Abstract
Calcium-based kidney stone disease is a highly prevalent and morbid condition, with an often complicated and multifactorial aetiology. An abundance of research on the role of specific vitamins (B6, C and D) in stone formation exists, but no consensus has been reached on how these vitamins influence stone disease. As a consequence of emerging research on the role of the gut microbiota in urolithiasis, previous notions on the contribution of these vitamins to urolithiasis are being reconsidered in the field, and investigation into previously overlooked vitamins (A, E and K) was expanded. Understanding how the microbiota influences host vitamin regulation could help to determine the role of vitamins in stone disease.
Subject terms: Urology, Microbiome, Renal calculi
In this Perspective article, the authors summarize the roles of vitamins in calcium-containing kidney stone disease, and hypothesize implications for the gut microbiota in altering vitamin homeostasis by modulating vitamin absorption, production and chemical modification.
Introduction
Kidney stone disease affects ~0.2–20% of the world’s population and places a considerable clinical and economic burden on global health systems1–3. In North America, ~10% of people will have experienced a kidney stone episode in their lifetime4–6. Urinary stone disease is also a highly recurrent condition, with up to 50% of patients developing a recurrent stone in the absence of preventative treatment7. Modern medical treatment is available, but kidney stones still result in substantial patient morbidity, including pain and renal colic, chronic kidney disease, infections and urosepsis8. Consequently, the annual cost of direct care to treat patients with nephrolithiasis exceeds $10 billion in the USA alone9. Amongst patients with stone disease, 3.1 million workdays are estimated to be lost to hospitalizations and ambulatory care, further contributing to the financial burden indirectly caused by this disease10,11. The prevalence of the disease is expected to inflate owing to the increased global incidence of nephrolithiasis; thus, additional research focused on disease aetiology is needed, with the aim of mitigating patient morbidity and associated expenses1,6,12,13.
The largest proportion of kidney stones contain calcium oxalate (60–75%) or calcium phosphate (15–20%)4,14,15. Other less common stone compositions include uric acid, struvite and cystine4,14,15. The formation of renal calculi is not completely understood, but is predicted to proceed along the path of crystal nucleation, growth, aggregation and fixation within the kidney, where the crystal can further grow into a macroscopic stone16. The presence of stone-forming substrates or inhibitors at any point in this process can promote or hamper crystal formation17. Randall’s plaques or plugs — which are calcium phosphate deposits found in the papillary interstitium or collecting ducts, respectively — are hypothesized to serve as a nidus for crystal attachment and subsequent stone development18. Additionally, the components of these deposits can induce reactive oxygen species (ROS) and inflammation, which damage the epithelium and promote further crystal deposition — a process that is probably accelerated by crystalluria19.
Diet has long been a risk factor for stone disease20–22. Results from empirical analysis showed that the typical Western diet produces a urine chemical profile that is more susceptible to calcium-based stone formation than that obtained with other nutritional regimes such as an ovo-lacto-vegetarian diet23, which is in part owing to decreased urine volume, pH and citrate levels. Western diet is characterized by increased consumption of animal protein, saturated fats and refined sugars, but reduced consumption of fruits and vegetables24. In turn, this diet shifts the gut microbiota to a predominantly proteolytic state, which increases uraemic toxin25 and endotoxin26 production. Together, these factors contribute to increased inflammation and to the development of metabolic diseases such as irritable bowel disease24,27.
Notably, the Western diet is depleted in dietary vitamins28. Vitamins have a long and complex history in kidney stone management, with some of the earliest reports on this topic dating back to the middle of the twentieth century29. Since then, numerous large epidemiological datasets such as the Nurses’ Health Study I and II and Health Professionals Follow-up have been analysed to assess the role of vitamins in stone disease, but with inconclusive and often conflicting results. The lack of consensus among different studies exemplifies the complicated nature of the relationship between stone disease and vitamin intake, which can be further complicated considering the role of the microbiota.
The potential role of the gut microbiota in stone disease is a relatively new research line30. The idea of a role of gut microbiome in stone disease is supported by the strong association between oral antibiotic use and incidence of kidney stones31. The initial attempts to mechanistically understand how bacteria influence nephrolithiasis focused on Oxalobacter formigenes, a Gram-negative bacterium that uses oxalate as the sole carbon source32. Results from an early study showed that the presence, whether indigenous or supplemented (for example, as a probiotic), of O. formigenes reduced urinary oxalate levels and could provide a protective effect against calcium oxalate stone formation33. However, this conjecture was challenged by results from subsequent studies showing that there was no difference in the abundance of Oxalobacter between stone formers and healthy individuals34–38. Instead, this bacterium might be part of a network of bacteria involved in host oxalate homeostasis39–41.
In subsequent studies, the role of gut microbiota as a whole has been assessed in stone disease, rather than focusing on individual bacterial types. In general, the gut microbiota of stone formers has repeatedly been shown to differ from that of non-stone-forming individuals, but the specific differences are often not consistent across studies35–37,40,42–44. Results from a 2020 study showed that the level of evidence for these differences varies according to bacterial taxa, but the microbiota of stone formers is likely to be depleted in short-chain fatty acid-producing bacteria and to have an elevated amount of bacteria associated with inflammation38,45. Results from a meta-analysis34 showed that the gut microbiota from stone formers had lower diversity than that of healthy individuals, but specific bacterial taxa that might be crucial in influencing stone disease were not identified. Additionally, stone composition was associated with the urinary tract microbiome but not with the gut microbiome; thus, the authors concluded that, based on this analysis, the urinary microbiome might influence the host environment to promote or inhibit stone formation34. In this report, some knowledge of the role of gut microbiome in stone disease is provided, but functional changes in bacteria metabolism that could differ between different species and strains in the gut were not fully considered. These functional changes in the microbiome are poorly captured by traditional 16S rRNA sequencing methods and are best addressed by shotgun metagenomic sequencing and metabolomics38.
The effect of nutrient intake on gut microbiota composition and the gut–kidney axis has also been studied39. Interestingly, all vitamin synthesis pathways have been identified in human faecal samples through shotgun metagenomic sequencing, suggesting that the microbiota might have some roles in vitamin acquisition by the host, including vitamins that were previously not considered as microbially derived46. Not all bacteria can produce these vitamins de novo, but in an adapted microbial network, bacteria might exchange resources in the extracellular “pantryome”47 to complete gaps in metabolic pathways. The “pantryome” concept is based on the idea that individual bacteria that lack some nutrients can take these nutrients from a pool of metabolites shared with other members of the microbiome and, in return, donate the excess metabolites back to the pool for other members to use. Metabolite sharing enables individual members of the gut microbiota to make up for functional absences in the metabolic pathways. Thus, an individual bacterium might not directly correlate with a disease state — as perceived from traditional 16S rRNA sequencing studies — and instead, the function of the entire community as a whole should be considered.
The contribution of the microbiota to vitamin absorption and activity has been overlooked in many current studies in which the role of the microbiota has been assessed in several diseases47. Gaps in the understanding of the role of vitamins and kidney stones have become particularly evident considering the uncertain history of vitamins and kidney stone disease, and the evidence that gut bacteria are able to synthesize these vitamins47,48.
In this Perspective, we discuss the potential roles of vitamins in stone disease (Box 1). Specifically, we focused on vitamins A, B6, C, D, E and K, considering the suspected roles of these vitamins in stone formation and potential modulation by the gut microbiota. We also provide a new perspective on the role of the microbiota as a local producer of vitamins and an overall regulator of vitamin bioavailability and function (Fig. 1).
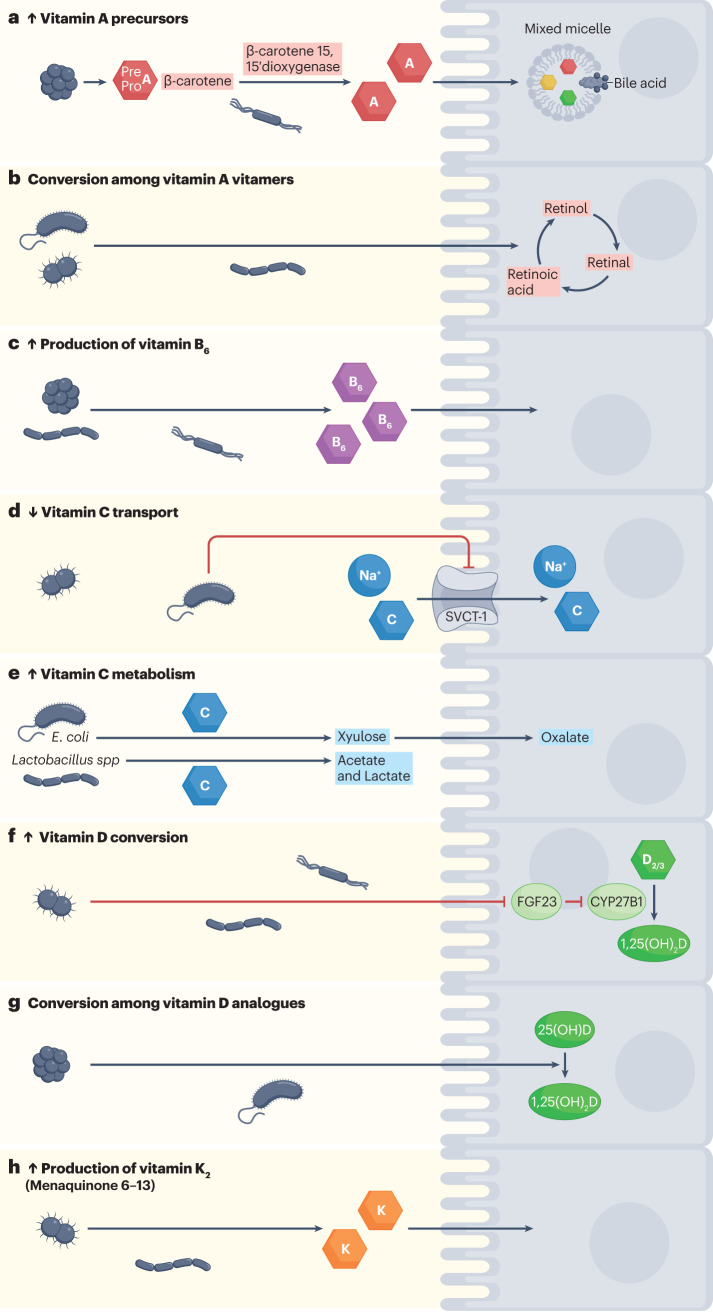
Fig. 1. Possible roles of the microbiota in host vitamin acquisition and homeostasis.
Members of the gut microbiota can a, produce vitamin A precursors and can convert β-carotene to retinoic acid71 and b, facilitate the interconversion among vitamin A vitamers75,77,78. c, Gut microbiota is also well known to be able to produce sufficient amounts of vitamin B6 (refs. 47,48,105,106). d, Lipopolysaccharide from Gram-negative microorganisms reduces SVCT-1 expression and, in turn, SVCT-1-mediated uptake of vitamin C167,170. e, Vitamin C is also metabolized by gut bacteria such as E. coli159,166 and Lactobacillus156 species to yield xylulose and short-chain fatty acids, respectively; host cells can further process xylulose to yield oxalate. f, Through local immune activation, the microbiota can increase the conversion of vitamin D into the active form 1,25(OH)2D by reducing the expression of FGF23, which normally inhibits CYP27B1-mediated conversion of vitamin D into active vitamin D213,214. g, Additionally, multiple members of the microbiota might have the ability to convert vitamin D analogues204–209. h, Humans also rely on gut bacteria for the production of vitamin K2 (refs. 47,303,304,306,307,312,313). SVCT, sodium-dependent vitamin C transporter 1; D2/3, vitamin D2 and vitamin D3; 25(OH)D, 25-hydroxyvitamin D; 1,25(OH)2D, 1,25-dihydroxyvitamin D.
Box 1 Vitamin functions potentially related to nephrolithiasis.
Systemic
Vitamin C and E act as non-specific antioxidants that protect host cells from oxidative damage and in turn might prevent potential calcium oxalate crystal attachment and kidney stone formation.
Vitamin K2 is needed for local activation of dephosphorylated-uncarboxylated matrix Gla protein (dp-ucMGP), a Gla family protein that sequesters free calcium and prevents soft-tissue calcification.
Liver
Vitamin B6 is a cofactor for the alanine-glyoxylate aminotransferase-mediated degradation of glyoxylate to glycine, instead of oxalate.
Intestines
Vitamin A can improve intestinal integrity, in turn reducing the translocation of dietary oxalate into the systemic circulation and pathogen colonization.
Vitamin D controls calcium homeostasis by modulating calcium absorption and excretion.
Immune system
Vitamin A and D act as immune boosters to maintain host immunity and reduce pathogen colonization, which could enhance gut barrier integrity, in turn reducing the absorption of lipopolysaccharide (LPS) and oxalate.
Kidneys
Vitamin D controls calcium homeostasis by modulating calcium absorption and excretion.
Bone
Vitamin D controls calcium homeostasis by modulating calcium absorption and excretion.
Vitamin A
Vitamin A is the generic descriptor for a group of lipid-soluble retinoids that are recognized by a β-ionone ring with an isoprenoid side chain49 (Fig. 2). Vitamers of vitamin A are named according to the functional group at the end of the isoprenoid chain. Dietary sources of vitamin A exist in the form of provitamin A (such as carotenoids) or preformed vitamin A (such as retinol and retinyl esters), which are obtained from the consumption of pigmented vegetables and animal products, respectively50. In the body, these substances are metabolized into the active forms of vitamin A, retinal and retinoic acid, by enzymes present in the intestines and liver50.
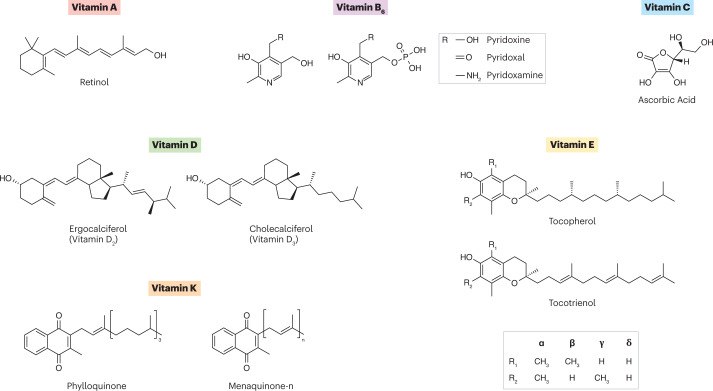
Fig. 2. Structures of vitamins discussed in this Perspective.
Vitamin A (all-trans retinol) contains a β-ionone ring with an isoprenoid side chain and a hydroxyl functional group. Vitamin B6 is the generic name for six vitamers defined based on the R group and phosphorylation status. Vitamin C (ascorbic acid) weakly resembles a cyclic sugar molecule. Vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol) structures are defined as secosteroids. Vitamin E contains four tocopherols and four tocotrienols that are assigned the Greek letters α, β, γ and δ based on the functional group in R1 and R2. Vitamin K structures have a characteristic 2-methyl-1,4-naphthoquinone structure and a unique side chain. Vitamin K1 (phylloquinone) has a phytyl side chain and vitamin K2 (menaquinone) has a polyisoprenyl side chain.
In rats, vitamin A deficiency has been shown to reduce urinary citrate and pH, and to increase the presence of urinary calculi51,52 (Table 1). Supplementation with high amounts of vitamin A (retinol) in a calcium oxalate stone rat model enhanced urinary citrate levels, increased urinary pH, and lowered the concentration of calcium and oxalate in renal tissues53. These changes observed in vitamin A-supplemented rats could be partially attributed to the ability of vitamin A to alter gene expression in cells in the collecting ducts, which have crucial roles in maintaining urinary pH54. The influence of vitamin A on urinary calculi formation has been corroborated by the evidence that vitamin A deficiency resulted in crystalluria in a small group of young boys (n = 37, 4–10 years old), but not in individuals with adequate levels of vitamin A (n = 8)55. Furthermore, vitamin A treatment in children with vitamin A deficiency ameliorated urinary risk factors for stones, including reduced calcium and oxalate and increased citrate and glycans levels55. In a case-controlled observational study, adult stone formers (n = 75) were shown to have lower circulating levels of vitamin A than healthy individuals (n = 25)56. However, this evidence was not confirmed in another study in adults, in which plasma vitamin A levels were not shown to be lower in calcium-oxalate stone formers than in healthy individuals51 (Table 1). An important aspect to consider when examining these results is that blood levels of vitamin A might not be a very sensitive measurement of vitamin A deficiency, as this vitamin is stored in the liver and released into the bloodstream only when needed57. Overall, evidence of a direct contribution of vitamin A to stone diseases is weak and limited to a few observational studies and preclinical models. Considering that vitamin A is a multifaceted metabolite that has many roles throughout body systems, which are directed by the present vitamers49, investigating how these indirect factors contribute to urolithiasis is worthwhile.
Table 1.
