Abstract
Background
Rotavirus vaccine performance appears worse in countries with high rotavirus genotype diversity. Evidence suggests diminished vaccine efficacy (VE) against G2P[4], which is heterotypic with existing monovalent rotavirus vaccine formulations. Most studies assessing genotype-specific VE have been underpowered and inconclusive.
Methods
We pooled individual-level data from 10 Phase II and III clinical trials of rotavirus vaccine containing G1 and P[8] antigens (RV1) conducted between 2000 and 2012. We estimated VE against both any-severity and severe (Vesikari score ≥11) rotavirus gastroenteritis (RVGE) using binomial and multinomial logistic regression models for non-specific VE against any RVGE, genotype-specific VE, and RV1-typic VE against genotypes homotypic, partially heterotypic, or fully heterotypic with RV1 antigens. We adjusted models for concomitant oral poliovirus and RV1 vaccination and the country's designated child mortality stratum.
Results
Analysis included 87 644 infants from 22 countries in the Americas, Europe, Africa, and Asia. For VE against severe RVGE, non-specific VE was 91% (95% confidence interval [CI]: 87–94%). Genotype-specific VE ranged from 96% (95% CI: 89–98%) against G1P[8] to 71% (43–85%) against G2P[4]. RV1-typic VE was 92% (95% CI: 84–96%) against partially heterotypic genotypes but 83% (67–91%) against fully heterotypic genotypes. For VE against any-severity RVGE, non-specific VE was 82% (95% CI: 75–87%). Genotype-specific VE ranged from 94% (95% CI: 86–97%) against G1P[8] to 63% (41–77%) against G2P[4]. RV1-typic VE was 83% (95% CI: 72–90%) against partially heterotypic genotypes but 63% (40–77%) against fully heterotypic genotypes.
Conclusions
RV1 VE is comparatively diminished against fully heterotypic genotypes including G2P[4].
Keywords: rotavirus, vaccines, clinical trials, efficacy, genotypes
Monovalent rotavirus vaccine is unambiguously less efficacious against heterotypic genotypes and specifically G2P[4]. Efficacy against G1P[8], the genotype homotypic with the vaccine is similar for any-severity and severe disease, although efficacy against other genotypes is lower against less-severe disease.
Before rotavirus vaccine introduction, rotavirus caused substantial morbidity and mortality among children <5 years old, with an estimated 2 million hospitalizations and 440 000 (interquartile range [IQR]:352 000–592 000) deaths annually among children in this age group worldwide [1]. Although nearly every child globally was infected by 5 years of age, nearly all deaths occurred in countries in sub-Saharan Africa and South Asia [1]. With the introduction of rotavirus vaccines into national immunization programs in 87 countries by the end of 2016 [2] , approximately 28 800 (95% uncertainty interval: 14 600–46 700) deaths were prevented in 2016 because of rotavirus vaccines [3]. However, children in low- and middle-income countries in sub-Saharan Africa and South Asia continue to be disproportionately affected by rotavirus gastroenteritis (RVGE) [3, 4]. Rotavirus vaccine efficacy and effectiveness estimates are consistently lower in these high rotavirus burden countries with high child mortality rates [5–7] , so vaccine-conferred protection is sub-optimal in higher-burden settings. Rotavirus vaccines also appear less effective against less severe disease [8].
In the pre-vaccine era, 5 rotavirus genotypes—G1P[8], G2P[4], G3P[8], G4P[8], and G9P[8]—were globally responsible for the bulk of human RVGE [9]. Importantly, 1 of these genotypes—G2P[4]—features a different genetic constellation for the other non-G and P rotavirus proteins (DS-1-like) than the other 4 genotypes (Wa-like) [10]. After introduction of GlaxoSmithKline's (GSK) Rotarix (RV1) rotavirus vaccine, which contains G1 and P[8] antigens [4], observational studies reported increased presence of G2P[4] among RVGE cases in vaccine-introducing countries; however, other non-introducing countries also reported a similar phenomenon [11]. Because high burden settings also feature greater rotavirus genotypic diversity, their higher prevalence of heterotypic genotypes may contribute to regional differences in rotavirus vaccine efficacy and effectiveness estimates [12]. In a meta-analysis, the five common pre-vaccine era genotypes were detected in >90% of rotavirus infections in North America, Europe, and Australia but detected in only 68% of infections in South America and Asia and 50% in Africa [9]. Meanwhile, uncommon, heterotypic genotypes like G2P[6] and G8P[6] each accounted for ∼10% of rotavirus detections in African countries [13].
