Abstract
Viral diseases are the most notorious infective agent(s) causing morbidity and mortality in every nook and corner for ages; viruses are active in host cells, and specific anti-virus medicines’ developments remain uncanny. In this century of the biological era, human viruses act predominantly as versatile spreaders. The infection of the present COVID-19 virus is up in the air; blithely, the integument of medicinal chemistry approaches, particularly bioactive derived phytocompounds could be helpful to control those human viruses, recognized in the last 100 years. Indeed, natural products are being used for various therapeutic purposes. The major bioactive phytocompounds are chemically containing coumarin, thiosulfonate, steroid, polysaccharide, tannin, lignin, proanthocyanidin, terpene, quinone, saponin, flavonoid, alkaloid, and polyphenol, that are documented for inhibitory action against several viral infections. Mostly, about 20–30% of plants from tropical or temperate regions are known to have some antiviral activity. This comprehensive analysis of bioactive-derived phytocompounds would represent a significant impact and might be helpful for antiviral research and the current state of viral treatments.
Keywords: Phytochemicals, Natural product, COVID-19, Antiviral, Viral infection disease, SAR studies
Introduction
For decades, active ingredients from fresh parts and solvent-processed plant extracts have been used in mainstream medicines in every culture. However, plant extracts have several secondary metabolites for therapeutic utility as pharmaceuticals, but today, herbal medicines are exploited for drug development cascades. Thus, it is important to know about potential phytoactive compounds with antiviral properties, in this pandemic COVID-19 time. Moreover, viral diseases (Huerta-Reyes et al. 2022) cause mild to severe acute human respiratory illnesses. The liver-damaging hepatitis A, B, and C viruses and several other viruses, including Zika, chikungunya, dengue, herpes simplex virus (HSV), and a few more, have caused consternation in human health in the last four decades. Indeed, viruses have comparatively faster-mutating rates, resulting in suitably surviving strains that pose significant hazards to humanity (Kapoor et al. 2017). There is a regular advent of novel and versatile viral strains that survive the hard preventive measures, which had been addressed by newly identified anti-viral agent(s) with lesser side effects/host toxicity, in the past.
Several lethal viruses have recently caused pandemics, and a few more drugs have been located/produced to effectively treat those viral diseases (Li et al. 2022; Mohanty et al. 2021; Issa et al. 2022; Beck et al. 2013). Most of the anti-viral medicines have detrimental failures with long-term drug resistance. Various plants are valuable assets, which are to be explored, especially in emerging resistant infectious diseases (Idriss et al. 2023a, b; Mukhtar et al. 2008). Undeniably, plant-derived phytoactive compounds are potential therapeutic metabolites with the power to obstruct, control viral uptake, attach to surface receptors, and compete for pathways of activation of intracellular signals (Mao et al. 2022; Ghosh et al. 2009; Kapoor et al. 2017; Khan et al. 2005; Mohapatra and Dar 2010).
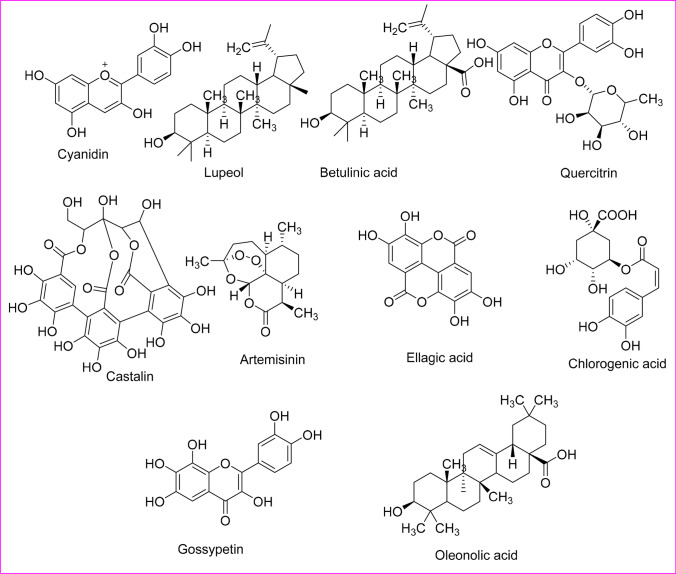
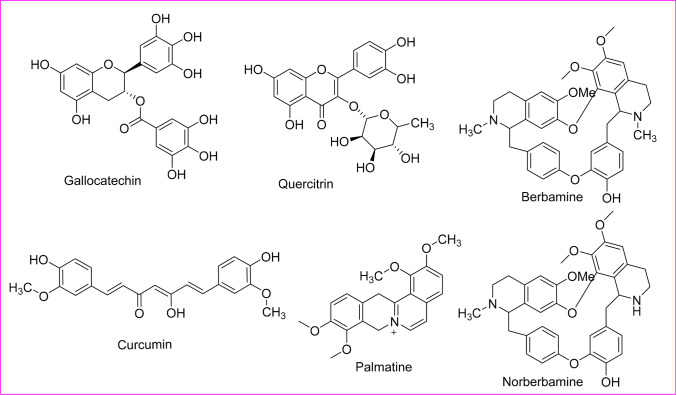
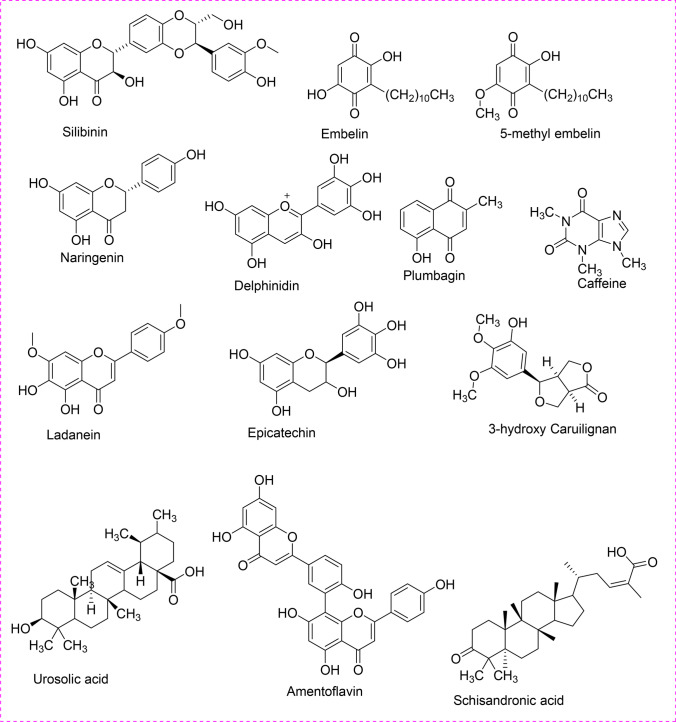
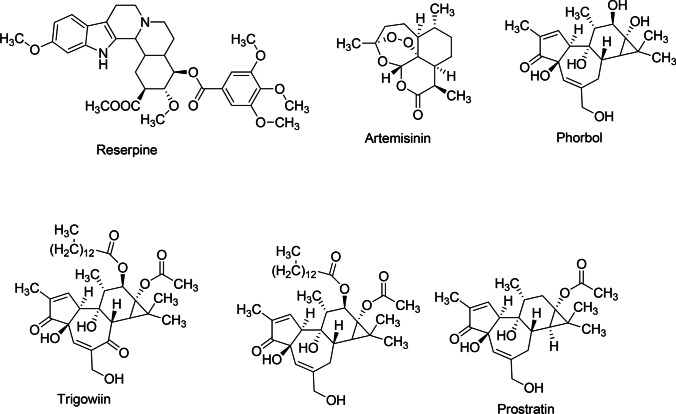
Furthermore, plant-derived phytoactive compounds are the most wanted items in pharmacology to investigate, extract active ingredients, and demonstrate the inherent therapeutic properties. Currently, phytochemicals are conjugated with nanoparticles to increase their efficacy against several diseases (Bakrim et al. 2022; Goktas et al. 2020), and a similar conjugated nanoparticles approach can be taken against infectious viral diseases to increase the potency of phytoactive compounds. However, only about 25% of phytocompounds are used as drugs (Cragg and Newman 2005; Kapoor et al. 2017). Moreover, since time immemorial, phytocompounds from around 2500 meditational plant species had been recorded as traditional medicines (De Smet 2002; Shinwari 2010) (Fig. 1). The major bioactive phytocompounds are coumarins, thiosulfonates, steroids, polysaccharides, tannins, lignins, proanthocyanidins, terpenes, quinones, saponins, flavonoids, alkaloids, and polyphenols, which are known to fight several viral infections (Kapoor et al. 2017). Mostly, about 20–30% of plants from tropical or temperate regions are known to have some antiviral activity (Roumy et al. 2020; Perez 2003). In this review, some bioactive phytochemicals with antiviral activity are described, which might be helpful for antiviral research.
Fig. 1.
