Abstract
Formation of anti-Toxoplasma gondii HSP70 (TgHSP70) antibody cross-reactive to mouse HSP70 (mHSP70) was observed in the sera of BALB/c (a resistant strain) and C57BL/6 (B6; a susceptible strain) mice after peroral infection with T. gondii cysts of the Fukaya strain. The levels of anti-mHSP70 immunoglobulin G (IgG) autoantibody production in B6 mice were higher than those in BALB/c mice. The isotype and subclass of IgG of anti-TgHSP70 monoclonal antibodies cross-reactive to mHSP70 were μ and γ3. Anti-mHSP70 autoantibody in T. gondii-infected BALB/c and B6 mice was shown to be produced by the CD5+ subset of B cells (B-1a cells) but not by conventional B cells (B-2 cells). The epitopes recognized by anti-mHSP70 autoantibody were located primarily in the C-terminal fragment of mHSP70.
Toxoplasma gondii, an obligate intracellular parasitic protozoan, causes a life-threatening toxoplasmosis in fetuses or in immunocompromised humans and mice, and the mechanisms of antigen presentation of T. gondii-infected cells have been analyzed (2, 45, 46, 48). We have previously demonstrated a potential role of human heat shock cognate protein 70 (hHSC70) in antigen processing and presentation of T. gondii-infected cells to CD4+ cytotoxic T lymphocytes in humans (44) and have reported anti-T. gondii HSP70 (TgHSP70) antibody formation in T. gondii-infected BALB/c and B6 mice (23).
Members of the HSP family have been shown to have important functions as (i) intracellular detergents for aggregated and denatured molecules formed as a result of exposure of cells to physical stressors and (ii) molecular chaperones in peptide and protein transport between cell organelles (14, 35, 41). Of the HSP family members, HSP70 has been shown to be a major immunodominant antigen in bacterial and parasite infections as well as the preferred target of the humoral and cell-mediated immune responses to infection (7, 28, 49). The nucleic acid sequence of HSP70 is known to be highly homologous among different species. Indeed, the sequence of TgHSP70 is 68.1 and 74.1% homologous to those of mouse HSP70 (mHSP70) and hHSC70, respectively (6, 8, 47, 50); thus, it is possible that the high homology of HSP70 between T. gondii and hosts could induce autoreactive immune responses in T. gondii-infected hosts. Autoantibody induction against host components has been reported in infection with other parasites such as Trypanosoma cruzi (16), Plasmodium falciparum (21), Schistosoma mansoni (15), and Onchocerca volvulus (27). In particular, Mattei et al. demonstrated that sera from humans infected with malaria recognized the human HSP70, indicating that autoantibodies directed against host HSP70 could be induced by the homologous parasite protein (21). In contrast, humans infected with Echinococcus granulosus and Leishmania braziliensis did not induce autoimmune reactivity against homologous hHSP70, although specific antibodies reactive with parasite HSP70 were detected (4, 34). No such anti-host HSP70 autoantibody was induced in dogs during viscero-cutaneous leishmaniasis either (31). Thus, it seems that anti-HSP70 autoantibody formation is not often observed in parasite infection.
On the other hand, the existence of autoreactive B cells specific for HSP70 has been reported in both experimental and human autoimmune diseases (3, 29, 32, 37, 38). Furthermore, it has been found that CD5+ B cells (B-1 cells, specifically B-1a cells), which differ from conventional (CD5−) B cells (B-2 cells) are particularly predisposed to autoantibody production (9, 11, 13, 24).
In this study, we demonstrated the production of anti-TgHSP70 antibody cross-reactive to self mHSP70 and showed that B-1a cells are responsible for anti-mHSP70 autoantibody formation in T. gondii-infected BALB/c and B6 mice.
MATERIALS AND METHODS
Mice and T. gondii strain.
Eight-week-old female BALB/c (H-2d) and B6 (H-2b) mice were purchased from SLC (Hamamatsu, Japan). BALB/c and B6 mice were perorally infected with various numbers of T. gondii cysts of the Fukaya strain as previously described (20, 23). At 1, 3, 5, 7, and 9 weeks postinfection, mice were bled via the tail vein. Sera were collected, and antibody production was tested by enzyme-linked immunosorbent assay (ELISA) and Western blotting.
Cloning and expression of rmHSP70.
The total RNA of B6 lymphoma (RMA) cells was prepared by a single-step guanidine isothiocyanate-phenol-chloroform extraction method (TRIzol; GIBCO BRL, Gaithersburg, Md.). Oligonucleotide primers were designed based on the mHSP70 cognate DNA sequence (GenBank accession number M19141) with appropriate flanking restriction enzyme digestion sites to facilitate cloning. Preparation of cDNA and PCR for the amplification of mHSP70 cDNAs were performed using a Takara RNA kit with avian myeloblastosis virus reverse transcriptase (RT) (Takara Shuzo Co., Kyoto, Japan). The sequence of the sense and antisense PCR primers used were 5′-GGCTCGAGCATATGATGTCTAAGGGACCTGCA-3′ and 5′-GGGGATCCTTAATCCACCTCTTCAATGG-3′, respectively. Thirty-five cycles of PCR were performed, each cycle consisting of 1 min of denaturation at 94°C, 1 min of annealing at 54°C, and 2 min of elongation at 72°C. For molecular cloning of the PCR fragments, RT-PCR products of mHSP70 from RMA cells were inserted into the pBC KS(+) phagemid vector (Stratagene, La Jolla, Calif.). To synthesize recombinant mHSP70 (rmHSP70), the mHSP70 cDNA was excised from pBC KS(+) by digestion with appropriate restriction enzymes and ligated into the expression vector pET-15b (Novagen, Madison, Wis.). The resulting constructs were then used to transform Escherichia coli strain BL21(DE), and the synthesis of recombinant protein was induced with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG). The recombinant His6-HSP70 protein (74 kDa) was then purified from the extract of transformed BL21(DE) by nickel chelate affinity chromatography according to the manufacturer's instructions. The purified His6-tagged protein isolated from the transformed BL21(DE) cells was analyzed by sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis (SDS-PAGE) and was stained with Coomassie blue. Cloning and expression of recombinant TgHSP70 (rTgHSP70) were previously described (23).
Western blotting and ELISA.
