Abstract
The aetiology of nodding syndrome remains unclear, and comprehensive genotyping and phenotyping data from patients remain sparse. Our objectives were to characterize the phenotype of patients with nodding syndrome, investigate potential contributors to disease aetiology, and evaluate response to immunotherapy.
This cohort study investigated members of a single-family unit from Lamwo District, Uganda. The participants for this study were selected by the Ugandan Ministry of Health as representative for nodding syndrome and with a conducive family structure for genomic analyses. Of the eight family members who participated in the study at the National Institutes of Health (NIH) Clinical Center, three had nodding syndrome.
The three affected patients were extensively evaluated with metagenomic sequencing for infectious pathogens, exome sequencing, spinal fluid immune analyses, neurometabolic and toxicology testing, continuous electroencephalography and neuroimaging. Five unaffected family members underwent a subset of testing for comparison. A distinctive interictal pattern of sleep-activated bursts of generalized and multifocal epileptiform discharges and slowing was observed in two patients. Brain imaging showed two patients had mild generalized cerebral atrophy, and both patients and unaffected family members had excessive metal deposition in the basal ganglia. Trace metal biochemical evaluation was normal. CSF was non-inflammatory and one patient had CSF-restricted oligoclonal bands. Onchocerca volvulus-specific antibodies were present in all patients and skin snips were negative for active onchocerciasis. Metagenomic sequencing of serum and CSF revealed hepatitis B virus in the serum of one patient. Vitamin B6 metabolites were borderline low in all family members and CSF pyridoxine metabolites were normal. Mitochondrial DNA testing was normal. Exome sequencing did not identify potentially causal candidate gene variants.
Nodding syndrome is characterized by a distinctive pattern of sleep-activated epileptiform activity. The associated growth stunting may be due to hypothalamic dysfunction. Extensive testing years after disease onset did not clarify a causal aetiology. A trial of immunomodulation (plasmapheresis in two patients and intravenous immunoglobulin in one patient) was given without short-term effect, but longer-term follow-up was not possible to fully assess any benefit of this intervention.
Keywords: epilepsy, global health, genetic, autoimmune, infectious
Soldatos et al. describe the deep phenotyping of a family with nodding syndrome, a cryptogenic childhood-onset epileptic encephalopathy identified in Northern Uganda and South Sudan. They investigate potential contributors to disease aetiology and evaluate the short-term response to immunotherapy.
Introduction
Nodding syndrome is a distinct childhood-onset epileptic encephalopathy occurring in temporal and geographical clusters in the Republic of South Sudan, Uganda and Tanzania.1 The disease is characterized by head nodding episodes, defined as repetitive involuntary drops of the head on at least two occasions.1 Video EEG recordings have suggested that these episodes are most consistent with atonic seizures2; however, many patients develop other seizure types over time.3 In addition to seizures, affected children may develop associated psychomotor decline and growth retardation.4
Extensive epidemiological investigations have revealed an association between nodding syndrome and the presence of antibodies to the parasite Onchocerca volvulus.1,5–8 Recent cohort studies revealed a temporal and dose-dependent correlation between O. volvulus infection and subsequent development of epilepsy in paediatric patients.9,10 Interventions by the government of Uganda to reduce both the vector and parasite are correlated to reductions in the incidence of nodding syndrome, the overall incidence of epilepsy11 and the lack of emergence of new cases of nodding syndrome.12 Although an association between epilepsy and O. volvulus infection has been purported for over 50 years,13–17 direct evidence of a causal relationship between O. volvulus and epilepsy is lacking. Further, the clinical phenotype of nodding syndrome has never been fully characterized.
Therefore, to both further characterize the clinical manifestations of nodding syndrome and investigate its underlying aetiology, a collaborative effort between the Ministry of Health in Uganda and the United States National Institutes of Health (NIH) and Centers for Disease Control and Prevention (CDC) was initiated to study an affected family at the NIH Clinical Center (NIH-CC). Here we describe the in-depth clinical phenotyping, immunological profiling and genotyping of three persons affected with nodding syndrome.
Materials and methods
Patient selection and clinical samples
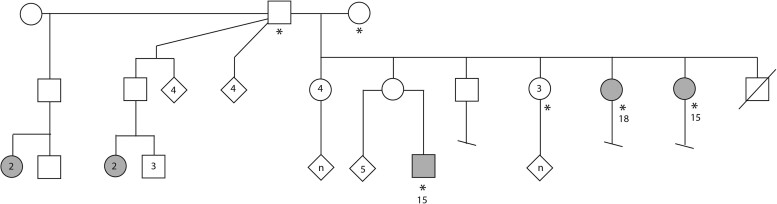
Clinicians at the NIH-CC evaluated a family of the Acholi tribe of Northern Uganda that was selected by the Ugandan Ministry of Health as representative for nodding syndrome and had a family structure conducive for genetic analysis (Fig. 1). Due to regional conflict, in 1997 this family left their village and lived in camps for internally displaced persons. This unprecedented study involved a huge amount of effort and planning over several months. A formal plan for this project was written and entitled, ‘Proposal to Evaluate Families with nodding syndrome at the National institutes of Health: A collaborative effort between the Center for Disease Control, National Institutes of Health and Uganda’. As noted below, the consenting process for subjects was performed repeatedly. Following the official policy of the National Human Genome Research Institute’s Institutional Review Board (IRB), a short form of the consent was translated into Acholi, certified and signed by the subjects. The consenting process was managed in close consultation with the IRB Chair. IRB approval was also obtained from the United States Center for Disease Control and Prevention and the Uganda Joint Clinical Research Center.
Figure 1.
Pedigree of family unit examined at the NIH. The symbols with an asterisk indicate those individuals examined at the NIH. These include the patriarch and matriarch of the family, three unaffected daughters, two daughters with nodding syndrome and one grandson with nodding syndrome. Open symbols are unaffected individuals and shaded symbols are those with nodding syndrome. Circles indicate female, squares indicate males and the diamond icons indicate gender not specified. Numbers within symbol indicate the number of individuals represented by that shape and the letter ‘n’ indicates an unknown number, but more than one, of individuals being represented by that shape. Numbers outside the circle indicate age of the patient when seen at the NIH.
