Abstract
Background
National guidelines recommend antiviral treatment for children with influenza at high risk for complications regardless of symptom duration. Little is known about concordance of clinical practice with this recommendation.
Methods
We performed a cross-sectional study of outpatient children (aged 1–18 years) at high risk for complications who were diagnosed with influenza during the 2016–2019 influenza seasons. High-risk status was determined using an existing definition that includes age, comorbidities, and residence in a long-term care facility. The primary outcome was influenza antiviral dispensing within 2 days of influenza diagnosis. We determined patient- and provider-level factors associated with guideline-concordant treatment using multivariable logistic regression.
Results
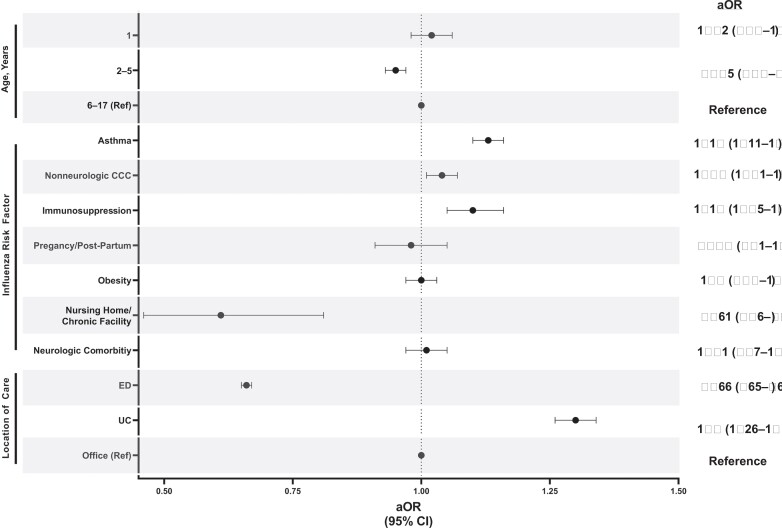
Of the 274 213 children with influenza at high risk for influenza complications, 159 350 (58.1%) received antiviral treatment. Antiviral treatment was associated with the presence of asthma (aOR, 1.13; 95% confidence interval [CI], 1.11–1.16), immunosuppression (aOR, 1.10; 95% CI, 1.05–1.16), complex chronic conditions (aOR, 1.04; 95% CI, 1.01–1.07), and index encounter in the urgent care setting (aOR, 1.3; 95% CI, 1.26–1.34). Factors associated with decreased odds of antiviral treatment include age 2–5 years compared with 6–17 years (aOR, 0.95; 95% CI, .93–.97), residing in a chronic care facility (aOR, .61; 95% CI, .46–.81), and index encounter in an emergency department (aOR, 0.66; 95% CI, .63–.71).
Conclusions
Among children with influenza at high risk for complications, 42% did not receive guideline-concordant antiviral treatment. Further study is needed to elucidate barriers to appropriate use of antivirals in this vulnerable population.
Keywords: influenza, oseltamivir, pediatrics, antiviral
Among children with influenza at high risk for complications, 42% did not receive guideline-concordant antiviral treatment. Antiviral treatment varied according to a number of patient- and provider-level factors. Further study is needed to elucidate barriers to appropriate use of antivirals in children.
In the United States, approximately 25 000 children are hospitalized annually with influenza, accounting for up to 10% of all pediatric hospitalizations during the winter season [1, 2]. Complications of influenza can be life-threatening and include secondary bacterial infections, sepsis, neurologic complications such as meningitis, and death. Young children and those with underlying comorbidities are at higher risk for hospitalization, influenza complications, and death [3–8].
The preponderance of published data supports the use of antivirals, specifically oseltamivir, to treat influenza, especially in children at high risk for complications. Oseltamivir acts as neuraminidase inhibitor to prevent viral replication and reduce infectivity. Oseltamivir use is reported to decrease symptom duration, hospitalizations, hospital length of stay, and complications [1, 9–22]. Meta-analyses have concluded that oseltamivir is also associated with a decreased risk of otitis media [21, 22] and less antibiotic use in children [21].
The American Academy of Pediatrics (AAP), the Infectious Disease Society of America (IDSA), and the Centers for Disease Control and Prevention (CDC) state that oseltamivir or other influenza antiviral treatment may be considered in healthy, symptomatic children within 48 hours of symptoms onset [23, 24]. Importantly, the AAP, IDSA, and CDC strongly recommend antiviral treatment in all children at high risk for influenza complications regardless of duration of symptoms at diagnosis [23, 24].
