Abstract
Background
Participants with human immunodeficiency virus (HIV) seroconversion in The Ring Study, a phase 3 trial of dapivirine vaginal ring (DVR), or in the open-label extension trial dapivirine ring extended access and monitoring (DREAM) were offered enrollment in an observational cohort study (IPM 007) to assess clinical presentation and response to antiretroviral therapy (ART).
Methods
Participants’ HIV infection was managed at local treatment clinics according to national treatment guidelines. IPM 007 study visits occurred 3 and 6 months after enrollment and every 6 months thereafter. Assessments included plasma HIV-1 RNA, CD4+ T-cell counts, and recording of HIV/AIDS-associated events and antiretroviral use. Post hoc virology analyses were performed for participants identified with virologic failure.
Results
One hundred fifty-one of 179 eligible participants (84.4%) enrolled into IPM 007; 103 had previously received the DVR in the Ring or DREAM studies, and 48 had received placebo in The Ring Study. HIV-1 RNA and CD4+ T-cell counts after 12 months’ follow-up were similar for participants who used the DVR in The Ring Study and DREAM, compared to those who received placebo. Of the 78 participants with a study visit approximately 6 months after ART initiation, 59 (75.6%) had HIV-1 RNA <40 copies/mL (The Ring Study: placebo: 13/23 [56.5%]; DVR: 32/39 [82.1%]; DREAM [DVR]: 14/16 [87.5%]). Post hoc virology analysis indicated that genotypic patterns observed at virologic failure were as expected of a nonnucleoside reverse transcriptase inhibitor (NNRTI)–based regimen.
Conclusions
Seroconversion during DVR use did not negatively affect clinical presentation or treatment outcome. Mutation patterns at virologic failure were in line with individuals failing an NNRTI-based regimen.
Clinical Trials Registration
Keywords: HIV, prevention, dapivirine, seroconversion, virology
Data from IPM 007 demonstrated that seroconversion during use of the dapivirine vaginal ring did not negatively affect clinical presentation or treatment outcome. Mutation patterns at treatment failure were consistent with failure on an NNRTI-based regimen.
A silicone matrix vaginal ring containing 25 mg dapivirine (a human immunodeficiency virus type 1 [HIV-1] nonnucleoside reverse transcriptase inhibitor [NNRTI]) reduced the risk of HIV-1 infection in women in 2 randomized placebo-controlled trials, The Ring Study (protocol IPM 027) and A Study to Prevent Infection with a Ring for Extended Use (ASPIRE) (protocol MTN-020) [1, 2]. In The Ring Study, the dapivirine vaginal ring (DVR) demonstrated a statistically significantly reduced risk of HIV-1 infection compared to placebo, with an overall risk reduction of 35.1% (95% confidence interval [CI]: 9.1%–53.6%) relative to placebo [3]. In the dapivirine ring extended access and monitoring (DREAM) open-label extension trial (protocol IPM 032), an HIV-1 incidence rate of 1.8 (95% CI: 1.1–2.9) per 100 person-years was observed, which is 62% lower than the simulated placebo rate based on bootstrap sampling of participants in the placebo group of The Ring Study, matched for research center, age, and presence of sexually transmitted infections at enrollment. The higher response rate in DREAM is most likely explained by increased product adherence due to knowledge of proven efficacy and safety [4].
Long-term follow-up of participants who acquired HIV in prevention trials is important for assessing if there are differences in antiretroviral (ARV) treatment response and development of resistance post–antiretroviral therapy (ART) initiation among participants previously exposed to an ARV or ARV-containing microbicide compared to placebo [5, 6]. This is particularly important when NNRTIs are used for prevention, as they generally have a lower genetic barrier to resistance compared to other ARV classes [7]. Indeed, several studies have shown that single-dose nevirapine, used to prevent mother-to-child transmission of HIV-1, can select for NNRTI-resistant virus [8, 9]. The results from the Optimal Combination Therapy After Nevirapine Exposure (OCTANE) trial demonstrated that archiving of virus with resistant mutations might affect response during later exposure to the relevant ARV [10].
The proportions of participants with NNRTI resistance-associated mutations (RAMs) observed at seroconversion in both The Ring Study (14.8%) (Steytler et al, unpublished data) and ASPIRE (11.0%) [11] were broadly consistent with a survey of transmitted HIV-1 drug resistance mutations in South Africa over a contemporaneous time period [12]. Although the proportions of participants with NNRTI RAMs in the DVR and placebo groups were similar, there was a statistically nonsignificant imbalance in the virus encoding E138A in The Ring Study (Steytler et al, unpublished data). No imbalance between study arms for virus encoding E138A was observed in the ASPIRE trial [11]. The E138A variant is a common polymorphism in subtype C HIV-1 isolates from southern Africa [13]. It is associated with potential or low-level resistance to the NNRTIs etravirine and rilpivirine (both close analogues of dapivirine), but it is not associated with resistance to efavirenz and nevirapine [13–15].
Here we describe the results of study IPM 007, an observational cohort study of HIV clinical presentation, response to ARV treatment, and virologic outcomes in participants who seroconverted during The Ring Study and DREAM.
METHODS
Study Design and Participants
Women who seroconverted (defined as previously described) [1, 2] in The Ring Study and DREAM were offered enrollment into IPM 007, a follow-up cohort study of HIV infection. IPM 007 was an observational study and women were referred to local treatment clinics for management of their HIV infection and ARV treatment according to national treatment guidelines in place at the time.
