Abstract
Background
Long-term persistence of Ebola virus (EBOV) in immunologically privileged sites has been implicated in recent outbreaks of Ebola virus disease (EVD) in Guinea and the Democratic Republic of Congo. This study was designed to understand how the acute course of EVD, convalescence, and host immune and genetic factors may play a role in prolonged viral persistence in semen.
Methods
A cohort of 131 male EVD survivors in Liberia were enrolled in a case-case study. “Early clearers” were defined as those with 2 consecutive negative EBOV semen test results by real-time reverse-transcription polymerase chain reaction (rRT-PCR) ≥2 weeks apart within 1 year after discharge from the Ebola treatment unit or acute EVD. “Late clearers” had detectable EBOV RNA by rRT-PCR >1 year after discharge from the Ebola treatment unit or acute EVD. Retrospective histories of their EVD clinical course were collected by questionnaire, followed by complete physical examinations and blood work.
Results
Compared with early clearers, late clearers were older (median, 42.5 years; P < .001) and experienced fewer severe clinical symptoms (median 2, P = .006). Late clearers had more lens opacifications (odds ratio, 3.9 [95% confidence interval, 1.1–13.3]; P = .03), after accounting for age, higher total serum immunoglobulin G3 (IgG3) titers (P = .005), and increased expression of the HLA-C*03:04 allele (0.14 [.02–.70]; P = .007).
Conclusions
Older age, decreased illness severity, elevated total serum IgG3 and HLA-C*03:04 allele expression may be risk factors for the persistence of EBOV in the semen of EVD survivors. EBOV persistence in semen may also be associated with its persistence in other immunologically protected sites, such as the eye.
Keywords: Ebola, persistence, semen
Specific risk factors among male survivors, including decreased acute Ebola virus disease severity, intraocular lens opacifications, elevated total serum immunoglobulin G3, and expression of the HLA-C*03:04 allele, may help predict the likelihood of prolonged Ebola virus persistence in semen.
Ebola virus disease (EVD) caused by Ebola virus species Zaire ebolavirus (EBOV) predominantly manifests as an acute infection with a mortality rate of 70%–90% if untreated. After recovery from acute EVD, a subset of patients may go on to develop persistent EBOV in immunologically privileged sites, such as the brain, eyes, spinal cord, and male reproductive system [1, 2].
Male survivors of EVD who experienced no residual signs or symptoms of EVD, or other evidence of disease recrudescence can also transmit EBOV sexually to their partners [3]. EBOV has historically been detected by real-time reverse-transcription polymerase chain reaction (rRT-PCR) in the semen of EVD survivors within 3 months of their discharge from an Ebola treatment unit (ETU), with most men clearing the virus from semen within 1 year [4, 5]. In a subset of men, viral RNA was shown to persist in semen >1 year from ETU discharge, with one study documenting rRT-PCR detection of EBOV 40 months after illness onset [6]. Although EBOV has been cultured from semen up to 82 days after illness onset, semen of survivors can remain infectious for a much longer period of time [3]. Genetic sequencing suggests that the 2021 EVD outbreak in Guinea was linked to persistent infection from a survivor of the 2014 West Africa EVD outbreak [7].
Prior studies have attempted to identify risk factors for the persistence of EBOV in the semen of EVD survivors. One study found that older patients (>40 years) demonstrated prolonged viral RNA persistence in semen [8]. Another cohort study conducted among EVD survivors in Sierra Leone observed that higher viremia during acute EVD was associated with prolonged EBOV persistence in semen [9]. However, whether additional host epidemiologic, clinical, and immunologic risk factors may play a role in the persistence of EBOV in semen is unknown.
To this end, the Liberian Ministry of Health (MoH), the Men’s Health Screening Program (MHSP), and the US Centers for Disease Control and Prevention (CDC) enrolled a cohort of 131 male survivors from the 2014–2016 EVD outbreak to identify risk factors for EBOV RNA persistence in semen. Herein, we report the complete study results and discuss key findings.
METHODS
Men’s Health Screening Program
MHSP was established by the Liberian MoH in July 2015. The program provided male EVD survivors with semen testing for EBOV RNA by rRT-PCR and behavioral counseling on safe sex practices in accordance with World Health Organization (WHO) guidance [8, 10]. As described elsewhere, men were eligible to enroll in MHSP if they were aged ≥15 years and could provide an ETU discharge certificate [8, 11]. Because laboratory confirmation was not available for all patients with suspected EVD owing to insufficient laboratory capacity during the height of the EVD outbreak in Liberia, program eligibility was later expanded to include those without an ETU discharge certificate but who self-reported experiencing an EVD-compatible illness after contact with someone with confirmed EVD whose illness could be corroborated by witnesses. Participants “graduated” from the program after receiving 2 consecutive semen tests results that did not detect the presence of EBOV RNA with rRT-PCR [8].
Between June 2015 and June 2016, semen specimens were tested using the CDC’s emergency use authorization NP (nucleoprotein) and VP40 rRT-PCR EBOV assays [12, 13]. In August 2016, in an effort to harmonize rRT-PCR testing platforms used to test semen in Liberia, MHSP switched to the GeneXpert CE-IVD EBOV assay (Cepheid), a 2-target assay designed to detect the EBOV glycoprotein and nucleoprotein.
