Abstract
The aggregation substance (AS) of Enterococcus faecalis, encoded on sex pheromone plasmids, is a surface-bound glycoprotein that mediates aggregation between bacteria thereby facilitating plasmid transfer. Sequencing of the pAD1-encoded Asa1 revealed that this surface protein contains two RGD motifs which are known to ligate integrins. Therefore, we investigated the influence of AS on the interaction of E. faecalis with human monocyte-derived macrophages which constitutively express β2 integrins (e.g., CD18). AS was found to cause a greater-than-fivefold increase in enterococcal adherence to macrophages and a greater-than-sevenfold increase in phagocytosis. Adherence was mediated by an interaction between the RGD motif and the integrin CD11b/CD18 (complement receptor type 3) as demonstrated by inhibition studies with monoclonal antibodies and RGD peptide. AS-bearing enterococci were significantly more resistant to macrophage killing during the first 3 h postinfection, probably due to inhibition of the respiratory burst as indicated by reduced concentrations of superoxide anion.
Enterococci are gram-positive cocci which inhabit the gastrointestinal tract as well as the vagina and the oral cavity. Enterococcus faecalis accounts for 90% of human enterococcal infections, the most common being urinary tract infections, followed by abdominal infections, wound infections, bacteremia, and infective endocarditis (31, 39). Although infections due to E. faecalis have increased substantially during the last 10 years, the understanding of virulence mechanisms is still limited (24). One of the postulated virulence factors is the aggregation substance (AS), a sex pheromone plasmid-encoded surface protein which promotes the conjugative transfer of sex pheromone plasmids by formation of mating aggregates between donor and recipient cells (6, 13, 52). DNA sequencing of the structural gene for the pAD1-encoded AS revealed the presence of two Arg-Gly-Asp (RGD) sequences (16); RGD is a well-known motif recognized by a family of eukaryotic receptors, the integrins (38). Integrins consist of noncovalently linked α and β chains and are expressed on leukocytes, thrombocytes, endothelium, and various epithelial cells (21, 37, 42). Our group first suggested an interaction of AS with integrins, since we found that AS augmented adherence to porcine renal tubular cells which could be inhibited competitively by an RGD-Ser (RGDS) peptide (26). This hypothesis was corroborated by in vitro experiments with human polymorphonuclear leukocytes (PMN) which demonstrated that AS promotes opsonin-independent binding of E. faecalis via a β2 integrin-mediated mechanism (46). It is assumed that many enterococcal infections are endogenous, originating from the intestinal tract (25, 51). Wells et al. speculated that macrophages may serve as a vehicle facilitating translocation from the intestinum into the lymph system and bloodstream (49, 50). However, this can occur only if enterococci are able to survive within macrophages. Indeed, Gentry-Weeks et al. demonstrated that E. faecalis can survive for a prolonged period in mouse peritoneal macrophages and that this ability is not affected by cytolysin or gelatinase (17). However, the influence of AS on E. faecalis survival in macrophages was not studied.
Therefore, we investigated the influence of pAD1-encoded Asa1 on adherence, phagocytosis, and survival of E. faecalis within human macrophages. Asa1 was found to significantly augment adherence and internalization by macrophages via an interaction with the integrin CD11b/CD18 (complement receptor type 3 [CR3], macrophage-1 antigen [Mac-1]). Our results suggest that AS-positive enterococci outlived phagocytosis significantly better than the AS-negative strain by inhibition of the respiratory burst.
(This work was presented in part at the 98th General Meeting of the American Society for Microbiology, Atlanta, Ga., 17 to 21 May 1998.)
MATERIALS AND METHODS
Isolation and culture of human macrophages.
Human monocytes were purified from buffy coats on Ficoll-Paque (Pharmacia, Freiburg, Germany) and Percoll (Sigma Chemicals, Munich, Germany) gradients as described previously (54). For better separation of lymphocytes and monocytes, the osmolarity of the Percoll gradients was modified (density, 1.068 g/ml; 335 mosM) by mixing 4.81 parts of Percoll, 0.95 part of 10× phosphate-buffered saline (PBS; BioWhittaker, Verviers, Belgium), and 4.24 parts of distilled water (3, 54). Cells were cultured in 12.5% human AB serum (PAA, Linz, Austria) in Teflon beakers (Nalge Co., Rochester, N.Y.). After 5 to 8 days, when monocytes had matured into macrophages, cells were washed twice with PBS and were resuspended in HAP buffer (PBS containing 3 mM glucose, 0.5 mg of human serum albumin per ml [Sigma], and 0.3 U of aprotinin per ml [Sigma]) to a final concentration of 2.5 × 105/ml. The resultant cell suspension contained ≥90% macrophages as determined by light scatter and CD14 expression in a cytofluorograph (FACScan; Becton Dickinson, Heidelberg, Germany). Cell viability was >98%, as assessed by the trypan blue exclusion test.
Bacterial strains.
The E. faecalis strains used in this study are listed in Table 1 and have previously been described in detail (33). The deletion derivatives of the asa1 gene reside on the shuttle vector pWM401 and are pheromone controlled via complementing pAM944, a Tn917 derivative of pAD1 defective in asa1. Enterococci were maintained on Todd-Hewitt agar (THB; Oxoid, Basingstroke, Hants, England) supplemented with 10 μg of erythromycin (EM; Sigma) and 10 μg of chloramphenicol (CM; Sigma) per ml as indicated. For experiments, enterococci were grown in fresh THB at 37°C with gentle shaking. To induce expression of AS, synthetic sex pheromone cAD1 was added to bacterial suspensions with an optical density at 600 nm (OD600) of 0.2 at concentrations exceeding the minimal inducing concentration by 100-fold. After incubation for 2 to 3 h, bacteria were harvested, washed, and resuspended in PBS. Just before use, enterococcal suspensions were gently sonicated, usually with 80 W continuously for 20 s at 15°C (Branson sonifier W-450; Branson Ultrasonics Corp., Danbury, Conn.) to disrupt bacterial clumps. Electron microscopy and adherence assays confirmed that sonication influenced neither the structure of the cell surface nor the adhesion characteristics.
TABLE 1.