The family members were enrolled in protocol 76-HG-0238, ‘Diagnosis and Treatment of Patients with Inborn Errors of Metabolism or Other Genetic Disorders’, as part of the National Human Genome Research Institute (NGHRI) Undiagnosed Diseases Program.18–20 The protocol was approved by the NGHRI IRB at the NIH.
Consenting and preparations in Uganda
The CDC officials worked closely with the Ugandan Health Minister and her office and the Ugandan officials were intimately involved in the consent process and the ultimate approval of the investigation. They, in turn, had close interactions with the local community leaders in Gulu to help identify the family and patients for this study. The consenting process involved translation of the entire study in the local language, creation of a flip chart that had drawings of each of the procedures and explanation of all potential side effects, etc. The process was approved by the NIH IRB and the Ugandan Health Ministry and was performed by the Ugandan team. The socioeconomic and educational status of the family represented a significant challenge to ensuring that informed consent was obtained. Hence, in collaboration with our Uganda Ministry of Health colleagues, we took all reasonable measures to ensure that the family understood the process and procedures and that true informed consent was adequately obtained.
Transport to NIH
The patients were accompanied by a local physician (B.O.T.) and a nurse (J.A.-K.) from Uganda who oversaw the entire hospital stay and procedures. CDC made all the logistical arrangements for travel, which included obtaining visas, clothes, luggage and hotel stay in Kampala. The NIH covered the costs for airline travel, hospital stay and all evaluations, as well as on-campus housing for the accompanying family members. NIH officials received the family at the airport and transported them to the NIH Clinical Center.
Consenting and preparation at NIH
When the patients arrived at NIH they were re-consented by the study team using an interpreter and the short consent form translated into Acholi. Members of the IRB were present in the room to observe the consenting process. Clinical protocol 76-HG-0238 is employed by the NIH Undiagnosed Diseases Program and allows for all the investigations and treatments conducted on these patients. This program routinely evaluates international patients, although the arrangements and preparations for bringing the nodding syndrome patients far surpassed anything we had previously encountered. The NIH nutrition department, social workers, nurses and support staff formed a team of nearly 50 people, meeting periodically over 1–2 months prior to the arrival of the patients to discuss each step of the study. The entire team was assigned to the family’s care full-time.
They were admitted to the NIH-CC for 2 weeks. Anthropometrics were assessed using CDC growth charts. Formal neurocognitive assessment was possible in only one of the three patients. Blood, stool, skin biopsies and urine samples were obtained (Supplementary Table 1) for nutritional, metabolic, infectious, immune, toxicology and genetic testing and CSF was analysed for immune profile and neurotransmitters. Neurocognitive assessment was performed on the most mildly affected proband who was able to participate in such testing with the help of an Acholi interpreter.
The patients underwent long-term EEG monitoring and MRI with contrast using a 3 T scanner. Accompanying unaffected family members served as controls and were investigated with EEG and MRI. The brain MRI was performed on a 3 T magnet along with MR spectroscopy. Sagittal T1-weighted, axial T2-weighted, axial diffusion tensor imaging (DTI), axial balanced fast field-echo (BFFE), axial Fluid attenuated inversion recovery (FLAIR), sagittal susceptibility-weighted imaging (SWI) and axial magnetization-prepared rapid gradient echo (MP-RAGE) sequences were obtained. Coronal short tau inversion recovery (STIR), coronal T2-weighted and coronal FLAIR images of the temporal lobes were also acquired. Post-contrast axial FLAIR and sagittal 3D fast field-echo (3D FFE) images were performed. Single voxel point resolved spectroscopy with time of echo (TE) = 38 ms and time of repetition (TR) = 2000 ms was performed at four locations in the brain: superior cerebellar vermis, left centrum semiovale, midline parietal grey matter and left thalamus. As part of their evaluation, each patient underwent long-term EEG monitoring with a 27-channel digital EEG with time-locked video and single-lead EKG. The EEG electrodes were placed according to the internationally accepted 10–20 system of electrode placement. In addition, subtemporal F9, F10, T9, T10, P9 and P10 electrodes were placed for better localization of temporal lobe activity. Background EEG and clinical events were reviewed using at least three standard montages by a board-certified clinical neurophysiologist (S.I.).
Infectious diseases and autoantibody testing
In addition to standard clinical assays, metagenomics was performed on total RNA extracted from 250 μl of serum and 250 μl of CSF from each of the three probands, as well as reference serum samples from the five unaffected family members as previously described21,22 (Supplementary material).
IgG and IgG4 antibodies to the Onchocerca-specific antigens Ov16, OvFAR1 and OvMSA1 and Ov tropomyosin were measured as described previously.1,23,24 Antibodies to human leiomodin-1 and human DJ1 from the sera were determined by immunoblotting (Supplementary material).
DNA sequence analysis
Genomic DNA was extracted from whole blood using the Gentra Puregene Blood kit (Qiagen) based on manufacturer instructions. Single nucleotide polymorphism (SNP) microarray analysis and exome sequencing were performed (Supplementary material).
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its Supplementary material.
Results
Case descriptions
Case 1
This 15-year-old girl was the first child in the family to develop nodding syndrome, in 2007, at approximately 8 years of age. Prenatal history was notable for amniotic fluid leak at 5 months’ gestation. Prior to seizure onset, she was cognitively normal with normal milestones and attended primary school. Shortly after the onset of nodding spells she developed generalized tonic–clonic seizures. She was treated with carbamazepine at age 8 years and valproate since age 13 years. However, she continued to have more than one generalized seizure per week with occasional clusters of up to five seizures in a day. The nodding episodes occurred about once a month and had no triggers. Since 10 years of age, she had progressive cognitive decline as evidenced by loss of independence in her activities of daily living.
Evaluation revealed small stature and microcephaly (Supplementary Table 2). Her neurological examination was notable for encephalopathy; she was not communicative, would not follow directions and had blunted affect, frequent spells of staring and catatonia and behavioural dysregulation with intermittent aggression. Frontal release signs were present. She had multiple breakthrough seizures.
Case 2
This 18-year-old female had failure to thrive since birth with developmental delays (walking and speaking) and learning difficulties resulting in withdrawal from school at Grade 4. Between 5 and 6 years of age, she experienced deterioration in memory. Head nodding began at age 11 years, 3 months after her younger sister (Case 1) developed symptoms. She was treated with carbamazepine for 5 years, followed by valproate. Despite treatment, she experienced generalized tonic–clonic seizures every 2 months and nodding episodes twice weekly triggered by eating and cold temperatures. Since seizure onset, her parents reported a deterioration in physical strength but no further cognitive decline.
