Abstract
We examined the structural components of pertussis toxin that are required for efficient export from Bordetella pertussis via the Ptl system, a member of the type IV family of macromolecular transporters. First, we constructed a strain of B. pertussis that contains a functional Ptl system but does not produce pertussis toxin. Plasmids which express either the S1 subunit or the B oligomer were then introduced into this strain. We found that the B oligomer of the toxin is not secreted in the absence of the S1 subunit. Conversely, the S1 subunit is also not secreted by a Ptl-mediated mechanism in the absence of the B oligomer. Thus, an assembled holotoxin is required for Ptl-mediated export of pertussis toxin from B. pertussis.
Proper targeting of many protein toxins produced by gram-negative bacteria requires secretion of the toxin into the extracellular milieu. This transport process, while essential for toxin action, is complex and usually requires a number of bacterial accessory proteins. One such secreted toxin is pertussis toxin (PT), an essential virulence factor that is exported from Bordetella pertussis with the help of a set of nine accessory proteins known as the Ptl proteins (30).
PT is an oligomeric protein composed of five different subunits, S1 through S5 (22, 23, 29). Structurally, PT belongs to the A-B class of bacterial toxins. Its enzymatically active A component is the S1 subunit which catalyzes the ADP ribosylation of GTP-binding regulatory proteins that are involved in signal transduction in the eukaryotic cell (12, 16). To manifest its biological effects, S1 must associate with the B oligomer, which binds receptors on the eukaryotic cell and mediates the translocation of S1 into the cell (15, 29). The B oligomer is a ring-shaped structure that consists of one copy each of subunits S2, S3, and S5 and two copies of subunit S4 (26, 29). The assembled holotoxin has a molecular mass of 105 kDa (23, 29).
The subunits of PT are encoded by the ptx genes that are located in a large operon which also contains the genes encoding the Ptl proteins (30). Once the PT subunits are synthesized, they must fold, assemble, and cross the inner and outer bacterial membranes before interacting with the eukaryotic cell. However, the mechanistic details and sequence of events in the pathway of PT assembly and secretion are not well understood. Because the DNA sequence of the PT structural genes indicates that each subunit is synthesized with a signal peptide (22, 23), it has been suggested that the individual subunits cross the inner membrane in an unfolded state through a Sec-like pathway. Transport across the outer membrane is then thought to be completed with the assistance of the Ptl proteins; which are members of a rapidly growing family of proteins, known as type IV transporters, that facilitate the export of proteins and/or DNA from bacterial cells (7, 30, 32).
Fundamental questions remain regarding the nature of the interactions between the Ptl proteins and PT. For example, it has not been established whether the Ptl proteins function by transporting individual PT subunits or the assembled holotoxin. In this report, we have examined the structural elements of PT required for Ptl-mediated secretion in order to better understand the mechanistic details of the transport process.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and plasmids.
The strains of B. pertussis and Escherichia coli and the plasmids used in this study are listed in Table 1. B. pertussis strains were grown at 37°C on Bordet-Gengou (BG) agar or in Stainer-Scholte liquid medium. For liquid cultures, cells that had been grown on BG agar plates were resuspended in 20 ml of Stainer-Scholte medium to yield an A550 of 0.2. The strains were then grown in shaking culture for 48 h to an A550 of 1.2 to 1.5.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5α | F−φ80dlacZΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17(rK−, mK+) phoA supE44λ− thi-1 gyrA96 relA1 | GIBCO BRL |
| SM10λpir | thi thr leu tonA lacY supE recA::RP4-2-Tc::Mu λpirR6K | 25 |
| B. pertussis | ||
| BP536 | Wild-type, nalidixic acid-resistant, Smr derivative of Tohama I | 28 |
| BP536Δptx | BPH101, referred to as BP536Δptx in this study to emphasize the mutation; BP536 with a 2.6-kb deletion in the ptx genes, from nucleotide 936 to nucleotide 3514 | This study |
| BP536ptlC::tet | BP536 with a 540-bp deletion in ptlC, from nucleotide 5198 to nucleotide 5738, and an inserted Tetr cassette | 8 |
| BP536Δptxptl | BP536 with a 115-kb deletion in the ptx-ptl genes, from nucleotide 425 to nucleotide 11971 | This study |
| BPM3171 | BP338 with a Tn5 lac transposon inserted near the end of ptlC; kanamycin resistant (Kmr) | 30, 31 |
| Plasmids | ||
| pSZH1 | pUC19 containing nucleotides 1 to 1316 of the ptx region | 13 |
| pGEM-11Zf(+) | Ampicillin-resistant (Ampr) cloning vector | Promega |
| pTH13 | pGEM-11Zf(+) containing nucleotides 1 to 935 of the ptx region | This study |
| pTH14 | pGEM-11Zf(+) containing nucleotides 1 to 935 of the ptx region, followed by nucleotides 3515 to 4574 of the ptx-ptl region | This study |
| pSS1129 | Genr Ampr Sms allelic exchange vector | 27 |
| pTH16 | pSS1129 containing nucleotides 1 to 935 of the ptx region, followed by nucleotides 3515 to 4574 of the ptx-ptl region | This study |
| pRK2013 | ColE1 Tra+ Kmr helper plasmid | 10 |
| pSZH4 | pUC19 containing the entire ptx-ptl region | 13 |
| pDMC28 | pSS1129 containing nucleotides 1 to 424 of the ptx region, followed by nucleotides 11972 to 13025 of the ptl region | This study |
| pUFR047 | Broad-host-range (IncW) vector; Mob+lacZα+ Genr | 9 |
| pRK415 | Broad-host-range (IncP1) vector; Tetr | 17 |
| pDMC36 | pUFR047 containing the ptx-ptl promoter region and ptxS1 | This study |
| pTH19 | pRK415 containing the ptx-ptl promoter region and ptxS1 | This study |
| pSK10 | pUFR047 containing the ptx-ptl promoter region, ptxS1, and ptlH | 20 |
| pUC19 | Ampr cloning vector | GIBCO BRL |
| pTH18 | pUFR047 containing ptxS2, ptxS4, ptxS5, and ptxS3 under lacZ promoter control | This study |
| p2959 | Source of phoA | Scott Stibitz |
| pTH22 | pUFR047 containing phoA | This study |
Construction of an in-frame deletion in the PT structural genes.
