Abstract
Background
Nicotine receptor partial agonists may help people to stop smoking by a combination of maintaining moderate levels of dopamine to counteract withdrawal symptoms (acting as an agonist) and reducing smoking satisfaction (acting as an antagonist). This is an update of a Cochrane Review first published in 2007.
Objectives
To assess the effectiveness of nicotine receptor partial agonists, including varenicline and cytisine, for smoking cessation.
Search methods
We searched the Cochrane Tobacco Addiction Group's Specialised Register in April 2022 for trials, using relevant terms in the title or abstract, or as keywords. The register is compiled from searches of CENTRAL, MEDLINE, Embase, and PsycINFO.
Selection criteria
We included randomised controlled trials that compared the treatment drug with placebo, another smoking cessation drug, e‐cigarettes, or no medication. We excluded trials that did not report a minimum follow‐up period of six months from baseline.
Data collection and analysis
We followed standard Cochrane methods. Our main outcome was abstinence from smoking at longest follow‐up using the most rigorous definition of abstinence, preferring biochemically validated rates where reported. We pooled risk ratios (RRs), using the Mantel‐Haenszel fixed‐effect model. We also reported the number of people reporting serious adverse events (SAEs).
Main results
We included 75 trials of 45,049 people; 45 were new for this update. We rated 22 at low risk of bias, 18 at high risk, and 35 at unclear risk.
We found moderate‐certainty evidence (limited by heterogeneity) that cytisine helps more people to quit smoking than placebo (RR 1.30, 95% confidence interval (CI) 1.15 to 1.47; I2 = 83%; 4 studies, 4623 participants), and no evidence of a difference in the number reporting SAEs (RR 1.04, 95% CI 0.78 to 1.37; I2 = 0%; 3 studies, 3781 participants; low‐certainty evidence). SAE evidence was limited by imprecision. We found no data on neuropsychiatric or cardiac SAEs.
We found high‐certainty evidence that varenicline helps more people to quit than placebo (RR 2.32, 95% CI 2.15 to 2.51; I2 = 60%, 41 studies, 17,395 participants), and moderate‐certainty evidence that people taking varenicline are more likely to report SAEs than those not taking it (RR 1.23, 95% CI 1.01 to 1.48; I2 = 0%; 26 studies, 14,356 participants). While point estimates suggested increased risk of cardiac SAEs (RR 1.20, 95% CI 0.79 to 1.84; I2 = 0%; 18 studies, 7151 participants; low‐certainty evidence), and decreased risk of neuropsychiatric SAEs (RR 0.89, 95% CI 0.61 to 1.29; I2 = 0%; 22 studies, 7846 participants; low‐certainty evidence), in both cases evidence was limited by imprecision, and confidence intervals were compatible with both benefit and harm.
Pooled results from studies that randomised people to receive cytisine or varenicline showed that more people in the varenicline arm quit smoking (RR 0.83, 95% CI 0.66 to 1.05; I2 = 0%; 2 studies, 2131 participants; moderate‐certainty evidence) and reported SAEs (RR 0.67, 95% CI 0.44 to 1.03; I2 = 45%; 2 studies, 2017 participants; low‐certainty evidence). However, the evidence was limited by imprecision, and confidence intervals incorporated the potential for benefit from either cytisine or varenicline. We found no data on neuropsychiatric or cardiac SAEs.
We found high‐certainty evidence that varenicline helps more people to quit than bupropion (RR 1.36, 95% CI 1.25 to 1.49; I2 = 0%; 9 studies, 7560 participants), and no clear evidence of difference in rates of SAEs (RR 0.89, 95% CI 0.61 to 1.31; I2 = 0%; 5 studies, 5317 participants), neuropsychiatric SAEs (RR 1.05, 95% CI 0.16 to 7.04; I2 = 10%; 2 studies, 866 participants), or cardiac SAEs (RR 3.17, 95% CI 0.33 to 30.18; I2 = 0%; 2 studies, 866 participants). Evidence of harms was of low certainty, limited by imprecision.
We found high‐certainty evidence that varenicline helps more people to quit than a single form of nicotine replacement therapy (NRT) (RR 1.25, 95% CI 1.14 to 1.37; I2 = 28%; 11 studies, 7572 participants), and low‐certainty evidence, limited by imprecision, of fewer reported SAEs (RR 0.70, 95% CI 0.50 to 0.99; I2 = 24%; 6 studies, 6535 participants). We found no data on neuropsychiatric or cardiac SAEs.
We found no clear evidence of a difference in quit rates between varenicline and dual‐form NRT (RR 1.02, 95% CI 0.87 to 1.20; I2 = 0%; 5 studies, 2344 participants; low‐certainty evidence, downgraded because of imprecision). While pooled point estimates suggested increased risk of SAEs (RR 2.15, 95% CI 0.49 to 9.46; I2 = 0%; 4 studies, 1852 participants) and neuropsychiatric SAEs (RR 4.69, 95% CI 0.23 to 96.50; I2 not estimable as events only in 1 study; 2 studies, 764 participants), and reduced risk of cardiac SAEs (RR 0.32, 95% CI 0.01 to 7.88; I2 not estimable as events only in 1 study; 2 studies, 819 participants), in all three cases evidence was of low certainty and confidence intervals were very wide, encompassing both substantial harm and benefit.
Authors' conclusions
Cytisine and varenicline both help more people to quit smoking than placebo or no medication. Varenicline is more effective at helping people to quit smoking than bupropion, or a single form of NRT, and may be as or more effective than dual‐form NRT. People taking varenicline are probably more likely to experience SAEs than those not taking it, and while there may be increased risk of cardiac SAEs and decreased risk of neuropsychiatric SAEs, evidence was compatible with both benefit and harm. Cytisine may lead to fewer people reporting SAEs than varenicline. Based on studies that directly compared cytisine and varenicline, there may be a benefit from varenicline for quitting smoking, however further evidence could strengthen this finding or demonstrate a benefit from cytisine.
Future trials should test the effectiveness and safety of cytisine compared with varenicline and other pharmacotherapies, and should also test variations in dose and duration. There is limited benefit to be gained from more trials testing the effect of standard‐dose varenicline compared with placebo for smoking cessation. Further trials on varenicline should test variations in dose and duration, and compare varenicline with e‐cigarettes for smoking cessation.
Plain language summary
Can medications like varenicline and cytisine (nicotine receptor partial agonists) help people to stop smoking and do they cause unwanted effects?
Key messages
· Varenicline can help people to stop smoking for at least 6 months. Evidence shows it works better than bupropion and using only one type of nicotine replacement therapy (e.g. only patches). Quit rates might be similar to using more than one type of nicotine replacement therapy at the same time (e.g. patches and gum together).
· Cytisine can help people to stop smoking for at least 6 months. It may work as well as varenicline, but future evidence may show that while it helps, it is not quite as helpful as varenicline.
· Future studies should test the effectiveness and safety of cytisine compared with varenicline and other stop‐smoking medications, and should also investigate giving cytisine or varenicline at different doses and for different lengths of time.
What are 'nicotine receptor partial agonists'?
Smoking tobacco is extremely bad for people’s health. For people who smoke, quitting is the best thing they can do to improve their health. Many people find it difficult to quit smoking. Nicotine receptor partial agonists (NRPAs) are a type of medication used to help people to stop smoking. They help to reduce the withdrawal symptoms people experience when they stop smoking, like cravings and unpleasant mood changes. They also reduce the pleasure people usually experience when they smoke. The most widely‐available treatment in this drug type is varenicline. Cytisine is another, similar medication. They may cause unwanted effects such as feeling sick (nausea) and other stomach problems, difficulties sleeping, abnormal dreams, and headache. They may also lead to potentially serious unwanted effects, such as suicidal thoughts, heart problems and raised blood pressure.
What did we want to find out?
We wanted to find out if using NRPAs can help people to quit smoking, and if they cause unwanted effects. We wanted to know:
· how many people stopped smoking for at least 6 months; and
· how many people had unwanted effects.
What did we do?
We searched for studies that investigated NRPAs used to help people quit smoking. People in the studies had to be chosen at random to receive an NRPA, or another NRPA, placebo (medication like the NRPA but with no active ingredients) or no treatment. They had to be adult tobacco smokers who wanted to stop smoking.
What did we find?
We found 75 studies that compared NRPAs with:
· placebo or no medicine;
· nicotine replacement therapy, such as patches or gum;
· bupropion (another medicine to help people stop smoking);
· another NRPA;
· e‐cigarettes.
The USA hosted the most studies (28 studies). Other studies took place in a range of countries across the world, some in several countries.
Main results
People are more likely to stop smoking for at least six months using varenicline than using placebo (41 studies, 17,395 people), bupropion (9 studies, 7560 people), or just one type of nicotine replacement therapy, like patches alone (11 studies, 7572 people). They may be just as likely to quit as people using two or more kinds of nicotine replacement therapy, like patches and gum together (5 studies, 2344 people).
Cytisine probably helps more people to stop smoking than placebo (4 studies, 4623 people) and based on studies that compared cytisine with varenicline (2 studies, 2131 people), there may be a benefit from varenicline for quitting smoking, however further evidence could strengthen this finding or show a benefit from cytisine.
For every 100 people using varenicline to stop smoking, 21 to 25 might successfully stop, compared with only 18 of 100 people using bupropion, 18 of 100 people using a single form of nicotine‐replacement therapy, and 20 of 100 using two or more kinds of nicotine‐replacement therapy. For every 100 people using cytisine to stop smoking, 18 to 23 might successfully stop.
The most common unwanted effect of varenicline is nausea, but this is mostly at mild or moderate levels and usually clears over time. People taking varenicline likely have an increased chance of a more serious unwanted effect that could result in going to hospital, however these are still rare (2.7% to 4% of people on varenicline, compared with 2.7% of people without) and may include many that are unrelated to varenicline. People taking cytisine may also have a slightly increased chance of serious unwanted effects compared with people not taking it, but this may be less likely compared with varenicline.
What are the limitations of the evidence?
The evidence for some of our results is very reliable. We’re very confident that varenicline helps people to quit smoking better than many alternatives. We’re less sure of some other results because fewer or smaller studies provided evidence.
Several results suggest one treatment is better or less harmful than another, but the opposite could still be true.
How up to date is the evidence?
The evidence is up to date to 29 April 2022.
Summary of findings
Summary of findings 1. Varenicline versus placebo or no medication for smoking cessation.
| Varenicline versus placebo or no medication for smoking cessation | ||||||
| Patient or population: people who smoke tobacco Setting: smoking cessation clinics, hospitals, universities and other research centres Intervention: varenicline Comparison: placebo or no medication | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo or no medication | Corresponding risk with varenicline | |||||
|
Smoking abstinence at longest follow‐up (6+ months) (varenicline vs placebo) |
99 per 1000 |
230 per 1000 (213 to 249) |
RR 2.32 (2.15 to 2.51) |
17,395 (41 studies) | ⊕⊕⊕⊕a High |
|
|
SAEs (varenicline vs placebo or no medication) |
27 per 1000 |
33 per 1000 (27 to 40) |
RR 1.23 (1.01 to 1.48) |
14,356 (26 studies) | ⊕⊕⊕⊝b Moderate |
|
|
Neuropsychiatric SAEs (varenicline vs placebo or no medication) |
11 per 1000 |
10 per 1000 (7 to 14) |
RR 0.89 (0.61 to 1.29) |
7846 (22 studies) | ⊕⊕⊝⊝c Low |
|
|
Cardiac SAEs (varenicline vs placebo or no medication) |
11 per 1000 |
13 per 1000 (8 to 20) |
RR 1.20 (0.79 to 1.84) |
7151 (18 studies) | ⊕⊕⊝⊝c Low |
|
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). The assumed risk in the comparison group is calculated as the median risk in control groups. CI: confidence interval; RR: risk ratio; SAE: serious adverse event | ||||||
|
GRADE Working Group grades of evidence
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aModerate heterogeneity detected, however all but three studies showed positive effect of varenicline, so we did not downgrade on this basis.
bDowngraded one level because of imprecision: CI incorporates no clinical difference as well as clinically significant harm.
cDowngraded two levels because of imprecision: CI incorporates clinically significant benefit and clinically significant harm.
Summary of findings 2. Cytisine versus placebo or no medication for smoking cessation.
| Cytisine versus placebo or no medication for smoking cessation | ||||||
| Patient or population: people who smoke tobacco Setting: smoking cessation clinics, hospitals, universities and other research centres Intervention: cytisine Comparison: placebo or no medication | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo or no medication | Corresponding risk with cytisine | |||||
|
Smoking abstinence at longest follow‐up (6+ months) (cytisine vs placebo) |
158 per 1000 |
205 per 1000 (181 to 232) |
RR 1.30 (1.15 to 1.47) |
4623 (4 studies) | ⊕⊕⊕⊝a Moderate |
|
|
SAEs (cytisine vs placebo or no medication) |
46 per 1000 |
48 per 1000 (36 to 63) |
RR 1.04 (0.78 to 1.37) |
3781 (3 studies) | ⊕⊕⊝⊝b Low |
|
|
Neuropsychiatric SAEs (cytisine vs placebo or no medication) |
No data | No data | No data | No data | No data | |
|
Cardiac SAEs (cytisine vs placebo or no medication) |
No data | No data | No data | No data | No data | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). The assumed risk in the comparison group is calculated as the median risk in control groups. CI: confidence interval; RR: risk ratio; SAE: serious adverse event | ||||||
|
GRADE Working Group grades of evidence
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level because of heterogeneity: I2 = 83%. bDowngraded two levels because of imprecision: CI incorporates clinically significant benefit and clinically significant harm.
Summary of findings 3. Cytisine versus varenicline for smoking cessation.
| Cytisine versus varenicline for smoking cessation | ||||||
| Patient or population: people who smoke tobacco Setting: community, community pharmacy, participants' homes Intervention: cytisine Comparison: varenicline | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with varenicline | Corresponding risk with cytisine | |||||
|
Smoking abstinence at longest follow‐up (6+ months) |
132 per 1000 |
109 per 1000 (87 to 138) |
RR 0.83 (0.66 to 1.05) |
2131 (2 studies) | ⊕⊕⊕⊝a Moderate |
|
|
SAEs |
49 per 1000 |
33 per 1000 (21 to 50) |
RR 0.67 (0.44 to 1.03) |
2017 (2 studies) | ⊕⊕⊝⊝b Low |
|
|
Neuropsychiatric SAEs |
No data | No data | No data | No data | No data | |
|
Cardiac SAEs |
No data | No data | No data | No data | No data | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). The assumed risk in the comparison group is calculated as the median risk in control groups. CI: confidence interval; RR: risk ratio; SAE: serious adverse event | ||||||
|
GRADE Working Group grades of evidence
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level because of imprecision: CI incorporates no difference as well as clinically significant harm. bDowngraded two level because of imprecision: CI incorporates no difference as well as clinically significant benefit, and number of events in analysis very low (n = 82).
Summary of findings 4. Cytisine versus nicotine replacement therapy for smoking cessation.
| Cytisine versus nicotine replacement therapy for smoking cessation | ||||||
| Patient or population: people who smoke tobacco Setting: participants' homes (participants were callers to a national Quitline) Intervention: cytisine Comparison: nicotine replacement therapy (NRT) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with NRT | Corresponding risk with cytisine | |||||
|
Smoking abstinence at longest follow‐up (6+ months) |
153 per 1000 |
218 per 1000 (173 to 275) |
RR 1.43 (1.13 to 1.80) |
1310 (1 study) | ⊕⊕⊝⊝a,b Low |
|
|
SAEs |
60 per 1000 |
68 per 1000 (45 to 104) |
RR 1.15 (0.76 to 1.75) |
1310 (1 study) | ⊕⊝⊝⊝a,c Very low |
|
|
Neuropsychiatric SAEs |
No data | No data | No data | No data | No data | |
|
Cardiac SAEs |
No data | No data | No data | No data | No data | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). The assumed risk in the comparison group is calculated as the median risk in control groups. CI: confidence interval; NRT: nicotine replacement therapy; RR: risk ratio; SAE: serious adverse event | ||||||
|
GRADE Working Group grades of evidence
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level because of risk of bias: sole study at high risk. bDowngraded one level because of imprecision: fewer than 300 events in the analysis. cDowngraded two levels because of imprecision: CI incorporates clinically significant benefit and clinically significant harm.
Summary of findings 5. Varenicline versus bupropion for smoking cessation.
| Varenicline versus bupropion for smoking cessation | ||||||
| Patient or population: people who smoke tobacco Setting: smoking cessation clinics, hospitals, universities and other research centres Intervention: varenicline Comparison: bupropion | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with bupropion | Corresponding risk with varenicline | |||||
|
Smoking abstinence at longest follow‐up (6+ months) |
177 per 1000 |
241 per 1000 (222 to 264) |
RR 1.36 (1.25 to 1.49) |
7560 (9 studies) | ⊕⊕⊕⊕ High |
|
|
SAEs |
20 per 1000 |
18 per 1000 (12 to 27) |
RR 0.89 (0.61 to 1.31) |
5317 (5 studies) | ⊕⊕⊝⊝a Low |
|
|
Neuropsychiatric SAEs |
2 per 1000 |
2 per 1000 (0 to 16) |
RR 1.05 (0.16 to 7.04) |
866 (2 studies) | ⊕⊕⊝⊝a Low |
|
|
Cardiac SAEs |
0 per 1000 |
0 per 1000 (0 to 0) |
RR 3.17 (0.33 to 30.18) |
866 (2 studies) | ⊕⊕⊝⊝a Low |
|
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). The assumed risk in the comparison group is calculated as the median risk in control groups. CI: confidence interval; RR: risk ratio; SAE: serious adverse event | ||||||
|
GRADE Working Group grades of evidence
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels because of imprecision: CI incorporates clinically significant benefit and clinically significant harm.
Summary of findings 6. Varenicline versus nicotine replacement therapy monotherapy for smoking cessation.
| Varenicline versus nicotine replacement therapy (NRT) monotherapy for smoking cessation | ||||||
| Patient or population: people who smoke tobacco Setting: smoking cessation clinics, hospitals, universities and other research centres Intervention: varenicline Comparison: nicotine replacement therapy (NRT) monotherapy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with NRT monotherapy | Corresponding risk with varenicline | |||||
|
Smoking abstinence at longest follow‐up (6+ months) |
180 per 1000 |
225 per 1000 (205 to 247) |
RR 1.25 (1.14 to 1.37) |
7572 (11 studies) | ⊕⊕⊕⊕ High |
|
|
SAEs |
9 per 1000 |
6 per 1000 (5 to 9) |
RR 0.70 (0.50 to 0.99) |
6535 (6 studies) | ⊕⊕⊝⊝a Low |
No events in two studies |
|
Neuropsychiatric SAEs |
Not estimable (no events in analysis) |
Not estimable (no events in analysis) |
Not estimable (no events in analysis) |
137 (1 study) | ||
|
Cardiac SAEs |
Not estimable (no events in analysis) |
Not estimable (no events in analysis) |
Not estimable (no events in analysis) |
137 (1 study) | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). The assumed risk in the comparison group is calculated as the median risk in control groups. CI: confidence interval; NRT: nicotine replacement therapy; RR: risk ratio; SAE: serious adverse event | ||||||
|
GRADE Working Group grades of evidence
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels because of imprecision: fewer than 150 events in the analysis.
Summary of findings 7. Varenicline versus combination nicotine replacement therapy for smoking cessation.
| Varenicline versus combination nicotine replacement therapy for smoking cessation | ||||||
| Patient or population: people who smoke tobacco Setting: smoking cessation clinics, hospitals, universities and other research centres Intervention: varenicline Comparison: combination nicotine replacement therapy (NRT) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with combination NRT | Corresponding risk with varenicline | |||||
|
Smoking abstinence at longest follow‐up (6+ months) |
195 per 1000 |
199 per 1000 (170 to 234) |
RR 1.02 (0.87 to 1.20) |
2344 (5 studies) | ⊕⊕⊝⊝a Low |
|
|
SAEs |
2 per 1000 |
5 per 1000 (1 to 20) |
RR 2.15 (0.49 to 9.46) |
1852 (4 studies) | ⊕⊕⊝⊝b Low |
|
|
Neuropsychiatric SAEs |
0 per 1000 |
0 per 1000 (0 to 0) |
RR 4.69 (0.23 to 96.50) |
764 (2 studies) | ⊕⊕⊝⊝b Low |
Only one study reported any events |
|
Cardiac SAEs |
2 per 1000 |
1 per 1000 (0 to 19) |
RR 0.32 (0.01 to 7.88) |
819 (2 studies) | ⊕⊕⊝⊝b Low |
Only one study reported any events |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). The assumed risk in the comparison group is calculated as the median risk in control groups. CI: confidence interval; NRT: nicotine replacement therapy; RR: risk ratio; SAE: serious adverse event | ||||||
|
GRADE Working Group grades of evidence
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels because of imprecision: confidence intervals include the potential for clinically significant benefit from either intervention. bDowngraded two levels because of imprecision: CI incorporates clinically significant benefit and clinically significant harm.
Summary of findings 8. Varenicline versus e‐cigarettes for smoking cessation.
| Varenicline versus e‐cigarettes for smoking cessation | ||||||
| Patient or population: people who continued to smoke tobacco following acute coronary syndrome Setting: hospital Intervention: varenicline Comparison: e‐cigarettes | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with e‐cigarettes | Corresponding risk with varenicline | |||||
|
Smoking abstinence at longest follow‐up (6+ months) |
148 per 1000 | 481 per 1000 (179 to 1000) |
RR 3.25 (1.21 to 8.71) |
54 (1 study) | ⊕⊝⊝⊝a,b Very low |
|
|
SAEs |
Not estimable (no events in analysis) |
Not estimable (no events in analysis) |
Not estimable (no events in analysis) |
54 (1 study) | ||
|
Neuropsychiatric SAEs |
Not estimable (no events in analysis) |
Not estimable (no events in analysis) |
Not estimable (no events in analysis) |
54 (1 study) | ||
|
Cardiac SAEs |
Not estimable (no events in analysis) |
Not estimable (no events in analysis) |
Not estimable (no events in analysis) |
54 (1 study) | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). The assumed risk in the comparison group is calculated as the median risk in control groups. CI: confidence interval; RR: risk ratio; SAE: serious adverse event | ||||||
|
GRADE Working Group grades of evidence
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels because of imprecision: fewer than 150 events in the analysis. bDowngraded one level because of risk of bias: sole study at high risk.
Background
Description of the condition
Smoking is the main preventable cause of morbidity and premature death worldwide, killing more than 8 million people each year (WHO 2022). It is also a leading cause of health inequalities (ASH 2019). Quitting tobacco smoking significantly reduces risk of tobacco‐related disease and death (USDHHS 2020). There are a range of interventions available to help people quit smoking, including different kinds of behavioural and pharmacological support, but even with the most effective interventions, long‐term quit rates remain relatively low (Livingstone‐Banks 2022a; Rigotti 2022).
Description of the intervention and how it might work
Nicotine receptor partial agonists are a family of drugs that aim to mitigate the addictiveness of tobacco by binding to the α4β2 nicotinic acetylcholine receptor (the receptor that mediates nicotine dependence through released dopamine). When bound to the receptor, a partial agonist prompts the receptor to release dopamine in the way nicotine would, and prevents nicotine from tobacco from binding to the receptor. This reduces nicotine withdrawal symptoms and reduces the rewarding effects of tobacco. There are two main nicotine receptor partial agonists: varenicline and cytisine. A third drug, dianicline, was developed but unfavourable results led to its withdrawal from further development (Kirchhoff 2009).
Varenicline was developed by Pfizer Inc in 1997 (Coe 2005), and was approved as a prescription‐only aid to smoking cessation in 2006 by the American Food and Drug Administration under the trade name Chantix, and by the European Medicines Evaluation Agency under the trade name Champix. In July 2007 it was approved by the National Institute for Health and Clinical Excellence (NICE) for prescribing by the UK National Health Service (ASH 2006; NICE 2007). In 2021, the World Health Organization added varenicline to its Essential Medicines List (WHO 2021). Post‐marketing surveillance raised subsequent concerns about possible links between varenicline and major health risks, including suicidal ideation and behaviour, depression, and serious adverse cardiovascular events (FDA 2008), which led to an FDA warning label in 2009. This warning was removed in 2016 after a large trial found no evidence to support the concerns (EAGLES 2016). In 2021, Pfizer announced a recall of varenicline because it exceeded acceptable intake limits of a nitrosamine impurity, called N‐nitroso‐varenicline. While this is believed to only be temporary, it has led to shortages at the time of writing.
Cytisine was developed in Bulgaria in the 1960s, and is less widely available than varenicline (Foulds 2004; Tutka 2005; Tutka 2006). Its original manufacturer, Sopharma Pharmaceuticals, developed their phytoproduct from the plant Cytisus Laburnum L. (Golden Rain). Although cytisine is not licensed and available for use as a smoking cessation aid across most countries outside Eastern Europe, it works by the same mechanism as varenicline and it is available for substantially less cost (Tutka 2019; Gotti 2021). An important difference between the treatments is that standard treatment with cytisine lasts 25 days, compared with 12 weeks for varenicline.
Why it is important to do this review
The scale of the impact on health from tobacco worldwide makes it imperative that we continue to develop our understanding of smoking cessation interventions. While the effectiveness of varenicline for smoking cessation is well established, substantial questions remain about different doses and durations of treatment, and what impact they have on how effective varenicline is at helping people to quit smoking.
Varenicline is a front‐line smoking cessation medication in many countries, and its current shortage poses a substantial challenge for tobacco control strategies around the world. Learning more about how effective and safe cytisine is for smoking cessation may inform decisions about whether to licence it in countries that have historically relied on varenicline.
This is an update of a Cochrane Review first published in 2007, and most recently updated in 2016. The previous update found high‐certainty evidence of a benefit from varenicline, but only included a limited number of studies testing cytisine for smoking cessation (Cahill 2016). New evidence comparing cytisine with placebo and with varenicline warranted an update of this review.
Objectives
To assess the effectiveness of nicotine receptor partial agonists, including varenicline and cytisine, for smoking cessation.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and cluster‐RCTs. We did not include quasi‐randomised studies, in which the allocation sequence is not truly random, for example, studies where participant date of birth determines participant allocation.
Types of participants
We included studies that recruited adult tobacco smokers. Studies testing nicotine receptor partial agonists to help smokeless tobacco users to quit, or as a relapse prevention intervention among people who are already abstinent from smoking tobacco, are covered in separate Cochrane Reviews (Ebbert 2011a; Livingstone‐Banks 2019; Livingstone‐Banks 2022b).
Types of interventions
Selective nicotine receptor partial agonists, including cytisine, dianicline and varenicline (or any other in this class of drug as they reach Phase 3 trial stage), compared with placebo, no medication, or another smoking cessation pharmacotherapy (including nicotine replacement therapy, bupropion, electronic cigarettes, and other nicotine receptor partial agonists). We also included studies that compared different doses and regimes of eligible treatments. Lobeline is covered in an earlier Cochrane Review (Stead 2003). We only included studies that tested the effect of nicotine receptor partial agonists for smoking cessation and not studies focused on harm reduction, which is covered in a separate Cochrane Review (Lindson‐Hawley 2016).
Types of outcome measures
Primary outcomes
Abstinence from smoked tobacco at longest follow‐up, at least six months from study baseline. We used the strictest definition of abstinence reported in each study (e.g. prolonged or continuous over point prevalence), and where available, we favoured biochemically validated over self‐reported abstinence. We only included studies that measured abstinence from tobacco smoking at six months or longer from baseline.
Number of participants who experienced the following adverse events: nausea, insomnia, abnormal dreams, headache, depression, and suicidal ideation
Number of participants who experienced serious adverse events as defined by the authors of included studies
Number of participants who experienced neuropsychiatric serious adverse events
Number of participants who experienced cardiac serious adverse events.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Tobacco Addiction Group's Specialised Register for studies, using relevant terms (e.g. 'cytisine' or 'Tabex' or 'dianicline' or 'varenicline' or 'nicotine receptor partial agonist') in the title or abstract, or as keywords. This Register has been developed from electronic searching of the Cochrane Central Register of Controlled trials (CENTRAL), MEDLINE, Embase, and PsycINFO, together with handsearching of specialist journals, conference proceedings and reference lists of previous trials and overviews. The most recent search of the Register was on 29 April 2022, and included reports of trials indexed in CENTRAL, 2022, Issue 3; MEDLINE (via OVID) to update 20220405; Embase (via OVID) to week 202214; PsycINFO (via OVID) to update 20220404, all from inception. See the Cochrane Tobacco Addiction Group Website for details of the search strategies for these databases. The search strategy for this specific review is listed in Appendix 1. We did not place any limits on our searches (e.g. by language, year of publication, or publication format).
Searching other resources
Our search of the Cochrane Tobacco Addiction Group Specialised Register also covered ongoing and unpublished trials included in the following databases, as these are indexed in CENTRAL.
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov, searched via CENTRAL); and
World Health Organization (WHO) International Clinical Trials Registry Platform (apps.who.int/trialsearch, searched via CENTRAL).
We also checked the reference lists of included studies for potentially eligible trials.
Data collection and analysis
Selection of studies
For this update we screened the search results in two stages using the software Covidence. Two review authors (of JL‐B, AT, AH and NL) independently screened the title and abstract of each study found in our searches. We then reviewed the full text of all potentially eligible reports in duplicate. At each stage, we resolved any disagreement through discussion and if needed by referring to a third review author. We noted the reasons for study exclusion at full‐text stage for our PRISMA diagram illustrating the flow of studies (Liberati 2009).
Data extraction and management
Two review authors (of JL‐B, AT, AH, LH, TRF, and KT) independently extracted the following information about each included study in duplicate, using a prepiloted data extraction form. We resolved disagreement through discussion and if needed by referring to a third review author.
Country and setting (e.g. primary care, community, hospital outpatient/inpatient)
Method of recruiting participants
Definition of smoker used
Methods of randomisation and allocation, and blinding of study personnel, participants and assessors
Demographic characteristics of participants (e.g. average age, sex, average cigarettes per day)
Intervention and control description (dose, provider, duration, number of visits, etc.)
Outcomes including definition of abstinence used, and biochemical validation of cessation
Proportion of participants with follow‐up data
Any adverse events
Declarations of interest and sources of study funding
Assessment of risk of bias in included studies
We assessed each included study using Cochrane's RoB 1 tool for the following domains of risk (Higgins 2011).
Random sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding of participants and study personnel and blinding/objectivity of outcome assessment (performance bias and detection bias)
Incomplete outcome data (attrition bias)
Selective reporting (reporting bias)
Other potential risks of bias. For this domain, we assessed and reported any forms of bias present in studies that did not fall under one of the above domains. Where no relevant form of other potential bias was found, we left this field blank.
Two review authors (of JL‐B, AT, AH, LH, TRF, and KT) independently judged each study as at low, unclear, or high risk of bias for each domain, justifying judgements using information from the study report. We resolved disagreements in judgements through discussion and by referral to a third review author where needed.
Measures of treatment effect
We present estimates of effects for individual studies using risk ratios (RRs), calculated as ((number of events in intervention condition/intervention denominator)/(number of events in control condition/control denominator)), with a 95% confidence interval (CI). An RR greater than one indicates a higher rate of outcome (either smoking abstinence or adverse events) in the intervention group than in the control group.
Unit of analysis issues
As cluster‐randomised trials are eligible for inclusion in this review, there is the potential for unit of analysis issues. Where required, we adjusted for clustering using an intraclass correlation, either from the study in question or from a similar study. Where studies compared more than one eligible intervention arm with a non‐intervention control, we either pooled intervention arms together (assuming they did not differ in pharmacotherapy given) or added them separately to the meta‐analysis and split the control group data evenly between them, to avoid double‐counting any participants in the analysis.
Dealing with missing data
We conducted our analyses on an intention‐to‐treat basis, including all participants in the study arms to which they were randomised, regardless of whether they received the intervention. We counted participants lost to follow‐up as continuing smoking, which is standard in the field (West 2005). Where study reports lacked the information needed for our analyses, we tried to contact study authors to ask for this information. Attempts to contact study authors are recorded in the Characteristics of included studies tables.
Assessment of heterogeneity
To investigate heterogeneity, we used the I2 statistic, given by the formula [(Q ‐ df)/Q] × 100%, where Q is the Chi2 statistic and df is its degrees of freedom (Higgins 2003). This describes the percentage of variability in effect estimates that is due to heterogeneity rather than to sampling error (chance). We interpreted the I2 result using the following overlapping bands (Deeks 2022):
0% to 40%: might not be important
30% to 60%: may represent moderate heterogeneity
50% to 90%: may represent substantial heterogeneity
75% to 100%: considerable heterogeneity
Where we found moderate to substantial heterogeneity, we investigated further using subgroup analyses based on study characteristics decided upon through review author consensus. In the event of considerable unexplained statistical heterogeneity (i.e. I2 =75% or over), we evaluated whether it was still appropriate to report a pooled result (Deeks 2022).
Assessment of reporting biases
For the two smoking cessation comparisons with 10 or more studies, we assessed the risk of reporting bias using a funnel plot. Regardless of the number of studies included, we considered the possibility of reporting bias in our discussion.
Data synthesis
We conducted a narrative summary of the included studies and, where more than one study reported an outcome comparing an eligible intervention with placebo, no medication, another eligible intervention, or the same intervention delivered with a different dose or regime, we conducted meta‐analyses to pool data from sufficiently similar studies using a Mantel‐Haenszel fixed‐effect model to calculate pooled RRs with 95% CIs.
Subgroup analysis and investigation of heterogeneity
Where studies compared an intervention pharmacotherapy with either placebo or no medication and there was substantial heterogeneity, we considered subgrouping analyses based on comparator and using the I2 statistic to test for difference between subgroups and decide whether to report an overall pooling or by subgroup only.
Sensitivity analysis
We conducted sensitivity analyses testing the effect of removing studies we judged to be at high risk of bias to see if those studies affected the overall result. In analyses where we pooled studies that compared an intervention with either placebo or no medication but did not subgroup, we conducted sensitivity analyses testing removing studies comparing against no medication. In our comparison of varenicline versus placebo we also conducted a further ad hoc sensitivity analysis to explore the high level of heterogeneity, removing studies that used an extended treatment course of 24 or 52 weeks rather than the 12 weeks of the other studies.
Because we were primarily interested in whether there is evidence that varenicline works differently for disease‐specific populations and people in specific subgroups and healthcare settings, we conducted sensitivity analyses, treating studies in these populations and settings as subgroups of the main analyses and using the I2 statistic to test for subgroup differences.
Summary of findings and assessment of the certainty of the evidence
Following standard Cochrane methods, we produced summary of findings tables for smoking abstinence at longest follow‐up and all of our serious adverse events outcomes for each comparison of varenicline or cytisine with placebo or another pharmacotherapy (Schünemann 2022). Two review authors (JLB, NL) assessed the certainty of the evidence using the five GRADE considerations (risk of bias, inconsistency, imprecision, indirectness, and publication bias (Schünemann 2013).
Results
Description of studies
Results of the search
Our literature searches for this update found 810 studies (from 885 records). After we removed duplicates, 682 studies remained for title and abstract screening. We ruled out 544 studies at this stage, leaving 138 studies for full‐text screening. From this, we identified 45 new included studies and 20 new ongoing studies, combined with studies from previous updates of this review, this resulted in a total of 75 included studies of 45,049 people and 28 ongoing studies. See Figure 1 for PRISMA diagram detailing study flow (Liberati 2009). For this update, we contacted authors of four studies and received additional results data.
1.
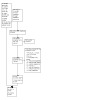
Study flow diagram of searches for 2022 update
For this update, we excluded 14 previously included studies because they focused on relapse prevention (these studies are covered in a separate review; Livingstone‐Banks 2019), or had previously been included for data on harms but did not fully meet our inclusion criteria.
Included studies
Full details of the included studies are given in the Characteristics of included studies tables.
Cytisine
Eight studies in just under 9000 people investigated cytisine as a smoking cessation drug. Four studies compared cytisine with placebo, two with varenicline, one with nicotine replacement therapy (NRT), and one with no medication. Pastorino 2022 also compared longer with shorter duration cytisine. Studies tested cytisine at a dose of 9 mg per day for 20 to 25 days, except for Pastorino 2022, which gave cytisine for 40 and 84 days in different arms.
Studies were conducted in Australia, Bangladesh and Pakistan, Italy, East Germany, Kyrgyzstan, New Zealand, and Poland. Two studies took place in smoking cessation clinics, and two in the community. Walker 2014 recruited people who contacted a national smoking quitline. Vinnikov 2008 was set in a Kyrgyz mining company, and Dogar 2020 took place in tuberculosis treatment centres. Pastorino 2022 recruited heavy smokers participating in a lung‐screening trial.
Varenicline
Sixty‐eight studies of over 37,000 people tested varenicline for smoking cessation. This excludes two studies that compared varenicline with cytisine, which are described above.
Setting
Twenty‐eight studies were conducted in the USA, four in Canada, three in China, three in Japan, two in France, two in Greece, two in India, two in Turkey, one in Australia, one in Iran, one in Denmark, one in Finland, one in Israel, and one in Spain. Fifteen studies took place internationally, in between two and 15 countries. The studies were conducted in smoking cessation clinics, hospitals, universities and other research centres.
Participants
Participants in the majority of trials were adult smokers, willing to make a quit attempt. Several trials were conducted in clinical subgroups, including hospital inpatients (Carson‐Chahhoud 2020; Hong 2015; Le Mao 2020; Steinberg 2011; Windle 2018; Wong 2012), and disease‐specific patient groups: cardiovascular disease (Rigotti 2010; Windle 2018); chronic obstructive pulmonary disease (Hong 2015; Le Mao 2020; Tashkin 2011; Yang 2016); HIV (Ashare 2019; Mercie 2018); asthma (Westergaard 2015); substance use disorder (Nahvi 2014a; Stein 2013); alcohol dependence (Hurt 2018; O'Malley 2018; Zawertailo 2020); depression (Anthenelli 2013; Cinciripini 2018); and bipolar/schizophrenia, schizoaffective disorder (Chengappa 2014; Williams 2012). EAGLES 2016 enrolled two cohorts of adult smokers with and without histories of psychiatric disorders, including primary affective disorders (70%), anxiety disorders (19%), psychotic disorders (9.5%) and personality disorders (0.6%). Gonzales 2014 recruited people who had previously used varenicline in an unsuccessful quit attempt. De Dios 2012 and Ebbert 2016 tested varenicline in light smokers.
Interventions
Forty‐seven trials used the standard 12‐week regimen of varenicline, routinely titrating the first week up to the recommended daily dose of 1 mg twice a day. Nakamura 2007, Nides 2006 and Oncken 2006 tested 1 mg per day, and Niaura 2008 allowed participants to regulate their own dosage throughout the treatment phase. Ebbert 2015 and Stein 2013 tested a 24‐week regimen, and Williams 2007 tested 52 weeks.
Comparators
Forty‐five studies compared varenicline with placebo and five with no medication. Of the 14 studies that compared varenicline with NRT, 12 randomised participants to receive single‐form NRT and five to a combination of two or more forms of NRT (3 studies tested varenicline against both NRT monotherapy and combination NRT). Ten studies compared varenicline with bupropion.
Seven studies compared standard varenicline with either a lower dose (4 studies) or a longer duration (3 studies).
Outcomes
All studies measured smoking cessation at least six months after study baseline. Follow‐up lengths ranged from six months to two years. Many studies biochemically validated abstinence using either exhaled carbon monoxide, or salivary or urinary cotinine.
Thirty‐eight studies measured adverse events, including nausea, insomnia, abnormal dreams, headache, depression, and suicidal ideation. Twenty‐eight measured serious adverse events, neuropsychiatric serious adverse events and cardiac serious adverse events.
Dianicline
One trial investigated dianicline. It was set in 22 sites across six European countries (Tonstad 2011). Dianicline was administered as a 40 mg tablet twice a day for seven weeks, with brief counselling at each contact. Final follow‐up of the participants was at 26 weeks, with self‐reported abstinence verified by expired carbon monoxide and by plasma cotinine samples.
Study funding
Of the trials included in this review, 35 received funds from pharmaceutical companies with interests in the treatment being tested, 13 received free study medications, and four trials without pharmaceutical support had authors who had received funds for other work. Fifteen studies reported no conflicts, and two did not report study funding or author declarations of interests. This is significant because a recent analysis found that authors of opinion pieces on varenicline who reported financial ties to the pharmaceutical industry (as a conflict of interest or funding source) were more likely to minimise the cardiovascular and psychiatric risk of varenicline compared to those without conflicts of interest or industry funding (odds ratio 4.00, 95% CI 1.32 to 12.16 for cardiovascular risk; odds ratio 8.51, 95% CI 3.79 to 19.11 for psychiatric risk; Fabbri 2022).
Excluded studies
We list 95 potentially eligible but ultimately excluded studies, along with reasons for exclusion, in the Characteristics of excluded studies tables. Common reasons for exclusion were following up with participants for less than six months, not randomising participants, testing an eligible intervention for an ineligible purpose (smoking reduction or alcohol dependence), or testing another intervention as an adjunct to an eligible one.
For this update we excluded 14 studies that were included in the previous version of the review. Evins 2014, Tonstad 2006, Tønnesen 2013, and NCT00828113 recruited already abstinent participants and tested varenicline for relapse prevention, a topic covered in a different Cochrane Review (Livingstone‐Banks 2019). We excluded Hajek 2015 because follow‐up was under six months. Brandon 2011, Ebbert 2011b, Faessel 2009, Fagerström 2010, Garza 2011, Hughes 2011, McClure 2013, Meszaros 2013 and Mitchell 2012 were previously included for data on harms only but did not meet all of our prespecified inclusion criteria.
We did not find sufficient information to include or exclude two studies. These are listed in Characteristics of studies awaiting classification.
Ongoing studies
We found 28 eligible ongoing studies, some with multiple relevant comparisons. Studies that tested varenicline compared it with placebo (11 studies), no medication (two studies), bupropion (one study), NRT (seven studies), e‐cigarettes (one study), and different doses or regimes of varenicline (six studies). Studies that tested cytisine compared it with placebo (two studies), varenicline (two studies), NRT (one study), and e‐cigarettes (one study).
Studies were set in various populations and setting, including HIV (three studies), hospital and perioperative patients (three studies), cardiovascular disease (three studies), substance abuse (two studies), mental health (two studies), and single studies in lung cancer, diabetes, chronic obstructive pulmonary disease, adolescents, and e‐cigarette users who smoke.
Further details of the ongoing studies are given in the Characteristics of ongoing studies tables.
Risk of bias in included studies
Overall, we judged 22 studies to be at low risk of bias (low risk of bias across all domains), 18 at high risk of bias (high risk of bias in at least one domain), and the remaining 35 at unclear risk of bias. Our judgements on the risks of bias of all the included studies are summarised in Figure 2 and Figure 3, and reasons for the judgements are detailed in the Characteristics of included studies tables.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
Allocation
We separately assessed allocation bias resulting from randomisation sequence generation and from allocation concealment. We judged 29 studies to be at unclear risk of allocation bias as a result of insufficient information about randomisation sequence generation. The remaining studies were judged at low risk, with none deemed to be at high risk.
We judged one study to be at high risk of allocation bias as a result of inadequate concealment of randomisation because that study's participant allocation was unblinded. We judged 34 studies to be at unclear risk because there was insufficient information to make a judgement. We judged the remaining studies to be at low risk.
Blinding
We judged 10 studies to be at high risk of performance or detection bias because they were open‐label studies without blinding of participant allocation. We judged 13 studies to be at unclear risk because there was insufficient information to make a judgement. The remaining studies were deemed at low risk.
Incomplete outcome data
We judged five studies to be at high risk of attrition bias, four because of either high levels of attrition or highly differential attrition rates between study arms, and one that did not provide a baseline number of participants, and reported only those followed up at 12 months as a denominator (Benli 2017). We judged 20 studies to be at unclear risk because of insufficient reporting of follow‐up rates for us to make a judgement. The remaining studies were deemed at low risk.
Selective reporting
We judged two studies to be at high risk of reporting bias. In Nakamura 2007, continuous abstinence rates for all participants were reported, but demographics, withdrawal and craving measures, and point‐prevalence abstinence were reported for the nicotine‐dependent subset of participants only. The trial registry entry for Tuisku 2016 planned a 12‐month follow‐up, which was not reported in their results paper. However, it is possible that this may be reported in a subsequent paper. We judged 18 studies to be at unclear risk because there was insufficient information to make a judgement; typically because no protocol or trial registry entry was available. We judged the remaining studies to be at low risk.
Other potential sources of bias
We judged two studies to be at high risk of bias for other reasons. For Ioakeimidis 2018, we found only an abstract and poster, which reported different quit rates in the e‐cigarette arm. Zincir 2013 reported that no participants experienced adverse events, which is unlikely given standard definitions of adverse events.
We judged five studies to be at unclear risk of bias from other sources. In Rose 2013, there was a minor unexplained reporting disparity, with different denominators given for the varenicline arm. Walker 2014 supplied cytisine for free, while NRT users had to pay a nominal charge (NZD 3 for an 8‐week course of each NRT item). Le Mao 2020 reported that their small sample size was because of premature interruption of pharmaceutical funding. Cinciripini 2013 began comparing nortriptyline with bupropion, but after three months nortriptyline was changed to varenicline. In Chengappa 2014, four participants in each arm received bupropion for depression. Three out of 15 varenicline quitters and one out of three placebo quitters were on long‐term bupropion.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8
See summary of findings tables (Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8).
Cytisine versus placebo or no medication
Smoking cessation
We pooled five studies that compared cytisine to either placebo or no medication, subgrouping by comparator type (placebo versus no medication). We found evidence of a substantial subgroup difference (I2 = 97.3%; Analysis 1.1) and so present the subgroup effects separately here. Four studies, including 4623 participants, compared standard dose cytisine (9 mg per day) with placebo. More people successfully quit smoking in the cytisine arm (RR 1.30, 95% CI 1.15 to 1.47; moderate‐certainty evidence; Analysis 1.1.1), but there was a high level of heterogeneity (I2 = 83%). We present the pooled estimate despite this heterogeneity as all point estimates suggested a benefit from cytisine. Pastorino 2022 compared standard‐dose cytisine with no medication. This three‐arm trial tested different durations of cytisine (40 days and 84 days), so we split the control arm. More people randomised to receive cytisine successfully quit than in the no‐medication arm (RR 4.44, 95% CI 3.06 to 6.46; I2 = 0%; 869 participants; Analysis 1.1.2).
1.1. Analysis.

Comparison 1: Cytisine vs placebo or no medication, Outcome 1: Abstinence at longest follow‐up
We were unable to conduct our planned sensitivity analysis removing studies at high risk of bias because we judged all studies comparing with placebo to be at low or unclear risk and the sole study comparing with no medication to be at high risk.
Adverse events
None of the studies in this comparison measured our prespecified adverse events outcomes, so we pooled all non‐serious adverse events. Results from four studies of 4052 participants showed that more people randomised to receive cytisine reported experiencing non‐serious adverse events than those randomised to receive placebo or no medication (RR 1.22, 95% CI 1.07 to 1.39; I2 = 0%; Analysis 1.2). However, a sensitivity analysis removing one study comparing cytisine with no medication resulted in a confidence interval that crossed the null (RR 1.19, 95% CI 0.97 to 1.46; I2 = 0%; 3 studies, 3183 participants).
1.2. Analysis.

Comparison 1: Cytisine vs placebo or no medication, Outcome 2: Adverse events
Serious adverse events
Results from three studies of 3781 participants comparing cytisine with placebo or no medication showed no evidence of difference in the number who experienced serious adverse events (RR 1.04, 95% CI 0.78 to 1.37; I2 = 0%; low‐certainty evidence; Analysis 1.3). A sensitivity analysis removing one study comparing cytisine with no medication did not affect the interpretation of this result (RR 1.15, 95% CI 0.79 to 1.67; I2 = 0%; 2 studies, 3012 participants). None of the studies measured neuropsychiatric or cardiac serious adverse events.
1.3. Analysis.

Comparison 1: Cytisine vs placebo or no medication, Outcome 3: Serious adverse events
Cytisine: variations in usage
Pastorino 2022 compared 40 days and 84 days of cytisine, and found that more people successfully quit on the longer treatment, although confidence intervals did cross the null, indicating the potential for no difference in the effects (RR 1.28, 95% CI 0.98 to 1.67; 480 participants; Analysis 2.1).
2.1. Analysis.

Comparison 2: Cytisine: longer vs shorter duration, Outcome 1: Abstinence at longest follow‐up
Cytisine versus varenicline
Smoking cessation
Two studies including 2131 people compared standard‐dose cytisine (9 mg per day) with standard‐dose varenicline (2 mg per day). The point estimate showed more people quitting in the varenicline arm, but confidence intervals indicate imprecision and incorporate the potential for no difference or slight benefit from cytisine (RR 0.83, 95% CI 0.66 to 1.05; I2 = 0%; moderate‐certainty evidence; point estimate favours varenicline; Analysis 3.1).
3.1. Analysis.

Comparison 3: Cytisine vs varenicline, Outcome 1: Abstinence at longest follow‐up
Adverse events
Two studies of 2017 participants found that people randomised to receive cytisine were less likely to report experiencing nausea (RR 0.41, 95% CI 0.33 to 0.50; I2 = 0%; Analysis 3.2) and abnormal dreams (RR 0.60, 95% CI 0.50 to 0.73; I2 = 58%; Analysis 3.3) than those in the varenicline arm.
3.2. Analysis.

Comparison 3: Cytisine vs varenicline, Outcome 2: Nausea
3.3. Analysis.

Comparison 3: Cytisine vs varenicline, Outcome 3: Abnormal dreams
The same two studies of 2017 participants, found no evidence of clear differences between the cytisine and varenicline arms in the number of people experiencing insomnia (RR 0.90, 95% CI 0.73 to 1.10; I2 = 68%; Analysis 3.4), headaches (RR 1.02, 95% CI 0.79 to 1.33; I2 = 0%; Analysis 3.5), and suicidal ideation (RR 0.33, 95% CI 0.01 to 8.02; I2 not estimable as events only in 1 study; Analysis 3.7). However, in all cases confidence intervals indicated imprecision, and the potential for more adverse events when using either treatment.
3.4. Analysis.

Comparison 3: Cytisine vs varenicline, Outcome 4: Insomnia
3.5. Analysis.

Comparison 3: Cytisine vs varenicline, Outcome 5: Headache
3.7. Analysis.

Comparison 3: Cytisine vs varenicline, Outcome 7: Suicidal ideation
One study of 679 participants did not find evidence of a clear difference between cytisine and varenicline arms in the number of people experiencing depression (RR 3.04, 95% CI 0.12 to 74.47; Analysis 3.6); however, this result should also be treated with caution because of substantial imprecision.
3.6. Analysis.

Comparison 3: Cytisine vs varenicline, Outcome 6: Depression
Serious adverse events
Two studies of 2017 participants compared the number of people in cytisine and varenicline arms reporting experiencing serious adverse events. The point estimate showed that fewer people in the cytisine arm reported serious adverse events (RR 0.67, 95% CI 0.44 to 1.03; I2 = 45%; low‐certainty evidence; point estimate favours cytisine; Analysis 3.8), but confidence intervals did incorporate the potential for no difference. Neither study measured neuropsychiatric or cardiac serious adverse events.
3.8. Analysis.

Comparison 3: Cytisine vs varenicline, Outcome 8: SAEs
Cytisine versus nicotine replacement therapy
Smoking cessation
Walker 2014 provided participants with cytisine, compared with an eight‐week course of NRT, supplied in the form of vouchers that required redemption by participants. Participants in the cytisine arm also received vouchers for NRT to use after their initial 25‐day course of cytisine, and study authors reported that at week one 26 participants were using NRT obtained through the vouchers; only 19 participants used NRT and cytisine concomitantly. This study found that more people in the cytisine arm successfully quit than in the NRT arm (RR 1.43, 95% CI 1.13 to 1.80; 1310 participants; low‐certainty evidence; Analysis 4.1).
4.1. Analysis.

Comparison 4: Cytisine vs NRT, Outcome 1: Abstinence at longest follow‐up
Adverse events
Nausea was the only one of our adverse event outcomes measured. Walker 2014 reported that, compared with people randomised to receive NRT, those in the cytisine arm were more likely to report experiencing nausea (RR 15.00, 95% CI 3.60 to 62.51; 1310 participants; Analysis 4.2).
4.2. Analysis.

Comparison 4: Cytisine vs NRT, Outcome 2: Nausea
Serious adverse events
Walker 2014 did not find evidence of a difference in the rate of serious adverse events between those randomised to receive cytisine or NRT (RR 1.15, 95% CI 0.76 to 1.75; 1310 participants; very low‐certainty evidence; Analysis 4.3). Walker 2014 did not measure neuropsychiatric or cardiac serious adverse events.
4.3. Analysis.

Comparison 4: Cytisine vs NRT, Outcome 3: SAEs
Varenicline versus placebo or no medication
Smoking cessation
We pooled studies that compared varenicline to either placebo or no medication, subgrouping by comparator type (placebo or no medication). We found evidence of a substantial subgroup difference (I2 = 95.8%; Analysis 5.1) and so present the subgroup effects separately here. Forty‐six studies compared standard‐dose varenicline (2 mg per day) with either placebo or no medication. Our meta‐analysis found that more people successfully quit smoking when randomised to receive varenicline compared with placebo (RR 2.32, 95% CI 2.15 to 2.51; I2 = 60%, 41 studies, 17,395 participants; high‐certainty evidence; Analysis 5.1.1) or with no medication (RR 1.57, 95% CI 1.37 to 1.80; I2 = 95%; 5 studies, 1050 participants; Analysis 5.1.2). Despite the substantial heterogeneity in the latter subgroup we present the pooled estimate as all the individual study point estimates suggested a benefit of varenicline.
5.1. Analysis.

Comparison 5: Varenicline 2 mg per day vs placebo or no medication, Outcome 1: Abstinence at longest follow‐up
A sensitivity analysis removing studies at high risk of bias did not reduce the heterogeneity found in the varenicline versus placebo analysis or the interpretation of the effect. However, removing three studies that used an extended treatment course of 24 or 52 weeks rather than the 12 weeks of the other studies, resulted in a minor impact, reducing the I2 statistic to 53%. We were unable to conduct a sensitivity analysis removing high risk of bias studies for the varenicline versus no medication analysis, as we judged all studies to be at high risk.
King 2022 tested varenicline combined with NRT patch against NRT patch with a varenicline placebo. We did not include this study in our analysis, but it showed no clear evidence of a difference in quit rates as a result of adding varenicline (RR 0.94, 95% CI 0.51 to 1.72; 122 participants). However, confidence intervals incorporated the possibilities of both an increased and a decreased quit rate, as well as no difference.
Adverse events
Studies comparing varenicline with placebo or no medication found that people randomised to receive varenicline were more likely to report experiencing nausea (RR 2.61, 95% CI 2.44 to 2.80; I2 = 79%; 36 studies, 17,080 participants; Analysis 5.2), insomnia (RR 1.37, 95% CI 1.27 to 1.47; I2 = 29%; 35 studies, 16,803 participants; Analysis 5.3), abnormal dreams (RR 1.82, 95% CI 1.67 to 1.97; I2 = 70%; 32 studies, 16,211 participants; Analysis 5.4), and headaches (RR 1.11, 95% CI 1.03 to 1.19; I2 = 30%; 31 studies, 16,326 participants; Analysis 5.5). Statistical heterogeneity was substantial in our analyses for nausea and abnormal dreams, but we decided to present the pooled estimate because the point estimates of individuals studies were almost entirely in the same direction.
5.2. Analysis.

Comparison 5: Varenicline 2 mg per day vs placebo or no medication, Outcome 2: Nausea
5.3. Analysis.

Comparison 5: Varenicline 2 mg per day vs placebo or no medication, Outcome 3: Insomnia
5.4. Analysis.

Comparison 5: Varenicline 2 mg per day vs placebo or no medication, Outcome 4: Abnormal dreams
5.5. Analysis.