IPM 007 was conducted from 4 October 2012 through 2 May 2019 at the same research centers as The Ring Study and DREAM. Participant follow-up was expected to continue for at least 12 months after enrollment. Study visits were scheduled at months 3 and 6 after enrollment and every 6 months thereafter. As ARV treatment was initiated by local treatment clinics, independent of IPM 007, study visits and timepoints for assessment of ARV were not aligned and generally occurred later than defined response assessment points.
Samples for HIV-1 RNA and CD4+ T-cell count determination were taken at every visit and data on HIV/AIDS-associated events recorded. ARV treatment data (initiation dates, regimen, and adherence) based on participant report were recorded. Physical examinations were performed at all visits, but as no investigational product was used, adverse event monitoring or safety laboratory testing was not performed. Routine sample storage at each visit for potential HIV-1 susceptibility testing was not originally planned. However, storage was mandated in the IPM 007 Protocol Version 2.0 Amendment 2.0, dated 16 January 2017, which was fully implemented by September 2017.
The study was registered with ClinicalTrials.gov (NCT01618058) and conducted in compliance with the Declaration of Helsinki and International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. All local regulatory and legal requirements were followed. The study protocol was approved by independent ethics committees at all research centers. Written informed consent was provided by all participants.
Data Presentation and Analysis
Data are presented across 5 groups: The Ring Study treatment arm (DVR or placebo), DREAM participants (DVR), a total DVR group, and all participants from both trials.
The study primary endpoint was HIV-1 RNA levels at 12 months after enrollment. Clinical presentation was evaluated through assessment of changes in World Health Organization (WHO) disease stage [16]. HIV-1 RNA levels and CD4+ T-cell count at 3 and/or 6 months postenrollment and every 6 months thereafter, and time to ARV initiation, were also determined. Response to ARV treatment was evaluated by assessing plasma HIV-1 RNA approximately 6, 12, and 24 months after treatment, maximizing visit and ARV commencement date alignment.
A post hoc virology analysis defined the virology population as all enrolled participants with a visit after at least 6 months (180 days) of ART. Virologic response was defined as HIV-1 RNA <200 copies/mL at all visits after 6 months’ treatment; virologic failure included participants with no response, defined as HIV-1 RNA ≥200 copies/mL after 180-days’ self-reported uninterrupted treatment and those with rebound, defined as HIV-1 RNA ≥200 copies/mL at any visit after achieving <200 copies/mL after at least 180 days of ARV treatment. Samples for HIV susceptibility testing were available only if an analysis point was met at or after approximately September 2017. Descriptive statistics were used for all analyses.
Virology Methods
Population-based genotyping, using sequencing assays optimized for local HIV-1 subtypes, was performed at the Bio-Analytical Research Corporation laboratory in South Africa as previously described [17].
Testing was performed on all plasma samples with HIV-1 RNA >200 copies/mL, collected at the time of seroconversion from The Ring Study and DREAM, and at/after virologic failure (as defined for the post hoc virology analysis) in IPM 007.
Next-generation sequencing (NGS) of available samples was performed at the Microbicide Trials Network (MTN) Virology Core Laboratory, Pittsburgh, using an Illumina platform–based NGS assay with unique molecular identifiers to sequence reverse transcriptase (RT) codons 81–149 and 152–212 of the HIV-1 RT gene as previously described [5]. HIV-1 RT RAMs were identified using the Stanford HIV-1 Drug Resistance Database algorithm, version 8.4 [8, 9].
Phenotypic susceptibility testing on full-length RT plasma-derived HIV-1 was also performed at the MTN Core Virology Laboratory using a validated laboratory-developed TZM-bl luciferase-based single cycle drug susceptibility assay as previously described [5]. Fold-change (FC) values were determined relative to a parallel determination of 50% in vitro concentration of the NNRTIs dapivirine, nevirapine, efavirenz, etravirine, and rilpivirine in a wild-type recombinant virus.
RESULTS
Participant Disposition
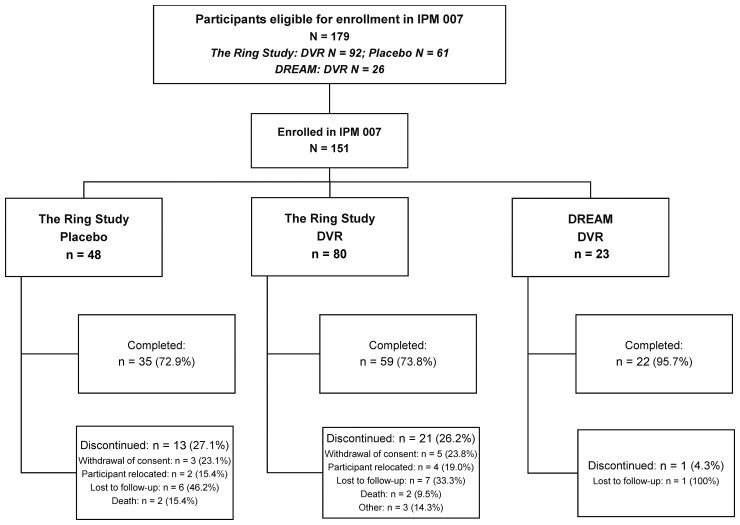
Of the 179 participants with confirmed HIV-1 seroconversion during The Ring Study (randomized 2:1 DVR:placebo ring) and DREAM, 151 (84.4%) participants enrolled in IPM 007. Of these, 128 had seroconverted during The Ring Study (DVR: 80/151 [53.0%]; placebo: 48/151 [31.8%]) and 23 (23/151 [15.2%]) during DREAM. The mean follow-up was 36.35 and 37.2 months for The Ring Study DVR and placebo participants, respectively, and 12.79 months for DREAM participants. Thirty-five of the 151 (23.2%) participants withdrew from the study early (Figure 1).