Previous reports suggest that oseltamivir and other influenza antivirals may be underutilized in children [25–31]. For example, despite recommendations that all children hospitalized with influenza receive oseltamivir, almost 25% of children do not receive it during their hospitalization [32–34]. However, the frequency of outpatient oseltamivir use in children at high risk for influenza complications in the United States is unknown. We sought to determine the prevalence of and factors associated with guideline-concordant antiviral treatment in outpatient children at high risk for influenza complications in the United States.
METHODS
Study Design and Population
We conducted a cross-sectional study using the MarketScan Medicaid database (IBM Watson Health, Armonk, NY), which includes data on paid Medicaid claims for all covered healthcare encounters, including both outpatient and inpatient settings [35]. We included children aged 1–18 years at high risk for influenza complications with an influenza diagnosis (Supplementary Table 1) between 2016 and 2019. Included children were continuously enrolled (≥12 months) in Medicaid or the Children’s Health Insurance Program fee-for-service and managed care plans from 12–15 states (depending on the year) within all geographic regions of the United States. Those evaluated in the emergency department (ED) were included only if they were discharged from the ED and not if they were admitted to the hospital directly from the ED. We required continuous enrollment during the 12 months prior to the qualifying diagnosis in order to comprehensively capture healthcare utilization and accurately classify comorbidities. Exclusion criteria included hospitalization at the time of influenza diagnosis (eg, hospitalization and diagnosis on the same day), prior influenza infection diagnosis within 30 days, radiology or laboratory visit with an International Classification of Diseases, 10th Edition (ICD-10), Clinical Modification, claim for influenza, and infants aged <1 year. Radiology and laboratory visit diagnoses were excluded as these are not typically provider encounters and reflect carryover diagnoses from provider encounters. Infants were excluded because oseltamivir had not been approved by the US Food and Drug Administration (FDA) in all children aged <1 year during the study period. This study of deidentified data was considered exempt from review by the Vanderbilt University Medical Center Institutional Review Board.
Influenza Infection and Season Definition
Outpatient influenza infections were identified using ICD-10 diagnoses codes [33, 36] for influenza (Supplementary Table 1). Recent studies demonstrate that ICD-10 codes have greater validity than ICD-9 codes for influenza infection [37–40] and influenza ICD-10 codes have been successfully implemented as influenza surveillance systems [40, 41]. The influenza season was defined as the period from 1 October through 30 April in 2016–2017 and 2017–2018. The 2018–2019 influenza season encompassed 1 October through 31 December due to lack of available data in calendar year 2019.
Children at High Risk for Influenza Complications
Children at high risk for influenza complications were identified using the AAP/IDSA definitions for high-risk children (Table 1) and included age <5 years, chronic neurological conditions (neurological conditions defined using the pediatric complex chronic conditions [CCCs] classification system version 2) [42], asthma diagnosis, pregnancy or post-partum status, obesity diagnosis [43], residing in a nursing home or other chronic care facility, CCCs (categorized as immunosuppressive CCC and nonneurologic CCCs), neurologic comorbidity (defined as presence of a neurologic CCC or high-intensity neurologic impairment diagnosis [44]; Supplementary Table 2) [42]. The neurologic comorbidity definition comprised both CCC and high-intensity neurologic impairment diagnosis to comprehensively capture children with neurologic comorbidities as this is an especially high-risk population at increased risk for morbidity and mortality with influenza disease [33, 45–47]. A look-back period of 1 year prior to influenza diagnosis was used to identify high-risk comorbid conditions. Indigenous American and Alaska Native race and ethnicity were not considered as they are not separately identifiable in the race/ethnicity categorization in the database. Children with long-term aspirin use were excluded due to the low frequency of aspirin use in children. Hospitalization was excluded as a risk factor given that the database lacks inpatient medication dispensing information.
Table 1.