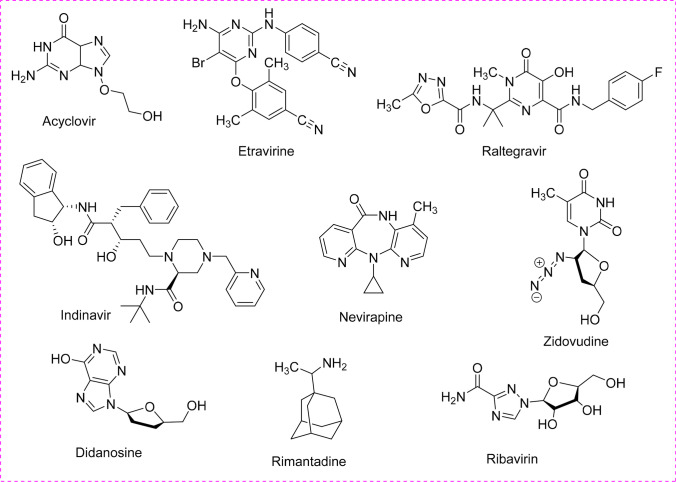
Synthetic commercially available several classes of antiviral drugs
Utility of phytoactive compound for emerging viral diseases
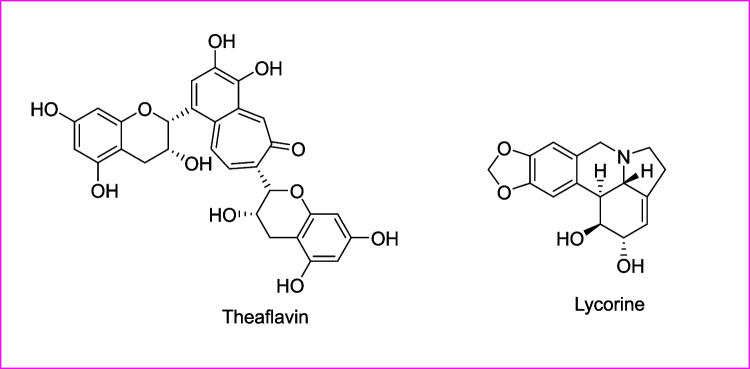
Emerging diseases from viral infections, especially in this era, are a serious challenge to humanity. Phytochemicals with broad-spectrum anti-viral effects could be used to discover novel drugs for such dreadful infectious diseases causing a pandemic. Some examples such as Lindera aggregate, Pyrrosia lingua, Artemisia annua, Myrica cerifera, Curcuma longa, Rheum palmatum, Phaseolus vulgaris, Amaranthus tricolor, Citri reticulatae pericarpium, Erigeron breviscapus, Fraxinus sieboldiana, Hyptis atrorubens Poit, Camellia sinensis, Phyllanthus emblica, Lycoris radiate, Glycyrrhiza uralensis, Glycyrrhiza radix, Psorothamnus arborescens, and Scutellaria baicalensis appeared to have a broad-spectrum antiviral activity. Among these, Lycoris radiate (Li et al. 2005; Mani et al. 2020) and Nigella sativa (Idrees et al. 2021) had shown powerful anti-severe acute respiratory syndrome-related coronavirus (SARS-CoV or SARSr-CoV) activity. Several plant-derived phytoactive compounds previously known to have broad-spectrum antiviral effects could unveil new drugs for newly emerging novel viral strains (Table 1) (Fig. 2). Lycorine from L. radiate had shown a high efficiency against SARS-CoV (EC50 of 15.7 ± 1.2 nM), with more than 900 selectivity index. In this context, L. radiate has toxic effects at low levels (Kretzing et al. 2011; Mani et al. 2020). Theaflavin a phytoactive compound found in Camellia sinensis had been shown in silico effectively against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) strains (Lung et al. 2020; Mani et al. 2020). The phytoactive compounds from all these above plants could be screened for SARS-CoV-2.
Table 1.
List of phytoactive compounds’ inhibitory action against viral diseases
| Sl. No | Plant name | Phytoactive compound | Effective against | Reference |
|---|---|---|---|---|
| 1 | Actinodaphne hookeri | Actinophnine | HSV-1 | (Perez 2003) |
| 2 | Flindersia fournieri and Flindersia amboinensis | Alkaloids | ZIKV | (Byler et al. 2016) |
| 3 | Atropa belladona L | Atropine | All enveloped viruses | (Perez 2003) |
| 4 | Euodia roxburghiana | Buchapine | HIV-1 | (Perez 2003) |
| 5 | Ophiorrhiza mungos | Camptothecin, 10-methoxycamptothecin | HHV | (Perez 2003) |
| 6 | Commelina communis | Homonojirimycin | H1N1 | (Ghildiyal et al. 2020; Zhang et al. 2013) |
| 7 | Carnavalia ensiformis L | Canavanin | Influenza virus, Semliki Forest virus | (Perez 2003) |
| 9 | Theobroma cacao L | Caffeine | Coxsackie-virus, Echonovirus, Herpes, Poliovirus, vaccinia, influenza virus | (Perez 2003) |
| 10 | Cassia siamea | Cassiarin D | ZIKV | (Byler et al. 2016) |
| 11 | Chelidonium majus L | Chelidonine | HHV, influenza virus | (Perez 2003) |
| 12 | Cordyceps militaris | Cordycepin | picornavirus, poliovirus, vaccinia, newcastle disease virus, HSV, influenza viruses | (Perez 2003) |
| 13 | Mammea americana and Tabernaemontana cymosa | Coumarin |
CHIKV DENV |
(Gómez-Calderón et al. 2017) |
| 14 | Cryptocarya pleurosperma | Cryptopleurine | HSV-1 | (Perez 2003) |
| 15 | Curcuma longa | Curcumin | EBOV | (Baikerikar 2017) |
| 16 | Buchenavia capitata | O-Demethyl-buchenavianine | HIV | (Perez 2003) |
| 17 | Cephaelis ipecacuanha | Emetine | HHV, Semliki Forest virus | (Perez 2003) |
| 18 | Camellia sinensis | Epigallocathechin-3-gallate | HBV | (Ben-Shabat et al. 2020) |
| 19 | Fagara zanthoxyloides Lam | Fagaronine | All retrovirus | (Perez 2003) |
| 20 | Flindersia acuminata | Flinderole A | ZIKV | (Byler et al. 2016) |
| 21 | Peganum harmala | Harmaline, harmine | HSV-1 | (Perez 2003) |
| 22 | Clivia miniata | Lycorine | several RNA and DNA viruses | (Perez 2003) |
| 23 | Lycoris radiata | Lycorine | SARS-CoV | (Mani et al. 2020) |
| 24 | Boenninghausenia sessilicarpa | Leptodactylone | SARS-CoV | (Mani et al. 2020; Yang et al. 2007b) |
| 25 |
Vaccinium macrocarpon Calamus scipionum Allium sativum |
Myricetin | SARS-CoV | (Mani et al. 2020) |
| 26 | Scutellaria baicalensis | Scutellarein | SARS-CoV | (Mani et al. 2020) |
| 27 | Ancistrocladus korupensis D | Michellamines D and F | HIV | (Perez 2003) |
| 28 | Camptotheca acuminata | 10-Methoxycamptothecin | Adenovirus, herpes, and vaccinia viruses | (Perez 2003) |
| 29 | Polyathia oliveri | Oliverine | HSV-1 | (Perez 2003) |
| 30 | Stephania japonica | Oxostephanine | HSV-1 | (Montanha et al. 1995; Perez 2003) |
| 31 | Pachypodanthium staudti | Pachystaudine | HSV-1 | (Montanha et al. 1995; Perez 2003) |
| 32 | Papaver somniferum | Papaverine | Cytomegalovirus (CMV), measles, HIV, and several other viruses | (Manske and Holmes 2014; Perez 2003) |
| 33 | Cephaelis acuminata | Psychotrine | HIV-1 | (Perez 2003) |
| 34 | Schumanniophyton magnificum | Schumannificine |
HIV HSV |
(Mukherjee 2019; Perez 2003) |
| 35 | Camellia sinensis | Theaflavin | SARS-CoV-2 | (Lung et al. 2020; Mani et al. 2020) |
| 36 |
Arachniotus aureus (Eidam) Schoeter and Aspergillus terreus |
Aranotin, gliotoxin | Poliovirus, rhinovirus, influenza virus, para-influenza virus type 3 | (Perez 2003) |
| 37 | Castanospermum australe | Castanospermine, Australine | HIV | (Perez 2003) |
| 38 |
Catharanthus roseus L. G. Don C. lanceus Pich |
Leurocristina, periformyline, perivine, vincaleucoblastine | Poliovirus, vaccinia, influenza viruses | (Perez 2003) |
| 39 | Berberis vulgaris | Columbamine, berberine, palmitine | HIV-1 | (Manske and Holmes 2014; Perez 2003) |
| 40 | Buxus sempervirens | Buxamine E, cyclobuxamine H | HIV-1 | (Perez 2003) |
| 41 | Jatropha multifida L | Multifidone, multifidanol, multifidenol, and jatrophone | Influenza virus | (Shoji et al. 2017) |
| 42 | Andrographis paniculata, Curcuma longa, Gynostemma pentaphyllum, Kaempferia parviflora, Psidium guajava | 5,7-Dimethoxyflavone, Trimethylapigenin, Tetramethylluteolin | Influenza virus | (Sornpet et al. 2017) |
| 43 | Scutellaria baicalensis | Baicalin | DENV | (Kapoor et al. 2017; Moghaddam et al. 2014) |
| 44 | Glycyrrhiza inflate | Chalcones | Influenza virus | (Dao et al. 2011; Kapoor et al. 2017) |
| 45 | Aglaia sp. | Dammarenolic acid | Retroviruses | (Esimone et al. 2010) |
| 46 | Croton mauritianus | Decanoylphorbol-13 acetate | CHIKV | (Corlay et al. 2014) |
| 47 | Phyllanths urinaria | Excoecariani, loliolide | HSV- 2, Hepatitis C (HCV) | (Chung et al. 2016) |
| 48 | Ziziphus jujuba | Jubanines | Porcine epidemic diarrhea virus (PEDV) | (Kang et al. 2015) |
| 49 | Swietenia macrophylla | Limonoids | HCV | (Cheng et al. 2014) |
| 50 | Camellia japonica | Oleanane | PDEV | (Yang et al. 2015) |
| 51 | Embelia ribes | Quercetin | HCV | (Kapoor et al. 2017) |
| 52 | Bupleurum kaoi | Saikosaponins | HCV | (Lin et al. 2015) |
| 53 | Rheum palmatum | Sennoside A | HIV-1 | (Esposito et al. 2016) |
| 54 | Aglaia foveolata | Silvestrol | EBOV | (Biedenkopf et al. 2017) |
| 55 | Schisandra micrantha | SJP-L-5 | HIV-1 | (Bai et al. 2015) |
| 56 | Tanacetum vulgare | Swerilactones |
HSV-1 HSV-2 |
(Álvarez et al. 2015) |
