Abstract
Beryllium is associated with a human pulmonary granulomatosis characterized by an accumulation of CD4+ T cells in the lungs and a heightened specific lymphocyte proliferative response to beryllium (Be) with gamma interferon (IFN-γ) release (i.e., a T helper 1 [Th1] response). While an animal model of Be sensitization is not currently available, Be has exhibited adjuvant effects in animals. The effects of Be on BALB/c mice immunized with soluble leishmanial antigens (SLA) were investigated to determine if Be had adjuvant activity for IFN-γ production, an indicator of the Th1 response. In this strain of Leishmania-susceptible BALB/c mice, a Th2 response is normally observed after in vivo SLA sensitization and in vitro restimulation with SLA. If interleukin-12 (IL-12) is given during in vivo sensitization with SLA, markedly increased IFN-γ production and decreased IL-4 production are detected. We show here that when beryllium sulfate (BeSO4) was added during in vivo sensitization of BALB/c mice with SLA and IL-12, significantly increased IFN-γ production and decreased IL-4 production from lymph node and spleen cells were detected upon in vitro SLA restimulation. No specific responses were observed to Be alone. Lymph node and spleen cells from all mice proliferated strongly and comparably upon in vitro restimulation with SLA and with SLA plus Be; no differences were noted among groups of mice that received different immunization regimens. In vivo, when Be was added to SLA and IL-12 for sensitization of BALB/c mice, more effective control of Leishmania infection was achieved. This finding has implications for understanding not only the development of granulomatous reactions but also the potential for developing Be as a vaccine adjuvant.
Beryllium is the lightest metal, with an atomic weight of 4 and a molecular weight of 9. Its use as a pure metal in aerospace and nuclear reactors, as an oxide, and as an alloy has led to the development among industrial workers of a granulomatous disease due to a delayed-type hypersensitivity to beryllium (31). Chronic beryllium disease (CBD), as it is known, is characterized by activation and maturation of macrophages (Mφ) along with recruitment and expansion of CD4+ T lymphocytes (7, 24, 42). Patients with CBD, who have been exposed to and sensitized against beryllium salts in the workplace, exhibit specific peripheral blood and alveolar lymphocyte proliferative responses to Be (31, 33). Be-stimulated cells from CBD patients express gamma interferon (IFN-γ) but not interleukin-4 (IL-4) (44), indicative of a T helper 1 (Th1) response. These immunological activities lead to the formation of noncaseating granulomas in the lungs.
In addition to the role of Be as an antigen, adjuvant activities have been observed in animals. In rats, increased levels of immunoglobulin A (IgA) antibodies were noted when killed brucella organisms or sheep red blood cells were injected with soluble beryllium hydroxide [Be(OH)2]; biliary and serum antibodies to bovine serum albumin were detected only when bovine serum albumin was given with beryllium adjuvant (13). A twofold increase in protection against trichostrongylus infection of rabbits was observed when immunization with nematode proteins was combined with Be (51). In addition, Be was better than complete Freund's adjuvant for increasing the levels of rabbit anti-nematode antibodies (50). The heightened antibody levels in rats and rabbits can be due to either Th1 or Th2 responses.
Murine and human studies have differentiated activated T cells into two distinct populations, Th1 and Th2 (1, 27, 40). Various factors drive T cells into these subsets, characterized by distinct patterns of proinflammatory cytokine secretion and immunoglobulin induction. Th1 cells are marked by increased IFN-γ and IL-2 production, which induces protective cell-mediated immunity and delayed-type hypersensitivity reactions. In contrast, Th2 responses are typically characterized by increased IL-4 production and are involved in helminthic inflammations and the induction of B cells to secrete isotype- and antigen-specific immunoglobulin molecules.
The adjuvant properties of Be have not yet been investigated with respect to either Th1 or Th2 responses. Since Be-sensitized BAL cells from CBD patients secrete significant amounts of Th1 cytokines, we hypothesize that the adjuvant effects of Be would favor the production of IFN-γ and implicate the Th1 response. In the present study, we utilized the Leishmania-BALB/c model to determine whether Be has specific adjuvant activity that would favor the production of IFN-γ. In this model, the protozoan parasite Leishmania major causes a nonhealing cutaneous lesion in BALB/c mice; cells from these mice develop a Th2 response with low IFN-γ and high IL-4 production after in vitro stimulation. However, if these mice are immunized with soluble leishmanial antigen (SLA) combined with IL-12 and/or IFN-γ, they become able to control leishmanial infection and a Th1-like response is observed with increased IFN-γ production and decreased IL-4 production (2, 38). In this study, we showed that Be can act as an adjuvant to promote the production of IFN-γ and enhance the properties of IL-12 to protect against infection in otherwise Leishmania-susceptible mice.
MATERIALS AND METHODS
Mice.
Female BALB/cByJ mice, age 5 to 8 weeks, were obtained from The Jackson Laboratory (Bar Harbor, Maine). The mice were housed in the University Laboratory Animal Resources Center at the University of Pennsylvania School of Veterinary Medicine. The specific-pathogen-free animal colony was screened regularly for the presence of murine pathogens and consistently tested negative.
Parasites and immunogens.
A clone of L. major (WHO MHOM/IL/80/Friedlin) was grown in Grace's insect cell culture medium (Life Technologies, Grand Island, N.Y.) supplemented with 20% fetal bovine serum (HyClone, Inc., Logan, Utah) and 2 mM glutamine. As described previously (32), stationary-phase promastigotes were harvested and metacyclic-stage parasites were lectin selected using Arachis hypogae agglutinin (Sigma Chemical Co., St. Louis, Mo.). The mice were challenged with 105 purified metacyclic promastigotes in the hind footpads. SLA was prepared as described previously (39). Soluble beryllium sulfate (BeSO4) was obtained from Brush Wellman (Elmore, Ohio). Recombinant IL-12 (4.4 × 106 U/mg) was a generous gift of Stanley Wolf (Genetics Institute, Cambridge, Mass.).
Immunization protocol.
