Abstract
Background
Staphylococcus aureus represents the leading cause of complicated bloodstream infections among persons who inject drugs (PWID). Standard of care (SOC) intravenous (IV) antibiotics result in high rates of treatment success but are not feasible for some PWID. Transition to oral antibiotics may represent an alternative treatment option.
Methods
We evaluated all adult patients with a history of injection drug use hospitalized from January 2016 through December 2021 with complicated S. aureus bloodstream infections, including infective endocarditis, epidural abscess, vertebral osteomyelitis, and septic arthritis. Patients were compared by antibiotic treatment (standard of care intravenous [SOC IV] antibiotics, incomplete IV therapy, or transition from initial IV to partial oral) using the primary composite endpoint of death or readmission from microbiologic failure within 90 days of discharge.
Results
Patients who received oral antibiotics after an incomplete IV antibiotic course were significantly less likely to experience microbiologic failure or death than patients discharged without oral antibiotics (P < .001). There was no significant difference in microbiologic failure rates when comparing patients who were discharged on partial oral antibiotics after receiving at least 10 days of IV antibiotics with SOC regimens (P > .9).
Conclusions
Discharge of PWID with partially treated complicated S. aureus bacteremias without oral antibiotics results in high rates of morbidity and should be avoided. For PWID hospitalized with complicated S. aureus bacteremias who have received at least 10 days of effective IV antibiotic therapy after clearance of bacteremia, transition to oral antibiotics with outpatient support represents a potential alternative if the patient does not desire SOC IV antibiotic therapy.
Keywords: substance abuse, opioid use disorder, endocarditis, osteomyelitis, Staphylococcus aureus
For patients with injection drug use associated Staphylococcus aureus bacteremia who struggle to complete prolonged courses of intravenous (IV) antibiotics, transition to oral antibiotics is safest when patients complete at least 10 days of IV antibiotics following clearance of bacteremia.
Staphylococcus aureus is the most common pathogen in serious injection drug use–related infections such as infective endocarditis, osteomyelitis, epidural abscess, and septic arthritis [1–3]. The current standard of care for complicated S. aureus bacteremia is prolonged courses of intravenous (IV) antibiotics for 4 to 6 weeks [4, 5]. However, a 4- to 6-week course of antibiotics for persons who inject drugs (PWID) can be challenging [6–8]. PWID are frequently considered ineligible for outpatient parenteral antibiotic therapy (OPAT) [9], and often choose to leave the hospital or skilled nursing facilities prior to completing a multiweek course of IV antibiotic therapy as inpatients [10, 11]. Transition to oral antibiotic regimens may represent an attractive treatment strategy.
The consensus surrounding IV-only therapy for invasive S. aureus infections has recently come under increased scrutiny following the publication of several large, randomized controlled trials of bacteremia, osteomyelitis, and infective endocarditis [12–14]. Iversen et al. demonstrated that transition to oral antibiotics is safe and effective for patients with infective endocarditis; however, their study notably did not identify any methicillin-resistant S. aureus (MRSA) infections and only included 5 PWID [14]. A quasi-experimental study evaluating transition to high-dose oral trimethoprim-sulfamethoxazole yielded similar results while including a number of MRSA infections [15]. Li et al. demonstrated that transition to oral antibiotics is safe and effective for patients with osteomyelitis; however, this study excluded patients with any associated bacteremia [13]. Taken together, these studies suggest that oral step-down therapy may be reasonable for some invasive infections after initial IV antibiotic therapy has stabilized patients and cleared their bacteremia. However, there are limited data on partial oral antibiotic treatment for many of the more complex clinical syndromes associated with S. aureus bacteremia in PWID.
PWID represent a unique population in infectious diseases. Clinicians must assess anticipated antibiotic adherence, feasibility of outpatient monitoring, potential drug–drug interactions with medications for opioid use disorder, and access to outpatient follow-up care. The aim of this retrospective cohort study was to compare the effectiveness of standard of care (SOC) IV antibiotic regimens to incomplete IV antibiotic therapy or transition to partial oral antibiotic therapy for PWID with complicated S. aureus bloodstream infections.
METHODS
Study Design and Patient Population
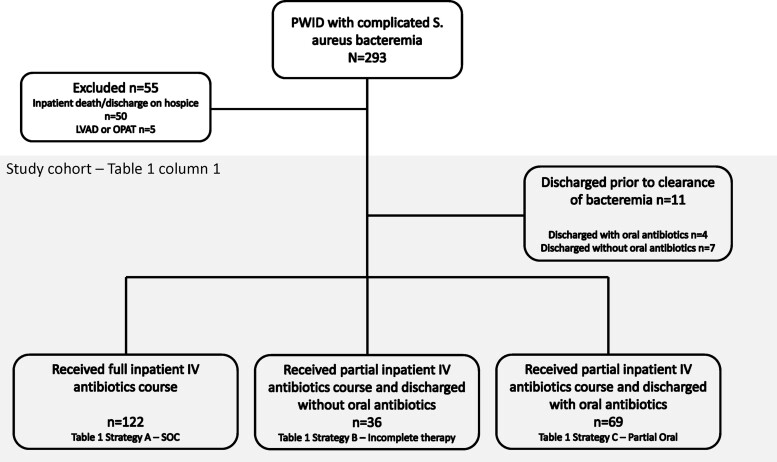
We completed a retrospective cohort analysis of patients admitted to Barnes Jewish Hospital in St. Louis, Missouri, with S. aureus bloodstream infections and a history of active or recent injection drug use (IDU). Patients were identified from microbiology blood culture results that were positive for S. aureus from January 2016 to December 2021, and all charts were reviewed for IDU history as described previously [16]. Patients were included if they had at least 1 positive blood culture for S. aureus, a history of active or recent IDU on chart review, evidence of either infective endocarditis, septic arthritis, epidural abscess, and/or vertebral osteomyelitis, as diagnosed in infectious diseases (ID) consult notes, and if they survived to hospital discharge. Patients were excluded if they died during the index hospitalization, were discharged on OPAT, or had a left ventricular assist device. Only index hospitalizations for S. aureus bloodstream infections were included (Figure 1). For patients with multiple hospitalizations for S. aureus bloodstream infections over the 5-year study period, only the earliest hospitalization with an S. aureus bloodstream infection was included for each discrete clinical episode that were at least 90 days apart.
