Abstract
Background
Heroin use may work synergistically with human immunodeficiency virus (HIV) infection to cause greater immune dysregulation than either factor alone. Unraveling how this affects end-organ disease is key as it may play a role in the excess mortality seen in people with HIV (PWH) who use heroin despite access to care and antiretroviral therapy.
Methods
This is a prospectively enrolled, cross-sectional study of adults with and without HIV who use and do not use heroin using (18)F-fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) to compare tissue-specific inflammation including aortic (target-to-background ratio [TBR]), splenic, and bone marrow (standardized uptake value [SUV]).
Results
A total of 120 participants were enrolled. The unadjusted mean difference in aortic TBR was 0.43 between HIV-positive [HIV+] heroin+ and HIV+ heroin-negative [heroin−] (P = .02); however, among HIV−, aortic TBR was similar regardless of heroin-use status. Further, HIV-by-heroin-use status interaction was significant (P = .02), indicating that the relationship between heroin use and higher aortic TBR depended on HIV status. On the other hand, both HIV (1.54 vs 1.68; P = .04, unadjusted estimated means for HIV+ vs HIV−) and heroin use were associated with lower bone marrow SUV, although the effect of heroin depended on sex (heroin-use-by-sex interaction, P = .03). HIV-by-heroin-use interaction was not significant for splenic or bone marrow SUV.
Conclusions
Aortic inflammation was greatest in PWH who use heroin, but paradoxically, bone marrow activity was the least in this group, suggesting complex and possibly divergent pathophysiology within these different end organs.
Keywords: HIV, heroin, inflammation, aorta, FDG-PET/CT
Among participants with HIV, heroin use was associated with the greatest vascular but lowest bone marrow inflammation as measured by FDG-PET/CT. These findings suggest complex and possibly divergent pathophysiology within these different end organs.
Despite antiretroviral therapy (ART) improvements, people with human immunodeficiency virus (PWH) continue to have increased morbidity and mortality compared with the general population often due to non–AIDS-related events [1, 2]. Beyond traditional risk factors and toxicity associated with ART, persistent inflammation and heightened immune activation have been clearly linked with cardiometabolic comorbidities in PWH [3–8].
We and others have linked injection drug use, specifically heroin use, to heightened microbial translocation, systemic inflammation, and immune activation in people with and without human immunodeficiency virus (HIV) [9–11]. This is relevant because, even in those surviving for 10 years on ART, PWH whose transmission risk was injection drug use are approximately 2.5 times more likely to die from all causes [12]. High-risk lifestyle, bacterial infection, or hepatitis C are likely contributors; however, cardiometabolic events driven by chronic immune activation may be playing an important role [13, 14]. This is highly relevant in the context of HIV where mechanisms of immune activation may overlap and work synergistically.
The mechanisms of heightened immune activation in PWH are multifactorial. Persistent low-level HIV replication occurring in lymph nodes or the gastrointestinal tract, inflammatory lipids, and coinfection with other viruses such as hepatitis B or cytomegalovirus are likely contributors [15–17]. Further, damage to the gastrointestinal tract allows microbial products to enter the lamina propria and systemic circulation, leading to immune dysfunction [18–20].
This study aims to evaluate vascular inflammation, an early step in atherosclerosis, by measuring aortic uptake of (18)F-fluorodeoxyglucose (FDG) by positron emission tomography/computed tomography (PET/CT) in people with and without HIV who use and do not use heroin. Using FDG-PET/CT is an established noninvasive means to quantify vascular inflammation, specifically metabolically active proinflammatory macrophages, as well as cardiovascular disease (CVD) risk [21]. We hypothesized that a synergistic relationship exists between HIV and heroin use, such that vascular inflammation would be greater in PWH who use heroin than in people with either risk factor alone. Additional aims include evaluating inflammation in the hematopoietic organs, including spleen and bone marrow.
METHODS
Study Population
This is a cross-sectional analysis of entry visit data from the Impact of Heroin on Immune ACTIVATion in HIV (ACTIVATE) Study. The ACTIVATE study is a prospective cohort study designed to examine the effect of heroin use on innate and acquired immune activation and vascular inflammation in PWH who actively use heroin (HIV+ heroin+) and age-, sex-, and CD4+ cell count–matched PWH who have never used heroin (HIV+ heroin−) at University Hospitals Cleveland Medical Center and MetroHealth Medical Center in Cleveland, Ohio. People without HIV who actively use heroin (HIV− heroin+) and those who have never used heroin (HIV− heroin−) were enrolled for a single visit for cross-sectional comparisons. Adults aged 18 years and older who reported currently injecting or snorting heroin for at least 1 month with cumulative past use of at least 12 months or no heroin use ever were included. Participants with HIV were required to have HIV-1 RNA of 400 copies/mL or less, be on stable antiretrovirals for at least 12 weeks, and have cumulative duration of ART of at least 12 months if on ART. Presence of an autoimmune disease, cancer, infection, pregnancy, diabetes mellitus, CVD, liver transaminases more than 2.5 times the upper normal limits, hemoglobin less than 9 g/dL, or creatinine clearance by Cockcroft-Gault less than 30 mL/minute were exclusionary. All participants provided written informed consent. The study protocol and informed consent were approved by the institutional review boards at University Hospitals of Cleveland and the MetroHealth System. The first 120 participants enrolled were included in this analysis.
