Abstract
Background
In 2018, the municipal Sexual Health Clinic in Seattle, implemented trans-inclusive questions about sexual behavior, anatomy, gender-affirming surgeries, and sexually transmitted infection (STI) symptoms in the clinic’s computer-assisted self-interview (CASI) to improve care for transgender and nonbinary (TNB) patients.
Methods
We calculated test positivity, the proportion of TNB patient visits that received testing for human immunodeficiency virus (HIV); syphilis; pharyngeal, rectal, and urogenital gonorrhea (GC); and chlamydia (CT) before (5/2016–12/2018) and after (12/2018–2/2020) implementation of new CASI questions, and the proportion of asymptomatic patients who received anatomic site–specific screening based on reported exposures.
Results
There were 434 TNB patients with 489 and 337 clinic visits during each period, respectively. Nonbinary patients assigned male at birth (AMAB) had the highest prevalence of GC (10% pharyngeal, 14% rectal, 12% urogenital). Transgender women, transgender men, and nonbinary people AMAB had a high prevalence of rectal CT (10%, 9%, and 13%, respectively) and syphilis (9%, 5%, and 8%). Asymptomatic transgender women, transgender men, and nonbinary patients AMAB were more likely to receive extragenital GC/CT screening compared with nonbinary patients assigned female at birth. After implementation of trans-inclusive questions, there was a 33% increase in the number of annual TNB patient visits but no statistically significant increase in HIV/STI testing among TNB patients.
Conclusions
TNB people had a high prevalence of extragenital STIs and syphilis. Implementation of trans-inclusive medical history questions at a clinic that serves cisgender and transgender patients was feasible and important for improving the quality of affirming and inclusive sexual healthcare.
Keywords: transgender, nonbinary, extragenital STI, STI screening, CASI
Transgender and nonbinary patients had a high prevalence of extragenital sexually transmitted infections and syphilis. Incorporating trans-inclusive medical history questions at a clinic that serves cisgender and transgender patients was feasible and important for expanding access to inclusive sexual healthcare.
Transgender and nonbinary (TNB) people experience disproportionately high rates of sexually transmitted infections (STIs) [1,2]. However, there is limited information on the prevalence of extragenital STIs (ie, throat and rectal infections) and screening rates among TNB people [1,3–5]. A study of 6 jurisdictions within the STD (Sexually Transmitted Diseases) Surveillance Network found a higher prevalence of extragenital infections (15% rectal and 7% pharyngeal chlamydia [CT], 12% rectal and 9% pharyngeal gonorrhea [GC]) compared with urogenital infections (1% CT, 4% GC) among 626 TNB patients [6]. This study also found that transgender men and women received less frequent extragenital testing (48% and 62%) compared with urogenital testing (83% and 78%), despite the prevalence at extragenital sites being higher than at urogenital sites [6].
The majority of extragenital and cervicovaginal GC/CT infections are asymptomatic, thus, identifying and treating these infections requires testing patients who are asymptomatic (ie, STI screening) [7–12]. Prior to the 2021 STI Treatment Guidelines, the Centers for Disease Control and Prevention recommended that clinicians screen TNB patients based on their current anatomy and sexual behaviors [13]. The recently published 2021 guidelines provide more specific recommendations. These include annual STI screening for transgender women who have had vaginoplasty at all exposed sites (eg, oral, anal, or vaginal) and sexually active transgender men and nonbinary people age <25 years if they have a cervix. The updated guidelines also recommend using a cervical swab, rather than urine specimen, to screen for cervicovaginal infections among transgender men who have had a metoidioplasty with urethral lengthening and have not had a vaginectomy [14]. Therefore, it is important for healthcare providers who conduct STI screening to ask clinically relevant and trans-inclusive questions about STI-related symptoms and anatomy due to the diversity of gender-affirming surgical procedures that are desired by and accessible to TNB people [15]. It is also important to inquire about sexual behaviors to ascertain anatomic sites of exposure [16,17].
In 2018, the municipal Sexual Health Clinic in Seattle, Washington, incorporated trans-inclusive medical history questions about sexual behavior, sex partners, current anatomy, gender-affirming surgeries, and STI symptoms into the clinic’s computer-assisted self-interview (CASI) intake questionnaire to improve the quality of human immunodeficiency virus (HIV)/STI care for TNB patients.
In this study, our aim was to describe response patterns to the new trans-inclusive medical history questions, determine whether systematic collection of these data through a CASI increased the proportion of TNB patients who received HIV/STI testing, and calculate the test positivity for extragenital and urogenital STIs.
METHODS
Study Population and Setting
The Public Health–Seattle and King County (PHSKC) Sexual Health Clinic (SHC) provides HIV/STI testing and treatment on a drop-in and sliding fee basis. We conducted a cross-sectional analysis of data collected as part of routine care from patients who attended the SHC between 5 May 2016 and 28 February 2020. All new English-speaking patients who presented to the clinic were asked to complete a CASI. For this study, we restricted our analysis to all patient visits related to a new health concern and excluded follow-up appointments that occurred within 30 days of the index appointment.