| 57 | Humulus lupulus | Xanthohumol | BVDV | (Kapoor et al. 2017) |
| 58 | Artocarpus lakoocha | Oxyresveratrol | HSV-1 HSV-2 | (Kapoor et al. 2017) |
| 59 | Bupleurum kaoi | Saikosaponin b2 | HCV | (Xu et al. 2014) |
| 60 | Citrus reticulate | Tangeretin and nobiletin | RSV | (Nothias-Scaglia et al. 2014) |
| 61 | Euphorbia amygdaloides spp. and semiperfoliata | Compound 3 |
CHIKV HIV-1 HIV-2 |
(Kapoor et al. 2017) |
| 62 | Glycyrrhiza radix | Glycyrrhizic acid | EBV | (Kapoor et al. 2017) |
| 63 | Houttuynia cordata | Quercetin 3rhamnoside | Influenza virus | (Kapoor et al. 2017) |
| 64 | Limonium sinense | Samarangenin B | HSV-1 | (Huang et al. 2014) |
| 65 | Liriope platyphylla | LPRP-Et-97543 | HBV | (Kapoor et al. 2017) |
| 66 | Melia azedarach L | Tetranortriterpenoid 1-cinnamoyl-3, 11-dihydroxymeliacarpin |
VSV HSV-1 |
(Kapoor et al. 2017) |
| 67 | Prunella vulgaris | Lignin–carbohydrate complex | HSV-1 HSV-2 | (Kapoor et al. 2017) |
| 68 | Pterocarya stenoptera | Pterocarnin A | HSV-2 | (Wahyuni et al. 2014) |
| 69 | Ruta angustifolia | Chalepin and pseudane IX | HCV | (Cui et al. 2014) |
| 70 | Saururus chinensis | Manassantin B | EBV | (Li et al. 2005) |
| 71 | Schefflera heptaphylla | Dicaffeoylquinic acids | RSV | (Kapoor et al. 2017) |
| 72 | Scoparia dulcis L | Scopadulcic acid B | HSV-1 | (Kapoor et al. 2017) |
| 73 | Scutellaria baicalensis | 5,7,4′ trihydroxy-8- methoxyflavone | Influenza virus | (Qin et al. 2011) |
| 74 | Tanacetum vulgare | Spiroketalenol ether derivative |
HSV-1 HSV-2 |
(Bauer and Brönstrup 2014) |
| 75 | Veratrum sabadilla | Sabadinine | SARS-CoV 3CL Protease | (Toney et al. 2004) |
| 76 | Isatis indigotica | Sinigrin | SARS-CoV 3CL Protease | (Lin et al. 2015) |
|
Chinese medicinal plants including a. Forsythiae fructus b. Mori cortex c. Mori follum d. Chrysanthemi flos e. Farfarae flos f. Lonicerae japonicae flos g. Peucedani radix h. Rhizoma fagopyri cymosi i. Tamaricis cacumen j. Erigeron breviscapus k. Radix bupleuri l. Coptidis rhizome m. Houttuyniae herba |
Betulinic acid Coumaroyltyramine Cryptotanshinone Desmethoxyreserpine Dihomo-c-linolenic Dihydrotanshinone Kaempferol Lignan Moupinamide N-cis-feruloyltyramine Quercetin Sugiol Tanshinone IIa |
SARS-CoV-2 | (Zhang et al. 2020) |
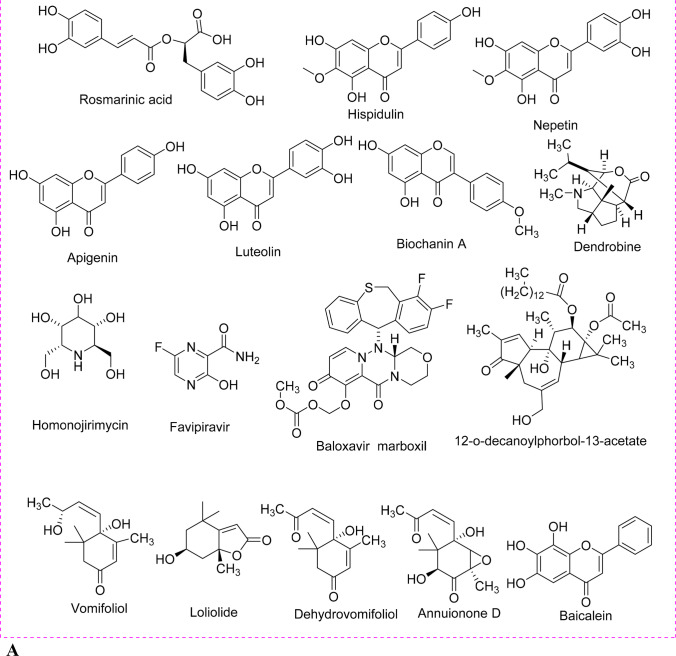
Fig. 2.
Structure of the phytoactive derivative for emerging viral infectious diseases
Phytoactive compounds for influenza
Influenza is the upper respiratory tract disease, involving seasonal or climatic occurrences from avian and other zoonotic origins, causing global 3 to 5 million morbid cases annually (Krammer et al. 2018). Previous and recent research shows a wide range of phytochemicals could prevent the influenza virus both in vitro (Eggers et al. 2022; Yang et al. 2022; Idriss et al. 2023a, b; Mothana et al. 2006; Pantev et al. 2006; Prajoubklang et al. 2005) and in vivo (Ivanova et al. 2005; Prahoveanu et al. 1985). Moreover, flavonoids derived from aerial parts of Salvia plebeia were proven effective against influenza A (H1N1) neuraminidase, inhibiting the replication cycle (Bang et al. 2016). An alkaloid extracted from Commelina communis, the homonojirimycin (HNJ), had been reported to have strong anti-H1N1 activity in vivo and good survival rates in infected mice (Ghildiyal et al. 2020; Zhang et al. 2013). Investigations were shown as the potential anti-influenza activity by several phytochemicals (Table 1) (Fig. 3A–C), such as saponins, polyphenols, glucosides, flavonoids, and alkaloids (Cock and Vuuren 2020; Wang et al. 2006). This phytochemical would be capable of producing possible phytoactive compounds to control the influenza virus in the future.
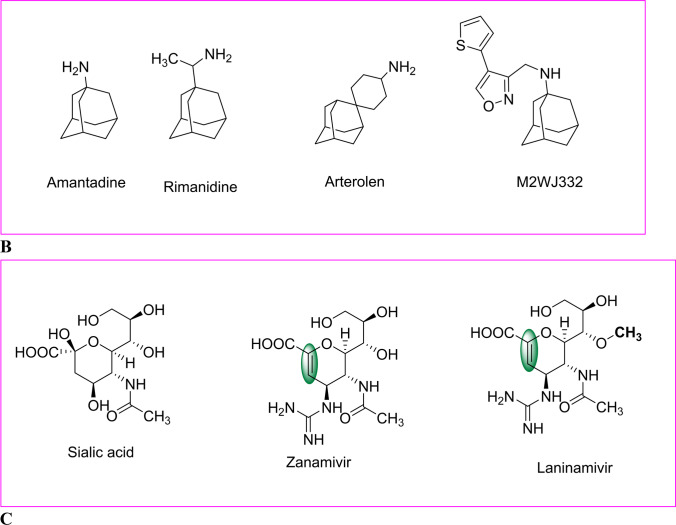
Fig. 3.
A Reported active compounds for inhibitory action against influenza viruses. B Structure of Adamantane derivative for inhibitory action against anti-influenza. C Structure of the sialic acid derivative for inhibitory action against anti-influenza
Role of phytoactive compound for HIV/AIDS
Human immunodeficiency virus (HIV) infects specifically CD4+ T cells (helper T cells) weakening the immune system over the infection time, thereby causing the problem of acquired immunodeficiency syndrome (AIDS). Out of the two main types of HIVs, HIV-1 is more potent and more contagious than HIV-2 and is the major cause of HIV contagion, worldwide. There are about 38 million people infected with HIV worldwide (WHO 2023). Recent literature suggested the use of phytoactive compounds could be affordable and proficient in inhibiting the HIV-1 progression (Table 1) (Fig. 4). These also ease toxicity issues of treatment, as phytochemicals do not accumulate significantly in human organs and can be easily digested and eliminated out of the body. The Canadian AIDS Treatment Information Exchange (CATIE) has summarized a list of potent medicinal plants with advantageous effects for HIV individuals. This list also provides identification and herbal products for controlling HIV and associated infections (Mukhtar et al. 2008). One such antiretroviral phytoactive compound could be derived from Terminalia catappa, Jatropha curcas, Cordia spinescens, Hyptis lantanifolia, and Etrapteris macrocarpa possessing anti-HIV properties (Matsuse et al. 1998). These are flavonoids cyanidin, ellagitannins, gallotannins, and tannins (Dwevedi et al. 2016). Griffithsin a lectin derived from Griffithsia sp., a red alga that inhibited the viral entry into the host cell. Similarly, cyanovirin-N derived from several cyanobacteria showed comparable inhibition of HIV entry. Mirabamide A and neamphamide A extracted from different sponges show anti-HIV activity even in low concentrations. Triterpene betulinic acid derivative bevirimat derived from Chinese traditional herbs have been shown in vitro to inhibit HIV with enhancing efficacy.
Fig. 4.
Structure of the phytoactive compounds for HIV/AIDS
Phytoactive compound for herpesvirus
Human herpes viruses (HHVs) family has several varieties of DNA viruses, out of which herpes simplex virus 1 and 2 (HSV-1, HSV-2) cause the majority of infection. Other notorious HHVs, which cause infection are HHV-3, HHV-4, HHV-5, HHV-6A, HHV-6B, HHV-7, and HHV-8. Aqueous extract of Podophyllum peltatum L. prohibits HSV-1 in vitro (Bedows and Hatfield 1982). Acetone extract of Phyllanthus urinaria and Phyllanthus emblica L. (Euphorbiaceae) could also inhibit HSV-2 and HSV-1 (Xiang et al. 2011; Yang et al. 2007a, b). Even extract of few other medicinal plants is effective, which suggest that phytoactive compounds have potential anti-HSV activity (Table 1) (Fig. 5). There is always a pursuit for anti-viral drugs, which could vanquish virus-resistant or repress the emergence of resistance viral strains. One such example is the roots of Carissa edulis Vahl (Apocynaceae) also showed a significant anti-HSV activity in vitro and in vivo (Mukhtar et al. 2008; Tolo et al. 2006). In recent studies, extract of Ginkgo biloba had been shown with potential anti-HSV activities suggesting G. biloba could supplement current therapies for HSV (Sochocka et al. 2019).
Fig. 5.