Cohort Demographic and Clinical Characteristics
| Characteristic | All Patients With an Influenza Diagnosis and at High Risk for Complications | No Antiviral Treatment, N (%) | Antiviral Treatment, N (%) |
|---|---|---|---|
| N | 274 213 | 114 863 (41.9) | 159 350 (58.1) |
| Age | |||
| ȃMedian (interquartile range), years | 4 (3–9) | 4 (3–8) | 4 (3–9) |
| ȃȃ1 | 19 725 (7.2) | 8530 (43.2) | 11 195 (56.8) |
| ȃȃ2–5 | 145 124 (52.9) | 63 436 (43.7) | 81 688 (56.3) |
| ȃȃ6–11 | 67 693 (24.7) | 26 632 (39.3) | 41 061 (60.7) |
| ȃȃ12–17 | 41 671 (15.2) | 16 265 (39) | 25 406 (61) |
| Male gender | 146 709 (53.5) | 60 738 (41.4) | 85 971 (58.6) |
| Race/Ethnicity | |||
| ȃNon-Hispanic White | 125 049 (45.6) | 53 130 (42.5) | 71 919 (57.5) |
| ȃNon-Hispanic Black | 87 306 (31.8) | 36 336 (41.6) | 50 970 (58.4) |
| ȃHispanic | 23 262 (8.5) | 7984 (34.3) | 15 278 (65.7) |
| ȃOther | 10 929 (4) | 4510 (41.3) | 6419 (58.7) |
| ȃUnknown | 27 667 (10.1) | 12 903 (46.6) | 14 764 (53.4) |
| Risk factor | |||
| ȃAge <5 years | 164 849 (60.1) | 71 966 (62.7) | 92 883 (58.3) |
| ȃComplex chronic conditions | 26 986 (9.8) | 10 937 (40.5) | 16 049 (59.5) |
| ȃAsthma | 67 593 (24.6) | 25 694 (38) | 41 899 (62) |
| ȃImmunosuppressed condition | 7235 (2.6) | 2874 (39.7) | 4361 (60.3) |
| ȃPregnancy or post-partum | 2907 (1.1) | 1236 (42.5) | 1671 (57.5) |
| ȃObesity | 39 778 (14.5) | 16 147(40.6) | 23 631 (59.4) |
| ȃNursing homes and other chronic care facilities | 191 (.1) | 96 (50.3) | 95 (49.7) |
| ȃNeurologic comorbidity | 15 262 (5.6) | 6297 (41.3) | 8965 (58.7) |
| Location of care | |||
| ȃEmergency department | 93 829 (34.2) | 46 019(49) | 47 810 (51) |
| ȃUrgent care | 24 164 (8.8) | 8190 (33.9) | 15 974 (66.1) |
| ȃOffice | 156 220 (57) | 60 654 (38.8) | 95 566 (61.2) |
| Hospitalization ≤7 days of diagnosis | 843 (.3) | 458 (.4) | 385 (.2) |
Outcomes
The primary outcome was antiviral treatment defined as a dispensed pharmacy claim for any influenza antiviral (oseltamivir or zanamivir) within 2 calendar days of influenza diagnosis. Peramivir was excluded due to its limited use in the outpatient setting as an intravenous medication, and baloxavir was excluded due to its FDA approval late in the study period and limited inclusion Medicaid formularies. Antivirals were identified using a National Drug Code. Our secondary analysis focused on identifying factors associated with influenza antiviral prescribing. These included (determined a priori) category of known risk factors for influenza complications (age <5 years, asthma, CCC, immunosuppressed condition, neurologic comorbidity, pregnancy or post-partum, obesity, nursing homes and other chronic care facilities), influenza season, and location of care (office, urgent care, and ED).
Statistical Analyses
Demographic characteristics were summarized using frequencies and percentages for categorical variables and geometric means with standard deviations for continuous variables. The proportion of children at high risk for complications with antiviral prescribing was calculated by dividing the number of high-risk individuals with a qualifying influenza diagnosis and antiviral dispensing by the total number of high-risk individuals with a qualifying influenza diagnosis. Factors independently associated with antiviral treatment were identified a priori and evaluated using multivariable logistic regression incorporating the following covariates: age, sex, race/ethnicity, AAP/IDSA risk factor category, concurrent lower respiratory infection (defined as the presence of a pneumonia, empyema, or lower respiratory tract diagnosis [48]), concurrent antibiotic use, number of CCCs, influenza season, and location of care (office, urgent care, and ED). Adjusted odds ratios (aORs) for influenza risk factors and location of care were generated using logistic regression. All analyses were performed using SAS version 9.4 (SAS Institute, Inc, Cary, NC), and P < .05 was considered statistically significant.
RESULTS
Study Population
Of 601 847 children with an influenza diagnosis, 274 213 were identified as high risk for influenza complications and included in the study. Median age was 4 years (interquartile range, 3–9), and the majority of included children were male, of White race or ethnicity, and were cared for in the office setting. Individuals who received antiviral treatment were less likely to be hospitalized within 7 days of diagnosis compared with those who did not receive antiviral treatment (P < .001). Additional demographic and clinical characteristics can be found in Table 1.
Prevalence of Antiviral Treatment
Of the 274 213 individuals at high risk for influenza complications, 159 350 (58.1%) received antiviral treatment. Of the antivirals prescribed, oseltamivir was used almost exclusively (159 344, 99.9%). There were statistically significant differences in the proportion of children prescribed antivirals by influenza season (Table 2).
Table 2.