Some evidence suggests that rotavirus vaccines are generally less effective against heterotypic genotypes (G- and/or P-protein different from vaccine antigens) [12], but other data are conflicting or inconclusive [14]. Estimates of lower effectiveness against heterotypic genotypes are imprecise, even in meta-analyses, possibly stemming from a lack of power for genotype-specific analyses in individual studies. Furthermore, many studies assessing genotype-specific protection either occurred in regions with low genotype diversity or had only a few genotypes circulating during the study's conduct [15]. To date, studies reporting genotype-specific vaccine protection have not been definitive. We accessed and pooled multi-country data from 10 Phase II and III RV1 clinical trials to address the lack of power from individual studies and estimate type-specific rotavirus vaccine efficacy (VE).
METHODS
We pooled individual-level data collected on infants enrolled in 10 GSK Phase II and III clinical trials of RV1 conducted between 2000 and 2012. The randomized, double-blind, placebo-controlled trials all enrolled only healthy infants and used similar inclusion/exclusion criteria, vaccination schedules, definitions for the acute gastroenteritis outcome, stool sample collection timings, and stool sample testing and genotyping procedures (Supplementary Table 1). Four trials collected stool samples for all episodes of acute gastroenteritis (any-severity trials), 5 trials collected stool samples for episodes of acute gastroenteritis resulting in hospitalization and/or administration of oral rehydration therapy, and 1 trial collected stool samples for medically-attended acute gastroenteritis episodes. One trial included multiple RV1 treatment arms with different CCID50 concentrations, but infants assigned to an RV1 arm with CCID50 < 106.0 were excluded from analysis.
Protocol-specified follow-up ranged from 1 to 2 years after receipt of the final study dose, so, for consistency across trials, we restricted analysis to data collected during the first year of life. We created 2 pooled datasets: 1 including data from all trials and 1 including data only from any-severity trials. Data were deidentified and obfuscated by GSK before sharing. The Emory University Institutional Review Board determined this study did not meet the definition of human subjects research requiring approval. Data management and analyses were conducted in R software (version 4.0.3).
Statistical Analysis
We included infants in VE analyses if they received 2 study doses per their assigned treatment arm, did not experience RVGE until at least 14 days after the second study dose, and attended at least 1 study visit after the second study dose. For the 2 trials featuring a 3-dose regimen of 1 placebo dose followed by 2 RV1 doses, we considered the 2 RV1 doses as the 2 relevant doses. Our 2 outcomes of interest were any-severity RVGE, defined as an episode of gastroenteritis where the collected stool sample was rotavirus-positive by both enzyme-linked immunosorbent assay (ELISA) and polymerase chain reaction (PCR), and severe RVGE, defined as the subset of any-severity RVGE with a Vesikari score ≥11 [16].
For type-specific VE analyses, RVGE episodes were classified in 2 ways: based on the specific G- and P-protein detected (genotype classification) and based on whether the G- and P-proteins detected were the same as those in RV1 (RV1-typic classification). Genotype categories used were G1P[8], G2P[4], G9P[8], and non-G1P[8] detections (which included other single G-proteins detected in combination with P[8] alone). RV1-typic categories used were homotypic (G1P[8] detected, since RV1 only contains G1 and P[8] antigens), partially heterotypic (either G1 or P[8] detected in combination with a single non-P[8] protein or non-G1 protein, respectively), and fully heterotypic (no G1 or P[8] detected).
We first estimated non-specific VE against any-severity RVGE (using only the any-severity trial data) and against severe RVGE (using first the any-severity trial data, then the entire pooled dataset) using binomial logistic regression models. We estimated type-specific VE against any-severity RVGE (using only the any-severity trial data) and severe RVGE (using first the any-severity trial data, then the entire pooled dataset) using multinomial logistic regression models. We additionally conducted a multinomial logistic regression sieve analysis, as described by Gilbert et al [17], to estimate VE against G2P[4] and G9P[8] relative to G1P[8]. Outcome categories for multinomial models were unordered.