A 2.6-kb segment of the ptx region, extending from nucleotide 936 through nucleotide 3514 and corresponding to the second half of ptxS1, ptxS2, ptxS4, and ptxS5 and 84% of ptxS3, was deleted from the B. pertussis chromosome (Fig. 1) by homologous recombination as follows. Nucleotides 1 to 935 of the ptx region were excised as an SstI-SalI fragment from pSZH1, a plasmid in which a PCR fragment consisting of nucleotides 1 to 1316 had been inserted into pUC19 (13). The SstI-SalI fragment, which included the ptx-ptl promoter region and approximately half of ptxS1, was inserted into pGEM11Zf+, resulting in pTH13. Using PCR, as described previously (14), nucleotides 3515 to 4574 of the ptx-ptl region were amplified as a SalI-BamHI fragment, using the upstream primer 5′-CTCCGCCAGGTCGACGTACGCGTCCACGTCAGCAAGGAA (9 nucleotides, followed by a SalI site [underlined], and nucleotides 3515 to 3538 of the ptx region) and the downstream primer 5′-TGCAATGGATCCAAAGCGCGACCT (nucleotides 4580 to 4557 of the ptl region; BamHI site is underlined). The amplified DNA fragment, which included the last 114 nucleotides of ptxS3, ptlA, ptlB, and a small portion of ptlC, was inserted into the SalI-BamHI site of pTH13, generating pTH14. The EcoRI-BamHI fragment from pTH14, corresponding to nucleotides 1 to 935 of the ptx region, followed by nucleotides 3515 to 4574 of the ptx-ptl region, was inserted into pSS1129, a vector which cannot replicate in B. pertussis (27), generating pTH16. Next, pTH16 was transformed into E. coli DH5α and transferred to B. pertussis BP536 by triparental conjugation using E. coli DH5α containing the helper plasmid pRK2013 (10) as described previously (2). The BP536 cells in which pTH16 had integrated into the chromosome by homologous recombination were selected on BG plates containing gentamicin (10 μg/ml) and a colicin B-enriched bacterial lysate (gift of Diana Amsbaugh, Food and Drug Administration) which was prepared from the colicin-producing E. coli strain DM1187(pCLB1) as previously described (4). Colicin B was used to counterselect against E. coli. Gentamicin resistance (Genr) was conferred by integration of pTH16 into the chromosome. A second homologous recombination was achieved by selection on BG agar plates containing streptomycin (100 μg/ml), resulting in loss of the plasmid which contained the rpsL gene, encoding streptomycin sensitivity (Sms). Strains in which the two homologous recombination events occurred on opposite sides of the deleted segment should have sustained the intended deletion.
FIG. 1.
The ptx-ptl region. The structure and nucleotide numbering system of the ptx-ptl region (30) through a portion of ptlC are shown. Pr indicates the location of the ptx-ptl promoter. The DNA segment deleted in BP536Δptx is indicated.
Streptomycin-resistant (Smr) exconjugants sustaining the 2.6-kb deletion in the ptx genes were identified by PCR as follows. PCR was performed using template DNA from streptomycin resistant exconjugants, an upstream oligonucleotide primer (5′-CGCGAGCTCCCGGCCGGCACCATCCCGCATACGTGTTGG) which included the sequence corresponding to nucleotides 271 to 300 of the ptx region (underlined), and a downstream oligonucleotide primer (5′-TGCAATGGATCCAAAGCGCGACCT) corresponding to nucleotides 4580 to 4557 of the ptl region. Using template DNA from strains sustaining the 2.6-kb deletion, PCR should have resulted in an amplified product of 1.7 kb. In contrast, using template DNA from strains not sustaining the deletion, these oligonucleotide primers theoretically would define a 4.3-kb product. A strain which resulted in a 1.7-kb product, which we designated BPH101, was selected for further characterization. Throughout this report, we will refer to this strain as BP536Δptx to emphasize the type of mutation that the strain carries.
BP536Δptx was expected to produce the Ptl proteins, but not the PT subunits, except possibly a truncated S1 protein or a mutant protein consisting of the N-terminal half of S1 and the C-terminal end of S3. To test this, a cell extract of BP536Δptx was first examined by immunoblotting using a polyclonal antibody specific for PtlF (14). As expected, in BP536Δptx, PtlF was readily detected at levels similar to that observed in wild-type B. pertussis (data not shown). In an attempt to visualize the S1 subunit, BP536Δptx was examined by immunoblotting using monoclonal antibody 3CX4, which recognizes a conformational epitope on the S1 subunit, and monoclonal antibody X2X5, which recognizes a linear epitope located near the N-terminal end of S1 (19). No stable forms of S1 were detected by immunoblotting using either of these monoclonal antibodies (data not shown).
