Abstract
During the early months of the COVID-19 pandemic, the international medical product supply chain was tight, causing breaks in the availability of neuromuscular blocking agents essential for the treatment of patients in intensive care units. The present study describes the pharmaceutical development of an injectable 2 mg/mL solution of pancuronium bromide (PC) in a very short lapse of time. The sterile solution was compounded into a good manufacturing practice grade A clean room, filtered (0.2 µm) and filled into 10 mL type I glass, manually sealed with bromobutyl rubber stoppers. A novel HPLC-MS stability indicating method for pancuronium quantification and its degradation product was developed and validated. This fast, sensitive and straightforward method was used to study the stability of the formulation using a semi-predictive method, enabling a very fast attribution of a temporary shelf-life, which was confirmed by a classic prospective stability study. The production line and the analytical tools set-up were performed in six weeks and the semi-predictive stability study was conducted in 90 days, allowing us to predict a shelf life, which was successfully confirmed by prospective study. In conclusion, using innovative methods, we were able to rapidly overcome the shortage of a critical drug.
Keywords: Pancuronium, Drug stability, Drug compounding, Accelerated stability study, High-performance liquid chromatography, Mass spectrometry
Graphical abstract

1. Introduction
The COVID-19 pandemic has challenged the medical product supply chain causing a shortage of certain critical active pharmaceutical ingredients (API) such as neuromuscular blocking agents (NMBA). During the early stages of the crisis, there was a shortage of the NMBA API found in the most frequently prescribed medicines, e.g. cisatracurium besilate, atracurium dibesylate, rocuronium bromide, and pancuronium bromide (PC). The latter was one of the first synthesised ammonium steroids and since has been surpassed by others due to a risk of prolonged neuromuscular blockade (Hansen-Flaschen et al., 1993; Andersen et al., 1988), causing a worldwide shortage less intense than that of other NMBA API. In this context, and with a surge of patients requiring neuromuscular blockade, the Pharmacy department of our regional university hospital undertook the pharmaceutical development of an injectable 2 mg/mL solution of PC (similar to Pavulon; Schering-Plough, Eragny-sur-Epte, France). This included the development of novel analytical methods and the assessment of stability, which aimed to improve the tools currently available. Such a method should be able to identify and quantify the API and its degradation products. Throughout its storage in a saline-buffered solution, PC is hydrolysed into two major compounds: 3-desacetylpancuronium (3-dPC) and 17-desacetylpancuronium (17-dPC); both of which are also hydrolysed into 3,17-didesacetylpancuronium (3,17-ddPC). The nomenclature of these four compounds of interest differs between pharmacopoeias, which is presented in Table 1 . The analytical method proposed by the European Pharmacopoeia (pH. Eur.; thin layer chromatography [TLC]) and the United States Pharmacopeia (USP; ion chromatography with suppressed conductivity detection) can quantify 17-dPC (Ph. Eur impurity A, USP related compound C); that proposed by the USP can quantify 3-dPC (USP related compound B) and 3,17-ddPC (USP related compound A). TLC can also quantify impurity D (which is not a degradation product), however, it has low sensitivity and a bad selectivity; ion chromatography with suppressed conductivity detection is marginally better but sensitivity and selectivity are improvable. More recently, Zecevic et al., in 2002, published an easy method for the quantification of PC in Pavulon based on high-performance liquid chromatography (HPLC) coupled with ultraviolet (UV) detection (Zečević et al., 2002); it cannot, however, be used to investigate the stability of PC injectable drugs because it does not separate and quantify 3,17-ddPC from the other compounds. It is also of note that several methods have been published to quantify NMBA in biological matrices: fluorometry, HPLC coupled with mass spectrometry (MS), or gas chromatography with nitrogen sensitive detection (Furuta et al., 1988; Cirimele et al., 2003; Huang et al., 2021), but these are unable to quantify the four relevant compounds for the assessment of the stability of PC injectable drugs. The stability-indicating quantitative analytical method was used to study the stability of the compounded formulation. In an emergency context, two methods were applied to assess the stability: a semi-predictive method (based on the Arrhenius equation and the kinetics equations developed during the 20th century), allowing a very fast assessment, and a prospective method (classical method) as a confirmation of the prediction (Šesták and Berggren, 1971; KC Waterman and Adami, 2005; Merienne et al., 2020; Clénet, 2018).
Table 1.
Identity (CAS, USP, Ph. Eur) of PC and its impurities.
| Compound name | CAS | Ph.Eur | U.S.P |
|---|---|---|---|
| Pancuronium | 16974–53–1 | / | / |
| 3-desacetylpancuronium | 43021–44–9 | Impurity B | Related compound B |
| 17-desacetylpancuronium (dacuronium) | 27115–86–2 | Impurity A | Related compound C |
| 3,17-didesacetylpancuronium | 43021–46–1 | Impurity C | Related compound A |
Herein we describe the semi-manual compounding process, the quantitative HPLC-MS stability-indicating method for PC and its degradation products as well as its validation, the evaluation of microbiological and physicochemical stability, and the prediction of chemical stability of the PC and its impurities using the semi-predictive method.