In all of these models, we included covariates for RV1 vaccination (yes or no), oral poliovirus vaccination concomitant with RV1 vaccination (yes or no), and the country's child mortality stratum (high, low, or very low); countries were assigned to a stratum using the 2002 World Health Report [18]. This variable was included to control for unmeasured confounders of the relationship between RV1-conferred immunity and susceptibility to RVGE, as many of these confounders are correlated with a country's child mortality stratum.
We conducted 4 sensitivity analyses for our modeling approaches. In the first sensitivity analysis, we estimated genotype-specific VE using a series of binomial logistic regression models to assess if the multinomial regression forced VE against some genotypes to be lower based on the non-specific VE. In the second sensitivity analysis, we used the country's WHO region in lieu of the country's child mortality stratum. Because child mortality stratum and WHO region were perfectly correlated in the any-severity trial data, this analysis was only necessary for estimation of VE against severe RVGE using all pooled data. For the third sensitivity analysis, we slightly changed how the RV1-typic categories were defined. In our main analyses, GXP[6] detections (ie, G-type uncharacterized) composed a substantial number of RVGE episodes and were classified as fully heterotypic, representing the assumption that no G1 co-occurred with P[6]. We classified GXP[6] as only partially heterotypic in our sensitivity analysis, representing the other extreme assumption that these were all G1P[6]. The fourth sensitivity analysis followed the main analysis and also included model adjustment for specific trial.
RESULTS
A total of 87 644 infants from 22 countries and 10 RV1 trials were included in our analysis of VE against severe RVGE using the entire pooled dataset. Just under half of participants (n = 42 947) were female, 62% (n = 54 663) were from countries with low child mortality rates, and 23% (n = 19 818) were from countries with high child mortality rates. Classification of countries into strata by child mortality rates are in Supplementary Table 2. The distribution of these characteristics did not appear to differ by treatment group (Table 1). Among infants assigned to RV1 (n = 46 649), 17% received oral poliovirus vaccine on the same day that RV1 was administered (concomitant OPV) (Table 1).
Table 1.
Characteristics of Infants Included in Vaccine Efficacy Analyses
| Characteristic, n (%)a | Severe RVGE Analysis | Any-severity RVGE Analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| Placebo (n = 40 995) | RV1 (n = 46 649) | Placebo (n = 4476) | RV1 (n = 7527) | |||||
| Sex | ||||||||
| Female | 20 058 | (49) | 22 889 | (49) | 2205 | (49) | 3627 | (48) |
| Male | 20 937 | (51) | 23 760 | (51) | 2271 | (51) | 3900 | (52) |
| Country's child mortality stratum | ||||||||
| High | 9001 | (22) | 10 817 | (23) | 1564 | (35) | 3329 | (44) |
| Low | 26 193 | (64) | 28 470 | (61) | 1586 | (35) | 1592 | (21) |
| Very low | 5801 | (14) | 7362 | (16) | 1326 | (30) | 2606 | (35) |
| Country's WHO region | ||||||||
| African Region | 1569 | (4) | 3337 | (7) | 1564 | (35) | 3329 | (44) |
| Region of the Americas | 30 034 | (73) | 32 354 | (69) | 0 | (0) | 0 | (0) |
| European region | 2321 | (6) | 3620 | (8) | 1326 | (30) | 2606 | (35) |
| Western Pacific region | 7071 | (17) | 7338 | (16) | 1586 | (35) | 1592 | (21) |
| Concomitant OPV and RV1 | ||||||||
| Yes | 0 | (0) | 7758 | (17) | 0 | (0) | 3480 | (46) |
| No | 40 995 | (100) | 38 891 | (83) | 4476 | (100) | 4047 | (54) |
Data from all 10 trials were included in the severe RVGE analysis. Data from the 4 trials that collected stool samples for gastroenteritis of any severity were included in the any-severity RVGE analysis.
Abbreviations: OPV, Oral poliovirus vaccine; RV1, Monovalent rotavirus vaccine; RVGE, rotavirus gastroenteritis; WHO, World Health Organization.
Percentages may not sum to 100 due to rounding.
