Abstract
Background
The CLEAR Trial demonstrated that a multisite body decolonization regimen reduced post-discharge infection and hospitalization in methicillin-resistant Staphylococcus aureus (MRSA) carriers. Here, we describe decolonization efficacy.
Methods
We performed a large, multicenter, randomized clinical trial of MRSA decolonization among adult patients after hospital discharge with MRSA infection or colonization. Participants were randomized 1:1 to either MRSA prevention education or education plus decolonization with topical chlorhexidine, oral chlorhexidine, and nasal mupirocin. Participants were swabbed in the nares, throat, axilla/groin, and wound (if applicable) at baseline and 1, 3, 6, and 9 months after randomization. The primary outcomes of this study are follow-up colonization differences between groups.
Results
Among 2121 participants, 1058 were randomized to decolonization. By 1 month, MRSA colonization was lower in the decolonization group compared with the education-only group (odds ration [OR] = 0.44; 95% confidence interval [CI], .36–.54; P ≤ .001). A similar magnitude of reduction was seen in the nares (OR = 0.34; 95% CI, .27–.42; P < .001), throat (OR = 0.55; 95% CI, .42–.73; P < .001), and axilla/groin (OR = 0.57; 95% CI, .43–.75; P < .001). These differences persisted through month 9 except at the wound site, which had a relatively small sample size. Higher regimen adherence was associated with lower MRSA colonization (P ≤ .01).
Conclusions
In a randomized, clinical trial, a repeated post-discharge decolonization regimen for MRSA carriers reduced MRSA colonization overall and at multiple body sites. Higher treatment adherence was associated with greater reductions in MRSA colonization.
Keywords: MRSA, decolonization, clinical trial, post-discharge
We examined microbiologic outcomes of the Changing Lives by Eradicating Antibiotic Resistance Trial, a large, randomized, multicenter, post-hospitalization decolonization clinical trial, and found that serial post-discharge decolonization with topical chlorhexidine, nasal mupirocin, and oral chlorhexidine significantly decreased methicillin-resistant Staphylococcus aureus colonization at key body sites for 9 months post-enrollment.
Staphylococcus aureus remains a common cause of healthcare-associated infections and the most common pathogen responsible for device and procedural infections [1]. As the dominant resistant form, methicillin-resistant S. aureus (MRSA) infections cause or complicate 278 000 hospitalizations annually in the United States, including 56 000 septic events, and 19 000 MRSA-related deaths [2].
Furthermore, hospitalized MRSA-colonized or MRSA-infected persons are at high risk for post-discharge MRSA infection [3–5]. MRSA carriers from a tertiary care hospital were reported to have a 14% risk of post-discharge MRSA infection in the subsequent year associated with a 9% attributable risk of death [3]. Others have estimated that 23.5/10 000 hospital admissions are associated with a post-discharge MRSA infection [6]. The Centers for Disease Control and Prevention estimated that 79% of community-onset healthcare-associated MRSA infections occurred among patients hospitalized in the prior year [7].
MRSA prevention studies have largely focused on hospitalized patients where decolonization protocols with chlorhexidine have reduced infection risk among surgical patients [8, 9] and in the intensive care unit setting [10–12]. The Changing Lives by Eradicating Antibiotic Resistance (CLEAR) Trial was a randomized, controlled, clinical trial of repeated decolonization vs standard of care among adult MRSA carriers discharged from acute care hospitals. In the CLEAR Trial, it was found that decolonization reduced the main outcomes of MRSA infection by 30% and all-cause infection by 17% compared with education alone [13]. Here, we describe the efficacy of this decolonization regimen on nasal, oropharyngeal, and skin MRSA colonization.
METHODS
Study Design
The CLEAR Trial was a previously published, unblinded, randomized, controlled, superiority trial comparing a twice-monthly 5-day decolonization regimen that involved chlorhexidine bathing, oral chlorhexidine rinse, and intranasal mupirocin plus patient education vs patient education alone following discharge from acute care hospitals [13]. Here, we describe the impact of the trial on the secondary outcome of MRSA colonization. This study was approved by a centralized institutional review board at the University of California–Irvine.
Recruitment
Details of the CLEAR Trial have been previously published [13]. In brief, participants were adult (aged ≥18 years) inpatients with microbiologically confirmed MRSA colonization or infection at several Southern California hospitals. Informed written consent to participate in the post-discharge trial was obtained from all participants or legal representatives. Inclusion criteria included being able to bathe or shower regularly, either independently or with the aid of a caregiver [13]. Exclusion criteria included allergy to study products and moribund state unlikely to survive hospitalization. A full list of inclusion and exclusion criteria are listed in Supplementary Table 1.
Randomization and Intervention
Participants were randomized at a 1:1 ratio to the standard-of-care group or the intervention group using a stratification scheme described previously [13]. The standard-of-care education group participants received education on enhanced hygiene to prevent MRSA infection. The intervention group received the same education plus nasal 2% mupirocin, 4% rinse-off chlorhexidine body wash, and 0.12% chlorhexidine mouth wash to use Monday–Friday twice monthly (every other week) for 6 months.
Initial and Follow-up Visits and Laboratory Studies
Participants underwent an initial in-person evaluation prior to, or shortly after, hospital discharge (baseline visit) and also had in-person follow-up visits at 1, 3, 6, and 9 months (M1, M3, M6, and M9 visits). During each in-person visit, participants completed a risk factor survey to collect demographic, socioeconomic, medical, and behavioral history and were swabbed at up to 4 body sites: anterior nares, pharyngeal arches, bilateral groin and axilla (using a single swab), and open wounds (if present). Premoistened cotton tip swabs (BD BBL CultureSwab) were used for sampling, and all samples were processed within 48 hours of collection for detection of MRSA using selective media: SPECTRA MRSA plate (Remel, Lenexa, KS). A final 12-month follow-up phone visit was performed without sampling.
Participants in the decolonization group provided self-reported adherence estimates for the topical chlorhexidine, nasal mupirocin, and chlorhexidine oral rinse using a standardized survey during the M1, M3, and M6 visits (no adherence assessment was done at the M9 visit because the decolonization intervention lasted only through the M6 visit). Participant adherence was trichotomized into 3 groups: full adherence (all prescribed doses taken), partial adherence (at least some of prescribed doses taken), and nonadherence (no prescribed doses taken).
