalyst spot (Figure 4c). In a recent paper, Zhang et al.449 conducted exhaustive high-throughput experiments by means of the SG-TC for rapid screening of a library of OER catalysts based on a Mn–Co–Fe–Ni multicomponent metal oxide system prepared by the inkjet printing in O2-free 1 M KOH solution (Figure 4d). A potential program was used to accurately measure the OER activity using square wave voltammetry up to 0.7 V vs Ag/AgCl (OER activity) and alternating potentiostatic steps at 0.3 V vs Ag/AgCl (no OER activity), while the SECM Pt tip (10 μm) was polarized to detect the evolved O2. Using this method, Mn5Co10Fe30Ni55Ox was found to be the most active OER catalyst. It can be concluded that the SG-TC mode of SECM is a powerful tool allowing for high-throughput investigations of the OER properties of catalyst libraries under operando conditions. The formation of O2 bubbles remains an intrinsic challenge in operando OER investigation as continuous bubbles formed on the surface electrically insulate the active sites. The blockage of the surface results in variations in activity, increased overpotentials, change in apparent solution resistance and leads to a localized convection effect.454 Due to this issue, the SG-TC mode of SECM is generally limited to the investigation of the local electrocatalytic OER activity at low current densities to avoid the blockage of the surfaces (tip and sample). Chen et al.455 reported an interesting example of an SECM study using the operando SG-TC mode to interrogate the evolution of gas bubbles on the surface of OER catalysts, once the impact of bubbles formation and growth is a relevant feature. The authors studied the OER on RuO2 and some industrial O2-forming catalysts and used a Pt UME to measure the evolved O2. The authors employed the so-called “‘noise mode”’ of SECM to investigate how O2 bubbles were formed and departed from the surface. The noise of the tip current is used to deconvolute the contribution of the local formation of bubbles, correlated to the rate of oxygen evolution reaction.450Figure 4e presents an example of SG-TC-SECM images of OER activity (left) and the tip current noise (right) for the same area of an OER catalyst. The local formation of bubbles across the surface of the catalyst and their departure could be monitored, which is an influencing aspect of OER electrodes that should not be overlooked.
SECM in operando SG-TC mode has also been demonstrated to study the surface of OER catalysts. For example, Konkena et al.456 used the SECM in the SG-TC mode to study OER electrocatalysis of NiPS3@NiOOH core–shell heterostructures in an O2-saturated 0.1 M KOH solution. By combining the SECM results with Raman spectroscopy, SEM, and XPS measurements, the high OER activity of the NiPS3 nanosheets is attributed to a high density of accessible active metallic edges and defect sites due to a structural disorder. Sun et al.451 demonstrated the capability of the spatial resolution of operando SG-TC-SECM to assess the local electrocatalytic OER activity of semi-2D NiO nanosheets. A spatial resolution of 15 nm was achieved by using a Pt nanotip. The FB mode with a ferrocene redox mediator was used to obtain topographic information (Figure 4f(I,III)) and SG-TC-SECM mapped the local OER activity to ascertain the higher activity of the edges (up to 200 times higher) as compared to the fully coordinated surfaces (Figure 4f(II,IV)). Note that the FB mode was employed to determine the topographic contribution and it is not related to the analysis of the OER electroactivity.
Additionally, the operando SG-TC mode of SECM has demonstrated its versatility for studying of different reactions in the case of bifunctional electrocatalysts. For example, Gao et al.452 employed the SG-TC mode to investigate a Ni foam-based monolithic electrode with high performance for both HER and OER in neutral media. Figure 4g shows a scheme in which SG-TC-SECM was explored to correlatively study both the OER and HER. Other recent examples studying bifunctional electrocatalysts for the OER and ORR have been presented by Chakrabarty et al. and Lu et al., who used the SG-TC mode to investigate the local OER in alkaline media on flower-like ZnCo2O4 grafted onto reduced graphene oxide457 and a Co–B,N,S–graphene composite at different temperatures,458 respectively. Moreover, the SECM tip is not limited to the detection of evolved O2 during the OER. Iffelsberger et al.459 employed a Pt UME (25 μm) to monitor reactive oxygen species (ROS) evolved during water oxidation at Pt and boron-doped diamond (BDD) macroelectrodes in acid media (0.2 M HClO4). Forced convection was enabled by high precision stirring to form a stable diffusion layer of electrochemically produced species and different ROS species. Counihan et al.460 also employed this strategy to study water oxidation at a BDD surface in different electrolytes (Figure 5a). Besides O2, the Au-UME tip was used to detect other evolved products like ROS. The OER products (O2, H2O2, and ROS) can be selectively collected at the tip by carefully selecting the applied potential. The study indicated that the condition to evolve more reactive species is at pH 11 in sulfate, nitrate, and perchlorate electrolytes. Moreover, the results correlated the OER products with the chemical heterogeneity of the BDD surface. To characterize the surface, the surface interrogation mode of SECM (SI-SECM) was employed as a correlative measurement. The SI-SECM mode was able to quantify the surface coverage and the chemical characteristics of surface intermediates (mainly adsorbed OH).
Figure 5.
(a) SG-TC-SECM experiments with a BDD electrode during water oxidation: tip current responses at different tip potentials (top) corresponding to evolved products during voltammetric sweeps of the BDD substrate (bottom) (I). The electrolyte was 0.1 M sulfate, pH 2; the scan rate was 50 mV/s. Comparison of tip currents in 0.1 M sulfate electrolyte at various pH values with the tip biased to reduce oxygen at 0.0 V vs RHE (II). Comparison of collection currents in pure water and 0.1 M electrolyte, buffered at pH 11 for various electrolytes containing different anions. The tip was biased to collect ROS to 1.4 V vs RHE. Asterisks denote features on cathodic and anodic substrate scans (III). Reproduced with permission from ref (460). Copyright 2019 John Wiley and Sons. (b) Scheme of the through-tip illumination approach to perform SPECM experiments with high resolution. Reproduced from ref (196). Copyright 2017 American Chemical Society (c) Scheme of SG-TC-SECM to study the photoelectrochemical water oxidation at Nb-doped TiO2 under through tip illumination (left), SECM OER reactivity map of a specific area of the 0.5% doped Nb:TiO2 crystal in a 0.1 M phosphate buffer (pH 7). ETip = −0.9 V; ES = 0 V vs Hg/HgSO4 (right). Reproduced from ref (199). Copyright 2019 American Chemical Society (d) Schematic of the investigation of potential-dependent interfacial charge-transfer kinetics of PEC water oxidation at TiO2 nanorods using SECM: reduction of IrCl62– by photogenerated electrons at OCP (I); simultaneous oxidation of water and IrCl63– by photogenerated holes when ESub = 0.46–1.66 V (vs RHE) (II). Reproduced from ref (461). Copyright 2021 American Chemical Society. (e) Scheme of the SG-TC-SECM combined with Raman data acquisition (left) and potential profile where the UME is held at a constant potential (−0.6 V) while the sample electrode (WE1) is modulated with discrete potential steps in the positive direction. Reproduced from ref (462). Copyright 2017 American Chemical Society. (f) Scheme depicting the principle of n-EC-STM. (I) Without a reaction occurring (“off”) the EC-STM signal is stable. (II) An ongoing reaction (‘‘on”) will increase the noise level of the STM signal. The noise is most distinct when the tip is placed over an active site compared to an inactive site. Reproduced with permission from ref (463). Copyright 2021 Elsevier.
SI-SECM provides relevant mechanistic and kinetic information, which is not easily accessible by other spectroscopic and voltammetric techniques.137 The SI-SECM mode is widely employed for the study of OER electrocatalyst surfaces, because it enables the identification of different active sites within the same OER catalyst,464 the quantification of short-lived reaction intermediates,134,465 the detection of different surface metal oxidation states466 and the calculation of kinetic rate constants of the active sites.467 Nevertheless, the use of SI-SECM to interrogate the surface toward the OER, is not considered operando as the electrochemical modulation of the substrate surface and the signals registered from the tip are taken at separate times.
In parallel to the study of OER at TMOs, SECM has also been extensively employed in the study of photoelectrochemical systems. Local activity studies are generally performed by the operando SG-TC mode, whereby the generation of O2 at photoanodes is monitored by ORR at the tip (Figure 5b), while the interrogated surface is illuminated.196 In 2008, the Bard group introduced an operando SG-TC mode of SECM for rapid screening of photocatalysts arrays.468 An optical fiber modified with a Pt-plated Au ring that locally illuminated the spots and the tip detected the evolved O2 by performing ORR. The spots consisted of BiVO4 and Zn-doped BiVO4 with various ratios of Bi:V:Zn. Later the same strategy was used to study arrays of photoelectrocatalysts for water oxidation469 in different BiVO4-based systems containing various third element compositions, showing that the doping rate with 5–10% of tungsten noticeably enhanced the properties of BiVO4 as photoanode. A similar approach was used to show the enhancement of the OER activity by depositing Pt and Co3O4 on a semiconductor film based on 5% W-doped BiVO4 (BiVW-O).470 However, the Au-coated optical fiber provided low lateral resolution in the photo-SECM analysis. The resolution was improved later by a simple and promising approach introduced by Conzuelo et al.,196 where a light fiber was coupled to the backside of a 25 μm diameter Pt-disk UME. The SECM tip acted simultaneously as an electrochemical probe and a light guide, which minimized interferences from light screening, reflections, and scattering. The feasibility of this strategy was demonstrated by the investigation of n-type semiconductor-based photoanodes for water splitting such as BiVO4, Mo-doped BiVO4, and CoOx on Mo-doped BiVO4, all deposited on FTO, as well as sharp-edged TiO2. Later, the group of Mirkin reported nanoscale resolution using the through-tip illumination approach.199,408 Water oxidation at a 0.5% Nb-doped:TiO2 rutile (110) single crystal photoelectrocatalyst (Figure 5c). In the work by Sarkar et al.,471 the same method was employed to study the photoelectrochemical water oxidation of TiO2 nanorods. Interestingly, they used a TEM finder grid as conductive support, which allowed complementing the local photo-SG-TC-SECM findings with the atomic-scale structural and bonding information obtained by transmission electron microscopy (TEM), to address the limitations in the SPECM resolution. Despite the nanoscale resolution achieved, one limiting issue was still the mechanical interaction of the rigid optical fiber during the tip motion. In a recently published paper, Askarova and colleagues472 have reported an improved experimental setup in which the tip is mechanically decoupled from the fiber and the light is delivered to the back of the tip capillary using a complex lens system, with no significant losses in intensity.
Li and Pan461 published a study using the SECM with three modes of SPECM (FB, RC, and SG-TC) in operando conditions to study the properties of TiO2 nanorods coated on FTO as photoanode toward photoelectrochemical water oxidation. The FB mode and RC modes were used to study the interfacial charge-transfer kinetics using the redox mediator IrCl62–/IrCl63–. The SG-TC mode was employed to study the OER activity of the substrate by detecting O2 with the Pt UME tip. Figure 5d provides a graphical summary of the investigation conducted, with a scheme for each one of those different processes. A paper recently published by Iffelsberger, Ng, and Pumera utilizing the SG-TC-photo-SECM is worth noting.473 Photoelectrocatalysis of TiO2 toward different reactions of interest like OER, HER, and the chlorine evolution reaction (CER) were studied. Cyclic voltammograms were registered at the TiO2-modified substrate to select the potential windows where the different reactions take place. Simultaneously a Pt UME (25 μm diameter) was polarized to specific potentials to convert the corresponding species: H2, O2, Cl2, or ROS. In addition to the local analysis, activity maps were registered, with and without illumination, for the different reactions by selectively applying potentials at the substrate and monitoring the corresponding product at the polarized tip. This SG-TC study concluded important findings, such as a heterogeneity of the activity and selectivity of the TiO2 surface despite the apparently uniform composition according to the morphological characterization. Illumination dramatically changes the selectivity of the competing oxygen and chlorine evolution reaction. The capabilities of the SG-TC mode of SECM as well as the correlative SG-TC and FB modes in SPECM measurements were demonstrated to interrogate materials with respect to the OER in a high-throughput mode. Additionally, the SG-TC-SECM mode has also been combined with other techniques to study the OER, providing spectroscopic or morphological information during the reaction or, in other words, in operando conditions. Steimecke et al.462 reported a Raman/SECM methodology to investigate OER at Ni and Ni/Fe thin-film electrodes deposited on ITO in 0.1 M KOH solution. During the OER, a UME was placed closely above the substrate to detect the evolved O2, while a Raman microscope probed the same spot from below (Figure 5e).462 The authors were able to obtain information about the OER activity and the structural changes tof the materials during the reaction. In 2021, Ju and collaborators474 used the SG-TC-SECM mode coupled with AFM to study the effect on the OER electrocatalysis caused by intercalating polypyrrole (ppy) in a NiFe-layered double hydroxide (NiFe-LDH). A Pt-nanoelectrode tip detected O2 while OER was conducted at the substrate. At the same time, the topography was visualized by AFM, which permitted to decouple the topographic and electrochemical information. The combination of both techniques allowed to demonstrate that the as-prepared NiFe-LDH-ppy exhibited a twice as high catalytic current density than the bare NiFe-LDH.
Most SEPM techniques used to study the OER under operando conditions are based on SECM, particularly on the SG-TC mode. Recently, SECCM was coupled to a UV radiation source to probe the photoelectrochemical reactivity of TiO2 nanotubes toward the OER. The operando SECCM measurements were performed under dark and light conditions to reveal photoelectroactive sites of the photoanode material. The high-resolution SECCM maps did not show a clear difference between the reactivity on the top and wall regions TiO2 nanotubes. However, this work demonstrated the capability of coupling a method for modulating the surface activity while simultaneously using SECCM to monitor the differences in the OER activity.364 To the best of our knowledge, SICM has not been used for the investigation of the OER in operando conditions. We understand that the reason is due to the intrinsic limitation of SICM of not being operando by default. Several works have been published in recent years based on the operando EC-STM technique. Stumm et al.475 investigated the structural properties of layered cobalt oxides on Au(111) in a potential window from OCP to the OER region by EC-STM. EC-STM is proved capable of providing structural information with high resolution directly under potential control. Hence, such measurements are considered operando studies since the EC-STM images are registered while a potential is applied to the substrate. Combining the results of EC-STM with cyclic voltammetry and EC online inductively coupled plasma mass spectrometry; the structure, mobility, and stability of a material can be analyzed. Kluge et al. reported for the first time the suitability of the noise mode of EC-STM under reaction conditions (“noise” or n-EC-STM) to study electrochemical reactions.53,428 For example, n-EC-STM was employed to study the OER activity of an amorphous iridium oxide surface, which is formed during the electrochemical cycling of an Ir(111) single crystal.463 With this mode, active areas can be detected when the noise level of the STM signal increases, in contrast to the case of inactive sites (Figure 5f). An electrochemical reaction in the gap between the tip and the surface can locally and continuously rearrange the electrolyte structure and change its composition, which influences the tunneling barrier. By doing so, the authors could monitor local OER activity in operando conditions while analyzing simultaneously the surface morphology.
Table 3. Summary of Experimental Conditions Made in OER Studies with SEPMs.
| SEPM | mode | substrate for OER | tip | tip reaction | ref |
|---|---|---|---|---|---|
| SECM | SG-TC | polycrystalline Ti | Pt disk UME (Ø, 10 μm) | ORR | (445) |
| SECM | SG-TC | Ni | Pt disk UME (Ø, 100 μm) | pH sensor: | (446) |
| Pt + OH– → PtOH + e– | |||||
| PtOH + OH– → PtO + H2O + e– | |||||
| SECM | SG-TC | Ni-based materials and Ni(OH)2 | Pt disk UME (Ø, 10 μm) | ORR | (447) |
| graphite, glassy carbon, ITO | |||||
| SECM | SG-TC | La0.6Sr0.4Fe0.6Co0.4O3 and RuO2 | Pt disk UME (Ø, 25 μm) | ORR | (444) |
| SECM | SG-TC | different perovskites La0.6Sr0.4FeO3, La0.6Sr0.4FeO3, La0.74Sr0.2Fe0.8Co0.2O3, and RuO2 in double-barrel microcavity electrodes (Ø = 100 μm) | Pt disk UME (Ø, 50 μm) | ORR | (448) |
| SECM | SG-TC | array of Sn1–xIrxO2 mixed oxides (x = from 0 to 1) | Au disk UME (Ø, 100 μm) shielded with an outer Au layer | ORR | (453) |
| SECM | SG-TC | array of Sn1–xIrxO2 mixed oxides | Au disk UME (Ø, 100 μm) | ORR | (95) |
| SECM | SG-TC | Mn–Co–Fe–Ni multicomponent metal oxides in an array of FTO as substrate | Pt disk UME (Ø, 10 μm) | ORR | (449)a |
| Mn5Co10Fe30Ni55Ox as the best OER performing material | |||||
| SECM | SG-TC (noise mode) | RuO2 | Pt disk UME (Ø, 25 μm) | ORR-current noise measurement at the tip due to gas bubble departure | (455) |
| O2-evolving industrial catalyst | |||||
| SECM | SG-TC | NiPS3@NiOOH core–shell heterostructure | Pt disk-nanoelectrode | ORR | (456) |
| SECM | SG-TC | NiO nanosheets on HOPG | Pt disk-nanoelectrode (Ø, 80 nm) | ORR | (451)a |
| SECM | SG-TC | Ni foam-based monolithic electrode | Pt disk UME | ORR/HOR | (452)a |
| SECM | SG-TC (OER) | Co–B,N,S–graphene composite | Pt disk UME (Ø, 25 μm) | ORR | (458)a |
| SECM | SG-TC | flower-like ZnCo2O4 grafted onto the reduced graphene oxide | Pt disk UME (Ø, 25 μm) | ORR/(H2O2 oxidation) | (457)a |
| SECM | SG-TC | Pt | Pt disk UME (Ø, 25 μm) | ORR (Etip = 0.3 V vs Ag/AgCl) | (459)a |
| BDD | ROS reduction (Etip = 1 V vs Ag/AgCl) | ||||
| SECM | SG-TC SI (no operando) | polycrystalline BDD | Au disk UME (Ø, 25 μm) | ORR (0.0 V vs RHE) | (460)a |
| Pt disk UME (Ø, 25 μm) | H2O2 → O2 (>1.0 V vs RHE) | ||||
| ROS reduction (0.4–1.4 V vs RHE) | |||||
| SPECM | SG-TC | BiVO4 | Pt-plated Au ring optical fiber | ORR | (468) |
| Zn-doped BiVO4 (different ratios Bi:V:Zn) | ring diameter = 275 μm | redox couple: | |||
| [Fe(CN)6]3– + e– → [Fe(CN)6]4– | |||||
| E° = 0.45 V vs Ag/AgCl | |||||
| SPECM | SG-TC | BiVO4-based photocatalysts containing various third element compositions | Pt-plated Au ring optical fiber | ORR | (469) |
| ring diameter = 275 μm | |||||
| SPECM | SG-TC | Pt and Co3O4 supported on 5% W-doped bismuth vanadate film | Pt-plated Au ring optical fiber | ORR | (470) |
| ring diameter = 275 μm | |||||
| SPECM | SG-TC | BiVO4 | Pt disk UME (Ø, 25 μm) through-tip illumination | ORR | (196) |
| Mo-doped BiVO4 | |||||
| CoOx on Mo-doped BiVO4 | |||||
| sharp-edged TiO2 | |||||
| SPECM | SG-TC | 0.5% doped Nb:TiO2 (110) rutile single crystals | Pt (Ø, 580 nm) through-tip illumination | ORR | (199)a |
| redox couple: | |||||
| FeMe(OH)+ + e– → FeMe(OH) | |||||
| E°′ = −0.2 V vs Hg/HgSO4 | |||||
| SPECM | SG-TC | TiO2 nanorods supported on a conductive (Au) TEM grid | Pt (Ø, 115 nm) through-tip illumination | ORR | (471)a |
| redox couple: | |||||
| FeMe(OH)+ + e– → FeMe(OH) | |||||
| E°′ = −0.2 V vs Hg/HgSO4 | |||||
| SPECM | SG-TC | BiVO4 0.5% doped Nb:TiO2 (110) rutile single crystals | Pt (Ø, 110 nm) through-tip illumination decoupled from scanning by a system with lenses | ORR | (472)a |
| redox couple: | |||||
| FeMe(OH)+ + e– → FeMe(OH) | |||||
| E°′ = −0.2 V vs Hg/HgSO4 | |||||
| SPECM | SG-TC | TiO2 nanorods-coated FTO UMEs (Ø = 25 μm) | Pt disk UME (Ø, 25 μm) | ORR | (461)a |
| FB | redox couple: | ||||
| RC | IrCl62– + e– → IrCl63– | ||||
| E° = 0.68 V vs Ag/AgCl | |||||
| SPECM | SG-TC | TiO2 | Pt disk UME (Ø, 25 μm) | ORR | (473)a |
| Raman/SECM | SG-TC | Ni and Ni/Fe thin films | Pt | ORR | (462) |
| AFM/SECM | SG-TC | polypyrrol intercalated in NiFe-LDH deposited on Au-on-Si | Pt-coated nanoelectrode tip (Ø, ∼25 nm) | ORR | (474)a |
| SECCM/optical probe | SECCM/UV source | TiO2 nanotube | SECCM capillary (Ø, 20–100 nm) | photocurrent due to OER occurring at the sample surface. | (364)a |
| EC-STM | noise mode | highly oriented pyrolytic graphite (HOPG) | ripped Pt/Ir alloy wire (Pt80/Ir20); insulated with Apiezon wax | tunneling current | (53)a |
| EC-STM | constant tip potential | cobalt oxide on Au(111) | W STM tip insulated with Apiezon wax | tunneling current | (475)a |
| EC-STM | noise mode | Ir oxide layer after anodically annealed Ir(111) single crystal | ripped Pt/Ir alloy wire (Pt80/Ir20); insulated with Apiezon wax | tunneling current | (463)a |
Works published in the last 5 years.
3.3. Investigation of HER Activity at the Substrate Surface
The hydrogen evolution reaction (HER) is a fundamental process in electrocatalysis that plays an important role in water electrolyzers, the chlor-alkali industry, and chlorate cells.476 Furthermore, H2 occupies a very important niche as an indispensable raw material in petroleum refining (hydrocracking and desulfurization), production of steel, aluminum, and ammonia (Haber Bosch process) as well as in the transformation of CO2 into valuable chemical feedstocks. Besides the highest gravimetric energy density of H2, HER has attracted great attention because the process yields highly pure H2 (>99.999%) at fast production rates under mild reaction conditions, and it has a low pollution margin; thus HER is pegged as a potential alternative to fossil fuels.477−480
The HER is a classic example of a two-electron-transfer process where protons and water are reduced to H2 in acidic and alkaline media, respectively. Mechanistically, HER involves three basic steps; the Volmer reaction, which results in the adsorption of a proton on the electrode surface, followed either by the Heyrovsky and/or the Tafel reaction to produce H2.477,478
Although generally regarded as a fast reaction compared to the OER, the very slow kinetics of HER in alkaline media has revived a renewed interest to understand the fundamental parameters governing the reaction. In alkaline media, HER undergoes more complex steps of adsorption and dissociation of water in an environment full of hydroxyl (OH–) disturbances. Furthermore, it is known that the HER kinetics strongly depends on the electronic properties of the electrode material and the nature of electrolyte used.477 To this end, operando SEPMs come in handy as they provide a direct way to disclose the HER kinetics at active sites with high mass transport conditions, hard to achieve with others techniques.477Scheme 5 summarizes the strategy for HER studies with operando SEPM. Most works explored the strategy to perform HER on the catalyst surface while HOR is carried out by the SEPM tip.
Scheme 5. Schematic Drawing Representing the Main Operando SEPM Configurations for HER Interrogation.
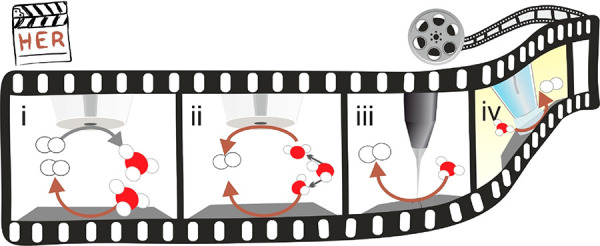
The SEPM tip is employed as spectator while the catalyst is modulated to perform HER (brown arrow): (i) SG-TC-SECM case, where the tip oxidizes the H2 (HOR, gray arrow); (ii) HER is also performed at the SECM tip; (iii) EC-STM mapping of a polarized surface; (iv) evaluating the electroactivity of semiconductors towards HER in dark and light conditions by SECCM.
The work by Selzer et al. is perhaps the earliest study to use SECM operated in the FB mode for the study of electron-transfer processes during proton reduction by methyl viologen radical cations at a Pt electrode, although not under operando conditions.481 The same approach was used later to study HER kinetics at Au NPs,482 Pd nanoparticles capped with 4-dimethylaminopyridine (Pd-DMAP),483 and 2D assemblies of Pd on an inert support.484 Although not conducted under operando conditions, these studies have contributed to the established framework presently used for HER investigations using SECM.485 Initially, operando SECM use was applied to fundamentals studies of HER at platinum substrates. SECM was essential to achieve high mass-transport conditions to quantify kinetic parameters of the HER on microelectrodes, while the substrate was polarized to HOR. Zhou and co-workers486 used the operando SECM to calculate the H2 oxidation rate on Pt in the presence of adsorbed halide anions. Zoski487 employed the same approach to calculate HOR kinetic parameters at Pt and Ir. Later, Fernandez and co-workers488 detailed the use of SECM to reveal the potential-dependence transition between Volmer–Tafel and Volmer–Heyrovsky steps and mechanistic routes on Pt and Au SECM tips. Leveraging this framework, Li and colleagues reported the kinetics of HER at newly identified active sites that consist of S vacancies on the basal plane of MoS2, by utilizing two approaches of SG-TC mode in tandem with finite difference method (FDM) modeling. In the first experiment, the MoS2 substrate potential was swept from 0 V to −0.7 V vs Ag/AgCl to generate hydrogen continuously, and the hydrogen was then oxidized at a Pt SECM tip. In the second study, the substrate potential was switched between the open circuit potential (OCP) and the negative potentials for the HER by dual-potential pulses. Figure 6a,b shows schemes of the adopted strategy using the SG-TC setup and the complementary simulation for the spatial distribution of the hydrogen concentration.485 The reliability of the SECM measurement were confirmed by making four replicates of the experiment on the same substrate, and complementary measurements using a three-electrode electrochemical compression cell. Results were found to be consistent despite variations in electrolytes and sample dimensions.485 The same SG-TC strategy with a Pt SECM tip to oxidize the evolved hydrogen was used to study the HER activity at a MoSx catalyst in a flow cell under flow and stationary conditions,489 individual Au NPs (on HOPG/polyphenylene),490 in situ growth of NiCoP grains on Ti3C2Tx MXenes (NiCoP@MXene) supported on HOPG,491 and active-metal frameworks (Al2(OH)2-TCPP and Cu-based MOF HKUST-1).492
Figure 6.
(a) Schematic of the SECM measurement setup for operation in the SG-TC mode. The top trapezoid represents a Pt UME tip, and the bottom a strained MoS2 monolayer with S vacancies functions as the working electrode. H2 is generated at the MoS2 substrate through HER and subsequently collected and oxidized at the Pt UME tip through HOR, Schematic of the complementary COMSOL simulation for the spatial distribution of hydrogen concentration (CR/mM). The left side shows the tip and substrate 2D configuration used in the simulation. (b) Right side shows a representative calculated CR distribution (condition: SV-MoS2 potential at −0.6 V and Pt UME tip potential at 0 V in 0.1 M HClO4). Left inset: zoomed-in view of CR near the tip–substrate gap. Bottom inset: color bar of CR. Reproduced from ref (485). Copyright 2016 American Chemical Society. (c) Schematic of a Pd-modified microelectrode positioned at a second Pt macroelectrode; i–t curve of H2 absorption at the Pd microelectrode (red) was recorded for the duration of the cyclic voltammogram recorded at the Pt macroelectrode (gray); for clarity, the time axis is not plotted. (d) Schematic of a Pd-modified microelectrode positioned over the insulating sheath, i–t curve H2 absorption at the Pd-modified microelectrode (red) and cyclic voltammogram recorded at the Pt macroelectrode (gray). Reproduced with permission from ref (493). Copyright 2022 John Wiley & Sons, Ltd. (e) Schematic illustration of the FB, RC, and SG-TC detection modes. Reproduced with permission from ref (111). Copyright 2020 Elsevier. (f) Schematic illustration of the SPECM examination of the (photo)electrochemical HER at the cross section of a 3D Cu photoelectrode doped with Al2O3 as a refractory; composed optical and scanning (photo)electrochemical images of the cross section of an Al2O3/3DCu photoelectrode for the HER, (g) composed optical and SG/TC image of the HER without light and (h) with illumination. Reproduced with permission from ref (496). Copyright 2022 American Chemical Society. (i) EC-STM images of He ion-treated MoS2 for the condition when HER is hindered (−600 mV, “HER off”) and (j) when HER is provoked (−800 mV, “HER on”); high-resolution n-EC-STM measurements on HOPG for HER “on” conditions. Reproduced with permission from ref (288). Copyright 2019 Springer Nature. The positions of the line scans below the images are marked in the main image as lines of the corresponding color (black, red, or blue). (k) Active sites which show the highest tunneling currents (white color) are located in the vicinity of a step edge. In addition, the line scan confirms that the sites of the highest tunneling current are indeed located near the step edge (marked by a gray line). (l) Active sites are detected with even higher resolution, confirming their location near step edges. The line scans below indicate the second honeycomb away from the step edge being active, active sites are detected near the defective area, i.e., a deviation from the perfectly ordered carbon lattice (marked by a white circle). (m) Line scan below compares an inactive (black) to an active (red) step edge. Besides, the noise level increase at the defect sites is shown in blue. Reproduced with permission from ref (499). Copyright 2021 Royal Society of Chemistry..
Kund and co-workers fabricated a Pd-modified dealloyed Au–Ni microelectrode tip, which they used for the detection of H2 under operando conditions at a Pt UME substrate during HER.493 In this study, the tip potential was kept constant (2 V vs Ag/AgCl quasi-RE) to oxidize H2 (HOR) while cycling the substrate potential (0.1 V to −1.2 V vs Ag/AgCl quasi-RE). Figure 6c,d is a schematic of a Pd-modified microelectrode positioned above the spot where H2 is evolved and over an insulating surface as control measurement. The capability of the proposed probe for the quantification of H2 at heterogenized Co-based HER catalysts under illumination was demonstrated.493 Visibile et al. proposed the use of a cavity microelectrode modified with the studied materials in the TG-SC mode as a simple and fast method for screening different semiconductor materials (core–shell CuI/CuO2 to estimate their efficiencies toward H2 (or O2) production under photocorrosion conditions.494 When a photocathodic material was used, the potential of the Pt substrate was held constant at the value for hydrogen oxidation, while when a photoanodic powder was under study at the value for oxygen reduction. The tip potential was scanned by recording a LSV under pulsed illumination in the potential window specific to the material. The cavity, filled with the photomaterial of interest, produced either hydrogen or oxygen which was then detected by the underlying Pt substrate.494 Jamali et al. successfully investigated the catalytic activity of photoreduced Pt particles on an immobilized multilayer assembly of unmodified photosystem I (PSI) from spinach toward the HER with the SG-TC mode.495 During the imaging process, the tip and substrate were polarized at 0.0 V and −1.0 V vs Ag/AgCl, respectively. For the first time the potency of operando SECM to image the catalytic activity of photoreduced Pt particles on the surface of a photoactive protein film was demonstrated.495 Asserghine and colleagues combined three detection modes to investigate the effect of cathodic polarization on the surface reactivity of a thin TiO2 film toward the HER for biomaterial application. The FB, RC, and SG-TC modes as depicted in Figure 6e were used to acquire information on the interfacial electrochemical behavior at the substrate. The FB mode was used to monitor proton reduction and H intercalation into the TiO2 film, and the SG-TC mode was used to monitor H2 by its oxidation.111 In another study the amperometric and potentiometric modes of SECM were combined to obtain correlative information about the corrosion of nitinol in saline solution before and after anodic polarization. The potentiometric mode was used to map the pH distribution in the electrolyte volume adjacent to the nitinol surface corresponding to the Ni2+ discharge.29
Very recently, Iffelsberger and colleagues used SPECM in the SG-TC mode to study HER and OER on 3D-printed Cu electrodes doped with Al2O3 (Al2O3/3DCu). Figure 6f is a schematic illustration of the setup used in the study. The SPECM HER images were recorded under illumination with a 365 nm light source to invoke HER or without illumination, and a Pt UME SECM tip biased at 1.06 V vs RHE was used to oxidize the substrate-generated H2. Figure 6g,h shows the optical and SPECM images of the cross-section of the substrate during HER. The authors showed that the activity of the substrate is localized at the interface between the sintered Cu and the Al2O3 microcrystals.496 Hill and co-workers342,497 employed the operando optical source/SECCM (see Scheme 5iv) to correlate photocatalytic activity with the structure of p-type WSe2. The high-resolution photocurrent maps revealed a high rate for HER in regions with short steps on the nanosheet. In another work, the SECCM tip was used to locally invoke micrometer-sized corrosion spots on a WSe2 nanosheet. Afterward, operando SECCM photocurrent maps under intermittent illumination showed a high HER activity on the defect edges.