Antiviral Treatment by Influenza Season
| Treatment | All Patients | 2016–2017a | 2017–2018a | 2018–2019b | P Value |
|---|---|---|---|---|---|
| Any | 159 350 (58.1%) | 52 499 (62.9%) | 60 182 (60.6%) | 46 669 (51%) | <.001 |
| Oseltamivir | 159 344 (58.1%) | 52 498 (62.9%) | 60 179 (60.6%) | 46 667 (51%) | <.001 |
| Zanamivir | 7 (0%) | 2 (0%) | 3 (0%) | 2 (0%) | .931 |
Influenza season defined as 1 October–30 April.
Influenza season defined as 1 October–31 December.
Factors Associated With Antiviral Treatment
Factors associated with antiviral treatment in children at high risk for influenza complications included the presence of an asthma diagnosis, immunosuppression, other CCCs, and treatment in the urgent care setting (compared with the office setting; Figure 1). Factors associated with decreased odds of antiviral treatment included age 2–5 years compared with age 6–17 years, residing in a chronic care facility, and being evaluated in an ED (Figure 1). To determine whether children aged <5 years without comorbidities were driving the lower odds of antiviral treatment, we compared antiviral use in children aged <5 years with and without CCCs and found similar rates of antiviral use (59.4% vs 57.9%, respectively; P < .001; Supplementary Table 3).
Figure 1.
Adjusted odds of receiving antiviral treatment.
DISCUSSION
In this large, multicenter study of antiviral treatment among children with influenza at high risk for influenza complications, we found that 4 in 10 children with an influenza diagnosis and at high risk for influenza complications did not receive antiviral therapy as recommended broadly by national organizations and treatment guidelines. Prescribing was higher among children with selected comorbidities but less common in children aged 2–5 years and in those residing in a chronic care facility or being evaluated in the ED.
Our finding of limited use of antiviral treatment in children and adolescents at high risk for influenza complications is concerning. The AAP, IDSA, and CDC recommend treatment based on strong evidence that high-risk children disproportionately experience poor influenza outcomes [24, 49]. For example, children with underlying neurologic or neuromuscular conditions are more likely to experience respiratory failure, shock, encephalopathy, and death from influenza compared with children without these conditions [33, 45–47]. Children with nonneurologic medical conditions are also more likely to require hospitalization, mechanical ventilation, and intensive care and to develop neurologic complications [11, 24, 46]. Children aged <2 years are at higher risk of hospitalization and death from influenza compared with older children and adolescents [24]. There is strong evidence that guideline-concordant antiviral therapy improves outcomes in this population. A meta-analysis of 11 randomized, controlled trials found that antiviral therapy was associated with an approximately 65% reduction in influenza-related complications in high-risk children [22]. In hospitalized children, antiviral treatment has been associated with decreased prevalence of respiratory failure and death, as well as intensive care unit and overall hospital length of stay [18, 50, 51]. In children with CCCs, antiviral use was associated with significant reductions in pneumonia, otitis media, and hospitalization [11, 49]. There is a wealth of data that support the high-risk status in the pediatric population for other risk factors such as pregnancy, asthma, immunosuppression, obesity, and others. We did find that certain high-risk groups (those with immunocompromised conditions, asthma, and nonneurologic CCCs) did have a greater odds of treatment [24, 52, 53]. However, given these data, our finding that a substantial proportion of high-risk children with influenza do not receive antiviral treatment is alarming and provides a high-value target for care improvement.
We identified several factors associated with decreased odds of guideline-concordant antiviral treatment in high-risk children that may provide targeted areas for improvement. High-risk children aged 2–5 years, those residing in a chronic care facility, and those cared for in the ED were less likely to receive antiviral treatment. The underlying reasons for these groups receiving less treatment are multifactorial and likely include patient-, clinician-, and systems-level factors. For example, for a child being discharged from the ED, a common patient-level factor may be severity of illness. Children who present with mild symptoms or during convalescence of illness may be prescribed less antivirals. The spectrum of illness and risk tolerance experienced by providers (eg, general pediatricians, ED providers) varies and may also lead to differential prescribing. An ED provider may be less inclined to treat a patient being discharged home who is late in the disease course and improving without treatment. An example of a systems-level factor is that the capability and accuracy of influenza testing may vary by provider type and location of care. However, establishing a diagnosis through testing is not a requirement to treat with oseltamivir, although it is recommended in high-risk individuals. Finally, it is probable that some of the decreased dispensed pharmacy claims for antivirals were due to pharmacies being closed in the evening or patient improvement by the time caregivers were able to pick up their prescriptions, rather than lack of prescribing by providers. These situations may all ultimately lead to fewer dispensed pharmacy claims for influenza antivirals despite a diagnosis of influenza.