Statistical Analyses
Overall and body site-specific colonization proportions were calculated for each group by visit and compared between groups using χ2 tests. Odds ratios and confidence intervals were calculated using standard techniques. In accordance with the trial design, MRSA colonization proportions were also evaluated in 4 subgroups between baseline and month 6 (Hispanics, non-Hispanics, recent surgery patients, and nursing home residents). To understand predictors of persistent colonization, we performed multivariable generalized linear mixed effects models to assess predictors of colonization at months 1, 3, 6, and 9, accounting for clustering on the participant, age, gender, race/ethnicity, insurance status, nursing home residence, comorbidities, and treatment allocation. Independent variables assessed included adherence reported at each visit as time-varying covariate. Models were assessed for overall colonization and body site–specific colonization whereby adherence was limited to the body site–specific product (eg, mupirocin for the outcome of nasal colonization; chlorhexidine body wash for axilla/groin colonization; and chlorhexidine mouth wash for throat colonization).
RESULTS
A total of 2121 participants were enrolled, with 1063 patients randomized to the education-only group and 1058 patients to the decolonization group. The majority of hospital enrollment cultures were from nasal surveillance (n = 1182, 56%), followed by wound (n = 625, 29%), respiratory (n = 89, 4.2%), blood (n = 74, 3.5%), urine (n = 63, 3.0%), bone/joint (n = 29, 1.4%), and other (n = 59, 2.8%). Participant characteristics were similar between study groups (Table 1). Median age was 56.0 years (range, 18.1–97.4; mean 55.9 years with standard deviation = 17). The most common comorbidities included diabetes (40%), chronic obstructive pulmonary disease (20%), and immunocompromised state (19%; Table 1). Visit completion was 76% at M1 (78% in the education group vs 74% in the decolonization group, P = .04), 72% at M3 (73% vs 70%, P = .12), 66% at M6 (68% vs 64%, P = .06), and 61% at M9 (62% vs 60%, P = .34).
Table 1.
Demographic Characteristics of Study Participants at Recruitment Hospitalization
| Characteristic | Decolonization Group, | Education Group, | P Value |
|---|---|---|---|
| N (%) | N (%) | ||
| N | 1058 | 1063 | |
| Age, mean (SD), years | 56 (17) | 56 (17) | .78 |
| Male | 565 (53.4) | 583 (54.8) | .51 |
| Hispanic | 339 (32.0) | 339 (31.9) | .94 |
| Racea | .87 | ||
| ȃWhite | 840 (80.4) | 844 (80.2) | |
| ȃBlack | 132 (12.6) | 124 (11.8) | |
| ȃAsian | 47 (4.5) | 58 (5.5) | |
| ȃAmerican Indian | 6 (0.6) | 6 (0.6) | |
| ȃOther | 20 (1.9) | 21 (2.0) | |
| Primary insurancea | .48 | ||
| ȃMedicaid | 378 (38.7) | 408 (41.3) | |
| ȃMedicare | 124 (12.7) | 132 (13.4) | |
| ȃPrivate | 283 (28.9) | 259 (26.2) | |
| ȃOther | 193 (19.7) | 188 (19.0) | |
| Less than high school education | 231 (22.5) | 210 (20.4) | .59 |
| Bathe daily or every other day | 927 (89.7) | 926 (89.3) | .73 |
| Bathing assistance needed | 224 (22.1) | 200 (19.5) | .15 |
| Comorbiditiesb | |||
| ȃDiabetes | 462 (43.8) | 424 (39.9) | .08 |
| ȃChronic obstructive pulmonary disease | 203 (19.4) | 212 (20.1) | .70 |
| ȃCongestive heart failure | 149 (14.3) | 145 (13.7) | .73 |
| ȃCancer | 161 (15.4) | 153 (14.5) | .56 |
| ȃRenal disease | 134 (12.7) | 140 (13.2) | .74 |
| ȃCerebrovascular disease | 104 (10.0) | 115 (10.9) | .48 |
| ȃLiver disease | 91 (8.7) | 81 (7.7) | .39 |
| Charlson comorbidity score, mean (SD) | 1.7 (1.6) | 1.7 (1.6) | .49 |
| Enrollment MRSA source | .79 | ||
| ȃNaresc | 602 (56.9) | 580 (54.6) | |
| ȃWound | 305 (28.8) | 320 (30.1) | |
| ȃRespiratory | 45 (4.3) | 44 (4.1) | |
| ȃBlood | 31 (2.9) | 43 (4.0) | |
| ȃUrine | 33 (3.1) | 30 (2.8) | |
| ȃBone/Joint | 13 (1.2) | 16 (1.5) | |
| ȃOther | 29 (2.7) | 30 (2.8) | |
| Recruitment hospitalizationd | |||
| ȃHospitalized in prior yearb | 598 (57.4) | 595 (56.9) | .80 |
| ȃNursing home stay in prior yearb | 168 (16.2) | 165 (15.8) | .84 |
| ȃIntensive care unit stay | 206 (19.7) | 188 (17.8) | .27 |
| ȃSurgery | 399 (38.2) | 392 (37.2) | .63 |
| ȃDecolonizing agents | 81 (7.8) | 92 (8.7) | .40 |
| ȃMupirocin | 76 (7.3) | 78 (7.4) | .89 |
| ȃChlorhexidine body wash | 5 (0.5) | 14 (1.3) | .06 |
| ȃMRSA infectione | 438 (41.4) | 447 (42.1) | .76 |
| ȃWound at discharge | 588 (56.3) | 587 (55.6) | .77 |
| ȃMedical device at discharge | 307 (23.7) | 320 (30.3) | .63 |
| ȃDischarged to nursing home | 116 (11.0) | 120 (11.3) | .81 |
Parts of this table have been published previously [13].
Abbreviations: MRSA, methicillin-resistant Staphylococcus aureus; SD, standard deviation.
Reflects responses to the survey question among participants. Not all participants responded to every question.
Data reflect a positive response to either a survey question or chart review. Not all participants responded to every question, and not all enrollment charts were received from recruiting hospitals despite a signed release request (N = 21 missing).