Construction of an in-frame deletion in the PT structural genes and the ptl genes.
Virtually the entire ptx-ptl region, extending from nucleotide 425 through nucleotide 11971, was deleted from the B. pertussis chromosome by using homologous recombination, as follows. pSZH4, which contains the entire ptx-ptl region (13), was digested with Asp718I, resulting in excision of nucleotides 425 to 11971 of the ptx-ptl region. The remaining vector was religated, and the EcoRI-HindIII fragment encompassing nucleotides 1 to 424 of the ptx region, followed by nucleotides 11972 to 13025 of the ptl region, was inserted into pSS1129. The resultant vector, pDMC28, was transformed into E. coli SM10λpir and introduced into BP536ptlC::tet by biparental mating. BP536ptlC::tet has a deletion in a portion of ptlC, extending from nucleotide 5198 to nucleotide 5738, with a tetracycline resistance (Tetr) cassette inserted in place of the deleted segment (8). Cells in which pDMC28 had integrated into the chromosome by homologous recombination were selected on BG agar plates containing nalidixic acid (50 μg/ml) and gentamicin (10 μg/ml). Next, colonies were plated onto BG agar containing streptomycin (100 μg/ml) to select for loss of the plasmid by homologous recombination. Finally, loss of tetracycline resistance was used as a marker for the strain sustaining a deletion in the ptx-ptl region, which we designated BP536Δptxptl. The deleted segment included the ptx-ptl promoter region, all of the ptx genes, and the ptl genes except for the extreme 3′ end of ptlH.
Introduction of the genes encoding S1 and the B oligomer in trans into BP536Δptx and BP536Δptxptl.
Plasmids capable of expressing the S1 subunit were constructed as follows. Nucleotides 1 to 1316 of the ptx region, including the ptx-ptl promoter through the end of ptxS1, were inserted into pUFR047 (9) and pRK415 (17), broad-host-range plasmids which can replicate in B. pertussis, generating pDMC36 and pTH19, respectively. First, nucleotides 1 to 1316 of the ptx region were excised from pSK10 (20) as a HindIII-XbaI fragment and inserted into pRK415, resulting in pTH19. The HindIII-XbaI fragment from pSK10 was inserted into pUC19 and then excised as a HindIII-SstI fragment. The HindIII-SstI fragment was then inserted into pUFR047, resulting in pDMC36.
A plasmid capable of expressing the subunits of the B oligomer was next constructed. Nucleotides 1356 to 3628 of the ptx region, encompassing the genes encoding the subunits of the B oligomer, were inserted downstream of the lacZ promoter in pUFR047, as follows. Using PCR as described previously (14), two DNA fragments were generated. The first fragment consisted of ptxS2 and the first 44 nucleotides of ptxS4, preceded by a sequence for a ribosomal binding site. The upstream primer was 5′-CTCCGCGAATTCTAATTAATTAAAGGAAACAGCGATGCCGATCGACCGCAAGACGCTC (6 nucleotides followed by an EcoRI site, stop codons in three reading frames, nucleotides AGGA representing a ribosomal binding site, 7 nucleotides, and nucleotides 1356 to 1379 of the ptx region). The downstream primer was 5′-CCCTGTCCCGGGGCGGTGGTTCGAGT (nucleotides 2073 to 2048 of the ptx region; XmaI site is underlined). The second DNA fragment consisted of the remaining nucleotides of ptxS4, ptxS5, and ptxS3. The upstream primer was 5′-ACTCGAACCACCGCCCCGGGACAGGG (nucleotides 2048 to 2073 of the ptx region; XmaI site is underlined). The downstream primer was 5′-CTCCGCAAGCTTTCAGCATATCGACGCTGCCGGGTT (6 nucleotides, followed by a HindIII site, and nucleotides 3628 to 3605 of the ptx region). The first DNA fragment was inserted into the EcoRI-XmaI site of pUC19. The second DNA fragment was inserted into the XmaI-HindIII site of the resultant vector. Next, the two fragments were excised as one DNA segment and inserted into pUFR047, using EcoRI and HindIII. In the resultant vector, pTH18, the genes encoding the subunits of the B oligomer were situated downstream from the lacZ promoter.
Each of the plasmids pDMC36, pTH19, and pTH18 was transformed into E. coli DH5α. pDMC36 (pUFR047 containing the ptx-ptl promoter and ptxS1) and pTH18 (pUFR047 containing the genes encoding the B oligomer) were separately introduced into BP536Δptx by triparental conjugation as described previously (2). Exconjugants were selected on BG agar containing gentamicin (10 μg/ml) and streptomycin (100 μg/ml). The same procedures were used to introduce plasmids pDMC36 and pTH18 separately into BP536Δptxptl.
Next, pTH19, containing the ptx-ptl promoter region and ptxS1 in pRK415, was introduced into BP536Δptx(pTH18) via triparental conjugation. Exconjugants were selected on BG agar containing gentamicin (10 μg/ml), tetracycline (10 μg/ml), and streptomycin (100 μg/ml). The resultant strain, BP536Δptx(pTH18)(pTH19), contained ptxS1 and the genes encoding the B oligomer subunits on two separate plasmids. In the same manner, pTH19 was introduced into BP536Δptxptl(pTH18).
Expression of phoA in BPM3171.