Our findings are similar to those in the adult population. A meta-analysis of 26 studies found a wide range in use of antiviral treatment among adults with influenza [26]. A recent study of a population that included both adults and children revealed that approximately 40% of individuals at high risk for influenza complications did not receive treatment [25]. The reason for low prescribing overall and for high-risk children in particular is likely multifactorial. Qualitative studies reveal oseltamivir underprescribing by physicians is driven by concerns of its risk–benefit profile [54]. Concern surrounding the effectiveness of oseltamivir, overall, as well as the safety of oseltamivir, in particular, neuropsychiatric adverse events, may mitigate prescribing efforts among providers [26, 55]. Given controversary surrounding manufacturer data transparency regarding oseltamivir effectiveness [56], it is also possible that some prescribers do not routinely prescribe influenza antivirals. For these same reasons, parental skepticism on the safety and effectiveness of oseltamivir may lead to caregivers not filling the prescription [26].
Our results must be considered in the context of several limitations. First, it is possible that some Medicaid enrollees may have supplemental commercial insurance or pay for certain prescriptions out of pocket, which would not be captured in these data. However, the high cost of oseltamivir in this public insurance mitigates this concern. Our findings will generalize best to children with only Medicaid only insurance. Second, we identified antiviral use by the presence of a pharmacy claim, which indicates a prescription was dispensed but we cannot identify if a prescription was written but never filled. As mentioned previously, this limitation would miss patients who may have improved without antiviral treatment and whose caregivers opted not to fill the prescription, despite adherence to guidelines by providers. Third, we used ICD-10 diagnosis codes to identify influenza infections, the validity of which is not well characterized in the outpatient setting in the United States; it is possible there is misclassification of influenza diagnoses. We likely have underestimated cases of influenza as pediatric patients without influenza testing may be diagnosed with influenza-like illness or viral illness in the outpatient setting. Fourth, we used ICD-10 codes to identify children with obesity. Diagnosis codes have high specificity but relatively low sensitivity for capturing children with obesity [57, 58]. Therefore, we underestimated the prevalence of obesity in our study, which would likely bias our results toward a null finding. Fifth, the Truven MarketScan database does not contain zip code–level data. There may be differences in prescribing by urban/rural status (rural, metropolitan, urban regions) that could not be accounted for in the analysis. We were unable to identify all children and adolescents at high risk for influenza complications including those with prolonged aspirin use, those of Indigenous American or Alaska heritage, and hospitalized patients. Furthermore, the Truven database contains limited sociodemographic information, including limited race and ethnicity data. Therefore, we were unable to perform a rigorous evaluation on the role of race and ethnicity in antiviral use among high-risk children. Further study in needed to evaluate race, ethnicity, and other sociodemographic factors in the management of children with influenza. Finally, in an unadjusted analysis, we found a statistically significant difference in hospitalization among children exposed to oseltamivir. Additional rigorous evaluation of the relationship between oseltamivir and risk of hospitalization is warranted to determine the accuracy of this association after adjusting for other clinical factors. Nevertheless, these findings highlight a need to improve care for children at high risk for influenza complications, especially those in chronic care facilities, cared for in the ED, and young children.
CONCLUSIONS
Among children with influenza at high risk for complications, 42% did not receive guideline-recommended antiviral treatment. Antiviral treatment varied according to a number of patient- and provider-level factors. Future studies should evaluate why certain high-risk individuals were less likely to receive antiviral treatment. Further study is needed to elucidate the role of provider and caregiver perceptions, preferences, and other determinants to improve guideline-concordant use of antivirals in these vulnerable populations.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
James W Antoon, Monroe Carell Jr. Children’s Hospital at Vanderbilt, Nashville, Tennessee, USA; Division of Hospital Medicine, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Matt Hall, Children’s Hospital Association, Lenexa, Kansas, USA.
James A Feinstein, Department of Pediatrics, Adult and Child Consortium for Health Outcomes Research & Delivery Science, Children's Hospital Colorado, University of Colorado, Aurora, Colorado, USA.
Kathryn E Kyler, Department of Pediatrics, Division of Hospital Medicine, Children's Mercy Hospitals and Clinics, Kansas City, Missouri, USA.
Samir S Shah, Divisions of Hospital Medicine and Infectious Diseases, Cincinnati Children's Hospital Medical Center & Department of Pediatrics, University of Cincinnati College of Medicine, Cincinnati, Ohio, USA.
Sonya Tang Girdwood, Divisions of Hospital Medicine and Clinical Pharmacology, Cincinnati Children's Hospital Medical Center & Department of Pediatrics, University of Cincinnati College of Medicine, Cincinnati, Ohio, USA.
Jennifer L Goldman, Department of Pediatrics, Division of Clinical Pharmacology, Children's Mercy Hospitals and Clinics, Kansas City, Missouri, USA; Department of Pediatrics, Division of Infectious Diseases, Children's Mercy Hospitals and Clinics, Kansas City, Missouri, USA.