2. Materials and methods
2.1. Formulation of PC injectable solution
The formulation of 2 mg/mL PC injectable solution is presented in Table 2 . PC dibromide API was supplied by AMRI Pharmaceutical (Amri Italy SRL, Milan, Italy), sodium acetate trihydrate by Cooper (Melun, France), and water for injection and sodium chloride 20% by CDM Lavoisier (La Chaussée-Saint-Victor, France). After mixing, 5 mL aliquots of the solution were filtered (0.2 µm) and automatically filled by a BAXA Repeater Pump (Baxter Corporation, Deerfield, Illinois, USA) into 10 mL sterile depyrogenated colourless type I SCHOTT glass vials (Eurofins CDMO, Martillac, France) hermetically sealed with bromobutyl rubber stoppers (RayDyLyo®; ARaymondlife, Saint-Égrève, France). All the manufacturing steps were performed in a grade A clean room accordingly to good manufacturing practice.
Table 2.
Composition of PC injectable solution 2 mg/mL in the present study.
| Qualitative and quantitative composition | |
|---|---|
| Pancuronium dibromide | 12.00 mg |
| Sodium acetate trihydrate | 19.08 mg |
| Sodium chloride 20% | 0.24 mL |
| Water for injection | qsp 6 mL |
| Colourless type I glass | 1 |
| Bromobutyl rubber stopper | 1 |
2.2. Chemicals and reagents for HPLC
European Pharmacopeia Reference Standard PC (P0250000), USP Pancuronium bromide Related Compound A, USP Pancuronium bromide Related Compound B, USP Pancuronium bromide Related Compound C, and ammonium formate were supplied from Merck/Sigma-Aldrich (Darmstadt, Germany). Formic acid 99%, ammonium formate, acetonitrile, anhydrous acetic acid and ultrapure ULC-MS grade water were supplied by Biosolve Chimie (Dieuze, France).
2.3. Calibration and validation standard solutions
Accurate 2 mg/mL stock calibration solutions of PC were prepared from the CRS powder diluted in ultrapure water; this was then dilued in the mobile phase to form calibration standards at 1 µg/mL, 2 µg/mL, and 3 µg/mL. The stock validation solutions of PC 4 mg/mL were prepared by mixing 80 mg of PC API with 64 mg sodium acetate trihydrate, 80 µL sodium chloride 20%, 100 µL anhydrous acetic acid, and 20 mL of water for injection, to mimic the matrix of PC injectable solution; this was then diluted in the mobile phase to form validation standards at 1.5 µg/mL, 2 µg/mL, and 2.5 µg/mL. Stock solutions of each impurity (A, B, and C) at 20 µg/mL were prepared in mobile phase; these were then further diluted to form standard solutions at 2 µg/mL and 0.2 µg/mL.
2.4. Chromatography apparatus and conditions
The Agilent 1290 Infinity Quaternary LC System (Agilent Technologies, Santa Clara, CA, USA) was used in the present study. It was composed of a binary pump, an integrated vacuum degasser, a thermostated column compartment (30 °C), an autosampler, and a mass spectrometer. A Kinetex 2.6 µm C18 100 Å, 100×2.1 mm LC Column (Phenomenex, Torrance, CA, USA) was used as the stationary phase. The mobile phase A was an 5 mM ammonium formate buffer (pH = 3), and the mobile phase B was acetonitrile with 10% of mobile phase A. The following gradient was applied: starting at 90% A / 10% B, increasing linearly to 10% A / 90% B over 2 min and then maintained for between 2 and 3 min, and then decrease to 90% A / 10% B between 3 and 4 min. The flow rate was 0.5 mL/min and the injection volume was 2 µL.
2.5. Mass spectrometry apparatus and conditions
The mass spectrometer system used as a detector was an Agilent SQD 6125 (Agilent Technologies) with an electrospray ionisation source in positive ion mode. Desolvation was performed using N2 gas at 350 °C and 12 L/min. The capillary voltage was set at 3 kV.
2.6. Impurities and degradations products
PC is hydrolysed into two major compounds: 3-desacetylpancuronium (3-dPC) and 17-desacetylpancuronium (17-dPC); both of which are also hydrolysed into 3,17-didesacetylpancuronium (3,17-ddPC; Fig. 1 ). PC and its impurities (A, B, and C accordingly to the Ph. Eur) were analysed and compared to degradations products. The forced degradation of PC was investigated by acidic (0.1 – 1 M HCl for 3 h) and alkaline (0.01 – 0.1 M NaOH for 1 h) treatments, by heating (80 °C for 96 h), under oxidative conditions (3% H2O2 for 24 h) as well as under UVB (246 nm for 24 h) and UVA (340 nm for 24 h) conditions. Identity, origin, chromatographic properties and mass spectrometry settings including names (USP and PE), Chemical Abstract Service (CAS) numbers, retention times, ions, voltage of fragmentor, gain, dwell of each molecule are presented in Table 3 .
Fig. 1.
Degradation pathways of PC in aqueous solution, molecular weight and LogP of PC, 3-dPC, 17-dPC and 3,17-ddPC, estimated from Pubchem.
Table 3.