By law, California requires hospitals to screen 5 patient groups for MRSA on hospital admission (patients who are transferred from a nursing home, hospitalized in the past 30 days, on hemodialysis, undergoing imminent surgery, and admitted to an intensive care unit).
Data reflect chart review from received medical records. Not all recruiting hospitals released participants’ medical records to the study despite a signed release request (N = 21 missing).
Reflects primary study outcome based on Centers for Disease Control and Prevention criteria.
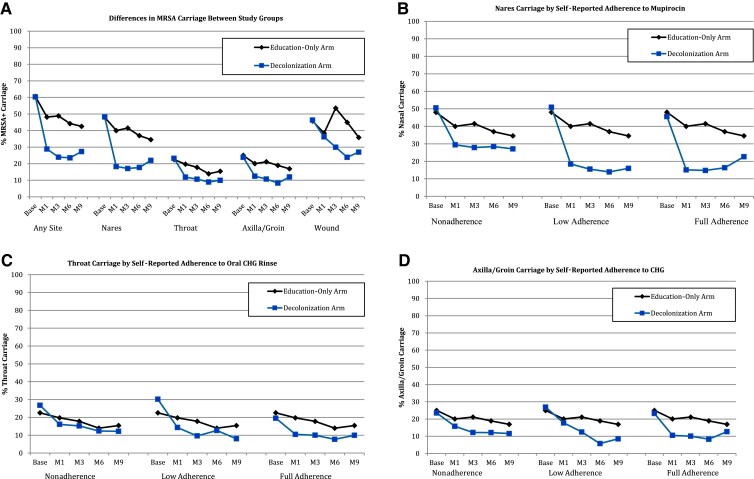
At enrollment, all participants had recent hospital cultures for MRSA per eligibility criteria. Swabs taken after enrollment were performed from the nose, throat, axilla/groin, and wound (if any) and revealed similar proportions of participants who were positive for MRSA: 60% in the decolonization group and 61% in the education group (P = .86). Site-specific baseline colonization at the nares, throat, and axilla did not differ between groups (Table 2). On all follow-up visits, MRSA colonization was higher in the education group vs the decolonization group: M1: 48%, 399 of 828 vs 29%, 226 of 783, P ≤ .001; M3: 49%, 381 of 780 vs 24%, 177 of 739, P ≤ .001; M6: 44%, 319 of 721 vs 24%, 159 of 675, P ≤ .001; and M9: 43%, 282 of 663 vs 27%, 174 of 636, P ≤ .001; Figure 1A). Similar colonization differences were seen in the nares, throat, and axilla/groin (Figure 1B–D). Figure 2 illustrates similar differences in MRSA colonization between groups in the subset of participants who completed all visits.
Table 2.
Methicillin-Resistant Staphylococcus aureus Colonization Differences Between Treatment Groups at Baseline and Follow-up Visits
| Body site | Decolonization,% (N/D) | Education, % (N/D) | Decolonization Group | Education vs Decolonization |
|---|---|---|---|---|
| Baseline | Baseline vs Follow-up, P Value | Groups at Each Visit, P Value | ||
| Any site | 60.3 (629/1044) | 60.6 (633/1044) | .86 | |
| Nares | 48.0 (501/1044) | 47.9 (500/1044) | .96 | |
| Axilla/Groin | 23.6 (246/1044) | 24.7 (258/1044) | .53 | |
| Throat | 22.6 (236/1044) | 22.0 (230/1044) | .75 | |
| Wound | 46.3 (101/218) | 45.8 (87/190) | .91 | |
| Month 1 Follow-up | ||||
| Any site | 28.9 (226/783) | 48.2 (625/1611) | <.001 | <.001 |
| Nares | 18.3 (143/783) | 39.9 (330/828) | <.001 | <.001 |
| Axilla/Groin | 12.4 (97/783) | 19.9 (165/828) | <.001 | <.001 |
| Throat | 11.8 (92/783) | 19.4 (161/828) | <.001 | <.001 |
| Wound | 36.2 (34/94) | 38.5 (40/104) | .1 | .74 |
| Month 3 Follow-up | ||||
| Any site | 24.0 (177/739) | 48.9 (558/780) | <.0001 | <.0001 |
| Nares | 17.1 (126/739) | 41.4 (323/780) | <.0001 | <.0001 |
| Axilla/Groin | 10.7 (79/739) | 21.2 (165/780) | <.0001 | <.0001 |
| Throat | 10.6 (78/739) | 17.7 (138/780) | <.0001 | <0.0001 |
| Wound | 30.0 (15/50) | 53.5 (38/71) | .03 | .01 |
| Month 6 Follow-up | ||||
| Any site | 23.6 (159/675) | 44.2 (319/721) | <.0001 | <.0001 |
| Nares | 17.6 (119/675) | 36.9 (266/721) | <.0001 | <.0001 |
| Axilla/Groin | 8.3 (56/675) | 18.9 (136/721) | <.0001 | <.0001 |
| Throat | 8.89 (60/675) | 13.87 (100/721) | <.0001 | 0.0035 |
| Wound | 23.91 (11/46) | 45 (27/60) | .0052 | .025 |
| Month 9 Follow-up | ||||
| Any site | 27.36 (174/636) | 42.53 (282/663) | <.0001 | <.0001 |
| Nares | 22.01 (140/636) | 34.54 (229/663) | <.0001 | <.0001 |
| Axilla/Groin | 11.95 (76/636) | 16.89 (112/663) | <.0001 | .01 |
| Throat | 9.91 (63/636) | 15.38 (102/663) | <.0001 | .003 |
| Wound | 27.03 (10/37) | 35.85 (19/53) | .02 | .38 |
N/D, numerator/denominator.
Figure 1.