The rRT-PCR results were interpreted as described elsewhere [8]. Semen specimens were deemed positive if both EBOV targets amplified within the respective cycles of replication, 40 for the CDC assay and 45 for the GeneXpert assay. Specimens were deemed negative if neither target was amplified over the course of the reaction. Specimens were considered indeterminate if only one of the EBOV targets showed positive amplification during rRT-PCR. Finally, specimens tested using the CDC assay that did not demonstrate amplification of either EBOV target or RNase P of >30 reaction cycles were deemed to be of poor quality, and re-collection was requested. Specimens tested using the GeneXpert assay were deemed to be poor quality if either of the internal controls (specimen processing control or GeneXpert internal controls) failed, and re-collection was requested.
Case-Case Study Participant Selection
Study investigators reviewed the rRT-PCR results for all MHSP participants and divided them into 3 groups: early, late, and indeterminate clearers, based on the dynamics of the participants’ semen clearance before enrollment in the study. “Early clearers” were participants who had 2 consecutive negative EBOV RNA semen test results by rRT-PCR, collected ≥2 weeks apart through MHSP, within 1 year after discharge from the ETU or acute illness. “Late clearers” were participants who produced ≥1 semen specimen with detectable EBOV RNA by rRT-PCR >1 year after ETU discharge or acute illness. The 1-year cutoff was chosen to correspond to the 2016 WHO interim guidance stating that safer sex practices should be practiced for ≥12 months if semen testing is not available [10]. “Indeterminate clearers” were those who produced ≥2 poor-quality semen specimens and could not be classified as early or late clearers. By definition, all early, late, and indeterminate clearers enrolled in the case-case study met this definition before study entry. All MHSP participants (graduates and current enrollees) who had provided ≥2 semen samples were offered enrollment in the study. In total, 3 early clearers were offered enrollment for each late clearer. The study protocol was approved by the CDC and Liberia MoH institutional review boards and by Meharry Medical College.
Study Components
A detailed medical questionnaire, including demographics, social history, and family and personal medical history, was administered to all study participants. In addition, information was collected regarding their acute EVD illness, including symptoms, duration of illness, treatments received, and disease severity. Disease severity was ascertained through the collection of self-reported signs and symptoms of severe disease. Self-reported signs of severe EVD were defined as hemorrhage, hiccups, and/or seizures [14]. Self-reported symptoms of severe EVD were defined as ≥1 of the following: being too sick to get to the toilet, being too sick to drink water, or being delirious. Participants were also asked about symptoms after recovery from acute EVD, including sexual dysfunction, which was assessed using the standardized Sexual Health Inventory for Men (SHIM) [15]. A medical provider completed a physical examination for all participants, including vital signs and head, ear, nose, throat, cardiac, lung, abdominal, genital, neurologic, extremity, vascular, lymphatic, and skin examination. A complete anterior and posterior chamber ocular examination with tonometry and visual field testing was also performed.
Blood, stool, urine, urethral, dried blood spot, and semen specimens were collected from all participants in 2017 and 2018. Laboratory testing of these specimens was performed as described in the Supplementary Materials.
Laboratory Methods
Following collection, specimens were transported and maintained at −20°C or colder. One aliquot of blood and urine was sent to Eternal Love Winning Africa hospital in Monrovia, Liberia for hemoglobin A1C testing, complete cell blood count, and urinalysis. Whole blood from the second aliquot was used to spot filter paper for dried blood spot testing and HLA typing. The remaining blood, urine, stool, urethral, and semen specimens were transported to the United States for further testing (Supplementary Materials). Details on viral RNA quantification used in MHSP have been published elsewhere [8].
Statistical Methods
Questionnaire and laboratory data were entered and maintained in a Microsoft Access database. Nonparametric comparative analysis of medians between groups with continuous variables was performed using the Kruskal–Wallis test, and χ2 and Fisher exact tests were performed to compare binary variables as appropriate. Logistic regression was used to identify univariate associations with late clearance and further adjust each for age. RStudio version 1.2.5042 and Stata16-SE software were used for statistical analysis.
RESULTS
In total, 131 MHSP participants were enrolled in the case-case study, including all 30 eligible late clearers, 91 of the 100 eligible early clearers, and 10 of the 18 eligible indeterminate clearers (Table 1). The median age among all participants was 36 years (range 19–69). Late clearers were significantly older than early clearers (median age, 42.5 vs 33 years, respectively; P < .001). The median time from ETU discharge or acute EVD to study enrollment was 898 days (range, 622–1309 days) (Table 1). The longest documented duration of positivity was 852 days after ETU discharge or acute EVD (median, 447.5 days). There was no difference between late and early clearers in the length of ETU hospitalization or in the time from ETU discharge or acute EVD to study enrollment.
Table 1.