One microgram each of rTgHSP70, rmHSP70, Fukaya tachyzoite lysates containing TgHSP70 (72 kDa), and RMA lysates containing mHSP70 (72 kDa) were denatured by boiling in SDS sample buffer and then subjected to SDS-PAGE under reducing conditions. After electrophoresis, separated proteins were electroblotted onto a nitrocellulose membrane (Amersham, Buckinghamshire, England) as previously described (44). Blots were blocked with 10% skim milk in Tris-buffered saline (pH 7.6) containing 0.1% Tween 20 (TBST), probed with anti-TgHSP70 monoclonal antibody (MAb) for 2 h, washed four times in TBST, incubated with biotinylated rabbit anti-mouse immunoglobulin G (IgG) antibody (Sigma Biosciences, St. Louis, Mo.) diluted 1:1,000 for 2 h, and incubated with horseradish peroxidase-conjugated streptavidin (Sigma) diluted 1:1,000 for 30 min. Protein bands were visualized with an enhanced chemiluminescence detection system (Amersham, Arlington Heights, Ill.) according to the manufacturer's specifications.
Detection of anti-TgHSP70 antibody and anti-mHSP70 autoantibody in sera of T. gondii-infected mice was done by ELISA using rTgHSP70 and rmHSP70 as described previously (23). The peroxidase-conjugated anti-mouse polyvalent immunoglobulin (IgG, IgA, and IgM) antiserum (Sigma) was used as a second antibody. The sera of T. gondii-infected BALB/c and B6 mice were diluted at 1:50, 1:100, 1:200, 1:400, 1:800, 1:1,600, and 1:3,200, and reactivity of the diluted sera against rTgHSP70 and rmHSP70 was analyzed by ELISA.
Production of anti-TgHSP70 MAbs.
The spleen cells of T. gondii-infected BALB/c mice were fused with hypoxanthine-aminopterin-thymidine-sensitive P3U1 cells at a 1-to-5 ratio using 45% polyethylene glycol (molecular weight, 4,000; Sigma). For cloning, the culture supernatants of the hybridomas were tested by ELISA using rTgHSP70 as a target antigen.
Antibody adsorption.
Sera of T. gondii-infected BALB/c or B6 mice 4 weeks after infection were diluted 1:800 with phosphate-buffered saline (PBS) and were incubated for 1 h on ice in a plastic plate coated with 60 μg of either rTgHSP70 or rmHSP70; then the preadsorbed sera were harvested. As a control, the sera diluted 1:800 with PBS were similarly adsorbed in a plastic plate coated with 10 mg of bovine serum albumin (BSA) per ml. The preadsorbed sera were used for the ELISA targeting rTgHSP70 and rmHSP70. The titration of the sera of T. gondii-infected BALB/c and B6 mice unadsorbed and preadsorbed with rTgHSP70, rmHSP70, or BSA was analyzed at dilutions of 1:100, 1:200, 1:400, 1:800, 1:1,600, and 1:3,200 by ELISA.
Flow cytometric analysis.
Peritoneal exudate cells (PECs) of BALB/c and B6 mice before or 3 days after T. gondii infection were harvested. After deletion of the adherent cells by incubating PECs in a plastic bottle, the supernatants containing the nonadherent cells were collected and washed with chilled PBS containing 2% fetal calf serum and 0.05% NaN3. The cells were stained with R-phycoerythrin-conjugated rat anti-mouse CD5 (Ly-1) MAb (Ly1) and fluorescein isothiocyanate-conjugated rat anti-mouse CD45R/B220 MAb (PharMingen, San Diego, Calif.) by incubation for 30 min at 4°C. After washing, the stained cells were analyzed on a FACScan (Becton Dickinson).
Purification and culturing of CD5+ B cells.
To eliminate T cells, single-cell suspensions of PECs from BALB/c and B6 mice 3 days after T. gondii infection were incubated with microbeads conjugated with anti-CD90 MAb (Thy1.2; Miltenyi Biotec, Auburn, Calif.) and passed through a magnetic field (VarioMACS separator system; Miltenyi Biotec). Subsequently, CD5+ B cells were positively enriched from T-cell-depleted PECs by using microbeads conjugated with anti-CD5 MAb (Miltenyi Biotec). CD5+ and CD5− B-cell fractions of PECs were resuspended in RPMI 1640 culture medium supplemented with 5% fetal calf serum, 2-mercaptoethanol, and antibiotics and were cultured for 3 days at 37°C in a 96-well microplate at 105 cells/well. Production of anti-TgHSP70 antibody and anti-mHSP70 autoantibody in the supernatants was tested by ELISA. Titration of the culture supernatant of CD5+ and CD5− B cells against rTgHSP70 and rmHSP70 was analyzed at dilutions of 1:2, 1:4, 1:8, 1:16, and 1:32.
Statistics.
Differences between mean values were analyzed by unpaired Student's t test. P values less than 0.05 were considered statistically significant.
RESULTS
T. gondii cyst dose-dependent kinetics of anti-TgHSP70 antibody production in T. gondii-infected mice.
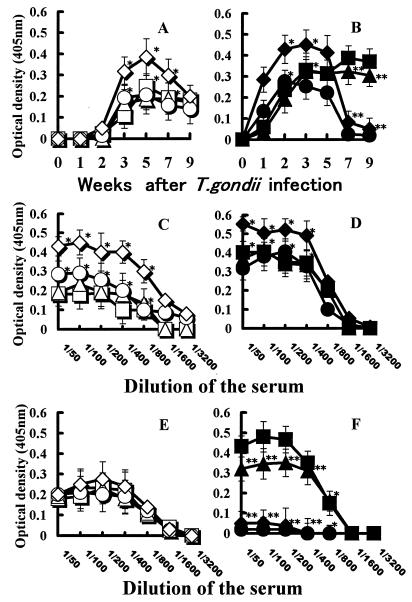
BALB/c (resistant) and B6 (susceptible) mice were perorally infected with various numbers of T. gondii Fukaya cysts (4, 6, 8, or 10 cysts/mouse), and production in the serum of antibody against TgHSP70 was tested weekly (Fig. 1). BALB/c mice infected with T. gondii began to produce anti-TgHSP70 antibody from 2 weeks after infection. The level of anti-TgHSP70 antibody formation in the sera of BALB/c mice infected with 10 cysts reached a peak at 5 weeks and then gradually decreased. However, the levels of anti-TgHSP70 antibody in the sera of BALB/c mice infected with four, six, and eight cysts reached a plateau at 3 to 5 weeks, and then the plateau persisted for more than 9 weeks (Fig. 1A). Titration data for anti-TgHSP70 antibody in the sera of BALB/c mice 3 and 9 weeks postinfection are shown in Fig. 1C and E, respectively.
FIG. 1.