Operando EC-STM has also been used to shed light on the complex HER mechanism. Mitterreiter et al.288 demonstrated that the edges of single MoX2 flakes showed high catalytic activity for HER, whereas their terraces were inactive. The authors accomplished this by using EC-STM to visualize the activity of mechanically exfoliated, few-layer MoS2 and MoSe2 using a Pt/Ir alloy wire insulated with Apiezon wax as the EC-STM tip. Operated in the constant-current mode, EC-STM images were taken at different potentials from −0.60 to −0.90 V and −500 to −800 V vs Pt for MoS2 and MoSe2 layers, respectively. The setup allowed for a direct comparison of the HER activity at the edge sites and the basal planes. The authors also introduced lattice defects to the basal planes by bombarding large areas of the MoS2 samples with accelerated He ions (30 keV), and Figure 6i,j compares the EC-STM data for different electrode potentials corresponding to the “HER off” (−0.60 V vs Pt) and “HER on” (−0.80 V vs Pt) conditions, respectively.288 Kosmala and colleagues developed a method to extract quantitative information from the noise in the tunneling current which they successfully correlated to the faradaic occurrences at single atomic sites.498 The substrates were made of a graphene monolayer covering either a (111)-oriented Pt single crystal (Gr/Pt(111)), or a few-monolayer Fe film on Pt(111) (Gr/Fe(n ML)/Pt(111)). Under operando conditions and using a tungsten wire as an STM tip, the authors monitored the nanoscale HER events occurring at the substrate in real-time with atomic resolution. They demonstrated that the macroscopic electrocatalytic activity as observed in standard LSV experiments did not only originate from the presence of the Gr/Fe interface, but also stemmed from defects like carbon vacancies filled by iron atoms, and bent Gr layers covering metal step edges.498 Kluge et al.499 employed the n-EC-STM technique427 to study undoped HOPG under HER conditions and showed that HER activity was due to step edges and defects rather than defect-free terraces. The authors augmented their experimental results with DFT calculations to determine the energetics of hydrogen adsorption at these sites. Figure 6k–m shows the high-resolution n-EC-STM measurements on HOPG for HER “on” conditions.499
Table 4. Summary of Experimental Conditions Made in HER Studies with SEPMs.
| SEPM | mode | substrate for HER | tip | tip reaction | ref |
|---|---|---|---|---|---|
| SECM | SG-TC | MoS2 catalyst | Pt disk UME (Ø, 25 μm) | H2 oxidation | (485) |
| SECM | SG-TC | MoSx catalysts | Pt disk UME (Ø, 25 μm) | H2 oxidation | (489)a |
| SECM | SG-TC | Au NPs | Pt disk UME (Ø, 66 μm) | H2 oxidation | (490) |
| SECM | SG-TC | NiCoP@Mxene on HOPG | Pt disk UME (Ø, 80 μm) | H2 oxidation | (491)a |
| SECM | SG-TC and RC | Al2(OH)2-TCPP and Cu-based MOF HKUST-1 | Pt disk UME (Ø, 10 μm) | H2 oxidation | (492)a |
| SECM | SG-TC | Pt, Co-based HER catalysts | Pd-modified dealloyed Au–Ni microelectrodes | H2 detection | (493)a |
| SPECM | TG-SC | core–shell CuI/CuO, CuI and TiO2 in cavity (modified tip) | cavid-filled Au (Ø, 25 μm) | H2 or O2 evolution | (494)a |
| SECM | SG-TC (not mentioned in paper) | photoreduced platinum particles (on PSI) | Pt disk UME (Ø, 2 μm) | H2 detection (oxidation) | (495) |
| SPECM | SG-TC | Al2O3/3DCu | Pt disk UME (Ø, 25 μm) | H2 oxidation | (496)a |
| SECM | SG-TC | AuNPs (on HOPG/polyphenylene) | Pt disk nanoelectrode (Ø, 120 nm) | H2 oxidation | (80) |
| SECM | FB, RC, and SG-TC | thin TiO2 film | Pt disk UME (Ø, 25 μm) | H2 oxidation | (111)a |
| SECM | SG-TC | nitinol | Pt disk UME (Ø, 30 μm) | H2 oxidation | (29)a |
| SECCM/optical probe | SECCM/light source | WSe2 nanosheet | SECCM capillary (Ø, 200–300 nm) | photocurrent due to HER on the sample surface | (497)a |
| SECCM/optical probe | SECCM/light source | WSe2 nanosheet | SECCM capillary (Ø, 500 nm) | photocurrent due to HER on the sample surface | (342)a |
| EC-STM | constant-current mode | MoS2 and MoSe2 Flakes | ripped Pt/Ir alloy wire (Pt80/Ir20) | tunneling current | (288)a |
| EC-STM | constant-current mode | Gr/Fe (0.6 ML)/Pt(111) | W wire | tunneling current | (498)a |
| n-EC-STM | constant-current mode | HOPG | ripped Pt/Ir alloy wire | tunneling current | (499)a |
Works published in the last 5 years.
3.4. Investigation of CO2RR Activity at the Sample Surface
The electrochemical reduction of CO2 (CO2RR) into value-added chemicals using renewable electricity is considered a promising approach to decrease CO2 emissions, an eco-friendly alternative to fossil fuels as the feedstock of chemicals, and also solve the intermittency challenge of renewable electricity generation.500−502 As an added advantage, the electrochemical CO2 reduction process can be controlled by adjusting the applied potential and proceeds under ambient temperature and pressure.503 These factors make scale-up applications of this technology accessible.
The CO2RR is a multielectron cathodic reaction comprising coupled multielectron/multiproton transfer pathways and generally follows three key steps:503−505 the first is the chemisorption of CO2 at the surface of the electrocatalyst, followed by the activation of the molecule by migration of electron and/or proton with subsequent cleavage of the C–O bond and/or formation of C–H bonds (or intermediates). The final step involves the rearrangement or coupling of the intermediates to form products and their subsequent desorption from the surface of the electrocatalyst. Although great research attention is given to CO2RR, unravelling the pathways of the process remains a task to be achieved.503 Among the main reactions discussed in this review (ORR, OER, and HER), the CO2RR is the most complicated reaction. Whereas the CO2RR reaction pathway to C1 products (CO, CH4, HCOO–, and CH3OH) is simple and well established, that of multicarbon or C2+ products (C2H4, C2H5OH, CH3COOH, n-C3H7OH, etc.), which are known to possess higher volumetric energy densities and are key organic synthons for the synthesis of long-chain hydrocarbon fuels and oxygenates, is complex and remains ambiguous.503 The probable factors for the high difficulty in the selectively of CO2 to C2+ products are the high energy barrier of the CO2•– intermediate formation which is believed to be the key intermediate of the first step; competition between the C–C bond, and C–H and C–O bond formations; multiple coupling steps of electrons and protons; deactivation of catalysts by intermediates, byproducts, and impurities from the electrolyte; and the competitive HER. Furthermore, the much lower energy efficiency and partial reduction of the current density observed for C2+ products are stalling the practical application of CO2 conversion to C2+ products technology in commercial electrolyzers506 For a detailed discourse on the CO2RR mechanism, the reader is hereby directed to some good reviews.500,503,506
Considering the bottlenecks, the goal of research has been to prepare electrocatalysts that can facilitate the CO2RR at low overpotentials, and selectively generate desirable products at high current densities over sustained periods, while avoiding the formation of unwanted byproducts.507 So far, Cu-based materials remain the only known electrocatalysts which are close to fitting the criterion concerning the formation of C2+ products with acceptable efficiency, although an overpotential of almost 1 V is required and a quite broad mixture of major and minor products are formed during the CO2RR.507,508 Recent efforts to optimize CO2RR electrocatalysts have generally involved exploring a variety of material compositions and morphologies as well as experimental conditions. For example, the effects of transition metal catalysts,509 alloying,510 meso- and nanostructuring,511,512 and electrolyte engineering513 on the activity and selectivity of the CO2RR process have been reported. Furthermore, theoretical approaches have been utilized to provide atomistic insights into the reaction mechanism and the nature of active sites (mainly on Cu), and in situ spectroscopic methods have been used to detect reaction intermediates during CO2RR studies.514,515 Despite these advances, the CO2RR mechanism remains a difficult nut to crack, thus necessitating the urgency for advanced high-throughput electrochemical and electroanalytical techniques, that can probe the CO2 reduction process as well as screen electrocatalysts, especially under reaction or operando conditions. As SEPMs have become powerful tools employed to demystify interfacial phenomena on electrocatalysts at reaction conditions and have afforded better insights into the surface structure, composition, and oxidation state, as well as adsorbed intermediates. Compared to the traditional RRDE and other conventional electrochemical approaches, SEPMs are the go-to techniques for high-throughput and high-precision measurement and have been used in CO2RR investigations.516 Generally, CO2RR studies with operando SEPMs, involves polarizing a substrate (catalyst) to invoke the CO2RR while a concurrently biased tip monitors the CO2RR-induced changes at the interface, such as products or local pH changes. Scheme 6 summarizes the main operando SEPM approaches for investigating CO2RR. The following paragraphs present state-of-the-art utilization of operando SEPMs as qualitative and/or quantitative electrochemical tools employed to probe interfacial dynamics during CO2RR electrocatalysis. It is worth mentioning that the SECM is possibly the most explored technique for operando investigations in CO2RR electrocatalysis.
Scheme 6. Schematic Drawing Representing the Main Operando SEPM Configurations for CO2RR Interrogation.
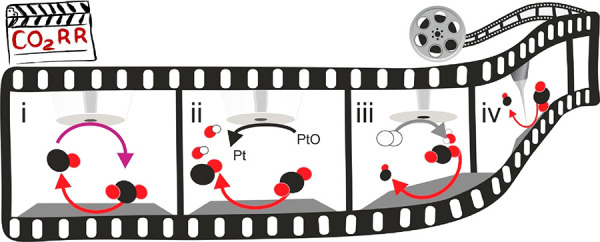
The catalyst is polarized to perform CO2RR (red arrows), while the SEPM tip is positioned as a spectator, where the tip response depends on the actuator method. (i) SG-TC-SECM with the tip polarized to oxidize CO (CO2RR product). (ii) SECM tip monitors the local OH– activity variation using the Pt/PtO potential. (iii) SG-TC-SECM tip registers H2 oxidation (gray arrow). (iv) EC-STM mapping of a polarized surface performing CO2RR.
The work by Sreekanth and Phani,516 in 2014 is perhaps the foremost operando SECM study that was conducted to unravel the interfacial processes on CO2RR electrocatalyst. The authors used the SG-TC-SECM mode to monitor the selective CO2 conversion to formate in CO2-saturated KHCO3 solution at metal electrodes (Au, Pd, Ag, and thin films of Hg and Bi deposited on GC) using a Pt UME as the SECM tip. During the study, the substrate was biased at potentials to reduce HCO3– and CO2, while the Pt tip potential was cycled (−0.6 to 0.9 V vs Ag/AgCl/sat. KCl) to oxidize the substrate-generated products (HCOO– and CO). Figure 7a–d shows the scheme of the SECM setup, approach curves and the corresponding tip voltammograms registered during the polarization of a Au substrate. The local SECM interrogation showed that the reduction of bicarbonate resulted mainly in formate formation in CO2-saturated KHCO3 solution whereas at lower pH values (e.g., pH 6.8 and 6.5) and higher potential, CO2RR produced only CO at the Au substrate.516 The same SG-TC strategy was replicated in later studies to monitor the electrocatalytic activity of metal-free B-doped graphene,517 to investigate the role of surface roughness and interfacial pH on product selectivity at Au substrates,518 monitor the activities of Au, Cu, and Ag electrodes toward CO2RR in nonmetal cation containing electrolytes,519 to study the influence of electrolytes containing metal cations on the performance of a Au electrode,520 and the performance of electrochemically reduced In2O3 to In0–In2O3 composite521 toward the CO2RR.
Figure 7.
(a) Schematic of the SG-TC mode for CO2/bicarbonate reduction–formate oxidation, (b) negative feedback, (c) positive feedback approach curves, (d) cyclic voltammetric responses of the Pt UME (10 μm)-tip to HCOO– generated at the Au substrate in 0.1 M KHCO3. Reproduced with permission from ref (516). Copyright 2014 Royal Society of Chemistry. CV-SECM scans (tip scan rate: 0.05 V s–1) for a catalyst array at a substrate potential of −1.5 V vs Ag/AgCl: (e) forward scan, (f) backward scan. The black arrow indicates the starting scan position of the tip. CV: scan rate 1 V s–1, potential range: 1.2 to −1.0 V vs Ag/AgCl. Tip–substrate distance: 100 μm, tip scan rate 100 μm s–1. Electrolyte: 0.1 M KHCO3 saturated with CO2 at atmospheric pressure, 293 K. Reproduced with permission from ref (522). Copyright 2020 Springer Nature. Schematic representation of the SG-TC mode (g) where a Pt-UME probes CO and H2 while CO2RR occurs on a Au substrate, (h) functionalized Au-UME used for monitoring pH changes. Reproduced from ref (524). Copyright 2020 American Chemical Society. (i) Local H2O and OH– activities modulated by the competing HER and CO2RR are monitored using a precisely positioned Pt nanoelectrode. The PtO reduction peak potential changes with the different ion activities established inside the three-phase boundary of the GDE at varying reaction rates. Reproduced with permission from ref (526). Copyright 2021 John Wiley and Sons. (j) Setup of the Raman-coupled scanning electrochemical microscope, (k,l) selected cyclic voltammograms of the Pt UME in 10 μm tip-to-sample distance to the Cu2O microcrystal at various substrate potentials (Esubstrate from −0.1 to −1.05 V); the arrows in (k) indicate the decrease of currents with decreasing substrate potential, whereas in (l), the curve progression is indicated for −1 V, (m) selected corresponding in situ Raman spectra, the asterisk indicates a peak from the quartz substrate. Reproduced with permission from ref (186). Copyright 2022 John Wiley and Sons. (n) Low-resolution (200 nm × 200 nm) and zoomed-in (2 nm × 2 nm) operando EC-STM images at −0.9 V in 0.1 M KOH. Experimental parameters: bias voltage = −300 mV. Tunneling current for low-resolution images = 2 nA; for high-resolution images = 5 nA. Reproduced from ref (530). Copyright 2014 American Chemical Society..
Mayer et al. investigated the potential of SECM for electrocatalytic screening of Sn/SnOx-based catalysts for the reduction of CO2 to formate.522 The authors employed SG-TC-SECM scans on an array composed of three Sn/SnOx catalysts, with the substrate biased at a constant potential (−1.5 V vs Ag/AgCl) while recording the tip CVs (1.2 to −1.0 V vs Ag/AgCl). Figure 7e,f shows CV-SECM scans for a catalyst array at a substrate potential of −1.5 V vs Ag/AgCl. The authors recommended the combination of CV-SECM scans with local surface analysis techniques like XPS, to map local hotspots and compositional inhomogeneities on catalyst surfaces.522 Kim et al. fabricated a Pt- and Sn-modified Pt SECM tip to selectively monitor the production of CO during the CO2RR on highly dispersed Au NPs on carbon black (Au NPs/CB) substrate using the operando SG-TC-SECM mode. The substrate was polarized at cathodic potentials to induce the reduction of CO2 while the tip was biased at +0.5 V vs SCE to oxidize the CO generated at the Au NPs/CB substrate. The authors noted that the SG-TC mode coupled with the electrochemical CO microsensor tip was an effective method for the detection of CO during CO2RR at low overpotentials potential.523 Monteiro and co-workers also employed a Pt UME tip and a functionalized gold SECM tip to probe CO and H2 electrooxidation.524 In this work, the pH evolution in the substrate diffusion layer was monitored during CO2RR at a Au surface. Figure 7g,h is a scheme of the SG-TC mode employed to detect CO and H2 and the functionalized Au UME used to measure the pH. The two peaks often observed during CO oxidation were due to diffusion limitation by CO and OH–.524
All of the above-cited CO2RR studies have been carried out in an aqueous phase. Aqueous-phase reactors, however, have some practical limitations that reduce conversion rates and energy efficiencies of the CO2RR process.525 The use of reactors that operate using CO2 delivered to the cathode in the vapor/gas phase, such as those using gas diffusion electrodes (GDEs), have been successfully used to counterbalance the limitations of aqueous-phase reactors, where CO2 is dissolved in the electrolyte.525 GDEs are known to exhibit interconnected pore channels, which enable the formation of the triple-phase boundary between gaseous CO2, liquid electrolyte, and solid catalyst, making the intraporous electrolyte modulations close to the CO2RR sites very tantalizing.525,526 In 2021, the first experimental report was published describing monitoring of the local OH– and H2O activities using a Pt nanoelectrode as SECM tip, which was positioned close to a Ag-based GDE during the CO2RR (Figure 7i).526 The authors achieved this by potentiodynamically cycling the positioned tip in a potential range between Pt oxide formation and Pt oxide reduction (0.60 to −1.1 V vs Ag/AgCl/3 M KCl), while the Ag-based GDE was kept at cathodic potentials to induce the CO2RR. High turnover HER/CO2RR at a GDE leads to modulations of the alkalinity at the local electrolyte/electrode interface.526 The same shear-force-based tip positioning technique was used to simultaneously monitor pH changes and the topography with high resolution.527 The SG-TC mode was used to study the effect of catalyst loading and CO2 pressure on the activity of a Au GDEs toward CO2 reduction to CO. During the experiment, the diffusion-limited CO oxidation current was constantly recorded at the positioned Au-nanoelectrode close to the GDE while the tip was scanned across the loading gradient. At the same time, the potential applied to the substrate and the CO2 back-pressure was varied. This allowed for a detailed evaluation of the interplay between catalyst loading and CO2 back-pressure with respect to the optimum performance of the studied GDEs. An optimum local catalyst loading was necessary to achieve high activities and the optimum loading also depended directly on the CO2 back-pressure.527 The same approach527 was employed to study the activity and selectivity of Ag core/porous Cu shell NPs in a H-cell and a GDE cell configuration,528 and to monitor the local OH– ion activity of PTFE-modified GDEs of a series of metal–organic framework (MOF) derived CuxOyCz catalysts.529
Recently, Steimecke et al.186 combined SECM with Raman microscopy to investigate the activity of a single cuprous oxide microcrystal electrochemically deposited on a transparent ITO substrate (Cu2O/ITO) toward CO2RR. Figure 7j is a scheme of the Raman-coupled SECM setup that allowed Raman measurements from the backside of the ITO electrode while the SG-TC-SECM mode was run simultaneously. This setup allowed to simultaneously obtain electrochemical and spectroscopic information with high spatial resolution and as complementary data sets from the very same location of the electrode (Figure 7k–m). The Cu2O/ITO substrate was continuously polarized from −0.1 to −1.05 V vs Ag/AgCl/KClsat (50 mV step–1) to induce the CO2RR while recording CVs at a Pt SECM tip which was biased potentiodynamically (20 mV s–1) between −0.5 to 0.9 V vs Ag/AgCl/KClsat to detect the substrate-generated products. Raman spectra revealed that the reduction of Cu2O crystal to Cu occurred when the substrate was polarized at −0.2 V vs Ag/AgCl/KClsat. The ITO electrode had no relevant contribution to the CO2RR and as such can be a useful substrate material although its application as a transparent electrode material for optical probing was limited to potential values above −1 V vs Ag/AgCl/KClsat.186
SECM in the SG-TC mode has been the most employed SEPM methodology for the operando investigation of CO2RR. Nevertheless, EC-STM was also used to study the interfacial dynamics of electrocatalysts. Kim et al. used operando EC-STM to investigate the Cu(100)-like behavior of polycrystalline Cu (Cu(pc)) during CO2RR.530 Previous investigations suggested that Cu(pc) exhibited Cu(100)-like behavior in that it generated ethylene as a major product.531 The authors explain the unexpected product selectivity observed for Cu(pc) by operando EC-STM images (Figure 7n), demonstrating that during CO2RR (at −0.9 V vs SHE, 0.1 M KOH) Cu(pc) facets undergo reconstruction to a pure single-crystal with a Cu(100) surface.530 This study epitomizes the potency of operando studies to reveal the structure-composition-activity correlation sought after during electrocatalysis. Phan and colleagues also employed operando EC-STM to reveal the dynamics of the morphological evolution of polycrystalline Cu (p-Cu) and graphene-covered polycrystalline Cu(g-Cu) during the CO2RR. The study unveiled a drastic reconstruction of p-Cu to nanocuboids at negative potentials and even in halide-free electrolytes. They also demonstrated the protective character of a single graphene layer on Cu against the massive reconstruction at operando conditions. Their results showed that Cu exhibited a similar intrinsic activity when normalized by the electrochemically active area which opens a new prospect to reinterpret the mechanism of nanostructured Cu-based materials without the presence of oxidized species or halides.532
Table 5. Summary of Experimental Conditions Made in CO2RR Studies with SEPMs.
| SEPM | mode | substrate | tip | tip reaction(s) | ref |
|---|---|---|---|---|---|
| SECM | SG-TC | Au, Pd, Ag, and thin films of Hg and Bi on GC | Pt disk UME (Ø, 10 μm) | CO and HCO2– oxidation | (516) |
| SECM | SG-TC | B-doped graphene | Pt disk UME (Ø, 10 μm) | HCO2– oxidation | (517) |
| SECM | SG-TC | Au (varying roughness) | Pt disk UME (Ø, 10 μm) | CO oxidation | (518)a |
| SECM | SG-TC | Au, Cu, and Ag | Pt disk UME (Ø, 10 μm) | CO oxidation | (519)a |
| SECM | SG-TC | Au electrode | Pt disk UME (Ø, 50 μm) | CO oxidation | (520)a |
| SECM | SG-TC | In0–In2O3 composites | Pt disk UME (Ø, 200 μm) | CO oxidation | (521) |
| SECM | SG-TC | Sn/SnOx-based catalysts | Pt disk UME (Ø, 10 μm) | detection of HCOO–, CO, and H2 | (522)a |
| SECM | SG-TC | Au NPs/CB | Pt- and Sn-modified Pt SECM tip (Ø, 76 μm) | CO oxidation | (523)a |
| SECM | SG-TC | Au UME | Pt- and Au-modified pH sensor | CO oxidation and pH monitoring | (524)a |
| SECM | SG-TC | Ag-based GDE | Pt nanoelectrode | CO oxidation and pH monitoring | (526)a |
| SECM | SG-TC (SF-SECM) | Au-based GDEs | Au disk UME (Ø, 2 μm) | CO oxidation | (527)a |
| SECM | SG-TC (SF-SECM) | Ag core/porous Cu shell NPs | Pt disk UME | reduction of PtO | (528)a |
| SECM | SG-TC (SF-SECM) | MOF-derived CuxOyCz | Pt disk UME (Ø, 1 μm) | reduction of PtO | (529)a |
| Raman-SECM | SG-TC | Cu2O/ITO | Pt disk UME (Ø, 10 μm) | HCO2– and H2O2 oxidation | (186)a |
| EC-STM | constant current | Cu(pc) | W (Ø, 25 μm) | tunneling current | (530) |
| EC-STM | constant current | p-Cu and g-Cu | ripped Pt/Ir wire | tunneling current | (532)a |
Works published in the last 5 years.
3.5. SEPM Tip in the Investigation of Other Reactions for Electrolyzers and Bioelectrocatalysis Interests
In this section, we demonstrate the versatility and potency of operando SECM for the investigation of other important reactions. The electrochemical chlorine evolution reaction (CER) is a significant anodic reaction in chlor-alkali electrolysis.533 Cl2 gas is an important precursor for many crucial industrial processes including pharmaceuticals, polymer synthesis, pulp and paper, disinfectant production, and wastewater treatment.533,534 Zeradjanin et al. employed operando SECM in the SG-TC and RC modes as an analytical tool to detect and visualize the local electrocatalytic activity of dimensionally stable anodes (DSA) toward the chlorine evolution from brine.535 The same techniques were used later to monitor the local activity of CER on Ti–Ru–Ir mixed metal oxide DSA surfaces.536
Reactive intermediates play an important role in many electrochemical processes and SECM is well adapted to monitor their formation during electrocatalysis.537 Chang and Bard reported the detection of the short-lived Sn(III) intermediate and the mechanism of Sn(IV)/Sn(II) electroreduction in bromide media by cyclic voltammetry and operando SECM.538
The Bard group employed the TG-SC mode to capture the unstable intermediate CO2•– to study the mechanism of CO2RR. This demonstrates the capacity of the SECM to detect and characterize short-lived species which can dimerize, undergo disproportionation, and react with proton donors and even mild oxidants.539 This work highlighted the advantage of SECM over fast-scan cyclic voltammetry for the study of fast reaction intermediates in that it helps to overcome the limitations of double-layer charging and adsorbed species observed in fast-scan CV. SECM allows for measurements at steady-state while transient currents from adsorbed species do not perturb the measurements. The biochemical and enzymatic activities of substrates have also been monitored by the SECM. Wijayawardhana et al. examined the biochemical activity of beads that were modified with antimouse antibodies using the SG-TC mode by oxidizing 4-aminophenol formed in the alkaline phosphatase-catalyzed hydrolysis of 4-aminophenyl phosphate at the surface of the beads, using a Pt microdisk electrode (10 μm diameter) as a SECM tip.540
Maciejewska and co-workers employed the SG-TC-SECM mode to monitor the localized enzymatic activity of enzyme/polymer spots made of resydrol and glucose oxidase on glass surfaces.541 During the study, they evaluated the complex interplay between glucose and ascorbic acid in a glucose oxidase-based amperometric biosensor.541 In a closely related study, the SG-TC mode was used to monitor the localized sensor response of glucose oxidase immobilized within a polymer matrix in a spot of about 300–400 μm diameter.542 Karnicka et al. monitored the spatial distribution of the biocatalytic activity of bilirubin oxidase/Os-complex modified redox polymer toward ORR with RC-SECM.107 Fernández et al. used the TG-SC-SECM to perform a high-throughput study on the ORR activity of arrays of “wired” bilirubin oxidase and laccase enzymes.543 The arrays contained spots with different ternary mixtures of enzyme, cross-linker, and redox polymers, and the goal was to find the optimal composition. During the study, a 25 μm diameter Pt tip was polarized to produce locally O2 by OER while the substrate was held at 0.3 and 0.4 V vs Ag/AgCl (3 M KCl) to trigger the enzymatic ORR activity of the different composites.
Operando SECM operating in the SG-TC mode was used to analyze the reaction mechanism of the electrooxidation of glycerol at copper surfaces in NaOH solutions. A potential sweep from 0 to 0.8 V vs Ag/AgCl was applied to the substrate for glycerol electrooxidation while the tip was kept constant at 0.2 V vs Ag/AgCl to monitor the formation of electroactive species, e.g., reduction of Cu(III) species.544
Chronoamperometry and micropipette delivery/substrate collection (MD/SC) mode of SECM was used to investigate the electrocatalytic activity of 5 different metallic nanoparticles (Pt100, Pt75Pd25, Pt50Pd50, Pt25Pd75, and Pd100) toward formic acid oxidation in the presence of simultaneous ORR.545 The Au UME SECM tip was kept at a potential negative enough to perform ORR under steady-state conditions, and SECM images were collected at 3 different potential values, (0.3 V, 0.5 and 0.7 V vs RHE). The authors demonstrated SECM as a fast and powerful technique for studying the O2 crossover effect in different electrocatalysts and for identifying highly selective electrocatalyst candidates for mixed-reactant fuel cells.545
Operando SECM operated in the SG-TC mode was employed to investigate substrate-generated H2O2 by using a highly stable and selective ultramicrosensor (made of Prussian Blue modified with films of iron and nickel hexacyanoferrates) as a tip. A Au surface was biased to generate H2O2 while the selective tip was employed for imaging the distribution of H2O2.546 The RC and SG-TC modes were also applied to investigate the electrochemical activities of various AuPd compositions deposited onto ITO toward H2O2 and FcMeOH+ reduction reactions.547 Tomlinson et al. reported the first use of electrochemical imaging to identify the defect and defect-free areas in single crystal boron-doped diamond (BDD) electrodes. Intermittent contact SG-TC-SECM was successfully used to detect defects in single crystal BDD electrodes by measuring variations in the tip current in correlation with changes in the boron dopant levels in the materials.548 Leonard and Bard reported a new TG-SC mode approach that allowed them to acquire information by the separation of partial currents at multireactional electrochemical interfaces, which they employed to study HER at a Mn tip.101 During the measurement, the Mn tip potential was scanned from −1.5 to 0 V vs Ag/AgCl to invoke HER, while the substrate potential was held at +0.1 V vs Ag/AgCl to capture solely the tip-generated H2 (HOR). By this approach, the authors investigated HER on the Mn surface, a reaction that has not been directly studied owing to the highly corrosive nature of Mn. The approach curve showed positive feedback for H2 production at the tip and H2 oxidation at the Pt substrate, and at very close distances, ∼100% collection efficiency was obtained, highlighting the powerful sensitivity of SECM measurements.101 HOR electrocatalysts for fuel-cell applications have also been studied with SECM. Kim et al. studied the HOR activity of Pt NPs electrodeposited on highly oriented pyrolytic graphite (HOPG). They used the operando TG-SC mode to generate H2 at the Pt-nanoelectrode tip (134 nm of diameter), while HOR was performed at the polarized substrate (Pt NPs on HOPG) with large effective rate constant (higher than 2 cm/s) caused by the effective mass transfer rate.549
The mechanism for the electrosynthesis of nanoparticles has also been probed using operando SECM. Miranda Vieira and co-workers employed operando SECM for the local electrosynthesis of Ag2O nanocubes. The TG-SC mode was used to electro-generate Ag+ at an anodic potential (+0.3 V) using a Ag sacrificial electrode as SECM tip while the substrate (Au electrode) was biased at a cathodic potential (−0.4 V) to induce ORR producing the OH– needed for Ag2O nanocubes formation. The nanocubes were also detected at the Au-UME substrate by nanoimpact coulometry using the electroreduction of Ag2O to Ag.550 This approach showed how electrochemical impact in SECM can be applied to reveal NP formation and growth mechanism.
Another interesting study that exemplifies the capability of operando SECM to study electrocatalytic processes was reported by He and co-workers, who studied the electrochemical reductions of NO3– to NO2– and NO2– to NH3 on a Cu–Co(OH)2 catalyst using various in situ characterization techniques including operando SECM. During the operando SECM measurements, the Cu–Co(OH)2 catalyst substrate was polarized at −0.12 V (vs RHE) to trigger the NO3RR while CV scans were carried out at the Pt-UME (SECM tip, diameter of ∼1 μm) between −0.12 and 1.58 V (vs RHE) to identify NO2– (at 0.06 V vs RHE) and NH3 (at 0.76 V vs RHE).551 The authors showed that the NO2– was selectively formed on the Cu layer and then diffuses to the near by Co(OH)2 layer, where the NO2– is further reduced to NH3.
The potential of FB mode SECM for investigating surface charge transfer properties of semiconductors used as photoelectrodes was introduced by Horrocks, Mirkin, and Bard already in 1994.121,552 The substrate (biased or not) is illuminated to generate reactive charge carriers. A redox mediator in the electrolyte is converted at the polarized tip and the so-transformed species further interact with the photogenerated charge carriers of the substrate, giving a negative or positive feedback current as the tip response. The FB mode of SECM in these cases is interpreted as an operando tool since simultaneously reactive species generated at the substrate surface under illumination are indirectly monitored at the SECM tip. Such a strategy generates information about the surface processes that are directly correlated to photoelectrocatalysis, which occurs in a short time regime, highlighting the spatial and temporal capability of operando SECM investigation. Zhang and co-workers553 used the FB mode to investigate the charge-transfer kinetics at the photoelectrode/electrolyte interface of the photocatalysts BiVO4 and Mo-doped BiVO4 using the redox couple [Fe(CN)6]3–/[Fe(CN)6]4– as a mediator. The researchers concluded that the introduction of the Mo6+ ion into BiVO4 can facilitate light-induced OER and suppress the interfacial back reaction at the photoanode/electrolyte interface. A similar approach was employed to study charge-transfer dynamics on the surface of BiVO4 and BiVO4/NiFe-LDH.554 This work showed that the back transfer of electrons is suppressed when adding the NiFe-LDH cocatalyst onto the BiVO4 semiconductor surface, explaining the enhanced water oxidation properties of the combined material.
