Abstract
Background
Next-generation sequencing (NGS) is increasingly used for periprosthetic joint infection (PJI) diagnosis, but its clinical utility is poorly defined. Shotgun metagenomic sequencing (sNGS) has been reported to identify PJI pathogens undetected by culture in sonicate fluid. However, sNGS is complex and costly. Here, 16S ribosomal RNA (rRNA) gene-based targeted metagenomic sequencing (tNGS) was compared to sNGS of sonicate fluid for microbial detection and identification in patients with total hip arthroplasty (THA) and total knee arthroplasty (TKA) failure.
Methods
A convenience sample of sonicate fluids derived from patients who had undergone THA or TKA removal, enriched with culture negative PJI cases, was tested. Samples had been previously tested by sNGS. For tNGS, samples were extracted, amplified by polymerase chain reaction targeting the V1 to V3 regions of the 16S rRNA gene, and sequenced on an Illumina MiSeq.
Results
A total of 395 sonicate fluids, including 208 from subjects with PJI, were studied. Compared with sonicate fluid culture, tNGS had higher positive percent agreement (72.1 vs 52.9%, P < .001), detecting potential pathogens in 48.0% of culture-negative PJIs. There was no difference between the positive percent agreement of tNGS (72.1%) and sNGS (73.1%, P = .83).
Conclusions
16S rRNA gene-based tNGS is a potential diagnostic tool for PJI pathogen identification in sonicate fluid from failed THAs and TKAs in culture-negative cases, with similar performance characteristics to sNGS.
Keywords: 16S rRNA PCR/sequencing, next generation sequencing, periprosthetic joint infection
Targeted and shotgun metagenomic sequencing of sonicate fluid are interchangeable for periprosthetic joint infection (PJI) diagnosis. Targeted metagenomic sequencing should be considered in culture-negative PJI.
Primary total hip and knee arthroplasty incidence is rising in the United States, and revision surgery frequency is increasing proportionally; revision occurs because of periprosthetic joint infection (PJI) or noninfectious aseptic failure (NIAF) [1]. PJI is a devastating complication, occurring in 1% to 2% of total hip or knee arthropathies [1, 2], and requiring further surgery and prolonged antibiotic administration [2]. Identifying a causative pathogen is helpful for diagnosis and, most importantly, to guide appropriate management; however, 5% to 35% of PJIs are culture-negative [2]. Culture-negative PJI can be associated with antecedent antimicrobial therapy, inadequate use of diagnostics, or the presence of fastidious microorganisms. Recently, PJI diagnosis has evolved, with improved tissue culture methods [3], sonication to sample biofilms on implant surfaces [4], and molecular diagnostics, especially polymerase chain reaction (PCR) assays; yet, many cases remain microbiologically negative.
Previously, we showed that 16S ribosomal RNA (rRNA) gene PCR followed by Sanger sequencing on sonicate fluid had a sensitivity of 70% and specificity of 98%, equivalent to sonicate fluid culture [5]. A limitation of the approach was the inability to decipher polymicrobial infection and 16S rRNA copy variants, and limited assay sensitivity because of a cycle threshold value cutoff being used to select samples for sequencing. Cazanave et al evaluated sonicate fluid from 434 subjects undergoing resection arthroplasties (144 with PJI) using a multiplex PCR assay; sonicate fluid multiplex PCR was more sensitive than tissue culture (P = .04) and, among patients receiving antibiotics in the 2 weeks before surgery, had higher sensitivity than sonicate fluid culture (88% and 70%, respectively; P = .01) [6]. Because multiplex PCR can only detect predefined targets, however, not all pathogens are detected.