(A and B) Antibody production specific for TgHSP70 in T. gondii-infected mice depends on the number of infecting T. gondii Fukaya cysts. BALB/c (A) and B6 (B) mice were perorally infected with 4, 6, 8, or 10 T. gondii Fukaya cysts. Production of antibody against TgHSP70 in the sera of T. gondii-infected BALB/c and B6 mice was tested weekly by ELISA by using rTgHSP70 protein as an antigen. (C to F) Titration analysis of anti-TgHSP70 antibody in sera of mice infected with different number of T. gondii cysts. The sera of BALB/c and B6 mice 3 (C and D) and 9 (E and F) weeks postinfection were diluted as described in Materials and Methods. Titration of anti-TgHSP70 antibody in sera of BALB/c and B6 mice infected with 4, 6, 8, and 10 cysts was measured by ELISA. Symbols represent sera of BALB/c mice infected with 4 (□), 6 (▵), 8 (○), or 10 (◊) cysts and sera of B6 mice infected with 4 (■), 6 (▴), 8 (●) or 10 (⧫) cysts of T. gondii Fukaya. Three to five mice were used for each experimental group, and the experiment was repeated three times. Data from a representative experiment are shown. ∗, P < 0.05; ∗∗, P < 0.01.
B6 mice infected with T. gondii Fukaya cysts produced anti-TgHSP70 antibody from 1 week after T. gondii infection. The level of anti-TgHSP70 antibody in the sera of B6 mice infected with 8 to 10 cysts reached a peak at 2 to 3 weeks and then decreased to the background level at 7 weeks after infection. On the other hand, the level of anti-TgHSP70 antibody in the sera of B6 mice infected with four to six cysts reached a plateau at 3 weeks, and then the plateau persisted for more than 9 weeks (Fig. 1B). The titration data of anti-TgHSP70 antibody in the sera of T. gondii-infected B6 mice 3 weeks postinfection are shown in Fig. 1D. Titration data for anti-TgHSP70 antibody in the sera of B6 mice 9 weeks after infection are shown in Fig. 1F. The levels of anti-TgHSP70 antibody in the sera of B6 mice infected with six or four cysts were significantly high, whereas no meaningful amount of antibody was observed in the sera of B6 mice infected with 8 or 10 cysts 9 weeks postinfection.
Anti-mHSP70 autoantibody formation in T. gondii-infected mice.
Because of the high homology in nucleotide sequences between TgHSP70 and mHSP70, we next examined the possibility of anti-mHSP70 autoantibody formation in T. gondii-infected mice. Both BALB/c and B6 mice were perorally infected with five T. gondii Fukaya cysts, and the formation of antibody reactive with mHSP70 in the sera of BALB/c and B6 mice was examined weekly.
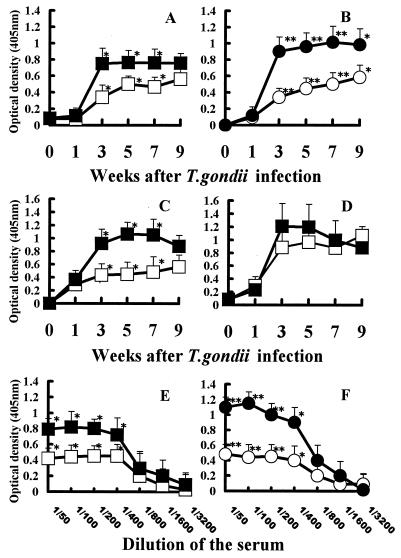
The level of anti-mHSP70 autoantibody, like that of anti-TgHSP70 antibody, markedly increased in both BALB/c and B6 mice after T. gondii infection (Fig. 2A and B). Anti-mHSP70 autoantibody formation in B6 mice increased from 1 week and reached a plateau at 3 weeks. Then autoantibody formation persisted for more than 9 weeks after infection. Anti-mHSP70 autoantibody formation of BALB/c mice reached a plateau at 5 weeks and then persisted until the end of the study. The patterns of antibody formation for mHSP70 were similar to those for TgHSP70 in both BALB/c and B6 mice. The levels of anti-mHSP70 autoantibody in the sera of B6 mice were higher than those in the sera of BALB/c mice. Similarly, the levels of anti-TgHSP70 antibody of B6 mice were higher than those of BALB/c mice. The titration data for anti-mHSP70 autoantibody and anti-TgHSP70 antibody in the sera of BALB/c and B6 mice 5 weeks after T. gondii infection are shown in Fig. 2E and F.
FIG. 2.
(A and B) Anti-mHSP70 autoantibodies as well as anti-TgHSP70 antibodies were produced in T. gondii-infected BALB/c and B6 mice. BALB/c and B6 mice were perorally infected with five T. gondii cysts of the Fukaya strain. By using rmHSP70 and rTgHSP70 protein as antigens, formation of anti-mHSP70 autoantibody (A) and anti-TgHSP70 antibody (B) in the sera of infected BALB/c and B6 mice was tested weekly by ELISA. (C and D) Isotype specificity of anti-mHSP70 autoantibody generated by T. gondii-infected mice. BALB/c and B6 mice were perorally infected with five T. gondii cysts of the Fukaya strain. Production of anti-mHSP70 IgG autoantibodies (C) or anti-mHSP70 IgM autoantibodies (D) in the sera of T. gondii-infected BALB/c and B6 mice was tested weekly by ELISA using alkaline phosphatase-conjugated anti-mouse IgG antibody or anti-mouse IgM antibody as the secondary antibody. (E and F) Titration analysis of anti-mHSP70 autoantibody and anti-TgHSP70 antibody in the sera of T. gondii-infected BALB/c and B6 mice. After dilution of the sera of BALB/c and B6 mice 5 weeks after T. gondii infection as described in Materials and Methods, the titration of anti-mHSP70 autoantibody (E) and anti-TgHSP70 antibody (F) was analyzed by ELISA. Symbols: □, anti-mHSP70 autoantibodies in BALB/c mice; ■, anti-mHSP70 autoantibodies in B6 mice; ○, anti-TgHSP70 antibodies in BALB/c mice; ●, anti-TgHSP70 antibodies in B6 mice. ∗, P < 0.05; ∗∗, P < 0.01.
Anti-mHSP70 IgG (Fig. 2C) and IgM (Fig. 2D) autoantibodies were produced in T. gondii-infected BALB/c and B6 mice. The level of anti-mHSP70 IgG autoantibody of B6 mice increased from 1 week after infection. Autoantibody formation reached a peak at 5 weeks and then gradually decreased. However, the level of anti-mHSP70 IgG autoantibody in BALB/c mice increased more gradually and was always lower than that in B6 mice. In contrast, similar patterns of anti-mHSP70 IgM autoantibody formation were observed in BALB/c and B6 mice.
Cross-reactivity of anti-TgHSP70 antibody with mHSP70.