Comparison 5: Varenicline 2 mg per day vs placebo or no medication, Outcome 5: Headache
Studies comparing varenicline with placebo or no medication did not find clear evidence of difference in the numbers of participants who reported experiencing depression (RR 1.05, 95% CI 0.91 to 1.20; I2 = 0%; 32 studies, 15,922 participants; Analysis 5.6), and found fewer people reporting suicidal ideation in the varenicline arm (RR 0.69, 95% CI 0.44 to 1.08; I2 = 0%; 22 studies, 12,343 participants; Analysis 5.7). However, confidence intervals indicated imprecision, and included the potential for harm as well as no difference.
5.6. Analysis.

Comparison 5: Varenicline 2 mg per day vs placebo or no medication, Outcome 6: Depression
5.7. Analysis.

Comparison 5: Varenicline 2 mg per day vs placebo or no medication, Outcome 7: Suicidal ideation
We conducted sensitivity analyses removing studies comparing varenicline with no medication rather than placebo, but this had no substantial impact on heterogeneity or results.
Serious adverse event
Serious adverse events
Twenty‐six studies of 14,356 participants found that more people randomised to receive varenicline reported experiencing serious adverse events than those randomised to receive placebo or no medication (RR 1.23, 95% CI 1.01 to 1.48; I2 = 0%; moderate‐certainty evidence; Analysis 5.8). Absolute rates for serious adverse events were 3.3% and 2.7% in varenicline and control arms respectively. King 2022 tested varenicline combined with NRT patch against NRT patch with a varenicline placebo. We did not include this study in our analysis, but it reported two participants with serious adverse events in the varenicline arm and none in the NRT‐alone arm. A sensitivity analysis removing one study comparing varenicline with no medication had no substantial impact on this result.
5.8. Analysis.

Comparison 5: Varenicline 2 mg per day vs placebo or no medication, Outcome 8: SAEs
Neuropsychiatric serious adverse events
The point estimate from pooling 22 studies of 7846 people showed that fewer people reported experiencing neuropsychiatric serious adverse events in the varenicline arm compared with placebo or no medication (RR 0.89, 95% CI 0.61 to 1.29; I2 = 0%; low‐certainty evidence; Analysis 5.9). However confidence intervals demonstrated imprecision, also encompassing the possibility of more neuropsychiatric serious adverse events in the varenicline arm. A sensitivity analysis removing one study comparing varenicline with no medication had no substantial impact on this result.
5.9. Analysis.

Comparison 5: Varenicline 2 mg per day vs placebo or no medication, Outcome 9: Neuropsychiatric SAEs (not deaths)
Cardiac serious adverse events
The point estimate from pooling 18 studies of 7151 people showed that more people reported experiencing cardiac serious adverse events in the varenicline arm compared with placebo or no medication (RR 1.20, 95% CI 0.79 to 1.84; I2 = 0%; low‐certainty evidence; Analysis 5.10). However confidence intervals demonstrated imprecision, also encompassing the possibility of no difference or fewer serious adverse events in the varenicline arm. A sensitivity analysis removing one study comparing varenicline with no medication had no substantial impact on this result.
5.10. Analysis.

Comparison 5: Varenicline 2 mg per day vs placebo or no medication, Outcome 10: Cardiac SAEs, including deaths
Varenicline: variations in usage
Low‐dose varenicline versus placebo
Four studies tested varenicline at doses lower than standard (under 2 mg per day). Three studies tested 1 mg per day compared with placebo and still found that more people quit in the varenicline arm (RR 1.87, 95% CI 1.35 to 2.60; 906 participants; Analysis 6.1.1). There was substantial heterogeneity (I2 = 71%), however in all cases point estimates favoured varenicline.
6.1. Analysis.

Comparison 6: Varenicline: variations in usage, Outcome 1: Low‐dose varenicline vs placebo
Niaura 2008 allowed participants to choose their own dose of varenicline, ranging between 0.5 mg and 2.0 mg daily, and found more people in the varenicline arm quit than in the placebo arm (RR 2.92, 95% CI 1.57 to 5.41; 320 participants; Analysis 6.1.2).
Higher‐dose varenicline versus lower‐dose varenicline
Four studies compared varenicline at 2 mg per day with 1 mg per day and did not provide clear evidence of a difference in how many people quit (RR 1.12, 95% CI 0.97 to 1.30; 1563 participants; I2 = 44%; Analysis 6.2.1); however confidence intervals encompassed potential benefit and a slight disadvantage of the higher dose. Nides 2006 also compared other dosages and also did not find clear evidence of a difference in quit rates among participants randomised to receive 2 mg compared with 0.3 mg per day (RR 1.84 95% CI 0.89 to 3.84; 253 participants; Analysis 6.2.2) or 1 mg compared with 0.3 mg per day (RR 0.71, 95% CI 0.28 to 1.81; 254 participants; Analysis 6.2.3). However, in both cases confidence intervals were wide and may indicate benefit and harm of higher doses.
6.2. Analysis.

Comparison 6: Varenicline: variations in usage, Outcome 2: Higher‐dose varenicline versus lower‐dose varenicline
Longer‐duration varenicline versus standard‐duration varenicline
Three studies tested extended durations of varenicline compared with standard duration of varenicline (12 weeks). We found no clear evidence of a difference as a result of extending varenicline treatment to 24 weeks (RR 0.97, 95% CI 0.77 to 1.23; I2 = 17%; 2 studies, 1458 participants; Analysis 6.3.1) or 52 weeks (RR 1.30, 95% CI 0.70 to 2.43; 1 study; 107 participants; Analysis 6.3.2). However, the confidence intervals indicate imprecision and uncertainty in the point estimates.
6.3. Analysis.

Comparison 6: Varenicline: variations in usage, Outcome 3: Longer vs standard duration varenicline
Six weeks versus one week of varenicline preloading
Bohadana 2020 tested varenicline with a preloading period of six weeks before quit date against the standard one week of preloading and found that more people in the six‐week preloading arm quit than in the one‐week arm (RR 5.60, 95% CI 2.24 to 14.02; 242 participants).
Varenicline in specific patient groups
Studies testing varenicline against placebo or no medication in specific patient populations did not find any clear evidence of varenicline working differently in these groups than in the general population.
Analyses found more people successfully quitting in the varenicline arm than in control in studies of people with cardiovascular disease (RR 1.88, 95% CI 1.44 to 2.47; I2 = 81%; 2 studies, 1006 participants; Analysis 7.1); schizophrenia, bipolar disorder, or another psychiatric disorder (RR 2.26, 95% CI 1.78 to 2.86; I2 = 0%; 3 studies, 2245 participants; Analysis 7.4); depression (RR 2.17, 95% CI 1.45 to 3.24; I2 = 0%; 2 studies, 745 participants; Analysis 7.5); HIV (RR 1.96, 95% CI 1.06 to 3.63; I2 = 0%; 2 studies, 427 participants; Analysis 7.8); and chronic obstructive pulmonary disease (RR 1.47, 95% CI 1.28 to 1.69; I2 = 94%; 4 studies of 860 participants; Analysis 7.2).
7.1. Analysis.

Comparison 7: Varenicline (vs placebo or no medication) in specific patient groups, Outcome 1: Cardiovascular disease
7.4. Analysis.

Comparison 7: Varenicline (vs placebo or no medication) in specific patient groups, Outcome 4: Schizophrenia/bipolar/psychiatric disorder
7.5. Analysis.

Comparison 7: Varenicline (vs placebo or no medication) in specific patient groups, Outcome 5: Depression
7.8. Analysis.

Comparison 7: Varenicline (vs placebo or no medication) in specific patient groups, Outcome 8: HIV
7.2. Analysis.

Comparison 7: Varenicline (vs placebo or no medication) in specific patient groups, Outcome 2: COPD
Treating these trials as a subgroup of the main analysis (Analysis 5.1), testing for subgroup differences showed no evidence that varenicline works differently from in the general population: cardiovascular disease, P = 0.24, I2 = 26.2%; psychiatric disorders, P = 0.40, I2 = 0%; depression, P = 0.92, I2 = 0%; HIV, P = 0.69, I2 = 0%). Testing the COPD result for subgroup difference initially showed evidence that varenicline may work differently, with lower effectiveness, in this population (P < 0.00001, I2 = 96.8%), however this difference disappeared when we performed a sensitivity analysis removing studies comparing varenicline with no medication rather than placebo (P = 1.00, I2 = 0%).
Analyses presented inconclusive evidence for three patient populations: asthma (RR 1.25, 95% CI 0.38 to 4.14; 1 study; 52 participants; Analysis 7.3), substance use disorder (RR 3.72, 95% CI 0.50 to 27.59; I2 = 0%; 2 studies, 294 participants; Analysis 7.6), and alcohol dependence (RR 3.01, 95% CI 0.92 to 9.92; I2 = 54%; 3 studies, 195 participants; Analysis 7.7). However, point estimates all favoured varenicline, and wide confidence intervals were likely the result of very low numbers of events. Treating the trials as a subgroup of the main analysis (Analysis 5.1) and testing for subgroup differences showed no evidence that varenicline works differently in these populations (asthma: P = 0.76, I2 = 0.4%; substance use disorder: P = 0.61, I2 = 0%; alcohol dependence: P = 0.70, I2 = 0.4%).
7.3. Analysis.

Comparison 7: Varenicline (vs placebo or no medication) in specific patient groups, Outcome 3: Asthma
7.6. Analysis.

Comparison 7: Varenicline (vs placebo or no medication) in specific patient groups, Outcome 6: Substance use disorder/ methadone‐maintained at 24 weeks
7.7. Analysis.

Comparison 7: Varenicline (vs placebo or no medication) in specific patient groups, Outcome 7: Alcohol‐dependence
Varenicline in specific settings or subgroups
Six studies of 1324 participants tested varenicline against placebo or no medication among hospital inpatients and perioperative patients and found that more people successfully quit in the varenicline arm (RR 1.27, 95% CI 1.12 to 1.43; I2 = 58%; Analysis 8.1). Treating these trials as a subgroup of the main analysis (Analysis 5.1), which includes studies conducted in both clinical and community settings such as cessation clinics and university campuses, and testing for subgroup differences did show evidence of subgroup difference, with lower effectiveness (P < 0.00001; I2 = 98.6%). Heterogeneity remained when we performed a sensitivity analysis removing studies comparing varenicline with no medication rather than placebo (P < 0.00001; I2 = 96%).
8.1. Analysis.

Comparison 8: Varenicline in specific settings/subgroups, Outcome 1: Hospital inpatients/perioperative patients
Gonzales 2014 tested varenicline against placebo among people who had previously used varenicline for two weeks or more, at least three months prior to admission to the study, and had not successfully quit but were motivated to try again. This single study found that more people successfully quit in the varenicline arm (RR 6.15, 95% CI 2.98 to 12.70; 494 participants; Analysis 8.2).
8.2. Analysis.

Comparison 8: Varenicline in specific settings/subgroups, Outcome 2: Smokers with a previous quit attempt on varenicline
Two studies of 114 participants tested varenicline against placebo among light smokers and found that more people successfully quit in the varenicline arm (RR 4.16, 95% CI 1.58 to 10.97; I2 = 0%; Analysis 8.3). Treating these trials as a subgroup of the main analysis (Analysis 5.1) and testing for subgroup differences showed no evidence that varenicline works differently in this population (P = 0.20, I2 = 39.3%).
8.3. Analysis.

Comparison 8: Varenicline in specific settings/subgroups, Outcome 3: Light or heavy smokers
Varenicline versus bupropion
Smoking cessation
Nine studies of 7560 participants compared varenicline with bupropion and found that more people quit smoking when using varenicline (RR 1.36, 95% CI 1.25 to 1.49; I2 = 0%; high‐certainty evidence; Analysis 9.1).
9.1. Analysis.

Comparison 9: Varenicline vs bupropion, Outcome 1: Abstinence at longest follow‐up
Johns 2017b randomised 300 participants to receive varenicline, bupropion, or both. They did not provide any useable results data but reported that more people quit in the combined varenicline and bupropion arm compared with the bupropion‐ or varenicline‐alone arms. However, only an abstract was available for this study, and without further information, this result should be treated with caution.
Adverse events
Studies comparing varenicline with bupropion found that people randomised to receive varenicline were more likely to report experiencing nausea (RR 2.46, 95% CI 2.20 to 2.75; I2 = 0%; 4 studies, 5808 participants; Analysis 9.2), abnormal dreams (RR 1.56, 95% CI 1.39 to 1.76; I2 = 0%; 4 studies, 5808 participants; Analysis 9.4), and headache (RR 1.24, 95% CI 1.06 to 1.45; I2 = 13%; 3 studies, 4888 participants; Analysis 9.5). However, people randomised to receive varenicline were less likely to report experiencing insomnia (RR 0.84, 95% CI 0.75 to 0.93; I2 = 75%; 6 studies, 6789 participants; Analysis 9.3).
9.2. Analysis.

Comparison 9: Varenicline vs bupropion, Outcome 2: Nausea
9.4. Analysis.

Comparison 9: Varenicline vs bupropion, Outcome 4: Abnormal dreams
9.5. Analysis.

Comparison 9: Varenicline vs bupropion, Outcome 5: Headache
9.3. Analysis.

Comparison 9: Varenicline vs bupropion, Outcome 3: Insomnia
Two studies of 4210 people did not find evidence a difference between those randomised to receive varenicline or bupropion in reported rates of depression (RR 0.90, 95% CI 0.35 to 2.35; I2 = 0%; Analysis 9.6) or suicidal ideation (RR 1.99, 95% CI 0.18 to 21.93; I2 not estimable as events only in 1 study; Analysis 9.7).
9.6. Analysis.

Comparison 9: Varenicline vs bupropion, Outcome 6: Depression
9.7. Analysis.

Comparison 9: Varenicline vs bupropion, Outcome 7: Suicidal ideation
Serious adverse events
Serious adverse events
Five studies of 5317 people did not find evidence of a clear difference in the number of people reporting experiencing serious adverse events (RR 0.89, 95% CI 0.61 to 1.31; I2 = 0%; low‐certainty evidence; Analysis 9.8).
9.8. Analysis.

Comparison 9: Varenicline vs bupropion, Outcome 8: SAEs
Neuropsychiatric serious adverse events
Two studies of 866 people did not find evidence of a clear difference in the number of people reporting experiencing neuropsychiatric serious adverse events (RR 1.05, 95% CI 0.16 to 7.04; I2 = 10%; low‐certainty evidence; Analysis 9.9), though confidence intervals were very wide.
9.9. Analysis.

Comparison 9: Varenicline vs bupropion, Outcome 9: Neuropsychiatric SAEs (not deaths)
Cardiac serious adverse events
Two studies of 866 people did not find evidence of a clear difference in the number of people reporting experiencing cardiac serious adverse events (RR 3.17, 95% CI 0.33 to 30.18; I2 = 0%; low‐certainty evidence; Analysis 9.10), though confidence intervals were very wide.
9.10. Analysis.

Comparison 9: Varenicline vs bupropion, Outcome 10: Cardiac SAEs, including deaths
Varenicline versus nicotine replacement therapy (NRT) monotherapy
Smoking cessation
Eleven studies of 7572 participants compared varenicline with NRT monotherapy and found that more people quit smoking in the varenicline arm (RR 1.25, 95% CI 1.14 to 1.37; I2 = 28%; high‐certainty evidence; Analysis 10.1).
10.1. Analysis.

Comparison 10: Varenicline vs NRT monotherapy, Outcome 1: Abstinence at longest follow‐up
Adverse events
Studies comparing varenicline with NRT monotherapy found that people randomised to receive varenicline were more likely to report experiencing nausea (RR 2.69, 95% CI 2.41 to 3.01; I2 = 59%; 6 studies, 6500 participants; Analysis 10.2) and headache (RR 1.14, 95% CI 1.01 to 1.28; I2 = 69%; 4 studies, 6287 participants; Analysis 10.5). However, they did not find evidence of a clear difference in the number of people reporting experiencing insomnia (RR 1.08, 95% CI 0.96 to 1.21; I2 = 42%; 5 studies, 6319 participants; Analysis 10.3), abnormal dreams (RR 0.93, 95% CI 0.83 to 1.05; I2 = 67%; 4 studies, 5803 participants; Analysis 10.4), or depression (RR 0.94, 95% CI 0.76 to 1.16; I2 = 0%; 3 studies, 5541 participants; Analysis 10.6). The pooled point estimate from two studies found a higher rate of suicidal ideation among participants in the varenicline arm (RR 5.00, 95% CI 0.87 to 28.77; I2 = 0%; 2 studies, 4876 participants; Analysis 10.7), but confidence intervals were very wide and incorporated the potential for no difference or reduced harm. Statistical heterogeneity was substantial in several analyses, but we decided to present the pooled estimate because the point estimates of individuals studies were almost entirely in the same direction in each analysis.
10.2. Analysis.

Comparison 10: Varenicline vs NRT monotherapy, Outcome 2: Nausea
10.5. Analysis.

Comparison 10: Varenicline vs NRT monotherapy, Outcome 5: Headache
10.3. Analysis.

Comparison 10: Varenicline vs NRT monotherapy, Outcome 3: Insomnia
10.4. Analysis.

Comparison 10: Varenicline vs NRT monotherapy, Outcome 4: Abnormal dreams
10.6. Analysis.

Comparison 10: Varenicline vs NRT monotherapy, Outcome 6: Depression
10.7. Analysis.

Comparison 10: Varenicline vs NRT monotherapy, Outcome 7: Suicidal ideation
Serious adverse events
Serious adverse events
Six studies of 6535 people comparing varenicline with NRT monotherapy found that people randomised to receive varenicline were less likely to report experiencing serious adverse events (RR 0.70, 95% CI 0.50 to 0.99; I2 = 24%; low‐certainty evidence; Analysis 10.8).
10.8. Analysis.

Comparison 10: Varenicline vs NRT monotherapy, Outcome 8: SAEs
Neuropsychiatric serious adverse events
Only one study measured neuropsychiatric serious adverse events, and reported no events in either arm (Rohsenow 2017; 137 participants).
Cardiac serious adverse events
Only one study measured cardiac serious adverse events, and reported no events in either arm (Rohsenow 2017; 137 participants).
Varenicline versus combination NRT
Smoking cessation
Five studies of 2344 participants compared varenicline with combination NRT and did not detect evidence of a clear difference in the number of people who quit smoking, although confidence intervals indicate imprecision, which reduces our certainty in the effect (RR 1.02, 95% CI 0.87 to 1.20; I2 = 0%; low‐certainty evidence; Analysis 11.1).
11.1. Analysis.

Comparison 11: Varenicline vs combination NRT, Outcome 1: Abstinence at longest follow‐up
Adverse events
Studies comparing varenicline with combination NRT found that people randomised to receive varenicline were more likely to report experiencing nausea (RR 1.76, 95% CI 1.45 to 2.15; I2 = 47%; 3 studies, 1609 participants; Analysis 11.2), insomnia (RR 1.40, 95% CI 1.15 to 1.70; I2 = 82%; 3 studies, 1609 participants; Analysis 11.3), and abnormal dreams (RR 1.59, 95% CI 1.22 to 2.08; 1 study; 549 participants; Analysis 11.4). However, they did not find evidence of a clear difference in the number of people reporting experiencing headache (RR 0.98, 95% CI 0.78 to 1.23; I2 = 0%; 3 studies, 1609 participants; Analysis 11.5), depression (RR 1.08, 95% CI 0.83 to 1.40; I2 = 82%; 3 studies, 1609 participants; Analysis 11.6), or suicidal ideation (RR 0.94, 95% CI 0.06 to 14.79; I2 not estimable as events only in 1 study; 2 studies, 764 participants; Analysis 11.7). Statistical heterogeneity was substantial in several analyses, but we decided to present the pooled estimate because the point estimates of individual studies were almost entirely in the same direction.
11.2. Analysis.

Comparison 11: Varenicline vs combination NRT, Outcome 2: Nausea
11.3. Analysis.

Comparison 11: Varenicline vs combination NRT, Outcome 3: Insomnia
11.4. Analysis.

Comparison 11: Varenicline vs combination NRT, Outcome 4: Abnormal dreams
11.5. Analysis.

Comparison 11: Varenicline vs combination NRT, Outcome 5: Headache
11.6. Analysis.

Comparison 11: Varenicline vs combination NRT, Outcome 6: Depression
11.7. Analysis.

Comparison 11: Varenicline vs combination NRT, Outcome 7: Suicidal ideation
Serious adverse events
Serious adverse events
Pooled data from four studies of 1852 people showed more people in the varenicline arm reporting serious adverse events compared with combination NRT (RR 2.15, 95% CI 0.49 to 9.46; I2 = 0%; low‐certainty evidence; Analysis 11.8). However, confidence intervals were very wide and included the potential for no difference or reduced risk.
11.8. Analysis.

Comparison 11: Varenicline vs combination NRT, Outcome 8: SAEs
Neuropsychiatric serious adverse events
Two studies of 764 people reported the number of people reporting neuropsychiatric serious adverse events. While the point estimate suggested participants receiving varenicline were more likely to report experiencing neuropsychiatric serious adverse events, confidence intervals were extremely wide, and incorporated both benefit and harm (RR 4.69, 95% CI 0.23 to 96.50; I2 not estimable as events only in 1 study; low‐certainty evidence; Analysis 11.9).
11.9. Analysis.

Comparison 11: Varenicline vs combination NRT, Outcome 9: Neuropsychiatric SAEs (not deaths)
Cardiac serious adverse events
Two studies of 819 people reported the number of people reporting cardiac serious adverse events. While the point estimate suggested that participants receiving varenicline were less likely to report experiencing cardiac serious adverse events, confidence intervals were very wide, and incorporated both benefit and harm (RR 0.32, 95% CI 0.01 to 7.88; I2 not estimable as events only in 1 study; low‐certainty evidence; Analysis 11.10).
11.10. Analysis.

Comparison 11: Varenicline vs combination NRT, Outcome 10: Cardiac SAEs, including deaths
Varenicline versus e‐cigarettes
Smoking cessation
One study of 54 participants compared varenicline with e‐cigarettes and found that more people quit smoking in the varenicline arm (RR 3.25, 95% CI 1.21 to 8.71; very low‐certainty evidence; Analysis 12.1).
12.1. Analysis.

Comparison 12: Varenicline vs e‐cigarettes, Outcome 1: Abstinence at longest follow‐up
Adverse events
The only one of our adverse event outcomes reported by this study was nausea. While the point estimate suggested that participants receiving varenicline were more likely to report experiencing nausea, confidence intervals were extremely wide, and incorporated both benefit and harm (RR 3.00, 95% CI 0.33 to 27.06; Analysis 12.2).
12.2. Analysis.

Comparison 12: Varenicline vs e‐cigarettes, Outcome 2: Nausea
Serious adverse events
While the study reported serious adverse events, neuropsychiatric serious adverse events, and cardiac serious adverse events as outcomes, they reported no events among study participants in either arm.
Dianicline versus placebo
Smoking cessation
Tonstad 2011 (602 participants) compared dianicline with placebo and did not detect evidence of a clear difference in the number of people who quit smoking (RR 1.20, 95% CI 0.82 to 1.75; Analysis 13.1), however, results were imprecise and confidence intervals encompass the possibility of benefit and harm of dianicline.
13.1. Analysis.

Comparison 13: Dianicline vs placebo, Outcome 1: Abstinence at longest follow‐up
Adverse events
Tonstad 2011 reported that more people randomised to receive dianicline reported experiencing nausea (RR 2.83, 95% CI 1.88 to 4.27; 602 participants; Analysis 13.2) and depression (RR 8.05, 95% CI 1.01 to 63.99; 602 participants; Analysis 13.4) than those in the placebo arm. They did not detect a difference in the number of people reporting experiencing headaches (RR 1.23, 95% CI 0.82 to 1.85; 602 participants; Analysis 13.3). The study did not measure our other adverse event outcomes.
13.2. Analysis.

Comparison 13: Dianicline vs placebo, Outcome 2: Nausea
13.4. Analysis.

Comparison 13: Dianicline vs placebo, Outcome 4: Depression
13.3. Analysis.

Comparison 13: Dianicline vs placebo, Outcome 3: Headache
Serious adverse events
Tonstad 2011 did not detect a difference in rates of serious adverse events (RR 1.01, 95% CI 0.20 to 4.95; 602 participants; Analysis 13.5) or cardiac serious adverse events (RR 1.01, 95% CI 0.06 to 16.02; 602 participants; Analysis 13.6) between participants in the dianicline and placebo study arms. However, in both instances confidence intervals were very wide and included the potential for both harm and benefit. The study did not measure neuropsychiatric serious adverse events.
13.5. Analysis.

Comparison 13: Dianicline vs placebo, Outcome 5: Serious adverse events
13.6. Analysis.

Comparison 13: Dianicline vs placebo, Outcome 6: Cardiac SAEs, including deaths
Discussion
Summary of main results
This review includes eight studies that investigated cytisine use in just under 9000 people, 68 studies that investigated varenicline use in over 37,000 people, and one study that investigated dianicline use in 602 people. Forty‐five of these studies are new to this update.
We found moderate‐certainty evidence that cytisine probably helps more people to quit smoking than placebo. While people randomised to receive cytisine were more likely to experience adverse events than those in the placebo arm, low‐certainty evidence gave no clear indication of an increased risk of serious adverse events. We found no data on neuropsychiatric or cardiac serious adverse events.
Our analysis did not find definitive evidence of a difference in cessation rates between cytisine and varenicline. The point estimate favoured varenicline, but this moderate‐certainty evidence is subject to imprecision and may change as more evidence becomes available. A component network meta‐analysis that is currently underway may reveal a more certain result by also employing data from indirect comparisons (Lindson 2022). Although the point estimates in the separate analyses comparing varenicline with placebo and cytisine with placebo did differ (with the varenicline analysis producing a higher risk ratio) the issue with this type of indirect comparison is it does not adjust for potential differences in baseline event rates. In our analysis, Dogar 2020 was the largest study that compared cytisine with placebo. It recruited people diagnosed with pulmonary tuberculosis who, as part of the behavioural aspect of the intervention, were informed of the dangers of continued tobacco use in people with tuberculosis. This made for a highly motivated population, who also happened to smoke fewer cigarettes per day than in other cytisine trials. These two characteristics may have contributed to the higher placebo arm quit rates in the cytisine studies and minimised the benefit gained from pharmacotherapy. These factors may also explain why the results from Dogar 2020 are less compelling than those of the other studies, and may also account for the statistical heterogeneity in the cytisine versus placebo analysis (I2 = 83%).
Participants in the cytisine arm were less likely to experience nausea or abnormal dreams than those in the varenicline arm, and there was no evidence of a difference in rates of insomnia, headache, depression, or suicidal ideation. The same studies provided low‐certainty evidence of fewer people experiencing serious adverse events in the cytisine arm compared with varenicline. However, in all cases, confidence intervals indicated imprecision, and the potential for more adverse events when using either treatment. We found no data on neuropsychiatric or cardiac serious adverse events.
Low‐certainty evidence suggested that cytisine may help more people to quit than NRT monotherapy (Walker 2014). However, after the initial 25‐day course, participants in the cytisine arm also received vouchers for NRT. This may distort the results as some participants in the cytisine arm may have in fact received two pharmacotherapies, but study authors reported that few participants in the cytisine arm used their NRT vouchers. Low‐certainty evidence did not show a difference in the number of people reporting serious adverse events. We found no data on neuropsychiatric or cardiac serious adverse events.
Evidence on the effect of different lengths of cytisine treatment was sparse and inconclusive.
There is high‐certainty evidence that varenicline increases the chances of successful smoking cessation by more than two‐fold compared with placebo. Since the previous version of this review was published (Cahill 2016), this estimate has remained stable, despite the growing inclusion of pragmatic trials in real‐world settings and those conducted in particular groups of smokers previously excluded from clinical trials, such as those in lower‐ and middle‐income countries, and in disease‐specific populations.
We also found high‐certainty evidence that varenicline helped more people to quit than bupropion, or NRT monotherapy, with no clear evidence of difference between varenicline and bupropion in rates of serious adverse events, neuropsychiatric serious adverse events, or cardiac serious adverse events (all low‐certainty evidence), and low‐certainty evidence suggesting reduced risk of serious adverse events compared with NRT. We found no data comparing varenicline with NRT monotherapy for neuropsychiatric or cardiac serious adverse events.
Low‐certainty evidence did not show a difference in quit rates compared with combination NRT, and while low‐certainty evidence suggested potentially increased risk of serious adverse events and neuropsychiatric serious adverse events, and reduced risk of cardiac serious adverse events, in all cases confidence intervals were very wide, encompassing both substantial harm and benefit.
One small study of 54 people provided very‐low certainty evidence of more people quitting with varenicline than with e‐cigarettes (Ioakeimidis 2018); however this study was at high risk of bias and imprecise due to few events, and while they reported serious adverse events, neuropsychiatric serious adverse events, and cardiac serious adverse events as outcomes, they reported no events among study participants in either arm. Studies that tested varenicline versus placebo in specific populations and settings did not demonstrate varenicline working differently than it does in the general population in disease‐specific groups of patients (e.g. cardiovascular, chronic obstructive pulmonary disease, HIV, schizophrenia and psychiatric disorders, depression, alcohol dependence), or in specific subgroups or settings (e.g. hospital inpatients, light‐smokers, smokers who failed to quit on varenicline previously).
Analyses found evidence of increased rates of adverse events such as nausea, insomnia, abnormal dreams, and headache among people randomised to receive varenicline compared with placebo. However, we found no clear evidence of increased rates of depression or suicidal ideation, although confidence intervals indicated imprecision, and the potential for more or fewer adverse events when using varenicline compared with control. Moderate‐certainty serious adverse event data suggest there may be a 23% increased risk of such events among the varenicline groups compared with the controls. However, serious adverse events were still rare (2.7% to 4% of people on varenicline, compared with 2.7% of people without) and this finding is based on simple counts across the trials of participants reporting one or more such events, thus not distinguishing between events attributed and those unrelated to treatment. We did not find evidence of an increased risk of neuropsychiatric serious adverse events, but pooled results did suggest a potential increased risk of cardiac serious adverse events, although again these results were subject to imprecision, and we deemed this evidence to be low certainty because of its compatibility with both increased and decreased risk of harm.
One trial compared dianicline with placebo for smoking cessation and the results were inconclusive (Tonstad 2011).
Overall completeness and applicability of evidence
We conducted systematic searches of multiple online databases, including clinical trials registries and followed Cochrane methods for screening. We therefore expect that any published trials we have missed will be through chance rather than systematic error. We were able to assess publication bias for two comparisons by constructing funnel plots: varenicline versus placebo (Figure 4), and varenicline versus NRT monotherapy (Figure 5). Figure 5 appears to show a small amount of asymmetry that may suggest a lack of smaller trials with negative findings. However, the number of studies is still very low with few smaller studies, and so we cannot treat this as definitive evidence of publication bias. These were the only cessation comparisons with enough studies to construct a funnel plot, so we were unable to assess publication bias for other comparisons. As such, we cannot ignore the possibility of publication bias for some comparisons in this review.
4.

Funnel plot of comparison 6: varenicline (1.0 mg 2/d) vs placebo, outcome: 6.1 abstinence at longest follow‐up
5.

Funnel plot of comparison 13: varenicline vs nicotine replacement therapy monotherapy, outcome: 13.1 abstinence at longest follow‐up
The benefits of varenicline for smoking cessation is now well established, with the point estimate remaining unchanged as more studies (including non‐manufacturer‐ (Pfizer) funded trials) accumulate. Trials are now being conducted and reported in areas where the evidence is less comprehensive, such as testing cytisine (against placebo and varenicline), and testing varenicline in specific populations and settings, and in variations of treatment dose or duration.
Quality of the evidence
We judged the evidence comparing varenicline with placebo for smoking cessation to be of high certainty. While we detected moderate heterogeneity (I2 = 60%), all but three studies had a point estimate that favoured varenicline over placebo, so we did not downgrade on this basis. The effectiveness of varenicline for smoking cessation has remained constant through several updates of this review, and we think it is unlikely to change with further evidence. We judged the evidence on serious adverse events to be of moderate certainty, downgraded because of imprecision. For cardiac and neuropsychiatric serious adverse events, we downgraded evidence to low because of imprecision. Despite respectable numbers of studies and participants in each analysis, because of the rarity of these kinds of adverse events, there were very few events in the analyses, with some studies reporting no events at all.
We judged the evidence comparing cytisine with placebo to be of moderate certainty for smoking cessation and low certainty for serious adverse events, downgraded due to substantial unexplained heterogeneity and imprecision, respectively. We judged the evidence comparing cytisine with varenicline for smoking cessation and serious adverse events to be of moderate and low certainty, respectively, downgraded for imprecision in both cases.
We judged the evidence comparing varenicline with bupropion and NRT monotherapy for smoking cessation to be of high certainty, and with combination NRT to be of low certainty, limited by imprecision. Only one small study compared varenicline with e‐cigarettes for smoking cessation, and we graded this evidence as of very low certainty because of imprecision and risk of bias.
Potential biases in the review process
We have followed standard Cochrane methodology, which endeavours to minimise biases in the review process, so we are confident that any errors in our data will be through chance rather than systematic error. However, it is impossible to rule out individual errors in the review process.
A potential limitation to our review is that for data on harms we relied on adverse events and serious adverse events as defined by papers reporting included studies. This does not take into account whether those events were genuinely attributable to the tested interventions. Further, we only considered the number of participants reporting these events, which does not account for people who experienced more than one event.
Another potential limitation is that the majority of varenicline trials reported in this review received either funding or study medication from Pfizer Inc, the manufacturers of varenicline. Evidence from systematic reviews suggests that industry‐funded trials, although conducted to a high standard, are more likely to have outcomes favourable to the product sponsor than studies with other sponsors (Etter 2007; Walsh 2011). However, we deem the provision of study medication less likely to amount to the kind of sponsorship that may bias results, and modern trials increasingly report funders and medication providers as having no involvement in trial conduct or decision making.
Agreements and disagreements with other studies or reviews
Reviews of controlled studies of cytisine have focused upon its potential as an established and affordable aid to smoking cessation (Etter 2006; Etter 2008; Tutka 2005; Tutka 2006; Tutka 2008). Many of the early cytisine studies excluded from this review are discussed and evaluated in Etter 2006, who concluded that cytisine may be effective for smoking cessation. A systematic review and network meta‐analysis (Leaviss 2014), compared the benefits and cost‐effectiveness of cytisine (2 trials: Vinnikov 2008; West 2011), with varenicline (21 trials). While the analysis found both treatments to be effective for smoking cessation, cytisine delivered more quality‐adjusted life‐years at a lower cost than varenicline. Cytisine was also associated with lower rates of headache and nausea than varenicline. Our analyses on harms, using direct evidence, found lower rates of nausea, but did not find evidence of difference in rates of headache. A recent review of cytisine found similar results to ours, though with a slightly higher point estimate for smoking cessation (Tutka 2019). This is likely because they had broader inclusion criteria than our review, and included studies with shorter follow‐up periods.
A Cochrane overview and network meta‐analysis of a number of pharmacological interventions for smoking cessation assessed 12 Cochrane Reviews published to November 2012 (Cahill 2013), and therefore drew on the previous version of this review. Comparisons between varenicline, bupropion and single‐treatment NRT found varenicline to be superior to both treatments (OR 1.59, 95% credible interval 1.29 to 1.96 and OR 1.57, 95% credible interval 1.29 to 1.91, respectively). Varenicline demonstrated comparable benefits for smoking cessation to combination NRT (OR 1.06, 95% credible interval 0.75 to 1.48), but the number of NRT trials informing this comparison was low (9 trials). This review is currently being updated (Lindson 2022). A 2012 network meta‐analysis (Mills 2012), comparing high‐dose and combination NRT versus varenicline and versus bupropion across 146 RCTs, found varenicline (11 trials) to be superior to placebo and to bupropion at all time points, and similar in benefits for smoking cessation to standard and to high‐dose NRT, in line with our findings. A more recent systematic review with network meta‐analysis reported similar findings to ours, reporting a benefit from varenicline compared with placebo (OR 2.69, 95% CI 2.27 to 3.19), bupropion (OR 1.46, 95% CI 1.18 to 1.81), and standard‐dose NRT (OR 1.32, 95% CI 1.05 to 1.65; Thomas 2020). However, they did not find evidence of increased rates of serious adverse events amongst those randomised to receive varenicline compared with placebo (OR 1.09, 95% CI 0.91 to 1.34), contrary to the increased risk we detected. This may be due to their choice of a random‐effects rather than fixed‐effect model for their analysis. They did not find clear evidence of a difference in cardiac serious adverse events (OR 0.76, 95% CI 0.41 to 1.25) and neuropsychiatric serious adverse events (OR 0.96, 95% CI 0.76 to 1.21), which is in line with our findings.
An earlier systematic review and meta‐analysis of 39 RCTs (10,761 participants) by the same team assessed the risk of neuropsychiatric adverse events among users of varenicline (Thomas 2015). In line with our findings, the authors found no clear evidence of an increased risk of suicide or attempted suicide (Peto odds ratio (OR) 1.67, 95% CI 0.33 to 8.57), suicidal ideation (Peto OR 0.58, 95% CI 0.28 to 1.20), depression (Peto OR 0.96, 95% CI 0.75 to 1.22) or death (Peto OR 1.05, 95% CI 0.47 to 2.38) associated with varenicline. Lee 2016 compared cardiovascular serious adverse event rates between people randomised to receive varenicline or placebo. They did not find evidence of increased risk of cardiovascular serious adverse events (RR 1.03, 95% CI 0.72 to 1.49) in 38 trials of 12,706 people. This result was consistent among cardiovascular (RR 1.04, 95% CI 0.57 to 1.89) and non‐cardiovascular patients (RR 1.03, 95% CI 0.64 to 1.64).
Authors' conclusions
Implications for practice.
Cytisine is likely to help more people to quit smoking than placebo or no medication.
Varenicline at standard dosage (1.0 mg twice a day) increased the chances of successful long‐term smoking cessation by more than two‐fold compared with placebo. We did not find evidence that varenicline is less effective in any of the specific populations we investigated.
Varenicline is more effective at helping people to quit smoking than bupropion, or a single form of nicotine replacement therapy, and may be as effective as or more effective than dual‐form nicotine replacement therapy.
People taking varenicline are probably more likely to experience serious adverse events than those not taking it, and while there may be increased risk of cardiac serious adverse events and decreased risk of neuropsychiatric serious adverse events, evidence was compatible with both benefit and harm.
Cytisine may lead to fewer people reporting serious adverse events than varenicline. There may be a benefit from varenicline for quitting smoking, however further evidence could strengthen this finding or demonstrate a benefit from cytisine.
Implications for research.
Future trials should test the effectiveness and safety of cytisine compared with varenicline and other pharmacotherapies, and should also test variations in dose and duration.
There is limited benefit to be gained from more trials testing the effect of standard dose varenicline compared with placebo for smoking cessation.
Further varenicline trials should test the effect of variations in dose and duration and preloading varenicline before quitting, and may be useful in specific populations and settings where there is a plausible rationale that the effect may differ.
What's new
| Date | Event | Description |
|---|---|---|
| 4 May 2023 | New search has been performed | New searches conducted 29 April 2022 adding 45 new studies |
| 4 May 2023 | New citation required and conclusions have changed | New searches conducted 29 April 2022. Analyses and conclusions updated |
History
Protocol first published: Issue 3, 2006 Review first published: Issue 1, 2007
| Date | Event | Description |
|---|---|---|
| 31 January 2016 | New citation required and conclusions have changed | Additional comparisons. Analyses expanded and restructured. SAE information updated |
| 31 January 2016 | New search has been performed | 39 trials of varenicline now included |
| 16 May 2013 | Amended | Minor change made to labelling on forest plot. |
| 14 March 2012 | New search has been performed | Seven new included studies (5 varenicline, 1 cytisine, 1 dianicline) and 14 new excluded studies added, plus safety data. |
| 14 March 2012 | New citation required and conclusions have changed | Safety profile modified, as new possible cardiovascular and psychiatric adverse events information incorporated. Efficacy findings unchanged but confirmed. |
| 13 January 2011 | Amended | Vinnikov trial of cytisine added to Studies awaiting Classification, for inclusion in next update. |
| 8 November 2010 | New search has been performed | Six new RCTs added; sources of funding added for all trials. Ongoing trials section expanded. |
| 8 November 2010 | New citation required and conclusions have changed | Surveillance data and secondary analyses do not support fears about safety. Efficacy conclusions strengthened but unchanged. |
| 17 July 2008 | Amended | Date of last search amended (2007 corrected to 2008); Source of support added. |
| 12 May 2008 | New citation required and conclusions have changed | Three new included trials, switch in the MA metric from OR to RR, updated background section and new safety information. |
| 15 March 2008 | New search has been performed | New search conducted. |
| 30 August 2007 | Amended | Converted to new review format. |
| 15 November 2006 | New citation required and conclusions have changed | Substantive amendment. |
Acknowledgements
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure and Cochrane Programme Grant funding to the Cochrane Tobacco Addiction Group. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service, or the Department of Health and Social Care.
We are very grateful for the contributions of everyone who has provided information or assistance to previous versions of this review. For this most recent update we would like to thank previous authors, including Kate Cahill, as well as Kevin Gray, Nathanial Baker, Marc Steinberg, Ugo Pastorino, Omara Dogar, Ada Keding, and Kamran Siddiqui for providing additional results data on included studies. We are also grateful to Aditi Hombali for helping with data extraction and Min Gao for translating and extracting Chinese‐language studies.
The following people conducted the editorial process for the most recent update:
Sign‐off Editor (final editorial decision): Lisa Bero, Cochrane Editorial Board,
• Managing Editor (selected peer reviewers, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Sam Hinsley, Cochrane Central Editorial Service
• Editorial Assistant (conducted editorial policy checks and supported editorial team): Leticia Rodrigues, Cochrane Central Editorial Service
• Copy Editor (copy editing and production): Denise Mitchell, Cochrane Evidence Production and Methods Directorate
• Peer‐reviewers (provided comments and recommended an editorial decision): Jennifer Hilgart, Cochrane Evidence Production and Methods Directorate (methods), Steve MacDonald, Cochrane Australia (search), Michael B. Steinberg, MD, MPH; Rutgers Center for Tobacco Studies and Robert Wood Johnson Medical School (clinical), Ryan J Courtney, UNSW Sydney, National Drug and Alcohol Research Centre (clinical), Timothy B. Baker Department of Medicine University of Wisconsin School of Medicine and Public Health (clinical).
Appendices
Appendix 1. Search strategy
CTAG Specialised Register (CRS web)
1. (cytisine or Tabex or dianicline or varenicline or champix or chantix):TI,AB,MH,EMT,XKY,KY,KW 2. MeSH DESCRIPTOR Nicotine WITH AG AI 3. MeSH DESCRIPTOR Nicotinic Agonists 4. MeSH DESCRIPTOR Nicotinic Antagonists 5. nicotinic agonist*:TI,AB,MH,EMT,XKY,KY,KW 6. nicotinic antagonist*:TI,AB,MH,EMT,XKY,KY,KW 7. nicotin* NEAR2 partial:TI,AB,MH,EMT,XKY,KY,KW #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7
Appendix 2. Glossary of tobacco‐related terms
| Term | Definition |
| Abstinence | A period of being quit, i.e. stopping the use of cigarettes or other tobacco products. May be defined in various ways; see also: point prevalence abstinence; prolonged abstinence; continuous/sustained abstinence |
| Biochemical verification | Also called 'biochemical validation' or 'biochemical confirmation' A procedure for checking a tobacco user's report that he or she has not smoked or used tobacco. It can be measured by testing levels of nicotine or cotinine or other chemicals in blood, urine, or saliva, or by measuring levels of carbon monoxide in exhaled breath or in blood. |
| Bupropion | A pharmaceutical drug originally developed as an antidepressant, but now also licensed for smoking cessation; trade names Zyban, Wellbutrin (when prescribed as an antidepressant) |
| Carbon monoxide (CO) | A colourless, odourless, highly poisonous gas found in tobacco smoke and in the lungs of people who have recently smoked, or (in smaller amounts) in people who have been exposed to tobacco smoke. May be used for biochemical verification of abstinence. |
| Cessation | Also called 'quitting' The goal of treatment to help people achieve abstinence from smoking or other tobacco use, also used to describe the process of changing behaviour. |
| Continuous abstinence | Also called 'sustained abstinence' A measure of cessation often used in clinical trials involving avoidance of all tobacco use since the quit day until the time the assessment is made. The definition occasionally allows for lapses. This is the most rigorous measure of abstinence. |
| 'Cold turkey' | Quitting abruptly, and/or quitting without behavioural or pharmaceutical support. |
| Craving | A very intense urge or desire (to smoke) See: Shiffman 2004 |
| Dopamine | A neurotransmitter in the brain that regulates mood, attention, pleasure, reward, motivation and movement |
| Efficacy | Also called 'treatment effect' or 'effect size' The difference in outcome between the experimental and control groups |
| Harm reduction | Strategies to reduce harm caused by continued tobacco/nicotine use, such as reducing the number of cigarettes smoked, or switching to different brands or products, e.g. potentially reduced exposure products (PREPs), smokeless tobacco |
| Lapse/slip | Terms sometimes used for a return to tobacco use after a period of abstinence. A lapse or slip might be defined as a puff or two on a cigarette. This may proceed to relapse, or abstinence may be regained. Some definitions of continuous, sustained or prolonged abstinence require complete abstinence, but some allow for a limited number or duration of slips. People who lapse are very likely to relapse, but some treatments may have their effect by helping people recover from a lapse. |
| nAChR | Neural nicotinic acetylcholine receptors Areas in the brain that are thought to respond to nicotine, forming the basis of nicotine addiction by stimulating the overflow of dopamine |
| Nicotine | An alkaloid derived from tobacco, responsible for the psychoactive and addictive effects of smoking |
| Nicotine replacement therapy (NRT) | A smoking cessation treatment in which nicotine from tobacco is replaced for a limited period by pharmaceutical nicotine. This reduces the craving and withdrawal experienced during the initial period of abstinence while users are learning to be tobacco‐free. The nicotine dose can be taken through the skin, using patches, by inhaling a spray, or by mouth using gum or lozenges. |
| Outcome | Often used to describe the result being measured in trials that is of relevance to the review. For example smoking cessation is the outcome used in reviews of ways to help smokers quit. The exact outcome in terms of the definition of abstinence and the length of time that has elapsed since the quit attempt was made may vary from trial to trial. |
| Pharmacotherapy | A treatment using pharmaceutical drugs, e.g. nicotine replacement therapy, bupropion |
| Point prevalence abstinence (PPA) | A measure of cessation based on behaviour at a particular point in time, or during a relatively brief specified period, e.g. 24 hours, 7 days. It may include a mixture of recent and long‐term quitters. cf. prolonged abstinence, continuous abstinence |
| Prolonged abstinence | A measure of cessation that typically allows a 'grace period' following the quit date (usually of about two weeks), to allow for slips/lapses during the first few days when the effect of treatment may still be emerging. See: Hughes 2003 |
| Relapse | A return to regular smoking after a period of abstinence |
| Secondhand smoke | Also called passive smoking or environmental tobacco smoke (ETS) A mixture of smoke exhaled by smokers and smoke released from smouldering cigarettes, cigars, pipes, bidis, etc. The smoke mixture contains gases and particulates, including nicotine, carcinogens and toxins. |
| Self‐efficacy | The belief that one will be able to change one's behaviour, e.g. to quit smoking |
| SPC [Summary of Product Characteristics] | Advice from the manufacturers of a drug, agreed with the relevant licensing authority, to enable health professionals to prescribe and use the treatment safely and effectively. |
| Tapering | A gradual decrease in dose at the end of treatment, as an alternative to abruptly stopping treatment |
| Titration | A technique of dosing at low levels at the beginning of treatment, and gradually increasing to full dose over a few days, to allow the body to get used to the drug. It is designed to limit side effects. |
| Withdrawal | A variety of behavioural, affective, cognitive and physiological symptoms, usually transient, which occur after use of an addictive drug is reduced or stopped. See: Shiffman 2004 |
Data and analyses
Comparison 1. Cytisine vs placebo or no medication.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Abstinence at longest follow‐up | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1.1 Cytisine vs placebo | 4 | 4623 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.15, 1.47] |
| 1.1.2 Cytisine vs no medication | 1 | 869 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.44 [3.06, 6.46] |
| 1.2 Adverse events | 4 | 4052 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [1.07, 1.39] |
| 1.3 Serious adverse events | 3 | 3781 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.78, 1.37] |
Comparison 2. Cytisine: longer vs shorter duration.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Abstinence at longest follow‐up | 1 | 470 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.98, 1.67] |
| 2.1.1 84 days vs 40 days | 1 | 470 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.98, 1.67] |
Comparison 3. Cytisine vs varenicline.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Abstinence at longest follow‐up | 2 | 2131 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.66, 1.05] |
| 3.2 Nausea | 2 | 2017 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.33, 0.50] |
| 3.3 Abnormal dreams | 2 | 2017 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.50, 0.73] |
| 3.4 Insomnia | 2 | 2017 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.73, 1.10] |
| 3.5 Headache | 2 | 2017 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.79, 1.33] |
| 3.6 Depression | 1 | 679 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.04 [0.12, 74.47] |
| 3.7 Suicidal ideation | 2 | 2017 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.02] |
| 3.8 SAEs | 2 | 2017 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.44, 1.03] |
Comparison 4. Cytisine vs NRT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Abstinence at longest follow‐up | 1 | 1310 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [1.13, 1.80] |
| 4.2 Nausea | 1 | 1310 | Risk Ratio (M‐H, Fixed, 95% CI) | 15.00 [3.60, 62.51] |
| 4.3 SAEs | 1 | 1310 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.76, 1.75] |
Comparison 5. Varenicline 2 mg per day vs placebo or no medication.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Abstinence at longest follow‐up | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1.1 Varenicline vs placebo | 41 | 17395 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.32 [2.15, 2.51] |
| 5.1.2 Varenicline vs no medication | 5 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.37, 1.80] |
| 5.2 Nausea | 36 | 17080 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.61 [2.44, 2.80] |
| 5.3 Insomnia | 35 | 16803 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.27, 1.47] |
| 5.4 Abnormal dreams | 32 | 16211 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [1.67, 1.97] |
| 5.5 Headache | 31 | 16326 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [1.03, 1.19] |
| 5.6 Depression | 32 | 15922 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.91, 1.20] |
| 5.7 Suicidal ideation | 22 | 12343 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.44, 1.08] |
| 5.8 SAEs | 26 | 14356 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [1.01, 1.48] |
| 5.9 Neuropsychiatric SAEs (not deaths) | 22 | 7846 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.61, 1.29] |
| 5.10 Cardiac SAEs, including deaths | 18 | 7151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.79, 1.84] |
Comparison 6. Varenicline: variations in usage.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Low‐dose varenicline vs placebo | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1.1 1 mg per day | 3 | 946 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [1.35, 2.60] |
| 6.1.2 As desired | 1 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.92 [1.57, 5.41] |
| 6.2 Higher‐dose varenicline versus lower‐dose varenicline | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.2.1 2 mg per day vs 1 mg per day | 4 | 1563 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.97, 1.30] |
| 6.2.2 2 mg per day vs 0.3 mg per day | 1 | 253 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.84 [0.89, 3.84] |
| 6.2.3 1 mg per day vs 0.3 mg per day | 1 | 254 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.28, 1.81] |
| 6.3 Longer vs standard duration varenicline | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.3.1 24 weeks vs 12 weeks | 2 | 1458 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.77, 1.23] |
| 6.3.2 52 weeks vs 12 weeks | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.70, 2.43] |
| 6.4 6 week vs 1 week preloading | 1 | 242 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.60 [2.24, 14.02] |
6.4. Analysis.

Comparison 6: Varenicline: variations in usage, Outcome 4: 6 week vs 1 week preloading
Comparison 7. Varenicline (vs placebo or no medication) in specific patient groups.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Cardiovascular disease | 2 | 1006 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [1.44, 2.47] |
| 7.2 COPD | 4 | 860 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [1.28, 1.69] |
| 7.3 Asthma | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.38, 4.14] |
| 7.4 Schizophrenia/bipolar/psychiatric disorder | 3 | 2245 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.26 [1.78, 2.86] |
| 7.5 Depression | 2 | 745 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.17 [1.45, 3.24] |
| 7.6 Substance use disorder/ methadone‐maintained at 24 weeks | 2 | 294 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.72 [0.50, 27.59] |
| 7.7 Alcohol‐dependence | 3 | 195 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.01 [0.92, 9.92] |
| 7.8 HIV | 2 | 427 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [1.06, 3.63] |
Comparison 8. Varenicline in specific settings/subgroups.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Hospital inpatients/perioperative patients | 6 | 1324 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [1.12, 1.43] |
| 8.2 Smokers with a previous quit attempt on varenicline | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.3 Light or heavy smokers | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.3.1 Light smokers | 2 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.16 [1.58, 10.97] |
Comparison 9. Varenicline vs bupropion.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Abstinence at longest follow‐up | 9 | 7560 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [1.25, 1.49] |
| 9.2 Nausea | 4 | 5808 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 [2.20, 2.75] |
| 9.3 Insomnia | 6 | 6789 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.75, 0.93] |
| 9.4 Abnormal dreams | 4 | 5808 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.39, 1.76] |
| 9.5 Headache | 3 | 4888 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [1.06, 1.45] |
| 9.6 Depression | 2 | 4210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.35, 2.35] |
| 9.7 Suicidal ideation | 2 | 4210 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [0.18, 21.93] |
| 9.8 SAEs | 5 | 5317 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.61, 1.31] |
| 9.9 Neuropsychiatric SAEs (not deaths) | 2 | 866 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.16, 7.04] |
| 9.10 Cardiac SAEs, including deaths | 2 | 866 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.17 [0.33, 30.18] |
Comparison 10. Varenicline vs NRT monotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Abstinence at longest follow‐up | 11 | 7572 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.14, 1.37] |
| 10.2 Nausea | 6 | 6500 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.69 [2.41, 3.01] |
| 10.3 Insomnia | 5 | 6319 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.96, 1.21] |
| 10.4 Abnormal dreams | 4 | 5803 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.83, 1.05] |
| 10.5 Headache | 4 | 6287 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [1.01, 1.28] |
| 10.6 Depression | 3 | 5541 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.76, 1.16] |
| 10.7 Suicidal ideation | 2 | 4876 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.00 [0.87, 28.77] |
| 10.8 SAEs | 6 | 6535 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.50, 0.99] |
| 10.9 Neuropsychiatric SAEs (not deaths) | 1 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 10.10 Cardiac SAEs, including deaths | 1 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
10.9. Analysis.

Comparison 10: Varenicline vs NRT monotherapy, Outcome 9: Neuropsychiatric SAEs (not deaths)
10.10. Analysis.

Comparison 10: Varenicline vs NRT monotherapy, Outcome 10: Cardiac SAEs, including deaths
Comparison 11. Varenicline vs combination NRT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Abstinence at longest follow‐up | 5 | 2344 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.87, 1.20] |
| 11.2 Nausea | 3 | 1609 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [1.45, 2.15] |
| 11.3 Insomnia | 3 | 1609 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [1.15, 1.70] |
| 11.4 Abnormal dreams | 1 | 549 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [1.22, 2.08] |
| 11.5 Headache | 3 | 1609 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.78, 1.23] |
| 11.6 Depression | 3 | 1609 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.83, 1.40] |
| 11.7 Suicidal ideation | 2 | 764 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.06, 14.79] |
| 11.8 SAEs | 4 | 1852 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.15 [0.49, 9.46] |
| 11.9 Neuropsychiatric SAEs (not deaths) | 2 | 764 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.69 [0.23, 96.50] |
| 11.10 Cardiac SAEs, including deaths | 2 | 819 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.88] |
Comparison 12. Varenicline vs e‐cigarettes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 12.1 Abstinence at longest follow‐up | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.25 [1.21, 8.71] |
| 12.2 Nausea | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.33, 27.06] |
| 12.3 SAEs | 1 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 12.4 Neuropsychiatric SAEs (not deaths) | 1 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 12.5 Cardiac SAEs, including deaths | 1 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
12.3. Analysis.

Comparison 12: Varenicline vs e‐cigarettes, Outcome 3: SAEs
12.4. Analysis.

Comparison 12: Varenicline vs e‐cigarettes, Outcome 4: Neuropsychiatric SAEs (not deaths)
12.5. Analysis.

