Abstract
Background
The impact of adopting a race-free estimated glomerular filtration rate (eGFR) creatinine (eGFRcr) equation on racial differences in chronic kidney disease (CKD) progression among people with human immunodeficiency virus (PWH) is unknown.
Methods
We defined eGFR stages using the original race-adjusted Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) eGFRcr equation and the new race-free CKD-EPI eGFRcr equation. We then estimated 5-year probabilities of transitioning from baseline kidney function to more advanced eGFR stages and examined the association of race (black vs white) with rates of CKD progression using Markov models.
Results
With the race-adjusted eGFRcr equation, black participants (n = 31 298) had a lower risk of progressing from eGFR stage 1 to 2 (hazard ratio [HR], 0.77; 95% confidence interval [CI], .73–.82), an equal risk of progressing from stage 2 to 3 (1.00; .92–.07) and a 3-fold risk of progressing from stage 3 to 4 or 5 (3.06; 2.60–3.62), compared with white participants (n = 27 542). When we used the race-free eGFRcr equation, 16% of black participants were reclassified into a more severe eGFR stage at baseline. The reclassified black individuals had a higher prevalence of CKD risk factors than black PWH who were not reclassified. With the race-free eGFRcr equation, black participants had a higher risk of disease progression across all eGFR stages than white participants.
Conclusions
The original eGFRcr equation systematically masked a subgroup of black PWH who are at high-risk of CKD progression. The new race-free eGFRcr equation unmasks these individuals and may allow for earlier detection and management of CKD.
Keywords: race, eGFR, CKD, ESKD, HIV
Adoption of the new race-free 2021 CKD-EPI eGFR equation identified more Black individuals with HIV as having chronic kidney disease (CKD) and revealed a higher risk of CKD progression. This may allow for earlier detection and management of CKD risk.
Black Americans living with human immunodeficiency virus (HIV) have a higher incidence of end-stage kidney disease (ESKD) than other people with HIV (PWH) and the general population [1]. Studies have reported that black and white individuals have similar prevalences of earlier stages of chronic kidney disease (CKD), suggesting that black Americans with established CKD progress to ESKD more rapidly than their white counterparts [2 , 3]. Therefore, early identification of individuals at risk of rapid CKD progression is imperative to implement targeted interventions that may halt or slow the progression of kidney disease.
Measured glomerular filtration rate (GFR) is the “gold standard” for evaluating kidney function, but measurement protocols are too complex for routine clinical practice [4]. Instead, measured GFR is estimated from equations that incorporate serum creatinine, body size, age, sex, and race [5]. The inclusion of race—a nonbiological social construct—in the estimated glomerular filtration rate (eGFR) equations has been previously justified on the basis that the race coefficient improved the equations’ accuracy and precision in predicting measured GFR [5]. This coefficient systematically assigns higher kidney function for all black individuals, and it may hinder clinicians’ ability to identify individuals at higher risk of CKD progression. The leading nephrology professional societies recently recommended immediate implementation of a new Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) eGFR creatinine equation (eGFRcr) refit without the race variable in all laboratories in the United States [6, 7].
Recent cross-sectional studies have shown the impact of adopting the new race-free equation on CKD prevalence in the United States [7, 8]. However, clinical decisions and monitoring for CKD progression rely on longitudinal assessments of kidney function. It is unclear whether the association of race with CKD progression is dependent on the use of the race coefficient in eGFRcr equations and whether adopting the race-free equation will reduce bias in the estimated association of race with CKD progression among PWH. To fill this knowledge gap, we sought to examine the differences in probability and rate of kidney function decline at each eGFR stage among black versus white PWH and to examine how those relative differences were altered after adopting the race-free 2021 eGFRcr equation. We hypothesized that adopting a race-free eGFRcr equation would reveal a consistently higher risk of CKD progression across all levels of baseline kidney function among black compared with white individuals.
METHODS
Study Population
We included PWH who are part of the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD), a multisite collaboration involving >20 clinical and interval cohort studies in the United States and Canada [9], Each participating site has obtained institutional review board approval. The institutional review board of the University of California, San Francisco, approved the present study.
We included participants enrolled in NA-ACCORD between 1 January 2005, and 31 December 2014, who had a baseline eGFR measurement. Enrollment criteria include ≥1 follow-up visit after enrollment in an interval cohort and ≥2 HIV clinical care visits in a clinical cohort. The baseline date was defined as the date of enrollment in NA-ACCORD or the beginning of the cohort eGFR observation window, whichever came last. Participants were followed up from the baseline until death, the end of the cohort observation window, or administrative censoring on 31 December 2015, whichever came first. We excluded participants with baseline ESKD, defined as an eGFR <15 mL/min/1.73 m2 or the need for dialysis. We also excluded those who were missing a baseline CD4+ cell count or did not have ≥2 serum creatinine or HIV viral load measures during follow-up (Supplementary Figure 1).
