Abstract
Modern antiretroviral therapy (ART) has increased longevity of people with HIV and shifted the age distribution of the HIV pandemic upward toward that of the general population. This positive development has also led to concerns about premature and/or accelerated neurocognitive and physical ageing due to the combined effects of chronic HIV, accumulating comorbidities, adverse effects or possible toxicities of ART and biological ageing. Here we present results of comprehensive assessments over 12 years of 402 people with HIV in the CNS HIV ART Effects Research (CHARTER) programme, who at follow-up were composed of younger (<60 years) and older (≥60 years) subgroups.
Over the 12 years, ART use and viral suppression increased in both subgroups as did systemic and psychiatric comorbidities; participants in both subgroups also evidenced neurocognitive decline beyond what is expected in typical ageing. Contrary to expectations, all these adverse effects were comparable in the younger and older CHARTER subgroups, and unrelated to chronological age. Neurocognitive decline was unrelated to HIV disease or treatment characteristics but was significantly predicted by the presence of comorbid conditions, specifically diabetes, hypertension, chronic pulmonary disease, frailty, neuropathic pain, depression and lifetime history of cannabis use disorder. These results are not consistent with premature or accelerated neurocognitive ageing due to HIV itself but suggest important indirect effects of multiple, potentially treatable comorbidities that are more common among people with HIV than in the general population.
Good medical management of HIV disease did not prevent these adverse outcomes, and increased attention to a range of comorbid conditions in people with HIV may be warranted in their care.
Keywords: HIV, neurologic complications, cognition, brain
In a longitudinal study following more than 400 people with HIV (people with HIV) over 12 years, Heaton et al. find that cognitive decline is not associated with chronological age, but with comorbidities. This is inconsistent with premature cognitive ageing due to HIV, and suggests that comorbidity management may prevent cognitive decline in people with HIV.
See Cysique and Brew (https://doi.org/10.1093/brain/awad035) for a scientific commentary on this article.
See Cysique and Brew (https://doi.org/10.1093/brain/awad035) for a scientific commentary on this article.
Introduction
Advances in antiretroviral therapy (ART) have greatly reduced the medical morbidity associated with HIV infection. Well treated HIV disease with suppression of viral replication has become the norm in much of the world. Consequently, people with HIV are living much longer and the age distribution of the HIV pandemic has progressively shifted higher.
Like other AIDS-defining illnesses, HIV-associated dementia (HAD) has become much less common in the era of modern ART. Nevertheless, the prevalence of milder forms of neurocognitive disorders in people with HIV remains high,1 even within the context of suppressive ART.2–4 Reasons for persistence of milder neurocognitive disorders in well treated people with HIV likely include continuing systemic inflammation that may be due to production of HIV RNA and proteins in infected cells, translocation of microbial products across the damaged gut barrier, and the side effects or possible toxicities of ART itself.5–7 These same mechanisms may synergize with immune senescence to increase risks for non-infectious, age-related comorbidities in people with HIV.8–10 Substantial evidence supports the conclusion that ageing with HIV results in higher prevalence and incidence of several medical comorbidities that also increase risk for neurocognitive impairment in the general population. These include diabetes, hypertension, cardiovascular disease, chronic pulmonary disease (CPD), chronic liver disease, depression, frailty, and metabolic syndrome.6,11–15,16,17
Atypical ageing in people with HIV has been described as (i) ‘accelerated ageing’, in which risks for age-related conditions increase more rapidly with time in people with HIV than in the general population; (ii) ‘accentuated ageing’, in which HIV infection is associated with increased prevalence of various conditions at all ages, but without a widening of the gap over time between infected and uninfected individuals as they age; or (iii) ‘premature ageing’, in which age-related conditions appear earlier in people with HIV, and then show either typical or accelerated risk trajectories over time. Adequately addressing these distinctions requires large, longitudinal studies that follow demographically comparable people across the lifespan who either do or do not have HIV infection. Limited evidence exists regarding the possible types of atypical neurocognitive ageing in people with HIV.
In a recent review of neurocognitive studies that focused on age effects in people with HIV, Aung et al.18 determined that a meta-analysis was not possible because of major methodological differences between studies. Age influenced neurocognitive outcomes in most of the 31 studies that had a cross-sectional design. Of the six longitudinal studies, one19 focused on clinical diagnoses and did not report any longitudinal neurocognitive results; the authors reported an age effect on transition to worse neurocognitive status, but this was attributable to older people with HIV who were not taking ART. Analyses from the Multicenter AIDS Cohort Study (MACS) found evidence of accelerated neurocognitive decline over 5 years in older people with HIV, but only on one of three tests (Trailmaking, Part B).20 A more recent MACS study analysed 5 years of neurocognitive data from large samples of males with and without HIV and considered medical and psychiatric comorbidities in the results.21 The older subgroup of males with HIV evidenced worse outcomes on two of five neurocognitive domains (episodic memory and motor) within the context of advanced HIV disease, but the authors did not analyse neurocognitive change over time (i.e. an Age × Time interaction). In a 1-year follow-up study, Seider et al.22 reported a significant Age × Time interaction on one of four episodic memory scores, with older people with HIV doing worse. In another 1-year follow-up using a neurocognitive summary score, Sheppard et al.23 found no evidence of accelerated neurocognitive ageing in HIV. Finally, in a 4-year follow-up of 30 people with HIV (15 older) and 25 HIV controls, Haynes et al.24 found an HIV × Age interaction on global neurocognitive change and concluded that this needs to be replicated in larger samples. Excellent work in this area has also been reported from international settings.25–31 In summary, available studies are inconclusive, but suggest accelerated neurocognitive ageing among people with HIV, highlighting the need for longer term evidence of changes with comprehensive neurocognitive testing and consideration of relevant comorbidities that are common in people with HIV.
The current study was designed to fill this gap, by analysing 12-year changes in neurocognitive function in older and younger people with HIV who initially received comprehensive neurocognitive and neuromedical examinations in the multisite CNS HIV Antiretroviral Therapy Effects Research (CHARTER) programme between 2003 and 2007. Effects of age and Age × Time interactions were examined, together with associated medical and psychiatric comorbidities. We expected that older CHARTER participants (≥60 years at follow-up) would evidence greater neurocognitive decline over 12 years, in comparison with those who were younger (<60 years). These age effects were expected to remain after accounting for declines that may be expected based on typical ageing. In addition, we sought to examine the degree to which trajectories of neurocognitive change over 12 years could be predicted or accounted for by presence of comorbid neuromedical conditions (e.g. cardiovascular, metabolic) and psychiatric conditions (e.g. depression) that also were expected to become more prevalent at older ages.
Materials and methods
Overall CHARTER design
CHARTER is a prospective, observational study conducted at six US academic medical centres: The Johns Hopkins University, Baltimore, Maryland, the University of Texas Medical Branch, Galveston, Texas, the Icahn School of Medicine at Mount Sinai, New York, New York, the University of California, San Diego, the University of Washington, Seattle, and Washington University at St Louis, Missouri. The institutional review board at each site approved the research, and every participant provided written informed consent. In addition to HIV infection, eligibility criteria included the ability to undergo a structured clinical interview and comprehensive neuromedical, neurobehavioural, and laboratory assessments. Prospective participants were excluded only for active opportunistic infections, major psychiatric disorders, or active substance use disorders that would interfere with their ability to participate in a full-day assessment. Assessments were standardized across sites and performed by trained personnel.
Procedures
We planned to re-contact and recruit 400 of the 1597 CHARTER participants to undergo a comprehensive re-evaluation 12 years after their baseline assessment. Baseline assessments occurred between 2003 and 2007, and the follow-up assessments between 2015 and 2020. The neuromedical and neurobehavioural evaluations at the second time point included all the components of the baseline assessment and added new assessments for the Fried Frailty Index (FFI), the Framingham Cardiovascular Risk and 10-year Stroke Risk Indexes, as well as collection of more detailed information about non-ART medication use. Recruitment targeted two age groups defined as having follow-up ages as either younger than 60 or at least 60 years old to facilitate analyses of age-group effects, time effects, and Group × Time interactions.