Data Collection and Measures
Throughout the entire study period, gender and sex assigned at birth were ascertained using the same 2-step question that included nonbinary/genderqueer and write-in response options (Supplementary Table 1) [18]. From 5 May 2016 to 19 December 2018, the CASI intake questionnaire was used to ascertain sexual behavior, gender of sex partners, and STI symptoms only for cisgender patients. During this period, if a patient’s response to the 2-step question indicated that they were transgender or nonbinary, the CASI intake questionnaire ended, and a provider was given a paper form to be completed via in-person interview with the patient to ascertain their current anatomy, gender-affirming medical history, sexual exposure history, and STI symptoms. However, data collection through the paper form was incomplete and inconsistent, and fewer than one-third of TNB patients had any data collected from this form. In addition, some TNB patients and community members requested that clinic and data collection procedures be the same for both cisgender and TNB patients.
In response to these requests and with the goal of facilitating affirming and inclusive healthcare experiences at the SHC, trans-inclusive medical history questions about sexual behavior, gender of sex partners, current anatomy, gender-affirming surgeries, and STI symptoms were integrated into the CASI intake questionnaire. From 20 December 2018 to 28 February 2020, these data were collected electronically for all patients, both cisgender and TNB. During this period, if a patient’s response to the 2-step question indicated that they were TNB, they were asked check-all-that-apply questions about their current anatomy, history of gender-affirming genital surgeries, and current hormone use. The clinic does not ask patients about other affirming surgical procedures (such as facial or “top” surgery) since they are not relevant to care at the SHC. The CASI then used conditional branching logic to assess STI-related symptoms in all patients based on their self-reported current anatomy and gender-affirming procedures. A complete list of question-and-response options are provided in Supplementary Table 1.
Clinic policy is to test cisgender men who have sex with men (MSM) and TNB patients who have sex with cisgender men for pharyngeal and rectal GC and CT if they report those sites of exposure, regardless of reported symptoms. Rectal exposures include receptive anal intercourse in the past 12 months; pharyngeal exposure includes performing oral sex within the last 2 months. Patients with a vagina should receive urogenital GC/CT testing if they report receptive vaginal sex, regardless of reported symptoms. Patients with a penis only receive urogenital GC/CT testing if they are symptomatic or report exposure to a partner with GC/CT. Persons who do not meet the above criteria are tested/screened based on provider discretion. Providers at the clinic recommend that all patients are screened for syphilis and HIV. The clinic uses a combination of self-collected and provider-collected specimens, based on shared patient–provider decision-making.
The clinic uses nucleic acid amplification tests (Aptima Combo 2, Hologic, San Diego, CA) to diagnose urogenital (urine or vaginal swab) and extragenital GC/CT infections. For symptomatic patients, urethral GC could also be diagnosed using urethral Gram stain and/or culture. Syphilis (primary, secondary, or latent) is diagnosed by a combination of clinical assessment and the following tests. All patients receive rapid plasma regain testing with the Treponema pallidum particle agglutination assay used for confirmatory testing; symptomatic patients and asymptomatic patients with a known syphilis exposure also receive a rapid syphilis test, while patients with a chancre also tested using darkfield microscopy. All HIV testing was done in the PHSKC laboratory using fourth-generation HIV enzyme immunoassay (BioRad GS HIV Combo Ag/Ab EIA, Hercules, CA). Cisgender and transgender MSM are also offered rapid HIV antibody tests (INSTI, bioLytical Laboratories, British Columbia).
Statistical Analyses
The unit of analysis was clinic visits for a new health concern. First, we describe the response patterns to the new CASI questions related to anatomy and gender-affirming surgical procedures. We consider the nonresponse rate as a proximal measure of acceptability since people may be less likely to respond to items perceived to be intrusive or about sensitive topics [19].
Using data from the entire study period, we estimated the proportion of TNB patients who were tested for GC/CT by anatomic site, syphilis, and HIV before (May 2016–December 2018) and after (December 2018–February 2020) the incorporation of the new trans-inclusive medical history questions. We used a 2-sided χ2 test to test for differences in these proportions. We also report the proportion of TNB patients who received a test and who had a positive test result. Using data collected after 20 December 2018, we examined the proportion of asymptomatic TNB patients who received GC/CT screening based on reported exposures by anatomic site, as defined above. We were unable to assess changes in STI screening (ie, testing in patients without symptoms) following the incorporation of trans-inclusive sexual health questions because the clinic did not systematically collect data on STI-related symptoms for TNB patients prior to 20 December 2018. All analyses were conducted in R version 3.4.2 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
There were 434 unique TNB patients with 826 clinic visits during the study period. A total of 489 visits by 298 patients occurred before and 337 visits by 214 patients occurred after the incorporation of trans-inclusive medical history questions into the CASI intake questionnaire; 78 patients attended the clinic during both periods. Most TNB patient visits were nonbinary people (41% assigned male at birth [AMAB]; 17% assigned female at birth [AFAB]), 24% were transgender women, 13% were transgender men, and 5% had another gender not listed. Additional demographics are reported in Supplementary Table 2.