In vivo studies to investigate the role of vitamins in stone disease
| Vitamin | Organism | Study group | Main findings | Ref. |
|---|---|---|---|---|
| Vitamin A | Animal models | Healthy male Wistar rats (young and old) | Vitamin A deficiency resulted in reduced urinary citrate and reduced pH | 51 |
| Healthy female Sprague–Dawley rats | Vitamin A deficiency led to increased renal calculi formation | 52 | ||
| Male Wistar rats with induced hyperoxaluria (oral 0.5% ethylene glycol) | Rats receiving oral supplementation with retinol had increased urinary pH and citrate but no change in renal accumulation of calcium and oxalate) compared with unsupplemented lithogenic rats | 53a | ||
| Human participants | Adult calcium oxalate stone formers and healthy individuals (male and female) | Plasma levels of retinol were not different between sex-matched stone formers and healthy individuals | 51 | |
| Male paediatric patients with vitamin A deficiency (Bitot’s spots) and healthy individuals | Paediatric patients with vitamin A deficiency had increased urinary calcium and oxalate, reduced citrate and increased presence of crystalluria. In these children, oral treatment with 24,000 IU vitamin A palmitate for 10 days reduced urinary oxalate and calcium to the level of healthy children and increased citrate (but not the level of healthy children) | 55 | ||
| Adult stone formers and healthy individuals (male and female) | Stone formers had lower plasma vitamin A than healthy individuals (case–control study) | 56 | ||
| Vitamin B6 | Animal models | Healthy male rats | Rats fed pyridoxine HCl had lower urinary oxalate than untreated rats | 89 |
| Healthy male Wistar rats | Vitamin B6-deficient rats developed calcium oxalate and calcium phosphate crystals in the renal tract | 90 | ||
| Human participants | Health care workers (adult male and female participants) | Meta-analysis of observational data suggests that vitamin B6 intake is not associated with the risk of kidney stones in both males and females (follow-up study) | 86 | |
| Health care workers (adult male participants) | Vitamin B6 intake is not associated with the risk of kidney stones (prospective study) | 87 | ||
| Health care workers (adult female participants) | High doses of vitamin B6 are associated with a reduced risk of kidney stones (prospective study) | 88 | ||
| Adults with a history of recurrent renal calculi (male participants) | Oral pyridoxine (250–500 mg/day) for up to 18 months reduced urinary oxalate | 93 | ||
| Adult recurrent calcium oxalate stone formers (male and female participants) | Oral magnesium oxide (300 mg/day) with pyridoxine-HCl (10 mg/day) for up to 120 days reduced urinary oxalate | 94 | ||
| Adult patients from a urology stone clinic (male and female participants) | Retrospective dietary intervention and oral pyridoxine (50–100 mg/day) for up to 1 year reduced urinary oxalate and calcium oxalate supersaturation | 95 | ||
| Adult patients with idiopathic calcium oxalate calculi (unknown gender) | Oral pyridoxine for up to 6 weeks reduced urinary oxalate and glycolate | 96 | ||
| Adult calcium oxalate stone formers (male and female participants) | Oral pyridoxine (60 mg/day) for 28 days did not change urinary oxalate levels | 97 | ||
| Adult patients with recurrent idiopathic hypocitraturia (male and female participants) | Oral UriKind-KM6 (1,100 mg potassium citrate, 375 mg magnesium citrate, and 20 mg pyridoxine-HCl) taken every 8 h for 24 h did not change urinary oxalate levels | 98 | ||
| Adult patients with recurrent idiopathic calcium stone formation and healthy individuals (male participants) | Stone formers had less serum vitamin B6 than healthy individuals. Oral pyridoxine (300 mg/day) for 7 days did not change urinary oxalate levels in either group | 99 | ||
| Adult patients with genetically confirmed primary hyperoxaluria type I (male and female participants) | Oral pyridoxine-HCl (5–20 mg/kg/day) for 24 weeks reduced urinary oxalate | 100 | ||
| Vitamin C | Animal models | Male Wistar rats with induced hyperoxaluria (oral 12% ethylene glycol) | Rats treated with oral vitamin C (100 mg/day) for 2 weeks had no change in renal crystals compared with untreated rats | 145 |
| Male Sprague–Dawley rats with induced hyperoxaluria (oral 2% hydroxyl L-proline) | Rats treated with intravenous vitamin C (500 mg/kg) alone for up to 20 days had reduced calcium oxalate dihydrate crystals in urine, but the formation of other crystal types was unchanged compared with unsupplemented rats | 146 | ||
| Human participants | Health care workers (adult male participants) | Vitamin C intake is not associated with the risk of kidney stones (prospective study) | 87 | |
| Health care workers (adult female participants) | Vitamin C intake is not associated with the risk of kidney stones (prospective study) | 88 | ||
| Health care workers (adult male and female participants) | Total and supplemental vitamin C intake was associated with an increased risk of kidney stones in men but not in women (observational follow-up study) | 122 | ||
| Health care workers (adult male participants) | Men who consumed >1,000 mg/day of vitamin C were at a higher risk of stone formation than men who consumed 90 mg/day (observational follow-up study) | 123 | ||
| Individuals representative of the Swedish male population (adult male participants) | Men who took only vitamin C supplements were more likely to develop kidney stones than men who did not take the supplement (prospective study) | 125 | ||
| Healthy adults (male and female participants) | Oral treatment with 1 g/day of vitamin C did not change urinary oxalate levels, but 5 g/day and 10 g/day of vitamin C increased urinary oxalate | 128 | ||
| Adult calcium oxalate stone formers and healthy individuals (male and female participants) | Treatment with oral vitamin C (5 g/day) for 2 days significantly increased urinary oxalate in both groups compared with placebo (P < 0.01) | 129 | ||
| Adult calcium oxalate stone formers and healthy individuals (male and female participants) | Oral vitamin C (2 g/day) significantly increased oxalate absorption and urinary oxalate in stone formers but not in healthy individuals (P < 0.05) | 130 | ||
| Health care workers (adult male and female participants) | Participants who consumed >1 g/day total of vitamin C had excreted a significantly higher amount of urinary oxalate than participants consuming <1 g/day (trend P < 0.001) | 131 | ||
| Adult calcium stone-formers and healthy individuals (male and female participants) | Oral vitamin C (1 g/day and 2 g/day) for 3 days increased urinary oxalate | 132 | ||
| Adults with sensitivity to acidic foods (male and female participants) | Oral supplementation with vitamin C (500 mg/day) and vitamin C metabolites for 10 days resulted in a lower urinary oxalate than supplementation with vitamin C alone | 136 | ||
| Adult healthy individuals (male and female participants) | Parenteral vitamin C (100 mg/day and 200 mg/day) increased urinary oxalate in a dose-dependent manner | 138 | ||
| One adult woman admitted to the hospital with a complex medical history | Treatment with two doses of intravenous vitamin C (unknown concentration) at an alternative medicine clinic led to development of renal calculi (case report) | 139 | ||
| Two adult men admitted to hospital for SARS-CoV-2 | Patients receiving 50 mg/kg vitamin C four times per day developed renal calculi (case reports) | 140 | ||
| Two adults admitted to the hospital for thermal injuries (one man and one woman) | Patients treated with intravenous vitamin C (66 mg/kg/h) for 18 h and 20 h, respectively, showed calcium oxalate crystals in the kidney (case report) | 141 | ||
| Adult hospitalized patients receiving a high dose of intravenous vitamin C (male and female participants) | High-dose intravenous vitamin C did not cause renal calculi (prospective study) | 142 | ||
| Patients with various conditions receiving high-dose intravenous vitamin C | Scoping review of the harms of high-dose intravenous vitamin C. Renal calculi formation was observed in only one case study | 143 | ||
| Vitamin D | Human participants | Adult kidney stone formers and healthy individuals (male and female participants) | Calcium-containing stone formers had higher 1,25(OH)2D levels than healthy individuals, and hypercalciuria stone formers had higher 25(OH)D levels than normocalciuria stone formers and healthy individuals (meta-analysis) | 188 |
| Adult kidney stone formers and healthy individuals (male and female participants) | Long-term vitamin D supplementation resulted in increased risk of hypercalciuria but not increased risk of stone formation compared with placebo (meta-analysis) | 189 | ||
| Health care workers (adult male and female participants) | Vitamin D intake was not associated with kidney stone formation (meta-analysis) | 190 | ||
| Adult patients with a history of calcium kidney stones and individuals with no history of kidney stones from a urology clinic (male and female participants) | Calcium-containing stone formers had lower levels of circulating 25(OH)D than non-stone formers | 191 | ||
| Adult patients with history of calcium kidney stones and individuals with no history of kidney stones (male and female participants) | Serum levels of 25(OH)D were higher in calcium-containing stone formers than in non-stone formers (retrospective study) | 192 | ||
| Adult kidney stone formers and healthy individuals (male and female participants) | Participants with urolithiasis had an increased prevalence of vitamin D deficiency compared with individuals without urolithiasis (prospective case–control study) | 193 | ||
| Vitamin E | Animal models | Male Sprague–Dawley rats with induced hyperoxaluria (oral 2% hydroxyl L-proline) | Rats treated with intraperitoneal vitamin E (200 mg/kg) alone or in combination with intravenous vitamin C (500 mg/kg) for up to 20 days had reduced calcium oxalate crystals in urine | 146 |
| Male Sprague–Dawley rats with induced hyperoxaluria (oral 150 mg/day ethylene glycol) | Rats treated with an excess (2,000 U/kg) of vitamin E had fewer renal calculi and reduced oxidative stress markers after receiving ethylene glycol than rats receiving adequate (100 U vitamin E/kg) amounts of vitamin E and vitamin E-deficient rats | 230 | ||
| Male Wistar rats with induced hyperoxaluria (oral 0.75% ethylene glycol) | Rats receiving two intraperitoneal injections per week of 200 mg/kg vitamin E for 6 weeks had a reduced Tiselius Risk index for stone oxidative stress markers than unsupplemented rats | 231 | ||
| Male Wistar rats receiving lithogenic diet (calculi-inducing diet) | Rats receiving 400 mg/kg of vitamin E had increased production of antioxidant enzymes and reduced markers of lipid peroxidation compared with control rats | 232 | ||
| Male Sprague–Dawley rats with induced hyperoxaluria (oral 2% hydroxyl L-proline) | Rats treated with intraperitoneal vitamin E (200 mg/kg) alone or in combination with intravenous vitamin C (500 mg/kg) for up to 20 days had reduced calcium oxalate crystals in urine | 146 | ||
| Human participants | Adult calcium oxalate stone formers and healthy individuals (male and female participants) | Plasma levels of tocopherol were higher in sex-matched stone formers than in healthy individuals | 51 | |
| Adult stone formers and healthy individuals (male and female participants) | Stone formers had lower plasma vitamin E than healthy individuals (case–control study) | 56 | ||
| Adult patients with lithiasis (male participants) | Stone formers had significantly (P < 0.05) lower levels of vitamin E than reference values observed in healthy individuals | 233 | ||
| Adult patients with fat malabsorption with or without kidney stones (male and female participants) | Vitamin E levels were lower and markers of oxidative damage were higher in stone formers than in non-stone formers | 234 | ||
| Adult calcium oxalate stone formers and healthy individuals (male and female participants) | Stone formers had lower vitamin E and increased markers of oxidative damage than healthy individuals | 235 | ||
| Adult stone formers and healthy individuals (male and female participants) | Stone formers receiving vitamin E (400 mg/day) for 9 months produced Tamm–Horsfall protein with an increased inhibitory activity against calcium oxalate crystals compared with stone formers who did not receive vitamin E | 238 | ||
| Vitamin K | Animal models | Male Sprague–Dawley rats with induced hyperoxaluria (oral 0.75% ethylene glycol) | Rats treated with vitamin K2 for 6 weeks showed reduced crystal deposition compared with untreated rats | 290 |
| Human participants | Paediatric patients with bladder stones (male participants) | Gla residues found in amino acids isolated from calcium oxalate and hydroxyapatite stones | 276 | |
| Adult patients with kidney stones | Gla residues found in amino acids isolated from calcium oxalate and hydroxyapatite stones | 277 | ||
| Adult patients with calcium oxalate kidney stones and non-stone formers | Reduced number of Gla residues and lower crystal affinity of a human glycoprotein (nephrocalcin) in stone formers than in non-stone formers | 280 | ||
| Adults from Flemish population (male and female participants) | Increased levels of plasma dp-ucMGP were associated with an increased risk of stone formation (Mendelian randomization study) | 291 | ||
| Adult first-time stone formers and healthy individuals (male and female participants) | No change in serum levels of dp-ucMGP in stone formers compared with healthy individuals (prospective study) | 292 |
HCl, hydrochloric acid; Dp-ucMGP, dephosphorylated-uncarboxylated Matrix Gla protein; Gla, γ-carboxyglutamate. aNo statistics performed in the study.
Vitamin A has the notable ability to improve intestinal integrity by effectively reducing gut permeability. Indeed, a weakly positive correlation between serum retinol levels and gut barrier integrity has been observed in children58,59. Specifically, children with ≤0.25 mg/l serum retinol showed impaired gut barrier function, as determined by the mannitol/lactulose test58,59. Results from in vitro experiments in human intestinal epithelial cell lines showed that all-trans retinoic acid can improve gut integrity as observed through increased transepithelial electrical resistance, reduced permeability, and increased expression of the tight junction protein ZO-1 (refs. 60,61). In rodent models, dietary retinoids were shown to be involved in the proliferation and differentiation of the intestinal epithelium62,63. Moreover, vitamin A can bolster immune activity and limit the colonization of pathogenic bacteria that damage the intestinal epithelium through interaction with the resident intestinal immune system64. Considering that oxalate is absorbed paracellularly in the absence of calcium65,66, reduced intestinal permeability could reduce oxalate uptake and, consequently, the formation of calcium oxalate stones. This hypothesis is further supported by data showing that in obese mice with increased intestinal permeability, gut absorption of oxalate was elevated, independently of dietary intake67. Gut barrier integrity is required to prevent the passive translocation of bacterial endotoxins (such as lipopolysaccharides) into the bloodstream; in the kidneys, these toxins can induce local damage and further increase stone burden by providing a site for crystal adherence68. This hypothesis has been partially supported by the observation that non-infectious calcium oxalate stones contain various levels of endotoxins69. These endotoxins could have originated from resident bacteria of the kidney, but gut-derived endotoxins were also shown to be able to reach the kidneys and induce local inflammation70.
The interactions between host vitamin A homeostasis and the gut microbiota are quite complex (Table 2). Many bacteria can make carotenoids, but these pigments cannot necessarily be converted into useable retinoids71,72. Exogenous β-carotene, a highly abundant carotenoid found in vegetables, is cleaved to become all-trans retinal by β-carotene 15,15′-dioxygenase73. Most of the bacteria known to express this enzyme are found in marine and environmental locations74; however, β-carotene 15,15′-dioxygenase has also been found in human faecal samples, and is predicted, using homologous sequences, to be harboured by Clostridium spp., Streptococcus spp., Enterococcus spp. and Prevotella marshii, in which β-carotene 15,15′-dioxygenase might also function to enhance salt tolerance75,76. A notorious foodborne pathogen, Bacillus cereus, possesses a multi-functional aldehyde dehydrogenase that is capable of converting both retinoic acid and retinal to retinol77. Future determination of gut microbiota members that harbour additional multifunctional enzymes implicated in the conversion of vitamin A vitamers could provide meaningful insights into the role of these bacteria in mediating vitamin A absorption and function. In addition to these direct systems, the microbiota has an overall influence on retinoid homeostasis, highlighted by an increase in retinoic acid and a decrease in retinol and retinyl palmitate observed in germ-free mice compared with conventionally reared mice78. The authors of this study identified a novel mechanism through which bacteria of the Clostridia class can inhibit retinoic acid synthesis in mouse intestinal epithelial cells; additionally, this work highlighted the intimate relationship between the host’s micronutrient status and the host’s microorganisms78. Future work should be aimed at investigating whether commensal bacteria alter the vitamin A profile in stone formers and the potential consequences of these alterations in stone disease.
Table 2.
Studies to assess the role of the gut microbiota in vitamin homeostasis
| Vitamin | Main findings | Role of microbiota in vitamin homeostasis | Ref. |
|---|---|---|---|
| All vitamins | All vitamin synthesis pathways are found in the human faecal metagenome | Synthesis of vitamins | 46 |
| Vitamin A | Bacteria synthesize many types of carotenoids that might be converted into vitamin A | Source of vitamin A | 71 |
| A gene encoding β-carotene 15,15′-dioxygenase is predicted to be present in the genomes of Clostridium spp., Streptococcus spp., Enterococcus spp. and Prevotella marshii | Potential conversion among vitamin A vitamers | 75 | |
| Bacillus cereus expresses a multi-functional aldehyde dehydrogenase that can convert retinoic acid and retinal into retinol | Conversion among vitamin A vitamers | 77 | |
| Bacteria in the Clostridia class can inhibit mouse retinoic acid synthesis | Reduction in circulating vitamin A | 78 | |
| Vitamin B6 | Several vitamin B6 genes were found to be present in members of the gut microbiotaa | Potential source of vitamin B6 | 47 |
| The gut microbiota is predicted to produce over 80% of the recommended dietary intake of vitamin B6 | Source of vitamin B6 | 48 | |
| Microbiota transplant from wild type to vitamin B6-genetically deficient mice restored circulating levels of vitamin B6 | Source of vitamin B6 | 105 | |
| Numerous bacteria from the gut microbiota have functional pathways for vitamin B6 synthesis | Source of vitamin B6 | 106 | |
| Vitamin C | Lactobacillus helveticus CICC 22171 requires vitamin C to grow | Catabolism of vitamin C | 155 |
| Lacticaseibacillus rhamnosus GG metabolizes vitamin C to lactate, acetate and CO2 | Metabolism of vitamin C into other metabolites | 156 | |
| Escherichia coli K-12 can ferment L-ascorbate to D-xylulose 5-phosphate through the ula gene clustera | Metabolism of vitamin C into other metabolites | 159 | |
| Streptococcus pneumoniae encodes for part of the ula gene cluster | Catabolism of vitamin C | 161 | |
| Salmonella enterica encodes for part of the ula gene cluster | Catabolism of vitamin C | 162 | |
| Shigella flexneri encodes for part of the ula gene cluster | Catabolism of vitamin C | 163 | |
| Enterococcus faecalis V583 has homologs of the ula gene cluster and can grow on vitamin C | Catabolism of vitamin C | 164 | |
| Klebsiella pneumoniae 13882 encodes for part of the ula gene cluster and metabolizes L-ascorbate | Catabolism of vitamin C | 165 | |
| The Escherichia coli ascorbate transporter has a high affinity for vitamin C | Catabolism of vitamin C | 166 | |
| LPS released by E. coli 0111:B4 reduces the expression of SVCT1 and SVCT2 in Caco-2 cells and in mice, reducing the uptake of vitamin C | Reduced absorption of vitamin C | 167 | |
| Infection with enteropathogenic E. coli E2348/69 reduces the expression of SVCT1 and SVCT2, increases the expression of SVCT inhibitory miRNA (miR103a, miR141, miR200a) and reduces vitamin C uptake in mice | Reduced absorption of vitamin C | 170 | |
| Vitamin D | In men, serum 1,25(OH)2D partly explained the variance in α-diversity (within sample diversity) and β-diversity (between sample diversity and was associated with a change in abundance of 12 taxa | Potential metabolism of vitamin D | 202 |
| Serum 25(OH)D was associated with certain bacterial taxa and thought to mediate vitamin D absorption | Microbiota potentially mediates vitamin D absorption | 203 | |
| Streptomyces sclerotialus FERM BP-1370 can convert 25(OH)D into 1,25(OH)2D through cytochrome P-450 | Conversion among vitamin D vitamers | 204 | |
| The gene encoding Streptomyces griseolus P450SU-1 (CYP105A1) converts vitamin D2 and D3 to 25(OH)D forms and converts 25(OH)D3 to 1,25(OH)2D3 | Conversion among vitamin D vitamers | 205 | |
| Pseudonocardia autotrophica NBRC 12743 converts vitamin D3 into 25(OH)D3 or 1,25(OH)2D3 through vdh | Conversion among vitamin D vitamers | 206 | |
| Bacillus lehensis G1 converts 1α-hydroxyvitamin D3 into 1,25(OH)2D3 through CYP107CB2 | Conversion among vitamin D vitamers | 207 | |
| Bacillus lehensis G1 converts vitamin D3 to 25(OH)D3 through CYP107CB2 | Conversion among vitamin D vitamers | 208 | |
| Ruminococcus torques encodes for a gene that shows partial homology with human CYP27A1a | Potential conversion among vitamin D vitamers | 209 | |
| Germ-free mice have lower titres of circulating vitamin D than conventional mice, and have increased titres of vitamin D after colonization with the pathogen Citrobacter rodentium, which regulates immunity to promote vitamin D absorption | Altered vitamin D absorption | 213 | |
| Butyrate (microbiota metabolite) enhances VDR production and binding of 1,25(OH)2D3 to VDR | Altered absorption and function of vitamin D | 219 | |
| Administration of Limosilactobacillus reuteri NCIMB 30242 increased serum 25(OH)D levels in otherwise healthy adults with hypercholesterolaemia | Increased absorption of vitamin D | 223 | |
| Co-administration of Lacticaseibacillus paracasei DG and vitamin D3 increases serum 25(OH)D levels in mice | Increased absorption of vitamin D | 225 | |
| Vitamin E | α-tocopherol quinone and α-tocopherol quinol are found in many types of bacterial cells | Potential source of vitamin E | 240 |
| Mice receiving antibiotics in combination with vitamin E had increased titres of vitamin E compared with mice only receiving vitamin E | Altered absorption of vitamin E | 244 | |
| Chickens receiving antibiotics in combination with vitamin E acetate had increased titres of vitamin E compared with chickens only receiving vitamin E acetate | Altered absorption of vitamin E | 245 | |
| Mice monocolonized with Lactobacillus acidophilus NCFM had reduced titres of α-tocopherol acetate in the small intestine compared with germ-free controls | Altered absorption of vitamin E | 251 | |
| Certain members of the gut microbiota express a bile salt hydrolase that can deconjugate bile acids and potentially alter the absorption of fat-soluble vitamins | Potentially altered absorption of vitamin E | 248 | |
| Vitamin K | Several members of the gut microbiota express vitamin K2 genesa | Potential source of vitamin K2 | 47 |
| Veillonella spp., Bacteroides spp. and Eubacterium lentum produce menaquinones with different side chain length | Source of vitamin K2 | 303 | |
| Prevotella spp. produce menaquinones with different side chain length | Sources of vitamin K2 | 304 | |
| Members of the gut microbiota have incomplete pathways for menaquinone synthesis | Potential source of vitamin K2 | 306 | |
| Members of the gut microbiota have incomplete pathways for menaquinone use | Potential metabolism of vitamin K2 | 307 | |
| Increased abundance of specific members of the gut microbiota associated with faecal vitamers of vitamin K2 | Potential source of vitamin K2 | 308 | |
| Increased abundance of specific members of the gut microbiota associated with faecal vitamers of vitamin K2 | Potential source of vitamin K2 | 309 | |
| Bacteroides spp. were higher in postmenopausal women with high blood vitamin K2 than women with low blood vitamin K2 | Potential source of vitamin K2 | 310 | |
| The diversity of the gut microbiota of patients with Crohn’s disease correlated negatively with vitamin K deficiency (as measured by inactive osteocalcin) | Potential source of vitamin K2 | 311 | |
| Healthy participants taking antibiotics had lower circulating menaquinones than participants who did not take antibiotics (measured post-mortem) | Potential source of vitamin K2 | 312 | |
| Mice given antibiotics had reduced caecal vitamin K2 compared with controls | Potential source of vitamin K2 | 313 |
LPS, lipopolysaccharide; VDR, vitamin D receptor. aReview article.
Vitamin B6
The generic vitamin B6 complex encompasses multiple structurally similar water-soluble vitamers: pyridoxal, pyridoxine and pyridoxamine, and the phosphorylated derivatives pyridoxal 5′-phosphate, pyridoxine 5′-phosphate and pyridoxamine 5′-phosphate (Fig. 2). Each phosphorylated vitamer can act as an enzymatic cofactor79, but pyridoxal 5′-phosphate is the most biologically active80. However, the term vitamin B6 commonly refers to pyridoxine only. Pyridoxine is a hydroxy methylpyridine with the characteristic methyl group at position 2, a hydroxy group at position 3, and two hydroxymethyl groups at positions 4 and 5 (ref. 81). Mammals cannot produce vitamin B6, which, therefore, must be obtained through plant-based foods or produced by bacteria48,82. In the body, dietary pyridoxine is converted into pyridoxine 5′-phosphate and pyridoxal 5′-phosphate, and is involved in >140 biochemical reactions in humans83, including a role as a cofactor for the synthesis of amino acids and neurotransmitters. Specifically, vitamin B6 acts as a cofactor for the enzyme alanine-glyoxylate aminotransferase (AGT), which is used in the conversion of glyoxylate to glycine84. A deficiency in this vitamin results in the reduction of AGT activity, which might lead to decreased glyoxylate detoxification and, in turn, increased conversion to oxalate, similar to what happens in primary hyperoxaluria type 1 (PH1, which results from mutations in AGXT). Increasing the amount of oxalate circulating in the body is a known risk factor for the development of calcium oxalate kidney stones85. Coherent with this hypothesis, pyridoxine supplementation was shown to be useful in the prevention of kidney stones86–88. However, conflicting results have generated a debate around this topic.
Some of the earliest and most compelling evidence that links vitamin B6 deficiency to calcium oxalate kidney stone development stems from work in feline and murine models (Table 1). In these studies, animals with B6 deficiency had increased urinary oxalate and incidence of kidney stones compared with non-vitamin B6-deficient animals89,90. Vitamin B deficiency was also associated with marked renal damage in cell line models, which contributed to crystal adherence and growth, and might have resulted from the excessive formation of endogenous oxalate85,89–91. Attempts to replicate these findings in humans have yielded conflicting results that question the role of this vitamin in kidney stone disease86. In two large-scale studies, the Health Professional Follow-up Study and the Nurses’ Health Study I92, the health outcomes of >40,000 men and women, respectively, were monitored for 6–14 years87,88. The intake of vitamins and kidney stone incidence were among the parameters included in the investigation. In men, no association between vitamin B6 consumption and the risk of idiopathic stone formation was observed87. However, among women, the consumption of >40 mg/day of vitamin B6 was associated with a lower incidence of kidney stones (relative risk (RR): 0.75, 95% CI 0.52–1.09) compared with women who consumed <3 mg/day of vitamin B6 (RR: 1)88. Unfortunately, the strength of these findings is questionable, as results from a follow-up study involving the same cohorts after >10 years from these results showed no protective effect of vitamin B6 against stone disease in either men or women86. Vitamin B6 given to stone formers was shown to reduce urinary oxalate93–96 in some studies, but, in other studies, no influence of vitamin B6 on urinary oxalate was observed97–99 (Table 1). These disparities could explain why no conclusion has been reached on the use of vitamin B6 to mitigate calcium oxalate stone disease; additional studies are needed to determine the effect of vitamin B6 on urinary oxalate.
