Abstract
Background
Chronic pain is common in adults, and often has a detrimental impact upon physical ability, well‐being, and quality of life. Previous reviews have shown that certain antidepressants may be effective in reducing pain with some benefit in improving patients’ global impression of change for certain chronic pain conditions. However, there has not been a network meta‐analysis (NMA) examining all antidepressants across all chronic pain conditions.
Objectives
To assess the comparative efficacy and safety of antidepressants for adults with chronic pain (except headache).
Search methods
We searched CENTRAL, MEDLINE, Embase, CINAHL, LILACS, AMED and PsycINFO databases, and clinical trials registries, for randomised controlled trials (RCTs) of antidepressants for chronic pain conditions in January 2022.
Selection criteria
We included RCTs that examined antidepressants for chronic pain against any comparator. If the comparator was placebo, another medication, another antidepressant, or the same antidepressant at different doses, then we required the study to be double‐blind. We included RCTs with active comparators that were unable to be double‐blinded (e.g. psychotherapy) but rated them as high risk of bias. We excluded RCTs where the follow‐up was less than two weeks and those with fewer than 10 participants in each arm.
Data collection and analysis
Two review authors separately screened, data extracted, and judged risk of bias. We synthesised the data using Bayesian NMA and pairwise meta‐analyses for each outcome and ranked the antidepressants in terms of their effectiveness using the surface under the cumulative ranking curve (SUCRA). We primarily used Confidence in Meta‐Analysis (CINeMA) and Risk of Bias due to Missing Evidence in Network meta‐analysis (ROB‐MEN) to assess the certainty of the evidence. Where it was not possible to use CINeMA and ROB‐MEN due to the complexity of the networks, we used GRADE to assess the certainty of the evidence.
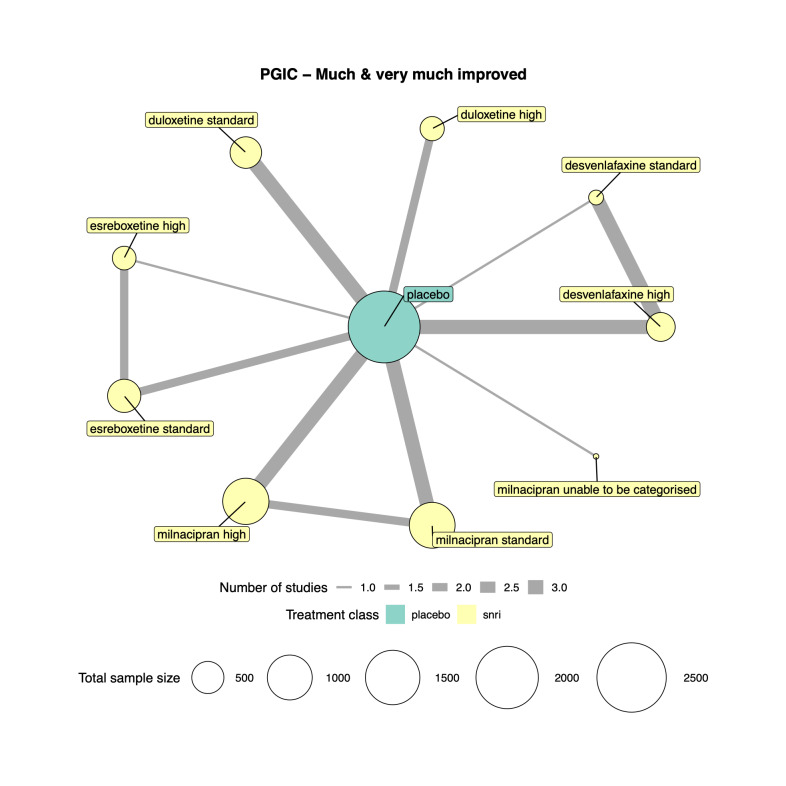
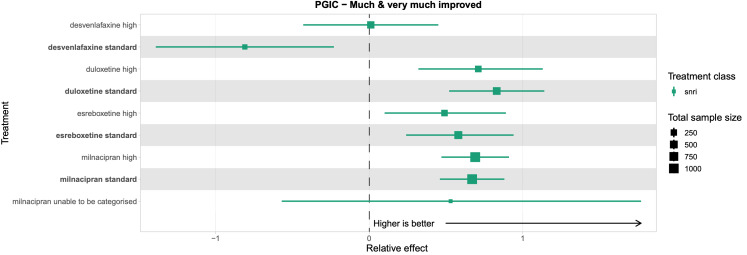
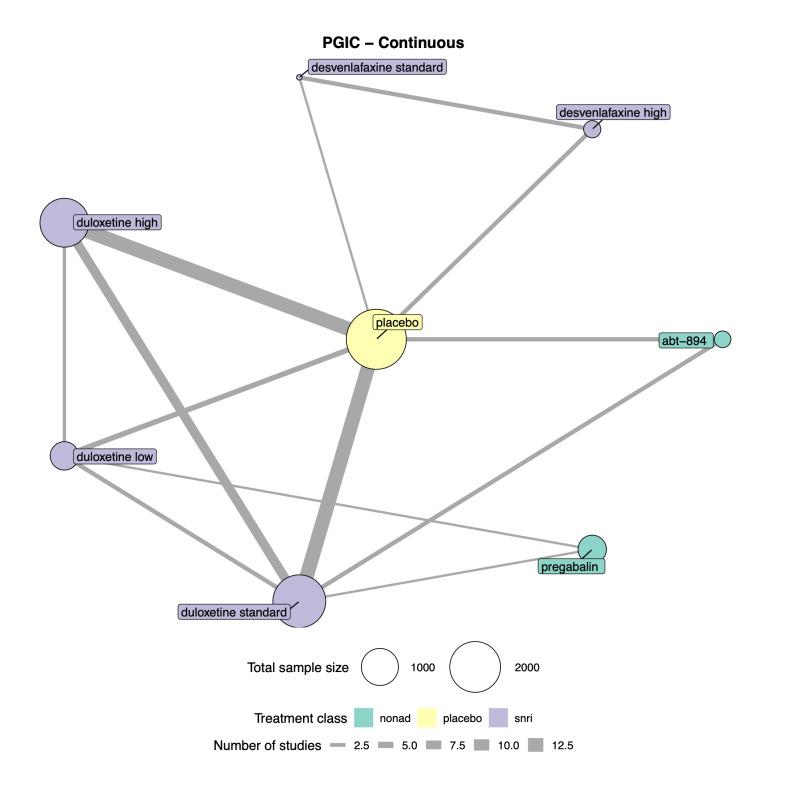
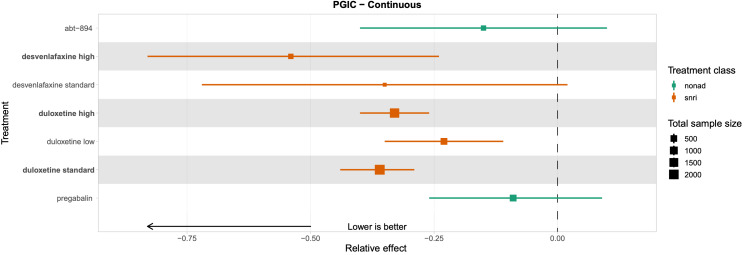
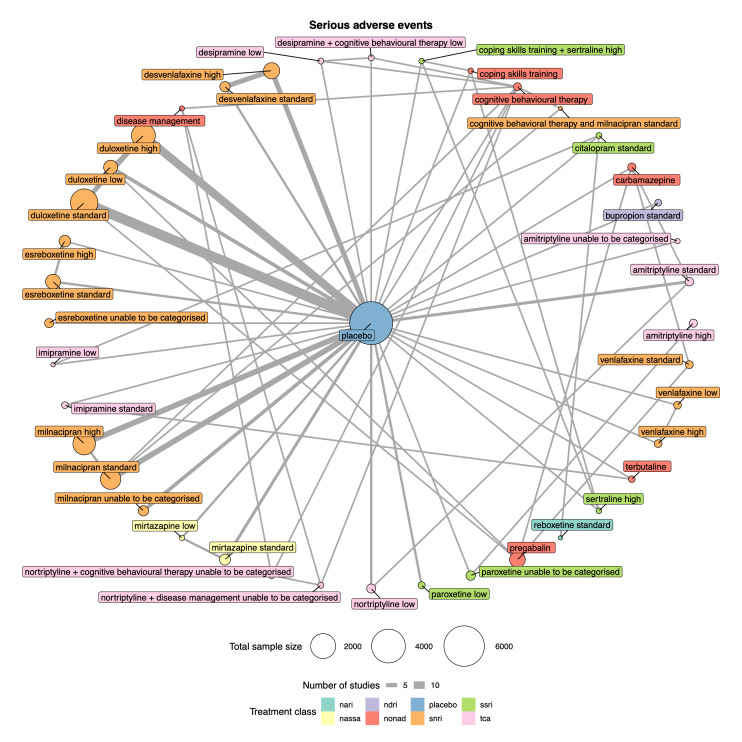
Our primary outcomes were substantial (50%) pain relief, pain intensity, mood, and adverse events. Our secondary outcomes were moderate pain relief (30%), physical function, sleep, quality of life, Patient Global Impression of Change (PGIC), serious adverse events, and withdrawal.
Main results
This review and NMA included 176 studies with a total of 28,664 participants. The majority of studies were placebo‐controlled (83), and parallel−armed (141). The most common pain conditions examined were fibromyalgia (59 studies); neuropathic pain (49 studies) and musculoskeletal pain (40 studies). The average length of RCTs was 10 weeks. Seven studies provided no useable data and were omitted from the NMA. The majority of studies measured short‐term outcomes only and excluded people with low mood and other mental health conditions.
Across efficacy outcomes, duloxetine was consistently the highest‐ranked antidepressant with moderate‐ to high‐certainty evidence. In duloxetine studies, standard dose was equally efficacious as high dose for the majority of outcomes. Milnacipran was often ranked as the next most efficacious antidepressant, although the certainty of evidence was lower than that of duloxetine. There was insufficient evidence to draw robust conclusions for the efficacy and safety of any other antidepressant for chronic pain.
Primary efficacy outcomes
Duloxetine standard dose (60 mg) showed a small to moderate effect for substantial pain relief (odds ratio (OR) 1.91, 95% confidence interval (CI) 1.69 to 2.17; 16 studies, 4490 participants; moderate‐certainty evidence) and continuous pain intensity (standardised mean difference (SMD) −0.31, 95% CI −0.39 to −0.24; 18 studies, 4959 participants; moderate‐certainty evidence). For pain intensity, milnacipran standard dose (100 mg) also showed a small effect (SMD −0.22, 95% CI −0.39 to 0.06; 4 studies, 1866 participants; moderate‐certainty evidence). Mirtazapine (30 mg) had a moderate effect on mood (SMD −0.5, 95% CI −0.78 to −0.22; 1 study, 406 participants; low‐certainty evidence), while duloxetine showed a small effect (SMD −0.16, 95% CI −0.22 to −0.1; 26 studies, 7952 participants; moderate‐certainty evidence); however it is important to note that most studies excluded participants with mental health conditions, and so average anxiety and depression scores tended to be in the 'normal' or 'subclinical' ranges at baseline already.
Secondary efficacy outcomes
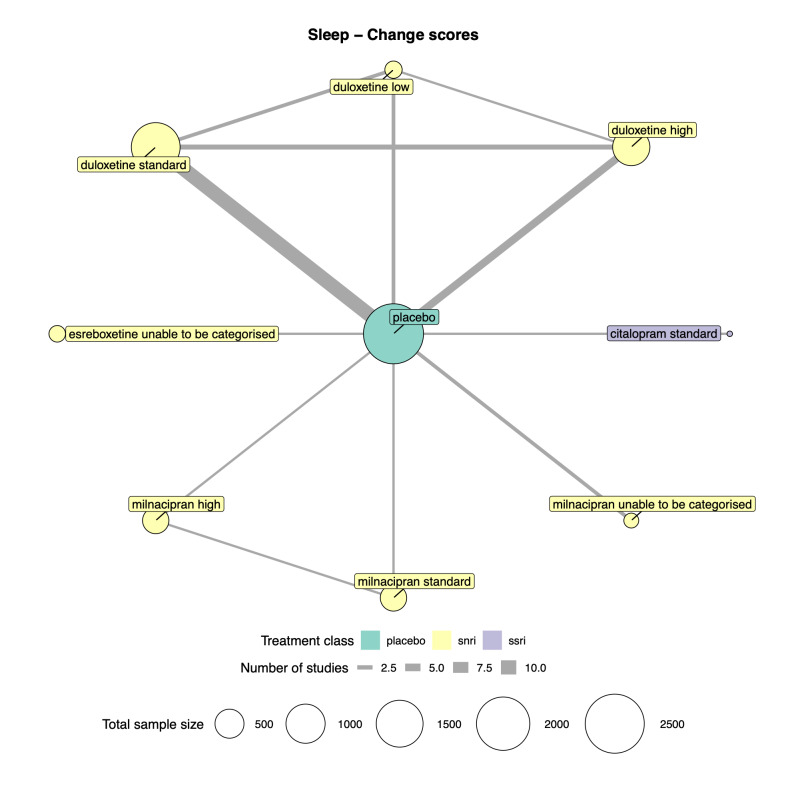
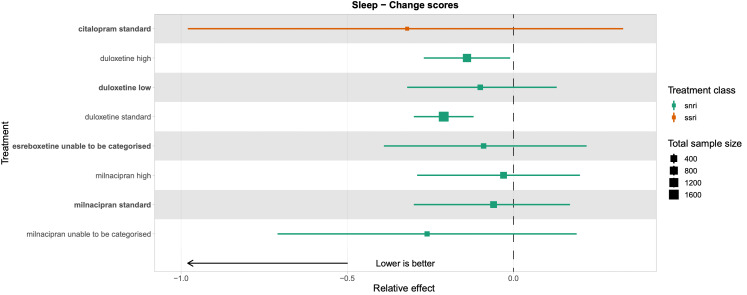
Across all secondary efficacy outcomes (moderate pain relief, physical function, sleep, quality of life, and PGIC), duloxetine and milnacipran were the highest‐ranked antidepressants with moderate‐certainty evidence, although effects were small. For both duloxetine and milnacipran, standard doses were as efficacious as high doses.
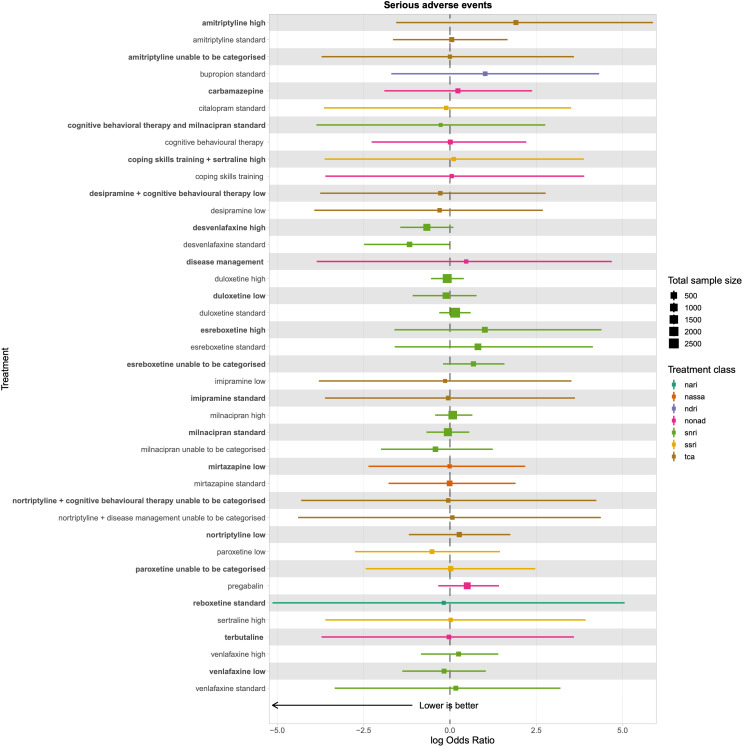
Safety
There was very low‐certainty evidence for all safety outcomes (adverse events, serious adverse events, and withdrawal) across all antidepressants. We cannot draw any reliable conclusions from the NMAs for these outcomes.
Authors' conclusions
Our review and NMAs show that despite studies investigating 25 different antidepressants, the only antidepressant we are certain about for the treatment of chronic pain is duloxetine. Duloxetine was moderately efficacious across all outcomes at standard dose. There is also promising evidence for milnacipran, although further high‐quality research is needed to be confident in these conclusions. Evidence for all other antidepressants was low certainty. As RCTs excluded people with low mood, we were unable to establish the effects of antidepressants for people with chronic pain and depression. There is currently no reliable evidence for the long‐term efficacy of any antidepressant, and no reliable evidence for the safety of antidepressants for chronic pain at any time point.
Keywords: Adult, Humans, Antidepressive Agents, Antidepressive Agents/therapeutic use, Chronic Pain, Chronic Pain/drug therapy, Duloxetine Hydrochloride, Milnacipran, Network Meta-Analysis, Pain Management, Randomized Controlled Trials as Topic
Plain language summary
How effective are antidepressants used to treat chronic pain and do they cause unwanted effects?
Key messages
• We are only confident in the effectiveness of one antidepressant: duloxetine. We found that a standard dose (60 mg) was effective, and that there is no benefit to using a higher dose.
• We are uncertain about unwanted effects for any antidepressant as the data for this were very poor. Future research should address this.
• In clinical practice for chronic pain, a standard dose of duloxetine may be considered before trying other antidepressants.
• Adopting a person‐centred approach is critical. Pain is a very individual experience and certain medications may work for people even while the research evidence is inconclusive or unavailable. Future studies should last longer and focus on unwanted effects of antidepressants.
What is chronic pain?
Chronic pain is pain of any kind that lasts for more than three months. Over one‐third of people across the world experience chronic pain. This often affects people's mood and well‐being, and their ability to work and carry out daily tasks.
How do antidepressants treat chronic pain?
Antidepressants are medications originally developed to treat depression. Different types of antidepressants work in different ways. Antidepressants that work in the same way are grouped into classes. The most common classes are selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants (TCAs), and serotonin‐noradrenalin reuptake inhibitors (SNRIs). Research suggests that antidepressants may be effective for pain because the same chemicals that affect mood might also affect pain.
What did we want to find out?
We wanted to find out if antidepressants were effective for managing chronic pain and whether they cause unwanted effects.
What did we do?
We searched for studies that compared any antidepressant with any other treatment for any type of chronic pain (except headache). We compared all the treatments against each other using a statistical method called network meta‐analysis. This method allows us to rank the effectiveness of the different antidepressants from best to worst.
What did we find?
We found 176 studies including 28,664 people with chronic pain. These studies investigated 89 different types or combinations of treatment. Studies mainly investigated the effect of antidepressants on three different types of pain: fibromyalgia (59 studies), nerve pain (49 studies), and musculoskeletal pain (e.g. osteoarthritis or low back pain; 40 studies). The most common antidepressant classes investigated were SNRIs (74 studies), TCAs (72 studies), and SSRIs (34 studies). The most common antidepressants investigated were: amitriptyline (a TCA; 43 studies); duloxetine (an SNRI; 43 studies), and milnacipran (an SNRI; 18 studies). Of the 146 studies that reported where their funding came from, pharmaceutical companies funded 72 studies. The average study lasted 10 weeks.
Most of the studies compared an antidepressant with a placebo (which looks like the real medicine but doesn’t have any medicine in it), but some studies compared an antidepressant against a different type of medicine, a different antidepressant, a different type of treatment (like physiotherapy), or different doses of the same antidepressant.
Most of the studies in this review reported information on pain relief and unwanted effects. Fewer studies reported on quality of life, sleep, and physical function.
Main results
• Duloxetine probably has a moderate effect on reducing pain and improving physical function. It was the antidepressant that we have the most confidence in. Higher doses of duloxetine probably provided no extra benefits than standard doses. For every 1000 people taking standard‐dose duloxetine, 435 will experience 50% pain relief compared with 287 who will experience 50% pain relief taking placebo.
• Milnacipran may reduce pain, but we are not as confident in this result as duloxetine because there were fewer studies with fewer people involved.
• Most studies excluded people with mental health conditions, meaning that participants were already in the 'normal' ranges for anxiety and depression at the beginning of studies. This limited our analysis for mood. Mirtazapine and duloxetine may improve mood, but we are very uncertain about the results.
• We do not know about unwanted effects of using antidepressants for chronic pain; there are not enough data to be certain about the results.
What are the limitations of the evidence?
There are still a number of questions that we were unable to answer:
• Aside from duloxetine and milnacipran, we do not have confidence in the results from any other antidepressant included in this review because there are not enough studies.
• We do not know whether antidepressants are effective at treating pain in the long term. The average length of studies was 10 weeks.
• There was no reliable evidence on the safety of taking antidepressants for chronic pain, both short‐ and long‐term.
• We do not know how effective antidepressants are for people with both chronic pain and depression as the most studies excluded participants with depression and anxiety.
How up to date is this evidence?
This review is up to date to January 2022.
Summary of findings
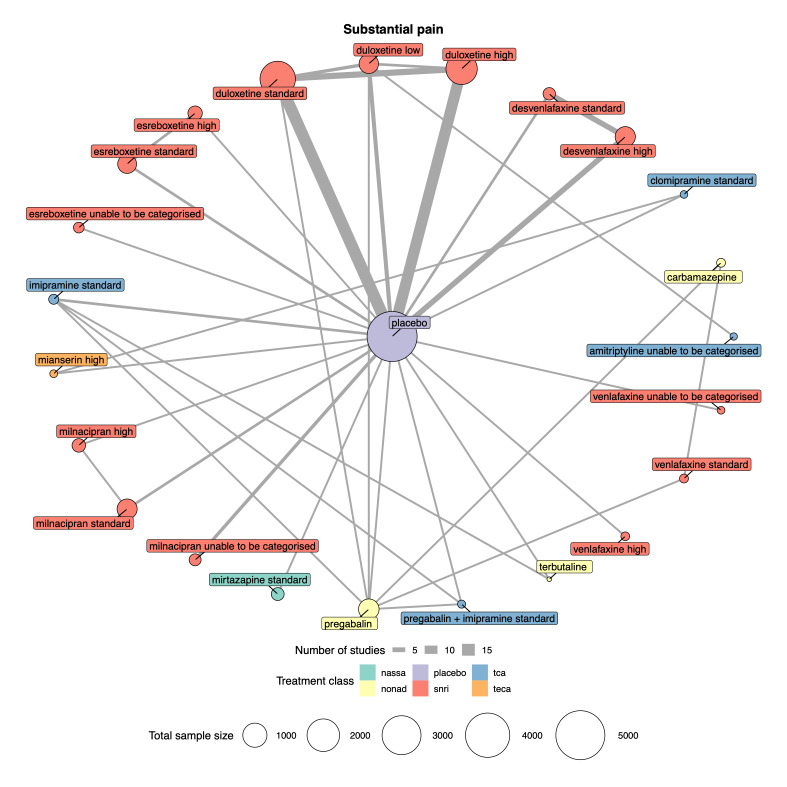
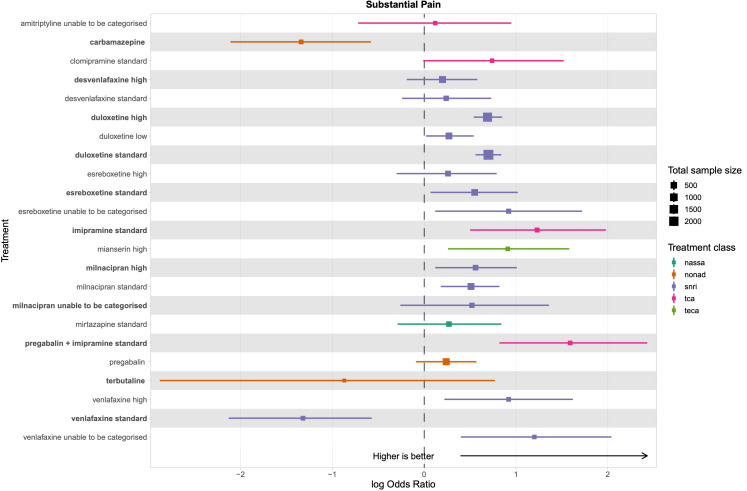
Summary of findings 1. Substantial pain relief summary of findings.
| Estimates of effects, credible intervals, and certainty of the evidence for substantial pain relief in people with chronic pain | |||||||
| Bayesian network meta‐analysis summary of findings table | |||||||
|
Patient or population: people with chronic pain Interventions: desvenlafaxine high dose (≥ 50 mg); duloxetine low dose (< 60 mg), standard dose (60 mg), and high dose (> 60 mg); esreboxetine standard dose (4‐8 mg) and high dose (≥ 8 mg); milnacipran standard dose (100 mg) and high dose (> 100 mg); mirtazapine standard dose (30 mg) Comparator (reference): placebo Outcome: substantial pain relief (≥ 50% reduction in pain intensity from baseline) as measured on various scales including 0‐10 VAS, 0‐100 VAS, and the Brief Pain Inventory Direction: higher is better (i.e. more people reporting substantial pain relief) | |||||||
|
Total studies: 42 Total participants: 14,626 |
Relative effect (OR and 95% CI) |
Anticipated absolute effect (event rate)* | Certainty of the evidence (CINeMA) |
Ranking** (2.5% to 97.5% credible interval) |
Interpretation of findings | ||
| With placebo | With intervention | Difference | |||||
|
Duloxetine standard dose RCTs: 16 Participants: 4490 |
1.91 (1.69 to 2.17) |
592/2061 287 per 1000 |
1058/2429 435 per 1000 |
148 more per 1000 | Moderatea | 8 (5 to 12) |
Equivalent to NNTB of 7.1 |
|
Duloxetine high dose RCTs: 14 Participants: 3692 |
1.91 (1.66 to 2.21) |
431/1855 232 per 1000 |
674/1837 366 per 1000 |
134 more per 1000 | Moderatea | 8 (5 to 12) |
Equivalent to NNTB of 7.4 |
|
Milnacipran high dose RCTs: 1 Participants: 384 |
1.64 (1.04 to 2.58) |
38/145 262 per 1000 |
88/239 368 per 1000 |
106 more per 1000 | Very lowa,b | 11 (4 to 19) |
Equivalent to NNTB of 9.4 |
|
Esreboxetine standard dose RCTs: 1 Participants: 828 |
1.72 (1.13 to 2.62) |
33/275 120 per 1000 |
105/553 190 per 1000 |
70 more per 1000 | Lowa | 11 (4 to 19) |
Equivalent to NNTB of 14 |
|
Milnacipran standard dose RCTs: 2 Participants: 1298 |
1.65 (1.28 to 2.13) |
130/654 199 per 1000 |
187/644 290 per 1000 |
91 more per 1000 | Lowa,c | 12 (6 to 18) |
Equivalent to NNTB of 11 |
|
Mirtazapine standard dose RCTs: 1 Participants: 422 |
1.30 (0.79 to 2.15) |
33/211 156 per 1000 |
41/211 194 per 1000 |
39 more per 1000 | Lowe | 15 (6 to 21) |
Not significantly different from placebo |
|
Duloxetine low dose RCTs: 6 Participants: 1116 |
1.71 (1.36 to 2.20) |
150/523 287 per 1000 |
242/593 407 per 1000 |
120 more per 1000 | Moderatea,b,c | 16 (11 to 20) |
Equivalent to NNTB of 8.3 |
|
Esreboxetine high dose RCTs: 1 Participants: 555 |
1.29 (0.79 to 2.11) |
33/275 120 per 1000 |
42/280 150 per 1000 |
30 more per 1000 | Very lowa,b | 16 (7 to 22) |
Not significantly different from placebo |
|
Desvenlafaxine high dose RCTs: 2 Participants: 870 |
1.19 (0.83 to 1.70) |
51/215 237 per 1000 |
177/655 270 per 1000 |
33 more per 1000 | Very lowa,b | 17 (11 to 21) |
Not significantly different from placebo |
|
Network meta‐analysis‐summary of findings table definitions *Anticipated absolute effect. Anticipated absolute effect compares 2 risks by calculating the difference between the risk of the intervention group with the risk of the control group. ** Mean rank and credible intervals are presented. CI: confidence interval; CINeMA: Confidence in Network Meta‐Analysis; NNTB: number needed to treat for an additional beneficial outcome; OR: odds ratio; RCT: randomised controlled trial; VAS: visual analogue scale The number of participants for each antidepressant reflects the total number of participants taking the antidepressant or placebo from the studies in the network meta‐analysis. | |||||||
|
CINeMA grades of confidence in the evidence High: further research is unlikely to change our confidence in the estimate of effect. Moderate: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low: we are very uncertain about the estimate. | |||||||
aDowngraded due to within‐study bias. bDowngraded due to imprecision in the estimate. cDowngraded due to heterogeneity in the estimate. dDowngraded due to incoherence in the network. eDowngraded due to a small number of trials and participants; we cannot draw reliable conclusions.
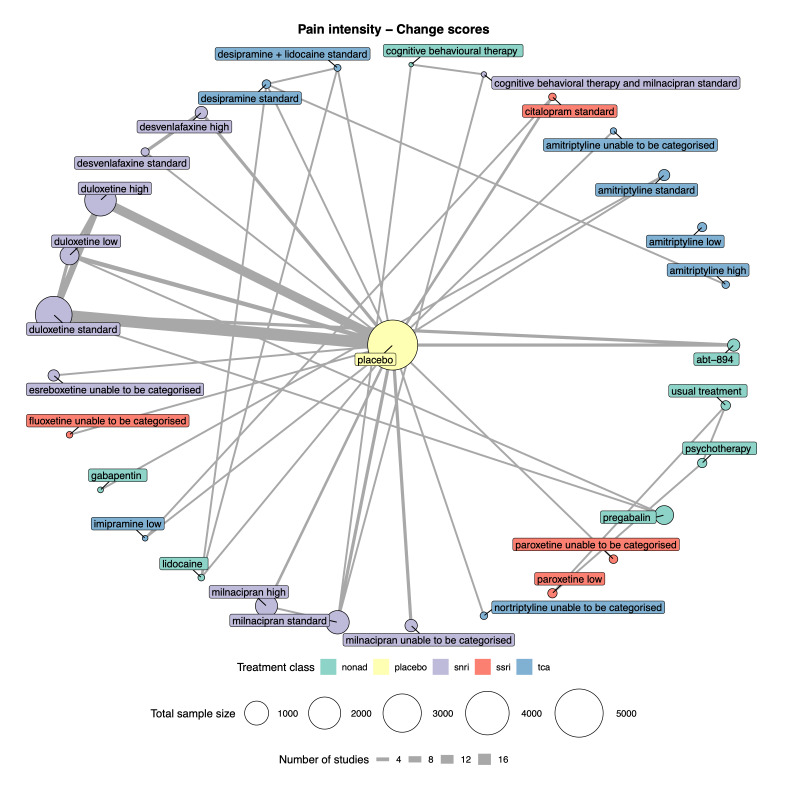
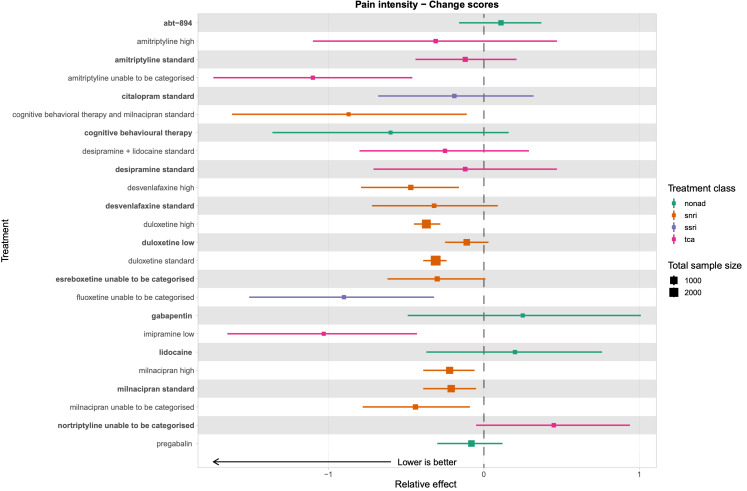
Summary of findings 2. Pain intensity summary of findings.
| Estimates of effects, credible intervals, and certainty of the evidence for pain intensity in people with chronic pain | |||||||
| Bayesian network meta‐analysis summary of findings table | |||||||
|
Patient or population: people with chronic pain Interventions: duloxetine low dose (< 60 mg), standard dose (60 mg), and high dose (> 60 mg); milnacipran standard dose (100 mg) and high dose (> 100 mg) Comparator (reference): placebo Outcome: change in pain intensity, as measured on multiple scales including 0‐10 VAS, 0‐100 VAS, Brief Pain Inventory, and the Short‐form McGill Pain Questionnaire Direction: lower is better (i.e. a greater reduction in pain intensity) | |||||||
|
Total studies: 50 Total participants: 14,926 |
Relative effect | Anticipated absolute effect (event rate) | Certainty of the evidence (CINeMA) |
Ranking* (2.5% to 97.5% credible interval) |
Interpretation of findings** | ||
| With placebo | With intervention | Difference | |||||
|
Duloxetine high dose RCTs: 14 Participants: 3683 |
‐ | ‐ | ‐ |
SMD −0.37 (−0.45 to −0.28) |
Lowa,b | 9 (8 to 13) |
Small to moderate effect |
|
Duloxetine standard dose RCTs: 18 Participants: 4959 |
‐ | ‐ | ‐ |
SMD −0.31 (−0.39 to −0.24) |
Moderateb | 11 (10 to 15) |
Small to moderate effect |
|
Milnacipran high dose RCTs: 2 Participants: 1670 |
‐ | ‐ | ‐ |
SMD −0.22 (−0.40 to −0.05) |
Lowa,c | 14 (12 to 19) |
Small effect |
|
Milnacipran standard dose RCTs: 4 Participants: 1866 |
‐ | ‐ | ‐ |
SMD −0.22 (−0.39 to −0.06) |
Moderatea,b | 14 (12 to 20) |
Small effect |
|
Duloxetine low dose RCTs: 6 Participants: 1104 |
‐ | ‐ | ‐ |
SMD −0.11 (−0.25 to 0.03) |
Moderatea,c | 17 (12 to 21) |
Not significantly different from placebo |
|
Network meta‐analysis‐summary of findings table definitions *Mean rank and credible intervals are presented. **SMD interpretation based on clinical judgement and in line with Cohen 1988 and the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2022) as small (0.2), moderate (0.5) and large (0.8). CI: confidence interval; CINeMA: Confidence in Network Meta‐Analysis; RCT: randomised controlled trial; SMD: standardised mean difference; VAS: visual analogue scale The number of participants for each antidepressant reflects the total number of participants taking the antidepressant or placebo from the studies in the network meta‐analysis. | |||||||
|
CINeMA grades of confidence in the evidence High: further research is unlikely to change our confidence in the estimate of effect. Moderate: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low: we are very uncertain about the estimate. | |||||||
aDowngraded due to within‐study bias. bDowngraded due to imprecision in the estimate. cDowngraded due to heterogeneity in the estimate. dDowngraded due to incoherence in the network. eDowngraded due to a small number of trials and participants; we cannot draw reliable conclusions.
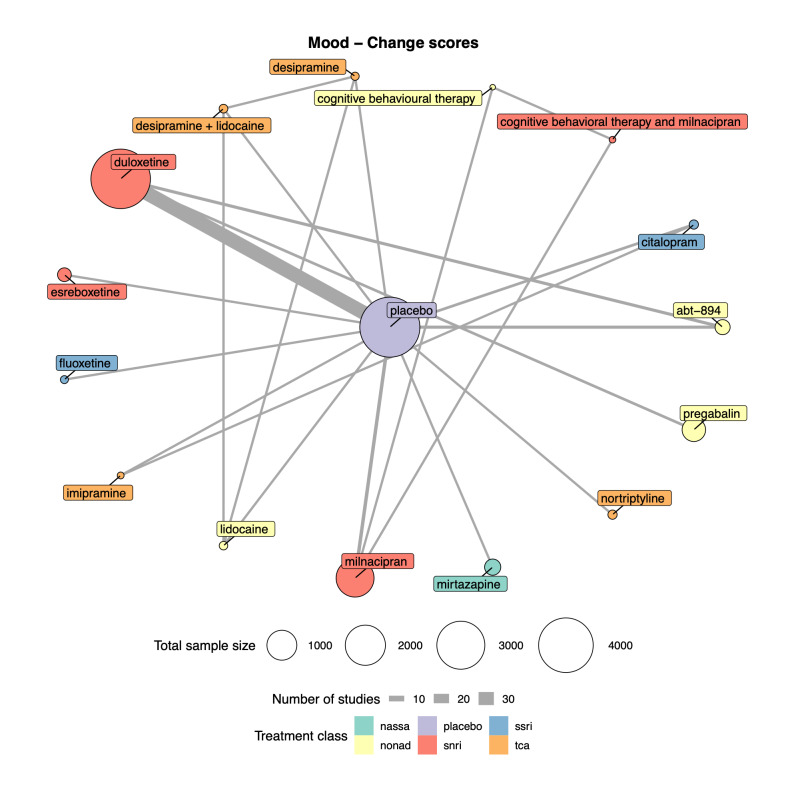
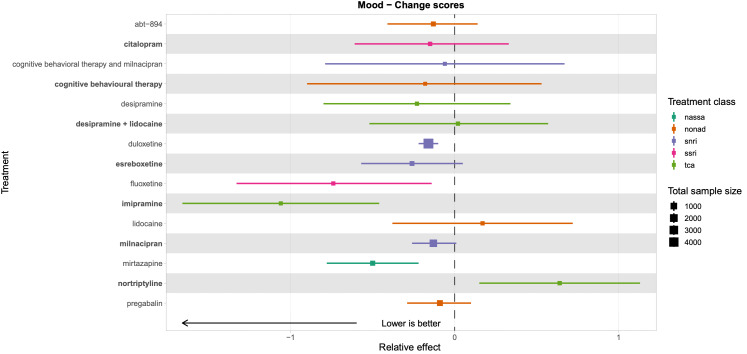
Summary of findings 3. Mood summary of findings.
| Estimates of effects, credible intervals, and certainty of the evidence of antidepressants on mood in people with chronic pain | |||||||
| Bayesian network meta‐analysis summary of findings table | |||||||
|
Patient or population: people with chronic pain Interventions: duloxetine (all doses combined), milnacipran (all doses combined), mirtazapine (all doses combined) Comparator (reference): placebo Outcome: change in mood (depression, anxiety, distress) scores as measured on various scales including the Beck Anxiety Inventory, Beck Depression Inventory, SF‐36 Mental Component Score, and the SF‐36 Mental Health Subscale Direction: lower is better (i.e. a greater reduction of distress, depression, or anxiety) | |||||||
|
Total studies: 38 Total participants: 12,985 |
Relative effect | Anticipated absolute effect (event rate) | Certainty of the evidence (CINeMA) |
Ranking* (2.5% to 97.5% credible interval) |
Interpretation of findings** | ||
| With placebo | With intervention | Difference | |||||
|
Mirtazapine RCTs: 1 Participants: 406 |
‐ | ‐ | ‐ |
SMD −0.5 (−0.78 to −0.22) |
Lowe | 4 (2 to 7) | Moderate effect |
|
Duloxetine RCTs: 26 Participants: 7952 |
‐ | ‐ | ‐ |
SMD −0.16 (−0.22 to −0.1) |
Moderatea | 8 (5 to 11) | Small effect |
|
Milnacipran RCTs: 5 Participants: 3109 |
‐ | ‐ | ‐ |
SMD −0.13 (−0.26 to 0.01) |
Moderatea,c | 9 (5 to 13) | Not significantly different from placebo |
|
Network meta‐analysis‐summary of findings table definitions *Mean rank and credible intervals are presented. **SMD interpretation based on clinical judgement and in line with Cohen 1988 and the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2022) as small (0.2), moderate (0.5) and large (0.8). CI: confidence interval; CINeMA: Confidence in Network Meta‐Analysis; RCT: randomised controlled trial; SMD: standardised mean difference The number of participants for each antidepressant reflects the total number of participants taking the antidepressant or placebo from the studies in the network meta‐analysis. | |||||||
|
CINeMA grades of confidence in the evidence High: further research is unlikely to change our confidence in the estimate of effect. Moderate: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low: we are very uncertain about the estimate. | |||||||
aDowngraded due to within‐study bias. bDowngraded due to imprecision in the estimate. cDowngraded due to heterogeneity in the estimate. dDowngraded due to incoherence in the network. eDowngraded due to a small number of trials and participants; we cannot draw reliable conclusions.
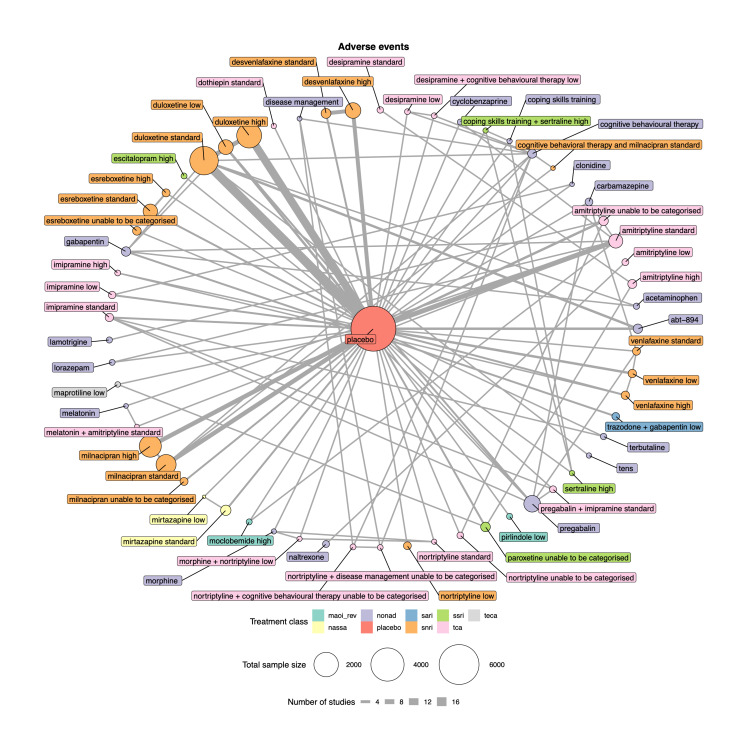
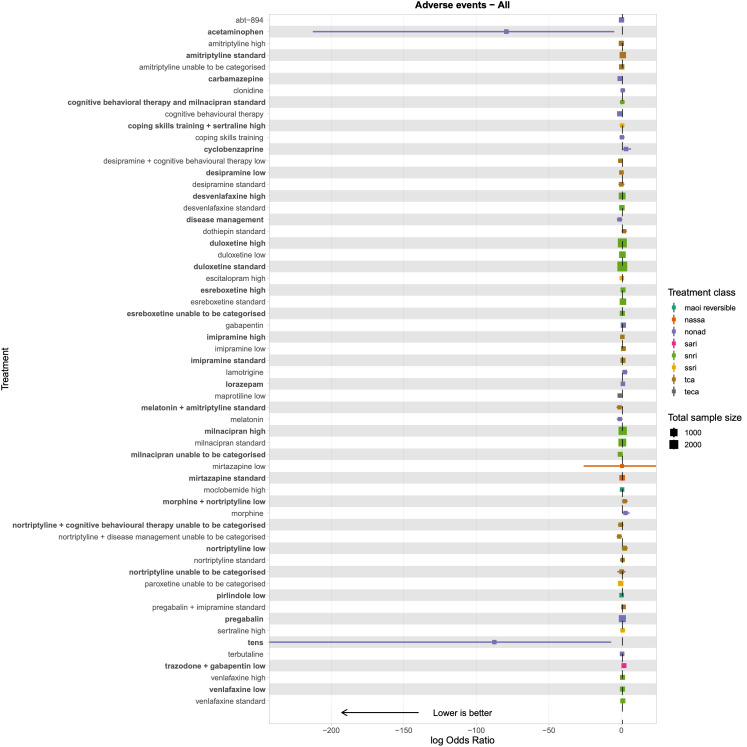
Summary of findings 4. Adverse events summary of findings.
| Estimates of effects, credible intervals, and certainty of the evidence for adverse events with antidepressants in people with chronic pain | |||||||
| Bayesian network meta‐analysis summary of findings table | |||||||
|
Patient or population: people with chronic pain Interventions: amitriptyline standard dose (25‐75 mg); desvenlafaxine high dose (> 50 mg); duloxetine low dose (< 60 mg), standard dose (60 mg), and high dose (> 60 mg); milnacipran standard dose (100 mg) and high dose (> 100 mg); mirtazapine standard dose (30 mg) Comparator (reference): placebo Outcome: adverse events (as reported per study) Direction: lower is better (i.e. fewer people reporting adverse events) | |||||||
|
Total studies: 93 Total participants: 22,558 |
Relative effect (OR and 95% CI) |
Anticipated absolute effect (event rate)* | Certainty of the evidence (GRADE) |
Ranking** (2.5% to 97.5% credible interval) |
Interpretation of findings | ||
| With placebo | With intervention | Difference | |||||
|
Desvenlafaxine high dose RCTs: 2 Participants: 905 |
1.67 (0.92 to 2.41) |
174/220 791 per 1000 |
590/685 863 per 1000 |
72 more per 1000 | Very lowa,b,c | 30 (16 to 48) | Not significantly different from placebo |
|
Mirtazapine standard dose RCTs: 2 Participants: 457 |
1.70 (0.48 to 2.91) |
135/228 592 per 1000 |
162/229 712 per 1000 |
120 more per 1000 | Very lowb,c | 31 (11 to 52) | Not significantly different from placebo |
|
Duloxetine standard dose RCTs: 20 Participants: 4998 |
1.88 (1.58 to 2.17) |
1259/2164 582 per 1000 |
1883/2834 723 per 1000 |
142 more per 1000 | Very lowa,b | 33 (24 to 42) | Equivalent NNTH is 7.0 |
|
Milnacipran standard dose RCTs: 8 Participants: 2491 |
1.92 (1.37 to 2.46) |
930/1235 753 per 1000 |
1039/1256 854 per 1000 |
101 more per 1000 | Very lowa,b,c | 33 (20 to 45) | Equivalent NNTH is 10 |
|
Duloxetine high dose RCTs: 10 Participants: 4000 |
1.93 (1.64 to 2.23) |
1199/1912 627 per 1000 |
1587/2088 764 per 1000 |
137 more per 1000 | Very lowa,b | 34 (24 to 43) | Equivalent NNTH is 7.03 |
|
Duloxetine low dose RCTs: 6 Participants: 1031 |
2.03 (1.45 to 2.62) |
271/437 620 per 1000 |
325/594 768 per 1000 |
148 more per 1000 | Very lowa,b | 35 (21 to 47) | Equivalent NNTH is 7.0 |
|
Milnacipran high dose RCTs: 7 Participants: 2837 |
2.44 (1.89 to 2.98) |
930/1264 736 per 1000 |
1294/1573 872 per 1000 |
136 more per 1000 | Very lowa,b | 39 (25 to 50) | Equivalent NNTH is 6.8 |
|
Amitriptyline standard dose RCTs: 10 Participants: 997 |
2.66 (2.14 to 3.19) |
250/479 522 per 1000 |
351/518 744 per 1000 |
222 more per 1000 | Very lowa,b,e | 41 (28 to 51) | Equivalent NNTH is 4.5 |
|
Esreboxetine standard dose RCTs: 1 Participants: 783 |
2.92 (1.90 to 3.93) |
85/227 374 per 1000 |
315/556 636 per 1000 |
262 more per 1000 | Very lowa,b,c,e | 42 (21 to 56) | Equivalent NNTH is 3.8 |
|
Network meta‐analysis‐summary of findings table definitions * Anticipated absolute effect. Anticipated absolute effect compares two risks by calculating the difference between the risk of the intervention group with the risk of the control group. ** Mean ranks and credible intervals are presented. CI: confidence interval; NNTH: number needed to treat for an additional harmful outcome; OR: odds ratio; RCT: randomised controlled trial The number of participants for each antidepressant reflects the total number of participants taking the antidepressant or placebo from the studies in the network meta‐analysis. | |||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||||
aDowngraded due to within‐study bias. bDowngraded due to imprecision in the estimate. cDowngraded due to heterogeneity in the estimate. dDowngraded due to incoherence in the network. eDowngraded due to a small number of trials and participants; we cannot draw reliable conclusions.
Background
Description of the condition
Chronic pain is common in adults internationally, and is defined as pain lasting or recurring for three months or longer (IASP 2019). Chronic pain can be a primary condition or can occur in the context of a disease (Treede 2019).
Chronic pain and its impact on an individual is generally assessed via self‐report. It is estimated that about one in five adults worldwide experience pain that is moderate or severe in its intensity and lasts three months or more (Moore 2014), however estimates vary and may be higher. For example, reviews of chronic pain in the UK suggest that between a third and a half of the population experience chronic pain (Fayaz 2016); and a review of chronic low back pain in Africa reported the annual prevalence as 57% (Morris 2018). Some populations are more likely to experience chronic pain: older adults, women, people not in employment due to ill health and disability, and people with comorbidities (Mills 2019). Social circumstances are particularly influential; people in low socio‐economic circumstances are not only more likely to experience chronic pain, but also report higher levels of severity and disability (Mills 2019).
The impact of chronic pain is similar across conditions, despite the different aetiologies. Globally, chronic pain accounts for the highest number of years lived with disability, and affects individuals’ daily lives, society and healthcare services (Breivik 2006; Rice 2016). Chronic pain accounts for up to one in five general practice consultations each year in Europe, Africa and Asia (European Pain Federation 2016; Jordan 2010; Morris 2018). Chronic pain is also one of the global leading causes for sickness absence and people being unable to work (Bevan 2012; Office for National Statistics 2019).
On an individual level, chronic pain can severely impair a person's quality of life, including physical functioning, mood, sleep, and ability to work outside the home (Breivik 2006). It has also been long‐established that chronic pain influences a person's mood; depression is estimated to be three to four times more prevalent in people with chronic pain than those without (Gureje 1998; Sullivan 1992; Tunks 2008). Depression is characterised by persistent feelings of sadness or low mood, loss of pleasure in activities, fatigue, loss of motivation, changes in appetite and having thoughts of suicide or self‐harm (American Psychiatric Association 2013). People have reported that experiencing only a few depressive symptoms can be both distressing and disabling; therefore, it is important to address these as effectively as possible (NICE 2009a). Depression and chronic pain are complex to address in both research and clinical practice, as many of the symptoms of chronic pain can overlap with those of depression (for example, fatigue and loss of motivation or pleasure in activities). Furthermore, the content of depressive thoughts and the antecedents of feelings of sadness experienced by people in chronic pain may differ to those experienced in people with depression but without pain. It is important to identify differences in pain‐related distress (i.e. individuals with chronic pain experiencing low mood because of their pain) and clinical depression, which may reflect on the prevalence statistics reported above.
Successful treatment of chronic pain can result in significant improvements in quality of life, including anxiety and depression (Goesling 2013; Moore 2010a; Moore 2014). A systematic review identified that for people with fibromyalgia, reductions in pain intensity of 50% or more is associated with self‐reports of sleep, fatigue and depression reverting to normative values (Moore 2014). Therefore, efficacious treatment of the pain condition is essential for improvement of both pain and mood, in addition to potential improvements in sleep, physical function and quality of life. There are many different treatments aimed at reducing and managing chronic pain, including analgesic medication, physiotherapy, self‐management guidance, exercise, psychological therapy, antidepressants, pain management clinics and surgery. The use of these depends upon the pain condition, severity of pain, individual characteristics, availability of services and national policy and guidelines.
Description of the intervention
Antidepressants are medicines developed and used primarily for the treatment of clinical depression. A network meta‐analysis (NMA) of the 21 most common antidepressants has shown that they are efficacious in the treatment of acute major depression, particularly severe depression (Cipriani 2018).
Antidepressants are grouped into different classes based on their chemical structure and presumed mechanism of action. The most common classes are:
tricyclic antidepressants (TCAs): amitriptyline, desipramine, imipramine, nortriptyline, and others;
selective serotonin reuptake inhibitors (SSRIs): citalopram, sertraline, fluoxetine, and others;
serotonin norepinephrine reuptake inhibitors (SNRIs): duloxetine, levomilnacipran, milnacipran, venlafaxine, and others;
-
monoamine oxidase inhibitors (MAOIs):
irreversible: phenelzine, tranylcipromine, izocarboxazid, and others;
reversible: brofaramine, moclobemide, tyrima, and others.
Antidepressants are recommended for first‐line treatment of depression, but can also be used 'off‐label' in clinical practice to treat other conditions, including chronic pain (British National Formulary 2022a). Prescriptions of antidepressants are relatively common in patients with chronic pain internationally; for example, 12.3% of people with chronic low back pain in Portugal report taking antidepressants for pain relief (Gouveia 2017; Kurita 2012). Recent guidance from the National Institute for Health and Care Excellence (NICE) recommends the use of duloxetine, amitriptyline, fluoxetine, paroxetine, citalopram and sertraline in the management of chronic primary pain (NICE 2020). Amitriptyline and duloxetine are also recommended as first‐line treatments for neuropathic pain in primary care (NICE 2019). Both of these guidelines recommend these antidepressants regardless of a person's mood. However, other guidelines contradict this, for example antidepressants can be prescribed for people with a chronic physical health condition only if they are also experiencing moderate to severe depression (NICE 2009b), but they are not recommended at all for the treatment of chronic low back pain (without sciatica; NICE 2017). The NICE guidelines for chronic primary pain recommend antidepressants as the only pharmacological intervention to manage chronic primary pain (NICE 2021).
These guidelines only reviewed the evidence from head‐to‐head trials, and subsequently recommend six antidepressants with no hierarchy: amitriptyline, citalopram, duloxetine, fluoxetine, paroxetine, or sertraline. Therefore, guidance for clinicians is mixed and unclear. Furthermore, as antidepressants can be prescribed for treating mood or pain, the proportions of antidepressants prescribed to people with chronic pain for the primary aim to reduce pain or improve mood is unknown.
There are also risks in the prescription of antidepressants. Adverse events such as dizziness, headache, nausea, ejaculation disorder, weight loss, tremor, sweating and insomnia, have been found by randomised controlled trials (RCTs) to be more common in people taking antidepressants compared with those taking placebo (Riediger 2017; Sinyor 2020). Use of antidepressants is associated with an increased risk of falls, fractures, all‐cause mortality, and stroke in older adults (aged 65 and over), and self‐harm and suicide in both younger adults (aged 20 to 64) and older adults (Coupland 2011; Coupland 2015). Antidepressants also increase the risk of onset of seizures (Hill 2015); and the potential for gastrointestinal bleeding with SSRIs is widely recognised (Jiang 2015). Therefore, long‐term use of antidepressants for people with chronic pain is expected to be associated with potential for harms at the population level.
How the intervention might work
Antidepressants were originally developed to treat depression. Most antidepressants work by targeting monoamine neurotransmitters associated with mood and emotion and their receptors in the nervous system. These receptors, such as 5‐hydroxytryptamine receptors, are activated by many neurotransmitters including serotonin, dopamine, adrenaline and noradrenaline (Harmer 2017). Antidepressants prevent the neurotransmitters from being absorbed into neurons, which prolongs their activity in synapses. The process by which this relieves depression is not fully understood, but research currently focuses on theories of neurochemical changes and neuroplasticity (Harmer 2017). Additionally, depending upon the class, the effect of antidepressants may be delayed, with reported clinical improvement often taking weeks to occur (Harmer 2017; Tylee 2007).
Antidepressants are also often used to manage chronic pain. Antidepressants are reported to offer an analgesic response in people with pain without depression, particularly for neuropathic pain, but also for some people with fibromyalgia, osteoarthritis, and back pain. It is theorised that the body's pain response systems travelling to and from the brainstem involve the noradrenergic neurotransmitters (Taylor 2017). Therefore, by increasing the amount of serotonin and noradrenaline in the nervous system, this may subsequently block pain signals at the peripheral, spinal, and supraspinal levels, reducing perceived pain; particularly in neuropathic pain (Finnerup 2021; Kremer 2018).
Additionally, a part of the brain called the locus coeruleus may have an analgesic effect on pain in the body (Llorca‐Torralba 2016). Signals from this part of the brain are sent when the body reacts to a stimulus, such as pain, and noradrenaline is released into the dorsal horn in the spine to block receptors. Animal studies have shown that when pain signals are continuously received, as is the case in chronic pain, this analgesic response lessens over time, and noradrenaline is then not released (Llorca‐Torralba 2016; Obata 2017). However, when antidepressants are given, the analgesic response from the locus coeruleus is restored (Alba‐Delgado 2012; Llorca‐Torralba 2016).
Why it is important to do this review
To date, there have been no NMAs investigating all antidepressants for all chronic pain conditions. There is no evidence comparing classes of antidepressants to each other in the management of chronic pain, as identified by the recent NICE guidelines (NICE 2020). Therefore, in the absence of any one RCT comparing the efficacy and safety of all antidepressants for chronic pain, a NMA is required to assess their relative effectiveness.
Previous Cochrane Reviews have investigated the efficacy of individual antidepressants in improving individual chronic pain conditions, and where possible by dose. There is no high‐quality evidence to support or refute the use of amitriptyline, milnacipran, nortriptyline, venlafaxine, desipramine or imipramine for management of neuropathic pain (Derry 2015a; Derry 2015b; Gallagher 2015; Hearn 2014a; Hearn 2014b; Moore 2015), principally because of limited numbers of small studies with some high risks of bias. This is despite amitriptyline being recommended as a first‐line treatment for neuropathic pain in primary care in guidelines for the UK, Canada and the International Association for the Study of Pain (Bates 2019; Finnerup 2015; Moulin 2014; NICE 2019). However, there is moderate‐quality evidence that duloxetine is efficacious for diabetic peripheral neuropathy at doses of 60 mg and 120 mg (Lunn 2014).
For fibromyalgia, Cochrane Reviews of antidepressants show that there is no unbiased evidence that amitriptyline, desvenlafaxine, venlafaxine or SSRIs are superior to placebo (Walitt 2015; Welsch 2018). There is low‐quality evidence that duloxetine and milnacipran have some benefit in improving patients’ global impression of change (PGIC) and providing an improvement in pain relief of 30% or more, but no clinical benefit over placebo for improvement in pain relief of 50% or more, health‐related quality of life or fatigue (Welsch 2018). Similarly, for mirtazapine, there is evidence for improvement in pain relief of 30% or more, and reduction of mean pain intensity and sleep problems, but this evidence is of low to medium quality, and there is no benefit for improvement in pain relief of 50% or more, PGIC, 20% improvement of health‐related quality of life, reduction of fatigue or reduction in negative mood (Welsch 2015).
Only one Cochrane Review has investigated the use of antidepressants for low back pain, and it found no clear evidence to support the use of any antidepressants (Urquhart 2008). A more recent systematic review supports these conclusions (Koes 2018). However, when analysed using the baseline observation carried forward imputation method for missing data, pooled individual patient data analyses of RCTs have shown duloxetine and etoricoxib to be effective in reducing pain for pain conditions including chronic low back pain (Moore 2010b; Moore 2014). These distributions were bimodal; participants generally responded very well or very poorly, with few in between (Moore 2014).
These previous reviews have shown that there is no evidence comparing the data across all antidepressants and pain conditions. Through our review and network meta‐analysis, we intend to compare all these antidepressants across pain conditions, and identify whether certain classes or doses of antidepressants are useful in the management of pain and mood for people with chronic pain, and for certain chronic pain conditions. As antidepressants are also associated with a number of side effects, we will compare the proportion of adverse events occurring with the use of different antidepressants (including different classes of antidepressants, different types of antidepressants, and different dose regimes) within populations living with chronic pain.
There is evidence that people with chronic pain may be experiencing pain‐related distress rather than clinical depression, although both conditions can present with similar symptoms (Rusu 2016). The distinction between pain‐related distress and depression is particularly important as primary care practitioners are often given contradictory guidance: they are encouraged to better detect depression (Mitchell 2009; Nuyen 2005), whilst avoiding over‐medicalisation of distress and thus over‐treatment (Dowrick 2013; Mulder 2008). This is important as antidepressants can be prescribed for both the management of pain and mood (e.g. clinical depression) in people with chronic pain. This review aimed to clarify this guidance as, unlike previous reviews in this area, we intended to investigate whether there were differences dependent upon whether the antidepressants were prescribed to primarily treat mood or pain.
Objectives
To assess the comparative efficacy and safety of antidepressants for adults with chronic pain (except headache) by:
assessing the efficacy of antidepressants by type, class and dose in improving pain, mood, physical function, sleep, quality of life and PGIC;
assessing the number of adverse events and serious adverse events for antidepressants by type, class and dose;
ranking antidepressants for efficacy of treating pain, mood and adverse events.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs that compared any antidepressant with any comparator. RCTs are the best design to minimise bias when evaluating the effectiveness of an intervention. We followed the guidance in the Cochrane Handbook for Systematic Reviews of Interventions for the inclusion of cross‐over RCTs, which requires inclusion of this type of study unless there is a justifiable reason not to (McKenzie 2020). The risk in this review was that washout periods between the periods of the study would not be long enough for carry‐over effects from the antidepressants or comparators to be sufficiently minimised. Therefore, we only included cross‐over trials with washout periods of at least five times the length of the antidepressant half‐life (this was calculated individually for each antidepressant).
The most common comparators we anticipated finding in the literature were: the same antidepressant at a different dose; a different antidepressant; placebo (both active and inert); other medications for pain management purposes (e.g. pregabalin, gabapentin); analgesics; psychological therapy (e.g. cognitive behavioural therapy, acceptance and commitment therapy); exercise; physiotherapy; multidisciplinary pain programmes; herbal medicines and nutraceuticals (e.g. St John’s Wort); and acupuncture. Where the comparator was a placebo, antidepressant, analgesic or other medication for pain management purposes, these studies were required to be double‐blind. We included studies that examined any dose of antidepressants, with a study duration of at least two weeks and minimum of 10 participants per arm. We excluded non‐randomised studies, case reports, experimental studies, clinical observations and prevention studies.
Types of participants
We included adults (aged 18 years or older) reporting primary or secondary pain in any part of their body (except headache) as their primary complaint, that matched the International Association for the Study of Pain (IASP) definition of chronic pain (i.e. at least three months' duration; IASP 2019). We included all studies regardless of the severity of participants' chronic pain, although we extracted whether severity was part of the inclusion criteria of the individual studies. We excluded studies where the participants' primary complaint was headache or migraine, as this had been covered in previous Cochrane Reviews (Williams 2020). Although this condition does fit within the IASP criteria, the diagnosis, classification and treatment of primary and secondary headache are often different from that of other pain conditions; and clinical trials are primarily aimed at prevention of further headaches or migraines rather than symptomatic treatment. We included participants with multiple health conditions as long as the chronic pain condition was the focus of the trial.
Types of interventions
Decision set
We included any antidepressant at any dose, for any indication, but used primarily for treatment of people with chronic pain and compared to placebo or active intervention. We included antidepressants grouped into the following classes.
Tricyclic antidepressants (TCAs): amitriptyline, clomipramine, imipramine, trimipramine, doxepin, desipramine, protriptyline, nortriptyline, dothiepin, lofepramine, and others
Selective serotonin reuptake inhibitors (SSRIs): fluvoxamine, fluoxetine, paroxetine, sertraline, citalopram, escitalopram, zimelidine and others
Serotonin‐noradrenaline reuptake inhibitors (SNRIs): venlafaxine, milnacipran, duloxetine, and others
-
Monoamine oxidase inhibitors (MAOIs):
irreversible: phenelzine, tranylcipromine, izocarboxazid, and others;
reversible: brofaramine, moclobemide, tyrima, and others
-
Other antidepressants
Noradrenaline reuptake inhibitors (NARIs): reboxetine, atomoxetine, and others
Noradrenaline and dopamine reuptake inhibitors (NDRIs): amineptine, bupropion, and others
Noradrenergic and specific serotonergic antidepressants (NaSSAs) including tetracyclic antidepressants (TeCA) such as: mirtazapine, mianserin, maprotiline, and others
Serotonin antagonist and reuptake inhibitors (SARIs): trazodone, and others
Unclassified: agomelatine, vilazodone, and others
We categorised doses of included antidepressants into low, standard, and high doses. These are displayed in Table 5. As the majority of antidepressants are not licensed for pain, we based our judgements on the recommendations of daily doses for clinical depression in the British National Formulary (British National Formulary 2022a). The judgements were made by clinical authors of the review; initially by the clinical pharmacist and then approved by discussion with a psychiatrist and anaesthetist.
1. Antidepressant dose categorisation.
| Antidepressant | Total daily dosage | ||
| Low | Standard | High | |
| Amitriptyline | < 25 mg | 25‐75 mg | > 75 mg |
| Bupropion | n/aa | 150‐300 mg | > 300 mg |
| Citalopram | < 20 mg | 20 mg | 40 mg |
| Clomipramine | < 30 mg | 30‐150 mg | > 150 mg |
| Desipramine | < 100 mg | 100‐200 mg | > 200 mg |
| Desvenlafaxine | n/ab | 50 mg | > 50 mg |
| Dothiepin (dosulepin) | < 75 mg | 75‐150 mg | > 150 mg |
| Doxepin | < 75 mg | 75‐150 mg | > 150 mg |
| Duloxetine | < 60 mg | 60 mg | > 60 mg |
| Escitalopram | < 10 mg | 10 mg | 20 mg |
| Esreboxetine | n/ac | 4‐8 mg | > 8 mg |
| Fluoxetine | < 20 mg | 20‐40 mg | > 40 mg |
| Imipramine | < 75 mg | 75‐150 mg | > 150 mg |
| Nortriptyline | < 75 mg | 75‐100 mg | > 100 mg |
| Maprotiline | 150 mg | 300 mg | > 300 mg |
| Mianserin | < 30 mg | 30‐40 mg | > 40 mg |
| Milnacipran | < 100 mg | 100 mg | > 100 mg |
| Mirtazapine | < 30 mg | 30 mg | > 30 mg |
| Moclobemide | 150 mg | 300 mg | 600 mg |
| Paroxetine | < 20 mg | 20 mg | 50 mg |
| Pirlindole | < 225 mg | 225‐300 mg | > 300 mg |
| Reboxetine | < 8 mg | 8 mg | > 8 mg |
| Sertraline | n/ad | 50 mg | > 50 mg |
| Trazodone | < 150 mg | 150‐300 mg | > 300 mg |
| Trimipramine | < 75 mg | 75‐150 mg | > 150 mg |
| Venlafaxine | < 75 mg | 75‐150 mg | > 150 mg |
| Zimelidine | < 300 mg | 300 mg | > 300 mg |
aLowest dose form is 150 mg.
bDesvenlafaxine is not available in UK, lowest dose form is 50 mg.
cEsreboxetine is not available in UK, and no doses lower than 4 mg have been used in trials.
d50 mg is both the initial and standard dose, no recommendations of lower doses in the British National Formulary.
Standard doses were the recommended doses for depression in adults. Low doses were those listed as initial doses (where a standard range is specified), the dose for elderly patients, or any dose below the standard dose (where no range was specified). High doses were those listed at the upper range of standard dose ranges, or above the standard dose where no range is specified. Where studies included flexible dosing across multiple categories and did not report mean dose, we labelled them as ‘unable to be categorised’.
Supplementary sets
We included studies with any active comparator. We included studies where the antidepressant is combined with another intervention, as long as there was an arm solely for the other intervention, so we were able to isolate the effects of the antidepressant (e.g. antidepressant + drug versus drug). We did not include combination studies where there was no way to isolate the effects of an antidepressant (e.g. antidepressant A + drug versus antidepressant B). For this review we assumed that any participant who met the inclusion criteria was, in principle, equally likely to be randomised to any of the eligible antidepressants; however, we acknowledge there may have been differences in patients’ expectations of treatment and outcomes depending upon which antidepressant was studied.
Types of outcome measures
We anticipated that there would be a variety of outcome measures used throughout the literature. Due to the distinction between distress and depression discussed above, this review used the term 'mood' as an outcome, to include depression that is diagnosed, mood that is measured via self‐report, and distress.
For pain and mood, where applicable we also dichotomised outcomes into pain relief or improvement of 50% or greater, in line with the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) guidance, to indicate substantial improvement (Dworkin 2008). Where possible, we planned separate NMAs to compare antidepressants to the comparators immediately post‐intervention, at short‐term follow‐up (12 weeks or less post‐treatment) and long‐term follow up (over 12 weeks post‐treatment). Where studies included multiple follow‐up time points, we took the most recent time point within each period. If multiple measures were used for the same outcome (e.g. for continuous pain intensity both a 0 to 10 numerical rating scale and the McGill Pain Questionnaire (Melzack 1975) were reported), then we extracted from the most valid, reliable, and widely used measure in the field.
Primary outcomes
Substantial pain relief: proportion of participants (number and percentage of total and per arm) reporting at least 50% reduction in pain intensity from baseline, irrespective of pain measurement method (e.g. visual analogue scale, numerical rating scale)
Pain intensity: continuous data from any measures of pain intensity or severity (e.g. visual analogue scale or validated measures such as Brief Pain Inventory)
Mood: continuous data from any measures of mood (e.g. visual analogue scale, Hospital Anxiety and Depression Scale)
Adverse events: the proportion of participants (number of percentage of total and per arm) reporting adverse events
Secondary outcomes
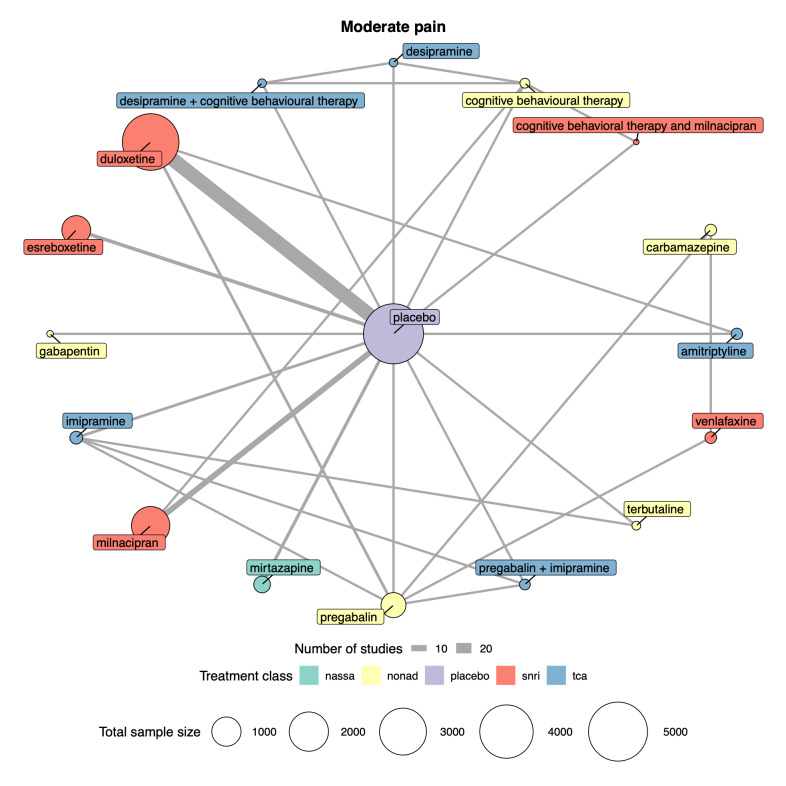
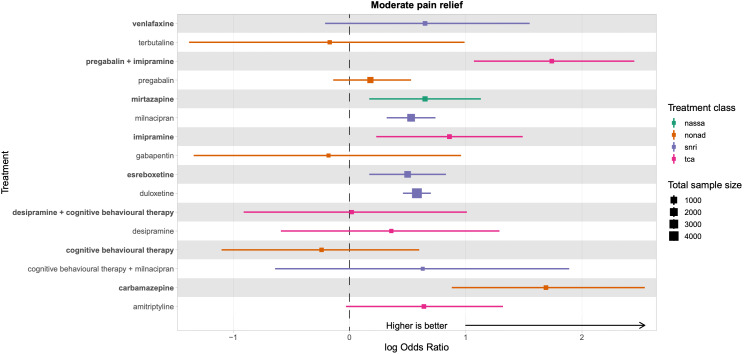
Moderate pain relief: the proportion of participants (number and percentage of total and per arm) reporting at least 30% reduction in pain intensity from baseline, irrespective of pain measurement method (e.g. visual analogue scale, numerical rating scale).
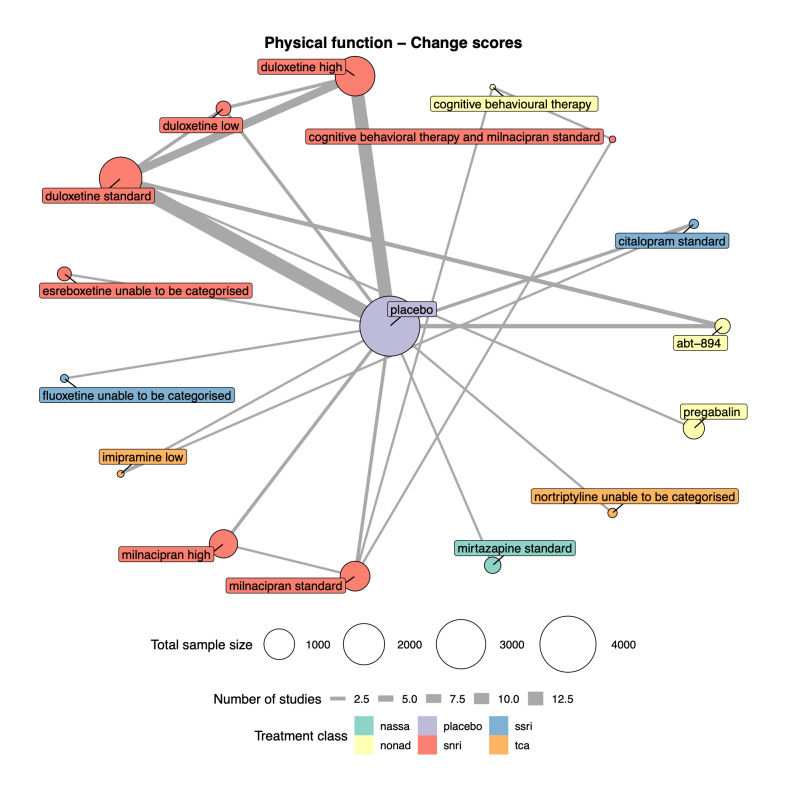
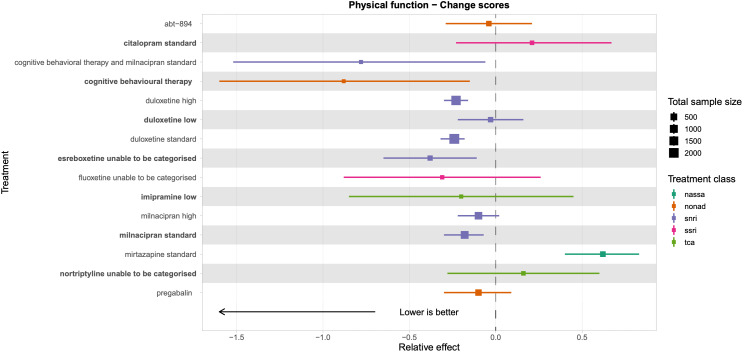
Physical function: continuous data from any measures of physical movement and disability, e.g. numerical rating scale, SF‐36 Physical Component Score)
Sleep: continuous data from any measures of quality of sleep, including insomnia, restfulness, etc. (e.g. Brief Pain Inventory, Jenkins Sleep Scale)
Quality of life: continuous data from any measure of quality of life (e.g. numerical rating scale, EQ‐5D)
Patient Global Impression of Change (PGIC): the proportion of participants (number and percentage of total and per arm) reporting "much" and "very much" improved on the PGIC scale, and continuous data from the PGIC scale.
Serious adverse events: the proportion of participants (number of percentage of total and per arm) reporting serious adverse events).
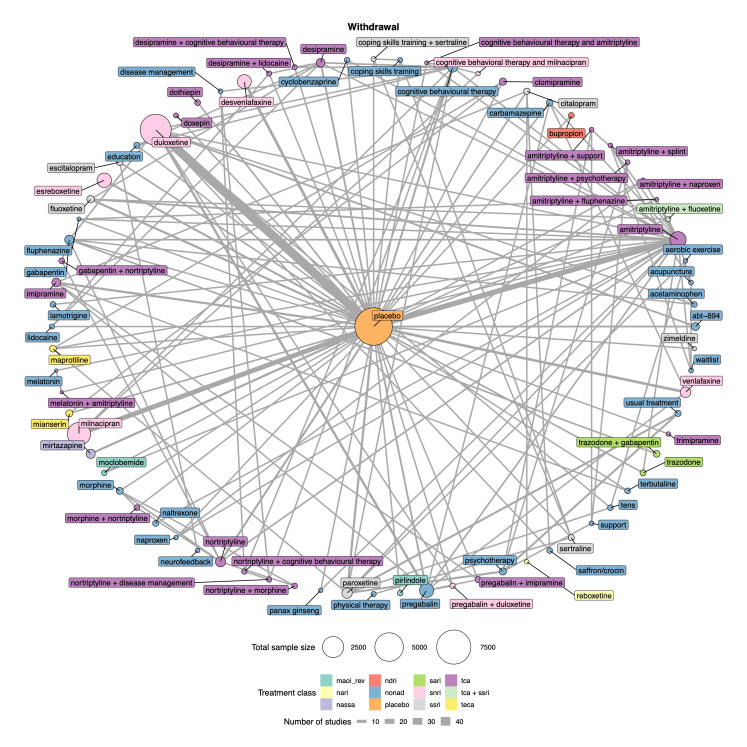
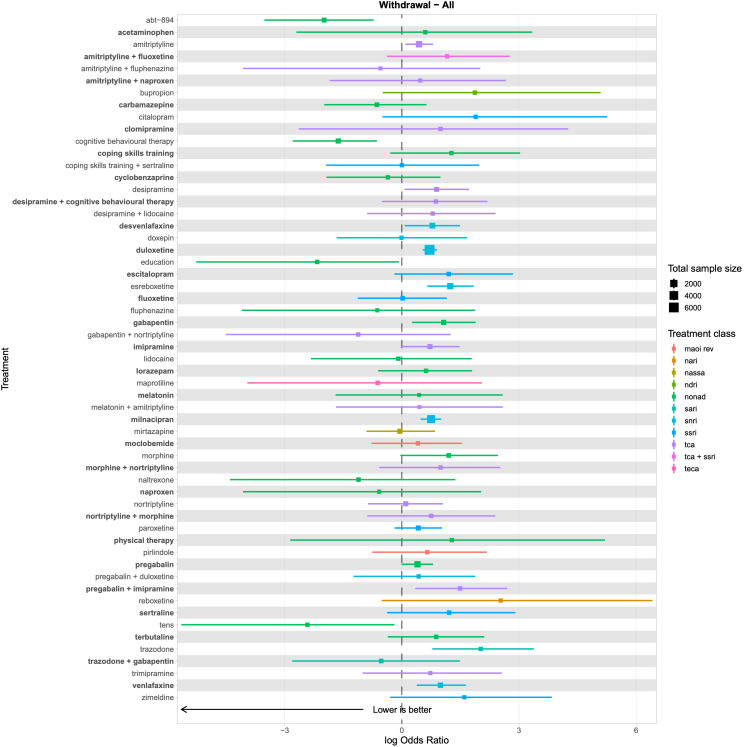
Withdrawal: the proportion of participants (number and percentage of total and per arm) withdrawing for any reason.
Search methods for identification of studies
This search was last run on 4 January 2022.
Electronic searches
We searched the following databases, without language restrictions.
The Cochrane Central Register of Controlled Trials (CENTRAL; 2021, Issue 12) via the Cochrane Library (searched 4 January 2022)
MEDLINE and MEDLINE In‐Process (via OVID) ‐ 1946 to 4 January 2022
Embase (via OVID) ‐ 1974 to 4 January 2022
CINAHL (via EBSCO) ‐ 1981 to December 2021
LILACS (via Birme ‐ 1982 to Dec 2021)
PsycINFO (via EBSCO)) ‐ 1872 to 4 January 2022
AMED (via OVID) ‐ 1985 to December 2021
We tailored searches to individual databases. The search strategies used can be found in Appendix 1. The search strategy was developed by the Cochrane Pain, Palliative and Supportive Care (PaPaS) Review Group’s Information Specialist and was independently peer‐reviewed. The PaPaS Information Specialist performed the searches.
Searching other resources
We searched ClinicalTrials.govand the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished and ongoing studies. In addition, we searched grey literature, checked reference lists of reviews and retrieved articles for additional studies, and performed citation searches on key articles. We contacted study authors for additional information where necessary.
Data collection and analysis
Selection of studies
Two review authors (HB and CF) independently determined eligibility of each study identified by the search. Review authors independently eliminated studies that clearly did not satisfy inclusion criteria, and obtained full copies of the remaining studies. HB and CF read these studies independently to select relevant studies, and in the event of a disagreement, third and fourth authors adjudicated (TP and CE). We did not anonymise the studies in any way before assessment. We have included a PRISMA flow chart that shows the status of identified studies (Moher 2009), as recommended in Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2022). We included studies in the review irrespective of whether measured outcome data were reported in a 'useable' way. We recorded reasons for exclusion of any ineligible studies at the full‐text stage.
Data extraction and management
Two review authors (HB and CF) independently extracted data using a standard piloted form and checked for agreement before entry into Review Manager Web (RevMan Web 2023). In the event of disagreement, third and fourth authors (TP and CE) adjudicated. We collated multiple reports of the same study, so that each study rather than each report was the unit of interest in the review. We collected characteristics of the included studies in sufficient detail to populate the table of 'Characteristics of included studies'. We extracted the following information.
Study design: authors, publication year and journal, duration, sponsorship, conflicts of interest, aim (pain or emotional functioning), design, number of treatment arms, setting, missing data methods, power calculation used, definition of chronic pain, minimum level of pain for entry, inclusion and exclusion criteria
Setting
Participant characteristics: overall number, number in each arm, withdrawal (total, per arm and by sex), type of participant, chronic pain conditions, sex, age, baseline differences
Intervention: type of antidepressant, class, dose (freeform and dichotomised), route of administration, duration
Comparator(s): type (e.g. placebo, psychological therapy), description (if placebo medication: active or inert, appearance, taste, smell, titration, number of tablets), type and class (if other antidepressant), doses, route of administration, length, intensity (if physical or psychological comparator)
Outcomes (data from all time points reported in the study): domain (e.g. pain, physical functioning), measure, measure validation, baseline data, results for each time point, effect sizes
Adverse events and withdrawals (proportion overall and per arm): any, serious, withdrawal due to adverse event, withdrawal due to lack of efficacy
Assessment of risk of bias in included studies
Two review authors (HB and CF) independently assessed risk of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), with any disagreements resolved by discussion. We completed a risk of bias table for each included study using the Cochrane risk of bias tool (RoB 1) in Review Manager 5 (Review Manager 2020).
We assessed the following for each study.
-
Random sequence generation (checking for possible selection bias). We assessed the method used to generate the allocation sequence as being at:
low risk of bias (any truly random process, e.g. random number table; computer random number generator); or
unclear risk of bias (method used to generate sequence not clearly stated).
We excluded studies using a non‐random process (e.g. odd or even date of birth; hospital or clinic record number).
-
Allocation concealment (checking for possible selection bias). The method used to conceal allocation to interventions prior to assignment determines whether intervention allocation could have been foreseen in advance of or during recruitment, or changed after assignment. We assessed the methods as being at:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered, sealed, opaque envelopes); or
unclear risk of bias (method not clearly stated).
We will exclude studies that do not conceal allocation (e.g. open list).
-
Blinding of participants and personnel (checking for possible performance bias). Due to the inclusion of studies using any comparator, our review will contain both double‐blinded RCTs and those studies in which double‐blinding is not possible (i.e. RCTs of psychological therapy or acupuncture). In the RCTs that are double‐blinded, we assessed the methods used to blind study participants and personnel from knowledge of which intervention a participant received in the double‐blind trials. We assessed methods as being at:
low risk of bias (the study states that it was blinded and describes the method used to achieve blinding, such as identical tablets matched in appearance or smell, or a double‐dummy technique); or
unclear risk of bias (the study states that it was blinded but does not provide an adequate description of how this was achieved).
Studies in which double‐blinding was not possible due to the comparator will be considered to have high risk of bias.
-
Blinding of outcome assessment (checking for possible detection bias). We assessed the methods used to blind study participants and outcome assessors from knowledge of which intervention a participant received. We assessed the methods as being at:
low risk of bias (the study has a clear statement that outcome assessors were unaware of treatment allocation, and ideally describes how this was achieved);
unclear risk of bias (the study states that outcome assessors were blind to treatment allocation but it lacks a clear statement on how this was achieved); or
high risk of bias (the outcome assessment was not blinded).
-
Selective reporting (checking for reporting bias). We assessed whether primary and secondary outcome measures were pre‐specified and whether these were consistent with those reported. We assessed the methods as being at:
low risk of bias (study protocol is available with pre‐specified measures);
unclear risk of bias (insufficient information available to permit a judgement of high or low risk of bias); or
high risk of bias (not all of the study’s prespecified primary outcomes have been reported; one or more primary outcomes have been reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not prespecified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review have been reported incompletely so that they cannot be entered in a meta‐analysis; the study report failed to include results for a key outcome that would be expected to have been reported for such a study).
-
Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data). We assessed the methods used to deal with incomplete data as being at:
low risk of bias (no missing outcome data; reasons for missing outcome data are unlikely to be related to the true outcome; missing outcome data are balanced in numbers across intervention groups, with similar reasons for missing data across groups; missing data have been imputed using 'baseline observation carried forward’ (BOCF) analysis);
unclear risk of bias (insufficient reporting of attrition/exclusions to permit a judgement of low or high risk of bias (e.g. number randomised not stated; no reasons for missing data provided; or the study did not address this outcome)); or
high risk of bias (the reason for missing outcome data is likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; ‘as‐treated’ analysis was done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation; use of 'last observation carried forward’ (LOCF) without the addition of any other low risk of bias methods).
Other bias. We assessed any other potential sources of bias that were not included in the other domains.
We considered studies to be at high risk of bias overall if they met the criteria for high risk of bias in any of the above domains.
Measures of treatment effect
For the outcomes measuring continuous data (pain intensity, mood, physical function, sleep, quality of life, and PGIC continuous), studies reported data as either post‐intervention scores (the mean scores at the end of the intervention period) or change scores (mean change from baseline score). We conducted separate analyses for these. As is common in pain management studies, for all outcomes (apart from PGIC) studies used a broad range of scales to measure the outcomes. Therefore, once data were extracted, we converted them into standardised mean difference (SMD) with 95% confidence intervals (CIs). We interpreted SMD as small (0.2), moderate (0.5) and large (0.8), in line with Cohen 1988 and the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022a). For outcomes with dichotomous data (substantial pain relief, adverse events, moderate pain relief, PGIC much/very much improved, serious adverse events, and withdrawal), we used odds ratios (OR) with 95% CIs.
Unit of analysis issues
For most RCTs, we did not encounter any unit of analysis complexities as participants were randomised to different study arms, allowing direct analysis. For cross‐over RCTs, if the results for the first period (prior to cross‐over) were reported, we extracted these in an attempt to avoid cross‐over effects. If the results from the first period were not reported then we extracted the final study results, provided there was a sufficient washout period of at least five times the length of the antidepressant half‐life (minimum washout period length calculated separately for each antidepressant). The majority of cross‐over trials reported the combined effects of both periods (only one study reported first period and second period effects separately), therefore we analysed cross‐over trials using these combined effects. Our search did not return any cluster‐RCTs that met our inclusion criteria.
Dealing with missing data
For all missing study‐level statistical data relevant to our outcomes we first tried to contact the authors of the study. If we could not get the data from the authors, then we followed the guidance from the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2022). If standard deviations were missing then we used the Review Manager calculator (RevMan Web 2023) to calculate these from other data reported in the study. We did not impute any data, but assessed each study’s risk of bias due to missing data.
Assessment of heterogeneity
We assessed heterogeneity within the network meta‐analyses using the Tau statistic, in line with the guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2022). We assessed heterogeneity using Confidence in Meta‐Analysis (CINeMA) software, which calculated the Chi² test and the I² statistic for each pairwise comparison on each outcome (Nikolakopoulou 2020). As outlined in the Cochrane Handbook for Systematic Reviews of Interventions, we interpreted the I² statistic as follows (Deeks 2022).
0% to 40%: might not be important
30% to 50%: may represent moderate heterogeneity
50% to 90%: may represent substantial heterogeneity
75% to 100%: considerable heterogeneity
We took into account the magnitude and strength of effects when assessing heterogeneity.
Assessment of the transitivity assumption
We carefully scrutinised transitivity, which is the key underlying assumption of NMA. Transitivity requires studies to be similar on average across all factors that might alter treatment effects other than the intervention comparison being made (Chaimani 2022). To address this, we only included studies with similar clinical populations (i.e. participants reporting pain lasting at least three months; Furukawa 2016). Previous research, combined with review authors' clinical experience and knowledge, identified variables that could potentially influence our primary outcome:
pain condition;
age;
pain intensity at baseline;
depressive severity at baseline;
treatment duration; and
dosing schedule.
We explored the impact of these factors by assessing the indirectness of the network.
The inclusion of placebo and concerns about its potential to violate the transitivity assumption have been highlighted in general (Cipriani 2013), and particularly in depression studies (Rutherford 2009). Therefore, we explicitly compared placebo‐controlled studies with those that provide head‐to‐head evidence as a form of validation of the network.
Assessment of reporting biases
We assessed reporting biases using the Cochrane risk of bias tool (RoB 1) in Review Manager 5 (Review Manager 2020), by checking for study protocols and pre‐specified outcomes (as detailed in the Assessment of risk of bias in included studies section). We also used funnel plots for pairwise analyses for antidepressants where more than 10 studies were available, as advised in the Cochrane Handbook for Systematic Reviews of Interventions (Page 2022). Funnel plots were drawn using ROB‐MEN, which is part of CINeMA, and used to assess the significant small study effects via funnel plot asymmetry.