Figure 1.
Flowchart for development of retrospective cohort. Abbreviations: IV, intravenous; LVAD, left ventricular assist device; OPAT, outpatient parenteral antibiotic therapy; S. aureus, Staphylococcus aureus; SOC, standard of care.
Data Collection
Patient demographics, substance use history, infection type, care characteristics, and outcomes were reviewed in the electronic medical record. Patient comorbidities were captured using the Elixhauser comorbidity index [17]. Duration of bacteremia was defined as the number of days between the first positive blood culture and the last positive blood culture (inclusive of both the first and last date). Prolonged bacteremia was defined as 5 or more days of documented S. aureus bacteremia before sustained negative blood cultures. Physicians (L .R. M., M. J. D., N. L. C., N. S. N., S. L., S .S.) performed manual chart review of ID consult notes, echocardiography reports, imaging, and microbiology data to identify type of clinical syndrome. Patients were divided into 3 antibiotic treatment strategy groups;
Strategy A “SOC”: standard of care IV antibiotics; patients who completed an SOC IV antibiotic regimen during their inpatient admission as recommended by ID consult notes;
Strategy B “incomplete IV”: incomplete IV antibiotic therapy; patients who left the hospital before completing IV antibiotic therapy and did not receive any oral antibiotics on discharge; and
Strategy C “partial oral”: transition to partial oral antibiotic therapy; patients who left the hospital on oral antibiotics either through a patient-directed discharge or against medical advice discharge before completing a SOC IV antibiotic regimen.
The planned antibiotic duration was determined by chart review of infectious disease consultation notes and discharge summaries. The date of effective IV antibiotic therapy used to calculate duration of antibiotics before discharge was determined as the date of both source control (ie, laminectomy, joint washout, or heart valve replacement surgery if applicable) and/or blood culture negativity, whichever was achieved later. Physicians reviewed discharge prescriptions and postdischarge clinic follow-up notes to identify the type of oral antibiotics prescribed. The majority of patients in strategy C (partial oral) participated in a previously published postdischarge support program that focused on antibiotic adherence and substance use disorder care by providing patient's with access to health coaches, case management, close ID clinic follow-up, and free antibiotics for uninsured patients [18]. Patient-reported antibiotic adherence for PWID on oral antibiotics who participated in this program was abstracted from the chart where available. All subsequent admissions within 90 days after discharge were reviewed by 2 study physicians to identify if death or readmission was related to microbiologic failure. If there was no consensus, a third physician reviewed the case and readmission was discussed as a group to determine if it met criteria for the primary outcome.
The primary outcome was microbiological treatment failure at 90 days. This endpoint was defined as a composite readmission within 90 days of discharge that was related to the initial S. aureus infection with either ongoing infection without any significant change, or development of new clinical worsening including isolation of S. aureus from any sterile site, or death during a subsequent hospital stay associated with microbiologic failure. Common reasons for readmissions which were not considered to represent microbiologic failure included nonfatal drug overdose, noninfectious medical issues like gunshot wounds, normal spontaneous vaginal delivery, suicidal ideation, complications of diabetes, or readmissions for new infectious complications from IDU with a different pathogen.
Statistical Analysis
Descriptive analysis was performed using the baseline characteristics, as well as the primary and secondary outcomes. Categorical variables were summarized as percentages and continuous variables as the median and interquartile range (IQR). Group comparisons were investigated using the Kruskal–Wallis test for continuous variables and the χ2 test (or variants thereof) for categorical variables. Uni- and multivariable logistic regression analysis was used to determine the factors associated with treatment failure with the appropriate outcome as the dependent variable. We included all the explicative variables that were clinically relevant or that have been previously associated with poor outcomes in the univariable analysis; demographics, comorbidities, health insurance status, type of infection, MRSA versus MSSA, prolonged bacteremia, addiction medicine consultation, medications for opioid use disorder, and antibiotic treatment group. Those variables significant at the 10% level in univariable analyses were included in the multivariable models with forward selection and backward deletion used to determine the most parsimonious model. Inverse probability weights were included in the models to adjust for baseline covariate imbalance between the respective patient groups.
All analyses were performed using SAS (version 9.4; SAS Institute Inc.) and R version 4.1.1 (R foundation for Statistical Computing, Vienna, Austria). P values <.05 were considered statistically significant with pairwise comparisons were not corrected for multiple testing (unless stated otherwise).
Subgroup Analysis
We performed a subgroup analysis comprised of patients who had completed at least 10 days of IV antibiotic therapy after clearance of bacteremia and source control, similar to the minimum duration recommended in the POET trial [14].
Patient Consent Statement
The Washington University School of Medicine Human Research Protection Office approved this study under institutional review board 202110099. Informed consent was not required for this study according to the Human Research Protection Office regulations given its minimal risk and retrospective observational study design.