Similar to what was observed for oxalate kidney stones, incongruous results regarding the role of vitamin B6 in primary hyperoxaluria were reported. Oral supplementation of vitamin B6 was shown to reduce urinary oxalate by ~25% in ~50% of patients with primary hyperoxaluria, but a notable variability in the efficacy was observed100. This variability was attributed to different AGT mutations — with the best response to treatment observed in patients harbouring the G508A mutation — although the reason for this evidence is not understood. Perhaps, in non-responders, underlying factors limit the conversion of pyridoxine to the active form pyridoxal 5′-phosphate100. In most studies including patients with either primary hyperoxaluria or calcium oxalate stones, vitamin B6 was supplemented with the pyridoxine vitamer; however, results from in vitro studies in cell line models suggest that the effect of pyridoxine on AGT function can differ among hosts harbouring different AGTX genetic variants101, and that pyridoxal or pyridoxamine vitamers could be more beneficial than pyridoxine in hosts harbouring rare AGT variants102. Together, these data showed that the efficacy of AGT could be mediated by the B6 vitamer, which is used as a cofactor.
The variability in responsiveness to vitamin B6 could also be influenced by the microbial enzymes present in the gastrointestinal tract99,100,103,104. The gut microbiome can potentially modulate the bioavailability or vitamers of vitamin B6 that are absorbed (Table 2). A microbiota transplant from a wild type mouse to mice genetically deficient for EPHB6 — encoding a receptor tyrosine kinase of the EPH family — has been shown to restore circulating pyridoxal 5′-phosphate levels, which had a reduced abundance in the microbiome of these mice105. This effect could be ascribed to the evidence that many microorganisms (including Acinetobacter spp., most Bacteroides spp., Bifidobacterium spp., Klebsiella spp., Prevotella spp., some Clostridium spp. and some Ruminococcus spp.) have all the enzymes to produce pyridoxine de novo48,106. In other bacteria, enzymatic pathways to produce pyridoxine are incomplete, and metabolite sharing between microorganisms is required to obtain pyridoxine48,106. In total, ~85% of the human dietary reference intake of vitamin B6 is predicted to be sourced from the gut microbiota48. Preliminary results showed a reduction in the abundance of vitamin B6-related genes in the gut microbiota of stone formers inferred through 16S rRNA gene sequencing43. Vitamin B6 vitamers are also important to sustain a healthy gut microbiome. For example, Faecalibacterium prausnitzii — a presumed beneficial species that is decreased in stone formers38 — is an auxotroph for pyridoxal but not pyridoxamine, highlighting the importance of vitamin B6 vitamers in preserving the health of bacterial species106,107.
Results from a cross-sectional study showed a 27% decrease in in serum pyridoxal 5′-phosphate concentration in calcium stone formers compared with healthy individuals (P < 0.0001), even after pyridoxine supplementation99. This study is single-centred, but highlights the importance of assessing the blood concentration of B6 vitamers in future works to determine whether vitamers reach the circulation upon supplementation. Results from works on primary hyperoxaluria indicate that B6 vitamers could have differential consequences for the activity of AGT, and, ultimately, for urinary oxalate101,102. Bacteria typically make pyridoxal, pyridoxine and pyridoxamine, which can be used as reactants but, sometimes, are also the final products of specific pathways in bacterial vitamin B6 synthesis108. Thus, ingested or synthesized pyridoxal might be converted through incomplete pathways and metabolite sharing occurring in the “pantryome”47 into other vitamers that might be responsible for the different urinary oxalate titres observed across studies. Considering these hypotheses, determining how the composition of the microbiota relates to the circulating vitamer titres in stone formers and non-stone formers would be an interesting area to explore.
Vitamin C
Vitamin C is the common name for L-ascorbic acid and L-dehydroascorbic acid, the oxidized form of ascorbic acid. These vitamins are water soluble and have a structure that weakly resembles a cyclic sugar molecule (Fig. 2). Differently from most animals, humans lack a functional gluconolactone oxidase, the enzyme required for the biosynthesis of ascorbic acid, and, therefore, are unable to produce this vitamin de novo109. The main sources of dietary vitamin C are fruits and vegetables, including tomatoes, potatoes, bell peppers and citrus fruits. Dietary vitamin C is readily absorbed in the intestine through sodium-dependent vitamin C transporters (SVCT1 and SVCT2)110. However, vitamin C is a water-soluble vitamin and, therefore, only limited amounts of vitamin C are stored in the body, whereas the majority of this vitamin is eliminated through urinary excretion111. For this reason, ascorbic acid must be consumed regularly to effectively counter the rapid excretion of vitamin C and maintain sufficient levels in the body112. Owing to the rapid excretion of vitamin C, dietary intake of vitamin C is better captured by blood and urine measurements of ascorbic acid, whereas vitamin C intracellular content would provide a realistic overview of bodily vitamin C status113. The prevalence of vitamin C deficiency is low in high-income countries and increasing in low-income and middle-income countries114.
In the body, vitamin C acts as a non-specific antioxidant and protein cofactor, and might prevent infection115,116. A combination of genetic investigations and radiolabelling studies indicated that dehydroascorbic acid, 2,3-diketogulonic acid and oxalate are the major metabolites produced from the catabolism of vitamin C in mammals111,117. Thus, increased oxalate in the urine might, in part, depend on vitamin C metabolism, although the role of vitamin C as a risk factor for kidney stone development is strongly debated118 (Table 1).
Canadian Urological Association clinical guidelines continue to recommend limiting vitamin C intake to <1,000 mg/day to theoretically prevent hyperoxaluria, whereas limiting vitamin C intake is not mentioned in the American Urology Association or European Association of Urology guidelines for stone disease119–121. However, evidence for the role of vitamin C in stone disease is conflicting. In one of the earliest studies dating back to the 1990s — the Health Professionals Follow-up Study — no difference in the relative risk of stone formation was reported in men who consumed ≥1,000 mg/day of vitamin C (from foods and supplements) compared with men who consumed <250 mg/day of vitamin C87. These findings were confirmed after 2 years in women, in the Nurses’ Health Study I88. Conversely, in a follow-up study in which the same cohorts were monitored >10 years after these studies, both total and supplemental vitamin C intake was significantly associated with an increased risk of kidney stones in men (P = 0.005), but not in women (P = 0.01)122,123. Importantly, in these follow-up studies, a baseline of 90 mg/day, instead of the previously used 250 mg/day, was used to reflect changes in the Recommended Dietary Allowance (minimum dietary intake to meet nutritional requirement) of vitamin C; thus, the statistically significant results reported in the follow-up studies were probably partially ascribable to the definition of adequate vitamin C intake124. A report on the Cohort of Swedish Men, which is a population of >45,000 men aged 45–79 years recruited in 1997 and undergoing follow-up monitoring in 2008/2009 and 2019, showed that men who specifically took vitamin C supplements, but not multivitamins containing vitamin C, were nearly twice as likely to develop stones than men who did not take the supplements125. These long-term studies rely on the voluntary recording of dietary and supplement intake; thus, several confounding factors — the most obvious of which is dietary misreporting — could contribute to the inconsistencies observed across these studies126. Regardless of these discrepancies, evidence hints at the possibility that the role of ascorbic acid in stone disease could be sex specific. Evidence from a review127 in which vitamin C data from multiple studies were summarized showed that women typically had higher blood levels of vitamin C than men. The mechanisms underlying this difference have not been determined, but the reduction in circulating vitamin C in men could be hypothesized to be the result of increased metabolism to oxalate; however, additional data are required to support this speculation.
In a study in which the effect of vitamin C on urinary oxalate was investigated128, marginal increases in urinary oxalate were observed after treatment with up to 10 g/day of ascorbic acid in healthy individuals. In other studies, small but consistent increases in urinary oxalate were also shown upon supplementation with >1,000 mg/day of vitamin C in both healthy individuals and patients with kidney stones129–132. Interestingly, calcium oxalate stone formers seemed to have larger increases in urinary oxalate upon oral administration of ascorbic acid than non-stone formers, suggesting that individual patient factors are likely to contribute to stone disease129,130,132. Whether oral ascorbic acid is converted into oxalate in the gastrointestinal tract before absorption is unclear, but results from studies in which the absorption of 13C2-oxalate was investigated showed that calcium oxalate stone formers absorbed more oxalate than healthy individuals, suggesting that increased oxalate absorption could be a driving factor for calcium oxalate stone disease133,134.
Caution should be taken when interpreting the results from these studies because the non-enzymatic conversion of urinary vitamin C into oxalate can occur rapidly and during the analysis phase128,135. However, results from a small-scale crossover study in adults with sensitivity to acidic foods showed that the intake of vitamin C in combination with downstream metabolites (excluding oxalate) resulted in a smaller increase in urinary oxalate than vitamin C alone, suggesting that the observed increases in urinary oxalate are likely to be enzymatic in nature136. The rate at which vitamin C is converted into oxalate can be hypothesized to be variable among individuals, but oral administration is associated with confounding variables, such as the gut microbiota, which can metabolize vitamin C, making it difficult to infer meaningful conclusions about the fate of supplemented vitamin C. Intravenous administration of ascorbic acid bypasses the gastrointestinal tract and directly enters the bloodstream, providing a more accurate representation of how vitamin C reacts in circulation than that obtained with oral administration. Both high and low doses of intravenous ascorbic acid increase urinary oxalate137,138, and the formation of renal calculi with intravenous vitamin C has been documented in multiple case studies139–141. However, in a large prospective study, no renal stones were observed in patients receiving intravenous vitamin C, also in patients with a history of stones142. This evidence is corroborated by a scoping review143, in which the review of 73 studies showed that intravenous vitamin C did not result in the development of renal calculi. In only one case report analysed in this scoping review143 one patient developed renal calculi after intravenous treatment of vitamin C at an alternative medicine clinic139. The majority of evidence shows that intravenous vitamin C increases urinary oxalate, but does not necessarily result in the formation of renal calculi. However, results from studies in which oral consumption of vitamin C was used point towards some indication that vitamin C can increase the risk of renal calculi, although this evidence is not strong. The rationale behind a stronger support towards an involvement in stone formation for vitamin C received orally rather than intravenously is difficult to determine. Perhaps the route of consumption could be the missing component in understanding how vitamin C could directly contribute to stone disease, but additional research is needed to fill in this gap.
An overlooked aspect of vitamin C with regard to stone disease is the unique ability of this vitamin to act as a water-soluble, non-specific antioxidant. This evidence first emerged from a study in which vitamin C alone or in combination with vitamin E (α-tocopherol) was shown to ameliorate ROS generation and oxalate-induced markers of oxidative damage in renal tubule cell lines144. However, the combination approach was the most effective at decreasing the toxicity of calcium oxalate crystals144. These claims have been refuted through in vitro experiments with Madin–Darby canine kidney cells and in vivo experiments using male Wistar rats in which no effect of vitamin C as an effective antioxidant to reduce calcium-oxalate-associated ROS generation was shown145. However, in another study in which a Sprague–Dawley rat model was used146, vitamin C alone was shown to be able reduce the abundance of calcium oxalate dihydrate crystals at some time points, and of some — but not all — markers of oxidative damage; however, the abundance of calcium oxalate monohydrate crystals was unchanged146. The contrasting outcomes among these two studies145,146 could be explained by important differences in the experimental approach used. For example, Wistar rats were used in one study145, and received oral vitamin C treatment along with ethylene glycol administered in the drinking water; conversely, male Sprague–Dawley rats were used in the other study146, and were treated with intravenous vitamin C, whereas hydroxyl L-proline was added to the drinking water. In addition to the metabolic shifts caused by ethylene glycol and hydroxyl L-proline147, Sprague–Dawley rats might be more susceptible to kidney stones than Wistar rats148. Thus, additional experimental evidence is needed to determine whether vitamin C can mitigate oxidative damage from kidney stones and whether these effects are enhanced by combination therapy with vitamin E.
The ability of oral vitamin C to be converted into oxalate yields the potential to exacerbate stone disease, but this observation is inconsistent among individuals. Thus, other factors that mediate vitamin C homeostasis in humans must exist in the gastrointestinal tract (Table 2). The gut microbiome can synthesize vitamin C, but the amount of vitamin C synthesized through this route is minimal compared with that coming from dietary sources149–151. However, oral consumption of vitamin C can potentially alter the microbiota because of the ability of specific members of the microbiota to metabolize ascorbic acid152–154. Some lactobacilli can convert vitamin C155,156 into acetate and lactate, which are non-toxic metabolites that increase microbiome function through bioenergetic pathways47,157 and might promote the colonization of these oxalate-degrading bacteria158. Moreover, using the ula (utilization of L-ascorbic acid) gene cluster159, bacteria such as Escherichia coli can metabolize ascorbic acid to D-xylulose, which can be absorbed and further broken down into oxalate160. This same ula gene cluster is also found in pathogenic bacteria such as Streptococcus pneumoniae161, Salmonella spp.162, Shigella flexneri163, Enterococcus faecalis164 and Klebsiella pneumoniae165. The E. coli ascorbic acid transporter also shows a higher affinity for ascorbic acid than the mammalian SVCT1, suggesting that the bacteria can compete with the host for vitamin C166. Additionally, E. coli can reduce host uptake of ascorbic acid through the release of lipopolysaccharides (LPS)167, which increases NF-κB-dependent production of TNF, which in turn reduces the expression of sodium-dependent vitamin C transporters SVCT1 and SVCT2 through the inhibition of SLC23A1 and SLC23A2 promoters (encoding SVCT1 and SVCT2, respectively)168,169. Pathogenic E. coli also increases the expression of multiple host microRNAs that directly block the production of SVCT1 and SVCT2 (refs. 170–172). Taken together, these results show that the composition of the microbiota seems to potentially have a role in the metabolism of vitamin C before this vitamin is even absorbed by the host. The microbiota of stone formers is more commonly enriched in pathogenic bacteria that typically harbour the ula gene cluster compared with the microbiota of non-stone formers, and these individuals showed a larger increase in oxalate levels when receiving oral vitamin C than non-stone formers; thus, whether the microbiota of these individuals is converting oral vitamin C into a metabolite that is readily absorbed and easily converted into oxalate would be interesting to postulate. Moreover, the extent to which commensal bacteria influence vitamin C absorption and subsequent stone formation is unknown, and is an important knowledge gap in this area, as these microorganisms constitute a large proportion of the microbiota.
Vitamin D
Vitamin D is the generic identifier that refers to a group of fat-soluble secosteroids with crucial biological properties. In humans, vitamin D is primarily found as naturally synthesized vitamin D2 (ergocalciferol) or vitamin D3 (cholecalciferol), but other forms also exist (Fig. 2). For example, vitamin D4 (22-dihydroergocalciferol), another naturally derived form of vitamin D, is present in some fungi. Synthetic versions like vitamin D5 (1α-hydroxy-24-ethyl-cholecalciferol) have also been produced and tested as an antitumour therapeutics. Generally, vitamin D is obtained from diet, supplements and ultraviolet light exposure from the sun. Oily fish, mushrooms, egg yolks and fortified milk are the best dietary sources for vitamin D2 and D3 (ref. 173). Natural synthesis of vitamin D3 requires epidermal exposure to ultraviolet B radiation; however, the efficiency of this production relies on the time and intensity of sunlight exposure, and on skin pigmentation174. Regardless of the synthesis method, both vitamin D2 and D3 are transported throughout the body through blood circulation to be processed for local use (Fig. 3). The vitamers go to the liver for hydroxylation to the storage form, 25-hydroxyvitamin D (shortened to 25(OH)D)173. In the kidneys, 25(OH)D is converted to the active form calcitriol (1,25(OH)2D), which can travel to effector locations to perform different functions173. Both 25(OH)D and 1,25(OH)2D can be actively degraded to maintain the necessary titres of vitamin D173.
Fig. 3. Vitamin D metabolic pathway.
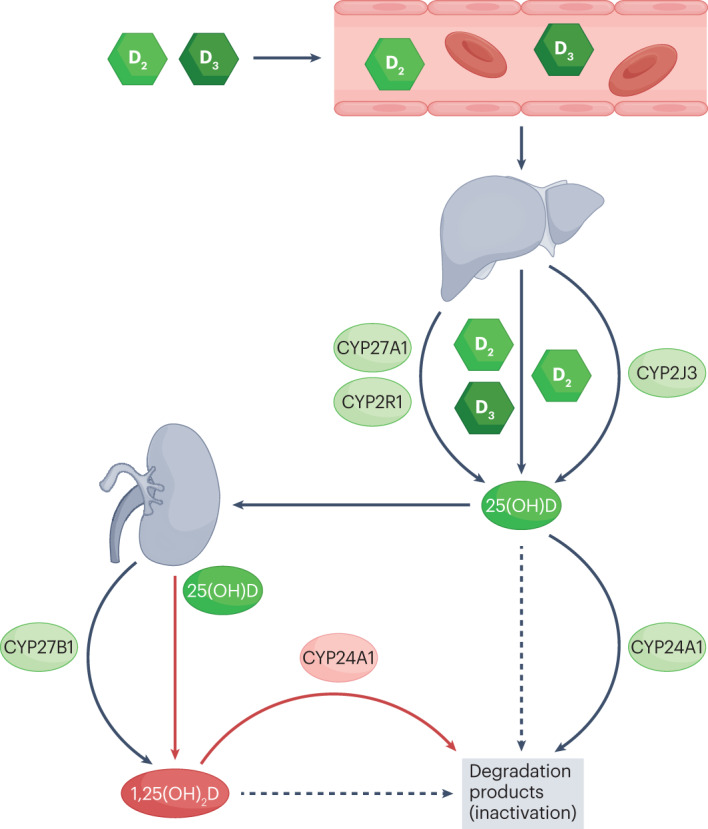
Vitamin D2 and D3 — synthesized by the skin or acquired from ingestion — are circulated to the liver, where the hydroxylation of these vitamins by CYP27A1, CYP2R1 or CYP2J3 occurs. Vitamin D2 and D3 are converted into ergocalciferol (25-hydroxyergocalciferol) and calcifediol (25-hydroxycholecalciferol), respectively. Together, these products are referred to as 25-hydroxyvitamin D (shortened to 25(OH)D). In the proximal convoluted tubules of the kidneys and in some immune cells (not shown), 25(OH)D is converted into the active form calcitriol (also known as 1,25 dihydroxyvitamin D (1,25(OH)2D)), through hydroxylation by CYP27B1. Both 25(OH)D and 1,25(OH)2D can be degraded by CYP24A1 to maintain the necessary titres of vitamin D. CYP, cytochrome P450.
In humans, the active form of vitamin D regulates multiple bodily processes, but is mostly known for a role in modulating the absorption, excretion and storage of calcium. To facilitate active transcellular absorption of calcium, 1,25(OH)2D binds the vitamin D receptor (VDR) in the intestinal epithelium, stimulating the uptake of dietary calcium in epithelial cells, where calcium can bind calcium-binding proteins175,176. These proteins transport calcium to the basolateral side of the epithelium, from which calcium is actively pumped into the bloodstream175,176. Calcium can also be absorbed paracellularly, but this phenomenon is dependent on the strength of the calcium gradient, and usually occurs in a vitamin D-deficient state175,176. Conversely, vitamin D has minor roles in influencing calcium excretion from the kidneys177. The majority (~70%) of calcium reabsorption in the kidney occurs paracellularly in the proximal tubule178,179. The next most common area of reabsorption is the distal tubule, in which calcium is reabsorbed by vitamin D-mediated transcellular transport178,180. The process of distal tubule reabsorption is regulated by 1,25(OH)2D-mediated increased expression of apical calcium receptor proteins (transient receptor potential cation channel subfamily V member 5 (TRPV5)181 and TRPV6 (ref. 182)), intracellular calcium-binding proteins (calbindin-D28K181,183), and basolateral calcium transporters (Na+/Ca2+ exchanger 1 (NCX1) and plasma membrane Ca2+ ATPase 1b (PMCA1b)183). Together, these proteins facilitate distal tubule calcium reabsorption179. Additionally, vitamin D participates in a feedback loop with parathyroid hormone (PTH) to influence control over renal calcium reabsorption179. Briefly, low serum calcium triggers the production of PTH, which increases the production of 1,25(OH)2D in the kidney and, in turn, increases renal calcium reabsorption179.
Vitamin D modulates the dietary absorption and renal reabsorption of calcium, and also acts on bone cells to regulate calcium homeostasis and bone integrity. Bone metabolism is tightly controlled by osteoblasts (bone-forming cells) and osteoclasts (bone-absorbing cells), which are regulated by vitamin D signalling through VDR. Mice lacking functional VDR showed reduced serum calcium, which resulted in reduced bone mineral density compared with heterozygous VDR mice, highlighting the importance of vitamin D in calcium absorption and bone integrity184. VDR can also act directly on bone cells, as mice harbouring an osteoblast-specific knockout of Vdr showed increased bone mass compared with mice with functional Vdr185. Active vitamin D also stimulates the differentiation of osteoclasts186, in turn promoting normal bone resorption187.