Retention time (min), ions, mass spectrometer conditions (fragmentor, gain, dwell) and origin of PC and its impurities.
| Compound name | Retention time (min) | Ion (m/z) | Fragmentor (V) | Gain | Dwell (ms) | Acidic | Alkaline | Heat | UV - Oxidation |
|---|---|---|---|---|---|---|---|---|---|
| PC | 1.40 | 286.2 | 110 | 1.0 | 230 | / | / | / | / |
| 3-dPC |
1.35 | 265.2 | 110 | 1.0 | 80 | + | + | + | – |
| 17-dPC (dacuronium) | 1.45 | 265.2 | 110 | 1.0 | 80 | + | + | + | – |
| 3,17-ddPC | 1.15 | 244.2 | 110 | 1.0 | 110 | + | + | + | – |
2.7. Assay validation procedure
Assay validation was performed according to the guidelines of the International Conference for Harmonization (ICH), the European Society of Hospital Pharmaceutical Technologies (GERPAC), and the French Society of Pharmaceuticals Sciences and Techniques (SFSTP) “Harmonization of strategies for the validation of quantitative analytical procedures”; protocol V2 was selected for this validation (Hubert et al., 2007; Sautou et al., 2013). The following criteria were evaluated: selectivity, response function (calibration curve), linearity, trueness, precision (repeatability and intermediate precision), accuracy profile, as well as the limit of detection (LOD) and the limit of quantification (LOQ).
2.8. Accelerated predictive stability
Immediately after compounding the bottles were stored in climatic chambers (Meditest H1300; Froilabo, Lyon, France). The chambers were regulated in temperature and relative humidity (RH) as recommended by the ICH: 5 ± 3 °C; 25 ± 2 °C / 60% ± 5% RH; 40 ± 2 °C, 75% ± 5% RH as well as in custom environmental conditions: 60 ± 2 °C. Quantitative analysis of PC and its degradation products by HPLC-MS was performed (n = 5) immediately after compounding and after different durations of exposure in normal and accelerated conditions as presented in Table 4 .
Table 4.
Experimental design of the predictive stability study for PC formulation. Storage in ICH and non ICH conditions and time of storage. RH: Relative Humidity (%).
| Storage condition | Duration of storage (days) |
|---|---|
| 5 °C ± 3 °C (ICH) | 0; 30; 90; 180; 250; 365 |
| 25 °C ± 2 °C, 60% RH ± 5% RH (ICH) | 0; 30; 45; 60; 75; 90; 250; 365 |
| 40 °C ± 2 °C, 75% RH ± 5% RH (ICH) | 0; 15; 30; 45; 60; 75; 90; 250 |
| 60 °C ± 2 °C (non ICH) | 0; 15; 30; 45; 60 |
2.9. Modelling of PC chemical degradation kinetics and formation of impurities, as well as time-to-failure prediction
The datasets related to the degradation of PC and its degradation products were fitted to kinetic degradation models using the PREDISTAB software that automates the semi-predictive methods (KC Waterman and Adami, 2005). Two approaches were tested: isoconversional and model screening. The fit of the model was evaluated using the Bayesian Information Criterion (BIC), the Akaike Information Criterion (AIC), the Relative Mean Square Error (RMSE), and the determination coefficient R². The best-fitted model was then used to predict the long-term degradation rate of PC over 24 months and the appearance of its degradations products, allowing the attribution of shelf life to the preparation.
2.10. Other physicochemical and microbiological controls
The other physicochemical controls of the PC injectable drug included visual aspect, pH measurement (pH metre; Mettler Toledo, Greifensee, Switzerland), determination of osmolality (Osmopro®; Advanced Instrument, Norwood, MA, USA) and particulate contamination by subvisible particles (2.9.19 Ph. Eur monograph, 2020 method 1: light obscuration particle count test, HACH—HIAC 9703+, Beckman Coulter Life Sciences, Brea, CA, USA). The microbiological controls were carried out by membrane filtration using a Steritest closed filtration device (Millipore, Merck KGaA, Darmstadt, Germany) and peptone water 0.1% as rinse fluid, according to the 2.6.1 Ph. Eur monograph, 2020. In addition, a fast-detection colorimetric method using BactAlert® 3D method and FA PLUS aerobic as well as FN PLUS anaerobic culture bottles (BioMerieux, Lyon, France).