Aged-matched mice were injected in the left and right footpads with a final inoculating volume of 50 μl for each footpad. The final concentrations of the solutions used for the inoculations were as follows: (i) 1 mg of SLA per ml, (ii) 1 mg of SLA per ml plus 2 mM BeSO4, (iii) 1 mg of SLA per ml plus 0.25 μg of IL-12, and (iv) 1 mg of SLA per ml plus 0.25 μg of IL-12 and 2 mM BeSO4. In one set of experiments, two other doses of IL-12 (0.1 and 0.5 μg) were used to make up the additional solutions of (v) 1 mg of SLA per ml plus 0.1 μg of IL-12, (vi) 1 mg of SLA per ml plus 0.5 μg of IL-12, (vii) 1 mg of SLA per ml plus 0.1 μg of IL-12 and 2 mM BeSO4, and (viii) 1 mg of SLA per ml plus 0.5 μg of IL-12 and 2 mM BeSO4. In the in vivo challenge experiments, mice were initially immunized with 50 μl in the right footpads only and the concentrations of BeSO4 and IL-12 were twice those indicated above. Further, the 0.5-μg dose of IL-12 was replaced with 1.0 μg, giving the mice in group vi 1 mg of SLA per ml plus 1.0 μg of IL-12 (group ix) and those in group viii 1 mg of SLA per ml plus 1.0 μg of IL-12 and 2 mM BeSO4 (group x). Equal volumes of sterile phosphate-buffered saline (PBS) were injected into one group of mice as a negative control for the in vivo challenge experiments. Mice were checked regularly for signs of Be toxicity, especially at the site of injection, and no abnormalities were noted.
In vitro restimulations.
Popliteal lymph nodes (LNs) and spleens (SPLs) were removed 7 days after immunization. LNs and SPLs from mice in the same group were combined. Single-cell suspensions were made with glass tissue homogenizers (Wheaton; Fisher, Pittsburgh, Pa.). LN cells were washed twice (Dulbecco's minimal essential medium [DMEM]-low glucose, 2% fetal bovine serum, 2 mM glutamine, 100 U of penicillin 6-phosphate per ml, 100 μg of streptomycin sulfate per ml, 25 mM HEPES) before culture. SPL cells were also treated with lysing solution (0.144 M NH4Cl, 0.017 M Tris-HCl [pH 7.65]) to eliminate red blood cells. The cells were resuspended at 2.5 × 106 cells/ml in complete tissue culture medium (CTCM) (DMEM-high glucose, 10% fetal bovine serum, 2 mM glutamine, 100 U of penicillin 6-phosphate per ml, 100 μg of streptomycin sulfate per ml, 25 mM HEPES, 50 nM 2-mercaptoethanol). LN and SPL cells were cultured in CTCM alone or with concanavalin A (ConA) (10 μg/ml; Sigma Chemical Co.), SLA (0.1 mg/ml), BeSO4 (1 μM), or BeSO4 plus SLA. Cells (2.5 × 105 per well) were seeded into 96-well, round-bottom tissue culture plates. Restimulation assays were done in triplicate or quadruplicate for each condition. Supernatants from 72-h LN and SPL cell cultures were harvested for IL-4 and IFN-γ analysis. Levels of proliferation were assayed by tritiated-thymidine incorporation after 3 days of stimulation. LN and SPL cell cultures were pulsed with [methyl-3H]thymidine at a final concentration of 12 μCi/ml (specific activity, 60 Ci/mmol [ICN Biomedicals, Costa Mesa, Calif.]). After 14 h, the cells were harvested on the Tomtec Harvester 96 Mach II cell harvester (Wallac Inc., Gaithersburg, Md.), and radioactive β-emission was counted on the Wallac Betaplate 1205 scintillation counter.
Cytokine measurement.
Supernatants from 3-day-stimulated LN and SPL cells were analyzed for IFN-γ and IL-4 by specific two-site enzyme-linked immunosorbent assays (ELISAs) as previously described (19–22). The ELISA for each condition was performed in duplicate. The levels of IFN-γ and IL-4 were calculated by comparison with a standard curve generated with recombinant murine IFN-γ (1 U = 153 pg) (provided by Genentech, South San Francisco, Calif.) or recombinant murine IL-4 (1 U = 45 pg) (provided by DNAX, Palo Alto, Calif.). ELISA sensitivities for IFN-γ and IL-4 were 0.025 ng/ml and 3.0 U/ml, respectively.
In vivo challenge experiments.
Eight groups of mice (four or five mice per group) received initial-immunization solutions described above at a final volume of 50 μl in the right footpads only. Ten days later, secondary-immunization solutions diluted in PBS to a final volume of 25 μl were injected subcutaneously in the left flank region. The concentrations of BeSO4 and IL-12 were adjusted so that the mice received the same doses of these reagents as in the initial immunizations. Mice injected with PBS alone served as negative controls. Two weeks after the secondary booster immunizations, each mouse was challenged with 105 purified metacyclic L. major promastigotes into the right footpad. The size of the parasitic lesion was determined by measuring the thickness of the infected right footpad with a dial caliper (L. S. Starrett Co., Athol, Mass.) and then subtracting the thickness of the contralateral uninfected footpad. The mice were monitored for 54 days, at the end of which parasitic load in the footpad lesions and SPLs was determined. The total number of parasites in the lesions was determined by limiting-dilution analysis (46). Briefly, the infected foot of each mouse was extracted and the parasites were released from the lesions with a tissue homogenizer after removing the skin of the footpads. Parasites from individual mouse footpad lesions were cultured in 1:10 serial dilutions in complete parasite medium (Grace's insect cell culture medium supplemented with 20% fetal calf serum). Eight days later, parasitic growth in limiting dilutions was visually scored as having growth or no growth.
Statistical analyses.
An unpaired Student t test was performed to determine the significance of LN and SPL cell yields among mice receiving different immunizations, and paired Student t tests were used to establish the significance of the ELISA results. The F test was used to compare the sizes of footpad lesions among mice with various immunizations. A P value of less than 0.05 was used to indicate significance.
RESULTS
Beryllium promotes an IFN-γ response to SLA in LN and SPL cells of BALB/c mice primed with SLA and IL-12.