Differences in MRSA carriage between study groups (all patients). A, The proportion of overall and site-specific MRSA colonization among trial participants by decolonization and education groups. Note that the intervention lasted 6 months total, so that month 9 data represent colonization 3 months post-discontinuation of decolonization agents (treatment group only). Also note that not all participants had wounds amenable to culture. MRSA colonization at any site was significantly different between the groups at months 1, 3, 6, and 9. Colonization at nares (B), throat (C), and axilla/groin (D) by group at each follow-up time point stratified by adherence of each corresponding product (B), nasal iodophor; (C), chlorhexidine body wash; (D), chlorhexidine mouthwash. In a repeated measures model, differences in colonization prevalence at the nares, throat, and axilla/groin were significant between the education-only group and each of the 3 strata of adherence in the intervention group (P < .01 for all comparisons; see text for details). Abbreviations: base, baseline; CHG, chlorhexidine gluconate; M1, month 1; M3, month 3; M6, month 6; M9, month 9; MRSA, methicillin-resistant Staphylococcus aureus.
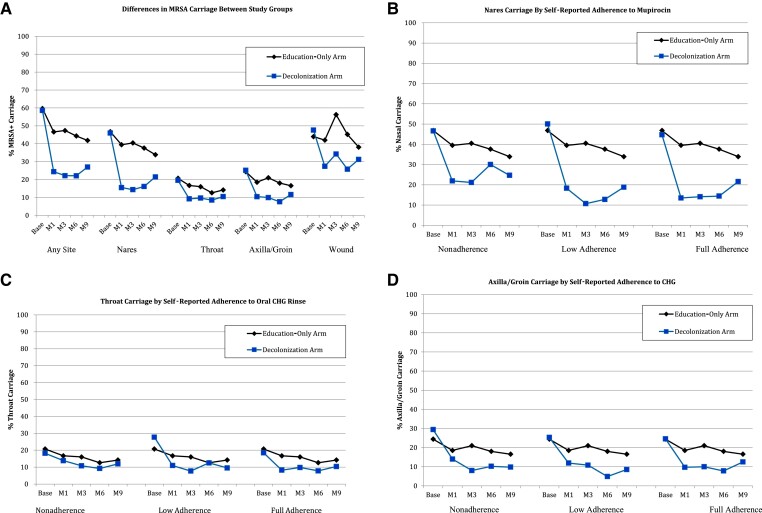
Figure 2.
Differences in MRSA carriage between study groups in the subgroup of participants who completed all visits. MRSA colonization prevalence is shown for the decolonization and education-only groups among only patients who completed all visits (n = 1134). Note that the intervention lasted 6 months total, so that month 9 data represent colonization 3 months post-discontinuation of decolonization agents (treatment group only). Also note that not all participants had wounds amenable to culture. Differences in colonization prevalence were significant (P < .001) at months 1, 3, 6, and 9 (see text). Comparisons of any colonization (A) and colonization in the nares (B), throat (C), and axilla/groin (D) at each time point stratified by adherence of each corresponding product (B, nasal iodophor; C, topical chlorhexidine gluconate; D, oral chlorhexidine gluconate mouthwash). In a repeated measures model, differences in colonization prevalence at the nares, throat, and axilla/groin were significant between the education-only group and each of the 3 strata of adherence in the intervention group (P < .01 for all comparisons; see text for details). Abbreviations: base, baseline; CHG, chlorhexidine gluconate; M1, month 1; M3, month 3; M6, month 6; M9, month 9; MRSA, methicillin-resistant Staphylococcus aureus.
At the M1 visit, overall MRSA colonization was lower in the decolonization group compared with the education group (OR = 0.44; 95% confidence interval [CI], .36–.54; P < .001). Significant reductions were seen in the nares (OR = 0.34; 95% CI, .27–.42; P < .001), throat (OR = 0.55; 95% CI, .42–.73; P < .001), and axilla/groin (OR = 0.57; 95% CI, .43–.75; P < .001). At the M6 visit, overall MRSA colonization remained lower in the decolonization group for nares (OR = 0.37; 95% CI, .28–.47; P < .001), throat (OR = 0.61; 95% CI, .43–.85; P = .003), axilla/groin (OR = 0.39; 95% CI, .28–.57; P < .001), and wounds (OR = 0.38; 95% CI, .16–.90; P = .02). At the M9 visit, overall MRSA colonization remained lower in the decolonization group for nares (OR = 0.53; 95% CI, .42–.68; P < .001), throat (OR = 0.60; 95% CI, .43–.85; P = .003), axilla/groin (OR = 0.67; 95% CI, .49–.91; P = .01), but not for wounds (OR = 0.66; 95% CI, .26–1.66; P = .38).
Among prespecified trial subgroups, MRSA colonization significantly decreased among Hispanics, non-Hispanics, recent surgery participants, and nursing home residents when comparing the decolonization group to the education group (P < .01 for comparisons at all time points; Table 3). There were also differences in colonization among diabetics, nondiabetics, participants on hemodialysis, and those not on hemodialysis at all time points except among participants with hemodialysis at month 9, although the sample size of that population was relatively small (Table 3).
Table 3.