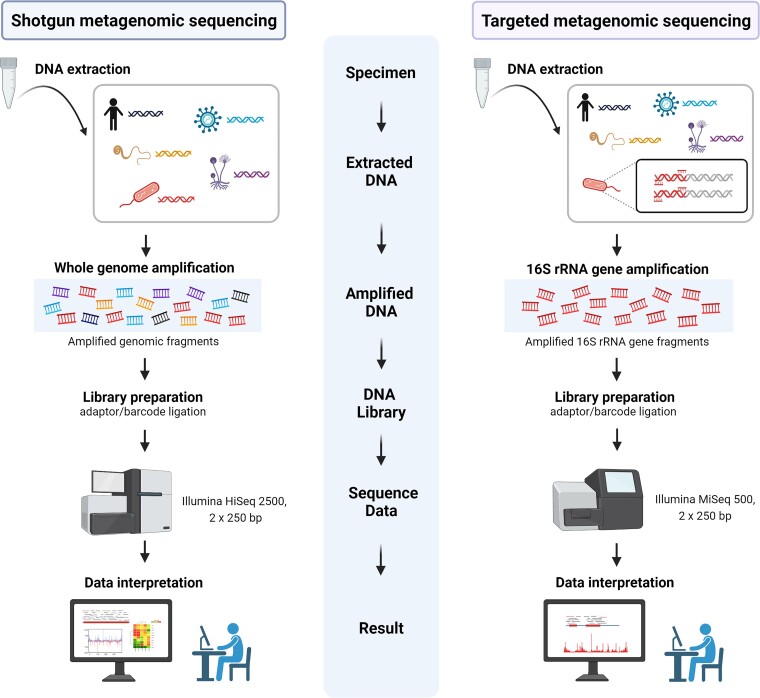
Next-generation sequencing (NGS) interrogates thousands to billions of DNA fragments in parallel, allowing microbial detection and identification using comprehensive reference databases and appropriate quality practices. Two general NGS approaches are applied to PJI: 16S rRNA gene-based targeted and shotgun metagenomic sequencing (sNGS) (Figure 1). The 2 approaches are increasingly used for diagnosis of many infectious diseases, including PJI, sepsis, and infections with unusual pathogens [7–12]. 16S rRNA gene-based targeted metagenomic sequencing (tNGS), which revolutionized study of the human gastrointestinal microbiome more than a decade ago [13, 14], enriches a specific microbial sequence region before library preparation, potentially maximizing sensitivity and shortening turnaround time. We showed that 16S rRNA gene-based tNGS on sonicate fluid from 105 patients (47 with PJI) undergoing revision arthroplasty was more sensitive than culture (85% vs 77%, respectively) with similar specificity (98% vs 100%, respectively) for elbow PJI diagnosis [8]. This approach showed low false positivity and good concordance with culture when applying strict diagnostic criteria. Additionally, it improved turnaround time; however, it is unable to identify fungi, viruses, or parasites. sNGS sequences all nucleic acids in given specimens, providing unbiased microorganism detection. We previously reported analysis of sNGS on 408 sonicate fluid samples (213 with PJI) generated from resected prostheses [7]. sNGS detected microorganisms identified by culture, as well as those not detected by culture and enabled discovery of a novel cause of PJI, Metamycoplasma salivarium [15]. Nevertheless, sNGS has limitations, including sequencing human and contaminant microbial DNA, high cost, long turnaround time, and complex data analysis. Recent reports suggest potential utility of NGS-based metagenomic approaches as PJI diagnostic tools [7–10, 16–18]. Here, we compared the diagnostic performance of 16S rRNA gene-based tNGS analysis of 395 sonicate fluid samples generated from removed hip and knee arthroplasties to sNGS performed from our previous study (Figure 1) [7].
Figure 1.
Workflow comparing shotgun and targeted metagenomic sequencing. This figure was created using BioRender.
METHODS
Samples
Sonicate fluid samples derived from patients who underwent resection of prosthetic hip or knee components between 2011 and 2016 from a previous sNGS study were studied [7]; samples were collected under Mayo Clinic Institutional Review Board protocol 09-00808. Sonicate fluids were prepared using a vortexing/sonication method described in the Supplementary Methods and stored at –80°C.
Definitions
Samples were classified as PJI or NIAF according to Infectious Diseases Society of America (IDSA) diagnostic criteria [19]. Sonicate fluid cultures with ≥20 colony-forming units/10 mL were considered culture-positive. Tissue cultures were considered positive if 2 or more cultures grew the same microorganism. When comparing results of polymicrobial detections, if the portfolio of microorganisms detected by the 2 methods was not identical, results were considered discordant.
Nucleic Acid Extraction and PCR Amplification for tNGS
DNA was extracted on a MagNA Pure 96 system (Roche Diagnostics). Extracted DNA was amplified by PCR on a LightCycler 480II (Roche Diagnostics) using dual priming oligonucleotides targeting the 16S rRNA V1-V3 regions. See Supplementary Methods for details.