Data synthesis
We undertook separate NMAs for each outcome. NMAs combine information (evidence) from both direct comparisons of interventions within RCTs, and indirect comparisons across studies based on a common placebo comparator (Caldwell 2005; Jansen 2011). Direct comparisons (direct evidence) occur when two or more interventions are compared head to head in a study; in the absence of head‐to‐head comparisons, interventions can be indirectly compared (indirect evidence).
We analysed the data for all primary and secondary outcomes using Bayesian random‐effects NMAs implemented using the R (r-project.org) package multinma (Phillippo 2022). Where dose was included in the network, we categorised them (low, standard, high) and incorporated them as separate nodes. Where a study had multiple arms investigating different doses of the same antidepressant that fall into in the same category (e.g. two different low doses), we did not combine them; by using the multinma package we were able to keep these as separate arms in the analysis.
We fitted random‐effects models using broad normal prior distributions for the treatment effects, and study‐specific intercepts and a half‐normal prior for the heterogeneity standard deviation. We used four chains, each with 2000 iterations and 1000 post‐warm up draws per chain.
We explored network connectivity via network plots. In the network plot, for treatment‐only models, the nodes represent each intervention. In treatment‐dose models, the antidepressant nodes represent the antidepressant and dose (low, standard, high). The colour of the node represents the antidepressant class, and the "nonad" label refers to all interventions that were not an antidepressant. The size of each node represents the combined sample size of participants from all studies investigating that intervention, and the thickness of the lines represents the number of studies for that comparison. The forest plots present the estimates and credible intervals for each intervention in the network, with reference to placebo.
We assessed convergence using the potential scale reduction factor for each parameter, ensured that effective sample sizes were sufficiently large (Vehtari 2021), and verified that there were no divergent transitions (Betancourt 2015). We explored heterogeneity by fitting connected networks for treatment, treatment‐dose, class, risk of bias, and condition where network geometry allowed sufficient connectivity (Dias 2013).
We assessed model fit using mean residual deviance, and explored inconsistency through unrelated mean‐effect models (UME) and node‐splitting where network geometry allowed (Dias 2013a). We used dev‐dev plots, which compare residual deviance contributions from each model, to explore inconsistency. The data points are plotted against a line of equality; points on the line fit equally well under either model, whereas points above or below the line indicate better fits for one of the two models (Phillippo 2022). Node‐splitting plots present the evidence of direct, indirect, and combined evidence on the same plot to allow comparisons.
We reported effect estimates and cumulative posterior ranks of effect alongside strength of evidence assessment using GRADE (Schünemann 2013).
To rank the treatments for each outcome by probability of best treatment, we used the surface under the cumulative ranking curve (SUCRA) and the mean ranks. We reported relative effects and mean rank of treatments and plotted cumulative rankograms showing the range of rankings of different treatments for each outcome.
We used the deviance information criterion (DIC) to compare the different models for reporting (treatment only, treatment‐dose, class and, change score and post‐intervention studies for contrast‐based models) to assess their parsimony. Substantive differences in DIC (> 5) or models with marginally lower DIC but lower Tau and fewer studies with residual deviance greater than 3 in combination were deemed superior. We selected models to report on the basis of parsimony, minimisation of inconsistency (identified via UME and node‐splitting models), residual deviance and heterogeneity (measured as Tau). This approach balanced clinical exploration of results and the risk of overfitting (Dias 2013).
NMA, UME and node‐splitting models were implemented in multinma in R (version 4·1.3). Further details of the modelling framework are described by Phillippo 2018; Phillippo 2022.
Subgroup analysis and investigation of heterogeneity
Where data allowed, we performed subgroup analyses for the following factors.
Class of antidepressant (SSRI, SNRI, TCA, MAOI, etc.)
Type of pain condition
We used a Bayesian random‐effects NMA to account for expected heterogeneity and variation in the data. These methods allowed the uncertainty inherent in the between‐study variance component to be reflected in effect estimate precision. We performed these subgroup analyses by building separate models, however this was dependent on the geometry and connectedness of the networks.
Due to sparsity of data, we were unable to perform subgroup analyses for the following factors for any outcome.
-
Aim of the study (i.e. whether the intervention is aimed at pain or mood)
Only one study had a main aim of addressing mood (Richards 2015)
-
Baseline level of depression (none, mild, moderate, severe, as defined by the individual measure criteria)
Upon examination, the average scores for the five most commonly used scales (Beck Depression Inventory, Brief Pain Inventory Mood Item, SF‐36 Mental Component Score, SF‐36 Mental Health Subscale, and Hamilton Depression Rating Scale) were all in the none/minimal ranges.
Sensitivity analysis
We could only undertake analysis by risk of bias judgement (high and not high) for substantial pain relief. We were unable to perform sensitivity analyses for any outcome that compared active placebo to inert placebo, as in total only nine studies used an active placebo.
Summary of findings and assessment of the certainty of the evidence
To assess the certainty of the NMA, we primarily used the CINeMA framework (Nikolakopoulou 2020). In contrast to the NMAs in this review, which were conducted within a Bayesian framework, CINeMA operates within a frequentist framework using the netmeta package in R (Rücker 2017). The CINeMA framework considers the impact of certain issues within NMAs on clinical decision making made from the results. This framework is based on GRADE, and considers the following six domains specific to NMA (Nikolakopoulou 2020).
-
Within‐study bias (impact of risk of bias in the included studies)
CINeMA assesses the impact of risk of bias by combining the study's risk of bias (as judged by the review authors using a risk of bias tool) with its contribution to the network meta‐analysis.
-
Reporting bias (publication and other reporting biases)
Reporting bias in CINeMA is categorised as either 'suspected' or 'undetected'. Suspected reporting bias is when the review methods do not take into account unpublished data, the meta‐analysis is based on a small number of positive early findings, or treatments are exclusively studied in industry‐funded studies. Undetected reporting bias is when data from unpublished studies has been identified and findings agree, when prospective trial registration has been completed and there are no deviations from protocols, and comparisons of estimates between small and large studies agree.
-
Indirectness (relevance to the research question, addressing transitivity)
Each study in the NMA is evaluated according to its relevance to the research question. Study‐level judgements are combined with the percentage contribution of the study to the network. This approach assesses potential transitivity issues in the NMA.
-
Imprecision (the precision of the NMA, by combining direct with indirect evidence)
Relevant treatment effects that represent a minimal clinically important difference (MCID) are defined and the range of clinical equivalence is produced (the value of the MCID either side of the line of no effect). CINeMA then compares the treatment effects included in the 95% CI to the range of clinical equivalence. If the 95% CI of a treatment effect crosses the range of clinical equivalence, then it is considered to have major concerns of imprecision. If the 95% CI of a treatment effect only crosses one side of the range of equivalence then there are no concerns of imprecision.
-
Heterogeneity (variability in the results of studies)
CINeMA accounts for both heterogeneity between studies by comparing the confidence and prediction intervals of a treatment effect. When confidence and prediction intervals indicate the same effect, then there is no evidence of heterogeneity; conversely if a prediction interval leads to a different conclusion than the CIs then there is evidence of heterogeneity.
-
Incoherence (agreement between the results of direct and indirect evidence)
This is the variation between direct and indirect evidence in the network and also an assessment of transitivity. CINeMA compares the 95% CIs of the estimates of the direct and indirect estimates. If both of these estimates lie on the same side of the range of clinical equivalence, then there are no concerns about incoherence.
The CINeMA framework results in the review authors summarising the judgements across the domains into the four domains of GRADE (high certainty, moderate certainty, low certainty, very low certainty).
For outcomes where we were unable to use CINeMA due to the complexity of the network (adverse events, serious adverse events, and withdrawal), we used GRADE. The GRADE system considers the following five considerations to assess the certainty of the body of evidence for each outcome.
Serious or very serious study limitations (risk of bias)
Important or serious inconsistency of results
Some or major indirectness of evidence
Serious or very serious imprecision
Probability of publication bias
The GRADE system results in the assignment of one of the following grades to the evidence.
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different.
Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
Two authors (HB and GS) independently interpreted the findings, and collaboratively made the final judgements across all outcomes. To present our findings, we have produced separate summary of findings tables for all outcomes. We have used the template summary of findings tables designed for NMAs (Yepes‐Nuñez 2019). Due to the scale of the analyses, we only included antidepressants that had 200 or more participants in total receiving the antidepressant in the write‐ups and summary of findings tables. This decision was made to ensure quality and certainty of the final results and conclusions. We based this decision through reference of the tiers of evidence for pain research; Tier 2 uses data from at least 200 participants (Wiffen 2016).
Results
Description of studies
Results of the search
We ran the original search on 6 May 2020, and the top‐up search on 4 January 2022. Both searches searched six databases and clinicaltrials.gov. The original search returned 21,569 records, and the top‐up search returned 1814 records for a total of 23,383. After removing duplicates, we screened the titles and abstracts of 16,569 records. From this, we excluded 15,738 records, leaving 831 full‐text records. After full‐text screening, we included 176 studies. The study flow diagram is presented in Figure 1.
1.

PRISMA flow diagram of studies found, screened, and included
Included studies
In total, we included 176 studies in the review, with a total of 28,664 adult participants with a mean age of 50.6 years.
There were a variety of study designs across studies.
Antidepressant versus placebo (83 studies, e.g. Hudson 2021)
Antidepressant versus active comparator (22 studies, e.g. Enomoto 2018)
Antidepressant versus the same antidepressant at different doses versus placebo (17 studies, e.g. Arnold 2012b)
Antidepressant versus active comparator versus combined antidepressant + active comparator (13 studies, e.g. Ang 2013)
Antidepressant versus active comparator versus placebo (9 studies, e.g. Rowbotham 2012)
Antidepressant versus different antidepressant (9 studies, e.g. Kaur 2011)
Antidepressant versus active comparator versus combined antidepressant + active comparator versus placebo (8 studies, e.g. Gilron 2016)
Antidepressant versus different antidepressant versus placebo (7 studies, e.g. Heymann 2001)
Antidepressant versus different antidepressant versus active comparator (4 studies, e.g. Boyle 2012)
Antidepressant versus the same antidepressant at different doses (2 studies, e.g. Chappell 2009a)
Antidepressant versus same antidepressants at different doses versus different antidepressant versus different antidepressant at different doses versus placebo (1 study, Atkinson 2007)
Antidepressant versus different antidepressant versus combined antidepressants versus placebo (1 study, Goldenberg 1996)
Most studies were parallel‐arm design (141 studies) compared to cross‐over design (35 studies).
Studies mainly included participants with only one type of chronic pain.
59 studies included fibromyalgia
49 studies included neuropathic pain
40 studies included musculoskeletal pain
Nine studies included primary pain syndromes (not including fibromyalgia) that is, described only as 'somatoform' or 'idiopathic' pain
Six studies included gastrointestinal pain
Four studies included non‐cardiac chest pain
Two studies included burning mouth syndrome
Two studies included visceral pain
One study included atypical facial pain
One study included phantom limb pain
One study included pelvic pain
Two studies included participants with any type of chronic pain.
Most studies were funded by pharmaceutical companies.
72 studies were fully funded by pharmaceutical companies.
Five studies were partially funded by pharmaceutical companies.
67 studies were funded through non‐pharmaceutical means, mainly government, charity, or institutional funding.
32 studies did not report the source of funding.
Most studies had a primary aim of reducing pain.
144 studies had a primary aim of reducing pain.
Two studies had a primary aim of treating mood.
Eight studies had a primary aim of treating both pain and mood.
22 studies had other primary aims (e.g. sleep, other symptoms).
Studies ranged in length from two weeks to nine months, with an average length of 10 weeks.
Only six studies followed up with participants after participants finished taking the study treatment (Creed 2003; Kayiran 2010; NCT00066937; Sencan 2004; Tanum 1996; Zitman 1990). The follow‐up time points ranged from four weeks post‐treatment to one year post‐treatment.
Seven studies with a total of 156 participants provided no useable data and were therefore omitted from the NMAs (Atkinson 2007; Engel 1998; Kalso 1996; Ozerbil 2006; Sarzi Puttini 1988; Tasmuth 2002; Ward 1986).
Of the 176 studies and 28,664 participants, the number of participants receiving each antidepressant (not including combined interventions) are as follows.
Amitriptyline: 1843 (43 studies)
Bupropion: 54 (1 study)
Citalopram: 97 (5 studies)
Clomipramine: 124 (2 studies)
Desipramine: 336 (7 studies)
Desvenlafaxine: 884 (2 studies)
Dothiepin: 55 (3 studies)
Doxepin: 30 (2 studies)
Duloxetine: 6362 (43 studies)
Escitalopram: 93 (3 studies)
Esreboxetine: 978 (2 studies)
Fluoxetine: 277 (11 studies)
Imipramine: 300 (7 studies)
Maprotiline: 135 (4 studies)
Mianserin: 107 (2 studies)
Milnacipran: 3110 (18 studies)
Mirtazapine: 255 (2 studies)
Moclobemide: 42 (1 study)
Nortriptyline: 374 (7 studies)
Paroxetine: 422 (9 studies)
Pirlindole: 50 (1 study)
Reboxetine: 18 (1 study)
Sertraline: 91 (3 studies)
Trazodone: 63 (3 studies)
Trimipramine: 18 (1 study)
Venlafaxine: 489 (8 studies)
Zimeldine: 10 (1 study)
In total, 9854 participants received a placebo across 130 studies.
Excluded studies
We excluded a total of 655 references with reasons throughout the course of this review. The main reasons for exclusion were as follows.
Duplicate records (including trial registrations): 144 records
Not chronic pain condition: 71 records
Not accessible (primarily conference abstracts): 92 records
Pooled analysis: 50 records
Open‐label: 42 records
Fewer than 10 participants per arm: 22 records
Single‐blind: 15 records
Washout period not more than five lengths of antidepressant half‐life: 11
Reasons for exclusion other than these are reported in the Characteristics of excluded studies section.
We categorised 15 studies as 'awaiting classification' due to uncertainties regarding blinding or pain duration (Characteristics of studies awaiting classification), and 26 studies are ongoing (Characteristics of ongoing studies).
Risk of bias in included studies
Risk of bias findings from the included studies are shown in Figure 2 and Figure 3. Overall, we rated 116 of 176 studies as 'high risk', and 60 as 'not high risk'. However, of the 60 studies not rated as high risk, 29 had three or more domains rated as 'unclear'.
2.

Risk of bias of included studies by domain
3.

Risk of bias of included studies by study
Allocation
We did not assess any studies as high risk of bias for sequence generation or allocation concealment. For sequence generation, we judged 95 studies to be at low risk, and 81 studies as unclear. For allocation concealment, we judged 75 studies to have satisfactory procedures and rated them as low risk and the other 101 studies we rated as unclear. We rated only 64 studies as low risk of bias for both sequence generation and allocation concealment.
Blinding
For this review, we required studies comparing antidepressants with other antidepressants, different doses of the same antidepressant, or other pharmacological interventions to be double‐blind. We accepted that some interventions could not be blinded by their nature (e.g. psychological therapy, physiotherapy). These studies were included but judged to be high risk of bias for both blinding of participants, and blinding of outcomes assessors. Seventeen studies were of non‐pharmacological interventions and therefore rated high risk of bias for both domains. As this review is focused on pain, all outcomes were self‐reported by participants, and therefore judgements were often the same for both domains. In total, we rated 106 studies as low risk for both domains, and 49 studies as unclear for both domains. Low risk of bias was achieved in studies by study drugs appearing identical, having matched or sham dosing schedules across all arms, and using active placebos that mimic the side effects of antidepressants.
Incomplete outcome data
We rated the majority of studies as high risk of bias for incomplete outcome data; 102 studies were high risk. Studies were high risk primarily due to only using the last‐observation‐carried‐forward imputation method, reporting data only on participants who completed the study, or having significantly unequal attrition across arms. We rated 37 studies as low risk of bias; these studies either had no or very little attrition, or used appropriate imputation methods such as baseline‐observation‐carried‐forward or multiple imputation. We rated 37 studies as unclear, due to not clearly specifying missing data methods.
Selective reporting
We could not find protocols or trial registrations for the majority of studies. We rated 108 studies as unclear risk of bias, due to missing protocols or trial registrations published retrospectively, after the study had begun. We rated 44 studies as low risk of bias; outcomes and analyses in the published papers matched prospective protocols or registrations. We rated 24 studies as high risk of bias. Four of these studies were never published in journal articles, and data were extracted from trials registries (29060/433; NCT00066937; NCT01225068; NCT01510457). For the other studies rated as high risk of bias, there were discrepancies between the protocols and published papers that we judged to be of significant risk of bias (e.g. protocol states that outcomes would be collected that were not reported).
Other potential sources of bias
We did not identify any other sources of bias for 145 studies. We rated 17 studies as unclear risk of bias; primarily due to data not being presented in numerical form, or being reported in a different method to the protocol (e.g. percentage change rather than post‐intervention). We rated 14 studies as high risk of bias for the following reasons:
No published, peer‐reviewed articles (29060/433; NCT00066937; NCT01225068; NCT01510457)
Washout periods and tapering issues (Bateman 2013; Gilron 2016)
Poor reporting with mistakes in article (Hammody 2015)
Insufficient power (Morello 1999)
Significant differences at baseline (Razazian 2014)
Selection bias prior to participation (Spinhoven 2010)
Significant differences between published article and trial registry (Trugman 2014; Zabihiyeganeh 2021)
Using a potential intervention as a placebo (Zitman 1990)
We found some evidence of publication bias in one analysis (duloxetine versus placebo for substantial pain relief), as identified from funnel plots (used to assess small study effects as a proxy for publication bias).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Overview
The following sections detail the results of the NMAs for all outcomes included in the review. Due to the scale of the analysis, we only include antidepressants with more than 200 participants in the write‐ups and summary of findings tables. Each outcome has a table listing all the interventions included in the NMA. Antidepressants with fewer than 200 participants, and non‐antidepressant interventions are also included in figures for completeness and context.
For all outcomes, we made decisions on which networks to report in this results section. For all outcomes, we considered treatment and treatment‐dose networks. For continuous outcomes, we considered both change scores and post‐intervention scores networks. For each outcome we have reported the most robust and reliable network. The details of these decisions are reported in Appendix 2. The networks that we have not reported in this manuscript are available in the supplemental file.
The sections are reported in order of primary and secondary outcomes.
Primary outcomes:
Substantial pain relief
Pain intensity
Mood
Adverse events
Secondary outcomes:
Moderate pain relief
Physical function
Sleep
Quality of life
Patient Global Impression of Change (PGIC): proportion of participants reporting "much" and "very much" improved, and continuous scores
Serious adverse events
Withdrawal
Primary outcomes
Summary of findings tables are provided for substantial pain relief (Table 1); pain intensity (Table 2); mood (Table 3); and adverse events (Table 4).
Substantial pain relief (50% reduction)
We report the treatment‐dose network for substantial pain relief, as it was the model with the least heterogeneity and had no evidence of inconsistency.
We included 42 RCTs with a total of 14,626 participants (range in study from 47 to 1108). There were 25 different interventions, and some comparisons were informed only by direct evidence from one study. Table 6 shows the number of RCTs and total number of participants for each antidepressant dose included in the analysis. We could not include data from two studies due to disconnected networks. There were no concerns regarding model fit based on residual deviance and convergence diagnostics. The network diagram is presented in Figure 4, and the forest plot in Figure 5.
2. Substantial pain ‐ overview of interventions in the NMA.
| Treatment | RCTs | Participants |
| Antidepressants with ≥ 200 participants | ||
| Desvenlafaxine high dose | 2 | 655 |
| Duloxetine low dose | 6 | 593 |
| Duloxetine standard dose | 15 | 2429 |
| Duloxetine high dose | 14 | 1837 |
| Esreboxetine standard dose | 1 | 553 |
| Esreboxetine high dose | 1 | 280 |
| Milnacipran standard dose | 2 | 644 |
| Milnacipran high dose | 1 | 239 |
| Mirtazapine standard dose | 1 | 211 |
| Antidepressants with < 200 participants (excluded from summaries) | ||
| Amitriptyline dose unable to be categorised | 1 | 58 |
| Clomipramine standard dose | 1 | 62 |
| Desvenlafaxine standard dose | 2 | 194 |
| Esreboxetine dose unable to be categorised | 1 | 133 |
| Imipramine standard dose | 2 | 113 |
| Mianserin high dose | 2 | 89 |
| Imipramine + pregabalin standard dose | 1 | 69 |
| Venlafaxine standard dose | 1 | 86 |
| Venlafaxine high dose | 1 | 82 |
| Venlafaxine dose unable to be categorised | 1 | 64 |
| Non‐antidepressant interventions (excluded from summaries) | ||
| Carbamazepine | 1 | 85 |
| Pregabalin | 4 | 678 |
| Terbutaline | 1 | 39 |
|
RCT: randomised controlled trial Participant numbers reflect the total number of participants receiving the antidepressant. | ||
4.
Substantial pain relief network plot. NASSA: noradrenergic and specific serotonergic antidepressants; SNRI: serotonin noradrenalin reuptake inhibitors; SSRI: selective serotonin reuptake inhibitors; TCA: tricyclic antidepressants; nonad: non‐antidepressants
5.
Substantial pain relief forest plot (log odds ratio with credible intervals). NASSA: noradrenergic and specific serotonergic antidepressants; SNRI: serotonin noradrenalin reuptake inhibitors; SSRI: selective serotonin reuptake inhibitors; TCA: tricyclic antidepressants; nonad: non‐antidepressants
The top‐ranked antidepressants for substantial pain relief are shown in Table 7. Duloxetine standard dose and duloxetine high dose were the highest‐ranked antidepressants for substantial pain relief, and equally efficacious in comparison to placebo (OR 1.91, 95% CI 1.69 to 2.17 and OR 1.91, 95% CI 1.66 to 2.21, respectively). Milnacipran high dose (OR 1.64, 95% CI 1.04 to 2.58) and esreboxetine standard dose (OR 1.72, 95% CI 1.13 to 2.62) were also equally ranked, but less effective than duloxetine standard dose and duloxetine high dose. Mirtazapine standard dose, esreboxetine high dose, and desvenlafaxine high dose showed no significant difference in comparison to placebo.
3. Top‐ranked antidepressants for substantial pain relief.
| Antidepressant |
Odds ratio (95% CI) |
Mean rank | Credible intervals | |
| 2.5% | 97.5% | |||
| Duloxetine standard dose | 1.91 (1.69 to 2.17) |
8.3 | 5 | 12 |
| Duloxetine high dose | 1.91 (1.66 to 2.21) |
8.5 | 5 | 12 |
| Milnacipran high dose | 1.64 (1.04 to 2.58) |
10.9 | 4 | 19 |
| Esreboxetine standard dose | 1.72 (1.13 to 2.62) |
11.0 | 4 | 19 |
| Milnacipran standard dose | 1.65 (1.28 to 2.13) |
11.8 | 6 | 18 |
| Mirtazapine standard dose | 1.30 (0.79 to 2.15) |
15.4 | 6 | 21 |
| Duloxetine low dose | 1.71 (1.36 to 2.20) |
15.7 | 11 | 20 |
| Esreboxetine high dose | 1.29 (0.79 to 2.11) |
15.7 | 7 | 22 |
| Desvenlafaxine high dose | 1.19 (0.83 to 1.70) |
16.8 | 11 | 21 |
| CI: confidence interval | ||||
A visual representation of the cumulative rankings for every treatment included in the analysis and did not substantially alter interpretation of relative effects or mean rank credible intervals. The unrelated mean‐effect model had similar deviance information criteria to the dose‐treatment model, with no evidence of inconsistency. We confirmed this with node‐splitting models for all nine comparisons where it was possible to compare direct and indirect evidence. The comparison of pregabalin with placebo had the smallest Bayesian P value (P = 0.3) indicative of inconsistency where direct evidence suggests underestimation of the effect of pregabalin based on a single study. These figures are available in the supplemental file. The availability of a consistent evidence‐network precluded the need for exploration of transitivity violations.
Exploration of heterogeneity
Despite the risk of over‐fitting, we summarise results for multiple models because of the importance of substantial pain as an outcome for patients, clinicians, and overall quality of life. The full results of all models are reported in the supplemental file.
Class
We generated a network by aggregating treatment into classes. The analysis included four antidepressant classes: SNRI, TCA, TeCA, and NaSSA, however we could not draw any reliable conclusions about class differences due to inconsistency and overlapping credible intervals.
Condition
Studies reported substantial pain included neuropathic, fibromyalgia, musculoskeletal, primary, and gastrointestinal pain conditions. However, only neuropathic and fibromyalgia pain conditions had connected networks. We could not derive reliable treatment rankings for neuropathic pain, as the unrelated mean‐effect models and node‐splitting indicated inconsistency. For fibromyalgia, although the network geometry precluded analysis of inconsistency, esreboxetine, milnacipran, and duloxetine were relatively equally ranked: esreboxetine (mean rank = 2.02, 97.5% credible interval = 1 to 4); milnacipran (mean = 2.30, 97.5% credible interval = 1 to 4); duloxetine (2.48, 97.5% credible interval = 1 to 4).
Risk of bias
We conducted a sensitivity analysis to explore the effect of removing studies at high risk of bias. We rated 15 studies as low risk of bias. The model of the resulting network was unstable with divergent transitions indicating problems with model convergence. Unrelated mean‐effects models and the dev‐dev plot did not identify inconsistency, but we could not confirm this by node splitting due to network geometry. Results were consistent with the treatment‐dose model. The two best‐ranked antidepressants were esreboxetine (mean rank = 3.73. 97.5% credible interval = 2 to 7), and duloxetine (mean = 4.64, 97.5% credible interval = 3 to 6).
CINeMA
In addition to fitting multiple models to explore heterogeneity and utilising unrelated mean‐effects and node‐splitting models to explore inconsistency, we undertook further analysis of pairwise direct evidence and network evidence (excluding multi‐arm studies of dose) to facilitate strength of evidence assessment using CINeMA.
The design‐by‐treatment test showed no inconsistency between direct and indirect evidence (Chi² = 14.069, P = 0.296), although duloxetine low dose and desvenlafaxine high dose had high I² statistic values (73.6% and 65.8%) indicating heterogeneity. We rated duloxetine low, standard, and high doses as moderate certainty. We rated all other antidepressant doses as low, or very low certainty, primarily due to major concerns regarding studies at high risk of bias, imprecision (estimates crossing zero), and a small number of RCTs and participants contributing to the estimates.
Pain intensity
For pain intensity, we report the change‐score treatment‐dose network, as it was more robust than the other networks, with low heterogeneity and no indications of inconsistency.
Results
We included 49 RCTs with a total of 14,504 participants (range from 26 to 1191). We removed one study from this analysis due to implausible results (Miki 2016). Twenty‐eight studies compared against placebo, nine were studies with a head‐to‐head comparison with another active comparator, and 12 were dose‐comparison studies. There were 21 different interventions, and some comparisons were informed only by direct evidence from one study. Table 8 shows the number of RCTs and total number of participants for each intervention included in the analysis. There were no concerns regarding model fit. The network diagram is presented in Figure 6 and the forest plot in Figure 7.
4. Overview of interventions in pain intensity change‐score analysis.
| Treatment | RCTs | Participants |
| Antidepressants with ≥ 200 participants | ||
| Duloxetine low dose | 6 | 560 |
| Duloxetine standard dose | 18 | 2727 |
| Duloxetine high dose | 14 | 1925 |
| Milnacipran standard dose | 4 | 943 |
| Milnacipran high dose | 2 | 823 |
| Antidepressants with < 200 participants (excluded from summaries) | ||
| Amitriptyline high dose | 1 | 38 |
| Amitriptyline low dose | 1 | 70 |
| Amitriptyline standard dose | 2 | 130 |
| Amitriptyline dose unable to be categorised | 1 | 24 |
| Citalopram standard dose | 2 | 38 |
| Desipramine standard dose | 2 | 59 |
| Desipramine standard dose + lidocaine | 1 | 30 |
| Desvenlafaxine standard dose | 1 | 49 |
| Desvenlafaxine high dose | 1 | 175 |
| Esreboxetine dose unable to be categorised | 1 | 133 |
| Fluoxetine dose unable to be categorised | 1 | 25 |
| Imipramine low dose | 1 | 18 |
| Milnacipran dose unable to be categorised | 2 | 176 |
| Nortriptyline dose unable to be categorised | 1 | 38 |
| Paroxetine low dose | 1 | 74 |
| Paroxetine dose unable to be categorised | 1 | 58 |
| Non‐antidepressant interventions (excluded from summaries) | ||
| ABT‐894 | 1 | 170 |
| Cognitive behavioural therapy | 1 | 15 |
| Gabapentin | 1 | 19 |
| Lidocaine | 1 | 27 |
| Pregabalin | 2 | 550 |
| Psychotherapy | 1 | 74 |
| Usual treatment | 1 | 79 |
|
RCT: randomised controlled trial Participant numbers reflect the total number of participants receiving the antidepressant. | ||
6.
Pain intensity network diagram. SNRI: serotonin noradrenalin reuptake inhibitors; SSRI: selective serotonin reuptake inhibitors; TCA: tricyclic antidepressants; nonad: non‐antidepressants
7.
Pain intensity forest plot (standardised mean difference with credible intervals). SNRI: serotonin noradrenalin reuptake inhibitors; SSRI: selective serotonin reuptake inhibitors; TCA: tricyclic antidepressants; nonad: non‐antidepressants
Ranking of antidepressants
The top‐ranked antidepressants for pain intensity change scores are shown in Table 9. Duloxetine high and standard dose were the highest‐ranked antidepressants for pain intensity, with small to moderate effects (SMD −0.37, 95% CI −0.45 to −0.28 and SMD −0.31, 95% CI −0.39 to −0.24, respectively). Milnacipran high and standard doses had a small effect (SMD −0.22, 95% CI −0.40 to −0.05). Duloxetine low dose showed no significant difference in comparison to placebo.
5. Top‐ranked antidepressants for pain intensity change scores.
| Standardised mean difference (95% CI) | Mean rank | Credible intervals | ||
| 2.5% | 97.5% | |||
| Duloxetine high dose | ‐0.37 (‐0.45 to ‐0.28) |
9.3 | 8 | 13 |
| Duloxetine standard dose | ‐0.31 (‐0.39 to ‐0.24) |
11.1 | 10 | 15 |
| Milnacipran high dose | ‐0.22 (‐0.40 to ‐0.05) |
14.0 | 12 | 19 |
| Milnacipran standard dose | ‐0.22 (‐0.39 to ‐0.06) |
14.2 | 12 | 20 |
| Duloxetine low dose | ‐0.11 (‐0.25 to 0.03) |
17.0 | 12 | 21 |
| CI: confidence interval | ||||
A visual representation of the cumulative rankings for every intervention included in the analysis did not alter interpretation. The unrelated mean‐effect model had similar deviance information criteria to the dose‐treatment model, with no evidence of inconsistency. We confirmed this with node‐splitting models for all nine comparisons where it was possible to compare direct and indirect evidence. The lowest Bayesian P value was for the comparison of duloxetine standard dose compared to duloxetine high dose (0.08). These figures are available in the supplemental files (link provided in Appendix 3).
Condition and risk of bias
We were unable to undertake further NMAs of condition or risk of bias due to small sample sizes, network geometry and the risk of over‐fitting, but these were examined in pairwise analyses and network analysis (excluding multi‐dose arms) in CINeMA to inform strength of evidence assessment.
CINeMA
The design‐by‐treatment test showed no inconsistency between direct and indirect evidence (Chi² = 8.34; P = 0.82), although duloxetine standard dose and milnacipran standard dose had high I² statistic values (65.3% and 67.7%) indicating heterogeneity. We had moderate certainty in the estimates for duloxetine low, standard, and milnacipran standard doses. We rated all other antidepressant doses as low certainty due to major concerns regarding studies at high risk of bias and imprecision (estimates crossing zero).
Mood
For mood, we report the change‐score treatment network as this was the most robust and reliable network, with low heterogeneity and no indications of inconsistency.
Results
We included 38 RCTs with a total of 12,985 participants (range from 42 to 1191). Twenty‐two studies compared against placebo only, six were multi‐arm studies with another active comparator, nine were comparing the same antidepressant in different doses, and one compared two antidepressants together. There were 16 different interventions, and some comparisons were informed only by direct evidence from one study. We rated 23 studies as high risk of bias. At baseline, the average scores for the five most commonly used scales (Beck Depression Inventory, Brief Pain Inventory Mood Item, SF‐36 Mental Component Score, SF‐36 Mental Health Subscale, and Hamilton Depression Rating Scale) were all in the none or minimal ranges. We could not include data from one study due to disconnected networks. There were no concerns regarding model fit. An overview of the interventions in the analysis is given in Table 10. The network diagram is presented in Figure 8 and the forest plot is presented in Figure 9.
6. Overview of interventions in mood change‐score analysis.
| Treatment | RCTs | Participants |
| Antidepressants with ≥ 200 participants | ||
| Duloxetine | 26 | 4837 |
| Milnacipran | 5 | 1753 |
| Mirtazapine | 1 | 204 |
| Antidepressants with < 200 participants (excluded from summaries) | ||
| Citalopram | 2 | 38 |
| Desipramine | 1 | 27 |
| Desipramine + lidocaine | 1 | 32 |
| Esreboxetine | 1 | 126 |
| Fluoxetine | 1 | 25 |
| Imipramine | 1 | 18 |
| Milnacripran + cognitive behavioural therapy | 1 | 17 |
| Nortriptyline | 1 | 38 |
| Paroxetine | 1 | 59 |
| Non‐antidepressant interventions (excluded from summaries) | ||
| ABT‐894 | 1 | 166 |
| Cognitive behavioural therapy | 1 | 15 |
| Pregabalin | 2 | 548 |
| Psychotherapy | 1 | 58 |
| Usual treatment | 1 | 63 |
| RCT: randomised controlled trial | ||
8.
Mood network diagram. NASSA: noradrenergic and specific serotonergic antidepressants; SNRI: serotonin noradrenalin reuptake inhibitors; SSRI: selective serotonin reuptake inhibitors; TCA: tricyclic antidepressants; nonad: non‐antidepressants
9.
Mood forest plot (standardised mean difference with credible intervals). NASSA: noradrenergic and specific serotonergic antidepressants; SNRI: serotonin noradrenalin reuptake inhibitors; SSRI: selective serotonin reuptake inhibitors; TCA: tricyclic antidepressants; nonad: non‐antidepressants
Ranking of antidepressants
The top‐ranked antidepressants for mood change scores are shown in Table 11. Mirtazapine was the highest‐ranked antidepressant for mood with a moderate effect (SMD −0.5, 95% CI −0.78 to −0.22), based on one RCT. Duloxetine and milnacipran were equally ranked. Duloxetine showed very small effects (SMD −0.16, 95% CI −0.22 to −0.1), and milnacipran showed no difference in comparison to placebo.
7. Top‐ranked antidepressants for mood change‐score analysis.
| Standardised mean difference (95% CI) | Mean rank | Credible intervals | ||
| 2.5% | 97.5% | |||
| Mirtazapine | −0.5 (−0.78 to −0.22) |
3.7 | 2 | 7 |
| Duloxetine | −0.16 (−0.22 to −0.1) |
8.0 | 5 | 11 |
| Milnacipran | −0.13 (−0.26 to 0.01) |
8.9 | 5 | 13 |
| CI: confidence interval | ||||
A visual representation of the cumulative rankings for every intervention included in the analysis did not alter interpretation of the results. This figure is available the supplemental files (link provided in Appendix 3). The unrelated mean effect model had similar deviance information criteria to the dose treatment model, with no evidence of inconsistency.
Class, condition, and risk of bias
We did not undertake further analyses because of small sample sizes, network geometry and the risk of over‐fitting but pairwise and NMA (excluding multi‐dose study) were performed in CINeMA to inform strength of evidence assessment.
CINeMA
The design‐by‐treatment test showed no evidence of inconsistency (Chi² = 1.83, P = 0.4), and all I² statistic values were below 40%, despite the analysis being unable to run node‐splitting. We rated both duloxetine and milnacipran as moderate certainty; there were no domains indicating major concern. We rated mirtazapine as having low‐certainty evidence, as the estimates were formed from only one study.
Adverse events
For adverse events we report the treatment‐dose network. There were similar levels of heterogeneity and inconsistency across networks but we were able to run node‐splitting models for treatment dose.
Results
We included 93 RCTs with a total of 22,558 participants. Of all the studies in the network, 47 studies compared antidepressants only against placebo, 27 were multi‐arm studies with another active comparator, 15 were dose‐comparison studies, and four compared two antidepressants to each other. We rated 62 studies as high risk of bias. There were 60 different interventions, and some comparisons were informed only by direct evidence from one study. We could not include data from one study due to disconnected networks. There were no concerns regarding model fit. Of the 60 interventions included in the network, only nine met the criteria of 200 or more participants to be included in the summary. An overview of all the interventions included in the network is given in Table 12. The network diagram is presented in Figure 10, and the forest plot is presented in Figure 11.
8. Overview of interventions in adverse event treatment‐dose analysis.
| Treatment | RCTs | Participants |
| Antidepressants with ≥ 200 participants | ||
| Amitriptyline standard dose | 10 | 518 |
| Desvenlafaxine high dose | 2 | 685 |
| Duloxetine high dose | 15 | 2088 |
| Duloxetine low dose | 6 | 594 |
| Duloxetine standard dose | 20 | 2834 |
| Esreboxetine standard dose | 1 | 556 |
| Milnacipran high dose | 7 | 1573 |
| Milnacipran standard dose | 8 | 1256 |
| Mirtazapine standard dose | 1 | 229 |
| Antidepressants with < 200 participants (excluded from summaries) | ||
| Amitriptyline low dose | 1 | 67 |
| Amitriptyline standard dose + melatonin | 1 | 21 |
| Amitriptyline high dose | 2 | 150 |
| Amitriptyline dose unable to be categorised | 5 | 175 |
| Desipramine low dose | 1 | 38 |
| Desipramine low dose + cognitive behavioural therapy | 1 | 37 |
| Desipramine standard dose | 1 | 54 |
| Desvenlafaxine standard dose | 2 | 199 |
| Dothiepin standard dose | 1 | 30 |
| Escitalopram high dose | 1 | 41 |
| Esreboxetine high dose | 1 | 107 |
| Esreboxetine dose unable to be categorised | 1 | 134 |
| Imipramine low dose | 2 | 85 |
| Imipramine standard dose | 2 | 121 |
| Imipramine standard dose + pregabalin | 1 | 69 |
| Imipramine high dose | 1 | 40 |
| Maprotiline low dose | 1 | 33 |
| Milnacipran standard dose + cognitive behavioural therapy | 1 | 20 |
| Milnacipran dose unable to be categorised | 2 | 105 |
| Mirtazapine low dose | 1 | 13 |
| Moclobemide high dose | 1 | 43 |
| Nortriptyline low dose | 1 | 99 |
| Nortriptyline low dose + morphine | 1 | 28 |
| Nortriptyline standard dose | 1 | 28 |
| Nortriptyline dose unable to be categorised | 2 | 61 |
| Nortriptyline dose unable to be categorised + cognitive behavioural therapy | 1 | 41 |
| Nortriptyline dose unable to be categorised + disease management | 1 | 37 |
| Paroxetine unable to be categorised | 3 | 186 |
| Pirlindole low dose | 1 | 45 |
| Sertraline high dose | 1 | 30 |
| Sertraline high dose + coping skills training | 1 | 28 |
| Trazadone low dose + gabapentin | 1 | 94 |
| Venlafaxine low dose | 3 | 123 |
| Venlafaxine standard dose | 2 | 106 |
| Venlafaxine high dose | 2 | 122 |
| Non‐antidepressant interventions (excluded from summaries) | ||
| ABT‐894 | 1 | 172 |
| Acetaminophen (paracetamol) | 1 | 50 |
| Carbamazepine | 2 | 99 |
| Clonidine | 1 | 20 |
| Cognitive behavioural therapy | 4 | 155 |
| Coping skills training | 1 | 29 |
| Cyclobenzaprine | 1 | 42 |
| Disease management | 1 | 24 |
| Gabapentin | 4 | 175 |
| Lamotrigine | 1 | 46 |
| Lorazepam | 1 | 41 |
| Melatonin | 1 | 21 |
| Morphine | 1 | 28 |
| Naltrexone | 1 | 67 |
| TENS | 1 | 30 |
| Terbutaline | 1 | 51 |
|
RCT: randomised controlled trial; TENS: transcutaneous electrical nerve stimulation Participant numbers reflect the total number of participants receiving the antidepressant. | ||
10.
Adverse events network diagram. MAOI_rev: monoamine oxidase inhibitors (reversible); NASSA: noradrenergic and specific serotonergic antidepressants; SNRI: serotonin noradrenalin reuptake inhibitors; SSRI: selective serotonin reuptake inhibitors; TCA: tricyclic antidepressants; TECA: tetracyclic antidepressants; nonad: non‐antidepressants
11.
Adverse events forest plot (log odds ratio with credible intervals). MAOI_rev: monoamine oxidase inhibitors (reversible); NASSA: noradrenergic and specific serotonergic antidepressants; SNRI: serotonin noradrenalin reuptake inhibitors; SSRI: selective serotonin reuptake inhibitors; TCA: tricyclic antidepressants; TECA: tetracyclic antidepressants; nonad: non‐antidepressants
Ranking of antidepressants
The ranking of the nine antidepressants with 200 or more participants is given in Table 13. Data for adverse events were sparse, and studies were underpowered. All antidepressants with over 200 participants in the antidepressant arm were closely ranked. Desvenlafaxine and mirtazapine were the highest‐ranked antidepressants, with no significant difference compared to placebo (OR 1.67, 95% CI 0.92 to 2.41 and OR 1.70, 95% CI 0.48 to 2.91, respectively). The evidence for both of these antidepressant doses was based on only two studies each. Duloxetine standard dose, milnacipran standard dose, and duloxetine high dose were equally ranked. Duloxetine low dose, milnacipran high dose, amitriptyline standard dose, and esreboxetine standard dose were the lowest‐ranked antidepressants, with all odds ratios greater than 2.
9. Top‐ranked antidepressants for adverse events analysis.
| Odds ratio (95% CI) | Mean rank | Credible intervals | ||
| 2.5% | 97.5% | |||
| Desvenlafaxine high dose | 1.67 (0.92 to 2.41) |
30.4 | 16 | 48 |
| Mirtazapine standard dose | 1.70 (0.48 to 2.91) |
31.1 | 11 | 52 |
| Duloxetine standard dose | 1.88 (1.58 to 2.17) |
32.7 | 24 | 42 |
| Milnacipran standard dose | 1.92 (1.37 to 2.46) |
33.2 | 20 | 45 |
| Duloxetine high dose | 1.93 (1.64 to 2.23) |
33.5 | 24 | 43 |
| Duloxetine low dose | 2.03 (1.45 to 2.62) |
35.0 | 21 | 47 |
| Milnacipran high dose | 2.44 (1.89 to 2.98) |
38.9 | 25 | 50 |
| Amitriptyline standard dose | 2.66 (2.14 to 3.19) |
41.0 | 28 | 51 |
| Esreboxetine standard dose | 2.92 (1.90 to 3.93) |
41.5 | 21 | 56 |
| CI: confidence interval | ||||
A visual representation of the cumulative rankings for every intervention included in the analysis did not alter interpretation. We further investigated inconsistency through unrelated mean‐effect models and node‐splitting models for all 30 comparisons where it was possible to compare direct and indirect evidence. There was evidence of inconsistency in unrelated mean‐effects models but not node‐splitting. These figures are available in the supplemental files (link provided in Appendix 3). However, multiple divergent transition warnings indicate the potential for inconsistency to be poorly estimated in the latter models.
Class, condition, and risk of bias
Our overall model of adverse events is problematic due to divergent transitions, low effective sample sizes and inconsistency in unrelated mean‐effects model. We were unable to undertake further exploration of class, condition and risk of bias given the high uncertainty in overall effects.
CINeMA
We were unable to use CINeMA for this outcome due to complexity of the network. Therefore, two review authors (HB and GS) made the judgements based on GRADE and CINeMA domains and the available results. We judged all antidepressants and doses as very low certainty primarily due to concerns with within‐study bias, and imprecision in the network.
Secondary outcomes
Moderate pain relief (30% reduction)
For moderate pain relief we report the treatment network as this model had low heterogeneity and no evidence of inconsistency. We present the summary of findings for moderate pain relief in Table 14.
10. Moderate pain summary of findings.
| Estimates of effects, credible intervals, and certainty of the evidence for moderate pain relief in people with chronic pain | |||||||
| Bayesian network meta‐analysis summary of findings table | |||||||
|
Patient or population: people with chronic pain Interventions: mirtazapine, duloxetine, milnacipran. all doses were combined for each antidepressant. Comparator (reference): placebo Outcome: moderate pain relief (defined as 30% reduction in pain intensity from baseline to post‐intervention; measured on a range of scales including 0‐10 VAS, 0‐100 VAS, and hort‐form McGill Pain Questionnaire Direction: Higher is better (i.e. more people reporting moderate pain relief) | |||||||
|
Total studies: 40 Total participants: 14,208 |
Relative effect (OR and 95% CI) |
Anticipated absolute effect (event rate)* | Certainty of the evidence (CINeMA) |
Ranking** (2.5% to 97.5% credible interval) |
Interpretation of findings | ||
| With placebo | With intervention | Difference | |||||
|
Mirtazapine RCTs: 2 Participants: 462 |
1.92 (1.45 to 2.39) |
70/224 313 per 1000 |
112/238 466 per 1000 |
154 more per 1000 | Lowe | 7 (3 to 13) |
Equivalent NNTB is 6.5 |
|
Duloxetine RCTs: 24 Participants: 7833 |
1.79 (1.67 to 1.91) |
1324/3271 405 per 1000 |
2469/4562 549 per 1000 |
144 more per 1000 | Moderatea | 7 (4 to 11) |
Equivalent NNTB is 6.9 |
|
Milnacipran RCTs: 7 Participants: 3056 |
1.7 (1.48 to 1.92) |
347/1128 308 per 1000 |
825/1928 430 per 1000 |
123 more per 1000 | Moderatea | 8 (4 to 12) |
Equivalent NNTB is 8.1 |
|
Esreboxetine RCTs: 2 Participants: 1374 |
1.65 (1.32 to 1.98) |
107/409 262 per 1000 |
356/965 369 per 1000 |
107 more per 1000 | Lowa,e | 9 (4 to 13) |
Equivalent NNTB is 9.3 |
|
Network meta‐analysis‐summary of findings table definitions * Anticipated absolute effect. Anticipated absolute effect compares two risks by calculating the difference between the risk of the intervention group with the risk of the control group. ** Mean rank and credible intervals are presented. CI: confidence interval; CINeMA: Confidence in Network Meta‐Analysis; NNTB: number needed to treat for an additional beneficial outcome; OR: odds ratio; RCT: randomised controlled trial; VAS: visual analogue scale The number of participants for each antidepressant reflects the total number of participants taking the antidepressant or placebo from the studies in the network meta‐analysis. | |||||||
|
CINeMA grades of confidence in the evidence High: further research is unlikely to change our confidence in the estimate of effect. Moderate: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low: we are very uncertain about the estimate. | |||||||
aDowngraded due to within‐study bias. bDowngraded due to imprecision in the estimate. cDowngraded due to heterogeneity in the estimate. dDowngraded due to incoherence in the network. eDowngraded due to a small number of trials and participants; we cannot draw reliable conclusions.
Results
We included 40 RCTs with a total of 14,208 participants (range from 37 to 1025). Twenty studies compared against placebo, eight were multi‐arm studies with another active comparator, 11 were dose‐comparison studies, and one study compared two antidepressants head to head. There were 17 different interventions, and some comparisons were informed only by direct evidence from one study. We rated 25 studies as high risk of bias. There were no concerns regarding model fit. The network diagram is presented in Figure 12, and the forest plot is presented in Figure 13. An overview of the interventions included in the analysis is given in Table 15.
12.
Moderate pain relief network diagram. NASSA: noradrenergic and specific serotonergic antidepressants; SNRI: serotonin noradrenalin reuptake inhibitors; TCA: tricyclic antidepressants; nonad: non‐antidepressants
13.
Moderate pain relief forest plot. NASSA: noradrenergic and specific serotonergic antidepressants; SNRI: serotonin noradrenalin reuptake inhibitors; TCA: tricyclic antidepressants; nonad: non‐antidepressants
11. Overview of all interventions in the moderate pain relief analysis.
| Treatment | RCTs | Participants |
| Antidepressants with ≥ 200 participants | ||
| Duloxetine | 24 | 4562 |
| Esreboxetine | 2 | 965 |
| Milnacipran | 7 | 1928 |
| Mirtazapine | 2 | 238 |
| Antidepressants with < 200 participants (excluded from summaries) | ||
| Amitriptyline | 2 | 80 |
| Desipramine | 1 | 37 |
| Desipramine + cognitive behavioural therapy | 1 | 37 |
| Imipramine | 2 | 113 |
| Imipramine + pregabalin | 1 | 69 |
| Venlafaxine | 1 | 86 |
| Non‐antidepressant interventions (excluded from summaries) | ||
| Carbamazepine | 2 | 85 |
| Cognitive behavioural therapy | 2 | 53 |
| Gabapentin | 1 | 22 |
| Pregabalin | 4 | 680 |
| Terbutaline | 1 | 39 |
|
RCT: randomised controlled trial Participant numbers reflect the total number of participants receiving the antidepressant. | ||
Ranking of antidepressants
The top‐ranked antidepressants for moderate pain relief are shown in Table 16. All antidepressants with more than 200 participants in the antidepressant arm showed an effect for moderate pain relief, and were very closely ranked. Mirtazapine was the highest‐ranked antidepressant (OR 1.92, 95% CI 1.45 to 2.39), followed by duloxetine (OR 1.79, 95% CI 1.67 to 1.91), milnacipran (OR 1.70, 95% CI 1.48 to 1.92) and esreboxetine (OR 1.65, 95% CI 1.32 to 1.98).
12. Top‐ranked antidepressants moderate pain relief.
| Odds ratio (95% CI) | Mean rank | Credible intervals | ||
| 2.5% | 97.5% | |||
| Mirtazapine | 1.92 (1.45 to 2.39) |
6.9 | 3 | 13 |
| Duloxetine | 1.79 (1.67 to 1.91) |
7.4 | 4 | 11 |
| Milnacipran | 1.7 (1.48 to 1.92) |
8.2 | 4 | 12 |
| Esreboxetine | 1.65 (1.32 to 1.98) |
8.7 | 4 | 13 |
| CI: confidence interval | ||||
A visual representation of the cumulative rankings for every intervention included in the analysis did not alter interpretation. The unrelated mean‐effect model showed no evidence of inconsistency. We confirmed this with node‐splitting models for all nine comparisons where it was possible to compare direct and indirect evidence. The comparison of duloxetine and placebo had the lowest Bayesian P value (0.18) with indirect evidence indicative of a larger effect than direct evidence. These figures are available in the supplemental files (link provided in Appendix 3).
Exploration of heterogeneity
We also explored the impact of including dose in the model. There was low heterogeneity (Tau = 0.11), and whilst there was no evidence of inconsistency in unrelated mean‐effects and node‐splitting models, there were several divergent transitions. The analysis showed similar rankings of antidepressants to the treatment‐only model, with mirtazapine, duloxetine, and milnacipran remaining the highest‐ranked across doses. The full results of all the analyses are reported in the supplemental files (link provided in Appendix 3).
Class
Three classes were included in the treatment ‐only analysis: NaSSA, SNRI, and TCA. Only the NaSSA and SNRI classes had over 200 participants in the analyses. SNRI was the highest‐ranked class (logOR: 0.56; CrI: 0.45 to 0.60) followed by NaSSA (logOR: 0.67; CrI: 0.11 to 1.23).
Condition and risk of bias
We were unable to undertake further NMAs due to small sample size, network geometry and risk of over‐fitting; but pairwise and NMA excluding multi‐dose studies were undertaken to inform strength of evidence assessment using CINeMA.
CINeMA
The design‐by‐treatment test showed no evidence of inconsistency between the direct and indirect evidence in the network (Chi² = 2.65, P = 0.62), and only esreboxetine had an I² statistic value of above 40% (44.6%). We rated duloxetine and milnacipran as moderate certainty, while we downgraded mirtazapine and esreboxetine due to low numbers of studies and participants.
Physical function
For physical function, we report the change‐score treatment‐dose network as it had lower heterogeneity than other models and no inconsistency. We present the summary of findings for physical function in Table 17.
13. Physical function summary of findings.
| Estimates of effects, credible intervals, and certainty of the evidence of antidepressants on physical function in people with chronic pain | |||||||
| Bayesian network meta‐analysis summary of findings table | |||||||
|
Patient or population: people with chronic pain Interventions: duloxetine standard dose (60 mg) and high dose (> 60 mg); milnacipran standard dose (100 mg) and high dose (> 100 mg); mirtazapine standard dose (30 mg) Comparator (reference): placebo Outcome: change in physical function (lower scores are better) from a range of measures, including Fibromyalgia Impact Questionnaire and the SF‐36 Direction: lower is better (i.e. a greater improvement in physical function and disability) | |||||||
|
Total studies: 32 Total participants: 11,760 |
Relative effect | Anticipated absolute effect (event rate) | Certainty of the evidence (CINeMA) |
Ranking* (2.5% to 97.5% credible interval) |
Interpretation of findings** | ||
| With placebo | With intervention | Difference | |||||
|
Duloxetine standard dose RCTs: 15 Participants: 3887 |
‐ | ‐ | ‐ |
SMD −0.24 (−0.32 to −0.18) |
High | 6 (3 to 8) |
Small effect |
|
Duloxetine high dose RCTs: 13 Participants: 3503 |
‐ | ‐ | ‐ |
SMD −0.23 (−0.30 to −0.16) |
Moderatea | 6 (2 to 9) |
Small effect |
|
Milnacipran standard dose RCTs: 3 Participants: 1840 |
‐ | ‐ | ‐ |
SMD −0.18 (−0.30 to −0.07) |
Moderatea | 7 (4 to 11) |
Small effect |
|
Milnacipran high dose RCTs: 2 Participants: 1670 |
‐ | ‐ | ‐ |
SMD −0.1 (−0.22 to 0.07) |
Very lowa,c | 9 (6 to 13) |
Not significantly different from placebo |
|
Mirtazapine standard dose RCTs: 1 Participants: 204 |
‐ | ‐ | ‐ |
SMD 0.62 (0.11 to 0.69) |
Very lowe | 16 (15 to 16) |
Moderate to large effect |
|
Network meta‐analysis‐summary of findings table definitions ** Mean rank and credible intervals are presented. **SMD interpretation based on clinical judgement and in line with Cohen 1988 and the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2022) as small (0.2), moderate (0.5) and large (0.8). CI: confidence interval; CINeMA: Confidence in Network Meta‐Analysis; RCT: randomised controlled trial; SMD: standardised mean difference; VAS: visual analogue scale The number of participants for each antidepressant reflects the total number of participants taking the antidepressant or placebo from the studies in the network meta‐analysis. | |||||||
|
CINeMA grades of confidence in the evidence High: further research is unlikely to change our confidence in the estimate of effect. Moderate: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low: we are very uncertain about the estimate. | |||||||
aDowngraded due to within‐study bias. bDowngraded due to imprecision in the estimate. cDowngraded due to heterogeneity in the estimate. dDowngraded due to incoherence in the network. eDowngraded due to a small number of trials and participants; we cannot draw reliable conclusions.
Results
We included 32 RCTs with a total of 11,760 participants (range from 42 to 1025). Twenty studies compared against placebo, four were head‐to‐head studies with another active comparator, seven were dose‐comparison studies, and one was a direct head‐to‐head comparison between two different antidepressants. There were 18 different interventions, and some comparisons were informed only by direct evidence from one study. We rated 21 studies as high risk of bias. We did not need to remove any studies due to disconnected networks. There were no concerns regarding model fit. The network diagram is presented in Figure 14, the forest plot of placebo comparisons in Figure 15, and Table 18 shows the number of RCTs and total number of participants for each intervention included in the analysis.
14.
Physical function network diagram. NASSA: noradrenergic and specific serotonergic antidepressants; SNRI: serotonin noradrenalin reuptake inhibitors; SSRI: selective serotonin reuptake inhibitors; TCA: tricyclic antidepressants; nonad: non‐antidepressants
15.
Physical function forest plot (standardised mean difference with credible intervals). NASSA: noradrenergic and specific serotonergic antidepressants; SNRI: serotonin noradrenalin reuptake inhibitors; SSRI: selective serotonin reuptake inhibitors; TCA: tricyclic antidepressants; nonad: non‐antidepressants
14. Overview of all interventions in the physical function analysis.
| Treatment | RCTs | Participants |
| Antidepressants with ≥ 200 participants | ||
| Duloxetine high dose | 13 | 1831 |
| Duloxetine standard dose | 14 | 2157 |
| Milnacipran high dose | 2 | 823 |
| Milnacipran standard dose | 3 | 930 |
| Mirtazapine standard dose | 1 | 204 |
| Antidepressants with < 200 participants (excluded from summaries) | ||
| Citalopram standard dose | 2 | 38 |
| Duloxetine low dose | 2 | 150 |
| Esreboxetine dose unable to be categorised | 1 | 126 |
| Fluoxetine | 1 | 25 |
| Imipramine | 1 | 18 |
| Milnacipran standard + cognitive behavioural therapy | 1 | 17 |
| Nortriptyline dose unable to be categorised | 1 | 38 |
| Paroxetine low dose | 1 | 59 |
| Non‐antidepressant interventions (excluded from summaries) | ||
| ABT‐894 | 1 | 166 |
| Cognitive behavioural therapy | 1 | 15 |
| Pregabalin | 1 | 401 |
| Psychotherapy | 1 | 58 |
| Usual treatment | 1 | 63 |
|
RCT: randomised controlled trial Participant numbers reflect the total number of participants receiving the antidepressant. | ||
Ranking of antidepressants
The top‐ranked antidepressants for physical function change scores are shown in Table 19. Duloxetine standard dose (SMD −0.24, 95% CI −0.32 to −0.18), duloxetine high dose (SMD −0.23, 95% CI 0.30 to 0.16), and milnacipran standard dose (SMD −0.18, 95% CI −0.30 to −0.07) were the highest‐ranked antidepressants with small effects. Duloxetine standard dose and duloxetine high doses were equally effective. Milnacipran high dose showed no significant difference compared to placebo (SMD −0.10, 95% CI −0.22 to 0.07). Mirtazapine standard dose was the lowest‐ranked antidepressant (SMD 0.62, 95% CI 0.11 to 0.69).
15. Top‐ranked antidepressants for physical function change‐score analysis.
| Standardised mean difference (95% CI) | Mean rank | Credible intervals | ||
| 2.5% | 97.5% | |||
| Duloxetine standard | −0.24 (−0.32 to −0.18) |
5.5 | 3 | 8 |
| Duloxetine high | −0.23 (−0.30 to −0.16) |
6.0 | 2 | 9 |
| Milnacipran standard | −0.18 (−0.30 to −0.07) |
7.3 | 4 | 11 |
| Milnacipran high | −0.10 (−0.22 to 0.07) |
9.5 | 6 | 13 |
| Mirtazapine standard | 0.62 (0.11 to 0.69) |
15.9 | 15 | 16 |
| CI: confidence interval | ||||
A visual representation of the cumulative rankings for every intervention included in the analysis did not alter interpretation. We performed node‐splitting models for all four comparisons where it was possible to compare direct and indirect evidence. The lowest Bayesian P value was for the comparison of duloxetine high dose compared to placebo, where direct evidence showed a larger effect than indirect evidence (P = 0.07). These figures are available in the supplemental files (link provided in Appendix 3).
Class
We included four classes of antidepressants in the analysis: SNRI, SSRI, TCA, and NaSSA, however due to interventions including combinations of drugs, we could not analyse models including class.
Condition and risk of bias
We were unable to undertake further NMAs due to small sample sizes, network geometry and the risk of over‐fitting.
CINeMA
The design‐by‐treatment test showed no evidence of inconsistency between the direct and indirect evidence (Chi² = 6.45, P = 0.69), and no antidepressants had an I² statistic value of over 40%, although values could not be generated for mirtazapine. We rated duloxetine and milnacipran as moderate certainty, downgraded only due to some concerns with within‐study bias. We downgraded esreboxetine and mirtazapine further to low due to the small number of studies and participants included in the analyses.
Sleep
For sleep, we report the change‐score treatment‐dose network as this was the most robust and reliable model. We present summary of findings for sleep in presented Table 20.
16. Sleep summary of findings.
| Estimates of effects, credible intervals, and certainty of the evidence of antidepressants on sleep in people with chronic pain | |||||||
| Bayesian network meta‐analysis summary of findings table | |||||||
|
Patient or population: people with chronic pain Interventions: duloxetine standard dose (60 mg) and high dose (> 60 mg); milnacipran standard dose (100 mg) and high dose (> 100 mg) Comparator (reference): placebo Outcome: change in sleep as measured on various scales, primarily Brief Pain Inventory Sleep Item Direction: lower is better (i.e. greater improvement in sleep compared to baseline) | |||||||
|
Total studies: 18 Total participants: 6301 |
Relative effect | Anticipated absolute effect (event rate) | Certainty of the evidence (CINeMA) |
Ranking* (2.5% to 97.5% credible interval) |
Interpretation of findings** | ||
| With placebo | With intervention | Difference | |||||
|
Duloxetine standard RCTs: 11 Participants: 2615 |
‐ | ‐ | ‐ |
SMD −0.21 (−0.30 to −0.12) |
Moderatea,d | 3 (1 to 6) |
Small effect |
|
Duloxetine high RCTs: 6 Participants: 1494 |
‐ | ‐ | ‐ |
SMD −0.14 (−0.27 to −0.01) |
Very lowa,c,d | 4 (2 to 7) |
Small effect |
|
Milnacipran standard RCTs: 1 Participants: 799 |
‐ | ‐ | ‐ |
SMD −0.06 (−0.30 to 0.17) |
Very lowa,c,d,e | 6 (2 to 9) |
Not significantly different from placebo |
|
Milnacipran high RCTs: 1 Participants: 797 |
‐ | ‐ | ‐ |
SMD −0.03 (−0.29 to 0.20) |
Very lowa,c,d,e | 7 (2 to 9) |
Not significantly different from placebo |
|
Network meta‐analysis‐summary of findings table definitions * Mean rank and credible intervals are presented. **SMD interpretation based on clinical judgement and in line with Cohen 1988 and the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2022) as small (0.2), moderate (0.5) and large (0.8). CI: confidence interval; CINeMA: Confidence in Network Meta‐Analysis; RCT: randomised controlled trial; SMD: standardised mean difference The number of participants for each antidepressant reflects the total number of participants taking the antidepressant or placebo from the studies in the network meta‐analysis. | |||||||
|
CINeMA grades of confidence in the evidence High: further research is unlikely to change our confidence in the estimate of effect. Moderate: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low: we are very uncertain about the estimate. | |||||||
aDowngraded due to within‐study bias. bDowngraded due to imprecision in the estimate. cDowngraded due to heterogeneity in the estimate. dDowngraded due to incoherence in the network. eDowngraded due to a small number of trials and participants; we cannot draw reliable conclusions.
Results
We included 18 RCTs with a total of 6301 participants (range from 42 to 1195). Twelve studies compared against placebo and six were dose‐comparison studies. There were eight different interventions, and some comparisons were informed only by direct evidence from one study. We rated nine studies as high risk of bias overall. There were no concerns regarding model fit. The network diagram is presented in Figure 16, the forest plot for placebo comparison is presented in Figure 17, and an overview of all interventions included in the analysis is given in Table 21.
16.
Sleep network diagram. SNRI: serotonin noradrenalin reuptake inhibitors; SSRI: selective serotonin reuptake inhibitors
17.
Sleep forest plot (standardised mean difference with credible intervals). SNRI: serotonin noradrenalin reuptake inhibitors; SSRI: selective serotonin reuptake inhibitors
17. Overview of all interventions in the sleep analysis.
| Treatment | RCTs | Participants |
| Antidepressants with ≥ 200 participants | ||
| Duloxetine standard dose | 11 | 1640 |
| Duloxetine high dose | 6 | 891 |
| Milnacipran standard dose | 1 | 398 |
| Milnacipran high dose | 1 | 396 |
| Antidepressants with < 200 participants (excluded from summaries) | ||
| Citalopram standard dose | 1 | 21 |
| Duloxetine low dose | 1 | 141 |
| Esreboxetine unable to be categorised | 1 | 126 |
| Milnacipran unable to be categorised | 1 | 97 |
|
RCT: randomised controlled trial Participant numbers reflect the total number of participants receiving the antidepressant. | ||
Ranking of antidepressants
The top‐ranked antidepressants for sleep are displayed in Table 22. Duloxetine standard and high doses were the highest‐ranked antidepressants, and the only antidepressants to show a significant effect when compared to placebo, although the effects were small (standard dose: SMD −0.21, 95% CI −0.30 to −0.12; high dose: SMD −0.14, 95% CI −0.27 to −0.01). Milnacipran standard dose (SMD −0.06, 95% CI −0.30 to 0.17) and high dose (SMD −0.03, 95% CI −0.29 to 0.20) showed no significant difference in comparison to placebo.
18. Top‐ranked antidepressants for sleep change‐score analysis.
| Standardised mean difference (95% CI) | Mean rank | Credible intervals | ||
| 2.5% | 97.5% | |||
| Duloxetine standard | −0.21 (−0.30 to −0.12) |
3.0 | 1 | 6 |
| Duloxetine high | −0.14 (−0.27 to −0.01) |
4.4 | 2 | 7 |
| Milnacipran standard | −0.06 (−0.30 to 0.17) |
6.0 | 2 | 9 |
| Milnacipran high | −0.03 (−0.29 to 0.20) |
6.6 | 2 | 9 |
| CI: confidence interval | ||||
A visual representation of the cumulative rankings for every intervention included in the analysis did not alter interpretations. Node‐splitting models had divergent transitions and indicated inconsistency for the comparison of high and standard dose duloxetine (P 0.02). We therefore downgraded the strength of evidence for the duloxetine high dose estimate. These figures are available in the supplemental files (link provided in Appendix 3).
Exploration of heterogeneity
Class, condition and risk of bias
Although there were two different classes in the network (SNRI and SSRI), SSRI was only represented by one study using citalopram with 21 participants; therefore only SNRI crossed the threshold of 200 participants. We did not explore condition and risk of bias further using NMA because of concerns about sample size, network geometry and the risk of over‐fitting.
CINeMA
The design‐by‐treatment test showed no evidence of inconsistency between the direct and indirect evidence in the network (Chi²= 7.39, P = 0.4) despite the concerns identified in node‐splitting models. No antidepressants had I² statistic values of above 40%, although we could not calculate values for milnacipran high or standard doses. We rated only duloxetine as moderate certainty, downgraded from high due to some concerns about within‐study bias and inconsistency from the NMA. We rated duloxetine high dose, milnacipran high dose, and milnacipran standard dose as very low certainty. We downgraded duloxetine high dose due to major concerns regarding within‐study bias and incoherence. We downgraded milnacipran standard and high doses due to major concerns regarding within‐study bias, and some concerns regarding imprecision, heterogeneity, and inconsistency. Of note, both milnacipran doses analyses were informed by the same study.
Quality of life
For quality of life we report the post‐intervention treatment network, as this was the network with the lowest heterogeneity. We present summary of findings for quality of life in Table 23.
19. Quality of life summary of findings.
| Estimates of effects, credible intervals, and certainty of the evidence of antidepressants on quality of life in people with chronic pain | |||||||
| Bayesian network meta‐analysis summary of findings table | |||||||
|
Patient or population: people with chronic pain Interventions: duloxetine, esreboxetine. All doses were combined for each antidepressant. Comparator (reference): placebo Outcome: quality of life (post‐intervention scores) as reported on various scales including the EQ5D and the Fibromyalgia Impact Questionnaire Direction: higher is better (i.e. a greater improvement in quality of life compared to baseline) | |||||||
|
Total studies: 19 Total participants: 3103 |
Relative effect | Anticipated absolute effect (event rate) | Certainty of the evidence (CINeMA) |
Ranking* (2.5% to 97.5% credible interval) |
Interpretation of findings** | ||
| With placebo | With intervention | Difference | |||||
|
Esreboxetine RCTs: 1 Participants: 998 |
‐ | ‐ | ‐ |
SMD −0.30 (−1.24 to 0.64) |
Very lowe | 8 (1 to 21) |
Not significantly different from placebo |
|
Duloxetine RCTs: 6 Participants: 867 |
‐ | ‐ | ‐ |
SMD 0.02 (−0.56 to 0.58) |
Lowa,e | 12 (4 to 20) |
Not significantly different from placebo |
|
Network meta‐analysis‐summary of findings table definitions * Mean rank and credible intervals are presented **SMD interpretation based on clinical judgement and in line with Cohen 1988 and the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2022) as small (0.2), moderate (0.5) and large (0.8). CI: confidence interval; CINeMA: Confidence in Network Meta‐Analysis; RCT: randomised controlled trial; SMD: standardised mean difference The number of participants for each antidepressant reflects the total number of participants taking the antidepressant or placebo from the studies in the network meta‐analysis. | |||||||
|
CINeMA grades of confidence in the evidence High: further research is unlikely to change our confidence in the estimate of effect. Moderate: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low: we are very uncertain about the estimate. | |||||||
aDowngraded due to within‐study bias. bDowngraded due to imprecision in the estimate. cDowngraded due to heterogeneity in the estimate. dDowngraded due to incoherence in the network. eDowngraded due to a small number of trials and participants; we cannot draw reliable conclusions.
Results
We included 19 RCTs with a total of 3103 participants (range from 30 to 998). Five studies compared against placebo, 11 were multi‐arm studies with another active comparator, two were direct head‐to‐head comparisons of different antidepressants, and one was a dose‐comparison study. There were 23 different interventions, and some comparisons were informed only by direct evidence from one study. We could not include data from one study due to disconnected networks. We rated 13 studies as high risk of bias overall. There were no concerns regarding model fit. The network diagram is presented in Figure 18 and the forest plot is presented in Figure 19. An overview of the interventions included in the analysis is presented in Table 24.
18.
Quality of life network diagram. SNRI: serotonin noradrenalin reuptake inhibitors; SSRI: selective serotonin reuptake inhibitors; TCA: tricyclic antidepressants; nonad: non‐antidepressants
19.
Quality of life forest plot (standardised mean difference with credible intervals). SNRI: serotonin noradrenalin reuptake inhibitors; SSRI: selective serotonin reuptake inhibitors; TCA: tricyclic antidepressants; nonad: non‐antidepressants
20. Overview of all interventions in the quality‐of‐life post‐intervention analysis.
| Treatment | RCTs | Participants |
| Antidepressants with ≥ 200 participants | ||
| Duloxetine | 6 | 306 |
| Esreboxetine | 1 | 736 |
| Antidepressants with < 200 participants (excluded from summaries) | ||
| Amitriptyline | 181 | |
| Amitriptyline + fluoxetine | 1 | 19 |
| Amitriptyline + melatonin | 1 | 21 |
| Amitriptyline + splint | 1 | 23 |
| Desipramine | 135 | |
| Duloxetine + pregabalin | 1 | 39 |
| Fluoxetine | 61 | |
| Fluoxetine + melatonin | 1 | 50 |
| Imipramine | 42 | |
| Milnacipran | 53 | |
| Nortriptyline | 36 | |
| Non‐antidepressant interventions (excluded from summaries) | ||
| ABT‐894 | 1 | 169 |
| Acupuncture | 1 | 28 |
| Cognitive behavioural therapy | 199 | |
| Education | 1 | 66 |
| Melatonin | 1 | 48 |
| Pregabalin | 1 | 63 |
| Saffron | 1 | 23 |
| Terbutaline | 1 | 40 |
| Waitlist | 1 | 21 |
|
RCT: randomised controlled trial Participant numbers reflect the total number of participants receiving the antidepressant. | ||
Ranking of antidepressants
The top‐ranked antidepressants for quality of life are displayed in Table 25. Neither esreboxetine nor duloxetine showed a significant difference compared to placebo for quality of life (SMD −0.30, 95% CI −1.24 to 0.64 and SMD 0.02, 95% CI −0.56 to 0.58, respectively).
21. Top‐ranked antidepressants for quality‐of‐life analysis.
| Standardised mean difference (95% CI) | Mean rank | Credible intervals | ||
| 2.5% | 97.5% | |||
| Esreboxetine | −0.30 (−1.24 to 0.64) |
8.2 | 1 | 21 |
| Duloxetine | 0.02 (−0.56 to 0.58) |
12.1 | 4 | 20 |
| CI: confidence interval | ||||
A visual representation of the cumulative rankings for every intervention included in the analysis did not alter interpretations. Node‐splitting models were undertaken for all 13 comparisons where it was possible to compare direct and indirect evidence. The comparison with the lowest Bayesian P value (0.16) was fluoxetine compared to amitriptyline. These figures are available in the supplemental files (link provided in Appendix 3). Unrelated mean‐effects models also failed to identify inconsistency.
Exploration of heterogeneity
We explored models including both treatment and dose; this model had higher heterogeneity (Tau = 0.67) and similar residual deviance to that of the treatment‐only model.
Class, condition and risk of bias
We were unable to generate meaningful networks including class, condition, and risk of bias. Only one class had antidepressants with over 200 participants (SNRI). Small sample sizes, network geometry and the risk of over‐fitting precluded analyses of condition and risk of bias.
CINeMA
The design‐by‐treatment test showed evidence of significant inconsistency between the direct and indirect evidence in the network (Chi² = 80.27, P = 0.00) despite node‐splitting and unrelated mean‐effect models indicating no concern. The I² statistic value for duloxetine showed evidence of heterogeneity (I²= 67.2%) and could not be calculated for esreboxetine. Therefore, we rated duloxetine as having low‐certainty evidence (downgraded due to within‐study bias, heterogeneity, and inconsistency) and esreboxetine as very low‐certainty evidence (downgraded due to within‐study bias, inconsistency, and low numbers of studies).
Patient Global Impression of Change (PGIC)
PGIC was reported in two ways: as a continuous score, and as the proportion of participants scoring one (very much improved) and two (much improved). We include both of these results.
PGIC much and very much improved
For PGIC much and very much improved we report the treatment‐dose network as this had low heterogeneity with no inconsistency. We present summary of findings for PGIC much or very much improved in Table 26.
22. Patient Global Impression of Change much/very much improved summary of findings.
| Estimates of effects, credible intervals, and certainty of the evidence of antidepressants on Patient Global Impression of Change in people with chronic pain | |||||||
| Bayesian network meta‐analysis summary of findings table | |||||||
|
Patient or population: people with chronic pain Interventions: desvenlafaxine high dose (> 50 mg); duloxetine standard dose (60 mg) and high dose (> 60 mg); esreboxetine standard dose (4‐8 mg) and high dose (> 8 mg); milnacipran standard dose (100 mg) and high dose (> 100 mg) Comparator (reference): Placebo Outcome: Patient Global Impression of Change (PGIC) – people reporting much or very much improved (i.e. 1 or 2 on the 7‐point PGIC scale) Direction: higher is better (i.e. more people reporting much or very much improved from baseline) | |||||||
|
Total studies: 12 Total participants: 6995 |
Relative effect (OR and 95% CI) |
Anticipated absolute effect (event rate)* | Certainty of the evidence (CINeMA) |
Ranking** (2.5% to 97.5% credible interval) |
Interpretation of findings | ||
| With placebo | With intervention | Difference | |||||
|
Duloxetine standard dose RCTs: 3 Participants: 974 |
2.29 (1.98 to 2.60) |
215 per 1000 106/493 |
382 per 1000 184/481 |
170 more per 1000 | Moderatea | 2 (1 to 6) |
Equivalent to NNTB of 5.9 |
|
Duloxetine high dose RCTs: 2 Participants: 567 |
2.03 (1.62 to 2.44) |
250 per 1000 70/280 |
404 per 1000 113/287 |
154 more per 1000 | Very lowa,e | 4 (1 to 7) |
Equivalent to NNTB of 6.5 |
|
Milnacipran high dose RCTs: 3 Participants: 2057 |
1.99 (1.77 to 2.21) |
282 per 1000 280/992 |
439 per 1000 480/1065 |
157 more per 1000 | Lowa | 4 (1 to 7) |
Equivalent to NNTB of 6.4 |
|
Milnacipran standard dose RCTs: 3 Participants: 2098 |
1.95 (1.73 to 2.17) |
303 per 1000 320/1055 |
459 per 1000 462/1043 |
156 more per 1000 | Moderatea | 4 (1 to 7) |
Equivalent to NNTB of 6.4 |
|
Esreboxetine standard dose RCTs: 1 Participants: 811 |
1.79 (1.44 to 2.14) |
291 per 1000 80/275 |
423 per 1000 226/536 |
133 more per 1000 | Very lowa,e | 5 (1 to 7) |
Equivalent to NNTB of 7.5 |
|
Esreboxetine high dose RCTs: 1 Participants: 550 |
1.63 (1.24 to 2.02) |
291 per 1000 80/275 |
401 per 1000 110/275 |
110 more per 1000 | Very lowa,e | 6 (2 to 8) |
Equivalent to NNTB of 9.1 |
|
Desvenlafaxine high dose RCTs: 1 Participants: 528 |
1.01 (0.58 to 1.44) |
429 per 1000 54/126 |
431 per 1000 173/402 |
2 more per 1000 | Very lowa,b,e | 8 (6 to 9) |
Not significantly different from placebo |
|
Network meta‐analysis‐summary of findings table definitions * Anticipated absolute effect. Anticipated absolute effect compares two risks by calculating the difference between the risk of the intervention group with the risk of the control group. ** Mean rank and credible intervals are presented. CI: confidence interval; CINeMA: Confidence in Network Meta‐Analysis; NNTB: number needed to treat for an additional beneficial outcome; OR: odds ratio; PGIC: Patient Global Impression of Change; RCT: randomised controlled trial; VAS: visual analogue scale The number of participants for each antidepressant reflects the total number of participants taking the antidepressant or placebo from the studies in the network meta‐analysis. | |||||||
|
CINeMA grades of confidence in the evidence High: further research is unlikely to change our confidence in the estimate of effect. Moderate: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low: we are very uncertain about the estimate. | |||||||
aDowngraded due to within‐study bias. bDowngraded due to imprecision in the estimate. cDowngraded due to heterogeneity in the estimate. dDowngraded due to incoherence in the network. eDowngraded due to a small number of trials and participants; we cannot draw reliable conclusions.
Results
We included 12 RCTs with a total of 6995 participants (range from 43 to 1025). Eight studies compared against placebo and four were dose‐comparison studies. There were nine different interventions, and some comparisons were informed only by direct evidence from one study. We judged seven studies to be high risk of bias. There were no concerns regarding model fit. The network diagram is presented in Figure 20, and the forest plot is presented in Figure 21. An overview of all interventions included in the analysis is given in Table 27.
20.
Patient Global Impression of Change much/very much improved network diagram. SNRI: serotonin noradrenalin reuptake inhibitors
21.
Patient Global Impression of Change much/very much improved forest plot. SNRI: serotonin noradrenalin reuptake inhibitors; SSRI: selective serotonin reuptake inhibitors; TCA: tricyclic antidepressants; nonad: non‐antidepressants
23. Overview of all interventions in the Patient Global Impression of Change much/very much improved analysis.
| Treatment | RCTs | Participants |
| Antidepressants with ≥ 200 participants | ||
| Desvenlafaxine high dose | 1 | 402 |
| Duloxetine high dose | 2 | 287 |
| Duloxetine standard dose | 3 | 481 |
| Esreboxetine high dose | 1 | 275 |
| Esreboxetine standard dose | 1 | 536 |
| Milnacipran high dose | 3 | 1065 |
| Milnacipran standard dose | 3 | 1043 |
| Antidepressants with < 200 participants (excluded from summaries) | ||
| Desvenlafaxine standard dose | 1 | 131 |
| Milnacipran dose unable to be categorised | 1 | 79 |
|
RCT: randomised controlled trial Participant numbers reflect the total number of participants receiving the antidepressant. | ||
Ranking of antidepressants
The top‐ranked antidepressants for PGIC much and very much improved are presented in Table 28. Duloxetine standard dose was the highest‐ranked antidepressant for PGIC much and very much improved, with a large effect (OR 2.29, 95% CI 1.98 to 2.60). Duloxetine high dose (OR 2.03, 95% CI 1.62 to 2.44), milnacipran high dose (OR 1.99, 95% CI 1.77 to 2.21), and milnacipran standard dose (OR 1.95, 95% CI 1.73 to 2.17) were the next highest‐ranked antidepressants. Both esreboxetine doses showed a smaller effect (standard: OR 1.79, 95% CI 1.44 to 2.14; high: OR 1.63, 95% CI 1.24 to 2.02), but were among the lowest‐ranked antidepressants. Desvenlafaxine high dose showed no significant effects when compared to placebo (OR 1.01, 95% CI 0.58 to 1.44).
24. Top‐ranked antidepressants for Patient Global Impression of Change much/very much improved analysis.
| Odds ratio (95% CI) | Mean rank | Credible intervals | ||
| 2.5% | 97.5% | |||
| Duloxetine standard dose | 2.29 (1.98 to 2.60) |
2.3 | 1 | 6 |
| Duloxetine high dose | 2.03 (1.62 to 2.44) |
3.5 | 1 | 7 |
| Milnacipran high dose | 1.99 (1.77 to 2.21) |
3.6 | 1 | 7 |
| Milnacipran standard dose | 1.95 (1.73 to 2.17) |
3.9 | 1 | 7 |
| Esreboxetine standard dose | 1.79 (1.44 to 2.14) |
4.7 | 1 | 7 |
| Esreboxetine high dose | 1.63 (1.24 to 2.02) |
5.6 | 2 | 8 |
| Desvenlafaxine high dose | 1.01 (0.58 to 1.44) |
8.2 | 6 | 9 |
| CI: confidence interval | ||||
A visual representation of the SUCRA rankings for every intervention included in the analysis did not alter interpretation. The unrelated mean‐effect model had no evidence of inconsistency. We were only able to compare direct and indirect evidence for milnacipran standard versus milnacipran high dose with a Bayesian P value of 0.66, indicative of no inconsistency. These figures are available the supplemental files (link provided in Appendix 3)..
Exploration of heterogeneity
Class, condition and risk of bias: we were unable to include class, condition, and risk of bias in the models. For class, all the antidepressants included in the model were SNRI. For condition and risk of bias, the sparse network geometry created disconnected networks with small sample sizes and high risk of over‐fitting.
CINeMA: the design‐by‐treatment test showed no evidence of inconsistency (Chi² = 0.35, P = 0.84), and no antidepressants had I² statistic values of over 40%. We rated the majority of the evidence to be very low certainty, due to within‐study bias and low study and participant numbers. We rated milnacipran high dose as low certainty, downgraded due to major concerns of within‐study bias. We rated milnacipran and duloxetine standard dose as moderate certainty, only downgraded due to concerns about within‐study bias.
PGIC continuous
For PGIC continuous we report the treatment‐dose network as it had low heterogeneity and the most clinical utility. We present the summary of findings in Table 29.
25. Patient Global Impression of Change continuous summary of findings.
| Estimates of effects, credible intervals, and certainty of the evidence of antidepressants on Patient Global Impression of Change in people with chronic pain | |||||||
| Bayesian network meta‐analysis summary of findings table | |||||||
|
Patient or population: people with chronic pain Interventions: duloxetine low dose (< 60 mg), standard dose (60 mg), and high dose (> 60 mg) Comparator (reference): placebo Outcome: Patient Global Impression of Change (PGIC) measured continuously on the PGIC 1‐7 scale Direction: lower is better (1 on the scale represents ‘very much improved’, 7 represents ‘very much worse’) | |||||||
|
Total studies: 24 Total participants: 8415 |
Relative effect | Anticipated absolute effect (event rate) | Certainty of the evidence (CINeMA) |
Ranking* (2.5% to 97.5% credible interval) |
Interpretation of findings | ||
| With placebo | With intervention | Difference | |||||
|
Duloxetine standard dose RCTs: 14 Participants: 3847 |
‐ | ‐ | ‐ | SMD −0.36 (−0.44 to −0.29) |
Moderated | 3 (1 to 4) |
Small to moderate effect |
|
Duloxetine high dose RCTs: 14 Participants: 3520 |
‐ | ‐ | ‐ | SMD −0.33 (−0.40 to −0.26) |
Moderated | 3 (2 to 5) |
Small to moderate effect |
|
Duloxetine low dose RCTs: 5 Participants: 1097 |
‐ | ‐ | ‐ | SMD −0.23 (−0.35 to −0.11) |
Moderatea,d | 5 (3 to 6) |
Small effect |
|
NMA‐SoF table definitions *Mean rank and credible intervals are presented. **SMD interpretation based on clinical judgement and in line with Cohen 1988 and the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2022) as small (0.2), moderate (0.5) and large (0.8). CI: confidence interval; CINeMA: Confidence in Network Meta‐Analysis; RCT: randomised controlled trial; SMD: standardised mean difference The number of participants for each antidepressant reflects the total number of participants taking the antidepressant or placebo from the studies in the network meta‐analysis. | |||||||
|
CINeMA grades of confidence in the evidence High: further research is unlikely to change our confidence in the estimate of effect. Moderate: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low: we are very uncertain about the estimate. | |||||||
aDowngraded due to within‐study bias. bDowngraded due to imprecision in the estimate. cDowngraded due to heterogeneity in the estimate. dDowngraded due to incoherence in the network. eDowngraded due to a small number of trials and participants; we cannot draw reliable conclusions.
Results
We included 24 RCTs with a total of 8415 participants (range from 194 to 804). Twelve studies compared against only placebo, three were multi‐arm studies with another active comparator, and nine were dose‐comparison studies. There were seven different interventions, and some comparisons were informed only by direct evidence from one study. We judged 15 studies as high risk of bias overall. There were no concerns regarding model fit. The network diagram is presented in Figure 22, and the forest plot of placebo comparisons is presented in Figure 23. An overview of all the interventions included in the analysis is given in Table 30.
22.
Patient Global Impression of Change continuous network diagram. SNRI: serotonin noradrenalin reuptake inhibitors; nonad: non‐antidepressants
23.
Patient Global Impression of Change continuous forest plot (standardised mean difference with credible intervals). SNRI: serotonin noradrenalin reuptake inhibitors; nonad: non‐antidepressants
26. Overview of all interventions in the Patient Global Impression of Change continuous analysis.
| Treatment | RCTs | Participants |
| Antidepressants with ≥ 200 participants | ||
| Duloxetine low dose | 5 | 554 |
| Duloxetine standard dose | 14 | 2183 |
| Duloxetine high dose | 14 | 1838 |
| Antidepressants with < 200 participants (excluded from summaries) | ||
| Desvenlafaxine high dose | 1 | 184 |
| Desvenlafaxine standard dose | 1 | 54 |
| Non‐antidepressant interventions (excluded from summaries) | ||
| ABT‐394 | 1 | 172 |
| Pregabalin | 2 | 552 |
|
RCT: randomised controlled trial Participant numbers reflect the total number of participants receiving the antidepressant. | ||
Ranking of antidepressants
The top‐ranked antidepressants for PGIC continuous are presented in Table 31. Duloxetine standard and high doses were the highest‐ranked antidepressants, with a small to moderate effect (SMD −0.36, 95% CI −0.44 to −0.29 and SMD −0.33, 95% CI −0.40 to −0.26, respectively). Duloxetine low dose was the lowest‐ranked antidepressant with a small effect (SMD −0.23, 95% CI −0.35 to −0.11).
27. Top‐ranked antidepressants for Patient Global Impression of Change continuous analysis.
| Standardised mean difference (95% CI) | Mean rank | Credible intervals | ||
| 2.5% | 97.5% | |||
| Duloxetine standard | −0.36 (−0.44 to −0.29) |
2.7 | 1 | 4 |
| Duloxetine high | −0.33 (−0.40 to −0.26) |
3.4 | 2 | 5 |
| Duloxetine low | −0.23 (−0.35 to −0.11) |
5.0 | 3 | 6 |
| CI: confidence interval | ||||
A visual representation of the cumulative rankings for every intervention included in the analysis did not alter interpretations. Both unrelated mean‐effect models and node‐splitting models showed evidence of inconsistency. The highest Bayesian P value (0.03) suggested that direct evidence overestimated the effectiveness of high‐dose duloxetine versus placebo compared to indirect evidence, resulting in strength of evidence downgrading. These figures are available in the supplemental files (link provided in Appendix 3).
Exploration of heterogeneity
Class, condition, and risk of bias: we were unable to run models including class, condition, and risk of bias. We were unable to analyse class as there was only one class present in the network (SNRI). We were unable to analyses condition and risk of bias due to the high risk of over‐fitting.
CINeMA: the design‐by‐treatment test showed no evidence of inconsistency between the direct and indirect evidence in the network (Chi² = 14.98, P = 0.13), and no antidepressants had an I² statistic value higher than 40%. We rated duloxetine standard and high doses as moderate certainty as a result of incoherence. We downgraded duloxetine low dose to moderate certainty due to some concerns regarding within‐study bias in addition to network inconsistency.
Serious adverse events
For serious adverse events we report the treatment‐dose model. Both treatment and treatment‐dose models had studies with high levels of imprecision; treatment‐dose was selected for reporting due to its clinical utility. We present the summary of findings in Table 32.