Comparison 12: Varenicline vs e‐cigarettes, Outcome 5: Cardiac SAEs, including deaths
Comparison 13. Dianicline vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 13.1 Abstinence at longest follow‐up | 1 | 602 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.82, 1.75] |
| 13.2 Nausea | 1 | 602 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.83 [1.88, 4.27] |
| 13.3 Headache | 1 | 602 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.82, 1.85] |
| 13.4 Depression | 1 | 602 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.05 [1.01, 63.99] |
| 13.5 Serious adverse events | 1 | 602 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.20, 4.95] |
| 13.6 Cardiac SAEs, including deaths | 1 | 602 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.06, 16.02] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Anthenelli 2013.
| Study characteristics | ||
| Methods | Country: USA (9 centres) and international (24 centres, across Bosnia & Herzogovina, Croatia, Germany, Hungary, Romania, Russian Federation, Spain)
Setting: academic clinical trial centres and SC clinics Aim: to assess the efficacy and safety of 12 weeks of varenicline treatment or placebo for SC, with 40 weeks of non‐treatment follow‐up, in adults with current or past depression (MDD) Study design: double‐blind placebo‐controlled RCT Dates conducted: March 2010 ‐ June 2012 |
|
| Participants | 525 adult smokers, aged 18‐75, smoking at least 10 CPD, motivated to quit, diagnosed with unipolar MDD without psychotic features. 37% male, mean age 46, average CPD at baseline 22, mean FTND 5.9. Allocated to varenicline (256) or placebo (269)
Exclusion criteria: current or past diagnosis of dementia, schizophrenia, schizoaffective disorder, or other psychotic disorder, bipolar I disorder, bipolar II disorder. People with antisocial, schizotypal, or any other personality disorder severe enough to compromise their ability to comply with the study requirements Current use of either bupropion or nortriptyline |
|
| Interventions |
All participants received manual‐guided SC support, telephone support and one‐to‐one 10‐min counselling by the same person where possible. Participants in both groups could reduce the dosage if they wished TQD was set for week 1 visit Treatment period was 12 weeks. Visits at screening, baseline, weekly for weeks 1‐12, and then at weeks 13, 16, 24, 32, 40, 52 (or early termination); phone calls at weeks 14, 20, 28, 36, 44 and 48. Weekly pill counts to assess adherence Safety data were reviewed regularly by an external independent data safety monitoring committee |
|
| Outcomes | Primary: CO‐confirmed CAR for weeks 9‐12 Secondary: CO‐confirmed CAR for weeks 9‐24, 9‐52; 7‐day PPA at weeks 12, 24, 52; AEs and SAEs Verification: CO < 10 ppm |
|
| Notes | New for 2016 update Study funding: "This study was funded by Pfizer. Dr. Anthenelli’s writing of this manuscript was funded, in part, by a Department of Veterans Affairs Merit Review award (NEUA‐003‐08S) and by a National Institute on Alcohol Abuse and Alcoholism grant (AA019720). Dr. Morris was supported, in part, by grants from the University of California, San Francisco, Smoking Cessation Leadership Center, and Colorado Department of Public Health and Environment. Drs. Ramey, Tsilkos, Russ, and Yunis and Ms. Dubrava are employees of Pfizer. Editorial support was provided by Abegale Templar, PhD, of Engage Scientific and funded by Pfizer." Author declaration of interests: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Eligible participants were randomly assigned to varenicline or placebo in a 1:1 ratio by using a computer generated, 4‐block randomization scheme at each site." |
| Allocation concealment (selection bias) | Low risk | "Randomization was stratified by antidepressant medication use at baseline (any vs. none) and baseline Montgomery–Åsberg Depression Rating Scale (MADRS) score (11 vs. 11) (32). Investigators obtained participant identification numbers and randomized study drug assignments by using a Web‐based or telephone call‐in computerized drug management system." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The study drug was supplied in blinded bottles by the sponsor to the study sites, where they were dispensed according to computerized instructions." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 68.4% of varenicline group completed study (lost 15.6% in treatment and 16% in follow‐up); 66.6% of placebo group completed study (lost 21.9% in treatment and 11.5% in follow‐up) |
| Selective reporting (reporting bias) | Low risk | Results reported for all outcomes pre‐specified in NCT record. |
Ashare 2019.
| Study characteristics | ||
| Methods | Country: USA Setting: not explicitly reported, likely at home and in hospital Aim: to evaluate the safety and efficacy of varenicline for SC among people living with HIV Study design: parallel placebo‐controlled phase 3 RCT Dates conducted: October 2012‐September 2018 |
|
| Participants | 179 adult smokers all with a confirmed HIV diagnosis, currently smoking and motivated to quit. All treated with antiretroviral therapy with HIV loads < 1000 copies/mL and CD4+ counts > 200 cells/mm3. 32% female, mean age 48.6, average CPD at baseline 11.5 | |
| Interventions |
All participants received 6 sessions over 9 weeks of interactive behavioural support in person or by telephone. Treatment period was 12 weeks. |
|
| Outcomes | Primary: 7‐day PPA, CO‐confirmed, at weeks 12 and 24 Secondary: CA and time to relapse |
|
| Notes | New for 2022 update Funding source: "National Institute on Drug Abuse, Penn Center for AIDS Research, Penn Mental Health AIDS Research Center. Pfizer provided medication and placebo free of charge" Declaration of interests: "Dr. Schnoll has provided consultation to Pfizer, GlaxoSmithKline, and Curaleaf. Dr. Gross serves on a Pfizer Data and Safety Monitoring Board for a drug unrelated to smoking or HIV. Dr. Ashare has an investigator‐initiated grant from Novo Nordisk for a drug unrelated to the current study." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Eligible participants were randomized 1:1 by a computer‐generated protocol provided by the study statistician to the University of Pennsylvania’s Investigational Drug Service (IDS)" |
| Allocation concealment (selection bias) | Low risk | Randomisation conducted separately by the University of Pennsylvania’s Investigational Drug Service (IDS), and all other study personnel were blinded. Therefore, concealment was likely adequate. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Abstinence was biochemically validated and "All participants and study personnel, aside from IDS [Investigational Drug Service], were blinded from treatment arm allocation throughout the trial." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates were similar between study arms. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes from trial registry entry all reported in published results |
Aubin 2008.
| Study characteristics | ||
| Methods | Country: Belgium, France, Netherlands, UK, USA
Setting: 24 research centres Aim: to compare the efficacy of varenicline with nicotine patch, both open‐label Dates conducted: January 2005‐June 2006 Study design: open‐label randomised trial |
|
| Participants | 757 healthy adults, recruited from SC clinics or by local advertising, aged 18‐75, weight > 45.5 kg, BMI 15‐38, smoking ≥ 15 CPD. Varenicline arm 378, NRT arm 379. Mean age 42.9, 49.2% men, 93% white. Mean CPD 22.7. Previous use of nicotine patch 47.4%, previous use of bupropion 20%. Mean FTND 5.5. Exclusion criteria: standard pharmacotherapy trial criteria, + participants must not have been in a varenicline trial in previous year, or used NRT in previous 6 months | |
| Interventions |
All participants received Clearing the Air booklet at baseline, and brief counselling (≤ 10 min) at each clinic visit or by phone. TQD was at week 1 visit. Weekly visits throughout treatment phase, plus a phone call 3 days post‐TQD In follow‐up phase, clinic visits at weeks 13, 16, 24, 32, 40, 48 and 52, plus brief phone calls at weeks 14, 20, 28, 36 and 44 |
|
| Outcomes | CO‐confirmed CAR for last 4 weeks' treatment (varenicline weeks 9‐12, NRT weeks 8‐11) CO‐confirmed CAR at weeks 9‐24 and 9‐52 (varenicline) and 8‐24 and 8‐52 (NRT) 7‐day PPA at EoT and at weeks 24 and 52 Other outcomes: weight change, withdrawal symptoms (using MNWS and mCEQ), AEs Validation was by expired CO ≤ 10 ppm Dropouts and losses to follow‐up were included in the analyses as continuing smokers (ITT analysis) Attrition in treatment phase was 17.3% varenicline, 20.3% NRT. Losses to follow‐up 17% in each group 65.7% of varenicline and 62.2% of NRT groups completed study | |
| Notes | New for 2008 update Study funding: study funded by Pfizer Author declaration of interests: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Using a central computer‐generated sequence" |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Using an open‐label design". However, comparators were two active treatments, minimising risk of performance bias, and abstinence was biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Missing CO data were assumed to be < 10 ppm provided other conditions were met", i.e. no NRT other than prescribed patches. Missing = negative assumption reduced successes by 1 in each group |
| Selective reporting (reporting bias) | Low risk | All predicted outcomes fully reported |
Baker 2016.
| Study characteristics | ||
| Methods | Country: USA
Setting: 2 University sites in Wisconsin (Madison, Milwaukee) Aim: to compare the efficacy of varenicline with nicotine patch, and with combination NRT (C‐NRT) Dates conducted: May 2012‐November 2015 Study design: open‐label randomised trial (no placebo) |
|
| Participants | 1086 healthy adults, recruited from participants in the ongoing Wisconsin Smokers Health Study or by media and community outreach, aged 17+, smoking ≥ 5 CPD, motivated to quit
Varenicline arm 424, nicotine patch arm 241, combination NRT arm 421 Mean age 48.1, 47.9% men, 67% white. Mean CPD 17. Mean FTND 4.8 Exclusion criteria: standard pharmacotherapy trial criteria, CO < 4 ppm, no suicide attempts in previous 5 years, or current suicidal ideation, diagnosis or treatment of psychoses in previous 10 years |
|
| Interventions |
All participants received counselling (20 min at visits 1, 2 and 3, and 10 min by phone and at visits 4, 5) at 1 week pre‐TQD and at TQD, weeks 1, 4, 12 post‐TQD, plus phone call at week 8 In follow‐up phase, participants were contacted at weeks 26 and 52 by phone |
|
| Outcomes | All comparisons were based on varenicline and C‐NRT versus patch (reference arm), and on varenicline versus C‐NRT CO‐confirmed PPA at week 26 CO‐confirmed PA from day 7 post‐TQD to day 181 CO‐confirmed PPA at weeks 4, 12, 52 Other outcomes: adherence, withdrawals, AEs Validation was by expired CO ≤ 9 ppm and ≤ 5 ppm Dropouts and losses to follow‐up were included in the analyses as continuing smokers (ITT analysis) Withdrawal rates were 8.3% varenicline, 6.2% nicotine patch, 3.1% C‐NRT |
|
| Notes | The trial was funded by grant 5R01HL109031 from National Heart, Lung, and Blood Institute, and by grant K05CA139871 from the National Cancer Insitute New for 2016 update Author declaration of interests: "All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Stein reports receipt of data and safety monitoring board honoraria from Lilly and Abbott. No other disclosures were reported." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐based randomisation" |
| Allocation concealment (selection bias) | High risk | "Treatment assignment was unblinded" (open‐label) |
| Blinding (performance bias and detection bias) All outcomes | High risk | "Treatment assignment was unblinded" (open‐label) "The follow‐up telephone assessments were intended to be blinded, but a database search by interviewers could have revealed treatment assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low rates of attrition, ITT analysis used |
| Selective reporting (reporting bias) | Low risk | All predicted outcomes reported, protocol available |
Baker 2021.
| Study characteristics | ||
| Methods | Country: USA Setting: 2 research clinics in Wisconsin Aim: to compare combinations of varenicline plus the nicotine or placebo patch vs combinations used for either 12 weeks (standard duration) or 24 weeks (extended duration) Study design: double‐blind, 2 × 2 factorial RCT |
|
| Participants | 1251 adult smokers, smoking at least ≥ 5 CPD and motivated to quit. 54% female, mean age 49.1, average CPD at baseline 16 | |
| Interventions |
All participants received a total of 1.5 h (6 x 15 min over 10 weeks) of interactive behavioural support, either as face‐to‐face consultations or via telephone. Treatment period was either 12 or 24 weeks |
|
| Outcomes | Primary outcome: CO‐confirmed self‐reported 7‐day PPA at 52 weeks Secondary outcome: prolonged CO‐confirmed self‐reported abstinence ‐ from day 7‐week 52 after TQD) Verification: CO ≤ 5 ppm |
|
| Notes | New for 2022 update. Study funding: "Pfizer supplied the study with free active and placebo varenicline as per an investigator‐initiated research agreement. This research was supported by grant 5R01HL109031 from the National Heart, Lung, and Blood Institute and grant K05CA139871 from the National Cancer Institute (both awarded to the University of Wisconsin Center for Tobacco Research and Intervention)." Author declaration of interests: "Dr Baker reported receiving personal fees from the National Cancer Institute for editing a monograph and grants from the National Cancer Institute. Dr Bolt reported receiving grants from the National Institutes of Health. Dr Fiore reported receiving personal fees from the National Cancer Institute. No other disclosures were reported." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of software (SASProcPlanversion9.4) to generate randomisation schedule. Quote: "Participants were randomized to 1 of 2 levels of the 2 experimental factors (medication type and medication duration) via a database that used stratified permuted block randomization." |
| Allocation concealment (selection bias) | Unclear risk | Allocation process not reported (how interventions (active or placebo pills and patches) were put into the letters and sent to participants) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and study personnel blinded to allocation. Abstinence biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up < 50% overall, loss similar across treatment arms, missing observations were assumed to reflect current smoking |
| Selective reporting (reporting bias) | Low risk | Outcomes pre‐specified in trial registry all reported in results paper |
Benli 2017.
| Study characteristics | ||
| Methods | Study design: RCT Country: Turkey Setting: SC clinic Recruitment method: participants applied to the SC clinic directly by calling the Turkish Ministry of Health's 'stop smoking' helpline and making an appointment. |
|
| Participants | An unspecified number of participants were randomised. 405 participants were analysed. 17.5% female; average age 35.2; average age 35.2; average CPD 23; mean FTND 6.3 | |
| Interventions |
Common components: behavioural therapy support with a biopsychosocial approach |
|
| Outcomes | SC: 7‐day PPA at 12 months. Validated by a CO level ≤ 5 ppm | |
| Notes | Funding source: no funding Author conflicts of interest: the authors declare that they have no competing interests Unclear from study reporting if study is a genuine trial or a follow‐up of treatment in a clinic. However, treatment was randomly allocated, so we have treated this study as a trial. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Patients who were to receive the medication were randomly determined by the medication support center in order to provide a constant distribution rate of varenicline and bupropion and so that physicians would not be aware of the medication distribution.” Comment: no further detail is provided. |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Patients who were to receive the medication were randomly determined by the medication support center in order to provide a constant distribution rate of varenicline and bupropion and so that physicians would not be aware of the medication distribution.” Comment: no further detail is provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: “Patients who were to receive the medication were randomly determined by the medication support center in order to provide a constant distribution rate of varenicline and bupropion and so that physicians would not be aware of the medication distribution” Comment: some attempt appears to have been made to blind physicians to group assignment, however no further detail is given, so it is unclear whether participants and outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only those followed up at 12 months are included in analysis |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
Bohadana 2020.
| Study characteristics | ||
| Methods | Country: Israel Setting: Pulmonary Institute of Shaare Zedek Medical Center (SZMC), Jerusalem, Israel, in participants' home Aim: to assess whether varenicline preloading for 6 weeks prior to the TQD reduced pre‐quit smoke intake and enhanced 6‐month abstinence outcomes compared with the standard 1‐week preloading. Study design: parallel placebo‐controlled RCT |
|
| Participants | 242 daily smokers, smoking ≥ 10 CPD, and had smoked for ≥ 5 pack years, and were motivated to quit. 27% female, mean age 48, average CPD at baseline 25 | |
| Interventions |
TQD was 6 weeks post‐initiation of treatment. |
|
| Outcomes | Primary: 24‐week biochemically verified CAR from weeks 6–30 Secondary: 23‐week CAR from 1‐week post‐TQD (week 7) to week 30; 7‐day PPA at week 30 Biochemically validated: expired CO ≤ 5 ppm and urine cotinine equivalent concentration ≤ 1 mg/mL |
|
| Notes | New for 2022 update Study funding: "2013 Global Research Award for Nicotine Dependence (GRAND) supported by Pfizer, Inc. (#WI182915)" Author declaration of interests: "None" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomisation was accomplished by extracting a systematic sample via a pre‐prepared list of alternate allocations" |
| Allocation concealment (selection bias) | Unclear risk | "These procedures were performed by the randomisation monitor (VP) in a secure room where the study medication, provided free of charge by Pfizer Inc., NY, was stored in identically packaged, blinded bottles containing varenicline or placebo. Participants were referred to the secure room where they were randomly allocated (in a 1:1 ratio) to the experimental group." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind during preloading phase (open‐label afterwards as both groups were receiving identical treatments). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition similar in both groups, overall < 50% |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as in clinical trials record |
Bolliger 2011.
| Study characteristics | ||
| Methods | Countries: Brazil, Colombia, Costa Rica, Egypt, Jordan, Lebanon, Mexico, Saudi Arabia, South Africa, United Arab Emirates, Venezuela
Setting: 42 research centres (51.2% Latin America, 30.6% Africa, 18.2% Middle East) Aim: to test the efficacy and tolerability of varenicline in regions not previously exposed to SC RCTs of varenicline Dates conducted: April 2008‐August 2009 Study design: double‐blind placebo‐controlled RCT |
|
| Participants | 593 adults, recruited from SC clinics, aged 18‐75, weight > 45.5 kg, BMI 15‐38, smoking ≥ 10 CPD, motivated to quit. Randomised to varenicline 394 (390 got medication), or placebo 199 (198 got medication). Mean age 43.5, 63.6% men, mean CPD 23.8, mean FTND 6.0. 55% had no prior quit attempt Exclusion criteria: standard pharmacotherapy trial criteria, + participants must not have used NRT, bupropion, clonidine or nortriptyline in previous 6 months | |
| Interventions |
Treatment period was 12 weeks. All participants received You can quit smoking self‐help booklet (available in English, Spanish, Portugese and Arabic) at baseline, and brief counselling (≤ 10 min) at each clinic or telephone contact. TQD set for week 1. Clinic visits at weeks 2, 3, 4, 6, 8, 10 and 12 throughout treatment phase, plus a phone call 3 days post‐TQD In follow‐up phase, clinic visits at weeks 13, 16, 20 and 24, plus brief phone calls at weeks 14, 18 and 22 |
|
| Outcomes | Primary outcome: CO‐validated CAR at 9‐12 weeks Secondary outcomes: CO‐validated CAR at 9‐24 weeks; 7‐day PPA at weeks 12 and 24 Other outcomes: AEs, clinically significant changes in vital signs, SAEs Abstinence was assessed using the Nicotine‐Use Inventory (NUI); validation was by expired CO ≤ 10 ppm Dropouts and losses to follow‐up were included in the analyses as continuing smokers (ITT analysis). [4 (V) 1 (P) who did not receive allocated intervention re‐included in denominators in this analysis.] Attrition in treatment phase was 11.2% (V) and 20.6% (P); in follow‐up phase 2.5% (V) and 0.5% (P) | |
| Notes | New for 2012 update The study was funded and managed by Pfizer Inc Author declaration of interests: "(1) the institutions of Drs. Bolliger, Issa, Posadas‐Valay, and Safwat received financial support from Pfizer for the clinical trial; (2) Drs. Bolliger, Issa, Posadas‐Valay, and Safwat received no financial support from Pfizer for the submitted work; (3) Drs. Bolliger, Issa, Posadas‐Valay, and Safwat have specified relationships with Pfizer that might have an interest in the submitted work in the previous 3 years, including investigator payments, consulting honoraria, and grants; (4) their spouses, partners, and children have no financial relationships that may be relevant to the submitted work; and (5) Drs. Bolliger, Issa, Posadas‐Valay, and Safwat have no nonfinancial interests that may be relevant to the submitted work. Drs. Bolliger, Issa, Posadas‐Valay, and Safwat did not receive financial support with respect to the writing or development of the manuscript. Dr. Abreu, Mr. Correia, Dr. Park, and Mr. Chopra are employees of, and stockholders in, Pfizer." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Using a block randomization within each site, eligible participants were randomly assigned in a 2:1 ratio to receive varenicline or placebo" |
| Allocation concealment (selection bias) | Low risk | "a web‐based or telephone call‐in drug management system directed by the sponsor" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "All of the study personnel and participants were blinded to treatment assignment until the end of the nontreatment follow‐up phase" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts and attrition fully reported |
| Selective reporting (reporting bias) | Low risk | All predicted and expected outcomes reported |
Carson‐Chahhoud 2020.
| Study characteristics | ||
| Methods | Country: Australia
Setting: respiratory, cardiology, neurology, vascular and general medicine wards of 3 Adelaide (South Australia) hospitals
Aim: to evaluate efficacy and safety of varenicline + quitline counselling vs quitline counselling alone in people admitted with smoking‐related acute illnesses
Study design: phase II/III open‐label single‐blind RCT
Dates conducted: August 2008‐December 2011 Study name: Smoking Termination Opportunity for inPatients (STOP) |
|
| Participants | 392 adult smokers, aged 18‐75, smoking 10 CPD+, willing to quit, admitted with acute smoking‐related illnesses; randomised to varenicline + counselling (196) or counselling alone (196) Mean age 53, 32% women, 96% white, mean CPD 25, mean FTND 5.6 Exclusion criteria: standard pharmacotherapy criteria, acute or pre‐existing psychiatric illness, history of psychosis or suicidal ideation, use of varenicline in past 12 months |
|
| Interventions |
Both groups received Quit SA 5A behavioural counselling, i.e. maximum of 8 calls over 3 months. Also booklet Quit because you can, + stickers and fridge magnets. Participants had to set a TQD within first 2 weeks Contacts were attempted with all participants at days 3 and 5, weeks 1, 2, 3, 4, 12 (EoT). Additional contacts at weeks 26 and 52 |
|
| Outcomes | Primary outcome: self‐reported CAR (< 5 cigarettes in total) (2 weeks‐12 months) Secondary outcomes: CAR at 4, 12 and 26 weeks. 7‐day PPA each week for first 4 weeks; craving; reduced hospital bed utilisation; reduction in healthcare costs CO validation ≤ 10 ppm used only in "a random sub‐set of subjects" |
|
| Notes | Partially funded by the Department of Respiratory Medicine, Queen Elizabeth Hospital, Adelaide, SA New for 2012 update (study ID was Smith 2012; changed for 2015 update) Author declaration of interests: "The authors have read the journal’s policy and have the following potential competing interests: KVCC was paid an honorarium and provided with economy airfares and accommodation by Pfizer Australia to present at the 2019 Smoking Exchange Summit in New South Wales where she spoke about ‘culturalspecific issues in smoking cessation’ and as an invited panellist in a plenary session about ‘a national approach to smoking cessation’. In 2017 she received an honorarium and provided with economy airfares and accommodation to speak about 12‐month results of the STOP trial at the annual Pfizer Australia conference in New South Wales, Hunter Valley. This does not alter our adherence to PLOS ONE policies on sharing data and materials. There are no patents, products in development or marketed products associated with this research to declare." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A pre‐defined, central, computer‐generated randomization sequence was used to assign subjects in a 1:1 ratio to either 12 weeks of treatment with varenicline plus Quitline‐counseling or 12 weeks of Quitline‐counseling alone." |
| Allocation concealment (selection bias) | Low risk | "using opaque, sealed envelopes with consecutive numbers" |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label design. Attempt at single‐blinding (statistical investigator). "Participants and investigators were not blinded to treatment assignment". "Randomization and allocation concealment were performed by respiratory staff independent of the study". Biochemical validation of abstinence conducted in a random subset of participants via exhaled CO levels ≤ 10 ppm |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Missing data from questionnaire (e.g., a question missed when administering follow‐up) were randomly imputed via a computer programme" 84% varenicline completed the study at 52 weeks, vs 82% in the placebo group |
| Selective reporting (reporting bias) | Unclear risk | Reuslts unavailable for some secondary outcomes prespecified in NCT record, however results may still be forthcoming in future publications |
Chen 2020.
| Study characteristics | ||
| Methods | Country: USA Aim: to compare the efficacies of cNRT and varenicline with one another and with placebo, as a function of the rs16969968 genotype Dates: May 2015‐August 2019 Study design: genotype‐stratified randomised, double‐blind, placebo‐controlled clinical trial |
|
| Participants | 822 current smokers, smoking > 5 CPD, with exhaled CO ≥ 8 ppm. 54.6% female, mean age 46.5, baseline average CPD 17.6 | |
| Interventions |
Randomisation was stratified by rs16969968 genotype in blocks of 6 patients (2 per treatment per block) |
|
| Outcomes | Primary: 7‐day PPA at week 12 Secondary: 7‐day PPA with CO verification at 6 months; 7‐day PPA at 1 year by self‐report; AEs, and adherence Abstinence was defined as no self‐reported smoking for at least 7 days before the assessment with biochemical verification (CO < 8 ppm) for those self‐reporting abstinence. |
|
| Notes | New for 2022 update Funding by National Institute on Drug Abuse, Siteman Cancer Center and NCI Cancer Center Support Grant. Pfizer supplied the study medication free of charge. Author declaration of interests: "L.‐S.C. received free supply of study medication (varenicline and placebo) for this research project via an investigator‐initiated research agreement (IIR) from Pfizer. This free Pfizer product constitutes the support for this study. Pfizer supports the Principal Investigator to exercise the academic freedom and encourages publication of study results whether or not they are favorable for the Pfizer Product. R.M.C. or a member of his family owns stock in Pfizer Inc. L.J.B. is listed as an inventor on Issued US Patent 8,080,371 “Markers for Addiction” covering the use of certain single nucleotide polymorphisms in determining the diagnosis, prognosis, and treatment of addiction, and served as a consultant for the pharmaceutical company Pfizer Inc. (New York City, NY) in 2008. The spouse of N.L.S. is also listed as an inventor on Issued US Patent 8,080,371 “Markers for Addiction.” All other authors declared no competing interests for this work." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "SAS Version 9 statistical software will be used to generate the random assignment table stratified by CHRNA5 genotype rs16969968" From protocol |
| Allocation concealment (selection bias) | Low risk | "The group assignment and genotype will be coded to ensure that the double blind is maintained, and the interface will prevent staff from having access to the participant’s assignment and genotype until after the baseline and post treatment assessments have been completed." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo‐controlled and matched contact between groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition < 50% and similar between arms |
| Selective reporting (reporting bias) | Low risk | Results for all prespecified outcomes reported |
Chengappa 2014.
| Study characteristics | ||
| Methods | Country: Pittsburgh, USA
Setting: 2 outpatient clinics, Western Psychiatric Institute and Clinic; and Dubois Medical Regional Center, Pennsylvania Aim: to assess the efficacy and safety of varenicline to assist in SC among patients with bipolar disorder who were euthymic and motivated to quit smoking Study design: double‐blind placebo‐controlled RCT Dates conducted: February 2010‐March 2013 |
|
| Participants | 60 outpatient smokers with DSM‐IV‐diagnosed bipolar disorder, aged 18‐65, stable state or on medication, willing to quit in the next 30 days, 10+ CPD; randomised to varenicline (31) or placebo (29) Mean age 46, 69% women, 66% white, mean CPD 18.1, mean FTND 6.2 Exclusions: bupropion use (for SC); usual pharmacological criteria |
|
| Interventions |
All participants received 15‐min SC counselling at each visit. CO tested and pill counts at each visit. Participants in both groups could reduce the dosage if they wished. TQD was set for week 2 onwards (i.e. full dosage reached) Treatment period was 12 weeks. Weekly pill counts to assess adherence Safety data were reviewed blind monthly by an external independent data safety and monitoring board (DSMB) |
|
| Outcomes | Primary: 7‐day PPA, CO‐verified, at 12 weeks Secondary outcomes: 7‐day PPA at 24 weeks; CA at 12, 24 weeks Validation: CO < 10 ppm |
|
| Notes | New for 2016 update Study funding: "The National Institute of Mental Health (NIMH) of the NIH, under award R21MH087928 (Dr Chengappa), provided the main funding for this study. Pfizer provided drug/placebo and an investigator‐initiated grant, WS‐515343 (Dr Chengappa). These monies channeled through the University of Pittsburgh were used to offset costs of study procedures, participant payments, and a percentage of the time and effort of research staff and faculty salaries." Author declaration of interests: "Dr Turkin has served on the speaker’s bureau of Forest, Sunovion, and Otsuka; and owns shares of Pfizer stock. Dr George has received investigator‐initiated and contract research support from Pfizer, served as a consultant to Novartis, and received honoraria from National Institutes of Health (NIH) and American College of Neuropsychopharmacology. Drs Chengappa, Perkins, Brar, and Levine, and Mss Schlicht and Hetrick and have no financial disclosures with regards to this study." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated, other than "stratified by gender" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The treatment assignment was blinded to participating subjects, raters, investigators and statisticians" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 24 participants in each group completed treatment phase, and 24 (77%) and 20 (69%) completed full study in varenicline and placebo groups respectively Data were analysed using ITT with LOCF |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes from trial registry all reported |
| Other bias | Unclear risk | 8 participants (4 in each arm) were on bupropion for depression; 3/15 varenicline quitters and 1/3 placebo quitters were on long‐term bupropion |
Cinciripini 2013.
| Study characteristics | ||
| Methods | Country: Houston, TX, USA
Setting: University of Texas MD Anderson Cancer Center Aim: to assess the relative efficacy of varenicline and bupropion SR plus intensive counselling on SC and emotional functioning Study design: double‐blind placebo‐controlled RCT Dates conducted: August 2006‐October 2007 |
|
| Participants | 294 volunteer smokers, aged 18‐65, 5+ CPD, fluent in English, no uncontrolled chronic illness, baseline CO > 6 ppm. Mean age 44, 39% women, 66% white, mean CPD 20, mean FTND 4.5, mean baseline CO 24.5 ppm. Allocated to varenicline (86), bupropion (102) or placebo (106) Exclusions: usual pharma exclusions, current or history of psychotic disorder, moderate or high risk of suicidality, contra‐indications to varenicline or bupropion |
|
| Interventions |
Blinded study physician could adjust dosages to reduce side effects if required throughout study All participants got intensive counselling, i.e. 6 x in‐person 30‐min individual counselling sessions and 4 x 15‐min phone calls during treatment phase, based on MI techniques. During follow‐up, each participant got a 15‐min in‐person visit at 3 months and 6 months, and a 15‐min phone call at 4 months |
|
| Outcomes | Primary: PA at EoT Secondary: PA at 3 months post‐quit, 6 months post‐quit; CA at 3 months post‐quit, 6 months post‐quit; 7‐day PPA at EoT, 3 months post‐quit, 6 months post‐quit Validation: CO < 10 ppm. Self‐reported abstainers were asked to send a salivary cotinine sample (< 15 ng/mL) by post |
|
| Notes | New for 2016 update Funding: NIDA grant DA017073, NCI grant P50CA70907; varenicline supplied by Pfizer Author declaration of interests: "Dr Cinciripini served on the scientific advisory board of Pfizer, conducted educational talks sponsored by Pfizer on SC (2006‐2008), and has received grant support from Pfizer." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Adaptive randomization (minimization) was used to stratify the groups for sex, race/ethnicity, history of depression, and baseline smoking rate." |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Abstinence was biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to treatment and follow‐up reported, and key variables with significant differences (FTND, years of education) identified between those who stayed in and those who left. ITT analysis conducted. By 6 months, 21/86 for varenicline (24.4%), 29/102 for bupropion (28.4%) and 30/106 for placebo (28.3%) had been lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Abstinence outcomes reported as prespecified in trial registry record |
| Other bias | Unclear risk | Study began as nortriptyline vs bupropion; 3 months later, 19 people had been recruited to bupropion and 18 to placebo; nortriptyline was replaced as the target treatment by varenicline. The nortriptyline phase group (cohort 1) had 19 days of medication and 3 counselling sessions before TQD, whereas varenicline phase group (cohort 2) had 12 days of medication and 2 counselling sessions before TQD. No differences were found between the 2 cohorts, nor between overall findings and cohort 2 findings. Study authors therefore combined both groups into a single study cohort for analysis. |
Cinciripini 2018.
| Study characteristics | ||
| Methods | Country: USA Setting: hospital‐based outpatient clinic Aim: to determine if varenicline and bupropion combination treatment would result in higher abstinence rates than varenicline alone Study design: double‐blind, parallel‐group RCT |
|
| Participants | 386 adult smokers who were "moderately dependent", and motivated to quit. 41.56% female, mean age 48.97, baseline average CPD 19.66 | |
| Interventions |
Participants were randomised in a 3:1 ratio of active treatment:placebo All smokers received 13 x 15‐min in‐person individual behavioral SC support, and 2 brief supportive telephone calls. |
|
| Outcomes | Primary: PA at the 12‐month follow‐up as a function of treatment, specifically between the 2 active treatment groups Secondary: PA at EoT and the 6‐month follow‐up; all other abstinence definitions; nicotine withdrawal, affect; smoking reinforcement/satisfaction; sleep problems Self‐reported abstinence was validated by exhaled CO < 4 ppm |
|
| Notes | New for 2022 update Study funding: "United States National Institutes of Health (NIH) grant R01DA024709 (PI Cinciripini) and by The University of Texas MD Anderson’s Cancer Center Support Grant CA016672, funded by the National Cancer Institute (NCI)." Author declaration of interests: "Dr. Cinciripini served on the scientific advisory board of Pfizer Pharmaceuticals, conducted educational talks sponsored by Pfizer on SC (2006–2008), and has received grant support and medication support from Pfizer. Dr. Karam‐Hage participated in two multi‐site Pfizer‐funded trials and received varenicline from Pfizer to conduct 4 NIH funded trials. Pfizer (New York, NY) provided the active and matching placebo varenicline capsules. All other authors declare that they have no conflicts of interest." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Group assignment was generated with an algorithm developed and managed by study data managers, whose role was limited to data quality and integrity management. |
| Allocation concealment (selection bias) | Low risk | Allocation method not reported but likely low risk of selection bias "Participants were enrolled onto the trial by study staff […] Participants, medical and research staff who interacted with participants, and the study investigators were blinded to group assignment" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo controlled. "Participants, medical and research staff who interacted with participants, and the study investigators were blinded to group assignment". Cessation was biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete loss to follow‐up = 28%, similar loss to follow‐up across groups (36%, 29%, 23%) |
| Selective reporting (reporting bias) | Low risk | Outcomes on Clinicaltrials.gov record are reported |
Courtney 2021.
| Study characteristics | ||
| Methods | Country: Australia Setting: in the community Aim: to determine if cytisine is non‐inferior to varenicline regarding SC Study design: non‐inferiority, outcome‐blinded, open‐label RCT |
|
| Participants | 1452 adult smokers, motivated to quit. 51% female, mean age 43, baseline average CPD 18. Recruited via advertisements and SC telephone line | |
| Interventions |
No placebo control group. All participants received up to 6 telephone calls providing interactive behavioural support. |
|
| Outcomes | Primary: 6‐month CA verified using a CO breath test at 7‐month follow‐up Secondary: self‐reported 3‐ and 6‐month CA; 7‐day PPA at 4 weeks after baseline and at 4‐month and 7‐month follow‐up; cigarette consumption at each follow‐up |
|
| Notes | New for 2022 update Funding by Australian National Health and Medical Research Council, and the Australian government (under the Substance Misuse Prevention and Service Improvements Fund). Author declaration of interests: "Dr McRobbie reported receiving honoraria from Pfizer for speaking at smoking cessation meetings and attending advisory board meetings. Drs McRobbie and Walker reported previously receiving cytisine from Sopharma for the conduct of a noninferiority trial of cytisine vs nicotine replacement therapy. Dr Tutka reported serving as consultant to Aflofarm, which is a manufacturer of cytisine. Dr Mendelsohn reported receiving funding from Pfizer Australia, GlaxoSmithKline, and Johnson & Johnson Pacific for teaching, consulting, serving on an advisory board, and conference expenses. Dr Kwan reported receiving speaking fees from Pfizer. Dr Walker reported receiving cytisine from Achieve Life Sciences for the conduct of a noninferiority trial of cytisine (Tabex) vs varenicline; receiving investigator‐initiated grants and smoking cessation medication (varenicline) and matching placebo from Pfizer for the conduct of a relapse prevention trial in patients with chronic obstructive pulmonary disease who smoke; and serving as a consultant for and receiving honoraria and travel support for speaking at research meetings from Achieve Life Sciences and Pfizer (manufacturers of smoking cessation medications). Dr Gartner reported receiving grants from the Australian Research Council, Metro South Health Service, Central Queensland Hospital and Health Service, Arthritis Australia, and the HIV Foundation Queensland. Dr Ferguson reported previously serving as a consultant to Pfizer and GlaxoSmithKline Consumer Healthcare on matters relating to smoking cessation and harm minimization; having been a member of a scientific advisory board for Johnson & Johnson; receiving researcher‐initiated project grant funding from Pfizer (through the Grand initiative); and having provided consulting services to JUUL Labs Inc while working as a consultant for Pinney Associates. Dr Zwar reported receiving honoraria from Pfizer and GlaxoSmithKline for advice on smoking cessation education programs and for conference expenses. Dr West reported serving as a consultant to Pfizer, which manufactures varenicline, and receiving grants from Pfizer. Dr Farrell reported receiving unrestricted research funding from Mundipharma, Seqirus, and Indivior. No other disclosures were reported." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A data management system (UNICOM Intelligence) located at the social research center was used to assign a unique randomization number to study participants using a pregenerated randomization list embedded in the system" |
| Allocation concealment (selection bias) | Low risk | "A data management system (UNICOM Intelligence) located at the social research center was used to assign a unique randomization number to study participants using a pregenerated randomization list embedded in the system. Only an independent statistician located at the social research center had access to the pregenerated randomization list. After the baseline computer‐assisted telephone interview, the datamanagement system was used to randomly assign each participant to 1 of the treatment groups in a 1:1 ratio (Figure 1)." The permuted block randomization used unequal block sizes of 12 and 16. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Open‐label study; however, both groups received active SC treatment. Abstinence was biochemically validated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates were < 50% and similar between arms. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes from protocol reported in results paper |
Cox 2022.
| Study characteristics | ||
| Methods | Study design: RCT Country: USA Recruitment method: through media and physician referral Double‐blind parallel‐group RCT |
|
| Participants | 500 adult African Americans who smoked 5+ CPD, motivated to quit. Baseline average CPD: 12.5. Average age 52. 87% female | |
| Interventions |
All participants received culturally relevant individualised counselling over 16 weeks |
|
| Outcomes | 7‐day PPA at 6 months biochemically validated by saliva cotinine 15 ng/mL | |
| Notes | Study funding: “This work was funded by the National Institute on Drug Abuse (R01DA035796; Cox). Pfizer Global Pharmaceuticals and Pfizer Global Medical Grants provided study medication. Analytical chemistry was supported in part by P30 DA 012393 from the National Institute on Drug Abuse and S10 RR 026437 from the National Center for Research Resources (Benowitz). Dr Tyndale is supported by the Canada Research Chairs Program (Canada Research Chair in Pharmacogenomics). Dr Ahluwalia is supported by P20GM130414, a National Institutes of Health– funded Centers of Biomedical Research Excellence. […] The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.” Author declaration of interests: “Dr Nollen reported receiving grants from the National Institutes of Health during the conduct of the study and nonfinancial support from Pfizer, which has provided study medication for this and other studies, outside the submitted work. Dr Mayo reported receiving grants from the National Institutes of Health during the conduct of the study. Dr Faseru reported receiving grants from the National Institutes of Health during the conduct of the study. Dr Ellerbeck reported receiving grants from the National Institute on Drug Abuse during the conduct of the study. Dr Tyndale reported consulting for Quinn Emanuel Urquhart & Sullivan and Ethimos and receiving grant support from the National Institutes of Health and Canadian Institutes of Health Research during the conduct of the study for work on other projects. Dr Benowitz reported receiving personal fees from Pfizer for serving on an advisory committee and Achieve Life Sciences for serving on a data and safety monitoring board and being a paid expert witness in litigation against tobacco companies outside the submitted work. Dr Ahluwalia reported receiving personal fees from Respira Technologies as a consultant and equity owner outside the submitted work. No other disclosures were reported” |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "participants were assigned to receive varenicline or placebo in a 3:2 ratio via computergenerated individual random assignment to allow more participants to receive active treatment and concurrently strengthen evaluation of medication adverse events (Figure 1). Randomization was stratified by smoking level (≤10 or >10 cigarettes/d) and sex. A block size of 10 was used to generate the randomization schema." |
| Allocation concealment (selection bias) | Low risk | "The university pharmacy bottled study medication, per randomization code, labelled with participant ID numbers to ensure that study staff, investigators, and participants were blinded to treatment assignment." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Abstinence was biochemically validated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates similar between study arms |
| Selective reporting (reporting bias) | Low risk | Outcomes pre‐specified in NCT record are reported in results paper |
De Dios 2012.
| Study characteristics | ||
| Methods | Country: Rhode Island and Massachussetts, USA
Setting: Butler Hospital, RI Aim: to assess the relative efficacy of varenicline and NRT on SC in Latino light smokers (< 10 CPD) Study design: feasibility double‐blind placebo‐controlled 3‐arm RCT Dates conducted: April 2010‐July 2010 |
|
| Participants | 32 Latino volunteer light smokers (≤ 10 CPD), aged 18+, willing to set a quit date. Mean age 42, 53.1% women, mean CPD 7.6, mean FTND 2.9. Allocated to varenicline (10), NRT (11), placebo (11) Exclusions: usual pharmacological conditions, on NRT or smokeless tobacco, history of suicide attempts, chronic or acute psychiatric disorder, employed as a pilot, driver or heavy machinery operator |
|
| Interventions |
All participants received a 30‐min face‐to‐face "culturally informed" SC behavioural intervention, + a non‐tailored self‐help brochure, all available in both English and Spanish. All participants were compensated for attendance, and could receive travel vouchers if necessary |
|
| Outcomes | Primary: 7‐day PPA at 6 months Secondary: 7‐day PPA at weeks 1, 2, 1 month, 2 months, 3 months, 4 months; adherence Validation: CO < 5 ppm; salivary cotinine (not for the NRT group) > 10 mg/mL AEs not reported in detail, although study reports that "There was no pattern that suggested a higher side‐effect profile for those in the varenicline group" |
|
| Notes | New for 2016 update Funding: NCI grant R01CA0129226‐S1 (De Dios); NIDA grant K24‐DA000512 (Stein); NIDA grant R01‐DA1234, NCI grant K07‐CA95623 (Stanton) Author declaration of interests: "The authors of this article do not have any conflicts of interest to declare." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Study personnel and participants in the two‐pill groups (varenicline and varenicline‐placebo) were blinded to treatment condition. The research pharmacy maintained the study blind." NRT group could not be blinded to treatment; outcome assessment blinding not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up were fully reported; per protocol and ITT analyses conducted |
| Selective reporting (reporting bias) | Unclear risk | Protocol unavailable |
Dogar 2020.
| Study characteristics | ||
| Methods | Country: Bangladesh and Pakistan Setting: 32 tuberculosis treatment centres, and at home Aim: to assess the effectiveness and safety of cytisine as a SC aid in patients with tuberculosis Study design: randomised, double‐blind, placebo‐controlled trial |
|
| Participants | 2472 adult daily smokers with pulmonary tuberculosis diagnosed in the previous 4 weeks, and motivated to quit smoking. 24.1% female, mean age 42.5, average CPD at baseline 11 | |
| Interventions |
Randomisation was 1:1 across trial arms. All participants received interactive in‐person behavioural support, which included a 5‐min session on the day of enrolment, and a 10‐min session on the quit date. |
|
| Outcomes | Primary: CA at 6 months, defined as self‐report (of not having used > 5 cigarettes, bidis, a water pipe, or smokeless tobacco products since the quit date) Abstinence was confirmed biochemically by a breath CO < 10 ppm. Secondary: CA at 12 months; PPA at weeks 5 and 12, and 6 and 12 months; early lapses; clinical tuberculosis score; chest X‐ray grade, sputum smear microscopy; adherence to tuberculosis treatment; tuberculosis treatment outcome; Mood and Physical Symptoms Scale score; Strength of Urges To Smoke scores; time to first use of tobacco product of the day |
|
| Notes | New for 2022 update Funding by European Union Horizon 2020 research and innovation programme. Aflofarm provided the study drug cytisine at no cost. Author declaration of interests: "Dogar 2020: DK reports an unrestricted grant from Pfizer in 2009 for an investigator initiated trial on the effectiveness of practice nurse counselling and varenicline for SC in primary care (Dutch Trial Register NTR3067). EK reports participation in clinical studies by Pfizer, and has received grants from Pfizer, before and during the conduct of the study. KS reports a research grant from Pfizer to study the effects of varenicline on waterpipe SC. All other authors declare no competing interests." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation plus stratification; Randomisation lists were pregenerated by the trial statistician at York Trials Unit (University of York). |
| Allocation concealment (selection bias) | Low risk | "Randomisation lists were pregenerated by the trial statistician at York Trials Unit (University of York) and held securely at the offices of the study partners (ARK Foundation [Dhaka, Bangladesh] and The Initiative [Islamabad, Pakistan]) for sequential allocation by masked trial coordinating staff." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Abstinence was biochemically validated and investigators, clinicians, and patients were masked to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates were very low and similar between study arms. |
| Selective reporting (reporting bias) | Low risk | Pre‐specified outcomes are reported. |
EAGLES 2016.
| Study characteristics | ||
| Methods | Countries: Argentina, Australia, Brazil, Bulgaria, Canada, Chile, Denmark, Finland, Germany, Mexico, New Zealand, Russian Federation, Slovakia, South Africa, Spain, USA
Setting: multiple research centres Aim: to evaluate the efficacy of varenicline, bupropion SR, nicotine patch and placebo for SC, and to assess how far this is moderated by the presence of psychiatric disorders Dates conducted: November 2011‐January 2015 Study design: phase 4 triple‐dummy, double‐blind placebo‐controlled parallel‐group RCT Study name: EAGLES (Evaluating Global Events in a Global Smoking Cessation Study) |
|
| Participants | Treatment‐seeking adult smokers, aged 18‐75, smoking at least 10 CPD, with exhaled CO > 10 ppm at screening. Participants in the psychiatric disorder cohort had to have a current or lifetime stable psychiatric diagnosis, confirmed by Structured Clinical Interview for DSM‐IV disorders, i.e. no acute exacerbation in the previous 6 months, no changes to treatment for 3 months, not imminently likely to change treatment, and not at risk of self‐harm. Allocation for the phychiatric cohort was balanced across 4 diagnostic group disorders, i.e. mood, anxiety, psychotic, personality 44% men, mean age 46, mean CPD 20.7, mean FTND 5.8 Exclusions: past or current diagnosis of schizophreniform or delusional disorders, all delirium, dementia, and other cognitive disorders, and all substance‐induced disorders (other than nicotine) In the psychiatric disorders group, 70% had primary affective disorders, 19% anxiety disorders, 9.5% psychotic disorders, 0.6% personality disorders, and at least ⅓ were taking psychotropic medications Participants were grouped by the presence (4116) or absence (4028) of a history of psychiatric disorders Psychiatric disorders: varenicline 1032; bupropion 1033, NRT patch 1025, placebo 1026 No psychiatric disorders: varenicline 1005; bupropion 1001, NRT patch 1013, placebo 1009 Safety analyses were conducted in cohorts of 4074 (psychiatric) and 3984 (non‐psychiatric) |
|
| Interventions |
All participants received counselling (up to 10 min) at all contacts, and were encouraged to complete all visits even if treatment was discontinued Participants were monitored at weeks 1‐6, 8, 12, 13, 16, 20, 24; contacts were up to 15 face‐to‐face visits and 11 telephone visits |
|
| Outcomes | At least 1 SAE of anxiety depression, feeling abnormal, or hostility, and/or moderate or severe AE of agitation, aggression, delusions, hallucinations, homicidal ideation, mania, panic paranoia, psychosis, suicidal ideation/behaviour/completed 4‐week abstinence confirmed by CO < 10 ppm at weeks 9‐12, and 15‐week abstinence at weeks 9‐24 In the non‐psychiatric cohort, 78.9% completed treatment, and 78.4% completed the study In the psychiatric cohort, 74.2% completed treatment, and 77.8% completed the study |
|
| Notes | Trial was funded by Pfizer and GlaxoSmithKline Author declaration of interests: "RMA reports receiving grants from Pfizer and Alkermes, and providing consulting and advisory board services to Pfizer, Arena Pharmaceuticals, and Cerecor. RMA’s writing of this manuscript was supported, in part, by National Institute on Alcohol Abuse and Alcoholism grant numbers U01 AA013641 and R01 AA019720; National Institute on Drug Abuse/Veterans Affairs Cooperative Studies numbers 1031 and 1032; and Veterans Affairs Merit Award number NEUA‐003‐08S. NLB reports providing consulting and advisory board services to Pfizer and GlaxoSmithKline, and having been a paid expert witness in litigation against tobacco companies. RW reports receiving grants from Pfizer, Johnson & Johnson, and GlaxoSmithKline, and receiving personal fees for advisory board services from Pfizer and GlaxoSmithKline. RW’s salary is funded by Cancer Research UK. AEE reports receiving grants from Pfizer and Forum Pharmaceuticals, and receiving personal fees for advisory board services from Pfizer and Reckitt Benckiser. AEE’s writing of the manuscript was supported by a National Institute on Drug Abuse Career Award in Patient‐Oriented Research, number K24 DA030443. LSA, TM, DL, and CR are employees and stockholders of Pfizer. JA is an employee of GlaxoSmithKline and stockholder of that company. AK is a PAREXEL employee working on behalf of GlaxoSmithKline. The opinions expressed in this Article are the authors’ own, and do not necessarily reflect the views of their employers." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated randomisation schedule ... using a block size of 8 (1:1:1:1 ratio) for each of the 20 diagnosis by region combinations" |
| Allocation concealment (selection bias) | Low risk | "Investigators obtained participant identification numbers via a web‐based or telephone call‐in drug management system" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Study product kit codes did not allow deciphering of randomised treatment or block size. As such, participants, investigators, and research personnel were masked to treatment assignment" "The triple dummy design feature required participants to take study medication as masked tablets dispensed in separate varenicline and bupropion pill bottles each with matching placebo along with either applying active or placebo patches on a daily basis" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All losses fully accounted for; ITT analysis conducted throughout |
| Selective reporting (reporting bias) | Low risk | All protocol‐reported outcomes were addressed. |
Ebbert 2015.
| Study characteristics | ||
| Methods | Country: 65 centres in 10 countries: USA (14), Australia (4), Canada (6), Czech Republic (7), Egypt (3), Germany (7), Japan (6), Mexico (4), Taiwan (7), UK (7)
Setting: clinics, hospitals, academic research centres Aim: to determine the efficacy and safety of varenicline for increasing smoking abstinence rates through smoking reduction Study design: double‐blind placebo‐controlled multinational RCT Study name: Reduce to Quit Dates conducted: July 2011‐July 2013 |
|
| Participants | 1510 adult smokers, unwilling to quit abruptly (within the next month), aged 18+, smoking mean 10+ CPD, interested in trying to quit within 3 months. Mean age 44.5, 43.7% women, mean CPD 20.7, mean FTND 5.5. Allocated to varenicline (760) or placebo (750) Exclusions: suicidal behaviour in previous 2 years or history of suicide attempts; major depression, anxiety; diagnosis of psychosis, panic disorder, post‐traumatic stress disorder, schizophrenia |
|
| Interventions |
All participants asked to reduce their smoking rate by 50% by week 4, by 75%+ by week 8, and 100% by week 12. Individual 10‐min counselling at each visit (18 face‐to‐face and 10 phone calls), + a copy of Clearing the air: quit smoking today |
|
| Outcomes | Primary: CAR at weeks 15‐24 Secondary: CAR at weeks 21‐24, 15‐52, 21‐52; 7‐day PPA at weeks 24, 52 Validation: CO < 10 ppm |
|
| Notes | New for 2016 update Funding: Pfizer Author declaration of interests: "The authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Ebbert reports receiving grants from JHP Pharmaceuticals, Orexigen, Pfizer, and the National Institutes of Health; and receiving personal fees from GlaxoSmithKline during the conduct of the study. Dr Hughes reports receiving personal fees from Alere/Free and Clear, Cicatelli, DLA Piper, Dorrffermeyer, Embera, Equinox, GlaxoSmithKline, Healthwise, Nicoventures, Pfizer, Pro Ed, Publicis, Selecta, and nonfinancial support from Swedish Match. Dr West reports receiving grants, personal fees, and nonfinancial support from Pfizer, GlaxoSmithKline, and Johnson & Johnson. His salary is funded by a Centre grant from Cancer Research UK. Dr Rennard reports being an advisory board member for A2B Bio, Almirall, Dalichi Sankyo, Novartis, Nycomed, and Pfizer; consulting for Almirall, APT Pharma/Britnall, AstraZeneca, Boehringer Ingelheim, Chiesi, CSL Behring, Decision Resource, Dunn Group, Easton Associates, Gerson, GlaxoSmithKline, MedImmune, Novartis, Pearl, Roche, Takeda, and Theravance; receiving lecture fees from CME Incite, Forest, Novis, PriMed, and Takeda; receiving grants from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Johnson & Johnson, Novartis, and Otsuka. Drs Russ, McRae, Yu, Dutro, Park, and Ms Treadow are employees and stockholders of Pfizer." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomized to receive varenicline or placebo for 24 weeks of treatment in a 1:1 ratio using a computer generated block randomization schedule within site" |