We observed an increase in both the proportion of unique patients who were TNB (2.7% and 3.3%, P = .025) as well as an increase in clinic visits by TNB people (2.5% and 3.2%, P < .001). This corresponded with a 33% increase in the mean number of annual TNB patient visits per year following inclusion of the trans-inclusive questions, after adjusting for an overall secular increase in visits by cisgender patients (Supplementary Table 3).
Response Patterns
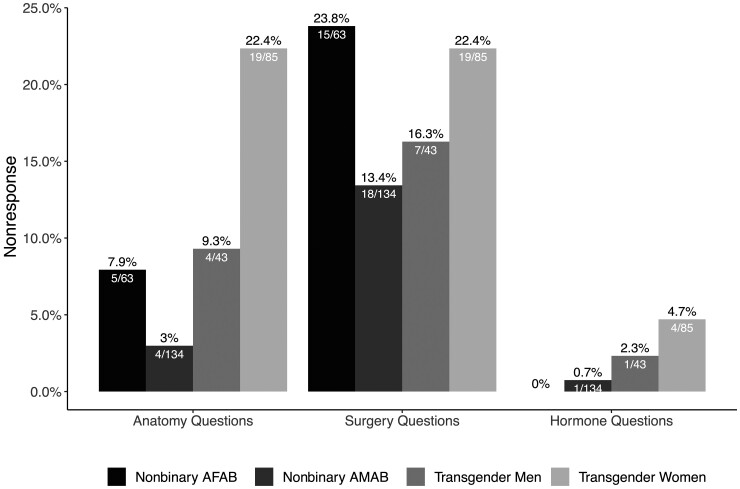
Overall, 89% (301 of 337) of TNB patients responded to questions about their current anatomy; 7% (n = 25) preferred to discuss their anatomy with their provider, 3% (n = 11) did not respond to this question. Eighty-one percent (274 of 337) of TNB patients responded to questions about past surgical procedures, and 19% (n = 63) did not respond to this question. Last, 95% (319 of 337) responded to a question about current hormone use, and 5% (18 of 337) did not respond to this question. Transgender women were most likely to prefer to discuss their anatomy with a clinician or not respond to questions about current anatomy or surgeries (Figure 1).
Figure 1.
Proportion of transgender and nonbinary patients who did not respond to questions about current anatomy, gender-affirming genital surgeries, and current hormone use. Nonresponse to the question about anatomy includes both participants who indicated they preferred to discuss their anatomy with their provider and those who did not respond to the questions. the 95% confidence intervals are reported in Supplementary Table 5. Abbreviations: AFAB, assigned female at birth; AMAB, assigned male at birth.
Most patients (94%, 258 of 274) reported having no surgical procedures; 7 reported hysterectomy, 4 oophorectomy, 1 vaginectomy, 4 orchiectomy, and 5 reproductive procedures not listed that are generally not considered to be gender-affirming procedures (eg, loop electrical excision procedure). Among patients who reported 1 or more surgeries, all responses to the questions about current anatomy were consistent with reported surgical procedures. For example, transgender men who reported having an oophorectomy and hysterectomy also reported having a vagina (and not having a cervix, uterus, or ovaries). All response patterns are provided in Supplementary Table 4.
STI/HIV Testing
After incorporating trans-inclusive questions into the CASI, we did not observe a statistically significant increase in HIV/STI testing. There was a trend toward increased pharyngeal GC/CT testing among nonbinary patients AFAB (35% vs 48%), transgender men (45% vs 61%), and transgender women (62% vs 74%), although they were not statistically significant (Table 1). We observed a similar trend in rectal GC/CT testing among transgender women (57% vs 71%) as well as HIV and syphilis testing among transgender men (69% vs 81% and 66% vs 79%), although these were also not statistically significant.
Table 1.