28. Serious adverse events summary of findings.
| Estimates of effects, credible intervals, and certainty of the evidence for serious adverse events with antidepressants in people with chronic pain | |||||||
| Bayesian network meta‐analysis summary of findings table | |||||||
|
Patient or population: people with chronic pain Interventions: desvenlafaxine high dose (> 50 mg); duloxetine low dose (< 60 mg), standard dose (60 mg), and high dose (> 60 mg); esreboxetine standard dose (4‐8 mg) and high dose (> 8 mg); milnacipran standard dose (100 mg), high dose (> 100 mg), and dose unable to be categorised; mirtazapine standard dose (30 mg) Comparator (reference): placebo Outcome: serious adverse events (events that are life‐threatening or resulting in: hospitalisation, persistent or significant disability, or death) as reported per study Direction: lower is better (i.e. fewer people having serious adverse events) | |||||||
|
Total studies: 71 Total participants: 19304 |
Relative effect (OR and 95% CI) |
Anticipated absolute effect (event rate)* | Certainty of the evidence (GRADE) |
Ranking** (2.5% to 97.5% credible interval) |
Interpretation of findings | ||
| With placebo | With intervention | Difference | |||||
|
Desvenlafaxine high dose RCTs: 2 Participants: 912 |
0.51 (‐0.27 to 1.29) |
12/221 54 per 1000 |
20/691 28 per 1000 |
26 fewer per 1000 | Very lowa,b,c | 11 (4 to 24) |
Not significantly different from placebo |
|
Milnacipran dose unable to be categorised RCTs: 3 Participants: 272 |
0.66 (‐0.95 to 2.27) |
3/69 43 per 1000 |
5/203 29 per 1000 |
14 fewer per 1000 | Very lowa,b,c | 15 (2 to 36) |
Not significantly different from placebo |
|
Duloxetine low dose RCTs: 4 Participants: 935 |
0.89 (‐0.05 to 1.83) |
11/462 24 per 1000 |
9/473 21 per 1000 |
3 fewer per 1000 | Very lowa,b,c | 19 (6 to 32) |
Not significantly different from placebo |
|
Duloxetine high dose RCTs: 12 Participants: 3404 |
0.92 (0.43 to 1.41) |
33/1601 21 per 1000 |
40/1803 19 per 1000 |
2 fewer per 1000 | Very lowa,b,c | 19 (9 to 29) |
Not significantly different from placebo |
|
Milnacipran standard dose RCTs: 7 Participants: 2474 |
0.94 (0.31 to 1.57) |
22/1234 18 per 1000 |
21/1240 17 per 1000 |
1 fewer per 1000 | Very lowa,b,c | 19 (9 to 31) |
Not significantly different from placebo |
|
Mirtazapine standard dose RCTs: 3 Participants: 484 |
0.99 (‐0.83 to 2.81) |
3/241 12 per 1000 |
3/243 12 per 1000 |
0 fewer per 1000 | Very lowb,c | 10 (3 to 38) |
Not significantly different from placebo |
|
Milnacipran high dose RCTs: 7 Participants: 2826 |
1.08 (0.55 to 1.61) |
28/1257 22 per 1000 |
35/1569 24 per 1000 |
2 more per 1000 | Very lowa,b,c | 22 (11 to 32) |
Not significantly different from placebo |
|
Duloxetine standard dose RCTs: 15 Participants: 4589 |
1.16 (0.71 to 1.61) |
34/1082 16 per 1000 |
52/2507 19 per 1000 |
3 more per 1000 | Very lowa,b,c | 23 (13 to 32) |
Not significantly different from placebo |
|
Esreboxetine standard dose RCTs: 1 Participants: 833 |
2.25 (‐0.69 to 5.19) |
1/277 4 per 1000 |
3/556 8 per 1000 |
4 more per 1000 | Very lowa,b,c,e | 27 (4 to 41) |
Not significantly different from placebo |
|
Esreboxetine high dose RCTs: 1 Participants: 558 |
2.75 (‐0.35 to 5.85) |
1/277 4 per 1000 |
2/281 10 per 1000 |
6 more per 1000 | Very lowa,b,c,e | 28 (4 to 41) |
Not significantly different from placebo |
|
Network meta‐analysis‐summary of findings table definitions * Anticipated absolute effect. Anticipated absolute effect compares two risks by calculating the difference between the risk of the intervention group with the risk of the control group. ** Mean rank and credible intervals are presented. CI: confidence interval; OR: odds ratio; RCT: randomised controlled trial The number of participants for each antidepressant reflects the total number of participants taking the antidepressant or placebo from the studies in the network meta‐analysis. | |||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||||
aDowngraded due to within‐study bias. bDowngraded due to imprecision in the estimate. cDowngraded due to heterogeneity in the estimate. dDowngraded due to incoherence in the network. eDowngraded due to a small number of trials and participants; we cannot draw reliable conclusions.
Results
We included 71 RCTs with a total of 19,304 participants (range from 26 to 1025). Thirty‐nine studies compared against placebo, 12 compared against another active comparator, 15 were dose‐comparison studies, and four studies compared two different antidepressants against each other. There were 31 different interventions, and some comparisons were informed only by direct evidence from one study. We judged 45 studies as high risk of bias. We could not include data from three studies due to disconnected networks. There were no concerns regarding model fit. The network diagram is presented in Figure 24, and the forest plot of placebo comparisons is presented in Figure 25. An overview of all interventions included in the analysis is given in Table 33.
24.
Serious adverse events network diagram. NARI: noradrenaline reuptake inhibitors; NDRI: Noradrenaline and dopamine reuptake inhibitors; NASSA: noradrenergic and specific serotonergic antidepressants; SNRI: serotonin noradrenaline reuptake inhibitors; SSRI: selective serotonin reuptake inhibitors; TCA: tricyclic antidepressants; nonad: non‐antidepressants
25.
Serious adverse events forest plot (log odds ratio with credible intervals). NARI: noradrenaline reuptake inhibitors; NDRI: Noradrenaline and dopamine reuptake inhibitors; NASSA: noradrenergic and specific serotonergic antidepressants; SNRI: serotonin noradrenaline reuptake inhibitors; SSRI: selective serotonin reuptake inhibitors; TCA: tricyclic antidepressants; nonad: non‐antidepressants
29. Overview of all interventions in the serious adverse events analysis.
| Treatment | RCTs | Participants |
| Antidepressants with ≥ 200 participants | ||
| Desvenlafaxine high dose | 2 | 691 |
| Duloxetine high dose | 12 | 1803 |
| Duloxetine low dose | 4 | 473 |
| Duloxetine standard dose | 15 | 2507 |
| Esreboxetine high dose | 1 | 281 |
| Esreboxetine standard dose | 1 | 556 |
| Milnacipran high dose | 7 | 1569 |
| Milnacipran standard dose | 7 | 1240 |
| Milnacipran dose unable to be categorised | 3 | 203 |
| Mirtazapine standard dose | 3 | 243 |
| Antidepressants with < 200 participants (excluded from summaries) | ||
| Amitriptyline high dose | 1 | 96 |
| Amitriptyline low dose | 1 | 32 |
| Amitriptyline standard dose | 3 | 114 |
| Amitriptyline dose unable to be categorised | 1 | 25 |
| Bupropion standard dose | 1 | 54 |
| Citalopram standard dose | 2 | 34 |
| Desipramine low dose | 1 | 38 |
| Desipramine + cognitive behavioural therapy | 1 | 37 |
| Desvenlafaxine standard dose | 2 | 199 |
| Esreboxetine dose unable to be categorised | 1 | 134 |
| Imipramine low dose | 1 | 18 |
| Imipramine standard dose | 1 | 51 |
| Milnacipran standard + cognitive behavioural therapy | 1 | 17 |
| Mirtazapine low dose | 1 | 26 |
| Nortriptyline low dose | 2 | 137 |
| Nortriptyline unable to be categorised | 1 | 56 |
| Nortriptyline unable to be categorised + cognitive behavioural therapy | 1 | 41 |
| Nortriptyline unable to be categorised + disease management | 1 | 37 |
| Paroxetine low dose | 2 | 62 |
| Paroxetine dose unable to be categorised | 2 | 152 |
| Reboxetine standard dose | 1 | 18 |
| Sertraline high dose | 1 | 30 |
| Trazadone + gabapentin | 1 | 94 |
| Venlafaxine high dose | 1 | 82 |
| Venlafaxine low dose | 1 | 82 |
| Venlafaxine standard dose | 1 | 86 |
| Non‐antidepressant interventions (excluded from summaries) | ||
| Carbamazepine | 2 | 99 |
| Cognitive behavioural therapy | 3 | 72 |
| Coping skills training | 1 | 29 |
| Disease management | 1 | 24 |
| Gabapentin | 2 | 56 |
| Nabilone | 1 | 32 |
| Pregabalin | 3 | 643 |
| Terbutaline | 1 | 51 |
|
RCT: randomised controlled trial Participant numbers reflect the total number of participants receiving the antidepressant. | ||
Ranking of antidepressants
The top‐ranked antidepressants for serious adverse events are displayed in Table 34. Data for serious adverse events were very sparse, and studies were generally underpowered to detect rare events. No antidepressants showed any significant difference when compared with placebo, and the confidence intervals were very wide.
30. Top‐ranked antidepressants for serious adverse events analysis.
| Odds ratio (95% CI) | Mean rank | Credible intervals | ||
| 2.5% | 97.5% | |||
| Desvenlafaxine high dose | 0.51 (−0.27 to 1.29) |
11.4 | 4 | 24 |
| Milnacipran dose unable to be categorised | 0.66 (−0.95 to 2.27) |
15.5 | 2 | 36 |
| Duloxetine low dose | 0.89 (−0.05 to 1.83) |
18.5 | 6 | 32 |
| Duloxetine high dose | 0.92 (0.43 to 1.41) |
18.8 | 9 | 29 |
| Milnacipran standard dose | 0.94 (0.31 to 1.57) |
19.3 | 9 | 31 |
| Mirtazapine standard dose | 0.99 (−0.83 to 2.81) |
10.0 | 3 | 38 |
| Milnacipran high dose | 1.08 (0.55 to 1.61) |
21.6 | 11 | 32 |
| Duloxetine standard dose | 1.16 (0.71 to 1.61) |
22.8 | 13 | 32 |
| Esreboxetine standard dose | 2.25 (−0.69 to 5.19) |
26.7 | 4 | 41 |
| Esreboxetine high dose | 2.75 (−0.35 to 5.85) |
28.3 | 4 | 41 |
| CI: confidence interval | ||||
We undertook a visual representation of the cumulative rankings for every intervention included in the analysis. The unrelated mean‐effect model had no evidence of inconsistency. We confirmed this with node‐splitting models for all 16 comparisons where it was possible to compare direct and indirect evidence. The lowest Bayesian P value (0.07) was for the comparison of pregabalin and low‐dose duloxetine. These figures are available in the supplemental files (link provided in Appendix 3).
Class, condition, and risk of bias: we were unable to undertake further analysis of class, condition, or risk of bias in networks due to small sample sizes, network geometry and the risk of over‐fitting.
CINeMA: we were unable to use CINeMA for this outcome due to complexity of the network. Therefore, two review authors (HB and GS) made the judgements based on GRADE and CINeMA domains and the available results. We judged all antidepressants and doses as very low certainty, primarily due to concerns with within‐study bias, heterogeneity, and imprecision in the network.
Withdrawal
For withdrawal, we report the treatment network. Although this model has high heterogeneity, we determined that including dose would increase the network complexity to a point where analysis would be infeasible. We present the summary of findings in Table 35.
31. Withdrawal summary of findings.
| Estimates of effects, credible intervals, and certainty of the evidence for withdrawal from studies in people with chronic pain | |||||||
| Bayesian network meta‐analysis summary of findings table | |||||||
|
Patient or population: people with chronic pain Interventions: amitriptyline, desipramine, desvenlafaxine, duloxetine, esreboxetine, milnacipran, mirtazapine, nortriptyline, paroxetine, venlafaxine. All doses were combined for each antidepressant. Comparator (reference): placebo Outcome: withdrawal from the study (for any reason) Direction: lower is better (i.e. fewer people withdrawing from studies) | |||||||
|
Total studies: 152 Total participants: 28120 |
Relative effect (OR and 95% CI) |
Anticipated absolute effect (event rate)* | Certainty of the evidence (GRADE) |
Ranking** (2.5% to 97.5% credible interval) |
Interpretation of findings | ||
| With placebo | With intervention | Difference | |||||
|
Nortriptyline RCTs: 7 Participants: 612 |
0.54 (0.09 to 1.17) |
101 per 1000 | 57 per 1000 |
44 fewer per 1000 (111 fewer to 15 more) |
Very lowa,b | 13 (5 to 26) |
Not significantly different from placebo |
|
Mirtazapine RCTs: 3 Participants: 510 |
0.99 (0.34 to 1.64) |
120 per 1000 | 119 per 1000 |
1 fewer per 1000 (76 fewer to 63 more) |
Very lowb,c | 28 (11 to 52) |
Not significantly different from placebo |
|
Amitriptyline RCTs: 34 Participants: 2126 |
1.12 (0.85 to 1.39) |
138 per 1000 | 152 per 1000 |
14 more per 1000 (18 fewer to 44 more) |
Very lowa,b,c | 31 (20 to 43) |
Not significantly different from placebo |
|
Duloxetine RCTs: 45 Participants: 10140 |
1.20 (1.06 to 1.34) |
207 per 1000 | 239 per 1000 |
32 more per 1000 (10 more to 52 more) |
Lowa,b | 33 (24 to 43) |
Equivalent to NNTH of 31 |
|
Desvenlafaxine RCTs: 2 Participants: 1105 |
1.25 (0.82 to 1.68) |
450 per 1000 | 506 per 1000 |
56 more per 1000 (48 fewer to 129 more) |
Very lowa,b,c | 35 (19 to 53) |
Not significantly different from placebo |
|
Milnacipran RCTs: 17 Participants: 5088 |
1.34 (1.12 to 1.56) |
254 per 1000 | 314 per 1000 |
59 more per 1000 (22 more to 93 more) |
Very lowa,b | 38 (27 to 49) |
Equivalent to NNTH of 17 |
|
Venlafaxine RCTs: 6 Participants: 624 |
140 (0.91 to 1.89) |
158 per 1000 | 208 per 1000 |
50 more per 1000 (12 fewer to 104 more) |
Very lowa,b,c | 40 (21 to 59) |
Not significantly different from placebo |
|
Esreboxetine RCTs: 2 Participants: 1389 |
1.42 (1.01 to 1.83) |
251 per 1000 | 322 per 1000 |
71 more per 1000 (2 more to 129 more) |
Very lowa,b,c | 41 (23 to 56) |
Equivalent to NNTH of 31 |
|
Desipramine RCTs: 4 Participants: 368 |
1.57 (1.02 to 2.12) |
196 per 1000 | 276 per 1000 |
81 more per 1000 (3 more to 145 more) |
Very lowa,b,c | 44 (24 to 61) |
Equivalent to NNTH of 14 |
|
Paroxetine RCTs: 9 Participants: 568 |
1.68 (1.23 to 2.12) |
173 per 1000 | 260 per 1000 |
87 more per 1000 (32 more to 134 more) |
Very lowa,b | 46 (28 to 60) |
Equivalent to NNTH of 11 |
|
Network meta‐analysis‐summary of findings table definitions * Anticipated absolute effect. Anticipated absolute effect compares two risks by calculating the difference between the risk of the intervention group with the risk of the control group. ** Mean and credible intervals are presented. Rank statistics is defined as the probabilities that a treatment out of n treatments in a network meta‐analysis is the best, the second, the third and so on until the least effective treatment. CI: confidence interval; NNTH: number needed to treat for an additional harmful outcome; OR: odds ratio; RCT: randomised controlled trial The number of participants for each antidepressant reflects the total number of participants taking the antidepressant or placebo from the studies in the network meta‐analysis. | |||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||||
aDowngraded due to within‐study bias. bDowngraded due to imprecision in the estimate. cDowngraded due to heterogeneity in the estimate. dDowngraded due to incoherence in the network. eDowngraded due to a small number of trials and participants; we cannot draw reliable conclusions.
Results
We included 152 RCTs with a total of 28,120 participants (range from 24 to 1025). Seventy‐three studies compared against placebo, 47 were multi‐arm studies with another active comparator, 18 were dose‐comparison studies, and 14 were head‐to‐head studies comparing two different antidepressants. There were 77 different interventions, and some comparisons were informed only by direct evidence from one study. We rated 106 studies as high risk of bias. We could not include data from two studies due to disconnected networks. There were no concerns regarding model fit. We present the network diagram in Figure 26, and the forest plot of placebo comparisons in Figure 27. We give an overview of all interventions included in the analysis in Table 36.
26.
Withdrawal network diagram. MAOI_rev: monoamine oxidase inhibitors (reversible); NARI: noradrenaline reuptake inhibitors; NDRI: noradrenaline and dopamine reuptake inhibitors; NASSA: noradrenergic and specific serotonergic antidepressants; SNRI: serotonin noradrenaline reuptake inhibitors; SSRI: selective serotonin reuptake inhibitors; TCA: tricyclic antidepressants; TECA: tetracyclic antidepressants; nonad: non‐antidepressants
27.
Withdrawal forest plot (log odds ratio with credible intervals). MAOI_rev: monoamine oxidase inhibitors (reversible); NARI: noradrenaline reuptake inhibitors; NDRI: Noradrenaline and dopamine reuptake inhibitors; NASSA: noradrenergic and specific serotonergic antidepressants; SNRI: serotonin noradrenaline reuptake inhibitors; SSRI: selective serotonin reuptake inhibitors; TCA: tricyclic antidepressants; TECA: tetracyclic antidepressants; nonad: non‐antidepressants
32. Overview of all interventions in the withdrawal analysis.
| Treatment | RCTs | Participants |
| Antidepressants with ≥ 200 participants | ||
| Amitriptyline | 34 | 1326 |
| Desipramine | 4 | 230 |
| Desvenlafaxine | 2 | 885 |
| Duloxetine | 45 | 6082 |
| Esreboxetine | 2 | 978 |
| Imipramine | 5 | 240 |
| Milnacipran | 17 | 3090 |
| Mirtazapine | 3 | 269 |
| Nortriptyline | 7 | 374 |
| Paroxetine | 9 | 389 |
| Venlafaxine | 6 | 409 |
| Antidepressants with < 200 participants (excluded from summaries) | ||
| Amitriptyline + fluoxetine | 1 | 31 |
| Amitriptyline + fluphenazine | 1 | 12 |
| Amitriptyline + naproxen | 1 | 19 |
| Amitriptyline + psychotherapy | 1 | 26 |
| Amitriptyline + splint | 1 | 24 |
| Amitriptyline + support | 1 | 26 |
| Bupropion | 1 | 54 |
| Citalopram | 4 | 76 |
| Clomipramine | 2 | 124 |
| Cognitive behavioural therapy and milnacipran | 1 | 20 |
| Cognitive behavioural therapy and amitriptyline | 1 | 12 |
| Coping skills training + sertraline | 1 | 28 |
| Desipramine + cognitive behavioural therapy | 1 | 37 |
| Desipramine + lidocaine | 1 | 34 |
| Dothiepin | 2 | 55 |
| Doxepin | 1 | 30 |
| Escitalopram | 3 | 86 |
| Fluoxetine | 6 | 140 |
| Fluphenazine | 1 | 13 |
| Gabapentin + nortriptyline | 1 | 56 |
| Maprotiline | 3 | 98 |
| Melatonin + amitriptyline | 1 | 21 |
| Mianserin | 2 | 109 |
| Moclobemide | 1 | 43 |
| Morphine + nortriptyline | 1 | 55 |
| Nortriptyline + cognitive behavioural therapy | 1 | 41 |
| Nortriptyline + disease management | 1 | 37 |
| Nortriptyline + morphine | 1 | 52 |
| Pirlindole | 1 | 45 |
| Pregabalin + duloxetine | 1 | 41 |
| Pregabalin + imipramine | 1 | 73 |
| Reboxetine | 1 | 18 |
| Sertraline | 2 | 66 |
| Trazodone | 3 | 63 |
| Trazodone + gabapentin | 2 | 94 |
| Trimipramine | 1 | 18 |
| Zimeldine | 1 | 17 |
| Non‐antidepressant interventions (excluded from summaries) | ||
| ABT‐894 | 1 | 172 |
| Acetaminophen (paracetamol) | 1 | 50 |
| Acupuncture | 1 | 24 |
| Aerobic exercise | 1 | 20 |
| Carbamazepine | 2 | 99 |
| Cognitive behavioural therapy | 7 | 333 |
| Coping skills training | 1 | 29 |
| Cyclobenzaprine | 1 | 42 |
| Disease management | 1 | 24 |
| Education | 1 | 71 |
| Gabapentin | 6 | 269 |
| Lamotrigine | 1 | 53 |
| Lidocaine | 1 | 33 |
| Melatonin | 1 | 21 |
| Morphine | 2 | 107 |
| Naltrexone | 1 | 67 |
| Naproxen | 1 | 19 |
| Neurofeedback | 1 | 20 |
| Panax ginseng | 1 | 19 |
| Physical therapy | 1 | 34 |
| Pregabalin | 9 | 919 |
| Psychotherapy | 2 | 116 |
| Saffron/crocin | 2 | 53 |
| Support | 1 | 24 |
| TENS | 1 | 50 |
| Terbutaline | 1 | 51 |
| Usual treatment | 1 | 70 |
| Waitlist | 1 | 24 |
|
RCT: randomised controlled trial; TENS: transcutaneous electrical nerve stimulation Participant numbers reflect the total number of participants receiving the antidepressant. | ||
Ranking of antidepressants
The ranking of antidepressants with over 200 participants in order of highest‐ranked to lowest‐ranked is presented in Table 37. Nortriptyline was the highest‐ranked antidepressant. Nortriptyline, mirtazapine, amitriptyline, desvenlafaxine, and venlafaxine all showed no significant difference compared to placebo for withdrawal. Duloxetine, milnacipran, esreboxetine, desipramine, and paroxetine all showed significant effects, ranging from small to moderate.
33. Top‐ranked antidepressants for withdrawal analysis.
| Antidepressant | Odds ratio (95% CI) | Mean rank | Credible intervals | |
| 2.5% | 97.5% | |||
| Nortriptyline | 0.54 (0.09 to 1.17) |
13.3 | 5 | 26 |
| Mirtazapine | 0.99 (0.34 to 1.64) |
27.8 | 11 | 52 |
| Amitriptyline | 1.12 (0.85 to 1.39) |
30.9 | 20 | 43 |
| Duloxetine | 1.20 (1.06 to 1.34) |
33.4 | 24 | 43 |
| Desvenlafaxine | 1.25 (0.82 to 1.68) |
35.3 | 19 | 53 |
| Milnacipran | 1.34 (1.12 to 1.56) |
38.4 | 27 | 49 |
| Venlafaxine | 1.40 (0.91 to 1.89) |
39.9 | 21 | 59 |
| Esreboxetine | 1.42 (1.01 to 1.83) |
40.6 | 23 | 56 |
| Desipramine | 1.57 (1.02 to 2.12) |
43.8 | 24 | 61 |
| Paroxetine | 1.68 (1.23 to 2.12) |
46.3 | 28 | 60 |
| CI: confidence interval | ||||
A visual representation of the cumulative rankings for every intervention included in the analysis is given in the supplemental files (link provided in Appendix 3). We were unable to draw any very reliable conclusions due to all antidepressants having wide, overlapping credible intervals.
Exploration of heterogeneity
Due to the complexity and geometry of the network, we were only able to examine models including class, and were unable to examine condition or risk of bias.
Class
We included 10 classes of antidepressant in the analysis: SNRI, SSRI, TCA, MAOI reversible, NARI, NaSSA, NDRI, SARI, TCA+SSRI, and TeCA. There was slightly higher heterogeneity than the treatment‐only model (Tau = 0.33), but no evidence of inconsistency in the unrelated mean effects models. Half of the classes had fewer than 200 participants, leaving SNRI, SSRI, TCA, NaSSA, and TeCA with reliable sample sizes. The rankings of these classes are presented in Table 38.
34. Top‐ranked antidepressant classes for withdrawal analysis.
| Class | Antidepressant | Participants | Mean rank | Credible intervals | |
| 2.5% | 97.5% | ||||
| NaSSA | Mirtazapine | 242 | 3.61 | 1 | 10 |
| TCA | Amitriptyline Clomipramine Desipramine Dothiepin Doxepin Imipramine Nortriptyline |
2593 | 4.33 | 2 | 7 |
| SNRI | Duloxetine Esreboxetine Milnacipran Venlafaxine |
7804 | 6.24 | 4 | 9 |
| TeCA | Maprotiline Mianserin |
207 | 6.96 | 2 | 11 |
| SSRI | Citalopram Escitalopram Fluoxetine Paroxetine Sertraline Zimeldine |
713 | 7.7 | 4 | 10 |
| NaSSA: noradrenergic and specific serotonergic antidepressant; SNRI: serotonin‐noradrenalin reuptake inhibitors; SSRI: selective serotonin reuptake inhibitors; TCA: tricyclic antidepressants; TeCA: tetracyclic antidepressants | |||||
CINeMA
We were unable to use CINeMA for this outcome due to complexity of the network. Therefore, two review authors (HB and GS) made the judgements based on GRADE and CINeMA domains and the available results. We judged all antidepressants except duloxetine as very low certainty, primarily due to concerns with within‐study bias, heterogeneity, and imprecision in the network. We rated duloxetine as low certainty, as the only antidepressant without major concerns due to imprecision.
Discussion
Summary of main results
Overall
We report an NMA of 176 double‐blind RCTs that investigated antidepressants for chronic pain. Studies included 28,664 adult participants with a mean age of 50.6 years. The majority of studies investigated antidepressants from three classes: SNRI (74 studies); TCA (72 studies); and SSRI (34 studies). There was a variety of study designs, however the majority of studies were placebo‐controlled (83 studies). The remainder compared an antidepressant against an active comparator with no placebo (22 studies) or compared two or more different doses of the same antidepressant with a placebo arm (17 studies). Most studies were parallel‐arm design (141 studies) compared to cross‐over design (35 studies). Studies mainly included participants with only one type of chronic pain: 59 studies included participants with fibromyalgia; 49 neuropathic pain; 40 musculoskeletal pain; and 26 included participants with other conditions (e.g. gastrointestinal, primary pain conditions, non‐cardiac chest pain etc.). Finally, 72 studies were fully funded by pharmaceutical companies. Thirty‐two studies did not report the source of funding.
Seven studies, with a total of 156 participants, provided no useable data and were therefore omitted from the NMAs. At the time of writing the review, the majority of antidepressants are not licenced for use in chronic pain. Only amitriptyline and duloxetine are indicated for types of chronic pain in the British National Formulary; amitriptyline for neuropathic pain, and duloxetine for diabetic neuropathy (British National Formulary 2022b; British National Formulary 2022c).
The following results are based on NMA. One study (Vrethem 1997), reported the results separately according to the type of pain condition. This study was stratified into two to include the results for both conditions.
Primary efficacy outcomes
For the primary efficacy outcomes (substantial pain relief, pain intensity, and mood) duloxetine was consistently the highest‐ranked antidepressant that had data from over 200 participants in total across studies, and the only antidepressant with robust evidence that showed an effect with moderate‐certainty evidence. For substantial pain and pain intensity, standard‐dose duloxetine was as efficacious as high‐dose duloxetine. For pain intensity and mood, milnacipran also showed reliable effectiveness, with moderate‐certainty evidence. At a class level, SNRIs were the only class to have an effect with reliable evidence. For pain intensity, we removed one study that showed improbable effects from the data extracted from the published article (Miki 2016). We emailed the study authors for clarification but received no response.
Secondary efficacy outcomes
Across all the secondary efficacy outcomes (moderate pain relief, physical function, sleep, quality of life, and PGIC) duloxetine and milnacipran were the highest‐ranked and most trustworthy antidepressants respectively. Very few other antidepressants included over 200 participants, and those that did were ranked as very low certainty. For both duloxetine and milnacipran, standard doses were as effective as high doses, although effects for both were small.
Safety
We extracted adverse event, serious adverse event, and withdrawal data from the studies included in the review. The data for these outcomes were poor. Although we have reported the ranking of antidepressants in the summary of findings tables, the quality and certainty of this evidence for all antidepressants and doses is very low, and we cannot draw any reliable conclusions from the analyses.
Overall completeness and applicability of evidence
We were able to draw some conclusions about the effectiveness and rankings of antidepressants in the efficacy and safety of treating chronic pain. The evidence is particularly lacking for long‐term outcomes and safety data.
Participants
The sample of participants in the included studies was mostly female (68.3%) and had a mean age of 50.6 years. Most studies had a minimum pain intensity inclusion criterion, with 92 studies requiring participants to score 4 or higher on a 0 to 10 scale or equivalent at baseline, and most participants reported experiencing pain for over one year.
Our inclusion criteria for participants was strict, we required the study population to have had pain for three months or longer. If this timeframe was not explicitly reported by the study or required for a diagnosis of the pain condition, then we excluded it. Therefore, we excluded six studies from our full‐text screening with a study population described as having a ‘chronic’ pain condition without information regarding duration. This may mean that we excluded other relevant studies, but we believe the number of studies to be affected by this to be minimal.
Interventions
There were 89 different interventions included in the review, 26 of which were antidepressants. We included all interventions that matched the inclusion criteria regardless of dose, formulation, and route of administration. Only four antidepressants were investigated in over 10 studies. The only antidepressant that had robust studies and evidence is duloxetine, with 43 studies and a total of 11,608 participants randomised. Participants in duloxetine studies accounted for over a third of all the participants included in this review. Milnacipran also showed some reliable evidence across outcomes, with 11 studies and a total of 5083 participants. Forty‐three studies, with a total of 3372 participants, investigated amitriptyline, although the certainty of this evidence was very low, and only three studies randomised over 200 participants. Fluoxetine was the fourth antidepressant to be included in more than 10 studies, but the quality and certainty of the evidence was very low, with 11 studies including 630 participants in total. All other antidepressants were included in fewer than 10 studies.
Study designs and comparisons
A variety of study designs were used by studies included in the review. Half the studies included in the review were two‐arm, parallel‐designed studies comparing antidepressant to placebo (89 out of 176 studies). There were also dose‐comparison studies, comparisons against active comparators, combined antidepressant interventions (e.g. antidepressant + psychological therapy), and a number of studies included multiple types of these comparisons. Some of the combined antidepressant comparisons precluded full analysis in the NMA as we were unable to isolate the effects of the antidepressant alone. There were few head‐to‐head studies comparing two antidepressants with a placebo arm for reference.
The majority of studies provided useable data for the primary efficacy outcomes; 131 studies measured pain intensity, and 87 measured mood. Although these figures represent the majority of studies, it is evident that a large number of studies in chronic pain do not report these key outcomes. In the review, over half of studies did not measure mood, and almost a third did not measure or report pain intensity. Despite the 2005 publication of the IMMPACT guidelines for core outcomes of chronic pain studies (Dworkin 2008), only 44 and 43 studies reported the proportion of participants achieving 50% and 30% pain relief, respectively. For the secondary outcomes, around a third of studies reported physical function, less than a quarter reported sleep, and only a quarter reported quality of life.
All outcomes aside from withdrawal used self‐reported measures. There was considerable heterogeneity in the outcome measures used across all outcomes such that SMD was required for the continuous outcomes. Additionally, studies reported a mix of change scores (change in outcome from baseline to post‐intervention) and post‐intervention scores. As we had to use SMD, this meant that we could not build one NMA that included all data for each outcome; rather we were required to build both change‐score and post‐intervention‐score models and subsequently decide which model to report for each outcome. Typically, larger studies, funded by pharmaceutical companies, reported change scores, whilst smaller studies reported post‐intervention scores. Future reviews would benefit from studies reporting both types of scores, so that results can be combined for a holistic evidence synthesis. We found that the data for the safety outcomes were particularly poor; adverse events were reported in various different ways across studies, and studies were often not powered adequately or lasted long enough to detect events.
Mood
As antidepressants are primarily designed and used to manage depression, and low mood is a common comorbidity with chronic pain, we planned to explore their impact upon mood in this analysis in several ways.
First, we planned to undertake a subgroup analysis exploring whether there were any differences in outcomes between studies reporting a main aim of targeting pain compared to those reporting a main aim of targeting mood. We were unable to undertake this analysis as only two studies had a main aim of targeting mood. In contrast, 144 studies had a main aim of targeting pain.
Second, we planned to undertake analyses examining differences in outcomes for studies stratified by levels of depression at baseline (none, mild, moderate, and severe as defined by the diagnostic tools used). The majority of studies excluded participants with diagnoses of major depressive disorder and other mental health conditions. Because of this, baseline measures of depression or anxiety, or both, failed to exceed average scores of mild depression at baseline.
As we were unable to undertake these analyses, we are unable to assess the effect of depression and mood on the outcomes of the NMA, and unable to draw any meaningful conclusions regarding the mood outcome.
Timing
Most of the studies included in this review were of short duration: the average length of the study from baseline to post‐intervention was 10 weeks. We planned to undertake analyses at several time points:
post‐intervention (immediately at the end of the treatment period);
short‐term follow‐up (< 12 weeks after the treatment had finished);
long‐term follow‐up (≥ 12 weeks after the treatment had finished).
We were only able to undertake analyses at the post‐intervention time point as only a small number of studies had follow‐up periods of any length after the intervention had been completed (6/176 studies). Therefore, we are unable to draw any conclusions regarding the long‐term efficacy and safety of using antidepressants for chronic pain.
Ongoing studies
We categorised 26 studies as 'ongoing', which are investigating the following antidepressants.
Duloxetine (12 studies)
Amitriptyline (4 studies)
Citalopram (2 studies)
Venlafaxine (2 studies)
Agomelatine (1 study)
Bupropion (1 study)
Clomipramine (1 study)
Fluoxetine (1 study)
Mianserin (1 study)
Nortriptyline (1 study)
The ongoing studies are investigating the following pain conditions.
Neuropathic pain (9 studies)
Osteoarthritis (6 studies)
Low back pain (4 studies)
Chest pain (2 studies)
Facial pain (2 studies)
Irritable bowel syndrome (1 study)
Mastalgia (1 study)
Phantom limb pain (1 study)
Considering their context, we do not anticipate that the evidence from these studies will have a significant impact on the findings of this review. We consider our results for duloxetine, neuropathic pain, and musculoskeletal pain to be robust ‐ the addition of these results are unlikely to change this. These studies may contribute to conclusions for amitriptyline if the sample sizes are large enough; we were unable to include amitriptyline in the write‐up of the review as often there were not more than 200 participants from the studies.
Quality of the evidence
Overall quality
We assessed the quality of the evidence using CINeMA (Nikolakopoulou 2020) (and ROB‐MEN (Chiocchia 2021), and GRADE (Schünemann 2013), where appropriate). Across the outcomes, the only antidepressant with consistently robust evidence is duloxetine, followed by milnacipran. We judged all other antidepressants as having low‐ or very low‐certainty evidence. The most common reasons for downgrading comparisons were within‐study bias, imprecision in the NMA (wide credible intervals), and small numbers of studies and participants. Additionally, we graded all evidence for safety as very low certainty due to heterogeneity, imprecision, and sparsity of data.
Risk of bias
Overall, the risk of bias for included studies was relatively high. Using RoB 1 resulted in 116 studies being defined as high risk of bias overall. We often downgraded evidence due to within‐study bias across antidepressants and outcomes. There are several points relating to risk of bias to be discussed. The common method of deciding the overall rating of a study’s risk of bias stipulates that if any one domain is high risk, then the whole study is rated as high risk of bias. As we included studies that compared antidepressants to other active comparators, this included interventions whose designs inherently require participants and study staff to be unblinded (e.g. psychological therapies). To be consistent with other studies in the review, we rated these as high risk of bias for the blinding domains, but it has been recognised previously that these domains are not appropriate for these interventions, and in previous reviews these domains have been omitted (Williams 2020).
Additionally, we found that a number of studies simply do not report the information needed to make a judgement. Of the 60 studies rated as ‘not high’ risk of bias, over half had three or more domains judged as ‘unclear’. Therefore, this raises concerns as to the reporting quality of these studies, an ongoing problem in health research (Pirosca 2022). There is a number of clinical trial reporting guidelines available which these studies have not abided by, which suggests that some of the studies may have been rated as high risk of bias if the correct information had been provided.
Heterogeneity
We found substantial heterogeneity in direct comparisons and entire networks across outcomes when including all doses of each treatment together in the NMAs. Where this was evident, splitting treatments by dose categories removed heterogeneity for most outcomes. Therefore, most of the outcomes were analysed using a split‐dose model. Further exploration of heterogeneity by including antidepressant class and pain condition had to be balanced against the risk of over‐fitting multiple models (Dias 2013). The decision process for this is discussed within each outcome results section.
Imprecision
Imprecision was a problem across most of our NMAs. Of the 26 different antidepressants included in our review, only four were used in more than 10 studies. Although we included all treatments in each analysis, for each outcome we graded any study with fewer than 200 participants in the antidepressant arms as very low by default and excluded these from the written summaries and summary of findings tables. The remaining networks were generally robust at a network level, but problems remained with network connectivity relying on single studies. Imprecision was a major problem for safety data, particularly adverse events, and serious adverse events, meaning that we cannot be sure of the true effect for these outcomes.
Inconsistency
For each outcome, we used unrelated mean‐effect and node‐splitting models to assess inconsistency in treatment and split treatment‐dose networks. Network geometry was generally adequate to allow both unrelated mean‐effect models and node‐splitting models to be used to assess discrepancy between direct and indirect evidence. Where discrepancies were identified, we considered the potential for transitivity assumption violations in strength‐of‐evidence assessments and model choice. On some occasions the distributions of estimates from direct and indirect evidence were wide due to low power, or we were unable to make important comparisons due to an absence of head‐to‐head studies. In these circumstances, transitivity assumption violations cannot be discounted. In general, there was sufficient evidence to identify discrepancy between direct and indirect evidence ‐ and such discrepancies were rare ‐ especially considering the size of the networks and the potential diversity of participants across pain conditions.
Publication bias
We used ROB‐MEN to assess publication bias in the review (Chiocchia 2021). For the primary outcomes, we were only able to produce funnel plots for the duloxetine‐placebo comparison as it was the only comparison with over 10 studies. These funnel plots showed some evidence of publication bias, and therefore the comparisons were rated as ‘some concerns’. As all other antidepressants tended to report small effects with small numbers of studies and participants, we judged all comparisons to have ‘some concerns’.
Potential biases in the review process
We minimised the potential for bias in the review process as much as possible. We published our protocol through the Cochrane Library and followed this for the review process (Birkinshaw 2021). We had an extensive search strategy that included six databases, and also searched clinical trials registries for unpublished and ongoing studies. The chance of a missed study is minimal, and even more minimal is the chance of any missed study having a substantial effect on the overall results.
Two review authors completed screening, data extraction, and risk of bias assessments in duplicate and independently, with all disagreements resolved by discussion. Where possible, we contacted study authors to request missing data, but their response rate was low. Where the study was registered in a clinical trials registry, we collected data that were not reported in the published paper from the results section of the registry.
We used CINeMA (Nikolakopoulou 2020) and ROB‐MEN (Chiocchia 2021) to assess our confidence in the results. Two review authors made the final interpretation and judgements in discussion.
Agreements and disagreements with other studies or reviews
To our knowledge, this is the only NMA that has examined all antidepressants for all types of chronic pain; previous reviews in this topic area have focused solely on one pain condition, or one antidepressant, or have examined efficacy by drug, dose, and pain condition. There have been a number of systematic reviews and meta‐analyses over the past decade examining antidepressants for different types of pain conditions, the majority of which were Cochrane Reviews.
For neuropathic pain, multiple reviews have shown there is no high‐quality or high‐certainty evidence for the efficacy of amitriptyline, desipramine, imipramine, milnacipran, nortriptyline, or venlafaxine (Derry 2015a; Derry 2015b; Gallagher 2015; Hearn 2014a; Hearn 2014b; Moore 2015). However, there was moderate‐certainty evidence that duloxetine is efficacious for diabetic peripheral neuropathy (Lunn 2014). For fibromyalgia, reviews show that there was no unbiased evidence that amitriptyline, desvenlafaxine, venlafaxine, or SSRIs were better than placebo, but there is low‐certainty evidence that duloxetine, milnacipran, and mirtazapine are efficacious (Walitt 2015; Welsch 2018). Finally, for musculoskeletal pain, two reviews found no clear evidence to support the use of antidepressants for low back pain (Koes 2018; Urquhart 2008), though a recent systematic review and meta‐analysis showed moderate‐certainty evidence for SNRIs for low back pain (Ferreira 2021). The majority of studies in Ferreira and colleagues' review and meta‐analysis investigated chronic low back pain, although acute low back pain studies were also included.
Although we were unable to examine the outcomes by condition, our results are broadly in line with previous reviews. We found no high‐quality or high‐certainty evidence for the efficacy of amitriptyline, desipramine, desvenlafaxine, imipramine, mirtazapine, nortriptyline, or venlafaxine in any of our outcomes. Our review and NMA found that duloxetine had robust evidence and was the highest rated antidepressant for the majority of outcomes. For most outcomes, milnacipran was the second most efficacious antidepressant, although the certainty of evidence ranged between very low and moderate. For outcomes where a treatment‐dose model was used, standard and high doses of both duloxetine and milnacipran were equally effective.
Authors' conclusions
Implications for practice.
For people with chronic pain
Research from randomised controlled trials suggests that duloxetine is more effective than other antidepressants (including amitriptyline) for management of chronic pain. For people with chronic pain considering trying an antidepressant for pain relief, it may be worth trying duloxetine first before other antidepressants. However, it is important to acknowledge that there is no 'one size fits all' with both antidepressants and pain. Adopting a person‐centred approach is critical.
For clinicians
Amitriptyline was not among the highest‐ranked antidepressants in terms of efficacy for either substantial pain relief or reduction in pain intensity. The evidence suggests that generic duloxetine could be the first option when considering the use antidepressants for chronic pain management. Additionally, for duloxetine there is often no benefit to using a high dose; using a standard dose (60 mg) is often as effective as using a high dose (> 60 mg). We were unable to be certain about the adverse events and harms for any antidepressant, so this is important to consider when prescribing antidepressants for chronic pain.
For policy makers
A full analysis of international guidelines is out of scope, but the National Institute for Health and Care Excellence (NICE) guidelines for the treatment of chronic primary pain recommends antidepressants as the only pharmacological treatment option (NICE 2021). In these guidelines, NICE specifically recommend amitriptyline, citalopram, duloxetine, fluoxetine, paroxetine, or sertraline, with no recommendations regarding dose. Our review and analyses found only moderate‐ to high‐certainty evidence for duloxetine in the management of chronic pain, evidence for amitriptyline, citalopram, fluoxetine, paroxetine or sertraline was low quality and of very low certainty.
For funders of the intervention
Currently, amitriptyline is the most common and first‐line antidepressant prescribed for the management of chronic pain; however, there are no large, high‐quality studies to support this position. There is also a lack of head‐to‐head studies where multiple antidepressants are compared in the same study. It is important to recognise that there are no long‐term safety data available for any antidepressant used for chronic pain treatment, and that collection and reporting of these data during trials is essential.
Implications for research.
General implications
For all antidepressants aside from duloxetine, there is a lack of high‐quality, robust studies to establish effectiveness and safety. Amitriptyline and milnacipran particularly require further research; amitriptyline because it is the most common antidepressant prescribed for chronic pain management, and milnacipran because it has consistently ranked equivalent or very close to duloxetine.
Serotonin‐noradrenalin reuptake inhibitors (SNRIs) as a class require further research. Duloxetine and milnacipran were consistently the highest‐ranked antidepressants across outcomes. Research to identify and explore the mechanisms underpinning the effectiveness of these antidepressants is required.
The relationship between chronic pain and depression deserves further attention. It is common in studies of analgesics to exclude participants with comorbid mental health disorders such as clinical depression, anxiety, or psychosis. As a consequence, we know nothing of the effects of antidepressants on pain in these populations. Further, depression and anxiety are common consequences of chronic pain, and often co‐exist. Although the dosing schedules of anti‐depressant medicines are different when prescribed for analgesia rather than depression (typically smaller) there is a possibility of dual effect, but this is not possible to study in these trials.
Design implications
Longer trials are required: there is no evidence regarding the long‐term efficacy or safety of using antidepressants for the treatment of chronic pain. This is critical as it is likely that patients will be prescribed antidepressants for long periods of time, and currently we do not know if there are likely to be any harms related to this.
Head‐to‐head trials between antidepressants are required to accurately measure the effects of antidepressants for chronic pain.
Larger sample sizes: there is no need for small trials; sufficient sizes are required to establish effect.
There is a need for pragmatic trials with more complex designs to address changes in medication. Pragmatic trial designs that account for individual difference have been recommended for over a decade (Moore 2010c), yet the majority of studies are still designed as two‐arm placebo‐controlled trials.
Measurement implications
There is now guidance on the optimal conduct and reporting of clinical trials, and specific guidance on the reporting of pain trials, the Consolidated Standards of Reporting Trials (CONSORT; Schulz 2010), and Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT; Dworkin 2008). These recommendations should be adhered to in order to reduce research waste and efficiently inform clinical decision making.
Where applicable, both post‐intervention and change scores should be reported to enable comprehensive evidence synthesis.
If trials are reporting responder analyses (e.g. 50% pain relief), then they should also report the continuous data, to reduce the chance of Type 1 errors. Some studies in our review only reported responder analyses and could not be included in the counterpart continuous measures.
Adverse events should be reported following the CONSORT guidelines, as highlighted many times previously (Edwards 1999; Phillips 2019).
History
Protocol first published: Issue 4, 2021
Acknowledgements
Cochrane Review Group funding acknowledgement: this project was funded by the National Institute for Health Research (NIHR) via Cochrane Infrastructure funding to Cochrane Pain, Palliative and Supportive Care (PaPaS). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
We acknowledge and thank the NIHR Health Technology Assessment programme (HTA) for funding this review.
We thank Joanne Abbott (Information Specialist for Cochrane PaPaS Review Group) for developing and running the search strategy, and Iris Gordon (Information Specialist for Cochrane Eyes and Vision Review Group) for peer reviewing the search strategy.
We thank the peer reviewers of the protocol Dr Sarah Nevitt, Kevin Pacheco‐Barrios MD, Dr Christina Abdel Shaheed, and Prof Amanda C de C Williams, and consumer reviewers Harrison Nelson and Stella O’Brien.
Editorial and peer‐reviewer contributions
Cochrane Pain, Palliative and Supportive Care (PaPaS) supported the authors in the development of this review.
The following people conducted the editorial process for this article.
Sign‐off Editor (final editorial decision): Dr Neil O'Connell, PaPaS Co‐ordinating Editor, and Reader at Brunel University London
Contact Editor: Bethan Copsey, Leeds Institute of Clinical Trials Research, University of Leeds, UK
Managing Editor (selected peer reviewers, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Anna Erskine and Jessica Thomas (Oxford University Hospitals (OUH) NHS Foundation Trust, Oxford, UK)
Assistant Managing Editor (conducted editorial checks and supported editorial team): Kerry Harding (Oxford University Hospitals (OUH) NHS Foundation Trust, Oxford, UK)
Information Specialist (searching support): Joanne Abbott (Oxford University Hospitals (OUH) NHS Foundation Trust, Oxford, UK)
Peer reviewers: Brian Duncan (consumer reviewer, Psychology); Dr Ye Jin, Mental Health centre, Yingkou, Liaoning, China (clinical reviewer); Giovanni Ferreira, Institute for Musculoskeletal Health & Sydney Musculoskeletal Health, The University of Sydney (clinical reviewer); José A López‐López, Department of Basic Psychology and Methodology, Faculty of Psychology and Speech Therapy, University of Murcia, Murcia (Spain) (clinical reviewer); Rachel Richardson (Cochrane Evidence Production and Methods Directorate), Sofia Tsokani (Cochrane Evidence Production and Methods Directorate).
Copy‐editing (initial copy‐edit and final proofread): Denise Mitchell (Cochrane Evidence Production and Methods Directorate)
Appendices
Appendix 1. Search strategies
MEDLINE
1. pain/ or exp abdominal pain/ or exp arthralgia/ or exp back pain/ or breakthrough pain/ or cancer pain/ or exp chest pain/ or chronic pain/ or earache/ or eye pain/ or facial pain/ or flank pain/ or glossalgia/ or exp headache/ or mastodynia/ or metatarsalgia/ or exp musculoskeletal pain/ or exp neck pain/ or neuralgia/ or exp nociceptive pain/ or pain, intractable/ or exp pain, postoperative/ or pain, referred/ or exp pelvic pain/ or renal colic/ 2. pain.tw. 3. (headache* or migraine* or fibromyalgia* or neuralgia*).tw. 4. Fibromyalgia/ 5. 1 or 2 or 3 or 4 6. exp ANTIDEPRESSIVE AGENTS/ 7. exp MONOAMINE OXIDASE INHIBITORS/ 8. exp NEUROTRANSMITTER UPTAKE INHIBITORS/ 9. ((serotonin or norepinephrine or noradrenaline or neurotransmitter* or dopamin*) and (uptake or reuptake or re uptake)).tw. 10. (noradrenerg* or antiadrenergic or anti adrenergic or SSRI* or SNRI* or NARI* or SARI* or NDRI* or TCA* or tricyclic* or tetracyclic* or heterocyclic or pharmacotherap* or psychotropic).tw. 11. (antidpress* or anti‐depress*).tw. 12. (MAOI* or RIMA).tw. 13. monoamine oxidase inhibit*.tw. 14. (Agomelatine or Amoxapine or Amineptine or Amitriptylin* or Amitriptylinoxide or Atomoxetine or Befloxatone or Benactyzine or Brofaromin*).tw. 15. (Bupropion or Amfebutamone or Butriptylin* or Caroxazone or Cianopramin* or Cilobamin* or Cimoxatone or Citalopram or Chlorimipramin* or Clomipramin* or Chlomipramin* or Clomipramine).tw. 16. (Clorgyline or Clovoxamin* or "CX157" or Tyrima or Tririma or Demexiptilin* or Deprenyl or Desipramin* or Pertofrane or Desvenlafaxine or Dibenzepin or Diclofensin* or Dimetacrin* or Dosulepin or Dothiepin or Doxepin or Duloxetine or Desvenlafaxine or "DVS‐233" or Escitalopram or Etoperidone or Femoxetin* or Fluotracen or Fluoxetine or Fluvoxamin*).tw. 17. (Hyperforin or Hypericum or St John* or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Isocarboxazid* or Levomilnacipran or Lofepramin* or "Lu AA21004" or Vortioxetine or "Lu AA24530" or Tedatioxetine or "LY2216684" or Edivoxetine or Maprotilin* or Medifoxamin* or Melitracen or Metapramin* or Mianserin or Milnacipran or Minaprin* or Mirtazapin* or Moclobemide).tw. 18. (Nefazodone or Nialamide or Nitroxazepine or Nomifensin* or Norfenfluramin* or Nortriptylin* or Noxiptilin* or Opipramol or Paroxetine or Phenelzine or Pheniprazine or Pipofezine or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin* or Quinupramine or Reboxetine or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromin* or Trazodone or Trimipramin* or Tryptophan* or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Zalospirone).tw. 19. or/6‐18 20. randomized controlled trial.pt. 21. controlled clinical trial.pt. 22. randomized.ab. 23. placebo.ab. 24. drug therapy.fs. 25. randomly.ab. 26. trial.ab. 27. or/20‐26 28. exp animals/ not humans.sh. 29. 27 not 28 30. 5 and 19 and 29 31. limit 30 to "all adult (19 plus years)"
Cochrane Central Register of Controlled Trials (CENTRAL)
#1 MeSH descriptor: [Antidepressive Agents] explode all trees
#2 MeSH descriptor: [Monoamine Oxidase Inhibitors] explode all trees
#3 MeSH descriptor: [Neurotransmitter Uptake Inhibitors] explode all trees
#4 (((serotonin or norepinephrine or noradrenaline or neurotransmitter* or dopamin*) and (uptake or reuptake or re uptake))):ti,ab,kw (Word variations have been searched)
#5 ((noradrenerg* or antiadrenergic or anti adrenergic or SSRI* or SNRI* or NARI* or SARI* or NDRI* or TCA* or tricyclic* or tetracyclic* or heterocyclic or pharmacotherap* or psychotropic)):ti,ab,kw (Word variations have been searched)
#6 (antidpress* or anti‐depress*):ti,ab,kw (Word variations have been searched)
#7 (MAOI* or RIMA):ti,ab,kw (Word variations have been searched)
#8 (monoamine oxidase inhibit*):ti,ab,kw (Word variations have been searched)
#9 ((Agomelatine or Amoxapine or Amineptine or Amitriptylin* or Amitriptylinoxide or Atomoxetine or Befloxatone or Benactyzine or Brofaromin*)):ti,ab,kw (Word variations have been searched)
#10 ((Bupropion or Amfebutamone or Butriptylin* or Caroxazone or Cianopramin* or Cilobamin* or Cimoxatone or Citalopram or Chlorimipramin* or Clomipramin* or Chlomipramin* or Clomipramine)):ti,ab,kw (Word variations have been searched)
#11 ((Clorgyline or Clovoxamin* or "CX157" or Tyrima or Tririma or Demexiptilin* or Deprenyl or Desipramin* or Pertofrane or Desvenlafaxine or Dibenzepin or Diclofensin* or Dimetacrin* or Dosulepin or Dothiepin or Doxepin or Duloxetine or Desvenlafaxine or "DVS‐233" or Escitalopram or Etoperidone or Femoxetin* or Fluotracen or Fluoxetine or Fluvoxamin*)):ti,ab,kw (Word variations have been searched)
#12 ((Hyperforin or Hypericum or St John* or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Isocarboxazid* or Levomilnacipran or Lofepramin* or "Lu AA21004" or Vortioxetine or "Lu AA24530" or Tedatioxetine or "LY2216684" or Edivoxetine or Maprotilin* or Medifoxamin* or Melitracen or Metapramin* or Mianserin or Milnacipran or Minaprin* or Mirtazapin* or Moclobemide)):ti,ab,kw (Word variations have been searched)
#13 ((Nefazodone or Nialamide or Nitroxazepine or Nomifensin* or Norfenfluramin* or Nortriptylin* or Noxiptilin* or Opipramol or Paroxetine or Phenelzine or Pheniprazine or Pipofezine or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin* or Quinupramine or Reboxetine or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromin* or Trazodone or Trimipramin* or Tryptophan* or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Zalospirone)):ti,ab,kw (Word variations have been searched)
#14 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13
#15 ((headache* or migraine* or fibromyalgia* or neuralgia*)):ti,ab,kw (Word variations have been searched)
#16 (pain):ti,ab,kw (Word variations have been searched)
#17 MeSH descriptor: [Fibromyalgia] this term only
#18 MeSH descriptor: [Abdominal Pain] explode all trees
#19 MeSH descriptor: [Arthralgia] explode all trees
#20 MeSH descriptor: [Back Pain] explode all trees
#21 MeSH descriptor: [Back Pain] this term only
#22 MeSH descriptor: [Cancer Pain] this term only
#23 MeSH descriptor: [Chest Pain] explode all trees
#24 MeSH descriptor: [Chronic Pain] this term only
#25 MeSH descriptor: [Earache] this term only
#26 MeSH descriptor: [Eye Pain] this term only
#27 MeSH descriptor: [Facial Pain] this term only
#28 MeSH descriptor: [Flank Pain] this term only
#29 MeSH descriptor: [Glossalgia] this term only
#30 MeSH descriptor: [Headache] explode all trees
#31 MeSH descriptor: [Mastodynia] this term only
#32 MeSH descriptor: [Metatarsalgia] this term only
#33 MeSH descriptor: [Musculoskeletal Pain] explode all trees
#34 MeSH descriptor: [undefined] explode all trees
#35 MeSH descriptor: [Neuralgia] this term only
#36 MeSH descriptor: [Nociceptive Pain] explode all trees
#37 MeSH descriptor: [Pain, Intractable] this term only
#38 MeSH descriptor: [Pain, Postoperative] explode all trees
#39 MeSH descriptor: [Pain, Referred] this term only
#40 MeSH descriptor: [Pelvic Pain] explode all trees
#41 MeSH descriptor: [Renal Colic] this term only
#42 #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41
#43 #14 and #42
Embase
1. *pain/ or exp abdominal pain/ or exp arthralgia/ or exp back pain/ or *breakthrough pain/ or *cancer pain/ or exp chest pain/ or *chronic pain/ or *earache/ or *eye pain/ or *facial pain/ or *flank pain/ or *glossalgia/ or exp headache/ or *mastodynia/ or *metatarsalgia/ or exp musculoskeletal pain/ or exp neck pain/ or *neuralgia/ or exp nociceptive pain/ or *pain, intractable/ or exp pain, postoperative/ or pain, referred/ or exp pelvic pain/ or *renal colic/
2. pain.tw.
3. (headache* or migraine* or fibromyalgia* or neuralgia*).tw.
4. Fibromyalgia/
5. 1 or 2 or 3 or 4
6. exp ANTIDEPRESSIVE AGENTS/
7. exp MONOAMINE OXIDASE INHIBITORS/
8. exp NEUROTRANSMITTER UPTAKE INHIBITORS/
9. ((serotonin or norepinephrine or noradrenaline or neurotransmitter* or dopamin*) and (uptake or reuptake or re uptake)).tw.
10. (noradrenerg* or antiadrenergic or anti adrenergic or SSRI* or SNRI* or NARI* or SARI* or NDRI* or TCA* or tricyclic* or tetracyclic* or heterocyclic or pharmacotherap* or psychotropic).tw.
11. (antidpress* or anti‐depress*).tw.
12. (MAOI* or RIMA).tw.
13. monoamine oxidase inhibit*.tw.
14. (Agomelatine or Amoxapine or Amineptine or Amitriptylin* or Amitriptylinoxide or Atomoxetine or Befloxatone or Benactyzine or Brofaromin*).tw.
15. (Bupropion or Amfebutamone or Butriptylin* or Caroxazone or Cianopramin* or Cilobamin* or Cimoxatone or Citalopram or Chlorimipramin* or Clomipramin* or Chlomipramin* or Clomipramine).tw.
16. (Clorgyline or Clovoxamin* or "CX157" or Tyrima or Tririma or Demexiptilin* or Deprenyl or Desipramin* or Pertofrane or Desvenlafaxine or Dibenzepin or Diclofensin* or Dimetacrin* or Dosulepin or Dothiepin or Doxepin or Duloxetine or Desvenlafaxine or "DVS‐233" or Escitalopram or Etoperidone or Femoxetin* or Fluotracen or Fluoxetine or Fluvoxamin*).tw.
17. (Hyperforin or Hypericum or St John* or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Isocarboxazid* or Levomilnacipran or Lofepramin* or "Lu AA21004" or Vortioxetine or "Lu AA24530" or Tedatioxetine or "LY2216684" or Edivoxetine or Maprotilin* or Medifoxamin* or Melitracen or Metapramin* or Mianserin or Milnacipran or Minaprin* or Mirtazapin* or Moclobemide).tw.
18. (Nefazodone or Nialamide or Nitroxazepine or Nomifensin* or Norfenfluramin* or Nortriptylin* or Noxiptilin* or Opipramol or Paroxetine or Phenelzine or Pheniprazine or Pipofezine or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin* or Quinupramine or Reboxetine or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromin* or Trazodone or Trimipramin* or Tryptophan* or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Zalospirone).tw.
19. or/6‐18
20. random$.tw.
21. factorial$.tw.
22. crossover$.tw.
23. cross over$.tw.
24. cross‐over$.tw.
25. placebo$.tw.
26. (doubl$ adj blind$).tw.
27. (singl$ adj blind$).tw.
28. assign$.tw.
29. allocat$.tw.
30. volunteer$.tw.
31. Crossover Procedure/
32. double‐blind procedure.tw.
33. Randomized Controlled Trial/
34. Single Blind Procedure/
35. 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34
36. (animal/ or nonhuman/) not human/
37. 35 not 36
38. 5 and 19 and 37
39. limit 38 to (adult <18 to 64 years> or aged <65+ years>)
AMED
1. *pain/ or exp abdominal pain/ or exp arthralgia/ or exp back pain/ or *breakthrough pain/ or *cancer pain/ or exp chest pain/ or *chronic pain/ or *earache/ or *eye pain/ or *facial pain/ or *flank pain/ or *glossalgia/ or exp headache/ or *mastodynia/ or *metatarsalgia/ or exp musculoskeletal pain/ or exp neck pain/ or *neuralgia/ or exp nociceptive pain/ or *pain, intractable/ or exp pain, postoperative/ or pain, referred/ or exp pelvic pain/ or *renal colic/
2. pain.tw.
3. (headache* or migraine* or fibromyalgia* or neuralgia*).tw.
4. Fibromyalgia/
5. 1 or 2 or 3 or 4
6. exp ANTIDEPRESSIVE AGENTS/
7. exp MONOAMINE OXIDASE INHIBITORS/
8. exp NEUROTRANSMITTER UPTAKE INHIBITORS/
9. ((serotonin or norepinephrine or noradrenaline or neurotransmitter* or dopamin*) and (uptake or reuptake or re uptake)).tw.
10. (noradrenerg* or antiadrenergic or anti adrenergic or SSRI* or SNRI* or NARI* or SARI* or NDRI* or TCA* or tricyclic* or tetracyclic* or heterocyclic or pharmacotherap* or psychotropic).tw.
11. (antidpress* or anti‐depress*).tw.
12. (MAOI* or RIMA).tw.
13. monoamine oxidase inhibit*.tw.
14. (Agomelatine or Amoxapine or Amineptine or Amitriptylin* or Amitriptylinoxide or Atomoxetine or Befloxatone or Benactyzine or Brofaromin*).tw.
15. (Bupropion or Amfebutamone or Butriptylin* or Caroxazone or Cianopramin* or Cilobamin* or Cimoxatone or Citalopram or Chlorimipramin* or Clomipramin* or Chlomipramin* or Clomipramine).tw.
16. (Clorgyline or Clovoxamin* or "CX157" or Tyrima or Tririma or Demexiptilin* or Deprenyl or Desipramin* or Pertofrane or Desvenlafaxine or Dibenzepin or Diclofensin* or Dimetacrin* or Dosulepin or Dothiepin or Doxepin or Duloxetine or Desvenlafaxine or "DVS‐233" or Escitalopram or Etoperidone or Femoxetin* or Fluotracen or Fluoxetine or Fluvoxamin*).tw.
17. (Hyperforin or Hypericum or St John* or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Isocarboxazid* or Levomilnacipran or Lofepramin* or "Lu AA21004" or Vortioxetine or "Lu AA24530" or Tedatioxetine or "LY2216684" or Edivoxetine or Maprotilin* or Medifoxamin* or Melitracen or Metapramin* or Mianserin or Milnacipran or Minaprin* or Mirtazapin* or Moclobemide).tw.
18. (Nefazodone or Nialamide or Nitroxazepine or Nomifensin* or Norfenfluramin* or Nortriptylin* or Noxiptilin* or Opipramol or Paroxetine or Phenelzine or Pheniprazine or Pipofezine or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin* or Quinupramine or Reboxetine or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromin* or Trazodone or Trimipramin* or Tryptophan* or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Zalospirone).tw.
19. or/6‐18
20. (random* or factorial* or placebo* or assign* or allocat* or crossover).tw.
21. (cross adj over*).tw.
22. (trial* and (control* or comparative)).tw.
23. ((blind* or mask*) and (single or double or triple or treble)).tw.
24. (treatment adj arm*).tw.
25. (control* adj group*).tw.
26. (phase adj (III or three)).tw.
27. (versus or vs).tw.
28. rct.tw.
29. RANDOM ALLOCATION/
30. DOUBLE BLIND METHOD/
31. placebos/
32. randomized controlled trials/
33. 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32
34. 5 and 19 and 33
35. exp adult/
36. 34 and 35
PsycINFO
S29 S20 AND S28
S28 S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27
S27 (singl* OR doubl* OR trebl* OR tripl*) N3 (blind* OR mask*)
S26 clinical N3 trial* OR research N3 design OR evaluat* N3 stud* OR prospectiv* N3 stud*
S25 placebo* OR random* OR "comparative stud*"
S24 DE "Followup Studies"
S23 DE "Placebo"
S22 DE "Treatment Outcomes" OR DE "Psychotherapeutic Outcomes" OR DE "Side Effects (Treatment)" OR DE "Treatment Compliance" OR DE "Treatment Duration" OR DE "Treatment Refusal" OR DE "Treatment Termination" OR DE "Treatment Withholding"
S21 DE "Treatment Effectiveness Evaluation"
S20 S15 AND S19
S19 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S16 OR S17 OR S18
S18 DE "Neurotransmitter Uptake Inhibitors" OR DE "Atomoxetine" OR DE "Serotonin Norepinephrine Reuptake Inhibitors" OR DE "Serotonin Reuptake Inhibitors"
S17 DE "Monoamine Oxidase Inhibitors" OR DE "Iproniazid" OR DE "Isocarboxazid" OR DE "Moclobemide" OR DE "Nialamide" OR DE "Pargyline" OR DE "Phenelzine" OR DE "Pheniprazine" OR DE "Tranylcypromine"
S16 DE "Antidepressant Drugs" OR DE "Bupropion" OR DE "Citalopram" OR DE "Fluoxetine" OR DE "Fluvoxamine" OR DE "Iproniazid" OR DE "Isocarboxazid" OR DE "Lithium Carbonate" OR DE "Methylphenidate" OR DE "Mianserin" OR DE "Moclobemide" OR DE "Molindone" OR DE "Nefazodone" OR DE "Nialamide" OR DE "Nomifensine" OR DE "Paroxetine" OR DE "Phenelzine" OR DE "Pheniprazine" OR DE "Pipradrol" OR DE "Serotonin Norepinephrine Reuptake Inhibitors" OR DE "Sertraline" OR DE "Sulpiride" OR DE "Tranylcypromine" OR DE "Trazodone" OR DE "Tricyclic Antidepressant Drugs" OR DE "Venlafaxine" OR DE "Zimeldine"
S15 S12 OR S13 OR S14
S14 DE "Fibromyalgia"
S13 pain OR ( headache* or migraine* or fibromyalgia* or neuralgia* )
S12 DE "Pain" OR DE "Aphagia" OR DE "Back Pain" OR DE "Chronic Pain" OR DE "Headache" OR DE "Myofascial Pain" OR DE "Neuralgia" OR DE "Neuropathic Pain" OR DE "Somatoform Pain Disorder"
S11 PAIN
S10 (Nefazodone or Nialamide or Nitroxazepine or Nomifensin* or Norfenfluramin* or Nortriptylin* or Noxiptilin* or Opipramol or Paroxetine or Phenelzine or Pheniprazine or Pipofezine or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin* or Quinupramine or Reboxetine or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromin* or Trazodone or Trimipramin* or Tryptophan* or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Zalospirone)
S9 (Hyperforin or Hypericum or St John* or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Isocarboxazid* or Levomilnacipran or Lofepramin* or "Lu AA21004" or Vortioxetine or "Lu AA24530" or Tedatioxetine or "LY2216684" or Edivoxetine or Maprotilin* or Medifoxamin* or Melitracen or Metapramin* or Mianserin or Milnacipran or Minaprin* or Mirtazapin* or Moclobemide)
S8 (Clorgyline or Clovoxamin* or "CX157" or Tyrima or Tririma or Demexiptilin* or Deprenyl or Desipramin* or Pertofrane or Desvenlafaxine or Dibenzepin or Diclofensin* or Dimetacrin* or Dosulepin or Dothiepin or Doxepin or Duloxetine or Desvenlafaxine or "DVS‐233" or Escitalopram or Etoperidone or Femoxetin* or Fluotracen or Fluoxetine or Fluvoxamin*)
S7 (Bupropion or Amfebutamone or Butriptylin* or Caroxazone or Cianopramin* or Cilobamin* or Cimoxatone or Citalopram or Chlorimipramin* or Clomipramin* or Chlomipramin* or Clomipramine)
S6 (Agomelatine or Amoxapine or Amineptine or Amitriptylin* or Amitriptylinoxide or Atomoxetine or Befloxatone or Benactyzine or Brofaromin*)
S5 monoamine oxidase inhibit*
S4 MAOI* or RIMA
S3 antidpress* or anti‐depress*
S2 (noradrenerg* or antiadrenergic or anti adrenergic or SSRI* or SNRI* or NARI* or SARI* or NDRI* or TCA* or tricyclic* or tetracyclic* or heterocyclic or pharmacotherap* or psychotropic)
S1 ((serotonin or norepinephrine or noradrenaline or neurotransmitter* or dopamin*) and (uptake or reuptake or re uptake))
CINAHL
S31 S4 AND S18 AND S30
S30 S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29
S29 TX allocat* random*
S28 (MH "Quantitative Studies")
S27 (MH "Placebos")
S26 TX placebo*
S25 TX random* allocat*
S24 (MH "Random Assignment")
S23 TX randomi* control* trial*
S22 TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) )
S21 TX clinic* n1 trial*
S20 PT Clinical trial
S19 (MH "Clinical Trials+")
S18 S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17
S17 (Nefazodone or Nialamide or Nitroxazepine or Nomifensin* or Norfenfluramin* or Nortriptylin* or Noxiptilin* or Opipramol or Paroxetine or Phenelzine or Pheniprazine or Pipofezine or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin* or Quinupramine or Reboxetine or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromin* or Trazodone or Trimipramin* or Tryptophan* or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Zalospirone)
S16 (Hyperforin or Hypericum or St John* or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Isocarboxazid* or Levomilnacipran or Lofepramin* or "Lu AA21004" or Vortioxetine or "Lu AA24530" or Tedatioxetine or "LY2216684" or Edivoxetine or Maprotilin* or Medifoxamin* or Melitracen or Metapramin* or Mianserin or Milnacipran or Minaprin* or Mirtazapin* or Moclobemide)
S15 (Clorgyline or Clovoxamin* or "CX157" or Tyrima or Tririma or Demexiptilin* or Deprenyl or Desipramin* or Pertofrane or Desvenlafaxine or Dibenzepin or Diclofensin* or Dimetacrin* or Dosulepin or Dothiepin or Doxepin or Duloxetine or Desvenlafaxine or "DVS‐233" or Escitalopram or Etoperidone or Femoxetin* or Fluotracen or Fluoxetine or Fluvoxamin*)
S14 (Bupropion or Amfebutamone or Butriptylin* or Caroxazone or Cianopramin* or Cilobamin* or Cimoxatone or Citalopram or Chlorimipramin* or Clomipramin* or Chlomipramin* or Clomipramine)
S13 (Agomelatine or Amoxapine or Amineptine or Amitriptylin* or Amitriptylinoxide or Atomoxetine or Befloxatone or Benactyzine or Brofaromin*)
S12 monoamine oxidase inhibit*
S11 MAOI* or RIMA
S10 antidpress* or anti‐depress*
S9 (noradrenerg* or antiadrenergic or anti adrenergic or SSRI* or SNRI* or NARI* or SARI* or NDRI* or TCA* or tricyclic* or tetracyclic* or heterocyclic or pharmacotherap* or psychotropic)
S8 ((serotonin or norepinephrine or noradrenaline or neurotransmitter* or dopamin*) and (uptake or reuptake or re uptake))
S7 (MH "Neurotransmitter Uptake Inhibitors+")
S6 (MH "Monoamine Oxidase Inhibitors+")
S5 (MH "Antidepressive Agents+")
S4 S1 OR S2 OR S3
S3 (MH "Fibromyalgia")
S2 pain OR ( headache* or migraine* or fibromyalgia* or neuralgia* )
S1 (MH "Pain+")
LILACS
headache$ or migraine$ or fibromyalgia$ or neuralgia$ or pain [Words] and (Nefazodone or Nialamide or Nitroxazepine or Nomifensin$ or Norfenfluramin$ or Nortriptylin$ or Noxiptilin$ or Opipramol or Paroxetine or Phenelzine or Pheniprazine or Pipofezine or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin$ or Quinupramine or Reboxetine or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Thozalinone or Tianeptin$ or Toloxatone or Tranylcypromin$ or Trazodone or Trimipramin$ or Tryptophan$ or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Zalospirone) or (Hyperforin or Hypericum or St John$ or Imipramin$ or Iprindole or Iproniazid$ or Ipsapirone or Isocarboxazid$ or Levomilnacipran or Lofepramin$ or "Lu AA21004" or Vortioxetine or "Lu AA24530" or Tedatioxetine or "LY2216684" or Edivoxetine or Maprotilin$ or Medifoxamin$ or Melitracen or Metapramin$ or Mianserin or Milnacipran or Minaprin$ or Mirtazapin$ or Moclobemide) or (Clorgyline or Clovoxamin$ or "CX157" or Tyrima or Tririma or Demexiptilin$ or Deprenyl or Desipramin$ or Pertofrane or Desvenlafaxine or Dibenzepin or Diclofensin$ or Dimetacrin$ or Dosulepin or Dothiepin or Doxepin or Duloxetine or Desvenlafaxine or "DVS‐233" or Escitalopram or Etoperidone or Femoxetin$ or Fluotracen or Fluoxetine or Fluvoxamin$) or (Bupropion or Amfebutamone or Butriptylin$ or Caroxazone or Cianopramin$ or Cilobamin$ or Cimoxatone or Citalopram or Chlorimipramin$ or Clomipramin$ or Chlomipramin$ or Clomipramine) or (Agomelatine or Amoxapine or Amineptine or Amitriptylin$ or Amitriptylinoxide or Atomoxetine or Befloxatone or Benactyzine or Brofaromin$) or ((serotonin or norepinephrine or noradrenaline or neurotransmitter$ or dopamin$) and (uptake or reuptake or re uptake)) or (noradrenerg$ or antiadrenergic or anti adrenergic or SSRI$ or SNRI$ or NARI$ or SARI$ or NDRI$ or TCA$ or tricyclic$ or tetracyclic$ or heterocyclic or pharmacotherap$ or psychotropic) or (antidpress$ or anti‐depress$ or MAOI$ or RIMA or monoamine oxidase inhibit$) [Words] and randomised OR randomized OR randomisation OR randomization OR trial OR placebo OR blind OR "phase 3" OR "phase III" [Words]
Appendix 2. Network meta‐analysis reporting decisions
Overview
This appendix details the decisions made in the reporting of the network meta‐analyses (NMAs) in the results section of the review. For each network we took into account heterogeneity, inconsistency, and network geometry.
Substantial pain relief (50% reduction)
Networks – which model is the best fit?
Our primary analysis was a Bayesian network meta‐analysis including treatment. This analysis had high heterogeneity (Tau = 0.26) and inconsistency in both unrelated mean effects and node‐splitting models. We also explored networks that separated treatments into different doses, conditions and risk of bias categories and aggregated treatment by class. These networks resulted in models that had similar heterogeneity and variable indications for inconsistency but the model that included antidepressant dose reduced the estimate of heterogeneity by half (Tau = 0.11) and there was no indication of inconsistency. Therefore, the results are based on the treatment‐dose model.
Pain intensity
Change scores and post‐intervention
Studies in the review reported pain intensity results in two ways: change scores and post‐intervention scores. Fifty studies with 14,926 participants reported change scores, 74 studies with 7703 participants reported post‐intervention scores. As these two types of scores cannot be combined directly, we selected model‐data combinations on the basis of parsimony, minimisation of inconsistency (identified via unrelated mean‐effect models (UME) and node‐splitting models), residual deviance and heterogeneity (measured as Tau) to minimise the risk of over‐fitting.
Networks – which model is the best fit?
For both change‐score and post‐intervention analyses, we generated networks and models based on treatment and treatment dose.
Change
The treatment analysis had low heterogeneity (Tau = 0.17) and low inconsistency in the UME model, however node‐splitting models could not be run due to inappropriate network geometry. Models including dose had lower heterogeneity (Tau = 0.10) and no indications for inconsistency in both UME and node‐splitting models.
Post‐intervention scores
The treatment analysis had high heterogeneity (Tau = 2.06) compared to change‐score analysis and inconsistency in the UME model, that suggest it is not possible to fit a robust model to the data. Models including dose continued to have higher heterogeneity than the change‐score analysis (Tau = 0.46), and high residual deviance across multiple studies suggesting that a robust model is unlikely to fit the data. UME models continued to show inconsistency between direct and indirect evidence, although node‐splitting models showed no inconsistency within studies.
Mood
Change scores and post‐intervention
Studies in the review reported pain intensity results in two ways: change scores and post‐intervention scores. Thirty‐eight studies with 12,985 participants reported change scores, 46 studies with 3885 participants reported post‐intervention scores. As these two types of scores cannot be combined, we reported the most appropriate and robust model for the data.
Networks – which model is the best fit?
For both change‐score and post‐intervention analyses, the primary analysis was a Bayesian NMA including treatment.
Change
The treatment analysis had low heterogeneity (Tau = 0.09), with no inconsistency in the UME model. We were unable to run node‐splitting models due to the network geometry as the majority of the network is formed from two‐arm placebo‐controlled studies. As the treatment‐only analysis had low heterogeneity and no inconsistency, no further analyses were undertaken.
Post‐intervention
This analysis had moderate heterogeneity (Tau = 0.69), with high residual deviance across multiple studies. UME models showed inconsistency between direct and indirect evidence, although node‐splitting models showed no inconsistency within studies. We were unable to run any further analyses including any covariates due to small sample sizes, network geometry and the risk of over‐fitting.
Adverse events
Networks – which model is the best fit?
Our primary analysis was a Bayesian NMA including treatment. This analysis had high heterogeneity (Tau = 0.49), with the UME model indicating high inconsistency and divergent transitions within the network. We were unable to run node‐splitting models due to network geometry. Models including dose continued to have high heterogeneity (Tau = 0.59), and the UME model showed high inconsistency, similar to the treatment‐only model. There continued to be divergent transitions within the network and low effective sample sizes, however the node‐splitting models were able to run and showed no evidence of inconsistency. Due to the network geometry and inappropriateness of running extra models, no further analyses including other covariates were run. The results are based on the treatment‐dose model, due to similar levels of heterogeneity and inconsistency, and the ability to run node‐splitting models.
Moderate pain relief
Networks – which model is the best fit?
Our primary analysis was a Bayesian NMA including treatment. This analysis had low heterogeneity (Tau = 0.13) and no evidence of inconsistency in both UME and node‐splitting models. Therefore, the results are based on a model including treatment only. Divergent transitions suggested unstable models when analysing treatment‐dose networks.
Physical function
Change scores and post‐intervention
Studies in the review reported physical function results in two ways: change scores and post‐intervention scores. Thirty‐two studies with 11,760 participants reported change scores, while 30 studies with 3645 participants reported post‐intervention scores. As these two types of scores cannot be combined, we reported the most appropriate and robust model for the data.
Networks – which model is the best fit?
For both change score and post‐intervention score analyses, the primary analysis was a Bayesian NMA including treatment.
Change scores
Our primary analysis was a Bayesian NMA including treatment. This analysis had low heterogeneity (Tau = 0.05), and there was little evidence of inconsistency in the UME model or node‐splitting models. Using a model including dose resulted in lower heterogeneity (Tau = 0.04) and no major indications for inconsistency from both unrelated mean effect and node‐splitting models.
Post‐intervention scores
Our primary analysis was a Bayesian NMA including treatment. This analysis had moderate heterogeneity, higher than that of the change score analysis (Tau = 0.69) with no inconsistency in both UME and node‐splitting models. Models including dose increased the heterogeneity (Tau = 0.82) but continued to show no evidence of inconsistency.
Sleep
Change scores and post‐intervention
Studies in the review reported sleep results in two ways: change scores and post‐intervention scores. Eighteen studies with 6301 participants reported change scores, while 18 studies with 1921 participants reported post‐intervention scores. As these two types of scores cannot be combined, we reported the most appropriate and robust model for the data.
Networks – which model is the best fit?
For both change‐score and post‐intervention score analyses, the primary analysis was a Bayesian NMA including treatment.
Change scores
Our primary analysis was a Bayesian NMA including treatment. This analysis had low heterogeneity (Tau = 0.06), but due to the star‐shaped network geometry we were unable to explore inconsistency using node‐splitting models in the treatment‐only network. Models including dose also had low heterogeneity (Tau = 0.11) and no indications for inconsistency in UME but node‐splitting models indicated inconsistency, although these parameter estimates may be unreliable due to divergent transitions.
Post‐intervention scores
The primary analysis was a Bayesian NMA including treatment. This analysis had low heterogeneity (Tau = 0.12) and no inconsistency in both UME and node‐splitting models, although there were three divergent transitions. Models including dose had slightly higher heterogeneity (Tau = 0.16), but the network was disconnected requiring four studies to be removed, and there were 12 divergent transitions.
Model used
Comparing the post‐intervention and change‐score analyses shows that the change‐score treatment network is more robust and reliable than the post‐intervention network as models without divergent transitions were generated. Therefore, the results are based on a model of change scores including both treatment and dose. Results for the treatment‐only model are available in the supplemental files (link provided in Appendix 3).
Quality of life
Change scores and post‐intervention
Studies in the review reported pain intensity results in two ways: change scores and post‐intervention scores. Twenty‐seven studies with 9693 participants reported change scores, 19 studies with 3103 participants reported post‐intervention scores. As these two types of scores cannot be combined, we reported the most appropriate and robust model for the data.
Networks – which model is the best fit?
For both change‐score and post‐intervention analyses, the primary analysis was a Bayesian NMA including treatment.
Change scores
The treatment‐only analysis had high heterogeneity (Tau = 0.87), with no evidence of inconsistency in UME and node‐splitting models. Models including dose continued to have higher heterogeneity (0.76), with some evidence of inconsistency in the node‐splitting models for milnacipran.
Post‐intervention scores
The treatment‐only analysis had moderate heterogeneity (Tau = 0.55) and no evidence of inconsistency in both UME and node‐splitting models, although some residual deviance was present on multiple studies. Models including dose had higher heterogeneity (Tau = 0.67) with similar levels of residual deviance.
Model used
Comparing the post‐intervention and change‐score analyses shows that the post‐intervention score treatment network has lower heterogeneity than the change‐score treatment‐dose network. Therefore, the results are based on a model of post‐intervention scores including treatment. The results of the change‐score analyses are available in the supplemental files (link provided in Appendix 3).
Patient Global Impression of Change (PGIC)
PGIC much/very much improved
Networks – which model is the best fit?
Our primary analysis was a Bayesian NMA including treatment. This analysis had low heterogeneity (Tau = 0.12) and no evidence inconsistency in both UME and node‐splitting models. However, there were several divergent transitions. Models including dose reduced the heterogeneity (Tau = 0.08) and continued to show no indications for inconsistency. There was only one divergent transition in this model. Therefore, the results are based on a model including treatment and dose. The results of the treatment‐only model are included in the appendices.
PGIC continuous
Networks – which model is the best fit?
Our primary analysis was a Bayesian NMA including treatment. This analysis had low heterogeneity (Tau = 0.05) but some evidence of inconsistency in both UME and node‐splitting models. Models including dose continued to have low heterogeneity (Tau = 0.05) and evidence of inconsistency. As the models were very similar, we decide to use the treatment‐dose model for clinical utility. The results for the treatment‐only model are available in the supplemental files (link provided in Appendix 3).
Serious adverse events
Networks – which model is the best fit?
Our primary analysis was a Bayesian NMA including treatment. This analysis had low heterogeneity (Tau = 0.13) and no inconsistency in both UME and node‐splitting models. Including dose into the model did not alter the level of heterogeneity (Tau = 0.16), and continued to have no inconsistency in the UME and node‐splitting models. Both treatment‐only and treatment‐dose models had multiple studies with high residual deviance and imprecision. As both models were very similar, we decided to use the treatment‐dose model due to clinical utility. The results for treatment only are available in the supplemental files (link provided in Appendix 3).
Withdrawal
Networks – which model is the best fit?
Our primary analysis was a Bayesian NMA including treatment. This analysis had high residual deviance and relatively high heterogeneity (Tau = 0.23). We were unable to examine the model using node‐splitting models due to the network geometry, as a large proportion of the model was formed of single study connections only. We decided to use this treatment model for the analysis despite the relatively high heterogeneity, as including dose or condition would increase network complexity and dilute already weakly informative edges.