Changes in Methicillin-Resistant Staphylococcus aureus Carriage in Selected Subgroups
| Body site | MRSA Carriers Decolonization Group,% (N/D) | MRSA Carriers Education Group, % (N/D) | Decolonization Group, Baseline vs Follow-up: P Value | Education vs Decolonization Groups at Each Visit: P Value |
|---|---|---|---|---|
| Baseline | ||||
| Hispanic, nursing home resident | 58.3 (14/24) | 63.6 (14/22) | .71 | |
| Hispanic, non-nursing home resident | 53.3 (168/315) | 52.1 (162/311) | .75 | |
| Non-Hispanic, nursing home resident | 62.9 (56/89) | 63.8 (60/94) | .90 | |
| Non-Hispanic, non-nursing home resident | 63.5 (391/616) | 64.3 (397/617) | .75 | |
| Recent surgery at time of enrollment | 51.1 (201/393) | 51.9 (200/385) | .82 | |
| Diabetes | 62.1 (283/456) | 59.4 (246/414) | .42 | |
| No diabetes | 58.8 (344/585) | 61.4 (386/629) | .36 | |
| Hemodialysis | 67.4 (89/132) | 62.8 (86/137) | .42 | |
| No hemodialysis | 59.2 (538/909) | 60.3 (546/906) | .63 | |
| Month 1 follow-up | ||||
| Hispanic, nursing home resident | 31.6 (6/19) | 88.2 (15/17) | .08 | .001 |
| Hispanic, non-nursing home resident | 22.0 (56/254) | 44.4 (111/250) | <.001 | <.001 |
| Non-Hispanic, nursing home resident | 35.4 (23/65) | 46.3 (37/80) | <.001 | .18 |
| Non-Hispanic, non-nursing home resident | 31.7 (141/445) | 49.1 (236/481) | <.001 | <.001 |
| Recent surgery at time of enrollment | 22.3 (67/300) | 38.9 (119/306) | <.001 | <.001 |
| Diabetes | 33.8 (120/355) | 44.9 (149/332) | <.001 | .003 |
| No diabetes | 24.8 (106/428) | 50.4 (250/496) | <.001 | <.001 |
| Hemodialysis | 30.8 (28/91) | 49.5 (52/105) | <.001 | .008 |
| No hemodialysis | 28.6 (198/692) | 48.0 (347/723) | <.001 | <.001 |
| Month 3 follow-up | ||||
| Hispanic, nursing home resident | 31.6 (6/19) | 76.5 (13/17) | .08 | .007 |
| Hispanic, non-nursing home resident | 18.1 (45/249) | 44.8 (107/239) | <.001 | <.001 |
| Non-Hispanic, nursing home resident | 39.3 (22/56) | 55.1 (38/69) | .005 | .07 |
| Non-Hispanic, non-nursing home resident | 25.1 (104/415) | 49.0 (223/455) | <.001 | <.001 |
| Recent surgery at time of enrollment | 18.6 (54/291) | 41.3 (119/288) | <.001 | <.001 |
| Diabetes | 25.9 (88/340) | 49.4 (156/316) | <.001 | <.001 |
| No diabetes | 22.3 (89/399) | 48.5 (225/464) | <.001 | <.001 |
| Hemodialysis | 23.3 (21/90) | 52.1 (50/96) | <.001 | <.001 |
| No hemodialysis | 24.0 (156/649) | 48.4 (331/684) | <.001 | <.001 |
| Month 6 follow-up | ||||
| Hispanic, nursing home resident | 29.4 (5/17) | 73.3 (11/15) | .06 | .01 |
| Hispanic, non-nursing home resident | 19.5 (44/226) | 35.9 (80/223) | <.001 | <.001 |
| Non-Hispanic, nursing home resident | 27.5 (14/51) | 41.7 (25/60) | <.001 | .11 |
| Non-Hispanic, non-nursing home resident | 25.2 (96/381) | 48.0 (203/423) | <.001 | <.001 |
| Recent surgery at time of enrollment | 21.3 (57/268) | 36.6 (102/279) | <.001 | <.001 |
| Diabetes | 24.1 (74/307) | 43.2 (128/296) | <.001 | <.001 |
| No diabetes | 23.1 (85/368) | 44.9 (191/425) | <.001 | <.001 |
| Hemodialysis | 22.5 (18/80) | 47.7 (41/86) | <.001 | <.001 |
| No hemodialysis | 23.7 (141/595) | 43.8 (278/635) | <.001 | <.001 |
| Month 9 follow-up | ||||
| Hispanic, nursing home resident | 30.8 (4/13) | 64.3 (9/14) | .11 | .08 |
| Hispanic, non-nursing home resident | 21.5 (46/214) | 37.7 (80/212) | <.001 | <.001 |
| Non-Hispanic, nursing home resident | 32.0 (16/50) | 45.8 (22/48) | .0005 | .16 |
| Non-Hispanic, non-nursing home resident | 30.1 (108/359) | 44.0 (171/389) | <.001 | <.001 |
| Recent surgery at time of enrollment | 24.8 (64/258) | 38.0 (98/258) | <.001 | .001 |
| Diabetes | 28.4 (82/289) | 46.3 (126/272) | <.001 | <.001 |
| No diabetes | 26.5 (92/347) | 39.9 (156/391) | <.001 | <.001 |
| Hemodialysis | 36.5 (27/74) | 49.4 (38/77) | <.001 | .11 |
| No hemodialysis | 26.2 (147/562) | 41.6 (244/586) | <.001 | <.001 |
Abbreviation: MRSA, methicillin-resistant Staphylococcus aureus.
Among participants in the decolonization group, self-reported product adherence to chlorhexidine body wash, chlorhexidine mouthwash, and mupirocin was 82%, 79%, and 80% at M1; 88%, 87%, and 85% at M3; and 88%, 86%, and 85% at M6, respectively. At M6, study participants’ adherence to chlorhexidine body wash, chlorhexidine mouthwash, and mupirocin, respectively, was grouped as follows: 12%, 14%, and 15% participants were nonadherent; 16%, 13%, and 20% were partially adherent; and 73%, 74%, and 65% were fully adherent. For all subgroups of adherence at all time points (M1 through M6), site-specific colonization was significantly lower for the education-only group for all comparisons (P < .01 for all comparisons; Figure 1B–D).
In the multivariate model, factors associated with MRSA colonization at month 9 included Medicaid insurance (OR = 1.43; 95% CI, 1.20–1.70; P < .001) and cancer (OR = 1.23; 95% CI, 1.05–1.60; P = .02). Decolonization group (OR = 0.60; 95% CI, .52–.69; P < .001) and Hispanic ethnicity (OR = 0.66; 95% CI, .56–.79; P < .001) were inversely associated with MRSA colonization.
DISCUSSION
The CLEAR Trial demonstrated that post-discharge decolonization of the nares, throat, and skin reduced MRSA infection and all-cause infection in MRSA carriers in the year following hospitalization [13]. This analysis identified significant reductions in MRSA colonization in the nares, throat, and skin and overall colonization associated with the decolonization strategy.
Here, we describe the efficacy of a MRSA decolonization regimen using widely available chlorhexidine antiseptic products plus mupirocin as a common nasal antimicrobial agent. Our results affirm the efficacy of self-administration of anti-MRSA topical products by patients and/or their caregivers after hospital discharge. Other investigations have examined the ability of outpatients to perform decolonization, for example, among patients on maintenance hemodialysis and prior to major surgical procedures [9, 14, 15]. This trial provides a randomized, controlled investigation to examine decolonization in MRSA carriers (colonized or infected) following hospital discharge. While a prior investigation examined the impact of polyhexanide-based topical decolonization combined with thrice daily mupirocin for 5 days, the study was not randomized and examined only 77 post-discharge patients [16]. In that study, decolonization was successful in >50% of participants, although the efficacy in the outpatient post-discharge subgroup was not described. Our findings demonstrate that verbal and written instructions, which were provided by trained research associates [13], are a feasible mechanism for educating patients on how to perform decolonization. Participants were able to carry out these instructions successfully, despite many of them being of older age with a high prevalence of comorbidities.
