Abstract
1H-indol-2,3-dione (isatin) class of biologically active compounds have analgesic, anti-microbial, anti-inflammatory, anti-tubercular, anti-proliferative properties, and is also useful for the treatment of SARS-CoV. Schiff bases containing isatin moiety are known to have broad spectrum of biological activities like anti-viral, anti-tubercular, anti-fungal, and anti-bacterial. In this work, several Schiff base derivatives have been synthesized using two methods (synthetic and microwave) by reacting isatin with o-phenylenediamine. The synthesized compounds were structurally characterized and their in-vivo antimicrobial activity was tested against Gram-negative and Gram-positive bacteria using the inhibition zone method. Several newly synthesized isatin derivatives were found effective as antimicrobial agents and showed good potency (compounds 3c, 3d, 6a, 6b, 6d). Compound 3c displayed higher antimicrobial activity than standard drug (Amoxicillin) against Staphylococcus aureus at higher concentration (16 μg/mL) and against Escherichia coli at lower concentration (1 μg/mL).
Keywords: isatin, o-phenylenediamine, chemotherapeutic, amoxicillin, antimicrobial activity, antibacterial
Introduction
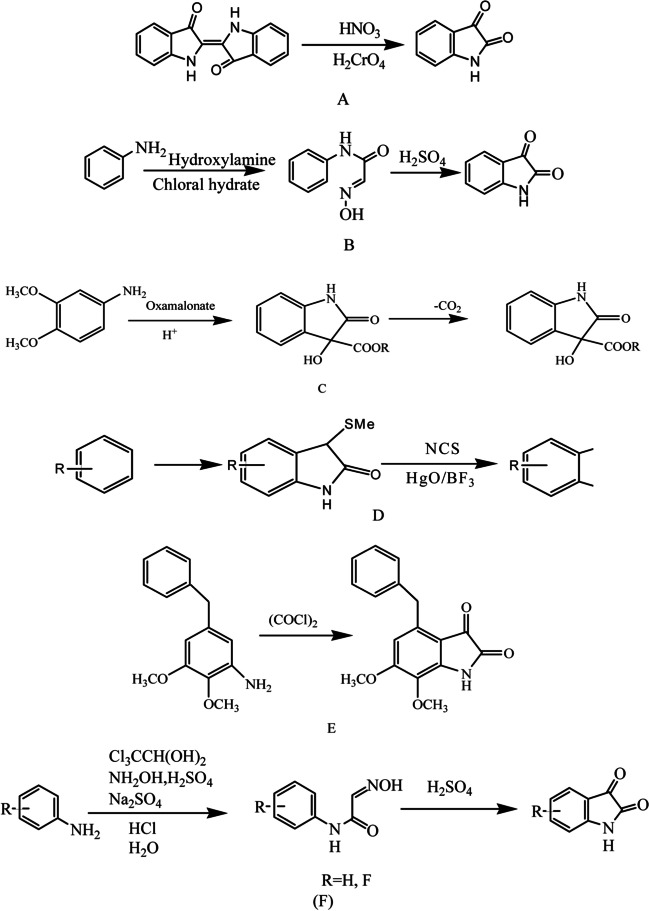
The discovery of antibiotics has been proved to be a blessing in the medical science field still limitation is resistance, there has emerged the need for new potent antibacterial agents. It is the need of time to improve the existing anti-microbials and develop new ones. In particular, isatin scaffold possesses analgesic, antimicrobial, anti-inflammatory, anti-tubercular, anti-proliferative, and many other properties. Isatin (1H-indole-2,3-dione) is a moiety of interest for medicinal chemists for the synthesis of heterocyclic compounds such as quinolines and indoles. It is an indole derivative that comprises two cyclic carbonyl groups (one five-membered and other six-membered, a good moiety for chemical conversion rings) and contains two carbonyl groups at 2nd and 3rd positions and nitrogen at 1st position (Fig. 1). It was discovered and synthesized by Laurent and Erdman (Fig. 2A) in 1841 by oxidizing indigo using nitric acid and chromic acids. Other scientists have discovered several different methods to synthesize isatins, including T. Sandmeyer (Fig. 2B) (the oldest and most widely used pathway to isatin which gives a good yield, although it is effective for small analog), Martinet (Fig. 2C), Gassman (Fig. 2D) and Stolle (Fig. 2E). Subsequently, G. S. Hiers and C. S. Marvel (Fig. 2F) modified Sandmeyer’s method in which aniline or substituted aniline was treated with hydrochloride, chloral hydrate, sulfate, or salts of hydroxylamine, which was made in a solution of sodium sulfate and then cyclization reaction proceeded in the presence of conc. H2SO4 [1–5].