A total of 12 003 infants were included in our analysis of VE against any-severity RVGE. Infants came from 9 countries and 4 RV1 trials where stool samples were collected and tested for all episodes of gastroenteritis (any-severity trials). Almost half (n = 5832) were female, 41% (n = 4893) were from countries with high child mortality rates, and 33% (n = 3932) were from countries with very low child mortality rates. The distribution of infants in the RV1 group between child mortality strata was similar to the overall distribution, but placebo group infants were split more evenly between strata (Table 1). Child mortality stratum was also highly correlated with WHO region (Supplementary Table 2). Among infants assigned to RV1 (n = 7527), 46% received OPV concomitant with RV1 (Table 1).
Rotavirus Types
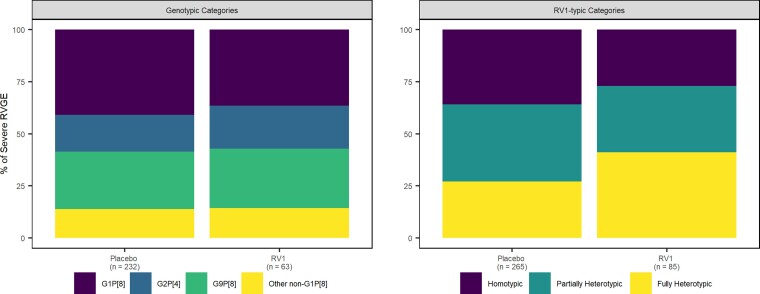
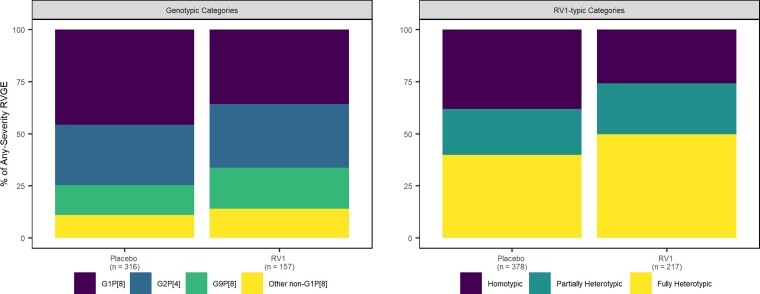
In all pooled data, the homotypic genotype (ie G1P[8]) was detected in 41% of the 232 severe single-genotype RVGE cases in the placebo group and in 37% of the 63 severe single-genotype RVGE cases in the RV1 group (Figure 1, Supplementary Table 3). Similar proportions of G2P[4] and non-G1P[8] occurred in the two groups (placebo: 18% and 14%, respectively; RV1: 21% and 14%, respectively) (Figure 1, Supplementary Table 3). Homotypic, partially heterotypic, and fully heterotypic genotypes occurred in similar proportions among the 265 severe RVGE cases in the placebo group, but the 85 cases in the RV1 group had a greater proportion of fully heterotypic genotypes (Figure 1, Supplementary Table 3). Similar distributions of genotype categories and RV1-typic categories occurred for the 595 single-genotype any-severity cases and the 233 single-genotype severe cases in the any-severity trial data (Figure 2, Supplementary Table 3). RVGE cases with multiple G- or P-types detected are not included in the total case numbers above and were excluded from all analyses; for this reason, 14 cases (11 in the placebo group) were excluded from all pooled data. Twenty-one cases (12 in the placebo group) were excluded from the any-severity trial data. Classifications of rotavirus genotypes into homotypic and heterotypic categories are located in Supplementary Table 4. Genotypic and RV1-typic distributions varied by trial (Supplementary Figures 1 and 2), child mortality stratum (Supplementary Figures 3 and 5), and WHO region (Supplementary Figures 4 and 5).
Figure 1.
Percent of severe (Vesikari score ≥11) RVGE cases in each genotype category (left) and RV1-typic category (right) by treatment arm. Abbreviations: RV1-typic, similarity to monovalent rotavirus vaccine antigens; RVGE, rotavirus gastroenteritis.
Figure 2.
Percent of any-severity RVGE cases in each genotype category (left) and RV1-typic category (right) by treatment arm. Abbreviations: RV1-typic, similarity to monovalent rotavirus vaccine antigens; RVGE, rotavirus gastroenteritis.