| Allocation concealment (selection bias) | Low risk | "Investigators obtained participant identification numbers and treatment group assignments through a web‐based or telephone call‐in drug management system" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Participants, investigators, and research personnel were blinded to randomization until after the database was locked" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses fully reported. ITT analyses conducted for efficacy (760 varenicline, 750 placebo), and treated denominators for safety outcomes (751 varenicline, 742 placebo) |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes from trial registry reported in results paper |
Ebbert 2016.
| Study characteristics | ||
| Methods | Country: USA Setting: community Aim: to evaluate the efficacy of varenicline for smoking abstinence among light smokers Study design: placebo‐controlled RCT |
|
| Participants | 93 participants, all light smokers (defined as 5‐10 CPD), and motivated to quit. 60% female, mean age 37, baseline mean CPD 7.7 | |
| Interventions |
All participants received in‐person interactive behavioural support, consisting of 6 x 10‐min sessions |
|
| Outcomes | Primary: 7‐day PPA rate at week 12. Biochemically verified with exhaled C0 < 8 ppm Secondary: PA |
|
| Notes | Funding and medication provided by Pfizer Author declaration of interests: "JOE reports grants from Pfizer during the conduct of the study and grants from Takeda, the US Department of Defense, and the NIH outside the submitted work. RTH reports grants from Pfizer during the conduct of the study and grants from the NIH outside the submitted work. JTH reports grant from Pfizer during the conduct of the study. ITC and DRS declare no conflicts of interest." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computer‐generated randomization sequence assigned participants in a 1:1 ratio to treatment conditions" |
| Allocation concealment (selection bias) | Low risk | "Pharmacy personnel dispensed study medication into containers labeled according to study identification numbers. Study participants, investigators, and pharmacy staff were blinded to treatment assignment." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Study participants, investigators, and pharmacy staff were blinded to treatment assignment." Abstinence biochemically validated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study attrition rates were high and varied significantly between study arms: " Study completion rates were 62% (28/45) in the varenicline group and 42% (20/48) in the placebo group." |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported in methods are reported in results. However, trial protocol or registry entry unavailable |
Fouz‐Roson 2017.
| Study characteristics | ||
| Methods | Country: Spain Setting: Stop‐Smoking Clinic Aim: to test for differences between standard 1‐ and 0.5‐mg doses of varenicline in smoking abstinence, adherence and side effects Study design: open‐label randomised parallel‐group controlled trial with 1‐year follow‐up |
|
| Participants | 484 adult smokers aged between 20‐80. 40% female, mean age 50.67 years and a smoking history of 37.5 pack‐years. 60.5% had already made at least one attempt to quit previously. Exclusion criteria were: advanced neoplastic disease, advanced chronic kidney disease, pregnancy, breastfeeding or inclusion in a fertility programme |
|
| Interventions |
All participants received 6 sessions of interactive behavioural support, as face‐to‐face cognitive behavioural therapy sessions. Participants also had access to telephone support as needed. |
|
| Outcomes | Primary: continuous self‐reported abstinence during 1 year, verified biochemically with exhaled CO < 6 ppm Secondary: adherence and side effects |
|
| Notes | New for 2022 update Funding research grant awarded by the Fundación Neumosur, within the ‘Asociación de Neumología y Cirugía Torácica del SUR’, partial funding by Grifols Author declaration of interests: "All the authors are grateful for the research grant awarded by the Fundación Neumosur within the ‘Asociación de Neumología y Cirugía Torácica del SUR’, as well as the funding made available to the Respiratory Unit of the Virgen Macarena University Hospital by Grifols. Research Ethics Committee Registry. C.P.‐ C.I. 2217 (promotor code and internal code)." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was conducted by a statistician from our hospital, who generated a list of study group assignments for 460 patients, using computer‐generated blocks of 10, with MAS software version 2.1". |
| Allocation concealment (selection bias) | Unclear risk | Concealment not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Study open label, but abstinence was biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates were very low in both study arms. |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registry entry found |
Gonzales 2006.
| Study characteristics | ||
| Methods | Country: USA
Setting: 19 research centres Aim: to test the efficacy and safety of varenicline for SC Dates conducted: June 2003‐April 2005 Study design: double‐blind placebo‐controlled parallel‐group RCT |
|
| Participants | 1025 healthy adult volunteers, recruited through media advertising. Allocated to varenicline (352), bupropion (329) or placebo (344). 54% men, 79% white, mean age 42.4, mean CPD 21, mean FTND score 5.3. No significant differences between groups at baseline Exclusion criteria: standard pharmacotherapy trial criteria, + use of tobacco products other than cigarettes; use of NRT, clonidine, nortriptyline within last month; BMI < 15 or > 38 or weight < 45.5 kg; any prior use of bupropion or varenicline | |
| Interventions |
Treatment period was 12 weeks. All participants received Clearing the Air self‐help booklet at baseline, and brief counselling (≤ 10 min) at each clinic visit. Weekly visits throughout treatment phase, plus a phone call 3 days post‐TQD In follow‐up phase, clinic visits at weeks 13, 24, 36, 44 and 52, plus brief phone calls at weeks 16, 20, 28, 32, 40 and 48 |
|
| Outcomes | Primary outcome: CO‐validated CAR at 9‐12 weeks Secondary outcomes: CO‐validated CAR at 9‐24 weeks and 9‐52 weeks; 7‐day PPA at weeks 12, 24 and 52 Other outcomes: weight change, withdrawal symptoms (using MNWS, QSU‐brief and mCEQ), AEs Validation was by expired CO ≤ 10 ppm Dropouts and losses to follow‐up were included in the analyses as continuing smokers (ITT analysis) Attrition in treatment phase was 31.5%, losses to follow‐up 16% of treatment completers | |
| Notes | This trial had the same aims and study design as Jorenby 2006 Study funding: "This study was supported by Pfizer Inc, which provided funding, study drug and placebo, and monitoring" Author declaration of interests: "Dr Gonzales reports having received research contracts from Pfizer, SanofiAventis, GlaxoSmithKline, and Nabi Biopharmaceuticals; consulting fees and honoraria from Pfizer, SanofiAventis, and GlaxoSmithKline; and owning 5 shares of Pfizer stock. Dr Rennard reports having had or currently having a number of relationships with companies who provide product and/or services relevant to outpatient management of chronic obstructive pulmonary disease. These relationships include serving as a consultant for Adams, Almirall, Altana, Array Biopharma, AstraZeneca, Aventis, Biolipox, Centocor, Dey, Critical Therapeutics, GlaxoSmithKline, Johnson & Johnson,Merck, Novartis,Ono Pharma,Otsuka, RJ Reynolds, Roche, Sankyo, Schering‐Plough, Scios, and Wyeth; advising regarding clinical trials for Altana, AstraZeneca, Aventis, Centocor, GlaxoSmithKline, Novartis, Pfizer, and Philip Morris; and speaking at continuing medical education programs and performing funded research both at basic and clinical levels for Altana, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, and Novartis. Dr Nides reports having received research grants, consulting fees, and honoraria from Pfizer, SanofiAventis, and GlaxoSmithKline. Dr Oncken reports having received research grants, consulting fees, and honoraria from Pfizer; receiving, at no cost, nicotine replacement and placebo products from GlaxoSmithKline for SC studies; and receiving honoraria from Pri‐Med. Drs Azoulay, Watsky, Gong, Williams, and Reeves and Mr Billing report owning Pfizer stock or having stock options in Pfizer" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "predefined ... computer‐generated randomization sequence", 1:1:1, using block size of 6, stratified by centre |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Participants and investigators were blinded to drug treatment assignments[, and] ... were not encouraged to guess their treatment assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Considered abstinent if, at next non‐missed visit, they reported no smoking... Missing CO but otherwise OK considered abstinent, except at end of study, where all criteria had to be present |
| Selective reporting (reporting bias) | Low risk | All expected and predicted outcomes covered |
Gonzales 2014.
| Study characteristics | ||
| Methods | Country: 37 centres in 8 countries: USA (8), Australia (4), Belgium (4), Canada (4), Czech Republic (4), France (3), Germany (5), UK (5)
Setting: clinics, hospitals, academic research centres Aim: to evaluate the efficacy and safety of retreatment with varenicline in smokers who had taken varenicline for ≥ 2 weeks in a previous SC attempt Study design: double‐blind placebo‐controlled multinational RCT Dates conducted: December 2010‐November 2012 |
|
| Participants | 498 adult smokers (varenicline 251, placebo 247) with previous use of 2+ weeks of varenicline at least 3 months prior to screening, aged 18+, CPD 10+, motivated to quit. Mean age 47.5, 50.4% women, 93% white, mean CPD 20.5, mean FTND 5.5 | |
| Interventions |
Brief (< 10 min) counselling at each contact. TQD set for week 1 visit. Clinic visits at weeks 1, 2, 3, 4, 6, 8, 9, 10, 11, 12; 13, 16, 24, 32, 40, 48, 52. Brief phone calls at weeks 5, 7, 14, 20, 36, 44. Dosage could be halved if intolerable |
|
| Outcomes | Primary: CAR at weeks 9‐12, 9‐52 Secondary: CAR at weeks 9‐24; 7‐day PPA at weeks 12, 24, 52 Validation: CO < 10 ppm |
|
| Notes | New for 2016 update Funding: Pfizer Author declaration of interests: "D.G. reports grants, and nonfinancial and other support from Pfizer during the conduct of the study; and grants, stock ownership, and nonfinancial and other support from Pfizer, grants from Nabi Biopharmaceuticals, and personal fees and nonfinancial support from GlaxoSmithKline outside the submitted work. P.H. reports grants from Queen Mary University of London during the conduct of the study and grants and personal fees from Pfizer, McNeil, GlaxoSmithKline, and Novartis outside the submitted work. L.P. reports grants from Pfizer outside the submitted work. K.N. reports funding and nonfinancial support from Pfizer during the conduct of the study and personal fees from Pfizer (Belgium) outside the submitted work, for serving as an advisory board member and for lectures. L.‐J.T, T.D.M., and J.T. are employees of Pfizer." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Eligible participants were randomly assigned to receive either varenicline or placebo at a 1:1 ratio for 12 weeks of drug treatment using computer‐generated block randomization within each site" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up fully reported. ITT analyses conducted |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes from trial registry reported in results paper |
Gray 2019.
| Study characteristics | ||
| Methods | Country: USA Setting: university outpatient clinic Aim: to evaluate whether varenicline, when added to brief cessation counselling, is efficacious and safe for SC in adolescents Study design: parallel double‐blind placebo‐controlled RCT |
|
| Participants | 157 treatment‐seeking adolescent current smokers, aged 14‐21, and motivated to quit. 40% female, mean age 19.4, baseline average CPD 11.3. Participants must have smoked daily for at least 6 months and had at least one failed attempt at quitting. Exclusion criteria: history of mood or psychotic disorder, suicidality, homicidality, or significant hostility or aggression; substance dependence other than nicotine, unstable medical disorder, pregnancy, breastfeeding, or use of medications with SC efficacy; known hypersensitivity to varenicline |
|
| Interventions |
Participants were randomised in a 1:1 ratio. All participants received in‐person interactive behavioural support weekly for 12 weeks. |
|
| Outcomes | Primary: 7‐day abstinence at the end of the 12 weeks of treatment. Confirmed with urine‐cotinine level Secondary: weekly abstinence throughout active treatment; abstinence at post‐treatment follow‐up visits; time to first 7‐day abstinence |
|
| Notes | New for 2022 update. Study authors provided additional results data upon request Funding by grants from then NIH, and varenicline and placebo were supplied at no cost by Pfizer. Author declaration of interests: "Dr Gray reported consulting for Pfizer, Inc, and receiving grant support from the National Institutes of Health (NIH). Mr Baker reported receiving grant support from the NIH. Dr McClure reported receiving grant support from the NIH. Dr Tomko reported receiving grant support from the NIH during the conduct of the study and outside the submitted work. Dr Squeglia reported receiving grant support from the NIH. Dr Saladin reported receiving grant support from the NIH. Dr Carpenter reported consulting for Pfizer, Inc, during the conduct of the study. No other disclosures were reported." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated randomised, but randomisation method not specified |
| Allocation concealment (selection bias) | Unclear risk | Concealment not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Trial described as double blinded and equivalent face to face contact identical between study arms. Abstinence biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates were similar between study arms |
| Selective reporting (reporting bias) | Low risk | Pre‐specified outcomes reported |
Heydari 2012.
| Study characteristics | ||
| Methods | Country: Tehran, Iran
Setting: SC clinics in the Tobacco Prevention and Control Research Centre, Shahid Beheshti University of Medical Sciences Aim: to evaluate the effectiveness of varenicline in the Iranian community of tobacco quitters and compare it with other treatment methods Study design: 3‐arm randomised parallel clinical study Dates conducted: 2009‐2010 Analysis: 91 participants per group were required |
|
| Participants | 272 treatment‐seeking participants: brief advice (91), NRT (92), varenicline (89). 41.2% women, mean age 42.5 years, mean FTND 5.5 | |
| Interventions |
All participants were managed by the same physician. All received brief (5 min) education and counselling at 4 x weekly sessions. TQD was day 14 |
|
| Outcomes | Abstinence at 6 months and 12 months, in person or by phone, verified by expired CO (cut‐off value not given) | |
| Notes | Funding: Masih Daneshvari Hospital Research Institute, Tehran New for 2016 update Author declaration of interests: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Smokers who attended the clinic for help in quitting were divided randomly" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label; blinding of outcome assessors not reported, but abstinence biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition: "Participants entered the study of their own accord and none left the study" |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes from trial registry reported in results paper |
Hong 2015.
| Study characteristics | ||
| Methods | Country: China Setting: hospital Study design: parallel RCT |
|
| Participants | 300 COPD inpatients/perioperative patients, all smokers. 3% female, mean age 58 | |
| Interventions |
All participants received interactive behavioural support, either in person or on the telephone |
|
| Outcomes | Self‐reported CA at 24 weeks | |
| Notes | Study funding: not reported Author declaration of interests: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated randomised but methods not reported |
| Allocation concealment (selection bias) | Unclear risk | Concealment not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study reported no participants lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Protocol unavailable |
Hurt 2018.
| Study characteristics | ||
| Methods | Country: USA Study design: phase II/III randomised quadruple‐blind placebo‐controlled trial Study period: July 2011‐April 2013 |
|
| Participants | 33 adult alcohol‐dependent smokers | |
| Interventions | Varenicline 1 mg 2 x day for 12 weeks vs placebo | |
| Outcomes | PA at 12 weeks (EoT), and at 6 months Abstinence self‐reported and biochemically validated by exhaled CO | |
| Notes | Study results posted on www.clinicaltrials.gov May 2014 Study funding: "This current study was supported by grant R21 DA30645 to Dr Richard D. Hurt and Pfizer IIR to Dr J. Taylor Hays" Author declaration of interests: "R.T.H. reports research grants from Pfizer and the NIH. J.T.H. reports research grants from Pfizer. J.O.E. reports grants from Pfizer during the conduct of the study and grants from Takeda, the U.S. Department of Defense and the NIH outside the submitted work. R.D.H. reports a research grant from the NIH. All other others have nothing to disclose" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomized" but method not reported |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Pharmacy personnel dispensed study medication into containers labeled with study identification numbers. Study participants, investigators, and pharmacy staff were blinded to treatment assignment." Abstinence biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High attrition rates: 4/16 varenicline group (1 withdrawal, 3 lost), 12/17 placebo group (7 withdrawals, 5 lost) |
| Selective reporting (reporting bias) | Low risk | Pre‐specified outcomes from NCT record all reported in results paper |
Ikonomidis 2017.
| Study characteristics | ||
| Methods | Country: Greece Setting: hospital SC clinic Study design: parallel RCT Recruitment method: approached everyone attending SC clinic |
|
| Participants | 188 adults who smoke and were motivated to quit. Average age 50. 45% female. Baseline CPD 30 | |
| Interventions |
No behavioural support |
|
| Outcomes | SC at 52 weeks, verified by CO < 10 ppm | |
| Notes | Study funding: "The study was funded by a grant from the Hellenic Cardiac Society" Author declaration of interests: "The authors declared they do not have anything to disclose regarding conflict of interest with respect to this manuscript." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "One hundred eighty‐eight current smokers were randomized to varenicline or nicotine replacement treatment (NRT) for a 3‐month period" No further information provided |
| Allocation concealment (selection bias) | Unclear risk | "One hundred eighty‐eight current smokers were randomized to varenicline or nicotine replacement treatment (NRT) for a 3‐month period" No further information provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Both groups received active treatments and SC was biochemically validated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition rates unclear |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
Ioakeimidis 2018.
| Study characteristics | ||
| Methods | Country: Greece Setting: hospital Aim: to compare the SC efficacy and safety of e‐cigarettes vs varenicline among patients who continue to smoke after acute coronary syndrome Study design: parallel RCT |
|
| Participants | 54 smokers, smoking ≥ 10 CPD and expressing motivation to quit. All had previous acute coronary syndrome (mean time interval between acute coronary syndrome and study entry 6 ± 2.7 months) Baseline average CPD 12 |
|
| Interventions |
All participants received "low intensity" interactive behavioural support. |
|
| Outcomes | Primary: PPA, defined by self‐report of complete abstinence in the 7 days before the 24‐week clinic visit. Abstinence was not biochemically verified. | |
| Notes | New for 2022 update Study funding: not reported Author declaration of interests: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided on randomisation methods |
| Allocation concealment (selection bias) | Unclear risk | No information provided on randomisation methods |
| Blinding (performance bias and detection bias) All outcomes | Low risk | 2 effective treatments provided and equal amounts of contact between arms |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not specified |
| Selective reporting (reporting bias) | Unclear risk | Abstract/poster only so not able to judge |
| Other bias | High risk | Abstract and poster only. 2 different figures presented for quit rate in e‐cigarettes arm (no difference in those presented in varenicline arm) between abstract and poster. Poster percentage aligns with figure, so using that (16.5%) as opposed to abstract figure (32.5%). Contacted study authors but no reply. Calculated n quit based on percentages but unclear what denominators were; e‐cigarettes calculates back to whole number for e‐cigarettes but not for varenicline |
Johns 2017a.
| Study characteristics | ||
| Methods | Study design: RCT Country: India Setting: not reported Recruitment method: not reported |
|
| Participants | 200 people who smoked with one of the following: previous lung disease, a family history of lung cancer, past cancer treatment, lowered immunity, previous smoking‐related cancers, exposure to certain chemicals and radon gas | |
| Interventions |
|
|
| Outcomes | PPA at 52 weeks, verified by exhaled CO < 10 ppm | |
| Notes | Study funding: not reported Author declaration of interests: not reported Abstract only |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition rates not reported |
| Selective reporting (reporting bias) | Unclear risk | Both continuous and PPA collected but only point prevalence reported (abstract only) |
Johns 2017b.
| Study characteristics | ||
| Methods | Study design: RCT Country: India Setting and recruitment method not specified |
|
| Participants | 300 participants randomised | |
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Funding source: none specified Author conflicts of interest: none specified Abstract only. Insufficient data to add to MA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States trial was randomised, no further detail given |
| Allocation concealment (selection bias) | Unclear risk | No relevant information given |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | States only that the study was "double‐blind", no further detail given |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No relevant information given |
| Selective reporting (reporting bias) | Unclear risk | Protocol unavailable |
Jorenby 2006.
| Study characteristics | ||
| Methods | Country: USA
Setting: 14 research centres Aim: to test the efficacy and safety of varenicline for SC Dates conducted: June 2003‐March 2005 Study design: double‐blind placebo‐controlled RCT |
|
| Participants | 1027 healthy adult volunteers. Allocated to varenicline (344), bupropion (342) or placebo (341). 58% men, 84% white, mean age 43.3, mean CPD 22, mean FTND score 5.3. No significant differences between groups at baseline Exclusion criteria: standard pharmacotherapy trial criteria, + use of tobacco products other than cigarettes; use of NRT, clonidine, nortriptyline within last month; BMI < 15 or > 38 or weight < 45.5 kg; any prior use of bupropion or varenicline | |
| Interventions |
Treatment period was 12 weeks. All participants received brief counselling (≤ 10 min) at each clinic visit Weekly visits throughout treatment phase, plus a phone call 3 days post‐TQD In follow‐up phase, clinic visits at weeks 13, 24, 36, 44 and 52, plus brief phone calls at weeks 16, 20, 28, 32, 40 and 48 |
|
| Outcomes | Primary outcome: CO‐validated CAR at 9‐12 weeks Secondary outcomes: CO‐validated CAR at 9‐24 weeks and 9‐52 weeks; 7‐day PPA at weeks 12, 24 and 52 Other outcomes: weight change, withdrawal symptoms (using MNWS, QSU‐brief and mCEQ), AEs Validation was by expired CO ≤ 10 ppm Dropouts and losses to follow‐up were included in the analyses as continuing smokers (ITT analysis) Attrition in treatment phase was 29.3%, losses to follow‐up 8% of treatment completers | |
| Notes | This trial had the same aims and study design as Gonzales 2006. Study funding: "The data reported in this article were derived from a clinical trial sponsored by Pfizer Inc, which provided funding, study drug and placebo, and monitoring." Author declaration of interests: "Dr Jorenby reported receiving research support from Pfizer, Nabi Biopharmaceutical, Sanofi‐Aventis and consulting fees from Nabi Biopharmaceutical. Dr Hays reported receiving a research grant from Pfizer. Dr Rigotti reported receiving research grant funding and consulting fees from GlaxoSmithKline, which markets smoking cessation medications, and Pfizer and Sanofi‐Aventis, which are developing smoking cessation medications. Dr Rigotti also reported receiving consulting fees from Merck, which is developing smoking cessation medications." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated list" |
| Allocation concealment (selection bias) | Low risk | "completed centrally ... and sites used an electronic system to assign participants to treatment" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "in a double‐blind manner" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | CA for missed visits: if self‐reported abstinent at next visit, assumed abstinent, except at week 52 visit when all criteria had to be met |
| Selective reporting (reporting bias) | Low risk | All expected and predicted outcomes covered |
King 2022.
| Study characteristics | ||
| Methods | Country: USA Setting: 2 outpatient clinics Aim: to determine whether combined treatment with varenicline tartrate and nicotine patch improves CA from cigarette smoking among smokers who drink heavily Study design: double‐blind, placebo‐controlled, superiority RCT |
|
| Participants | 122 current smokers participants, all alcohol‐dependent. Eligible participants smoked between 5 and 30 CPD and drank heavily (> 14 drinks per week for men or > 7 drinks per week for women and ≥ 1 heavy drinking day (defined as > 5 drinks per occasion for men or > 4 drinks per occasion for women) per month for the past year) and were motivated to quit. 45.1% female, mean age 44, baseline average CPD 11.8 | |
| Interventions |
Intervention duration was 12 weeks. Participants were randomised in a 1:1 ratio. All participants received 2x interactive behavioural support sessions 2 weeks apart. |
|
| Outcomes | Primary: self‐reported cigarette CA through weeks 9‐12. Abstinence was biochemically confirmed at the week 12 study visit with exhaled CO < 10 ppm. Secondary: frequency of weekly drinking and weekly heavy drinking during the study period |
|
| Notes | New for 2022 update Funding by grant P30 CA14599 from the University of Chicago Medicine Comprehensive Cancer Center, Global Research Award for Nicotine Dependence from Pfizer, Women’s Board of the University of Chicago Cancer Research Foundation. Author declaration of interests: "Dr King reported receiving grants from Pfizer during the conduct of the study and personal fees from the Respiratory Health Association outside the submitted work. Dr de Wit reported being on the board of directors of PharmAla Biotech; being a scientific advisor to Awakn Life Sciences, Gilgamesh Pharmaceuticals, and Schedule I Therapeutics; and receiving research support from the Beckley Foundation outside the submitted work. Dr Grant reported receiving grants from Biohaven Pharmaceuticals, Otsuka Pharmaceutical Company, and Promentis Pharmaceuticals outside the submitted work. No other disclosures were reported." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A 1:1 randomization list was generated by the data manager using a random number generator..." |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo‐controlled, matched behavioural support and biochemically validated cessation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates low and similar between study arms |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported either in published results paper or on trial registry website |
Le Mao 2020.
| Study characteristics | ||
| Methods | Country: France Setting: hospital, home Aim: to demonstrate that, in smoker patients hospitalised for COPD exacerbation, early initiation of varenicline for 12 weeks, combined with an intensive counselling, is associated with a higher CA rate as compared to intensive counselling alone Study design: multicentre, prospective, double‐blind, randomised trial |
|
| Participants | 81 smokers hospitalised for COPD exacerbation. Participants must have been hospitalised for at least 24 h, and smoked ≥ 10 CPD during the last year, and were motivated to quit smoking. 39.5% female, mean age 56.8, baseline average CPD 23.1 Exclusion criteria: patients who used concomitant treatment for SC, past history of severe depression requiring therapy drugs within 5 years or/with ≥ 2 episodes of severe depression requiring medication, attempted suicide |
|
| Interventions |
Participants were randomised on a 1:1 ratio. All participants received interactive behavioural support in person (weeks 1, 4, 8, 12, 26, 52) and on the telephone (weeks 2, 18, 34, 42). |
|
| Outcomes | Primary: CA rate at week 52, defined by the rate of participants who presented the all following criteria: SC for weeks 8‐12, exhaled CO level ≤ 10 ppm at each clinic visit. < 6 cigarettes to weeks 12‐52. complete SC during the 7 previous days before week 52 Secondary CA rate at weeks 12 and 26; punctual abstainer rate (PAR) defined as patient’s rate who did not smoke within 7 days prior to follow‐up visit with exhaled CO level of < 10 ppm at weeks 12, 26 and 52; AEs; nicotine substitute consumption |
|
| Notes | New for 2022 update Funding by "Programme Hospitalier de Recherche Clinique", Ministère de la Santé, Pfizer. Author declaration of interests: "All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr. Couturaud reports having received research grant support from Pfizer and fees for board memberships or symposia from Bayer and Astra Zeneca and having received travel support from Bayer, Daiichi Sankyo, Leo Pharma, Intermune and Actelion. Dr. Le Mao declares he has no conflict of interest related to this research. Dr. Tromeur declares she has no conflict of interest related to this research. Dr. Paleiron declares he has no conflict of interest related to this research. Dr. Sanchez reports having received research grant support from Bayer, Daiichi Sankyo and Portola Pharmaceuticals, and fees or non‐financial support for consultancy activities from Actelion, GlaxoSmithKline, Boehringer Ingelheim and Chiesi. Dr Gagnadoux declares he has no conflict of interest related to this research. Dr Jouneau reports grants from AIRB, Boehringer Ingelheim, LVL, Novartis and Roche, and personal fees from Actelion, AIRB, AstraZeneca, BMS, Boehringer Ingelheim, Chiesi, GSK, LVL, Mundipharma, Novartis, Pfizer and Roche. Dr A. Magnan reports personal fees and non‐financial support from GlaxoSmithKline, Novartis, Boehringer Ingelheim, AstraZeneca, Stallergnes, ALK, MundiPharma, Teva, Menarini and Meda Pharma, during the past 5 years. Dr Hayem‐Vannimenus declares she has no conflict of interest related to this research. Dr Dansou declares she has no conflict of interest related to this research. Dr Proust reports fees for consulting from Novartis and personal fees or nonfinancial support from AstraZeneca, Boehringer, Chiesi, Mundifarma, Glaxo‐Smith‐Klein, Novartis, Pearl, Portola, Roche, Sanofi, and Teva. Ms Dion declares he has no conflict of interest related to this research. Dr Larhantec declares he has no conflict of interest related to this research. Ms Le Brestec declares she has no conflict of interest related to this research. Dr. Dewitte declares he has no conflict of interest related to this research. Dr. Roche declares he has no conflict of interest related to this research. Dr Leroyer reports having received research grant support from Pfizer and fees for board memberships or symposia from Bayer and Astra Zeneca and having received travel support from Bayer, Daiichi Sankyo, Leo Pharma, Intermune and Actelion. [...] The funding source was not involved in designing or conducting the study, collecting, managing, analysing or interpreting the data, preparing, reviewing or approving the manuscript, or deciding to submit this for publication. An academic steering committee assumed overall responsibility for all these steps. Dr Couturaud takes responsibility for data access and integrity of the data." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Investigators obtained patient randomisation numbers and treatment group assignments through a central computerised internet‐based system." |
| Allocation concealment (selection bias) | Low risk | "Investigators obtained patient randomisation numbers and treatment group assignments through a central computerised internet‐based system." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Abstinence biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates similar between study arms |
| Selective reporting (reporting bias) | Low risk | Study registered: NCT01694732. Prespecified outcomes reported |
| Other bias | Unclear risk | "The main limitation of our study is the small sample size due to premature interruption in relation with the premature interruption of pharmaceutical funding (the study was co‐financed by an institutional French grant and pharmaceutical grant)." |
Lerman 2015.
| Study characteristics | ||
| Methods | Country: USA and Canada Setting: community Aim: to evaluate whether a genetically informed biomarker of nicotine clearance, the nicotine metabolite ratio (NMR; 3′‐hydroxycotinine:cotinine), predicts response to NRT or varenicline for SC Study design: 4‐site NMR‐stratified multicentre, placebo‐controlled RCT |
|
| Participants | 1246 smokers, aged 18–65 years old and reported smoking ≥ 10 CPD for ≥ 6 months (verified with exhaled CO > 10 ppm). 44% female, mean age 46, baseline average CPD 18 | |
| Interventions |
Participants were assigned in a 1:1:1 ratio, by NMR group. All participants received interactive behavioural support. This included 1 in‐person session pre‐quit, and 4 x 15‐min follow‐up telephone calls. |
|
| Outcomes | Primary: 7‐day PPA at EoT (week 11) to estimate the pharmacological effect by NMR group during the medication period. Abstinence was biochemically verified with exhaled CO < 8 ppm. Secondary end points were side‐effects, withdrawal symptoms, and 6‐month and 12‐month quit rates |
|
| Notes | New for 2022 update Funding by National Institute on Drug Abuse, National Cancer Institute, National Human Genome Research institute, National Institute on General Medical sciences, Abramson Cancer Centre at the University of Pennsylvania, Commonwealth of Pennsylvania Department of Health, Canadian Institutes of Health Research, Canada Foundation for Innovation, Ontario Ministry of Research and Innovation. Pfizer Inc. provided varenicline and placebo pills at no cost. Author declaration of interests: "Lerman received study medication and placebo, as well as support for medication packaging, from Pfizer. She has also consulted to Gilead, and has been a paid expert witness in litigation against tobacco companies. Cinciripini served on the scientific advisory board of Pfizer Pharmaceuticals, conducted educational talks sponsored by Pfizer on smoking cessation from 2006‐2008, and has received grant support from Pfizer. Schnoll received medication and placebo free of charge from Pfizer for a different project, and has consulted to Pfizer and GlaxoSmithKline. George has had both investigator‐initiated and industry‐sponsored grants from Pfizer in the past 12 months, and serves on a Data Monitoring Committee for Novartis. Benowitz has served as a consultant to several pharmaceutical companies that market smoking cessation medications and has been a paid expert witness in litigation against tobacco companies. Tyndale has acted as a consultant to pharmaceutical companies, primarily on smoking cessation. The remaining authors report no conflicts of interest." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Biostatistician, independent of study investigators, developed the randomisation procedure which was integrated into a centralised data management system. Subjects were randomised to the treatment arms in a 1:1:1 ratio. Randomisation was stratified by baseline NMR status and study site, and blocked in blocks of 12 patients (4/treatment block) to ensure approximate balance" |
| Allocation concealment (selection bias) | Low risk | Allocation performed using centralised data management system |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Abstinence biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates similar between study arms |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported |
Littlewood 2017.
| Study characteristics | ||
| Methods | Country: USA Setting: at home with in‐person study visits (unclear setting) Aim: to evaluate the effectiveness of varenicline for SC, and examine the influence of psychological factors on treatment outcome Study design: double‐blind, placebo‐controlled, RCT |
|
| Participants | 205 current cigarette smokers interested in quitting, 34% female, mean age 34, baseline average CPD 16 | |
| Interventions |
Participants were randomised in a 1:1 ratio. Interventions lasted 12 weeks. All participants received in‐person interactive behavioural support, comprising a 30‐min baseline counselling session and a 10‐20‐min follow‐up at weeks 2, 6, and 12. |
|
| Outcomes |
|
|
| Notes | New for 2022 update Funding by National Institute on Drug Abuse (NIDA) 1R01DA025074‐01A2. Author declaration of interests: "The authors declare that they have no conflict of interest." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Urn‐randomisation used |
| Allocation concealment (selection bias) | Low risk | "In order to maintain the blind, the pharmacist controlled the pre‐generated urn randomization schedule and packed varenicline tablets in opaque capsules with microcrystalline cellulose, an inert powder commonly used in packaging medications." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and staff measuring the outcome were blinded to allocation. Abstinence biochemically validated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 52% of participants lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No trial registration but reports expected outcomes |
Mercie 2018.
| Study characteristics | ||
| Methods | Country: France Setting: 30 HIV clinics in French hospitals Aim: to assess the efficacy and safety of varenicline with counselling to aid SC in people living with HIV Study design: randomised, parallel, double‐blind, multicentre, placebo‐controlled phase 3 trial |
|
| Participants | 248 people living with HIV who had smoked at least 10 CPD for ≥ 1 year. Participants were motivated to stop smoking, were not dependent on another psychoactive substance. Participants with no history of depression or suicide attempt were eligible. 17% female, mean age 45, baseline number of CPD 20 | |
| Interventions | Participants were randomised on a 1:1 ratio. Intervention duration was 12 weeks
All participants received interactive, in‐person, behavioural support comprising 10‐15 sessions over 1 year |
|
| Outcomes | Primary: proportion of smokers continuously abstinent from week 9‐week 48, biochemically verified by exhaled CO < 10 ppm | |
| Notes | New for 2022 update Funding by ANRS, Emerging Infectious diseases, Pfizer. Author declaration of interests: "The institution of JR has received funds from Institut National de la Santé et de la Recherche Médicale (Inserm)‐France Recherche Nord et Sud Sida‐hiv hépatites (ANRS). XD has received grant support from Pfizer. J‐MM is a member of scientific advisory boards of Merck laboratories, Gilead, Bristol‐Myers Squibb, ViiV Healthcare, and Janssen and has received grant support from Merk laboratories and Gilead. BS has received honoraria for seminars from Merck laboratories, Gilead, and Janssen and support for the IAS 2014 conference from Merck laboratories. The institution of CF and GC has received grant support from Inserm‐ANRS and Pfizer. GC has received grant support for International Workshop on HIV and Hepatitis Observational Databases from Gilead, Tibotec‐Janssen, Roche, Merck laboratories, Janssen Pharmaceuticals, Boehringer Ingelheim, Bristol‐Myers Squibb, GlaxoSmithKline, ViiV Healthcare, Mylan, Abbvie, and Abbott and grant support for ongoing clinical trials of Inserm‐ANRS from Gilead, Tibotec‐Janssen, Merck laboratories, Boehringer Ingelheim, and Abbott. All other authors declare no competing interests." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation (1:1) was done centrally via electronic case report software (CS software, Ennov‐Clinsight), on the basis of a list generated with SAS software, version 9.2 (PROC PLAN procedure, block size 8)" |
| Allocation concealment (selection bias) | Low risk | "Only the trial statistician (JA) had access to the randomisation list during the trial." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | CO ≤ 10 ppm and "Patients and investigators were masked to treatment group allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "71 (58%) participants in the varenicline group versus 81 (65%) in the placebo group completed follow‐up at 48 weeks" |
| Selective reporting (reporting bias) | Unclear risk | Not all prespecified outcomes reported in results paper. However, authors state "Other objectives described in the protocol will be reported elsewhere". |
Nahvi 2014a.
| Study characteristics | ||
| Methods | Country: USA
Setting: 3 urban outpatient clinics for substance use disorder (SUD) in the Bronx, NY Aim: to test the efficacy and safety of varenicline for SC among opioid‐dependent people on a maintenance regimen Study design: randomised quadruple‐blind controlled trial Dates conducted: August 2009‐September 2011 |
|
| Participants | 112 smokers in methadone treatment for substance abuse, aged 18+, CPD 5+, motivated to quit within next 6 months. Allocated 57 varenicline, 55 placebo. 52% women, 54% Hispanic, mean CPD 15, mean FTND 4 | |
| Interventions |
All participants set a TQD 1 week after treatment began. All were offered structured, brief (≤ 10 min) individual in‐person counselling by a physician or tobacco specialist at baseline and at 2‐, 4‐, 8‐ and 12‐week visits. All participants were also offered free quitline support |
|
| Outcomes | 7‐day PPA at 12 and 24 weeks Validation: expired CO < 8 ppm |
|
| Notes | New for 2016 update Funding: National Center for Research Resources grant UL1 RR025750 to SN, and the National Institute on Drug Abuse grants K23 DA025736 to SN and R25 DA023021 to SN and JHA Author declaration of interests: "The authors have no connection with the tobacco, alcohol, pharmaceutical or gaming industries or anybody substantially funded by one of these organizations. The Albert Einstein College of Medicine Office of Biotechnology and Business Development receives funding from Pfizer, which manufactures varenicline; neither that office nor Pfizer had any role in the study design, analyses or decision to submit the manuscript for publication. None of the authors have direct or indirect funding from Pfizer" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Treatment group allocation was computer‐generated, and stratified by the three clinic sites in blocks of six within each stratum" |
| Allocation concealment (selection bias) | Low risk | "a central data manager concealed the allocation sequence using a password‐protected file, assigned subjects to treatment groups and faxed pre‐printed medication orders to the study pharmacist. The pharmacist prepared the research medication by compounding varenicline tablets or placebo lactose powder to create identical‐appearing capsules. The pharmacist marked medication bottles with subjects’ study identification numbers, and delivered medications for individual study subjects to clinical sites, where they were distributed to each subject by the research assistant" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The pharmacist prepared the research medication by compounding varenicline tablets or placebo lactose powder to create identical‐appearing capsules. The pharmacist marked
medication bottles with subjects’ study identification numbers, and delivered medications for individual study subjects to clinical sites, where they were distributed to each subject by the research assistant" "All subjects, research assistants, counsellors and physicians were blinded to treatment assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses during treatment (varenicline 6, placebo 9) and during follow‐up (varenicline 2, placebo 3) fully reported; ITT analyses conducted |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes from trial registry reported in results paper |
Nakamura 2007.
| Study characteristics | ||
| Methods | Country: Japan
Setting: 19 study sites
Aim: to test efficacy, safety and tolerability of 3 doses of varenicline over 12 weeks Dates conducted: not stated Study design: double‐blind, placebo‐controlled, parallel‐group RCT |
|
| Participants | 619 healthy Japanese adult volunteers, aged 20‐75, smoking ≥ 10 CPD. Allocated to varenicline 0.25 mg x 2/day (153), 0.5 mg x 2/day (156), 1.0 mg x 2/day (156) or placebo x 2/day (154). 1 participant withdrew before treatment, and is excluded from ITT denominator. 1 road traffic accident death removed from varenicline group at 52 weeks Participants stratified by level of nicotine dependence, measured by Tobacco Dependence Screener scale (≥ 5) and by FTND. 515 (83.3%) classified as nicotine‐dependent Demographic data only supplied for nicotine‐dependent group (515/618): 75% men, mean age 39.8, mean CPD 24, mean FTND score 5.6 Exclusion criteria: standard pharmacotherapy trial criteria, + use of NRT within last 30 days, use of pipe tobacco, snuff, chewing tobacco, cigars within last 30 days and throughout trial | |
| Interventions |
Treatment period 12 weeks, 1st week titrated dosage. All participants received a SC booklet Clearing the Air at baseline, + brief counselling (≤ 10 min) at each clinic visit. Weekly visits throughout treatment phase, plus a 5‐min phone call at TQD and +3 days post‐TQD In follow‐up phase, clinic visits at weeks 13, 16, 24, 36, 44 and 52, plus brief phone calls at weeks 20, 28, 32, 40 and 48 |
|
| Outcomes | Primary outcome: CO‐validated CAR at 9‐12 weeks Secondary outcomes: CO‐validated CAR at 9‐24 weeks and 9‐52 weeks; 7‐day PPA at weeks 2, 12, 24 and 52 Validation was by expired CO ≤ 10 ppm Other outcomes: withdrawal symptoms (using MNWS, QSU‐brief and mCEQ), AEs Dropouts and losses to follow‐up were included in the analyses as continuing smokers (ITT analysis) Attrition in treatment phase was 6.4%, losses to follow‐up 11.4% of treatment completers (excluding 1 death) | |
| Notes | Trial was funded by Pfizer Inc
New for 2008 update Author declaration of interests: "Dr. Nakamura has received research contracts from Pfizer Japan Inc. (Tokyo, Japan), Novartis Pharma K.K. (Tokyo, Japan), and Sanofi‐Aventis K.K. (Tokyo, Japan), and a research grant from Pfizer Research Foundation (Tokyo, Japan). Dr. Oshima has received research contracts from Pfizer Japan Inc" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated list of random numbers" |
| Allocation concealment (selection bias) | Low risk | "randomized to 1 of the 4 treatment groups in a 1:1:1:1 ratio using a central procedure" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "double‐blinding of subjects and investigators was maintained throughout the study". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No comment on level or handling of missing data |
| Selective reporting (reporting bias) | High risk | CA rates for all participants reported, but demographics, withdrawal and craving measures, and PPA for nicotine‐dependent group only |
NCT01162239.
| Study characteristics | ||
| Methods | Country: USA Setting: outpatient medical care Aim: to assess the efficacy of the relapse prevention treatment to other extended treatments, such as health education, brief contact, and varenicline Study design: open‐label parallel 4‐arm RCT |
|
| Participants | 271 adult smokers, smoking 5+ CPD, who have all completed 12‐week course of varenicline + counselling 38.9% female, mean age 48.9. | |
| Interventions |
All participants in all trial arms receive 12 weeks of varenicline treatment at standard dosage of 2 x 0.5 mg/day |
|
| Outcomes | Primary: 7‐day PPA at weeks 12, 24, 52, 64, 104 Biochemically verified with exhaled CO at < 8 ppm Secondary: comparison of combined extended vs brief treatment at weeks 24 and 52 |
|
| Notes | New for 2022 update Funding by Pfizer (manufacturer‐funded study), and National Institute on Drug Abuse Author declaration of interests: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided on randomisation method (trial registry only) |
| Allocation concealment (selection bias) | Unclear risk | No information provided (trial registry only) |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label (and no placebo used in standard varenicline duration arm) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up < 50% and similar between arms |
| Selective reporting (reporting bias) | Low risk | No evidence of selected reporting. All abstinence outcomes reported as planned |
Niaura 2008.
| Study characteristics | ||
| Methods | Country: USA
Setting: 5 research centres Aim: to test the efficacy and safety of varenicline in smokers allowed to modify their own dosage regimen Dates conducted: December 2001‐June 2003 Study design: double‐blind placebo‐controlled RCT |
|
| Participants | 320 healthy adult volunteers, aged 18‐65, smoking ≥ 10 CPD. Allocated to varenicline (160), or placebo (160) 52% men, 91% white, mean age 42, mean CPD 22, mean FTND score 5.4 Exclusion criteria: standard pharmacotherapy trial criteria, + use of NRT within last 3 months | |
| Interventions |
Treatment period 12 weeks, 1st week titrated dosage up to 0.5 mg x 2/day. All participants received a SC booklet Clearing the Air at baseline, + brief counselling (≤ 10 min) at each clinic visit. Weekly visits throughout treatment phase In follow‐up phase, clinic visits at weeks 13, 24, and 52 weeks, plus monthly phone calls between visits |
|
| Outcomes | Primary outcome: CAR at 4‐7, 9‐12 and 9‐52 weeks Validation was by expired CO ≤ 10 ppm Secondary outcomes: CO‐confirmed CAR at 9‐24 weeks; CO‐confirmed 7‐day PPA Other outcomes: mean modal dosage; withdrawal symptoms (using MNWS, QSU‐brief and mCEQ), AEs Dropouts and losses to follow‐up were included in the analyses as continuing smokers (ITT analysis) Attrition in treatment phase was 22% in varenicline group and 29% in placebo group; losses to follow‐up by week 52 were 36% from varenicline group and 43% from placebo group | |
| Notes | The trial was funded by Pfizer Inc
New for 2010 update Author declaration of interests: " KEW, KRR, and CBB are employees of Pfizer and have stock or stock options in Pfizer. RN has received consulting fees from Pfizer, GlaxoSmithKline, Sanofi‐Aventis, Merck, Constella, and LLC. DEJ has received consulting fees from Nabi Biopharmaceutical and receives research support from Pfizer, Nabi Biopharmaceutical, and Sanofi‐Aventis. FTL serves on speakers’ bureaus for Pfizer and Merck and is a consultant on an advisory panel with Pfizer. JTH received grant support from Pfizer. JEP received grant support from Merck, DepoMed, Pfizer, Novartis, Takeda, Sanofi‐Aventis, Symbollon, TAP, and GlaxoSmithKline. Editorial support was provided by Ray Beck, Jr, PhD of Envision Pharma and was funded by Pfizer, Inc. " |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly permuted blocks and a pseudo‐random number generator" |
| Allocation concealment (selection bias) | Low risk | "participants were assigned in a 1:1 ratio to varenicline treatment or placebo in the numerical order that they were accepted to the study" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "double‐blind" but no further information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data imputed if prior and subsequent abstinence confirmed, otherwise assumed still smoking |
| Selective reporting (reporting bias) | Low risk | All expected and predicted outcomes covered |
Nides 2006.
| Study characteristics | ||
| Methods | Country: USA
Setting: 7 research centres
Aim: to test efficacy, tolerability and safety of 3 doses of varenicline over 6 weeks Dates conducted: February 2000‐January 2003 Study design: phase 2 double‐blind placebo‐controlled RCT |
|
| Participants | 638 healthy volunteer smokers, aged 18‐65, smoking at least 10 CPD on average. 48% men, 87% white, average age 42, average CPD 20, mean FTND 5.5. Allocated to varenicline group 1 (128), group 2 (128), group 3 (127), bupropion (128), placebo (127) Exclusion criteria: standard pharmacotherapy trial criteria, + use of bupropion within previous 12 months, use of NRT within past 3 months | |
| Interventions |
All groups received self‐help booklet Clearing the Air at baseline, + brief (≤ 10 min) counselling at weekly clinic visits throughout treatment phase. At each visit smoking status reported and verified; lab samples taken at screening, baseline and weeks 1, 2, 4, 6 and 7 Follow‐up phase (optional): clinic visits at weeks 12, 24, 52 for brief counselling, smoking status and vital signs. Phone calls every 4 weeks from week 16 |
|
| Outcomes | Primary outcome: continuous verified 4‐week abstinence for any part of treatment period Secondary outcomes: CQR weeks 4‐7; CQR from week 4 to weeks 12, 24, and 52 Other outcomes: weight change; reduction of craving and withdrawal using MNWS, QSU‐brief and mCEQ; AEs Validation was by expired CO ≤ 10 ppm Trial report ITT analysis based on numbers treated (N = 626); for consistency our MA used numbers randomised (N = 638). Attrition was 30% during treatment period, 25% of follow‐up consenters lost during follow‐up phase | |
| Notes | Previous users of bupropion > 12 months before were not excluded, unlike Gonzalez and Jorenby trials; prior use ranged from 13% to 20.6% across groups
Denominator in trial report is all treated; we have used all randomised in our MA
The trial was funded by Pfizer Inc Author declaration of interests: "Dr Nides has received research grants, consulting fees, and honoraria from Pfizer, Sanofi‐Aventis, and GlaxoSmithKline. Dr Oncken has received research grants, consulting fees, and honoraria from Pfizer; received, at no cost, nicotine replacement and placebo products from GlaxoSmithKline for smoking cessation studies; and received honoraria from Pri‐Med. Dr Gonzales reports having received research contracts from Pfizer, Sanofi‐Aventis, GlaxoSmithKline and Nabi Biopharmaceuticals; consulting fees and honoraria from Pfizer, Sanofi‐Aventis, and GlaxoSmithKline; and owning 5 shares of Pfizer stock. Dr Rennard has had or currently has a number of relationships with companies that provide product and/or services relevant to outpatient management of chronic obstructive pulmonary disease. These relationships include serving as a consultant (Adams, Almirall, Altana, Array Biopharma, AstraZeneca, Aventis, Biolipox, Centocor, Dey, Critical Therapeutics, GlaxoSmithKline, Johnson & Johnson, Merck, Novartis, Ono Pharma, Otsuka, RJ Reynolds, Roche, Sankyo, Schering‐Plough, Scios, and Wyeth); advising regarding clinical trials (Altana, AstraZeneca, Aventis, Centocor, GlaxoSmithKline, Novartis, Pfizer, and Philip Morris); speaking at continuing medical education programs; and performing funded research at both basic and clinical levels (Altana, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, and Novartis). He owns no stock in any pharmaceutical companies. Drs Watsky and Reeves and Mr Anziano are employees of Pfizer and own Pfizer stock or have stock options." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated using a method of randomly permuted blocks and a pseudo‐random number generator" |
| Allocation concealment (selection bias) | Low risk | "assigned ... medication to subjects in numerical order of acceptance into the study" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "double‐blind", "to preserve treatment blinding" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information |
| Selective reporting (reporting bias) | Low risk | All expected and predicted outcomes covered |
O'Malley 2018.
| Study characteristics | ||
| Methods | Country: USA Setting: home, 2 x outpatient substance abuse treatment and research facilities Aim: to test the efficacy of varenicline with medical management for patients with alcohol use disorder and comorbid smoking seeking alcohol treatment, and to evaluate the secondary effects on smoking abstinence Study design: phase 2, randomised, double‐blind, parallel‐group, placebo‐controlled trial |
|
| Participants | 131 adult smokers with alcohol use disorder. Eligible participants met alcohol‐dependence criteria and reported heavy drinking (≥ 5 drinks for men and ≥ 4 drinks for women) ≥ 2 times per week and smoking ≥ 2 times per week. 29.8% female, mean age 42.7, baseline average CPD 11.4 Exclusion criteria: current, clinically significant disease or abnormality; diagnosis of a serious psychiatric illness; current suicidal ideation or lifetime history of suicidal behavior; risk of aggression; current diagnosis of drug dependence; risk of clinically significant alcohol withdrawal; medications in the past 3 months to treat alcohol or tobacco dependence; psychotropic medications in the past month. Women of childbearing age could not be pregnant or nursing and had to be practicing effective contraception. |
|
| Interventions |
Intervention duration was 16 weeks. Participants did not receive any behavioural support for SC ‐ solely for alcohol use reduction. |
|
| Outcomes |
|
|
| Notes | New for 2022 update Funding by grants from the National Institutes of Health and by the State of Connecticut Department of Mental Health and Addiction Services. Pfizer provided varenicline and placebo pills at no cost. Author declaration of interests: "Dr O’Malley reported having been a consultant or an advisory board member for Alkermes, Amygdala, Arkeo, Cerecor, Mitsubishi Tanabe, Opiant, Pfizer; a member of the American Society of Clinical Psychopharmacology Alcohol Clinical Trials Initiative supported by Abbott, Amygdala, Ethypharm, Lilly, Lundbeck, Otsuka, Pfizer, Arbor Pharmaceuticals, and Indivior; a coinvestigator on studies receiving donated medications from Astra Zeneca, Novartis; a site principal investigator for a multisite trial by Lilly; and a scientific panel member for Hazelden Foundation. Dr Petrakis reported being a consultant to Alkermes. Dr Fucito reported registering with the US Patent and Trademark Office the name and content of a web‐based program to help with sleeping and drinking (ie, Call it a Night). No other disclosures were reported" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization list was generated by our study statistician (R.G.) and was implemented through a web‐based system (Endpoint Systems)." |