Study Evaluations
FDG-PET/CT
Imaging was performed using methods described previously [22]. In brief, FDG was injected and, 3 hours later, images were acquired on a Philips Gemini TF Big Bore PET/CT scanner (Philips Healthcare, Andover, MA, USA). Images were analyzed offline using MIM version 6.6.6 by a reader blinded to participant clinical characteristics (S. E. J.). Standard uptake dose values (SUVs) were defined per standard convention as decay-corrected tissue concentration of FDG (in kBq/g) divided by injected dose per body weight (in kBq/g). “Mean-max” aortic target-to-background ratio (TBR) was defined as the average aortic SUVmax measured on consecutive axial slices through the ascending aorta from the sinus of valsalva to the aortic arch divided by the average of superior vena cava blood pool SUVmean measured from the same axial slices (mean aortic SUVmax ÷ mean superior vena cava SUVmean = mean-max aortic TBR). While both mean-max and mean-mean measures are widely used, most HIV studies have used mean-max [8, 23, 24]. We chose mean-max as our primary dependent variable of interest.
The borders of the spleen were traced using a 3-dimensional region-of-interest tool to determine spleen SUVmean as our dependent variable of interest for the spleen. For bone marrow, the SUV tool was used to include all of the bone marrow up to but not including the walls of the vertebrae to find the SUVmean for each slice from the sternum down 4 concentric thoracic vertebrae. The mean of the means or bone marrow SUVmean was calculated as our dependent variable of interest for bone marrow. Spleen SUVmean and SUVmax (r = 0.8; P < .01) and bone marrow SUVmean and SUVmax (r = 0.97; P < .01) were highly correlated.
Statistical Analysis
Differences between groups were compared using Wilcoxon rank-sum, Kruskal-Wallis, or general linear models for continuous variables and chi-square, Fisher's exact, or Pearson's exact chi-square tests for categorical variables, as appropriate. For the continuous FDG-PET/CT outcomes (aortic TBR, splenic, and bone marrow SUV), 3 a priori comparisons were made: (1) HIV+ heroin+ versus HIV+ heroin−, to test the heroin effect in the context of HIV infection; (2) HIV+ heroin + versus HIV− heroin+, to test the HIV effect in the context of heroin use; and (3) HIV− heroin+ versus HIV− heroin−, to test the heroin effect without HIV infection. To assess if there is a synergistic relationship between HIV and heroin use on the FDG-PET/CT outcomes, we used linear mixed models with a random intercept that included HIV status, heroin-use status, and an HIV-by-heroin-use interaction term. Adjusted models also included age, sex, race, pooled cohort equations atherosclerotic cardiovascular disease (ASCVD) risk score, body mass index (BMI), hepatitis C status, and self-reported substance use. Shapiro-Wilk’s test statistic was used to test the null hypothesis of normality of residuals; leverage greater than [(2 × number of predictors + 2)/n] and Cook's D cutoff greater than (4/n) were used to assess outcome values with large error variance. Extreme observations (n = 7) were set to missing. All 2- and 3-way interactions were assessed and Tukey-Kramer method was used to address multiple comparisons. P values less than .05 were considered statistically significant and all analyses were computed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
Demographic and Clinical Characteristics
From July 2017 to March 2021, 120 participants were enrolled (24 HIV+ heroin+, 59 HIV− heroin+, 21 HIV+ heroin−, 16 HIV− heroin−). Demographics and clinical characteristics are shown in Table 1. Overall, the median (interquartile range [IQR]) age was 41 (33–52) years and 73% were men. There were differences in race/ethnicity distribution among the groups (P < .01 overall). In the groups that use heroin, there were more participants of Hispanic ethnicity regardless of race (23% vs 5%; P = .02), more current/past smokers (100% vs 62%; P < .01), and more participants with active hepatitis C (54% vs 0%; P < .01), and BMI was lower (24 vs 27 kg/m2; P < .01). Polysubstance use was common. Of those who use heroin, 57% also reported currently using marijuana, 37% cocaine, and 13% amphetamines. Supplementary Table 1 shows results of urine drug screen analysis by group.
Table 1.