Fig. 1.

Chemical structure of isatin.
Fig. 2.
Methods of isatin synthesis by methods of (A) Erdman and Laurent, (B) T. Sandmeyer, (C) Martinet, (D) Gassman, (E) Stolle, and (F) G. S. Hiers and C. S. Marvel.
Isatin and endogenous indoles have been found in the body fluids (adrenaline hormone) and mammalian tissues. It has been identified in urine, blood, and tissue using GC-MS and, more recently by HPLC with ultraviolet detector [1, 2]. Plants of genuses such as Isatis tinctoria, Couroupita guianensisisatis, and Calanthe discolor [4, 6–8] also contain isatin. It is also secreted from the Bufo frog’s parotid gland [5, 9]. It is one of the constituents of coal tar [3]. It has been reported that isatin possesses diverse biological properties such as antioxidant [10, 11], anti-inflammatory [12, 13], antimicrobial (bacterial, viral, fungal) [14–16], anti-tuberculosis [17, 18], anticancer [19, 20], anticonvulsant [21, 22], anti-HIV [23, 24] and many more. Isatin is also effective in treating coronavirus [25–27] and hepatitis C [27]. In addition to biological activities, it has several industrial uses as corrosion inhibitors, dyes, and fluorescence sensors [1].
Subsequent studies suggested that N-alkylation and substitution in the 5th position of isatin with strong electron-donating groups/atoms like bromine, fluorine, or chlorine produce a series of more active compounds compared to the parent compound [28]. Isatin substitution at 3rd position with aromatic or substituted aromatic ring contributes to antimicrobial activity. The phenyl ring moieties, heterocyclic rings, and alphabetic system have been other possible substitutions at 3rd position [29].
Chaithanya, et al. (2019) designed twenty new isatin derivatives by reacting with 2-methyl-benzimidazole and benzimidazole; and intermediates were obtained by condensing chloroacetyl chloride and o-phenylenediamine, and then designed compounds were screened for antimicrobial activity and only a few showed potent antifungal anti-bacterial activity (Fig. 3) [30].
Fig. 3.
Chemical structure of isatin benzimidazole derivatives.
Alsalihi, et al. (2018) synthesized several Schiff base derivatives by condensing isatin with 3-amino acetophenone in presence of KOH and then tested synthesized compounds for antibacterial activity, and a few were found potent (Fig. 4) [31].
Fig. 4.

Chemical structure of Schiff base derivatives of isatin.
Almutairi, et al. (2017) synthesized indole isatin derivatives using GAA as a catalyst [32]. The synthesized compounds were checked for antibacterial activity, and some were found potent (Fig. 5).
Fig. 5.

Chemical structure of indole isatin derivatives.
Bogdano, et al. (2015) designed Schiff base derivatives by condensing isatin and o-Phenylenediamine in the presence of ethanol, and the synthesized compound was tested for antibacterial activity and a few compounds were found to be potent (Fig. 6) [33].
Fig. 6.

Structure of Schiff base derivatives of isatin with o-phenylenediamine.
Alsalihi, et al. (2015) designed some new Schiff base derivatives of isatin using the microwave radiation method. The synthesized compounds were tested for antibacterial activity against Gram-negative and Gram-positive bacteria (Fig. 7) and were found potent [34].
Fig. 7.

Structure of Schiff base derivatives of isatin.
Singh, et al. (2010) designed some new Schiff and Mannich base derivatives of isatin in the presence of secondary amine and formaldehyde. The synthesized compounds were tested for antimicrobial activity and some were found more potent than the standard drug (Fig. 8) [35].
Fig. 8.

Structure of Schiff and Mannich base derivatives of isatin.
