Abstract
In recent years, the number of cancer survivors has been increasing each year due to advances in the early diagnosis and treatment of cancer. Cancer survivors present a variety of physical and psychological complications due to cancer and its treatment. Physical exercise is an effective nonpharmacological treatment for complications in cancer survivors. Furthermore, recent evidence has shown that physical exercise improves the prognosis of cancer survivors. The benefits of physical exercise have been widely reported, and guidelines for physical exercise for cancer survivors have been published. These guidelines recommend that cancer survivors engage in moderate- or vigorous-intensity aerobic exercises and/or resistance training. However, many cancer survivors have a poor commitment to physical exercise. In the future, it is necessary to promote physical exercise among cancer survivors through outpatient rehabilitation and community support.
Keywords: Cancer survivor, Physical exercise, Complication, Prognosis
The number of cancer cases is increasing every year. It is estimated that 19.3 million people worldwide were diagnosed with cancer in 20201) and 28.4 million people will be newly diagnosed in 20401). In Japan, there were approximately 1,012,000 new cancer cases in 20202). In recent years, advances in cancer screening and treatment have led to improved survival rates and an increase in the number of cancer survivors2,3). Furthermore, about half of the growing number of cancer survivors are elderly adults2). The number of elderly cancer survivors will continue to grow as the population ages.
Complications for Cancer Survivors
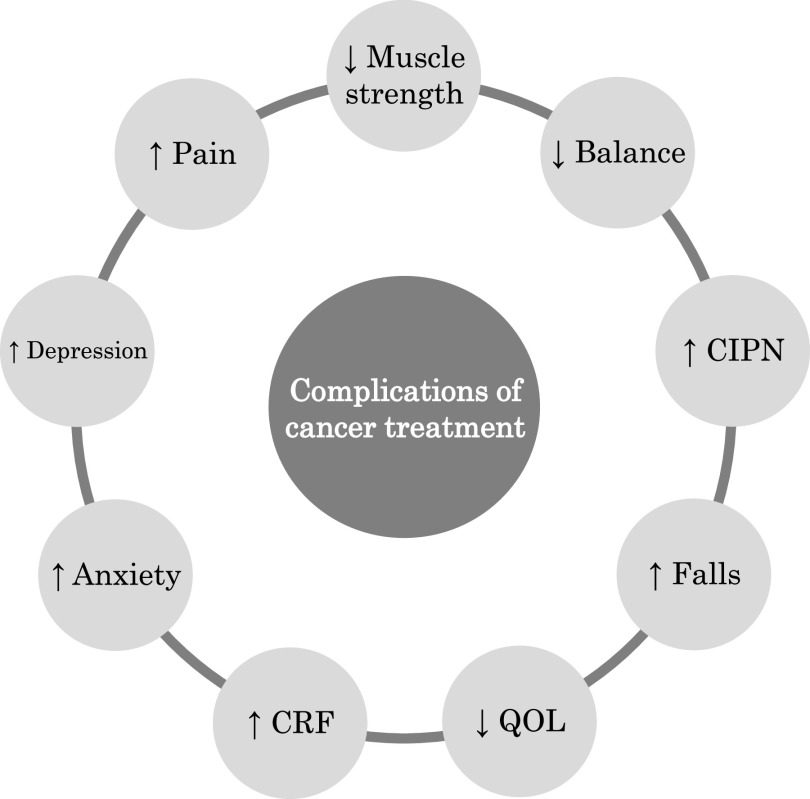
Cancer treatment is selected based on the type of tumor, site, stage, and pathological diagnosis. In addition, cancer treatment can affect nontarget tissues4) and cancer cells also adversely affect bodily functions5). Therefore, cancer survivors experience various complications due to the effects of cancer and its treatment (Fig. 1).
Fig. 1. Various complications of cancer treatment in cancer survivors.
CIPN, chemotherapy-induced peripheral neuropathy; QOL, quality of life; CRF, cancer-related fatigue
A previous study has reported breast cancer survivors to have lower upper body muscle strength than healthy controls6). Another study reported that 26 cancer survivors had significantly lower hand grip strength than age-matched healthy controls7). These reports indicate that cancer survivors may have reduced muscle strength compared to healthy controls.