| Allocation concealment (selection bias) | Low risk | "The randomization list was generated by our study statistician (R.G.) and was implemented through a web‐based system (Endpoint Systems)." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Participants, treatment providers, and research staff were blind to the assignment throughout the study" and abstinence biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "N=94 (71.8%) provided data at the 12 month follow‐up" Similar between arms |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported |
Oncken 2006.
| Study characteristics | ||
| Methods | Country: USA
Setting: 10 research centres
Aim: to evaluate efficacy and safety of 4 varenicline dose regimens Dates conducted: not stated Study design: phase 2 double‐blind placebo‐controlled RCT |
|
| Participants | 647 healthy volunteer smokers, aged 18‐65, smoking at least 10 CPD. 49.5% men, 80% white, average CPD 21, mean FTND 5.5. Allocated to group 1 (129), group 2 (130), group 3 (129), group 4 (130) or placebo (129) Exclusion criteria: standard pharmacotherapy trial criteria, + use of NRT or bupropion within last 3 months; use of marijuana or tobacco other than cigarettes with last month | |
| Interventions |
All groups received self‐help booklet at baseline, + brief (≤ 10 min) counselling at weekly clinic visits throughout treatment phase, and phone call 3 days post‐TQD. At each visit smoking status reported and CO verified; vital signs, weight and AEs. Urine, blood tests and ECGs at screening, baseline, weeks 1, 2, 4, 7 and 12 Follow‐up phase: smoking status + CO measured at weeks 13, 24, 52; self‐reported status by phone at weeks 16, 20, 28, 32, 36, 40, 44 |
|
| Outcomes | Primary outcome: continuous verified 4‐week abstinence at weeks 4‐7 and 9‐12 Secondary outcomes: continuous verified abstinence at weeks 2‐12 and 9‐52; 7‐day PPA throughout treatment phase and at weeks 12, 24 and 52 Other outcomes: weight change; craving and withdrawal changes using MNWS and mCEQ; AEs Validation was by expired CO ≤ 10 ppm Cessation analyses were ITT (all participants randomised), while tolerability and safety analyses were based only on those known to have used the intervention drug (N = 627). Attrition was 27% during treatment phase, and 22% of follow‐up consenters lost in follow‐up phase | |
| Notes | For cessation analyses, titrated and non‐titrated results were reported separately and pooled. 24‐week continuous cessation data supplied by study authors
The trial was funded by Pfizer Inc Author declaration of interests: "Dr Oncken has received research grants, consulting fees, and honoraria from Pfizer; nicotine replacement and placebo products from GlaxoSmithKline at no cost for smoking cessation studies; and honoraria from Pri‐Med. Dr Gonzales has received research contracts, consulting fees, and honoraria from Pfizer, Sanofi‐Aventis, and GlaxoSmithKline and owns 5 shares of Pfizer stock that he received as a gift from his parents. Dr Rennard has had or currently has a number of relationships with companies who provide products and/or services relevant to outpatient management of chronic obstructive pulmonary disease. These relationships include serving as a consultant (for Adams, Almirall, Altana, Array Biopharma, AstraZeneca, Aventis, Biolipox, Centocor, Dey, Critical Therapeutics, GlaxoSmithKline, Johnson & Johnson, Merck, Novartis, Ono Pharma, Otsuka, RJ Reynolds, Roche, Sankyo, Schering‐Plough, Scios, and Wyeth), advising regarding clinical trials (Altana, AstraZeneca, Aventis, Centocor, GlaxoSmithKline, Novartis, Pfizer, and Philip Morris), speaking at continuing medical education programs and performing funded research at both basic and clinical levels (Altana, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, and Novartis). He does not own any stock in any pharmaceutical companies. Dr Nides has received research grants, consulting fees, and honoraria from Pfizer, Sanofi‐Avenits, and GlaxoSmithKline. Drs Watsky and Reeves and Messrs Billing and Anziano are employees of Pfizer and own Pfizer stock or hold Pfizer stock options." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | "Eligible subjects were randomly assigned to 1 of 5 groups" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Subjects and investigators were blinded to the study drug treatment [, and] were not encouraged to guess their treatment assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing COs or visits OK if confirmed abstinent before and after missed measure |
| Selective reporting (reporting bias) | Low risk | All expected and predicted outcomes covered |
Pastorino 2022.
| Study characteristics | ||
| Methods | Study design: RCT Country: Italy Setting: community Recruitment method: volunteers were recruited through press releases, social networks, television, and other media. |
|
| Participants | 869 participants in a lung‐screening trial who smoked. 44% female. Median age 60. Heavy smokers | |
| Interventions |
|
|
| Outcomes | CA at 52 weeks ‐ verified by CO ≤ 9 ppm | |
| Notes | Study funding: “Funded by the Scientific Directorate Fondazione IRCCS Istituto Nazionale Tumori, Italian Association for Cancer Research AIRC (AIRC 5x1000 ID 12162, extension 2017‐2020), Investigation Grant from the Foundation AIRC for the Research on Cancer (AIRC IG 2019, ID 23244), and Ricerca Corrente of the Italian Ministry of Health. The work of SG is partially supported by an Investigation Grant from the Foundation AIRC for the Research on Cancer (AIRC IG 2021, ID 25987) and by the Italian League Against Cancer (LILT, Milan). The fundings had no role in the design of the study; collection, analysis, and interpretation of data; writing of the manuscript, or the decision concerning submission.” Author declaration of interests: "We declare no competing interests." Study authors provided additional results data upon request. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated randomised but method not specified |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | A placebo was not used in the arm that did not receive cytisine. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition rates under 50% however data are not available on how many were followed up in the cytisine arms when they were split by length of treatment ‐ thus we can not see if differential attrition occurred |
| Selective reporting (reporting bias) | Low risk | Prespecified smoking‐related outcomes reported |
Qin 2021.
| Study characteristics | ||
| Methods | Study design: RCT Country: China Setting: China–Japan Friendship hospital in Beijing Recruitment method: via a trial site, a hotline of SC, advertisements in the community from February 2019‐June 2020 |
|
| Participants | 136 smokers diagnosed with COPD randomised; 2.9% female, average age 62; average CPD 19.21; FTND mode 0‐3 | |
| Interventions |
Common components, quote: "Participants received a counseling session for more than 60 min when they began medication at week 0, and they also received up to 10 min of counseling at weeks 1, 2, 4, 6, 9, 12, and 24." |
|
| Outcomes | SC: abstinence at 24 weeks (no further details given). Validated by CO ≤ 10 ppm | |
| Notes | Study funding: "This study was supported by the Capital Health Development Research Project in China (Grant No. 2018‐2‐4066), the National Natural Science Foundation of China (Grant No. 81720108001) and the National Key R&D Program of China (Grant No. 2017YFC1309400)." Author declaration of interests: "The authors report no conflicts of interest in this work." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A biostatistician, independent of the study used Proc Plan in SAS version 9.4 (SAS Institute) to generate a table of random digit to randomly assign the numbers to the two groups. (the number of the random seed is 87,654,321)." |
| Allocation concealment (selection bias) | Low risk | Quote: "To ensure random concealment, the group information assigned to each participant was put in a sealed, and opaque envelope." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Because of the different medication packaging, only statisticians were blinded to medication allocation however, all participants received an active evidence‐based smoking cessation pharmacotherapy." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Only 8/68 and 7/68 lost in the varenicline and bupropion arms respectively." |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes from trial registry reported |
Rennard 2012.
| Study characteristics | ||
| Methods | Countries: Argentina, Brazil, Canada, China, Czech Republic, France, Germany, Hungary, Italy, Korea, Mexico, Taiwan, UK, USA
Setting: 33 research centres
Aim: to evaluate efficacy and safety of varenicline allowing a self‐selected quit date Dates conducted: September 2008‐December 2009 Study design: double‐blind placebo‐controlled RCT |
|
| Participants | 659 healthy volunteer smokers, aged 18‐75, motivated to quit, smoking at least 10 CPD. 60% men, mean age 43, 68% white, mean CPD 21, mean FTND 5.5, 66% had tried to quit at least once before. Allocated to varenicline (493) or placebo (166) Exclusion criteria: standard pharmacotherapy trial criteria, + use of NRT, bupropion, clonidine or nortriptyline within last 3 months, ever used varenicline; use of marijuana or tobacco other than cigarettes with last month | |
| Interventions |
Participants could choose their own quit date between days 8 and 35 Treatment period was 12 weeks. All participants received Clearing the Air: Quit smoking today booklet at baseline, + brief counselling (≤ 10 min) at each clinic visit. Weekly visits throughout treatment phase, and in follow‐up phase clinic visits at weeks 13, 16, 20 and 24. Phone calls at weeks 14, 18 and 22 |
|
| Outcomes | Primary outcome: CO‐validated CAR at 9‐12 weeks Secondary outcomes: CO‐validated CAR at 9‐24 weeks; 7‐day PPA at weeks 12 and 24 Other outcomes: AEs, SAEs; timing and number of quit attempts Validation was by expired CO ≤ 10 ppm Dropouts and losses to follow‐up were included in the analyses as continuing smokers (ITT analysis) Attrition to end of study (24 weeks) was 12.4% from varenicline, 20.5% from placebo | |
| Notes | New for 2012 update Additional information supplied by the study authors The study was funded and managed by Pfizer Inc Author declaration of interests: "SR, JH, PC, EK, and TR did not receive any financial support with respect to the writing or development of this manuscript. Funding from Pfizer Inc. has been received by the authors or their institutions for the following in the 36 months prior to submission: research grants (JH, PC, EK, and TR); advisory board membership (SR and JH); reimbursement for travel and accommodation expenses (SR, PC, EK, and TR); lectures/speaker bureau (PC); consultancy (EK); and development of educational materials (EK and TR). CA, LSA, and CR are employees of Pfizer Inc. JH has received consulting fees from several non‐profit and for‐profit entities that develop or promote smoking cessation products or that advocate for tobacco control measures." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "a predefined, central, computer‐generated randomization sequence...assigned subjects in a 3:1 ratio". Block size: 4, stratified by centre |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Triple‐blind (participant, care‐giver, investigator) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts and attrition rates fully reported |
| Selective reporting (reporting bias) | Low risk | All predicted and expected outcomes reported |
Rigotti 2010.
| Study characteristics | ||
| Methods | Country: 15 countries in Europe, Asia, Americas
Setting: 39 research centres
Aim: to evaluate efficacy and safety of varenicline in patients with stable CVD Dates conducted: February 2006‐August 2008 Study design: phase 3 double‐blind placebo‐controlled RCT |
|
| Participants | 714 adult smokers, aged 35‐75, smoking at least 10 CPD, with stable CVD and motivated to quit. 79% men, 80% white, mean CPD 22, mean FTND 5.6. Allocated to varenicline (355) or placebo (359), stratified by site Exclusion criteria: standard pharmacotherapy trial criteria, + use of NRT or bupropion within previous month. All had been diagnosed for at least 2 months with CVD, but not hypertension alone | |
| Interventions |
Both groups received brief (≤ 10 min) counselling at weekly clinic visits throughout treatment phase, and phone call 3 days post‐TQD. At each visit smoking status reported and CO verified; vital signs, weight and AEs. Urine, blood tests and ECGs at screening, baseline, weeks 12 and 52 Follow‐up phase: smoking status + CO measured at weeks 13, 16, 24, 32, 40 and 52; counselling and self‐reported status by phone at weeks 14, 20, 28, 36 and 44 |
|
| Outcomes | Primary outcome: CO‐validated CAR at weeks 9‐12 Secondary outcomes: CO‐validated CAR at weeks 9‐52 and 9‐24; 7‐day PPA at weeks 12, 24 and 52 Other outcomes: AEs; SAEs; cardiovascular events; changes in blood pressure and heart rate Validation was by expired CO ≤ 10 ppm Cessation analyses were ITT (all participants randomised minus deaths), while tolerability and safety analyses were based only on those known to have used the intervention drug (N = 703). Attrition was 17.5% from the varenicline group and 20.3% from the placebo group during treatment phase, and 14.9% varenicline and 19.5% placebo who did not complete the study. This includes 2 deaths in the varenicline group and 5 in the placebo group by 52‐week follow‐up | |
| Notes | New for 2010 update Study funding: "This study was funded by Pfizer Inc. Editorial support for the development of this manuscript was provided by Alexandra Bruce, PhD, of UBC Scientific Solutions and was funded by Pfizer Inc." Author declaration of interests: "Drs Rigotti, Pipe, Benowitz, and Tonstad have consulted for Pfizer. Dr Rigotti has been the site principal investigator for clinical trials of smoking cessation medications funded by Pfizer, sanofi‐aventis, and Nabi Biopharmaceuticals. Dr Pipe has received educational and research support in the past from Bristol‐ Myers Squibb, Johnson & Johnson, GlaxoSmithKline, and Merrell‐Dow. Drs Benowitz and Tonstad served on the scientific planning committee for this study and have been paid consultants to Pfizer and other pharmaceutical companies that are developing and/or marketing smoking cessation medications. Dr Benowitz has been a paid expert witness in litigation against tobacco companies. At the time of the study, his family owned a small amount of Pfizer stock, but no longer does. Dr Tonstad has been the site principal investigator for clinical trials of smoking cessation medication and other medications funded by Pfizer and other pharmaceutical companies. Dr Arteaga is a statistical director at Pfizer Inc, supporting the varenicline studies. Dr Garza is a senior medical director of clinical research and development at Pfizer Inc, and the medical monitor for this study. The other authors report no conflicts." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The study sponsor conducted the randomization centrally using a computer‐generated list that prespecified the order of treatment allocation" |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Described as "double‐blind" (participants and study implementation). Cardiovascular outcomes "were reviewed separately and adjudicated under blinded conditions by an independent event committee made up of 3 board‐certified cardiologists" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analyses conducted; participants who missed a visit but had validated abstinence at next visit were considered continuously abstinent. But 52‐week status had to be attended and confirmed |
| Selective reporting (reporting bias) | Low risk | All expected and predicted outcomes covered |
Rohsenow 2017.
| Study characteristics | ||
| Methods | Country: USA Setting: university offices in Rhode Island, USA Aim: to compare varenicline with transdermal nicotine (NRT) for smokers with current substance use disorders (SUD) for effects on smoking abstinence Study design: double‐blind double‐placebo‐controlled randomised design, stratifying by MDD history |
|
| Participants | 137 adult smokers in substance‐use disorder treatment, who were substance abstinent < 12 months, and smoked 10+ CPD for the past 6 months. 47% female, mean age 39.6, baseline CPD 19.5 Participants did not need to motivated to quit Exclusion criteria: hallucinations/delusions, current SC treatment, contraindications for either medication, using medications affected by SC (antipsychotics, warfarin, theophylline and insulin), suicidal ideation, not willing to try to quit smoking and, substance use reported on the day of or before recruitment or positive breath alcohol at recruitment |
|
| Interventions |
|
|
| Outcomes | Primary: 7‐day smoking PPA at 3 months Secondary: 7‐day PPA at 6 months; quantity and frequency of smoking and substance use at 3 and 6 months; within‐treatment abstinence; medication adherence; depressive symptoms |
|
| Notes | New for 2022 update Funding by a personal grant 1R01DA024652 from the National Institute on Drug Abuse and Department of Veterans Affairs Author declaration of interests: "All authors report no financial interests or potential conflicts of interest except for the following: R.M.S. has received travel and honorarium from D&A Pharma and has received consultant fees from CT Laboratorie" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Urn randomization [32] to medication condition (1: 1 assignment) was stratified by gender, median score on the Fagerström Test for Nicotine Dependence [33] from a previous study [28] and history of MDD. The randomization program was run by the Project Manager, who concealed the assignment from all other staff." |
| Allocation concealment (selection bias) | Low risk | "Urn randomization [32] to medication condition (1: 1 assignment) was stratified by gender, median score on the Fagerström Test for Nicotine Dependence [33] from a previous study [28] and history of MDD. The randomization program was run by the Project Manager, who concealed the assignment from all other staff." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind trial with placebo patch and placebo tablets used (double placebo). Abstinence biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 23/60 and 25/77 did not complete follow‐ups for primary outcome at 3 months but information not stated for 6 months; however multiple imputation sensitivity analysis has been done (supplementary materials) which the study authors claim did not change the conclusion |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes from trial registry reported |
Rose 2013.
| Study characteristics | ||
| Methods | Country: USA
Setting: Duke University Medical Center, Durham, NC Aim: "Given the safety and tolerability profile of nicotine replacement therapy, our rationale in this study was to use nicotine replacement therapy as an initial line of treatment, and then identify early on which smokers are unlikely to benefit from nicotine alone" Study design: randomised double‐blind parallel‐arm adaptive treatment trial in 2 phases Dates conducted: not stated |
|
| Participants | 606 adult smokers, motivated to quit, aged 18‐65, mean CPD 10+ for 3 years, expired CO level 10+ ppm. 46% women, 63% white, mean CPD 21.7, mean FTND 5.8. Participants could receive up to USD 320 for study participation | |
| Interventions | 2‐phase study All participants seen weekly for 2 weeks before TQD, and attended 4‐6 sessions after the TQD. At each session, participant received brief (< 15 min) support, + clinical trial materials. Smoking diaries, expired CO, withdrawal symptoms and reports of AEs were collected each time. Participants were recontacted at 6 months, and those reporting abstinence were invited to return to give a CO sample All participants were given open‐label active NRT patch, either 42 mg/day (baseline CO > 30 ppm) or 21 mg/day (baseline CO < 30 ppm) for 2 weeks; dose reductions allowed if side effects dictated. At 1 week, participants were classified as 'responders' (reduced ad lib smoking by > 50%, CO‐verified) or 'non‐responders' (< 50%)
Non‐lapsers (N = 130) remained on open‐label NRT throughout study duration All participants received dummy (placebo) versions of the other 2 treatments as well as their own active treatment. 47 participants were excluded from the analysis (27 Phase 1, 20 Phase 2) because of using contra‐indicated medications during the study or failing to meet other entry requirements. 1 individual died before EoT, and 1 was excluded for extreme CO change from the mean sample range |
|
| Outcomes | Primary: CAR at weeks 8‐11 Secondary: CA from TQD for 11 weeks (EoT); 7‐day PPA at 6 months: CA from TQD to 6 months Validation: CO ≤ 10 ppm AEs and SAEs (reported, but not by treatment group) |
|
| Notes | Phase 1 and Phase 2 groups combined for varenicline vs NRT analysis New for 2016 update Study funding: "Supported by a grant to Duke University from Philip Morris USA. Nicotine patches were donated by GlaxoSmithKline. The companies had no role in the planning or execution of the study, data analysis, or publication of results." Author declaration of interests: "Dr. Rose has served as a consultant for Targacept and Philip Morris USA and has a patent purchase agreement with Philip Morris International. Both authors have received research funding from Philip Morris USA." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses fully reported; exclusions for protocol violations or contra‐indicated medicines. 1 death and 1 'rogue' CO reading excluded |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes from trial registry all reported |
| Other bias | Unclear risk | Unexplained disparity between CONSORT (N = 103) and Results table (N = 108) denominators for rescue varenicline group |
Scharfenberg 1971.
| Study characteristics | ||
| Methods | Country: East Germany Aim: to test the efficacy of cytisine for SC Setting: SC clinic, Magdeburg, July‐December 1967 Study design: double‐blind placebo‐controlled randomised trial |
|
| Participants | 1214 smokers recruited from 1452 applicants through smoking clinics and via initial press releases. 88.2% male. 2.5% of participants smoked < 10 CPD, 42.4% 10‐20 CPD, 48.9% 21‐30 CPD, 5.2% > 30 CPD 40.4% had smoked > 20 years. 40.6% had tried to quit at least once before Randomised to cytisine (607) or placebo (607) Exclusion criteria not stated (214 volunteers excluded at initial screening) | |
| Interventions |
Behavioural support: none |
|
| Outcomes | Self‐reported abstinence at 4 weeks, 6 months and 2 years ITT analysis. Attrition rate 34% by longest follow‐up | |
| Notes | Study funding: not reported Author declaration of interests: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | "a numbered pouch" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
Schnoll 2019.
| Study characteristics | ||
| Methods | Country: USA Setting: unclear Aim: to determine if extended‐use varenicline should be considered for treating tobacco use among cancer patients Study design: placebo‐controlled parallel RCT |
|
| Participants | 207 adult smokers with a diagnosis of cancer or a recurrence within the past 5 years, and smoking ≥ 5 cigarettes per week. Participants were motivated to quit. 50.7% female, mean age 58.5, and baseline average CPD 13.4 Exclusion criteria: daily use of nicotine products other than cigarettes, unstable substance abuse/dependence in the last year |
|
| Interventions |
Participants were randomised 1:1. All participants had 7 x SC counselling sessions over 24 weeks (4 x in‐person, 3 x by telephone). |
|
| Outcomes | Primary: 7‐day biochemically confirmed abstinence at weeks 24 and 52 | |
| Notes | New for 2022 update Funding by R01 CA165001 and K24 DA045244. Pfizer provided medication and placebo free of charge. Author declaration of interests: "Drs. Schnoll and Hitsman receive medication and placebo free from, and provide consultation to, Pfizer. Drs. Schnoll and Dr. Kalhan have consulted for GlaxoSmithKline. Dr. Schnoll consults with Curaleaf." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The study statistician provided a randomization procedure (1:1) to the Penn Investigational Drug Service (IDS), which distributed pills." |
| Allocation concealment (selection bias) | Low risk | "The study statistician provided a randomization procedure (1:1) to the Penn Investigational Drug Service (IDS), which distributed pills. All personnel, except IDS, were blinded to assignment." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Objective verification and participants blinded during the second 12 weeks (first 12 weeks were open‐label as all participants received varenicline) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 77% and 75% in the 2 arms completed study according to flow‐chart |
| Selective reporting (reporting bias) | Low risk | Outcomes are reported as planned on clinical trials record |
Stein 2013.
| Study characteristics | ||
| Methods | Country: USA
Setting: 9 methadone‐maintained treatment centres in New England Aim: "[to] test varenicline versus placebo, and include a comparison condition of combination nicotine replacement therapy" Study design: randomised 3‐armed double‐blind controlled trial Dates conducted: December 2008‐January 2012 Analysis: sample sizes of 132 (varenicline) and 44 (placebo) estimated to give 80% power to detect quit rates of 20% and 2.5% respectively; the study was not powered to detect differences between varenicline and combination NRT |
|
| Participants | 315 adult methadone‐maintained smokers, smoking 10+ CPD, willing to set a quit date within the 1st week Allocated 3:1:3 to varenicline (137): placebo (45): combination NRT (133). Mean age 39.9, 47.6% women, 78.5% white, mean CPD 20, mean FTND 5.7 | |
| Interventions |
All participants received a standardised 15‐min session of advice to quit (5As model: Ask, Advise, Assess, Assist, and Arrange), and were asked to set a TQD for 8 days' time. All made monthly visits for support and top‐up medication. Participants were paid USD 30 for the baseline assessment and USD 40 for the 6‐month assessment |
|
| Outcomes | Primary: 7‐day PPA at 6 months Secondary: CA from week 2 to 6 months; for non‐quitters: CPD reduction in the 28 days prior to 6‐month assessment Validation: CO < 8 ppm; urinary cotinine in varenicline and placebo participants claiming abstinence |
|
| Notes | Funding: "Funding for this study was provided by the National Cancer Institute (RO1 CA129226). Dr. Stein is a recipient of a NIDA Mid‐Career Investigator Award (K24 DA00512). NCI and NIDA had no further role in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication." New for 2016 update Author declaration of interests: "No conflict declared" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Participants were randomized to treatment after completing the baseline assessment". No further information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "double‐blind"; research assistants were "blind to participant group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to treatment and follow‐up reported; ITT analyses conducted |
| Selective reporting (reporting bias) | Low risk | Outcomes prespecified in trial registry reported |
Steinberg 2011.
| Study characteristics | ||
| Methods | Country: New Jersey, USA
Setting: Robert Wood Johnson Hospital (584‐bed, university‐based)
Aim: to evaluate efficacy and safety of varenicline in hospital inpatients Dates conducted: August 2007‐March 2009 Study design: phase III triple‐blind pilot RCT |
|
| Participants | 79 adult smokers, aged 18+, smoking 10+ CPD; randomised to varenicline (40) or placebo (39) 59% men, mean age: 51, 72% white, 57% > 20 CPD, 40% FTND > 6 Admission diagnoses 57% CVD, 14% orthopaedic, 13% pulmonary, 16% other Exclusion criteria: standard pharmacotherapy criteria, + current use of any SC medications |
|
| Interventions |
Initial visit by Clinic Co‐ordinator of local Tobacco Dependence Program for 5‐10 min counselling Subsequent sessions of 15‐min post‐discharge After discharge, data collection sessions at 4, 12 and 26 weeks, + 1 phone call at 2 weeks with research nurse USD 25 gift card for attendance at each follow‐up visit |
|
| Outcomes | Primary outcome: 7‐day PPA at 26 weeks Secondary outcomes: 7‐day PPA at 4, 12 weeks. Repeated PPA at 4, 12 and 24 weeks. AEs and SAEs; withdrawal and craving on MNWS; motivation; CPD; utilisation of outpatient services; composite medical outcome Validation: CO validation ≤ 8 ppm. Self‐report accepted if unable to attend |
|
| Notes | Study was funded and support by Robert Wood Johnson Foundation and Pfizer Repeated PPA at 4, 12 and 24 weeks used as strictest definition of abstinence and included in main MA New for 2012 update Author declaration of interests: "Dr. Steinberg had previously received honoraria for educational programs from Pfizer (2006–2009). The other authors declare that they have no conflicts of interest to disclose." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomized in a 1:1 ratio through centralized telephone randomization process by the study statistician and hospital research pharmacist" |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The subject, research nurse, and treatment staff were blinded to treatment assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analysis conducted; unvalidated smoking status included where ascertained for non‐attenders, but % of unvalidated status not reported |
| Selective reporting (reporting bias) | Low risk | All expected and predicted outcomes covered, except for detailed identification of SAEs |
Steinberg 2018.
| Study characteristics | ||
| Methods | Country: USA Setting: community Aim: to conduct a proof‐of‐concept RCT of varenicline for smokers willing to reduce, but not quit smoking Study design: parallel, placebo‐controlled RCT |
|
| Participants | 53 current adult smokers, smoking at least 10 CPD for the past 6‐months, and interested in cutting down, but not in quitting in the next 30 days. 49.2% female, mean age 44.3, baseline average CPD 15 Exclusion criteria: any past use of varenicline, current use of bupropion/nortriptyline/nicotine preparations, use of tobacco products other than cigarettes more than once per month, currently receiving tobacco use disorder counselling, alcohol use disorder, drug abuse, psychosis, depression or suicidal ideation |
|
| Interventions |
Participants were randomised on a 1:1 ratio. All participants received in‐person interactive behavioural support, which comprised 3 x 20‐min and 1 x 35‐min counselling visits |
|
| Outcomes | Relevant outcomes
|
|
| Notes | New for 2022 update. Abstinence data were provided upon request by study authors Funding by Global Research Award for Nicotine Dependence (GRAND) grant #WS777117 – An independent competitive grants program supported by Pfizer. Pfizer had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication. Author declaration of interests: "MLS and JMW have consulted for and received unrestricted educational grants and research grant support from Pfizer. SE‐L has no conflicts to declare." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomized to active or placebo varenicline using an urn randomization procedure" |
| Allocation concealment (selection bias) | Unclear risk | Concealment not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Abstinence biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates 12% in placebo arm and 29% in varenicline arm |
| Selective reporting (reporting bias) | Low risk | Abstience a prespecified outcome, but results not published. However, abstinence data were provided upon request by study authors |
Tashkin 2011.
| Study characteristics | ||
| Methods | Country: USA (17 centres), Spain (3 centres), France (4 centres), Italy (3 centres)
Setting: 27 research centres Aim: to test efficacy and safety of varenicline in smokers with COPD Dates conducted: May 2006‐April 2009 Study design: double‐blind placebo‐controlled RCT |
|
| Participants | 504 adult smokers with mild‐to‐moderate COPD, aged 35+, smoking 10+ CPD, motivated to quit; allocated to varenicline (250), or placebo (254). 62% men, mean age 57, CPD 24‐25, FTND score 5.9‐6.2 Treatment groups were comparable at baseline Exclusion criteria: standard pharmacotherapy trial criteria, + treatment with systemic corticosteroids or hospitalised for COPD in previous 4 weeks | |
| Interventions |
Both groups received SC educational booklet, + brief (≤ 10 min) counselling at weekly clinic visits throughout treatment phase, and phone call 3 days post‐TQD. At each visit smoking status reported and CO verified; throughout treatment and at week 52 lung function, respiratory symptoms, weight, blood pressure, pulse, temperature, ECGs, haematology and serum chemistry assessed, + AEs Follow‐up phase: smoking status + CO measured at weeks 13, 16, 24, 32, 40, 48 and 52; counselling and self‐reported status by phone at weeks 14, 20, 28, 36 and 44 |
|
| Outcomes | Primary outcome: CO‐validated CAR at weeks 9‐12 Secondary outcomes: CO‐validated CAR at weeks 9‐52 and 9‐24; 7‐day PPA at weeks 12, 24 and 52 Other outcomes: AEs; SAEs; weight change Validation was by expired CO ≤ 10 ppm Cessation analyses were ITT (all participants randomised), while tolerability and safety analyses were based only on those known to have used the intervention drug (N = 499). Attrition was 17% in the varenicline group and 24% in the placebo group during treatment phase, and 29% varenicline and 38% placebo who did not complete the study. This includes 2 deaths in the varenicline group and 1 in the placebo group | |
| Notes | The study was funded by Pfizer Inc
New for 2010 update Author declaration of interests: "The authors have reported to the CHEST the following conflicts of interest: Dr Tashkin received grant support from Pfizer Inc and Nabi Pharmaceuticals and fees for attending advisory board meetings from Pfizer Inc. Dr Hays received a research grant from Pfizer Inc for the conduct of the clinical trial described in this manuscript. In the past 3 years, Dr Rennard has been a consultant or a member of an advisory board for Able Associates, Adelphi Research, Almirall/Prescott, APT Pharma/Britnall, Aradigm, AstraZeneca, Boehringer Ingelheim, Chiesi, CommonHealth, Consult Complete, COPDForum, Data‐Monitor, Decision Resources, Defined Health, Dey, Dunn Group, Eaton Associates, Equinox, Gerson, GlaxoSmithKline, Infomed, KOL Connection, M Pankove, MedaCorp, MDRx Financial, Mpex, Novartis, Nycomed, Oriel Therapeutics, Otsuka, Pennside Partners, Pfizer Inc (varenicline), Pharma Ventures, Pharmaxis, Price Waterhouse, Propagate, Pulmatrix, Reckner Associates, Recruiting Resources, Roche, Schlesinger Medical, SciMed, Sudler and Hennessey, TargeGen, Theravance, UBC, Uptake Medical, and VantagePoint Management. Dr Rennard has lectured for the American Thoracic Society, AstraZeneca, Boehringer Ingelheim, California Allergy Society, Creative Educational Concept, France Foundation, Information TV, Network for Continuing Ed, Novartis, Pfizer, and SOMA and has received industry‐sponsored grants from AstraZeneca, Biomarck, Centocor, Mpex, Nabi Pharmaceuticals, Novartis, and Otsuka. Ms Ma and Drs Lawrence and Lee are all employees of Pfizer Inc, own Pfizer Stock, and have Pfizer stock options." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "participants were randomized" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "double blind" but details not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | All expected and predicted outcomes covered |
Tonstad 2011.
| Study characteristics | ||
| Methods | Countries: France, Spain, Belgium, Sweden, Denmark, Norway
Setting: 22 research centres Aim: to test the efficacy and safety of dianicline for SC Dates conducted: June 2006‐June 2007 Study design: double‐blind placebo‐controlled parallel group RCT Study name: EURODIAN study |
|
| Participants | 602 healthy adult volunteers, smoking 10+ CPD within previous 2 months, aged 18+; allocated to dianicline (300), or placebo (302). 42% men, mean age 45, mean CPD 21, mean previous quit attempts 3.4, mean FTND score 5.75. Treatment groups were comparable at baseline Exclusion criteria: standard pharmacotherapy trial criteria, plus any quit attempt in previous 3 months, any use of bupropion, NRT, tobacco other than cigarettes 3+ times in previous 3 months | |
| Interventions |
TQD was set for days 3‐7 following baseline visit All participants received standardised brief counselling (≤ 10 min, based on Smoke‐Free and Living It) at each visit Weekly visits throughout weeks 1‐7, then (for treatment completers) at weeks 8, 10, 14, 18, 22 and 26 Smoking status and brief advice at each visit Participants completed smoking diaries |
|
| Outcomes | Primary outcome: CO‐confirmed CAR for weeks 4‐7
Secondary outcomes: CO‐confirmed CAR at 26 weeks. PPA weeks 4‐7
Validation by expired CO < 10 ppm (all visits) and plasma cotinine ≤ 8 μg/L (weeks 4 and 7)
Other outcomes: AEs, SAEs; craving and withdrawal symptoms
Dropouts and losses to follow‐up were included in the analyses as continuing smokers (ITT analysis) 25.2% dianicline and 23% placebo participants did not complete the study. AE‐related dropouts were 4.3% dianicline and 7.6% placebo |
|
| Notes | New for 2012 update The trial was funded by Sanofi‐Aventis. "The sponsor did not play a role in writing of the manuscript" Author declaration of interests: "Serena Tonstad and Philip Tønnesen have received honoraria for lectures and advising from sanofi‐aventis, Pfizer, Novartis, and GlaxoSmithKline, all manufacturers of drugs for smoking cessation." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "a predefined, central, and computer‐generated randomization accessed through an Interactive Voice Response System assigned participants on a 1:1 ratio" |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Participants and investigators were blinded to drug treatment assignments" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts fully reported |
| Selective reporting (reporting bias) | Low risk | All expected and predicted outcomes covered |
Tsai 2007.
| Study characteristics | ||
| Methods | Country: Taiwan and Korea
Setting: 5 sites in each country Aim: to test the efficacy and safety of varenicline for SC in Taiwanese and Korean smokers Dates conducted: February 2005‐March 2006 Study design: double‐blind placebo‐controlled RCT |
|
| Participants | 250 healthy adult volunteers, motivated to quit, aged 18‐75; allocated to varenicline (126), or placebo (124). 89% men, mean age 40.3, BMI < 15 or > 38 or weight < 45.5 kg, mean CPD 24, mean FTND score 5.1 Treatment groups were comparable at baseline Exclusion criteria: standard pharmacotherapy trial criteria | |
| Interventions |
Treatment period 12 weeks, 1st week titrated dosage. All participants received a SC booklet Clearing the Air at baseline, + brief counselling (≤ 10 min) at each clinic visit. Clinic visits at baseline and at weeks 1, 2, 3, 4, 6, 8, 10, 12, plus a 5‐min phone call at +3 days post‐TQD, and at weeks 5, 7, 9, 11 In follow‐up phase, clinic visits at weeks 13, 16, 20, 24 plus brief phone calls at weeks 14, 18, 22 |
|
| Outcomes | Primary outcome: CO‐validated CAR at 9‐12 weeks Secondary outcomes: CO‐validated CAR at 9‐24 weeks; 7‐day PPA at weeks 12 and 24 Validation was by expired CO ≤ 10 ppm Other outcomes: withdrawal symptoms (using MNWS, QSU‐brief and mCEQ), AEs Dropouts and losses to follow‐up were included in the analyses as continuing smokers (ITT analysis) Attrition in treatment phase was 2.8%, losses to follow‐up 2.5% of treatment completers | |
| Notes | Trial was funded by Pfizer Inc
New for 2008 update Author declaration of interests: "Drs. Tsai and Cho have been members of Pfizer‐sponsored advisory panels and, together with Drs. Cheng, Kim, and Hsueh, were investigators for a Pfizer‐sponsored clinical trial." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly permuted blocks" (block size=4) |
| Allocation concealment (selection bias) | Low risk | Web‐ and telephone‐based assignment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants, investigators, study staff and sponsor personnel |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information, but very high compliance rates |
| Selective reporting (reporting bias) | Unclear risk | All expected and predicted outcomes covered |
Tsukahara 2010.
| Study characteristics | ||
| Methods | Country: Japan
Setting: cessation clinic in Fukuoka University Hospital Aim: to test the efficacy and safety of varenicline for SC in Japanese smokers Dates conducted: August 2008‐November 2009 Study design: open‐label RCT Study name: the VN‐SEESAW Study |
|
| Participants | 32 adult smokers, motivated to quit, allocated to varenicline (16) or nicotine patch (16). 75% men, mean age 46, mean CPD 28 (varenicline), 25 (patch), mean Brinkman index score (CPD x years smoking) 702. 71% had tried to quit previously, and 7% had used nicotine patches before Standard pharmacotherapy trial exclusion criteria, plus attendance at any SC clinic during previous 12 months | |
| Interventions |
No non‐treatment or placebo control group Varenicline group received 8 clinic visits and nicotine group 5 visits over 12 weeks, with 5 brief counselling sessions (≤ 10 min) |
|
| Outcomes | CO‐confirmed CAR at 9‐12 weeks, and self‐reported at 9‐24 weeks by phone interview Validation by expired CO < 8 ppm at 12 weeks, but not at 24 weeks Other outcomes: safety and tolerability by week 12, using MNWS at weeks 2, 4, 8 and 12. Also used Stress Check List and Strait‐trait Anxiety Inventory Dropouts and losses to follow‐up were included in the analyses as continuing smokers (ITT analysis) Attrition in treatment phase was 12.5% from each group | |
| Notes | The study was supported by the Japanese Ministry of Education, Science and Culture, Fukuoka University and FU‐Global program
New for 2010 update Author declaration of interests: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "by computer" allocating men: women 3:1 to reflect Japanese smoking prevalence (M: 40%, F: 12%) |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and personnel were not blinded to treatment. However, both groups received an active licensed SC treatment and there is no reason to believe that participants would have favoured one over the other. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Low risk | All expected and predicted outcomes covered |
Tuisku 2016.
| Study characteristics | ||
| Methods | Country: Finland Setting: community Aim: to investigate the efficacy of varenicline and the nicotine patch as a SC aid in volunteer daily smokers in their twenties Study design: parallel, placebo‐controlled RCT |
|
| Participants | 288 current smokers aged 18‐26 year‐old, who had smoked daily for at least the past month, and smoked ≥ 100 cigarettes in their life. Participants were motivated to quit. 50.2% of participants were female, median age was 21, and average CPD at baseline 14 Exclusion criteria: current drug or alcohol abuse, known allergy towards medications used in the study, lactation, pregnancy or intention to become pregnant during the study period |
|
| Interventions | Light smokers were randomly assigned into 2 groups:
Heavy smokers were randomly assigned into two groups:
|
|
| Outcomes | Primary: self‐reported smoking abstinence at week 12 Secondary: self‐reported smoking abstinence at weeks 4 and 26; self‐reported abstinence verified by saliva cotinine level at week 12 |
|
| Notes | New for 2022 update Funding by Ministry of Social Affairs and Health, Finland; Finnish Research Foundation of the Pulmonary Disease; Finnish Medical Society Duodecim Author declaration of interests: "The authors declare no conflict of interest." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "After assessment… at the baseline visit, simple randomisation with a computer‐generated random list… was used to allocate study subjects into the different treatment groups” |
| Allocation concealment (selection bias) | Unclear risk | No information reported on allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Abstinence was self‐reported, however placebo or another licensed treatment were used as comparators and behavioural support was balanced between groups. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates were similar between study arms. |
| Selective reporting (reporting bias) | High risk | It appears 12‐month follow‐up was planned (NCT01531049) but is not reported in the published paper. |
Tulloch 2016.
| Study characteristics | ||
| Methods | Country: Canada Setting: community Aim: to evaluate smoking abstinence with standard nicotine patch, extended use of combined formulations of NRT, or varenicline Study design: parallel RCT |
|
| Participants | 737 smokers, including those with medical and psychiatric comorbidities, motivated to quit. 46.6% female, mean age 48.61, baseline average CPD 23.2 Exclusion criteria: use of NRT or varenicline for > 72 consecutive h in the past month; serious cardiac arrhythmias or a myocardial infarction or cerebral vascular accident within the previous 10 days; severe or unstable angina pectoris; end‐stage renal disease or use of cimetidine; alcohol or substance abuse in the previous 3 months; unstable psychiatric symptoms; an inability to understand English or French. Women were excluded if pregnant, lactating, or likely to become pregnant in the next year. |
|
| Interventions |
All participants received 6 x 15‐min cessation counselling interactive behavioural support from experienced nurses. |
|
| Outcomes | Primary: CO‐confirmed CA rates from weeks 5–52. Secondary: CA rates from weeks 5–10 and 5–22; CO‐confirmed 7‐day PPA at weeks 10, 22, and 52 |
|
| Notes | New for 2022 update Funding by the Heart and Stroke Foundation of Ontario. Study authors have previously received research grants and delivered educational presentation for Pfizer. Author declaration of interests: "AP and RR have received research grants from Pfizer. AP and BR have been paid for developing and delivering educational presentations for Pfizer. AP is on the advisory board for Pfizer and Johnson & Johnson." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization sequence was generated using a computer generated algorithm in Statistical Analysis Software (SAS program) by a researcher not involved in the study and blinded to the identity of participants." |
| Allocation concealment (selection bias) | Low risk | "Randomization numbers were placed in opaque, sealed, and consecutively numbered envelopes and opened following the completion of the baseline data collection." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Open‐label with higher intensity medication provided in the combination NRT arm than the nicotine patch arm. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition was < 50% and comparable between arms. |
| Selective reporting (reporting bias) | Low risk | Abstinence was reported as stated in trial register (NCT01623505) |
Vinnikov 2008.
| Study characteristics | ||
| Methods | Country: Kyrgyzstan
Setting: Mining company (Kumtor Operating Company) Aim: to test the efficacy and safety of cytisine for SC in a workplace setting Study design: double‐blind placebo‐controlled parallel‐group RCT |
|
| Participants | 197 adult smokers, aged 20+, smoking at least 15 CPD, no prior use of cytisine, and motivated to quit Randomised to cytisine (100) or placebo (97). 26 (15 cytisine, 11 placebo) who took no medication were excluded from trial report 97% men, mean age 39, mean CPD 22, mean FTND 5.3, 86% had tried to quit previously; mean previous quit attempts 3.3 Exclusion criteria: standard pharmacotherapy trial criteria |
|
| Interventions | Tabex tablets (1.5 mg cytisine):
Placebo tablets, same regimen Treatment period was 25 days, with TQD Day 5. All participants received "behavior counselling" (no further detail) |
|
| Outcomes | Primary outcome: CO‐validated CAR from day 5 to week 8 Secondary outcome: CO‐validated CAR from day 5 to week 26 Validation was by expired CO ≤ 8 ppm Other outcomes: change in health‐related QoL measures, changes in body weight, AEs, SAEs Attrition to 8 weeks was 6 in cytisine group and 7 in placebo group; to 26 weeks 10 in cytisine group and 16 in placebo group | |
| Notes | New for 2012 update Additional information supplied by the study author Study funding: not reported Author declaration of interests: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was done by independent statistician in an Excel programme and the randomization key was kept by an independent person" |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Nor patients neither investigators did not know where Tabex and where placebo were"; "follow‐up was blind" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 26 participants who did not take a single treatment dose were excluded from denominators by authors (restored to our MAs) |
| Selective reporting (reporting bias) | Low risk | Expected and predicted outcomes reported |
Walker 2014.
| Study characteristics | ||
| Methods | Country: New Zealand
Setting: National Quitline
Aim: "a non‐inferiority trial to investigate whether cytisine was at least as effective as nicotine‐replacement‐therapy"
Study design: parallel‐group non‐inferiority RCT Dates conducted: March 2011‐February 2013 |
|
| Participants | 1310 daily smokers, callers to the NZ National Quitline, aged 18+, motivated to quit. Allocated to cytisine (655) or to open‐label NRT (655). Mean age 38, 57% women, 33% NZ Maori, mean CPD 19, mean FTND 5.4 | |
| Interventions |
All participants received standard Quitline support, i.e. average 3 x 10‐15‐min calls over 8 weeks |
|
| Outcomes | Self‐reported CAR (5 cigarettes or fewer) at 1 month CAR and 7‐day PPA (no smoking) at 1 week, 1 month, 2 months and 6 months. AEs Validation: none used |
|
| Notes | Funding by Health Research Council of New Zealand; cytisine supplied at no cost by Sopharma New for 2016 update Author declaration of interests: "Dr. McRobbie reports receiving lecture fees from Johnson & Johnson and grant support from Pfizer. No other potential conflict of interest relevant to this article was reported." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly allocated, by computer ... in a 1:1 ratio" |
| Allocation concealment (selection bias) | Low risk | "Randomization was stratified with the use of minimization according to sex, ethnicity (Maori, Pacific Islander, or non‐Maori and non–Pacific Islander), and cigarette dependence, which was determined by means of the Fagerström Test of Cigarette Dependence, in which smokers were assigned to one of two groups: those with scores of 5 or lower, indicating lower dependence, and those with scores greater than 5, indicating greater dependence" |
| Blinding (performance bias and detection bias) All outcomes | High risk | "Participants and researchers collecting outcome data were aware of treatment allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses fully reported. By 6 months, 182 cytisine participants (28%) lost to follow‐up, and 16 withdrawals; 173 NRT participants (26%) lost to follow‐up, and 14 withdrawals. 19 cytisine users crossed over to NRT, and 1 NRT user crossed over to cytisine. ITT analyses conducted |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes from protocol all reported |
| Other bias | Unclear risk | Cytisine was supplied free, while NRT users had to pay a nominal charge (NZD 3 for an 8‐week course of each NRT item). |
Walker 2021.
| Study characteristics | ||
| Methods | Country: New Zealand Setting: community pharmacy (for drug distribution), participants' homes Aim: to determine whether cytisine was at least as effective as varenicline in supporting smoking abstinence for ≥ 6 months in New Zealand indigenous Māori or whānau (extended‐family) of Māori Study design: open‐label, parallel, RCT |
|
| Participants | 679 adult daily smokers who identified as Māori or whānau of Māori, motivated to quit in the next 2 weeks, and were eligible for subsidised varenicline. 69.6% female, mean age 43, baseline average CPD 15.5 Exclusion criteria: pregnant/breastfeeding; currently using SC medication (including e‐cigarettes); enrolled in another cessation programme/study; used varenicline or cytisine in the previous 12 months; known hypersensitivity; self‐reported moderate/severe renal impairment; treatment for active/latent tuberculosis; heart attack, stroke or severe angina within the previous 2 weeks; uncontrolled high blood pressure; history of seizures |
|
| Interventions |
All participants received low‐intensity cessation behavioural support from the prescribing doctor and community stop‐smoking services or a research assistant. |
|
| Outcomes | Primary: CA at 6 months (verified with exhaled CO < 9 ppm) Secondary outcomes measured at 1, 3, 6 and 12 months post‐quit date included: self‐reported CA, 7‐day PPA, CPD, time to (re)lapse, AEs, treatment adherence/compliance and acceptability, nicotine withdrawal/urge to smoke and healthcare utilization/health‐related QoL |
|
| Notes | New for 2022 update Funding by the Health Research Council of NZ. Manufacturer supplied the treatment free of charge. Author declaration of interests: "All authors report grants from the Health Research Council of New Zealand and non‐financial support from Achieve Life Sciences during the conduct of the study. N. W., C.B.and M.V. V.P. report grants from Pfizer, grants from the Health Research Council of New Zealand, outside the submitted work; and has previously undertaken two trials of e‐cigarettes for smoking cessation [with e‐cigarettes purchased from a NZ e‐cigarette on‐line retailer (NZVAPOR, https://www.nzvapor.com/), e‐liquid for one trial purchased from Nicopharm, Australia (https://www. nicopharm.com.au/) and nicotine patches supplied by the NZ Government via their contract with Novartis (Sydney, Australia)]. Neither NZVAPOR nor Nicopharm have links with the tobacco industry. C.B. also reports personal fees from the Moffat Cancer Center, University of Florida, USA, and personal fees from Virginia Commonwealth University, USA outside the submitted work. J.B. also reports personal fees from New Zealand Ministry of Health Natural Health Products (NHPs) Regulations Subcommittee on the Permitted Substances List (member of subcommittee 2016–17), non‐financial support from Uppsala Monitoring Centre, Sweden (who manages the technical and scientific aspects of the WHO Programme for International Drug Monitoring); honorary consultant and herbal safety signal reviewer (2004–current), outside the submitted work. None of the above parties had any role in the design, conduct, analysis or interpretation of the trial findings or writing of the resulting publication." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Block randomization was undertaken, with varying block sizes. The randomization sequence was prepared by the trial statistician using R and loaded into the REDCap database, which was the accessed by the study doctor via a computer at the point of randomization." |
| Allocation concealment (selection bias) | Low risk | "Block randomization was undertaken, with varying block sizes. The randomization sequence was prepared by the trial statistician using R and loaded into the REDCap database, which was the accessed by the study doctor via a computer at the point of randomization." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Abstinence was verified by a researcher or community‐based cessation provider, using standardized exhaled carbon monoxide (CO) measurement with a Bedfont Smokerlyzer". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 39% of participants in both groups were followed up at 6 months (12 months is unclear) although these numbers do not match main results table. Methods say multiple imputation used but not clear what effect this had on findings. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported match the trial protocol paper |
Wang 2009.
| Study characteristics | ||
| Methods | Country: China (10 sites), Singapore (3 sites), Thailand (2 sites) Aim: to test the efficacy and safety of varenicline for SC in Chinese, Singaporean and Thai smokers Dates conducted: not stated Study design: double‐blind placebo‐controlled RCT |
|
| Participants | 333 healthy adult volunteers, aged 18‐75; allocated to varenicline (165), or placebo (168). 97% men, mean age 39, BMI > 15 and < 38 or weight > 45.5 kg, mean CPD 20, mean FTND score 5.4. Treatment groups were comparable at baseline. 58% had never tried to quit before Exclusion criteria: standard pharmacotherapy trial criteria, plus any use of NRT or bupropion in previous 6 months | |
| Interventions |
Treatment period 12 weeks, 1st week titrated dosage. All participants received a SC booklet at baseline, + brief counselling (≤ 10 min) at each clinic visit, except for weeks 5 and 7, when counselling was conducted by phone In follow‐up phase, clinic visits at weeks 13, 16, 20, 24 plus brief phone calls at weeks 14, 18, 22. Dosing and CO checked at each visit, and lab samples taken at weeks 12 and 24 |
|
| Outcomes | Primary outcome: CO‐confirmed CAR for weeks 9‐12 Secondary outcomes: CO‐confirmed CAR for weeks 9‐24; 7‐day PPA at 24 weeks Validation by expired CO < 10 ppm Other outcomes: AEs; long‐term quit rates Dropouts and losses to follow‐up were included in the analyses as continuing smokers (ITT analysis) Attrition in treatment phase was 3.0% in varenicline group, and 3.6% in placebo group. By week 24, 4.2% of had dropped out of each group | |
| Notes | The trial was funded by Pfizer Inc
New for 2010 update Author declaration of interests: "Pfizer Inc. funded the study and was involved with its design, analysis and writing the manuscript. All authors had complete access to all relevant data. Dahlia Garza and Simon Davies are employees of Pfizer Inc., and therefore hold shares in the company. Editorial support was provided by Aideen Young, PhD, of UBC Scientific Solutions and funded by Pfizer Inc. None of the other authors hold shares in any companies. Chen Wang and Dan Xiao are affiliated with the WHO Collaborating Centre for Tobacco or Health. WHO had no role in the study’s funding, design, analysis or write‐up." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "eligible subjects were randomized in a 1:1 ratio" |
| Allocation concealment (selection bias) | Unclear risk | See above |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "double‐blind", but no further information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information, but very high compliance rates |
| Selective reporting (reporting bias) | Low risk | All expected and predicted outcomes covered |
West 2011.
| Study characteristics | ||
| Methods | Country: Poland
Setting: SC clinic in Warsaw Aim: to test the efficacy and safety of cytisine for SC with minimal counselling and support Dates conducted: December 2007‐September 2010 Study design: single‐centre, double‐blind placebo‐controlled parallel‐group RCT |
|
| Participants | 740 healthy adults, smoking 10+ CPD, motivated to quit. Randomised to cytisine (370) or placebo (370) 46.5% men, mean age 48, mean CPD 23, prior quit attempts 82%, mean FTND 6.2 Exclusions were current psychiatric disorder or any medical condition contraindicated on cytisine label |
|
| Interventions | Tabex tablets (1.5 mg cytisine):
Placebo tablets, same regimen Treatment period was 25 days. Quitting advice, randomisation and drugs dispensed at baseline visit; phone calls at TQD + 1 week later (+ optional clinic visit). Clinic visit 4 weeks post‐TQD, then phone calls at 6 months and 12 months, with visit to confirm abstinence if claimed. Behavioural support was minimal, to simulate likelihood of real‐world conditions in countries where Tabex is available |