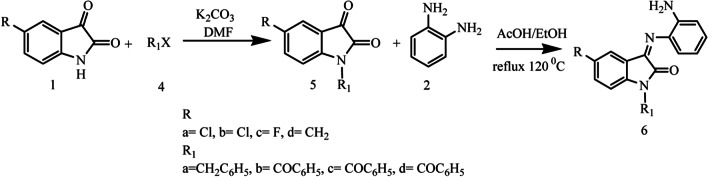
Since isatin derivatives are known to affect CNS [36] and show several biological activities, in the present study we have designed and synthesized isatin derivatives of 3-(2-aminophenylimino)-5-substituted-indoline-2-ones 3 (a–d) and 6 (a–d) by reacting isatin with o-phenylenediamine. The synthesized compounds were characterization by spectral techniques and screened for antimicrobial activity against selected bacteria.
Experimental Part
Materials and Methods
All reagents and chemicals used were of the analytical grade. Melting points were determined using the melting point apparatus (Buchi). The infrared spectra were recorded as KBr pellets on Agilent Technologies FT-IR (Cary 660) spectrophotometer. The NMR spectra were recorded on Bruker NMR spectrometer (300 MHz) using DMSO-d6 as solvent. The mass spectra were obtained on Bruker MS-632 instrument equipped with an electrospray ionization (ESI) system, using nebulizing gas (nitrogen) at a pressure of 0.3 bar and a flow rate of 4 L/min, at voltages in a range from –500 to 4500 V. The completion of all reactions was checked by method of thin-layer chromatography (TLC).
Synthesis of Isatin Derivatives
Preparation of 3-(2-aminophenylimino)-5-substituted-indolin-2-ones (3a–3d). Equimolar (0.01 mole) amounts of 5-substituted isatin and o-phenylenediamine were taken in 30 mL of ethanol, 5-10 drops of glacial acetic acid was added to the reaction mixture, the reaction was refluxed at 120°C, and then the mixture was kept overnight for the formation of precipitate. The precipitate was filtered and recrystallized from methanol and chloroform. The reaction was monitored with the help of TLC using hexane/ethyl acetate (1:1) solvent mixture (Fig. 9) [37, 38].
Fig. 9.
Synthesis of designed isatin derivatives 3a–3d.
Preparation of 3-(2-aminophenylimino)-N-5-substitutedindolin-2-ones (6a–6d). Atwo-step reaction was employed to form final compounds. In step 1, equimolar (0.01 mole) amounts of 5-substituted isatin and KOH were taken in 5 mL of DMF and the reaction mixture was stirred for 10 min at room temperature. Then, halohydrocarbon (0.01 mole) was added dropwise, the mixture was exposed to microwave radiation and the reaction was heated for 5 min at 80°C (400 W), then heated further for 5 min at the same temperature, and finally cooled for 5 min under fan. The reaction mixture was poured on ice so that the precipitate was formed and then it was filtered. The completion of the reaction was checked with the help of TLC using hexane/ethyl acetate (1:1) solvent mixture. In step 2, equimolar amounts of intermediate (0.003 moles) and o-phenylenediamine (0.003 moles) were taken in 30 mL of ethanol, 5 – 10 drops of glacial acetic acid were added, and the reaction was refluxed at 120°C. After reflux, the mixture was cooled overnight and the obtained precipitate was recrystallized from methanol and chloroform. The reaction was monitored with the help of TLC using hexane/ethyl acetate (1:1) solvent mixture (Fig. 10) [33, 37, 38].
Fig. 10.
Synthesis of designed isatin derivatives (6a–6d).
Antimicrobial Activity Assay
All the designed compounds were screened for in vitro antibacterial activity in Luria agar medium. The antibacterial activities of the compounds were evaluated against Gram-positive bacteria (Staphylococcus aureus MTCC-6908) and Gram-negative bacteria (Escherichia coli MTCC-433), using Amoxicillin as a standard drug that acts as antimicrobial agent by inhibition of bacterial cell wall.
Growth inhibition zone determination. Antimicrobial activities of compounds 3a–3d and 6a–6d were evaluated using the growth inhibition zone method [39]. According to this, various dilutions of synthesized compounds (1, 2, 4, 8 and 16 μg/mL) were prepared from the stock of 100 μg/mL in dimethyl sulfoxide for determining the growth inhibition zone size. Visibility of the zone was enhanced using 1% methylene blue dye solution. The test bacteria were swabbed over the plates. The plates were incubated at 37°C. Diameters of the growth inhibition zones were measured after 24 h treatment with reference to Amoxicillin as standard drug for antibacterial activity.