16S rRNA Gene-based tNGS
tNGS was done as previously described [8]. PCR products were processed according to the manufacturer’s protocol for library preparation, normalization, and sequencing (Illumina 2013). Libraries were sequenced using a 2 × 250 V2 nano kit on an Illumina MiSeq (Illumina). Subsequently, demultiplexed results were analyzed using Pathogenomix (Santa Cruz, CA) bioinformatics software for microorganism identification. See Supplementary Methods for details.
tNGS Interpretation
Identification was reported when the sequence was a clear match, not detected in the negative control, and not a known contaminant (Supplementary Table 1). In addition, reportable microorganism abundance had to be >10% of total read abundance. The BLAST tool in National Center for Biotechnology Information was used to search for matches to sequences not identified and to obtain more detailed taxomonic information. See Supplementary Methods for details.
Statistical Analysis
Data were analyzed using MedCalc statistical Software (version 18.11.3). Because there is no reference standard to compare with NGS, results were described in terms of positive percent agreement (PPA) and negative percent agreement (NPA) rather than sensitivity and specificity [20]. PPA and NPA of sonicate fluid culture, tissue culture, tNGS, and sNGS, determined based on whether the test reflected a PJI diagnosis, were compared using McNemar test. Receiver operating characteristic (ROC) curve analysis was performed, and area under the ROC curve (AUC) calculated to evaluate diagnostic accuracy of different methods. A P value of less than .05 was considered statistically significant.
RESULTS
A total of 408 sonicate fluids from the same subjects in our previous sNGS study was considered for inclusion in the study. Thirteen were excluded because insufficient specimen was available for analysis. Finally, 395 subjects were included in the study, of whom 208 (53%) were categorized as having PJI and 187 (47%) as having NIAF (Supplementary Figure 1). Demographic and clinical characteristics and classification criteria are summarized in Table 1. Among PJI subjects, 150 (72%, 150/208) tested tNGS positive; 2 NIAF subjects (1%, 2/187) also tested tNGS positive. Microorganisms detected by tNGS are summarized in Supplementary Table 2. A total of 172 microorganisms were detected by tNGS from 152 cases, including 17 cases with polymicrobial detections (Supplementary Table 3).
Table 1.
Demographic and Clinical Characteristics of Infected and Noninfected Patients
| Characteristics | NIAF (n = 187) |
PJI (n = 208) |
|---|---|---|
| Age, y, median (range) | 65 (18–89) | 66 (30–93) |
| Male, No. (%) | 84 (44.9) | 112 (53.8) |
| Site of arthroplastya, No. (%) | … | … |
| ȃKnee | 148 (79.1) | 122 (58.7) |
| ȃHip | 39 (20.9) | 86 (41.3) |
| Underlying joint disordera, No. (%) | … | … |
| ȃOsteoarthritis | 131(70.1) | 157 (75.5) |
| ȃBone fracture or trauma | 24 (12.8) | 19 (9.1) |
| ȃInflammatory joint disorderb | 11 (5.9) | 13 (6.3) |
| ȃAvascular bone necrosis | 3 (1.6) | 1 (0.5) |
| ȃOthersc | 26 (13.9) | 21 (10.1) |
| Underlying risks, No. (%) | … | … |
| ȃBMI >30 | 114 (61.0) | 126 (60.6) |
| ȃDiabetes mellitus | 19 (10.2) | 43 (20.7) |
| ȃLong-term use of immunosuppressive agent therapy | 9 (4.8) | 14 (6.7) |
| IDSA PJI criteria, No. | … | … |
| ȃPurulence visible intraoperatively | 0 | 129 |
| ȃSinus tract | 0 | 50 |
| ȃTwo positive cultures of the same microorganism | 0 | 107 |
| ȃAcute inflammation on histopathology | 2 of 163 | 111 of 143 |
| Antibiotics within 4 wks before surgery, No. (%) | 10 (5.3) | 128 (61.5) |
| Laboratory, median (IQR) | … | … |
| ȃHemoglobin (g/dL) | 13.6 (12.5–14.7) | 11.7 (10.4–13.0) |
| ȃLeukocyte count (109/L) | 6.9 (5.8–8.3) | 7.8 (6.4–9.8) |
| ȃErythrocyte sedimentation rate (mm/h) | 9 (4–19) | 4122 (24–60) |
| ȃC-reactive protein (mg/L) | 3.8 (3.0–7.7) | 32 (13.3–60.2) |
Abbreviations: BMI, body mass index; IDSA, Infectious Diseases Society of America; NIAF, non-infectious arthroplasty failure; PJI, periprosthetic joint infection.