|
| Outcomes | Primary: CO‐validated abstinence 12 months after EoT. Abstinence defined as smoking < 5 cigarettes during preceding 6 months, and none in week before visit Secondary outcomes: sustained CO‐validated abstinence at 6‐month follow‐up; 2‐week PPA at 4 weeks; 7‐day PPA at 12 months Validation was expired CO < 10 ppm Attrition: 79 (cytisine) and 89 (placebo) participants were lost to follow‐up over 12 months. Drug discontinuation or reduction rates similar in both groups: 6.2% for cytisine and 4.6% for placebo Other outcomes: AEs, SAEs |
|
| Notes | New for 2012 update The trial was funded by the UK National Prevention Research Initiative, Cancer Research UK, and the National Institute for Health Research Author declaration of interests: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "performed by a statistician at Sopharma, who generated a list of study‐group assignments for 740 participants with nQuery Advisor software. Assignments were made in variable block sizes of either 20 (10 cytisine, 10 placebo) or 10 (5 and 5)" |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Trial staff and participants were unaware of the group assignments and the randomization scheme" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts and attrition fully reported |
| Selective reporting (reporting bias) | Low risk | All expected and predicted outcomes reported |
Westergaard 2015.
| Study characteristics | ||
| Methods | Country: Denmark Aim: to evaluate the effect of varenicline on tobacco cessation in young smokers suffering from asthma Dates conducted: not stated Study design: double‐blind placebo‐controlled RCT |
|
| Participants | 52 young (aged 19‐40) smokers with asthma, randomised to varenicline (26) or placebo (26). CPD ≥ 10; FTND 5.6 | |
| Interventions |
No further details |
|
| Outcomes | Primary: presumed PPA at 12 weeks Secondary: presumed PPA at 0, 6, 24 weeks Validation by expired CO < 10 ppm Also assessed asthma symptom score, general health quality score (15D) and methacholine challenge |
|
| Notes | Study funding: "The Respiratory Research Unit has received financial support from Pfizer Inc. [WS807136] in relation to the conduction of the present study. Furthermore, the Varenicline and placebo tablets were provided by Pfizer Inc. Pfizer Inc. has not been involved in study design, in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication." Author declaration of interests: not reported Author supplied further details |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. ""randomized, placebo‐controlled, double‐blinded trial" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated. "double‐blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated; ITT analysis conducted |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
Williams 2007.
| Study characteristics | ||
| Methods | Country: USA and Australia
Setting: 9 research centres (8 USA, 1 Australia) Aim: to test the safety of long‐term (12 months) use of varenicline in smokers trying to quit Study design: double‐blind placebo‐controlled RCT Dates conducted: October 2003‐March 2005 |
|
| Participants | 377 adult smokers, aged 18‐75, smoking at least 10 CPD. 49.9% men, 88.6% white, average CPD at baseline 23, mean FTND 5.5 in treatment group, 6.05 in control group. Allocated to varenicline (251) or placebo (126) Exclusion criteria: standard pharmacotherapy trial criteria, + no use of NRT, antidepressants, antipsychotics, naltrexone during study period | |
| Interventions |
All participants received booklet Clearing the Air. Brief counselling (≤ 10 min) at each visit TQD was 1st day of week 1 visit (7‐10 days post‐randomisation) Treatment period was 52 weeks. Weekly visits throughout weeks 1‐8, then every 4 weeks to week 52, + week 53 assessment Blood and urine samples taken at screening, baseline, weeks 2, 12, 24, 36, 52 (or early termination) Complete physical exam at baseline, weeks 24 and 52; BP, pulse and weight measured at all visits, ECG at screening, baseline, weeks 2, 24 and 52 (or early termination) |
|
| Outcomes | Primary outcome: safety of smokers treated continuously with varenicline over 52 weeks, measured at week 53 by level and tolerability of AEs and incidence of SAEs Secondary outcome: 7‐day CO‐verified PPA at all clinic visits (expired CO ≤ 10 ppm) Other outcomes: weight change; changes in vital signs Attrition was 46.2% in varenicline group, 53.2% in control group by end of study | |
| Notes | This was a safety study, with cessation rates collected as a secondary outcome
The trial was funded and conducted by Pfizer Inc
In the first version of this review, this trial appeared as Reeves 2006 (unpublished data) Author declaration of interests: "Kathryn E. Williams, Karen R. Reeves, Clare B. Billing, Jr, Ann M. Pennington, and Jason Gong are all employees of Pfizer and were involved in designing the study, data monitoring, data management, statistical analysis, and interpretation of the results, as well as the drafting, editing, and reviewing of this manuscript" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation 2:1 varenicline to placebo. No detailed information reported |
| Allocation concealment (selection bias) | Unclear risk | No information reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing CO and/or visit taken as smokers |
| Selective reporting (reporting bias) | Low risk | Primary outcome was safety, so minimal cessation data |
Williams 2012.
| Study characteristics | ||
| Methods | Countries: Canada, USA
Setting: 12 sites
Aim: to evaluate primarily safety, but also efficacy of varenicline in smokers with schizophrenia or schizoaffective disorders Dates conducted: May 2008‐April 2010 Study design: double‐blind placebo‐controlled RCT. Sample size [120] was considered sufficient to detect a between‐group difference in 7‐day PPA "for a medium effect size" |
|
| Participants | 128 adults, diagnosed with stable schizophrenia or schizoaffective disorders, smoking at least 15 CPD and motivated to quit. Randomised to varenicline (85) or placebo (43). 77% men aged 18‐75 | |
| Interventions |
Weekly clinic visits, for safety and efficacy, ≤ 30‐min counselling sessions; after treatment phase, clinic visits at weeks 13, 16, 20, 24, with brief phone calls at weeks 14, 18 and 22. Follow‐up sessions included brief (≤ 10 min) counselling. AEs collected to 30 days after treatment, and neuropsychiatric AEs to week 24 |
|
| Outcomes | Primary outcome: N of participants with AEs and SAEs from baseline to 30 days after EoT (12 weeks). N of participants with psychiatric AEs, including suicidal ideation or behaviour Secondary outcomes: CO‐confirmed PPA at weeks 12 and 24; 50%+ reduction in CPD; change in CPD from baseline. Assessments on mood and psychiatric scales Validation was by exhaled CO ≤ 10 ppm Dropouts in treatment phase: 14 (varenicline), 3 (placebo); follow‐up phase: 10 (varenicline), 3 (placebo) 1 varenicline participant died during follow‐up phase |
|
| Notes | The study was funded by Pfizer New for 2012 update Author declaration of interests: "Dr Williams has received research support from the National Institutes of Health (NIH [National Institute of Mental Health and National Institute on Drug Abuse]) and Pfizer and has received further support from Pfizer for advisory board membership and product support. Dr Anthenelli provides consultancy and/or advisory services for Pfizer and GlaxoSmithKline, and his laboratory receives funding support from the National Institute on Alcohol Abuse and Alcoholism, the Department of Veterans Affairs, Pfizer, Nabi Biopharmaceuticals, and sanofi‐aventis. He has also received honoraria from Pfizer. Dr Morris has received research support from Pfizer. Drs Thompson and Yunis and Ms Treadow are employees of and shareholders in Pfizer. Dr George has received consulting fees from Pfizer, sanofi‐aventis, Novartis, Eli Lilly, Prepharm, AstraZeneca, and Janssen; has received research support from Pfizer and Sepracor; and has received grant support relevant to the study medication from the NIH, Canadian Institutes of Health Research, Canada Foundation for Innovation, and the Ontario Mental Health Foundation" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Subjects were randomized (2:1) to varenicline or placebo ... and were stratified according to antipsychotic medication type (typical vs atypical)." |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Abstinence biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not yet reported |
| Selective reporting (reporting bias) | Low risk | Outcomes prespecified in trial registry reported |
Windle 2018.
| Study characteristics | ||
| Methods | Country: 40 centres in USA and Canada
Setting: hospitals Aim: to determine the efficacy and safety of varenicline for increasing smoking abstinence rates through smoking reduction Study design: double‐blind placebo‐controlled multicentre RCT Study name: evaluation of varenicline in SC for patients post‐acute coronary syndrome (EVITA) Dates conducted: not stated |
|
| Participants | 302 adult smokers, aged 18+, smoking 10+ CPD, interested in trying to quit, hospitalised in USA or Canada for acute coronary syndrome (MI or unstable angina). Mean age 55, 25% women, mean CPD 21.5 Allocated to varenicline (151) or placebo (151) Exclusions: excessive alcohol, history of panic disorder, psychosis, bipolar disease, dementia, renal or hepatic impairment, current or recent drug use, history of suicidal ideation/attempt or family history of suicide |
|
| Interventions |
Medication was begun in hospital. All participants received low‐intensity counselling Follow‐up at weeks 1, 2 and 8 by phone, and clinic visits at weeks 4, 12, 24 and 52 |
|
| Outcomes | Primary: 7‐day PPA at week 24 Secondary: CAR at all follow‐up visits, 7 day PPA at other follow‐up visits, ≥ 50% reduction in CPD Measures of side effects and SAEs Validation: CO ≤ 10 ppm |
|
| Notes | New for 2016 update Study funding: "EVITA was an investigator‐initiated trial that received funding and the study drug and placebo from Pfizer Inc. Pfizer had no role in the design, conduct, analysis, interpretation of data, or reporting of the EVITA trial." Author declaration of interests: "Shamir Mehta reports funding from AstraZeneca, Boston Scientific, Bayer and Abbott. Beth Abramson has received grants or research support from AstraZeneca and Sanofi; honoraria from Amgen, AstraZeneca, Boehringer Ingelheim, Bristol–Myers Squibb, Novartis, Fournier, Merck, Pfizer, Servier and Sanofi; and consulting fees from Amgen, Bayer, Boehringer Ingelheim, Sanofi and Servier. She authored Heart Health for Canadians. Mark Eisenberg, Payam Dehghani, François Grondin and Mina Madan received honoraria from Pfizer for providing continuing medical education on SC. No other competing interests were declared" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomized to either varenicline or matching placebo... Randomization was performed by enrolling center personnel and stratified by center using a computer‐generated list of permuted blocks of 2 and 4" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Described as "double‐blind", but no further detail. Abstinence biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses fully reported; ITT analyses conducted |
| Selective reporting (reporting bias) | Low risk | Outcomes prespecified in trial registry reported |
Wong 2012.
| Study characteristics | ||
| Methods | Country: Canada
Setting: 2 Toronto hospitals Aim: "to determine the effectiveness and safety of a perioperative SC intervention including varenicline and counseling versus placebo and counseling to increase short‐ and long‐term abstinence in surgical patients" Study design: randomised placebo‐controlled double‐blind trial Dates conducted: June 2008‐November 2010 |
|
| Participants | 286 non‐cardiac elective surgery patients, smoking 10+ CPD, no abstinence > 3 months in last year, scheduled for surgery in the next 8‐30 days. Allocated to varenicline (151) or placebo (135). Mean age 52.6, 47% women, mean CPD 17.4, mean FTND 4.8 | |
| Interventions |
All participants received 2 standardised 15‐min counselling sessions by researchers, 1 in pre‐op clinic and 1 24 h after surgery, supplemented by written materials. All participants retained the same counsellor throughout the process Weekly counselling phone calls for 4 weeks, and at the end of 8 weeks. From 3‐12 months, phone calls every 4 weeks for smoking status, nicotine dependence, stage of change, CPD, brief (< 5 min) counselling. TQD was set for 24 h before surgery, and medication begun 7 days before TQD Participants were invited to visit the hospital at 3, 6, and 12 months, for assessment and testing. Participants unable to visit the hospital were sent a self‐test urinary kit |
|
| Outcomes | 7‐day PPA at 12 months; abstinence on TQD; 7‐day PPA at 3 months and 6 months. Self‐reported changes in CPD and stage of change at 3, 6 and 12 months Validation: expired CO and urinary cotinine (cut‐offs not given) |
|
| Notes | Supported by Canadian academic institutes and Pfizer Canada New for 2016 update Author declaration of interests: "Dr. Chapman holds the GSK‐CIHR Research Chair in Respiratory Health Care Delivery. Dr. Wong is a recipient of a Merit Research Award from the Department of Anesthesia at the University of Toronto, Toronto, Ontario, Canada." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Smokers were randomly assigned to receive varenicline (Pfizer Inc., Kirkland, Quebec, Canada) or matching placebo using a computer‐generated randomization list at each center. A stratified randomization with blocks of 40, based on the smoker’s stage of change, was employed because the stage of change may predict successful abstinence from smoking." |
| Allocation concealment (selection bias) | Low risk | "The patient assignments were placed into sequentially numbered, opaque sealed envelopes, and were kept by an independent research pharmacist at each center who was not involved with patient care or outcome assessments. For each patient, the research pharmacist opened the envelope and provided the research coordinator with the medication or placebo (lactose, identical in appearance) according to the randomization schedule." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The patients, healthcare personnel, and research staff were blinded to the randomization throughout the study period." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses fully reported: varenicline: 6 discontinued treatment, 11 discontinued follow‐up. Placebo: 6 discontinued treatment, 10 discontinued follow‐up. ITT analyses conducted |
| Selective reporting (reporting bias) | Low risk | Outcomes prespecified in trial registry reported |
Yang 2016.
| Study characteristics | ||
| Methods | Country: China Setting: hospital Study design: parallel randomised trial |
|
| Participants | 78 male smokers, motivated to quit, with a diagnosis of COPD Mean age 58, baseline average CPD 5 |
|
| Interventions |
|
|
| Outcomes | PA at 24 weeks. Self‐reported | |
| Notes | New for 2022 update Study funding: not reported Author declaration of interests: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated randomised but methods not reported |
| Allocation concealment (selection bias) | Unclear risk | Concealment not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition rate unclear |
| Selective reporting (reporting bias) | Unclear risk | Protocol unavailable |
Zawertailo 2020.
| Study characteristics | ||
| Methods | Country: Canada Setting: inpatient or outpatient treatment clinic Aim: to evaluate if varenicline would be a safe and efficacious treatment for tobacco dependence in people with alcohol dependence Study design: randomised, placebo‐controlled, double‐blind pilot study |
|
| Participants | 31 daily dependent smokers, aged 18‐65 years, also in treatment for alcohol dependence. Eligible participants smoked at least 10 CPD; and scored ≥ 3 on the Fagerstrom Test of Nicotine Dependence (FTND). 26.7% were female, mean age 44.6, baseline average CPD 18.4 Exclusion criteria: serious medical condition requiring immediate investigation or treatment, current pregnancy or breastfeeding, current psychiatric disorder excluding alcohol use disorder and tobacco dependence, any known contraindication to using varenicline, inability to provide informed written consent, or inability or unwillingness to attend weekly study visits for 12 consecutive weeks |
|
| Interventions |
All participants received weekly individual in‐person SC counselling for 12 weeks |
|
| Outcomes | Primary: abstinence from smoking (defined as no smoking during the last 4 weeks) at the EoT. Self‐reported abstinence was biochemically verified using exhaled CO of < 10 ppm Secondary: abstinence from smoking at 6 months, biochemically confirmed using exhaled CO of < 10 ppm |
|
| Notes | New for 2022 update Funding by Pfizer and the Ministry of Health, the Canadian Institutes of Health Research Author declaration of interests: "L.Z. is currently receiving investigator‐initiated funding from Pfizer, Inc, and peer‐reviewed grant funding from the Ontario Ministry of Health and the Canadian Institutes of Health Research. B.L.F. has obtained funding from Pfizer (GRAND Awards, including salary support) for investigator‐initiated projects. P.S. has received funding from the Ontario Ministry of Health, Canadian Institutes of Health Research, Canadian Centre on Substance Abuse, Public Health Agency of Canada, Pfizer, Inc/Canada, Ontario Lung Association, and Canadian Cancer Society Research Institute. He has received honoraria for speaking engagements and consulting from Pfizer Canada, Inc, ABBVie, Bristol‐Myers Squibb, Evidera, Inc, Johnson & Johnson Group of Companies, Medcan Clinic, Inflexxion, Inc, V‐CC Systems, Inc, MedPlan Communications, Kataka Medical Communications, Miller Medical Communications, NVision Insight Group, Sun Life Financial, and Myelin & Associates For the remaining authors, no conflicts of interest are declared." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated by the CAMH Research Pharmacy", |
| Allocation concealment (selection bias) | Low risk | "Both participants and study staff were blinded to the randomization assignment" with allocation performed by the CAMH Research Pharmac, who were not involved in recruitment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Both participants and study staff were blinded to the randomization assignment." "Self‐reported abstinence at each weekly visit was confirmed with expired CO of less than 10 ppm using a Bedfont Smokelyzer." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5/15 (placebo) and 4/16 (varenicline) dropped out. 21/31 participants attended 6 months follow‐up |
| Selective reporting (reporting bias) | Low risk | Pre‐specified outcomes reported |
Zhang 2022.
| Study characteristics | ||
| Methods | Study design: RCT Country: Canada Recruitment method (quote): "The primary method of recruitment was by word of mouth and Facebook advertisement. Interested participants were directed to the study website, where they could indicate their consent for participation and complete eligibility questionnaires" |
|
| Participants | 2461 smokers; 56% female, average age 46.5, 46.9% of bupropion group and 44.9% of varenicline group smoked 11‐20 CPD | |
| Interventions |
Common components: weekly motivational emails |
|
| Outcomes |
|
|
| Notes | Study funding: "This research was funded by Global Research Awards for Nicotine Dependence (GRAND), a peer‐reviewed research grant competition funded by Pfizer Pharmaceuticals [Zawertailo (GRAND2012) WS2391913]. The study’s funder had no role in study design, data collection, data analysis, data interpretation, or writing of the report, and had no decision to submit the article for publication. Dr. Le Foll is supported by a clinician‐scientist award from the Department of Family and Community Medicine and by the Addiction Psychiatry Chair of the Department of Psychiatry of University of Toronto. Dr. Selby is supported by a clinician‐scientist award from the Department of Family and Community Medicine and CAMH." Author declaration of interests: "All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organization for the submitted work; PS reports receiving funding and/ or honoraria from Pfizer Inc./Canada, Shoppers Drug Mart, Bhasin Consulting Fund Inc., Patient‐Centered Outcomes Research Institute, ABBVie, and Bristol‐Myers Squibb; BLF and LZ both receive support from Pfizer Global Research Awards in Nicotine Dependence (GRAND) Award Program; there are no other relationships or activities that could appear to have influenced the submitted work. BLF also reports grants from Brainsway, grants from Bioprojet, grants from Alkermes, grants from Canopy, grants from ACS, non‐financial support from Aurora, outside the submitted work." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study quote: "Participants were randomly assigned to one of two medication arms (varenicline or bupropion) using permuted‐block randomization in a 1:1 ratio in blocks of 100." Protocol quote: "The randomization process will be computerized." |
| Allocation concealment (selection bias) | Low risk | Protocol quote: "The randomization process will be computerized." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Study quote: "Participants were not blinded to treatment since their health care provider signing the prescription form was required to know which drug was being prescribed to their patient." However, both study groups received active smoking cessation pharmacotherapy treatment. The abstinence outcome was not biochemically verified, however, study arms received the same behavioural support. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The overall number of participants lost at the 52‐week follow‐up was > 50% in each condition. However, a complete case sensitivity analysis (section 3.5.2) did not change findings at the 52‐week follow‐up (i.e. no difference between conditions) |
| Selective reporting (reporting bias) | Unclear risk | Not all prespecified outcomes from trial registry reported in main results paper. However, SC outcomes are reported. |
Zincir 2013.
| Study characteristics | ||
| Methods | Study design: RCT Country: Turkey Setting: outpatient SC clinic in a hospital Recruitment method: patients who presented at the SC outpatient clinic were included in the study on a voluntary basis |
|
| Participants | 300 participants randomised; average age: 45.8 in those who stopped smoking and 40.8 in those who continued smoking; average boxes of cigarettes per year: 23.62 in those who stopped smoking and 23.26 in those who continued smoking; mean FTND: 5.9 in those who stopped smoking and 6.7 in those who continued smoking | |
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Funding: not reported Author declaration of interests: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “…they were randomized to the pharmacological therapy groups” Comment: no further information given |
| Allocation concealment (selection bias) | Unclear risk | Quote: “…they were randomized to the pharmacological therapy groups" Comment: no further information given |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: “This was a naturalistic clinical follow‐up study." Comment: those involved in the study were therefore unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 300 participants were randomised and 251 completed the study. Therefore 49/300 (16.3%) were lost to follow‐up overall. However, it is impossible to establish the number lost to follow‐up by group. |
| Selective reporting (reporting bias) | Unclear risk | Protocol unavailable |
| Other bias | High risk | Quote: “no adverse event was reported during the study”. This is highly unlikely to be correct when considering standard definitions of AEs. There is no explanation of how AEs were assessed in this study. In addition, the wording of the paper makes the final follow‐up slightly unclear. After discussion we judged final follow‐up to be 24‐28 weeks from study start, although quit rates are higher than would be expected at this time point. |
AE: adverse event; BMI: Body Mass Index (kg/m2); CA: continuous abstinence; CAR: Continuous Abstinence Rate; cNRT: combined nicotine replacement therapy; CO: carbon monoxide; COPD: chronic obstructive pulmonary disease; CPD: cigarettes per day; CQR: continuous quit rate; CVD: cardiovascular disease; DSM‐IV:Diagnostic and Statistical Manual of Mental Disorders, 4th Edition; ECG: electrocardiogram; EoT: end of treatment; FTND: Fagerström Test for Nicotine Dependence; ITT: intention‐to‐treat; LOCF: last observation carried forward; MA: meta‐analysis; MDD: major depressive disorder; MI: motivational interviewing; MIMS: Monthly Index of Medical Specialities; mCEQ: Modified Cigarette Evaluation Questionnaire; MNWS: Minnesota Nicotine Withdrawal Scale; NCT: National Clinical Trials (ClincialTrials.gov registry); NRT: nicotine replacement therapy; PA: prolonged abstinence; PPA: point‐prevalence abstinence; ppm: parts per million; QoL: quality of life; QSU‐brief: Brief Questionnaire of Smoking Urges; RCT: randomised controlled trial; SAE: serious adverse event; SC: smoking cessation; TQD: target quit date
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Brandon 2011 | Short‐term (15 days) RCT, to test craving and psychological reward; cessation was not an outcome. Previously included but excluded for 2023 update |
| Bullen 2018 | Study tested effect of e‐cigarettes as an adjunct to varenicline |
| Burstein 2006 | RCT of tolerability and safety of varenicline in 24 elderly (≥ 65) smokers for 1 week. Not a cessation trial |
| Chantix 2006 | Tested NRT alone or varenicline + NRT for 12 days to test safety and side effects of co‐administration. Not a cessation trial |
| Cui 2012 | Open‐label non‐randomised pre/post study of 36 HIV+ participants; all received varenicline |
| Dezee 2013 | RCT in which all participants were given varenicline; intervention tested was in‐person vs internet counselling |
| Dutra 2012 | 53 participants with schizophrenia given varenicline + cognitive behavioural therapy. Abstinence assessed at 12 weeks (end of treatment) |
| Ebbert 2009a | Open‐label, single‐arm Phase II study, for safety and efficacy of varenicline plus bupropion |
| Ebbert 2009b | Cohort analysis of 104 participants on varenicline + NRT and 135 participants treated prior to release of varenicline (93% used NRT) |
| Ebbert 2011b | Pilot study of varenicline for smokeless tobacco users. 12‐week outcome (EoT) reported, not long‐term post‐treatment. Previously included but excluded for 2023 update |
| Ebbert 2014 | RCT in which all participants were given varenicline; the intervention being tested was bupropion vs placebo. See also Hong 2011 |
| Evins 2014 | Relapse prevention study that recruited already abstinence participants. Previously included but excluded for 2023 update |
| Faessel 2009 | Outcomes were safety, tolerability and pharmacokinetics, not smoking cessation. Previously included but excluded for 2023 update |
| Fagerström 2010 | 431 smokeless tobacco users in Norway and Sweden, randomised to varenicline or placebo; CAR assessed at 12 and 26 weeks. Previously included but excluded for 2023 update |
| Falk 2014 | Varenicline was used for alcohol reduction, not for smoking |
| Fatemi 2013 | 3‐arm RCT of varenicline, bupropion and placebo; only assessed to end of treatment (12 weeks). |
| Ferketich 2012 | Pilot study of varenicline vs NRT; participants could choose their treatment; intervention being tested was the addition of a lung cancer screening programme |
| Ferketich 2013 | Safety of varenicline among smokers enrolled in the Lung HIV study. Participants could choose varenicline or NRT, and were only followed for 3 months. |
| Fertig 2015 | Study testing effect of varenicline on alcohol dependence |
| Frye 2013 | Participants followed only until EoT (12 weeks) |
| Fucito 2011 | Primary outcome was effects on drinking behaviour. Smoking status only measured at end of study (8 weeks) |
| Garza 2011 | 110 abstinent smokers treated with varenicline or placebo, to assess incidence and severity of neuropsychiatric symptoms; not a cessation trial. Previously included but excluded for 2023 update |
| Granatowicz 1976 | No control group |
| Gray 2012 | Pilot study of varenicline vs bupropion in older adolescents; outcome was reduction rather than cessation, and participants were only followed for 3 months. |
| Hajek 2011 | 101 smokers randomised to preloaded varenicline or placebo; abstinence not measured beyond 12 weeks |
| Hajek 2013 | All were given varenicline, with the intervention tested being the addition of a NRT patch. Only followed to 3 months |
| Hajek 2015 | Follow‐up under 6 months. Previously included but excluded for 2023 update |
| Hartwell 2014 | Varenicline for drinking and smoking; smoking topography and pharmacogenetics rather than smoking cessation |
| Hawk 2012 | RCT of extended pre‐TQD varenicline vs standard regimen; all participants got varenicline, and were followed only until end of treatment (12 weeks) |
| Hong 2011 | Secondary analysis to Ebbert 2014, looking at depression in recipients of varenicline + bupropion vs varenicline alone |
| Hoogsteder 2014 | All participants were given open‐label varenicline; the intervention being tested was the addition of NicVAX. |
| Hsueh 2014 | Open‐label cohort study of smokers taking varenicline or NRT |
| Hsueh 2021 | Study not randomized. Participants chose their own treatment. |
| Hughes 2011 | 218 smokers not ready to quit assigned to varenicline or placebo for 2‐8 weeks for cigarette reduction. Previously included but excluded for 2023 update |
| IRCT20100127003210N | Study not randomised |
| Jain 2014 | RCT of smokeless tobacco users. Follow‐up of 12 weeks |
| Jennings 2014 | Only followed to 16 weeks |
| Jiménez‐Ruiz 2013 | Not an RCT |
| Kempe 1967 | Observational uncontrolled study |
| Koegelenberg 2014 | All participants took varenicline; the intervention being tested was the addition of NRT. |
| Maliszewski 1972 | Uncontrolled study of 14 smokers on a 25‐day course of cytisine (Tabex); followed up for 2 weeks |
| Marakulin 1984 | Follow‐up 6 weeks |
| McClure 2013 | Laboratory study following an RCT of varenicline in a programmed lapse; abstinence only to 4 weeks. Previously included but excluded for 2023 update |
| McColl 2008 | RCT of varenicline's potential as an abuse drug in smokers and non‐smokers; not a smoking cessation trial |
| McNaughton 2013 | All participants received varenicline; the intervention being tested, as a relapse prevention aid, was interactive voice response phone calls |
| Meszaros 2013 | Pilot study (10 participants, only 4 completers), only followed to 3 months; objective was reduction, not cessation. Previously included but excluded for 2023 update |
| Metelitsa 1987 | Uncontrolled study |
| Mitchell 2012 | Varenicline was for drinking reduction, not smoking cessation; only followed for 12 weeks. Previously included but excluded for 2023 update |
| Mocking 2013 | 7‐day administration of varenicline for emotional and cognitive processing in non‐smokers |
| Monova 2004 | Follow‐up was 60 days |
| Nahvi 2014b | Follow‐up under 6 months |
| Nahvi 2020 | Study tested an intervention for improving adherence to varenicline |
| NCT00387946 | Follow‐up under 6 months |
| NCT00502216 | Study of varenicline and naltrexone for tolerability and weight gain in smokers, not cessation |
| NCT00554840 | Follow‐up under 6 months |
| NCT00828113 | Relapse prevention study that recruited already abstinent participants. Previously included but excluded for 2023 update |
| NCT01093937 | Follow‐up under 6 months |
| NCT01413516 | Follow‐up under 6 months |
| NCT01532232 | Study terminated |
| NCT01574703 | Tobacco abstinence not measured |
| NCT01592695 | Different levels of behavioural support between study arms |
| NCT01639560 | Follow‐up under 6 months |
| NCT01771627 | Follow‐up under 6 months |
| NCT01772641 | Follow‐up under 6 months |
| NCT01806779 | All participants got varenicline; the addition of bupropion was the intervention being tested. |
| NCT01892813 | Different levels of behavioural support between study arms |
| NCT02048917 | Follow‐up under 6 months |
| NCT02147132 | Cross‐over trial |
| NCT02271919 | Follow‐up under 6 months |
| NCT02501265 | Study testing the addition of bupropion to a subset of participants receiving varenicline |
| NCT03709823 | Follow‐up under 6 months |
| Nides 2021 | Follow‐up under 6 months |
| Nollen 2011 | All participants received varenicline, but half received extended counselling and half a single session. Cessation only measured to 3 month end point |
| Ostrovskaia 1994 | Uncontrolled study |
| Park 2011 | RCT of 49 smokers with lung cancer randomised to varenicline or placebo; follow‐up only for 12 weeks to EoT |
| Patterson 2010 | Short‐term (3‐week) study of propensity to relapse with working memory deficits after 10 days of varenicline |
| Paun 1968 | Controlled trial of cytisine (Tabex) (366 smokers) vs placebo (239 smokers) but followed only for 8 weeks. Observational study of 230 cytisine‐users followed for 26 weeks, but no comparator group |
| Pfeifer 2019 | Did not measure smoking cessation as an outcome |
| Pfizer 2006 | Follow‐up under 6 months |
| Poling 2010 | RCT of varenicline in 31 methadone‐maintained smokers; trial lasted 3 months, and reduction was an outcome of interest (only 3‐month abstinence was reported) |
| Ramon 2014 | RCT in which all participants received varenicline; intervention being tested was the addition of NRT |
| Rose 2014 | RCT of varenicline versus varenicline + bupropion, in smokers who had failed to quit on NRT. All got varenicline |
| Schlienz 2014 | 4 weeks treatment with varenicline; outcome was impact on behavioural economic indices, not smoking cessation |
| Schmidt 1974 | Non‐randomised trial |
| Schnoll 2011 | RCT of open‐label varenicline + counselling; intervention being tested was recruitment strategies, not smoking cessation |
| Shim 2011 | 60 smokers with schizophrenia randomised to varenicline or placebo for 8 weeks; assessment at end of treatment, reduction but not abstinence rates reported |
| Sicras‐Mainar 2010 | Multicentre observational non‐randomised non‐controlled study |
| Smith 2013 | Follow‐up under 6 months |
| Stapleton 2008 | Non‐randomised trial |
| Stoyanov 1972 | Observational study with no comparator group and short but unstated length of follow‐up |
| Swan 2010 | All participants were given varenicline (treated as an included study for 2012 update) |
| Tonstad 2006 | Relapse prevention study that recruited already abstinent participants. Previously included but excluded for 2023 update |
| Tønnesen 2013 | Relapse prevention study that recruited already abstinent participants. Previously included but excluded for 2023 update. |
| Weiner 2011 | Follow‐up under 6 months |
| Zatonski 2006 | Uncontrolled observational study |
CAR: Continuous Abstinence Rate; EoT: end of treatment; NRT: nicotine replacement therapy; RCT: randomised controlled trial; TQD: target quit date
Characteristics of studies awaiting classification [ordered by study ID]
Wiratmoko 2013.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Abstract only; further details awaited |
Yujie 2014.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Abstract only; further details awaited |
Characteristics of ongoing studies [ordered by study ID]
Berlin 2019.
| Study name | Randomised trial of electronic cigarettes with or without nicotine in smoking cessation (ECSMOKE) |
| Methods | Randomised, placebo‐controlled, double‐blind, double‐dummy, multicentre, parallel‐group trial |
| Participants | At least 650 adults smoking at least 10 CPD in the past year, motivated to quit, aged 18‐70 |
| Interventions |
|
| Outcomes | Continuous smoking abstinence rate during the last 4 weeks of the treatment period. Validated by self‐report and expired CO < 8 ppm at 12 and 24 weeks after treatment initiation |
| Starting date | 17 October 2018 |
| Contact information | Ivan Berlin, +33142161678, ivan.berlin@aphp.fr |
| Notes | Study funding: Programme Hospitalier de Recherche Clinique (PHRC) National 2015, Ministry of Health, France |
Caponnetto 2019.
| Study name | Efficacy of smoking cessation with varenicline plus counselling for e‐cigarettes users (VAREVAPE) |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel 2‐group trial |
| Participants | 140 participants that exclusively smoke e‐cigarettes daily for > 3 months 140 'dual users' that use e‐cigarettes daily for > 3 months, who also smoke at least one combustible CPD All motivated to quit |
| Interventions |
|
| Outcomes | Continuous smoking abstinence rate between weeks 9‐24. Validated by self‐report and expired CO < 10 ppm, and saliva cotinine levels < 7 ng/mL |
| Starting date | June 2019 |
| Contact information | Pasquale Caponnetto: p.caponnetto@unict.it |
| Notes | Study funding: Pfizer, GRAND, Global Research Award for Nicotine Dependence |
ChiCTR1900021400.
| Study name | Individual tobacco cessation research based on nicotine metabolite ratio in smoking patients with chronic obstructive pulmonary disease: a randomized controlled trial |
| Methods | Parallel‐group RCT |
| Participants | 224 participants aged 18‐85 years who meet the diagnostic criteria for both COPD and tobacco dependency, who have been smoking for > 5 years, with at least 10 CPD for the past 12 months. Exhaled CO > 10 ppm |
| Interventions |
|
| Outcomes | Smoking abstinence rate. Nicotine metabolite ratio |
| Starting date | 18 February 2019 |
| Contact information | Xiao Dan, +86138111374263, danxiao@263.net |
| Notes | Study funding: The China‐Japan Friendship Hospital |
IRCT20200719048133N1.
| Study name | Efficacy of varenicline for smoking cessation among persistent smokers after coronary artery revascularization: a randomized placebo controlled clinical trial |
| Methods | Double‐blind, randomised, placebo‐controlled trial |
| Participants | 600 participants that are male, > 18 years, and a current daily smoker of ≥ 5 CPD. Must also have a history of coronary artery revascularisation 9‐12 months prior to the study |
| Interventions |
|
| Outcomes | Continuous smoking abstinence rate at the end of 26 weeks' follow‐up, by self‐report and validated by a close relative |
| Starting date | 22 August 2020 |
| Contact information | Masoumeh Lotfi Tokaldany, +982188029256, lotfi213366@yahoo.co.uk |
| Notes | Study funding: Tehran University of Medical Sciences |
Lawson 2021.
| Study name | Extended pre‐quit varenicline to assist in quitting smoking (EVarQuit) |
| Methods | Parallel group, placebo‐controlled RCT |
| Participants | 320 participants, smoking at least 10 CPD for the past 6 months, expired CO > 7 ppm, motivated to quit |
| Interventions |
|
| Outcomes | Continous smoking abstinence at 12 and 26 weeks post‐quit date, self‐report but bioverified |
| Starting date | 01 October 2017 |
| Contact information | Larry Hawk, lhawk@buffalo.edu |
| Notes | Study funding: state University of New York at Buffalo, National Cancer Institute, Pfizer |
NCT00906386.
| Study name | Methadone maintenance treatment and smoking cessation (MMTASC) |
| Methods | Randomised, double‐blind, placebo‐controlled trial |
| Participants | 112 people who smoke and are on methadone maintenance for opioid dependence |
| Interventions | Varenicline 1.0 mg x 2/day vs placebo for 12 weeks |
| Outcomes | 7‐day PPA at 26 weeks |
| Starting date | May 2009 |
| Contact information | Milan Khara |
| Notes | Current status unknown |
NCT01243203.
| Study name | Smoking cessation program in the preadmission clinic: the use of a teachable moment |
| Methods | Double‐blind, parallel‐group RCT |
| Participants | 300 adults scheduled for elective surgery, aged 18+, smoking 10+ CPD |
| Interventions | Varenicline vs placebo |
| Outcomes | Abstinence at 24 and 52 weeks |
| Starting date | November 2007 |
| Contact information | Francis Chung |
| Notes | Study funding: Pfizer |
NCT01312909.
| Study name | Smoking cessation study in healthy adolescent smokers |
| Methods | Phase IV randomised, triple‐blind, placebo‐controlled trial |
| Participants | 300 healthy adolescents (12‐19 years) smoking at least 5 CPD, with at least 1 failed quit attempt |
| Interventions | Varenicline 1 mg x 2/day vs varenicline 0.5 mg x 2/day vs placebo |
| Outcomes | CA at weeks 9‐12, 9‐24, 9‐52; 7‐day PPA at weeks 12, 24, 52; reduction in CPD |
| Starting date | April 2011 |
| Contact information | Pfizer Inc |
| Notes | Study funding: Pfizer |
NCT01800019.
| Study name | The Canadian HIV Quit Smoking Trial: tackling the co‐morbidities of depression and cardiovascular disease in HIV+ smokers (CANQUIT) |
| Methods | Open‐label, 4‐arm, factorial RCT |
| Participants | 256 adults who are HIV+ and smoke, 5+ CPD, willing to set a quit date |
| Interventions |
|
| Outcomes | 7‐day PPA and 4‐week CA at week 48, CO‐verified |
| Starting date | January 2014 |
| Contact information | Louise Balfour, PhD, Ottawa Hospital Research Institute |
| Notes | Study funding: CIHR Research Operating Grant |
NCT02106637.
| Study name | Early in‐hospital initiation of pharmacotherapy for smoking cessation, patients after ACS |
| Methods | Double‐blind, parallel‐group RCT |
| Participants | 300 adult smokers with ACS |
| Interventions | Varenicline vs placebo |
| Outcomes | CA at 1 month, 6 months, 1 year after hospitalisation; SAE rate |
| Starting date | June 2014 |
| Contact information | Ilan Goldenberg, MD, Ilan.Goldenberg@sheba.health.gov.il |
| Notes |
NCT02162849.
| Study name | Reward sensitivity and pharmacotherapy for smoking cessation |
| Methods | Double‐blind, parallel‐group RCT |
| Participants | 90 adults who smoke, 5+ CPD |
| Interventions | Varenicline + placebo patch vs nicotine patch + placebo tablet; all get behavioural counselling |
| Outcomes | CA at EoT, 3 months and 6 months |
| Starting date | April 2015 |
| Contact information | Paul Cinciripini |
| Notes |
NCT02378714.
| Study name | Behavioral activation and varenicline for smoking cessation in depressed smokers |
| Methods | Parallel‐group, placebo‐controlled RCT |
| Participants | Adults who smoke with MDD |
| Interventions |
|
| Outcomes | Bioverified PPA at 27 weeks; AE and SAE rates |
| Starting date | 24 July 2015 |
| Contact information | Brian Hitsman, Ph.D. Northwestern University |
| Notes | Study funding: 1R01CA184211‐01A1 (U.S. NIH Grant/Contract) |
NCT02460900.
| Study name | Optimizing smoking cessation for people with HIV/AIDS who smoke |
| Methods | Randomised factorial trial |
| Participants | Adults who smoke and have HIV/AIDS |
| Interventions |
|
| Outcomes | 7‐day PPA at 24 weeks |
| Starting date | July 2016 |
| Contact information | Principal Investigators: Seth Himelhoch, MD, MPH and Deanna Kelly, PharmD, University of Maryland School of Medicine |
| Notes |
NCT02856581.
| Study name | Reducing surgical complications in patients with newly diagnosed lung cancer who smoke cigarettes |
| Methods | Parallel group, placebo‐group, phase III, RCT |
| Participants | 23 participants, aged > 18, with a new diagnosis of lung cancer with surgery scheduled 10 days‐12 weeks after randomisation. Have smoked daily/nearly every day for previous 6 months, and at least 1 puff in the previous 7 days. Motivated to quit |
| Interventions |
|
| Outcomes |
|
| Starting date | 29 September 2017 |
| Contact information | Ivana Croghan, ivana.croghan@mayo.adu |
| Notes | Study funding: Alliance for Clinical Trials in Oncology, National Cancer institute, Pfizer |
NCT02991781.
| Study name | Combined bio‐ and neuro‐ feedback vs. varenicline use for smoking cessation |
| Methods | RCT |
| Participants | Unemployed adults < 35 who smoke (subgroup of a larger trial population) |
| Interventions |
|
| Outcomes | Abstinence at 2 years |
| Starting date | January 2017 |
| Contact information | Principal Investigator: Panos Bamidis, Ass. Prof, Medical School, Aristotle University of Thessaloniki |
| Notes |
NCT03365362.
| Study name | Achieving smoking cessation milestones in opioid treatment patients: a randomised 2x2 factorial trial of directly observed and long‐term varenicline |
| Methods | 2 x 2 factorial, placebo‐controlled, RCT |
| Participants | 450 participants, aged > 18, currently smoking at least 5 CPD and > 100 in their lifetime, motivated to quit, receiving methadone or buprenorphine in an opioid treatment programme > 3 months |
| Interventions |
|
| Outcomes |
|
| Starting date | 25 October 2018 |
| Contact information | Shadi Nahvi, snahvi@montefiore.org |
| Notes | Study funding: Albert Einstein College of Medicine, NIH, Pfizer, National Institute on Drug Abuse |
NCT03557294.
| Study name | Varenicline OTC trial on efficacy and safety (VOTC) |
| Methods | Parallel‐group, placebo‐controlled, RCT |
| Participants | 307 participants, aged ≥ 21, daily smoking with exhaled CO > 10 ppm or positive urine cotinine, motivated to quit |
| Interventions |
|
| Outcomes | Smoking abstinence, validated by self‐report and exhaled CO |
| Starting date | 07 May 2018 |
| Contact information | Not reported |
| Notes | Study funding: Arizona State University, National Institute on Drug abuse, Los Angeles Clinical Trials, University of Nevada, Reno, Pfizer |
NCT04011280.
| Study name | Novel pharmacotherapy approaches in smokers with serious mental illness |
| Methods | Phase IV, double‐blind, randomised, parallel‐group RCT |
| Participants | 60 participants, 18‐70 years, with a diagnosis of bipolar disorder or schizophrenia spectrum disorder, smoke > 10 CPD, exhaled CO > 10 ppm, motivated to quit |
| Interventions |
|
| Outcomes | Primary: feasibility of combining ACT (Acceptance and Commitment Therapy) with different varenicline regimens Secondary: nicotine‐metabolite ratio |
| Starting date | 15 July 2019 |
| Contact information | Benjamin McKenna, bmckenna@ucsd.edu |
| Notes | Study funding: University of California, San Diego, Veterans Medical Research Foundation, University of California |
NCT04015414.
| Study name | Varenicline versus cytisine for smoking cessation in the primary care setting in Croatia and Slovenia ‐ a randomised controlled trial |
| Methods | Parallel‐group, RCT |
| Participants | 380 participants, > 18 years, currently smoking, motivated to quit |
| Interventions |
|
| Outcomes | Self‐reported 7‐day abstinence from tobacco (primary outcome: 12‐weeks following TQD) |
| Starting date | 14 July 2020 |
| Contact information | Stjepan Oreskovin, +385912858247, sooreskov@gmail.com Jeffrey Ashburner, 617/724‐3828, jasburner@mgh.harvard.edu |
| Notes | Study funding: University of Zagreb, University of Ljubliana School of Medicine, University of Zagreb School of Medicine, Harvard Medical School |
NCT04188873.
| Study name | Optimized chronic care for smokers: developing and implementing integrated clinical systems interventions in primary care ‐ cessation trial |
| Methods | 2x2x2x2 factorial design, RCT |
| Participants | 608 participants, > 18 years, smoking > 4 CPD for the previous 6 months |
| Interventions | Factorial trial with the following arms:
|
| Outcomes | Complete 7‐day abstinence ‐ 12‐months post‐TQD, validated by exhaled CO < 5 ppm |
| Starting date | 10 December 2020 |
| Contact information | Megan Piper, 608‐265‐4572, mep@ctri.wisc.edu Stevens Smith, 608‐262‐7563, sss@ctri.wisc.edu |
| Notes | Study funding: University of Wisconsin, Madison, National Cancer Institute |
NCT04525755.
| Study name | A translational randomized clinical trial of varenicline sampling to promote smoking cessation and scalable treatment dissemination |
| Methods | Parallel‐group, open‐label RCT |
| Participants | 648 participants, > 18 years, daily smoker (25+ days/previous month, and > 5 CPD), smoking for > 1, motivated to quit |
| Interventions |
|
| Outcomes | Primary: 7‐day self‐reported abstinence Secondary: reduction in smoking by 50%, any quit attempts, use of smoking cessation medication |
| Starting date | 08 February 2021 |
| Contact information | Lisa Coles, 843‐876‐2291, colesl@musc.edu Amy Boatright, 843‐876‐2440, boatright@musc.edu |
| Notes | Study funding: Medical University of South Carolina |
NCT04604509.
| Study name | PISCES I: Precision implemented smoking cessation evaluation study |
| Methods | Parallel‐group, open‐label RCT |
| Participants | 2010 participants, > 18 years, smoking > 5 CPD, motivated to quit |
| Interventions |
All groups have behavioural counselling |
| Outcomes | Primary: EoT 7‐day PPA, expired CO, abstinence at 12 weeks |
| Starting date | 04 August 2020 |
| Contact information | Paul Cinciripini, 713‐745‐3822, pcinciri@mdanderson.org |
| Notes | Study funding: MD Anderson Cancer Center, National Cancer Institute |
NCT05102123.
| Study name | PREVENT: PeRiopEratiVE smokiNg cessaTion Trial |
| Methods | 2x2 factorial, RCT |
| Participants | 1720 participants, > 18 years, scheduled to undergo surgery within 28 days, currently smoking (> 100 cigarettes over lifetime and smoke every day in last 30 days) |
| Interventions |
|
| Outcomes | Primary: complete abstinence from quit date to 6 months post‐randomisation, verified by exhaled CO < 10 ppm |
| Starting date | 01 April 2022 |
| Contact information | Emily Di Sante, 905‐297‐3479, emily.disante@phri.ca Jessica Vincent, 905‐297‐3479 ext 40635, jessica.vincent@phri.ca |
| Notes | Study funding: Population Health Research Institute |
NCT05311085.
| Study name | Cytisine and e‐cigarettes with supportive text‐messaging for smoking cessation (Cess@Tion) |
| Methods | RCT |
| Participants | Adults who smoke |
| Interventions |
|
| Outcomes | CA at 6 months |
| Starting date | May 2022 |
| Contact information | Natalie Walker, PhD, n.walker@auckland.ac.nz Chris Bullen, PhD MBChB, c.bullen@auckland.ac.nz |
| Notes |
Reid 2010.
| Study name | Varenicline versus transdermal nicotine patch for smoking cessation in patients with coronary heart disease |
| Methods | Randomised open‐label trial |
| Participants | 60 adult smokers |
| Interventions | Varenicline or NRT patch for 12 weeks |
| Outcomes | CO‐confirmed CA for weeks 12‐26 |
| Starting date | April 2009 |
| Contact information | Robert Reid |
| Notes |
Russo 2021.
| Study name | Efficacy and safety of smoking cessation with varenicline tartrate in diabetic smokers (DIASMOKE) |
| Methods | Randomised, double‐blind, placebo‐controlled trial |
| Participants | 300 adults with type 2 diabetes, who smoke |
| Interventions | Varenicline 1 mg x 2/day for 12 weeks vs placebo |
| Outcomes | CA at week 24 and week 52; AEs |
| Starting date | June 2011 |
| Contact information | Riccardo Polosa |
| Notes | Study funding: supported by grant WS5086648 from GRAND (Global Research Award for Nicotine Dependence), an independently reviewed competitive grants programme funded by Pfizer Inc. |
TCTR20180312001.
| Study name | Efficacy safety and health‐related quality of life (HRQoL) of cytisine in smoking cessation |
| Methods | Double‐blind, RCT |
| Participants | 130 participants, 18‐65 years, current smoker > 10 CPD, motivated to quit |
| Interventions |
|
| Outcomes |
|
| Starting date | 01 May 2018 |
| Contact information | Sunee Lertsinudom, 081‐6617237, lsunee@kku.ac.th |
| Notes | Study funding: Government Pharmaceuticsl Organization, Thailand |
Tindle 2020.
| Study name | STudying Partial‐agonists for Ethanol and Tobacco Elimination in Russians with HIV (St PETER HIV) |
| Methods | Parallel group, placebo‐controlled, RCT |
| Participants | 400 participants with HIV and alcohol dependence, who smoke |
| Interventions |
|
| Outcomes | Smoking cessation at 12 months Percent heavy drinking days in past 30 days |
| Starting date | 19 July 2017 |
| Contact information | hilary.tindle@vumc.org |
| Notes | Study funding: "This work was supported by grants from the National Institute on Alcohol Abuse and Alcoholism (NI AAA) in support of URBAN ARCH :U01AA020780 , U24AA020779, U24AA020778 ; and by the Providence/ Boston Center for AIDS Research ( P30AI042853 ) and Tennessee Center for AIDS Research ( P30AI110527 ). The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health." |
ACS: acute coronary syndrome; AE: adverse event; CA: continuous abstinence; CIHR: Canadian Institutes of Health Research; C‐NRT: combined nicotine replacement therapy; CO: carbon monoxide; COPD: chronic obstructive pulmonary disease; CPD: cigarettes per day; e‐cigarette: electronic cigarette; MDD: major depressive disorder; NRT: nicotine replacement therapy; PPA: point‐prevalence abstinence; ppm: parts per million; QoL: quality of life; RCT: randomised controlled trial; SAE: serious adverse event; TQD: target quit date
Differences between protocol and review
For this update we did some restructuring of the meta‐analyses and summary of findings tables to better present the evidence available, including adding analyses of adverse events for comparisons of varenicline with other pharmacotherapies. We excluded previously included studies as these studies recruited already‐abstinent participants (Evins 2014; Tonstad 2006; Tønnesen 2013; NCT00828113), and because follow‐up was under six months (Hajek 2015). We also excluded Brandon 2011; Ebbert 2011b; Faessel 2009; Fagerström 2010; Garza 2011; Hughes 2011; McClure 2013; Meszaros 2013; Mitchell 2012, which had previously been included for data on harms only but do not meet all inclusion criteria.
Contributions of authors
JL‐B led this review update, updating the text and analyses. JL‐B, AT, AH, and NL screened for new studies and JL‐B, AT, AH, LH, TRF, and KT performed data extraction and study evaluation. All authors contributed to text and findings, and approved the final version of the review.
Sources of support
Internal sources
-
Nuffiled Department of Primary Care Health Sciences, University of Oxford, UK
Editorial base for the Cochrane Tobacco Addiction Group
External sources
-
National Institute for Health and Care Research, UK
Infrastructure and programme grant funding for the Cochrane Tobacco Addiction Group
Declarations of interest
JL‐B is employed by the University of Oxford to work as a Managing Editor for the Cochrane Tobacco Addiction Review Group. He was not involved in the editorial process for this review. Core infrastructure funding for the Cochrane Tobacco Addiction Group is provided by the NIHR to the University of Oxford. JLB has no conflicts of interest.
TF: none known
KT published an opinion piece on varenicline (Davies 2017). KT is on the Specialist Register in the UK as a Consultant in Public Health Medicine, employed by the University of Bristol as an Associate Professor in Public Health Medicine, and currently holds Honorary status as a Consultant in Public Health at South Gloucestershire Council, and is Clinical Director for the NIHR CRN West of England. KT has no conflicts of interest.
AH: none known
AT: none known
LH: none known
NL is employed by the University of Oxford to work as a Managing Editor for the Cochrane Tobacco Addiction Review Group. She was not involved in the editorial process for this review. Core infrastructure funding for the Cochrane Tobacco Addiction Group is provided by the NIHR to the University of Oxford. NL has written pieces for The Conversation on the findings of Cochrane Reviews assessing the effects of treatments for smoking cessation. These are evidence‐based and not based on personal opinion. NL receives funding from CRUK and the NIHR (a part of the NHS) who both have interests in people stopping smoking and run educational campaigns, and in the latter case provide treatment to encourage people to stop smoking. NL has no conflicts of interest.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Anthenelli 2013 {published and unpublished data}
- Anthenelli RM, Morris C, Ramey TS, Dubrava SJ, Tsilkos K, Russ C, et al. Effects of varenicline on smoking cessation in adults with stably treated current or past major depression: a randomized trial. Annals of Internal Medicine 2013;159(6):390-400. [CENTRAL: 921009] [EMBASE: 2013581859] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Doran N, Dubrava S, Anthenelli RM. Effects of varenicline, depressive symptoms, and region of enrollment on smoking cessation in depressed smokers. Nicotine & Tobacco Research 2018;21(2):156-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01078298. Safety and efficacy of 12 weeks of varenicline for smoking cessation in smokers with depression. clinicaltrials.gov/ct2/show/NCT01078298 (first received 2 March 2010). [ClinicalTrials.gov ID NCT01078298]
Ashare 2019 {published data only}
- Ashare RL, Thompson M, Serrano K, Leone F, Metzger D, Frank I, et al. Placebo-controlled randomized clinical trial testing the efficacy and safety of varenicline for smokers with HIV. Drug and Alcohol Dependence 2019;200:26-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashare RL, Wileyto EP, Logue-Chamberlain E, Gross R, Tyndale RF, Lerman C, et al. Patterns of lapses and recoveries during a quit attempt using varenicline and behavioral counseling among smokers with and without HIV. Psychology of Addictive Behaviors 2021;35(7):788-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer AM, Hosie Quinn M, Lubitz SF, Flitter A, Ashare RL, Leone FT, et al. Medication adherence and rate of nicotine metabolism are associated with response to treatment with varenicline among smokers with HIV. Addictive Behaviors 2021;112:106638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubitz SF, Flitter A, Ashare RL, Thompson M, Leone F, Gross R, et al. Improved clinical outcomes among persons with HIV who quit smoking. AIDS Care 2020;32(10):1217-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01710137. A placebo controlled trial of varenicline for smoking among those with HIV/AIDS. ClinicalTrials.gov/show/NCT01710137 (first received 6 October 2019).
- Quinn MH, Bauer AM, Flitter A, Lubitz SF, Ashare RL, Thompson M, et al. Correlates of varenicline adherence among smokers with HIV and its association with smoking cessation. Addictive Behaviors 2020;102:106151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson M, Schnoll R, Serrano K, Leone F, Gross R, Collman R G, et al. The effect of varenicline on mood and cognition in smokers with HIV. Psychopharmacology 2020;237(4):1223-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aubin 2008 {published data only}
- Aubin H-J, Bobak A, Britton JR, Oncken C, Billing CB, Gong J, et al. Authors' reply [to JE Rose]. Thorax 2008;63(8):752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubin H-J, Bobak A, Britton JR, Oncken C, Billing CB, Gong J, et al. Authors' reply [to T Hillman]. Thorax 2008;63(8):752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubin H-J, Bobak A, Britton JR, Oncken C, Billing CB, Gong J, et al. Varenicline versus transdermal nicotine patch for smoking cessation: results from a randomised, open-label trial. Thorax 2008;1:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aveyard P. The place of varenicline in smoking cessation treatment. Thorax 2008;63(8):666-8. [DOI] [PubMed] [Google Scholar]
- Hillman T, Rajakulasingam K, Bhowmik A. Clinically significant outcomes in smoking cessation. Thorax 2008;63(8):752. [PubMed] [Google Scholar]
- Rose JE. Pre-cessation varenicline treatment vs post-cessation NRT: an uneven playing field. Thorax 2008;63(8):751. [PubMed] [Google Scholar]
Baker 2016 {published data only}