Result
Compounds 3a–3d have been synthesized using a single- step condensation reaction (Fig. 9). Other series of compounds (6a–6d) were synthesized in two steps: first N-alkylation and then condensation (Fig. 10). The synthesized isatin derivatives were structurally characterized by spectral methods including FT-IR, 1H NMR, and MS. The prepared compounds were also evaluated in vitro for their antimicrobial activity.
Analytical Data
3-(2-Aminophenylimino)-indoline-2-one (3a). Yield, 28.52%; crystalline bright yellow compound; m.p.. 235 – 237°C; FT-IR (KBr, νmax, cm-1) 3417 (N–H), 3064 (Ar C–H), 2827 – 2968 (C-H), 1710 (CO), 1617 (C=N), 1596-1574 (R-NH2), 1135 – 1209 (C-O); 1H-NMR (DMSO-d6, δ, ppm): 8.05 (s, 1H) 7.36 (s, 1H), 7.07 (s, 1H), 7.59 (dd, 2H, J1=8.4, J2=0.5 Hz), 7.34 (s, 1H), 7.36 (s, 1H), 7.39 (s, 2H), 3.37 (s, 2H); mass spectroscopy (ESI-MS): m/z 238 [M+1]+.
3-(2-Aminophenylimino)-5-methylindoline-2-one (3b). Yield, 30.32%; crystalline bright yellow compound; m.p.243 – 245°C; FT-IR (KBr, νmax, cm-1) 3126 (N–H), 3021 (Ar C–H), 2841-2711 (C-H), 1716 (C=O), 1655 (C=N), 1467 (R-NH2), 1392 (CH3), 1135 – 1244 (C-O); 1H-NMR (DMSO-d6, δ, ppm) 8.02 (s, 1H), 7.43 (d, 2H, J=8.2 Hz), 7.58 (dd, 3H, J1=8.4, J2=0.5 Hz), 7.74 (s, 1H), 7.78 (s, 1H), 3.37 (s, 3H), 2.47 (s, 2H); mass spectroscopy (ESI-MS): m/z 252 [M+1]+.
3-(2-Aminophenylimino)-5-chloroindoline-2-one (3c). Yield, 26.81%; orange crystalline compound; m.p. 263 – 265°C; FT-IR (KBr, νmax, cm-1) 3126 (N–H), 3021 (Ar C–H), 2841 – 2711 (C-H), 1716 (C=O), 1665 (C=N), 1467 (R-NH2), 1119-1278 (C-O), 752 (R-Cl); 1H-NMR (DMSO-d6, δ, ppm) 8.05 (s, 1H), 7.57 (dd, 1H, J1=8.5 , J2=1.7 Hz), 7.65 (dd, 2H, J1=8.5, J2=0.5 Hz), 7.72 (s, 1H), 7.75 (s, 1H), 7.79 (s, 1H), 7.81 (s, 1H), 3.34 (s, 2H); mass spectroscopy(ESI-MS): m/z 272 [M+1]+.
3-(2-Aminophenylimino)-5-fluoroindoline-2-one (3d). Yield, 32.56%; crystalline yellowish orange compound; m.p. 258 – 260°C; FT-IR (KBr, νmax, cm-1) 3372 (N–H), 3097 (Ar C–H), 2853 – 2897 (C-H), 1700 (C=O), 1657 (C=N), 1528 – 1574 (R-NH2), 1288 – 1165 (C-O); 1H-NMR (DMSO-d6, δ, ppm) 8.03 (s, 1H) 7.02 (d, 1H, J=8.4 Hz), 7.29-7.34 (d, 2H, J=8.4 Hz), 7.51 (s, 1H), 7.74 (s, 1H), 7.76 (s, 1H), 7.80 (s, 1H), 3.30 (s, 2H); mass spectroscopy (ESI-MS): m/z 256 [M+1]+.