Physical examination showed stunted growth, kyphosis, hypophonia, anxious affect, paucity of speech with delayed responses, bilateral dysmetria, end-gaze nystagmus, slow horizontal saccades but normal gait including tandem walking. No nodding spells were captured on EEG despite titration off valproate. She was found to have central adrenal insufficiency and secondary amenorrhea due to hypogonadotropic hypogonadism. Pituitary MRI showed an overall normal pituitary gland size although slightly below average for age (6.6 mm height convex compared to median of 7 mm).25 Bone age was consistent with the chronological age and skeletal survey was normal. This patient’s insulin growth factor type-1 was slightly suppressed and her growth hormone levels were normal, which in the setting of her symmetric short stature was considered consistent with an undernourished state. Prealbumin was low at 16 mg/dl (reference range 20–40 mg/dl.) Total and free carnitine concentrations were moderately reduced, with no accumulation of esterified species, indicative of mild secondary carnitine deficiency.
Case 3
This 15-year-old male had onset of nodding syndrome the same year as his aunts (Case 1 and Case 2), at age 8 years. He was treated with carbamazepine, followed by phenytoin with folic acid. He developed generalized tonic–clonic seizures, which occurred for approximately one year, until 13 years of age when treatment was initiated with valproate. With this treatment the generalized tonic–clonic seizures stopped and the almost daily nodding episodes were reduced to a few times monthly. Growth was relatively spared. He had a mild left upper extremity intention tremor without any other neurological abnormalities. He was the only affected patient able to participate in formal neuropsychological testing (Supplementary Table 3), albeit with the caveat of significant cultural differences, foreign environment, multiple medical procedures and discontinuous schooling.
This patient had two episodes of nodding while at the NIH, neither captured on EEG. One episode preceded a prolonged complex partial seizure; the other occurred as he was eating a meal during an outing. Single-lead EKG showed long pauses up to 9 s during one of the nights. Prolonged Holter cardiac monitoring was normal. Polysomnography showed occasional central apnoeas without significant desaturations. Echocardiogram showed mild–moderate pulmonary valve regurgitation.
Electrophysiology
Case 1
Interictal EEG showed diffusely slow background and multifocal epileptiform discharges. Multiple seizure types were also recorded; 16 semiologically similar seizures occurred consisting of altered awareness, ictal cry and agitation. EEG showed diffuse attenuation at onset evolving to bitemporal beta then alpha activity with somewhat variable evolution. Onset in three of these was maximal in the temporal region, one on the right, one on the left, one bitemporally; one of these proceeded to secondary generalization. Three additional seizures were recorded with different semiologies and electrographic correlates. One electrographic seizure was recorded consisting of diffuse rhythmic theta activity maximal in the bilateral posterior temporal regions. A second was characterized by asymmetric tonic elevation of both upper extremities with superimposed clonic movements, followed by secondary generalization; ictal EEG onset was characterized by rhythmic alpha activity in the right posterior quadrant. A third atypical seizure consisted of several seconds of brief clonic jerks involving all four extremities, which obscured the underlying EEG recording.
Case 2
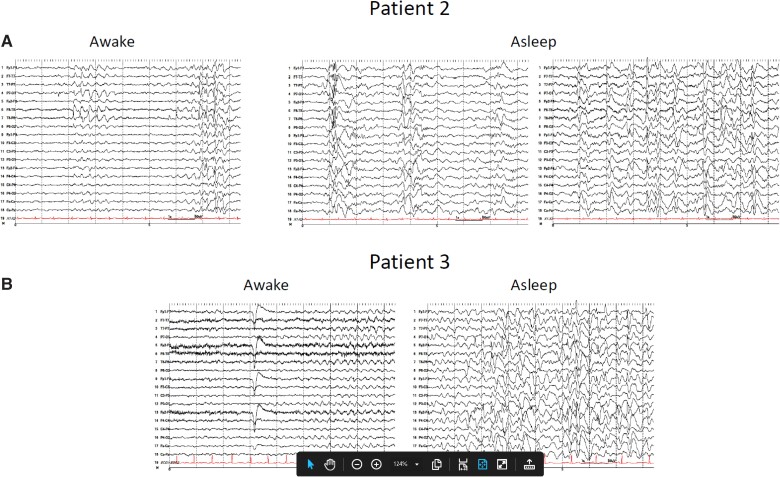
Interictal EEG showed mild to moderate diffuse and/or multifocal background slowing with generalized and multifocal epileptiform discharges occurring most frequently in the temporal regions. Epileptiform activity was significantly activated during sleep (Fig. 2) with frequent bursts of diffuse high-amplitude irregular polymorphic slowing with intermixed poorly localized spikes and polyspike and wave activity lasting up to 90 s. An appearance similar to repetitive epileptiform K complexes or dyshormia, consisting of high-amplitude diffuse slow waves and spindle-like activity with intermixed spikes, was also observed.
Figure 2.
EEG recordings including sleep in patients with nodding syndrome. Patients underwent long-term EEG monitoring with a 27-channel digital EEG with time-locked video and single-lead EKG. The EEG electrodes were placed according to the internationally accepted 10–20 system of electrode placement. In addition, subtemporal F9, F10, T9, T10, P9 and P10 electrodes were placed for better localization. The EEG events were reviewed with at least three standard montages. (A) Interictal EEG performed on Patient 2 while awake (left) shows slow background activity with superimposed focal slowing and sharp waves in the temporal regions, at times becoming more diffuse. While asleep (right), generalized and multifocal spikes and polyspike and wave activity are present, frequently embedded within K complexes. (B) Interictal EEG performed on Patient 3 while awake (left) shows diffuse background slowing, at times with runs of semirhythmic or rhythmic theta activity. While asleep (right), findings similar to Patient 2 are observed.
Case 3
No clinical or electrographic seizures were recorded during 10 days of long-term video-EEG monitoring. However, interictal findings were similar to those of Case 2, with mild to moderate diffuse and/or multifocal background slowing with multifocal epileptiform discharges, most frequently occurring in the left temporal region, with sleep recordings again notable for the characteristic activation of frequent bursts of diffuse high-amplitude irregular polymorphic slowing with intermixed spikes (Fig. 2).