Carlos G Grijalva, Division of Pharmacoepidemiology, Departments of Health Policy and Biomedical Informatics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Derek J Williams, Monroe Carell Jr. Children’s Hospital at Vanderbilt, Nashville, Tennessee, USA; Division of Hospital Medicine, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Notes
Disclaimer. The content presented here is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Financial support. J. W. A. (K23 AI168496), C. G. G. (K24 AI148459), and D. J. W. (R01 AI125642) were supported by the National Institute for Allergy and Infectious Diseases (NIAID) of the NIH. J. A. F. (K23 HD091295) and S. T.-G. (K12 HD028827) were supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the NIH.
References
- 1. Miyakawa R, Barreto NB, Kato RM, Neely MN, Russell CJ. Early use of anti-influenza medications in hospitalized children with tracheostomy. Pediatrics 2019; 143:e20182608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rolfes MA, Foppa IM, Garg S, et al. Annual estimates of the burden of seasonal influenza in the United States: a tool for strengthening influenza surveillance and preparedness. Influenza Other Respir Viruses 2018; 12:132–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blyth CC, Macartney KK, McRae J, et al. Influenza epidemiology, vaccine coverage and vaccine effectiveness in children admitted to sentinel Australian hospitals in 2017: results from the PAEDS-FluCAN Collaboration. Clin Infect Dis 2019; 68:940–8. [DOI] [PubMed] [Google Scholar]
- 4. Burton C, Vaudry W, Moore D, et al. Burden of seasonal influenza in children with neurodevelopmental conditions. Pediatr Infect Dis J 2014; 33:710–4. [DOI] [PubMed] [Google Scholar]
- 5. Chaves SS, Perez A, Farley MM, et al. The burden of influenza hospitalizations in infants from 2003 to 2012, United States. Pediatr Infect Dis J 2014; 33:912–9. [DOI] [PubMed] [Google Scholar]
- 6. Gill PJ, Ashdown HF, Wang K, et al. Identification of children at risk of influenza-related complications in primary and ambulatory care: a systematic review and meta-analysis. Lancet Respir Med 2015; 3:139–49. [DOI] [PubMed] [Google Scholar]
- 7. Hardelid P, Verfuerden M, McMenamin J, Gilbert R. Risk factors for admission to hospital with laboratory-confirmed influenza in young children: birth cohort study. Eur Respir J 2017; 50:1700489. [DOI] [PubMed] [Google Scholar]
- 8. Tuckerman J, Misan S, Crawford NW, Marshall HS. Influenza in children with special risk medical conditions: a systematic review and meta-analysis. Pediatr Infect Dis J 2019; 38:912–9. [DOI] [PubMed] [Google Scholar]
- 9. Lee JJ, Smith M, Bankhead C, et al. Oseltamivir and influenza-related complications in children: a retrospective cohort in primary care. Eur Respir J 2020; 56:1902246. [DOI] [PubMed] [Google Scholar]
- 10. Heinonen S, Silvennoinen H, Lehtinen P, et al. Early oseltamivir treatment of influenza in children 1-3 years of age: a randomized controlled trial. Clin Infect Dis 2010; 51:887–94. [DOI] [PubMed] [Google Scholar]
- 11. Piedra PA, Schulman KL, Blumentals WA. Effects of oseltamivir on influenza-related complications in children with chronic medical conditions. Pediatrics 2009; 124:170–78. [DOI] [PubMed] [Google Scholar]
- 12. Falagas ME, Koletsi PK, Vouloumanou EK, Rafailidis PI, Kapaskelis AM, Rello J. Effectiveness and safety of neuraminidase inhibitors in reducing influenza complications: a meta-analysis of randomized controlled trials. J Antimicrob Chemother 2010; 65:1330–46. [DOI] [PubMed] [Google Scholar]
- 13. Dai Z, Zhang L, Yu Q, Liu L, Yang M, Fan K. Early administration of oseltamivir within 48 hours after onset of flulike symptoms can reduce the risk of influenza B virus-associated pneumonia in hospitalized pediatric patients with influenza B virus infection. Pediatr Infect Dis J 2020; 39:e20–2. [DOI] [PubMed] [Google Scholar]
- 14. Jefferson T, Jones MA, Doshi P, et al. Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database Syst Rev 2014; 2014:CD008965. [DOI] [PubMed] [Google Scholar]
- 15. Jefferson T, Jones M, Doshi P, Spencer EA, Onakpoya I, Heneghan CJ. Oseltamivir for influenza in adults and children: systematic review of clinical study reports and summary of regulatory comments. BMJ 2014; 348:g2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aoki FY, Macleod MD, Paggiaro P, et al. Early administration of oral oseltamivir increases the benefits of influenza treatment. J Antimicrob Chemother 2003; 51:123–29. [DOI] [PubMed] [Google Scholar]
- 17. Butler CC, van der Velden AW, Bongard E, et al. Oseltamivir plus usual care versus usual care for influenza-like illness in primary care: an open-label, pragmatic, randomised controlled trial. Lancet 2020; 395:42–52. [DOI] [PubMed] [Google Scholar]