Bacterial strains useda
| Strain | No. | AS | Antibiotics | Reference(s) |
|---|---|---|---|---|
| OG1X and OG1X(pAM944) | I | None, EM | 14, 23 | |
| OG1X(pAM721) and OG1X(pAM944/pWHH6) | II | EM, EM/CM | 22, 33 | |
| OG1X(pAM944/pWHH6) (ΔPvuII, 0.2 kb) | III | EM/CM | 33 | |
| OG1X(pAM944/pWHH6) (ΔEcoRI, 0.6 kb) | IV | EM/CM | 33 | |
| OG1X(pAM944/pWHH6) (ΔXhoII, 1.3 kb) | V | EM/CM | 33 | |
| OG1X(pAM944/pWHH6) (ΔPst, 1.1 kb) | VI | EM/CM | 33 | |
| OG1X(pAM944/pWHH6) (ΔPstI, 1.3 kb) | VII | EM/CM | 33 | |
| OG1X(pAM944/pWHH6) (ΔPstI, 2.4 kb) | VIII | EM/CM | 33 |
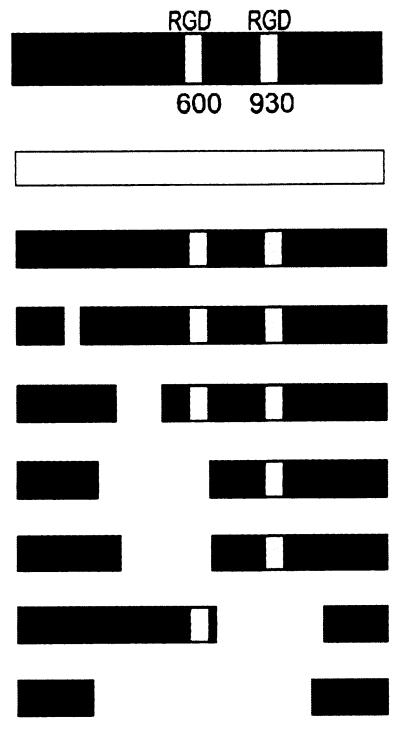
The localization of deletions in mutants III to VIII is demonstrated in relation to a schematic presentation of the AS Asa1 exposed on the cell surface.
Labeling of bacteria.
For some experiments, bacteria were labeled with fluorescein isothiocyanate (FITC, 1 mg/ml; Sigma) as described previously (36). Subsequently, the bacteria were washed three times with PBS and adjusted to a final concentration of 108 bacteria/ml in HAP buffer as determined by measuring the OD600. Plating of FITC-labeled bacteria on Mueller-Hinton agar and adherence assays with native bacteria demonstrated that the labeling with FITC affected neither viability nor adhesion characteristics of the tested strains.
Adherence assay.
Five microliters of macrophage suspension (2.5 × 105/ml) was incubated in 60-well Terasaki culture plates (Nunc, Naperville, Ill.) for 45 min at 37°C and 5% CO2 (54). To eliminate unbound cells, wells were washed twice with PBS before FITC-labeled enterococci (2.5 × 107 to 1 × 108 CFU/ml; 5 μl/well) were added for 15 to 60 min at 37°C. Subsequently, the wells were washed five times to remove nonadherent bacteria. The attachment of bacteria within 60 min was scored as an adherence index (AI) which includes both the adherent and internalized bacteria (4). The AI was defined as the mean number of bacteria on 100 macrophages counted by fluorescence microscopy in a 40× field using an inverted microscope (IMT2-RFL; Olympus Optical Co., Hamburg, Germany). Values for three replicate wells were averaged; all assays were performed in quadruplicate.
For inhibition studies, adherent macrophages were preincubated for 20 min at 37°C with 5 μl of PBS containing the peptides Arg-Gly-Asp-Ser (RGDS; Sigma) and Arg-Ala-Asp-Ser (RADS; kindly supplied by R. Süßmuth, Department of Organic Chemistry, Eberhard-Karls-University, Tuebingen, Germany) or one of the following monoclonal antibodies (MAbs): IB4 (anti-CD18, mouse immunoglobulin G2a [IgG2a], kindly provided by E. Tuomanen, Department of Infectious Diseases, St. Jude Medical Hospital, Memphis, Tenn.) (53), ICRF44 (anti-CD11b, mouse IgG1; Serotec Ltd., Oxford, England) (29), DF1524 (anti-CD11a, mouse IgG2b; Serotec) (10), or 3.9 (anti-CD11c, mouse IgG1; Serotec) (19, 29). The mouse MAbs IgG2aκ (Sigma), IgG2b (Serotec), and IgG1 (Serotec) served as negative controls. Thereafter, 5 μl of FITC-labeled bacteria in HAP buffer (2.5 × 107 CFU/ml) was added for the actual binding assay. All assays were performed in triplicate.
Internalization of E. faecalis.
Cells were examined after 15 and 60 min of incubation with FITC-labeled enterococci. To distinguish between intracellular and extracellular bacteria, the green fluorescence of extracellular enterococci was quenched by addition of ethidium bromide (Sigma) at a final concentration of 50 μg/ml. In this assay, intracellular bacteria fluoresce green whereas extracellular bacteria fluoresce red (12). For each data point, at least 200 macrophages in each of double cultures were scored under a fluorescence microscope (Axioplan2; Zeiss, Jena, Germany). All assays were done in duplicate.
CL assay.
Free-oxygen radical formation by macrophages in response to E. faecalis was studied by lucigenin-enhanced chemiluminescence (CL) (2, 48) measured with a MicroLumat LB96P (EG&G Berthold, Bad Wildbad, Germany) at 37°C. Fifty microliters of lucigenin (bis-N-methyl acridinium nitrate, 2.5 × 10−4 M, Sigma) was added to 100 μl of suspended macrophages (0.5 × 106 cells) in a 96-well microtiter plate and placed in the detection chamber for 15 min for temperature equilibration. To activate the CL reaction, 50 μl of bacterial suspension was added at multiplicities of infection (MOIs) of 10:1, 20:1, and 50:1. Unopsonized zymosan (final concentrations, 0.04, 0.08, and 0.2 mg/ml; Sigma) boiled for 15 min in a water bath was used as control stimulus (43). CL response was recorded as relative light units (RLU) at 2-min intervals for 120 min. Initial CL activity induced by enterococci was expressed by integrated responses over a 15-min period from the start of the reaction. All experiments were carried out in triplicate. Control wells containing macrophages in buffer alone showed only weak spontaneous generation of CL (mean, 16 RLU). All data were corrected for this baseline CL.
Intracellular survival of E. faecalis.