RESULTS
Patient demographics and infection characteristics are presented in Table 1. Substance use characteristics and MRSA prevalence at baseline were not significantly different between groups. Groups differed in rates of infective endocarditis (P = .007), and the Elixhauser comorbidity score (P = .004), both of which were highest in strategy A (SOC). Duration of bacteremia differed across all groups with a marginally shorter mean duration seen among patients in strategy B (incomplete IV) (P = .03); however, there was no significant difference in pairwise comparisons between patients in strategy A (SOC) or strategy C (partial oral) for either prolonged bacteremia (A vs C: 44/122 (36.1%) vs 25/69 (36.2%), P > .9) or duration of bacteremia (median 3 days, IQR 1–6 for both strategy A and C; P = .9). The loss to follow-up rate was lowest in strategy C (partial oral), likely influenced by the concurrent implementation of a postdischarge support program at our institution [18].
Table 1.
Demographics of PWID Admitted for Complicated S. aureus Bacteremia, by Antibiotic Treatment Group
| n (%) Median [IQR] | All Patients n = 238 | Characteristics of Patients Who Were Discharged Following Clearance of S. aureus Bacteremia Grouped by Antibiotic Treatment Strategy, n = 227 | A vs B vs C P Value | ||
|---|---|---|---|---|---|
| Strategy A Completed Inpatient IV [Standard of Care] n = 122 | Strategy B Partial IV, No Oral Antibiotics [Incomplete Therapy] n = 36 | Strategy C Partial IV, Partial Oral Antibiotics [Partial Oral] n = 69 | |||
| Age, y | 35 [31, 42] | 35 [30, 42] | 32 [30, 40] | 37 [32, 44] | .03 |
| Male | 126 (52.9) | 62 (50.8) | 24 (66.7) | 36 (52.2) | .2 |
| White | 171 (71.8) | 85 (69.7) | 24 (66.7) | 55 (79.7) | .2 |
| Unstable housing | 48 (20.2) | 19 (15.6) | 7 (19.4) | 22 (31.9) | .03 |
| Insurance: self-pay | 77 (32.4) | 29 (23.8) | 16 (44.4) | 25 (36.2) | .03 |
| Substance use characteristicsa | |||||
| Injection opioid use | 218 (91.6) | 114 (93.4) | 31 (86.1) | 62 (89.9) | .4 |
| Injection Methamphetamine use | 84 (35.3) | 43 (35.2) | 15 (41.7) | 22 (31.9) | .6 |
| Injection cocaine | 38 (16.0) | 16 (13.1) | 9 (25.0) | 11 (15.9) | .2 |
| Comorbidities | |||||
| Hepatitis C infection | 157 (66.0) | 77 (63.1) | 29 (80.6) | 45 (65.2) | .1 |
| Number of Elixhauser comorbidities | 6 [4, 8] | 7 [5, 9] | 5 [3, 6] | 5 [4, 8] | .002 |
| Type of clinical syndrome caused by S. aureus bacteremiab | |||||
| Infective endocarditis | 154 (64.7) | 90 (73.8) | 22 (61.1) | 36 (52.1) | .007 |
| Epidural abscess | 35 (14.7) | 16 (13.1) | 5 (11.6) | 14 (20.3) | .4 |
| Septic arthritis | 56 (23.5) | 25 (20.5) | 10 (27.8) | 20 (29.0) | .2 |
| Vertebral osteomyelitis | 46 (19.3) | 18 (14.8) | 9 (25.0) | 16 (23.2) | .2 |
| S. aureus bacteremia characteristics | |||||
| Prolonged bacteremia, 5+ d | 77 (32.4) | 44 (36.1) | 6 (16.7) | 25 (36.2) | .08 |
| Duration of bacteremia, d | 3 [1, 6] | 3 [1, 6] | 2 [1, 3] | 3 [1, 6] | .03 |
| Methicillin-resistant S. aureus | 99 (41.6) | 48 (39.3%) | 18 (41.7%) | 32 (46.4%) | .6 |
| Inpatient care received | |||||
| Duration of IV abx before discharge, d | 34 [14, 42] | 42 [42, 42] | 15 [4, 27] | 18 [7, 32] | <.001 |
| Length of stay, d | 39 [17, 48] | 47 [43, 52] | 18 [5, 31] | 26 [8, 35] | - |
| % IV antibiotic course completed in the hospital | 100 [38, 100] | 100% [100, 100] | 37% [11, 71] | 46% [17, 76] | - |
| Addiction medicine consultation | 145 (60.9) | 81 (66.4) | 14 (38.9) | 48 (69.6) | .001 |
| Medications for opioid use Disorder | |||||
| none Buprenorphine methadone | 98 (41.2) 79 (33.2) 61 (25.6) |
40 (32.8) 47 (38.5) 35 (28.7) |
24 (66.7) 4 (11.1) 8 (22.2) |
25 (36.2) 37 (39.1) 17 (24.6) |
.004 |
| Lost to care | 31 (13.0) | 18 (14.8) | 8 (22.2) | 5 (7.2) | .09 |
| Primary endpoint Composite outcome microbiologic failure or death within 90 d of discharge | 38 (16.7) | 13 (10.7) | 16 (44.4) | 9 (13.0) | <.001 |
| Secondary endpoint, all-cause readmission within 90 d of discharge | 47 (19.7) | 38 (31.1%) | 19 (52.8%) | 18 (26.1%) | .02 |
Abbreviations: abx, antibiotics; IV, intravenous; PWID, persons who inject drugs; S. aureus, Staphylococcus aureus.
Patients may report more than one type of substance use.
Patients may present with multiple concurrent serious injection related infections.
In terms of primary endpoint among patients who had cleared bacteremia before discharge, patients in strategy B were the most likely across all groups to experience microbiologic failure or death within 90 days postdischarge (P < .001). In contrast, strategies A (SOC) and C (partial oral) had comparable levels of the primary outcome (A vs C: 13/122 (10.7%) vs 9/69 (13.0%), P = .6). The median duration of oral antibiotics prescribed in strategy C (partial oral) was 21 days (IQR 9–33). Evidence on using partial oral antibiotics for MRSA bacteremia is very limited, thus we further analyzed if there was any influence of MRSA vs MSSA infection on primary outcome rates in patients receiving strategy C (partial oral). Although the data were not adequately powered to study this outcome, we observed no significant difference whether partial oral antibiotics were used to treat MRSA (4/32 [12.5%]) or MSSA (5/37 [13.5%], P > .9).