Proportion of Transgender and Nonbinary Patients Who Were Tested for HIV and Sexually Transmitted Infections by Anatomic Site Before (May 2016–December 2018) and After (December 2018–February 2020) the Inclusion of Trans-inclusive Sexual Health Questions in the Computer-Assisted Self-Interview Intake Questionnaire
| Proportion Who Were Tested | |||||
|---|---|---|---|---|---|
| Gender and Anatomic Site–Specific Test | May 2016–December 2018 | December 2018–February 2020 | |||
| n | % (95% CI) | n | % (95% CI) | P-value | |
| Overall | |||||
| ȃN | 489 | 337 | |||
| ȃPharyngeal GC/CT | 310 | 63.4 (59.1–67.7) | 234 | 69.4 (64.5–74.4) | .085 |
| ȃRectal GC/CT | 253 | 51.7 (47.3–56.2) | 192 | 57 (51.7–62.3) | .158 |
| ȃUrogenital GC/CT | 224 | 45.8 (41.4–50.2) | 160 | 47.5 (42.1–52.8) | .688 |
| ȃSyphilis | 394 | 80.6 (77.1–84.1) | 256 | 76 (71.4–80.5) | .133 |
| ȃHIV | 381 | 77.9 (74.2–81.6) | 256 | 76 (71.4–80.5) | .568 |
| Nonbinary people assigned female at birth | |||||
| ȃN | 83 | 63 | |||
| ȃPharyngeal GC/CT | 29 | 34.9 (24.7–45.2) | 30 | 47.6 (35.3–60.0) | .169 |
| ȃRectal GC/CT | 11 | 13.3 (6–20.5) | 10 | 15.9 (6.8–24.9) | .835 |
| ȃUrogenital GC/CT | 71 | 85.5 (78–93.1) | 55 | 87.3 (79.1–95.5) | .950 |
| ȃSyphilis | 67 | 80.7 (72.2–89.2) | 51 | 81 (71.3–90.6) | 1.000 |
| ȃHIV | 68 | 81.9 (73.6–90.2) | 49 | 77.8 (67.5–88) | .680 |
| Nonbinary people assigned male at birth | |||||
| ȃN | 203 | 134 | |||
| ȃPharyngeal GC/CT | 162 | 79.8 (74.3–85.3) | 108 | 80.6 (73.9–87.3) | .969 |
| ȃRectal GC/CT | 143 | 70.4 (64.2–76.7) | 102 | 76.1 (68.9–83.3) | .308 |
| ȃUrogenital GC/CT | 63 | 31 (24.7–37.4) | 44 | 32.8 (24.9–40.8) | .820 |
| ȃSyphilis | 170 | 83.7 (78.7–88.8) | 100 | 74.6 (67.3–82) | .056 |
| ȃHIV | 155 | 76.4 (70.5–82.2) | 99 | 73.9 (66.4–81.3) | .699 |
| Transgender men | |||||
| ȃN | 62 | 43 | |||
| ȃPharyngeal GC/CT | 28 | 45.2 (32.8–57.5) | 26 | 60.5 (45.9–75.1) | .179 |
| ȃRectal GC/CT | 18 | 29 (17.7–40.3) | 14 | 32.6 (18.6–46.6) | .865 |
| ȃUrogenital GC/CT | 45 | 72.6 (61.5–83.7) | 30 | 69.8 (56–83.5) | .925 |
| ȃSyphilis | 41 | 66.1 (54.3–77.9) | 34 | 79.1 (66.9–91.2) | .221 |
| ȃHIV | 43 | 69.4 (57.9–80.8) | 35 | 81.4 (69.8–93) | .246 |
| Transgender women | |||||
| ȃN | 110 | 85 | |||
| ȃPharyngeal GC/CT | 68 | 61.8 (52.7–70.9) | 63 | 74.1 (64.8–83.4) | .097 |
| ȃRectal GC/CT | 63 | 57.3 (48–66.5) | 60 | 70.6 (60.9–80.3) | .078 |
| ȃUrogenital GC/CT | 30 | 27.3 (18.9–35.6) | 29 | 34.1 (24–44.2) | .382 |
| ȃSyphilis | 90 | 81.8 (74.6–89) | 65 | 76.5 (67.5–85.5) | .460 |
| ȃHIV | 90 | 81.8 (74.6–89) | 69 | 81.2 (72.9–89.5) | 1.000 |
Abbreviations: CI, confidence interval; CT, chlamydia; GC, gonorrhea; HIV, human immunodeficiency virus.
STI/HIV Test Positivity
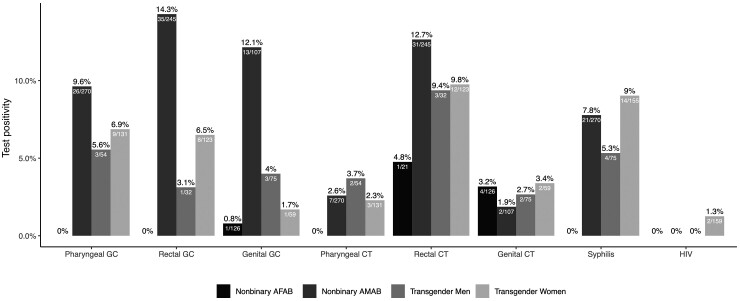
Nonbinary patients AMAB had the highest prevalence of GC (10% pharyngeal, 14% rectal, 12% urogenital; Figure 2). Transgender women, transgender men, and nonbinary people AMAB had a high prevalence of rectal CT (10%, 9%, and 13%, respectively) and syphilis (9%, 5%, and 8%, respectively). All TNB patients had similar levels of pharyngeal (range, 2%–4%) and genital CT (range, 2%–3%). Nonbinary patients AFAB had the lowest prevalence of STIs, with only 1 case of genital GC (1%), 1 case of rectal CT (5%), and 4 cases of genital CT (3%). Last, only 8 patients had prevalent HIV, and only 2 of 159 (1%) transgender women had a new positive HIV test result. There were no differences in HIV/STI positivity between the 2 time periods.