4. Summary and Future Prospective
A comprehensive review of state-of-the-art advances made with SEPM to understand interfacial processes aims on demonstrating the versatility of SEPMs to probe the catalytic activity of various substrates, understand structure dynamics, establish structure/composition–activity–selectivity relations, and disclose reaction mechanisms. The potential of SEPM is shown through the various local studies conducted with SECM, SICM, EC-STM, and SECCM. The working principle of each SEPM technique, their scope of applicability, advantages, and limitations are discussed. In addition, the concept of operando SEPM is delineated, i.e., the use of the SEPM tip to probe local surface property changes while tailoring the surface reactivity independently with another method.
Furthermore, the use of operando SEPM to investigate electrocatalytic reactions (ORR, OER, HER, and CO2RR) is presented systematically to demonstrate the extensive and exciting trajectory of operando SEPMs studies. In the application section, the uniqueness and utility of operando SEPMs have been portrayed in the many research studies that were reviewed. Beyond the general advantages of micro/nanoelectrochemistry, the use of operando SEPMs makes it possible to identify active sites, disclose reaction pathways, quantify reaction intermediates, and study the effects of the reaction environment on electrocatalytic processes. Moreover, the use of hybrid SEPM techniques for the extraction of additional information during the electrochemical reaction has been shown. Evidently, most operando SEPM studies were done using SECM and EC-STM, which are intrinsically operando SEPM techniques.
Although SECCM and SICM were not yet intensively explored for operando studies, the techniques have been shown as a powerful tool for local investigations in single-entity and sub-entity studies because of its high-resolution (some nm) and high-throughput features. However, the flexibility of the pipette-SEPM techniques still need improvement to allow easy and simultaneous coupling with other methods to enable operando investigations. We encourage the community on exploring operando SICM for charge mapping of electrodes surface, as well as the coupled SECCM-hybrid methods to investigate simultaneously single entity catalysts.
Finally, we advocate the incorporation of simulations and machine learning into general SEPM studies. This will pave the way for systematic benchmarking and design of electrocatalysts.
Glossary
Abbreviations
- AC
alternating current
- AFM
atomic force microscopy
- BDD
boron-doped diamond
- C1
single carbon-containing CO2RR products
- C2+
multiple carbon-containing CO2RR products
- CB
carbon black
- CD
constant distance
- CE
counter electrode
- CER
chlorine evolution reaction
- CNT
carbon nanotubes
- CO2RR
CO2 reduction reaction
- CoTPP
5,10,15,20-tetraphenyl-21H,23H-porphine cobalt(II)
- CV
cyclic voltamogramm
- DC
direct current
- DEMS
differential electrochemical mass spectrometry
- DFT
density functional theory
- DSA
dimensionally stable anode
- EC-STM
electrochemical scanning tunneling microscopy
- EQCM
quartz crystal microbalance
- FB
feedback
- FTIR
Fourier transform infrared spectroscopy
- FTO
fluorine-doped tin oxide
- GC
glassy carbon
- GDE
gas diffusion electrode
- Gr
graphene
- HER
hydrogen evolution reaction
- HOPG
highly oriented pyrolytic graphite
- HOR
hydrogen oxidation reaction
- ICR
ion current rectification
- ITO
indium tin oxide
- LDH
layered double hydroxide
- LEIS
local electrochemical impedance spectroscopy
- LSV
linear sweep voltammogram
- MCE
microcavity electrodes
- ML
monolayer
- MOF
metal–organic framework
- MWCNTs
multiwalled carbon nanotubes
- NC
nitrogen-doped carbon
- n-EC-STM
noise electrochemical scanning tunneling microscopy
- NHE
normal hydrogen electrode
- NO3RR
nitrate reduction
- NP
nanoparticle
- OCP
open circuit potential
- OER
oxygen evolution reaction
- ORR
oxygen reduction reaction
- pc
polycrystalline
- PSI
photosystem I
- QRCE
quasi-reference counter electrode
- RC
redox competition
- RDE
rotating disk electrode
- RE
reference electrode
- rGO
reduced graphene oxide composite
- RHE
reversible hydrogen electrode
- ROS
reactive oxygen species
- RRDE
rotating ring-disk electrode
- SAM
self-assembled monolayer
- SCE
saturated calomel electrode
- SC-TG
substrate collection–tip generation
- SECCM
scanning electrochemical cell microscopy
- SECM
scanning electrochemical microscopy
- SEI
solid-electrolyte interphase
- SEM
scanning electron microscope
- SEPM
scanning electrochemical probe microscopy
- SF
shear force
- SHE
standard hydrogen electrode
- SI
surface interrogation
- SICM
scanning ion conductance microscopy
- SMCM
scanning micropipette contact method
- SNEI
single nanoparticle electrochemical impact
- SPECM
scanning photoelectrochemical microscopy
- SPR
surface plasmon resonance
- STM
scanning tunneling microscopy
- STS
scanning tunneling spectroscopy
- SVET
scanning vibrating electrode technique
- TC-SG
tip collection–substrate generation
- TEM
transmission electron microscopy
- TMO
transition metal oxide
- TOF
turnover frequency
- UME
ultra-microelectrode
- WE
working electrode
- XPS
X-ray photoelectron spectroscopy
Biographies
Carla S. Santos received her Ph.D. (2019) degree from the University of Sao Paulo (Brazil) under the supervision of Prof. Mauro Bertotti. During her Ph.D., she worked on applying micro- and nanoelectrochemistry to study metabolism processes of cells and organisms. Since 2020, she has been a research fellow in the Schuhmann group at Ruhr-Universität Bochum. Her current research interests are focused on studying interfacial processes of materials for batteries employing scanning electrochemical probe microscopies.
Bright N. Jaato holds a B.Sc. and an M.Phil. in chemistry from the University of Ghana. In 2018, he was a Commonwealth Split-Site Scholar at the University of Cambridge, UK, where he studied and carried out research in “Applied Photochemistry and Nanotechnology for use in Water Purification” under the supervision of Prof. R. Vasant Kumar. Bright is currently a doctoral researcher in the Andronescu group at the University of Duisburg-Essen. His research focuses on the optimization of reactor designs for CO2 electroreduction.
Ignacio Sanjuán Moltó completed his Ph.D. in electrochemistry (2020) at the University of Alicante under the supervision of Vicente Montiel Leguey and Eduardo Expósito Rodríguez. During this time, he studied different electrochemical techniques to treat the reject water of an electrodialysis plant in collaboration with the company Global Omnium. Currently, he is a postdoctoral researcher in the group of Corina Andronescu at the University of Duisburg-Essen. His research focuses on the electrochemical CO2 reduction, specifically on preparation of electrocatalysts and fabrication of gas diffusion electrodes.
Wolfgang Schuhmann studied chemistry at the University of Karlsruhe and completed his Ph.D. degree in 1986 at the Technical University of Munich. After finishing his habilitation at Technical University of Munich in 1993, he was appointed Professor for Analytical Chemistry at the Ruhr University Bochum in 1996. His research interests cover a broad spectrum of different fields of electrochemistry, including biosensors, biofuel cells, batteries, photoelectrochemistry, electrocatalysts for energy conversion including high-entropy materials, scanning electrochemical microscopy, scanning electrochemical cell microscopy, in situ electrochemistry-spectroscopy techniques, micro- and nanoelectrochemistry, among others.
Corina Andronescu received her B.Sc. and M.Sc. from the University Politehnica of Bucharest in 2009 and 2011, respectively, where she also obtained her Ph.D. in 2014 in chemical engineering. In 2016 she joined the group of Prof. W. Schuhmann (Ruhr University Bochum), first as a postdoctoral researcher and later as a group leader. In December 2018, she was appointed Junior Professor at the University of Duisburg-Essen, where she leads the Electrochemical Catalysis group within the Faculty of Chemistry. The research interests include fundamental and applied topics, focusing on understanding electrocatalysts and electrode design for CO2 electroreduction and alcohol electrooxidation, paired electrolysis, ranging to high-throughput materials discovery and single entity electrochemistry.
Author Contributions
# C.S.S., B.N.J., and I.S. contributed equally. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. CRediT: Carla Santana Santos writing-original draft, writing-review & editing; Bright Nsolebna Jaato writing-original draft, writing -review & editing; Ignacio Sanjuán writing-original draft, writing-review & editing; Wolfgang Schuhmann conceptualization, funding acquisition, project administration, writing-review & editing; Corina Andronescu conceptualization, funding acquisition, supervision, writing-review & editing.
C.A., I.S.M., and B.N.J. acknowledge funding by the BMBF in the framework of the NanoMatFutur project “MatGasDif” (03XP0263). C.S.S. and W.S. acknowledge funding from the European Union’s Horizon 2020 research and innovation program under the Grant Agreement No. 861962 (NanoBat), from the European Research Council (ERC) under the Grant Agreement No. 833498 (CasCat), and by the European Innovation Council (EIC) under the Grant Agreement No. 101046742 (MeBattery). C.A. and W.S. acknowledge funding by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) in the framework of the CRC247-388390466.
The authors declare no competing financial interest.
Special Issue
Published as part of the Chemical Reviewsvirtual special issue “Operando and In Situ Studies in Catalysis and Electrocatalysis”.
References
- Masa J.; Andronescu C.; Schuhmann W. Electrocatalysis as the Nexus for Sustainable Renewable Energy: The Gordian Knot of Activity, Stability, and Selectivity. Angew. Chem., Int. Ed. 2020, 59, 15298–15312. 10.1002/anie.202007672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long C.; Han J.; Guo J.; Yang C.; Liu S.; Tang Z. Operando Toolbox for Heterogeneous Interface in Electrocatalysis. Chem. Catalysis 2021, 1, 509–522. 10.1016/j.checat.2021.07.012. [DOI] [Google Scholar]
- Zhang H.; Zhu M.; Schmidt O. G.; Chen S.; Zhang K. Covalent Organic Frameworks for Efficient Energy Electrocatalysis: Rational Design and Progress. Adv. Energy Sustainability Res. 2021, 2, 2000090. 10.1002/aesr.202000090. [DOI] [Google Scholar]
- Kumar A.; Vashistha V. K.; Das D. K.; Ibraheem S.; Yasin G.; Iqbal R.; Nguyen T. A.; Gupta R. K.; Rasidul Islam M. M-N-C-Based Single-Atom Catalysts for H2, O2 & CO2 Electrocatalysis: Activity Descriptors, Active Sites Identification, Challenges and Prospects. Fuel 2021, 304, 121420. 10.1016/j.fuel.2021.121420. [DOI] [Google Scholar]
- Campos-Roldán C. A.; Jones D. J.; Rozière J.; Cavaliere S. Platinum-Rare Earth Alloy Electrocatalysts for the Oxygen Reduction Reaction: A Brief Overview. ChemCatChem. 2022, 14, e202200334 10.1002/cctc.202200334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.; Peltier C. R.; Zeng R.; Schimmenti R.; Li Q.; Huang X.; Yan Z.; Potsi G.; Selhorst R.; Lu X.; et al. Electrocatalysis in Alkaline Media and Alkaline Membrane-Based Energy Technologies. Chem. Rev. 2022, 122, 6117–6321. 10.1021/acs.chemrev.1c00331. [DOI] [PubMed] [Google Scholar]
- Masa J.; Schuhmann W. Electrocatalysis and Bioelectrocatalysis – Distinction Without a Difference. Nano Energy 2016, 29, 466–475. 10.1016/j.nanoen.2016.04.007. [DOI] [Google Scholar]
- Liu F.; Shi C.; Guo X.; He Z.; Pan L.; Huang Z.-F.; Zhang X.; Zou J.-J. Rational Design of Better Hydrogen Evolution Electrocatalysts for Water Splitting: A Review. Adv. Sci. 2022, 9, 2200307 10.1002/advs.202200307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirth A. A.; Niece B. K. Electrochemical Applications of in Situ Scanning Probe Microscopy. Chem. Rev. 1997, 97, 1129–1162. 10.1021/cr960067y. [DOI] [PubMed] [Google Scholar]
- Chen S.; Kucernak A. Electrocatalysis under Conditions of High Mass Transport Rate: Oxygen Reduction on Single Submicrometer-Sized Pt Particles Supported on Carbon. J. Phys. Chem. B 2004, 108, 3262–3276. 10.1021/jp036831j. [DOI] [Google Scholar]
- Rao S. R.Physical Chemistry of Interfaces:Surface Chemistry of Froth Flotation; Springer: Boston, MA, 2004. [Google Scholar]
- Brett C. M.; Brett A. M. O.. Electrochemistry:Principles, Methods and Applications; Oxford University Press: New York, 1994. [Google Scholar]
- Yang Y.; Xiong Y.; Zeng R.; Lu X.; Krumov M.; Huang X.; Xu W.; Wang H.; DiSalvo F. J.; Brock J. D.; et al. Operando Methods in Electrocatalysis. ACS Catal. 2021, 11, 1136–1178. 10.1021/acscatal.0c04789. [DOI] [Google Scholar]
- Chee S. W.; Lunkenbein T.; Schlogl R.; Cuenya B. R. In Situ and Operando Electron Microscopy in Heterogeneous Catalysis-Insights into Multi-scale Chemical Dynamics. J. Condens. Matter Phys. 2021, 33, 153001. 10.1088/1361-648X/abddfd. [DOI] [PubMed] [Google Scholar]
- Zuo S.; Wu Z.-P.; Zhang H.; Lou X. W. Operando Monitoring and Deciphering the Structural Evolution in Oxygen Evolution Electrocatalysis. Adv. Energy Mater. 2022, 12, 2103383. 10.1002/aenm.202103383. [DOI] [Google Scholar]
- Zhu Y.; Wang J.; Chu H.; Chu Y.-C.; Chen H. M. In Situ/Operando Studies for Designing Next-Generation Electrocatalysts. ACS Energy Lett. 2020, 5, 1281–1291. 10.1021/acsenergylett.0c00305. [DOI] [Google Scholar]
- Li J.; Gong J. Operando Characterization Techniques for Electrocatalysis. Energy Environ. Sci. 2020, 13, 3748–3779. 10.1039/D0EE01706J. [DOI] [Google Scholar]
- Timoshenko J.; Roldan Cuenya B. In Situ/Operando Electrocatalyst Characterization by X-ray Absorption Spectroscopy. Chem. Rev. 2021, 121, 882–961. 10.1021/acs.chemrev.0c00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.; Zhao H.; Kang S. D.; Lim K.; Chen C.-C.; Yu Y.-S.; Braatz R. D.; Shapiro D. A.; Hong J.; Toney M. F.; et al. Fictitious Phase Separation in Li Layered Oxides Driven by Electro-Autocatalysis. Nat. Mater. 2021, 20, 991–999. 10.1038/s41563-021-00936-1. [DOI] [PubMed] [Google Scholar]
- Rüscher M.; Herzog A.; Timoshenko J.; Jeon H. S.; Frandsen W.; Kühl S.; Roldan Cuenya B. Tracking Heterogeneous Structural Motifs and the Redox Behaviour of Copper-Zinc Nanocatalysts for the Electrocatalytic CO2 Reduction using Operando Time Resolved Spectroscopy and Machine Learning. Catal. Sci. Technol. 2022, 12, 3028–3043. 10.1039/D2CY00227B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oja S. M.; Wood M.; Zhang B. Nanoscale Electrochemistry. Anal. Chem. 2013, 85, 473–486. 10.1021/ac3031702. [DOI] [PubMed] [Google Scholar]
- Li Y.; Ning X.; Ma Q.; Qin D.; Lu X. Recent Advances in Electrochemistry by Scanning Electrochemical Microscopy. TrAC - Trends Anal. Chem. 2016, 80, 242–254. 10.1016/j.trac.2016.02.002. [DOI] [Google Scholar]
- Wittstock G.; Burchardt M.; Pust S. E.; Shen Y.; Zhao C. Scanning Electrochemical Microscopy for Direct Imaging of Reaction Rates. Angew. Chem., Int. Ed. 2007, 46, 1584–1617. 10.1002/anie.200602750. [DOI] [PubMed] [Google Scholar]
- Bentley C. L.; Edmondson J.; Meloni G. N.; Perry D.; Shkirskiy V.; Unwin P. R. Nanoscale Electrochemical Mapping. Anal. Chem. 2019, 91, 84–108. 10.1021/acs.analchem.8b05235. [DOI] [PubMed] [Google Scholar]
- Zoski C. G. Nanoscale Scanning Electrochemical Microscopy:Emerging Advances in Applications and Theory. Curr. Opin. Electrochem. 2017, 1, 46–52. 10.1016/j.coelec.2017.01.002. [DOI] [Google Scholar]
- Polcari D.; Dauphin-Ducharme P.; Mauzeroll J. Scanning Electrochemical Microscopy: A Comprehensive Review of Experimental Parameters from 1989 to 2015. Chem. Rev. 2016, 116, 13234–13278. 10.1021/acs.chemrev.6b00067. [DOI] [PubMed] [Google Scholar]
- Barton Z. J.; Rodríguez-López J. Emerging Scanning Probe Approaches to the Measurement of Ionic Reactivity at Energy Storage Materials. Anal. Bioanal. Chem. 2016, 408, 2707–2715. 10.1007/s00216-016-9373-7. [DOI] [PubMed] [Google Scholar]
- Daviddi E.; Shkirskiy V.; Kirkman P. M.; Robin M. P.; Bentley C. L.; Unwin P. R. Nanoscale Electrochemistry in a copper/aqueous/oil Three-Phase System: Surface Structure-Activity-Corrosion Potential Relationships. Chem. Sci. 2021, 12, 3055–3069. 10.1039/D0SC06516A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asserghine A.; Medvidović-Kosanović M.; Stanković A.; Nagy L.; Souto R. M.; Nagy G. A Scanning Electrochemical Microscopy Characterization of the Localized Corrosion Reactions Occurring on Nitinol in Saline Solution after Anodic Polarization. Sens. Actuators B Chem. 2020, 321, 128610. 10.1016/j.snb.2020.128610. [DOI] [Google Scholar]
- Kunze J.; Maurice V.; Klein L. H.; Strehblow H.-H.; Marcus P. In situ STM Study of the Duplex Passive Films formed on Cu(111) and Cu(001) in 0.1 M NaOH. Corros. Sci. 2004, 46, 245–264. 10.1016/S0010-938X(03)00140-9. [DOI] [Google Scholar]
- Eckhard K.; Erichsen T.; Stratmann M.; Schuhmann W. Frequency-Dependent Alternating-Current Scanning Electrochemical Microscopy (4D AC-SECM) for Local Visualisation of Corrosion Sites. Chem.—Eur. J. 2008, 14, 3968–3976. 10.1002/chem.200701861. [DOI] [PubMed] [Google Scholar]
- Chen R.; Alanis K.; Welle T. M.; Shen M. Nanoelectrochemistry in the Study of Single-Cell Signaling. Anal. Bioanal. Chem. 2020, 412, 6121–6132. 10.1007/s00216-020-02655-z. [DOI] [PubMed] [Google Scholar]
- Bergner S.; Wegener J.; Matysik F.-M. Simultaneous Imaging and Chemical Attack of a Single Living Cell Within a Confluent Cell Monolayer by means of Scanning Electrochemical Microscopy. Anal. Chem. 2011, 83, 169–174. 10.1021/ac1021375. [DOI] [PubMed] [Google Scholar]
- Beaulieu I.; Kuss S.; Mauzeroll J.; Geissler M. Biological Scanning Electrochemical Microscopy and its Application to Live Cell Studies. Anal. Chem. 2011, 83, 1485–1492. 10.1021/ac101906a. [DOI] [PubMed] [Google Scholar]
- Nagy L.; Nagy G. Application of Scanning Electrochemical Microscopy in Bioanalytical Chemistry. Bioanal. Rev. 2016, 6, 281–339. 10.1007/11663_2016_5. [DOI] [Google Scholar]
- Nebel M.; Grützke S.; Diab N.; Schulte A.; Schuhmann W. Microelectrochemical Oisualization of Oxygen Consumption of Single Living Cells. Faraday Discuss. 2013, 164, 19–32. 10.1039/c3fd00011g. [DOI] [PubMed] [Google Scholar]
- Santos C. S.; Macedo F.; Kowaltowski A. J.; Bertotti M.; Unwin P. R.; Marques da Cunha F.; Meloni G. N. Unveiling the Contribution of the Reproductive System of Individual Caenorhabditis elegans on Oxygen Consumption by Single-Point Scanning Electrochemical Microscopy Measurements. Anal. Chim. Acta 2021, 1146, 88–97. 10.1016/j.aca.2020.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos C. S.; Kowaltowski A. J.; Bertotti M. Single Cell Oxygen Mapping (SCOM) by Scanning Electrochemical Microscopy Uncovers Heterogeneous Intracellular Oxygen Consumption. Sci. Rep. 2017, 7, 11428. 10.1038/s41598-017-11956-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M.; Wang L.; Xie Z.; Zhao L.; Zhang X.; Zhang M. High-Content Label-Free Single-Cell Analysis with a Microfluidic Device Using Programmable Scanning Electrochemical Microscopy. Anal. Chem. 2021, 93, 12417–12425. 10.1021/acs.analchem.1c02507. [DOI] [PubMed] [Google Scholar]
- Actis P.; Tokar S.; Clausmeyer J.; Babakinejad B.; Mikhaleva S.; Cornut R.; Takahashi Y.; López Córdoba A.; Novak P.; Shevchuck A. I.; et al. Electrochemical Nanoprobes for Single-Cell Analysis. ACS Nano 2014, 8, 875–884. 10.1021/nn405612q. [DOI] [PubMed] [Google Scholar]
- Wittstock G.; Schuhmann W. Formation and Imaging of Microscopic Enzymatically Active Spots on an Alkanethiolate-Covered Gold Electrode by Scanning Electrochemical Microscopy. Anal. Chem. 1997, 69, 5059–5066. 10.1021/ac970504o. [DOI] [Google Scholar]
- Liu D.; Shadike Z.; Lin R.; Qian K.; Li H.; Li K.; Wang S.; Yu Q.; Liu M.; Ganapathy S.; et al. Review of Recent Development of In Situ/Operando Characterization Techniques for Lithium Battery Research. Adv. Mater. 2019, 31, 1806620. 10.1002/adma.201806620. [DOI] [PubMed] [Google Scholar]
- Daboss S.; Rahmanian F.; Stein H. S.; Kranz C. The Potential of Scanning Electrochemical Probe Microscopy and Scanning Droplet Cells in Battery Research. Electrochem. Sci. Adv. 2022, 2, e2100122 10.1002/elsa.202100122. [DOI] [Google Scholar]
- Kempaiah R.; Vasudevamurthy G.; Subramanian A. Scanning Probe Microscopy Based Characterization of Battery Materials, Interfaces, and Processes. Nano Energy 2019, 65, 103925. 10.1016/j.nanoen.2019.103925. [DOI] [Google Scholar]
- Krueger B.; Balboa L.; Dohmann J. F.; Winter M.; Bieker P.; Wittstock G. Solid Electrolyte Interphase Evolution on Lithium Metal Electrodes Followed by Scanning Electrochemical Microscopy Under Realistic Battery Cycling Current Densities. ChemElectroChem. 2020, 7, 3590–3596. 10.1002/celc.202000441. [DOI] [Google Scholar]
- Ventosa E. Why Nanoelectrochemistry is Necessary in Battery Research?. Curr. Opin. Electrochem. 2021, 25, 100635. 10.1016/j.coelec.2020.09.002. [DOI] [Google Scholar]
- Kumatani A.; Matsue T. Recent Advances in Scanning Electrochemical Microscopic Analysis and Visualization on Lithium-ion Battery Electrodes. Curr. Opin. Electrochem. 2020, 22, 228–233. 10.1016/j.coelec.2020.07.010. [DOI] [Google Scholar]
- Santos C. S.; Botz A.; Bandarenka A. S.; Ventosa E.; Schuhmann W. Correlative Electrochemical Microscopy for the Elucidation of the Local Ionic and Electronic Properties of the Solid Electrolyte Interphase in Li-Ion Batteries. Angew. Chem., Int. Ed. 2022, 61, e202202744 10.1002/anie.202202744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conzuelo F.; Grützke S.; Stratmann L.; Pingarrón J. M.; Schuhmann W. Interrogation of Immunoassay Platforms by SERS and SECM after Enzyme-Catalyzed Deposition of Silver Nanoparticles. Microchim. Acta 2016, 183, 281–287. 10.1007/s00604-015-1654-x. [DOI] [Google Scholar]
- Fortin E.; Mailley P.; Lacroix L.; Szunerits S. Imaging of DNA Hybridization on Microscopic Polypyrrole Patterns using Scanning Electrochemical Microscopy (SECM): The HRP Bio-Catalyzed Oxidation of 4-Chloro-1-Naphthol. Analyst 2006, 131, 186–193. 10.1039/B504711K. [DOI] [PubMed] [Google Scholar]
- Lei R.; Stratmann L.; Schäfer D.; Erichsen T.; Neugebauer S.; Li N.; Schuhmann W. Imaging Biocatalytic Activity of Enzyme–Polymer Spots by Means of Combined Scanning Electrochemical Microscopy/Electrogenerated Chemiluminescence. Anal. Chem. 2009, 81, 5070–5074. 10.1021/ac900192n. [DOI] [PubMed] [Google Scholar]
- Kluge R. M.; Psaltis E.; Haid R. W.; Hou S.; Schmidt T. O.; Schneider O.; Garlyyev B.; Calle-Vallejo F.; Bandarenka A. S. Revealing the Nature of Active Sites on Pt-Gd and Pt-Pr Alloys During the Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2022, 14, 19604–19613. 10.1021/acsami.2c03604. [DOI] [PubMed] [Google Scholar]
- Haid R. W.; Kluge R. M.; Schmidt T. O.; Bandarenka A. S. In-Situ Detection of Active Sites for Carbon-Based Bifunctional Oxygen Reduction and Evolution Catalysis. Electrochim. Acta 2021, 382, 138285. 10.1016/j.electacta.2021.138285. [DOI] [Google Scholar]
- Bard A. J.; Fan F. R. F.; Kwak J.; Lev O. Scanning Electrochemical Microscopy. Introduction and Principles. Anal. Chem. 1989, 61, 132–138. 10.1021/ac00177a011. [DOI] [Google Scholar]
- Kalinin S. V.; Dyck O.; Balke N.; Neumayer S.; Tsai W.-Y.; Vasudevan R.; Lingerfelt D.; Ahmadi M.; Ziatdinov M.; McDowell M. T.; et al. Toward Electrochemical Studies on the Nanometer and Atomic Scales: Progress, Challenges, and Opportunities. ACS Nano 2019, 13, 9735–9780. 10.1021/acsnano.9b02687. [DOI] [PubMed] [Google Scholar]
- Kalinin S. V.; Strelcov E.; Belianinov A.; Somnath S.; Vasudevan R. K.; Lingerfelt E. J.; Archibald R. K.; Chen C.; Proksch R.; Laanait N.; et al. Big, Deep, and Smart Data in Scanning Probe Microscopy. ACS Nano 2016, 10, 9068–9086. 10.1021/acsnano.6b04212. [DOI] [PubMed] [Google Scholar]
- Franklin M. J.; White D. C.; Isaacs H. S. Pitting Corrosion by Bacteria on Carbon Steel, Determined by the Scanning Vibrating Electrode Technique. Corros. Sci. 1991, 32, 945–952. 10.1016/0010-938X(91)90014-G. [DOI] [Google Scholar]
- Ishikawa M.; Morita M.; Matsuda Y. In Situ Scanning Vibrating Electrode Technique for Lithium Metal Anodes. J. Power Sources 1997, 68, 501–505. 10.1016/S0378-7753(97)02524-X. [DOI] [Google Scholar]
- Montemor M. F.; Pinto R.; Ferreira M. Chemical Composition and Corrosion Protection of Silane Films Modified with CeO2 Nanoparticles. Electrochim. Acta 2009, 54, 5179–5189. 10.1016/j.electacta.2009.01.053. [DOI] [Google Scholar]
- Xiao X.; Bard A. J. Observing Single Nanoparticle Collisions at an Ultramicroelectrode by Electrocatalytic Amplification. J. Am. Chem. Soc. 2007, 129, 9610–9612. 10.1021/ja072344w. [DOI] [PubMed] [Google Scholar]
- Kwon S. J.; Fan F.-R. F.; Bard A. J. Observing Iridium Oxide (IrOx) Single Nanoparticle Collisions at Ultramicroelectrodes. J. Am. Chem. Soc. 2010, 132, 13165–13167. 10.1021/ja106054c. [DOI] [PubMed] [Google Scholar]
- Stuart E. J. E.; Tschulik K.; Batchelor-McAuley C.; Compton R. G. Electrochemical Observation of Single Collision Events: Fullerene Nanoparticles. ACS Nano 2014, 8, 7648–7654. 10.1021/nn502634n. [DOI] [PubMed] [Google Scholar]
- Stevenson K. J.; Tschulik K. A Materials Driven Approach for Understanding Single Entity Nano Impact Electrochemistry. Curr. Opin. Electrochem. 2017, 6, 38–45. 10.1016/j.coelec.2017.07.009. [DOI] [Google Scholar]
- Kazinczi R.; Szöcs E.; Kálmán E.; Nagy P. Novel Methods for Preparing EC-STM Tips. Appl. Phys. A: Mater. Sci. Process. 1998, 66, S535–S538. 10.1007/s003390051197. [DOI] [Google Scholar]
- Salerno M. Coating of Tips for Electrochemical Scanning Tunneling Microscopy by Means of Silicon, Magnesium, and Tungsten Oxides. Rev. Sci. Instrum. 2010, 81, 093703. 10.1063/1.3484191. [DOI] [PubMed] [Google Scholar]