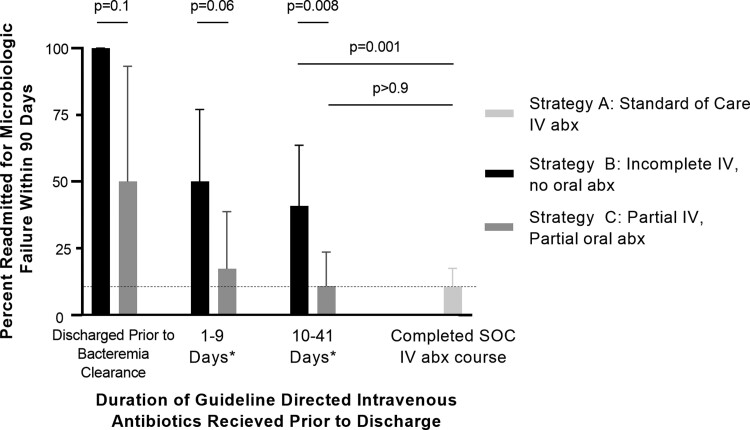
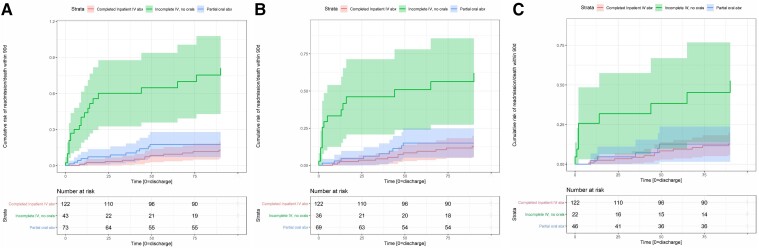
Duration of IV antibiotics received before discharge was associated with a duration-dependent effect on infection outcome (Figure 2). Discharge before clearance of bacteremia universally resulted in microbiologic failure for patients discharged without antibiotics, and results remained poor even for patients discharged with oral antibiotics, with 2 of 4 patients readmitted for microbiologic failure. However, in the subgroup of patients who received at least 10 days of IV antibiotics before transition to oral antibiotics, outcomes were not significantly different between strategy C (partial oral) and strategy A (SOC) (Figure 2). In terms of time from discharge to failure, Figure 3 shows the Nelson-Aalen cumulative hazard ratio for all patients (Figure 3A), patients who cleared their bacteremia before discharge (Figure 3B), and patients who were discharged after a minimum of 10 days of effective IV antibiotic therapy after clearance of bacteremia and source control (Figure 3C). In all cases, patients discharged without oral antibiotics had the highest rate of microbiologic failure.
Figure 2.
Rates of microbiologic failure within 90 d after discharge by duration of effective IV antibiotic therapy received before discharge. *Days of IV antibiotic therapy received after clearance of bacteremia and source control. Confidence intervals are 95% Clopper-Pearson confidence intervals. P values determined by Fisher exact test, not corrected for multiple testing. Abbreviations: abx, antibiotics; IV, intravenous; SOC, standard of care.
Figure 3.
Nelson-Aalen cumulative hazard plot by antibiotic treatment group of (A) all patients, (B) patients who achieved clearance of bacteremia before discharge, and (C) patients who received at least 10 d of effective IV antibiotic treatment after clearance of bacteremia and/or source control before discharge. Abbreviation: IV, intravenous.
Excluding those who did not clear their bacteremia before discharge, the only strong independent predictors of an increased risk of microbiologic failure in multivariable models were being in strategy B (incomplete IV therapy) (adjusted odds ratio [aOR] 7.9 compared with strategy A, 95% confidence interval, 2.9–21.6), P < .001, Table 2) and paraplegia (aOR 7.8 [2.1, 28.6], P = .002).
Table 2.
Variables Associated With Primary Outcome Among PWID With Complicated S. aureus Bacteremia
| Variable | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | |
| Patient demographicsa | ||||||
| Insurance | ||||||
| ȃManaged care | 1.0 | Reference | 1 | |||
| ȃMedicaid | NS | (reference) | NS | |||
| ȃMedicare | 3.9 | (0.8–18.8) | 0.09 | (1.1–33.0) | .04 | |
| ȃSelf-pay | NS | 5.9 | NS | |||
| Inpatient care | ||||||
| OUD treatment | ||||||
| ȃNone | 1 | |||||
| ȃBuprenorphine | (reference) | (0.2, 1.0) | .04 | … | … | NS |
| ȃMethadone | .04 | NS | ||||
| Addiction medicine consult | 0.4 | (0.2–0.8) | .01 | … | … | NS |
| Duration of IV antibiotics before Discharge: | ||||||
| ȃBacteremic at discharge; | 4.5 | (1.0–20.8) | .05.01 | … | … | … |
| ȃ1-9 days effective IV abx | 0.1 | (0.02–0.5) | <.001 | |||
| ȃ10+ days effective IV abx | 0.1 | (0.01–0.3) | <.001 | |||
| ȃCompleted Inpatient IV abx | 0.03 | (0.01–0.1) | ||||
| Elixhauser comorbiditiesa | ||||||
| Fluid and electrolyte disorders | 0.5 | (0.2–0.9) | .03 | 0.4 | (0.2–1.0) | .06 |
| Paraplegia | 6.9 | (2.3–20.5) | <.001 | 7.8 | (2.1–28.6) | .002 |
| Infection characteristicsa | ||||||
| Septic arthritis | 2.4 | (1.2–5.1) | .02 | 2.3 | (1.0–5.4) | .06 |
| Antibiotic treatment group | ||||||
| Completed inpatient IV | 1.0 (reference) | 1.0 (reference) | … | … | ||
| Partial IV, partial oral | 1.3 | (0.5–3.1) | .6 | - | - | NS |
| Partial IV, no oral | 6.7 | (2.8–16.0) | <.001 | 7.9 | (2.9–21.6) | <.001 |
Abbreviations: abx, antibiotics; CI, confidence interval; IV, intravenous; NS, not significant; OR, oods ratio; OUD, opioid use disorder; PWID, persons who inject drugs; S. aureus, Staphylococcus aureus.