Figure 2.
HIV and sexually transmitted infection positivity among transgender and nonbinary patients attending the sexual health clinic in Seattle, Washington, May 2016–February 2020 (N = 871). The 95% confidence intervals are reported in Supplementary Table 6. Abbreviations: AFAB, assigned female at birth; AMAB, assigned male at birth; CT, chlamydia; GC, gonorrhea; HIV, human immunodeficiency virus; .
Among patients (both symptomatic and asymptomatic) who tested positive for GC/CT at any anatomic site, there was a higher prevalence of genital and extragenital coinfection among transgender men (50%, 4 of 8) and nonbinary patients AFAB (80%, 4 of 5) compared with transgender women (4%, 1 of 23) and nonbinary patients AMAB (18%, 12 of 67; Table 2). Among transgender women and nonbinary patients AMAB, the majority of GC/CT infections were only at extragenital sites (87%, 20 of 23; and 78%, 52 of 67, respectively).
Table 2.
Prevalence of Extragenital and Genital Gonorrhea/Chlamydia Coinfection Among All Transgender and Nonbinary Patients, May 2016–February 2020
| Nonbinary People Assigned Female at Birth | Nonbinary People Assigned Male at Birth | Transgender Men | Transgender Women | P-value | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | ||
| N visitsa | 122 | 273 | 78 | 141 | |||||
| Any gonorrhea/chlamydia | 5 | 4.1 (.6–7.6) | 67 | 24.5 (19.4–29.6) | 8 | 10.3 (3.5–17) | 23 | 16.3 (10.2–22.4) | <.001 |
| ȃGenital onlyb | 1 | 20.0 (.0–55.1) | 2 | 3.0 (.0–7.1) | 1 | 12.5 (.0–35.4) | 2 | 8.7 (.0–20.2) | <.001 |
| ȃExtragenital onlyb | 0 | 0.0 (.0–0.0) | 52 | 77.6 (67.6–87.6) | 3 | 37.5 (4.0–71) | 20 | 87.0 (73.2–100.7) | |
| ȃGenital and extragenital coinfectionb | 4 | 80.0 (44.9–115.1) | 12 | 17.9 (8.7–27.1) | 4 | 50.0 (15.4–84.6) | 1 | 4.3 (.0–12.7) | |
Includes patients from both time periods who were both symptomatic and asymptomatic, from May 2016 to February 2020.
Abbreviation: CI, confidence interval.
Restricted to visits that tested for gonorrhea/chlamydia for at least 1 anatomic site.
Proportion of all gonorrhea/chlamydia infections.
Screening for Asymptomatic STIs
Among TNB patients who reported sex with a cisgender man, nearly all (95%) reported a pharyngeal exposure (Table 3). Nonbinary patients AMAB and transgender women were more likely to report a rectal exposure (87% and 80%) than nonbinary patients AFAB and transgender men (31% and 42%, P < .001). Most nonbinary patients AFAB and transgender men (94% and 90%) and 7% of transgender women reported a vaginal exposure.
Table 3.
Proportion of Asymptomatic Transgender and Nonbinary Patients Who Received Anatomic Site–Specific Screening for Gonorrhea and Chlamydia Based on Their Reported Exposures and Sex With a Cisgender Man, December 2018–February 2020
| Nonbinary People Assigned Female at Birth | Nonbinary People Assigned Male at Birth | Transgender Men | Transgender Women | P-value | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | ||
| N visits | 63 | 134 | 43 | 85 | |||||
| Reported sex with a cisgender man | 48 | 76.2 (65.7–86.7) | 121 | 90.3 (85.3–95.3) | 31 | 72.1 (58.7–85.5) | 79 | 92.9 (87.5–98.4) | .001 |
| Pharyngeal GC/CT | |||||||||
| ȃReported pharyngeal exposurea | 47 | 97.9 (93.9–100.0) | 120 | 99.2 (97.6–100.0) | 29 | 93.5 (84.9–100.0) | 68 | 86.1 (78.4–93.7) | .001 |
| ȃScreenedb | 23 | 48.9 (34.6–63.2) | 104 | 86.7 (80.6–92.7) | 21 | 72.4 (56.1–88.7) | 55 | 80.9 (71.5–90.2) | <.001 |
| Rectal GC/CT | |||||||||
| ȃReported rectal exposurea | 15 | 31.3 (18.1–44.4) | 105 | 86.8 (80.7–92.8) | 13 | 41.9 (24.6–59.3) | 63 | 79.7 (70.9–88.6) | <.001 |
| ȃSymptomaticc and reported rectal exposure | 0 | 0.0 (.0–0.0) | 7 | 6.7 (1.9–11.4) | 1 | 7.7 (.0–22.2) | 5 | 7.9 (1.3–14.6) | .738 |
| ȃAsymptomatic and reported rectal exposure | 15 | 100.0 (100.0–100.0) | 98 | 93.3 (88.6–98.1) | 12 | 92.3 (77.8–100.0) | 58 | 92.1 (85.4–98.7) | .738 |
| ȃScreenedb | 3 | 20 (.0–40.2) | 84 | 85.7 (78.8–92.6) | 10 | 83.3 (62.2–100.0) | 48 | 82.8 (73–92.5) | <.001 |
| Vaginal GC/CT | |||||||||
| ȃReported vaginal exposurea | 45 | 93.8 (86.9–100.6) | 0 | 0.0 (.0–0.0) | 28 | 90.3 (79.9–100.0) | 5 | 6.3 (1.0–11.7) | <.001 |
| ȃSymptomaticc and reported vaginal exposure | 13 | 28.9 (15.6–42.1) | 0 | 0.0 (.0–0.0) | 9 | 32.1 (14.8–49.4) | 0 | 0.0 (.0–0.0) | .335 |
| ȃAsymptomatic and reported vaginal exposure | 32 | 71.1 (57.9–84.4) | 0 | 0.0 (.0–0.0) | 19 | 67.9 (50.6–85.2) | 5 | 100.0 (100.0–100.0) | .335 |
| ȃScreenedb | 28 | 87.5 (76.0–99.0) | 0 | 0.0 (.0–0.0) | 16 | 84.2 (67.8–100.0) | 3 | 60 (17.1–102.9) | .297 |