Structure of the phytoactive compounds against herpes virus
Phytoactive compound for hepatitis
Hepatitis is an inflammatory state of the liver that can provoke an array of health disorders and can be lethal. The five main strains of the hepatitis virus are type A, B, C, D, and E. However, types B and C are the most fatal, as those cause chronic diseases such as cirrhosis of the liver, cancer, and ultimately mortality (Roumy et al. 2020). Approximately 325 million people are living with hepatitis B and/or C worldwide by WHO, 2023. Clinical studies on different species of Phyllanthus sp. have revealed its anti-hepatitis B virus (HBV) properties, which inhibit HBV polymerase activity and mRNA transcription (Ravikumar et al. 2011; Lee et al. 1996; Ott et al. 1997; Wang et al. 2006). Oenanthe javanica (Apiaceae) had been shown as a potent inhibitor of hepatitis B e-antigen (HBeAg) and hepatitis B surface antigen (HBsAg) (Wang et al. 2005). Similarly, Gymnema sylvestre R. Br. (Apocynaceae) inhibited HBsAgs binding, with the additional inhibition of HBV DNA polymerase (Siddiqui et al. 2017). Acanthus ilicifolius L. (Acantheceae) lowered transaminase by reducing HBV-prompted liver damages (Siddiqui et al. 2017; Wei et al. 2015). Sometimes, combinations of extracts of different medicinal plants were tried for better effects in particular therapies. One such example was the combination of phytoactive compounds of the reishi mushroom, Ganoderma lucidum and Sophorae flavescentis (Fabaceae), which proved to have a powerful anti-HBV effect in vitro and in vivo (Li et al. 2005) (Table 1) (Fig. 6).
Fig. 6.
Structure of the phytoactive compounds against hepatitis
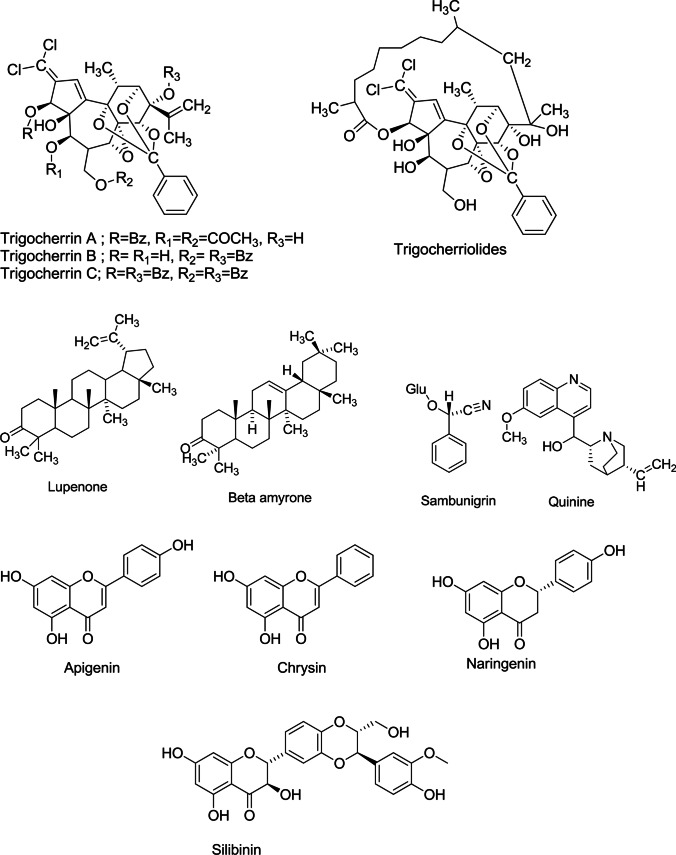
Phytoactive compound for chikungunya
Chikungunya virus (CHIKV) is causing surging “ignored” tropical infectious Arbovirus disease that causes a high fever and intense pain in joints (Arthralgia). It was first reported in 1952, and approximately, it has infected over 2 million people since 2005 by WHO, 2023. It has no specific anti-viral treatment, but the focus has been to relieve the symptoms. Diterpenoid type “trigowiin A” extracted from Trigonostemon howii (Euphorbiaceae) exhibited a moderate anti-CHIKV activity, and another unique indigenous plant Trigonostemon cherrieri (Euphorbiaceae) had a moderate to fair anti-CHIKV control (Bhakat and Soliman 2015). However, phytoactive compounds extracted from Croton mauritianus (Euphorbiaceae) leaves had some promising anti-chikungunya effects (Corlay et al. 2014). A coumarin derived from Mammea americana (Calophyllaceae) and Tabernaemontana cymosa (Apocynaceae) showed 100% inhibition for both chikungunya and dengue virus (Gómez-Calderón et al. 2017). Hexane extract of Santalum album (Santalaceae), Trichosanthes dioica (Cucurbitaceae), Chrysopogon zizanioides (Poaceae), and Andrographis paniculata (Acantheceae) contains coumarins, which is potential in inhibiting viral growth (Table 1) (Fig. 7). Therefore, these are mostly used for the treatment of both the chikungunya and dengue viruses (Ghildiyal et al. 2020).
Fig. 7.
Structure of the phytoactive compounds for chikungunya
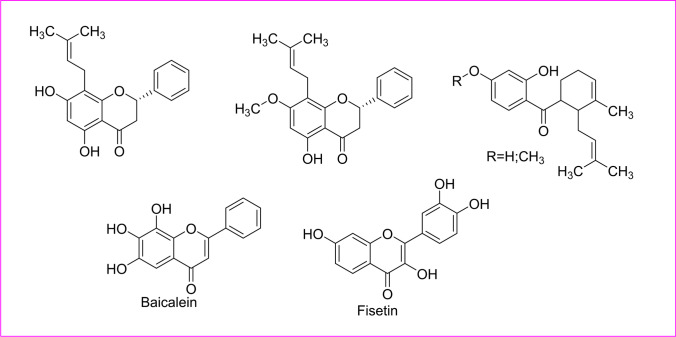
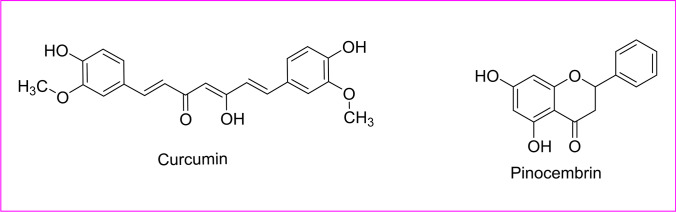
Phytoactive compound for dengue
Dengue virus (DENV) has emerged as one of the virulent tropical and subtropical infectious arbovirus causing a disease, which causes a wide range of diseases ranging from subclinical disease to acute flu-like signs. It was first recorded during the 1950s, and now, approximately 100–400 million people are infected each year by WHO, 2023. It has no specific anti-viral treatment, but the focus has been to reduce fever and pain along with maintenance of the infected person’s body fluid volume. It has four serotypes, meaning a person can be infected four times. An Ayurvedic polyherbal concoction called Nilavembu Kudineer (NVK) with Andrographis paniculata as the main constituent and other extracts from Mollugo cerviana (Molluginaceae), Piper nigrum (Piperaceae), Zingeber officinale (Zingeberaceae), Cyperus rotandus (Cyperaceae), Trichosanthes cucumerina, Santalum album, Vetiveria zizanioides (Piperaceae), and V. zizanioides Hedyotis diffusa and Artemisia capillaris had been reported to show good anti-DENV and anti-CHIKV during infection in vitro (Mao et al. 2022; Jain et al. 2020). Baicalein and fisetin phytoactive compounds had been shown to impede replication at different stages. Furthermore, flavonoids obtained from Tephrosia madrensis (Cucurbitaceae), T. viridiflora, and T. crassifolia showed a 70% inhibition on dengue viruses (Sánchez et al. 2000). Acetone-soluble leaf extract of Pavetta tomentosa (Rubiaceae) and Tarenna asiatica (Rubiaceae) had also been recently reported for the potential anti-dengue effects (Pratheeba et al. 2019) (Table 1) (Fig. 8).
Fig. 8.
Structure of the phytoactive compounds for dengue
Phytoactive compound for Zika
Zika virus (ZIKV) is another resurging tropical infectious, Arbovirus disease causes mild fever, malaise conjunctivitis, rash, and joint and muscle pain. This also increased the risk of neurologic disorders such as myelitis, neuropathy, and the Guillain-Barré syndrome. It was first reported in monkeys in 1947 and thereafter in humans in 1952 in Uganda. It can also be transmitted through sexual contact, organ transplantation, from mother to fetus during pregnancy, and transfusion of blood and blood products by WHO, 2023. It has no specific anti-viral treatment, but in recent years, researchers have detected curcumin and pinocembrin found in turmeric, and tea extracts from Hedyotis diffusa and Artemisia capillaris are found to have replication repressive effect (Mao et al. 2022), respectively in having anti-ZIKV effects (Akbar et al. 2018; Lee et al. 2019). Extract from endemic plants, Aphloia theiformis (Aphloiceae) and Psiloxylon mauritianum (Myrtaceae), found at Reunion Island, inhibited ZIKV adherence to cell surface makings by both anti-ZIKV and anti-DENV (Clain et al. 2019, 2018). Hexane extracts of Tontelea micrantha (Celastraceae) were also reported to have a promising result of anti-ZIKA effect with no cytotoxicity in vitro (Ferreira et al. 2019) (Table 1) (Fig. 9).
Fig. 9.
Structure of the phytoactive compounds for Zika
Phytoactive compound for Ebola
Ebola virus disease (EVD) is a rare but severe, often fatal resurging infectious disease, which causes the hemorrhagic fever. It was first reported in 1976, and in the recent 2018–2019 outbreak, it caused an average fatality rate of about 50%. It can be transmitted from human to human by blood or body fluids by WHO, 2023. As there is no proven treatment, only supportive care and experimental Ebola vaccine have improved the survival rate. WHO and Centers for Disease Control and Prevention (CDC) have estimated rate t as label 4 virus (requiring biosafety level 4-equivalent containment) by CDC, 2009. Leaf and bark extracts of Aglaia foveolata (fam. Mahogany) and aqueous extracts of Prunella vulgaris (Lamiaceae) had shown to have an anti-EVD effect (Ben-Shabat et al. 2020; Kapoor et al. 2017). In silico-based drug design had shown bioactive compounds extracted from Andrographis paniculata (Acanthaceae), Fumaria indica (Papaveraceae), and Adhatoda vasica (Acanthaceae) which had shown the best docking conformations of the ligands against Ebola virus (EBOV)-glycoprotein-and-host-cell-proteins, suggesting a possible anti-EVD from these plants (Shaikh et al. 2019). Curcumin found in turmeric had proven to be effective anti-EBOV (Baikerikar 2017) (Table 1) (Fig. 10).