Primary Outcome
The primary outcome was the 5-year probability of transitioning from baseline kidney function to more advanced stages of CKD. We estimated kidney function in 2 ways, using the original 2009 CKD-EPI eGFRcr equation [5] and the race-free 2021 CKD-EPI eGFRcr equation [7].
eGFR stages were classified according to Kidney Disease Improving Global Outcomes (KDIGO) guidelines [10] and included the following: stage 1, eGFR ≥90 mL/min/1.73 m2; stage 2, 60–89 mL/min/1.73 m2; stage 3, 30–59 mL/min/1.73 m2; and stages 4 and 5, <30 mL/min/1.73 m2. Kidney function stages were defined based on ≥2 eGFR values for ≥90 days or the last available eGFR. Mortality data were obtained via linkages to vital statistics registries, including the National Death Index, as well as physician report, and/or from the decedent’s survivors [11].
Race
Race and ethnicity were measured via self-report and abstraction from health records. Categories included, American Indian, Asian, black, Hispanic, multiracial, Pacific Islander, white, and not specified.
Covariate Definitions
Covariates included potential confounders of the association of race with CKD progression and death. We dichotomized calendar period (2005–2009 and 2010–2015) to account for temporal changes in healthcare practice patterns that may affect kidney function (eg, angiotensin-converting enzyme inhibitor [ACEI] or angiotensin-receptor blocker [ARB] use, blood pressure goal guidelines). Demographic variables included age and sex. Comorbid conditions included hepatitis C virus (HCV) coinfection (defined as antibody or RNA positivity), hypertension (defined by diagnosis code or prescription of antihypertension medications), diabetes mellitus (defined by diagnosis code, use of diabetic medications, or hemoglobin A1c ≥6.5%), cardiovascular disease (defined by diagnosis codes for myocardial infarction, non–myocardial infarction coronary artery disease, cerebrovascular accident, peripheral vascular disease, transient ischemic attack, or vasculitis), and history of AIDS (defined by clinical diagnosis). Medications of interest that may potentially confound the association of race and eGFR included antiretroviral therapy (ART) [12], and ACEI/ARB. HIV viral load suppression was defined as an HIV-1 RNA level <400 copies/mL.
Statistical Analysis
We first summarized demographic and clinical characteristics across racial categories using mean (standard deviation [SD]) or percentage with frequency, as appropriate. We then examined clinical characteristics for black participants who were reclassified to more advanced baseline eGFR stages after adoption of the race-free 2021 CKD-EPI eGFRcr equation. All descriptive analyses were conducted using SAS 9.4 software (SAS Institute).
Estimation of Transition Through eGFR Stages or Death
We conducted 2 analyses in parallel. In the first analysis, we determined eGFR stages using the original 2009 CKD-EPI eGFRcr equation, while the second analysis determined the eGFR stages using the race-free 2021 CKD-EPI eGFRcr equation. We first calculated eGFR using every outpatient serum creatinine measure and categorized each measure according to KDIGO eGFR stages [10]. We then identified all instances of confirmed eGFR stage, which we defined as having ≥2 eGFR measures >90 days apart that fell below an eGFR stage threshold without a contradictory intervening measure. If the last observed stage was within 90 days of the study exit date, we included this stage regardless of whether the measure was confirmed. Date of death was considered the last observation time for those who died during the study. Participants who were still alive at study exit and had no eGFR measures within the preceding 90 days were assigned a “censored state.”
We then used multistate Markov models to estimate rates of transition from baseline kidney function to successive stages of kidney disease or death [13]. Our Markov models assumed that each individual started at their baseline kidney function and followed an irreversible path through each successive stage of kidney disease (states), ending with the last observed eGFR stage or death (absorbing state) (Supplementary Figure 2). The multistate models were time-homogeneous models, in which the probability of being in any state depended only on the most recent state occupied. In addition, the models “true” underlying progression of kidney disease, and a second hidden Markov component to account for error inherent in biomarker measures (eg, serum creatinine and eGFR) that might lead to misclassification of the true underlying state value. The true underlying state of disease is modeled as a latent variable process which generates the data we observe (analogous to a random effect mode for characterizing the heterogeneity and variability in an unobserved process) [13].
The 5-state Markov multistate model consisted of the following: eGFR stage 1 (state 1), eGFR stage 2 (state 2), eGFR stage 3 (state 3), eGFR stage 4/5 (state 4), and death (state 5). The model assumed that instantaneous transitions could occur in a stepwise manner, for example, progression from state 1 to state 4 required passing through each successive state (ie, progression from eGFR stage 1 to 4/5 required passing through stages 2 and 3 [Supplementary Figure 2].) The multistate model accounted for death as a competing risk [14] by modeling death as an absorbing state to which participants could enter from any stage of kidney function [15].
The multistate model included race as the primary independent variable and adjusted for the following potential confounders: calendar period, age, sex, HIV viral load, baseline eGFR, history of diabetes, hypertension, cardiovascular disease, HCV, and AIDS diagnosis. HIV viral load was modeled using log10–based categories. Age was updated annually, and baseline eGFRs were mean centered for this analysis. Because of computational limitations within our system, we were unable to accommodate all covariates (eg, the use of ACEI/ARB or ART) in the final model, despite attempting all recommended solutions [16].