- Dong C.; Meng G.; Saji S. E.; Gao X.; Zhang P.; Wu D.; Pan Y.; Yin Z.; Cheng Y. Simulation-Guided Nanofabrication of High-Quality Practical Tungsten Probes. RSC Adv. 2020, 10, 24280–24287. 10.1039/D0RA03967E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binnig G.; Rohrer H.; Gerber C.; Weibel E. Surface Studies by Scanning Tunneling Microscopy. Phys. Rev. Lett. 1982, 49, 57–61. 10.1103/PhysRevLett.49.57. [DOI] [Google Scholar]
- Bard A. J.; Mirkin M. V.; Unwin P. R.; Wipf D. O. Scanning Electrochemical Microscopy. 12. Theory and Experiment of the Feedback Mode with Finite Heterogeneous Electron-Transfer Kinetics and Arbitrary Substrate Size. J. Phys. Chem. 1992, 96, 1861–1868. 10.1021/j100183a064. [DOI] [Google Scholar]
- Ishimatsu R.; Kim J.; Jing P.; Striemer C. C.; Fang D. Z.; Fauchet P. M.; McGrath J. L.; Amemiya S. Ion-selective Permeability of an Ultrathin Nanoporous Silicon Membrane as Probed by Scanning Electrochemical Microscopy Using Micropipet-supported ITIES Tips. Anal. Chem. 2010, 82, 7127–7134. 10.1021/ac1005052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danis L.; Snowden M. E.; Tefashe U. M.; Heinemann C. N.; Mauzeroll J. Development of Nano-Disc electrodes for Application as Shear Force Sensitive Electrochemical Probes. Electrochim. Acta 2014, 136, 121–129. 10.1016/j.electacta.2014.05.047. [DOI] [Google Scholar]
- Kranz C. Recent Advancements in Nanoelectrodes and Nanopipettes used in Combined Scanning Electrochemical Microscopy Techniques. Analyst 2014, 139, 336–352. 10.1039/C3AN01651J. [DOI] [PubMed] [Google Scholar]
- Katemann B. B.; Schuhmann W. Fabrication and Characterization of Needle-Type Pt-Disk Nanoelectrodes. Electroanalysis 2002, 14, 22–28. . [DOI] [Google Scholar]
- Hansma P. K.; Drake B.; Marti O.; Gould S. A.; Prater C. B. The Scanning Ion-Conductance Microscope. Science 1989, 243, 641–643. 10.1126/science.2464851. [DOI] [PubMed] [Google Scholar]
- Snowden M. E.; Güell A. G.; Lai S. C. S.; McKelvey K.; Ebejer N.; O’Connell M. A.; Colburn A. W.; Unwin P. R. Scanning Electrochemical Cell Microscopy: Theory and Experiment for Quantitative High Resolution Spatially-Resolved Voltammetry and Simultaneous Ion-Conductance Measurements. Anal. Chem. 2012, 84, 2483–2491. 10.1021/ac203195h. [DOI] [PubMed] [Google Scholar]
- Morris C. A.; Chen C.-C.; Baker L. A. Transport of Redox Probes through Single Pores Measured by Scanning Electrochemical-Scanning Ion Conductance Microscopy (SECM-SICM). Analyst 2012, 137, 2933–2938. 10.1039/c2an16178h. [DOI] [PubMed] [Google Scholar]
- Binnig G.; Rohrer H. Scanning Tunneling Microscopy. Surf. Sci. 1983, 126, 236–244. 10.1016/0039-6028(83)90716-1. [DOI] [Google Scholar]
- Binnig G.; Quate C. F.; Gerber C. Atomic Force Microscope. Phys. Rev. Lett. 1986, 56, 930–933. 10.1103/PhysRevLett.56.930. [DOI] [PubMed] [Google Scholar]
- Kwak J.; Bard A. J. Scanning Electrochemical Microscopy. Theory of the Feedback Mode. Anal. Chem. 1989, 61, 1221–1227. 10.1021/ac00186a009. [DOI] [Google Scholar]
- Sun P.; Mirkin M. V. Kinetics of Electron-Transfer Reactions at Nanoelectrodes. Anal. Chem. 2006, 78, 6526–6534. 10.1021/ac060924q. [DOI] [PubMed] [Google Scholar]
- Sun T.; Yu Y.; Zacher B. J.; Mirkin M. V. Scanning Electrochemical Microscopy of Individual Catalytic Nanoparticles. Angew. Chem., Int. Ed. 2014, 53, 14120–14123. 10.1002/anie.201408408. [DOI] [PubMed] [Google Scholar]
- Sonnenfeld R.; Hansma P. K. Atomic-Resolution Microscopy in Water. Science 1986, 232, 211–213. 10.1126/science.232.4747.211. [DOI] [PubMed] [Google Scholar]
- Lai S. C. S.; Dudin P. V.; Macpherson J. V.; Unwin P. R. Visualizing Zeptomole (Electro)Catalysis at Single Nanoparticles within an Ensemble. J. Am. Chem. Soc. 2011, 133, 10744–10747. 10.1021/ja203955b. [DOI] [PubMed] [Google Scholar]
- Izquierdo J.; Knittel P.; Kranz C. Scanning Electrochemical Microscopy: An Analytical Perspective. Anal. Bioanal. Chem. 2018, 410, 307–324. 10.1007/s00216-017-0742-7. [DOI] [PubMed] [Google Scholar]
- Mirkin M. V.; Sun T.; Yu Y.; Zhou M. Electrochemistry at One Nanoparticle. Acc. Chem. Res. 2016, 49, 2328–2335. 10.1021/acs.accounts.6b00294. [DOI] [PubMed] [Google Scholar]
- Lazenby R.; White R. Advances and Perspectives in Chemical Imaging in Cellular Environments using Electrochemical Methods. Chemosensors 2018, 6, 24. 10.3390/chemosensors6020024. [DOI] [Google Scholar]
- Caniglia G.; Kranz C. Scanning Electrochemical Microscopy and its Potential for Studying Biofilms and Antimicrobial Coatings. Anal. Bioanal. Chem. 2020, 412, 6133–6148. 10.1007/s00216-020-02782-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F.; Unwin P. R.; Bard A. J. Scanning Electrochemical Microscopy. 16. Study of Second-Order Homogeneous Chemical Reactions via the Feedback and Generation/Collection Modes. J. Phys. Chem. 1992, 96, 4917–4924. 10.1021/j100191a036. [DOI] [Google Scholar]
- Lefrou C.; Cornut R. Analytical Expressions for Quantitative Scanning Electrochemical Microscopy (SECM). ChemPhysChem 2010, 11, 547–556. 10.1002/cphc.200900600. [DOI] [PubMed] [Google Scholar]
- Wipf D. O.; Bard A. J. Scanning Electrochemical Microscopy VII. Effect of Heterogeneous Electron-Transfer Rate at the Substrate on the Tip Feedback Current. J. Electrochem. Soc. 1991, 138, 469–474. 10.1149/1.2085612. [DOI] [Google Scholar]
- Cornut R.; Lefrou C. New Analytical Approximations for Negative Feedback Currents with a Microdisk SECM Tip. J. Electroanal. Chem. 2007, 604, 91–100. 10.1016/j.jelechem.2007.02.029. [DOI] [Google Scholar]
- Zampardi G.; Ventosa E.; La Mantia F.; Schuhmann W. In Situ Visualization of Li-ion Intercalation and Formation of the Solid Electrolyte Interphase on TiO2 Based Paste Electrodes using Scanning Electrochemical Microscopy. Chem. Commun. 2013, 49, 9347–9349. 10.1039/c3cc44576c. [DOI] [PubMed] [Google Scholar]
- Lee C.; Kwak J.; Anson F. C. Application of Scanning Electrochemical Microscopy to Generation/Collection Experiments with High Collection Efficiency. Anal. Chem. 1991, 63, 1501–1504. 10.1021/ac00014a030. [DOI] [Google Scholar]
- Martin R. D.; Unwin P. R. Scanning Electrochemical Microscopy Kinetics of Chemical Reactions Following Electron-Transfer Measured with the Substrate-Generation–Tip-Collection Mode. J. Chem. Soc., Faraday Trans. 1998, 94, 753–759. 10.1039/a707984b. [DOI] [Google Scholar]
- Tefashe U. M.; Loewenstein T.; Miura H.; Schlettwein D.; Wittstock G. Scanning Electrochemical Microscope Studies of Dye Regeneration in Indoline (D149)-Sensitized ZnO Photoelectrochemical Cells. J. Electroanal. Chem. 2010, 650, 24–30. 10.1016/j.jelechem.2010.09.014. [DOI] [Google Scholar]
- Minguzzi A.; Battistel D.; Rodríguez-López J.; Vertova A.; Rondinini S.; Bard A. J.; Daniele S. Rapid Characterization of Oxygen-Evolving Electrocatalyst Spot Arrays by the Substrate Generation/Tip Collection Mode of Scanning Electrochemical Microscopy with Decreased O2 Diffusion Layer Overlap. J. Phys. Chem. C 2015, 119, 2941–2947. 10.1021/jp510651f. [DOI] [Google Scholar]
- Park H. S.; Kweon K. E.; Ye H.; Paek E.; Hwang G. S.; Bard A. J. Factors in the Metal Doping of BiVO4 for Improved Photoelectrocatalytic Activity as Studied by Scanning Electrochemical Microscopy and First-Principles Density-Functional Calculation. J. Phys. Chem. C 2011, 115, 17870–17879. 10.1021/jp204492r. [DOI] [Google Scholar]
- Rastgar S.; Wittstock G. Characterization of Photoactivity of Nanostructured BiVO4 at Polarized Liquid–Liquid Interfaces by Scanning Electrochemical Microscopy. J. Phys. Chem. C 2017, 121, 25941–25948. 10.1021/acs.jpcc.7b09550. [DOI] [Google Scholar]
- Fernández J. L.; Bard A. J. Scanning Electrochemical Microscopy 50. Kinetic Study of Electrode Reactions by the Tip Generation-Substrate Collection Mode. Anal. Chem. 2004, 76, 2281–2289. 10.1021/ac035518a. [DOI] [PubMed] [Google Scholar]
- Fernández J. L.; Bard A. J. Scanning Electrochemical Microscopy. 47. Imaging Electrocatalytic Activity for Oxygen Reduction in an Acidic Medium by the Tip Generation-Substrate Collection Mode. Anal. Chem. 2003, 75, 2967–2974. 10.1021/ac0340354. [DOI] [PubMed] [Google Scholar]
- Johnson L.; Walsh D. A. Tip Generation–Substrate Collection–Tip Collection Mode Scanning Electrochemical Microscopy of Oxygen Reduction Electrocatalysts. J. Electroanal. Chem. 2012, 682, 45–52. 10.1016/j.jelechem.2012.06.024. [DOI] [Google Scholar]
- Leonard K. C.; Bard A. J. The Study of Multireactional Electrochemical Interfaces via a Tip Generation/Substrate Collection Mode of Scanning Electrochemical Microscopy: The Hydrogen Evolution Reaction for Mn in Acidic Solution. J. Am. Chem. Soc. 2013, 135, 15890–15896. 10.1021/ja407395m. [DOI] [PubMed] [Google Scholar]
- Zhang Q.; Ye Z.; Zhu Z.; Liu X.; Zhang J.; Cao F. Separation and Kinetic Study of Iron Corrosion in Acidic Solution via a Modified Tip Generation/Substrate Collection Mode by SECM. Corros. Sci. 2018, 139, 403–409. 10.1016/j.corsci.2018.05.021. [DOI] [Google Scholar]
- Conzuelo F.; Stratmann L.; Grützke S.; Pingarrón J. M.; Schuhmann W. Detection and Quantification of Sulfonamide Antibiotic Residues in Milk using Scanning Electrochemical Microscopy. Electroanalysis 2014, 26, 481–487. 10.1002/elan.201300577. [DOI] [Google Scholar]
- Pitta Bauermann L.; Schuhmann W.; Schulte A. An Advanced Biological Scanning Electrochemical Microscope (Bio-SECM) for Studying Individual Living Cells. Phys. Chem. Chem. Phys. 2004, 6, 4003–4008. 10.1039/b405233a. [DOI] [Google Scholar]
- Sklyar O.; Wittstock G. Numerical Simulations of Complex Nonsymmetrical 3D Systems for Scanning Electrochemical Microscopy using the Boundary Element Method. J. Phys. Chem. B 2002, 106, 7499–7508. 10.1021/jp020301q. [DOI] [Google Scholar]
- Eckhard K.; Chen X. X.; Turcu F.; Schuhmann W. Redox Competition Mode of Scanning Electrochemical Microscopy (RC-SECM) for Visualisation of Local Catalytic Activity. Phys. Chem. Chem. Phys. 2006, 8, 5359–5365. 10.1039/b609511a. [DOI] [PubMed] [Google Scholar]
- Karnicka K.; Eckhard K.; Guschin D. A.; Stoica L.; Kulesza P. J.; Schuhmann W. Visualisation of the Local Bio-Electrocatalytic Activity in Biofuel Cell Cathodes by Means of Redox Competition Scanning Electrochemical Microscopy (RC-SECM). Electrochem. Commun. 2007, 9, 1998–2002. 10.1016/j.elecom.2007.05.015. [DOI] [Google Scholar]
- Okunola A. O.; Nagaiah T. C.; Chen X.; Eckhard K.; Schuhmann W.; Bron M. Visualization of Local Electrocatalytic Activity of Metalloporphyrins Towards Oxygen Reduction by Means of Redox Competition Scanning electrochemical microscopy (RC-SECM). Electrochim. Acta 2009, 54, 4971–4978. 10.1016/j.electacta.2009.02.047. [DOI] [Google Scholar]
- Ivanauskas F.; Morkvenaite-Vilkonciene I.; Astrauskas R.; Ramanavicius A. Modelling of Scanning Electrochemical Microscopy at Redox Competition Mode using Diffusion and Reaction Equations. Electrochim. Acta 2016, 222, 347–354. 10.1016/j.electacta.2016.10.179. [DOI] [Google Scholar]
- González-García Y.; García S. J.; Hughes A. E.; Mol J. A Combined Redox-Competition and Negative-Feedback SECM Study of Self-Healing Anticorrosive Coatings. Electrochem. Commun. 2011, 13, 1094–1097. 10.1016/j.elecom.2011.07.009. [DOI] [Google Scholar]
- Asserghine A.; Medvidović-Kosanović M.; Nagy L.; Souto R. M.; Nagy G. A Study of the Electrochemical Reactivity of Titanium under Cathodic Polarization by Means of Combined Feedback and Redox Competition Modes of Scanning Electrochemical Microscopy. Sens. Actuators B Chem. 2020, 320, 128339. 10.1016/j.snb.2020.128339. [DOI] [Google Scholar]
- Morkvenaite-Vilkonciene I.; Ramanaviciene A.; Ramanavicius A. Redox Competition and Generation-Collection Modes Based Scanning Electrochemical Microscopy for the Evaluation of Immobilised Glucose Oxidase-Catalysed Reactions. RSC Adv. 2014, 4, 50064–50069. 10.1039/C4RA08697J. [DOI] [Google Scholar]
- Morkvenaite-Vilkonciene I.; Ramanaviciene A.; Genys P.; Ramanavicius A. Evaluation of Enzymatic Kinetics of GOx-based Electrodes by Scanning Electrochemical Microscopy at Redox Competition Mode. Electroanalysis 2017, 29, 1532–1542. 10.1002/elan.201700022. [DOI] [Google Scholar]
- Zinovicius A.; Morkvenaite-Vilkonciene I.; Ramanaviciene A.; Rozene J.; Popov A.; Ramanavicius A. Scanning Electrochemical Impedance Microscopy in Redox-Competition Mode for the Investigation of Antibodies Labelled with Horseradish Peroxidase. Materials 2021, 14, 4301. 10.3390/ma14154301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhard K.; Schuhmann W. Localised Visualisation of O2 Consumption and H2O2 Formation by Means of SECM for the Characterisation of Fuel Cell Catalyst Activity. Electrochim. Acta 2007, 53, 1164–1169. 10.1016/j.electacta.2007.02.028. [DOI] [Google Scholar]
- Nebel M.; Erichsen T.; Schuhmann W. Constant-Distance Mode SECM as a Tool to Visualize Local Electrocatalytic Activity of Oxygen Reduction Catalysts. Beilstein J. Nanotechnol. 2014, 5, 141–151. 10.3762/bjnano.5.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrotte O.; Boudet A.; Limani N.; Bergonzo P.; Zribi B.; Scorsone E.; Jousselme B.; Cornut R. Steady-State Electrocatalytic Activity Evaluation with the Redox Competition Mode of Scanning Electrochemical Microscopy: A Gold Probe and a Boron-Doped Diamond Substrate. ChemElectroChem. 2020, 7, 4633–4640. 10.1002/celc.202001088. [DOI] [Google Scholar]
- Nishizawa M.; Takoh K.; Matsue T. Micropatterning of HeLa Cells on Glass Substrates and Evaluation of Respiratory Activity Using Microelectrodes. Langmuir 2002, 18, 3645–3649. 10.1021/la011576k. [DOI] [Google Scholar]
- Shiku H.; Shiraishi T.; Ohya H.; Matsue T.; Abe H.; Hoshi H.; Kobayashi M. Oxygen Consumption of Single Bovine Embryos Probed by Scanning Electrochemical Microscopy. Anal. Chem. 2001, 73, 3751–3758. 10.1021/ac010339j. [DOI] [PubMed] [Google Scholar]
- Yasukawa T.; Kaya T.; Matsue T. Characterization and Imaging of Single Cells with Scanning Electrochemical Microscopy. Electroanalysis 2000, 12, 653–659. . [DOI] [Google Scholar]
- Horrocks B. R.; Mirkin M. V.; Pierce D. T.; Bard A. J.; Nagy G.; Toth K. Scanning Electrochemical Microscopy. 19. Ion-Selective Potentiometric Microscopy. Anal. Chem. 1993, 65, 1213–1224. 10.1021/ac00057a019. [DOI] [Google Scholar]
- Baranski A. S.; Szulborska A. High Frequency Impedance Measurements at Ultramicroelectrodes. Electrochim. Acta 1996, 41, 985–991. 10.1016/0013-4686(95)00429-7. [DOI] [Google Scholar]
- Eckhard K.; Schuhmann W. Alternating Current Techniques in Scanning Electrochemical Microscopy (AC-SECM). Analyst 2008, 133, 1486–1497. 10.1039/b806721j. [DOI] [PubMed] [Google Scholar]
- Bandarenka A. S.; Eckhard K.; Maljusch A.; Schuhmann W. Localized Electrochemical Impedance Spectroscopy: Visualization of Spatial Distributions of the Key Parameters Describing Solid/Liquid Interfaces. Anal. Chem. 2013, 85, 2443–2448. 10.1021/ac303490t. [DOI] [PubMed] [Google Scholar]
- Zou F.; Thierry D.; Isaacs H. S. A High-Resolution Probe for Localized Electrochemical Impedance Spectroscopy Measurements. J. Electrochem. Soc. 1997, 144, 1957–1965. 10.1149/1.1837729. [DOI] [Google Scholar]
- Estrada-Vargas A.; Bandarenka A.; Kuznetsov V.; Schuhmann W. In Situ Characterization of Ultrathin Films by Scanning Electrochemical Impedance Microscopy. Anal. Chem. 2016, 88, 3354–3362. 10.1021/acs.analchem.6b00011. [DOI] [PubMed] [Google Scholar]
- Rodríguez-López J.; Alpuche-Avilés M. A.; Bard A. J. Interrogation of Surfaces for the Quantification of Adsorbed Species on Electrodes: Oxygen on Gold and Platinum in Neutral Media. J. Am. Chem. Soc. 2008, 130, 16985–16995. 10.1021/ja8050553. [DOI] [PubMed] [Google Scholar]
- Rodríguez-López J.; Bard A. J. Scanning Electrochemical Microscopy: Surface Interrogation of Adsorbed Hydrogen and the Open Circuit Catalytic Decomposition of Formic Acid at Platinum. J. Am. Chem. Soc. 2010, 132, 5121–5129. 10.1021/ja9090319. [DOI] [PubMed] [Google Scholar]
- Papaderakis A.; Tsiplakides D.; Balomenou S.; Sotiropoulos S. Probing the Hydrogen Adsorption Affinity of Pt and Ir by Surface Interrogation Scanning Electrochemical Microscopy (SI-SECM). Electrochem. Commun. 2017, 83, 77–80. 10.1016/j.elecom.2017.09.003. [DOI] [Google Scholar]
- Liang Z.; Ahn H. S.; Bard A. J. A Study of the Mechanism of the Hydrogen Evolution Reaction on Nickel by Surface Interrogation Scanning Electrochemical Microscopy. J. Am. Chem. Soc. 2017, 139, 4854–4858. 10.1021/jacs.7b00279. [DOI] [PubMed] [Google Scholar]
- Simpson B. H.; Rodríguez-López J. Redox Titrations via Surface Interrogation Scanning Electrochemical Microscopy at an Extended Semiconducting Surface for the Quantification of Photogenerated Adsorbed Intermediates. Electrochim. Acta 2015, 179, 74–83. 10.1016/j.electacta.2015.04.128. [DOI] [Google Scholar]
- Huang C. C.; Wang Q.; Xiang D. B.; Shao H. B. Surface Interrogation Mode of Scanning Electrochemical Microscopy for Oxidation Study of Adsorbed CO Generated from Serine on Platinum. Chin. Chem. Lett. 2011, 22, 1481–1484. 10.1016/j.cclet.2011.07.017. [DOI] [Google Scholar]
- Wang Q.; Rodríguez-López J.; Bard A. J. Reaction of Br2 with Adsorbed CO on Pt, Studied by the Surface Interrogation Mode of Scanning Electrochemical Microscopy. J. Am. Chem. Soc. 2009, 131, 17046–17047. 10.1021/ja907626t. [DOI] [PubMed] [Google Scholar]
- Park H. S.; Leonard K. C.; Bard A. J. Surface Interrogation Scanning Electrochemical Microscopy (SI-SECM) of Photoelectrochemistry at a W/Mo-BiVO4 Semiconductor Electrode: Quantification of Hydroxyl Radicals During Water Oxidation. J. Phys. Chem. C 2013, 117, 12093–12102. 10.1021/jp400478z. [DOI] [Google Scholar]
- Zigah D.; Rodríguez-López J.; Bard A. J. Quantification of Photoelectrogenerated Hydroxyl Radical on TiO2 by Surface Interrogation Scanning Electrochemical Microscopy. Phys. Chem. Chem. Phys. 2012, 14, 12764–12772. 10.1039/c2cp40907k. [DOI] [PubMed] [Google Scholar]
- Ma Y.; Shinde P. S.; Li X.; Pan S. High-Throughput Screening and Surface Interrogation Studies of Au-Modified Hematite Photoanodes by Scanning Electrochemical Microscopy for Solar Water Splitting. ACS Omega 2019, 4, 17257–17268. 10.1021/acsomega.9b01907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz J.; Yu M.; Tüysüz H.; Harms C.; Dyck A.; Wittstock G. Coulometric Titration of Active Sites at Mesostructured Cobalt Oxide Spinel by Surface Interrogation Mode of Scanning Electrochemical Microscopy. J. Phys. Chem. C 2020, 124, 7737–7748. 10.1021/acs.jpcc.9b11114. [DOI] [Google Scholar]
- Kim J. Y.; Ahn H. S.; Bard A. J. Surface Interrogation Scanning Electrochemical Microscopy for a Photoelectrochemical Reaction: Water Oxidation on a Hematite Surface. Anal. Chem. 2018, 90, 3045–3049. 10.1021/acs.analchem.7b04728. [DOI] [PubMed] [Google Scholar]
- Sugimura H.; Uchida T.; Kitamura N.; Shimo N.; Masuhara H. Direct-Mode Scanning Electrochemical Microscopy with Three electrodes: Application to Fluorescent Micropattern Formation. J. Electroanal. Chem. 1993, 361, 57–63. 10.1016/0022-0728(93)87038-W. [DOI] [Google Scholar]
- Schwamborn S.; Stoica L.; Neugebauer S.; Reda T.; Schmidt H.-L.; Schuhmann W. Local Modulation of the Redox State of p-Nitrothiophenol Self-Assembled Monolayers Using the Direct Mode of Scanning Electrochemical Microscopy. ChemPhysChem 2009, 10, 1066–1070. 10.1002/cphc.200900118. [DOI] [PubMed] [Google Scholar]
- Danieli T.; Mandler D. Local Surface Patterning by Chitosan-Stabilized Gold Nanoparticles using the Direct Mode of Scanning Electrochemical Microscopy (SECM). J. Solid State Electrochem. 2013, 17, 2989–2997. 10.1007/s10008-013-2194-0. [DOI] [Google Scholar]
- Stratmann L.; Clausmeyer J.; Schuhmann W. Non-destructive Patterning of Carbon Electrodes by using the Direct Mode of Scanning Electrochemical Microscopy. ChemPhysChem 2015, 16, 3477–3482. 10.1002/cphc.201500585. [DOI] [PubMed] [Google Scholar]
- Han L.; Hu Z.; Sartin M. M.; Wang X.; Zhao X.; Cao Y.; Yan Y.; Zhan D.; Tian Z.-Q. Direct Nanomachining on Semiconductor Wafer By Scanning Electrochemical Microscopy. Angew. Chem., Int. Ed. 2020, 59, 21129–21134. 10.1002/anie.202008697. [DOI] [PubMed] [Google Scholar]
- Evans S. A.; Brakha K.; Billon M.; Mailley P.; Denuault G. Scanning Electrochemical Microscopy (SECM): Localized Glucose Oxidase Immobilization via the Direct Electrochemical Microspotting of Polypyrrole–Biotin Films. Electrochem. Commun. 2005, 7, 135–140. 10.1016/j.elecom.2004.11.019. [DOI] [Google Scholar]
- Matrab T.; Combellas C.; Kanoufi F. Scanning Electrochemical Microscopy for the Direct Patterning of a Gold Surface with Organic Moities Derived from Iodonium Salt. Electrochem. Commun. 2008, 10, 1230–1234. 10.1016/j.elecom.2008.06.006. [DOI] [Google Scholar]
- Eifert A.; Mizaikoff B.; Kranz C. Advanced Fabrication Process for Combined Atomic Force-Scanning Electrochemical Microscopy (AFM-SECM) Probes. Micron (Oxford, England: 1993) 2015, 68, 27–35. 10.1016/j.micron.2014.08.008. [DOI] [PubMed] [Google Scholar]
- O’Connell M. A.; Wain A. J. Mapping Electroactivity at Individual Catalytic Nanostructures using High-Resolution Scanning Electrochemical-Scanning Ion Conductance Microcopy. Anal. Chem. 2014, 86, 12100–12107. 10.1021/ac502946q. [DOI] [PubMed] [Google Scholar]
- Etienne M.; Anderson E. C.; Evans S. R.; Schuhmann W.; Fritsch I. Feedback-Independent Pt Nanoelectrodes for Shear Force-Based Constant-Distance Mode Scanning Electrochemical Microscopy. Anal. Chem. 2006, 78, 7317–7324. 10.1021/ac061310o. [DOI] [PubMed] [Google Scholar]
- Adam C.; Kanoufi F.; Sojic N.; Etienne M. Shearforce Positioning of Nanoprobe Electrode Arrays for Scanning Electrochemical Microscopy Experiments. Electrochim. Acta 2015, 179, 45–56. 10.1016/j.electacta.2015.04.140. [DOI] [Google Scholar]
- Ballesteros Katemann B.; Schulte A.; Schuhmann W. Constant-Distance Mode Scanning Electrochemical Microscopy (SECM) - Part I: Adaptation of a Non-Optical Shear-Force-Based Positioning Mode for SECM Tips. Chemistry 2003, 9, 2025–2033. 10.1002/chem.200204267. [DOI] [PubMed] [Google Scholar]
- Hussien E. M.; Schuhmann W.; Schulte A. Shearforce-Based Constant-Distance Scanning Electrochemical Microscopy as Fabrication Tool for Needle-Type Carbon-Fiber Nanoelectrodes. Anal. Chem. 2010, 82, 5900–5905. 10.1021/ac100738b. [DOI] [PubMed] [Google Scholar]
- Nebel M.; Eckhard K.; Erichsen T.; Schulte A.; Schuhmann W. 4D Shearforce-Based Constant-Distance Mode Scanning Electrochemical Microscopy. Anal. Chem. 2010, 82, 7842–7848. 10.1021/ac1008805. [DOI] [PubMed] [Google Scholar]
- Izquierdo J.; Fernández-Pérez B. M.; Eifert A.; Souto R. M.; Kranz C. Simultaneous Atomic Force - Scanning Electrochemical Microscopy (AFM-SECM) Imaging of Copper Dissolution. Electrochim. Acta 2016, 201, 320–332. 10.1016/j.electacta.2015.12.160. [DOI] [Google Scholar]
- Kranz C.; Friedbacher G.; Mizaikoff B.; Lugstein A.; Smoliner J.; Bertagnolli E. Integrating an Ultramicroelectrode in an AFM Cantilever: Combined Technology for Enhanced Information. Anal. Chem. 2001, 73, 2491–2500. 10.1021/ac001099v. [DOI] [PubMed] [Google Scholar]
- Kueng A.; Kranz C.; Lugstein A.; Bertagnolli E.; Mizaikoff B. Integrated AFM-SECM in Tapping Mode: Simultaneous Topographical and Electrochemical Imaging of Enzyme Activity. Angew. Chem., Int. Ed. 2003, 42, 3238–3240. 10.1002/anie.200351111. [DOI] [PubMed] [Google Scholar]
- Zampardi G.; Klink S.; Kuznetsov V.; Erichsen T.; Maljusch A.; La Mantia F.; Schuhmann W.; Ventosa E. Combined AFM/SECM Investigation of the Solid Electrolyte Interphase in Li-Ion Batteries. ChemElectroChem. 2015, 2, 1607–1611. 10.1002/celc.201500085. [DOI] [Google Scholar]
- Takahashi Y.; Shevchuk A. I.; Novak P.; Murakami Y.; Shiku H.; Korchev Y. E.; Matsue T. Simultaneous Noncontact Topography and Electrochemical Imaging by SECM/SICM Featuring Ion Current Feedback Regulation. J. Am. Chem. Soc. 2010, 132, 10118–10126. 10.1021/ja1029478. [DOI] [PubMed] [Google Scholar]
- Etienne M.; Schulte A.; Mann S.; Jordan G.; Dietzel I. D.; Schuhmann W. Constant-Distance Mode Scanning Potentiometry. 1. Visualization of Calcium Carbonate Dissolution in Aqueous Solution. Anal. Chem. 2004, 76, 3682–3688. 10.1021/ac0349227. [DOI] [PubMed] [Google Scholar]
- Etienne M.; Dierkes P.; Erichsen T.; Schuhmann W.; Fritsch I. Constant-Distance Mode Scanning Potentiometry. High Resolution pH Measurements in Three-Dimensions. Electroanalysis 2007, 19, 318–323. 10.1002/elan.200603735. [DOI] [Google Scholar]
- Wain A. J.; Pollard A. J.; Richter C. High-resolution electrochemical and topographical imaging using batch-fabricated cantilever probes. Anal. Chem. 2014, 86, 5143–5149. 10.1021/ac500946v. [DOI] [PubMed] [Google Scholar]
- Eckhard K.; Shin H.; Mizaikoff B.; Schuhmann W.; Kranz C. Alternating Current (AC) Impedance Imaging with Combined Atomic Force Scanning Electrochemical Microscopy (AFM-SECM). Electrochem. Commun. 2007, 9, 1311–1315. 10.1016/j.elecom.2007.01.027. [DOI] [Google Scholar]
- Etienne M.; Lhenry S.; Cornut R.; Lefrou C. Optimization of the Shearforce Signal for Scanning Electrochemical Microscopy and Application for Kinetic Analysis. Electrochim. Acta 2013, 88, 877–884. 10.1016/j.electacta.2012.09.063. [DOI] [Google Scholar]
- Etienne M.; Moulin J.-P.; Gourhand S. Accurate Control of the Electrode Shape for High Resolution Shearforce Regulated SECM. Electrochim. Acta 2013, 110, 16–21. 10.1016/j.electacta.2013.03.096. [DOI] [Google Scholar]
- Tefashe U. M.; Wittstock G. Quantitative Characterization of Shear Force Regulation for Scanning Electrochemical Microscopy. C. R. Chim. 2013, 16, 7–14. 10.1016/j.crci.2012.03.011. [DOI] [Google Scholar]
- Schulte A.; Nebel M.; Schuhmann W. Single Live Cell Topography and Activity Imaging with the Shear-Force-Based Constant-Distance Scanning Electrochemical Microscope. Methods Enzymol. 2012, 504, 237–254. 10.1016/B978-0-12-391857-4.00012-4. [DOI] [PubMed] [Google Scholar]
- Holder M. N.; Gardner C. E.; Macpherson J. V.; Unwin P. R. Combined Scanning Electrochemical-Atomic Force Microscopy (SECM-AFM): Simulation and Experiment for Flux-Generation at Un-Insulated Metal-Coated Probes. J. Electroanal. Chem. 2005, 585, 8–18. 10.1016/j.jelechem.2005.07.004. [DOI] [Google Scholar]