Fig. 10.
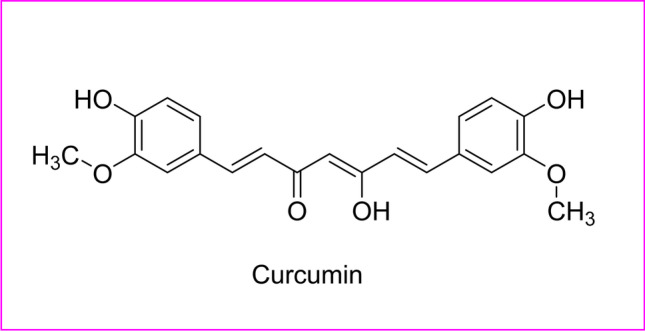
Structure of the active compounds for Ebola
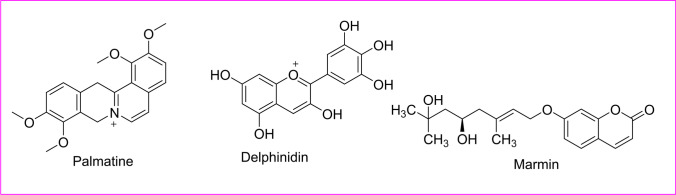
Phytoactive compound for Marburg
Marburg virus disease (MVD) is a rare but highly virulent infectious disease that causes hemorrhagic fever with a fatality rate of 88%. Its Ebola virus belongs to the Filoviridae family of viruses and was first reported in 1967. Both the diseases are clinically similar and highly potent to cause histrionic outbreaks with high casualty rates by WHO, 2023. It had been declared as label 4 virus by WHO and CDC in 2009. The in silico-based drug design had shown bioactive compounds extracted from Murraya koenigii (Rutaceae) leaf had shown the best docking confirmations for 6 proteins of Marburg virus (MARV) (Taj et al. 2018). Bioactive compounds such as palmatine, squalene chloride, delphinidin, and marmin show the maximum binding energy for MARV protein in silico (Badoni et al. 2015) (Fig. 11).
Fig. 11.
Structure of the active compounds for Marburg
Mechanism of action and effect of anti-viral phytoactive compounds
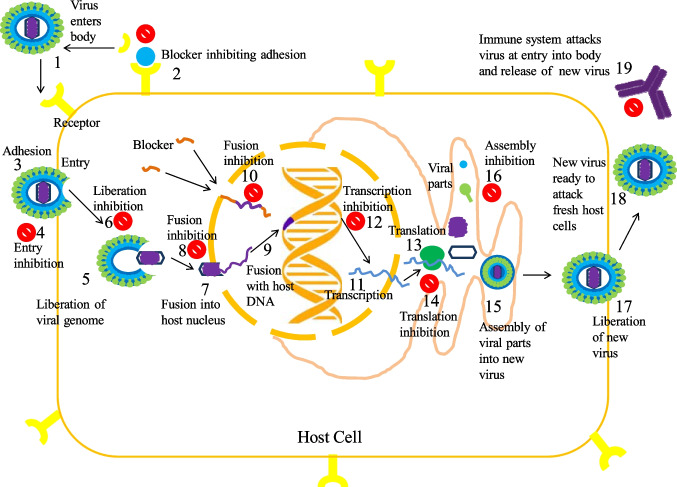
Viruses contain a genome (DNA or RNA) covered by a protein capsule, which stores a few enzymes and in some may be covered by a lipid envelope. The main concept behind the anti-viral drug is to recognize viral proteins or parts of viral proteins, that can be deactivated/weakened/destroyed, obstructing viral replication by inhibiting the entry of the virus into the host cell in controlling the viral uptake, attaching to surface receptors, and by competing for pathways of activation of intracellular signals (Ghosh et al. 2009; Kapoor et al. 2017; Khan et al. 2005; Mohapatra and Dar 2010). For a drug to have the broad-spectrum effect, the viral targets should also be common for many strains of a virus or at least, for different species in the same family. Creating secure and potent anti-viral drugs is tough, as viruses tend to replicate in a host cell (hard to find viral targets without damaging host cell), mutate fast, viral variation, and in the long term develop resistance. Each virus has a specific life cycle depending on the strain, but those all follow a specific pattern: (1) adhesion to host cell, (2) liberation of the viral genome and perhaps enzymes into the host cell, (3) replication of viral parts by subjugating host-cell machinery, (4) congregation of viral parts into full viral particles, and (5) liberation of viral particles to infect fresh host cells (Fig. 12).
Fig. 12.
Schematic representation of the mechanism of action and effect of anti-viral phytoactive compounds through virus life cycle
One of the strategies is to inhibit the adhesion of the virus to the host cell. This would be achieved by blocking receptor sites on host cells by anti-virus-associated protein (VAP) antibodies. Inhibiting the entry by a powerful entry-inhibitor or entry-blocker agent may not only impede the spreading of the virus within an infected person but also the dissemination from an infected person to an uninfected person is stopped. Eventually, this will result in difficulties for the virus to develop resistance, as those would not use host-cell machinery to reproduce and mutate. Even research has been conducted for blocking sockets on the viral surface that checks the un-coating process. The next strategy is to focus on methods virus use to synthesize Alarcón by subjugating host-cell machinery. The target is to block at the label of transcription, translation, and post-translational modifications (Alarcón et al. 1988; Deas et al. 2005; Ryu and Lee 2003). Thereafter, the next step is to inhibit the congregation of viral parts into full viral particles and releases (Sodeik et al. 1994). The final and important step is to stimulate the immune system to attack viruses by generating antibodies and interferon (Samuel 2001) (Fig. 12).
Structural activity relationship studies of antiviral action of phytocompounds
Many flavonoids including baicalein, fisetin, quercetin, and naringenin had been used to control the replication of several viruses from which, baicalein deteriorated the structure of the Japanese encephalitis virus (JSV) and interfered the viral absorption, but quercetin had no other antiviral action with JSV. As per the structural concerning of these flavonoids, quercetin, fisetin, and kaempferol, the latter is chemically a flavonol (3-hydroxy flavone) derivative, whereas baicalein had the flavone structure that makes it less polar than flavonol derived glycosides. The flavonoids with less hydrophilic give more response against JSV. Similarly, the flavylium salt dalphinidin had shown having more binding affinity with a MARV protein that was proven by a molecular docking study. Flavanone-derived glycoside, naringenin, had been a potent antiviral with action against dengue fever. Chemically, α-β unsaturated β-diketone, aromatic methoxy, and the phenolic system in the curcumin structure are responsible for antiviral action against Ebola and Zika viruses. The diketone system of curcumin was converted into enolates that were readily deprotonated and acted as an acceptor in Michael’s nucleophilic addition reaction. For the enhancing of the aqueous solubility of curcumin, the chemical modification of α-β unsaturated β-diketone moiety led to producing a potent antiviral candidate. The presence of endoperoxide, a lactone group in artemisinin structure, and their semisynthetic derivatives might be responsible for a good antiviral action against the chickenguniya virus. Additionally, the trigocherrin A (dechlorinated methylene diterpene orthoester) had exhibited a potent and selective effect against the chikungunya virus in Vero cells. Overall, concisely, for the essential structural activity study of phyto-oils for the inhibition of different viruses, the presence of polyphenolic moiety in coumarin or chromone fused ring system, dihydrochalcone group, lactone derived endoperoxide group, or pentacyclic triterpenoid saponin within the ring system containing benzoquinone ring and gallate of catechin must be helpful.
Conclusions and future perspectives
In the realm of targeted therapies, there is a necessity for some low-cost, potent, and safe anti-viral drugs/therapies with the least side effects. Therefore, the suitable phytoactive compound(s) can be effective in developing such anti-virals, as selective non-toxic plant products would be metabolized and excreted without increasing any cell toxicity. Collaborative work can be done in the future by combining new as, the reported anti-viral phytoactive compounds with already approved drugs for enhanced and durable effectiveness. Phytochemicals can be converted to nanosuspensions, nanoparticles, nanocapsules, microspheres, solid dispersions, crystals, micelles, self-nanoemulsifying and self-microemulsifying drug delivery systems (SNEDDS and SMEDDS), for improved targeted delivery, extended activity, and superior effects, as seen against in “Mainstream medicines.” Moreover, every country should encourage exclusive research to explore in and around various biodiversity-rich zones and contact ethnomedicine practitioners to identify more potential phytoactive compounds with anti-viral effects, for the global issues of viral diseases.
Acknowledgements
The authors are thankful to the Institute of Medical Sciences & SUM Hospital, School of Pharmaceutical Sciences, Siksha O Anusandhan Deemed to be University, Bhubaneswar, Odisha, India.
Author contribution
SM, CRS: formal analysis, data curation, software, and validation. SM, CRS, SKP: writing of this manuscript and critical revision. RNP: edited the whole manuscript. All authors read and approved the final manuscript. The authors confirm that no paper mill and artificial intelligence was used.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Swati Sucharita Mohanty, Email: swatimohanty16@gmail.com.
Chita Ranjan Sahoo, Email: chitaranjan.biotech@gmail.com.
Sudhir Kumar Paidesetty, Email: sairampaidesetty@gmail.com.
Rabindra Nath Padhy, Email: rnpadhy54@gmail.com.