Demographics and Clinical Characteristics by Group
| HIV+ Heroin+ (n = 24) | HIV+ Heroin− (n = 21) | HIV− Heroin+ (n = 59) | HIV− Heroin− (n = 16) | P Value Overall | P Value Heroin+ vs Heroin− | P Value HIV+ vs HIV− | |
|---|---|---|---|---|---|---|---|
| Age, y | 47.1(33.1, 55.4) | 49.6(38.8, 53.8) | 38(32.7, 46.6) | 41.6(33.9, 51.4) | .18 | .22 | .04 |
| Male, n (%) | 18 (75) | 15 (71) | 40 (68) | 14 (88) | <.01 | <.01 | .28 |
| Race/ethnicity, n (%) | <.01 | <.01 | <.01 | ||||
| ȃWhite, not Hispanic | 12 (50) | 3 (14) | 41 (69) | 14 (88) | |||
| ȃBlack, not Hispanic | 3 (13) | 15 (71) | 6 (10) | 1 (6) | |||
| ȃOther, not Hispanic | 0 (0) | 2 (10) | 2 (3) | 0 (0) | |||
| ȃHispanic, regardless of race | 9 (38) | 1 (5) | 10 (17) | 1 (6) | |||
| Current or past smoker, n (%) | 24 (100) | 16 (76) | 59 (100) | 7 (44) | <.01 | <.01 | .88 |
| BMI, kg/m2 | 23.3 (21.8, 25.4) | 27.2 (23.7, 28.4) | 24.6 (22.5, 28.1) | 28.2 (25.9, 29.7) | .01 | <.01 | .28 |
| Hypertension diagnosis, n (%) | 3 (13) | 6 (29) | 8 (14) | 1 (6) | .25 | .42 | .23 |
| LDL cholesterol, mg/dL | 88 (70, 106) | 106 (76, 126) | 93 (75, 113) | 117 (92, 133) | .02 | <.01 | .5 |
| HDL cholesterol, mg/dL | 40 (35, 45) | 44 (32, 54) | 46 (37, 53) | 49 (40, 55) | .1 | .36 | .06 |
| On statin, n (%) | 0 (0) | 8 (38) | 1 (2) | 0 (0) | <.01 | <.01 | <.01 |
| ASCVD risk | 4.3 (3.4, 6.8) | 4.8 (3.1, 6.2) | 3.6 (1.9, 5.6) | 1.2 (1, 5.1) | .02 | .71 | .01 |
| Chronic hepatitis C, n (%) | 13 (54) | 0 (0) | 32 (54) | 0 (0) | <.01 | <.01 | .13 |
| CD4+ count, cells/mm3 | 562 (276, 784) | 767 (621, 1036) | … | … | .02 | ||
| Nadir CD4+ count, cells/mm3 | 251 (99, 523) | 212 (110, 280) | … | … | .37 | ||
| HIV RNA ≤400 copies/mL, n (%) | 22 (92) | 21 (100) | … | … | .18 | ||
| HIV duration, y | 11.6 (6.7, 19.3) | 14.1 (8.1, 19) | … | … | .72 | ||
| Antiretroviral duration, y | 6.1 (2.3, 13.1) | 9.1 (7.2, 13.8) | … | … | .08 | ||
| Heroin route, n (%) | .51 | ||||||
| ȃIntravenous | 18 (75) | … | 48 (81) | … | |||
| ȃIntranasal | 6 (25) | … | 11 (19) | … | |||
| Current substance use, n (%) | |||||||
| ȃMarijuana | 12 (50) | 16 (76) | 35 (59) | 4 (25) | .01 | .79 | .28 |
| ȃCocaine | 9 (38) | 2 (10) | 22 (37) | 2 (13) | .03 | <.01 | .38 |
| ȃAmphetamines | 3 (13) | 0 (0) | 8 (14) | 0 (0) | .14 | .02 | .46 |
| ȃHallucinogens | 0 (0) | 0 (0) | 1 (2) | 1 (6) | .39 | .55 | .27 |
N = 120. Values shown are median (interquartile range) and frequency (column %) for continuous and categorical variables, respectively. P values are from Kruskal-Wallis tests for continuous variables and chi-square, Fisher's exact, or Pearson's exact chi-square tests for categorical variables, as appropriate. Current substance use was assessed by self-report.
Abbreviations: ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; HDL, high density lipoprotein; HIV, human immunodeficiency virus; LDL, low density lipoprotein.
Among participants with HIV, the median duration of HIV was 13 years and 96% had HIV-1 RNA of less than 400 copies/mL. Heroin users with HIV were less likely to be Black and non-Hispanic (13% vs 71%; P < .01), and had lower current (562 vs 767 cells/mm3; P = .02) but similar nadir CD4+ counts (251 vs 212 cells/mm3; P = .37).