BALB/c mice were immunized as described in Materials and Methods for groups i through iv. After 7 days, the SPLs and LNs were harvested. At the time of organ harvest, LNs of mice immunized with Be (groups ii and iv) were visibly larger while the SPLs showed no obvious differences. Cell yields were determined after pooling the organs by groups and creation of single-cell suspensions (Table 1). LNs from all the mice that were given Be yielded significantly greater numbers of cells than did those from the mice that were not (P < 0.05). No significant differences were noted in cell yields from SPLs (P > 0.3 as determined by the Student t tests). Cells were cultured with SLA, Be, SLA with Be, and the mitogen ConA (as positive control), and 72-h proliferation levels were measured by tritiated-thymidine uptake. LN and SPL cells from all four groups of mice proliferated extremely well in response to the SLA and to ConA, as expected for the four groups of mice (Table 2 and data not shown); however, among the four groups, no significant differences in the proliferative capacities were observed. None of the cells responded to Be alone, and Be cocultured with SLA had no additive effects on the proliferative responses to SLA alone.
TABLE 1.
LN and SPL cell yields after immunizationa
| Groupb | 106 cell yieldc from:
|
|
|---|---|---|
| LN | SPL | |
| i (n = 5) | 11.4 ± 1.6 | 78.4 ± 23.5 |
| ii (n = 4) | 26.5 ± 3.7d | 56.1 ± 5.4 |
| iii (n = 3) | 12.9 ± 2.8 | 106.0 ± 33.0 |
| iv (n = 3) | 30.5 ± 6.2d | 99.1 ± 12.9 |
BALB/c mice were inoculated in each footpad as described in Materials and Methods for groups i through iv. Seven days later, LNs and SPLs were harvested from three or five mice per group per experiment and cell counts were determined. Unpaired Student t tests were performed for all statistical analyses to compare differences in LN and SPL yields among the four groups.
n, number of experiments.
Cell yields are shown as mean yield per mouse ± standard error of the mean.
P < 0.05 compared to comparable LN cells immunized without beryllium.
TABLE 2.
LN cell proliferative responses after immunizationa
| Groupb | LN cell response (cpm) after stimulation withc:
|
||||
|---|---|---|---|---|---|
| Control | ConA | SLA | SLA + Be | Be | |
| i | 1,401 ± 113 | 75,732 ± 4,806 | 28,503 ± 2,001 | 30,207 ± 776 | 1,252 ± 438 |
| ii | 1,384 ± 117 | 110,482 ± 17,498 | 36,241 ± 2,317 | 40,176 ± 908 | 1,411 ± 249 |
| iii | 2,335 ± 362 | 85,877 ± 2,008 | 48,916 ± 1,990 | 47,706 ± 1,485 | 2,173 ± 338 |
| iv | 1,788 ± 158 | 117,732 ± 7,318 | 54,177 ± 4,461 | 47,590 ± 6,664 | 1,600 ± 109 |
Mice were immunized as described in Table 1, footnote a. Seven days after immunization, LNs and SPLs were harvested from three or five mice per group per experiment, cells were pooled, and single-cell suspensions were made for each group and plated at 2.5 × 105 cells per well. Cells were restimulated for 3 days with ConA, SLA (20 μg), BeSO4 (100 μg), or SLA plus BeSO4. Cell proliferation was measured by determining the tritiated-thymidine uptake after 12 to 18 h. The results are representative of one complete experiment where LN cells from all four groups of mice were stimulated with ConA, SLA, SLA plus Be, and Be. The LN cell proliferative responses to ConA and SLA stimulations were assayed in at least two other independent experiments, and the results were comparable to those presented in this table.
Groups i to iv as defined in Materials and Methods.
Results are means ± standard errors of the mean of triplicate determinations.
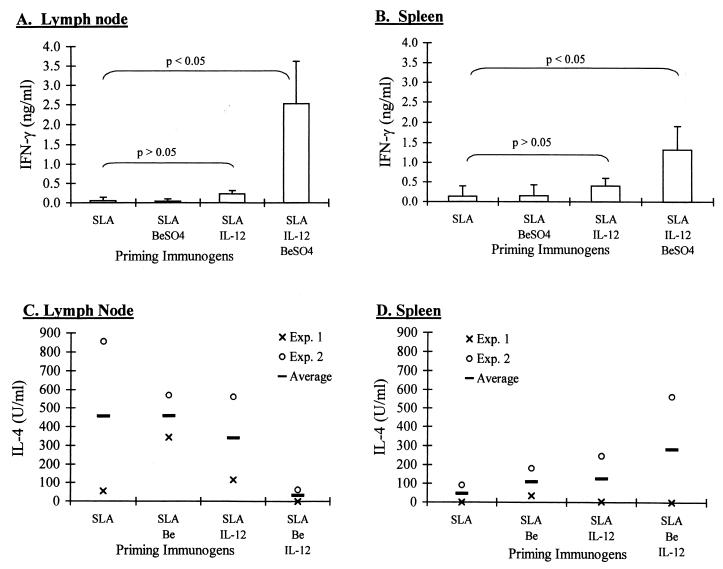
Since Be has been shown to induce a Th1 response in humans, the phenotypes of the immune response in these four groups of mice were determined by analyzing the cytokine profiles of the restimulated LN and SPL cells. The levels of IFN-γ and IL-4 secreted by cells were measured by ELISA. SLA-restimulated LN and SPL cells from mice immunized with SLA alone and with SLA plus Be produced IFN-γ at background levels, comparable to the amount produced by unstimulated LN cells (Fig. 1A and B). Cells from mice immunized with SLA plus IL-12 produced slightly higher but not significant levels of IFN-γ compared to background. Immunizing with Be and IL-12 plus SLA, however, significantly (P < 0.05) increased IFN-γ secretions from SLA-restimulated LN cells compared to background and all other groups (Fig. 1A). For SPL cells, the outcomes were not as dramatic (Fig. 1B). While the amounts of IFN-γ produced by restimulated SPL cells from SLA-, Be-, and IL-12-sensitized mice were significantly greater than those produced by cells from mice sensitized with SLA alone and SLA plus Be, they were not as significantly different (P = 0.06) compared to the amounts produced by SLA- and IL-12-sensitized SPL cells.
FIG. 1.
IFN-γ and IL-4 secretion after SLA restimulation of LN and SPL cells of BALB/c mice primed with the same combinations of SLA, IL-12, and BeSO4 as in Table 1. LN cells (A and C) and SPL cells (B and D) were harvested and restimulated with SLA. Supernatants from restimulated cells were harvested 3 days after culture, and cytokine levels were measured by sandwich ELISA in duplicate. IFN-γ production was increased in LN cells (A) and SPL cells (B) of mice primed with SLA, IL-12, plus BeSO4 upon in vitro restimulation with SLA. Paired Student t tests were performed for statistical analyses. The mean of three separate experiments is illustrated in panels A and B, each with three to five mice per immunization group. Only two experiments were done, with three to five mice for each condition, in panels C and D.