Cancer survivors may also have impaired balance function. In a study by Schmitt et al., cancer survivors exhibited longer mediolateral root mean square and increased center of pressure velocity compared to healthy controls8). Another previous study reported that cancer survivors had lower Mini-Balance Evaluation Systems Test scores and longer Timed Up and Go test times compared to healthy controls7,9). With eyes open, the area of the center of pressure was significantly larger in cancer survivors than in healthy controls9). These findings indicate that cancer survivors have impaired balance function compared to healthy individuals. This lower balance function in cancer survivors may be related to chemotherapy-induced peripheral neuropathy (CIPN). CIPN presents primarily with sensory and motor neuropathy in the hands and feet10). A meta-analysis of 31 studies of 4179 cancer survivors showed that the prevalence of CIPN is 68.1% at one month, 60.0% at three months, and 30.0% at six months after chemotherapy11). CIPN also negatively affects physical functions such as upper and lower extremity function12,13), gait12,14), and balance function14,15), and is one of the main concerns of cancer survivors.
Cancer survivors present with various physical symptoms, including pain, fatigue, and anxiety. The prevalence of pain in cancer survivors is 39.3% after curative treatment, 55.0% during anticancer therapy, and 66.4% during advanced/metastatic/terminally ill stages16). Cancer-related fatigue (CRF) is prevalent among cancer survivors and may continue to be experienced long after treatment17). Additionally, a study of the prevalence of anxiety and depression in 1154 adult cancer survivors showed that at six months post diagnosis, the prevalence of anxiety and depression was 22% and 13%, respectively, and then 21% and 13%, respectively, at 12 months18).
Cancer survivors’ complications negatively affect their quality of life (QOL). Previous studies have shown that cancer survivors have a lower QOL than healthy controls19), and there is a significant association between muscle strength and QOL19). Kober et al. reported that cancer survivors with peripheral neuropathy had poorer balance function and lower QOL scores than those without peripheral neuropathy, particularly in the physical function domain13). On the other hand, another study reported that balance function in cancer survivors might have little effect on QOL20). Therefore, cancer survivors’ muscle strength may more significantly impact their QOL than their balance function.
Elderly cancer survivors, as well as adult cancer survivors, present a variety of complications. In a previous study, 3766 elderly endometrial cancer survivors had more difficulty with walking and/or balance than an age- and race-matched group of women with no cancer history21). Another report showed that elderly breast cancer survivors had lower short physical performance battery scores, longer chair stand times, and lower grip strength than women of the same age without cancer22). Thus, elderly cancer survivors may have poorer physical function than healthy elderly adults.
Falls are a significant problem for elderly cancer survivors. A systematic review of falls in elderly cancer survivors reported a fall rate of 1.52%–3.41% per 1000 patient days for inpatients and 39%–64% for outpatients23). Furthermore, falls in elderly cancer survivors have been shown to impact subsequent cancer treatment negatively23).
In summary, adult cancer survivors present with various complications and reduced QOL due to cancer and its treatment. In addition, elderly cancer survivors have poor physical function and a higher risk of falls.
Effects of Physical Exercise on Complications
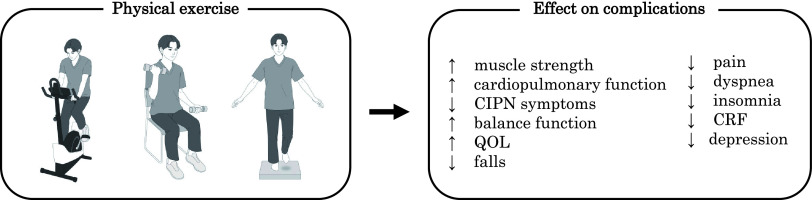
Physical exercise is an effective nonpharmacological treatment for complications in cancer survivors (Fig. 2). A systematic review of 16 randomized controlled trials (RCTs) of cancer survivors undergoing active treatment showed that physical exercise, including aerobic exercise and/or resistance training, improved muscle strength more than usual care24). Another meta-analysis, including 48 RCTs with cancer survivors (n = 3632), showed that physical exercise significantly improved cardiopulmonary function25). In that study, 56% of physical exercise was aerobic25).