Demographics, Medical History, and Ebola virus disease Clinical History
| Characteristic | Early Clearers (n = 91) | Late Clearers (n = 30) | ORa | aORa | Indeterminate Clearers (n = 10) |
|---|---|---|---|---|---|
| Demographics | |||||
| ȃAge, median (range), y | 33 (19–54) | 42.5 (19–69) | 1.1b | … | 30 (18–62) |
| ȃTime from ETU discharge or acute EVD to blood collection, median (range), d | 894 (622–1288) | 921 (851–1309) | 1.0 | 1.0 | 904 (825–1236) |
| ȃSemen ever tested positive for EBOV RNA during enrollment in MHSP, no. | 8 | 30 | … | … | 0 |
| ȃPossesses an ETU discharge certificate, no. | 91 | 29 | … | … | 10 |
| ȃListed in MoH registry of laboratory-confirmed survivors, no. | 59 | 19 | … | … | 2 |
| ȃStill enrolled in MHSP at start of case-case study, no. | 0 | 0 | … | … | 0 |
| ȃNo. of children, median (range) | |||||
| ȃȃTotal no. | 2 (0–9) | 3 (0–10) | 1.3b | 0.9 | 1.5 (0–13) |
| ȃȃNo. after EVD | 0 (0–2) | 0 (0–1) | 0.4 | 0.5 | 0 (0–4) |
| Ebola virus serology | |||||
| ȃSerum IgM positive | 4 | 2 | 1.6 | 1.3 | 0 |
| ȃSerum IgG positive | 71 | 26 | 3.3 | 2.6 | 3 |
| Clinical course | |||||
| ȃDuration of acute illness, median (range), d | 7 (1–30) | 7 (1–21) | 1 | 1 | 5.5 (0–15) |
| Received convalescent plasma, no. (%) | 6 (7) | 0 | … | … | 0 |
| Received ZMapp, no. (%) | 3 (3) | 1 (3) | 0.7 | 0.3 | 0 |
| Treated by a traditional healer, no. (%) | 1 (1) | 3 (10) | 10.4b | 12.2b | 0 |
| Reported EVD symptoms, no. (%) | |||||
| ȃFever | 85 (93) | 23 (77) | 0.23b | 0.39 | 7 (70) |
| ȃVomiting | 71 (78) | 18 (60) | 0.42 | 0.63 | 1 (10) |
| ȃDiarrhea | 70 (77) | 22 (73) | 0.83 | 1.3 | 6 (60) |
| ȃAny hemorrhagec | 26 (29) | 3 (10) | 0.28b | 0.28 | 2 (20) |
| ȃHiccups | 37 (41) | 7 (23) | 0.44 | 0.51 | 3 (30) |
| ȃAbdominal pain | 51 (56) | 16 (53) | 0.9 | 0.75 | 6 (60) |
| ȃTesticular pain | 14 (15) | 3 (10) | 0.61 | 0.73 | 0 (0) |
| ȃToo sick to get to the toilet | 42 (46) | 7 (25) | 0.39 | 0.43 | 3 (30) |
| ȃToo sick to drink water | 25 (27) | 5 (17) | 0.55 | 0.72 | 2 (20) |
| ȃDelirious | 69 (76) | 16 (55) | 0.39b | 0.26b | 6 (60) |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; EBOV, Ebola virus; ETU, Ebola treatment unit; EVD, Ebola virus disease; MHSP, Men’s Health Screening Program; MoH, Ministry of Health; OR, odds ratio.
ORs and aORs computed to compare early and late clearers, with aORs adjusted for age.
Significant at P < .05.
Epistaxis, gingival bleeding, hematemesis, or hematochezia.
Testing for EBOV-specific immunoglobulin M and G (IgM and IgG) antibodies was available for 126 study participants (Table 1) and has been reported elsewhere [11]. The semen of all early clearers and indeterminate clearers, provided at the time of enrollment into the case-case study, tested negative for EBOV RNA. The semen of 2 of the 28 late clearers tested positive for EBOV RNA at the time of enrollment into the case-case study; the remainder tested negative.
Compared with early clearers, late clearers were less likely to experience fever (odds ratio [OR], 0.23 [95% confidence interval (CI), .07–.76), hemorrhage (0.28 [.08–.99]), or delirium (0.39 [.16–.94]) (Table 1). Late clearers were also less likely to experience any sign or symptom of severe disease (OR, 0.19 [95% CI, .07–.55). This association was still seen when the analysis was restricted to those with serologic evidence of EVD infection (OR, 0.25 [95% CI, .08–.84). Disease severity was not found to be associated with age (β = 0.6; P = .4).
At physical examination, late clearers were more likely to have lens opacifications (OR, 3.9 [95% CI, 1.1–13.3]), after accounting for age (Table 2). This finding was more pronounced when the analysis was restricted to those with serologic evidence of EVD infection (OR, 10.8 [95% CI, 3.3–35.6]). No other significant ocular differences were detected, including a composite value for active uveitis including eye pain, vision changes, eye redness, floaters, and photophobia.
Table 2.