3-(2-Aminophenylimino)-1-benzyl-5-chloroindoline-2-one (6a). Yield, 49.03%; light yellow crystalline compound; m.p. 228 – 230°C; FT-IR (KBr, νmax, cm-1) 3098 (N–H), 3025 (Ar C–H), 2843 (C-H), 1615 (C=O), 1616 (C=N), 1467 (R-NH2), 1119 – 1278 (C-O), 797 (R-Cl), 751 (CH2); 1H-NMR (DMSO-d6, δ, ppm) 8.05 (s, 1H), 7.57 (t, 1H, J=7.7 Hz), 7.70 (dd, 3H, J1=7.9, J2=0.6 Hz), 7.7 (s, 3H), 7.71 (s, 2H), 7.81 (s, 2H), 7.84 (s, 2H), 3.34 (s, 2H); mass spectroscopy ESI-MS): m/z 362 [M+1]+.
3-(2-Aminophenylimino)-N-benzoyl-5chloroindoline-2-one (6b). Yield, 51.29%; crystalline light yellow compound; m.p. 287 – 290°C; FT-IR (KBr, νmax, cm-1) 3285 (N–H), 3095 (Ar C–H), 2833 – 2895 (C-H), 1647 (C=N), 1567 (NH) ,1427-1453 (R-NH2), 1361 – 1394 [CH(CH3)2], 1196 – 1275 (C-O), 750 (R-Cl), 725 (CH2); 1H-NMR (DMSO-d6, δ, ppm) 8.1 (s, 1H), 7.19 (t, 1H, J=7.5 Hz), 7.37 (d, 3H, J =8.5 Hz), 7.48 (d, 2H, J=1.1 Hz), 7.75 (dd, 3H, J1=8,0, J2=1.6 Hz), 6.86 (d, 2H, J=7.4 Hz), 3.95 (s, 1H), 3.09 (s, 1H); mass spectroscopy (ESI-MS): m/z 376 [M+1]+.
3-(2-Aminophenylimino)-N-benzoyl-5fluoroindoline-2-one (6c). Yield, 42.61%; crystalline light yellow compound; m.p. 138 – 140°C; FT-IR (KBr, νmax, cm1) 3070 (N–H), 3006 (Ar C–H), 2558 – 2835 (C-H), 1687 (C=N), 1494 (NH), 1423 (R-NH2), 1325 (CH(CH3)2), 1127 – 1229 (C-O), 707 (CH2); 1H-NMR (DMSO-d6, δ, ppm) 8.13 (s, 1H), 7.26 (t, 1H, J=7.5 Hz), 7.27 (dd, 3H, J1=8.5, J2=1.6 Hz), 7.3 (s, 1H), 7.52 (s, 1H), 7.69 (s, 1H), 7.74 (s, 1H), 5.71 (s, 2H), 3.85 (s, 1H), 3.19 (s, 2H); mass spectroscopy (ESI-MS): m/z 360 [M+1]+.
3-(Aminophenylimino)-N-benzoyl-5methylindoline-2-one (6d). Yield, 46.76%; crystalline light yellow compound; m.p. 158 – 160°C; FT-IR (KBr, νmax, cm1) 3071 (N–H), 3010 (Ar C–H), 2558 – 2834 (C-H), 1686 (C=N), 1583 (NH), 1453 – 1423 (R-NH2), 1325 (CH(CH3)2), 1127 – 1295 (C-O), 707 (CH2); 1H-NMR (DMSO-d6, δ, ppm) 8.12 (s, 2H), 7.25 (t, 1H, J=7.5 Hz), 7.51 (dd, 2H, J1=8.5, J2=1.4 Hz), 7.46 (s, 2H), 7.48 (s, 2H), 7.59 (s, 1H), 7.72 (s, 1H), 6.01 (s, 2H), 2.5 (s, 4H); mass spectroscopy (ESI-MS): m/z 356 [M+1]+.
Antimicrobial Activity Assay
All synthesized compounds were screened for in vitro antibacterial activity in the Luria agar medium. The antibacterial activities of compounds were evaluated against Gram-positive bacteria (Staphylococcus aureus MTCC-6908) and Gram-negative bacteria (Escherichia coli MTCC-433) (Tables 1, 2 and Fig. 11, 12).
Table 1.