Figure 1.
Participant disposition. Percentages for the primary reasons for participant discontinuation from the study are based on the number of participants who discontinued participation from the study early in each treatment group. All other percentages are expressed as the percentage of the total number of participants enrolled in each treatment group. Abbreviations: DREAM, dapivirine ring extended access and monitoring; DVR, dapivirine vaginal ring.
Study Entry Characteristics
The mean age of the 151 participants enrolled was 26.6 years, and the majority (140/151 [92.7%]) were single. All participants were Black. Twenty-seven (17.9%) participants were on ARV treatment at enrollment into IPM 007 (Table 1). The mean time from seroconversion to IPM 007 enrollment was similar for all participant groups (The Ring Study: DVR: 134.2 days; placebo: 140 days; DREAM: 128.9 days).
Table 1.
Participant Entry Characteristics
| Characteristic | Parent Trial | Total DVR |
All Participants | ||
|---|---|---|---|---|---|
| The Ring Study Placebo |
The Ring Study DVR |
DREAM DVR |
|||
| No. of participants enrolled | 48 | 80 | 23 | 103 | 151 |
| Age, y | |||||
| ȃ18–21 | 9 (18.8) | 16 (20.0) | 0 | 16 (15.5) | 25 (16.6) |
| ȃ22–25 | 18 (37.5) | 30 (37.5) | 6 (26.1) | 36 (35.0) | 54 (35.8) |
| ȃ26–30 | 14 (29.2) | 18 (22.5) | 10 (43.5) | 28 (27.2) | 42 (27.8) |
| ȃ31–35 | 2 (4.2) | 9 (11.3) | 5 (21.7) | 14 (13.6) | 16 (10.6) |
| ȃ36 and above | 5 (10.4) | 7 (8.8) | 2 (8.7) | 9 (8.7) | 14 (9.3) |
| Marital status | |||||
| ȃMarried | 3 (6.3) | 4 (5.0) | 1 (4.3) | 5 (4.9) | 8 (5.3) |
| ȃSingle | 44 (91.7) | 75 (93.8) | 21 (91.3) | 96 (93.2) | 140 (92.7) |
| ȃSeparated | 1 (2.1) | 1 (1.3) | 1 (4.3) | 2 (1.9) | 3 (2.0) |
| On ARVsa | 2 (4.2) | 8 (10) | 17 (73.9) | 25 (24.3) | 27 (17.9) |
Data are presented as No. (%). Percentages are expressed as the percentage of the total number of participants enrolled in each treatment group.
Abbreviations: ARV, antiretroviral drug; DREAM, dapivirine ring extended access and monitoring; DVR, dapivirine vaginal ring.
As reported by participant.
Four participants did not have a genotype performed successfully during the parent trial. Virus from 25 of the remaining 147 (17.0%) participants had NNRTI RAMs detected prior to enrollment in IPM 007 and initiation of ARV treatment (NNRTI RAMs: The Ring Study: DVR: 17/78 [21.8%]; placebo: 5/48 [10.4%]; DREAM: 3/21 [14.3%]). One participant had an accessory nucleoside reverse transcriptase inhibitor RAM (E44D) (Table 2).
Table 2.
Resistance-Associated Mutations Detected Following Human Immunodeficiency Virus Type 1 (HIV-1) Infection at Any Time Point After HIV-1 Infection in The Ring Study and DREAM
| Resistance Mutation | Parent Trial | Total DVR (n = 103) |
All Participants (N = 151) |
||
|---|---|---|---|---|---|
| The Ring Study Placebo (n = 48) |
The Ring Study DVR (n = 80) |
DREAM DVR (n = 23) |
|||
| Participants with a genotype result | 48 (100) | 78 (97.5) | 21 (91.3) | 99 (96.1) | 147 (97.4) |
| Participants with NNRTI resistance mutations | |||||
| ȃNone | 43 (89.6) | 61 (76.3) | 18 (78.3) | 79 (76.7) | 120 (80.8) |
| ȃAny | 5 (10.4) | 17 (21.3) | 3 (13.0) | 20 (19.4) | 27 (16.6) |
| ȃ1 mutation | 3 (6.3) | 15 (18.8) | 3 (13.0) | 18 (17.5) | 21 (13.9) |
| ȃ2 mutations | 1 (2.1) | 2 (2.5) | 0 | 2 (1.9) | 3 (2.0) |
| ȃ≥3 mutations | 1 (2.1) | 0 | 0 | 0 | 1 (0.7) |
| Individual NNRTI mutational patterns | |||||
| A98G | 1 (2.1) | 2 (2.5) | 1 (4.3) | 3 (2.9) | 4 (2.6) |
| ȃK101E | 1 (2.1) | 0 | 1 (4.3) | 1 (1.0) | 2 (1.3) |
| ȃK103N | 1 (2.1) | 2 (2.5) | 0 | 2 (1.9) | 3 (2.0) |
| ȃE138A | 0 | 10 (12.5) | 1 (4.3) | 11 (10.7) | 11 (7.3) |
| ȃG190GA | 0 | 1 (1.3) | 0 | 1 (1.0) | 1 (0.7) |
| ȃK101E, E138A | 0 | 1 (1.3) | 0 | 1 (1.0) | 1 (0.7) |
| ȃK103KN, V106VM | 0 | 1 (1.3) | 0 | 1 (1.0) | 1 (0.7) |
| ȃV106M, Y188C | 1 (2.1) | 0 | 0 | 0 | 1 (0.7) |
| ȃV108I, Y181C, H221Y | 1 (2.1) | 0 | 0 | 0 | 1 (0.7) |
| Participants with NRTI resistance mutations | |||||
| ȃE44D | 0 | 1 (1.3) | 0 | 1 (1.0) | 1 (0.7) |
| Participants with PI major resistance mutations | |||||
| ȃM46L | 0 | 2 (2.5) | 1 (4.3) | 3 (2.9) | 3 (2.0) |
Data are presented as No. (%). Percentages are expressed as the percentage of the total number of participants enrolled in each treatment group. Human immunodeficiency virus type 1 drug resistance–associated mutations were defined according to the Stanford HIV-1 Drug Resistance Database version 8.4, dated 16 June 2017 [8, 9].