The patients had 1 or more underlying joint disorders.
Inflammatory joint disorders include rheumatoid arthritis and psoriasis.
Other reasons for underlying joint disorder include polymyalgia rheumatica, osteosarcoma, septic arthritis, developmental dysplasia, and unknown reasons.
Performance of 16S rRNA tNGS Compared with Sonicate Fluid Culture
Compared with sonicate fluid culture, PPA of 16S rRNA tNGS was higher (72.1% vs 52.9%, P < .001), although notably the cohort studied was enriched for culture-negative cases. NPA of the 2 methods was not different (98.9% and 100%, P = .16) (Table 2).
Table 2.
Comparison of Diagnostic Performance of Microbiologic Tests
| Methods | Positive Number | PPA (%) (95% CI) |
NPA (%) (95% CI) |
|
|---|---|---|---|---|
| PJI (n = 208) |
NIAF (n = 187) |
|||
| SF culture | 110 | 0 | 52.9 (46.1─59.6) |
100 (98.0─100) |
| Tissue culture | 107 | 0 | 51.4 (44.7─58.2) |
100 (98.0─100) |
| SF tNGS | 150 | 2 | 72.1 (65.7─77.8) |
98.9 (96.2─99.8) |
| SF sNGS | 152 | 7 | 73.1 (66.7─78.7) |
96.3 (92.5─98.2) |
Abbreviations: CI, confidence interval; NIAF, noninfectious arthroplasty failure; NPA, negative percent agreement; PJI, periprosthetic joint infection; PPA, positive percent agreement; SF, sonicate fluid; sNGS, shotgun metagenomic sequencing; tNGS, 16S ribosomal RNA gene-based targeted metagenomic sequencing.
Sonicate Fluid Culture-Positive PJI
Of 110 culture-positive PJI cases, microorganisms were detected by tNGS in 103 (93.6%), including a single microorganism detected in 88 subjects and multiple microorganisms in 15. The most common microorganism identified with both methods was Staphylococcus species. tNGS results were concordant with sonicate fluid culture in 90 cases (81.8%), with additional potential pathogens detected in 9 (8.2%) cases. Three of these 9 were known polymicrobial infections based on culture.
In 12 (10.9%) culture-positive PJI cases, the microorganism isolated in culture was not detected using tNGS (Table 3). Five were polymicrobial infections in which at least 1 microorganism was found in tNGS. The other 7 cases included 2 Candida albicans PJI cases, 2 Staphylococcus epidermidis PJI cases, and 1 case each of Mycobacterium abscessus, Corynebacterium jeikeium, and Cutibacterium acnes PJI (Supplementary Figure 2). Two of the 5 bacterial cases had been treated with antibiotics before surgery.
Table 3.
Performance of Sonicate Fluid 16S Ribosomal RNA Gene-based Targeted Metagenomic Sequencing (tNGS) Versus Sonicate Fluid Culture
| Case Classification | Number of Samples | Identical Findings | New Microorganisms Detected by tNGS | Organisms not Identified by tNGS |
|---|---|---|---|---|
| NIAF | 187 | 185 (98.9%) | 2 (1.1%) | NA |
| Culture-positive PJI | 110 | 90 (81.8%) | 9 (8.2%) | 12 (10.9%) |
| Culture-negative PJI | 98 | 51 (52.0%) | 47 (48.0%)a | NA |
Data shown are the no. (%) of samples in which identical findings or discrepant findings between sonicate fluid tNGS and sonicate fluid culture were observed.
Abbreviations: NA, not applicable; NIAF, noninfectious arthroplasty failure; PJI, periprosthetic joint infection.
Forty-nine microorganisms were detected by tNGS in 47 culture-negative PJIs.
Sonicate Fluid Culture-Negative PJI
Among the 98 culture-negative PJI cases, 49 potential pathogens were found by tNGS in 47 subjects (48.0%) (Table 3). Commonly identified microorganisms were Staphylococcus aureus complex (13/49, 26.5%), Streptococcus agalactiae (4/49, 8.2%), and Streptococcus dysgalactiae (4/49, 8.2%). Of the new identifications, 12 had the same microorganisms identified by intraoperative tissue culture. tNGS detected potential pathogens in 35 culture-negative PJI cases.