As a control for cell lysis which could result in release of PT subunits into culture supernatants, we utilized BPM3171, a B. pertussis strain which has a transposon inserted in ptlC (30, 31). This strain produces all of the PT subunits but lacks a functional Ptl transport system. By expressing phoA encoding periplasmically active alkaline phosphatase in BPM3171, we were able to compare the relative amounts of PT subunits and alkaline phosphatase activity in the supernatant and cellular material of cultures. Alkaline phosphatase activity observed in the culture supernatant was indicative of cell lysis. We introduced a plasmid containing phoA into BPM3171 as follows. The plasmid p2959, which contains an altered phoA gene preceded by the sequence for a ribosomal binding site and flanked by EcoRI sites, was obtained from Scott Stibitz (Food and Drug Administration). The phoA gene contained in this plasmid has three point mutations but is still able to express active alkaline phosphatase. The mutations consist of a change in the start codon of phoA from GTG to ATG and silent point mutations in the two EcoRI sites of phoA. Using EcoRI, the phoA gene was excised from p2959 and inserted into pUFR047. Insertion of the phoA gene in the same orientation as the lacZ promoter of pUFR047 was demonstrated by restriction enzyme analysis with SphI. The resultant plasmid, pTH22, was transformed into E. coli SM10λpir and transferred into BPM3171 by triparental conjugation. Exconjugants were selected on BG agar containing gentamicin (10 μg/ml) and kanamycin (25 μg/ml).
Alkaline phosphatase assay.
Cells and supernatant fractions from liquid cultures of BPM3171(pTH22) were collected by centrifugation at 17,000 × g for 10 min. Cells were resuspended in a volume of 1.0 M Tris-HCl, pH 8.0, equivalent to the volume of the supernatant. A volume of 0.5 ml of 1.0 M Tris-HCl, pH 8.0, was added to 0.5 ml of the cell suspensions and supernatants or appropriate dilutions in the same buffer. Cells were permeabilized by the addition of 30 μl of chloroform and 30 μl of 0.1% sodium dodecyl sulfate (SDS), followed by vortexing. Supernatants were treated in the same manner. The assay for alkaline phosphatase was performed essentially as described by Brickman and Beckwith (3). After the addition of 0.1 ml of 0.4% para-nitrophenylphosphate, samples were incubated at room temperature for 15 min, at which time 0.1 ml of 1 M K2HPO4 was added to stop the reactions. The optical density of each sample was read at 420 and 550 nm. The relative amount of alkaline phosphatase activity in the cells and supernatant of BPM3171(pTH22) was determined based on the ratio of the respective dilution factors which resulted in the same amount of absorbance by p-nitrophenol (A420, corrected for light scattering by cell debris).
Immunoblot analysis of cell extracts and culture supernatants.
Cells and supernatant fractions from liquid cultures were collected by centrifugation of 5 ml of the culture at 17,000 × g for 10 min. Cells were resuspended in 5 ml of phosphate-buffered saline (pH 7.4). Samples of cell extracts (100 μl or as otherwise specified) and supernatants (400 μl or as otherwise specified) were precipitated with 2.3 times the volume of 100% ethanol by incubating in a dry ice-ethanol bath for 30 min. After centrifugation, the precipitates were suspended in 15 μl of SDS sample buffer.
Samples were subjected to SDS-polyacrylamide gel electrophoresis, performed essentially as described by Laemmli (21), using 15% polyacrylamide gels. After electrophoresis, proteins were electrophoretically transferred to nitrocellulose as previously described (14). Immunoblot analysis was performed as previously described (5) with monoclonal antibody 3CX4 (18) to visualize the S1 subunit and monoclonal antibody P11B10 (11) to visualize the S2 subunit.
RESULTS
Development of a system to assess transport of PT and its individual components.
In order to determine whether the Ptl system functions by facilitating transport of the individual PT subunits or the assembled holotoxin, we developed a system which we could use to analyze Ptl-mediated transport of the individual components of PT, the S1 subunit or the B oligomer. First, we constructed a strain which does not produce PT subunits but which contains a functional Ptl transport system. We did this by introducing a large in-frame deletion into the ptx region of the wild-type strain BP536 extending from midway in ptxS1 through essentially the end of ptxS3, as described in Materials and Methods. A schematic diagram depicting the deletion in this strain, BP536Δptx, is shown in Fig. 1. Individual plasmids capable of expressing either S1 or the B oligomer were then constructed. The ptx-ptl promoter region followed by ptxS1 was inserted into the broad-host-range plasmids pUFR047 and pRK415 to generate pDMC36 and pTH19, respectively. The genes encoding the subunits of the B oligomer were inserted behind the lacZ promoter in pUFR047 to generate pTH18. These plasmids were then introduced individually or together into BP536Δptx, and secretion of PT was examined.
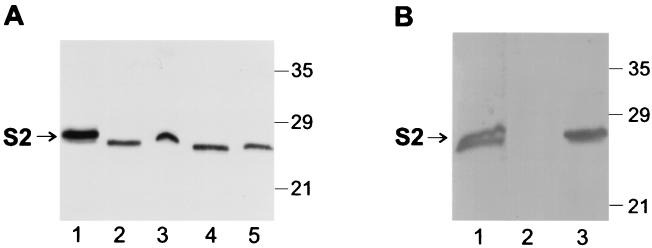
In order to verify that the Ptl proteins of BP536Δptx were functional, we compared secretion of PT from BP536Δptx(pTH18)(pTH19) with that of wild-type BP536. As shown in Fig. 2A, when both S1 and the B oligomer were expressed in BP536Δptx, the relative amount of S2 (detected by immunoblot analysis) which was secreted into the culture supernatant was similar to that observed in wild-type B. pertussis. This finding confirmed that BP536Δptx produced a functional Ptl system.