- Takahashi Y.; Hirano Y.; Yasukawa T.; Shiku H.; Yamada H.; Matsue T. Topographic, Electrochemical, and Optical Images Captured using Standing Approach Mode Scanning Electrochemical/Optical Microscopy. Langmuir 2006, 22, 10299–10306. 10.1021/la0611763. [DOI] [PubMed] [Google Scholar]
- Takahashi Y.; Shevchuk A. I.; Novak P.; Babakinejad B.; Macpherson J.; Unwin P. R.; Shiku H.; Gorelik J.; Klenerman D.; Korchev Y. E.; et al. Topographical and Electrochemical Nanoscale Imaging of Living Cells using Voltage-Switching Mode Scanning Electrochemical Microscopy. Proc. Natl. Acad. Sci. U.S.A. 2012, 109, 11540–11545. 10.1073/pnas.1203570109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte A.; Nebel M.; Schuhmann W. Single Live Cell Topography and Activity Imaging with the Shear-Force-Based Constant-Distance Scanning Electrochemical Microscope. Methods Enzymol. 2012, 504, 237–254. 10.1016/B978-0-12-391857-4.00012-4. [DOI] [PubMed] [Google Scholar]
- Jantz D. T.; Balla R. J.; Huang S.-H.; Kurapati N.; Amemiya S.; Leonard K. C. Simultaneous Intelligent Imaging of Nanoscale Reactivity and Topography by Scanning Electrochemical Microscopy. Anal. Chem. 2021, 93, 8906–8914. 10.1021/acs.analchem.1c01248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirabal A.; Calabrese Barton S. Numerical Correction of In Situ AFM-SECM Measurements. Anal. Chem. 2021, 93, 12495–12503. 10.1021/acs.analchem.1c00770. [DOI] [PubMed] [Google Scholar]
- Wei C.; Bard A. J.; Kapui I.; Nagy G.; Tóth K. Scanning Electrochemical Microscopy. 32. Gallium Ultramicroelectrodes and their Application in Ion-Selective Probes. Anal. Chem. 1996, 68, 2651–2655. 10.1021/ac9512362. [DOI] [PubMed] [Google Scholar]
- Filotás D.; Fernández-Pérez B. M.; Kiss A.; Nagy L.; Nagy G.; Souto R. M. Double Barrel Microelectrode Assembly to Prevent Electrical Field Effects in Potentiometric SECM Imaging of Galvanic Corrosion Processes. J. Electrochem. Soc. 2018, 165, C270–C277. 10.1149/2.0671805jes. [DOI] [Google Scholar]
- Filotás D.; Fernández-Pérez B. M.; Izquierdo J.; Kiss A.; Nagy L.; Nagy G.; Souto R. M. Improved Potentiometric SECM Imaging of Galvanic Corrosion Reactions. Corros. Sci. 2017, 129, 136–145. 10.1016/j.corsci.2017.10.006. [DOI] [Google Scholar]
- Filotás D.; Fernández-Pérez B. M.; Nagy L.; Nagy G.; Souto R. M. Multi-Barrel Electrodes Containing an Internal Micro-Reference for the Improved Visualization of Galvanic Corrosion Processes in Magnesium-Based Materials using Potentiometric Scanning Electrochemical Microscopy. Sens. Actuators B Chem. 2019, 296, 126625. 10.1016/j.snb.2019.126625. [DOI] [Google Scholar]
- Shin M.; Jeon C. Frequency-Distance Responses in SECM-EQCM: A Novel Method for Calibration of the Tip-Sample Distance. Bull. Korean Chem. Soc. 1998, 19, 1227–1232. [Google Scholar]
- Cliffel D. E.; Bard A. J. Scanning Electrochemical Microscopy. 36. A combined Scanning Electrochemical Microscope-Quartz Crystal Microbalance Instrument for Studying Thin Films. Anal. Chem. 1998, 70, 1993–1998. 10.1021/ac971217n. [DOI] [PubMed] [Google Scholar]
- Gollas B.; Bartlett P. N.; Denuault G. An Instrument for Simultaneous EQCM Impedance and SECM Measurements. Anal. Chem. 2000, 72, 349–356. 10.1021/ac990796o. [DOI] [PubMed] [Google Scholar]
- Hess C.; Borgwarth K.; Heinze J. Integration of an Electrochemical Quartz Crystal Microbalance into a Scanning Electrochemical Microscope for Mechanistic Studies of Surface Patterning Reactions. Electrochim. Acta 2000, 45, 3725–3736. 10.1016/S0013-4686(00)00465-5. [DOI] [Google Scholar]
- Blanc C.; Pébère N.; Tribollet B.; Vivier V. Galvanic Coupling Between Copper and Aluminium in a Thin-Layer Cell. Corros. Sci. 2010, 52, 991–995. 10.1016/j.corsci.2009.11.023. [DOI] [Google Scholar]
- Gabrielli C.; Joiret S.; Keddam M.; Perrot H.; Portail N.; Rousseau P.; Vivier V. Development of a Coupled SECM-EQCM Technique for the Study of Pitting Corrosion on Iron. J. Electrochem. Soc. 2006, 153, B68. 10.1149/1.2161574. [DOI] [Google Scholar]
- Gabrielli C.; Joiret S.; Keddam M.; Perrot H.; Portail N.; Rousseau P.; Vivier V. A SECM Assisted EQCM Study of Iron Pitting. Electrochim. Acta 2007, 52, 7706–7714. 10.1016/j.electacta.2007.03.008. [DOI] [Google Scholar]
- Clausmeyer J.; Nebel M.; Grützke S.; Kayran Y. U.; Schuhmann W. Local Surface Modifications Investigated by Combining Scanning Electrochemical Microscopy and Surface-Enhanced Raman Scattering. ChemPlusChem. 2018, 83, 414–417. 10.1002/cplu.201800031. [DOI] [PubMed] [Google Scholar]
- Gossage Z. T.; Schorr N. B.; Hernández-Burgos K.; Hui J.; Simpson B. H.; Montoto E. C.; Rodríguez-López J. Interrogating Charge Storage on Redox Active Colloids via Combined Raman Spectroscopy and Scanning Electrochemical Microscopy. Langmuir 2017, 33, 9455–9463. 10.1021/acs.langmuir.7b01121. [DOI] [PubMed] [Google Scholar]
- Hatfield K. O.; Gole M. T.; Schorr N. B.; Murphy C. J.; Rodríguez-López J. Surface-Enhanced Raman Spectroscopy-Scanning Electrochemical Microscopy: Observation of Real-Time Surface pH Perturbations. Anal. Chem. 2021, 93, 7792–7796. 10.1021/acs.analchem.1c00888. [DOI] [PubMed] [Google Scholar]
- Steimecke M.; Araújo-Cordero A. M.; Dieterich E.; Bron M. Probing Individual Cuprous Oxide Microcrystals towards Carbon Dioxide Reduction by using In Situ Raman-coupled Scanning Electrochemical Microscopy. ChemElectroChem. 2022, 9, e202101221 10.1002/celc.202101221. [DOI] [Google Scholar]
- Szunerits S.; Knorr N.; Calemczuk R.; Livache T. New Approach to Writing and Simultaneous Reading of Micropatterns: Combining Surface Plasmon Resonance Imaging with Scanning Electrochemical Microscopy (SECM). Langmuir 2004, 20, 9236–9241. 10.1021/la0492557. [DOI] [PubMed] [Google Scholar]
- Xiang J.; Guo J.; Zhou F. Scanning Electrochemical Microscopy Combined with Surface Plasmon Resonance: Studies of Localized Film Thickness Variations and Molecular Conformation Changes. Anal. Chem. 2006, 78, 1418–1424. 10.1021/ac051601h. [DOI] [PubMed] [Google Scholar]
- Xin Y.; Gao Y.; Guo J.; Chen Q.; Xiang J.; Zhou F. Real-Time Detection of Cu2+ Sequestration and Release by Immobilized Apo-Metallothioneins using SECM Combined with SPR. Biosens. Bioelectron. 2008, 24, 369–375. 10.1016/j.bios.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Wang L.; Kowalik J.; Mizaikoff B.; Kranz C. Combining Scanning Electrochemical Microscopy with Infrared Attenuated Total Reflection Spectroscopy for in situ Studies of Electrochemically Induced Processes. Anal. Chem. 2010, 82, 3139–3145. 10.1021/ac9027802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.; Kranz C.; Mizaikoff B. Monitoring Scanning Electrochemical Microscopy Approach Curves with Mid-Infrared Spectroscopy: Toward a Novel Current-Independent Positioning Mode. Anal. Chem. 2010, 82, 3132–3138. 10.1021/ac902781h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan F.-R. F.; Cliffel D.; Bard A. J. Scanning Electrochemical Microscopy. 37. Light Emission by Electrogenerated Chemiluminescence at SECM Tips and Their Application to Scanning Optical Microscopy. Anal. Chem. 1998, 70, 2941–2948. 10.1021/ac980107t. [DOI] [Google Scholar]
- Kanoufi F.; Cannes C.; Zu Y.; Bard A. J. Scanning Electrochemical Microscopy. 43. Investigation of Oxalate Oxidation and Electrogenerated Chemiluminescence across the Liquid–Liquid Interface. J. Phys. Chem. B 2001, 105, 8951–8962. 10.1021/jp0108667. [DOI] [Google Scholar]
- Miao W.; Choi J.-P.; Bard A. J. Electrogenerated Chemiluminescence 69: The Tris(2,2’-bipyridine)ruthenium(II), (Ru(bpy)3(2+))/tri-n-propylamine (TPrA) System Revisited- A new Route Involving TPrA*+ Cation Radicals. J. Am. Chem. Soc. 2002, 124, 14478–14485. 10.1021/ja027532v. [DOI] [PubMed] [Google Scholar]
- Boldt F.-M.; Heinze J.; Diez M.; Petersen J.; Börsch M. Real-time pH Microscopy Down to the Molecular Level by Combined Scanning Electrochemical Microscopy/Single-Molecule Fluorescence Spectroscopy. Anal. Chem. 2004, 76, 3473–3481. 10.1021/ac049635x. [DOI] [PubMed] [Google Scholar]
- Conzuelo F.; Sliozberg K.; Gutkowski R.; Grützke S.; Nebel M.; Schuhmann W. High-Resolution Analysis of Photoanodes for Water Splitting by Means of Scanning Photoelectrochemical Microscopy. Anal. Chem. 2017, 89, 1222–1228. 10.1021/acs.analchem.6b03706. [DOI] [PubMed] [Google Scholar]
- Zhao F.; Plumeré N.; Nowaczyk M. M.; Ruff A.; Schuhmann W.; Conzuelo F. Interrogation of a PS1-Based Photocathode by Means of Scanning Photoelectrochemical Microscopy. Small 2017, 13, 1604093. 10.1002/smll.201604093. [DOI] [PubMed] [Google Scholar]
- Shinde P. S.; Peng X.; Wang J.; Ma Y.; McNamara L. E.; Hammer N. I.; Gupta A.; Pan S. Rapid Screening of Photoanode Materials Using Scanning Photoelectrochemical Microscopy Technique and Formation of Z-Scheme Solar Water Splitting System by Coupling p- and n-type Heterojunction Photoelectrodes. ACS Appl. Energy Mater. 2018, 1, 2283–2294. 10.1021/acsaem.8b00381. [DOI] [Google Scholar]
- Bae J. H.; Nepomnyashchii A. B.; Wang X.; Potapenko D. V.; Mirkin M. V. Photo-Scanning Electrochemical Microscopy on the Nanoscale with Through-Tip Illumination. Anal. Chem. 2019, 91, 12601–12605. 10.1021/acs.analchem.9b03347. [DOI] [PubMed] [Google Scholar]
- Zhao F.; Hartmann V.; Ruff A.; Nowaczyk M. M.; Rögner M.; Schuhmann W.; Conzuelo F. Unravelling Electron Transfer Processes at Photosystem 2 Embedded in an Os-Complex Modified Redox Polymer. Electrochim. Acta 2018, 290, 451–456. 10.1016/j.electacta.2018.09.093. [DOI] [Google Scholar]
- James P.; Casillas N.; Smyrl W. H. Simultaneous Scanning Electrochemical and Photoelectrochemical Microscopy by Use of a Metallized Optical Fiber. J. Electrochem. Soc. 1996, 143, 3853–3865. 10.1149/1.1837308. [DOI] [Google Scholar]
- Chen C.-C.; Zhou Y.; Baker L. A. Scanning Ion Conductance Microscopy. Annu. Rev. Anal. Chem. 2012, 5, 207–228. 10.1146/annurev-anchem-062011-143203. [DOI] [PubMed] [Google Scholar]
- Edwards M. A.; Williams C. G.; Whitworth A. L.; Unwin P. R. Scanning Ion Conductance Microscopy: A Model for Experimentally Realistic Conditions and Image Interpretation. Anal. Chem. 2009, 81, 4482–4492. 10.1021/ac900376w. [DOI] [PubMed] [Google Scholar]
- Rheinlaender J.; Schäffer T. E. Image Formation, Resolution, and Height Measurement in Scanning Ion Conductance Microscopy. J. Appl. Phys. 2009, 105, 094905. 10.1063/1.3122007. [DOI] [Google Scholar]
- Zhu C.; Huang K.; Siepser N. P.; Baker L. A. Scanning Ion Conductance Microscopy. Chem. Rev. 2021, 121, 11726–11768. 10.1021/acs.chemrev.0c00962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheinlaender J.; Geisse N. A.; Proksch R.; Schäffer T. E. Comparison of Scanning Ion Conductance Microscopy with Atomic Force Microscopy for Cell Imaging. Langmuir 2011, 27, 697–704. 10.1021/la103275y. [DOI] [PubMed] [Google Scholar]
- Takahashi Y.; Murakami Y.; Nagamine K.; Shiku H.; Aoyagi S.; Yasukawa T.; Kanzaki M.; Matsue T. Topographic Imaging of Convoluted Surface of Live Cells by Scanning Ion Conductance Microscopy in a Standing Approach Mode. Phys. Chem. Chem. Phys. 2010, 12, 10012–10017. 10.1039/c002607g. [DOI] [PubMed] [Google Scholar]
- Happel P.; Thatenhorst D.; Dietzel I. D. Scanning Ion Conductance Microscopy for Studying Biological Samples. Sensors 2012, 12, 14983–15008. 10.3390/s121114983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korchev Y. E.; Gorelik J.; Lab M. J.; Sviderskaya E. V.; Johnston C. L.; Coombes C. R.; Vodyanoy I.; Edwards C. R. Cell Volume Measurement Using Scanning Ion Conductance Microscopy. Biophys. J. 2000, 78, 451–457. 10.1016/S0006-3495(00)76607-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelik J.; Zhang Y.; Shevchuk A. I.; Frolenkov G. I.; Sánchez D.; Lab M. J.; Vodyanoy I.; Edwards C. R. W.; Klenerman D.; Korchev Y. E. The Use of Scanning Ion Conductance Microscopy to Image A6 Cells. Mol. Cell. Endocrinol. 2004, 217, 101–108. 10.1016/j.mce.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Seifert J.; Rheinlaender J.; Novak P.; Korchev Y. E.; Schäffer T. E. Comparison of Atomic Force Microscopy and Scanning Ion Conductance Microscopy for Live Cell Imaging. Langmuir 2015, 31, 6807–6813. 10.1021/acs.langmuir.5b01124. [DOI] [PubMed] [Google Scholar]
- Böcker M.; Muschter S.; Schmitt E. K.; Steinem C.; Schäffer T. E. Imaging and Patterning of Pore-Suspending Membranes with Scanning Ion Conductance Microscopy. Langmuir 2009, 25, 3022–3028. 10.1021/la8034227. [DOI] [PubMed] [Google Scholar]
- McKelvey K.; Perry D.; Byers J. C.; Colburn A. W.; Unwin P. R. Bias Modulated Scanning Ion Conductance Microscopy. Anal. Chem. 2014, 86, 3639–3646. 10.1021/ac5003118. [DOI] [PubMed] [Google Scholar]
- Pastré D.; Iwamoto H.; Liu J.; Szabo G.; Shao Z. Characterization of AC Mode Scanning Ion-Conductance Microscopy. Ultramicroscopy 2001, 90, 13–19. 10.1016/S0304-3991(01)00096-1. [DOI] [PubMed] [Google Scholar]
- Chen C.-C.; Derylo M. A.; Baker L. A. Measurement of Ion Currents Through Porous Membranes with Scanning Ion Conductance Microscopy. Anal. Chem. 2009, 81, 4742–4751. 10.1021/ac900065p. [DOI] [PubMed] [Google Scholar]
- Shkirskiy V.; Kang M.; McPherson I. J.; Bentley C. L.; Wahab O. J.; Daviddi E.; Colburn A. W.; Unwin P. R. Electrochemical Impedance Measurements in Scanning Ion Conductance Microscopy. Anal. Chem. 2020, 92, 12509–12517. 10.1021/acs.analchem.0c02358. [DOI] [PubMed] [Google Scholar]
- Happel P.; Hoffmann G.; Mann S. A.; Dietzel I. D. Monitoring Cell Movements and Volume Changes with Pulse-Mode Scanning Ion Conductance Microscopy. J. Microsc. 2003, 212, 144–151. 10.1046/j.1365-2818.2003.01248.x. [DOI] [PubMed] [Google Scholar]
- Mann S. A.; Hoffmann G.; Hengstenberg A.; Schuhmann W.; Dietzel I. D. Pulse-Mode Scanning Ion Conductance Microscopy - A Method to Investigate Cultured Hippocampal Cells. J. Neurosci. Meth. 2002, 116, 113–117. 10.1016/S0165-0270(02)00023-7. [DOI] [PubMed] [Google Scholar]
- Liu S.; Dong Y.; Zhao W.; Xie X.; Ji T.; Yin X.; Liu Y.; Liang Z.; Momotenko D.; Liang D.; et al. Studies of Ionic Current Rectification using Polyethyleneimines Coated Glass Nanopipettes. Anal. Chem. 2012, 84, 5565–5573. 10.1021/ac3004852. [DOI] [PubMed] [Google Scholar]
- Sa N.; Lan W.-J.; Shi W.; Baker L. A. Rectification of Ion Current in Nanopipettes by External Substrates. ACS Nano 2013, 7, 11272–11282. 10.1021/nn4050485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan W.-J.; Edwards M. A.; Luo L.; Perera R. T.; Wu X.; Martin C. R.; White H. S. Voltage-Rectified Current and Fluid Flow in Conical Nanopores. Acc. Chem. Res. 2016, 49, 2605–2613. 10.1021/acs.accounts.6b00395. [DOI] [PubMed] [Google Scholar]
- He X.; Zhang K.; Li T.; Jiang Y.; Yu P.; Mao L. Micrometer-Scale Ion Current Rectification at Polyelectrolyte Brush-Modified Micropipets. J. Am. Chem. Soc. 2017, 139, 1396–1399. 10.1021/jacs.6b11696. [DOI] [PubMed] [Google Scholar]
- Bard A. J., Mirkin M. V., Eds. Scanning Electrochemical Microscopy, 3rd ed.; Taylor and Francis, 2001. [Google Scholar]
- Deng X. L.; Takami T.; Son J. W.; Kang E. J.; Kawai T.; Park B. H. Effect of Concentration Gradient on Ionic Current Rectification in Polyethyleneimine Modified Glass Nano-Pipettes. Sci. Rep. 2014, 4, 4005. 10.1038/srep04005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G.-C.; Gao M.-J.; Chen W.; Hu X.-Y.; Song L.-B.; Liu B.; Zhao Y.-D. pH-Modulated Ion-Current Rectification in a Cysteine-Functionalized Glass Nanopipette. Electrochem. Commun. 2018, 97, 6–10. 10.1016/j.elecom.2018.09.017. [DOI] [Google Scholar]
- Macazo F. C.; White R. J. Bioinspired Protein Channel-Based Scanning Ion Conductance Microscopy (Bio-SICM) for Simultaneous Conductance and Specific Molecular Imaging. J. Am. Chem. Soc. 2016, 138, 2793–2801. 10.1021/jacs.5b13252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lab M. J.; Bhargava A.; Wright P. T.; Gorelik J. The Scanning Ion Conductance Microscope for Cellular Physiology. Am. J. Physiol. Heart Circ. 2013, 304, H1–11. 10.1152/ajpheart.00499.2012. [DOI] [PubMed] [Google Scholar]
- Zhou L.; Zeng Y.; Baker L. A.; Hou J. A Proposed Route to Independent Measurements of Tight Junction Conductance at Discrete Cell Junctions. Tissue Barriers 2015, 3, e1105907 10.1080/21688370.2015.1105907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevchuk A.; Tokar S.; Gopal S.; Sanchez-Alonso J. L.; Tarasov A. I.; Vélez-Ortega A. C.; Chiappini C.; Rorsman P.; Stevens M. M.; Gorelik J.; et al. Angular Approach Scanning Ion Conductance Microscopy. Biophys. J. 2016, 110, 2252–2265. 10.1016/j.bpj.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelik J.; Gu Y.; Spohr H. A.; Shevchuk A. I.; Lab M. J.; Harding S. E.; Edwards C. R.; Whitaker M.; Moss G. W.; Benton D. C.; et al. Ion Channels in Small Cells and Subcellular Structures Can Be Studied with a Smart Patch-Clamp System. Biophys. J. 2002, 83, 3296–3303. 10.1016/S0006-3495(02)75330-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anariba F.; Anh J. H.; Jung G.-E.; Cho N.-J.; Cho S.-J. Biophysical Applications of Scanning Ion Conductance Microscopy (SICM). Mod. Phys. Lett. B 2012, 26, 1130003. 10.1142/S0217984911300031. [DOI] [Google Scholar]
- Pan R.; Hu K.; Jia R.; Rotenberg S. A.; Jiang D.; Mirkin M. V. Resistive-Pulse Sensing Inside Single Living Cells. J. Am. Chem. Soc. 2020, 142, 5778–5784. 10.1021/jacs.9b13796. [DOI] [PubMed] [Google Scholar]
- McKelvey K.; Kinnear S. L.; Perry D.; Momotenko D.; Unwin P. R. Surface Charge Mapping with a Nanopipette. J. Am. Chem. Soc. 2014, 136, 13735–13744. 10.1021/ja506139u. [DOI] [PubMed] [Google Scholar]
- Perry D.; Paulose Nadappuram B.; Momotenko D.; Voyias P. D.; Page A.; Tripathi G.; Frenguelli B. G.; Unwin P. R. Surface Charge Visualization at Viable Living Cells. J. Am. Chem. Soc. 2016, 138, 3152–3160. 10.1021/jacs.5b13153. [DOI] [PubMed] [Google Scholar]
- Page A.; Perry D.; Young P.; Mitchell D.; Frenguelli B. G.; Unwin P. R. Fast Nanoscale Surface Charge Mapping with Pulsed-Potential Scanning Ion Conductance Microscopy. Anal. Chem. 2016, 88, 10854–10859. 10.1021/acs.analchem.6b03744. [DOI] [PubMed] [Google Scholar]
- Perry D.; Page A.; Chen B.; Frenguelli B. G.; Unwin P. R. Differential-Concentration Scanning Ion Conductance Microscopy. Anal. Chem. 2017, 89, 12458–12465. 10.1021/acs.analchem.7b03543. [DOI] [PubMed] [Google Scholar]
- Fuhs T.; Klausen L. H.; Sønderskov S. M.; Han X.; Dong M. Direct Measurement of Surface Charge Distribution in Phase Separating Supported Lipid Bilayers. Nanoscale 2018, 10, 4538–4544. 10.1039/C7NR09522H. [DOI] [PubMed] [Google Scholar]
- Cremin K.; Jones B. A.; Teahan J.; Meloni G. N.; Perry D.; Zerfass C.; Asally M.; Soyer O. S.; Unwin P. R. Scanning Ion Conductance Microscopy Reveals Differences in the Ionic Environments of Gram-Positive and Negative Bacteria. Anal. Chem. 2020, 92, 16024–16032. 10.1021/acs.analchem.0c03653. [DOI] [PubMed] [Google Scholar]
- Perry D.; Al Botros R.; Momotenko D.; Kinnear S. L.; Unwin P. R. Simultaneous Nanoscale Surface Charge and Topographical Mapping. ACS Nano 2015, 9, 7266–7276. 10.1021/acsnano.5b02095. [DOI] [PubMed] [Google Scholar]
- Zhu C.; Zhou L.; Choi M.; Baker L. A. Mapping Surface Charge of Individual Microdomains with Scanning Ion Conductance Microscopy. ChemElectroChem. 2018, 5, 2986–2990. 10.1002/celc.201800724. [DOI] [Google Scholar]
- Wang Y.; Shan X.; Wang S.; Tao N.; Blanchard P.-Y.; Hu K.; Mirkin M. V. Imaging Local Electric Field Distribution by Plasmonic Impedance Microscopy. Anal. Chem. 2016, 88, 1547–1552. 10.1021/acs.analchem.5b04382. [DOI] [PubMed] [Google Scholar]
- Teahan J.; Perry D.; Chen B.; McPherson I. J.; Meloni G. N.; Unwin P. R. Scanning Ion Conductance Microscopy: Surface Charge Effects on Electroosmotic Flow Delivery from a Nanopipette. Anal. Chem. 2021, 93, 12281–12288. 10.1021/acs.analchem.1c01868. [DOI] [PubMed] [Google Scholar]
- Tao L.; Qiao M.; Jin R.; Li Y.; Xiao Z.; Wang Y.; Zhang N.; Xie C.; He Q.; Jiang D.; et al. Bridging the Surface Charge and Catalytic Activity of a Defective Carbon Electrocatalyst. Angew. Chem., Int. Ed. 2019, 58, 1019–1024. 10.1002/anie.201810207. [DOI] [PubMed] [Google Scholar]
- Comstock D. J.; Elam J. W.; Pellin M. J.; Hersam M. C. Integrated ultramicroelectrode-Nanopipet Probe for Concurrent Scanning Electrochemical Microscopy and Scanning Ion Conductance Microscopy. Anal. Chem. 2010, 82, 1270–1276. 10.1021/ac902224q. [DOI] [PubMed] [Google Scholar]
- Zhou Y.; Chen C.-C.; Weber A. E.; Zhou L.; Baker L. A. Potentiometric-Scanning Ion Conductance Microscopy. Langmuir 2014, 30, 5669–5675. 10.1021/la500911w. [DOI] [PubMed] [Google Scholar]
- Chen C.-C.; Zhou Y.; Morris C. A.; Hou J.; Baker L. A. Scanning Ion Conductance Microscopy Measurement of Paracellular Channel Conductance in Tight Junctions. Anal. Chem. 2013, 85, 3621–3628. 10.1021/ac303441n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadappuram B. P.; McKelvey K.; Al Botros R.; Colburn A. W.; Unwin P. R. Fabrication and Characterization of Dual Function Nanoscale pH-Scanning Ion Conductance Microscopy (SICM) Probes for High Resolution pH Mapping. Anal. Chem. 2013, 85, 8070–8074. 10.1021/ac401883n. [DOI] [PubMed] [Google Scholar]
- Rothery A. M.; Gorelik J.; Bruckbauer A.; Yu W.; Korchev Y. E.; Klenerman D. A Novel Light Source for SICM-SNOM of Living Cells. J. Microsc. 2003, 209, 94–101. 10.1046/j.1365-2818.2003.01122.x. [DOI] [PubMed] [Google Scholar]
- Clarke R. W.; White S. S.; Zhou D.; Ying L.; Klenerman D. Trapping of Proteins Under Physiological Conditions in a Nanopipette. Angew. Chem., Int. Ed. 2005, 44, 3747–3750. 10.1002/anie.200500196. [DOI] [PubMed] [Google Scholar]
- Lyon A. R.; MacLeod K. T.; Zhang Y.; Garcia E.; Kanda G. K.; Lab M. J.; Korchev Y. E.; Harding S. E.; Gorelik J. Loss of T-tubules and other Changes to Surface Topography in Ventricular Myocytes from Failing Human and Rat Heart. Proc. Natl. Acad. Sci. U.S.A. 2009, 106, 6854–6859. 10.1073/pnas.0809777106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergner S.; Vatsyayan P.; Matysik F.-M. Recent Advances in High Resolution Scanning Electrochemical Microscopy of Living Cells - A Review. Anal. Chim. Acta 2013, 775, 1–13. 10.1016/j.aca.2012.12.042. [DOI] [PubMed] [Google Scholar]
- Zhou Y.; Saito M.; Miyamoto T.; Novak P.; Shevchuk A. I.; Korchev Y. E.; Fukuma T.; Takahashi Y. Nanoscale Imaging of Primary Cilia with Scanning Ion Conductance Microscopy. Anal. Chem. 2018, 90, 2891–2895. 10.1021/acs.analchem.7b05112. [DOI] [PubMed] [Google Scholar]
- Bednarska J.; Novak P.; Korchev Y.; Rorsman P.; Tarasov A. I.; Shevchuk A. Release of Insulin Granules by Simultaneous, High-Speed Correlative SICM-FCM. J. Microsc. 2021, 282, 21–29. 10.1111/jmi.12972. [DOI] [PubMed] [Google Scholar]
- Zhou L.; Zhou Y.; Baker L. A. Measuring Ions with Scanning Ion Conductance Microscopy. Electrochem. Soc. Interface 2014, 23, 47–52. 10.1149/2.F05142if. [DOI] [Google Scholar]
- Bednarska J.; Pelchen-Matthews A.; Novak P.; Burden J. J.; Summers P. A.; Kuimova M. K.; Korchev Y.; Marsh M.; Shevchuk A. Rapid Formation of Human Immunodeficiency Virus-Like Particles. Proc. Natl. Acad. Sci. U.S.A. 2020, 117, 21637–21646. 10.1073/pnas.2008156117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbritter J.; Repphun G.; Vinzelberg S.; Staikov G.; Lorenz W. J. Tunneling Mechanisms in Electrochemical STM - Distance and Voltage Tunneling Spectroscopy. Electrochim. Acta 1995, 40, 1385–1394. 10.1016/0013-4686(95)00038-G. [DOI] [Google Scholar]
- Kuznetsov A. M.; Ulstrup J. Mechanisms of in Situ Scanning Tunnelling Microscopy of Organized Redox Molecular Assemblies. J. Phys. Chem. A 2000, 104, 11531–11540. 10.1021/jp993635x. [DOI] [Google Scholar]
- Matsushima H.; Lin S.-W.; Morin S.; Magnussen O. M. In situ video-STM Studies of the Mechanisms and Dynamics of Electrochemical Bismuth Nanostructure Formation on Au. Faraday Discuss. 2016, 193, 171–185. 10.1039/C6FD00086J. [DOI] [PubMed] [Google Scholar]
- Gewirth A. A.; Bard A. J. In Situ Scanning Tunneling Microscopy of the Anodic Oxidation of Highly Oriented Pyrolytic Graphite Surfaces. J. Phys. Chem. 1988, 92, 5563–5566. 10.1021/j100331a006. [DOI] [Google Scholar]
- Itaya K.; Tomita E. Scanning Tunneling Microscope for Electrochemistry - A New Concept for the in situ Scanning Tunneling Microscope in Electrolyte Solutions. Surf. Sci. 1988, 201, L507–L512. 10.1016/0039-6028(88)90489-X. [DOI] [Google Scholar]
- Lustenberger P.; Rohrer H.; Christoph R.; Siegenthaler H. Scanning Tunneling Microscopy at Potential Controlled Electrode Surfaces in Electrolytic Environment. J. Electroanal. Chem. 1988, 243, 225–235. 10.1016/0022-0728(88)85043-5. [DOI] [Google Scholar]
- Lev O.; Fan F.-R.; Bard A. J. The Application of Scanning Tunneling Microscopy to In Situ Studies of Nickel Electrodes under Potential Control. J. Electrochem. Soc. 1988, 135, 783–784. 10.1149/1.2095751. [DOI] [Google Scholar]
- Hachiya T.; Honbo H.; Itaya K. Detailed Underpotential Deposition of Copper on Gold(III) in Aqueous Solutions. J. Electroanal. Chem. 1991, 315, 275–291. 10.1016/0022-0728(91)80076-3. [DOI] [Google Scholar]
- Nagahara L. A.; Thundat T.; Lindsay S. M. Preparation and Characterization of STM Tips for Electrochemical Studies. Rev. Sci. Instrum. 1989, 60, 3128–3130. 10.1063/1.1140590. [DOI] [Google Scholar]
- Hudson J. E.; Abruña H. D. STM and ECSTM Study of the Formation and Structure of Self-Assembling Osmium Complexes on Pt(111). J. Phys. Chem. 1996, 100, 1036–1042. 10.1021/jp951708g. [DOI] [Google Scholar]
- Güell A. G.; Díez-Pérez I.; Gorostiza P.; Sanz F. Preparation of Reliable Probes for Electrochemical Tunneling Spectroscopy. Anal. Chem. 2004, 76, 5218–5222. 10.1021/ac035150h. [DOI] [PubMed] [Google Scholar]
- Pobelov I. V.; Li Z.; Wandlowski T. Electrolyte Gating in Redox-Active Tunneling Junctions - An Electrochemical STM Approach. J. Am. Chem. Soc. 2008, 130, 16045–16054. 10.1021/ja8054194. [DOI] [PubMed] [Google Scholar]