IL-4, an indicator of a Th2-type immune response, was secreted from SLA-restimulated lymph node cells of mice in immunization groups i to iii (Fig. 1C). LN cells from mice in group iv, however, produced much less IL-4. This synergistic effect of Be and IL-12 in down-regulating IL-4 production was not seen in SPL cells (Fig. 1D). Interestingly, IL-4 production appeared to be increased in one of two experiments in SLA-restimulated SPL cells from SLA-, Be-, and IL-12-sensitized mice despite a down-regulation in the lymph node.
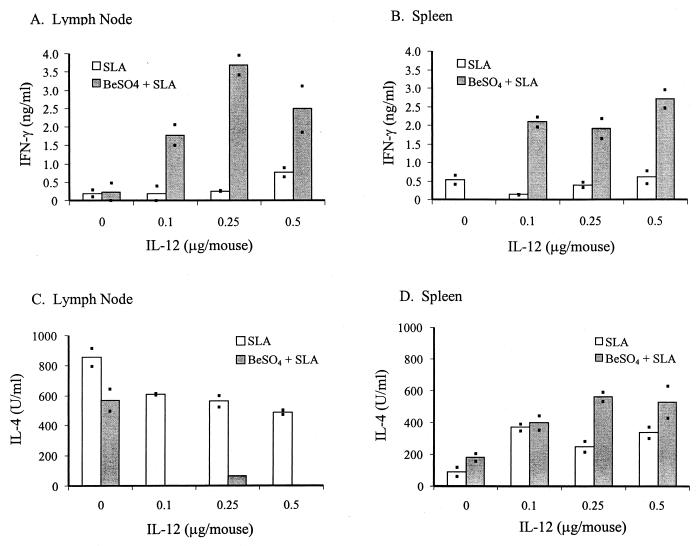
This synergistic effect of IL-12 and Be was also observed with different doses of IL-12. Eight groups of mice (three mice per group) were immunized as described in Materials and Methods for groups i through viii. Seven days later, LNs and SPLs were harvested and the cells were cultured and restimulated as described in Materials and Methods. Priming with Be and a range of IL-12 concentrations increased IFN-γ production in SLA-stimulated LN and SPL cells compared to immunizations without Be (Fig. 2A and B). Similar to Figure 1, priming with Be and different IL-12 doses plus SLA led to a decrease in IL-4 production by SLA-stimulated LN cells but not SPL cells (Fig. 2C and D). In contrast to the LN cells, SPL cells produced comparable levels of IL-4 regardless of whether they were harvested from mice primed with or without Be (Fig. 2D). These results once again suggest that beryllium exerts a specific adjuvant effect that is characterized by increased IFN-γ and decreased IL-4 production. This effect was present in LNs, while only increased IFN-γ production was seen in SPLs.
FIG. 2.
Priming with BeSO4 and different concentrations of IL-12 on IFN-γ production in LN and SPL cells upon in vitro restimulation with SLA. Eight groups of mice (three mice per group) were immunized (groups i to viii as described in Materials and Methods). LN and SPL cells from these mice were prepared, and cytokine productions were analyzed as described for Fig. 1. Each bar represents the amount IFN-γ (A and B) or IL-4 (C and D) secreted by SLA-restimulated LN (A and C) or SPL (B and D) cells of a mouse group. Open bars represent cytokines produced by cells from mice immunized with SLA and IL-12; shaded bars represent cytokines produced by cells from mice immunized with SLA, IL-12, plus BeSO4. Cytokine production was measured in duplicate (represented by the points) by ELISA, and the results were averaged as illustrated by the histograms.
Immunizations with beryllium and SLA plus IL-12 allow mice to control parasitic infections.
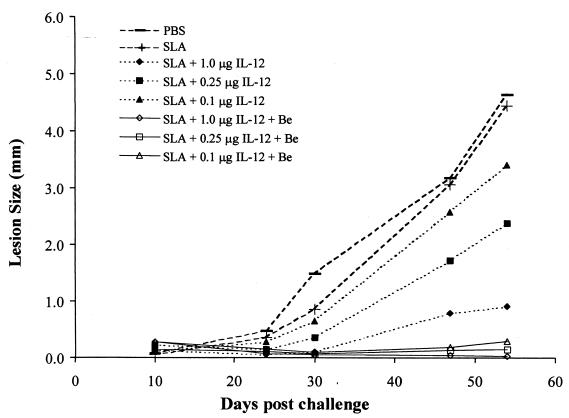
To investigate the effects of Be in vivo, mice were immunized as described in Materials and Methods for groups i, iii, iv, v, vii, ix, and x, along with a PBS control. The mice received secondary booster inoculations with the same regimens and were later challenged with virulent parasites. Figure 3 depicts the course of infection compiled from determining lesion sizes in the footpads. Four weeks after parasite challenge, the control mice (those immunized with PBS or SLA) exhibited gross pathology of inflamed footpads. The lesions were large compared to those from mice immunized with SLA plus IL-12 (Fig. 3). The lesion sizes correlated with the amount of IL-12 the mice received; i.e., mice immunized with the largest dose of IL-12 plus SLA developed smaller lesions than did those immunized with lower doses. Strikingly, mice immunized with SLA, IL-12, plus Be developed minimal to almost no lesions. This was seen in all the mice immunized with Be, regardless of the dose of IL-12 they initially received. More specifically, by day 31, all animals immunized with IL-12 or with IL-12 plus Be had significantly smaller lesions than did the SLA or PBS controls, except for the mice sensitized with 0.1 μg of IL-12 (group v) (P < 0.01). Also, 31 days after infection, all animals initially immunized with Be plus 0.1 or 0.25 μg of IL-12 (group v) had smaller lesions than did animals immunized with these concentrations of IL-12 alone (groups iii and v) (P < 0.01). By day 54, the animals immunized with Be and the highest dose of IL-12 (1.0 μg) (group x) had significantly smaller lesions than did animals immunized with 1.0 μg of IL-12 alone (group ix) (P < 0.01). By week 8 of infection, control mice and mice initially immunized with SLA and the lower doses of IL-12 had necrotic paws while mice immunized with SLA plus IL-12 and Be appeared normal with nondistinguishable footpads.