FIG. 2.
Immunoblot analysis of S2 in cell extracts and culture supernatants of B. pertussis strains. Samples of culture supernatants (400 μl) and cell extracts (100 μl) were prepared as described in Materials and Methods. Samples were then subjected to SDS-polyacrylamide gel electrophoresis and immunoblot analysis using monoclonal antibody P11B10 to visualize the S2 subunit of PT. Positions of molecular size markers are indicated at the right in kilodaltons. The arrow indicates the protein band corresponding to S2. (A) Lane 1, purified PT, 0.25 μg; lane 2, BP536Δptx(pTH18)(pTH19) supernatant; lane 3, BP536Δptx(pTH18)(pTH19) cell extract; lane 4, BP536 supernatant; lane 5, BP536 cell extract. (B) Lane 1, purified PT, 0.25 μg; lane 2, BP536Δptxptl(pTH18)(pTH19) supernatant; lane 3, BP536Δptxptl(pTH18)(pTH19) cell extract.
In order to verify that the secretion of PT from BP536Δptx(pTH18)(pTH19) was mediated by the Ptl proteins, we introduced pTH18 and pTH19 into the strain BP536Δptxptl, which contains an 11.5-kb deletion of the ptx-ptl region and thus does not have a functional Ptl system. As expected, when both S1 and the B oligomer were expressed in BP536Δptxptl, the S2 remained cell associated; none was detected in the culture supernatant (Fig. 2B).
In the absence of S1, the B oligomer is inefficiently secreted.
Next, by immunoblot analysis, we examined secretion of S2 in BP536Δptx containing only pTH18, the plasmid into which the genes encoding the subunits of the B oligomer were inserted. This strain, BP536Δptx(pTH18), produces a functional Ptl system and the B oligomer subunits but no S1. To optimize our ability to detect S2 in the culture supernatant, we loaded four times more supernatant relative to cellular material on the SDS-polyacrylamide gel used for the immunoblot. As shown in Fig. 3, in BP536Δptx(pTH18), S2 was readily detected in the cellular material (lane 3) but was barely detectable in the supernatant (lane 2). Compared with wild-type B. pertussis, secretion of S2 in BP536Δptx(pTH18) was extremely inefficient (Fig. 3; compare lanes 2 and 3 to lanes 6 and 7). As expected, when pTH18 was introduced into BP536Δptxptl, S2 was not detected in the supernatant; all of the S2 remained cell associated (Fig. 3, lanes 4 and 5). When we examined BP536Δptx(pTH18) and BP536Δptxptl(pTH18) for secretion of S4 using monoclonal antibody 6DX3 (18) as a probe, we obtained results analogous to those for S2 (data not shown). Our findings indicate that in the absence of S1, secretion of the B oligomer is extremely inefficient.
FIG. 3.
Immunoblot analysis of S2 in cell extracts and culture supernatants of B. pertussis strains. Samples of culture supernatants (600 μl) and cell extracts (150 μl) were prepared as described in Materials and Methods. Samples were then subjected to SDS-polyacrylamide gel electrophoresis and immunoblot analysis using monoclonal antibody P11B10 to visualize the S2 subunit of PT. Positions of molecular size markers are indicated at the right in kilodaltons. The arrow indicates the protein band corresponding to S2. Lane 1, purified PT, 0.25 μg; lane 2, BP536Δptx(pTH18) supernatant; lane 3, BP536Δptx(pTH18) cell extract; lane 4, BP536Δptxptl(pTH18) supernatant; lane 5, BP536Δptxptl(pTH18) cell extract; lane 6, BP536 supernatant; lane 7, BP536 cell extract.
In the absence of the B oligomer, S1 is not secreted by the Ptl transport system.
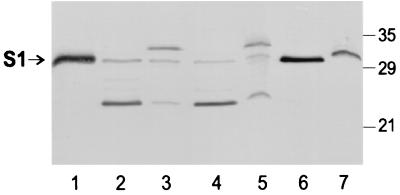
We also examined secretion of S1 from BP536Δptx and BP536Δptxptl, each harboring pDMC36, a plasmid into which was inserted the coding sequence for the ptx-ptl promoter region followed by ptxS1. The backbone used for this plasmid was the broad-host-range vector pUFR047, the same as that used for pTH18. As before, to optimize our ability to detect S1 in the culture supernatant, we loaded four times more supernatant relative to cellular material on the SDS-polyacrylamide gel used for the immunoblot. In wild-type B. pertussis, BP536, full-length S1 was readily detected in the culture supernatant (Fig. 4, lane 6). In the wild-type strain, the efficiency of secretion of S1 (Fig. 4, lanes 6 and 7) was similar to that observed for the B oligomer (Fig. 2A, lanes 4 and 5). Cultures of BP536Δptx(pDMC36), which should express S1 and the Ptl proteins, but not the B oligomer, exhibited S1 in both the culture supernatant and cellular material (Fig. 4, lanes 2 and 3). In this strain, relatively little full-length S1 was detected. The latter finding was not surprising because S1 is known to be susceptible to proteolysis, particularly when not associated with the B oligomer (6). A lower-molecular-weight form of S1 that likely represents a commonly observed degradation product (6) was the predominant form of S1 observed in the supernatant (Fig. 4, lane 2); a small amount of this degradation product was also observed in the cellular material (Fig. 4, lane 3). The predominant form of S1 observed in the cellular material migrated slightly slower than full-length S1 (Fig. 4, lane 3). This form of S1 has been observed previously in a wild-type strain of B. pertussis carrying a plasmid encoding S1 (20) and may represent a form of S1 in which the signal peptide, which has a predicted molecular weight of ∼3,800, was not removed because high-level production from the plasmid may have exceeded the normal processing capability of the cell. By visual examination, the total amount of S1 detected in 400 μl of the supernatant of BP536Δptx(pDMC36) was at least as great as the total amount detected in 100 μl of the cell extract (Fig. 4, compare lanes 2 and 3), indicating that at least 20% of the total S1 content of the culture was found in the supernatant. In order to determine whether S1 observed in the supernatant was actively secreted by a Ptl-mediated mechanism, we examined cultures of BP536Δptxptl(pDMC36). We found that the relative amounts of the various forms of S1 in the supernatant and cellular material were identical to that seen for BP536Δptx(pDMC36) (Fig. 4, lanes 4 and 5). Since BP536Δptxptl(pDMC36) lacks the Ptl transport system, any S1 that had been released into the supernatant could not have been secreted via the Ptl transport system. This finding indicated that even though BP536Δptx(pDMC36) produces a functional Ptl transport system, the S1 observed in the culture supernatant of this strain is not secreted via the Ptl system.