Participants
The goal was to balance participants across the six CHARTER sites and two age ranges (<60 and 60+ at follow-up). The final sample included 402 people with HIV, with the following baseline characteristics: mean (standard deviation, SD) age = 43.5(7.75); education 13.1(2.62); 76.4% Male; 185 African ancestry, 43 Hispanic ethnicity, 167 non-Hispanic Whites, and seven from other race/ethnicity. The mean (SD) verbal IQ estimates based on the Wide Range Achievement Test-III (WRAT-III) Oral Reading score was 92.1 (16.2). 247 of the participants had an AIDS diagnosis at baseline; median duration of HIV infection was 9.80 years (IQR 4.40, 14.5), current CD4+ T-cell count was 453 (279, 640), and nadir CD4+ T-cell count was 172 (30.2, 310). Also at baseline, 297 (73.9%) were on ART, with 187 (45.8%) having plasma HIV RNA ≤ 50 copies/ml (61.8% if on ART) and 308 (70.3%) CSF HIV RNA ≤ 50 copies/ml (87.3% if on ART). Mean (SD) total duration of ART use was 59.4 (49.9) months. The representation by study site was 57 for Seattle, 69 for St. Louis, 66 for Galveston, 71 for Baltimore, 66 for New York and 73 for San Diego. Mean follow-up time was 12.4 years [interquartile range (IQR): 12.1–13.0 years].
To assess the representativeness of the current longitudinal cohort, we compared their baseline characteristics with those of the 1195 CHARTER participants who were not re-assessed after 12 years. The two groups were not significantly different in most respects, including age, race, ethnicity, sex, premorbid verbal IQ estimate, estimated duration of HIV infection, current ART use, CD4+ T-cell count, the presence of most comorbid medical (e.g. HCV co-infection, diabetes, CPD) and psychiatric conditions (e.g. lifetime diagnosis of major depressive disorder, alcohol use disorder, substance use disorders). However, differences did occur. For example, the group in the current analysis had slightly more education (13.1 versus 12.5 years), were more likely to have HIV RNA ≤ 50 copies/ml in plasma (45.8% versus 38.7%) and CSF (70.3% versus 63.8%), had longer duration of ART exposure (59.4 versus 53.7 months), had better baseline neuropsychiatric comorbidity ratings (11.2% versus 17.0% with a confounding level of comorbidities, as defined by the Frascati criteria32), higher serum albumin (4.24 versus 4.16 mg/dl), and were less likely to have a lifetime methamphetamine use disorder (13.5% versus 18.7%), but more likely to have hypertension (18.7% versus 13.7%) or hyperlipidaemia (9.7% versus 3.9%).
Neurobehavioural assessments
At both time points, all participants completed a comprehensive neurocognitive test battery that assessed seven neurocognitive domains that are commonly affected by HIV (administration time 2–2.5 h).1 The best available normative standards were used, which correct for effects of age, education, sex, and race/ethnicity. Test scores were automatically converted to demographically corrected standard scores (T-scores). These were then converted to deficit scores, which were summarized as a Global Deficit Score (GDS), which reflects the number and severity of neurocognitive impairments on the entire test battery. A GDS ≥ 0.50 indicates global neurocognitive impairment that is consistent with Frascati criteria32 and indicates that, on average, the person was at least mildly impaired on at least half of the neurocognitive test measures in the battery.33,34
To determine neurocognitive change, we generated a z-score for each of 15 neurocognitive test scores, using published, regression-based normative data that correct for factors that affect test-retest changes in neurocognitively stable people.35 These z-scores reflect how well or poorly the person performed at follow-up, relative to expectations for someone with the same baseline neurocognitive and other relevant characteristics (including age and other demographics). The z-scores were then averaged to provide a regression-based global change score (GCS), with negative values indicating worse change than would be expected based on baseline test results, demographics and test-retest interval.35 GCS can be considered as a continuous variable or a categorical variable, with the bottom 5% of the GCS distribution of the normative sample defining the ‘decliner’ range.35,36 Neurocognitive change and decliner status from baseline was generated for the 12-year follow-up visit.
Psychiatric diagnoses were assessed using the computer-assisted Composite International Diagnostic Interview (CIDI), a structured instrument widely used in psychiatric research. The CIDI classifies current and lifetime diagnoses of mood disorders and substance use disorders, as well as other psychiatric disorders. Current mood was assessed with the Beck Depression Inventory II (BDI-II) values > 13 indicate at least mildly depressed mood.
Neuromedical and laboratory assessments
Neuromedical examination procedures included medical history, structured neurological and medical examination, as well as collection of blood, CSF and urine samples. These procedures were performed by trained physicians, nurse practitioners, nurses, or research associates. The staff performing neuromedical and neurocognitive assessments were certified by the Coordinating Center (University of California, San Diego). The following clinical factors were evaluated using structured interviews and laboratory assessments: current ART regimen and duration of use, HIV RNA in blood and CSF, current and nadir CD4+ T-cell count, and estimated duration of HIV infection. Clinical examination for signs of distal sensory polyneuropathy (DSP) consisted of assessments for distal reduction or loss bilaterally of ankle reflexes or sensation (vibration, sharp and light touch) in the legs and feet. DSP was defined as two or more of these signs bilaterally. Distal neuropathic pain was defined as burning, aching, or shooting symptoms in the distal legs and feet, and was classified into five grades of clinician-rated pain severity DSP based on participant report: none, slight (occasional, fleeting), mild (frequent), moderate (frequent, disabling), and severe (constant, daily, disabling, requiring analgesic medication or other pain medication). Blood was collected by venipuncture and CSF by lumbar puncture. HIV RNA was quantified by reverse transcription polymerase chain reaction ultrasensitive assay [nominal lower quantification limit, 50 copies/ml (Amplicor; Roche Diagnostic Systems)]. CD4+ T-cell count at the time of assessment was measured by flow cytometry. Participants were asked to recall their lowest prior CD4+ T-cell count, and the nadir was defined as the lower of this value or the measured CD4+ T-cell count.
Medical comorbidities were assessed based on medical history, medication list, and laboratory studies; these included diabetes mellitus, CPD (either chronic obstructive pulmonary disease or asthma), chronic renal disease, and hypertension (based on self-report or use of antihypertensive medications). Comorbidity indexes assessed were the Charlson Comorbidity Index (CCI37), Veterans Administration Comorbidity Score (VACS38), the Framingham cardiovascular risk (FCR39), and the Framingham stroke risk score.40 Framingham indexes were assessed only at the 12-year follow-up examination.
Frailty was defined by the FFI41; as meeting at least three of the following criteria: (i) unintentional weight loss more than 10 pounds in the prior year; (ii) grip strength by hand dynamometer less than the lowest 20th percentile by sex/BMI; (iii) self-reported exhaustion; (iv) low self-reported physical activity by the International Physical Activity Questionnaire42 (less than 383 kcal/week for males, less than 270 kcals/week for females); and (v) 15 feet walk time <20th percentile of normative sample,41 stratified by sex and height. Prefrail individuals met one or two of these criteria. Frailty assessments were added to the 12-year follow-up examinations but were not available at baseline.