Abbreviations: DREAM, Dapivirine Ring Access and Monitoring; DVR, dapivirine vaginal ring; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Clinical Presentation
One hundred thirty-three participants (88.1%) had a 12-month visit recorded, and HIV-1 RNA and CD4+ T-cell measurements were available for 132 participants. Overall (irrespective of ARV treatment), 42.9% participants who had used the DVR in The Ring Study had HIV-1 RNA <200 copies/mL at the 12-month follow-up visit, compared with 30.0% of participants who had used a placebo ring. Of those who had used the DVR in DREAM, 95.5% had HIV-1 RNA <200 copies/mL. Mean and median log-transformed HIV-1 RNA values were similar for the participants who used the DVR or a placebo ring in The Ring Study, with lower values recorded for participants who used the DVR in DREAM. All but 2 of the 64 participants who were not on ARVs at the 12-month follow-up visit had HIV-1 RNA ≥200 copies/mL (Table 3). A sensitivity analysis of HIV-1 RNA concentrations 12 months after seroconversion in the Ring and DREAM trials (ie, ignoring any delay in enrollment into IPM 007) showed similar results (Supplementary Table 1).
Table 3.
Human Immunodeficiency Virus Type 1 RNA at the 12-Month Follow-up Visit
| Characteristic | Parent Trial | Total DVR (n = 103) |
All Participants (N = 151) |
||
|---|---|---|---|---|---|
| The Ring Study Placebo (n = 48) |
The Ring Study DVR (n = 80) |
DREAM DVR (n = 23) |
|||
| Participants with month 12 follow-up after enrollment | 41 (85.4) | 70 (87.5) | 22 (95.7) | 92 (89.3) | 133 (88.1) |
| All participants, irrespective of ARV treatment | |||||
| ȃNo. | 40 | 70 | 22 | 92 | 132 |
| ȃHIV-1 RNA <40 copies/mL | 10 (25.0) | 28 (40.0) | 20 (90.9) | 48 (52.2) | 58 (43.9) |
| ȃHIV-1 RNA 40 to <200 copies/mL | 2 (5.0) | 2 (2.9) | 1 (4.5) | 3 (3.3) | 5 (3.8) |
| ȃHIV-1 RNA ≥200 copies/mL | 28 (70.0) | 40 (57.1) | 1 (4.5) | 41 (44.6) | 69 (52.3) |
| ȃLog HIV-1 RNA, mean | 3.47 | 2.98 | 1.76 | 2.69 | 2.92 |
| ȃLog HIV-1 RNA, median | 3.99 | 2.95 | 1.59 | 1.59 | 2.68 |
| ȃLog HIV-1 RNA, range | 1.6–5.8 | 1.6–5.6 | 1.6–5.0 | 1.6–5.6 | 1.6–5.8 |
| Participants not on ARV treatmenta | |||||
| ȃNo. | 25 | 37 | 2 | 39 | 64 |
| ȃHIV-1 RNA <40 copies/mL | 0 | 1 (2.7)b | 1 (50) | 2 (5.1) | 2 (3.1) |
| ȃHIV-1 RNA 40 to <200 copies/mL | 0 | 0 | 0 | 0 | 0 |
| ȃHIV-1 RNA ≥200 copies/mL | 25 (100) | 36 (97.3) | 1 (50.0) | 37 (94.9) | 62 (96.9) |
| ȃLog HIV-1 RNA, mean | 4.24 | 4.03 | 3.28 | 4.00 | 4.09 |
| ȃLog HIV-1 RNA, median | 4.23 | 4.07 | 3.28 | 4.07 | 4.16 |
| ȃLog HIV-1 RNA, range | 2.9–5.8 | 1.6–5.6 | 1.6–5.0 | 1.6–5.6 | 1.6–5.8 |
Data are presented as No. (%) unless otherwise indicated. Percentages for the participants with 12 months of follow-up are based on the number of participants enrolled in each treatment group. All other percentages are expressed as the percentage of the total number of participants with plasma HIV-1 RNA levels at 12 months in each treatment group.
Abbreviations: ARV, antiretroviral; DREAM, dapivirine ring extended access and monitoring; DVR, dapivirine vaginal ring; HIV-1, human immunodeficiency virus type 1.
As reported by the participant.
Participant is a suspected elite controller: HIV-1 RNA remained <40 copies/mL throughout.