The invasion of bacteria into macrophages was quantified by a standard antibiotic protection assay (15). Briefly, 0.5 × 106 macrophages were seeded into 96-well plates and allowed to form a confluent monolayer. Enterococci were resuspended in HAP buffer and added to each well at an MOI of 10:1 for 60 min at 37°C and 5% CO2. After washing (time zero), residual extracellular bacteria were killed by incubating with HAP buffer containing 12% heat-inactivated normal human serum, supplemented with 10 μg of gentamicin (Sigma) per ml and 100 μg of penicillin (Sigma) per ml (44), for 2.5 h. Cells were washed again, and antibiotic-free HAP buffer was added. Subsequently, macrophages were washed eight times with PBS and lysed for 3 to 5 min with 0.1% Triton X-100 (Sigma). To assess the number of viable intracellular bacteria and to confirm complete elimination of extracellular bacteria after incubation with antibiotics, serial dilutions of cell lysates and tissue culture supernatants were plated on Mueller-Hinton blood agar (Heipha, Heidelberg, Germany). Intracellular killing was expressed as the percent reduction of the initial number of viable intracellular bacteria (11, 28, 41).
Statistics.
Data were expressed as mean ± standard deviation of the indicated number of experiments. Differences between groups were tested by Student's t test for paired samples and were considered significant for P values of ≤0.05.
RESULTS
Adherence of E. faecalis to human macrophages.
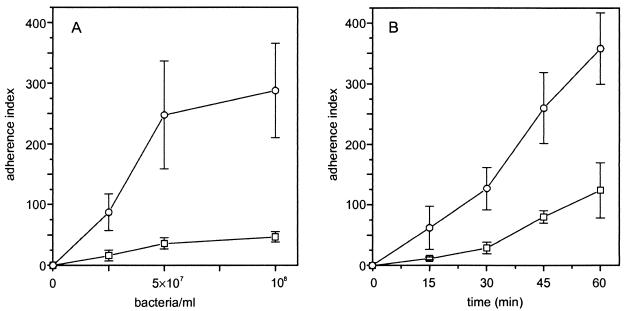
To investigate if AS promotes adherence to macrophages, the constitutively AS-expressing strain OG1X(pAM721) and the AS-negative strain OG1X were tested for their ability to adhere to macrophages. As shown in Fig. 1A, adherence of both strains occurred in a concentration-dependent manner. After incubation for 15 minutes, expression of AS augmented adherence to macrophages by more than fivefold for all tested concentrations. At bacterial densities of >108/ml, adherent bacteria were too numerous to count. Figure 1B demonstrates that a time dependence was observed for incubation times between 15 and 60 min during which binding of both strains increased in a linear fashion. Since the sex pheromone plasmid of E. faecalis OG1X(pAM721) codes not only for AS but also for cytolysin, we studied the influence of this trait on adherence to macrophages by comparing the binding capacity of the cytolysin-positive strain OG1X(pAM944), which produces an AS without a membrane anchor so that AS is shed from the bacterial cell surface, with that of the cytolysin- and AS-negative strain OG1X. The fact that both strains showed the same poor adhesion to macrophages [OG1X(pAM944) AI, 10.5 ± 6.1 and 117 ± 46 after 15 and 60 min, respectively; OG1X AI, 11.5 ± 2.6 and 122 ± 55 after 15 min and 60 min, respectively] indicates that cytolysin does not affect adherence to human macrophages.
FIG. 1.
Effect of bacterial concentration and time on adherence of E. faecalis to human macrophages. (A) Macrophages were incubated with various concentrations of enterococci at 37°C for 15 min. The number of FITC-labeled bacteria bound to 100 macrophages is expressed as the AI. Points represent mean values ± standard deviations of four independent assays with four wells. (B) Adherent macrophages were incubated with 2.5 × 107 enterococci/ml for 15 to 60 min. Data represent means ± standard deviations of four independent assays with three wells. Symbols: ○, E. faecalis OG1X(pAM721) (AS positive); □, E. faecalis OG1X (AS negative).
Interestingly, enterococci were not equally distributed among phagocytes. After 15 min of incubation at an MOI of 10:1 with the AS-positive E. faecalis strain OG1X(pAM721), 39% ± 7.6% of phagocytes were found to be associated with enterococci, with a mean of 16 bacteria per cell. In contrast, after incubation with the AS-negative strain OG1X, only 7% ± 2.1% of macrophages were associated with enterococci, with a mean of 3.5 bacteria per cell (n = 4).
Localization of macrophage binding sites within AS.
To assess which regions of the AS are involved in adherence to macrophages, binding capabilities of E. faecalis constructs with various in-frame deletions within the structural gene asa1 were compared with those of the AS-positive E. faecalis strain OG1X(pAM944/pWHH6) and the AS-negative strain OG1X(pAM944) (Fig. 2). Larger deletions within the N-terminal half of AS (mutants IV, V, and VI) caused a decrease in bacterial adherence of >40%. As expected, a smaller deletion in the N terminus distant from both RGD sequences (mutant III) had a minor effect, so that adherence of this strain was still threefold higher than that of the AS-negative strain OG1X(pAM944) (P = 0.005). Likewise, a deletion in the C-terminal half of the AS (mutant VII) resulted in a small decrease of ∼10% in adherence to macrophages, and binding was still 3.3-fold higher than that for OG1X(pAM944) (P < 0.01). Mutant VIII, lacking an extended region within the N- and C-terminal halves of AS, including both RGD motifs, showed the same poor adhesion as E. faecalis OG1X(pAM944). The facts that among the mutants only strains III and VII did not show significantly reduced binding compared to the AS-positive strain OG1X(pAM944/pWHH6) (P > 0.05) and that mutant III had a significantly better binding capacity than mutant V (P < 0.01) indicate that the N-terminal RGD motif and the adjacent N-terminal region are essential for macrophage binding. Interestingly, mutant IV, which contains the N-terminal RGD sequence, did not adhere significantly better than the comparable strains lacking the N-terminal RGD (mutants V and VI), suggesting that the N-terminal amino acids are essential for adequate presentation of this motif. Incubation of macrophages with enterococcal mutants for 60 min gave similar results for all tested strains (data not shown).
FIG. 2.
Adherence of E. faecalis mutants with various deletions in the structural gene asa1 in comparison to the AS-negative strain OG1X(pAM944) and the AS-positive strain OG1X(pAM944/pWHH6). Adherent macrophages were incubated with 2.5 × 107 enterococci per ml for 15 min. Columns represent mean values ± standard deviations of four independent assays with three wells. ∗, P < 0.05 compared with OG1X(pAM944).