Fig. 2. Effects of physical exercise on complications in cancer survivors.
CIPN, chemotherapy-induced peripheral neuropathy; QOL, quality of life; CRF, cancer-related fatigue
The effectiveness of physical exercise for CIPN has also been evaluated. A systematic review and meta-analysis of five RCTs found that physical exercise significantly improved mean CIPN scores26). The physical exercises included resistance, balance, sensorimotor-based, and nerve gliding exercises26). In contrast, another meta-analysis found no effect of physical exercise on CIPN symptoms27). That study showed that physical exercise significantly positively affected physical function (muscle strength, balance function) and QOL in cancer survivors with CIPN27).
The effects of physical exercise on physical symptoms in cancer survivors have been investigated. A meta-analysis of 10 RCTs found that physical exercise significantly improved physical symptoms such as fatigue, pain, dyspnea, and insomnia in cancer survivors compared to a healthy control group28). The types of physical exercise included aerobic, resistance, stretching, and walking28). A meta-analysis on the effects of pharmacotherapy, psychotherapy, and physical exercise on CRF was conducted29), and showed that physical exercise significantly improved CRF compared to other treatments29). In studies of physical exercise (n = 69), 36 involved aerobic exercises, 13 involved anaerobic exercises, and 20 involved a combination of aerobic and anaerobic exercises29). Another meta-analysis of 37 RCTs of 2929 cancer survivors showed that physical exercise reduced depressive symptoms compared to standard treatment30). Exercise modalities included walking, yoga, stationary cycling, resistance bands, and weight machines30).
Several studies have reported that physical exercise is an effective intervention for improving the QOL of cancer survivors. A meta-analysis of 16 RCTs showed that physical exercise significantly improved the QOL of cancer survivors compared to usual care31). Outcomes associated with QOL, such as fatigue and physical function, were also reported to improve with physical exercise31). In addition, another meta-analysis showed that physical exercise for cancer survivors during and after treatment significantly improved QOL and physical function compared to that for the control group32). Among the forms of exercise delivery in that study, supervised physical exercise was shown to have the largest positive impact on QOL and physical function32). Another study investigated the effects of physical exercise on various domains of cancer survivors’ QOL33), and the results showed that physical exercise improved global QOL, physical QOL, role QOL, and emotional QOL33).
There is a lack of evidence on the effect of physical exercise on falls, a concern in elderly cancer survivors34). Currently, trials of physical exercise interventions are being tested in elderly cancer survivors to assess their ability to prevent falls35).
In summary, many studies have suggested that physical exercise may improve muscle strength, cardiopulmonary function, CIPN symptoms, balance function, pain, dyspnea, insomnia, CRF, depression, and QOL in cancer survivors. Further research is needed on the effects of physical exercise on falls.
Prognostic Impact of Physical Exercise
Physical exercise has been shown to improve prognosis in cancer survivors (Fig. 3). Studies in the 2000s showed that physical activity improved the prognosis of breast and colorectal cancer survivors. For example, participation in physical activity after diagnosis reduced recurrence by 24% and mortality by 45% in breast cancer survivors36). Similarly, recurrence and mortality were reduced by 40% and 63%, respectively, among colorectal cancer survivors37).
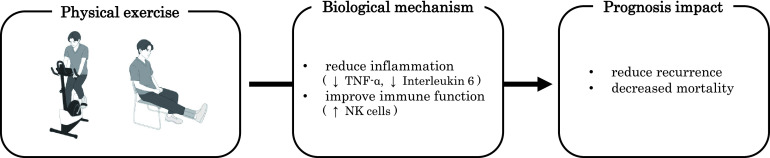
Fig. 3. Prognostic impact of physical exercise.