- 18. Coffin SE, Leckerman K, Keren R, Hall M, Localio R, Zaoutis TE. Oseltamivir shortens hospital stays of critically ill children hospitalized with seasonal influenza: a retrospective cohort study. Pediatr Infect Dis J 2011; 30:962–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hsu J, Santesso N, Mustafa R, et al. Antivirals for treatment of influenza: a systematic review and meta-analysis of observational studies. Ann Intern Med 2012; 156:512–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Muthuri SG, Venkatesan S, Myles PR, et al. Effectiveness of neuraminidase inhibitors in reducing mortality in patients admitted to hospital with influenza A H1N1pdm09 virus infection: a meta-analysis of individual participant data. Lancet Respir Med 2014; 2:395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Qiu S, Shen Y, Pan H, Wang J, Zhang Q. Effectiveness and safety of oseltamivir for treating influenza: an updated meta-analysis of clinical trials. Infect Dis (Lond) 2015; 47:808–19. [DOI] [PubMed] [Google Scholar]
- 22. Malosh RE, Martin ET, Heikkinen T, Brooks WA, Whitley RJ, Monto AS. Efficacy and safety of oseltamivir in children: systematic review and individual patient data meta-analysis of randomized controlled trials. Clin Infect Dis 2018; 66:1492–500. [DOI] [PubMed] [Google Scholar]
- 23. Committee on Infectious Diseases . Recommendations for prevention and control of influenza in children, 2021–2022. Pediatrics 2021; 148:e2021053745. [DOI] [PubMed] [Google Scholar]
- 24. Grohskopf LA, Alyanak E, Ferdinands JM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices, United States, 2021–22 influenza season. MMWR Recomm Rep 2021; 70:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stewart RJ, Flannery B, Chung JR, et al. Influenza antiviral prescribing for outpatients with an acute respiratory illness and at high risk for influenza-associated complications during 5 influenza seasons—United States, 2011–2016. Clin Infect Dis 2018; 66:1035–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Smith LE, D'Antoni D, Jain V, Pearce JM, Weinman J, Rubin GJ. A systematic review of factors affecting intended and actual adherence with antiviral medication as treatment or prophylaxis in seasonal and pandemic flu. Influenza Other Respir Viruses 2016; 10:462–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mueller MR, Smith PJ, Baumbach JP, et al. Influenza testing and antiviral prescribing practices among emergency department clinicians in 9 states during the 2006 to 2007 influenza season. Ann Emerg Med 2010; 55:32–9. [DOI] [PubMed] [Google Scholar]
- 28. Hersh AL, Maselli JH, Cabana MD. Changes in prescribing of antiviral medications for influenza associated with new treatment guidelines. Am J Public Health 2009; 99:S362–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Havers F, Thaker S, Clippard JR, et al. Use of influenza antiviral agents by ambulatory care clinicians during the 2012–2013 influenza season. Clin Infect Dis 2014; 59:774–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Havers F, Flannery B, Clippard JR, et al. Use of influenza antiviral medications among outpatients at high risk for influenza-associated complications during the 2013–2014 influenza season. Clin Infect Dis 2015; 60:1677–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shim SJ, Chan M, Owens L, Jaffe A, Prentice B, Homaira N. Rate of use and effectiveness of oseltamivir in the treatment of influenza illness in high-risk populations: a systematic review and meta-analysis. Health Sci Rep 2021; 4:e241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stockmann C, Byington CL, Pavia AT, et al. Limited and variable use of antivirals for children hospitalized with influenza. JAMA Pediatr 2017; 171:299–301. [DOI] [PubMed] [Google Scholar]
- 33. Antoon JW, Hall M, Herndon A, et al. Prevalence, risk factors, and outcomes of influenza-associated neurologic complications in children. J Pediatr 2021; 239:32–38 e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Solis-Garcia G, Chacon-Pascual A, Gonzalez Martinez F, et al. Neurologic complications in children hospitalized with influenza infections: prevalence, risk factors and impact on disease severity. Pediatr Infect Dis J 2020; 39:789–93. [DOI] [PubMed] [Google Scholar]
- 35. Feinstein JA, Hall M, Antoon JW, et al. Chronic medication use in children insured by Medicaid: a multistate retrospective cohort study. Pediatrics 2019; 143: e20183397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Antoon JW, Williams DJ, Thurm C, et al. The COVID-19 pandemic and changes in healthcare utilization for pediatric respiratory and nonrespiratory illnesses in the United States. J Hosp Med 2021; 16:294–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moore HC, Lehmann D, de Klerk N, et al. How accurate are International Classification of Diseases-10 diagnosis codes in detecting influenza and pertussis hospitalizations in children? J Pediatric Infect Dis Soc 2014; 3:255–60. [DOI] [PubMed] [Google Scholar]
- 38. Amodio E, Tramuto F, Costantino C, et al. Diagnosis of influenza: only a problem of coding? Med Princ Pract 2014; 23:568–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hamilton MA, Calzavara A, Emerson SD, et al. Validating International Classification of Disease 10th Revision algorithms for identifying influenza and respiratory syncytial virus hospitalizations. PLoS One 2021; 16:e0244746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. US Armed Forces Health Surveillance Center. Influenza-like illness. 2015; 9. https://www.health.mil/Reference-Center/Publications/2015/10/01/Influenza-Like-Illness. Accessed 29 April 2022.