The relationship between vitamin D-mediated calcium homeostasis and the risk of kidney stone disease is a well-studied topic, but the conclusions are still not clear179 (Table 1). In a meta-analysis188 of five studies including stone formers and healthy individuals, calcium-containing stone formers were shown to have increased titres of circulating 1,25(OH)2D compared with non-stone formers (P = 0.002), but no significant difference in 25(OH)D levels was observed between the two groups (P = 0.56). One consequence of this increase in 1,25(OH)2D is that calcium-containing stone formers might be at a substantially increased risk of hypercalcaemia, which could result in hypercalciuria. However, stone-forming individuals with hypercalciuria had increased titres of 25(OH)D but similar 1,25(OH)2D levels compared with stone formers with normocalciuria and with healthy individuals188. Thus, only specific vitamers of vitamin D seem to potentially have a role in hypercalciuria. Results from multiple meta-analyses have shown that vitamin D supplementation increases blood calcium levels but does not enhance the risk of stone disease189,190. Results from one of the meta-analyses189 in which the effect of long-term supplementation with vitamin D on urolithiasis was investigated concluded that vitamin D supplements do not pose a risk of stone formation (P = 0.10)189. However, consideration should be given to the fact that one of the nine studies included in this meta-analysis accounted for 73% of the weighted decision and that large confidence intervals were reported in the included studies, which could imply that responders and non-responders might exist189. In summary, vitamin D seems to modulate urinary calcium levels, but might not necessarily contribute to the risk of stone formation, potentially owing to the multifaceted nature of stone disease.
In three studies191–193 that were not included in the meta-analysis188, 25(OH)D levels were slightly decreased in stone formers and a higher proportion of individuals with vitamin D deficiency (as measured by 25(OH)D) was observed among stone formers than among non-stone formers, indicating that vitamin D deficiency could still be prevalent in calcium-containing stone formers. Clinically, hesitancy exists with regard to treating vitamin D deficiency in stone formers because of the preconceived idea that increasing vitamin D will exacerbate stone disease through hypercalciuria, although this hypothesis has been contradicted by experimental evidence189,190,194. However, vitamin D has important roles throughout the body, which might also affect stone formation. The manifestations of vitamin D deficiency include increased inflammation and oxidative damage, which can aggravate stone disease195. Moreover, vitamin D status (as measured by 25(OH)D) correlates negatively with PTH levels196; thus, low 25(OH)D could result in increased PTH, which will activate bone resorption and induce hypercalcaemia and subsequent hypercalciuria, potentially increasing the risk of stone formation197. Results from multiple studies have shown that low bone mineral density is a risk factor for stone disease, but the serum levels of vitamin D were not addressed in these studies198,199. A reduction in vitamin D could also lead to reduced VDR signalling, which is essential for the control of hundreds of genes, including those involved in immunity, the cell cycle, DNA-damage repair, and metabolism200,201. These processes are not explicitly linked to stone disease, but further research could help us to understand whether vitamin D could indirectly influence calcium-containing stone disease through the regulation of these processes.
The apparent inconsistencies regarding the effect of vitamin D on stone formation leave the field open to debate about the relationship between vitamin D and disease aetiology. The majority of work in which the role of 25(OH)D in urinary calcium titres has been investigated produced very conflicting evidence about the function of 25(OH)D in hypercalciuria and in stone disease. Perhaps, the active vitamer 1,25(OH)2D could provide additional information on this relationship, considering the multifaceted role of this vitamer in human physiology. Thus, in future studies assessing vitamin D status in stone formation, both 25(OH)D and 1,25(OH)2D should be considered.
The role of the gut microbiota in vitamin D metabolism has not been investigated with regard to stone disease, but has begun to be studied in other fields. Results from a study including octogenarian men showed that circulating levels of active vitamin D (1,25(OH)2D), but not 25(OH)D, are associated with an increase in alpha diversity of the gut microbiota202. However, in a large genome-wide association study, a correlation between 25(OH)D levels and a higher or lower abundance of specific gut bacterial species was shown; unfortunately, 1,25(OH)2D was not evaluated in this study203. The diversity of microbial metabolism within the human intestine and the contribution of partial metabolic pathways to the ‘pantryome’47 might partially explain the variations in vitamin D status observed in stone formers. The role of commensal microorganisms in vitamin D metabolism is a relatively unexplored topic, although bacteria might modulate vitamin D homeostasis through direct and indirect mechanisms (Table 2). Specifically, multiple environmental bacteria in the Actinomycetia class possess a cytochrome P-450 (CYP) enzyme capable of converting vitamin D into 25(OH)D (storage form) or 1,25(OH)2D (active form)204–206. The soil isolate Bacillus lehensis G1 was also shown to possess CYP enzymes that can convert vitamin D3 into 25(OH)D3, suggesting that this function is present in multiple lineages of bacteria207,208. The genes encoding these unique CYP enzymes have not been found in the genomic pool of human intestinal bacteria; however, a protein having partial homology with human CYP27A1 was identified in Ruminococcus torques, which is part of the human gut microbiota209. The potential conversion of vitamin D into 25(OH)D by bacteria in the gut might lead to an increase in circulating vitamin D levels owing to the potentially increased bioavailability of 25(OH)D210. Conversely, some bacteria might also reduce the bioavailability of vitamin D. Mycobacterium tuberculosis, although not part of the gut microbiota, can infect humans and degrade vitamin D through CYP124 for immune evasion in human phagocytic cells211,212.
Gut microorganisms can also indirectly affect vitamin D activity. In support of this hypothesis, germ-free mice have reduced titres of vitamin D analogues in the circulation owing to increased levels of fibroblast growth factor 23 (FGF23), a protein that inhibits CYP27B1 expression and, therefore, the conversion from 25(OH)D to 1,25(OH)2D213,214. Upon re-colonization with a defined set of eight commensal microorganisms, these mice experienced a reduction in FGF23 levels and a subsequent increase in circulating vitamin D213. Additionally, colonization of these mice with an enteric pathogen resulted in excessive inflammation and reduction of vitamin D levels until the inflammation passed, indicating that infection-induced inflammation can also regulate vitamin D status213. This evidence is corroborated by other works in which circulating vitamin D was shown to be inversely correlated with TNF levels215,216, a marker of inflammation that acts through the NF-κB signalling pathway to decrease the expression of colonic vitamin D receptors217. These observations support the hypothesis that members of the microbiota can indirectly induce a vitamin D receptor dysfunction in an attempt to shut off the host immune system, which results in reduced uptake of vitamin D218.
Beneficial microorganisms in the gut have the ability to affect the downstream function of vitamin D in the host through the production of metabolites. For example, the short chain fatty acid butyrate, which is produced by beneficial microorganisms in the gut microbiota, increases VDR expression, effectively enhancing the binding of active vitamin D to VDR219,220. Butyrate-induced upregulation of VDR, in combination with sufficient vitamin D levels, can reduce inflammation and combat pathogen insult, which could restore the microbiota of stone formers to a healthy and non-inflammatory status, similar to that of non-stone formers221. In line with these observations, results from a genome-wide association study showed that genetic variants of the human VDR locus can influence the composition of the gut microbiota, potentially through the gut–liver axis and the modulation of bile acid metabolism222.
Certain bacteria seem to also modulate vitamin D acquisition. In a study in which Limosilactobacillus reuteri (formally Lactobacillus reuteri) NCIMB 30242 was given to adults with hypercholesterolaemia, increased blood levels of 25(OH)D were observed223. Vitamin D is absorbed through micelles in a similar manner to cholesterol; thus, the authors proposed that the bacterium could alter the bile acid metabolism and in turn affect fat-soluble vitamin absorption, but this hypothesis was not confirmed, and the true mechanism remains unknown223,224. A suggested mechanism for Limosilactobacillus reuteri-mediated vitamin D absorption is that bacteria can solubilize vitamin D through the production of biosurfactants. Lacticaseibacillus paracasei DG (formally Lactobacillus paracasei) and Lacticaseibacillus rhamnosus GG (formally Lactobacillus rhamnosus) were shown to create a stable suspension of vitamin D3 in water225. L. paracasei DG co-administered with vitamin D3 to mice daily for 1 week resulted in a higher titres of 25(OH)D than that observed in control mice receiving vitamin D3 alone, indicating that the bacterium might improve vitamin D absorption225. These results have not been confirmed in humans, but the microbiota contains many important enzymes that could mediate the absorption of vitamin D and other fat-soluble compounds226. Results from these studies, although not yet confirmed in humans, also suggest that inflammation-associated microorganisms might reduce vitamin D absorption, that short-chain fatty-acid-producing bacteria might improve vitamin D absorption, and that specific bacteria can convert between vitamers, which, together, point towards a potential role of the gut microbiota in modulating vitamin D homeostasis. Considering the increased abundance of inflammation-associated bacteria and the reduction of short-chain fatty-acid-producing bacteria in stone formers38,45, determining how the gut microbiota in stone formers could influence vitamin D homeostasis and subsequent stone formation is worthy of investigation.
Vitamin E
Vitamin E is an umbrella term for a group of four tocopherols and four tocotrienols; tocopherols have a saturated (phytyl) side chain, whereas tocotrienols have an unsaturated (farnesyl) chain227 (Fig. 2). Different methylation patterns on the remaining sites of the chromanol double-ring result in different vitamers of these chemicals, denoted by the Greek letters α, β, γ or δ;227 α-tocopherol is the most biologically active form228. The majority of vitamin E is derived from foods or supplements. Vitamin E contains different vitamer profiles, but nuts, seeds, cooking oils, fish and animal meat account for the largest contribution of dietary vitamin E intake in humans227.
Vitamin E functions as a fat-soluble antioxidant that scavenges free radicals and limits the toxic effects of these radicals, many of which are involved in stone formation229. Rats exposed to various calcium oxalate stone-promoting diets and concurrently receiving exogenous vitamin E showed a reduction in crystal deposition146,230,231. Conversely, an increased degree of calcium oxalate crystal binding was observed in the kidneys of rats receiving a diet deficient in dietary vitamin E230. Exogenous vitamin E is presumed to contribute to the reduction of oxidative stress that occurs with stone deposition. This hypothesis has been supported experimentally in studies in which lithogenic rats fed vitamin E had reduced lipid peroxidation and oxidative stress compared with rats not receiving vitamin E146,230. Rats supplemented with vitamin E also showed an increased activity of enzymes with antioxidant abilities (such as superoxide dismutase, catalase and glutathione reductase)230,232. From these preclinical models, vitamin E can be speculated to detoxify a broad range of ROS and ultimately reduce the necessity for enzymatic detoxification, in turn preserving the active forms and increasing the perceived activity of antioxidant enzymes.
Reduced concentrations of plasma vitamin E (as measured by tocopherol) is commonly observed in the blood of human stone formers56,233–235 (Table 1). However, in one study, plasma concentration of tocopherol was found to be increased in stone formers51. In this study, the highest levels of vitamin E compared with other studies were reported in stone formers (16.4 mg/l) and the second highest in non-stone formers (12.0 mg/l)51. The detection method in this study51 was similar to that used in other studies233,234; thus, the reason why such relatively high values were reported is difficult to ascertain, and the existence of a population-based effect on blood levels of vitamin E might be hypothesized. Similar to what is observed in rats, human stone formers often have increased levels of markers of oxidative stress and a reduction in enzymes involved in oxidative protection235,236. Oxidative stress damages the epithelial tissue, and might also impair urinary proteins, such as the Tamm–Horsfall protein, which protects against calcium-based stone formation237. Tamm–Horsfall protein isolated from individuals with hyperoxaluria shows compromised ability to inhibit calcium oxalate crystallization during in vitro assays compared with the protein isolated from age-matched and sex-matched healthy individuals238. Tamm–Horsfall protein isolated from patients in the hyperoxaluria group receiving antioxidant vitamin E supplements partially recovered function compared with that isolated from participants with hyperoxaluria receiving placebo238. Notably, different post-translational modifications were observed in the Tamm–Horsfall protein between patients with hyperoxaluria receiving vitamin E orally and patients with hyperoxaluria receiving placebo238. The most substantial change reported in the Tamm–Horsfall protein isolated from patients with hyperoxaluria receiving vitamin E was a reduction in the sialic acid content, which is known to promote calcium oxalate aggregation in vitro, indicating a reduced ability to inhibit calcium-based stone formation in these patients239. Oxidative stress seems to modulate the post-translational modification of the Tamm–Horsfall protein and reduce the efficacy of this protein to inhibit stone formation. Thus, the ability of vitamin E to detoxify ROS, in addition to reducing epithelial damage, could also preserve the integrity of renal inhibitor proteins.
The interactions between vitamin E and the gut microbiota are less characterized than interactions reported for other vitamins, although vitamin E derivatives (α-tocopherol quinone and α-tocopherol quinol) are present in many bacterial cells240 (Table 2). To date, the biosynthesis of vitamin E has only been characterized in plants and cyanobacteria241. However, through bioengineering of microorganisms, vitamin E synthesis has been extended to Saccharomyces cerevisiae and E. coli242,243. Indigenous bacteria of the gut microbiota have not been observed to directly synthesize vitamin E for host use, but these bacteria might have a role in modulating vitamin E absorption. In support of this idea, mice receiving antibiotics together with vitamin E had higher serum vitamin E titres than mice receiving vitamin E alone244. This effect was only observed when mice were supplemented with vitamin E before sacrifice. Chickens treated with antibiotics showed increased absorption of α-tocopheryl acetate, but this result was not recapitulated with γ-tocopherol, suggesting that different mechanisms of absorption based on the conjugates of the vitamer exist245. Taking these findings together, microbiota seems to have an effect on vitamin E bioavailability, but additional work is needed to confirm these observations.
The mechanism of vitamin E absorption is not completely elucidated, but is thought to be similar to the mechanism observed for other dietary lipids, according to which the vitamin is emulsified and incorporated into mixed micelles made of phospholipids that are conjugated to bile acids before passive diffusion or receptor-mediated endocytosis246. Both unconjugated and conjugated bile acids can form micelles, although conjugated micelles are more soluble than unconjugated ones247. Some intestinal commensals, such as bacteria in the Bacteroides, Clostridium, Bifidobacterium and Listeria genera, in addition to the Lactobacillaceae family, possess a bile salt hydrolase that deconjugates bile acids248. In the deconjugated form, bile acids have poor solubility and do not typically participate in micelle formation and, therefore, do not solubilize fat-soluble nutrients effectively, ultimately promoting malabsorption of these nutrients247,249,250. Fat-soluble vitamin absorption mediated by bile acid solubility could explain how vitamin E is absorbed; however, mice monocolonized with Lactobacillus acidophilus NCFM, a human intestinal isolate and probiotic strain that encodes for bile salt hydrolase and is unable to degrade α-tocopherol acetate in vitro, had reduced α-tocopherol acetate intestine titres compared with germ-free controls251. The authors concluded that L. acidophilus NCFM mice had increased absorption of α-tocopherol acetate despite the presence of a bile salt hydrogenase, but these results were not confirmed for hepatic or blood vitamin E titres251. Mice receiving L. acidophilus NCFM had an altered bile acid profile, marked by an increase in the unconjugated bile acid cholic acid, which the authors proposed to activate pancreatic bile acid-dependent carboxyl ester lipase to mediate the absorption of vitamin E acetate251,252. Unfortunately, the levels of deconjugated bile salts such as deoxycholate and lithocholate were not determined251. These findings highlight the unclear nature of fat-soluble vitamin absorption, but the deconjugation of bile acids by bacteria could impair the absorption of other fat-soluble vitamins in addition to vitamin E. This topic should be of general interest because most of the other vitamins, including vitamins A, D and K, are also fat soluble. Considering the increased rates of metabolic disease253 in stone formers — which can result in vitamin E malabsorption254 — and the crucial roles of vitamin E as a potential antioxidant in mitigating stone formation, it would be interesting to investigate the potential role of gut microbiota in these metabolic conditions and in reducing the absorption of crucial fat-soluble vitamins such as vitamin E.
Vitamin K
Vitamin K refers to a group of lipid-soluble vitamins with a characteristic 2-methyl-1,4-naphthoquinone structure (Fig. 2). Specifically, three main vitamers of vitamin K exist and differ based on the side chain structure, function and location in the human body255. Vitamin K1 (phylloquinone) is a plant-derived K vitamin that is sourced from leafy green vegetables. This vitamin exists as a single structural variant typically found in the liver, and participates in the coagulation response256. Vitamin K2 (menaquinone), which can be produced by both bacteria and mammals, exists as multiple structural analogues based on the number of isoprenoid side chain units256. These side chains can exist in 1 to 15 repeats (named MK-1–MK-15), although most research to date has focused on MK-4 and MK-7 (ref. 256). Interestingly, vitamin K2 variants with long chains have increased bioavailability and half-life compared with short-chain variants, which potentially increases the efficacy of these variants in reducing aberrant soft-tissue calcification257–259. Vitamin K2 is similar to vitamin K1 in terms of liver function, but can also circulate throughout the body and have additional roles in health. The third vitamer, vitamin K3 (menadione), lacks a side chain group and is synthetically produced256. Menadione is rapidly converted into menaquinone in the liver260.
Vitamers of vitamin K function throughout the body to reduce soft-tissue calcification through the activation of carboxyglutamate (Gla) proteins, osteocalcin and Matrix Gla protein (MGP). These proteins are commonly localized to bone cells and are stimulated by the active form of vitamin D261–263. After production, these proteins are post-translationally modified by γ-glutamyl carboxylase, which activates the protein by converting glutamate residues to Gla residues and, in turn, increases the overall negative charge enabling the protein to interact with calcium264. Vitamins K1 and K2 can both be used as co-factors for γ-glutamyl carboxylase, whereas vitamin K3 shows no activity in this reaction265. However, the presence of vitamin K1 is restricted to the liver, and sufficient concentrations of local vitamin K are required for Gla protein activation; thus, vitamin K2 is most likely to be responsible for mitigating aberrant calcification.
MGP is a unique member of the Gla family, which is expressed systemically266, differently from osteocalcin, which is only expressed by osteoblasts267. This unique expression pattern suggests that MGP might directly modulate soft-tissue calcification. Specifically, increased titres of dephosphorylated-uncarboxylated MGP (dp-ucMGP), the inactive form of this protein, have been associated with increased levels of vascular calcification in individuals with chronic kidney disease268–270 and type 2 diabetes271. A positive correlation between the amount of bodily dp-ucMGP and aortic stiffness has also been observed in individuals considered healthy272. Calcium-containing stone formers have increased degrees of aortic calcification compared with non-stone formers273,274; thus, MGP status could have a role in mediating calcium-containing stone formation.
Nephrocalcin and prothrombin are other Gla family proteins that might have roles in stone disease. Nephrocalcin is typically found in the kidneys and has four isoforms that differ by Gla content: form A has three Gla residues; forms B and C have two Gla residues; and form D does not have any Gla residues256. Prothrombin is a clotting protein that is mainly produced by the liver but is also expressed in the kidneys275. Similar to MGP, both nephrocalcin and prothrombin require vitamin K for carboxylation and subsequently sequestration of calcium ions256. The idea that Gla proteins could be implicated in calcium-containing stone disease came from the observation of Gla residues in amino acids extracted from renal stones composed of calcium oxalate and hydroxyapatite, but not from struvite stones276,277. Results from in vitro studies suggest that nephrocalcin and prothrombin278 inhibit calcium oxalate crystallization, and that the inhibitory effect of higher γ-carboxylation states of nephrocalcin on stone formation is greater than the inhibitory effect of lower γ-carboxylation states of nephrocalcin279. In one report, calcium oxalate stone formers were shown to produce urinary nephrocalcin with fewer Gla residues and lower crystal affinity than non-stone formers280. Nephrocalcin isolated from calcium oxalate stones had comparable amounts of Gla residues with urinary nephrocalcin279, suggesting that nephrocalcin participates in stone formation and few Gla residues could lead to stone formation281. With the exception of this study, human trials to assess the role of γ-carboxylation states of nephrocalcin and prothrombin in stone disease are scarce, and this area of research holds great potential.
Considering the role of vitamin K2 in the local γ-carboxylation, understanding the vitamin K2 status in stone formers would be interesting. Vitamin K is required for MGP activation; thus, circulating titres of dp-ucMGP have been proposed as a novel marker of vitamin K status282. Circulating titres of vitamin K2 might be more appropriate than dp-ucMGP to differentiate between K1 and K2 (ref. 283). To date, no long-term trials to assess dietary vitamin K2 intake — similar to studies carried out with vitamins B6, C and D — are available, partly because evidence of an association between vitamin K2 and stone disease is still preliminary, and also because dietary intake of this vitamin is restricted to supplements and certain fermented foods. However, similar to what is observed with other dietary trials, these long-term diet studies might not be the best course of action to understand how menaquinones influence stone burden owing to the difficulty in accurately assessing menaquinone intake. Interestingly, some indication for the role of vitamin K deficiency in stone formation comes from the observation that stone formers have many of the comorbidities that are associated with high dp-ucMGP, such as renal dysfunction284, chronic kidney disease268–270, hypertension285, obesity286 and diabetes271. High dp-ucMGP depends on vitamin K status; thus, these comorbidities might suggest that vitamin K deficiency is common in stone formers. Renal cells exposed to calcium oxalate have increased MGP expression287,288, which can reduce the formation of calcium oxalate289, indicating that MGP could inhibit calcium-containing stone formation. Male Sprague–Dawley rats treated with vitamin K1 (which can be converted to MK-4) showed reduced crystal formation after 6 weeks of supplementation290. To date, the relationship between circulating dp-ucMGP (which presumably derives from vitamin K deficiency) and nephrolithiasis in humans has been assessed in only two studies. In a Mendelian randomization study291, increased levels of circulating plasma dp-ucMGP were associated with an increased risk of stone disease (P < 0.05). In a prospective study including first-time kidney stone formers and matched non-stone formers292, no association between serum dp-ucMGP and stone incidence or recurrence was observed (P = 0.66). The reasons for the different outcomes of these studies are difficult to ascertain, and multicentred studies are needed to confirm the validity of the findings. However, these results highlight that the relationship between dp-ucMGP and stone disease is probably more complex than initially thought, especially considering the fact that the activation of dp-ucMGP is also influenced by genetic variants291,293–295. Perhaps the quantification of urinary dp-ucMGP in combination with blood analysis could clarify the status of dp-ucMGP in the local renal environment and the relationship of dp-ucMGP status with kidney stone formation. Considering that the composition of Randall’s plaques is highly similar to that of vascular calcifications, the role of vitamin K status and Gla protein modification in renal calculi development warrants further investigation.