TNF-α, tumor necrosis factor-α; NK, natural killer
Since the 2010s, more systematic reviews and meta-analyses have been reported. A meta-analysis of 136 studies found evidence that cancer survivors with higher physical activity levels after diagnosis had reduced cancer-specific mortality38). In addition, a meta-analysis of RCTs on physical exercise among cancer survivors has been investigated39). Eight RCTs were included in the meta-analysis, and interventions were roughly classified into 2 categories: short-term interventions in the hospital (two weeks in duration) and outpatient or home-based interventions (2–8 months in duration)9). All physical exercises were aerobic and/or resistance training39). A meta-analysis revealed that physical exercise reduced cancer survivors’ risk of recurrence by about 48% and the risk of mortality by about 24%39).
The biological mechanisms for the prognostic effects of physical exercise have not yet been established. In 2016, the antitumor effects of physical exercise were demonstrated in animal studies40). In a mouse model, six weeks of physical exercise reduced the incidence and growth of several tumors (melanoma, liver cancer, and lung cancer)40). In addition, physical exercise was shown to regulate tumor growth by increasing the number of natural killer (NK) cells40). The mobilization of NK cells involved interleukin-6, released from muscles as a result of physical exercise40).
Studies of cancer survivors suggest that physical exercise may maintain NK cell function41). However, there is currently limited evidence to support the effects of physical exercise on NK cells in cancer survivors42). On the other hand, evidence for an anti-inflammatory effect of physical exercise in cancer survivors is becoming more evident43). A meta-analysis of 26 RCTs showed that physical exercise reduces inflammatory markers, particularly C-reactive protein levels and tumor necrosis factor-α production43). The anti-inflammatory effects of physical exercise were more pronounced in prostate and breast cancer survivors43), and the most effective physical exercise program was a combination of aerobic and resistance training43).
In summary, physical exercise has been shown to influence the prognosis of cancer survivors. Recently, meta-analyses using RCTs, not only cohort studies, have been conducted, and the evidence in this area is growing. On the other hand, the biological mechanisms of the exercise effect have not been established and require further study. Cancer recurrence is a significant concern for most cancer survivors44). Therefore, physical therapists need to provide physical exercise to improve the prognosis of cancer survivors in the future.
Physical Exercise Guidelines for Cancer Survivors
The American Cancer Society (ACS) and the American College of Sports Medicine (ACSM) have published recommendations on physical exercise for cancer survivors45–49). This chapter presents several guidelines (Fig. 4).
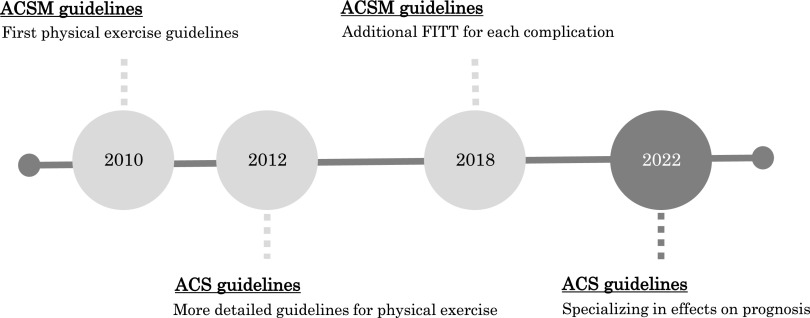
Fig. 4. History of physical exercise guidelines for cancer survivors.
ACSM, American College of Sports Medicine; FITT, frequency, intensity, time, and type; ACS, American Cancer Society
In 2010, the ACSM developed the first physical exercise guidelines for cancer survivors46). These guidelines reported strong evidence that physical exercise improves physical fitness, physical functioning, QOL, and CRF. However, there was insufficient evidence to establish a recommended amount of physical exercise. Therefore, based on the Physical Activity Guidelines for Americans, the guidelines recommend at least 150 minutes of aerobic exercise per week and resistance training at least two days per week.
In 2012, the ACS published more detailed recommendations for physical exercise47). Adult cancer survivors are recommended to perform 150 minutes per week of aerobic exercise at a moderate intensity or 75 minutes per week at a vigorous intensity, plus resistance training for major muscle groups at least two days per week. Elderly cancer survivors should follow the recommendations of adult cancer survivors if possible, but if there are limitations, it is recommended that they exercise whenever possible and avoid prolonged physical inactivity.