Adherence to study products was not 100%, as would be expected given that most patients’ adherence to any treatment is imperfect [17]. The mean self-reported adherence to the 3 study products was 79%–88%, which was likely overestimated since patients’ self-reported adherence typically overestimates true adherence [18]. Nevertheless, the relationship between higher adherence and lower subsequent colonization indicates 3 things. First, the findings strongly support the validity of the self-reported measure given the observed “dose-dependent” relationship between adherence and colonization. Second, the findings suggest that our decolonization strategy is effective, even in the partially adherent. Third, these data suggest that decolonization outcomes may be further enhanced by additional educational or other interventions to improve adherence and successful clearance or infection reduction due to the sizable minority (15%) that reported nonadherence with at least 1 decolonization product. Of note, in a single-center study of post-discharge MRSA decolonization, adherence to decolonization regimens was very poor (14%) [19]. We also found that patients who had Medicaid insurance or cancer were more likely to be MRSA-colonized at subsequent study visits. The reasons for these differences are unclear, although persons with Medicaid are of lower socioeconomic status, and previous studies have found a link between this and MRSA colonization [20]. Cancer is a known risk factor, likely due to repeated exposures to the healthcare system [21, 22], although it is unclear why this relationship was not seen in other groups with repeated exposures (hemodialysis, nursing home residence). Increased likelihood of colonization clearance was independently associated with Hispanic ethnicity, although reasons for this association are unclear and should be confirmed in other studies.
Decolonization efficacy differed slightly by body site. Overall, at month 6, MRSA colonization significantly decreased in the decolonization group compared with the control group by more than 60%. Nasal and wound colonization were similarly reduced by 63% and 62%, respectively, followed by skin carriage by 55% and throat carriage by 39%. Nasal mupirocin decolonization success is no surprise as it has been demonstrated repeatedly and consistently [23]. Skin decolonization has been used widely in studies of MRSA prevention in conjunction with nasal mupirocin and has the added benefit of reducing infection due to pathogens other than S. aureus [24]. In our trial, the magnitude of reduction of MRSA skin colonization was similar to that of nares.
Throat decolonization, however, is less well studied. Oral 0.12% chlorhexidine mouthwash is the gold standard in periodontal hygiene, including oral care in ventilated patients [25, 26]. However, data on chlorhexidine pharyngeal MRSA decolonization are relatively sparse and largely limited to hospitalized patients [27]. While throat colonization with MRSA and S. aureus can be substantial [28–30], it was similar to the baseline skin colonization in our population (22%–23%). Notably, the 80% reported adherence with chlorhexidine mouthwash only generated half the odds of throat clearance compared with the effect of chlorhexidine body wash on skin clearance. More studies are needed to assess effective methods for eradicating throat colonization and to quantify the increased value beyond mupirocin and chlorhexidine body wash.
It is worth highlighting that the reductions in MRSA clearance were sustained over time, even after the decolonization protocol ended. Overall MRSA colonization in the decolonization group at follow-up visits at month 3, 6, and even 9 (3 months after the decolonization protocol ended) were similar or slightly lower than MRSA clearance gains noted 1 month into the regimen. Sustained decolonization was seen at all individual body sites evaluated (nares, throat, axilla/groin, and wound). These data are consistent with the fact that participants continued to be adherent to the decolonization regimens, despite the time and effort that the treatments impose. Alternately, our findings may suggest that there is a cumulative decolonization effect that may mitigate any waning of medication adherence, as suggested by the persistent benefit seen 3 months after decolonization was stopped. This effect may be due to achieving permanent clearance vs MRSA suppression. Similar long-lasting effects have been reported in some studies of medication adherence [31–33].
There are limitations to our study. First, the frequency and duration of the decolonization regimen, 5 days twice monthly for 6 months, was selected as the protocol for the CLEAR Trial. It is not known whether more frequent administration may be more effective or, alternatively, so burdensome that it would lower adherence to the regimen. Nevertheless, the reduction in the odds of MRSA colonization by more than half and the associated infection reduction seen in the CLEAR Trial’s primary outcomes suggest that this is a highly successful decolonization regimen [13]. Second, since we gave all interventions (chlorhexidine body wash, chlorhexidine mouth wash, nasal mupirocin) synchronously, it is unclear if individual components, such as oral chlorhexidine, are truly needed to reduce decolonization. Finally, we did not perform strain typing on MRSA isolates. It is possible that some of the decolonization “failures” were actually colonization with a new strain, a phenomenon that has been observed in studies of decolonization [34, 35]. Nevertheless, even if colonization with new strains occurred, such findings would further confirm the need for repeated decolonization of colonizing strains in the post-hospitalization setting.
There are strengths to our study. First, our study is the first randomized trial to evaluate MRSA decolonization after hospital discharge. Second, our trial included an oral decolonization component that was generally lacking in prior studies of decolonization and may be an important neglected reservoir of S. aureus colonization. Finally, a third strength is the very large sample size and diverse patient population, which includes relatively healthy persons and those with multiple comorbidities, younger and older persons, and those who are colonized and those who are infected at hospital discharge.
In summary, we found that a self-performed periodic 6-month regimen of chlorhexidine body wash, chlorhexidine mouth wash, and nasal mupirocin was highly effective at persistently reducing MRSA colonization by more than 50% among MRSA carriers discharged from hospitals. These findings demonstrate that a home decolonization strategy is a practical and feasible means to reduce MRSA colonization in the nares, throat, and skin during a time when patients are highly vulnerable to infection. The reduction in colonization reinforces the previously reported trial findings of significantly reduced MRSA infections and all-cause infections in the year following discharge [13] and strongly suggests that the benefits were driven by reduction in MRSA colonization at multiple body sites.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support . This study was funded by the Agency for Healthcare Research and Quality (R01HS019388 to S. S. Huang). J. A. M. was supported in part by National Institutes of Health/National Center for Research Resources/National Center for Advancing Translational Sciences (NIH/NCRR/NCATS) (grant KL2TR000122 to the University of California–Los Angeles Clinical and Translational Science Institute). J. A. M., L. G. M., R. S., S. S. Huang, and L. H. report support for this work in the form of production donations from Medline, Sage, and Xttrium. R. S., S. S. Huang, and L. H. also report support for this work in the form of production donations from Molnlycke.