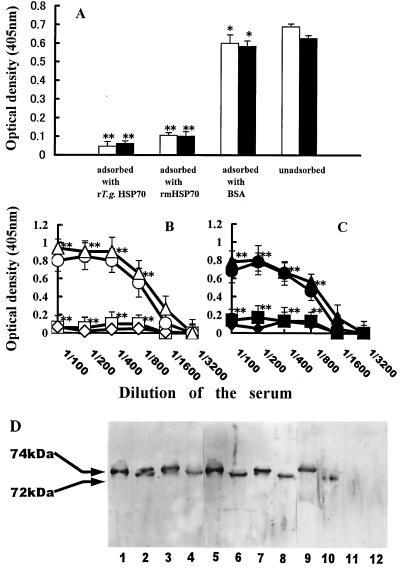
Next, the cross-reactivity of anti-TgHSP70 antibody with mHSP70 was analyzed by adsorption of serum with rTgHSP70 or rmHSP70. When the sera of T. gondii-infected BALB/c and B6 mice were adsorbed with rTgHSP70, the preadsorbed sera reacted with neither rTgHSP70 nor rmHSP70. Conversely, sera adsorbed with rmHSP70 reacted with neither rmHSP70 nor rTgHSP70. On the other hand, sera preadsorbed with BSA reacted with both rTgHSP70 and rmHSP70, indicating that the reactivity of the sera was specifically adsorbed with either rTgHSP70 and rmHSP70 (Fig. 3A). As shown in Fig. 3B and C, the titration data for anti-TgHSP70 antibody and anti-mHSP70 autoantibody of unadsorbed and adsorbed sera with rTgHSP70, rmHSP70, or BSA revealed that the majority of anti-TgHSP70 antibodies cross-reacted with mHSP70.
FIG. 3.
(A) Anti-mHSP70 autoantibody of T. gondii-infected mice recognized cross-reactive antigenic determinants shared by rTgHSP70 and rmHSP70. Sera of T. gondii-infected B6 mice were adsorbed with either rTgHSP70, rmHSP70, or BSA on ice as described in Materials and Methods, and then reactivities of the unadsorbed or preadsorbed sera with rTgHSP70 (open column) or rmHSP70 (closed column) were tested by ELISA. ∗, P > 0.05; ∗∗, P < 0.01 compared with the unadsorbed group. (B and C) Titration analysis of the unadsorbed and adsorbed sera of T. gondii-infected BALB/c and B6 mice with rTgHSP70, rmHSP70, or BSA against rTgHSP70 and rmHSP70. The diluted sera of T. gondii-infected mice were adsorbed with either rTgHSP70, rmHSP70, or BSA on ice as described in Materials and Methods. Titration of the sera unadsorbed (▵ or ▴) and adsorbed with either rTgHSP70 (□ or ■), rmHSP70 (◊ or ⧫), or BSA (○ or ●) against rTgHSP70 (B) and rmHSP70 (C) was analyzed by ELISA. ∗∗, P < 0.01. (D) Western blotting analysis of anti-mHSP70 autoantibody in T. gondii-infected mice. rTgHSP70 (lanes 1, 5, and 9) and TgHSP70 of Fukaya tachyzoite lysates (lanes 2, 6, and 10), rmHSP70 (lanes 3, 7, and 11), and mHSP70 of murine lymphoma line RMA lysates (lanes 4, 8, and 12) were separated by SDS-PAGE and then transferred onto nitrocellulose membranes. The membranes were probed with the sera of T. gondii-infected B6 mice (lanes 1 to 4), cross-reactive anti-TgHSP70 MAb TgCR 18 (lanes 5 to 8), and non-cross-reactive anti-TgHSP70 MAb TgNCR A5 (lanes 9 to 12).
Furthermore, the sera of T. gondii-infected BALB/c and B6 mice were shown by Western blotting to react not only with rTgHSP70 and TgHSP70 from lysates of Fukaya tachyzoites (Fig. 3D, lanes 1 and 2) but also with rmHSP70 and with natural mHSP70 from lysates of the murine lymphoma cell line RMA (Fig. 3D, lanes 3 and 4). Thus, anti-TgHSP70 antibodies in the sera of T. gondii-infected mice reacted with natural mHSP70 as well as rmHSP70.
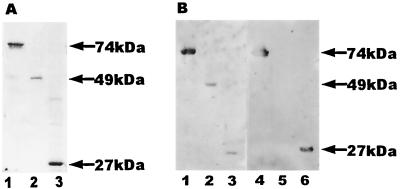
To examine the specificity of the anti-TgHSP70 antibody, we generated anti-TgHSP70 MAbs by establishing hybridomas from T. gondii-infected BALB/c mice. Two types of anti-TgHSP70 MAbs were obtained (Table 1). One type (TgCR 16, 18, and 20) was cross-reactive with mHSP70 (Fig. 3D, lanes 7 and 8), and the other (TgNCR A5, C1, and C2) was not (Fig. 3D, lanes 11 and 12). The cross-reactive MAbs reacted with the C-terminal fragment of both TgHSP70 and mHSP70. The epitope specificities of the cross-reactive MAbs were analyzed by Western blotting. Full-length rmHSP70 and N-terminal and C-terminal fragments of rmHSP70 were analyzed by SDS-PAGE (Fig. 4A). Sera of BALB/c and B6 mice obtained 8 weeks after T. gondii infection reacted with full-length rmHSP70 and N-terminal and C-terminal fragments of rmHSP70 (Fig. 4B, lanes 1 to 3). However, cross-reactive MAbs reacted with the full length (Fig. 4B, lane 4) and the C-terminal fragment (Fig. 4B, lane 6) but not with the N-terminal fragment (Fig. 4B, lane 5) of rmHSP70. Thus, the cross-reactive antigenic determinants of mHSP70 locate in both the N- and C-terminal regions of mHSP70, although three out of six MAb clones were shown to be cross-reactive with C-terminal region of mHSP70.
TABLE 1.
Specificity of each anti-TgHSP70 MAb tested by ELISA
| MAb | Isotype | Binding of MAb with recombinant proteina
|
|||||
|---|---|---|---|---|---|---|---|
| rTgHSP70
|
rmHSP70
|
||||||
| Full length | N terminal | C terminal | Full length | N terminal | C terminal | ||
| TgNCR A5 | γ1 | + | + | − | − | − | − |
| TgNCR C1 | γ2b | + | − | + | − | − | − |
| TgNCR C2 | γ2a | + | − | + | − | − | − |
| TgCR 16 | μ | + | − | + | + | − | + |
| TgCR 18 | μ | + | − | + | + | − | + |
| TgCR 20 | γ3 | + | − | + | + | − | + |
The specificity of each anti-TgHSP70 MAb was tested by ELISA using full- length rTgHSP70 and rmHSP70 and N-terminal and C-terminal fragments of each. The results were measured using a plate reader with a 405-nm filter and are shown as positive (+) or negative (−) reactions in ELISA.