Vascular Inflammation by HIV and Heroin-Use Status
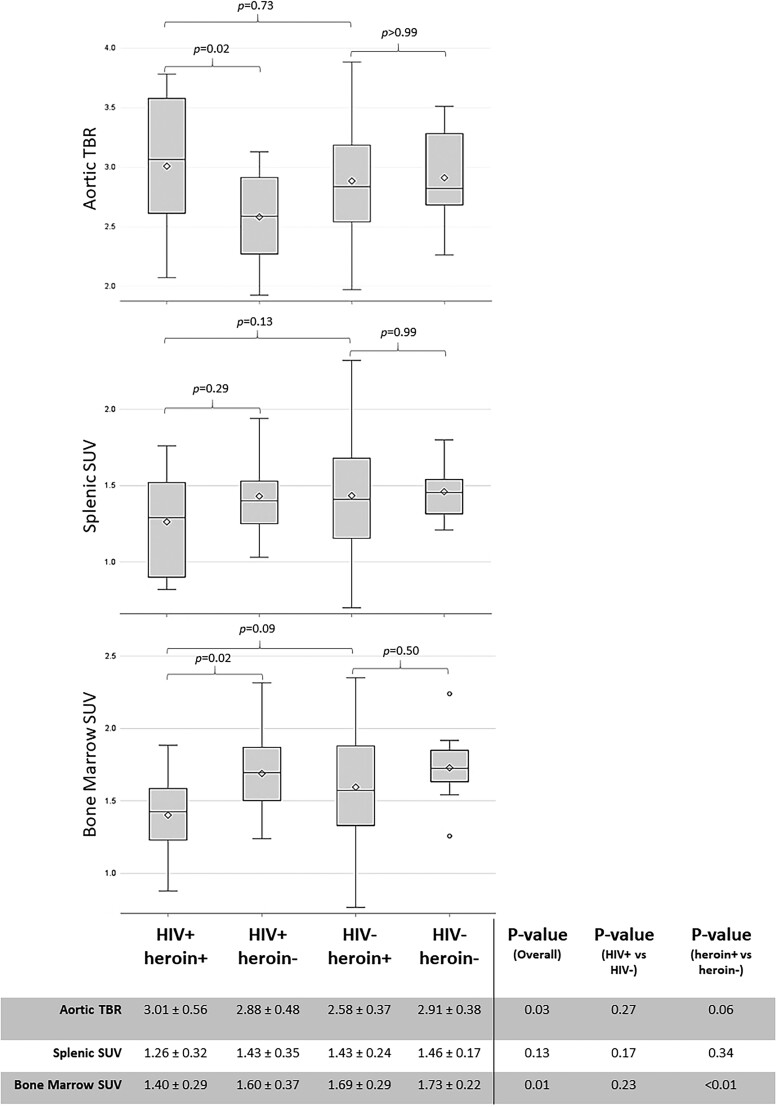
Figure 1 shows unadjusted aortic TBR results in the 4 groups. Based on the 3 a priori between-group comparisons made, using heroin was associated with higher aortic TBR in the context of HIV infection only (unadjusted mean difference aortic TBR: 0.43 between HIV+ heroin+ and HIV+ heroin− [P = .02] and −.03 between HIV− heroin+ vs HIV− heroin− [P > .99]). Further, aortic TBR was similar among all participants using heroin regardless of HIV status (unadjusted mean difference: 0.12 HIV+ heroin+ and HIV− heroin+; P = .73). In the model including HIV and heroin-use status, the HIV-by-heroin-use interaction was significant (ρ = 0.45; P = .02), indicating that the effect of heroin use on aortic TBR was greater in participants with HIV. The interaction remained significant after adjustment for ASCVD risk score, hepatitis C, and BMI (ρ = 0.44; P = .03), and after adjustment for amphetamines, cocaine, smoking, and alcohol (P ≤ .03 for all). The interaction was no longer significant in models adjusted for demographics only (age, sex, race) (ρ = 0.34; P = .12) or marijuana use (ρ = 0.34; P = .1). Participants who self-reported current marijuana use had −0.19 lower aortic TBR than nonusers (P < .05). (See Supplementary Tables 2 and 3A for linear mixed models described above.) None of the systemic inflammation markers previously described [11] were associated with aortic TBR in this study population (data not shown).
Figure 1.
Boxplots of aortic, splenic and bone marrow uptake by (18)F-fluorodeoxyglucose positron emission tomography/computed tomography by group. Values shown are unadjusted mean standard deviation. Abbreviations: HIV, human immunodeficiency virus; SUV, standardized uptake value; TBR, target-to-background ratio.