- Li Z.; Liu Y.; Mertens S. F. L.; Pobelov I. V.; Wandlowski T. From Redox Gating to Quantized Charging. J. Am. Chem. Soc. 2010, 132, 8187–8193. 10.1021/ja102754n. [DOI] [PubMed] [Google Scholar]
- Bodappa N.; Fluch U.; Fu Y.; Mayor M.; Moreno-García P.; Siegenthaler H.; Wandlowski T. Controlled Assembly and Single Electron Charging of Monolayer Protected Au144 Clusters: An Electrochemistry and Scanning Tunneling Spectroscopy Study. Nanoscale 2014, 6, 15117–15126. 10.1039/C4NR03793F. [DOI] [PubMed] [Google Scholar]
- Rudnev A. V.; Pobelov I. V.; Wandlowski T. Structural Aspects of Redox-Mediated Electron Tunneling. J. Electroanal. Chem. 2011, 660, 302–308. 10.1016/j.jelechem.2010.11.014. [DOI] [Google Scholar]
- Herrera S.; Adam C.; Ricci A.; Calvo E. J. Electrochemical Gating of Single Osmium Molecules Tethered to Au Surfaces. J. Solid State Electrochem. 2016, 20, 957–967. 10.1007/s10008-015-2983-8. [DOI] [Google Scholar]
- Wolfschmidt H.; Baier C.; Gsell S.; Fischer M.; Schreck M.; Stimming U. STM, SECPM, AFM and Electrochemistry on Single Crystalline Surfaces. Materials 2010, 3, 4196–4213. 10.3390/ma3084196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi Q.; Farver O.; Ulstrup J. Long-Range Protein Electron Transfer Observed at the Single-Molecule Level: In Situ Mapping of Redox-Gated Tunneling Resonance. Proc. Natl. Acad. Sci. U.S.A. 2005, 102, 16203–16208. 10.1073/pnas.0508257102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer A.; Ding X.; Bandarenka A. S.; Kunze-Liebhäuser J. The Potential of Zero Charge and the Electrochemical Interface Structure of Cu(111) in Alkaline Solutions. J. Phys. Chem. C 2021, 125, 5020–5028. 10.1021/acs.jpcc.0c09289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessandrini A.; Gerunda M.; Canters G. W.; Verbeet M.; Facci P. Electron Tunnelling Through Azurin is Mediated by the Active Site Cu Ion. Chem. Phys. Lett. 2003, 376, 625–630. 10.1016/S0009-2614(03)01020-0. [DOI] [Google Scholar]
- Ryan M. P.; Newman R. C.; Thompson G. E. An STM Study of the Passive Film Formed on Iron in Borate Buffer Solution. J. Electrochem. Soc. 1995, 142, L177–L179. 10.1149/1.2050035. [DOI] [Google Scholar]
- Dıez-Pérez I.; Gorostiza P.; Sanz F. Direct Evidence of the Electronic Conduction of the Passive Film on Iron by EC-STM. J. Electrochem. Soc. 2003, 150, B348. 10.1149/1.1580823. [DOI] [Google Scholar]
- Dıez-Pérez I.; Sanz F.; Gorostiza P. In situ studies of metal passive films. Curr. Opin. Solid State Mater. Sci. 2006, 10, 144–152. 10.1016/j.cossms.2007.01.002. [DOI] [Google Scholar]
- Zamborini F. P.; Crooks R. M. Corrosion Passivation of Gold by n -Alkanethiol Self-Assembled Monolayers: Effect of Chain Length and End Group. Langmuir 1998, 14, 3279–3286. 10.1021/la971121o. [DOI] [Google Scholar]
- Alessandrini A.; Corni S.; Facci P. Unravelling Single Metalloprotein Electron Transfer by Scanning Probe Techniques. Phys. Chem. Chem. Phys. 2006, 8, 4383–4397. 10.1039/b607021c. [DOI] [PubMed] [Google Scholar]
- Wang M.; Bugarski S.; Stimming U. Topological and Electron-Transfer Properties of Glucose Oxidase Adsorbed on Highly Oriented Pyrolytic Graphite Electrodes. J. Phys. Chem. C 2008, 112, 5165–5173. 10.1021/jp071122h. [DOI] [Google Scholar]
- Artés J. M.; Díez-Pérez I.; Sanz F.; Gorostiza P. Direct Measurement of Electron Transfer Distance Decay Constants of Single Redox Proteins by Electrochemical Tunneling Spectroscopy. ACS Nano 2011, 5, 2060–2066. 10.1021/nn103236e. [DOI] [PubMed] [Google Scholar]
- Artés J. M.; López-Martínez M.; Giraudet A.; Díez-Pérez I.; Sanz F.; Gorostiza P. Current-Voltage Characteristics and Transition Voltage Spectroscopy of Individual Redox Proteins. J. Am. Chem. Soc. 2012, 134, 20218–20221. 10.1021/ja3080242. [DOI] [PubMed] [Google Scholar]
- Yagati A. K.; Lee J.-Y.; Nam E.-S.; Cho S.; Choi J. W. Electrochemical Scanning Tunneling Microscopy Analysis on Protein Based Electronic Devices. Sci. Adv. Mater. 2017, 9, 102–121. 10.1166/sam.2017.3003. [DOI] [Google Scholar]
- Hahn C.; Hatsukade T.; Kim Y.-G.; Vailionis A.; Baricuatro J. H.; Higgins D. C.; Nitopi S. A.; Soriaga M. P.; Jaramillo T. F. Engineering Cu surfaces for the electrocatalytic conversion of CO2: Controlling selectivity toward oxygenates and hydrocarbons. Proc. Natl. Acad. Sci. U.S.A. 2017, 114, 5918–5923. 10.1073/pnas.1618935114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y.; Pfisterer J. H. K.; McLaughlin D.; Csoklich C.; Seidl L.; Bandarenka A. S.; Schneider O. Electrochemical Scanning Probe Microscopies in Electrocatalysis. Small Methods 2019, 3, 1800387. 10.1002/smtd.201800387. [DOI] [Google Scholar]
- Gu J.-Y.; Cai Z.-F.; Wang D.; Wan L.-J. Single-Molecule Imaging of Iron-Phthalocyanine-Catalyzed Oxygen Reduction Reaction by in Situ Scanning Tunneling Microscopy. ACS Nano 2016, 10, 8746–8750. 10.1021/acsnano.6b04281. [DOI] [PubMed] [Google Scholar]
- Mitterreiter E.; Liang Y.; Golibrzuch M.; McLaughlin D.; Csoklich C.; Bartl J. D.; Holleitner A.; Wurstbauer U.; Bandarenka A. S. In-Situ Visualization of Hydrogen Evolution Sites on Helium Ion Treated Molybdenum Dichalcogenides Under Reaction Conditions. npj 2D Mater. Appl. 2019, 3, 25. 10.1038/s41699-019-0107-5. [DOI] [Google Scholar]
- Inaba M.; Siroma Z.; Funabiki A.; Ogumi Z.; Abe T.; Mizutani Y.; Asano M. Electrochemical Scanning Tunneling Microscopy Observation of Highly Oriented Pyrolytic Graphite Surface Reactions in an Ethylene Carbonate-Based Electrolyte Solution. Langmuir 1996, 12, 1535–1540. 10.1021/la950848e. [DOI] [Google Scholar]
- Inaba M.; Siroma Z.; Kawatate Y.; Funabiki A.; Ogumi Z. Electrochemical Scanning Tunneling Microscopy Analysis of the Surface Reactions on Graphite Basal Plane in Ethylene Carbonate-Based Solvents and Propylene Carbonate. J. Power Sources 1997, 68, 221–226. 10.1016/S0378-7753(96)02555-4. [DOI] [Google Scholar]
- Wang X.; Cai Z.-F.; Wang D.; Wan L.-J. Molecular Evidence for the Catalytic Process of Cobalt Porphyrin Catalyzed Oxygen Evolution Reaction in Alkaline Solution. J. Am. Chem. Soc. 2019, 141, 7665–7669. 10.1021/jacs.9b01229. [DOI] [PubMed] [Google Scholar]
- Wang X.; Cai Z.-F.; Wang Y.-Q.; Feng Y.-C.; Yan H.-J.; Wang D.; Wan L.-J. In Situ Scanning Tunneling Microscopy of Cobalt-Phthalocyanine-Catalyzed CO2 Reduction Reaction. Angew. Chem., Int. Ed. 2020, 59, 16098–16103. 10.1002/anie.202005242. [DOI] [PubMed] [Google Scholar]
- Yoshimoto S.; Honda Y.; Ito O.; Itaya K. Supramolecular Pattern of Fullerene on 2D Bimolecular ″Chessboard″ Consisting of Bottom-Up Assembly of Porphyrin and Phthalocyanine Molecules. J. Am. Chem. Soc. 2008, 130, 1085–1092. 10.1021/ja077407p. [DOI] [PubMed] [Google Scholar]
- Seidl L.; Martens S.; Ma J.; Stimming U.; Schneider O. In Situ Scanning Tunneling Microscopy Studies of the SEI Formation on Graphite Electrodes for Li(+)-Ion Batteries. Nanoscale 2016, 8, 14004–14014. 10.1039/C6NR00825A. [DOI] [PubMed] [Google Scholar]
- Treutler T. H.; Wittstock G. Combination of an Electrochemical Tunneling Microscope (ECSTM) and a Scanning Electrochemical Microscope (SECM): Application for Tip-Induced Modification of Self-Assembled Monolayers. Electrochim. Acta 2003, 48, 2923–2932. 10.1016/S0013-4686(03)00357-8. [DOI] [Google Scholar]
- Kim Y.-G.; Javier A.; Baricuatro J. H.; Torelli D.; Cummins K. D.; Tsang C. F.; Hemminger J. C.; Soriaga M. P. Surface Reconstruction of Pure-Cu Single-Crystal Electrodes Under CO-Reduction Potentials in Alkaline Solutions: A Study by Seriatim ECSTM-DEMS. J. Electroanal. Chem. 2016, 780, 290–295. 10.1016/j.jelechem.2016.09.029. [DOI] [Google Scholar]
- Williams C. G.; Edwards M. A.; Colley A. L.; Macpherson J. V.; Unwin P. R. Scanning Micropipet Contact Method for High-Resolution Imaging of Electrode Surface Redox Activity. Anal. Chem. 2009, 81, 2486–2495. 10.1021/ac802114r. [DOI] [PubMed] [Google Scholar]
- Ebejer N.; Schnippering M.; Colburn A. W.; Edwards M. A.; Unwin P. R. Localized High Resolution Electrochemistry and Multifunctional Imaging: Scanning Electrochemical Cell Microscopy. Anal. Chem. 2010, 82, 9141–9145. 10.1021/ac102191u. [DOI] [PubMed] [Google Scholar]
- Ebejer N.; Güell A. G.; Lai S. C. S.; McKelvey K.; Snowden M. E.; Unwin P. R. Scanning Electrochemical Cell Microscopy: A Versatile Technique for Nanoscale Electrochemistry and Functional Imaging. Annu. Rev. Anal. Chem. 2013, 6, 329–351. 10.1146/annurev-anchem-062012-092650. [DOI] [PubMed] [Google Scholar]
- Wahab O. J.; Kang M.; Unwin P. R. Scanning Electrochemical Cell Microscopy: A Natural Technique for Single Entity Electrochemistry. Curr. Opin. Electrochem. 2020, 22, 120–128. 10.1016/j.coelec.2020.04.018. [DOI] [Google Scholar]
- Martín-Yerga D.; Costa-García A.; Unwin P. R. Correlative Voltammetric Microscopy: Structure-Activity Relationships in the Microscopic Electrochemical Behavior of Screen Printed Carbon Electrodes. ACS sensors 2019, 4, 2173–2180. 10.1021/acssensors.9b01021. [DOI] [PubMed] [Google Scholar]
- Yamamoto T.; Ando T.; Kawabe Y.; Fukuma T.; Enomoto H.; Nishijima Y.; Matsui Y.; Kanamura K.; Takahashi Y. Characterization of the Depth of Discharge-Dependent Charge Transfer Resistance of a Single LiFePO4 Particle. Anal. Chem. 2021, 93, 14448–14453. 10.1021/acs.analchem.1c02851. [DOI] [PubMed] [Google Scholar]
- Gao R.; Edwards M. A.; Qiu Y.; Barman K.; White H. S. Visualization of Hydrogen Evolution at Individual Platinum Nanoparticles at a Buried Interface. J. Am. Chem. Soc. 2020, 142, 8890–8896. 10.1021/jacs.0c02202. [DOI] [PubMed] [Google Scholar]
- Chen C.-H.; Ravenhill E. R.; Momotenko D.; Kim Y.-R.; Lai S. C. S.; Unwin P. R. Impact of Surface Chemistry on Nanoparticle-Electrode Interactions in the Electrochemical Detection of Nanoparticle Collisions. Langmuir 2015, 31, 11932–11942. 10.1021/acs.langmuir.5b03033. [DOI] [PubMed] [Google Scholar]
- Ustarroz J.; Kang M.; Bullions E.; Unwin P. R. Impact and Oxidation of Single Silver Nanoparticles at Electrode Surfaces:One Shot Versus Multiple Events. Chem. Sci. 2017, 8, 1841–1853. 10.1039/C6SC04483B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha P.; Hill J. W.; Walmsley J. D.; Hill C. M. Probing Electrocatalysis at Individual Au Nanorods via Correlated Optical and Electrochemical Measurements. Anal. Chem. 2018, 90, 12832–12839. 10.1021/acs.analchem.8b03360. [DOI] [PubMed] [Google Scholar]
- Valavanis D.; Ciocci P.; Meloni G. N.; Morris P.; Lemineur J.-F.; McPherson I. J.; Kanoufi F.; Unwin P. R. Hybrid Scanning Electrochemical Cell Microscopy-Interference Reflection Microscopy (SECCM-IRM): Tracking Phase Formation on Surfaces in Small Volumes. Faraday Discuss. 2022, 233, 122–148. 10.1039/D1FD00063B. [DOI] [PubMed] [Google Scholar]
- Cheng L.; Jin R.; Jiang D.; Zhuang J.; Liao X.; Zheng Q. Scanning Electrochemical Cell Microscopy Platform with Local Electrochemical Impedance Spectroscopy. Anal. Chem. 2021, 93, 16401–16408. 10.1021/acs.analchem.1c02972. [DOI] [PubMed] [Google Scholar]
- Momotenko D.; Byers J. C.; McKelvey K.; Kang M.; Unwin P. R. High-Speed Electrochemical Imaging. ACS Nano 2015, 9, 8942–8952. 10.1021/acsnano.5b02792. [DOI] [PubMed] [Google Scholar]
- Brunet Cabré M.; Djekic D.; Romano T.; Hanna N.; Anders J.; McKelvey K. Microscale Electrochemical Cell on a Custom CMOS Transimpedance Amplifier for High Temporal Resolution Single Entity Electrochemistry. ChemElectroChem. 2020, 7, 4724–4729. 10.1002/celc.202001083. [DOI] [Google Scholar]
- Blount B.; Juarez G.; Wang Y.; Ren H. iR Drop in Scanning Electrochemical Cell Microscopy. Faraday Discuss. 2022, 233, 149–162. 10.1039/D1FD00046B. [DOI] [PubMed] [Google Scholar]
- Li Y.; Morel A.; Gallant D.; Mauzeroll J. Ag+ Interference from Ag/AgCl Wire Quasi-Reference Counter Electrode Inducing Corrosion Potential Shift in an Oil-Immersed Scanning Micropipette Contact Method Measurement. Anal. Chem. 2021, 93, 9657–9662. 10.1021/acs.analchem.1c01045. [DOI] [PubMed] [Google Scholar]
- Hill J. W.; Hill C. M. Directly Visualizing Carrier Transport and Recombination at Individual Defects Within 2D Semiconductors. Chem. Sci. 2021, 12, 5102–5112. 10.1039/D0SC07033E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert C. L.; Hill C. M. Electrochemically Probing Exciton Transport in Monolayers of Two-Dimensional Semiconductors. Faraday Discuss. 2022, 233, 163. 10.1039/D1FD00052G. [DOI] [PubMed] [Google Scholar]
- Fu Z.; Hill J. W.; Parkinson B.; Hill C. M.; Tian J. Layer and Material-Type Dependent Photoresponse in WSe2 /WS2 Vertical Heterostructures. 2D Mater. 2022, 9, 015022. 10.1088/2053-1583/ac3c9c. [DOI] [Google Scholar]
- Güell A. G.; Ebejer N.; Snowden M. E.; McKelvey K.; Macpherson J. V.; Unwin P. R. Quantitative Nanoscale Visualization of Heterogeneous Electron Transfer Rates in 2D Carbon Nanotube Networks. Proc. Natl. Acad. Sci. U.S.A. 2012, 109, 11487–11492. 10.1073/pnas.1203671109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güell A. G.; Meadows K. E.; Dudin P. V.; Ebejer N.; Byers J. C.; Macpherson J. V.; Unwin P. R. Selection, Characterisation and Mapping of Complex Electrochemical Processes at Individual Single-Walled Carbon Nanotubes: The Case of Serotonin Oxidation. Faraday Discuss. 2014, 172, 439–455. 10.1039/C4FD00054D. [DOI] [PubMed] [Google Scholar]
- Aaronson B. D. B.; Chen C.-H.; Li H.; Koper M. T. M.; Lai S. C. S.; Unwin P. R. Pseudo-Single-Crystal Electrochemistry on Polycrystalline Electrodes:Visualizing Activity at Grains and Grain Boundaries on Platinum for the Fe2+/Fe3+ Redox Reaction. J. Am. Chem. Soc. 2013, 135, 3873–3880. 10.1021/ja310632k. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Gordon E.; Ren H. Mapping the Nucleation of H2 Bubbles on Polycrystalline Pt via Scanning Electrochemical Cell Microscopy. J. Phys. Chem. Lett. 2019, 10, 3887–3892. 10.1021/acs.jpclett.9b01414. [DOI] [PubMed] [Google Scholar]
- Yule L. C.; Daviddi E.; West G.; Bentley C. L.; Unwin P. R. Surface Microstructural Controls on Electrochemical Hydrogen Absorption at Polycrystalline Palladium. J. Electroanal. Chem. 2020, 872, 114047. 10.1016/j.jelechem.2020.114047. [DOI] [Google Scholar]
- Mariano R. G.; McKelvey K.; White H. S.; Kanan M. W. Selective Increase in CO2 Electroreduction Activity at Grain-Boundary Surface Terminations. Science 2017, 358, 1187–1192. 10.1126/science.aao3691. [DOI] [PubMed] [Google Scholar]
- Mariano R. G.; Kang M.; Wahab O. J.; McPherson I. J.; Rabinowitz J. A.; Unwin P. R.; Kanan M. W. Microstructural Origin of Locally Enhanced CO2 Electroreduction Activity on Gold. Nat. Mater. 2021, 20, 1000–1006. 10.1038/s41563-021-00958-9. [DOI] [PubMed] [Google Scholar]
- Bentley C. L.; Unwin P. R. Nanoscale Electrochemical Movies and Synchronous Topographical Mapping of Electrocatalytic Materials. Faraday Discuss. 2018, 210, 365–379. 10.1039/C8FD00028J. [DOI] [PubMed] [Google Scholar]
- Patten H. V.; Lai S. C. S.; Macpherson J. V.; Unwin P. R. Active sites for Outer-Sphere, Inner-Sphere, and Complex Multistage Electrochemical Reactions at Polycrystalline Boron-Doped Diamond Electrodes (pBDD) Revealed with Scanning Electrochemical Cell Microscopy (SECCM). Anal. Chem. 2012, 84, 5427–5432. 10.1021/ac3010555. [DOI] [PubMed] [Google Scholar]
- Ando T.; Asai K.; Macpherson J.; Einaga Y.; Fukuma T.; Takahashi Y. Nanoscale Reactivity Mapping of a Single-Crystal Boron-Doped Diamond Particle. Anal. Chem. 2021, 93, 5831–5838. 10.1021/acs.analchem.1c00053. [DOI] [PubMed] [Google Scholar]
- Liu D.-Q.; Chen C.-H.; Perry D.; West G.; Cobb S. J.; Macpherson J. V.; Unwin P. R. Facet-Resolved Electrochemistry of Polycrystalline Boron-Doped Diamond Electrodes: Microscopic Factors Determining the Solvent Window in Aqueous Potassium Chloride Solutions. ChemElectroChem. 2018, 5, 3028–3035. 10.1002/celc.201800770. [DOI] [Google Scholar]
- Maddar F. M.; Lazenby R. A.; Patel A. N.; Unwin P. R. Electrochemical Oxidation of Dihydronicotinamide Adenine Dinucleotide (NADH): Comparison of Highly Oriented Pyrolytic Graphite (HOPG) and Polycrystalline Boron-Doped Diamond (pBDD) Electrodes. Phys. Chem. Chem. Phys. 2016, 18, 26404–26411. 10.1039/C6CP05394G. [DOI] [PubMed] [Google Scholar]
- Aaronson B. D. B.; Lai S. C. S.; Unwin P. R. Spatially Resolved Electrochemistry in Ionic Liquids: Surface Structure Effects on Triiodide Reduction at Platinum Electrodes. Langmuir 2014, 30, 1915–1919. 10.1021/la500271f. [DOI] [PubMed] [Google Scholar]
- Daviddi E.; Chen Z.; Beam Massani B.; Lee J.; Bentley C. L.; Unwin P. R.; Ratcliff E. L. Nanoscale Visualization and Multiscale Electrochemical Analysis of Conductive Polymer Electrodes. ACS Nano 2019, 13, 13271–13284. 10.1021/acsnano.9b06302. [DOI] [PubMed] [Google Scholar]
- Wahab O. J.; Kang M.; Meloni G. N.; Daviddi E.; Unwin P. R. Nanoscale Visualization of Electrochemical Activity at Indium Tin Oxide Electrodes. Anal. Chem. 2022, 94, 4729–4736. 10.1021/acs.analchem.1c05168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley C. L.; Agoston R.; Tao B.; Walker M.; Xu X.; O’Mullane A. P.; Unwin P. R. Correlating the Local Electrocatalytic Activity of Amorphous Molybdenum Sulfide Thin Films with Microscopic Composition, Structure, and Porosity. ACS Appl. Mater. Interfaces 2020, 12, 44307–44316. 10.1021/acsami.0c11759. [DOI] [PubMed] [Google Scholar]
- Lai S. C. S.; Patel A. N.; McKelvey K.; Unwin P. R. Definitive Evidence for Fast Electron Transfer at Pristine Basal Plane Graphite from High-Resolution Electrochemical Imaging. Angew. Chem., Int. Ed. 2012, 51, 5405–5408. 10.1002/anie.201200564. [DOI] [PubMed] [Google Scholar]
- Zhang G.; Kirkman P. M.; Patel A. N.; Cuharuc A. S.; McKelvey K.; Unwin P. R. Molecular Functionalization of Graphite Surfaces: Basal Plane Versus Step Edge Electrochemical Activity. J. Am. Chem. Soc. 2014, 136, 11444–11451. 10.1021/ja505266d. [DOI] [PubMed] [Google Scholar]
- E S. P.; Kim Y.-R.; Perry D.; Bentley C. L.; Unwin P. R. Nanoscale Electrocatalysis of Hydrazine Electro-Oxidation at Blistered Graphite Electrodes. ACS Appl. Mater. Interfaces 2016, 8, 30458–30466. 10.1021/acsami.6b10940. [DOI] [PubMed] [Google Scholar]
- Payne N. A.; Mauzeroll J. Identifying Nanoscale Pinhole Defects in Nitroaryl Layers with Scanning Electrochemical Cell Microscopy. ChemElectroChem. 2019, 6, 5439–5445. 10.1002/celc.201901394. [DOI] [Google Scholar]
- Güell A. G.; Ebejer N.; Snowden M. E.; Macpherson J. V.; Unwin P. R. Structural Correlations in Heterogeneous Electron Transfer at Monolayer and Multilayer Graphene Electrodes. J. Am. Chem. Soc. 2012, 134, 7258–7261. 10.1021/ja3014902. [DOI] [PubMed] [Google Scholar]
- Yu Y.; Zhang K.; Parks H.; Babar M.; Carr S.; Craig I. M.; van Winkle M.; Lyssenko A.; Taniguchi T.; Watanabe K.; et al. Tunable Angle-Dependent Electrochemistry at Twisted Bilayer Graphene with Moiré Flat Bands. Nat. Chem. 2022, 14, 267–273. 10.1038/s41557-021-00865-1. [DOI] [PubMed] [Google Scholar]
- Thompson A. C.; Simpson B. H.; Lewis N. S. Macroscale and Nanoscale Photoelectrochemical Behavior of p-Type Si(111) Covered by a Single Layer of Graphene or Hexagonal Boron Nitride. ACS Appl. Mater. Interfaces 2020, 12, 11551–11561. 10.1021/acsami.9b21134. [DOI] [PubMed] [Google Scholar]
- Kumatani A.; Miura C.; Kuramochi H.; Ohto T.; Wakisaka M.; Nagata Y.; Ida H.; Takahashi Y.; Hu K.; Jeong S.; et al. Chemical Dopants on Edge of Holey Graphene Accelerate Electrochemical Hydrogen Evolution Reaction. Adv. Sci. 2019, 6, 1900119. 10.1002/advs.201900119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabré M. B.; Paiva A. E.; Velický M.; Colavita P. E.; McKelvey K. Electrochemical Detection of Isolated Nanoscale Defects in 2D Transition Metal Dichalcogenides. J. Phys. Chem. C 2022, 126, 11636. 10.1021/acs.jpcc.2c01656. [DOI] [Google Scholar]
- Bentley C. L.; Kang M.; Maddar F. M.; Li F.; Walker M.; Zhang J.; Unwin P. R. Electrochemical Maps and Movies of the Hydrogen Evolution Reaction on Natural Crystals of Molybdenite (MoS2): Basal vs. Edge Plane Activity. Chem. Sci. 2017, 8, 6583–6593. 10.1039/C7SC02545A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J. W.; Fu Z.; Tian J.; Hill C. M. Locally Engineering and Interrogating the Photoelectrochemical Behavior of Defects in Transition Metal Dichalcogenides. J. Phys. Chem. C 2020, 124, 17141–17149. 10.1021/acs.jpcc.0c05235. [DOI] [Google Scholar]
- Bentley C. L.; Kang M.; Unwin P. R. Nanoscale Structure Dynamics within Electrocatalytic Materials. J. Am. Chem. Soc. 2017, 139, 16813–16821. 10.1021/jacs.7b09355. [DOI] [PubMed] [Google Scholar]
- Takahashi Y.; Kobayashi Y.; Wang Z.; Ito Y.; Ota M.; Ida H.; Kumatani A.; Miyazawa K.; Fujita T.; Shiku H.; et al. High-Resolution Electrochemical Mapping of the Hydrogen Evolution Reaction on Transition-Metal Dichalcogenide Nanosheets. Angew. Chem., Int. Ed. 2020, 59, 3601–3608. 10.1002/anie.201912863. [DOI] [PubMed] [Google Scholar]
- Tao B.; Unwin P. R.; Bentley C. L. Nanoscale Variations in the Electrocatalytic Activity of Layered Transition-Metal Dichalcogenides. J. Phys. Chem. C 2020, 124, 789–798. 10.1021/acs.jpcc.9b10279. [DOI] [Google Scholar]
- Bentley C. L.; Andronescu C.; Smialkowski M.; Kang M.; Tarnev T.; Marler B.; Unwin P. R.; Apfel U.-P.; Schuhmann W. Local Surface Structure and Composition Control the Hydrogen Evolution Reaction on Iron Nickel Sulfides. Angew. Chem., Int. Ed. 2018, 57, 4093–4097. 10.1002/anie.201712679. [DOI] [PubMed] [Google Scholar]
- Choi M.; Siepser N. P.; Jeong S.; Wang Y.; Jagdale G.; Ye X.; Baker L. A. Probing Single-Particle Electrocatalytic Activity at Facet-Controlled Gold Nanocrystals. Nano Lett. 2020, 20, 1233–1239. 10.1021/acs.nanolett.9b04640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G.; Hao L.; Li H.; Zhang K.; Yu X.; Li D.; Zhu X.; Hao D.; Ma Y.; Ma L. Topography Mapping with Scanning Electrochemical Cell Microscopy. Anal. Chem. 2022, 94, 5248–5254. 10.1021/acs.analchem.1c04692. [DOI] [PubMed] [Google Scholar]
- Georgescu N. S.; Robinson D. A.; White H. S. Effect of Nonuniform Mass Transport on Nanobubble Nucleation at Individual Pt Nanoparticles. J. Phys. Chem. C 2021, 125, 19724–19732. 10.1021/acs.jpcc.1c05020. [DOI] [Google Scholar]
- Liu Y.; Jin C.; Liu Y.; Ruiz K. H.; Ren H.; Fan Y.; White H. S.; Chen Q. Visualization and Quantification of Electrochemical H2 Bubble Nucleation at Pt, Au, and MoS2 Substrates. ACS sensors 2021, 6, 355–363. 10.1021/acssensors.0c00913. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Lu X.; Peng Y.; Chen Q. Electrochemical Visualization of Gas Bubbles on Superaerophobic Electrodes Using Scanning Electrochemical Cell Microscopy. Anal. Chem. 2021, 93, 12337–12345. 10.1021/acs.analchem.1c02099. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Gordon E.; Ren H. Mapping the Potential of Zero Charge and Electrocatalytic Activity of Metal-Electrolyte Interface via a Grain-by-Grain Approach. Anal. Chem. 2020, 92, 2859–2865. 10.1021/acs.analchem.9b05502. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Li M.; Ren H. Voltammetric Mapping of Hydrogen Evolution Reaction on Pt Locally via Scanning Electrochemical Cell Microscopy. ACS Meas. Sci. Au 2022, 2, 304–308. 10.1021/acsmeasuresciau.2c00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocci P.; Lemineur J.-F.; Noël J.-M.; Combellas C.; Kanoufi F. Differentiating Electrochemically Active Regions of Indium Tin Oxide Electrodes for Hydrogen Evolution and Reductive Decomposition Reactions. An In Situ Optical Microscopy Approach. Electrochim. Acta 2021, 386, 138498. 10.1016/j.electacta.2021.138498. [DOI] [Google Scholar]
- Shkirskiy V.; Yule L. C.; Daviddi E.; Bentley C. L.; Aarons J.; West G.; Unwin P. R. Nanoscale Scanning Electrochemical Cell Microscopy and Correlative Surface Structural Analysis to Map Anodic and Cathodic Reactions on Polycrystalline Zn in Acid Media. J. Electrochem. Soc. 2020, 167, 041507. 10.1149/1945-7111/ab739d. [DOI] [Google Scholar]
- Jin R.; Cheng L.; Lu H.; Zhuang J.; Jiang D.; Chen H.-Y. High Spatial Resolution Electrochemical Microscopic Observation of Enhanced Charging under Bias at Active Sites of N-rGO. ACS Appl. Energy Mater. 2021, 4, 3502–3507. 10.1021/acsaem.0c03237. [DOI] [Google Scholar]
- Yule L. C.; Shkirskiy V.; Aarons J.; West G.; Bentley C. L.; Shollock B. A.; Unwin P. R. Nanoscale Active Sites for the Hydrogen Evolution Reaction on Low Carbon Steel. J. Phys. Chem. C 2019, 123, 24146–24155. 10.1021/acs.jpcc.9b07216. [DOI] [Google Scholar]
- Ustarroz J.; Ornelas I. M.; Zhang G.; Perry D.; Kang M.; Bentley C. L.; Walker M.; Unwin P. R. Mobility and Poisoning of Mass-Selected Platinum Nanoclusters during the Oxygen Reduction Reaction. ACS Catal. 2018, 8, 6775–6790. 10.1021/acscatal.8b00553. [DOI] [Google Scholar]
- Tetteh E. B.; Banko L.; Krysiak O. A.; Löffler T.; Xiao B.; Varhade S.; Schumacher S.; Savan A.; Andronescu C.; Ludwig A.; et al. Zooming-In – Visualization of Active Site Heterogeneity in High Entropy Alloy Electrocatalysts using Scanning Electrochemical Cell Microscopy. Electrochem. Sci. Adv. 2022, 2, e2100105 10.1002/elsa.202100105. [DOI] [Google Scholar]
- Tetteh E. B.; Löffler T.; Tarnev T.; Quast T.; Wilde P.; Aiyappa H. B.; Schumacher S.; Andronescu C.; Tilley R. D.; Chen X.; et al. Calibrating SECCM Measurements by Means of a Nanoelectrode Ruler. The Intrinsic Oxygen Reduction Activity of PtNi Catalyst Nanoparticles. Nano Res. 2022, 15, 1564–1569. 10.1007/s12274-021-3702-7. [DOI] [Google Scholar]
- Mariano R. G.; Wahab O. J.; Rabinowitz J. A.; Oppenheim J.; Chen T.; Unwin P. R.; Dincǎ M. Thousand-Fold Increase in O2 Electroreduction Rates with Conductive MOFs. ACS Cent. Sci. 2022, 8, 975–982. 10.1021/acscentsci.2c00509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X.; Li M.; Peng Y.; Xi X.; Li M.; Chen Q.; Dong A. Direct Probing of the Oxygen Evolution Reaction at Single NiFe2O4 Nanocrystal Superparticles with Tunable Structures. J. Am. Chem. Soc. 2021, 143, 16925–16929. 10.1021/jacs.1c08592. [DOI] [PubMed] [Google Scholar]