3. Results
3.1. Quantification assay validation and forced degradations
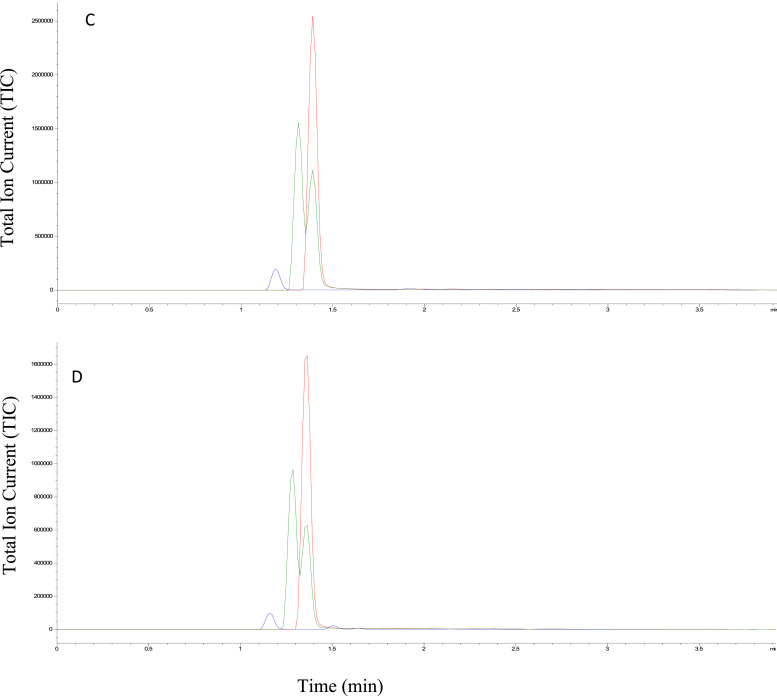
The PC calibration curve was linear over the range of concentrations (R² = 0.985). Repeatability was 4.68%, reproducibility 4.52%, and trueness −1.01%. The accuracy profile criteria were met (λ = 10%; β = 80%). The three degradation products, 3-dPC, 17-dPC and 3,17-ddPC, which were also the Ph. Eur impurities A, B and C, appeared after acid (Fig. 2 B), basic (Fig. 2C) and thermal exposure (Fig. 2D). No notable degradation product was identified after H2O2 or UVA/B exposure.
Fig. 2.
Typical chromatogram of PC solution and its degradation products (A) in a freshly made solution, (B) after 3 h of HCl 0.1 M exposure, (C) after 1 hour of NaOH 0.1 M exposure and (D) after 96 h at 80 °C. The PC is quantified at m/z = 286.2, Rt = 1.40 min (red chromatogram). The 3-dPC and 17-dPC are quantified at m/z = 265.2, Rt = 1.35 and 1.45 respectively (green chromatogram). Finally, the 3,17-ddPC is quantified at m/z = 244.2, Rt = 1.15 mn (blue chromatogram).
3.2. Formulation development and implementation of production line and quality controls
The interval between the initial request for the formulation to the first batch release was six weeks.
3.3. Modelling of PC chemical degradation kinetics and formation of impurities
The best fitted models were (i) 1D diffusion model for PC (BIC = 672.63, AIC = 662.08, RMSE = 4.17 and R² = 0.96), (ii) power of N = 2 for 3-dPC (BIC = −282.89, AIC = −292.47, RMSE = 0.05 and R² = 0.99), (iii) Johnson-Mehl-Avrami-Erofeyev-Kholmogorov (JMAEK) with N = 3 for 17-dPC (BIC = −233.82, AIC = 243.40, RMSE = 0.06 and R² = 0.98), and (iv) power of N = 3 for 3,17-ddPC (BIC = −339.23, AIC = −348.29, RMSE = 0.67, and R² = 0.99; Table 5 ). These were all retained, apart from the 1D diffusion model for PC for which the order 3 model was chosen despite fitting less well (for example, AIC = 675.93 and RMSE = 4.42); in a homogenous phase (liquid) the order 3 model makes more sense than a diffusion reaction model since the latter is rather a heterogeneous phase reaction. The data measured and modelled were visually well correlated, excepted for the 3,17-ddPC (Fig. 3 ); for the latter, even the best fitted model does not seem to have sufficient correlation with measured values (Fig. 3D).
Table 5.
Modelling results for the four compounds.
| Model | BIC | AIC | RMSE | R² | |
|---|---|---|---|---|---|
| PC | Order 0 | 732.34 | 721.69 | 5.26 | 0.93 |
| Order 1 | 711.87 | 701.21 | 5.39 | 0.94 | |
| Order 2 | 692.78 | 682.16 | 4.54 | 0.95 | |
| Order 3 | 686.55 | 675.93 | 4.42 | 0.95 | |
| Order N | 690.87 | 677.69 | 4.39 | 0.95 | |
| JMAEK n = 3 | 835.98 | 825.36 | 8.48 | 0.81 | |
| JMAEK n = 5 | 869.75 | 859.13 | 9.84 | 0.74 | |
| Power n = 2 | 814.21 | 803.59 | 7.72 | 0.85 | |
| Power n = 3 | 848.64 | 838.02 | 8.98 | 0.79 | |
| 1D Diffusion | 672.63 | 662.08 | 4.17 | 0.96 | |
| 3D Diffusion | 677.42 | 666.80 | 4.24 | 0.96 | |
| 2D Contraction | 716.38 | 705.76 | 8.62 | 0.93 | |
| 3D Contraction | 713.05 | 702.43 | 4.94 | 0.94 | |
| Autocatalytic | 866.94 | 856.32 | 9.63 | 0.75 | |
| 3-dPC | Order 0 | −230.65 | −240.23 | 0.07 | 0.98 |
| Order 1 | −135.12 | −144.70 | 0.10 | 0.94 | |
| Order 2 | −93.44 | −103.02 | 0.13 | 0.90 | |
| Order 3 | −70.04 | −79.62 | 0.16 | 0.87 | |