Splenic and Bone Marrow Inflammation by HIV and Heroin-Use Status
Splenic SUV was similar between HIV+ heroin+ and HIV+ heroin− (unadjusted mean difference: −0.17; P = .29), HIV+ heroin+ and HIV− heroin+ (unadjusted mean difference: −0.17; P = .13), and HIV− heroin+ and HIV− heroin− (unadjusted mean difference: −0.03; P = .99) (see Figure 1). Further, the HIV-by-heroin-use interaction was not significant (ρ = −0.14; P = .27) in the bivariable model. However, there was some evidence that HIV status, but not heroin-use status, was associated with splenic SUV (ρ = −0.12 [P = .06] and ρ = −0.09 [P = .16] for HIV status and heroin-use status, respectively). Participants with HIV had 9% lower splenic SUV compared with those without HIV (1.35 vs 1.47; P = .06; unadjusted estimated means for HIV+ vs HIV−). The association between HIV status and splenic SUV was similar in models adjusted for ASCVD risk score, BMI, and hepatitis C (ρ = −0.12; P = .03), demographics only (age, sex, and race) (ρ = −0.13; P = .05), and after adjustment for amphetamines, cocaine, marijuana, and alcohol (P ≤ .06 for all). HIV status was no longer significant in the model adjusted for smoking only (ρ = −0.1; P = .13).
Based on the 3 a priori between-group comparisons made, using heroin was associated with lower bone marrow SUV in the context of HIV only (unadjusted mean difference bone marrow SUV: −0.29 between HIV+ heroin+ and HIV+ heroin− [P = .02] and −0.13 [P = .50] for HIV− heroin+ vs HIV− heroin−). Further, bone marrow SUV was similar among all participants using heroin regardless of HIV status (unadjusted mean difference: −0.19 HIV+ heroin+ and HIV− heroin+; P = .09). In the model with HIV and heroin-use status, the HIV-by-heroin-use interaction was not significant (ρ = −0.15; P = .27). However, both HIV status and heroin use status were associated with bone marrow SUV (ρ = −0.14 [P = .04] and ρ = −0.21 [P < .01], respectively). Participants with HIV had 9% lower bone marrow SUV compared with those without HIV (1.54 vs 1.68; P = .04; unadjusted estimated means for HIV+ vs HIV−). The association with HIV status remained in models adjusted for demographics only (age, sex, race, and heroin-use-by-sex interaction) (ρ = −0.15; P = .03), and after adjustment for amphetamines, cocaine, marijuana, smoking, and alcohol (P ≤ .04 for all), but not when adjusted for ASCVD risk score, BMI, and hepatitis C (ρ = −0.11; P = .11). The association between heroin use and bone marrow SUV was modified by sex (heroin-use-by-sex interaction ρ = 0.43; P < .01). Among men, bone marrow SUV was similar regardless of heroin-use status (1.54 vs 1.67; P = .39; adjusted estimated means for heroin+ vs heroin−); however, for women, bone marrow SUV was significantly lower in those who used heroin (1.37 vs 1.93; P < .01; adjusted estimated means for heroin+ vs heroin−). Supplementary Figure 1 shows bone marrow SUV by group further subdivided by sex. (See Supplementary Tables 2, 3B, and 3C for models described above.)
DISCUSSION
To our knowledge, this is the first study to assess the effect of heroin use on FDG-PET/CT–measured arterial inflammation and splenic/bone marrow uptake. We have shown that using heroin is associated with higher vascular inflammation, but only in the setting of concurrent HIV infection. Paradoxically, among participants with HIV, heroin use was also associated with lower bone marrow inflammation. The low bone marrow uptake was particularly apparent among women who use heroin.
One key finding is that, among participants with HIV, those who use heroin had greater vascular inflammation than those who do not use heroin. Because differences in vascular inflammation were not seen based on heroin-use status overall, it is apparent that risk factors such as hepatitis C and smoking cannot be entirely to blame, as these factors were highly prevalent in heroin users, regardless of HIV status. Furthermore, even adjustment for ASCVD risk score did not change the results, suggesting that the risk extends beyond traditional CVD risk factors. Adjustment for concurrent substance use also did not change the results, with the exception of current marijuana use. Interestingly, in the general population, recent evidence suggests that marijuana may have detrimental effects on CVD risk. Tetrahydrocannabinol binds to a receptor called CB1 on cells in the brain, heart, and vasculature. Regular marijuana use causes inappropriate activation of CB1, which can cause inflammation and atherosclerosis, and it is associated with obesity and diabetes [25]. This opposing evidence suggests further study on the effect of marijuana on vascular inflammation is necessary in PWH.