Using the final adjusted Markov models, we estimated the 5-year transition probabilities from baseline kidney function to successive eGFR stages or death. The estimated number of individuals in each eGFR stage was estimated by carrying the last confirmed eGFR stage before or at 5-year follow-up. The Markov models also estimated the independent association (hazard ratio [HR] and 95% confidence interval [CI]) of race with rate of transitioning to successive eGFR stages or death, comparing black and white participants. All multistate models analyses were conducted using R software, version 3.6.0, via the multistate models package [16].
RESULTS
Study Population
We included 69 125 participants who had data on longitudinal eGFR, HIV viral load and CD4+ cell counts (Supplementary Figure 1). Of the participants, 31 298 (45%) were black, 27 452 (40%) were white, and 10 375 (15%) were in the “other” category, including 444 (0.64%) self-identifying as American Indian, 282 (0.41%) as Asian, 457 (0.66%) as Asian/Pacific Islander, 8032 (11.6%) as Hispanic, 228 (0.33%) as multiracial, 127 (0.18%) as Pacific Islander, and 715 (1.03%) not specified. Supplementary Table 1 presents the demographic and clinical characteristics of these groups. Henceforth, we focus our presentation on black and white participants, because participants in the “other” race category represent groups for whom eGFR equations have not been well validated [17].
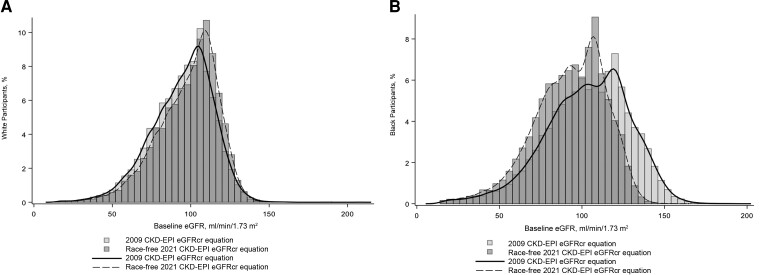
The mean baseline eGFR (SD) for black participants was 103.4 (25.4) mL/min/1.73 m2 with the 2009 CKD-EPI eGFRcr equation and 92.6 (21.7) mL/min/1.73 m2 with the race-free 2021 CKD-EPI eGFRcr equation (Figure 1). The mean baseline eGFR (SD) for white participants was 94.7 (19.6) mL/min/1.73 m2 with the 2009 CKD-EPI eGFRcr equation and 98.2 (19.1) mL/min/1.73 m2 with the race-free 2021 CKD-EPI eGFRcr equation (Figure 1). Baseline CD4+ count and HIV viral load were similar in black and white participants (Table 1). Use of ART and suppressed HIV viral load were less prevalent among black participants than among white participants. In contrast, HCV infection, hypertension, diabetes, and ACEI/ARB use were more prevalent among black participants .
Figure 1.
A, Baseline estimated glomerular filtration rate (eGFR) distribution among white (A) and black (B) participants using the original 2009 Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) eGFR creatinine (eGFRcr) equation and the new race-free 2021 CKD-EPI eGFRcr equation.
Table 1.
Summary of Baseline Demographic and Clinical Characteristics in the North American AIDS Cohort Collaboration on Research and Design, Stratified by Racea
| Characteristic | Participants by Race, No (%)b | |
|---|---|---|
| Black (n = 31 298) |
White (n = 27 542) | |
| Age, mean (SD), y | 44.4 (11.6) | 45.4 (11.3) |
| Female sex | 6810 (21.8) | 2258 (8.2) |
| BMIc | ||
| Underweight (<18.5) | 816 (2.6) | 571 (2.1) |
| Normal weight (≥18.5 to <25.0) | 11 373 (36.3) | 11 384 (41.3) |
| Overweight (≥25.0 to <30) | 8830 (28.2) | 8725 (31.7) |
| Obese (≥30.0) | 5810 (18.6) | 3924 (14.2) |
| Hypertension | 9189 (29.4) | 5643 (20.5) |
| Diabetes | 2885 (9.2) | 1670 (6.1) |
| CVD | 1333 (4.3) | 1353 (4.9) |
| MI (acute/history/unspecified) | 339 (1.1) | 385 (1.4) |
| CAD (non-MI) | 899 (2.9) | 1057 (3.8) |
| CVA (acute/history/unspecified) | 309 (1.0) | 192 (0.7) |
| PVD | 36 (0.1) | 32 (0.1) |
| TIA | 87 (0.3) | 92 (0.3) |
| Vasculitis | 1 (0.0) | 1 (0.0) |
| HCV positive | 5975 (19.1) | 3631 (13.2) |
| CD4+ cell count, mean (SD), cells/µL | 00.6 (286.3) | 444.6 (288.9) |
| HIV viral load, mean (SD), log10 copies/mL | 3.5 (1.2) | 3.4 (1.2) |
| HIV viral load suppression (<400 copies/mL) | 12 574 (40.2) | 13 346 (48.5) |
| History of AIDS | 4952 (15.8) | 4809 (17.5) |
| ART use | 19 688 (62.9) | 19 319 (70.1) |
| ACEI/ARB use | 5577 (17.8) | 3575 (13.0) |
Abbreviations: ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin-receptor blocker; ART, antiretroviral therapy; BMI, body mass index; CAD, coronary artery disease; CVA, cerebrovascular accident; CVD, cardiovascular disease; HCV, hepatitis C virus; HIV, human immunodeficiency virus; MI, myocardial infarction; NA-ACCORD, North American AIDS Cohort Collaboration on Research and Design; PVD, peripheral vascular disease; SD, standard deviation; TIA, transient ischemic attack.