- Baker TB, Piper ME, Stein JH, Smith SS, Bolt DM, Fraser DL, et al. Effects of nicotine patch vs varenicline vs combination nicotine replacement therapy on smoking cessation at 26 weeks. JAMA 2016;315(4):371-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborti Y, Coffman DL, Piper ME. Time-varying mediation of pharmacological smoking cessation treatments on smoking lapse via craving, cessation fatigue, and negative mood. Nicotine & Tobacco Research 2022;24(10):1548-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye JT, Johnson AL, Baker TB, Piper ME, Cook JW. Searching for personalized medicine for binge drinking smokers: smoking cessation using varenicline, nicotine patch, or combination nicotine replacement therapy. Journal of Studies on Alcohol and Drugs 2020;81(4):426-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye JT, Piper ME, Baker TB, Cook JW. Searching for personalized medicine for heavy drinking smokers: smoking cessation using varenicline, nicotine patch, or combination nicotine replacement therapy. Alcoholism: Clinical and Experimental Research Conference: 42nd Annual Scientific Meeting of the Research Society on Alcoholism 2019;43 Suppl 1:247A. [Google Scholar]
- Kim N, McCarthy DE, Piper ME, Baker TB. Comparative effects of varenicline or combination nicotine replacement therapy versus patch monotherapy on candidate mediators of early abstinence in a smoking cessation attempt. Addiction 2020;116(4):926-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy DE, Versella MV. Quitting failure and success with and without using medication: latent classes of abstinence and adherence to nicotine monotherapy, combination therapy, and varenicline. Nicotine & Tobacco Research 2019;21(11):1488-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlam TR, Baker TB, Smith SS, Cook JW, Piper ME. Anxiety sensitivity and distress tolerance in smokers: relations with tobacco dependence, withdrawal, and quitting success. Nicotine & Tobacco Research 2020;22(1):58-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baker 2021 {published data only}
- Baker TB, Piper ME, Smith SS, Bolt DM, Stein JH, Fiore MC. Effects of combined varenicline with nicotine patch and of extended treatment duration on smoking cessation: a randomized clinical trial. JAMA 2021;326(15):1485-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03176784. UW Quitting Using Intensive Treatment Study (QUITS). clinicaltrials.gov/show/NCT03176784 (first received 6 June 2017).
Benli 2017 {published data only}
- Benli AR, Erturhan S, Oruc MA, Kalpakci P, Sunay D, Demirel Y. A comparison of the efficacy of varenicline and bupropion and an evaluation of the effect of the medications in the context of the smoking cessation programme. Tobacco Induced Diseases 2017;15(10):1-8. [DOI: 10.1186/s12971-017-0116-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bohadana 2020 {published data only}
- Bohadana A, Freier-Dror Y, Peles V, Babai P, Izbicki G. Extending varenicline preloading to 6 weeks facilitates smoking cessation: a single-site, randomised controlled trial: six-week varenicline preloading for smoking cessation. eClinicalMedicine 2020;19(100228):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izbicki G, Freier Dror Y, Bohadana A. Extended, 6 weeks, varenicline preloading: does it facilitate smoking reduction and cessation? a randomized double-blind, placebo-controlled study. American Journal of Respiratory and Critical Care Medicine Conference: 2019 International Conference of the American Thoracic Society 2019;199(9):A2378. [Google Scholar]
- NCT02634281. Extended (6-Week) varenicline preloading: does it facilitate smoking reduction and cessation? https://clinicaltrials.gov/show/NCT02634281 (first received 18 December 2015).
Bolliger 2011 {published data only}
- Bolliger CT, Issa JS, Posadas-Valay R, Safwat T, Abreu P, Correia EA, et al. Effects of varenicline in adult smokers: a multinational, 24-week, randomized, double-blind, placebo-controlled study. Clinical Therapeutics 2011;33(4):465-77. [CENTRAL: 799588] [PMID: ] [DOI] [PubMed] [Google Scholar]
Carson‐Chahhoud 2020 {published and unpublished data}
- Brinn M, Daziel K, Carson K, Labiszewski N, Esterman A, Smith B. Cost effectiveness of an inpatient smoking cessation intervention for patients with tobacco related illnesses (Stop Trial): a multi-centre RCT [Abstract]. Respirology 2013;18 Suppl 2:16 [O027]. [CENTRAL: 849080] [EMBASE: 71010964] [Google Scholar]
- Carson K, Brinn M, Peters M, Fitridge R, Koblar S, Jannes J, et al. Superiority of a course of varenicline tartrate plus counselling over counselling alone for smoking cessation: a 24-month randomised controlled trial for inpatients. Respirology 2016;21 Suppl 2:45. [DOI] [PubMed] [Google Scholar]
- Carson K, Smith BJ, Peters MJ, Veale AJ, Esterman AJ. Superiority of a course of varenicline tartrate plus counselling over counselling alone for smoking cessation: A 24-month randomized controlled trial for inpatients. Respirology 2015;20 Suppl 3:115. [Google Scholar]
- Carson K, Smith BJ, Peters MJ, Veale AJ, Esterman AJ. Superiority of a course of varenicline tartrate plus counselling over counselling alone for smoking cessation: a 24-month randomized controlled trial for inpatients. Respirology 2015;20:115. [DOI] [PubMed] [Google Scholar]
- Carson KV, Brinn MP, Smith BJ, Garside CG, Goldsworthy SJ, Fitridge RA. Varenicline tartrate and counselling versus counselling alone in a randomised controlled trial for inpatient smoking cessation: 3 month interim results [Abstract]. Respirology 2010;15 Suppl 1:A30. [Google Scholar]
- Carson KV, Smith BJ, Brinn MP, Peters MJ, Fitridge R, Koblar SA, et al. Safety of varenicline tartrate and counseling versus counseling alone for smoking cessation: a randomized controlled trial for inpatients (STOP study). Nicotine & Tobacco Research 2014;16(11):1495-502. [CENTRAL: 1036725] [EMBASE: 2014920189] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Carson-Chahhoud KV, Smith BJ, Peters MJ, Brinn MP, Ameer F, Singh K, et al. Two-year efficacy of varenicline tartrate and counselling for inpatient smoking cessation (STOP study): a randomized controlled clinical trial. PloS One 2020;15(4):e0231095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnin K, Carson K, Brinn M, Jannes J, Ameer F, Esterman A, et al. Triggers resulting in relapse: cohort analysis from the smoking termination opportunity for inpatients (STOP) trial [Abstract]. Respirology 2014;19 Suppl 2:40 [TO 076]. [CENTRAL: 996495] [EMBASE: 71605471] [Google Scholar]
- Smith B, Carson K, Dalziel K, Brinn M, Moayeri F, Clarke P, et al. Cost effectiveness of inpatient initiated varenicline tartrate (VT) plus counselling compared to counselling alone: 2 year follow-up of the Smoking Termination Opportunity for inPatients (STOP) study. European respiratory journal. Conference: European Respiratory Society Annual Congress 2016. United Kingdom 2016;48:PA4600. [Google Scholar]
- Smith BJ, Carson KV, Brinn MP, Labiszewski NA, Peters MJ, Fitridge R, et al. Smoking termination opportunity for in patients (STOP): superiority of a course of varenicline tartrate plus counselling over counselling alone for smoking cessation: a 12-month randomised controlled trial for inpatients. Thorax 2013;68(5):485-6. [CENTRAL: 849081] [EMBASE: 2013242202] [NCT01141855/ClinicalTrials.gov] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Smith BJ, Peters MJ, Fitridge RA, Esterman AJ, Litt JC, Horowitz JD, et al. Varenicline tartrate and counselling versus counselling alone in a randomised controlled trial for inpatient smoking cessation: 6 month interim results. Conference: American Thoracic Society 2011 International Conference, May 13-18 2011.
Chen 2020 {published data only}
- Chen LS, Baker TB, Miller JP, Bray M, Smock N, Chen J, et al. Genetic variant in CHRNA5 and response to varenicline and combination nicotine replacement in a randomized placebo-controlled trial. Clinical Pharmacology and Therapeutics 2020;108(6):1315-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02351167. Genetically informed smoking cessation trial. ClinicalTrials.gov/show/NCT02351167 (first received 30 January 2015).
Chengappa 2014 {published and unpublished data}
- Chengappa KN, Perkins KA, Brar JS, Schlicht PJ, Turkin SR, Hetrick ML, et al. Varenicline for smoking cessation in bipolar disorder: a randomized, double-blind, placebo-controlled study. Journal of Clinical Psychiatry 2014;75(7):765-72. [CENTRAL: 1014641] [DOI] [PubMed] [Google Scholar]
- Chengappa KN. A randomized, double-blind, placebo-controlled clinical trial of varenicline in persons with bipolar disorder motivated to quit smoking. Bipolar Disorders 2015;17:38. [Google Scholar]
- Forrest PE, Brinson AJ, Gannon JM, George TP, Perkins KA, Chengappa KN. An association between the use of hypnotics and quit status in the treatment of nicotine dependence with varenicline in bipolar disorder. Journal of Clinical Psychopharmacology 2015;35(2):199-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01010204. Varenicline treatment for smoking cessation in patients with bipolar disorder (BEST). clinicaltrials.gov/ct2/show/NCT01010204 (first received 9 November 2009). [ClinicalTrials.gov ID NCT01010204]
Cinciripini 2013 {published and unpublished data}
- Cinciripini PM, Green CE, Robinson JD, Karam-Hage M, Engelmann JM, Minnix JA, et al. Benefits of varenicline vs. bupropion for smoking cessation: a Bayesian analysis of the interaction of reward sensitivity and treatment. Psychopharmacology 2017;234(11):1769-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinciripini PM, Karam-Hage M. Randomised controlled trial: study suggests varenicline safe and effective among adults with stable depression. Evidence-Based Medicine 2014;19(3):92. [EMBASE: 2014382469] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinciripini PM, Robinson JD, Karam-Hage M, Minnix JA, Lam C, Versace F, et al. Effects of varenicline and bupropion sustained-release use plus intensive smoking cessation counseling on prolonged abstinence from smoking and on depression, negative affect, and other symptoms of nicotine withdrawal. JAMA Psychiatry 2013;70(5):522-33. [CENTRAL: 863881] [EMBASE: 2013278203] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Engelmann J M, Xian J, Minnix J A, Lam C Y, Karam-Hage M, et al. Pharmacological intervention and abstinence in smokers undergoing cessation treatment: a psychophysiological study. International Journal of Psychophysiology 2018;123:25-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays JT. Varenicline may reduce negative effect while aiding smoking cessation. Evidence-Based Medicine 2014;19(1):23. [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT00507728. Pharmacogenetics, emotional reactivity and smoking. ClinicalTrials.gov/ct2/NCT00507728 (first received 26 July 207). [ClinicalTrials.gov ID NCT00507728]
Cinciripini 2018 {published data only}
- Cinciripini PM, Minnix JA, Green CE, Robinson JD, Engelmann JM, Versace F, et al. An RCT with the combination of varenicline and bupropion for smoking cessation: clinical implications for front line use. Addiction 2018;113(9):1673-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00943618. Combining varenicline and bupropion for smoking cessation. ClinicalTrials.gov/ct2/NCT00943618 (first received 22 July 2009).
Courtney 2021 {published data only}
- ACTRN12616001654448. A non-inferiority trial of cytisine versus varenicline for smoking cessation. http://www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12616001654448 (first received 30 November 2016).
- Courtney RJ, McRobbie H, Tutka P, Weaver NA, Petrie D, Mendelsohn CP, et al. Effect of cytisine vs varenicline on smoking cessation: a randomized clinical trial. JAMA 2021;326(1):56-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D, Farrell M, McRobbie H, Tutka P, Petrie D, West R, et al. The effectiveness, safety and cost-effectiveness of cytisine versus varenicline for smoking cessation in an Australian population: a study protocol for a randomized controlled non-inferiority trial. Addiction 2019;114(5):923-33. [DOI] [PubMed] [Google Scholar]
Cox 2022 {published data only}
- Cox LS, Nollen NL, Mayo MS, Faseru B, Greiner A, Ellerbeck EF, et al. Effect of varenicline added to counseling on smoking cessation among African American daily smokers: the Kick It at Swope IV randomized clinical trial. JAMA 2022;327(22):2201-9. [DOI: 10.1001/jama.2022.8274.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebmann EP. Dissertation Abstracts International: Section B: The Sciences and Engineering. Vol. 82. 2021. [Google Scholar]
- NCT02360631. Advancing tobacco use treatment for African American smokers. ClinicalTrials.gov/show/NCT02360631 (first received 10 February 2015).
De Dios 2012 {published data only}
- De Dios MA, Anderson BJ, Stanton C, Audet DA, Stein M. Project Impact: a pharmacotherapy pilot trial investigating the abstinence and treatment adherence of Latino light smokers. Journal of Substance Abuse Treatment 2012;43(3):322-30. [CENTRAL: 836798] [EMBASE: 2012562711] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dogar 2020 {published data only}
- Boeckmann M, Nohavova I, Dogar O, Kralikova E, Pankova A, Zvolska K, et al. Protocol for the mixed-methods process and context evaluation of the TB & Tobacco randomised controlled trial in Bangladesh and Pakistan: a hybrid effectiveness-implementation study. BMJ Open 2018;8(3):e019878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogar O, Barua D, Boeckmann M, Elsey H, Fatima R, Gabe R, et al. The safety, effectiveness and cost-effectiveness of cytisine in achieving six-month continuous smoking abstinence in tuberculosis patients—protocol for a double-blind, placebo-controlled randomized trial. Addiction 2018;113(9):1716-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogar O, Keding A, Gabe R, Marshall AM, Huque R, Barua D, et al. Cytisine for smoking cessation in patients with tuberculosis: a multicentre, randomised, double-blind, placebo-controlled phase 3 trial. Lancet Global Health 2020;8(11):e1408-17. [DOI] [PubMed] [Google Scholar]
- ISRCTN43811467. Tuberculosis (TB) and tobacco. www.isrctn.com/ISRCTN43811467 (first received 23 March 2016).
- Siddiqi K, Keding A, Marshall AM, Dogar O, Li J, Huque R, et al. Effect of quitting smoking on health outcomes during treatment for tuberculosis: secondary analysis of the TB & Tobacco Trial. Thorax 2021;7:74-8. [DOI] [PubMed] [Google Scholar]
EAGLES 2016 {published and unpublished data}
- Anthenelli R, Benowitz N, West R, St Aubin L, McRae T, Lawrence D, et al. Reports of suicidal ideation and behavior in the EAGLES trial. Proceedings of the Society for Research on Nicotine and Tobacco, USA 3rd - 5th March 2016;SYM5D:8. [Google Scholar]
- Anthenelli RM, Benowitz NL, West R, St Aubin L, McRae T, Lawrence D, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet 2016;387(10037):2507-20. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Anthenelli RM, Gaffney M, Benowitz NL, West R, McRae T, Russ C, et al. Predictors of neuropsychiatric adverse events with smoking cessation medications in the randomized controlled EAGLES trial. Journal of General Internal Medicine 2019;34(6):862-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayers CR, Heffner JL, Russ C, Lawrence D, McRae T, Evins AE, et al. Efficacy and safety of pharmacotherapies for smoking cessation in anxiety disorders: subgroup analysis of the randomized, active- and placebo-controlled EAGLES trial. Depression and Anxiety 2020;37(3):247-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker CL, Pietri G. A cost-effectiveness analysis of varenicline for smoking cessation using data from the EAGLES trial. ClinicoEconomics and outcomes research 2018;10:67-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard E, Jackson SE, Anthenelli RM, Benowitz NL, Aubin LS, McRae T, et al. Estimation of risk of neuropsychiatric adverse events from varenicline, bupropion and nicotine patch versus placebo: secondary analysis of results from the EAGLES trial using Bayes factors. Addiction (Abingdon, England) 2021;116(10):2816-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz N, Evins AE, West R, St Aubin L, McRae T, Lawrence D, et al. EAGLES trial: study design and neuropsychiatric safety results. In: Society for Research on Nicotine and Tobacco, USA 3rd - 5th March. Vol. SYM5B. 2016:7.
- Benowitz NL, Pipe A, West R, Hays JT, Tonstad S, McRae T, et al. Cardiovascular safety of varenicline, bupropion, and nicotine patch in smokers: a randomized clinical trial. JAMA Internal Medicine 2018;178(5):622-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa JB, Lawrence D, McKenna BS, Gaznick N, Saccone PA, Dubrava S, et al. Psychiatric co-morbidity and multi-morbidity in the EAGLES trial: descriptive correlates and associations with neuropsychiatric adverse events, treatment adherence, and smoking cessation. Nicotine & Tobacco Research 2021;23(10):1636-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbert J, Jimenez-Ruiz C, Dutro M P, Fisher M, Li J, Hays J T. Frequently reported adverse events with smoking cessation medications: post hoc analysis of a randomized trial. Mayo Clinic Proceedings 2021;96(7):1801-11. [DOI] [PubMed] [Google Scholar]
- Evins AE, Benowitz NL, West R, Russ C, McRae T, Lawrence D, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with psychotic, anxiety, and mood disorders in the EAGLES trial. Journal of Clinical Psychopharmacology 2019;39(2):108-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evins AE, West R, Benowitz NL, Russ C, Lawrence D, McRae T, et al. Efficacy and safety of pharmacotherapeutic smoking cessation aids in schizophrenia spectrum disorders: subgroup analysis of EAGLES. Psychiatric Services (Washington, D.C.) 2021;72(1):7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffner JL, Evins AE, Russ C, Lawrence D, Ayers CR, McRae T, et al. Safety and efficacy of first-line smoking cessation pharmacotherapies in bipolar disorders: subgroup analysis of a randomized clinical trial. Journal of Affective Disorders 2019;256:267-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01456936. A phase 4, randomized, double blind, active and placebo controlled multicenter study evaluating the neuropsychiatric safety and efficacy of 12 weeks varenicline tartrate 1mg BID and bupropion hydrochloride 150 mg BID for smoking cessation in subjects with and without a history of psychiatric disorders [EAGLES]. clinicaltrials.gov/ct2/show/NCT01456936 (first received 21 October 2011).
- Nollen NL, Ahluwalia JS, Sanderson Cox L, Okuyemi K, Lawrence D, Samuels L, et al. Assessment of racial differences in pharmacotherapy efficacy for smoking cessation: secondary analysis of the EAGLES randomized clinical trial. JAMA Network Open 2021;4(1):e2032053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska J, Benowitz N, West R, Anthenelli R. Evaluating adverse events in a global smoking cessation study (EAGLES): a randomized controlled trial comparing the safety and efficacy of the first-line smoking cessation aids in smokers with and without psychiatric disorders. Proceedings of the Society for Research on Nicotine and Tobacco, USA 3rd - 5th March 2016;SYM5:7. [Google Scholar]
- Prochaska J. Neuropsychiatric risk concerns in the context of smoking, quitting, and cessation pharmacotherapy use. Proceedings of the Society for Research on Nicotine and Tobacco, USA 3rd - 5th March 2016;SYM5A:7. [Google Scholar]
- West R, Benowitz N, Evins AE, St Aubin L, McRae T, Lawrence D, et al. Relative efficacy of varenicline, bupropion SR, and nicotine transdermal patch in aiding smoking cessation in the EAGLES trial. Proceedings of the Society for Research on Nicotine and Tobacco, USA 3rd - 5th March 2016;SYM5C:8. [Google Scholar]
- West R, Evins AE, Benowitz NL, Russ C, McRae T, Lawrence D, et al. Factors associated with the efficacy of smoking cessation treatments and predictors of smoking abstinence in EAGLES. Addiction 2018;113(8):1507-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox C, Oskooilar N, Guevarra K, Linh Tong M, Grosz D, Morrissey J, et al. A double-blind, active-and placebo-controlled evaluation of the neuropsychiatric safety and efficacy of varenicline and bupropion for smoking cessation in subjects with (pre-existing) psychiatric disorders: an objective blinded analysis. Neuropsychopharmacology 2015;40:S260. [Google Scholar]
Ebbert 2015 {published and unpublished data}
- Bastian H. Comment on Ebbert 2015 'Reduce to Quit' trial. www.ncbi.nlm.nih.gov/pubmed/?term=25688780 Feb 18 2015 (accessed 11 June 2015).
- Ebbert JO, Hughes JR, West RJ, Rennard SI, Russ C, McRae TD, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA 2015;313(7):687-94. [CENTRAL: 1050651] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbert JO, Hughes JR, West RJ. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. Journal of Vascular Surgery 2015;313(7):687-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01370356. A study to evaluate the efficacy and safety of varenicline compared to placebo for smoking cessation through reduction. clinicaltrials.gov/ct2/show/NCT01370356 (first received 9 June 2011). [ClinicalTrials.gov ID NCT01370356]
- Nakamura M, Abe M, Ohkura M, Treadow J, Yu CR, Park PW. Efficacy of varenicline for cigarette reduction before quitting in Japanese smokers: a subpopulation analysis of the reduce to quit trial. Clinical Therapeutics 2017;39(4):863-72. [DOI] [PubMed] [Google Scholar]
- Wilcox C, Grosz D, Tong M L, Morrissey J, De Francisco D, Guevarra K, et al. Smoking cessation through reduction: does it enhance or diminish successful quitting? Neuropsychopharmacology 2014;39:S343-4. [Google Scholar]
Ebbert 2016 {published data only}
- Ebbert JO, Croghan IT, Hurt RT, Schroeder DR, Hays JT. Varenicline for smoking cessation in light smokers. Nicotine & Tobacco Research 2016;18(10):2031-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fouz‐Roson 2017 {published data only}
- Fouz-Roson N, Montemayor-Rubio T, Almadana-Pacheco V, Montserrat-Garcia S, Gomez-Bastero AP, Romero-Munoz C, et al. Effect of 0.5 mg versus 1 mg varenicline for smoking cessation: a randomized controlled trial. Addiction 2017;112(9):1610-9. [DOI] [PubMed] [Google Scholar]
- Roson NF, Panadero-Paz C, Almadana-Pacheco V, Benito-Bernaldez C, Rodriguez-Martin PJ, Montemayor-Rubio T. Influence of psychiatric disorders in patients treated with varenicline. European Respiratory Journal 2017;50:PA4478. [Google Scholar]
- Roson NF, Panadero-Paz C, Benito-Bernaldez C, Rodriguez-Martin PJ, Almadana-Pacheco V, Montemayor-Rubio T. Influence of respiratory and cardiovascular diseases on smoking cessation. European Respiratory Journal 2017;50 Suppl 61:PA2987. [Google Scholar]
- Roson NF, Panadero-Paz C, Benito-Bernaldez C, Rodriguez-Martin PJ, Romero-Munoz C, Rubio TM. Short-term low-dose vs standard-dose varenicline therapy for smoking cessation: a randomized controlled trial. European Respiratory Journal 2017;50:PA4480. [Google Scholar]
Gonzales 2006 {published data only}
- Chen LS, Baker TB, Jorenby D, Piper M, Saccone N, Johnson E, et al. Genetic variation (CHRNA5), medication (combination nicotine replacement therapy vs. varenicline), and smoking cessation. Drug and Alcohol Dependence 2015;154:278-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales D, Jorenby DE, Brandon T, Arteaga C, Lee TC. Delayed quitting, lapse recovery and long-term outcomes for quitters taking varenicline, bupropion and placebo. Society for Research on Nicotine and Tobacco Annual Meeting, Portland OR, Feb 27-March 1st 2008.
- Gonzales D, Jorenby DE, Brandon TH, Arteaga C, Lee TC. Emergent adverse psychiatric symptoms by therapy during 12 weeks of treatment with varenicline, bupropion SR, or placebo for smoking cessation. Society for Research on Nicotine and Tobacco Europe: Rome, September 23-26 2008.
- Gonzales D, Jorenby DE, Brandon TH, Arteaga C, Lee TC. Immediate versus delayed quitting and rates of relapse among smokers treated successfully with varenicline, bupropion SR or placebo. Addiction 2010;105(11):2002-13. [DOI: 10.1111/j.1360-0443.2010.03058.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB et al. Varenicline, an α4ß2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation. JAMA 2006;296(1):47-55. [DOI] [PubMed] [Google Scholar]
- Hays JT, Leischow SJ, Lawrence D, Lee TC. Adherence to treatment for tobacco dependence: association with smoking abstinence and predictors of adherence. Nicotine & Tobacco Research 2010;12(6):574-81. [DOI] [PubMed] [Google Scholar]
- Heffner JL, Lee TC, Arteaga C, Anthenelli RM. Predictors of post-treatment relapse to smoking in successful quitters: pooled data from two phase III varenicline trials. Drug and Alcohol Dependence 2010;109:120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KC, Nahoopii R, Said Q, Dirani R, Brixner D. An employer-based cost-benefit analysis of a novel pharmacotherapy agent for smoking cessation. Journal of Occupational and Environmental Medicine 2007;49(4):453-60. [DOI] [PubMed] [Google Scholar]
- Nides M, Glover ED, Reus VI, Christen AG, Make BJ, Billing CB, et al. Varenicline versus bupropion SR or placebo for smoking cessation: a pooled analysis. American Journal of Health Behavior 2008;32(6):664-75. [DOI] [PubMed] [Google Scholar]
- O'Brien CP. A new medication for the worst addiction. Current Psychiatry Reports 2007;9(5):347-8. [PubMed] [Google Scholar]
- Ravva P, Gastonguay MR, French JL, Tensfeldt TG, Faessel HM. Quantitative assessment of exposure-response relationships for the efficacy and tolerability of varenicline for smoking cessation. Clinical Pharmacology and Therapeutics 2010;87(3):336-44. [DOI] [PubMed] [Google Scholar]
- Tonstad S. Practical implementation of varenicline as an aid to smoking cessation in clinical practice. Pneumologia 2009;58(3):167-74. [PubMed] [Google Scholar]
- West R, Baker CL, Cappelleri JC, Bushmakin AG. Effect of varenicline and bupropion on craving, nicotine withdrawal symptoms, and rewarding effects of smoking during a quit attempt. UK National Smoking Cessation Conference Proceedings, London, June 2007 2007.
- Xenakis JG, Kinter ET, Ishak KJ, Ward AJ, Marton JP, Willke RJ, et al. A discrete-event simulation of smoking-cessation strategies based on varenicline pivotal trial data. PharmacoEconomics 2011;29:497-510. [DOI] [PubMed] [Google Scholar]
Gonzales 2014 {published and unpublished data}
- Gonzales D, Hajek P, Pliamm L, Nackaerts K, Tseng L-J, McRae TD, et al. Retreatment with varenicline for smoking cessation in smokers who have previously taken varenicline: a randomized, placebo-controlled trial. Clinical Pharmacology and Therapeutics 2014;96(3):390-6. [CENTRAL: 1014162] [EMBASE: 2014565570] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01244061. A multi-national study to assess how effective and safe the smoking cessation medicine varenicline is in smokers who have already tried varenicline in the past as a prescription medicine from their usual healthcare provider. clinicaltrials.gov/ct2/show/NCT01244061 (first received 19th November 2010). [ClinicalTrials.gov ID NCT01244061]
Gray 2019 {published data only}
- Gray KM, Baker NL, McClure EA, Tomko RL, Squeglia LM, Saladin ME, et al. Efficacy and safety of varenicline for adolescent smoking cessation: a randomized clinical trial. JAMA Pediatrics 2019;173(12):1146-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure EA, Baker NL, Hood CO, Tomko RL, Squeglia LM, Flanagan JC, et al. Cannabis and alcohol co-use in a smoking cessation pharmacotherapy trial for adolescents and emerging adults. Nicotine & Tobacco Research 2019;22(8):1374-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01509547. A randomized controlled trial of varenicline for adolescent smoking cessation. ClinicalTrials.gov/show/NCT01509547 (first received 13th January 2012).
Heydari 2012 {published data only}138901111878N2
- Heydari G, Talischi F, Tafti SF, Masjedi MR. Quitting smoking with varenicline: parallel, randomised efficacy trial in Iran. International Journal of Tuberculosis and Lung Disease 2012;16(2):268-72. [CENTRAL: 814663] [EMBASE: 2012026893] [PMID: ] [DOI] [PubMed] [Google Scholar]
- IRCT138901111878N2. Evaluation of the effect of champ-ix in smoking cessation in smokers in Tehran. www.irct.ir/trial/1473 (first received 12 October 2010).
Hong 2015 {published data only}
- Hong H, Wang W, Chen L. Study on effect of 5A intervention method combined with varenicline for smoking cessation of COPD patients in stable stage. Chinese Nursing Research 2015;29(2C):667-70. [Google Scholar]
Hurt 2018 {unpublished data only}
- Hurt RT, Ebbert JO, Croghan IT, Schroeder DR, Hurt RD, Hays JT. Varenicline for tobacco-dependence treatment in alcohol-dependent smokers: a randomized controlled trial. Drug and Alcohol Dependence 2018;184:12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01347112. Varenicline treatment for active alcoholic smokers. clinicaltrials.gov/ct2/show/NCT01347112 (first received 4 May 2011).
Ikonomidis 2017 {published data only}
- Ikonomidis I, Kourea K, Vlastos D, Marinou M, Vlachos S, Varoudi M, et al. Varenicline vs. nicotine replacement therapy: a prospective study of changes in arterial stiffness, endothelial glycocalyx and oxidative stress in smokers during 1 year follow-up. European Heart Journal 2016;Suppl 1 European Society of Cardiology, ESC Congress 2016. Italy. 37:553-4. [Google Scholar]
- Ikonomidis I, Marinou M, Vlastos D, Kourea K, Andreadou I, Liarakos N, et al. Effects of varenicline and nicotine replacement therapy on arterial elasticity, endothelial glycocalyx and oxidative stress during a 3-month smoking cessation program. Atherosclerosis 2017;262:123-30. [DOI] [PubMed] [Google Scholar]
Ioakeimidis 2018 {published data only}
- Ioakeimidis N, Vlachopoulos C, Georgakopoulos C, Abdelrasoul M, Skliros N, Katsi V, et al. Smoking cessation rates with varenicline and electronic cigarettes in relapsed smokers with a history of acute coronary syndrome. European Heart Journal 2018;Suppl 1: European Society of Cardiology Congress, ESC 2018. Germany. 39:242. [Google Scholar]
Johns 2017a {published data only}
- Johns D. Randomised controlled trial comparing varenicline plus counselling and brief counselling alone on smoking cessation in patients prone to lung cancer using carbon monoxide moniter. Supportive Care in Cancer 2017;2 Suppl 1: 2017 international MASCC/ISOO symposium: supportive care in cancer. USA:S52. [Google Scholar]
Johns 2017b {published data only}
- Johns DA. The efficacy of combination therapy with varenicline and bupropion for smoking cessation. Annals of Oncology 2017;Suppl 2: 7th European Lung Cancer Conference, ELCC 2017. Switzerland. 28:iii6-7. [Google Scholar]
Jorenby 2006 {published data only}
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, et al. Efficacy of varenicline, an α4ß2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation. JAMA 2006;296(1):56-63. [DOI] [PubMed] [Google Scholar]
- O'Brien CP. A second varenicline trial. Current Psychiatry Reports 2007;9(5):348-8. [PubMed] [Google Scholar]
King 2022 {published data only}
- King A, Vena A, Wit H, Grant JE, Cao D. Effect of combination treatment with varenicline and nicotine patch on smoking cessation among smokers who drink heavily: a randomized clinical trial. JAMA Network Open 2022;5(3):e220951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02859142. Varenicline augmentation of patch outcomes in heavy drinkers' smoking cessation. clinicaltrials.gov/show/NCT02859142 (first received 8 August 2016).
Le Mao 2020 {published data only}
- Le Mao R, Tromeur C, Paleiron N, Sanchez O, Gagnadoux F, Jouneau S, et al. Effect of early initiation of varenicline on smoking cessation in COPD patients admitted for exacerbation: the save randomized clinical trial. COPD 2020;17(1):7-14. [DOI] [PubMed] [Google Scholar]
- NCT01694732. Efficacy of varenicline associated with intensive counselling versus placebo of varenicline associated with intensive counselling on smoking cessation at the acute phase of an exacerbation of chronic obstructive pulmonary disease (COPD). A multicenter randomized double-blind trial. ClinicalTrials.gov/show/NCT01694732 (first received 27 September 2012).
- Tromeur C, Le Mao R, Couturand F. Effect of varenicline on smoking cessation in COPD patients recovering from exacerbation: a randomized trial. Fundamental & Clinical Pharmacology 2018;32 Suppl 1:32. [Google Scholar]
Lerman 2015 {published data only}
- Ashare RL, Lerman C, Tyndale RF, Hawk LW, George TP, Cinciripini P, et al. Sleep disturbance during smoking cessation: withdrawal or side effect of treatment? Journal of Smoking Cessation 2017;12(2):63-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashare RL, Wileyto EP, Logue-Chamberlain E, Gross R, Tyndale RF, Lerman C, et al. Patterns of lapses and recoveries during a quit attempt using varenicline and behavioral counseling among smokers with and without HIV. Psychology of Addictive Behaviors 2021;35(7):788-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenoweth MJ, Novalen M, Hawk LWJ, Schnoll RA, George TP, Cinciripini PM, et al. Known and novel sources of variability in the nicotine metabolite ratio in a large sample of treatment-seeking smokers. Cancer Epidemiology, Biomarkers & Prevention 2014;23(9):1773-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenoweth MJ, Schnoll RA, Novalen M, Hawk LW Jr, George TP, Cinciripini PM, et al. The nicotine metabolite ratio is associated with early smoking abstinence even after controlling for factors that influence the nicotine metabolite ratio. Nicotine & Tobacco Research 2016;18(4):491-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman C, Schnoll RA, Hawk LW Jr, Cinciripini P, George TP, Wileyto EP, et al. Use of the nicotine metabolite ratio as a genetically informed biomarker of response to nicotine patch or varenicline for smoking cessation: a randomised, double-blind placebo-controlled trial. Lancet Respiratory Medicine 2015;3(2):131-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01314001. Pharmacogenetics of nicotine addiction treatment. clinicaltrials.gov/ct2/show/NCT01314001 (first received 14 March 2011).
- Peng AR, Schnoll R, Hawk LW Jr, Cinciripini P, George TP, Lerman C, et al. Predicting smoking abstinence with biological and self-report measures of adherence to varenicline: impact on pharmacogenetic trial outcomes. Drug and Alcohol Dependence 2018;190:72-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng AR, Swardfager W, Benowitz NL, Ahluwalia JS, Lerman C, Nollen NL, et al. Impact of early nausea on varenicline adherence and smoking cessation. Addiction 2020;115(1):134-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng AR. Measures of adherence to varenicline: impact on smoking cessation outcomes. Dissertation Abstracts International 2021;82. [Google Scholar]
- Robinson JD, Li L, Chen M, Lerman C, Tyndale RF, Schnoll RA, et al. Evaluating the temporal relationships between withdrawal symptoms and smoking relapse. Psychology of Addictive Behaviors 2019;33(2):105-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyndale RF, Zhu AZ, George TP, Paul C, Hawk L, Schnoll R, et al. Lack of associations of CHRNA5-A3-B4 genetic variants with smoking cessation treatment outcomes in Caucasian smokers despite associations with baseline smoking. Plos One 2015;10(5):e0128109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Littlewood 2017 {published data only}
- Bidwell LC, Karoly HC, Hutchison KE, Bryan AD. ADHD symptoms impact smoking outcomes and withdrawal in response to Varenicline treatment for smoking cessation. Drug and Alcohol Dependence 2017;179:18-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinnamon Bidwell L, Karoly H, Bryan A, Hutchison K. ADHD symptoms moderate the efficacy of verenacline in a randomized controlled trial. Neuropsychopharmacology 2016;41:S278. [Google Scholar]
- Littlewood RA, Claus ED, Wilcox CE, Mickey J, Arenella PB, Bryan AD, et al. Moderators of smoking cessation outcomes in a randomized-controlled trial of varenicline versus placebo. Psychopharmacology 2017;234(23-24):3417-29. [DOI] [PubMed] [Google Scholar]
Mercie 2018 {published data only}
- Mercie P, Arsandaux J, Katlama C, Ferret S, Beuscart A, Spadone C, et al. Efficacy and safety of varenicline for smoking cessation in people living with HIV in France (ANRS 144 Inter-ACTIV): a randomised controlled phase 3 clinical trial. Lancet HIV 2018;5(3):e126-35. [DOI] [PubMed] [Google Scholar]
- Mercie P, Roussillon C, Katlama C, Beuscart A, Ferret S, Wirth N, et al. Varenicline vs placebo for smoking cessation: ANRS 144 Inter-ACTIV randomized trial. Topics in Antiviral Medicine 2015;23:56-7. [Google Scholar]
- NCT00918307. Efficacy and safety of varenicline amongst HIV-infected patients (Inter-ACTIV). ClinicalTrials.gov/show/NCT00918307 (first received 11 June 2009).
Nahvi 2014a {published and unpublished data}
- Griffin JL, Segal KS, Nahvi S. Barriers to telephone quitline use among methadone-maintained smokers. Nicotine & Tobacco Research 2015;17(8):931-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01027754. Smoking cessation treatment for methadone maintenance patients. clinicaltrials.gov/ct2/show/NCT01027754 (first received 9 December 2009). [ClinicalTrials.gov ID NCT01027754]
- Nahvi S, Ning Y, Segal KS, Richter KP, Arnsten JH. Varenicline efficacy and safety among methadone maintained smokers: a randomized placebo-controlled trial. Addiction 2014;109(9):1554-63. [CENTRAL: 997902] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nakamura 2007 {published data only}
- Fagerström K, Nakamura M, Cho H, Tsa S, Wang C, Davies S, et al. Varenicline treatment for smoking cessation in Asian populations: a pooled analysis of placebo-controlled trials conducted in six Asian countries. Current Medical Research & Opinion 2010;26(9):2165-73. [DOI] [PubMed] [Google Scholar]
- Igarashi A, Takuma H, Fukada T, Tsutani K. Cost-utility analysis of varenicline, an oral smoking-cessation drug, in Japan. Pharmacoeconomics 2009;27(3):247-61. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Oshima A, Fujimoto Y, Maruyama N, Ishibashi T, Reeves KR. Efficacy and tolerability of nicotinic varenicline, an alpha4beta2 acetylcholine receptor partial agonist, in a 12-week, randomized, placebo-controlled, dose-response study with 40-week follow-up for smoking cessation in Japanese smokers. Clinical Therapeutics 2007;29(6):1040-56. [CENTRAL: 611262] [EMBASE: 2007380498] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Nakamura M, Oshima A, Ohkura M, Arteaga C, Suwa K. Predictors of lapse and relapse to smoking in successful quitters in a varenicline post hoc analysis in Japanese smokers. Clinical Therapeutics 2014;36(6):918-27. [CENTRAL: 995377] [EMBASE: 2014542011] [DOI] [PubMed] [Google Scholar]
NCT01162239 {published data only}
- NCT01162239. Maintaining nonsmoking. ClinicalTrials.gov/show/NCT01162239 (first received 14 July 2010).
Niaura 2008 {published data only}
- Niaura R, Taylor Hays J, Jorenby DE, Leone FT, Pappas JE, Reeves KR, et al. The efficacy and safety of varenicline for smoking cessation using a flexible dosing strategy in adult smokers: a randomized controlled trial. Current Medical Research and Opinion 2008;24(7):1931-41. [clinicaltrials.gov ID: NCT00150228] [DOI] [PubMed] [Google Scholar]
Nides 2006 {published data only}
- Nides M, Glover ED, Reus VI, Christen AG, Make BJ, Billing CB, et al. Varenicline versus bupropion SR or placebo for smoking cessation: a pooled analysis. American Journal of Health Behavior 2008;32(6):664-75. [DOI] [PubMed] [Google Scholar]
- Nides M, Oncken C, Gonzalez D, Rennard S, Watsky EJ, Anziano R, et al. Smoking cessation with varenicline, a selective α4β2 nicotinic receptor partial agonist. Archives of Internal Medicine 2006;166:1561-8. [DOI] [PubMed] [Google Scholar]
- Oncken C, Watsky E, Reeves K, Anziano R. Varenicline is efficacious and well tolerated in promoting smoking cessation: results from a 7-week, randomized, placebo- and bupropion-controlled trial [POS1-047]. Society for Research on Nicotine and Tobacco 11th Annual Meeting, 20-23 March 2005; Prague, Czech Republic 2005.
O'Malley 2018 {published data only}
- Bold KW, Zweben A, Fucito LM, Piepmeier ME, Muvvala S, Wu R, et al. Longitudinal findings from a randomized clinical trial of varenicline for alcohol use disorder with comorbid cigarette smoking. Alcoholism, Clinical and Experimental Research 2019;43(5):937-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01553136. 1/2-multi-site study: varenicline treatment of alcohol dependent smokers. ClinicalTrials.gov/show/NCT01553136 (first received 13 March 2012).
- O'Malley SS, Zweben A, Fucito LM, Wu R, Piepmeier ME, Ockert DM, et al. Effect of varenicline combined with medical management on alcohol use disorder with comorbid cigarette smoking: a randomized clinical trial. JAMA Psychiatry 2018;75(2):129-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oncken 2006 {published data only}
- Oncken C, Gonzales D, Nides M, Rennard S, Watsky E, Billing CB, et al. Efficacy and safety of the novel selective nicotinic acetylcholine receptor partial agonist, varenicline, for smoking cessation. Archives of Internal Medicine 2006;166(15):1571-7. [CENTRAL: 567305] [EMBASE: 2006391945] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Oncken C, Watsky E, Reeves K, Anziano R. Smoking cessation with varenicline, a selective nicotinic receptor partial agonist: results from a phase 2 study [POS1-046]. Society for Research on Nicotine and Tobacco 11th Annual Meeting, 20-23 March 2005; Prague, Czech Republic 2005.
Pastorino 2022 {published and unpublished data}
- Pastorino U, Ladisa V, Trussardo S, Sabia F, Rolli L, Valsecchi C, et al. Cytisine therapy improved smoking cessation in the randomized SMILE lung cancer screening trial. Journal of Thoracic Oncology 2022;17(11):1276-86. [DOI: 10.1016/j.jtho.2022.07.007.] [DOI] [PubMed] [Google Scholar]
Qin 2021 {published data only}
- ChiCTR1900021400. Individual tobacco cessation research based on nicotine metabolite ratio in smoking patients with chronic obstructive pulmonary disease: a randomized controlled trial. https://trialsearch.who.int/Trial2.aspx? TrialID=ChiCTR1900021400 (first received 19 February 2019).
- Qin R, Liu Z, Zhou X, Cheng A, Cui Z, Li J, et al. Adherence and efficacy of smoking cessation treatment among patients with COPD in China. International Journal of Chronic Obstructive Pulmonary Disease 2021;16:1203-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rennard 2012 {published and unpublished data}
- Hughes JR, Russ C, Messig MA. Association of deferring a quit attempt with smoking cessation success: a secondary analysis. Journal of Substance Abuse Treatment 2014;46(2):264-7. [CENTRAL: 911546] [EMBASE: 2013742404] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Hughes JR, Russ CI, Arteaga CE, Rennard SI. Efficacy of a flexible quit date versus an a priori quit date approach to smoking cessation: a cross-study analysis. Addictive Behaviors 2011;36(12):1288-91. [CENTRAL: 830553] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Rennard S, Hughes J, Cinciripini PM, Kralikova E, Raupach T, Arteaga C, et al. A randomized placebo-controlled trial of varenicline for smoking cessation allowing flexible quit dates. Nicotine & Tobacco Research 2012;14(3):343-50. [CENTRAL: 831332] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rigotti 2010 {published data only}
- Ockene I, Salmoirago-Blotcher E. Varenicline for smoking cessation in patients with coronary heart disease [editorial]. Circulation 2010;121(2):188-90. [DOI] [PubMed] [Google Scholar]
- Rigotti N, Pipe A, Benowitz N, Arteaga C, Garza D, Tonstad S, et al. A randomized trial of varenicline for smoking cessation in patients with cardiovascular disease: analysis of efficacy by baseline characteristics. Circulation 2010;Conference:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigotti NA, Pipe AL, Benowitz NL, Arteaga C, Garza D, Tonstad S. Efficacy and safety of varenicline for smoking cessation in patients with cardiovascular disease. Circulation 2010;121(2):221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigotti NA, Pipe AL, Benowitz NL, Arteaga C, Garza D, Tonstad S. Response to letter regarding article, efficacy and safety of varenicline for smoking cessation in patients with cardiovascular disease: a randomized trial. Circulation 2010;122(9):e446. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rohsenow 2017 {published data only}
- Martin RA, Rohsenow DJ, Tidey JW. Smokers with opioid use disorder may have worse drug use outcomes after varenicline than nicotine replacement. Journal of Substance Abuse Treatment 2019;104:22-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00756275. Varenicline and motivational advice for smokers with substance use disorders. ClinicalTrials.gov/ct2/show/NCT00756275 (first received 22 September 2008). [ClinicalTrials.gov ID NCT00756275]
- Rohsenow D, Tidey JW, Martin RA, Colby S, Monti PM. Varenicline versus nicotine patch plus brief advice for sober smokers in substance treatment. Drug and Alcohol Dependence 2015;156:e191-2. [Google Scholar]
- Rohsenow DJ, Tidey JW, Martin RA, Colby SM, Monti PM. Varenicline helps smokers with SUD stop smoking without harming recovery (POS5-63). Society for Research on Nicotine and Tobacco 21st Annual Meeting February 25-28 Philadelphia 2015.
- Rohsenow DJ, Tidey JW, Martin RA, Colby SM, Swift RM, Leggio L, et al. Varenicline versus nicotine patch with brief advice for smokers with substance use disorders with or without depression: effects on smoking, substance use and depressive symptoms. Addiction 2017;112(10):1808-20. [DOI] [PubMed] [Google Scholar]
Rose 2013 {unpublished data only}
- NCT00894166. Evaluation of a tailored smoking cessation treatment algorithm based on initial treatment response and genotype (ConNIC3). clinicaltrials.gov/ct2/show/NCT00894166 (first received 6 May 2009).
- Rose JE, Behm FM. Adapting smoking cessation treatment according to initial response to precessation nicotine patch. American Journal of Psychiatry 2013;170(8):860-7. [CENTRAL: 873084] [EMBASE: 2013550171] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl GR, Walther D, Musci R, Fisher C, Anthony JC, Storr CL, et al. Smoking quit success genotype score predicts quit success and distinct patterns of developmental involvement with common addictive substances. Molecular Psychiatry 2014;19(1):50-4. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Scharfenberg 1971 {published data only}
- Benndorf S, Kempe G, Scharfenberg G, Wendekamm R, Winkelvoss E. Results of smoking cessation treatment with cytisin (Tabex®) [Ergebnisse der medikamentösen raucherentwöhnung mit cytisin (Tabex®)]. Das Deutsche Gesundheitwesen 1968;23(44):2092-6. [PubMed] [Google Scholar]
- Benndorf S, Scharfenberg G, Kempe G, Wendekamm R, Winkelvoss E. Smoking cessation treatment with cytisin (Tabex®): half-yearly outcomes for former smokers abstinent at four weeks from beginning of treatment [Medikamentöse raucherentwöhnung mit cytisin (Tabex®): ergebnisse der halbjahresbefragung bei den vier wochen nach kurbeginn abstinenten ehemaligen rauchern]. Das Deutsche Gesundheitwesen 1970;24:774-6. [PubMed] [Google Scholar]
- Benndorf S, Scharfenberg G, Kempe G, Winkelvoss E, Wendekamm R. Further reports on a double blind trial of the Bulgarian cytisine compound Tabex® on 1214 smokers wishing to quit and practical experience in conducting clinics for such smokers [Weitere mitteilungen über einen doppelten blindversuch mit dem cytisinhaltigen bulgarischen präparat Tabex an 1214 entwöhnungswilligen rauchern und praktische erfahrungen bei der durchfürung einer sprechstunde für entwöhnungswillige raucher]. Das Deutsche Gesundheitswesen 1969;24:1135-40. [Google Scholar]
- Scharfenberg G, Benndorf S, Kempe G. Cytisine (Tabex®) as a treatment for smoking cessation [Cytisin (Tabex®) als medikamentöse raucherentwöhnungshilfe]. Das Deutsche Gesundheitwesen 1971;26(10):463-5. [PubMed] [Google Scholar]
Schnoll 2019 {published data only}
- Carroll AJ, Veluz-Wilkins AK, Blazekovic S, Kalhan R, Leone FT, Wileyto El, et al. Cancer-related disease factors and smoking cessation treatment: analysis of an ongoing clinical trial. Psycho-oncology 2018;27(2):471-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford G, Weisbrot J, Bastian J, Flitter A, Jao NC, Carroll A, et al. Predictors of varenicline adherence among cancer patients treated for tobacco dependence and its association with smoking cessation. Nicotine & Tobacco Research 2019;21(8):1135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May JR, Jao NC, McCarter K, Klass E, Pearman T, Leone F, et al. Change in health-related quality of life among individuals with cancer undergoing smoking cessation treatment involving varenicline. Oncology Nursing Forum 2021;48(1):112-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01756885. Extended duration varenicline for smoking among cancer patients: a clinical trial. ClinicalTrials.gov/show/NCT01756885 (first received 28 December 2012).
- Schnoll R, Leone F, Veluz-Wilkins A, Miele A, Hole A, Jao N C, et al. A randomized controlled trial of 24 weeks of varenicline for tobacco use among cancer patients: efficacy, safety, and adherence. Psycho-oncology 2019;28(3):561-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stein 2013 {unpublished data only}
- De Dios MA, Anderson BJ, Caviness CM, Stein MD. Early quit days among methadone-maintained smokers in a smoking cessation trial. Nicotine & Tobacco Research 2014;16(11):1463-9. [CENTRAL: 1042497] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00790569. Varenicline versus nicotine replacement for methadone-maintained smokers. https://clinicaltrials.gov/ct2/show/NCT00790569 (first received 13 November 2008).
- Stein MD, Caviness CM, Kurth ME, Audet D, Olson J, Anderson BJ. Varenicline for smoking cessation among methadone-maintained smokers: a randomized clinical trial. Drug and Alcohol Dependence 2013;133(2):486-93. [CENTRAL: 870955] [EMBASE: 2013694276] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Steinberg 2011 {published data only}
- Steinberg MB, Randall J, Greenhaus S, Schmelzer AC, Richardson DL, Carson JL. Tobacco dependence treatment for hospitalized smokers: a randomized, controlled, pilot trial using varenicline. Addictive Behaviors 2011;36(12):1127-32. [DOI] [PubMed] [Google Scholar]
Steinberg 2018 {published data only}
- NCT01308736. Varenicline-aided cigarette reduction in smokers not ready to quit. ClinicalTrials.gov/show/NCT01308736 (first received 4 March 2011).
- Steinberg ML, Lu SE, Williams JM. Varenicline for smoking reduction in smokers not yet ready to quit: a double-blind, proof-of-concept randomized clinical trial. Addictive Behaviors 2018;84:20-6. [DOI] [PubMed] [Google Scholar]
Tashkin 2011 {published data only}
- Antoniu SA, Trofor AC. Varenicline for smoking cessation intervention in chronic obstructive pulmonary disease. Expert Opinion on Pharmacotherapy 2011;12(16):2595-7. [DOI] [PubMed] [Google Scholar]
- Kotz D, Van Schayck OC. What justifies a placebo-controlled trial of varenicline for smoking cessation in patients with COPD? [comment]. Chest 2011;139:968-9. [DOI] [PubMed] [Google Scholar]
- Tashkin DP, Rennard S, Hays JT, Ma W, Lawrence D, Lee TC. Effects of varenicline on smoking cessation in mild-to-moderate COPD: a randomized controlled trial. Chest 2011;139:591-9. [DOI] [PubMed] [Google Scholar]
- Tashkin DP, Rennard S, Taylor Hays J, Lawrence D, Marton JP, Lee TC. Lung function and respiratory symptoms in a 1-year randomized smoking cessation trial of varenicline in COPD patients. Respiratory Medicine 2011;105(11):1682-90. [CENTRAL: 811557] [6378] [PMID: ] [DOI] [PubMed] [Google Scholar]
Tonstad 2011 {published data only}
- Tonstad S, Holme I, Tønnesen P. Dianicline, a novel α4β2 nicotinic acetylcholine receptor partial agonist, for smoking cessation: a randomized placebo-controlled clinical trial. Nicotine & Tobacco Research 2011;13(1):1-6. [DOI] [PubMed] [Google Scholar]
Tsai 2007 {published data only}
- Fagerström K, Nakamura M, Cho H, Tsa S, Wang C, Davies S, et al. Varenicline treatment for smoking cessation in Asian populations: a pooled analysis of placebo-controlled trials conducted in six Asian countries. Current Medical Research & Opinion 2010;26(9):2165-73. [DOI] [PubMed] [Google Scholar]
- Tsai S-T, Cho H-J, Cheng H-S, Kim C-H, Hsueh K-C, Billing CB, et al. A randomized, placebo-controlled trial of varenicline, a selective α4β2 nicotinic acetylcholine receptor partial agonist, as a new therapy for smoking cessation in Asian smokers. Clinical Therapeutics 2007;29(6):1027-39. [DOI] [PubMed] [Google Scholar]
Tsukahara 2010 {published data only}
- Fujiwara H. Smoking is a disease and smokers are patients. Circulation Journal 2010;74(4):628-9. [DOI] [PubMed] [Google Scholar]
- Tsukahara H, Noda K, Keijiro S. A randomized controlled open comparative trial of varenicline vs nicotine patch in adult smokers. Circulation Journal 2010;74(4):771-8. [DOI] [PubMed] [Google Scholar]
Tuisku 2016 {published data only}
- NCT01531049. Smoking cessation in young adults in Northern Finland. ClinicalTrials.gov/show/NCT01531049 (first received 10 February 2012).
- Tuisku A, Salmela M, Nieminen P, Toljamo T. Varenicline and nicotine patch therapies in young adults motivated to quit smoking: a randomized, placebo-controlled, prospective study. Basic & Clinical Pharmacology & Toxicology 2016;119(1):78-84. [DOI] [PubMed] [Google Scholar]
Tulloch 2016 {published data only}
- Clyde M, Pipe A, Els C, Reid R, Fu A, Clark A, et al. Nicotine metabolite ratio and smoking outcomes using nicotine replacement therapy and varenicline among smokers with and without psychiatric illness. Journal of Psychopharmacology 2018;32(9):979-85. [DOI] [PubMed] [Google Scholar]
- Clyde M, Pipe A, Els C, Reid R, Tulloch H. Factor structure of the Smoking Cessation Self-Efficacy Questionnaire among smokers with and without a psychiatric diagnosis. Psychology of Addictive Behaviors 2017;31(2):162-70. [DOI] [PubMed] [Google Scholar]
- NCT01623505. Reducing cardiovascular disease by combining smoking cessation pharmacotherapy and behavioural counselling. clinicaltrials.gov/ct2/show/NCT01623505 (first received 20 June 2012).
- Tulloch H, Pipe A, Els C, Aitken D, Clyde M, Corran B, et al. Flexible and extended dosing of nicotine replacement therapy or varenicline in comparison to fixed dose nicotine replacement therapy for smoking cessation: rationale, methods and participant characteristics of the FLEX trial. Contemporary Clinical Trials 2014;38(2):304-13. [CENTRAL: 999078] [EMBASE: 2014493992] [DOI] [PubMed] [Google Scholar]
- Tulloch HE, Pipe AL, Clyde MJ, Reid RD, Els C. The quit experience and concerns of smokers with psychiatric illness. American Journal of Preventive Medicine 2016;50(6):709-18. [DOI] [PubMed] [Google Scholar]
- Tulloch HE, Pipe AL, Els C, Clyde MJ, Reid RD. Flexible, dual-form nicotine replacement therapy or varenicline in comparison with nicotine patch for smoking cessation: a randomized controlled trial. BMC Medicine 2016;14(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang KM, Clyde M, Pipe A, Reid R, Els C, Tulloch HE. Do women and men differ in baseline smoking characteristics and quit rates following treatment with smoking cessation medications? A secondary analysis of the flex study. Journal of Cardiopulmonary Rehabilitation and Prevention. Conference: 2018 Fall Conference of the Canadian Association of Cardiovascular Prevention and Rehabilitation, CACPR 2018. Canada 2018;38(6):E21. [Google Scholar]
Vinnikov 2008 {published data only}
- Vinnikov D, Brimkulov N, Burjubaeva A. A double-blind, randomised, placebo-controlled trial of cytisine for smoking cessation in medium-dependent workers. Journal of Smoking Cessation 2008;3(1):57-62. [Google Scholar]
Walker 2014 {published data only}
- Walker N, Howe C, Bullen C, McRobbie H, Glover M, Parag V, et al. Study protocol for a non-inferiority trial of cytisine versus nicotine replacement therapy in people motivated to stop smoking. BMC Public Health 2011;11(1):880. [CENTRAL: 814611] [EMBASE: 22104038] [6430] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker N, Howe C, Glover M, McRobbie H, Barnes J, Nosa V, et al. Cytisine versus nicotine for smoking cessation. New England Journal of Medicine 2014;371(25):2353-62. [CENTRAL: 1037839] [EMBASE: 2014610901] [DOI] [PubMed] [Google Scholar]
Walker 2021 {published data only}
- NCT02957786. Cytisine versus varenicline for smoking cessation. clinicaltrials.gov/show/NCT02957786 (first received 8 November 2016).
- Walker N, Smith B, Barnes J, Verbiest M, Kurdziel T, Parag V, et al. Cytisine versus varenicline for smoking cessation for Maori (the indigenous people of New Zealand) and their extended family: protocol for a randomized non-inferiority trial. Addiction 2019;114(2):344-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker N, Smith B, Barnes J, Verbiest M, Parag V, Pokhrel S, et al. Cytisine versus varenicline for smoking cessation in New Zealand indigenous Maori: a randomized controlled trial. Addiction 2021;116(10):2847-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2009 {published data only}
- Fagerström K, Nakamura M, Cho H, Tsa S, Wang C, Davies S, et al. Varenicline treatment for smoking cessation in Asian populations: a pooled analysis of placebo-controlled trials conducted in six Asian countries. Current Medical Research & Opinion 2010;26(9):2165-73. [DOI] [PubMed] [Google Scholar]
- Wang C, Xiao D, Chan KP, Pothirat C, Garza D, Davies S. Varenicline for smoking cessation: a placebo-controlled, randomized study. Respirology 2009;14(3):384-92. [clinicaltrials.gov ID: NCT00371813] [DOI] [PubMed] [Google Scholar]
West 2011 {published data only}37568749
- West R, Zatonski W, Cedzynska M, Lewandowska D, Pazik J, Aveyard PA, et al. Placebo-controlled trial of cytisine for smoking cessation. New England Journal of Medicine 2011;365:1193-200. [Current Controlled Trials ID: ISRCTN37568749] [DOI] [PubMed] [Google Scholar]
- West R, Zatonski W. Cytisine increased smoking cessation in adults. Annals of Internal Medicine 2012;156(2):JC1-6. [CENTRAL: 897954] [EMBASE: 2012033460] [DOI] [PubMed] [Google Scholar]
Westergaard 2015 {published data only}
- Westergaard CG, Porsbjerg C, Backer V. The effect of smoking cessation on airway inflammation in young asthma patients. Clinical & Experimental Allergy 2013;44:353-61. [DOI] [PubMed] [Google Scholar]
- Westergaard CG, Porsbjerg C, Backer V. The effect of varenicline on smoking cessation in a group of young asthma patients. American Journal of Respiratory and Critical Care Medicine 2014;189:A1091. [DOI] [PubMed] [Google Scholar]
- Westergaard CG, Porsbjerg C, Backer V. The effect of varenicline on smoking cessation in a group of young asthma patients. Respiratory Medicine 2015;109(11):1416-22. [DOI: 10.1016/j.rmed.2015.07.017] [DOI] [PubMed] [Google Scholar]
Williams 2007 {published and unpublished data}
- Reeves K, Watsky E, Williams K, Azoulay S, Billing B, Gong J. The safety of varenicline taken for 52 weeks for smoking cessation [RPOS3-54]. Society for Research on Nicotine and Tobacco 12th Annual Conference Orlando Fla, USA 2006.
- Spangler JG, Williams KE, Reeves KR, Billing CB, Pennington AM, Gong J. Comment and reply: a double-blind study evaluating the long-term safety of varenicline for smoking cessation. Current Medical Research and Opinion 2008;24(2):577-9. [DOI] [PubMed] [Google Scholar]
- Williams KE, Reeves KR, Billing CB, Pennington AM, Gong J. A double-blind study evaluating the long-term safety of varenicline for smoking cessation. Current Medical Research and Opinion 2007;23(4):793-801. [DOI] [PubMed] [Google Scholar]
Williams 2012 {unpublished data only}
- NCT00644969. Smoking cessation study for patients with schizophrenia or schizoaffective disorder. clinicaltrials.gov/ct2/show/NCT00644969 (first received 27 March 2008).
- Williams JM, Anthenelli RM, Morris CD, Treadow J, Thompson JR, Yunis C, et al. A randomized, double-blind, placebo-controlled study evaluating the safety and efficacy of varenicline for smoking cessation in patients with schizophrenia or schizoaffective disorder. Journal of Clinical Psychiatry 2012;73(5):654-60. [CENTRAL: 836870] [EMBASE: 2012321245] [PMID: ] [DOI] [PubMed] [Google Scholar]