The metabolic syndrome was defined as having any three of the following: (i) fasting glucose > 110 mg/dl; (ii) waist circumference >102 cm for males or >88 cm for females; (iii) serum triglycerides > 150 mg/dl; (iv) serum high density lipoprotein < 40 mg/dl for males or < 50 mg/dl for females; and (v) blood pressure >130/85 mm.43,44
Statistical analysis
Demographic and clinical characteristics were summarized by the age group and visit as number (percentage), mean (SD), or median (IQR). Pairwise multiple comparisons were conducted using the two-sample t-test and Fisher’s exact test for numeric and binary outcomes, respectively, and the Benjamini Hochberg procedure was then used to control for multiple comparisons. For coding variables with highly skewed or otherwise non-normal distributions, we either transformed them (e.g. log10) to improve the symmetry of their distribution or used alternative approaches such as categorical analysis, which gave substantially similar results. Longitudinal analyses first considered as dependent variables demographics, HIV disease and treatment characteristics, clinical chemistries, medical comorbidities and psychiatric and substance use comorbidities. The change of continuous and binary dependent variables over visit were compared between the younger (<60 years) and the older (≥60 years) groups using mixed-effects models and mixed-effects logistic models, respectively, with fixed effects of age group, visit and their interaction, and a subject-specific random intercept. Univariable regression models were used to determine associations with neurocognitive change from baseline to the 12-year follow-up (i.e. the GCS) and demographic and clinical variables. Variables predictive of neurocognitive decline with P-levels ≤ 0.15 in the univariable analyses were introduced into multivariable models for more optimal 12-year predictions of change. Backward stepwise selection was used to determine a final, reduced model that predicted GCS. Finally, new comorbidity indexes were generated using combinations of predictors of negative neurocognitive outcomes for each visit. The new baseline comorbidity index (range 0–6) included BDI-II > 13, hypertension, CPD, lifetime cannabis use disorder, serum aspartate transaminase >40, and serum total protein <7.1 (7.1 was identified as a threshold value by recursive partitioning to classify neurocognitive decline). The new 12-year follow-up comorbidity index (range 0–6) included BDI-II > 13, diabetes, CPD, frailty, HCT (male <41%, female < 36%), and lifetime cannabis use disorder. Proportions of neurocognitive decline were compared between the categories of the index (four categories in baseline index: 0, 1, 2, 3+; five categories for 12-year follow-up: 0, 1, 2, 3, 4+) using Fisher’s exact test; the reason for considering more categories at the 12-year point is that more participants were found to have 4 + of the comorbidities then. Also, separate simple linear regression was used to determine the association of GCS and the indexes at baseline and follow-up. In addition, we compared the new indexes with previously published indexes of risks for abnormal ageing (i.e. the VACS Index and CCI for ageing in people with HIV, and the Framingham Stroke and Cardiovascular Disease Indexes) using multiple regression. All statistical analyses were performed using R version 3.6.3, 2020. The significance level α was set to 5%.
Data availability
The data that support the findings of this study are available to the public on request from the National NeuroAIDS Tissue Consortium (NNTC) Data Coordinating Center (https://nntc.org/content/requests), which coordinates requests for data and biospecimens for the CHARTER resource. The data are not available without a formal request since the NNTC/CHARTER Steering Committee reviews requests for scientific merit and tracks and reports use of the resource to funding agencies.
Results
Tables 1–3 describe and compare baseline and follow-up characteristics of CHARTER participants in the two age groups. Age groups were comparable in sex, race, ethnicity, and in premorbid Verbal IQ estimates (WRAT-III), but the older group did have somewhat higher education levels (13.5 versus 12.8 years). Frequency of ART use was similar at each time point; the younger group had a shorter duration of ART use, as well as a lower ART regimen CNS penetration effectiveness rating, and was less likely to have plasma HIV RNA ≤ 200 copies/ml at baseline. Age group comparisons on medical comorbidities revealed that the older group had more HCV co-infection, hyperlipidaemia, DSP, and neuropathic pain at both time points, and more hypertension at follow-up. Age groups did not significantly differ at either time point in diabetes, CPD, metabolic syndrome or frailty. While composite risk indexes (CCI, VACS and Framingham) had higher values in the older group, age is included in their calculation so differences should be interpreted accordingly. Finally, the younger group was more likely to have lifetime methamphetamine use disorder at both time points.
Table 1.
Demographic characteristics, neurocognitive performance and depressive symptoms at baseline and 12 years later of the two age groups
| Younger (<60 years) | Older (≥60 years) | Age group | Visit | Age group × Visit | Group contrastsa | |||
|---|---|---|---|---|---|---|---|---|
| (n = 260) | (n = 142) | P-value | P-value | P-value | ||||
| Characteristic | Baseline (1) | 12 years (2) | Baseline (3) | 12 years (4) | ||||
| Age, years | 39.2 (5.30) | 51.8 (5.47) | 51.5 (4.59) | 64.4 (4.34) | <0.001 | <0.001 | 0.018 | 1 < 2,3; 4 > 2,3 |
| Education, years | 12.8 (2.53) | Unchanged | 13.5 (2.73) | Unchanged | 0.005 | No test | No test | 1 < 3 |
| Sex, male | 197 (75.8%) | Unchanged | 110 (77.5%) | Unchanged | 0.81 | No test | No test | No diff. |
| Race, Black | 118 (45.4%) | Unchanged | 67 (47.2%) | Unchanged | 0.75 | No test | No test | No diff. |
| Ethnicity, Hispanic | 32 (12.3%) | Unchanged | 11 (7.8%) | Unchanged | 0.18 | No test | No test | No diff. |
| WRAT-III | 92.3 (15.3) | 92.5 (15.4) | 91.6 (17.7) | 92.1 (18.2) | 0.68 | 0.63 | 0.57 | No diff. |
| Global Deficit Scoreb | 0.50 (0.50) | 0.53 (0.57) | 0.51 (0.46) | 0.55 (0.54) | 0.59 | 0.88 | 0.82 | No diff. |
| Global impairment | 116 (44.6%) | 104 (40.5%) | 65 (45.8%) | 65 (46.4%) | 0.81 | 0.25 | 0.39 | No diff. |
| BDI-II | 13.5 (10.8) | 9.55 (9.35) | 13.2 (11.0) | 9.83 (10.0) | 0.77 | <0.001 | 0.66 | 1 > 2; 3 > 4 |
| BDI-II >13 | 109 (41.9%) | 71 (28.1%) | 60 (42.3%) | 40 (29.0%) | 0.95 | <0.001 | 0.99 | 1 > 2; 3 > 4 |
Values are mean (SD) or n (%). BDI = Beck Depression Inventory; No diff. = no difference; WRAT = Wide Range Achievement Test-III.
Four pairwise comparisons (1 versus 2, 3 versus 4, 1 versus 3, and 2 versus 4) were performed and adjusted using the Benjamini Hochberg procedure.
Square root transformation.
Table 2.
HIV characteristics and clinical lab assays
| Younger (<60 years) | Older (≥60 years) | Age group | Visit | Age group × Visit | Group contrastsa | |||
|---|---|---|---|---|---|---|---|---|
| (n = 260) | (n = 142) | |||||||
| Characteristic | Baseline (1) | 12 years (2) | Baseline (3) | 12 years (4) | P-value | P-value | P-value | |
| AIDS diagnosis | 155 (59.6%) | 192 (73.8%) | 91 (64.1%) | 104 (73.2%) | 0.87 | <0.001 | <0.001 | 1 < 2; 3 < 4 |
| Duration of HIV, years | 8.9 (6.3) | 21.6 (6.5) | 11.5 (6.0) | 24.3 (6.1) | <0.001 | <0.001 | 0.084 | 1 < 2,3; 4 > 2,3 |
| CD4+ T cells, /µl | 470 (291) | 621 (366) | 510 (279) | 581 (276) | 0.16 | <0.001 | 0.071 | 1 < 2; 3 < 4 |
| Nadir CD4+ T cells, /µlb | 210 (212) | 147 (158) | 189 (176) | 154 (139) | 0.42 | <0.001 | 0.002 | 1 > 2; 3 > 4 |
| Taking ART | 184 (70.8%) | 249 (96.1%) | 113 (79.6%) | 135 (95.7%) | 0.056 | <0.001 | 0.32 | 1 < 2; 3 < 4 |
| Duration of ART (months)b | 52.0 (47.7) | 173 (73.2) | 72.9 (51.0) | 202 (75.0) | <0.001 | <0.001 | 0.028 | 1 < 2,3; 4 > 2,3 |
| CPE | 7.8 (2.0) | 8.0 (2.5) | 8.4 (2.2) | 7.7 (2.2) | 0.030 | 0.37 | 0.019 | 3 > 1,4 |
| Plasma VL ≤200 cp/mlc,d | 123 (66.8%) | 241 (92.7%) | 89 (78.8%) | 127 (94.1%) | 0.064 | <0.001 | 0.98 | 1 < 2,3; 3 < 4 |
| CSF VL ≤200 cp/mlc,d,e,f | 129 (87.9%) | 120 (94.5%) | 79 (96.3%) | 64 (100.0%) | 0.29 | 0.034 | 0.15 | 1,2 < 3,4 |
| Serum AST, mg/dl | 39.5 (32.2) | 29.1 (26.4) | 36.6 (18.3) | 27.7 (18.4) | 0.30 | <0.001 | 0.68 | 1 > 2; 3 > 4 |
| Serum total protein, mg/dl | 7.81 (0.80) | 7.47 (0.84) | 7.88 (0.89) | 7.43 (0.81) | 0.38 | <0.001 | 0.21 | 1 > 2; 3 > 4 |
| Serum albumin, mg/dl | 4.23 (0.44) | 4.30 (0.46) | 4.26 (0.44) | 4.25 (0.52) | 0.60 | 0.058 | 0.19 | No diff. |
| Serum creatinine, mg/dl | 0.94 (0.55) | 1.20 (0.04) | 0.96 (0.24) | 1.26 (0.96) | 0.89 | <0.001 | 0.61 | 1 < 2; 3 < 4 |
| Serum glucose, mg/dl | 96.3 (26.8) | 101 (37.8) | 96.9 (31.0) | 107 (54.9) | 0.88 | 0.14 | 0.26 | No diff. |
| Haematocrit (%) | 41.2 (4.27) | 42.1 (4.85) | 40.9 (3.94) | 41.2 (5.66) | 0.39 | 0.009 | 0.34 | 1 < 2 |
Values are mean (SD) or n (%). ART = antiretroviral treatment; AST = aspartate transaminase; CPE = CNS penetration effectiveness; No diff. = no difference.