In other fields of study, oral supplementation with menaquinones seems to reduce circulating dp-ucMGP296–299, and reduces the degree of vascular calcification297,300,301, but the effects of these supplements on kidney stones are unknown. In most of these studies MK-7 was used, which has a larger number of isoprenoid side chain units than MK-4, another commonly used menaquinone. MK-7 has been shown to have increased half-life and enhanced bioavailability257,259 at the potential cost of reduced efficiency compared with MK-4 (ref. 265). Additional work is needed to determine whether vitamin K2 supplements will reduce the risk of calcium-containing stone formation, but particular interest could be focused on the ability of large menaquinones to mitigate stone formation, considering the unique pharmacology of these vitamers.
Mammals can produce only one form of menaquinone, and the process occurs through the conversion of dietary vitamin K1 into MK-4 mediated by the UbiA prenyltransferase domain-containing protein 1 (UBIAD1)302. However, the gut microbiota is able to produce medium- to long-chain menaquinones (MK-7–MK-13). The de novo production of menaquinones occurs in many bacteria including Bacteroides (which produce MK-9–MK-11), Prevotella (producing MK-11–MK-13) and Veillonella (which produce MK-6 and MK-7)47,303,304 (Table 2). These bacteria use chorismate as a precursor molecule for the conversion to menaquinone through the classical menaquinone pathway (Men operon) or the futalosine pathway (Mqn operon)305,306. Other gut bacteria contain incomplete menaquinone synthesis pathways and rely on metabolite sharing from the pantryome to produce vitamin K2 (refs. 47,306,307). Results from multiple studies have shown that blood profiles of menaquinones are directly altered by the composition of the human gut microbiota308–310. Generally, an increase in the proportion of menaquinone-producing bacteria leads to a rise in the blood content of menaquinones308–310. Moreover, results from an observational study including patients with Crohn’s disease311 showed a negative correlation between vitamin K deficiency (as measured by serum titres of the inactive Gla protein, undercarboxylated-osteocalcin (ucOC)) and alpha diversity of the gut microbiota (as measured by Chao1). This correlation suggests that the microbiota could contribute to a person’s vitamin K status. Disruption of the gut microbiota with antibiotics decreases serum and caecal vitamin K2 levels312,313. Together, results from these studies suggest that the microbiota has a role in producing an adequate supply of menaquinones in humans, which could be used to activate dp-ucMGP and effectively reduce the occurrence of aberrant calcification. In a meta-analysis of studies in which the microbiota of stone formers was assessed34, some species of Prevotella were found to be more abundant in the gut microbiota of stone formers than in healthy individuals, whereas other species of the genus were more prevalent in non-stone formers than in stone formers. Considering that members of Prevotella can produce menaquinones, species-specific influence from this genus on vitamin K2 production could be explored. Further consideration of the understanding of menaquinone production in the microbiome of stone formers could help to clarify the role of vitamin K2 production in the aetiology of stone disease.
Future directions
The current understanding of how vitamins influence calcium-containing kidney stone disease is insufficient. Thus, conflicting observations have polarized the literature and clinical guidelines without any strong justification. Considering that vitamins are multifunctional cofactors that participate in a myriad of processes throughout the body, the idea of understanding the roles of vitamins in stone disease can be discouraging. This picture is further complicated considering the ability of gut microbiome to modulate vitamin titres, absorption and function. However, these important relationships can be deduced through rigorously defined experiments and clinical trials.
Different future directions can be proposed to begin to address the role of vitamins in stone disease. First, properly conducted multicentred studies are needed to address a multitude of potential confounding variables in calcium-containing stone formers compared with healthy individuals. In these trials, participants should be stratified based on features such as diet, stone type and sex to truly discern the differences between stone formers and non-stone formers. Crucial research outcomes from these studies would include quantifying vitamer concentrations of vitamins A, B6, C, D, E or K, which might have roles in kidney stone disease. In these studies, gut microbiota should also be analysed through a systems-level approach including metagenomic, metabolomic and/or metatranscriptomic analyses, corroborated by mechanistic studies to gather a complete understanding of the biochemical networks harboured within the gut microbiota, with the aim of identifying differences in the gut microbiota of stone formers versus non-stone formers. Additionally, well-designed in vitro and in vivo studies to examine the effect of vitamer and probiotic supplementation on both the gut microbiota and calcium oxalate stone formation could be used to further delineate the microbiome–vitamin–stone axis. Together, these approaches would help to describe the causal factors of stone disease, including the influence of vitamins and the gut microbiome. Second, in vitro and in vivo models should be established based on differential features observed in stone formers compared with healthy individuals to deduce the mechanisms through which these hypothetical alterations would participate in stone formation. Germ-free models should be used to investigate the role of the gut microbiota in stone disease, and the influence of the indigenous microbiota on stone formation in the model organisms should also be assessed. However, results from these studies must be interpreted with caution, as model organisms harbour a microbiome that is vastly different from that of humans and possess a unique physiology that might not be representative of vitamin acquisition and stone formation in humans. Model organisms are not ideal for these reasons, but might still help to provide some insight into how specific microorganisms could contribute to stone formation and facilitate the development of new ideas. Third, mechanistic findings from preclinical studies will need to be confirmed in humans to support or induce changes to clinical guidelines or patient care. Trials to validate findings obtained in preclinical models will need to be multicentred and span a diverse patient cohort taking into account patient sex, diet, stone type, ethnicity and medical comorbidities. In the hypothesis that mechanistic findings would be confirmed in humans, model organisms could be used to develop therapeutics, but patient data would ultimately be needed to drive these discoveries into the clinic.
Conclusions
Some useful information can be found by analysing the incongruencies of the multiple studies in which the role of vitamins in kidney stone disease was investigated (Box 1). Specifically, vitamin A seems to improve urinary parameters that mitigate stone formation and reduce intestinal permeability, which might reduce oxalate absorption. Some evidence that vitamin B6 can alter metabolism to reduce oxalate production exists, but is debated owing to conflicting results, as vitamin B6 has been shown to either be able to reduce stone formation or to have no effect on stone formation. Vitamin C might be converted to oxalate more proficiently in men than in women, indicating that a sex-specific effect on oxalate production from vitamin C might exist. Vitamin C has antioxidant roles in both men and women, which could be protective from stone disease; however, conflicting results in preclinical models suggest that vitamin C could be ineffective at preventing stone formation. Vitamin D has historically been recognized for the ability to increase blood calcium levels, although this phenomenon does not directly contribute to stone formation; however, additional work is needed to investigate the role of this vitamin in stone formation beyond calcium homeostasis, as vitamin D has roles in multiple pathways throughout the body, including immunity, cell signalling, DNA repair and metabolism. Vitamin E acts as a non-selective antioxidant that can detoxify free radicals, which can reduce crystal adherence to the renal epithelium and maintain the integrity of endogenous urinary protein inhibitors. Vitamin K2 is capable of activating dp-ucMGP, which can sequester free calcium and might be able to reduce aberrant calcification systemically. However, considering the multifunctional role of these vitamins in human health, unravelling the individual role in stone disease will be challenging. This Perspective ultimately discusses observations in the literature in an attempt to promote a new direction in a field in which prior research has not been definitive.
Perhaps, the microbiota, which is able to produce, modify and influence the absorption of vitamins (Fig. 1) can be the missing piece to this puzzle. In this Perspective, the surface of how the microbiota influences vitamin homeostasis in the human host is merely scratched, as many of these pathways are not fully characterized, and exist in other microorganisms (such as fungi or archaea) that inhabit the intestine. The functional capacity of the microbiota should be preserved through careful clinical antimicrobial stewardship and other factors such as diet to minimize any potential collateral damage caused by the indiscriminate depletion of microbial cross-feeding networks from which the host also benefits. An increased understanding of the mechanisms underpinning kidney stone disease and the development of new preventative strategies are needed to provide patients with effective therapeutic options, and to help mitigate the substantial clinical and economic burden of the growing prevalence of urinary stone disease.
Acknowledgements
This work is supported by a Lawson Health Research Institute Internal Research Fund, a Northeastern Section of the American Urological Association Datta G. Wagle Young Investigator Grant, and a Canadian Institutes of Health Research Canada Graduate Scholarship–Doctoral (175836).
Author contributions
J.A.C., G.A.S. and K.F.A. researched data for the article. All authors contributed substantially to discussion of the content. J.A.C., G.A.S., K.F.A. and J.P.B. wrote the article. All authors reviewed and/or edited the manuscript before submission.
Peer review
Peer review information
Nature Reviews Urology thanks A. Miller, A. Ticinesi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Romero V, Akpinar H, Assimos DG. Kidney stones: a global picture of prevalence, incidence, and associated risk factors. Rev. Urol. 2010;12:e86–e96. [PMC free article] [PubMed] [Google Scholar]
- 2.Wang W, et al. Prevalence of kidney stones in mainland China: a systematic review. Sci. Rep. 2017;7:41630. doi: 10.1038/srep41630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turney BW, Reynard JM, Noble JG, Keoghane SR. Trends in urological stone disease. BJU Int. 2012;109:1082–1087. doi: 10.1111/j.1464-410X.2011.10495.x. [DOI] [PubMed] [Google Scholar]
- 4.Moe OW. Kidney stones: pathophysiology and medical management. Lancet. 2006;367:333–344. doi: 10.1016/S0140-6736(06)68071-9. [DOI] [PubMed] [Google Scholar]
- 5.Scales CD, Smith AC, Hanley JM, Saigal CS, Urologic Diseases in America Project. Prevalence of kidney stones in the United States. Eur. Urol. 2012;62:160–165. doi: 10.1016/j.eururo.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill AJ, et al. Incidence of kidney stones in the United States: the continuous national health and nutrition examination survey. J. Urol. 2022;207:851–856. doi: 10.1097/JU.0000000000002331. [DOI] [PubMed] [Google Scholar]
- 7.Rule AD, et al. The ROKS nomogram for predicting a second symptomatic stone episode. J. Am. Soc. Nephrol. 2014;25:2878–2886. doi: 10.1681/ASN.2013091011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rule AD, Lieske JC, Pais VM. Management of kidney stones in 2020. J. Am. Med. Assoc. 2020;323:1961–1962. doi: 10.1001/jama.2020.0662. [DOI] [PubMed] [Google Scholar]
- 9.Scales CD, et al. Urinary stone disease: advancing knowledge, patient care, and population health. Clin. J. Am. Soc. Nephrol. 2016;11:1305–1312. doi: 10.2215/CJN.13251215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saigal CS, Joyce G, Timilsina AR, Urologic Diseases in America Project. Direct and indirect costs of nephrolithiasis in an employed population: opportunity for disease management? Kidney Int. 2005;68:1808–1814. doi: 10.1111/j.1523-1755.2005.00599.x. [DOI] [PubMed] [Google Scholar]
- 11.Hyams ES, Matlaga BR. Economic impact of urinary stones. Transl. Androl. Urol. 2014;3:278–283. doi: 10.3978/j.issn.2223-4683.2014.07.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yasui T, Iguchi M, Suzuki S, Kohri K. Prevalence and epidemiological characteristics of urolithiasis in Japan: national trends between 1965 and 2005. Urology. 2008;71:209–213. doi: 10.1016/j.urology.2007.09.034. [DOI] [PubMed] [Google Scholar]
- 13.Edvardsson VO, Indridason OS, Haraldsson G, Kjartansson O, Palsson R. Temporal trends in the incidence of kidney stone disease. Kidney Int. 2013;83:146–152. doi: 10.1038/ki.2012.320. [DOI] [PubMed] [Google Scholar]
- 14.Singh P, et al. Stone composition among first-time symptomatic kidney stone formers in the community. Mayo Clin. Proc. 2015;90:1356–1365. doi: 10.1016/j.mayocp.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lieske JC, et al. Stone composition as a function of age and sex. Clin. J. Am. Soc. Nephrol. 2014;9:2141–2146. doi: 10.2215/CJN.05660614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ratkalkar VN, Kleinman JG. Mechanisms of stone formation. Clin. Rev. Bone Min. Metab. 2011;9:187–197. doi: 10.1007/s12018-011-9104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chmiel JA, et al. High-throughput in vitro gel-based plate assay to screen for calcium oxalate stone inhibitors. Urol. Int. 2022;106:616–622. doi: 10.1159/000519842. [DOI] [PubMed] [Google Scholar]
- 18.Randall A. An hypothesis for the origin of renal calculus. N. Engl. J. Med. 1936;214:234–242. doi: 10.1056/NEJM193602062140603. [DOI] [Google Scholar]
- 19.Khan SR. Reactive oxygen species, inflammation and calcium oxalate nephrolithiasis. Transl. Androl. Urol. 2014;3:256–276. doi: 10.3978/j.issn.2223-4683.2014.06.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferraro PM, Bargagli M, Trinchieri A, Gambaro G. Risk of kidney stones: influence of dietary factors, dietary patterns, and vegetarian-vegan diets. Nutrients. 2020;12:E779. doi: 10.3390/nu12030779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Alessandro C, et al. Which diet for calcium stone patients: a real-world approach to preventive care. Nutrients. 2019;11:E1182. doi: 10.3390/nu11051182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heilberg IP, Goldfarb DS. Optimum nutrition for kidney stone disease. Adv. Chronic Kidney Dis. 2013;20:165–174. doi: 10.1053/j.ackd.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Siener R, Hesse A. The effect of different diets on urine composition and the risk of calcium oxalate crystallisation in healthy subjects. Eur. Urol. 2002;42:289–296. doi: 10.1016/S0302-2838(02)00316-0. [DOI] [PubMed] [Google Scholar]
- 24.Statovci D, Aguilera M, MacSharry J, Melgar S. The impact of Western diet and nutrients on the microbiota and immune response at mucosal interfaces. Front. Immunol. 2017;8:838. doi: 10.3389/fimmu.2017.00838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Candeliere F, et al. Indole and p-cresol in feces of healthy subjects: concentration, kinetics, and correlation with microbiome. Front. Mol. Med. 2022;2:1–13. doi: 10.3389/fmmed.2022.959189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pendyala S, Walker JM, Holt PR. A high-fat diet is associated with endotoxemia that originates from the gut. Gastroenterology. 2012;142:1100–1101.e2. doi: 10.1053/j.gastro.2012.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zinöcker MK, Lindseth IA. The Western diet-microbiome-host interaction and its role in metabolic disease. Nutrients. 2018;10:E365. doi: 10.3390/nu10030365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cordain L, et al. Origins and evolution of the Western diet: health implications for the 21st century. Am. J. Clin. Nutr. 2005;81:341–354. doi: 10.1093/ajcn.81.2.341. [DOI] [PubMed] [Google Scholar]
- 29.Freedman P. Renal colic and persistent hypercalcuria following self-administration of vitamin D. Lancet. 1957;272:668–669. doi: 10.1016/S0140-6736(57)91125-X. [DOI] [PubMed] [Google Scholar]
- 30.Whiteside SA, Razvi H, Dave S, Reid G, Burton JP. The microbiome of the urinary tract — a role beyond infection. Nat. Rev. Urol. 2015;12:81–90. doi: 10.1038/nrurol.2014.361. [DOI] [PubMed] [Google Scholar]
- 31.Tasian GE, et al. Oral antibiotic exposure and kidney stone disease. J. Am. Soc. Nephrol. 2018;29:1731–1740. doi: 10.1681/ASN.2017111213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dawson KA, Allison MJ, Hartman PA. Isolation and some characteristics of anaerobic oxalate-degrading bacteria from the rumen. Appl. Environ. Microbiol. 1980;40:833–839. doi: 10.1128/aem.40.4.833-839.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duncan SH, et al. Oxalobacter formigenes and its potential role in human health. Appl. Environ. Microbiol. 2002;68:3841–3847. doi: 10.1128/AEM.68.8.3841-3847.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kachroo N, et al. Meta-analysis of clinical microbiome studies in urolithiasis reveal age, stone composition, and study location as the predominant factors in urolithiasis-associated microbiome composition. mBio. 2021;12:e0200721. doi: 10.1128/mBio.02007-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ticinesi A, et al. Understanding the gut-kidney axis in nephrolithiasis: an analysis of the gut microbiota composition and functionality of stone formers. Gut. 2018;67:2097–2106. doi: 10.1136/gutjnl-2017-315734. [DOI] [PubMed] [Google Scholar]
- 36.Magwira CA, et al. Diversity of faecal oxalate-degrading bacteria in black and white South African study groups: insights into understanding the rarity of urolithiasis in the black group. J. Appl. Microbiol. 2012;113:418–428. doi: 10.1111/j.1365-2672.2012.05346.x. [DOI] [PubMed] [Google Scholar]
- 37.Tang R, et al. 16S rRNA gene sequencing reveals altered composition of gut microbiota in individuals with kidney stones. Urolithiasis. 2018;46:503–514. doi: 10.1007/s00240-018-1037-y. [DOI] [PubMed] [Google Scholar]
- 38.Al, K. Characterizing the role of the microbiome in kidney stone disease (Western University, 2020).