Appendix 3. Statistical analyses
Where additional analyses and the supplemental files are referred to in the text, these are available on the Open Science Framework (https://osf.io/ka5hr). For additional statistical queries please contact Gavin Stewart (gavin.stewart@newcastle.ac.uk).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
29060/433.
| Study characteristics | ||
| Methods | Design: parallel Duration: 8 weeks Assessment: baseline and post‐intervention Country: Belgium |
|
| Participants | Pain condition: fibromyalgia Population: people with fibromyalgia and depressive symptoms Minimum pain intensity: ≥ 4 on 0‐10 VAS Inclusion criteria
Exclusion criteria
Total participants randomised: 52 Age in years (mean): 45 Gender: 12/45 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Placebo
Paroxetine 20 mg
|
|
| Outcomes | AEs SAEs Withdrawal |
|
| Missing data methods | ITT but no method reported | |
| Funding source | Pharamaceutical: GlaxoSmithKline | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information given regarding blinding procedures |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported outcomes from participants but unsure of blinding measures |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Use ITT for primary outcome but don't report imputation method. Completer‐only analysis for the secondary outcomes Attrition Total: 13/52 (25.0%) Placebo: 5/26 (19.2%) Paroxetine 20 mg: 8/26 (30.8%) |
| Selective reporting (reporting bias) | High risk | State a number of measures that they will collect but don't report findings for (Abnormal Involuntary Movement Scale, fatigue VAS). No protocol, no publication |
| Other bias | High risk | Not published ‐ just a scientific summary on GSK registry. Trial ran in 1995 but only posted in 2005 |
Abou‐Raya 2012.
| Study characteristics | ||
| Methods | Design: parallel Duration: 16 weeks Assessment: baseline and post‐intervention Country: Egypt |
|
| Participants | Pain condition: knee OA Population: older adults (aged ≥ 65) with knee OA Minimum pain duration: ≥ 40 on 0 ‐100 scale Inclusion criteria
Exclusion criteria
Total participants randomised: 288 Age in years (mean, SD): 68.5 (SD NR) Gender: 241/288 were female |
|
| Interventions | Duloxetine
Placebo
|
|
| Outcomes | Pain intensity Physical function Mood AE SAE Withdrawal |
|
| Missing data methods | ITT but method not specified | |
| Funding source | Non‐pharmaceutical: sponsored by University of Alexandria, Egypt | |
| Conflicts of interest | Author conflicts of interest NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised by a clinical pharmacist using a computerised random number list. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed in sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, and placebo was identical to duloxetine. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes by blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | States ITT but no imputation method reported Attrition: Total: 34/288 (11.8%) Placebo: 13/144 (9.0%) Duloxetine 60 mg: 21/144 (14.6%) |
| Selective reporting (reporting bias) | Unclear risk | Protocol registered retrospectively: https://clinicaltrials.gov/show/NCT01425827 2011: Pain is the only stated outcome in the trial registry |
| Other bias | Low risk | No other sources of bias were identified. |
Agger 2017.
| Study characteristics | ||
| Methods | Design: parallel Duration: 15 weeks Assessment: baseline and post‐intervention (15 weeks) Country: Denmark |
|
| Participants | Pain condition: multiorgan bodily distress syndrome (including fibromyalgia, IBS, and non‐cardiac chest pain) Population: adults aged 20‐50 with a diagnosis of chronic multi‐organ bodily distress syndrome Minimum pain duration: no Inclusion criteria
Exclusion criteria
Total participants randomised: 139 Age in years (mean, SD): NR Gender: 94/139 were female |
|
| Interventions | Imipramine
Placebo
|
|
| Outcomes | AE Withdrawal |
|
| Missing data methods | ITT but imputation method NR | |
| Funding source | Non‐pharmaceutical: The Research Clinic for Functional Disorders, Aarhus University Hospital, Denmark | |
| Conflicts of interest | JLA, AS, LKG, JSJ, and PKF declare no competing interests. TSJ reports personal fees from Pfizer and Mundipharma, outside the submitted work. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation code was generated by a trained, but independent employee at the hospital pharmacy at Aarhus University Hospital through a web‐based system. |
| Allocation concealment (selection bias) | Low risk | Coded (numbered) packs of study drug and matched placebo were produced according to the randomisation schedule by the hospital pharmacy. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded study, with medications over‐encapsulated by the hospital pharmacy to ensure identical appearance |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes by blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT but no imputation method given Attrition: Total: 21/139 (15.1%) Placebo: 15/68 (22.1%) Imipramine 25‐75 mg: 13/70 (18.6%) |
| Selective reporting (reporting bias) | High risk | Published article reports slightly different registered outcomes to those mentioned in the protocol: https://clinicaltrials.gov/ct2/show/NCT01518634 They registered they will measure VAS for pain and the FIC checklist but do not report VAS and use a different checklist. |
| Other bias | Low risk | No other sources of bias were identified. |
Ahmed 2016.
| Study characteristics | ||
| Methods | Design: cross‐over Duration: 6 weeks Assessment: baseline and post‐intervention (6 weeks) Country: USA |
|
| Participants | Pain condition: fibromyalgia Population: adults with fibromyalgia and clinically significant sleep disturbance Inclusion criteria
Exclusion criteria
Total participants randomised: 19 Age in years (mean, SD): 49.2 Gender: 17/19 were female |
|
| Interventions | Milnacipran
Placebo
|
|
| Outcomes | Sleep Quality of life Pain intensity AE SAE Withdrawal |
|
| Missing data methods | LOCF | |
| Funding source | Pharmaceutical: Forest Research Institute, New Jersey, USA | |
| Conflicts of interest | Authors indicated no other financial conflicts of interest | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers were used for enrollment and allocation to sequence (1:1): milnacipran → placebo or placebo → milnacipran |
| Allocation concealment (selection bias) | Low risk | The investigator, clinical staff, participants, and the study sponsor were blinded to sequence allocation. A noninvolved staff member generated the random allocation sequence and kept an electronic copy in a secure location. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study drug was supplied as masked tablets of milnacipran and matching placebos. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes self‐reported by blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only use LOCF and unbalanced dropout Attrition: Total: 4/19 (21.1%) Placebo: 1/19 (5.5%) Milnacipran 100 mg: 3/19 (16.7%) |
| Selective reporting (reporting bias) | Low risk | Trial registered prospectively: https://clinicaltrials.gov/show/NCT01234675 Study outcomes reported match those in the protocol |
| Other bias | Low risk | No other sources of bias were identified |
Alcoff 1982.
| Study characteristics | ||
| Methods | Design: parallel Duration: 8 weeks Assessment: baseline and post‐intervention (8 weeks) Country: USA |
|
| Participants | Pain condition: low back pain Population: adults with chronic low back pain Inclusion criteria
Exclusion criteria NR, but the following potential participants were excluded:
Total participants randomised: 50 Age in years (mean, SD): NR Gender: 24/50 were female Pain duration (categorical): < 2 years (n = 8), 2–4 years (n = 6), > 4 years (n = 14) |
|
| Interventions | Imipramine
Placebo
|
|
| Outcomes | Withdrawal | |
| Missing data methods | NR | |
| Funding source | Non‐pharmaceutical: the Bureau of Medicine and Surgery, Department of the Navy, Clinical Investigation Program, USA | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not enough information ‐ just says 'randomly assigned' |
| Allocation concealment (selection bias) | Unclear risk | Only the pharmacist knew the treatment allocation, but unclear how this was allocated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, placebo was identical in appearance |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes by blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing data methods NR and unequal attrition between arms Attrition: Total: 9/50 (18.0%) Placebo attrition: 2/22 (9.1%) Imipramine 150 mg: 7/28 (25.0%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias identified |
Allen 2014.
| Study characteristics | ||
| Methods | Design: parallel Duration: 13 weeks Assessment: baseline and post‐intervention (13 weeks) Country: USA |
|
| Participants | Pain condition: diabetic peripheral neuropathy Population: adults with diabetic peripheral neuropathy Inclusion criteria
Exclusion criteria
Total participants randomised: 412 Age in years (mean, SD): 60.3 (SD NR) Gender: 108/412 were female Pain duration: NR |
|
| Interventions | Desvenlafaxine 50 mg
Desvenlafaxine 100 mg
Desvenlafaxine 200 mg
Desvenlafaxine 400 mg
Placebo
|
|
| Outcomes | Pain intensity 50% pain reduction PGIC AE SAE Withdrawal |
|
| Missing data methods | LOCF | |
| Funding source | Pharmaceutical: sponsored by Wyeth, company now owned by Pfizer | |
| Conflicts of interest | Rob Allen, is a former Pfizer employee currently working as an independent consultant. Suna Barlas, is a Pfizer employee. Uma Sharma, is a former Pfizer employee and currently works at MMS Holdings Inc. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blinding of all patients and site personnel to treatment allocation was ensured by using a computerised randomisation/enrollment system to assign participant numbers and study drug package numbers |
| Allocation concealment (selection bias) | Low risk | Study drug package numbers were produced by the computer‐randomised system |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear as to whether placebo was identical to desvenlafaxine medication |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported outcomes by participants, but unclear blinding procedures regarding medication appearance |
| Incomplete outcome data (attrition bias) All outcomes | High risk | LOCF and unbalanced attrition across arms Attrition: Total: 107/412 (30.0%) Placebo: 15/90 (16.7%) Desvenlafaxine 50 mg: 12/63 (19.0%) Desvenlafaxine 100 mg: 18/87 (20.7%) Desvenlafaxine 200 mg: 31/99 (31.3%) Desvenlafaxine 400 mg: 27/69 (39.1%) |
| Selective reporting (reporting bias) | Unclear risk | The 2 stated in the protocol are reported in the paper, but the article also reports other outcomes that were not included in the protocol. |
| Other bias | Low risk | No other sources of bias identified |
Allen 2017.
| Study characteristics | ||
| Methods | Design: parallel Duration: 15 weeks (intended to be 27 weeks but terminated early) Assessment: baseline, study termination (15 weeks) Country: USA |
|
| Participants | Pain condition: fibromyalgia Population: adults with fibromyalgia Inclusion criteria
Exclusion criteria
Total participants randomised: 697 Age in years (mean, SD): NR Gender: NR Pain duration: NR |
|
| Interventions | Desvenlafaxine 50 mg
Desvenlafaxine 100 mg
Desvenlafaxine 200 mg
Desvenlafaxine 400 mg
Placebo
|
|
| Outcomes | Pain intensity 50% pain reduction PGIC AE SAE Withdrawal |
|
| Missing data methods | LOCF | |
| Funding source | Pharmaceutical: Wyeth Research, now incorportated into Pfizer | |
| Conflicts of interest | Rob Allen, MD, is a former Pfizer employee currently working as an independent consultant. Suna Barlas, PhD, is a Pfizer employee. Uma Sharma, PhD, is a former Pfizer employee and currently works at MMS Holdings Inc. | |
| Notes | Study terminated early (at 15 weeks instead of 27 weeks) due to interim efficacy analysis not meeting the preplanned efficacy criteria | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not enough information ‐ just says 'randomly assigned' |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information on matching appearance or dosing schedules of antidepressants and placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported outcomes by participants, but blinding information unclear |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study terminated, so missing data from all time points past 15 weeks. LOCF. Very high attrition across all arms before study termination. Attrition: Total: 445/697 (63.8%) Placebo: 84/130 (67.7%) Desvenlafaxine 50 mg: 87/136 (66.0%) Desvenlafaxine 100 mg: 81/140 (62.0%) Desvenlafaxine 200 mg: 100/142 (76.0%) Desvenlafaxine 400 mg: 93/149 (67.0%) |
| Selective reporting (reporting bias) | Low risk | Protocol stated the interim analyses |
| Other bias | Low risk | Study terminated early, but this was due to interim efficacy analyses not meeting the prespecified criteria. |
Anderberg 2000.
| Study characteristics | ||
| Methods | Design: parallel Duration: 16 weeks Assessment: baseline and post‐intervention Country: Sweden |
|
| Participants | Pain condition: fibromyalgia Population: women with fibromyalgia Minimum pain duration: no Inclusion criteria
Exclusion criteria
Total participants randomised: 40 Age in years (mean, SD): 48.6 (7.5) Gender: 40/40 were female Pain duration: 11.9 (7.0) years average duration of fibromyalgia |
|
| Interventions | Citalopram
Placebo
|
|
| Outcomes | AE Withdrawal |
|
| Missing data methods | ITT but no method reported | |
| Funding source | The study was supported by grants from H. Lundbeck AB, the Söderström Königska Foundation, the Swedish Association of Physicians, the Märta and Nicke Nasvell Foundation, the Swedish Health Insurance System, the Uppsala County Council and ‘Förenade Liv’ Mutual Group Life Insurance Company, Stockholm, Sweden and the Swedish Medical Research Council (21X‐9523) | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information given |
| Allocation concealment (selection bias) | Low risk | Randomisation was made at a separate agency, and the investigator had a coded list. Included patients were given consecutive code numbers. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information given regarding appearance of placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not enough information given |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Unequal attrition, states ITT but no method given Attrition: Total: 5/40 (12.5%) Placebo: 1/19 (5.26%) Citalopram 30‐40 mg: 4/21 (19.1%) |
| Selective reporting (reporting bias) | Low risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified. |
Ang 2013.
| Study characteristics | ||
| Methods | Design: parallel Duration: 21 weeks Assessment: baseline and post‐intervention (21 weeks) Country: USA |
|
| Participants | Pain condition: fibromyalgia Population: adults with fibromyalgia Minimum pain intensity: ≥ 4 out of 10 Inclusion criteria
Exclusion criteria
Total participants randomised: 58 Age in years (mean, SD): 46.59 (10.39) Gender: 54/58 were female Pain duration: average duration since fibromyalgia of 12.07 (10.04) years |
|
| Interventions | CBT
CBT + milnacipran
Milnacipran + education
|
|
| Outcomes | Pain intensity Moderate pain relief Physical function Quality of life Depression AEs SAEs Withdrawal |
|
| Missing data methods | NR | |
| Funding source | National Institute of Arthritis and Musculoskeletal and Skin Diseases (Grant number: 1R21AR056046‐01A2). The authors thank Forest Research Institute for providing the active drug and placebo. | |
| Conflicts of interest | Authors state no conflicts of interest to declare | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Does not report how the participants were randomised ‐ just says "participants were randomised to one of the three treatment arms". |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The professionals delivering the CBT and education sessions are authors on the paper, and would have been unblinded to participant selection |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants likely to identify which psychological therapy group they were in, and study authors did not report participants' identification of group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No missing data methods reported Attrition: Total: 9/58 (15.5%) CBT: 4/19 (21.1%) CBT + milnacipran: 3/20 (15.0%) Education + milnacipran: 2/19 (10.5%) |
| Selective reporting (reporting bias) | High risk | Stated in the protocol that they would measure participants' identification of group assignment but NR. |
| Other bias | Low risk | No other sources of bias were identified |
Aragona 2005.
| Study characteristics | ||
| Methods | Design: parallel Duration: 8 weeks Assessment: baseline and post‐intervention Country: Italy |
|
| Participants | Pain condition: somatoform DSM‐IV‐TR pain disorder Population: people with somatoform DSM‐IV‐TR pain disorder Minimum pain intensity: no Inclusion criteria
Exclusion criteria
Total participants randomised: 35 Age in years (mean, SD): NR Gender: 21/35 were female Pain duration: NR |
|
| Interventions | Citalopram
Reboxetine
|
|
| Outcomes | Pain intensity Depression AEs SAEs Withdrawal |
|
| Missing data methods | LOCF | |
| Funding source | NR | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned using random tables |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information given regarding appearance of medications |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given regarding appearance of medications |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Used LOCF as imputation method, high attrition Attrition: Total: 6/35 (17.1%) Citalopram 40 mg: 6/17 (35.3%) Reboxetine 8 mg: 9/18 (50.0%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified |
Arnold 2002.
| Study characteristics | ||
| Methods | Design: parallel Duration: 12 weeks Assessment: baseline and post‐intervention Country: USA |
|
| Participants | Pain condition: fibromyalgia Population: women with fibromyalgia Minimum pain intensity: no Inclusion criteria
Exclusion criteria
Total participants randomised: 60 Age in years (mean, SD): 46 (11) Gender: 46/46 were female Pain duration: average duration of fibromyalgia was 11 (9) years |
|
| Interventions | Fluoxetine
Placebo
|
|
| Outcomes | Pain intensity Quality of life Depression Physical function Withdrawal |
|
| Missing data methods | LOCF | |
| Funding source | Pharmaceutical: Eli Lilly | |
| Conflicts of interest | NR, but authors in other papers have declared conflicts of interest regarding involvement with Eli Lilly | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not enough information ‐ just says that participants were 'randomly assigned' |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical capsules and titration process |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High attrition and used LOCF Attrition: Total: 23/60 (38.3%) Placebo: 12/30 (40.0%) Fluoxetine 10‐ 30 mg: 11/30 (36.7%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified |
Arnold 2004.
| Study characteristics | ||
| Methods | Design: parallel Duration: 12 weeks Assessment: baseline and post‐intervention (12 weeks) Country: USA |
|
| Participants | Pain condition: fibromyalgia Population: adults with fibromyalgia with and without MDD Minimum pain intensity: ≥ 4 out of 10 Inclusion criteria
Exclusion criteria
Total participants randomised: 207 Age in years (mean, SD): Gender: 184/200 were female Pain duration: NR |
|
| Interventions | Duloxetine
Placebo
|
|
| Outcomes | Pain intensity Quality of life Physical function Mood PGIC AEs Withdrawal |
|
| Missing data methods | Mixed‐effects model and LOCF | |
| Funding source | Pharmaceutical ‐ Eli Lilly | |
| Conflicts of interest | Drs Crofford and Arnold have received consulting fees or honoraria in the last 2 years from Eli Lilly and Company (DrCrawford USD 10,000, Dr Arnold USD 10,000). In addition to the authors employed by Eli Lilly and Company listed above, Dr Goldstein's wife is employed by Eli Lilly and Company | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Assignment to treatment groups was determined by a computer‐generated random sequence. |
| Allocation concealment (selection bias) | Low risk | Participants were allocated using an interactive voice response system. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Matched appearance and dosing schedule |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High attrition, unequal reasons for dropout, used LOCF Attrition: Total: 83/207 (40.1%) Placebo: 37/103 (35.9%) Duloxetine 120 mg: 46/104 (44.2%) |
| Selective reporting (reporting bias) | Unclear risk | Trial registration was retrospective. |
| Other bias | Low risk | No other sources of bias were identified. |
Arnold 2005.
| Study characteristics | ||
| Methods | Design: parallel Duration: 12 weeks Assessment: baseline and post‐intervention Country: USA |
|
| Participants | Pain condition: fibromyalgia Population: women with fibromyalgia, with and without MDD Minimum pain intensity: ≥ 4 out of 10 Inclusion criteria
Exclusion criteria
Total participants randomised: 354 Age in years (mean, SD): 49.6 (10.9) Gender: 354/354 were female Pain duration: NR |
|
| Interventions | Duloxetine 60 mg
Duloxetine 120 mg
Placebo
|
|
| Outcomes | Pain intensity Quality of life Physical function Mood Moderate pain relief Substantial pain relief PGIC AEs SAEs Withdrawal |
|
| Missing data methods | LOCF | |
| Funding source | Pharmaceutical ‐ Eli Lilly | |
| Conflicts of interest | NR, but have declared CoIs in other papers with this sponsor/funder | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information regarding randomisation procedures given |
| Allocation concealment (selection bias) | Unclear risk | No information regarding allocation concealment given |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information regarding matched dose schedules or identical appearance of medications given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported outcomes from participants, uncertain about blinding procedures |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High attrition and significantly different reasons for dropout between groups. Used LOCF Attrition: Total: 138/354 (39.0%) Placebo: 52/121 (43.0%) Duloxetine 60 mg: 41/117 (35.0%) Duloxetine 120 mg: 45/115 (39.1%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified. |
Arnold 2010a.
| Study characteristics | ||
| Methods | Design: parallel Duration: 8 weeks Assessment: baseline and post‐intervention (8 weeks) Country: USA |
|
| Participants | Pain condition: fibromyalgia Population: adults with fibromyalgia Minimum pain intensity: ≥ 40 on 0‐100 VAS Inclusion criteria
Exclusion criteria
Total participants randomised: 268 Age in years (mean, range): 50 (20‐84) Gender: 239/268 were female Pain duration in years (mean, range): 7 (0‐46.8) |
|
| Interventions | Esreboxetine
Placebo
|
|
| Outcomes | Pain intensity Quality of life Sleep Mood Physical function Moderate pain relief Substantial pain relief PGIC AEs SAEs |
|
| Missing data methods | LOCF | |
| Funding source | Pharmaceutical ‐ Pfizer | |
| Conflicts of interest | Dr Arnold has received grants/research support from Allergan, Boehringer Ingelheim, Cypress Biosciences Inc., Forest Laboratories Inc., Eli Lilly and Company, Pfizer Inc., Sanofi‐Aventis, and Wyeth Pharmaceuticals. She has been a consultant for Allergan, AstraZeneca, Boehringer Ingelheim, Cypress Biosciences, Forest Laboratories, Eli Lilly and Company, Organon, Pfizer, sanofi‐aventis, Sepracor, Takeda, Theravance, Inc., DCB, Vivus, Inc., and Wyeth. She has served on speakers' bureaus for Forest Laboratories, Eli Lilly and Company, and Pfizer. Drs Chatamra, Hirsch, and Stoker were employees of Pfizer at the time of the study. They have indicated that they have no other conflicts of interest with regard to the content of this article. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation to treatment groups was performed according to a computer‐generated randomisation code. |
| Allocation concealment (selection bias) | Low risk | Allocation was managed through a centralised telerandomisation system. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo matched appearance and dose |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Equal attrition (20%), LOCF supplemented by BOCF Attrition: Total: 55/267 (20.5%) Placebo: 27/133 (20.3%) Esreboxetine: 27/134 (20.1%) |
| Selective reporting (reporting bias) | Unclear risk | Some changes in what were secondary or primary outcomes, not 100% lining up with protocol but primary outcome remains the same |
| Other bias | Low risk | No other sources of bias were identified. |
Arnold 2010b.
| Study characteristics | ||
| Methods | Design: parallel Duration: 12 weeks Assessment: baseline and post‐intervention (12 weeks) Country: USA and Canada |
|
| Participants | Pain condition: fibromyalgia Population: adults with fibromyalgia Minimum pain intensity: ≥ 40 on 0‐100 VAS Inclusion criteria
Exclusion criteria
Total participants randomised: 1025 Age in years (mean, SD): NR Gender: 977/1025 were female Pain duration in years (mean): 10.8 |
|
| Interventions | Milnacipran
Placebo
|
|
| Outcomes | Pain intensity Physical function Mood Quality of life Moderate pain relief Substantial pain relief PGIC AEs SAEs Withdrawal |
|
| Missing data methods | BOCF | |
| Funding source | Pharmaceutical ‐ Forest Laboratories | |
| Conflicts of interest | Dr Arnold has received consulting fees, speaking fees, and/or honoraria from Cypress Bioscience, Wyeth, Boehringer Ingelheim, Allergan, Takeda, UCB, Theravance, AstraZeneca, and Sanofi‐Aventis (less than USD 10,000 each) and from Eli Lilly, Pfizer, and Forest Laboratories (> USD 10,000 each) and has received research support from Eli Lilly, Cypress Bioscience, Wyeth, Boehringer In‐gelheim, Allergan, Forest Laboratories, and Pfizer. Drs R. M. Gendreau and J. F. Gendreau own stock or stock options in Cypress Bioscience. Drs Palmer and Wang own stock or stock options in Forest Laboratories. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation assignment by computer code in blocks of 4 |
| Allocation concealment (selection bias) | Low risk | Assignment to treatment groups was conducted centrally (i.e. at the study level) using an interactive voice response system. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical placebo appearance and matched dosing schedule |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar attrition across both arms, BOCF used for imputation Attrition: Total: 309/1025 (30.1%) Placebo: 150/509 (29.5%) Milnacipran 100 mg: 159/516 (30.8%) |
| Selective reporting (reporting bias) | Low risk | Trial registered prospectively on clinicaltrials.gov |
| Other bias | Low risk | No other sources of bias were identified. |
Arnold 2010c.
| Study characteristics | ||
| Methods | Design: parallel Duration: 12 weeks Assessment: baseline and post‐intervention (12 weeks) Country: USA and Puerto Rico |
|
| Participants | Pain condition: fibromyalgia Population: adults with fibromyalgia Minimum pain intensity: ≥ 4 out of 10 Inclusion criteria
Exclusion criteria
Total participants randomised: 530 Age in years (mean, SD): 50.2 (11.1) Gender: 494/530 were female Pain duration in years: NR |
|
| Interventions | Duloxetine
Placebo
|
|
| Outcomes | Pain intensity Mood Physical function Moderate pain relief Substantial pain relief PGIC AEs SAEs Withdrawal |
|
| Missing data methods | LOCF and MMRM | |
| Funding source | Pharmaceutical ‐ Lilly USA LLC | |
| Conflicts of interest | Dr Mease has received grants/research support from Eli Lilly and Company; Pfizer, Inc; Cypress Bioscience, Inc; Forest Laboratories, Inc; Allergan; Fralex; and Boehringer Ingelheim. He has been a consultant for Eli Lilly and Company; Pfizer, Inc; Cypress Bioscience, Inc; Forest Laboratories, Inc; Allergan; Fralex; Boehringer Ingelheim; Pierre Fabre; and Wyeth; and he is on the Speakers Bureau of Pfizer, Inc. Dr Arnold has received grants/research support from Eli Lilly and Company; Pfizer, Inc; Cypress Bioscience, Inc; Boehringer Ingelheim; and Forest Laboratories, Inc, and received honoraria as a consultant to Eli Lilly and Company; Pfizer, Inc; Cypress Bioscience, Inc; Boehringer Ingelheim; Forest Laboratories, Inc; Allergan; Takeda; UCB Inc.; Theravance; AstraZeneca; Sanofi‐Aventis; and Grunenthal. Drs Mohs, Ahl, Gaynor, and Wohlreich are all employees and stockholders in Eli Lilly andCompany. Dr Wang is a former employee of Lilly USA, LLC. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Allocation was managed using an Interactive Voice Response System. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Good blinding procedures, identical appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | HIgh attrition but equal, ITT with LOCF and BOCF Attrition: Total: 167/530 (31.5%) Placebo: 80/267 (30.0%) Duloxetine 60‐120 mg: 87/263 (33.1%) |
| Selective reporting (reporting bias) | Low risk | Trial registered prospectively and all outcomes reported |
| Other bias | Low risk | No other sources of bias were identified. |
Arnold 2012a.
| Study characteristics | ||
| Methods | Design: parallel Duration: 12 weeks Assessment: baseline and post‐intervention (12 weeks) Country: USA |
|
| Participants | Pain condition: fibromyalgia Population: adults with fibromyalgia Minimum pain intensity: ≥ 4 out of 10 Inclusion criteria
Exclusion criteria
Total participants randomised: 308 Age in years (mean): 51 Gender: 95.2% were female Pain duration in years (mean): 6.5 |
|
| Interventions | Duloxetine
Placebo
|
|
| Outcomes | Pain intensity Mood Quality of life Moderate pain relief Substantial pain relief PGIC Adverse SAE Withdrawal |
|
| Missing data methods | LOCF and BOCF | |
| Funding source | Pharmaceutical ‐ Eli Lilly | |
| Conflicts of interest | B.A.P. and S.Z. are full time employees and stockholders at Eli Lilly and Company. L.M.A. has received grants from and/or is a consultant for Eli Lilly and Company, Pfizer Inc, Cypress Bioscience Inc, Forest Laboratories, Boehringer Ingelheim, Novartis, Takedo, Grunenthal and Daiichi Sankyo | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Aloocation was managed using an interactive voice response system. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind with placebo identical appearance to duloxetine |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition and reasons for withdrawal similar across groups. Mix of methods for missing data including LOCF and BOCF Attrition: Total: 77/308 (25.0%) Placebo: 31/110 (28.2%) Duloxetine 30 mg: 29/121 (23.9%) |
| Selective reporting (reporting bias) | Low risk | Trial registered prospectively, outcomes match those predefined |
| Other bias | Low risk | No other sources of bias were identified. |
Arnold 2012b.
| Study characteristics | ||
| Methods | Design: parallel Duration: 14 weeks Assessment: baseline and post‐intervention Country: USA and Canada |
|
| Participants | Pain condition: fibromyalgia Population: adults with fibromyalgia Minimum pain intensity: ≥ 40 out of 100 Inclusion criteria
Exclusion criteria
Total participants randomised: 1122 Age in years (mean, range): 50 (19‐84) Gender: 1009/1122 were female Pain duration in years (mean, range): 7 (0‐55) |
|
| Interventions | Esreboxetine 4 mg
Esreboxetine 8 mg
Esreboxetine 10 mg
Placebo
|
|
| Outcomes | Pain intensity Physical function Mood Quality of life Moderate pain relief Substantial pain relife PGIC AEs SAEs Withdrawal |
|
| Missing data methods | LOCF with BOCF as a sensitivity analysis on pain outcomes | |
| Funding source | Pharmaceutical ‐ Pfizer | |
| Conflicts of interest | Dr Arnold has received consulting fees from Eli Lilly, Cypress Bioscience, Forest Laboratories, Takeda, AstraZeneca, Sanofi‐Aventis, Grunenthal, Johnson & Johnson, and Daiichi Sankyo (less than USD 10,000 each) and from Pfizer (> USD 10,000); she has received research grants from Eli Lilly, Pfizer, Cypress Bioscience, Boehringer Ingelheim, Forest Laboratories, Novartis, and Takeda. Dr Hirsch owns stock or stock options in AstraZeneca. Dr Sanders owns stock or stock options in Pfizer and AstraZeneca. Drs Ellis and Hughes own stock or stock options in Pfizer. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eligible patients were then randomised, according to a computer‐generated pseudorandom code, in a 1:1:1:1 ratio |
| Allocation concealment (selection bias) | Low risk | A centralised telerandomisation system was used to manage the allocation of treatment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients received esreboxetine or matching placebo once daily in the form of round, light grey tablets; all of the tablets were identical in appearance, to preserve blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High dropout across arms and significant differences in rates between placebo and intervention arms Attrition: Total: 406/1122 (36.2%) Placebo: 76/278 (27.3%) Esreboxetine 4 mg: 103/277 (37.2%) Esreboxetine 8 mg: 111/284 (39.1%) Esreboxetine 10 mg: 108/283 (38.2%) |
| Selective reporting (reporting bias) | High risk | Primary outcomes were switched on the trial registry. Protocol states they will collect and report HADS, SDI, and Sleep Interference but not published |
| Other bias | Low risk | No other sources of bias were identified. |
Ash 1999.
| Study characteristics | ||
| Methods | Design: parallel Duration: 10 weeks Assessment: baseline and post‐intervention Country: UK |
|
| Participants | Pain condition: RA Population: women with RA and depression Minimum pain intensity: NR Inclusion criteria:
Exclusion criteria
Total participants randomised: 48 Age in years (mean, SD): NR Gender: 48/48 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Dothiepin
Placebo
|
|
| Outcomes | Pain intensity Mood Physical function Withdrawal |
|
| Missing data methods | ITT but does not state imputation methods | |
| Funding source | Pharmaceutical ‐ Boots | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States random allocation but no method given |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind with identical appearing antidepressants and placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Over 40% of participants did not compelte the study due to lack of effect or intolerable side effects. Attrition: Total: 21/48 (43.75%) Placebo: 10/23 (43.5%) Dothiepin 75 to 150 mg: 11/25 (44.0%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified. |
Atkinson 1998.
| Study characteristics | ||
| Methods | Design: parallel Duration: 8 weeks Assessment: baseline and post‐intervention Country: USA |
|
| Participants | Pain condition: low back pain Population: men with chronic low back pain Minimum pain intensity: NR Inclusion criteria
Exclusion criteria
Total participants randomised: 78 Age in years (mean, SD): 47.13 (10.65) Gender: 0/78 were female Pain duration in years (mean, SD): 14.81 years |
|
| Interventions | Nortriptyline:
Placebo:
|
|
| Outcomes | Pain intensity Physical function Mood Quality of life AEs Withdrawal |
|
| Missing data methods | ITT, but no methods of imputation given | |
| Funding source | Non‐pharmaceutical: United States Department of Veteran's Affairs and the National Institutes of Health | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned using a random number table |
| Allocation concealment (selection bias) | Low risk | Randomisation performed by research pharmacist not involved in any other aspects of the trial |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded with identical appearing placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 26% of participants withdrew. LOCF imputation only. Attrition: Total: 21/78 (26.9%) Placebo: 11/40 (27.5%) Nortriptyline 25 to 100 mg: 10/38 (26.3%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found. |
| Other bias | Low risk | No other sources of bias were identified. |
Atkinson 1999.
| Study characteristics | ||
| Methods | Design: parallel Duration: 8 weeks Assessment: baseline and post‐intervention Country: USA |
|
| Participants | Pain condition: low back pain Population: adults with low back pain Minimum pain intensity: NR Inclusion criteria
Exclusion criteria
Total participants randomised: 103 Age in years (mean, SD): NR Gender: 40/103 were female Pain duration in years (mean, SD): 14.5 (11.1) |
|
| Interventions | Maprotiline 50‐150 mg
Paroxetine 10 to 30 mg
Placebo (diphenhydramine 37.5 mg)
|
|
| Outcomes | Pain intensity AEs Withdrawal |
|
| Missing data methods | ITT using LOCF | |
| Funding source | Non‐pharmaceutical: funded by the United States Department of Veterans Affairs and the National Institutes of Health | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using a random number table |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed by a research pharmacist not otherwise involved in the trial |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded, active placebo, all capsules had identical appearance |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes by blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT using LOCF. Unequal dropout across arms Attrition Total: 29/103 (28.2%) Maprotiline 50‐150 mg: 13/33 (39.4%) Paroxetine 10‐30 mg: 12/34 (35.3%) Placebo: 4/36 (11.1%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found. Only report data for primary outcome despite collecting post‐intervention data for other outcomes |
| Other bias | Low risk | No other sources of bias were identified |
Atkinson 2007.
| Study characteristics | ||
| Methods | Design: parallel Duration: 12 weeks Assessment: baseline and post‐intervention Country: USA |
|
| Participants | Pain condition: low back pain Population: adults with low back pain Minimum pain intensity: NR Inclusion criteria
Exclusion criteria
Total participants randomised: 121 Age in years (mean, SD): NR Gender: NR Pain duration in years (mean, SD): NR |
|
| Interventions | Placebo (benzotropine mesylate 0.5 mg)
Desipramine 50 mg
Desipramine 100 mg
Desipramine 150 mg
Fluoxetine 20 mg
Fluoxetine 40 mg
Fluoxetine 60 mg
|
|
| Outcomes | No useable data provided | |
| Missing data methods | ITT with LOCF | |
| Funding source | Non‐pharmaceutical: US Department of Veterans Affairs | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using a computerised random number generator |
| Allocation concealment (selection bias) | Low risk | Randomisation was completed by a research pharmacist not involved in other aspects of the study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded, double‐dummy design, no significant difference in participants guessing allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes by blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT with LOCF Attrition: Total: 38/121 (31.4%) Attrition by arm NR |
| Selective reporting (reporting bias) | Unclear risk | Only 1 outcome prespecified in protocol. Do not perform their original analysis |
| Other bias | Low risk | No other sources of bias were identified. |
Bansal 2009.
| Study characteristics | ||
| Methods | Design: cross‐over Duration: 5 weeks Assessment: baseline and post‐intervention Country: India |
|
| Participants | Pain condition: diabetic peripheral neuropathy Population: adults with diabetic peripheral neuropathy Minimum pain intensity: ≥ 50 on 0‐100 VAS Inclusion criteria
Exclusion criteria
Total participants randomised: 51 Age in years (median, range): 54.5 (48‐61) Gender: 25/44 completers were female Pain duration in months (mean, IQR): 12 (3‐24) |
|
| Interventions | Pregabalin
Amitriptyline
|
|
| Outcomes | Pain intensity PGIC Withdrawal |
|
| Missing data methods | ITT but no method of imputation reported | |
| Funding source | Funding: NR | |
| Conflicts of interest | No conflicts of interest to declare | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using a random number table |
| Allocation concealment (selection bias) | Low risk | Blinding and randomisation were carried out by an independent person unrelated to the study, while drug administration and patient assessment were carried out by the investigator. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded, identical appearing tablets |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes by blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | States ITT but no method of imputation reported Attrition: Total: 7/51 (13.7%) Amitriptyline 10‐50 mg: 3/25 (12.0%) Pregabalin 75‐300 mg: 4/26 (15.4%) |
| Selective reporting (reporting bias) | Unclear risk | Trial registration, but registered retrospectively |
| Other bias | Low risk | No other sources of bias were identified. |
Bateman 2013.
| Study characteristics | ||
| Methods | Design: parallel Duration: 10 weeks Assessment: baseline and post‐intervention Country: USA |
|
| Participants | Pain condition: fibromyalgia Population: people with fibromyalgia who did not respond to duloxetine Minimum pain intensity: VAS pain score ≥ 40 mm/100 mm Inclusion criteria
Exclusion criteria
Total participants randomised: 107 Age in years (mean): 48.6 Gender: 92/107 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Placebo
Milnacipran 50‐200 mg
|
|
| Outcomes | Pain intensity Quality of life Moderate pain relief Substantial pain relief PGIC AEs SAEs Withdrawal |
|
| Missing data methods | ITT with LOCF | |
| Funding source | Pharmaceutical ‐ Forest Laboratories Inc. | |
| Conflicts of interest | LB has received research support and speaker fees from Forest Laboratories, Inc. and Forest Research Institute, Inc. RHP, JMT, and YL are full‐time employees of Forest Research Institute, Inc., a wholly owned subsidiary of Forest Laboratories, Inc., and hold stock in the parent company. This study was supported by Forest Laboratories, Inc. The authors thank Allan Spera at Forest Research Institute, Inc. for his contributions to the study and development of this paper. The authors also thank Mildred Bahn at Prescott Medical Communications Group (Chicago, IL, USA) for medical writing assistance supported by Forest Research Institute, Inc. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information given |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Included small placebo arm to ensure blinding, matched dosing schedule but no information given regarding appearance |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported outcomes from participants, but unsure of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT with LOCF and high attrition Attrition Total: 45/107 (42.1%) Placebo: 10/21 (47.6%) Milnacipran 50‐200 mg: 35/86 (40.7%) |
| Selective reporting (reporting bias) | Low risk | Primary measures match those listed prospectively in trial registry |
| Other bias | High risk | Placebo group spent first week still taking duloxetine while active drug group had no down taper between taking duloxetine and milnacipran |
Bird 2000.
| Study characteristics | ||
| Methods | Design: parallel Duration: 8 weeks Assessment: baseline and post‐intervention Countries: UK, Ireland, Germany, Italy, and Belgium |
|
| Participants | Pain condition: RA Population: adults with RA and depression Minimum pain intensity: NR Inclusion criteria
Exclusion criteria
Total participants randomised: 191 Age in years (mean): 54.8 Gender: 150/191 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Paroxetine 20‐40 mg
Amitriptyline 75‐150 mg
|
|
| Outcomes | Mood PGIC AEs SAEs Withdrawal |
|
| Missing data methods | ITT but imputation method not specified | |
| Funding source | Pharmaceutical ‐ educational grant from SmithKline Beecham | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information given |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded ‐ double‐dummy dosing schedule |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | States ITT but no information regarding imputation method given Attrition Total: 37/191 (19.4%) Paroxetine 20‐40 mg: 18/95 (18.9%) Amitriptyline 75‐150 mg: 20/105 (19.0%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified. |
Boyle 2012.
| Study characteristics | ||
| Methods | Design: parallel Duration: 4 weeks Assessment: baseline and post‐intervention Country: UK |
|
| Participants | Pain condition: diabetic peripheral neuropathy Population: adults with diabetic peripheral neuropathy Minimum pain intensity: NR Inclusion criteria
Exclusion criteria
Total participants randomised: 83 Age in years (mean, SD): 65.1 (8.9) Gender: 26/83 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Pregabalin 600 mg
Amitriptyline 75 mg
Duloxetine 120 mg
|
|
| Outcomes | Pain intensity Sleep Physical function Mood Withdrawal |
|
| Missing data methods | ITT but imputation methods not specified | |
| Funding source | Pharmaceutical ‐ Pfizer | |
| Conflicts of interest | This study was funded by an investigator‐led research grant, which was awarded by Pfizer Ltd. J.B. received an honorarium to present the research findings internally to a Pfizer consultancy board. D.K.received consultancy fees and honoraria from Eli Lilly, Novo Nordisk, Abbott Diabetes Care, and Roche, companies providing medicine and monitoring equipment used by participants in this study. No other potential conflicts of interest relevant to this article were reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not specified |
| Allocation concealment (selection bias) | Unclear risk | Procedure for allocation not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Matched dosing, but no information given regarding appearance of tablets |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported pain outcomes, but not enough information regarding blinding procedures |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Missing data methods: NR Attrition Total: 18/83 (21.7%) Pregabalin 300 mg: 5/27 (18.5%) Amitriptyline 75 mg: 4/28 (14.3%) Duloxetine 120 mg: 0/28 (0.0%) |
| Selective reporting (reporting bias) | Low risk | Pre‐registered protocol lists primary outcomes |
| Other bias | Low risk | No other sources of bias were identified. |
Branco 2010.
| Study characteristics | ||
| Methods | Design: parallel Duration: 17 weeks Assessment: baseline and post‐intervention Country: Czech Republic, Denmark, Finland, France, Germany, Italy, Norway, Poland, Portugal, Romania, Spain, Sweden, UK |
|
| Participants | Pain condition: fibromyalgia Population: adults with fibromyalgia Minimum pain intensity: baseline VAS pain intensity rating between 40 and 90 (0‐100 scale) Inclusion criteria
Exclusion criteria
Total participants randomised: 884 Age in years (mean): 48.4 Gender: 826 were female Pain duration in years (mean): 9.5 |
|
| Interventions | Placebo
Milnacipran 200 mg
|
|
| Outcomes | Pain intensity Quality of life Physical function Mood PGIC AEs SAEs Withdrawal |
|
| Missing data methods | ITT with LOCF, and BOCF sensitivity analyses | |
| Funding source | Pharmaceutical ‐ Pierre Fabre Medicament, France | |
| Conflicts of interest | Dr Branco has received grant support as an investigator and consultant for Pierre Fabre Medicament. Drs Zachrisson and Perrot have served as speakers and consultants for Pierre Fabre Medicament. Dr Mainguy is an employee and shareholder of Pierre Fabre Medicament. Medical writing assistance provided by Prescott Medical Communications Group was supported by Pierre Fabre Medicament. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedure not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Matched dosing but no information regarding appearance of drugs |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported outcomes from participants, but unsure of blinding procedures |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT with LOCF and BOCF as sensitivity analysis. More dropouts in antidepressant arm related to side‐effects Attrition Total: 206/882 (23.3%) Placebo: 79/449 (17.6%) Milnacipran 200 mg: 127/435 (29.2%) |
| Selective reporting (reporting bias) | Unclear risk | Primary outcome matches trial registry, but secondary outcomes not listed |
| Other bias | Low risk | No other sources of bias were identified. |
Braz 2013.
| Study characteristics | ||
| Methods | Design: parallel Duration: 12 weeks Assessment: baseline and post‐intervention Country: Brazil |
|
| Participants | Pain condition: fibromyalgia Population: women with fibromyalgia Minimum pain intensity: NR Inclusion criteria
Exclusion criteria
Total participants randomised: 52 Age in years (mean): 43.2 Gender: 52/52 were female Pain duration in months (mean): 43.8 |
|
| Interventions | Placebo
Amitriptyline 25 mg
Panax ginseng 100 mg
|
|
| Outcomes | Withdrawal | |
| Missing data methods | Completer‐only analysis | |
| Funding source | NR | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation procedure not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedure not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, drugs identical appearance |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported measures completed by blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer‐only analysis, unequal attrition Attrition Total: 14/52 (26.9%) Placebo: 4/17 (23.5%) Amitriptyline 25 mg: 3/16 (18.8%) Panax ginseng 100 mg: 7/19 (36.8%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified. |
Calderon 2011.
| Study characteristics | ||
| Methods | Design: parallel Duration: 7 weeks Assessment: baseline, post‐intervention, follow‐up (4 weeks after post‐intervention) Country: Brazil |
|
| Participants | Pain condition: orofacial pain Population: women with temporomandibular disorders Minimum pain intensity: ≥ 40 mm on a 0‐100 mm VAS Inclusion criteria
Exclusion criteria
Total participants randomised: 47 Age in years (mean, range): 35.6 (17‐52) Gender: 47/47 were female Pain duration in months (mean, range): 72.35 (6‐384) |
|
| Interventions | Placebo
Placebo + CBT
Amitriptyline 25 mg
Amitriptyline 25 mg + CBT
|
|
| Outcomes | Withdrawal | |
| Missing data methods | Completer‐only analysis | |
| Funding source | Non‐pharmaceutical ‐ Ministry of Education in Brazil | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using the website www.randomization.com |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of CBT group participants cannot be blinded. When comparing the placebo versus amitriptyline group(s) there was no description of whether pills were matched. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self‐reported outcomes from unblinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No clear explanation of reasons for withdrawal and from which group, not clear on handling missing data or group sizes in outcomes Attrition Total: 10/47 (21.3%) Placebo: 2/13 (15.4%) CBT: 2/11 (18.2%) Amitriptyline 25 mg: 2/11 (18.2%) CBT + amitriptyline 25 mg: 4/12 (33.3%) |
| Selective reporting (reporting bias) | Unclear risk | No trial registration or protocol found |
| Other bias | Low risk | No other sources of bias were identified. |
Cannon 1994.
| Study characteristics | ||
| Methods | Design: parallel Duration: 3 weeks Assessment: baseline and post‐intervention Country: USA |
|
| Participants | Pain condition: non‐cardiac chest pain Population: adults with non‐cardiac chest pain Minimum pain intensity: NR Inclusion criteria
Exclusion criteria
Total participants randomised: 60 Age in years (mean, range): 50 (29‐72) Gender: 40/60 were female Pain duration in months (mean, range): 53 (3‐175) |
|
| Interventions | Placebo
Clonidine 0.2 mg
Imipramine 50 mg
|
|
| Outcomes | AEs | |
| Missing data methods | NR | |
| Funding source | Funding: NR | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation procedure not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedure not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, matched dosing schedules and identical appearance |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information given regarding dropout or missing data analyses ‐ could be that everyone completed the trial but NR Attrition NR |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified. |
Cardenas 2002.
| Study characteristics | ||
| Methods | Design: parallel Duration: 6 weeks Assessment: baseline and post‐intervention Country: USA |
|
| Participants | Pain condition: pain resulting from spinal cord injury Population: adults with persistent pain from spinal cord injury Minimum pain intensity: average of ≥ 3 out of 10 over the last month Inclusion criteria
Exclusion criteria
Total participants randomised: 84 Age in years (mean): 41.5 Gender: 17/84 were female Pain duration in months (mean): 168.3 |
|
| Interventions | Placebo (benztropine mesylate 0.5 mg)
Amitriptyline 10‐125 mg
|
|
| Outcomes | Pain intensity Mood Physical function AEs Withdrawal |
|
| Missing data methods | ITT but no imputation method specified | |
| Funding source | Non‐pharmaceutical ‐ National Institutes of Health | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not specified |
| Allocation concealment (selection bias) | Low risk | Random assignment to treatment group and provision of medication was done by the University of Washington Medical Center Pharmacy Investigational Drug Services. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, active placebo, identical appearance |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Low levels of dropout, not significantly different across both arms. ITT analysis but no imputation method specified Attrition Total: 11/84 (13.1%) Benzotropine mesylate 0.5 mg: 3/40 (7.5%) Amitriptyline 10‐125 mg: 8/44 (18.2%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified. |
Carette 1986.
| Study characteristics | ||
| Methods | Design: parallel Duration: baseline and post‐intervention Assessment: 9 weeks Country: Canada |
|
| Participants | Pain condition: primary fibrositis/fibromyalgia Population: people with fibromyalgia Minimum pain intensity: NR Inclusion criteria
Exclusion criteria
Total participants randomised: 70 Age in years (mean): 41 Gender: 54/70 were female Pain duration in months (mean): 84 |
|
| Interventions | Placebo
Amitriptyline 10‐50 mg
|
|
| Outcomes | Pain intensity AEs Withdrawal |
|
| Missing data methods | Completer analysis only | |
| Funding source | Non‐pharmaceutical ‐ Arthritis Grant | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedure not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Although the study used double‐blind procedures, the authors noted that 70% of the amitriptyline participants experienced side effects that, in some cases, unblinded participants and research staff. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self‐reported outcomes from participants who may have been unblinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer analysis only Attrition Total: 11/70 (15.7%) Placebo: 4/32 (11.1%) Amitriptyline 10‐50 mg: 7/27 (20.6%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified. |
Carette 1994.
| Study characteristics | ||
| Methods | Design: parallel Duration: 25 weeks Assessment: baseline and post‐intervention Country: Canada |
|
| Participants | Pain condition: fibromylagia Population: adults with fibromyalgia Minimum pain intensity: ≥ 4 on a 0‐10 VAS Inclusion criteria
Exclusion criteria
Total participants randomised: 208 Age in years (mean): 44.9 Gender: 199/208 were female Pain duration in months (mean): 92.6 |
|
| Interventions | Placebo
Amitriptyline 25‐50 mg
Cyclobenzaprine 20‐30 mg
|
|
| Outcomes | Pain intensity Physical function Mood AEs Withdrawal |
|
| Missing data methods | ITT but no imputation methods stated | |
| Funding source | Partly pharmaceutical ‐ supported by grants from the Canadian Arthritis Society and Merck Frosst Canada | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using a random number table |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedure not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Matched dosing but no information on appearance. Not enough information about physician blinding as there were some physician‐reported measures. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Mostly self‐reported outcomes from participants. Not enough information regarding blinding procedures |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Do not clearly report reasons for withdrawal and numbers between groups. State ITT but no methods specified Attrition Total: 52/208 (25.0%) Placebo: 14/84 (33.3%) Amitriptyline 50 mg: 14/82 (16.7%) Cyclobenzaprine 20‐30 mg: 24/42 (29.3%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified. |
Caruso 1987.
| Study characteristics | ||
| Methods | Design: parallel Duration: 8 weeks Assessment: baseline, week 2, week 4, post‐intervention Country: Italy |
|
| Participants | Pain condition: fibromyalgia Population: adults with fibromyalgia Minimum pain intensity: NR Inclusion criteria
Exclusion criteria: NR Total participants randomised: 60 Age in years (mean): 46 Gender: 52/60 were female Pain duration in years (mean): 5.7 |
|
| Interventions | Placebo
Dothiepin 75 mg
|
|
| Outcomes | AEs Withdrawal |
|
| Missing data methods | NR | |
| Funding source | Funding: NR | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomsiation methods not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind with matched dosing and identical appearance of tablets |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes by blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer analysis only Attrition Total: 8/60 (13.3%) Placebo: 4/30 (13.3%) Dothiepin 75 mg: 4/30 (13.3%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified. |
Chappell 2008.
| Study characteristics | ||
| Methods | Design: parallel Duration: 27 weeks Assessment: baseline and post‐intervention Country: USA, Germany, Spain, Sweden, UK |
|
| Participants | Pain condition: fibromyalgia Population: adults with fibromyalgia Minimum pain intensity: none Inclusion criteria
Exclusion criteria
Total participants randomised: 330 Age in years (mean, SD): 50.5 (10.7) Gender: 308/330 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Placebo
Duloxetine
|
|
| Outcomes | Pain intensity Quality of life Mood Physical function Moderate pain relief Substantial pain relief PGIC AEs Withdrawal |
|
| Missing data methods | ITT with LOCF | |
| Funding source | Pharamaceutical ‐ Eli Lilly and Boehringer Ingelheim GmbH | |
| Conflicts of interest | Drs Chappell, Detke, and D'Souza are employees and stockholders of Eli Lilly and Company. Dr Wiltse is a former employee of Eli Lilly and Company. Dr Spaeth is a consultant to Allergan, Eli Lilly, Jazz, and Pierre Fabre Medicament, and is on the speaker bureaus of Eli Lilly and Pierre Fabre Medicament. Dr Bradley is a consultant for Eli Lilly, Pfizer, and Forest; has received grant/research support from the National Institutes of Health, the Agency for Healthcare Research and Quality, Eli Lilly, Pfizer, and the American Fibromyalgia Syndrome Association; has received honoraria from Eli Lilly, Pfizer, Forest, and the Society for Women's Health Research; is a member of the speaker/advisory board for Pfizer; and has received royalties from UpToDate Rheumatology. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Assignment to treatment groups was determined by a computer‐generated random sequence within each study centre, stratified by MDD status |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedure not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical appearance tablets |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Significant difference in participants withdrawing due to lack of efficacy (higher in placebo). Uses ITT with LOCF Attrition Total: 126/330 (38.2%) Placebo: 65/168 (38.7%) Duloxetine 60‐120 mg: 61/162 (37.7%) |
| Selective reporting (reporting bias) | Low risk | Outcomes match those listed in trial registration record |
| Other bias | Low risk | No other sources of bias were identified. |
Chappell 2009a.
| Study characteristics | ||
| Methods | Design: parallel Duration: 8 weeks open‐label, 52 weeks double‐blind Assessment: baseline and post‐intervention Country: Argentina, Australia, Brazil, Canada, Mexico, Poland, Taiwan, USA |
|
| Participants | Pain condition: fibromyalgia Population: adults with fibromyalgia Minimum pain intensity: ≥ 4 on 0‐10 VAS Inclusion criteria
Exclusion criteria
Total participants randomised in double‐blind phase: 307 Age in years (mean, SD): 49 (11.07) Gender: 335/350 were female (including those in the open‐label phase) Pain duration in years (mean, SD): NR |
|
| Interventions | Duloxetine 60 mg
Duloxetine 120 mg
|
|
| Outcomes | Pain intensity Quality of life Physical function Sleep Mood PGIC AEs SAEs Withdrawal |
|
| Missing data methods | ITT with LOCF | |
| Funding source | Pharmaceutical ‐ Eli Lilly and Co and Boehringer Ingelheim | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation procedure not specified |
| Allocation concealment (selection bias) | Low risk | Patients were allocated using an interactive voice response system that was accessed via telephone by each investigator. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | States double‐blind but not enough information on medication i.e. appearance or number of tablets |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported outcomes from participants, but not enough information regarding blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Use ITT with LOCF. Similar but significant attrition rates in both arms Attrition Total: 112/307 (36.5%) Duloxetine 60 mg: 33/104 (31.7%) Duloxetine 120 mg: 79/203 (38.9%) |
| Selective reporting (reporting bias) | Unclear risk | Protocol not very detailed, report matched domains but did not register measures and time points, etc |
| Other bias | Low risk | No other sources of bias were identified. |
Chappell 2009b.
| Study characteristics | ||
| Methods | Design: parallel Duration: 13 weeks Assessment: baseline and post‐intervention Country: USA, Puerto Rico, Romania |
|
| Participants | Pain condition: knee OA Population: adults aged ≥ 40 with knee OA Minimum pain intensity: ≥ 4 on 24‐h 0‐10 VAS Inclusion criteria
Exclusion criteria
Total participants randomised: 231 Age in years (mean): 62.3 Gender: 151/231 were female Pain duration in years (mean): 9 |
|
| Interventions | Placebo
Duloxetine
|
|
| Outcomes | Pain intensity Sleep Quality of life Mood Physical function Moderate pain relief Substantial pain relief PGIC AEs SAEs Withdrawal |
|
| Missing data methods | ITT with LOCF, and MMRM | |
| Funding source | Pharmaceutical ‐ Eli Lilly | |
| Conflicts of interest | NR, but authors are employed by Eli Lilly and declare CoIs in other papers. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using a computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Participants were allcoated using an Interactive Voice Response System (IVRS). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded, matched dosing and identical appearance, smell, and taste of capsules |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT with LOCF, BOCF, and MMRM Attrition Total: 58/231 (25.1%) Placebo: 24/120 (20.0%) Duloxetine 60‐120 mg: 34/111 (30.6%) |
| Selective reporting (reporting bias) | Low risk | Outcomes and procedures match those listed prospectively in trial registration |
| Other bias | Low risk | No other sources of bias were identified. |
Chappell 2011.
| Study characteristics | ||
| Methods | Design: parallel Duration: 13 weeks Assessment: baseline and post‐intervention Country: USA, Canada, Greece, Russia, Sweden |
|
| Participants | Pain condition: knee OA Population: adults aged ≥ 40 with knee OA Minimum pain intensity: ≥ 4 on 0‐10 VAS Inclusion criteria
Exclusion criteria
Total participants randomised: 256 Age in years (mean): 62.5 Gender: 196/256 were female Pain duration in years (mean): 7.4 |
|
| Interventions | Placebo
Duloxetine
|
|
| Outcomes | Pain intensity Physical function Moderate pain relief Substantial pain relief PGIC AEs SAEs Withdrawal |
|
| Missing data methods | ITT with LOCF, sensitivity analysis of primary outcome with BOCF and modified‐BOCF | |
| Funding source | Pharmaceutical ‐ Eli Lilly | |
| Conflicts of interest | This study was sponsored by Eli Lilly and Company, Indianapolis, IN, USA. Drs Chappell, Skljarevski, Desaiah, Liu‐Seifert, and Ms Zhang are employees and stockholders of Eli Lilly and Company. Drs Belenkov and Brown were participating investigators in the conduct of this study and received funding from Eli Lilly and Company. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Assignment to treatment was determined by a computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Participants were allocated using an interactive voice response system to ensure blinding. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | States double‐blind and matched dosing, but don't mention drug appearance |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported outcomes from participants, but unsure of blinding procedures |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition rates may be influenced by group allocation: "Significantly more patients in the duloxetine group (n = 24, 18.8%) discontinued from the study due to adverse events (P = 0.002) than patients in the placebo group (n = 7, 5.5%)." Used LOCF, BOCF, mBOCF, ITT to handle/impute missing data Attrition Total: Placebo: 17/128 (13.3%) Duloxetine 60‐120 mg: 35/128 (27.3%) |
| Selective reporting (reporting bias) | High risk | Data not presented on outcomes that were non‐significant |
| Other bias | Low risk | No other sources of bias were identified. |
Clauw 2008.
| Study characteristics | ||
| Methods | Design: parallel Duration: 15 weeks Assessment: baseline and post‐intervention Country: USA |
|
| Participants | Pain condition: fibromyalgia Population: adults with fibromyalgia Minimum pain intensity: ≥ 40 on 0‐100 VAS Inclusion criteria
Exclusion criteria
Total participants randomised: 1207 Age in years (mean): 50.2 Gender: 1151/1207 were female Pain duration in years (mean): 9.7 |
|
| Interventions | Placebo
Milnacipran 100 mg
Milnacipran 200 mg
|
|
| Outcomes | Pain intensity Moderate pain relief Physical function Mood Quality of life Sleep PGIC AEs SAEs Withdrawal |
|
| Missing data methods | ITT with LOCF and BOCF | |
| Funding source | Pharmaceutical ‐ Forest Research Institute, Inc. and Cypress Bioscience, Inc. | |
| Conflicts of interest | This research was financially supported by Forest Research Institute, Inc., Jersey City, New Jersey, and Cypress Bioscience, Inc., San Diego, California. The study drug was manufactured by Pierre Fabre Medicament, Boulogne, France. Drug supply and data collection were managed by Forest Research Institute. The study was designed and conducted under the supervision of Drs Gendreau, Palmer, and Clauw. The manuscript was prepared with the editorial assistance of Prescott Medical Communications Group, Chicago, Illinois, under the supervision of Dr Clauw. Dr Clauw has received grant support from Cypress Bioscience, Inc., and serves as a consultant to Cypress Bioscience, Forest Laboratories, and Pierre Fabre Medicament, all of which are involved in the development of milnacipran for fibromyalgia. He also acts as a consultant to Eli Lilly and Company, Pfizer Inc., Procter & Gamble, and Wyeth Pharmaceuticals. He has owned stock in Cypress Bioscience. Dr Mease has received research grant support from Allergan, Inc.; Cypress Bioscience; Forest Laboratories; Fralex Therapeutics Inc.; Jazz Pharmaceuticals; Eli Lilly; Pfizer; and Wyeth. Drs Palmer and Wang are employees of Forest Research Institute and own stock in Forest Laboratories. Dr Gendreau is an employee of Cypress Bioscience and owns stock in that company. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation lists for each site were generated by a computer program. |
| Allocation concealment (selection bias) | Low risk | Assignments made via an interactive voice response system |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, matched dosing, and identical appearance of tablets |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High attrition rates, did not adhere to the mentioned plan with handling and reporting missing data Attrition Total: Placebo: 115/405 (28.4%) Milnacipran 100 mg: 137/401 (34.2%) Milnacipran 200 mg: 144/401 (35.9%) |
| Selective reporting (reporting bias) | Unclear risk | Trial registration available but did not specify outcome measures ‐ just outcomes |
| Other bias | Low risk | No other sources of bias were identified. |
Creed 2003.
| Study characteristics | ||
| Methods | Design: parallel Duration: 12 weeks Assessment: baseline, post‐intervention, follow‐up 1 year post‐intervention Country: UK |
|
| Participants | Pain condition: IBS Population: adults with IBS Minimum pain intensity: Inclusion criteria
Exclusion criteria
Total participants randomised: 257 Age in years (mean): 43.3 Gender: 205/257 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Psychotherapy
Paroxetine 20 mg/day
Usual treatment
|
|
| Outcomes | Pain intensity Physical function Mood Withdrawal |
|
| Missing data methods | ITT, data imputed using SOLAS (data imputation software) | |
| Funding source | Non‐pharmaceutical ‐ Medical Research Council and UK North West Regional Health Authority Research and Development Directorate | |
| Conflicts of interest | F Creed has consultancy links with Lilly. He has received payment for sitting on an advisory panel. All other authors declare that they have no competing interests. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by a computer‐generated series of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | When patients had been assessed and accepted into the trial, they were then allocated to a treatment group by the trial administrator using the next slot on the appropriate randomisation list. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and researchers unable to be blinded to due to nature of psychotherapy |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self‐reported outcomes from unblinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unequal attrition. State ITT and data imputed by SOLAS ‐ but no explanation given Attrition Total: 69/257 (26.8%) Psychotherapy: 26/85 (30.6%) Paroxetine 20 mg: 32/86 (37.2%) Usual treatment: 11/86 (12.8%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified. |
de Zanette 2014.
| Study characteristics | ||
| Methods | Design: parallel Duration: 6 weeks Assessment: baseline and post‐intervention Country: Brazil |
|
| Participants | Pain condition: fibromyalgia Population: women with fibromyalgia Minimum pain intensity: ≥ 50 on 0‐100 VAS Inclusion criteria
Exclusion criteria
Total participants randomised: 63 Age in years (mean): 48.9 Gender: 63/63 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Melatonin 10 mg
Amitriptyline 25 mg
Melatonin 10 mg + amitriptyline 25 mg
|
|
| Outcomes | Pain intensity Quality of life Sleep AEs Withdrawal |
|
| Missing data methods | ITT with LOCF | |
| Funding source | Non‐pharmaceutical ‐ multiple Brazilian governmental agencies | |
| Conflicts of interest | The authors declare that there are no financial or other relationships that might lead to CoIs involving any of the following arrangements: financial relationship to the work, employees of a company, consultants for a company, stockholders of the company, members of a speakers' bureau or any other form of financial compensation. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods: NR |
| Allocation concealment (selection bias) | Low risk | Sealed, sequentially numbered envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, double‐dummy trial, identical appearance of tablets |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | States ITT but no method of imputation specified. Low attrition Attrition Total: 6/63 (9.5%) Melatonin 10 mg: 2/21 (9.5%) Amitriptyline 25 mg: 2/21 (9.5%) Melatonin + amitriptyline: 2/21 (9.5%) |
| Selective reporting (reporting bias) | Low risk | Outcomes match those listed on trial registration |
| Other bias | Low risk | No other sources of bias were identified. |
Dickens 2000.
| Study characteristics | ||
| Methods | Design: parallel Duration: 8 weeks Assessment: baseline and post‐intervention Country: UK |
|
| Participants | Pain condition: low back pain Population: adults with chronic low back pain and depression Minimum pain intensity: NR Inclusion criteria
Exclusion criteria
Total participants randomised: 98 Age in years (mean, SD): 45.2 (10.2) Gender: 53/98 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Placebo
Paroxetine 20 mg
|
|
| Outcomes | Pain intensity Physical function Mood |
|
| Missing data methods | ITT with LOCF | |
| Funding source | Pharmaceutical: SmithKline Beecham | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation of order of treatment allocation was achieved using a computer‐generated randomisation list in which treatments were balanced. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered treatment packs containing the medication were held in and distributed by the hospital pharmacy. The packs were allocated to consecutive participants in strict sequential order. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, appearance of both placebo and antidepressant was identical |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT with LOCF, but very low dropout Attrition Total: 6/98 (6.1%) Attrition per arm NR |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified. |
Drossman 2003.
| Study characteristics | ||
| Methods | Design: parallel Duration: 12 weeks Assessment: baseline and post‐intervention Country: USA and Canada |
|
| Participants | Pain condition: functional bowel disorders Population: women with moderate to severe functional bowel disorders Minimum pain intensity: NR Inclusion criteria
Exclusion criteria
Total participants randomised: 431 Age in years (mean, SD): 38.6 (12.0) Gender: 431/431 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Placebo
Desipramine 150 mg
CBT
Education
|
|
| Outcomes | Pain intensity Quality of life Withdrawal |
|
| Missing data methods | ITT but imputation method not specified | |
| Funding source | Non‐pharmaceutical: supported by a research grant from the National Institutes of Health (RO1‐DK49334). | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by computer |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to be double‐blinded across all study arms due to the nature of CBT |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self‐reported outomes from unblinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | States ITT but method not specified Attrition Total: 123/431 (28.5%) Placebo: 16/72 (22.2%) Desipramine 150 mg: 49/144 (34.0%) CBT: 33/144 (22.9%) Education: 25/71 (35.2%) |
| Selective reporting (reporting bias) | Unclear risk | Protocol registered but no outcome measures listed |
| Other bias | Low risk | No other sources of bias were identified |
Eberhard 1988.
| Study characteristics | ||
| Methods | Design: parallel Duration: 6 weeks Assessment: baseline and post‐intervention Country: Sweden |
|
| Participants | Pain condition: idiopathic pain syndromes Population: adults with idiopathic pain syndromes Minimum pain intensity: NR Inclusion criteria
Exclusion criteria
Total participants randomised: 70 Age in years (mean, SD): 50.3 (12.5) Gender: 51/70 were female Pain duration in years (range): 0.5‐28 |
|
| Interventions | Maprotiline 25‐150 mg
Clomipramine 25‐150 mg
|
|
| Outcomes | Pain intensity Mood Withdrawal |
|
| Missing data methods | None ‐ completer‐only analysis | |
| Funding source | NR | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information regarding sequence generation given |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical appearing tablets with matched dosing |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer analysis only Attrition Total: 18/70 (25.7%) Maprotiline 25‐150 mg: 5/30 (16.7%) Clomipramine 25‐150 mg: 13/40 (32.5%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias identified |
Engel 1998.
| Study characteristics | ||
| Methods | Design: cross‐over Duration: 15 weeks (6 weeks per cross‐over period) Assessment: baseline and post‐intervention Country: USA |
|
| Participants | Pain condition: pelvic pain Population: women with chronic pelvic pain Minimum pain intensity: no Inclusion criteria
Exclusion criteria
Total participants randomised: 25 Age in years (mean, SD): 29.0 (7.2) Gender: 25/25 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Placebo
Sertraline 100 mg
|
|
| Outcomes | No useable data were able to be extracted from the study. | |
| Missing data methods | Completer analysis only | |
| Funding source | NR | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information on blinding procedures |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported outcomes but unsure of blinding procedures |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Completer analysis but low dropout Attrition Total: 2/25 (8%) Attrition per arm NR |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias identified |
Enomoto 2018.
| Study characteristics | ||
| Methods | Design: parallel Duration: 12 weeks Assessment: baseline and post‐intervention Country: Japan |
|
| Participants | Pain condition: peripheral diabetic neuropathy Population: adults with peripheral diabetic neuropathy Minimum pain intensity: ≥ 4 on 0‐10 VAS Inclusion criteria
Exclusion criteria
Total participants randomised: 303 Age in years (mean, SD): 59.6 (9.03) Gender: 83/303 were female Pain duration in years (mean, SD): 4.59 (4.25) |
|
| Interventions | Pregabalin 300‐600 mg
Duloxetine 40‐60 mg
|
|
| Outcomes | Pain intensity Quality of life Mood Moderate pain relief Substantial pain relief PGIC AEs SAEs Withdrawal |
|
| Missing data methods | MMRM | |
| Funding source | Pharmaceutical: Eli Lilly Japan | |
| Conflicts of interest | HE, SF, MT and AY are employees of Eli Lilly Japan K.K. AN is an employee of Shionogi & Co. Ltd., and MF, MI and TT are employees and minor stockholders of Shionogi & Co. Ltd. LA is an employee of Eli Lilly Turkey. SF and LA hold shares in Eli Lilly and Company. HY reports speaking fees from Nippon Boehringer Ingelheim Co. Ltd., Eli Lilly Japan K.K., Shionogi & Co. Ltd., Sanwa Kagaku Kenkyusyo Co. Ltd., and Sumitomo Dainippon Pharma Co. Ltd., and consulting fees from Shionogi & Co. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were assigned to duloxetine or pregabalin in a 1:1 ratio via a computer‐generated random sequence. |
| Allocation concealment (selection bias) | Low risk | Patients were assigned via a computer‐generated random sequence using an interactive web response system. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, study drugs were identical in appearance and followed a matched dosing schedule |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | In the statistical analysis plan in their protocol they mention they will handle missing data and impute using LOCF and BOCF but this is not mentioned anywhere in the paper. State MMRM Attrition Total: 36/303 (11.9%) Pregabalin 300‐600 mg: 21/151 (13.9%) Duloxetine 40‐60 mg: 15/152 (9.9%) |
| Selective reporting (reporting bias) | Low risk | All outcomes prospectively listed on clinicaltrials.gov before trial started |
| Other bias | Low risk | No other sources of bias were identified |
Enteshari‐Moghaddam 2019.
| Study characteristics | ||
| Methods | Design: parallel Duration: 12 weeks Assessment: baseline to post‐intervention Country: Iran |
|
| Participants | Pain condition: knee OA Population: adults with moderate‐severe knee OA Minimum pain intensity: ≥ 5 on 0‐10 VAS Inclusion criteria
Exclusion criteria
Total participants randomised: 150 Age in years (mean): 54.4 Gender: 110/150 were female Pain duration in years (mean): 8.44 |
|
| Interventions | Paracetamol 2000 mg
Duloxetine 60 mg
Gabapentin 600 mg
|
|
| Outcomes | AEs Withdrawal |
|
| Missing data methods | All participants completed the trial | |
| Funding source | Non‐pharmaceutical: Ardabil University of Medical Sciences | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using random number blocks. |
| Allocation concealment (selection bias) | Low risk | Participants were allocated to an arm using sealed, opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | States double‐blind but does not report blinding procedures |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported outcomes by participants, but unsure of blinding procedures |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the trial Attrition: none |
| Selective reporting (reporting bias) | High risk | Do not report quality of life as stated in protocol |
| Other bias | Low risk | No other sources of bias were found |
Forssell 2004.
| Study characteristics | ||
| Methods | Design: cross‐over Duration: 4 weeks Assessment: baseline to post‐intervention Country: Finland |
|
| Participants | Pain condition: atypical facial pain Population: adults with atypical facial pain Minimum pain intensity: ≥ 3 on 0‐10 scale Inclusion criteria
Exclusion criteria
Total participants randomised: 30 Age in years (median, range): 52 (38‐66) Gender: 12/30 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Placebo
Venlafaxine 37.5‐70 mg
|
|
| Outcomes | Pain intensity Mood Withdrawal |
|
| Missing data methods | Completer analysis only | |
| Funding source | Non‐pharmaceutical: funded by Helsinki University Central Hospital Research Fund | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Allocation procedure not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind with identical appearance and matched dosing schedules |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer‐only analysis Attrition Total: 10/30 (33.3%) Venlafaxine 37.5‐70 mg: 6/30 (20.0%) Placebo: 4/30 (13.3%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified. |
Foster 2010a.
| Study characteristics | ||
| Methods | Design: parallel Duration: 12 weeks Assessment: baseline and post‐intervention Country: USA |
|
| Participants | Pain condition: vulvodynia Population: women with vulvodynia Minimum pain intensity: ≥ 4 on 0‐10 VAS Inclusion criteria
Exclusion criteria
Total participants randomised: 133 Age in years (mean): 30.4 Gender: 133/133 were female Pain duration in years (range): 4.4‐6.5 |
|
| Interventions | Placebo
Lidocaine 5% cream
Desipramine 150 mg
Desipramine 150 mg and lidocaine 5% cream
|
|
| Outcomes | Pain intensity Mood Withdrawal |
|
| Missing data methods | ITT with LOCF | |
| Funding source | Non‐pharamaceutical: supported by grant RO‐1 HD040123‐05 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health | |
| Conflicts of interest | The study authors did not report any potential CoIs. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using permuted block randomisation scheme by means of a computer‐based random numbers generator. |
| Allocation concealment (selection bias) | Low risk | Drug assignments were determined by the Department of Biostatistics. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical‐appearing pills and creams, matched dosing with active drug treatment for both tablets and creams |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT with LOCF. 4 x higher number of dropouts in desipramine+lidocaine arm than placebo Attrition Total: 21/133 (15.8%) Placebo: 2/33 (6.1%) Lidocaine 5%: 5/33 (15.2%) Desipramine 150 mg: 6/33 (18.2%) Desipramine 150 mg + lidocaine 5%: 6/34 (17.7%) |
| Selective reporting (reporting bias) | Low risk | All outcomes listed prospectively on clinicaltrials.gov |
| Other bias | Low risk | No other sources of bias were identified. |
Foster 2010b.
| Study characteristics | ||
| Methods | Design: parallel Duration: 12 weeks Assessment: baseline and post‐intervention Country: USA and Canada |
|
| Participants | Pain condition: Interstitial Cystitis/Painful Bladder Syndrome Population: people with painful bladder pain with no prior treatment experience for IC/PBS. Minimum pain intensity: ≥ 3 on 0‐10 VAS Inclusion criteria
Exclusion criteria
Total participants randomised: 271 Age in years (median): 38 Gender: 216/271 were female Pain duration in years (mean): 6.4 |
|
| Interventions | Placebo
Amitriptyline 25‐75 mg
|
|
| Outcomes | Pain intensity AEs Withdrawal |
|
| Missing data methods | ITT but do not specify missing data methods | |
| Funding source | Non‐pharmaceutical: National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), collaborator: University of Pennsylvania | |
| Conflicts of interest | Dr Foster reports having no conflicts. Dr Hanno reports Astellas, Pfizer, and Trillium. Dr Nickel reports receiving consulting fees from Merck, Glaxo‐Smith‐Kline, Pfizer, Ortho Women's Health, Farr Labs, Watson, Medtronic, NeurAxon, Genyous Biomed and research support from Merck, Glaxo‐Smith Kline, Allergan, Watson, Pfizer and American Medical Systems. Dr C. Yang reports Medtronic. Dr Chai reports Pfizer and Allergan. Dr Kusek reports holding stock in deCode Genetics. No other potential COI relevant to this manuscript was reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | States double‐blind but no information given regarding study drug appearance |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported outcomes from participants but unsure of blinding procedures |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | State ITT but no imputation methods specified Attrition Total: 40/271 (14.8%) Placebo: 17/136 (12.5%) Amitriptyline 25‐75 mg: 23/135 (17.0%) |
| Selective reporting (reporting bias) | High risk | Primary outcome reported according to protocol, not all secondary outcomes reported. Added new outcomes into the outcome measures under methods but never report the outcome for these. |
| Other bias | Low risk | No other sources of bias were identified. |
Frakes 2011.
| Study characteristics | ||
| Methods | Design: parallel Duration: 8 weeks Assessment: baseline to post‐intervention Country: USA and Puerto Rico |
|
| Participants | Pain condition: knee OA Population: adults over 40 with OA who have not responded to NSAIDs Minimum pain intensity: ≥ 4 on 0‐10 VAS Inclusion criteria:
Exclusion criteria
Total participants randomised: 524 Age in years (mean, SD): 61 (9.2) Gender: 299/524 were female Pain duration in years (mean, SD): 9.5 (8.9) |
|
| Interventions | Placebo
Duloxetine 60‐120 mg
|
|
| Outcomes | Pain intensity Moderate pain relief Substantial pain relief Physical function Mood Sleep PGIC AEs SAEs Withdrawal |
|
| Missing data methods | ITT, modified ITT, BOCF, LOCF, MMRM | |
| Funding source | Pharmaceutical: Eli Lilly | |
| Conflicts of interest | "At the time this manuscript was written, E.P.F., R.C.R., and M.M.W. were full‐time employees of Eli Lilly and/or one of its subsidiaries and were minor stockholders of Eli Lilly and Company. M.C.H. currently receives research support from the National Institutes of Health; is a consultant for Abbott Laboratories, Amgen, Astra‐Zeneca Pharmaceutical Co., Bioiberica S.A., Bristol Myers Squibb Company, Covidien, Eli Lilly and Company, EMD Serono, Inc., Genentech/Roche, Iroko Pharmaceuticals, Merck & Co. Inc., NiCox S.A., Pfizer Inc., Pozen Inc., Rand Corporation, Smith & Nephew, TransPharma Medical Ltd, and UCB Inc.; is a member or chair of DSMB, National Eye Institute, Novartis Pharma A.G., Savient Pharmaceuticals Inc., and Stryker Biotech LLC; and is a member of the medical advisory board and owns stock in Theralogixx, LLC. TDB is a full‐time employee of i3 Data Services, a division of InVentiv Health Company. She was contracted by Eli Lilly for writing services. CMRO peer reviewers may have received honoraria for their review work. The peer reviewers on this manuscript have disclosed that they have no relevant financial relationships." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods were not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures were not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | States double‐blind but no information on drug appearance and dosing schedules |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported outcomes from participants but unsure of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT with LOCF, BOCF, and mBOCF Attrition Total: 136/524 (30.0%) Placebo: 61/260 (23.5%) Duloxetine 60‐120 mg: 75/264 (28.4%) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported match those registered prospectively on clincaltrials.gov |
| Other bias | Low risk | No other sources of bias were identified. |
Gao 2010.
| Study characteristics | ||
| Methods | Design: parallel Duration: 12 weeks Assessment: baseline and post‐intervention Country: China |
|
| Participants | Pain condition: diabetic peripheral neuropathy Population: adults with diabetic peripheral neuropathy Minimum pain intensity: ≥ 4 on 0‐10 VAS Inclusion criteria
Exclusion criteria
Total participants randomised: 215 Age in years (mean): 59.3 Gender: 14/215 were female Pain duration in years (mean): 3.2 |
|
| Interventions | Placebo
Duloxetine 60 to 120 mg
|
|
| Outcomes | Pain intensity Sleep Mood Quality of life Moderate pain relief Substantial pain relief PGIC AEs SAEs Withdrawal |
|
| Missing data methods | ITT with LOCF | |
| Funding source | Pharmaceutical: Eli Lilly and Boehringer Ingelheim Pharmaceuticals | |
| Conflicts of interest | Drs Vladimir Skljarevski, Durisala Desaiah, Zhang Shu‐yu, and Zhang Qi are employees and stockholders of Eli Lilly and Company. All other authors from China were the investigators and received funding from Eli Lilly and Company for conducting this study. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind with identical placebo and matched dosing schedule |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT with LOCF Attrition Total: 36/215 (16.7%) Placebo: 17/109 (15.6%) Duloxetine 60‐120 mg: 19/106 (17.9%) |
| Selective reporting (reporting bias) | Low risk | All outcomes registered prospectively on clinicaltrials.gov |
| Other bias | Low risk | No other sources of bias identified |
Gao 2015.
| Study characteristics | ||
| Methods | Design: parallel Duration: 12 weeks Assessment: baseline and post‐intervention Country: China |
|
| Participants | Population: adults with diabetic peripheral neuropathy Minimum pain intensity: ≥ 4 on 0‐10 VAS Inclusion criteria
Exclusion criteria
Total participants randomised: 405 Age in years (mean, SD): 61.4 (9.5) Gender: 223/405 were female Pain duration in years (mean, SD): 3.3 (3.6) |
|
| Interventions | Placebo
Duloxetine 60 mg
|
|
| Outcomes | Pain intensity Physical function Sleep Moderate pain relief Substantial pain relief PGIC AEs SAEs Withdrawal |
|
| Missing data methods | MMRM, ITT with LOCF | |
| Funding source | Pharmaceutical: Eli Lilly | |
| Conflicts of interest | Drs Gao, Guo, Han, Li, Yang, and Qu have no conflicts of interest. Drs Due~nas, Yue, Wang, Skljarevski, and Raskin are employees and minor shareholders of Eli Lilly | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | States double‐blind but no information regarding study drugs appearance etc |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported outcomes from participants, but unsure of blinding procedures |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT with LOCF Attrition Total: 56/405 (13.2%) Placebo: 26/202 (12.9%) Duloxetine 60 mg: 30/203 (14.8%) |
| Selective reporting (reporting bias) | Low risk | All outcomes listed prospectively to trial on clinicaltrials.gov |
| Other bias | Low risk | No other sources of bias were identified |
Gillving 2021.
| Study characteristics | ||
| Methods | Design: Denmark Duration: 5 weeks Assessment: baseline and post‐intervention Country: cross‐over |
|
| Participants | Pain condition: painful polyneuropathy of any aetiology Population: people with painful polyneuropathy Minimum pain intensity: ≥ 4 on 0‐10 VAS Inclusion criteria:
Exclusion criteria:
Total participants randomised: 51 Age in years (median, range): 59 (20‐76) Gender: 22/51 were female Pain duration in years (median, range): 40 (10‐156) |
|
| Interventions | Placebo
Terbutaline 5‐15 mg
Imipramine 30‐150 mg
|
|
| Outcomes | Pain intensity Quality of life Sleep Substantial pain relief Moderate pain relief AEs SAEs Withdrawal |
|
| Missing data methods | ITT with LOCF | |
| Funding source | Non‐pharmaceutical: grants from Danish Regions (Grant no. 14/217) and the Research Foundation of Odense University Hospital. S.S. Gylfadottir was funded by a grant from the Novo Nordic Foundation (Grant no. 14OC0011633). | |
| Conflicts of interest | The study authors have no conflicts of interest to declare | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised through a computer‐generated randomisation list. |
| Allocation concealment (selection bias) | Low risk | Participants were consecutively allocated to the next available randomisation number. The study drugs were packed in containers marked with a randomisation number and treatment period by the hospital pharmacy. Sealed, opaque envelopes containing the treatment sequence for each participant were present at the study sites for emergency situations. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical study drugs and double‐dummy design |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT with LOCF Attrition Total: 12/51 (23.5%) Placebo: 3/51 (5.9%) Terbutaline 5‐15 mg: 5/51 (9.8%) Imipramine 30‐150 mg: 4/51 (7.8%) |
| Selective reporting (reporting bias) | Low risk | Trial registered prospectively and outcomes matched those registered |
| Other bias | Low risk | No other sources of bias were identified |
Gilron 2009.
| Study characteristics | ||
| Methods | Design: cross‐over Duration: 6 weeks Assessment: baseline and post‐intervention Country: Canada |
|
| Participants | Pain condition: neuropathic pain from diabetic peripheral neuropathy or post‐herpetic neuralgia Population: adults with diabetic polyneuropathy or post‐herpetic neuralgia Minimum pain intensity: ≥ 4 on 0‐10 VAS Inclusion criteria
Exclusion criteria
Total participants randomised: 56 Age in years (median, range): diabetic peripheral: 61 (53‐69); post‐herpetic: 68 (65‐73) Gender: 21/56 were female Pain duration in years (median, range): diabetic peripheral: 5.2 (3.4); post‐herpetic: 2.8 (4.3) |
|
| Interventions | Gabapentin ≤ 3600 mg
Nortriptyline ≤ 100 mg
Nortriptyline ≤ 100 mg and gabapentin ≤ 3600 mg
|
|
| Outcomes | Pain intensity Sleep Mood Physical fucntion SAEs Withdrawal |
|
| Missing data methods | ITT but no method specified | |
| Funding source | Non‐pharmaceutical: Canadian Institutes of Health Research (grant numbers MCT‐69422 and MSH‐55041) | |
| Conflicts of interest | IG has received honoraria for consulting or being a member of an advisory board, or both for Pfizer. RLH has received research grant support from Pfizer. All other authors declare that they have no conflicts of interest. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using computer randomisation of the 3 sequences in blocks of 3. |
| Allocation concealment (selection bias) | Low risk | A trial pharmacist prepared a concealed allocation schedule, and the pharmacist had no further involvement in the trial. Patients were assigned in turn to the next consecutive number, and the corresponding series of study drugs was dispensed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, double‐dummy design |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT with LOCF, unequal dropout Attrition Total: 11/56 (19.6%) Gabapentin ≤ 3600 mg: 8/56 (14.3%) Nortriptyline ≤ 100 mg: 1/56 (1.8%) Gabapentin ≤ 3600 mg + nortriptyline ≤ 100 mg: 2/56 (3.6%) |
| Selective reporting (reporting bias) | Unclear risk | Can't find global pain relief reported in study (was stated in prospective ISRCTN registration). In the protocol, the Profile of Mood State questionnaire was listed as a secondary outcome but it is NR. |
| Other bias | Low risk | No other sources of bias identified |
Gilron 2015.
| Study characteristics | ||
| Methods | Design: cross‐over Duration: 6 weeks Assessment: baseline to post‐intervention Country: Canada |
|
| Participants | Pain condition: any chronic neuropathic pain Population: adults with chronic peripheral neuropathic pain Minimum pain intensity: ≥ 4 on 0‐10 VAS Inclusion criteria
Exclusion criteria
Total participants randomised: 52 Age in years (median, range): 66 (49‐80) Gender: 14/52 were female Pain duration in years (mean, SD): 6.1 (6.4) |
|
| Interventions | Morphine ≤ 100 mg
Nortriptyline
Nortriptyline and morphine
|
|
| Outcomes | Pain intensity Mood Sleep Moderate pain relief Substantial pain relief Withdrawal |
|
| Missing data methods | NR | |
| Funding source | Part funded by pharmaceutical: "This work was supported by CIHR (Canadian Institutes of Health Research) Grant #MCT‐94187 and a CIHR‐Pfizer Rx&D Collaborative Research Investigator Program (CIHR Grant #MSH‐55041)." | |
| Conflicts of interest | I. Gilron has received support from Adynxx, TARIS Biomedical, AstraZeneca, Pfizer, and Johnson & Johnson and has received grants from the Canadian Institutes of Health Research, Physicians' Services Incorporated Foundation, and Queen's University. R. R. Holden has received research funding from the Canadian Institutes of Health Research, the Social Sciences and Humanities Research Council of Canada, the American Foundation for Suicide Prevention, and Queen's University. A. C. Jackson has received grants from the Canadian Institutes of Health Research, Research Manitoba (formerly the Manitoba Health Research Council), and the University of Manitoba. The remaining authors have no conflicts of interest to declare. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using computer randomisation of the 3 sequences in blocks of 3. |
| Allocation concealment (selection bias) | Low risk | A trial pharmacist prepared a concealed allocation schedule, and the pharmacist had no further involvement in the trial. Patients were assigned in turn to the next consecutive number, and the corresponding series of study drugs was dispensed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, double‐dummy design |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Unequal attrition across arms, states ITT but no imputation methods specified Attrition Total: 16/52 (30.8%) Morphine: 9/52 (17.3%) Nortriptyline ≤ 100 mg: 2/52 (3.9%) Nortriptyline + morphine: 7/52 (13.5%) |
| Selective reporting (reporting bias) | Low risk | All outcomes prospectively reported on ISRCTN.com |
| Other bias | Low risk | No other sources of bias were identified |
Gilron 2016.
| Study characteristics | ||
| Methods | Design: cross‐over Duration: 6 weeks Assessment: baseline and post‐intervention Country: Canada |
|
| Participants | Pain condition: fibromyalgia Population: people with fibromyalgia Minimum pain intensity: ≥ 4 on 0‐10 VAS Inclusion criteria
Exclusion criteria
Total participants randomised: 41 Age in years (median, range): 56 (20‐71) Gender: 36/41were female Pain duration in years (mean, SD): NR |
|
| Interventions | Placebo
Pregabalin ≤ 450 mg
Duloxetine ≤ 120 mg
Pregabalin ≤ 450 mg + duloxetine ≤ 120 mg
|
|
| Outcomes | Pain intensity Quality of life Physical function Mood Sleep Withdrawal |
|
| Missing data methods | NR | |
| Funding source | Part funded by pharmaceutical: "This work was supported by CIHR (Canadian Institutes ofHealth) Grant CIHR‐MOP‐106489 and a CIHR‐Pfizer R&D Collaborative Research Investigator Program (CIHR Grant MSH‐55041)." | |
| Conflicts of interest | I. Gilron has received support from Adynxx, Taris Biomedical, Astra Zeneca, Pfizer, and Johnson & Johnson and has received grants from the Canadian Institutes of Health Research, Physicians' Services Incorporated Foundation, and Queen's University. L. E. Chaparro received a John J. Bonica Training Fellowship from the International Association for the Study of Pain and also financial support from the Queen's University Department of Anesthesiology and Perioperative Medicine. R. R. Holden has received research funding from the Canadian Institutes of Health Research, the Social Sciences and Humanities Research Council of Canada, the American Foundation for Suicide Prevention, and Queen's University. R. Milev has received financial support and research grants from CIHR, Ontario Brain Institute, Ontario Mental Health Foundation, Lundbeck, Lilly, Sunovion, BMS, Otsuka, Pfizer, Paladin, and Merck. T. Towheed has received financial support from Abbvie and Bristol‐Meyers‐Squibb and research funding from the Canadian Institutes of Health Research. D. D. Shore. and S. Walker received no external financial support. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using computer randomisation of the 3 sequences in blocks of 3. |
| Allocation concealment (selection bias) | Low risk | A trial pharmacist prepared a concealed allocation schedule, and the pharmacist had no further involvement in the trial. Patients were assigned in turn to the next consecutive number, and the corresponding series of study drugs was dispensed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, matched dosing schedule and double dummy design |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on missing data methods Attrition Total: 8/41 (19.5%) Placebo: 1/41 (2.4%) Pregabalin ≤ 450 mg: 1/41 (2.4%) Duloxetine ≤ 120 mg: 3/41 (7.32%) Pregabalin ≤ 450 mg + duloxetine ≤ 120 mg: 4/41 (9.76%) |
| Selective reporting (reporting bias) | Low risk | Everything as reported in prospectively registered protocol |
| Other bias | High risk | Taper and washout period were combined, only 1 day complete washout. They state that "primary analysis revealed no significant effects of sequence or carryover, but effects of period and treatment were significant". |
Ginsberg 1996.
| Study characteristics | ||
| Methods | Design: parallel Duration: 8 weeks Assessment: baseline, 4 weeks (halfway point), post‐intervention Country: Belgium |
|
| Participants | Pain condition: fibromyalgia Population: people with fibromyalgia Minimum pain intensity: no Inclusion criteria
Exclusion criteria
Total participants randomised: 51 Age in years (mean): 46 Gender: 38/51 were female Pain duration in years (mean): 3.2 |
|
| Interventions | Placebo
Amitriptyline 25 mg
|
|
| Outcomes | Pain intensity Sleep AEs Withdrawal |
|
| Missing data methods | ITT but no information regarding imputation methods given | |
| Funding source | NR | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information given, just says patients were "randomised" |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, identical study drugs and matched dosing |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | State ITT but no imputation methods reported Attrition Total: 6/51 (11.8%) Placebo: 3/25 (12.0%) Amitriptyline 25 mg: 3/26 (11.5%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias identified |
Ginsberg 1998.
| Study characteristics | ||
| Methods | Design: parallel Duration: 4 weeks Assessment: baseline and post‐intervention Country: Belgium |
|
| Participants | Pain condition: fibromyalgia Population: people with fibromyalgia Minimum pain intensity: no Inclusion criteria
Exclusion criteria
Total participants randomised: 100 Age in years (mean): 39.8 Gender: 85/100 were female Pain duration in months (mean): 34.7 |
|
| Interventions | Placebo
Pirlindole 150 mg
|
|
| Outcomes | Pain intensity Mood AEs Withdrawal |
|
| Missing data methods | NR | |
| Funding source | NR | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | States double‐blind, but no information regarding study drugs' appearance |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported outcomes from participants, but unsure of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer analysis only. Unclear with number randomised, completer analysis and no clear explanation of when and who withdrew. Attrition Total: 39/100 (39.0%) Placebo: 22/44 (50.0%) Pirlindole 150 mg: 17/45 (37.8%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias identified |
Goldenberg 1986.
| Study characteristics | ||
| Methods | Design: parallel Duration: 6 weeks Assessment: baseline and post‐intervention Country: USA |
|
| Participants | Pain condition: fibromyalgia Population: adults with fibromyalgia Minimum pain intensity: ≥ 4 on 0‐10 VAS Inclusion criteria
Exclusion criteria
Total participants randomised: 62 Age in years (mean, range): 43.8 (21‐69) Gender: 59/62 were female Pain duration in years (mean, range): 3.5 (0.25‐20) |
|
| Interventions | Amitriptyline 50 mg + naproxen 1000 mg
Placebo + naproxen 1000 mg
Amitriptyline 50 mg + placebo
Placebo + placebo
|
|
| Outcomes | Withdrawal | |
| Missing data methods | Completer analysis | |
| Funding source | Partly pharmaceutical: supported by grants from the Arthritis Foundation, Multipurpose Arthritis Center grant no. AM‐20613, and a clinical investigator grant from Syntex Co. | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐dummy design, but no information on study drug appearance and dosing |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported outcomes from participants, but unsure of blinding procedures |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Completer analysis but very low dropout Attrition Total: 4/62 (6.5%) Attrition per arm NR |
| Selective reporting (reporting bias) | High risk | Only present the data for the groups that had significant differences. No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified |
Goldenberg 1996.
| Study characteristics | ||
| Methods | Design: cross‐over Duration: 6 weeks Assessment: baseline and post‐intervention Country: USA |
|
| Participants | Pain condition: fibromyalgia Population: adults with fibromyalgia Minimum pain intensity: ≥ 30 on 0‐100 VAS Inclusion criteria
Exclusion criteria
Total participants randomised: 31 Age in years (mean, SD): 43.2 (9.1) Gender: 28/31 were female Pain duration/fibromyalgia symptoms in months (mean, SD): 72.6 (48.1) |
|
| Interventions | Placebo
Amitriptyline 25 mg + placebo
Fluoxetine 20 mg + placebo
Amitriptyline 25 mg + fluoxetine 20 mg
|
|
| Outcomes | Pain intensity Quality of life Sleep Mood Withdrawal |
|
| Missing data methods | Completer analysis only | |
| Funding source | Non‐pharmaceutical: Lot Page Fund, Newton‐Wellesley Hospital, Newton | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using a table of random numbers |
| Allocation concealment (selection bias) | Low risk | Randomisation and allocation was performed in the hospital pharmacy |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical tablets and double‐dummy to match dosing schedules across groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer‐only analysis, unequal attrition across arms and high overall dropout Attrition Total: 12/31 (38.7%) Placebo: 1/31 (3.2%) Amitriptyline 25 mg: 1/31 (3.2%) Fluoxetine 20 mg: 4/31 (12.9%) Amitriptyline 25 mg + fluoxetine 20 mg: 5/31 (16.1%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified |
Goldman 2010.
| Study characteristics | ||
| Methods | Design: parallel Duration: 6 weeks Assessment: baseline and post‐intervention Country: USA |
|
| Participants | Pain condition: arm pain Population: people with persistent arm pain from repetitive use Minimum pain intensity: ≥ 3 on 0‐10 VAS Inclusion criteria
Exclusion criteria
Total participants randomised: 118 Age in years (mean): 37.5 Gender: 66/118 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Placebo
Amitriptyline 25 mg
|
|
| Outcomes | Pain intensity Sleep Mood AEs SAEs Withdrawal |
|
| Missing data methods | ITT with LOCF | |
| Funding source | Non‐pharmaceutical: "This study was supported by Grants 1RO1 AT 00402‐01 and 1K24 AT 004095 from the National Center for Complementary and Alternative Medicine (NCCAM) at the National Institutes of Health, USA" | |
| Conflicts of interest | "No author had or now has any financial interest in any for‐profit organisation related to the treatment of patients with repetitive strain injuries or related disabling conditions. Dr Rose Goldman sometimes serves as a paid expert witness, independent medical examiner, and/or consultant in workers' compensation and disability cases that might involve musculoskeletal problems and repetitive strain injuries." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using a permuted block randomisation design |
| Allocation concealment (selection bias) | Low risk | Participants were allocated using assignments sealed in sequentially numbered opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical study drugs and matched dosing |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT with LOCF but low attrition Attrition Total: 12/118 (10.2%) Placebo: 4/59 (6.8%) Amitriptyline 25 mg: 8/59 (13.6%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified. |
Goldstein 2005.