Inhibition of adherence to macrophages by the RGDS peptide.
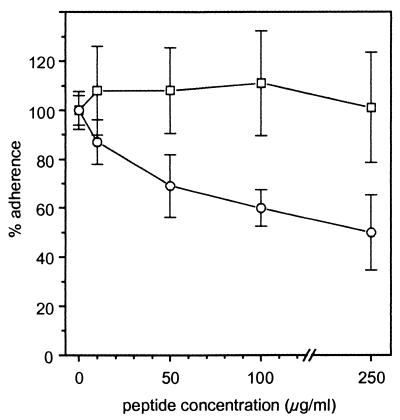
To determine if the RGD motifs of the AS mediate the interaction with macrophages, cells were preincubated with the peptide RGDS for 15 min before enterococci were added. As shown in Fig. 3, the RGDS peptide blocked binding of AS-positive E. faecalis OG1X(pAM721) in a concentration-dependent manner with a 50% ± 15% reduction at 250 μg/ml. In contrast, the RGDS peptide did not influence the macrophage binding of AS-negative strain OG1X. The specificity of inhibition of adherence by RGDS was tested by experiments employing the homologous control peptide RADS, in which the glycine is replaced with the structurally similar alanine. In contrast to RGDS, RADS peptide used at concentrations up to 250 μg/ml exerted no effect either on the adherence of AS-positive E. faecalis OG1X(pAM721) (AI, 104% ± 30% of peptide-free control) or on binding of AS-negative E. faecalis OG1X (AI, 90% ± 3%).
FIG. 3.
Effect of RGDS on adherence of E. faecalis OG1X and OG1X(pAM721). Adherent macrophages were preincubated with various concentrations of peptide for 15 min prior to exposure to bacteria (2.5 × 107 cells/ml) for 15 min. The 100% values without peptide are 98 ± 6 adherent bacteria per 100 macrophages for OG1X(pAM721) and 13 ± 1 for OG1X. Values are expressed as percentages of the mean value ± standard deviation from three independent experiments with three wells. Symbols: ○, OG1X(pAM721); □, OG1X.
Inhibition of adherence to macrophages by anti-CD18 MAb IB4.
To find out if adherence was mediated through interaction of AS with the β2 integrins which are constitutively expressed on macrophages, we first examined the inhibitory effect of the MAb IB4 against CD18 on the binding of E. faecalis OG1X and OG1X(pAM721). Pretreatment of macrophages adherent to Terasaki plates with IB4 significantly decreased AS-dependent adherence of OG1X(pAM721) in a concentration-dependent manner, while the binding of OG1X was not affected (Fig. 4). Isotype-specific antibodies at concentrations from 10 to 250 μg/ml, which served as the negative control, did not compete with both strains for binding to macrophages (data not shown).
FIG. 4.
Inhibition of enterococcal attachment by pretreatment with anti-CD18 MAb IB4. Macrophages were preincubated with different concentrations of IB4 for 15 min at 37°C before bacteria were added. Data are expressed as percentages of the mean value ± standard deviation from three independent experiments with three wells. The 100% value represents adherent bacteria per 100 macrophages without addition of antibody and is 151 ± 10 for OG1X(pAM721) and 9 ± 0.2 for OG1X. Isotype-specific antibody which served as negative control had no effect (not shown). ○, OG1X(pAM721); □, OG1X.
Inhibition of adherence to human macrophages by anti-CD11b MAb.
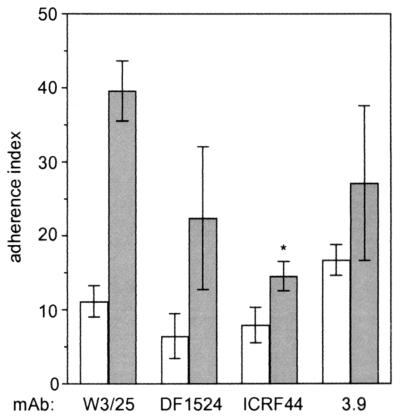
To examine which of the CD18 integrins are involved in the interaction with AS-positive enterococci, human macrophages were preincubated with MAbs against the integrin α-chains CD11a, CD11b, and CD11c. As shown in Fig. 5, MAbs against CD11b markedly decreased AS-dependent adhesion of E. faecalis to 37% of that of the control (P < 0.01). In contrast, antibodies against CD11a and CD11c did not significantly decrease adhesion compared to isotype-specific control antibodies.
FIG. 5.
Inhibition of E. faecalis binding to macrophages by MAbs against CD11a (DF1524), CD11b (ICRF44), and CD11c (MAb 3.9). Macrophages were pretreated with MAbs for 15 min at 37°C prior to incubation with enterococcal strains OG1X and OG1X(pAM721). Data are expressed as mean values ± standard deviations (n = 3) of the AI described in the legend to Fig. 1. Open bars, OG1X; shaded bars, OG1X(pAM721). ∗, P < 0.05 versus control MAb IgG1 (W3/25).
Internalization of E. faecalis in macrophages.
To distinguish internalized from bound bacteria on the surface of macrophages, extracellular bacteria were quenched by the addition of ethidium bromide so that fluorescence of extracellular FITC-labeled prokaryotes switched from green to red. Microscopic quantification of internalized bacteria revealed that the AS also promoted phagocytosis by macrophages. After an incubation for 15 min with a bacterium-cell ratio of 10:1, 4.4 ± 1.2 bacteria of the AS-negative strain OG1X and 35 ± 9.0 bacteria of the AS-positive strain OG1X(pAM721) bacteria were internalized per 100 macrophages. After 60 min, 54 ± 12.4 bacteria of OG1X and 237 ± 54 bacteria of OG1X(pAM721) were found to be intracellular.
CL response of human macrophages to E. faecalis.