FIG. 3.
Effect of immunization with BeSO4 and IL-12 on footpad swelling after L. major infection in BALB/c mice. Eight groups of mice (four or five mice per group) were immunized: PBS (control), SLA (group i), SLA and 0.1 μg of IL-12 (group v), SLA and 0.25 μg of IL-12 (group iii), SLA and 1.0 μg of IL-12 (group ix), SLA plus BeSO4 and 0.1 μg of IL-12 (group vii), SLA plus BeSO4, and 0.25 μg of IL-12 (group iv), and SLA plus BeSO4, and 1.0 μg of IL-12 (group x). As described in Materials and Methods, the mice were boosted with the same regimen 7 days after initial immunizations and challenged with parasites 2 weeks thereafter. For all lesion sizes greater than 1.0 mm, the average coefficient of variance was 0.27 (range, 0.14 to 0.37). For all lesion sizes less than 1.0 mm, the average standard deviation was 0.14 (range, 0.00 to 0.58). Each data point represents the mean of lesion sizes for four or five mice. The F test was used for comparisons.
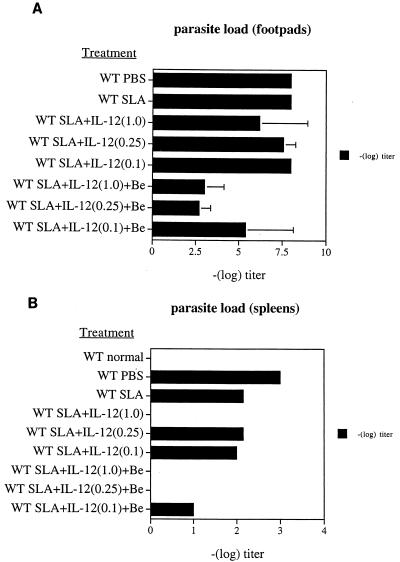
The parasitic load was quantified in the footpads and SPLs of these mice by limiting-dilution analysis 8 weeks after Leishmania challenge. Beryllium had no effect on the in vitro growth of parasites in the limiting-dilution assay (data not shown). Footpad lesions from control mice (those immunized with PBS or SLA alone) contained more than 108 parasites. These mice were unable to control or eliminate parasites from the lesions (Fig. 4A). This was also seen in mice immunized with SLA and the lowest dose of IL-12. Mice immunized with SLA and higher doses of IL-12 were better able to control parasite proliferation. Moreover, mice immunized with SLA, IL-12, and Be were best able to control and eliminate parasites, as demonstrated by the lower parasite burden in their footpad lesions. Similar results were seen in the SPL (Fig. 4B). With the addition of beryllium to the immunization regimens that also included SLA and IL-12, mice were better able to control parasitic expansion and eliminate parasites from the lesions upon Leishmania challenge.
FIG. 4.
Parasitic load in footpads (A) and SPLs (B) of mice immunized after L. major infection. The experiment was done as described in Materials and Methods and in the legend to Fig. 3. Parasitic quantification in panel A represents the mean for four or five mice, and error bars denote standard deviation.
DISCUSSION
In this study, we demonstrated that beryllium has an adjuvant effect with IL-12 against SLA in the induction of IFN-γ up-regulation and IL-4 down-regulation, characteristic of a Th1 type of immune response. In vitro SLA restimulation of LN cells from mice immunized with Be, IL-12, plus SLA led to the production of significant amounts of IFN-γ, although no specific immune response to beryllium was observed. Immunization with the similar regimens was able to give these mice the ability to suppress lesion development and control parasitic expansion upon Leishmania promastigote challenge. Beryllium alone had no significant effects, but it had an adjuvant effect with IL-12 in promoting IFN-γ production in BALB/c mice. Immunizations with Be, SLA, plus IL-12 generated a more efficacious response than did immunizations with only SLA plus IL-12. This was observed in the increased IFN-γ production by in vitro SLA-restimulated lymphocytes and splenocytes and in the ability of lower doses of IL-12 to suppress lesion development in the presence of Be.
We have shown for the first time that beryllium has adjuvant activities for the production of IFN-γ, a Th1 cytokine; prior studies have demonstrated only nonspecific adjuvant actions of beryllium. In Parkes mice, Be stimulated the production of immunoglobulin G2b by spleen cells (21, 22). Immunization of BALB/c mice with human gamma globulin in the presence of BeSO4 increased IL-5, IL-6, and IL-2 mRNA production from splenocytes (47). Expansion of the T-cell zone was found in Swiss CD-1 mice 12 to 24 h after injection of Be (26). These studies also demonstrated that beryllium exhibited similar effects to synthetic polyribonucleotide complexes, poly(A-U). The adjuvant activities of poly(A-U) induce a rapid release of IFN-γ from Th1 cells when it is administered with antigen and increase the number of antibody-forming plasma cells (14, 18, 28). It is not known whether poly(A-U) or beryllium directly increases IFN-γ production. Moreover, it is unlikely that the up-regulation of IFN-γ is the sole cause of the adjuvant activities of Be. Our data show that vigorous adjuvant effects were seen when IL-12 was present in the initial sensitization. We speculate that Be may enhance the ability of IL-12 to promote IFN-γ production. However, no difference in the amount of IFN-γ produced was detected when naive BALB/c spleen cells were cultured with Be and IL-12 compared to cultures with IL-12 alone (23).
L. major is an obligate intracellular protozoan that infects mammalian Mφ. In Leishmania-resistant mice (C3H, C57BL/6, B10.02, and 129 strains), IL-12, produced soon after infection, induces the production of IFN-γ by natural killer (NK) cells (36). IFN-γ initiates the differentiation of CD4+ cells toward the Th1 phenotype, drives further IFN-γ production by T cells, and feeds back to Mφs for further production of IL-12. This cascade serves to establish a strong Th1 or cell-mediated immune response, which stimulates Mφ leishmanicidal activities and is the means by which resistant mice resolve their infectious lesions (35, 37, 38). In susceptible mice (BALB/c), the inability to heal Leishmania infection is associated with a persistent IL-4-dominated Th2 response and an inability to generate an effective Th1 response (15). Furthermore, the inability to up-regulate the expression of IL-12 receptors renders naive cells unable to mount a Th1 response, and mice eventually die due to uncontrollable parasitic expansion (19, 37). One role that beryllium may play to promote the production of IFN-γ and perhaps the Th1 response in our model may involve the cytokines or cytokine receptors that are critical for Th1 responses. However, stimulating activated peritoneal Mφ with Be did not directly elicit IL-12 production (23). Beryllium must have other modulatory effects on the expression of other proinflammatory cytokines and/or their receptors that would promote leishmanicidal responses.