Only variables that were statistically significant in univariate analysis are listed; statistical significance is included at the 5% level.
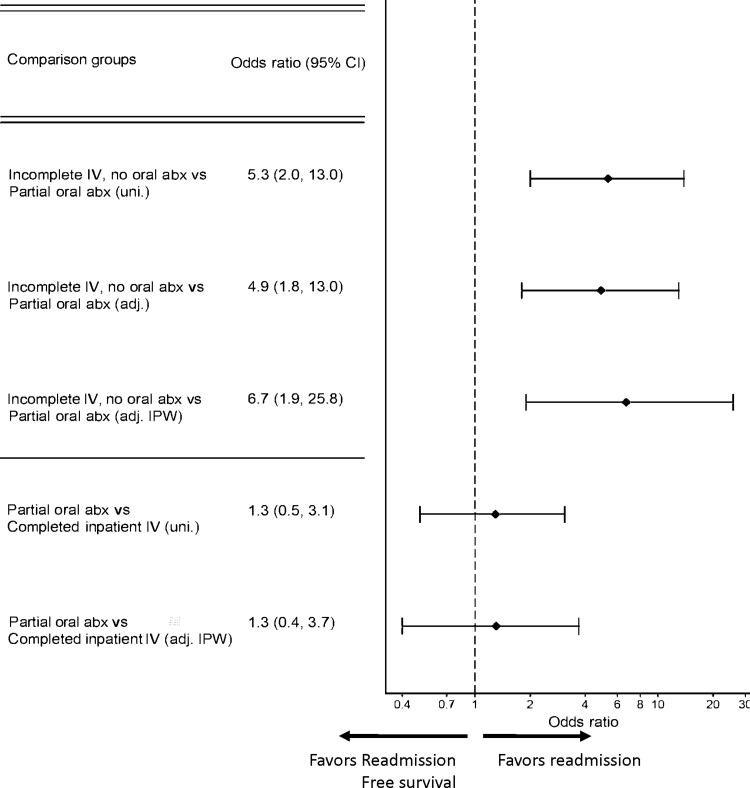
When evaluating patients who left the hospital before completion of IV antibiotics (strategies B and C), there was a significantly higher risk of microbiologic failure associated with strategy B (incomplete IV) compared with strategy C (partial oral), and this difference persisted in inverse probability weighted models adjusted for baseline covariate imbalance (aOR 6.7 [1.9, 25.8], P = .005, Table 3, Figure 4). There was no significant difference in outcomes between strategy A (SOC) and strategy C (partial oral) (inverse probability weighted aOR 1.3 [0.4, 3.7], P = .7).
Table 3.
Inverse Propensity Weighting Comparisons of Primary Endpoint (Microbiologic Failure at 90 d) by Antibiotics Strategy
| Comparison Groups | A Completed Inpatient IV [Gold standard] |
B Partial IV, No Oral |
C Partial IV, Partial Oral |
P Value |
|---|---|---|---|---|
| Comparing microbiologic failure at 90 d between patients who discharged before completing IV antibiotics (strategy B vs C) | … | 16 (44.4) [N = 36] |
9 (13.0) [N = 69] |
.001 |
| - Inverse probability weighted | … | aOR 6.7 (1.9, 25.8) | 1 (reference) | .005 |
| Comparing microbiologic failure at 90 d between patients who completed standard of care (strategy A) vs partial oral antibiotics (strategy C) | 13 (10.7) [N = 122] |
… | 9 (13.0) [N = 69] |
.8 |
| - Inverse probability weighted | 1 (reference) | … | aOR 1.3 (0.4, 3.7) | .7 |
| Subgroup analysis: Including only those with ≥10 d effective IV antibiotic therapy before discharge | ||||
| Comparing microbiologic failure at 90 d between patients who discharged before completing IV antibiotics (strategy B vs C) | … | 9 (40.9) [N = 22] |
5 (10.9) [N = 46] |
.004 |
| - Inverse probability weighted | … | aOR 5.4 (1.2, 24.0) | 1 (reference) | .03 |
| Comparing microbiologic failure at 90 d between patients who completed standard of care (strategy A) vs partial oral antibiotics (strategy C) | 13 (10.7) [N = 122] |
… | 5 (10.9) [N = 46] |
.9 |
| - Inverse probability weighted | 1 (reference) | … | aOR 1.1 (0.3, 3.9) | .9 |
Abbreviations: aOR, adjusted odds ratio; IV, intravenous.
Figure 4.
Forest plot of estimates from logistic regression analyses of subgroups. Abbreviations: abx, antibiotics; adj., adjusted; CI, confidence interval; IPW, inverse probability weighted model; uni., univariable.
Subgroup Analyses
Our subgroup analysis found that patients in strategy C (partial oral) who had received at least 10 days of effective IV antibiotic therapy vs. strategy A (SOC) had similar results (Figure 4).