Performance of 16S rRNA tNGS Compared with sNGS
Results of sNGS have been published [7]. sNGS on sonicate fluid was positive in 152 (73.1%) PJIs and 7 (3.7%) NIAFs (Table 2). PPA of tNGS and sNGS of sonicate fluid were 72.1 and 73.1%, respectively (P = .83), and NPA were 98.9% and 96.3%, respectively (P = .09).
Sonicate Fluid Culture-Positive PJI
Of 110 culture-positive PJI cases, the 2 metagenomic methods detected microorganisms in 92 (83.6%) cases (Table 4). Six microorganisms (5.5%) were identified by tNGS, but not by sNGS. Three were polymicrobial infections with at least 1 microorganism was found by both tNGS and sNGS. For 2 cases, Pseudomonas aeruginosa was found by sonicate fluid culture, intraoperative tissue culture, and tNGS, but not sNGS. In one case of S. epidermidis PJI, tNGS additionally detected S. aureus complex (Supplementary Figure 3).
Table 4.
Performance of Sonicate Fluid 16S Ribosomal RNA Gene-based Targeted Metagenomic Sequencing (tNGS) Versus Sonicate Fluid Shotgun Metagenomic Sequencing (sNGS)
| Case Classification | Number of Samples | Identical Findings | Organisms Identified by tNGS but not sNGS | Organisms Identified by sNGS but not tNGS |
|---|---|---|---|---|
| NIAF | 187 | 178 (95.2%) | 2 (1.1%) | 7 (3.7%) |
| Culture-positive PJI | 110 | 92 (83.6%) | 6 (5.5%) | 13 (11.8%)a |
| Culture-negative PJI | 98 | 76 (77.6%) | 12 (12.2%)b | 11 (11.2%)c |
Data are the no. (%) of samples in which identical findings or discrepant findings between sonicate fluid tNGS and sonicate fluid sNGS were observed.
Abbreviations: NIAF, noninfectious arthroplasty failure; PJI, periprosthetic joint infection.
Fourteen microorganisms detected by sNGS but not by tNGS in 13 cases.
Thirteen microorganisms detected by tNGS but not by sNGS in 12 culture-negative PJIs.
Thirteen microorganisms detected by sNGS but not by tNorGS in 11 culture-negative PJIs.
In 13 cases (11.8%), microorganisms identified by sNGS were not identified by tNGS. In 8 of 13 cases, at least 1 microorganism was found by both culture and tNGS, with more microorganisms detected using sNGS. For the other 5 cases (3 S. epidermidis, 1 C. albicans, and 1 C. acnes PJI), undetected microorganisms by tNGS were found by culture and sNGS.
Sonicate Fluid Culture-Negative PJI
Among the 98 culture-negative PJI cases, potential pathogens were detected in 47 (48.0%) by tNGS and 45 (45.9%) by sNGS. In the 76 cases (77.6%), metagenomic results were concordant (Table 4). Microorganisms identified by both metagenomic approaches included known PJI pathogens; the most commonly identified microorganism was Staphylococcus species (35.3%, 12/34). Twelve cases (12.2%) had positive tNGS results, but were negative both by sNGS and culture. In 2 cases, the same microorganisms (1 case each of S. aureus and P. aeruginosa) were also identified in intraoperative tissue culture.
In 11 cases, microorganisms were detected by sNGS but not by tNGS or culture. Three were Candida cases, in 2 of which the same microorganisms were identified by intraoperative tissue cultures. There were 42 culture-negative cases in which both tNGS and sNGS were negative; intraoperative tissue culture was positive in 4 cases.
Analysis of NIAF Cases
Of 187 NIAF cases, microorganisms were suggested in 9 cases by NGS; 2 by tNGS and 7 by sNGS (Table 4). The two microorganisms detected by tNGS were found in specimens yielding cycle threshold values <34 cycles and a high abundance relative to total reads (96.8% for Bacteroides fragilis and 97.8% for Streptococcus mitis group). The 7 potential pathogens (3 S. aureus, 2 Streptococcus sanguinis, and 2 C. acnes) detected by sNGS were described previously [7].