RV1 Efficacy
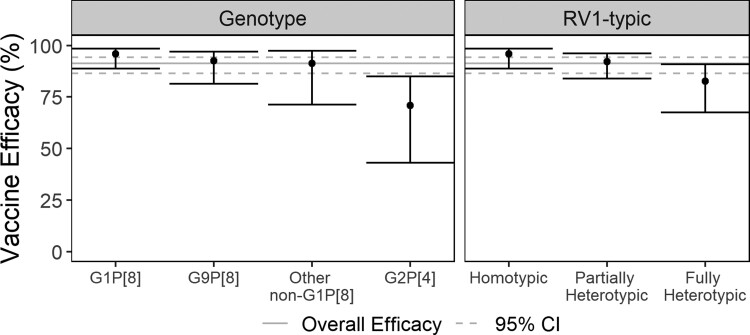
Non-specific VE against severe RVGE was 91% when estimated using all pooled data and when estimated using only any-severity trial data (all pooled data 95% confidence interval [CI]: 87–94%; any-severity trial data 95% CI: 85–96%). Genotype-specific VE estimates from the model fit to all pooled data ranged from 96% (95% CI: 89–98%) against G1P[8] and 92% (95% CI: 81–97%) against G9P[8] to 71% (95% CI: 43–85%) against G2P[4] (Figure 3, Supplementary Table 5). Based on the sieve analysis, RV1 protection against severe RVGE caused by G9P[8] was similar to its protection against homotypic G1P[8] (odds ratio [OR]: 1.61; 95% CI: 0.41–6.37). Vaccinated infants who experienced severe RVGE were 9.18 times (95% CI: 2.14–39.34) more likely to be infected by G2P[4] than by G1P[8].
Figure 3.
Vaccine efficacy against severe RVGE caused by specific rotavirus genotypes (left) and genotypic similarity to monovalent rotavirus vaccine antigens (right). All pooled data were used for these estimates. Gray lines represent the overall efficacy (solid line) and its associated 95% CI (dashed lines). I-bars represent 95% CIs for each type-specific efficacy estimate. Abbreviations: CI, confidence interval; RVGE, rotavirus gastroenteritis.
VE against severe RVGE caused by partially heterotypic genotypes (92% [95% CI: 84–96%]) was similar to VE against the homotypic genotype, but VE against fully heterotypic genotypes was lower (83% [95% CI: 67–91%])(Figure 3, Supplementary Table 5). Genotype-specific and RV1-typic VE estimates from the model fit to any-severity trial data followed a similar pattern (Supplementary Table 5). Similar estimates of type-specific VE against severe RVGE were obtained when adjusting for WHO region in lieu of child mortality stratum (Supplementary Table 6).
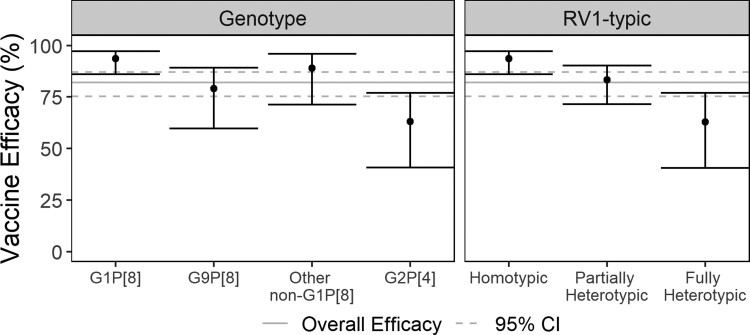
Non-specific VE against any-severity RVGE was 82% (95% CI: 75–87%). Genotype-specific VE estimates ranged from 94% (95% CI: 86–97%) against G1P[8] to 79% (95% CI: 60–89%) against G9P[8] and 63% (95% CI: 41–77%) against G2P[4] (Figure 4, Supplementary Table 5). VE estimates were precise (ie narrow 95% CI width) for VE against G1P[8] but less so for VE estimates against all other genotypes. Based on the sieve analysis, RV1 was more protective against any-severity RVGE caused by G1P[8] than by either G9P[8]- or G2P[4]-caused RVGE (G9P[8] OR: 3.43 [95% CI:1.22–9.62]; G2P[4] OR: 4.52 [95% CI: 1.15–17.71]). VE against any-severity RVGE was lower (83% [95% CI: 72–90%]) against partially heterotypic genotypes compared to the homotypic genotype but lowest (63% [95% CI: 40–77%]) against fully heterotypic genotypes (Figure 4, Supplementary Table 5). Our sensitivity analyses for genotype-specific VE estimated with a series of binomial logistic regressions (Supplementary Table 7), for RV1-typic recategorization of GXP[6] (Supplementary Table 8), and for adjustment for specific trial (Supplementary Table 9) yielded similar type-specific estimates for VE against both any-severity and severe RVGE.