This analysis was restricted to patients who attended the sexual health clinic from December 2018 through February 2020 and who reported sex with a cisgender man in the past year. During this period, clinic policy was to screen asymptomatic transgender and nonbinary patients if they reported an anatomic site–specific exposure and sex with a cisgender man in the last year. Among patients with a penis, we were unable to assess the proportion who received urogenital screening for asymptomatic infection based on their reported exposures because clinic policy is to only provide urogenital GC/CT tests if they are symptomatic based on an evaluation of Public Health–Seattle and King County Sexual Health Clinic data.
Abbreviations: CI, confidence interval; CT, chlamydia; GC, gonorrhea.
Pharyngeal exposure is defined as performing oral genital sex in the past 2 months. Rectal exposure is defined as receptive anal sex in the past 12 months. Vaginal exposure is defined as receptive vaginal sex in the past 12 months.
The proportion of asymptomatic patients with an anatomic site–specific exposure that received GC/CT testing. All patients who reported a pharyngeal exposure are included in the denominators for the proportion of patients who received pharyngeal screening.
Rectal symptoms are defined as self-reported pain, discomfort, or discharge from the rectum. Vaginal symptoms are defined as self-reported abnormal vaginal discharge, pain, or burning during urination.
Among asymptomatic patients who were eligible for screening according to clinic guidelines, we observed that rectal GC/CT screening was similar among transgender women, transgender men, and nonbinary patients AMAB (83%, 83%, and 86%, respectively). Pharyngeal GC/CT screening was slightly lower among transgender men (72%) compared with transgender women (81%) and nonbinary patients AMAB (87%). Nonbinary patients AFAB were significantly less likely to receive extragenital GC/CT screening than other groups (49% pharyngeal and 20% rectal) despite reporting pharyngeal/rectal exposures and partnering with cisgender men. There were no statistically significant differences in vaginal GC/CT screening by gender.
DISCUSSION
TNB people attending the public health SHC in Seattle had a high prevalence of extragenital STIs and syphilis, and STI test positivity varied by gender and anatomic site. Incorporating trans-inclusive medical history questions about sexual behavior, current anatomy, gender-affirming surgeries, and STI symptoms into a CASI intake questionnaire allowed us to determine whether asymptomatic TNB patients received screening based on their anatomy and sexual exposures. Overall, a high proportion of transgender women, transgender men, and nonbinary patients AMAB who reported an exposure received anatomic site–specific GC/CT screening, although extragenital screening was low for nonbinary people AFAB.
Similar to prior studies conducted among transgender men and women, we observed that TNB patients had a higher prevalence of extragenital GC/CT infections compared with urogenital infections [6]. To our knowledge, this is one of the first studies to report on extragenital STIs among nonbinary people. Notably, nonbinary people AMAB had the highest prevalence of extragenital GC and rectal CT. Nonbinary people AMAB also had a high prevalence of urogenital GC/CT and syphilis. In contrast, nonbinary patients AFAB had the lowest prevalence of STIs. However, this low prevalence may be an underestimate due to the low rates of extragenital screening among nonbinary people AFAB. This highlights the need to collect data on nonbinary identities and that stratifying nonbinary patients by their sex assigned at birth may be important for identifying disparities in access to care and for characterizing the epidemiology of HIV/STIs, the prevalence of asymptomatic extragenital infections, and their clinical significance.
Contrary to our hypothesis, adding trans-inclusive sexual and medical history questions in the CASI did not appear to increase testing rates among TNB patients. Prior to updating the CASI, collection of these data by clinicians via a paper form was incomplete; however, implementing these questions in the CASI may not have significantly impacted clinician practices regarding testing offered to TNB patients. Small sample sizes may be a partial explanation for our null result since we observed statistically nonsignificant trends toward increased testing among some TNB people. However, even if inclusion of these new questions in the CASI did not change clinic testing patterns, our primary goal was to be responsive to community requests for more inclusive clinic procedures and to facilitate affirming healthcare experiences for TNB patients at our clinic.