Antibiotics used, along with patient self-reported antibiotic adherence data obtained through chart review, are shown in Table 4. Although the sample size was not powered to compare different treatment regimens, no specific treatment regimens resulted in a noticeably higher failure rate. Self-reported antibiotic adherence could be assessed in 53 of 73 patients discharged on partial oral antibiotic therapy, whereas 20 patients (31.5%) had incomplete data on antibiotic adherence. There was a higher but nonsignificantly different rate of self-reported antibiotic noncompliance in patients who were prescribed dual oral antibiotic therapy (P = .7).
Table 4.
Table of Type of Oral Antibiotics Used
| Antibiotic Class and Dosing | Primary Outcome (Microbiologic Failure at 90 days) | Self-reported Adherence Abstracted from Medical Record through Chart Review | ||
|---|---|---|---|---|
| Self-reported Adherence | Self-reported Nonadherence | No Adherence Data Available | ||
| Beta-lactamsa | 0/8 (0%) | 6 | 1 | 1 |
| Clindamycin 450 mg QID | 1/3 (33%) | 2 | 0 | 1 |
| Doxycycline 100 mg BID | 6/37 (16%) | 23 | 5 | 9 |
| Ciprofloxacin 750 mg BID | 0/2 (0%) | 1 | 0 | 1 |
| Linezolid 600 mg BID | 3/15 (20%) | 9 | 2 | 4 |
| Rifampinb 450 mg BID | 1/3 (33%) | 1 | 1 | 1 |
| Trimethoprim-sulfamethoxazole 2 DS BID | 4/26 (15%) | 15 | 4 | 7 |
| Comparison of dual- vs single-antibiotic class therapy | ||||
| Single-agent therapy | 7/52 (13%) | 33 | 3 | 16 |
| Dual-agent therapyc | 4/21 (19%) | 12 | 5 | 4 |
Abbreviations: BID, twice per day; DS, double strength; QID, 4 times per day.
Includes amoxicillin-clavulanate 875 mg BID, cephalexin 500 mg QID, cefadroxil 1000 mg BID, and dicloxacillin 1000 mg QID.
Rifampin was never used as single-agent therapy.
Patients who received dual-agent therapy are listed for both categories.
DISCUSSION
Our data suggest that when faced with a patient who no longer wishes to receive SOC IV antibiotics for treatment of their complicated S. aureus infection, providing a transition to oral antibiotics with a hospital-based outpatient antibiotics support program, significantly reduces the risk of microbiologic failure or death compared with discharge without any additional antibiotic treatment. For patients discharged on partial oral antibiotics, success rates were highest when patients received at least 10 days of IV antibiotic therapy after clearance of bacteremia, similar to durations study participants received in the POET trial [14]. The observed rate of microbiologic failure in both patients who received SOC IV antibiotic therapy and those receiving partial oral antibiotic therapy after at least 10 days of IV antibiotics is consistent with rates described in the broader population [19–21]. These data suggest that oral antibiotics may represent an effective treatment for complicated S. aureus bacteremias in PWID with endocarditis, epidural abscess, vertebral osteomyelitis, or septic arthritis, who have had adequate source control, and received at least 10 days of IV antibiotics after clearance of bacteremia. These findings are consistent with other literature that shows partial oral antibiotics are effective in smaller cohorts of IDU-associated endocarditis [22]. However, our cohort represents an important addition to the literature as it includes a significant proportion of infections secondary to MRSA.
The choice of oral antibiotic regimens in PWID with invasive S. aureus infections often presents unique challenges compared with the treatment of other populations. Factors confounding their care include high rates of unstable housing [23], low health literacy rates [24], and low rates of health insurance [25]. Identifying optimal antibiotic therapy regimens for this population may require balancing the need for medication adherence against the existing evidence on antibiotic treatment options. For example, oral antibiotic treatment options used previously for infective endocarditis [14] have relied heavily on adjunctive rifampin which may not be feasible for many PWID who may be on methadone or receiving direct acting antivirals for hepatitis C treatment. Similarly, many of the previously proposed endocarditis regimens required dosing 3 or 4 times a day [14], which may be more challenging in populations with limited health literacy [26]. In contrast, the OVIVA trial [13] included several antibiotic regimens with once or twice daily dosing with a single antibiotic which may prove easier for many PWID to achieve optimal antibiotic adherence. Although not powered to assess individual regimens, our data suggest that several oral antibiotic regimens with twice-daily dosing including doxycycline, linezolid, cefadroxil, and trimethoprim-sulfamethoxazole may be potential options for patients in whom pill burden and medication nonadherence is a significant concern.
The increasing movement for OPAT programs to support PWID will enhance patient access to SOC IV antibiotic treatment and represents an important advancement in infectious diseases care for PWID [27–29]. However, it is likely that even at institutions with expanded access to OPAT, not all PWID may be eligible, either because of physician-perceived barriers, lack of safe and stable housing, lack of health insurance, or limited access to outpatient follow-up [30, 31]. For some patients, there may also be benefits to avoiding the complexities of OPAT. Multidisciplinary conferences for coordinating prolonged antibiotic therapy for PWID, allow for both patients and providers to identify patient-centered antibiotic treatment options [32]. Physicians caring for PWID who decline SOC IV antibiotic treatment and desire to leave the hospital before completion of IV antibiotics should engage patients in shared decision making about the risks and benefits of partial oral antibiotic therapy. Key aspects of this discussion include the consistent adherence needed while on oral antibiotics, the importance of completing the full duration of oral antibiotic therapy and following up in postdischarge clinic visits.