Neuroimaging
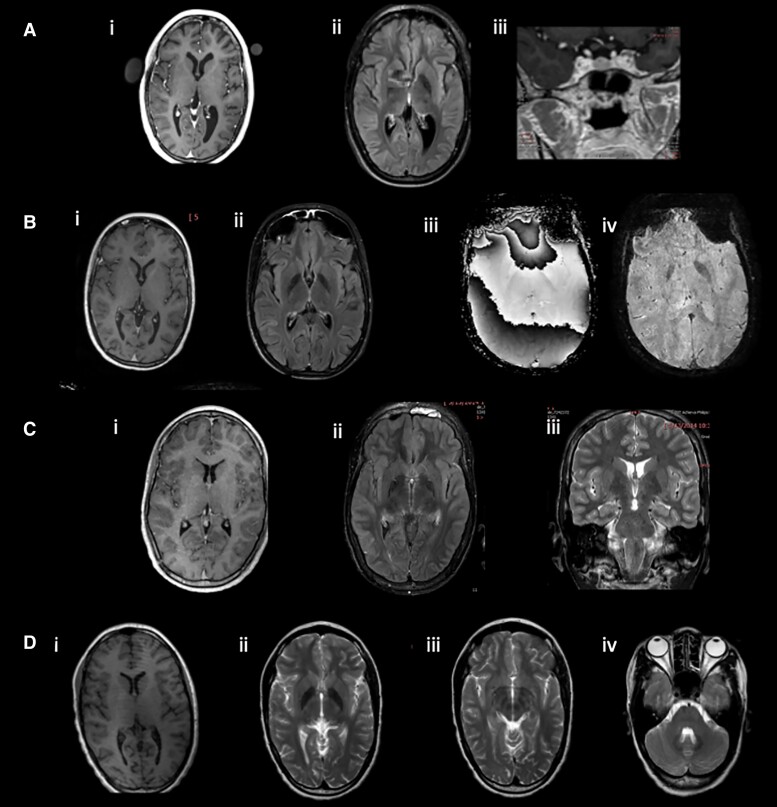
Cases 1 and 2 had mild generalized cerebral atrophy and Case 3 had asymmetric hippocampi with slight atrophy on the left. Case 1 had mild cerebellar atrophy. The patriarch had mild cerebral atrophy greater than typical for age, mild cerebellar atrophy, white matter lesions presumed to arise from small vessel vasculopathy, and a small cyst in the right hippocampus. The matriarch had mild cerebral atrophy greater than expected for age. Similar to the three affected children, all five of the relatives had enhanced deposition of metal in the globi pallidi (Fig. 3), substantia nigra and red nuclei. In the patriarch, the heads of the caudate nuclei and the putamina were involved, while in the other four, the dentate nuclei were involved.
Figure 3.
Brain MRI findings in patients with nodding syndrome and an unaffected sibling. (A) Patient 1: (i) cerebral atrophy and mild colpocephaly; (ii) deposition of metal in globus pallidus more than typical for age; (iii) possible pituitary lesion. (B) Patient 2: (i) slight cerebral atrophy; (ii) deposition of metal in globus pallidus more than typical for age; (iii and iv) this signal change represents metal based upon the direction of the phase shift on SWI sequences. (C) Patient 3: (i) normal cerebral volume; (ii) deposition of metal in globus pallidus more than typical for age; (iii) subtle asymmetry of the hippocampi with left being smaller. (D) Unaffected sibling: (i) minimal bilateral colpocephaly; (i) deposition of metal more than typical for age in globus pallidus; (iii) deposition of metal in the substantia nigra and red nucleus; (iv) deposition of metal in the cerebellar dentate nuclei.
CSF neurotransmitters and metabolites
In all three affected family members, CSF was non-inflammatory, including a normal neopterin which is a surrogate marker of innate immune activation.26 Case 2 had CSF-restricted oligoclonal bands suggestive of intrathecal immune activation (Table 1). Metabolites of serotonin, catecholamines and dopamine were measured in all patients.24 5-Hydroxyindoleacetic acid was decreased in Case 1, while homovanillic acid was decreased in Cases 1 and 2, possibly correlating with some neuronal loss given the mild atrophy on MRI. CSF amino acids, lactate, tetrahydrobiopterin and 5-methyltetrahydrofolate were normal. CSF tau was not elevated in the two cases examined (Case 2, 67.1 pg/ml and Case 3, 84.3 pg/ml, reference healthy donors, 82.5 pg/ml).
Table 1.
Inflammatory and metabolic testing in CSF of patients with nodding syndrome
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| White blood cell count | 3 | 3 | 1 |
| Cytology | Normal | Rare foamy macrophages | Normal |
| Red blood cell count | 0 | 1 | 1 |
| Protein (mg/dl) | 21 | 19 | 15 |
| Glucose (mg/dl) | 63 | 47 | 50 (serum 71) |
| IgG index | Normal | Normal | Normal |
| Albumin quotient | Normal | Low | Low |
| Oligoclonal bands | Negative | Positive | Partially identical in CSF and serum |
| Lactate | Normal | Normal | Normal |
| Amino acid profile | ↑alanine | No diagnostic pattern | No diagnostic pattern |
| 5-Hydroxyindoleacetic acid (ref 67–189 nmol/l) | 35 ↓ | 79 | 95 |
| Homovanillic acid (ref 167–563 nmol/l) | 79 ↓ ↓ | 124 ↓ | 273 |
| 3-O-Methyldopa (ref <100 nmol/l) | 8 | 13 | 11 |
| Neopterin (ref 8–33 nmol/l) | 19 | 15 | 23 |
| Tetrahydrobiopterin (ref 9–32 nmol/l) | 14 | 19 | 18 |
| 5-Methyltetrahydrofolate (ref 40–120 nmol/l) | 49 | 50 | 47 |
| Pyridoxal 5-phosphate (ref 10–37 nmol/l) | 19 | 16 | 18 |
| Biomarkers for folinic acid-/pyridoxine-responsive seizures (Antiquitin, ALDH7A1) | Not detected | Not detected | Not detected |
Toxicology
Trace metals were not increased in urine (antimony, arsenic, barium, beryllium, cadmium, cobalt, caesium, manganese, molybdenum, lead, platinum, strontium, thallium, tin, tungsten, uranium) or blood (lead, cadmium, manganese, mercury, selenium) in any of the affected patients. The matriarch and unaffected Sibling 1 had increased urinary strontium; unaffected Sibling 2 had increased urinary cobalt, urinary strontium, blood manganese; and unaffected Sibling 3 had increased urinary cobalt.