In 2019, the ACSM released new physical exercise recommendations for cancer survivors48). These guidelines provided recommendations and frequency, intensity, time, and type (FITT) for various complications in cancer survivors. Specifically, they reported strong evidence to support the effectiveness of physical exercise for anxiety, depressive symptoms, CRF, health-related QOL, lymphedema, and physical function. The guidelines also provided moderate evidence for bone health and sleep and insufficient evidence for cardiotoxicity, CIPN, cognitive function, falls, nausea, pain, sexual function, and treatment tolerance. For FITT, the introductions focused mainly on outcomes in the strong evidence category. For most, 30 minutes of aerobic exercise three times a week is an effective exercise prescription. Resistance training, which consists of two or more sets of 8–15 repetitions at 60% or more of the maximum number of repetitions per session at least twice a week, has also been equally effective. Because cancer survivors have sequelae and comorbidities, individualized goals are recommended.
In 2022, the ACS published the latest recommendations for physical exercise49). The purpose of these ACS guidelines was to provide recommendations for physical exercise (including nutrition) to improve prognosis. For physical exercise, the studies included systematic reviews, meta-analyses, cohort studies, and RCTs (sample sizes of at least 200 participants) published in 2018 or later. The review revealed that nine studies met the inclusion criteria and that physical exercise is recommended for breast, colorectal, and prostate cancer survivors to improve prognosis. There is currently limited evidence for the prognostic impact of physical exercise on survivors of gynecologic, lung, hematologic, and pediatric cancers. In addition, these guidelines recommend 150–300 minutes per week for adult cancer survivors at moderate intensity, 75–150 minutes of physical exercise at vigorous intensity, and resistance training at least two days per week. Children and adolescents should perform at least one hour of moderate- or vigorous-intensity physical exercise daily. Compared to the 2012 ACS guidelines, the recommended amount of physical exercise has been increased, and recommendations for cancer survivors in the adolescent and young adult generation have been added.
In summary, the guidelines for physical exercise for cancer survivors recommend that physical exercise should include aerobic exercise, resistance training, or a combination of both. In recent years, the guidelines have also shown that physical exercise can improve the prognosis of cancer survivors. Recommended physical exercise is provided, but cancer survivors have various complications and should be evaluated individually.
Future Directions
Physical exercise is a critical nonpharmacological therapy to improve complications and prognosis in cancer survivors. However, most cancer survivors lack compliance with physical exercise50–52). A previous study reported that in 102 cancer survivors, the amount of physical exercise significantly decreased after cancer diagnosis and remained decreased during treatment50). Another report indicated that only 38% of 72 cancer survivors were able to meet 90 to 150 minutes of moderate to vigorous exercise per week and only 10% were able to perform resistance training twice per week51). The amount of physical exercise over ten years for 631 breast cancer survivors was also studied, and the results showed that 7.8% met the guidelines for physical exercise at all follow-up periods52). Thus, many cancer survivors lack compliance with physical exercise.
ACSM guidelines recommend supervised physical exercise for cancer survivors48). However, in Japan, outpatient rehabilitation for cancer survivors is not covered by medical fees, and the implementation rate of outpatient rehabilitation is 39.1%53). Therefore, a medical fee revision is needed to promote outpatient cancer rehabilitation.
Elderly cancer survivors have limited mobility54). Therefore, community support is essential for cancer survivors. However, only 39.1% of the hospitals in Japan designated as cooperative cancer treatment centers have regional cooperation53), and only 9.8% have established a regional cooperation path53). In the future, regional cooperation should be promoted to support cancer survivors regarding physical exercise.