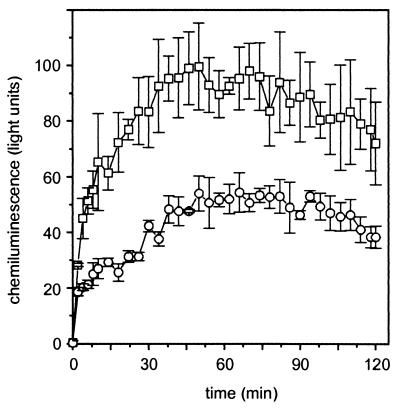
The ability to generate microbicidal reactive oxygen derivatives in response to a panel of stimuli, the so-called respiratory or oxidative burst, is a well-known characteristic of phagocytes that usually accompanies phagocytosis (1). Our interest was focused on superoxide anions (·O2−), because generation of these radicals by NADPH oxidase reflects the initiation of the respiratory burst (8, 43). To investigate the kinetics of ·O2− production in human macrophages in response to AS-bearing and -lacking E. faecalis, we analyzed the CL arising from the reductive cleavage of lucigenin (45). As shown in Fig. 6, both strains (MOI, 10:1) induced a respiratory burst activity in macrophages with a continuous increase in CL over a period of ∼60 min. However, the AS-positive strain induced a significantly lower CL compared to the AS-negative strain, which could also be observed at bacterium-cell ratios up to 50:1 as evaluated by integration (45% ± 2.4% at an MOI of 10:1; 59% ± 3% at an MOI of 50:1). Interestingly, although both enterococcal strains, like zymosan, induced respiratory burst activity in a concentration-dependent manner, the AS-positive strain, even when added at an MOI of 50:1, induced a lower CL response (integral of 704 ± 33 RLU·min) than the AS-negative strain added at an MOI of 10:1 (753 ± 71 RLU·min). Some strains of E. faecalis are known to produce superoxide (20). Therefore, we examined the superoxide production by the AS-expressing strain OG1X(pAM721) and the AS-negative strains OG1X and OG1X(pAM944) at bacterial concentrations used in this assay. All three strains did produce very low amounts of superoxide (maxima of 18, 19, and 14 RLU, respectively).
FIG. 6.
Respiratory burst activity of human macrophages in response to E. faecalis as measured by lucigenin-enhanced CL. Macrophages (5 × 106/ml) were first incubated in medium containing lucigenin. At time zero, enterococci (5 × 107/ml) were added and CL (light units) was measured at 2-min intervals over a time period of 120 min. Data are mean values ± standard deviations of three wells. The graphs show representative results of one of three experiments. Wells without bacteria served as negative controls. Control values ranged from 8 to 25 light units and were subtracted from test data. ○, AS-positive OG1X(pAM721); □, AS-negative OG1X.
Intracellular survival of E. faecalis in human macrophages.
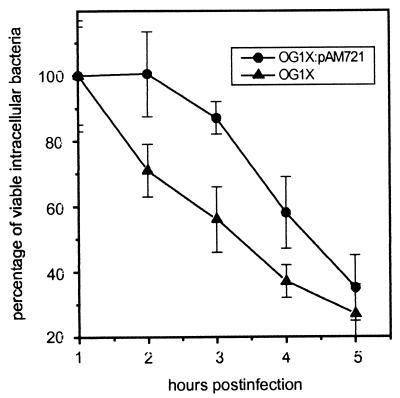
The AS-mediated inhibition of ·O2− production during phagocytosis of E. faecalis suggested a reduced killing of AS-bearing enterococci by macrophages. Therefore, we studied the intracellular survival of E. faecalis strains OG1X and OG1X(pAM721) in human macrophages up to 24 h after internalization. During the first 3 h, the viability of the AS-positive strain OG1X(pAM721) was not decreased significantly (P > 0.05), whereas viable cells of the AS-negative strain OG1X were already reduced to 56% ± 10% of the initial values (P < 0.001) (Fig. 7). However, at later time points, the killing rates of both strains were found to be similar (data not shown), suggesting an AS-mediated advantage particularly in short-term survival.
FIG. 7.
Time course of intracellular survival of AS-positive E. faecalis OG1X(pAM721) and AS-negative E. faecalis OG1X within human macrophages. Macrophages (2 × 104) were allowed to ingest bacteria (2 × 105) for 1 h. Extracellular bacteria were removed by washing and killed by penicillin and gentamicin. The results represent the mean ± standard deviation percent viable intracellular bacteria per 105 macrophages of three independent experiments with two wells.
DISCUSSION
A substantial portion of infections due to E. faecalis occur as a consequence of bacterial translocation from the natural habitat, the intestine, into tissue as well as the lymphatic and blood systems (25). In order to survive in this environment, E. faecalis must be able to evade host cellular defense mechanisms such as killing by neutrophil granulocytes, monocytes, and macrophages. Recently, Gentry-Weeks et al. have shown that E. faecalis survives better in mouse peritoneal macrophages for 72 h than do other members of the intestinal flora, such as Lactococcus lactis and a nonpathogenic strain of Escherichia coli (17). However, it was not clear which virulence factor contributed to this feature, since cytolysin and gelatinase did not influence intracellular survival. Another potential virulence factor of E. faecalis is the AS, a surface protein which mediates adherence between enterococci but also between E. faecalis and renal tubular cells (26) and enterocytes (34). Sequencing of the pAD1-encoded AS revealed two RGD motifs which are known to interact with β2 integrins (16), a family of eukayotic adhesion molecules which are constitutively expressed on macrophages (42). Therefore, we speculated that the AS could interact with macrophages, thereby promoting enterococcal adherence, phagocytosis, and perhaps intracellular survival.
In this study, we have shown that the pAD1-encoded AS Asa1 increased opsonin-independent binding of E. faecalis to human monocyte-derived macrophages by more than fivefold. Studies with mutants containing various in-frame deletions within the asa1 gene indicated that macrophage binding was not mediated by the C-terminal half of the adhesin, as shown by the fact that removal of 441 amino acids in this region, including the C-terminal RGD sequence (corresponding to ΔPstI1.3, mutant VII), reduced macrophage binding only slightly. In contrast, deletions within the N-terminal half resulted in a significant reduction of adherence to macrophages. The only exception was a derivative strain bearing a small (73-amino-acid) deletion within the N terminus separated by 375 intact amino acids from the first RGD motif (mutant III). This strain was significantly more adherent than a strain lacking the N-terminal RGD motif (mutant V). Moreover, the same strain showed stronger adherence than the derivative which also possessed the N-terminal RGD but only 30 N-terminus-adjacent amino acids (mutant IV). This stresses the importance of the N-terminal RGD motif and the N-terminus-adjoining amino acids which are likely to be necessary for adequate presentation of the RGD motif. Muscholl-Silberhorn demonstrated with the same strains that the C-terminal half of AS does not play an essential role in bacterial clumping either (33). Comparison of his results from the clumping assay with the data presented here reveal that bacterial clumping does correlate with the ability to adhere to macrophages, suggesting that aggregation facilitates adhesion or that both features are mediated by related domains.