Four pairwise comparisons (1 versus 2, 3 versus 4, 1 versus 3, and 2 versus 4) were performed and adjusted using the Benjamini Hochberg procedure.
Square root transformation.
On ART.
The 200 cp/ml value is based on prescribing guidelines.
CSF was collected in a subgroup.
Bayesian maximum a posteriori approach.
Table 3.
Comorbid conditions at baseline and 12 years later of the two age groups
| Younger (<60 years) | Older (≥60 years) | Age group | Visit | Age group × Visit | Group contrastsa | |||
|---|---|---|---|---|---|---|---|---|
| (n = 260) | (n = 142) | |||||||
| Characteristic | Baseline (1) | 12 years (2) | Baseline (3) | 12 years (4) | P-value | P-value | P-value | |
| HCV seropositive | 50 (19.2%) | 84 (32.4%) | 45 (31.7%) | 62 (44.0%) | 0.013 | <0.001 | 0.43 | 1 < 2,3; 4 > 2,3 |
| Diabetes | 18 (6.9%) | 46 (17.8%) | 8 (5.6%) | 36 (25.5%) | 0.82 | <0.001 | 0.073 | 1 < 2; 3 < 4 |
| Hypertension | 42 (16.2%) | 121 (46.7%) | 33 (23.2%) | 87 (61.7%) | 0.091 | <0.001 | 0.46 | 1 < 2; 3 < 4; 2 < 4 |
| Hyperlipidaemia | 19 (7.3%) | 89 (34.4%) | 20 (14.1%) | 70 (49.6%) | 0.039 | <0.001 | 0.94 | 1 < 2,3; 4 > 2,3 |
| CPD | 23 (8.8%) | 49 (18.9%) | 14 (9.9%) | 33 (23.4%) | 0.60 | <0.001 | 0.55 | 1 < 2; 3 < 4 |
| Neuropathic pain | 67 (25.8%) | 82 (31.9%) | 52 (36.9%) | 64 (45.7%) | 0.019 | 0.059 | 0.81 | 1 < 3; 2 < 4 |
| Distal sensory neuropathy | 50 (27.2%) | 91 (50.3%) | 53 (60.2%) | 86 (78.2%) | <0.001 | <0.001 | 0.75 | 1 < 2,3; 4 > 2,3 |
| Charlson Comorbidity Index | 7.47 (3.37) | 9.65 (3.31) | 8.57 (3.35) | 11.3 (3.14) | 0.002 | <0.001 | 0.047 | 1 < 2,3; 4 > 2,3 |
| VACS Indexb | 18.1 (15.0) | 24.4 (16.4) | 25.1 (14.7) | 36.8 (18.3) | <0.001 | <0.001 | 0.12 | 1 < 2,3; 4 > 2,3 |
| Framingham CVD Risk | No data | 14.4 (12.0) | No data | 23.0 (14.8) | <0.001 | No test | No test | 2 < 4 |
| Framingham Stroke Risk | No data | 5.48 (5.00) | No data | 11.7 (11.0) | <0.001 | No test | No test | 2 < 4 |
| Metabolic syndrome | 191 (73.5%) | 181 (69.9%) | 112 (78.9%) | 100 (71.4%) | 0.22 | 0.28 | 0.46 | No diff. |
| Prefrail | No data | 118 (45.7%) | No data | 68 (48.2%) | 0.32 | No test | No test | No diff. |
| Frail | No data | 14 (5.4%) | No data | 15 (10.6%) | 0.069 | No test | No test | No diff. |
| Lifetime MDD | 128 (49.4%) | 168 (65.4%) | 64 (45.4%) | 80 (57.1%) | 0.39 | <0.001 | 0.39 | 1 < 2; 3 < 4 |
| Current MDD | 37 (14.3%) | 16 (6.69%) | 14 (9.93%) | 12 (9.38%) | 0.21 | 0.003 | 0.082 | 1 > 2 |
| Lifetime alcohol disorder | 132 (51.0%) | 149 (58.0%) | 79 (56.0%) | 81 (57.9%) | 0.92 | <0.001 | <0.001 | 1 < 2 |
| Current alcohol disorder | 4 (1.54%) | 3 (1.26%) | 1 (0.71%) | 1 (0.78%) | 0.48 | 0.78 | 0.85 | No diff. |
| Lifetime cannabis disorder | 77 (29.7%) | 89 (34.6%) | 32 (22.7%) | 38 (27.1%) | 0.69 | <0.001 | 0.79 | 1 < 2; 3 < 4 |
| Current cannabis disorder | 7 (2.70%) | 1 (0.42%) | 1 (0.71%) | 1 (0.78%) | 0.27 | 0.10 | 0.21 | No diff. |
| Lifetime meth disorder | 42 (16.2%) | 50 (19.5%) | 12 (8.5%) | 12 (8.6%) | 0.42 | 0.014 | 0.16 | 1 > 3; 2 > 1,4 |
| Current meth disorder | 1 (0.38%) | 0 (0.00%) | 1 (0.71%) | 0 (0.00%) | 0.67 | 1.00 | 1.00 | No diff. |
| Lifetime any substance use disorder | 186 (71.8%) | 201 (78.2%) | 99 (70.2%) | 102 (72.9%) | 0.85 | <0.001 | 0.25 | 1 < 2 |
| Current any substance use disorder | 22 (8.5%) | 5 (2.1%) | 4 (2.8%) | 3 (2.3%) | 0.039 | 0.003 | 0.14 | 1 > 2 |
| Positive urine drug screen | 49 (19.1%) | 53 (20.8%) | 27 (19.0%) | 16 (11.6%) | 0.99 | 0.57 | 0.059 | No diff. |
Values are mean (standard deviation) or n (%). CVD = cardiovascular disease; MDD = major depressive disorder; meth = methamphetamine; No diff. = no difference.
Four pairwise comparisons (1 versus 2, 3 versus 4, 1 versus 3, and 2 versus 4) were performed and adjusted using the Benjamini Hochberg procedure.
Square root transformation.
The clearest and most consistent findings were the 12-year differences apparent for both age groups: at follow-up, both groups had lower nadir CD4+ T-cell counts, more AIDS diagnoses, more HCV co-infection, and more diabetes, hypertension, hyperlipidaemia, CPD, DSP, and lifetime cannabis use disorder and major depressive disorder. Clinically favourable changes at follow-up included that many more participants were taking ART with more frequent HIV suppression and higher CD4+ T-cell counts and had modestly improved clinical lab indicators (hepatic aspartate transaminase, total protein, and creatinine; haematocrit in the younger group), and less currently depressed mood (BDI-II). The only Age group × Time interactions of possible consequence were unfavourable for the younger group: nadir CD4+ T-cell count declined more, and lifetime alcohol use disorder increased more than in the older group at follow-up. Importantly, and contrary to expectations, cross-sectional GDS and rate of neurocognitive impairment were high for both groups at both time points but did not evidence significant differences between the age groups or the time points and did not show an Age group × Time interaction.
Neurocognitive change
The primary neurocognitive outcome was the GCS, a continuous variable reflecting direction and degree of 12-year neurocognitive change. The mean (SD) GCS for the total group was −0.28 (0.064), which was not significantly different between the two age groups [linear regression coefficient = 0.011 (−0.012, 0.14), P = 0.87] or related to age as a continuous variable [coefficient = 0.003 (−0.005, 0.011, P = 0.48)], although the GCS attempts to account for the influence of age. Thus, on average, neurocognitive performance declined modestly over 12 years, regardless of age or age group.