Figure 4.
Vaccine efficacy against any-severity RVGE caused by specific rotavirus genotypes (left) and genotypic similarity to monovalent rotavirus vaccine antigens (right). Only any-severity trial data were used for these estimates. Gray lines represent the overall efficacy (solid line) and its associated 95% CI (dashed lines). I-bars represent 95% CIs for each type-specific estimate. Abbreviations: CI, confidence interval; RVGE, rotavirus gastroenteritis.
DISCUSSION
Our results provide robust evidence from controlled trials that RV1 is less efficacious against heterotypic rotavirus genotypes and, specifically, G2P[4], than against G1P[8]. RV1 was similarly effective against severe RVGE caused by homotypic and partially heterotypic genotypes. G1P[8]-specific VE estimates against any-severity RVGE and against severe RVGE were similar, although protection against other genotypes follows the documented phenomenon of lower VE against less-severe RVGE.
Our results are consistent with prior pooled analyses and meta-analyses, but ours might be considered more conclusive because of the quantity of data we analyzed from several studies occurring over a broad span of time. In a previous pooled analysis of 5 RV1 trials encompassing either the first year of life or a full rotavirus season, VE against G2P[4] was slightly lower than VE against G1P[8] [19]. However, the prior analysis and the individual studies were underpowered to reach firm conclusions about diminished VE against the G2P[4] genotype, and some of the trials (which we did not include in our pooled data) used a lower viral titer than the current commercialized formulation.
Results from a meta-analysis of genotype-specific vaccine effectiveness from post-licensure case-control studies were similarly suggestive of lowered effectiveness against G2P[4] and other heterotypic genotypes for both RV1 and Merck's RotaTeq formulation (RV5), which contains G1, G2, G3, G4, and P[8] antigens [20]. However, this meta-analysis was also limited by the small sample sizes and imprecise estimates from individual studies. Given our results for RV1 VE against G2P[4], RV5 VE may also be diminished against G2P[4], although perhaps less so since the G2 antigen is in its formulation.
Several countries have reported increased detection of G2P[4] after RV1 introduction [21–23], although whether this is due to inherent evolutionary mechanism or vaccine-induced selective pressures is unclear. G12P[8] has also become more common globally in the post-vaccine era [24]. A recent full-genome analysis of 13 years of G2P[4] strains collected in South Africa suggested that the distinct pre- and post-vaccine era lineages were a consequence of natural evolutionary dynamics [25]. If G2P[4] and other heterotypic genotypes become more and more prevalent and existing vaccines are less effective against them, rotavirus immunization programs may experience diminishing returns. Our findings highlight the need for continued development of more cross-protective rotavirus vaccines.
Our results are subject to several limitations. We were unable to estimate VE against more than three specific rotavirus genotypes due to limited genotypic diversity in the pooled data and small case numbers for some genotypes. The non-G1P[8] genotype category was an attempt to mitigate this limitation, as this category was only partially heterotypic with the G1 and P[8] antigens in the RV1 formulation. VE against genotypes common in the post-vaccine era, but not pre-vaccine era, could not be evaluated because of the time during which the trials were conducted. Further work should evaluate VE against newer, common genotypes like G12P[8] as more genotypes emerge but vaccine formulations remain the same. We also could not evaluate enteric co-infections, which would result in underestimated VE [26, 27]. However, as our goal is comparing genotype-specific and RV1-typic VE, the general trends of our comparisons likely hold even with underestimation. Finally, the 2 types of models that we fit (binomial and multinomial logistic regression models) both make strong assumptions about the nature of RV1 efficacy. Binomial regression to estimate non-specific VE assumes that VE is the same against all genotypes. Meanwhile, because multinomial regression estimates VE for every rotavirus type separately, robust data for each genotype of interest are necessary for model specification and narrow confidence intervals. An ideal balance between the 2 approaches would shrink VE estimates for genotypes with sparse data towards the overall VE estimate, whereas VE estimates for genotypes with more robust data would align closely with their respective estimates from a multinomial model.