References
- Akbar MU, Zia KM, Nazir A, Iqbal J, Ejaz SA, Akash MSH. Pluronic-based mixed polymeric micelles enhance the therapeutic potential of curcumin. AAPS PharmSciTech. 2018 doi: 10.1208/s12249-018-1098-9. [DOI] [PubMed] [Google Scholar]
- Alarcón B, González ME, Carrasco L. Megalomycin C, a macrolide antibiotic that blocks protein glycosylation and shows antiviral activity. FEBS Lett. 1988 doi: 10.1016/0014-5793(88)80732-4. [DOI] [PubMed] [Google Scholar]
- Álvarez ÁL, Habtemariam S, Abdel Moneim AE, Melón S, Dalton KP, Parra F. A spiroketal-enol ether derivative from Tanacetum vulgare selectively inhibits HSV-1 and HSV-2 glycoprotein accumulation in vero cells. Antiviral Res. 2015 doi: 10.1016/j.antiviral.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Badoni H, Painuli S, Semwal P. In silico screening of phytoactive components against Junin, Hanta, Dengue, Marburg and Ebola Viruses. J Chem Pharm Res. 2015;7:209–224. [Google Scholar]
- Bai R, Zhang XJ, Li YL, Liu JP, Zhang HB, Xiao WL, Pu JX, Sun HD, Zheng YT, Liu LX. SJP-L-5, a novel small-molecule compound, inhibits HIV-1 infection by blocking viral DNA nuclear entry Microbe-host interactions and microbial pathogenicity. BMC Microbiol. 2015 doi: 10.1186/s12866-015-0605-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baikerikar S. Curcumin and natural derivatives inhibit Ebola viral proteins: an in silico approach. Pharmacognosy Res. 2017 doi: 10.4103/pr.pr_30_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakrim S, Aboulaghras S, El Menyiy N, El Omari N, Assaggaf H, Lee LH, Montesano D, Gallo M, Zengin G, Aldhaheri Y, Bouyahya A. Phytochemical compounds and nanoparticles as phytochemical delivery systems for Alzheimer's disease management. Molecules. 2022;27(24):9043. doi: 10.3390/molecules27249043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang S, Quy Ha TK, Lee C, Li W, Oh WK, Shim SH (2016) Antiviral activities of compounds from aerial parts of Salvia plebeia R. Br. J Ethnopharmacol. 10.1016/j.jep.2016.09.030 [DOI] [PubMed]
- Bauer A, Brönstrup M. Industrial natural product chemistry for drug discovery and development. Nat Prod Rep. 2014 doi: 10.1039/c3np70058e. [DOI] [PubMed] [Google Scholar]
- Beck CR, Sokal R, Arunachalam N, Puleston R, Cichowska A, Kessel A, Zambon M, Nguyen-Van-Tam JS (2013) Neuraminidase inhibitors for influenza: a review and public health perspective in the aftermath of the 2009 pandemic. Influenza Other Respi. Viruses. 10.1111/irv.12048 [DOI] [PMC free article] [PubMed]
- Bedows E, Hatfield GM. An investigation of the antiviral activity of Podophyllum peltatum. J Nat Prod. 1982 doi: 10.1021/np50024a015. [DOI] [PubMed] [Google Scholar]
- Ben-Shabat S, Yarmolinsky L, Porat D, Dahan A (2020) Antiviral effect of phytochemicals from medicinal plants: applications and drug delivery strategies. Drug Deliv Transl Res. 10.1007/s13346-019-00691-6 [DOI] [PMC free article] [PubMed]
- Bhakat S, Soliman MES. Chikungunya virus (CHIKV) inhibitors from natural sources: a medicinal chemistry perspective. J Nat Med. 2015 doi: 10.1007/s11418-015-0910-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedenkopf N, Lange-Grünweller K, Schulte FW, Weißer A, Müller C, Becker D, Becker S, Hartmann RK, Grünweller A. The natural compound silvestrol is a potent inhibitor of Ebola virus replication. Antiviral Res. 2017;137:76–81. doi: 10.1016/j.antiviral.2016.11.011. [DOI] [PubMed] [Google Scholar]
- Byler KG, Ogungbe IV, Setzer WN. In-silico screening for anti-Zika virus phytochemicals. J Mol Graph Model. 2016 doi: 10.1016/j.jmgm.2016.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng YB, Chien YT, Lee JC, Tseng CK, Wang HC, Lo IW, Wu YH, Wang SY, Wu YC, Chang FR. Limonoids from the seeds of swietenia macrophylla with inhibitory activity against dengue virus 2. J Nat Prod. 2014 doi: 10.1021/np5002829. [DOI] [PubMed] [Google Scholar]
- Chung CY, Liu CH, Burnouf T, Wang GH, Chang SP, Jassey A, Tai CJ, Tai CJ, Huang CJ, Richardson CD, Yen MH, Lin CC, Lin LT. Activity-based and fraction-guided analysis of Phyllanthus urinaria identifies loliolide as a potent inhibitor of hepatitis C virus entry. Antiviral Res. 2016 doi: 10.1016/j.antiviral.2016.03.012. [DOI] [PubMed] [Google Scholar]
- Clain E, Haddad JG, Koishi AC, Sinigaglia L, Rachidi W, Desprès P, Duarte dos Santos CN, Guiraud P, Jouvenet N, El Kalamouni C. The polyphenol-rich extract from psiloxylon mauritianum, an endemic medicinal plant from reunion island, inhibits the early stages of dengue and zika virus infection. Int J Mol Sci. 2019 doi: 10.3390/ijms20081860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clain E, Sinigaglia L, Koishi AC, Gorgette O, Gadea G, Viranaicken W, Krejbich-Trotot P, Mavingui P, Desprès P, Dos Santos CND, Guiraud P, Jouvenet N, El Kalamouni C (2018) Extract from Aphloia theiformis, an edible indigenous plant from Reunion Island, impairs Zika virus attachment to the host cell surface. Sci Rep. 10.1038/s41598-018-29183-2 [DOI] [PMC free article] [PubMed]
- Cock IE, Van Vuuren SF. The traditional use of southern African medicinal plants in the treatment of viral respiratory diseases: a review of the ethnobotany and scientific evaluations. J Ethnopharmacol. 2020 doi: 10.1016/j.jep.2020.113194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corlay N, Delang L, Girard-Valenciennes E, Neyts J, Clerc P, Smadja J, Guéritte F, Leyssen P, Litaudon M. Tigliane diterpenes from Croton mauritianus as inhibitors of chikungunya virus replication. Fitoterapia. 2014 doi: 10.1016/j.fitote.2014.05.015. [DOI] [PubMed] [Google Scholar]
- Cragg GM, Newman DJ (2005) Biodiversity: a continuing source of novel drug leads, in: Pure and applied chemistry. 10.1351/pac200577010007
- Cui H, Xu B, Wu T, Xu J, Yuan Y, Gu Q. Potential antiviral lignans from the roots of saururus chinensis with activity against epstein-barr virus lytic replication. J Nat Prod. 2014 doi: 10.1021/np400757k. [DOI] [PubMed] [Google Scholar]
- Dao TT, Nguyen PH, Lee HS, Kim E, Park J, Lim SI, Oh WK. Chalcones as novel influenza A (H1N1) neuraminidase inhibitors from Glycyrrhiza inflata. Bioorg Med Chem Lett. 2011;21:294–298. doi: 10.1016/j.bmcl.2010.11.016. [DOI] [PubMed] [Google Scholar]
- De Smet PAGM. Herbal remedies. N Engl J Med. 2002;347:2046–2056. doi: 10.1056/NEJMra020398. [DOI] [PubMed] [Google Scholar]
- Deas TS, Binduga-Gajewska I, Tilgner M, Ren P, Stein DA, Moulton HM, Iversen PL, Kauffman EB, Kramer LD, Shi P-Y. Inhibition of flavivirus infections by antisense oligomers specifically suppressing viral translation and RNA replication. J Virol. 2005 doi: 10.1128/jvi.79.8.4599-4609.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwevedi A, Dwivedi R, Sharma Y (2016) Exploration of phytochemicals found in Terminalia sp. and their antiretroviral activities. Pharmacogn Rev. 10.4103/0973-7847.194048 [DOI] [PMC free article] [PubMed]
- Eggers M, Jungke P, Wolkinger V, Bauer R, Kessler U, Frank B. Antiviral activity of plant juices and green tea against SARS-CoV-2 and influenza virus. Phytother Res. 2022;36(5):2109–2115. doi: 10.1002/ptr.7431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esimone CO, Eck G, Nworu CS, Hoffmann D, Uberla K, Proksch P. Dammarenolic acid, a secodammarane triterpenoid from Aglaia sp. shows potent anti-retroviral activity in vitro. Phytomedicine. 2010;17:540–547. doi: 10.1016/j.phymed.2009.10.015. [DOI] [PubMed] [Google Scholar]
- Esposito F, Carli I, Del Vecchio C, X L, Corona A, Grandi N, Piano D, Maccioni E, Distinto S, Parolin C, Tramontano E (2016) Sennoside A, derived from the traditional chinese medicine plant Rheum L., is a new dual HIV-1 inhibitor effective on HIV-1 replication. Phytomedicine. 10.1016/j.phymed.2016.08.001 [DOI] [PubMed]
- Ferreira FL, Hauck MS, Duarte LP, de Magalhães JC, da Silva LSM, Pimenta LPS, Lopes JCD, Mercadante‑Simões MO, Vieira Filho SA (2019) Zika virus activity of the leaf and branch extracts of Tontelea micrantha and its hexane extracts phytochemical study. J Braz Chem Soc. 10.21577/0103-5053.20180210
- Ghildiyal R, Prakash V, Chaudhary VK, Gupta V, Gabrani R, (2020) Phytochemicals as antiviral agents: recent updates, in: Plant-derived bioactives: production, properties and therapeutic applications. 10.1007/978-981-15-1761-7_12
- Ghosh T, Chattopadhyay K, Marschall M, Karmakar P, Mandal P, Ray B. Focus on antivirally active sulfated polysaccharides: from structure-activity analysis to clinical evaluation. Glycobiology. 2009 doi: 10.1093/glycob/cwn092. [DOI] [PubMed] [Google Scholar]