Consistent with viral load findings, median CD4+ T-cell counts were similar for participants who used the DVR or placebo in The Ring Study, while the median was greater for DREAM participants (Table 4). During the study, the most advanced WHO stage recorded remained stage 1 for >80% of participants, with no clinically significant differences between the groups (Supplementary Table 2). The median time to ARV initiation was similar for participants who used the DVR or placebo ring in The Ring Study (482 and 496 days, respectively). Participants who used the DVR in DREAM initiated treatment sooner after seroconversion (median, 48 days).
Table 4.
CD4+ T-Cell Counts at the 12-Month Follow-up Visit
| Characteristic | Parent Trial | Total DVR (n = 103) |
All Participants (N = 151) |
||
|---|---|---|---|---|---|
| The Ring Study Placebo (n = 48) |
The Ring Study DVR (n = 80) |
DREAM DVR (n = 23) |
|||
| Participants with month 12 follow-up after enrollment | 41 (85.4) | 70 (87.5) | 22 (95.7) | 92 (89.3) | 133 (88.1) |
| All participants, irrespective of ARV treatment | |||||
| ȃNo. | 40 | 70 | 22 | 92 | 132 |
| ȃCD4+ T-cell count <200 cells/μL | 2 (5.0) | 0 | 0 | 0 | 2 (1.5) |
| ȃCD4+ T-cell count 200–500 cells/μL | 20 (50.0) | 29 (41.4) | 2 (9.1) | 31 (33.7) | 51 (38.6) |
| ȃCD4+ T-cell count >500 cells/μL | 18 (45.0) | 41 (58.6) | 20 (90.9) | 61 (66.3) | 79 (59.8) |
| ȃMedian CD4+ T-cell count, cells/μL (range) | 486 (199–1219) |
563 (251–1378) |
755.5 (357–1137) |
605.5 (251–1378) |
570 (199–1378) |
| Participants not on ARV treatmenta | |||||
| ȃNo. | 25 | 37 | 2 | 39 | 64 |
| ȃCD4+ T-cell count <200 cells/μL | 1 (4.0) | 0 | 0 | 0 | 1 (1.6) |
| ȃCD4+ T-cell count 200–500 cells/μL | 15 (60.0) | 19 (51.4) | 1 (50.0) | 20 (51.3) | 35 (54.7) |
| ȃCD4+ T-cell count >500 cells/μL | 9 (36.0) | 18 (48.6) | 1 (50.0) | 19 (48.7) | 28 (43.8) |
| ȃMedian CD4+ T-cell count, cells/μL (range) | 477.0 (199–821) |
497.0 (251–1378) |
530 (368–692) |
497.0 (251–1378) |
493.5 (199–1378) |
Data are presented as No. (%) unless otherwise indicated. Percentages are expressed as the percentage of the total number of participants enrolled in each treatment group.
Abbreviations: ARV, antiretroviral; DREAM, dapivirine ring extended access and monitoring; DVR, dapivirine vaginal ring.
As per participant report.
Response to ARV Treatment
One hundred twenty-two (122/151 [80.8%]) participants received ARVs at some point during IPM 007. All participants received an NNRTI-based regimen (120 participants received efavirenz, and 2 nevirapine). The proportion of participants with HIV-1 RNA <40 copies/mL and <200 copies/mL at approximately 6, 12, and 24 months after ARV initiation are summarized in Table 5. The response rate for The Ring Study DVR was not lower than that for the placebo group at any time point (Table 5). Participants who seroconverted in the DREAM trial had the highest response.
Table 5.
Response to Antiretroviral Treatment
| Characteristic | Parent Trial | Total DVR |
All Participants | ||
|---|---|---|---|---|---|
| The Ring Study Placebo |
The Ring Study DVR |
DREAM DVR |
|||
| Participants with follow-up approximately 6 mo after ARV therapy initiationa | |||||
| ȃNo. | 23 | 39 | 16 | 55 | 78 |
| ȃHIV-1 RNA <40 copies/mL | 13 (56.5) | 32 (82.1) | 14 (87.5) | 46 (83.6) | 59 (75.6) |
| ȃHIV-1 RNA 40 to <200 copies/mL | 2 (8.7) | 2 (5.1) | 1 (6.3) | 3 (5.5) | 5 (6.4) |
| ȃHIV-1 RNA ≥200 copies/mL | 8 (34.8) | 5 (12.8) | 1 (6.3) | 6 (10.9) | 14 (17.9) |
| Participants with follow-up approximately 12 mo after ARV therapy initiationa | |||||
| ȃNo. | 27 | 49 | 19 | 68 | 95 |
| ȃHIV-1 RNA <40 copies/mL | 21 (77.8) | 40 (81.6) | 18 (94.7) | 58 (85.3) | 79 (83.2) |
| ȃHIV-1 RNA 40 to <200 copies/mL | 0 | 2 (4.1) | 1 (5.3) | 3 (4.4) | 3 (3.2) |
| ȃHIV-1 RNA ≥200 copies/mL | 6 (22.2) | 7 (14.3) | 0 | 7 (10.3) | 13 (13.7) |
| Participants with follow-up approximately 24 mo after ARV therapy initiationa | |||||
| ȃNo. | 26 | 39 | 4 | 43 | 69 |
| ȃHIV-1 RNA <40 copies/mL | 16 (61.5) | 31 (79.5) | 4 (100) | 35 (81.4) | 51 (73.9) |
| ȃHIV-1 RNA 40 to <200 copies/mL | 1 (3.8) | 2 (5.1) | 0 | 2 (4.7) | 3 (4.3) |
| ȃHIV-1 RNA ≥200 copies/mL | 9 (34.6) | 6 (15.4) | 0 | 6 (14.0) | 15 (21.7) |
Data are presented as No. (%) unless otherwise indicated. Percentages are expressed as the percentage of the total number of participants with plasma HIV-1 RNA levels in each visit/treatment group.