Laboratory Test Results and Ophthalmologic Examination Findings for Early and Late Clearers
| Test Findings | Early Clearers, No. (%) | Late Clearers, No. (%) | OR | aORa | Indeterminate Clearers, No. (%) |
|---|---|---|---|---|---|
| Abnormal eye examination findings | |||||
| ȃConjunctiva | 27 (29) | 8 (27) | 0.9 | 0.4 | 2 (20) |
| ȃAnterior chamber | 1 (1) | 1 (3) | 3.1 | 1.5 | 0 |
| ȃPupil | 3 (3) | 3 (10) | 3.1 | 1.6 | 0 |
| ȃLens | 7 (8) | 13 (43) | 8.7b | 3.9b | 2 (20) |
| ȃVitreous | 1 (1) | 1 (3) | 3.1 | 1.3 | 1 (10) |
| ȃFundus | 21 (23) | 5 (17) | 0.7 | 0.4 | 1 (10) |
| ȃElevated intraocular pressure | 3 (3) | 3 (10) | 3.3 | 2 | 0 |
| Abnormal endocrine testing findings | |||||
| ȃCortisol (units) | 24 (28) | 2 (8) | 0.22b | 0.24 | 3 (33) |
| ȃFSH | 5 (6) | 2 (8) | 1.4 | 0.5 | 1 (11) |
| ȃGrowth hormone | 7 (8) | 5 (19) | 2.6 | 3.4 | 1 (11) |
| ȃLH | 18 (21) | 8 (31) | 1.7 | 2 | 6 (66) |
| ȃProlactin | 7 (8) | 2 (8) | 1 | 1.2 | 2 (22) |
| ȃPSA | 2 (2) | 3 (11) | 5.4 | 1.6 | 0 |
| ȃSHBG | 2 (2) | 0 | … | … | 0 |
| ȃ% Free testosterone | 4 (5) | 2 (10) | 1.8 | 2 | 0 |
| ȃFree testosterone | 2 (2) | 3 (12) | 5.5 | 3.3 | 0 |
| ȃTestosterone | 7 (8) | 2 (8) | 1 | 1.3 | 1 (11) |
| ȃThyroid-stimulating hormone (TSH) | 0 | 1 (4) | … | … | 0 |
| ȃFree T3 | 9 (10) | 3 (12) | 1.1 | 0.8 | 1 (11) |
| ȃFree T4 | 0 | 0 | … | … | 0 |
| Hemoglobinopathy | |||||
| ȃNo sickle trait | 77 (85) | 24 (86) | 1.1 | 0.5 | 9 (100) |
| ȃSickle trait | 12 (13) | 4 (14) | 1.1 | 2.5 | 0 |
| ȃHgbC | 0 | 0 | … | … | 0 |
| ȃ β-Thalassemia | 1 (1) | 0 | … | … | 0 |
| ȃO-Arab | 0 | 0 | … | … | 0 |
| Infectious disease testing | |||||
| ȃChlamydia | 1 (1) | 1 (3) | 3.1 | 3.8 | 1 (10) |
| ȃGonorrhea | 0 | 1 (3) | … | … | 0 |
| ȃHIV Ag/Ab | 5 (6) | 1 (4) | 0.6 | 0.4 | 0 |
| ȃHepatitis B Virus immunity (vaccination) | 3 (3) | 5 (19) | 6.4b | 8.5b | 2 (22) |
| ȃHepatitis B Virus immune (natural infection) | 34 (39) | 7 (26) | 0.6 | 0.6 | 2 (22) |
| ȃHepatitis B Virus—HBV core Ab only | 32 (37) | 11 (41) | 1.2 | 0.9 | 4 (50) |
| ȃHepatitis C Virus RNA | 0 | 0 | … | … | 0 |
| ȃActive P. falciparum malaria | 6 (7) | 2 (7) | 1 | 1.1 | 4 (40) |
| ȃMild/past P. falciparum malaria | 3 (3) | 2 (7) | 2.1 | 3.8 | 0 |
| Stool ova and parasites (hookworm, Ascaris, Trichuris, Hymenolepis, Taenia, or Schistosoma mansoni) | 0 | 0 | … | … | 0 |
Abbreviations: Ab, antibody; Ag, antigen; aOR, adjusted odds ratio; FSH, follicle-stimulating hormone; HgbC, hemoglobin C trait; HIV, human immunodeficiency virus; LH, luteinizing hormone; OR, odd ratio; P. falciparum, Plasmodium falciparum; PSA, prostate-specific antigen; SHBG, sex hormone–binding globulin; T3, triiodothyronine; T4, thyroxine.
ORs adjusted for age.
Significant at P < .05.
No significant associations were found for any other components of the physical examination; medical history, including smoking, alcohol, and drug use; standardized SHIM score; or number of children after accounting for age. No significant associations were identified for hematologic or endocrine laboratory results or autoimmune disease markers (Supplementary Table).
Although 13 participants were identified as having active or recent Plasmodium falciparum infection, no differences were identified between groups. Other identified infections included 6 participants with human immunodeficiency virus (HIV), 2 with chlamydia, and 1 with gonorrhea; however, these infections showed no association with late clearance. Forty-three participants (36%) had evidence of immunity to hepatitis B virus due to natural infection, 10 (8%) had evidence of immunity due to vaccination, and 9 (8%) had chronic hepatitis B infection. Participants with immunity due to vaccination were 8.5 times more likely to be late clearers, after accounting for age (OR, 8.5 [95% CI, 1.7–43.7]). Sixteen participants had sickle cell trait, 1 had β-thalassemia, and 1 had O-Arab trait; no traits were associated with late clearance. Compared with early clearers, late clearers had increased expression of 1 of 77 HLA alleles tested: HLA-C*03:04 (OR, 0.14 [95% CI, .02–.70]; P = .007). This finding was still seen when the analysis was restricted to early and late clearers with serologic evidence of EVD infection (OR, 0.06 [95% CI, .001–.54]; P = .003).