FIG. 4.
Epitope specificities of anti-mHSP70 autoantibody produced in T. gondii-infected mice. (A) Full length (lane 1), N-terminal fragment (lane 2), and C-terminal fragment (lane 3) of rmHSP70 were analyzed by SDS-PAGE. (B) Western blotting analysis of epitope specificities of mHSP70. Full length (lanes 1 and 4), N-terminal region (lanes 2 and 5), and C-terminal region (lanes 3 and 6) were separated by SDS-PAGE and then transferred onto nitrocellulose membranes. The membranes were probed with the sera of T. gondii-infected B6 mice (lanes 1 to 3) and anti-TgHSP70 MAb TgCR 18 (lanes 4 to 6). The data are representative of three independent experiments.
Production of anti-self HSP70 autoantibody by B-1a cells in T. gondii-infected mice.
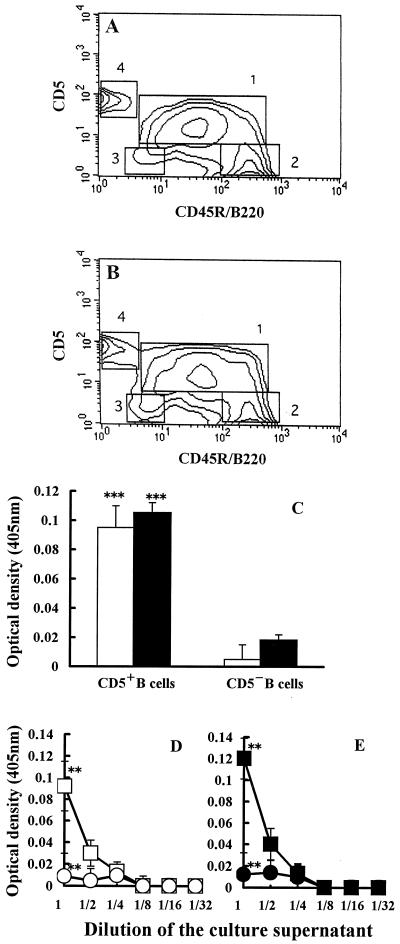
To examine the B-cell subset(s) which produced anti-mHSP70 autoantibody in T. gondii-infected mice, PECs of T. gondii-infected mice were double stained with anti-mCD5 (Ly1) MAb and anti-mCD45R/B220 MAb. The percentages of CD5+ (B-1a) cells and CD5− (B-2) cells in PECs of uninfected mice were 56.12 and 24.35%, respectively (Fig. 5A). The proportion of B-1a cells in T. gondii-infected mice was 71.33%, whereas the proportion of B-2 cells was 14.31% (Fig. 5B). Thus, the percentage of B-1a cells was markedly increased in PECs of T. gondii-infected mice.
FIG. 5.
(A and B) Flow cytometric analysis of CD5+ B cells in PECs. The percentages of B-1a cell (square 1), conventional B-cell (square 2), B-1b cell (square 3), and T-cell (square 4) populations in PECs of uninfected mice (A) or T. gondii-infected (B) mice were analyzed by FACScan as described in Materials and Methods. The proportions of B220-expressing cells are shown on the x axis, and those of CD5-expressing cells are shown on the y axis. The data are representative of three independent experiments. (C) Anti-mHSP70 autoantibody formation by B-1a cells. B-1a and B-2 cells from PECs of T. gondii-infected mice were sorted by magnet-conjugated anti-CD5 MAb and anti-B220 MAb. After 3 days of incubation at 37°C, formation of anti-TgHSP70 antibody (open column) and anti-mHSP70 autoantibody (closed column) in the culture supernatant of B-1a and B-2 cells was analyzed by ELISA (C). ∗∗∗, P < 0.005 compared with the culture supernatant of B-2 cells. (D and E) Titration analysis of anti-TgHSP70 antibody and anti-mHSP70 autoantibody in culture supernatants of B-1a and B-2 cells. B-1a and B-2 cells from PECs of T. gondii-infected mice were sorted, incubated, and diluted as described in Materials and Methods. Titration of the culture supernatants of B-1a (CD5+; □ or ■) and B-2 (CD5−; ○ or ●) cells against rTgHSP70 (D) and rmHSP70 (E) was tested by ELISA. ∗∗, P < 0.01.
The production of anti-self HSP70 autoantibody by B-1a and B-2 cells in PECs of T. gondii-infected mice was examined in vitro. High levels of anti-mHSP70 autoantibody were produced by B-1a cells but not by B-2 cells (Fig. 5C).
These data indicated that anti-mHSP70 autoantibodies were predominantly produced by B-1a cells in T. gondii-infected mice.
DISCUSSION
Dose-dependent antibody kinetics.
We demonstrated that the kinetic patterns of anti-TgHSP70 antibody production were related to the number of T. gondii cysts used for peroral infection in B6 mice. Anti-TgHSP70 antibody production was observed transiently after infection with high doses (8 to 10 cysts) of T. gondii, while the production of anti-TgHSP70 antibody persisted for more than 9 weeks when mice were infected with only 4 to 6 cysts. However, the dose-dependent kinetic pattern of formation of antibody to TgHSP70 was not related to the number of cysts used for infection in BALB/c mice, and the antibody formation responses of BALB/c mice were weaker than those of B6 mice. We speculate that exposure of mice to high doses of antigen might induce high levels of anti-TgHSP70 antibody production, leading to immunocomplex formation, which induces immunosuppression in T. gondii-infected mice (36, 39). Alternatively, although host immunoresponses were activated by low-dose T. gondii infection, high-dose infection induced suppression of host immunoresponses. There may be different thresholds of immunoresponse and immunosuppression induced by T. gondii-derived molecules. It has been reported that TgHSP70 suppresses host immunoresponses to T. gondii infection when TgHSP70 is injected into mice (23). Thus, TgHSP70 is one of the candidate molecules for a down-regulator of antibody formation.
Anti-mHSP70 autoantibody formation.
Both BALB/c and B6 mice produced anti-mHSP70 autoantibodies in the serum after peroral T. gondii infection, and the kinetic pattern of anti-mHSP70 autoantibody formation was similar to that of anti-TgHSP70 antibody formation. In spite of high homology, HSP70 has been shown to be an immunodominant antigen (7, 22, 37, 40, 51) and to induce self-HSP70-reactive autoantibody formation when the breakdown of immunotolerance against self-HSP70 is triggered. Actually, TgHSP70, which has 68% homology in nucleic acid sequences with mHSP70, has been shown to have strong antigenicity in T. gondii-infected mice (23), and anti-TgHSP70 antibodies that cross-react with mHSP70 were detected in the sera of T. gondii-infected mice. Furthermore, the stress and inflammation of the infection increased the synthesis of self-HSP70 (1, 38). Indeed, mRNA expression of mHSP70 was upregulated after T. gondii infection in mice (data not shown). Thus, we hypothesize that anti-TgHSP70 antibodies produced after T. gondii infection cross-react with mHSP70, which is upregulated by the stress of the infection.