| Study characteristics | ||
| Methods | Design: parallel Duration: 12 weeks Assessment: baseline and post‐intervention Country: USA |
|
| Participants | Pain condition: painful diabetic neuropathy Population: people with painful diabetic neuropathy Minimum pain intensity: ≥ 4 on 0‐10 VAS Inclusion criteria
Exclusion criteria
Total participants randomised: 457 Age in years (mean, SD): 60.1 (10.9) Gender: 176/457 were female Pain duration in years (mean, SD): 3.7 (3.8) |
|
| Interventions | Placebo
Duloxetine 20 mg
Duloxetine 60 mg
Duloxetine 120 mg
|
|
| Outcomes | Pain intensity Physical function Quality of life Mood Substantial pain relief PGIC Withdrawal |
|
| Missing data methods | ITT with LOCF | |
| Funding source | Pharmaceutical: Eli Lilly and Company and PRN Consulting | |
| Conflicts of interest | Authors are employees and/or stockholders of Eli Lilly and Company. David J. Goldstein, MD, PhD, is a consultant for Eli Lilly and Company | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using a computer‐generated random sequence. |
| Allocation concealment (selection bias) | Low risk | Participants were allocated using an Interactive Voice Response System. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information given on blinding procedures in regard to medication, although reported as double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported outcomes from participants, but unsure of blinding procedures |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT with LOCF Attrition Total: 113/457 (24.7%) Placebo: 28/115 (24.4%) Duloxetine 20 mg: 24/115 (20.9%) Duloxetine 60 mg: 28/114 (24.6%) Duloxetine 120 mg: 33/113 (29.2%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified |
González‐Viejo 2005.
| Study characteristics | ||
| Methods | Design: parallel Duration: physical therapy 3 weeks; sertraline 24 weeks Assessment: baseline and post‐intervention Country: Spain |
|
| Participants | Pain condition: fibromyalgia Population: women with fibromyalgia Minimum pain intensity: no Inclusion criteria
Exclusion criteria
Total participants randomised: 70 Age in years (mean, SD): 47.5 (4) Gender: 70/70 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Physical therapy
Sertraline 50 mg
|
|
| Outcomes | Pain intensity Sleep Withdrawal |
|
| Missing data methods | All participants completed the trial | |
| Funding source | NR | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to be double‐blind due to the nature of interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self‐reported outcomes from unblinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the trial Attrition None |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified |
Goodkin 1990.
| Study characteristics | ||
| Methods | Design: parallel Duration: 6 weeks Assessment: baseline and post‐intervention Country: USA |
|
| Participants | Pain condition: low back pain Population: adults with low back pain Minimum pain intensity: ≥ 4 on 0‐10 VAS Inclusion criteria
Exclusion criteria
Total participants randomised: 42 Age in years (mean, SD): 53.6 (12.9) Gender: 16/42 were female Pain duration in years (mean, SD): 20.3 (16.0) |
|
| Interventions | Placebo
Trazodone ≤ 600 mg
|
|
| Outcomes | Pain intensity Physical function Mood Withdrawal |
|
| Missing data methods | ITT with LOCF | |
| Funding source | Partly funded by pharmaeutical: "This work was supported by NIH grants MH18764 and MH16744 and NIMH Mental Health Clinical Research Center grant MH41115, a grant from the Procter and Gamble Company, a grant from the Stanford University Health Sciences Research and Development Fund, and a grant from the Western Research and Development Office of the Veterns Administration." | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not specified |
| Allocation concealment (selection bias) | Low risk | Participants were randomised to either trazodone or placebo groups by the Stanford University pharmacist who never interacted with participants |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical appearance of study drugs and matched dosing schedule |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT with LOCF Attrition Total: 13/42 (31.0%) Placebo: 4/20 (20.0%) Trazodone ≤ 600 mg: 9/22 (40.9%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified. |
Gould 2020.
| Study characteristics | ||
| Methods | Design: parallel Duration: 12 weeks Assessment: baseline and post‐intervention Country: USA |
|
| Participants | Pain condition: low back pain Population: adults with low back pain Minimum pain intensity: ≥ 4 on 0‐10 VAS Inclusion criteria
Exclusion criteria
Total participants randomised: 142 Age in years (mean, SD): 55.8 (11.7) Gender: 15/142 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Placebo (benzotropine mesylate 0.125 mg)
Desipramine
Placebo (benzotropine mesylate 0.125 mg) + CBT
Desipramine + CBT
|
|
| Outcomes | Pain intensity Physical function Moderate pain relief AEs SAEs Withdrawal |
|
| Missing data methods | ITT with LOCF | |
| Funding source | Non‐pharmaceutical: VA Office of Research and Development Collaborator: University of California, San Diego | |
| Conflicts of interest | The study authors have no conflicts of interest to declare | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | To minimise the risk of bias in treatment assignment, randomisation using a random number generator (www.randomizer.org) was conducted by a VA San Diego Healthcare System Clinical Research Pharmacy (author S.D.F.), who alone held the key |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Double‐blinding across all arms not possible due to the nature of CBT |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self‐reported outcomes from unblinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT with LOCF. Attrition unequal across arms Attrition Total: Placebo: 9/33 (27.3%) Desipramine 20‐60 mg: 11/38 (29.0%) Placebo + CBT: 7/34 (20.6%) Desipramine 20‐60 mg + CBT: 16/37 (43.2%) |
| Selective reporting (reporting bias) | Unclear risk | Mention in published paper that other outcomes were measured and reported in the protocol (which they don't seem to be) and that they were NR in the publication as it was not in keeping with the study hypothesis/aim |
| Other bias | Low risk | No other sources of bias were identified |
Grace 1985.
| Study characteristics | ||
| Methods | Design: parallel Duration: 12 weeks Assessment: baseline, 4 weeks, 8 weeks, and post‐intervention Country: Canada |
|
| Participants | Pain condition: RA Population: adults with RA Minimum pain intensity: no Inclusion criteria
Exclusion criteria: NR Total participants randomised: 36 Age in years (mean, range): 58 (27‐76) Gender: 29/36 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Placebo
Amitriptyline 50‐75 mg
|
|
| Outcomes | Withdrawal | |
| Missing data methods | Completer‐only analysis | |
| Funding source | NR | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical tablets with matched dosing |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes by blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer analysis only Attrition Total: 8/36 (22.2%) Placebo: 4/18 (22.2%) Amitriptyline 50‐75 mg: 4/18 (22.2%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified. |
Graff‐Radford 2000.
| Study characteristics | ||
| Methods | Design: parallel Duration: 8 weeks Assessment: baseline to post‐intervention Country: USA |
|
| Participants | Pain condition: post‐herpetic neuralgia Population: adults with post‐herpetic neuralgia Minimum pain intensity: no Inclusion criteria
Exclusion criteria: NR Total participants randomised: 50 Age in years (mean, SD): 72.9 (10.1) Gender: 22/50 were female Pain duration in months (mean, SD): 33.4 (29.5) |
|
| Interventions | Amitriptyline ≤ 200 mg
Amitriptyline ≤ 200 mg + fluphenazine ≤ 3 mg
Fluphenazine ≤ 3 mg
Placebo (glycopyrrolate)
|
|
| Outcomes | Pain intensity Mood Withdrawal |
|
| Missing data methods | Completer analysis only | |
| Funding source | Non‐pharmaceutical: "This study was supported by a grant from the National Institute of Health/National Institute of Dental Research (1RO3DE10086‐01)" | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical appearing study drugs, double‐dummy design |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Completer analysis but only 1 person withdrew Attrition Total: 1/50 (2.0%) Amitriptyline 12.5‐200 mg: 1/12 (8.3%) Amitriptyline 12.5‐200 mg + fluphenazine 1‐3 mg: 0/12 (0.0%) Fluphenazine 1‐3 mg: 0/13 (0.0%) Placebo: 0/13 (0.0%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified. |
Hadianfard 2012.
| Study characteristics | ||
| Methods | Design: parallel Duration: 8 weeks Assessment: baseline, 2 weeks, 4 weeks, post‐intervention, follow‐up (10 months post‐intervention) Country: Iran |
|
| Participants | Pain condition: fibromyalgia Population: women ≤ 65 with fibromyalgia Minimum pain intensity: ≥ 4 on 0‐10 VAS Inclusion criteria
Exclusion criteria
Total participants randomised: 30 Age in years (mean): 44 Gender: 30/30 were female Pain duration in months (mean): 81.2 |
|
| Interventions | Acupuncture
Fluoxetine 20 mg
|
|
| Outcomes | Pain intensity Quality of life Mood Physical function |
|
| Missing data methods | ITT but methods not specified | |
| Funding source | Non‐pharmaceutical: Shiraz University of Medical Sciences research project No. 88‐5035 | |
| Conflicts of interest | "We declare no conflict of interest. This article is from Shiraz University of Medical Sciences research project No. 88‐5035" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using a computer‐generated random sequence of the numbers |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. Lead author was the acupuncturist |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self‐reported outcomes from unblinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | States ITT but no imputation methods reported Attrition NR |
| Selective reporting (reporting bias) | Unclear risk | Protocol registered retrospectively |
| Other bias | Low risk | No other sources of bias were identified. |
Hameroff 1984.
| Study characteristics | ||
| Methods | Design: parallel Duration: 6 weeks Assessment: baseline and post‐intervention Country: USA |
|
| Participants | Pain condition: chronic cervical and/or lumbar spine pain Population: adults with chronic cervical and/or lumbar spine pain and depression Minimum pain intensity: no Inclusion criteria
Exclusion criteria: NR Total participants randomised: 60 Age in years (mean): 48.7 Gender: 28/60 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Placebo
Doxepin ≤ 300 mg
|
|
| Outcomes | Withdrawal | |
| Missing data methods | NR | |
| Funding source | NR | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information, just says "patients were randomly assigned to one of two treatment groups" |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Says double‐blind but no information regarding procedures |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported outcomes from participants, but unsure of blinding procedures |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only report completer analysis. 50% more dropout in placebo arm than intervention Attrition Total: 9/60 (15.0%) Placebo: 6/30 (20.0%) Doxepin ≤ 300 mg: 3/30 (10.0%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified |
Hammody 2015.
| Study characteristics | ||
| Methods | Design: parallel Duration: 12 weeks Assessment: baseline, week 4, week 8, post‐intervention Country: Iraq |
|
| Participants | Pain condition: fibromyalgia Population: adults with fibromyalgia Minimum pain intensity: no Inclusion criteria
Exclusion criteria
Total participants randomised: 123 Age in years (mean, SD): NR Gender: NR Pain duration in years (mean, SD): NR |
|
| Interventions | Pregabalin 75 mg
Amitriptyline 25 mg
|
|
| Outcomes | Pain intensity Quality of life |
|
| Missing data methods | NR | |
| Funding source | NR | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | States double‐blind, but no information regarding blinding procedures |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported outcomes from participants, but unsure of blinding procedures |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High attrition, analysis of per‐protocol population Attrition Total: 45/123 (36.6%) Attrition per arm NR |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | High risk | Poorly reported ‐ mistakes throughout document, figures not really adding up and tables wrongly titled |
Hannonen 1998.
| Study characteristics | ||
| Methods | Design: parallel Duration: 12 weeks Assessment: baseline and post‐intervention Country: Finland |
|
| Participants | Pain condition: fibromyalgia Population: women with fibromyalgia Minimum pain intensity: ≥ 4 on 0‐10 VAS Inclusion criteria
Exclusion criteria
Total participants randomised: 130 Age in years (mean): 48.7 Gender: 130/130 were female Pain duration in years (mean): 8.2 |
|
| Interventions | Placebo
Moclobemide 450‐600 mg
Amitriptyline 25‐37.5 mg
|
|
| Outcomes | Pain intensity Sleep Mood Physical function AEs Withdrawal |
|
| Missing data methods | States ITT but no methods reported | |
| Funding source | Partly supported by pharmaceutical: "The financial support by Roche Oy, Finland, is gratefully acknowledged." | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods NR |
| Allocation concealment (selection bias) | Low risk | The randomisation was organised centrally with sequentially numbered envelopes consisting of blocks of 6 |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded, study drugs were identical, double‐dummy design |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes by blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | States ITT but no methods reported Attrition Total: 38/130 (29.2%) Moclobemide 450‐600 mg: 13/43 (30.2%) Amitriptyline 25‐37.5 mg: 10/42 (23.8%) Placebo: 15/45 (33.3%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias identified |
Heymann 2001.
| Study characteristics | ||
| Methods | Design: parallel Duration: 8 weeks Assessment: baseline and post‐intervention Country: Brazil |
|
| Participants | Pain condition: fibromyalgia Population: women with fibromyalgia Minimum pain intensity: no Inclusion criteria
Exclusion criteria
Total participants randomised: 118 Age in years (mean): 50.5 Gender: 118/118 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Placebo
Amitriptyline 25 mg
Nortriptyline 25 mg
|
|
| Outcomes | Quality of life SAEs Withdrawal |
|
| Missing data methods | Completer analysis | |
| Funding source | NR | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using random number tables |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures NR |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, all study drugs were identical in appearance and packaging |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Much higher attrition in the placebo group than intervention groups. No missing data methods; report only completer analysis Attrition Total: 12/118 (10.2%) Amitriptyline 25 mg: 3/40 (7.5%) Nortriptyline 25 mg: 2/38 (5.3%) Placebo: 7/40 (17.5%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified |
Holbech 2015.
| Study characteristics | ||
| Methods | Design: cross‐over Duration: 5 weeks Assessment: baseline and post‐intervention Country: Denmark |
|
| Participants | Pain condition: polyneuropathy Population: adults with polyneuropathy Minimum pain intensity: ≥ 4 on 0‐10 VAS Inclusion criteria
Exclusion criteria
Total participants randomised: 73 Age in years (mean, range): 59.3 (29‐82) Gender: 28/73 were female Pain duration in months (mean, range): 63.5 (9 ‐24) |
|
| Interventions | Placebo
Pregabalin 300 mg
Imipramine 75 mg
Pregabalin 300 mg + imipramine 75 mg
|
|
| Outcomes | Moderate pain relief Substantial pain relief AEs Withdrawal |
|
| Missing data methods | ITT with LOCF and per‐protocol analysis | |
| Funding source | Partly funded by pharmaceutical: "This was an investigator‐initiated trial supported by Pfizer with a grant of USD 52080 (grant no: WS368802). The trial was also supported by a grant from Odense University Hospital." | |
| Conflicts of interest | F. W. Bach reports to have been compensated as an Investigator in clinical trials on neuropathic pain sponsored by Pfizer and Grunenthal. N. B. Finnerup reports personal fees from Pfizer, grants and personal fees from Grunenthal, personal fees from Astellas, personal fees from Norpharma, grants from EU/EFPIA; outside the submitted work. T. S. Jensen reports to be on Advisory Board for Pfizer, Grunenthal, and Orion. The other authors have no conflicts of interest to declare. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised through a computer‐generated randomisation list. |
| Allocation concealment (selection bias) | Low risk | Participants were allocated using sealed, opaque envelopes containing the treatment sequence. The randomisation plan was generated by a person at the hospital pharmacy at Odense University Hospital, who was not otherwise involved in the conduct of the trial. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical appearing study drugs, double‐dummy design |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT with LOCF Attrition Total: 23/70 (32.9%) Placebo: 5/73 (6.9%) Pregabalin 300 mg: 5/73 (6.9%) Imipramine 75 mg: 4.73 (5.5%) Pregabalin 300 mg + imipramine 75 mg: 9/73 (13.0%) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported prospectively on clinicaltrials.gov |
| Other bias | Low risk | No other sources of bias were identified |
Hudson 2021.
| Study characteristics | ||
| Methods | Design: parallel Duration: 14 weeks Assessment: baseline and post‐intervention Country: New Zealand |
|
| Participants | Pain condition: knee OA Population: people with knee OA on a stable analgesic regime Minimum pain intensity: ≥ 20 out of 50 on WOMAC pain subscale Inclusion criteria
Exclusion criteria
Total participants randomised: 205 Age in years (mean): 64.5 Gender: 87/205 were female Pain duration in years (mean): 7.6 |
|
| Interventions | Placebo
Nortriptyline ≤ 100 mg
|
|
| Outcomes | Pain intensity Physical function Mood AEs SAE Withdrawal |
|
| Missing data methods | Imputation using multivariate normal multiple imputation | |
| Funding source | Non‐pharmaceutical: project grant from the Health Research Council of New Zealand (reference number: 14/152). | |
| Conflicts of interest | The authors have declared no competing interests | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised with a 1:1 allocation, computer‐generated randomisation list with blocks of varying size (1‐4) was prepared by the study statistician (https://cran.r‐project. org/web/packages/blockrand/index.html). |
| Allocation concealment (selection bias) | Low risk | The contracted pharmacist will determine which group of participants, A or B, will be allocated to receive nortriptyline. The study medication (nortriptyline or identical placebo) will be packaged in identical containers. Each container will be pre‐labelled (by the study pharmacist contracted to provide the study medication) with a study identifier according to randomisation schedule. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identically appearing study drugs, matched dosing schedules |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Very low dropout with no data collection at follow‐ up (4/205). Multiple imputation for missing data Attrition Total: 4/205 (2.0%) Placebo: 1/103 (1.0%) Nortriptyline 25‐100 mg: 3/102 (2.9%) |
| Selective reporting (reporting bias) | Low risk | Published trial protocol: https://trialsjournal.biomedcentral.com/track/pdf/10.1186/s13063‐015‐0961‐1.pdf. All outcomes reported or reasons for no further analysis given. Although there was an error collecting data at baseline for the first 24 participants, this was reported and accounted for in the analysis. |
| Other bias | Low risk | No other sources of bias were identified. |
Hussain 2011.
| Study characteristics | ||
| Methods | Design: parallel Duration: 8 weeks Assessment: baseline and post‐intervention Country: Iraq |
|
| Participants | Pain condition: fibromyalgia Population: people aged between 18‐65 with early diagnosed fibromyalgia Minimum pain intensity: no Inclusion criteria
Exclusion criteria
Total participants randomised: 101 Age in years (mean, SD): 38.8 Gender: 95/101 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Melatonin 5 mg + placebo
Fluoxetine 20 mg + placebo
Fluoxetine 20 mg + melatonin 3 mg
Fluoxetine 20 mg + melatonin 5 mg
|
|
| Outcomes | Pain intensity Quality of life Mood Physical function |
|
| Missing data methods | NR | |
| Funding source | Non‐pharmaceutical: the present data were abstracted from PhD theses submitted to the Department of Pharmacology and Toxicology, College of Pharmacy, University of Baghdad. "The authors gratefully thank the College of Pharmacy for supporting the project." | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐dummy dosing used, but no information given regarding appearance of capsules. Also no information regarding AEs given. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported outcomes from participants, but uncertain of blinding procedures |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on withdrawal. No missing data methods reported |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified. |
Isomeri 1993.
| Study characteristics | ||
| Methods | Design: parallel Duration: 12 weeks Assessment: baseline and post‐intervention Country: Finland |
|
| Participants | Pain condition: fibromyalgia Population: people with fibromyalgia Minimum pain intensity: no Inclusion criteria
Exclusion criteria
Total participants randomised: 51 Age in years (mean): 43.7 Gender: 39/51 were female Pain duration in years (mean): 7.9 |
|
| Interventions | Physiotherapy and amitriptyline 25 mg
Physical fitness training
Physical fitness training and amitriptyline 25 mg
|
|
| Outcomes | Withdrawal | |
| Missing data methods | NR | |
| Funding source | Non‐pharmaceutical: supported by grants from the Rheumatism Research Foundation | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods NR |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures NR |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not able to be double‐blinded due to nature of interventions. Doesn't mention sham dosing or placebo for group not receiving amitriptyline |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self‐reported outcomes from unblinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer‐only analysis, no information given about withdrawal reasons Attrition Total: 6/51 (11.8%) Physiotherapy + amitriptyline 25 mg: 1/17 (5.9%) Physical fitness training: 2/17 (11.8%) Physical fitness training + amitriptyline 25 mg: 3/17 (17.7%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified. |
Iwaki 2020.
| Study characteristics | ||
| Methods | Design: parallel Duration: 12 weeks Assessment: baseline and post‐intervention Country: Japan |
|
| Participants | Pain condition: pain in Parkinson's disease Population: adults with Parkinson's disease experiencing associated pain Minimum pain intensity: no Inclusion criteria
Exclusion criteria
Total participants randomised: 47 Age in years (mean): 68.0 Gender: 25/47 were female Pain duration in years (mean): 2.3 |
|
| Interventions | Placebo
Duloxetine 40 mg
|
|
| Outcomes | Pain intensity Mood SAEs Withdrawal |
|
| Missing data methods | Not specified | |
| Funding source | Pharmaceutical: funding was provided by Ehime University under a contract with Shionogi & Co. Ltd (pharmaceutical company). | |
| Conflicts of interest | The authors have no COI to report | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods NR |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | States double‐blinded but no information on appearance of study drugs |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported outcomes from participants, but uncertain of blinding procedures |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Much higher attrition in antidepressant arm than placebo. Unsure of imputation methods used Attrition Total: 9/46 (19.6%) Placebo: 2/23 (8.7%) Duloxetine 40 mg: 7/23 (30.4%) |
| Selective reporting (reporting bias) | Low risk | Outcomes match those in protocol |
| Other bias | Low risk | No other sources of bias were identified |
Johansson 1979.
| Study characteristics | ||
| Methods | Design: parallel Duration: 4 weeks Assessment: baseline and post‐intervention Country: Sweden |
|
| Participants | Pain condition: chronic pain conditions Population: people hospitalised at the Department of Neurology, University of Umeå with chronic pain syndromes Minimum pain intensity: no Inclusion criteria
Exclusion criteria: NR Total participants randomised: 40 Age in years (range): 25‐65 Gender: 23/40 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Placebo
Zimelidine 200 mg
|
|
| Outcomes | Pain intensity Withdrawal |
|
| Missing data methods | Completer analysis | |
| Funding source | NR | |
| Conflicts of interest | NR | |
| Notes | Zimelidine has been banned worldwide due to serious, sometimes fatal, cases of central and/or peripheral neuropathy known as Guillain‐Barré syndrome and due to a peculiar hypersensitivity reaction involving many organs including skin exanthema, flu‐like symptoms, arthralgias, and sometimes eosinophilia. Additionally, zimelidine was found to cause an increase in suicidal ideation and/or attempts among depressive patients. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were then according to a randomisation list given tablets of identical form, color and taste, containing either Zimelidine 25 mg or a placebo according to a fixed dose regimen" |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, matched dosing and appearance of study drugs |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | HIgh attrition in Zimeldine arm. Reported compeleter analysis only, with no missing data methods Attrition Total: 8/20 (40.0%) Placebo: 3/11 (27.3%) Zimeldine 200 mg: 5/9 (55.6%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | No other sources of bias were identified. |
Joharchi 2019.
| Study characteristics | ||
| Methods | Design: parallel Duration: 12 weeks Assessment: baseline, 4 weeks, 8 weeks, post‐intervention Country: Iran |
|
| Participants | Pain condition: diabetic peripheral neuropathy Population: type 2 diabetic adults aged ≥ 40 and ≤ 65 with diabetic peripheral neuropathic pain Minimum pain intensity: ≥ 40 on 0‐100 VAS Inclusion criteria
Exclusion criteria
Total participants randomised: 180 Age in years (mean): 54.48 Gender: 109/180 were female Pain duration in years (mean): 3.8 |
|
| Interventions | Duloxetine 30‐60 mg
Pregabalin 150‐300 mg
|
|
| Outcomes | Pain intensity AEs Withdrawal |
|
| Missing data methods | Completer analysis | |
| Funding source | Non‐pharmaceutical: part of a PhD project ‐ financially supported by “Research Department of theSchool of Medicine Shahid Beheshti University of Medical Sciences(SBUMS)” (Grant No 13/587). | |
| Conflicts of interest | The authors declare that they have no COI. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not specified: Just states "randomly divided into 2 groups" |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Used "similar" capsules but participants in pregabalin arm took 2 capsules a day compared to 1 a day for duloxetine |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported outcomes from blinded participants, unsure of blinding procedures |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Higher attirition in duloxetine group than pregabalin, completer analysis only Attrition Total: 36/180 (20.0%) Duloxetine 30‐60 mg: 24/90 (26.7%) Pregabalin 150‐300 mg: 12/90 (13.3%) |
| Selective reporting (reporting bias) | Low risk | Protocol registered prospectively to study with outcome measures |
| Other bias | Low risk | No other sources of bias were identified. |
Jose 2007.
| Study characteristics | ||
| Methods | Design: cross‐over Duration: 6 weeks Assessment: baseline, 2 weeks, 4 weeks, post‐intervention Country: India |
|
| Participants | Pain condition: diabetic peripheral neuropathy Population: adults with type 2 diabetes and diabetic peripheral neuropathy Minimum pain intensity: ≥ 50 on 0‐100 scale Inclusion criteria
Exclusion criteria
Total participants randomised: 75 Age in years (median, range): 56 (50‐62) Gender: 30/75 were female Pain duration in years (median, range): 12 (4‐24) |
|
| Interventions | Lamotrigine 50‐200 mg
Amitriptyline 10‐50 mg
|
|
| Outcomes | Pain intensity AEs Withdrawal |
|
| Missing data methods | ITT with LOCF | |
| Funding source | NR | |
| Conflicts of interest | None declared | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using random number tables by block randomisation |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedure unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, study drugs appeared identical and matched dosing schedules |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Use ITT and LOCF. Unequal attrition across arms ‐ 100% of participants who completely dropped out did so from the 1st period in one intervention arm. Attrition Total: 7/53 (13.2%) Lamotrigine 50‐200 mg: 0/53 (0.0%) Amitriptyline 10‐50 mg: 7/53 (13.2%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified. |
Kalso 1996.
| Study characteristics | ||
| Methods | Design: cross‐over Duration: 4 weeks Assessment: baseline, 2 weeks, post‐intervention Country: Finland |
|
| Participants | Pain condition: cancer‐related neuropathic pain Population: women with chronic neuropathic pain following treatment for breast cancer Minimum pain intensity: no Inclusion criteria
Exclusion criteria: NR Total participants randomised: 20 Age in years (median, range): 56 (39‐72) Gender: 20/20 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Placebo
Amitriptyline 100 mg
|
|
| Outcomes | The article reported no useable data | |
| Missing data methods | Completer‐only analysis | |
| Funding source | Non‐pharmaceutical: The study was supported by the Academy of Finland (E.K., T.T.), the Paulo Foundation, Finland (E.K.) and the Centre for International Mobility (T.T.). | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedure not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | States double‐blind and matched dosing but doesn't specify other blinding procedures |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported outcomes from participants, but unsure of blinding procedures |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer‐only analysis. Withdrawal information doesn't specify in which period the participants withdrew Attrition Total: 5/20 (25.0%) Attrition per arm NR |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified. |
Katz 2005.
| Study characteristics | ||
| Methods | Design: cross‐over Duration: 7 weeks Assessment: baseline and post‐intervention Country: USA |
|
| Participants | Pain condition: low back pain Population: adults with chronic low back pain Minimum pain intensity: no Inclusion criteria
Exclusion criteria
Total participants randomised: 54 Age in years (mean, SD): 50.6 (10.7) Gender: 26/54 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Placebo
Bupropion 300 mg
|
|
| Outcomes | Pain intensity SAEs Withdrawal |
|
| Missing data methods | ITT with LOCF | |
| Funding source | Partly funded by pharmaceutical: "Supported in part by an investigator‐initiated research grant from GlaxoSmithKline" | |
| Conflicts of interest | Supported in part by an investigator‐initiated research grant from GlaxoSmithKline to R.H.D., who has also received research support, consulting fees, or lecture honoraria in the past year from Abbott Laboratories, Eli Lilly & Co., Endo Pharmaceuticals, EpiCept Corporation, NeurogesX, Novartis Pharmaceuticals, Organon, Ortho‐McNeil Pharmaceutical, Pfizer, Purdue Pharma, Ranbaxy Corporation, Reliant Pharmaceuticals, Renovis, and UCB Pharma. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using a computer‐generated list of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures NR |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | States double‐blind and used same dosing for placebo as intervention but no information given regarding other blinding procedures |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported outcomes from participants but uncertain of blinding procedures |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT with LOCF Attrition Total: 14/54 (25.9%) Placebo: 5/54 (9.3%) Bupropion 300 mg: 9/54 (16.7%) |
| Selective reporting (reporting bias) | High risk | "Several other health‐related quality‐of‐life measures of physical and emotional functioning were administered, but these data were not analyzed because of the absence of significant beneficial effects on the pain intensity and relief outcome measures." |
| Other bias | Unclear risk | Participants tapering off of bupropion reported having AEs from reducing the medication, which could have lasted the washout period, but this is not explored further in the article. |
Kaur 2011.
| Study characteristics | ||
| Methods | Design: cross‐over Duration: 6 weeks Assessment: baseline and post‐intervention Country: India |
|
| Participants | Pain condition: diabetic peripheral neuropathy Population: adults with type 2 diabetes and diabetic peripheral neuropathy Minimum pain intensity: ≥ 50 on 0‐100 VAS Inclusion criteria
Exclusion criteria
Total participants randomised: 65 Age in years (median, IQR): 52.5 (48.2–62) Gender: 31/65 were female Pain duration in years (median IQR): 8 (6–36) |
|
| Interventions | Amitriptyline 10‐50 mg
Duloxetine 20‐60 mg
|
|
| Outcomes | Moderate pain relief Substantial pain relief |
|
| Missing data methods | Unclear regarding methods | |
| Funding source | NR | |
| Conflicts of interest | No potential conflicts of interest relevant to this study were reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using computer‐generated randomisation of blocks of 4 |
| Allocation concealment (selection bias) | Low risk | The drug packets were administered to patients serially according to the patient’s reporting sequence. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Says double‐blind but no information given regarding procedure e.g. appearance of tablets |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported outcomes by participants but not enough information given regarding blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear reporting of withdrawals and analysis. State ITT analysis but only including those 58 who completed the study Attrition Total: 7/65 (10.8%) Attrition per arm NR |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified. |
Kayiran 2010.
| Study characteristics | ||
| Methods | Design: parallel Duration: 4 or 8 weeks Assessment: baseline, 2 weeks (mid‐intervention), 4 weeks (post‐intervention for neurofeedback), 8 weeks (post‐intervention for escitalopram), week 16 (follow‐up), week 24 (follow‐up) Country: Turkey |
|
| Participants | Pain condition: fibromyalgia Population: women aged 16‐49 with fibromyalgia Minimum pain intensity: no Inclusion criteria
Exclusion criteria
Total participants randomised: 40 Age in years (mean): 32.1 Gender: 40/40 were female Pain duration in years (mean): 4.8 |
|
| Interventions | Neurofeedback
Escitalopram 10 mg
|
|
| Outcomes | Pain Mood Withdrawal |
|
| Missing data methods | NR | |
| Funding source | NR | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods NR |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures NR |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Can't be double‐blind due to neurofeedback intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self‐reported outcomes but participants weren't blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Methods NR but low attrition Attrition Total: 4/40 (10.0%) Neurofeedback: 2/20 (10.0%) Esciptalopram 10 mg: 2/20 (10.0%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified |
Keefe 2011.
| Study characteristics | ||
| Methods | Design: parallel Duration: 34 weeks Assessment: baseline, 10 weeks, post‐intervention Country: USA |
|
| Participants | Pain condition: non‐cardiac chest pain Population: people who had presented to medical care with complaints of non‐cardiac chest pain Minimum pain intensity: no Inclusion criteria
Exclusion criteria
Total participants randomised: 115 Age in years (mean, SD): 48 (12) Gender: 77/115 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Placebo
Sertraline ≤ 200 mg
Coping skills training + placebo
Sertraline ≤ 200 mg + coping skills training
|
|
| Outcomes | Pain intensity Mood Physical function AEs SAEs Withdrawal |
|
| Missing data methods | ITT but no method specified | |
| Funding source | Non‐pharmaceutical: "This study was supported by a grant from NIMH (R01 MH63429)" | |
| Conflicts of interest | The authors on this manuscript report no COI. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using a randomisation table |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedure NR |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to be double‐blind across all arms due to the nature of coping skills training intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self‐reported outcomes from unblinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | More participants withdrew from the coping skills training+sertraline arm than other arms. Did not report missing data methods Attrition Total: Placebo: 6/28 (21.4%) Sertraline ≤ 200 mg: 5/30 (16.7%) Coping skills training: 8/29 (28.0%) Coping skills training + sertraline ≤ 200 mg: 12/28 (42.9%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified |
Khoromi 2007.
| Study characteristics | ||
| Methods | Design: cross‐over Duration: 9 weeks Assessment: baseline and post‐intervention Country: USA |
|
| Participants | Pain condition: chronic lumbar root pain Population: people with lumbar radiculopathy Minimum pain intensity: ≥ 4 on 0‐10 VAS Inclusion criteria
Exclusion criteria
Total participants randomised: 55 Age in years (median, range): 53 (19‐65) Gender: 25/55 were female Pain duration in years (median, range): 5 (0.3‐37) |
|
| Interventions | Placebo (benzotropine ≤ 1 mg)
Morphine ≥ 15 and ≤ 90 mg
Nortriptyline ≥ 25 and ≤ 100 mg
Morphine ≥ 15 and ≤ 90 mg + nortriptyline ≥ 25 and ≤ 100 mg
|
|
| Outcomes | Pain intensity Mood Physical function AEs Withdrawal |
|
| Missing data methods | Completer‐only analysis | |
| Funding source | Non‐pharmaceutical: "This study was supported by an intramural grant from the National Institute of Dental and Craniofacial Research." | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned by random numbers within blocks of four to 1 of 4 treatment sequences specified by a Latin square. |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures NR |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical appearing study drugs, sham dosing |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer‐only analysis. High attrition overall Attrition Total: 27/55 (49.1%) Placebo: 9/55 (16.4%) Morphine 15‐90 mg: 9/55 (16.4%) Nortriptyline 25‐100 mg: 3/55 (5.5%) Morphine 15‐90 mg + nortriptyline 25‐100 mg: 6/55 (10.9%) |
| Selective reporting (reporting bias) | Low risk | All outcomes registered prospectively on clinicaltrials.gov |
| Other bias | Low risk | No other sources of bias were identified. |
Kim 2013.
| Study characteristics | ||
| Methods | Design: cross‐over Duration: 6 weeks Assessment: baseline and post‐intervention Country: USA |
|
| Participants | Pain condition: fibromyalgia Population: adults with fibromyalgia Minimum pain intensity: no Inclusion criteria
Exclusion criteria
Total participants randomised: 20 Age in years (mean, SD): 47.6 (9.1) Gender: 18/20 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Placebo
Milnacipran 12.5‐200 mg
|
|
| Outcomes | AEs SAEs |
|
| Missing data methods | ITT with LOCF | |
| Funding source | Pharmaceutical: "This study was supported by Forest Laboratories through an Investigator‐Initiated Award." | |
| Conflicts of interest | "Dr Marks has served as a consultant to Forest, Dey, Gilead, and TTK; has received grant/research support from Bristol‐Myers Squibb, Dov, Eli Lilly, Endo, GlaxoSmithKline, Janssen, Johnson & Johnson, Pfizer, Saegis, Sepracor, and Somaxon; and has served on the speakers or advisory boards of Alkermes, Bristol‐Myers Squibb, Dey, Pfizer, and Sunovion. Dr Masand has served as a consultant to Forest, Lundsbeck, Merck, Pfizer, and Sunovion; has received grant/research support from Forest; has received honoraria from or served on the speakers or advisory boards of Forest, GlaxoSmithKline, Merck, Pfizer, and Sunovion; and is a stock shareholder in Global Medical Education. Dr Millet has received grant/research support from Forest. Dr Keefe has served as a consultant to Abbvie, Akebia, Amgen, Asubio, BiolineRx, Biomarin, Boehringer‐Ingelheim, Eli Lilly, EnVivo, Lundbeck, Merck, Mitsubishi, Novartis, Otsuka, Pfizer, Roche, Shire, Sunovion, Takeda, and Targacept; has received grant/research support from Feinstein Institute for Medical Research, GlaxoSmithKline, National Institute of Mental Health, PsychoGenics, Research Foundation for Mental Hygiene, and Singapore Medical Research Council; is a stock shareholder in NeuroCog Trials; and has received royalties from the Brief Assessment of Cognition in Schizophrenia (BACS) and MATRICS Battery (BACS Symbol Coding). Dr Patkar has served as a consultant to Dey, Forest, Gilead, and TTK; has received grant/research support from Dey, Duke Endowment, Envivo, Forest, Janssen, Lundbeck, National Institutes of Health (National Institute on Drug Abuse/National Institute on Alcohol Abuse and Alcoholism), Pfizer, Shire, Sunovion, and Titan; and has served on the speakers or advisory boards of Alkermes, BristolMyers Squibb, Dey, Pfizer, and Sunovion. Dr Kim and Mss Rele and Yerramsetty report no conflicts of interest related to the subject of this article. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Says double‐blinded but no specific information given regarding identical medication |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported outcomes by participants but not enough information regarding blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Authors state that the same 20 participants completed both phases of study (20) but LOCF numbers are 31. Not clear about ITT, imputation or handling of missing data Attrition Not clearly reported, unable to establish total attrition and attrition per arm |
| Selective reporting (reporting bias) | Unclear risk | Protocol lists pain, fatigue and cognition prospectively but doesn't mention any of the secondary measures. A lot of missing outcomes |
| Other bias | Unclear risk | Data NR in numerical forms ‐ all secondary outcomes are classified e.g. "transient change" which has no interpretation |
Konno 2016.
| Study characteristics | ||
| Methods | Design: parallel Duration: 14 weeks Assessment: baseline and post‐intervention Country: Japan |
|
| Participants | Pain condition: low back pain Population: adults aged 20‐80 with chronic low back pain Minimum pain intensity: ≥ 4 on 0‐10 scale Inclusion criteria
Exclusion criteria
Total participants randomised: 458 Age in years (mean): 58.9 Gender: 237/458 were female Pain duration in years (mean): 10.1 |
|
| Interventions | Placebo
Duloxetine 60 mg
|
|
| Outcomes | Pain intensity Sleep Quality of life Physical function Mood Moderate pain relief Substantial pain relief PGIC AEs SAEs Withdrawal |
|
| Missing data methods | MMRM and LOCF, BOCF as sensitivity analysis for pain | |
| Funding source | Pharmaceutical: Shionogi & Co. Ltd., Eli Lilly Japan K.K., and Eli Lilly and Company funds were received in support of this work. | |
| Conflicts of interest | Relevant financial activities outside the submitted work: consultancy, employment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using a stochastic minimisation procedure |
| Allocation concealment (selection bias) | Low risk | An "investigator in charge of blinding" randomly assigned participants to a treatment arm based on an assignment table. This assignment table was sealed and was inaccessible to all parties until after the clinical report was finalised. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical study drugs and matched dosing |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes by blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition rates. Mixture of analyses for primary outcome including MMRM, LOCF and BOCF. Results were the same across all missing data analyses. Attrition Total: 49/458 (10.7%) Placebo: 26/226 (11.5%) Duloxetine 60 mg: 23/232 (9.9%) |
| Selective reporting (reporting bias) | Low risk | All outcomes were prospectively registered on clinicaltrials.gov |
| Other bias | Low risk | No other sources of bias were identified |
Lee 2010.
| Study characteristics | ||
| Methods | Design: cross‐over Duration: 4 weeks Assessment: baseline and post‐intervention Country: South Korea |
|
| Participants | Pain condition: functional chest pain Population: adults aged 20‐29 with functional chest pain Minimum pain intensity: no Inclusion criteria
Exclusion criteria
Total participants randomised: 50 Age in years (mean, SD): 23.5 (1.9) Gender: 6/50 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Placebo
Venlafaxine 75 mg
|
|
| Outcomes | Pain intensity Mood Physical function AEs Withdrawal |
|
| Missing data methods | Unclear | |
| Funding source | Not financially supported | |
| Conflicts of interest | No potential competing interests | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using a computer‐generated random list |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed using a sealed opaque envelope technique |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, study drugs had identical appearance and matched dosage |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Completer analysis but low attrition Attrition Total: 4/25 (16.0%) Placebo: 3/25 (12.0%) Venlafaxine 75 mg: 1/25 (4.0%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified. |
Lee 2016.
| Study characteristics | ||
| Methods | Design: cross‐over Duration: 6 weeks Assessment: baseline and post‐intervention Country: USA |
|
| Participants | Pain condition: RA Population: adults aged ≥ 24 with RA in ≥ 5 body pain sites Minimum pain intensity: ≥ 4 on the BPI short form, ≥ 5 on the Regional Pain Scale Inclusion criteria
Exclusion criteria
Total participants randomised: 43 Age in years (mean): 54.0 Gender: 25/43 were female Pain duration in years (mean, SD): 11.29 |
|
| Interventions | Placebo:
Milnacipran 100 mg
|
|
| Outcomes | Pain intensity Moderate pain relief AEs SAEs Withdrawal |
|
| Missing data methods | ITT with LOCF | |
| Funding source | Pharmaceutical: "This work was conducted with support from Forest Research Institute, NIH‐NIAMS K23AR057578, NIH‐NIAMS K24 AR055989, Harvard Catalyst" | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised via a random number generator, with 4 participants per block. |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedure not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, study drugs had identical appearance and matched dosing |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Says they will use ITT and LOCF, but only report results from completers Attrition Total: 9/41 (22.0%) Placebo: 3/41 (7.3%) Milnacipran 100 mg: 6/41 (14.6%) |
| Selective reporting (reporting bias) | High risk | Outcomes stated in the methods section of the paper are NR. Protocol changed on clinicaltrials.gov to remove some outcomes |
| Other bias | Low risk | No other sources of bias were identified. |
Leijon 1989.
| Study characteristics | ||
| Methods | Design: cross‐over Duration: 4 weeks Assessment: baseline, week 1, week 2, week 3, post‐intervention Country: Sweden |
|
| Participants | Pain condition: central post‐stroke pain Population: adults with central post‐stroke pain Minimum pain intensity: no Inclusion criteria
Exclusion criteria:
Total participants randomised: 15 Age in years (mean, range): 66 (53‐74) Gender: 3/15 were female Pain duration in months (mean, range): 54 (11‐154) |
|
| Interventions | Placebo
Amitriptyline 75 mg
Carbamazepine 800 mg
|
|
| Outcomes | Pain intensity PGIC AEs SAEs Withdrawal |
|
| Missing data methods | NR | |
| Funding source | Non‐pharmaceutical: "The study was supported by grants from the County Council of Ostergotland and the Swedish Association of the Neurologically Disabled" | |
| Conflicts of interest | None reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information given apart from "randomised" |
| Allocation concealment (selection bias) | Low risk | Separate pharmacy team performed randomisation and allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, study drugs had identical appearance and matched dosing. Investigators were also blinded ‐ separate neurologists were consulted for side‐effects. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data methods, but only 1 person withdrew Attrition Total: 1/15 (6.7%) Placebo: 0/15 (0.0%) Amitriptyline 75 mg: 0/15 (0.0%) Carbamazepine 800 mg: 1/14 (7.1%) |
| Selective reporting (reporting bias) | Low risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified. |
Lipone 2020.
| Study characteristics | ||
| Methods | Design: parallel Duration: 8 weeks Assessment: baseline and post‐intervention Country: Czech Republic, Hungary, and Poland |
|
| Participants | Pain condition: painful diabetic neuropathy Population: people with painful diabetic neuropathy Minimum pain intensity: ≥ 4 on 0‐10 scale Inclusion criteria
Exclusion criteria
Total participants randomised: 142 Age in years (mean): 62.7 Gender: 68/142 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Placebo + gabapentin 2400 mg
Trazodone 30 mg + gabapentin 2400 mg
Trazodone 60 mg + gabapentin 2400 mg
|
|
| Outcomes | Pain intensity Substantial pain relief AEs SAEs Withdrawal |
|
| Missing data methods | ITT with LOCF | |
| Funding source | Pharmaceutical: "This study was sponsored by Angelini Pharma S.p.A. (S. Palomba, Pomezia, Rome, Italy)." | |
| Conflicts of interest | Giorgio Cruccu received personal fees for advisory boards or consultancy from Angelini, Grunenthal, and Lilly, and personal fees for educational activity by PTS Global Services. Andrea Truini received honoraria for speaking at symposia or research financial supports from Alpha‐Sigma, Angelini Pharma, Epitech, FB Health, Pfizer, and Grunenthal. Edvard Ehler has no conflicts of interest that are directly relevant to the content of this study; however, his institution received a fee for conducting the clinical trial from Angelini Pharma S.p.A. Marcin Nastaj and Ilona Palka‐Kisielowska received principal investigator fees from Angelini Pharma S.p.A. Fabrizio Calisti, Agnese Cattaneo, Alessandro Comandini, Alessandra Del Vecchio, Giorgio Di Loreto, Paola Lipone, and Ilena Pochiero are full‐time employees of Angelini Pharma S.p.A. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised in a 1:1:1 ratio to the 3 parallel groups, based on a computer‐generated sequence |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures NR |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Double‐blinding was maintained throughout all treatment periods by using a TRZ [trazodone] solution matching PLB [placebo] solution and the same dosing regimen for all groups in terms of timing and number of drops." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes by blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT with LOCF Attrition Total: 38/142 (26.8%) Gabapentin 2400 mg: 13/48 (27.1%) Trazodone 30 mg + gabapentin 2400 mg: 10/43 (23.3%) Trazodone 60 mg + gabapentin 2400 mg: 15/51 (29.4%) |
| Selective reporting (reporting bias) | Unclear risk | Do not report a lot of the secondary outcomes clearly, the baseline or the post‐intervention, these are also NR in the trial registry |
| Other bias | Low risk | No other sources of bias were identified. |
Loldrup 1989.
| Study characteristics | ||
| Methods | Design: parallel Duration: 6 weeks Assessment: baseline and post‐intervention Country: Denmark |
|
| Participants | Pain condition: idiopathic pain syndromes: (a) tension headache, (b) burning mouth syndrome (oral dysaesthesia), (c) abdominal pain (gastroscopy negative for ulcer), and (d) low back pain Population: people with idiopathic pain syndromes Minimum pain intensity: no Inclusion criteria
Exclusion criteria
Total participants randomised: 253 Age in years (median, range): 51.0 (17‐80) Gender: 185/253 were female Pain duration in years (median, range): 60.0 (6‐636) |
|
| Interventions | Placebo
Clomipramine 75‐150 mg
Mianserin 30‐60 mg
|
|
| Outcomes | Substantial pain Withdrawal |
|
| Missing data methods | Completer analysis only | |
| Funding source | Non‐pharmaceutical: "This study was financially supported by: Danish Medical Research Council, Danish Medical Research Council‐Region‐III, Kleins legat, Geerd Jorgensens fond, Lundbeck Fonden, Mimi and Victor Larsens Fond, Danish Dental Association (FUT‐foundation), Bryde Nielsen Fond, P. Carl Petersens Fond, Ciba Geigy A/S, and Organon." | |
| Conflicts of interest | "Per Bech has occasionally over the past 3 years until August 2008 received funding from and been speaker or member of advisory boards for pharmaceutical companies with an interest in drug treatment of affective disorders (Astra‐Zeneca, Lilly, H. Lundbeck A/S, Lundbeck Foundation, Organon). All other authors declare that they have no conflicts of interests." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised by use of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedure not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical study drugs and dosing |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer analysis only Attrition Total: 75/253 (29.6%) Placebo: 15/87 (17.2%) Clomipramine 75‐100 mg: 28/84 (33.3%) Mianserin 30‐60 mg: 28/82 (34.2%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified. |
Luo 2009.
| Study characteristics | ||
| Methods | Design: parallel Duration: 8 weeks Assessment: baseline, week 1, week 2, week 4, post‐intervention Country: China |
|
| Participants | Pain condition: persistent somatoform pain disorder Population: people aged 18‐65 with persistent somatoform pain disorder Minimum pain intensity: no Inclusion criteria
Exclusion criteria
Total participants randomised: 80 Age in years (mean, SD): 40.96 (12.69) Gender: 46/80 were female Pain duration in months (mean, SD): 21.02 (9.02) |
|
| Interventions | Placebo
Fluoxetine 20 mg
|
|
| Outcomes | Pain intensity | |
| Missing data methods | ITT with LOCF | |
| Funding source | Non‐pharmaceutical: "This research was supported by Shanghai Science and Technology Committee." | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedure not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical study drugs, matched dosing schedule |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT with LOCF Attrition No withdrawal data reported |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified. |
Maarrawi 2018.
| Study characteristics | ||
| Methods | Design: parallel Duration: 8 weeks Assessment: baseline and post‐intervention Country: Lebanon |
|
| Participants | Pain condition: chronic neck pain Population: people with chronic neck pain Minimum pain intensity: no Inclusion criteria
Exclusion criteria
Total participants randomised: 332 Age in years (mean, SD): 44.23 (11.39) Gender: 190/332 were female Pain duration in years (mean, SD): 15.4 (4.86) |
|
| Interventions | Placebo
Amitriptyline 5 mg
|
|
| Outcomes | Pain intensity | |
| Missing data methods | Completer‐only analysis | |
| Funding source | Non‐pharmaceutical: "This study was supported by the Council of Research of the Saint Joseph University of Beirut – Lebanon (FM201)" | |
| Conflicts of interest | No conflicts of interest | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised via the block randomisation method, computer‐generated via www.randomization.com) |
| Allocation concealment (selection bias) | Low risk | Randomisation was centralised by a staff nurse (who had never seen the patient) not otherwise involved in the study and noted the group of each participant next to the number assigned to him. The same staff nurse delivered the corresponding medication to each patient. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, study drugs were identical with matched dosing schedule |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer‐only analysis. Withdrawal rate rate was ~17% Attrition Total: 58/332 (17.5%) Placebo: 25/166 (15.1%) Amitriptyline 5 mg: 33/166 (19.9%) |
| Selective reporting (reporting bias) | Low risk | All outcomes match up with those prospectively registered on clinicaltrials.gov |
| Other bias | Unclear risk | Unclear reporting in the publication, especially in relation to sample size and withdrawal |
Macfarlane 1986.
| Study characteristics | ||
| Methods | Design: parallel Duration: 12 weeks Assessment: baseline and post‐intervention Country: Canada |
|
| Participants | Pain condition: RA Population: adults with RA and elevated self‐reported depression Minimum pain intensity: no Inclusion criteria
Exclusion criteria: NR Total participants randomised: 36 Age in years (mean, SD): 59.15 Gender: 27/36 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Placebo
Trimipramine 75 mg
|
|
| Outcomes | Pain intensity Mood Withdrawal |
|
| Missing data methods | NR | |
| Funding source | NR | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedure not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical study drugs |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on handling missing data, report 9 participants withdrew in the text but 10 in the table Attrition Total: 9/36 (25.0%) Placebo: 4/18 (22.2%) Trimipramine 25‐75 mg: 5/18 (27.8%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Unclear risk | Not a lot of information on methods, short publication so not enough information to assess whether a further risk of bias exists |
Mahmoud 2021.
| Study characteristics | ||
| Methods | Design: parallel Duration: 16 weeks Assessment: baseline and post‐intervention Country: Egypt |
|
| Participants | Pain condition: neck pain Population: adults with chronic neck pain Minimum pain intensity: no Inclusion criteria
Exclusion criteria
Total participants randomised: 80 Age in years (mean): 46.6 Gender: 52/80 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Amitripyline 5 mg
Amitripyline 10 mg
|
|
| Outcomes | Pain intensity Withdrawal |
|
| Missing data methods | Completer‐only analysis | |
| Funding source | Non‐pharmaceutical: "This work was funded in part by Fayoum University Hospitals (Fayoum, Egypt) and by the authors’ personal resources." | |
| Conflicts of interest | The authors declare that there are no conflicts of interest regarding the publication of this paper. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple randomisation using a randomisation table created by a computer software program |
| Allocation concealment (selection bias) | Low risk | Participants were allocated using sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind with identical study drugs |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer analysis only Attrition Total: 10/80 (12.5%) Amitriptyline 5 mg: 5/40 (12.5%) Amitriptyline 10 mg: 5/40 (12.5%) |
| Selective reporting (reporting bias) | Unclear risk | Outcomes published match trial registry though retrospectively registered |
| Other bias | Unclear risk | Confusing reporting of primary outcome. In the text, it says that neck pain (as measured by the Neck Pain Driving Index) decreased by 71.9% ± 13.4% in the 10 mg group, which was greater than the decrease in the 5 mg group (47.3% ± 17.3%). However in the figure it says that the decreases were 48.3% for 5 mg and 68.2% for 10 mg. |
Majdinasab 2019.
| Study characteristics | ||
| Methods | Design: parallel Duration: 8 weeks Assessment: baseline and post‐intervention Country: Iran |
|
| Participants | Pain condition: painful diabetic peripheral polyneuropathy Population: adults with painful diabetic peripheral polyneuropathy Minimum pain intensity: ≥ 40 on 0‐100 VAS Inclusion criteria
Exclusion criteria
Total participants randomised: 104 Age in years (mean): 60.3 Gender: 50/104 were female Pain duration in years (mean): 3.75 |
|
| Interventions | Gabapentin 300‐900 mg
Duloxetine 30‐60 mg
|
|
| Outcomes | Pain intensity Sleep AEs Withdrawal |
|
| Missing data methods | NR | |
| Funding source | Non‐pharmaceutical: "This study was funded by Ahvaz Jundishapur University of Medical Sciences (grant number IR.AJUMS.REC.1395.78)" | |
| Conflicts of interest | Dr Nastaran Majdinasab, Dr Hossein Kaveyani, and Dr Mojgan Azizi have received research grants from Ahvaz Jundishapur University of Medical Sciences (grant number IR.AJUMS.REC.1395.78). The authors report no other conflicts of interest in this work. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using the 4 block randomised method (equalised 4‐blocks). |
| Allocation concealment (selection bias) | Low risk | "The medications of this study were first made similar to each other by a doctor who had no role in the collection and analysis of data and then sufficient amounts were packed into packets A and B and were given to the researcher." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Used identical drugs and placebos and packets but: "Before the commencement of the study, the side effects of the medications were explained to the patients " could then allow participants to know what they're experiencing and which medication it comes from. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported outcomes from participants, but unsure of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Method of missing data not specified Attrition Total: 16/104 (15.4%) Gabapentin 300‐900 mg: 11/52 (21.2%) Duloxetine 30‐60 mg: 5/52 (9.6%) |
| Selective reporting (reporting bias) | Unclear risk | Protocol registered retrospectively |
| Other bias | Unclear risk | Errors in publication between tables |
Masand 2009.
| Study characteristics | ||
| Methods | Design: parallel Duration: 12 weeks Assessment: baseline and post‐intervention Country: USA |
|
| Participants | Pain condition: IBS Population: adults aged 18‐75 with IBS Minimum pain intensity: no Inclusion criteria
Exclusion criteria
Total participants randomised: 72 Age in years (mean): 49.0 Gender: 63/72 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Placebo
Paroxetine 12.5‐50 mg
|
|
| Outcomes | SAEs Withdrawal |
|
| Missing data methods | ITT with LOCF | |
| Funding source | Pharmaceutical: This study was supported by a collaborative research grant from GlaxoSmithKline. | |
| Conflicts of interest | Dr Masand is a consultant for Bristol‐Myers Squibb Company, Cephalon, Inc., Eli Lilly and Company, Forest Pharmaceutical Laboratories Inc., GlaxoSmithKline, Janssen Pharmaceutica, Jazz Pharmaceuticals, Organon, Inc., Pfizer Inc., U.S. Pharmaceuticals Group., Targacept Inc., and Wyeth Pharmaceuticals. He is on the speaker's bureau of Astra‐Zeneca, Bristol‐Myers Squibb Company, Forest Pharmaceutical Laboratories, Inc., GlaxoSmithKline, Janssen Pharmaceutica, Pfizer Inc., U.S. Pharmaceuticals Group., and Wyeth Pharmaceuticals. He has received research support from AstraZeneca Pharmaceuticals, Bristol‐Myers Squibb Company, Cephalon, Inc.., Eli Lilly and Company, Forest Pharmaceutical Laboratories Inc., GlaxoSmithKline, Ortho McNeil Pharmaceutical, Inc., Janssen Pharmaceutica, and Wyeth Pharmaceuticals, and is an employee of i3CME. Dr Patkar is a consultant for Bristol‐Myers Squibb Company, GlaxoSmithKline, and Reckitt Benckiser; he is on the speaker's bureau of Bristol‐Myers Squibb Company, GlaxoSmithKline, and Reckitt Benckiser, and has received research support from National Institutes of Health, AstraZeneca Pharmaceuticals, Bristol‐Myers Squibb Company, Forest Pharmaceuticals, Inc., GlaxoSmithKline, Janssen Pharmaceutica, McNeil Consumer and Specialty Inc., Organon, Inc., Jazz Pharmaceuticals, and Pfizer Inc., U.S. Pharmaceuticals Group. Dr Pae has received research support from GlaxoSmithKline. Mr. Krulewicz is an employee of GlaxoSmithKline and owns common stock in the company. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not described |
| Allocation concealment (selection bias) | Low risk | Participants were allocated using an Interactive Voice Response System |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, matching drug appearance and identical dosing schedules |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT with LOCF Attrition Total: 14/72 (19.4%) Placebo: 8/36 (22.2%) Paroxetine 12.5‐50 mg: 6/36 (16.7%) |
| Selective reporting (reporting bias) | High risk | Trial registry lists quality of life and IBS symptoms as outcomes but these are NR. Beck Depression Index and Beck Anxiety Index are NR for all participants, divided into samples with or without history of anxiety and depression. |
| Other bias | Low risk | No other sources of bias were identified |
Matthey 2013.
| Study characteristics | ||
| Methods | Design: parallel Duration: 7 weeks Assessment: baseline and post‐intervention Country: Switzerland |
|
| Participants | Pain condition: fibromyalgia Population: adult women with fibromyalgia Minimum pain intensity: ≥ 40 on 0‐100 VAS Inclusion criteria
Exclusion criteria
Total participants randomised: 80 Age in years (mean): 49.7 Gender: 80/80 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Placebo
Milnacipran 100‐200 mg
|
|
| Outcomes | Pain intensity Quality of life Physical function Mood Sleep Withdrawal |
|
| Missing data methods | ITT wit h LOCF | |
| Funding source | Pharmaceutical: This trial was supported by a grant from Pierre Fabre Médicament. | |
| Conflicts of interest | "Each author certifies that he or she, or a member of his or her immediate family, has no commercial association, (i.e., consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might post a COI in connection with the submitted manuscript." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomisation list was computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Allocation of treatments was done by the investigator according to the chronological order of the occurring visit 2 |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | State double‐blind but not enough information about study drug appearance and dosing |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported outcomes from participants, but unsure of blinding procedures |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT with LOCF, high attrition Attrition Total: 37/80 (46.3%) Placebo: 16/40 (40.0%) Milnacipran 100‐200 mg: 21/40 (52.5%) |
| Selective reporting (reporting bias) | Unclear risk | No changes to protocol, but it's registered 2 years after trial start |
| Other bias | Low risk | No other sources of bias were identified. |
Max 1988.
| Study characteristics | ||
| Methods | Design: cross‐over Duration: each cross‐over period was 6 weeks Assessment: baseline and post‐cross‐over period Country: USA |
|
| Participants | Pain condition: post‐herpetic neuralgia Population: adults with post‐herpetic neuralgia Minimum pain intensity: no Inclusion criteria
Exclusion criteria
Total participants randomised: 58 Age in years (median, range): 72 (25‐86) Gender: 27/58 were female Pain duration in months (median, range): 19 months (3 months‐25 years) |
|
| Interventions | Placebo
Amitriptyline 12.5‐150 mg
Lorazepam 0.5‐6 mg
|
|
| Outcomes | AEs | |
| Missing data methods | Unclear | |
| Funding source | Non‐pharmaceutical: from the Neurobiology and Anaesthesiology Branch, National Institute of Dental Research (Drs Max, Gracely, Smoller, and Dubner). and the Nursing Department, Clinical Center (Ma. Schnfer and Me. Culnane), National Institutes of Health, Bethesda, MD | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Says double‐blind but no information about this was given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported outcomes from participants, but unsure of blinding procedures |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information regarding missing data reported. 41 completed both of the treatment periods for their group, but authors report data on 58 |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias identified |
Max 1992.
| Study characteristics | ||
| Methods | Design: cross‐over Duration: each cross‐over period was 6 weeks Assessment: baseline and post‐cross‐over period Country: USA |
|
| Participants | Pain condition: diabetic peripheral neuropathy Population: type 1 or 2 diabetic adults with diabetic peripheral neuropathy Minimum pain intensity: no Inclusion criteria
Exclusion criteria
Total participants randomised: 54 Age in years (median, range): 58 (20‐84) Gender: 21/54 were female Pain duration in years (median, range): 3 (0.5‐12) |
|
| Interventions | Desipramine 12.5‐150 mg
Amitriptyline 12.5‐150 mg
|
|
| Outcomes | Pain intensity AEs |
|
| Missing data methods | Completer‐only analysis | |
| Funding source | NR | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | States double‐blind but no information regarding procedures e.g. study drug appearance |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported outcomes from participants, but unsure of blinding process |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer analysis only Attrition Total: 16/54 (29.6%) Attrition per arm NR |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Unclear risk | Complicated trial design between 2 studies. Of the 54 participants in the desipramine vs amitriptyline study only 29 were randomised into it. |
Mease 2009.
| Study characteristics | ||
| Methods | Design: parallel Duration: 27 weeks Assessment: baseline, 15 weeks, post‐intervention Country: USA |
|
| Participants | Pain condition: fibromyalgia Population: adults with fibromyalgia Minimum pain intensity: ≥ 50 on 0‐100 scale Inclusion criteria
Exclusion criteria
Total participants randomised: 888 Age in years (mean, SD): 49.5 Gender: 849/888 were female Pain duration in years (mean, SD): 5.6 |
|
| Interventions | Placebo
Milnacripran 100 mg
Milnacripran 200 mg
|
|
| Outcomes | Pain intensity Moderate pain relief Substantial pain relief PGIC AEs SAEs Withdrawal |
|
| Missing data methods | ITT with LOCF, sensitivity analyses with BOCF | |
| Funding source | Pharmaceutical: supported by Forest Laboratories, Inc., New York, New York, and CypressBioscience, Inc., San Diego, California, USA | |
| Conflicts of interest | Dr Mease has received research grant support from Pfizer Inc, Cypress Bioscience, Inc., Forest Laboratories, Inc., Eli Lilly and Company, Allergan, Wyeth Pharmaceuticals, Jazz Pharmaceuticals, and Fralex Therapeutics. Dr Clauw has received grant support from Cypress Bioscience, Inc. and serves as a consultant to Cypress Bioscience, Inc, Forest Laboratories, Inc., Pierre Fabre Medicament, Pfizer Inc, Eli Lilly and Company, Wyeth Pharmaceuticals, and Proctor and Gamble. Dr Mease was an investigator of this study and a consultant; Dr Clauw was a consultant for this study. As consultants, Drs Mease and Clauw were involved in the study design, analysis of results, and preparation of the manuscript. Drs Gendreau, Rao, and Kranzler are employees of Cypress Bioscience, Inc. Drs Chen and Palmer are employees of Forest Laboratories, Inc |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedure not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind with study drugs identical and matched dosing |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Very high attrition rate, especially for the high‐dose milnacipran. Use a mix of imputation methods including LOCF, BOCF and completers, but not all of the data for this are presented in the paper Attrition Total: 376/888 (42.3%) Placebo: 78/223 (35.0%) Milnacipran 100 mg: 96/224 (42.9%) Milnacipran 200 mg: 202/441 (45.8%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified. |
Miki 2016.
| Study characteristics | ||
| Methods | Design: parallel Duration: 12 weeks Assessment: baseline and post‐intervention Country: Japan |
|
| Participants | Pain condition: fibromyalgia Population: Japanese adults aged between 20‐64 with fibromyalgia Minimum pain intensity: ≥ 40 on 0‐100 scale Inclusion criteria
Exclusion criteria
Total participants randomised: 430 Age in years (mean): 45.2 Gender: 347/430 were female Pain duration in years (mean): 4.4 |
|
| Interventions | Placebo
Mirtazapine 30 mg
|
|
| Outcomes | Pain intensity Mood Quality of life Physical function Moderate pain relief Substantial pain relief AEs Severe AEs Withdrawal |
|
| Missing data methods | NR | |
| Funding source | Pharmaceutical: funded by Meiji Seika Pharma Co, Ltd. | |
| Conflicts of interest | The authors have no conflicts of interest to declare. K. Miki, M. Murakami, H. Oka, K. Osada received honorarium from Meiji Seika Pharma Co, Ltd. K. Onozawa and S. Yoshida are employees of Meiji Seika Pharma Co, Ltd. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was done by a computer‐generated allocation sequence |
| Allocation concealment (selection bias) | Low risk | Allocation was delivered by a telephone randomisation service (randomisation manager) not involved in participant recruitment or treatment to ensure allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical study drugs with matched dosing |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT but methods not specified Attrition Total: 48/430 (11.2%) Placebo: 23/215 (10.7%) Mirtazapine 30 mg: 25/215 (11.6%) |
| Selective reporting (reporting bias) | Unclear risk | Protocol published prospectively but no outcomes specified apart from "amount of change from baseline" |
| Other bias | High risk | Pain intensity change scores reported in the paper do not seem to be correct. When calculated into SMD, an SMD of over 4 resulted, which is improbable. Emailed study authors for clarification but no response, and no correction found. |
Morello 1999.
| Study characteristics | ||
| Methods | Design: cross‐over Duration: cross‐over periods were 6 weeks Assessment: baseline and post‐intervention Country: USA |
|
| Participants | Pain condition: diabetic peripheral neuropathy Population: type 1 and 2 diabetic veterans with diabetic peripheral neuropathy pain Minimum pain intensity: no Inclusion criteria
Exclusion criteria
Total participants randomised: 25 Age in years (mean, SD): 60.4 (10.8) Gender: 1/25 were female Pain duration in years (mean, SD): 5.7 (4.2) |
|
| Interventions | Gabapentin 900‐1800 mg
Amitriptyline 25‐75 mg
|
|
| Outcomes | Pain intensity AEs Withdrawal |
|
| Missing data methods | Completer analysis only | |
| Funding source | NR | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedure not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind medications, same dosing schedules and appearance |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes by blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer‐only analysis Attrition Total: 4/25 (16.0%) Gabapentin 900‐1800 mg: 2/25 (8.0%) Amitriptyline 25‐75 mg: 2/25 (8.0%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | High risk | Post hoc power analysis and report needing sample of 280 to detect effect, they have 25 participants randomised |
Muller 2008.
| Study characteristics | ||
| Methods | Design: parallel Duration: 12 weeks Assessment: baseline and post‐intervention Country: South Africa |
|
| Participants | Pain condition: multisomatoform disorder Population: people aged 18‐65 with somatoform symptoms and medically unexplained symptoms Minimum pain intensity: no Inclusion criteria
Exclusion criteria
Total participants randomised: 51 Age in years (mean, SD): 39.6 Gender: 46/51 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Placebo
Escitalopram 10‐20 mg
|
|
| Outcomes | Pain intensity Mood Physical function Withdrawal |
|
| Missing data methods | ITT with LOCF | |
| Funding source | Pharmaceutical: this study was funded by H. Lundbeck A/S | |
| Conflicts of interest | At the time this study was conducted, Professor Stein, Professor Seedat and Dr Muller were funded by the Medical Research Council of South Africa | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly assigned via computer‐generated randomisation lists |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedure not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, with study drugs identical and matched dosing schedule |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes by blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT and LOCF, but only 1 person withdrew Attrition Total: 1/51 (2.0%) Placebo: 0/26 (0.0%) Escitalopram 10‐20 mg: 1/25 (4.0%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified. |
Murakami 2015.
| Study characteristics | ||
| Methods | Design: parallel Duration: 14 weeks Assessment: baseline and post‐intervention Country: Japan |
|
| Participants | Pain condition: fibromyalgia Population: adults aged 20‐75 with fibromyalgia Minimum pain intensity: ≥ 4 on 0‐10 scale Inclusion criteria
Exclusion criteria
Total participants randomised: 393 Age in years (mean, SD): 48.7 (11.9) Gender: 321/393 were female Pain duration in years (mean): 5.6 |
|
| Interventions | Placebo
Duloxetine 60 mg
|
|
| Outcomes | Pain intensity Mood Sleep Quality of life Physical function PGIC AEs SAEs Withdrawal |
|
| Missing data methods | ITT with LOCF, MMRM, and sensitivity analyses using BOCF and WOCF | |
| Funding source | Pharmaceutical: Shionogi & Co. Ltd., Eli Lilly Japan K.K., and Eli Lilly & Company provided funding for the study | |
| Conflicts of interest | HM and TO are employees of Shionogi & Co. Ltd. LA is an employee of Eli Lilly Japan K.K. MM, KO, and KN have provided consultancy services and MM and KO received compensation from Shionogi & Co. Ltd. for their participation in this study. MM, KO, and KN did not receive any compensation for their input into this study. The authors confirm that there are no non‐financial competing interests to declare in relation to this article. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were assigned randomly to receive duloxetine or placebo in a 1:1 ratio, using a web‐based patient registration system (ACRONET Corp., Tokyo, Japan) with a stochastic minimisation procedure. |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedure not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, study drugs had identical appearance, packaging, and labelling, matched dosing schedule |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Although there was attrition, sensitivity analyses of the primary outcomes with LOCF, BOCF, and WOCF showed no signficiant differences. Attrition Total: 78/393 (19.9%) Placebo: 48/198 (24.4%) Duloxetine 60 mg: 30/196 (15.3%) |
| Selective reporting (reporting bias) | Low risk | All outcomes listed prospectively on clinicaltrials.gov |
| Other bias | Low risk | No other sources of bias were identified. |
Nabi 2021.
| Study characteristics | ||
| Methods | Design: parallel Duration: 12 weeks Assessment: baseline, 4 weeks, post‐intervention Country: Iran |
|
| Participants | Pain condition: painful diabetic neuropathy Population: adults with type I or type II diabetes and a diagnosis of diabetic peripheral neuropathic pain Minimum pain intensity: ≥ 4 on 0‐10 scale Inclusion criteria
Exclusion criteria
Total participants randomised: 72 Age in years (mean, SD): 57.71 (7.43) Gender: 29/72 were female Pain duration in months (mean, SD): 22.46 (9.52) |
|
| Interventions | TENS
Duloxetine ‐ 60 mg
|
|
| Outcomes | Pain intensity AEs Withdrawal |
|
| Missing data methods | Completer analysis only ‐ 12 participants discontinued treatment due to intolerability and were replaced with new cases. | |
| Funding source | Study was not financially supported | |
| Conflicts of interest | The study authors reported no conflicts of interest. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using http://www.graphpad.com/quickcalcs/index.cfm. |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to be blinded due to the nature of TENS |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self‐reported outcomes from unblinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer analysis only. 12 participants dropped out, all in the duloxetine arm due to side effects. Participants who dropped out were replaced with new participants, and their data not analysed. Attrition Total: 12/72 (16.7%) TENS: 0/30 (0.0%) Duloxetine 60 mg: 12/42 (28.6%) |
| Selective reporting (reporting bias) | Low risk | Trial registered prospectively and outcomes match |
| Other bias | Low risk | No other sources of bias were identified. |
Natelson 2015.
| Study characteristics | ||
| Methods | Design: parallel Duration: 8 weeks Assessment: baseline and post‐intervention Country: USA |
|
| Participants | Pain condition: fibromyalgia Population: adults aged 18‐68 with fibromyalgia Minimum pain intensity: no Inclusion criteria
Exclusion criteria
Total participants randomised: 34 Age in years (mean, SD): 46.8 Gender: 33/34 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Placebo
Milnacipran 100 mg
|
|
| Outcomes | Pain intensity AEs SAEs Withdrawal |
|
| Missing data methods | Completer‐only analysis | |
| Funding source | Part funded by pharmaceutical: "This work was supported by a Forest Laboratories Investigator‐initiated grant to B.H.N., and, in part, by National Institutes of Mental Health (NIMH) grant R01 MH100005 to D.C.S." | |
| Conflicts of interest | "J.D.C. has been a speaker for Pfizer, Forest, Bristol Myers Squibb, Glaxo Smith Kline, Eli Lilly, and Sunovion. He has received grants from Pfizer Pharmaceuticals, GSK, Corcept, and Neurocrine. There were no other conflicts of interest in doing this research. This work was supported by a Forest Laboratories Investigator‐initiated grant to B.H.N., and, in part, by National Institutes of Mental Health (NIMH) grant R01 MH100005 to D.C.S. The sources of funding had no involvement in any of the aspects of running this study, analyzing the data, or preparing this manuscript." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not specified |
| Allocation concealment (selection bias) | Low risk | Mount Sinai Beth Israel Pharmacy dispensed the drug or placebo according to the randomisation list in sequential order. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, with identical study drugs and matched dosage schedule |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer‐only analysis Attrition Total: 8/34 (23.5%) Placebo: 4/17 (23.5%) Milnacipran 100 mg: 4/17 (23.5%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified |
NCT00066937.
| Study characteristics | ||
| Methods | Design: parallel Duration: 8 weeks Assessment: baseline, post‐intervention, 3‐month follow‐up, 6‐month follow‐up Country: USA |
|
| Participants | Pain condition: temporomandibular joint disorders Population: adults aged 18‐65 with temporomandibular joint disorders Minimum pain intensity: no Inclusion criteria
Exclusion criteria
Total participants randomised: 140 Age in years (mean, SD): 37.2 (11.5) Gender: 28/140 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Placebo (benztropine mesylate) + CBT
Nortriptyline + CBT
Placebo (benztropine mesylate) + management
Nortriptyline + management
|
|
| Outcomes | Pain intensity Mood AEs SAEs Withdrawal |
|
| Missing data methods | Completer analysis | |
| Funding source | Non‐pharmaceutical: Johns Hopkins University. Collaborator: National Institute of Dental and Craniofacial Research (NIDCR) | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Although there are matched active placebos/interventions there is not enough information to determine blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported outcomes from participants, but unsure of blinding procedures |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer‐only analysis Attrition Total: 24/140 (17.1%) CBT: 5/38 (13.2%) Nortriptyline 25‐150 mg + CBT: 3/41 (7.3%) Disease management: 5/24 (20.8%) Nortriptyline 25‐150 mg + disease management: 11/37 (29.7%) |
| Selective reporting (reporting bias) | High risk | Changed primary outcome from physical and psychosocial function to "pain" |
| Other bias | High risk | Not published, all information and data extracted from trial registration: https://clinicaltrials.gov/ct2/show/study/NCT00066937 |
NCT01225068.
| Study characteristics | ||
| Methods | Design: parallel Duration: 6 weeks Assessment: baseline and post‐intervention Country: USA |
|
| Participants | Pain condition: low back pain Population: adults aged 18‐70 with chronic neuropathic low back pain Minimum pain intensity: ≥ 50 on 0‐100 scale Inclusion criteria
Exclusion criteria
Total participants randomised: 40 Age in years (mean, SD): 47.7 (10.3) Gender: 21/40 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Placebo
Milnacipran 100‐200 mg
|
|
| Outcomes | AEs SAEs Withdrawal |
|
| Missing data methods | Completer analysis | |
| Funding source | Partly funded by pharmaceutical: sponsor: Northwestern University; Collaborators: Forest Laboratories; Shirley Ryan Ability; Lab Best Practice | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical study drugs with matched dosing schedules |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer‐only analysis Attrition Total: 5/40 (12.5%) Placebo: 1/20 (5.0%) Milnacipran 100‐200 mg: 4/20 (20.0%) |
| Selective reporting (reporting bias) | High risk | Originally had lots of outcome measures listed: effect size of outcome measures, VAS pain, BPI, McGill Pain Questionnaire and Physical Activity measurement), but only VAS pain reported |
| Other bias | High risk | Not published. All info and results extracted from trial registration: https://clinicaltrials.gov/ct2/show/study/NCT01225068 |
NCT01510457.
| Study characteristics | ||
| Methods | Design: parallel Duration: approximately 8 weeks Assessment: baseline and post‐intervention Country: USA |
|
| Participants | Pain condition: knee OA Population: adults with knee OA Minimum pain intensity: ≥ 4 on 0‐10 scale Inclusion criteria
Exclusion criteria
Total participants randomised: 46 Age in years (mean, SD): 56 (8) Gender: 23/46 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Placebo
Milnacipran 100 mg‐200 mg
|
|
| Outcomes | Pain intensity Mood Physical function AEs SAEs Withdrawal |
|
| Missing data methods | Completer analysis only | |
| Funding source | Pharmaceutical: Forest Laboratories | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinded procedures, but 0 placebo participants reported AEs and 34% of milnacipran participants did report AEs, somewhat unblinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported outcomes from participants, unsure of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer‐only analysis Attrition Total: 8/46 (17.4%) Placebo: 5/17 (29.4%) Milnacipran 100‐200 mg: 3/29 (10.3%) |
| Selective reporting (reporting bias) | Unclear risk | As we're using all outcomes from trial they report all registered outcomes BUT they first posted the trial in 2012, the trial started in 2010. |
| Other bias | High risk | Not published ‐ trial registry report only |
Nørregaard 1995.
| Study characteristics | ||
| Methods | Design: parallel Duration: 8 weeks Assessment: baseline and post‐intervention Country: Denmark |
|
| Participants | Pain condition: fibromyalgia Population: people with fibromyalgia Minimum pain intensity: no Inclusion criteria
Exclusion criteria
Total participants randomised: 43 Age in years (mean, SD): 49 Gender: NR Pain duration in years (mean, SD): NR |
|
| Interventions | Placebo
Citalopram 20‐40 mg
|
|
| Outcomes | Pain intensity Mood Physical function Sleep |
|
| Missing data methods | ITT but methods NR | |
| Funding source | Pharmaceutical: "This work was supported by funding from H. Lundbeck A/S." | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical study drugs with sham dosing |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | State ITT but no methods specified Attrition Total: 10/43 (23.3%) Attrition per arm NR |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified |
Otto 2008.
| Study characteristics | ||
| Methods | Design: cross‐over Duration: 5 weeks Assessment: baseline and post‐intervention Country: Denmark |
|
| Participants | Pain condition: polyneuropathy Population: adults aged 20‐80 with chronic polyneuropathy Minimum pain intensity: ≥ 4 on 0‐10 scale Inclusion criteria
Exclusion criteria
Total participants randomised: 48 Age in years (median, range): 62 (37–74) Gender: 12/48 were female Pain duration in months (median, range): 48 (8–180) |
|
| Interventions | Placebo
Escitalopram 20 mg
|
|
| Outcomes | Pain intensity Sleep Mood AEs Withdrawal |
|
| Missing data methods | ITT with LOCF | |
| Funding source | Partly pharmaceutical: Odense University Hospital The work behind this study was supported by unrestricted grants from H. Lundbeck A/S and Gruenenthal GmbH and a grant from the Danish Clinical Intervention Research Academy. | |
| Conflicts of interest | This was an investigator‐initiated study and neither company was responsible for the creation of the study protocol, the data analysis, data interpretation, or writing of the manuscript. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Assignment to treatment sequence was random via a computer‐generated randomisation code with a block size of 4. |
| Allocation concealment (selection bias) | Low risk | The randomisation plan was generated by a person in the hospital pharmacy at Odense University Hospital who was not involved in the conduct of the trial. The study drugs were packed in boxes marked with randomisation number and treatment period by the hospital pharmacy. Patients were enrolled by the investigators and, after the baseline period, numbered consecutively by the investigators and treated with the study drugs with the corresponding randomisation number. Sealed opaque envelopes with the treatment sequence for each patient for emergency situations were present at the study sites. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical study drugs with matched dosing |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT with LOCF Attrition Total: 10/41 (24.4%) Placebo: 4/41 (9.8%) Escitalopram 20 mg: 6/41 (14.6%) |
| Selective reporting (reporting bias) | High risk | Some outcomes mentioned prospectively on clinicaltrials.gov NR e.g. different subtypes of pain and quality of life |
| Other bias | Low risk | No other sources of bias were identified |
Ozerbil 2006.
| Study characteristics | ||
| Methods | Design: cross‐over Duration: 2 weeks Assessment: baseline and post‐intervention Country: Turkey |
|
| Participants | Pain condition: fibromyalgia Population: adult women aged 20‐60 with fibromyalgia Minimum pain intensity: no Inclusion criteria
Exclusion criteria
Total participants randomised: 15 Age in years (mean, SD): NR Gender: 15/15 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Amitriptyline 25 mg + placebo
Fluoxetine 20 mg + placebo
|
|
| Outcomes | The study provided no useable data | |
| Missing data methods | NR | |
| Funding source | NR | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using randomisation tables |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedure not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, dummy dosing technique and identical tablets |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Researcher blinding ‐ not enough information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information regarding missing data or withdrawal given |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias identified |
Pakfetrat 2019.
| Study characteristics | ||
| Methods | Design: parallel Duration: 11 weeks Assessment: baseline, 3 weeks, 7 weeks, post‐intervention Country: Iran |
|
| Participants | Pain condition: burning mouth syndrome Population: people with burning mouth syndrome Minimum pain intensity: ≥ 5 on 0‐10 VAS Inclusion criteria
Exclusion criteria
Total participants randomised: 47 Age in years (mean): 50.9 Gender: 32/47 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Crocin (saffron) 30 mg
Citalopram 20 mg
|
|
| Outcomes | Pain intensity Mood Withdrawal |
|
| Missing data methods | No participants withdrew | |
| Funding source | Non‐pharmaceutical: "We are thankful to the Vice Chancellor of Mashhad University of Medical Sciences for providing financial support for this study". | |
| Conflicts of interest | "The authors declare that they have no conflict of interest in this research." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation methods not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Mention similar package and pill appearance but citalopram is being taken once daily and saffron twice daily so it's not completely identical in dosing schedule. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of participants who completed self reported measures is unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants withdrew |
| Selective reporting (reporting bias) | Unclear risk | Could not access trial registration |
| Other bias | Low risk | No other sources of bias were identified |
Patkar 2007.
| Study characteristics | ||
| Methods | Design: parallel Duration: 12 weeks Assessment: baseline and post‐intervention Country: USA |
|
| Participants | Pain condition: fibromyalgia Population: people aged 18‐65 with fibromyalgia and depression Minimum pain intensity: ≥ 5 on 0‐10 scale Inclusion criteria
Exclusion criteria
Total participants randomised: 116 Age in years (mean, SD): 48.5 Gender: 109/116 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Placebo
Paroxetine 12.5‐62.5 mg
|
|
| Outcomes | Pain intensity AEs SAEs Withdrawal |
|
| Missing data methods | ITT with LOCF | |
| Funding source | Pharmaceutical: this work was supported by a Collaborative Research Grant from GlaxoSmithKline | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation (1:1) was determined by the Investigational Drug Service through a computer‐generated sequence. |
| Allocation concealment (selection bias) | Low risk | The trial staff obtained the randomisation assignment over the phone at screening. The allocation sequence was concealed from the staff before and after assignment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, study drugs identical |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT with LOCF. Double the number of people withdrew from intervention arm than placebo Attrition Total: 30/116 (25.9%) Placebo: 10/58 (17.2%) Paroxetine 12.5‐62.5 mg: 20/58 (34.5%) |
| Selective reporting (reporting bias) | High risk | Differs from protocol, though primary outcome remains the same In the protocol on clinicaltrials.gov it says that they will report change from baseline in BDI/BAI, but they do not. |
| Other bias | Low risk | No other sources of bias were identified. |
Petzke 2013.
| Study characteristics | ||
| Methods | Design: parallel Duration: 13 weeks Assessment: baseline and post‐intervention Country: UK, Sweden, and Germany |
|
| Participants | Pain condition: fibromyalgia Population: right‐handed women, 18–55 years of age, with fibromyalgia Minimum pain intensity: ≥ 40 on 0‐100 VAS Inclusion criteria
Exclusion criteria
Total participants randomised: 92 Age in years (mean, SD): 44.2 Gender: 92/92 were female Pain duration in years (mean, SD): 11.1 |
|
| Interventions | Placebo
Milnacipran 200 mg
|
|
| Outcomes | AEs SAEs Withdrawal |
|
| Missing data methods | MMRM for pain, NR for other outcomes | |
| Funding source | Pharmaceutical: this study (EudraCT # 2004‐004249‐16) was financed and performed in collaboration with the pharmaceutical company Pierre Fabre. | |
| Conflicts of interest | This study (EudraCT # 2004‐004249‐16) was financed and performed in collaboration with the pharmaceutical company Pierre Fabre. There are no other conflicts of interest. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedure not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical study drugs with matched dosing |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Mix of different methods used for missing data. More participants discontinued in intervention arm due to AEs than placebo arm Attrition Total: 22/92 (23.9%) Placebo: 8/46 (17.4%) Milnacipran: 13/46 (28.3%) |
| Selective reporting (reporting bias) | High risk | Lots of outcomes reported in the EudraCT registration recorded at baseline and 12 weeks that are NR in the results on there or published papers. |
| Other bias | Low risk | No other sources of bias were identified |
Pickering 2018.
| Study characteristics | ||
| Methods | Design: parallel Duration: 4 weeks Assessment: baseline and post‐interventions Country: France |
|
| Participants | Pain condition: fibromyalgia Population: adult women with fibromyalgia Minimum pain intensity: no Inclusion criteria
Exclusion criteria
Total participants randomised: 54 Age in years (mean, SD): 46.7 (10.6) Gender: 54/54 were female Pain duration in months (mean): 71.9 |
|
| Interventions | Placebo
Milnacipran 100 mg
|
|
| Outcomes | Pain intensity Moderate pain relief AEs SAEs Withdrawal |
|
| Missing data methods | Completer analysis only | |
| Funding source | Non‐pharmaceutical: "We thank the Apicil foundation for their financial support" | |
| Conflicts of interest | The study authors report no conflicts of interest in this work. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation sequence was generated using random blocks. |
| Allocation concealment (selection bias) | Low risk | Treatment allocation followed a predefined randomisation plan and was conducted by a person independent from the protocol. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical study drugs with matched dosing |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer analysis only Attrition: Total: 6/54 (11.1%) Placebo: 1/29 (3.5%) Milnacipran 100 mg: 5/25 (20.0%) |
| Selective reporting (reporting bias) | Low risk | Study protocol published: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4393595/ All outcomes matched those published |
| Other bias | Low risk | No other sources of bias were identified. |
Pilowsky 1990.
| Study characteristics | ||
| Methods | Design: parallel Duration: 12 weeks Assessment: baseline and post‐intervention Country: Australia |
|
| Participants | Pain condition: chronic, intractable 'psychogenic' pain Population: patients with chronic, intractable ‘psychogenic’ pain Minimum pain intensity: no Inclusion criteria
Exclusion criteria
Total participants randomised: 129 Age in years (mean): 42.26 Gender: 80/129 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Amitriptyline + pyschotherapy
Amitriptyline + support
Placebo + psychotherapy
Placebo + support
|
|
| Outcomes | Pain intensity Withdrawal |
|
| Missing data methods | Completer analysis | |
| Funding source | Non‐pharmaceutical: "We are indebted to the National Health and Medical Research Council who provided generous support for the conduct of this study." | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised to 1 of 4 treatment groups with the use of a table of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedure not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of blinding procedures between psychotherapy/support and amitriptyline/placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Very different intervention experience for those undergoing psychotherapy versus simple support. Triallists attempt to control for effects of contact in therapy by having clincian support, but participants would be aware of their intervention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer analysis only. Original numbers of participants in arms not given, and withdrawal only given in percentages. No reasons given for withdrawal Attrition Total: 28/129 (21.7%) Amitriptyline ≤ 150 mg + psychotherapy: 6/26 (24%) Amitriptyline ≤ 150 mg + support: 7/26 (25%) Psychotherapy: 5/26 (19%) Support: 7/24 (31%) |
| Selective reporting (reporting bias) | High risk | Some outcome measures mentioned in the methods don't seem to be reported in the results section (e.g. McGill pain questionnaire). No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified. |
Pirbudak 2003.
| Study characteristics | ||
| Methods | Design: parallel Duration: 9 months Assessment: baseline, 2 weeks, 6 weeks, 3 months, 6 months, post‐intervention Country: Turkey |
|
| Participants | Pain condition: low back pain Population: people aged > 35 years with chronic low back pain Minimum pain intensity: no Inclusion criteria
Exclusion criteria
Total participants randomised: 92 Age in years (mean, SD): 49.1 Gender: 62/92 were female Pain duration in months (median, range): 16.5 (6‐48) |
|
| Interventions | Epidural injection + placebo
Epidural injection + amitriptyline (10‐50 mg)
|
|
| Outcomes | Pain intensity Physical function |
|
| Missing data methods | Unclear | |
| Funding source | NR | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Says double‐blinded, but doesn't specify information about the medication |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported outcomes from participants, but unsure of blinding procedures |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No missing data methods and no withdrawal data reported, seems like all participants completed but unclear |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified. |
Rani 1996.
| Study characteristics | ||
| Methods | Design: parallel Duration: 4 weeks Assessment: baseline, week 1, week 2, week 3, post‐intervention Country: India |
|
| Participants | Pain condition: chronic pain syndrome Population: 27 presented with low back pain, 16 with OA, 8 with fibromyalgia, and 8 with RA Minimum pain intensity: ≥ 60 on 0‐100 VAS Inclusion criteria
Exclusion criteria
Total participants randomised: 59 Age in years (mean, SD): 40 Gender: 36/59 were female Pain duration in months (mean): 25.3 |
|
| Interventions | Placebo
Amitriptyline 25 mg
Fluoxetine 20 mg
|
|
| Outcomes | Pain intensity Withdrawal |
|
| Missing data methods | Unclear | |
| Funding source | Pharmaceutical: supported by Natco Pharma Limited, India | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical study drugs with matched dosing |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of missing data handling/impute methods, it seems like no participants dropped out but this is unclear |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified |
Raskin 2005.