Conclusions
Cancer survivors present not only physical complications, such as low muscle strength and balance function, but also psychological complications, such as anxiety, depression, and CRF, as a result of cancer and its treatment. Furthermore, these complications negatively affect QOL. Elderly cancer survivors likewise present a variety of complications, and falls are a significant problem. Physical exercise can improve physical and psychological complications in cancer survivors. Recently, physical exercise has even been shown to improve the prognosis of cancer survivors. The ACSM and ACS guidelines for cancer survivors recommend physical exercises such as aerobic exercise and resistance training to improve complications and prognosis. However, most cancer survivors lack compliance with physical exercise. To promote physical exercise among cancer survivors, inpatient rehabilitation alone is insufficient. In the future, outpatient rehabilitation and community support should be used to promote physical exercise among cancer survivors after discharge.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1). Sung H, Ferlay J, et al.: Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71: 209–249. [DOI] [PubMed] [Google Scholar]
- 2). National Cancer Center [Internet] . Cancer statistics in Japan-2021 [updated 2021 Apr 23; cited 2022 Sep 13] Available from: https://ganjoho.jp/public/qa_links/report/statistics/pdf/cancer_statistics_2021.pdf (in Japanese) [Google Scholar]
- 3). Matsuda T, Ajiki W, et al.: Population-based survival of cancer patients diagnosed between 1993 and 1999 in Japan: a chronological and international comparative study. Jpn J Clin Oncol. 2011; 41: 40–51. [DOI] [PubMed] [Google Scholar]
- 4). Chen Y, Jungsuwadee P, et al.: Collateral damage in cancer chemotherapy: oxidative stress in nontargeted tissues. Mol Interv. 2007; 7: 147–156. [DOI] [PubMed] [Google Scholar]
- 5). Fearon KC: Cancer cachexia and fat-muscle physiology. N Engl J Med. 2011; 365: 565–567. [DOI] [PubMed] [Google Scholar]
- 6). Harrington S, Padua D, et al.: Comparison of shoulder flexibility, strength, and function between breast cancer survivors and healthy participants. J Cancer Surviv. 2011; 5: 167–174. [DOI] [PubMed] [Google Scholar]
- 7). Morishita S, Hirabayashi R, et al.: Assessment of the mini-balance evaluation systems test, timed up and go test, and body sway test between cancer survivors and healthy participants. Clin Biomech (Bristol, Avon). 2019; 69: 28–33. [DOI] [PubMed] [Google Scholar]
- 8). Schmitt AC, Repka CP, et al.: Comparison of posture and balance in cancer survivors and age-matched controls. Clin Biomech (Bristol, Avon). 2017; 50: 1–6. [DOI] [PubMed] [Google Scholar]
- 9). Morishita S, Mitobe Y, et al.: Differences in balance function between cancer survivors and healthy subjects: a pilot study. Integr Cancer Ther. 2018; 17: 1144–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Miltenburg NC, Boogerd W: Chemotherapy-induced neuropathy: a comprehensive survey. Cancer Treat Rev. 2014; 40: 872–882. [DOI] [PubMed] [Google Scholar]
- 11). Seretny M, Currie GL, et al.: Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain. 2014; 155: 2461–2470. [DOI] [PubMed] [Google Scholar]
- 12). Winters-Stone KM, Horak F, et al.: Falls, functioning, and disability among women with persistent symptoms of chemotherapy-induced peripheral neuropathy. J Clin Oncol. 2017; 35: 2604–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Kober KM, Mazor M, et al.: Phenotypic characterization of paclitaxel-induced peripheral neuropathy in cancer survivors. J Pain Symptom Manage. 2018; 56: 908–919.E3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Monfort SM, Pan X, et al.: Gait, balance, and patient-reported outcomes during taxane-based chemotherapy in early-stage breast cancer patients. Breast Cancer Res Treat. 2017; 164: 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Kneis S, Wehrle A, et al.: Balance impairments and neuromuscular changes in breast cancer patients with chemotherapy-induced peripheral neuropathy. Clin Neurophysiol. 2016; 127: 1481–1490. [DOI] [PubMed] [Google Scholar]
- 16). van den Beuken-van Everdingen MH, Hochstenbach LM, et al.: Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. J Pain Symptom Manage. 2016; 51: 1070–1090.E9. [DOI] [PubMed] [Google Scholar]