Anti-mHSP70 autoantibody production by B-1 cells.
The isotypes of anti-TgHSP70 MAbs which cross-reacted with mHSP70 were IgM and IgG3, while those of non-cross-reactive MAbs were IgG1, IgG2a, and IgG2b. It has recently been argued that B-1 cells are involved in mucosal immunity and autoimmunity because B-1 cells preferentially produce IgM, IgA, and IgG3 antibodies, which have broad specificities against bacterial and self antigens (9, 13, 24). Anti-mHSP70 autoantibodies were secreted in the culture supernatant of B-1 cells isolated from PECs of T. gondii-infected mice. Recently, Qin et al. indicated that B-1 cells, like germinal center B cells, could express recombinase-activating genes 1 and 2 (RAG1 and RAG2) and undergo secondary V(D)J recombination of immunoglobulin genes (30), and the data suggested that the secondary immunoglobulin gene rearrangements were important in development of autoreactive antibodies. Moreover, by using a germ line gene-encoded specificity, Hayakawa et al. demonstrated that self-antigen can positively influence B-cell fate, selecting B cells bearing an appropriate light-chain partner and generating a B-cell pool with an autoreactive specificity (10). Furthermore, it has recently been shown that in transgenic mice, B-1 but not B-2 cells are selected by the strength of signals through B-cell receptors triggered by self antigens (19, 43). It was reported that under certain conditions the cross-linking of surface immunoglobulin on B cells may lead to development of a B-1 cell phenotype on B-2 cells (5). The administration of lipopolysaccharides of gram-negative bacteria activated B-1 cells in the peritoneal cavities and lamina propria of the gut (25). Thus, additional factors such as infections, cytokines (interleukins 5 and 10), and the costimulatory help (B7-CD28/CTLA-4 and CD40/CD40L) of Th2 cells are required for the activation of autoreactive B cells (24). Also, Karras et al. showed that signaling pathways involving STAT3 proteins control B-1 cell growth and development (17). Our data suggest that the expression of mHSP70, which is upregulated by T. gondii infection, promotes B-1 cell accumulation, and a significant proportion of anti-mHSP70 autoantibody is produced by B-1 cells in T. gondii-infected mice.
Cross-reactive epitopes of mHSP70.
The cDNA sequences of the full length, N-terminal fragment, and C-terminal fragment of TgHSP70 are 68.1, 68.5, and 66.0%, respectively, homologous to those of mHSP70. In our study, the epitopes recognized by cross-reactive anti-TgHSP70 MAbs were predominantly located in the C-terminal region of mHSP70. This is in agreement with the study of Kumar and Zheng, who reported that a peptide corresponding to the GGMP repeat sequence in the C-terminal region of P. falciparum HSP70 was recognized by more than 75% of sera from immunized mice (18). Similarly, Renia et al. reported that the antigenic epitope of P. falciparum HSP70 preferentially recognized by sera from immune monkeys was located in a less conserved region of HSP70 (33). Similarly, immunodominant B-cell epitopes of L. donovani HSP70 (42), S. japonicum or S. mansoni HSP70 (12), and Mycobacterium leprae HSP70 (26) were also shown to map at the C-terminal region of HSP70, indicating that the C-terminal region of HSP70 is the immunodominant site. On the other hand, B-cell epitopes of anti-HSP70 autoantibodies produced in sera of hosts with malaria were shown to exist not only in the C-terminal but also in the N-terminal region of HSP70 (21). In our studies, the sera of T. gondii-infected mice reacted with not only the C-terminal but also the N-terminal fragment of mHSP70, although three out of six MAb clones were shown to be cross-reactive with the C-terminal region of mHSP70. The MAbs cross-reactive with the N-terminal region of mHSP70 might be obtained by establishing larger numbers of MAb clones.
It is likely that self HSP70 expression is not the primary event triggering autoimmunity but rather is an event subsequent to the tissue damage induced by the autoimmune process itself, which is accompanied by inflammation. The mechanisms triggering breakdown of the immunotolerance of B-1 cells producing anti-self HSP70 autoantibody and the pathogenic significance of such anti-mHSP70 autoantibodies in T. gondii-infected mice remain to be clarified.
ACKNOWLEDGMENTS
This work was supported in part by a Grant-in-Aid for Scientific Research of Health and Welfare and a grant from the Ministry of Education, Science, Sports and Culture, Japan.