| Study characteristics | ||
| Methods | Design: parallel Duration: 12 weeks Assessment: baseline and post‐intervention Country: "Worldwide" |
|
| Participants | Pain condition: diabetic peripheral neuropathic pain Population: type 1 or 2 diabetic adults with diabetic peripheral neuropathic pain Minimum pain intensity: ≥ 4 on 0‐10 scale Inclusion criteria
Exclusion criteria
Total participants randomised: 348 Age in years (mean, SD): 58.8 (10.1) Gender: 186/348 were female Pain duration in years (mean, SD): 4.3 (4.2) |
|
| Interventions | Placebo
Duloxetine 60 mg
Duloxetine 120 mg
|
|
| Outcomes | Pain intensity Mood Sleep Moderate pain relief Substantial pain relief PGIC AEs SAEs Withdrawal |
|
| Missing data methods | ITT with LOCF | |
| Funding source | Pharmaceutical: funded by Eli Lilly | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Assignment to treatment groups was determined by a computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Allocation to treatment groups using an Interactive Voice Response System |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical study drugs with matched dosing |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT with LOCF. Significantly more people in the duloxetine 120 mg arm dropped out due to AE than other arm Attrition Total: 52/348 (14.9%) Placebo: 16/116 (13.8%) Duloxetine 60 mg: 15/116 (12.9%) Duloxetine 120 mg: 21/116 (18.1%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified |
Razazian 2014.
| Study characteristics | ||
| Methods | Design: parallel Duration: 4 weeks Assessment: baseline, 2 days, 7 days, 14 days, 1 week post‐intervention Country: Iran |
|
| Participants | Pain condition: diabetic polyneuropathy Population: adults with diabetic polyneuropathy referred to diabetic clinic Minimum pain intensity: ≥ 40 on 0‐100 scale Inclusion criteria
Exclusion criteria
Total participants randomised: 257 Age in years (mean, SD): 56.3 (10.4) Gender: 156/257 were female Pain duration in months (mean, SD): 23.5 (2.5) |
|
| Interventions | Carbamazepine 400 mg
Pregabalin 150 mg
Venlafaxine 150 mg
|
|
| Outcomes | Moderate pain relief Substantial pain relief Sleep Mood AEs SAEs Withdrawal |
|
| Missing data methods | NR | |
| Funding source | Non‐pharmaceutical: Kermanshah Univesity Of Medical Science | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised via a computer‐generated randomisation schedule |
| Allocation concealment (selection bias) | Low risk | Investigators and participants were blinded to the treatments by preprinted medication code labels. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | States double‐blind but drugs not identical and dosage schedule differs between participants: venlafaxine taken as tablet twice daily, pregabalin as capsule once daily and carbamazepine twice daily as tablet |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self‐reported outcomes from participants, but not strict blinding procedures |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No methods for dealing with missing data specified, think they present completer analysis. Attrition Total: 33/257 (12.8%) Carbamazepine 400 mg: 7/85 (8.2%) Pregabalin 150 mg: 9/86 (10.5%) Venlafaxine 150 mg: 17/86 (19.8%) |
| Selective reporting (reporting bias) | High risk | Protocol not very clear, mention reporting 30th day as outcome time point but in article it's the 35th day. Did not mention work interference as outcome but have included it in paper, mention primary outcome will be measured with "PPI" and VAS but seems that PPI NR in article. Protocol registered on IRCT while recruiting participants, only 2 outcomes specified. |
| Other bias | High risk | Significant difference in VAS pain between groups at baseline |
RBR‐5dsrhv.
| Study characteristics | ||
| Methods | Design: parallel Duration: 16 weeks Assessment: baseline and post‐intervention Country: Brazil |
|
| Participants | Pain condition: temporomandibular pain Population: women aged 18‐59 with chronic temporomandibular pain Minimum pain intensity: intensity of muscle pain ≥ 7 on a 0‐10 VAS Inclusion criteria
Exclusion criteria
Total participants randomised: 96 Age in years (mean): 35.9 Gender: 96/96 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Waitlist
Amitriptyline 10 mg
Amitriptyline 10 mg + splint
Acupuncture
|
|
| Outcomes | Pain intensity Mood Quality of life Sleep Withdrawal |
|
| Missing data methods | Completer‐only analysis | |
| Funding source | Non‐pharmaceutical: thanks the CAPES scholarship for fund | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised by means of random numbers generated by computer |
| Allocation concealment (selection bias) | Low risk | The randomisation of patients in the 4 groups was carried out by means of opaque and sealed envelopes encoded by 'A', 'B', 'C' or 'D', prepared by a researcher without contact with the other procedures. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not possible to blind the participants and the clinicians due to the nature of the interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self‐reported outcomes from unblinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer analysis only. Unequal attrition ‐ many more participants withdrew from the acupuncture group than the other groups. Attrition Total: 18/96 (18.75%) Waitlist: 3/24 (12.5%) Amitriptyline 10 mg: 3/24 (12.5%) Amitriptyline 10 mg + splint: 1/24 (4.2%) Acupuncture: 11/24 (45.8%) |
| Selective reporting (reporting bias) | Unclear risk | Trial was registered on the Brazilian Registry of Clinical Trials after completion. |
| Other bias | Unclear risk | Extracted from a doctoral thesis translated from Portuguese ‐ can't find published papers |
Richards 2015.
| Study characteristics | ||
| Methods | Design: parallel Duration: 12 weeks Assessment: baseline and post‐intervention Country: USA |
|
| Participants | Pain condition: pain from spinal cord injury Population: adults aged 18‐64 with spinal cord injury and dysthymia/major depression Minimum pain intensity: no Inclusion criteria
Exclusion criteria
Total participants randomised: 123 Age in years (mean, SD): 40 (11) Gender: 31/123 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Placebo
Venlafaxine 37.5 ‐ 225 mg
|
|
| Outcomes | Substantial pain relief Mood Withdrawal |
|
| Missing data methods | NR | |
| Funding source | Non‐pharmaceutical: the contents of this article were developed under a grant from the Department of Education, National Institute on Disability and Rehabilitation Research (grant no. H133A060107). | |
| Conflicts of interest | Supported by Pfizer in the form of study drug (0600B1‐4439). Study authors report no CoIs. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was computer‐generated by the study biostatistician |
| Allocation concealment (selection bias) | Low risk | Drug allocation handled by outside pharmacy "The investigational drug service at the lead center (University of Washington) trained and coordinated pharmacists at all sites, provided randomisation logs, and supplied active and placebo drug encapsulated into blinded study drug A and B." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical study drugs, matched dosing |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Missing data methods not specified Attrition Total: 29/123 (23.6%) Placebo: 14/59 (23.7%) Venlafaxine 37.5‐300 mg: 15/64 (23.4%) |
| Selective reporting (reporting bias) | Low risk | Outcomes as listed on the main trial registration (https://clinicaltrials.gov/ct2/show/study/NCT00592384) |
| Other bias | Low risk | No other sources of bias were identified. |
Rintala 2007.
| Study characteristics | ||
| Methods | Design: cross‐over Duration: each cross‐over period was 8 weeks Assessment: baseline and post‐cross‐over period Country: USA |
|
| Participants | Pain condition: chronic neuropathic pain following spinal cord injury Population: adults with a spinal cord injury at least 12 months ago with chronic neuropathic pain Minimum pain intensity: ≥ 5 on 0‐10 scale Inclusion criteria
Exclusion criteria
Total participants randomised: 38 Age in years (completers; mean, SD): 42.6 (12.6) Gender (completers): 2/38 were female Pain duration in years (completers; mean, SD): 7.3 (7.7) |
|
| Interventions | Gabapentin ≤ 3600 mg
Amitriptyline ≤ 150 mg
Placebo (diphenhydramine ≤ 75 mg)
|
|
| Outcomes | Pain intensity Moderate pain relief Withdrawal |
|
| Missing data methods | Completer‐only analysis | |
| Funding source | Non‐pharmaceutical: supported by the Department of Veterans Affairs, Veterans Health Administration, Rehabilitation Research and Development Service (grant no. B2573R) | |
| Conflicts of interest | "No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit upon the author(s) or upon any organisation with which the author(s) is/are associated" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The order of this assignment within the sets of 6 was based on a table of random numbers, and varied from set to set. |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedure not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Matched dosing regime, active comparator used as placebo, and identical capsules for medication |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer analysis only. High levels of attrition Attrition Total: 16/38 (42.1%) Gabapentin ≤ 3600 mg: 12/38 (31.6%) Amitriptyline ≤ 150 mg: 10/38 (26.3%) Placebo: 13/38 (34.2%) As this is a cross‐over study, some participants only withdrew from one period of the study, not the study as a whole, therefore, the numbers of participants withdrawing per arm does not match the total numbers of participants withdrawing. |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias identified |
Robinson 2004.
| Study characteristics | ||
| Methods | Design: parallel Duration: 6 weeks Assessment: baseline and post‐intervention Country: USA |
|
| Participants | Pain condition: phantom/residual limb pain Population: amputees with chronic phantom limb/residual limb pain Minimum pain intensity: ≥ 2 on 0‐10 scale Inclusion criteria
Exclusion criteria
Total participants randomised: 39 Age in years (mean, SD): 44.9 Gender: 5/20 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Placebo (benztropine mesylate 0.5 mg)
Amitriptyline
|
|
| Outcomes | Pain Mood Physical function Withdrawal |
|
| Missing data methods | ITT but no methods specified | |
| Funding source | Non‐pharmaceutical: supported by the National Institutes of Health, National Institute of Child Health and Human Development, National Institute of Neurological Disorders and Stroke (grant no. 1PO1 HD/NS33988) | |
| Conflicts of interest | "No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit upon the authors(s) or upon any organisation with which the author(s) is/are associated." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not specified |
| Allocation concealment (selection bias) | Low risk | Provision of medication was done by the Harborview Medical Center Pharmacy Investigational Drug Services. Medication was provided to each participant on a weekly basis by the study nurse or by mail for participants who lived far from the study center. A 7‐day supply of medication was provided to each participant each week in identical gelatin capsules placed in a plastic holder (Mediset), so that study personnel and participants were blind to medication assignment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical study medication |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data methods reported, but low withdrawal Attrition Total: 2/39 (5.1%) Placebo: 0/19 (0.0%) Amitriptyline ≤ 125 mg: 2/20 (10.0%) |
| Selective reporting (reporting bias) | Unclear risk | Outcomes not registered in protocol and protocol registered retrospectively |
| Other bias | Low risk | No other sources of bias were identified |
Rowbotham 2004.
| Study characteristics | ||
| Methods | Design: parallel Duration: 6 weeks Assessment: baseline and post‐intervention Country: USA |
|
| Participants | Pain condition: diabetic peripheral neuropathy Population: type 1 or 2 diabetic adults with diabetic peripheral neuropathy Minimum pain intensity: ≥ 40 on 0‐10 scale Inclusion criteria
Exclusion criteria
Total participants randomised: 245 Age in years (mean): 59 Gender: 99/245 were female Pain duration in weeks (mean): 252.6 |
|
| Interventions | Placebo
Venlafaxine 75 mg
Venlafaxine 150/225 mg
|
|
| Outcomes | Substantial pain relief AEs SAEs Withdrawal |
|
| Missing data methods | ITT with LOCF | |
| Funding source | Pharmaceutical: support for this study was provided by Wyeth Research, Collegeville, Pennsylvania. | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinded bottles and capsules, identical dosing schedules between groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT with LOCF Attrition Total: 43/245 (17.6%) Placebo: 12/81 (14.8%) Venlafaxine 75 mg: 13/82 (15.9%) Venlafaxine 150‐225 mg: 18/82 (22.0%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified. |
Rowbotham 2005.
| Study characteristics | ||
| Methods | Design: parallel Duration: 6 weeks Assessment: baseline and post‐intervention Country: USA |
|
| Participants | Pain condition: post‐herpetic neuralgia Population: people aged > 40 with post‐herpetic neuralgia Minimum pain intensity: no Inclusion criteria
Exclusion criteria
Total participants randomised: 47 Age in years (mean, range): 72 (40‐84) Gender: 27/47 were female Pain duration in months (mean, range): 42 (3‐168) |
|
| Interventions | Desipramine 25‐150 mg
Amitriptyline 25‐150 mg
Fluoxetine 10‐60 mg
|
|
| Outcomes | Withdrawal | |
| Missing data methods | ITT but no methods | |
| Funding source | Non‐pharmaceutical: supported by NIH program project grant NINDS 21445 and NINDS K24 NS02164 | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods NR |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures NR |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, double‐dummy design |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes by blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Says modified ITT but doesn't mention imputation method. Higher attrition in the fluoxetine arm than other arms Attrition Total: 9/47 (19.2%) Desipramine 25‐150 mg: 2/15 (13.3%) Amitriptyline 25‐150 mg: 2/17 (11.8%) Fluoxetine 10‐60 mg: 5/15 (33.3%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registry found |
| Other bias | Low risk | No other sources of bias were identified. |
Rowbotham 2012.
| Study characteristics | ||
| Methods | Design: parallel Duration: 8 weeks Assessment: baseline and post‐intervention Country: USA, Canada, France, Germany, Italy, Mexico, Puerto Rico |
|
| Participants | Pain condition: diabetic peripheral neuropathy Population: diabetic adults with diabetic peripheral neuropathic pain Minimum pain intensity: ≥ 4 on 0‐10 scale Inclusion criteria
Exclusion criteria
Total participants randomised: 280 Age in years (mean, SD): NR Gender: 128/280 were female Pain duration in years (mean): 4.68 |
|
| Interventions | Placebo
Duloxetine 60 mg
ABT‐894 2 mg
ABT‐894 4 mg
ABT‐894 8 mg
|
|
| Outcomes | Pain intensity Physical function Mood Quality of life PGIC AEs Withdrawal |
|
| Missing data methods | ITT with LOCF | |
| Funding source | Pharmaceutical: Abbott Laboratories: AbbVie (prior sponsor, Abbott) | |
| Conflicts of interest | These studies were sponsored by Abbott Laboratories. Dr Rowbotham has served as a consultant to Abbott, Adynxx, Afferent Pharmaceuticals, Allergan, Arcion, Bristol Meyers Squibb, Cardiome, Flexion, Kyowa Hakko Kirin, Neurotherapeutics Pharma, NuvoResearch, Xenon, Xenoport, and Zalicus. Dr Stacey has received grant support from NeurogesX and Pfizer, and has served as a consultant to AstraZeneca, Boehringer Ingelheim, Endo Pharmaceuti‐cals, NeurogesX, and Pfizer. Dr Arslanian has no conflicts of interest to declare. Dr Zhou is an employee of Abbott. Drs Nothaft, Duan, Best, and Pritchett are employees of Abbott and hold Abbott stock and stock options. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised 1:1 to each treatment arm using a randomisation schedule that was generated before study start. |
| Allocation concealment (selection bias) | Low risk | Patients were allocated to each treatment arm via an interactive voice response system. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not clear on blinding procedures regarding study drug appearance and dosing |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported outcomes from participants, but unsure of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | States ITT but no methods reported Attrition Total: 43/280 (15.4%) Placebo: 7/51 (13.7%) Duloxetine 60 mg: 13/57 (22.8%) ABT‐894 2 mg: 8/61 (13.1%) ABT‐894 4 mg: 8/56 (14.3%) ABT‐894 8 mg: 7/55 (12.7%) |
| Selective reporting (reporting bias) | Low risk | Everything as listed in the protocol |
| Other bias | Low risk | No other sources of bias were identified. |
Russell 2008.
| Study characteristics | ||
| Methods | Design: parallel Duration: 28 weeks Assessment: baseline, 3 months, post‐intervention Country: USA and Puerto Rico |
|
| Participants | Pain condition: fibromyalgia Population: adults with fibromyalgia with or without MDD Minimum pain intensity: ≥ 4 on 0‐10 scale Inclusion criteria
Exclusion criteria
Total participants randomised: 520 Age in years (mean, SD): 51.02 (10.87) Gender: 492/520 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Placebo
Duloxetine 20 mg then 60 mg
Duloxetine 60 mg
Duloxetine 120 mg
|
|
| Outcomes | Pain intensity Quality of life Physical function Mood Moderate pain relief Substantial pain relief PGIC AEs SAEs Withdrawal |
|
| Missing data methods | ITT with LOCF | |
| Funding source | Pharmaceutical: this work was sponsored by Eli Lilly and Company and Boehringer Ingelheim GmbH. | |
| Conflicts of interest | Drs Chappell, Detke, Kajdasz, Walker, and Wohlreich are employees and stockholders of Eli Lilly and Company. Drs Arnold, Mease, Russell, and Smith were Principal Investigators at sites conducting the trial. Their sites received funds for participating in the research study. Dr Arnold has received grants/research support from Eli Lilly and Company, Pfizer Inc, Cypress Biosciences Inc, Wyeth Pharmaceuticals, Sanofi‐Aventis, Boehringer Ingelheim, Allergan, and Forest; she has been a consultant for Eli Lilly and Company, Pfizer Inc, Cypress Biosciences Inc, Wyeth Pharmaceuticals, Sanofi‐Aventis, Boehringer Ingelheim, Sepracor, Forest Laboratories Inc, Allergan, Vivus Inc, and Organon; and she is on the Speakers Bureau of Eli Lilly and Company and Pfizer, Inc. Dr Mease has received grants/research support from Eli Lilly and Company, Pfizer Inc, Cypress Bioscience, Forest, Allergan, Fralex, and Boehringer Ingelheim; he has been a consultant for Eli Lilly and Company, Pfizer Inc, Cypress Bioscience, Forest, Allergan, Fralex, Boehringer Ingelheim, Pierre Fabre, and Wyeth; and he is on the Speakers Bureau of Pfizer Inc. Dr Russell has received grants/research support from the National Institutes of Health, RGK Foundation of Austin Texas, The National Fibromyalgia Association, Autoimmune Technologies, LLC, New Orleans, Louisiana, LKB World (Southern France), Pfizer Central Research, Eli Lilly and Company, Orphan Medical/Jazz, Grutnenthal GmbH, Allergan, and Schwarz; and he is on medical advisory boards of Pfizer Inc, Eli Lilly and Company, Jazz Pharmaceutical, Gruenthal GmbH, and Allergan. Dr Smith has received grants/research support from Abbott, Allergan, AstraZeneca, Bristol‐Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, Johnson and Johnson, Merck, Ortho‐McNeil, Pfizer Inc, Minster, Novartis, Novo Nordisk, Orexigen, Shionogi, Schwarz, Vernalis, and Wyeth; he has been a consultant or on advisory boards of Allergan, Eli Lilly and Company, was previously on a medical advisory board for Eli Lilly and Compant, GlaxoSmithKline and Merck. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Assignment to treatment groups was determined by a computer‐generated random sequence |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | All participants only took 1 dose daily to maintain blinding. No information about appearance, taste etc. Possibly some participants in the 20/60 arm would become unblinded with the increase in dose. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported outcomes but unsure of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT with LOCF. High attrition Attrition Total: 24/520 (46.7%) Placebo: 72/144 (50.0%) Duloxetine 20 mg then 60 mg: 35/79 (44.3%) Duloxetine 60 mg: 68/150 (45.3%) Duloxetine 120 mg: 68/147 (46.3%) |
| Selective reporting (reporting bias) | High risk | Not completely clear in all outcome measures to be used ‐ only domains ‐ in the protocol. In the trial registry results submitted by study authors: they show they've measured the same outcomes with multiple scales (Hamilton Depression Rating Scale and BDI‐II) have also measured further outcomes like BPI interference but do not report these. Have reported significant results in the trial report. |
| Other bias | Low risk | No other sources of bias were identified. |
Sarzi Puttini 1988.
| Study characteristics | ||
| Methods | Design: parallel Duration: 4 weeks Assessment: baseline and post‐intervention Country: Italy |
|
| Participants | Pain condition: RA Population: adults with RA and with or without depression Minimum pain intensity: ≥ 50 on 0‐100 scale Inclusion criteria
Exclusion criteria: NR Total participants randomised: 60 Age in years (mean, SD): NR Gender: 52/60 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Placebo
Dothiepin 75 mg
|
|
| Outcomes | Study provided no useable data | |
| Missing data methods | Completer analysis | |
| Funding source | NR | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation methods not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Says matched dosing schedules but not other information given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported outcomes from participants but unsure of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer analysis only Attrition Total: 10/60 (16.7%) Attrition per arm NR |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified |
Schukro 2016.
| Study characteristics | ||
| Methods | Design: cross‐over Duration: 4 weeks Assessment: baseline and post‐intervention Country: Austria |
|
| Participants | Pain condition: chronic low back pain with a neuropathic component Population: adults with chronic low back pain with a neuropathic component Minimum pain intensity: ≥ 5 on 0‐10 scale Inclusion criteria
Exclusion criteria
Total participants randomised: 41 Age in years (mean, SD): 57.9 (13.4) Gender: 21/41 were female Pain duration in months (mean, SD): 18 (6–70) |
|
| Interventions | Placebo
Duloxetine ≤ 120 mg
|
|
| Outcomes | Pain intensity Physical function Mood Substantial pain relief AEs Withdrawal |
|
| Missing data methods | ITT with LOCF | |
| Funding source | Non‐pharmaceutical: this study was supported by the Medical Scientific Fund of the Mayor of the City of Vienna, Vienna, Austria | |
| Conflicts of interest | The study authors declare no competing interests. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was computer‐assisted and stratified according to age and sex. |
| Allocation concealment (selection bias) | Low risk | Study drugs and placebo were packaged in blue opaque capsules, which were manufactured by the hospital pharmacy of the Medical University of Vienna, and administered according to the assignment code, which was held by an independent study nurse. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical study drugs with matched dosing |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT with LOCF. High attrition Attrition Total: 20/41 (48.8%) Placebo: 6/41 (14.6%) Duloxetine 120 mg: 6/41 (14.6%) 7 participants dropped out after randomisation but before starting to take study medication, and 1 participant dropped out between study periods. Therefore the total withdrawal is 20, while only 12 participants' withdrawal can be attributed to an arm. |
| Selective reporting (reporting bias) | High risk | Says in protocol registered on clinicaltrials.gov that participant data from the BDI will be collected at screening, week 4 and week 10, but in the paper it was only used as a screening tool. |
| Other bias | Low risk | No other sources of bias were identified. |
Scudds 1989.
| Study characteristics | ||
| Methods | Design: cross‐over Duration: 4 weeks per cross‐over period Assessment: baseline and post‐cross‐over period Country: Canada |
|
| Participants | Pain condition: fibrositis (fibromyalgia) Population: adults with fibromyalgia Minimum pain intensity: no Inclusion criteria
Exclusion criteria
Total participants randomised: 39 Age in years (completers; mean, SD): 39.9 (10.2) Gender (completers): 32/39 were female Pain duration in years (completers; mean, SD): 5.1 (4.6) |
|
| Interventions | Placebo
Amitriptyline 50 mg
|
|
| Outcomes | Withdrawal | |
| Missing data methods | Completer‐only analysis | |
| Funding source | Non‐pharmaceutical: "Supported in part by The Arthritis Society Studentship S‐198 to R.A. Scudds and NSERC Grant AO 392 10" | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures NR |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical study drugs, matched dosing |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Completer analysis but low dropout Attrition Total: 3/39 (7.7%) Placebo: 2/39 (5.13%) Amitriptyline 50 mg: 1/39 (2.6%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified. |
Sencan 2004.
| Study characteristics | ||
| Methods | Design: parallel Duration: 6 weeks Assessment: baseline, post‐intervention, follow‐up (6 months) Country: Turkey |
|
| Participants | Pain condition: fibromyalgia Population: women aged 18‐50 with fibromyalgia Minimum pain intensity: no Inclusion criteria
Exclusion criteria
Total participants randomised: 60 Age in years (mean): 34.5 Gender: 60/60 were female Pain duration in years (mean): 5.4 |
|
| Interventions | Aerobic exercise
Paroxetine 20 mg
Placebo TENS
|
|
| Outcomes | Pain intensity Mood |
|
| Missing data methods | No participants withdrew | |
| Funding source | NR | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants unable to be blinded due to the nature of interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self‐reported outcomes from unblinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants withdrew during the trial period |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified |
Shakiba 2018.
| Study characteristics | ||
| Methods | Design: parallel Duration: 8 weeks Assessment: baseline, 4 weeks, post‐intervention Country: Iran |
|
| Participants | Pain condition: fibromyalgia Population: adults aged 18‐60 with fibromyalgia Minimum pain intensity: ≥ 40 on 0‐100 scale Inclusion criteria
Exclusion criteria
Total participants randomised: 54 Age in years (mean): 41.98 Gender: 34/54 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Saffron 15 mg
Duloxetine 30 mg
|
|
| Outcomes | Pain intensity Mood Quality of life Withdrawal |
|
| Missing data methods | ITT with LOCF | |
| Funding source | Non‐pharmaceutical: "This study was supported by Tehran University of Medical Sciences (TUMS) through a grant to Prof. Shahin Akhondzadeh (Grant number 31842)." | |
| Conflicts of interest | "The authors of this manuscript declare that they have no COI. TUMS had no role in the design, conduct, data collection, analysis, data interpretation, manuscript preparation, review, final approval, or decision to submit this paper for publication." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation to either saffron or the duloxetine arm, was carried out in a 1:1 ratio through computerised random number generation by an independent person. |
| Allocation concealment (selection bias) | Low risk | Treatment allocation concealment was achieved using sequentially numbered sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Saffron capsules were identical to duloxetine in shape, size, texture, odour, and colour. Medications were distributed by an independent investigational drug pharmacist. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | State that they use ITT with LOCF, but then the n in tables is completers Attrition Total: 8/54 (14.8%) Saffron 15 mg: 4/27 (14.8%) Duloxetine 30 mg: 4/27 (14.8%) |
| Selective reporting (reporting bias) | Low risk | Outcomes listed prospectively: https://en.irct.ir/trial/940 |
| Other bias | Low risk | No other sources of bias were identified |
Sindrup 2003.
| Study characteristics | ||
| Methods | Design: cross‐over Duration: each cross‐over period lasted 4 weeks Assessment: baseline, post‐cross‐over period Country: Denmark |
|
| Participants | Pain condition: polyneuropathy Population: adults aged 20‐70 with painful polyneuropathy Minimum pain intensity: ≥ 4 on 0‐10 scale Inclusion criteria
Exclusion criteria
Total participants randomised: 40 Age in years (mean, range): 56 (31‐69) Gender: 9/40 were female Pain duration in months (mean, range): 51 (6‐300) |
|
| Interventions | Placebo
Venlafaxine 225 mg
Imipramine 150 mg
|
|
| Outcomes | AEs | |
| Missing data methods | Completer analysis | |
| Funding source | Non‐pharmaceutical: supported by the Danish National Research Council (NASTRA grant no. 42820) and the local research foundation at Odense University Hospital. | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Assignment to one of the 6 possible treatment sequences was random via a computer‐generated randomisation code. |
| Allocation concealment (selection bias) | Low risk | "The study drugs were packed in boxes marked with patient number and treatment period. After the baseline period, the patients were numbered consecutively and were treated with the study drugs with the corresponding randomisation number. Sealed envelopes with treatment sequence for each patient were present at the study sites for emergency situations." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy technique, matching study drugs and package appearance |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes by blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT with LOCF Attrition Total: 7/40 (17.5%) Not clear in which arm withdrawals occurred |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified. |
Skljarevski 2009.
| Study characteristics | ||
| Methods | Design: parallel Duration: 13 weeks Assessment: baseline and post‐intervention Country: USA and Argentina |
|
| Participants | Pain condition: low back pain Population: adult patients with non‐radicular chronic low back pain Minimum pain intensity: ≥ 4 on 0‐10 scale Inclusion criteria
Exclusion criteria
Total participants randomised: 404 Age in years (mean, SD): 53.9 (14.1) Gender: 232/404 were female Pain duration in years (mean, SD): 11.7 (11.4) |
|
| Interventions | Placebo
Duloxetine 20 mg
Duloxetine 60 mg
Duloxetine 120 mg
|
|
| Outcomes | Pain intensity Sleep Physical function Quality of life Mood PGIC Moderate pain relief Substantial pain relief AEs SAEs Withdrawal |
|
| Missing data methods | ITT with LOCF | |
| Funding source | Pharmaceutical: study design, funding and drugs were supplied by Eli Lilly and Company. | |
| Conflicts of interest | Authors V. Skljarevski, M. Ossanna, H. Liu‐Seifert, Q. Zhang, A. Chappell, S. Iyengar and M. Detke are or were at the time of submission employees of Eli Lilly and Company and may be minor shareholders. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly assigned by a computer‐generated random sequence. |
| Allocation concealment (selection bias) | Low risk | Participants were allocated using an Interactive Voice Response System. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical study drugs, matched dosing |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes by blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High attrition with significantly more missing in higher dose arm due to AEs. Use ITT and LOCF Attrition Total: 137/404 (33.9%) Placebo: 35/117 (29.9%) Duloxetine 20 mg: 16/59 (27.1%) Duloxetine 60 mg: 36/116 (31.0%) Duloxetine 120 mg: 50/112 (44.6%) |
| Selective reporting (reporting bias) | Low risk | Primary outcomes specified prospectively on clincialtrials.gov along with data not presented in paper |
| Other bias | Unclear risk | Some baseline differences in important variables: pain history but not imbalanced in a way which favours treatment |
Skljarevski 2010a.
| Study characteristics | ||
| Methods | Design: parallel Duration: 13 weeks Assessment: baseline and post‐intervention Country: Brazil, France, Germany, Mexico, and Netherlands |
|
| Participants | Pain condition: low back pain Population: adults with chronic low back pain Minimum pain intensity: ≥ 4 on 0‐10 scale Inclusion criteria
Exclusion criteria
Total participants randomised: 236 Age in years (mean): 51.5 Gender: 144/236 were female Pain duration in years (mean): 9.2 |
|
| Interventions | Placebo
Duloxetine 60‐120 mg
|
|
| Outcomes | Pain intensity Mood Physical function Quality of life Sleep PGIC Moderate pain relief Substantial pain relief AEs SAEs Withdrawal |
|
| Missing data methods | ITT with LOCF | |
| Funding source | Pharmaceutical: Eli Lilly and Company | |
| Conflicts of interest | Drs Skljarevski, Desaiah, Liu‐Seifert, Zhang, Chappell, and Iyengar are employees and stockholders of Eli Lilly and Company. Dr Detke was a full‐time employee and a major stock holder of Eli Lilly andCompany until March 2009 and is currently a full‐time employee and a major stock holder of Medavante Corporation. Dr Atkinson serves on Lilly Pain Advisory Board. Dr Backonja serves on Lilly Pain Advisory Board and in addition performed clinical trials and received research funding from Allergan, Astellas, Johnson and Johnson, Lilly,Merck, NeurogesX, and Pfizer Corporate/Industry and Foundation funds were received in support of this work. One or more of the author(s) has/have received or will receive benefits for personal or professional use from a commercial party related directly or indirectly to the subject of this manuscript: e.g., honoraria, gifts, consultancies, royalties, stocks, stock options, decision‐making position. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures not specified; only mention voice centralised system for allocating participants to higher dose, not when randomising and allocating all sample |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | States double blind, matched dosing but no information on study drug appearance |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported outcomes from participants, but uncertain of blinding procedures |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT with LOCF. Some data are not the same in the protocol and the paper: more AEs reported on clinicaltrials.gov than in the paper. Participants did have significant differences as to why they have missing data: duloxetine group had significantly more withdrawals due to AEs. Attrition Total: 54/236 (22.9%) Placebo: 23/121 (19.0%) Duloxetine 60‐120 mg: 31/115 (27.0%) |
| Selective reporting (reporting bias) | Low risk | All outcomes match those registered on clinicaltrials.gov |
| Other bias | Low risk | No other sources of bias identified |
Skljarevski 2010b.
| Study characteristics | ||
| Methods | Design: parallel Duration: 12 weeks Assessment: baseline and post‐intervention Country: Germany, Netherlands, Poland, Russia, Spain and USA |
|
| Participants | Pain condition: low back pain Population: adults with chronic low back pain Minimum pain intensity: ≥ 4 on 0‐10 scale Inclusion criteria
Exclusion criteria
Total participants randomised: 401 Age in years (mean): 54.1 Gender: 246/401 were female Pain duration in years (mean): 8.3 |
|
| Interventions | Placebo
Duloxetine 60 mg
|
|
| Outcomes | Pain intensity Sleep Mood Physical function Quality of life Moderate pain relief Substantial pain relief PGIC AEs SAEs Withdrawal |
|
| Missing data methods | ITT with LOCF, BOCF for sensitivity analyses of primary outcome | |
| Funding source | Pharmaceutical: Eli Lilly and Company | |
| Conflicts of interest | Drs Skljarevski and Desaiah, Ms Zhang, and Ms Alaka are employees of Eli Lilly and Company and hold company stocks. Drs Palacios, Miazgowski, and Patrick were study investigators and received funding from Eli Lilly and Company, Indianapolis, Indiana. These external authors had access to the data relevant to this manuscript. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | States double‐blind but no information on study drug appearance or matched dosing |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reprorted outcomes from participants, but uncertain of blinding procedures |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Used ITT with LOCF but for primary outcome used BOCF and mBOCF for sensitivity analysis. Results using all methods of imputation were significant. Attrition Total: 98/401 (24.4%) Placebo: 47/203 (23.2%) Duloxetine 60 mg: 51/198 (25.8%) |
| Selective reporting (reporting bias) | Low risk | Published outcomes match protocol |
| Other bias | Unclear risk | In the trial registry they've registered 2 research sites in Brazil but have just not mentioned it anywhere after, no reason for excluding those centres stated |
Smith 2013.
| Study characteristics | ||
| Methods | Design: cross‐over Duration: cross‐over periods were 5 weeks Assessment: baseline and post‐cross‐over period Country: USA |
|
| Participants | Pain condition: neuropathic pain caused by chemotherapy Population: adults aged ≥ 25 with cancer and neuropathic pain after completing chemotherapy Minimum pain intensity: ≥ 4 on 0‐10 scale Inclusion criteria
Exclusion criteria
Total participants randomised: 231 Age in years (mean, SD): 59 (10.5) Gender: 138/231 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Placebo
Duloxetine 60 mg
|
|
| Outcomes | Pain intensity Moderate pain relief Substantial pain relief Quality of life SAEs Withdrawal |
|
| Missing data methods | Completer analysis | |
| Funding source | Non‐pharmaceutical: This study was supported by grant CA31946 from the NCI Division of Cancer Prevention, the Alliance Statistics and Data Center, and the Alliance Chairman | |
| Conflicts of interest | "Disclosures: all authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Smith reported receiving support from CALGB/Alliance for travel to meetings. Dr Paskett reported institutional support from CALGB/Alliance for travel to meetings. Dr Ahles reported receiving support from CALBG/Alliance for travel to meetings. Dr Fadul reported pending institutional grants from Genentech. Dr Gilman reported institutional and direct grants pending from the NCI [National Cancer Institute]. No other financial disclosures were made." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation, provided by the CALGB/Alliance Statistical Center, was stratified by neurotoxic drug class (taxanes vs platinums) and by pain risk (high risk vs no risk). A computer‐generated kit number was used to order the blinded study drug from a distribution center." |
| Allocation concealment (selection bias) | Low risk | "A computer‐generated kit number was used to order the blinded study drug from a distribution center. Drug labels were applied to the capsule bottles at the distribution center before being mailed to study sites; thus, all patients and personnel were blinded to the treatment assignment." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical study drugs with matched dosing |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes by blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Mention ITT and imputation, but only report completer analysis Attrition Total: 33/230 (14.3%) Placebo: 12/111 (10.8%) Duloxetine 60 mg: 21/109 (19.3%) |
| Selective reporting (reporting bias) | Low risk | Outcomes match those on prospective trial registration on clinicaltrials.gov |
| Other bias | Low risk | No other sources of bias were identified. |
Sofat 2017.
| Study characteristics | ||
| Methods | Design: parallel Duration: 12 weeks Assessment: baseline and post‐intervention Country: UK |
|
| Participants | Pain condition: hand OA Population: adults aged 40–75 with hand OA Minimum pain intensity: ≥ 5 on 0‐10 scale Inclusion criteria
Exclusion criteria
Total participants randomised: 65 Age in years (mean, SD): NR Gender: 52/65 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Placebo
Pregabalin 300 mg
Duloxetine 60 mg
|
|
| Outcomes | Pain intensity Physical function Mood Withdrawal |
|
| Missing data methods | ITT with LOCF | |
| Funding source | Non‐pharmaceutical: "This work was supported by The Rosetrees’ Trust, grant number M11‐F1, by the UK National Institute of Health (NIHR) Clinical Research Network and an NIHR Clinical Academic Fellowship to MR" | |
| Conflicts of interest | Disclosure: the authors report no conflicts of interest in this work. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not specified |
| Allocation concealment (selection bias) | Low risk | The random allocation sequence, with a block size of nine, was generated by the manufacturer and implemented through sequentially numbered containers. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical study drugs, matched dosing |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT with LOCF Attrition Total: 13/65 (20.0%) Placebo: 3/22 (13.6%) Pregabalin 300 mg: 5/22 (22.7%) Duloxetine 60 mg: 5/21 (23.8%) |
| Selective reporting (reporting bias) | High risk | 2 protocols found registed, which have different primary outcomes. The protocol was submitted 2.5 years after recruitment started. |
| Other bias | Unclear risk | Small baseline difference in groups "prior analgesic use", there was slightly less paracetamol (acetaminophen) use at baseline before enrollment in the duloxetine group than in the pregabalin and placebo groups, but for other NSAIDs and opiates, analgesic use was similar in all 3 groups. |
Spinhoven 2010.
| Study characteristics | ||
| Methods | Design: parallel Duration: 16 weeks Assessment: baseline, mid‐intervention, post‐intervention Country: Netherlands |
|
| Participants | Pain condition: non‐cardiac chest pain Population: adult cardiology outpatients with a diagnosis of non‐cardiac chest pain Minimum pain intensity: no Inclusion criteria
Exclusion criteria
Total participants randomised: 69 Age in years (mean): 55.9 Gender: 32/69 were female Pain duration in years (mean): 5.4 |
|
| Interventions | Placebo
Paroxetine 10‐40 mg
CBT
|
|
| Outcomes | Pain intensity Mood Withdrawal |
|
| Missing data methods | ITT with LOCF | |
| Funding source | Partly funded by pharmaceutical: supported by a grant of the Dutch Heart Foundation (grant nr. 1998B209) and an unconditional educational grant of Glaxo Smith Kline. | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using random permuted blocks with a length of 6. |
| Allocation concealment (selection bias) | Unclear risk | States allocation by pharmacists not involved in trial, but procedure not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding across arms not possible due to nature of CBT intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self‐reported outcomes from unblinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT with LOCF and unequal attrition Attrition Total: 11/69 (15.9%) Placebo: 4/23 (17.4%) Paroxetine 10‐40 mg: 7/23 (30.4%) CBT: 0/23 (0.0%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | High risk | Selection bias: 379 (80%) of patients approached refused participation due to potential of being put on paroxetine |
Srinivasan 2021.
| Study characteristics | ||
| Methods | Design: cross‐over Duration: cross‐over periods lasted 6 weeks Assessment: baseline and post‐cross‐over period Country: India |
|
| Participants | Pain condition: painful diabetic neuropathy Population: adults aged 18‐75 with type 2 diabetes and painful diabetic neuropathy Minimum pain intensity: ≥ 50 on 0‐100 scale Inclusion criteria
Exclusion criteria
Total participants randomised: 67 Age in years (mean, SD): 49 (4) Gender: 32/67 were female Pain duration in years (mean, SD): 28 (6) |
|
| Interventions | Naltrexone 4‐8 mg
Amitriptyline 10‐50 mg
|
|
| Outcomes | PGIC AEs Withdrawal |
|
| Missing data methods | ITT with multiple imputation | |
| Funding source | Non‐pharmaceutical: postgraduate Institute of Medical Education and Research | |
| Conflicts of interest | The authors have no COI pertaining to this study. The authors are thankful to M/s. Sun Pharmaceutical Industries Limited, Mumbai (India), and M/s. Wockhardt Pharmaceuticals, Mumbai (India), for providing the pure naltrexone active pharmaceutical ingredient and amitriptyline tablets, respectively, for this study. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation codes were generated by a random block randomisation method using the “random allocation software.” |
| Allocation concealment (selection bias) | Low risk | "The blinding and allocation concealment was maintained by labeling the container with the serial numbers provided for each randomisation code by a person not related to the trial. The drugs were dispensed by an investigator who was neither involved in screening nor involved in evaluating the end points of the study." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical appearance and dosing |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Multiple imputation techniques (multivariate imputation by chained equations) was used to deal with the missing values for ITT. Low dropouts, balanced across arms Attrition Total: 7/67 (10.5%) Naltrexone 2‐4 mg: 2/67 (3.0%) Amitriptyline 25‐50 mg: 5/67 (7.5%) |
| Selective reporting (reporting bias) | Unclear risk | Trial registered retrospectively |
| Other bias | Low risk | No other sources of bias were identified. |
Staud 2015.
| Study characteristics | ||
| Methods | Design: parallel Duration: 6 weeks Assessment: baseline and post‐intervention Country: USA |
|
| Participants | Pain condition: fibromyalgia Population: adults with fibromyalgia Minimum pain intensity: ≥ 4 on 0‐10 VAS Inclusion criteria
Exclusion criteria
Total participants randomised: 61 Age in years (mean, SD): NR Gender: 56/61 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Placebo
Milnacipran 100 mg
|
|
| Outcomes | Pain intensity Mood Withdrawal |
|
| Missing data methods | ITT with LOCF | |
| Funding source | Pharmaceutical: This study was supported by an investigator‐initiated grant from Forest Laboratories. All study drugs were provided by Forest Laboratories. | |
| Conflicts of interest | Funded by an investigator‐initiated grant from Forest Laboratories. All study drugs were provided by Forest Laboratories. The sponsors of this trial had no role in planning and implementing the study, and in the analysis of the data. They were not involved in the writing of this report. None of the authors have any financial or other relationships that might lead to a COI. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using Research Randomizer (http://www.randomizer.org/) |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedure not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical study drugs with matched dosing |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Similar attrition in both arms, report that they will use LOCF for missing data but then state that as "missing data did not result in different conclusions, we report only the results of uncorrected analyses", so completer analysis. Attrition Total: 26/62 (41.9%) Placebo: 5/23 (21.7%) Milnacipran 100 mg: 6/23 (26.1%) 15 participants (8 milnacipran, 7 placebo) withdrew post‐randomisation prior to receiving study medication, so were not included in the arm‐specific totals above. No reasons were given for the withdrawals of these 15 participants. |
| Selective reporting (reporting bias) | High risk | Protocol only lists mechanical and heat hyperalgesia and clinical pain as outcomes. Doesn't specify how these will be collected or the other measures used in the study. |
| Other bias | High risk | Create a second baseline essentially: a lot of participants withdrew after randomisation and so the authors ignore that in final analysis and only include those who came back for a second study visit. |
Suttiruksa 2016.
| Study characteristics | ||
| Methods | Design: parallel Duration: 13 weeks Assessment: baseline, 5 weeks, 9 weeks, post‐intervention Country: Thailand |
|
| Participants | Pain condition: fibromyalgia Population: Thai adults with fibromyalgia Minimum pain intensity: ≥ 40 on 0‐100 VAS Inclusion criteria
Exclusion criteria
Total participants randomised: 40 Age in years (mean): 44.7 Gender: 40/40 were female Pain duration in years (mean): 3.5 |
|
| Interventions | Placebo
Mirtazapine 15 mg
Mirtazapine 30 mg
|
|
| Outcomes | Pain intensity Mood Physical function SAEs Withdrawal |
|
| Missing data methods | ITT but no methods specified | |
| Funding source | Non‐pharmaceutical: This work was supported by the Office of theHigher Education Commission, Thailand through a grant in the program “Strategic Scholarships for Frontier Research Network for the PhD Program, Thai Doctoral degree”. | |
| Conflicts of interest | The authors declare that there is no COI in this research. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The patients were allocated using a block size of 3 in a ratio of 1:1:1 with parallel assignment to 1 of 3 groups, using a pharmacy‐controlled randomisation process with a random number table. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered identical containers that were administered serially |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical study drugs, matched dosing |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes by blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT but no methods specified Attrition Total: 8/40 (20.0%) Placebo: 3/13 (23.1%) Mirtazapine 15 mg: 2/13 (15.4%) Mirtazapine 30 mg: 3/14 (21.4%) |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol registered online is for multiple studies (https://clinicaltrials.gov/ct2/show/NCT00919295) |
| Other bias | Low risk | No other sources of bias were identified |
Talley 2008.
| Study characteristics | ||
| Methods | Design: parallel Duration: 12 weeks Assessment: baseline and post‐intervention Country: Australia |
|
| Participants | Pain condition: IBS Population: people with IBS Minimum pain intensity: ≥ 3 on 0‐10 scale Inclusion criteria
Exclusion criteria
Total participants randomised: 51 Age in years (mean, SD): NR Gender: NR Pain duration in years (mean, SD): NR |
|
| Interventions | Placebo
Imipramine 50 mg
Citalopram 40 mg
|
|
| Outcomes | Pain intensity Mood Physical function SAEs Withdrawal |
|
| Missing data methods | ITT with LOCF | |
| Funding source | Non‐pharmaceutical: this work was supported by the National Health and Medical Research Council of Australia (Dr Talley, PI). | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised to one of the 3 treatment arms using a computer‐generated random list. |
| Allocation concealment (selection bias) | Low risk | Concealed allocation was assured by a central drug distribution from the hospital pharmacy. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical study drugs, double‐dummy design |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT with LOCF. Very unequal attrition between arms, high attrition for imipramine Attrition Total: 17/51 (33.3%) Placebo: 3/16 (18.8%) Imipramine 50 mg: 9/18 (50.0%) Citalopram 40 mg: 5/17 (29.4%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified. |
Tammiala‐Salonen 1999.
| Study characteristics | ||
| Methods | Design: parallel Duration: 8 weeks Assessment: baseline and post‐intervention Country: Finland |
|
| Participants | Pain condition: burning mouth syndrome Population: women with burning mouth syndrome Minimum pain intensity: ≥ 30 on 0‐100 VAS Inclusion criteria
Exclusion criteria: NR Total participants randomised: 37 Age in years (mean, range): 58.6 (39‐71) Gender: 37/37 were female Pain duration in years (mean, range): 2.8, (6 months‐20 years) |
|
| Interventions | Placebo
Trazodone 200 mg
|
|
| Outcomes | Withdrawal | |
| Missing data methods | Completer analysis | |
| Funding source | Non‐pharmaceutical: the study was supported by the Finnish Dental Society. | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical study drugs, matched dosing |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer analysis only. Unequal attrition ‐ more participants withdrew due to AEs in the intervention arm than the placebo arm Attrition Total: 9/37 (24.3%) Placebo: 2/19 (10.5%) Trazodone 200 mg: 7/18 (38.9%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified. |
Tanum 1996.
| Study characteristics | ||
| Methods | Design: parallel Duration: 7 weeks Assessment: baseline, 3 weeks, post‐intervention, follow‐up (4 weeks after taper) Country: Norway |
|
| Participants | Pain condition: functional gastrointestinal disorder Population: adults aged 18‐70 functional gastrointestinal disorder Minimum pain intensity: no Inclusion criteria:
Exclusion criteria
Total participants randomised: 49 Age in years (mean): 37.3 Gender: 32/49 were female Pain duration in years (mean, SD): 8.3 (9.2) |
|
| Interventions | Placebo
Mianserin
|
|
| Outcomes | Pain intensity Substantial pain relief Withdrawal |
|
| Missing data methods | ITT with LOCF | |
| Funding source | Non‐pharmaceutical: the study was on request supported by an educational grant from NV Organon, Oss, The Netherlands | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical study drugs, matched dosing |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT with LOCF, but low attrition Attrition Total: 2/49 (4.1%) Placebo: 0/22 (0.0%) Mianserin 120 mg: 2/27 (7.4%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias identified |
Tasmuth 2002.
| Study characteristics | ||
| Methods | Design: cross‐over Duration: cross‐over periods were 4 weeks Assessment: baseline and post‐cross‐over period Country: Finland |
|
| Participants | Pain condition: neuropathic pain following breast cancer treatment Population: women with neuropathic pain following treatment of breast cancer Minimum pain intensity: moderate severity (no numerical scale) Inclusion criteria
Exclusion criteria
Total participants randomised: 15 Age in years (mean, range): 55 (37‐72) Gender: 15/15 were female Pain duration in months (mean, range): 20 (18‐26) |
|
| Interventions | Placebo
Venlafaxine ≤ 75 mg
|
|
| Outcomes | The study provided no useable data | |
| Missing data methods | NR | |
| Funding source | Non‐pharmacetucal: financial support was received from the Helsinki University Central Hospital Research Funds. | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Hospital pharmacy performed the randomisation using computer‐generated numbers |
| Allocation concealment (selection bias) | Unclear risk | States that hospital pharmacy performed randomisation but not how this was allocated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | States double‐blind, matched dosing, but no information regarding appearance of study drugs |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported outcomes by blinded participants, but unsure of blinding methods |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Low attrition, but not explained fully (i.e. during which period dropout happened) Attrition Total: 2/15 (13.3%) Attrition per arm NR |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified. |
Tesfaye 2013.
| Study characteristics | ||
| Methods | Design: parallel Duration: 8 weeks Assessment: baseline to post‐intervention Country: Australia, Canada, Croatia, France, Germany, Greece, Italy, South Korea, Mexico, Netherlands, Poland, Spain, Sweden, Switzerland, Turkey, UK |
|
| Participants | Pain condition: diabetic neuropathy Population: adults with diabetes type 1 or 2 and diabetic neuropathy Minimum pain intensity: ≥ 4 on 0‐10 scale Inclusion criteria
Exclusion criteria
Total participants randomised: 811 Age in years (mean): 61.7 Gender: 356/804 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Duloxetine 60 mg
Pregabalin 300 mg
|
|
| Outcomes | Pain intensity Physical function Mood Moderate pain relief Substantial pain relief PGIC AEs SAEs Withdrawal |
|
| Missing data methods | ITT with LOCF | |
| Funding source | Pharmaceutical: The sponsor, Eli Lilly & Company (Indianapolis, IN, USA), was involved in study design, in the collection, analysis, and interpretation of data, in the writing of the manuscript, and in the decision to submit the paper for publication. | |
| Conflicts of interest | The sponsor, Eli Lilly & Company (Indianapolis, IN, USA), was involved in study design, in the collection, analysis, and interpretation of data, in the writing of the manuscript, and in the decision to submit the paper for publication.
Stefan Wilhelm, Alexander Schacht, and Vladimir Skljarevski own stock in and are Lilly employees. Alberto Lledo, former Lilly employee, owns Lilly stocks. Solomon Tesfaye, Thomas T√∂lle, Didier Bouhassira, Giorgio Cruccu, and Rainer Freynhagen have received economic compensation for participation in the Lilly EU Pain Advisory Board. Solomon Tesfaye declares having received honoraria for invited lectures from Eli Lilly & Company and Pfizer Inc. Thomas Tolle reports consultancy and invited lectures for Grunenthal, Mundipharma, Biogen Idec, Hexal, Pfizer Inc., Janssen‐Cilag, Astellas, Pharmaleads, Boehringer‐Ingelheim, Eli Lilly & Company, and Esteve. Didier Bouhassira has served on the Speakers‚Äô Bureau for Eli Lilly & Company, Pfizer Inc., and Astellas, and has worked as a consultant to Eli Lilly & Company, Pfizer Inc., Sanofi‐Aventis, SanofiPasteur‐MSD, Astra Zeneca, and Astellas and has received research support from Pfizer Inc. Giorgio Cruccu has received fees for advisory boards and for lectures by Astellas, Eli Lilly & Company, and Pfizer Inc. Rainer Freynhagen has received consultancy and speaker fees in the past 12 months from Astellas, Epionics, Grunenthal, Forrest Research, HRA, Eli Lilly & Company, and Pfizer. All authors have made substantial contribution to conception and design of the COMBO‐DN study, or analysis or interpretation of the data or revising the manuscript critically for important intellectual content. Alberto Lledo was responsible for generating the primary hypothesis of the study and reviewed the manuscript critically. Solomon Tesfaye, Thomas Tolle, Didier Bouhassira, Giorgio Gruccu, and Rainer Freynhagen were involved in the early conception of the study, the selection of the primary and secondary objectives and the final review of the manuscript. Alexander Schacht was responsible for building the final statistical plan. Stefan Wilhelm and Alexander Schacht were responsible for data collection and extraction and completion of the final study report. Solomon Tesfaye and Stefan Wilhelm wrote the primary version of the manuscript and Vladimir Skljarevski reviewed the manuscript critically with regard to interpretation of the data. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "At the start of the initial therapy period, patients were randomised in a 1:1:1:1 ratio to 4 parallel groups stratified by site, based on a computer‐generated sequence" |
| Allocation concealment (selection bias) | Low risk | Participants were allocated using a centralised interactive voice response system. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical study drugs, matched dosing |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT with LOCF Attrition Total: 138/811 (17.1%) Pregabalin 300 mg: 70/403 (17.4%) Duloxetine 60 mg: 68/401 (17.0%) |
| Selective reporting (reporting bias) | Low risk | Outcomes match trial registration |
| Other bias | Low risk | No other sources of bias identified |
Trugman 2014.
| Study characteristics | ||
| Methods | Design: parallel Duration: 7 weeks Assessment: baseline and post‐intervention Country: USA |
|
| Participants | Pain condition: fibromyalgia Population: adults with fibromyalgia Minimum pain intensity: no Inclusion criteria
Exclusion criteria
Total participants randomised: 321 Age in years (mean): 49.2 Gender: 264/321 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Placebo
Milnacipran
|
|
| Outcomes | AEs SAEs Withdrawal |
|
| Missing data methods | Unclear | |
| Funding source | Pharmaceutical: The study was sponsored by Forest Laboratories Inc. in collaboration with Cypress Bioscience Inc. (acquired by Royalty Pharma). | |
| Conflicts of interest | J.M.T., R.H.P. and Y.M. are all full‐time employees with Forest Research Institute Inc., a wholly owned subsidiary of Forest Laboratories Inc. CMRO peer reviewers may have received honoraria for their review work. The peer reviewers on this manuscript have disclosed that they have no relevant financial relationships. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods NR |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures NR |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear blinding of study drugs, says "matched" but no other information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported outcomes from participants, uncertain of blinding procedures |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear missing data methods Attrition Total: 75/321 (23.4%) Placebo: 25/111 (22.5%) Milnacipran 200 mg: 50/210 (23.8%) |
| Selective reporting (reporting bias) | Low risk | Primary outcomes match those registered prospectively on clinicaltrials.gov |
| Other bias | High risk | Outcomes extracted from published paper, but these are very different to what's registered in the trial registry results |
Tétreault 2016.
| Study characteristics | ||
| Methods | Design: parallel Duration: 16 weeks Assessment: baseline and post‐intervention Country: USA |
|
| Participants | Pain condition: knee OA Population: adults aged 45‐80 with knee OA Minimum pain intensity: ≥ 5 on 0‐10 scale Inclusion criteria
Exclusion criteria
Total participants randomised: 40 Age in years (mean, SD): 58.7 (7.6) Gender: 21/40 were female Pain duration in years (mean, SD): 10.54 (9.1) |
|
| Interventions | Placebo
Duloxetine 60 mg
|
|
| Outcomes | Pain intensity Physical function Mood AEs SAEs |
|
| Missing data methods | Completer‐only analysis | |
| Funding source | Partly pharmaceutical: Eli Lilly Pharmaceuticals (IIT number: F1J‐US‐XO61). This research was also partially supported by grants from National Institute of Neurological Disorders and Stroke, ninds.nih.gov (NS035115), and National Center for Complementary and Integrative Health, nccih.nih.gov (AT007987) of the US National Institutes of Health. PT was supported by postdoctoral fellowships from the Canadian Institutes of Health Research (CIHR), cihr‐irsc.gc.ca. | |
| Conflicts of interest | No financial or other relationships that might lead to a COI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical study drugs, matched dosing |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer analysis only Attrition Total: 21/60 (35.0%) Attrition per arm NR |
| Selective reporting (reporting bias) | Low risk | Outcomes match those in the protocol |
| Other bias | Low risk | No other sources of bias identified |
Uchio 2018.
| Study characteristics | ||
| Methods | Design: parallel Duration: 14 weeks Assessment: baseline and post‐intervention Country: Japan |
|
| Participants | Pain condition: knee OA Population: adults aged 40‐80 with chronic knee pain due to OA Minimum pain intensity: ≥ 4 on 0‐10 scale Inclusion criteria
Exclusion criteria
Total participants randomised: 354 Age in years (mean): 65.9 Gender: 274/354 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Placebo
Duloxetine 60 mg
|
|
| Outcomes | Pain intensity Sleep Quality of life Mood Physical function Moderate pain relief Substantial pain relief PGIC AEs SAEs Withdrawal |
|
| Missing data methods | ITT with LOCF and BOCF | |
| Funding source | Pharmaceutical: Eli Lilly and Company and Shionogi | |
| Conflicts of interest | TT is an employee of and owns stock in Shionogi Co. Ltd. HE, SF, NS, and HT are employees of Eli Lilly Japan K.K. SF and HE own stock in Eli Lilly and Company. YU has been a member of a Board of Directors and Speakers' Bureau and had a consulting role with Eli Lilly Japan K.K. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised participants an Interactive Web Response System and stochastic minimisation method |
| Allocation concealment (selection bias) | Low risk | Participants were allocated using an Interactive Web Response System |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical capsules for study drugs, matched doses |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes by blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition. Missing data were imputed using the LOCF, BOCF, or the modified BOCF. These findings were consistent for all missing data imputation methods. Attrition Total: 31/354 (8.8%) Placebo: 14/176 (8.0%) Duloxetine 60 mg: 17/178 (10.0%) |
| Selective reporting (reporting bias) | Low risk | Some results for outcomes (BDI, Patient Global Assessment of Illness) reported on clinicaltrials.gov but not in the paper |
| Other bias | Low risk | No other sources of bias were identified. |
Urquhart 2018.
| Study characteristics | ||
| Methods | Design: parallel Duration: 24 weeks Assessment: baseline, 3 months, post‐intervention Country: Australia |
|
| Participants | Pain condition: low back pain Population: people aged 18‐75 with chronic non‐specific low back pain Minimum pain intensity: no Inclusion criteria
Exclusion criteria
Total participants randomised: 146 Age in years (mean, SD): 54.8 (13.7) Gender: 53/146 were female Pain duration in years (mean): 14.3 |
|
| Interventions | Placebo (benzotropine mesylate 1 mg)
Amitriptyline 25 mg
|
|
| Outcomes | Pain intensity Physical function Mood Quality of life AEs Withdrawal |
|
| Missing data methods | ITT using multiple imputation with chained equations | |
| Funding source | Non‐pharmaceutical: "This work was supported by theNational Health and Medical Research Council(NHMRC, Australia, ID 1024401). Drs Urquhart, Wluka, and Wang are recipients of NHMRC Career Development Fellowships (Clinical Level 1 No.1011975; Clinical Level 2 No. 1063574; Clinical Level1 No. 1065464, respectively)" | |
| Conflicts of interest | None reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was based on computer‐generated random numbers prepared by a statistician who had no involvement in trial conduct." |
| Allocation concealment (selection bias) | Low risk | "The use of a central allocation that involved pharmacy‐controlled randomisation ensured that the allocation could not be accessed by research personnel." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, active placebo, identical appearance, matched dosing |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes by blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Uses multiple imputation by chained equations, presents comparisons with no multiple imputation Attrition Total: 28/146 (19.2%) Placebo: 15/74 (20.3%) Amitriptyline 25 mg: 13/72 (18.1%) |
| Selective reporting (reporting bias) | Low risk | Matches protocol. Explains why Descriptor Differential Scale is NR (participants had difficulty filling it in) |
| Other bias | Low risk | No other sources of bias |
Vahedi 2005.
| Study characteristics | ||
| Methods | Design: parallel Duration: 12 weeks Assessment: baseline, post‐intervention, follow‐up (4 weeks post‐intervention) Country: Iran |
|
| Participants | Pain condition: IBS Population: people with pain and constipation‐predominant IBS Minimum pain intensity: no Inclusion criteria
Exclusion criteria
Total participants randomised: 44 Age in years (mean, SD): 34.9 (10.0) Gender: 27/44 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Placebo
Fluoxetine 20 mg
|
|
| Outcomes | Withdrawal | |
| Missing data methods | No participants withdrew | |
| Funding source | Non‐pharmaceutical: This study was supported by a grant from the Digestive Disease Research Center of Tehran University of Medical Sciences. | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly assigned according to a computer‐generated randomisation table |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures NR |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical study drugs, matched dosing |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes by blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants withdrew |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified. |
Van Ophoven 2004.
| Study characteristics | ||
| Methods | Design: parallel Duration: 16 weeks Assessment: baseline and post‐intervention Country: Germany |
|
| Participants | Pain condition: interstitial cystitis Population: adults with interstitial cystitis Minimum pain intensity: Inclusion criteria
Exclusion criteria
Total participants randomised: 50 Age in years (mean, SD): NR Gender: 44/50 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Placebo
Amitriptyline ≤ 100 mg
|
|
| Outcomes | Pain intensity AEs SAEs Withdrawal |
|
| Missing data methods | NR | |
| Funding source | NR | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods NR |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures NR |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Identical study medication, amitriptyline arm could self‐titrate, no information given about whether this was matched for placebo arm |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported outcomes from participants, uncertain of blinding procedures |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Completer‐only analysis, but ≤ 5% dropout Attrition Total: 2/50 (4.0%) Placebo: 1/25 (4.0%) Amitriptyline ≤ 100 mg: 1/25 (4.0%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified. |
Ventafridda 1987.
| Study characteristics | ||
| Methods | Design: parallel Duration: 15 days Assessment: baseline and post‐intervention Country: Italy |
|
| Participants | Pain condition: chronic pain syndromes from deafferentation and with oncological pain with deafferentation component Population: adults aged 34‐79 with cancer pain and other painful syndromes with deafferentation component Minimum pain intensity: no Inclusion criteria
Exclusion criteria
Total participants randomised: 45 Age in years (range) 34‐79 Gender: NR Pain duration in years (mean, SD): NR |
|
| Interventions | Amitriptyline 75 mg
Trazodone 225 mg
|
|
| Outcomes | Withdrawal | |
| Missing data methods | Completer‐only analysis | |
| Funding source | NR | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods NR |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures NR |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical appearance of study drugs |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer analysis with ~30% dropout Attrition Total: 14/45 (31.1%) Amitriptyline 75 mg: 4/22 (18.2%) Trazodone 225 mg: 10/23 (43.5%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Unclear risk | Unclear ‐ data other than withdrawal not presented in any useable way, no tables only figures |
Vitton 2004.
| Study characteristics | ||
| Methods | Design: parallel Duration: 12 weeks Assessment: baseline and post‐intervention Country: USA |
|
| Participants | Pain condition: fibromyalgia Population: adults aged 18‐70 with fibromyalgia Minimum pain intensity: ≥ 10 on a 20‐point logarithmic pain scale (Gracely scale) Inclusion criteria
Exclusion criteria
Total participants randomised: 125 Age in years (mean, SD): 47.0 (11.1) Gender: 122/125 were female Pain duration in years (mean, SD): 4.1 (4.2) years |
|
| Interventions | Placebo
Milnacipran ≤ 200 mg (one dose)
Milnacipran ≤ 200 mg (2 doses)
|
|
| Outcomes | Pain intensity Sleep Moderate pain relief Substantial pain relief PGIC SAEs Withdrawal |
|
| Missing data methods | ITT with LOCF | |
| Funding source | Pharmaceutical: supported by Cypress Biosciences, San Diego, California | |
| Conflicts of interest | Drs M.Gendreau, J. Gendreau, and J. Kranzler are employees of Cypress Biosciences. Drs Clauw, Gracely, and Williams are paid consultants for and shareholders in Cypress Biosciences. Drs Mease and Thorn are consultants for Cypress Biosciences. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using a randomisation table. |
| Allocation concealment (selection bias) | Low risk | Participants were allocated using an automated telephone response system. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical study drugs, matched dosing, double‐dummy design |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT with LOCF Attrition Total: 35/125 (28.0%) Placebo: 7/28 (25.0%) Milnacipran 200 mg: 14/46 (30.4%) Milnacipran 400 mg: 14/51 (27.5%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Unclear risk | Imbalance in prevalence of depression at baseline but no further information on whether controlled for or which group had more/less |
Vollmer 2014.
| Study characteristics | ||
| Methods | Design: parallel Duration: 6 weeks Assessment: baseline and post‐intervention Country: Belgium, Canada, Poland and the USA |
|
| Participants | Pain condition: central neuropathic pain due to multiple sclerosis Population: adults with multiple sclerosis experiencing chronic neuropathic pain Minimum pain intensity: ≥ 4 on 0‐10 scale Inclusion criteria
Exclusion criteria
Total participants randomised: 239 Age in years (mean): 51.8 Gender: 179/239 were female Pain duration in years (mean): 6.9 |
|
| Interventions | Placebo
Duloxetine 60 mg
|
|
| Outcomes | Pain intensity Quality of life Mood Sleep Moderate pain relief Substantial pain relief PGIC AEs SAEs Withdrawal |
|
| Missing data methods | ITT with LOCF and BOCF as sensitivity analysis | |
| Funding source | Pharmaceutical: Eli Lilly and Company | |
| Conflicts of interest | Dr Robinson was a full‐time employee and shareholder of Eli Lilly and Company at the time this study was conducted. Dr Robinson is a current employee of AbbVie. Author TLV is a consultant and/or advisory board member with Lilly and has received grants from and is involved in research supported by Lilly. Authors RCR and SKM are current employees and/or stockholders of Lilly. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods NR |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures NR |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, matched doses, but no information regarding study drugs appearance etc |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported outcomes from participants, unclear regarding blinding procedures |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT with LOCF, BOCF for sensitivity analysis of primary outcome ‐ no significant differences. Low attriton Attrition Total: 30/239 (12.6%) Placebo: 12/121 (9.9%) Duloxetine 60 mg: 18/118 (15.3%) |
| Selective reporting (reporting bias) | Low risk | All outcomes pre‐specified on clinicaltrials.gov prospectively. |
| Other bias | Low risk | No other sources of bias identified |
Vranken 2011.
| Study characteristics | ||
| Methods | Design: parallel Duration: 8 weeks Assessment: baseline and post‐intervention Country: Netherlands |
|
| Participants | Pain condition: neuropathic pain caused by spinal cord injury or stroke Population: people with severe neuropathic pain caused by spinal cord injury or stroke Minimum pain intensity: ≥ 6 on 0‐10 scale Inclusion criteria
Exclusion criteria
Total participants randomised: 48 Age in years (mean, SD): NR Gender: NR Pain duration in years (mean, SD): NR |
|
| Interventions | Placebo
Duloxetine 60‐120 mg
|
|
| Outcomes | Pain intensity Quality of life Physical function Mood PGIC Withdrawal |
|
| Missing data methods | State ITT but no methods | |
| Funding source | Non‐pharmaceutical: "Academic Medical Center": assuming the author's institution: Medical Center Alkmaar | |
| Conflicts of interest | There are no conflicts of interest. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using computerised random sampling (clorandm.exe) |
| Allocation concealment (selection bias) | Low risk | At baseline each coded medication bottle was supplied by the hospital pharmacist to the blinded treating physician. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical study drugs, sham dosing of placebo to match intervention arm |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition. State ITT but do not report methods Attrition Total: 4/48 (8.3%) Placebo: 1/24 (4.2%) Duloxetine 60‐120 mg: 3/24 (12.5%) |
| Selective reporting (reporting bias) | Low risk | Matches what's registered in protocol: https://www.trialregister.nl/trial/1125 |
| Other bias | Low risk | No other sources of bias were identified. |
Vrethem 1997.
| Study characteristics | ||
| Methods | Design: cross‐over Duration: each cross‐over period lasted 4 weeks Assessment: baseline, mid‐intervention (8‐14 days), post‐cross‐over period Country: Sweden |
|
| Participants | Pain condition: polyneuropathy (diabetic and non‐diabetic) Population: adults with painful polyneuropathy. 19 had diabetic polyneuropathy, 18 had non‐diabetic polyneuropathy Minimum pain intensity: no Inclusion criteria
Exclusion criteria
Total participants randomised: 37 Age in years (range): 35‐83 Gender: 19/37 were female Pain duration in years (range): 6‐168 |
|
| Interventions | Placebo
Amitriptyline 75 mg
Maprotiline 75 mg
|
|
| Outcomes | Pain intensity | |
| Missing data methods | NR | |
| Funding source | Non‐pharmaceutical: This work was supported by grants from The Medical Research Council, project no. 9058, The Swedish Association of Neurologically Disabled, The County Council of Ostergotland, and The University Hospital of Linkoping | |
| Conflicts of interest | NR | |
| Notes | This study reported results separately for participants with and without neuropathic pain caused by diabetes. Therefore, in the NMA we separated the study into 2 to include the 2 sets of results for both populations. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods NR |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures NR |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical study drugs, double‐dummy design |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No clear information regarding withdrawal, no information regarding missing data methods Attrition Total: 4/37 (10.8%) Attrition per arm NR |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified |
Wang 2017.
| Study characteristics | ||
| Methods | Design: parallel Duration: 13 weeks Assessment: baseline and post‐intervention Country: China |
|
| Participants | Pain condition: knee or hip OA Population: adults aged ≥ 40 with knee or hip OA Minimum pain intensity: ≥ 4 on 0‐10 scale Inclusion criteria
Exclusion criteria
Total participants randomised: 407 Age in years (mean): 60.5 Gender: 311/407 were female Pain duration in years (mean): 7.99 |
|
| Interventions | Placebo
Duloxetine 60 mg
|
|
| Outcomes | Pain intensity Physical function Mood Sleep Moderate pain relief Substantial pain relief PGIC AEs SAEs Withdrawal |
|
| Missing data methods | MRMM, ITT with LOCF | |
| Funding source | Pharmaceutical: Eli Lilly and Company | |
| Conflicts of interest | Drs Guochun Wang, LiQi Bi, Xiangpei Li, Zhijun Li, Dongbao Zhao, Jinwei Chen, and Dongyi He had no conflicts of interest to report. Drs Hector Due nas, Li Yue, Chia‐Ning Wang, and Vladimir Skljarevski, are employees and minor shareholders of Eli Lilly and Company. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Assignment to treatment groups was determined by a computer‐generated random sequence using an interactive web‐response system (IWRS)." |
| Allocation concealment (selection bias) | Low risk | "The IWRS was used to assign investigational product packages to each patient throughout this study." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical study drugs, matched dosing |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low levels of attrition. Used ITT with both MMRM and LOCF Attrition Total: 65/407 (16.0%) Placebo: 26/202 (12.9%) Duloxetine 60 mg: 39/205 (19.0%) |
| Selective reporting (reporting bias) | Low risk | All outcomes match those registered prospectively on clinicaltrials.gov. |
| Other bias | Low risk | No other sources of bias were identified. |
Ward 1986.
| Study characteristics | ||
| Methods | Design: parallel Duration: 4 weeks Assessment: baseline and post‐intervention Country: USA |
|
| Participants | Pain condition: low back pain Population: adults with chronic low back pain and diagnosed with depressive mood (major affective disorder, unipolar depression, dysthymic disorder) Minimum pain intensity: ≥ 4 on 0‐10 scale Inclusion criteria
Exclusion criteria
Total participants randomised: NR Age in years (mean): 40.2 Gender: 17/35 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Doxepin
Desipramine
|
|
| Outcomes | Study reports no useable data | |
| Missing data methods | Completer analysis | |
| Funding source | NR | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods NR |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures NR |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information given regarding blinding procedures |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported outcomes by participants but unsure of blinding procedures |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer analysis only. Do not report number of participants randomised, reasons for dropouts, from which arms, etc. |
| Selective reporting (reporting bias) | High risk | No protocol or trial registration found. Didn't plan to combine data from both arms until they found no significant differences between arms. |
| Other bias | Unclear risk | Combined data from both arms in the paper |
Ware 2010.
| Study characteristics | ||
| Methods | Design: cross‐over Duration: each cross‐over period lasted 2 weeks Assessment: baseline and post‐cross‐over period Country: Canada |
|
| Participants | Pain condition: fibromyalgia Population: adults with fibromyalgia and self‐reported chronic insomnia Minimum pain intensity: no Inclusion criteria
Exclusion criteria
Total participants randomised: 32 Age in years (mean, SD): 49.5 (11.2) Gender: 26/32 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Nabilone 0.5‐1.0 mg
Amitriptyline 10‐20 mg
|
|
| Outcomes | SAEs | |
| Missing data methods | Unclear | |
| Funding source | Pharmaceutical: supported by an unrestricted grant from Valeant (Canada) Inc. | |
| Conflicts of interest | MAW and MAF have received honoraria from Valeant Canada for CME activities. YS and LJ have no conflicts to declare. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomisation schedule was prepared (ralloc procedure, Stata version 8.0, Houston, TX) using randomly assigned block sizes ranging from 2 to 8." |
| Allocation concealment (selection bias) | Low risk | "The schedule was kept by the study pharmacist away from the investigators. Study subjects were consecutively assigned to treatment order by the study nurse based on the randomisation schedule. A coded script was given to the subject with instructions on the use of the allocated treatment. The subject then collected the medication from the study pharmacy and began taking the medication the same night." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical opaque capsules for both nabilone and amitriptyline |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes by blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear ‐ no missing data methods reported Attrition Total: 3/32 (9.4%) Attrition per arm NR |
| Selective reporting (reporting bias) | Low risk | All outcomes are prespecified in protocol on clinicaltrials.gov |
| Other bias | Low risk | No other sources of bias identified |
Watson 1992.
| Study characteristics | ||
| Methods | Design: cross‐over Duration: each cross‐over period lasted 5 weeks Assessment: baseline and post‐cross‐over period Country: Canada |
|
| Participants | Pain condition: post‐herpetic neuralgia Population: adults with post‐herpetic neuralgia Minimum pain intensity: pain of at least moderate severity (disagreeable, unpleasant, uncomfortable) for at least one half of the day; no numerical values Inclusion criteria
Exclusion criteria
Total participants randomised: 35 Age in years (median, range): 71 (55‐85) Gender: 17/35were female Pain duration in months (median, range): 14 months (4 months‐7 years) |
|
| Interventions | Amitriptyline
Maprotiline
|
|
| Outcomes | Withdrawal | |
| Missing data methods | Unclear | |
| Funding source | Non‐pharmaceutical: The study was funded by Physicians’ Services Incorporated (PSI) Grant PSI: 88‐17. | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods NR |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures NR |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, double‐dummy design |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes by blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Completer analysis but very low dropout Attrition Total: 3/35 (8.6%) Amitriptyline 37.5‐150 mg: 2/35 (5.7%) Maprotiline 50‐150 mg: 1/35 (2.9%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found. Lots of measures mentioned in the methods have no data given in results, just a sentence description |
| Other bias | Low risk | No other sources of bias were identified |
Watson 1998.
| Study characteristics | ||
| Methods | Design: cross‐over Duration: each cross‐over period lasted 5 weeks Assessment: baseline and post‐cross‐over period Country: Canada |
|
| Participants | Pain condition: post‐herpetic neuralgia Population: adults with post‐herpetic neuralgia Minimum pain intensity: pain of at least moderate severity (disagreeable, unpleasant, uncomfortable) for at least one half of the day; no numerical values Inclusion criteria
Exclusion criteria
Total participants randomised: 33 Age in years (mean, SD): NR Gender: NR Pain duration in months (median): 13 months |
|
| Interventions | Nortriptyline
Amitriptyline
|
|
| Outcomes | AEs Withdrawal |
|
| Missing data methods | Unclear | |
| Funding source | NR | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Individuals were randomised by telephone at another site by computer. |
| Allocation concealment (selection bias) | Low risk | The sequence was concealed in sequential, numbered, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical study drugs |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes by blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data methods unclear, but only 1 participant withdrew Attrition Total: 2/33 (6.1%) Amitriptyline 10‐160 mg: 1/33 (3.0%) Nortriptyline 10‐150 mg: 1/33 (3.0%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified |
Wernicke 2006.
| Study characteristics | ||
| Methods | Design: parallel Duration: 12 weeks Assessment: baseline and post‐intervention Country: USA |
|
| Participants | Pain condition: diabetic peripheral neuropathic pain Population: type 1 and 2 diabetic adults with diabetic peripheral neuropathic pain Minimum pain intensity: ≥ 4 on 0‐10 scale Inclusion criteria
Exclusion criteria
Total participants randomised: 334 Age in years (mean, SD): 60.7 (10.6) Gender: 130/334 were female Pain duration in years (mean, SD): 3.8 (4.4) |
|
| Interventions | Placebo
Duloxetine 60 mg
Duloxetine 120 mg
|
|
| Outcomes | Pain intensity Mood Quality of life Physical function Moderate pain relief Substantial pain relief PGIC AEs SAEs Withdrawal |
|
| Missing data methods | ITT with LOCF | |
| Funding source | Pharmaceutical: research for this study was funded by Eli Lilly and Company | |
| Conflicts of interest | "Authors (J.F.W., D.N.D., A.W., S.I., J.R.) are employees and stockholders of Eli Lilly and Company. P.T. and Y.L.P. are former employees of Eli Lilly and Company. J.F.W., Y.L.P., P.T., and J.R. hold equity in Eli Lilly and Company in excess of USD 10,000." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Assignment to a treatment group was determined by a computer‐generated random sequence. |
| Allocation concealment (selection bias) | Low risk | Participants were allocated using an interactive voice response system (IVRS). |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information on blinding procedures for study drugs, appearance, dosing etc |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported outcomes from participants, uncertain of blinding procedures |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT with LOCF Attrition Total: 86/334 (25.8%) Placebo: 23/108 (21.3%) Duloxetine 60 mg: 29/114 (25.4%) Duloxetine 120 mg: 34/112 (30.4%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias were identified |
Wolfe 1994.
| Study characteristics | ||
| Methods | Design: parallel Duration: 6 weeks Assessment: baseline, 3 weeks, post‐intervention Country: USA |
|
| Participants | Pain condition: fibromyalgia Population: women aged 21‐70 with fibromyalgia Minimum pain intensity: ≥ 1 on a 0‐3 VAS Inclusion criteria
Exclusion criteria: NR Total participants randomised: 42 Age in years (mean): 50.5 Gender: 42/42 were female Pain duration in years (mean, SD): 12.8 |
|
| Interventions | Placebo
Fluoxetine 20 mg
|
|
| Outcomes | Pain intensity Sleep Physical function Mood Withdrawal |
|
| Missing data methods | Completer‐only analysis (but ITT with LOCF for depression?) | |
| Funding source | Pharmaceutical: supported by a grant from Lilly Research Laboratories, Inc, Indianapolis, lN, USA | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Assignment was made by the use of a computer‐generated random number table. |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding procedures reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported outcomes from participants, uncertain of blinding procedures |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Seem to only report completer analysis for each time point (with possible ITT and LOCF for depression?). Imbalanced withdrawal between groups (double in placebo compared to fluoxetine). Attrition Total: 18/42 (42/9%) Placebo: 12/21 (57.1%) Fluoxetine 20 mg: 6/21 (28.6%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias identified |
Yasuda 2011.
| Study characteristics | ||
| Methods | Design: parallel Duration: 12 weeks Assessment: baseline and post‐intervention Country: Japan |
|
| Participants | Pain condition: diabetic neuropathic pain Population: adults aged 20‐80 with diabetic neuropathic pain Minimum pain intensity: ≥ 4 on 0‐10 scale Inclusion criteria
Exclusion criteria
Total participants randomised: 339 Age in years (mean, SD): 60.8 (10.0) Gender: 82/339 were female Pain duration in years (mean, SD): 4.3 (4.1) |
|
| Interventions | Placebo
Duloxetine 40 mg
Duloxetine 60 mg
|
|
| Outcomes | Pain intensity Mood Sleep Moderate pain relief Substantial pain relief PGIC AEs SAEs Withdrawal |
|
| Missing data methods | MMRM, LOCF | |
| Funding source | Pharmaceutical: This study is financially supported by Shionogi & Co.Ltd., Eli Lilly Japan K.K., and Eli Lilly and Company. | |
| Conflicts of interest | The study authors have no COI to declare. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Before randomisation, an assigning table was prepared using Create Key Code 3.3. Patients were randomly assigned to duloxetine 40 or 60 mg or placebo groups in a 1:1:2 ratio by stochastic minimisation allocation taking into account the following 4 factors: (i) weekly mean of 24‐h average pain score at baseline < or ‡6; (ii) duration of diabetic neuropathy < or ‡2 years; (iii) type 1 or type 2 diabetes mellitus; and (iv) each study center." |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedure not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Says double‐blind but procedures not specified. No information on drug or packaging concealment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported outcomes from participants but unsure of blinding procedures |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | LOCF, MMRM. Low attrition Attrition Total: 44/339 (13.0%) Placebo: 17/167 (10.2%) Duloxetine 40 mg: 13/86 (15.1%) Duloxetine 60 mg: 14/86 (16.3%) |
| Selective reporting (reporting bias) | Unclear risk | Did not include depression outcome in publication, other than that, everything lines up with protocol |
| Other bias | Low risk | No other sources of bias were identified. |
Yeephu 2013.
| Study characteristics | ||
| Methods | Design: parallel Duration: 13 weeks Assessment: baseline and post‐intervention Country: Thailand |
|
| Participants | Pain condition: fibromyalgia Population: Thai adults with fibromyalgia Minimum pain intensity: ≥ 40 on 0‐100 scale Inclusion criteria
Exclusion criteria
Total participants randomised: 40 Age in years (mean, SD): 44.66 (10.77) Gender: 40/40 were female Pain duration in years (mean, SD): 3.44 (2.71) |
|
| Interventions | Placebo
Mirtazapine 15 mg
Mirtazapine 30 mg
|
|
| Outcomes | Moderate pain relief PGIC AEs SAEs Withdrawal |
|
| Missing data methods | ITT with LOCF, BOCF | |
| Funding source | Non‐pharmaceutical: This study was supported by a scholarship from the Commission on Higher Education Staff Development Project for the Joint PhD Program in Biopharmaceutical Sciences, Thailand. | |
| Conflicts of interest | Study authors reported no conflicts of interest. | |
| Notes | Same study as Suttiruksa 2016 ‐ however different outcomes were reported in the two papers. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The patients were allocated using a block size of 3 in a ratio of 1:1:1 with parallel assignment to 1 of 3 groups using a pharmacy‐controlled randomisation process. |
| Allocation concealment (selection bias) | Low risk | Participants were allocated with sequentially numbered identical containers. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical study drugs |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcomes from blinded participants |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | State that they use LOCF and BOCF measures, but don't present the numbers of participants in each of these analyses. Low attrition rates across all arms. Attrition Total: 8/40 (20.0%) Placebo: 3/13 (23.1%) Mirtazapine 15 mg: 2/13 (15.4%) Mirtazapine 30 mg: 3/14 (21.4%) |
| Selective reporting (reporting bias) | Low risk | All outcomes in protocol reported either in this article or Suttiruksa 2016 |
| Other bias | Low risk | No other sources of bias were identified |
Yucel 2005.
| Study characteristics | ||
| Methods | Design: parallel Duration: 8 weeks Assessment: baseline and post‐intervention Country: Turkey |
|
| Participants | Pain condition: neuropathic pain of any cause Population: people aged between 20 and 70 with neuropathic pain Minimum pain intensity: ≥ 4 on 0‐10 scale Inclusion criteria
Exclusion criteria
Total participants randomised: 60 Age in years (mean): 50.2 Gender: 33/60 were female Pain duration in years (mean, SD): NR |
|
| Interventions | Placebo
Venlafaxine 75 mg
Venlafaxine 150 mg
|
|
| Outcomes | AEs SAEs Withdrawal |
|
| Missing data methods | Completer‐only analysis | |
| Funding source | Pharmaceutical: This study was supported by a grant from Wyeth Ilaclari A.S., Istanbul, Turkey. | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Says double‐blinded but no information regarding blinding procedures given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported outcomes from participants but unsure of blinding procedures |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only report completer analysis, but very low attrition Attrition Total: 5/60 (8.3%) Placebo: 1/20 (5.0%) Venlafaxine 75 mg: 1/20 (5.0%) Venlafaxine 150 mg: 3/20 (15.0%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | Low risk | No other sources of bias identified |
Zabihiyeganeh 2021.
| Study characteristics | ||
| Methods | Design: parallel Duration:10 weeks Assessment: baseline and post‐intervention Country: Iran |
|
| Participants | Pain condition: fibromyalgia Population: women aged 18‐65 with fibromyalgia Minimum pain intensity: no Inclusion criteria
Exclusion criteria
Total participants randomised: 128 Age in years (mean): 42.5 Gender: 128/128 were female Pain duration in years (mean): 3.9 |
|
| Interventions | CBT
Duloxetine 60 mg
|
|
| Outcomes | Pain intensity Quality of life AEs Withdrawal |
|
| Missing data methods | ITT with LOCF | |
| Funding source | Non‐pharmaceutical: This study was funded by Iran University of Medical Sciences under the Grant code of 32415. | |
| Conflicts of interest | The study authors declare that they have no confict of interest. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear ‐ state that was perfomed via a random number list, but also that participants were allocated depending upon order of referral: "the frst 64 random numbers were assigned to the CBT group, and the following 64 random numbers were assigned to the duloxetine group" |
| Allocation concealment (selection bias) | Unclear risk | Allocation methods unclear (see random sequence generation) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to be blinded due to nature of CBT intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self‐reported outcomes from unblinded participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT with LOCF Attrition Total: 23/128 (18.0%) CBT: 12/64 (18.8%) Duloxetine 60 mg: 11/64 (17.2%) |
| Selective reporting (reporting bias) | Unclear risk | Prospectively registered protocol (https://en.irct.ir/trial/24406). Primary outcomes match but secondary outcomes (FIQ, Widespread Pain Index) not registered, no plan of analysis |
| Other bias | High risk | In the protocol they state a third group, a control group with no treatment, but this isn't mentioned anywhere in the paper. |
Zitman 1990.