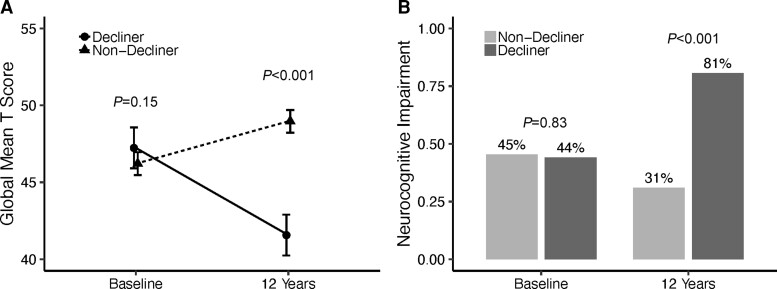
The GCS also can be used to define change status of individuals (decliner, stable or improver) using a 90% confidence interval with the published normative data.35,36 Based upon this metric, 23.7% declined, 70.0% remained stable, and 6.2% improved. Figure 1A shows the mean of demographically corrected T-scores on the total test battery for decliners and non-decliners at baseline and then after 12 years. The group’s mean T-scores were similar at baseline (P > 0.10) but were substantially different at the 12-year follow-up (P = 2.2 × 10−16), with decliners doing much worse over time. Figure 1B shows the neurocognitive impairment rates for these two groups at the two time points. As in Fig. 1A for mean T-scores, these impairment rates were quite similar at baseline (P = 0.83) but very different after 12 years (P = 2.9 × 10−18); in fact, the impairment rate dropped by about 15% for the non-decliners (45% to 31%), and almost doubled for the decliners (44% to 81%).
Figure 1.
Change by decliner status. (A) Global T-score by decliner status at baseline and 12 years (values are mean and 95% confidence interval). (B) Global neurocognitive impairment by decliner status at baseline and 12 years (values are observed proportions).
To further assess neurocognitive changes among the decliners, we compared their impairment classifications at baseline and 12-years using GDSs with correction for ‘practice effects’.35 Consistent with prior normative classifications for GDS, we considered GDS < 0.50 as unimpaired, 0.50–0.99 as mildly impaired, 1.00–1.49 as mildly-to-moderately impaired, 1.50–1.99 as moderately impaired, 2.00–2.49 as moderately-to-severely impaired, and ≥2.50 as severely impaired. None of the participants had severe impairment at baseline, but 5.4% progressed to this level after 12 years. Some (39.8%) progressed from unimpaired to at least mildly impaired (follow-up range mild to severe), and 19.4% progressed from at least mild impairment to a worse category of impairment at 12 years. The remainder (35.4%) stayed in the same category even though their performance declined. This can occur when a participant’s performance begins in the better end of the range of values for that category and then declines into the worse end of the range.
Baseline and 12-year predictors of neurocognitive change
Univariable linear regressions identified the following baseline participant characteristics that predicted worse GCS over 12 years with a P-value < 0.15: hypertension (P = 0.08); CPD (P = 0.12); elevated hepatic aspartate transaminase (P = 0.003); lower serum total protein (P = 0.049); current major depressive disorder (P = 0.04); BDI-II score > 13 (P = 0.012); lifetime substance use disorder (any drug) (P = 0.041); lifetime cannabis use disorder (P = 0.036); and lifetime cocaine use disorder (P = 0.12). Multivariable linear regression modelling revealed that the following baseline variables most strongly and independently predicted worse GCS over the 12-year follow-up, with a model R2 of 0.050 (P = 0.0034): hypertension, CPD, BDI-II > 13, lifetime cannabis use disorder, higher serum hepatic aspartate transaminase, and lower serum protein. BDI-II > 13 and current major depressive disorder had comparable performance in the model but BDI-II > 13 was chosen because of its ease of calculation.
The following participant characteristics at the 12-year assessment were associated with worse GCS with a P-value < 0.15: diabetes (P = 0.016); CPD (P = 0.001); neuropathic pain (P = 0.019); current major depressive disorder (P = 0.01); BDI-II > 13 (P < 0.001); lifetime any substance use disorder (P = 0.042); lifetime cannabis use disorder (P = 0.024); lower haematocrit (P = 0.001), pre-frailty (P = 0.007), frailty (<0.001), and higher VACS index values (P = 0.077). The best multivariable prediction model using variables collected at the 12-year point included diabetes, CPD, lower haematocrit, BDI-II > 13, the combination of prefrailty and frailty, and lifetime history of cannabis use disorder; the model R2 was 0.11 (P < 0.001).
Worse GCS value after 12 years was not associated with baseline or 12-year HIV disease or treatment variables (AIDS diagnosis, nadir or current CD4+ T-cell count, ART use, or HIV suppression in plasma or CSF), CCI, or demographic variables, although as noted the GCS attempts to account for the influence of age, sex, education, race and ethnicity using normative data. Similarly, the follow-up Framingham 10-year stroke risk and Framingham CVD risk indexes were not associated with worse GCS values, although they were only available at follow-up. The VACS index at follow-up had trend significance as a univariable predictor of GCS but did not contribute to the best multivariable models.
To better understand the influence of age on GCS, we analysed interactions between age group and many of the variables in Tables 1–3. This analysis identified a statistically significant interaction between age group and plasma HIV suppression at baseline. The nature of the interaction was that the older and younger groups had comparable GCS values if their plasma HIV RNA was undetectable but older people who had detectable plasma HIV RNA at baseline had greater neurocognitive decline over time than younger people who had detectable plasma HIV RNA at baseline.
Baseline and 12-year comorbidity indexes predicting worse neurocognitive change
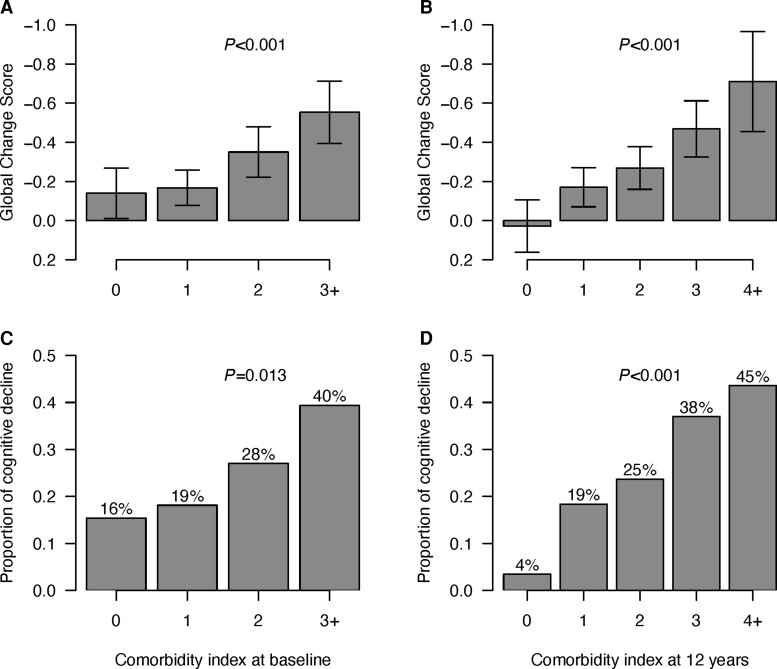
Based only upon categorical predictors in the two optimal prediction models, we created two new indexes that are simply the counts of the categorical risk conditions at baseline (hypertension, CPD, BDI-II > 13, lifetime cannabis use disorder, AST > 40, and total serum protein <7.1) and at 12 years (diabetes, CPD, the combination of pre-frailty and frailty, BDI-II > 13, low haematocrit (<42% for males, < 37% for females), and lifetime cannabis use disorder). Figure 2 shows worse mean GCS values for each successive level of the two indexes (0, 1, 2, and 3+ at baseline, and 0, 1, 2, 3 and 4+ for 12-year follow-up). Effect sizes (Cohen’s d values) for GCS differences across baseline index values were −0.045 for index 0 to 1, −0.34 from index 0 to 2, and −0.67 for 0 to 3+; at follow-up, effect sizes were −0.33 from follow-up index 0 to 1, −0.49 from 0 to 2, −0.82 from 0 to 3, and −1.22 from 0 to 4+. Numbers of participants at these respective index levels were 80, 139, 111 and 66 for the baseline index, and 59, 113, 116, 74, and 39 for the follow-up index. Figure 2 also shows the percentage with increasing index scores who were neurocognitive decliners over the 12 years.