REFERENCES
- 1.Adhuna, Salotra P, Mukhopadhyay B, Bhatnagar R. Modulation of macrophage heat shock proteins (HSPs) expression in response to intracellular infection by virulent and avirulent strains of Leishmania donovani. Biochem Mol Biol Int. 1997;43:1265–1275. doi: 10.1080/15216549700205091. [DOI] [PubMed] [Google Scholar]
- 2.Aosai F, Yang T H, Ueda M, Yano A. Isolation of naturally processed peptides from a Toxoplasma gondii-infected human B lymphoma cell line that are recognized by cytotoxic T lymphocytes. J Parasitol. 1994;80:260–266. [PubMed] [Google Scholar]
- 3.Appetecchia M, Castelli M, Delpino A. Anti-heat shock proteins autoantibodies in autoimmune thyroiditis. Preliminary study. J Exp Clin Cancer Res. 1997;16:395–400. [PubMed] [Google Scholar]
- 4.Colebrook A L, Lightowlers M W. Serological reactivity to heat shock protein 70 in patients with hydatid disease. Parasite Immunol. 1997;19:41–46. doi: 10.1046/j.1365-3024.1997.d01-141.x. [DOI] [PubMed] [Google Scholar]
- 5.Cong Y Z, Rabin E, Wortis H H. Treatment of murine CD5− B cells with anti-Ig, but not LPS, induces surface CD5: two B-cell activation pathways. Int Immunol. 1991;3:467–476. doi: 10.1093/intimm/3.5.467. [DOI] [PubMed] [Google Scholar]
- 6.Dworniczak B, Mirault M E. Structure and expression of a human gene coding for a 71 kd heat shock ‘cognate’ protein. Nucleic Acids Res. 1987;15:5181–5197. doi: 10.1093/nar/15.13.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garbe T R. Heat shock proteins and infection: interactions of pathogen and host. Experientia. 1992;48:635–639. doi: 10.1007/BF02118308. [DOI] [PubMed] [Google Scholar]
- 8.Giebel L B, Dworniczak B P, Bautz E K. Developmental regulation of a constitutively expressed mouse mRNA encoding a 72-kDa heat shock-like protein. Dev Biol. 1988;125:200–207. doi: 10.1016/0012-1606(88)90073-5. [DOI] [PubMed] [Google Scholar]
- 9.Hardy R R, Hayakawa K. Development and physiology of Ly-1 B and its human homolog, Leu-1 B. Immunol Rev. 1986;93:53–79. doi: 10.1111/j.1600-065x.1986.tb01502.x. [DOI] [PubMed] [Google Scholar]
- 10.Hayakawa K, Asano M, Shinton S A, Gui M, Allman D, Stewart C L, Silver J, Hardy R R. Positive selection of natural autoreactive B cells. Science. 1999;285:113–116. doi: 10.1126/science.285.5424.113. [DOI] [PubMed] [Google Scholar]
- 11.Hayakawa K, Hardy R R, Herzenberg L A. Peritoneal Ly-1 B cells: genetic control, autoantibody production, increased lambda light chain expression. Eur J Immunol. 1986;16:450–456. doi: 10.1002/eji.1830160423. [DOI] [PubMed] [Google Scholar]
- 12.Hedstrom R, Culpepper J, Schinski V, Agabian N, Newport G. Schistosome heat-shock proteins are immunologically distinct host-like antigens. Mol Biochem Parasitol. 1988;29:275–282. doi: 10.1016/0166-6851(88)90082-5. [DOI] [PubMed] [Google Scholar]
- 13.Herzenberg L A, Stall A M, Lalor P A, Sidman C, Moore W A, Parks D R, Herzenberg L A. The Ly-1 B cell lineage. Immunol Rev. 1986;93:81–102. doi: 10.1111/j.1600-065x.1986.tb01503.x. [DOI] [PubMed] [Google Scholar]
- 14.Hightower L E. Heat shock, stress protein, chaperones, and proteotoxicity. Cell. 1991;66:191–197. doi: 10.1016/0092-8674(91)90611-2. [DOI] [PubMed] [Google Scholar]
- 15.Humphries D, Vella A T, Pearce E J. Increased CD4+ T cell-dependent anti-erythrocyte antibody levels following the onset of parasite egg production in Schistosoma mansoni infected mice. Parasite Immunol. 1994;16:469–477. doi: 10.1111/j.1365-3024.1994.tb00375.x. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan D, Ferrari I, Bergami P L, Mahler E, Levitus G, Chiale P, Hoebeke J, Van Regenmortel M H, Levin M J. Antibodies to ribosomal P proteins of Trypanosoma cruzi in Chagas disease possess functional autoreactivity with heart tissue and differ from anti-P autoantibodies in lupus. Proc Natl Acad Sci USA. 1997;94:10301–10306. doi: 10.1073/pnas.94.19.10301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karras J G, Wang Z, Huo L, Howard R G, Frank D A, Rothstein T L. Signal transducer and activator of transcription-3 (STAT3) is constitutively activated in normal, self-renewing B-1 cells but only inducibly expressed in conventional B lymphocytes. J Exp Med. 1997;185:1035–1042. doi: 10.1084/jem.185.6.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar N, Zheng H. Evidence for epitope-specific thymus-independent response against a repeat sequence in a protein antigen. Immunology. 1998;94:28–34. doi: 10.1046/j.1365-2567.1998.00486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lam K P, Rajewsky K. B cell antigen receptor specificity and surface density together determine B-1 versus B-2 cell development. J Exp Med. 1999;190:471–477. doi: 10.1084/jem.190.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo W, Aosai F, Ueda M, Yamashita K, Shimizu K, Sekiya S, Yano A. Kinetics in parasite abundance in susceptible and resistant mice infected with an avirulent strain of Toxoplasma gondii by using quantitative competitive PCR. J Parasitol. 1997;83:1070–1074. [PubMed] [Google Scholar]
- 21.Mattei D, Scherf A, Bensaude O, da Silva L P. A heat shock-like protein from the human malaria parasite Plasmodium falciparum induces autoantibodies. Eur J Immunol. 1989;19:1823–1828. doi: 10.1002/eji.1830191010. [DOI] [PubMed] [Google Scholar]
- 22.Multhoff G, Botzler C, Issels R. The role of heat shock proteins in the stimulation of an immune response. Biol Chem. 1998;379:295–300. [PubMed] [Google Scholar]
- 23.Mun H S, Aosai F, Yano A. Role of Toxoplasma gondii HSP70 and Toxoplasma gondii HSP30/bag1 in antibody formation and prophylactic immunity in mice experimentally infected with Toxoplasma gondii. Microbiol Immunol. 1999;43:471–479. doi: 10.1111/j.1348-0421.1999.tb02430.x. [DOI] [PubMed] [Google Scholar]
- 24.Murakami M, Honjo T. Involvement of B-1 cells in mucosal immunity and autoimmunity. Immunol Today. 1995;16:534–539. doi: 10.1016/0167-5699(95)80047-6. [DOI] [PubMed] [Google Scholar]
- 25.Murakami M, Tsubata T, Shinkura R, Nisitani S, Okamoto M, Yoshioka H, Usui T, Miyawaki S, Honjo T. Oral administration of lipopolysaccharides activates B-1 cells in the peritoneal cavity and lamina propria of the gut and induces autoimmune symptoms in an autoantibody transgenic mouse. J Exp Med. 1994;180:111–121. doi: 10.1084/jem.180.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peake P W, Britton W J, Davenport M P, Roche P W, McKenzie K R. Analysis of B-cell epitopes in the variable C-terminal region of the Mycobacterium leprae 70-kilodalton heat shock protein. Infect Immun. 1993;61:135–141. doi: 10.1128/iai.61.1.135-141.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petralanda I, Piessens W F. Pathogenesis of onchocercal dermatitis: possible role of parasite proteases and autoantibodies to extracellular matrix proteins. Exp Parasitol. 1994;79:177–186. doi: 10.1006/expr.1994.1077. [DOI] [PubMed] [Google Scholar]