Abbreviations: ARV, antiretroviral; DREAM, dapivirine ring extended access and monitoring; DVR, dapivirine vaginal ring; HIV-1, human immunodeficiency virus type 1.
As per participant report.
Virology Analysis
At least 6 months’ uninterrupted efavirenz-based ARV treatment was received by 113 of 151 (74.8%) enrolled participants (The Ring Study: placebo: 34/48; DVR: 59/80; DREAM: 20/23); none changed therapy during the study. Virologic response was observed in 82 of 113 (72.6%) participants (The Ring Study: placebo: 20/34 [58.8%]; DVR: 42/59 [71.2%]; DREAM: 20/20 [100%]). Fourteen of the 31 failures showed initial viral suppression with later rebound (placebo: 7/14 [50.0%]; DVR: 7/17 [41.2%]) and 12 of the failures showed virologic suppression at the termination visit (placebo: 5/14 [35.7%]; DVR: 7/17 [41.2%]).
Nineteen participants with ≥180 days’ treatment had NNRTI RAMs at seroconversion (The Ring Study: DVR: n = 11; placebo: n = 5; DREAM: n = 3). Ten of the 14 (71.4%) DVR and 4 of the 5 (80.0%) placebo participants had HIV-1 RNA <200 copies/mL on study termination. This included 6 of 8 (75.0%) DVR participants enrolled with virus encoding E138A as a lone NNRTI RAM. Viruses without NNRTI RAMs at seroconversion did not show any detrimental response among those with prior DVR use compared to placebo ring use (The Ring Study: DVR: 37/48 [77.1%]; placebo: 17/31 [54.8%]; DREAM: 17/17 [100%]).
Of the failure viruses, population-based genotyping was available for 10 of 14 placebo-treated (4 failed prior to the protocol amendment) and 10 of 17 DVR-treated (4 failed prior to the protocol amendment and 3 had missing samples) participants. Although a slightly greater proportion of participants in The Ring Study DVR group had mutations at failure, numbers were too small to draw conclusions. Emerging mutations were consistent with failure during efavirenz use, with K103N the most prevalent (Table 6).
Table 6.
Summary of Nonnucleoside Reverse Transcriptase Inhibitor and Nucleoside Reverse Transcriptase Inhibitor Resistance-Associated Mutations on Failure in The Ring Studya
| Resistance Mutation | The Ring Study Placebo |
The Ring Study DVR |
|---|---|---|
| Population-based genotyping, No. | 10 | 10 |
| With no mutations | 4 (40.0) | 2 (20.0) |
| With any mutations | 6 (60.0) | 8 (80.0) |
| With new NNRTI RAMs | 4 (40.0) | 6 (60.0) |
| ȃPlus NRTI RAMs | 3 (30.0) | 2 (20.0) |
| Emergent major mutationsb | ||
| ȃL100I | 1/1 | 0/0 |
| ȃK101E | 0/0 | 1/1 |
| ȃK103N | 3/5 | 4/5 |
| ȃV106M | 0/1 | 2/2 |
| ȃE138A | 0/0 | 0/2 |
| ȃG190A | 0/0 | 1/1 |
| ȃM230L | 1/1 | 0/0 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: DVR, dapivirine vaginal ring; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; RAM, resistance-associated mutation.
NNRTI and NRTI patterns are provided in Supplementary Table 3.
NGS analysis was successful in 9 samples from each group. In addition to the population-based genotyping-detected mutations, 1 each of minority species K103N (1% prevalence) and V108I (3% prevalence) and of K103N (33% prevalence), E138G (4% prevalence) and G190A (5% prevalence) were observed in the placebo and DVR groups, respectively.
Phenotypic susceptibility determinations were further limited by test failures (results: DVR: n = 6; placebo: n = 7). Fold-changes did not indicate any reduction in dapivirine susceptibility compared to wild-type in viruses with no mutation or 1 mutation, whereas virus with 2 mutations showed moderate FCs (FC range: DVR: 5.25–13.1; placebo: 2.13–41.7). One virus with 3 mutations (K103N, E138A, P225PH) from the DVR group had greater FC (>473) (Supplementary Table 4). Viruses with 1 or more mutation had high-level resistance to efavirenz and nevirapine (FC: efavirenz: >19.8 to >21.9; nevirapine: 43.3 to >2102), but FC <10 for etravirine and rilpivirine (Supplementary Table 5).
DISCUSSION
Evaluation of HIV-1 RNA, CD4+ T-cell counts, and WHO disease stage showed no differences in participants who used the DVR in The Ring Study or DREAM, compared to those who used a placebo ring. HIV-1 RNA and CD4+ T-cell values were similar between the DVR and placebo groups from The Ring Study, while DREAM participants had lower HIV-1 RNA and higher CD4+ T-cell counts at the 12-month follow-up visit.
Time to initiation of ARV treatment was similar between the placebo and DVR groups from The Ring Study but was significantly shorter for DREAM participants.