Most (89%) patients responded to the check-all-that-apply question that assessed current anatomy, suggesting that it may be slightly more acceptable or perceived as less intrusive than the question about prior gender-affirming genital surgeries, to which 81% of patients provided a response. Nonetheless, incorporating trans-inclusive questions into a clinical intake form may be a simple intervention to facilitate patient–provider conversations and improve the provision of affirming sexual healthcare. Although we were unable to directly assess this in the present study, we did observe statistically significant increases in the proportion of TNB patients as well as the proportion and number of clinic visits by TNB people, which may be suggestive that the clinic was perceived to be more welcoming to TNB patients. Future qualitative research is needed to better understand why TNB patients perceive these questions to be acceptable or unacceptable/intrusive and how it impacts their experience of receiving care at our clinic.
There are several limitations to our current approach. Among individuals who were not tested, we were unable to determine if testing was not offered/ordered by the clinician or if the test was declined by patients. We also were unable to exclude patient visits that were for HIV preexposure prophylaxis (PrEP) follow-up and management from our analysis, which may result in a slight overestimate of screening rates in our clinic population. Notably, more than one-third of patients who reported having no surgeries only reported having a penis/phallus or vagina/front hole and did not indicate having additional reproductive anatomy (eg, a cervix or testes). These response patterns may be due, in part, to the check-all-that-apply format or the use of medical terminology. In addition, the current survey relies on biomedical terms and does not allow patients to choose the language/terms used to reference their anatomy/body and does not incorporate gender-affirming language options [20,21]. There is some evidence that TNB patients at the clinic may nonetheless be using affirming language to describe their anatomy instead of biomedical terminology (eg, transgender men who reported no prior surgeries and reported having a penis/phallus; Supplementary Table 4) [22].
Recent studies have demonstrated the importance of trans-inclusive language when talking about sexual behaviors and anatomy [23–25]. One study conducted among transmasculine people found that few providers (27%) had ever asked about their preferred language for their genitalia/anatomy and that only 65% of participants wanted a provider to use biomedical or clinical terminology [24]. There exists only 1 published example of a CASI that facilitates linguistic self-determination. Moseson et al developed a customizable electronic survey that allows TNB people AFAB to determine what words are used to refer to their reproductive anatomy [25]. Further research is needed to develop and validate survey items for ascertaining sexual health for TNB people.
Overall, our findings demonstrate the feasibility of implementing trans-inclusive questions at a clinic that serves both cisgender and transgender patients. Given the high prevalence of STIs observed among TNB patients, creation of trans-affirming environments at low-cost, low-barrier public sexual health clinics is critical for improving the quality of care and expanding access to inclusive, timely, and affordable sexual healthcare for TNB people.
Supplementary Material
Contributor Information
Diana M Tordoff, Department of Epidemiology, University of Washington, Seattle, Washington, USA.
Julia C Dombrowski, Department of Epidemiology, University of Washington, Seattle, Washington, USA; Public Health–Seattle and King County HIV/STD Program, Seattle, Washington, USA; Department of Medicine, University of Washington, Seattle, Washington, USA.
Meena S Ramchandani, Public Health–Seattle and King County HIV/STD Program, Seattle, Washington, USA; Department of Medicine, University of Washington, Seattle, Washington, USA.
Lindley A Barbee, Public Health–Seattle and King County HIV/STD Program, Seattle, Washington, USA; Department of Medicine, University of Washington, Seattle, Washington, USA.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Dr Matthew Golden for reviewing and providing feedback on this article.
Financial support. D. M. T. receives support from the National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (F31AI152542).