Genetic studies
We examined known disease-causing genes associated with microcephaly as well as epilepsy (Supplementary Table 4). Given that the patriarch of this family had affected children from multiple partnerships, we prioritized the following Mendelian inheritance models: autosomal dominant inheritance with reduced penetrance or a very common recessive allele that could be pseudo-dominant. Genetic testing also included oligonucleotide microarray, which did not reveal regions of homozygosity suggesting consanguinity, nor gene deletions or duplications. All the exomes were deleted in the entire 3′-UTR of C16orf58 and the last intron of SLC5A2 had a large deletion. This is either an artefact of alignment or represents an Acholi ethnic common variant. Mitochondrial DNA sequencing on Case 2 did not show pathogenic mutations.
As the reduced penetrance model did not produce bioinformatically interesting variant candidates, an analysis of the affected individuals with a less-stringent filtration was performed by categorizing the variants with the following criteria: gnomAD Homozygote Count ≤25, HGMD Disease-Causing Mutations Only and ClinVar Pathogenic Variants Only. There were 12 common variants among the affected individuals in SLC2A1, ARID1B, CLN8, STXBP1, SCN8A, POLG and CLN3. These variants were ranked as benign candidates or variants of unknown significance.
Infectious disease testing
Dual skin snips were negative for O. volvulus by PCR. Antibodies to Zika virus and dengue virus were negative, but all samples were positive for chikungunya virus antibodies (Supplementary Fig. 1). Two probands were measles virus non-immune, the third proband had equivocal measles virus serology.
Metagenomics revealed that the serum sample from Case 3 contained sequences that were 100% identical to hepatitis B and G viruses. That patient did have elevations in transaminases. The CSF from Case 3 contained no hepatitis B virus sequences and only eight reads that aligned to hepatitis G virus out of 16 006 028 paired-end sequences. All other serum and CSF samples contained only common skin flora and reagent contaminants. There were no reads that aligned to the genomes for O. volvulus, Zika virus, dengue virus or measles virus.
Antibodies to Onchocerca volvulus and potential autoantigens
Assays for serum antibodies to O. volvulus (Ov)-specific proteins were performed on all subjects, cases and unaffected family members and for the CSF in the three affected subjects. All individuals had IgG and IgG4 antibodies to Ov16 and IgG antibodies to OvTmy1 (Supplementary Table 5). Significant amounts of both IgG and IgG4 against OvFAR1/OvMSA1 were also found. There was no Ov-specific antibody in the CSF from Cases 1 and 2 and minimal antibody from Case 3. Autoantibodies to leiomodin-1 and DJ-1 were also assessed (Supplementary Table 5). There was no evidence of local CSF antibody production.
Immunotherapy and other treatments
Due to the presumed diagnosis of autoimmune epilepsy, we treated the patients with immunotherapy.27,28 Case 1 had refractory seizures and encephalopathy and Case 2 had possible hypothalamic dysfunction and CNS-restricted oligoclonal bands. Both were treated with five cycles of plasmapheresis administered every other day. Case 3 was treated empirically with intravenous immunoglobulin 2 mg/kg divided over 2 days.
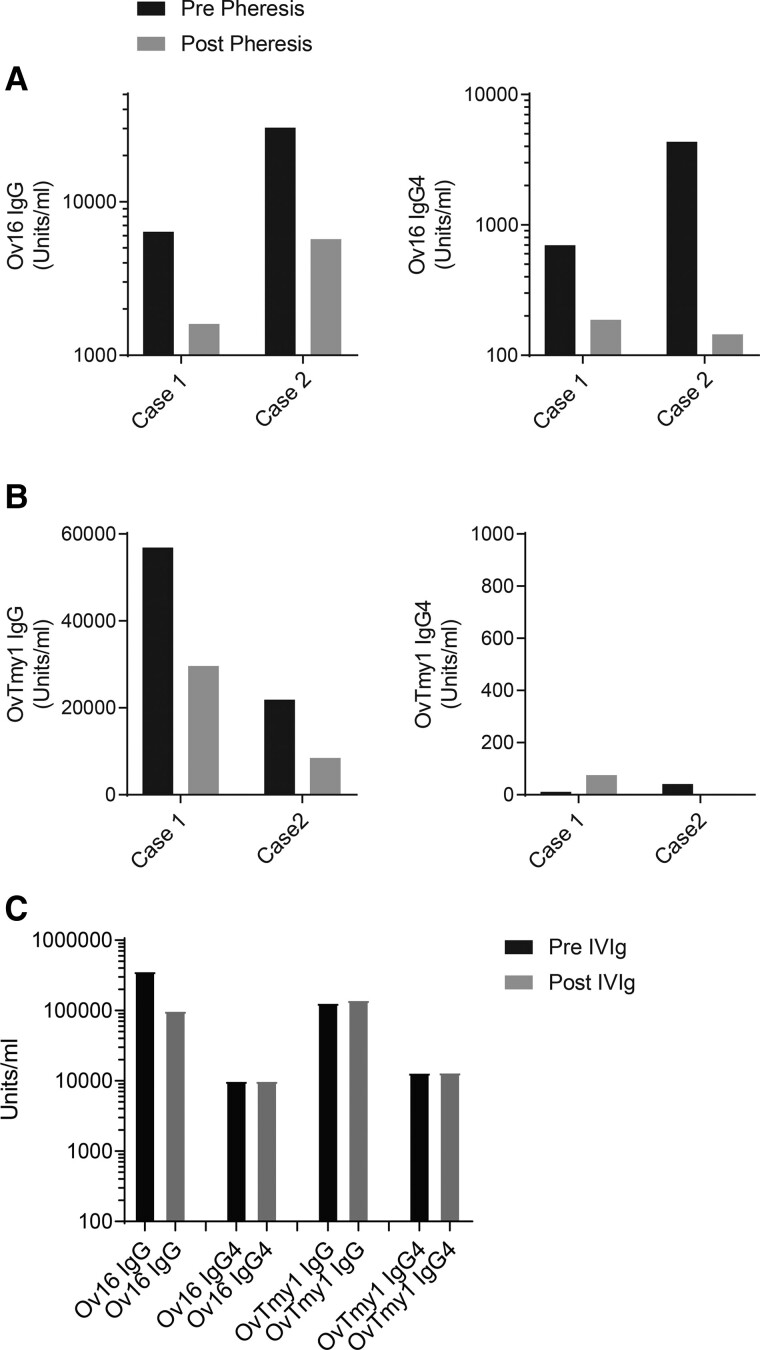
To assess the efficacy of the plasmapheresis and/or the IVIG on the levels of Ov-specific antibodies, serum levels post-treatment were measured and compared to those pre-treatment. In the two individuals receiving plasmapheresis, the serum levels of Ov16- and OvTMY1-specific antibodies all decreased following five plasmapheresis cycles (Fig. 4). There was no effect of IVIG on the serum levels of these same antibodies. During the 2-week hospital stay, there was no change in the EEG with immunomodulation; longer-term follow-up on discharge was not available.