| JMAEK n = 3 | −266.52 | −276.10 | 0.05 | 0.99 | |
| JMAEK n = 5 | −199.87 | −209.41 | 0.11 | 0.97 | |
| Power n = 2 | −282.89 | −292.47 | 0.05 | 0.99 | |
| Power n = 3 | −193.39 | −202.97 | 0.08 | 0.97 | |
| 1D Diffusion | −123.30 | −132.83 | 0.11 | 0.93 | |
| 3D Diffusion | −84.24 | −93.82 | 0.14 | 0.88 | |
| 2D Contraction | −171.44 | −181.02 | 0.09 | 0.96 | |
| 3D Contraction | −157.47 | −167.05 | 0.09 | 0.95 | |
| Autocatalytic | −209.64 | −219.22 | 0.24 | 0.74 | |
| 17-dPC | Order 0 | −228.20 | −237.78 | 0.06 | 0.98 |
| Order 1 | −160.01 | −169.59 | 0.09 | 0.95 | |
| Order 2 | −113.39 | −123.39 | 0.12 | 0.92 | |
| Order 3 | −87.01 | −96.59 | 0.14 | 0.89 | |
| JMAEK n = 3 | −233.82 | −243.40 | 0.06 | 0.98 | |
| JMAEK n = 5 | −167.62 | −177.20 | 0.09 | 0.96 | |
| Power n = 2 | −190.76 | −200.33 | 0.08 | 0.96 | |
| Power n = 3 | −127.81 | −137.39 | 0.11 | 0.93 | |
| 1D Diffusion | −139.76 | −149.34 | 0.10 | 0.94 | |
| 3D Diffusion | −101.00 | −110.58 | 0.13 | 0.90 | |
| 2D Contraction | −195.06 | −204.64 | 0.08 | 0.97 | |
| 3D Contraction | −182.49 | −192.07 | 0.08 | 0.96 | |
| Autocatalytic | −176.52 | −186.09 | 0.24 | 0.77 | |
| 3.17-ddPC | Order 0 | −160.65 | −169.70 | 0.22 | 0.94 |
| Order 1 | −122.00 | −131.05 | 0.16 | 0.86 | |
| Order 2 | −101.90 | −110.95 | 0.15 | 0.83 | |
| Order 3 | −89.29 | −98.34 | 0.15 | 0.81 | |
| JMAEK n = 3 | −286.59 | −295.64 | 0.18 | 0.90 | |
| JMAEK n = 5 | −324.67 | −333.72 | 0.20 | 0.88 | |
| Power n = 2 | −312.52 | −321.57 | 0.41 | 0.99 | |
| Power n = 3 | −339.23 | −348.29 | 0.67 | 0.99 | |
| 1D Diffusion | −97.30 | −106.35 | 0.17 | 0.85 | |
| 3D Diffusion | −84.24 | −93.82 | 0.16 | 0.81 | |
| 2D Contraction | −137.58 | −146.63 | 0.17 | 0.89 | |
| 3D Contraction | −131.77 | −140.82 | 0.15 | 0.88 | |
| Autocatalytic | −329.61 | −338.67 | 0.20 | 0.88 |
JMAEK is the nucleation model of Johnson-Mehl-Avrami-Erofeyev-Kholmogorov. BIC is the Bayesian Information Criterion. AIC is the Akaike Information Criterion. RMSE is the Root Mean Square Error and R² is the coefficient of determination.
Fig. 3.
Kinetics profiles of (A) PC degradation and (B) 3-dPC, (C) 17-dPC and (D) 3,17-ddPC formation from dosage forms stored at (∆) 5 °C ± 3 °C; (○) 25 °C ± 2 °C, 60% RH ± 5% RH; (◊) 40 °C ± 2 °C, 75% RH ± 5% RH; (□) 60 °C ± 2 °C. Each data is the mean ± standard deviation of five experimental determinations.
3.4. Time to failure prediction
Using the kinetics equations generated previously, PC degradation and impurities formation was modelled at 5 °C ± 3 °C; the models, generated by 90 days of experiments, allowed the accurate prediction of the kinetics of PC degradation and the apparition of its degradation products (Fig. 4 ). The PC reached its threshold of 90% of initial concentration after 6.6 years (2 400 days), while the 3-dPC no more than (NMT) threshold was reached after 2.7 years (1 000 days), 17-dPC 4.2 years (1 525 days), and 3,17-ddPC after 7.8 years (2 850 days). As the first degradation product reached its NMT threshold at 2.7 years, a chemical stability of 2.5 years can be attributed to the preparation (Table 6 ).
Fig. 4.
Predicted kinetics profiles and confidence interval (5%) of (A) PC, (B) 3-dPC, (C) 17-dPC, and (D) 3,17-ddPC at 5 °C ± 3 °C over 1000 days. Crosses represent the measured results after 365 days and 730 days.
Table 6.