Macrophage binding could be inhibited competitively by preincubation of the cells with MAbs against CD18 and CD11b as well as by an RGDS peptide, indicating that adherence is mediated by an interaction between the integrin CR3, which is constitutively expressed on macrophages, and the RGD motif of AS. This finding is consistent with results reported by Vanek et al. (46), who demonstrated that nonopsonized AS-expressing E. faecalis cells bind to human neutrophils via a CR3-dependent mechanism. However, the fact that AS-positive enterococci did not bind to CR3-bearing CHO-Mac-1 cells and that adherence to PMNs was also inhibited by antibodies against integrin-associated protein (IAP) and l-selectin suggests that AS-mediated binding to PMNs requires other adhesion molecules besides CR3. Since RGD-containing peptides were shown not to bind directly to purified CR3 but rather to other integrins (47), such as the αVβ3/IAP complex which subsequently interferes with CR3 (56), it is possible that the RGD motifs of AS also interact with this complex on macrophages. However, this remains to be determined. It should be stressed that adhesion of E. faecalis to human macrophages is mediated not only by AS, since AS-negative strains do also adhere, although binding is significantly less than that of AS-positive strains. Interestingly, cytolysin, another postulated virulence factor of E. faecalis, did not affect macrophage binding, as demonstrated by comparing adhesion of the AS-negative, cytolysin-negative strain OG1X with that of the AS-negative, cytolysin-positive strain OG1X(pAM944).
Bacteria and human parasites have been shown to bind, opsonin independently, monocytes and macrophages via CR3 (18). It was hypothesized that they utilize this mechanism since binding to CR3 can promote entry into the macrophage without inducing a respiratory burst (32, 55), thereby preventing oxygen-dependent killing. Microscopical examination of macrophages infected with E. faecalis at an MOI of 10:1 for 15 min revealed that the AS augmented phagocytosis by ∼700%, indicating that AS-mediated uptake of enterococci occurs very fast. The AS-positive strain was significantly more resistant to intracellular killing during the first 3 h postinfection, although killing rates were found to be similar at later time points. Our data are in accordance to results presented by Rakita et al. (35), who demonstrated that unopsonized E. faecalis bearing AS had a better survival rate after being phagocytosed by PMNs and macrophages than enterococci lacking AS. With neutrophils they showed that the failure of PMNs to kill AS-positive E. faecalis was not due to a lack of PMN activation, as shown by surface expression of Mac-1 and the Mac-1 activation epitope and shedding of l-selectin, but probably was due to a modification of phagosomal maturation.
The fate of phagocytosed microorganisms depends at least in part on activation of the respiratory burst (9). Various bacteria, such as Erysipelothrix rhusiopathiae (41), Salmonella enterica serovar Typhi (30), and Brucella abortus (27), suppress the oxidative burst or induce a low-level oxidative burst, resulting in successful intracellular survival. The data presented in this report show that the constitutively AS-expressing E. faecalis strain OG1X(pAM721) elicited a significantly weaker respiratory burst than the isogenic AS-negative strain OG1X, as determined by measuring the superoxide anion production, although phagocytosis rates of AS-bearing enterococci were eightfold higher. Since the AS-containing strain itself produces neglectably low amounts of superoxide but significantly more than the AS-free strain, the possibility that this effect was due to bacterial superoxide can be ruled out. This corroborates the hypothesis that internalization of microorganisms by macrophages via CR3 inhibits the respiratory burst. In PMNs an analogous mechanism has not been described, and experiments with neutrophils showed that the presence of AS resulted in increased superoxide and phagosomal oxidant production (35). To our knowledge, this is the first report of a bacterium that invades macrophages by its RGD motif using a CR3-dependent mechanism resulting in a reduced respiratory burst and improved intracellular survival.
In vivo, the AS of E. faecalis was demonstrated to be a virulence factor in rabbit models of endocarditis, resulting in increased vegetation weights (5) and a higher mortality (40). The increased uptake and resistance to killing by PMNs and macrophages allow enterococci to persist in the cardiac valve vegetation intracellularly, thereby being protected from antibiotics. Sex pheromone plasmid-containing E. faecalis cells have been found more frequently in clinical isolates from patients with bacteremia and wound infections than from stool specimens of healthy volunteers and hospitalized patients (7), indicating that AS functions also as a virulence factor in humans.
ACKNOWLEDGMENTS
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (RO977/2-1, Wi731/6-1) and the Interdisziplinäres Forschungszentrum (IZKF) to E.R, R.W., and R.M.
REFERENCES
- 1.Babior B M. Oxygen-dependent microbial killing by phagocytes (first of two parts) N Engl J Med. 1978;298:659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- 2.Baxter M A, Leslie R G, Reeves W G. The stimulation of superoxide anion production in guinea-pig peritoneal macrophages and neutrophils by phorbol myristate acetate, opsonized zymosan and IgG2-containing soluble immune complexes. Immunology. 1983;48:657–665. [PMC free article] [PubMed] [Google Scholar]
- 3.Boyum A. Separation of lymphocytes, granulocytes, and monocytes from human blood using iodinated density gradient media. Methods Enzymol. 1984;108:88–102. doi: 10.1016/s0076-6879(84)08076-9. [DOI] [PubMed] [Google Scholar]