Comparison of Diagnostic Values of Different Methods
Diagnostic performance of the different methods was assessed using ROC analysis. AUC values of sonicate fluid culture, tissue culture, tNGS, and sNGS were 0.764 (95% confidence interval [CI], .719–.805), 0.757 (95% CI, .712–.799), 0.855 (95% CI, .817–.888), and 0.847 (95% CI, .807–.881), respectively (Figure 2). Both tNGS and sNGS showed AUCs >0.80, higher than sonicate fluid culture and tissue culture. By pairwise comparison, the AUC of tNGS was higher than that of sonicate fluid and tissue culture (P < .001 for both); however, the difference between tNGS and sNGS was not statistically significant (P = .559).
Figure 2.
ROC curves for different diagnostic methods. Abbreviations: AUC, area under the ROC (receiver operating characteristic) curve; SF, sonicate fluid; sNGS, shotgun metagenomic sequencing; tNGS, 16S ribosomal RNA gene-based targeted metagenomic sequencing.
Analysis of Antimicrobial Exposure
Antibiotic treatment had been administrated before surgery in 128 PJI cases, among which sonicate fluid culture was positive in 64 and tNGS in 96 (P < .0001).
DISCUSSION
PJI diagnosis can be challenged by the presence of nonviable and/or nonculturable microorganisms. Metagenomic sequencing is providing new tools for defining potential causative microorganisms of PJI [7–10, 15–18]. There are 2 common metagenomic approaches, tNGS and sNGS; to our knowledge, they have not been previously compared in PJI. The diagnostic value of 16S rRNA gene-based tNGS was compared with sNGS for PJI detection. It was shown that tNGS could be a potential diagnostic tool for pathogen identification in culture-negative PJI, with similar performance characteristics to sNGS. There was no significant difference in PPA observed between tNGS and sNGS (72.1% vs 73.1%, P = .83); tNGS identified potential pathogens in 48.0% of culture-negative PJI cases, similar to sNGS (45.9%, P = .77).
There have been several studies exploring the diagnostic value of NGS for hip or knee PJI [7, 9, 10, 17, 18]. Although these studies applied different diagnostic criteria, sample types, and sequencing methods, they show NGS analysis to be more sensitive compared with conventional culture in detecting potential PJI pathogens. The sensitivity and specificity of NGS on sonicate fluid and/or synovial fluid have been reported to be between 63% and 96% and 73% and 96%, respectively. Tarabichi et al published a study using tNGS to diagnose PJI [10]. They compared tNGS with culture performed on synovial fluid and/or tissue in 65 hip or knee revision arthroplasties and showed tNGS to be more sensitive (89%) than culture, but specificity was low (73%). The high false-positivity rate was due to reporting of any bacteria detected by sequencing. In this study, expert interpretation was performed with a clear definition of positive and negative, yielding lower false positivity (2 of 187).
Additionally, the possibility of combining sonicate fluid culture, tissue culture, and each of tNGS or sNGS was evaluated (Supplementary Table 4). When sonicate fluid culture, tissue culture, and tNGS were evaluated in combination, PPA was higher (78.9%) than for individual tests and NPA was similar (98.9%) to individual tests.
Because NGS is culture-independent, it can detect difficult-to-detect microorganisms if microbial DNA is present in the specimen. Detection rates using NGS have been reported to range from 9% to 100% in culture-negative PJI [7, 9, 10, 17, 18]. In the present study, tNGS detected a microorganism in 48.0% of culture-negative PJIs. The tNGS detection rate was 75.0% (96/128) among PJIs with prior antibiotic exposure and 56.3% (36/64) in culture-negative PJIs with preoperative antibiotic treatment; tNGS was less affected by previous antibiotic administration than was sonicate fluid culture (P < .0001).
Microorganisms have been suspected to play a potential role in prosthetic joint failure believed to be aseptic. There were 2 interesting cases in which potential pathogens were detected by tNGS in the NIAF group. One patient with B. fragilis had been on clindamycin as chronic oral antimicrobial suppression for 4 years because of prior right THA infection with Prevotella loescheii with no evidence of infection recurrence before revision surgery. Bacteroides loescheii was renamed as P. loescheii and there is a possibility that the B. fragilis identified by sequencing was misidentified in previous testing as P. loescheii (or was copresent with P. loescheii but not recovered in culture). The other case with S. mitis group also had a long history of THA infection with a viridans group Streptococcus species and had been treated with cephalexin for a year through revision surgery. There was no evidence of infection at revision surgery; the tNGS result may represent residual bacteria or DNA.