Other racial categories are shown in Supplementary Table 1.
Data represent no. (%) of participants unless otherwise specified.
BMI calculated as weight in kilograms divided by height in meters squared.
CKD Reclassification at Baseline Among Black Individuals With Use Race-Adjusted Versus Unadjusted eGFRcr Equation
After adoption of the race-free 2021 CKD-EPI eGFRcr equation, 4977 black participants (15.9%) were reclassified into more advanced eGFR stages at baseline (Table 2). The likelihood of being reclassified into worse eGFR stage among black participants varied by eGFR category and was highest for those with an eGFR ≥90 mL/min/1.73 m2 (stage 1) at baseline (19% reclassification) and lowest for those with an eGFR of 30–60 mL/min/1.73 m2 (stage 3) at baseline (4% reclassification) (Table 2). Participants who were reclassified to lower baseline eGFR categories had a higher prevalence of CKD risk factors (eg, hypertension and diabetes) than those who were not reclassified (Table 3).
Table 2.
Baseline Estimated Glomerular Filtration (eGFR) Stage Reclassification of Black Participants After Adoption of the Race-Free 2021 Chronic Kidney Disease Epidemiology Collaboration eGFR Creatinine Equationa
| eGFR Stage per 2009 CKD-EPI eGFRcr Equation | eGFR Stage per Race-Free 2021 CKD-EPI eGFRcr Equation, No. (%) of Participants | ||||
|---|---|---|---|---|---|
| Stage 1 (n = 18 141) |
Stage 2 (n = 10 736) |
Stage 3 (n = 2127) |
Stage 4 (n = 275) |
Stage 5 (n = 19) | |
| Stage 1 (n = 22 311) | 18 141 (81) | 4170 (19) | |||
| Stage 2 (n = 7299) | 0 | 6566 (90) | 733 (10) | 0 | 0 |
| Stage 3 (n = 1449) | 0 | 0 | 1394 (96) | 55(4) | 0 |
| Stage 4 (n = 239) | 0 | 0 | 0 | 220 (92) | 19 (8) |
Abbreviations: CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; eGFRcr, eGFR creatinine.
eGFR stages were defined as follows: stage 1, eGFR ≥90 mL/min/1.73 m2; stage 2, 60–89 mL/min/1.73 m2; stage 3, 30–59 mL/min/1.73 m2; stage 4, 15–29 mL/min/1.73 m2; and stage 5, <15 mL/min/1.73 m2.
Table 3.
Comparison of Baseline Characteristics Among Black Participants Within Each Estimated Glomerular Filtration Rate (eGFR) Category by Reclassification Status after Adoption of the Race-Free 2021 Chronic Kidney Disease Epidemiology Collaboration eGFR Creatinine Equation
| Variable | Participants by eGFR Stage, %a | |||||||
|---|---|---|---|---|---|---|---|---|
| Stage 1: No Change (n = 18 141) | Stage 1 → 2 (n = 4170) |
Stage 2: No Change (n = 6566) | Stage 2 → 3 (n = 733) |
Stage 3: No Change (n = 1394) |
Stage 3 → 4 (n = 55) |
Stage 4: No Change (n = 220) |
Stage 4 → 5 (n = 19) |
|
| Death during study | 11 | 12 | 17 | 23 | 32 | 49 | 44 | 53 |
| Age, mean (SD), y | 41 (11) | 46 (10) | 50 (10) | 52 (10) | 55 (11) | 54 (12) | 52 (12) | 55 (10) |
| Hypertension | 21 | 30 | 40 | 55 | 65 | 78 | 71 | 95 |
| HCV positive | 17 | 19 | 23 | 27 | 26 | 18 | 27 | 47 |
| Diabetes | 7 | 8 | 11 | 17 | 21 | 35 | 22 | 37 |
| CVD | 3 | 4 | 6 | 9 | 12 | 16 | 14 | 26 |
| MI | 1 | 1 | 2 | 4 | 4 | 7 | 4 | 5 |
| CAD (non-MI) | 2 | 3 | 4 | 6 | 8 | 11 | 9 | 26 |
| CVA | 1 | 1 | 1 | 2 | 3 | 6 | 4 | 0 |
| PVD | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| TIA | 0 | 0 | 0 | 0 | 1 | 2 | 2 | 0 |
| Vasculitis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| History of AIDS | 15 | 15 | 16 | 23 | 22 | 24 | 21 | 37 |
Abbreviations: CAD, coronary artery disease; CKD-EPI Chronic Kidney Disease Epidemiology Collaboration; CVA, cerebrovascular accident; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; HCV, hepatitis C virus; MI, myocardial infarction; PVD, peripheral vascular disease; SD, standard deviation; TIA, transient ischemic attack.