Best-fitted models and parameters, specifications and time-to-failure for each compound.
| Best-fitted model | Specifications | Time to failure at 5 °C ± 3 °C (Days – Years) | |
|---|---|---|---|
| PC |
Order 3 ∝(t) = ∝(0)−∝(0)*(1 −(1 −(2kt))−0,5) |
α(t) ≥ 90% | 2 400 – 6.6 |
| 3-dPC |
Power of N = 2 ∝′(t) = ∝′(0)−∝′(0)*(kt)2 |
NMT 3.0% | 1 000 – 2.7 |
| 17-dPC |
JMAEK N = 3 ∝′(t) = ∝′(0)−∝′(0)*(1−exp(−(kt)3))) |
NMT 2.0% | 1 525 – 4.2 |
| 3.17-ddPC |
Power of N = 3 ∝′(t) = ∝′(0)−∝′(0)*(kt)3 |
NMT 0.4% | 2 850 – 7.8 |
α’ is a conversion of the impurities data apparition as:.
; Acceptance criteria for impurities are based on USP.
JMAEK is the nucleation model of Johnson-Mehl-Avrami-Erofeyev-Kholmogorov.
3.5. Other physicochemical and microbiological analysis results
The physicochemical assays (appearance, pH, osmolality, PC and impurities content and sub-visible particles) all conformed to their specifications, except for the mean PC content measured at 90 days at 5 °C (2.25 mg/mL). This out of specification (OOS) result was investigated, revealing a potential human error; this was confirmed by subsequent results at days 180, 365 and 730 that were all within specification. All microbiological analyses of the study met the specifications (Table 7 ).
Table 7.
Overall results of the stability study at 5 °C ± 3 °C for 2 years and at 25 °C ± 2 °C for 90 days.
| Specifications | Day 0 | Day 30 | Day 90 | Day 180 | Day 365 | Day 730 | |||
|---|---|---|---|---|---|---|---|---|---|
| 5 °C ± 3 °C | 25 °C ± 2 °C | 5 °C ± 3 °C | 25 °C ± 2 °C | 5 °C ± 3 °C | 5 °C ± 3 °C | 5 °C ± 3 °C | |||
| Appearance | Colourless (C). Limpid (L). Other (O) | C. L | C. L | C. L | C. L | C. L | C. L | C. L | C. L |
| pH | 3.50 - 4.50 | 3.78 | 3.78 | 3.84 | 4.00 | 3.88 | 4.00 | 4.05 | 4.01 |
| Osmolality (mOsm/kg) | 342 - 418 | 416 | 389 | 416 | 387 | 386 | 392 | 394 | 398 |
| Mean PC content (mg/mL) | 1.80 - 2.20 | 2.13 | 2.04 | 2.12 | 2.25 | 1.99 | 2.12 | 2.05 | 2.00 |
| Mean 3-dPC content (% of AUC(PC)) |
NMT 3.0% | 0.02 | 0.04 | 0.97 | 0.12 | 2.54 | 0.4 | 0.99 | 2.02 |
| Mean 17-dPC content (% of AUC(PC)) |
NMT 2.0% | < 0.01 | 0.05 | 0.28 | 0.10 | 0.96 | 0.3 | 0.43 | 0.68 |
| Mean 3.17-ddPC content (% of AUC(PC)) |
NMT 0.4% | < 0.01 | < 0.01 | 0.04 | < 0.01 | 0.03 | < 0.01 | < 0.01 | 0.06 |
| Subvisible particles ≥ 10 µm /mL |
≤ 968.00 | 18.8 | / | / | / | / | / | 27.12 | 15.72 |
| Subvisible particles ≥ 25 µm / mL |
≤ 97.00 | 0.9 | / | / | / | / | / | 1.85 | 0.98 |
| Sterility | Sterile | Sterile | Sterile | Sterile | Sterile | Sterile | Sterile | Sterile | Sterile |
| Bacterial endotoxin content (EU/mL) | < 100 | < 0.5 | < 0.5 | < 0.5 | < 0.5 | < 0.5 | < 0.5 | < 0.5 | < 0.5 |
NMT: No More Than.
4. Discussion
Our main objective was to implement in a very brief time a production line of PC 2 mg/mL for intravenous administration during the first weeks of the COVID-19 pandemic. The manufacturing process and the analytical quantitative HPLC-MS method were implemented within six weeks in our pharmacy, and using the semi-predictive method shelf life was attributed within 90 days.