- 41. Buda S, Tolksdorf K, Schuler E, Kuhlen R, Haas W. Establishing an ICD-10 code based SARI-surveillance in Germany—description of the system and first results from five recent influenza seasons. BMC Public Health 2017; 17:612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr 2014; 14:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Antoon JW, Grijalva CG, Thurm C, et al. Factors associated with COVID-19 disease severity in US children and adolescents. J Hosp Med 2021; 16:603–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Thomson JE, Feinstein JA, Hall M, Gay JC, Butts B, Berry JG. Identification of children with high-intensity neurological impairment. JAMA Pediatr 2019; 173:989–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Blanton L, Peacock G, Cox C, Jhung M, Finelli L, Moore C. Neurologic disorders among pediatric deaths associated with the 2009 pandemic influenza. Pediatrics 2012; 130:390–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Keren R, Zaoutis TE, Bridges CB, et al. Neurological and neuromuscular disease as a risk factor for respiratory failure in children hospitalized with influenza infection. JAMA 2005; 294:2188–94. [DOI] [PubMed] [Google Scholar]
- 47. Smith M, Peacock G, Uyeki TM, Moore C. Influenza vaccination in children with neurologic or neurodevelopmental disorders. Vaccine 2015; 33:2322–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Williams DJ, Shah SS, Myers A, et al. Identifying pediatric community-acquired pneumonia hospitalizations: accuracy of administrative billing codes. JAMA Pediatr 2013; 167:851–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jefferson T, Jones MA, Doshi P, et al. Neuraminidase inhibitors for preventing and treating influenza in adults and children. Cochrane Database Syst Rev 2014; 4:CD008965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Launes C, Garcia-Garcia JJ, Jordan I, Martinez-Planas A, Selva L, Munoz-Almagro C. 2009 influenza A H1N1 infections: delays in starting treatment with oseltamivir were associated with a more severe disease. Pediatr Infect Dis J 2011; 30:622–5. [DOI] [PubMed] [Google Scholar]
- 51. Louie JK, Yang S, Samuel MC, Uyeki TM, Schechter R. Neuraminidase inhibitors for critically ill children with influenza. Pediatrics 2013; 132:e1539–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Siston AM, Rasmussen SA, Honein MA, et al. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA 2010; 303:1517–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Centers for Disease C and Prevention . Deaths related to 2009 pandemic influenza A (H1N1) among American Indian/Alaska natives—12 states, 2009. MMWR Morb Mortal Wkly Rep 2009; 58:1341–4. [PubMed] [Google Scholar]
- 54. Nowak GJ, Sheedy K, Bursey K, Smith TM, Basket M. Promoting influenza vaccination: insights from a qualitative meta-analysis of 14 years of influenza-related communications research by US Centers for Disease Control and Prevention (CDC). Vaccine 2015; 33:2741–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Harrington R, Adimadhyam S, Lee TA, Schumock GT, Antoon JW. The relationship between oseltamivir and suicide in pediatric patients. Ann Fam Med 2018; 16:145–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Payne D. Tamiflu: the battle for secret drug data. BMJ 2012; 345:e7303. [DOI] [PubMed] [Google Scholar]
- 57. Woo JG, Zeller MH, Wilson K, Inge T. Obesity identified by discharge ICD-9 codes underestimates the true prevalence of obesity in hospitalized children. J Pediatr 2009; 154:327–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Katzow M, Homel P, Rhee K. Factors associated with documentation of obesity in the inpatient setting. Hosp Pediatr 2017; 7:731–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.