Growth Inhibition Zone Diameters (mm) of Synthesized Compounds and Standard Drug Tested against Staphylococcus aureus
| Conc. mg/mL | 3a | 3b | 3c | 3d | 6a | 6b | 6c | 6d | Standard |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 8 ± 0.57 | 8.5 ± 0.86 | 9.5 ± 0.28 | 7.5 ± 0.28 | 6 ± 0.57 | 8 ± 0.57 | 7.5 ± 0.29 | 8 ± 0.57 | 10 ± 0.57 |
| 2 | 10.5 ± 0.86 | 10 ± 0.57 | 11 ± 1.15 | 10 ± 0.57 | 8 ± 0.57 | 9.83 ± 0.44 | 9.83 ± 0.44 | 9.5 ± 0.28 | 10.5 ± 0.86 |
| 4 | 11.37 ± 0.36 | 11.37 ± 0.21 | 11.25 ± 0.43 | 9.37 ± 0.43 | 9.37 ± 0.44 | 10.12 ± 0.07 | 10.25 ± 0.29 | 10.37 ± 0.21 | 11.75 ± 0.43 |
| 8 | 12.5 ± 0.86 | 12 ± 0.5 | 13 ± 1.15 | 13 ± 1.15 | 10 ± 0.57 | 11.5 ± 0.57 | 11.5 ± 0.29 | 12.5 ± 0.28 | 14 ± 0.57 |
| 16 | 13.5 ± 0.28 | 13.5 ± 0.86 | 16.5 ± 0.28 | 12.5 ± 0.28 | 13.5 ± 0.28 | 13.5 ± 0.28 | 12.5 ± 0.29 | 13.5 ± 0.28 | 15 ± 1.15 |
Data are expressed as mean ± SD (n = 3)
Table 2.
Growth Inhibition Zone Size (mm) of Synthesized Compounds and Standard Drug Tested against Escherichia coli
| Conc. μg/mJ | 3a | 3b | 3c | 3d | 6a | 6b | 6c | 6d | Standard |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 10 ± 0.57 | 8.5 ± 0.28 | 10 ± 0.57 | 9.5 ± 0.28 | 7.5 ± 0.28 | 8.5 ± 0.28 | 8.5 ± 0.29 | 6.5 ± 0.28 | 8.5 ± 0.86 |
| 2 | 10.5 ± 0.28 | 10 ± 0.57 | 11.5 ± 0.28 | 10.5 ± 0.86 | 9.83 ± 0.44 | 9.5 ± 0.28 | 9.5 ± 0.29 | 8 ± 0.58 | 10 ± 0.57 |
| 4 | 11.5 ± 0.28 | 10.5 ± 0.28 | 11.37 ± 0.07 | 11.25 ± 0.25 | 10.37 ± 0.07 | 9.79 ± 0.39 | 10.12 ± 0.07 | 9.37 ± 0.56 | 11.75 ± 0.14 |
| 8 | 12 ± 0.57 | 12 ± 0.57 | 12.5 ± 0.28 | 13.5 ± 0.28 | 12.5 ± 0.28 | 12.5 ± 0.28 | 12.5 ± 0.29 | 13.5 ± 0.29 | 13.5 ± 0.28 |
| 16 | 13.5 ± 0.28 | 13 ± 0.57 | 13.5 ± 0.28 | 15.16 ± 1.16 | 14.5 ± 0.28 | 13.5 ± 0.28 | 14 ± 0.58 | 13 ± 1.16 | 16 ± 0.57 |
Data are expressed as mean ± SD (n = 3)
Fig. 11.

Antibacterial activity of synthesized compounds and standard drug tested against Staphylococcus aureus.
Fig. 12.
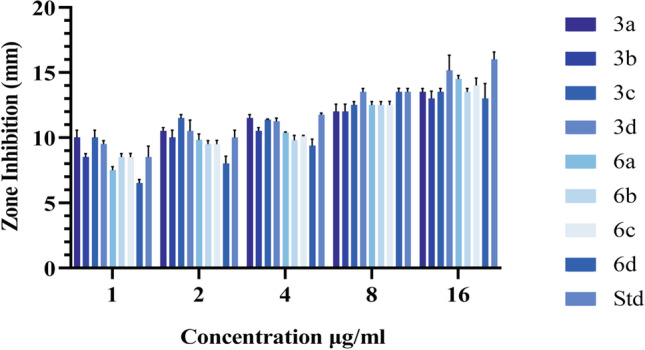
Antibacterial activity of synthesized compound and standard drug tested against Escherichia coli.