Data represent percentage of participants unless otherwise specified. Arrows indicate reclassification to another eGFR stage. The stages were defined as follows: stage 1, eGFR ≥90 mL/min/1.73 m2; stage 2, 60–89 mL/min/1.73 m2; stage 3, 30–59 mL/min/1.73 m2; stage 4, 15–29 mL/min/1.73 m2; and stage 5, <15 mL/min/1.73 m2.
Five-Year Probabilities of eGFR Stage Transition
Overall, black participants had slightly shorter follow-up time compared with white participants (median [interquartile range], 7.2 [3.0–10.3] vs 8.1 [4.2–10.7] years); however, the mean number of eGFR measurements were similar in black and white participants (median, 20 [10–36] and 20 [10–34], respectively). Table 4 shows the predicted 5-year probabilities for all possible transitions and the estimated prevalence of each eGFR stage or death at 5 years. When black and white participants were compared, the 5-year probabilities of transitioning from baseline kidney function to each successive stage differed substantially according to whether kidney function was estimated with the 2009 CKD-EPI eGFRcr equation or with the race-free 2021 CKD-EPI eGFRcr equation (Table 4).
Table 4.
Conditional 5-Year State Transition Probabilities, Stratified by Race and Use of Race Coefficient
| Baseline kidney Function | Transition Probability at 5-y Follow-upa | ||||
|---|---|---|---|---|---|
| eGFR Stage 1 | eGFR Stage 2 | eGFR Stage 3 | eGFR Stage 4/5 | Death | |
| Black participants | |||||
| 2009 CKD-EPI eGFRcr equation | (n = 14 540) | (n = 5323) | (n = 943) | (n = 358) | (n = 2557) |
| ȃeGFR stage 1 (n = 22 311) | 0.768 | 0.194 | 0.008 | 0.001 | 0.029 |
| ȃeGFR stage 2 (n = 7299) | 0 | 0.882 | 0.066 | 0.009 | 0.043 |
| ȃeGFR stage 3 (n = 1449) | 0 | 0 | 0.632 | 0.177 | 0.192 |
| ȃeGFR stage 4 (n = 239) | 0 | 0 | 0 | 0.478 | 0.522 |
| Race-free 2021 CKD-EPI eGFRcr equation | (n = 11 681) | (n = 7677) | (n = 1409) | (n = 385) | (n = 2553) |
| ȃeGFR stage 1 (n = 18 141) | 0.698 | 0.259 | 0.010 | 0.001 | 0.033 |
| ȃeGFR stage 2 (n = 10 736) | 0 | 0.890 | 0.064 | 0.005 | 0.041 |
| ȃeGFR stage 3 (n = 2127) | 0 | 0 | 0.734 | 0.111 | 0.156 |
| ȃeGFR stage 4 (n = 275) | 0 | 0 | 0 | 0.426 | 0.574 |
| White participants | |||||
| 2009 CKD-EPI eGFRcr equation | (n = 11 761) | (n = 6470) | (n = 1042) | (n = 86) | (n = 1843) |
| ȃeGFR stage 1 (n = 17 415) | 0.713 | 0.242 | 0.011 | 0.0003 | 0.034 |
| ȃeGFR stage 2 (n = 8688) | 0 | 0.881 | 0.074 | 0.003 | 0.042 |
| ȃeGFR stage 3 (n = 1353) | 0 | 0 | 0.802 | 0.053 | 0.145 |
| ȃeGFR stage 4 (n = 86) | 0 | 0 | 0 | 0.292 | 0.708 |
| Race-free 2021 CKD-EPI eGFRcr equation | (n = 13 195) | (n = 5332) | (n = 756) | (n = 76) | (n = 1843) |
| ȃeGFR stage 1 (n = 19 177) | 0.764 | 0.197 | 0.007 | 0.0002 | 0.032 |
| ȃeGFR stage 2 (n = 7279) | 0 | 0.893 | 0.063 | 0.003 | 0.042 |
| ȃeGFR stage 3 (n = 1011) | 0 | 0 | 0.797 | 0.058 | 0.145 |
| ȃeGFR stage 4 (n = 0) | 0 | 0 | 0 | 0.298 | 0.71 |
Abbreviations: CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; eGFRcr, eGFR creatinine.
This table shows 5-year transition probabilities across all baseline eGFR stages. For example, a black participant with eGFR stage 1 at baseline with use of the 2009 CKD-EPI eGFRcr equation has a 0.768 probability of remaining in that state and a 0.194 probability of transitioning to stage 2. eGFR stages were defined as follows: stage 1, eGFR ≥90 mL/min/1.73 m2; stage 2, 60–89 mL/min/1.73 m2; stage 3, 30–59 mL/min/1.73 m2; stage 4, <30 mL/min/1.73 m2.