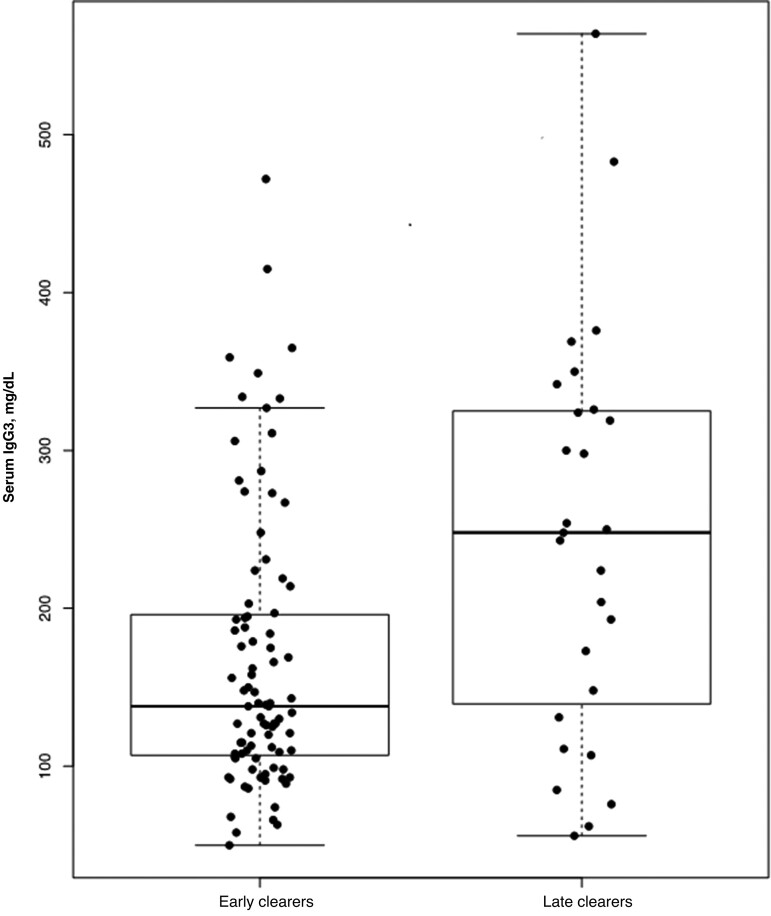
No clinically significant differences in median total serum IgG1, IgG2, or IgG4 were observed between groups. However, late clearers had a higher median total serum IgG3 level (P = .005; Figure 1), including after adjustment for age (P = .009). This finding was still seen when the analysis was restricted to those with serologic evidence of EVD infection (P = .008). This association was also independent of the time from participant discharge from ETU or from acute EVD to serum sample collection (median, 900 days; range 622–1309 days). Nine participants, all early clearers, were found to have elevated total serum IgG3 levels >1.5 times the third quartile over the interquartile range. When these 9 participants were compared with the remaining 79 early clearers, they did not have significant differences in their age, time from ETU discharge or acute EVD to obtaining their second negative semen EBOV RNA result, occurrence of severe acute EVD disease, presence of ocular symptoms during acute EVD, presence of abnormal visual acuity at study enrollment, presence of post-EVD arthralgia, or EBOV IgM/IgG serostatus.
Figure 1.
Box plot of serum total immunoglobulin G3 (IgG3) levels in early versus late clearers.
Indeterminate Clearers
The median time from acute illness to enrollment was 904 days. Indeterminate clearers produced a median of 3 poor-quality samples and produced poor-quality samples for a median of 158.5 days. Compared with early and late clearers, indeterminate clearers were more likely to have abnormally elevated levels of luteinizing hormone (OR, 6.7 [95% CI, 1.6–28.6]) but had no other differences on laboratory evaluation. No significant differences were found between indeterminate, early, and late clearers regarding age, illness severity, number of children after their illness, physical examination, or SHIM score.
DISCUSSION
Identifying EVD survivors at greatest risk for viral persistence in semen is important to understanding the pathophysiology of EBOV in the testes and to preventing sexual transmission. Older age has been associated with late clearance of EBOV RNA from semen in 2 studies [8, 9, 16]. One study of EVD survivors in Sierra Leone found an association between severe disease, as defined by an rRT-PCR cycle threshold (Ct) value of <27 (using blood collected during the acute phase of infection as the sample matrix), and late clearance of EBOV RNA from semen [8, 9].
Our study found an association between older age and late clearance. It also found an association between nonsevere EVD and late clearance of EBOV RNA from semen. This discordance compared with prior findings may be in part because we used a symptom-based definition of severe disease as reported by participants, rather than a single Ct value from blood during acute illness. We chose this definition for 2 reasons. First, viral load varies over the course of illness, and therefore a single time point may be insufficient to characterize the overall severity of disease. Second, severe EVD is likely a function of both viral characteristics (eg, viral load, species) and the immune response to infection including the generation of proinflammatory cytokines, chemokines, and growth factors [17–19]. In this context, our findings suggest that a less severe disease course may result in a more permissive immunologic environment that provides greater opportunities for the virus to enter and persist in immunologically protected sites, such as the testes.
Uveal infection with EBOV resulting in uveitis is a complication that can develop during the convalescent period of EVD [1] and can lead to cataract formation, posterior synechiae, and vision loss if not treated. The association we found between lens opacification and late clearance of EBOV was independent of age, based on logistic regression adjusting for age. This finding suggests that individuals with EBOV persistence in semen may also have EBOV persistence in other immunologically protected sites, such as the eye.
Late clearers expressed the HLA-C*03:04 allele more than early clearers. Previous studies have found that HIV and hepatitis C virus undergo escape from killer immunoglobulinlike receptor (KIR) DL3+ natural killer (NK) cells via promotion of inhibitory binding of KIR2DL3 to HLA-C*03:04-expressing cells [20, 21]. A similar mechanism has been described for other HLA-C–restricted viral epitopes in Lassa virus escape from KIR2DL2+ NK cells [22]. While associations with these specific KIRs have not been described in EBOV infection, up-regulation of other KIR genes has been previously associated with fatal outcomes in acute EVD [18, 23]. Although our study did not directly evaluate the role of NK cells in EVD survivors, it is possible that similar mechanisms of viral escape in individuals with HLA-C*03:04 may predispose them to experience EBOV persistence in immunologically protected sites.