FIG. 4.
Immunoblot analysis of S1 in cell extracts and culture supernatants of B. pertussis strains. Samples of culture supernatants (400 μl) and cell extracts (100 μl) were prepared as described in Materials and Methods. Samples were then subjected to SDS-polyacrylamide gel electrophoresis and immunoblot analysis using monoclonal antibody 3CX4 to visualize the S1 subunit of PT. Positions of molecular size markers are indicated at the right in kilodaltons. The arrow indicates the protein band corresponding to full-length S1. Lane 1, purified PT, 0.25 μg; lane 2, BP536Δptx(pDMC36) supernatant; lane 3, BP536Δptx(pDMC36) cell extract; lane 4, BP536Δptxptl(pDMC36) supernatant; lane 5, BP536Δptxptl(pDMC36) cell extract; lane 6, BP536 supernatant; lane 7, BP536 cell extract.
We next set out to address whether the amount of S1 that we observed in the culture supernatant of strains that did not exhibit Ptl-mediated secretion could be accounted for by lysis of the cells. In order to examine this question, we utilized strain BPM3171, which has a transposon inserted in ptlC and therefore lacks a functional Ptl transport system (30, 31). Examination of the supernatant and cellular fraction of BPM3171 indicated that the ratio of S1 in the supernatant compared to the cellular fraction was similar to that observed with BP536Δptx(pDMC36); that is, approximately 20% of the total S1 subunit content was found in the supernatant (data not shown). We next introduced a plasmid which expresses the phoA gene encoding alkaline phosphatase, an enzyme that normally localizes to the bacterial periplasmic space, into this strain to assess cell lysis. Using the same conditions described above, we obtained cellular material and the supernatant from a culture of BPM3171 expressing phoA and examined serial dilutions of these fractions for alkaline phosphatase activity. We detected alkaline phosphatase activity in both the cellular material and supernatant, at a relative ratio of approximately 8:1. This finding indicated that at least some of the S1 detected in the culture supernatant of B. pertussis strains lacking a functional Ptl transport system may be due to cell lysis.
DISCUSSION
In this study, we developed a dual plasmid system to study the requirement of holotoxin assembly in Ptl-mediated secretion of PT. Using this system, we were able to examine the secretion of the S1 subunit or the B oligomer, individually, in a strain that contains a functional Ptl system. Our results indicate that only the holotoxin form of PT is efficiently released from the bacteria by a Ptl-mediated mechanism. Neither the individual S1 subunit nor the B oligomer is properly exported by the Ptl system in the absence of the other.
Our work also suggests that secretion of PT from B. pertussis may not be an efficient process in that significant quantities of PT subunits remained cell associated, even in wild-type strains of B. pertussis. As seen in Fig. 2A, as much as 50 to 75% of the toxin subunits remained cell associated in the wild-type strain (note that four times more supernatant volume was loaded onto the polyacrylamide gel compared to cellular material). Similar ratios of secreted versus cell-associated PT have been reported previously (20, 30).
In the absence of the S1 subunit, the B oligomer is very inefficiently secreted. Previously, two groups of investigators addressed the question of whether the B oligomer can be secreted in the absence of S1 but reported conflicting results. Both studies were conducted before the discovery of the Ptl system; therefore, neither group investigated the role of the Ptl proteins in the secretion process. Pizza et al. reported that the S1 subunit is required for efficient secretion of PT (24). They constructed B. pertussis mutants encoding an S1 subunit with one or more amino acid substitutions which altered the conformation of the S1 subunit and decreased its stability substantially. In these strains, the subunits comprising the B oligomer were secreted into the culture medium with much lower efficiency compared to PT in wild-type B. pertussis. In contrast, Antoine and Locht (1) reported that the B oligomer was efficiently secreted in the culture medium in the absence of the S1 subunit. These investigators constructed B. pertussis mutants with either an alteration in the cysteine residue at position 41 of S1 or with a deletion in the carboxy-terminal region of S1. The carboxy-terminal deletions encompassed as many as 48 amino acid residues, or approximately 20% of the mature form of S1, after cleavage of the N-terminal signal peptide. In all of the mutant strains, S1 could not be detected, but the S2 subunit, presumably as a component of the B oligomer, was efficiently synthesized and readily detected in the culture supernatant. Our results are consistent with those of Pizza et al. (24). Several potential explanations for the discrepancies in results exist. For example, in the study by Antoine and Locht, while the mutant S1 subunits could not be detected (1), the possibility exists that they transiently associated with the B oligomer for amounts of time sufficient for export to occur and were then rapidly degraded. Also, these workers did not examine whether significant cell lysis might have occurred under the growth conditions that were used.