Figure 2.
GCS and decliner status by comorbidity indices at baseline and at 12 Years. (A) GCS by number of comorbidities at baseline. (B) GCS by number of comorbidities at 12 years. (C) Proportion of decliners by number of comorbidities at baseline. (D) Proportion of decliners by number of comorbidities at 12 years. Values in A and B are mean and 95% confidence intervals. In C and D, values are proportions.
When these indexes of categorical comorbidity counts were included in multivariable models along with previously published comorbidity indexes (VACS index and CCI at both baseline and follow-up, and Framingham CVD risk and 10-year stroke risk at follow-up), the current indexes remained highly significant predictors of the GCS, whereas the other indexes did not improve the models.
Comorbidity treatment information
To further assess the influence of the medical comorbid conditions that were associated with GCS, we also analysed the use of prescribed drugs that treat these conditions. Use of all prescribed drugs (including ART) was collected at the 12-year assessments but only drugs in specific categories (e.g. antidepressants, ART) were collected at baseline. As a result, we focused analyses on the more comprehensive lists of prescribed drugs taken at the 12-year follow-up. At that time, 111 participants had a BDI-II > 13 and 56 were taking an antidepressant, 82 participants had a diagnosis of diabetes and 46 were taking an antidiabetic drug, 208 had a diagnosis of hypertension and 158 were taking an antihypertensive drug, and 82 had a diagnosis of CPD with 39 taking a bronchodilator. For those who had a BDI-II > 13, antidepressant therapy was associated with worse GCS (P = 0.004). However, for those with CPD, being treated with bronchodilators related to better GCS over the 12 years (P = 0.05). Treatment at the 12-year time point was not significantly related to GCS for hypertension (P = 0.21), diabetes (P = 0.76), or hyperlipidaemia (P = 0.85). Being prescribed opiates at follow-up was associated with worse GCS (P = 0.017), but it did not explain additional variance in GCS as a covariate in the best multivariable model above.
Discussion
We evaluated changes in neurocognitive performance and a wide range of potential medical and psychiatric predictors in 402 older and younger people with HIV. The participants were representative of the larger group of CHARTER participants who were originally recruited from across the USA 12 years before. At follow-up, the age of over 35% of the sample was 60 years or older. The overall goal was to determine the nature and predictors of neurocognitive decline in people with HIV who were ageing with HIV. We originally hypothesized that, as people with HIV enter their seventh decade, the combined risks of longer exposure to HIV and potentially toxic effects of ART, together with a larger number of ageing-related medical comorbidities, would result in greater decline than for younger participants.
On average, the total CHARTER cohort evidenced a modest neurocognitive decline over the 12-year follow-up, beyond what would be expected based on typical ageing. In addition, 23.7% had substantial decline that is greater than the 95th percentile of a published, normative sample and resulted in a substantial increase in neurocognitive impairment at follow-up. Contrary to expectations, however, the observed neurocognitive decline was not different for the older and younger age groups and was not significantly related to age as a continuous variable.
Both younger and older groups of people with HIV were much more likely to be taking suppressive ART after 12 years, and had improved CD4+ T-cell count, suggesting good medical management of their HIV disease. Their clinical chemistries and mood ratings also improved. On the other hand—and regardless of age group—the prevalence of AIDS increased at follow-up, nadir CD4+ T-cell count declined, and multiple neuromedical comorbidities increased. The magnitude of these increases in comorbidity burdens, and the fact that they were not different for the older and younger groups, were contrary to expectations, and could have resulted from a relatively age-independent vulnerability in people with HIV (e.g. due to persistent inflammation) as well as unmeasured adverse lifestyle risk factors or suboptimal medical attention to disorders other than HIV infection.
Prior cross-sectional analyses of the complete CHARTER cohort at baseline focused on neurocognitive impairment, not decline from prior, documented neurocognitive performance.35 At that time, the most powerful predictors of neurocognitive impairment were non-HIV-related neuropsychiatric comorbidities (e.g. seizure disorder, prolonged loss of consciousness32), as well as nadir CD4+ T-cell count < 200/µl and presence of detectable HIV RNA in plasma35; these HIV-associated risks for cross-sectional neurocognitive impairment are consistent with other reports (e.g. Tozzi et al.4). A prior CHARTER longitudinal study focused on neurocognitive decline over 3 years in 436 people with HIV, identifying that the strongest independent predictors were being off ART at follow-up, low serum albumin, low haematocrit, more depressed mood, severe neuropsychiatric comorbidity ratings, a lifetime history of methamphetamine use disorder and Hispanic ethnicity.36
Since these earlier studies were completed, CHARTER participants have improved control of their HIV infection, such that almost all were on suppressive ART at follow-up. The lack of association between neurocognitive change and HIV disease characteristics may be because nearly all participants were virally suppressed on ART at their most recent assessment. While the legacy effect of prior severe immune suppression previously related to neurocognitive impairment at baseline in CHARTER and other studies,4,35,45 our analyses did not identify it as a risk factor for neurocognitive decline in well treated people with HIV.
While neither age itself nor HIV disease or ART characteristics were associated with the observed neurocognitive decline over 12 years in the CHARTER cohort, several medical and psychiatric comorbidities continued to accumulate, regardless of age, and did predict worse neurocognitive change over 12 years. At baseline, at follow-up, or at both time points, these included cardiovascular risk factors (hypertension and diabetes), CPD, neuropathic pain, prefrailty/frailty, depression, anaemia, low serum protein, and evidence of liver injury. Cardiovascular risk factors have been previously associated with neurocognitive impairment in people with HIV (e.g. Becker et al.,46 Valcour et al.,47 Wright et al.48), and with adverse medical outcomes. In a recent surveillance report from New York City that tracked results from 2005 to 2019, age-adjusted rates of non-HIV medical conditions, and in particular cardiovascular disease, have now substantially overtaken HIV conditions as causes of death in people with HIV.49 This study provides the longest period of observation linking cardiovascular risks to neurocognitive trajectories in people with HIV.50
Depression also frequently has been associated with cross-sectional neurocognitive impairment in people with HIV.13 Consistent with our results over a longer period, a recent longitudinal study reported that cumulative BDI-assessed depressive burden over an average of almost 4 years related to significantly worse neurocognitive trajectories.51
At both time points, a lifetime (not current) history of cannabis use disorder and ‘any’ substance use disorder also were associated with neurocognitive decline. These findings should be interpreted in the context of current substance use disorders being rare among CHARTER participants at both baseline and 12-year evaluations, whereas lifetime prevalence increased over the follow-up period. Thus, substance use disorders that occurred between the assessments could have posed risks for CNS injury. In that regard, cannabis has anti-inflammatory effects,52–55 which could be beneficial for persistent inflammation in people with HIV. This may be true, however, only when cannabis is used in moderation. Cannabis use that is substantial enough to support a diagnosis of cannabis use disorder (e.g. it interferes with everyday functioning) may overwhelm the benefits of moderate use.56 While lifetime use disorders of stimulants and ‘any’ substances related to worse neurocognitive trajectories, both weakened in multivariable models.
Regarding the putative pathological ageing with HIV, the current findings are limited by the lack of a demographically comparable comparison group of people without HIV. Even though a large epidemiological literature supports an increased prevalence and incidence of ageing-related medical comorbidities in people with HIV (e.g. Turrini et al.,15 Feinstein et al.,17), the lack of a comparison group here prevents us from confidently concluding that the longitudinal comorbidity findings reported here are specific to HIV. Nevertheless, the absence of age effects on the high rates of reported comorbid conditions, which increased over time and without Age × Time interactions, would be more consistent with ‘accentuated’ rather than ‘accelerated’ ageing. Notably as well, the same lack of age effects applied to neurocognitive changes over the 12-year follow-up, which instead were linked to comorbidities at both time points.
This study has other important limitations.