PWID discharged on oral antibiotics for complicated S. aureus bacteremia should receive multidisciplinary care during both the hospitalization and immediate postdischarge period. In our experience, many PWID struggle with navigating the healthcare system, and outpatient support is required to ensure that patients both initiate and tolerate antibiotic prescriptions. Outreach by healthcare team members can help address any cost issues for antibiotics, while also trouble-shooting common side effects such as nausea that might otherwise result in premature cessation of antibiotics. These simple interventions along with close clinic follow-up are essential to help minimize subsequent treatment failures.
This study has several important limitations. This was a single-center, retrospective study performed at an academic institution with access to addiction medicine physicians over a period in which there was an increasing emphasis on multidisciplinary care including outpatient support for patients discharged on oral antibiotics; this may not be available at all institutions. There also was a higher loss to follow-up in patients discharged without oral antibiotics, which may lead to an underestimation of risk of death in that cohort. Additionally, we have excluded any patients that died before discharge, potentially favoring SOC IV antibiotics, and have used the date of discharge as a standard starting point for calculating a 90-day follow-up period, which may lead to immortal time bias. Methodologically, we attempted to adjust for baseline covariate imbalance by fitting models including inverse probability weights, but this cannot overcome underlying systematic unmeasured confounding between the groups. Last, patients in this cohort were immunocompetent, and younger than the average aged patient with non–PWID-associated S. aureus infections, and many of the S. aureus strains causing infections in this cohort have been previously identified as having fewer virulence factors and supra-antigenic toxins than what is often seen in non-PWID associated S. aureus infections [16].
CONCLUSIONS
The SOC for S. aureus bacteremias complicated by septic arthritis, vertebral osteomyelitis, epidural abscess, or infective endocarditis, is a multiweek course of IV antibiotics [4, 5]. We firmly believe that SOC regimens should continue to be offered to all PWID with complicated S. aureus bacteremias. However, we recognize that for many patients this option is not desired or feasible and would significantly reduce their quality of life. Our data suggest that incomplete antibiotic therapy should be avoided at all costs, and that transition to oral antibiotics should be offered to PWID who decline SOC IV antibiotics, with the best outcomes observed in patients who are able to complete at least 10 days of effective in-house IV antibiotic therapy after clearance of bacteremia. These findings should be incorporated into treatment guidelines to caution against discharging PWID with partially treated infections without offering them outpatient oral antibiotic therapy.
Contributor Information
John A Wildenthal, Medical Scientist Training Program, Washington University in St. Louis School of Medicine, St. Louis, Missouri, USA; Division of Infectious Diseases, Washington University in St. Louis School of Medicine, St. Louis, Missouri, USA; Department of Computational and Systems Biology, Washington University in St. Louis School of Medicine, St. Louis, Missouri, USA.
Andrew Atkinson, Department of Infectious Diseases, Bern University Hospital, Inselspital, University of Bern, Bern, Switzerland.
Sophia Lewis, Division of Infectious Diseases, Washington University in St. Louis School of Medicine, St. Louis, Missouri, USA.
Sena Sayood, Division of Infectious Diseases, Washington University in St. Louis School of Medicine, St. Louis, Missouri, USA.
Nathanial S Nolan, Division of Infectious Diseases, Washington University in St. Louis School of Medicine, St. Louis, Missouri, USA.
Nicolo L Cabrera, Division of Infectious Diseases, Washington University in St. Louis School of Medicine, St. Louis, Missouri, USA.
Jonas Marschall, Division of Infectious Diseases, Washington University in St. Louis School of Medicine, St. Louis, Missouri, USA.
Michael J Durkin, Division of Infectious Diseases, Washington University in St. Louis School of Medicine, St. Louis, Missouri, USA.
Laura R Marks, Division of Infectious Diseases, Washington University in St. Louis School of Medicine, St. Louis, Missouri, USA.
Notes
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Author Contributions. L. M. and J. A. W. conceptualized and designed the study. A. A. and J. A. W. conducted the statistical analysis. L. R. M., M. J. D., N. L. C., N. S. N., S. L., and S. S performed all chart review. All authors had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors contributed to the writing and critical revision of the report. All authors contributed to the data acquisition, data analysis, or data interpretation and reviewed and approved the final version.
Financial support. This work was also supported by the National Institutes of Health under grant numbers KL2TR002346 and K23DE029514. A. A. was partially supported for this work by the Swiss National Science Foundation grant number CRSK-3_190977/1.
Patient consent. This study was approved and granted a waiver of consent by the Washington University institutional review board before any research activities were performed.