- 39.Ticinesi A, et al. Calcium oxalate nephrolithiasis and gut microbiota: not just a gut-kidney axis. A nutritional perspective. Nutrients. 2020;12:548. doi: 10.3390/nu12020548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller AW, Choy D, Penniston KL, Lange D. Inhibition of urinary stone disease by a multi-species bacterial network ensures healthy oxalate homeostasis. Kidney Int. 2019;96:180–188. doi: 10.1016/j.kint.2019.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu M, et al. Microbial genetic and transcriptional contributions to oxalate degradation by the gut microbiota in health and disease. Elife. 2021;10:e63642. doi: 10.7554/eLife.63642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stern JM, et al. Evidence for a distinct gut microbiome in kidney stone formers compared to non-stone formers. Urolithiasis. 2016;44:399–407. doi: 10.1007/s00240-016-0882-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suryavanshi MV, et al. Hyperoxaluria leads to dysbiosis and drives selective enrichment of oxalate metabolizing bacterial species in recurrent kidney stone endures. Sci. Rep. 2016;6:34712. doi: 10.1038/srep34712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suryavanshi MV, Bhute SS, Gune RP, Shouche YS. Functional eubacteria species along with trans-domain gut inhabitants favour dysgenic diversity in oxalate stone disease. Sci. Rep. 2018;8:16598. doi: 10.1038/s41598-018-33773-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stanford J, Charlton K, Stefoska-Needham A, Ibrahim R, Lambert K. The gut microbiota profile of adults with kidney disease and kidney stones: a systematic review of the literature. BMC Nephrol. 2020;21:215. doi: 10.1186/s12882-020-01805-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arumugam M, et al. Enterotypes of the human gut microbiome. Nature. 2011;473:174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Daisley BA, et al. Emerging connections between gut microbiome bioenergetics and chronic metabolic diseases. Cell Rep. 2021;37:110087. doi: 10.1016/j.celrep.2021.110087. [DOI] [PubMed] [Google Scholar]
- 48.Magnúsdóttir S, Ravcheev D, de Crécy-Lagard V, Thiele I. Systematic genome assessment of B-vitamin biosynthesis suggests co-operation among gut microbes. Front. Genet. 2015;6:148. doi: 10.3389/fgene.2015.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Al Tanoury Z, Piskunov A, Rochette-Egly C. Vitamin A and retinoid signaling: genomic and nongenomic effects. J. Lipid Res. 2013;54:1761–1775. doi: 10.1194/jlr.R030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tanumihardjo SA, et al. Biomarkers of nutrition for development (BOND) — vitamin A review. J. Nutr. 2016;146:1816S–1848SS. doi: 10.3945/jn.115.229708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grases F, Garcia-Gonzalez R, Genestar C, Torres JJ, March JG. Vitamin A and urolithiasis. Clin. Chim. Acta. 1998;269:147–157. doi: 10.1016/S0009-8981(97)00198-8. [DOI] [PubMed] [Google Scholar]
- 52.Munday JS, et al. Cystitis, pyelonephritis, and urolithiasis in rats accidentally fed a diet deficient in vitamin A. J. Am. Assoc. Lab. Anim. Sci. 2009;48:790–794. [PMC free article] [PubMed] [Google Scholar]
- 53.Bardaoui M, Sakly R, Neffati F, Najjar MF, El Hani A. Effect of vitamin A supplemented diet on calcium oxalate renal stone formation in rats. Exp. Toxicol. Pathol. 2010;62:573–576. doi: 10.1016/j.etp.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 54.Wong YF, et al. Endogenous retinoic acid activity in principal cells and intercalated cells of mouse collecting duct system. PLoS One. 2011;6:e16770. doi: 10.1371/journal.pone.0016770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kancha RK, Anasuya A. Contribution of vitamin A deficiency to calculogenic risk factors of urine: studies in children. Biochem. Med. Metab. Biol. 1992;47:1–9. doi: 10.1016/0885-4505(92)90002-G. [DOI] [PubMed] [Google Scholar]
- 56.Kato J, et al. Lipid peroxidation and antioxidant vitamins in urolithiasis. Indian. J. Clin. Biochem. 2007;22:128–130. doi: 10.1007/BF02912895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Conaway HH, Henning P, Lerner UH. Vitamin a metabolism, action, and role in skeletal homeostasis. Endocr. Rev. 2013;34:766–797. doi: 10.1210/er.2012-1071. [DOI] [PubMed] [Google Scholar]
- 58.Chen P, et al. Association of vitamin A and zinc status with altered intestinal permeability: analyses of cohort data from northeastern Brazil. J. Health Popul. Nutr. 2003;21:309–315. [PubMed] [Google Scholar]
- 59.Quadro L, et al. Retinol and retinol-binding protein: gut integrity and circulating immunoglobulins. J. Infect. Dis. 2000;182(Suppl. 1):S97–S102. doi: 10.1086/315920. [DOI] [PubMed] [Google Scholar]
- 60.Li Y, et al. Retinoic acid facilitates toll-like receptor 4 expression to improve intestinal barrier function through retinoic acid receptor beta. Cell Physiol. Biochem. 2017;42:1390–1406. doi: 10.1159/000479203. [DOI] [PubMed] [Google Scholar]
- 61.Yamada S, Kanda Y. Retinoic acid promotes barrier functions in human iPSC-derived intestinal epithelial monolayers. J. Pharmacol. Sci. 2019;140:337–344. doi: 10.1016/j.jphs.2019.06.012. [DOI] [PubMed] [Google Scholar]
- 62.Wang JL, Swartz-Basile DA, Rubin DC, Levin MS. Retinoic acid stimulates early cellular proliferation in the adapting remnant rat small intestine after partial resection. J. Nutr. 1997;127:1297–1303. doi: 10.1093/jn/127.7.1297. [DOI] [PubMed] [Google Scholar]
- 63.Jijon HB, et al. Intestinal epithelial cell-specific RARα depletion results in aberrant epithelial cell homeostasis and underdeveloped immune system. Mucosal Immunol. 2018;11:703–715. doi: 10.1038/mi.2017.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Iyer N, Vaishnava S. Vitamin A at the interface of host–commensal–pathogen interactions. PLoS Pathog. 2019;15:e1007750. doi: 10.1371/journal.ppat.1007750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mulay SR, et al. Oxalate-induced chronic kidney disease with its uremic and cardiovascular complications in C57BL/6 mice. Am. J. Physiol. Renal Physiol. 2016;310:F785–F795. doi: 10.1152/ajprenal.00488.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Knauf F, et al. Net intestinal transport of oxalate reflects passive absorption and SLC26A6-mediated secretion. J. Am. Soc. Nephrol. 2011;22:2247–2255. doi: 10.1681/ASN.2011040433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bashir M, et al. Enhanced gastrointestinal passive paracellular permeability contributes to the obesity-associated hyperoxaluria. Am. J. Physiol. Gastrointest. Liver Physiol. 2019;316:G1–G14. doi: 10.1152/ajpgi.00266.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yoo J-Y, et al. LPS-induced acute kidney injury is mediated by Nox4-SH3YL1. Cell Rep. 2020;33:108245. doi: 10.1016/j.celrep.2020.108245. [DOI] [PubMed] [Google Scholar]
- 69.McAleer IM, Kaplan GW, Bradley JS, Carroll SF, Griffith DP. Endotoxin content in renal calculi. J. Urol. 2003;169:1813–1814. doi: 10.1097/01.ju.0000061965.51478.79. [DOI] [PubMed] [Google Scholar]
- 70.Li J, Moturi KR, Wang L, Zhang K, Yu C. Gut derived-endotoxin contributes to inflammation in severe ischemic acute kidney injury. BMC Nephrol. 2019;20:16. doi: 10.1186/s12882-018-1199-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nupur LNU, et al. ProCarDB: a database of bacterial carotenoids. BMC Microbiol. 2016;16:96. doi: 10.1186/s12866-016-0715-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ram S, Mitra M, Shah F, Tirkey SR, Mishra S. Bacteria as an alternate biofactory for carotenoid production: a review of its applications, opportunities and challenges. J. Funct. Foods. 2020;67:103867. doi: 10.1016/j.jff.2020.103867. [DOI] [Google Scholar]
- 73.Olson JA, Hayaishi O. The enzymatic cleavage of beta-carotene into vitamin A by soluble enzymes of rat liver and intestine. Proc. Natl Acad. Sci. USA. 1965;54:1364–1370. doi: 10.1073/pnas.54.5.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim Y-S, Park C-S, Oh D-K. Retinal production from beta-carotene by beta-carotene 15,15′-dioxygenase from an unculturable marine bacterium. Biotechnol. Lett. 2010;32:957–961. doi: 10.1007/s10529-010-0239-3. [DOI] [PubMed] [Google Scholar]
- 75.Culligan EP, Sleator RD, Marchesi JR, Hill C. Metagenomic identification of a novel salt tolerance gene from the human gut microbiome which encodes a membrane protein with homology to a brp/blh-family β-carotene 15,15′-monooxygenase. PLoS One. 2014;9:e103318. doi: 10.1371/journal.pone.0103318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Culligan EP, Sleator RD, Marchesi JR, Hill C. Functional metagenomics reveals novel salt tolerance loci from the human gut microbiome. ISME J. 2012;6:1916–1925. doi: 10.1038/ismej.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hong S-H, et al. Alternative biotransformation of retinal to retinoic acid or retinol by an aldehyde dehydrogenase from Bacillus cereus. Appl. Environ. Microbiol. 2016;82:3940–3946. doi: 10.1128/AEM.00848-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grizotte-Lake M, et al. Gut commensals suppress epithelial cell retinoic acid synthesis to regulate intestinal interleukin-22 activity and prevent microbial dysbiosis. Immunity. 2018;49:1103–1115.e6. doi: 10.1016/j.immuni.2018.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hellmann H, Mooney S. Vitamin B6: a molecule for human health? Molecules. 2010;15:442–459. doi: 10.3390/molecules15010442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Amadasi A, et al. Pyridoxal 5′-phosphate enzymes as targets for therapeutic agents. Curr. Med. Chem. 2007;14:1291–1324. doi: 10.2174/092986707780597899. [DOI] [PubMed] [Google Scholar]
- 81.Harris SA, Folkers K. Synthetic vitamin B6. Science. 1939;89:347. doi: 10.1126/science.89.2311.347. [DOI] [PubMed] [Google Scholar]
- 82.Rosenberg J, Ischebeck T, Commichau FM. Vitamin B6 metabolism in microbes and approaches for fermentative production. Biotechnol. Adv. 2017;35:31–40. doi: 10.1016/j.biotechadv.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 83.Percudani R, Peracchi A. The B6 database: a tool for the description and classification of vitamin B6-dependent enzymatic activities and of the corresponding protein families. BMC Bioinforma. 2009;10:273. doi: 10.1186/1471-2105-10-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pey AL, Albert A, Salido E. Protein homeostasis defects of alanine-glyoxylate aminotransferase: new therapeutic strategies in primary hyperoxaluria type I. Biomed. Res. Int. 2013;2013:687658. doi: 10.1155/2013/687658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sun X-Y, Gan Q-Z, Ouyang J-M. Calcium oxalate toxicity in renal epithelial cells: the mediation of crystal size on cell death mode. Cell Death Discov. 2015;1:1–8. doi: 10.1038/cddiscovery.2015.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ferraro PM, Taylor EN, Gambaro G, Curhan GC. Vitamin B6 intake and the risk of incident kidney stones. Urolithiasis. 2018;46:265–270. doi: 10.1007/s00240-017-0999-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Curhan GC, Willett WC, Rimm EB, Stampfer MJ. A prospective study of the intake of vitamins C and B6, and the risk of kidney stones in men. J. Urol. 1996;155:1847–1851. doi: 10.1016/S0022-5347(01)66027-0. [DOI] [PubMed] [Google Scholar]
- 88.Curhan GC, Willett WC, Speizer FE, Stampfer MJ. Intake of vitamins B6 and C and the risk of kidney stones in women. J. Am. Soc. Nephrol. 1999;10:840–845. doi: 10.1681/ASN.V104840. [DOI] [PubMed] [Google Scholar]
- 89.Gershoff SN, Faragalla FF, Nelson DA, Andrus SB. Vitamin B6 deficiency and oxalate nephrocalcinosis in the cat. Am. J. Med. 1959;27:72–80. doi: 10.1016/0002-9343(59)90062-2. [DOI] [PubMed] [Google Scholar]
- 90.Di Tommaso L, et al. Renal calcium phosphate and oxalate deposition in prolonged vitamin B6 deficiency: studies on a rat model of urolithiasis. BJU Int. 2002;89:571–575. doi: 10.1046/j.1464-410X.2002.02670.x. [DOI] [PubMed] [Google Scholar]
- 91.Aihara K, Byer KJ, Khan SR. Calcium phosphate-induced renal epithelial injury and stone formation: involvement of reactive oxygen species. Kidney Int. 2003;64:1283–1291. doi: 10.1046/j.1523-1755.2003.00226.x. [DOI] [PubMed] [Google Scholar]
- 92.Colditz GA. The Nurses’ Health Study: a cohort of US women followed since 1976. J. Am. Med. Women’s Assoc. 1995;50:40–44. [PubMed] [Google Scholar]
- 93.Mitwalli A, Ayiomamitis A, Grass L, Oreopoulos DG. Control of hyperoxaluria with large doses of pyridoxine in patients with kidney stones. Int. Urol. Nephrol. 1988;20:353–359. doi: 10.1007/BF02549567. [DOI] [PubMed] [Google Scholar]
- 94.Rattan V, Sidhu H, Vaidyanathan S, Thind SK, Nath R. Effect of combined supplementation of magnesium oxide and pyridoxine in calcium-oxalate stone formers. Urol. Res. 1994;22:161–165. doi: 10.1007/BF00571844. [DOI] [PubMed] [Google Scholar]
- 95.Ortiz-Alvarado O, et al. Pyridoxine and dietary counseling for the management of idiopathic hyperoxaluria in stone-forming patients. Urology. 2011;77:1054–1058. doi: 10.1016/j.urology.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 96.Balcke P, Schmidt P, Zazgornik J, Kopsa H, Minar E. Pyridoxine therapy in patients with renal calcium oxalate calculi. Proc. Eur. Dial. Transpl. Assoc. 1983;20:417–421. [PubMed] [Google Scholar]
- 97.Revúsová V, et al. The evaluation of some biochemical parameters in pyridoxine-treated calcium oxalate renal stone formers. Urol. Int. 1977;32:348–352. doi: 10.1159/000280150. [DOI] [PubMed] [Google Scholar]
- 98.Reddy SVK, Shaik AB, Bokkisam S. Effect of potassium magnesium citrate and vitamin B-6 prophylaxis for recurrent and multiple calcium oxalate and phosphate urolithiasis. Korean J. Urol. 2014;55:411–416. doi: 10.4111/kju.2014.55.6.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kaelin A, Casez J-P, Jaeger P. Vitamin B6 metabolites in idiopathic calcium stone formers: no evidence for a link to hyperoxaluria. Urol. Res. 2004;32:61–68. doi: 10.1007/s00240-003-0386-2. [DOI] [PubMed] [Google Scholar]
- 100.Hoyer-Kuhn H, et al. Vitamin B6 in primary hyperoxaluria I: first prospective trial after 40 years of practice. Clin. J. Am. Soc. Nephrol. 2014;9:468–477. doi: 10.2215/CJN.06820613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fargue S, Knight J, Holmes RP, Rumsby G, Danpure CJ. Effects of alanine:glyoxylate aminotransferase variants and pyridoxine sensitivity on oxalate metabolism in a cell-based cytotoxicity assay. Biochim. Biophys. Acta. 2016;1862:1055–1062. doi: 10.1016/j.bbadis.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Oppici E, et al. Pyridoxamine and pyridoxal are more effective than pyridoxine in rescuing folding-defective variants of human alanine:glyoxylate aminotransferase causing primary hyperoxaluria type I. Hum. Mol. Genet. 2015;24:5500–5511. doi: 10.1093/hmg/ddv276. [DOI] [PubMed] [Google Scholar]
- 103.Yoshii K, Hosomi K, Sawane K, Kunisawa J. Metabolism of dietary and microbial vitamin B family in the regulation of host immunity. Front. Nutr. 2019;6:48. doi: 10.3389/fnut.2019.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nakano H, Gregory JF. Pyridoxine and pyridoxine-5′-β-D-glucoside exert different effects on tissue B-6 vitamers but similar effects on β-glucosidase activity in rats. J. Nutr. 1995;125:2751–2762. doi: 10.1093/jn/125.11.2751. [DOI] [PubMed] [Google Scholar]
- 105.Li Y, et al. The gut microbiota regulates autism-like behavior by mediating vitamin B6 homeostasis in EphB6-deficient mice. Microbiome. 2020;8:120. doi: 10.1186/s40168-020-00884-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Soto-Martin EC, et al. Vitamin biosynthesis by human gut butyrate-producing bacteria and cross-feeding in synthetic microbial communities. mBio. 2020;11:e00886–20. doi: 10.1128/mBio.00886-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Heinken A, et al. Functional metabolic map of Faecalibacterium prausnitzii, a beneficial human gut microbe. J. Bacteriol. 2014;196:3289–3302. doi: 10.1128/JB.01780-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Richts B, Commichau FM. Underground metabolism facilitates the evolution of novel pathways for vitamin B6 biosynthesis. Appl. Microbiol. Biotechnol. 2021;105:2297–2305. doi: 10.1007/s00253-021-11199-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nishikimi M, Yagi K. Molecular basis for the deficiency in humans of gulonolactone oxidase, a key enzyme for ascorbic acid biosynthesis. Am. J. Clin. Nutr. 1991;54:1203S–1208S. doi: 10.1093/ajcn/54.6.1203s. [DOI] [PubMed] [Google Scholar]
- 110.Lykkesfeldt J, Tveden-Nyborg P. The pharmacokinetics of vitamin C. Nutrients. 2019;11:E2412. doi: 10.3390/nu11102412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Naidu KA. Vitamin C in human health and disease is still a mystery? An overview. Nutr. J. 2003;2:7. doi: 10.1186/1475-2891-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hellman L, Burns JJ. Metabolism of L-ascorbic acid-1-C14 in man. J. Biol. Chem. 1958;230:923–930. doi: 10.1016/S0021-9258(18)70515-2. [DOI] [PubMed] [Google Scholar]
- 113.Emadi-Konjin P, Verjee Z, Levin AV, Adeli K. Measurement of intracellular vitamin C levels in human lymphocytes by reverse phase high performance liquid chromatography (HPLC) Clin. Biochem. 2005;38:450–456. doi: 10.1016/j.clinbiochem.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 114.Rowe S, Carr AC. Global vitamin C status and prevalence of deficiency: a cause for concern? Nutrients. 2020;12:E2008. doi: 10.3390/nu12072008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Valdés F. Vitamin C. Actas Dermosifiliogr. 2006;97:557–568. doi: 10.1016/S0001-7310(06)73466-4. [DOI] [PubMed] [Google Scholar]
- 116.Hemilä H. Vitamin C and infections. Nutrients. 2017;9:E339. doi: 10.3390/nu9040339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Smirnoff N. Ascorbic acid metabolism and functions: a comparison of plants and mammals. Free Radic. Biol. Med. 2018;122:116–129. doi: 10.1016/j.freeradbiomed.2018.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Johnston CS. Biomarkers for establishing a tolerable upper intake level for vitamin C. Nutr. Rev. 1999;57:71–77. doi: 10.1111/j.1753-4887.1999.tb06926.x. [DOI] [PubMed] [Google Scholar]
- 119.Bhojani N, et al. Update — 2022 Canadian Urological Association guideline: evaluation and medical management of the kidney stone patient. Can. Urol. Assoc. J. 2022;16:175–188. doi: 10.5489/cuaj.7872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pearle MS, et al. Medical management of kidney stones: AUA guideline. J. Urol. 2014;192:316–324. doi: 10.1016/j.juro.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 121.Skolarikos, A. et al. EAU guidelines on urolithiasis. in EAU Guidelines. Edn. presented at the EAU Annual Congress Amsterdam (EAU Guidelines Office, 2022).