Statistical Analysis
The obtained values were expressed as mean ± standard deviation and statistical analysis was carried out by one-way ANOVA, P < 0.05 was considered significant, and the graphs were made using Graph pad Prism 9.2 software.
Discussion
The goal of medicinal chemistry is to develop new therapeutic agents. Isatin derivatives are known to have various effects on the brain and infections. Hence, this is an important class of bioactive compounds displaying caspase inhibitor, antibacterial, and antiproliferative activity. Isatin is a multipurpose substrate that can be cast-off to prepare a large variety of heterocyclic compounds like indoles and quinolines and can be used in drug synthesis. Hence, knowledge of the structure and activity of isatin derivatives may provide an effective and cheaper regimen against various bacteria, fungi, and viruses.
This biological study showed that halogenation at 5th position of the initial compound increases its activity which leads to the discovery of new derivatives. The activity of compounds was found to be more potent against Gram-positive bacteria as compared with Gram-negative bacteria (3c, 3d, 6a, 6b, 6d). Compound 3c displayed higher antimicrobial activity than standard drug (amoxicillin) against Staphylococcus aureus at higher concentration (16 μg/mL) and against Escherichia coli at lower concentration (1 μg/mL). The synthesized derivatives of isatin act by mixed mechanism as antimicrobial agent, that is by (i) inhibition of bacterial cell wall and (ii) inhibition of bacterial cell fusion. Further studies on isatin derivatives are required to develop effective and cheaper regimens.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
The authors would like to thank honorable vice-chancellor for providing facilities to carry out this research work.
References
- 1.Chahal VW, Nirwan S, Kakkar R. Med. Chem. Commun. 2019;10(3):351–368. doi: 10.1039/C8MD00585K. [DOI] [Google Scholar]
- 2.Visagaperumal D, Ezekwem JE, Munji H, Chandy V. Pharma Tutor. 2018;6(5):38–47. doi: 10.29161/PT.v6.i5.2018.38. [DOI] [Google Scholar]
- 3.Shukla PK, Singh MP, Patel R. J. Appl. Pharm. Sci. Res. 2018;1(2):16–22. [Google Scholar]
- 4.Grewa SA. Int. J. Pharm. Sci. Res. 2014;6(1):1–8. [Google Scholar]
- 5.Silva VB. J. Braz. Chem. Soc. 2013;24(5):707–720. [Google Scholar]
- 6.Premanathan M, Radhakrishnan S, Kulangiappar K, et al. Indian J. Med Res. 2012;136(5):822–826. [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshikawa M, Murakami T, Kishi A, et al. Chem. Pharm. Bull. 1998;46(5):886–888. doi: 10.1248/cpb.46.886. [DOI] [PubMed] [Google Scholar]
- 8.Bergman J, Lindstrom JO, Tilstam U. Tetrahedron. 1985;41(14):2879–2881. doi: 10.1016/S0040-4020(01)96609-8. [DOI] [Google Scholar]
- 9.da Silva JFM, Garden SJ, Pinto AC. J. Braz. Chem. Soc. 2001;12(3):273–324. doi: 10.1590/S0103-50532001000300002. [DOI] [Google Scholar]
- 10.Andreani A, Burnelli S, Granaiola M, et al. Eur. J. Med. Chem. 2010;45:1374–1378. doi: 10.1016/j.ejmech.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 11.F. Sonmez, Z. Gunesli, Z. B. Kurt, et al., Mol. Divers., 1 – 16 (2019). [DOI] [PubMed]
- 12.R. Jarapula, K. Gangarapu, S. Manda, and S. Rekulapally, Int. J. Med. Chem., 1 – 9 (2016). [DOI] [PMC free article] [PubMed]