When looking at ARV response for participants with study visits at approximately 6, 12, and 24 months after treatment initiation, the proportion of participants with HIV-1 RNA <40 copies/mL and <200 copies/mL for the DVR groups was similar to, or higher than the proportions in the placebo group. This is consistent with the results of the MTN-015 study, which evaluated clinical progression and ARV responses in participants who seroconverted in the ASPIRE study [18]. The use of different cutoffs to define virologic suppression makes comparison across studies difficult; however, the proportion of participants with HIV-1 RNA <200 copies/mL compares favorably with data from the 2017 South African National HIV Survey, which indicated that 87.3% of people living with HIV and receiving ARVs in South Africa had HIV-1 RNA <1000 copies/mL [19]. Data from a study in Uganda indicate that 95% of adults had HIV-1 RNA <1000 copies/mL after 12 months on first-line ARV regimens [20]. Overall, this indicates that participants who seroconverted in The Ring Study and DREAM, irrespective of treatment assignment, achieved ARV treatment responses in line with response rates reported in the general population of people with HIV in these regions.
The most likely explanation for the lower HIV-1 RNA and higher CD4+ T-cell counts at the 12-month follow-up visit, as well as improved ARV responses in DREAM participants, is the implementation of the universal test-and-treat (UTT) model in South Africa in September 2016 and in Uganda in February 2017. Prior to implementation of the UTT model, ARVs were initiated at a CD4+ count of <500 cells/μL [21, 22]. Due to the later trial initiation date of DREAM compared to The Ring Study, the implementation of the UTT strategy was in place for a greater proportion of the participants who seroconverted in DREAM.
The virologic findings indicate that DVR use at the time of infection was not detrimental to the later response to first-line NNRTI-based treatment during study IPM 007. Furthermore, while numbers were small, participants with virus-encoded mutations at seroconversion showed high proportions with virologic response. Importantly, the presence of E138A at seroconversion, the most prevalent mutation observed in The Ring Study, did not appear to reduce response to efavirenz-based regimens. The genotypic patterns, including additional mutations observed using NGS, observed at failure, were as expected of a first-generation NNRTI-based regimen, in most instances driven by K103N [8, 9]. Consistent with this, the failure viruses showed greater FC to efavirenz and nevirapine, than to dapivirine or the related drugs, etravirine and rilpivirine.
This study had several key limitations. In common with all follow-up cohort studies, selection processes (ie, seroconversion during The Ring Study and DREAM, and enrollment in DREAM and IPM 007) may bias the population being observed. Furthermore, any differences between treatment groups in markers of clinical progression, time to treatment initiation, and treatment response, are difficult to interpret, due to a change in the WHO ARV treatment guidelines to the UTT model during the study, the limited follow-up period, and the nonalignment of visits with time of seroconversion. For the virology analysis, the definition used to identify virologic response was applied retrospectively to reflect the more stringent endpoint used in the MTN-015 study [18]. The limited visit schedule, and challenges in aligning the visit schedule with timing of ARV initiation at external clinics, meant it was not possible to obtain timely confirmation of virologic failure and limited the number of available population-based genotype results. Additionally, dates of ARV initiation and adherence were based on participant reports and could not be verified. Finally, limited samples were available for NGS and phenotypic susceptibility analysis due to the late protocol amendment mandating the storage of samples.
Despite these limitations, the as-observed analyses provide confidence that the prior use of DVR to reduce the risk of HIV-1 infection should not result in detrimental effects on clinical presentation or treatment outcomes during later treatment with first-generation NNRTI-based regimens. Although integrase inhibitor–based regimens are now preferred for initial treatment [23], NNRTIs might still be an option when integrase inhibitors cannot be used or after failure of earlier regimens. These results demonstrate that becoming infected with HIV while using the DVR is unlikely to have any deleterious effect on management of HIV infection in the longer term.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copy edited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
John Steytler, International Partnership for Microbicides South Africa NPC, Johannesburg, South Africa.
Elna van der Ryst, Independent Consultant, Kent.
Charles Craig, Research Virology Consulting Ltd, Cambridgeshire, United Kingdom.
Ben Van Baelen, BVB Clin Consult BVBA, Herent, Belgium.
Jeremy Nuttall, International Partnership for Microbicides, Silver Spring, Maryland, USA.
Neliëtte van Niekerk, International Partnership for Microbicides South Africa NPC, Johannesburg, South Africa.
John Mellors, Microbicide Trials Network Virology Core Laboratory, University of Pittsburgh, Pennsylvania, USA.
Urvi Parikh, Microbicide Trials Network Virology Core Laboratory, University of Pittsburgh, Pennsylvania, USA.
Carole Wallis, Bio-Analytical Research Corporation Laboratory, Johannesburg, South Africa.
Notes
Acknowledgments. The authors thank the women who participated in this study for their motivation and dedication and the communities that supported this work. Acknowledgment is given to the IPM 007 clinical affairs team as well as the clinical operations teams at the research centers for their contributions to data collection during the study.
Data sharing. De-identified individual participant data from this study will be available to researchers with a methodologically sound proposal. Data requestors will need to sign a data access agreement. Publication concept proposal forms should be directed to mconradie@ipmglobal.org to gain access.
Financial support. This work was supported by the Danish Ministry of Foreign Affairs; the Flanders Ministry of Foreign Affairs; Irish Aid; the Ministry of Foreign Affairs of the Netherlands; the Norwegian Agency for Development Cooperation; UK Aid from the UK government's Foreign Commonwealth and Development Office United Kingdom Ministerie van Buitenlandse Zaken Netherlands Departement Buitenlandse Zaken Belguim; the US President’s Emergency Plan for AIDS Relief in partnership with the United States Agency for International Development (USAID); and the Bill & Melinda Gates Foundation. J. M. reports research grants to the University of Pittsburgh from the National Institutes of Health (NIH) in support of this work. J. N., J. S., and N. v. N. report support for this work as full-time employees of the International Partnership for Microbicides (IPM). U. P. reports support for this work made to institution from IPM.