- 17). Bower JE: Cancer-related fatigue: mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. 2014; 11: 597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18). Boyes AW, Girgis A, et al.: Prevalence and predictors of the short-term trajectory of anxiety and depression in the first year after a cancer diagnosis: a population-based longitudinal study. J Clin Oncol. 2013; 31: 2724–2729. [DOI] [PubMed] [Google Scholar]
- 19). Morishita S, Tsubaki A, et al.: Cancer survivors exhibit a different relationship between muscle strength and health-related quality of life/fatigue compared to healthy subjects. Eur J Cancer Care (Engl). 2018; 27: e12856. [DOI] [PubMed] [Google Scholar]
- 20). Morishita S, Hirabayashi R, et al.: Relationship between balance function and QOL in cancer survivors and healthy subjects. Medicine (Baltimore). 2021; 100: e27822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Anderson C, Olshan A, et al.: Falls, walking or balance problems, and limitations in activities of daily living (ADLs) among older endometrial cancer survivors. Support Care Cancer. 2022; 30: 6339–6351. [DOI] [PubMed] [Google Scholar]
- 22). Winters-Stone KM, Medysky ME, et al.: Patient-reported and objectively measured physical function in older breast cancer survivors and cancer-free controls. J Geriatr Oncol. 2019; 10: 311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23). Sattar S, Haase K, et al.: Falls in older adults with cancer: an updated systematic review of prevalence, injurious falls, and impact on cancer treatment. Support Care Cancer. 2021; 29: 21–33. [DOI] [PubMed] [Google Scholar]
- 24). Stene GB, Helbostad JL, et al.: Effect of physical exercise on muscle mass and strength in cancer patients during treatment: a systematic review. Crit Rev Oncol Hematol. 2013; 88: 573–593. [DOI] [PubMed] [Google Scholar]
- 25). Scott JM, Zabor EC, et al.: Efficacy of exercise therapy on cardiorespiratory fitness in patients with cancer: a systematic review and meta-analysis. J Clin Oncol. 2018; 36: 2297–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26). Lin WL, Wang RH, et al.: The effects of exercise on chemotherapy-induced peripheral neuropathy symptoms in cancer patients: a systematic review and meta-analysis. Support Care Cancer. 2021; 29: 5303–5311. [DOI] [PubMed] [Google Scholar]
- 27). Guo S, Han W, et al.: Effects of exercise on chemotherapy-induced peripheral neuropathy in cancer patients: a systematic review and meta-analysis. J Cancer Surviv. 2022; s11764- 022-01182-3. [DOI] [PubMed] [Google Scholar]
- 28). Nakano J, Hashizume K, et al.: Effects of aerobic and resistance exercises on physical symptoms in cancer patients: a meta-analysis. Integr Cancer Ther. 2018; 17: 1048–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29). Mustian KM, Alfano CM, et al.: Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: a meta-analysis. JAMA Oncol. 2017; 3: 961–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30). Brown JC, Huedo-Medina TB, et al.: The efficacy of exercise in reducing depressive symptoms among cancer survivors: a meta-analysis. PLoS One. 2012; 7: e30955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31). Gerritsen JK, Vincent AJ: Exercise improves quality of life in patients with cancer: a systematic review and meta-analysis of randomised controlled trials. Br J Sports Med. 2016; 50: 796–803. [DOI] [PubMed] [Google Scholar]
- 32). Sweegers MG, Altenburg TM, et al.: Which exercise prescriptions improve quality of life and physical function in patients with cancer during and following treatment? a systematic review and meta-analysis of randomised controlled trials. Br J Sports Med. 2018; 52: 505–513. [DOI] [PubMed] [Google Scholar]
- 33). Fukushima T, Nakano J, et al.: Effects of aerobic, resistance, and mixed exercises on quality of life in patients with cancer: a systematic review and meta-analysis. Complement Ther Clin Pract. 2021; 42: 101290. [DOI] [PubMed] [Google Scholar]