- Goktas Z, Zu Y, Abbasi M, Galyean S, Wu D, Fan Z, Wang S. Recent advances in nanoencapsulation of phytochemicals to combat obesity and its comorbidities. J Agric Food Chem. 2020;68(31):8119–8131. doi: 10.1021/acs.jafc.0c00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Calderón C, Mesa-Castro C, Robledo S, Gómez S, Bolivar-Avila S, Diaz-Castillo F, Martínez-Gutierrez M. Antiviral effect of compounds derived from the seeds of Mammea americana and Tabernaemontana cymosa on dengue and chikungunya virus infections. BMC Complement Altern Med. 2017 doi: 10.1186/s12906-017-1562-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TJ, Tsai YC, Chiang SY, Wang GJ, Kuo YC, Chang YC, Wu YY, Wu YC. Anti-viral effect of a compound isolated from Liriope platyphylla against hepatitis B virus in vitro. Virus Res. 2014 doi: 10.1016/j.virusres.2014.07.015. [DOI] [PubMed] [Google Scholar]
- Huerta-Reyes M, Gaitán-Cepeda LA, Sánchez-Vargas LO. Punica granatum as anticandidal and anti-HIV agent: an HIV oral cavity potential drug. Plants. 2022;11(19):2622. doi: 10.3390/plants11192622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idriss H, Siddig B, González-Maldonado P, Elkhair HM, Alakhras AI, Abdallah EM, Elzupir AO, Sotelo PH. Effect of the phytochemical agents against the SARS-CoV and some of them selected for application to COVID-19: a mini-review. Curr Pharm Biotechnol. 2023 doi: 10.2174/1389201021666200703201458. [DOI] [PubMed] [Google Scholar]
- Idrees M, Khan S, Memon NH, Zhang Z (2021) Effect of the Phytochemical Agents against the SARS-CoV and Some of them Selected for Application to COVID-19: A Mini-Review. Curr Pharm Biotechnol. 22:444–450 Available at: https://pubmed.ncbi.nlm.nih.gov/32619167/. Accessed 6 May 2023 [DOI] [PubMed]
- Idriss H, Siddig B, González-Maldonado P, Elkhair HM, Alakhras AI, Abdallah EM, Elzupir AO, Sotelo PH. Inhibitory activity of Saussurea costus extract against bacteria, candida, herpes, and SARS-CoV-2. Plants. 2023;12(3):460. doi: 10.3390/plants12030460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa SS, Sokornova SV, Zhidkin RR, Matveeva TV. The main protease of SARS-CoV-2 as a target for phytochemicals against coronavirus. Plants. 2022;11(14):1862. doi: 10.3390/plants11141862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova E, Toshkova R, Serkedjieva J. A plant polyphenol-rich extract restores the suppressed functions of phagocytes in influenza virus-infected mice. Microbes Infect. 2005 doi: 10.1016/j.micinf.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Jain J, Kumar A, Narayanan V, Ramaswamy RS, Sathiyarajeswaran P, Shree Devi MS, Kannan M, Sunil S. Antiviral activity of ethanolic extract of Nilavembu Kudineer against dengue and chikungunya virus through in vitro evaluation. J Ayurveda Integr Med. 2020 doi: 10.1016/j.jaim.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang KB, Ming G, Kim GJ, Ha TKQ, Choi H, Oh WK, Sung SH. Jubanines F-J, cyclopeptide alkaloids from the roots of Ziziphus jujuba. Phytochemistry. 2015 doi: 10.1016/j.phytochem.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor R, Sharma B, Kanwar SS (2017) Antiviral phytochemicals: an overview. Biochem Physiol Open Access. 10.4172/2168-9652.1000220
- Khan MTH, Ather A, Thompson KD, Gambari R. Extracts and molecules from medicinal plants against herpes simplex viruses. Antiviral Res. 2005 doi: 10.1016/j.antiviral.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Krammer F, Smith GJD, Fouchier RAM, Peiris M, Kedzierska K, Doherty PC, Palese P, Shaw ML, Treanor J, Webster RG, García-Sastre A (2018) Influenza Nat Rev Dis Prim. 10.1038/s41572-018-0002-y [DOI] [PMC free article] [PubMed]
- Kretzing S, Abraham G, Seiwert B, Ungemach FR, Krügel U, Regenthal R. Dose-dependent emetic effects of the Amaryllidaceous alkaloid lycorine in beagle dogs. Toxicon. 2011 doi: 10.1016/j.toxicon.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Lee CD, Ott M, Thyagarajan SP, Shafritz DA, Burk RD, Gupta S. Phyllanthus amarus down-regulates hepatitis B virus mRNA transcription and replication. Eur J Clin Invest. 1996 doi: 10.1046/j.1365-2362.1996.410595.x. [DOI] [PubMed] [Google Scholar]
- Lee JL, Loe MWC, Lee RCH, Chu JJH. Antiviral activity of pinocembrin against Zika virus replication. Antiviral Res. 2019 doi: 10.1016/j.antiviral.2019.04.003. [DOI] [PubMed] [Google Scholar]
- Li Y, Wu Y, Li S, Li Y, Zhang X, Shou Z, Gu S, Zhou C, Xu D, Zhao K, Tan S. Identification of phytochemicals in Qingfei Paidu decoction for the treatment of coronavirus disease 2019 by targeting the virus-host interactome. Biomed Pharmacother. 2022;156:113946. doi: 10.1016/j.biopha.2022.113946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, But PPH, Ooi VEC (2005) Antiviral activity and mode of action of caffeoylquinic acids from Schefflera heptaphylla (L.) Frodin. Antiviral Res. 10.1016/j.antiviral.2005.06.004 [DOI] [PubMed]
- Lin LT, Chung CY, Hsu WC, Chang SP, Hung TC, Shields J, Russell RS, Lin CC, Li CF, Yen MH, Tyrrell DLJ, Lin CC, Richardson CD. Saikosaponin b2 is a naturally occurring terpenoid that efficiently inhibits hepatitis C virus entry. J Hepatol. 2015 doi: 10.1016/j.jhep.2014.10.040. [DOI] [PubMed] [Google Scholar]
- Lung J, Lin YS, Yang YH, Chou YL, Shu LH, Cheng YC, Liu HT, Wu CY. The potential chemical structure of anti-SARS-CoV-2 RNA-dependent RNA polymerase. J Med Virol. 2020 doi: 10.1002/jmv.25761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani JS, Johnson JB, Steel JC, Broszczak DA, Neilsen PM, Walsh KB, Naiker M. Natural product-derived phytochemicals as potential agents against coronaviruses: A review. Virus Res. 2020 doi: 10.1016/j.virusres.2020.197989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manske RHF, Holmes HL (2014) The Alkaloids: Chemistry and Physiology 534
- Mao ZQ, Minakawa N, Moi ML. Novel antiviral efficacy of Hedyotis diffusa and Artemisia capillaris extracts against dengue virus, Japanese encephalitis virus, and Zika virus infection and immunoregulatory cytokine signatures. Plants. 2022;11(19):2589. doi: 10.3390/plants11192589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuse IT, Lim YA, Hattori M, Correa M, Gupta MP. A search for anti-viral properties in Panamanian medicinal plants. The effects on HIV and its essential enzymes. J Ethnopharmacol. 1998;64(1):15–22. doi: 10.1016/s0378-8741(98)00099-3. [DOI] [PubMed] [Google Scholar]
- Moghaddam E, Teoh BT, Sam SS, Lani R, Hassandarvish P, Chik Z, Yueh A, Abubakar S, Zandi K (2014) Baicalin, a metabolite of baicalein with antiviral activity against dengue virus. Sci Rep. 10.1038/srep05452 [DOI] [PMC free article] [PubMed]
- Mohanty SS, Sahoo CR, Padhy RN (2021) Targeting some enzymes with repurposing approved pharmaceutical drugs for expeditious antiviral approaches against newer strains of COVID-19. AAPS PharmSciTech. 22(6):214. Published 2021 Aug 10. 10.1208/s12249-021-02089-5 [DOI] [PMC free article] [PubMed]
- Mohapatra S, Dar L (2010) Emerging and remerging viral infections in India. J Int Med Sci Acad 23:33–36. Available at: https://www.researchgate.net/publication/288187987
- Montanha JA, Amoros M, Boustie J, Girre L. Anti-herpes virus activity of aporphine alkaloids. Planta Med. 1995;61:419–424. doi: 10.1055/s-2006-958128. [DOI] [PubMed] [Google Scholar]
- Mothana RAA, Mentel R, Reiss C, Lindequist U (2006) Phytochemical screening and antiviral activity of some medicinal plants from the island Soqotra. Phyther Res. 10.1002/ptr.1858 [DOI] [PubMed]
- Mukherjee PK (2019) Antiviral evaluation of herbal drugs, in: Quality control and evaluation of herbal drugs. Elsevier, pp. 599–628. 10.1016/B978-0-12-813374-3.00016-8
- Mukhtar M, Arshad M, Ahmad M, Pomerantz RJ, Wigdahl B, Parveen Z. Antiviral potentials of medicinal plants. Virus Res. 2008 doi: 10.1016/j.virusres.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothias-Scaglia LF, Retailleau P, Paolini J, Pannecouque C, Neyts J, Dumontet V, Roussi F, Leyssen P, Costa J, Litaudon M. Jatrophane diterpenes as inhibitors of chikungunya virus replication: structure-activity relationship and discovery of a potent lead. J Nat Prod. 2014 doi: 10.1021/np500271u. [DOI] [PubMed] [Google Scholar]
- Ott M, Thyagarajan SP, Gupta S. Phyllanthus amarus suppresses hepatitis B virus by interrupting interactions between HBV enhancer I and cellular transcription factors. Eur J Clin Invest. 1997 doi: 10.1046/j.1365-2362.1997.2020749.x. [DOI] [PubMed] [Google Scholar]