Windle 2018 {published data only}
- Dehghani P, Habib B, Windle SB, Roy N, Old W, Grondin FR, et al. Smokers and postcessation weight gain after acute coronary syndrome. Journal of the American Heart Association 2017;6(4):e004785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg MJ, Windle SB, Roy N, Old W, Grondin F, Bata I, et al. Varenicline for smoking cessation in hospitalized patients with acute coronary syndrome. Circulation 2016;133(1):21-30. [DOI] [PubMed] [Google Scholar]
- NCT00794573. Evaluation of varenicline (Champix) in smoking cessation for patients post-acute coronary syndrome (EVITA) trial (EVITA). clinicaltrials.gov/ct2/show/NCT00794573 (first received 20 November 2008).
- Windle SB, Bata I, Madan M, Abramson BL, Eisenberg MJ. A randomized controlled trial of the efficacy and safety of varenicline for smoking cessation after acute coronary syndrome: design and methods of the Evaluation of Varenicline in Smoking Cessation for Patients Post-Acute Coronary Syndrome trial. American Heart Journal 2015;170(4):635-40. [DOI] [PubMed] [Google Scholar]
- Windle SB, Dehghani P, Roy N, Old W, Grondin FR, Bata I, et al. Smoking abstinence 1 year after acute coronary syndrome: follow-up from a randomized controlled trial of varenicline in patients admitted to hospital. CMAJ : Canadian Medical Association Journal 2018;190(12):E347-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle SB, Dehghani P, Roy N, Old WD, Grondin FR, Bata I, et al. Sustained smoking abstinence 12 months after acute coronary syndrome: follow-up from a randomized controlled trial of varenicline in hospitalized patients. Canadian Journal of Cardiology. Conference: 69th Annual Meeting of the Canadian Cardiovascular Society. Canada. 2016;10 Suppl 1:S109-10. [Google Scholar]
Wong 2012 {unpublished data only}
- NCT00937508. Smoking cessation program in the pre-admission clinic. clinicaltrials.gov/ct2/show/NCT00937508 (first received 13 July 2009).
- Wong J, Abrishami A, Yang Y, Zaki A, Friedman Z, Selby P, et al. A perioperative smoking cessation intervention with varenicline: a double-blind, randomized, placebo-controlled trial. Anesthesiology 2012;117(4):755-64. [CENTRAL: 835869] [EMBASE: 2012567287] [PMID: ] [DOI] [PubMed] [Google Scholar]
Yang 2016 {published data only}
- Yang DX, Gu CJ, Ni L, Li N, Li QY, Zhou JP. Assessment of efficacy of medication combined with WeChat platform for quitting smoking in patients with chronic obstructive pulmonary disease. Journal of Shanghai Jiaotong University (Medical Science) 2016;36(3):385-9. [Google Scholar]
Zawertailo 2020 {published data only}
- NCT01286584. Varenicline in residential treatment (ViRT). clinicaltrials.gov/ct2/show/NCT01286584 (first received 31 January 2011).
- Zawertailo L, Ivanova A, Ng G, Le Foll B, Selby P. Safety and efficacy of varenicline for smoking cessation in alcohol-dependent smokers in concurrent treatment for alcohol use disorder: a pilot, randomized placebo-controlled trial. Journal of Clinical Psychopharmacology 2020;40(2):130-6. [DOI] [PubMed] [Google Scholar]
Zhang 2022 {published data only}
- NCT02146911. The MATCH (Medication Aids for Tobacco Cessation and Health) study. clinicaltrials.gov/show/NCT02146911 (first received 26 May 2014).
- Zawertailo L, Mansoursadeghi-Gilan T, Zhang H, Hussain S, Le Foll B, Selby P. Varenicline and bupropion for long-term smoking cessation (the MATCH Study): protocol for a real-world, pragmatic, randomized controlled trial. JMIR Research Protocols 2018;7(10):e10826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Mansoursadeghi-Gilan T, Hussain S, Veldhuizen S, Le Foll B, Selby P, et al. Evaluating the effectiveness of bupropion and varenicline for smoking cessation using an internet-based delivery system: a pragmatic randomized controlled trial (MATCH study). Drug and Alcohol Dependence 2022;232:109312. [DOI] [PubMed] [Google Scholar]
Zincir 2013 {published data only}
- Zincir SB, Zincir S, Kaymak E, Sunbul EA. Comparison of the effectiveness of varenicline, extended-release bupropion and nicotine replacement therapy on the success and the maintenance of a smoking cessation program. Bulletin of Clinical Psychopharmacology 2013;23(3):224-30. [CENTRAL: 914104] [EMBASE: 2013628205] [Google Scholar]
References to studies excluded from this review
Brandon 2011 {published data only}
- Brandon TH, Drobes DJ, Unrod M, Heckman BW, Oliver JA, Roetzheim RC, et al. Varenicline effects on craving, cue reactivity, and smoking reward. Psychopharmacology 2011;218(2):391-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bullen 2018 {published data only}
- Bullen C, Verbiest M, Galea-Singer S, Kurdziel T, Laking G, Newcombe D, et al. The effectiveness and safety of combining varenicline with nicotine e-cigarettes for smoking cessation in people with mental illnesses and addictions: study protocol for a randomised-controlled trial. BMC Public Health 2018;18(1):596. [DOI] [PMC free article] [PubMed] [Google Scholar]
Burstein 2006 {published data only}
- Burstein AH, Fullerton T, Clark DJ, Faessel HM. Pharmacokinetics, safety, and tolerability after single and multiple oral doses of varenicline in elderly smokers. Journal of Clinical Pharmacology 2006;46:1234-40. [DOI] [PubMed] [Google Scholar]
Chantix 2006 {published data only}
- Pfizer Inc. Chantix prescribing information. www.chantix.com/content/Prescribing_Information.jsp (accessed 10 February 2007).
Cui 2012 {published data only}
- Cui Q, Robinson L, Elston D, Smaill F, Cohen J, Quan C, et al. Safety and tolerability of varenicline tartrate (Champix®/Chantix®) for smoking cessation in HIV-infected subjects: a pilot open-label study. AIDS Patient Care and STDs 2012;26(1):12-19. [DOI: 10.1089/apc.2011.0199] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dezee 2013 {published data only}
- Cowan CM, Wink JS, DeZee KJ. Use of the patient health questionnaire-2 to predict suicidal ideations in patients taking varenicline. American Journal on Addictions / American Academy of Psychiatrists in Alcoholism and Addictions 2012;21(4):356-62. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Dezee KJ, Wink JS, Cowan CM. Internet versus in-person counseling for patients taking varenicline for smoking cessation. Military Medicine 2013;178(4):401-5. [CENTRAL: 959429] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dutra 2012 {published data only}
- Dutra SJ, Stoeckel LE, Carlini SV, Pizzagalli DA, Evins AE. Varenicline as a smoking cessation aid in schizophrenia: effects on smoking behavior and reward sensitivity. Psychopharmacology 2012;219(1):25-34. [DOI: 10.1007/s00213-011-2373-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ebbert 2009a {published data only}
- Ebbert JO, Croghan IT, Sood A, Schroeder DR, Hays JT, Hurt RD. Varenicline and bupropion sustained-release combination therapy for smoking cessation. Nicotine & Tobacco Research 2009;11(3):234-9. [DOI] [PubMed] [Google Scholar]
Ebbert 2009b {published data only}
- Ebbert JO, Burke MV, Hays JT, Hurt RD. Combination treatment with varenicline and nicotine replacement therapy. Nicotine & Tobacco Research 2009;11(5):572-6. [DOI] [PubMed] [Google Scholar]
Ebbert 2011b {published data only}
- Ebbert JO, Croghan IT, North F, Schroeder DR. A pilot study to assess smokeless tobacco use reduction with varenicline. Nicotine & Tobacco Research 2011;13(9):820-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ebbert 2014 {unpublished data only}
- Ebbert JO, Hatsukami DK, Croghan IT, Schroeder DR, Allen SS, Hays JT, et al. Combination varenicline and bupropion SR for tobacco-dependence treatment in cigarette smokers: a randomized trial. JAMA 2014;311(2):155-63. [CENTRAL: 921055] [EMBASE: 2014041620] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00935818. Varenicline and bupropion for smoking cessation (CHANBAN). clinicaltrials.gov/ct2/show/NCT00935818 (first received 9 July 2009).
Evins 2014 {published and unpublished data}
- Cather C, Hoeppner S, Pachas G, Pratt S, Achtyes E, Cieslak KM, et al. Improved depressive symptoms in adults with schizophrenia during a smoking cessation attempt with varenicline and behavioral therapy. Journal of Dual Diagnosis 2017;13(3):168-78. [DOI] [PubMed] [Google Scholar]
- Evins AE, Cather C, Pratt SA, Pachas GN, Hoeppner SS, Goff DC, et al. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. JAMA 2014;311(2):145-54. [CENTRAL: 921056] [EMBASE: 2014041619] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evins AE. Extended duration pharmacotherapy with varenicline prevents relapse to smoking in adult smokers with schizophrenia. Neuropsychopharmacology 2013;38:S63-4. [CENTRAL: 993941] [EMBASE: 71278012] [Google Scholar]
- NCT00621777. A study of varenicline for prevention of relapse to smoking in patients with schizophrenia. clinicaltrials.gov/ct2/show/NCT00621777 (first received 22 February 2008).
- Pachas GN, Cather C, Pratt SI, Hoeppner B, Nino J, Carlini SV, et al. Varenicline for smoking cessation in schizophrenia: safety and effectiveness in a 12-week open-label trial. Journal of Dual Diagnosis 2012;8(2):117-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorndike AN, Achtyes ED, Cather C, Pratt S, Pachas GN, Hoeppner SS, et al. Weight gain and 10-year cardiovascular risk with sustained tobacco abstinence in smokers with serious mental illness: a subgroup analysis of a randomized trial. Journal of Clinical Psychiatry 2016;77(3):e320-6. [DOI] [PubMed] [Google Scholar]
- Thorndike AN, Hoeppner SS, Cather C, Pachas GN, Achtyes ED, Evins AE. Weight gain and cardiovascular risk reduction associated with tobacco abstinence in smokers with serious mental illness. Circulation 2015;131:AP008. [DOI] [PubMed] [Google Scholar]
Faessel 2009 {published data only}
- Faessel H, Ravva P, Williams K. Pharmacokinetics, safety, and tolerability of varenicline in healthy adolescent smokers: a multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clinical Therapeutics 2009;31:177-89. [clinicaltrials.gov ID: NCT00463918] [DOI] [PubMed] [Google Scholar]
Fagerström 2010 {published data only}
- Fagerström K, Gilljam H, Metcalfe M, Tonstad S, Messig M. Stopping smokeless tobacco with varenicline: randomised double blind placebo controlled trial. BMJ 2010;341:c6549. [DOI: 10.1136/bmjc6549] [DOI] [PMC free article] [PubMed] [Google Scholar]
Falk 2014 {published data only}
- Falk DE, Litten RZ, Ryan ML, Fertig JB. A double-blind, placebo-controlled trial assessing the efficacy of varenicline tartrate for alcohol dependence. Alcoholism, Clinical and Experimental Research 2014;38 Suppl s1:139A. [CENTRAL: 993967] [EMBASE: 71503638] [Google Scholar]
- Litten RZ, Ryan ML, Fertig JB, Falk DE, Johnson B, Dunn KE, et al. A double-blind, placebo-controlled trial assessing the efficacy of varenicline tartrate for alcohol dependence. Journal of Addiction Medicine 2013;7(4):277-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fatemi 2013 {published data only}
- Fatemi SH, Yousefi MK, Kneeland RE, Liesch SB, Folsom TD, Thuras PD. Antismoking and potential antipsychotic effects of varenicline in subjects with schizophrenia or schizoaffective disorder: a double-blind placebo and bupropion-controlled study. Schizophrenia Research 2013;146(1-3):376-8. [CENTRAL: 877011] [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT01111149. Varenicline and smoking cessation in schizophrenia (VSCS). clinicaltrials.gov/ct2/show/NCT01111149 (first received 27 April 2010).
Ferketich 2012 {published data only}
- Ferketich AK, Otterson GA, King M, Hall N, Browning KK, Wewers ME. A pilot test of a combined tobacco dependence treatment and lung cancer screening program. Lung Cancer 2012;76(2):211-5. [CENTRAL: 814368] [EMBASE: 2012198269] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferketich 2013 {published data only}
- Ferketich AK, Diaz P, Browning KK, Lu B, Koletar SL, Reynolds NR, et al. Safety of varenicline among smokers enrolled in the lung HIV study. Nicotine & Tobacco Research 2013;15(1):247-54. [EMBASE: 2012756151] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fertig 2015 {published data only}
- Fertig JB, Ryan ML, Falk DE, Litten RZ. Moderators of the varenicline treatment effect in a double-blind, placebo controlled trial for alcohol dependence. Alcoholism: Clinical and Experimental Research 2015;39:80A. [DOI] [PMC free article] [PubMed] [Google Scholar]
Frye 2013 {published data only}
- Frye MA, Ebbert JO, Prince CA, Lineberry TW, Geske JR, Patten CA. A feasibility study of varenicline for smoking cessation in bipolar patients with subsyndromal depression. Journal of Clinical Psychopharmacology 2013;33(6):821-3. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fucito 2011 {published data only}
- Fucito LM, Toll BA, Wu R, Romano DM, Tek C, O'Malley SS. A preliminary investigation of varenicline for heavy drinking smokers. Psychopharmacology 2011;215(4):655-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garza 2011 {published data only}
- Garza D, Murphy M, Tseng L-J, Riordan HJ, Chatterjee A. A double-blind randomized placebo-controlled pilot study of neuropsychiatric adverse events in abstinent smokers treated with varenicline or placebo. Biological Psychiatry 2011;69:1075-82. [DOI] [PubMed] [Google Scholar]
Granatowicz 1976 {published data only}
- Granatowicz J. Smoking cessation through the use of cytisine and other therapy. World Smoking Health 1976;1:8-11. [Google Scholar]
Gray 2012 {published data only}
- Gray KM, Carpenter MJ, Lewis AL, Klintworth EM, Upadhyaya HP. Varenicline versus bupropion XL for smoking cessation in older adolescents: a randomized, double-blind pilot trial. Nicotine & Tobacco Research 2012;14(2):235-9. [CENTRAL: 830824] [EMBASE: 2012065214] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hajek 2011 {published data only}
- Dhelaria RK, Rothberg M. Is varenicline effectiveness declining in randomized trials? Archives of Internal Medicine 2011;171(19):1770-1. [DOI] [PubMed] [Google Scholar]
- Hajek P, McRobbie H, Myers K, Stapleton J, Dhanji AR. Is varenicline effectiveness declining in randomized trials? - Reply. Archives of Internal Medicine 2011;171(19):1771-2. [DOI] [PubMed] [Google Scholar]
- Hajek P, McRobbie HJ, Myers KE, Stapleton J, Dhanji A-R. Use of varenicline for 4 weeks before quitting smoking. Archives of Internal Medicine 2011;171(8):770-7. [DOI] [PubMed] [Google Scholar]
- Simon JA. Smoking cessation interventions: a primer for physicians. Archives of Internal Medicine 2011;171(8):777-8. [DOI] [PubMed] [Google Scholar]
Hajek 2013 {published data only}
- Hajek P, Smith KM, Dhanji A-R, McRobbie H. Is a combination of varenicline and nicotine patch more effective in helping smokers quit than varenicline alone? A randomised controlled trial. BMC Medicine 2013;11(1):140. [CENTRAL: 963647] [EMBASE: 2013375051] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hajek 2015 {published data only}
- Hajek P, McRobbie H, Myers Smith K, Phillips A, Cornwall D, Dhanji AR. Erratum: increasing varenicline dose in smokers WHO do not respond to the standard dosage: a randomized clinical trial (JAMA internal medicine (2015) 175: 2 (266-271)). JAMA Internal Medicine 2016;176(1):143. [DOI] [PubMed] [Google Scholar]
- Hajek P, McRobbie H, Myers Smith K, Phillips A, Cornwall D, Dhanji AR. Increasing varenicline dose in smokers who do not respond to the standard dosage: a randomized clinical trial. JAMA Internal Medicine 2015;175(2):266-71. [CENTRAL: 1047635] [EMBASE: 2015718793] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hartwell 2014 {published data only}
- Hartwell EE, Roche DJ, Ray LA. Pharmacogenetics of naltrexone and varenicline in heavy drinking smokers. Alcoholism, Clinical and Experimental Research 2014;38 Suppl s1:223A. [CENTRAL: 993964] [EMBASE: 71503974] [Google Scholar]
- Roche DJ, Bujarski S, Hartwell E, Green R, Ray LA. Combined varenicline and naltrexone treatment reduces smoking topography intensity in heavy-drinking smokers. Pharmacology, Biochemistry, and Behavior 2015;134:92-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hawk 2012 {published and unpublished data}
- Hawk LW, Ashare RL, Lohnes SF, Schlienz NJ, Rhodes JD, Tiffany ST, et al. The effects of extended pre-quit varenicline treatment on smoking behavior and short-term abstinence: a randomized clinical trial. Clinical Pharmacology and Therapeutics 2012;91(2):172-80. [CENTRAL: 814229] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00835900. An alternative dosing schedule for varenicline for smoking cessation. clinicaltrials.gov/ct2/show/NCT00835900 (first received 4 February 2009).
Hong 2011 {unpublished data only}
- Hong LE, Thaker GK, McMahon RP, Summerfelt A, Rachbeisel J, Fuller RL, et al. Effects of moderate-dose treatment with varenicline on neurobiological and cognitive biomarkers in smokers and nonsmokers with schizophrenia or schizoaffective disorder. Archives of General Psychiatry 2011;68(12):1195-206. [CENTRAL: 810278] [6348] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00492349. Varenicline adjunctive treatment in schizophrenia. clinicaltrials.gov/ct2/show/NCT00492349 (first received 27 June 2007).
Hoogsteder 2014 {published data only}
- Hoogsteder PH, Kotz D, Van Spiegel PI, Viechtbauer W, Van Schayck OC. Efficacy of the nicotine vaccine 3'-AmNic-rEPA (NicVAX) co-administered with varenicline and counselling for smoking cessation: a randomized placebo-controlled trial. Addiction 2014;109(8):1252-9. [CENTRAL: 1000793] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hsueh 2014 {published data only}
- Hsueh K-C, Hsueh S-C, Chou M-Y, Pan L-F, Tu M-S, McEwen A, et al. Varenicline versus transdermal nicotine patch: a 3-year follow-up in a smoking cessation clinic in Taiwan. Psychopharmacology 2014;231(14):2819-23. [CENTRAL: 1050988] [EMBASE: 2014439532] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hsueh 2021 {published data only}
- Hsueh KC, Tang PL, McRobbie H. Effectiveness of varenicline versus combination nicotine replacement therapy for smoking cessation: one-year outcomes in a smoking cessation clinic in Taiwan. Nicotine & Tobacco Research 2021;23(7):1094-102. [DOI] [PubMed] [Google Scholar]
Hughes 2011 {published data only}
- Hughes JR, Rennard SI, Fingar JR, Talbot SK, Callas PW, Fagerström KO. Efficacy of varenicline to prompt quit attempts in smokers not currently trying to quit: a randomized placebo-controlled trial. Nicotine & Tobacco Research 2011;13(10):955-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00595868. Efficacy of varenicline in ambivalent smokers. ClinicalTrials.gov/ct2/show/NCT00595868 2007 (accessed 2 January 2016).
IRCT20100127003210N {published data only}
- IRCT20100127003210N. Cytisine versus nicotine for smoking cessation in hospitalized psychiatric patients. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20100127003210N16 (first received 2019).
Jain 2014 {published data only}
- Jain R, Jhanjee S, Jain V, Gupta T, Mittal S, Goelz P, et al. A double-blind placebo-controlled randomized trial of varenicline for smokeless tobacco dependence in India. Nicotine & Tobacco Research 2014;16(1):50-7. [CENTRAL: 1000326] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jennings 2014 {published data only}22073647
- Jennings C, Kotseva K, De Bacquer D, Hoes A, Velasco J, Brusaferro S, et al. Effectiveness of a preventive cardiology programme for high CVD risk persistent smokers: the EUROACTION PLUS varenicline trial. European Heart Journal 2014;35(21):1411-20. [CENTRAL: 996426] [EMBASE: 2014386940] [PMID: ] [DOI] [PubMed] [Google Scholar]
Jiménez‐Ruiz 2013 {published data only}
- Jiménez-Ruiz CA, Barrios M, Peña S, Cicero A, Mayayo M, Cristóbal M, et al. Increasing the dose of varenicline in patients who do not respond to the standard dose. Mayo Clinic Proceedings 2013;88(12):1443-5. [DOI] [PubMed] [Google Scholar]
Kempe 1967 {published data only}
- Bacvarov VI. Smoking cessation through medication: remarks on G. Scharfenberg, E Winkelvoss and S. Benndorf, Munch Med Wschr. 109 (1967) 1687-1689. Munchener Medizinische Wochenschrift 1967;109(50):2663-5. [PubMed] [Google Scholar]
- Kempe G. Observation about the Bulgarian medicine for smoking withdrawal Tabex, produced by Pharmachim-Sofia. Savr Med 1967;18(4):355-6. [Google Scholar]
Koegelenberg 2014 {published data only}
- Koegelenberg CF. Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation: A randomized clinical trial. [German]. Zeitschrift fur Gefassmedizin 2014;11(3):26. [CENTRAL: 994156] [EMBASE: 2014813108] [PMID: ] [DOI] [PubMed] [Google Scholar]
Maliszewski 1972 {published data only}
- Maliszewski L, Straczynski A. Therapeutic use of Tabex. Wiadomoscie Lekarskie 1972;25(24):2207-10. [PubMed] [Google Scholar]
Marakulin 1984 {published data only}
- Marakulin VS, Komarov VM, Chuprin VV. Treatment of nicotinism [Ob opyte lecheniia nikotinizma]. Voenno-Meditsinskii Zhurnal 1984;1:55-8. [PubMed] [Google Scholar]
McClure 2013 {unpublished data only}
- McClure EA, Vandrey RG, Johnson MW, Stitzer ML. Effects of varenicline on abstinence and smoking reward following a programmed lapse. Nicotine & Tobacco Research 2013;15(1):139-48. [CENTRAL: 873078] [EMBASE: 2012756138] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00944554. Varenicline for relapse prevention. ClinicalTrials.gov/ct2/ 2009 (accessed 2 November 2010).
McColl 2008 {published data only}
- McColl SL, Burstein AH, Reeves KR, Billing CB, Stolar M, Sellers EM. Human abuse liability of the smoking cessation drug varenicline in smokers and nonsmokers. Clinical Therapeutics and Pharmacology 2008;83(4):607-14. [DOI] [PubMed] [Google Scholar]
McNaughton 2013 {published data only}
- McNaughton B, Frohlich J, Graham A, Young QR. Extended interactive voice response telephony (IVR) for relapse prevention after smoking cessation using varenicline and IVR: a pilot study. BMC Public Health 2013;13:824. [CENTRAL: 1015643] [DOI] [PMC free article] [PubMed] [Google Scholar]
Meszaros 2013 {published data only}
- Meszaros ZS, Abdul-Malak Y, Dimmock JA, Wang D, Ajagbe TO, Batki SL. Varenicline treatment of concurrent alcohol and nicotine dependence in schizophrenia: a randomized, placebo-controlled pilot trial. Journal of Clinical Psychopharmacology 2013;33(2):243-7. [CENTRAL: 908808] [EMBASE: 2013155799] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Meszaros ZS, Abdul-Malak Y, Dimmock JA, Wang D, Batki SL. Varenicline treatment of alcohol and nicotine dependence in schizophrenia: problems encountered in a pilot trial. American Journal on Addictions / American Academy of Psychiatrists in Alcoholism and Addictions 2012;21(4):393-4. [CENTRAL: 834600] [EMBASE: 70815316] [Google Scholar]
- NCT00727103. Varenicline treatment in alcohol and nicotine dependent patients with schizophrenia. clinicaltrials.gov/ct2/show/NCT00727103 (first received 1 August 2008).
Metelitsa 1987 {published data only}
- Metelitsa VI. Pharmacological agents in controlling smoking. Biulleten Vsesoiuznogo Kardiologicheskogo Nuachnogo Tsentra AMN SSSR 1987;10(1):109-12. [PubMed] [Google Scholar]
Mitchell 2012 {published data only}
- Mitchell JM, Teague CH, Kayser AS, Bartlett SE, Fields HL. Varenicline decreases alcohol consumption in heavy-drinking smokers. Psychopharmacology 2012;223(3):299-306. [CENTRAL: 845347] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mocking 2013 {published data only}
- Mocking RJ, Patrick Pflanz C, Pringle A, Parsons E, McTavish SF, Cowen PJ, et al. Effects of short-term varenicline administration on emotional and cognitive processing in healthy, non-smoking adults: a randomized, double-blind, study. Neuropsychopharmacology 2013;38(3):476-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Monova 2004 {unpublished data only}
- Monova A, Monova D, Petrov V, Peneva E, Todorova M, Petrova M. Final report on double blind, placebo controlled, randomized clinical study for evaluation of efficacy, safety and tolerability of Tabex in patients with chronic nicotinism (tabacism): TAB - SPH - 04014. Sopharma plc unpublished report 2004.
Nahvi 2014b {published data only}
- Nahvi S, Segal KS, Litwin AH, Arnsten JH. Rationale and design of a randomized controlled trial of varenicline directly observed therapy delivered in methadone clinics. Addiction Science & Clinical Practice 2014;9:9. [CENTRAL: 1037892] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nahvi 2020 {published data only}
- Nahvi S, Adams TR, Ning Y, Zhang C, Arnsten JH. Effect of varenicline directly observed therapy versus varenicline self-administered therapy on varenicline adherence and smoking cessation in methadone-maintained smokers: a randomized controlled trial. Addiction 2020;116(4):902-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00387946 {unpublished data only}
- NCT00387946. Efficacy and safety of dianicline treatment as an aid to smoking cessation in cigarette smokers (AMERIDIAN). clinicaltrials.gov/ct2/show/NCT00387946 (first received 13 October 2006).
NCT00502216 {unpublished data only}
- NCT00502216. Naltrexone and varenicline: weight gain and tolerability in smokers. ClinicalTrials.gov/show/NCT00502216 (first received 17 July 2007).
NCT00554840 {unpublished data only}
- NCT00554840. Comparison of varenicline and placebo for smoking cessation in schizophrenia. ClinicalTrials.gov/ct2/show/NCT00554840 (first received 7 November 2007).
NCT00828113 {unpublished data only}
- NCT00828113. Long-term varenicline treatment for smoking cessation. clinicaltrials.gov/ct2/show/NCT00828113 (first received 23 January 2009).
NCT01093937 {unpublished data only}
- NCT01093937. Study of varenicline for smoking cessation/reduction in patients with bipolar disorder. clinicaltrials.gov/ct2/show/NCT01093937 (first received 26 March 2010).
NCT01413516 {published data only}
- NCT01413516. A two-part pilot study of dosing, safety and efficacy of varenicline initiated during an acute smoke-free hospitalization and continued post-hospitalization. clinicaltrials.gov/show/NCT01413516 (first received 10 August 2011).
NCT01532232 {published data only}
- NCT01532232. Treatment of tobacco dependence in breast cancer patients: a randomized trial of varenicline (Chantix). ClinicalTrials.gov/show/NCT01532232 (first received 14 February 2012).
NCT01574703 {unpublished data only}
- NCT01574703. Study to evaluate cardiac assessments following different treatments of smoking cessation medications in subjects with and without psychiatric disorders [CATS]. ClinicalTrials.gov/show/NCT01574703 (first received 10 April 2012).
NCT01592695 {published data only}
- NCT01592695. Tailored tobacco cessation program for rural veterans with comorbid depression, alcoholism or obesity. ClinicalTrials.gov/show/NCT01592695 (first received 7 May 2012).
NCT01639560 {unpublished data only}
- NCT01639560. Varenicline for light smokers (ChanLight). clinicaltrials.gov/ct2/show/NCT01639560 (first received 12 July 2012).
NCT01771627 {published data only}
- NCT01771627. Pilot study of varenicline vs. nicotine patch delivered by a telephone quitline to promote smoking cessation. ClinicalTrials.gov/show/NCT01771627 (first received 18 January 2013).
NCT01772641 {published data only}
- NCT01772641. A combination of scheduled reduced smoking with varenicline to enhance cessation. ClinicalTrials.gov/show/NCT01772641 (first received 21 January 2013).
NCT01806779 {unpublished data only}
- NCT01806779. Combination bupropion/varenicline for smoking cessation in male smokers. ClinicalTrials.gov/show/NCT01806779 (first received 7 March 2013).
NCT01892813 {published data only}
- NCT01892813. Tailored tobacco intervention. ClinicalTrials.gov/show/NCT01892813 (first received 4 July 2013).
NCT02048917 {published data only}
- NCT02048917. Optimization of smoking cessation strategies in community cancer programs for newly diagnosed lung and head and neck cancer patients. Clinicaltrials.gov/show/NCT02048917 (first received 29 January 2014). [CENTRAL: 1013511]
NCT02147132 {published data only}
- NCT02147132. A pilot randomized, placebo-controlled, crossover study of the effect of the nicotine nasal spray and varenicline on cigarette smoking following methadone dosing in methadone-maintained patients. ClinicalTrials.gov/show/NCT02147132 (first received 26 May 2014).
NCT02271919 {published data only}
- NCT02271919. Varenicline and combined nicotine replacement therapy (NRT) for initial smoking cessation and rescue Treatment in smokers: a randomized pilot trial. ClinicalTrials.gov/show/NCT02271919 (first received 22 October 2014).
NCT02501265 {published data only}
- NCT02501265. Adaptive pharmacotherapy for smoking cessation. https://clinicaltrials.gov/show/NCT02501265 (first received 17 July 2015).
NCT03709823 {published data only}
- NCT03709823. Trial of cytisine in adult smokers. clinicaltrials.gov/ct2/show/NCT03709823 (first received 17 October 2018).
Nides 2021 {published data only}
- Nides M, Rigotti NA, Benowitz N, Clarke A, Jacobs C. A multicenter, double-blind, randomized, placebo-controlled phase 2b trial of cytisinicline in adult smokers (the ORCA-1 trial). Nicotine & Tobacco Research 2021;23(10):1656-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nollen 2011 {published data only}
- Buchanan TS, Berg CJ, Cox LS, Nazir N, Benowitz NL, Yu L, et al. Adherence to varenicline among African American smokers: an exploratory analysis comparing plasma concentration, pill count, and self-report. Nicotine & Tobacco Research 2012;14(9):1083-91. [CENTRAL: 834603] [EMBASE: 2012554324] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nollen NL, Cox LS, Nazir N, Ellerbeck EF, Owen A, Pankey S, et al. A pilot clinical trial of varenicline for smoking cessation in Black smokers. Nicotine & Tobacco Research 2011;13(9):868-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ostrovskaia 1994 {published data only}
- Ostrovskaia TP. Results of clinical investigation of anti-nicotine drug patches. Meditsinskaia Tekhnika 1994;3:42-3. [PubMed] [Google Scholar]
Park 2011 {published data only}
- Park ER, Japuntich S, Temel J, Lanuti M, Pandiscio J, Hilgenberg J, et al. A smoking cessation intervention for thoracic surgery and oncology clinics. Journal of Thoracic Oncology 2011;6(6):1059-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Patterson 2010 {unpublished data only}
- NCT00948649. Effects of Chantix on relapse prevention for smoking cessation. clinicaltrials.gov/ct2/show/NCT00948649 (first received 29 July 2009).
- Patterson F, Jepson C, Loughead J, Perkins K, Strasser AA, Siegel S, et al. Working memory deficits predict short-term smoking resumption following brief abstinence. Drug and Alcohol Dependence 2010;106(1):61-4. [CENTRAL: 731184] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Strasser AA, Loughead J, Perkins KA, Gur RC, et al. Varenicline improves mood and cognition during smoking abstinence. Biological Psychiatry 2009;65(2):144-9. [CENTRAL: 687104] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnick ND, Strasser AA, Phillips JM, Jepson C, Patterson F, Frey JM, et al. Mouse model predicts effects of smoking and varenicline on event-related potentials in humans. Nicotine & Tobacco Research 2010;12(6):589-97. [CENTRAL: 751949] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Paun 1968 {published data only}
- Paun D, Franze J. Smoking cessation with cytisine 'Tabex' tablets [Raucherentwöhnung mit cytisinhaltigen "Tabex" tabletten]. Sonderduck Aus Das Deutsche Gesundheitwesen 1968;23(44):2088-91. [PubMed] [Google Scholar]
- Paun D, Franze Y. Tabex: registering and treatment of smokers with chronic bronchitis in the consultation against tobacco smoking - Berlin. Medico-Biologic Information 1970;3:15-9. [Google Scholar]
Pfeifer 2019 {published data only}
- Pfeifer P, Fehr C. Efficacy of varenicline in patients with severe alcohol dependence: a pilot double-blind randomized and controlled study. Journal of Clinical Psychopharmacology 2019;39(4):398-402. [DOI] [PubMed] [Google Scholar]
Pfizer 2006 {unpublished data only}
- Pfizer Inc. Flexible dosing trial. NDA 21-928 [reported in CDER 2006] 2006.
Poling 2010 {published data only}
- Poling J, Rounsaville B, Gonsai K, Severino K, Sofuoglu M. The safety and efficacy of varenicline in cocaine using smokers maintained on methadone: a pilot study. American Journal on Addictions 2010;19:401-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramon 2014 {published data only}
- Ramon JM, Morchon S, Baena A, Masuet-Aumatell C. Combining varenicline and nicotine patches: a randomized controlled trial study in smoking cessation. BMC Medicine 2014;12(1):172. [CENTRAL: 1066789] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rose 2014 {unpublished data only}
- NCT01303861. Concurrent bupropion/varenicline for smoking cessation (ConNic4). clinicaltrials.gov/ct2/show/NCT01303861 (first received 25 February 2011).
- Rose JE, Behm FM. Combination treatment with varenicline and bupropion in an adaptive smoking cessation paradigm. American Journal of Psychiatry 2014;171(11):1199-205. [CENTRAL: 1021652] [EMBASE: 2014905509] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE, Behm FM. Combination varenicline/bupropion treatment benefits male NRT-nonresponders. Society for Research on Nicotine and Tobacco 19th Annual Meeting March 13-16 Boston MA 2013:261.
Schlienz 2014 {published data only}
- Schlienz NJ, Hawk LWJ, Tiffany ST, O'Connor RJ, Mahoney MC. The impact of pre-cessation varenicline on behavioral economic indices of smoking reinforcement. Addictive Behaviors 2014;39(10):1484-90. [CENTRAL: 993961] [EMBASE: 2014413435] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schmidt 1974 {published data only}
- Schmidt F. Medical support of nicotine withdrawal. Report on a double blind trial in over 5000 smokers (author's transl) [Medikamentose unterstutzung der raucherentwohnung: bericht uber versuche an uber 5000 rauchern im doppelblindversuch]. Munch Med Wachr 1974;116(11):557-64. [PubMed] [Google Scholar]
Schnoll 2011 {published data only}
- Schnoll RA, Cappella J, Lerman C, Pinto A, Patterson F, Wileyto EP, et al. A novel recruitment message to increase enrollment into a smoking cessation treatment program: preliminary results from a randomized trial. Health Communication 2011;26(8):735-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shim 2011 {published data only}
- Shim JC, Jung D, Oh M, Kong B, Ha T, Cho D, et al. Varenicline treatment for smoking cessation in people with schizophrenia: a randomized double-blind placebo-controlled trial. Schizophrenia Bulletin 2011;37:320-1. [Google Scholar]
- Shim JC, Jung DU, Jung SS, Seo YS, Cho DM, Lee JH, et al. Adjunctive varenicline treatment with antipsychotic medications for cognitive impairments in people with schizophrenia: a randomized double-blind placebo-controlled trial. Neuropsychopharmacology 2012;37(2):660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sicras‐Mainar 2010 {unpublished data only}
- Sicras-Mainar A, Navarro-Artieda R, Diaz-Cerezo S, Sanz de Burgoa V. Effectiveness of varenicline compared with bupropion and nicotine replacement therapy (NRT) for smoking cessation in two smoking specialized units in the primary care setting. Society for Research on Nicotine and Tobacco Europe Conference: Bath, UK, 6-9 October 2010.
Smith 2013 {unpublished data only}
- NCT00802919. Varenicline for cognitive deficits and cigarette smoking in schizophrenia. ClinicalTrials.gov/ct2/show/NCT00802919 (first received 5 December 2008).
- Smith RC, Amiaz R, Mei S, Maayan L, Jin H, Boules S, et al. Varenicline effects on smoking, cognition, and psychiatric symptoms in schizophrenia. Neuropsychopharmacology 2013;38:S364-5. [CENTRAL: 990503] [EMBASE: 71278474] [Google Scholar]
Stapleton 2008 {published data only}
- Stapleton JA, Watson L, Spirling LI, Smith R, Milbrandt A, Ratcliffe M, et al. Varenicline in the routine treatment of tobacco dependence: a pre-post comparison with nicotine replacement therapy and an evaluation in those with mental illness. Addiction 2008;103(1):146-54. [DOI] [PubMed] [Google Scholar]
Stoyanov 1972 {published data only}
- Stoyanov S, Yanachkova M. On the therapeutic effectiveness and tolerability of Tabex [Bulgarian]. Savremenna Medicina 1972;23(6):30-3. [Google Scholar]
Swan 2010 {published data only}
- Catz SL, Jack LM, McClure JB, Javitz HS, Deprey M, Zbikowski SM, et al. Adherence to varenicline in the COMPASS smoking cessation intervention trial. Nicotine & Tobacco Research 2011;13(5):361-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halperin AC, McAfee TA, Jack LM, Catz SL, McClure JB, Deprey TM, et al. Impact of symptoms experienced by varenicline users on tobacco treatment in a real world setting. Journal of Substance Abuse Treatment 2009;36:428-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure JB, Swan GE, Catz SL, Jack L, Javitz H, McAfee T, et al. Smoking outcome by psychiatric history after behavioral and varenicline treatment. Journal of Substance Abuse and Treatment 2010;38:394-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure JB, Swan GE, Jack L, Catz SL, Zbikowski SM, McAfee TA, et al. Mood, side-effects and smoking outcomes among persons with and without probably lifetime depression taking varenicline. Journal of General Internal Medicine 2009;24:563-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan GE, McClure JB, Jack LM, Zbikowski SM, Javitz HS, Catz SL, et al. Behavioral counseling and varenicline treatment for smoking cessation. American Journal of Preventive Medicine 2010;38(5):482-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zbikowski SM, Jack LM, McClure JB, Deprey M, Javitz HS, McAfee TA, et al. Utilization of services in a randomized trial testing phone- and web-based interventions for smoking cessation. Nicotine & Tobacco Research 2011;13(5):319-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tonstad 2006 {published data only}
- Bolin K, Mörk A-C, Wilson K. Smoking-cessation therapy using varenicline: the cost-utility of an additional 12-week course of varenicline for the maintenance of smoking abstinence. Journal of Evaluation in Clinical Practice 2009;15:478-85. [DOI] [PubMed] [Google Scholar]
- Hajek P, Tønneson P, Arteaga C, Russ C, Tonstad S. Varenicline in prevention of relapse to smoking: effect of quit pattern on response to extended treatment. Addiction 2009;104:1597-602. [DOI] [PubMed] [Google Scholar]
- Knight C, Howard P, Baker CL, Marton JP. The cost-effectiveness of an extended course (12 + 12 weeks) of varenicline compared with other available smoking cessation strategies in the United States: an extension and update to the BENESCO model. Value in Health 2010;13(2):209-14. [DOI] [PubMed] [Google Scholar]
- Knight CJ, Howard PA, Baker CL. An evaluation of the cost-effectiveness of an extended course of varenicline in preventing smokers who have quit from relapsing [PSM3]. Value in Health 2007;10(6):A472. [Google Scholar]
- Lee JH, Jones PG, Bybee K, O'Keefe JH. A longer course of varenicline therapy improves smoking cessation rates. Preventive Cardiology 2008;11(4):210-4. [DOI] [PubMed] [Google Scholar]
- O'Brien CP. Varenicline as maintenance therapy. Current Psychiatry Reports 2007;9(5):348-8. [DOI] [PubMed] [Google Scholar]
- Tonstad S, Tønnesen P, Hajek P, Williams KE, Billing CB, Reeves KR. Effect of maintenance therapy with varenicline on smoking cessation: a randomized controlled trial. JAMA 2006;296(1):64-71. [clinicaltrials.gov ID: NCT00143286] [DOI] [PubMed] [Google Scholar]
Tønnesen 2013 {published and unpublished data}
- NCT00977249. Varenicline for long-term NRT users. clinicaltrials.gov/ct2/show/NCT00977249 (first received 15 September 2009).
- Tønnesen P, Mikkelsen K. Varenicline to stop long-term nicotine replacement use: a double-blind, randomized, placebo-controlled trial. Nicotine & Tobacco Research 2013;15(2):419-27. [CENTRAL: 861602] [EMBASE: 2013064611] [PMID: ] [DOI] [PubMed] [Google Scholar]
Weiner 2011 {published data only}
- Weiner E, Buchholz A, Coffay A, Liu F, McMahon RP, Buchanan RW, et al. Varenicline for smoking cessation in people with schizophrenia: a double blind randomized pilot study. Schizophrenia Research 2011;129:94-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zatonski 2006 {unpublished data only}
- Zatonski W, Cedzynska M, Przewozniak K, Karpinska E, Lewandowska D, Pstrucha E, et al. An open label observational study of herbal cytisine (Tabex) as an aid to smoking cessation [POS1-058]. Society for Research on Nicotine and Tobacco 11th Annual Meeting, 20-23 March 2005; Prague, Czech Republic 2005.
- Zatonski W, Cedzynska M, Tutka P, West R. An uncontrolled trial of cytisine (Tabex) for smoking cessation. Tobacco Control 2006;15(6):481-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Wiratmoko 2013 {published data only}
- Wiratmoko MR, Yunus F, DSusanto A, Ginting TT, Kekalih A. Efficacy of varenicline, an nicotinic acetylcholine receptor partial agonist, vs placebo for smoking cessation. A randomized controlled trial. Respirology 2013;18 Suppl 4:66 [OS205]. [CENTRAL: 989836] [EMBASE: 71371580] [Google Scholar]
Yujie 2014 {published data only}
- Yujie W, Huliang L. Efficacy and safety of varenicline for smoking cessation in patients with CAD undergoing PCI. Journal of the American College of Cardiology 2014;64(16 Suppl 1):C207. [CENTRAL: 1054389] [EMBASE: 71665049] [Google Scholar]
References to ongoing studies
Berlin 2019 {published data only}
- Berlin I, Dautzenberg B, Lehmann B, Palmyre J, Liegey E, De Rycke Y, et al. Randomised, placebo-controlled, double-blind, double-dummy, multicentre trial comparing electronic cigarettes with nicotine to varenicline and to electronic cigarettes without nicotine: the ECSMOKE trial protocol. BMJ Open 2019;9(5):e028832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03630614. Randomized trial of electronic cigarettes with or without nicotine in smoking cessation. clinicaltrials.gov/ct2/show/NCT03630614 (first received 15 August 2018).
Caponnetto 2019 {published data only}
- Caponnetto P, Maglia M, Polosa R. Efficacy of smoking cessation with varenicline plus counselling for e-cigarettes users (VAREVAPE): a protocol for a randomized controlled trial. Contemporary Clinical Trials Communications 2019;15:100412. [DOI] [PMC free article] [PubMed] [Google Scholar]
ChiCTR1900021400 {published data only}
- ChiCTR1900021400. Individual tobacco cessation research based on nicotine metabolite ratio in smoking patients with chronic obstructive pulmonary disease: a randomized controlled trial. www.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR1900021400 2019.
IRCT20200719048133N1 {published data only}
- IRCT20200719048133N1. Effect of varenicline in smoking cessation after invasive treatment of coronary artery disease. en.irct.ir/trial/49723 (first received 23 July 2020).
Lawson 2021 {published data only}
- Lawson SC, Gass JC, Cooper RK Jr, Tonkin SS, Colder CR, Mahoney MC, et al. The impact of three weeks of pre-quit varenicline on reinforcing value and craving for cigarettes in a laboratory choice procedure. Psychopharmacology 2021;238(2):599-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03262662. EVarQuit: extended pre-quit varenicline to assist in quitting smoking. clinicaltrials.gov/show/NCT03262662 (first received 25 August 2017).
NCT00906386 {unpublished data only}
- NCT00906386. Methadone maintenance treatment and smoking cessation (MMTASC). clinicaltrials.gov/ct2/show/NCT00906386 (first received 21 May 2009).
NCT01243203 {published data only}
- NCT01243203. Smoking cessation program in the preadmission clinic: the use of a teachable moment. ClinicalTrials.gov/show/NCT01243203 (first received 18 November 2010).
NCT01312909 {unpublished data only}
- NCT01312909. Smoking cessation study in healthy adolescent smokers. clinicaltrials.gov/ct2/show/NCT01312909 (first received 11 March 2011).
NCT01800019 {published data only}
- NCT01800019. The Canadian HIV quit smoking trial: tackling the co-morbidities of depression and cardiovascular disease in HIV+ smokers. ClinicalTrials.gov/show/NCT01800019 (first received 27 February 2013).
NCT02106637 {published data only}
- NCT02106637. Early in-hospital initiation of pharmacotherapy for smoking cessation, concomitant with nurse-led support, in patients after an acute coronary syndrome (ACS). ClinicalTrials.gov/show/NCT02106637 (first received 8 April 2014).
NCT02162849 {published data only}
- NCT02162849. The effects of behavioral counseling plus nicotine replacement therapy (NRT) or varenicline on smoking cessation among smokers high and low in intrinsic reward sensitivity. ClinicalTrials.gov/show/NCT02162849 (first received 13 June 2014).
NCT02378714 {published data only}
- NCT02378714. Behavioral activation and varenicline for smoking cessation in depressed smokers. clinicaltrials.gov/show/NCT02378714 (first received 4 March 2015).
NCT02460900 {published data only}
- NCT02460900. Optimizing smoking cessation for people with HIV/AIDS who smoke. clinicaltrials.gov/show/NCT02460900 (first received 3 June 2015).
NCT02856581 {published data only}
- NCT02856581. Management of tobacco treatment intervention in reducing surgical complications in patients with newly diagnosed lung cancer who smoke cigarettes. clinicaltrials.gov/show/NCT02856581 (first received 5 August 2016).
NCT02991781 {published data only}
- NCT02991781. Combined bio- and neuro- feedback vs. varenicline use for smoking cessation. clinicaltrials.gov/show/NCT02991781 (first received 14 December 2016).
NCT03365362 {published data only}
- NCT03365362. A trial of directly observed and long-term varenicline. clinicaltrials.gov/show/NCT03365362 (first received 7 December 2017).
NCT03557294 {published data only}
- NCT03557294. Varenicline OTC trial on efficacy and safety. clinicaltrials.gov/show/NCT03557294 (first received 15 June 2018).
NCT04011280 {published data only}
- NCT04011280. Novel pharmacotherapy approaches in smokers with serious mental illness. clinicaltrials.gov/show/NCT04011280 first received 8 July 2019).
NCT04015414 {published data only}
- NCT04015414. Varenicline versus cytisine for smoking cessation in primary care setting. clinicaltrials.gov/show/NCT04015414 (first received 11 July 2019).
NCT04188873 {published data only}
- NCT04188873. Cessation screening project. clinicaltrials.gov/show/NCT04188873 (first received 6 December 2019).
NCT04525755 {published data only}
- NCT04525755. STARS (Smoking Treatment And Remote Sampling) study. clinicaltrials.gov/show/NCT04525755 (first received 25 August 2020).
NCT04604509 {published data only}
- NCT04604509. Nicotine replacement therapy, counseling, varenicline, and bupropion for smoking cessation, the PISCES I trial. clinicaltrials.gov/show/NCT04604509 (first received 27 October 2020).
NCT05102123 {published data only}
- NCT05102123. PeRiopEratiVE smokiNg cessaTion Trial (PREVENT). clinicaltrials.gov/show/NCT05102123 (first recieved 1 November 2021).
NCT05311085 {published data only}
- NCT05311085. Cytisine and e-cigarettes with supportive text-messaging for smoking cessation (Cess@Tion). clinicaltrials.gov/show/NCT05311085 (first received 5 April 2022).
Reid 2010 {unpublished data only}
- NCT00959972. Varenicline versus transdermal nicotine patch for smoking cessation in patients with coronary heart disease. clinicaltrials.gov/ct2/show/NCT00959972 (first received 17 August 2009).
- Reid RD, Armstrong A, McDonnell L, Aitken DA, Robert L, LaRue A, et al. Varenicline versus transdermal nicotine patch for smoking cessation in patients with coronary heart disease: a pilot randomized trial. Canadian Journal of Cardiology 2010;26 Suppl D:53D. [EMBASE: 70674067] [Google Scholar]
Russo 2021 {unpublished data only}
- EUCTR2009-017599-26-IT. Efficacy and safety of smoking cessation with varenicline tartrate in diabetic smokers: a double-blind, placebo-controlled, randomized trial. www.clinicaltrialsregister.eu/ctr-search/search?query=2009-017599-26 (first received 22 January 2010).
- NCT01387425. Efficacy and safety of smoking cessation with varenicline tartrate in diabetic smokers (DIASMOKE). clinicaltrials.gov/ct2/show/NCT01387425 (first received 4 July 2011).
- Russo C, Caponnetto P, Cibella F, Maglia M, Alamo A, Campagna D, et al. A double blind randomized controlled trial investigating efficacy and safety of varenicline for smoking cessation in patients with type 2 diabetes: study protocol. Internal & Emergency Medicine 2021;16(7):1823-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
TCTR20180312001 {published data only}
- TCTR20180312001. Efficacy safety and health-related quality of life (HRQoL) of cytisine in smoking cessation. www.who.int/trialsearch/Trial2.aspx?TrialID=TCTR20180312001 (first received 2017).
Tindle 2020 {published data only}
- NCT02797587. Studying partial-agonists for ethanol and tobacco elimination in Russians with HIV (St PETER HIV). clinicaltrials.gov/show/NCT02797587 (first received 13 June 2016).
- Tindle HA, Freiberg MS, Gnatienko N, Blokhina E, Cheng DM, Yaroslavtseva T, et al. Design of a randomized controlled trial of smoking cessation medications for alcohol reduction among HIV-positive heavy drinkers and daily smokers in St. Petersburg, Russia. Contemporary Clinical Trials Communications 2020;19:100625. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
ASH 2006
- Raw M, McNeill A, Arnott D. Varenicline: guidance for health professionals on a new prescription-only stop smoking medication. www.ash.org.uk/html/cessation/ASHVareniclineguidance.pdf (accessed November 2006).
ASH 2019
- Actions on Smoking and Health (ASH). Briefing: Health inequalities and smoking. ash.org.uk/uploads/ASH-Briefing_Health-Inequalities.pdf 2019.
Cahill 2013
- Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database of Systematic Reviews 2013, Issue 5. Art. No: CD009329. [DOI: 10.1002/14651858.CD009329.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Coe 2005
- Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, et al. Varenicline: an alpha4beta2 nAChR nicotinic receptor partial agonist for smoking cessation. Journal of Medicinal Chemistry 2005;48:3474-7. [DOI] [PubMed] [Google Scholar]
Davies 2017
- Davies NM, Thomas KH. The Food and Drug Administration and varenicline: should risk communication be improved? Addiction 2017;112(4):555-8. [DOI: 10.1111/add.13592.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2022
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Ebbert 2011a
- Ebbert JO, Montori VM, Erwin PJ, Stead LF. Interventions for smokeless tobacco use cessation. Cochrane Database of Systematic Reviews 2011, Issue 2. Art. No: CD004306. [DOI: 10.1002/14651858.CD004306.pub4] [DOI] [PubMed] [Google Scholar]
Etter 2006
- Etter J-F. Cytisine for smoking cessation: a literature review and a meta-analysis. Archives of Internal Medicine 2006;166:1-7. [DOI] [PubMed] [Google Scholar]
Etter 2007
- Etter JF, Burri M, Stapleton J. The impact of pharmaceutical company funding on results of randomized trials of nicotine replacement therapy for smoking cessation: a meta-analysis. Addiction 2007;102(5):815-22. [DOI] [PubMed] [Google Scholar]
Etter 2008
- Etter JF, Lukas RJ, Benowitz NL, West R, Dresler CM. Cytisine for smoking cessation: a research agenda. Drug and Alcohol Dependence 2008;92(1-3):3-8. [DOI] [PubMed] [Google Scholar]
Fabbri 2022
- Fabbri A, Nejstgaard CH, Grundy Q, Bero L, Dunn AG, Mohammad A, et al. Association between conflicts of interest and authors’ positions on harms of varenicline: a cross-sectional analysis. Journal of General Internal Medicine 2022;37(2):290-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
FDA 2008
- US Food and Drug Administration. FDA issues Public Health Advisory on Chantix. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2008/ucm116849.htm (accessed 3 September 2015).
Foulds 2004
- Foulds J, Burke M, Steinberg M, Williams JM, Ziedonis DM. Advances in pharmacotherapy for tobacco dependence. Expert Opinion on Emerging Drugs 2004;9(1):39-53. [DOI] [PubMed] [Google Scholar]
Gotti 2021
- Gotti C, Clementi F. Cytisine and cytisine derivatives. More than smoking cessation aids. Pharmacological Research 2021;170:105700. [DOI: 10.1016/j.phrs.2021.105700] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;7414:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook.
Hughes 2003
- Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine & Tobacco Research 2003;5(1):13-25. [PubMed] [Google Scholar]
Kirchhoff 2009
- Kirchhoff VD, Nguyen HT, Soczynska JK, Woldeyohannes H, McIntyre RS. Discontinued psychiatric drugs in 2008. Expert Opinion on Investigational Drugs 2009;18(10):1431-43. [DOI] [PubMed] [Google Scholar]
Leaviss 2014
- Leaviss J, Sullivan W, Ren S, Everson-Hock E, Stevenson M, Stevens JW, et al. What is the clinical effectiveness and cost-effectiveness of cytisine compared with varenicline for smoking cessation? a systematic review and economic evaluation. Health Technology Assessment 2014;18(33):1-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2016
- Sterling LH, Windle SB, Filion KB, Touma L, Eisenberg MJ. Varenicline and adverse cardiovascular events: a systematic review and meta-analysis of randomized controlled trials. Journal of the American Heart Association 2016;5:e002849. [DOI: 10.1161/JAHA.115.002849] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLOS One 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lindson 2022
- Lindson N, Theodoulou A, Livingstone-Banks J, Aveyard P, Fanshawe TR, Ordóñez-Mena JM, et al. Pharmacological and electronic cigarette interventions for smoking cessation in adults: component network meta‐analyses. Cochrane Database of Systematic Reviews 2022, Issue 3. Art. No: CD015226. [DOI: 10.1002/14651858.CD015226] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lindson‐Hawley 2016
- Lindson-Hawley N, Hartmann-Boyce J, Fanshawe TR, Begh R, Farley A, Lancaster T. Interventions to reduce harm from continued tobacco use. Cochrane Database of Systematic Reviews 2016, Issue 10. Art. No: CD005231. [DOI: 10.1002/14651858.CD005231.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Livingstone‐Banks 2019
- Livingstone-Banks J, Norris E, Hartmann-Boyce J, West R, Jarvis M, Chubb E, et al. Relapse prevention interventions for smoking cessation. Cochrane Database of Systematic Reviews 2019, Issue 10. Art. No: CD003999. [DOI: 10.1002/14651858.CD003999.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Livingstone‐Banks 2022a
- Livingstone-Banks J, Lindson N, Hartmann-Boyce J, Aveyard P. Effects of interventions to combat tobacco addiction: Cochrane update of 2019 and 2020 reviews. Addiction 2022;117(6):1573-88. [DOI: 10.1111/add.15769] [DOI] [PubMed] [Google Scholar]
Livingstone‐Banks 2022b
- Livingstone-Banks J, Siddiqui F, Croucher R, Mehrotra R, Vidyasagaran A, Siddiqi K. Interventions for smokeless tobacco use cessation. Cochrane Database of Systematic Reviews 2022, Issue 1. Art. No: CD015314. [DOI: 10.1002/14651858.CD015314] [DOI] [Google Scholar]
Mills 2012
- Mills EJ, Wu P, Lockhart I, Thorlund K, Puhan M, Ebbert JO. Comparisons of high-dose and combination nicotine replacement therapy, varenicline and bupropion for smoking cessation: a systematic review and multiple-treatment meta-analysis. Annals of Medicine 2012;44(6):588-97. [DOI] [PubMed] [Google Scholar]
NICE 2007
- National Institute for Health and Clinical Excellence. Varenicline for smoking cessation. www.nice.org.uk/nicemedia/pdf/TA123Guidance.pdf (accessed 14th April 2008) 2007.
Rigotti 2022
- Rigotti NA, Kruse GR, Livingstone-Banks J, Hartmann-Boyce J. Treatment of tobacco smoking: a review. JAMA 2022;327(6):566-77. [10.1001/jama.2022.0395] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Schünemann 2022
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Shiffman 2004
- Shiffman S, West R, Gilbert D. Recommendations for the assessment of tobacco craving and withdrawal in smoking cessation trials. Nicotine & Tobacco Research 2004;6(4):599-614. [DOI] [PubMed] [Google Scholar]
Stead 2003
- Stead LF, Hughes JR. Lobeline for smoking cessation. Cochrane Database of Systematic Reviews 2003, Issue 3. Art. No: CD000124. [DOI: 10.1002/14651858.CD000124] [DOI] [PubMed] [Google Scholar]
Thomas 2015
- Thomas KH, Martin RM, Knipe DW, Higgins JP, Gunnell D. Risk of neuropsychiatric adverse events associated with varenicline: systematic review and meta-analysis. BMJ 2015;350:h1109. [PROSPERO 2014:CRD42014009224] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomas 2020
- Thomas, KH, Dalili, MN, López-López, JA, Keeney, E, Phillippo, DM, Munafò, MR, et al. Comparative clinical effectiveness and safety of tobacco cessation pharmacotherapies and electronic cigarettes: a systematic review and network meta-analysis of randomized controlled trials. Addiction 2020;117:861-76. [DOI: 10.1111/add.15675] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tutka 2005
- Tutka P, Zatoński W. Cytisine for the treatment of nicotine addiction: from a molecule to therapeutic efficacy. Pharmacological Reports 2005;58:777-98. [PubMed] [Google Scholar]
Tutka 2006
- Tutka P, Mróz T, Zatoński W. Cytisine - renaissance of well known alkaloid. Pharmacological aspects of efficacy in the treatment of tobacco dependence [Cytyzyna – renesans znanego alkaloidu. Aspekty farmakologiczne zastosowania w leczeniu uzależnienia od nikotyny]. Farmakoterapia w Psychiatrii i Neurologii 2006;1:33-9. [Google Scholar]
Tutka 2008
- Tutka P. Nicotinic receptor partial agonists as novel compounds for the treatment of smoking cessation. Expert Opinion on Investigational Drugs 2008;17(10):1473-85. [DOI] [PubMed] [Google Scholar]
Tutka 2019
- Tutka P, Vinnikov D, Courtney RJ, Benowitz NL. Cytisine for nicotine addiction treatment: a review of pharmacology, therapeutics and an update of clinical trial evidence for smoking cessation. Addiction 2019;114(11):1951-69. [DOI: 10.1111/add.14721] [DOI] [PubMed] [Google Scholar]
USDHHS 2020
- US Department of Health and Human Services. Smoking cessation: a report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2020. [Google Scholar]
Walsh 2011
- Walsh RA. Australia's experience with varenicline: usage, costs and adverse reactions [letter]. Addiction 2011;106:449-52. [DOI] [PubMed] [Google Scholar]
West 2005
- West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction 2005;100:299-303. [DOI] [PubMed] [Google Scholar]
WHO 2021
- World Health Organization. Two new tobacco cessation medicines added to the WHO essential medicines list. www.who.int/news/item/05-11-2021-two-new-tobacco-cessation-medicines-added-to-the-who-essential-medicines-list (accessed 16 November 2022).
WHO 2022
- World Health Organization. World Health Organization fact sheets: Tobacco. www.who.int/en/news-room/fact-sheets/detail/tobacco (accessed 16 November 2022).
References to other published versions of this review
Cahill 2007
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database of Systematic Reviews 2007, Issue 1. Art. No: CD006103. [DOI: 10.1002/14651858.CD006103.pub2] [DOI] [PubMed] [Google Scholar]
Cahill 2008
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database of Systematic Reviews 2008, Issue 3. Art. No: CD006103. [DOI: 10.1002/14651858.CD006103.pub3] [DOI] [PubMed] [Google Scholar]
Cahill 2010
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database of Systematic Reviews 2010, Issue 2. Art. No: CD006103. [DOI: 10.1002/14651858.CD006103.pub5] [DOI] [PubMed] [Google Scholar]
Cahill 2012
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database of Systematic Reviews 2012, Issue 4. Art. No: CD006103. [DOI: 10.1002/14651858.CD006103.pub6] [DOI] [PubMed] [Google Scholar]
Cahill 2016
- Cahill K, Lindson-Hawley N, Thomas KH, Fanshawe TR, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database of Systematic Reviews 2016, Issue 5. Art. No: CD006103. [DOI: 10.1002/14651858.CD006103.pub7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hey 2006
- Hey K, Lancaster T, Bala M. Nicotine receptor partial agonists for smoking cessation. Cochrane Database of Systematic Reviews 2006, Issue 3. Art. No: CD006103. [DOI: 10.1002/14651858.CD006103] [DOI] [PubMed] [Google Scholar]