The effect of beryllium on cytokine profiles during Leishmania infection in mice may be compared to the cytokine profiles of patients with CBD and individuals exposed or sensitized to beryllium. In the serum and bronchoalveolar lavage fluid (BALF) of CBD patients and individuals sensitized to Be, several cytokines and their receptors have been detected at significantly higher than normal levels. The increase in production of Th1 cytokines (IFN-γ and IL-2) but not Th2 cytokines (IL-4) in the Be-stimulated BAL cells of CBD patients is the most convincing evidence that Be stimulates a Th1-type immune response in humans (44). Significantly increased TNF-α and IL-6 protein and mRNA levels have also been detected by reverse transcription-PCR in BALF and serum of patients (5, 44, 45). TNF-α and IL-6, along with IL-1β, are critical proinflammatory mediators in granulomatous diseases. Combinations of IL-1, IL-6, and TNF-α have enhancing activities on other cytokines, particularly the secretion of IL-2 from established effector T cells (20). Increased IL-1β protein and mRNA levels in human monocytic THP-1 cells have been detected after incubation with Be (10). Also, in the serum and/or BALF of CBD patients, higher levels of soluble TNF receptor I (45) and the α subunit of the IL-2 receptor, crucial for T-cell proliferation and activation, have been demonstrated (44). The effects of Be on antigen-stimulated IFN-γ production may influence multiple cytokine cascades and feedback loops, in addition to the most notable IFN-γ/IL-12 loop between Mφ and Th1 lymphocytes.
Aside from the presence of specific cytokines, other factors may be modulated by beryllium to favor the cell-mediated response. Beryllium up-regulated the murine major histocompatibility complex expression in peritoneal Mφs as did lipopolysaccharides and complete Freund's adjuvant (4). This may modify antigen presentation by increasing the exposure of antigen to T cells. In BALF cells from CBD patients, while Be failed to up-regulate HLA expression, it did inhibit the IL-10-mediated down-regulation of HLA-DR expression (43). Beryllium may also be involved in modulating the amount of antigen detected by the immune system. Granulomas (aggregates of monocyte-derived epithelioid cells, Langhan giant cells, and lymphocytes [30]) formed as a result of the presence of Be near the sites of injection (17, 48; unpublished observations) may be capable of concentrating antigens for gradual release, leading to IFN-γ production and a Th1 response. Indeed, low doses of antigen released slowly have allowed the development of increased effectiveness of the immune system (6, 25). Beryllium has also been demonstrated to rapidly recruit effector cells such as neutrophils near the site of response (4) and to stimulate the migration of peripheral blood lymphocytes in chemotaxis chambers in a Be salt-specific manner (34). The cell types that constitute the visibly larger LNs in our immunized mice, as well as the chemoattractants and chemokines released from the Be-induced granulomas, remain to be determined.
Beryllium may also exert its effects at the molecular level. It may bind to the major histocompatibility complex-associated peptide complex and alter the affinity of the T-cell receptor, as shown with the metals gold and nickel (12, 41). If the result of antigen processing can discriminate between the production of Th1 and Th2 cytokine subsets and if Be can alter the type of antigenic peptides produced, then a potential mechanism for the adjuvant effect of Be would be that it binds to L. major peptides and modifies T-cell recognition to favor Th1 cytokine production. Beryllium may interfere with the activities of intracellular enzymes. It can complex with fluoride to form BeF3−, which is isomorphous to a phosphate group, creating an analog to mimic the γ-phosphate group and to place proteins in the active conformation. This has been described for beryllium in Gt proteins (transducin), tubulin, actin, myosin, mitochondrial ATPase, p21ras, cdc42, adenylate kinase, and inositol phosphatase (3, 8, 9, 11, 16). Perhaps the ability of Be to interfere with various signaling events directs the immune system toward the Th1 cytokine response.
The mechanism by which beryllium serves as an adjuvant for IFN-γ (a Th1 cytokine), shown here for the first time, is obscure. Nevertheless, the finding has several important implications. First, the adjuvant properties of beryllium may be important for our understanding of why only a Th1 immune response to beryllium has been observed in humans. This may have significant implications for the mechanisms of granulomatous disease in general. Second, the ability of Be to synergize with IL-12 to promote Th1 cytokine production may have clinical relevance for the development of safe and effective immunization protocols. The vaccines produced by recombinant DNA technology or attenuated or inactive bacteria are often poorly immunogenic, creating a need for safe and effective adjuvants (29, 49). The ability of beryllium to synergize with IL-12 may be an important factor in the development of such adjuvants.
ACKNOWLEDGMENTS
This work was supported by grants AI-35914 to P. Scott and HL-48210 to M. Rossman from the National Institutes of Health.
We thank Yvonne Paterson and Giorgio Trinchieri for critical review of the manuscript and Mary McNichol for clerical assistance. We also thank Stanley Wolf for the gift of recombinant IL-12.