- Pantev A, Ivancheva S, Staneva L, Serkedjieva J (2006) Biologically active constituents of a polyphenol extract from Geranium sanguineum L. with anti-influenza activity. Zeitschrift fur Naturforsch. - Sect. C J Biosci. 10.1515/znc-2006-7-807 [DOI] [PubMed]
- Perez GRM (2003) Antiviral activity of compounds isolated from plants. Pharm Biol 41:107–157. https://www.researchgate.net/publication/250188204_Antiviral_Activity_of_Compounds_Isolated_From_Plants. Accessed 4 May 2023
- Prahoveanu E, Eşanu V, Anton G, Frunzulicǎ S (1985) The effect of an aqueous horse-radish extract, applied as such or in association with caffeine, on experimental influenza in mice. Virologie 37:121–123. Available at: http://www.ncbi.nlm.nih.gov/pubmed/4036004. Accessed 4 May 2023 [PubMed]
- Prajoubklang A, Sirithunyalug B, Charoenchai P, Suvannakad R, Sriubolmas N, Piyamongkol S, Kongsaeree P, Kittakoop P. Bioactive deoxypreussomerins and dimeric naphthoquinones from Diospyros ehretioides fruits: Deoxypreussomerins may not be plant metabolites but may be from fungal epiphytes or endophytes. Chem Biodivers. 2005 doi: 10.1002/cbdv.200590108. [DOI] [PubMed] [Google Scholar]
- Pratheeba T, Taranath V, Sai Gopal DVR, Natarajan D. Antidengue potential of leaf extracts of Pavetta tomentosa and Tarenna asiatica (Rubiaceae) against dengue virus and its vector Aedes aegypti (Diptera: Culicidae) Heliyon. 2019 doi: 10.1016/j.heliyon.2019.e02732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin N, Li CB, Jin MN, Shi LH, Duan HQ, Niu WY. Synthesis and biological activity of novel tiliroside derivants. Eur J Med Chem. 2011 doi: 10.1016/j.ejmech.2011.07.059. [DOI] [PubMed] [Google Scholar]
- Ravikumar YS, Ray U, Nandhitha M, Perween A, Naika HR, Khanna N, Das S. Inhibition of hepatitis C virus replication by herbal extract: Phyllanthus amarus as potent natural source. Virus Res. 2011;158(1–2):89–97. doi: 10.1016/j.virusres.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Roumy V, Ruiz L, Macedo JCR, Gutierrez-Choquevilca AL, Samaillie J, Encinas LA, Mesia WR, Cotrina HER, Rivière C, Sahpaz S, Bordage S. Viral hepatitis in the Peruvian Amazon: ethnomedical context and phytomedical resource. J Ethnopharmacol. 2020;255:112735. doi: 10.1016/j.jep.2020.112735. [DOI] [PubMed] [Google Scholar]
- Ryu KJ, Lee SW. Identification of the most accessible sites to ribozymes on the hepatitis C virus internal ribosome entry site. J Biochem Mol Biol. 2003 doi: 10.5483/bmbrep.2003.36.6.538. [DOI] [PubMed] [Google Scholar]
- Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev. 2001 doi: 10.1128/CMR.14.4.778-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez I, Gómez-Garibay F, Taboada J, Ruiz BH (2000) Antiviral effect of flavonoids on the dengue virus. Phyther Res. 10.1002/(SICI)1099-1573(200003)14:2<89::AID-PTR569>3.0.CO;2-C [DOI] [PubMed]
- Shaikh F, Zhao Y, Alvarez L, Iliopoulou M, Lohans C, Schofield CJ, Padilla-Parra S, Siu SWI, Fry EE, Ren J, Stuart DI. Structure-based in silico screening identifies a potent Ebolavirus inhibitor from a traditional Chinese medicine library. J Med Chem. 2019 doi: 10.1021/acs.jmedchem.8b01328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinwari ZK (2010) Medicinal plants research in Pakistan. J Med Plants Res 4:161–176. Available at: https://academicjournals.org/article/article1380378394_Shinwari.pdf. Accessed 4 May 2023
- Shoji M, Woo SY, Masuda A, Win NN, Ngwe H, Takahashi E, Kido H, Morita H, Ito T, Kuzuhara T (2017) Anti-influenza virus activity of extracts from the stems of Jatropha multifida Linn.collected in Myanmar. BMC Complement. Altern Med. 10.1186/s12906-017-1612-8 [DOI] [PMC free article] [PubMed]
- Siddiqui MH, Alamri SA, Al-Whaibi MH, Hussain Z, Ali HM, El-Zaidy ME. A mini-review of anti-hepatitis B virus activity of medicinal plants. Biotechnol Biotechnol Equip. 2017;31:9–15. doi: 10.1080/13102818.2016.1240593. [DOI] [Google Scholar]
- Sochocka M, Sobczyński M, Ochnik M, Zwolińska K, Leszek J. Hampering herpesviruses HHV-1 and HHV-2 infection by extract of ginkgo biloba (EGb) and its phytochemical constituents. Front Microbiol. 2019 doi: 10.3389/fmicb.2019.02367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodeik B, Griffiths G, Ericsson M, Moss B, Doms RW. Assembly of vaccinia virus: effects of rifampin on the intracellular distribution of viral protein p65. J Virol. 1994 doi: 10.1128/jvi.68.2.1103-1114.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sornpet B, Potha T, Tragoolpua Y, Pringproa K (2017) Antiviral activity of five Asian medicinal pant crude extracts against highly pathogenic H5N1 avian influenza virus. Asian Pac J Trop Med. 10.1016/j.apjtm.2017.08.010 [DOI] [PubMed]
- Taj R, Rao SV, Tulasi DP, Nisha R, Pavithra KBS. In silico studies on dengue and Marburg viral proteins with selected Murraya koenigii leaves constituents. World J Pharm Res. 2018;7:1290–1304. [Google Scholar]
- Tolo FM, Rukunga GM, Muli FW, Njagi ENM, Njue W, Kumon K, Mungai GM, Muthaura CN, Muli JM, Keter LK, Oishi E, Kofi-Tsekpo MW. Anti-viral activity of the extracts of a Kenyan medicinal plant Carissa edulis against herpes simplex virus. J Ethnopharmacol. 2006;104:92–99. doi: 10.1016/j.jep.2005.08.053. [DOI] [PubMed] [Google Scholar]
- Toney JH, Navas-Martín S, Weiss SR, Koeller A. Sabadinine: a potential non-peptide anti-severe acute-respiratory-syndrome agent identified using structure-aided design. J Med Chem. 2004;47:1079–1080. doi: 10.1021/jm034137m. [DOI] [PubMed] [Google Scholar]
- Wahyuni TS, Widyawaruyanti A, Lusida MI, Fuad A, Soetjipto, Fuchino H, Kawahara N, Hayashi Y, Aoki C, Hotta H (2014) Inhibition of hepatitis C virus replication by chalepin and pseudane IX isolated from Ruta angustifolia leaves. Fitoterapia. 10.1016/j.fitote.2014.10.011 [DOI] [PubMed]
- Wang WN, Yang XB, Liu HZ, Huang ZM, Wu GX. Effect of Oenanthe javanica flavone on human and duck hepatitis B virus infection. Acta Pharmacol Sin. 2005 doi: 10.1111/j.1745-7254.2005.00055.x. [DOI] [PubMed] [Google Scholar]
- Wang X, Jia W, Zhao A, Wang X (2006) Anti-influenza agents from plants and traditional Chinese medicine. Phytother Res 20:335–341. Available at: https://pubmed.ncbi.nlm.nih.gov/16619359/. Accessed 4 May 2023 [DOI] [PubMed]
- Wei PH, Wu SZ, Mu XM, Xu B, Su QJ, Wei JL, Yang Y, Qin B, Xie ZC (2015) Effect of alcohol extract of Acanthus ilicifolius L. on anti-duck hepatitis B virus and protection of liver. J Ethnopharmacol. 10.1016/j.jep.2014.10.050 [DOI] [PubMed]
- WHO (2023) HIV. Available at: https://www.who.int/data/gho/data/themes/hiv-aids. Accessed 4 May 2023
- Xiang Y, Pei Y, Qu C, Lai Z, Ren Z, Yang K, Xiong S, Zhang Y, Yang C, Wang D, Liu Q. In vitro anti-herpes simplex virus activity of 1,2,4,6-tetra-O-galloyl-β-D-glucose from Phyllanthus emblica L. (Euphorbiaceae) Phytother Res. 2011;25(7):975–982. doi: 10.1002/ptr.3368. [DOI] [PubMed] [Google Scholar]
- Xu JJ, Wu X, Li MM, Li GQ, Yang YT, Luo HJ, Huang WH, Chung HY, Ye WC, Wang GC, Li YL. Antiviral activity of polymethoxylated flavones from “ guangchenpi”, the edible and medicinal pericarps of citrus reticulata ‘chachi’. J Agric Food Chem. 2014 doi: 10.1021/jf404310y. [DOI] [PubMed] [Google Scholar]
- Yang QY, Tian XY, Fang WS. Bioactive coumarins from Boenninghausenia sessilicarpa. J Asian Nat Prod Res. 2007 doi: 10.1080/10286020500382397. [DOI] [PubMed] [Google Scholar]
- Yang JL, Ha TKQ, Dhodary B, Pyo E, Nguyen NH, Cho H, Kim E, Oh WK. Oleanane triterpenes from the flowers of Camellia japonica inhibit porcine epidemic diarrhea virus (PEDV) replication. J Med Chem. 2015 doi: 10.1021/jm501567f. [DOI] [PubMed] [Google Scholar]
- Yang M, Wang Y, Yue Y, Liang L, Peng M, Zhao M, Chen Y, Cao X, Li W, Li C, Zhang H. Traditional Chinese medicines as effective agents against influenza virus-induced pneumonia. Biomed Pharmacother. 2022;153:113523. doi: 10.1016/j.biopha.2022.11352. [DOI] [PubMed] [Google Scholar]
- Yang CM, Cheng HY, Lin TC, Chiang LC, Lin CC (2007a) Hippomanin A from acetone extract of Phyllanthus urinaria inhibited HSV-2 but not HSV-1 infection in vitro. Phyther Res. 10.1002/ptr.2232 [DOI] [PubMed]
- Zhang GB, Zhang B, Zhang XX, Bing FH. Homonojirimycin, an alkaloid from dayflower inhibits the growth of influenza A virus in vitro. Acta Virol. 2013 doi: 10.4149/av_2013_01_85. [DOI] [PubMed] [Google Scholar]
- Zhang DH, Wu KL, Zhang X, Deng SQ, Peng B (2020) In silico screening of Chinese herbal medicines with the potential to directly inhibit 2019 novel coronavirus. J Integr Med. 10.1016/j.joim.2020.02.005 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.