- 122.Ferraro PM, Curhan GC, Gambaro G, Taylor EN. Total, dietary, and supplemental vitamin C intake and risk of incident kidney stones. Am. J. Kidney Dis. 2016;67:400–407. doi: 10.1053/j.ajkd.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Taylor EN, Stampfer MJ, Curhan GC. Dietary factors and the risk of incident kidney stones in men: new insights after 14 years of follow-up. J. Am. Soc. Nephrol. 2004;15:3225–3232. doi: 10.1097/01.ASN.0000146012.44570.20. [DOI] [PubMed] [Google Scholar]
- 124.Institute of Medicine (US) Panel on dietary antioxidants and related compounds. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids (National Academies Press (US), 2000). [PubMed]
- 125.Thomas LDK, Elinder C-G, Tiselius H-G, Wolk A, Akesson A. Ascorbic acid supplements and kidney stone incidence among men: a prospective study. JAMA Intern. Med. 2013;173:386–388. doi: 10.1001/jamainternmed.2013.2296. [DOI] [PubMed] [Google Scholar]
- 126.Subar AF, et al. Using intake biomarkers to evaluate the extent of dietary misreporting in a large sample of adults: the OPEN study. Am. J. Epidemiol. 2003;158:1–13. doi: 10.1093/aje/kwg092. [DOI] [PubMed] [Google Scholar]
- 127.Travica N, et al. The contribution of plasma and brain vitamin C on age and gender-related cognitive differences: a mini-review of the literature. Front. Integr. Neurosci. 2020;14:47. doi: 10.3389/fnint.2020.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wandzilak TR, D’Andre SD, Davis PA, Williams HE. Effect of high dose vitamin C on urinary oxalate levels. J. Urol. 1994;151:834–837. doi: 10.1016/S0022-5347(17)35100-5. [DOI] [PubMed] [Google Scholar]
- 129.Traxer O, Huet B, Poindexter J, Pak CYC, Pearle MS. Effect of ascorbic acid consumption on urinary stone risk factors. J. Urol. 2003;170:397–401. doi: 10.1097/01.ju.0000076001.21606.53. [DOI] [PubMed] [Google Scholar]
- 130.Chai W, Liebman M, Kynast-Gales S, Massey L. Oxalate absorption and endogenous oxalate synthesis from ascorbate in calcium oxalate stone formers and non-stone formers. Am. J. Kidney Dis. 2004;44:1060–1069. doi: 10.1053/j.ajkd.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 131.Taylor EN, Curhan GC. Determinants of 24-hour urinary oxalate excretion. Clin. J. Am. Soc. Nephrol. 2008;3:1453–1460. doi: 10.2215/CJN.01410308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Baxmann AC, De O G Mendonça C, Heilberg IP. Effect of vitamin C supplements on urinary oxalate and pH in calcium stone-forming patients. Kidney Int. 2003;63:1066–1071. doi: 10.1046/j.1523-1755.2003.00815.x. [DOI] [PubMed] [Google Scholar]
- 133.Hesse A, Schneeberger W, Engfeld S, Von Unruh GE, Sauerbruch T. Intestinal hyperabsorption of oxalate in calcium oxalate stone formers: application of a new test with [13C2]oxalate. J. Am. Soc. Nephrol. 1999;10(Suppl. 14):S329–S333. [PubMed] [Google Scholar]
- 134.Sikora P, et al. [13C2]oxalate absorption in children with idiopathic calcium oxalate urolithiasis or primary hyperoxaluria. Kidney Int. 2008;73:1181–1186. doi: 10.1038/ki.2008.63. [DOI] [PubMed] [Google Scholar]
- 135.Chalmers AH, Cowley DM, McWhinney BC. Stability of ascorbate in urine: relevance to analyses for ascorbate and oxalate. Clin. Chem. 1985;31:1703–1705. doi: 10.1093/clinchem/31.10.1703. [DOI] [PubMed] [Google Scholar]
- 136.Moyad MA, et al. Vitamin C with metabolites reduce oxalate levels compared to ascorbic acid: a preliminary and novel clinical urologic finding. Urol. Nurs. 2009;29:95–102. [PubMed] [Google Scholar]
- 137.Robitaille L, et al. Oxalic acid excretion after intravenous ascorbic acid administration. Metabolism. 2009;58:263–269. doi: 10.1016/j.metabol.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Peña de la Vega L, Lieske JC, Milliner D, Gonyea J, Kelly DG. Urinary oxalate excretion increases in home parenteral nutrition patients on a higher intravenous ascorbic acid dose. JPEN J. Parenter. Enter. Nutr. 2004;28:435–438. doi: 10.1177/0148607104028006435. [DOI] [PubMed] [Google Scholar]
- 139.Cossey LN, Rahim F, Larsen CP. Oxalate nephropathy and intravenous vitamin C. Am. J. Kidney Dis. 2013;61:1032–1035. doi: 10.1053/j.ajkd.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 140.Fontana F, et al. Oxalate nephropathy caused by excessive vitamin C administration in 2 patients with COVID-19. Kidney Int. Rep. 2020;5:1815–1822. doi: 10.1016/j.ekir.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Buehner M, et al. Oxalate nephropathy after continuous infusion of high-dose vitamin C as an adjunct to burn resuscitation. J. Burn. Care Res. 2016;37:e374–e379. doi: 10.1097/BCR.0000000000000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Prier M, Carr AC, Baillie N. No reported renal stones with intravenous vitamin C administration: a prospective case series study. Antioxidants. 2018;7:E68. doi: 10.3390/antiox7050068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yanase F, et al. Harm of IV high-dose vitamin C therapy in adult patients: a scoping review. Crit. Care Med. 2020;48:e620–e628. doi: 10.1097/CCM.0000000000004396. [DOI] [PubMed] [Google Scholar]
- 144.Thamilselvan V, Menon M, Thamilselvan S. Oxalate at physiological urine concentrations induces oxidative injury in renal epithelial cells: effect of α-tocopherol and ascorbic acid. BJU Int. 2014;114:140–150. doi: 10.1111/bju.12642. [DOI] [PubMed] [Google Scholar]
- 145.Fishman AI, et al. Preventive effect of specific antioxidant on oxidative renal cell injury associated with renal crystal formation. Urology. 2013;82:489.e1–7. doi: 10.1016/j.urology.2013.03.065. [DOI] [PubMed] [Google Scholar]
- 146.Jaturakan O, et al. Combination of vitamin E and vitamin C alleviates renal function in hyperoxaluric rats via antioxidant activity. J. Vet. Med. Sci. 2017;79:896–903. doi: 10.1292/jvms.17-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tzou DT, Taguchi K, Chi T, Stoller ML. Animal models of urinary stone disease. Int. J. Surg. 2016;36:596–606. doi: 10.1016/j.ijsu.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 148.Tannehill-Gregg SH, et al. Strain-related differences in urine composition of male rats of potential relevance to urolithiasis. Toxicol. Pathol. 2009;37:293–305. doi: 10.1177/0192623309332990. [DOI] [PubMed] [Google Scholar]
- 149.Pham VT, Dold S, Rehman A, Bird JK, Steinert RE. Vitamins, the gut microbiome and gastrointestinal health in humans. Nutr. Res. 2021;95:35–53. doi: 10.1016/j.nutres.2021.09.001. [DOI] [PubMed] [Google Scholar]
- 150.Chambial S, Dwivedi S, Shukla KK, John PJ, Sharma P. Vitamin C in disease prevention and cure: an overview. Indian. J. Clin. Biochem. 2013;28:314–328. doi: 10.1007/s12291-013-0375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chang Y-L, et al. A screen of Crohn’s disease-associated microbial metabolites identifies ascorbate as a novel metabolic inhibitor of activated human T cells. Mucosal Immunol. 2019;12:457–467. doi: 10.1038/s41385-018-0022-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yang Q, et al. Role of dietary nutrients in the modulation of gut microbiota: a narrative review. Nutrients. 2020;12:381. doi: 10.3390/nu12020381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Otten AT, et al. Vitamin C supplementation in healthy individuals leads to shifts of bacterial populations in the gut — a pilot study. Antioxidants. 2021;10:1278. doi: 10.3390/antiox10081278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pham VT, et al. Effects of colon-targeted vitamins on the composition and metabolic activity of the human gut microbiome — a pilot study. Gut Microbes. 2021;13:1875774. doi: 10.1080/19490976.2021.1875774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yao C, Chou J, Wang T, Zhao H, Zhang B. Pantothenic acid, vitamin C, and biotin play important roles in the growth of Lactobacillus helveticus. Front. Microbiol. 2018;9:1194. doi: 10.3389/fmicb.2018.01194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Linares D, Michaud P, Delort A-M, Traïkia M, Warrand J. Catabolism of L-ascorbate by Lactobacillus rhamnosus GG. J. Agric. Food Chem. 2011;59:4140–4147. doi: 10.1021/jf104343r. [DOI] [PubMed] [Google Scholar]
- 157.Stuivenberg G, Daisley B, Akouris P, Reid G. In vitro assessment of histamine and lactate production by a multi-strain synbiotic. J. Food Sci. Technol. 2021 doi: 10.1007/s13197-021-05327-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chamberlain CA, Hatch M, Garrett TJ. Metabolomic profiling of oxalate-degrading probiotic Lactobacillus acidophilus and Lactobacillus gasseri. PLoS One. 2019;14:e0222393. doi: 10.1371/journal.pone.0222393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yew WS, Gerlt JA. Utilization of L-ascorbate by Escherichia coli K-12: assignments of functions to products of the yjf-sga and yia-sgb operons. J. Bacteriol. 2002;184:302–306. doi: 10.1128/JB.184.1.302-306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Holmes RP, Assimos DG. Glyoxylate synthesis, and its modulation and influence on oxalate synthesis. J. Urol. 1998;160:1617–1624. doi: 10.1016/S0022-5347(01)62363-2. [DOI] [PubMed] [Google Scholar]
- 161.Afzal M, Shafeeq S, Henriques-Normark B, Kuipers OP. UlaR activates expression of the ula operon in Streptococcus pneumoniae in the presence of ascorbic acid. Microbiology. 2015;161:41–49. doi: 10.1099/mic.0.083899-0. [DOI] [PubMed] [Google Scholar]
- 162.Martinez-Sanguiné AY, et al. Salmonella enterica serovars Dublin and Enteritidis comparative proteomics reveals differential expression of proteins involved in stress resistance, virulence, and anaerobic metabolism. Infect. Immun. 2021;89:e00606–e00620. doi: 10.1128/IAI.00606-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mai S-NT, et al. The evolutionary history of Shigella flexneri serotype 6 in Asia. Microb. Genom. 2021;7:000736. doi: 10.1099/mgen.0.000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mehmeti I, Solheim M, Nes IF, Holo H. Enterococcus faecalis grows on ascorbic acid. Appl. Env. Microbiol. 2013;79:4756–4758. doi: 10.1128/AEM.00228-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Campos E, et al. The yiaKLX1X2PQRS and ulaABCDEFG gene systems are required for the aerobic utilization of L-ascorbate in Klebsiella pneumoniae strain 13882 with L-ascorbate-6-phosphate as the inducer. J. Bacteriol. 2008;190:6615–6624. doi: 10.1128/JB.00815-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhang Z, Aboulwafa M, Smith MH, Saier MH. The ascorbate transporter of Escherichia coli. J. Bacteriol. 2003;185:2243–2250. doi: 10.1128/JB.185.7.2243-2250.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Subramanian VS, Sabui S, Moradi H, Marchant JS, Said HM. Inhibition of intestinal ascorbic acid uptake by lipopolysaccharide is mediated via transcriptional mechanisms. Biochim. Biophys. Acta Biomembr. 2018;1860:556–565. doi: 10.1016/j.bbamem.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Subramanian VS, Sabui S, Subramenium GA, Marchant JS, Said HM. Tumor necrosis factor alpha reduces intestinal vitamin C uptake: a role for NF-κB-mediated signaling. Am. J. Physiol. Gastrointest. Liver Physiol. 2018;315:G241–G248. doi: 10.1152/ajpgi.00071.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Subramanian VS, Teafatiller T, Agrawal A, Kitazawa M, Marchant JS. Effect of lipopolysaccharide and TNFα on neuronal ascorbic acid uptake. Mediat. Inflamm. 2021;2021:4157132. doi: 10.1155/2021/4157132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Heskett CW, et al. Enteropathogenic Escherichia coli infection inhibits intestinal ascorbic acid uptake via dysregulation of its transporter expression. Dig. Dis. Sci. 2021;66:2250–2260. doi: 10.1007/s10620-020-06389-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Subramanian VS, Sabui S, Marchant JS, Said HM. MicroRNA-103a regulates sodium-dependent vitamin C transporter-1 expression in intestinal epithelial cells. J. Nutr. Biochem. 2019;65:46–53. doi: 10.1016/j.jnutbio.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sangani R, et al. MicroRNAs-141 and 200a regulate the SVCT2 transporter in bone marrow stromal cells. Mol. Cell Endocrinol. 2015;410:19–26. doi: 10.1016/j.mce.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jones G. Extrarenal vitamin D activation and interactions between vitamin D2, vitamin D3, and vitamin D analogs. Annu. Rev. Nutr. 2013;33:23–44. doi: 10.1146/annurev-nutr-071812-161203. [DOI] [PubMed] [Google Scholar]
- 174.Holick MF, et al. Photosynthesis of previtamin D3 in human skin and the physiologic consequences. Science. 1980;210:203–205. doi: 10.1126/science.6251551. [DOI] [PubMed] [Google Scholar]
- 175.Slepchenko BM, Bronner F. Modeling of transcellular Ca transport in rat duodenum points to coexistence of two mechanisms of apical entry. Am. J. Physiol. Cell Physiol. 2001;281:C270–C281. doi: 10.1152/ajpcell.2001.281.1.C270. [DOI] [PubMed] [Google Scholar]
- 176.Diaz de Barboza G, Guizzardi S, Tolosa de Talamoni N. Molecular aspects of intestinal calcium absorption. World J. Gastroenterol. 2015;21:7142–7154. doi: 10.3748/wjg.v21.i23.7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Fleet JC. The role of vitamin D in the endocrinology controlling calcium homeostasis. Mol. Cell Endocrinol. 2017;453:36–45. doi: 10.1016/j.mce.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lassiter WE, Gottschalk CW, Mylle M. Micropuncture study of renal tubular reabsorption of calcium in normal rodents. Am. J. Physiol. 1963;204:771–775. doi: 10.1152/ajplegacy.1963.204.5.771. [DOI] [Google Scholar]
- 179.Moor MB, Bonny O. Ways of calcium reabsorption in the kidney. Am. J. Physiol. Renal Physiol. 2016;310:F1337–F1350. doi: 10.1152/ajprenal.00273.2015. [DOI] [PubMed] [Google Scholar]
- 180.Bindels RJ, Hartog A, Timmermans J, Van Os CH. Active Ca2+ transport in primary cultures of rabbit kidney CCD: stimulation by 1,25-dihydroxyvitamin D3 and PTH. Am. J. Physiol. 1991;261:F799–F807. doi: 10.1152/ajprenal.1991.261.5.F799. [DOI] [PubMed] [Google Scholar]
- 181.Hoenderop JGJ, van der Kemp AWCM, Urben CM, Strugnell SA, Bindels RJM. Effects of vitamin D compounds on renal and intestinal Ca2+ transport proteins in 25-hydroxyvitamin D3-1α-hydroxylase knockout mice. Kidney Int. 2004;66:1082–1089. doi: 10.1111/j.1523-1755.2004.00858.x. [DOI] [PubMed] [Google Scholar]
- 182.Nijenhuis T, Hoenderop JGJ, van der Kemp AWCM, Bindels RJM. Localization and regulation of the epithelial Ca2+ channel TRPV6 in the kidney. J. Am. Soc. Nephrol. 2003;14:2731–2740. doi: 10.1097/01.ASN.0000094081.78893.E8. [DOI] [PubMed] [Google Scholar]
- 183.Hoenderop JGJ, et al. Modulation of renal Ca2+ transport protein genes by dietary Ca2+ and 1,25-dihydroxyvitamin D3 in 25-hydroxyvitamin D3-1α-hydroxylase knockout mice. FASEB J. 2002;16:1398–1406. doi: 10.1096/fj.02-0225com. [DOI] [PubMed] [Google Scholar]
- 184.Xue Y, Fleet JC. Intestinal vitamin D receptor is required for normal calcium and bone metabolism in mice. Gastroenterology. 2009;136:1317–1327, e1-2. doi: 10.1053/j.gastro.2008.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yamamoto Y, et al. Vitamin D receptor in osteoblasts is a negative regulator of bone mass control. Endocrinology. 2013;154:1008–1020. doi: 10.1210/en.2012-1542. [DOI] [PubMed] [Google Scholar]
- 186.Kitazawa R, Mori K, Yamaguchi A, Kondo T, Kitazawa S. Modulation of mouse RANKL gene expression by Runx2 and vitamin D3. J. Cell Biochem. 2008;105:1289–1297. doi: 10.1002/jcb.21929. [DOI] [PubMed] [Google Scholar]
- 187.Mori T, et al. The vitamin D receptor in osteoblast-lineage cells is essential for the proresorptive activity of 1α,25(OH)2D3 in vivo. Endocrinology. 2020;161:bqaa178. doi: 10.1210/endocr/bqaa178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hu H, et al. Association between circulating vitamin D level and urolithiasis: a systematic review and meta-analysis. Nutrients. 2017;9:E301. doi: 10.3390/nu9030301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Malihi Z, Wu Z, Stewart AW, Lawes CM, Scragg R. Hypercalcemia, hypercalciuria, and kidney stones in long-term studies of vitamin D supplementation: a systematic review and meta-analysis. Am. J. Clin. Nutr. 2016;104:1039–1051. doi: 10.3945/ajcn.116.134981. [DOI] [PubMed] [Google Scholar]
- 190.Ferraro PM, Taylor EN, Gambaro G, Curhan GC. Vitamin D intake and the risk of incident kidney stones. J. Urol. 2017;197:405–410. doi: 10.1016/j.juro.2016.08.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Girón-Prieto MS, et al. Analysis of vitamin D deficiency in calcium stone-forming patients. Int. Urol. Nephrol. 2016;48:1243–1246. doi: 10.1007/s11255-016-1290-3. [DOI] [PubMed] [Google Scholar]
- 192.Ticinesi A, et al. Idiopathic calcium nephrolithiasis and hypovitaminosis D: a case-control study. Urology. 2016;87:40–45. doi: 10.1016/j.urology.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 193.Dholakia K, Selvaraj N, Ragavan N. Prevalence of vitamin D inadequacy in urolithiasis patients. Cureus. 2021;13:e15379. doi: 10.7759/cureus.15379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Tang J, Chonchol MB. Vitamin D and kidney stone disease. Curr. Opin. Nephrol. Hypertens. 2013;22:383–389. doi: 10.1097/MNH.0b013e328360bbcd. [DOI] [PubMed] [Google Scholar]
- 195.Tavasoli S, Taheri M. Vitamin D and calcium kidney stones: a review and a proposal. Int. Urol. Nephrol. 2019;51:101–111. doi: 10.1007/s11255-018-1965-z. [DOI] [PubMed] [Google Scholar]
- 196.Martins JS, Palhares MDO, Teixeira OCM, Gontijo Ramos M. Vitamin D status and its association with parathyroid hormone concentration in Brazilians. J. Nutr. Metab. 2017;2017:9056470. doi: 10.1155/2017/9056470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Silva BC, Bilezikian JP. Parathyroid hormone: anabolic and catabolic actions on the skeleton. Curr. Opin. Pharmacol. 2015;22:41–50. doi: 10.1016/j.coph.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Prochaska M, Taylor E, Vaidya A, Curhan G. Low bone density and bisphosphonate use and the risk of kidney stones. Clin. J. Am. Soc. Nephrol. 2017;12:1284–1290. doi: 10.2215/CJN.01420217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Taguchi K, et al. Low bone mineral density is a potential risk factor for symptom onset and related with hypocitraturia in urolithiasis patients: a single-center retrospective cohort study. BMC Urol. 2020;20:174. doi: 10.1186/s12894-020-00749-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Carlberg C, Seuter S, Heikkinen S. The first genome-wide view of vitamin D receptor locations and their mechanistic implications. Anticancer. Res. 2012;32:271–282. [PubMed] [Google Scholar]
- 201.Bikle DD. Extraskeletal actions of vitamin D. Ann. N. Y. Acad. Sci. 2016;1376:29–52. doi: 10.1111/nyas.13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Thomas RL, et al. Vitamin D metabolites and the gut microbiome in older men. Nat. Commun. 2020;11:5997. doi: 10.1038/s41467-020-19793-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Yang X, et al. Causal relationship between gut microbiota and serum vitamin D: evidence from genetic correlation and Mendelian randomization study. Eur. J. Clin. Nutr. 2022;76:1017–1023. doi: 10.1038/s41430-021-01065-3. [DOI] [PubMed] [Google Scholar]
- 204.Sasaki J, Mikami A, Mizoue K, Omura S. Transformation of 25- and 1 alpha-hydroxyvitamin D3 to 1α, 25-dihydroxyvitamin D3 by using Streptomyces sp. strains. Appl. Environ. Microbiol. 1991;57:2841–2846. doi: 10.1128/aem.57.10.2841-2846.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Sawada N, et al. Conversion of vitamin D3 to 1α,25-dihydroxyvitamin D3 by Streptomyces griseolus cytochrome P450SU-1. Biochem. Biophys. Res. Commun. 2004;320:156–164. doi: 10.1016/j.bbrc.2004.05.140. [DOI] [PubMed] [Google Scholar]
- 206.Fujii Y, et al. Purification, characterization, and directed evolution study of a vitamin D3 hydroxylase from Pseudonocardia autotrophica. Biochem. Biophys. Res. Commun. 2009;385:170–175. doi: 10.1016/j.bbrc.2009.05.033. [DOI] [PubMed] [Google Scholar]
- 207.Ang SS, et al. Molecular characterization, modeling and docking of CYP107CB2 from Bacillus lehensis G1, an alkaliphile. Comput. Biol. Chem. 2015;56:19–29. doi: 10.1016/j.compbiolchem.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 208.Ang SS, et al. Biochemical characterization of the cytochrome P450 CYP107CB2 from Bacillus lehensis G1. Protein J. 2018;37:180–193. doi: 10.1007/s10930-018-9764-z. [DOI] [PubMed] [Google Scholar]
- 209.Yamamoto EA, Jørgensen TN. Relationships between vitamin D, gut microbiome, and systemic autoimmunity. Front. Immunol. 2019;10:3141. doi: 10.3389/fimmu.2019.03141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Jakobsen J. Bioavailability and bioactivity of vitamin D3 active compounds — which potency should be used for 25-hydroxyvitamin D3? Int. Congr. Ser. 2007;1297:133–142. doi: 10.1016/j.ics.2006.08.026. [DOI] [Google Scholar]
- 211.Vasilevskaya AV, et al. Identification of Mycobacterium tuberculosis enzyme involved in vitamin D and 7-dehydrocholesterol metabolism. J. Steroid Biochem. Mol. Biol. 2017;169:202–209. doi: 10.1016/j.jsbmb.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 212.Varaksa T, et al. Metabolic fate of human immunoactive sterols in Mycobacterium tuberculosis. J. Mol. Biol. 2021;433:166763. doi: 10.1016/j.jmb.2020.166763. [DOI] [PubMed] [Google Scholar]
- 213.Bora SA, Kennett MJ, Smith PB, Patterson AD, Cantorna MT. The gut microbiota regulates endocrine vitamin D metabolism through fibroblast growth factor 23. Front. Immunol. 2018;9:408. doi: 10.3389/fimmu.2018.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Shimada T, et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J. Bone Min. Res. 2004;19:429–435. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- 215.Peterson CA, Heffernan ME. Serum tumor necrosis factor-alpha concentrations are negatively correlated with serum 25(OH)D concentrations in healthy women. J. Inflamm. 2008;5:10. doi: 10.1186/1476-9255-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Du J, Wei X, Ge X, Chen Y, Li YC. Microbiota-dependent induction of colonic Cyp27b1 is associated with colonic inflammation: implications of locally produced 1,25-dihydroxyvitamin D3 in inflammatory regulation in the colon. Endocrinology. 2017;158:4064–4075. doi: 10.1210/en.2017-00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Chen Y, et al. MicroRNA-346 mediates tumor necrosis factor α-induced downregulation of gut epithelial vitamin D receptor in inflammatory bowel diseases. Inflamm. Bowel Dis. 2014;20:1910–1918. doi: 10.1097/MIB.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Waterhouse JC, Perez TH, Albert PJ. Reversing bacteria-induced vitamin D receptor dysfunction is key to autoimmune disease. Ann. N. Y. Acad. Sci. 2009;1173:757–765. doi: 10.1111/j.1749-6632.2009.04637.x. [DOI] [PubMed] [Google Scholar]
- 219.Gaschott T, Stein J. Short-chain fatty acids and colon cancer cells: the vitamin D receptor–butyrate connection. Recent Results Cancer Res. 2003;164:247–257. doi: 10.1007/978-3-642-55580-0_18. [DOI] [PubMed] [Google Scholar]