- 34). Williams AD, Bird ML, et al.: Exercise for reducing falls in people living with and beyond cancer. Cochrane Database Syst Rev. 2018; 10: CD011687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). Sattar S, Haase KR, et al.: Feasibility and efficacy of a remotely delivered fall prevention exercise program for community-dwelling older adults with cancer: protocol for the STABLE trial. J Geriatr Oncol. 2022; 13: 1273–1280. [DOI] [PubMed] [Google Scholar]
- 36). Holmes MD, Chen WY, et al.: Physical activity and survival after breast cancer diagnosis. JAMA. 2005; 293: 2479–2486. [DOI] [PubMed] [Google Scholar]
- 37). Meyerhardt JA, Heseltine D, et al.: Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol. 2006; 24: 3535–3541. [DOI] [PubMed] [Google Scholar]
- 38). Friedenreich CM, Stone CR, et al.: Physical activity and mortality in cancer survivors: a systematic review and meta-analysis. JNCI Cancer Spectr. 2020; 4: pkz080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39). Morishita S, Hamaue Y, et al.: Effect of exercise on mortality and recurrence in patients with cancer: a systematic review and meta-analysis. Integr Cancer Ther. 2020; 19: 1534735420917462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40). Pedersen L, Idorn M, et al.: Voluntary running suppresses tumor growth through epinephrine- and IL-6-dependent NK cell mobilization and redistribution. Cell Metab. 2016; 23: 554–562. [DOI] [PubMed] [Google Scholar]
- 41). Toffoli EC, Sweegers MG, et al.: Effects of physical exercise on natural killer cell activity during (neo)adjuvant chemotherapy: a randomized pilot study. Physiol Rep. 2021; 9: e14919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42). Valenzuela PL, Saco-Ledo G, et al.: Exercise training and natural killer cells in cancer survivors: current evidence and research gaps based on a systematic review and meta-analysis. Sports Med Open. 2022; 8: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43). Khosravi N, Stoner L, et al.: Exercise training, circulating cytokine levels and immune function in cancer survivors: a meta-analysis. Brain Behav Immun. 2019; 81: 92–104. [DOI] [PubMed] [Google Scholar]
- 44). Foster C, Wright D, et al.: Psychosocial implications of living 5 years or more following a cancer diagnosis: a systematic review of the research evidence. Eur J Cancer Care (Engl). 2009; 18: 223–247. [DOI] [PubMed] [Google Scholar]
- 45). Doyle C, Kushi LH, et al.: Nutrition and physical activity during and after cancer treatment: an American Cancer Society guide for informed choices. CA Cancer J Clin. 2006; 56: 323–353. [DOI] [PubMed] [Google Scholar]
- 46). Schmitz KH, Courneya KS, et al.: American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010; 42: 1409–1426. [DOI] [PubMed] [Google Scholar]
- 47). Rock CL, Doyle C, et al.: Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012; 62: 242–274. [DOI] [PubMed] [Google Scholar]
- 48). Campbell KL, Winters-Stone KM, et al.: Exercise guidelines for cancer survivors: consensus statement from International Multidisciplinary Roundtable. Med Sci Sports Exerc. 2019; 51: 2375–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49). Rock CL, Thomson CA, et al.: American Cancer Society nutrition and physical activity guideline for cancer survivors. CA Cancer J Clin. 2022; 72: 230–262. [DOI] [PubMed] [Google Scholar]
- 50). Chan A, Ports K, et al.: Barriers and facilitators to exercise among adult cancer survivors in Singapore. Support Care Cancer. 2022; 30: 4867–4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51). Smith-Turchyn J, Allen L, et al.: Characterizing the exercise behaviour, preferences, barriers, and facilitators of cancer survivors in a rural Canadian community: a cross-sectional survey. Curr Oncol. 2021; 28: 3172–3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52). Mason C, Alfano CM, et al.: Long-term physical activity trends in breast cancer survivors. Cancer Epidemiol Biomarkers Prev. 2013; 22: 1153–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53). Fukushima T, Tsuji T, et al.: Cancer rehabilitation provided by designated cancer hospitals in Japan: the current state of outpatient setting and coordination after discharge. Prog Rehabil Med. 2022; 7: 20220006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54). Keating NL, Nørredam M, et al.: Physical and mental health status of older long-term cancer survivors. J Am Geriatr Soc. 2005; 53: 2145–2152. [DOI] [PubMed] [Google Scholar]