References
- 1. Sredl M, Fleischauer AT, Moore Z, Rosen DL, Schranz AJ. Not just endocarditis: hospitalizations for selected invasive infections among persons with opioid and stimulant use diagnoses-North Carolina, 2010–2018. J Infect Dis 2020; 222:S458–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jackson KA, Bohm MK, Brooks JT, et al. Invasive methicillin-resistant Staphylococcus aureus infections among persons who inject drugs - six sites, 2005–2016. MMWR Morbidity Mortality Weekly Rep 2018; 67:625–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. See I, Gokhale RH, Geller A, et al. National public health burden estimates of endocarditis and skin and soft-tissue infections related to injection drug use: a review. J Infect Dis 2020; 222:S429–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52: e18–55. [DOI] [PubMed] [Google Scholar]
- 5. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis. Antimicrob Ther Manag Complic 2015;132:1435–86. [DOI] [PubMed] [Google Scholar]
- 6. Simon R, Snow R, Wakeman S. Understanding why patients with substance use disorders leave the hospital against medical advice: a qualitative study. Substance Abuse 2020; 41:519–25. [DOI] [PubMed] [Google Scholar]
- 7. Rapoport AB, Fine DR, Manne-Goehler JM, Herzig SJ, Rowley CF. High inpatient health care utilization and charges associated with injection drug use-related infections: a cohort study, 2012–2015. Open For Infect Dis 2021; 8:ofab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McNeil R, Small W, Wood E, Kerr T. Hospitals as a ‘risk environment': an ethno-epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med (1982) 2014; 105:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rapoport AB, Fischer LS, Santibanez S, Beekmann SE, Polgreen PM, Rowley CF. Infectious diseases physicians’ perspectives regarding injection drug use and related infections, United States, 2017. Open Forum Infectious Diseases 2018; 5:ofy132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ashraf B, Hoff E, Brown LS, et al. Health care utilization patterns for patients with a history of substance use requiring OPAT. Open Forum Infect Dis 2021; 8:ofab540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Appa A, Adamo M, Le S, et al. Patient-directed discharges among persons who use drugs hospitalized with invasive Staphylococcus aureus infections: opportunities for improvement. Am J Med 2022; 135:91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wald-Dickler N, Holtom PD, Phillips MC, et al. Oral is the new IV. Challenging decades of blood and bone infection dogma: a systematic review. Am J Med 2022; 135:369–79.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li H-K, Rombach I, Zambellas R, et al. Oral versus intravenous antibiotics for bone and joint infection. N Engl J Med 2019; 380:425–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Iversen K, Ihlemann N, Gill SU, et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med 2019; 380:415–24. [DOI] [PubMed] [Google Scholar]
- 15. Tissot-Dupont H, Gouriet F, Oliver L, et al. High-dose trimethoprim-sulfamethoxazole and clindamycin for Staphylococcus aureus endocarditis. Int J Antimicrob Agents 2019; 54:143–8. [DOI] [PubMed] [Google Scholar]
- 16. Marks LR CJ, Wildenthal JA, Wallace M/A, et al. Staphylococcus aureus injection drug use-associated bloodstream infections are propagated by community outbreaks of diverse lineages. Commun Med 2021; 1:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care 1998; 36:8–27. [DOI] [PubMed] [Google Scholar]
- 18. Lewis S, Liang SY, Schwarz ES, et al. Patients with serious injection drug use-related infections who experience patient-directed discharges on oral antibiotics have high rates of antibiotic adherence but require multidisciplinary outpatient support for retention in care. Open Forum Infect Dis 2022; 9:ofab633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hamad Y, Connor L, Bailey TC, George IA. Outcomes of outpatient parenteral antimicrobial therapy with ceftriaxone for methicillin-susceptible Staphylococcus aureus bloodstream infections-a single-center observational study. Open Forum Infect Dis 2020; 7:ofaa341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li J, Echevarria KL, Hughes DW, Cadena JA, Bowling JE, LewisJS, 2nd. Comparison of cefazolin versus oxacillin for treatment of complicated bacteremia caused by methicillin-susceptible Staphylococcus aureus. Antimicrob Agents Chemother 2014; 58:5117–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rao SN, Rhodes NJ, Lee BJ, et al. Treatment outcomes with cefazolin versus oxacillin for deep-seated methicillin-susceptible Staphylococcus aureus bloodstream infections. Antimicrob Agents Chemother 2015; 59:5232–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miller K, Evans E, Sheridan KR, et al. Partial oral antibiotic treatment of endocarditis in patients who inject drugs: a case series. JAC Antimicrob Resist 2022; 4:dlac008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hotton A, Mackesy-Amiti ME, Boodram B. Trends in homelessness and injection practices among young urban and suburban people who inject drugs: 1997–2017. Drug Alcohol Depend 2021; 225:108797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rolova G, Gavurova B, Petruzelka B. Health literacy, self-perceived health, and substance use behavior among young people with alcohol and substance use disorders. Int J Environ Res Public Health 2021; 18:4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lewis R, Baugher AR, Finlayson T, Wejnert C, Sionean C, Group NHBSS . Healthcare access and utilization among persons who inject drugs in medicaid expansion and nonexpansion states: 22 United States cities, 2018. J Infect Dis 2020; 222:S420–S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Davis TC, Wolf MS, BassPF, 3rd, et al. Literacy and misunderstanding prescription drug labels. Ann Intern Med 2006; 145:887–94. [DOI] [PubMed] [Google Scholar]
- 27. Price CN, Solomon DA, Johnson JA, Montgomery MW, Martin B, Suzuki J. Feasibility and safety of outpatient parenteral antimicrobial therapy in conjunction with addiction treatment for people who inject drugs. J Infect Dis 2020; 222:S494–s8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fanucchi LC, Walsh SL, Thornton AC, Nuzzo PA, Lofwall MR. Outpatient parenteral antimicrobial therapy plus buprenorphine for opioid use disorder and severe injection-related infections. Clin Infect Dis 2020; 70:1226–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ho J, Archuleta S, Sulaiman Z, Fisher D. Safe and successful treatment of intravenous drug users with a peripherally inserted central catheter in an outpatient parenteral antibiotic treatment service. J Antimicrob Chemother 2010; 65:2641–4. [DOI] [PubMed] [Google Scholar]
- 30. Jawa R, Rozansky H, Clemens D, Fagan M, Walley AY. Rethinking home-based outpatient parenteral antibiotic therapy for persons who inject drugs: an opportunity for change in the time of COVID-19. J Addict Med 2022; 16:e70–e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Eaton EF, Mathews RE, Lane PS, et al. A 9-point risk assessment for patients who inject drugs and require intravenous antibiotics: focusing inpatient resources on patients at greatest risk of ongoing drug use. Clin Infect Dis 2019; 68:1041–3. [DOI] [PubMed] [Google Scholar]
- 32. Sikka MK, Gore S, Vega T, Strnad L, Gregg J, Englander H. “OPTIONS-DC”, a feasible discharge planning conference to expand infection treatment options for people with substance use disorder. BMC Infect Dis 2021; 21:772. [DOI] [PMC free article] [PubMed] [Google Scholar]