References
- 1. Nel A, Van Niekerk N, Kapiga S, et al. Safety and efficacy of a dapivirine vaginal ring for HIV prevention in women. N Engl J Med 2016; 375:2133–43. [DOI] [PubMed] [Google Scholar]
- 2. Baeten JM, Palanee-Phillips T, Brown ER, et al. Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. N Engl J Med 2016; 375:2121–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. European Medicines Agency . Summary of product characteristics—dapivirine vaginal ring. Available at: https://www.ema.europa.eu/en/documents/outside-eu-product-information/dapivirine-vaginal-ring-25-mg-product-information_en-0.pdf. Accessed 14 June 2022.
- 4. Nel A, Van Niekerk N, Van Baelen B, et al. Safety, adherence, and HIV-1 seroconversion among women using the dapivirine vaginal ring (DREAM): an open-label, extension study. Lancet HIV 2021; 8:e77–86. [DOI] [PubMed] [Google Scholar]
- 5. Parikh UM, Mellors JW. Should we fear resistance from tenofovir/emtricitabine preexposure prophylaxis? Curr Opin HIV AIDS 2016; 11:49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grant RM, Liegler T. Weighing the risk of drug resistance with the benefits of HIV preexposure prophylaxis. J Infect Dis 2015; 211:1202–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mackie N Resistance to non-nucleoside reverse transcriptase inhibitors. In: Geretti AM, ed. Antiretroviral resistance in clinical practice. London, UK: Mediscript, 2006. [PubMed] [Google Scholar]
- 8. Jackson JB, Becker-Pergola G, Guay LA, et al. Identification of the K103N resistance mutation in Ugandan women receiving nevirapine to prevent vertical transmission. AIDS 2000; 14:F111–5. [DOI] [PubMed] [Google Scholar]
- 9. Cunningham CK, Chaix ML, Rekacewicz C, et al. Development of resistance mutations in women receiving standard antiretroviral therapy who received intrapartum nevirapine to prevent perinatal human immunodeficiency virus type 1 transmission: a substudy of pediatric AIDS Clinical Trials Group protocol 316. J Infect Dis 2002; 186:181–8. [DOI] [PubMed] [Google Scholar]
- 10. Lockman S, Hughes MD, McIntyre J, et al. Antiretroviral therapies in women after single-dose nevirapine exposure. N Engl J Med 2010; 363:1499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Parikh UM, Penrose KJ, Heaps AL, et al. HIV-1 drug resistance among individuals who seroconverted in the ASPIRE dapivirine ring trial. J Int AIDS Soc 2021; 24:e25833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Steegen K, Carmona S, Bronze M, et al. Moderate levels of pre-treatment HIV-1 antiretroviral drug resistance detected in the first South African National Survey. PLoS One 2016; 11:e0166305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sluis-Cremer N, Jordan MR, Huber K, et al. E138a in HIV-1 reverse transcriptase is more common in subtype C than B: implications for rilpivirine use in resource-limited settings. Antiviral Res 2014; 107:31–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lui TF, Shafer RW. Web resources for HIV type 1 genotypic-resistance test interpretation. Clin Infect Dis 2006; 42:1608–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shafer RW. Rationale and uses of a public HIV drug-resistance database. J Infect Dis 2006; 194(Suppl 1):S51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. World Health Organization. Interim WHO clinical staging of HIV/AIDS and HIV/AIDS case definitions for surveillance—African region 2005. Available at: https://apps.who.int/iris/handle/10665/69058. Accessed 30 June 2022.
- 17. Wallis CL, Papathanasopoulos MA, Lakhi S, et al. Affordable in-house antiretroviral drug resistance assay with good performance in non-subtype B HIV-1. J Virol Methods 2010; 163:505–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Riddler SA, Balkus JE, Parikh UM, et al. Clinical and virologic outcomes following initiation of antiretroviral therapy among seroconverters in the microbicide trials network-020 phase III trial of the dapivirine vaginal ring. Clin Infect Dis 2019; 69:523–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Simbayi LC, Zuma K, Zungu N, et al. South African National HIV prevalence, incidence, behaviour and communication survey, 2017. Cape Town, South Africa: HSRC Press, 2019. [Google Scholar]
- 20. Ssemwanga D, Asio J, Watera C, et al. Prevalence of viral load suppression, predictors of virological failure and patterns of HIV drug resistance after 12 and 48 months on first line antiretroviral therapy: a national cross-sectional survey in Uganda. J Antimicrob Chemother 2020; 75:1280–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. South Africa Department of Health . Implementation of the universal test and treat strategy for HIV positive patients and differentiated care for stable patients.2016. Available at: https://sahivsoc.org/Files/22%208%2016%20Circular%20UTT%20%20%20Decongestion%20CCMT%20Directorate.pdf. Accessed 30 June 2022.
- 22. World Health Organization. Progress report 2016: prevent HIV, test and treat all. WHO support for country impact. 2016. Available at: https://apps.who.int/iris/handle/10665/251713. Accessed 30 June 2022.
- 23. World Health Organization. Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach. Geneva, Switzerland: World Health Organization, 2021. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.