Whereas the 16S rRNA NGS only allows detection of bacteria, sNGS can also identify fungi, viruses, and parasites. In the present study, there were 3 fungal PJI cases in which microorganisms were detected by sNGS, but unsurprisingly not by tNGS; 2 cases of C. albicans were also detected by sonicate fluid culture and in intraoperative tissue culture. One case of Candida parapsilosis PJI had <20 colony-forming units/10 mL in sonicate fluid culture and was detected in intraoperative tissue culture. This 16S rRNA gene sequencing limitation might be assessed by combining tNGS with microbial culture or by incorporating a fungal sequencing target.
Of note, most reads generated from sNGS were human, requiring steps for microbial enrichment. Only specific bacterial regions of interest are sequenced using the described 16S rRNA gene-based approach; data analysis is more complex with sNGS compared with tNGS [21]. There were 6 culture-positive PJI cases detected by tNGS, but not sNGS. Of these, 2 were P. aeruginosa PJI with the organism also found in sonicate fluid and tissue culture. In our previous study, we determined that certain P. aeruginosa strains might be degraded during the MoIYsis microbial enrichment step, a tool used to enhance sNGS results that was not used for tNGS. Additionally, tNGS has a turnaround time of approximately 2 days, compared with sNGS which takes 2 to 6 days [7]. tNGS may be a more cost-effective way for NGS uptake in clinical laboratories because of the more labor-intensive workflow and high reagent cost associated with sNGS.
One NGS challenge is the possibility of false positivity from background bacteria and contamination; it can be difficult to distinguish between infection, colonization, and contamination [21]. Standardized NGS bioinformatics analyses and data interpretation is needed. Because of the potential for false-positive results, metagenomic techniques require well-trained laboratorians for appropriate result interpretation.
There are some limitations to this study. This study had a retrospective design, studying samples that had been stored for several years. Prospective studies are needed to negate the effect of specimen age on results. The IDSA diagnostic criteria are imperfect and may have resulted in case misclassification. The data set was enriched for culture-negative cases. The lack of significant differences in PPA and NPV between tNGS and sNGS may be the result of type II error. Additionally, a limitation of the tNGS technique is that amplified DNA may not be generated from highly fragmented starting material (eg, pathogen DNA with a fragmented 16S rRNA gene V1-V3 region); sNGS may be better suited for fragmented samples.
In conclusion, there was no difference in performance between tNGS and sNGS for PJI diagnosis. With the advantages of turnaround time, cost, and ease of data analysis compared with sNGS, tNGS may be a preferred supplement to culture-based methods, when conventional culture fails to yield a pathogen.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Hyo-Lim Hong, Department of Laboratory Medicine and Pathology, Division of Clinical Microbiology, Mayo Clinic, Rochester, Minnesota, USA; Department of Internal Medicine, Daegu Catholic University School of Medicine, Daegu, Republic of Korea.
Laure Flurin, Department of Laboratory Medicine and Pathology, Division of Clinical Microbiology, Mayo Clinic, Rochester, Minnesota, USA; Department of Intensive Care, University Hospital of Guadeloupe, Pointe-à-Pitre, France.
Matthew J Thoendel, Division of Public Health, Infectious Diseases, and Occupational Medicine, Mayo Clinic, Rochester, Minnesota, USA.
Matthew J Wolf, Department of Laboratory Medicine and Pathology, Division of Clinical Microbiology, Mayo Clinic, Rochester, Minnesota, USA.
Matthew P Abdel, Department of Orthopedic Surgery, Mayo Clinic, Rochester, Minnesota, USA.
Kerryl E Greenwood-Quaintance, Department of Laboratory Medicine and Pathology, Division of Clinical Microbiology, Mayo Clinic, Rochester, Minnesota, USA.
Robin Patel, Department of Laboratory Medicine and Pathology, Division of Clinical Microbiology, Mayo Clinic, Rochester, Minnesota, USA; Division of Public Health, Infectious Diseases, and Occupational Medicine, Mayo Clinic, Rochester, Minnesota, USA.
Notes
Acknowledgments. We are grateful for the assistance of the Clinical Microbiology Laboratory staff at the Mayo Clinic (Rochester, Minnesota). We also thank Sang Gyu, Kwak (Department of Medical Statistics, Daegu Catholic University School of Medicine) for assistance with statistical analysis.