We found an association between delayed EBOV RNA clearance in semen and elevated median total serum IgG3. In addition, elevated total serum IgG3 levels were detected an average of 936 days after acute EVD. Aside from its recognized role in the early immune response to infection and in acute rheumatic fever, elevated total IgG3 is not known to be associated with another disease entity [24]. In a 2021 study evaluating monoclonal antibodies recovered from EVD survivors, IgG3 antibodies induced high-level antibody-dependent enhancement [25]. Accordingly, it may be plausible that elevated serum IgG3 antibodies in late clearers may have induced antibody-dependent enhancement and promoted entry and persistence of EBOV into the testes. Prospective longitudinal sampling and analysis of EVD survivors could provide greater clarity on the timing and nature of changes in total and EBOV-specific IgG subclasses, including IgG3.
The current study is subject to several limitations. First, our assessment of EVD severity was reliant on patient recollection of their symptoms years after ETU discharge and thus differed from other studies that have used Ct values as surrogates for severe illness [9]. Second, owing to the logistical challenges of obtaining serial serum samples from EVD survivors, our data do not provide any comprehensive profile over a longitudinal period. Prospective, longitudinal analysis of EVD survivors may provide greater clarity on our findings, including IgG subclass dynamics. Third, we did not identify any markers for viral persistence based on basic metabolic, hematologic, liver function, endocrine, or infectious disease testing. Although prior vaccination against hepatitis B virus appeared to be associated with late clearance, given the small numbers of vaccinated individuals the significance of this result is difficult to interpret. Fourth, our analysis of HLA alleles did not include adjustment for multiple tests, which could lead to a spurious conclusion. However, the association of HLA-C*03:04 positivity with late clearance appears to be biologically plausible. Fifth, although the role of immune-based therapies in modifying the risk of EBOV persistence is unclear, recent evidence suggests that it may be associated with delayed clearance [26]. We found the opposite, that more early clearers (n = 9) received immune-based therapies (convalescent plasma or ZMapp) than late clearers (n = 1), although this differences was not statistically significant. Finally, because blood specimens were collected several years after recovery and at a single time point, these findings may not precisely represent the full spectrum of the convalescent immune response.
Programs that offer semen testing to EVD survivors are operational for a limited amount of time, with most typically closing by 18 months after the end of an EVD outbreak. However, the risk of EBOV transmission from survivors may exceed this, as witnessed by the recent EVD outbreak in Guinea [27]. While it may be impractical to monitor male EVD survivors in convalescence indefinitely, it may be easier to provide longer-term monitoring for a smaller, select group of “higher-risk” survivors.
We found that older age, nonsevere disease, lens opacifications, elevated serum IgG3 levels, and HLA-C*03:04 expression are all associated with prolonged EBOV persistence in male EVD survivors. Further corroboration of these findings and identification of any additional host determinants for prolonged EBOV persistence could help determine who should receive semen testing and counseling beyond the established follow-up period. Such efforts would benefit survivors and their sexual partners by clarifying their individual long-term risk of EBOV transmission. They may also counter the stigma that some EVD survivors, their partners, and family members face by providing a scientific basis for ongoing semen testing. In addition, they could benefit the EVD response community by targeting limited resources for outbreak prevention toward higher-risk survivors. Finally, future findings may help elucidate the underlying mechanisms of viral persistence in immunologically protected sites, which may in turn provide useful therapeutic targets for the eradication of EBOV in the convalescent period.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online.
Notes
Acknowledgments. We thank Laura Youngblood; participants and staff of the Men’s Health Screening Program; administrators at Redemption Hospital, Phebe Hospital, and Tellewoyan Hospital; and the Liberian Ministry of Health.
Disclaimer. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government. This research was supported in part by the Intramural Research Program of the National Institutes of Health, Frederick National Laboratory, Center for Cancer Research. The views expressed in this article are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
Financial support. This was work was supported by funds from the CDC Foundation and the Centers for Disease Control and Prevention (CDC) and by federal funds from the Frederick National Laboratory for Cancer Research (contract HHSN261200800001E).
Supplementary Material
Contributor Information
Jonathan Dyal, Epidemic Intelligence Service, Centers for Disease Control and Prevention, Atlanta, Georgia, USA; Division of High-Consequence Pathogens and Pathology, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Aaron Kofman, Epidemic Intelligence Service, Centers for Disease Control and Prevention, Atlanta, Georgia, USA; Division of High-Consequence Pathogens and Pathology, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Jomah Z Kollie, Men’s Health Screening Program, Monrovia, Liberia.
John Fankhauser, ELWA Hospital, Samaritan’s Purse, Monrovia, Liberia.
Romeo Orone, ELWA Hospital, Samaritan’s Purse, Monrovia, Liberia.
Moses J Soka, ELWA Hospital, Samaritan’s Purse, Monrovia, Liberia.
Uriah Glaybo, Men’s Health Screening Program, Monrovia, Liberia.
Armah Kiawu, Men’s Health Screening Program, Monrovia, Liberia.
Edna Freeman, Men’s Health Screening Program, Monrovia, Liberia.
Giovanni Giah, Men’s Health Screening Program, Monrovia, Liberia.
Henry D Tony, Men’s Health Screening Program, Monrovia, Liberia.
Mylene Faikai, Men’s Health Screening Program, Monrovia, Liberia.
Mary Jawara, Men’s Health Screening Program, Monrovia, Liberia.
Kuku Kamara, Men’s Health Screening Program, Monrovia, Liberia.