CONCLUSIONS
Overall, our findings indicate that RV1 is meaningfully less effective against fully heterotypic genotypes and G2P[4] specifically than it is against G1P[8] and partially heterotypic genotypes. Because many of the non-G1, P[8] genotypes (the majority of partially heterotypic-genotypes detected) feature the same Wa-like genetic backbone as circulating G1P[8], although G2P[4] features the distinctly different DS-1-like genetic backbone [10], our results could be a manifestation of diminished efficacy against DS-1-like rotaviruses. Whether the outer capsid proteins (the G- and P-proteins) or the entirety of the genotype constellation (Wa-like or DS-1-like) matters more for rotavirus VE is unclear. Regardless, our use of a large, pooled data set resolves the primary obstacle of insufficient power when attempting to draw conclusions about VE against different rotavirus genotypes. The notably reduced VE against the G2P[4] genotype is unambiguous.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Avnika B Amin, Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta, Georgia, USA; Department of Biostatistics and Bioinformatics, Rollins School of Public Health, Emory University, Atlanta, Georgia, USA.
Jacqueline E Tate, Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Lance A Waller, Department of Biostatistics and Bioinformatics, Rollins School of Public Health, Emory University, Atlanta, Georgia, USA.
Timothy L Lash, Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta, Georgia, USA.
Benjamin A Lopman, Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta, Georgia, USA.
Notes
Acknowledgments. The authors thank GlaxoSmithKline for enabling access to the data and www.clinicalstudydatarequest.com for facilitating the initial proposal and data request submission.
Disclaimer. The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention (CDC).
Financial support. Time contributions for A. B. A. and B. A. L. were funded by the National Institute of Allergy and Infectious Diseases (R01 GR111026 and R01 AI148127). B. A. L. reports support from NIH/NIGMS R01 GM124280. GlaxoSmithKline funded the original trials and were involved with original study design and data collection. The funders had no role in the secondary data analysis, interpretation, writing of the report, or the decision to submit the paper for publication. The authors were not paid to write this article by a pharmaceutical company or other agency.
References
- 1. Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass RI. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis 2003; 9:565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. International Vaccine Access Center . Universal vaccine introduction over time. Available at:https://view-hub.org/map/?set=universal-vaccine-introduction-over-time&group=vaccine-introduction&category=rv. Accessed 14 July 2021.
- 3. Troeger C, Khalil IA, Rao PC, et al. Rotavirus vaccination and the global burden of rotavirus diarrhea among children younger than 5 years. JAMA Pediatr 2018; 172:958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burke RM, Tate JE, Kirkwood CD, Steele AD, Parashar UD. Current and new rotavirus vaccines. Curr Opin Infect Dis 2019; 32:435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jonesteller CL, Burnett E, Yen C, Tate JE, Parashar UD. Effectiveness of rotavirus vaccination: a systematic review of the first decade of global postlicensure data, 2006–2016. Clin Infect Dis 2017; 65:840–50. [DOI] [PubMed] [Google Scholar]
- 6. Soares-Weiser K, Bergman H, Henschke N, Pitan F, Cunliffe N. Vaccines for preventing rotavirus diarrhoea: vaccines in use. Cochrane Database Syst Rev 2019; 2019:CD008521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clark A, van Zandvoort K, Flasche S, et al. Efficacy of live oral rotavirus vaccines by duration of follow-up: a meta-regression of randomised controlled trials. Lancet Infect Dis 2019; 19:717–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Velázquez RF, Linhares AC, Muñoz S, et al. Efficacy, safety and effectiveness of licensed rotavirus vaccines: a systematic review and meta-analysis for Latin America and the Caribbean. BMC Pediatr 2017; 17:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Santos N, Hoshino Y. Global distribution of rotavirus serotypes/genotypes and its implication for the development and implementation of an effective rotavirus vaccine. Rev Med Virol 2005; 15:29–56. [DOI] [PubMed] [Google Scholar]