One possibility for the finding of higher vascular inflammation in participants who use heroin but only in the context of HIV is that, because injection drug use and HIV are both associated with heightened systemic inflammation, their effects on endothelial cell activation and vascular inflammation are synergistic [14, 26]. Systemic inflammation related to injection drug use is likely multifactorial, with contributions from injection intensity, injection-related injury, infection, heroin type, injection practices, other high-risk behaviors, and environmental factors such as low individual and community socioeconomic status [14, 27–29]. While there is limited research quantifying this heightened systemic inflammation, data from the AIDS Linked to the IntraVenous Experience Study suggest that injection intensity is associated with elevated high-sensitivity C-reactive protein (hsCRP) in a dose–response fashion [14]. In the same cohort, interleukin-6 (IL-6) levels were higher in participants injecting at least daily compared with those with no injection in the last 6 months. HIV infection, even despite virologic suppression, has been associated with elevation of similar proinflammatory cytokines [30]. While this is a theoretical risk, this synergistic effect was not apparent in our study population as we have previously reported [11]. The 1 important exception was that the proportion of patrolling monocytes (CD14dimCD16+) were lowest in participants with HIV who used heroin [11]. These cells have the unique responsibility of patrolling the vasculature along the endothelium and play a role in mitigating damage [31, 32]. However, low numbers of patrolling monocytes in peripheral blood may not reflect low numbers in the vasculature.
Another consideration is that both HIV and opioids may lead to increased microbial translocation across a leaky gut, resulting in greater immune dysregulation than with either insult alone. It is clear that HIV-associated immune activation stems from depletion of CD4+ T cells in gut-associated lymphoid tissue, alteration in intestinal integrity, and resultant microbial translocation [18–20]. There is limited research regarding the effect of opioids on gut integrity [10]; however, alteration of the gut microbiome is a possible mechanism [33]. Further study using colonic tissue and of the microbiome are needed.
In this study we also evaluated splenic and bone marrow inflammation. Splenic and bone marrow FDG uptake quantifies hematopoietic tissue activation by measuring glycolysis of highly metabolic cells including immune system cells. In opposition to the effect seen in the aorta, heroin use was associated with lower bone marrow inflammation, a finding that was particularly apparent in women. These findings are paradoxical because in other disease processes such as post-acute coronary syndrome and other inflammatory diseases such as psoriasis splenic and bone marrow uptake are positively correlated with arterial wall uptake [34–36]. For example, both splenic and bone marrow FDG uptakes were increased in participants with psoriasis compared with healthy controls and were correlated with coronary artery plaque characteristics [35]. Further, after myocardial infarction, the so-called cardiosplenic axis has been used to predict risk of future CVD events [34]. It is not clear why there should be divergent effects in the arterial wall and hematopoietic tissues based on heroin-use status. However, in a cross-sectional study evaluating immunologic correlates of both arterial and lymph node inflammation measured by FDG-PEG/CT in PWH, the authors suggested these 2 sites did not share underlying pathways of immune activation as measures of HIV disease activity and persistence were associated with lymph node, but not arterial, inflammation [23]. Both endogenous and exogenous opioids are capable of suppressing both the innate and acquired immune system [37, 38]. It is possible that the divergent effects relate to the distribution of opioid receptors or the effect of opioids on expression of cytokines/chemokines in different compartments [39].
Our study has some limitations. In this cross-sectional study, we can only infer association and not causation. Further, given the limited sample size, it was not possible to adjust for all potential confounders using a single model for each outcome. However, it should be noted that most studies reporting on FDG-PET/CT within the context of HIV have even smaller sample sizes (N < 100).
In conclusion, in the first study to assess the effect of heroin use on FDG-PET/CT–measured arterial and splenic/bone marrow inflammation, we found that, among participants with HIV but not in uninfected controls, those who use heroin had the greatest vascular but lowest bone marrow inflammation. Low bone marrow inflammation was associated with heroin use overall, but particularly in women. These findings suggest complex and possibly divergent pathophysiology within these different end organs.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Corrilynn O Hileman, Department of Medicine, Division of Infectious Disease, MetroHealth Medical Center, Cleveland, Ohio, USA; School of Medicine, Case Western Reserve University, Cleveland, Ohio, USA.
Jared C Durieux, Department of Medicine and Pediatrics, Division of Infectious Disease, University Hospitals Cleveland Medical Center, Cleveland, Ohio, USA.
Scott E Janus, Harrington Heart and Vascular Institute, University Hospitals Cleveland Medical Center, Cleveland, Ohio, USA.
Emily Bowman, School of Health and Rehabilitation Sciences, Division of Medical Laboratory Science, The Ohio State University, Columbus, Ohio, USA.
Aaren Kettelhut, School of Health and Rehabilitation Sciences, Division of Medical Laboratory Science, The Ohio State University, Columbus, Ohio, USA.
Trong-Tuong Nguyen, Department of Medicine, Division of Infectious Disease, MetroHealth Medical Center, Cleveland, Ohio, USA.