Financial support. Research reported in this publication was supported in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award number R01AR056647. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1. Premkumar A, Kolin DA, Farley KX, et al. Projected economic burden of periprosthetic joint infection of the hip and knee in the United States. J Arthroplasty 2021; 36:1484–9.e3. [DOI] [PubMed] [Google Scholar]
- 2. Tande AJ, Patel R. Prosthetic joint infection. Clin Microbiol Rev 2014; 27:302–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peel TN, Dylla BL, Hughes JG, et al. Improved diagnosis of prosthetic joint infection by culturing periprosthetic tissue specimens in blood culture bottles. mBio 2016; 7:e01776-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Trampuz A, Piper KE, Jacobson MJ, et al. Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med 2007; 357:654–63. [DOI] [PubMed] [Google Scholar]
- 5. Gomez E, Cazanave C, Cunningham SA, et al. Prosthetic joint infection diagnosis using broad-range PCR of biofilms dislodged from knee and hip arthroplasty surfaces using sonication. J Clin Microbiol 2012; 50:3501–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cazanave C, Greenwood-Quaintance KE, Hanssen AD, et al. Rapid molecular microbiologic diagnosis of prosthetic joint infection. J Clin Microbiol 2013; 51:2280–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thoendel MJ, Jeraldo PR, Greenwood-Quaintance KE, et al. Identification of prosthetic joint infection pathogens using a shotgun metagenomics approach. Clin Infect Dis 2018; 67:1333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Flurin L, Wolf MJ, Greenwood-Quaintance KE, Sanchez-Sotelo J, Patel R. Targeted next generation sequencing for elbow periprosthetic joint infection diagnosis. Diagn Microbiol Infect Dis 2021; 101:115448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ivy MI, Thoendel MJ, Jeraldo PR, et al. Direct detection and identification of prosthetic joint infection pathogens in synovial fluid by metagenomic shotgun sequencing. J Clin Microbiol 2018; 56:e00402-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tarabichi M, Shohat N, Goswami K, et al. Diagnosis of periprosthetic joint infection: the potential of next-generation sequencing. J Bone Joint Surg Am 2018; 100:147–54. [DOI] [PubMed] [Google Scholar]
- 11. Rodino KG, Wolf MJ, Sheldon S, et al. Detection of tick-borne bacteria from whole blood using 16S ribosomal RNA gene PCR followed by next-generation sequencing. J Clin Microbiol 2021; 59:e03129-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fida M, Wolf MJ, Hamdi A, et al. Detection of pathogenic bacteria from septic patients using 16S ribosomal RNA gene-targeted metagenomic sequencing. Clin Infect Dis 2021; 73:1165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dethlefsen L, Huse S, Sogin ML, et al. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol 2008; 6:e280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu GD, Lewis JD, Hoffmann C, et al. Sampling and pyrosequencing methods for characterizing bacterial communities in the human gut using 16S sequence tags. BMC Microbiol 2010; 10:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thoendel M, Jeraldo P, Greenwood-Quaintance KE, et al. A novel prosthetic joint infection pathogen, Mycoplasma salivarium, identified by metagenomic shotgun sequencing. Clin Infect Dis 2017; 65:332–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Namdari S, Nicholson T, Abboud J, et al. Comparative study of cultures and next-generation sequencing in the diagnosis of shoulder prosthetic joint infections. J Shoulder Elbow Surg 2019; 28:1–8. [DOI] [PubMed] [Google Scholar]
- 17. Wang CX, Huang ZD, Fang XY, Li WB, Yang B, Zhang WM. Comparison of broad-range polymerase chain reaction and metagenomic next-generation sequencing for the diagnosis of prosthetic joint infection. Int J Infect Dis 2020; 95:8–12. [DOI] [PubMed] [Google Scholar]
- 18. Kildow BJ, Ryan SP, Danilkowicz R, et al. Next-generation sequencing not superior to culture in periprosthetic joint infection diagnosis. Bone Joint J 2021; 103b:26–31. [DOI] [PubMed] [Google Scholar]
- 19. Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2013; 56:e1–25. [DOI] [PubMed] [Google Scholar]
- 20. McAdam AJ. Sensitivity and specificity or positive and negative percent agreement? A micro-comic strip. J Clin Microbiol 2017; 55:3153–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chiang AD, Dekker JP. From the pipeline to the bedside: advances and challenges in clinical metagenomics. J Infect Dis 2020; 221:S331–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.