- 10. Matthijnssens J, Ciarlet M, McDonald SM, et al. Uniformity of rotavirus strain nomenclature proposed by the Rotavirus Classification Working Group (RCWG). Arch Virol 2011; 156:1397–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bibera GL, Chen J, Pereira P, Benninghoff B. Dynamics of G2P[4] strain evolution and rotavirus vaccination: a review of evidence for Rotarix. Vaccine 2020; 38:5591–600. [DOI] [PubMed] [Google Scholar]
- 12. Parker EP, Ramani S, Lopman BA, et al. Causes of impaired oral vaccine efficacy in developing countries. Future Microbiol 2018; 13:97–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Todd S, Page NA, Steele AD, Peenze I, Cunliffe NA. Rotavirus strain types circulating in Africa: review of studies published during 1997–2006. J Infect Dis 2010; 202:S34–42. [DOI] [PubMed] [Google Scholar]
- 14. Madhi SA, Cunliffe NA, Steele D, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med 2010; 362:289–98. [DOI] [PubMed] [Google Scholar]
- 15. Lamberti LM, Ashraf S, Walker CLF, Black RE. A systematic review of the effect of rotavirus vaccination on diarrhea outcomes among children younger than 5 years. Pediatr Infect Dis J 2016; 35:992–8. [DOI] [PubMed] [Google Scholar]
- 16. Ruuska T, Vesikari T. Rotavirus disease in Finnish children: use of numerical scores for clinical severity of diarrhoeal episodes. Scand J Infect Dis 1990; 22:259–67. [DOI] [PubMed] [Google Scholar]
- 17. Gilbert P, Self S, Rao M, Naficy A, Clemens J. Sieve analysis: methods for assessing from vaccine trial data how vaccine efficacy varies with genotypic and phenotypic pathogen variation. J Clin Epidemiol 2001; 54:68–85. [DOI] [PubMed] [Google Scholar]
- 18. World Health Organization . List of member states by WHO region and mortality stratum. In: The World Health Report 2002: Reducing Risks, Promoting Health Life. 233–5.
- 19. De Vos B, Han HH, Bouckenooghe A, et al. Live attenuated human rotavirus vaccine, RIX4414, provides clinical protection in infants against rotavirus strains with and without shared G and P genotypes: integrated analysis of randomized controlled trials. Pediatr Infect Dis J 2009; 28:261–6. [DOI] [PubMed] [Google Scholar]
- 20. Cates J, Amin AB, Tate JE, Lopman B, Parashar U. Do rotavirus strains affect vaccine effectiveness? A systematic review and meta-analysis. Pediatr Infect Dis J 2021; 40:1135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zeller M, Heylen E, Tamim S, et al. Comparative analysis of the RotarixTM vaccine strain and G1P[8] rotaviruses detected before and after vaccine introduction in Belgium. PeerJ 2017; 5:e2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vizzi E, Piñeros OA, Oropeza MD, et al. Human rotavirus strains circulating in Venezuela after vaccine introduction: predominance of G2P[4] and reemergence of G1P[8]. Virol J 2017; 14:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Al-Ayed MSZ, Asaad AM, Qureshi MA, Hawan AA. Epidemiology of group A rotavirus infection after the introduction of monovalent vaccine in the National Immunization Program of Saudi Arabia. J Med Virol 2017; 89:429–34. [DOI] [PubMed] [Google Scholar]
- 24. Dóró R, László B, Martella V, et al. Review of global rotavirus strain prevalence data from six years post vaccine licensure surveillance: is there evidence of strain selection from vaccine pressure? Infect Genet Evol J Mol Epidemiol Evol Genet Infect Dis 2014; 28:446–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mwangi PN, Page NA, Seheri ML, et al. Evolutionary changes between pre- and post-vaccine South African group A G2P[4] rotavirus strains, 2003–2017. Microb Genom 2022; 8:000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mokomane M, Tate JE, Steenhoff AP, et al. Evaluation of the influence of gastrointestinal co-infections on rotavirus vaccine effectiveness in Botswana. Pediatr Infect Dis J 2018; 37:e58–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Praharaj I, Platts-Mills JA, Taneja S, et al. Diarrheal etiology and impact of coinfections on rotavirus vaccine efficacy estimates in a clinical trial of a monovalent human–bovine (116E) oral rotavirus vaccine, Rotavac, India. Clin Infect Dis 2019; 69:243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.