| Study characteristics | ||
| Methods | Design: parallel Duration: 6 weeks Assessment: baseline, 2 weeks, post‐intervention, follow‐up (6 weeks post‐intervention) Country: Netherlands |
|
| Participants | Pain condition: chronic pain of various origins Population: adults aged 30‐60 with chronic pain of various origins Minimum pain intensity: no Inclusion criteria
Exclusion criteria
Total participants randomised: 49 Age in years (mean, SD): 45.2 (1.3) Gender: 20/49 were female Pain duration in years (mean, SD): 5.1 (3.4) |
|
| Interventions | Placebo (riboflavin 15 mg)
Amitriptyline 75 mg + placebo (riboflavin 15 mg)
|
|
| Outcomes | Pain intensity Mood Withdrawal |
|
| Missing data methods | Completer analysis | |
| Funding source | NR | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedures not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Says double‐blind but no information given regarding appearance of tablets etc. Also the 12‐week follow‐up was open‐label and participants could choose what they wanted. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported outcomes, but unsure of blinding conditions |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No missing data methods given, completer analysis only Attrition Total: 10/49 (20.4%) Placebo: 4/24 (16.7%) Amitriptyline 75 mg: 6/25 (24.0%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration found |
| Other bias | High risk | A lot of imbalances at baseline. Authors class vitamin B as a placebo, but this could have a beneficial effect on mood. |
ACR: American College of Rheumatology; AE: adverse events; ARA: American Rheumatism Association; BAI: Beck Anxiety Inventory; BDI: Beck Depression Inventory; BMI: body mass index; BOCF: baseline observation carried forward; BPI: Brief Pain Inventory; CBT: cognitive behavioural therapy; CoI: conflict of interest; DMS‐IV:Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; DSM‐IV‐TR:Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision; ECG: electrocardiogram; FIC: functional impairment checklist; FIQ: Fibromyalgia Impact Questionnaire; FM: fibromyalgia; GAD: generalised anxiety disorder; HADS: Hospital Anxiety and Depression Scale; IBS: irritable bowel syndrome; ICD‐10:International Classification of Diseases 10th Revision; IQR: interqurtile range; ITT: intention‐to‐treat; LOCF: last observation carried forward; MADRS: Montgomery–Åsberg Depression Rating Scale; MAOI: monoamine oxidase inhibitors; mBOCF: mean baseline observation carried forward; MDD: major depressive disorder; MINI: Mini International Neuropsychiatric Interview; MMRM: mixed models for repeated measures; MNSI: Michigan Neuropathy Screening Instrument; NaRI: noradrenaline reuptake inhibitors; NaSSA: noradrenergic and specific serotonergic antidepressant; NR: not reported; NRS: numerical rating scale; NSAID: non‐steroidal anti‐inflammatory drug; OA: osteoarthritis; ODI: Oswestry Disability Index; PGIC: Patient Global Impression of Change; RA: rheumatoid arthritis; SAE: serious adverse events; SARI: serotonin antagonist and reuptake inhibitors; SD: standard deviation; SDI: Sleep Disorders Inventory; SNRI: serotonin‐noradrenalin reuptake inhibitors; SSRI: selective serotonin reuptake inhibitors; TCA: tricyclic antidepressants; TeCA: tetracyclic antidepressants; TENS: transcutaneous electrical nerve stimulation; VAS: visual analogue scale; WOCF: worst observation carried forward; WOMAC: Western Ontario and McMaster Universities Osteoarthritis pain scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Amelin 1991 | One combined arm, no comparator |
| Amr 2010 | Prevention not treatment |
| Arnold 2014 | All participants currently taking antidepressants |
| Avan 2018 | Not chronic pain |
| Beaumont 1980 | Study invalidated ‐ paper describes an attempt at a trial of clomipramine and a matching placebo which failed |
| Braak 2011 | Condition does not meet chronic pain criteria |
| Carette 1995 | Cross‐over trial ‐ no washout period |
| ChiCTR‐TRC‐12001968 | Pain inclusion criteria not met |
| ChiCTR‐TRC‐12001969 | Pain inclusion criteria not met |
| ChiCTR2000030195 | Pain treatment/prevention post‐surgery |
| Chitsaz 2009 | Pain inclusion criteria not met |
| CTRI/2015/05/005791 | Pain inclusion criteria not met |
| Daghaghzadeh 2015 | Pain inclusion criteria not met |
| Dinat 2015 | Not chronic pain |
| Ehrnrooth 2001 | Not chronic pain |
| EUCTR2005‐005555‐17‐NL | Study terminated due to insufficient clinical response |
| EUCTR2006‐003656‐38‐GB | Study was prematurely ended, but no reason given |
| EUCTR2006‐005506‐32‐DK | Trial registration says prematurely ended, but no reason given |
| EUCTR2009‐013061‐26‐FI | Study prematurely ended due to poor recruitment |
| EUCTR2016‐003146‐89‐GB | No antidepressant‐only arm |
| EUCTR2017‐003307‐21‐NL | Pain inclusion criteria not met |
| EUCTR2018‐000133‐12‐GB | No antidepressant‐only arm |
| EUCTR2019‐003437‐42‐DK | Study terminated but reason not given |
| Farshchian 2018 | Not chronic pain |
| Frank 1988 | Washout period not > 5 half‐lifes of antidepressant |
| Gardela 1991 | Not chronic pain |
| Gelijkens 2014 | Not chronic pain |
| Ghadir 2011 | Pain inclusion criteria not met |
| Goldenberg 2010 | Participants re‐randomised partway through study |
| Gomez‐Perez 1985 | No antidepressant‐only arm, just a combined arm |
| Greenbaum 1987 | Pain inclusion criteria not met |
| Henry 2018 | Not chronic pain |
| IRCT201506171647N4 | Pain inclusion criteria not met |
| IRCT20170829035966N1 | Pain inclusion criteria not met |
| IRCT20191210045685N1 | Treatment/prevention of pain post‐surgery |
| ISRCTN16086699 | Pain inclusion criteria not met |
| ISRCTN63671932 | Pain inclusion criteria not met |
| Kaosombatwattana 2015 | Pain inclusion criteria not met |
| Kautio 2008 | Not chronic pain |
| Khalilian 2021 | Pain inclusion criteria not met |
| Khosrawi 2018 | Not chronic pain |
| Kieburtz 1998 | Not chronic pain |
| Kishore‐Kumar 1990 | Washout period not > 5 half‐lifes of antidepressant |
| Kreiter 2021 | Pain inclusion criteria not met |
| Kroenke 2006 | Pain inclusion criteria not met |
| Kuiken 2003 | Pain inclusion criteria not met |
| Kvinesdal 1984 | Cross‐over study ‐ no washout period |
| Ladabaum 2010 | Pain inclusion criteria not met |
| Lara Muñoz 1986 | Effect of amitriptyline on the pain relief provided by other analgesics, not the effect of amitriptyline itself |
| Li 2019 | Pain inclusion criteria not met |
| Matsuoka 2019a | Not chronic pain |
| Max 1987 | Cross‐over study ‐ no washout period |
| Max 1991 | Cross‐over study ‐ no washout period |
| McQuay 1992 | Cross‐over study ‐ no washout period |
| Mishra 2012 | Not chronic pain |
| NCT00006157 | Pain inclusion criteria not met |
| NCT00189059 | Study terminated but reason not given |
| NCT00191919 | Somatic symptoms of depression, not chronic pain condition |
| NCT00283842 | Study terminated for business reasons |
| NCT00592384 | Pain inclusion criteria not met |
| NCT00610909 | Pain inclusion criteria not met |
| NCT00619983 | Study terminated due to poor recruitment |
| NCT00625833 | Study terminated due to insufficient clinical response |
| NCT00696787 | Study terminated by sponsor |
| NCT00754793 | Study terminated due to poor recruitment |
| NCT00945945 | Study invalidated ‐ study drugs were mislabelled, participants who were supposed to receive placebo actually received duloxetine and vice versa. |
| NCT01116531 | Study withdrawn |
| NCT01173055 | Experimental pain |
| NCT01268709 | Pain inclusion criteria not met |
| NCT01288937 | Study terminated due to poor recruitment |
| NCT01359514 | Pain prevention rather than treatment |
| NCT01359826 | Principle Investigator left institution and unable to locate any study documents |
| NCT01377038 | Study withdrawn due to funding issues |
| NCT01451606 | Study terminated due to poor recruitment |
| NCT01471379 | Terminated due to recruitment difficulties |
| NCT01579279 | Study terminated but no reason given |
| NCT01869907 | Pain inclusion criteria not met |
| NCT01910259 | Pain inclusion criteria not met |
| NCT02650544 | Pain inclusion criteria not met |
| NCT02970591 | No specific antidepressant, just "optimised management", which could include an antidepressant option |
| NCT03364075 | Study terminated due to recruitment issues |
| NCT03522207 | Study terminated due to short‐staffing |
| NCT04747314 | Antidepressant arm is not one single antidepressant, it's a mixture |
| Nickel 2005 | No antidepressant only arm, just a combined arm |
| Panerai 1990 | Cross‐over study ‐ no washout period |
| Parker 2003 | No antidepressant‐only arm |
| Parkman 2013 | Pain inclusion criteria not met |
| Pilowsky 1982 | Cross‐over study ‐ no washout period |
| Pilowsky 1995 | All participants received antidepressant |
| Poulsen 1987 | Unable to determine trial length |
| Raja 2002 | Participants could take different antidepressants/comparators, no comparisons per drug |
| Rajagopalan 1998 | Pain inclusion criteria not met |
| Saxe 2009 | Results from discontinuation phase of trial |
| Seddighnia 2020 | Pain inclusion criteria not met |
| Selvarajah 2018 | No antidepressant‐only arm |
| Semenchuk 2001 | No washout period |
| Strauss 2019 | Not chronic pain |
| Tadyon Najafabadi 2019 | Pain inclusion criteria not met |
| Tondlova 2002 | Not chronic pain |
| van Houdenhove 1992 | Washout period not > 5 half‐lifes of natidepressant |
| Varia 2000 | Not chronic pain |
| Vork 2018 | Pain inclusion criteria not met |
| Wang 2014 | Pain inclusion criteria not met |
Characteristics of studies awaiting classification [ordered by study ID]
ACTRN12620000656932.
| Methods | Double‐blind, parallel‐arm, placebo‐controlled RCT |
| Participants | Unclear from trial registration whether this is acute or chronic pain Inpatients and outpatients with diagnoses of cancer and neuropathic pain (probable or definite neuropathic pain by IASP criteria) Pain related to cancer with a worst pain score of ≥ 4 on BPI item 3 (worst pain intensity) score in the past 24 h Neuropathic Pain on LANSS ≥ 12 Taking stable regular analgesics within 72 hours before commencing on the study. Target: 160 |
| Interventions | Duloxetine 30/day orally for 7 days, then increase to 60 mg/day for 7 days, then downward titrate to 30 mg/day for 7 days Pregabalin 50/day orally for 3 days, 150 mg/day for 4 days, then 300 mg/day for 7 days, then downward titration to 150 mg/day for 4 days, and 50 mg/day for 3 days |
| Outcomes | Pain intensity Anxiety Depression Daily opioid use |
| Notes |
Brown 2015.
| Methods | Double‐blind, placebo‐controlled, parallel, 2‐arm RCT |
| Participants | Patients with multiple sclerosis n = 38 |
| Interventions | Duloxetine Placebo |
| Outcomes | Pain PGIC Depression Quality of life Sleep |
| Notes | Unable to ascertain pain duration, unsure if chronic |
Chandra 2006.
| Methods | Double‐blind, parallel, head‐to‐head, 2‐arm RCT 9 weeks |
| Participants | Adult PHN patients 8 weeks of postherpetic neuralgia pain after healing of rash Pain intensity of at least 40 mm on a 100 mm VAS at screening and at randomisation Average pain score of at least 4 on the Likert scale during the baseline week n = 70 |
| Interventions | Gabapentin Nortriptyline Flexibly dosed to maximum tolerated dose |
| Outcomes | Pain intensity Sleep |
| Notes | Unable to ascertain pain duration ‐ not sure if chronic |
Cánovas Martínez 2009.
| Methods | Parallel RCT 3 months |
| Participants | 60 patients with severe neuropathic pain (VAS > 6) |
| Interventions | Duloxetine Placebo |
| Outcomes | Pain intensity Symptom relief |
| Notes | Unable to ascertain blinding |
Di 2019.
| Methods | Parallel, 2‐arm RCT |
| Participants | Patients with severe cancer pain and depression n = 46 |
| Interventions | Oxycontin + amitriptyline Oxycontin |
| Outcomes | Cancer pain Depression |
| Notes | Unable to ascertain blinding Unable to ascertain pain duration ‐ unclear if chronic |
Hammack 2002.
| Methods | Double‐blind, placebo‐controlled, cross‐over, RCT |
| Participants | Patients who had received CDDP (cisplatin) chemotherapy, and have had painful paresthaesiae for at least 1 month attributed to CDDP neuropathy. n = 51 |
| Interventions | Nortriptyline 100 mg Placebo |
| Outcomes | Pain Sleep Quality of life AEs |
| Notes | Unable to ascertain pain duration ‐ inclusion criteria only says at least 1 month |
Jia 2006.
| Methods | Double‐blind, placebo‐controlled, double‐dummy, parallel, 2‐arm RCT 2 weeks |
| Participants | Patients with painful peripheral diabetic neuropathy n = 132 |
| Interventions | Carbamazepine 0.2 mg Venlafaxine 50 mg |
| Outcomes | Pain intensity Quality of life Mood Sleep AEs |
| Notes | Unable to establish pain duration, unsure if chronic |
Keskinbora 2006.
| Methods | Double‐blind, comparative, parallel, 2‐arm RCT 4 weeks |
| Participants | Patients with neuropathic pain n = 46 |
| Interventions | Gabapentin Amitriptyline |
| Outcomes | Pain sensations Satisfaction |
| Notes | States chronic pain, but no duration reported in article, so unable to confirm chronic |
Riesner 2008.
| Methods | Double‐blind, placebo‐controlled, parallel, 2‐arm RCT 8 weeks |
| Participants | People with knee and hip OA |
| Interventions | Fluvoxamine 50‐150 mg Placebo |
| Outcomes | Pain WOMAC total score PGIC AEs |
| Notes | Unable to ascertain pain duration, unsure if chronic |
Salehifar 2020.
| Methods | Double‐blind, comparative, parallel, 2‐arm RCT 6 weeks |
| Participants | Patients with breast cancer who had a ≥ grade 1 neuropathy and who had score ≥ 4 neuropathic pain severity based on the VAS |
| Interventions | Pregabalin 150 mg Duloxetine 60 mg |
| Outcomes | Pain Sensory neuropathy grade |
| Notes | Unable to ascertain pain duration ‐ unclear if chronic |
Shabbir 2011.
| Methods | Parallel, 3‐arm RCT |
| Participants | Patients with peripheral diabetic neuropathy for at least 6 months duration, an average pain score ≥ 4 (on an 11‐point, Likert‐like NRS; 0 = “no pain” to 10 = “worst possible pain”) over a 7‐day baseline period |
| Interventions | Amitriptyline Pregabalin Placebo Flexibly dosed depending upon tolerance |
| Outcomes | Pain intensity 50% pain relief |
| Notes | Unable to ascertain blinding |
Shlay 1998.
| Methods | Comparative RCT |
| Participants | Patients with HIV‐ associated, symptomatic, lower‐extremity peripheral neuropathy n = 250 |
| Interventions | Acupuncture Amitriptyline 75 mg Placebo |
| Outcomes | Pain intensity |
| Notes | Unable to establish pain duration, unsure if chronic |
Taghizadeh 2020.
| Methods | Comparative, 2‐arm RCT 12 weeks |
| Participants | Women with mastalgia n = 62 |
| Interventions | Fluoxetine Tamoxifen |
| Outcomes | Pain intensity |
| Notes | Unable to ascertain blinding, and duration of pain |
Xu 2006.
| Methods | Comparative, 2‐arm RCT 4 weeks |
| Participants | Patients with primary fibromyalgia syndrome n = 46 |
| Interventions | Amitriptyline 25 mg ~ 50 mg Paroxetine 10 mg ~ 20 mg |
| Outcomes | Pain intensity AEs |
| Notes | Unable to ascertain blinding |
Zakerkish 2017.
| Methods | Double‐blind, placebo‐controlled, parallel, 2‐arm RCT 6 weeks |
| Participants | Patients with diabetic peripheral neuropathy n = 134 |
| Interventions | Duloxetine 30‐60 mg Placebo |
| Outcomes | Pain intensity 50% pain relief AEs |
| Notes | Unable to establish pain duration, unclear if chronic |
AE: adverse event; BPI: Brief Pain Inventory; IASP: International Association for the Study of Pain; LANSS: Leeds Assessment of Neuropathic Symptoms and Signs; NRS: numeric rating scale; OA: osteoarthritis; PGIC: Patient Global Impression of Change; RCT: randomised controlled trial; VAS: visual analogue scale; WOMAC: Western Ontario and McMaster Universities Osteoarthritis pain scale
Characteristics of ongoing studies [ordered by study ID]
ACTRN12619000878178.
| Study name | A randomised controlled trial of venlafaxine to treat patients with knee osteoarthritis pain |
| Methods | Double‐blind, parallel‐arm, placebo‐controlled, 2‐arm RCT |
| Participants |
|
| Interventions | Venlafaxine; 75 mg daily for 4 weeks and then increase to 150 mg daily for next 8 weeks Placebo |
| Outcomes | Pain intensity Physical function Quality of life painDETECT score Anxiety Pain catastrophising Pain disability Depression Responders (using OMERACT‐OARSI criteria) |
| Starting date | 20 June 2019 |
| Contact information | Dr Feng Pan Menzies Institute for Medical Research 17 Liverpool Street Hobart Tasmania 7000 Australia Feng.Pan@utas.edu.au |
| Notes |
ACTRN12619001082190.
| Study name | Venlafaxine compared to duloxetine for the treatment of osteoarthritis pain: A double‐blind, randomised, non‐inferiority trial |
| Methods | Double‐blind, parallel arm, antidepressant head‐to‐head, 2‐arm RCT |
| Participants | Men and women at least 40 years old who have radiographic evidence of knee OA and meet the ACR clinical criteria for the diagnosis of knee OA
A history of knee pain for > 14 days of each month for ≥ 3 months
A BPI average pain rating of at least 4/10 at the time of initial screening Target: 146 |
| Interventions | Venlafaxine 75 mg for 1 week, then 150 mg for 7 weeks Duloxetine 30 mg for 1 week, then 60 mg for 7 weeks |
| Outcomes | Pain intensity Anxiety Depression Physical function Quality of life PGIC Moderate pain relief (30% reduction) Substantial pain relief (50% reduction) |
| Starting date | 6 August 2019 |
| Contact information | Dr David Rice Waitemata Pain Services, Level 10, North Shore Hospital, 124 Shakespeare Road, Takapuna, Auckland 0622, New Zealand david.rice@aut.ac.nz |
| Notes |
Ammitzboll 2021.
| Study name | A mechanism based proof of concept study of the effects of duloxetine in the treatment of patients with osteoarthritic knee pain |
| Methods | Double‐blind, crossover, placebo‐controlled, 2‐arm trial |
| Participants | Men and women between 40 and 75 years of age Patients with knee OA based on disease diagnostic criteria Self‐reported pain intensities ≥ 5 cm on a 0‐10 cm VAS when asked to assess the worst pain within the last 24 hours Knee pain for at least 14 days per month for the last 3 months before study entry |
| Interventions | Patients will be randomised to 1 of 2 treatment sequences:
The 2 treatment periods of 14 weeks each are separated by a washout period of 2 weeks and include a 2‐week titration period. |
| Outcomes | Pressure Pain Threshold |
| Starting date | 13 January 2020 |
| Contact information | Kristian Kjær Petersen, Aalborg University |
| Notes |
ChiCTR1900027038.
| Study name | Synergistic analgesia of duloxetine in phantom limb pain of amputees from bone tumors: a randomized controlled trial |
| Methods | Unclear on blinding or chronic pain from trial registration Placebo‐controlled, 2‐arm, RCT |
| Participants | Bone tumour patients, phantom limb pain after amputation Aged 18‐65 Target: 120 |
| Interventions | Duloxetine 60 mg Placebo |
| Outcomes | Pain intensity |
| Starting date | 29 October 2019 |
| Contact information | Shuang Jiang 44 Xiaoheyan Road, Dadong District, Shenyang, Liaoning, China 110042 jiangshuang@cancerhosp‐ln‐cmu.com |
| Notes |
CTRI/2018/10/015944.
| Study name | A comparative evaluation of duloxetine and gabapentin in painful diabetic neuropathy: a randomised control trial |
| Methods | Double‐blind, placebo‐controlled, parallel, 2‐arm RCT 12 weeks |
| Participants |
Target: 86 |
| Interventions | Duloxetine 60 mg daily Gabapentin 300 mg daily |
| Outcomes | Pain intensity Diabetic neuropathy symptom score |
| Starting date | 22 October 2018 |
| Contact information | Dr Sameer Khasbage Department of Pharmacology Basni 2 AIIMS Jodhpur Rajasthan 342005 Jodhpur, Rajasthan, India samkhasbage@gmail.com |
| Notes |
CTRI/2018/10/015983.
| Study name | Effectiveness of vitamin D as a supplement with conventional therapy in the treatment of diabetic peripheral neuropathy ‐ a randomized controlled clinical trial |
| Methods | Double‐blind, placebo‐controlled, parallel, 2‐arm RCT 3 months |
| Participants |
Target: 80 |
| Interventions | Amitriptyline 25 mg Vitamin D |
| Outcomes | Vitamin D levels Pain intensity Quality of life |
| Starting date | 10 October 2018 |
| Contact information | Dr Melvin George Department of Pharmacology SRM Medical College Hospital and Research Centre SRM Institute of Science and Technology (SRMIST) Kattankulathur 603203 Kancheepuram, Tamil Nadu, India melvingeorge2003@gmail.com |
| Notes |
CTRI/2021/02/031068.
| Study name | A randamized double‐blind comparative study evaluating the efficacy of a combination of pregabalin and duloxetine versus pregabalin alone and the modulation of mRNA expression of PPARG and Akt genes in patients of painful diabetic peripheral neuropathy |
| Methods | Unclear blinding from trial registration 2‐arm, combination vs antidepressant‐only RCT 12 weeks |
| Participants |
Target: 60 |
| Interventions | Combination of tablet pregabalin 75 mg and tablet duloxetine 30 mg pregabalin 75 mg twice a day orally |
| Outcomes | Sleep Pain Physical function Modulation of mRNA expression of PPARG and Akt gene |
| Starting date | 08 February 2021 |
| Contact information | Dr Ashok Kumar Department of Anaesthesia and Critical Care, second floor University College of Medical Sciences and GTB Hospital, Dilshad Garden, Delhi 110095 East, Delhi, India profashoksaxena2@gmail.com |
| Notes |
CTRI/2021/03/031875.
| Study name | Efficacy of duloxetine in patients with central post‐stroke pain: a randomised double blind placebo controlled study |
| Methods | Double‐blind, placebo‐controlled, parallel, 2‐arm, RCT 4 weeks |
| Participants |
Target: 82 |
| Interventions | Duloxetine: 30 mg in the night every day and followed up at 2 weeks if no response, i.e. decrease in NRS score < 2 then the dose is doubled and again followed up after 2 weeks Placebo: the similar appearing placebo tablets are given at night every day and followed up at 2 weeks, if no response, i.e. decrease in NRS score < 2 the dose is doubled and again followed up after 2 weeks |
| Outcomes | Pain intensity Disability PGIC |
| Starting date | 10 March 2021 |
| Contact information | Dr Rameshwar Nath Chaurasiya Department of Neurology, Institue of Medical Sciences, Banaras Hindu University, 221005 Varanasi, Uttar Pradesh, India goforrameshwar@gmail.com |
| Notes |
EUCTR2019‐000243‐27‐DK.
| Study name | The effect of bupropion in peripheral neuropathic pain. A randomized, double‐blind, placebo‐controlled study |
| Methods | Double‐blind, placebo‐controlled, parallel, 2‐arm RCT |
| Participants |
Target: 90 |
| Interventions | Bupropion 150 mg Placebo |
| Outcomes | Pain intensity Pain modulation PGIC Neuropathic pain symptoms Suicide ideation |
| Starting date | 28 January 2019 |
| Contact information | Neuromuscular Clinic J.B.Winsløws Vej 4 5000 Odense Denmark soeren.sindrup@rsyd.dk |
| Notes |
EUCTR2019‐000324‐17‐GB.
| Study name | Amitriptyline at low‐dose and titrated for irritable bowel syndrome as second‐line treatment (the ATLANTIS study): a double‐blind placebo‐controlled trial ‐ the ATLANTIS study |
| Methods | Double‐blind, placebo‐controlled, parallel, 2‐arm RCT |
| Participants | Unclear about pain chronicity from trial registration
Target: 518 |
| Interventions | Amitriptyline 10 mg Placebo |
| Outcomes | IBS symptoms Anxiety Depression Quality of life Health care use Ability to work |
| Starting date | 07 November 2019 |
| Contact information | Dr Heather Cook CTRU, University of Leeds LS2 9JT Leeds United Kingdom Atlantis@leeds.ac.uk |
| Notes |
EUCTR2019‐001202‐14‐NL.
| Study name | CiPA Trial: effect of citalopram on chest pain in patients with achalasia |
| Methods | Double‐blind, placebo‐controlled, parallel, 2‐arm RCT 6 weeks |
| Participants | Diagnosed with achalasia type 1 or 2, confirmed by high‐resolution manometry Recurrent chest pain
Target: 68 |
| Interventions | Citalopram Placebo |
| Outcomes | Pain intensity and frequency Quality of life Anxiety Depression AEs |
| Starting date | 18 April 2019 |
| Contact information | Research Team Meibergdreef 9 1105 AZ Amsterdam Netherlands j.m.schuitenmaker@amc.uva.nl |
| Notes |
EUCTR2021‐002288‐24‐NL.
| Study name | Effect of citalopram on chest pain in patients with functional chest pain ‐ Ci‐FCP |
| Methods | Double‐blind, placebo‐controlled, parallel, 2‐arm RCT 12 weeks |
| Participants |
Target: 52 |
| Interventions | Citalopram Placebo |
| Outcomes | Reduction in chest pain Chest pain severity and frequency Quality of life Depression Anxiety AEs |
| Starting date | 27 July 2021 |
| Contact information | Research Team Meibergdreef 9 1105 AZ Amsterdam Netherlands t.kuipers1@amsterdamumc.nl |
| Notes | Potentially linked to Euctr 2019? |
IRCT20110413006186N13.
| Study name | A comparison of the effectiveness of transcutaneous electrical nerve stimulation and duloxetine on diabetic peripheral neuropathic pain |
| Methods | Parallel, 2‐arm RCT |
| Participants | Patients with type I or II diabetes mellitus, with diabetic neuropathic pain
Resistant to usual drug treatments, at least for 6 months
Minimum Pain Rating ≥ 4 based on NRS Target: 60 |
| Interventions | TENS Duloxetine 60 mg |
| Outcomes | Pain intensity |
| Starting date | 22 June 2019 |
| Contact information | Dr Bahram Naderi Nabi Poursina Hospital 4193713189 Rasht Iran (Islamic Republic of) naderi_bahram@yahoo.com |
| Notes |
IRCT20200205046381N1.
| Study name | Comparing the analgesic effect of fluoxetine and vitamin E with vitamin E only in mastalgia due to fibrocystic breast disease |
| Methods | Double‐blind, double‐dummy, parallel, 2‐arm RCT 8 weeks |
| Participants |
Target: 70 |
| Interventions | Vitamin E + fluoxetine: 600 units of vitamin E daily and 10 mg of fluoxetine Vitamin E: 600 units of vitamin E and placebo daily |
| Outcomes | Pain intensity |
| Starting date | 20 March 2020 |
| Contact information | Sheida Shabanian Hajar Hospital of Shahrekord University of Medical Sciences, Parastar street, Shahrekord, Iran 818718791 Shahrekord Iran (Islamic Republic of) shabanian@skums.ac.ir |
| Notes |
IRCT20200620047852N1.
| Study name | Comparing the analgesic effect of agomelatin versus placebo in combination with pregabalin in patients with chronic low back pain: a randomized, double‐blinded study |
| Methods | Double‐blind, double‐dummy, parallel, 2‐arm RCT |
| Participants |
|
| Interventions | Pregabalin 75mg twice daily + agomelatine 25 mg Pregabalin 75 mg twice daily + placebo |
| Outcomes | Pain Anxiety Depression Quality of life Disability |
| Starting date | 06 July 2020 |
| Contact information | Shayan Amiri No 24, First West Street, 24 Metres Boulvard, Saadat Abad, Tehran, Iran, 1998667133 Tehran, Iran (Islamic Republic of) Amiri.shayan23@gmail.com |
| Notes |
NCT00981149.
| Study name | Duloxetine for treatment of painful temporomandibular joint disorder |
| Methods | Double‐blind, placebo‐controlled, parallel, 2‐arm RCT 6 weeks |
| Participants | n = 24 Unclear whether chronic pain from trial registration
|
| Interventions | Duloxetine 30 mg Placebo |
| Outcomes | Pain intensity |
| Starting date | May 2009 |
| Contact information | |
| Notes |
NCT03249558.
| Study name | Effect of combined morphine and duloxetine on chronic pain |
| Methods | Double‐blind, combination + double‐dummy, parallel, 3‐arm RCT 10 weeks |
| Participants |
Target: 135 |
| Interventions | Morphine 60 mg + duloxetine 60 mg Morphine 60 mg + placebo Duloxetine 60 mg + placebo |
| Outcomes | Opioid dose Pain intensity |
| Starting date | 01 February 2018 |
| Contact information | Karina de Sousa kdesousa1@mgh.harvard.edu |
| Notes |
NCT03324035.
| Study name | Treatment of neuropathic pain in leprosy: a randomized double blind controlled study |
| Methods | Double‐blind, placebo‐controlled, parallel, 2‐arm RCT |
| Participants |
n = 102 |
| Interventions | Amitriptyline, flexible doses varying from 25‐75 mg Placebo, flexible doses from 1‐3 capsules |
| Outcomes | 30% pain relief Pain intensity Neuropathic pain symptoms Quality of life AEs |
| Starting date | 01 March 2017 |
| Contact information | Daniel Ciampi Araujo de Andrade, MD, PhD, Principal Investigator, Pain Center coordinator, Department of Neurology, University of Sao Paulo, São Paulo, Brazil, University of Sao Paulo |
| Notes |
NCT04704453.
| Study name | Phase II randomized controlled study aiming to evaluate the interest of Qutenza in patients with head and neck cancer in remission and with sequelae neuropathic pain |
| Methods | Double‐blind, parallel, 2‐arm RCT 9 months |
| Participants | Unclear pain duration from trial registration
Target: 130 |
| Interventions | Capsaïcin patch (Qutenza) 8% Amitriptyline flexibly dosed 25‐75 mg |
| Outcomes | Pain intensity Neuropathic pain symptoms Quality of life AEs |
| Starting date | 28 April 2021 |
| Contact information | Antoine Boden 05 31 15 57 91 boden.antoine@iuct‐oncopole.fr |
| Notes |
NCT04727502.
| Study name | Comparison of duloxetine versus pregabalin in post‐mastectomy pain syndrome: a randomized controlled trial |
| Methods | Double‐blind, comparative, parallel, 2‐arm RCT 12 weeks |
| Participants | Patients with 3 months of chronic neuropathic pain after breast surgery Target: 70 |
| Interventions | Duloxetine 30 mg Pregabalin 150 mg |
| Outcomes | Pain intensity |
| Starting date | 20 December 2020 |
| Contact information | Mohamed Abdel Wadod, MD +201006645981 m_wadod@yahoo.com |
| Notes |
PACTR202001764151121.
| Study name | Efficacy of clomipramine for chronic lumbar radicular pain: a randomized clinical trial |
| Methods | Double‐blind, placebo‐controlled, parallel, 2‐arm RCT |
| Participants |
Target: 62 |
| Interventions | Clomipramine Placebo |
| Outcomes | Pain intensity Neuropathic pain symptoms Walking Disability Anxiety Depression |
| Starting date | 27 May 2019 |
| Contact information | Redouane Abouqal Faculty of Medicine and Pharmacy, Impasse Souissi, Rabat, Morocco Redouane.abouqal@yahoo.fr |
| Notes |
RBR‐6pqx4n.
| Study name | Efficacy of duloxetine in chronic temporomandibular disorder: a randomized clinical trial |
| Methods | Double‐blind, placebo‐controlled, parallel, 2‐arm RCT 12 weeks |
| Participants | Temporomandibular disorder Presence of pain for at least 3 months |
| Interventions | Duloxetine 60 mg Placebo |
| Outcomes | Pain intensity Sleep Psychosocial profile Mechanical somatosensory profile |
| Starting date | 01 October 2018 |
| Contact information | Dyna Mara Araújo Oliveira Ferreira Al. Octávio Pinheiro Brisola, 9‐75 17012‐901 Bauru Brazil dyna.mara@hotmail.com |
| Notes |
Reckziegel 2017.
| Study name | Imaging pain relief in osteoarthritis (IPRO): protocol of a double‐blind randomised controlled mechanistic study assessing pain relief and prediction of duloxetine treatment outcome |
| Methods | Double‐blind, placebo‐controlled, parallel, 2‐arm RCT |
| Participants | Chronic knee pain with radiographically defined OA changes (Kellgren Lawrence ≥ grade 2) Aged ≥ 35 n = 77 |
| Interventions | Duloxetine 60 mg Placebo |
| Outcomes | Experimental pain Functional magnetic resonance imaging |
| Starting date | December 2014 |
| Contact information | University of Nottingham ‐ School of Medicine ‐ Radiological Sciences Nottingham, Nottinghamshire, United Kingdom, NG7 2UH |
| Notes |
TCTR20190303001.
| Study name | A comparison of analgesic efficacy between amitriptyline and mianserin in chronic low back pain patients: a randomized double‐blind controlled trial |
| Methods | Double‐blind, placebo‐controlled, parallel, 2‐arm RCT |
| Participants |
Target: 60 |
| Interventions | Amitriptyline 10‐50 mg Mianserin 10‐50 mg |
| Outcomes | Pain intensity Quality of life |
| Starting date | 01 November 2018 |
| Contact information | Suratsawadee Wangnamthip Bangkok Noi 10700 Bangkok Thailand suratsawadee.wang@gmail.com |
| Notes |
TCTR20210311009.
| Study name | Comparison effectiveness of nortriptyline and placebo in the treatment of chronic osteoarthritis knee |
| Methods | Uncertain of blinding from trial registry |
| Participants |
Target: 200 |
| Interventions | Nortriptyline 25 mg Placebo |
| Outcomes | WOMAC total score Pain intensity |
| Starting date | 29 May 2019 |
| Contact information | Krittamuk Ompornnuwat 681 Samsen road, Vajira hospital, 20300 10300 Dusit Thailand krittamuk@nmu.ac.th |
| Notes |
Wluka 2021.
| Study name | Knee osteoarthritis pain study (KOPS) |
| Methods | Double‐blind, parallel arm, placebo controlled 2‐arm RCT 12 weeks |
| Participants | Adults aged 40‐75 with knee OA as defined by the ACR clinical and radiographic criteria Pain intensity of ≥ 30 on 0‐100 pain scale |
| Interventions | Amitriptyline 25 mg Placebo |
| Outcomes | Pain intensity WOMAC total score Moderate pain relief (30% reduction) Substantial pain relief (50% reduction) |
| Starting date | 07 July 2015 |
| Contact information | Mrs Aruna Kartik Department of Epidemiology and Preventive Medicine, Monash University, Alfred Hospital, Commercial Road, Melbourne, VIC 3004, Australia jointstudy@monash.edu |
| Notes |
ACR: American College of Rheumatology; AE: adverse event; BPI: Brief Pain Inventory; IBS: irritable bowel syndrome; MNSI: Michigan Neuropathy Screening Instrument; NRS: numeric rating scale; NSAID: non‐steroidal anti‐inflammatory drug; OA: osteoarthritis; OMERACT‐OARSI: Outcome Measures in Rheumatology‐Osteoarthritis Research Society International; PGIC: Patient Global Impression of Change; RCT: randomised controlled trial; s; TENS: transcutaneous electrical nerve stimulation; VAS: visual analogue scale;
Differences between protocol and review
There are a number of differences between the protocol and the review (Birkinshaw 2021).
-
Updating of the background section
We removed the International Association for the Study of Pain (IASP) pain categories from the background, as there is currently discourse about the clinical usefulness of primary pain, and we subsequently did not categorise pain types into these. If we were to have used the IASP categories, then a number of distinct pain conditions (e.g. fibromyalgia, low back pain) would have been combined, whereas there is evidence for these types of conditions being kept separate to evaluate the effects.
We reference National Institute for Health and Care Excellence (NICE) guidelines to the background which were not published at time of protocol publication.
We have updated the literature in the How the intervention might work section for clarity, and to reflect current understanding and theories.
-
Methods
We reported continuous pain intensity as an outcome, which was not included in the published version of the protocol. This was originally in the protocol, and was removed accidentally during the protocol editing process.
We separated adverse events and serious adverse events outcomes as they are defined differently, and we assessed them using separate NMAs. Therefore, we moved serious adverse events to a secondary outcome.
We rated studies that imputed missing data using the 'last observation carried forward' method as high risk of bias, unless attrition was very low. This rule was not explained in the protocol.
We stated that we would present the primary outcomes on a 0 to 100 scale. As outcomes were reported on a wide variety of scales, this was not possible. Instead, we have reported the 'number needed to treat for an additional beneficial outcome' and 'number needed to treat for an additional harmful outcome' in the summary of findings tables.
We planned to use threshold analysis to analyse how much evidence needed to be added for our conclusions to change. We did not undertake threshold analysis in the review as we judged the majority of evidence to be low or very low certainty; and therefore it is already likely that new evidence will affect the conclusions.
We have added in the criteria for antidepressant doses, being categorised as 'low', 'standard', or 'high', and clarified how we included dose in the analysis in the 'data synthesis' section (moved from the subgroup analysis section). This was done as networks would not converge when using dose as a continuous measure.
We omitted the 'Other bias' domain from the protocol accidentally ‐ we did assess for this in our risk of bias assessments, and so have included this in the methods under the risk of bias section.
We reordered parts of the methods section regarding sensitivity analyses for clarification: we moved assessment of consistency to data synthesis and added further information regarding the sensitivity analyses to the sensitivity analysis section. We did this because the assessment of consistency was part of our main analysis methods, not as a standalone sensitivity analysis.
Contributions of authors
TP, PC, CE, MS, GS, and SW conceived, designed, and gained funding for the review. HB co‐ordinated the review. HB and CF were responsible for screening and selection of studies from the search results, data extraction, and risk of bias assessments. HB cleaned the data for analysis. PC, MS, and SW were responsible for decisions requiring clinical knowledge. GS undertook data analysis through network meta analyses, with support from DP. HB and GS assessed the certainty of the evidence using CINeMA. HB drafted the manuscript. All authors contributed to the interpretation of findings, and writing and editing of the manuscript. TP will be responsible for the update of this review.
Sources of support
Internal sources
No sources of support provided
External sources
-
NIHR Health Technology Assessment programme, UK
This review is funded by the NIHR Health Technology Assessment programme through a grant awarded to Professor Tamar Pincus (Award ID: NIHR128782).
-
National Institute for Health Research (NIHR), UK
Cochrane Infrastructure funding to the Cochrane Pain, Palliative and Supportive Care Review Group (PaPaS)
Declarations of interest
Hollie Birkinshaw: none known
Claire Friedrich: none known
Peter Cole is a Consultant in anaesthesia and pain medicine and manages people with chronic pain.
Christopher Eccleston: none known
Andrew Moore: none known
David Phillippo: none known
Marc Serfaty is a Consultant Psychiatrist and manages people with mental health conditions.
Gavin Stewart: none known
Simon White: none known
Tamar Pincus had one consultancy advisory meeting with Reckitt Benckiser Group PLC in February 2020. Reckitt Benckiser Group PLC are a multinational company that produce consumer goods, including pharmacological products such as analgesics. Tamar Pincus was asked to deliver an advisory talk to the company about psychological factors that might compromise randomised controlled trials. The University of Southampton was paid for her time. This talk did not cover the use of antidepressants.
New
References
References to studies included in this review
29060/433 {published data only}
- 29060/433. Treatment of fibromyalgia: a randomized, double-blind, placebo-controlled study of paroxetine, a selective serotonin re-uptake inhibitor. https://www.gsk-studyregister.com/en/trial-details/?id=29060/433 (first received 1 December 2005).
Abou‐Raya 2012 {published data only}
- Abou-Raya S, Abou-Raya A, Helmii M. Duloxetine for the management of pain in older adults with knee osteoarthritis: randomised placebo-controlled trial. Age and Ageing 2012;41(5):646-52. [DOI: 10.1093/ageing/afs072] [DOI] [PubMed] [Google Scholar]
Agger 2017 {published data only}
- Agger JL, Schröder A, Gormsen LK, Jensen JS, Jensen TS, Fink PK. Imipramine versus placebo for multiple functional somatic syndromes (STreSS-3): a double-blind, randomised study. Lancet Psychiatry 2017;4(5):378-88. [DOI: 10.1016/S2215-0366(17)30126-8] [DOI] [PubMed] [Google Scholar]
Ahmed 2016 {published data only}
- Ahmed M, Aamir R, Jishi Z, Scharf MB. The effects of milnacipran on sleep disturbance in fibromyalgia: a randomized, double-blind, placebo-controlled, two-way crossover study. Journal of Clinical Sleep Medicine 2016;12(1):79-86. [DOI: 10.5664/jcsm.5400] [DOI] [PMC free article] [PubMed] [Google Scholar]
Alcoff 1982 {published data only}
- Alcoff J, Jones E, Rust P, Newman R. Controlled trial of imipramine for chronic low back pain. Family Practice 1982;14(5):841-6. [PubMed] [Google Scholar]
Allen 2014 {published data only}
- Allen R, Sharma U, Barlas S. Clinical experience with desvenlafaxine in treatment of pain associated with diabetic peripheral neuropathy. Journal of Pain Research 2014;7:339-51. [DOI: 10.2147/JPR.S55682] [DOI] [PMC free article] [PubMed] [Google Scholar]
Allen 2017 {published data only}
- Allen R, Sharma U, Barlas S. Clinical experience with desvenlafaxine in treatment of patients with fibromyalgia syndrome. Clinical Pharmacology in Drug Development 2017;6(3):224-33. [DOI: 10.1002/cpdd.271] [DOI] [PubMed] [Google Scholar]
Anderberg 2000 {published data only}
- Anderberg UM, Marteinsdottir I, Knorring L. Citalopram in patients with fibromyalgia—a randomized, double-blind, placebo-controlled study. European Journal of Pain 2000;4(1):27-35. [DOI: 10.1053/eujp.1999.0148] [DOI] [PubMed] [Google Scholar]
Ang 2013 {published data only}
- Ang DC, Jensen MP, Steiner JL, Hilligoss J, Gracely RH, Saha C. Combining cognitive-behavioral therapy and milnacipran for fibromyalgia: a feasibility randomized-controlled trial. Clinical Journal of Pain 2013;29(9):747-54. [DOI: 10.1097/AJP.0b013e31827a784e] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aragona 2005 {published data only}
- Aragona M, Bancheri L, Perinelli D, Tarsitani L, Pizzimenti A, Conte A, et al. Randomized double-blind comparison of serotonergic (Citalopram) versus noradrenergic (Reboxetine) reuptake inhibitors in outpatients with somatoform, DSM-IV-TR pain disorder. European Journal of Pain 2005;9(1):33-8. [DOI: 10.1016/j.ejpain.2004.03.003] [DOI] [PubMed] [Google Scholar]
Arnold 2002 {published data only}
- Arnold LM, Hess EV, Hudson JI, Welge JA, Berno SE, Keck PE Jr. A randomized, placebo-controlled, double-blind, flexible-dose study of fluoxetine in the treatment of women with fibromyalgia. American Journal of Medicine 2002;112(3):191-7. [DOI: 10.1016/s0002-9343(01)01089-0] [DOI] [PubMed] [Google Scholar]
Arnold 2004 {published data only}
- Arnold LM, Lu Y, Crofford LJ, Wohlreich M, Detke MJ, Iyengar S, et al. A double-blind, multicenter trial comparing duloxetine with placebo in the treatment of fibromyalgia patients with or without major depressive disorder. Arthritis & Rheumatology 2004;50(9):2974-84. [DOI: 10.1002/art.20485] [DOI] [PubMed] [Google Scholar]
Arnold 2005 {published data only}
- Arnold LM, Rosen A, Pritchett YL, D'Souza DN, Goldstein DJ, Iyengar S, et al. A randomized, double-blind, placebo-controlled trial of duloxetine in the treatment of women with fibromyalgia with or without major depressive disorder. Pain 2005;119(1-3):5-15. [DOI: 10.1016/j.pain.2005.06.031] [DOI] [PubMed] [Google Scholar]
Arnold 2010a {published data only}
- Arnold LM, Chatamra K, Hirsch I, Stoker M. Safety and efficacy of esreboxetine in patients with fibromyalgia: an 8-week, multicenter, randomized, double-blind, placebo-controlled study. Clinical Therapeutics 2010;32(9):1618-32. [DOI: 10.1016/j.clinthera.2010.08.003] [DOI] [PubMed] [Google Scholar]
Arnold 2010b {published data only}
- Arnold LM, Gendreau RM, Palmer RH, Gendreau JF, Wang Y. Efficacy and safety of milnacipran 100 mg/day in patients with fibromyalgia: results of a randomized, double-blind, placebo-controlled trial. Arthritis & Rheumatology 2010;62(9):2745-56. [DOI: 10.1002/art.27559] [DOI] [PubMed] [Google Scholar]
Arnold 2010c {published data only}
- Arnold LM, Clauw D, Wang F, Ahl J, Gaynor PJ, Wohlreich MM. Flexible dosed duloxetine in the treatment of fibromyalgia: a randomized, double-blind, placebo-controlled trial. Journal of Rheumatology 2010;37(12):2578-86. [DOI: 10.3899/jrheum.100365] [DOI] [PubMed] [Google Scholar]
Arnold 2012a {published data only}
- Arnold LM, Zhang S, Pangallo BA. Efficacy and safety of duloxetine 30 mg/d in patients with fibromyalgia: a randomized, double-blind, placebo-controlled study. Clinical Journal of Pain 2012;28(9):775-81. [DOI: 10.1097/AJP.0b013e3182510295] [DOI] [PubMed] [Google Scholar]
Arnold 2012b {published data only}
- Arnold LM, Hirsch I, Sanders P, Ellis A, Hughes B. Safety and efficacy of esreboxetine in patients with fibromyalgia: a fourteen-week, randomized, double-blind, placebo-controlled, multicenter clinical trial. Arthritis & Rheumatology 2012;64(7):2387-97. [DOI: 10.1002/art.34390] [DOI] [PubMed] [Google Scholar]
Ash 1999 {published data only}
- Ash G, Dickens CM, Creed FH, Jayson MI, Tomenson B. The effects of dothiepin on subjects with rheumatoid arthritis and depression. Rheumatology 1999;38(10):959-67. [DOI: 10.1093/rheumatology/38.10.959] [DOI] [PubMed] [Google Scholar]
Atkinson 1998 {published data only}
- Atkinson JH, Slater MA, Williams RA, Zisook S, Patterson TL, Grant I, et al. A placebo-controlled randomized clinical trial of nortriptyline for chronic low back pain. Pain 1998;76(3):287-96. [DOI: 10.1016/S0304-3959(98)00064-5] [DOI] [PubMed] [Google Scholar]
Atkinson 1999 {published data only}
- Atkinson JH, Slater MA, Wahlgren DR, Williams RA, Zisook S, Pruitt SD, et al. Effects of noradrenergic and serotonergic antidepressants on chronic low back pain intensity. Pain 1999;83(2):137-45. [DOI: 10.1016/s0304-3959(99)00082-2] [DOI] [PubMed] [Google Scholar]
Atkinson 2007 {published data only}
- Atkinson JH, Slater MA, Capparelli EV, Wallace MS, Zisook S, Abramson I, et al. Efficacy of noradrenergic and serotonergic antidepressants in chronic back pain: a preliminary concentration-controlled trial. Journal of Clinical Psychopharmacology 2007;27(2):135-42. [DOI: 10.1097/jcp.0b013e3180333ed5] [DOI] [PubMed] [Google Scholar]
Bansal 2009 {published data only}
- Bansal D, Bhansali A, Hota D, Chakrabarti A, Dutta P. Amitriptyline vs. pregabalin in painful diabetic neuropathy: a randomized double blind clinical trial. Diabetic Medicine 2009;26(10):1019-26. [DOI: 10.1111/j.1464-5491.2009.02806.x] [DOI] [PubMed] [Google Scholar]
Bateman 2013 {published data only}
- Bateman L, Palmer RH, Trugman JM, Lin Y. Results of switching to milnacipran in fibromyalgia patients with an inadequate response to duloxetine: a phase IV pilot study. Journal of Pain Research 2013;6:311-18. [DOI: 10.2147/JPR.S43395] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bird 2000 {published data only}
- Bird H, Broggini M. Paroxetine versus amitriptyline for treatment of depression associated with rheumatoid arthritis: a randomized, double blind, parallel group study. Journal of Rheumatology 2000;27(12):2791-7. [PubMed] [Google Scholar]
Boyle 2012 {published data only}
- Boyle J, Eriksson MEV, Gribble L, Gouni R, Johnsen S, Coppini DV, et al. Randomized, placebo-controlled comparison of amitriptyline, duloxetine, and pregabalin in patients with chronic diabetic peripheral neuropathic pain: impact on pain, polysomnographic sleep, daytime functioning, and quality of life. Diabetes Care 2012;35(12):2451-8. [DOI: 10.2337/dc12-0656] [DOI] [PMC free article] [PubMed] [Google Scholar]
Branco 2010 {published data only}
- Branco JC, Zachrisson O, Perrot S, Mainguy Y, Multinational Coordinator Study Group. A European multicenter randomized double-blind placebo-controlled monotherapy clinical trial of milnacipran in treatment of fibromyalgia. Journal of Rheumatology 2010;37(4):851-9. [DOI: 10.3899/jrheum.090884] [DOI] [PubMed] [Google Scholar]
Braz 2013 {published data only}
- Braz AS, Morais LC, Paula AP, Diniz MF, Almeida RN. Effects of Panax ginseng extract in patients with fibromyalgia: a 12-week, randomized, double-blind, placebo-controlled trial. Revista Brasileira de Psiquiatria [Brazilian Journal of Psychiatry] 2013;35(1):21-8. [DOI: 10.1016/j.rbp.2013.01.004] [DOI] [PubMed] [Google Scholar]
Calderon 2011 {published data only}
- Calderon PS, Tabaquim ML, Oliveira LC, Camargo AP, Netto TC, Conti PC. Effectiveness of cognitive-behavioral therapy and amitriptyline in patients with chronic temporomandibular disorders: a pilot study. Brazilian Dental Journal 2011;22(5):415-21. [DOI: 10.1590/s0103-64402011000500012] [DOI] [PubMed] [Google Scholar]
Cannon 1994 {published data only}
- Cannon RO 3rd, Quyyumi AA, Mincemoyer R, Stine AM, Gracely RH, Smith WB, et al. Imipramine in patients with chest pain despite normal coronary angiograms. New England Journal of Medicine 1994;330(20):1411-7. [DOI: 10.1056/NEJM199405193302003] [DOI] [PubMed] [Google Scholar]
Cardenas 2002 {published data only}
- Cardenas DD, Warms CA, Turner JA, Marshall H, Brooke MM, Loeser JD. Efficacy of amitriptyline for relief of pain in spinal cord injury: results of a randomized controlled trial. Pain 2002;96(3):365-73. [DOI: 10.1016/S0304-3959(01)00483-3] [DOI] [PubMed] [Google Scholar]
Carette 1986 {published data only}
- Carette S, McCain GA, Bell DA, Fam AG. Evaluation of amitriptyline in primary fibrositis. A double-blind, placebo-controlled study. Arthritis & Rheumatology 1986;29(5):655-9. [DOI: 10.1002/art.1780290510] [DOI] [PubMed] [Google Scholar]
Carette 1994 {published data only}
- Carette S, Bell MJ, Reynolds WJ, Haraoui B, McCain GA, Bykerk VP, et al. Comparison of amitriptyline, cyclobenzaprine, and placebo in the treatment of fibromyalgia: a randomized, double-blind clinical trial. Arthritis & Rheumatology 1994;37(1):32-40. [DOI: 10.1002/art.1780370106] [DOI] [PubMed] [Google Scholar]
Caruso 1987 {published data only}
- Caruso I, Sarzi Puttini PC, Boccassini L, Santandrea S, Locati M, Volpato R, et al. Double-blind study of dothiepin versus placebo in the treatment of primary fibromyalgia syndrome. Journal of International Medical Research 1987;15(3):154-9. [DOI: 10.1177/030006058701500305] [DOI] [PubMed] [Google Scholar]
Chappell 2008 {published data only}
- Chappell AS, Bradley LA, Wiltse C, Detke MJ, D'Souza DN, Spaeth M. A six-month double-blind, placebo-controlled, randomized clinical trial of duloxetine for the treatment of fibromyalgia. International Journal of General Medicine 2008;1:91‐102. [DOI: 10.2147/ijgm.s3979] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chappell 2009a {published data only}
- Chappell AS, Littlejohn G, Kajdasz DK, Scheinberg M, D'Souza DN, Moldofsky H. A 1-year safety and efficacy study of duloxetine in patients with fibromyalgia. Clinical Journal of Pain 2009;25(5):365-75. [DOI: 10.1097/AJP.0b013e31819be587] [DOI] [PubMed] [Google Scholar]
Chappell 2009b {published data only}
- Chappell AS, Ossanna MJ, Liu-Seifert H, Iyengar S, Skljarevski V, Li LC, et al. Duloxetine, a centrally acting analgesic, in the treatment of patients with osteoarthritis knee pain: a 13-week, randomized, placebo-controlled trial. Pain 2009;146(3):253-60. [DOI: 10.1016/j.pain.2009.06.024] [DOI] [PubMed] [Google Scholar]
Chappell 2011 {published data only}
- Chappell AS, Desaiah D, Liu-Seifert H, Zhang S, Skljarevski V, Belenkov Y, et al. A double-blind, randomized, placebo-controlled study of the efficacy and safety of duloxetine for the treatment of chronic pain due to osteoarthritis of the knee. Pain Practice 2011;11(1):33-41. [DOI: 10.1111/j.1533-2500.2010.00401.x] [DOI] [PubMed] [Google Scholar]
Clauw 2008 {published data only}
- Clauw DJ, Mease P, Palmer RH, Gendreau RM, Wang Y. Milnacipran for the treatment of fibromyalgia in adults: a 15-week, multicenter, randomized, double-blind, placebo-controlled, multiple-dose clinical trial. Clinical Therapeutics 2008;30(11):1988-2004. [DOI: 10.1016/j.clinthera.2008.11.009] [DOI] [PubMed] [Google Scholar]
Creed 2003 {published data only}
- Creed F, Fernandes L, Guthrie E, Palmer S, Ratcliffe J, Read N, et al, North of England IBS Research Group. The cost-effectiveness of psychotherapy and paroxetine for severe irritable bowel syndrome. Gastroenterology 2003;124(2):303-17. [DOI: 10.1053/gast.2003.50055] [DOI] [PubMed] [Google Scholar]
de Zanette 2014 {published data only}
- Zanette SA, Vercelino R, Laste G, Rozisky JR, Schwertner A, Machado CB, et al. Melatonin analgesia is associated with improvement of the descending endogenous pain-modulating system in fibromyalgia: a phase II, randomized, double-dummy, controlled trial. BMC Pharmacology and Toxicology 2014;15:40. [DOI: 10.1186/2050-6511-15-40] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dickens 2000 {published data only}
- Dickens C, Jayson M, Sutton C, Creed F. The relationship between pain and depression in a trial using paroxetine in sufferers of chronic low back pain. Psychosomatics 2000;41(6):490-9. [DOI: 10.1176/appi.psy.41.6.490] [DOI] [PubMed] [Google Scholar]
Drossman 2003 {published data only}
- Drossman DA, Toner BB, Whitehead WE, Diamant NE, Dalton CB, Duncan S, et al. Cognitive-behavioral therapy versus education and desipramine versus placebo for moderate to severe functional bowel disorders. Gastroenterology 2003;125(1):19-31. [DOI: 10.1016/s0016-5085(03)00669-3] [DOI] [PubMed] [Google Scholar]
Eberhard 1988 {published data only}
- Eberhard G, Knorring L, Nilsson HL, Sundequist U, Björling G, Linder H, et al. A double-blind randomized study of clomipramine versus maprotiline in patients with idiopathic pain syndromes. Neuropsychobiology 1988;19(1):25-34. [DOI: 10.1159/000118429] [DOI] [PubMed] [Google Scholar]
Engel 1998 {published data only}
- Engel CC Jr, Walker EA, Engel AL, Bullis J, Armstrong A. A randomized, double-blind crossover trial of sertraline in women with chronic pelvic pain. Journal of Psychosomatic Research 1998;44(2):203-7. [DOI: 10.1016/s0022-3999(97)00215-8] [DOI] [PubMed] [Google Scholar]
Enomoto 2018 {published data only}
- Enomoto H, Yasuda H, Nishiyori A, Fujikoshi S, Furukawa M, Ishida M, et al. Duloxetine in patients with diabetic peripheral neuropathic pain in Japan: a randomized, doubleblind, noninferiority comparative study with pregabalin. Journal of Pain Research 2018;11:1857-68. [DOI: 10.2147/JPR.S170646] [DOI] [PMC free article] [PubMed] [Google Scholar]
Enteshari‐Moghaddam 2019 {published data only}
- Enteshari-Moghaddam A, Azami A, Isazadehfar K, Mohebbi H, Habibzadeh A, Jahanpanah P. Efficacy of duloxetine and gabapentin in pain reduction in patients with knee osteoarthritis. Clinical Rheumatology 2019;38(10):2873-80. [DOI: 10.1007/s10067-019-04573-7] [DOI] [PubMed] [Google Scholar]
Forssell 2004 {published data only}
- Forssell H, Tasmuth T, Tenovuo O, Hampf G, Kalso E. Venlafaxine in the treatment of atypical facial pain: a randomized controlled trial. Journal of Orofacial Pain 2004;18(2):131-7. [PubMed] [Google Scholar]
Foster 2010a {published data only}
- Foster DC, Kotok MB, Huang L-S, Watts A, Oakes D, Howard FM, et al. Oral desipramine and topical lidocaine for vulvodynia: a randomized controlled trial. Obstetrics & Gynecology 2010;116(3):583-93. [DOI: 10.1097/AOG.0b013e3181e9e0ab] [DOI] [PMC free article] [PubMed] [Google Scholar]
Foster 2010b {published data only}
- Foster HE Jr, Hanno PM, Nickel JC, Payne CK, Mayer RD, Burks DA, et al. Effect of amitriptyline on symptoms in treatment naïve patients with interstitial cystitis/painful bladder syndrome. Journal of Urology 2010;183(5):1853‐58. [DOI: 10.1016/j.juro.2009.12.106] [DOI] [PMC free article] [PubMed] [Google Scholar]
Frakes 2011 {published data only}
- Frakes EP, Risser RC, Ball TD, Hochberg MC, Wohlreich MM. Duloxetine added to oral nonsteroidal anti-inflammatory drugs for treatment of knee pain due to osteoarthritis: results of a randomized, double-blind, placebo-controlled trial. Current Medical Research and Opinion 2011;27(12):2361-72. [DOI: 10.1185/03007995.2011.633502] [DOI] [PubMed] [Google Scholar]
Gao 2010 {published data only}
- Gao Y, Ning G, Jia W-P, Zhou Z-G, Xu Z-R, Liu Z-M, et al. Duloxetine versus placebo in the treatment of patients with diabetic neuropathic pain in China. Chinese Medical Journal 2010;123(22):3184-92. [PubMed] [Google Scholar]
Gao 2015 {published data only}
- Gao Y, Guo X, Han P, Li Q, Yang G, Qu S, et al. Treatment of patients with diabetic peripheral neuropathic pain in China: a double-blind randomised trial of duloxetine vs. placebo. International Journal of Clinical Practice 2015;69(9):957-66. [DOI: 10.1111/ijcp.12641] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gillving 2021 {published data only}
- Gillving M, Demant D, Holbech JV, Gylfadottir SS, Bach FW, Jensen TS, et al. A randomized, controlled trial of a β2-agonist in painful polyneuropathy. Pain 2021;162(5):1364‐73. [DOI: 10.1097/j.pain.0000000000002140] [DOI] [PubMed] [Google Scholar]
Gilron 2009 {published data only}
- Gilron I, Bailey JM, Tu D, Holden RR, Jackson AC, Houlden RL. Nortriptyline and gabapentin, alone and in combination for neuropathic pain: a double-blind, randomised controlled crossover trial. Lancet 2009;374(9697):1252-61. [DOI: 10.1016/S0140-6736(09)61081-3] [DOI] [PubMed] [Google Scholar]
Gilron 2015 {published data only}
- Gilron I, Tu D, Holden RR, Jackson AC, DuMerton-Shore D. Combination of morphine with nortriptyline for neuropathic pain. Pain 2015;156(8):1440-8. [DOI: 10.1097/j.pain.0000000000000149] [DOI] [PubMed] [Google Scholar]
Gilron 2016 {published data only}
- Gilron I, Chaparro LE, Tu D, Holden RR, Milev R, Towheed T, et al. Combination of pregabalin with duloxetine for fibromyalgia: a randomized controlled trial. Pain 2016;157(7):1532-40. [DOI: 10.1097/j.pain.0000000000000558] [DOI] [PubMed] [Google Scholar]
Ginsberg 1996 {published data only}
- Ginsberg F, Mancaux A, Joos E, Vanhove P, Famaey J-P. A randomized placebo-controlled trial of sustained-release amitriptyline in primary fibromyalgia. Journal of Musculoskeletal Pain 1996;4(3):37-47. [DOI: 10.1300/J094v04n03_05] [DOI] [Google Scholar]
Ginsberg 1998 {published data only}
- Ginsberg F, Joos E, Géczy J, Brahwyler J, Vandekerckhove K, Famaey J-P. A pilot randomized placebo-controlled study of pirlindole in the treatment of primary fibromyalgia. Journal of Musculoskeletal Pain 1998;6(2):5-17. [DOI: 10.1300/J094v06n02_02] [DOI] [Google Scholar]
Goldenberg 1986 {published data only}
- Goldenberg DL, Felson DT, Dinerman H. A randomized, controlled trial of amitriptyline and naproxen in the treatment of patients with fibromyalgia. Arthritis & Rheumatology 1986;29(11):1371‐7. [DOI: 10.1002/art.1780291110] [DOI] [PubMed] [Google Scholar]
Goldenberg 1996 {published data only}
- Goldenberg D, Mayskiy M, Mossey C, Ruthazer R, Schmid C. A randomized, double-blind crossover trial of fluoxetine and amitriptyline in the treatment of fibromyalgia. Arthritis & Rheumatology 1996;39(11):1852-9. [DOI: 10.1002/art.1780391111] [DOI] [PubMed] [Google Scholar]
Goldman 2010 {published data only}
- Goldman RH, Stason WB, Park SK, Kim R, Mudgal S, Davis RB, et al. Low-dose amitriptyline for treatment of persistent arm pain due to repetitive use. Pain 2010;149(1):117-23. [DOI: 10.1016/j.pain.2010.01.016] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldstein 2005 {published data only}
- Goldstein DJ, Lu Y, Detke M J, Lee TC, Iyengar S. Duloxetine vs. placebo in patients with painful diabetic neuropathy. Pain 2005;116(1-2):109-18. [DOI: 10.1016/j.pain.2005.03.029] [DOI] [PubMed] [Google Scholar]
González‐Viejo 2005 {published data only}
- González-Viejo MA, Avellanet M, Hernández-Morcuende MI. A comparative study of fibromyalgia treatment: ultrasonography and physiotherapy versus sertraline treatment [Étude comparative dans traitement de la fibromyalgie: ultrasons et kinésithérapie versus sertraline]. Annales de Réadaptation et de Médecine Physique 2005;48(8):610-5. [DOI: 10.1016/j.annrmp.2005.03.012] [DOI] [PubMed] [Google Scholar]
Goodkin 1990 {published data only}
- Goodkin K, Gullion CM, Agras WS. A randomized, double-blind, placebo-controlled trial of trazodone hydrochloride in chronic low back pain syndrome. Journal of Clinical Psychopharmacology 1990;10(4):269-78. [PubMed] [Google Scholar]
Gould 2020 {published data only}
- Gould HM, Atkinson JH, Chircop-Rollick T, D'Andrea J, Garfin S, Patel SM, et al. A randomized placebo-controlled trial of desipramine, cognitive behavioral therapy, and active placebo therapy for low back pain. Pain 2020;12:1341-9. [DOI: 10.1097/j.pain.0000000000001834] [DOI] [PubMed] [Google Scholar]
Grace 1985 {published data only}
- Grace EM, Bellamy N, Kassam Y, Buchanan WW. Controlled, double-blind, randomized trial of amitriptyline in relieving articular pain and tenderness in patients with rheumatoid arthritis. Current Medical Research and Opinion 1985;9(6):426-9. [DOI: 10.1185/03007998509109614] [DOI] [PubMed] [Google Scholar]
Graff‐Radford 2000 {published data only}
- Graff-Radford SB, Shaw LR, Naliboff BN. Amitriptyline and fluphenazine in the treatment of postherpetic neuralgia. Clinical Journal of Pain 2000;16(3):188-92. [DOI: 10.1097/00002508-200009000-00002] [DOI] [PubMed] [Google Scholar]
Hadianfard 2012 {published data only}
- Hadianfard MJ, Hosseinzadeh Parizi M. A randomized clinical trial of fibromyalgia treatment with acupuncture compared with fluoxetine. Iranian Red Crescent Medical Journal 2012;14(10):631. [PMC free article] [PubMed] [Google Scholar]
Hameroff 1984 {published data only}
- Hameroff SR, Weiss JL, Lerman JC, Cork RC, Watts KS, Crago BR, et al. Doxepin's effects on chronic pain and depression: a controlled study. Journal of Clinical Psychiatry 1984;45(3 Pt 2):47-53. [PubMed] [Google Scholar]
Hammody 2015 {published data only}
- Hammody EL, Matloub SY, Shihab SS. Pregabalin versus amitriptyline in the treatment of fibromyalgia patients (a double blind comparative study). Iraqi Postgraduate Medical Journal 2015;14(1):39-45. [Google Scholar]
Hannonen 1998 {published data only}
- Hannonen P, Malminiemi K, Yli-Kerttula U, Isomeri R, Roponen P. A randomized, double-blind, placebo-controlled study of moclobemide and amitriptyline in the treatment of fibromyalgia in females without psychiatric disorder. British Journal of Rheumatology 1998;37(12):1279-86. [DOI: 10.1093/rheumatology/37.12.1279] [DOI] [PubMed] [Google Scholar]
Heymann 2001 {published data only}
- Heymann RE, Helfenstein M, Feldman D. A double-blind, randomized, controlled study of amitriptyline, nortriptyline and placebo in patients with fibromyalgia. An analysis of outcome measures. Clinical and Experimental Rheumatology 2001;19(6):697-702. [PubMed] [Google Scholar]
Holbech 2015 {published data only}
- Holbech JV, Bach FW, Finnerup NB, Brosen K, Jensen TS, Sindrup SH. Imipramine and pregabalin combination for painful polyneuropathy: a randomized controlled trial. Pain 2015;156(5):958-66. [DOI: 10.1097/j.pain.0000000000000143] [DOI] [PubMed] [Google Scholar]
Hudson 2021 {published and unpublished data}
- Hudson B, Williman JA, Stamp LK, Alchin JS, Hooper GJ, Mangin D, et al. Nortriptyline for pain in knee osteoarthritis: a double-blind randomised controlled trial in New Zealand general practice. British Journal of General Practice 2021;71(708):e538‐46. [DOI: 10.3399/BJGP.2020.0797] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hussain 2011 {published data only}
- Hussain SA, Al Khalifa II, Jasim NA, Gorial FI. Adjuvant use of melatonin for treatment of fibromyalgia. Journal of Pineal Research 2011;50(3):267-71. [DOI: 10.1111/j.1600-079X.2010.00836.x] [DOI] [PubMed] [Google Scholar]
Isomeri 1993 {published data only}
- Isomeri R, Mikkelsson M, Latikka P, Kammonen K. Effects of amitriptyline and cardiovascular fitness training on pain in patients with primary fibromyalgia. Journal of Musculoskeletal Pain 1993;1(3-4):253-60. [Google Scholar]
Iwaki 2020 {published data only}
- Iwaki H, Ando R, Tada S, Nishikawa N, Tsujii T, Yamanishi Y, et al. A double-blind, randomized controlled trial of duloxetine for pain in Parkinson's disease. Journal of the Neurological Sciences 2020;414:116833. [DOI] [PubMed] [Google Scholar]
Johansson 1979 {published data only}
- Johansson F, Knorring L. A double-blind controlled study of seratonin uptake inhibitor (zimelidine) versus placebo in chronic pain patients. Pain 1979;7(1):69-78. [DOI] [PubMed] [Google Scholar]
Joharchi 2019 {published data only}
- Joharchi K, Memari M, Azargashb E, Saadat N. Efficacy and safety of duloxetine and pregabalin in Iranian patients with diabetic peripheral neuropathic pain: a double-blind, randomized clinical trial. Journal of Diabetes and Metabolic Disorders 2019;18(2):575-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jose 2007 {published data only}
- Jose VM, Bhansali A, Hota D, Pandhi P. Randomized double-blind study comparing the efficacy and safety of lamotrigine and amitriptyline in painful diabetic neuropathy. Diabetic Medicine 2007;24(4):377-83. [DOI: 10.1111/j.1464-5491.2007.02093.x] [DOI] [PubMed] [Google Scholar]
Kalso 1996 {published data only}
- Kalso E, Tasmuth T, Neuvonen PJ. Amitriptyline effectively relieves neuropathic pain following treatment of breast cancer. Pain 1996;64(2):293-302. [DOI: 10.1016/0304-3959(95)00138-7] [DOI] [PubMed] [Google Scholar]
Katz 2005 {published data only}
- Katz J, Pennella-Vaughan J, Hetzel RD, Kanazi GE, Dworkin RH. A randomized, placebo-controlled trial of bupropion sustained release in chronic low back pain. Journal of Pain 2005;6(10):656-61. [DOI: 10.1016/j.jpain.2005.05.002] [DOI] [PubMed] [Google Scholar]
Kaur 2011 {published data only}
- Kaur H, Hota D, Bhansali A, Dutta P, Bansal D, Chakrabarti A. A comparative evaluation of amitriptyline and duloxetine in painful diabetic neuropathy: a randomized, double-blind, cross-over clinical trial. Diabetes Care 2011;34(4):818-22. [DOI: 10.2337/dc10-1793] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kayiran 2010 {published data only}
- Kayiran S, Dursun E, Dursun N, Ermutlu N, Karamursel S. Neurofeedback intervention in fibromyalgia syndrome; a randomized, controlled, rater blind clinical trial. Applied Psychophysiology and Biofeedback 2010;35(4):293-302. [DOI: 10.1007/s10484-010-9135-9] [DOI] [PubMed] [Google Scholar]
Keefe 2011 {published data only}
- Keefe FJ, Shelby RA, Somers TJ, Varia I, Blazing M, Waters SJ, et al. Effects of coping skills training and sertraline in patients with non-cardiac chest pain: a randomized controlled study. Pain 2011;152(4):730-41. [DOI: 10.1016/j.pain.2010.08.040] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khoromi 2007 {published data only}
- Khoromi S, Cui L, Nackers L, Max MB. Morphine, nortriptyline and their combination vs. placebo in patients with chronic lumbar root pain. Pain 2007;130(1-2):66-75. [DOI: 10.1016/j.pain.2006.10.029] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2013 {published data only}
- Kim JL, Rele S, Marks DM, Masand PS, Yerramsetty P, Millet RA, et al. Effects of milnacipran on neurocognition, pain, and fatigue in fibromyalgia: a 13-week, randomized, placebo-controlled, crossover trial. Primary Care Companion for CNS Disorders 2013;15(6):PCC.13m01555. [DOI: 10.4088/PCC.13m01555] [DOI] [PMC free article] [PubMed] [Google Scholar]
Konno 2016 {published data only}
- Konno S, Oda N, Ochiai T, Alev L. Randomized, double-blind, placebo-controlled phase iii trial of duloxetine monotherapy in Japanese patients with chronic low back pain. Spine 2016;41(22):1709-17. [DOI: 10.1097/BRS.0000000000001707] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2010 {published data only}
- Lee H, Kim JH, Min B-H, Lee JH, Son HJ, Kim JJ, et al. Efficacy of venlafaxine for symptomatic relief in young adult patients with functional chest pain: a randomized, double-blind, placebo-controlled, crossover trial. American Journal of Gastroenterology 2010;105(7):1504-12. [DOI: 10.1038/ajg.2010.82] [DOI] [PubMed] [Google Scholar]
Lee 2016 {published data only}
- Lee YC, Massarotti E, Edwards RR, Lu B, Liu C, Lo Y, et al. Effect of milnacipran on pain in patients with rheumatoid arthritis with widespread pain: a randomized blinded crossover trial. Journal of Rheumatology 2016;43(1):38-45. [DOI: 10.3899/jrheum.150550] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leijon 1989 {published data only}
- Leijon G, Boivie J. Central post-stroke pain—a controlled trial of amitriptyline and carbamazepine. Pain 1989;36(1):27-36. [DOI: 10.1016/0304-3959(89)90108-5] [DOI] [PubMed] [Google Scholar]
Lipone 2020 {published data only}
- Lipone P, Ehler E, Nastaj M, Palka-Kisielowska I, Cruccu G, Truini A, et al. Efficacy and safety of low doses of trazodone in patients affected by painful diabetic neuropathy and treated with gabapentin: a randomized controlled pilot study. CNS Drugs 2020;34(11):1177-89. [DOI: 10.1007/s40263-020-00760-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Loldrup 1989 {published data only}
- Loldrup D, Langemark M, Hansen HJ, Olesen J, Bech P. Clomipramine and mianserin in chronic idiopathic pain syndrome. A placebo controlled study. Psychopharmacology 1989;99(1):1-7. [DOI: 10.1007/BF00634443] [DOI] [PubMed] [Google Scholar]
Luo 2009 {published data only}
- Luo Y-L, Zhang M-Y, Wu W-Y, Li C-B, Lu Z, Li Q-W. A randomized double-blind clinical trial on analgesic efficacy of fluoxetine for persistent somatoform pain disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry 2009;33(8):1522-5. [DOI: 10.1016/j.pnpbp.2009.08.013] [DOI] [PubMed] [Google Scholar]
Maarrawi 2018 {published data only}
- Maarrawi J, Abdel Hay J, Kobaiter-Maarrawi S, Tabet P, Peyron R, Garcia-Larrea L. Randomized double-blind controlled study of bedtime low-dose amitriptyline in chronic neck pain. European Journal of Pain 2018;22(6):1180-87. [DOI: 10.1002/ejp.1206] [DOI] [PubMed] [Google Scholar]
Macfarlane 1986 {published data only}
- Macfarlane JG, Jalali S, Grace EM. Trimipramine in rheumatoid arthritis: a randomized double-blind trial in relieving pain and joint tenderness. Current Medical Research and Opinion 1986;10(2):89-93. [DOI: 10.1185/03007998609110424] [DOI] [PubMed] [Google Scholar]
Mahmoud 2021 {published data only}
- Mahmoud AM, Ragab SG, Boules ML, Botros JM. Comparison between two low doses of amitriptyline in the management of chronic neck pain: a randomized, double-blind, comparative study. Pain Research & Management 2021;2021:8810178. [DOI: 10.1155/2021/8810178] [DOI] [PMC free article] [PubMed] [Google Scholar]
Majdinasab 2019 {published data only}
- Majdinasab N, Kaveyani H, Azizi M. A comparative double-blind randomized study on the effectiveness of duloxetine and gabapentin on painful diabetic peripheral polyneuropathy. Drug Design, Development and Therapy 2019;13:1985-92. [DOI: 10.2147/DDDT.S185995] [DOI] [PMC free article] [PubMed] [Google Scholar]
Masand 2009 {published data only}
- Masand PS, Pae C-U, Krulewicz S, Peindl K, Mannelli P, Varia IM, et al. A double-blind, randomized, placebo-controlled trial of paroxetine controlled-release in irritable bowel syndrome. Psychosomatics 2009;50(1):78-86. [DOI: 10.1176/appi.psy.50.1.78] [DOI] [PubMed] [Google Scholar]
Matthey 2013 {published data only}
- Matthey A, Cedraschi C, Piguet V, Besson M, Chabert J, Daali Y, et al. Dual reuptake inhibitor milnacipran and spinal pain pathways in fibromyalgia patients: a randomized, double-blind, placebo-controlled trial. Pain Physician 2013;16(5):E553-62. [PubMed] [Google Scholar]
Max 1988 {published data only}
- Max MB, Schafer SC, Culnane M, Smoller B, Dubner R, Gracely RH. Amitriptyline, but not lorazepam, relieves postherpetic neuralgia. Neurology 1988;38(9):1427-32. [DOI: 10.1212/wnl.38.9.1427] [DOI] [PubMed] [Google Scholar]
Max 1992 {published data only}
- Max MB, Lynch SA, Muir J, Shoaf SE, Smoller B, Dubner R. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. New England Journal of Medicine 1992;326(19):1250-6. [DOI: 10.1056/NEJM199205073261904] [DOI] [PubMed] [Google Scholar]
Mease 2009 {published data only}
- Mease PJ, Clauw DJ, Gendreau RM, Rao SG, Kranzler J, Chen W. The efficacy and safety or milnacipran for treatment of fibromyalgia. A randomized, double-blind, placebo-controlled trial. Journal of Rheumatology 2009;36(2):398‐409. [DOI: 10.3899/jrheum.080734] [DOI] [PubMed] [Google Scholar]
Miki 2016 {published data only}
- Miki K, Murakami M, Oka H, Onozawa K, Yoshida S, Osada K. Efficacy of mirtazapine for the treatment of fibromyalgia without concomitant depression: a randomized, double-blind, placebo-controlled phase IIa study in Japan. Pain 2016;157(9):2089-96. [DOI: 10.1097/j.pain.0000000000000622] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morello 1999 {published data only}
- Morello CM, Leckband SG, Stoner CP, Moorhouse DF, Sahagian GA. Randomized double-blind study comparing the efficacy of gabapentin with amitriptyline on diabetic peripheral neuropathy pain. Arch Intern Med 1999;159(16):1931-7. [DOI: 10.1001/archinte.159.16.1931] [DOI] [PubMed] [Google Scholar]
Muller 2008 {published data only}
- Muller JE, Wentzel I, Koen L, Niehaus DJ, Seedat S, Stein DJ. Escitalopram in the treatment of multisomatoform disorder: a double-blind, placebo-controlled trial. International Clinical Psychopharmacology 2008;23(1):43-8. [DOI: 10.1097/YIC.0b013e32825ea301] [DOI] [PubMed] [Google Scholar]
Murakami 2015 {published data only}
- Murakami M, Osada K, Mizuno H, Ochiai T, Alev L, Nishioka K. A randomized, double-blind, placebo-controlled phase III trial of duloxetine in Japanese fibromyalgia patients. Arthritis Research and Therapy 2015;17:224. [DOI: 10.1186/s13075-015-0718-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nabi 2021 {published data only}
- Nabi BN, Saberi A, Eghbali BB, Hosseininezhad M, Biazar G, Malekabadi AA, et al. Efficacy and safety of TENS and duloxetine in patients with painful diabetic neuropathy: a single blind randomized clinical trial. Journal of Advances in Medical and Biomedical Research 2021;29(136):286‐92. [Google Scholar]
Natelson 2015 {published data only}
- Natelson BH, Vu D, Mao X, Weiduschat N, Togo F, Lange G, et al. Effect of milnacipran treatment on ventricular lactate in fibromyalgia: a randomized, double-blind, placebo-controlled trial. Journal of Pain 2015;16(11):1211-9. [DOI: 10.1016/j.jpain.2015.08.004] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00066937 {published data only}
- NCT00066937. Comparison of psychological and pharmacological treatments for pain due to temporomandibular joint disorder (TMD) [Pain management in temporomandibular joint disorders]. https://clinicaltrials.gov/show/NCT00066937 (first received 8 August 2003).
NCT01225068 {published data only}
- NCT01225068. Effect of milnacipran in chronic neuropathic low back pain [An exploratory randomized placebo controlled trial of milnacipran in patients with chronic neuropathic low back pain]. https://clinicaltrials.gov/show/NCT01225068 (first received 20 October 2010).
NCT01510457 {published data only}
- NCT01510457. Milnacipran for chronic pain in knee osteoarthritis [Milnacipran for the pain, sensory sensitization and mood changes in knee osteoarthritis]. https://clinicaltrials.gov/show/NCT01510457 (first received 16 January 2012).
Nørregaard 1995 {published data only}
- Nørregaard J, Volkmann H, Danneskiold-Samstøe B. A randomized controlled trial of citalopram in the treatment of fibromyalgia. Pain 1995;61(3):445-9. [DOI: 10.1016/0304-3959(94)00218-4] [DOI] [PubMed] [Google Scholar]
Otto 2008 {published data only}
- Otto M, Bach FW, Jensen TS, Brøsen K, Sindrup SH. Escitalopram in painful polyneuropathy: a randomized, placebo-controlled, cross-over trial. Pain 2008;139(2):275-83. [DOI: 10.1016/j.pain.2008.04.012] [DOI] [PubMed] [Google Scholar]
Ozerbil 2006 {published data only}
- Ozerbil O, Okudan N, Gökbel H, Levendoğlu F. Comparison of the effects of two antidepressants on exercise performance of the female patients with fibromyalgia. Clinical Rheumatology 2006;25(4):495-7. [DOI: 10.1007/s10067-005-0076-2] [DOI] [PubMed] [Google Scholar]
Pakfetrat 2019 {published data only}
- Pakfetrat A, Talebi M, Dalirsani Z, Mohajery SA, Zamani R, Ghazi A. Evaluation of the effectiveness of crocin isolated from saffron in treatment of burning mouth syndrome: a randomized controlled trial. Avicenna Journal of Phytomedicine 2019;9(6):505-16. [DOI: 10.22038/AJP.2019.12764] [DOI] [PMC free article] [PubMed] [Google Scholar]
Patkar 2007 {published data only}
- Patkar AA, Masand PS, Krulewicz S, Mannelli P, Peindl K, Beebe KL, et al. A randomized, controlled, trial of controlled release paroxetine in fibromyalgia. American Journal of Medicine 2007;120(5):448-54. [DOI: 10.1016/j.amjmed.2006.06.006] [DOI] [PubMed] [Google Scholar]
Petzke 2013 {published data only}
- Petzke F, Jensen KB, Kosek E, Choy E, Carville S, Fransson P, et al. Using fMRI to evaluate the effects of milnacipran on central pain processing in patients with fibromyalgia. Scandinavian Journal of Pain 2013;4(2):65-74. [DOI: 10.1016/j.sjpain.2012.10.002] [DOI] [PubMed] [Google Scholar]
Pickering 2018 {published data only}
- Pickering G, Macian N, Delage N, Picard P, Cardot J-M, Sickout-Arondo S, et al. Milnacipran poorly modulates pain in patients suffering from fibromyalgia: a randomized double-blind controlled study. Drug Design, Development and Therapy 2018;12:2485-96. [DOI: 10.2147/DDDT.S162810] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pilowsky 1990 {published data only}
- Pilowsky I, Barrow CG. A controlled study of psychotherapy and amitriptyline used individually and in combination in the treatment of chronic intractable, 'psychogenic' pain. Pain 1990;40(1):3-19. [DOI: 10.1016/0304-3959(90)91045-K] [DOI] [PubMed] [Google Scholar]
Pirbudak 2003 {published data only}
- Pirbudak L, Karakurum G, Oner U, Gulec A, Karadasli H. Epidural corticosteroid injection and amitriptyline for the treatment of chronic low back pain associated with radiculopathy. Pain Clinic 2003;15(3):247-53. [DOI: 10.1163/156856903767650763] [DOI] [Google Scholar]
Rani 1996 {published data only}
- Rani PU, Naidu MU, Prasad VB, Rao TR, Shobha JC. An evaluation of antidepressants in rheumatic pain conditions. Anesthia & Analgesia 1996;83(2):371-5. [DOI: 10.1097/00000539-199608000-00029] [DOI] [PubMed] [Google Scholar]
Raskin 2005 {published data only}
- Raskin J, Pritchett YL, Wang F, D'Souza DN, Waninger AL, Iyengar S, et al. A double-blind, randomized multicenter trial comparing duloxetine with placebo in the management of diabetic peripheral neuropathic pain. Pain Medicine 2005;6(5):346-56. [DOI: 10.1111/j.1526-4637.2005.00061.x] [DOI] [PubMed] [Google Scholar]
Razazian 2014 {published data only}
- Razazian N, Baziyar M, Moradian N, Afshari D, Bostani A, Mahmoodi M. Evaluation of the efficacy and safety of pregabalin, venlafaxine, and carbamazepine in patients with painful diabetic peripheral neuropathy. A randomized, double-blind trial. Neurosciences 2014;19(3):192-8. [PMC free article] [PubMed] [Google Scholar]
RBR‐5dsrhv {published data only}
- RBR-5dsrhv. Comparation of treatments of temporomandibular joint problems [Comparative study of temporomandibular disfunction therapies: randomized blind controled clinical trials]. https://trialsearch.who.int/Trial2.aspx?TrialID=RBR-5dsrhv (first received 28 May 2018).