Thanks to the open science practices promoted by the many publishers and researchers (Besançon et al., 2021) the bibliographic analysis to choose the formulation and the quality control strategy was rapid. The first step was to implement a manual process of injectable sterile drug compounding according to GMP practices. This process was applied to PC compounding, but could be used for other injectable drugs in the context of scarcity of a medicinal product. In a second step, a novel HPLC-MS stability-indicating method was developed and validated. This method allowed the quantification of the PC and its degradation products (3-dPC, 17-dPC, and 3,17-ddPC) with very high sensitivity in a very short analysis time. Before development of this analytical tool, the only method that could quantify these four compounds was the USP method, using chromatography with conductivity detection (Bjorksten et al., 1990); the HPLC-MS method described herein is ultra-fast, easy, sensitive and specific. It was not necessary to use an internal standard thanks to a simple matrix; furthermore, the implementation of this method made it possible to carry out the quality control and stability study required to develop the formulation in a laboratory that was not equipped with a conductivity metre. In the final step, stability of the preparation was studied using the classical ICH method and the semi-predictive method. The latter was successfully applied in the present study; the models chosen for the extrapolation were the order 3 for PC, power of N = 2 for 3-dPC, JMAEK for 17-dPC, and power of N = 3 for 3,17-ddPC. With the exception of the model chosen for PC, these were the best-fitted in each case. For PC, a diffusion model had the best fit but also the second-best fit, however an order 3 model was chosen because diffusion models are used to describe reactions that involve a diffusion step that occurs in heterogeneous phases (the 1D diffusion model [parabolic law], the 2D model, and 3D model described by Jander and by Ginstling-Brounshtein (Okhotnikov and Babicheva, 1988; Jander, 1927; Ginstling and Brounshtein, 1950)). Herein, PC is dissolved in a homogeneous phase; it would therefore be conceptually debatable to use a diffusion model. In parallel, an absorption or adsorption phenomenon mechanism cannot be postulated, PC being classically formulated in a glass container. More generally, it is of note that while the diffusion models for PC had a relatively similar shelf-life to the model chosen (between 2 400 and 2 600 days at 5 °C), and the threshold was reached after 1 000 days due to the apparition of the 3-dPC (after extrapolation of the model at 5 °C) leading to a predicted shelf-life of 2.5 years. The mathematical model that best fit with the experimental data of 17-dPC is the JMAEK model. In this model of nucleation, it is suggested that a reaction initiated in one nucleus propagates in all directions of space until it encounters other nuclei with which it coalesces (Avrami, 1940).. This results in an instantaneous reaction rate that starts slowly, then accelerates, and finally decelerates during this merger. The resulting kinetic curve appears to be sigmoidal. This model can also fit to a multi-step reaction. The rate of 3-dPC appearance is correlated to a so-called power model. This type of reaction starts slowly and then accelerates abruptly. This power law correlates well with the experimental data for each temperature studied and allowed us to assign a shelf life to the preparation. Despite a strong correlation between experimental data and mathematical models, it is difficult to rely on these results to understand the real reaction mechanism of these two reactions as these two models can only be considered as approximations. To be precise, the generation of the primary degradation products (3-dPC and 17-dPC) and their simultaneous degradation to 3,17-ddPC should have been modelled. However, herein this was sufficient to assign a shelf life with accuracy. The prospective results of the stability study conducted with the classical ICH method confirmed the stability predicted for the PC and its impurities. This was despite an experimental design with storage conditions that may seem very aggressive (60 °C for a drug that is stored at 5 °C) as at this temperature secondary degradation may occur and bias the extrapolation of the results obtained at 5 °C. This would have resulted in a strong decorrelation between high and low temperatures, which would have led to the exclusion of the extreme points from the statistical analysis; this was not the case with PC. This semi-predictive methodology applied to the study of the stability of a generic drug, for which much published data exists, may be considered as a validated tool for the attribution of shelf life if the results match that reported elsewhere. For instance, the shelf life of commercial PC (Pavulon) is 2 years. The early information about the stability of the medicine is of crucial value in order to take the right decision during the development steps. A semi-predictive stability study approach makes possible to obtain reliable data about the evolution of an API in its pharmaceutical formulation earlier than a prospective confirmation of the stability in ICH long-term storage conditions, which does, however, remain mandatory. This is the direction in which the recommendations of the ICH are evolving, which, in a forthcoming revision of the Q1A-F and Q5C guidelines, opens up the possibility of using modern tools and strategies to improve product understanding.
5. Conclusion
In conclusion, using innovative methods, we were able to rapidly overcome the shortage of a critical drug. After the initial months of the crisis, a national network of injectable NMBA drug production was organised to ensure coverage of the needs of all areas of France; such networks were also implemented in different countries around the world (Roehr, 2020), underscoring the need to secure the industrial and hospital production capacity of drugs.
Credit author statement
Please find below the credit author statement :
Camille Merienne : Conceptualization, methodology, validation, alanysis, Writing. Samira Filali & Chloé Marchand : Conceptualization, methodology and validation. Benjamine Lapras : Metholodogy and software parts. Carole Paillet & Fabrice Pirot : Administration, supervision and methodology + ressources.
Data availability
No data was used for the research described in the article.