Even though the current follow-up cohort was comparable to the larger CHARTER sample at baseline, their findings are potentially subject to survivor biases: we do not have accurate information about deaths over the 12-year follow-up, and older people with HIV may have had higher mortality rates and comorbidity burdens that influenced their availability for this study. However, survivorship bias would be expected to attenuate comorbidity or neurocognitive changes over time, and not to increase them.
The project compared older people with HIV to younger people with HIV but did not include people without HIV. While the normative data used to calculate the GCS derive from people without HIV, the findings may not address whether the neurocognitive performance of people with HIV declines more than similar people without HIV.
Although the same, comprehensive neuromedical and neurobehavioural evaluations were performed at baseline and follow-up, these represent only two time points separated by 12 years. We have no information about when medical risk factors first presented or worsened, whether and when medical treatment may have been provided, and with what results. In particular, the prescription of medications for some comorbid conditions was not consistent across participants and likely differed regarding time of initiation, medication type, dose and adherence; thus, while medication effects on comorbid conditions associated with neurocognitive decline are important, they cannot be readily discerned in an observational study.
Our analyses of age effects were based on chronological age; indicators more closely related to biological ageing may have yielded different results. People with HIV appear to have older biological age than people without HIV, whether measured by telomere length,57 DNA methylation,58 brain MRI,59 or other methods.
This study lacked details about economic status, lifestyle factors (e.g. diet and exercise), and access to and use of healthcare services over the 12-year study period, which likely influenced medical and neurocognitive outcomes.
Finally, the project was performed in the USA and so the findings may best generalize to Americans with HIV who are in clinical care.
We devised two new comorbidity indexes that are easy-to-use and, with further validation in independent samples, may be helpful in clinical practice. The first index was constructed with baseline data and predicted future neurocognitive decline. The second was based on data collected at the 12-year follow-up and might be used to signal risk for current neurocognitive impairment (decline that has already happened). Both indexes are simple counts of presence or absence of comorbid conditions, and three of the components overlap: current depressed mood, CPD, and lifetime cannabis use disorder; the only differences are that hypertension and two routine blood chemistry results (serum AST and total protein) at baseline were independently predictive of neurocognitive change, and diabetes and lower haematocrit at follow-up were associated with past decline. Prefrailty or frailty at the follow-up visit also was associated with neurocognitive decline but the frailty assessment was not performed at baseline. Apart from lifetime (not current) cannabis use disorder, the other comorbidities are potentially treatable and may deserve increased attention in the clinical management of people with HIV.17 Although laboratory values are not as easy to use in indexes of binary variables such as these, they are typically available in clinical practice. As such, aspartate transaminase, serum protein and haematocrit, which predicted neurocognitive decline in our models, may deserve increased attention in medical care of ageing people with HIV.
Supplementary Material
Acknowledgements
The CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) group is affiliated with Johns Hopkins University; the Icahn School of Medicine at Mount Sinai; University of California, San Diego; University of Texas, Galveston; University of Washington, Seattle; Washington University, St. Louis; and is headquartered at the University of California, San Diego. We also gratefully acknowledge the contribution of Jules Levin of The National AIDS Treatment Advocacy Project.
Appendix 1
The CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) group
Full details are provided in the Supplementary material.
Robert K. Heaton, Scott L. Letendre, Ronald J. Ellis, Christine Fennema-Notestine, Rebecca Theilmann, Amanda Bischoff-Grethe, Bin Tang, Clint Cushman, Debra Cookson, Matthew Dawson, Donald Franklin Jr, Leah Rubin, Susan Morgello, Letty Mintz, J. Allen McCutchan, Christina Marra, Benjamin Gelman, Eleanor Head, David Clifford, Beau Ances, Muhammad Al-Lozi, Mengesha Teshome.
Contributor Information
Robert K Heaton, Department of Psychiatry, University of California San Diego, San Diego, CA 92093, USA.
Ronald J Ellis, Department of Psychiatry, University of California San Diego, San Diego, CA 92093, USA; Department of Neurosciences, University of California, San Diego, CA 92093, USA.
Bin Tang, Department of Psychiatry, University of California San Diego, San Diego, CA 92093, USA.
Christina M Marra, Department of Neurology, University of Washington, Seattle, WA 98104, USA.
Leah H Rubin, Department of Neurology, Johns Hopkins University, Baltimore, MD 21218, USA.
David B Clifford, Department of Neurology, Washington University at St. Louis, St. Louis, MO 63110, USA.
J Allen McCutchan, Department of Medicine, University of California San Diego, San Diego, CA 92093, USA.
Benjamin B Gelman, Department of Pathology, University of Texas Medical Branch, Galveston, TX 77555, USA; Department of Neuroscience and Cell Biology, University of Texas Medical Branch, Galveston, TX 77555, USA.
Susan Morgello, Department of Neurology, Icahn School of Medicine at Mount Sinai, New York, NY 10029, USA.
Donald R Franklin, Department of Psychiatry, University of California San Diego, San Diego, CA 92093, USA.
Scott L Letendre, Department of Psychiatry, University of California San Diego, San Diego, CA 92093, USA; Department of Medicine, University of California San Diego, San Diego, CA 92093, USA.
Funding
The authors gratefully acknowledge funding from the National Institute of Mental Health (R01 MH107345, P30 MH062512).
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Heaton RK, Franklin DR, Ellis RJ, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: Differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Simioni S, Cavassini M, Annoni JM, et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS. 2010;24:1243–1250. [DOI] [PubMed] [Google Scholar]
- 3. Tozzi V, Balestra P, Bellagamba R, et al. Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: Prevalence and risk factors. Research support, non-U.S. Gov’t. J Acquir Immune Defic Syndr. 2007;45:174–182. [DOI] [PubMed] [Google Scholar]
- 4. Tozzi V, Balestra P, Libertone R, Antinori A. Cognitive function in treated HIV patients. Neurobehav HIV Med. 2010;2:95–113. [Google Scholar]
- 5. Lanman T, Letendre S, Ma Q, Bang A, Ellis R. CNS Neurotoxicity of antiretrovirals. J Neuroimmune Pharmacol. 2021;16:130–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pathai S, Bajillan H, Landay AL, High KP. Is HIV a model of accelerated or accentuated aging? J Gerontol A Biol Sci Med Sci. 2014;69:833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Torres RA, Lewis W. Aging and HIV/AIDS: Pathogenetic role of therapeutic side effects. Lab Invest. 2014;94:120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deeks SG. HIV Infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deeks SG, Tracy R, Douek DC. Systemic effects of inflammation on health during chronic HIV infection. Immunity. 2013;39:633–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kirk JB, Goetz MB. Human immunodeficiency virus in an aging population, a complication of success. J Am Geriatr Soc. 2009;57:2129–2138. [DOI] [PubMed] [Google Scholar]
- 11. Bonfanti P, Giannattasio C, Ricci E, et al. HIV And metabolic syndrome: A comparison with the general population. J Acquir Immune Defic Syndr. 2007;45:426–431. [DOI] [PubMed] [Google Scholar]
- 12. Guaraldi G, Orlando G, Zona S, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis. 2011;53:1120–1126. [DOI] [PubMed] [Google Scholar]