- 28.Polla B S. Heat shock proteins in host-parasite interactions. Immunol Today. 1991;12:A38–A41. doi: 10.1016/S0167-5699(05)80011-8. [DOI] [PubMed] [Google Scholar]
- 29.Portig I, Pankuweit S, Maisch B. Antibodies against stress proteins in sera of patients with dilated cardiomyopathy. J Mol Cell Cardiol. 1997;29:2245–2251. doi: 10.1006/jmcc.1997.0463. [DOI] [PubMed] [Google Scholar]
- 30.Qin X F, Schwers S, Yu W, Papavasiliou F, Suh H, Nussenzweig A, Rajewsky K, Nussenzweig M C. Secondary V(D)J recombination in B-1 cells. Nature. 1999;397:355–359. doi: 10.1038/16933. [DOI] [PubMed] [Google Scholar]
- 31.Quijada L, Requena J M, Soto M, Alonso C. During canine viscero-cutaneous leishmaniasis the anti-Hsp70 antibodies are specifically elicited by the parasite protein. Parasitology. 1996;112:277–284. doi: 10.1017/s0031182000065793. [DOI] [PubMed] [Google Scholar]
- 32.Rauch S D, San Martin J E, Moscicki R A, Bloch K J. Serum antibodies against heat shock protein 70 in Meniere's disease. Am J Otol. 1995;16:648–652. [PubMed] [Google Scholar]
- 33.Renia L, Mattei D, Goma J, Pied S, Dubois P, Miltgen F, Nussler A, Matile H, Menegaux F, Gentilini M. A malaria heat-shock-like determinant expressed on the infected hepatocyte surface is the target of antibody-dependent cell-mediated cytotoxic mechanisms by nonparenchymal liver cells. Eur J Immunol. 1990;20:1445–1449. doi: 10.1002/eji.1830200706. [DOI] [PubMed] [Google Scholar]
- 34.Skeiky Y A, Benson D R, Guderian J A, Whittle J A, Bacelar O, Carvalho E M, Reed S G. Immune responses of leishmaniasis patients to heat shock proteins of Leishmania species and humans. Infect Immun. 1995;63:4105–4114. doi: 10.1128/iai.63.10.4105-4114.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Srivastava P K, Udono H, Blachere N E, Li Z. Heat shock proteins transfer peptides during antigen processing and CTL priming. Immunogenetics. 1994;39:93–98. doi: 10.1007/BF00188611. [DOI] [PubMed] [Google Scholar]
- 36.Takizawa F, Adamczewski M, Kinet J P. Identification of the low affinity receptor for immunoglobulin E on mouse mast cells and macrophages as Fc gamma RII and Fc gamma RIII. J Exp Med. 1992;176:469–475. doi: 10.1084/jem.176.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tishler M, Shoenfeld Y. Anti-heat-shock protein antibodies in rheumatic and autoimmune diseases. Semin Arthritis Rheum. 1996;26:558–563. doi: 10.1016/s0049-0172(96)80043-6. [DOI] [PubMed] [Google Scholar]
- 38.Tomer Y, Davies T F. Infection, thyroid disease, and autoimmunity. Endocrine Rev. 1993;14:107–120. doi: 10.1210/edrv-14-1-107. [DOI] [PubMed] [Google Scholar]
- 39.Tyagi P, Patil S A, Girdhar B K, Katoch K, Sengupta U. Suppressive effect of circulating immune complexes from leprosy patients on the lymphocyte proliferation induced by M. leprae antigens in healthy responders. Int J Lepr Other Mycobact Dis. 1992;60:562–569. [PubMed] [Google Scholar]
- 40.Udono H, Srivastava P K. Heat shock protein 70-associated peptides elicit specific cancer immunity. J Exp Med. 1993;178:1391–1396. doi: 10.1084/jem.178.4.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vanbuskirk A, Crump B L, Margoliash E, Pierce S K. A peptide binding protein having a role in antigen presentation is a member of the HSP70 heat shock family. J Exp Med. 1989;170:1799–1809. doi: 10.1084/jem.170.6.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wallace G R, Ball A E, MacFarlane J, el Safi S H, Miles M A, Kelly J M. Mapping of a visceral leishmaniasis-specific immunodominant B-cell epitope of Leishmania donovani Hsp70. Infect Immun. 1992;60:2688–2693. doi: 10.1128/iai.60.7.2688-2693.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watanabe N, Nisitani S, Ikuta K, Suzuki M, Chiba T, Honjo T. Expression levels of B cell surface immunoglobulin regulate efficiency of allelic exclusion and size of autoreactive B-1 cell compartment. J Exp Med. 1999;190:461–469. doi: 10.1084/jem.190.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang T H, Aosai F, Norose K, Mun H S, Yano A. Heat shock cognate protein 71-associated peptides function as an epitope for Toxoplasma gondii-specific CD4+ CTL. Microbiol Immunol. 1997;41:553–561. doi: 10.1111/j.1348-0421.1997.tb01891.x. [DOI] [PubMed] [Google Scholar]
- 45.Yang T H, Aosai F, Norose K, Ueda M, Yano A. Enhanced cytotoxicity of IFN-gamma-producing CD4+ cytotoxic T lymphocytes specific for T. gondii-infected human melanoma cells. J Immunol. 1995;154:290–298. [PubMed] [Google Scholar]
- 46.Yano A, Aosai F, Ohta M, Hasekura H, Sugane K, Hayashi S. Antigen presentation by Toxoplasma gondii-infected cells to CD4+ proliferative T cells and CD8+ cytotoxic cells. J Parasitol. 1989;75:411–416. [PubMed] [Google Scholar]
- 47.Yano A, Mun H S, Yang T H, Hata H, Kobayashi M, Norose K, Hayakawa S, Tagawa Y, Iwakura Y, Nakazaki S, Nakazaki Y, Sekiya S, Yamaura A, Kubosawa H, Yumoto N, Aosai F. Role of IFN-γ in effector mechanisms and pathogenicity of HSPs in mice and human infected with Toxoplasma gondii. In: Tada I, Kojima S, Tsuji M, editors. ICOPA IX: 9th International Congress of Parasitology. Bologna, Italy: Monduzzi Editore; 1998. pp. 457–466. [Google Scholar]
- 48.Yano A, Ohno S, Norose K, Baba T, Yamashita K, Aosai F, Segawa K. Antigen presentation by Toxoplasma-infected cells: antigen entry through cell membrane fusion. Int Arch Allergy Immunol. 1992;98:13–17. doi: 10.1159/000236159. [DOI] [PubMed] [Google Scholar]
- 49.Young D B, Mehlert A, Smith D. Stress proteins and infectious diseases. In: Morimoto R, Tissières A, Georgopoulos C, editors. Stress proteins in biology and medicine. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1990. pp. 131–166. [Google Scholar]
- 50.Yui K, Yano A. Humoral immune responses against members of the hsp70 family in Toxoplasma gondii. Jpn J Trop Med Hyg. 1998;26:305–318. [Google Scholar]
- 51.Zugel U, Kaufmann S H. Immune response against heat shock proteins in infectious diseases. Immunobiology. 1999;201:22–35. doi: 10.1016/s0171-2985(99)80044-8. [DOI] [PubMed] [Google Scholar]