Figure 4.
Immunomodulation results in decreases in antigen specific antibodies. (A) Serum antibodies to O. volvulus antigen-16 (Ov16) or (B) O. volvulus tropomyosin (OVTmy1) in two patients prior to (black bars) and after (grey bars) plasmapheresis. Total IgG antibodies specific for O. volvulus antigens are shown on the left and IgG4 antibodies are shown on the right. (C) Serum antibodies to OV16 (IgG and IgG4) and OVTmy1 (IgG and IgG4) in a patient prior to (black bars) and after (grey bars) administration of intravenous immunoglobulin (IVIg).
All three children received empirical treatment with ivermectin and albendazole as well as pyridoxine supplementation (Supplementary Table 6). Case 2, who was identified to have central adrenal insufficiency, was treated with prednisone for physiological replacement.
Discussion
The patients evaluated at the NIH-CC had clinical features of nodding syndrome similar to those previously described,1 including stunted growth and proportional microcephaly. Case 2 was found to have central adrenal insufficiency associated with hypogonadotropic hypogonadism, suggesting hypothalamic dysfunction, reminiscent of the description of Nakalanga dwarfism syndrome.29–32
Although we did not capture nodding spells, long-term EEG monitoring in this cohort did reveal distinctive interictal EEG findings not previously reported in the literature. Cases 2 and 3, who still experience monthly nodding spells, shared similar interictal EEG findings of diffuse generalized and multifocal epileptiform activity during wakefulness. Interictal epileptiform activity was significantly activated during sleep, with prolonged bursts of diffuse polymorphic slowing with intermixed focal and generalized spike or polyspike and wave activity. In contrast, the phenotype and EEG pattern of Case 1 was different, with interictal findings of diffuse background slowing and multifocal interictal epileptiform discharges but without prominent activation during sleep. Multiple seizure types were recorded in this patient, including seizures consisting primarily of altered awareness, as well as two seizures with bilateral limb tonic and clonic movements. These findings support the increasingly recognized concept that the atonic seizures characteristic of nodding syndrome are only part of these patients’ seizure spectrum33,34 and that a unique EEG abnormality during sleep may occur in patients with nodding syndrome. Future investigations should include EEG monitoring during sleep.
We examined patients for evidence of past or current infections, autoimmune processes, metabolic deficiencies and toxins and performed exome sequencing to identify genetic contributors to disease. Sequencing studies revealed only hepatitis B and G viruses in the serum of Case 3. None of the children had serological evidence of measles virus exposure, which has been suggested as a cause for nodding syndrome,35 and no measles virus RNA was detected in the CSF. All family members had antibodies to chikungunya virus. We recognize the caveat that these samples were collected and tested many years after illness onset, such that past infection remains a possibility.
There is an epidemiological association of nodding syndrome with O. volvulus infection1,5–11 and the patients we examined had evidence of past infection. However, the mechanism driving the relationship between seizures and the parasite remains unclear. One hypothesis is that neuronal damage occurs from neurotoxic O. volvulus antibodies that cross-react with leiomodin-1.36,37 All family members examined (affected and unaffected) had antibodies to leiomodin-1, although this does not exclude an age-related susceptibility. While the immunomodulatory therapy provided to the patients did not demonstrate any appreciable benefit during the short duration at the NIH, the lack of clinical response to immunomodulatory therapy does not preclude an immune aetiology of disease. As leiomodin-1 antibodies are toxic to newly formed neurons expressing leiomodin-1 in the cell membrane,37 it is possible that irreversible damage had already occurred and that once epileptogenesis has been established, immune responses are not required to perpetuate disease.
Environmental toxins, tau38 and nutritional deficiencies have also been implicated in the aetiology of nodding syndrome.2 Our extensive studies for heavy metals, micronutrients and metabolism revealed slightly increased levels of strontium in the unaffected siblings and borderline levels of pyridoxine in all family members. A previous study similarly found low pyridoxine levels in nodding syndrome patients and controls.5 No evidence of increased tau was present in the CSF of patients examined. Hence, we did not find evidence that these factors are related to the aetiology of the syndrome, with the limitation that we tested these cases many years after the onset of the illness.
Genomic analyses did not reveal any copy number variations, no evidence of consanguinity, and no mitochondrial pathogenic mutations. Exome sequencing did not reveal a causal mutation; however, variants of unknown significance were detected in the following genes associated with dominant epileptic encephalopathies: SLC2A1 associated with GLUT1 deficiency syndromes (our patients did not have CSF hypoglycorrhachia); and STXBP1 and SCN8A, which are associated with early infantile epileptic encephalopathy (the age of onset in our patients being much older). Our analyses are limited because of the absence of comparable genetic reference data from ethnically matched reference populations, such that the possibility of genetic factors cannot be excluded.
Supplementary Material
Acknowledgements
We thank the children and their families for participating in this study and the Ugandan government for their participation and ongoing support. We thank the nursing staff on the neurology inpatient unit and the NIH Children’s Inn staff for their care of these patients. We thank Thomas Markello (NHGRI) for his helpful insights into the genomic analyses of this family and Maryland Pao (NIMH) for her guidance on the mental health aspects of this family’s evaluation. We thank Rena Godfrey, Kevin O’Brien and Quentin Whitley (NHGRI) for clinical assistance with these patients. We thank Stephen Whitehead, Davis Gordon and Ted Pierson (NIAID/LVD) for performing the arboviral serological testing.