- 13. Morgello S, Gensler G, Sherman S, et al. Frailty in medically complex individuals with chronic HIV. AIDS. 2019;33:1603–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rodriguez-Penney AT, Iudicello JE, Riggs PK, et al. Co-morbidities in persons infected with HIV: Increased burden with older age and negative effects on health-related quality of life. AIDS Patient Care STDS. 2013;27:5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Turrini G, Chan SS, Klein PW, et al. Assessing the health status and mortality of older people over 65 with HIV. PLoS One. 2020;15:e0241833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yu B, Pasipanodya E, Montoya JL, et al. Metabolic syndrome and neurocognitive deficits in HIV infection. J Acquir Immune Defic Syndr. 2019;81:95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Feinstein MJ, Hsue PY, Benjamin LA, et al. Characteristics, prevention, and management of cardiovascular disease in people living with HIV: A scientific statement from the American heart association. Circulation. 2019;140:e98-e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aung HL, Aghvinian M, Gouse H, et al. Is there any evidence of premature, accentuated and accelerated aging effects on neurocognition in people living with HIV? A systematic review. AIDS Behav. 2021;25:917–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Larussa D, Lorenzini P, Cingolani A, et al. Highly active antiretroviral therapy reduces the age-associated risk of dementia in a cohort of older HIV-1-infected patients. AIDS Res Hum Retroviruses. 2006;22:386–392. [DOI] [PubMed] [Google Scholar]
- 20. Sacktor N, Skolasky RL, Cox C, et al. Longitudinal psychomotor speed performance in human immunodeficiency virus-seropositive individuals: Impact of age and serostatus. J Neurovirol. 2010;16:335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goodkin K, Miller EN, Cox C, et al. Effect of ageing on neurocognitive function by stage of HIV infection: Evidence from the multicenter AIDS cohort study. Lancet HIV. 2017;4:e411–e422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Seider TR, Luo X, Gongvatana A, et al. Verbal memory declines more rapidly with age in HIV infected versus uninfected adults. J Clin Exp Neuropsychol. 2014;36:356–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sheppard DP, Woods SP, Bondi MW, et al. Does older age confer an increased risk of incident neurocognitive disorders among persons living with HIV disease? Clin Neuropsychol. 2015;29:656–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Haynes BI, Pitkanen M, Kulasegaram R, et al. HIV: Ageing, cognition and neuroimaging at 4-year follow-up. HIV Med. 2018;19:376–385. [DOI] [PubMed] [Google Scholar]
- 25. Aung HL, Bloch M, Vincent T, et al. Cognitive ageing is premature among a community sample of optimally treated people living with HIV. HIV Med. 2021;22:151–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ciccarelli N, Fabbiani M, Baldonero E, et al. Effect of aging and human immunodeficiency virus infection on cognitive abilities. J Am Geriatr Soc. 2012;60:2048–2055. [DOI] [PubMed] [Google Scholar]
- 27. Damas J, Ledergerber B, Nadin I, et al. Neurocognitive course at 2-year follow-up in a Swiss cohort of people with well-treated HIV. AIDS. 2021;35:2469–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Haddow LJ, Sudre CH, Sokolska M, et al. Magnetic resonance imaging of cerebral small vessel disease in men living with HIV and HIV-negative men aged 50 and above. AIDS Res Hum Retroviruses. 2019;35:453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Joska JA, Dreyer AJ, Nightingale S, Combrinck MI, De Jager CA. Prevalence of HIV-1 infection in an elderly rural population and associations with neurocognitive impairment. AIDS. 2019;33:1765–1771. [DOI] [PubMed] [Google Scholar]
- 30. Milanini B, Allen I, Paul R, et al. Frequency and predictors of HIV-related cognitive impairment in east Africa: The Africa cohort study (AFRICOS). J Acquir Immune Defic Syndr. 2020;83:157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schouten J, Wit FW, Stolte IG, et al. Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: The AGEhIV cohort study. Clin Infect Dis. 2014;59:1787–1797. [DOI] [PubMed] [Google Scholar]
- 32. Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Blackstone K, Moore DJ, Franklin DR, et al. Defining neurocognitive impairment in HIV: Deficit scores versus clinical ratings. Clin Neuropsychol. 2012;26:894–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Carey CL, Woods SP, Gonzalez R, et al. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol. 2004;26:307–319. [DOI] [PubMed] [Google Scholar]
- 35. Cysique LA, Franklin D Jr, Abramson I, et al. Normative data and validation of a regression based summary score for assessing meaningful neuropsychological change. J Clin Exp Neuropsychol. 2011;33:505–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Heaton RK, Franklin DR Jr, Deutsch R, et al. Neurocognitive change in the era of HIV combination antiretroviral therapy: The longitudinal CHARTER study. Clin Infect Dis. 2015;60:473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. [DOI] [PubMed] [Google Scholar]
- 38. Justice AC, Modur SP, Tate JP, et al. Predictive accuracy of the veterans aging cohort study index for mortality with HIV infection: A north American cross cohort analysis. J Acquir Immune Defic Syndr. 2013;62:149–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. D’Agostino RB S, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: The framingham heart study. Circulation. 2008;117:743–753. [DOI] [PubMed] [Google Scholar]
- 40. Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: A risk profile from the framingham study. Stroke. 1991;22:312–318. [DOI] [PubMed] [Google Scholar]
- 41. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 42. Hallal PC, Victora CG. Reliability and validity of the international physical activity questionnaire (IPAQ). Med Sci Sports Exerc. 2004;36:556. [DOI] [PubMed] [Google Scholar]
- 43. Alberti KG, Zimmet P, Shaw J, Group IDFETFC . The metabolic syndrome–a new worldwide definition. Lancet. 2005;366:1059–1062. [DOI] [PubMed] [Google Scholar]
- 44. Saely CH, Rein P, Drexel H. The metabolic syndrome and risk of cardiovascular disease and diabetes: Experiences with the new diagnostic criteria from the international diabetes federation. Horm Metab Res. 2007;39:642–650. [DOI] [PubMed] [Google Scholar]
- 45. Munoz-Moreno JA, Fumaz CR, Ferrer MJ, et al. Nadir CD4 cell count predicts neurocognitive impairment in HIV-infected patients. AIDS Res Hum Retroviruses. 2008;24:1301–1307. [DOI] [PubMed] [Google Scholar]
- 46. Becker JT, Kingsley L, Mullen J, et al. Vascular risk factors, HIV serostatus, and cognitive dysfunction in gay and bisexual men. Neurology. 2009;73:1292–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Valcour VG, Sacktor NC, Paul RH, et al. Insulin resistance is associated with cognition among HIV-1-infected patients: The Hawaii aging with HIV cohort. J Acquir Immune Defic Syndr. 2006;43:405–410. [DOI] [PubMed] [Google Scholar]
- 48. Wright EJ, Grund B, Robertson K, et al. Cardiovascular risk factors associated with lower baseline cognitive performance in HIV-positive persons. Neurology. 2010;75:864–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. HIV Epidemiology Program. HIV Surveillance Annual Report, 2020. 2021.
- 50. Ellis RJ, Paolillo E, Saloner R, Heaton RK. Higher comorbidity burden predicts worsening neurocognitive trajectories in people with human immunodeficiency virus. Clin Infect Dis. 2022;74:1323–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Paolillo EW, Pasipanodya EC, Moore RC, et al. Cumulative burden of depression and neurocognitive decline among persons with HIV: A longitudinal study. J Acquir Immune Defic Syndr. 2020;84:304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bilkei-Gorzo A, Albayram O, Draffehn A, et al. A chronic low dose of Delta(9)-tetrahydrocannabinol (THC) restores cognitive function in old mice. Nat Med. 2017;23:782–787. [DOI] [PubMed] [Google Scholar]
- 53. Castro FOF, Silva JM, Dorneles GP, et al. Distinct inflammatory profiles in HIV-infected individuals under antiretroviral therapy using cannabis, cocaine or cannabis plus cocaine. AIDS. 2019;33:1831–1842. [DOI] [PubMed] [Google Scholar]
- 54. Cristino L, Bisogno T, Di Marzo V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat Rev Neurol. 2020;16:9–29. [DOI] [PubMed] [Google Scholar]
- 55. Yadav-Samudrala BJ, Fitting S. Mini-review: The therapeutic role of cannabinoids in neuroHIV. Neurosci Lett. 2021;750:135717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Selamoglu A, Langley C, Crean R, et al. Neuropsychological performance in young adults with cannabis use disorder. J Psychopharmacol. 2021;35:1349–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mehta SR, Iudicello JE, Lin J, et al. Telomere length is associated with HIV infection, methamphetamine use, inflammation, and comorbid disease risk. Drug Alcohol Depend. 2021;221:108639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Levine AJ, Quach A, Moore DJ, et al. Accelerated epigenetic aging in brain is associated with pre-mortem HIV-associated neurocognitive disorders. J Neurovirol. 2016;22:366–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cole JH, Underwood J, Caan MW, et al. Increased brain-predicted aging in treated HIV disease. Neurology. 2017;88:1349–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available to the public on request from the National NeuroAIDS Tissue Consortium (NNTC) Data Coordinating Center (https://nntc.org/content/requests), which coordinates requests for data and biospecimens for the CHARTER resource. The data are not available without a formal request since the NNTC/CHARTER Steering Committee reviews requests for scientific merit and tracks and reports use of the resource to funding agencies.