- 220.Sun J. VDR/vitamin D receptor regulates autophagic activity through ATG16L1. Autophagy. 2016;12:1057–1058. doi: 10.1080/15548627.2015.1072670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Huang F-C, Huang S-C. The combined beneficial effects of postbiotic butyrate on active vitamin D3-orchestrated innate immunity to Salmonella colitis. Biomedicines. 2021;9:1296. doi: 10.3390/biomedicines9101296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Wang J, et al. Genome-wide association analysis identifies variation in vitamin D receptor and other host factors influencing the gut microbiota. Nat. Genet. 2016;48:1396–1406. doi: 10.1038/ng.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Jones ML, Martoni CJ, Prakash S. Oral supplementation with probiotic L. reuteri NCIMB 30242 increases mean circulating 25-hydroxyvitamin D: a post hoc analysis of a randomized controlled trial. J. Clin. Endocrinol. Metab. 2013;98:2944–2951. doi: 10.1210/jc.2012-4262. [DOI] [PubMed] [Google Scholar]
- 224.Reboul E, et al. Vitamin D intestinal absorption is not a simple passive diffusion: evidences for involvement of cholesterol transporters. Mol. Nutr. Food Res. 2011;55:691–702. doi: 10.1002/mnfr.201000553. [DOI] [PubMed] [Google Scholar]
- 225.Castagliuolo I, et al. Co-administration of vitamin D3 and Lacticaseibacillus paracasei DG increase 25-hydroxyvitamin D serum levels in mice. Ann. Microbiol. 2021;71:42. doi: 10.1186/s13213-021-01655-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Semova I, et al. Microbiota regulate intestinal absorption and metabolism of fatty acids in the zebrafish. Cell Host Microbe. 2012;12:277–288. doi: 10.1016/j.chom.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Jiang Q. Natural forms of vitamin E as effective agents for cancer prevention and therapy. Adv. Nutr. 2017;8:850–867. doi: 10.3945/an.117.016329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Hosomi A, et al. Affinity for α-tocopherol transfer protein as a determinant of the biological activities of vitamin E analogs. FEBS Lett. 1997;409:105–108. doi: 10.1016/S0014-5793(97)00499-7. [DOI] [PubMed] [Google Scholar]
- 229.Khan SR. Reactive oxygen species as the molecular modulators of calcium oxalate kidney stone formation: evidence from clinical and experimental investigations. J. Urol. 2013;189:803–811. doi: 10.1016/j.juro.2012.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Thamilselvan S, Menon M. Vitamin E therapy prevents hyperoxaluria-induced calcium oxalate crystal deposition in the kidney by improving renal tissue antioxidant status. BJU Int. 2005;96:117–126. doi: 10.1111/j.1464-410X.2005.05579.x. [DOI] [PubMed] [Google Scholar]
- 231.Huang H-S, Chen J, Chen C-F, Ma M-C. Vitamin E attenuates crystal formation in rat kidneys: roles of renal tubular cell death and crystallization inhibitors. Kidney Int. 2006;70:699–710. doi: 10.1038/sj.ki.5001651. [DOI] [PubMed] [Google Scholar]
- 232.Santhosh Kumar M, Selvam R. Supplementation of vitamin E and selenium prevents hyperoxaluria in experimental urolithic rats. J. Nutr. Biochem. 2003;14:306–313. doi: 10.1016/S0955-2863(03)00033-0. [DOI] [PubMed] [Google Scholar]
- 233.Sakly R, Fekih M, Ben Amor A, Najjar MF, Mbazaa M. Possible role of vitamin A and E deficiency in human idiopathic lithiasis. Ann. Urol. 2003;37:217–219. doi: 10.1016/S0003-4401(03)00074-3. [DOI] [PubMed] [Google Scholar]
- 234.Siener R, Machaka I, Alteheld B, Bitterlich N, Metzner C. Effect of fat-soluble vitamins A, D, E and K on vitamin status and metabolic profile in patients with fat malabsorption with and without urolithiasis. Nutrients. 2020;12:E3110. doi: 10.3390/nu12103110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Tungsanga K, Sriboonlue P, Futrakul P, Yachantha C, Tosukhowong P. Renal tubular cell damage and oxidative stress in renal stone patients and the effect of potassium citrate treatment. Urol. Res. 2005;33:65–69. doi: 10.1007/s00240-004-0444-4. [DOI] [PubMed] [Google Scholar]
- 236.Huang H-S, Ma M-C, Chen C-F, Chen J. Lipid peroxidation and its correlations with urinary levels of oxalate, citric acid, and osteopontin in patients with renal calcium oxalate stones. Urology. 2003;62:1123–1128. doi: 10.1016/S0090-4295(03)00764-7. [DOI] [PubMed] [Google Scholar]
- 237.Mo L, et al. Tamm-Horsfall protein is a critical renal defense factor protecting against calcium oxalate crystal formation. Kidney Int. 2004;66:1159–1166. doi: 10.1111/j.1523-1755.2004.00867.x. [DOI] [PubMed] [Google Scholar]
- 238.Sumitra K, Pragasam V, Sakthivel R, Kalaiselvi P, Varalakshmi P. Beneficial effect of vitamin E supplementation on the biochemical and kinetic properties of Tamm-Horsfall glycoprotein in hypertensive and hyperoxaluric patients. Nephrol. Dial. Transpl. 2005;20:1407–1415. doi: 10.1093/ndt/gfh794. [DOI] [PubMed] [Google Scholar]
- 239.Viswanathan P, et al. Calcium oxalate monohydrate aggregation induced by aggregation of desialylated Tamm-Horsfall protein. Urol. Res. 2011;39:269–282. doi: 10.1007/s00240-010-0353-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Hughes PE, Tove SB. Occurrence of alpha-tocopherolquinone and alpha-tocopherolquinol in microorganisms. J. Bacteriol. 1982;151:1397–1402. doi: 10.1128/jb.151.3.1397-1402.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Sattler SE, Cahoon EB, Coughlan SJ, DellaPenna D. Characterization of tocopherol cyclases from higher plants and cyanobacteria. Evolutionary implications for tocopherol synthesis and function. Plant. Physiol. 2003;132:2184–2195. doi: 10.1104/pp.103.024257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Shen B, et al. Fermentative production of vitamin E tocotrienols in Saccharomyces cerevisiae under cold-shock-triggered temperature control. Nat. Commun. 2020;11:5155. doi: 10.1038/s41467-020-18958-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Albermann C, et al. Biosynthesis of the vitamin E compound delta-tocotrienol in recombinant Escherichia coli cells. Chembiochem. 2008;9:2524–2533. doi: 10.1002/cbic.200800242. [DOI] [PubMed] [Google Scholar]
- 244.Ran L, et al. Effects of antibiotics on degradation and bioavailability of different vitamin E forms in mice. Biofactors. 2019;45:450–462. doi: 10.1002/biof.1492. [DOI] [PubMed] [Google Scholar]
- 245.Knarreborg A, Lauridsen C, Engberg RM, Jensen SK. Dietary antibiotic growth promoters enhance the bioavailability of alpha-tocopheryl acetate in broilers by altering lipid absorption. J. Nutr. 2004;134:1487–1492. doi: 10.1093/jn/134.6.1487. [DOI] [PubMed] [Google Scholar]
- 246.Reboul E. Vitamin E bioavailability: mechanisms of intestinal absorption in the spotlight. Antioxidants. 2017;6:E95. doi: 10.3390/antiox6040095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Carey MC, Small DM. Micelle formation by bile salts. Physical-chemical and thermodynamic considerations. Arch. Intern. Med. 1972;130:506–527. doi: 10.1001/archinte.1972.03650040040005. [DOI] [PubMed] [Google Scholar]
- 248.Ridlon JM, Kang D-J, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 2006;47:241–259. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 249.Hofmann AF. The continuing importance of bile acids in liver and intestinal disease. Arch. Intern. Med. 1999;159:2647–2658. doi: 10.1001/archinte.159.22.2647. [DOI] [PubMed] [Google Scholar]
- 250.Łozińska N, Jungnickel C. Importance of conjugation of the bile salt on the mechanism of lipolysis. Molecules. 2021;26:5764. doi: 10.3390/molecules26195764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Roager HM, et al. Lactobacillus acidophilus NCFM affects vitamin E acetate metabolism and intestinal bile acid signature in monocolonized mice. Gut Microbes. 2014;5:296–303. doi: 10.4161/gmic.28806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Lombardo D, Guy O. Studies on the substrate specificity of a carboxyl ester hydrolase from human pancreatic juice. II. Action on cholesterol esters and lipid-soluble vitamin esters. Biochim. Biophys. Acta. 1980;611:147–155. doi: 10.1016/0005-2744(80)90050-9. [DOI] [PubMed] [Google Scholar]
- 253.West B, et al. Metabolic syndrome and self-reported history of kidney stones: the National Health and Nutrition Examination Survey (NHANES III) 1988–1994. Am. J. Kidney Dis. 2008;51:741–747. doi: 10.1053/j.ajkd.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 254.Barzegar-Amini M, et al. Association between serum vitamin E concentrations and the presence of metabolic syndrome: a population-based cohort study. Acta Biomed. 2021;92:e2021047. doi: 10.23750/abm.v92i3.9173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Halder M, et al. Vitamin K: double bonds beyond coagulation insights into differences between vitamin K1 and K2 in health and disease. Int. J. Mol. Sci. 2019;20:896. doi: 10.3390/ijms20040896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Mladěnka P, et al. Vitamin K — sources, physiological role, kinetics, deficiency, detection, therapeutic use, and toxicity. Nutr. Rev. 2022;80:677–698. doi: 10.1093/nutrit/nuab061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Sato T, Schurgers LJ, Uenishi K. Comparison of menaquinone-4 and menaquinone-7 bioavailability in healthy women. Nutr. J. 2012;11:93. doi: 10.1186/1475-2891-11-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Schurgers LJ, Vermeer C. Determination of phylloquinone and menaquinones in food. Effect of food matrix on circulating vitamin K concentrations. Haemostasis. 2000;30:298–307. doi: 10.1159/000054147. [DOI] [PubMed] [Google Scholar]
- 259.Schurgers LJ, Vermeer C. Differential lipoprotein transport pathways of K-vitamins in healthy subjects. Biochim. Biophys. Acta. 2002;1570:27–32. doi: 10.1016/S0304-4165(02)00147-2. [DOI] [PubMed] [Google Scholar]
- 260.Okano T, et al. Conversion of phylloquinone (Vitamin K1) into menaquinone-4 (Vitamin K2) in mice: two possible routes for menaquinone-4 accumulation in cerebra of mice. J. Biol. Chem. 2008;283:11270–11279. doi: 10.1074/jbc.M702971200. [DOI] [PubMed] [Google Scholar]
- 261.Price PA, Baukol SA. 1,25-Dihydroxyvitamin D3 increases synthesis of the vitamin K-dependent bone protein by osteosarcoma cells. J. Biol. Chem. 1980;255:11660–11663. doi: 10.1016/S0021-9258(19)70182-3. [DOI] [PubMed] [Google Scholar]
- 262.Fraser JD, Otawara Y, Price PA. 1,25-Dihydroxyvitamin D3 stimulates the synthesis of matrix gamma-carboxyglutamic acid protein by osteosarcoma cells. Mutually exclusive expression of vitamin K-dependent bone proteins by clonal osteoblastic cell lines. J. Biol. Chem. 1988;263:911–916. doi: 10.1016/S0021-9258(19)35439-0. [DOI] [PubMed] [Google Scholar]
- 263.Fraser JD, Price PA. Induction of Matrix Gla protein synthesis during prolonged 1,25-dihydroxyvitamin D3 treatment of osteosarcoma cells. Calcif. Tissue Int. 1990;46:270–279. doi: 10.1007/BF02555007. [DOI] [PubMed] [Google Scholar]
- 264.Dowd P, Hershline R, Ham SW, Naganathan S. Vitamin K and energy transduction: a base strength amplification mechanism. Science. 1995;269:1684–1691. doi: 10.1126/science.7569894. [DOI] [PubMed] [Google Scholar]
- 265.Buitenhuis HC, Soute BA, Vermeer C. Comparison of the vitamins K1, K2 and K3 as cofactors for the hepatic vitamin K-dependent carboxylase. Biochim. Biophys. Acta. 1990;1034:170–175. doi: 10.1016/0304-4165(90)90072-5. [DOI] [PubMed] [Google Scholar]
- 266.Fraser JD, Price PA. Lung, heart, and kidney express high levels of mRNA for the vitamin K-dependent Matrix Gla protein. Implications for the possible functions of Matrix Gla protein and for the tissue distribution of the gamma-carboxylase. J. Biol. Chem. 1988;263:11033–11036. doi: 10.1016/S0021-9258(18)37912-2. [DOI] [PubMed] [Google Scholar]
- 267.Moser SC, van der Eerden BCJ. Osteocalcin — a versatile bone-derived hormone. Front. Endocrinol. 2018;9:794. doi: 10.3389/fendo.2018.00794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Jaminon AMG, et al. Matrix Gla protein is an independent predictor of both intimal and medial vascular calcification in chronic kidney disease. Sci. Rep. 2020;10:6586. doi: 10.1038/s41598-020-63013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Schurgers LJ, et al. The circulating inactive form of matrix gla protein is a surrogate marker for vascular calcification in chronic kidney disease: a preliminary report. Clin. J. Am. Soc. Nephrol. 2010;5:568–575. doi: 10.2215/CJN.07081009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Kurnatowska I, et al. Plasma desphospho-uncarboxylated Matrix Gla protein as a marker of kidney damage and cardiovascular risk in advanced stage of chronic kidney disease. Kidney Blood Press. Res. 2016;41:231–239. doi: 10.1159/000443426. [DOI] [PubMed] [Google Scholar]
- 271.Liabeuf S, et al. Vascular calcification in patients with type 2 diabetes: the involvement of matrix Gla protein. Cardiovasc. Diabetol. 2014;13:85. doi: 10.1186/1475-2840-13-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Mayer O, et al. Desphospho-uncarboxylated Matrix Gla protein is associated with increased aortic stiffness in a general population. J. Hum. Hypertens. 2016;30:418–423. doi: 10.1038/jhh.2015.55. [DOI] [PubMed] [Google Scholar]
- 273.Fabris A, et al. The relationship between calcium kidney stones, arterial stiffness and bone density: unraveling the stone-bone-vessel liaison. J. Nephrol. 2015;28:549–555. doi: 10.1007/s40620-014-0146-0. [DOI] [PubMed] [Google Scholar]
- 274.Shavit L, et al. Vascular calcification and bone mineral density in recurrent kidney stone formers. Clin. J. Am. Soc. Nephrol. 2015;10:278–285. doi: 10.2215/CJN.06030614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Stapleton AM, Timme TL, Ryall RL. Gene expression of prothrombin in the human kidney and its potential relevance to kidney stone disease. Br. J. Urol. 1998;81:666–671. doi: 10.1046/j.1464-410x.1998.00620.x. [DOI] [PubMed] [Google Scholar]
- 276.Ogasawara K, Van Reen R, Ako H. γ-Carboxyglutamic acid, a component in human pediatric bladder stones containing calcium salts. J. Urol. 1987;137:349–352. doi: 10.1016/S0022-5347(17)44021-3. [DOI] [PubMed] [Google Scholar]
- 277.Lian JB, Prien EL, Glimcher MJ, Gallop PM. The presence of protein-bound gamma-carboxyglutamic acid in calcium-containing renal calculi. J. Clin. Invest. 1977;59:1151–1157. doi: 10.1172/JCI108739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Grover PK, Ryall RL. Inhibition of calcium oxalate crystal growth and aggregation by prothrombin and its fragments in vitro: relationship between protein structure and inhibitory activity. Eur. J. Biochem. 1999;263:50–56. doi: 10.1046/j.1432-1327.1999.00448.x. [DOI] [PubMed] [Google Scholar]
- 279.Kurutz JW, Carvalho M, Nakagawa Y. Nephrocalcin isoforms coat crystal surfaces and differentially affect calcium oxalate monohydrate crystal morphology, growth, and aggregation. J. Cryst. Growth. 2003;255:392–402. doi: 10.1016/S0022-0248(03)01308-3. [DOI] [Google Scholar]
- 280.Nakagawa Y, et al. Urine glycoprotein crystal growth inhibitors. Evidence for a molecular abnormality in calcium oxalate nephrolithiasis. J. Clin. Invest. 1985;76:1455–1462. doi: 10.1172/JCI112124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Nakagawa Y, Ahmed M, Hall SL, Deganello S, Coe FL. Isolation from human calcium oxalate renal stones of nephrocalcin, a glycoprotein inhibitor of calcium oxalate crystal growth. Evidence that nephrocalcin from patients with calcium oxalate nephrolithiasis is deficient in gamma-carboxyglutamic acid. J. Clin. Invest. 1987;79:1782–1787. doi: 10.1172/JCI113019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Dalmeijer GW, et al. Circulating Matrix Gla protein is associated with coronary artery calcification and vitamin K status in healthy women. J. Nutr. Biochem. 2013;24:624–628. doi: 10.1016/j.jnutbio.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 283.Akbulut AC, et al. Vitamin K2 needs an RDI separate from vitamin K1. Nutrients. 2020;12:1852. doi: 10.3390/nu12061852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Puzantian H, et al. Circulating dephospho-uncarboxylated Matrix Gla-protein Is associated With kidney dysfunction and arterial stiffness. Am. J. Hypertens. 2018;31:988–994. doi: 10.1093/ajh/hpy079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Chirinos JA, et al. Aldosterone, inactive matrix gla-protein, and large artery stiffness in hypertension. J. Am. Soc. Hypertens. 2018;12:681–689. doi: 10.1016/j.jash.2018.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Jespersen T, et al. Uncarboxylated matrix Gla-protein: a biomarker of vitamin K status and cardiovascular risk. Clin. Biochem. 2020;83:49–56. doi: 10.1016/j.clinbiochem.2020.05.005. [DOI] [PubMed] [Google Scholar]
- 287.Gao B, et al. Matrix Gla protein expression in NRK-52E cells exposed to oxalate and calcium oxalate monohydrate crystals. Urol. Int. 2010;85:237–241. doi: 10.1159/000314947. [DOI] [PubMed] [Google Scholar]
- 288.Khan A, Wang W, Khan SR. Calcium oxalate nephrolithiasis and expression of Matrix Gla protein in the kidneys. World J. Urol. 2014;32:123–130. doi: 10.1007/s00345-013-1050-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Goiko M, et al. Peptides of Matrix Gla protein inhibit nucleation and growth of hydroxyapatite and calcium oxalate monohydrate crystals. PLoS One. 2013;8:e80344. doi: 10.1371/journal.pone.0080344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Li Y, et al. Vitamin K1 inhibition of renal crystal formation through Matrix Gla protein in the kidney. Kidney Blood Press. Res. 2019;44:1392–1403. doi: 10.1159/000503300. [DOI] [PubMed] [Google Scholar]
- 291.Wei F-F, et al. The risk of nephrolithiasis is causally related to inactive Matrix Gla protein, a marker of vitamin K status: a Mendelian randomization study in a Flemish population. Nephrol. Dial. Transpl. 2018;33:514–522. doi: 10.1093/ndt/gfx014. [DOI] [PubMed] [Google Scholar]
- 292.Castiglione V, et al. Evaluation of inactive Matrix-Gla-protein (MGP) as a biomarker for incident and recurrent kidney stones. J. Nephrol. 2020;33:101–107. doi: 10.1007/s40620-019-00623-0. [DOI] [PubMed] [Google Scholar]
- 293.Gao B, et al. A polymorphism of Matrix Gla protein gene is associated with kidney stones. J. Urol. 2007;177:2361–2365. doi: 10.1016/j.juro.2007.01.118. [DOI] [PubMed] [Google Scholar]
- 294.Murugesan A, Kumar L, Janarthanan P. Status of single nucleotide polymorphism of Matrix Gla protein gene (rs4236) in nephrolithiasis: a preliminary study in Indian population. Int. J. Appl. Basic. Med. Res. 2018;8:38–41. doi: 10.4103/ijabmr.IJABMR_420_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Lu X, et al. A polymorphism of Matrix Gla protein gene is associated with kidney stone in the Chinese Han population. Gene. 2012;511:127–130. doi: 10.1016/j.gene.2012.09.112. [DOI] [PubMed] [Google Scholar]
- 296.Dalmeijer GW, et al. The effect of menaquinone-7 supplementation on circulating species of Matrix Gla protein. Atherosclerosis. 2012;225:397–402. doi: 10.1016/j.atherosclerosis.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 297.Knapen MHJ, et al. Menaquinone-7 supplementation improves arterial stiffness in healthy postmenopausal women. A double-blind randomised clinical trial. Thromb. Haemost. 2015;113:1135–1144. doi: 10.1160/TH14-08-0675. [DOI] [PubMed] [Google Scholar]
- 298.Westenfeld R, et al. Effect of vitamin K2 supplementation on functional vitamin K deficiency in hemodialysis patients: a randomized trial. Am. J. Kidney Dis. 2012;59:186–195. doi: 10.1053/j.ajkd.2011.10.041. [DOI] [PubMed] [Google Scholar]
- 299.Theuwissen E, et al. Low-dose menaquinone-7 supplementation improved extra-hepatic vitamin K status, but had no effect on thrombin generation in healthy subjects. Br. J. Nutr. 2012;108:1652–1657. doi: 10.1017/S0007114511007185. [DOI] [PubMed] [Google Scholar]
- 300.Nakamura E, Aoki M, Watanabe F, Kamimura A. Low-dose menaquinone-4 improves γ-carboxylation of osteocalcin in young males: a non-placebo-controlled dose–response study. Nutr. J. 2014;13:85. doi: 10.1186/1475-2891-13-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Inaba N, Sato T, Yamashita T. Low-dose daily intake of vitamin K2 (menaquinone-7) improves osteocalcin γ-carboxylation: a double-blind, randomized controlled trials. J. Nutr. Sci. Vitaminol. 2015;61:471–480. doi: 10.3177/jnsv.61.471. [DOI] [PubMed] [Google Scholar]
- 302.Nakagawa K, et al. Identification of UBIAD1 as a novel human menaquinone-4 biosynthetic enzyme. Nature. 2010;468:117–121. doi: 10.1038/nature09464. [DOI] [PubMed] [Google Scholar]
- 303.Fernandez F, Collins MD. Vitamin K composition of anaerobic gut bacteria. FEMS Microbiol. Lett. 1987;41:175–180. doi: 10.1111/j.1574-6968.1987.tb02191.x. [DOI] [Google Scholar]
- 304.Hayashi H, Shibata K, Sakamoto M, Tomita S, Benno Y. Prevotella copri sp. nov. and Prevotella stercorea sp. nov., isolated from human faeces. Int. J. Syst. Evol. Microbiol. 2007;57:941–946. doi: 10.1099/ijs.0.64778-0. [DOI] [PubMed] [Google Scholar]
- 305.Hiratsuka T, et al. An alternative menaquinone biosynthetic pathway operating in microorganisms. Science. 2008;321:1670–1673. doi: 10.1126/science.1160446. [DOI] [PubMed] [Google Scholar]
- 306.Ravcheev DA, Thiele I. Genomic analysis of the human gut microbiome suggests novel enzymes involved in quinone biosynthesis. Front. Microbiol. 2016;7:128. doi: 10.3389/fmicb.2016.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Fenn K, et al. Quinones are growth factors for the human gut microbiota. Microbiome. 2017;5:161. doi: 10.1186/s40168-017-0380-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Karl JP, et al. Fecal menaquinone profiles of overweight adults are associated with gut microbiota composition during a gut microbiota-targeted dietary intervention. Am. J. Clin. Nutr. 2015;102:84–93. doi: 10.3945/ajcn.115.109496. [DOI] [PubMed] [Google Scholar]
- 309.Karl JP, et al. Fecal concentrations of bacterially derived vitamin K forms are associated with gut microbiota composition but not plasma or fecal cytokine concentrations in healthy adults. Am. J. Clin. Nutr. 2017;106:1052–1061. doi: 10.3945/ajcn.117.155424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Ozaki D, Kubota R, Maeno T, Abdelhakim M, Hitosugi N. Association between gut microbiota, bone metabolism, and fracture risk in postmenopausal Japanese women. Osteoporos. Int. 2021;32:145–156. doi: 10.1007/s00198-020-05728-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Wagatsuma K, et al. Diversity of gut microbiota affecting serum level of undercarboxylated osteocalcin in patients with Crohn’s disease. Nutrients. 2019;11:1541. doi: 10.3390/nu11071541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Conly J, Stein K. Reduction of vitamin K2 concentrations in human liver associated with the use of broad spectrum antimicrobials. Clin. Invest. Med. 1994;17:531–539. [PubMed] [Google Scholar]
- 313.Luna M, et al. Components of the gut microbiome that influence bone tissue-level strength. J. Bone Min. Res. 2021;36:1823–1834. doi: 10.1002/jbmr.4341. [DOI] [PMC free article] [PubMed] [Google Scholar]