Race Differences in the Risk of CKD Progression
When the original 2009 CKD-EPI eGFRcr equation was used to define eGFR stages, black participants, compared with white participants, had a 23% lower risk of progressing from eGFR stage 1 to 2 (HR, 0.77; 95% CI, .73–.82) but a 3-fold risk of progressing from eGFR stage 3 to stage 4/5 (3.06; 2.60–3.62) (Table 5). In contrast, after adoption of the race-free 2021 CKD-EPI eGFRcr equation, black participants had a higher risk of disease progression across all levels of baseline kidney function compared with white participants (HR, 1.37 [95% CI, 1.30–1.45] for eGFR stage 1 to 2 transition, 1.07 [.99–1.16] for eGFR stage 2 to 3 transition, and 1.71 [1.45–2.02] for eGFR stage 3 to 4/5 transition).
Table 5.
Association of Race With Estimated Glomerular Filtration Rate (eGFR) Stage Transitions Defined With and Without eGFR Race Coefficient
| CKD Progression by eGFR Stage | HR for Black vs White Race (95% CI) | |
|---|---|---|
| 2009 CKD-EPI eGFRcr Equation | Race-Free 2021 CKD-EPI eGFRcr Equation | |
| Stage 1 to 2 | 0.77 (.73–.82) | 1.37 (1.30–1.45) |
| Stage 2 to 3 | 1.00 (0.92–1.07) | 1.07 (.99–.16) |
| Stage 3 to 4 | 3.06 (2.60–3.62) | 1.71 (1.45–2.02) |
Abbreviations: CI, confidence interval; CKD, chronic kidney disease; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; eGFRcr, eGFR creatinine; HR, hazard ratio.
Models adjusted for calendar period (2005–2009 and 2010–2015), age, sex, history of AIDS, hepatitis C, diabetes, hypertension, cardiovascular disease, baseline human immunodeficiency virus viral load, and baseline eGFR. eGFR stages were defined as follows: stage 1, eGFR ≥90 mL/min/1.73 m2; stage 2, 60–89 mL/min/1.73 m2; stage 3, 30–59 mL/min/1.73 m2; and stage 4, <30 mL/min/1.73 m2.
DISCUSSION
We examined the effect of adopting the new race-free 2021 CKD-EPI eGFRcr equation on risk estimates of CKD progression among PWH in North America. After adoption of this equation, 16% of black participants were reclassified into a more severe eGFR stage. Black participants had a higher risk of CKD progression than white participants across all baseline eGFR stages, but only when GFR was estimated with the race-free equation. In contrast, when using the original CKD-EPI eGFRcr with the race coefficient, black participants with eGFR stage 1 had a lower risk of disease progression than white participants. These findings imply that prior studies suggesting that black Americans experience accelerated rates of kidney disease progression only at later stages of CKD may have been biased by the race coefficient in eGFR [2]. More importantly, the use of the race coefficient in the original eGFRcr CKD-EPI equation systematically hides a subgroup of black individuals who are at high risk of CKD progression, and the immediate adoption of the race-free 2021 CKD-EPI eGFRcr equation [7]—as recently recommended [6]—unmasks this group.
To our knowledge, our study is the first longitudinal study to evaluate the effects of removing the race coefficient from eGFR equations among PWH, a population at increased risk of CKD. In reporting the race-free equation, CKD-EPI authors showed that >600 000 black Americans would be newly identified as having CKD [7]. A national study of US veterans showed that immediate adoption of the race-free 2021 CKD-EPI eGFRcr equation would identify 66 000 more black veterans with eGFR stages 3 to 4 than the original CKD-EPI eGFRcr equation [8]. Early identification of CKD is a necessary first step in any efforts to slow progression of disease. While identifying CKD early can facilitate disease modifying interventions in primary care settings [18], diagnosing advanced CKD can also prompt earlier referral for nephrology specialty care and enable listing for kidney transplantation [19]. To our knowledge, available studies reporting the impact of adopting the new race-free equation are all cross-sectional studies and based on GFR estimation at a single time point, but clinical decisions often rely on longitudinal assessments of kidney function. Thus, our study using multiple longitudinal eGFR measures is more representative of routine clinical practice and provides additional support for adopting race-free eGFR equations.
In the current study, using eGFR estimates with the race coefficient to define eGFR stages was associated with a lower probability of CKD progression at earlier eGFR stages, but higher likelihood at more advanced eGFR stages. Given the disproportionately higher rates of ESKD among black individuals, having a lower risk of disease progression for black compared with white individuals at any baseline kidney function seems biologically implausible. In fact, with adoption of the race-free 2021 CKD-EPI eGFRcr equation in our study, black individuals consistently had higher risk of disease progression across all eGFR stages, which aligns with the literature showing faster rate of kidney function decline among black individuals than their white counterparts [20, 21]. This has critical implications for clinical care because adoption of the new race-free equation can improve recognition and mitigation of disease progression among black patients. This is especially important in nephrology [22], where racial differences in ESKD incidence, particularly among PWH [1], are among the most dramatic examples of health disparities [23]. Thus, detecting and managing CKD at the earliest stages is a health justice imperative [18].