References
- 1. Van Gerwen OT, Jani A, Long DM, Austin EL, Musgrove K, Muzny CA. Prevalence of sexually transmitted infections and human immunodeficiency virus in transgender persons: a systematic review. Transgender Heal 2020; 5:90–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Becasen JS, Denard CL, Mullins MM, Higa DH, Sipe TA. Estimating the prevalence of HIV and sexual behaviors among the US transgender population: a systematic review and meta-analysis, 2006–2017. Am J Public Health 2018; 109:e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Man OM, Ramos WE, Vavala G, et al. Optimizing screening for anorectal, pharyngeal, and urogenital C. trachomatis and N. gonorrhoeae infections in at risk adolescents and young adults in New Orleans, Louisiana and Los Angeles, California, USA. Clin Infect Dis 2020; 73:e3201–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hiransuthikul A, Janamnuaysook R, Sungsing T, et al. High burden of chlamydia and gonorrhoea in pharyngeal, rectal and urethral sites among Thai transgender women: implications for anatomical site selection for the screening of STI. Sex Transm Infect 2019; 95:534–9. [DOI] [PubMed] [Google Scholar]
- 5. Johnson A, Reisner S, Mimiaga M, Garofalo R, Kuhns L. Prevalence and perceived acceptability of nongenital sexually transmitted infection testing in a cohort of young transgender women. LGBT Heal 2018; 5:381–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pitasi MA, Kerani RP, Kohn R, et al. Chlamydia, gonorrhea, and HIV infection among transgender women and transgender men attending clinics that provide STD services in six US cities. Sex Transm Dis 2018; 46:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Annan NT, Sullivan AK, Nori A, et al. Rectal chlamydia—a reservoir of undiagnosed infection in men who have sex with men. Sex Transm Infect 2009; 85:176–9. [DOI] [PubMed] [Google Scholar]
- 8. Kent CK, Chaw JK, Wong W, et al. Prevalence of rectal, urethral, and pharyngeal chlamydia and gonorrhea detected in 2 clinical settings among men who have sex with men: San Francisco, California, 2003. Clin Infect Dis 2005; 41:67–74. [DOI] [PubMed] [Google Scholar]
- 9. Morris SR, Klausner JD, Buchbinder SP, et al. Prevalence and incidence of pharyngeal gonorrhea in a longitudinal sample of men who have sex with men: the EXPLORE study. Clin Infect Dis 2006; 43:1284–9. [DOI] [PubMed] [Google Scholar]
- 10. Hunte T, Alcaide M, Castro J. Rectal infections with chlamydia and gonorrhoea in women attending a multiethnic sexually transmitted diseases urban clinic. Int J STD AIDS 2010; 21:819–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Platt R, Rice PA, McCormack WM. Risk of acquiring gonorrhea and prevalence of abnormal adnexal findings among women recently exposed to gonorrhea. JAMA 1983; 250:3205–9. [PubMed] [Google Scholar]
- 12. Martín-Sánchez M, Fairley CK, Ong JJ, et al. Clinical presentation of asymptomatic and symptomatic women who tested positive for genital gonorrhoea at a sexual health service in Melbourne, Australia. Epidemiol Infect 2020; 148:e240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Workowski KA, Bolan GA, Centers for Disease Control and Prevention . Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64:1–137. Available at:https://www.cdc.gov/std/tg2015/tg-2015-print.pdf. Accessed 16 November 2020. [PMC free article] [PubMed] [Google Scholar]
- 14. Centers for Disease Control and Prevention . Transgender and gender diverse persons. 2021. Available at: https://www.cdc.gov/std/treatment-guidelines/trans.htm. Accessed 27 July 2021.
- 15. James SE, Herman JL, Rankin S, et al. The report of the 2015 U.S. Transgender Survey. Washington, DC: National Center for Transgender Equality, 2016. Available at: http://www.transequality.org/sites/default/files/docs/usts/USTSFullReport-FINAL1.6.17.pdf. Accessed 11 December 2021. [Google Scholar]
- 16. Reisner SL, Deutsch MB, Bhasin S, et al. Advancing methods for US transgender health research. Curr Opin Endocrinol Diabetes Obes 2016; 23:198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reisner SL, Radix A, Deutsch MB. Integrated and gender-affirming transgender clinical care and research. J Acquir Immune Defic Syndr 2016; 72(Suppl 3):S235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tordoff DM, Morgan J, Dombrowski JC, Golden MR, Barbee LA. Increased ascertainment of transgender and non-binary patients using a two-step versus one-step gender identity intake question in a STD clinic setting. Sex Transm Dis 2019; 46:254––9.. [DOI] [PubMed] [Google Scholar]
- 19. Tourangeau R, Yan T. Sensitive questions in surveys. Psychol Bull 2007; 133:859–83. [DOI] [PubMed] [Google Scholar]
- 20. Zimman L. Trans people’s linguistic self-determination and the dialogic nature of identity. In: (Re)Presenting trans: linguistic, legal, and everyday perspectives. Wellington, New Zealand: Victoria University Press, 2017:226–48. [Google Scholar]
- 21. Zimman L. Transgender language reform: some challenges and strategies for promoting trans-affirming, gender-inclusive language. J Lang Discrim 2017; 1:84–105. [Google Scholar]
- 22. Zimman L. The discursive construction of sex: remaking and reclaiming the gendered body in talk about genitals among trans men. In: Zimman L, Davis J, Raclaw J, eds. Queer excursions: retheorizing binaries in language, gender, and sexuality. New York: Oxford University Press, 2014:13–34. [Google Scholar]
- 23. Tordoff DM, Haley SG, Shook Aet al. “Talk about bodies”: recommendations for using transgender-inclusive language in sex education curricula. Sex Roles 2020; 84:152–65. [Google Scholar]
- 24. Klein A, Golub SA. Enhancing gender-affirming provider communication to increase health care access and utilization among transgender men and trans-masculine non-binary individuals. LGBT Heal 2020; 7:292–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moseson H, Lunn MR, Katz A, et al. Development of an affirming and customizable electronic survey of sexual and reproductive health experiences for transgender and gender nonbinary people. PLoS One 2020; 15:e0232154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.