Contributor Information
Ariane Soldatos, National Institutes of Health Intramural Research Program, Bethesda, MD 20892, USA.
Thomas B Nutman, National Institutes of Health Intramural Research Program, Bethesda, MD 20892, USA.
Tory Johnson, National Institutes of Health Intramural Research Program, Bethesda, MD 20892, USA.
Scott F Dowell, Centers for Disease Control and Prevention, Atlanta, GA 30329, USA.
James J Sejvar, Centers for Disease Control and Prevention, Atlanta, GA 30329, USA.
Michael R Wilson, University of California San Francisco, San Francisco, CA 94143, USA; Weill Institute for Neurosciences, University of California San Francisco, San Francisco, CA 94158, USA.
Joseph L DeRisi, University of California San Francisco, San Francisco, CA 94143, USA; Chan Zuckerberg Biohub, San Francisco, CA 94158, USA.
Sara K Inati, National Institutes of Health Intramural Research Program, Bethesda, MD 20892, USA.
Catherine Groden, National Institutes of Health Intramural Research Program, Bethesda, MD 20892, USA.
Colleen Evans, National Institutes of Health Intramural Research Program, Bethesda, MD 20892, USA.
Elise M O’Connell, National Institutes of Health Intramural Research Program, Bethesda, MD 20892, USA.
Bernard Opar Toliva, Ministry of Health, Kampala, Republic of Uganda.
Jane R Aceng, Ministry of Health, Kampala, Republic of Uganda.
Josephine Aryek-Kwe, Ministry of Health, Kampala, Republic of Uganda.
Camilo Toro, National Institutes of Health Intramural Research Program, Bethesda, MD 20892, USA.
Constantine A Stratakis, National Institutes of Health Intramural Research Program, Bethesda, MD 20892, USA.
A Gretchen Buckler, National Institutes of Health Intramural Research Program, Bethesda, MD 20892, USA.
Cathy Cantilena, National Institutes of Health Intramural Research Program, Bethesda, MD 20892, USA.
Tara N Palmore, National Institutes of Health Intramural Research Program, Bethesda, MD 20892, USA.
Audrey Thurm, National Institutes of Health Intramural Research Program, Bethesda, MD 20892, USA.
Eva H Baker, National Institutes of Health Intramural Research Program, Bethesda, MD 20892, USA.
Richard Chang, National Institutes of Health Intramural Research Program, Bethesda, MD 20892, USA.
Harper Fauni, National Institutes of Health Intramural Research Program, Bethesda, MD 20892, USA.
David Adams, National Institutes of Health Intramural Research Program, Bethesda, MD 20892, USA.
Ellen F Macnamara, National Institutes of Health Intramural Research Program, Bethesda, MD 20892, USA.
C Christopher Lau, National Institutes of Health Intramural Research Program, Bethesda, MD 20892, USA.
May Christine V Malicdan, National Institutes of Health Intramural Research Program, Bethesda, MD 20892, USA.
Barbara Pusey-Swerdzewski, National Institutes of Health Intramural Research Program, Bethesda, MD 20892, USA.
Robert Downing, Uganda Virus Research Institute, Ministry of Health, Entebbe, Republic of Uganda.
Sudhir Bunga, Centers for Disease Control and Prevention, Atlanta, GA 30329, USA.
Jerry D Thomas, National Center for Environmental Health, Atlanta, GA 30341, USA.
William A Gahl, National Institutes of Health Intramural Research Program, Bethesda, MD 20892, USA.
Avindra Nath, National Institutes of Health Intramural Research Program, Bethesda, MD 20892, USA.
Funding
Funding by the Centers for Disease Control and Prevention, the Ministry of Health, Uganda, the Intramural Research Programs of NINDS and NHGRI, the Office of the Director of the NIH (Undiagnosed Diseases Program), as well as the Sandler and the William K. Bowes, Jr. Foundations (J.L.D. and M.R.W.), the Chan Zuckerberg Initiative (J.L.D.) and a Mentored Clinical Scientist Development award K08NS096117 from the National Institute for Neurological Disorders and Stroke (M.R.W.).
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Dowell SF, Sejvar JJ, Riek L, et al. . Nodding syndrome. Emerg Infect Dis. 2013;19:1374–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sejvar JJ, Kakooza AM, Foltz JL, et al. . Clinical, neurological, and electrophysiological features of nodding syndrome in Kitgum, Uganda: An observational case series. Lancet Neurol. 2013;12:166–174. [DOI] [PubMed] [Google Scholar]
- 3. Winkler AS, Wallner B, Friedrich K, et al. . A longitudinal study on nodding syndrome—A new African epilepsy disorder. Epilepsia. 2014;55:86–93. [DOI] [PubMed] [Google Scholar]
- 4. Piloya-Were T, Odongkara-Mpora B, Namusoke H, Idro R. Physical growth, puberty and hormones in adolescents with nodding syndrome; A pilot study. BMC Res Notes. 2014;7:858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Foltz JL, Makumbi I, Sejvar JJ, et al. . An epidemiologic investigation of potential risk factors for nodding syndrome in Kitgum district, Uganda. PLoS One. 2013;8:e66419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Konig R, Nassri A, Meindl M, et al. . The role of Onchocerca volvulus in the development of epilepsy in a rural area of Tanzania. Parasitology. 2010;137:1559–1568. [DOI] [PubMed] [Google Scholar]
- 7. Tumwine JK, Vandemaele K, Chungong S, et al. . Clinical and epidemiologic characteristics of nodding syndrome in Mundri County, southern Sudan. Afr Health Sci. 2012;12:242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Winkler AS, Friedrich K, Velicheti S, et al. . MRI Findings in people with epilepsy and nodding syndrome in an area endemic for onchocerciasis: An observational study. Afr Health Sci. 2013;13:529–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chesnais CB, Nana-Djeunga HC, Njamnshi AK, et al. . The temporal relationship between onchocerciasis and epilepsy: A population-based cohort study. Lancet Infect Dis. 2018;18:1278–1286. [DOI] [PubMed] [Google Scholar]