Ann K Avery, Department of Medicine, Division of Infectious Disease, MetroHealth Medical Center, Cleveland, Ohio, USA; School of Medicine, Case Western Reserve University, Cleveland, Ohio, USA.
Nicholas Funderburg, School of Health and Rehabilitation Sciences, Division of Medical Laboratory Science, The Ohio State University, Columbus, Ohio, USA.
Claire Sullivan, School of Medicine, Case Western Reserve University, Cleveland, Ohio, USA; Harrington Heart and Vascular Institute, University Hospitals Cleveland Medical Center, Cleveland, Ohio, USA.
Grace A McComsey, School of Medicine, Case Western Reserve University, Cleveland, Ohio, USA; Department of Medicine and Pediatrics, Division of Infectious Disease, University Hospitals Cleveland Medical Center, Cleveland, Ohio, USA.
Notes
Disclaimer. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Financial support. This work was supported by the National Institutes of Drug Abuse (NIDA) at the National Institutes of Health (NIH; grant number R01DA044576; to C. O. H. and G. A. M.) and the Clinical and Translational Science Collaborative of Cleveland (grant number UL1TR002548) from the National Center for Advancing Translational Sciences component of the NIH and NIH roadmap for Medical Research.
References
- 1. Teeraananchai S, Kerr SJ, Amin J, Ruxrungtham K, Law MG. Life expectancy of HIV-positive people after starting combination antiretroviral therapy: a meta-analysis. HIV Med 2017; 18(4):256–66. [DOI] [PubMed] [Google Scholar]
- 2. Farahani M, Mulinder H, Farahani A, et al. Prevalence and distribution of non-AIDS causes of death among HIV-infected individuals receiving antiretroviral therapy: a systematic review and meta-analysis. Int J STD AIDS 2017; 28(7):636–50. [DOI] [PubMed] [Google Scholar]
- 3. Mdodo R, Frazier EL, Dube SR, et al. Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: cross-sectional surveys. Ann Intern Med 2015; 162:335–44. [DOI] [PubMed] [Google Scholar]
- 4. Sabin CA, Reiss P, Ryom L, et al. Is there continued evidence for an association between Abacavir usage and myocardial infarction risk in individuals with HIV? A cohort collaboration. BMC Med 2016; 14:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. DADS Group; Friis-Moller N, Reiss P, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med 2007; 356:1723–35. [DOI] [PubMed] [Google Scholar]
- 6. Kelesidis T, Kendall MA, Yang OOHodis HN, Currier JS. Biomarkers of microbial translocation and macrophage activation: association with progression of subclinical atherosclerosis in HIV-1 infection. J Infect Dis 2012; 206(10):1558–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tang N, Sun B, Gupta A, Rempel H, Pulliam L. Monocyte exosomes induce adhesion molecules and cytokines via activation of NF-kappaB in endothelial cells. FASEB J 2016; 30(9):3097–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Subramanian S, Tawakol A, Burdo TH, et al. Arterial inflammation in patients with HIV. JAMA 2012; 308:379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Underwood ML, Nguyen T, Uebelhoer LS, Kunkel LE, Korthuis PT, Lancioni CL. Altered monocyte phenotype and dysregulated innate cytokine responses among people living with HIV and opioid-use disorder. AIDS 2020; 34(2):177–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ancuta P, Kamat A, Kunstman KJ, et al. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS One 2008; 3:e2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hileman CO, Bowman ER, Gabriel J, et al. Impact of heroin and HIV on gut integrity and immune activation. J Acquir Immune Defic Syndr 2022; 89:519–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Trickey A, May MT, Vehreschild J, et al. Cause-specific mortality in HIV-positive patients who survived ten years after starting antiretroviral therapy. PLoS One 2016; 11:e0160460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Piggott DA, Varadhan R, Mehta SH, et al. Frailty, inflammation, and mortality among persons aging with HIV infection and injection drug use. J Gerontol A Biol Sci Med Sci 2015; 70: 1542–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Salter ML, Lau B, Mehta SH, Go VF, Leng S, Kirk GD. Correlates of elevated interleukin-6 and C-reactive protein in persons with or at high risk for HCV and HIV infections. J Acquir Immune Defic Syndr 2013; 64(5):488–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chun TW, Nickle DC, Justement JS, et al. Persistence of HIV in gut-associated lymphoid tissue despite long-term antiretroviral therapy. J Infect Dis 2008; 197:714–20. [DOI] [PubMed] [Google Scholar]
- 16. Zidar DA, Juchnowski S, Ferrari B, et al. Oxidized LDL levels are increased in HIV infection and may drive monocyte activation. J Acquir Immune Defic Syndr 2015; 69:154–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Naeger DM, Martin JN, Sinclair E, et al. Cytomegalovirus-specific T cells persist at very high levels during long-term antiretroviral treatment of HIV disease. PLoS One 2010; 5:e8886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006; 12:1365–71. [DOI] [PubMed] [Google Scholar]