- Quast T.; Varhade S.; Saddeler S.; Chen Y.-T.; Andronescu C.; Schulz S.; Schuhmann W. Single Particle Nanoelectrochemistry Reveals the Catalytic Oxygen Evolution Reaction Activity of Co3O4 Nanocubes. Angew. Chem., Int. Ed. 2021, 60, 23444–23450. 10.1002/anie.202109201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarova M. V.; Amano F.; Nomura S.; Tateishi C.; Fukuma T.; Takahashi Y.; Korchev Y. E. Direct Electrochemical Visualization of the Orthogonal Charge Separation in Anatase Nanotube Photoanodes for Water Splitting. ACS Catal. 2022, 12, 1201–1208. 10.1021/acscatal.1c04910. [DOI] [Google Scholar]
- Tsujiguchi T.; Kawabe Y.; Jeong S.; Ohto T.; Kukunuri S.; Kuramochi H.; Takahashi Y.; Nishiuchi T.; Masuda H.; Wakisaka M.; et al. Acceleration of Electrochemical CO2 Reduction to Formate at the Sn/Reduced Graphene Oxide Interface. ACS Catal. 2021, 11, 3310–3318. 10.1021/acscatal.0c04887. [DOI] [Google Scholar]
- Jeong S.; Choi M.-H.; Jagdale G. S.; Zhong Y.; Siepser N. P.; Wang Y.; Zhan X.; Baker L. A.; Ye X. Unraveling the Structural Sensitivity of CO2 Electroreduction at Facet-Defined Nanocrystals via Correlative Single-Entity and Macroelectrode Measurements. J. Am. Chem. Soc. 2022, 144, 12673. 10.1021/jacs.2c02001. [DOI] [PubMed] [Google Scholar]
- Kirkman P. M.; Güell A. G.; Cuharuc A. S.; Unwin P. R. Spatial and Temporal Control of the Diazonium Modification of sp2 Carbon Surfaces. J. Am. Chem. Soc. 2014, 136, 36–39. 10.1021/ja410467e. [DOI] [PubMed] [Google Scholar]
- Lai S. C. S.; Lazenby R. A.; Kirkman P. M.; Unwin P. R. Nucleation, Aggregative Growth and Detachment of Metal Nanoparticles during Electrodeposition at Electrode Surfaces. Chem. Sci. 2015, 6, 1126–1138. 10.1039/C4SC02792B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker A. S.; Al Botros R.; Kinnear S. L.; Snowden M. E.; McKelvey K.; Ashcroft A. T.; Carvell M.; Joiner A.; Peruffo M.; Philpotts C.; et al. Combinatorial Localized Dissolution Analysis: Application to Acid-Induced Dissolution of Dental Enamel and the Effect of Surface Treatments. J. Colloid Interface Sci. 2016, 476, 94–102. 10.1016/j.jcis.2016.05.018. [DOI] [PubMed] [Google Scholar]
- Parker A. S.; Patel A. N.; Al Botros R.; Snowden M. E.; McKelvey K.; Unwin P. R.; Ashcroft A. T.; Carvell M.; Joiner A.; Peruffo M. Measurement of the Efficacy of Calcium Silicate for the Protection and Repair of Dental Enamel. J. Dent. 2014, 42, S21–S29. 10.1016/S0300-5712(14)50004-8. [DOI] [PubMed] [Google Scholar]
- Zhan D.; Yang D.; Zhu Y.; Wu X.; Tian Z.-Q. Fabrication and Characterization of Nanostructured ZnO Thin Film Microdevices by Scanning Electrochemical Cell Microscopy. Chem. Commun. 2012, 48, 11449–11451. 10.1039/c2cc35809c. [DOI] [PubMed] [Google Scholar]
- Majumdar P.; Gao R.; White H. S. Electroprecipitation of Nanometer-Thick Films of Ln(OH)3 Ln = La, Ce, and Lu at Pt Microelectrodes and Their Effect on Electron-Transfer Reactions. Langmuir 2022, 38, 8125–8134. 10.1021/acs.langmuir.2c01008. [DOI] [PubMed] [Google Scholar]
- McKelvey K.; O’Connell M. A.; Unwin P. R. Meniscus Confined Fabrication of Multidimensional Conducting Polymer Nanostructures with Scanning Electrochemical Cell Microscopy (SECCM). Chem. Commun. 2013, 49, 2986–2988. 10.1039/c3cc00104k. [DOI] [PubMed] [Google Scholar]
- Aaronson B. D. B.; Garoz-Ruiz J.; Byers J. C.; Colina A.; Unwin P. R. Electrodeposition and Screening of Photoelectrochemical Activity in Conjugated Polymers Using Scanning Electrochemical Cell Microscopy. Langmuir 2015, 31, 12814–12822. 10.1021/acs.langmuir.5b03316. [DOI] [PubMed] [Google Scholar]
- Oseland E. E.; Ayres Z. J.; Basile A.; Haddleton D. M.; Wilson P.; Unwin P. R. Surface Patterning of Polyacrylamide Gel using Scanning Electrochemical Cell Microscopy (SECCM). Chem. Commun. 2016, 52, 9929–9932. 10.1039/C6CC05153G. [DOI] [PubMed] [Google Scholar]
- Takahashi Y.; Kumatani A.; Munakata H.; Inomata H.; Ito K.; Ino K.; Shiku H.; Unwin P. R.; Korchev Y. E.; Kanamura K.; et al. Nanoscale Visualization of Redox Activity at Lithium-Ion Battery Cathodes. Nat. Commun. 2014, 5, 5450. 10.1038/ncomms6450. [DOI] [PubMed] [Google Scholar]
- E S. P.; Kang M.; Wilson P.; Meng L.; Perry D.; Basile A.; Unwin P. R. High Resolution Visualization of the Redox Activity of Li2O2 in Non-Aqueous Media: Conformal Layer vs. Toroid Structure. Chem. Commun. 2018, 54, 3053–3056. 10.1039/C7CC09957F. [DOI] [PubMed] [Google Scholar]
- Dayeh M.; Ghavidel M. R. Z.; Mauzeroll J.; Schougaard S. B. Micropipette Contact Method to Investigate High-Energy Cathode Materials by using an Ionic Liquid. ChemElectroChem. 2019, 6, 195–201. 10.1002/celc.201800750. [DOI] [Google Scholar]
- Inomata H.; Takahashi Y.; Takamatsu D.; Kumatani A.; Ida H.; Shiku H.; Matsue T. Visualization of Inhomogeneous Current Distribution on ZrO2-Coated LiCoO2 Thin-Film Electrodes using Scanning Electrochemical Cell Microscopy. Chem. Commun. 2019, 55, 545–548. 10.1039/C8CC08916G. [DOI] [PubMed] [Google Scholar]
- Tao B.; Yule L. C.; Daviddi E.; Bentley C. L.; Unwin P. R. Correlative Electrochemical Microscopy of Li-Ion (De)intercalation at a Series of Individual LiMn2 O4 Particles. Angew. Chem., Int. Ed. 2019, 58, 4606–4611. 10.1002/anie.201814505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y.; Yamashita T.; Takamatsu D.; Kumatani A.; Fukuma T. Nanoscale Kinetic Imaging of Lithium Ion Secondary Battery Materials using Scanning Electrochemical Cell Microscopy. Chem. Commun. 2020, 56, 9324–9327. 10.1039/D0CC02865G. [DOI] [PubMed] [Google Scholar]
- Kumatani A.; Takahashi Y.; Miura C.; Ida H.; Inomata H.; Shiku H.; Munakata H.; Kanamura K.; Matsue T. Scanning Electrochemical Cell Microscopy for Visualization and Local Electrochemical Activities of Lithium-Ion (De) Intercalation Process in Lithium-Ion Batteries Electrodes. Surf. Interface Anal. 2019, 51, 27–30. 10.1002/sia.6538. [DOI] [Google Scholar]
- Martín-Yerga D.; Kang M.; Unwin P. R. Scanning Electrochemical Cell Microscopy in a Glovebox: Structure-Activity Correlations in the Early Stages of Solid-Electrolyte Interphase Formation on Graphite. ChemElectroChem. 2021, 8, 4240–4251. 10.1002/celc.202101161. [DOI] [Google Scholar]
- Bentley C. L.; Kang M.; Unwin P. R. Scanning Electrochemical Cell Microscopy (SECCM) in Aprotic Solvents: Practical Considerations and Applications. Anal. Chem. 2020, 92, 11673–11680. 10.1021/acs.analchem.0c01540. [DOI] [PubMed] [Google Scholar]
- Gateman S. M.; Georgescu N. S.; Kim M.-K.; Jung I.-H.; Mauzeroll J. Efficient Measurement of the Influence of Chemical Composition on Corrosion: Analysis of an Mg-Al Diffusion Couple Using Scanning Micropipette Contact Method. J. Electrochem. Soc. 2019, 166, C624–C630. 10.1149/2.0681916jes. [DOI] [Google Scholar]
- Yule L. C.; Bentley C. L.; West G.; Shollock B. A.; Unwin P. R. Scanning Electrochemical Cell Microscopy: A Versatile Method for Highly Localised Corrosion Related Measurements on Metal Surfaces. Electrochim. Acta 2019, 298, 80–88. 10.1016/j.electacta.2018.12.054. [DOI] [Google Scholar]
- Yule L. C.; Shkirskiy V.; Aarons J.; West G.; Shollock B. A.; Bentley C. L.; Unwin P. R. Nanoscale Electrochemical Visualization of Grain-Dependent Anodic Iron Dissolution from Low Carbon Steel. Electrochim. Acta 2020, 332, 135267. 10.1016/j.electacta.2019.135267. [DOI] [Google Scholar]
- Aaronson B. D. B.; Byers J. C.; Colburn A. W.; McKelvey K.; Unwin P. R. Scanning Electrochemical Cell Microscopy Platform for Ultrasensitive Photoelectrochemical Imaging. Anal. Chem. 2015, 87, 4129–4133. 10.1021/acs.analchem.5b00288. [DOI] [PubMed] [Google Scholar]
- Strange L. E.; Yadav J.; Garg S.; Shinde P. S.; Hill J. W.; Hill C. M.; Kung P.; Pan S. Investigating the Redox Properties of Two-Dimensional MoS2 using Photoluminescence Spectroelectrochemistry and Scanning Electrochemical Cell Microscopy. J. Phys. Chem. Lett. 2020, 11, 3488–3494. 10.1021/acs.jpclett.0c00769. [DOI] [PubMed] [Google Scholar]
- Kim J.; Gewirth A. A. Mechanism of Oxygen Electroreduction on Gold Surfaces in Basic Media. J. Phys. Chem. B 2006, 110, 2565–2571. 10.1021/jp0549529. [DOI] [PubMed] [Google Scholar]
- Yeager E. Dioxygen Electrocatalysis: Mechanisms in Relation to Catalyst Structure. J. Mol. Catal. 1986, 38, 5–25. 10.1016/0304-5102(86)87045-6. [DOI] [Google Scholar]
- Vassilev P.; Koper M. T. M. Electrochemical Reduction of Oxygen on Gold Surfaces: A Density Functional Theory Study of Intermediates and Reaction Paths. J. Phys. Chem. C 2007, 111, 2607–2613. 10.1021/jp064515+. [DOI] [Google Scholar]
- Ge X.; Sumboja A.; Wuu D.; An T.; Li B.; Goh F. W. T.; Hor T. S. A.; Zong Y.; Liu Z. Oxygen Reduction in Alkaline Media: From Mechanisms to Recent Advances of Catalysts. ACS Catal. 2015, 5, 4643–4667. 10.1021/acscatal.5b00524. [DOI] [Google Scholar]
- Vielstich W.; Lamm A.; Gasteiger H. A.. Handbook of Fuel Cells:Fundamentals Technology and Applications Technology and Applications. Pt. 2; Wiley: Chichester, 2003. [Google Scholar]
- Greeley J.; Markovic N. M. The Road from Animal Electricity to Green Energy: Combining Experiment and Theory in Electrocatalysis. Energy Environ. Sci. 2012, 5, 9246. 10.1039/c2ee21754f. [DOI] [Google Scholar]
- Anastasijević N. A.; Vesović V.; Adžić R. R. Determination of the Kinetic Parameters of the Oxygen Reduction Reaction using the Rotating Ring-disk Electrode: Part I. Theory. J. Electroanal. Chem. 1987, 229, 305–316. 10.1016/0022-0728(87)85148-3. [DOI] [Google Scholar]
- Anastasijević N. A.; Vesović V.; Adžić R. R. Determination of the Kinetic Parameters of the Oxygen Reduction Reaction using the Rotating Ring-disk Electrode: Part II. Applications. J. Electroanal. Chem. 1987, 229, 317–325. 10.1016/0022-0728(87)85149-5. [DOI] [Google Scholar]
- Zinola C. F.; Arvia A. J.; Estiu G. L.; Castro E. A. A Quantum Chemical Approach to the Influence of Platinum Surface Structure on the Oxygen Electroreduction Reaction. J. Phys. Chem. 1994, 98, 7566. 10.1021/j100082a030. [DOI] [Google Scholar]
- Shen Y.; Träuble M.; Wittstock G. Detection of Hydrogen Peroxide Produced During Electrochemical Oxygen Reduction using Scanning Electrochemical Microscopy. Anal. Chem. 2008, 80, 750–759. 10.1021/ac0711889. [DOI] [PubMed] [Google Scholar]
- Fernández J. L.; Walsh D. A.; Bard A. J. Thermodynamic Guidelines for the Design of Bimetallic Catalysts for Oxygen Electroreduction and Rapid Screening by Scanning Electrochemical Microscopy. M-Co (M: Pd, Ag, Au). J. Am. Chem. Soc. 2005, 127, 357–365. 10.1021/ja0449729. [DOI] [PubMed] [Google Scholar]
- Wang X.; Li Z.; Qu Y.; Yuan T.; Wang W.; Wu Y.; Li Y. Review of Metal Catalysts for Oxygen Reduction Reaction: From Nanoscale Engineering to Atomic Design. Chem. 2019, 5, 1486–1511. 10.1016/j.chempr.2019.03.002. [DOI] [Google Scholar]
- Liu B.; Bard A. J. Scanning Electrochemical Microscopy. 45. Study of the Kinetics of Oxygen Reduction on Platinum with Potential Programming of the Tip. J. Phys. Chem. B 2002, 106, 12801–12806. 10.1021/jp026824f. [DOI] [Google Scholar]
- Sanchez-Sanchez C. M.; Bard A. J. Hydrogen Peroxide Production in the Oxygen Reduction Reaction at Different Electrocatalysts as Quantified by Scanning Electrochemical Microscopy. Anal. Chem. 2009, 81, 8094–8100. 10.1021/ac901291v. [DOI] [PubMed] [Google Scholar]
- Sánchez-Sánchez C. M.; Rodríguez-López J.; Bard A. J. Scanning Electrochemical Microscopy. 60. Quantitative Calibration of the SECM Substrate Generation/Tip Collection Mode and its use for the Study of the Oxygen Reduction Mechanism. Anal. Chem. 2008, 80, 3254–3260. 10.1021/ac702453n. [DOI] [PubMed] [Google Scholar]
- Zhang C.; Fan F.-R. F.; Bard A. J. Electrochemistry of Oxygen in Concentrated NaOH Solutions: Solubility, Diffusion Coefficients, and Superoxide Formation. J. Am. Chem. Soc. 2009, 131, 177–181. 10.1021/ja8064254. [DOI] [PubMed] [Google Scholar]
- Shen Y.; Träuble M.; Wittstock G. Electrodeposited Noble Metal Particles in Polyelectrolyte Multilayer Matrix as Electrocatalyst for Oxygen Reduction Studied using SECM. Phys. Chem. Chem. Phys. 2008, 10, 3635–3644. 10.1039/b802688b. [DOI] [PubMed] [Google Scholar]
- Noel J.-M.; Kostopoulos N.; Achaibou C.; Fave C.; Anxolabéhère-Mallart E.; Kanoufi F. Probing the Activity of Iron Peroxo Porphyrin Intermediates in the Reaction Layer during the Electrochemical Reductive Activation of O2. Angew. Chem., Int. Ed. 2020, 59, 16376–16380. 10.1002/anie.202004977. [DOI] [PubMed] [Google Scholar]
- Bae J. H.; Yu Y.; Mirkin M. V. Scanning Electrochemical Microscopy Study of Electron-Transfer Kinetics and Catalysis at Nanoporous Electrodes. J. Phys. Chem. C 2016, 120, 20651–20658. 10.1021/acs.jpcc.6b01620. [DOI] [Google Scholar]
- Barwe S.; Andronescu C.; Engels R.; Conzuelo F.; Seisel S.; Wilde P.; Chen Y.-T.; Masa J.; Schuhmann W. Cobalt Metalloid and Polybenzoxazine Derived Composites for Bifunctional Oxygen Electrocatalysis. Electrochim. Acta 2019, 297, 1042–1051. 10.1016/j.electacta.2018.12.047. [DOI] [Google Scholar]
- Sklyar O.; Ufheil J.; Heinze J.; Wittstock G. Application of the Boundary Element Method Numerical Simulations for Characterization of Heptode Ultramicroelectrodes in SECM Experiments. Electrochim. Acta 2003, 49, 117–128. 10.1016/j.electacta.2003.04.007. [DOI] [Google Scholar]
- Nagaiah T. C.; Maljusch A.; Chen X. X.; Bron M.; Schuhmann W. Visualization of the Local Catalytic Activity of Electrodeposited Pt-Ag Catalysts for Oxygen Reduction by means of SECM. ChemPhysChem 2009, 10, 2711–2718. 10.1002/cphc.200900496. [DOI] [PubMed] [Google Scholar]
- Schwamborn S.; Stoica L.; Chen X.; Xia W.; Kundu S.; Muhler M.; Schuhmann W. Patterned CNT Arrays for the Evaluation of Oxygen Reduction Activity by SECM. ChemPhysChem 2010, 11, 74–78. 10.1002/cphc.200900744. [DOI] [PubMed] [Google Scholar]
- Kundu S.; Nagaiah T. C.; Xia W.; Wang Y.; Van Dommele S.; Bitter J. H.; Santa M.; Grundmeier G.; Bron M.; Schuhmann W.; et al. Electrocatalytic Activity and Stability of Nitrogen-Containing Carbon Nanotubes in the Oxygen Reduction Reaction. J. Phys. Chem. C 2009, 113, 14302–14310. 10.1021/jp811320d. [DOI] [Google Scholar]
- Singh V.; Tiwari A.; Nagaiah T. C. Facet-Controlled Morphology of Cobalt Disulfide Towards Enhanced Oxygen Reduction Reaction. J. Mater. Chem. A 2018, 6, 22545–22554. 10.1039/C8TA06710D. [DOI] [Google Scholar]
- Lima A.; Lima A.; Meloni G.; Santos C.; Bertotti M. Perovskite-Type Oxides La0.6M0.4Ni0.6Cu0.4O3 (M = Ag, Ba, Ce) towards the Oxygen Reduction Reaction (ORR) in Alkaline Medium: Structural Aspects and Electrocatalytic Activity. J. Braz. Chem. Soc. 2021, 32, 665–674. 10.21577/0103-5053.20200220. [DOI] [Google Scholar]
- Okunola A.; Kowalewska B.; Bron M.; Kulesza P. J.; Schuhmann W. Electrocatalytic Reduction of Oxygen at Electropolymerized Films of Metalloporphyrins Deposited onto Multi-walled Carbon Nanotubes. Electrochim. Acta 2009, 54, 1954–1960. 10.1016/j.electacta.2008.07.077. [DOI] [Google Scholar]
- Nagaiah T. C.; Schäfer D.; Schuhmann W.; Dimcheva N. Electrochemically Deposited Pd-Pt and Pd-Au Codeposits on Graphite Electrodes for Electrocatalytic H2O2 Reduction. Anal. Chem. 2013, 85, 7897–7903. 10.1021/ac401317y. [DOI] [PubMed] [Google Scholar]
- Seiffarth G.; Steimecke M.; Walther T.; Kühhirt M.; Rümmler S.; Bron M. Mixed Transition Metal Oxide Supported on Nitrogen Doped Carbon Nanotubes - A Simple Bifunctional Electrocatalyst Studied with Scanning Electrochemical Microscopy. Electroanalysis 2016, 28, 2335–2345. 10.1002/elan.201600254. [DOI] [Google Scholar]
- Dobrzeniecka A.; Zeradjanin A.; Masa J.; Puschhof A.; Stroka J.; Kulesza P. J.; Schuhmann W. Application of SECM in Tracing of Hydrogen Peroxide at Multicomponent Non-Noble Electrocatalyst Films for the Oxygen Reduction Reaction. Catal. Today 2013, 202, 55–62. 10.1016/j.cattod.2012.03.060. [DOI] [Google Scholar]
- Silva S. M.; Aguiar L. F.; Carvalho R. M. S.; Tanaka A. A.; Damos F. S.; Luz R. C. S. A Glassy Carbon Electrode Modified with an Iron N4-Macrocycle and Reduced Graphene Oxide for Voltammetric Sensing of Dissolved Oxygen. Microchim. Acta 2016, 183, 1251–1259. 10.1007/s00604-016-1750-6. [DOI] [Google Scholar]
- Schulte W.; Liu S.; Plettenberg I.; Kuhri S.; Lüke W.; Lehnert W.; Wittstock G. Local Evaluation of Processed Membrane Electrode Assemblies by Scanning Electrochemical Microscopy. J. Electrochem. Soc. 2017, 164, F873–F878. 10.1149/2.0061709jes. [DOI] [Google Scholar]
- Botz A.; Clausmeyer J.; Öhl D.; Tarnev T.; Franzen D.; Turek T.; Schuhmann W. Local Activities of Hydroxide and Water Determine the Operation of Silver-Based Oxygen Depolarized Cathodes. Angew. Chem., Int. Ed. 2018, 57, 12285–12289. 10.1002/anie.201807798. [DOI] [PubMed] [Google Scholar]
- Kolagatla S.; Subramanian P.; Schechter A. Simultaneous Mapping of Oxygen Reduction Activity and Hydrogen Peroxide Generation on Electrocatalytic Surfaces. ChemSusChem 2019, 12, 2708–2714. 10.1002/cssc.201900656. [DOI] [PubMed] [Google Scholar]
- Hengstenberg A.; Kranz C.; Schuhmann W. Facilitated Tip-Positioning and Applications of Non-Electrode Tips in Scanning Electrochemical Microscopy using a Sheer Force Based Constant-Distance Mode. Chem.—Eur. J. 2000, 6, 1547–1554. . [DOI] [PubMed] [Google Scholar]
- O’Connell M. A.; Lewis J. R.; Wain A. J. Electrochemical Imaging of Hydrogen Peroxide Generation at Individual Gold Nanoparticles. Chem. Commun. 2015, 51, 10314–10317. 10.1039/C5CC01640A. [DOI] [PubMed] [Google Scholar]
- Cai Z.-F.; Wang X.; Wang D.; Wan L.-J. Cobalt-Porphyrin-Catalyzed Oxygen Reduction Reaction: A Scanning Tunneling Microscopy Study. ChemElectroChem. 2016, 3, 2048–2051. 10.1002/celc.201600435. [DOI] [Google Scholar]
- Pfisterer J. H. K.; Liang Y.; Schneider O.; Bandarenka A. S. Direct Instrumental Identification of Catalytically Active Surface Sites. Nature 2017, 549, 74–77. 10.1038/nature23661. [DOI] [PubMed] [Google Scholar]
- Haid R. W.; Kluge R. M.; Liang Y.; Bandarenka A. S. In Situ Quantification of the Local Electrocatalytic Activity via Electrochemical Scanning Tunneling Microscopy. Small Methods 2021, 5, 2000710 10.1002/smtd.202000710. [DOI] [PubMed] [Google Scholar]
- Yoshimoto S.; Tada A.; Suto K.; Itaya K. Adlayer Structures and Electrocatalytic Activity for O2 of Metallophthalocyanines on Au(111): In Situ Scanning Tunneling Microscopy Study. J. Phys. Chem. B 2003, 107, 5836–5843. 10.1021/jp027825a. [DOI] [Google Scholar]
- Yoshimoto S.; Tada A.; Itaya K. In Situ Scanning Tunneling Microscopy Study of the Effect of Iron Octaethylporphyrin Adlayer on the Electrocatalytic Reduction of O2 on Au(111). J. Phys. Chem. B 2004, 108, 5171–5174. 10.1021/jp049667o. [DOI] [Google Scholar]
- Liang Y.; McLaughlin D.; Csoklich C.; Schneider O.; Bandarenka A. S. The Nature of Active Centers Catalyzing Oxygen Electro-Reduction at Platinum Surfaces in Alkaline Media. Energy Environ. Sci. 2019, 12, 351–357. 10.1039/C8EE03228A. [DOI] [Google Scholar]
- Chen X.; Eckhard K.; Zhou M.; Bron M.; Schuhmann W. Electrocatalytic Activity of Spots of Electrodeposited Noble-Metal Catalysts on Carbon Nanotubes Modified Glassy Carbon. Anal. Chem. 2009, 81, 7597–7603. 10.1021/ac900937k. [DOI] [PubMed] [Google Scholar]
- Chen X.; Botz A. J. R.; Masa J.; Schuhmann W. Characterisation of Bifunctional Electrocatalysts for Oxygen Reduction and Evolution by Means of SECM. J. Solid State Electrochem. 2016, 20, 1019–1027. 10.1007/s10008-015-3028-z. [DOI] [Google Scholar]
- Suen N.-T.; Hung S.-F.; Quan Q.; Zhang N.; Xu Y.-J.; Chen H. M. Electrocatalysis for the Oxygen Evolution Reaction: Recent Development and Future Perspectives. Chem. Soc. Rev. 2017, 46, 337–365. 10.1039/C6CS00328A. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Zhou D.; Deng T.; He G.; Chen A.; Sun X.; Yang Y.; Miao P. Research Progress of Oxygen Evolution Reaction Catalysts for Electrochemical Water Splitting. ChemSusChem 2021, 14, 5359–5383. 10.1002/cssc.202101898. [DOI] [PubMed] [Google Scholar]
- Reier T.; Nong H. N.; Teschner D.; Schlögl R.; Strasser P. Electrocatalytic Oxygen Evolution Reaction in Acidic Environments - Reaction Mechanisms and Catalysts. Adv. Energy Mater. 2017, 7, 1601275. 10.1002/aenm.201601275. [DOI] [Google Scholar]
- Badreldin A.; Abusrafa A. E.; Abdel-Wahab A. Oxygen-Deficient Cobalt-Based Oxides for Electrocatalytic Water Splitting. ChemSusChem 2021, 14, 10–32. 10.1002/cssc.202002002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J.; Wei C.; Huang Z.-F.; Liu C.; Zeng L.; Wang X.; Xu Z. J. A Review on Fundamentals for Designing Oxygen Evolution Electrocatalysts. Chem. Soc. Rev. 2020, 49, 2196–2214. 10.1039/C9CS00607A. [DOI] [PubMed] [Google Scholar]
- Li J. Oxygen Evolution Reaction in Energy Conversion and Storage: Design Strategies Under and Beyond the Energy Scaling Relationship. Nano-micro Lett. 2022, 14, 112. 10.1007/s40820-022-00857-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazhayil A.; Vazhayal L.; Thomas J.; Ashok C S.; Thomas N. A Comprehensive Review on the Recent Developments in Transition Metal-Based Electrocatalysts for Oxygen Evolution Reaction. Appl. Surf. Sci. 2021, 6, 100184. 10.1016/j.apsadv.2021.100184. [DOI] [Google Scholar]
- Jamesh M.-I.; Sun X. Recent Progress on Earth Abundant Electrocatalysts for Oxygen Evolution Reaction (OER) in Alkaline Medium to Achieve Efficient Water Splitting – A Review. J. Power Sources 2018, 400, 31–68. 10.1016/j.jpowsour.2018.07.125. [DOI] [Google Scholar]
- Chen S.; Ma L.; Huang Z.; Liang G.; Zhi C. In Situ/Operando Analysis of Surface Reconstruction of Transition Metal-Based Oxygen Evolution Electrocatalysts. Cell Reports Physical Science 2022, 3, 100729. 10.1016/j.xcrp.2021.100729. [DOI] [Google Scholar]
- Galán-Mascarós J. R. Water Oxidation at Electrodes Modified with Earth-Abundant Transition-Metal Catalysts. ChemElectroChem. 2015, 2, 37–50. 10.1002/celc.201402268. [DOI] [Google Scholar]
- Maljusch A.; Ventosa E.; Rincón R. A.; Bandarenka A. S.; Schuhmann W. Revealing Onset Potentials using Electrochemical Microscopy to Assess the Catalytic Activity of Gas-Evolving Electrodes. Electrochem. Commun. 2014, 38, 142–145. 10.1016/j.elecom.2013.11.024. [DOI] [Google Scholar]
- Fushimi K.; Okawa T.; Seo M. A Scanning Electrochemical Microscopic Observation of Heterogeneous Oxygen Evolution on a Polycrystalline Titanium during Anodic Oxidation. Electrochemistry 2000, 68, 950–954. 10.5796/electrochemistry.68.950. [DOI] [Google Scholar]
- Kang J.; Yang Y.; Jiang F.; Shao H. Study on the Anodic Reaction of Ni in an Alkaline Solution by Transient pH Detection Based on Scanning Electrochemical Microscopy (SECM). Surf. Interface Anal. 2007, 39, 877–884. 10.1002/sia.2604. [DOI] [Google Scholar]
- Snook G. A.; Duffy N. W.; Pandolfo A. G. Detection of Oxygen Evolution from Nickel Hydroxide Electrodes Using Scanning Electrochemical Microscopy. J. Electrochem. Soc. 2008, 155, A262. 10.1149/1.2830837. [DOI] [Google Scholar]
- Botz A. J.; Nebel M.; Rincón R. A.; Ventosa E.; Schuhmann W. Onset Potential Determination at Gas-Evolving Catalysts by Means of Constant-Distance Mode Positioning of Nanoelectrodes. Electrochim. Acta 2015, 179, 38–44. 10.1016/j.electacta.2015.04.145. [DOI] [Google Scholar]
- Zhang T.; Wu Y.; Yu Y.; Li Y.; Qin G.; Li S. High Throughput Screening Driven Discovery of Mn5Co10Fe30Ni55Ox as Electrocatalyst for Water Oxidation and Electrospinning Synthesis. Appl. Surf. Sci. 2022, 588, 152959. 10.1016/j.apsusc.2022.152959. [DOI] [Google Scholar]
- Zeradjanin A. R.; Ventosa E.; Bondarenko A. S.; Schuhmann W. Evaluation of the Catalytic Performance of Gas-Evolving Electrodes Using Local Electrochemical Noise Measurements. ChemSusChem 2012, 5, 1905–1911. 10.1002/cssc.201200262. [DOI] [PubMed] [Google Scholar]
- Sun T.; Wang D.; Mirkin M. V.; Cheng H.; Zheng J.-C.; Richards R. M.; Lin F.; Xin H. L. Direct High-Resolution Mapping of Electrocatalytic Activity of Semi-Two-Dimensional Catalysts with Single-Edge Sensitivity. Proc. Natl. Acad. Sci. U.S.A. 2019, 116, 11618–11623. 10.1073/pnas.1821091116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X.; Chen Y.; Sun T.; Huang J.; Zhang W.; Wang Q.; Cao R. Karst Landform-Featured Monolithic Electrode for Water Electrolysis in Neutral Media. Energy Environ. Sci. 2020, 13, 174–182. 10.1039/C9EE02380A. [DOI] [Google Scholar]
- Minguzzi A.; Alpuche-Aviles M. A.; Rodríguez López J.; Rondinini S.; Bard A. J. Screening of Oxygen Evolution Electrocatalysts by Scanning Electrochemical Microscopy using a Shielded Tip Approach. Anal. Chem. 2008, 80, 4055–4064. 10.1021/ac8001287. [DOI] [PubMed] [Google Scholar]
- Angulo A.; van der Linde P.; Gardeniers H.; Modestino M.; Fernández Rivas D. Influence of Bubbles on the Energy Conversion Efficiency of Electrochemical Reactors. Joule 2020, 4, 555–579. 10.1016/j.joule.2020.01.005. [DOI] [Google Scholar]
- Chen X.; Maljusch A.; Rincón R. A.; Battistel A.; Bandarenka A. S.; Schuhmann W. Local Visualization of Catalytic Activity at Gas Evolving Electrodes using Frequency-Dependent Scanning Electrochemical Microscopy. Chem. Commun. 2014, 50, 13250–13253. 10.1039/C4CC06100D. [DOI] [PubMed] [Google Scholar]
- Konkena B.; Masa J.; Botz A. J. R.; Sinev I.; Xia W.; Koßmann J.; Drautz R.; Muhler M.; Schuhmann W. Metallic NiPS3@NiOOH Core–Shell Heterostructures as Highly Efficient and Stable Electrocatalyst for the Oxygen Evolution Reaction. ACS Catal. 2017, 7, 229–237. 10.1021/acscatal.6b02203. [DOI] [Google Scholar]
- Chakrabarty S.; Mukherjee A.; Su W.-N.; Basu S. Improved Bi-Functional ORR and OER Catalytic Activity of Reduced Graphene Oxide Supported ZnCo2O4 Microsphere. Int. J. Hydrog. 2019, 44, 1565–1578. 10.1016/j.ijhydene.2018.11.163. [DOI] [Google Scholar]
- Lu Z.; Yang Q.; Pan H.; Liu Z.; Huang X.; Chen X.; Niu L. Bifunctional Oxygen Electrocatalysis at Co-B,N,S-Graphene Composite Investigated by Scanning Electrochemical Microscopy at Variable Temperatures and its Application in Zn-Air Battery. Electrochim. Acta 2021, 389, 138751. 10.1016/j.electacta.2021.138751. [DOI] [Google Scholar]