Strengths of our study include a large sample size with a wide representation of PWH in North America. We also used a well-established cohort with extensive longitudinal measurements of kidney function, key risk factors and outcomes, which allowed for robust estimates of disease progression using multistate Markov models. We recognize some limitations, however. First, this study included PWH and thus cannot be generalized to individuals without HIV. However, because the race coefficient is independent of HIV status, we would anticipate a similar impact of adopting the race-free 2021 CKD-EPI eGFRcr equation in the general population. Second, we recognize that additional factors beyond the race coefficient, such as socioeconomic status, may complicate clinical care and kidney disease progression, and we encourage future studies to include rigorous measures of social determinants of disease. Third, the study does not have reference-standard measured GFR, precluding comparison of the predictive performances of the 2009 versus the race-free 2021 CKD-EPI eGFRcr equations. Fourth, although the combination of creatinine and cystatin C is the most accurate method of estimating kidney function [7], cystatin C data were not available in NA-ACCORD.
In conclusion, we report that after adopting the race-free 2021 CKD-EPI eGFRcr equation to estimate kidney function, black participants had a higher risk of kidney disease progression across all baseline eGFR stages than white participants. This suggests that the long-standing practice of automatically assigning a higher kidney function based purely on race may have systemically hindered our ability to detect a subgroup of black persons living with HIV at higher risk of CKD progression. Adoption of the race-free 2021 CKD-EPI eGFRcr equation unmasks this group and should be implemented immediately.
Supplementary Material
Contributor Information
Anthony N Muiru, Kidney Health Research Collaborative, Department of Medicine, University of California, San Francisco, California, USA; Division of Nephrology, Department of Medicine, University of California, San Francisco, California, USA.
Erin Madden, Kidney Health Research Collaborative, Department of Medicine, University of California, San Francisco, California, USA; San Francisco VA Health Care System, San Francisco, California, USA.
Rebecca Scherzer, Kidney Health Research Collaborative, Department of Medicine, University of California, San Francisco, California, USA; San Francisco VA Health Care System, San Francisco, California, USA.
Michael A Horberg, Kaiser Permanente Mid-Atlantic Permanente Research Institute, Rockville, Maryland, USA.
Michael J Silverberg, Kaiser Permanente Northern California, Oakland, California, USA.
Marina B Klein, Division of Infectious Diseases and Chronic Viral Illness Service, McGill University Health Centre, Montreal, Quebec, Canada.
Angel M Mayor, Retrovirus Research Center, Internal Medicine Department, Universidad Central del Caribe, School of Medicine, Bayamon, Puerto Rico, USA.
M John Gill, Department of Medicine, University of Calgary, Southern Alberta HIV Clinic, Calgary, Alberta, Canada.
Sonia Napravnik, Division of Infectious Diseases, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA.
Heidi M Crane, Division of Allergy and Infectious Diseases, Center for AIDS Research, University of Washington, Seattle, Washington, USA.
Vincent C Marconi, Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA; Department of Global Health, Rollins School of Public Health, Emory University, Atlanta, Georgia, USA.
John R Koethe, Division of Infectious Diseases, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Alison G Abraham, Department of Epidemiology, School of Public Health University of Colorado, Anschutz Medical Campus, Aurora, Colorado, USA; Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Keri N Althoff, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Gregory M Lucas, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Richard D Moore, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Michael G Shlipak, Kidney Health Research Collaborative, Department of Medicine, University of California, San Francisco, California, USA; San Francisco VA Health Care System, San Francisco, California, USA.
Michelle M Estrella, Kidney Health Research Collaborative, Department of Medicine, University of California, San Francisco, California, USA; Division of Nephrology, Department of Medicine, University of California, San Francisco, California, USA; San Francisco VA Health Care System, San Francisco, California, USA.
Notes
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial support. This work was supported by the National Institutes of Health (NIH; grants U01AI069918, F31AI124794, F31DA037788, G12MD007583, K01AI093197, K01AI131895, K23EY013707, K24AI065298, K24AI118591, K24DA000432, K24DA035684, KL2TR000421, N01CP01004, N02CP055504, N02CP91027, P30AI027757, P30AI027763, P30AI027767, P30AI036219, P30AI050409, P30AI050410, P30AI094189, P30AI110527, P30MH62246, R01AA016893, R01DA011602, R01DA012568, R01 AG053100, R01DA026770, R24AI067039, U01AA013566, U01AA020790, U01AI038855, U01AI038858, U01AI068634, U01AI068636, U01AI069432, U01AI069434, U01DA03629, U01DA036935, U10EY008057, U10EY008052, U10EY008067, U01HL146192, U01HL146193, U01HL146194, U01HL146201, U01HL146202, U01HL146203, U01HL146204, U01HL146205, U01HL146208, U01HL146240, U01HL146241, U01HL146242, U01HL146245, U01HL146333, U24AA020794, U54GM133807, UL1RR024131, UL1TR000004, UL1TR000083, Z01CP010214, and Z01CP010176); the US Centers for Disease Control and Prevention (contracts CDC-200-2006-18797 and CDC-200-2015-63931); the Agency for Healthcare Research and Quality (contract 90047713); the Health Resources and Services Administration (contract 90051652); the Canadian Institutes of Health Research (grants CBR-86906, CBR-94036, HCP-97105, and TGF-96118); the Ontario Ministry of Health and Long Term Care; the Government of Alberta, Canada; and the following NIH institutes: the National Institute of Allergy and Infectious Diseases, the National Cancer Institute, National Heart, Lung, and Blood Institute, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Human Genome Research Institute, the National Institute for Mental Health, the National Institute on Drug Abuse, the National Institute on Aging, the National Institute of Dental and Craniofacial Research, the National Institute of Neurological Disorders and Stroke, the National Institute of Nursing Research, the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Deafness and Other Communication Disorders, and the National Institute of Diabetes and Digestive and Kidney Diseases.