References
- Hansen-Flaschen J., Cowen J., Raps E.C. Neuromuscular blockade in the intensive care unit. Am. Rev. Respir. Dis. 1993;147(1):234–236. doi: 10.1164/ajrccm/147.1.234. [DOI] [PubMed] [Google Scholar]
- Andersen B.N., Madsen J.V., Schurizek B.A., Juhl B. Residual curarisation: a comparative study of atracurium and pancuronium. Acta. Anaesthesiol. Scand. 1988;32(2):79–81. doi: 10.1111/j.1399-6576.1988.tb02692.x. [DOI] [PubMed] [Google Scholar]
- Zečević M., Živanović L., Stojković A. Validation of a high-performance liquid chromatography method for the determination of pancuronium in Pavulon injections. J. Chromatogr. A. 2002;949(1–2):61–64. doi: 10.1016/S0021-9673(01)01552-7. [DOI] [PubMed] [Google Scholar]
- Furuta T., Canfell P.C., Castagnoli K.P., Sharma M.L., Miller R.D. Quantitation of pancuronium, 3-desacetylpancuronium, vecuronium, 3-desacetylvecuronium, pipecuronium and 3-desacetylpipecuronium in biological fluids by capillary gas chromatography using nitrogen-sensitive detection. J. Chromatogr. B. Biomed. Appl. 1988;427:41–53. doi: 10.1016/0378-4347(88)80103-8. Jan 1. [DOI] [PubMed] [Google Scholar]
- Cirimele V., Villain M., Pepin G., Ludes B., Kintz P. Screening procedure for eight quaternary nitrogen muscle relaxants in blood by high-performance liquid chromatography–electrospray ionization mass spectrometry. J. Chromatogr. B. 2003;5(1):107–113. doi: 10.1016/S1570-0232(03)00070-9. 789. [DOI] [PubMed] [Google Scholar]
- Huang Y., Hui T.A., Yunyang S.O., Bo C.H., Zhong H. Simultaneous determination of three quaternary ammonium muscle relaxants in blood by high performance liquid chromatography-tandem mass spectrometry. Chin. J. Chromatogr. 2021;7(7):695. doi: 10.3724/SP.J.1123.2020.09020. 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šesták J., Berggren G. Study of the kinetics of the mechanism of solid-state reactions at increasing temperatures. Thermochim. Acta. 1971;1(1):1–2. doi: 10.1016/0040-6031(71)85051-7. 3. [DOI] [Google Scholar]
- Waterman K.C., Adami R.C. Accelerated aging: prediction of chemical stability of pharmaceuticals. Int. J. Pharm. 2005:101–125. doi: 10.1016/j.ijpharm.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Merienne C., Marchand C., Filali S., Salmon D., Pivot C., Pirot F. Measurement, analysis and prediction of amoxicillin oral dose stability from integrated molecular description approach and accelerated predictive stability (APS) Pharm. Technol. Hosp. Pharm. 2020;1(1):5. doi: 10.1515/pthp-2020-0009. [DOI] [Google Scholar]
- Clénet D. Accurate prediction of vaccine stability under real storage conditions and during temperature excursions. Eur. J. Pharm. Biopharm. 2018;1(125):76–84. doi: 10.1016/j.ejpb.2018.01.005. [DOI] [PubMed] [Google Scholar]
- Hubert P., Nguyen-Huu J.J., Boulanger B., Chapuzet E., Cohen N., Compagnon P.A., Dewé W., Feinberg M., Laurentie M., Mercier N., Muzard G. Harmonization of strategies for the validation of quantitative analytical procedures: a SFSTP proposal–Part III. J. Pharm. Biomed. Anal. 2007;21(1):82–96. doi: 10.1016/j.jpba.2007.06.013. 45. [DOI] [PubMed] [Google Scholar]
- Sautou V., Brossard D., Chedru-Legros V., Crauste-Manciet S., Lagarce F., Odou P. Methodological guidelines for stability studies of hospital pharmaceutical preparations. SFPC. and. GERPAC. 2013;75:28–31. [Google Scholar]
- Waterman K.C., Adami R.C. Accelerated aging: prediction of chemical stability of pharmaceuticals. Int. J. Pharm. 2005;11(1–2):101–125. doi: 10.1016/j.ijpharm.2004.12.013. 293. [DOI] [PubMed] [Google Scholar]
- Besançon L., Peiffer-Smadja N., Segalas C., Jiang H., Masuzzo P., Smout C., Billy E., Deforet M., Leyrat C. Open science saves lives: lessons from the COVID-19 pandemic. BMC. Med. Res. Methodol. 2021;21(1):1–8. doi: 10.1186/s12874-021-01304-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorksten A.R., Beemer G.H., Crankshaw D.P. Simple high-performance liquid chromatographic method for the analysis of the non-depolarizing neuromuscular blocking drugs in clinical anaesthesia. J. Chromatogr. B. Biomed. Appl. 1990;533:241–247. doi: 10.1016/S0378-4347(00)82209-4. 1. [DOI] [PubMed] [Google Scholar]
- Okhotnikov V.B., Babicheva I.P. Initial stage in isothermal dehydration of vermiculite single crystals in vacuum. React. kinet. catal. lett. 1988;37(2):417–422. doi: 10.1007/BF02062093. [DOI] [Google Scholar]
- Jander W. Reactions in solid state at high temperatures: I, reaction rates of endothermic reactions. Z. Anorg. Allgem. Chec. 1927;163(1):1927. 30. [Google Scholar]
- Ginstling A.M., Brounshtein B.I. On diffusion kinetics in chemical reactions taking place in spherical powder grains. Zhur. Priklad. Khim. 1950;1:23. [Google Scholar]
- Avrami M. Kinetics of phase change. II transformation-time relations for random distribution of nuclei. J. Chem. Phys. B. 1940:212‑24. doi: 10.1063/1.1750631. [DOI] [Google Scholar]
- Roehr B. Bringing drug production home: how the US is rebuilding the drug supply chain after covid-19. Br. Med. J. 2020;370:21. doi: 10.1136/bmj.m3393. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.