- 10. Chesnais CB, Bizet C, Campillo JT, et al. . A second population-based cohort study in Cameroon confirms the temporal relationship between onchocerciasis and epilepsy. Open Forum Infect Dis. 2020;7:ofaa206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gumisiriza N, Mubiru F, Siewe Fodjo JN, et al. . Prevalence and incidence of nodding syndrome and other forms of epilepsy in onchocerciasis-endemic areas in northern Uganda after the implementation of onchocerciasis control measures. Infect Dis Poverty. 2020;9:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gumisiriza N, Kaiser C, Asaba G, et al. . Changes in epilepsy burden after onchocerciasis elimination in a hyperendemic focus of western Uganda: A comparison of two population-based, cross-sectional studies. Lancet Infect Dis. 2020;20:1315–1323. [DOI] [PubMed] [Google Scholar]
- 13. Jilek-Aall LM. Epilepsy in the Wapogoro tribe in Tanganyika. Acta Psychiat Scand. 1965;41:57–86. [Google Scholar]
- 14. Colebunders R, Hendy A, Mokili JL, et al. . Nodding syndrome and epilepsy in onchocerciasis endemic regions: Comparing preliminary observations from South Sudan and the democratic republic of the Congo with data from Uganda. BMC Res Notes. 2016;9:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kamgno J, Pion SD, Boussinesq M. Demographic impact of epilepsy in Africa: Results of a 10-year cohort study in a rural area of Cameroon. Epilepsia. 2003;44:956–963. [DOI] [PubMed] [Google Scholar]
- 16. Kaiser C, Pion SD, Boussinesq M. Case-control studies on the relationship between onchocerciasis and epilepsy: Systematic review and meta-analysis. PLoS Negl Trop Dis. 2013;7:e2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pion SD, Kaiser C, Boutros-Toni F, et al. . Epilepsy in onchocerciasis endemic areas: Systematic review and meta-analysis of population-based surveys. PLoS Negl Trop Dis. 2009;3:e461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gahl WA, Tifft CJ. The NIH undiagnosed diseases program: Lessons learned. JAMA. 2011;305:1904–1905. [DOI] [PubMed] [Google Scholar]
- 19. Gahl WA, Markello TC, Toro C, et al. . The National Institutes of Health Undiagnosed Diseases Program: Insights into rare diseases. Genet Med. 2012;14:51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gahl WA, Mulvihill JJ, Toro C, et al. . The NIH Undiagnosed Diseases Program and network: Applications to modern medicine. Mol Genet Metab. 2016;117:393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Doan T, Wilson MR, Crawford ED, et al. . Illuminating uveitis: Metagenomic deep sequencing identifies common and rare pathogens. Genome Med. 2016;8:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wilson MR, Suan D, Duggins A, et al. . A novel cause of chronic viral meningoencephalitis: Cache Valley virus. Ann Neurol. 2017;82:105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Burbelo PD, Leahy HP, Iadarola MJ, Nutman TB. A four-antigen mixture for rapid assessment of Onchocerca volvulus infection. PLoS Negl Trop Dis. 2009;3:e438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Santiago HC, Bennuru S, Boyd A, Eberhard M, Nutman TB. Structural and immunologic cross-reactivity among filarial and mite tropomyosin: Implications for the hygiene hypothesis. J Allergy Clin Immunol. 2011;127:479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wolpert SM, Molitch ME, Goldman JA, Wood JB. Size, shape, and appearance of the normal female pituitary gland. AJR Am J Roentgenol. 1984;143:377–381. [DOI] [PubMed] [Google Scholar]
- 26. Dale RC, de Sousa C, Chong WK, Cox TC, Harding B, Neville BG. Acute disseminated encephalomyelitis, multiphasic disseminated encephalomyelitis and multiple sclerosis in children. Brain 2000; 123(Pt 12):2407–2422. [DOI] [PubMed] [Google Scholar]
- 27. Husari KS, Dubey D. Autoimmune epilepsy. Neurotherapeutics. 2019;16:685–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Quek AM, Britton JW, McKeon A, et al. . Autoimmune epilepsy: Clinical characteristics and response to immunotherapy. Arch Neurol. 2012;69:582–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kipp W, Burnham G, Bamuhiiga J, Leichsenring M. The Nakalanga syndrome in Kabarole district, Western Uganda. Am J Trop Med Hyg. 1996;54:80–83. [DOI] [PubMed] [Google Scholar]
- 30. Foger K, Gora-Stahlberg G, Sejvar J, et al. . Nakalanga syndrome: Clinical characteristics, potential causes, and its relationship with recently described nodding syndrome. PLoS Negl Trop Dis. 2017;11:e0005201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Newell ED, Vyungimana F, Bradley JE. Epilepsy, retarded growth and onchocerciasis, in two areas of different endemicity of onchocerciasis in Burundi. Trans R Soc Trop Med Hyg. 1997;91:525–527. [DOI] [PubMed] [Google Scholar]
- 32. Marshall AJ, Cherry JK. Endocrine dysfunction in a Nakalanga dwarf. Trans R Soc Trop Med Hyg. 1961;55:188–191. [DOI] [PubMed] [Google Scholar]
- 33. Wamala JF, Malimbo M, Tepage F, et al. . Nodding syndrome may be only the ears of the hippo. PLoS Negl Trop Dis. 2015;9:e0003880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. de Polo G, Romaniello R, Otim A, Benjamin K, Bonanni P, Borgatti R. Neurophysiological and clinical findings on nodding syndrome in 21 South Sudanese children and a review of the literature. Seizure. 2015;31:64–71. [DOI] [PubMed] [Google Scholar]
- 35. Spencer PS, Mazumder R, Palmer VS, et al. . Environmental, dietary and case-control study of nodding syndrome in Uganda: A post-measles brain disorder triggered by malnutrition? J Neurol Sci. 2016;369:191–203. [DOI] [PubMed] [Google Scholar]
- 36. Johnson TP, Tyagi R, Lee PR, et al. . Nodding syndrome may be an autoimmune reaction to the parasitic worm Onchocerca volvulus. Sci Transl Med. 2017;9:eaaf6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nauen DW, Haffner MC, Kim J, et al. . Putative autoantigen leiomodin-1 is expressed in the human brain and in the membrane fraction of newly formed neurons. Pathogens. 2020;9:1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pollanen MS, Onzivua S, Robertson J, et al. . Nodding syndrome in Uganda is a tauopathy. Acta Neuropathol. 2018;136:691–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its Supplementary material.