References
- 1. Abraham AG, Althoff KN, Jing Y, et al. End-stage renal disease among HIV-infected adults in North America. Clin Infect Dis 2015; 60:941–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hsu CY, Lin F, Vittinghoff E, Shlipak MG. Racial differences in the progression from chronic renal insufficiency to end-stage renal disease in the United States. J Am Soc Nephrol 2003; 14:2902–7. [DOI] [PubMed] [Google Scholar]
- 3. Lucas GM, Lau B, Atta MG, Fine DM, Keruly J, Moore RD. Chronic kidney disease incidence, and progression to end-stage renal disease, in HIV-infected individuals: a tale of two races. J Infect Dis 2008; 197:1548–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stevens LA, Levey AS. Measured GFR as a confirmatory test for estimated GFR. J Am Soc Nephrol 2009; 20:2305–13. [DOI] [PubMed] [Google Scholar]
- 5. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Delgado C, Baweja M, Crews DC, et al. A unifying approach for GFR estimation: recommendations of the NKF-ASN Task Force on reassessing the inclusion of race in diagnosing kidney disease. Am J Kidney Dis 2022; 79:268–288 e1. [DOI] [PubMed] [Google Scholar]
- 7. Inker LA, Eneanya ND, Coresh J, et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med 2021; 385:1737–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gregg LP, Richardson P, Akeroyd J, Matheny M, Virani S, Navaneethan S. Effects of the 2021 CKD-EPI creatinine eGFR equation among a national US veteran cohort. Clin J Am Soc Nephrol 2021; 17:283–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gange SJ, Kitahata MM, Saag MS, et al. Cohort profile: the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD). Int J Epidemiol 2007; 36:294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kidney Disease Improving Global Outcomes Working Group. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group . KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3:4–4. [Google Scholar]
- 11. Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PloS One 2013; 8:e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Panel on Antiretroviral Guidelines for Adults and Adolescents . Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. US Department of Health and Human Services. 2019. Available at: https://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. Accessed 11 November 2019.
- 13. Jackson CH, Sharples LD, Thompson SG, Duffy SW, Couto E. Multistate Markov models for disease progression with classification error. J R Stat Soc 2003; 52:193–209. [Google Scholar]
- 14. Leffondré K, Touraine C, Helmer C, Joly P. Interval-censored time-to-event and competing risk with death: is the illness-death model more accurate than the Cox model? Int J Epidemiol 2013; 42:1177–86. [DOI] [PubMed] [Google Scholar]
- 15. Boucquemont J, Heinze G, Jager KJ, Oberbauer R, Leffondre K. Regression methods for investigating risk factors of chronic kidney disease outcomes: the state of the art. BMC Nephrol 2014; 15:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jackson C. Multi-state models for panel data: the msm package for R. J Stat SoftW 2011; 38:28. [Google Scholar]
- 17. Stevens LA, Claybon MA, Schmid CH, et al. Evaluation of the Chronic Kidney Disease Epidemiology Collaboration equation for estimating the glomerular filtration rate in multiple ethnicities. Kidney Int 2011; 79:555–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shlipak MG, Tummalapalli SL, Boulware LE, et al. The case for early identification and intervention of chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 2021; 99:34–47. [DOI] [PubMed] [Google Scholar]
- 19. Norris KC, Eneanya ND, Boulware LE. Removal of race from estimates of kidney function: first, do no harm. JAMA 2020; 325:135–137. [DOI] [PubMed] [Google Scholar]
- 20. Peralta CA, Katz R, DeBoer I, et al. Racial and ethnic differences in kidney function decline among persons without chronic kidney disease. J Am Soc Nephrol 2011; 22:1327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peralta CA, Vittinghoff E, Bansal N, et al. Trajectories of kidney function decline in young black and white adults with preserved GFR: results from the Coronary Artery Risk Development in Young Adults (CARDIA) study. Am J Kidney Dis 2013; 62:261–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bignall ONR 2nd, Crews DC. Stony the road we trod: towards racial justice in kidney care. Nat Rev Nephrol 2020; 17:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Norris K, Nissenson AR. Race, gender, and socioeconomic disparities in CKD in the United States. J Am Soc Nephrol 2008; 19:1261–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.