- 4.Bullock W E, Wright S D. Role of the adherence-promoting receptors, CR3, LFA-1, and p150, 95, in binding of Histoplasma capsulatum by human macrophages. J Exp Med. 1987;165:195–210. doi: 10.1084/jem.165.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chow J W, Thal L A, Perri M B, Vazquez J A, Donabedian S M, Clewell D B, Zervos M J. Plasmid-associated hemolysin and aggregation substance production contribute to virulence in experimental enterococcal endocarditis. Antimicrob Agents Chemother. 1993;37:2474–2477. doi: 10.1128/aac.37.11.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clewell D B. Bacterial sex pheromone-induced plasmid transfer. Cell. 1993;73:9–12. doi: 10.1016/0092-8674(93)90153-h. [DOI] [PubMed] [Google Scholar]
- 7.Coque T M, Patterson M E, Steckelberg J M, Murray B E. Incidence of hemolysin, gelatinase, and aggregation substance among enterococci isolated from patients with endocarditis and other infections and from feces of hospitalized and community-based persons. J Infect Dis. 1995;171:1223–1229. doi: 10.1093/infdis/171.5.1223. [DOI] [PubMed] [Google Scholar]
- 8.De B P, Schram E. Luminescent bioassays based on macrophage cell lines. Methods Enzymol. 1986;133:507–530. doi: 10.1016/0076-6879(86)33087-8. [DOI] [PubMed] [Google Scholar]
- 9.Densen P, Mandell G L. Phagocyte strategy vs. microbial tactics. Rev Infect Dis. 1980;2:817–838. doi: 10.1093/clinids/2.5.817. [DOI] [PubMed] [Google Scholar]
- 10.Diamond M S. Differential effects on leukocyte functions of CD11a, CD11b and CD18 mAb. In: Knapp W, editor. Leukocyte typing IV: white cell differentiation antigens. Oxford, United Kingdom: Oxford University Press; 1989. pp. 570–584. [Google Scholar]
- 11.Dijkmans B A, Leijh P C J, Braat A G P, van Furth R. Effect of bacterial competition on the opsonization, phagocytosis, and intracellular killing of microorganisms by granulocytes. Infect Immun. 1985;49:219–224. doi: 10.1128/iai.49.1.219-224.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drevets D A, Campbell P A. Macrophage phagocytosis: use of fluorescence microscopy to distinguish between extracellular and intracellular bacteria. J Immunol Methods. 1991;142:31–38. doi: 10.1016/0022-1759(91)90289-r. [DOI] [PubMed] [Google Scholar]
- 13.Dunny G M. Genetic functions and cell-cell interactions in the pheromone-inducible plasmid transfer system of Enterococcus faecalis. Mol Microbiol. 1990;4:689–696. doi: 10.1111/j.1365-2958.1990.tb00639.x. [DOI] [PubMed] [Google Scholar]
- 14.Ehrenfeld E E, Clewell D B. Transfer functions of the Streptococcus faecalis plasmid pAD1: organization of plasmid DNA encoding response to sex pheromone. J Bacteriol. 1987;169:3473–3481. doi: 10.1128/jb.169.8.3473-3481.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finlay B B, Falkow S. Comparison of the invasion strategies used by Salmonella cholerae-suis, Shigella flexneri and Yersinia enterocolitica to enter cultured animal cells: endosome acidification is not required for bacterial invasion or intracellular replication. Biochimie. 1988;70:1089–1099. doi: 10.1016/0300-9084(88)90271-4. [DOI] [PubMed] [Google Scholar]
- 16.Galli D, Lottspeich F, Wirth R. Sequence analysis of Enterococcus faecalis aggregation substance encoded by the sex pheromone plasmid pAD1. Mol Microbiol. 1990;4:895–904. doi: 10.1111/j.1365-2958.1990.tb00662.x. [DOI] [PubMed] [Google Scholar]
- 17.Gentry-Weeks C R, Karkhoff-Schweizer R, Pikis A, Estay M, Keith J M. Survival of Enterococcus faecalis in mouse peritoneal macrophages. Infect Immun. 1999;67:2160–2165. doi: 10.1128/iai.67.5.2160-2165.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoepelman A I, Tuomanen E I. Consequences of microbial adherence: directing host cell functions with adhesins. Infect Immun. 1992;60:1729–1733. doi: 10.1128/iai.60.5.1729-1733.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hogg N, Takacs L, Palmer D G, Selvendran Y, Allen C. The p150,95 molecule is a marker of human mononuclear phagocytes: comparison with expression of class II molecules. Eur J Immunol. 1986;16:240–248. doi: 10.1002/eji.1830160306. [DOI] [PubMed] [Google Scholar]
- 20.Huycke M M, Joyce W, Wack M F. Augmented production of extracellular superoxide by blood isolates of Enterococcus faecalis. J Infect Dis. 1996;173:743–746. doi: 10.1093/infdis/173.3.743. [DOI] [PubMed] [Google Scholar]
- 21.Hynes R O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 22.Ike Y, Clewell D B. Genetic analysis of the pAD1 pheromone response in Streptococcus faecalis, using transposon Tn917 as an insertional mutagen. J Bacteriol. 1984;158:777–783. doi: 10.1128/jb.158.3.777-783.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ike Y, Craig R A, White B A, Yagi Y, Clewell D B. Modification of Streptococcus faecalis sex pheromones after acquisition of plasmid DNA. Proc Natl Acad Sci USA. 1983;80:5369–5373. doi: 10.1073/pnas.80.17.5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jett B D, Huycke M M, Gilmore M S. Virulence of enterococci. Clin Microbiol Rev. 1994;7:462–478. doi: 10.1128/cmr.7.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson A P. The pathogenicity of enterococci. J Antimicrob Chemother. 1994;33:1083–1089. doi: 10.1093/jac/33.6.1083. [DOI] [PubMed] [Google Scholar]
- 26.Kreft B, Marre R, Schramm U, Wirth R. Aggregation substance of Enterococcus faecalis mediates adhesion to cultured renal tubular cells. Infect Immun. 1992;60:25–30. doi: 10.1128/iai.60.1.25-30.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kreutzer D L, Dreyfus L A, Robertson D C. Interaction of polymorphonuclear leukocytes with smooth and rough strains of Brucella abortus. Infect Immun. 1979;23:737–742. doi: 10.1128/iai.23.3.737-742.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leijh P C J, van den Barselaar M T, Daha M R, van Furth R. Stimulation of the intracellular killing of Staphylococcus aureus by monocytes: regulation by immunoglobulin G and complement components C3/C3b and B/Bb. J Immunol. 1982;129:332–337. [PubMed] [Google Scholar]
- 29.Malhotra V, Hogg N, Sim R B. Ligand binding by the p150,95 antigen of U937 monocytic cells: properties in common with complement receptor type 3 (CR3) Eur J Immunol. 1986;16:1117–1123. doi: 10.1002/eji.1830160915. [DOI] [PubMed] [Google Scholar]
- 30.Miller R M, Carbus J, Hornick R B. Lack of enhanced oxygen consumption by polymorphonuclear leukocytes on phagocytosis of virulent Salmonella typhi. Science. 1972;175:1010–1011. doi: 10.1126/science.175.4025.1010. [DOI] [PubMed] [Google Scholar]