Supplementary Material
Contributor Information
Loren G Miller, Infectious Disease Clinical Outcomes Research Unit, Division of Infectious Disease, Los Angeles Biomedical Research Institute, Harbor–UCLA Medical Center, Torrance, California, USA.
Raveena Singh, Division of Infectious Diseases and Health Policy Research Institute, University of California, Irvine School of Medicine, Irvine, California, USA.
Samantha J Eells, Infectious Disease Clinical Outcomes Research Unit, Division of Infectious Disease, Los Angeles Biomedical Research Institute, Harbor–UCLA Medical Center, Torrance, California, USA.
Daniel Gillen, Division of Infectious Diseases and Health Policy Research Institute, University of California, Irvine School of Medicine, Irvine, California, USA.
James A McKinnell, Infectious Disease Clinical Outcomes Research Unit, Division of Infectious Disease, Los Angeles Biomedical Research Institute, Harbor–UCLA Medical Center, Torrance, California, USA.
Steven Park, Division of Infectious Diseases and Health Policy Research Institute, University of California, Irvine School of Medicine, Irvine, California, USA.
Tom Tjoa, Division of Infectious Diseases and Health Policy Research Institute, University of California, Irvine School of Medicine, Irvine, California, USA.
Justin Chang, Division of Infectious Diseases and Health Policy Research Institute, University of California, Irvine School of Medicine, Irvine, California, USA.
Syma Rashid, Division of Infectious Diseases and Health Policy Research Institute, University of California, Irvine School of Medicine, Irvine, California, USA.
Raul Macias-Gil, Infectious Disease Clinical Outcomes Research Unit, Division of Infectious Disease, Los Angeles Biomedical Research Institute, Harbor–UCLA Medical Center, Torrance, California, USA.
Lauren Heim, Division of Infectious Diseases and Health Policy Research Institute, University of California, Irvine School of Medicine, Irvine, California, USA.
Adrijana Gombosev, Division of Infectious Diseases and Health Policy Research Institute, University of California, Irvine School of Medicine, Irvine, California, USA.
Diane Kim, Division of Infectious Diseases and Health Policy Research Institute, University of California, Irvine School of Medicine, Irvine, California, USA.
Eric Cui, Division of Infectious Diseases and Health Policy Research Institute, University of California, Irvine School of Medicine, Irvine, California, USA.
Jennifer Lequieu, Division of Infectious Diseases and Health Policy Research Institute, University of California, Irvine School of Medicine, Irvine, California, USA.
Chenghua Cao, Division of Infectious Diseases and Health Policy Research Institute, University of California, Irvine School of Medicine, Irvine, California, USA.
Suzie S Hong, Division of Infectious Diseases and Health Policy Research Institute, University of California, Irvine School of Medicine, Irvine, California, USA.
Ellena M Peterson, Division of Infectious Diseases and Health Policy Research Institute, University of California, Irvine School of Medicine, Irvine, California, USA.
Kaye D Evans, Division of Infectious Diseases and Health Policy Research Institute, University of California, Irvine School of Medicine, Irvine, California, USA.
Bryn Launer, Infectious Disease Clinical Outcomes Research Unit, Division of Infectious Disease, Los Angeles Biomedical Research Institute, Harbor–UCLA Medical Center, Torrance, California, USA.
Steven Tam, Division of Infectious Diseases and Health Policy Research Institute, University of California, Irvine School of Medicine, Irvine, California, USA.
Michael Bolaris, Infectious Disease Clinical Outcomes Research Unit, Division of Infectious Disease, Los Angeles Biomedical Research Institute, Harbor–UCLA Medical Center, Torrance, California, USA.
Susan S Huang, Division of Infectious Diseases and Health Policy Research Institute, University of California, Irvine School of Medicine, Irvine, California, USA.
References
- 1. Sievert DM, Ricks P, Edwards JR, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention 2009–2010. Infect Control Hosp Epidemiol 2013; 34:1–14. [DOI] [PubMed] [Google Scholar]
- 2. Klein E, Smith DL, Laxminarayan R. Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus. United States 1999–2005. Emerg Infect Dis 2007; 13: 1840–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang SS, Hinrichsen VL, Datta R, et al. Methicillin-resistant Staphylococcus aureus infection and hospitalization in high-risk patients in the year following detection. PLoS One 2011; 6:e24340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Datta R, Huang SS. Risk of infection and death due to methicillin-resistant Staphylococcus aureus in long-term carriers. Clin Infect Dis 2008; 47:176–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nelson RE, Evans ME, Simbartl L, et al. Methicillin-resistant Staphylococcus aureus colonization and pre- and post-hospital discharge infection risk. Clin Infect Dis 2019; 68:545–53. [DOI] [PubMed] [Google Scholar]
- 6. Avery TR, Kleinman KP, Klompas M, Aschengrau A, Huang SS. Inclusion of 30-day postdischarge detection triples the incidence of hospital-onset methicillin-resistant Staphylococcus aureus. Infect Control Hosp Epidemiol 2012; 33:114–21. [DOI] [PubMed] [Google Scholar]
- 7. Dantes R, Mu Y, Belflower R, et al. National burden of invasive methicillin-resistant Staphylococcus aureus infections. United States 2011. JAMA Intern Med 2013; 173:1970–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schweizer ML, Chiang HY, Septimus E, et al. Association of a bundled intervention with surgical site infections among patients undergoing cardiac, hip, or knee surgery. JAMA 2015; 313:2162–71. [DOI] [PubMed] [Google Scholar]
- 9. Bode LGM, Kluytmans JAJW, Wertheim HFL, et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med 2010; 362:9–17. [DOI] [PubMed] [Google Scholar]