- Iffelsberger C.; Raith T.; Vatsyayan P.; Vyskočil V.; Matysik F.-M. Detection and Imaging of Reactive Oxygen Species Associated with the Electrochemical Oxygen Evolution by Hydrodynamic Scanning Electrochemical Microscopy. Electrochim. Acta 2018, 281, 494–501. 10.1016/j.electacta.2018.05.115. [DOI] [Google Scholar]
- Counihan M. J.; Setwipatanachai W.; Rodríguez-López J. Interrogating the Surface Intermediates and Water Oxidation Products of Boron-Doped Diamond Electrodes with Scanning Electrochemical Microscopy. ChemElectroChem. 2019, 6, 3507–3515. 10.1002/celc.201900659. [DOI] [Google Scholar]
- Li X.; Pan S. Transparent Ultramicroelectrodes for Studying Interfacial Charge-Transfer Kinetics of Photoelectrochemical Water Oxidation at TiO2 Nanorods with Scanning Electrochemical Microscopy. Anal. Chem. 2021, 93, 15886–15896. 10.1021/acs.analchem.1c02598. [DOI] [PubMed] [Google Scholar]
- Steimecke M.; Seiffarth G.; Bron M. In Situ Characterization of Ni and Ni/Fe Thin Film Electrodes for Oxygen Evolution in Alkaline Media by a Raman-Coupled Scanning Electrochemical Microscope Setup. Anal. Chem. 2017, 89, 10679–10686. 10.1021/acs.analchem.7b01060. [DOI] [PubMed] [Google Scholar]
- Kluge R. M.; Haid R. W.; Bandarenka A. S. Assessment of Active Areas for the Oxygen Evolution Reaction on an Amorphous Iridium Oxide Surface. J. Catal. 2021, 396, 14–22. 10.1016/j.jcat.2021.02.007. [DOI] [Google Scholar]
- Ahn H. S.; Bard A. J. Surface Interrogation Scanning Electrochemical Microscopy of Ni(1-x)Fe(x)OOH (0 < x < 0.27) Oxygen Evolving Catalyst: Kinetics of the ″fast″ Iron Sites. J. Am. Chem. Soc. 2016, 138, 313–318. 10.1021/jacs.5b10977. [DOI] [PubMed] [Google Scholar]
- Arroyo-Currás N.; Bard A. J. Iridium Oxidation as Observed by Surface Interrogation Scanning Electrochemical Microscopy. J. Phys. Chem. C 2015, 119, 8147–8154. 10.1021/acs.jpcc.5b00106. [DOI] [Google Scholar]
- Jin Z.; Bard A. J. Surface Interrogation of Electrodeposited MnOx and CaMnO3 Perovskites by Scanning Electrochemical Microscopy: Probing Active Sites and Kinetics for the Oxygen Evolution Reaction. Angew. Chem., Int. Ed. 2021, 60, 794. 10.1002/anie.202008052. [DOI] [PubMed] [Google Scholar]
- Barforoush J. M.; Jantz D. T.; Seuferling T. E.; Song K. R.; Cummings L. C.; Leonard K. C. Microwave-assisted synthesis of a nanoamorphous (Ni 0.8,Fe 0.2) oxide oxygen-evolving electrocatalyst containing only “fast” sites. J. Mater. Chem. A 2017, 5, 11661–11670. 10.1039/C7TA00151G. [DOI] [Google Scholar]
- Lee J.; Ye H.; Pan S.; Bard A. J. Screening of Photocatalysts by Scanning Electrochemical Microscopy. Anal. Chem. 2008, 80, 7445–7450. 10.1021/ac801142g. [DOI] [PubMed] [Google Scholar]
- Ye H.; Lee J.; Jang J. S.; Bard A. J. Rapid Screening of BiVO4 -Based Photocatalysts by Scanning Electrochemical Microscopy (SECM) and Studies of Their Photoelectrochemical Properties. J. Phys. Chem. C 2010, 114, 13322–13328. 10.1021/jp104343b. [DOI] [Google Scholar]
- Ye H.; Park H. S.; Bard A. J. Screening of Electrocatalysts for Photoelectrochemical Water Oxidation on W-Doped BiVO 4 Photocatalysts by Scanning Electrochemical Microscopy. J. Phys. Chem. C 2011, 115, 12464–12470. 10.1021/jp200852c. [DOI] [Google Scholar]
- Sarkar S.; Wang X.; Hesari M.; Chen P.; Mirkin M. V. Scanning Electrochemical and Photoelectrochemical Microscopy on Finder Grids: Toward Correlative Multitechnique Imaging of Surfaces. Anal. Chem. 2021, 93, 5377–5382. 10.1021/acs.analchem.1c00358. [DOI] [PubMed] [Google Scholar]
- Askarova G.; Hesari M.; Wang C.; Mirkin M. V. Decoupling Through-Tip Illumination from Scanning in Nanoscale Photo-SECM. Anal. Chem. 2022, 94, 7169–7173. 10.1021/acs.analchem.2c00753. [DOI] [PubMed] [Google Scholar]
- Iffelsberger C.; Ng S.; Pumera M. Photoelectrolysis of TiO2 is Highly Localized and the Selectivity is Affected by the Light. Chem. Eng. J. 2022, 446, 136995. 10.1016/j.cej.2022.136995. [DOI] [Google Scholar]
- Ju M.; Cai R.; Ren J.; Chen J.; Qi L.; Long X.; Yang S. Conductive Polymer Intercalation Tunes Charge Transfer and Sorption-Desorption Properties of LDH Enabling Efficient Alkaline Water Oxidation. ACS Appl. Mater. Interfaces 2021, 13, 37063–37070. 10.1021/acsami.1c08429. [DOI] [PubMed] [Google Scholar]
- Stumm C.; Bertram M.; Kastenmeier M.; Speck F. D.; Sun Z.; Rodríguez-Fernández J.; Lauritsen J. V.; Mayrhofer K. J. J.; Cherevko S.; Brummel O.; et al. Structural Dynamics of Ultrathin Cobalt Oxide Nanoislands under Potential Control. Adv. Funct. Mater. 2021, 31, 2009923. 10.1002/adfm.202009923. [DOI] [Google Scholar]
- Tilak B. V.; Ramamurthy A. C.; Conway B. E. High Performance Electrode Materials for the Hydrogen Evolution Reaction from Alkaline Media. Proc. Indian Acad. Sci. (Chem. Sci.) 1986, 97, 359–393. 10.1007/BF02849200. [DOI] [Google Scholar]
- Dubouis N.; Grimaud A. The Hydrogen Evolution Reaction: From Material to Interfacial Descriptors. Chem. Sci. 2019, 10, 9165–9181. 10.1039/C9SC03831K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J.; Hu L.; Zhao P.; Lee L. Y. S.; Wong K.-Y. Recent Advances in Electrocatalytic Hydrogen Evolution Using Nanoparticles. Chem. Rev. 2020, 120, 851–918. 10.1021/acs.chemrev.9b00248. [DOI] [PubMed] [Google Scholar]
- Ferriday T. B.; Middleton P. H.; Kolhe M. L. Review of the Hydrogen Evolution Reaction—A Basic Approach. Energies 2021, 14, 8535. 10.3390/en14248535. [DOI] [Google Scholar]
- Anantharaj S.; Aravindan V. Developments and Perspectives in 3d Transition-Metal-Based Electrocatalysts for Neutral and Near-Neutral Water Electrolysis. Adv. Energy Mater. 2020, 10, 1902666. 10.1002/aenm.201902666. [DOI] [Google Scholar]
- Selzer Y.; Turyan I.; Mandler D. Studying Heterogeneous Catalysis by the Scanning Electrochemical Microscope (SECM): The Reduction of Protons by Methyl Viologen Catalyzed by a Platinum Surface. J. Phys. Chem. B 1999, 103, 1509–1517. 10.1021/jp9833348. [DOI] [Google Scholar]
- Zhang J.; Lahtinen R. M.; Kontturi K.; Unwin P. R.; Schiffrin D. J. Electron Transfer Reactions at Gold Nanoparticles. Chem. Commun. 2001, 1818–1819. 10.1039/b103458h. [DOI] [PubMed] [Google Scholar]
- Li F.; Bertoncello P.; Ciani I.; Mantovani G.; Unwin P. R. Incorporation of Functionalized Palladium Nanoparticles within Ultrathin Nafion Films: A Nanostructured Composite for Electrolytic and Redox-Mediated Hydrogen Evolution. Adv. Funct. Mater. 2008, 18, 1685–1693. 10.1002/adfm.200701447. [DOI] [Google Scholar]
- Li F.; Ciani I.; Bertoncello P.; Unwin P. R.; Zhao J.; Bradbury C. R.; Fermin D. J. Scanning Electrochemical Microscopy of Redox-Mediated Hydrogen Evolution Catalyzed by Two-Dimensional Assemblies of Palladium Nanoparticles. J. Phys. Chem. C 2008, 112, 9686–9694. 10.1021/jp8001228. [DOI] [Google Scholar]
- Li H.; Du M.; Mleczko M. J.; Koh A. L.; Nishi Y.; Pop E.; Bard A. J.; Zheng X. Kinetic Study of Hydrogen Evolution Reaction over Strained MoS2 with Sulfur Vacancies Using Scanning Electrochemical Microscopy. J. Am. Chem. Soc. 2016, 138, 5123–5129. 10.1021/jacs.6b01377. [DOI] [PubMed] [Google Scholar]
- Zhou J.; Zu Y.; Bard A. J Scanning Electrochemical Microscopy Part 39. The Proton/Hydrogen Mediator System and its Application to the Study of the Electrocatalysis of Hydrogen Oxidation. J. Electroanal. Chem. 2000, 491, 22–29. 10.1016/S0022-0728(00)00100-5. [DOI] [Google Scholar]
- Zoski C. G. Scanning Electrochemical Microscopy: Investigation of Hydrogen Oxidation at Polycrystalline Noble Metal Electrodes. J. Phys. Chem. B 2003, 107, 6401–6405. 10.1021/jp027436g. [DOI] [Google Scholar]
- Bonazza H. L.; Vega L. D.; Fernández J. L. Analysis of the Hydrogen Electrode Reaction Mechanism in Thin-Layer Cells. 2. Study of Hydrogen Evolution on Microelectrodes by Scanning Electrochemical Microscopy. J. Electroanal. Chem. 2014, 713, 9–16. 10.1016/j.jelechem.2013.11.027. [DOI] [Google Scholar]
- Iffelsberger C.; Wert S.; Matysik F.-M.; Pumera M. Catalyst Formation and In Operando Monitoring of the Electrocatalytic Activity in Flow Reactors. ACS Appl. Mater. Interfaces 2021, 13, 35777–35784. 10.1021/acsami.1c09127. [DOI] [PubMed] [Google Scholar]
- Sun T.; Yu Y.; Zacher B. J.; Mirkin M. V. Scanning Electrochemical Microscopy of Individual Catalytic Nanoparticles. Angew. Chem., Int. Ed. 2014, 53, 14120–14123. 10.1002/anie.201408408. [DOI] [PubMed] [Google Scholar]
- Niu H.-J.; Yan Y.; Jiang S.; Liu T.; Sun T.; Zhou W.; Guo L.; Li J. Interfaces Decrease the Alkaline Hydrogen-Evolution Kinetics Energy Barrier on NiCoP/Ti3C2Tx MXene. ACS Nano 2022, 16, 11049. 10.1021/acsnano.2c03711. [DOI] [PubMed] [Google Scholar]
- Liberman I.; Ifraemov R.; Shimoni R.; Hod I. Localized Electrosynthesis and Subsequent Electrochemical Mapping of Catalytically Active Metal–Organic Frameworks. Adv. Funct Materials 2022, 32, 2112517. 10.1002/adfm.202112517. [DOI] [Google Scholar]
- Kund J.; Romer J.; Oswald E.; Gaus A.-L.; Küllmer M.; Turchanin A.; Delius M.; Kranz C. Pd-Modified De-alloyed Au–Ni-Microelectrodes for In Situ and Operando Mapping of Hydrogen Evolution. ChemElectroChem. 2022, 9, e202200071 10.1002/celc.202200071. [DOI] [Google Scholar]
- Visibile A.; Baran T.; Rondinini S.; Minguzzi A.; Vertova A. Determining the Efficiency of Photoelectrode Materials by Coupling Cavity-Microelectrode Tips and Scanning Electrochemical Microscopy. ChemElectroChem. 2020, 7, 2440–2447. 10.1002/celc.202000432. [DOI] [Google Scholar]
- Jamali S. S.; Moulton S. E.; Tallman D. E.; Weber J.; Wallace G. G. Electro-Oxidation and Reduction of H2 on Platinum Studied by Scanning Electrochemical Microscopy for the Purpose of Local Detection of H2 Evolution. Surf. Interface Anal. 2015, 47, 1187–1191. 10.1002/sia.5874. [DOI] [Google Scholar]
- Iffelsberger C.; Rojas D.; Pumera M. Photo-Responsive Doped 3D-Printed Copper Electrodes for Water Splitting: Refractory One-Pot Doping Dramatically Enhances the Performance. J. Phys. Chem. C 2022, 126, 9016–9026. 10.1021/acs.jpcc.1c10686. [DOI] [Google Scholar]
- Hill J. W.; Hill C. M. Directly Mapping Photoelectrochemical Behavior within Individual Transition Metal Dichalcogenide Nanosheets. Nano Lett. 2019, 19, 5710–5716. 10.1021/acs.nanolett.9b02336. [DOI] [PubMed] [Google Scholar]
- Kosmala T.; Baby A.; Lunardon M.; Perilli D.; Liu H.; Durante C.; Di Valentin C.; Agnoli S.; Granozzi G. Operando Visualization of the Hydrogen Evolution Reaction with Atomic-Scale Precision at Different Metal–Graphene Interfaces. Nat. Catal. 2021, 4, 850–859. 10.1038/s41929-021-00682-2. [DOI] [Google Scholar]
- Kluge R. M.; Haid R. W.; Stephens I. E. L.; Calle-Vallejo F.; Bandarenka A. S. Monitoring the Active sites for the Hydrogen Evolution Reaction at Model Carbon Surfaces. Phys. Chem. Chem. Phys. 2021, 23, 10051–10058. 10.1039/D1CP00434D. [DOI] [PubMed] [Google Scholar]
- Nitopi S.; Bertheussen E.; Scott S. B.; Liu X.; Engstfeld A. K.; Horch S.; Seger B.; Stephens I. E. L.; Chan K.; Hahn C.; et al. Progress and Perspectives of Electrochemical CO2 Reduction on Copper in Aqueous Electrolyte. Chem. Rev. 2019, 119, 7610–7672. 10.1021/acs.chemrev.8b00705. [DOI] [PubMed] [Google Scholar]
- Pacala S.; Socolow R. Stabilization Wedges: Solving the Climate Problem for the Next 50 Years with Current Technologies. Science 2004, 305, 968–972. 10.1126/science.1100103. [DOI] [PubMed] [Google Scholar]
- De Luna P.; Hahn C.; Higgins D.; Jaffer S. A.; Jaramillo T. F.; Sargent E. H. What Would it take for Renewably Powered Electrosynthesis to Displace Petrochemical Processes?. Science 2019, 364, 364. 10.1126/science.aav3506. [DOI] [PubMed] [Google Scholar]
- Zhu D. D.; Liu J. L.; Qiao S. Z. Recent Advances in Inorganic Heterogeneous Electrocatalysts for Reduction of Carbon Dioxide. Adv. Mater. 2016, 28, 3423–3452. 10.1002/adma.201504766. [DOI] [PubMed] [Google Scholar]
- Rasul S.; Anjum D. H.; Jedidi A.; Minenkov Y.; Cavallo L.; Takanabe K. A Highly Selective Copper–Indium Bimetallic Electrocatalyst for the Electrochemical Reduction of Aqueous CO2 to CO. Angew. Chem., Int. Ed. 2015, 127, 2174–2178. 10.1002/ange.201410233. [DOI] [PubMed] [Google Scholar]
- Pei Y.; Zhong H.; Jin F. A Brief Review of Electrocatalytic Reduction of CO2—Materials, Reaction Conditions, and Devices. Energy Sci. Eng. 2021, 9, 1012–1032. 10.1002/ese3.935. [DOI] [Google Scholar]
- Zheng Y.; Vasileff A.; Zhou X.; Jiao Y.; Jaroniec M.; Qiao S.-Z. Understanding the Roadmap for Electrochemical Reduction of CO2 to Multi-Carbon Oxygenates and Hydrocarbons on Copper-Based Catalysts. J. Am. Chem. Soc. 2019, 141, 7646–7659. 10.1021/jacs.9b02124. [DOI] [PubMed] [Google Scholar]
- Kuhl K. P.; Cave E. R.; Abram D. N.; Jaramillo T. F. New Insights into the Electrochemical Reduction of Carbon Dioxide on Metallic Copper Surfaces. Energy Environ. Sci. 2012, 5, 7050. 10.1039/c2ee21234j. [DOI] [Google Scholar]
- Hori Y.; Kikuchi K.; Suzuki S. Production of CO and CH4 In Electrochemical Reduction of CO2 at Metal Electrodes in Aqueous Hydrogencarbonate Solution. Chem. Lett. 1985, 14, 1695–1698. 10.1246/cl.1985.1695. [DOI] [Google Scholar]
- Kuhl K. P.; Hatsukade T.; Cave E. R.; Abram D. N.; Kibsgaard J.; Jaramillo T. F. Electrocatalytic Conversion of Carbon Dioxide to Methane and Methanol on Transition Metal Surfaces. J. Am. Chem. Soc. 2014, 136, 14107–14113. 10.1021/ja505791r. [DOI] [PubMed] [Google Scholar]
- Yang H.-P.; Yue Y.-N.; Qin S.; Wang H.; Lu J.-X. Selective Electrochemical Reduction of CO2 to Different Alcohol Products by an Organically Doped Alloy Catalyst. Green Chem. 2016, 18, 3216–3220. 10.1039/C6GC00091F. [DOI] [Google Scholar]
- Appel A. M.; Bercaw J. E.; Bocarsly A. B.; Dobbek H.; DuBois D. L.; Dupuis M.; Ferry J. G.; Fujita E.; Hille R.; Kenis P. J. A.; et al. Frontiers, Opportunities, and Challenges in Biochemical and Chemical Catalysis of CO2 Fixation. Chem. Rev. 2013, 113, 6621–6658. 10.1021/cr300463y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. S.; Yoon Y.; Wuttig A.; Surendranath Y. Mesostructure-Induced Selectivity in CO2 Reduction Catalysis. J. Am. Chem. Soc. 2015, 137, 14834–14837. 10.1021/jacs.5b08259. [DOI] [PubMed] [Google Scholar]
- Rosen B. A.; Salehi-Khojin A.; Thorson M. R.; Zhu W.; Whipple D. T.; Kenis P. J. A.; Masel R. I. Ionic Liquid-Mediated Selective Conversion of CO2 to CO at Low Overpotentials. Science 2011, 334, 643–644. 10.1126/science.1209786. [DOI] [PubMed] [Google Scholar]
- Liu X.; Xiao J.; Peng H.; Hong X.; Chan K.; Nørskov J. K. Understanding Trends in Electrochemical Carbon Dioxide Reduction Rates. Nat. Commun. 2017, 8, 15438. 10.1038/ncomms15438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorova T. K.; Schreiber M. W.; Fontecave M. Mechanistic Understanding of CO2 Reduction Reaction (CO2RR) Toward Multicarbon Products by Heterogeneous Copper-Based Catalysts. ACS Catal. 2020, 10, 1754–1768. 10.1021/acscatal.9b04746. [DOI] [Google Scholar]
- Sreekanth N.; Phani K. L. Selective Reduction of CO2 to Formate through Bicarbonate Reduction on Metal Electrodes: New Insights Gained from SG/TC Mode of SECM. Chem. Commun. 2014, 50, 11143–11146. 10.1039/C4CC03099K. [DOI] [PubMed] [Google Scholar]
- Sreekanth N.; Nazrulla M. A.; Vineesh T. V.; Sailaja K.; Phani K. L. Metal-free Boron-Doped Graphene for Selective Electroreduction of Carbon Dioxide to Formic acid/Formate. Chem. Commun. 2015, 51, 16061–16064. 10.1039/C5CC06051F. [DOI] [PubMed] [Google Scholar]
- Narayanaru S.; Chinnaiah J.; Phani K. L.; Scholz F. pH dependent CO Adsorption and Roughness-Induced Selectivity of CO2 Electroreduction on Gold Surfaces. Electrochim. Acta 2018, 264, 269–274. 10.1016/j.electacta.2018.01.106. [DOI] [Google Scholar]
- Monteiro M. C. O.; Dattila F.; Hagedoorn B.; García-Muelas R.; López N.; Koper M. T. M. Absence of CO2 Electroreduction on Copper, Gold and Silver Electrodes without Metal Cations in Solution. Nat. Catal. 2021, 4, 654–662. 10.1038/s41929-021-00655-5. [DOI] [Google Scholar]
- Li J.; Zhang Z.; Hu W. Insight into the Effect of Metal Cations in the Electrolyte on Performance for Electrocatalytic CO2 Reduction Reaction. Energy Environ. Mater. 2022, 5, 1008–1009. 10.1002/eem2.12294. [DOI] [Google Scholar]
- Shaughnessy C. I.; Jantz D. T.; Leonard K. C. Selective Electrochemical CO2 Reduction to CO using in situ Reduced In2O3 nanocatalysts. J. Mater. Chem. A 2017, 5, 22743–22749. 10.1039/C7TA06570A. [DOI] [Google Scholar]
- Mayer F. D.; Hosseini-Benhangi P.; Sánchez-Sánchez C. M.; Asselin E.; Gyenge E. L. Scanning Electrochemical Microscopy Screening of CO2 Electroreduction Activities and Product Selectivities of Catalyst Arrays. Commun. Chem. 2020, 3, 155. 10.1038/s42004-020-00399-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.; Jo A.; Ha Y.; Lee Y.; Lee D.; Lee Y.; Lee C. Highly Dispersive Gold Nanoparticles on Carbon Black for Oxygen and Carbon Dioxide Reduction. Electroanalysis 2018, 30, 2861–2868. 10.1002/elan.201800555. [DOI] [Google Scholar]
- Monteiro M. C. O.; Jacobse L.; Koper M. T. M. Understanding the Voltammetry of Bulk CO Electrooxidation in Neutral Media through Combined SECM Measurements. J. Phys. Chem. Lett. 2020, 11, 9708–9713. 10.1021/acs.jpclett.0c02779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins D.; Hahn C.; Xiang C.; Jaramillo T. F.; Weber A. Z. Gas-Diffusion Electrodes for Carbon Dioxide Reduction: A New Paradigm. ACS Energy Lett. 2019, 4, 317–324. 10.1021/acsenergylett.8b02035. [DOI] [Google Scholar]
- Dieckhöfer S.; Öhl D.; Junqueira J. R. C.; Quast T.; Turek T.; Schuhmann W. Probing the Local Reaction Environment During High Turnover Carbon Dioxide Reduction with Ag-Based Gas Diffusion Electrodes. Chemistry 2021, 27, 5906–5912. 10.1002/chem.202100387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro M. C. O.; Dieckhöfer S.; Bobrowski T.; Quast T.; Pavesi D.; Koper M. T. M.; Schuhmann W. Probing the Local Activity of CO2 Reduction on Gold Gas Diffusion Electrodes: Effect of the Catalyst Loading and CO2 Pressure. Chem. Sci. 2021, 12, 15682–15690. 10.1039/D1SC05519D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junqueira J. R. C.; O’Mara P. B.; Wilde P.; Dieckhöfer S.; Benedetti T. M.; Andronescu C.; Tilley R. D.; Gooding J. J.; Schuhmann W. Combining Nanoconfinement in Ag Core/Porous Cu Shell Nanoparticles with Gas Diffusion Electrodes for Improved Electrocatalytic Carbon Dioxide Reduction. ChemElectroChem. 2021, 8, 4848–4853. 10.1002/celc.202100906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikdar N.; Junqueira J. R. C.; Dieckhofer S.; Quast T.; Braun M.; Song Y.; Aiyappa H. B.; Seisel S.; Weidner J.; Ohl D.; et al. A Metal–Organic Framework derived CuxOyCz Catalyst for Electrochemical CO2 Reduction and Impact of Local pH Change. Angew. Chem., Int. Ed. 2021, 60, 23427–23434. 10.1002/anie.202108313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.-G.; Baricuatro J. H.; Javier A.; Gregoire J. M.; Soriaga M. P. The Evolution of the Polycrystalline Copper Surface, first to Cu(111) and then to Cu(100), at a fixed CO2RR Potential: A Study by Operando EC-STM. Langmuir 2014, 30, 15053–15056. 10.1021/la504445g. [DOI] [PubMed] [Google Scholar]
- Schouten K. J. P.; Qin Z.; Pérez Gallent E.; Koper M. T. M. Two Pathways for the Formation of Ethylene in CO Reduction on Single-Crystal Copper Electrodes. J. Am. Chem. Soc. 2012, 134, 9864–9867. 10.1021/ja302668n. [DOI] [PubMed] [Google Scholar]
- Phan T. H.; Banjac K.; Cometto F. P.; Dattila F.; García-Muelas R.; Raaijman S. J.; Ye C.; Koper M. T. M.; López N.; Lingenfelder M. Emergence of Potential-Controlled Cu-Nanocuboids and Graphene-Covered Cu-Nanocuboids under Operando CO2 Electroreduction. Nano Lett. 2021, 21, 2059–2065. 10.1021/acs.nanolett.0c04703. [DOI] [PubMed] [Google Scholar]
- Lim T.; Jung G. Y.; Kim J. H.; Park S. O.; Park J.; Kim Y.-T.; Kang S. J.; Jeong H. Y.; Kwak S. K.; Joo S. H. Atomically Dispersed Pt-N4 sites as Efficient and Selective Electrocatalysts for the Chlorine Evolution Reaction. Nat. Commun. 2020, 11, 412. 10.1038/s41467-019-14272-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Liu Y.; Wiley D.; Zhao S.; Tang Z. Recent Advances in Electrocatalytic Chloride Oxidation for Chlorine Gas Production. J. Mater. Chem. A 2021, 9, 18974–18993. 10.1039/D1TA02745J. [DOI] [Google Scholar]
- Zeradjanin A. R.; Schilling T.; Seisel S.; Bron M.; Schuhmann W. Visualization of Chlorine Evolution at Dimensionally Stable Anodes by Means of Scanning Electrochemical Microscopy. Anal. Chem. 2011, 83, 7645–7650. 10.1021/ac200677g. [DOI] [PubMed] [Google Scholar]
- Zeradjanin A. R.; Menzel N.; Schuhmann W.; Strasser P. On the Faradaic Selectivity and the Role of Surface Inhomogeneity during the Chlorine Evolution Reaction on Ternary Ti-Ru-Ir Mixed Metal Oxide Electrocatalysts. Phys. Chem. Chem. Phys. 2014, 16, 13741–13747. 10.1039/C4CP00896K. [DOI] [PubMed] [Google Scholar]
- Noël J.-M.; Kanoufi F. Probing the Reactive Intermediate Species Generated during Electrocatalysis by Scanning Electrochemical Microscopy. Curr. Opin. Electrochem. 2022, 35, 101071. 10.1016/j.coelec.2022.101071. [DOI] [Google Scholar]
- Chang J.; Bard A. J. Detection of the Sn(III) Intermediate and the Mechanism of the Sn(IV)/Sn(II) Electroreduction Reaction in Bromide Media by Cyclic Voltammetry and Scanning Electrochemical Microscopy. J. Am. Chem. Soc. 2014, 136, 311–320. 10.1021/ja409958a. [DOI] [PubMed] [Google Scholar]
- Kai T.; Zhou M.; Duan Z.; Henkelman G. A.; Bard A. J. Detection of CO2•– in the Electrochemical Reduction of Carbon Dioxide in N,N-Dimethylformamide by Scanning Electrochemical Microscopy. J. Am. Chem. Soc. 2017, 139, 18552–18557. 10.1021/jacs.7b08702. [DOI] [PubMed] [Google Scholar]
- Wijayawardhana C. A.; Wittstock G.; Halsall H. B.; Heineman W. R. Spatially Addressed Deposition and Imaging of Biochemically Active Bead Microstructures by Scanning Electrochemical Microscopy. Anal. Chem. 2000, 72, 333–338. 10.1021/ac990977q. [DOI] [PubMed] [Google Scholar]
- Maciejewska M.; Schäfer D.; Schuhmann W. SECM Visualization of Spatial Variability of Enzyme-Polymer Spots. Part 2: Complex Interference Elimination by Means of Selection of Highest Sensitivity Sensor Substructures and Artificial Neural Networks. Electroanalysis 2006, 18, 1916–1928. 10.1002/elan.200603611. [DOI] [Google Scholar]
- Mauzeroll J.; Buda M.; Bard A. J.; Prieto F.; Rueda M. Detection of Tl(I) Transport through a Gramicidin–Dioleoylphosphatidylcholine Monolayer Using the Substrate Generation–Tip Collection Mode of Scanning Electrochemical Microscopy. Langmuir 2002, 18, 9453–9461. 10.1021/la0262710. [DOI] [Google Scholar]
- Fernández J. L.; Mano N.; Heller A.; Bard A. J. Optimization Of “Wired” Enzyme O2-Electroreduction Catalyst Compositions by Scanning Electrochemical Microscopy. Angew. Chem., Int. Ed. 2004, 43, 6355–6357. 10.1002/anie.200461528. [DOI] [PubMed] [Google Scholar]
- Dantas L. M. F.; De Souza A. P. R.; Castro P. S.; Paixão T. R. L. C.; Bertotti M. SECM Studies on the Electrocatalytic Oxidation of Glycerol at Copper Electrodes in Alkaline Medium. Electroanalysis 2012, 24, 1778–1782. 10.1002/elan.201200144. [DOI] [Google Scholar]
- Perales-Rondón J. V.; Herrero E.; Solla-Gullón J.; Sánchez-Sánchez C. M.; Vivier V. Oxygen Crossover Effect on Palladium and Platinum Based Electrocatalysts During Formic Acid Oxidation Studied by Scanning Electrochemical Microscopy. J. Electroanal. Chem. 2017, 793, 218–225. 10.1016/j.jelechem.2016.12.049. [DOI] [Google Scholar]
- Komkova M. A.; Holzinger A.; Hartmann A.; Khokhlov A. R.; Kranz C.; Karyakin A. A.; Voronin O. G. Ultramicrosensors Based on Transition Metal Hexacyanoferrates for Scanning Electrochemical Microscopy. Beilstein J. Nanotechnol. 2013, 4, 649–654. 10.3762/bjnano.4.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M.; Li Y.; Wu J.; Su T.; Zhang J.; Tang J. SECM Screening of the Catalytic Activities of AuPd Bimetallic Patterns Fabricated by Electrochemical Wet-Stamping Technique. J. Electroanal. Chem. 2016, 772, 96–102. 10.1016/j.jelechem.2016.03.007. [DOI] [Google Scholar]
- Tomlinson L. I.; Patten H. V.; Green B. L.; Iacobini J.; Meadows K. E.; McKelvey K.; Unwin P. R.; Newton M. E.; Macpherson J. V. Intermittent-Contact Scanning Electrochemical Microscopy (IC-SECM) as a Quantitative Probe of Defects in Single Crystal Boron Doped Diamond Electrodes. Electroanalysis 2016, 28, 2297–2302. 10.1002/elan.201600291. [DOI] [Google Scholar]
- Kim J.; Renault C.; Nioradze N.; Arroyo-Currás N.; Leonard K. C.; Bard A. J. Electrocatalytic Activity of Individual Pt Nanoparticles Studied by Nanoscale Scanning Electrochemical Microscopy. J. Am. Chem. Soc. 2016, 138, 8560–8568. 10.1021/jacs.6b03980. [DOI] [PubMed] [Google Scholar]
- Miranda Vieira M.; Lemineur J.-F.; Médard J.; Combellas C.; Kanoufi F.; Noël J.-M. Operando Analysis of the Electrosynthesis of Ag2O Nanocubes by Scanning Electrochemical Microscopy. Electrochem. Commun. 2021, 124, 106950. 10.1016/j.elecom.2021.106950. [DOI] [Google Scholar]
- He W.; Zhang J.; Dieckhöfer S.; Varhade S.; Brix A. C.; Lielpetere A.; Seisel S.; Junqueira J. R. C.; Schuhmann W. Splicing the Active Phases of Copper/Cobalt-Based Catalysts Achieves High-Rate Tandem Electroreduction of Nitrate to Ammonia. Nat. Commun. 2022, 13, 1129. 10.1038/s41467-022-28728-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haram S. K.; Bard A. J. Scanning Electrochemical Microscopy. 42. Studies of the Kinetics and Photoelectrochemistry of Thin Film CdS/Electrolyte Interfaces. J. Phys. Chem. B 2001, 105, 8192–8195. 10.1021/jp011068j. [DOI] [Google Scholar]
- Zhang B.; Zhang X.; Xiao X.; Shen Y. Photoelectrochemical Water Splitting System-A Study of Interfacial Charge Transfer with Scanning Electrochemical Microscopy. ACS Appl. Mater. Interfaces 2016, 8, 1606–1614. 10.1021/acsami.5b07180. [DOI] [PubMed] [Google Scholar]
- Yu Z.; Huang Q.; Jiang X.; Lv X.; Xiao X.; Wang M.; Shen Y.; Wittstock G. Effect of a Cocatalyst on a Photoanode in Water Splitting: A Study of Scanning Electrochemical Microscopy. Anal. Chem. 2021, 93, 12221–12229. 10.1021/acs.analchem.1c01235. [DOI] [PubMed] [Google Scholar]