Richards 2015 {published data only}
- Richards JS, Bombardier CH, Wilson CS, Chiodo AE, Brooks L, Tate DG, et al. Efficacy of venlafaxine XR for the treatment of pain in patients with spinal cord injury and major depression: a randomized, controlled trial. Archives of Physical Medicine and Rehabilitation 2015;96(4):680-9. [DOI: 10.1016/j.apmr.2014.11.024] [DOI] [PubMed] [Google Scholar]
Rintala 2007 {published data only}
- Rintala DH, Holmes SA, Courtade D, Fiess RN, Tastard LV, Loubser PG. Comparison of the effectiveness of amitriptyline and gabapentin on chronic neuropathic pain in persons with spinal cord injury. Archives of Physical Medicine and Rehabilitation 2007;88(12):1547-60. [DOI: 10.1016/j.apmr.2007.07.038] [DOI] [PubMed] [Google Scholar]
Robinson 2004 {published data only}
- Robinson LR, Czerniecki JM, Ehde DM, Edwards WT, Judish DA, Goldberg ML, et al. Trial of amitriptyline for relief of pain in amputees: results of a randomized controlled study. Archives of Physical Medicine and Rehabilitation 2004;85(1):1-6. [DOI: 10.1016/s0003-9993(03)00476-3] [DOI] [PubMed] [Google Scholar]
Rowbotham 2004 {published data only}
- Rowbotham MC, Goli V, Kunz NR, Lei D. Venlafaxine extended release in the treatment of painful diabetic neuropathy: a double-blind, placebo-controlled study. Pain 2004;110(3):697-706. [DOI: 10.1016/j.pain.2004.05.010] [DOI] [PubMed] [Google Scholar]
Rowbotham 2005 {published data only}
- Rowbotham MC, Reisner LA, Davies PS, Fields HL. Treatment response in antidepressant-naïve postherpetic neuralgia patients: double-blind, randomized trial. Journal of Pain 2005;6(11):741‐6. [DOI: 10.1016/j.jpain.2005.07.001] [DOI] [PubMed] [Google Scholar]
Rowbotham 2012 {published data only}
- Rowbotham MC, Arslanian A, Nothaft W, Duan WR, Best AE, Pritchett Y, et al. Efficacy and safety of the alpha4β2 neuronal nicotinic receptor agonist ABT-894 in patients with diabetic peripheral neuropathic pain. Pain 2012;153(4):862-8. [DOI: 10.1016/j.pain.2012.01.009] [DOI] [PubMed] [Google Scholar]
Russell 2008 {published data only}
- Russell IJ, Mease PJ, Smith TR, Kajdasz DK, Wohlreich MM, Detke MJ, et al. Efficacy and safety of duloxetine for treatment of fibromyalgia in patients with or without major depressive disorder: results from a 6-month, randomized, double-blind, placebo-controlled, fixed-dose trial. Pain 2008;136(3):432-44. [DOI: 10.1016/j.pain.2008.02.024] [DOI] [PubMed] [Google Scholar]
Sarzi Puttini 1988 {published data only}
- Sarzi Puttini P, Cazzola M, Boccassini L, Ciniselli G, Santandrea S, Caruso I, et al. A comparison of dothiepin versus placebo in the treatment of pain in rheumatoid arthritis and the association of pain with depression. Journal of International Medical Research 1988;16(5):331-7. [DOI: 10.1177/030006058801600502] [DOI] [PubMed] [Google Scholar]
Schukro 2016 {published data only}
- Schukro RP, Oehmke MJ, Geroldinger A, Heinze G, Kress H-G, Pramhas S. Efficacy of duloxetine in chronic low back pain with a neuropathic component: a randomized, double-blind, placebo-controlled crossover trial. Anesthesiology 2016;124(1):150-8. [DOI: 10.1097/ALN.0000000000000902] [DOI] [PubMed] [Google Scholar]
Scudds 1989 {published data only}
- Scudds RA, McCain GA, Rollman GB, Harth M. Improvements in pain responsiveness in patients with fibrositis after successful treatment with amitriptyline. Journal of Rheumatology Supplement 1989;19:98-103. [PubMed] [Google Scholar]
Sencan 2004 {published data only}
- Sencan S, Ak S, Karan A, Muslumanoglu L, Ozcan E, Berker E. A study to compare the therapeutic efficacy of aerobic exercise and paroxetine in fibromyalgia syndrome. Journal of Back and Musculoskeletal Rehabilitation 2004;17(2):57-61. [DOI: 10.3233/BMR-2004-17204] [DOI] [Google Scholar]
Shakiba 2018 {published data only}
- Shakiba M, Moazen-Zadeh E, Noorbala AA, Jafarinia M, Divsalar P, Kashani L, et al. Saffron (Crocus sativus) versus duloxetine for treatment of patients with fibromyalgia: a randomized double-blind clinical trial. Avicenna Journal of Phytomedicine 2018;8(6):513-23. [PMC free article] [PubMed] [Google Scholar]
Sindrup 2003 {published data only}
- Sindrup SH, Bach FW, Madsen C, Gram LF, Jensen TS. Venlafaxine versus imipramine in painful polyneuropathy: a randomized, controlled trial. Neurology 2003;60(8):1284-9. [DOI] [PubMed] [Google Scholar]
Skljarevski 2009 {published data only}
- Skljarevski V, Ossanna M, Liu-Seifert H, Zhang Q, Chappell A, Iyengar S, et al. A double-blind, randomized trial of duloxetine versus placebo in the management of chronic low back pain. European Journal of Neurology 2009;16(9):1041-8. [DOI: 10.1111/j.1468-1331.2009.02648.x] [DOI] [PubMed] [Google Scholar]
Skljarevski 2010a {published data only}
- Skljarevski V, Desaiah D, Liu-Seifert H, Zhang Q, Chappell AS, Detke MJ, et al. Efficacy and safety of duloxetine in patients with chronic low back pain. Spine 2010;35(13):E578-85. [DOI: 10.1097/BRS.0b013e3181d3cef6] [DOI] [PubMed] [Google Scholar]
Skljarevski 2010b {published data only}
- Skljarevski V, Zhang S, Desaiah D, Alaka KJ, Palacios S, Miazgowski T, et al. Duloxetine versus placebo in patients with chronic low back pain: a 12-week, fixed-dose, randomized, double-blind trial. Journal of Pain 2010;11(12):1282-90. [DOI: 10.1016/j.jpain.2010.03.002] [DOI] [PubMed] [Google Scholar]
Smith 2013 {published data only}
- Smith EM, Pang H, Cirrincione C, Fleishman S, Paskett ED, Ahles T, et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. Journal of the American Medical Association 2013;309(13):1359-67. [DOI: 10.1001/jama.2013.2813] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sofat 2017 {published data only}
- Sofat N, Harrison A, Russell MD, Ayis S, Kiely PD, Baker EH, et al. The effect of pregabalin or duloxetine on arthritis pain: a clinical and mechanistic study in people with hand osteoarthritis. Journal of Pain Research 2017;10:2437-49. [DOI: 10.2147/JPR.S147640] [DOI] [PMC free article] [PubMed] [Google Scholar]
Spinhoven 2010 {published data only}
- Spinhoven P, Van der Does AJ, Van Dijk E, Van Rood YR. Heart-focused anxiety as a mediating variable in the treatment of noncardiac chest pain by cognitive-behavioral therapy and paroxetine. Journal of Psychosomatic Research 2010;69(3):227-35. [DOI: 10.1016/j.jpsychores.2010.02.005] [DOI] [PubMed] [Google Scholar]
Srinivasan 2021 {published data only}
- Srinivasan A, Dutta P, Bansal D, Chakrabarti A, Bhansali AK, Hota D. Efficacy and safety of low-dose naltrexone in painful diabetic neuropathy: a randomized, double-blind, active-control, crossover clinical trial. Journal of Diabetes 2021;13(10):770-8. [DOI: 10.1111/1753-0407.13202] [DOI] [PubMed] [Google Scholar]
Staud 2015 {published data only}
- Staud R, Lucas YE, Price DD, Robinson ME. Effects of milnacipran on clinical pain and hyperalgesia of patients with fibromyalgia: results of a 6-week randomized controlled trial. Journal of Pain 2015;16(8):750-9. [DOI: 10.1016/j.jpain.2015.04.010] [DOI] [PubMed] [Google Scholar]
Suttiruksa 2016 {published data only}
- Suttiruksa S, Yeephu S, Prateepavanich P, Suthisisang C. Effects of mirtazapine on quality of life of Thai patients with fibromyalgia syndrome: a double-blind, randomized, placebo-controlled trial. Asian Biomedicine 2016;10(5):435-45. [DOI: 10.5372/1905-7415.1005.506] [DOI] [Google Scholar]
Talley 2008 {published data only}
- Talley NJ, Kellow JE, Boyce P, Tennant C, Huskic S, Jones M. Antidepressant therapy (imipramine and citalopram) for irritable bowel syndrome: a double-blind, randomized, placebo-controlled trial. Digestive Diseases and Sciences 2008;53(1):108-15. [DOI: 10.1007/s10620-007-9830-4] [DOI] [PubMed] [Google Scholar]
Tammiala‐Salonen 1999 {published data only}
- Tammiala-Salonen T, Forssell H. Trazodone in burning mouth pain: a placebo-controlled, double-blind study. Journal of Orofacial Pain 1999;13(2):83-8. [PubMed] [Google Scholar]
Tanum 1996 {published data only}
- Tanum L, Malt UF. A new pharmacologic treatment of functional gastrointestinal disorder. A double-blind placebo-controlled study with mianserin. Scandinavian Journal of Gastroenterology 1996;31(4):318-25. [DOI: 10.3109/00365529609006404] [DOI] [PubMed] [Google Scholar]
Tasmuth 2002 {published data only}
- Tasmuth T, Härtel B, Kalso E. Venlafaxine in neuropathic pain following treatment of breast cancer. European Journal of Pain 2002;6(1):17-24. [DOI: 10.1053/eujp.2001.0266] [DOI] [PubMed] [Google Scholar]
Tesfaye 2013 {published data only}
- Tesfaye S, Wilhelm S, Lledo A, Schacht A, Tölle T, Bouhassira D, et al. Duloxetine and pregabalin: high-dose monotherapy or their combination? the "COMBO-DN study"— a multinational, randomized, double-blind, parallel-group study in patients with diabetic peripheral neuropathic pain. Pain 2013;154(12):2616-25. [DOI: 10.1016/j.pain.2013.05.043] [DOI] [PubMed] [Google Scholar]
Tétreault 2016 {published and unpublished data}
- Tétreault P, Mansour A, Vachon-Presseau E, Schnitzer TJ, Apkarian AV, Baliki MN. Brain connectivity predicts placebo response across chronic pain clinical trials. PLOS Biology 2016;14(10):e1002570. [DOI: 10.1371/journal.pbio.1002570] [DOI] [PMC free article] [PubMed] [Google Scholar]
Trugman 2014 {published data only}
- Trugman JM, Palmer RH, Ma Y. Milnacipran effects on 24-hour ambulatory blood pressure and heart rate in fibromyalgia patients: a randomized, placebo-controlled, dose-escalation study. Current Medical Research and Opinion 2014;30(4):589-97. [DOI: 10.1185/03007995.2013.861812] [DOI] [PubMed] [Google Scholar]
Uchio 2018 {published data only}
- Uchio Y, Enomoto H, Alev L, Kato Y, Ishihara H, Tsuji T, et al. A randomized, double-blind, placebo-controlled phase III trial of duloxetine in Japanese patients with knee pain due to osteoarthritis. Journal of Pain Research 2018;11:809-21. [DOI: 10.2147/JPR.S164128] [DOI] [PMC free article] [PubMed] [Google Scholar]
Urquhart 2018 {published data only}
- Urquhart DM, Wluka AE, Van Tulder M, Heritier S, Forbes A, Fong C, et al. Efficacy of low-dose amitriptyline for chronic low back pain: a randomized clinical trial. JAMA Internal Medicine 2018;178(11):1474-81. [DOI: 10.1001/jamainternmed.2018.4222] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vahedi 2005 {published data only}
- Vahedi H, Merat S, Rashidioon A, Ghoddoosi A, Malekzadeh R. The effect of fluoxetine in patients with pain and constipation-predominant irritable bowel syndrome: a double-blind randomized-controlled study. Alimentary Pharmacology and Therapeutics 2005;22(5):381-5. [DOI: 10.1111/j.1365-2036.2005.02566.x] [DOI] [PubMed] [Google Scholar]
Van Ophoven 2004 {published data only}
- Van Ophoven A, Pokupic S, Heinecke A, Hertle L. A prospective, randomized, placebo controlled, double-blind study of amitriptyline for the treatment of interstitial cystitis. Journal of Urology 2004;172(2):533-6. [DOI: 10.1097/01.ju.0000132388.54703.4d] [DOI] [PubMed] [Google Scholar]
Ventafridda 1987 {published data only}
- Ventafridda V, Bonezzi C, Caraceni A, De Conno F, Guarise G, Ramella G, et al. Antidepressants for cancer pain and other painful syndromes with deafferentation component: comparison of amitriptyline and trazodone. Italian Journal of Neurological Sciences 1987;8(6):579-87. [DOI: 10.1007/BF02333665] [DOI] [PubMed] [Google Scholar]
Vitton 2004 {published data only}
- Vitton O, Gendreau M, Gendreau J, Kranzler J, Rao SG. A double-blind placebo-controlled trial of milnacipran in the treatment of fibromyalgia. Human Psychopharmacology: Clinical and Experimental 2004;19 Suppl 1:S27-35. [DOI: 10.1002/hup.622] [DOI] [PubMed] [Google Scholar]
Vollmer 2014 {published data only}
- Vollmer TL, Robinson MJ, Risser RC, Malcolm SK. A randomized, double-blind, placebo-controlled trial of duloxetine for the treatment of pain in patients with multiple sclerosis. Pain Practice 2014;14(8):732-44. [DOI: 10.1111/papr.12127] [DOI] [PubMed] [Google Scholar]
Vranken 2011 {published data only}
- Vranken JH, Hollmann MW, Van der Vegt MH, Kruis MR, Heesen M, Vos K, et al. Duloxetine in patients with central neuropathic pain caused by spinal cord injury or stroke: a randomized, double-blind, placebo-controlled trial. Pain 2011;152(2):267-73. [DOI: 10.1016/j.pain.2010.09.005] [DOI] [PubMed] [Google Scholar]
Vrethem 1997 {published data only}
- Vrethem M, Boivie J, Arnqvist H, Holmgren H, Lindström T, Thorell LH. A comparison of amitriptyline and maprotiline in the treatment of painful polyneuropathy in diabetics and nondiabetics. Clinical Journal of Pain 1997;13(4):313-23. [DOI: 10.1097/00002508-199712000-00009] [DOI] [PubMed] [Google Scholar]
Wang 2017 {published data only}
- Wang G, Bi L, Li X, Li Z, Zhao D, Chen J, et al. Efficacy and safety of duloxetine in Chinese patients with chronic pain due to osteoarthritis: a randomized, double-blind, placebo-controlled study. Osteoarthritis Cartilage 2017;25(6):832-8. [DOI: 10.1016/j.joca.2016.12.025] [DOI] [PubMed] [Google Scholar]
Ward 1986 {published data only}
- Ward NG. Tricyclic antidepressants for chronic low-back pain. Mechanisms of action and predictors of response. Spine 1986;11(7):661-5. [DOI: 10.1097/00007632-198609000-00003] [DOI] [PubMed] [Google Scholar]
Ware 2010 {published data only}
- Ware MA, Fitzcharles M-A, Joseph L, Shir Y. The effects of nabilone on sleep in fibromyalgia: results of a randomized controlled trial. Anesthesia & Analgesia 2010;110(2):604-10. [DOI: 10.1213/ANE.0b013e3181c76f70] [DOI] [PubMed] [Google Scholar]
Watson 1992 {published data only}
- Watson PN, Chipman M, Reed K, Evans RJ, Birkett N. Amitriptyline versus maprotiline in postherpetic neuralgia: a randomized, double-blind, crossover trial. Pain 1992;48(1):29-36. [DOI: 10.1016/0304-3959(92)90128-X] [DOI] [PubMed] [Google Scholar]
Watson 1998 {published data only}
- Watson CP, Vernich L, Chipman M, Reed K. Nortriptyline versus amitriptyline in postherpetic neuralgia: a randomized trial. Neurology 1998;51(4):1166-71. [DOI: 10.1212/wnl.51.4.1166] [DOI] [PubMed] [Google Scholar]
Wernicke 2006 {published data only}
- Wernicke JF, Pritchett YL, D'Souza DN, Waninger A, Tran P, Iyengar S, et al. A randomized controlled trial of duloxetine in diabetic peripheral neuropathic pain. Neurology 2006;67(8):1411-20. [DOI: 10.1212/01.wnl.0000240225.04000.1a] [DOI] [PubMed] [Google Scholar]
Wolfe 1994 {published data only}
- Wolfe F, Cathey MA, Hawley DJ. A double-blind placebo controlled trial of fluoxetine in fibromyalgia. Scandinavian Journal of Rheumatology 1994;23(5):255-9. [DOI: 10.3109/03009749409103725] [DOI] [PubMed] [Google Scholar]
Yasuda 2011 {published data only}
- Yasuda H, Hotta N, Nakao K, Kasuga M, Kashiwagi A, Kawamori R. Superiority of duloxetine to placebo in improving diabetic neuropathic pain: results of a randomized controlled trial in Japan. Journal of Diabetes Investigation 2011;2(2):132-9. [DOI: 10.1111/j.2040-1124.2010.00073.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yeephu 2013 {published data only}
- Yeephu S, Suthisisang C, Suttiruksa S, Prateepavanich P, Limampai P, Russell IJ. Efficacy and safety of mirtazapine in fibromyalgia syndrome patients: a randomized placebo-controlled pilot study. Annals of Pharmacotherapy 2013;47(7-8):921-32. [DOI: 10.1345/aph.1R725] [DOI] [PubMed] [Google Scholar]
Yucel 2005 {published data only}
- Yucel A, Ozyalcin S, Koknel Talu G, Kiziltan E, Yucel B, Andersen OK, et al. The effect of venlafaxine on ongoing and experimentally induced pain in neuropathic pain patients: a double blind, placebo controlled study. European Journal of Pain 2005;9(4):407-16. [DOI: 10.1016/j.ejpain.2004.09.009] [DOI] [PubMed] [Google Scholar]
Zabihiyeganeh 2021 {published data only}
- Zabihiyeganeh M, Kadijani AA, Afshar SV, Janbozorgi M, Akbari A, Mirzaei A. The effect of cognitive-behavioral therapy versus duloxetine on the laboratory indices of inflammation in fibromyalgia: a randomized controlled trial. Journal of Rational-Emotive & Cognitive-Behavior Therapy 202s;40:512–26. [DOI: 10.1007/s10942-021-00426-y] [DOI] [Google Scholar]
Zitman 1990 {published data only}
- Zitman FG, Linssen AC, Edelbroek PM, Stijnen T. Low dose amitriptyline in chronic pain: the gain is modest. Pain 1990;42(1):35-42. [DOI: 10.1016/0304-3959(90)91089-2] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Amelin 1991 {published data only}
- Amelin AV, Vasil'ev IN, Ignatov ID, Skoromets AA. The combined use of acupuncture and antidepressants for managing the spondylogenic lumbosacral pain syndrome. Farmakologiia i Toksikologiia [Pharmacology and Toxicology] 1991;54(5):12-3. [PubMed] [Google Scholar]
Amr 2010 {published data only}
- Amr YM, Yousef AA. Evaluation of efficacy of the perioperative administration of venlafaxine or gabapentin on acute and chronic postmastectomy pain. Clinical Journal of Pain 2010;26(5):381-5. [DOI: 10.1097/AJP.0b013e3181cb406e] [DOI] [PubMed] [Google Scholar]
Arnold 2014 {published data only}
- Arnold L, Sarzi-Puttini P, Arsenault P, Khan T, Bhadra Brown P, Clair A, et al. Pregabalin is effective irrespective of antidepressant class in fibromyalgia patients currently receiving antidepressant medication for comorbid depression. Journal of Pain 2014;15(4 Suppl 1):S75. [DOI: 10.1016/j.jpain.2014.01.307] [DOI] [Google Scholar]
Avan 2018 {published data only}
- Avan R, Janbabaei G, Hendouei N, Alipour A, Borhani S, Tabrizi N, et al. The effect of pregabalin and duloxetine treatment on quality of life of breast cancer patients with taxane-induced sensory neuropathy: a randomized clinical trial. Journal of Research in Medical Sciences 2018;23(1):52. [DOI: 10.4103/jrms.JRMS_1068_17] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beaumont 1980 {published data only}
- Beaumont G, Seldrup J. Comparative trial of clomipramine and placebo in the treatment of terminal pain. Journal of International Medical Research 1980;8 Suppl 3:67‐9. [PubMed] [Google Scholar]
Braak 2011 {published data only}
- Braak B, Klooker TK, Wouters MM, Lei A, den Wijngaard RM, Boeckxstaens GE. Randomised clinical trial: the effects of amitriptyline on drinking capacity and symptoms in patients with functional dyspepsia, a double-blind placebo-controlled study. Alimentary Pharmacology and Therapeutics 2011;34(6):638-48. [DOI: 10.1111/j.1365-2036.2011.04775.x] [DOI] [PubMed] [Google Scholar]
Carette 1995 {published data only}
- Carette S, Oakson G, Guimont C, Steriade M. Sleep electroencephalography and the clinical response to amitriptyline in patients with fibromyalgia. Arthritis & Rheumatology 1995;38(9):1211-7. [DOI: 10.1002/art.1780380906] [DOI] [PubMed] [Google Scholar]
ChiCTR2000030195 {published data only}
- ChiCTR2000030195. Duloxetine reduces postoperative pain and improves quality of life after single-segment lumbar fusion procedures in centrally sensitized patients: a prospective randomized controlled study. https://trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR2000030195 (first received 25 February 2020).
ChiCTR‐TRC‐12001968 {published data only}
- ChiCTR-TRC-12001968. Epidemiology survey and the effects of low-dose amitriptyline on intractable functional dyspepsia: a prospective, randomized, controlled study. https://trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR-TRC-12001968 (first received 13 February 2012).
ChiCTR‐TRC‐12001969 {published data only}
- ChiCTR-TRC-12001969. Incidence of refractory irritable bowel syndrome and the effects of low dose amitriptyline in the patients: a prospective, randomized-controlled trial. https://trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR-TRC-12001969 (first received 29 February 2012).
Chitsaz 2009 {published data only}
- Chitsaz A, Janghorbani M, Shaygannejad V, Ashtari F, Heshmatipour M, Freeman J. Sensory complaints of the upper extremities in multiple sclerosis: relative efficacy of nortriptyline and transcutaneous electrical nerve stimulation. Clinical Journal of Pain 2009;25(4):281-5. [DOI: 10.1097/AJP.0b013e318190862b] [DOI] [PubMed] [Google Scholar]
CTRI/2015/05/005791 {published data only}
- CTRI/2015/05/005791. Treatment of irritable bowel syndrome (IBS) by a plant based Ayurvedic formulation [Ayurvedic formulation developed for the prevention and management of irritable bowel syndrome (IBS)]. https://trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2015/05/005791 (first received 21 May 2015).
Daghaghzadeh 2015 {published data only}
- Daghaghzadeh H, Naji F, Afshar H, Sharbafchi MR, Feizi A, Maroufi M, et al. Efficacy of duloxetine add on in treatment of inflammatory bowel disease patients: a double-blind controlled study. Journal of Research in Medical Sciences 2015;20(6):595‐601. [DOI: 10.4103/1735-1995.165969] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dinat 2015 {published data only}
- Dinat N, Marinda E, Moch S, Rice AS, Kamerman PR. Randomized, double-blind, crossover trial of amitriptyline for analgesia in painful HIV-associated sensory neuropathy. PLOS One 2015;10(5):e0126297. [DOI: 10.1371/journal.pone.0126297] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ehrnrooth 2001 {published data only}
- Ehrnrooth E, Grau C, Zachariae R, Andersen J. Randomized trial of opioids versus tricyclic antidepressants for radiation-induced mucositis pain in head and neck cancer. Acta Oncologica 2001;40(6):745-50. [DOI: 10.1080/02841860152619179] [DOI] [PubMed] [Google Scholar]
EUCTR2005‐005555‐17‐NL {published data only}
- EUCTR2005-005555-17-NL. [S,S]-reboxetine add-on trial: a randomized, double-blind, placebo-controlled, multi-center trial of [S,S]-reboxetine in patients with postherpetic neuralgia (PHN) concomitantly treated with pregabalin. https://trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2005-005555-17-NL (first received 5 October 2006).
EUCTR2006‐003656‐38‐GB {published data only}
- EUCTR2006-003656-38-GB. A double-blind, randomised, parallel groups investigation into the effects of pregabalin, duloxetine and amitriptyline on aspects of pain, sleep, and next day performance in patients suffering from diabetic peripheral neuropathy. https://trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2006-003656-38-GB (first received 18 September 2006).
EUCTR2006‐005506‐32‐DK {published data only}
- EUCTR2006-005506-32-DK. Pain, anxiety and depression in neuropathic and non-neuropathic pain: effect of monoamine modulation. https://trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2006-005506-32-DK (first received 12 January 2007).
EUCTR2009‐013061‐26‐FI {published data only}
- EUCTR2009-013061-26-FI. Efficacy, safety, tolerability and pharmacokinetics of concomitant administration of tramadol with duloxetine or pregabalin: a randomized controlled flexible-dose study in patients with neuropathic pain. https://trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2009-013061-26-FI (first received 8 June 2009).
EUCTR2016‐003146‐89‐GB {published data only}
- EUCTR2016-003146-89-GB. Optimal pathway for treating neuropathic pain in diabetes mellitus [A multicentre, double-blind, centre-stratified multi-period crossover trial to evaluate the efficacy of the optimal pathway for treatIng neuropathic pain in diabetes mellitus (OPTION-DM)]. https://trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2016-003146-89-GB (first received 10 September 2018). [DOI] [PMC free article] [PubMed]
EUCTR2017‐003307‐21‐NL {published data only}
- EUCTR2017-003307-21-NL. Personalized treatment of functional dyspepsia with nortriptyline [Tailored treatment of functional dyspepsia with nortriptyline: a multi-center double-blind placebo-controlled trial (TENDER)]. https://trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2017-003307-21-NL (first received 19 December 2017).
EUCTR2018‐000133‐12‐GB {published data only}
- EUCTR2018-000133-12-GB. Efficacy and safety of products containing trazodone and gabapentin in patients affected by painful diabetic neuropathy [Efficacy and safety of fixed-dose combination (FDC) products containing trazodone and gabapentin in patients affected by painful diabetic neuropathy: randomized, controlled, dose finding study]. https://trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2018-000133-12-GB (first received 26 June 2018).
EUCTR2019‐003437‐42‐DK {published data only}
- EUCTR2019-003437-42-DK. The effect of duloxetine on pain sensitivity in patients with osteoarthritis [A mechanism based proof of concept study of the effects of duloxetine in the treatment of patients with osteoarthritic knee pain]. https://trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2019-003437-42-DK (first received 30 August 2019).
Farshchian 2018 {published data only}
- Farshchian N, Alavi A, Heydarheydari S, Moradian N. Comparative study of the effects of venlafaxine and duloxetine on chemotherapy-induced peripheral neuropathy. Cancer Chemotherapy and Pharmacology 2018;82(5):787-93. [DOI: 10.1007/s00280-018-3664-y] [DOI] [PubMed] [Google Scholar]
Frank 1988 {published data only}
- Frank RG, Kashani JH, Parker JC, Beck NC, Brownlee-Duffeck M, Elliott TR, et al. Antidepressant analgesia in rheumatoid arthritis. Journal of Rheumatology 1988;15(11):1632-8. [PubMed] [Google Scholar]
Gardela 1991 {published data only}
- Gardela G. Value of adjuvant treatment with imipramine for lumbosacral pain syndrome. Polski Tygodnik Lekarski 1991;46(30-31):544-6. [PubMed] [Google Scholar]
Gelijkens 2014 {published data only}
- Gelijkens V, Van Zundert J, De Vooght P, Vander Laenen M, Heylen R, Vanelderen P. The effectiveness of amitriptyline in the treatment of subacute lumbar radicular pain. European Journal of Anaesthesiology 2014;31:232. [Google Scholar]
Ghadir 2011 {published data only}
- Ghadir MR, Habibinejad H, Heidari A, Vahedi H. Doxepin is more effective than nortriptyline and placebo for the treatment of diarrhea-predominant irritable bowel syndrome: a randomized triple-blind placebo-controlled trial. Tehran University Medical Journal 2011;69(6):352‐8. [Google Scholar]
Goldenberg 2010 {published data only}
- Goldenberg DL, Clauw DJ, Palmer RH, Mease P, Chen W, Gendreau RM. Durability of therapeutic response to milnacipran treatment for fibromyalgia. Results of a randomized, double-blind, monotherapy 6-month extension study. Pain Medicine 2010;11(2):180-94. [DOI: 10.1111/j.1526-4637.2009.00755.x] [DOI] [PubMed] [Google Scholar]
Gomez‐Perez 1985 {published data only}
- Gomez-Perez FJ, Rull JA, Dies H, Rodriquez-Rivera JG, Gonzalez-Barranco J, Lozano-Castañeda O. Nortriptyline and fluphenazine in the symptomatic treatment of diabetic neuropathy. A double-blind cross-over study. Pain 1985;23(4):395-400. [DOI: 10.1016/0304-3959(85)90010-7] [DOI] [PubMed] [Google Scholar]
Greenbaum 1987 {published data only}
- Greenbaum DS, Mayle JE, Vanegeren LE, Jerome JA, Mayor JW, Greenbaum RB, et al. Effects of desipramine on irritable bowel syndrome compared with atropine and placebo. Digestive Diseases and Sciences 1987;32(3):257-66. [DOI: 10.1007/BF01297051] [DOI] [PubMed] [Google Scholar]
Henry 2018 {published data only}
- Henry NL, Unger JM, Schott A, Hansen L, Lew D, Wade JL, et al. A randomized placebo-controlled phase III study of duloxetine for treatment of aromatase inhibitor (AI)-associated musculoskeletal symptoms in women with early-stage breast cancer: SWOG S1202. Journal of Clinical Oncology 2014;32(15 Suppl 1):1. [Google Scholar]
- Henry NL, Unger JM, Schott AF, Fehrenbacher L, Flynn PJ, Prow D, et al. Randomized, placebo controlled trial of duloxetine for aromatase inhibitor (AI)-associated musculoskeletal symptoms (AIMSS) in early stage breast cancer (SWOG S1202). Cancer Research 2017;77(4 Suppl):S5-6. [DOI: 10.1158/1538-7445.SABCS16-S5-06] [DOI] [Google Scholar]
- Henry NL, Unger JM, Schott AF, Fehrenbacher L, Flynn PJ, Prow DM, et al. Randomized, multicenter, placebo-controlled clinical trial of duloxetine versus placebo for aromatase inhibitor-associated arthralgias in early-stage breast cancer: SWOG S1202. Journal of Clinical Oncology 2018;36(4):326-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry NL, Unger JM, Till C, Schott AF, Crew KD, Lew DL, et al. Association between body mass index (BMI) and response to duloxetine for aromatase inhibitor (AI)-associated musculoskeletal symptoms (AIMSS). Cancer Research 2018;79(4 Suppl 1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry NL, Unger JM, Till C, Schott AF, Crew KD, Lew DL, et al. Association between body mass index and response to duloxetine for aromatase inhibitor-associated musculoskeletal symptoms in SWOG S1202. Cancer 2019;125(12):2123-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
IRCT201506171647N4 {published data only}
- IRCT201506171647N4. The effect of mesalazine and nortriptyline in patients with irritable bowel syndrome with diarrhea: a randomized clinical trial [The effect of mesalazine and nortriptyline on improvement of severity and frequency of abdominal pain and stool frequency recorded daily in patients with irritable bowel syndrome with diarrhea: a randomized clinical trail]. https://trialsearch.who.int/Trial2.aspx?TrialID=IRCT201506171647N4 (first received 13 July 2015).
IRCT20170829035966N1 {published data only}
- IRCT20170829035966N1. Efficacy of laughter yoga and anti-anxiety drugs on irritable bowel syndrome. https://trialsearch.who.int/Trial2.aspx?TrialID=IRCT20170829035966N1 (first received 17 January 2018).
IRCT20191210045685N1 {published data only}
- IRCT20191210045685N1. Effectiveness of duloxetine in chronic low back pain [Effetiveness of duloxetine on severity of pain and quality of life in chronic low back pain in patients who had posterior spinal fixation (PSF)]. https://trialsearch.who.int/Trial2.aspx?TrialID=IRCT20191210045685N1 (first received 4 July 2020).
ISRCTN16086699 {published data only}
- ISRCTN16086699. MODULATE: a study to evaluate the effectiveness of either amitriptyline, ondansetron, loperamide, or dietary intervention (the low FODMAP diet) against standard dietary advice for the treatment of diarrhoea in patients with stable ulcerative colitis [Management of diarrhoea in ulcerative colitis: multi-arm multi-stage trial of low FODMAP diet, amitriptyline, ondansetron, or loperamide: MODULATE]. https://trialsearch.who.int/Trial2.aspx?TrialID=ISRCTN16086699 (first received 13 February 2020).
ISRCTN63671932 {published data only}
- ISRCTN63671932. Multicentre trial of combined cognitive behavioural therapy and antidepressant treatment in functional bowel disorders [Cognitive behavioural therapy and antidepressant treatment in functional bowel disorders: a multicentre randomised, parallel, three arm trial studying behavioural and medication impact]. https://trialsearch.who.int/Trial2.aspx?TrialID=ISRCTN63671932 (first received 30 January 2007).
Kaosombatwattana 2015 {published data only}
- Kaosombatwattana U, Pongprasobchai S, Limsrivilai J, Leelakusolvong S, Tanwandee T. Efficacy and safety of nortriptyline in functional dyspepsia in Asians: a randomized double-blind placebo-controlled trial. Gastroenterology 2015;148(4 Suppl 1):S822. [DOI] [PubMed] [Google Scholar]
Kautio 2008 {published data only}
- Kautio A-L, Haanpää M, Saarto T, Kalso E. Amitriptyline in the treatment of chemotherapy-induced neuropathic symptoms. Journal of Pain and Symptom Management 2008;35(1):31-9. [DOI: 10.1016/j.jpainsymman.2007.02.043] [DOI] [PubMed] [Google Scholar]
Khalilian 2021 {published data only}
- Khalilian A, Ahmadimoghaddam D, Saki S, Mohammadi Y, Mehrpooya M. A randomized, double-blind, placebo-controlled study to assess efficacy of mirtazapine for the treatment of diarrhea predominant irritable bowel syndrome. BioPsychoSocial Medicine 2021;15:3. [DOI: 10.1186/s13030-021-00205-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khosrawi 2018 {published data only}
- Khosrawi S, Khosravi M, Haghighat S, Akbari M. Duloxetine in the treatment of carpal tunnel syndrome: a pilot randomized clinical trial study. Journal of isfahan Medical School 2018;36(479):517‐23. [DOI: 10.22122/JIMS.V36I479.9795] [DOI] [Google Scholar]
Kieburtz 1998 {published data only}
- Kieburtz K, Simpson D, Yiannoutsos C, Max MB, Hall CD, Ellis RJ, et al. A randomized trial of amitriptyline and mexiletine for painful neuropathy in HIV infection. Neurology 1998;51(6):1682-8. [DOI: 10.1212/wnl.51.6.1682] [DOI] [PubMed] [Google Scholar]
Kishore‐Kumar 1990 {published data only}
- Kishore-Kumar R, Max MB, Schafer SC, Gaughan AM, Smoller B, Gracely RH, et al. Desipramine relieves postherpetic neuralgia. Clinical Pharmacology & Therapeutics 1990;47(3):305-12. [DOI: 10.1038/clpt.1990.33] [DOI] [PubMed] [Google Scholar]
Kreiter 2021 {published data only}
- Kreiter D, Drukker M, Mujagic Z, Vork L, Rutten BP, Os J, et al. Symptom-network dynamics in irritable bowel syndrome with comorbid panic disorder using electronic momentary assessment: a randomized controlled trial of escitalopram vs. placebo. Journal of Psychosomatic Research 2021;141:110351. [DOI: 10.1016/j.jpsychores.2020.110351] [DOI] [PubMed] [Google Scholar]
Kroenke 2006 {published data only}
- Kroenke K, Messina N 3rd, Benattia I, Graepel J, Musgnung J. Venlafaxine extended release in the short-term treatment of depressed and anxious primary care patients with multisomatoform disorder. Journal of Clinical Psychiatry 2006;67(1):72-80. [DOI: 10.4088/jcp.v67n0111] [DOI] [PubMed] [Google Scholar]
Kuiken 2003 {published data only}
- Kuiken SD, Tytgat GN, Boeckxstaens GE. The selective serotonin reuptake inhibitor fluoxetine does not change rectal sensitivity and symptoms in patients with irritable bowel syndrome: a double blind, randomized, placebo-controlled study. Clinical Gastroenterology and Hepatology 2003;1(3):219-28. [DOI] [PubMed] [Google Scholar]
Kvinesdal 1984 {published data only}
- Kvinesdal B, Molin J, Froland A, Gram LF. Imipramine treatment for painful diabetic neuropathy. Journal of the American Medical Association 1984;251(13):1727‐30. [PubMed] [Google Scholar]
Ladabaum 2010 {published data only}
- Ladabaum U, Sharabidze A, Levin TR, Zhao WK, Chung E, Bacchetti P, et al. Citalopram provides little or no benefit in nondepressed patients with irritable bowel syndrome. Clinical Gastroenterology and Hepatology 2010;8(1):42-8. [DOI: 10.1016/j.cgh.2009.09.008] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lara Muñoz 1986 {published data only}
- Lara Muñoz M, Navarro M, Guarneros A, Plancarte Sánchez R, Bayón A, Fuente JR. Potentiating effect of amitriptyline over analgesics in patients with cancer pain [Efecto potenciador de la amitriptilina sobre los analgésicos en pacientes con dolor oncológico]. Revista Mexicana de Anestesiología 1986;9(4):217-20. [Google Scholar]
Li 2019 {published data only}
- Li W-D, Jia L, Jiang S-M, Ping L, Xu M. The therapeutic effect of low-dose amitriptyline on patients with refractory diarrhea-predominant irritable bowel syndrome and its 1-year follow-up study. International Journal of Clinical and Experimental Medicine 2019;12(1):891-8. [Google Scholar]
Matsuoka 2019a {published data only}
- Matsuoka H, Ishiki H, Iwase S, Koyama A, Kawaguchi T, Kizawa Y, et al. Study protocol for a multi-institutional, randomised, double-blinded, placebo-controlled phase III trial investigating additive efficacy of duloxetine for neuropathic cancer pain refractory to opioids and gabapentinoids: the DIRECT study. BMJ Open 2017;7(8):e017280. [DOI: 10.1136/bmjopen-2017-017280] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka H, Iwase S, Miyaji T, Kawaguchi T, Ariyoshi K, Oyamada S, et al. Additive duloxetine for cancer-related neuropathic pain nonresponsive or intolerant to opioid-pregabalin therapy: a randomized controlled trial (JORTC-PAL08). Journal of Pain and Symptom Management 2019;58(4):645-53. [DOI: 10.1016/j.jpainsymman.2019.06.020] [DOI] [PubMed] [Google Scholar]
- Matsuoka H, Iwase S, Miyaji T, Kawaguchi T, Ariyoshi K, Oyamada S, et al. Predictors of duloxetine response in patients with neuropathic cancer pain: a secondary analysis of a randomized controlled trial-JORTC-PAL08 (DIRECT) study. Supportive Care in Cancer 2020;28(6):2931-9. [DOI: 10.1007/s00520-019-05138-9] [DOI] [PubMed] [Google Scholar]
Max 1987 {published data only}
- Max MB, Culnane M, Schafer SC, Gracely RH, Walther DJ, Smoller B, et al. Amitriptyline relieves diabetic neuropathy pain in patients with normal or depressed mood. Neurology 1987;37(4):589-96. [DOI: 10.1212/wnl.37.4.589] [DOI] [PubMed] [Google Scholar]
Max 1991 {published data only}
- Max MB, Kishore-Kumar R, Schafer SC, Meister B, Gracely RH, Smoller B, et al. Efficacy of desipramine in painful diabetic neuropathy: a placebo-controlled trial. Pain 1991;45(1):3-9. [DOI: 10.1016/0304-3959(91)90157-S] [DOI] [PubMed] [Google Scholar]
McQuay 1992 {published data only}
- McQuay HJ, Carroll D, Glynn CJ. Dose-response for analgesic effect of amitriptyline in chronic pain. Anaesthesia 1993;48(4):281-5. [DOI: 10.1111/j.1365-2044.1993.tb06943.x] [DOI] [PubMed] [Google Scholar]
- McQuay HJ, Carroll D, Glynn CJ. Low dose amitriptyline in the treatment of chronic pain. Anaesthesia 1992;47(8):646-52. [DOI: 10.1111/j.1365-2044.1992.tb02383.x] [DOI] [PubMed] [Google Scholar]
Mishra 2012 {published data only}
- Mishra S, Bhatnagar S, Goyal GN, Rana SP, Upadhya SP. A comparative efficacy of amitriptyline, gabapentin, and pregabalin in neuropathic cancer pain: a prospective randomized double-blind placebo-controlled study. American Journal of Hospice and Palliative Medicine 2012;29(3):177-82. [DOI: 10.1177/1049909111412539] [DOI] [PubMed] [Google Scholar]
NCT00006157 {published data only}
- NCT00006157. Treatment of functional bowel disorders [Multicenter trial of functional bowel disorders]. https://clinicaltrials.gov/ct2/show/NCT00006157 (first received 9 August 2000).
NCT00189059 {published data only}
- NCT00189059. Effects of amitriptyline for the treatment of pain on driving performance and cognition [Effects of pain and the treatment of pain with amitriptyline on driving performance, attentional capacity and psychomotor performance in chronic neuropathic pain patients]. https://clinicaltrials.gov/show/NCT00189059 (first received 16 September 2005).
NCT00191919 {published data only}
- NCT00191919. A randomized double blind study evaluating duloxetine in outpatients with MDD and pain [A ten-week, randomized, double-blind study evaluating the efficacy of duloxetine 60mg once daily versus placebo in outpatients with major depressive disorder and pain]. https://clinicaltrials.gov/show/NCT00191919 (first received 19 September 2005).
NCT00283842 {published data only}
- NCT00283842. Study evaluating desvenlafaxine succinate sustained-release (DVS SR) in adult outpatients with pain associated with diabetic peripheral neuropathy [A multicenter, randomized, double-blind, placebo-controlled, parallel-group, 13-week, adaptive-design study of 4 fixed oral doses of DVS SR in adult outpatients with pain associated with diabetic peripheral neuropathy]. https://clinicaltrials.gov/show/NCT00283842 (first received 30 January 2006).
NCT00592384 {published data only}
- NCT00592384. Project to improve symptoms and mood in people with spinal cord injury (PRISMS) [A controlled trial of venlafaxine XR for major depression after spinal cord injury: a multi-site study]. https://clinicaltrials.gov/show/NCT00592384 (first received 14 January 2008).
NCT00610909 {published data only}
- NCT00610909. Paroxetine - controlled release in the treatment of irritable bowel syndrome (IBS) [Single-site, double-blind, flexible-dose, placebo-controlled study of the efficacy, tolerability, & safety of paroxetine - controlled release in the treatment of irritable bowel syndrome (IBS)]. https://clinicaltrials.gov/show/NCT00610909 (first received 8 February 2008).
NCT00619983 {published data only}
- NCT00619983. Three way interaction between gabapentin, duloxetine, and donepezil in patients with diabetic neuropathy. https://clinicaltrials.gov/show/NCT00619983 (first received 21 February 2008).
NCT00625833 {published data only}
- NCT00625833. A trial of [S,S]-reboxetine In patients with chronic painful diabetic peripheral neuropathy [A randomized, double-blind placebo controlled trial of [S,S]-reboxetine in patients with chronic painful diabetic peripheral neuropathy]. https://clinicaltrials.gov/show/NCT00625833 (first received 28 February 2008).
NCT00696787 {published data only}
- NCT00696787. A study evaluating desvenlafaxine sustained release (DVS SR) in adult female outpatients with fibromyalgia [A multicenter, randomized, double-blind, placebo-controlled, pregabalin-referenced, parallel-group, adaptive design study of DVS SR in adult female outpatients with fibromyalgia syndrome]. https://clinicaltrials.gov/show/NCT00696787 (first received 13 June 2008).
NCT00754793 {published data only}
- NCT00754793. Sinusitis and facial pain disorders anti-depression trial (SFPAT). https://clinicaltrials.gov/show/NCT00754793 (first received 18 September 2008).
NCT00945945 {published data only}
- NCT00945945. A study of duloxetine in patients with osteoarthritis knee pain [A phase 3b study to assess the efficacy of duloxetine 60 mg once daily compared with placebo on the reduction of pain caused by osteoarthritis of the knee, in a 13-week, double-blind, randomized study]. https://clinicaltrials.gov/ct2/show/NCT00945945 (first received 24 July 2009).
NCT01116531 {published data only}
- NCT01116531. Efficacy, safety, tolerability and pharmacokinetics of concomitant administration of tramadol with duloxetine or pregabalin [Efficacy, safety, tolerability and pharmacokinetics of concomitant administration of tramadol with duloxetine or pregabalin: a randomized controlled flexible-dose study in patients with neuropathic pain]. https://clinicaltrials.gov/show/NCT01116531 (first received 5 May 2010).
NCT01173055 {published data only}
- NCT01173055. A study to evaluate the effects of milnacipran on pain processing and functional MRI in patients with fibromyalgia [A randomized, double-blind,placebo-controlled, two-way crossover study to evaluate the effect of milnacipran on pain processing and functional magnetic resonance imaging activation patterns in patients with fibromyalgia]. https://clinicaltrials.gov/show/NCT01173055 (first received 30 July 2010).
NCT01268709 {published data only}
- NCT01268709. Effect of doxepin and nortriptyline on irritable bowel syndrome. https://clinicaltrials.gov/show/NCT01268709 (first received 31 December 2010).
NCT01288937 {published data only}
- NCT01288937. A placebo controlled, randomized, double blind trial of milnacipran for the treatment of idiopathic neuropathy pain. https://clinicaltrials.gov/show/NCT01288937 (first received 3 February 2011).
NCT01359514 {published data only}
- NCT01359514. Mechanism-based choice of therapy for neuropathic pain [Mechanism-based choice of therapy for neuropathic pain: can treatments success in neuropathic post-operative pain be coupled to psychophysical pain modulation profile?]. https://clinicaltrials.gov/show/NCT01359514 (first received 24 May 2011).
NCT01359826 {published data only}
- NCT01359826. The effect of milnacipran on fatigue and quality of life in lupus patients [The effect of milnacipran on fatigue and quality of life in a lupus cohort]. https://clinicaltrials.gov/show/NCT01359826 (first received 25 May 2011).
NCT01377038 {published data only}
- NCT01377038. OASIS: osteoarthritis sensitivity integration study (OASIS) [Central pain mechanisms in osteoarthritis: a longitudinal cohort]. https://clinicaltrials.gov/show/NCT01377038 (first received 20 June 2011).
NCT01451606 {published data only}
- NCT01451606. Duloxetine for the treatment of chronic pelvic pain [Evaluating duloxetine's analgesic effectiveness in chronic pelvic pain]. https://clinicaltrials.gov/show/NCT01451606 (first received 13 October 2011).
NCT01471379 {published data only}
- NCT01471379. Milnacipran (Savella) in irritable bowel syndrome (IBS) [A randomized, double-blind, placebo-controlled study to assess the efficacy of milnacipran in the treatment of irritable bowel syndrome]. https://clinicaltrials.gov/show/NCT01471379 (first received 16 November 2011).
NCT01579279 {published data only}
- NCT01579279. A study comparing the efficacy and safety of ABT-652 to placebo in subjects with diabetic neuropathic pain [A multicenter, randomized, double-blind, placebo- and active-controlled study comparing the analgesic efficacy and safety of ABT-652 to placebo in subjects with diabetic neuropathic pain]. https://clinicaltrials.gov/show/NCT01579279 (first received 17 April 2012).
NCT01869907 {published data only}
- NCT01869907. Effect of minocycline on pain caused by nerve damage (EMON) [Effect of minocycline on neuropathic pain]. https://clinicaltrials.gov/show/NCT01869907 (first received 5 June 2013).
NCT01910259 {published data only}
- NCT01910259. MS-SMART: multiple sclerosis-secondary progressive multi-arm randomisation trial (MS-SMART) [A multi-arm phase IIB randomised, double blind placebo-controlled clinical trial comparing the efficacy of three neuroprotective drugs in secondary progressive multiple sclerosis]. https://clinicaltrials.gov/show/NCT01910259 (first received 29 July 2013). [DOI] [PMC free article] [PubMed]
NCT02650544 {published data only}
- NCT02650544. Efficacy and safety analyses of mirtazapine in NSCLC patients with depression [Efficacy and safety analyses of mirtazapine in the treatment of malignant tumor related depression: a phase II, placebo-controlled, randomized, double-blinded clinical trial in advanced non-small cell lung cancer patients]. https://clinicaltrials.gov/show/NCT02650544 (first received 8 January 2016).
NCT02970591 {published data only}
- NCT02970591. A comparison of three different treatment options for irritable bowel syndrome (CARIBS) [The role of carbohydrates in irritable bowel syndrome (CARIBS): protocol for a randomized controlled trial comparing three different treatment options]. https://clinicaltrials.gov/show/NCT02970591 (first received 22 November 2016).
NCT03364075 {published data only}
- NCT03364075. Genetic variants associated with low back pain and their response to treatment with duloxetine or propranolol [Genetic variants associated with the occurrence of localized low back pain or low back pain with widespread pain symptoms, and their response to treatment with duloxetine or propranolol]. https://clinicaltrials.gov/show/NCT03364075 (first received 6 December 2017).
NCT03522207 {published data only}
- NCT03522207. Accuracy and efficacy of trazodone (Desyrel) on sleep quality and pain management of TMD patient [Stabilisation de la qualité du sommeil chez le sujet en douleurs orofaciales chroniques - étude expérimentale en chassé croisé: trazodone/ placebo]. https://clinicaltrials.gov/show/NCT03522207 (first received 11 May 2018).
NCT04747314 {published data only}
- NCT04747314. Treating negative affect in low back pain patients (TNA-LBP) [Proof of concept study to treat negative affect in chronic low back pain]. https://clinicaltrials.gov/show/NCT04747314 (first received 10 February 2021).
Nickel 2005 {published data only}
- Nickel MK, Nickel C, Lahmann C, Mitterlehner FO, Tritt K, Leiberich PK, et al. Changes in instrumental activities of daily living disability after treatment of depressive symptoms in elderly women with chronic musculoskeletal pain: a double-blind, placebo-controlled trial. Aging Clinical and Experimental Research 2005;17(4):293-6. [DOI: 10.1007/BF03324613] [DOI] [PubMed] [Google Scholar]
Panerai 1990 {published data only}
- Panerai AE, Monza G, Movilia P, Bianchi M, Francucci BM, Tiengo M. A randomized, within-patient, cross-over, placebo-controlled trial on the efficacy and tolerability of the tricyclic antidepressants chlorimipramine and nortriptyline in central pain. Acta Neurologica Scandinavica 1990;82(1):34-8. [DOI: 10.1111/j.1600-0404.1990.tb01584.x] [DOI] [PubMed] [Google Scholar]
Parker 2003 {published data only}
- Parker JC, Smarr KL, Slaughter JR, Johnston SK, Priesmeyer ML, Hanson KD, et al. Management of depression in rheumatoid arthritis: a combined pharmacologic and cognitive-behavioral approach. Arthritis Care & Research 2003;49(6):766-77. [DOI: 10.1002/art.11459] [DOI] [PubMed] [Google Scholar]
Parkman 2013 {published data only}
- Parkman HP, Van Natta ML, Abell TL, McCallum RW, Sarosiek I, Nguyen L, et al. Effect of nortriptyline on symptoms of idiopathic gastroparesis: the NORIG randomized clinical trial. Journal of the American Medical Association 2013;310(24):2640-9. [DOI: 10.1001/jama.2013.282833] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pilowsky 1982 {published data only}
- Pilowsky I, Hallett EC, Bassett DL, Thomas PG, Penhall RK. A controlled study of amitriptyline in the treatment of chronic pain. Pain 1982;14(2):169‐79. [DOI: 10.1016/0304-3959(82)90097-5] [DOI] [PubMed] [Google Scholar]
Pilowsky 1995 {published data only}
- Pilowsky I, Spence N, Rounsefell B, Forsten C, Soda J. Out-patient cognitive-behavioural therapy with amitriptyline for chronic non-malignant pain: a comparative study with 6-month follow-up. Pain 1995;60(1):49-54. [DOI: 10.1016/0304-3959(94)00087-U] [DOI] [PubMed] [Google Scholar]
Poulsen 1987 {published data only}
- Poulsen DL, Hansen HJ, Langemark M, Olesen J, Bech P. Discomfort or disability in patients with chronic pain syndrome. Psychotherapy and Psychosomatics 1987;48(1‐4):60‐2. [DOI: 10.1159/000288032] [DOI] [PubMed] [Google Scholar]
Raja 2002 {published data only}
- Raja SN, Haythornthwaite JA, Pappagallo M, Clark MR, Travison TG, Sabeen S, et al. Opioids versus antidepressants in postherpetic neuralgia: a randomized, placebo-controlled trial. Neurology 2002;59(7):1015-21. [DOI: 10.1212/wnl.59.7.1015] [DOI] [PubMed] [Google Scholar]
Rajagopalan 1998 {published data only}
- Rajagopalan M, Kurian G, John J. Symptom relief with amitriptyline in the irritable bowel syndrome. Journal of Gastroenterology and Hepatology 1998;13(7):738-41. [DOI: 10.1111/j.1440-1746.1998.tb00723.x] [DOI] [PubMed] [Google Scholar]
Saxe 2009 {published data only}
- Saxe PA, Arnold LM, Gendreau RM, Spera A, Gendreau J, Wang Y. A randomized, double-blind, placebo-controlled clinical trial of milnacipran 100 mg/day for the management of fibromyalgia: results from a 2-week discontinuation phase. Arthritis and Rheumatism 2009;60:1421. [Google Scholar]
Seddighnia 2020 {published data only}
- Seddighnia A, Tadayon Najafabadi B, Ghamari K, Noorbala AA, Ebrahimi Daryani N, Kashani L, et al. Vortioxetine effects on quality of life of irritable bowel syndrome patients: a randomized, double-blind, placebo-controlled trial. Journal of Clinical Pharmacy and Therapeutics 2020;45(1):97-104. [DOI: 10.1111/jcpt.13032] [DOI] [PubMed] [Google Scholar]
Selvarajah 2018 {published data only}
- Selvarajah D, Petrie J, White D, Julious S, Bortolami O, Cooper C, et al. Multicentre, double-blind, crossover trial to identify the optimal pathway for treating neuropathic pain in diabetes mellitus (OPTION-DM): study protocol for a randomised controlled trial. Trials 2018;19(1):578. [DOI: 10.1186/s13063-018-2959-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Semenchuk 2001 {published data only}
- Semenchuk MR, Sherman S, Davis B. Double-blind, randomized trial of bupropion SR for the treatment of neuropathic pain. Neurology 2001;57(9):1583-8. [DOI: 10.1212/wnl.57.9.1583] [DOI] [PubMed] [Google Scholar]
Strauss 2019 {published data only}
- Strauss DH, Santhanam DR, McLean SA, Beaudoin FL. Study protocol for a randomised, double-blind, placebo-controlled clinical trial of duloxetine for the treatment and prevention of musculoskeletal pain: altering the transition from acute to chronic pain (ATTAC pain). BMJ Open 2019;9(3):e025002. [DOI: 10.1136/bmjopen-2018-025002] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tadyon Najafabadi 2019 {published data only}
- Tadyon Najafabadi B, Ghamari K, Kermany Ranjabari T, Noorbala AA, Ebrahimi Daryani N, Vanaki E, et al. Therapeutic effects of saffron (Crocus sativus) versus fluoxetine on irritable bowel syndrome: a double-blind randomized clinical trial. Advances in Integrative Medicine 2019;6(4):167-73. [DOI: 10.1016/j.aimed.2019.01.001] [DOI] [Google Scholar]
Tondlova 2002 {published data only}
- Tondlova H, Bastecky J. Citalopram and dosulepine in adjuvant treatment of oncological pain. Bolest 2002;5(4):247-52. [Google Scholar]
van Houdenhove 1992 {published data only}
- Van Houdenhove B, Verstraeten D, Onghena P, De Cuyper H. Chronic idiopathic pain, mianserin and 'masked' depression. Psychotherapy and Psychosomatics 1992;58(1):46-53. [DOI: 10.1159/000288609] [DOI] [PubMed] [Google Scholar]
Varia 2000 {published data only}
- Varia I, Logue E, O'Connor C, Newby K, Wagner HR, Davenport C, et al. Randomized trial of sertraline in patients with unexplained chest pain of noncardiac origin. American Heart Journal 2000;140(3):367-72. [DOI: 10.1067/mhj.2000.108514] [DOI] [PubMed] [Google Scholar]
Vork 2018 {published data only}
- Vork L, Mujagic Z, Drukker M, Keszthelyi D, Conchillo J, Hesselink M, et al. Randomized controlled trial of escitalopram vs placebo in irritable bowel syndrome (IBS) and comorbid panic disorder: comparison of retrospective symptom assessments and the real- time experience sampling methodology. Neurogastroenterology and Motility 2018;30 Suppl 1:166-7. [DOI: 10.1111/nmo.13423] [DOI] [Google Scholar]
Wang 2014 {published data only}
- Wang Y-J. Clinical efficacy of sertraline combined with otilonium bromide in irritable bowel syndrome patients. World Chinese Journal of Digestology 2014;23:3517‐20. [Google Scholar]
References to studies awaiting assessment
ACTRN12620000656932 {published data only}
- ACTRN12620000656932. Duloxetine and pregabalin for neuropathic cancer pain [A phase III, international, multi-centre, double-blind, dose increment, parallel-arm, randomised controlled trial of duloxetine versus pregabalin over 14 days for opioid unresponsive cancer-related neuropathic pain]. https://trialsearch.who.int/Trial2.aspx?TrialID=ACTRN12620000656932 (first received 5 June 2020). [DOI] [PMC free article] [PubMed]
Brown 2015 {published data only}
- Brown TR, Slee A. A randomized placebo-controlled trial of duloxetine for central pain in multiple sclerosis. International Journal of MS Care 2015;17(2):83-9. [DOI: 10.7224/1537-2073.2014-001] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cánovas Martínez 2009 {published data only}
- Cánovas Martínez L, Gómez Gutiérrez I, Castro Bande M, Peralta Espinosa E, Prieto Gutiérrez JM, Segado Jiménez I. Analgesic efficacy of the association of duloxetine plus pregabalin in neuropathic pain: experience in 60 patients. Revista de la Sociedad Española del Dolor 2009;16(7):381‐5. [Google Scholar]
Chandra 2006 {published data only}
- Chandra K, Shafig N, Pandhi P, Gupta S, Malhotra S. Gabapentin versus nortriptyline in post-herpetic neuralgia patients: a randomized, double-blind clinical trial—The GONIP Trial. International Journal of Clinical Pharmacology and Therapeutics 2006;44(8):358-63. [DOI: 10.5414/cpp44358] [DOI] [PubMed] [Google Scholar]
Di 2019 {published data only}
- Di C, Xu D. Effect of concomitant administration of oxycontin and amitriptyline on patients with severe cancer pain and depression. Tropical Journal of Pharmaceutical Research 2019;18(1):129-34. [DOI: 10.4314/tjpr.v18i1.19] [DOI] [Google Scholar]
Hammack 2002 {published data only}
- Hammack JE, Michalak JC, Loprinzi CL, Sloan JA, Novotny PJ, Soori GS, et al. Phase III evaluation of nortriptyline for alleviation of symptoms of cis-platinum-induced peripheral neuropathy. Pain 2002;98(1-2):195-203. [DOI: 10.1016/s0304-3959(02)00047-7] [DOI] [PubMed] [Google Scholar]
Jia 2006 {published data only}
- Jia H-Y, Li Q-F, Song D-P, Liu Y, Ran X. Effects of venlafaxine and carbamazepine for painful peripheral diabetic neuropathy: a randomized, double-blind and double-dummy, controlled multi-center trial. Chinese Journal of Evidence-Based Medicine 2006;6(5). [Google Scholar]
Keskinbora 2006 {published data only}
- Keskinbora K, Pekel AF, Aydinli I. Comparison of efficacy of gabapentin and amitriptyline in the management of peripheral neuropathic pain. Ağrı (Algoloji) Derneği'nin Yayın Organıdır [Journal of the Turkish Society of Algology] 2006;18(2):34-40. [PubMed] [Google Scholar]
Riesner 2008 {published data only}
- Riesner H-J, Zeitler C, Schreiber H, Wild A. Additional treatment in chronic pain syndrome due to hip and knee arthritis with the selective serotonin reuptake inhibitor fluvoxamine (Fevarin) [Eine additive Therapie chronischer Schmerzen bei fortgeschrittener Gon-/Coxarthrose mit dem selektiven Serotonin-Reuptake-Hemmer Fluvoxamin (Fevarin®)]. Zeitschrift für Orthopädie und Unfallchirurgie 2008;146(6):742-6. [DOI: 10.1055/s-2008-1039038] [DOI] [PubMed] [Google Scholar]
Salehifar 2020 {published data only}
- Salehifar E, Janbabaei G, Hendouei N, Alipour A, Tabrizi N, Avan R. Comparison of the efficacy and safety of pregabalin and duloxetine in taxane-induced sensory neuropathy: a randomized controlled trial. Clinical Drug Investigation 2020;40(3):249-57. [DOI: 10.1007/s40261-019-00882-6] [DOI] [PubMed] [Google Scholar]
Shabbir 2011 {published data only}
- Shabbir B, Shafi F, Mahboob F. Amitriptyline vs pregabalin in painful diabetic neuropathy a randomised placebo-based study. Pakistan Journal of Medical and Health Sciences 2011;5(4):745-7. [Google Scholar]
Shlay 1998 {published data only}
- Shlay JC, Chaloner K, Max MB, Flaws B, Reichelderfer P, Wentworth D, et al, for the Terry Beirn Community Programs for Clinical Research on AIDS. Acupuncture and amitriptyline for pain due to HIV-related peripheral neuropathy: a randomized controlled trial. Journal of the American Medical Association 1998;280(18):1590-5. [DOI: 10.1001/jama.280.18.1590] [DOI] [PubMed] [Google Scholar]
Taghizadeh 2020 {published data only}
- Taghizadeh M, Elahabadi I, Kamiab Z, Zaydabadi Nejad N, Mortazavi Lahijani M, Vazirinejad R, et al. A comparative study on the effects of fluoxetine and tamoxifen on the treatment of mastalgia: a randomized clinical trial. KOOMESH 2020;22(4):574-80. [Google Scholar]
Xu 2006 {published data only}
- Xu J, Cheng YQ, Lv SP, Li XZ, Feng R, Cui RM. A controlled study of paroxetine and amitriptyline in the treatment of primary fibromyalgia syndrome. Chinese Mental Health Journal 2006;20(8):542‐4. [Google Scholar]
Zakerkish 2017 {published data only}
- Zakerkish M, Amiri F, Nasab NM, Ghorbani A. Comparative efficacy of duloxetine versus nortriptyline in patients with diabetic peripheral neuropathic pain: a double blind randomized controlled trial. Iranian Red Crescent Medical Journal 2017;19(8):e59995. [DOI: 10.5812/ircmj.59995] [DOI] [Google Scholar]
References to ongoing studies
ACTRN12619000878178 {published data only}
- ACTRN12619000878178. Venlafaxine for pain reduction in patients with knee osteoarthritis [A randomised controlled trial of venlafaxine to treat patients with knee osteoarthritis pain]. https://trialsearch.who.int/Trial2.aspx?TrialID=ACTRN12619000878178 (first received 20 June 2019).
ACTRN12619001082190 {published data only}
- ACTRN12619001082190. Serotonin noradrenaline reuptake inhibitors (SNRI) medications for the treatment of osteoarthritis pain (STOP) trial [Venlafaxine compared to duloxetine for the treatment of osteoarthritis pain: a double-blind, randomised, non-inferiority trial]. https://trialsearch.who.int/Trial2.aspx?TrialID=ACTRN12619001082190 (first received 6 August 2019).
Ammitzboll 2021 {published data only}
- Ammitzbøll N, Arendt-Nielsen L, Bertoli D, Brock C, Olesen AE, Kappel A, et al. A mechanism-based proof of concept study on the effects of duloxetine in patients with painful knee osteoarthritis. Trials 2021;22(1):958. [DOI: 10.1186/s13063-021-05941-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
ChiCTR1900027038 {published data only}
- ChiCTR1900027038. Synergistic analgesia of duloxetine in phantom limb pain: a randomized controlled trial. https://trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR1900027038 (first received 29 October 2019).
CTRI/2018/10/015944 {published data only}
- CTRI/2018/10/015944. A study of gabapentin and duloxetine in painful diabetic neuropathy [A comparative evaluation of duloxetine and gabapentin in painful diabetic neuropathy: a randomised control trial]. https://trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2018/10/015944 (first received 8 October 2018).
CTRI/2018/10/015983 {published data only}
- CTRI/2018/10/015983. Vitamin D as an add on therapy in the treatment of diabetic peripheral neuropathy [Effectiveness of vitamin d as a supplement with conventional therapy in the treatment of diabetic peripheral neuropathy - a randomized controlled clinical trial]. https://trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2018/10/015983 (first received 10 October 2018).
CTRI/2021/02/031068 {published data only}
- CTRI/2021/02/031068. Comparing the benefit of tablet pregabalin with tablet duloxetine and only tablet pregabalin in nerve pain in diabetes and relation with PPARG and Akt gene [A randomized double-blind comparative study evaluating the efficacy of a combination of pregabalin and duloxetine versus pregabalin alone and the modulation of mRNA expression of PPARG and Akt genes in patients of painful diabetic peripheral neuropathy]. https://trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2021/02/031068 (first received 8 February 2021).
CTRI/2021/03/031875 {published data only}
- CTRI/2021/03/031875. Effect of duloxetine in central post-stroke pain [Efficacy of duloxetine in patients with central post-stroke pain: a randomised double blind placebo controlled study]. https://trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2021/03/031875 (first received 10 March 2021).
EUCTR2019‐000243‐27‐DK {published data only}
- EUCTR2019-000243-27-DK. Bupropion for the treatment of nerve pain. A randomized, double-blind study [The effect of bupropion in peripheral neuropathic pain. A randomized, double-blind, placebo-controlled study]. https://trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2019-000243-27-DK (first received 10 May 2021).
EUCTR2019‐000324‐17‐GB {published data only}
- EUCTR2019-000324-17-GB. Amitriptyline at low-dose and titrated for irritable bowel syndrome as second-line treatment (the ATLANTIS study): a double-blind placebo-controlled trial [Amitriptyline at low-dose and titrated for irritable bowel syndrome as second-line treatment (the ATLANTIS study): a double-blind placebo-controlled trial]. https://trialsearch.who.int/Trial2.aspx?TrialID=ISRCTN48075063 (first received 7 June 2019).
EUCTR2019‐001202‐14‐NL {published data only}
- EUCTR2019-001202-14-NL. Effect of an antidepressant on chest pain in patients with achalasia. https://trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2019-001202-14-NL (first received 18 April 2019).
EUCTR2021‐002288‐24‐NL {published data only}
- EUCTR2021-002288-24-NL. Effect of citalopram on chest pain in patients with chest pain of unkown origin [Effect of citalopram on chest pain in patients with functional chest pain - Ci-FCP]. https://trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2021-002288-24-NL (first received 27 July 2021).
IRCT20110413006186N13 {published data only}
- IRCT20110413006186N13. The effect of electric stimulation through skin and duloxetine on diabetic neuropathic pain [A comparison of the effectiveness of transcutaneous electrical nerve stimulation and duloxetine on diabetic peripheral neuropathic pain]. https://trialsearch.who.int/Trial2.aspx?TrialID=IRCT20110413006186N13 (first received 27 May 2019).
IRCT20200205046381N1 {published data only}
- IRCT20200205046381N1. Comparing the analgesic effect of fluoxetine and vitamin E with vitamin E only in breast pain due to fibrocystic breast disease. https://trialsearch.who.int/Trial2.aspx?TrialID=IRCT20200205046381N1 (first received 28 February 2020).
IRCT20200620047852N1 {published data only}
- IRCT20200620047852N1. Agomelatin in chronic low back pain [Comparing the analgesic effect of agomelatin versus placebo in combination with pregabalin in patients with chronic low back pain: a randomized, double-blinded study]. https://trialsearch.who.int/Trial2.aspx?TrialID=IRCT20200620047852N1 (first received 23 June 2020).
NCT00981149 {published data only}
- NCT00981149. Duloxetine for treatment of painful temporomandibular joint disorder. https://clinicaltrials.gov/show/NCT00981149 (first received 22 September 2009).
NCT03249558 {published data only}
- NCT03249558. Effect of combined morphine and duloxetine on chronic pain. https://clinicaltrials.gov/show/NCT03249558 (first received 15 August 2017).
NCT03324035 {published data only}
- NCT03324035. Treatment of neuropathic pain in leprosy. https://clinicaltrials.gov/show/NCT03324035 (first received 27 October 2017).
NCT04704453 {published data only}
- NCT04704453. Study to evaluate the interest of qutenza in patients with head and neck cancer in remission and with sequelae neuropathic pain. https://clinicaltrials.gov/show/NCT04704453 (first received 11 January 2021).
NCT04727502 {published data only}
- NCT04727502. Comparison of duloxetine versus pregabalin [Comparison of duloxetine versus pregabalin in post-mastectomy pain syndrome: a randomized controlled trial]. https://clinicaltrials.gov/show/NCT04727502 (first received 27 January 2021).
PACTR202001764151121 {published data only}
- PACTR202001764151121. Efficacy of clomipramine for chronic lumbar radicular pain: a randomized clinical trial. https://trialsearch.who.int/Trial2.aspx?TrialID=PACTR202001764151121 (first received 6 November 2019).
RBR‐6pqx4n {published data only}
- RBR-6pqx4n. Efficacy of duloxetine in chronic facial pain. https://trialsearch.who.int/Trial2.aspx?TrialID=RBR-6pqx4n (first received 3 September 2018).
Reckziegel 2017 {published data only}
- Reckziegel D, Bailey H, Cottam WJ, Tench CR, Mahajan RP, Walsh DA, et al. Imaging pain relief in osteoarthritis (IPRO): protocol of a double-blind randomised controlled mechanistic study assessing pain relief and prediction of duloxetine treatment outcome. BMJ Open 2017;7(6):e014013. [DOI: 10.1136/bmjopen-2016-014013] [DOI] [PMC free article] [PubMed] [Google Scholar]
TCTR20190303001 {published data only}
- TCTR20190303001. A comparison of analgesic efficacy between amitriptyline and mianserin in chronic low back pain patients: a randomized double-blind controlled trial. https://trialsearch.who.int/Trial2.aspx?TrialID=TCTR20190303001 (first received 3 April 2023).
TCTR20210311009 {published data only}
- TCTR20210311009. Comparison effectiveness of nortriptyline and placebo in the treatment of chronic osteoarthritis knee. https://trialsearch.who.int/Trial2.aspx?TrialID=TCTR20210311009 (first received 11 March 2021).
Wluka 2021 {published data only}
- Wluka AE, Urquhart DM, Teichtahl AJ, Hussain SM, Forbes A, Arnold C, et al. Effect of low-dose amitriptyline on reducing pain in clinical knee osteoarthritis compared to benztropine: study protocol of a randomised, double blind, placebo-controlled trial. BMC Musculoskeletal Disorders 2021;22(1):826. [DOI: 10.1186/s12891-021-04690-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Alba‐Delgado 2012
- Alba-Delgado C, Mico JA, Sánchez-Blázquez P, Berrocoso E. Analgesic antidepressants promote the responsiveness of locus coeruleus neurons to noxious stimulation: implications for neuropathic pain. Pain 2012;153(7):1438-49. [DOI: 10.1016/j.pain.2012.03.034] [DOI] [PubMed] [Google Scholar]
American Psychiatric Association 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition. Washington (DC): American Psychiatric Association, 2013. [Google Scholar]
Bates 2019
- Bates D, Schultheis C, Hanes MC, Jolly SM, Chakravarthy KV, Deer TR, et al. A comprehensive algorithm for the management of neuropathic pain. Pain Medicine 2019;20 Suppl 1:S2-12. [DOI: 10.1093/pm/pnz075] [DOI] [PMC free article] [PubMed] [Google Scholar]
Betancourt 2015
- Betancourt M, Girolami M. Hamiltonian Monte Carlo for hierarchical models. In: Upadhyay SK, Singh U, Dey DK, Loganathan A, editors(s). Current Trends in Bayesian Methodology With Applications. 1st edition. Boca Raton (FL): CRC Press, 2015:80-100. [Google Scholar]
Bevan 2012
- Bevan S. The impact of back pain on sickness absence in Europe. London (UK): Work Foundation; 2012 June.
Boulton 2005
- Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care 2005;28(4):956-62. [DOI: 10.2337/diacare.28.4.956] [DOI] [PubMed] [Google Scholar]
Breivik 2006
- Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life and treatment. European Journal of Pain 2006;10(4):287-333. [DOI: 10.1016/j.ejpain.2005.06.009] [DOI] [PubMed] [Google Scholar]
British National Formulary 2022a
- British National Formulary 79. bnf.nice.org.uk/ (accessed 29 June 2022).
British National Formulary 2022b
- British National Formulary - Amitriptyline hydrochloride. https://bnf.nice.org.uk/drugs/amitriptyline-hydrochloride/ (accessed 29 June 2022).
British National Formulary 2022c
- British National Formulary - Duloxetine. https://bnf.nice.org.uk/drugs/duloxetine/ (accessed 29 June 2022).
Caldwell 2005
- Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 2005;331(7521):897-900. [DOI: 10.1136/bmj.331.7521.897] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaimani 2022
- Chaimani A, Caldwell DM, Li T, Higgins JP, Salanti G. Chapter 11: Undertaking network meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Chiocchia 2021
- Chiocchia V, Nikolakopoulou A, Higgins JP, Page MJ, Papakonstantinou T, Cipriani A, et al. ROB-MEN: a tool to assess risk of bias due to missing evidence in network meta-analysis. BMC Medicine 2021;19(1):304. [DOI: 10.1186/s12916-021-02166-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cipriani 2013
- Cipriani A, Higgins JP, Geddes JR, Salanti G. Conceptual and technical challenges in network meta-analysis. Annals of Internal Medicine 2013;159(2):130-7. [DOI: 10.7326/0003-4819-159-2-201307160-00008] [DOI] [PubMed] [Google Scholar]
Cipriani 2018
- Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet 2018;391(10128):1357-66. [DOI: 10.1016/S0140-6736(17)32802-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis in the Behavioral Sciences. 2nd edition. Hillsdale (NJ): Lawrence Erlbaum Associates, Inc, 1988. [Google Scholar]
Coupland 2011
- Coupland C, Dhiman P, Barton G, Morriss R, Arthur A, Sach T, et al. A study of the safety and harms of antidepressant drugs for older people: a cohort study analysis using a large primary care database. Health Technology Assessment 2011;15(28):1-202. [DOI: 10.3310/hta15280] [DOI] [PubMed] [Google Scholar]
Coupland 2015
- Coupland C, Hill T, Morriss R, Arthur A, Moore M, Hippisley-Cox J. Antidepressant use and risk of suicide and attempted suicide or self harm in people aged 20 to 64: cohort study using a primary care database. BMJ 2015;350:h517. [DOI: 10.1136/bmj.h517] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2022
- Deeks JJ, Higgins JP, Altman DG, on behalf of the Cochrane Statistical Methods Group. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Derry 2015a
- Derry S, Phillips T, Moore RA, Wiffen PJ. Milnacipran for neuropathic pain in adults. Cochrane Database of Systematic Reviews 2015, Issue 7. Art. No: CD011789. [DOI: 10.1002/14651858.CD011789] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2015b
- Derry S, Wiffen PJ, Aldington D, Moore RA. Nortriptyline for neuropathic pain in adults. Cochrane Database of Systematic Reviews 2015, Issue 1. Art. No: CD011209. [DOI: 10.1002/14651858.CD011209.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dias 2013
- Dias S, Sutton AJ, Welton NJ, Ades AE. Evidence synthesis for decision making 3: heterogeneity—subgroups, meta-regression, bias, and bias-adjustment. Medical Decision Making 2013;33(5):618-40. [DOI: 10.1177/0272989X13485157] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dias 2013a
- Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. Evidence synthesis for decision making 4: Inconsistency in networks of evidence based on randomized controlled trials. Medical Decision Making 2013;33(5):641-56. [DOI: 10.1177/0272989X12455847] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dowrick 2013
- Dowrick C, Frances A. Medicalising unhappiness: new classification of depression risks more patients being put on drug treatment from which they will not benefit. BMJ 2013;347:f7140. [DOI: 10.1136/bmj.f7140] [DOI] [PubMed] [Google Scholar]
Dworkin 2008
- Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. Journal of Pain 2008;9(2):105-21. [DOI: 10.1016/j.jpain.2007.09.005] [DOI] [PubMed] [Google Scholar]
Edwards 1999
- Edwards JE, McQuay HJ, Moore RA, Collins SL. Reporting of adverse effects in clinical trials should be improved: lessons from acute postoperative pain. Journal of Pain and Symptom Management 1999;18(6):427-37. [DOI: 10.1016/s0885-3924(99)00093-7] [DOI] [PubMed] [Google Scholar]
European Pain Federation 2016
- European Pain Federation. Pain proposal: improving the current and future management of chronic pain. europeanpainfederation.eu/wp-content/uploads/2016/06/pain_proposal.pdf (accessed 1 July 2020).
Fayaz 2016
- Fayaz A, Croft P, Langford RM, Donaldson LJ, Jones GT. Prevalence of chronic pain in the UK: a systematic review and meta-analysis of population studies. BMJ Open 2016;6(6):e010364. [DOI: 10.1136/bmjopen-2015-010364] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferreira 2021
- Ferreira GE, McLachlan AJ, Lin C-W, Zadro JR, Abdel-Shaheed C, O’Keeffe M, et al. Efficacy and safety of antidepressants for the treatment of back pain and osteoarthritis: systematic review and meta-analysis. BMJ 2021;372:m4825. [DOI: 10.1136/bmj.m4825] [DOI] [PMC free article] [PubMed] [Google Scholar]
Finnerup 2015
- Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurology 2015;14(2):162-73. [DOI: 10.1016/S1474-4422(14)70251-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Finnerup 2021
- Finnerup NB, Kuner R, Jensen TS. Neuropathic pain: from mechanisms to treatment. Physiological Reviews 2021;101(1):259-301. [DOI: 10.1152/physrev.00045.2019] [DOI] [PubMed] [Google Scholar]
Free 2007
- Free ML. Cognitive Therapy in Groups: Guidelines and Resources for Practice. 2nd edition. Hoboken (NJ): John Wiley & Sons, 2007. [Google Scholar]
Furukawa 2016
- Furukawa TA, Salanti G, Atkinson LZ, Leucht S, Ruhe HG, Turner EH, et al. Comparative efficacy and acceptability of first-generation and second-generation antidepressants in the acute treatment of major depression: protocol for a network meta-analysis. BMJ Open 2016;6(7):e010919. [DOI: 10.1136/bmjopen-2015-010919] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gallagher 2015
- Gallagher HC, Gallagher RM, Butler M, Buggy DJ, Henman MC. Venlafaxine for neuropathic pain in adults. Cochrane Database of Systematic Reviews 2015, Issue 8. Art. No: CD011091. [DOI: 10.1002/14651858.CD011091.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goesling 2013
- Goesling J, Clauw DJ, Hassett AL. Pain and depression: an integrative review of neurobiological and psychological factors. Current Psychiatric Reports 2013;15(12):421. [DOI: 10.1007/s11920-013-0421-0] [DOI] [PubMed] [Google Scholar]
Gouveia 2017
- Gouveia N, Rodrigues A, Ramiro S, Eusébio M, Machado PM, Canhão H, et al. The use of analgesic and other pain‐relief drugs to manage chronic low back pain: results from a national survey. Pain Practice 2017;17(3):353-65. [DOI: 10.1111/papr.12455] [DOI] [PubMed] [Google Scholar]
Gureje 1998
- Gureje O, Korff M, Simon GE, Gater R. Persistent pain and well-being: a World Health Organization study in primary care. Journal of the American Medical Association 1998;280(2):147-51. [DOI: 10.1001/jama.280.2.147] [DOI] [PubMed] [Google Scholar]
Harmer 2017
- Harmer CJ, Duman RS, Cowen PJ. How do antidepressants work? New perspectives for refining future treatment approaches. Lancet Psychiatry 2017;4(5):409-18. [DOI: 10.1016/S2215-0366(17)30015-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hearn 2014a
- Hearn L, Moore RA, Derry S, Wiffen PJ, Phillips T. Desipramine for neuropathic pain in adults. Cochrane Database of Systematic Reviews 2014, Issue 9. Art. No: CD011003. [DOI: 10.1002/14651858.CD011003.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hearn 2014b
- Hearn L, Derry S, Phillips T, Moore RA, Wiffen PJ. Imipramine for neuropathic pain in adults. Cochrane Database of Systematic Reviews 2014, Issue 5. Art. No: CD010769. [DOI: 10.1002/14651858.CD010769.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA, editor(s), on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from https://training.cochrane.org/handbook/archive/v5.1/.
Higgins 2022a
- Higgins JP, Li T, Deeks JJ, editor(s). Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Hill 2015
- Hill T, Coupland C, Morriss R, Arthur A, Moore M, Hippisley-Cox J. Antidepressant use and risk of epilepsy and seizures in people aged 20 to 64 years: cohort study using a primary care database. BMC Psychiatry 2015;15:315. [DOI: 10.1186/s12888-015-0701-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
IASP 2019
- Bark A. Chronic pain has arrived in the ICD-11. www.iasp-pain.org/PublicationsNews/NewsDetail.aspx?ItemNumber=8340&navItemNumber=643 (accessed 1 July 2020).
Jansen 2011
- Jansen JP, Fleurence R, Devine B, Itzler R, Barrett A, Hawkins N, et al. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 1. Value in Health 2011;14(4):417-28. [DOI: 10.1016/j.jval.2011.04.002] [DOI] [PubMed] [Google Scholar]
Jiang 2015
- Jiang H-Y, Chen H-Z, Hu X-J, Yu ZH-, Yang W, Deng M, et al. Use of selective serotonin reuptake inhibitors and risk of upper gastrointestinal bleeding: a systematic review and meta-analysis. Clinical Gastroenterology and Hepatology 2015;13(1):42-50.e3. [DOI: 10.1016/j.cgh.2014.06.021] [DOI] [PubMed] [Google Scholar]
Jordan 2010
- Jordan KP, Kadam UT, Hayward R, Porcheret M, Young C, Croft P. Annual consultation prevalence of regional musculoskeletal problems in primary care: an observational study. BMC Musculoskeletal Disorders 2010;11:144. [DOI: 10.1186/1471-2474-11-144] [DOI] [PMC free article] [PubMed] [Google Scholar]
Klit 2009
- Klit H, Finnerup NB, Jensen TS. Central post-stroke pain: clinical characteristics, pathophysiology, and management. Lancet Neurology 2009;8(9):857-68. [DOI: 10.1016/S1474-4422(09)70176-0] [DOI] [PubMed] [Google Scholar]
Koes 2018
- Koes BW, Backes D, Bindels PJ. Pharmacotherapy for chronic non-specific low back pain: current and future options. Expert Opinion on Pharmacotherapy 2018;19(6):537-45. [DOI: ] [DOI] [PubMed] [Google Scholar]
Kremer 2018
- Kremer M, Yalcin I, Goumon Y, Wurtz X, Nexon L, Daniel D, et al. A dual noradrenergic mechanism for the relief of neuropathic allodynia by the antidepressant drugs duloxetine and amitriptyline. Journal of Neuroscience 2018;38(46):9934-54. [DOI: 10.1523/JNEUROSCI.1004-18.2018] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kurita 2012
- Kurita GP, Sjøgren P, Juel K, Højsted J, Ekholm O. The burden of chronic pain: a cross-sectional survey focussing on diseases, immigration, and opioid use. Pain 2012;153(12):2332-8. [DOI: 10.1016/j.pain.2012.07.023] [DOI] [PubMed] [Google Scholar]
Lefebvre 2022
- Lefebvre C, Glanville J, Briscoe S, Featherstone R, Littlewood A, Marshall C, et al. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Llorca‐Torralba 2016
- Llorca-Torralba M, Borges G, Neto F, Mico JA, Berrocoso E. Noradrenergic locus coeruleus pathways in pain modulation. Neuroscience 2016;338:93-113. [DOI: 10.1016/j.neuroscience.2016.05.057] [DOI] [PubMed] [Google Scholar]
Lunn 2014
- Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database of Systematic Reviews 2014, Issue 1. Art. No: CD007115. [DOI: 10.1002/14651858.CD007115.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
McKenzie 2020
- McKenzie JE, Brennan SE, Ryan RE, Thomson HJ, Johnston RV, Thomas J. Chapter 3: Defining the criteria for including studies and how they will be grouped for the synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook/archive/v6.1/chapter-03.
Melzack 1975
- Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain 1975;1(3):277-99. [DOI: 10.1016/0304-3959(75)90044-5] [DOI] [PubMed] [Google Scholar]
Mills 2019
- Mills SE, Nicolson KP, Smith BH. Chronic pain: a review of its epidemiology and associated factors in population-based studies. British Journal of Anaesthesia 2019;123(2):273-83. [DOI: 10.1016/j.bja.2019.03.023] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mitchell 2009
- Mitchell AJ, Vaze A, Rao S. Clinical diagnosis of depression in primary care: a meta-analysis. Lancet 2009;374(9690):609-19. [DOI: 10.1016/S0140-6736(09)60879-5] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2010a
- Moore RA, Straube S, Paine J, Phillips CJ, Derry S, McQuay HJ. Fibromyalgia: moderate and substantial pain intensity reduction predicts improvement in other outcomes and substantial quality of life gain. Pain 2010;149(2):360-4. [DOI: 10.1016/j.pain.2010.02.039] [DOI] [PubMed] [Google Scholar]
Moore 2010b
- Moore AR, Smugar SS, Wang H, Peloso PM, Gammaitoni A. Numbers-needed-to-treat analyses—do timing, dropouts, and outcome matter? Pooled analysis of two randomized, placebo-controlled chronic low back pain trials. Pain 2010;151(3):592-7. [DOI: 10.1016/j.pain.2010.07.013] [DOI] [PubMed] [Google Scholar]
Moore 2010c
- Moore RA, Derry S, McQuay HJ, Straube S, Aldington D, Wiffen P, et al, ACTINPAIN writing group of the IASP Special Interest Group (SIG) on Systematic Reviews in Pain Relief. Clinical effectiveness: an approach to clinical trial design more relevant to clinical practice, acknowledging the importance of individual differences. Pain 2010;149(2):173-6. [DOI: 10.1016/j.pain.2009.08.007] [DOI] [PubMed] [Google Scholar]
Moore 2014
- Moore RA, Cai N, Skljarevski V, Tölle TR. Duloxetine use in chronic painful conditions–individual patient data responder analysis. European Journal of Pain 2014;18(1):67-75. [DOI: 10.1002/j.1532-2149.2013.00341.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2015
- Moore RA, Derry S, Aldington D, Cole P, Wiffen PJ. Amitriptyline for neuropathic pain in adults. Cochrane Database of Systematic Reviews 2015, Issue 7. Art. No: CD008242. [DOI: 10.1002/14651858.CD008242.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morris 2018
- Morris LD, Daniels KJ, Ganguli B, Louw QA. An update on the prevalence of low back pain in Africa: a systematic review and meta-analyses. BMC Musculoskeletal Disorders 2018;19(1):196. [DOI: 10.1186/s12891-018-2075-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moulin 2014
- Moulin DE, Boulanger A, Clark AJ, Clarke H, Dai T, et al. Pharmacological management of chronic neuropathic pain: revised consensus statement from the Canadian Pain Society. Pain Research and Management 2014;19(6):328-35. [DOI: 10.1155/2014/754693] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mulder 2008
- Mulder RT. An epidemic of depression or the medicalization of distress? Perspectives in Biology and Medicine 2008;51(2):238-50. [DOI: 10.1353/pbm.0.0009] [DOI] [PubMed] [Google Scholar]
NICE 2009a
- National Institute for Health Care Excellence. Depression in adults: recognition and management. www.nice.org.uk/guidance/cg90 (accessed 1 July 2020). [PubMed]
NICE 2009b
- National Institute for Health Care Excellence. Depression in adults with a chronic physical health problem: recognition and management. www.nice.org.uk/guidance/cg91/chapter/1-Guidance (accessed 1 July 2020).
NICE 2017
- National Institute for Health Care Excellence. Low back pain and sciatica in over 16s. https://www.nice.org.uk/guidance/qs155 (accessed 1 July 2020). [PubMed]
NICE 2019
- National Institute for Health Care Excellence. Neuropathic pain in adults: pharmacological management in non-specialist settings. www.nice.org.uk/guidance/cg173 (accessed 1 July 2020). [PubMed]
NICE 2020
- National Institute for Health Care Excellence. Chronic pain in over 16s: assessment and management. www.nice.org.uk/guidance/gid-ng10069/documents/draft-guideline (accessed 1 September 2020).
NICE 2021
- National Institute for Health Care Excellence. Chronic pain (primary and secondary) in over 16s: assessment of all chronic pain and management of chronic primary pain. https://www.nice.org.uk/guidance/ng193 (accessed 29 June 2022). [PubMed]
Nikolakopoulou 2020
- Nikolakopoulou A, Higgins JP, Papakonstantinou T, Chaimani A, Del Giovane C, Egger M, et al. CINeMA: an approach for assessing confidence in the results of a network meta-analysis. PLOS Medicine 2020;17(4):e1003082. [DOI: 10.1371/journal.pmed.1003082] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nuyen 2005
- Nuyen J, Volkers, AC, Verhaak PF, Schellevis FG, Groenewegen PP, den Bos GA. Accuracy of diagnosing depression in primary care: the impact of chronic somatic and psychiatric co-morbidity. Psychological Medicine 2005;35(8):1185-95. [DOI: 10.1017/s0033291705004812] [DOI] [PubMed] [Google Scholar]
Obata 2017
- Obata H. Analgesic mechanisms of antidepressants for neuropathic pain. Internation Journal of Molecular Sciences 2017;18(11):2483. [DOI: 10.3390/ijms18112483] [DOI] [PMC free article] [PubMed] [Google Scholar]
Office for National Statistics 2019
- Office for National Statistics. Sickness absence in the UK. www.ons.gov.uk/employmentandlabourmarket/peopleinwork/labourproductivity/articles/sicknessabsenceinthelabourmarket/2018 (accessed 1 July 2020).
Page 2022
- Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Phillippo 2018
- Phillippo DM, Ades AE, Dias S, Palmer S, Abrams KR, Welton NJ. Methods for population-adjusted indirect comparisons in health technology appraisal. Medical Decision Making 2018;38(2):200-11. [DOI: 10.1177/0272989X17725740] [DOI] [PMC free article] [PubMed] [Google Scholar]
Phillippo 2022 [Computer program]
- multinma: Bayesian Network Meta-Analysis of Individual and Aggregate Data. Phillippo DM, Version R package version 0.4.2. David Phillippo, 2022. https://dmphillippo.github.io/multinma/. [DOI: 10.5281/zenodo.3904454] [DOI]
Phillips 2019
- Phillips R, Hazell L, Sauzet O, Cornelius V. Analysis and reporting of adverse events in randomised controlled trials: a review. BMJ Open 2019;9(2):e024537. [DOI: 10.1136/bmjopen-2018-024537] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pirosca 2022
- Pirosca S, Shiely F, Clarke M, Treweek S. Tolerating bad health research: the continuing scandal. Trials 2022;23(1):458. [DOI: 10.1186/s13063-022-06415-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
RevMan Web 2023 [Computer program]
- Review Manager Web (RevMan Web). Version 5.0.0. The Cochrane Collaboration, 2023. Available at revman.cochrane.org.
Rice 2016
- Rice AS, Smith BH, Blyth FM. Pain and the global burden of disease. Pain 2016;157(4):791-6. [DOI: 10.1097/j.pain.0000000000000454] [DOI] [PubMed] [Google Scholar]
Riediger 2017
- Riediger C, Schuster T, Barlinn K, Maier S, Weitz J, Siepmann T. Adverse effects of antidepressants for chronic pain: a systematic review and meta-analysis. Frontiers in Neurology 2017;8:307. [DOI: 10.3389/fneur.2017.00307] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rusu 2016
- Rusu AC, Santos R, Pincus T. Pain-related distress and clinical depression in chronic pain: a comparison between two measures. Scandinavian Journal of Pain 2016;12:62-7. [DOI: 10.1016/j.sjpain.2016.04.001] [DOI] [PubMed] [Google Scholar]
Rutherford 2009
- Rutherford BR, Sneed JR, Roose SP. Does study design influence outcome? The effects of placebo control and treatment duration in antidepressant trials. Psychotherapy and Psychosomatics 2009;78:172-81. [DOI: 10.1159/000209348] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rücker 2017 [Computer program]
- netmeta: Network meta-analysis using frequentist methods. Rücker G, Schwarzer G, Krahn U, König J, Version R package version 0.9-5. Rücker, 2017. https://cran.r-project.org/package=netmeta.
Schulz 2010
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Journal of Pharmacology and Pharmacotherapeutics 2010;1(2):100-7. [DOI: 10.4103/0976-500X.72352] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html (accessed prior to 4 May 2023).
Schünemann 2022
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Sinyor 2020
- Sinyor M, Cheung CP, Abraha HY, Lanctôt KL, Saleem M, Liu CS, et al. Antidepressant-placebo differences for specific adverse events in major depressive disorder: a systematic review. Journal of Affective Disorders 2020;267:185-90. [DOI: 10.1016/j.jad.2020.02.013] [DOI] [PubMed] [Google Scholar]
Sullivan 1992
- Sullivan MJ, Reesor K, Mikail S, Fisher RT. The treatment of depression in chronic low back pain: review and recommendations. Pain 1992;50:5-13. [DOI: 10.1016/0304-3959(92)90107-M] [DOI] [PubMed] [Google Scholar]
Taylor 2017
- Taylor BK, Westlund KN. The noradrenergic locus coeruleus as a chronic pain generator. Journal of Neuroscience Research 2017;95(6):1336-46. [DOI: 10.1002/jnr.23956] [DOI] [PMC free article] [PubMed] [Google Scholar]
Treede 2019
- Treede R-D, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, et al. Chronic pain as a symptom or a disease: the IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 2019;160(1):19-27. [DOI: 10.1097/j.pain.0000000000001384] [DOI] [PubMed] [Google Scholar]
Tunks 2008
- Tunks ER, Crook J, Weir R. Epidemiology of chronic pain with psychological comorbidity: prevalence, risk, course, and prognosis. Canadian Journal of Psychiatry 2008;53(4):224-34. [DOI: 10.1177/070674370805300403] [DOI] [PubMed] [Google Scholar]
Tylee 2007
- Tylee A, Walters P. Onset of action of antidepressants. BMJ 2007;334(7600):911-2. [DOI: 10.1136/bmj.39197.619190.80] [DOI] [PMC free article] [PubMed] [Google Scholar]
Urquhart 2008
- Urquhart DM, Hoving JL, Assendelft WW, Roland M, Tulder MW. Antidepressants for non‐specific low back pain. Cochrane Database of Systematic Reviews 2008, Issue 1. Art. No: CD001703. [DOI: 10.1002/14651858.CD001703.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vehtari 2021
- Vehtari A, Gelman A, Simpson D, Carpenter B, and Bürkner PC. Rank-normalization, folding, and localization: an improved method for assessing convergence of MCMC. Bayesian Analysis 2021;16(2):667-718. [DOI: 10.1214/20-ba122135.] [DOI] [Google Scholar]
Walitt 2015
- Walitt B, Urrútia G, Nishishinya MB, Cantrell SE, Häuser W. Selective serotonin reuptake inhibitors for fibromyalgia syndrome. Cochrane Database of Systematic Reviews 2015, Issue 6. Art. No: CD011735. [DOI: 10.1002/14651858.CD011735] [DOI] [PMC free article] [PubMed] [Google Scholar]
Welsch 2015
- Welsch P, Bernardy K, Derry S, Moore RA, Häuser W. Mirtazapine for fibromyalgia in adults. Cochrane Database of Systematic Reviews 2018, Issue 8. Art. No: CD012708. [DOI: 10.1002/14651858.CD012708.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Welsch 2018
- Welsch P, Üçeyler N, Klose P, Walitt B, Häuser W. Serotonin and noradrenaline reuptake inhibitors (SNRIs) for fibromyalgia. Cochrane Database of Systematic Reviews 2018, Issue 2. Art. No: CD010292. [DOI: 10.1002/14651858.CD010292.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wiffen 2016
- Wiffen P, Moore RA. Pain leads the way: the development of evidence-based medicine for pain relief. International Journal of Clinical Pharmacology and Therapeutics 2016;54(7):505-13. [DOI: 10.5414/CP202546] [DOI] [PubMed] [Google Scholar]
Williams 2020
- Williams AC, Fisher E, Hearn L, Eccleston C. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database of Systematic Reviews 2020, Issue 8. Art. No: CD007407. [DOI: 10.1002/14651858.CD007407.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolfe 2010
- Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care & Research 2010;62(5):600-10. [DOI] [PubMed] [Google Scholar]
Yepes‐Nuñez 2019
- Yepes-Nuñez JJ, Li S-A, Guyatt G, Jack SM, Brozek JL, Beyene J, et al. Development of the summary of findings table for network meta-analysis. Journal of Clinical Epidemiology 2019;115:1-13. [DOI: 10.1016/j.jclinepi.2019.04.018] [DOI] [PubMed] [Google Scholar]
Yunus 1983
- Yunus MB. Fibromyalgia syndrome: a need for uniform classification. Journal of Rheumatology 1983;10:841-4. [PubMed] [Google Scholar]
Zung 1965
- Zung WW. A self-rating depression scale. Archives of General Psychiatry 1965;12:63-70. [DOI: 10.1001/archpsyc.1965.01720310065008] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Birkinshaw 2021
- Birkinshaw H, Friedrich C, Cole P, Eccleston C, Serfaty M, Stewart G, et al. Antidepressants for pain management in adults with chronic pain: a network meta‐analysis. Cochrane Database of Systematic Reviews 2021, Issue 4. Art. No: CD014682. [DOI: 10.1002/14651858.CD014682] [DOI] [PMC free article] [PubMed] [Google Scholar]