REFERENCES
- 1.Abbas A K, Murphy K M, Sher A. Functional diversity of helper T lymphocytes. Nature. 1997;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 2.Afonso L C C, Scharton T M, Vieira L Q, Wysocka M, Trinchieri G, Scott P. The adjuvant effect of interleukin-12 in a vaccine against Leishmania major. Science. 1994;263:235–237. doi: 10.1126/science.7904381. [DOI] [PubMed] [Google Scholar]
- 3.Antonny B, Chabre M. Characterization of the aluminum and beryllium fluoride species which activate transducin, analysis of the binding and dissociation kinetics. J Biol Chem. 1992;267:6710–6718. [PubMed] [Google Scholar]
- 4.Behbehani K, Beller D I, Unanue E R. The effects of beryllium and other adjuvants on Ia expression by macrophages. J Immunol. 1985;134:2046–2049. [PubMed] [Google Scholar]
- 5.Bost T W, Riches D W H, Schumacher B, Carre P C, Khan T Z, Martinez J A B, Newman L S. Alveolar macrophages from patients with beryllium disease and sarcoidosis express increased levels of mRNA for tumor necrosis factor-α and interleukin-6 but not interleukin-1β. Am J Respir Cell Mol Biol. 1994;10:506–513. doi: 10.1165/ajrcmb.10.5.8179912. [DOI] [PubMed] [Google Scholar]
- 6.Bretscher P A, Wei G, Menon J N, Bielefeldt-Ohmann H. Establishment of stable, cell-mediated immunity that makes “susceptible” mice resistant to Leishmania major. Science. 1992;257:539–542. doi: 10.1126/science.1636090. [DOI] [PubMed] [Google Scholar]
- 7.Deodhar S D, Barna B, Van Ordstrand H S. A study of the immunologic aspects of chronic berylliosis. Chest. 1973;63:309–313. doi: 10.1378/chest.63.3.309. [DOI] [PubMed] [Google Scholar]
- 8.Diaz J F, Sillen A, Engelborghs Y. Equilibrium and kinetic study of the conformational transition toward the active state of p21Ha-ras, induced by the binding of BeF3- to the GDP-bound state, in the absence of GTPase-activating proteins. J Biol Chem. 1997;272:23138–23143. doi: 10.1074/jbc.272.37.23138. [DOI] [PubMed] [Google Scholar]
- 9.Faraci W S, Zorn S H, Bakker A V, Jackson E, Pratt K. Beryllium competitively inhibits brain myo-inositol monophosphatase, but unlike lithium does not enhance agonist-induced inositol phosphate accumulation. Biochem J. 1993;291:369–374. doi: 10.1042/bj2910369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galbraith G M P, Pandey J P, Schmidt M G, Arnaud P, Goust J-M. Tumor necrosis factor alpha gene expression in human monocytic THP-1 cells exposed to beryllium. Arch Environ Health. 1996;51:29–33. doi: 10.1080/00039896.1996.9935990. [DOI] [PubMed] [Google Scholar]
- 11.Garin J, Vignais P V. Characterization of the inhibition of rabbit muscle adenylate kinase by fluoride and beryllium ions. Biochemistry. 1993;32:6821–6826. doi: 10.1021/bi00078a004. [DOI] [PubMed] [Google Scholar]
- 12.Griem P, von Vultee C, Panthel K, Best S L, Sadler P J, Shaw C F., III T cell cross-reactivity to heavy metals: identical cryptic peptides may be presented from protein exposed to different metals. Eur J Immunol. 1998;28:1941. doi: 10.1002/(SICI)1521-4141(199806)28:06<1941::AID-IMMU1941>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 13.Hall J G, Spencer J O. Studies on the adjuvant actions of beryllium II. Systemic effects with particular reference to secretory immunity. Immunology. 1984;53:115–120. [PMC free article] [PubMed] [Google Scholar]
- 14.Han I H, Johnson A G. Regulation of the immune system by synthetic polynucleotides. VI. Amplification of the immune response in young and aging mice. J Immunol. 1976;117:423–427. [PubMed] [Google Scholar]
- 15.Heinzel F P, Sadick M D, Holaday B J, Coffman R L, Locksley R M. Reciprocal expression of interferon γ and interleukin 4 during the resolution or progression of murine leishmaniasis. J Exp Med. 1989;169:59. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffman G R, Nassar N, Oswald R E, Cerione R A. Fluoride activation of the Rho family GTP-binding protein Cdc42Hs. J Biol Chem. 1998;273:4392–4399. doi: 10.1074/jbc.273.8.4392. [DOI] [PubMed] [Google Scholar]
- 17.Huang H, Meyer K, Kubai L, Auerback R. An immune model of beryllium-induced pulmonary granulomata in mice. Lab Investig. 1992;67:138–146. [PubMed] [Google Scholar]
- 18.Johnson A G. Molecular adjuvants and immunomodulators: new approaches to immunization. Clin Microbiol Rev. 1994;7:277–289. doi: 10.1128/cmr.7.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones D, Elloso M M, Showe L, Williams D, Trinchieri G, Scott P. Differential regulation of both the IL-12 receptor β1 and β2 subunits during the innate immune response to Leishmania major. Infect Immun. 1998;66:3818. doi: 10.1128/iai.66.8.3818-3824.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joseph S B, Miner K T, Croft M. Augmentation of naïve, Th1 and Th2 effector CD4 responses by IL-6, IL-1, and TNF. Eur J Immunol. 1998;28:277–289. doi: 10.1002/(SICI)1521-4141(199801)28:01<277::AID-IMMU277>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 21.Karagouni E E, Hadjipetrou-Kourounakis L. Regulation of isotype immunoglobulin and interleukin production by adjuvants in vitro. J Clin Lab Immunol. 1990;33:29–39. [PubMed] [Google Scholar]
- 22.Karagouni E E, Hadjipetrou-Kourounakis L. Regulation of isotype immunoglobulin production by adjuvants in vivo. Scand J Immunol. 1990;31:745–754. doi: 10.1111/j.1365-3083.1990.tb02826.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee J Y, Taylor L, King B, Scott P, Rossman M. Beryllium: an adjuvant that promotes Th1 cytokines. Am J Respir Crit Care Med. 1997;155:A760. [Google Scholar]
- 24.Marx J J, Burrell R. Delayed hypersensitivity to beryllium compounds. J Immunol. 1973;111:590–598. [PubMed] [Google Scholar]