- 13.Prasad DB, Vasanthi R, Kanth CB, et al. IJBPR. 2012;3(1):182–187. [Google Scholar]
- 14.Jarrahpour A, Khalili D, Clercq DE, et al. Molecules. 2007;12:1720–1730. doi: 10.3390/12081720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sridhara KS, Saravanana M, Ramesh A. Eur. J. Med. Chem. 2001;36(7–8):615–625. doi: 10.1016/S0223-5234(01)01255-7. [DOI] [PubMed] [Google Scholar]
- 16.Abbas YS, Farag AA, Ammar AY, et al. Monatsh. Chem. 2013;144:1725–1733. doi: 10.1007/s00706-013-1034-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fadl AT, Jubair BSAF. Int. J. Res. Pharm. Sci. 2010;1(2):113–126. [Google Scholar]
- 18.Fadl AT, Jubair BSAF, Wafa AO. Eur. J. Med. Chem. 2010;45(10):4578–4586. doi: 10.1016/j.ejmech.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 19.Vine LK, Matesic L, Locke MJ, et al. Anticancer Agents. Med. Chem. 2009;9:397–414. doi: 10.2174/1871520610909040397. [DOI] [PubMed] [Google Scholar]
- 20.Meleddu R, Petrikaite V, Distinto S, et al. ACS Med. Chem. Lett. 2019;10:571–576. doi: 10.1021/acsmedchemlett.8b00596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verma M, Pandeya HNS, Singh NK, et al. Acta Pharm. 2004;54:49–56. [PubMed] [Google Scholar]
- 22.Mathur G, Nain S. Med. Chem. 2014;4(4):417–427. [Google Scholar]
- 23.Meleddu R, Distinto S, Corona A, et al. J. Enzyme Inhib. Med. Chem. 2017;32(1):130–136. doi: 10.1080/14756366.2016.1238366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sridhar SK, Pandeya SN, De Clercq ED. Bull. Chim. Farm. 2001;140(5):302–305. [PubMed] [Google Scholar]
- 25.Zhou L, Liu Y, Zhang W, et al. J. Med. Chem. 2006;49:3440–3443. doi: 10.1021/jm0602357. [DOI] [PubMed] [Google Scholar]
- 26.Chen LR, Wang CY, Lin WY, et al. Bioorg. Med. Chem. Lett. 2005;15:3058–3062. doi: 10.1016/j.bmcl.2005.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selvam P, Murgesh N, Chandramohan M, et al. Indian J. Pharm. Sci. 2008;70(1):91–94. doi: 10.4103/0250-474X.40339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nathani RB, Pandya SK, Jeni MM, et al. Der. Chem. Sinica. 2011;2(6):97–103. [Google Scholar]
- 29.Singh SG, Desta YZ. Chem. Inform. 2013;44(41):74–115. [Google Scholar]
- 30.Chaithanya B, Kasiviswanath VI, Chary PD. Bull. Chem. Soc. Ethiop. 2019;33(2):321–329. doi: 10.4314/bcse.v33i2.12. [DOI] [Google Scholar]
- 31.Alsalihi IE, Fahdawi ASA. Aro – Sci. J. Koya Univ. 2018;6(1):38–45. [Google Scholar]
- 32.S. M. Almutairia, S. A. Zakariab, P. P. Ignasiusc, et al., J. Mol. Struct., 1 – 33 (2017).
- 33.Bogdanov VA, Mironov FV. Russ. J. Gen. Chem. 2015;85(10):2413–2415. doi: 10.1134/S107036321510031X. [DOI] [Google Scholar]
- 34.A. El-Faham, N. W. Hozzein, M. A. M. Wadaan, et al., J. Chem., 1 – 8 (2015).
- 35.Singh KU, Pandeya NS, Singh A, et al. Int. J. Pharm. Sci. Drug. Res. 2010;2(2):151–154. [Google Scholar]
- 36.Phogat P, Singh PA. Cent. Nerv. Syst. Agents. Med. Chem. 2015;15(1):28–31. doi: 10.2174/1871524915666150213122246. [DOI] [PubMed] [Google Scholar]
- 37.Prakash CR, Raja S, Selvam PT, et al. Rasayan. J. Chem. 2009;2(4):960–968. [Google Scholar]
- 38.Hajare RA, Gaurkhede RM, Chinchole PP, et al. Asian J. Res. Chem. 2009;2(3):289–291. [Google Scholar]
- 39.Balouirin M, Sadiki M, Ibnsouda KS. J. Pharm. Anal. 2016;6:71–79. doi: 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]