- 10. Huang SS, Septimus E, Kleinman K, et al. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med 2013; 368:2255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Climo MW, Sepkowitz KA, Zuccotti G, et al. The effect of daily bathing with chlorhexidine on the acquisition of methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus, and healthcare-associated bloodstream infections: results of a quasi-experimental multicenter trial. Crit Care Med 2009; 37:1858–65. [DOI] [PubMed] [Google Scholar]
- 12. Milstone AM, Elward A, Song X, et al. Daily chlorhexidine bathing to reduce bacteraemia in critically ill children: a multicentre, cluster-randomised, crossover trial. Lancet 2013; 381:1099–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang SS, Singh R, McKinnell JA, et al. Decolonization to reduce postdischarge infection risk among MRSA carriers. N Engl J Med 2019; 380:638–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Perl TM, Cullen JJ, Wenzel RP, et al. Intranasal mupirocin to prevent postoperative Staphylococcus aureus infections. N Engl J Med 2002; 346:1871–7. [DOI] [PubMed] [Google Scholar]
- 15. Kang YC, Tai WC, Yu CC, Kang JH, Huang YC. Methicillin-resistant Staphylococcus aureus nasal carriage among patients receiving hemodialysis in Taiwan: prevalence rate, molecular characterization and de-colonization. BMC Infect Dis 2012; 12:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jahn B, Wassenaar TM, Stroh A. Integrated MRSA-management (IMM) with prolonged decolonization treatment after hospital discharge is effective: a single centre, non-randomised open-label trial. Antimicrob Resist Infect Control 2016; 5:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005; 353:487–97. [DOI] [PubMed] [Google Scholar]
- 18. Liu H, Golin CE, Miller LG, et al. A comparison study of multiple measures of adherence to HIV protease inhibitors. Ann Intern Med 2001; 134:968–77. [DOI] [PubMed] [Google Scholar]
- 19. Hellman MD, Davis KW, Hackworth Bet al. Implementing a postdischarge methicillin-resistant Staphylococcus aureus decolonization protocol within a Veterans Affairs health care system facility. Infect Control Hosp Epidemiol 2022. doi: 10.1017/ice.2021.225. [DOI] [PubMed] [Google Scholar]
- 20. Charlebois ED, Bangsberg DR, Moss NJ, et al. Population-based community prevalence of methicillin-resistant Staphylococcus aureus in the urban poor of San Francisco. Clin Infect Dis 2002; 34:425–33. [DOI] [PubMed] [Google Scholar]
- 21. Hidron AI, Kourbatova EV, Halvosa JS, et al. Risk factors for colonization with methicillin-resistant Staphylococcus aureus (MRSA) in patients admitted to an urban hospital: emergence of community-associated MRSA nasal carriage. Clin Infect Dis 2005; 41:159–66. [DOI] [PubMed] [Google Scholar]
- 22. Schaefer AM, McMullen KM, Mayfield JL, Richmond A, Warren DK, Dubberke ER. Risk factors associated with methicillin-resistant Staphylococcus aureus colonization on hospital admission among oncology patients. Am J Infect Control 2009; 37:603–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Coates T, Bax R, Coates A. Nasal decolonization of Staphylococcus aureus with mupirocin: strengths, weaknesses and future prospects. J Antimicrob Chemother 2009; 64:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang SS. Chlorhexidine-based decolonization to reduce healthcare-associated infections and multidrug-resistant organisms (MDROs): who, what, where, when, and why? J Hosp Infect 2019; 103:235–43. [DOI] [PubMed] [Google Scholar]
- 25. FDI Commission . Mouth rinses and dental caries. Int Dent J 2002; 52:337–45. [PubMed] [Google Scholar]
- 26. Jorgensen MG, Slots J. Antimicrobials in periodontal maintenance. J Dent Hyg 2001; 75:233–9. [PubMed] [Google Scholar]
- 27. Segers P, Speekenbrink RG, Ubbink DT, van Ogtrop ML, de Mol BA. Prevention of nosocomial infection in cardiac surgery by decontamination of the nasopharynx and oropharynx with chlorhexidine gluconate: a randomized controlled trial. JAMA 2006; 296:2460–6. [DOI] [PubMed] [Google Scholar]
- 28. Mertz D, Frei R, Jaussi B, et al. Throat swabs are necessary to reliably detect carriers of Staphylococcus aureus. Clin Infect Dis 2007; 45:475–7. [DOI] [PubMed] [Google Scholar]
- 29. Yang ES, Tan J, Eells S, Rieg G, Tagudar G, Miller LG. Body site colonization prevalence in patients with community-associated methicillin-resistant Staphylococcus aureus and other forms of Staphylococcus aureus and skin infections. Clin Microbiol Infect 2010; 16:425–31. [DOI] [PubMed] [Google Scholar]
- 30. Miller LG, Eells SJ, Taylor AR, et al. Staphylococcus aureus colonization among household contacts of patients with skin infections: risk factors, strain discordance, and complex ecology. Clin Infect Dis 2012; 54:1523–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Demyttenaere K. Compliance during treatment with antidepressants. J Affect Disord 1997; 43:27–39. [DOI] [PubMed] [Google Scholar]
- 32. Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther 2001; 23:1296–310. [DOI] [PubMed] [Google Scholar]
- 33. Antoniou T, Gomes T, Juurlink DN, Loutfy MR, Glazier RH, Mamdani MM. Trimethoprim-sulfamethoxazole-induced hyperkalemia in patients receiving inhibitors of the renin-angiotensin system: a population-based study. Arch Intern Med 2010; 170:1045–9. [DOI] [PubMed] [Google Scholar]
- 34. Wertheim HF, Verveer J, Boelens HA, van Belkum A, Verbrugh HA, Vos MC. Effect of mupirocin treatment on nasal, pharyngeal, and perineal carriage of Staphylococcus aureus in healthy adults. Antimicrob Agents Chemother 2005; 49:1465–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Doebbeling BN, Reagan DR, Pfaller MA, Houston AK, Hollis RJ, Wenzel RP. Long-term efficacy of intranasal mupirocin ointment. Arch Intern Med 1994; 154:1505–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.