In this study, we also determined that in the absence of the B oligomer, S1 is not exported by the Ptl system. In strains lacking the B oligomer, we observed identical partitioning of S1 in culture fractions as well as identical profiles of the different forms of S1, whether or not the Ptl proteins were produced. While it appears from Fig. 4 that significant amounts of S1 were found in the culture supernatants of strains lacking the B oligomer, these findings must be interpreted cautiously. We have shown that some of the S1 observed in the supernatant of BP536Δptx(pDMC36) was likely released by cell lysis. In contrast to this strain, in BP536Δptx(pTH18), cell lysis (if it occurred) did not cause an apparent release of the B oligomer into the culture supernatant. It is possible that upon cell lysis, the B oligomer nonspecifically adhered to the surface of the cells. Alternatively, expressing S1 from a plasmid may be more detrimental to the cells than expressing the B oligomer from a plasmid. The detection of S1 in the culture supernatant of strains lacking the B oligomer also must be interpreted in view of the lack of stability of the S1 subunit. The concentrations of proteases within the bacterial cell are likely much higher than those in the supernatant. Therefore, S1 may be rapidly degraded within the cell, but if released, possibly by cell lysis, its lifetime could increase significantly, giving a false impression of the relative amount of S1 secreted compared to that which remains cell associated. Moreover, previous work has demonstrated that the Ptl system is critical for export of S1 when it is part of the holotoxin (20). Finally, it appears that the S1 subunit and the B oligomer combine to form the holotoxin while still cell associated since the pattern of cell-associated S1 and its proteolytic fragments differ depending on whether the B oligomer is present. In the presence of the B oligomer, as in the wild-type B. pertussis strain shown in Fig. 4, lane 7, only full-length S1 was observed in the cellular material. In contrast, in strains lacking the B oligomer, proteolysis of cell-associated S1 was apparent (Fig. 4, lanes 3 and 5). Therefore, it seems unlikely that S1 would normally be secreted by a non-Ptl-mediated mechanism independently of the B oligomer and then combine with the B oligomer in the culture supernatant only after secretion of both moieties of the toxin.
In summary, we have found that the Ptl system must be capable of exporting a multisubunit complex in that only the assembled form of the toxin is efficiently released from the bacterial cell via a Ptl-mediated mechanism. Individual components of the toxin are not effectively secreted by this mechanism. These studies shed light on the structural requirements for the secretion of PT. Further studies are needed to ascertain the detailed nature of interactions that may occur between PT and the Ptl system.
ACKNOWLEDGMENTS
We thank David Cook for providing the strain BP536Δptxptl and Sally Hausman for assistance with construction of the strain BP536Δptx.
REFERENCES
- 1.Antoine R, Locht C. Roles of the disulfide bond and the carboxy-terminal region of the S1 subunit in the assembly and biosynthesis of pertussis toxin. Infect Immun. 1990;58:1518–1526. doi: 10.1128/iai.58.6.1518-1526.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barry E M, Weiss A A, Ehrmann I E, Gray M C, Hewlett E L, Goodwin M S M. Bordetella pertussis adenylate cyclase toxin and hemolytic activities require a second gene, cyaC, for activation. J Bacteriol. 1991;173:720–726. doi: 10.1128/jb.173.2.720-726.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brickman E, Beckwith J. Analysis of the regulation of Escherichia coli alkaline phosphatase synthesis using deletions and φ80 transducing phages. J Mol Biol. 1975;96:307–316. doi: 10.1016/0022-2836(75)90350-2. [DOI] [PubMed] [Google Scholar]
- 4.Bullock J O, Armstrong S K, Shear J L, Lies D P, McIntosh M A. Formation of ion channels by colicin B in planar lipid bilayers. J Membr Biol. 1990;114:79–95. doi: 10.1007/BF01869387. [DOI] [PubMed] [Google Scholar]
- 5.Burnette W N. “Western blotting”: electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981;112:195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- 6.Burns D L, Hausman S Z, Lindner W, Robey F A, Manclark C R. Structural characterization of pertussis toxin A subunit. J Biol Chem. 1987;262:17677–17682. [PubMed] [Google Scholar]
- 7.Christie P J. Agrobacterium tumefaciens T-complex transport apparatus: a paradigm for a new family of multifunctional transporters in eubacteria. J Bacteriol. 1997;179:3085–3094. doi: 10.1128/jb.179.10.3085-3094.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cook D M, Farizo K M, Burns D L. Identification and characterization of PtlC, an essential component of the pertussis toxin secretion system. Infect Immun. 1999;67:754–759. doi: 10.1128/iai.67.2.754-759.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeFeyter R, Yang Y, Gabriel D W. Gene-for-genes interactions between cotton R genes and Xanthomonas campestris pv. malvacearum avr genes. Mol Plant-Microbe Interact. 1993;6:225–237. doi: 10.1094/mpmi-6-225. [DOI] [PubMed] [Google Scholar]