- 19. Nazli A, Chan O, Dobson-Belaire WN, et al. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog 2010; 6:e1000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jiang W, Lederman MM, Hunt P, et al. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J Infect Dis 2009; 199:1177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tarkin JM, Joshi FR, Rudd JH. PET imaging of inflammation in atherosclerosis. Nat Rev Cardiol 2014; 11:443–57. [DOI] [PubMed] [Google Scholar]
- 22. Longenecker CT, Sullivan CE, Morrison J, et al. The effects of HIV and smoking on aortic and splenic inflammation. AIDS 2018; 32:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tawakol A, Ishai A, Li D, et al. Association of arterial and lymph node inflammation with distinct inflammatory pathways in human immunodeficiency virus infection. JAMA Cardiol 2017; 2:163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lawal IO, Ankrah AO, Popoola GO, Lengana T, Sathekge MM. Arterial inflammation in young patients with human immunodeficiency virus infection: a cross-sectional study using F-18 FDG PET/CT. J Nucl Cardiol 2019; 26(4):1258–65. [DOI] [PubMed] [Google Scholar]
- 25. Wei TT, Chandy M, Nishiga M, et al. Cannabinoid receptor 1 antagonist genistein attenuates marijuana-induced vascular inflammation. Cell 2022; 185:1676–93.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sinha A, Ma Y, Scherzer R, et al. Role of T-cell dysfunction, inflammation, and coagulation in microvascular disease in HIV. J Am Heart Assoc 2016; 5:e004243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Summers PJ, Struve IA, Wilkes MS, Rees VW. Injection-site vein loss and soft tissue abscesses associated with black tar heroin injection: a cross-sectional study of two distinct populations in USA. Int J Drug Policy 2017; 39:21–7. [DOI] [PubMed] [Google Scholar]
- 28. Kidd SE, Grey JA, Torrone EA, Weinstock HS. Increased methamphetamine. Injection drug, and heroin use among women and heterosexual men with primary and secondary syphilis—United States 2013-2017. MMWR Morb Mortal Wkly Rep 2019; 68:144–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Petersen KL, Marsland AL, Flory J, Votruba-Drzal E, Muldoon MF, Manuck SB. Community socioeconomic status is associated with circulating interleukin-6 and C-reactive protein. Psychosom Med 2008; 70(6):646–52. [DOI] [PubMed] [Google Scholar]
- 30. Masyuko SJ, Page ST, Polyak SJ, et al. Human immunodeficiency virus is associated with higher levels of systemic inflammation among Kenyan adults despite viral suppression. Clin Infect Dis 2021; 73:e2034– e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Auffray C, Fogg D, Garfa M, et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 2007;317:666–70. [DOI] [PubMed] [Google Scholar]
- 32. Thomas G, Tacke R, Hedrick CC, Hanna RN. Nonclassical patrolling monocyte function in the vasculature. Arterioscler Thromb Vasc Biol 2015; 35(6):1306–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cruz-Lebron A, Johnson R, Mazahery C, et al. Chronic opioid use modulates human enteric microbiota and intestinal barrier integrity. Gut Microbes 2021; 13:1946368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Emami H, Singh P, MacNabb M, et al. Splenic metabolic activity predicts risk of future cardiovascular events: demonstration of a cardiosplenic axis in humans. JACC Cardiovasc Imaging 2015; 8:121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Patel NH, Osborne MT, Teague H, et al. Heightened splenic and bone marrow uptake of (18)F-FDG PET/CT is associated with systemic inflammation and subclinical atherosclerosis by CCTA in psoriasis: an observational study. Atherosclerosis 2021; 339:20–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim EJ, Kim S, Kang DO, Seo HS. Metabolic activity of the spleen and bone marrow in patients with acute myocardial infarction evaluated by 18f-fluorodeoxyglucose positron emission tomographic imaging. Circ Cardiovasc Imaging 2014; 7(3):454–60. [DOI] [PubMed] [Google Scholar]
- 37. Wen S, Jiang Y, Liang S, Cheng Z, Zhu X, Guo Q. Opioids regulate the immune system: focusing on macrophages and their organelles. Front Pharmacol 2021; 12:814241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Eisenstein TK, Hilburger ME. Opioid modulation of immune responses: effects on phagocyte and lymphoid cell populations. J Neuroimmunol 1998; 83:36–44. [DOI] [PubMed] [Google Scholar]
- 39. Xu N, Wang Y, Zhao S, et al. Naltrexone (NTX) relieves inflammation in the collagen-induced-arthritis (CIA) rat models through regulating TLR4/NFkappaB signaling pathway. Int Immunopharmacol 2020; 79:106056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.