Samuel Kamara, Men’s Health Screening Program, Monrovia, Liberia.
Benjamin Flowers, Men’s Health Screening Program, Monrovia, Liberia.
Mohammed L Kromah, Men’s Health Screening Program, Monrovia, Liberia.
Rodel Desamu-Thorpe, Office of Public Health Preparedness and Response, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
James Graziano, Division of High-Consequence Pathogens and Pathology, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Shelley Brown, Division of High-Consequence Pathogens and Pathology, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Maria E Morales-Betoulle, Division of High-Consequence Pathogens and Pathology, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Deborah L Cannon, Division of High-Consequence Pathogens and Pathology, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Kaihong Su, Department of Pathology and Microbiology, University of Nebraska Medical Center, Omaha, Nebraska, USA.
Susanne L Linderman, Emory Vaccine Center, Emory University, Atlanta, Georgia, USA.
Mateusz Plucinski, Division of Parasitic Diseases and Malaria, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Eric Rogier, Division of Parasitic Diseases and Malaria, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Richard S Bradbury, Division of Parasitic Diseases and Malaria, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
W Evan Secor, Division of Parasitic Diseases and Malaria, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Katherine E Bowden, Division of STD Prevention, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Christi Phillips, Division of STD Prevention, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Mary N Carrington, Basic Science Program, Frederick National Laboratory for Cancer Research, National Cancer Institute, Frederick, Maryland, USA; Laboratory of Integrative Cancer Immunology, Center for Cancer Research, National Cancer Institute, Bethesda, Maryland, USA; Ragon Institute of MGH, Massachusetts Institute of Technology and Harvard University, Cambridge, Massachusetts, USA.
Yeon-Hwa Park, Basic Science Program, Frederick National Laboratory for Cancer Research, National Cancer Institute, Frederick, Maryland, USA.
Maureen P Martin, Basic Science Program, Frederick National Laboratory for Cancer Research, National Cancer Institute, Frederick, Maryland, USA.
Maria del Pilar Aguinaga, Department of Internal Medicine, Meharry Sickle Cell Center, Meharry Medical College, Nashville, Tennessee, USA; Department of Obstetrics and Gynecology, Meharry Sickle Cell Center, Nashville, Tennessee, USA.
Robert Mushi, Department of Internal Medicine, Meharry Sickle Cell Center, Meharry Medical College, Nashville, Tennessee, USA.
Dana L Haberling, Division of High-Consequence Pathogens and Pathology, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Elizabeth D Ervin, Division of High-Consequence Pathogens and Pathology, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
John D Klena, Division of High-Consequence Pathogens and Pathology, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Moses Massaquoi, Ministry of Health, Liberia.
Tolbert Nyenswah, Ministry of Health, Liberia.
Stuart T Nichol, Division of High-Consequence Pathogens and Pathology, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
David E Chiriboga, University of Massachusetts Medical School, Worcester, Massachusetts, USA.
Desmond E Williams, Center for Global Health, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Steven H Hinrichs, Department of Pathology and Microbiology, University of Nebraska Medical Center, Omaha, Nebraska, USA.
Rafi Ahmed, Emory Vaccine Center, Emory University, Atlanta, Georgia, USA.
Benjamin T Vonhm, National Public Health Institute of Liberia, Monrovia, Liberia.
Pierre E Rollin, Division of High-Consequence Pathogens and Pathology, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Lawrence J Purpura, Division of High-Consequence Pathogens and Pathology, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Mary J Choi, Division of High-Consequence Pathogens and Pathology, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
References
- 1. Varkey JB, Shantha JG, Crozier I, et al. Persistence of Ebola virus in ocular fluid during convalescence. N Engl J Med 2015; 372:2423–7. doi: 10.1056/nejmoa1500306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jacobs M, Rodger A, Bell DJ, et al. Late Ebola virus relapse causing meningoencephalitis: a case report. Lancet 2016; 388:498–503. doi: 10.1016/S0140-6736(16)30386-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Subissi L, Keita M, Mesfin S, et al. Ebola virus transmission caused by persistently infected survivors of the 2014–2016 outbreak in West Africa. J Infect Dis 2018; 218:S287–91. doi: 10.1093/infdis/jiy280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rowe AK, Bertolli J, Khan AS, et al. Clinical, virologic, and immunologic follow-up of convalescent Ebola hemorrhagic fever patients and their household contacts, Kikwit, Democratic Republic of the Congo. J Infect Dis 1999; 179:S28–35. doi: 10.1086/514318 [DOI] [PubMed] [Google Scholar]
- 5. Deen GF, Broutet N, Xu W, et al. Ebola RNA persistence in semen of Ebola virus disease survivors—final report. N Engl J Med 2017; 377:1428–37. doi: 10.1056/nejmoa1511410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Prevail III Study Group . A longitudinal study of Ebola sequelae in Liberia. N Engl J Med 2019; 380:924–34. doi: 10.1056/NEJMoa1805435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Keita AK, Koundouno FR, Faye M, et al. Resurgence of Ebola virus in 2021 in Guinea suggests a new paradigm for outbreaks. Nature 2021; 597:539–43. doi: 10.1038/s41586-021-03901-9 [DOI] [PubMed] [Google Scholar]
- 8. Soka MJ, Choi MJ, Baller A, et al. Prevention of sexual transmission of Ebola in Liberia through a national semen testing and counselling programme for survivors: an analysis of Ebola virus RNA results and behavioural data. Lancet Global Health 2016; 4:e736–43. doi: 10.1016/S2214-109X(16)30175-9 [DOI] [PubMed] [Google Scholar]
- 9. Thorson AE, Deen GF, Bernstein KT, et al. Persistence of Ebola virus in semen among Ebola virus disease survivors in Sierra Leone: a cohort study of frequency, duration, and risk factors. PLoS Med 2021; 18:e1003273. doi: 10.1371/journal.pmed.1003273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization . Interim advice on the sexual transmission of the Ebola virus disease. 2016. Available at: https://www.who.int/publications/m/item/interim-advice-on-the-sexual-transmission-of-the-ebola-virus-disease.