- 10.Figurski D H, Helinski D R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA. 1979;76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frank D W, Parker C D. Interaction of monoclonal antibodies with pertussis toxin and its subunits. Infect Immun. 1984;46:195–201. doi: 10.1128/iai.46.1.195-201.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilman A G. G proteins: transducers of receptor generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- 13.Hausman S Z, Cherry J D, Heininger U, Wirsing von Konig C H, Burns D L. Analysis of proteins encoded by the ptx and ptl genes of Bordetella bronchiseptica and Bordetella parapertussis. Infect Immun. 1996;64:4020–4026. doi: 10.1128/iai.64.10.4020-4026.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson F D, Burns D L. Detection and subcellular localization of three Ptl proteins involved in the secretion of pertussis toxin from Bordetella pertussis. J Bacteriol. 1994;176:5350–5356. doi: 10.1128/jb.176.17.5350-5356.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katada T, Tamura M, Ui M. The A promoter of islet-activating protein, pertussis toxin, as an active peptide catalyzing ADP-ribosylation of a membrane protein. Arch Biochem Biophys. 1983;224:290–298. doi: 10.1016/0003-9861(83)90212-6. [DOI] [PubMed] [Google Scholar]
- 16.Katada T, Ui M. Direct modification of the membrane adenylate cyclase system by islet-activating protein due to ADP-ribosylation of a membrane protein. Proc Natl Acad Sci USA. 1982;79:3129–3133. doi: 10.1073/pnas.79.10.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keen N T, Tamaki S, Kobayashi D, Trollinger D. Improved broad-host-range plasmids for DNA cloning in Gram-negative bacteria. Gene. 1988;70:191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- 18.Kenimer J G, Kim K J, Probst P G, Manclark C R, Burstyn D G, Cowell J L. Monoclonal antibodies to pertussis toxin: utilization as probes of toxin function. Hybridoma. 1989;8:37–51. doi: 10.1089/hyb.1989.8.37. [DOI] [PubMed] [Google Scholar]
- 19.Kim K J, Burnette W N, Sublett R D, Manclark C R, Kenimer J G. Epitopes on the S1 subunit of pertussis toxin recognized by monoclonal antibodies. Infect Immun. 1989;57:944–950. doi: 10.1128/iai.57.3.944-950.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kotob S I, Burns D L. Essential role of the consensus nucleotide-binding site of PtlH in secretion of pertussis toxin from Bordetella pertussis. J Bacteriol. 1997;179:7577–7580. doi: 10.1128/jb.179.23.7577-7580.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 22.Locht C, Keith J M. Pertussis toxin gene: nucleotide sequence and genetic organization. Science. 1986;232:1258–1264. doi: 10.1126/science.3704651. [DOI] [PubMed] [Google Scholar]
- 23.Nicosia A, Perugini M, Franzini C, Casagli M C, Borri M G, Antoni G, Almoni M, Neri P, Ratti G, Rappuoli R. Cloning and sequencing of the pertussis toxin genes: operon structure and gene duplication. Proc Natl Acad Sci USA. 1986;83:4631–4635. doi: 10.1073/pnas.83.13.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pizza M, Bugnoli M, Manetti R, Covacci A, Rappuoli R. The subunit S1 is important for pertussis toxin secretion. J Biol Chem. 1990;265:17759–17763. [PubMed] [Google Scholar]
- 25.Simon R, Priefer U, Puhler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Bio/Technology. 1983;1:784–790. [Google Scholar]
- 26.Stein P E, Boodhoo A, Armstrong G D, Cockle S A, Klein M H, Read R J. The crystal structure of pertussis toxin. Structure. 1994;2:45–57. doi: 10.1016/s0969-2126(00)00007-1. [DOI] [PubMed] [Google Scholar]
- 27.Stibitz S. Use of conditionally counterselectable suicide vectors for allelic exchange. Methods Enzymol. 1994;235:458–465. doi: 10.1016/0076-6879(94)35161-9. [DOI] [PubMed] [Google Scholar]
- 28.Stibitz S, Yang M-S. Subcellular localization and immunochemical detection of proteins encoded by the vir locus of Bordetella pertussis. J Bacteriol. 1991;173:4288–4296. doi: 10.1128/jb.173.14.4288-4296.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tamura M, Nogimori K, Murai S, Yajima M, Ito K, Katada T, Ui M, Ishii S. Subunit structure of islet-activating protein, pertussis toxin, in conformity with the A-B model. Biochemistry. 1982;21:5516–5522. doi: 10.1021/bi00265a021. [DOI] [PubMed] [Google Scholar]
- 30.Weiss A A, Johnson F D, Burns D L. Molecular characterization of an operon required for pertussis toxin secretion. Proc Natl Acad Sci USA. 1993;90:2970–2974. doi: 10.1073/pnas.90.7.2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weiss A A, Melton A R, Walker K E, Andraos-Selim C, Meidl J J. Use of the promoter fusion transposon Tn5 lac to identify mutations in Bordetella pertussis vir-regulated genes. Infect Immun. 1989;57:2674–2682. doi: 10.1128/iai.57.9.2674-2682.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winans S C, Burns D L, Christie P J. Adaptation of a conjugal transfer system for the export of pathogenic macromolecules. Trends Microbiol. 1996;4:64–68. doi: 10.1016/0966-842X(96)81513-7. [DOI] [PMC free article] [PubMed] [Google Scholar]