- 25.Menon J N, Bretscher P A. Characterization of the immunological memory state generated in mice susceptible to Leishmania major following exposure to low doses of L. major and resulting in resistance to a normally pathogenic challenge. Eur J Immunol. 1996;26:243–249. doi: 10.1002/eji.1830260138. [DOI] [PubMed] [Google Scholar]
- 26.Moatamed F, Karnovsky M J, Unanue E R. Early cellular responses to mitogens and adjuvants in the mouse spleen. Lab Investig. 1975;32:303–312. [PubMed] [Google Scholar]
- 27.Mossman T R, Cherwinski H, Bond M W, Giedlin M A, Coffman R L. Two types of murine helper T cell clone, I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 28.Odean M J, Frane C M, Van derVieren M, Tomai M A, Johnson A G. Involvement of gamma interferon in antibody enhancement by adjuvants. Infect Immun. 1990;58:427–432. doi: 10.1128/iai.58.2.427-432.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Hagan D. Recent advances in vaccine adjuvants for systemic and mucosal administration. J Pharm Pharmcol. 1997;49:1. doi: 10.1111/j.2042-7158.1998.tb03298.x. [DOI] [PubMed] [Google Scholar]
- 30.Rossman M D, Jones-Williams W. Immunopathogenesis of chronic beryllium disease. In: Rossman M D, Preuss O P, Powers M B, editors. Beryllium: biomedical and environmental aspects. Baltimore, Md: The Williams & Wilkins Co.; 1991. pp. 121–132. [Google Scholar]
- 31.Rossman M D, Kern J A, Elias J A, Cullen M R, Epstein P E. Proliferative response of bronchoalveolar lymphocytes to beryllium. Ann Intern Med. 1988;108:687–693. doi: 10.7326/0003-4819-108-5-687. [DOI] [PubMed] [Google Scholar]
- 32.Sacks D L, Hieny S, Sher A. Identification of cell surface carbohydrate and antigen changes between noninfective and infective developmental stages of Leishmania major promastigotes. J Immunol. 1985;135:564. [PubMed] [Google Scholar]
- 33.Saltini C, Winestock K, Kirby M, Pinkston P, Crystal R G. Maintenance of alveolitis in patients with chronic beryllium disease by beryllium-specific helper T cells. N Engl J Med. 1989;320:1103–1109. doi: 10.1056/NEJM198904273201702. [DOI] [PubMed] [Google Scholar]
- 34.Sawyer R T, Doherty D E, Schumacher B A, Newman L S. Beryllium-stimulated in vitro migration of peripheral blood lymphocytes. Toxicology. 1999;138:155–163. doi: 10.1016/s0300-483x(99)00101-8. [DOI] [PubMed] [Google Scholar]
- 35.Scharton-Kersten T, Afonso L C C, Wysocka M, Trinchieri G, Scott P. IL-12 is required for natural killer cell activation and subsequent T helper 1 cell development in experimental leishmaniasis. J Immunol. 1995;154:5320–5330. [PubMed] [Google Scholar]
- 36.Scharton-Kersten T, Scott P. The role of the innate immune response in Th1 cell development following Leishmania major infection. J Leukoc Biol. 1995;57:515–522. doi: 10.1002/jlb.57.4.515. [DOI] [PubMed] [Google Scholar]
- 37.Scott P. Differentiation, regulation, and death of T helper cell subsets during infection with Leishmania major. Immunol Res. 1998;17:229–238. doi: 10.1007/BF02786447. [DOI] [PubMed] [Google Scholar]
- 38.Scott P. IFN-γ modulates the early development of Th1 and Th2 responses in a murine model of cutaneous leishmaniasis. J Immunol. 1991;147:3149–3155. [PubMed] [Google Scholar]
- 39.Scott P, Pearce E, Natovita P, Sher A. Vaccination against cutaneous Leishmaniasis in a murine model. II. Immunologic properties of protective and nonprotective subfractions of a soluble promastigote extract. J Immunol. 1987;139:3118–3125. [PubMed] [Google Scholar]
- 40.Seder R A, Paul W E. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu Rev Immunol. 1994;12:635–673. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 41.Sinigaglia F. The molecular basis of metal recognition by T cells. J Investig Dermatol. 1994;102:398–401. doi: 10.1111/1523-1747.ep12372149. [DOI] [PubMed] [Google Scholar]
- 42.Sterner J H, Eisenbud M. Epidemiology of beryllium intoxication. Arch Ind Hyg Occup Med. 1961;4:123. [PubMed] [Google Scholar]
- 43.Tinkle S S, Kittle L A, Newman L S. Partial IL-10 inhibition of the cell mediated immune response in chronic beryllium disease. J Immunol. 1999;163:2747–2753. [PubMed] [Google Scholar]
- 44.Tinkle S S, Kittle L A, Schumacher B A, Newman L S. Beryllium induces IL-2 and IFN-γ in berylliosis. J Immunol. 1997;158:518–526. [PubMed] [Google Scholar]
- 45.Tinkle S S, Newman L S. Beryllium stimulated release of tumor necrosis factor-(alpha), interleukin-6, and their soluble receptors in chronic beryllium disease. Am J Respir Crit Care Med. 1997;156:1884–1891. doi: 10.1164/ajrccm.156.6.9610040. [DOI] [PubMed] [Google Scholar]
- 46.Titus R G, Marchand M, Boon T, Louis J A. A limiting dilution assay for quantifying Leishmania major in tissue of infected mice. Parasite Immunol. 1985;7:545–555. doi: 10.1111/j.1365-3024.1985.tb00098.x. [DOI] [PubMed] [Google Scholar]
- 47.Victoratos P, Yiangou M, Avramidis N, Hadjipetrou L. Regulation of cytokine gene expression by adjuvants in vivo. Clin Exp Immunol. 1997;109:569–578. doi: 10.1046/j.1365-2249.1997.4631361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Votto J J, Barton R W, Gionfriddo M A, Cole S R, McCormick J R, Thrall R S. A model of pulmonary granulomata induced by beryllium sulfate in the rat. Sarcoidosis. 1987;4:71–76. [PubMed] [Google Scholar]
- 49.Warren H S, Chedid L A. Future prospects for vaccine adjuvants. Crit Rev Immunol. 1998;8:83. [PubMed] [Google Scholar]
- 50.Wedrychowicz H, Bezubik B. Influence of adjuvants on immunity in rabbits vaccinated with infective larval somatic proteins of Trichostrongylus colubriformis. Vet Parasitol. 1990;37:273–284. doi: 10.1016/0304-4017(90)90010-9. [DOI] [PubMed] [Google Scholar]
- 51.Wedrychowicz H, Romanik I, Szczygielska E, Bezubik B. The effects of adjuvant and specific or non-specific vaccination on development of protective immunity of rabbits against Trichostrongylus colubriformis infection. Int J Parasitol. 1992;22:991–996. doi: 10.1016/0020-7519(92)90058-s. [DOI] [PubMed] [Google Scholar]