- 31.Moellering R C., Jr Emergence of Enterococcus as a significant pathogen. Clin Infect Dis. 1992;14:1173–1176. doi: 10.1093/clinids/14.6.1173. [DOI] [PubMed] [Google Scholar]
- 32.Mosser D M, Edmond M B. The third component of complement (C3) is responsible for the intracellular survival of Leishmania major. Nature. 1987;327:329–331. doi: 10.1038/327329b0. [DOI] [PubMed] [Google Scholar]
- 33.Muscholl-Silberhorn A. Analysis of the clumping-mediating domain(s) of sex pheromone plasmid pAD1-encoded aggregation substance. Eur J Biochem. 1998;258:515–520. doi: 10.1046/j.1432-1327.1998.2580515.x. [DOI] [PubMed] [Google Scholar]
- 34.Olmsted S B, Dunny G M, Erlandsen S L, Wells C L. A plasmid-encoded surface protein on Enterococcus faecalis augments its internalization by cultured intestinal epithelial cells. J Infect Dis. 1994;170:1549–1556. doi: 10.1093/infdis/170.6.1549. [DOI] [PubMed] [Google Scholar]
- 35.Rakita R M, Vanek N N, Jaques-Palaz K, Mee M, Mariscalco M M, Dunny G M, Snuggs M, Van Winkle W B, Simon S I. Enterococcus faecalis bearing aggregation substance is resistant to killing by human neutrophils despite phagocytosis and neutrophil activation. Infect Immun. 1999;67:6067–6075. doi: 10.1128/iai.67.11.6067-6075.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rozdzinski E, Tuomanen E. Interactions of bacteria with leukocyte integrins. Methods Enzymol. 1994;236:333–345. doi: 10.1016/0076-6879(94)36025-1. [DOI] [PubMed] [Google Scholar]
- 37.Ruoslahti E. Integrins. J Clin Invest. 1991;87:1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruoslahti E, Pierschbacher M D. New perspectives in cell adhesion: RGD and integrins. Science. 1987;238:491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- 39.Schaberg D R, Culver D H, Gaynes R P. Major trends in the microbial etiology of nosocomial infection. Am J Med. 1991;91:72S–75S. doi: 10.1016/0002-9343(91)90346-y. [DOI] [PubMed] [Google Scholar]
- 40.Schlievert P M, Gahr P J, Assimacopoulos A P, Dinges M M, Stoehr J A, Harmala J W, Hirt H, Dunny G M. Aggregation and binding substance enhance pathogenicity in rabbit models of Enterococcus faecalis endocarditis. Infect Immun. 1998;66:218–223. doi: 10.1128/iai.66.1.218-223.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shimoji Y, Yokomizo Y, Mori M. Intracellular survival and replication of Erysipelothrix rhusiopathiae within murine macrophages: failure of induction of the oxidative burst of macrophages. Infect Immun. 1996;64:1789–1793. doi: 10.1128/iai.64.5.1789-1793.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Springer T A. Adhesion receptors of the immune system. Nature. 1990;346:425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 43.Trush M A, Wilson M E, Van D K. The generation of chemiluminescence (CL) by phagocytic cells. Methods Enzymol. 1978;57:462–494. [Google Scholar]
- 44.Valenti W P, Benkel P, Rohde M, Chhatwal G S. Entry and intracellular survival of group B streptococci in J774 macrophages. Infect Immun. 1996;64:2467–2473. doi: 10.1128/iai.64.7.2467-2473.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van D K, Van S M, Castranova V. Measurement of phagocytosis and cell-mediated cytotoxicity by chemiluminescence. Methods Enzymol. 1986;132:498–507. doi: 10.1016/s0076-6879(86)32035-4. [DOI] [PubMed] [Google Scholar]
- 46.Vanek N N, Simon S I, Jaques-Palaz K, Mariscalco M M, Dunny G M, Rakita R M. Enterococcus faecalis aggregation substance promotes opsonin-independent binding to human neutrophils via a complement receptor type 3-mediated mechanism. FEMS Immunol Med Microbiol. 1999;26:49–60. doi: 10.1111/j.1574-695X.1999.tb01371.x. [DOI] [PubMed] [Google Scholar]
- 47.Van Strijp J A, Russell D G, Tuomanen E, Brown E J, Wright S D. Ligand specificity of purified complement receptor type 3: indirect effects of an Arg-Gly-Asp sequence. J Immunol. 1993;151:3324–3336. [PubMed] [Google Scholar]
- 48.Vieweg R, Leslie R G. Soluble immune complex triggering of a respiratory burst in macrophages: the role of complex aggregation at the phagocyte surface. Eur J Immunol. 1987;17:149–151. doi: 10.1002/eji.1830170126. [DOI] [PubMed] [Google Scholar]
- 49.Wells C L, Jechorek R P, Erlandsen S L. Evidence for the translocation of Enterococcus faecalis across the mouse intestinal tract. J Infect Dis. 1990;162:82–90. doi: 10.1093/infdis/162.1.82. [DOI] [PubMed] [Google Scholar]
- 50.Wells C L, Maddaus M A, Simmons R L. Proposed mechanisms for the translocation of intestinal bacteria. Rev Infect Dis. 1988;10:958–979. doi: 10.1093/clinids/10.5.958. [DOI] [PubMed] [Google Scholar]
- 51.Wells C L, Vande Westerlo E M A, Jechorek R P, Erlandsen S L. Effect of hypoxia on enterocyte endocytosis of enteric bacteria. Crit Care Med. 1996;24:985–991. doi: 10.1097/00003246-199606000-00019. [DOI] [PubMed] [Google Scholar]
- 52.Wirth R. The sex pheromone system of Enterococcus faecalis. More than just a plasmid-collection mechanism? Eur J Biochem. 1994;222:235–246. doi: 10.1111/j.1432-1033.1994.tb18862.x. [DOI] [PubMed] [Google Scholar]
- 53.Wright S D, Rao P E, Van Voorhis W C, Craigmyle L S, Iida K, Talle M A, Westberg E F, Goldstein G, Silverstein S. Identification of the C3bi receptor of human monocytes and macrophages by using monoclonal antibodies. Proc Natl Acad Sci. 1983;80:5699–5703. doi: 10.1073/pnas.80.18.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wright S D, Silverstein S. Tumor-promoting phorbol esters stimulate C3b and C3b′ receptor-mediated phagocytosis in cultured human monocytes. J Exp Med. 1982;156:1149–1164. doi: 10.1084/jem.156.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wright S D, Silverstein S. Receptors for C3b and C3bi promote phagocytosis but not the release of toxic oxygen from human phagocytes. J Exp Med. 1983;158:2016–2023. doi: 10.1084/jem.158.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou M, Brown E J. Leukocyte response integrin and integrin-associated protein act as a signal transduction unit in generation of a phagocyte respiratory burst. J Exp Med. 1993;178:1165–1174. doi: 10.1084/jem.178.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]