- 11. Kofman A, Linderman S, Su K, et al. Characteristics of Ebola virus disease survivor blood and semen in Liberia: serology and reverse transcription polymerase chain reaction (RT-PCR). Clin Infect Dis 2021; 73:e3641–6. doi: 10.1093/cid/ciaa1331 [DOI] [PubMed] [Google Scholar]
- 12. Centers for Disease Control and Prevention . Ebola Virus VP40 Real-time RT-PCR Assay [package insert from the Internet]. Available at: https://www.fda.gov/media/91142/download. Accessed 10 January 2021.
- 13. Centers for Disease Control and Prevention . Ebola Virus NP Real-time RT-PCR Assay. Published 1 November 2021. Available at: https://www.fda.gov/media/91097/download. Accessed 10 January 2021.
- 14. Hartley MA, Young A, Tran AM, et al. Predicting Ebola severity: a clinical prioritization score for Ebola virus disease. PLoS Negl Trop Dis 2017; 11:e0005265. doi: 10.1371/journal.pntd.0005265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cappelleri JC, Rosen RC. The Sexual Health Inventory for Men (SHIM): a 5-year review of research and clinical experience. Int J Impotence Res 2005; 17:307–19. doi: 10.1038/sj.ijir.3901327 [DOI] [PubMed] [Google Scholar]
- 16. Fischer WA, Brown J, Wohl DA, et al. Ebola virus ribonucleic acid detection in semen more than two years after resolution of acute Ebola virus infection. Open Forum Infect Dis 2017; 4(3):ofx155. doi: 10.1093/ofid/ofx155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Villinger F, Rollin PE, Brar SS, et al. Markedly elevated levels of interferon (IFN)-γ, IFN-α, interleukin (IL)-2, IL-10, and tumor necrosis factor-α associated with fatal Ebola virus infection. J Infect Dis 1999; 179:S188–91. doi: 10.1086/514283 [DOI] [PubMed] [Google Scholar]
- 18. Wauquier N, Becquart P, Padilla C, Baize S, Leroy EM. Human fatal Zaire Ebola virus infection is associated with an aberrant innate immunity and with massive lymphocyte apoptosis. PLoS Negl Trop Dis 2010; 4:e837. doi: 10.1371/journal.pntd.0000837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baize S, Leroy EM, Georges-Courbot MC, et al. Defective humoral responses and extensive intravascular apoptosis are associated with fatal outcome in Ebola virus-infected patients. Nat Med 1999; 5:423–6. doi: 10.1038/7422 [DOI] [PubMed] [Google Scholar]
- 20. Lunemann S, Martrus G, Hölzemer A, et al. Sequence variations in HCV core-derived epitopes alter binding of KIR2DL3 to HLA-C∗03:04 and modulate NK cell function. J Hepatol 2016; 65:252–8. doi: 10.1016/j.jhep.2016.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hölzemer A, Thobakgale CF, Jimenez Cruz CA, et al. Selection of an HLA-C*03:04-restricted HIV-1 p24 gag sequence variant is associated with viral escape from KIR2DL3+ natural killer cells: data from an observational cohort in South Africa. PLoS Med 2015; 12:e1001900. doi: 10.1371/journal.pmed.1001900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wauquier N, Padilla C, Becquart P, Leroy E, Vieillard V. Association of KIR2DS1 and KIR2DS3 with fatal outcome in Ebola virus infection. Immunogenetics 2010; 62:767–71. doi: 10.1007/s00251-010-0480-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wawina-Bokalanga T, Vanmechelen B, Lhermitte V, et al. Human diversity of killer cell immunoglobulin-like receptors and human leukocyte antigen class I alleles and Ebola virus disease outcomes. Emerging Infect Dis 2021; 27:76–84. doi: 10.3201/EID2701.202177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chung AW, Ho TKC, Hanson-Manful P, et al. Systems immunology reveals a linked IgG3–C4 response in patients with acute rheumatic fever. Immunol Cell Biol 2020; 98:12–21. doi: 10.1111/imcb.12298 [DOI] [PubMed] [Google Scholar]
- 25. Kuzmina NA, Younan P, Gilchuk P, et al. Antibody-dependent enhancement of Ebola virus infection by human antibodies isolated from survivors. Cell Rep 2018; 24:1802–15.e5. doi: 10.1016/j.celrep.2018.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu J, Trefry JC, Babka AM, et al. Ebola virus persistence and disease recrudescence in the brains of antibody-treated nonhuman primate survivors. Sci Transl Med 2022; 14. doi: 10.1126/scitranslmed.abi5229 [DOI] [PubMed] [Google Scholar]
- 27. World Health Organization . Ebola outbreak 2021—N’Zerekore, Guinea. 2021. Available at: https://www.who.int/emergencies/situations/ebola-2021-nzerekore-guinea. Accessed January 10, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.